-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaA Pre-mRNA-Splicing Factor Is Required for RNA-Directed DNA Methylation in
Cytosine DNA methylation is a stable epigenetic mark that is frequently associated with the silencing of genes and transposable elements (TEs). In Arabidopsis, the establishment of DNA methylation is through the RNA-directed DNA methylation (RdDM) pathway. Here, we report the identification and characterization of RDM16, a new factor in the RdDM pathway. Mutation of RDM16 reduced the DNA methylation levels and partially released the silencing of a reporter gene as well as some endogenous genomic loci in the DNA demethylase ros1-1 mutant background. The rdm16 mutant had morphological defects and was hypersensitive to salt stress and abscisic acid (ABA). Map-based cloning and complementation test led to the identification of RDM16, which encodes a pre-mRNA-splicing factor 3, a component of the U4/U6 snRNP. RNA-seq analysis showed that 308 intron retention events occurred in rdm16, confirming that RDM16 is involved in pre-mRNA splicing in planta. RNA-seq and mRNA expression analysis also revealed that the RDM16 mutation did not affect the pre-mRNA splicing of known RdDM genes, suggesting that RDM16 might be directly involved in RdDM. Small RNA expression analysis on loci showing RDM16-dependent DNA methylation suggested that unlike the previously reported putative splicing factor mutants, rdm16 did not affect small RNA levels; instead, the rdm16 mutation caused a decrease in the levels of Pol V transcripts. ChIP assays revealed that RDM16 was enriched at some Pol V target loci. Our results suggest that RDM16 regulates DNA methylation through influencing Pol V transcript levels. Finally, our genome-wide DNA methylation analysis indicated that RDM16 regulates the overall methylation of TEs and gene-surrounding regions, and preferentially targets Pol IV-dependent DNA methylation loci and the ROS1 target loci. Our work thus contributes to the understanding of RdDM and its interactions with active DNA demethylation.
Published in the journal: . PLoS Genet 9(9): e32767. doi:10.1371/journal.pgen.1003779
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1003779Summary
Cytosine DNA methylation is a stable epigenetic mark that is frequently associated with the silencing of genes and transposable elements (TEs). In Arabidopsis, the establishment of DNA methylation is through the RNA-directed DNA methylation (RdDM) pathway. Here, we report the identification and characterization of RDM16, a new factor in the RdDM pathway. Mutation of RDM16 reduced the DNA methylation levels and partially released the silencing of a reporter gene as well as some endogenous genomic loci in the DNA demethylase ros1-1 mutant background. The rdm16 mutant had morphological defects and was hypersensitive to salt stress and abscisic acid (ABA). Map-based cloning and complementation test led to the identification of RDM16, which encodes a pre-mRNA-splicing factor 3, a component of the U4/U6 snRNP. RNA-seq analysis showed that 308 intron retention events occurred in rdm16, confirming that RDM16 is involved in pre-mRNA splicing in planta. RNA-seq and mRNA expression analysis also revealed that the RDM16 mutation did not affect the pre-mRNA splicing of known RdDM genes, suggesting that RDM16 might be directly involved in RdDM. Small RNA expression analysis on loci showing RDM16-dependent DNA methylation suggested that unlike the previously reported putative splicing factor mutants, rdm16 did not affect small RNA levels; instead, the rdm16 mutation caused a decrease in the levels of Pol V transcripts. ChIP assays revealed that RDM16 was enriched at some Pol V target loci. Our results suggest that RDM16 regulates DNA methylation through influencing Pol V transcript levels. Finally, our genome-wide DNA methylation analysis indicated that RDM16 regulates the overall methylation of TEs and gene-surrounding regions, and preferentially targets Pol IV-dependent DNA methylation loci and the ROS1 target loci. Our work thus contributes to the understanding of RdDM and its interactions with active DNA demethylation.
Introduction
Cytosine methylation in eukaryotic cells is an epigenetic mark that plays important roles in diverse biological processes, such as the silencing of genes and transposons [1], [2], X inactivation [3], paramutation [4], and imprinting [5]. In plants, cytosine methylation can occur in all three sequence contexts: CG, CHG and CHH (H = A, T, or C). The Arabidopsis genome has 24% of CG, 6.7% of CHG and 1.7% of CHH sites methylated at the cytosine [6]. Maintenance of CG, CHG and CHH methylation is catalyzed by MET1, CMT3 and DRM2 enzymes, respectively [7]–[10]. Nevertheless, de novo cytosine methylation in all three sequence contexts can be catalyzed by DRM2 [10] in a pathway known as RNA-directed DNA methylation (RdDM) [11], [12]. In this pathway, a plant-specific RNA polymerase IV is recruited to transcribe transposons and some endogenous repeat loci and the transcripts are copied into double-stranded RNAs (dsRNAs) by RNA-DEPENDENT RNA POLYMERASE2 (RDR2) [13]–[16]. The dsRNAs are then processed into 24-nucleotide (nt) siRNA duplexes by DICER-LIKE 3 (DCL3) and the siRNAs were subsequently methylated at their 3′ ends by the RNA methylase HEN1 for stabilization [17], [18]. PolIV, RDR2, DCL3 and HEN1 are the key components for siRNA biogenesis and stability. In addition, the SNF2-like putative chromatin remodeling protein CLSY1 and the homeodomain transcription factor-like SHH1/DTF1, which interacts with Pol IV, assists in the Pol IV and RDR2-dependent siRNA biogenesis [19]–[21]. Following the methylation of the siRNA duplexes by HEN1, one strand of the siRNAs is loaded into AGO4 [22]. AGO4 interacts with the nascent transcript produced by Pol V, another plant-specific RNA polymerase, through base-pairing between the siRNA and nascent transcript [23]. Pol V transcription is facilitated by a complex formed by DRD1, DMS3 and RDM1 (termed DDR complex), and the transcripts serve as scaffolds for recruiting RdDM effector complex [24], [25]. AGO4 also interacts with the largest subunit NRPE1 of Pol V and KTF1, a homolog of yeast transcription elongation factor Spt5, to stabilize the association of AGO4 with the scaffold transcripts [26]–[28]. The RDM1 protein of DDR complex is associated with AGO4 and DRM2 and thus may help to recruit DRM2 to the region being transcribed by Pol V to catalyze DNA methylation [25], [29]. In addition to Pol V transcripts, Pol II-generated non-coding transcripts are also involved in the RdDM through recruiting the AGO4-containing effector complex [25], [30].
Besides AGO4, AGO6 and AGO9 are also involved in the RdDM, acting in a partially redundant manner with AGO4 [31], [32]. Recently, the DRM2 paralog DRM3 that is catalytically mutated was reported to play a role in the RdDM through promoting the activity of DRM2 [33]. More recently, a GHKL ATPase domain-containing protein DMS11 was identified as a new component of the RdDM machinery and was proposed to cooperate with DMS3 in the RdDM pathway by providing the missing ATPase function for DMS3 [34] or to regulate chromatin architecture [35]. Together, these results suggest that there is great complexity to the RdDM pathway and more components are likely required for modulating this important pathway. Moreover, the mechanisms through which Pol IV, Pol V and DRM2 are targeted to specific loci are still not fully understood.
In an attempt to identify genes involved in the RdDM, Ausin et al (2012) carried out a screen on T-DNA insertion lines by using FWA transgene silencing as a reporter system [36]. As a result, a splicing factor SR45 was identified and demonstrated to be required for the RdDM. Splicing factors are well known to be involved in the removal of introns from pre-mRNAs. In eukaryotic cells, pre-mRNA splicing takes place in a large multicomponent complex, called the splicesome, which is formed by ordered interactions of four small ribonucleoprotein particles (snRNPs), U1, U2, U4/U6 and U5 snRNPs, and numerous snRNP-associated proteins [37]. The U1 snRNP assembles with the 5′ splice site of pre-mRNA and recruits several splicing factors to form the commitment complex, and then the U2 snRNP interacts with the branch point of the introns to form the pre-spliceosome. Subsequently, the U5 snRNP associates with the U4/U6 snRNP to form a U4/U6.U5 tri-snRNP that assembles with the pre-spliceosome to form the mature spliceosome [37], [38]. SR45 encodes a serine/arginine-rich (SR) protein belonging to a conserved family of structurally and functionally related non-snRNP proteins. SR45 is suggested to help with the formation of the bridge between 5′ and 3′ splice sites in the early stage of spliceosome assembly through interacting with U1-70K and U2AF35b proteins [39], [40]. The mechanism of SR45 function in RdDM is not understood. Although the abundance of Pol IV-dependent siRNAs is decreased in sr45 mutant, it is still not known how SR45 is involved in the siRNA accumulation and it is possible that SR45 may play an indirect role through the splicing of genes that encode RdDM pathway components [36].
In this study, we report a U4/U6 snRNP associated protein RDM16, which is required for the RdDM. RDM16 encodes a pre-mRNA-splicing factor 3 (PRP3) and is involved in the pre-mRNA splicing in planta. The rdm16 mutation did not influence the pre-mRNA splicing of known RdDM genes, suggesting that RDM16 involvement in the RdDM might be direct. We also show that rdm16 did not affect the siRNA levels but decrease the Pol V transcripts, which suggested that RDM16 functions in a later step of RdDM through a different mechanism from that of SR45. In addition, we performed genome-wide DNA methylation analysis and found that the majority of loci whose methylation is RDM16-dependent overlapped with Pol IV - and ROS1-targeted loci. Together, our results shed new light on the RdDM pathway and the dynamic balance between RdDM and active DNA demethylation.
Results
Identification of the rdm16 mutant through the screening of ros1 suppressors
To identify components involved in the RdDM machinery, we carried out a forward genetics screen on a T-DNA-mutagenized population in the ros1 background, which contains the RD29A promoter-driven luciferase reporter gene (RD29A-LUC) as well as the 35S promoter-driven NPTII transgene (35S-NPTII) [41]. In this system, the RD29A-LUC transgene, endogenous RD29A and 35S-NPTII transgene are well expressed in wild-type plants under stress conditions, while mutations in ROS1, the 5-methylcytosine DNA glycosylase/DNA demethylase gene, led to the silencing of all the three genes. Based on reactivation of the RD29A-LUC in ros1, we have identified a number of genes required for RdDM [28], [29], [42]–[44]. In this study, we identified a new mutant, rdm16, as a suppressor of ros1 (Figure 1A). Mutation of RDM16 in the ros1 background caused a partial release of the silencing of RD29A-LUC under stress treatment. Nevertheless, the silencing of the RdDM-independent 35S-NPTII transgene was not released in rdm16ros1 compared to the ros1 single mutant (Figure S1A).
Fig. 1. Mutation of RDM16 partially releases the transcriptional silencing of RD29A-LUC transgene and endogenous RD29A in ros1 mutant background. 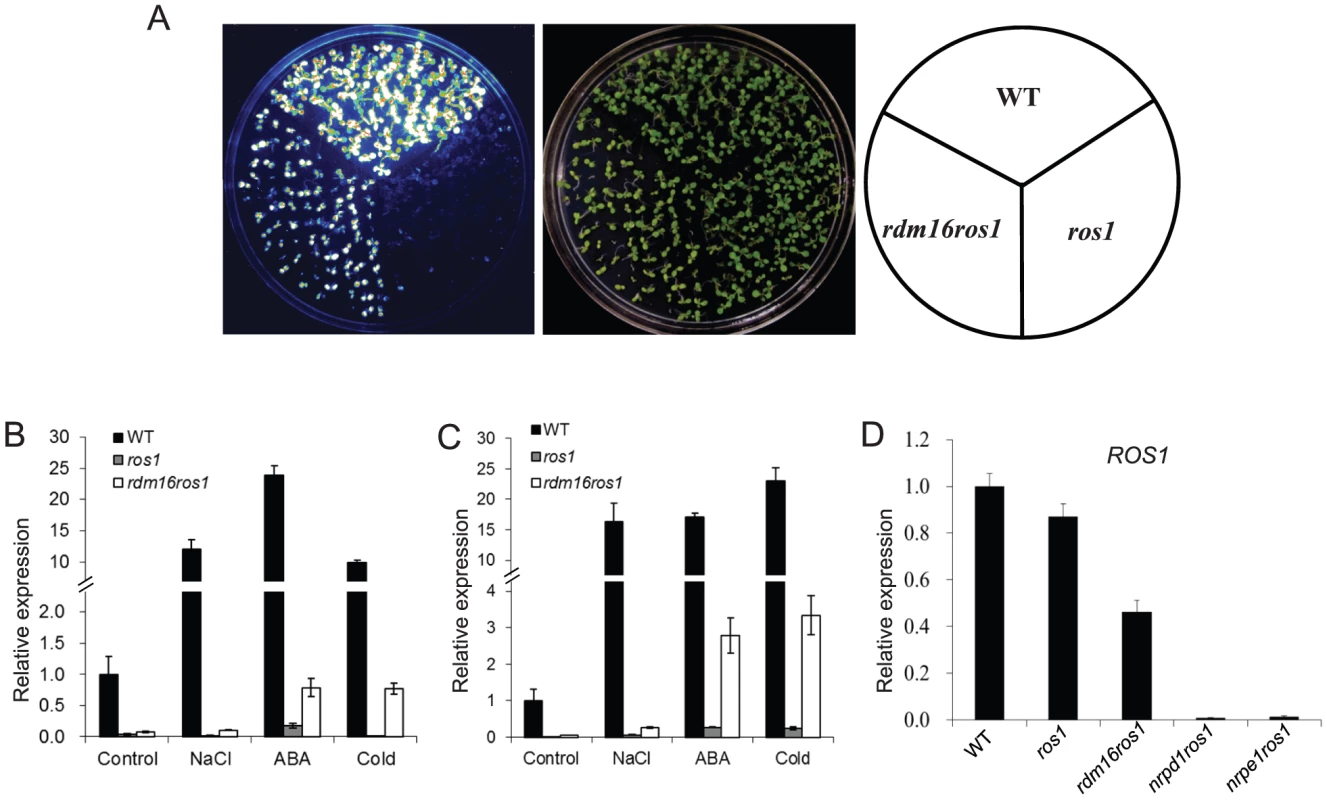
(A) Luminescence imaging of RD29A-LUC expression in WT, ros1 and rdm16ros1. (B–C) Real-time RT-PCR analysis of the expression of RD29A-LUC (B) and endogenous RD29A (C) in WT, ros1 and rdm16ros1 exposed to NaCl, abscisic acid (ABA) and cold (4°C) stresses. (D) Expression analysis of ROS1 in WT, ros1, rdm16ros1, nrpd1ros1 and nrpe1ros1. We also performed quantitative RT-PCR for the expression analysis of RD29A-LUC and endogenous RD29A under various stress conditions. The results showed that mutation of RDM16 reactivated the expression of both RD29A-LUC and endogenous RD29A under salt, ABA and cold treatments (Figures 1B and 1C).
The rdm16 mutation causes reduced DNA methylation and morphological and physiological defects
To examine the cause for the reactivation of RD29A-LUC expression in the rdm16ros1 mutant, we carried out DNA methylation analysis by bisulfite sequencing of WT, ros1 and rdm16ros1 mutants. Consistent with previous reports, the DNA methylation of both RD29A-LUC and endogenous RD29A promoters was detected at low levels in WT, while heavy cytosine methylation at all sequence contexts (CG, CHG and CHH) was found in ros1 (Figures 2A and 2B). In comparison with ros1, the high methylation of both RD29A-LUC and endogenous RD29A promoters was substantially decreased in the rdm16ros1 double mutant at all the three cytosine contexts CG, CHG and CHH. We also measured the DNA methylation in the 35S promoter and the results showed that the DNA methylation level of 35S promoter in rdm16ros1 was similar to that in ros1 (Figure S1B), which is consistent with the phenotype that mutation of RDM16 did not affect the silencing of 35S-NPTII transgene in ros1 (Figure S1A). Additionally, we also performed DNA methylation analysis on a ROS1-targeted endogenous locus, At4g18650 [45], and RdDM-targeted repeat loci, AtSN1, SoloLTR and MEA-ISR. Results showed that DNA methylation of At4g18650 promoter and AtSN1 was reduced in rdm16ros1 at all three cytosine contexts (CG, CHG and CHH) in comparison with that in ros1 (Figures 2C and 2D). The decreased level of DNA methylation in At4g18650 promoter was comparable to or even stronger in rdm16ros1 than that in nrpd1ros1 (Figure 2C). Mutation of RDM16 also reduced the DNA methylation at CHG sites of SoloLTR, while its effect on CG and CHH was not observed (Figure S2A). rdm16ros1 did not affect the DNA methylation at MEA-ISR locus (Figure S2B). Previous reports revealed that the expression of ROS1 is sensitive to RdDM mutations [29], [42]–[44]. Our expression analysis showed that like previously reported RdDM mutants, the expression of ROS1 was also reduced in rdm16ros1 (Figure 1D), which is consistent with a role of RDM16 in the RdDM pathway.
Fig. 2. Cytosine DNA methylation analysis of transgenic and endogenous loci in WT, ros1, rdm16ros1, nrpd1ros1 and nrpe1ros1 through bisulfite sequencing. 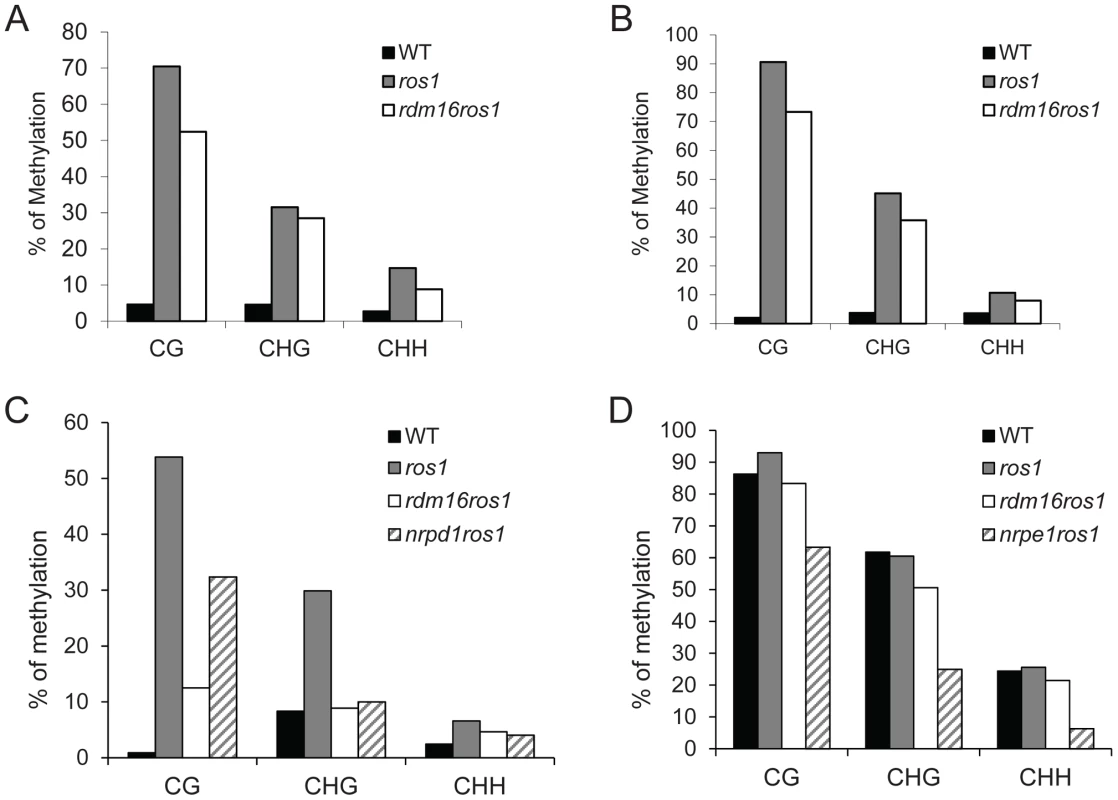
DNA methylation analysis of transgenic RD29A promoter (A), endogenous RD29A promoter (B), At4g18650 promoter (C) and AtSN1 (D). Mutation of RDM16 also caused morphological defects, including dwarf stature, smaller, rounded and wrinkled leaves, and smaller siliques (Figures 3A–3C). Furthermore, seed germination of rdm16ros1 mutant was hypersensitive to salt stress and abscisic acid (ABA) (Figures 3D–3F). Nevertheless, at the young seedling stage, rdm16ros1 did not show higher sensitivity to salt and ABA than ros1 and WT (Figures 3G and 3H).
Fig. 3. Morphological and physiological defects in rdm16ros1 compared to ros1 mutant. 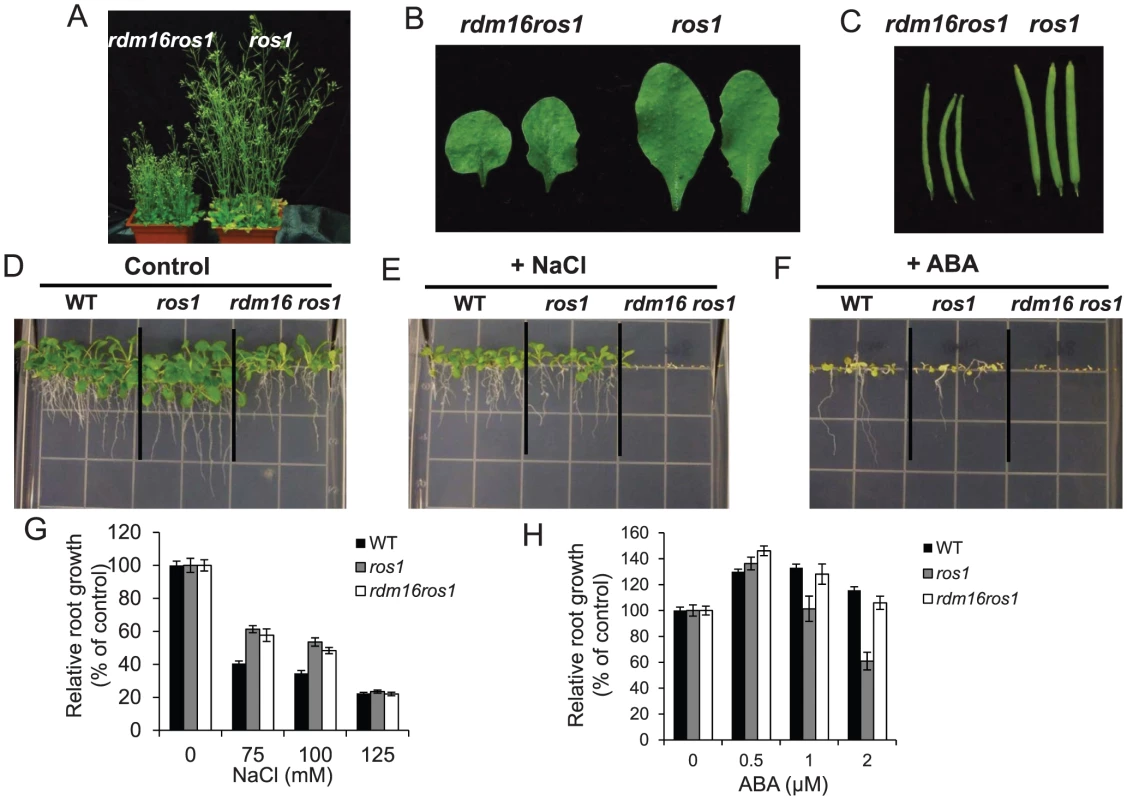
(A–C) Morphological comparison of rdm16ros1 with ros1 with respect to plant stature (A), leaves (B) and siliques (C). (D–F) WT and ros1, rdm16ros1 seed germination under control condition (D), 75 mM NaCl (E) and 0.5 µM ABA (F). (G–H) Seeds were germinated on a half-strength MS plate for 6 d and then the seedlings were exposed to different concentrations of NaCl (G) and ABA (H) for 7 d. Data are means ± SD (n = 15). Cloning of RDM16
To perform genetic analysis for rdm16ros1, we generated an F2 population from a cross between rdm16ros1 and ros1 and then observed the LUC signal and morphological defects. We found that reactivation of LUC expression was tightly linked to the morphological defects. Among 235 F2 plants examined, 59 plants showed both increased LUC signal and morphological defects, while the rest 176 plants showed low LUC signal and normal morphology. The ratio of plants with high LUC expression to plants with low LUC expression fitted to 1∶3 (P>0.95), suggesting that the increased LUC expression and morphological defects was controlled by a recessive mutation in a single nuclear gene.
To map the responsible gene, we used an F2 population derived from a cross between rdm16ros1 (C24 background) and ros1-4 (Col background) and selected plants with defective morphology and increased LUC signal for mapping. By using 25 indel markers across the genome and 74 plants, we were able to find the marker At124 at 11.3 Mb of chromosome 1 was linked to the RDM16 gene (Figure S3A). By developing three more polymorphic markers around At124, we mapped the gene between At120 and At124 on chromosome 1 with a recombination rate of 8.7% and 4.7% from the RDM16 gene, respectively. To further map the gene, we used a population of 632 plants and developed additional 6 polymorphic markers between the At120 and At124 markers. Through linkage analysis, we further mapped the RDM16 gene between the At126 and At130 markers, with 13 and 1 recombinants, respectively (Figure S3B). Three markers within the two markers At126 and At130 were tightly linked to the gene. As a result, the candidate region for RDM16 was defined to about 1.39 Mb (Figure S3B).
To clone the gene, we sequenced the whole genome of rdm16ros1 mutant by second-generation high throughput DNA sequencing. In the 1.39 Mb mapping interval, we found a gap in the promoter of At1g28060 in rdm16ros1 mutant, suggesting a deletion or insertion occurred in the region (Figure S4A). PCR analysis showed that the DNA fragment spanning the gap could not be amplified in rdm16ros1, whereas it was well amplified in WT and ros1 mutant (Figure S4B). These results suggested that there was a DNA fragment inserted into the At1g28060 promoter of rdm16ros1 mutant. To determine the DNA sequence inserted into the promoter, we performed TAIL-PCR analysis on both sides of the insertion. We detected partial sequence of At1g24590 from this analysis, although the full sequence of the insertion was not known (Figure S4C). In addition, we found that the insertion also caused a 45-bp deletion (-154 to -109 bp from ATG) in the promoter of At1g28060. To examine the effect of the mutation on the expression of At1g28060, we compared mRNA expression level of the gene between rdm16ros1 and ros1. In comparison with ros1, mRNA expression of the coding sequence of the gene was decreased by about 2 fold in rdm16ros1, and the expression of 5′UTR was greatly reduced (Figure S5). These results suggest that decreased expression of At1g28060 might be responsible for the observed phenotypes in rdm16ros1.
To confirm that At1g28060 is RDM16, two T-DNA insertion lines (rdm16-2 and rdm16-3) were obtained and characterized. rdm16-2 has a T-DNA insertion in the fifth intron of At1g28060 (Figures S6A and S6B). The T-DNA insertion did not abolish the expression of the full mRNA of the gene, but reduced its expression (Figures S6C and S6D). rdm16-2 also showed similar defective leaf phenotype to rdm16-1, including smaller, rounded and wrinkled leaves, although the morphological defects in rdm16-2 were stronger than those in rdm16-1 (Figure S6E). To examine whether rdm16-2 affected DNA methylation, we compared the DNA methylation level of AtSN1 between WT and rdm16-2 through bisulfite sequencing. Like rdm16-1, rdm16-2 also decreased DNA methylation at all three cytosine contexts (CG, CHG and CHH) in comparison with its WT (Figure S6F). Furthermore, the expression of ROS1 was reduced in rdm16-2 as well (Figure S6G).
The rdm16-3 mutant contains a T-DNA insertion in the fifth exon of At1g28060 (Figure S6A. Genomic PCR analysis with selfed progeny of a heterozygous plant of rdm16-3 showed that no homozygous rdm16-3 plants were found among 107 selfed progeny, suggesting that homozygous rdm16-3 mutant was lethal. In addition, we found wild-type and heterozygous plants at a 68∶39 ratio, distorting from the expected ratio of 1∶2, which suggested that the viability of male and/or female gametes of the heterozygous plants was affected. To test this hypothesis, we made reciprocal crosses between heterozygous rdm16-3/+ and SALK_057447C insertion line, and then analyzed the frequency of rdm16-3 haploid in the resultant F1 plants. The SALK_057447C line did not show morphological defects and its T-DNA insertion site could be used to determine whether the F1 plants are from real crosses. Of 107 real F1 plants with SALK_057447C line as the pollen donor, 17 plants were heterozygous for rdm16-3, whereas 90 plants showed wild-type RDM16. Among 82 F1 plants with rdm16-3/+ as the pollen donor, there were 24 heterozygous and 58 wild-type plants. These results indicated that knockout of RDM16 in both female and male gametes reduced their viability and the effect on female gametes was more severe.
To further confirm that the mutation in At1g28060 is responsible for the observed phenotype in rdm16ros1, we conducted a complementation test on rdm16ros1 by introducing a wild-type RDM16 with 1.4 kb promoter and the full genomic sequence of the gene into the mutant. In the complementation lines, the morphological defects including dwarf stature, smaller and deformed siliques, and defective leaves were fully complemented in the T2 generation (Figure 4A). We also analyzed the RD29A-LUC expression by luminescence imaging and DNA methylation level of both RD29A-LUC and endogenous RD29A promoters by bisulfite sequencing in the complementation lines. Results showed that the increased expression of RD29A-LUC and the reduced DNA methylation level of both promoters in rdm16ros1 were rescued in the complementation lines (Figures 4B–4D). Taken together, these results show that At1g28060 is RDM16 and the mutation in At1g28060 is responsible for the altered expression and DNA methylation of reporter gene and endogenous loci, and the defective plant development in rdm16ros1.
Fig. 4. Complementation test for the rdm16ros1 mutant. 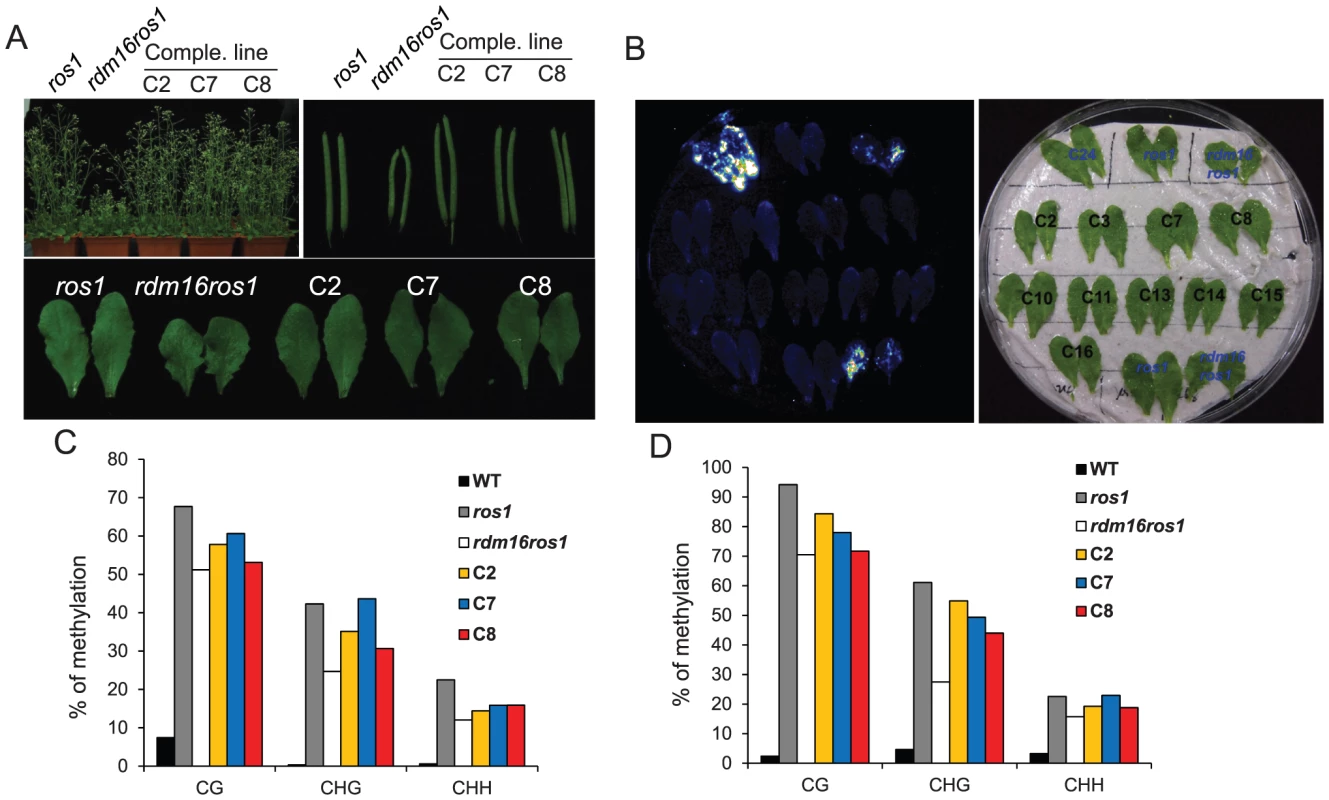
(A) All the morphological defects including dwarf stature, smaller silique and leaves in rdm16ros1 were rescued in three independent complementation lines. (B) Luminescence imaging of detached leaves exposed to 2% NaCl for 3 h. The increased expression of RD29A-LUC in rdm16ros1 was re-silenced in all complementation lines. (C–D) Cytosine DNA methylation analysis of transgenic RD29A promoter (C) and endogenous RD29A promoter (D) in WT, ros1, rdm16ros1 and complementation lines by bisulfite sequencing. RDM16 encodes a pre-mRNA splicing factor
RDM16 has six exons and five introns, encoding a peptide of 786 amino acids with a predicted molecular mass of 88.6 kD. BLAST searches indicated that RDM16 encodes a pre-mRNA-splicing factor 3, a component of U4/U6 snRNP protein complex. Domain analysis predicted that RDM16 has a pre-mRNA processing factor 3 (PRP3) domain and a DUF1115 domain with unknown function (Figure S7). RDM16 is conserved in eukaryotes and has putative orthologs in rice, human and yeast (Figure S7). For example, RDM16 exhibits 26.8% identity and 46.0% similarity at the amino acid level to human HPRP3 (accession number NP_004689), whose mutation results in autosomal dominant retinitis pigmentosa in humans [46]. RDM16 has 30.2% similarity to yeast Prp3 (accession number NP_010761), which is a U4/U6 snRNP protein necessary for the integrity of U4/U6 snRNP and U4/U6.U5 tri-snRNP [47]. In addition, there is a homologous protein, At3g55930, sharing 40.2% identity with RDM16, encoded in the Arabidopsis genome.
To examine whether RDM16 is indeed involved in pre-mRNA splicing, we carried out Illumina paired-end RNA-seq in WT (Col) and rdm16-2 mutant seedlings. A total of 51.5 million and 53.2 million reads for WT and rdm16-2 with the average length of 90 nucleotides were generated, respectively. Among them, there were 48.3 million and 49.6 million unique reads for WT and rdm16-2, respectively, mapped to the Arabidopsis genome. Analysis of intron-retention events in WT and rdm16-2 showed that 308 intron-retention events in 258 genes occurred in rdm16-2 mutant in comparison with WT (FDR<0.01) (Table S1). This result indicated that RDM16 is involved in pre-mRNA splicing in planta.
Our RNA-seq analysis also showed that there were 689 and 152 genes significantly upregulated (>2 fold) and downregulated (<2 fold) in the rdm16-2 mutant, respectively (Tables S2 and S3). Although the expression of so many genes was altered, no genes involved in the RdDM pathway were found in the list. Additionally, we did not find any genes involved in the RdDM pathway showing splicing defects in the mutant (Table S1). Therefore, it is unlikely that the altered DNA methylation in the rdm16 mutants was an indirect consequence of reduced expression or splicing defect of genes encoding RdDM components. To further exclude the possibility of RDM16 affecting the expression or splicing of the RdDM components, we carried out real-time RT-PCR and regular RT-PCR to examine the expression of the RdDM components among WT, ros1 and rdm16ros1 by using primers spanning introns of the genes. The real-time RT-PCR analysis showed that the expression of the genes involved in the RdDM pathway was not different in the three lines (Figure S8A). No splicing defects in any RdDM pathway genes were found in the rdm16ros1 mutant by the RT-PCR analysis (Figure S8B). Together, these results indicated that the involvement of RDM16 in RdDM was not an indirect effect of RDM16 on the expression or splicing of RdDM components.
Genome-wide effects of RDM16 on DNA methylation
To examine the effect of RDM16 on the Arabidopsis methylome, we performed whole genome bisulfite sequencing in WT, ros1, rdm16ros1 and nrpd1ros1 (4-week-old leaves). The average depth of sequenced methylomes is 15–18 with 0.3–0.5% error rates, which indicated a high quality of the data (Table S4). We analyzed the methylation levels of transposable elements (TEs), genes, and 2 kb upstream or downstream of the genes or TEs. Transposable elements are heavily methylated and show higher methylation levels than surrounding regions at all sequence contexts (Figure 5A, Figure S9A). In ros1 mutant, the overall CG and CHH methylation of TEs and surrounding regions were slightly increased in comparison with the wild-type. Mutation of RDM16 did not affect the CG and CHG methylation (Figure S9A). However, CHH methylation of both TEs and surrounding regions was substantially decreased in rdm16ros1 in comparison with ros1 or WT (Figure 5A).
Fig. 5. Average CHH methylation levels in transposable elements (TEs) (A) and genes (B). 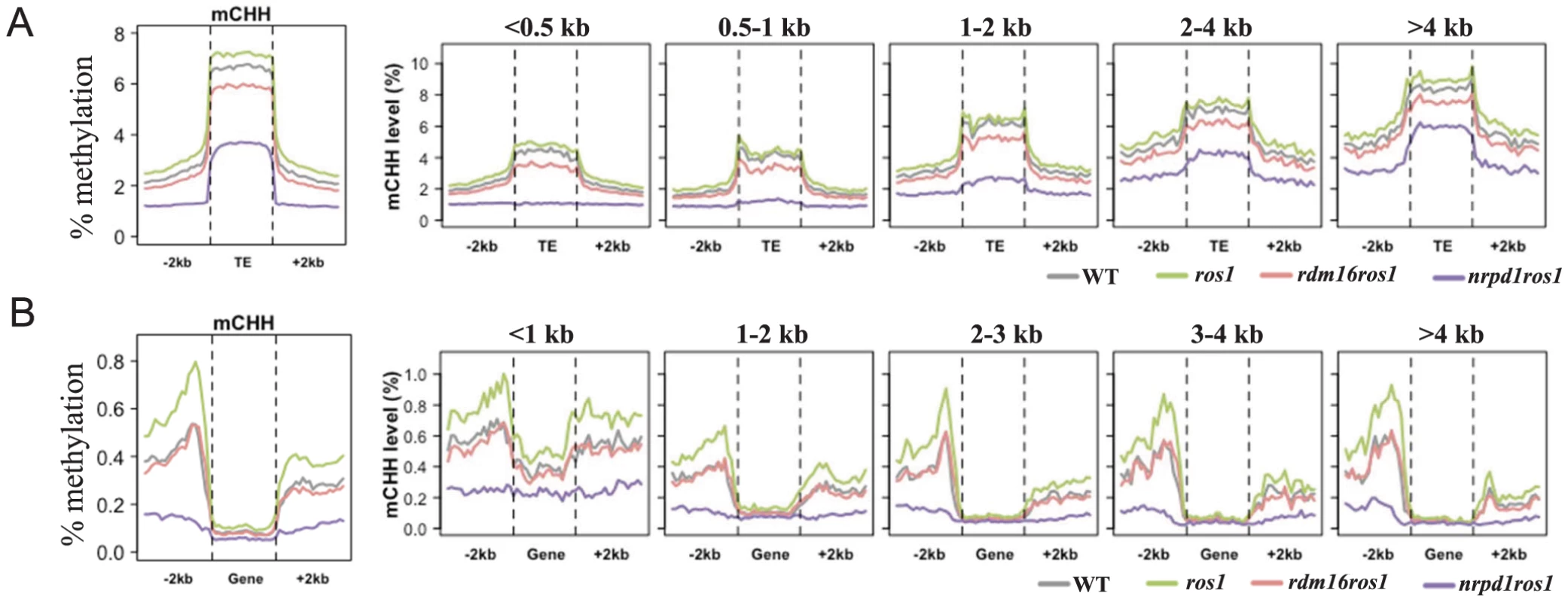
TEs and genes were divided into 5 groups based on their size for detailed comparison of the DNA methylation levels among WT, ros1, rdm16ros1 and nrpd1ros1. To investigate whether RDM16 has preference for TEs of different sizes, we divided the TEs into five groups based on the TE size and calculated average DNA methylation levels. The DNA methylation level of TEs was gradually elevated as the TE size increased. Mutation of RDM16 reduced the CHH methylation of all five groups of TEs, but did not influence CG and CHG methylation (Figure 5A, Figure S9A). This result indicates that RDM16 targets all TEs and does not have a preference for TEs of different sizes. The methylation pattern of TEs in rdm16ros1 is similar to nrpd1ros1, although the decrease in the level of DNA methylation was less dramatic in rdm16ros1 (Figure 5A).
In contrast to TEs, gene body methylation was mainly in the CG sequence context and the CG methylation is depleted towards both the 5′ and 3′ ends of genes (Figure S9B), which suggested that CG methylation at the 5′ and 3′ ends of the genes might not be compatible with Pol II-mediated transcription. Mutation of RDM16 did not affect overall DNA methylation of gene bodies in any sequence context (Figure 5B, Figure S9B). The methylation level of gene body was also not reduced in the nrpd1ros1 mutant, suggesting that the gene body methylation is largely independent of RdDM. Nevertheless, when we divided the genes into 5 groups with different gene sizes for the methylation analysis, we found that CHH methylation of short genes (<1 kb) was reduced in rdm16ros1 as well as nrpd1ros1 compared to ros1 mutant (Figure 5B, Figure S9B). Short genes have low CG methylation and increased CHH methylation, which suggested that RdDM pathway preferentially targets short genes, which results in elevated CHH methylation, and RDM16 is involved in the regulation of the DNA methylation of short genes. The CHG and CHH methylation levels of gene surrounding regions were greater than that of gene body, and 5′ promoter regions had higher methylation level than 3′ downstream region of genes in the wild-type (Figure 5B, Figure S9B). In ros1 mutant, the DNA methylation level was increased in all sequence contexts and the effect on CHH methylation was greater, suggesting that ROS1 targeted the promoters and downstream sequences of genes. Additionally, as gene size increased, the difference in CHH methylation in 3′ downstream regions of genes was decreased between WT and ros1 mutant (Figure 5B), which suggested that ROS1 preferentially targeted 3′ downstream regions of short genes. Mutation of RDM16 did not affect the CG methylation, but slightly reduced the CHG methylation and notably decreased the CHH methylation in both 5′ and 3′ regions of genes (Figure 5B, Figure S9B). In the rdm16ros1 mutant, the CHH methylation of 5′ and 3′ regions of genes of all sizes was reduced to a similar level as in WT, while in the nrpd1ros1 mutant the CHH methylation was further decreased (Figure 5B). These results suggest that ROS1 actively counteracts the effect of RDM16 and the RdDM pathway on the DNA methylation of 5′ promoters and 3′ downstream regions of genes.
We next identified differentially methylated regions (DMRs) in rdm16ros1 compared to ros1 mutant. There were 747 loci with decreased DNA methylation and 468 loci with increased DNA methylation in rdm16ros1 in comparison with ros1 mutant (Tables S5 and S6), while the number of hypomethylated and hypermethylated loci in nrpd1ros1 was 3929 and 1417, respectively (Tables S7 and S8). Of the 747 hypomethylated loci in rdm16ros1, 347 loci were located in intergenic regions, and most of the 347 loci were located within 1.5 kb promoter regions (Figures 6A and 6B), which suggested that RDM16 was preferentially involved in the regulation of DNA methylation of intergenic regions and potentially involved in regulating gene transcription. In contrast to RDM16, transposable elements (TE) are the main targets of NRPD1 (Figure 6A). Despite their different preferences, the hypomethylated loci of rdm16ros1 largely overlapped (77%) with those of nrpd1ros1 (Figure 6C). Moreover, the non-overlapping hypomethylated loci of rdm16ros1 also showed reduced DNA methylation levels in nrpd1ros1, especially at the CHH context. This result suggests that all RDM16 targets are influenced by the dysfunction of NRPD1, although some RDM16 targets were excluded from the list of NRPD1 targets due to the stringent cutoffs used to define differentially methylated regions. Interestingly, we noticed that the DNA demethylation enzyme ROS1 also preferentially targeted intergenic regions (Figure 6A). When comparing the 747 hypomethylated loci in rdm16ros1 with the hypermethylated loci in ros1, we found a striking overlap between them (Figure 6D). Similar to the case in nrpd1ros1, the nonoverlapping hypomethylated loci of rdm16ros1 also showed altered DNA methylation in ros1. Together, these results suggest that RDM16 is involved in the RdDM pathway and preferentially regulates the DNA methylation of loci that are targeted for demethylation by ROS1.
Fig. 6. Analysis of DMRs identified in rdm16ros1. 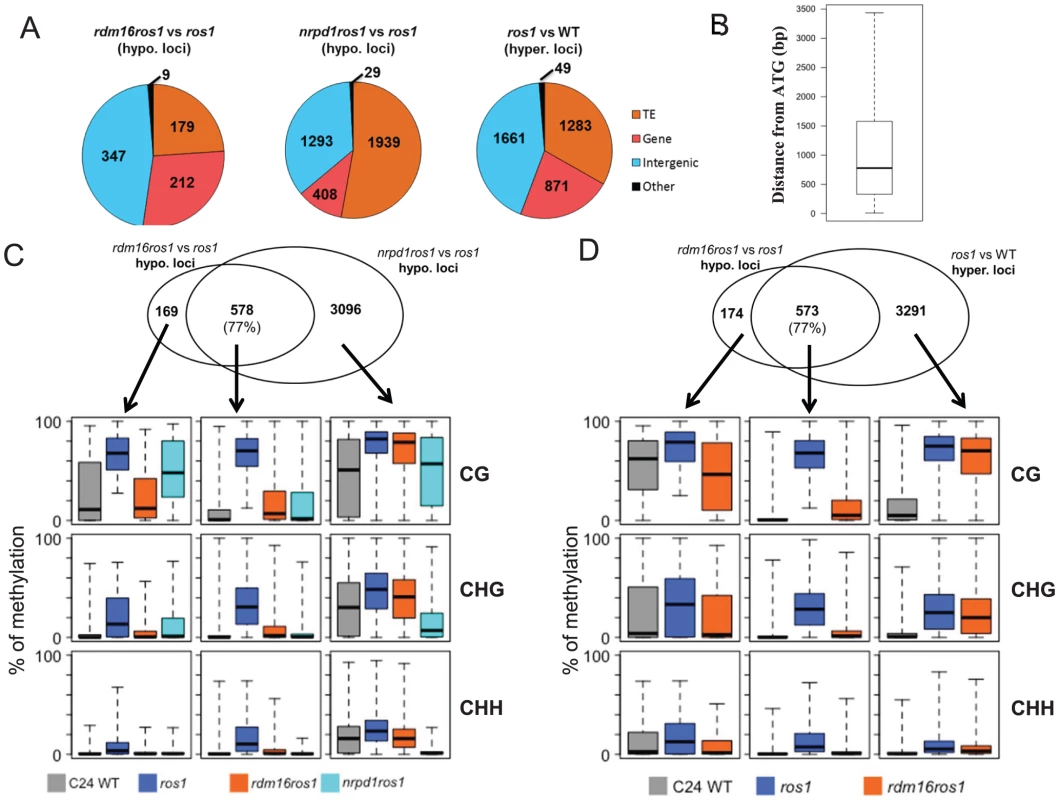
(A) Category of hypomethylated or hypermethylated loci in rdm16ros1, nrpd1ros1 or WT in comparison with ros1. (B) Distance distribution of intergenic DMRs relative to gene start codon. (C–D) Overlap of differentially methylated loci between rdm16ros1 and nrpd1ros1 (C), and between rdm16ros1 and ros1 (D). Boxplots represent methylation levels of each class of differentially methylated loci. To validate the whole-genome DNA methylation results, we selected 3 from the 747 hypomethylated loci for individual locus bisulfite sequencing. All three loci showed low levels of DNA methylation in WT, but became heavily methylated in ros1 at all sequence contexts (Figure 7). In the rdm16ros1 double mutant, the high DNA methylation was reduced compared to the ros1 single mutant, especially at the CHG and CHH contexts, although the decreased level of DNA methylation in rdm16ros1 was less prominent than in nrpd1ros1 (Figure 7).
Fig. 7. DNA methylation analysis of selected loci in WT, ros1, rdm16ros1 and nrpd1ros1. 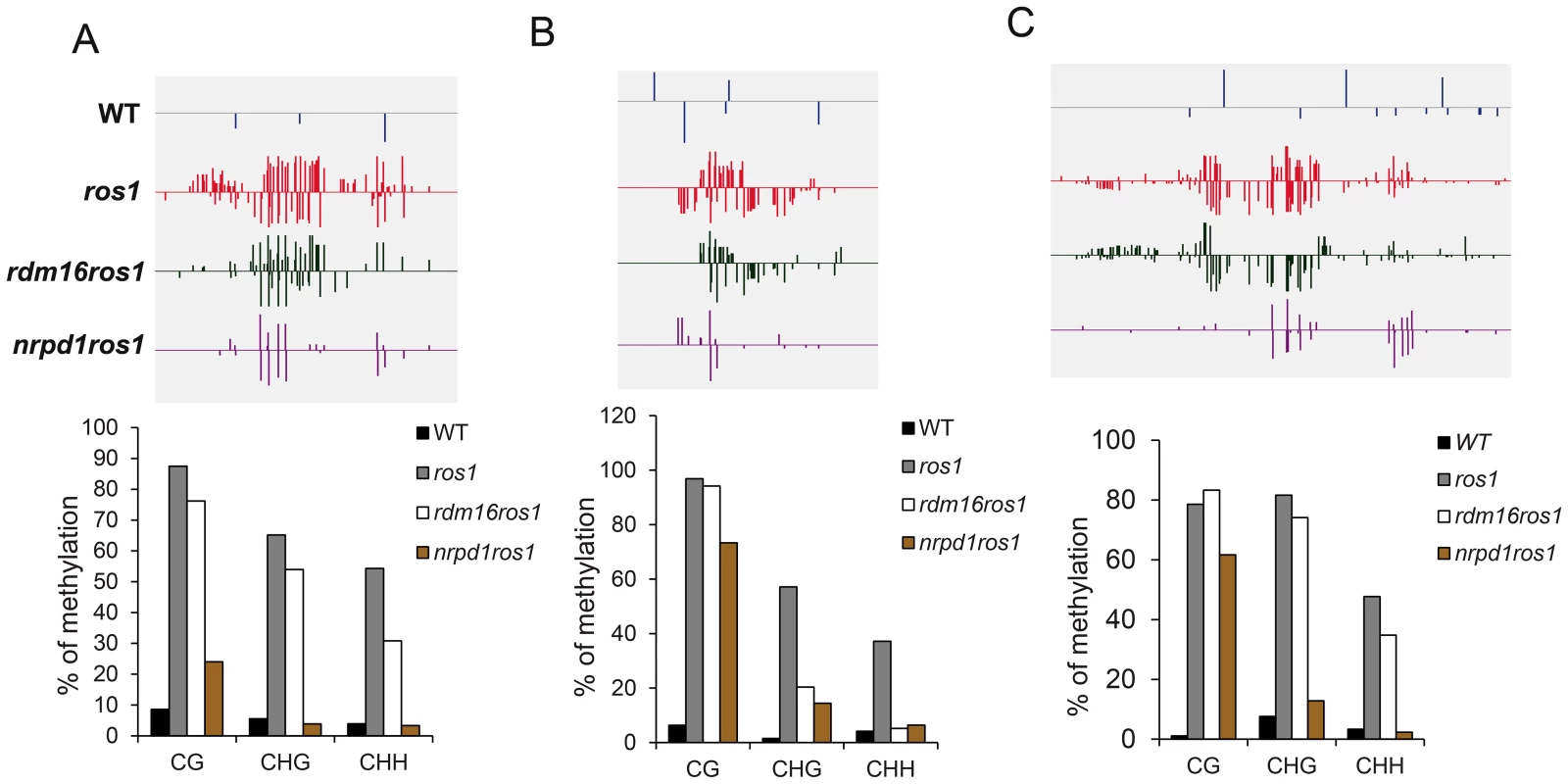
(A–C) DNA methylation levels of three loci. Upper panel, DNA methylation snapshot in IGV browser from whole-genome bisulfite sequencing data; Lower panel, individually bisulfite sequencing results. (A) At5g42940 promoter, (B) At5g35730 promoter, (C) At1g26400 promoter. The rdm16 mutation does not affect siRNA abundance
To investigate whether rdm16 may alter DNA methylation through influencing small RNA levels, we performed small RNA Northern blot analysis in WT, ros1, rdm16ros1, nrpd1ros1 and nrpe1ros1. The results showed that the rdm16 mutation did not affect the accumulation of any of the tested siRNAs compared to WT and ros1 controls, although the abundance of these tested siRNAs was greatly reduced in both nrpd1ros1 and nrpelros1 mutants (Figure 8). The targets of these siRNAs include the RD29A promoter, Solo-LTR and AtSN1 whose methylation levels were decreased in the rdm16ros1 mutant (Figure 2, Figure S2A). Therefore, these results suggest that RDM16 regulates DNA methylation not through influencing the siRNA abundance.
Fig. 8. siRNA accumulation was not affected by the mutation of RDM16. 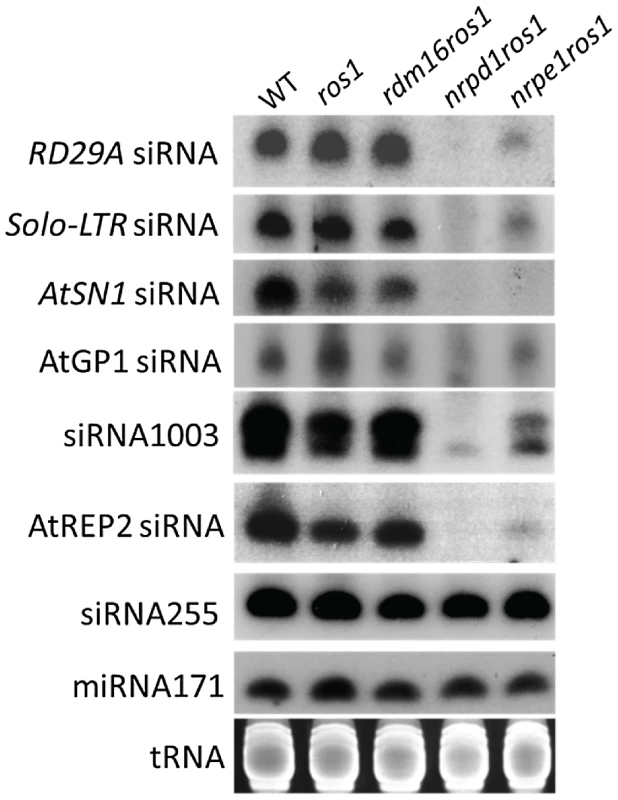
Small RNA Northern blotting of various siRNAs in WT, ros1, rdm16ros1, nrpd1ros1 and nrpe1ros1. tRNA, miRNA171 and siRNA255 were used as loading controls. RDM16 regulates Pol V transcript levels
The lack of effect of the rdm16 mutation on siRNA abundance suggests that RDM16 might function at a later step in the RdDM pathway. Therefore, we examined whether the rdm16 mutation may affect DNA methylation by influencing Pol V transcript levels. We selected 13 Pol V-dependent loci [48] including the RD29A-LUC promoter for analysis. Pol V-dependent transcripts of 8 of the 13 loci were detected in the C24 wild type under our conditions. Five of the 8 loci including the RD29A-LUC promoter displayed a decreased expression in rdm16ros1 compared to the WT and ros1 controls, although the reduction in expression level was less in rdm16ros1 than in nrpe1ros1 (Figure 9). All of the five loci with decreased Pol V transcripts in rdm16ros1 showed a reduced DNA methylation level to some extent in rdm16ros1 compared to ros1 (Figures 2A and 2B, Figure S10). We also compared the levels of the Pol V-dependent transcripts in Col WT, rdm16-2 and nrpe1-11. The results show that 8 loci had a decreased expression in rdm16-2 compared to WT, although the reduction in rdm16-2 was not as dramatic as in nrpe1-11 (Figure S11). These data suggest that RDM16 regulates DNA methylation through influencing Pol V transcripts.
Fig. 9. The rdm16 mutation reduced the levels of Pol V transcripts. 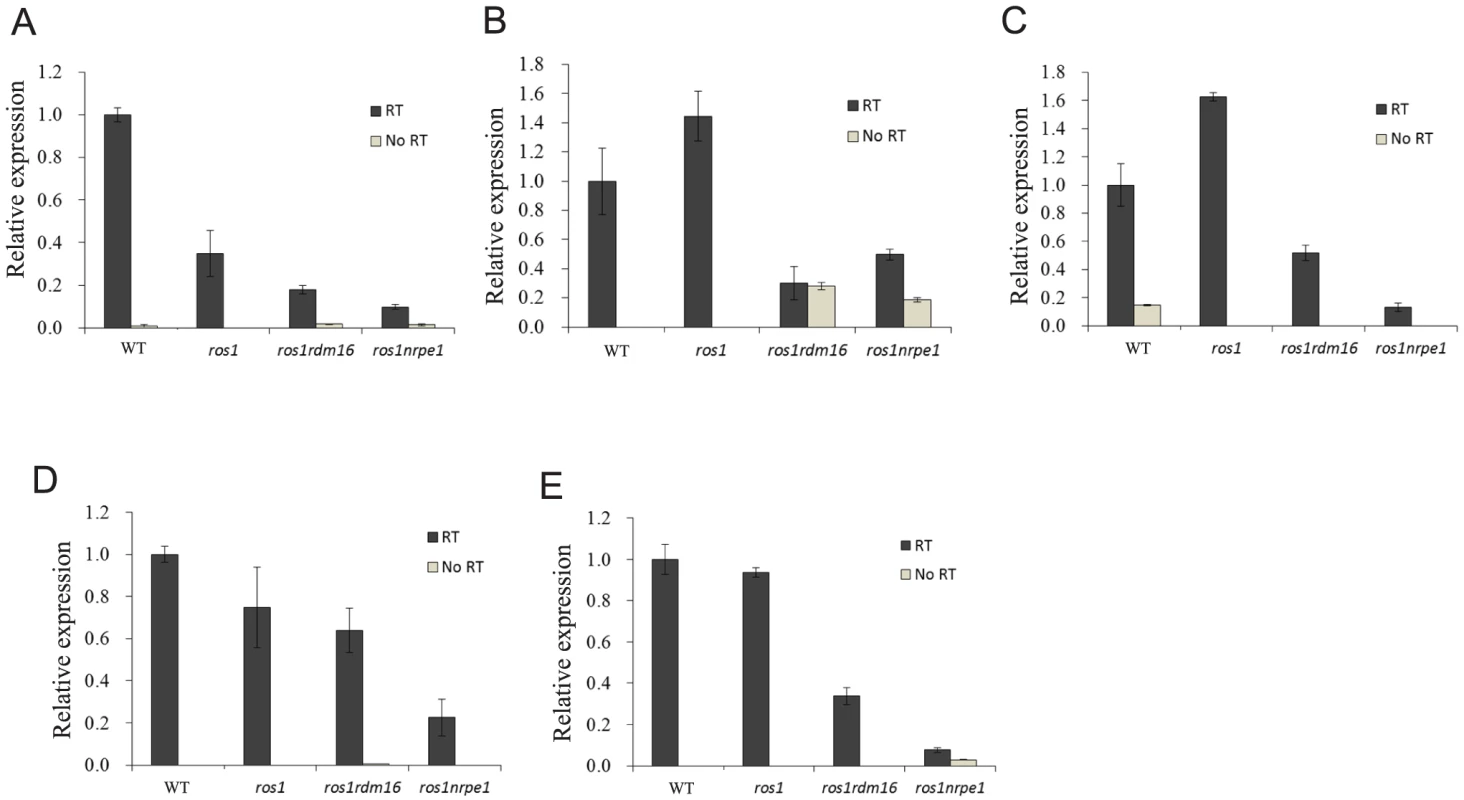
(A–E) Five Pol V-dependent loci showed decreased Pol V transcript levels in rdm16ros1 compared to WT and ros1 controls. (A) RD29A promoter, (B) IGN5, (C) IGN28, (D) IGN30, (E) IGN32. To investigate whether RDM16 may be associated with its target loci, we performed chromatin immunoprecipitation (ChIP) assays on Pol V target loci by using native promoter-driven RDM16-3xFlag or RDM16-3xHA transgenic lines. The RDM16-3xFlag or RDM16-3xHA construct complemented the defects of rdm16 mutant and we were able to detect tagged RDM16 protein in the transgenic lines by Western blot analysis (Figure S12), indicating that the tagged proteins are expressed and functional in vivo. The ChIP results show that RDM16 was enriched at all of the target loci tested in both RDM16-3xFlag and RDM16-3xHA transgenic lines (Figure 10), which indicated that RDM16 is associated with its target loci.
Fig. 10. ChIP analysis of RDM16 on Pol V targeted loci. 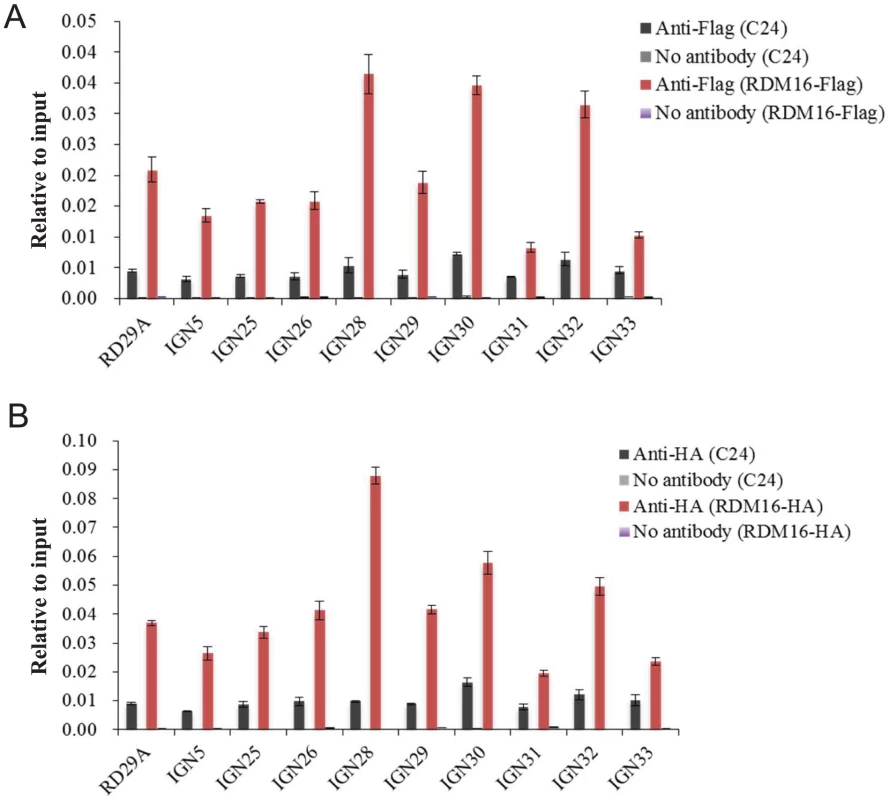
(A) ChIP analysis of RDM16-3xFlag transgenic lines using anti-Flag antibody. (B) ChIP analysis of RDM16-3xHA transgenic lines using anti-HA antibody. To examine the subnuclear localization patterns of RDM16, we used the RDM16-3xFlag transgenic line to perform immunolocalization assays. The results show that the RDM16 protein was dispersed throughout the nucleoplasm without any preferential accumulation at the Cajal body (Figure S13), based on the co-localization analysis between RDM16 and U2B, a maker for the Cajal body [26]. This localization pattern of RDM16 differs from that of ZOP1, a splicing factor recently reported to be involved in the RdDM pathway through affecting Pol IV-dependent siRNA accumulation [49]. In addition to the dispersed nucloplasmic localization, ZOP1 also preferentially accumulates in the Cajal body. The presence of ZOP1 but not RDM16 in the Cajal body is consistent with our hypothesis that RDM16 regulates DNA methylation through a different mechanism from those of reported splicing factors.”
Discussion
In this study, we isolated a new factor, RDM16, that functions in the RdDM pathway through a forward genetic screen. RDM16 is required for the transcriptional silencing and the increased DNA methylation of transgenic RD29A-LUC in ros1 mutant. Our genome-wide DNA methylation analysis showed that RDM16 also affects DNA methylation of TEs and gene surrounding regions globally and RDM16 preferentially influences NRPD1 - and ROS1-targted loci. RDM16 encodes a homolog of yeast Prp3 protein, which is a component of U4/U6 snRNP-associated protein and is involved in pre-mRNA-splicing in yeast [47]. Our RNA-seq data indicated that RDM16 is involved in the pre-mRNA-splicing in Arabidopsis (Table S1). None of the mis-spliced genes had changes in DNA methylation in the rdm16 mutant, suggesting that splicing and regulation of DNA methylation are two separate functions of RDM16. However, there is a possibility that RDM16 regulates DNA methylation by influencing expression and/or pre-mRNA splicing of genes involved in the RdDM pathway. Our RNA-seq and RT-PCR analysis indicated that RDM16 did not affect the expression or pre-mRNA splicing of genes encoding known RdDM components (Tables S1, S2, S3, Figure S7). These suggest that RDM16 involvement in the regulation of DNA methylation might be quite direct. To examine the mechanism of RDM16 in the RdDM, we compared the expression level of small RNAs and Pol V transcripts. However, unlike previously reported putative splicing factor sr45, mutation of RDM16 did not influence the small RNA accumulation but reduced the expression level of Pol V transcripts (Figures 8 and 9, Figure S11), which suggested that RDM16 regulates DNA methylation through a different mechanism from SR45.
RDM16 is critical for normal plant development, which is likely due to its role in the splicing of developmentally important genes. Knockout of RDM16 in both female and male gametes reduced their viability and knockout of both maternal and paternal RDM16 alleles in the embryo is lethal. Knockdown of RDM16 caused a series of developmental defects, including reduced plant stature, smaller leaves and siliques, rounded and wrinkled leaves (Figures 3A–C, Figure S6E). Furthermore, Knockdown of RDM16 led to an increased sensitivity to salt stress and ABA in seed germination (Figures 3D–F). Dysfunction of STA1, the U5 snRNP-associated pre-mRNA-splicing factor, also caused an increased sensitivity to ABA [50]. These suggest that hypersensitivity to ABA might be a common feature in the splicing factor-defective mutants. Nevertheless, there are differences in the physiological defects between the rdm16 and sta1 mutants. The hypersensitivity to chilling in sta1 mutant was not observed in the rdm16 mutant. On the other hand, sta1 did not have the phenotype of hypersensitivity to salt stress as rdm16 did. Moreover, unlike in sta1, the hypersensitivity to salt stress and ABA was not observed in the rdm16 mutant at the seedling stage (Figures 3G and 3H). Since both rdm16-1 and sta1-1 are weak mutant alleles but are not nulls, it is not clear whether the different physiological phenotypes between rdm16-1 and sta1-1 were due to the different mutations or due to the distinct targets of RDM16 and STA1.
In fission yeast, the formation of heterochromatin requires the RNAi machinery whose core components consist of Dicer (Dcr1), Argonaute (Ago1) and RNA-dependent RNA polymerase (Rdp1) [51], [52]. The pathway of RNA-induced heterochromatin formation in fission yeast parallels the RdDM pathway in Arabidopsis. In the fission yeast, Pol II transcribes the dg and dh repeats of centromeric regions and then Rdp1-containing RNA-dependent RNA polymerase complex (RDRC) and Dcr1 process the centromeric transcripts into siRNAs [51], [52]. The siRNA is loaded into Ago1 to form the RNA-induced transcriptional gene-silencing (RITS) complex that also contains the chromodomain protein Chp1 and GW-motif-containing protein Tas3. Through base-pairing of the siRNA with Pol II-produced nascent repeat transcripts, the RITS is specifically recruited to the target region and facilitate the recruitment of histone-modifying enzymes such as the H3K9 methyltransferase Clr4 to induce H3K9 methylation and the formation of heterochromatin [51], [52]. Recently, several splicing factors were reported to be involved in the RNAi-directed silencing process in fission yeast [53], [54]. Mutation of these splicing factors, but not the splicing itself, affects the accumulation of centromeric siRNAs and consequently the integrity of centromeric heterochromatin. Splicing factors are found to be co-purified with affinity-selected Cid12, a component of RDRC, and therefore it was proposed that splicing factors might be recruited to centromeric noncoding RNA through the recognition of dg intron sequence and then interact with RDRC to provide a platform for siRNA generation and finally facilitate the centromere repeat silencing [53], [54]. Among these splicing factors involved in the centromere repeat silencing, Prp3, the homolog of RDM16 in fission yeast, is not included. Nevertheless, Prp3 was co-immunoprecipitated with FLAG-tagged Cid12 and mutation of Prp3 led to the increased accumulation of dg transcripts [53], [54], which suggest that Prp3 is also involved in the processing of the centromeric transcripts into siRNAs, but the influence may not be strong enough to have an observable effect on the centromere repeat silencing in the mutant. In this report, we showed that RDM16, a homolog of yeast Prp3, is involved in the RdDM pathway in Arabidopsis. Mutation of RDM16 reduced the DNA methylation and released the transcriptional silencing of transgene and endogenous targets. However, rdm16 mutation did not influence the siRNA accumulation at DNA methylation-affected loci. These results suggest that RDM16 function in the RdDM pathway might adopt a different mechanism from the yeast splicing factors in the pathway of centromere repeat silencing.
Mutation of SR45 causes a decreased accumulation of siRNAs and the AGO4 protein level was also reduced, which suggested that SR45 acts at an early step in the RdDM pathway in siRNA generation [36]. Recently, Zhang et al. (2013) reported that ZOP1 and several other splicing factors are also involved in regulating DNA methylation through influencing siRNA abundance [49]. Since the rdm16 mutation did not cause a reduction in siRNA abundance, this suggests that RDM16 likely functions in a later step in RdDM. Indeed, mutation of RDM16 caused a reduction in the levels of Pol V transcripts. Dysfunction of Pol V not only abolishes the Pol V transcripts but also influences the siRNA abundance. However, the rdm16 mutation caused a decrease in Pol V transcript levels but did not affect the siRNA abundance, suggesting that the reduction in the levels of Pol V transcripts in rdm16 may not be strong enough to cause a decrease in the siRNA accumulation or that Pol V may function in the siRNA accumulation independently of its transcripts. As a splicing factor, RDM16 has a known role in RNA processing, so it is possible that RDM16 function in RdDM may involve an interaction with scaffold RNAs generated by Pol V or Pol II. Consistent with this hypothesis, our ChIP assays indicated that RDM16 is associated with Pol V target loci. The association of RDM16 with Pol V target loci might be mediated through the interaction of RDM16-containing complex with Pol V or nascent Pol V transcripts. Further work is required to determine whether RDM16 is associated with Pol V and/or can interact with Pol V transcripts. Regardless, the fact that SR45 [36] and ZOP1 [49] but not RDM16 are involved in siRNA accumulation suggests that the various splicing factors function at different steps in the RdDM pathway. This is consistent with our notion that RDM16 functions directly in RdDM, and further argues against the model that splicing factors function indirectly in RdDM by affecting the pre-mRNA splicing of genes encoding RdDM components.
Our analysis of the methylome of the rdm16 mutant showed that dysfunction of RDM16 affected the overall CHH methylation of TEs (Figure 5A), indicating an important role of RDM16 on the silencing of parasitic sequences. The overall methylation of gene body was not influenced by the mutation of any of the genes, ROS1, RDM16 or NRPD1 (Figure 5B). However, in short genes (<1 kb), mutation of all three genes affected the genic CHH methylation levels, and the methylation level was similar between rdm16ros1 and WT. These suggest that DNA methylation of short genes is largely dependent on the RdDM pathway and ROS1 demethylates the DNA methylation that is mainly contributed by RDM16 through the RdDM pathway. In the gene surrounding regions, mutation of ROS1 caused increased DNA methylation levels of both 5′ promoters and 3′ downstream regions of the genes, with the methylation peaks close to gene ends (Figure 5B), which suggested that ROS1 might be important for the regulation of DNA methylation of gene regulatory elements and thus likely affects gene transcription. Mutation of RDM16 in the ros1 background reduced the CHH methylation to the wild-type level. Furthermore, RDM16 target loci were largely overlapping with ROS1 target loci. The active DNA demethylation machinery may recognize features resulting from RdDM and thus preferentially demethylate RdDM target sequences. It would be interesting to investigate whether RDM16 or its associated proteins may be involved in somehow marking the RdDM target sequences for demethylation.
Materials and Methods
Plant materials, growth conditions and mutant screening
The wild-type C24 and ros1 mutant plants carry a homozygous RD29A promoter-driven luciferase transgene and a 35S promoter-driven NPTII transgene [41]. A T-DNA mutagenized ros1 population was generated and screened for suppressors of ros1 as described previously [43]. The rdm16ros1 mutant was obtained from this screening. rdm16-2 (CS861738), rdm16-3 (CS861738) and another T-DNA insertion line (SALK_057447C) have the Columbia-0 (Col) genetic background. Plants were grown in a growth chamber or controlled room at 23°C with 16 h of light and 8 h of darkness.
Cloning of RDM16
The rdm16ros1 was crossed to ros1 (C24 background) or ros1-4 (Col background, Salk_045303) for genetic analysis or molecular mapping. The selfed F2 plants were subjected to the observation of luminescence emission and morphological alterations. For the mapping of RDM16, 30 F2 plants with low luminescence signal and defective morphology were used to determine the location of RDM16 in the genome by using 25 indel polymorphic markers evenly distributed over the genome and then 74 F2 mutants and three more markers were used to map the RDM16 on the chromosome 1. For the fine mapping of RDM16, a total of 632 F2 plants were used and 6 additional markers between At120 and At124 markers were developed. As a result, RDM16 was finally mapped between At126 and At130 makers with a physical candidate region of 1.39 Mb. The second-generation high throughput DNA sequencing (Illumina) was carried out to search the mutation occurred in the rdm16ros1 mutant. There was a DNA fragment inserted in the promoter of At1g28060. TAIL-PCR technique was used to determine the inserted position and the sequence of the DNA fragment [55].
For the complementation test of rdm16ros1 mutant, a DNA fragment harboring 1.4 kb promoter, the gene and 0.7 kb downstream of stop codon of At1g28060 was amplified and finally cloned into a binary vector pORE-O2. The resultant vector was transformed into rdm16ros1 mutant through Agrobacterium-mediated floral dip method. T2 plants were genotyped by genomic PCR and plants harboring the transgene were subjected for morphological observations, luminescence imaging and bisulfite sequencing analysis. For the luminescence imaging, leaves of 4-week-old plants were detached and treated with 2% NaCl for 3 h in the light at 23°C, and then applied for the luminescence imaging.
Evaluation of the sensitivity to salt and ABA
For the sensitivity assay at germination stage, seeds of WT, ros1 and rdm16ros1 were sowed on a 1/2 MS plate containing 0, 75 mM NaCl or 0.5 µM ABA. The seed-containing plates were stratified at 4°C for 3 d and then exposed to a long day condition at 23°C. After 15 d treatment, the seedlings were photographed and compared. For the sensitivity assay at seedling stage, seeds were germinated and grown on a 1/2 MS plate for 6 d and then the seedlings were transferred to a 1/2 MS plate containing different concentrations of salt (0, 75, 100 or 125 mM NaCl) or ABA (0, 0.5, 1 or 2 µM ABA). After 7 d treatment, the root elongation was measured and compared.
Expression analysis of mRNA, small RNA and Pol V transcripts
To examine the expression of RD29A-LUC and endogenous RD29A, 12-day-old seedlings were exposed to various stress conditions: 0, 150 mM NaCl for 3 h, 50 µM ABA for 3 h or cold (4°C) for 2 d. After the treatment, the whole seedlings were sampled for RNA isolation. For the expression analysis of ROS1, RdDM genes and Pol V transcripts, leaves of plants with three weeks old were collected for RNA isolation. Total RNA was extracted using the RNeasy Mini Kit (Qiagen) and then treated with Turbo DNase (Ambion) for 45 min to remove the contaminated DNA. Total RNA was used for first strand cDNA synthesis using a SuperScript II kit (Invitrogen), following the manufacturer's instructions with an oligo(dT)12–18 or random primer. The derived cDNA was used as template for semiquantitative or real-time RT-PCR analysis. For small RNA accumulation analysis, 2-week-old seedlings of the wild-type, ros1, rdm16ros1, nrpd1ros1 and nrpe1ros1 were harvested for the small RNA extraction. The extraction method and northern blot analysis of small RNA have been described previously [43]. The primers or probes for the expression analysis are listed in Table S9.
DNA methylation analysis
Leaves of 4-week-old plants were collected for genomic DNA isolation. The DNA was extracted using the DNeasy Plant Mini Kit (Qiagen). To perform bisulfite sequencing, 80–100 ng of DNA was sodium-bisulfite converted and purified by using the BisulFlash DNA Modification Kit (EIPGENTEK). For the DNA methylation analysis, at least 15 clones at each sample were sequenced. The primers for the bisulfite sequencing are listed in Table S9.
High-throughput RNA sequencing analysis
Leaves of the wild-type (Col) and rdm16-2 plants with 4 weeks old were collected for RNA isolation. Total RNA was extracted using the RNeasy Mini Kit (Qiagen). The extracted RNA was sent to BGI (Shenzhen, China) for RNA-seq library preparation and whole transcriptome sequencing. The raw reads were aligned to the Arabidopsis genome (TAIR10, www.arabidopsis.org) by using TopHat program (http://tophat. cbcb.umd.edu). The assembling of the reads and the calculation of transcript abundance were performed by Cufflinks (http://cufflinks.cbcb.umd.edu). Transcripts that were differentially expressed in WT and rdm16-2 were identified by Cuffdiff, a part of the Cufflinks package. For the intron-retention analysis, reads located in intron regions were calculated in WT and rdm16-2 separately, and then degrees of differential expression were measured according to the method described by Audic et al. (1997) [56], which was constructed based on Poisson distribution eliminating the influence of sequencing depth. The introns with more than 95% read coverage and false discovery rate (FDR) <0.01 were regarded as intron-retention events.”
Whole-genome bisulfite sequencing and data analysis
DNA was extracted from leaves of 4-week-old plant and sent to BGI (Shenzhen, China) for bisulfite treatment, library preparation, and sequencing. For data analysis, adapter and low quality sequences (q<20) were trimmed and clean reads were mapped to a pseudo-C24 genome using BRAT-BW [57] allowing two mismatches. The pseudo-C24 genome was generated through the replacement of SNPs in the Col-0 genome with C24 variants (http://1001genomes.org/data/MPI/MPISchneeberger2011/releases/current//C24/Marker/C24.SNPs.TAIR9.txt). The method for identification of differentially methylated regions (DMRs) was according to Qian et al. (2012) with minor modifications [58]. In brief, differentially methylated cytosine (DMC) was identified if the p-value from the two-tailed Fisher's exact test was less than 0.05. DMRs were discovered using a sliding-window approach with 200 bp-window sliding at 50 bp intervals. A region with more than 2 DMCs was selected as an anchor region. The boundary of each anchor region was defined based on the locations of first and last DMCs in the region. If the distance between two anchor regions is less than 100 bp, they will be merged into one large region. DMRs were finally identified based on the regions with ≥100 bp, ≥5 DMCs, and absolute methylation difference of 0.3 for CG, 0.15 for CHG or 0.10 for CHH.
ChIP assays
Twelve-day-old seedlings of C24 and rdm16 mutant complementation lines with native promoter-driven RDM16-3xFLAG or RDM16-3xHA transgene were used for chromatin immunoprecipitation (ChIP) assays. The ChIP assays were performed according to a published protocol [59]. ChIP products were eluted into 50 µl of TE buffer, and a 2 µl aliquot was used for each qPCR reaction.
Immunolocalization
Nuclei were isolated from protoplasts of Arabidopsis young leaves according to a published protocol [60]. Nuclei were then fixed in 4% formaldehyde and applied to slides for immunolocalization assay as previously described [61]. After being treated with the blocking solution (3% BSA in PBS), the nuclei were then incubated with primary antibodies overnight at 4°C. Each primary antibody was properly diluted in the blocking solution. After washing the slides, secondary anti-mouse TRITC (Invitrogen) and anti-rabbit FITC (Invitrogen) were added and incubated at 37°C. Chromatin was counterstained with DAPI. Images were acquired by SPINNING DISK confocal microscopy and then analyzed with Volocity software.
Data deposition
The RNA-seq and whole-genome bisulfite sequencing data used in this paper have been deposited in the National Center for Biotechnology Information Gene Expression Omnibus (NCBI GEO) (http://www.ncbi.nlm.nih.gov/geo/) under accession numbers GSE44635 and GSE44417, respectively.
Supporting Information
Zdroje
1. LischD, BennetzenJL (2011) Transposable element origins of epigenetic gene regulation. Current Opinion in Plant Biology 14 : 156–161.
2. MirouzeM, PaszkowskiJ (2011) Epigenetic contribution to stress adaptation in plants. Current Opinion in Plant Biology 14 : 267–274.
3. HeardE (2004) Recent advances in X-chromosome inactivation. Current Opinion in Cell Biology 16 : 247–255.
4. ErhardKF, HollickJB (2011) Paramutation: a process for acquiring trans-generational regulatory states. Current Opinion in Plant Biology 14 : 210–216.
5. BauerMJ, FischerRL (2011) Genome demethylation and imprinting in the endosperm. Current Opinion in Plant Biology 14 : 162–167.
6. CokusSJ, FengSH, ZhangXY, ChenZG, MerrimanB, et al. (2008) Shotgun bisulphite sequencing of the Arabidopsis genome reveals DNA methylation patterning. Nature 452 : 215–219.
7. SazeH, ScheidOM, PaszkowskiJ (2003) Maintenance of CpG methylation is essential for epigenetic inheritance during plant gametogenesis. Nature Genetics 34 : 65–69.
8. HenikoffS, ComaiL (1998) A DNA methyltransferase homolog with a chromodomain exists in multiple polymorphic forms in Arabidopsis. Genetics 149 : 307–318.
9. CaoXF, JacobsenSE (2002) Locus-specific control of asymmetric and CpNpG methylation by the DRM and CMT3 methyltransferase genes. Proceedings of the National Academy of Sciences of the United States of America 99 : 16491–16498.
10. ChanSWL, HendersonIR, JacobsenSE (2005) Gardening the genome: DNA methylation in Arabidopsis thaliana. Nature Reviews Genetics 6 : 351–360.
11. WasseneggerM, HeimesS, RiedelL, SangerHL (1994) Rna-Directed De-Novo Methylation of Genomic Sequences in Plants. Cell 76 : 567–576.
12. MatzkeM, KannoT, ClaxingerL, HuettelB, MatzkeAJM (2009) RNA-mediated chromatin-based silencing in plants. Current Opinion in Cell Biology 21 : 367–376.
13. VaucheretH (2005) RNA polymerase IV and transcriptional silencing. Nature Genetics 37 : 659–660.
14. HerrAJ, JensenMB, DalmayT, BaulcombeDC (2005) RNA polymerase IV directs silencing of endogenous DNA. Science 308 : 118–120.
15. KasschauKD, FahlgrenN, ChapmanEJ, SullivanCM, CumbieJS, et al. (2007) Genome-wide profiling and analysis of Arabidopsis siRNAs. Plos Biology 5 : 479–493.
16. XieZX, JohansenLK, GustafsonAM, KasschauKD, LellisAD, et al. (2004) Genetic and functional diversification of small RNA pathways in plants. Plos Biology 2 : 642–652.
17. LiJJ, YangZY, YuB, LiuJ, ChenXM (2005) Methylation protects miRNAs and siRNAs from a 3′-end uridylation activity in Arabildopsis. Current Biology 15 : 1501–1507.
18. YuB, YangZY, LiJJ, MinakhinaS, YangMC, et al. (2005) Methylation as a crucial step in plant microRNA biogenesis. Science 307 : 932–935.
19. SmithLM, PontesO, SearleL, YelinaN, YousafzaiFK, et al. (2007) An SNF2 protein associated with nuclear RNA silencing and the spread of a silencing signal between cells in Arabidopsis. Plant Cell 19 : 1507–1521.
20. LiuJ, BaiG, ZhangCJ, ChenW, ZhouJX, et al. (2011) An atypical component of RNA-directed DNA methylation machinery has both DNA methylation-dependent and -independent roles in locus-specific transcriptional gene silencing. Cell Research 21 : 1691–1700.
21. LawJA, VashishtAA, WohlschlegelJA, JacobsenSE (2011) SHH1, a Homeodomain Protein Required for DNA Methylation, As Well As RDR2, RDM4, and Chromatin Remodeling Factors, Associate with RNA Polymerase IV. Plos Genetics 7: e1002195.
22. QiYJ, HeXY, WangXJ, KohanyO, JurkaJ, et al. (2006) Distinct catalytic and non-catalytic roles of ARGONAUTE4 in RNA-directed DNA methylation. Nature 443 : 1008–1012.
23. WierzbickiAT, ReamTS, HaagJR, PikaardCS (2009) RNA polymerase V transcription guides ARGONAUTE4 to chromatin. Nature Genetics 41 : 630–634.
24. LawJA, AusinI, JohnsonLM, VashishtAA, ZhuJK, et al. (2010) A Protein Complex Required for Polymerase V Transcripts and RNA-Directed DNA Methylation in Arabidopsis. Current Biology 20 : 951–956.
25. HaagJR, PikaardCS (2011) Multisubunit RNA polymerases IV and V: purveyors of non-coding RNA for plant gene silencing. Nature Reviews Molecular Cell Biology 12 : 483–492.
26. LiCF, PontesO, El-ShamiM, HendersonIR, BernatavichuteYV, et al. (2006) An ARGONAUTE4-containing nuclear processing center colocalized with Cajal bodies in Arabidopsis thaliana. Cell 126 : 93–106.
27. El-ShamiM, PontierD, LahmyS, BraunL, PicartC, et al. (2007) Reiterated WG/GW motifs form functionally and evolutionarily conserved ARGONAUTE-binding platforms in RNAi-related components. Genes & Development 21 : 2539–2544.
28. HeXJ, HsuYF, ZhuSH, WierzbickiAT, PontesO, et al. (2009) An Effector of RNA-Directed DNA Methylation in Arabidopsis Is an ARGONAUTE 4-and RNA-Binding Protein. Cell 137 : 498–508.
29. GaoZH, LiuHL, DaxingerL, PontesO, HeXJ, et al. (2010) An RNA polymerase II - and AGO4-associated protein acts in RNA-directed DNA methylation. Nature 465 : 106–U118.
30. ZhengBL, WangZM, LiSB, YuB, LiuJY, et al. (2009) Intergenic transcription by RNA Polymerase II coordinates Pol IV and Pol V in siRNA-directed transcriptional gene silencing in Arabidopsis. Genes & Development 23 : 2850–2860.
31. HaveckerER, WallbridgeLM, HardcastleTJ, BushMS, KellyKA, et al. (2010) The Arabidopsis RNA-Directed DNA Methylation Argonautes Functionally Diverge Based on Their Expression and Interaction with Target Loci. Plant Cell 22 : 321–334.
32. ZhengXW, ZhuJH, KapoorA, ZhuJK (2007) Role of Arabidopsis AGO6 in siRNA accumulation, DNA methylation and transcriptional gene silencing. Embo Journal 26 : 1691–1701.
33. HendersonIR, DelerisA, WongW, ZhongXH, ChinHG, et al. (2010) The De Novo Cytosine Methyltransferase DRM2 Requires Intact UBA Domains and a Catalytically Mutated Paralog DRM3 during RNA-Directed DNA Methylation in Arabidopsis thaliana. Plos Genetics 6: e1001182.
34. LorkovicZJ, NaumannU, MatzkeAJM, MatzkeM (2012) Involvement of a GHKL ATPase in RNA-Directed DNA Methylation in Arabidopsis thaliana. Current Biology 22 : 933–938.
35. MoissiardG, CokusSJ, CaryJ, FengS, BilliAC, et al. (2012) MORC family ATPases required for heterochromatin condensation and gene silencing. Science 336 : 1448–1451.
36. AusinI, GreenbergMVC, LiCF, JacobsenSE (2012) The splicing factor SR45 affects the RNA-directed DNA methylation pathway in Arabidopsis. Epigenetics 7 : 29–33.
37. JuricaMS, MooreMJ (2003) Pre-mRNA splicing: Awash in a sea of proteins. Molecular Cell 12 : 5–14.
38. KramerA (1996) The structure and function of proteins involved in mammalian pre-mRNA splicing. Annual Review of Biochemistry 65 : 367–409.
39. GolovkinM, ReddyASN (1999) An SC35-like protein and a novel serine/arginine-rich protein interact with Arabidopsis U1-70K protein. Journal of Biological Chemistry 274 : 36428–36438.
40. TanabeN, KimuraA, YoshimuraK, ShigeokaS (2009) Plant-specific SR-related protein atSR45a interacts with spliceosomal proteins in plant nucleus. Plant Molecular Biology 70 : 241–252.
41. GongZH, Morales-RuizT, ArizaRR, Roldan-ArjonaT, DavidL, et al. (2002) ROS1, a repressor of transcriptional gene silencing in Arabidopsis, encodes a DNA glycosylase/lyase. Cell 111 : 803–814.
42. HeXJ, HsuYF, ZhuSH, LiuHL, PontesO, et al. (2009) A conserved transcriptional regulator is required for RNA-directed DNA methylation and plant development. Genes & Development 23 : 2717–2722.
43. HeXJ, HsuYF, PontesO, ZhuJH, LuJ, et al. (2009) NRPD4, a protein related to the RPB4 subunit of RNA polymerase II, is a component of RNA polymerases IV and V and is required for RNA-directed DNA methylation. Genes & Development 23 : 318–330.
44. ZhengZM, XingY, HeXJ, LiWB, HuYL, et al. (2010) An SGS3-like protein functions in RNA-directed DNA methylation and transcriptional gene silencing in Arabidopsis. Plant Journal 62 : 92–99.
45. ZhuJ, KapoorA, SridharVV, AgiusF, ZhuJK (2007) The DNA glycosylase/lyase ROS1 functions in pruning DNA methylation patterns in Arabidopsis. Current Biology 17 : 54–59.
46. ChakarovaCF, HimsMM, BolzH, Abu-SafiehL, PatelRJ, et al. (2002) Mutations in HPRP3, a third member of pre-mRNA splicing factor genes, implicated in autosomal dominant retinitis pigmentosa. Human Molecular Genetics 11 : 87–92.
47. AnthonyJG, WeidenhammerEM, WoolfordJL (1997) The yeast Prp3 protein is a U4/U6 snRNP protein necessary for integrity of the U4/U6 snRNP and the U4/U6.U5 tri-snRNP. Rna-a Publication of the Rna Society 3 : 1143–1152.
48. WierzbickiAT, HaagJR, PikaardCS (2008) Noncoding transcription by RNA polymerase Pol IVb/Pol V mediates transcriptional silencing of overlapping and adjacent genes. Cell 135 : 635–648.
49. ZhangCJ, ZhouJX, LiuJ, MaZY, ZhangSW, et al. (2013) The splicing machinery promotes RNA-directed DNA methylation and transcriptional silencing in Arabidopsis. Embo Journal 32 : 1128–1140.
50. LeeBH, KapoorA, ZhuJ, ZhuJK (2006) STABILIZED1, a stress-upregulated nuclear protein, is required for pre-mRNA splicing, mRNA turnover, and stress tolerance in Arabidopsis. Plant Cell 18 : 1736–1749.
51. MoazedD (2009) Small RNAs in transcriptional gene silencing and genome defence. Nature 457 : 413–420.
52. GrewalSI (2010) RNAi-dependent formation of heterochromatin and its diverse functions. Current Opinion in Genetics & Development 20 : 134–141.
53. BayneEH, PortosoM, KaganskyA, Kos-BraunIC, UranoT, et al. (2008) Splicing factors facilitate RNAi-directed silencing in fission yeast. Science 322 : 602–606.
54. ChinenM, MoritaM, FukumuraK, TaniT (2010) Involvement of the spliceosomal U4 small nuclear RNA in heterochromatic gene silencing at fission yeast centromeres. J Biol Chem 285 : 5630–5638.
55. LiuYG, MitsukawaN, OosumiT, WhittierRF (1995) Efficient isolation and mapping of Arabidopsis thaliana T-DNA insert junctions by thermal asymmetric interlaced PCR. Plant J 8 : 457–463.
56. AudicS, ClaverieJM (1997) The significance of digital gene expression profiles. Genome Research 7 : 986–995.
57. HarrisEY, PontsN, Le RochKG, LonardiS (2012) BRAT-BW: efficient and accurate mapping of bisulfite-treated reads. Bioinformatics 28 : 1795–1796.
58. QianW, MikiD, ZhangH, LiuY, ZhangX, et al. (2012) A histone acetyltransferase regulates active DNA demethylation in Arabidopsis. Science 336 : 1445–1448.
59. SalehA, Alvarez-VenegasR, AvramovaZ (2008) An efficient chromatin immunoprecipitation (ChIP) protocol for studying histone modifications in Arabidopsis plants. Nat Protoc 3 : 1018–1025.
60. YooSD, ChoYH, SheenJ (2007) Arabidopsis mesophyll protoplasts: a versatile cell system for transient gene expression analysis. Nature Protocols 2 : 1565–1572.
61. OnoderaY, HaagJR, ReamT, NunesPC, PontesO, et al. (2005) Plant nuclear RNA polymerase IV mediates siRNA and DNA methylation-dependent heterochromatin formation. Cell 120 : 613–622.
Štítky
Genetika Reprodukční medicína
Článek Rapid Intrahost Evolution of Human Cytomegalovirus Is Shaped by Demography and Positive SelectionČlánek Common Variants in Left/Right Asymmetry Genes and Pathways Are Associated with Relative Hand SkillČlánek Manipulating or Superseding Host Recombination Functions: A Dilemma That Shapes Phage EvolvabilityČlánek Maternal Depletion of Piwi, a Component of the RNAi System, Impacts Heterochromatin Formation inČlánek Hsp104 Suppresses Polyglutamine-Induced Degeneration Post Onset in a Drosophila MJD/SCA3 ModelČlánek Cooperative Interaction between Phosphorylation Sites on PERIOD Maintains Circadian Period inČlánek VAPB/ALS8 MSP Ligands Regulate Striated Muscle Energy Metabolism Critical for Adult Survival inČlánek Histone Chaperone NAP1 Mediates Sister Chromatid Resolution by Counteracting Protein Phosphatase 2AČlánek A Link between ORC-Origin Binding Mechanisms and Origin Activation Time Revealed in Budding YeastČlánek Genotype-Environment Interactions Reveal Causal Pathways That Mediate Genetic Effects on PhenotypeČlánek Chromatin-Specific Regulation of Mammalian rDNA Transcription by Clustered TTF-I Binding SitesČlánek Meiotic Recombination in Arabidopsis Is Catalysed by DMC1, with RAD51 Playing a Supporting Role
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2013 Číslo 9- Akutní intermitentní porfyrie
- Farmakogenetické testování pomáhá předcházet nežádoucím efektům léčiv
- Růst a vývoj dětí narozených pomocí IVF
- IVF a rakovina prsu – zvyšují hormony riziko vzniku rakoviny?
- Pilotní studie: stres a úzkost v průběhu IVF cyklu
-
Všechny články tohoto čísla
- The Pathway Gene Functions together with the -Dependent Isoprenoid Biosynthetic Pathway to Orchestrate Germ Cell Migration
- Take Off, Landing, and Fly Anesthesia
- Nucleosome Assembly Proteins Get SET to Defeat the Guardian of Chromosome Cohesion
- Whole-Exome Sequencing Reveals a Rapid Change in the Frequency of Rare Functional Variants in a Founding Population of Humans
- Evidence Is Evidence: An Interview with Mary-Claire King
- Rapid Intrahost Evolution of Human Cytomegalovirus Is Shaped by Demography and Positive Selection
- Convergent Transcription Induces Dynamic DNA Methylation at Loci
- Environmental Stresses Disrupt Telomere Length Homeostasis
- Ultra-Sensitive Sequencing Reveals an Age-Related Increase in Somatic Mitochondrial Mutations That Are Inconsistent with Oxidative Damage
- Common Variants in Left/Right Asymmetry Genes and Pathways Are Associated with Relative Hand Skill
- Genetic and Anatomical Basis of the Barrier Separating Wakefulness and Anesthetic-Induced Unresponsiveness
- The Locus, Exclusive to the Ambulacrarians, Encodes a Chromatin Insulator Binding Protein in the Sea Urchin Embryo
- Binding of NF-κB to Nucleosomes: Effect of Translational Positioning, Nucleosome Remodeling and Linker Histone H1
- Manipulating or Superseding Host Recombination Functions: A Dilemma That Shapes Phage Evolvability
- Dynamics of DNA Methylation in Recent Human and Great Ape Evolution
- Functional Dissection of Regulatory Models Using Gene Expression Data of Deletion Mutants
- PAQR-2 Regulates Fatty Acid Desaturation during Cold Adaptation in
- N-alpha-terminal Acetylation of Histone H4 Regulates Arginine Methylation and Ribosomal DNA Silencing
- A Genome-Wide Systematic Analysis Reveals Different and Predictive Proliferation Expression Signatures of Cancerous vs. Non-Cancerous Cells
- Maternal Depletion of Piwi, a Component of the RNAi System, Impacts Heterochromatin Formation in
- miR-1/133a Clusters Cooperatively Specify the Cardiomyogenic Lineage by Adjustment of Myocardin Levels during Embryonic Heart Development
- Hsp104 Suppresses Polyglutamine-Induced Degeneration Post Onset in a Drosophila MJD/SCA3 Model
- Genome-Wide Analysis of Genes and Their Association with Natural Variation in Drought Tolerance at Seedling Stage of L
- Deep Resequencing of GWAS Loci Identifies Rare Variants in , and That Are Associated with Ulcerative Colitis
- Cooperative Interaction between Phosphorylation Sites on PERIOD Maintains Circadian Period in
- VAPB/ALS8 MSP Ligands Regulate Striated Muscle Energy Metabolism Critical for Adult Survival in
- Analysis of Genes Reveals Redundant and Independent Functions in the Inner Ear
- Predicting the Risk of Rheumatoid Arthritis and Its Age of Onset through Modelling Genetic Risk Variants with Smoking
- Histone Chaperone NAP1 Mediates Sister Chromatid Resolution by Counteracting Protein Phosphatase 2A
- A Shift to Organismal Stress Resistance in Programmed Cell Death Mutants
- Fragile Site Instability in Causes Loss of Heterozygosity by Mitotic Crossovers and Break-Induced Replication
- Tracking of Chromosome and Replisome Dynamics in Reveals a Novel Chromosome Arrangement
- The Condition-Dependent Transcriptional Landscape of
- Ago1 Interacts with RNA Polymerase II and Binds to the Promoters of Actively Transcribed Genes in Human Cancer Cells
- Nebula/DSCR1 Upregulation Delays Neurodegeneration and Protects against APP-Induced Axonal Transport Defects by Restoring Calcineurin and GSK-3β Signaling
- System-Wide Analysis Reveals a Complex Network of Tumor-Fibroblast Interactions Involved in Tumorigenicity
- Meta-Analysis of Genome-Wide Association Studies Identifies Six New Loci for Serum Calcium Concentrations
- and Are Required for Cellularization and Differentiation during Female Gametogenesis in
- Growth factor independent-1 Maintains Notch1-Dependent Transcriptional Programming of Lymphoid Precursors
- Whole Genome Sequencing Identifies a Deletion in Protein Phosphatase 2A That Affects Its Stability and Localization in
- An Alteration in ELMOD3, an Arl2 GTPase-Activating Protein, Is Associated with Hearing Impairment in Humans
- Genomic Identification of Founding Haplotypes Reveals the History of the Selfing Species
- Plasticity Regulators Modulate Specific Root Traits in Discrete Nitrogen Environments
- The IDD14, IDD15, and IDD16 Cooperatively Regulate Lateral Organ Morphogenesis and Gravitropism by Promoting Auxin Biosynthesis and Transport
- Stochastic Loss of Silencing of the Imprinted Allele, in a Mouse Model and Humans with Prader-Willi Syndrome, Has Functional Consequences
- The Prefoldin Complex Regulates Chromatin Dynamics during Transcription Elongation
- PKA Controls Calcium Influx into Motor Neurons during a Rhythmic Behavior
- A Pre-mRNA-Splicing Factor Is Required for RNA-Directed DNA Methylation in
- Cell-Type Specific Features of Circular RNA Expression
- The Uve1 Endonuclease Is Regulated by the White Collar Complex to Protect from UV Damage
- An Atypical Kinase under Balancing Selection Confers Broad-Spectrum Disease Resistance in Arabidopsis
- Genome-Wide Mutation Avalanches Induced in Diploid Yeast Cells by a Base Analog or an APOBEC Deaminase
- Extensive Divergence of Transcription Factor Binding in Embryos with Highly Conserved Gene Expression
- Bi-modal Distribution of the Second Messenger c-di-GMP Controls Cell Fate and Asymmetry during the Cell Cycle
- Cell Interactions and Patterned Intercalations Shape and Link Epithelial Tubes in
- A Link between ORC-Origin Binding Mechanisms and Origin Activation Time Revealed in Budding Yeast
- The Genome and Development-Dependent Transcriptomes of : A Window into Fungal Evolution
- SKN-1/Nrf, A New Unfolded Protein Response Factor?
- The Highly Prolific Phenotype of Lacaune Sheep Is Associated with an Ectopic Expression of the Gene within the Ovary
- Fusion of Large-Scale Genomic Knowledge and Frequency Data Computationally Prioritizes Variants in Epilepsy
- IL-17 Attenuates Degradation of ARE-mRNAs by Changing the Cooperation between AU-Binding Proteins and microRNA16
- An Enhancer Element Harboring Variants Associated with Systemic Lupus Erythematosus Engages the Promoter to Influence A20 Expression
- Genome Analysis of a Transmissible Lineage of Reveals Pathoadaptive Mutations and Distinct Evolutionary Paths of Hypermutators
- Type I-E CRISPR-Cas Systems Discriminate Target from Non-Target DNA through Base Pairing-Independent PAM Recognition
- Divergent Transcriptional Regulatory Logic at the Intersection of Tissue Growth and Developmental Patterning
- MEIOB Targets Single-Strand DNA and Is Necessary for Meiotic Recombination
- Transmission of Hypervirulence Traits via Sexual Reproduction within and between Lineages of the Human Fungal Pathogen
- Integration of the Unfolded Protein and Oxidative Stress Responses through SKN-1/Nrf
- Guanine Holes Are Prominent Targets for Mutation in Cancer and Inherited Disease
- Regulation of the Boundaries of Accessible Chromatin
- Natural Genetic Transformation Generates a Population of Merodiploids in
- Ablating Adult Neurogenesis in the Rat Has No Effect on Spatial Processing: Evidence from a Novel Pharmacogenetic Model
- Genotype-Environment Interactions Reveal Causal Pathways That Mediate Genetic Effects on Phenotype
- The Molecular Mechanism of a -Regulatory Adaptation in Yeast
- Phenotypic and Genetic Consequences of Protein Damage
- Recent Acquisition of by Baka Pygmies
- Fatty Acid Taste Signals through the PLC Pathway in Sugar-Sensing Neurons
- A Critical Role for PDGFRα Signaling in Medial Nasal Process Development
- Chromatin-Specific Regulation of Mammalian rDNA Transcription by Clustered TTF-I Binding Sites
- Meiotic Recombination in Arabidopsis Is Catalysed by DMC1, with RAD51 Playing a Supporting Role
- dTULP, the Homolog of Tubby, Regulates Transient Receptor Potential Channel Localization in Cilia
- Widespread Dysregulation of Peptide Hormone Release in Mice Lacking Adaptor Protein AP-3
- , a Direct Transcriptional Target, Modulates T-Box Factor Activity in Orofacial Clefting
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- A Genome-Wide Systematic Analysis Reveals Different and Predictive Proliferation Expression Signatures of Cancerous vs. Non-Cancerous Cells
- Recent Acquisition of by Baka Pygmies
- The Condition-Dependent Transcriptional Landscape of
- Histone Chaperone NAP1 Mediates Sister Chromatid Resolution by Counteracting Protein Phosphatase 2A
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



