-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Elongator Complex Influences Telomeric Gene Silencing and DNA Damage Response by Its Role in Wobble Uridine tRNA Modification
Elongator complex is required for formation of the side chains at position 5 of modified nucleosides 5-carbamoylmethyluridine (ncm5U34), 5-methoxycarbonylmethyluridine (mcm5U34), and 5-methoxycarbonylmethyl-2-thiouridine (mcm5s2U34) at wobble position in tRNA. These modified nucleosides are important for efficient decoding during translation. In a recent publication, Elongator complex was implicated to participate in telomeric gene silencing and DNA damage response by interacting with proliferating cell nuclear antigen (PCNA). Here we show that elevated levels of tRNALyss2UUU, tRNAGlns2UUG, and tRNAGlus2UUC, which in a wild-type background contain the mcm5s2U nucleoside at position 34, suppress the defects in telomeric gene silencing and DNA damage response observed in the Elongator mutants. We also found that the reported differences in telomeric gene silencing and DNA damage response of various elp3 alleles correlated with the levels of modified nucleosides at U34. Defects in telomeric gene silencing and DNA damage response are also observed in strains with the tuc2Δ mutation, which abolish the formation of the 2-thio group of the mcm5s2U nucleoside in tRNALysmcm5s2UUU, tRNAGlnmcm5s2UUG, and tRNAGlumcm5s2UUC. These observations show that Elongator complex does not directly participate in telomeric gene silencing and DNA damage response, but rather that modified nucleosides at U34 are important for efficient expression of gene products involved in these processes. Consistent with this notion, we found that expression of Sir4, a silent information regulator required for assembly of silent chromatin at telomeres, was decreased in the elp3Δ mutants.
Published in the journal: . PLoS Genet 7(9): e32767. doi:10.1371/journal.pgen.1002258
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1002258Summary
Elongator complex is required for formation of the side chains at position 5 of modified nucleosides 5-carbamoylmethyluridine (ncm5U34), 5-methoxycarbonylmethyluridine (mcm5U34), and 5-methoxycarbonylmethyl-2-thiouridine (mcm5s2U34) at wobble position in tRNA. These modified nucleosides are important for efficient decoding during translation. In a recent publication, Elongator complex was implicated to participate in telomeric gene silencing and DNA damage response by interacting with proliferating cell nuclear antigen (PCNA). Here we show that elevated levels of tRNALyss2UUU, tRNAGlns2UUG, and tRNAGlus2UUC, which in a wild-type background contain the mcm5s2U nucleoside at position 34, suppress the defects in telomeric gene silencing and DNA damage response observed in the Elongator mutants. We also found that the reported differences in telomeric gene silencing and DNA damage response of various elp3 alleles correlated with the levels of modified nucleosides at U34. Defects in telomeric gene silencing and DNA damage response are also observed in strains with the tuc2Δ mutation, which abolish the formation of the 2-thio group of the mcm5s2U nucleoside in tRNALysmcm5s2UUU, tRNAGlnmcm5s2UUG, and tRNAGlumcm5s2UUC. These observations show that Elongator complex does not directly participate in telomeric gene silencing and DNA damage response, but rather that modified nucleosides at U34 are important for efficient expression of gene products involved in these processes. Consistent with this notion, we found that expression of Sir4, a silent information regulator required for assembly of silent chromatin at telomeres, was decreased in the elp3Δ mutants.
Introduction
Elongator complex, first identified in Saccharomyces cerevisiae, consists of a core complex, Elp1–Elp3 and a sub-complex, Elp4–Elp6 [1]–[3]. Orthologs of Elp1 to Elp4 has been identified in higher eukaryotes and a six-subunit Elongator complex has been purified from humans [4]–[5]. In yeast, Elongator mutants display pleiotropic phenotypes in multiple cellular processes including RNA polymerase II transcription and exocytosis [1]–[3], [6]–[9]. A crucial observation in understanding the role of the yeast Elongator complex was the discovery of its requirement for formation of 5-carbamoylmethyl (ncm5) and 5-methoxycarbonylmethyl (mcm5) side chains of wobble uridines [10]. In yeast Elongator mutants, the formation of ncm5 and mcm5 side chains were abolished in the 11 tRNA species that normally contain one of these two side chains [10]–[12]. Elongator complex in C. elegans and A. thaliana is also required for formation of ncm5 and mcm5 side chains at wobble uridines [13]–[14]. When the ncm5 and mcm5 side chains were eliminated, the corresponding tRNA species acted less efficiently in translation [12]. Although lack of modifications at position 5 affects the decoding properties of many tRNAs, it appears that the pleiotropic phenotypes of Elongator mutants are predominantly due to decreased translational decoding by hypomodified and [15]. Simultaneous over-expression of hypomodified and , which both have the mcm5s2U modification at wobble position U34 in wild type strains, compensated all phenotypes observed in Elongator mutants including those in RNA polymerase II transcription and exocytosis without restoring formation of ncm5 and mcm5 side chains in tRNA [15]. These observations not only argue against a direct involvement of Elongator complex in other cellular processes than tRNA modification, but they also suggest that the mcm5 side chain is important for efficient translation of mRNAs encoding gene products critical for the processes in which Elongator mutants generate phenotypes.
In eukaryotes, the whole genome is packed into a nucleoprotein complex known as chromatin through which the genetic material is processed to regulate cellular processes including transcription, cell division, DNA replication and DNA repair [16]–[17]. Chromatin properties can be altered by the posttranscriptional modifications of histones including acetylation, methylation, phosphorylation and ubiquitination [16]. The Elp3 protein of Elongator complex contains a tentative histone acetyltransferase (HAT) domain in the C-terminal region and the histone acetylation levels are decreased in elp3 mutants [7]. However, the reduced histone acetylation levels in the elp3 mutant were restored by increased expression of and , indicating that the involvement of Elongator complex in chromatin remodeling is indirect [15]. In addition to the HAT domain, Elp3 contains an N-terminal region with sequence similarity to the radical S-adenosylmethionine (SAM) enzymes [18]. A recent report showed that Elongator mutants have a partial loss of telomeric gene silencing and are sensitive to DNA damage agents [19]. It was also observed that strains with different point mutations in the ELP3 gene, resulting in amino acid substitutions in the radical SAM and HAT domains, displayed differences in telomeric gene silencing and DNA damage response [19]. The participation of Elongator complex in telomeric gene silencing and DNA damage response was linked to its interaction with proliferating cell nuclear antigen (PCNA), a protein involved in DNA replication and DNA repair [19].
In this report, we demonstrate that defects observed in DNA damage response and telomeric gene silencing of yeast Elongator mutants are caused by the absence of wobble uridine tRNA modifications. So far, all phenotypes observed in yeast Elongator mutants can be explained by their influence on tRNA modification. We conclude that the primary role of Elongator complex in yeast is in formation of ncm5 and mcm5 side chains at U34 of tRNAs.
Results
Elevated levels of hypomodified tRNALyss2UUU, tRNAGlns2UUG, and tRNAGlus2UUC suppress defects in telomeric silencing and DNA damage response induced by Elongator mutants
In a recent report, Elongator mutants were shown to have decreased telomeric gene silencing, which was investigated by using an ura3-1 strain with a wild-type copy of the URA3 gene inserted near the left telomere of chromosome VII [19]. Cells with increased expression of Ura3 show reduced growth on plates containing 5-fluoroorotic acid (5-FOA) since the nontoxic 5-FOA is converted to the toxic 5-flurouracil by the URA3 gene product. In such a strain, 30–50% of the cell population are resistant to 5-FOA [20]. The URA3 gene was expressed in a population of cells in both wild type and elp3Δ strains (Figure 1A). However, the elp3Δ strain grew poorly on the 5-FOA containing plates compared to the wild type (Figure 1A), suggesting that telomeric gene silencing was decreased in the elp3Δ strain. Since we earlier showed that the primary function of Elongator complex is in formation of wobble uridine tRNA modifications, we investigated whether increased levels of hypomodified , and could suppress the defects in telomeric gene silencing of an elp3Δ strain. Over-expression of these tRNA species significantly improved the growth of the elp3Δ strain on 5-FOA plates (Figure 1B). The telomeric gene silencing defect of Elongator mutants was also investigated by using a color assay with the ADE2 marker inserted near the telomeric region. The elp3 mutant forms white color colonies due to loss of silencing of ADE2, which could be rescued by increased expression of , and (data not shown). This observation confirmed that Elongator mutants have a defect in telomeric gene silencing, which is caused by a translational dysfunction. The decreased telomeric silencing observed in other Elongator deletion mutants (elp1Δ, elp2Δ, elp4Δ, elp5Δ and elp6Δ) was also suppressed by elevated levels of , and (Figure 1C). Elongator mutants are also sensitive to DNA damaging agents, especially hydroxyurea (HU) [19] (Figure 2). Similar to the defect in telomeric gene silencing, the HU sensitivity of Elongator mutants was suppressed by elevated levels of , and (Figure 2). Collectively, these observations indicate that the reduced gene silencing in telomeric regions and the defect in DNA damage response of Elongator mutants is caused by inefficient translation due to lack of wobble uridine tRNA modifications.
Fig. 1. Increased levels of tRNALyss2UUU, tRNAGlns2UUG, and tRNAGlus2UUC suppress the telomeric silencing defect of Elongator mutants. 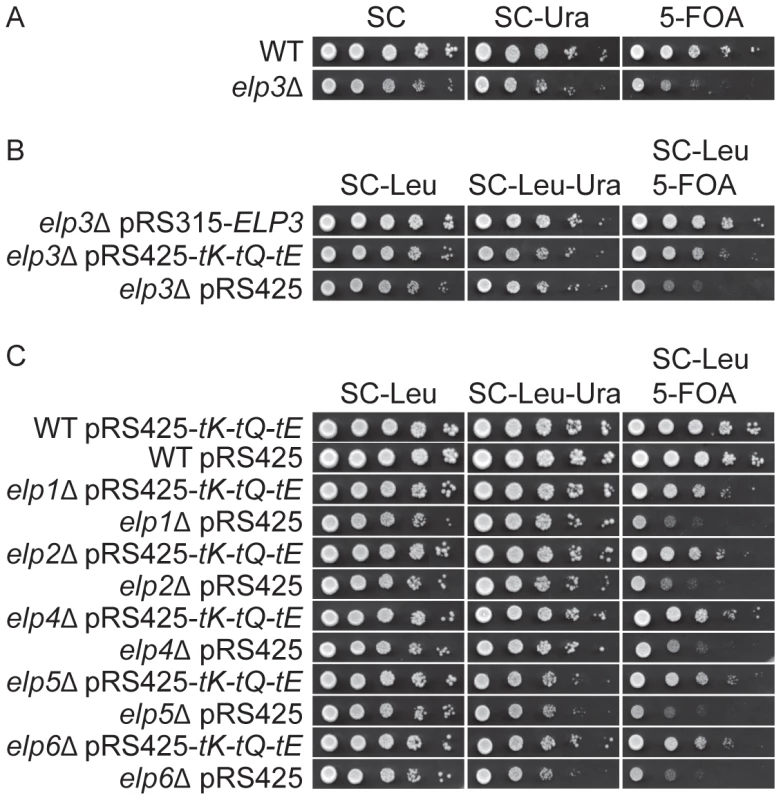
(A) The wild type (UMY2584) and elp3Δ (UMY3790) strains were 10-fold diluted, spotted on SC, SC-Ura and SC+5-FOA plates, and incubated at 30°C for 2 days. (B) The elp3Δ strain (UMY3790) with plasmids, pRS315-ELP3, pRS425-tK-tQ-tE or pRS425, were 10-fold diluted, spotted on SC-Leu, SC-Leu-Ura and SC-Leu+5-FOA plates, and incubated at 30°C for 2 days. (C) The wild type (UMY2584), elp1Δ (UMY3788), elp2Δ (UMY3789), elp4Δ (UMY3791), elp5Δ (UMY3792) and elp6Δ (UMY3793) with plasmids pRS425-tK-tQ-tE or pRS425 were treated as described in (B). Abbreviations for the tRNA genes encoding , and are tK, tQ and tE, respectively. Fig. 2. Elevated levels of tRNALyss2UUU, tRNAGlns2UUG, and tRNAGlus2UUC suppress the HU sensitivity induced by Elongator mutants. 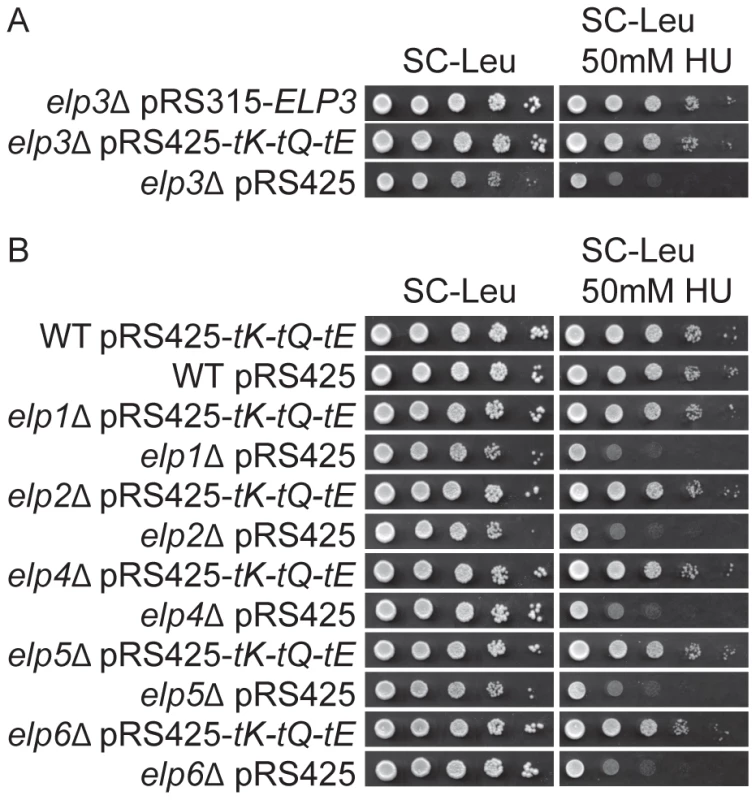
(A) The elp3Δ strain (UMY2843) carrying plasmids pRS315-ELP3, pRS425-tK-tQ-tE or pRS425 were 10-fold diluted, spotted on SC-Leu and SC-Leu+50 mM HU plates, and incubated at 30°C for 2 days. (B) The wild type (W303-1A), elp1Δ (UMY3783), elp2Δ (UMY3784), elp4Δ (UMY3785), elp5Δ (UMY3786) and elp6Δ (UMY3787) strains transformed with plasmids pRS425-tK-tQ-tE or pRS425 were assayed as described in (A). Abbreviations for the tRNA genes encoding tRNALysUUU, tRNAGlnUUG, and tRNAGluUUC are tK, tQ and tE, respectively. To investigate which of the , and species most efficiently suppressed the defects in telomeric silencing and DNA damage response of the elp3Δ strain, we introduced plasmids encoding these tRNAs independently or in various combinations into the mutant. Increased expression of alone could efficiently suppress the telomeric silencing defect and the HU-sensitivity of an elp3Δ strain (Figure S1). Simultaneous over-expression of , and gave a minor improvement in suppression of the telomeric gene silencing defect compared to over-expression of alone (Figure S1A). In the HU sensitivity assay, increased expression of together with improved the suppression compared to that of and was as good as elevated levels of , and (Figure S1B). These results indicate that certain open reading frames, encoding gene products critical for telomeric gene silencing and DNA damage response, might be enriched in AAA, CAA and GAA codons. Of these three codons, translation of AAA codons by seems to be most affected by lack of the mcm5 side chain.
Synergistic growth reduction and HU sensitivity of elp3Δ asf1Δ or elp3Δ rtt109Δ strains are compensated by increased expression of tRNALyss2UUU, tRNAGlns2UUG, and tRNAGlus2UUC
Asf1 functions as a histone chaperone to direct the histone acetyltransferase Rtt109 in substrate selection and stimulate its acetyltransferase activity [21]–[23]. The combination of elp3Δ asf1Δ or elp3Δ rtt109Δ mutations causes synergistic phenotypes to the strains, such as a more pronounced reduction in growth and increased sensitivity to HU (Figure 3 and Figure S2), which was suggested to be caused by loss of histone acetylation in the elp3Δ strain [19]. GCN5 encodes a histone acetyltransferase that acetylate H2B and H3 [24]–[25]. Previously it was shown that the elp3Δ gcn5Δ mutations generate a synergistic growth reduction [26]. However, increased levels of hypomodified tRNAs suppressed the synergistic growth reduction caused by the elp3Δ gcn5Δ mutations, and restore the histone acetylation levels in the elp3Δ mutant but not in the gcn5Δ strain [15]. When we over-expressed , and from a high copy vector in the elp3Δ asf1Δ or elp3Δ rtt109Δ double mutants, the growth reduction and HU sensitivity of the double mutants were similar to the defects observed in an asf1Δ or rtt109Δ strain, respectively (Figure 3 and Figure S2). These observations support the earlier conclusion that Elp3 is not directly required for histone acetylation [15].
Fig. 3. Increased levels of tRNALyss2UUU, tRNAGlns2UUG, and tRNAGlus2UUC bypass the phenotypes of asf1Δ elp3Δ double mutants. 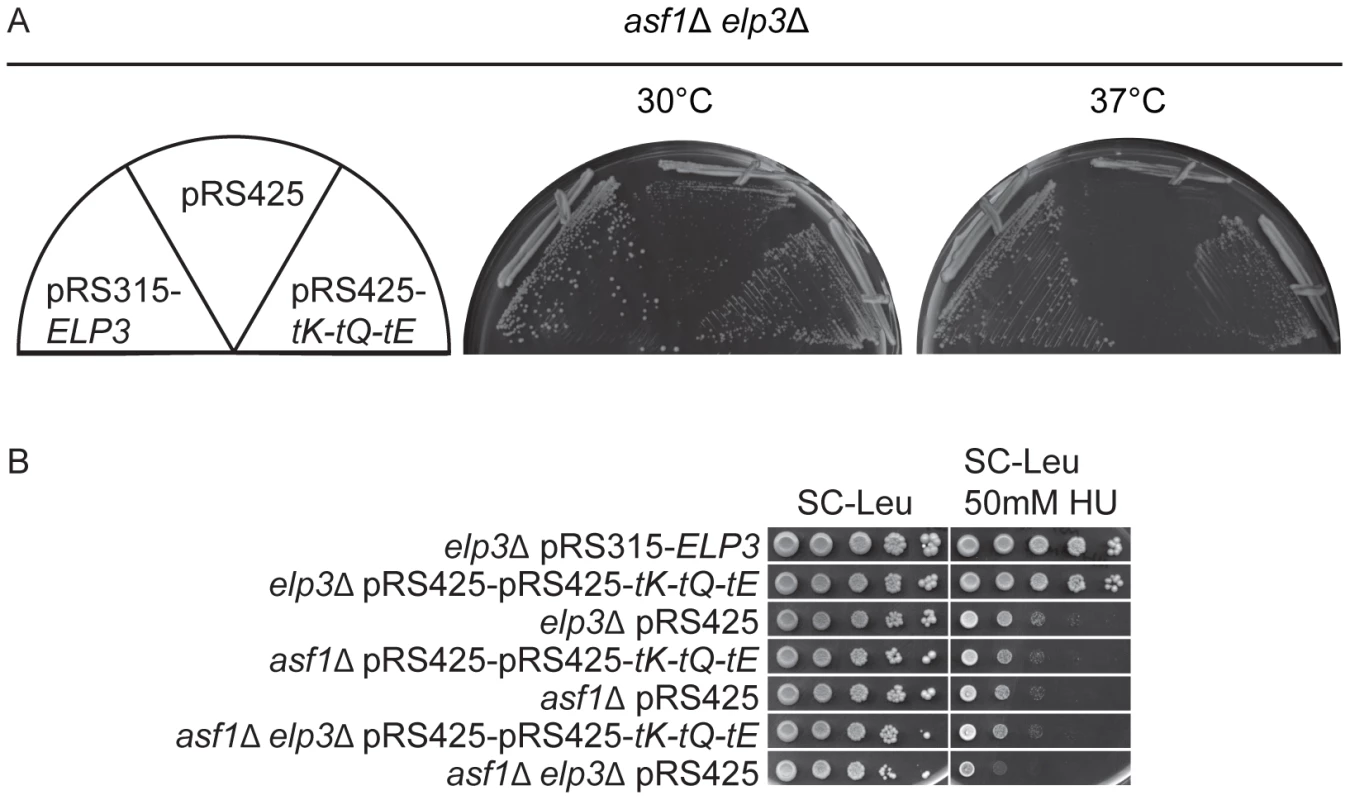
(A) The asf1Δ elp3Δ strain (UMY3805) was transformed with pRS315-ELP3, pRS425-tK-tQ-tE or pRS425. Transformants were streaked on SC-Leu plates and incubated at 30°C or 37°C for 2 days. (B) Ten fold dilutions of elp3Δ (UMY2843), asf1Δ (UMY3800) and asf1Δ elp3Δ (UMY3805) strains carrying either pRS425-tK-tQ-tE or pRS425 were spotted on SC-Leu and SC-Leu+50 mM HU plates, and incubated 4 days at 30°C. The elp3Δ (UMY2843) transformed with pRS315-ELP3 was used as control. Abbreviations for the tRNA genes encoding tRNALysUUU, tRNAGlnUUG, and tRNAGluUUC are tK, tQ and tE, respectively. Wobble uridine tRNA modification levels correlate to phenotypic variations generated by different mutant alleles of the ELP3 gene
Elp3 contains two conserved domains, a radical S-adenosylmethionine (SAM) domain in the N-terminal region and a putative histone acetyltransferase (HAT) domain located in C-terminal end (Figure 4A). Most strains expressing Elp3 proteins with amino acid substitutions in these two domains showed a reduction in telomeric gene silencing and HU resistance [19] (Figure 4). The elp3-C103A and elp3-G168R mutations did not influence telomeric gene silencing and HU sensitivity (Figure 4B and 4C) [19]. The elp3-Y540A and elp3-Y541A mutations partially reduced telomeric gene silencing and increased HU sensitivity but not as much as elp3Δ (Figure 4B and 4C) [19]. The remaining strains were similar as an elp3Δ null strain in telomeric gene silencing and HU sensitivity (Figure 4B and 4C) [19]. Moreover, all strains carrying individual mutations listed in Figure 4A except for elp3-C103A were resistant to Kluyveromyces lactis killer toxin (data not shown), indicating that these mutants have a defect in formation of wobble uridines tRNA modification [11].
Fig. 4. Strains carrying different ELP3 mutant alleles show decreased telomeric gene silencing and increased HU sensitivity. 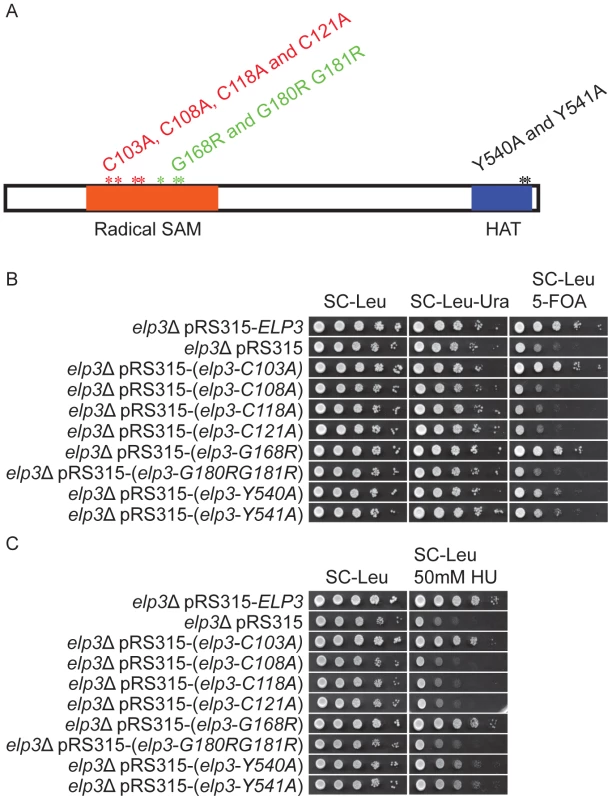
(A) Schematic drawing of the protein structure of Elp3. Orange box represents the radical-S-adenosyl methionine (Radical-SAM) domain and blue box indicates the location of the histone acetyltransferase (HAT) domain. Cysteine residues at position 103, 108, 118 or 121 were substituted with alanines. Glycine residues at position 168 or 180 together with 181 were replaced by arginines. Two tyrosine residues, positions 540 or 541, in the HAT domain were substituted with alanines. (B) The wild type and the different mutant alleles of the ELP3 gene, located in LEU2 containing vector pRS315, were transformed into the elp3Δ strain (UMY3790). The elp3Δ strain (UMY3790) carrying a pRS315 without insertion serves as control. The transformed yeast cells were spotted on SC-Leu, SC-Leu-Ura and SC-Leu+5-FOA plates, and incubated at 30°C for 2 days. (C) The elp3Δ strain (UMY2843) transformed with the same set of plasmids as in (B) were spotted on SC-Leu, SC-Leu+50 mM hydroxyurea plates, and incubated at 30°C for 2 days. To examine the status of wobble uridine tRNA modification in these elp3 mutants, total tRNAs from these mutants were isolated and analyzed by HPLC. The elp3-C103A and elp3-G168R mutants, which did not have defects in telomeric silencing and DNA damage response, had 96% and 51% mcm5s2U left, respectively (Figure 5, Table 1). Mutations in the HAT domain did not completely eliminate the formation of wobble uridine modifications, both elp3-Y540A and elp3-Y541A have 2 or 6% mcm5s2U left compared to the wild type (Figure 5, Table 1). In the rest of mutants, the mcm5 side chain formation was entirely abolished (Figure 5, Table 1). We conclude that phenotypes exhibited by elp3 mutants correlate with the levels of wobble uridine tRNA modification.
Fig. 5. HPLC analysis of total tRNAs isolated from mutants with different alleles of ELP3. 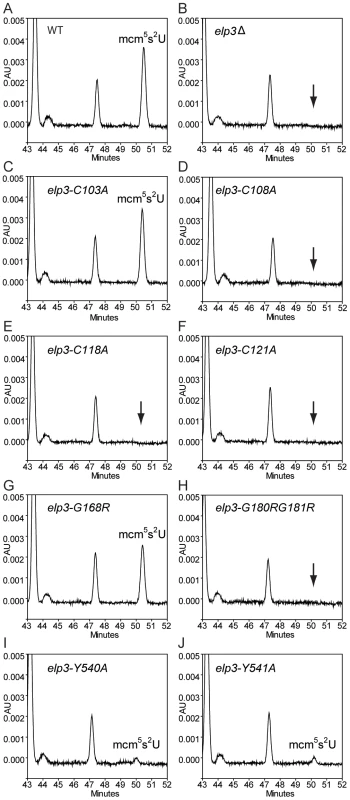
HPLC chromatograms of total tRNA isolated from SUP4 (UMY2894), elp3Δ SUP4 (UMY2915), elp3-C103A SUP4 (UMY3314), elp3-C108A SUP4 (UMY3315), elp3-C118A SUP4 (UMY3316), elp3-C121A SUP4 (UMY3317), elp3-G168R SUP4 (UMY3794), elp3-G180R G181R SUP4 (UMY3795), elp3-Y540A SUP4 (UMY3060) and elp3-Y541A SUP4 (UMY3061). Chromatograms were monitored at 314 nm. The parts of chromatograms between retention times 43 and 52 min are displayed. The arrows in B, D, E, F and H indicate the expected retention time of mcm5s2U. Tab. 1. Relative amounts of mcm5s2U analyzed by HPLC in various elp3 mutants. 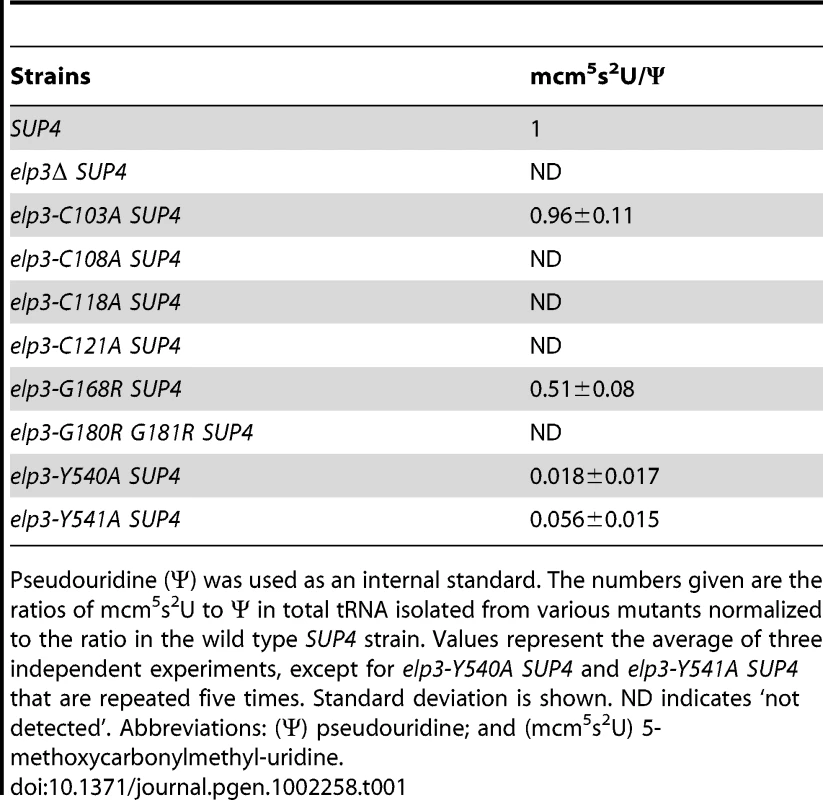
Pseudouridine (Ψ) was used as an internal standard. The numbers given are the ratios of mcm5s2U to Ψ in total tRNA isolated from various mutants normalized to the ratio in the wild type SUP4 strain. Values represent the average of three independent experiments, except for elp3-Y540A SUP4 and elp3-Y541A SUP4 that are repeated five times. Standard deviation is shown. ND indicates ‘not detected’. Abbreviations: (Ψ) pseudouridine; and (mcm5s2U) 5-methoxycarbonylmethyl-uridine. Different mcm5 modification levels correlate with ochre stop codon read through by a suppressor tRNA
Our observations suggest that phenotypes of Elongator mutants are caused by an inefficient translation due to lack of tRNA modification. If our model is correct, reduction in modification levels in elp3 mutants should result in decreased translation efficiency. To analyze whether the modification levels of different elp3 mutants listed in Table 1 influence translation efficiency, we used a dual-luciferase reporter system (Figure 6A) [27] to measure the ochre stop codon read through by a suppressor tRNA encoded by the SUP4 allele. The SUP4 allele encodes a suppressor with a G34 to U34 substitution in its anticodon. The U34 of this suppressor tRNA is modified at position 5 with a mcm side chain [10]. Presence of this modification improves the ability of the suppressor tRNA to read UAA ochre stop codons [10], [12].
Fig. 6. U34 modification levels influence ochre stop codon read through by a suppressor tRNA. 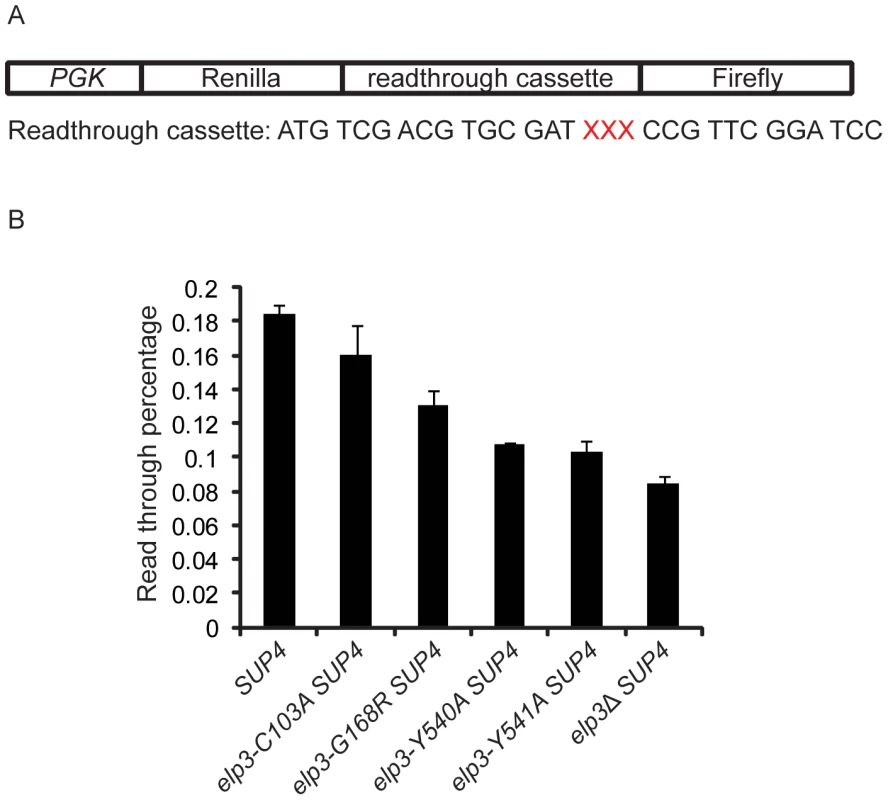
(A) Schematic drawing of the dual luciferase reporter system constructed by Keeling et al [27]. The sequence of the read through cassette between Renilla and firefly luciferase genes is shown. The XXX in red stands for either UAA in the assay plasmid or CAA in the control plasmid. (B) Read through levels of the UAA stop codon in SUP4 (UMY2894), elp3-C103A SUP4 (UMY3314), elp3-G168R SUP4 (UMY3794), elp3-Y541A SUP4 (UMY3060), elp3-Y541A SUP4 (UMY3061) and elp3Δ SUP4 (UMY2915). Values are ratios of Firefly to Renilla luciferase activities and based on three independent experiments. The error bars represent the standard deviation. Values were normalized to the wild type SUP4 (UMY2894), which was arbitrarily set to 1. In the dual-luciferase construct, the Renilla and firefly luciferase genes are separated by an UAA ochre stop codon [27]. Read through of the ochre stop codon was determined by calculating the ratio of firefly luciferase activity to Renilla luciferase activity. This ratio was compared to the value obtained from a control construct in which a CAA codon replaces the UAA stop codon (Figure 6A). Due to lack of mcm5 side chain in the SUP4 tRNA, the stop codon read through in the elp3Δ strain is reduced to 46% of wild type (t-test, p = 0.001), supporting that the mcm5 side chain is important for efficient decoding (Figure 6B). In the elp3-G168R mutant, in which the mcm5 side chain is reduced to 51%, the level of read through was significantly decreased compared to that in wild type (t-test, p = 0.008), but is higher than that observed in strains carrying the elp3-Y540A, elp3-Y541A or elp3Δ alleles (t-test, p = 0.04 and 0.03 respectively) (Figure 6B). In the elp3-Y540A and elp3-Y541A mutants, a small fraction of total tRNA was modified (2–6%) (Figure 5, Table 1), which contributed to an improvement of stop codon read through by the SUP4 suppressor tRNA compared to the elp3Δ strain (t-test, p = 0.004 and 0.006 respectively) (Figure 6B). In mutant alleles eliminating formation of the mcm5 side chain, no differences were observed in stop codon read through by the SUP4-encoded suppressor tRNA compared to the elp3 null mutant (Figure S3). These data show that reduced mcm5 modification levels correlate with decreased translational efficiency.
Defects in telomeric silencing and DNA damage response are also observed in strains unable to form the s2 group of mcm5s2U
Our findings that the defects in telomeric silencing and DNA damage response in Elongator mutants were bypassed by elevated levels of , and indicated that the mcm5 side chain in tRNA is critical for the expression of gene products in these two processes (Figure 1 and Figure 2). In addition to the mcm side chain at position 5 of U34, these three tRNAs also contain a 2-thio group forming mcm5s2U. Since the s2 group is also important for decoding [12], [15], [28], we hypothesized that strains deficient in formation of the 2-thio group might also display defects in telomeric silencing and DNA damage response as Elongator mutants. Tuc2 in yeast is required for the formation of the 2-thio group of the mcm5s2U nucleoside [15]. In a tuc2Δ strain, the formation of s2 group is abolished. As expected, telomeric gene silencing was decreased in the tuc2Δ strain (Figure 7A). This strain was also sensitive to 50 mM HU nearly to the same extent as observed in Elongator mutants (Figure 2 and Figure 7B). The defects in telomeric gene silencing and DNA damage response were completely suppressed by increased levels of , and (Figure 7). The phenotypes of Elongator and tuc2Δ mutants demonstrates that a translational dysfunction due to lack of U34 modifications in , and causes the defects in telomeric gene silencing and DNA damage response.
Fig. 7. The tuc2Δ strain is deficient in telomeric gene silencing and show increased HU sensitivity. 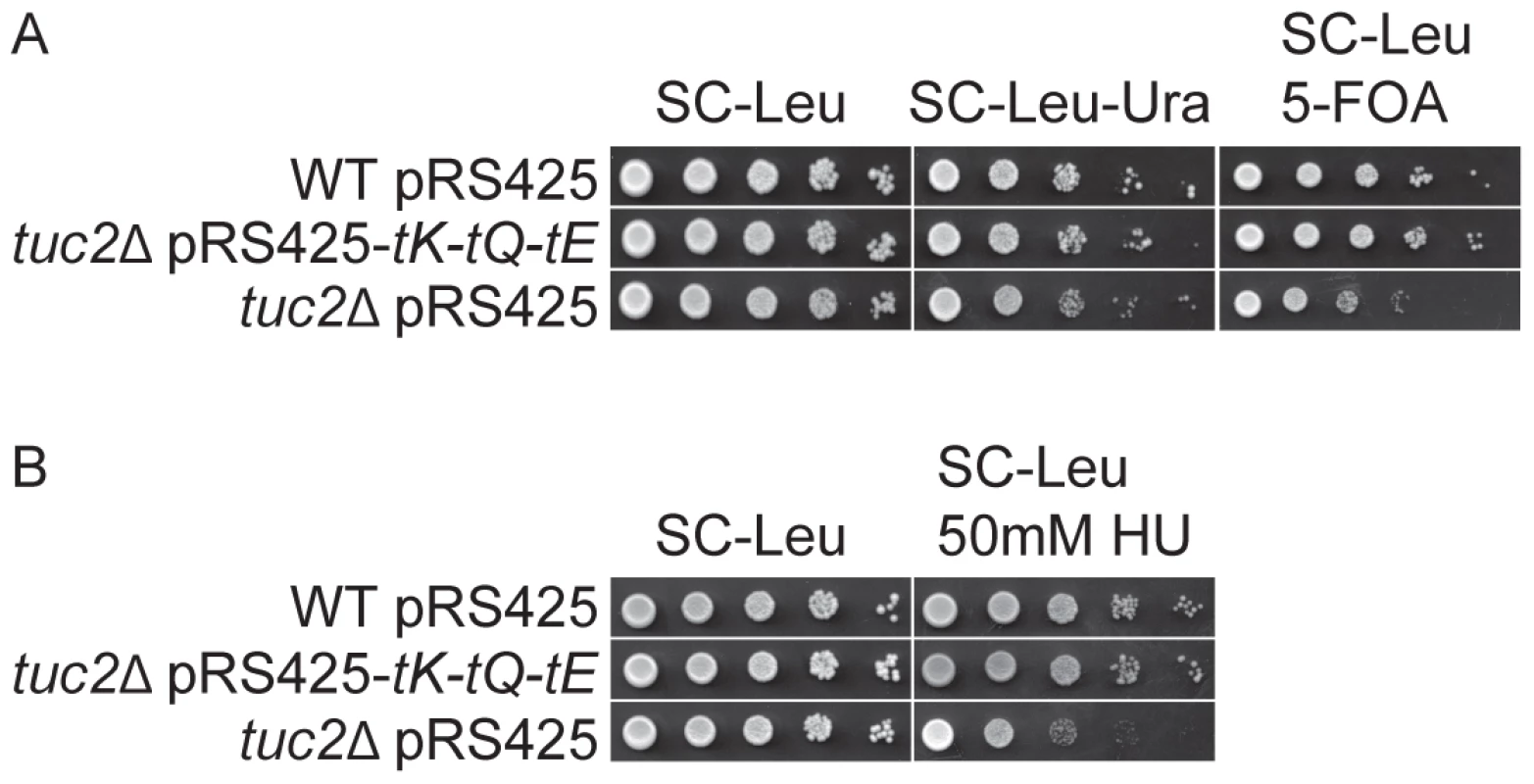
(A) The wild type strain (UMY2584) harboring plasmid pRS425 and the tuc2Δ mutant (UMY3804) harboring plasmids pRS425-tK-tQ-tE or pRS425 were assayed as described in Figure 1. (B) The wild type strain (UMY2067) harboring plasmid pRS425 and the tuc2Δ mutant (UMY3442) harboring plasmids pRS425-tK-tQ-tE or pRS425 were assayed as described in Figure 2. Abbreviations for the tRNA genes encoding , and are tK, tQ and tE, respectively. Sir4 expression is decreased in an elp3Δ strain
Among the three tRNA species responsible for the suppression of elp3Δ induced phenotypes, increased expression of gives the best suppression of the defect in telomeric gene silencing (Figure S1). Since decodes AAA codons, elimination of the mcm5 side chain from in the elp3Δ strain could influence the decoding efficiency of AAA codons. Therefore, we searched for open reading frames highly enriched in AAA codons (unpublished results). This analysis lead to the identification of SIR4, encoding a silent information regulator in yeast. Based on this observation, we hypothesized that the telomeric gene silencing defect of the elp3Δ mutant might be caused by decreased Sir4 expression. Accordingly, the Sir4 protein levels in the elp3Δ mutant were decreased to 34% of wild type (Figure 8A). The decreased Sir4 levels were restored to 80% of wild-type by increased expression of , and , and to 74% of wild-type by elevated levels of alone (Figure 8A and data not shown). We also observed that SIR4 mRNA levels were reduced to 76% of wild-type (Figure 8B), which cannot account for the decreased Sir4 protein levels. In addition, introducing the SIR4 gene on a high copy vector significantly suppressed the telomeric gene silencing defect of the elp3Δ strain, confirming that this defect seems to be caused by decreased Sir4 expression (Figure 8C). However, we do not exclude the possibility that there might be other open reading frames enriched in AAA codons whose translation is also affected and which might weaken silencing, directly or indirectly.
Fig. 8. Sir4 protein levels are decreased in the elp3Δ mutant. 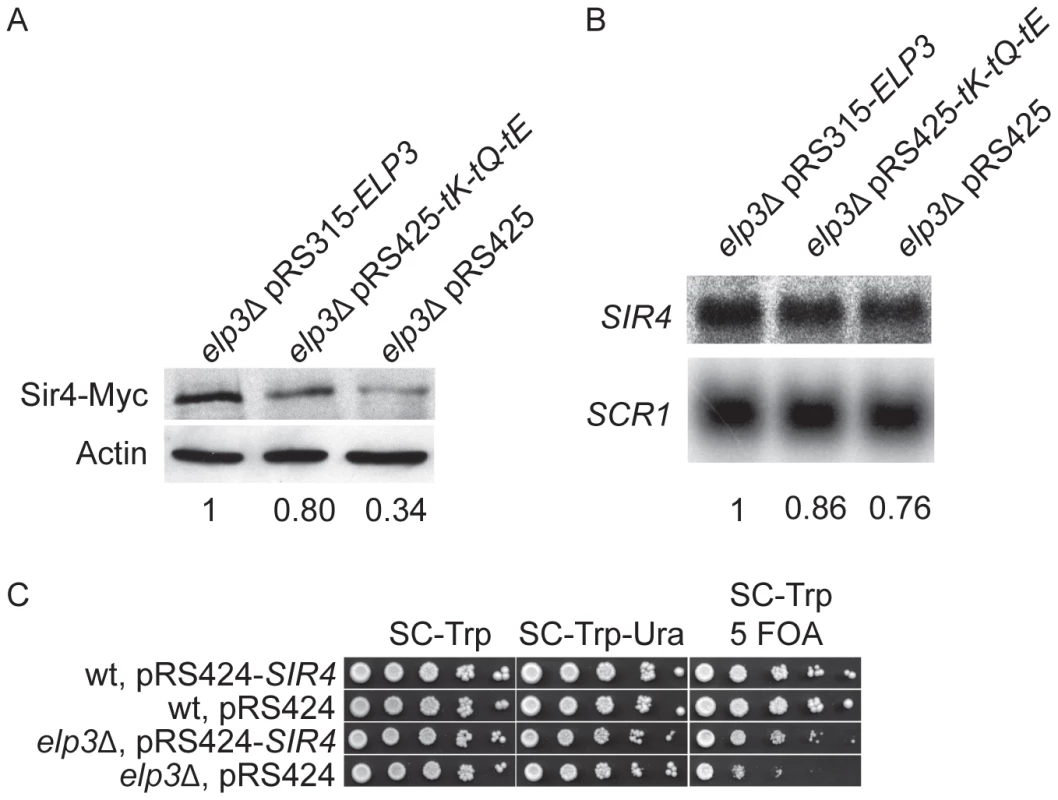
(A). Western blot analysis of Sir4-Myc protein levels in the elp3Δ strain transformed with plasmids pRS315-ELP3, pRS425-tK-tQ-tE or pRS425. The ratios of Sir4-Myc to Actin signals were calculated. The values are shown relative to elp3Δ pRS315-ELP3 strain, which was arbitrarily set to 1, and are the average of two independent experiments. (B). Northern blot analysis of SIR4 mRNA. The elp3Δ strain was transformed with plasmids pRS315-ELP3, pRS425-tK-tQ-tE or pRS425. Signals of SIR4 mRNAs were normalized to the non-coding SCR1 transcript. The values are shown relative to elp3Δ pRS315-ELP3 strain, which was arbitrarily set to 1, and are the average of two independent experiments. (C). The wild type strain (UMY2584) transformed with plasmids pRS424-SIR4 or pRS424, and the elp3Δ mutant (UMY3790) with plasmids pRS424-SIR4 or pRS424 were assayed as described in Figure 1. For A, B and C, representative figures are shown. Abbreviations for the tRNA genes encoding , and are tK, tQ and tE, respectively. Discussion
Elongator complex was initially identified by its apparent association with the elongating form of RNA polymerase II, implicating a role in PolII transcription [1]. However, its requirement in transcription was controversial based on its cytoplasmic localization and failure to detect this complex on actively transcribed genes [8], [29]–[30]. We discovered that Elongator complex was required for formation of mcm5 and ncm5 side chains at wobble uridines of tRNA [10]. The participation of Elongator complex in PolII transcription and exocytosis was indirect as elevated expression of hypomodified and could suppress previously reported phenotypes of Elongator mutants without restoring tRNA modification [15]. Recently, it was reported that Elongator complex modulates telomeric gene silencing and DNA damage response by its interaction with PCNA and its requirement for histone acetylation [19]. Since the histone acetylation defect of the elp3Δ mutant could be completely suppressed by increased expression of and [15], we assumed that Elongator complex indirectly participated in telomeric gene silencing and DNA damage response.
In this report, we show that the defects in telomeric gene silencing and DNA damage response in Elongator mutants were also suppressed by increased expression of hypomodified , and (Figure 1, Figure 2, and Figure S1). Thus, all phenotypes exhibited by Elongator mutants except the tRNA modification defect are overcome by elevated tRNA levels, indicating that the major function of this complex, at least in yeast, is in the formation of mcm5 and ncm5 side chains of wobble uridines. When , and were over-expressed in Elongator mutants, the HU sensitivity phenotype, but not the defect in telomeric gene silencing, was fully suppressed (Figure 1 and Figure 2). Since Elongator mutants affect the mcm5 and ncm5 side chain formation in 11 tRNA species, it is possible that poor translation of codons decoded by any of the other 8 hypo-modified tRNA species contributes to the defect in telomeric gene silencing, but not the HU sensitivity. In addition to the mcm side chain at position 5, U34 of , and are also thiolated at position 2. If our model is correct that the phenotypes observed in Elongator mutants are a consequence of inefficient translation, strains lacking the 2-thio group in , and will have similar phenotypes as Elongator mutants. We observed that the failure to form the 2-thio group in the tuc2Δ mutant resulted in defects in telomeric gene silencing and DNA damage response (Figure 7). These defects of the tuc2Δ mutant were completely suppressed by increased expression of , and . In addition, lack of the methyl ester in mcm5 side chain at wobble uridines in a trm9Δ strain has been linked to the defect of DNA damage response [31]. Thus, both mcm5 and s2 side chains of mcm5s2U containing tRNAs are important for efficient expression of gene products required for telomeric gene silencing and DNA damage response. These observations strongly suggest that Elongator complex influence these two processes by promoting efficient translation. Since increased expression of gives the best suppression of the telomeric gene silencing defect in Elongator mutants, we assumed genes encoding products important for this process are enriched in AAA codons. One such gene is SIR4. We demonstrate that Elongator mutants influence telomeric gene silencing by impairing efficient expression of SIR4. Even though we observed a slight reduction in SIR4 mRNA levels in the elp3Δ mutant, it cannot fully explain the decrease in Sir4 protein levels, and it is unclear if this reduction is caused by reduced transcription or increased decay of the poorly translated mRNA.
Recently, it was discovered that Elongator complex in C. elegans and A. thaliana is also required for formation of mcm5 and ncm5 side chains at wobble uridines of tRNA [13]–[14], indicating that this function of Elongator complex might be conserved in eukaryotes. In multicellular organisms, Elongator complex has also been linked to multiple processes including transcription, cytoplasmic kinase signaling and development [32]–[34]. Two recent articles suggested that Elongator complex was also required for α-tubulin acetylation and played a role in neurological processes in both mice and C. elegans [35]–[36]. In early developmental stages, C. elegans Elongator mutants have a decreased α-tubulin acetylation [36]. However, in adult Elongator mutant worms, normal levels of α-tubulin acetylation were observed, suggesting that Elongator complex is not absolutely required for acetylation of α-tubulin [13], [36]. Elongator mutants in C. elegans were also resistant to the acetylcholinesterase inhibitor aldicarb, indicating a reduced efficiency of synaptic exocytosis [13], [36]. However, a mutant allele of mec-12, which is completely missing α-tubulin acetylation, was not resistant to aldicarb, suggesting that the defect in synaptic exocytosis of Elongator mutants was not caused by reduced levels of α-tubulin acetylation [13]. Furthermore, mec-17 was discovered to be the α-tubulin acetylase in in Tetrahymena cells, C. elegans, zebrafish and mammalian cells, suggesting that Elongator might indirectly influence α-tubulin acetylation by modulating the expression of α-tubulin acetylase [37]. Based on these observations, it is tempting to speculate that the primary function of Elongator complex in multicellular organism is, as in yeast, in formation of wobble uridine tRNA modifications.
The Elp3 subunit in yeast has an N-terminal radical S-adenosylmethionine (SAM) domain and a C-terminal histone acetyltransferase (HAT) domain. In Methanocaldococcus jannaschii, the radical SAM domain of mjElp3 contains an iron sulfur cluster region and a region that binds SAM [38]. Cysteine residues at positions 96, 101 and 104 are critical for the FeS cluster formation in M. jannaschii [38]. When these corresponding cysteines at position 108, 118 and 121 in the yeast Elp3 were substituted with alanines, it eliminated the activity of yeast Elongator in formation of modified nucleosides at U34. In vitro, SAM can bind to M. jannaschii Elp3, but the binding of SAM to Elp3 from S. cerevisiae has not been detected [38]–[39]. However, when the conserved SAM binding sites (G180R G181R) in the radical SAM domain were mutated in yeast ELP3, a defect in formation of modified nucleosides was observed (Figure 5, Table 1). This observation shows that the FeS cluster and the SAM binding regions of the radical SAM domain of Elp3 are critical for the tRNA modification reaction. Substitution of glycine at position 168 to arginine, another conserved site located in the SAM binding region, reduced the wobble uridine tRNA modification to 51% of wild type (Figure 5, Table 1). In telomeric gene silencing and HU sensitivity assays, the elp3-G168R mutant displays the same phenotypes as a wild type strain suggesting that a 49% reduction in the levels of modified nucleosides do not cause phenotypes in telomeric gene silencing and DNA damage response. Two mutations in the HAT domain (Y540A and Y541A) of Elp3 did not entirely eliminate the formation of modified nucleosides at U34; 2 and 6% of mcm5s2U was detected in each mutant (Table 1). The residual level of modified nucleosides significantly improves the decoding capacity of the SUP4 encoded suppressor tRNA compared to the unmodified tRNA in the elp3 null mutant (Figure 6). This observation explains why the elp3-Y540A and elp3-Y541A mutants had increased telomeric silencing and reduced HU sensitivity compared to the elp3Δ strain (Figure 4).
Among the elp3 mutants described in Table 1, the elp3-G168R mutant, having 51% of modified nucleoside left (Figure 5 and Table 1), has the same phenotype as a wild type strain with respect to phenotypes in telomeric gene silencing and DNA damage response (Figure 4). However, this strain is resistant to killer toxin (data not shown), a phenotype tightly connected to wobble uridine tRNA modification [11]. The γ subunit of killer toxin is a tRNA endonuclease which cleaves tRNA at the anticodon region [11]. The mcm5 side chain at U34 of tRNA is important for the substrate recognition by γ toxin. In the elp3-G168R mutant, a fraction of the U34 tRNAs are missing the mcm5 side chain and the mutant is resistant to γ toxin (data not shown). However, the modified tRNAs in the elp3-G168R support the efficient expression of gene products required for telomeric gene silencing and DNA damage response. Thus, strains with tRNAs partially modified at U34 show weaker or no phenotypes compared to Elongator deficient strains.
In summary, the major function of Elongator complex in yeast is in formation of wobble uridine tRNA modifications and this function is probably conserved in eukaryotes. We suggest that when new phenotypes of Elongator mutants are discovered in yeast, an important first step is to investigate whether the phenotypes can be suppressed by over-expressing , and .
Materials and Methods
Yeast strains, media, and genetic procedures
All yeast strains used in this study are listed in Table S1. Yeast transformation, media, and genetic procedures have been described previously [40]. To generate elp null mutants in different strain backgrounds, chromosomal DNA from KanMX deleted elp mutants UMY2911 (elp1::KanMX4), UMY2913 (elp2::KanMX4), UMY2915 (elp3::KanMX4), UMY2917 (elp4::KanMX4), UMY2919 (elp5::KanMX6) and UMY2921 (elp6::KanMX4) served as templates. Primers were designed to amplify DNA fragments containing the KanMX cassette and 300–500 nt flanking sequences of each ELP gene. PCR products were transformed into either W303-1A or UMY2584, and the transformants were selected by using YEPD plates containing 200 µg/ml G418. The deletion mutants were verified by PCR. To introduce asf1::KanMX4 and rtt109::KanMX4 into W303 background, chromosomal DNAs from the corresponding mutants in the deletion collection (Open biosystems) were used as templates. Primers were designed to amplify the KanMX4 cassette and 500 nt flanking sequences. PCR products were transformed into diploid strain UMY3104 and transformants were selected on G418 containing plates. The asf1::KanMX4 and rtt109::KanMX4 strains were obtained by tetrad dissection after sporulation. To construct asf1::KanMX4 elp3::KanMX4 and rtt109::KanMX4 elp3::KanMX4, the elp3::KanMX4 strain was crossed with asf1::KanMX4 or rtt109::KanMX4 to generate the diploid and double mutants were obtained by tetrad dissection. To generate elp3::KanMX4 SIR4-13Myc-KanMX6 strain, the elp3::KanMX4 strain was crossed with SIR4-13Myc-KanMX6 strain. The diploid was sporulated and the elp3::KanMX4 SIR4-13Myc-KanMX6 strain was obtained by tetrad dissection.
A two-step gene replacement procedure was used to obtain strains with different mutant alleles of ELP3. Plasmids pABY1672 (elp3-C103A), pABY1673 (elp3-C108A), pABY1676 (elp3-C118A), pABY1677 (elp3-C121A), pABY1984 (elp3-G168R) and pABY1985 (elp3-G180R G181R) were digested with EcoRI and the linearized fragments were transformed into the UMY2894. Transformants were selected on SC-Ura plates and streaked on YEPD plates. Eight independent colonies on YEPD plates were picked and streaked on 5-FOA containing plates. The strains with elp3 mutant alleles except for elp3-C103A were identified by their resistance to killer toxin and confirmed by sequencing. In order to identify the elp3-C103A mutant, DNA isolated from several candidates were sequenced.
Plasmid constructions
Plasmids used in this study are listed in Table S2. The pRS306-ELP3 (pABY1554) was constructed previously [10] and used as DNA template for mutagenesis. Plasmids pABY1672 (elp3-C103A), pABY1673 (elp3-C108A), pABY1676 (elp3-C118A), pABY1677 (elp3-C121A), pABY1984 (elp3-G168R) and pABY1985 (elp3-G180R G181R) were generated by using Quickchange Lightning Multi Site-Directed mutagenesis kit according to the instruction manual (Agilent Technologies). Site-specific primers were designed by Agilent online service. To move mutant alleles of ELP3 to pRS315, pRS306-elp3 derivatives were digested using restriction enzymes BamHI and XhoI, and the excised fragments were cloned into the corresponding sites of pRS315. To generate pRS424-SIR4, SIR4 gene was amplified by PCR using W303-1A genomic DNA as template with oligos AAAA GAATTC TGTGA GTACATATAT CCGCAG and AAAA CTCGAG TTG GTATTTGATG GGTTGCTC. The PCR product was digested with EcoRI and XhoI, and cloned to the corresponding sites on pRS424.
tRNA isolation and HPLC analysis
Cells were grown at 30°C in 100 ml YEPD and harvested at OD600 = 1.5∼2. The cell pellet was resuspended in 3 ml 0.9% NaCl. The cell suspension was vortexed at room temperature for 30 minutes in the presence of 8 ml water-saturated phenol and vortexed for another 15 minutes after adding 0.4 ml chloroform. Centrifugation was carried out at 12000 g for 20 minutes. The water phase was collected and re-extracted with phenol. The final water phase was collected, mixed with 2.5 volume 99.5% ethanol and kept at −20°C for at least 3 hours. Total RNA was pelleted at 12000 g for 20 minutes. The RNA pellet was dissolved in 5 ml DE52 binding buffer (0.1 M Tris.HCl pH 7.4 and 0.1 M NaCl) and loaded onto the DE52 cellulose column. The column was washed twice with 7 ml DE52 binding buffer and the tRNA was eluted with 7 ml elution buffer (0.1 M Tris.HCl pH 7.4 and 1 M NaCl). The tRNA was precipitated with 0.7 volume of isopropanol at −20°C for at least 3 hours and pelleted by centrifugation at 12000 g for 20 minutes. The pellet was washed once with 70% ethanol and dissolved in 50 µl MQ. Purified tRNA was digested with Nuclease P1 for 16 hrs at 37°C and treated with bacterial alkaline phosphatase for 2 hours at 37°C. The hydrolysate was analyzed by high pressure liquid chromatography with a Develosil C-30 reverse-phase column as described [41].
Telomeric gene silencing and DNA damage response assays
To investigate the defect in telomeric gene silencing of Elongator mutants, 10-fold dilutions of freshly cultivated yeast cells were spotted on 5-FOA containing plates and control plates. Plates were incubated at 30°C for 2 days. To analyze the DNA damage response, 10 fold dilutions of freshly cultivated yeast cells were spotted on the plates containing 50 mM HU and control plates. The results were scored after 2 days of incubation at 30°C.
Dual-luciferase reporter assay
The luciferase activities were measured by GloMax 20/20 luminometer (Promega) and the dual-luciferase reporter assay system (Promega). Cells were grown to 0.5 OD600 and diluted 10 fold before use. 20 µl of diluted cell culture was mixed with 100 µl passive lysis buffer, vortexed for 12 seconds and 20 µl of cell lysate was used to determine the luciferase activity. Each culture was measured 3 times and 3 independent experiments were performed.
Western and Northern blotting
To determine the Sir4 protein levels, cells were grown at 30°C to OD600 = 0.5 before harvest. Cells were broken in breaking buffer (40 mM Hepes pH 7.3, 50 mM NH4Ac, 10 mM MgCl2 and 1 mM DTT) containing Complete Protease Inhibitor Cocktail Tablets (Roche Applied Science) by using FastPrep-24 homogenizer (MP biomedicals). 60 µg proteins were loaded in each lane. Mouse anti-Myc antibody (9E10) with a dilution 1∶1000 was used to detect recombinant proteins. The actin levels, used as an internal control, were detected using mouse anti-Act1 antibody (Thermo Scientific) at a 1∶2000 dilution. RNA levels were determined as previously described [42].
Supporting Information
Zdroje
1. OteroGFellowsJLiYde BizemontTDiracAM 1999 Elongator, a multisubunit component of a novel RNA polymerase II holoenzyme for transcriptional elongation. Mol Cell 3 109 118
2. WinklerGSPetrakisTGEthelbergSTokunagaMErdjument-BromageH 2001 RNA polymerase II elongator holoenzyme is composed of two discrete subcomplexes. J Biol Chem 276 32743 32749
3. KroganNJGreenblattJF 2001 Characterization of a six-subunit holo-elongator complex required for the regulated expression of a group of genes in Saccharomyces cerevisiae. Mol Cell Biol 21 8203 8212
4. HawkesNAOteroGWinklerGSMarshallNDahmusME 2002 Purification and characterization of the human elongator complex. J Biol Chem 277 3047 3052
5. KimJHLaneWSReinbergD 2002 Human Elongator facilitates RNA polymerase II transcription through chromatin. Proc Natl Acad Sci U S A 99 1241 1246
6. WittschiebenBOOteroGde BizemontTFellowsJErdjument-BromageH 1999 A novel histone acetyltransferase is an integral subunit of elongating RNA polymerase II holoenzyme. Mol Cell 4 123 128
7. WinklerGSKristjuhanAErdjument-BromageHTempstPSvejstrupJQ 2002 Elongator is a histone H3 and H4 acetyltransferase important for normal histone acetylation levels in vivo. Proc Natl Acad Sci U S A 99 3517 3522
8. RahlPBChenCZCollinsRN 2005 Elp1p, the yeast homolog of the FD disease syndrome protein, negatively regulates exocytosis independently of transcriptional elongation. Mol Cell 17 841 853
9. FrohloffFFichtnerLJablonowskiDBreunigKDSchaffrathR 2001 Saccharomyces cerevisiae Elongator mutations confer resistance to the Kluyveromyces lactis zymocin. EMBO J 20 1993 2003
10. HuangBJohanssonMJByströmAS 2005 An early step in wobble uridine tRNA modification requires the Elongator complex. RNA 11 424 436
11. LuJHuangBEsbergAJohanssonMJByströmAS 2005 The Kluyveromyces lactis gamma-toxin targets tRNA anticodons. RNA 11 1648 1654
12. JohanssonMJEsbergAHuangBBjörkGRByströmAS 2008 Eukaryotic wobble uridine modifications promote a functionally redundant decoding system. Mol Cell Biol
13. ChenCTuckSByströmAS 2009 Defects in tRNA modification associated with neurological and developmental dysfunctions in Caenorhabditis elegans elongator mutants. PLoS Genet 5 e1000561 doi:10.1371/journal.pgen.1000561
14. MehlgartenCJablonowskiDWrackmeyerUTschitschmannSSondermannD 2010 Elongator function in tRNA wobble uridine modification is conserved between yeast and plants. Mol Microbiol 76 1082 1094
15. EsbergAHuangBJohanssonMJByströmAS 2006 Elevated levels of two tRNA species bypass the requirement for elongator complex in transcription and exocytosis. Mol Cell 24 139 148
16. CamposEIReinbergD 2009 Histones: annotating chromatin. Annu Rev Genet 43 559 599
17. ClapierCRCairnsBR 2009 The biology of chromatin remodeling complexes. Annu Rev Biochem 78 273 304
18. SofiaHJChenGHetzlerBGReyes-SpindolaJFMillerNE 2001 Radical SAM, a novel protein superfamily linking unresolved steps in familiar biosynthetic pathways with radical mechanisms: functional characterization using new analysis and information visualization methods. Nucleic Acids Res 29 1097 1106
19. LiQFazlyAMZhouHHuangSZhangZ 2009 The elongator complex interacts with PCNA and modulates transcriptional silencing and sensitivity to DNA damage agents. PLoS Genet 5 e1000684 doi:10.1371/journal.pgen.1000684
20. GottschlingDEAparicioOMBillingtonBLZakianVA 1990 Position effect at S. cerevisiae telomeres: reversible repression of Pol II transcription. Cell 63 751 762
21. FillinghamJRechtJSilvaACSuterBEmiliA 2008 Chaperone control of the activity and specificity of the histone H3 acetyltransferase Rtt109. Mol Cell Biol 28 4342 4353
22. LinLJMinardLVJohnstonGCSingerRASchultzMC 2010 Asf1 can promote trimethylation of H3 K36 by Set2. Mol Cell Biol 30 1116 1129
23. TsubotaTBerndsenCEErkmannJASmithCLYangL 2007 Histone H3-K56 acetylation is catalyzed by histone chaperone-dependent complexes. Mol Cell 25 703 712
24. GrantPADugganLCoteJRobertsSMBrownellJE 1997 Yeast Gcn5 functions in two multisubunit complexes to acetylate nucleosomal histones: characterization of an Ada complex and the SAGA (Spt/Ada) complex. Genes Dev 11 1640 1650
25. EberharterASternerDESchieltzDHassanAYatesJR3rd 1999 The ADA complex is a distinct histone acetyltransferase complex in Saccharomyces cerevisiae. Mol Cell Biol 19 6621 6631
26. WittschiebenBOFellowsJDuWStillmanDJSvejstrupJQ 2000 Overlapping roles for the histone acetyltransferase activities of SAGA and elongator in vivo. EMBO J 19 3060 3068
27. KeelingKMLanierJDuMSalas-MarcoJGaoL 2004 Leaky termination at premature stop codons antagonizes nonsense-mediated mRNA decay in S. cerevisiae. RNA 10 691 703
28. BjörkGRHuangBPerssonOPByströmAS 2007 A conserved modified wobble nucleoside (mcm5s2U) in lysyl-tRNA is required for viability in yeast. RNA 13 1245 1255
29. PokholokDKHannettNMYoungRA 2002 Exchange of RNA polymerase II initiation and elongation factors during gene expression in vivo. Mol Cell 9 799 809
30. HuhWKFalvoJVGerkeLCCarrollASHowsonRW 2003 Global analysis of protein localization in budding yeast. Nature 425 686 691
31. BegleyUDyavaiahMPatilARooneyJPDiRenzoD 2007 Trm9-catalyzed tRNA modifications link translation to the DNA damage response. Mol Cell 28 860 870
32. ClosePHawkesNCornezICreppeCLambertCA 2006 Transcription impairment and cell migration defects in elongator-depleted cells: implication for familial dysautonomia. Mol Cell 22 521 531
33. HolmbergCKatzSLerdrupMHerdegenTJaattelaM 2002 A novel specific role for I kappa B kinase complex-associated protein in cytosolic stress signaling. J Biol Chem 277 31918 31928
34. NelissenHFleuryDBrunoLRoblesPDe VeylderL 2005 The elongata mutants identify a functional Elongator complex in plants with a role in cell proliferation during organ growth. Proc Natl Acad Sci U S A 102 7754 7759
35. CreppeCMalinouskayaLVolvertMLGillardMCloseP 2009 Elongator controls the migration and differentiation of cortical neurons through acetylation of alpha-tubulin. Cell 136 551 564
36. SolingerJAPaolinelliRKlossHScorzaFBMarchesiS 2010 The Caenorhabditis elegans Elongator complex regulates neuronal alpha-tubulin acetylation. PLoS Genet 6 e1000820 doi:10.1371/journal.pgen.1000820
37. AkellaJSWlogaDKimJStarostinaNGLyons-AbbottS 2010 MEC-17 is an alpha-tubulin acetyltransferase. Nature 467 218 222
38. ParaskevopoulouCFairhurstSALoweDJBrickPOnestiS 2006 The Elongator subunit Elp3 contains a Fe4S4 cluster and binds S-adenosylmethionine. Mol Microbiol 59 795 806
39. GreenwoodCSelthLADirac-SvejstrupABSvejstrupJQ 2009 An iron-sulfur cluster domain in Elp3 important for the structural integrity of elongator. J Biol Chem 284 141 149
40. BurkeDDawsonDStearnsT 2000 Methods in Yeast Genetics Cold Spring Harbor, NY Cold Spring Harbor Laboratory Press
41. BjörkGRJacobssonKNilssonKJohanssonMJByströmAS 2001 A primordial tRNA modification required for the evolution of life? EMBO J 20 231 239
42. HeFAmraniNJohanssonMJJacobsonA 2008 Chapter 6. Qualitative and quantitative assessment of the activity of the yeast nonsense-mediated mRNA decay pathway. Methods Enzymol 449 127 147
43. FiorentiniPHuangKNTishkoffDXKolodnerRDSymingtonLS 1997 Exonuclease I of Saccharomyces cerevisiae functions in mitotic recombination in vivo and in vitro. Mol Cell Biol 17 2764 2773
44. SikorskiRSHieterP 1989 A system of shuttle vectors and yeast host strains designed for efficient manipulation of DNA in Saccharomyces cerevisiae. Genetics 122 19 27
45. ChristiansonTWSikorskiRSDanteMSheroJHHieterP 1992 Multifunctional yeast high-copy-number shuttle vectors. Gene 110 119 122
Štítky
Genetika Reprodukční medicína
Článek Produces a Long Noncoding RNA and Has General Functions in the Germline, Stem Cells, and BrainČlánek Inference of Relationships in Population Data Using Identity-by-Descent and Identity-by-StateČlánek A Genome-Wide Meta-Analysis of Six Type 1 Diabetes Cohorts Identifies Multiple Associated LociČlánek Retrotransposon-Induced Heterochromatin Spreading in the Mouse Revealed by Insertional PolymorphismsČlánek Essential Roles of BCCIP in Mouse Embryonic Development and Structural Stability of ChromosomesČlánek The Receptor Tyrosine Kinase Alk Controls Neurofibromin Functions in Drosophila Growth and LearningČlánek PCNA Ubiquitination Is Important, But Not Essential for Translesion DNA Synthesis in Mammalian CellsČlánek Genome-Wide Association Study Identifies Four Loci Associated with Eruption of Permanent Teeth
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2011 Číslo 9- Akutní intermitentní porfyrie
- Farmakogenetické testování pomáhá předcházet nežádoucím efektům léčiv
- Růst a vývoj dětí narozených pomocí IVF
- Pilotní studie: stres a úzkost v průběhu IVF cyklu
- Intrauterinní inseminace a její úspěšnost
-
Všechny články tohoto čísla
- Temporal Trends in Results Availability from Genome-Wide Association Studies
- Association of eGFR-Related Loci Identified by GWAS with Incident CKD and ESRD
- Genetic Variants at Chromosomes 2q35, 5p12, 6q25.1, 10q26.13, and 16q12.1 Influence the Risk of Breast Cancer in Men
- Large-Scale Gene-Centric Analysis Identifies Novel Variants for Coronary Artery Disease
- Genetic Association for Renal Traits among Participants of African Ancestry Reveals New Loci for Renal Function
- Transcriptome Kinetics Is Governed by a Genome-Wide Coupling of mRNA Production and Degradation: A Role for RNA Pol II
- Conserved Regulation of p53 Network Dosage by MicroRNA–125b Occurs through Evolving miRNA–Target Gene Pairs
- Heterozygous Mutations of Are Associated with an Increased Risk of Isolated Metopic Craniosynostosis in Humans and Mice
- Study of FoxA Pioneer Factor at Silent Genes Reveals Rfx-Repressed Enhancer at and a Potential Indicator of Esophageal Adenocarcinoma Development
- Cholesterol Metabolism Is Required for Intracellular Hedgehog Signal Transduction
- Produces a Long Noncoding RNA and Has General Functions in the Germline, Stem Cells, and Brain
- Age-Dependent Recombination Rates in Human Pedigrees
- Sequence Conservation and Functional Constraint on Intergenic Spacers in Reduced Genomes of the Obligate Symbiont
- Sex Chromosome Mosaicism and Hybrid Speciation among Tiger Swallowtail Butterflies
- A Negative Feedback Loop That Limits the Ectopic Activation of a Cell Type–Specific Sporulation Sigma Factor of
- Phased Whole-Genome Genetic Risk in a Family Quartet Using a Major Allele Reference Sequence
- Mutations in or near the Transmembrane Domain Alter PMEL Amyloid Formation from Functional to Pathogenic
- Inactivation of Alters Melanosome Shape But Has Only a Subtle Effect on Visible Pigmentation
- Novel Interactions between Actin and the Proteasome Revealed by Complex Haploinsufficiency
- Germline Genetic Variants Disturbing the /LIN28 Double-Negative Feedback Loop Alter Breast Cancer Susceptibility
- Separation of Recombination and SOS Response in RecA Suggests LexA Interaction Sites
- Inference of Relationships in Population Data Using Identity-by-Descent and Identity-by-State
- Misregulation of Scm3p/HJURP Causes Chromosome Instability in and Human Cells
- A Noncoding Point Mutation of Causes Multiple Developmental Malformations and Obesity in Twirler Mice
- The Evolutionarily Conserved Longevity Determinants HCF-1 and SIR-2.1/SIRT1 Collaborate to Regulate DAF-16/FOXO
- A Genome-Wide Metabolic QTL Analysis in Europeans Implicates Two Loci Shaped by Recent Positive Selection
- Bacterial Communities of Diverse Species: Ecological Context of a Host–Microbe Model System
- A Genome-Wide Meta-Analysis of Six Type 1 Diabetes Cohorts Identifies Multiple Associated Loci
- Elongator Complex Influences Telomeric Gene Silencing and DNA Damage Response by Its Role in Wobble Uridine tRNA Modification
- Elevated Proteasome Capacity Extends Replicative Lifespan in
- Genome-Wide Analysis of Heteroduplex DNA in Mismatch Repair–Deficient Yeast Cells Reveals Novel Properties of Meiotic Recombination Pathways
- MicroRNA Predictors of Longevity in
- An Iterative Genetic and Dynamical Modelling Approach Identifies Novel Features of the Gene Regulatory Network Underlying Melanocyte Development
- Atypical AT Skew in Firmicute Genomes Results from Selection and Not from Mutation
- Retrotransposon-Induced Heterochromatin Spreading in the Mouse Revealed by Insertional Polymorphisms
- Genomic Analysis of QTLs and Genes Altering Natural Variation in Stochastic Noise
- The Abnormal Phenotypes of Cartilage and Bone in Calcium-Sensing Receptor Deficient Mice Are Dependent on the Actions of Calcium, Phosphorus, and PTH
- Cell Type–Specific Transcriptome Analysis Reveals a Major Role for and miR-200b in Mouse Inner Ear Morphogenesis
- Essential Roles of BCCIP in Mouse Embryonic Development and Structural Stability of Chromosomes
- IAP1-Mediated Ubiquitylation Controls Activation of the Initiator Caspase DRONC Independent of Protein Degradation
- VANG-1 and PRKL-1 Cooperate to Negatively Regulate Neurite Formation in
- The Receptor Tyrosine Kinase Alk Controls Neurofibromin Functions in Drosophila Growth and Learning
- Comparative and Functional Genomics of PD630 for Biofuels Development
- Identification of Type 1 Diabetes–Associated DNA Methylation Variable Positions That Precede Disease Diagnosis
- PCNA Ubiquitination Is Important, But Not Essential for Translesion DNA Synthesis in Mammalian Cells
- Genetic Effects at Pleiotropic Loci Are Context-Dependent with Consequences for the Maintenance of Genetic Variation in Populations
- Genome-Wide Association Study Identifies Four Loci Associated with Eruption of Permanent Teeth
- Bmp and Nodal Independently Regulate Expression to Maintain Unilateral Nodal Activity during Left-Right Axis Specification in Zebrafish
- Inter-Allelic Prion Propagation Reveals Conformational Relationships among a Multitude of [] Strains
- Emergence and Modular Evolution of a Novel Motility Machinery in Bacteria
- Histone Methyltransferase MET-2 Shields the Male X Chromosome from Checkpoint Machinery and Mediates Meiotic Sex Chromosome Inactivation
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Retrotransposon-Induced Heterochromatin Spreading in the Mouse Revealed by Insertional Polymorphisms
- The Evolutionarily Conserved Longevity Determinants HCF-1 and SIR-2.1/SIRT1 Collaborate to Regulate DAF-16/FOXO
- Genome-Wide Analysis of Heteroduplex DNA in Mismatch Repair–Deficient Yeast Cells Reveals Novel Properties of Meiotic Recombination Pathways
- Association of eGFR-Related Loci Identified by GWAS with Incident CKD and ESRD
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



