-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Cholesterol Metabolism Is Required for Intracellular Hedgehog Signal Transduction
We describe the rudolph mouse, a mutant with striking defects in both central nervous system and skeletal development. Rudolph is an allele of the cholesterol biosynthetic enzyme, hydroxysteroid (17-beta) dehydrogenase 7, which is an intriguing finding given the recent implication of oxysterols in mediating intracellular Hedgehog (Hh) signaling. We see an abnormal sterol profile and decreased Hh target gene induction in the rudolph mutant, both in vivo and in vitro. Reduced Hh signaling has been proposed to contribute to the phenotypes of congenital diseases of cholesterol metabolism. Recent in vitro and pharmacological data also indicate a requirement for intracellular cholesterol synthesis for proper regulation of Hh activity via Smoothened. The data presented here are the first in vivo genetic evidence supporting both of these hypotheses, revealing a role for embryonic cholesterol metabolism in both CNS development and normal Hh signaling.
Published in the journal: . PLoS Genet 7(9): e32767. doi:10.1371/journal.pgen.1002224
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1002224Summary
We describe the rudolph mouse, a mutant with striking defects in both central nervous system and skeletal development. Rudolph is an allele of the cholesterol biosynthetic enzyme, hydroxysteroid (17-beta) dehydrogenase 7, which is an intriguing finding given the recent implication of oxysterols in mediating intracellular Hedgehog (Hh) signaling. We see an abnormal sterol profile and decreased Hh target gene induction in the rudolph mutant, both in vivo and in vitro. Reduced Hh signaling has been proposed to contribute to the phenotypes of congenital diseases of cholesterol metabolism. Recent in vitro and pharmacological data also indicate a requirement for intracellular cholesterol synthesis for proper regulation of Hh activity via Smoothened. The data presented here are the first in vivo genetic evidence supporting both of these hypotheses, revealing a role for embryonic cholesterol metabolism in both CNS development and normal Hh signaling.
Introduction
Hedgehog (Hh) ligands have numerous and fundamental roles in both embryonic development [1], [2] and tumor biology [3]. Mammalian hedgehog proteins bind to the transmembrane receptor Patched (Ptc) and thereby relieve its repression of Smoothened (Smo). Intracellular transduction of Smo activity requires processing of GLI proteins. The primary cilium has shown to be an essential structural component for proper Hh signaling in mammals with dynamic localization of Ptc in response to binding of Sonic hedgehog (SHH; [4], [5], [6], [7]). Functional SHH signaling requires removal of PTC from the cilium, translocation of SMO to the cilium, and activation of SMO by an as yet unknown mechanism [5], [6], [8], [9].
It has been well established that cholesterol is an essential component of Hh signal transduction. Processing of the Hh ligand in the producing cell includes the covalent modification of cholesterol to the carboxyl end of the immature protein. Although cholesterol in Hh proteins is thought to facilitate the proper dispersal of SHH through the target field, it is not necessary for signal transduction [7]. More recently, however, metabolites of cholesterol and cholesterol biosynthetic pathway intermediates have been shown to have an intracellular role in SHH signal transduction. For example, pharmacological inhibition of cholesterol biosynthesis leads to defective responses to SHH ligand, independent of SHH processing, both in vitro and in vivo, with the in vivo effect mimicking Shh loss of function phenotypes [10], [11], [12]. Moreover, not only can cholesterol-derived oxysterols activate the Hh pathway in vitro [13], but treatment of cells with oxysterols has been shown to cause translocation of PTC and SMO to the cilium in the absence of SHH ligand [8].
Mutations in enzymes required for cholesterol biosynthesis are associated with a number of human diseases [14]. The best known is Smith-Lemli-Opitz Syndrome, in which patients have central nervous system (CNS) malformations, including holoprosencephaly and microcephaly, and skeletal defects (most often postaxial polydactyly) caused by mutations in 7-dehydrocholesterol reductase (DHCR7) [15]. Other disorders of cholesterol biosynthesis include desmosterolosis, lathosterolosis, X-linked dominant chondrodysplasia punctata (CDPX2), CHILD syndrome (congenital hemidysplasia with icthyosiform erythroderma or nevus and limb defects) and Greenberg skeletal dysplasia. Overlapping features of these disorders include abnormalities in the CNS, facial dysmorphisms, and skeletal defects, often including polydactyly or other digit patterning defects. Mouse models also exist for many of these disorders and have similar defects. The frequent occurrence in these human syndromes and mouse mutants of defects in neurodevelopment, craniofacial morphogenesis and skeletal growth and patterning has led to the proposal that abnormal Hh signaling may be the root cause of these embryological defects. This speculation is based principally on genetic experiments that have shown a role for Hh signaling in the development of all of these tissues, and the known role of sterol metabolism in Hh signal transduction. Despite this, there is relatively little direct evidence from these mouse models for a defect in Hh signaling.
Here we describe the phenotype of the rudolph mouse mutant, an ethyl-nitrosourea (ENU)-induced mutation in hydroxysteroid (17-beta) dehydrogenase 7 (Hsd17b7), which was the last enzyme of the cholesterol biosynthetic pathway to be identified and one of four proteins of the sterol-4-demethylase complex [16]. Rudolph mutants have severe developmental abnormalities in several tissues including the brain and appendicular skeleton. We find that tissues from rudolph mutants have an abnormal sterol profile consistent with impaired activity of the sterol-4-demethylase complex. We further demonstrate that the rudolph mutant has deficient responses to Hh signaling, both in vivo and in vitro. These results support the recently proposed model that functional intracellular sterol metabolism is required for proper cilia-mediated activation of the Hh signaling pathway.
Results
Rudolph mutants show defective growth and patterning of the CNS and appendicular skeleton
We recently recovered the rudolph mutation via an ENU mutagenesis screen designed to identify recessive mutations affecting development of the mammalian forebrain. Rudolph mutants were first ascertained by a blood spot on the end of the nose and their abnormally curved forelimbs (Figure 1B). The precursor to this nasal phenotype was sometimes evident at earlier stages as a blebbing of the craniofacial epithelium (Figure S1A). Examination of the embryonic skeleton revealed that all long bones of the appendicular skeleton were significantly shorter than those of wild-type littermates while the axial skeleton and ribs appeared normal. (Figure 1D, Figure S2, Table S1). Further analysis of the embryos revealed severe defects in CNS development. The telencephalic tissue was markedly reduced in size and highly disorganized in mutants at embryonic day (E) 16.5 (Figure 1F, 1H). Mutants had a smaller neurogenic ventricular zone and clumps of cells in the developing cortical plate. Similar defects were seen in the E16.5 retina and spinal cord (Figure 1J, Figure S3B). Initial cortical morphogenesis appeared largely normal (Figure S3D, S3F).
Fig. 1. Phenotypic characterization of rudolph mutants. 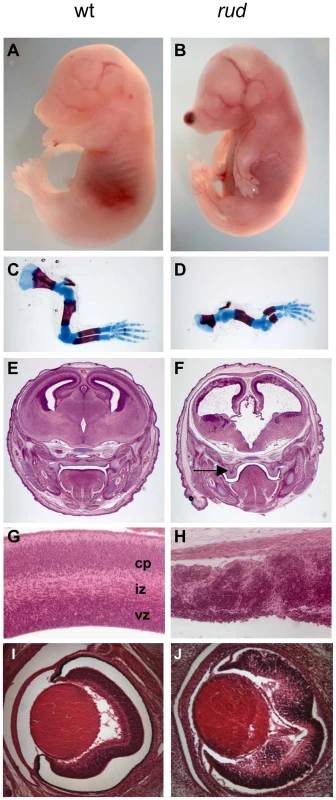
Rudolph mutants (B) are readily distinguishable from wild-type (A) littermates at E16.5 by their shortened, curved forelimbs. Some mutants have accumulations of blood in nasal blebs (B). Skeletal preparations of E16.5 limbs show significantly shorter long bones in mutants (D). The mutant forebrain at E16.5 (F) is severely affected with a reduction in tissue throughout. Cleft palate is also noted in mutants (arrow in F). While the wild-type cortex (G) has a readily identifiable ventricular zone (vz), intermediate zone (iz), and cortical plate (cp), closer examination of the dorsal cortex in the mutant reveals significant disorganization (H). Similar disorganization is seen in the rud retina (J). To further characterize the rudolph phenotype, we performed a molecular analysis of the cortical phenotype. We assessed cell proliferation at E14.5 by BrdU treatment of pregnant dams or immunostaining of embryos with Ki67 and found a marked decrease in proliferation in mutants (Figure 2B). Some of the mitoses we detected were seen as foci of BrdU-positive cells (inset in Figure 2B). We thus interpreted the clusters of cells seen histologically in the rud cortex to be neurogenic foci. To determine the cause of the reduced neuronal tissue, we assayed apoptosis at E14.5 using the TUNEL reaction and found increased levels of cell death in the mutant tissue, distributed throughout the cortex and enriched along the ventricular surface (Figure 2D). An increased level of cell death was not seen in non-neural tissue (data not shown). Decreased, disorganized neuronal proliferation and increased cell death were also evident at E12.5 (data not shown). Immunohistochemistry for TuJI to identify differentiated neurons at E14.5 showed a marked decrease in differentiation in mutants compared to wild-type (Figure 2F). In addition, foci of TuJI immunoreactivity appeared in regions of no differentiation, consistent with the disorganization of the cortex seen histologically. Disorganized proliferation was also evident in the developing rudolph retina, where we observed similarly abnormal neuronal differentiation, decreased cell proliferation and increased apoptosis, but at different stages of development. Whereas at E12.5 we saw no significant decrease in proliferation or apoptosis between wild-type and mutant (Figure 2H; data not shown), at E14.5 we found a decrease in BrdU incorporation in the mutant retina (Figure 2J) and an increase in apoptosis (Figure 2L). Furthermore, the pattern of neuronal disorganization we saw in the rud cortex was similarly evident in the rud retina at E14.5 (Figure 2N).
Fig. 2. Telencephalic and retinal development are abnormal in rudolph mutants. 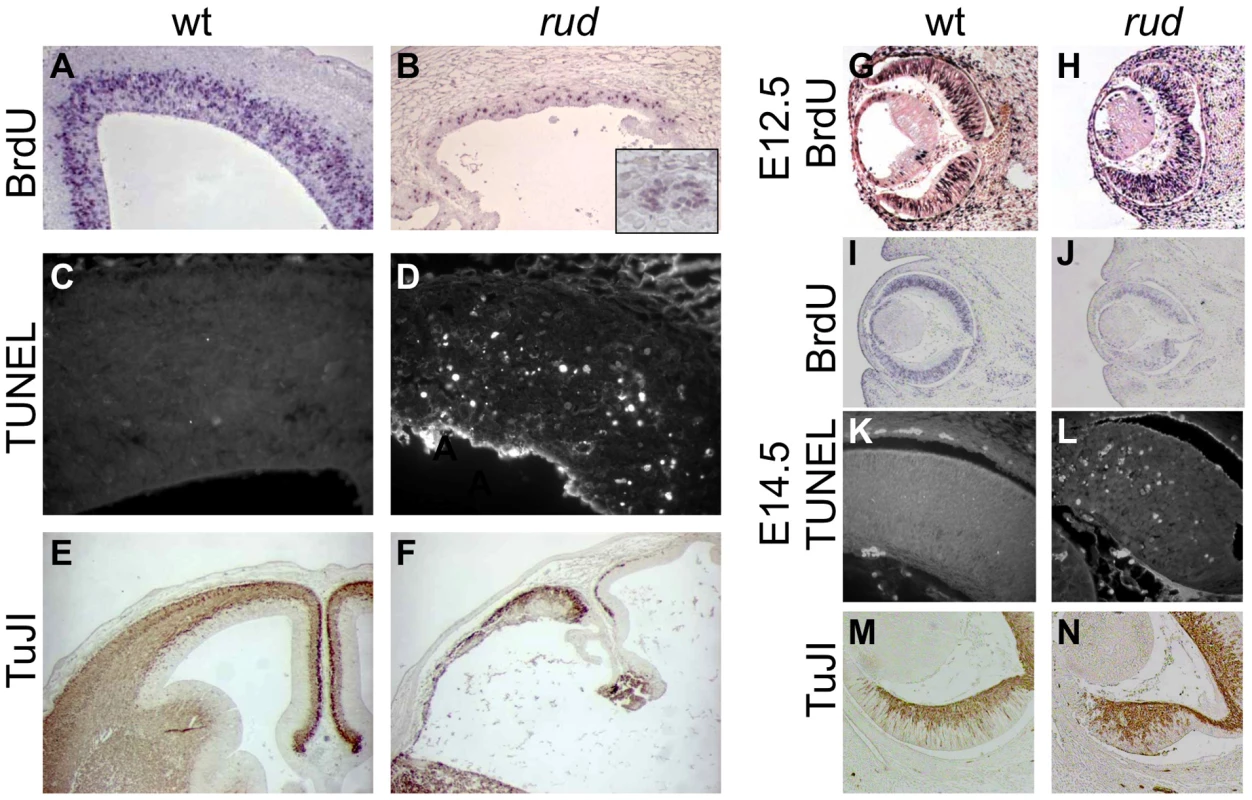
(A,B) BrdU labeling and immunohistochemistry shows a significant decrease in dividing cells in the rudolph cortex (A) as compared to the wild-type (A). The inset in B highlights the groups of dividing cells sometimes seen away from the ventricular zone. (C,D) Analysis of cell death using the TUNEL assay reveals a large increase in cell death in mutant tissue (B) with an enrichment of the death at the ventricular surface. (E,F) Neuronal differentiation as measured by TuJI immunohistochemistry is dramatically decreased in rud mutants (F). (G,H) BrdU labeling at E12.5 does not indicate a significant difference in rates of cell division in the rudolph retina at E12.5, but a significant decrease is seen at E14.5 (J). Similar to cortical development, the decreased proliferation is accompanied by an increase in apoptosis as shown by the TUNEL assay (K,L). Neuronal differentiation in the mutant retina is disorganized in mutant tissue (M) as compared to wild-type (N). Genetic background affects the rudolph phenotype
The phenotypes of the rudolph mutants exhibit a variability in severity that appears to be dependent on genetic background. For example, we noted increased blebbing on the developing head and limbs in embryos that came from a mixed background (Figure S1B–S1D). Embryos from our ENU screen have a mixed genetic background with contributions coming from both A/J (the mutagenized strain) and FVB mice (introduced as part of the outcross for mapping purposes). In this A/J; FVB background, embryos were recovered in approximately Mendelian ratios from E10.5–E18.5 (Table S2). No mutants have been recovered after birth, suggesting they are among the stillborn fetuses. Upon introducing the B6 background, the number of mutant embryos did not decrease significantly, but we then began to see a number of dead embryos from E11.5 and older (7.8%), an increase in the severity of the nasal blebbing (Figure S1A–S1C: 14.8%) and limb patterning defects (10.4%). Further introgression into the B6 background resulted in no significant decrease in recovery of mutant embryos but a large increase in the incidence of more severe blebbing (48.5% in pooled N1, N2, and N3B6 mice). Backcrossing to FVB rescued this defect, and blebbing is essentially absent in N3 FVB mice. The reduced fecundity of 129 mice limited our analysis of this genetic background, preventing any definitive comments on modifiers on the 129 background (Table S2).
Hsd17b7 is the gene mutated in rudolph embryos
We initially mapped the rudolph mutation to Chromosome 1 using a whole-genome 768-marker single nucleotide polymorphism (SNP) panel [17]. Examination of the 255 predicted and known genes in the region suggested Hsd17b7 as a candidate for further analysis because of its known function in cholesterol metabolism and its reported expression pattern in limb buds and the developing nervous system [16]. Sequencing of Hsd17b7 revealed a point mutation in the sixth intron, 27 base pairs upstream of the intron-exon boundary (Figure 3A). We analyzed transcripts by RT-PCR with primers spanning exon 7 and found the predominant PCR product in mutant tissue to be smaller than that of wild type (Figure 3B). Sequencing of this product revealed a precise excision of the seventh exon in the smaller PCR species. The loss of exon 7 was confirmed with primers in the sixth and seventh exons (Figure 3C). Interestingly, a small amount of this truncated transcript was present in wild-type cDNA, and, conversely, mutant tissues retained a very small fraction of the wild-type transcript (Figure 3B, 3C). We hypothesize that these RT-PCR products represent two naturally occurring forms of the Hsd17b7 transcript and that the rudolph ENU mutation affects the ratio of their abundances. The phenotypes we observe in the rudolph mutants appear to be somewhat tissue specific and have differing expressivity in different strains. However, the variation in the cDNA splicing pattern does not differ among different tissues examined (heart/lung, limbs, brain, or whole embryo) or depend on varying genetic backgrounds, suggesting that tissue specific transcription and variation in genetic background account for the variability in phenotype (Figure S1E).
Fig. 3. Hsd17b7 is the gene carrying the rudolph mutation. 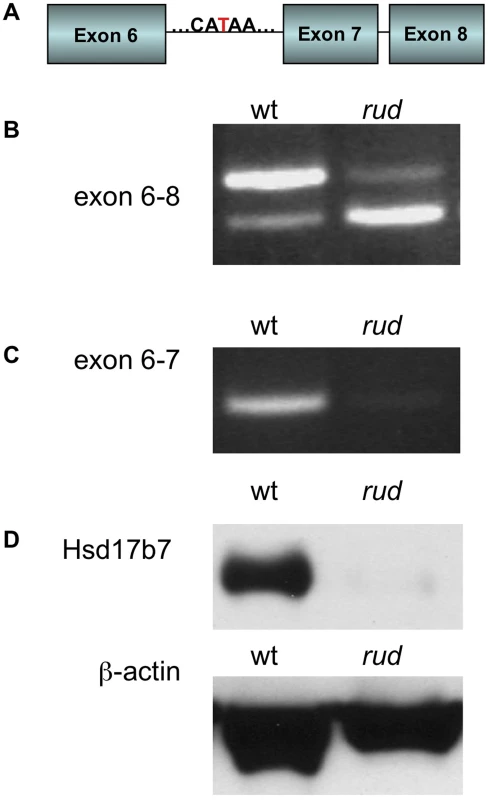
(A) Sequencing of the Hsd17b7 gene revealed a thymidine to adenosine mutation in the intron of rudolph mutants between the sixth and seventh exons. (B,C) RT-PCR analysis of wild-type and mutant transcripts with primers for exons 6–8 of the rudolph transcript give two PCR products. The larger species is found in low quantities in the rud mutant tissue. A smaller species, which lacks the seventh exon, was also amplified. RT-PCR with primers for the sixth and seventh exon results in very little amplification of mutant cDNA. (D) Immunoblotting from embryo lysates shows very little protein in rudolph mutants as compared to wild-type. Loss of the seventh exon of HSD17B7 is predicted to encode a protein with an in-frame deletion of 19 amino acids. To assess the effect of this splicing mutation, we tested HSD17B7 expression by Western immunoblot analysis and found only trace levels of protein in mutant tissue lysates (Figure 3D). Although the deleted seventh exon is part of a putative endoplasmic reticulum anchoring sequence, in vitro expression of a rud-GFP fusion protein results in deficient protein rather than mislocalization, suggesting that the rudolph deletion generates an unstable protein product (Figure S4). Embryos homozygous for a null allele of Hsd17b7 generated from a 129 genetic background do not survive past E10.5, suggesting the rudolph allele is likely a hypomorph [18], [19].
Sterol profiles are abnormal in rudolph tissues
We analyzed the sterols present in liver and brain tissue from wild-type, heterozygous, and rudolph embryos at E12.5 by gas chromatography-mass spectrophotometry (Figure 4) and found marked differences between wild-type and mutant tissues. The identified abnormalities in methylsterol abundances are consistent with reduced function of the Hsd1b7 enzyme in rudolph brain tissues (Table 1). Sterol species upstream of Hsd17b7 activity were present in increased amounts, the most prominent of which were the HSD17B7 substrates zymosterone and 4methyl-zymosterone, and a third ketosterol tentatively identified as methylcholest-7-en-3-one. Various mono - and dimethylsterols that do not normally accumulate in wild-type tissues were also present in increased amounts, including 4α-methyl-5 α-cholest-8-en-3β-ol, 4 α-methyl-5 α-cholest-7-en-3 β-ol, 4 α-methyl-cholesta-8(9),24-dien-3 β-ol, and 4,4-dimethyl-5 α-cholest-8(9),24-dien-3 β-ol. Desmosterol, a compound downstream of Hsd17b7, was reduced. The predominance of zymosterone (5 α-cholesta-8,24-dien-3-one) and 4 α-methylzymosterone in the brain compared to liver is in keeping with the known decreased activity of desmosterol reductase in the brain, especially fetal brain, and the retention of the 24-unsturated bond in certain sterol species in the normal brain. In wild-type brain, desmosterol (5 α-cholesta-5,24-dien-3β-ol) is the most abundant 24-unsaturated sterol.
Fig. 4. Sterol profiles. 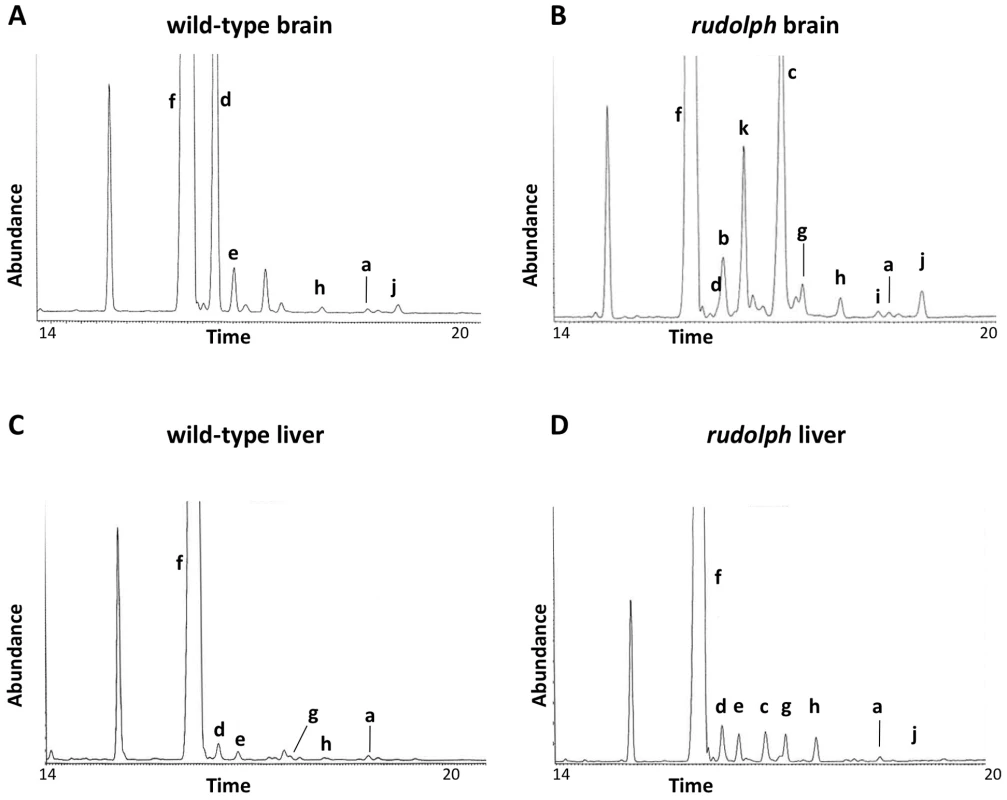
Brain (A,B) and liver (C,D) tissue from wild-type (A,C) and rudolph (B,D) E14.5 embryos were used to create sterol profiles by gas chromatography-mass spectrometry. Some compounds identified include lanosterol (a), zymosterone (b), 4α-methyl-zymosterone (c), desmosterol (d), lathosterol (e), cholesterol (f), 4α-methyl-5α-cholest-8-en-3β-ol (g), 4α-methyl-5α-cholest-7-en-3β-ol and 4α-methyl-cholesta-8(9),24-dien-3β-ol (h), 4,4′-dimethyl-5α-cholest-8-en-3β-ol (i), 4,4-dimethyl-5α-cholest-8(9),24-dien-3β-ol (j), and methylcholest-7-en-3-one (tentative) (k). Tab. 1. Analysis of sterols present in brain tissue. 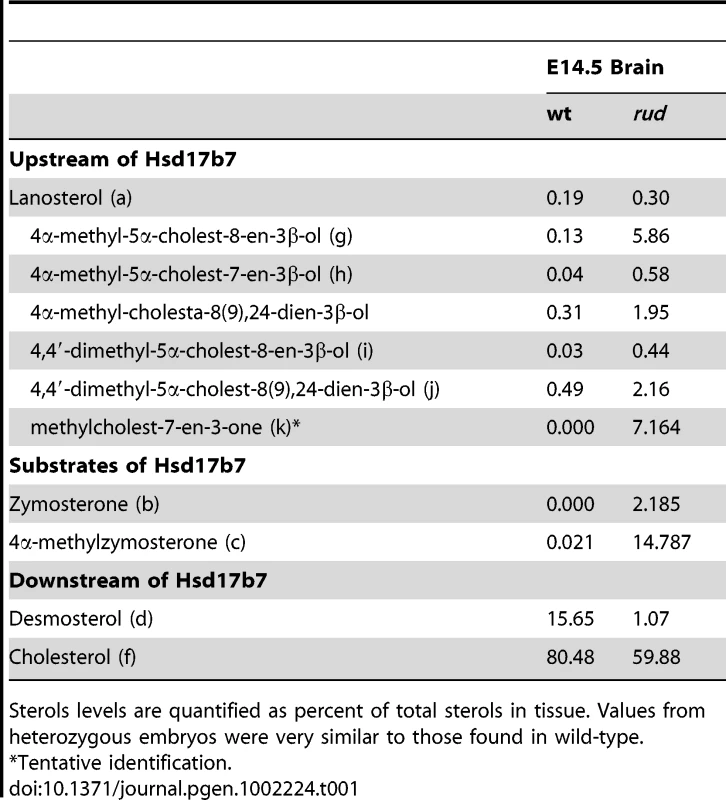
Sterols levels are quantified as percent of total sterols in tissue. Values from heterozygous embryos were very similar to those found in wild-type. Hedgehog signaling is disrupted in the rudolph mutant central nervous system
In view of the important role of Hh signaling in patterning of the limbs, face, and brain, and the genetic and cellular evidence for a role of cholesterol metabolism in this pathway, we hypothesized that Hh signaling is perturbed in the rudolph mutant. Furthermore, recent evidence specifically implicates intracellular sterols in the regulation of the subcellular localization of the Hh signaling components, Patched and Smoothened, supporting the possibility that an abnormal sterol profile in rudolph mutants could disrupt Hh signaling [5], [6], [8]. To assess this, we generated mice homozygous for the rudolph mutation that carried the Patched-lacZ gene, a transcriptional target of Gli2 and thus a reporter of Shh signaling activity. In these embryos, we found reduced levels of Ptc-lacZ in the developing brain at both E11.5 and E14.5 (Figure 5B, 5D; Figure S5; data not shown). We also noted decreased expression of another Shh target gene, Gli1, in the retina and brain of rudolph mutants at E14.5 (Figure 5F, Figure S5). Furthermore, analysis using quantitative RT-PCR demonstrated reduced Ptc mRNA in rudolph brain tissue compared to wild-type (63% of wild-type, p = 0.053, data not shown). All tissues with reduced SHH target gene expression have severe morphological abnormalities in the rudolph mutant and are known sites of Hh signaling. Because dorsal-ventral patterning of the neural tube also requires SHH signaling, we examined the dorsal-ventral character of the rudolph neural tube and found that, at both E10.5 (Figure S6A–S6N) and E12.5 (Figure S6O–S6BB), all immunohistochemical markers for cell fate we tested showed normal patterns of expression along the dorsal-ventral axis of the rudolph neural tube.
Fig. 5. Sonic hedgehog signal transduction is abnormal in rudolph embryonic nervous system and cells. 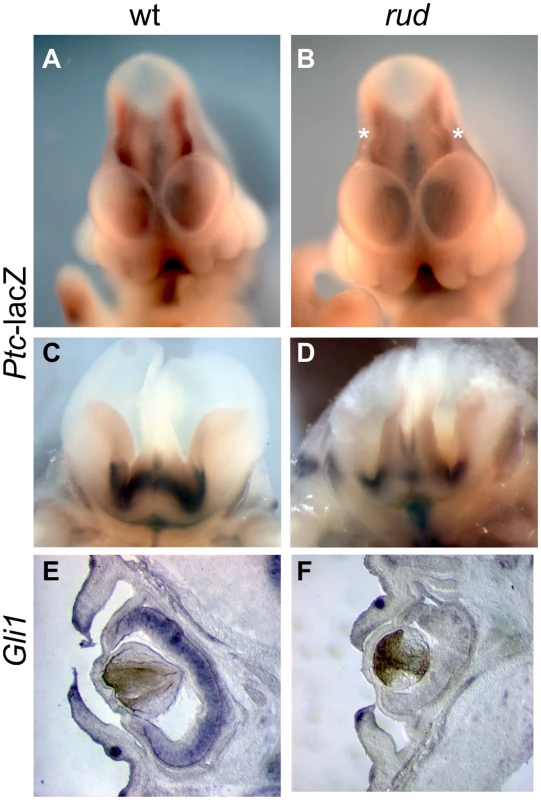
(A–D) Rudolph-Patched lacZ embryos at E11.5 (A,B) and E14.5 (C,D) show less Ptc-lacZ activity in rudolph mutant brain tissue (B,D) as compared to wild-type (A,C). Asterisks in B indicate the midbrain in the mutant where Ptc-lacZ is particulary reduced compared to wild-type. Section in situ hybridization shows less Gli1 expression in the rudolph retina at E14.5 (F) than in wild-type (E). Hedgehog signaling is disrupted in the rudolph mutant skeleton
We also observed limb patterning defects in mice from a mixed A/J, FVB, B6 (Figure 6A, 6B, Table S2). As Hh signaling is important for proper patterning, we examined Hh signaling in the developing limb bud. Normal patterning of the limb results in elevated Shh signaling in the posterior portion of the developing limb bud as compared to the anterior. We observed Ptc expression in rud mutants (n = 3) from a mixed background using both Ptc-lacZ expression and whole mount in situ hybridization and found one embryo with reduced Ptc expression in the posterior limb bud (Figure 6D) as compared to littermate control (Figure 6C). The variable penetrance of the limb patterning phenotype is consistent with an incompletely penetrant reduction in Shh activity in the developing limb bud. Hh signaling is also involved in long bone growth (where the relevant ligand is Indian hedgehog). We also see reduced expression of Ptc in the developing limb of rud mutants using either the Ptc-lacZ allele (Figure 6F) or an in situ riboprobe for Ptc (Figure 6H).
Fig. 6. Sonic hedgehog signal transduction is abnormal in rudolph skeletal elements. 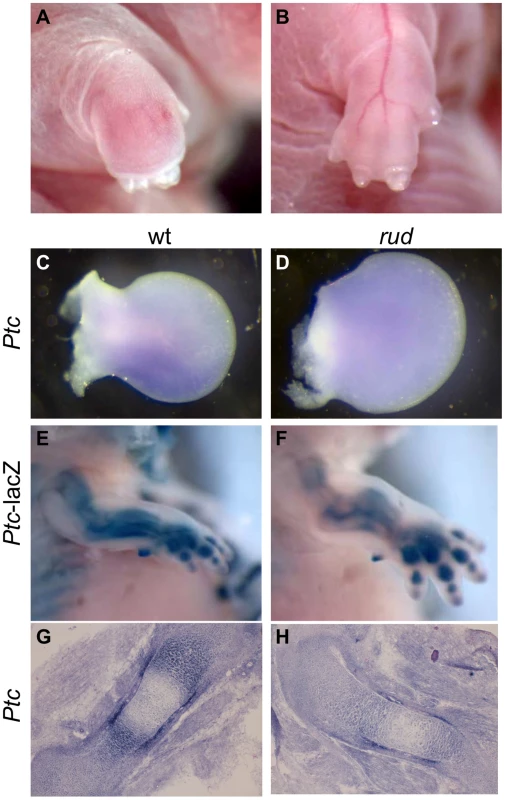
(A,B) Embryos from a mixed background show an incompletely penetrant limb patterning defect. Whole mount in situ hybridization shows a Ptc domain in the posterior portion of the developing wild-type limb bud (C), which can be lost in rudolph mutants (D). Hedgehog signaling is also decreased in the developing long bones of rudolph mutants (F,H) compared to wild-type (E,G) as shown by Ptc-lacZ (E,F) or section in situ hybridization (G,H). Hedgehog signaling is reduced in vitro upon reduction of Hsd17b7
To further determine if Hsd17b7 expression affects SHH signaling, we generated primary mouse embryonic fibroblasts (MEFs) from wild-type and rudolph embryos and assessed their response to added SHH protein. In wild-type MEFs, treatment with SHH protein resulted in increased cell proliferation and increased Ptc and Gli mRNA levels, which is consistent with the known role of SHH as a mitogen in several systems and with Ptc and Gli being direct targets of SHH signaling. In contrast, the effects of SHH treatment were blunted in mutant cells (Figure 7A–7C). We also generated MEFs from wild-type;Ptc-lacZ and rudolph;Ptc-lacZ embryos to measure SHH transcriptional activity via the accumulation of β-galactosidase. In this assay, wild-type;Ptc-lacZ MEFs responded to SHH treatment with increased β-galactosidase production, whereas rudolph;Ptc-lacZ MEFs did not (Figure 7D). Together these data suggest that rudolph mutant mice have reduced intracellular signal transduction distal to the binding of SHH ligand in the SHH signaling cascade.
Fig. 7. Sonic hedgehog signal transduction is abnormal in rudolph cells. 
In vitro analysis also shows a decreased response to hedgehog protein in mouse embryonic fibroblasts (MEFs). Wild-type, but not mutant, MEFs show increased cell growth upon treatment with SHH (A; *p = 0.001 for wt; n = 4). Quantitative RT-PCR analysis also shows a reduced response of Gli1 (B; *p = 0.002 for wt, p = 0.01 for rud; n = 3) and Patched (C; *p = 0.001 for wt; n = 3) upon SHH treatment in mutant MEFs. β-galactosidase accumulates in SHH-treated wild-type;Ptc-lacZ MEFs, but not rud;Ptc-lacZ MEFs (D; *p = 0.005 for wt; n = 4). (E) PzP53Med (Ptc-lacZ;p53 medulloblastoma) cells infected and clonally selected with control plKO.1 lentivirus respond to SHH treatment with accumulation of β-galactosidase (* p = 0.07 for wt; n = 4). Two independent clonal lines infected with Hsd17b7 RNAi constructs do not respond as robustly to SHH treatment. In a parallel approach, we used Pzp53MED cells [20], which are SHH-responsive cells carrying the Ptc-lacZ allele, to assess the role of Hsd17b7 in SHH signal transduction. We used lentiviral infection followed by clonal selection with plasmids for RNAi knockdown of Hsd17b7 to create cell lines with reduced levels of Hsd17b7 (approx. 80% reduced, data not shown) and then treated the lines with recombinant SHH, as done with the MEFs. Two independent RNAi clones did not induce significant β-galactosidase production upon SHH treatment while the control cell line responded robustly (Figure 7E).
Smoothened localization to the primary cilium is unaffected in the rudolph mutants
The decreased response to SHH protein in vitro shows that the signaling defect is downstream of ligand binding to cell surface receptors. Recent data have shown that treatment with oxysterols can cause a change in the subcellular localization of PTC and SMO protein [8], which is necessary but not sufficient for activation of the SMO protein [6], [21]. The Hsd17b7 phenotype may be caused by dysregulated sterol biosynthesis affecting the localization and/or active state of the Smo receptor. We therefore examined the localization of a SMO-GFP fusion protein in wild-type and rudolph MEFs upon treatment with SHH, but found no decreased mobility of the SMO-GFP to the cilia in the mutant MEFs (Figure 8). Wild-type MEFs without SHH treatment had SMO-GFP throughout the primary cilia in only a subset of cells examined (43.3%: n = 29/67 ciliated, transfected cells in two independent experiments). Upon addition of SHH, SMO-GFP was found throughout the cilium in the majority of cells examined (89.6%; 65/73 cells). Mutant MEFs behaved similarly, with untreated cells having 38.7% SMO-GFP positive cilia (24/62) and SHH treatment leading to 86.7% (72/83) of cells with SMO-GFP throughout the cilium. In addition to the increase in cells with SMO-GFP throughout the cilium upon SHH treatment, we also note that untreated cells often had SMO-GFP largely at the base of cilium. SHH treatment resulted in very few cells showing SMO-GFP localization to the base of the cilium, but rather throughout the length of the cilium. Taken together, these data suggest that the rudolph mutation does not affect the localization of SMO within the primary cilium in response to SHH treatment.
Fig. 8. SMO-GFP localization to the primary cilium is not affected in rudolph mutant MEFs. 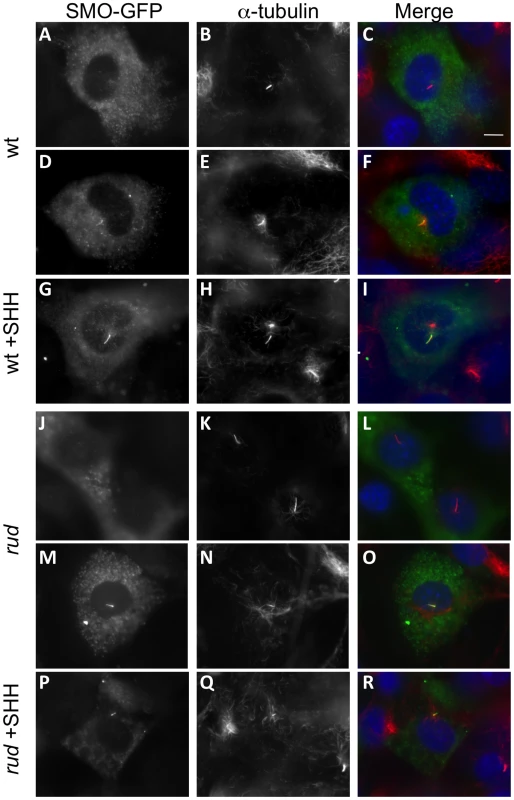
Wild-type (A–I) and rudolph (J–R) mutant MEFs were transfected with a SMO-GFP plasmid and acetylated α-tubulin immunocytochemistry was used to identify the primary cilium. SMO-GFP in untreated cells is seen outside the cilium (A–C, J–L), accumulating at the base of the axoneme (not shown), or throughout the cilium (D–F, M–O). Treatment with SHH protein localizes SMO-GFP throughout the cilium in the majority of cells observed (G–I, P–R). All images were taken at the same magnification and the scale bar in C represent 10 µm. Discussion
In this report, we describe the rudolph mouse mutant phenotype, which is caused by a mutation in the cholesterol biosynthetic enzyme Hsd17b7, the 3-ketosteroidreductase element of the sterol-4-demethylase complex. The rudolph phenotype includes marked abnormalities in the development of the nervous system and appendicular skeleton, which correlate with a decreased effectiveness of SHH signaling both in vivo and in vitro. Because sterols have been shown to affect SMO subcellular localization, we tested the movement of SMO to the primary cilium in response to SHH protein and found no defect in SMO trafficking. This suggests that the abnormality in rudolph affects the activation of SMO, resulting in a decreased response to SHH ligand binding.
Sterols in rudolph mutants
Our study of the sterols present in brain tissues from the mutant mice is consistent with reduced Hsd17b7 enzymatic function, as we observe an increase in compounds of the cholesterol biosynthetic pathway upstream of the Hsd17b7 enzyme. While no patient has yet been identified with a defect in Hsd17b7, increased levels of mono and dimethyl sterols have been reported in plasma and/or skin of patients with mutations in two other genes of the sterol-4-demethylase complex, SC4MOL (sterol C4-methyloxidase like) and NSDHL (NAD(P)H steroid dehydrogenase-like protein) [22], [23].
To explain the phenotype of the rudolph mouse we considered several possible metabolic effects, including 1) a decrease in the cellular level of cholesterol, 2) a decreased level of another product of Hsd17b7 enzymatic function, and 3) teratogenic effects of high levels of the cholesterol precursors detected in our study. Several lines of evidence suggest the last to be the major cause of the phenotype we observe. If simply a deficiency of the end-product, cholesterol, caused the rudolph phenotype, one might expect mice carrying mutations in the cholesterol biosynthetic pathway to resemble each other. This is not the case, since, despite some phenotypic overlap in adjacent disorders in the pathway, overall there are significant phenotypic differences across the spectrum of mouse models of human cholesterol biosynthetic disorders [24]. The best-characterized disorder of cholesterol biosynthesis is Smith-Lemli-Opitz syndrome [25], [26], caused by mutations in DHCR7, which encodes the 7-dehydrocholesterol reductase that converts 7-dehydrocholesterol to cholesterol [15], [27], [28]. Two mouse models with null alleles of Dhcr7 have abnormal phenotypes including cleft palate, but lack the striking brain phenotypes we found in the rudolph mutant [29], [30]. The Dhcr7 mutant mice have decreased cholesterol and increased 7-dehydrocholesterol levels in serum and tissues [29]. The enzyme immediately preceding DHCR7 is SC5DL (sterol C5-desaturase-like) which is deficient in human patients with lathosterolosis [31]. A null Scd5 allele in the mouse is a neonatal lethal with craniofacial and limb defects and decreased cholesterol similar to those of the Dhcr7-deficient mouse, but with increased levels of lathosterol (the substrate of Sc5d) in all tissues [32]. Given that the Dhcr7 and Scd5 mouse models have a significant decrease in cholesterol levels, which is not apparent in the rudolph mutant, and that their phenotypes do not resemble the rudolph phenotype, we conclude that cholesterol deficiency does not cause the distinctive embryological abnormalities of the rudolph mouse.
Nsdhl, another element of the sterol-4-demethylase complex, is the enzyme immediately preceding Hsd17b7 in the canonical cholesterol biosynthetic pathway [33]. NSDHL mutations in humans cause CHILD syndrome (congenital hemidysplasia with ichthyosiform erythroderma and limb defects), a rare X-linked dominant disorder with presumed lethality for CHILD causing alleles [34] and, with hypomorphic NSDHL mutations, CK syndrome, a form of X-linked mental retardation [22]. Mutations in Nsdhl are found in the Bare patches (Bpa) and Striated (Str) mice [35]. Analysis of tissue samples from Bpa/Str females showed an accumulation of 4-methyl and 4,4′-dimethyl sterol intermediates. Human mutations in EBP cause X-linked dominant chondrodysplasia punctata (CDPX2, Conradi-Hunermann syndrome [36]). Patients with CDPX2 usually have normal plasma total cholesterol levels but increased levels of other sterols, including 8-dehydrocholesterol and cholesta-8(9)-en-3β-ol [36], [37], [38], [39]. Tattered (Td) carries a missense mutation in the Ebp gene and phenotypically resembles the rudolph mutation most among all the known cholesterol biosynthetis mouse mutants. Male Td embryos die between E12.5 and birth and have defects in the skeleton and brain similar to those we describe here [40]. The sterol profile of heterozygous female mice includes elevated 8-dehydrocholestrol and choles-8(9)-en-3β-ol. All of these findings combined with our data suggest a model in which the accumulation of specific sterols leads to the defects we observe. One of the effects these inhibitory sterols may be to dampen the intracellular response to Shh signaling.
Rudolph mutants show similarities to other loss-of-function Shh phenotypes
Although rudolph mutants lack some of the classic features of the Shh null mice, such as holoprosencephaly, other more specific ablations of SHH signaling have some features resembling the rudolph phenotype. In particular, the skeletal defects we observe in the rudolph mutant are similar to those described in the Indian hedgehog (Ihh) and dispatched-1 (Disp1) loss of function mice and the conditional ablation of Smo from the developing skeleton [41], [42], [43]. As Ihh is the most active Hh ligand in development of long bones, we suggest that the similarities between rud and the HH-signaling mutants reflect the conservation of intracellular signaling transduction mechanisms between the different Hh ligands, and that these defects are due to an insufficient response to secreted IHH in the cartilage. In addition, the disorganized retina in rud mutants also resembles that seen in embryos with an ablation of Shh using a Thy1-Cre [44]. The role of Shh and Ihh as mitogens in retinal neuroblast proliferation has been established [45], and Shh, Ihh and Gli1 are expressed in retina at E12 and E13 [44], [46]. The difference in timing between the cortical and retinal defects (rudolph retinal molecular defects are seen at E14.5, but not E12.5, Figure S4) is consistent with the later expression pattern of Shh signaling components in the retina as compared to forebrain tissue.
The developing rudolph forebrain phenotype has both similarities to and differences from the known effects of Shh loss of function. The decreased cell proliferation and increased apoptosis we find are completely consistent with a role for Shh as a mitogen for the developing neural tissue and with the observations that blocking SHH function can lead to cell death [47], and that conditional ablation of Smo throughout the cortex by E9 using the Foxg1-Cre leads to increased cell death [48]. Emx1-Cre ablations of Shh and Smo in the dorsal telencephalon by E10 cause a smaller telencephalon featuring reduced proliferation and neuronal differentiation with increased cell death [49]. However, the striking disorganization of the cortex in rudolph mutants resembles more a loss of polarity phenotype, such as the cortical ablation of numb and numb-like [50]. Because Shh loss of function has not been demonstrated to directly affect polarity, we suggest that abnormalities of cortical signaling mechanisms other than Shh must be disrupted in the rudolph cortex to explain some of the developmental abnormalities of the CNS we observe. Because cortical dysplasia is not characteristic of any of the known human disorders of cholesterol biosynthesis, the extremely high CNS level of zymosterone, normally only a trace sterol in the brain, suggests that zymosterone or other 3-ketosterols in the rud brain could have a direct toxic effect or could otherwise impair neuronal differentiation.
Rudolph is a hypomorphic allele of Hsd17b7
Two reports have recently described the phenotypes of Hsd17b7 null allele mice [18], [19]. These embryos have major morphological abnormalities by E10.5, precluding direct comparison to the phenotypes studied here. The forebrain did appear smaller in the Hsd17b7 homozygous null embryos, and development does not proceed past E9.5. A sterol analysis performed in these mutants demonstrated increased Hsd17b7 enzyme substrates and unchanged cholesterol levels, similar to the results we report [19]. Maternal supply of cholesterol was also suggested to account for the normal embryonic cholesterol levels. Expression of Shh and Ptc was examined in the Hsd17b7 homozygous null embryos at E8.5, but the domain and levels of expression did not differ from wild-type [19].
The phenotypic differences between the rudolph and Shh mutants may be due to the hypomorphic nature of the rudolph allele. However, we also note that the rudolph phenotype in the CNS begins to emerge around E12.5, which is the time when the blood-brain barrier forms and synthesis of cholesterol within the CNS becomes separated from non-neural cholesterol synthesis [51], [52], [53], [54]. The developmental consequences of reduced sterol concentrations in the early embryo could be mitigated by the maternal circulation in view of evidence that, in rodents, substantial amounts of maternal cholesterol can be transported to the fetus through the placental-fetal interface [55], thus possibly compensating for a lack of early Hsd17b7 function in the initial patterning stages of embryonic development. We therefore propose that formation of the blood-brain barrier creates a neurodevelopmental compartment absolutely requiring endogenous Hsd17b7 function, which, when absent, results in the severe phenotypes we describe here.
Sterols in Hh signaling
Perhaps the most intriguing aspect of this study is that it is the first in vivo validation of several recent studies suggesting an intracellular role for sterols in SHH signaling [8], [11], [12]. These cholesterol intermediates have been demonstrated to have a role in the transduction of HH ligand signaling as well as the subcellular localization of HH signaling components. The fact that the mutant MEFs demonstrate normal Smoothened localization to the cilium, but a compromised response to SHH ligand, suggests that normal sterol concentrations are required for proper activation of Smoothened [6]. Alternatively, an inhibitory sterol may be present at increased levels, preventing the activation of the pathway.
Treatment with statins is a well-accepted method for lowering cholesterol levels in human patients by inhibiting HMG-CoA reductase (Hmgcr), the rate-limiting step in cholesterol biosynthesis. Statin treatment could also have significant effects on local concentrations of oxysterols generated from intermediates in the cholesterol biosynthetic pathway further downstream. Mouse Hmgcr mutants, which should genetically mimic a total block in the pathway at the site of action of statins, are not viable past implantation [56] and are therefore not informative in this regard. It will be important to study further the role of cholesterol intermediates and metabolites in various physiological settings and signaling paradigms. In doing so, we may find that statin treatments may be having unintended consequences in human health in sites of adult Hh activity, including adult neurogenesis.
Materials and Methods
Mouse husbandry and genotyping
Rudolph mice were originally generated by ENU mutagenesis of A/J mice and then outcrossed to FVB/J mice (both obtained from Jackson Labs, Bar Harbor, ME). Initial mapping was done with a whole genome SNP panel similar to one we previously described [17], and the mutation mapped to a 19.6 Mb interval on chromosome 1. Exon directed sequencing (including some flanking intronic sequence to identify mutations potentially affecting splicing) identified the rudolph mutation. Genotyping is done with either D1Mit454 or D1Mit524 microsatellite markers depending on strains involved. We maintained the colony with a combination of intercrossing and outcrossing to FVB. The C57BL/6J Ptc1-lacZ mouse was obtained from the Jackson laboratory and intercrossed with rud heterozygous mice;Ptc1-lacZ genotyping was done with standard lacZ primers. We have also performed backcrosses of the rud allele to mice on C57BL/6J and 129X1/SvJ backgrounds. All animals were housed in accordance with the Harvard Medical School ARCM regulations. Timed matings were checked for signs of copulation in the morning; vaginal plugs were noted and noon of that day was established as embryonic day (E) 0.5.
Histology and immunohistochemistry
Embryos used for histological analysis were fixed with Bouin's fixative for at least forty-eight hours and processed for paraffin embedding using a Leica TP1020 automated tissue processor. Sections were cut at a thickness of 14 µm and stained with hematoxylin and eosin using standard techniques. Microscopy was done with a Leica DC500 or Zeiss AxioImager with ApoTome. TUNEL assay was performed with the In Situ Cell Detection Kit, TMR Red, following the manufacturer's instructions (Roche). BrdU labeling was done with a BrdU Labeling and Injection Kit (Roche). The TuJI antibody (SIGMA) was used at 1∶500 for 2 hours at room temperature on paraffin sections with citrate buffer antigen retrieval. Neural tube immunohistochemistry was performed using standard methods with antibodies from the Developmental Studies Hybridoma Bank.
Skeletal measurements
To measure the size of the skeletal elements, embryos were stained for cartilage and bone using standard methods [57] and photographed. The length of each element was calculated using NIH Image J software, and units were converted to mm using standards.
In vitro analysis of SHH signaling
Mouse embryonic fibroblasts were generated using standard methods and plated at a density of 20,000 cells/cm2 in the presence or absence of 200 ng/mL SHH amino terminal peptide (R&D Systems). Cell number was determined with the CyQuant Cell Proliferation Assay (Invitrogen), and β-galactosidase production was measured with the Galacto-Light Plus System (Applied Biosystems). Assays were performed 48 hours after plating. Cell growth experiments were done with an initial culture of 6,000 cells in a 96-well plate.
RNAi clones
Lentiviral particles were made via transfection of 293T cells with plasmids including a plKO.1 control and a validated RNAi construct against mouse Hsd17b7 (Open Biosystems, Huntsville, AL; clone TRCN0000041646). PzP53Med cells [20] were infected with 293T supernatant containing lentivirus. After puromycin selection, resistant cells were plated at clonal density and individual clones were isolated, maintained and analyzed with qRT-PCR for Hsd17b7 levels. Control and knock-down clones were treated with SHH protein as described above.
RT-PCR analysis
Total RNA from either brains or MEF cultures was prepared with TRIZOL (Invitrogen) and cDNA was made with qScript cDNA synthesis kit (Quanta) or the SuperScript RTIII system (Invitrogen). Hsd17b7 transcripts were analyzed with both random hexamer primed cDNA and gene specific primed cDNA synthesis (primer: TTTTGGTACCTCAGCTCGGGTGATCCGATTTCTG). Hsd17b7 transcripts were analyzed with primers amplifying exons 6–8 (F: TCTGTATTCCAGTGTGATGTGC; R: CTTTTGGCCCGTGACGTAAT; 259 bp) or exons 6–7 (F: TCTGTATTCCAGTGTGATGTGC; R: CCACATTATGGGTAGGAGCAA ; 100 bp). Quantitative RT-PCR was done on a BioRad iCycler using either total RNA with Taqman probes (Applied BioSystems) or cDNA with Perfecta SYBR Green SuperMix (Quanta). SYBR-GREEN probes used were: Ptc-F (CCTGCAAACCATGTTCCAGTT ), Ptc-R (TCGTAGCCCCTGAAGTGTTCA) Gli1-F (CCAAGCCAACTTTATGTCAGGG), Gli1-R (AGCCCGCTTCTTTGTTAATTTGA), Gapdh-F (ACTCCACTCACGGCAAATTC), and Gapdh-R (TCTCCATGGTGGTGAAGACA).
In situ hybridization and LacZ staining
Section mount in situ hybridization was done as previously described [58] with hybridization at 60 degrees and with BM Purple (Roche) for visualization of riboprobes. Probes used are published: Gli1 [59] and Ptc [60]. Embryos were stained with lacZ using standard protocols [57] and then processed for paraffin histology as described above. Older embryos were fixed in 4% paraformaldehyde, cryoembedded in OCT, sectioned at 20 µm and stained on slides.
Westerns
Embryos were homogenized in 1% SDS Lysis Buffer and protein extracts were run on a 10% polyacrylamide gel. A rabbit polyclonal antibody was used for Hsd17b7 (1∶1000, overnight at 4 degrees C), and a mouse monoclonal anti-actin antibody (1∶5000, SIGMA, 60 minutes at room temperature) was used as a loading control.
GFP fusion proteins and transfections
Full-length mouse Hsd17b7 from wild-type and mutant tissue was initially cloned into a pENTR/D/TOPO vector (Invitrogen) and then into the pcDNA-DEST47 vector (Invitrogen). DNA for either Hsd17b7-GFP or rud-GFP was co-transfected with Sec61β-mCherry (gift of T. Kirchhausen) into NIH3T3 cells using Fugene (Roche) following manufacturer's instructions. 48 hours after transfection, cells were fixed with 4% paraformaldehyde and counter-stained with DAPI. SMO localization within ciliated MEFs was observed by transfection of 20–30% confluent MEFs plated on 0.4% gelatin or poly-lysine coated coverslips in 24-well plates with the pBabePuro-A1∶Smo∶GFP plasmid ([9], gift of A. McMahon). Confluent cells were treated overnight with SHH peptide 48 hours after transfection. Immunocytochemistry was done by fixing with 4% paraformaldehyde/0.2 TritonX-100 for 20 minutes and blocking with 1% BSA for 60 minutes. Antibodies used were acetylated α-tubulin (SIGMA) at 1∶2000 for 60 minutes at room temperature and Goat anti-mouse Alexa Flour 594 (Invitrogen) at 1∶500 for 30 minutes at room temperature. Cells were mounted with VectaShield (Vector Laboratories) and imaged on a Zeiss AxioImager.
Sterol analysis
Sterols were extracted from brain tissue as previously described with the addition of sterol-specific ions for the compounds of interest to this study [61]. Sterol levels are reported as a fraction of total sterols.
Supporting Information
Zdroje
1. FuccilloMJoynerALFishellG 2006 Morphogen to mitogen: the multiple roles of hedgehog signalling in vertebrate neural development. Nat Rev Neurosci 7 772 783
2. InghamPWMcMahonAP 2001 Hedgehog signaling in animal development: paradigms and principles. Genes Dev 15 3059 3087
3. Wechsler-ReyaRScottMP 2001 The developmental biology of brain tumors. Annu Rev Neurosci 24 385 428
4. EggenschwilerJTAndersonKV 2007 Cilia and developmental signaling. Annu Rev Cell Dev Biol 23 345 373
5. CorbitKCAanstadPSinglaVNormanARStainierDY 2005 Vertebrate Smoothened functions at the primary cilium. Nature
6. RohatgiRMilenkovicLCorcoranRBScottMP 2009 Hedgehog signal transduction by Smoothened: pharmacologic evidence for a 2-step activation process. Proc Natl Acad Sci U S A 106 3196 3201
7. HuangXLitingtungYChiangC 2007 Ectopic sonic hedgehog signaling impairs telencephalic dorsal midline development: implication for human holoprosencephaly. Hum Mol Genet 16 1454 1468
8. RohatgiRMilenkovicLScottMP 2007 Patched1 regulates hedgehog signaling at the primary cilium. Science 317 372 376
9. WangYZhouZWalshCTMcMahonAP 2009 Selective translocation of intracellular Smoothened to the primary cilium in response to Hedgehog pathway modulation. Proc Natl Acad Sci U S A 106 2623 2628
10. CooperMKPorterJAYoungKEBeachyPA 1998 Teratogen-mediated inhibition of target tissue response to Shh signaling. Science 280 1603 1607
11. CooperMKWassifCAKrakowiakPATaipaleJGongR 2003 A defective response to Hedgehog signaling in disorders of cholesterol biosynthesis. Nat Genet 33 508 513
12. CorcoranRBScottMP 2006 Oxysterols stimulate Sonic hedgehog signal transduction and proliferation of medulloblastoma cells. Proc Natl Acad Sci U S A 103 8408 8413
13. DwyerJRSeverNCarlsonMNelsonSFBeachyPA 2007 Oxysterols are novel activators of the hedgehog signaling pathway in pluripotent mesenchymal cells. J Biol Chem 282 8959 8968
14. HermanGE 2003 Disorders of cholesterol biosynthesis: prototypic metabolic malformation syndromes. Hum Mol Genet 12 Spec No 1 R75 88
15. WassifCAMaslenCKachilele-LinjewileSLinDLinckLM 1998 Mutations in the human sterol delta7-reductase gene at 11q12–13 cause Smith-Lemli-Opitz syndrome. Am J Hum Genet 63 55 62
16. MarijanovicZLaubnerDMollerGGegeCHusenB 2003 Closing the gap: identification of human 3-ketosteroid reductase, the last unknown enzyme of mammalian cholesterol biosynthesis. Mol Endocrinol 17 1715 1725
17. MoranJLBoltonADTranPVBrownADwyerND 2006 Utilization of a whole genome SNP panel for efficient genetic mapping in the mouse. Genome Res 16 436 440
18. ShehuAMaoJGiboriGBHalperinJLeJ 2008 Prolactin receptor-associated protein/17beta-hydroxysteroid dehydrogenase type 7 gene (Hsd17b7) plays a crucial role in embryonic development and fetal survival. Mol Endocrinol 22 2268 2277
19. JokelaHRantakariPLamminenTStraussLOlaR 2010 Hydroxysteroid (17beta) dehydrogenase 7 activity is essential for fetal de novo cholesterol synthesis and for neuroectodermal survival and cardiovascular differentiation in early mouse embryos. Endocrinology 151 1884 1892
20. BermanDMKarhadkarSSHallahanARPritchardJIEberhartCG 2002 Medulloblastoma growth inhibition by hedgehog pathway blockade. Science 297 1559 1561
21. WilsonCWChenMHChuangPT 2009 Smoothened adopts multiple active and inactive conformations capable of trafficking to the primary cilium. PLoS ONE 4 e5182 doi:10.1371/journal.pone.0005182
22. McLarrenKWSeversonTMdu SouichCStocktonDWKratzLE 2010 Hypomorphic temperature-sensitive alleles of NSDHL cause CK syndrome. Am J Hum Genet 87 905 914
23. HeMKratzLEMichelJJVallejoANFerrisL 2011 Mutations in the human SC4MOL gene encoding a methyl sterol oxidase cause psoriasiform dermatitis, microcephaly, and developmental delay. J Clin Invest
24. PorterFDHermanGE Malformation syndromes caused by disorders of cholesterol synthesis. J Lipid Res
25. IronsMEliasERSalenGTintGSBattaAK 1993 Defective cholesterol biosynthesis in Smith-Lemli-Opitz syndrome. Lancet 341 1414
26. TintGSIronsMEliasERBattaAKFriedenR 1994 Defective cholesterol biosynthesis associated with the Smith-Lemli-Opitz syndrome. N Engl J Med 330 107 113
27. FitzkyBUWitsch-BaumgartnerMErdelMLeeJNPaikYK 1998 Mutations in the Delta7-sterol reductase gene in patients with the Smith-Lemli-Opitz syndrome. Proc Natl Acad Sci U S A 95 8181 8186
28. MoebiusFFFitzkyBULeeJNPaikYKGlossmannH 1998 Molecular cloning and expression of the human delta7-sterol reductase. Proc Natl Acad Sci U S A 95 1899 1902
29. WassifCAZhuPKratzLKrakowiakPABattaileKP 2001 Biochemical, phenotypic and neurophysiological characterization of a genetic mouse model of RSH/Smith–Lemli–Opitz syndrome. Hum Mol Genet 10 555 564
30. FitzkyBUMoebiusFFAsaokaHWaage-BaudetHXuL 2001 7-Dehydrocholesterol-dependent proteolysis of HMG-CoA reductase suppresses sterol biosynthesis in a mouse model of Smith-Lemli-Opitz/RSH syndrome. J Clin Invest 108 905 915
31. Brunetti-PierriNCorsoGRossiMFerrariPBalliF 2002 Lathosterolosis, a novel multiple-malformation/mental retardation syndrome due to deficiency of 3beta-hydroxysteroid-delta5-desaturase. Am J Hum Genet 71 952 958
32. KrakowiakPAWassifCAKratzLCozmaDKovarovaM 2003 Lathosterolosis: an inborn error of human and murine cholesterol synthesis due to lathosterol 5-desaturase deficiency. Hum Mol Genet 12 1631 1641
33. RisleyJM 2002 Cholesterol Biosynthesis: Lanosterol to Cholesterol. Journal of Chemical Education 79 377 384
34. KonigAHappleRBornholdtDEngelHGrzeschikKH 2000 Mutations in the NSDHL gene, encoding a 3beta-hydroxysteroid dehydrogenase, cause CHILD syndrome. Am J Med Genet 90 339 346
35. LiuXYDangelAWKelleyRIZhaoWDennyP 1999 The gene mutated in bare patches and striated mice encodes a novel 3beta-hydroxysteroid dehydrogenase. Nat Genet 22 182 187
36. BravermanNLinPMoebiusFFObieCMoserA 1999 Mutations in the gene encoding 3 beta-hydroxysteroid-delta 8, delta 7-isomerase cause X-linked dominant Conradi-Hunermann syndrome. Nat Genet 22 291 294
37. HermanGEKelleyRIPurezaVSmithDKopaczK 2002 Characterization of mutations in 22 females with X-linked dominant chondrodysplasia punctata (Happle syndrome). Genet Med 4 434 438
38. HasCSeedorfUKannenbergFBruckner-TudermanLFolkersE 2002 Gas chromatography-mass spectrometry and molecular genetic studies in families with the Conradi-Hunermann-Happle syndrome. J Invest Dermatol 118 851 858
39. WhittockNVIzattLMannAHomfrayTBennettC 2003 Novel mutations in X-linked dominant chondrodysplasia punctata (CDPX2). J Invest Dermatol 121 939 942
40. DerryJMGormallyEMeansGDZhaoWMeindlA 1999 Mutations in a delta 8-delta 7 sterol isomerase in the tattered mouse and X-linked dominant chondrodysplasia punctata. jderry@immunex.com. Nat Genet 22 286 290
41. LongFJoengKSXuanSEfstratiadisAMcMahonAP 2006 Independent regulation of skeletal growth by Ihh and IGF signaling. Dev Biol 298 327 333
42. St-JacquesBHammerschmidtMMcMahonAP 1999 Indian hedgehog signaling regulates proliferation and differentiation of chondrocytes and is essential for bone formation. Genes Dev 13 2072 2086
43. TsiairisCDMcMahonAP 2008 Disp1 regulates growth of mammalian long bones through the control of Ihh distribution. Dev Biol 317 480 485
44. WangYPDakuboGHowleyPCampsallKDMazarolleCJ 2002 Development of normal retinal organization depends on Sonic hedgehog signaling from ganglion cells. Nat Neurosci 5 831 832
45. WallaceVARaffMC 1999 A role for Sonic hedgehog in axon-to-astrocyte signalling in the rodent optic nerve. Development 126 2901 2909
46. WangYDakuboGDThurigSMazerolleCJWallaceVA 2005 Retinal ganglion cell-derived sonic hedgehog locally controls proliferation and the timing of RGC development in the embryonic mouse retina. Development 132 5103 5113
47. AhlgrenSCBronner-FraserM 1999 Inhibition of sonic hedgehog signaling in vivo results in craniofacial neural crest cell death. Curr Biol 9 1304 1314
48. FuccilloMRalluMMcMahonAPFishellG 2004 Temporal requirement for hedgehog signaling in ventral telencephalic patterning. Development 131 5031 5040
49. KomadaMSaitsuHKinboshiMMiuraTShiotaK 2008 Hedgehog signaling is involved in development of the neocortex. Development 135 2717 2727
50. LiHSWangDShenQSchonemannMDGorskiJA 2003 Inactivation of Numb and Numblike in embryonic dorsal forebrain impairs neurogenesis and disrupts cortical morphogenesis. Neuron 40 1105 1118
51. JurevicsHMorellP 1995 Cholesterol for synthesis of myelin is made locally, not imported into brain. J Neurochem 64 895 901
52. TintGSYuHShangQXuGPatelSB 2006 The use of the Dhcr7 knockout mouse to accurately determine the origin of fetal sterols. J Lipid Res 47 1535 1541
53. JurevicsHAKidwaiFZMorellP 1997 Sources of cholesterol during development of the rat fetus and fetal organs. J Lipid Res 38 723 733
54. EdmondJKorsakRAMorrowJWTorok-BothGCatlinDH 1991 Dietary cholesterol and the origin of cholesterol in the brain of developing rats. J Nutr 121 1323 1330
55. LindegaardMLWassifCAVaismanBAmarMWasmuthEV 2008 Characterization of placental cholesterol transport: ABCA1 is a potential target for in utero therapy of Smith-Lemli-Opitz syndrome. Hum Mol Genet 17 3806 3813
56. OhashiKOsugaJTozawaRKitamineTYagyuH 2003 Early embryonic lethality caused by targeted disruption of the 3-hydroxy-3-methylglutaryl-CoA reductase gene. J Biol Chem 278 42936 42941
57. HoganBBeddingtonRCostantiniFLacyE 1994 Manipulating the Mouse Embryo New York Cold Spring Harbor Press
58. MurtaughLCChyungJHLassarAB 1999 Sonic hedgehog promotes somitic chondrogenesis by altering the cellular response to BMP signaling. Genes Dev 13 225 237
59. HuiCCSlusarskiDPlattKAHolmgrenRJoynerAL 1994 Expression of three mouse homologs of the Drosophila segment polarity gene cubitus interruptus, Gli, Gli-2, and Gli-3, in ectoderm - and mesoderm-derived tissues suggests multiple roles during postimplantation development. Dev Biol 162 402 413
60. GoodrichLVJohnsonRLMilenkovicLMcMahonJAScottMP 1996 Conservation of the hedgehog/patched signaling pathway from flies to mice: induction of a mouse patched gene by Hedgehog. Genes and Development 10 301 312
61. KelleyRI 1995 Diagnosis of Smith-Lemli-Opitz syndrome by gas chromatography/mass spectrometry of 7-dehydrocholesterol in plasma, amniotic fluid and cultured skin fibroblasts. Clin Chim Acta 236 45 58
Štítky
Genetika Reprodukční medicína
Článek Produces a Long Noncoding RNA and Has General Functions in the Germline, Stem Cells, and BrainČlánek Inference of Relationships in Population Data Using Identity-by-Descent and Identity-by-StateČlánek A Genome-Wide Meta-Analysis of Six Type 1 Diabetes Cohorts Identifies Multiple Associated LociČlánek Retrotransposon-Induced Heterochromatin Spreading in the Mouse Revealed by Insertional PolymorphismsČlánek Essential Roles of BCCIP in Mouse Embryonic Development and Structural Stability of ChromosomesČlánek The Receptor Tyrosine Kinase Alk Controls Neurofibromin Functions in Drosophila Growth and LearningČlánek PCNA Ubiquitination Is Important, But Not Essential for Translesion DNA Synthesis in Mammalian CellsČlánek Genome-Wide Association Study Identifies Four Loci Associated with Eruption of Permanent Teeth
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2011 Číslo 9- Akutní intermitentní porfyrie
- Farmakogenetické testování pomáhá předcházet nežádoucím efektům léčiv
- Růst a vývoj dětí narozených pomocí IVF
- Pilotní studie: stres a úzkost v průběhu IVF cyklu
- Intrauterinní inseminace a její úspěšnost
-
Všechny články tohoto čísla
- Temporal Trends in Results Availability from Genome-Wide Association Studies
- Association of eGFR-Related Loci Identified by GWAS with Incident CKD and ESRD
- Genetic Variants at Chromosomes 2q35, 5p12, 6q25.1, 10q26.13, and 16q12.1 Influence the Risk of Breast Cancer in Men
- Large-Scale Gene-Centric Analysis Identifies Novel Variants for Coronary Artery Disease
- Genetic Association for Renal Traits among Participants of African Ancestry Reveals New Loci for Renal Function
- Transcriptome Kinetics Is Governed by a Genome-Wide Coupling of mRNA Production and Degradation: A Role for RNA Pol II
- Conserved Regulation of p53 Network Dosage by MicroRNA–125b Occurs through Evolving miRNA–Target Gene Pairs
- Heterozygous Mutations of Are Associated with an Increased Risk of Isolated Metopic Craniosynostosis in Humans and Mice
- Study of FoxA Pioneer Factor at Silent Genes Reveals Rfx-Repressed Enhancer at and a Potential Indicator of Esophageal Adenocarcinoma Development
- Cholesterol Metabolism Is Required for Intracellular Hedgehog Signal Transduction
- Produces a Long Noncoding RNA and Has General Functions in the Germline, Stem Cells, and Brain
- Age-Dependent Recombination Rates in Human Pedigrees
- Sequence Conservation and Functional Constraint on Intergenic Spacers in Reduced Genomes of the Obligate Symbiont
- Sex Chromosome Mosaicism and Hybrid Speciation among Tiger Swallowtail Butterflies
- A Negative Feedback Loop That Limits the Ectopic Activation of a Cell Type–Specific Sporulation Sigma Factor of
- Phased Whole-Genome Genetic Risk in a Family Quartet Using a Major Allele Reference Sequence
- Mutations in or near the Transmembrane Domain Alter PMEL Amyloid Formation from Functional to Pathogenic
- Inactivation of Alters Melanosome Shape But Has Only a Subtle Effect on Visible Pigmentation
- Novel Interactions between Actin and the Proteasome Revealed by Complex Haploinsufficiency
- Germline Genetic Variants Disturbing the /LIN28 Double-Negative Feedback Loop Alter Breast Cancer Susceptibility
- Separation of Recombination and SOS Response in RecA Suggests LexA Interaction Sites
- Inference of Relationships in Population Data Using Identity-by-Descent and Identity-by-State
- Misregulation of Scm3p/HJURP Causes Chromosome Instability in and Human Cells
- A Noncoding Point Mutation of Causes Multiple Developmental Malformations and Obesity in Twirler Mice
- The Evolutionarily Conserved Longevity Determinants HCF-1 and SIR-2.1/SIRT1 Collaborate to Regulate DAF-16/FOXO
- A Genome-Wide Metabolic QTL Analysis in Europeans Implicates Two Loci Shaped by Recent Positive Selection
- Bacterial Communities of Diverse Species: Ecological Context of a Host–Microbe Model System
- A Genome-Wide Meta-Analysis of Six Type 1 Diabetes Cohorts Identifies Multiple Associated Loci
- Elongator Complex Influences Telomeric Gene Silencing and DNA Damage Response by Its Role in Wobble Uridine tRNA Modification
- Elevated Proteasome Capacity Extends Replicative Lifespan in
- Genome-Wide Analysis of Heteroduplex DNA in Mismatch Repair–Deficient Yeast Cells Reveals Novel Properties of Meiotic Recombination Pathways
- MicroRNA Predictors of Longevity in
- An Iterative Genetic and Dynamical Modelling Approach Identifies Novel Features of the Gene Regulatory Network Underlying Melanocyte Development
- Atypical AT Skew in Firmicute Genomes Results from Selection and Not from Mutation
- Retrotransposon-Induced Heterochromatin Spreading in the Mouse Revealed by Insertional Polymorphisms
- Genomic Analysis of QTLs and Genes Altering Natural Variation in Stochastic Noise
- The Abnormal Phenotypes of Cartilage and Bone in Calcium-Sensing Receptor Deficient Mice Are Dependent on the Actions of Calcium, Phosphorus, and PTH
- Cell Type–Specific Transcriptome Analysis Reveals a Major Role for and miR-200b in Mouse Inner Ear Morphogenesis
- Essential Roles of BCCIP in Mouse Embryonic Development and Structural Stability of Chromosomes
- IAP1-Mediated Ubiquitylation Controls Activation of the Initiator Caspase DRONC Independent of Protein Degradation
- VANG-1 and PRKL-1 Cooperate to Negatively Regulate Neurite Formation in
- The Receptor Tyrosine Kinase Alk Controls Neurofibromin Functions in Drosophila Growth and Learning
- Comparative and Functional Genomics of PD630 for Biofuels Development
- Identification of Type 1 Diabetes–Associated DNA Methylation Variable Positions That Precede Disease Diagnosis
- PCNA Ubiquitination Is Important, But Not Essential for Translesion DNA Synthesis in Mammalian Cells
- Genetic Effects at Pleiotropic Loci Are Context-Dependent with Consequences for the Maintenance of Genetic Variation in Populations
- Genome-Wide Association Study Identifies Four Loci Associated with Eruption of Permanent Teeth
- Bmp and Nodal Independently Regulate Expression to Maintain Unilateral Nodal Activity during Left-Right Axis Specification in Zebrafish
- Inter-Allelic Prion Propagation Reveals Conformational Relationships among a Multitude of [] Strains
- Emergence and Modular Evolution of a Novel Motility Machinery in Bacteria
- Histone Methyltransferase MET-2 Shields the Male X Chromosome from Checkpoint Machinery and Mediates Meiotic Sex Chromosome Inactivation
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Retrotransposon-Induced Heterochromatin Spreading in the Mouse Revealed by Insertional Polymorphisms
- The Evolutionarily Conserved Longevity Determinants HCF-1 and SIR-2.1/SIRT1 Collaborate to Regulate DAF-16/FOXO
- Genome-Wide Analysis of Heteroduplex DNA in Mismatch Repair–Deficient Yeast Cells Reveals Novel Properties of Meiotic Recombination Pathways
- Association of eGFR-Related Loci Identified by GWAS with Incident CKD and ESRD
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



