-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Tissue-Specific RNA Expression Marks Distant-Acting Developmental Enhancers
Up to 80% of mammalian genomes are actively transcribed, producing large numbers of non-coding RNAs without known functions. One particularly exciting category of such non-coding transcripts are the recently discovered enhancer RNAs (eRNAs) transcribed from distant-acting enhancer elements. Studies in cell-based paradigms suggest a functional requirement for such eRNA in enhancer-mediated gene regulation. In this study, we explored the in vivo expression dynamics of tissue-specific non-coding RNAs in embryonic mouse tissues via in-depth transcriptome profiling. Our results suggest that enhancers may be a predominant function associated with differentially expressed non-coding loci across developing tissues, and that differential eRNA expression signatures from total RNA-Seq can be used to identify uncharacterized tissue-specific in vivo enhancers independent of known epigenomic marks. Our results highlight the widespread and potentially important role of eRNAs in orchestrating gene expression and the necessity for functional studies in interpreting genome-wide enhancer predictions.
Published in the journal: . PLoS Genet 10(9): e32767. doi:10.1371/journal.pgen.1004610
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1004610Summary
Up to 80% of mammalian genomes are actively transcribed, producing large numbers of non-coding RNAs without known functions. One particularly exciting category of such non-coding transcripts are the recently discovered enhancer RNAs (eRNAs) transcribed from distant-acting enhancer elements. Studies in cell-based paradigms suggest a functional requirement for such eRNA in enhancer-mediated gene regulation. In this study, we explored the in vivo expression dynamics of tissue-specific non-coding RNAs in embryonic mouse tissues via in-depth transcriptome profiling. Our results suggest that enhancers may be a predominant function associated with differentially expressed non-coding loci across developing tissues, and that differential eRNA expression signatures from total RNA-Seq can be used to identify uncharacterized tissue-specific in vivo enhancers independent of known epigenomic marks. Our results highlight the widespread and potentially important role of eRNAs in orchestrating gene expression and the necessity for functional studies in interpreting genome-wide enhancer predictions.
Introduction
Development and function of mammalian tissues rely on the dynamic control of tissue-specific gene expression, a process largely regulated by distant-acting transcriptional enhancers [1]–[3]. Disruption of enhancer sequences can lead to severe phenotypes in mouse models [4]–[9]. Furthermore, population-scale genetic studies indicate that a large proportion of sequence variants associated with human diseases affect non-coding functions in the genome, of which enhancers are a major category [10]. Despite their functional relevance, the genome-scale identification of enhancers that are active in vivo in developmental and disease processes remains challenging. In principle, genome-wide profiling of enhancer-associated epigenomic marks (e.g. H3K27ac and CBP/p300) enables the genome-scale identification of enhancers predicted to be active in a given cell type or tissue [2], [3], [11]–[15]. However, none of these marks is unique to enhancer regions or found at all enhancers and ChIP-based technology has well-documented limitations with sensitivity and specificity [3], [14]–[16].
Recently, expression of short non-coding transcripts has been described as a feature of many enhancers with a possible tight correlation between cell type-specific enhancer activity and eRNA expression levels [17]–[20]. Using cap analysis of gene expression (CAGE) in a collection of human tissues and cell type, Andersson et al. [21] identified over 40,000 candidate enhancers marked by bidirectional capped RNA expression suggesting that RNA transcription can provide a complementary approach for de novo enhancer discovery. Anecdotal evidence suggests a functional requirement for such eRNAs in enhancer-mediated gene regulation [22], [23]. Regardless of the molecular mechanisms underlying eRNA-mediated regulatory functions, the prevalence of eRNA transcription at the whole transcriptome level in vivo and whether eRNA expression signatures can potentially be used as an independent mark for in vivo enhancer discovery remain poorly explored.
In this study, we compare eRNA expression profiles determined via total RNA sequencing across developmental mouse tissues and demonstrate highly tissue-specific genome-wide expression signatures of eRNAs in vivo. We find that eRNA expression globally correlates with tissue-specific enhancer activity and that RNAs transcribed from in vivo enhancers constitute a major proportion of tissue-specifically expressed non-coding RNAs. Finally, we demonstrate through application of reporter assays in transgenic mice that differential expression of eRNAs can correctly predict tissue-specific in vivo enhancer activities independent of other chromatin-associated marks.
Results
Tissue-specific eRNA expression in developing tissues
To test the hypothesis that eRNA transcription marks active in vivo enhancers in a tissue-specific manner, we first measured eRNA expression from 15 intergenic enhancers active in mouse embryonic forebrain or limb buds that were randomly selected from a larger collection of previously identified in vivo enhancers [24]. We assessed eRNA expression from each enhancer by quantitative RT-PCR across three different embryonic mouse tissues including forebrain, limb, and heart as a negative control (Figure 1). While baseline expression of each eRNA was detected in all three tissues, in 80% of cases eRNAs from tissue-specific enhancers showed highest expression in the predicted tissue compared with the other two tissues (12/15; p = 0.0006, Fisher's exact test), suggesting that eRNAs are commonly expressed from tissue-specific developmental enhancers with a quantitative relationship between eRNA transcription and tissue-specific enhancer activity.
Fig. 1. Tissue-specific eRNA expression at a subset of tissue-specific in vivo enhancers. 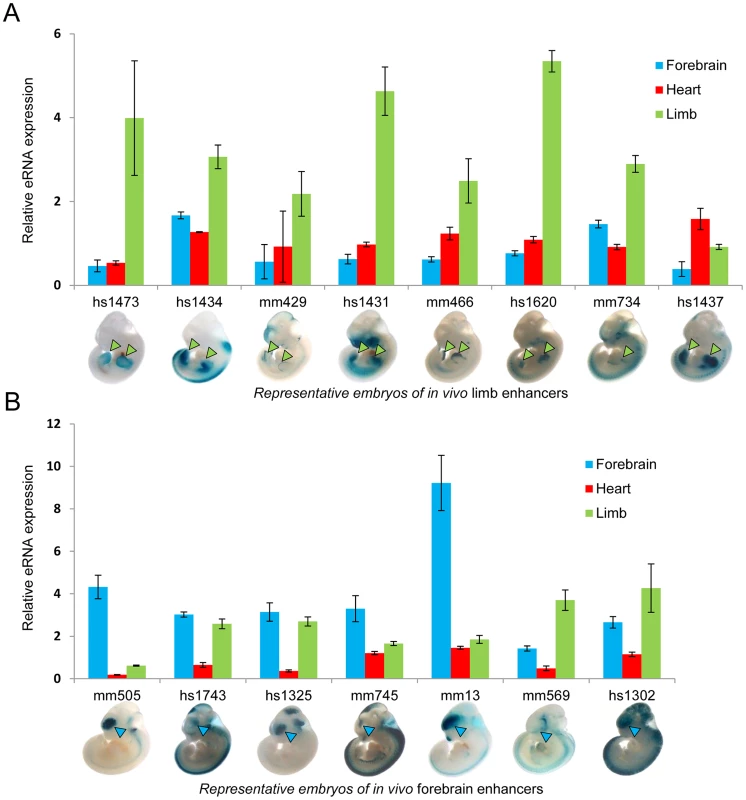
(A) The expression of eRNAs was quantified by RT-PCR for 8 randomly selected known limb enhancers in three tissues. (B) Tissue-specific eRNA expression from 7 known forebrain-specific enhancers. The expression of eRNAs were quantified by RT-PCR for 7 randomly selected forebrain enhancers in three tissues. Results from triplicate experiments were plotted (forebrain: blue; heart: red; limb: green). Error bars represent SEM. Representative LacZ-stained embryos at E11.5 from transgenic assays for individual elements are shown at the bottom. Arrowheads indicate reproducible LacZ staining patters in limb (green) or forebrain (blue). To study eRNA expression from in vivo enhancers beyond this small-scale qPCR screen, we examined genome-wide total RNA transcription in embryonic heart and limb, two tissues with different developmental origins and trajectories, and with divergent in vivo enhancer landscapes as assessed by epigenomic marks [25]–[27]. We extracted total RNA from limb and heart tissues microdissected at mouse embryonic day [E] 11.5. Following ribosomal RNA depletion, we used a strand-specific total RNA sequencing protocol to generate more than 200 million sequencing reads from each tissue (see Methods, Table S1). While the majority of sequencing reads (53% in heart, 60% in limb) mapped to annotated mouse cDNA sequences, a considerable proportion (38% in heart, 30% in limb) mapped to introns as well as intergenic regions, consistent with a possible association with in vivo enhancers. Examination of individual genomic loci containing known enhancers revealed examples of bidirectional tissue-specific eRNA expression from validated intergenic and intragenic enhancers consistent with their in vivo activity (Figure 2A and Figure S1). These results indicate widespread transcription from non-coding sequences in vivo and anecdotally support correlation of in vivo enhancer activity with tissue-specific eRNA transcription.
Fig. 2. Global eRNA expression profiles. 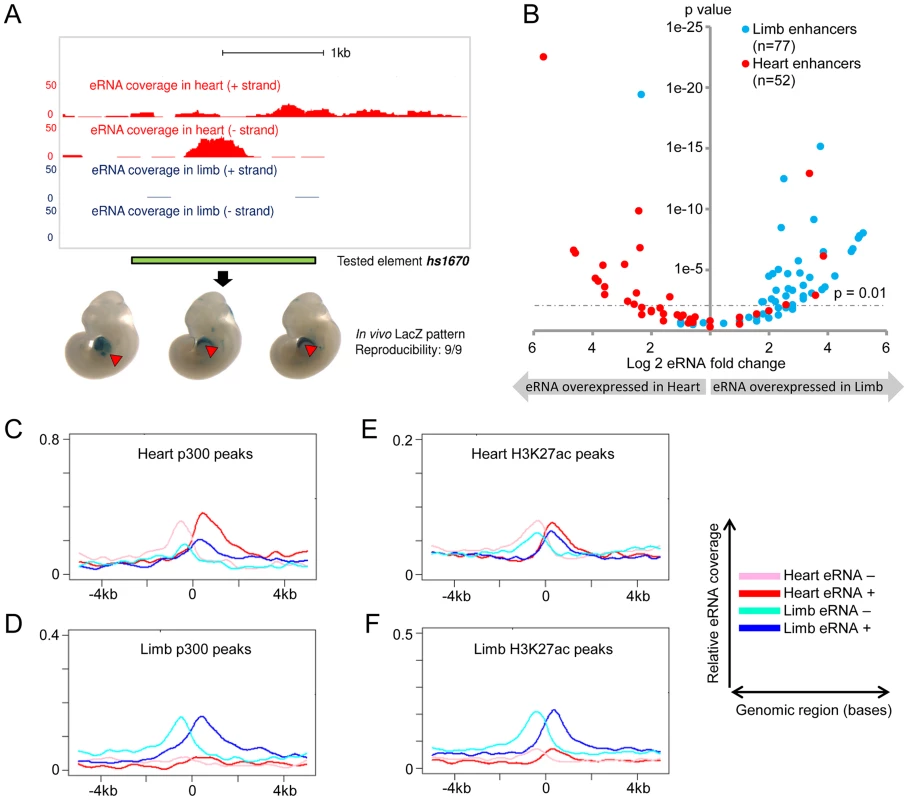
(A) Tissue- and strand-specific eRNA expression around a known heart enhancer (hs1670). Scales corresponding to read count are shown on the left. Genomic region cloned for the transgenic reporter assay is indicated by the green bar. Representative LacZ-stained embryos at E11.5 from transgenic assays for element hs1670 are shown at the bottom. Red arrowheads indicate reproducible LacZ staining pattern in heart. (B) Differential eRNA expression at known heart- or limb-specific enhancers correlates with the tissue-specificity of in vivo enhancer activities. Log2-transformed expression fold-changes of eRNAs arising from heart- (red) or limb-specific (cyan) enhancers are plotted against their associated p-value for each fold change (see Methods). (C–F) Cumulative strand-specific eRNA expression across candidate enhancers in a 10 kb window centered on p300 (C/D) or H3K27ac (E/F) ChIP-Seq peaks from the respective tissue. Sequencing reads mapped to forward strand (red in heart, blue in limb) or reverse strand (pink in heart, cyan in limb) are displayed separately. Expression of eRNA correlates with enhancer activity
In order to assess tissue-specific eRNA expression more systematically, we examined eRNA expression associated with a large collection of in vivo-validated tissue-specific enhancers [24], [26], [28] (http://enhancer.lbl.gov). To avoid confounding factors arising from the presence of pre-mRNAs, we restricted this analysis to intergenic in vivo enhancers (see Methods). We examined a total of 145 such enhancers that are active in heart or limb. In general, enhancers were substantially enriched in uniquely mapped reads, and they were nine times as likely as random non-coding regions to contain ten or more independent reads within 1 kb of the enhancer midpoint (p = 5.5E-108 based on background distribution; see Table S2 and Methods). While 41% of enhancers met this stringent threshold, overall 92% of enhancers showed evidence of at least weak transcription (≥1 uniquely mapped reads; p = 2.3E-15 based on background distribution, see Table S2 and Methods). Consistent with our small-scale sampling of enhancers by quantitative PCR (Figure 1), 79% of heart enhancers and 83% of limb enhancers showed higher eRNA expression in the tissue where enhancer activity was observed in vivo (Figure 2B; p<10−8, Fisher's exact test). We next examined tissue-derived RNA signatures at intergenic regions enriched for enhancer-associated p300 and H3K27ac epigenomic marks [27], [29] from the same tissues (see Methods). Similar to known in vivo enhancers, eRNA transcription was highly enriched around the center regions defined by ChIP-Seq, and tissue-specific eRNA expression patterns correlated with the predicted enhancer activity based on tissue-specific p300 or H3K27ac signature in the same tissues (Figure 2C–F; See Methods). This global correlation between tissue-specific eRNA expression and enhancer activity corroborates previous observations derived from CAGE analysis of human cell types and tissues [21] and supports the possibility that eRNA expression profiling from tissues may provide an effective approach for identifying tissue-specific in vivo enhancers.
De novo discovery of tissue-specific non-coding RNA expression
To explore the potential of eRNA profiling for de novo enhancer discovery, we first used a sliding window approach to identify candidate intergenic regions enriched for RNA expression. Known coding and intronic regions and unannotated transcripts were removed, which led to the identification of 3,422 and 3,775 intergenic regions in heart and limb, respectively, that showed marked RNA expression at a conservatively chosen threshold of ≥10 uniquely mapped reads (see Methods; Figure S2A–B and Table S2). These regions included 834 heart-specific and 1,078 limb-specific loci (tissue-specifically transcribed regions, TSTRs) that were differentially expressed in these two tissues (Figure 3A–B and Table S3). Most of these ∼2,000 TSTRs were located distal to the nearest transcription start site (Figure S2C). There is substantial overlap between TSTRs identified from developing mouse tissues in this study and candidate transcription start sites (TSSs) captured by CAGE from mouse cells and tissues [30]. Overall, 45% of heart TSTRs and 55% of limb TSTRs overlap with at least one CAGE-derived TSS candidate. This represents a strong enrichment compared to random control sequences (8% and 8.3%, respectively; p<4.3E-68, Fisher's exact test, see Methods), but also indicates that large numbers of additional enhancer candidates were identified by analysis of ex vivo tissue at relevant developmental stages. Tissue-specific expression of a panel of 22 candidate TSTRs was tested and in all cases confirmed by quantitative RT-PCR (Figure 3C–D, see Methods), demonstrating that these RNA-seq data sets accurately identified non-coding TSTRs across tissues.
Fig. 3. De novo identification of tissue-specifically transcribed regions. 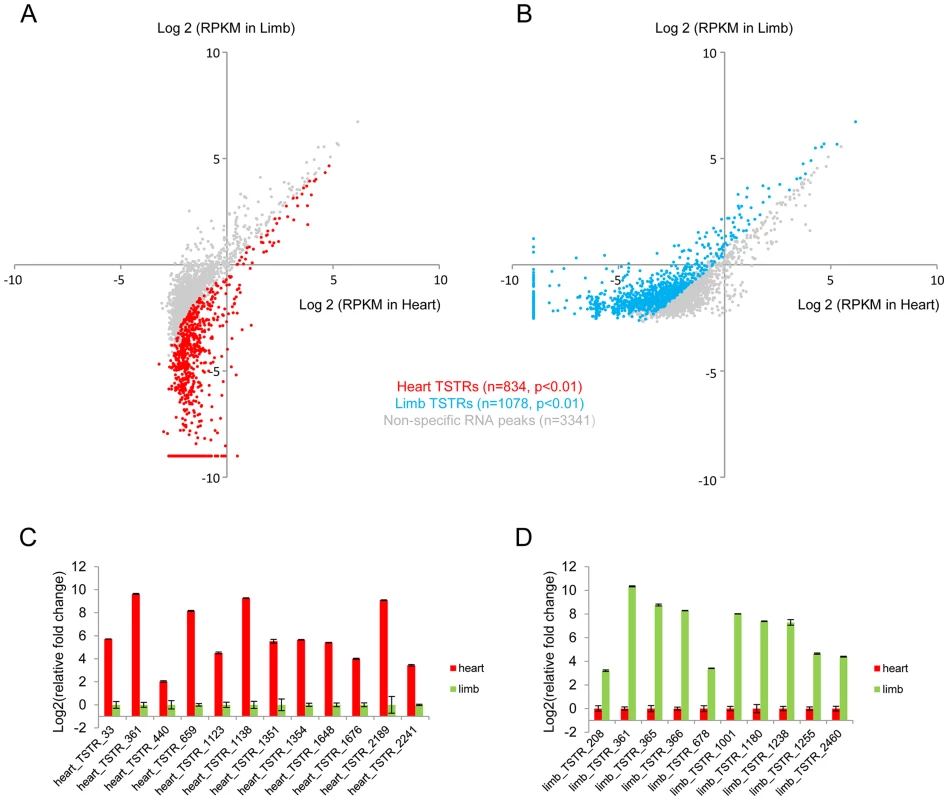
Dot plot showing all TSTRs identified by total RNA-Seq from heart (A) and limb (B) E11.5 tissues. Cyan and red dots indicate limb- or heart-specific TSTRs (p<0.01). Grey dots indicate RNA peaks without significant expression differences between the two tissues. RPKM<2−9 were arbitrarily set to 2−9 for visualization purposes (see Methods). A total of 22 candidate TSTRs were selected from heart (C) or limb (D) TSTRs. Tissue-specific RNA expression were quantified by RT-PCR by using total RNA samples from heart or limb tissues at E11.5 (see Methods). Error bars represent SEM. TSTRs are associated with candidate in vivo enhancers
To assess whether these TSTRs may represent in vivo enhancers, we first examined their evolutionary sequence constraint, a feature associated with many distant-acting enhancers [25], [31], [32]. We found that 69% and 73% of TSTRs in heart and limb, respectively, overlap with elements under evolutionary constraint as compared to 28% and 27% of random control sequences (p<2.0E-62, Fisher's exact test; Figure 4A and Figure S2D). Additionally, heart TSTRs are enriched near genes critical for cardiovascular and heart development, whereas limb TSTRs are enriched near genes involved in muscle tissue development and limb development/morphogenesis (Table 1). Heart and limb TSTRs are also enriched for different sets of transcription factor binding motifs related to development of the respective tissues compared with random genomic sequences (Table S5 and Table S6). Finally, we compared tissue-specific TSTR expression with mRNA levels of nearby genes in two tissues (see Methods). The strongest correlation was observed between TSTRs and their nearest genes (Pearson correlation: R = 0.68 for heart, R = 0.55 for limb), and decreased substantially for more distant genes (Figure 4B). These results support that TSTRs may represent regulatory elements coordinating the transcription of nearby genes.
Fig. 4. Intergenic regions marked by tissue-specific RNA expression may represent regulatory enhancer elements. 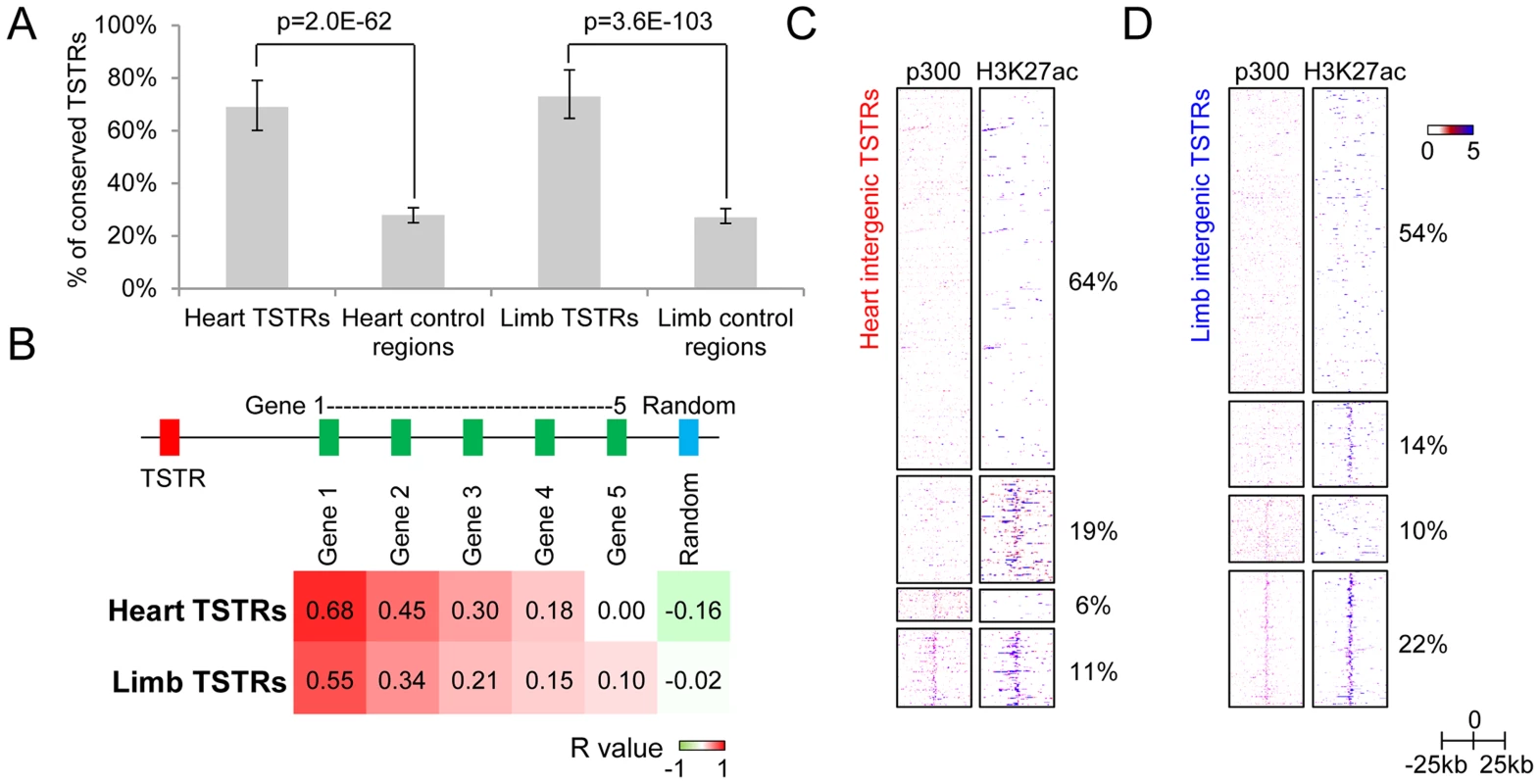
(A) Fraction of TSTRs or random control regions (all size normalized to 1 kb from center) that are under strong evolutionary constraint (30 vertebrate phastCons; see Methods). Error bars represent 95% binomial proportion confidence interval. (B) Heatmap of Pearson correlation coefficient between tissue-specificity of TSTRs and nearby genes (see Methods). Genes 1 to 5 indicates the first to the fifth closest genes to the corresponding TSTR regardless of strand. For comparison, correlation with random genes on the same chromosome as the TSTR is shown. (C and D) Heatmap of p300 binding and H3K27ac signal within a −25 kb to +25 kb window surrounding the center of all heart TSTRs (C) or all limb TSTRs (D). Each line represents a single TSTR for individual tissues, and color scale indicates the normalized signal from individual ChIP-Seq experiment (see Methods). Tab. 1. Top 10 GO biological processes enriched in genes nearby TSTRs (sorted by p-value). 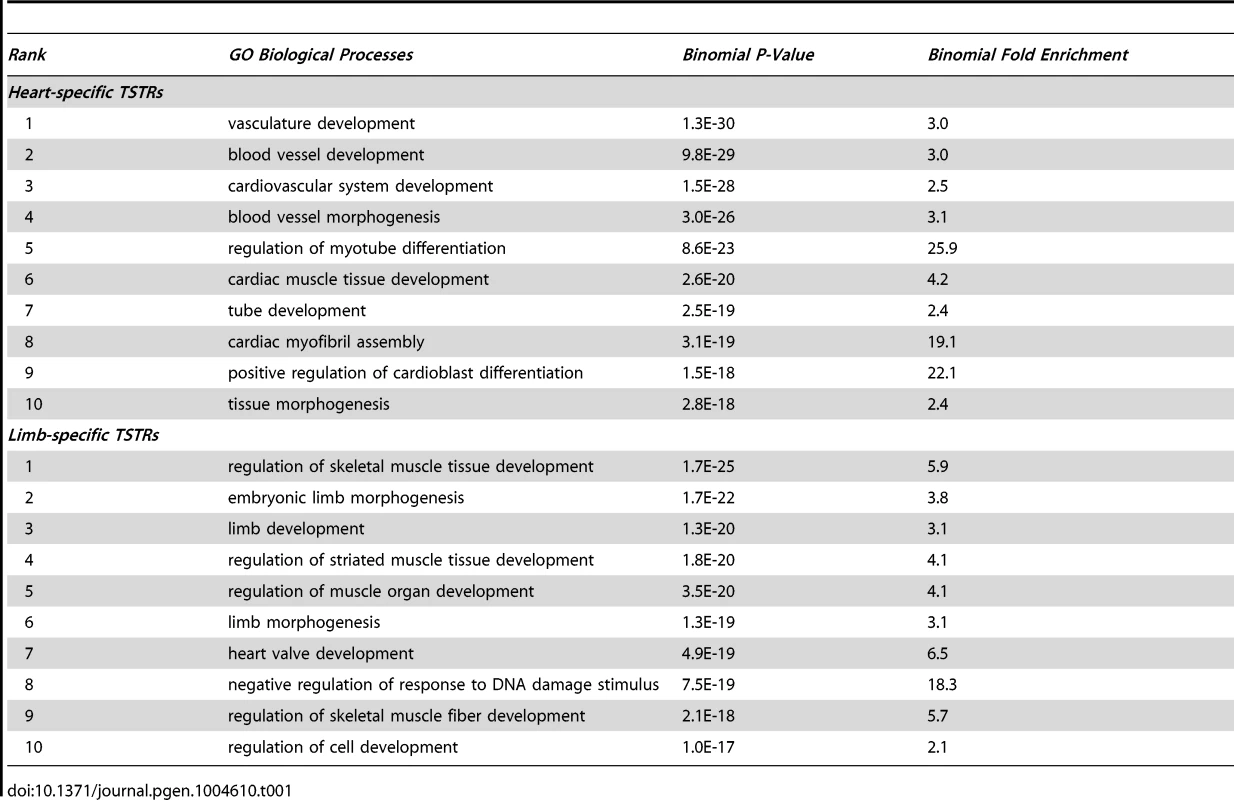
To evaluate the overlap of TSTRs with enhancer-associated epigenomic marks, we examined p300 and H3K27ac enrichment (Figure 4C–D). We find that 36% and 46% of heart and limb TSTRs are marked by p300 and/or H3K27ac. TSTRs with and without epigenomic enhancer marks show similar expression level and substantial evolutionary constraint (Figure 4A and Figure S3A–B). However, the transcription of TSTRs with enhancer marks tends to be more balanced in both directions, whereas TSTRs marked by tissue-specific RNAs only are more biased toward one direction (Figure S3C–D). In addition, TSTRs negative for p300 and/or H3K27ac are more distal to the nearest transcription start sites (Figure S3E). These results indicate a substantial overlap of extragenic TSTRs with enhancer-like regions. However, this does not exclude the possibility that subsets of the observed TSTRs represent other classes of regulatory elements or unannotated non-coding loci.
Transgenic validation of enhancer predictions based on TSTRs
To directly assess the potential of TSTRs identified by transcriptome profiling for the de novo discovery of tissue-specific in vivo enhancers, we used a transgenic mouse enhancer assay previously shown to reliably capture in vivo enhancer activity [25], [27], [33]. In an initial retrospective comparison, we found that heart - or limb-specific TSTRs overlap with 12 tested elements that had previously been examined due to increased conservation or enhancer associated epigenomic marks [24]. Of these elements, 9/12 (75%) were annotated as tissue-specific positive enhancers in vivo (Table S7, http://enhancer.lbl.gov). Next, we performed transgenic mouse assays for another set of 19 TSTRs that had not previously been tested (Table S8) and exhibited tissue-specific RNA expression. This panel included elements both with and without detectable p300 and/or H3K27ac signal in ChIP-Seq experiments (Table S8) that were chosen blind to the identity of nearby genes. Mouse genomic DNA for individual TSTRs with up to 2 kb of flanking sequence was cloned upstream of a minimal heat shock promoter fused to a lacZ reporter gene and transgenic mice were assayed by whole-mount staining for the expression of lacZ reporter at E11.5 [25] (see Methods). Only elements that drove reproducible reporter gene expression pattern in at least three embryos were considered positive enhancers. In total, 8/19 (42%) candidate enhancers predicted by tissue-specific RNA expression functioned as positive enhancers in vivo (Figure 5, Table S8 and Figure S4). In all cases, the observed tissue-specific in vivo enhancer activity was consistent with the tissue specificity of the corresponding TSTR. As representative examples, transgenic whole-mount embryos and transverse sections for elements mm1052, mm1018, mm1054 and mm1064 are shown in Figure 5. In these examples, reproducible LacZ reporter activities were detected in both atrial and ventricular regions of the heart (Figure 5A–C) and anterior regions of the fore - and hindlimb (Figure 5D). Combining the results from newly performed enhancer assays and retrospective comparisons with pre-existing in vivo data sets, 17 of 31 TSTRs (55%) represented in vivo enhancers, and for 15 of these 17 enhancers (88%) the tissue specificity of eRNA expression correctly predicted the in vivo enhancer activity patterns. These results support the general utility of eRNA profiling as an informative mark for in vivo enhancer prediction.
Fig. 5. Transgenic characterization of TSTRs for tissue-specific enhancer activity. 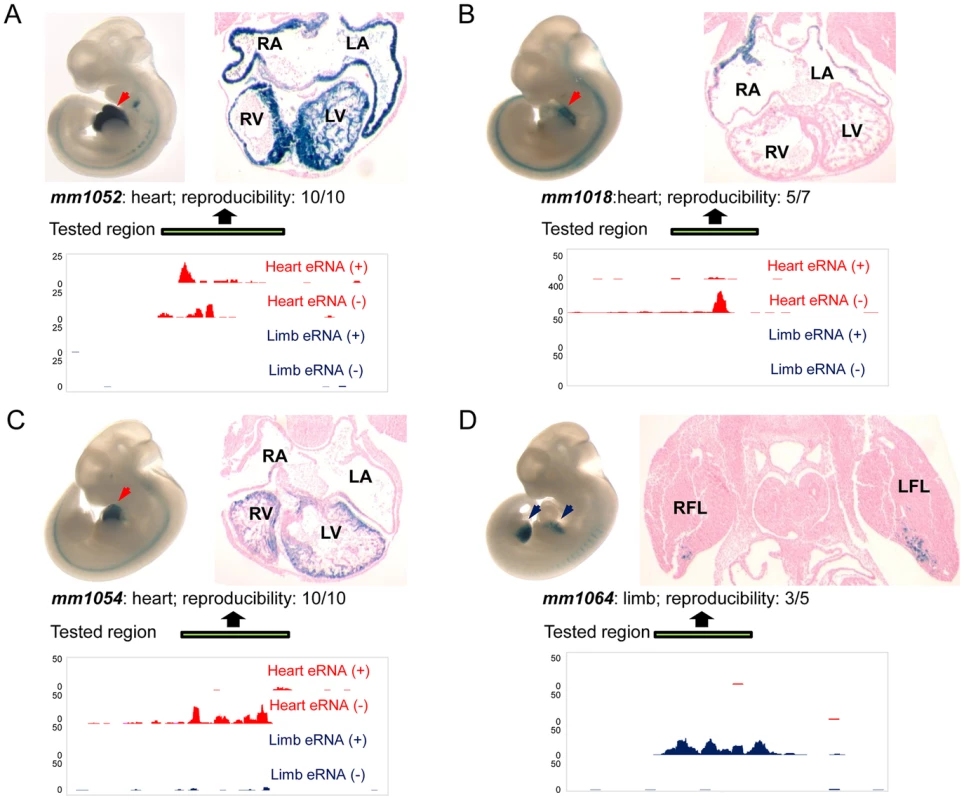
For each tested element, lateral views of whole-mount LacZ-stained embryos at E11.5 are shown in top left panels and transverse sections through heart or limb regions are shown in the top right panels. Arrowheads indicate reproducible LacZ staining pattern in heart (red) or limb (blue). Element ID and reproducibility of expression patterns are indicated at the bottom of the images. Strand-specific eRNA coverage of the tested regions in heart (red) or limb (blue) is shown in the bottom panels. Scales corresponding to read count are shown on the left of the coverage. Genomic regions cloned for the transgenic assay are indicated by green bars. (A) Enhancer element mm1052 with activity in both atrial and ventricular regions. (B) Enhancer element mm1018 shows activity in the right and left atrium. (C) Enhancer element 1054 with activity exclusively in the right and left ventricle. (D) Enhancer element mm1064 is active in the anterior domains of both forelimb and hindlimb, and only transverse section of forelimb is shown as an example. RA: right atrium; LA: left atrium; RV: right ventricle; LV: left ventricle; RFL; right forelimb; LFL: left forelimb. Transgenic results of all tested elements are available through the Vista Enhancer Browser (http://enhancer.lbl.gov). Discussion
Recent large-scale transcriptome studies suggest that up to 80% of mammalian genomes may be actively transcribed [34]–[37]. While many of these transcripts show differential expression signatures across cell types and tissues, the majority of non-coding transcripts have not been associated with in vivo functions. In the present study, we explored the in vivo expression dynamics of tissue-specific non-coding RNAs using a total RNA-Seq strategy that captures both coding and non-coding transcripts [18]. Our results suggest that the majority of enhancers show evidence of tissue-specific eRNA transcription. In addition, de novo identified tissue-specifically transcribed non-coding regions (TSTRs) showed major characteristics of canonical enhancers. These results indicate that enhancers are a predominant function associated with differentially expressed non-coding loci across developing tissues.
CAGE analysis from human cell lines and tissues showed that incorporating enhancer expression data can increase the validation rate of ENCODE enhancer predictions and that bidirectional capped RNA signatures can in principle be used to identify de novo cell-specific enhancers [21]. However, in the absence of sizable in vivo validation data sets, the quantitative correlation between tissue-specific eRNA expression and in vivo enhancer activity in mammalian developmental processes has remained unclear [21]. We have tested a set of 19 candidate enhancers predicted by tissue-specific RNA expression in transgenic mouse assays and 42% showed reproducible enhancer activity in vivo, demonstrating the general utility of eRNA-based enhancer prediction in a developmental mammalian system. Of note, two of the tissue-specific enhancers reported in this study (mm1052 and mm1061) did not overlap with any CAGE peaks collected from 399 mouse samples [30] despite the scope of the tissue and cell type panels examined in these previous studies. Considering the dynamics of the enhancer landscape in developing tissues and organs [29], it appears likely that many additional enhancers active during development will be identifiable by whole transcriptome analysis of tissues across different developmental stages.
While a substantial proportion of extragenic transcription appears linked to enhancer activity, our observation of several TSTRs that were not active in the transgenic enhancer reporter assays supports the hypothesis that eRNA-like transcripts can also originate from other non-coding elements, such as inactive enhancers. These observations are consistent with recent mechanistic studies on eRNAs showing that eRNA transcription precedes the establishment of H3K4me1/2 [38], suggesting that eRNA transcription may occur before enhancer activation. TSTRs without supportive p300/H3K27ac marks show significant, though slightly decreased conservation, less bi-directional transcription, and are more distal to the nearest coding genes (Figure S3), suggesting that they may have different biological functions. Consistent with this observation, a larger proportion of TSTRs with supportive p300/H3K27ac marks were active in vivo compared to TSTRs without such marks, although this difference was not significant at the sample size examined (p = 0.15, Fisher's exact test; Table S8). While the results of our study do not permit strong conclusions about the functionality of intergenic loci that exhibit transcription but no accompanying enhancer epigenomic signatures, it is possible that these regions are less likely to be active enhancers. Transcription may be occurring due to other processes or at a different class of regulatory element than active enhancers. Together, our data suggest that additional criteria such as bi-directional transcription, conservation and independent enhancer marks may further increase the performance of eRNA-based enhancer predictions. Nonetheless, considering the overall substantial correlation between TSTRs and tissue-specific in vivo enhancer activity, our results corroborate that short non-coding transcripts are commonly associated with the regulation of cell type - and tissue-specific gene expression.
Enhancer RNAs may be very unstable and sensitive to exosome degradation [21], [39], resulting in low steady-state level in cells. This may explain why eRNAs represent a small proportion of the transcriptome profile (Figure S2A), despite the large number of sites from which they originate. At current sequencing depth, many enhancers may still be missed (Figure S2B), which is consistent with the notion that a great proportion of mammalian genomes may be actively transcribed and cis-regulatory genomic elements may represent major sites of extragenic non-coding transcription [34]–[37], [39]. Recently, Andersson et al. showed that depletion of a co-factor of the exosome complex resulted in an over 3-fold average increase of eRNA abundance [21]. Thus, a combination of in-depth transcriptome profiling and exosome depletion may provide a more sensitive method for eRNA-based enhancer discovery.
Emerging evidence indicates that eRNA transcripts can be required for enhancer-mediated gene activation. Targeted knock-down of specific eRNAs has been shown to affect the expression of enhancer target genes in cell-based assays, providing a potential strategy for altering gene expression in experimental and therapeutic applications [22], [23], [40]. Through in-depth transcriptome profiling, we have shown extensive eRNA expression in developing tissues, as well as a global correlation of eRNA expression with tissue-specific in vivo enhancer activity. Our results highlight the widespread and potentially important role of eRNAs in orchestrating gene expression, providing support for the general feasibility of eRNA-based targeting of in vivo gene expression.
Methods
All procedures of this study involving animals were reviewed and approved by the Animal Welfare and Research Committee at Lawrence Berkeley National Laboratory.
Mouse tissue collection and RNA preparation
Embryonic heart or limb tissue was isolated from CD-1 strain mouse embryos at E11.5 by microdissection in cold PBS [27]. A single sample consisting of tissue pooled from multiple embryos was analyzed for either tissue. After washing, about 1 ml TRIzol reagent (Life Technologies, 15596-026) was added to every 100 mg of tissue sample, followed by homogenization using a glass dounce homogenizer. Total RNA from individual tissues were extracted following the manufacturer's instructions. Genomic DNA contamination was removed by using the TURBO DNA-free kit (Applied Biosystems, AM1907) following manufacture's protocol, and the RNA samples were stored at −80°C before further processing.
Illumina sequencing of total RNA
In order to perform the transcriptome analysis by Illumina sequencing, ribosomal RNAs was removed from total RNA (5∼10 µg per reaction) by using two rounds of the RiboMinus Eukaryote Kit for RNA-Seq (Life Technologies, A10837-08) following the manufacturer's instructions. The quality of total RNA after rRNA removal was analyzed on RNA 6000 Pico chip (Agilent, 5067-1513) to assure that rRNA contamination was less than 30%. 100 ng total RNA after rRNA removal were used to construct the individual sequencing libraries for Illumina sequencing. Strand-specific RNA-Seq libraries were created following in-house protocols. Briefly, RNA samples were fragmented with 10×Fragment buffer (Ambion, AM9938) to achieve an average fragment size of 200–300 nt. First strand cDNA synthesis was performed with random hexamer and Superscript II reverse transcriptase (Life Technologies, 18064-014). During the second strand synthesis, dUTP was used instead of dTTP to introduce strand-specificity. After adaptor ligation and size selection, the second strand containing dUTP was cleaved by AmpErase UNG (Life Technologies, N8080096). The resulting strand-specific cDNA was subjected to 12 cycles of PCR amplification and sequenced with HiSeq 2000 instrument. 50 sequencing cycles were carried out.
Data processing and de novo peak calling
Raw Illumina reads (50 bp) were first filtered using the Illumina CASAVA-1.8 FASTQ Filter module (http://cancan.cshl.edu/labmembers/gordon/fastq_illumina_filter/). The remaining sequence tags were mapped back to the mouse genome (NCBI build 37, mm9) using bowtie2 [41], and the alignments were extended to 200 bp in the 3′ direction to account for the average length of DNA fragments. Repetitively mapped reads were excluded from the following analysis. For de novo peak calling, a sliding window method EnrichedRegionMaker module from USEQ [42] was employed. For eRNA-based enhancer predictions, a conservative threshold of 10 or more reads (without considering strand specificity) was chosen based on the observation that in retrospective comparison with in vivo validated enhancers, 40.7% of enhancers met or exceeded this expression threshold, compared to 4.5% of random control regions (p = 5.5E-108, Table S2). Enriched regions overlapping with refGene, mouse mRNA, or ESTs (mm9) were also removed before the downstream analysis. This process was performed individually for heart and limb RNA-Seq data. To generate Figure S2B, 10% to 100% of sequencing reads were randomly selected from the raw sequencing data, and de novo peak calling was individually performed to identify the enriched intergenic regions.
Among raw enriched regions, tissue-specifically transcribed regions (TSTRs) were defined as non-coding regions with significantly higher expression in this tissue compared with the other tissue (p<0.01, two-proportion z-test; Figure 3A–B) [43] with the equation shown below:
where (n represents mappable reads within each TSTR in heart or limb, and N represent the total number of mappable reads excluding ribosomal regions in the corresponding tissue) and . RPKM<2−9 were arbitrarily set to 2−9 for visualization purposes in Figure 3A–B.Candidate transcription start sites (TSSs) marked by CAGE peaks were downloaded from http://fantom.gsc.riken.jp/5/ [30] and extended to 1 kb each side from the peak midpoint. For each TSTR (1 kb around the peak center), the overlapping candidate TSSs were identified by BEDTools [44]. Random control peaks were also generated using BEDTools with the same number and size of sequences and excluding known genes, mouse mRNAs and ESTs.
Enhancer predictions based on epigenomic marks
We compared tissue-derived RNA signatures at intergenic regions to enhancer-associated p300 [27] and H3K27ac marks from the same tissues and time-point. H3K27ac ChIP-Seq datasets are described in more detail in Nord et al. [29] and Attanasio et al. [45]. Candidate tissue-specific intergenic enhancers were predicted by ChIP-Seq of p300 (171 in heart, 656 in limb) or H3K27ac (6965 in heart, 2174 in limb) as described previously [27]. Briefly, uniquely aligned sequencing reads were extended to 300 bp in the 3′ direction. Enriched regions (peaks) were identified with MACS [46] (p≤1E-5) using matched input as controls. Peaks overlapping with repetitive regions, known genes, mouse mRNAs and ESTs were removed for further analysis.
Enhancer RNA coverage
Summary eRNA coverage plots were generated for p300 - and/or H3K27ac-derived intergenic enhancers within a 10 kb window, centering on the maximum ChIP-seq coverage. Using the mapped reads, normalized mean eRNA coverage values were calculated for 25 bp windows across the 10 kb regions scaled by total mapped reads. For mean calculations, only the 5th–95th percentiles were used to reduce the effect of outliers. Coverage was calculated separately for antisense and sense reads, and as a combined value. For the summary plots, a loess best fit line was plotted for each of the eRNA datasets (limb and heart), separating into sense and antisense reads (Figure 2C–F).
Conservation
Pre-computed conservation scores (phastCons scores) generated from 30 vertebrate genome alignments were download from the UCSC Genome Browser [47]. For each TSTR (1 kb around the peak center), the conservation score was defined as the most highly constrained overlapping phastCons element in the mouse mm9 genome. Random control peaks were generated using BEDTools with the same number and size of sequences and excluding known genes, mouse mRNAs and ESTs [44]. The percentages of TSTRs and random control regions overlapping phastCons elements were plotted in Figure 4A.
Heatmap generation
Tissue-specific TSTRs were classified as enriched in p300 and/or H3K27ac if the relative ChIP-seq coverage was equal to or greater than the 95th percentile of experiment background coverage estimated across 1 Mb of unique sequence. After classification, coverage heatmaps were generated for ChIP-seq data using normalized coverage values, with input corrections. Coverage was plotted for 25 bp windows centered on the peak RNA coverage and extending 25 kb on either side. For plotting purposes, coverage was centered and scaled using mean and SD in order to compare signal across datasets. TSTRs were organized as no H3K27ac and p300 signal, enriched in H3K27ac signal only, enriched in p300 signal only and enriched in both marks from the top to the bottom in Figure 4C–D.
Expression analysis
Known heart or limb enhancers were downloaded from Vista Enhancer Browser (http://enhancer.lbl.gov). For known enhancer regions, the expression level of individual eRNAs was defined as the mapped sequencing reads within a 2 kb window around the center of in vivo tested enhancers. For eRNAs only expressed in one tissue, the mapped number of reads was arbitrarily set to 1 in the other tissue in order to compute the absolute fold change for plotting purposes in Figure 2B. Fold change was defined as higher expression level divided by lower expression of each eRNA in two tissues. For the volcano plot, y axis represents p-value for the expression differences of each known enhancer, which was computed by two-proportion z-test [43].
Coverage of randomly selected control regions (excluding known genes, mRNA and ESTs) was also computed and iterated 100 times to estimate the genome-wide background based on normal distribution. The percentages of enhancers or the average percentage of control regions with indicated numbers of uniquely mapped reads in either tissue are listed in Table S2, as well as associated p-values.
After peak calling, for each individual TSTR, normalized RPKM (Reads Per Kilobase per Million mapped reads) was calculated in two tissues (heart and limb) with the raw mapped RNA-Seq data within a 2 kb window around the center of each TSTR. Then, a tissue-specificity index was computed as (s−u)/(s+u), in which s is the expression of TSTR in the matching tissue and u is its expression in the other tissue. The expression of mouse refGene (mm9) was also analyzed in the same way by computing the RPKM across annotated cDNA regions in two tissues.
The tissue-specific expression correlation between TSTRs and their nearby genes was computed as described [18] with minor modifications. Briefly, we paired each TSTR with the nearby genes. For each set of genes with the same ranked distance to TSTRs (the first to the fifth closest genes), genes were ranked based on tissue-specificity indices and grouped into 20 genes per bin. Average tissue-specificity indices from each bin were used to compute the correlation. The Pearson correlation between nearby genes and the corresponding TSTRs was conducted with the statistics module in the R package (http://cran.r-project.org/).
Gene ontology analysis
Gene ontology analysis for the genes near TSTR regions was performed by GREAT version 2.02 [48]. Enriched GO biological processes with a binomial p-value and fold enrichment were listed in Table 1.
Motif analysis
For TSTRs in heart and limb, enriched motifs were computed within a 2 kb window around the center of individual TSTRs by the motif finding module of HOMER (Hypergeometric Optimization of Motif EnRichment) [49]. Known motifs for transcription factors with a p-value less than 10−2 compared with random genomic sequences were reporter in Table S5 and Table S6.
Directionality analysis
For directionality analysis, the expression of individual TSTRs in sense and antisense strands was defined as the strand-specific mapped sequencing reads within a 2 kb window around the center of TSTRs in either heart or limb. Then the directionality index was defined as |f−r|/(f+r), in which f is the expression of TSTR in one strand and r is its expression in the other strand in the same tissue.
Expression validation in vivo
Total RNA was extracted from independently collected pools of heart or limb tissues with the same method as described before and synthesized into cDNA by reverse transcription using the SuperScript First-Strand Synthesis System (Invitrogen). Candidate TSTRs for RT-PCR validations were randomly selected from the top 30% differentially expressed regions ranked by Z scores. Expression analysis of candidate TSTRs was carried out by real-time PCR using gene-specific primers (Table S4) and KAPA SYBR FAST qPCR Master Mix (KAPA Biosystems) on a Roche LightCycler 480. All primers were designed in silico using Primer3 (http://primer3.wi.mit.edu/) and tested for amplification efficiency. Target gene expression was calculated with the 2−ΔΔCT method [50] and normalized to the Gapdh housekeeping gene.
In vivo transgenic validation
Candidate enhancers for in vivo testing were selected randomly from TSTRs with a p-value less than 0.01. The tested regions included up to 2 kb genomic DNA flanking the TSTRs on either sides. This general transgenic procedure has been described before [25], [27]. Briefly, the selected regions were PCR amplified from mouse genomic DNA and cloned into the Hsp68-promoter-LacZ reporter [51], [52]. Genomic coordinates and the PCR primers for the cloned regions are listed in Table 8. The transgenic embryos were assayed at E11.5 for expression patterns. A positive enhancer is defined as an element with reproducible expression pattern in at least three embryos resulting from independent transgenic integration events [27]. For histological analysis, selected embryos were embedded in paraffin and sectioned using standard methods.
Data access
RNA-seq data is available through GEO under accession number GSE58157. In vivo transgenic data is available through the Vista Enhancer Browser under the identifiers used throughout this study (http://enhancer.lbl.gov).
Supporting Information
Zdroje
1. OngCT, CorcesVG (2011) Enhancer function: new insights into the regulation of tissue-specific gene expression. Nat Rev Genet 12 : 283–293.
2. ViselA, RubinEM, PennacchioLA (2009) Genomic views of distant-acting enhancers. Nature 461 : 199–205.
3. BueckerC, WysockaJ (2012) Enhancers as information integration hubs in development: lessons from genomics. Trends Genet 28 : 276–284.
4. FurnissD, LetticeLA, TaylorIB, CritchleyPS, GieleH, et al. (2008) A variant in the sonic hedgehog regulatory sequence (ZRS) is associated with triphalangeal thumb and deregulates expression in the developing limb. Hum Mol Genet 17 : 2417–2423.
5. LetticeLA, HeaneySJ, PurdieLA, LiL, de BeerP, et al. (2003) A long-range Shh enhancer regulates expression in the developing limb and fin and is associated with preaxial polydactyly. Hum Mol Genet 12 : 1725–1735.
6. MasuyaH, SezutsuH, SakurabaY, SagaiT, HosoyaM, et al. (2007) A series of ENU-induced single-base substitutions in a long-range cis-element altering Sonic hedgehog expression in the developing mouse limb bud. Genomics 89 : 207–214.
7. SagaiT, HosoyaM, MizushinaY, TamuraM, ShiroishiT (2005) Elimination of a long-range cis-regulatory module causes complete loss of limb-specific Shh expression and truncation of the mouse limb. Development 132 : 797–803.
8. ViselA, ZhuY, MayD, AfzalV, GongE, et al. (2010) Targeted deletion of the 9p21 non-coding coronary artery disease risk interval in mice. Nature 464 : 409–412.
9. AttanasioC, NordAS, ZhuY, BlowMJ, LiZ, et al. (2013) Fine tuning of craniofacial morphology by distant-acting enhancers. Science 342 : 1241006.
10. AbecasisGR, AltshulerD, AutonA, BrooksLD, DurbinRM, et al. (2010) A map of human genome variation from population-scale sequencing. Nature 467 : 1061–1073.
11. CotneyJ, LengJ, OhS, DeMareLE, ReillySK, et al. (2012) Chromatin state signatures associated with tissue-specific gene expression and enhancer activity in the embryonic limb. Genome Res 22 : 1069–1080.
12. HeintzmanND, HonGC, HawkinsRD, KheradpourP, StarkA, et al. (2009) Histone modifications at human enhancers reflect global cell-type-specific gene expression. Nature 459 : 108–112.
13. ErnstJ, KheradpourP, MikkelsenTS, ShoreshN, WardLD, et al. (2011) Mapping and analysis of chromatin state dynamics in nine human cell types. Nature 473 : 43–49.
14. MastonGA, LandtSG, SnyderM, GreenMR (2012) Characterization of Enhancer Function from Genome-Wide Analyses. Annu Rev Genomics Hum Genet
15. NoonanJP, McCallionAS (2010) Genomics of long-range regulatory elements. Annu Rev Genomics Hum Genet 11 : 1–23.
16. ParkPJ (2009) ChIP-seq: advantages and challenges of a maturing technology. Nat Rev Genet 10 : 669–680.
17. WangD, Garcia-BassetsI, BennerC, LiW, SuX, et al. (2011) Reprogramming transcription by distinct classes of enhancers functionally defined by eRNA. Nature 474 : 390–394.
18. KimTK, HembergM, GrayJM, CostaAM, BearDM, et al. (2010) Widespread transcription at neuronal activity-regulated enhancers. Nature 465 : 182–187.
19. ZhuY, SunL, ChenZ, WhitakerJW, WangT, et al. (2013) Predicting enhancer transcription and activity from chromatin modifications. Nucleic Acids Res
20. HahN, MurakamiS, NagariA, DankoCG, KrausWL (2013) Enhancer transcripts mark active estrogen receptor binding sites. Genome Res 23 : 1210–1223.
21. AnderssonR, GebhardC, Miguel-EscaladaI, HoofI, BornholdtJ, et al. (2014) An atlas of active enhancers across human cell types and tissues. Nature 507 : 455–461.
22. LiW, NotaniD, MaQ, TanasaB, NunezE, et al. (2013) Functional roles of enhancer RNAs for oestrogen-dependent transcriptional activation. Nature 498 : 516–520.
23. MeloCA, DrostJ, WijchersPJ, van de WerkenH, de WitE, et al. (2013) eRNAs are required for p53-dependent enhancer activity and gene transcription. Mol Cell 49 : 524–535.
24. ViselA, MinovitskyS, DubchakI, PennacchioLA (2007) VISTA Enhancer Browser–a database of tissue-specific human enhancers. Nucleic Acids Res 35: D88–92.
25. PennacchioLA, AhituvN, MosesAM, PrabhakarS, NobregaMA, et al. (2006) In vivo enhancer analysis of human conserved non-coding sequences. Nature 444 : 499–502.
26. BlowMJ, McCulleyDJ, LiZ, ZhangT, AkiyamaJA, et al. (2010) ChIP-Seq identification of weakly conserved heart enhancers. Nat Genet 42 : 806–810.
27. ViselA, BlowMJ, LiZ, ZhangT, AkiyamaJA, et al. (2009) ChIP-seq accurately predicts tissue-specific activity of enhancers. Nature 457 : 854–858.
28. MayD, BlowMJ, KaplanT, McCulleyDJ, JensenBC, et al. (2011) Large-scale discovery of enhancers from human heart tissue. Nat Genet
29. NordAS, BlowMJ, AttanasioC, AkiyamaJA, HoltA, et al. (2013) Rapid and pervasive changes in genome-wide enhancer usage during mammalian development. Cell 155 : 1521–1531.
30. A promoter-level mammalian expression atlas. Nature 507 : 462–470.
31. ShenY, YueF, McClearyDF, YeZ, EdsallL, et al. (2012) A map of the cis-regulatory sequences in the mouse genome. Nature 488 : 116–120.
32. LiuY, LiuXS, WeiL, AltmanRB, BatzoglouS (2004) Eukaryotic regulatory element conservation analysis and identification using comparative genomics. Genome Res 14 : 451–458.
33. ViselA, PrabhakarS, AkiyamaJA, ShoukryM, LewisKD, et al. (2008) Ultraconservation identifies a small subset of extremely constrained developmental enhancers. Nat Genet 40 : 158–160.
34. CarninciP, YasudaJ, HayashizakiY (2008) Multifaceted mammalian transcriptome. Curr Opin Cell Biol 20 : 274–280.
35. CarninciP, KasukawaT, KatayamaS, GoughJ, FrithMC, et al. (2005) The transcriptional landscape of the mammalian genome. Science 309 : 1559–1563.
36. HangauerMJ, VaughnIW, McManusMT (2013) Pervasive transcription of the human genome produces thousands of previously unidentified long intergenic noncoding RNAs. PLoS Genet 9: e1003569.
37. DjebaliS, DavisCA, MerkelA, DobinA, LassmannT, et al. (2012) Landscape of transcription in human cells. Nature 489 : 101–108.
38. KaikkonenMU, SpannNJ, HeinzS, RomanoskiCE, AllisonKA, et al. (2013) Remodeling of the enhancer landscape during macrophage activation is coupled to enhancer transcription. Mol Cell 51 : 310–325.
39. NatoliG, AndrauJC (2012) Noncoding transcription at enhancers: general principles and functional models. Annu Rev Genet 46 : 1–19.
40. LamMT, ChoH, LeschHP, GosselinD, HeinzS, et al. (2013) Rev-Erbs repress macrophage gene expression by inhibiting enhancer-directed transcription. Nature 498 : 511–515.
41. LangmeadB, SalzbergSL (2012) Fast gapped-read alignment with Bowtie 2. Nat Methods 9 : 357–359.
42. NixDA, CourdySJ, BoucherKM (2008) Empirical methods for controlling false positives and estimating confidence in ChIP-Seq peaks. BMC Bioinformatics 9 : 523.
43. KalAJ, van ZonneveldAJ, BenesV, van den BergM, KoerkampMG, et al. (1999) Dynamics of gene expression revealed by comparison of serial analysis of gene expression transcript profiles from yeast grown on two different carbon sources. Mol Biol Cell 10 : 1859–1872.
44. QuinlanAR, HallIM (2010) BEDTools: a flexible suite of utilities for comparing genomic features. Bioinformatics 26 : 841–842.
45. AttanasioC, NordAS, ZhuY, BlowMJ, BiddieSC, et al. (2014) Tissue-specific SMARCA4 binding at active and repressed regulatory elements during embryogenesis. Genome Res
46. ZhangY, LiuT, MeyerCA, EeckhouteJ, JohnsonDS, et al. (2008) Model-based analysis of ChIP-Seq (MACS). Genome Biol 9: R137.
47. SiepelA, BejeranoG, PedersenJS, HinrichsAS, HouM, et al. (2005) Evolutionarily conserved elements in vertebrate, insect, worm, and yeast genomes. Genome Res 15 : 1034–1050.
48. McLeanCY, BristorD, HillerM, ClarkeSL, SchaarBT, et al. (2010) GREAT improves functional interpretation of cis-regulatory regions. Nat Biotechnol 28 : 495–501.
49. HeinzS, BennerC, SpannN, BertolinoE, LinYC, et al. (2010) Simple combinations of lineage-determining transcription factors prime cis-regulatory elements required for macrophage and B cell identities. Mol Cell 38 : 576–589.
50. LivakKJ, SchmittgenTD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 25 : 402–408.
51. KotharyR, ClapoffS, BrownA, CampbellR, PetersonA, et al. (1988) A transgene containing lacZ inserted into the dystonia locus is expressed in neural tube. Nature 335 : 435–437.
52. NobregaMA, OvcharenkoI, AfzalV, RubinEM (2003) Scanning human gene deserts for long-range enhancers. Science 302 : 413.
Štítky
Genetika Reprodukční medicína
Článek An Evolutionarily Conserved Role for the Aryl Hydrocarbon Receptor in the Regulation of MovementČlánek Requirement for Drosophila SNMP1 for Rapid Activation and Termination of Pheromone-Induced ActivityČlánek Co-regulated Transcripts Associated to Cooperating eSNPs Define Bi-fan Motifs in Human Gene NetworksČlánek Identification of a Regulatory Variant That Binds FOXA1 and FOXA2 at the Type 2 Diabetes GWAS LocusČlánek tRNA Modifying Enzymes, NSUN2 and METTL1, Determine Sensitivity to 5-Fluorouracil in HeLa CellsČlánek Derlin-1 Regulates Mutant VCP-Linked Pathogenesis and Endoplasmic Reticulum Stress-Induced ApoptosisČlánek A Genetic Assay for Transcription Errors Reveals Multilayer Control of RNA Polymerase II FidelityČlánek The Proprotein Convertase KPC-1/Furin Controls Branching and Self-avoidance of Sensory Dendrites inČlánek Regulation of p53 and Rb Links the Alternative NF-κB Pathway to EZH2 Expression and Cell SenescenceČlánek BMPs Regulate Gene Expression in the Dorsal Neuroectoderm of and Vertebrates by Distinct MechanismsČlánek Unkempt Is Negatively Regulated by mTOR and Uncouples Neuronal Differentiation from Growth Control
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2014 Číslo 9- Akutní intermitentní porfyrie
- Farmakogenetické testování pomáhá předcházet nežádoucím efektům léčiv
- Růst a vývoj dětí narozených pomocí IVF
- Hypogonadotropní hypogonadismus u žen a vliv na výsledky reprodukce po IVF
- Molekulární vyšetření pro stanovení prognózy pacientů s chronickou lymfocytární leukémií
-
Všechny články tohoto čísla
- Translational Regulation of the Post-Translational Circadian Mechanism
- An Evolutionarily Conserved Role for the Aryl Hydrocarbon Receptor in the Regulation of Movement
- Eliminating Both Canonical and Short-Patch Mismatch Repair in Suggests a New Meiotic Recombination Model
- Requirement for Drosophila SNMP1 for Rapid Activation and Termination of Pheromone-Induced Activity
- Co-regulated Transcripts Associated to Cooperating eSNPs Define Bi-fan Motifs in Human Gene Networks
- Targeted H3R26 Deimination Specifically Facilitates Estrogen Receptor Binding by Modifying Nucleosome Structure
- Role for Circadian Clock Genes in Seasonal Timing: Testing the Bünning Hypothesis
- The Tandem Repeats Enabling Reversible Switching between the Two Phases of β-Lactamase Substrate Spectrum
- The Association of the Vanin-1 N131S Variant with Blood Pressure Is Mediated by Endoplasmic Reticulum-Associated Degradation and Loss of Function
- Identification of a Regulatory Variant That Binds FOXA1 and FOXA2 at the Type 2 Diabetes GWAS Locus
- Regulation of Flowering by the Histone Mark Readers MRG1/2 via Interaction with CONSTANS to Modulate Expression
- The Actomyosin Machinery Is Required for Retinal Lumen Formation
- Plays a Conserved Role in Assembly of the Ciliary Motile Apparatus
- Hidden Diversity in Honey Bee Gut Symbionts Detected by Single-Cell Genomics
- Ribosome Rescue and Translation Termination at Non-Standard Stop Codons by ICT1 in Mammalian Mitochondria
- tRNA Modifying Enzymes, NSUN2 and METTL1, Determine Sensitivity to 5-Fluorouracil in HeLa Cells
- Causal Variation in Yeast Sporulation Tends to Reside in a Pathway Bottleneck
- Tissue-Specific RNA Expression Marks Distant-Acting Developmental Enhancers
- WC-1 Recruits SWI/SNF to Remodel and Initiate a Circadian Cycle
- Clonal Expansion of Early to Mid-Life Mitochondrial DNA Point Mutations Drives Mitochondrial Dysfunction during Human Ageing
- Methylation QTLs Are Associated with Coordinated Changes in Transcription Factor Binding, Histone Modifications, and Gene Expression Levels
- Differential Management of the Replication Terminus Regions of the Two Chromosomes during Cell Division
- Obesity-Linked Homologues and Establish Meal Frequency in
- Derlin-1 Regulates Mutant VCP-Linked Pathogenesis and Endoplasmic Reticulum Stress-Induced Apoptosis
- Stress-Induced Nuclear RNA Degradation Pathways Regulate Yeast Bromodomain Factor 2 to Promote Cell Survival
- The MAPK p38c Regulates Oxidative Stress and Lipid Homeostasis in the Intestine
- Widespread Genome Reorganization of an Obligate Virus Mutualist
- Trans-kingdom Cross-Talk: Small RNAs on the Move
- The Vip1 Inositol Polyphosphate Kinase Family Regulates Polarized Growth and Modulates the Microtubule Cytoskeleton in Fungi
- Myosin Vb Mediated Plasma Membrane Homeostasis Regulates Peridermal Cell Size and Maintains Tissue Homeostasis in the Zebrafish Epidermis
- GLD-4-Mediated Translational Activation Regulates the Size of the Proliferative Germ Cell Pool in the Adult Germ Line
- Genome Wide Association Studies Using a New Nonparametric Model Reveal the Genetic Architecture of 17 Agronomic Traits in an Enlarged Maize Association Panel
- Translational Regulation of the DOUBLETIME/CKIδ/ε Kinase by LARK Contributes to Circadian Period Modulation
- Positive Selection and Multiple Losses of the LINE-1-Derived Gene in Mammals Suggest a Dual Role in Genome Defense and Pluripotency
- Out of Balance: R-loops in Human Disease
- A Genetic Assay for Transcription Errors Reveals Multilayer Control of RNA Polymerase II Fidelity
- Altered Behavioral Performance and Live Imaging of Circuit-Specific Neural Deficiencies in a Zebrafish Model for Psychomotor Retardation
- Nipbl and Mediator Cooperatively Regulate Gene Expression to Control Limb Development
- Meta-analysis of Mutations in Autism Spectrum Disorders: A Gradient of Severity in Cognitive Impairments
- The Proprotein Convertase KPC-1/Furin Controls Branching and Self-avoidance of Sensory Dendrites in
- Hydroxymethylated Cytosines Are Associated with Elevated C to G Transversion Rates
- Memory and Fitness Optimization of Bacteria under Fluctuating Environments
- Regulation of p53 and Rb Links the Alternative NF-κB Pathway to EZH2 Expression and Cell Senescence
- Interspecific Tests of Allelism Reveal the Evolutionary Timing and Pattern of Accumulation of Reproductive Isolation Mutations
- PRO40 Is a Scaffold Protein of the Cell Wall Integrity Pathway, Linking the MAP Kinase Module to the Upstream Activator Protein Kinase C
- Low Levels of p53 Protein and Chromatin Silencing of p53 Target Genes Repress Apoptosis in Endocycling Cells
- SPDEF Inhibits Prostate Carcinogenesis by Disrupting a Positive Feedback Loop in Regulation of the Foxm1 Oncogene
- RRP6L1 and RRP6L2 Function in Silencing Regulation of Antisense RNA Synthesis
- BMPs Regulate Gene Expression in the Dorsal Neuroectoderm of and Vertebrates by Distinct Mechanisms
- Unkempt Is Negatively Regulated by mTOR and Uncouples Neuronal Differentiation from Growth Control
- Atkinesin-13A Modulates Cell-Wall Synthesis and Cell Expansion in via the THESEUS1 Pathway
- Dopamine Signaling Leads to Loss of Polycomb Repression and Aberrant Gene Activation in Experimental Parkinsonism
- Histone Methyltransferase MMSET/NSD2 Alters EZH2 Binding and Reprograms the Myeloma Epigenome through Global and Focal Changes in H3K36 and H3K27 Methylation
- Bipartite Recognition of DNA by TCF/Pangolin Is Remarkably Flexible and Contributes to Transcriptional Responsiveness and Tissue Specificity of Wingless Signaling
- The Olfactory Transcriptomes of Mice
- Muscular Dystrophy-Associated and Variants Disrupt Nuclear-Cytoskeletal Connections and Myonuclear Organization
- Interplay of dFOXO and Two ETS-Family Transcription Factors Determines Lifespan in
- Evidence for Widespread Positive and Negative Selection in Coding and Conserved Noncoding Regions of
- Genome-Wide Association Meta-analysis of Neuropathologic Features of Alzheimer's Disease and Related Dementias
- Rejuvenation of Meiotic Cohesion in Oocytes during Prophase I Is Required for Chiasma Maintenance and Accurate Chromosome Segregation
- Admixture in Latin America: Geographic Structure, Phenotypic Diversity and Self-Perception of Ancestry Based on 7,342 Individuals
- Local Effect of Enhancer of Zeste-Like Reveals Cooperation of Epigenetic and -Acting Determinants for Zygotic Genome Rearrangements
- Differential Responses to Wnt and PCP Disruption Predict Expression and Developmental Function of Conserved and Novel Genes in a Cnidarian
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Admixture in Latin America: Geographic Structure, Phenotypic Diversity and Self-Perception of Ancestry Based on 7,342 Individuals
- Nipbl and Mediator Cooperatively Regulate Gene Expression to Control Limb Development
- Genome Wide Association Studies Using a New Nonparametric Model Reveal the Genetic Architecture of 17 Agronomic Traits in an Enlarged Maize Association Panel
- Histone Methyltransferase MMSET/NSD2 Alters EZH2 Binding and Reprograms the Myeloma Epigenome through Global and Focal Changes in H3K36 and H3K27 Methylation
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání




