-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaType I-E CRISPR-Cas Systems Discriminate Target from Non-Target DNA through Base Pairing-Independent PAM Recognition
Discriminating self and non-self is a universal requirement of immune systems. Adaptive immune systems in prokaryotes are centered around repetitive loci called CRISPRs (clustered regularly interspaced short palindromic repeat), into which invader DNA fragments are incorporated. CRISPR transcripts are processed into small RNAs that guide CRISPR-associated (Cas) proteins to invading nucleic acids by complementary base pairing. However, to avoid autoimmunity it is essential that these RNA-guides exclusively target invading DNA and not complementary DNA sequences (i.e., self-sequences) located in the host's own CRISPR locus. Previous work on the Type III-A CRISPR system from Staphylococcus epidermidis has demonstrated that a portion of the CRISPR RNA-guide sequence is involved in self versus non-self discrimination. This self-avoidance mechanism relies on sensing base pairing between the RNA-guide and sequences flanking the target DNA. To determine if the RNA-guide participates in self versus non-self discrimination in the Type I-E system from Escherichia coli we altered base pairing potential between the RNA-guide and the flanks of DNA targets. Here we demonstrate that Type I-E systems discriminate self from non-self through a base pairing-independent mechanism that strictly relies on the recognition of four unchangeable PAM sequences. In addition, this work reveals that the first base pair between the guide RNA and the PAM nucleotide immediately flanking the target sequence can be disrupted without affecting the interference phenotype. Remarkably, this indicates that base pairing at this position is not involved in foreign DNA recognition. Results in this paper reveal that the Type I-E mechanism of avoiding self sequences and preventing autoimmunity is fundamentally different from that employed by Type III-A systems. We propose the exclusive targeting of PAM-flanked sequences to be termed a target versus non-target discrimination mechanism.
Published in the journal: . PLoS Genet 9(9): e32767. doi:10.1371/journal.pgen.1003742
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1003742Summary
Discriminating self and non-self is a universal requirement of immune systems. Adaptive immune systems in prokaryotes are centered around repetitive loci called CRISPRs (clustered regularly interspaced short palindromic repeat), into which invader DNA fragments are incorporated. CRISPR transcripts are processed into small RNAs that guide CRISPR-associated (Cas) proteins to invading nucleic acids by complementary base pairing. However, to avoid autoimmunity it is essential that these RNA-guides exclusively target invading DNA and not complementary DNA sequences (i.e., self-sequences) located in the host's own CRISPR locus. Previous work on the Type III-A CRISPR system from Staphylococcus epidermidis has demonstrated that a portion of the CRISPR RNA-guide sequence is involved in self versus non-self discrimination. This self-avoidance mechanism relies on sensing base pairing between the RNA-guide and sequences flanking the target DNA. To determine if the RNA-guide participates in self versus non-self discrimination in the Type I-E system from Escherichia coli we altered base pairing potential between the RNA-guide and the flanks of DNA targets. Here we demonstrate that Type I-E systems discriminate self from non-self through a base pairing-independent mechanism that strictly relies on the recognition of four unchangeable PAM sequences. In addition, this work reveals that the first base pair between the guide RNA and the PAM nucleotide immediately flanking the target sequence can be disrupted without affecting the interference phenotype. Remarkably, this indicates that base pairing at this position is not involved in foreign DNA recognition. Results in this paper reveal that the Type I-E mechanism of avoiding self sequences and preventing autoimmunity is fundamentally different from that employed by Type III-A systems. We propose the exclusive targeting of PAM-flanked sequences to be termed a target versus non-target discrimination mechanism.
Introduction
There are several prokaryotic defense systems that confer innate immunity against invading mobile genetic elements, such as receptor masking, blocking DNA injection, restriction/modification (R-M) and abortive infection (reviewed in [1]–[3]). In addition, half of the bacteria, and most of the archaea, contain CRISPR-Cas (Clustered Regularly Interspaced Short Palindromic Repeats/CRISPR-associated) defense systems, unique in being the only adaptive line of prokaryotic defense (reviewed in [4]–[7]). CRISPR-Cas systems provide adaptive immunity to the host by incorporating invader DNA sequences into chromosomal CRISPR loci [8]–[11]. The 30–40 nt invader-derived DNA sequences are separated by host-derived similarly-sized repeat sequences. Adjacent to a CRISPR locus, a set of cas genes can often be found that encode the protein machinery essential for CRISPR-immunity. The cas genes occur in characteristic combinations that serve as a classification criterion of CRISPR-Cas systems into three major types [12]. In Type I and Type III systems the long precursor CRISPR RNA (pre-crRNA) is processed by CRISPR specific endoribonucleases into small CRISPR RNAs (crRNAs) that contain a repeat sequence flaked by portions of the adjacent CRISPR repeat sequence [13]–[18]. In some CRISPR-Cas subtypes the crRNA undergoes further processing at the 3′ end [19], [20]. In Type II CRISPR-Cas systems the pre-crRNA is processed by RNase III [21]. The processed crRNA molecules then remain bound to one or more Cas proteins to guide recognition and cleavage of complementary nucleic acid sequences [22]–[27].
With the exception of Type III-B CRISPR-Cas systems, which cleave RNA [23], [24],[28], all other characterized CRISPR-Cas systems appear to target DNA [27], [29]–[32] and hence require a mechanism to avoid aberrant cleavage of genomic DNA, i.e. a mechanism to discriminate the genomic “self” DNA of a CRISPR cassette from the invader “non-self” DNA. The absence of such discrimination leads to a suicidal autoimmune response [33]–[35]. In R-M systems this problem is solved by modification of the genomic DNA and cleavage of unmodified invader DNA only (reviewed in [3]). For CRISPR-Cas systems on the other hand, the mechanism(s) of self versus non-self discrimination is only partially understood.
For the Type III-A system of Staphylococcus epidermidis autoimmunity is prevented through a mechanism that relies on sensing base pairing between the 5′-handle (the repeat-derived sequence at the 5′-end of the crRNA) and the corresponding portion of CRISPR repeat [36]. The Type III-A CRISPR-Cas system consists of nine cas genes (cas1, cas2, cas10, csm2, csm3, csm4, csm5, csm6, cas6) and a CRISPR with type-8 repeats [37]. After a primary processing step of the pre-crRNA, the resulting crRNAs are further matured through ruler-based cleavage from the 3′ end, yielding 43 and 37 nt crRNA species [20]. These mature crRNA species guide one or more Cas proteins (possibly a Csm-complex) to target DNA [32], presumably through base pairing between the crRNA spacer sequence and the complementary protospacer sequence. However, CRISPR-interference is inhibited when, in addition to base pairing over the spacer sequence, the 5′-handle also base pairs with the protospacer-flanking sequence of the target DNA [36]. In this manner, self-targeting of the CRISPR locus is avoided by default, since self-targeting inevitably leads to full base pairing of the 5′-handle of the crRNA with the CRISPR repeat sequence from which it is transcribed. In particular, the presence or absence of base pairing at three positions downstream of the protospacer (positions −2, −3, and −4 relative to the 3′-end of the protospacer) is decisive in discriminating self from non-self [36]. The molecular details of how base pairing at positions downstream of the protospacer are sensed, and whether it involves Cas proteins, is currently unknown.
Intriguingly, Type I systems contain di - or tri-nucleotide conserved motifs (protospacer adjacent motifs (PAM)) downstream of protospacers opposite of the crRNA 5′-handle [38]–[40] (Figure 1A and 2A). In the Type I-E CRISPR-Cas system, PAM sequences are recognized by ribonucleoprotein complex Cascade during target DNA binding [29], [41]. The Type I-E system of Escherichia coli K12 consists of 8 cas genes (cas3, cse1, cse2, cas7, cas5, cas6e, cas1, cas2) and two CRISPR loci with type-2 repeats [37]. The ribonucleoprotein complex Cascade is composed of a 61 nt crRNA, and five different Cas proteins in an uneven stoichiometry: Cse11Cse22Cas76Cas51Cas6e1 [22]. Cascade efficiently binds target DNA through an R-loop formed between the 32 nt spacer sequence of the crRNA and the protospacer sequence [22] (Figure 1A), with a binding affinity that is strongly dependent on the presence of one of the four functional PAM sequences [29], [41]. Whereas R-loop formation by Cascade involves the entire protospacer sequence [22], it is unknown whether the PAM nucleotides can participate in base pairing with the crRNA and, if so, how this influences CRISPR interference. Due to the fact that the last nucleotide from the repeat is derived from the PAM sequence during spacer acquisition [8], [11], [42], this nucleotide in the crRNA invariably has the potential to base pair with the −1 position of the PAM, and therefore might be involved in R-loop formation [8]. In contrast, the −2 and −3 positions of the PAM lack base pairing potential with the 5′-handle of the crRNA (Figure 2A). The 5′-handles of other Type I systems and 3′-handles of Type II also display limited base pairing potential with their cognate PAMs (Table S1), in principle allowing for a differential base pairing mechanism that defines self versus non-self. For Type I-F CRISPR-Cas systems, potential base pairing between PAM sequences and the 5′-handle of the crRNA was recently shown to affect CRISPR interference [43], suggesting that self versus non-self discrimination in this subtype may depend both on sensing PAM identity and on sensing differential base pairing with the crRNA repeat.
Fig. 1. Potential base pairing between the crRNA repeat regions and protospacer flanking regions does not affect CRISPR-interference. 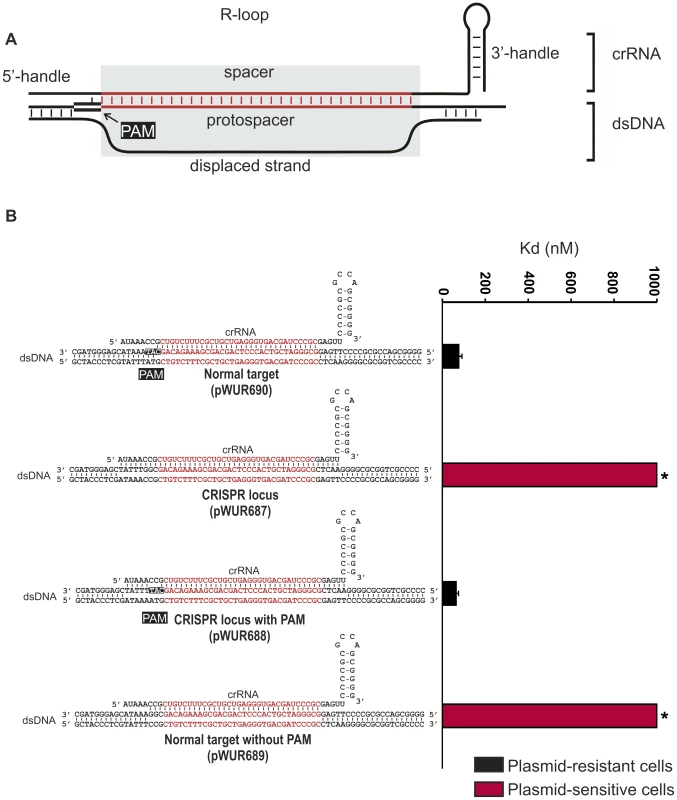
A) Model of the R-loop formed by Cascade during dsDNA binding. B) Cells expressing WT g8-Cascade and Cas3 are resistant to plasmids containing the CAT PAM adjacent to the g8 protospacer (black bars, transformation efficiency 6.7±1.5×105 cfu/µg DNA for plasmid pWUR690 and 6.8±0.9×105 cfu/µg DNA for plasmid pWUR688), but are susceptible to plasmid transformation when the g8 protospacer is flanked by a CGG PAM, which is fully complementary to the 5′-handle (red bars, transformation efficiency 4.2±0.9×108 cfu/µg DNA for plasmid pWUR687 and 4.5±0.8×108 cfu/µg DNA for plasmid pWUR689). Transformation efficiency for a control pUC19 plasmid is 6.2±1.1×108 cfu/µg DNA. The histogram shows the in vitro binding affinity of purified WT g8-Cascade for dsDNA containing the g8 protospacer flanked by sequences with a varying base pairing potential, as shown on the right. Asterisks indicate that the Kd value is >>1000 nM and the error bars represent the standard deviation of the mean. Fig. 2. Base pairing at the −1 position is not required for CRISPR-interference. 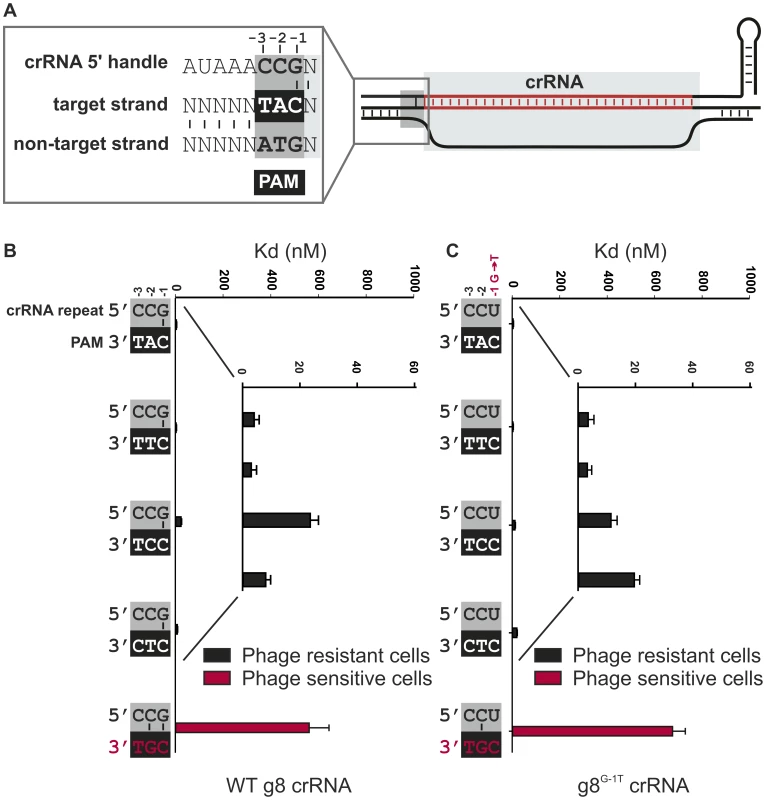
A) Model of the R-loop formed by Cascade during dsDNA binding. The nucleotide adjacent to the spacer sequence (the −1 position) has the potential to base pair with the first nucleotide of the PAM in the target strand of the DNA. B) Cells expressing WT g8-Cascade and Cas3 are resistant to M13 phage containing the CAT, CTT, CCT or CTC PAMs adjacent to the M13 protospacer (white font/black bars, e.o.p.<10−4), but not when containing the CGT PAM (red font/red bars, e.o.p. = 1). Note that in the figure the PAMs are oriented in 3′ to 5′direction to display base pairing potential with the last three nucleotides of the crRNA repeat. The in vitro binding affinity of purified WT g8-Cascade for dsDNA containing the g8 protospacer and each of the respective PAM mutants is shown in the adjacent histogram. C) Assays as in (B) using cells expressing the g8G-1T CRISPR, Cascade and Cas3, show that cells are resistant to M13 phage containing the CAT, CTT, CCT or CTC PAMs adjacent to the g8 protospacer (white font/black bars), but not when containing the CGT PAM (red font/red bars). The in vitro binding affinity of purified WT g8G-1T-Cascade for dsDNA containing the g8 protospacer and each of the respective PAM mutants is shown in the adjacent histogram. In (B) and (C) error bars represent the standard deviation of the mean. In Type I-E systems it has been shown that a loop structure (L1) of the Cse1 subunit of Cascade specifically interacts with the PAM sequence, a process that is thought to destabilize the double-stranded DNA of the target to allow for strand invasion during R-loop formation [44]. Since self DNA of the CRISPR locus does not contain PAM sequences, this mechanism would specifically direct Cascade to target DNA only. However, the observation that target DNA containing a PAM mutant triggers Cascade-dependent primed spacer acquisition in vivo suggests that PAM authentication may not be absolutely required for R-loop formation [11]. Indeed, negatively supercoiled DNA containing a protospacer with a mutant PAM can still be bound by Cascade, albeit with a lower affinity than the same target with wild-type PAM [29]. In line with this, it was suggested that during phage infection Cascade can overcome the absence of a bona fide PAM when Cascade expression levels are high and that the target flanking sequences could participate in this discrimination event [44]. This suggests that a differential base pairing mechanism may play a role in self versus non-self discrimination by Type I-E CRISPR-Cas systems. In agreement with this, it was suggested that complementarity between the crRNA repeat and the protospacer flanking sequence inhibits CRISPR-interference in the Type I-E system of Streptococcus thermophilus [45]. The mechanistic basis of such a differential base pairing mechanism could lie in a perturbation of Cse1-mediated PAM recognition by base pairing interactions between crRNA repeats and the PAM.
To study whether a differential base pairing mechanism plays a role in self versus non-self discrimination by the Type I-E system of E. coli K12, we have systematically mutated both the crRNA repeats and the protospacer-flanking sequences and determined the effects of these mutations and their combinations on CRISPR interference in vivo and target binding in vitro. The results of our analysis demonstrate that discrimination of self from non-self by Type I-E CRISPR-Cas systems occurs through a mechanism that is independent of base pairing between these sequences. Hence, the principal mechanism by which Type I-E systems discriminate self from non-self appears to be solely Cse1-mediated and as such is fundamentally different from the differential base pairing mechanism employed by Type III-A systems. While the mechanism employed by Type III-A is best described as being based on self-recognition (self versus non-self), the mechanism of Type I-E systems is instead based on target-recognition (target versus non-target). While Type III systems can differentiate between targets and non-targets in the absence of a PAM, Type I-E systems are fully PAM-dependent and discrimination cannot take place in the absence of a PAM.
Results
Self versus non-self discrimination by the Type III-A CRISPR-Cas system of S. epidermidis has been shown to rely on a differential base pairing mechanism [36]. As a result CRISPR-interference is specifically inhibited when protospacer sequences are flanked by CRISPR repeat sequences. To test whether this mechanism also applies to the Type I-E CRISPR-Cas system of E. coli K12, CRISPR-interference was tested against targets containing protospacers flanked by CRISPR repeat sequences. For these analyses, we have cloned the previously described g8 protospacer, from phage M13 [41], into the pUC19 plasmid and systematically mutated sequences adjacent to the protospacer. E. coli cells expressing Cascade, a g8 crRNA and Cas3 are resistant against transformation by a plasmid in which the g8 protospacer is flanked by a CAT PAM (Fig. 1B, pWUR690, approximately 1000-fold lower efficiency of transformation than a control pUC19 plasmid). In contrast, these cells are susceptible to plasmid transformation by plasmid pWUR687 in which the g8 protospacer is flanked by CRISPR repeat sequences (Figure 1B). However, the plasmid resistant phenotype can be restored by introducing a CAT PAM in the CRISPR repeat sequence flanking the protospacer (pWUR688), which alters the base pairing potential only at the −2 and −3 positions (Figure 1B). Plasmid pWUR689, which has the potential to base pair with g8 crRNA at positions −1, −2 and −3 (protospacer adjacent sequence is CGG) escapes CRISPR-interference from wild-type g8 crRNA expressing E. coli (Figure 1B). The observation that protospacer adjacent sequences complementary to the crRNA at positions −1, −2, and −3 avoid Cascade targeting suggest that base pairing at these positions may play a role in self avoidance.
To investigate whether avoidance of targeting is due to decreased binding affinities of Cascade for protospacers with mutations at the −1, −2, and −3 positions, we performed Electrophoretic Mobility Shift Assays using purified g8 crRNA-loaded Cascade. While high affinity binding could be demonstrated to dsDNA containing the g8 protospacer flanked by the CAT PAM (Figure 1B and S1), protospacers flanked by either CRISPR repeat sequences or a repeat-derived CGG sequence were bound with low affinity (Figure 1B and S1). This indicates that target versus non-target discrimination occurs at the level of Cascade affinity for dsDNA target sequences. Furthermore, the data also indicate that “self” DNA recognition may occur, as observed in Type III-A systems, through sensing differential base pairing between protospacer adjacent sequences and the 5′ handle of the crRNA.
To investigate if base pairing between the three nucleotides from the 5′-handle of the crRNA and the PAM is involved in discriminating self from non-self DNA we systematically mutated the corresponding nucleotides in the 5′-handle (i.e., −1, −2, and −3), and analyzed how these mutations affect CRISPR-based immunity against DNA targets flanked by various PAM sequences. Previously [29], four PAM sequences (CAT, CTT, CCT and CTC), have been reported to confer immunity on wild-type g8 crRNA expressing E. coli against phage M13 infection in vivo, and to give rise to high affinity DNA binding by g8 crRNA-bound Cascade in vitro (Figure 2B and Figure S2A). The last nucleotide of the 5′-handle of the crRNA (the −1 position) invariably has the potential to base pair with the PAM [8], while the −2 and −3 positions lack such base pairing potential (Figure 2A). The resulting configuration is distinct from the fully base-paired configuration that would form if base pairing in this region were the basis of self versus non-self discrimination.
To analyze whether base pairing at position −1 is required for CRISPR interference, a mutant CRISPR was constructed, yielding a g8 crRNA that lacks base pairing potential with the PAM at this position. This CRISPR, denoted g8G-1T carries a G-to-T substitution at position −1, within the repeat sequence. SDS-PAGE analysis of purified Cascade complexes containing either mutant or WT crRNA shows that these complexes have the same apparent stoichiometry, thereby confirming the integrity of the complex (Figure S4A). In addition, isolation of crRNA from these protein complexes shows that crRNA biogenesis is unaffected by the introduced mutation (Figure S4B). Interestingly, despite the absence of base pairing at the −1 position, cells expressing the mutant crRNA maintain the ability to block infection by M13 phages containing each of the four functional PAM sequences (Figure 2C). Consistently, high affinity binding by g8G-1T crRNA-containing Cascade to targets containing the g8 protospacer and the functional PAM variants was observed (Figure 2C and Figure S2B). However, as previously observed for the WT g8-crRNA-Cascade complex [29], a mutation at the −2 position of the PAM (i.e., CGT) neither confers resistance in vivo (efficiency of plaquing (e.o.p.) = 1) nor gives rise to high affinity DNA binding in vitro (Figure 2C, and Figure S2B). This PAM mutant potentially yields an additional base pair with the −2 position of the 5′-handle, both in the WT g8-crRNA-Cascade and the g8G-1T mutant complex (Figure 2BC). Hence, it appears that a base pair at position −2 may be the signal that a protospacer is located in “self” DNA and therefore should not be targeted.
To specifically test the role of base pairing at position −2 in CRISPR-immunity, we designed a synthetic CRISPR locus containing a C to A substitution at the −2 position of a CRISPR locus containing spacer sequences that target the g8 protospacer from M13 phage. The g8C-2A CRISPR mutation results in a slight effect on Cascade assembly, as the bands corresponding to Cse1 and Cse2 have modestly lower and higher intensities on an SDS-PAGE, respectively, as compared to wild-type g8-crRNA-Cascade (Figure S4). However, g8C-2A CRISPR RNA processing is unaffected (Figure S4). Importantly, the g8C-2A crRNA-guided Cascade complex has a slightly reduced affinity (60±12 nM) for dsDNA targets that have a canonical CTT PAM sequence, which has the potential to base pair at the −2 position of the mutant crRNA (Figure 3A, white PAM). Despite the potential of the mutant Cascade complex to establish an additional base pair, a partially resistant phenotype (e.o.p.∼10−2) is observed against phages carrying the canonical PAM (Figure 3A), which is consistent with the in vitro DNA binding experiments (Figure 3A and Figure S3A). Targets containing non-canonical PAM sequences are bound with more reduced affinities by the g8C-2A crRNA-guide Cascade complex and are not subject to CRISPR-interference in vivo (Figure 3A). The partial resistant phenotype of the g8C-2A mutant that is observed in combination with the canonical PAM indicates that potential base pairing at both positions −1 and −2 does not serve as a trigger for a non-targeting response.
Fig. 3. Base pairing at the −2 and −3 positions does not interfere with CRISPR-immunity. 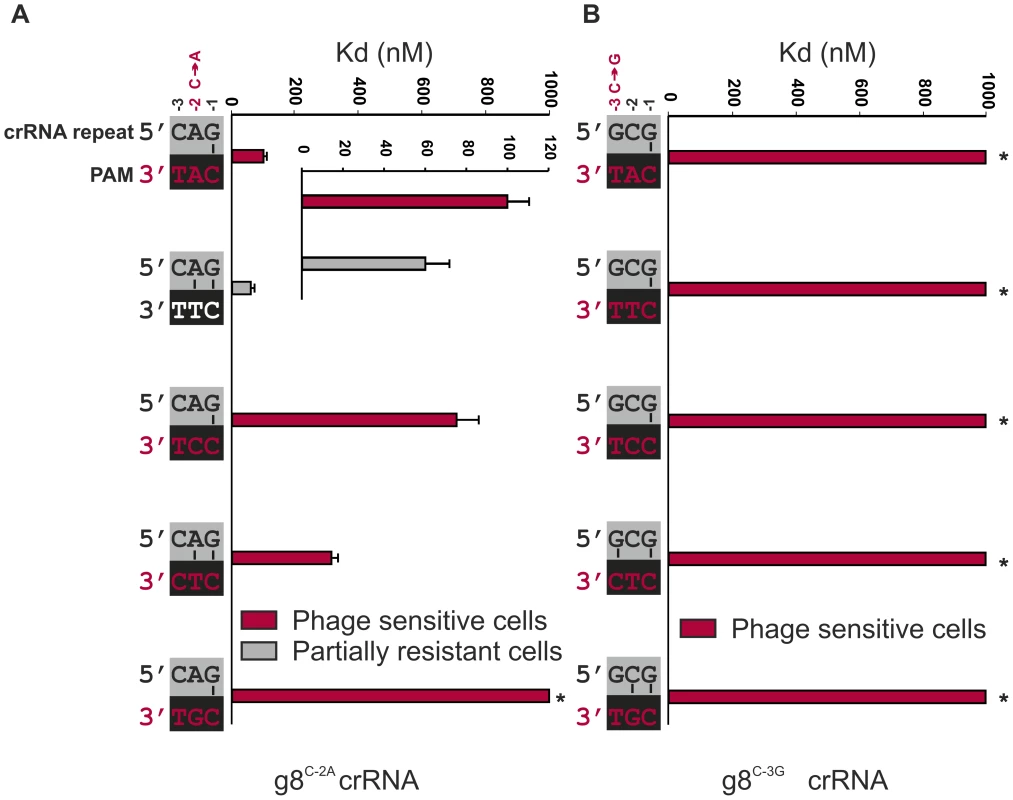
A) Cells expressing g8C-2A-Cascade and Cas3 are partially resistant to M13 phage containing the CTT PAM adjacent to the g8 protospacer (white font/grey bar, e.o.p.∼10−2), but not when containing the CAT, CCT, CTC or CGT PAM (red font/red bars). Note that in the figure the PAMs are oriented in 3′ to 5′direction to display base pairing potential with the last three nucleotides of the crRNA repeat. The in vitro binding affinity of purified WT g8C-2A-Cascade for dsDNA containing the g8 protospacer and each of the respective PAM mutants is shown in the adjacent histogram. B) Assays as in (A) using cells expressing the g8C-3G CRISPR, Cascade and Cas3, show that cells are not resistant to M13 phage containing the CAT, CTT, CCT, CTC or CGT PAMs adjacent to the g8 protospacer (red font/red bars). The in vitro binding affinity of purified WT g8C-3G-Cascade for dsDNA containing the M13 protospacer and each of the respective PAM mutants is shown in the adjacent histogram. Asterisks indicate that the Kd value is >>1000 nM and the error bars represent the standard deviation of the mean. To probe the importance of base pairing at the −3 position, an additional CRISPR mutant was designed, denoted g8C-3G, which carries a C to G mutation at the −3 position of the CRISPR repeat. Again, complex formation and crRNA biogenesis were unaffected by the mutation (Figure S4). Although the potential for base pairing with most PAM sequences remains the same, a dramatic decrease in both resistance against M13 phage in vivo and DNA binding by g8C-3G-Cascade in vitro is observed (Figure 3B and Figure S3B).
The combined results obtained with the three CRISPR mutants indicate that the repeat sequence itself rather than its base pairing potential with the protospacer flanking sequence affects PAM recognition. In order to have a more complete and unbiased analysis of the effects of adding or removing base pairing potential at positions −1, −2 and −3, we constructed 26 different PAM sequences adjacent to the g8 protospacer in the M13 phage genome (Figure 4A, white text on black background). All phages were viable as judged by their ability to infect host bacteria lacking the M13-targeting CRISPR (data not shown). The phages were tested for their ability to infect cells expressing each of the 21 different g8 crRNAs with mutated repeat sequences at positions −1, −2 and −3. Northern blot analysis showed that processing of mutant g8 crRNAs was unaffected (data not shown). The results reveal that only a small subset of CRISPR repeat mutants confer full phage resistance, and only in conjunction with the four previously validated functional PAM sequences (Fig. 4). When resistance was observed, it was independent of crRNA-PAM base pairing patterns, but rather appeared to be constrained by a limited number of allowed nucleotides at the −1, −2 and −3 positions of the 5′-handle, and a fixed number of PAM sequences.
Fig. 4. Synonymous mutations of the crRNA and the PAM do not affect self versus non-self discrimination. 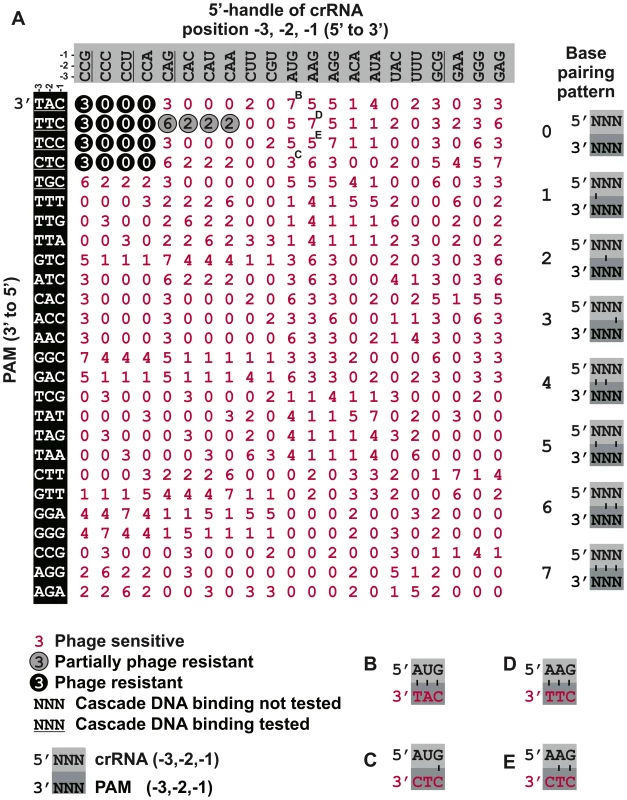
A) Infectivity of a library of M13 phage containing PAM mutants adjacent to the g8 protospacer was tested against cells expressing Cascade, Cas3 and g8 CRISPR containing mutations at the −1, −2 and −3 nucleotides of the CRISPR repeats. PAM mutations are shown on the left, with the PAM sequences indicated in the 3′ to 5′ direction. CRISPR repeat mutations at positions −1, −2 and −3 are indicated on the top in the 5′ to 3′ direction. Underscored sequences have been tested for binding affinity by EMSA. Base pairing potential between the PAM positions and the repeat is indicated using numbers (0–7) that correspond to a base pairing pattern that is shown in the panel on the right. A 0 signifies no base pairing, a 1 signifies base pairing at the −3 position, a 2 signifies base pairing at the −2 position, etc. Black circles with white digits indicate resistance against phage infection (e.o.p.<10−4), grey circles indicate partial resistance (e.o.p.∼10−2) and red digits without circle indicate susceptibility to phage infection (e.o.p. = 1), as determined by phage spot assays. Letters B, C, D, E indicate combinations shown in detail in the corresponding panels. B) Combination of g8C-3AC-2T CRISPR and M13 phage with CAT PAM, gives rise to full base pairing and a lack of resistance (red font). C) Combination of g8C-3AC-2T CRISPR and M13 phage with CTC PAM, gives rise to only base pairing at the −1 position and yields a lack of resistance (red font). D) Combination of g8C-3AC-2A CRISPR and M13 phage with CTT PAM gives rise to full base pairing and a lack of resistance (red font). E) Combination of g8C-3AC-2A CRISPR and M13 phage with CTC PAM gives rise to only two potential base pairs at the −1 and −2 positions and yields a lack of resistance (red font). Note in (A–E) PAMs are oriented in 3′ to 5′direction to display base pairing potential with the last three nucleotides of the crRNA repeat. Many 5′-handle mutants show a lack of resistance despite the presence of a bona fide PAM in the target and irrespective of the base pairing pattern (Figure S5). Efficient CRISPR-interference requires the presence of a cytosine at the −2 position of the crRNA repeat (Figure 4). Substitution of this position to guanidine or uracil interferes with CRISPR-defense. When this position is mutated to an adenosine, a partially resistant phenotype is observed during phage infection in conjunction with the canonical PAM, which is bound with the highest affinity by Cascade in vitro. Presumably this high affinity binding can compensate for the negative effects on DNA binding caused by mutations at the −2 position of the 5′-handle, leading to a partially phage resistant phenotype. Furthermore, CRISPR-mediated phage resistance requires a cytosine at the −3 position. The most likely explanation for the fact that some repeat mutants are not tolerated is that the Cascade subunits involved in binding the 5′-handle exhibit a level of sequence specificity.
Although combinations of fully complementary 5′-handles and protospacer flanking sequences do not lead to phage resistance in vivo, this appears to be base pairing independent (Figure S5), as restoring the wild-type base pairing pattern by altering protospacer flanking sequences fails to rescue the phage-sensitive phenotype. For example, the g8C-3A, C-2T CRISPR fails to provide resistance either against M13 phage with a fully complementary CAT PAM (Figure 4B) or against a CTC PAM mutant phage, which is complementary at the −1 position only (Figure 4C). A similar result is obtained when g8C-3A, C-2A CRISPR expressing cells are infected with CTT or CTC PAM phages (Figure 4D and E), indicating that the repeat sequence itself is affecting CRISPR-interference in these instances. Altogether, these data exclude the possibility that the Type I-E system makes use of a differential base pairing mechanism to inhibit self-targeting. The finding that the specificity of PAM recognition is unaffected by its potential to base pair with the 5′-handle is consistent with Cse1 being the only factor involved in PAM recognition [44].
To rule out the possibility that the specificity of PAM recognition by g8-Cascade variants depends on the expression levels of CRISPR-Cas components, the same analyses were performed with an engineered M13 targeting E. coli strain with cas genes fused to inducible promoters [12]. When repeat mutations were introduced into the genomic CRISPR cassette in this strain, identical results were obtained (Figure S6), showing that the data described here are expression level independent.
Previous studies on the S. thermophilus Type II-A CRISPR1/Cas system have revealed differences in PAM specificity and effectivity in either plasmid or phage interference assays [30],[45]. To test whether the Type I-E CRISPR/Cas system also displays assay-dependent differences in PAM utilization, we generated plasmids carrying the g8 protospacer (pG8) flanked by any of the 26 PAM mutants tested in the phage assays. Transformation of the pG8 variants into E. coli cells expressing Cascade, a g8 crRNA and Cas3 show that the four PAMs (CAT, CTT, CCT, and CTC) that provide interference during phage infection also affect plasmid transformation (resulting in a more than 1000-fold decrease in efficiency of transformation (e.o.t.)). Apart from these four PAMs, a non-consensus TTT PAM also yields a full resistance phenotype (Figure S7; >1000-fold decrease in e.o.t.), as has been observed before [8], while M13 phage carrying this non-consensus TTT PAM sequence escape interference (Figure 4A). In addition, ten non-consensus PAMs give rise to a partial resistance phenotype (Figure S7; e.o.t. <10−1 for CCA, CAA, GAT, CTG, and AGA PAMs; e.o.t. <10−2 for CTA, GTT, TAT, ATT and TTC PAMs), which is in line with previously reported partial resistance in S. thermophilus against transformation with a target plasmids carrying non-consensus PAMs [30]. The data show that PAM authentication during CRISPR-based protection is more promiscuous during plasmid transformation than during phage infection.
Discussion
CRISPR-Cas systems are the only prokaryotic adaptive immune systems described to date. Although initially thought of as a single system, we now know that these systems are structurally and mechanistically diverse. Here we have investigated whether a differential base pairing mechanism to discriminate self from non-self, as described for the Type III-A system of S. epidermidis, also applies to the Type I-E CRISPR-Cas system of E. coli K12. By systematically mutating the crRNA repeat sequence and the PAM positions, we demonstrate that this Type I-E system does not utilize the potential for base pairing between the 5′-handle and the protospacer flanking sequences to avoid self targeting.
The −1 position of crRNA has recently been shown to be invader-derived and hence invariably has the potential to base pair with cognate DNA, both in E. coli [8], [11], [42] and in S. thermophilus [45], [46]. This discovery suggested that base pairing at the −1 position would be critical for target recognition by Cascade, in the same way that nucleotides in the seed region (nucleotides +1 to +5, +7 and +8) are essential for target recognition [41]. However, our results clearly show that base pairing at position −1 is not essential for CRISPR-interference. It has recently been suggested that the −1 position of the CRISPR repeat could be considered part of the spacer [42]. However, this does not seem appropriate since this nucleotide does not appear to be involved in base pairing with the invading target sequence. The absence of a base pairing requirement for the −1 position might suggest that this position is not available for base pairing due to structural constraints.
The −2 position of the crRNA repeat requires the presence of a cytosine for efficient CRISPR-interference (Figure 4). When this position is mutated to an adenosine, a partially resistant phenotype is observed during phage infection in conjunction with the canonical PAM. Substitution of the −2 position to a guanidine or uracil renders the CRISPR-interference pathway non-functional. Interestingly, mutation of the −2 position to adenosine causes an apparent structural alteration of the Cascade complex. While most subunits are present in the same apparent stoichiometry in the mutant g8C-2A-Cascade as in the wild-type complex, the Cse1 subunit is underrepresented. This might suggest that Cse1 interacts with the −2 position of the repeat and that interaction with this base is important for efficient incorporation of Cse1 into the complex. Like the −2 position, the −3 position requires a cytosine for CRISPR-mediated phage resistance to be manifested. However, complex formation is unaffected in g8C-3G-Cascade (Figure S4A).
The −3, −2 and −1 positions are among the most conserved bases of type 2 repeats [37]. Although the current resolution of the Cascade structure does not allow us to confidently pinpoint the location of the −2 and −3 bases of the 5′-handle of the crRNA, these bases appear to be part of a 5′ hook-like structure that is primarily cradled by the last subunit of the Cas7 hexamer (i.e., Cas76) [47]. The arch of the crRNA may position the 5′ terminal nucleotides within bonding distance to residues in loop-1 of Cse1, which is consistent with the assembly defects reported for L1 mutations [44]. However, the resolution of the current Cascade structure and absence of density for L1 in the X-ray crystal structures of Cse1 prevent confident assignment of these interactions. Higher-resolution structures of the Cascade will be critical for a precise understanding how the crRNA and the Cas proteins are arranged in this complex.
In some CRISPR systems PAM sequences play an important role during different stages of CRISPR defense. In the Type I-E system of E. coli, PAM sequences are recognized by Cas1 and/or Cas2 during the selection of pre-spacers for integration into the CRISPR [9]. PAM motifs allow the CRISPR adaptation machinery to correctly orient newly acquired spacers into the CRISPR array [38], [48]–[50]. Interestingly, in Type I-E systems, the PAM selectivity of the CRISPR-adaptation machinery has co-evolved with that of the CRISPR-interference machinery, as the preference for the CTT PAM is observed both during Cas1/Cas2-dependent spacer integration [9] and during target DNA binding by Cascade [29]. In contrast, the E. coli I-F integration machinery appears to select for a PAM that overlaps but differs from the motif that yields optimal interference levels [43]. In this E. coli I-F subtype the PAM was found to be a GG motif at the −1 and −2 positions relative to the protospacer, while an overlapping, but different, motif (GG at the −2 and −3 positions) provided optimal interference levels [43]. The presence of a G at position −2 was both required and sufficient for interference. The I-F subtype of Pectobacterium atrosepticum on the other hand requires a GG motif immediately flanking the protospacer for interference, and mutagenesis of the G at position −1 to a T (which potentially base pairs with the repeat) gives rise to an escape phenotype [35]. Recently, a new nomenclature has been proposed that takes into account the differences in motif selectivity during spacer integration and CRISPR-interference [51].
PAMs have been shown to be important for CRISPR interference in various Type I and Type II CRISPR-Cas subtypes (e.g. Type I-A systems in S. solfataricus [40], Type I-B in Haloferax volcanii [39], Type I-E in E. coli [29], Type I-F in P. aeruginosa [52], E. coli [43] and P. atrosepticum [35], as well as in Type II-A and II-B systems of Streptococcus pyogenes and S. thermophilus [27], [30], [50], [53], [54]). Recently published x-ray crystal structures of the Cse1 subunit of Cascade [44], [55] have provided detailed insights into the molecular mechanism of Cascade-mediated recognition of the PAM. The well-conserved L1 loop of Cse1 was shown to directly interact with the PAM sequence and to enhance target DNA affinity in the presence of a bona fide PAM [44]. As such, the Cse1 subunit plays a crucial role in PAM authentication in Type I-E systems [44]. Our data indicate that PAM authentication occurs without the formation of base pairs between the 5′ handle of the crRNA and the PAM.
While Cascade-like complexes appear to be common components of Type I systems, the PAM-authenticating protein, Cse1, is unique to Type I-E systems. This could mean that other Cascade-like complexes, such as the aCascade (IA-Cascade) [25], IC-Cascade [17] the as yet unidentified ID-Cascade, and the Csy-complex (IF-Cascade) [26] may have their own specialized PAM-sensing proteins. It has been hypothesized that the large subunits of Type I systems (Cas8a1 and Cas8a2 (Type I-A), Cas8b (Type I-B), Cas8c (Type I-C), Cas10d (Type I-D), Cse1 (Type I-E), Csy1 (Type I-F)) are homologous to Cas10 proteins associated with the Type III systems [56], but these predictions await experimental verification. If these predictions are correct they may suggest that PAM recognition is carried out by the large subunit of other CRISPR-Cas subtypes.
Under native-like expression levels, the change in affinity of Cascade for a target resulting from the presence or absence of a PAM sequence appears to be sufficient to serve as a robust mechanism to discriminate non-self target sequences (i.e. protospacers flanked by a PAM) from non-target sequences (i.e. protospacers without PAM) in vivo [44]. Given the absence of PAM sequences in the CRISPR array, self DNA automatically falls into the non-target category and is not subject to interference. For Type III systems, on the other hand, no PAMs have yet been found, suggesting that these systems lack PAMs [23], [36]. For Type III-A systems it has been shown that differentiation between self DNA and non-self DNA relies on sensing differential complementarity between the 5′-handle of the crRNA and the protospacer-flanking sequence (Figure 5A) [36]. This discrimination mechanism is based on specific recognition of self DNA, and is therefore best described by the term self versus non-self discrimination (Figure 5A). Here we demonstrate that self-avoidance by the Type I-E system does not rely on potential base pairing between crRNA repeats and protospacer flanking sequence. Therefore, Cascade lacks the ability to specifically recognize self and relies on specific target DNA recognition through PAM authentication. We argue that PAM authentication is a “target versus non-target” discrimination mechanism (Figure 5B), which is fundamentally different from the “self versus non-self” discrimination mechanism employed by Type III-A systems. Either mechanism is sufficient to avoid targeting of the CRISPR locus on the host genome. In target versus non-target discrimination, self sequences within the CRISPR locus (i.e. spacers) automatically belong to the non-target class, since PAM sequences are absent in the CRISPR repeat. Likewise, in self versus non-self discriminating systems target sequences fall in the non-self class. It appears likely that PAM-sensing CRISPR-Cas systems all make use of target versus non-target discrimination. Unlike Type III systems, discrimination between targets and non-targets by Type I-E systems cannot take place in the absence of a PAM.
Fig. 5. Model of self versus non-self discrimination by Type III-A systems and target versus non-target discrimination by Type I-E systems. 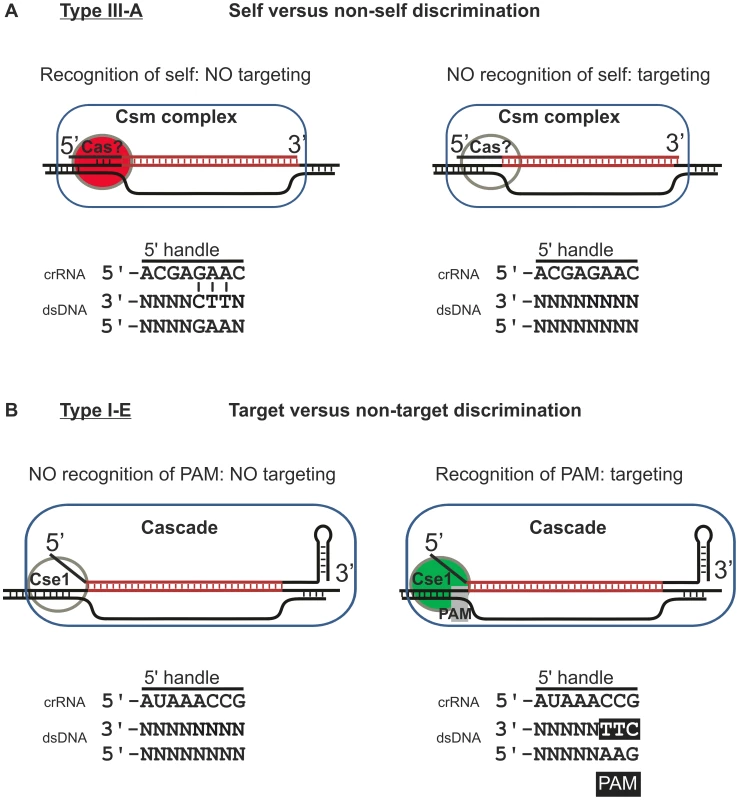
A) Model of the mechanism employed by Type III-A systems. Type III-A systems presumably target DNA through R-loop formation. The crRNA (here depicted as the 37 nt species) could be part of a ribonucleoprotein complex consisting of Cas proteins and a single crRNA (Csm complex). R-loop formation and subsequent interference is inhibited when base pairing occurs between the −2, −3 and −4 positions of the repeat sequence at the 5′ end of the crRNA and the sequence flanking the 3′ end of the protospacer. How this base pairing is monitored is currently unknown, but it might involve a Cas protein. B) Model of target versus non-target discrimination by Type I-E systems. Type I-E systems target DNA through R-loop formation. The 61 nt crRNA is part of the Cascade ribonucleoprotein complex. R-loop formation and subsequent Cas3-mediated cleavage of the target DNA are activated when a PAM is present at the −1, −2 and −3 positions, in the sequence flanking the 3′ end of the protospacer. The presence of a PAM is monitored through the Cse1 subunit. Both discrimination mechanisms, however, are not mutually exclusive. The Type I-F system of E. coli LF82 has been speculated to utilize both target versus non-target discrimination and self versus non-self discrimination [43], although this hypothesis awaits experimental verification by testing the effect of crRNA repeat mutagenesis on CRISPR interference. By having both mechanisms in place an additional level of security against self-targeting of the host genome could be warranted. The requirement for a more stringent protection against self-targeting could be related to the constitutive gene expression of the Type I-F in E. coli LF82 [43], whereas the expression of the Type I-E system of E. coli K12 is repressed under laboratory growth conditions [57], [58], [59].
The distinct mechanisms of self versus non-self discrimination of Type III-A and target versus non-target recognition of Type I-E have implications for the route that invaders can take to escape CRISPR-interference. While both systems can be evaded by making point mutations in the protospacer [41], [60], only the Type I-E system can be evaded by mutations outside the protospacer, specifically in the region containing the PAM. In contrast, escape from Type III-A interference through mutations outside the protospacer seems rather unlikely, as it would typically require three mutations to establish base pairing between the 5′ handle and the protospacer flank [36].
Materials and Methods
Bacterial strains, gene cloning, plasmids and vectors
E. coli BL21 (DE3) strains were used for Cascade purification. Novablue (DE3) cells supplemented with CRISPR plasmid and plasmids expressing cas genes and engineered K12 strains with cas genes fused to inducible promoters were used for phage sensitivity tests and transformation assays. A description of the plasmids and the strains used in this study can be found in the Supplementary Information (Table S1).
Protein expression and purification
Wildtype M13-Cascade was expressed in E. coli BL21 (DE3) and purified as described before [29], from pWUR408, pWUR514 and pWUR615 (Table S1). g8G-1T-Cascade, g8C-2A-Cascade, g8C-3G-Cascade, were expressed from pWUR408, pWUR514 and either pWUR680, pWUR682, or pWUR684, respectively (Table S1). pWUR680, pWUR682, and pWUR684 were generated by subcloning a synthetic CRISPR (Table S3 and Table S4, Geneart) into pACYC using EcoNI and Acc65I restriction sites. Although BL21 (DE3) contains genomic CRISPR loci, previous analyses by Mass Spectrometry have demonstrated that these expression and purification conditions yield homogeneous Cascade complexes loaded with crRNA species from the overexpression plasmids, and not from the chromosme [22].
Gel electrophoresis
Purified Cascade was separated on a 12% SDS-PAGE as described before [22], and stained using Coomassie Blue overnight, followed by destaining in Millipore water. Nucleic acids were isolated from purified Cascade complexes using an extraction with phenol∶chloroform∶isoamylalcohol (25∶24∶1) equilibrated at pH 8.0 (Fluka) and separated on a 6M urea 15% acrylamide gel, as described in [22], followed by staining with SybR safe (Invitrogen) in a 1∶10000 dilution in TAE for 30 minutes. Electrophoretic Mobility Shift Assays were performed as in [29], using the PAGE-purified oligonucleotides listed in Table S2, which were annealed and 5′-labeled with 32P γ-ATP (PerkinElmer) using T4 polynucleotide kinase (Fermentas). Determining the Kd of the Cascade target DNA interaction was performed as described in [41]. Briefly, the signals of unbound and bound probe were quantified using Quantity One software (Bio-Rad). The fraction of bound probe was plotted against the total Cascade concentration, and the data fitted by nonlinear regression analysis to the following equation: Fraction bound probe = [Cascade]total/(Kd+[Cascade]total).
Phage M13 mutagenesis
Mutations of PAM sequence preceding the g8 protospacer were introduced into the M13 phage genome by QuickChange Site-Directed Mutagenesis Kit (Stratagene) as described previously ([41]).
CRISPR repeat mutagenesis
Repeat mutant library was generated by QuikChange Site-Directed Mutagenesis Kit (Stratagene) according to manufacturer's protocol. The g8 CRISPR cassette plasmid targeting the M13 phage gene 8 (pWUR477-g8, described in [41]) was used as template. Mutations were introduced at positions −3, −2, or −1 of the repeat preceding the g8 spacer.
Phage infection studies
Cells sensitivity to wildtype and mutant M13 phages was determined by a spot test method as described [41] or using standard plaquing assay. Efficiency of plaquing was calculated as a ratio of the plaque number formed on a lawn of tested cells to the number of plaques on sensitive (non-targeting) cell lawn.
Transformation assay
K12 strains with cas genes fused to inducible promoters and g8 spacer in CRISPR were transformed with 10 ng of plasmid DNA by electroporation. Transformation efficiency was determined as colony forming units for transformants of targeting strain BW40119 (Table S1) per µg DNA. Plasmids containing the g8 protospacer and PAM mutants were ordered synthetically at Geneart, Germany.
Supporting Information
Zdroje
1. LabrieSJ, SamsonJE, MoineauS (2010) Bacteriophage resistance mechanisms. Nat Rev Microbiol 8 : 317–327.
2. BikardD, MarraffiniLA (2011) Innate and adaptive immunity in bacteria: mechanisms of programmed genetic variation to fight bacteriophages. Curr Opin Immunol 24 : 15–20.
3. WestraER, SwartsD, StaalsR, JoreM, BrounsSJJ, OostJvd (2012) The CRISPRs they are a-changin'-how prokaryotes generate adaptive immunity. Annu Rev Genet 46 : 311–339.
4. BhayaD, DavisonM, BarrangouR (2011) CRISPR-Cas systems in bacteria and archaea: versatile small RNAs for adaptive defense and regulation. Annu Rev Genet 45 : 273–297.
5. WiedenheftB, SternbergSH, DoudnaJA (2012) RNA-guided genetic silencing systems in bacteria and archaea. Nature 482 : 331–338.
6. TernsMP, TernsRM (2011) CRISPR-based adaptive immune systems. Curr Opin Microbiol 14 : 321–327.
7. RichterC, ChangJT, FineranPC (2012) Function and Regulation of Clustered Regularly Interspaced Short Palindromic Repeats (CRISPR)/CRISPR Associated (Cas) Systems. Viruses 4 : 2291–2311.
8. SwartsDC, MosterdC, van PasselMW, BrounsSJ (2012) CRISPR interference directs strand specific spacer acquisition. PloS one 7: e35888.
9. YosefI, GorenMG, QimronU (2012) Proteins and DNA elements essential for the CRISPR adaptation process in Escherichia coli. Nucleic Acids Res 40 : 5569–5576.
10. BarrangouR, FremauxC, DeveauH, RichardsM, BoyavalP, et al. (2007) CRISPR provides acquired resistance against viruses in prokaryotes. Science 315 : 1709–1712.
11. DatsenkoKA, PougachK, TikhonovA, WannerBL, SeverinovK, et al. (2012) Molecular memory of prior infections activates the CRISPR/Cas adaptive bacterial immunity system. Nat Commun 3 : 945.
12. MakarovaKS, HaftDH, BarrangouR, BrounsSJ, CharpentierE, et al. (2011) Evolution and classification of the CRISPR-Cas systems. Nat Rev Microbiol 9 : 467–477.
13. BrounsSJJ, JoreMM, LundgrenM, WestraER, SlijkhuisRJH, et al. (2008) Small CRISPR RNAs guide antiviral defense in prokaryotes. Science 321 : 960–964.
14. HaurwitzRE, JinekM, WiedenheftB, ZhouK, DoudnaJA (2010) Sequence - and structure-specific RNA processing by a CRISPR endonuclease. Science 329 : 1355–1358.
15. CarteJ, WangRY, LiH, TernsRM, TernsMP (2008) Cas6 is an endoribonuclease that generates guide RNAs for invader defense in prokaryotes. Gene Dev 22 : 3489–3496.
16. PrzybilskiR, RichterC, GristwoodT, ClulowJS, VercoeRB, et al. (2011) Csy4 is responsible for CRISPR RNA processing in Pectobacterium atrosepticum. RNA Biol 8 : 517–528.
17. NamKH, HaitjemaC, LiuX, DingF, WangH, et al. (2012) Cas5d protein processes pre-crRNA and assembles into a Cascade-like interference complex in subtype I-C/Dvulg CRISPR-Cas system. Structure 20 : 1574–84.
18. GarsideEL, SchellenbergMJ, GesnerEM, BonannoJB, SauderJM, et al. (2012) Cas5d processes pre-crRNA and is a member of a larger family of CRISPR RNA endonucleases. RNA 18 : 2020–2028.
19. HaleC, KleppeK, TernsRM, TernsMP (2008) Prokaryotic silencing (psi)RNAs in Pyrococcus furiosus. RNA 14 : 2572–2579.
20. Hatoum-AslanA, ManivI, MarraffiniLA (2011) Mature clustered, regularly interspaced, short palindromic repeats RNA (crRNA) length is measured by a ruler mechanism anchored at the precursor processing site. Proc Natl Acad Sci U S A 108 : 21218–21222.
21. DeltchevaE, ChylinskiK, SharmaCM, GonzalesK, ChaoY, et al. (2011) CRISPR RNA maturation by trans-encoded small RNA and host factor RNase III. Nature 471 : 602–607.
22. JoreMM, LundgrenM, van DuijnE, BultemaJB, WestraER, et al. (2011) Structural basis for CRISPR RNA-guided DNA recognition by Cascade. Nat Struct Mol Biol 18 : 529–536.
23. HaleCR, ZhaoP, OlsonS, DuffMO, GraveleyBR, et al. (2009) RNA-guided RNA cleavage by a CRISPR RNA-Cas protein complex. Cell 139 : 945–956.
24. ZhangJ, RouillonC, KerouM, ReeksJ, BruggerK, et al. (2012) Structure and mechanism of the CMR complex for CRISPR-mediated antiviral immunity. Mol Cell 45 : 303–313.
25. LintnerNG, KerouM, BrumfieldSK, GrahamS, LiuH, et al. (2011) Structural and functional characterization of an archaeal clustered regularly interspaced short palindromic repeat (CRISPR)-associated complex for antiviral defense (CASCADE). J Biol Chem 286 : 21643–21656.
26. WiedenheftB, van DuijnE, BultemaJB, WaghmareSP, ZhouK, et al. (2011) RNA-guided complex from a bacterial immune system enhances target recognition through seed sequence interactions. Proc Natl Acad Sci U S A 108 : 10092–10097.
27. JinekM, ChylinskiK, FonfaraI, HauerM, DoudnaJA, et al. (2012) A programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity. Science 337 : 816–821.
28. HaleCR, MajumdarS, ElmoreJ, PfisterN, ComptonM, et al. (2012) Essential features and rational design of CRISPR RNAs that function with the Cas RAMP Module Complex to cleave RNAs. Mol Cell 45 : 292–302.
29. WestraER, van ErpPB, KunneT, WongSP, StaalsRH, et al. (2012) CRISPR immunity relies on the consecutive binding and degradation of negatively supercoiled invader DNA by Cascade and Cas3. Mol Cell 46 : 595–605.
30. GarneauJE, DupuisME, VillionM, RomeroDA, BarrangouR, et al. (2010) The CRISPR/Cas bacterial immune system cleaves bacteriophage and plasmid DNA. Nature 468 : 67–71.
31. ManicaA, ZebecZ, TeichmannD, SchleperC (2011) In vivo activity of CRISPR-mediated virus defence in a hyperthermophilic archaeon. Mol Microbiol 80 : 481–491.
32. MarraffiniLA, SontheimerEJ (2008) CRISPR interference limits horizontal gene transfer in staphylococci by targeting DNA. Science 322 : 1843–1845.
33. EdgarR, QimronU (2010) The Escherichia coli CRISPR system protects from lambda lysogenization, lysogens, and prophage induction. J Bacteriol 192 : 6291–6294.
34. SternA, KerenL, WurtzelO, AmitaiG, SorekR (2010) Self-targeting by CRISPR: gene regulation or autoimmunity? Trends Genet 26 : 335–340.
35. VercoeRB, ChangJT, DyRL, TaylorC, GristwoodT, et al. (2013) Cytotoxic chromosomal targeting by CRISPR/Cas systems can reshape bacterial genomes and expel or remodel pathogenicity islands. PLoS Genet 9: e1003454.
36. MarraffiniLA, SontheimerEJ (2010) Self versus non-self discrimination during CRISPR RNA-directed immunity. Nature 463 : 568–571.
37. KuninV, SorekR, HugenholtzP (2007) Evolutionary conservation of sequence and secondary structures in CRISPR repeats. Genome Biol 8: R61.
38. MojicaFJM, Diez-VillasenorC, Garcia-MartinezJ, AlmendrosC (2009) Short motif sequences determine the targets of the prokaryotic CRISPR defence system. Microbiology 155 : 733–740.
39. FischerS, MaierLK, StollB, BrendelJ, FischerE, et al. (2012) An archaeal immune system can detect multiple protospacer adjacent motifs (PAMs) to target invader DNA. J Biol Chem 287 : 33351–33363.
40. GudbergsdottirS, DengL, ChenZ, JensenJV, JensenLR, et al. (2011) Dynamic properties of the Sulfolobus CRISPR/Cas and CRISPR/Cmr systems when challenged with vector-borne viral and plasmid genes and protospacers. Mol Microbiol 79 : 35–49.
41. SemenovaE, JoreMM, DatsenkoKA, SemenovaA, WestraER, et al. (2011) Interference by clustered regularly interspaced short palindromic repeat (CRISPR) RNA is governed by a seed sequence. Proc Natl Acad Sci U S A 108 : 10098–10103.
42. GorenMG, YosefI, AusterO, QimronU (2012) Experimental definition of a Clustered Regularly Interspaced Short Palindromic duplicon in Escherichia coli. J Mol Biol 423 : 14–16.
43. AlmendrosC, GuzmanNM, Diez-VillasenorC, Garcia-MartinezJ, MojicaFJ (2012) Target motifs affecting natural immunity by a constitutive CRISPR-Cas system in Escherichia coli. PLoS One 7: e50797.
44. SashitalDG, WiedenheftB, DoudnaJA (2012) Mechanism of foreign DNA selection in a bacterial adaptive immune system. Mol Cell 46 : 606–615.
45. SinkunasT, GasiunasG, WaghmareSP, DickmanMJ, BarrangouR, et al. (2013) In vitro reconstitution of Cascade-mediated CRISPR immunity in Streptococcus thermophilus. EMBO J 32 : 385–394.
46. Dupuis M, Moineau S (2013) Type II: Streptococcus thermophilus. In: Barrangou R, van der Oost J, editors. CRISPR-Cas Systems - RNA-mediated Adaptive Immunity in Bacteria and Archaea: Springer. pp. 171–200.
47. WiedenheftB, LanderGC, ZhouK, JoreMM, BrounsSJ, et al. (2011) Structures of the RNA-guided surveillance complex from a bacterial immune system. Nature 477 : 486–489.
48. ErdmannS, GarrettRA (2012) Selective and hyperactive uptake of foreign DNA by adaptive immune systems of an archaeon via two distinct mechanisms. Mol Microbiol 85 : 1044–1056.
49. WestraER, BrounsSJ (2012) The rise and fall of CRISPRs - dynamics of spacer acquisition and loss. Mol Microbiol 85 : 1021–1025.
50. Lopez-SanchezMJ, SauvageE, Da CunhaV, ClermontD, Ratsima HariniainaE, et al. (2012) The highly dynamic CRISPR1 system of Streptococcus agalactiae controls the diversity of its mobilome. Mol Microbiol 85 : 1057–1071.
51. ShahSA, ErdmannS, MojicaFJ, GarrettRA (2013) Protospacer recognition motifs: mixed identities and functional diversity. RNA biol 10 : 891–899.
52. CadyKC, Bondy-DenomyJ, HeusslerGE, DavidsonAR, O'TooleGA (2012) The CRISPR/Cas adaptive immune system of Pseudomonas aeruginosa mediates resistance to naturally occurring and engineered phages. J Bacteriol 194 : 5728–5738.
53. DeveauH, BarrangouR, GarneauJE, LabonteJ, FremauxC, et al. (2008) Phage response to CRISPR-encoded resistance in Streptococcus thermophilus. J Bacteriol 190 : 1390–1400.
54. MagadanAH, DupuisME, VillionM, MoineauS (2012) Cleavage of phage DNA by the Streptococcus thermophilus CRISPR3-Cas system. PLoS One 7: e40913.
55. MulepatiS, OrrA, BaileyS (2012) Crystal structure of the largest subunit of a bacterial RNA-guided immune complex and its role in DNA target binding. J Biol Chem 287 : 22445–22449.
56. MakarovaKS, AravindL, WolfYI, KooninEV (2011) Unification of Cas protein families and a simple scenario for the origin and evolution of CRISPR-Cas systems. Biol Direct 6 : 38.
57. PulU, WurmR, ArslanZ, GeissenR, HofmannN, et al. (2010) Identification and characterization of E. coli CRISPR-cas promoters and their silencing by H-NS. Mol Microbiol 75 : 1495–1512.
58. WestraER, PulU, HeidrichN, JoreMM, LundgrenM, et al. (2010) H-NS-mediated repression of CRISPR-based immunity in Escherichia coli K12 can be relieved by the transcription activator LeuO. Mol Microbiol 77 : 1380–1393.
59. PougachK, SemenovaE, BogdanovaE, DatsenkoKA, DjordjevicM, et al. (2010) Transcription, processing and function of CRISPR cassettes in Escherichia coli. Mol Microbiol 77 : 1367–1379.
60. MillenAM, HorvathP, BoyavalP, RomeroDA (2012) Mobile CRISPR/Cas-mediated bacteriophage resistance in Lactococcus lactis. PLoS One 7: e51663.
Štítky
Genetika Reprodukční medicína
Článek Rapid Intrahost Evolution of Human Cytomegalovirus Is Shaped by Demography and Positive SelectionČlánek Common Variants in Left/Right Asymmetry Genes and Pathways Are Associated with Relative Hand SkillČlánek Manipulating or Superseding Host Recombination Functions: A Dilemma That Shapes Phage EvolvabilityČlánek Maternal Depletion of Piwi, a Component of the RNAi System, Impacts Heterochromatin Formation inČlánek Hsp104 Suppresses Polyglutamine-Induced Degeneration Post Onset in a Drosophila MJD/SCA3 ModelČlánek Cooperative Interaction between Phosphorylation Sites on PERIOD Maintains Circadian Period inČlánek VAPB/ALS8 MSP Ligands Regulate Striated Muscle Energy Metabolism Critical for Adult Survival inČlánek Histone Chaperone NAP1 Mediates Sister Chromatid Resolution by Counteracting Protein Phosphatase 2AČlánek A Link between ORC-Origin Binding Mechanisms and Origin Activation Time Revealed in Budding YeastČlánek Genotype-Environment Interactions Reveal Causal Pathways That Mediate Genetic Effects on PhenotypeČlánek Chromatin-Specific Regulation of Mammalian rDNA Transcription by Clustered TTF-I Binding SitesČlánek Meiotic Recombination in Arabidopsis Is Catalysed by DMC1, with RAD51 Playing a Supporting Role
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2013 Číslo 9- Akutní intermitentní porfyrie
- Růst a vývoj dětí narozených pomocí IVF
- Farmakogenetické testování pomáhá předcházet nežádoucím efektům léčiv
- Pilotní studie: stres a úzkost v průběhu IVF cyklu
- IVF a rakovina prsu – zvyšují hormony riziko vzniku rakoviny?
-
Všechny články tohoto čísla
- The Pathway Gene Functions together with the -Dependent Isoprenoid Biosynthetic Pathway to Orchestrate Germ Cell Migration
- Take Off, Landing, and Fly Anesthesia
- Nucleosome Assembly Proteins Get SET to Defeat the Guardian of Chromosome Cohesion
- Whole-Exome Sequencing Reveals a Rapid Change in the Frequency of Rare Functional Variants in a Founding Population of Humans
- Evidence Is Evidence: An Interview with Mary-Claire King
- Rapid Intrahost Evolution of Human Cytomegalovirus Is Shaped by Demography and Positive Selection
- Convergent Transcription Induces Dynamic DNA Methylation at Loci
- Environmental Stresses Disrupt Telomere Length Homeostasis
- Ultra-Sensitive Sequencing Reveals an Age-Related Increase in Somatic Mitochondrial Mutations That Are Inconsistent with Oxidative Damage
- Common Variants in Left/Right Asymmetry Genes and Pathways Are Associated with Relative Hand Skill
- Genetic and Anatomical Basis of the Barrier Separating Wakefulness and Anesthetic-Induced Unresponsiveness
- The Locus, Exclusive to the Ambulacrarians, Encodes a Chromatin Insulator Binding Protein in the Sea Urchin Embryo
- Binding of NF-κB to Nucleosomes: Effect of Translational Positioning, Nucleosome Remodeling and Linker Histone H1
- Manipulating or Superseding Host Recombination Functions: A Dilemma That Shapes Phage Evolvability
- Dynamics of DNA Methylation in Recent Human and Great Ape Evolution
- Functional Dissection of Regulatory Models Using Gene Expression Data of Deletion Mutants
- PAQR-2 Regulates Fatty Acid Desaturation during Cold Adaptation in
- N-alpha-terminal Acetylation of Histone H4 Regulates Arginine Methylation and Ribosomal DNA Silencing
- A Genome-Wide Systematic Analysis Reveals Different and Predictive Proliferation Expression Signatures of Cancerous vs. Non-Cancerous Cells
- Maternal Depletion of Piwi, a Component of the RNAi System, Impacts Heterochromatin Formation in
- miR-1/133a Clusters Cooperatively Specify the Cardiomyogenic Lineage by Adjustment of Myocardin Levels during Embryonic Heart Development
- Hsp104 Suppresses Polyglutamine-Induced Degeneration Post Onset in a Drosophila MJD/SCA3 Model
- Genome-Wide Analysis of Genes and Their Association with Natural Variation in Drought Tolerance at Seedling Stage of L
- Deep Resequencing of GWAS Loci Identifies Rare Variants in , and That Are Associated with Ulcerative Colitis
- Cooperative Interaction between Phosphorylation Sites on PERIOD Maintains Circadian Period in
- VAPB/ALS8 MSP Ligands Regulate Striated Muscle Energy Metabolism Critical for Adult Survival in
- Analysis of Genes Reveals Redundant and Independent Functions in the Inner Ear
- Predicting the Risk of Rheumatoid Arthritis and Its Age of Onset through Modelling Genetic Risk Variants with Smoking
- Histone Chaperone NAP1 Mediates Sister Chromatid Resolution by Counteracting Protein Phosphatase 2A
- A Shift to Organismal Stress Resistance in Programmed Cell Death Mutants
- Fragile Site Instability in Causes Loss of Heterozygosity by Mitotic Crossovers and Break-Induced Replication
- Tracking of Chromosome and Replisome Dynamics in Reveals a Novel Chromosome Arrangement
- The Condition-Dependent Transcriptional Landscape of
- Ago1 Interacts with RNA Polymerase II and Binds to the Promoters of Actively Transcribed Genes in Human Cancer Cells
- Nebula/DSCR1 Upregulation Delays Neurodegeneration and Protects against APP-Induced Axonal Transport Defects by Restoring Calcineurin and GSK-3β Signaling
- System-Wide Analysis Reveals a Complex Network of Tumor-Fibroblast Interactions Involved in Tumorigenicity
- Meta-Analysis of Genome-Wide Association Studies Identifies Six New Loci for Serum Calcium Concentrations
- and Are Required for Cellularization and Differentiation during Female Gametogenesis in
- Growth factor independent-1 Maintains Notch1-Dependent Transcriptional Programming of Lymphoid Precursors
- Whole Genome Sequencing Identifies a Deletion in Protein Phosphatase 2A That Affects Its Stability and Localization in
- An Alteration in ELMOD3, an Arl2 GTPase-Activating Protein, Is Associated with Hearing Impairment in Humans
- Genomic Identification of Founding Haplotypes Reveals the History of the Selfing Species
- Plasticity Regulators Modulate Specific Root Traits in Discrete Nitrogen Environments
- The IDD14, IDD15, and IDD16 Cooperatively Regulate Lateral Organ Morphogenesis and Gravitropism by Promoting Auxin Biosynthesis and Transport
- Stochastic Loss of Silencing of the Imprinted Allele, in a Mouse Model and Humans with Prader-Willi Syndrome, Has Functional Consequences
- The Prefoldin Complex Regulates Chromatin Dynamics during Transcription Elongation
- PKA Controls Calcium Influx into Motor Neurons during a Rhythmic Behavior
- A Pre-mRNA-Splicing Factor Is Required for RNA-Directed DNA Methylation in
- Cell-Type Specific Features of Circular RNA Expression
- The Uve1 Endonuclease Is Regulated by the White Collar Complex to Protect from UV Damage
- An Atypical Kinase under Balancing Selection Confers Broad-Spectrum Disease Resistance in Arabidopsis
- Genome-Wide Mutation Avalanches Induced in Diploid Yeast Cells by a Base Analog or an APOBEC Deaminase
- Extensive Divergence of Transcription Factor Binding in Embryos with Highly Conserved Gene Expression
- Bi-modal Distribution of the Second Messenger c-di-GMP Controls Cell Fate and Asymmetry during the Cell Cycle
- Cell Interactions and Patterned Intercalations Shape and Link Epithelial Tubes in
- A Link between ORC-Origin Binding Mechanisms and Origin Activation Time Revealed in Budding Yeast
- The Genome and Development-Dependent Transcriptomes of : A Window into Fungal Evolution
- SKN-1/Nrf, A New Unfolded Protein Response Factor?
- The Highly Prolific Phenotype of Lacaune Sheep Is Associated with an Ectopic Expression of the Gene within the Ovary
- Fusion of Large-Scale Genomic Knowledge and Frequency Data Computationally Prioritizes Variants in Epilepsy
- IL-17 Attenuates Degradation of ARE-mRNAs by Changing the Cooperation between AU-Binding Proteins and microRNA16
- An Enhancer Element Harboring Variants Associated with Systemic Lupus Erythematosus Engages the Promoter to Influence A20 Expression
- Genome Analysis of a Transmissible Lineage of Reveals Pathoadaptive Mutations and Distinct Evolutionary Paths of Hypermutators
- Type I-E CRISPR-Cas Systems Discriminate Target from Non-Target DNA through Base Pairing-Independent PAM Recognition
- Divergent Transcriptional Regulatory Logic at the Intersection of Tissue Growth and Developmental Patterning
- MEIOB Targets Single-Strand DNA and Is Necessary for Meiotic Recombination
- Transmission of Hypervirulence Traits via Sexual Reproduction within and between Lineages of the Human Fungal Pathogen
- Integration of the Unfolded Protein and Oxidative Stress Responses through SKN-1/Nrf
- Guanine Holes Are Prominent Targets for Mutation in Cancer and Inherited Disease
- Regulation of the Boundaries of Accessible Chromatin
- Natural Genetic Transformation Generates a Population of Merodiploids in
- Ablating Adult Neurogenesis in the Rat Has No Effect on Spatial Processing: Evidence from a Novel Pharmacogenetic Model
- Genotype-Environment Interactions Reveal Causal Pathways That Mediate Genetic Effects on Phenotype
- The Molecular Mechanism of a -Regulatory Adaptation in Yeast
- Phenotypic and Genetic Consequences of Protein Damage
- Recent Acquisition of by Baka Pygmies
- Fatty Acid Taste Signals through the PLC Pathway in Sugar-Sensing Neurons
- A Critical Role for PDGFRα Signaling in Medial Nasal Process Development
- Chromatin-Specific Regulation of Mammalian rDNA Transcription by Clustered TTF-I Binding Sites
- Meiotic Recombination in Arabidopsis Is Catalysed by DMC1, with RAD51 Playing a Supporting Role
- dTULP, the Homolog of Tubby, Regulates Transient Receptor Potential Channel Localization in Cilia
- Widespread Dysregulation of Peptide Hormone Release in Mice Lacking Adaptor Protein AP-3
- , a Direct Transcriptional Target, Modulates T-Box Factor Activity in Orofacial Clefting
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- A Genome-Wide Systematic Analysis Reveals Different and Predictive Proliferation Expression Signatures of Cancerous vs. Non-Cancerous Cells
- Recent Acquisition of by Baka Pygmies
- The Condition-Dependent Transcriptional Landscape of
- Histone Chaperone NAP1 Mediates Sister Chromatid Resolution by Counteracting Protein Phosphatase 2A
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



