-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
The Proprotein Convertase KPC-1/Furin Controls Branching and Self-avoidance of Sensory Dendrites in
Sensory neurons receive input from other neurons or sample their environment through elaborate structures termed dendritic trees. The correct patterning of dendritic trees is crucial for the proper function of the nervous system, and ample evidence points to the involvement of dendritic defects in a wide range of neuropsychiatric diseases. However, we still do not understand fully how this process is regulated at the molecular level. We discovered an important role for the protein-processing enzyme KPC-1/furin in the development of touch-sensitive dendritic trees in the roundworm C. elegans. Animals lacking this enzyme show multiple defects in the size, shape and number of these dendritic branches as well as other neurons. We further show that the gene encoding KPC-1 is expressed widely in the nervous system and that it is required within the branching neuron to exert its function on dendritic growth. Finally, we reveal a genetic connection between KPC-1 function and genes of the menorin pathway, which was recently discovered to also play an essential role in dendrite development. Thus, our findings add new insight into the molecular understanding of dendrite formation.
Published in the journal: . PLoS Genet 10(9): e32767. doi:10.1371/journal.pgen.1004657
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1004657Summary
Sensory neurons receive input from other neurons or sample their environment through elaborate structures termed dendritic trees. The correct patterning of dendritic trees is crucial for the proper function of the nervous system, and ample evidence points to the involvement of dendritic defects in a wide range of neuropsychiatric diseases. However, we still do not understand fully how this process is regulated at the molecular level. We discovered an important role for the protein-processing enzyme KPC-1/furin in the development of touch-sensitive dendritic trees in the roundworm C. elegans. Animals lacking this enzyme show multiple defects in the size, shape and number of these dendritic branches as well as other neurons. We further show that the gene encoding KPC-1 is expressed widely in the nervous system and that it is required within the branching neuron to exert its function on dendritic growth. Finally, we reveal a genetic connection between KPC-1 function and genes of the menorin pathway, which was recently discovered to also play an essential role in dendrite development. Thus, our findings add new insight into the molecular understanding of dendrite formation.
Introduction
Multicellular organisms sense their environment through specialized nerve cells termed sensory neurons. The diversity in shape and structure that dendrites of sensory neurons (hereafter named ‘dendritic arbors’) assume reflects the variety of stimuli they receive [1]. Work over the past two decades has established that dendritic arbor development of sensory neurons relies on conserved molecular mechanisms [2], [3]. However, dendritic arbors do not only exist in the periphery, but also in the central nervous system where they function to receive synaptic input from other neurons. The molecules and mechanisms that orchestrate dendritic arbor development, e.g. cell adhesion molecules and molecular motor proteins, are often similar between the peripheral and central nervous system [see ref. 3 for these and more examples]. Importantly, failed dendrite development has been linked to neurological diseases of the central nervous system, ranging from Autism Spectrum Disorders (ASD) to Schizophrenia [4], [5].
The understanding of sensory dendrite morphogenesis has been greatly advanced through studies in the fly Drosophila melanogaster (reviewed in [3]). These studies revealed the importance of transcriptional cascades, cytoskeletal proteins, the secretory pathway, microtubular transport, and the basement membrane for development of the so-called dendritic arborization (da) neurons in flies [3], [6]–[8]. In the nematode Caenorhabditis elegans, the PVD mechanosensory neurons have been used to study development of dendritic arbors owing to their stereotypic branching patterns (Figure 1A,B) [9], [10]. A PVD cell on each side of the animal sends a primary dendrite both anteriorly and posteriorly that branches off perpendicular higher order branches in a stereotypical fashion (Figure 1A, B). The resulting structures resemble candelabra and have hence been named menorahs [11]. Like fly da neurons, transcriptional cascades and microtubule based motor proteins have been shown to play a role during PVD dendritic arbor formation suggesting that basic principles of dendritic arbor formation are evolutionarily conserved [12]–[14]. Recently, a tripartite complex consisting of the extracellular molecule MNR-1/menorin, the neural cell adhesion molecule SAX-7/L1CAM both of which act from the hypodermis (skin) and the leucine rich repeat DMA-1/LRR transmembrane receptor on PVD dendrites have been shown to be critical for PVD development [15], [16].
Fig. 1. KPC-1 is required for development of dendritic arbors. 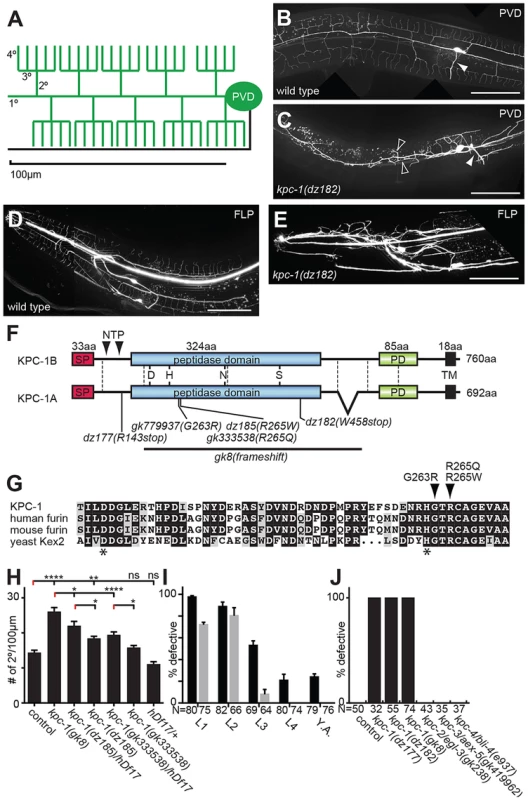
A Schematic of the PVD somatosensory neuron with dendrites (including primary/1°, secondary/2°, tertiary/3° and quaternary/4° branches) and the axon indicated in green and black, respectively. B–C Lateral view of an adult wild type (B) and kpc-1(dz182) mutant animal (C). PVD sensory neurons are visualized by a fluorescent reporter (wdIs52). Anterior is to the left and scale bars indicate 50 µm in all panels. An arrowhead denotes the PVD axon. Open arrow heads indicate ectopic 3° branches. D–E Images of an adult wild type animal (D) and a kpc-1(dz182) mutant animal (E). FLP sensory neurons are visualized by a fluorescent reporter (muIs32). F Schematic of KPC-1 with domains (SP: signal peptide, PD: P domain, TM: transmembrane domain) and exon boundaries (dashed lines) indicated. KPC-1 exists in a long and a short splice variant. All experiments were conducted with the short KPC-1A variant. Mutant alleles and predicted molecular changes are shown. NTP (N terminal propeptide) indicates cleavage sites that are necessary for autoproteolytic activation of the proprotein. G Partial sequence alignment of the peptidase domain in KPC-1 (NP_001021101), human furin (NP_002560.1), mouse furin (NP_001074923.1), and yeast Kex2 (NP_014161) protein. Asterisks indicate the aspartic acid and histidine of the catalytic triad. Alignments were obtained using Multalin (http://multalin.toulouse.inra.fr) and rendered with Boxshade (http://www.ch.embnet.org). For a Full alignment see Figure S1. H Quantification of the number of secondary branches in the genotypes indicated. hDf17 is a chromosome I deficiency allele that deletes the entire kpc-1 locus. Data are represented as mean +/− SEM. Statistical comparisons were performed using one-sided ANOVA with the Tukey correction. Statistical significance is indicated (*, P≤0.05; **, P≤0.01; ****, P≤0.0001, ns: not significant (P>0.05)). N = 22 animals (302 dendritic branches) of wild type control; N = 22 animals (548 dendritic branches) of kpc-1(gk8); N = 16 animals (331 dendritic branches) of kpc-1(dz185)/hDf17; N = 26 animals (461 dendritic branches) of kpc-1(dz185), N = 16 animals (292 dendritic branches) of kpc-1(gk333538)/hDf17, N = 26 animals (396 dendritic branches) of kpc-1(gk333538). I Quantification of animals with defects in PVD at the young adult stage following RNAi mediated knock down of kpc-1 (grey bars) or GFP as a control for RNAi efficacy (black bars) using the hypersensitive eri-1(mg366); wdIs52 reporter background at 15C°. All animals were assayed as 2 day old adults and the developmental stage at which knock down was initiated is shown. Animals were arbitrarily defined as defective, if they displayed excessive ectopic branching proximal to the cell body, coupled with complete lack of quaternary branches anterior to the vulva or, GFP was absent in PVD. Note, that while knock down of GFP initiated at late developmental stages is still effective, no effects are seen at these stages as a result of kpc-1 knock down. J Quantification of animals with defects in PVD in the genotypes indicated (See also Figure S6). Defective animals were defined as in I. Here we report the identification of the furin homolog kpc-1 in C. elegans as a factor that acts in concert with the ‘menorin’ pathway to shape sensory dendrite development. Furin is a serine protease of the proprotein convertase family, that following autocatalytic activation cleaves proteins at characteristic dibasic motifs (reviewed in [17]–[19]). We show that in C. elegans a reporter for kpc-1/furin is expressed broadly in the nervous system and is necessary for multiple neuronal positioning, neurite extension and branching events, including PVD dendritic growth and self-avoidance. Moreover, kpc-1 can act cell autonomously to shape PVD dendrites in a manner that is dependent on the ‘menorin’ pathway.
Results
In a screen for genes involved in PVD development [16] we also identified mutant alleles of the proprotein convertase kpc-1, which encodes the C. elegans furin homolog based on sequence similarity and domain organization [20], [21]. Mutant animals show no obvious morphological or defects in body size. However, they do display severely defective dendritic arbors in both PVD and the analogous anterior FLP neurons (Figure 1B–E). The identified recessive alleles include two nonsense (dz177, dz182) and a missense allele (dz185) (Figure 1F, Figure S1). We also obtained a deletion (gk8) and two additional missense alleles (gk333538, gk779937) (Figure 1F,G, Figure S1). The deletion and nonsense alleles truncate or delete the catalytic domain of KPC-1/furin, respectively, and their phenotypes seem indistinguishable in severity, suggesting that all three alleles represent strong if not complete loss of function alleles. The missense alleles dz185 and gk333538 are hypomorphic alleles because their phenotype was (1) less severe when compared to the presumptive gk8 null allele and (2) more severe when placed in trans to a deficiency (hdf17) that spans the genomic region of kpc-1/furin (Figure 1H). The missense mutations affect residues in a perfectly conserved alpha helix that positions the histidine of the catalytic triad in furin [22] (Figure 1G), suggesting that proteolytic activity of KPC-1/furin is necessary for PVD development.
To define when kpc-1/furin function is required during development we first conducted timed RNAi experiments as described [16]. We found that knock down of kpc-1/furin starting at early larval stages when PVD sensory dendrites begin to develop [13] resulted in robust defects in PVD in adult animals (Figure 1I). In contrast, knock down starting at the L3 stage resulted in weaker phenotypes in adults (Figure 1I). RNAi mediated knockdown initiated only 12–24 h later at the L4 larval stage failed to result in any PVD defects. Importantly, RNAi mediated knockdown of GFP in PVD neurons suggested that changes in RNAi efficacy cannot account for lack of defects upon induction of kpc-1 knock down at later larval stages (Figure 1I). Consistent with these observations we find defects in PVD dendrite arborization in kpc-1 mutant animals already during the L3 larval stage rather than later as a result of a maintenance defect (Figure S2). Collectively, these findings suggest that kpc-1/furin functions during the earlier stages of PVD development and may not play a major role in dendrite maintenance.
We next sought to determine whether kpc-1/furin functions in PVD neurons or in surrounding tissues such as the hypodermis (skin) or muscle. To this end we first conducted transgenic rescue experiments. We found rescue of the PVD defects in kpc-1 mutants when a kpc-1 cDNA was expressed under control of a PVD-specific heterologous promoter but not when expressed in muscle or the hypodermis (Figure 2A,B). Expression of this PVD-specific kpc-1 transgene in a wild-type background did not have any detectable effect on PVD architecture (Figure S3). To further investigate where kpc-1 may function we constructed transgenic animals that carried a kpc-1 reporter where a 5.8 Kb fragment of the endogenous upstream regulatory region of kpc-1 drives expression of green fluorescent protein. This transcriptional kpc-1prom5.8::GFP reporter was widely expressed from early developmental stages in the nervous system including in PVD (Figure 2C–E, Figure S4). We conclude that kpc-1/furin acts cell-autonomously to shape the dendritic arbors of PVD neurons.
Fig. 2. KPC-1 functions in PVD to shape dendritic arbors. 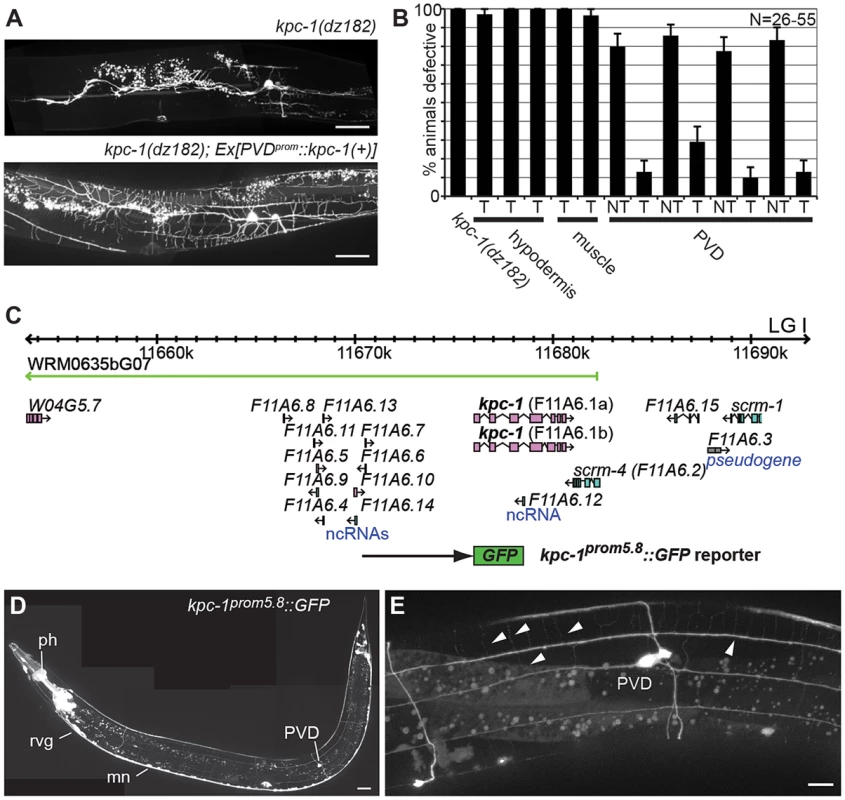
A Epifluorescence micrographs of kpc-1(dz182) mutant animals and transgenically rescued kpc-1(dz182) animals. Scale bars indicate 50 µm. B Quantification of transgenic rescue. Transgenic (T) and nontransgenic (NT) siblings were quantified in kpc-1 mutant animals in which a kpc-1 wild type DNA was driven from a hypodermal (dpy-7prom) [31], muscle (myo-3prom) [32] or PVD specific promoter (ser-2prom3) [9]. Defective animals were defined as in Figure 1I. C Schematic of the kpc-1 locus on linkage group (LG) I. The extent of the rescuing fosmid WRM0635bG07 is indicated in green as is the transcriptional kpc-1::GFP reporter that contains 5.8 kb of regulatory sequence upstream of the ATG (kpc-1prom5.8::GFP). A transcriptional reporter containing 2.8 kb of regulatory sequence (kpc-1prom2.8::GFP) was not expressed in PVD suggesting that regulatory elements for PVD expression reside between −2.8 kb and −5.8 kb (Figure S4). D Composite epifluorescent micrograph of an adult transgenic animal carrying the kpc-1prom5.8::GFP transgene. Expression is seen in the pharynx (ph) and the nervous system, e.g. the retrovesicular ganglion (rvg), ventral cord motor neurons (mn) and in PVD mechanosensory neurons. In addition, expression is seen in vulval epithelial tissues as well as the spermatheca (not shown here). A scale bar indicates 30 µm. E Higher magnification epifluorescent micrograph of an adult transgenic animal carrying the kpc-1prom5.8::GFP transgene. Expression is seen in PVD and its dendritic tree (arrowheads). A scale bar indicates 10 µm. The observed extensive neuronal expression of the kpc-1 reporter prompted us to examine other neuron types besides PVD for defects in migration, neurite extension and branching in kpc-1 mutant animals. We detected significant defects in multiple neuron types in kpc-1 mutants. For example, the sensory AQR neurons failed to branch appropriately in the nerve ring whereas VC motoneurons showed defects in the extension and formation of characteristic branches near the vulva (Figure 3). Yet, kpc-1 did not appear to be required for all branching processes because branching in AIY interneurons as a result of overexpression of the secreted kal-1 cell adhesion molecule [23] did not require kpc-1 and, AVL neurons showed ectopic branches but no defects in normal branch formation (Figure 3). Whereas DA/DB motoneurons did not display obvious defects, D-type motoneurons showed gaps in the dorsal cord, possibly due to defective neurite extension, and increased numbers of inappropriately positioned commissures on the left side of the animals (Figure 3). Similarly, AIY interneurons displayed a characteristic axonal ‘short stop’ phenotype, likely as a result of defects in extension [23]. In addition, we observed neuronal cell positioning defects of ALM touch receptor neurons and HSN motoneurons (Figure 3). Thus, kpc-1's function in nervous system development extends well beyond its role in dendrite morphogenesis and affects many but not all neurons in the major neuronal classes (sensory, motor and interneurons). Importantly, kpc-1 appears to be involved in both control of neurite branch formation and extension (see also below).
Fig. 3. kpc-1 displays defects in neurite branching, extension and cell positioning of certain sensory neurons, interneurons and motoneurons. 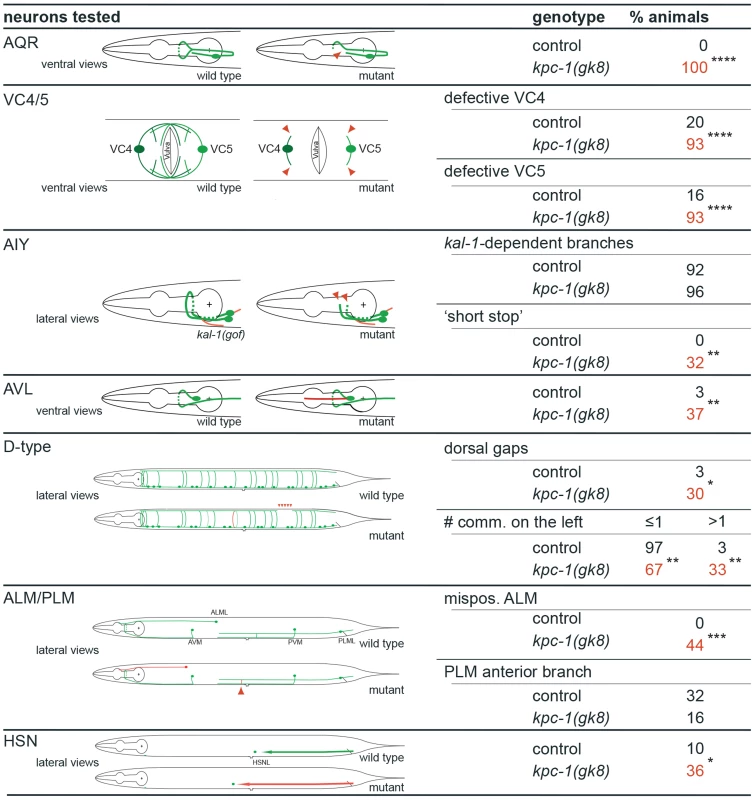
Summary of neuronal defects observed in several classes of neurons. Schematics of normal neuronal morphology is shown and contrasted with abnormal neuronal morphology indicated in red or with red arrowheads. N = 30 in all cases except AIY and ALM (N = 25). Statistical significance was calculated using Fisher's exact test and is indicated (*, P≤0.05; **, P≤0.01; ***, P≤0.001; ****, P≤0.0001, ns: not significant (P>0.05)). To define the function of kpc-1 in dendrite development in more detail we subjected mutants to a morphometric analysis of PVD dendrites. These studies showed an increase in secondary and ectopic tertiary branches with a concomitant decrease in the number of tertiary and quaternary branches in kpc-1 mutants (Figure 4A–F). The secondary branches in kpc-1 mutants often failed to reach the vicinity of the sublateral nerve cords (Figure 4F) where they normally bifurcate to form tertiary branches [13]. Instead, they frequently sprouted ectopic tertiary branches in places that normally do not support tertiary branches (Figure 4F). Interestingly, the average length of branches was significantly reduced in kpc-1/furin mutants (Figure 5A–E), as was the aggregate length of each branch order (2°, 3° and 4° branches) separately or combined (Figure S5). To distinguish whether this phenotype is a reflection of a decrease or increase in growth or retraction, we conducted time-lapse analyses of kpc-1 mutants and measured growth and retraction of secondary branches. We found significantly slowed growth in kpc-1 mutants compared to wild type animals whereas the speed of retraction remained unchanged (Figure 6, Movies S1 and S2). None of the observed PVD defects were shared in mutants of the three other proprotein convertases encoded in the C. elegans genome, including kpc-2/egl-3, kpc-3/aex-5, and kpc-4/bli-4 [20] (Figure 1J, Figure S6), suggesting that these genes play individually no role during PVD development.
Fig. 4. kpc-1 acts in a genetic pathway with mnr-1/menorin, sax-7/L1CAM, and the leucine rich repeat (LRR) transmembrane receptor dma-1. 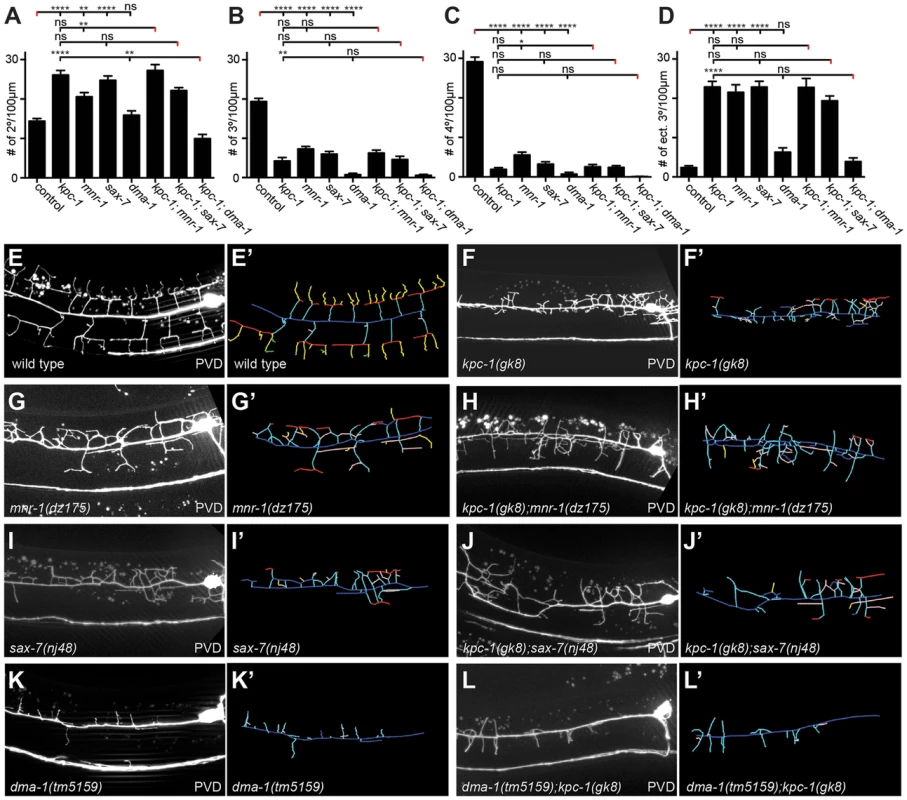
A–D Quantification of branch numbers in kpc-1(gk8), mnr-1(dz175), sax-7(nj48), and dma-1(tm5159) single and double mutant animals. Data are represented as mean +/− SEM of the number of branches found in the 100 µm segment immediately anterior to PVD cell body. Statistical comparisons were performed using one-sided ANOVA with the Tukey correction and statistical significance is indicated (*, P≤0.05; **, P≤0.01; ***, P≤0.001; ****, P≤0.0001, ns: not significant (P>0.05)). N = 21 animals (1371 dendritic branches) of wild type control; N = 21 animals (1158 dendritic branches) of kpc-1(gk8); N = 21 animals (1154 dendritic branches) of mnr-1(dz175); N = 22 animals (1251 dendritic branches) of sax-7(nj48), N = 22 animals (520 dendritic branches) of dma-1(tm5159), N = 19 animals (1119 dendritic branches) of kpc-1(gk8); mnr-1(dz175), N = 25 animals (1213 dendritic branches) of kpc-1(gk8); sax-7(nj48), N = 22 animals (319 dendritic branches) of kpc-1(gk8); dma-1(tm5159). Data for wild type control and kpc-1 are identical to Figure 1 and shown for comparison only. E–L maximum intensity projections of representative L3 larval animals of the genetic backgrounds as indicated, paired with schematics of the respective tracings (E′–L′). Traces were color coded as follows: dark blue: 1°, cyan: 2°, red: 3°, yellow: 4°, rose: ectopic 3°, dark blue: mispositioned 3° (combined with ectopic 3° for quantification purposes), green: 5°. Fig. 5. kpc-1 controls dendrite branch extension. 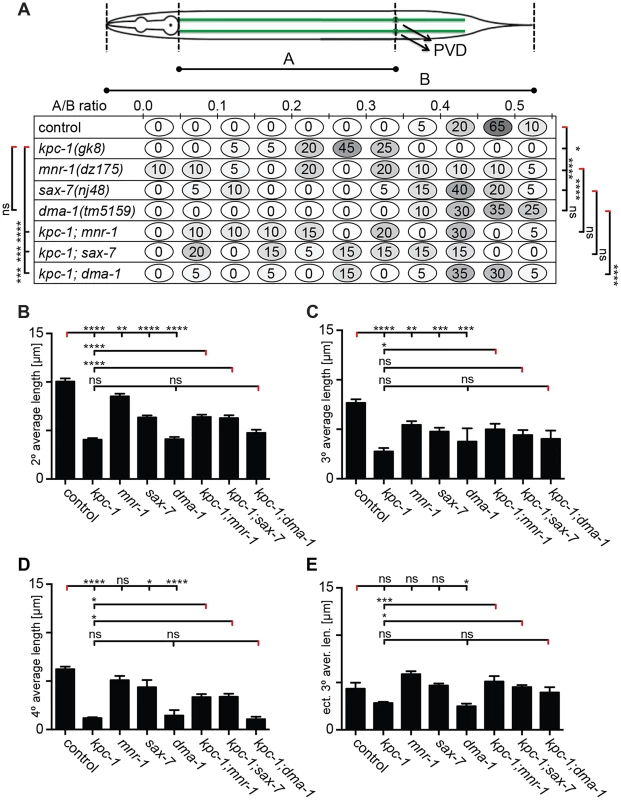
A Quantification of primary branch length, expressed as a ratio of A/B to normalize for animal size (as indicated in schematic) is shown for the genotypes indicated. Percentage of animals falling within a bin (of A/B ratio) is given (illustrated with the corresponding shade of grey). Statistical significance was calculated using the F test to compare variances (*, P≤0.05; **, P≤0.01; ***, P≤0.001; ****, P≤0.0001, ns: not significant (P>0.05). B–E Quantification of average branch length in kpc-1(gk8), mnr-1(dz175), sax-7(nj48), and dma-1(tm5159) single and double mutant animals. Data are represented as mean +/− SEM. Statistical comparisons were performed using one-sided ANOVA with the Tukey correction. Statistical significance is indicated (*, P≤0.05; **, P≤0.01; ***, P≤0.001; ****, P≤0.0001, ns: not significant (P>0.05)). Number of animals and dendrites scored as in legend to Figure 4. Fig. 6. Dendrites in kpc-1 display decreased speed of growth. 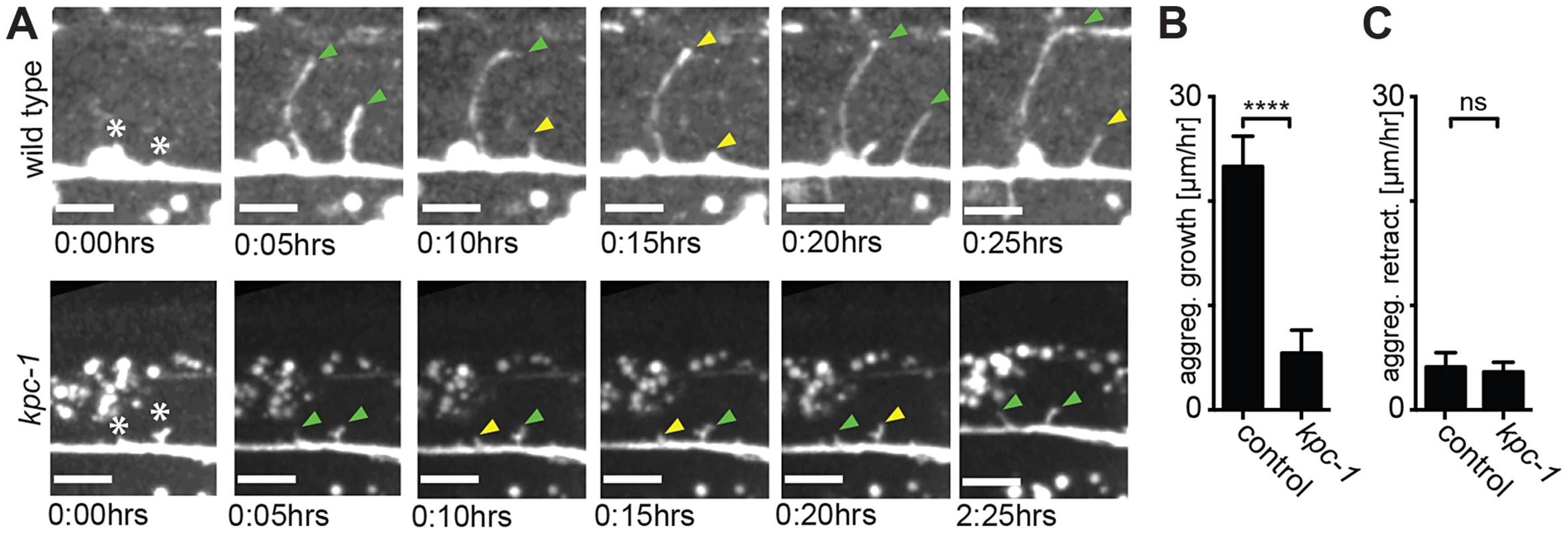
A Time-lapse still images of a wild type (upper panel) and a kpc-1(gk8) mutant animal (lower panel), indicating the times after imaging was begun at the L3 stage. Asterisks mark the place of budding secondary dendrites being measured. Green arrowhead: secondary dendrites in active dendritic growth. Yellow arrowhead: secondary dendrites undergoing retraction. Note the limited growth of dendrites in kpc-1 mutant animals even 2:25 hours after initiation. B–C Quantification of aggregate growth (B) and aggregate retraction speed (C) per hour in secondary branches from time lapse movies of the genotypes indicated. Data are represented as mean +/− SEM. Statistical comparisons were performed using the Student t-test and statistical significance indicated (***, P≤0.001; ns, not significant (P>0.05)). In the hypomorphic alleles kpc-1(gk333538) and kpc-1(dz185) we found that tertiary and possibly other dendritic branches appeared to show substantial overlap and an apparent lack of self-avoidance (Figure 7A,B). These observations could indicate bona fide defects in self-avoidance of dendrites. Alternatively, mutant dendrites could be growing erroneously in three dimensions into hypodermal tissues, rather than growing within a confined two-dimensional plane and directly avoiding each other. Such observations have recently been made for drosophila dendrite arborization neurons whose growth failed to be restricted to a two-dimensional plane in integrin mutants but rather occurred in three dimensions [7], [8]. To distinguish between these two possibilities, we optically sectioned animals bearing a hypomorphic mutation in kpc-1(dz185) and coded each optical section with a different color. Thus, dendrite processes that share a focal plane display the same color whereas processes in different focal planes will display different colors. These analyses suggested that dendrites touch and overlap in the same focal planes in kpc-1 hypomorphic mutant animals (Fig. 7C). Collectively, our data suggest that the normal function of kpc-1 is to promote the formation of tertiary and quaternary branches and to maintain self-avoidance between dendrites. In addition, kpc-1 functions in dendrite extension along the anterior-posterior and dorsal-ventral axes, likely by regulating the speed of growth.
Fig. 7. kpc-1 serves a function in dendritic self-avoidance. 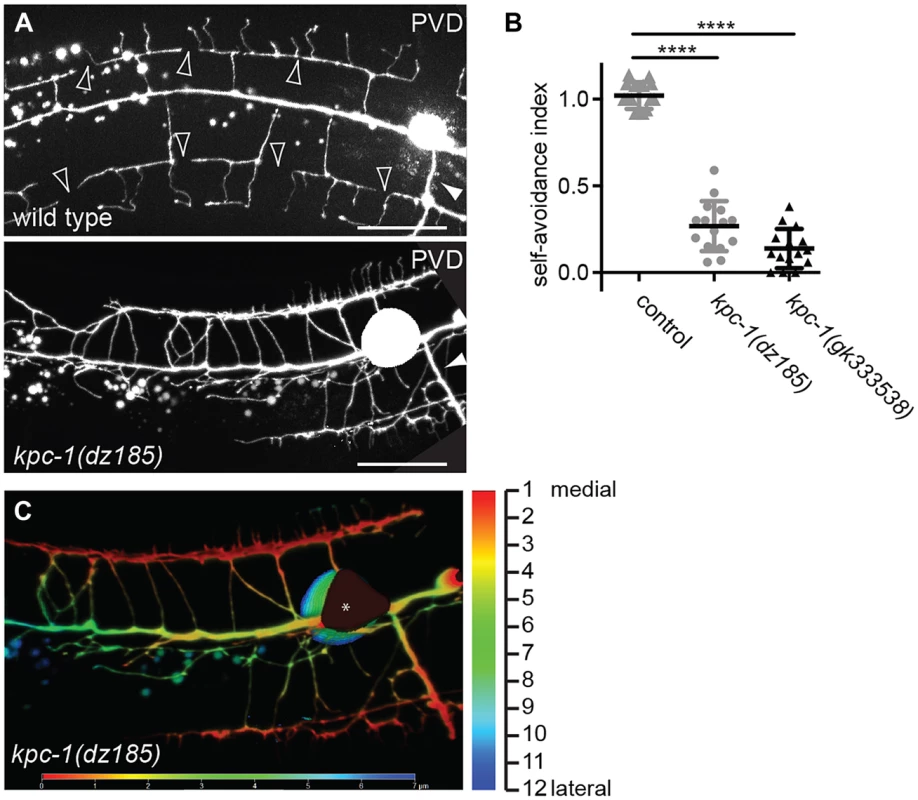
A Epifluorescent micrographs of a lateral view of wild type and kpc-1(dz185) hypomorphic animals. Filled white arrowheads indicate axons and empty white arrowheads gaps between tertiary dendrites, which are known to be maintained by self-avoidance [33]. Scale bar is 20 µm. B Quantification of self-avoidance index which was determined as previously described [34] and is defined as the ratio of the number of gaps between adjacent tertiary branches divided by the number of candelabra in a 100 µm segment anterior to the cell body. Statistical comparisons were performed using the Student t-test and statistical significance is indicated (****, P≤0.0001). C Maximum intensity projection of a Z-stack (lateral view of a kpc-1(dz815) mutant shown in A) in which each optical section (total of 12 sections over 7.2 µm equaling 0.6 µm/section) was color-coded with warmer to colder colors for medial to lateral sections of the animal, respectively. Note that the overlapping tertiary dendrites appear in the same focal plane. Since the width of dendritic branches is approximately 200 nm [35], these data suggest that tertiary dendrites are closely apposed (within 0.6 µm) or directly touching. An asterisk marks an area of the cell body that is saturated due to maximum intensity projection. The dendrite phenotypes in kpc-1 mutants were reminiscent of defects in mutants of the ‘menorin’ pathway, namely the cell surface molecule MNR-1/menorin, the neural cell adhesion molecule SAX-7/L1CAM and the leucine rich repeat containing transmembrane receptor DMA-1/LRR [15], [16]. We thus tested the genetic interactions between kpc-1/furin and each of these genes using the same morphometric criteria. Focusing first on the defects in branch formation in kpc-1/furin mutants (i.e. decreased tertiary and quaternary branching and increased secondary and ectopic tertiary branching) we found the kpc-1 mutant phenotype not further enhanced in double mutants with either mnr-1 or sax-7/L1CAM (Figure 4), suggesting that these genes act genetically in the same pathway. In contrast, dma-1 appeared generally epistatic to kpc-1 because the kpc-1; dma-1 double mutant looked more similar to the dma-1 single mutant (Figure 4). A possible exception was the reduced number of secondary branches in the kpc-1; dma-1 double compared to both single mutants and to wild type animals (Figure 4A). This synthetic phenotype reveals possibly a cryptic or parallel function for dma-1 in the formation of secondary branches that is only evident in the absence of kpc-1. To further investigate the genetic relationship between the menorin pathway and kpc-1, we used a previously described misexpression phenotype of mnr-1/menorin. When mnr-1/menorin is ectopically expressed in muscle, the resulting dendritic trees lack the characteristic menorah like morphology and, instead, display muscle-associated dendritic structures we named ‘baobabs’ because of their resemblance to baobab trees (Figure 8) [16]. We found that this mnr-1/menorin gain of function phenotype is fully dependent on kpc-1 (Figure 8) as it is on sax-7/L1CAM and dma-1/LRR [16], demonstrating that kpc-1 is required for the formation of mnr-1-dependent dendritic arbors. Taken together, these results suggest that for branch formation, kpc-1, mnr-1/menorin and sax-7/L1CAM act genetically in the same pathway and that dma-1/LRR is epistatic to kpc-1 in a similar way as it is to mnr-1/menorin and sax-7/L1CAM [16].
Fig. 8. MNR-1/menorin requires kpc-1 functions. 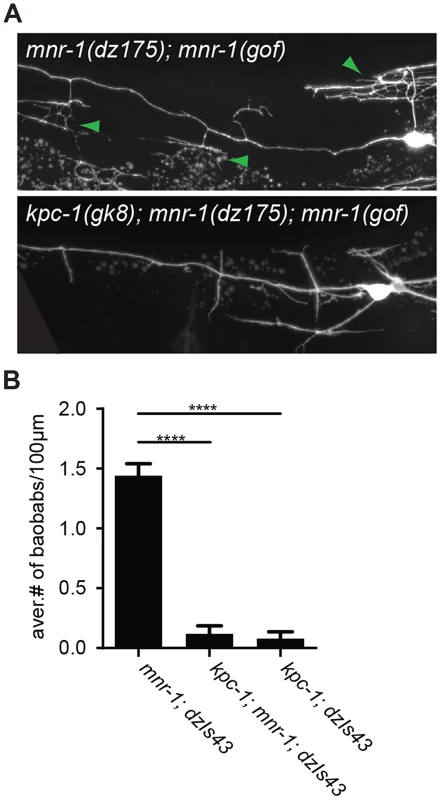
A Epifluorescent micrographs of mnr-1(dz175); mnr-1(gof)[dzIs43] animals with characteristic ‘baobab’-like dendritic arbors (green arrowheads, upper panel) [16] that are absent upon loss of kpc-1 (lower panel). B Quantification of ‘baobab’-like dendritic arbors. Mutant alleles used were mnr-1(dz175), dzIs43 (Is[myo-3prom::mnr-1]) and kpc-1(gk8). With regard to branch length, i.e. branch extension, the situation is slightly different. The kpc-1; dma-1 double mutant was indistinguishable from the kpc-1 and dma-1 single mutants, again demonstrating that both genes act genetically in the same pathway (Figure 5). In contrast, the reduced average length of higher-order branches in kpc-1 mutants was partially suppressed in double mutants with mnr-1/menorin or sax-7/L1CAM but not with dma-1/LRR (Figure 5B–E). Similar observations were made for the primary branches. Primary branch length was measured from cell body to pharynx border and normalized relative to total body length to account for possible variability in body size (Figure 5A, top). The shorter primary branch length in kpc-1 mutants was also partially suppressed by concomitant loss of mnr-1/menorin, sax-7/L1CAM, or dma-1/LRR (Figure 5A). Collectively, these results suggest that kpc-1 acts genetically in the same and, possibly in part, a parallel pathway to mnr-1/menorin and sax-7/L1CAM to control dendrite patterning.
Discussion
In a screen for genes required for patterning of the PVD somatosensory neuron, we isolated mutant alleles in kpc-1, which encodes the C. elegans furin homolog [20], [21]. The phenotypes in PVD patterning that we observed in kpc-1/furin mutants displayed striking similarities to mutants in the ‘menorin’ pathway, which comprises the three cell adhesion molecules MNR-1/menorin, SAX-7/L1CAM and DMA-1/LRR-TM [15], [16]. Our double mutant analyses revealed genetic interactions that were distinct for branch formation and extension. For branch formation, we suggest that kpc-1/furin acts in a genetic pathway with mnr-1/menorin, sax-7/L1CAM and dma-1/LRR. First, double mutants between kpc-1/furin and either mnr-1/menorin or sax-7/L1CAM were not more severe than the single mutants. Second, dma-1/LRR appeared generally more severe than the kpc-1/mnr-1 or kpc-1/sax-7 double mutants and epistatic to kpc-1/furin. Third, gain of function experiments demonstrated that mnr-1/menorin function requires kpc-1/furin. Overall, these genetic interactions are strikingly similar to those between mnr-1/menorin, sax-7/L1CAM and dma-1/LRR [15], [16] and suggest that kpc-1/furin, mnr-1/menorin and sax-7/L1CAM act in a linear pathway but that a parallel pathway may exist that also acts through dma-1/LRR during branch formation. For branch extension, the scenario is slightly different. First, kpc-1/furin mutant branch extension phenotypes were generally more similar to dma-1/LRR than to mnr-1/menorin or sax-7/L1CAM mutant phenotypes. Second, mutations in mnr-1/menorin or sax-7/L1CAM but not dma-1/LRR partially suppressed kpc-1/furin mutant branch extension phenotypes in higher order branches. This could suggest higher activity of a parallel pathway during extension that is normally inhibited by mnr-1/sax-7-function, but also requires dma-1/LRR. Taken together, the findings presented here suggest that kpc-1 collaborates with the menorin pathway to sculpt the PVD dendritic arbor through distinct genetic mechanisms for branch formation and extension.
Our experiments showed that kpc-1/furin functions cell autonomously to coordinate formation, extension and self-avoidance of PVD somatosensory dendritic branches. However, the functions of kpc-1/furin are not limited to shaping PVD and FLP neurons during development. First, a recent report described a function for KPC-1 in the remodeling of sensory dendrites of IL2s (a set of sensory neurons in the head of C. elegans) as a result of changes in environmental conditions [21]. This report provided also evidence for a role of kpc-1 in the patterning of PVD and FLP neurons. Second, our detailed survey of the neuroanatomy of several classes of neurons in kpc-1/furin mutants revealed that kpc-1/furin plays a more general role in nervous system development than previously acknowledged. For example, AIY interneurons display neurite extension defects and the D-type motoneurons exhibit gaps in the dorsal nerve cord that appear to be the result of defects in neurite extension. On the other hand, kpc-1/furin is required for the formation of certain characteristic neuronal branches like in AQR sensory neurons or VC4/5 motoneurons, while preventing ectopic branching in other neurons such as the AVL neuron. In addition, kpc-1 functions during cell migration of the touch neuron ALM and HSN motoneurons. How could kpc-1/furin regulate such seemingly diverse developmental processes as formation and extension of neurite branches or neuronal positioning? One hint may come from our time-lapse analyses, which established that PVD branches grow slower in kpc-1/furin mutants compared to wild type animals. It remains unclear how KPC-1/Furin controls branch formation, extension or the speed of growth in such diverse cellular contexts on a mechanistic level. One possibility is that KPC-1 regulates extracellular adhesion of the neuron/growth cone to the substrate either directly or indirectly to mediate these functions, much like it has been suggested for metalloproteases (reviewed in [24]).
An important question is hence to determine the target(s) that are proteolytically processed by KPC-1/furin. In vertebrates, furin or furin-like proteases are known to cleave members of the TGFbeta family of morphogens as well as neuropeptides in the secretory pathway [17], [18]. Yet, mutations in genes required for neuropeptide processing and secretion, including the EGL-21/carboxypeptidase E, the PAMN-1/peptidyl-α-hydroxyglycine-α-amidating lyase [25] or unc-31/CAPS which is required for dense core vesicle secretion [26] did not result in comparable defects in PVD (Figure S6). Similarly, neither mutations in genes of the TGFbeta pathway (including the TGFbeta ligands tig-2, dbl-1, unc-129 or the sole type II TGFbeta receptor daf-4) nor in the C. elegans homolog of the repulsive guidance molecule (RGM), known to be cleaved by furin [27] displayed similar defects in PVD (data not shown). Several of the genes that have been shown to be required for PVD development contain predicted cleavage sites for furin-like proprotein convertases (Table S3). Thus, a candidate gene approach testing those genes and alternative genetic or proteomic approaches will be required to identify the in vivo target(s) of KPC-1 that are important for PVD dendritic arborization.
Materials and Methods
Strains and genetics
Worms were grown on OP50 Escherichia coli-seeded nematode growth medium plates at 20°C. Strains used in this work include: N2 (wild type reference), kpc-1(dz177), kpc-1(dz182), kpc-1(dz185), kpc-1(gk333538), kpc-1(gk779937), kpc-1(gk8), sax-7(nj48), sax-7(dz156), mnr-1(dz175) and dma-1(tm5159). PVD neurons were visualized by the integrated transgene wdIs52 (Is[F49H12.4::GFP]) and FLP neurons with muIs32 (Is[mec-7prom::GFP]). Transgenic strains for cell-specific rescue were established by injecting the respective plasmids at 5 ng/µl together with rol-6 (su1006) at 50 ng/µl as a dominant injection marker into kpc-1(dz182). The transcriptional reporter kpc-1prom5.8::GFP was injected at 5 ng/µl together with ttx-3prom::mCherry at 5 ng/µl into N2 wild type animals. For details on strains and transgenesis see also Supplementary Text S1.
Identification of kpc-1
In a clonal F1 Ethyl methanesulfonate (EMS) screen [16] we identified three alleles of kpc-1. Two alleles with similar phenotypes, dz177 and dz182 were mapped and cloned using a one-step whole genome sequencing approach [28]. Within the mapped region both dz177 and dz182 carried nonsense mutations in kpc-1 on chromosome I at positions 11,676,957 (C to T) and 11,679,245 (G to A) (WS220), respectively. One additional allele, dz185 failed to complement dz182 for the PVD phenotype and contained a missense mutation in kpc-1 at position 11,678,076 (A to T); three additional alleles were obtained from the C. elegans strain collection: the gk8 deletion allele and the missense alleles gk333538 and gk779937, which change 11,678,078 (G to A) and 11,678,071 (G to A), respectively. Transgenic animals carrying a wild type copy of the kpc-1 locus (fosmid WRM0635bG07) fully rescued the PVD defect in dz182 mutants (Figure 2C). For details on mapping and identifying the molecular lesions of different alleles see Supplementary Text S1, and Tables S1 & S2.
Molecular cloning
The kpc-1 cDNA was amplified with gene specific primers from a N2 mixed stage cDNA sample and cloned KpnI/EcoRI downstream of the ttx-3promB regulatory element [29]. For the cell specific heterologous rescue the kpc-1 cDNA was placed under control of the dpy-7prom, myo-3prom, or ser-2prom3 promoters, respectively. The transcriptional reporter was constructed by cloning 5.8 kb upstream of the predicted kpc-1 translational start site into pPD95.75 (gift of A. Fire).
Imaging and quantification of branching
Synchronized starved L1 larvae were allowed to grow for 30 hrs (corresponding to mid - to late L4) at which time they were mounted and fluorescent images of immobilized animals (1–5 mM levamisol, Sigma) were captured using a Zeiss Axioimager Z1 Apotome. Z stacks were collected and maximum projections were used for tracing of dendrites as described [16]. For time lapse imaging, animals at the L3 stage (by gonadal development) were immobilized as described [30]. PVD neurons were imaged for six to eight hours in 5 min intervals starting at the beginning of secondary branch development. Z-projections (0.5 µm/step) spanning the focal depth of the neuron were collected using a 63× objective. At least four movies per genotype were obtained using an inverted Nikon TE2000-S microscope equipped with a Perkin-Elmer UltraVIEW spinning disk unit. Volocity software (version 6.2.1) was used to collect the raw files. Processing was carried out using the Image-J 1.46r software.
Supporting Information
Zdroje
1. HallDH, TreininM (2011) How does morphology relate to function in sensory arbors? Trends Neurosci 34 : 443–451.
2. ParrishJZ, EmotoK, KimMD, JanYN (2007) Mechanisms that regulate establishment, maintenance, and remodeling of dendritic fields. Annu Rev Neurosci 30 : 399–423.
3. JanYN, JanLY (2010) Branching out: mechanisms of dendritic arborization. Nat Rev Neurosci 11 : 316–328.
4. KaufmannWE, MoserHW (2000) Dendritic anomalies in disorders associated with mental retardation. Cereb Cortex 10 : 981–991.
5. KulkarniVA, FiresteinBL (2012) The dendritic tree and brain disorders. Mol Cell Neurosci 50 : 10–20.
6. CortyMM, MatthewsBJ, GrueberWB (2009) Molecules and mechanisms of dendrite development in Drosophila. Development 136 : 1049–1061.
7. HanC, WangD, SobaP, ZhuS, LinX, et al. (2012) Integrins Regulate Repulsion-Mediated Dendritic Patterning of Drosophila Sensory Neurons by Restricting Dendrites in a 2D Space. Neuron 73 : 64–78.
8. KimME, ShresthaBR, BlazeskiR, MasonCA, GrueberWB (2012) Integrins Establish Dendrite-Substrate Relationships that Promote Dendritic Self-Avoidance and Patterning in Drosophila Sensory Neurons. Neuron 73 : 79–91.
9. TsalikEL, NiacarisT, WenickAS, PauK, AveryL, et al. (2003) LIM homeobox gene-dependent expression of biogenic amine receptors in restricted regions of the C. elegans nervous system. Dev Biol 263 : 81–102.
10. HaleviS, McKayJ, PalfreymanM, YassinL, EshelM, et al. (2002) The C. elegans ric-3 gene is required for maturation of nicotinic acetylcholine receptors. EMBO J 21 : 1012–1020.
11. Oren-SuissaM, HallDH, TreininM, ShemerG, PodbilewiczB (2010) The fusogen EFF-1 controls sculpting of mechanosensory dendrites. Science 328 : 1285–1288.
12. SmithCJ, O'BrienT, ChatzigeorgiouM, SpencerWC, Feingold-LinkE, et al. (2013) Sensory Neuron Fates Are Distinguished by a Transcriptional Switch that Regulates Dendrite Branch Stabilization. Neuron 79 : 266–280.
13. SmithCJ, WatsonJD, SpencerWC, O'BrienT, ChaB, et al. (2010) Time-lapse imaging and cell-specific expression profiling reveal dynamic branching and molecular determinants of a multi-dendritic nociceptor in C. elegans. Dev Biol 345 : 18–33.
14. Aguirre-ChenC, BülowHE, KaprielianZ (2011) C. elegans bicd-1, homolog of the Drosophila dynein accessory factor Bicaudal D, regulates the branching of PVD sensory neuron dendrites. Development 138 : 507–518.
15. DongX, LiuOW, HowellAS, ShenK (2013) An extracellular adhesion molecule complex patterns dendritic branching and morphogenesis. Cell 155 : 296–307.
16. SalzbergY, Diaz-BalzacCA, Ramirez-SuarezNJ, AttreedM, TecleE, et al. (2013) Skin-Derived Cues Control Arborization of Sensory Dendrites in Caenorhabditis elegans. Cell 155 : 308–320.
17. ArtensteinAW, OpalSM (2011) Proprotein convertases in health and disease. N Engl J Med 365 : 2507–2518.
18. SeidahNG (2011) The proprotein convertases, 20 years later. Methods Mol Biol 768 : 23–57.
19. SeidahNG, PratA (2012) The biology and therapeutic targeting of the proprotein convertases. Nat Rev Drug Discov 11 : 367–383.
20. ThackerC, RoseAM (2000) A look at the Caenorhabditis elegans Kex2/Subtilisin-like proprotein convertase family. Bioessays 22 : 545–553.
21. SchroederNE, AndrowskiRJ, RashidA, LeeH, LeeJ, et al. (2013) Dauer-specific dendrite arborization in C. elegans is regulated by KPC-1/Furin. Curr Biol 23 : 1527–1535.
22. HenrichS, CameronA, BourenkovGP, KiefersauerR, HuberR, et al. (2003) The crystal structure of the proprotein processing proteinase furin explains its stringent specificity. Nat Struct Biol 10 : 520–526.
23. BülowHE, BerryKL, TopperLH, PelesE, HobertO (2002) Heparan sulfate proteoglycan-dependent induction of axon branching and axon misrouting by the Kallmann syndrome gene kal-1. Proc Natl Acad Sci U S A 99 : 6346–6351.
24. WeberS, SaftigP (2012) Ectodomain shedding and ADAMs in development. Development 139 : 3693–3709.
25. LiC, KimK (2008) Neuropeptides. WormBook 1–36.
26. BerwinB, FloorE, MartinTF (1998) CAPS (mammalian UNC-31) protein localizes to membranes involved in dense-core vesicle exocytosis. Neuron 21 : 137–145.
27. TassewNG, CharishJ, SeidahNG, MonnierPP (2012) SKI-1 and Furin generate multiple RGMa fragments that regulate axonal growth. Dev Cell 22 : 391–402.
28. MinevichG, ParkDS, BlankenbergD, PooleRJ, HobertO (2012) CloudMap: a cloud-based pipeline for analysis of mutant genome sequences. Genetics 192 : 1249–1269.
29. Altun-GultekinZ, AndachiY, TsalikEL, PilgrimD, KoharaY, et al. (2001) A regulatory cascade of three homeobox genes, ceh-10, ttx-3 and ceh-23, controls cell fate specification of a defined interneuron class in C. elegans. Development 128 : 1951–1969.
30. KimE, SunL, GabelCV, Fang-YenC (2013) Long-Term Imaging of Caenorhabditis elegans Using Nanoparticle-Mediated Immobilization. PLoS One 8: e53419.
31. GilleardJS, BarryJD, JohnstoneIL (1997) cis regulatory requirements for hypodermal cell-specific expression of the Caenorhabditis elegans cuticle collagen gene dpy-7. Mol Cell Biol 17 : 2301–2311.
32. OkkemaPG, HarrisonSW, PlungerV, AryanaA, FireA (1993) Sequence requirements for myosin gene expression and regulation in Caenorhabditis elegans. Genetics 135 : 385–404.
33. SmithCJ, WatsonJD, VanHovenMK, Colon-RamosDA, MillerDM3rd (2012) Netrin (UNC-6) mediates dendritic self-avoidance. Nat Neurosci 15 : 731–737.
34. LiuOW, ShenK (2012) The transmembrane LRR protein DMA-1 promotes dendrite branching and growth in C. elegans. Nat Neurosci 15 : 57–63.
35. AlbegA, SmithCJ, ChatzigeorgiouM, FeitelsonDG, HallDH, et al. (2011) C. elegans multi-dendritic sensory neurons: morphology and function. Mol Cell Neurosci 46 : 308–317.
36. ChatzigeorgiouM, YooS, WatsonJD, LeeWH, SpencerWC, et al. (2010) Specific roles for DEG/ENaC and TRP channels in touch and thermosensation in C. elegans nociceptors. Nat Neurosci 13 : 861–868.
Štítky
Genetika Reprodukční medicína
Článek An Evolutionarily Conserved Role for the Aryl Hydrocarbon Receptor in the Regulation of MovementČlánek Requirement for Drosophila SNMP1 for Rapid Activation and Termination of Pheromone-Induced ActivityČlánek Co-regulated Transcripts Associated to Cooperating eSNPs Define Bi-fan Motifs in Human Gene NetworksČlánek Identification of a Regulatory Variant That Binds FOXA1 and FOXA2 at the Type 2 Diabetes GWAS LocusČlánek tRNA Modifying Enzymes, NSUN2 and METTL1, Determine Sensitivity to 5-Fluorouracil in HeLa CellsČlánek Derlin-1 Regulates Mutant VCP-Linked Pathogenesis and Endoplasmic Reticulum Stress-Induced ApoptosisČlánek A Genetic Assay for Transcription Errors Reveals Multilayer Control of RNA Polymerase II FidelityČlánek Regulation of p53 and Rb Links the Alternative NF-κB Pathway to EZH2 Expression and Cell SenescenceČlánek BMPs Regulate Gene Expression in the Dorsal Neuroectoderm of and Vertebrates by Distinct MechanismsČlánek Unkempt Is Negatively Regulated by mTOR and Uncouples Neuronal Differentiation from Growth Control
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2014 Číslo 9- Akutní intermitentní porfyrie
- Růst a vývoj dětí narozených pomocí IVF
- Farmakogenetické testování pomáhá předcházet nežádoucím efektům léčiv
- Pilotní studie: stres a úzkost v průběhu IVF cyklu
- Vliv melatoninu a cirkadiálního rytmu na ženskou reprodukci
-
Všechny články tohoto čísla
- Translational Regulation of the Post-Translational Circadian Mechanism
- An Evolutionarily Conserved Role for the Aryl Hydrocarbon Receptor in the Regulation of Movement
- Eliminating Both Canonical and Short-Patch Mismatch Repair in Suggests a New Meiotic Recombination Model
- Requirement for Drosophila SNMP1 for Rapid Activation and Termination of Pheromone-Induced Activity
- Co-regulated Transcripts Associated to Cooperating eSNPs Define Bi-fan Motifs in Human Gene Networks
- Targeted H3R26 Deimination Specifically Facilitates Estrogen Receptor Binding by Modifying Nucleosome Structure
- Role for Circadian Clock Genes in Seasonal Timing: Testing the Bünning Hypothesis
- The Tandem Repeats Enabling Reversible Switching between the Two Phases of β-Lactamase Substrate Spectrum
- The Association of the Vanin-1 N131S Variant with Blood Pressure Is Mediated by Endoplasmic Reticulum-Associated Degradation and Loss of Function
- Identification of a Regulatory Variant That Binds FOXA1 and FOXA2 at the Type 2 Diabetes GWAS Locus
- Regulation of Flowering by the Histone Mark Readers MRG1/2 via Interaction with CONSTANS to Modulate Expression
- The Actomyosin Machinery Is Required for Retinal Lumen Formation
- Plays a Conserved Role in Assembly of the Ciliary Motile Apparatus
- Hidden Diversity in Honey Bee Gut Symbionts Detected by Single-Cell Genomics
- Ribosome Rescue and Translation Termination at Non-Standard Stop Codons by ICT1 in Mammalian Mitochondria
- tRNA Modifying Enzymes, NSUN2 and METTL1, Determine Sensitivity to 5-Fluorouracil in HeLa Cells
- Causal Variation in Yeast Sporulation Tends to Reside in a Pathway Bottleneck
- Tissue-Specific RNA Expression Marks Distant-Acting Developmental Enhancers
- WC-1 Recruits SWI/SNF to Remodel and Initiate a Circadian Cycle
- Clonal Expansion of Early to Mid-Life Mitochondrial DNA Point Mutations Drives Mitochondrial Dysfunction during Human Ageing
- Methylation QTLs Are Associated with Coordinated Changes in Transcription Factor Binding, Histone Modifications, and Gene Expression Levels
- Differential Management of the Replication Terminus Regions of the Two Chromosomes during Cell Division
- Obesity-Linked Homologues and Establish Meal Frequency in
- Derlin-1 Regulates Mutant VCP-Linked Pathogenesis and Endoplasmic Reticulum Stress-Induced Apoptosis
- Stress-Induced Nuclear RNA Degradation Pathways Regulate Yeast Bromodomain Factor 2 to Promote Cell Survival
- The MAPK p38c Regulates Oxidative Stress and Lipid Homeostasis in the Intestine
- Widespread Genome Reorganization of an Obligate Virus Mutualist
- Trans-kingdom Cross-Talk: Small RNAs on the Move
- The Vip1 Inositol Polyphosphate Kinase Family Regulates Polarized Growth and Modulates the Microtubule Cytoskeleton in Fungi
- Myosin Vb Mediated Plasma Membrane Homeostasis Regulates Peridermal Cell Size and Maintains Tissue Homeostasis in the Zebrafish Epidermis
- GLD-4-Mediated Translational Activation Regulates the Size of the Proliferative Germ Cell Pool in the Adult Germ Line
- Genome Wide Association Studies Using a New Nonparametric Model Reveal the Genetic Architecture of 17 Agronomic Traits in an Enlarged Maize Association Panel
- Translational Regulation of the DOUBLETIME/CKIδ/ε Kinase by LARK Contributes to Circadian Period Modulation
- Positive Selection and Multiple Losses of the LINE-1-Derived Gene in Mammals Suggest a Dual Role in Genome Defense and Pluripotency
- Out of Balance: R-loops in Human Disease
- A Genetic Assay for Transcription Errors Reveals Multilayer Control of RNA Polymerase II Fidelity
- Altered Behavioral Performance and Live Imaging of Circuit-Specific Neural Deficiencies in a Zebrafish Model for Psychomotor Retardation
- Nipbl and Mediator Cooperatively Regulate Gene Expression to Control Limb Development
- Meta-analysis of Mutations in Autism Spectrum Disorders: A Gradient of Severity in Cognitive Impairments
- The Proprotein Convertase KPC-1/Furin Controls Branching and Self-avoidance of Sensory Dendrites in
- Hydroxymethylated Cytosines Are Associated with Elevated C to G Transversion Rates
- Memory and Fitness Optimization of Bacteria under Fluctuating Environments
- Regulation of p53 and Rb Links the Alternative NF-κB Pathway to EZH2 Expression and Cell Senescence
- Interspecific Tests of Allelism Reveal the Evolutionary Timing and Pattern of Accumulation of Reproductive Isolation Mutations
- PRO40 Is a Scaffold Protein of the Cell Wall Integrity Pathway, Linking the MAP Kinase Module to the Upstream Activator Protein Kinase C
- Low Levels of p53 Protein and Chromatin Silencing of p53 Target Genes Repress Apoptosis in Endocycling Cells
- SPDEF Inhibits Prostate Carcinogenesis by Disrupting a Positive Feedback Loop in Regulation of the Foxm1 Oncogene
- RRP6L1 and RRP6L2 Function in Silencing Regulation of Antisense RNA Synthesis
- BMPs Regulate Gene Expression in the Dorsal Neuroectoderm of and Vertebrates by Distinct Mechanisms
- Unkempt Is Negatively Regulated by mTOR and Uncouples Neuronal Differentiation from Growth Control
- Atkinesin-13A Modulates Cell-Wall Synthesis and Cell Expansion in via the THESEUS1 Pathway
- Dopamine Signaling Leads to Loss of Polycomb Repression and Aberrant Gene Activation in Experimental Parkinsonism
- Histone Methyltransferase MMSET/NSD2 Alters EZH2 Binding and Reprograms the Myeloma Epigenome through Global and Focal Changes in H3K36 and H3K27 Methylation
- Bipartite Recognition of DNA by TCF/Pangolin Is Remarkably Flexible and Contributes to Transcriptional Responsiveness and Tissue Specificity of Wingless Signaling
- The Olfactory Transcriptomes of Mice
- Muscular Dystrophy-Associated and Variants Disrupt Nuclear-Cytoskeletal Connections and Myonuclear Organization
- Interplay of dFOXO and Two ETS-Family Transcription Factors Determines Lifespan in
- Evidence for Widespread Positive and Negative Selection in Coding and Conserved Noncoding Regions of
- Genome-Wide Association Meta-analysis of Neuropathologic Features of Alzheimer's Disease and Related Dementias
- Rejuvenation of Meiotic Cohesion in Oocytes during Prophase I Is Required for Chiasma Maintenance and Accurate Chromosome Segregation
- Admixture in Latin America: Geographic Structure, Phenotypic Diversity and Self-Perception of Ancestry Based on 7,342 Individuals
- Local Effect of Enhancer of Zeste-Like Reveals Cooperation of Epigenetic and -Acting Determinants for Zygotic Genome Rearrangements
- Differential Responses to Wnt and PCP Disruption Predict Expression and Developmental Function of Conserved and Novel Genes in a Cnidarian
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Admixture in Latin America: Geographic Structure, Phenotypic Diversity and Self-Perception of Ancestry Based on 7,342 Individuals
- Nipbl and Mediator Cooperatively Regulate Gene Expression to Control Limb Development
- Genome Wide Association Studies Using a New Nonparametric Model Reveal the Genetic Architecture of 17 Agronomic Traits in an Enlarged Maize Association Panel
- Histone Methyltransferase MMSET/NSD2 Alters EZH2 Binding and Reprograms the Myeloma Epigenome through Global and Focal Changes in H3K36 and H3K27 Methylation
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



