-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Variation, Sex, and Social Cooperation: Molecular Population Genetics of the Social Amoeba
Dictyostelium discoideum is a eukaryotic microbial model system for multicellular development, cell–cell signaling, and social behavior. Key models of social evolution require an understanding of genetic relationships between individuals across the genome or possibly at specific genes, but the nature of variation within D. discoideum is largely unknown. We re-sequenced 137 gene fragments in wild North American strains of D. discoideum and examined the levels and patterns of nucleotide variation in this social microbial species. We observe surprisingly low levels of nucleotide variation in D. discoideum across these strains, with a mean nucleotide diversity (π) of 0.08%, and no strong population stratification among North American strains. We also do not find any clear relationship between nucleotide divergence between strains and levels of social dominance and kin discrimination. Kin discrimination experiments, however, show that strains collected from the same location show greater ability to distinguish self from non-self than do strains from different geographic areas. This suggests that a greater ability to recognize self versus non-self may arise among strains that are more likely to encounter each other in nature, which would lead to preferential formation of fruiting bodies with clonemates and may prevent the evolution of cheating behaviors within D. discoideum populations. Finally, despite the fact that sex has rarely been observed in this species, we document a rapid decay of linkage disequilibrium between SNPs, the presence of recombinant genotypes among natural strains, and high estimates of the population recombination parameter ρ. The SNP data indicate that recombination is widespread within D. discoideum and that sex as a form of social interaction is likely to be an important aspect of the life cycle.
Published in the journal: . PLoS Genet 6(7): e32767. doi:10.1371/journal.pgen.1001013
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1001013Summary
Dictyostelium discoideum is a eukaryotic microbial model system for multicellular development, cell–cell signaling, and social behavior. Key models of social evolution require an understanding of genetic relationships between individuals across the genome or possibly at specific genes, but the nature of variation within D. discoideum is largely unknown. We re-sequenced 137 gene fragments in wild North American strains of D. discoideum and examined the levels and patterns of nucleotide variation in this social microbial species. We observe surprisingly low levels of nucleotide variation in D. discoideum across these strains, with a mean nucleotide diversity (π) of 0.08%, and no strong population stratification among North American strains. We also do not find any clear relationship between nucleotide divergence between strains and levels of social dominance and kin discrimination. Kin discrimination experiments, however, show that strains collected from the same location show greater ability to distinguish self from non-self than do strains from different geographic areas. This suggests that a greater ability to recognize self versus non-self may arise among strains that are more likely to encounter each other in nature, which would lead to preferential formation of fruiting bodies with clonemates and may prevent the evolution of cheating behaviors within D. discoideum populations. Finally, despite the fact that sex has rarely been observed in this species, we document a rapid decay of linkage disequilibrium between SNPs, the presence of recombinant genotypes among natural strains, and high estimates of the population recombination parameter ρ. The SNP data indicate that recombination is widespread within D. discoideum and that sex as a form of social interaction is likely to be an important aspect of the life cycle.
Introduction
The origin and maintenance of social cooperation is one of the most intriguing aspects of evolutionary history [1]. The evolution of cooperative interactions underlie some of the major evolutionary transitions, giving rise to phenomena as diverse as multicellularity [2], microbial sociality [3] and the development of animal societies [1]. For cooperation to be favored, the individual providing the assistance must gain fitness either directly (as in mutualism) or indirectly when it helps a relative, and thereby passes on more genes than it could alone [1], [4]. Kin selection theory indicates when altruistic helping should evolve, and predicts a dependence on the genetic relatedness among interactants. Social interactions may thus depend on patterns of genetic diversity either across the genome or at key loci and a molecular population genetic study of nucleotide variation can provide insights into the nature of social dynamics within species.
Dictyostelium discoideum has been a key model system for understanding the genetic basis of social behavior as well as multicellular development and cell-cell signaling [5], [6]. D. discoideum is a soil amoeba mostly distributed in temperate regions of the Northern hemisphere. The 34 Mb genome has been completely sequenced and is organized into six chromosomes that harbor ∼12,500 protein-coding genes [6]. Moreover, various molecular approaches are available to dissect molecular and cellular functions [5], facilitating a genetic analysis of cooperative behavior.
D. discoideum is notable for two social phases in its life cycle, one of which has received much more attention than the other (see Figure 1). A well-studied social phase of the life cycle occurs when starvation conditions lead to a transition from solitary lives as haploid single cells into swarming aggregates that eventually develop into multicellular fruiting bodies composed of haploid spores and stalk cells (see Figure 1) [7]–[12]. Aggregation of individual amoebae is mediated by the chemoattractant cAMP, which triggers the chemotactic movement of cells, the polarized secretion of more cAMP for signal relay, and the initiation of changes in developmental gene expression [5]. Up to 105 individual cells aggregate, leading to the formation of a fruiting body, with the sorus sitting on the top of the stalk. This structure contains cells encapsulated as spores that await dispersal and germination when conditions are favorable for single-cell growth. In this cooperative asexual structure, stalk cells perish and thus exhibit altruistic behavior towards the viable spores which they lift above the substrate where they are more likely to be dispersed [12].
Fig. 1. The life cycle of Dictyostelium discoideum. 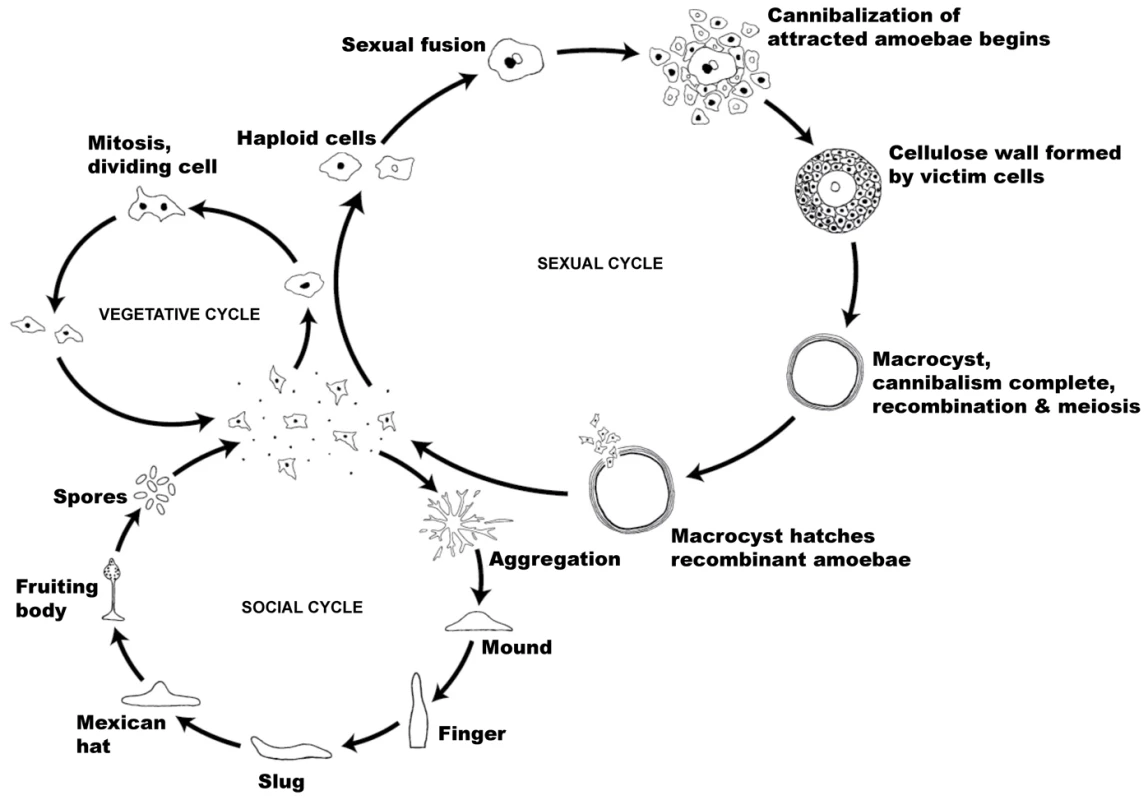
Most of its life, this haploid social amoeba undergoes the vegetative cycle, preying upon bacteria in the soil, and periodically dividing mitotically. When food is scarce, either the sexual cycle or the social cycle begins. Under the social cycle, amoebae aggregate and form a motile slug, which ultimately forms a fruiting body. Under the sexual cycle, amoebae aggregate and two cells of opposite mating types fuse, and then begin consuming the other attracted cells. Before they are consumed, some of the prey cells form a cellulose wall around the entire group. When cannibalism is complete, the giant diploid cell or macrocyst eventually undergoes recombination and meiosis. The differentiation of cell fate leads to cooperation as well as competition between individuals from distinct clones and dramatically increases the complexity of this system in the context of social behavior. Although social cooperation in D. discoideum strains has been largely studied in cells with the same genetic background, clones with different genetic backgrounds are known to co-occur in the field [13], [14]. It has been demonstrated, for example, that cheating behavior is commonly seen in these chimeric fruiting bodies comprised of disparate strains, in which selfish clones (cheaters) preferentially occupy a larger proportion of spores [12]. Moreover, the degree of kin discrimination has been reported to be positively correlated with genetic distance between strains [15], [16].
The second, little-studied social phase in this species is a sexual phase that represents an alternative albeit enigmatic response to starvation (see Figure 1) [17]–[19]. In the sexual phase, cell fusion from distinct (or sometimes similar) mating types is followed by cAMP-mediated attraction of other solitary cells that are cannibalistically consumed by the diploid zygote through massive phagocytosis. This leads to the formation of macrocysts, which represents an alternative mode of social behavior in D. discoideum, since neighboring solitary cells are attracted to the forming zygote, altruistically contribute to the formation of the macrocyst, and are then cannibalized for nutrition (see Figure 1). Being cannibalized may make sense when it benefits a member of ones own clone in the zygote, but it raises interesting potential social conflicts. In a mixture of two clones, the minority member would contribute equally to the zygote, but less than its fair share to the feeding of the zygote. In mixtures of more than two clones, cells unrelated to the zygote may be cannibalized against their interest.
Although the sexual phase is an interesting alternative social mode in D. discoideum, its role in the lifecycle of this species remains unclear [17]–[19]. The prevalence of sex in this social amoeba has never been determined, since germination of macrocysts to produce their haploid progeny in the laboratory has been infrequent [17], [20] and there is only one estimate of the zygotic recombination rate [20]. In contrast to other model genetic organisms such as yeast, fruit flies and C. elegans, the inability to conduct facile matings between strains in the laboratory has also hampered genetic analysis.
While D. discoideum is a key model species in the study of social behavior as well as development and cell-cell-signaling, very little is known about the evolutionary genetics of this organism, including the levels, patterns and distribution of nucleotide variation across the species' range. Understanding the nature of molecular variation in this social microbial species is particularly important given the key role genetic variation can play in the evolution and persistence of social interactions. Moreover, nucleotide variation data can be used to infer the role of sex in the lifecycle of D. discoideum in nature, by identifying the extent of recombination in the genome. Here we examine molecular diversity in gene fragments throughout the genome among natural strains collected in the eastern United States, and use these data to infer the molecular population genetics of this species. We find surprisingly low levels of variation in D. discoideum and use the distribution of single nucleotide polymorphisms (SNPs) to examine the role that recombination plays in shaping natural genetic diversity in this species. Analysis of the geographic distribution of mating types indicates that different types occur sympatrically, which likely facilitates sexual recombination and the decay of linkage disequilibrium observed in the SNP data. Evidence of recombination and our observation of greater discrimination against non-self during chimeric fruiting body formation in sympatric versus allopatric strains provides new insight into the evolution of cooperation in social microbes.
Results
Low level of variation in D. discoideum
A total of 137 gene fragments, approximately 400–600 bp in length, from across the Dictyostelium genome were chosen for sequencing, with ∼64.6 kb of sequence obtained for each strain. Ninety-four gene fragments are located on chromosome 4, while the remaining 43 are randomly distributed across the rest of the genome. We chose to sample more densely on chromosome 4 to allow for better inference of recombination and linkage disequilibrium (see Figure 2). For this chromosome, gene fragments were spaced at distances between 2.3 and 233.7 kb, with a mean spacing of 55.6 kb.
Fig. 2. Gene fragments and strain samples used in the study. 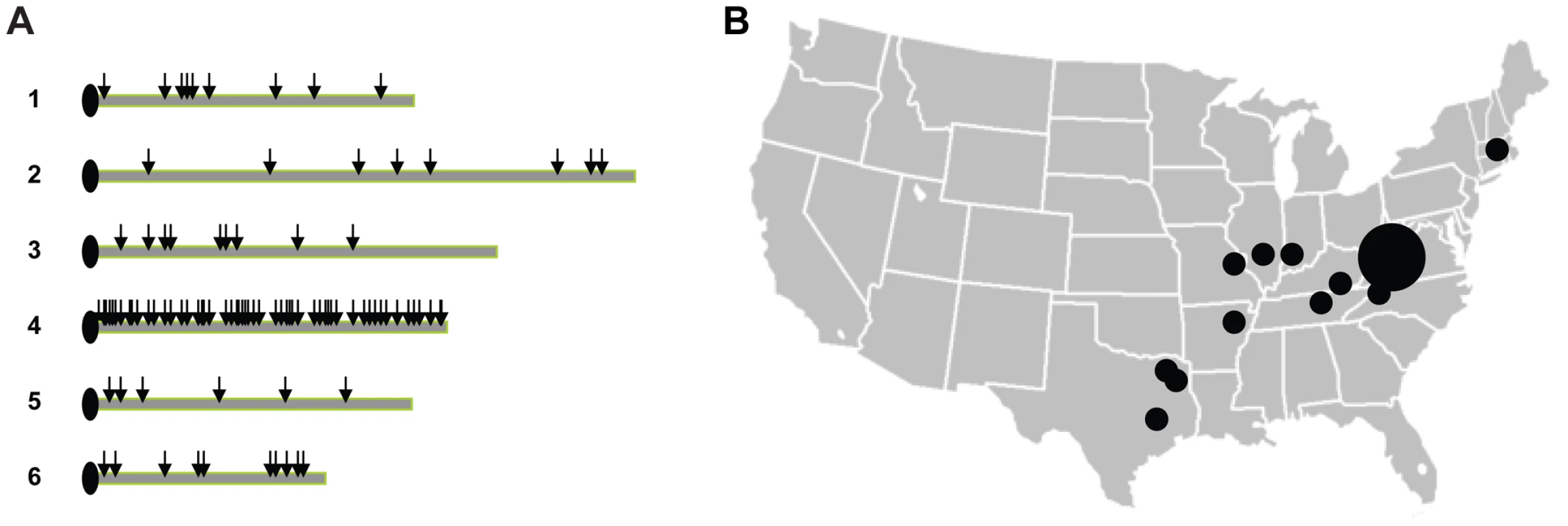
(A) Approximate locations of gene fragments used in the analysis (depicted by the arrows), with a higher density of fragments sequenced in chromosome 4. (B) Approximate locations of origins of the strains used in the study. The larger circle depicts the greater number of strains collected in sites in Mountain Lake, Virginia. We identified 184 SNPs in the sample, with an average of 1 SNP every ∼350 bps. The level of nucleotide variation is very low with the average number of pairwise differences per site, π, of 0.0008 (see Table 1). One-third of the fragments have no variation among the strains, while the most variable gene fragment had π = 0.0063 (see Figure 3). The level of variation for this haploid species is lower than humans (π = 0.001) and domesticated rice (π = 0.0015), two species that are known to have relatively low levels polymorphism [21], [22].
Fig. 3. Distribution of nucleotide variation and Tajima's D in gene fragments. 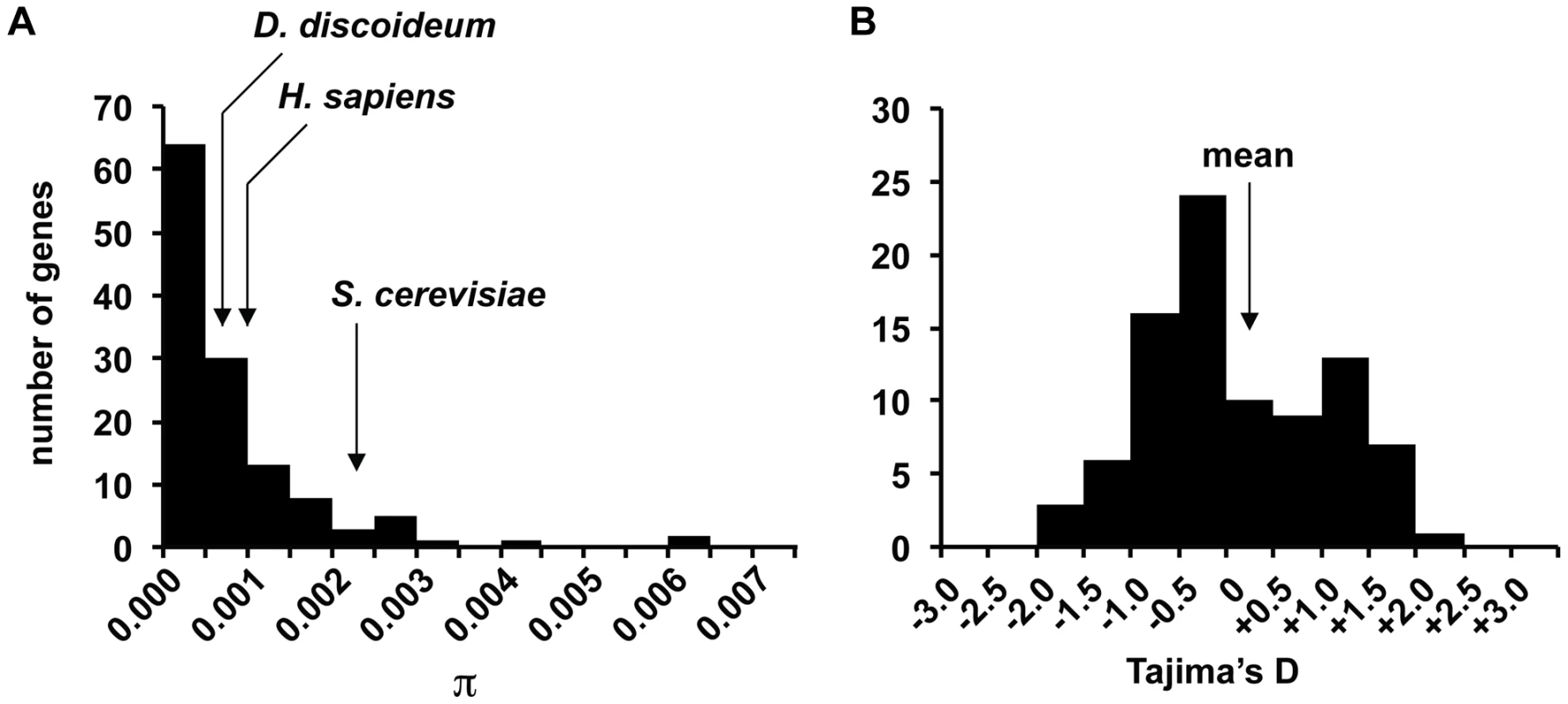
(A) The mean π for D. discoideum and various other species are indicated. (B) The mean Tajima's D for D. discoideum is indicated by the arrow. The smaller number of gene fragments plotted for Tajima's D is due to a large fraction of gene fragments with no variation and whose D estimate could not be determined. Tab. 1. Summary population genetic statistics for <i>D. discoideum</i>. 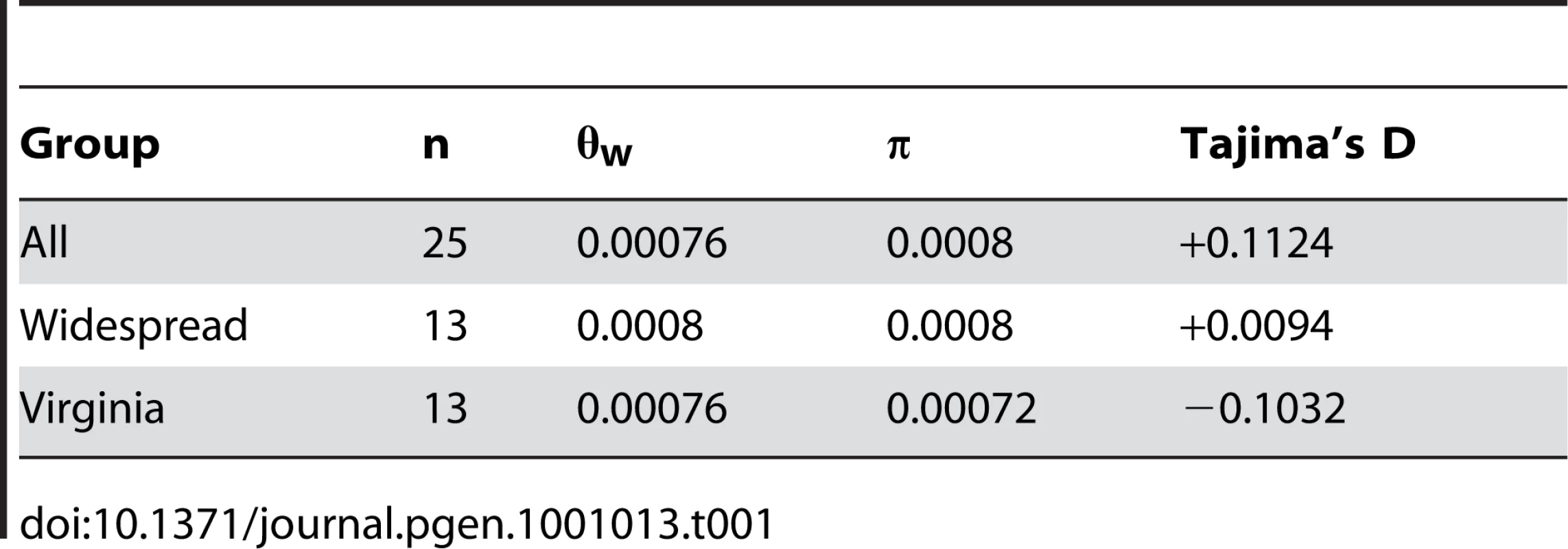
The low level of variation we observe could arise because half of our sampled isolates come from the Mountain Lake Biological Station, Virginia. We compared levels of variation in these strains (n = 13) to a geographically widespread sample (n = 13); the latter represents all of the non-Virginia strains, a randomly chosen Virginia strain, QS8, and the AX-4 laboratory strain (originally from North Carolina). The mean levels of nucleotide diversity are the same (π = 0.0008) whether we consider all the data or distinguish between the Virginia and geographically widespread samples.
The low level of genome-wide variation is consistent with a recent population bottleneck or possibly a population of small effective size that is at equilibrium between mutation and genetic drift. Recent changes in effective size may be detected with Tajima's D, a population genetic statistic based on the frequency of SNPs that is expected to deviate from zero when populations are not at equilibrium such as following expansion (D<0) or contraction (typically D>0) [23]. Tajima's D estimates for the gene fragments range from −1.5479 to +2.1043, with a mean of +0.1124 (see Figure 3), which is close to the neutral-equilibrium expectation of Tajima's D∼0 and consistent with a population that has not experienced dramatic changes in effective population size in recent history.
Absence of geographic structure in D. discoideum North American strains
The strains used in this study were collected across a wide geographic region in eastern North America, in addition to the Virginia site. A series of clustering analyses were performed to identify genetically similar strains and to identify potential geographic differentiation among strains.
An unrooted neighbor-joining tree based on concatenated sequenced fragments reveals several clusters of small numbers of strains with strong bootstrap support (see Figure 4). In most cases, each of the supported groups consists of strains from geographically distant locations such as the cluster of QS49 (Virginia) and QS37 (Texas) and a group consisting of QS34 (Indiana) and QS14 (Virginia). Furthermore, the Virginia strains are found throughout the tree with at least one Virginia strain found in all but one of the supported groups. A multiple correspondence analysis (MCA) of SNP variation, which can be considered as a generalization of principal component analysis (PCA) involving categorical (i.e., SNP) data [24] revealed a pattern of strain similarities comparable to the neighbor-joining analysis (see Figure S1). Visualization of the components in a 3-dimensional MCA plot also reveals that the 25 D. discoideum strains are generally found in the same clusters (see Figure S1) as observed in the neighbor-joining tree (unpublished observations), although we note that the true genealogical relationships among strains in these analyses may be obscured by recombination.
Fig. 4. Relationships between D. discoideum strains. 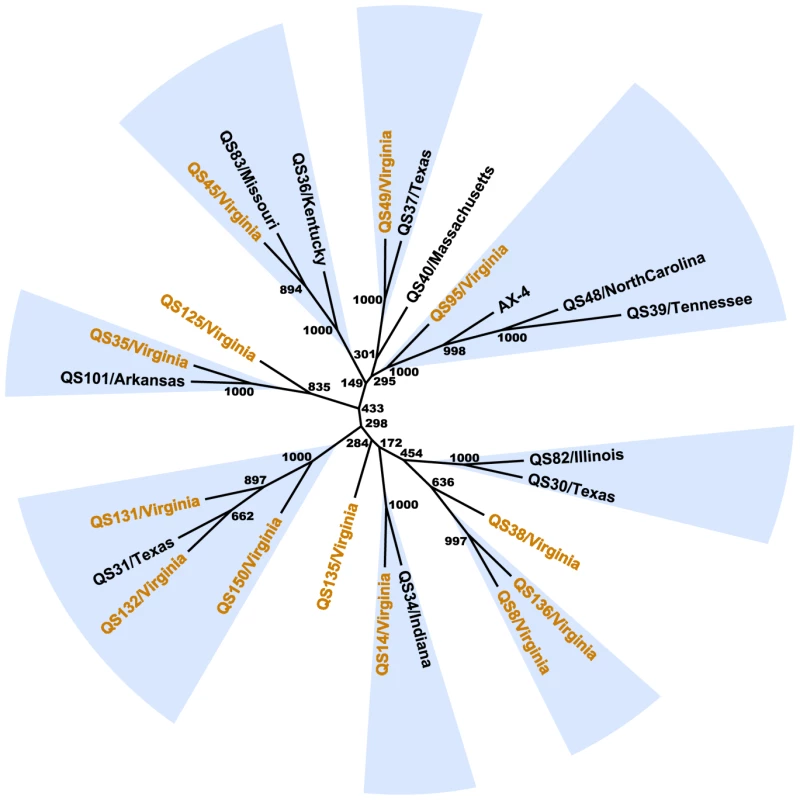
All STS fragment alignments were concatenated and clustering of strains were estimated with an unrooted neighbor-joining analysis as implemented in PHYLIP, with distances calculated using the Kimura 2-parameter model. Branch bootstrap estimates were obtained from 1,000 replicates. Groups of strains supported by >99% of the bootstrap replicates are highlighted in blue and strains from Virginia are colored in orange. Finally, a Bayesian clustering analysis based on a population genetic model implemented in the program STRUCTURE suggests that there are at least six and as many as eight clusters (i.e., the change in likelihood, Pr(X|K), begins to plateau at K = 6, and is maximized at K = 8 clusters)[see Figure S2]. However, we observe evidence of admixture among these groups [see Figure S1B], a result consistent with gene exchange (i.e., recombination) among these clusters (see below).
The clustering of strains from geographically distant locations and evidence of extensive admixture in the STRUCTURE analysis indicates no clear geographic differentiation of D. discoideum strains in our sample. The mean number of pairwise differences between the Virginia strains is similar to those from the geographically widespread sample (d = 42.732±17.59 in the Virginia sample versus d = 48.61±14.91 in the widespread panel). Moreover, we found no evidence for isolation-by-distance between strains using a Mantel test (r = 0.11, 0.05<P<0.11), and the Fst estimate between the Virginia site and the rest of the strains is very low (mean Fst = 0.023).
Relationship among SNP divergence, geographic distance, and fruiting body formation
Kin selection theory predicts that, when cost and benefit involved in a social interaction are fixed, individuals are expected to discriminate based on genetic relatedness and cooperate altruistically more frequently with close relatives. We examined whether kin discrimination is operating in our system by assessing whether strains discriminate in favor of kin (i.e., clonemates) during chimera formation and whether related strains, as measured by sequence similarity, cooperate more than more divergent strains.
We quantified the extent of kin discrimination by estimating relatedness of fruiting bodies relative to sample average (rfb) in various chimeric mixing experiments. In each experiment, two strains in the single-celled stage were mixed and allowed to form chimeric fruiting bodies with the proportions of each genotype determined in the initial cell mixture and in replicate fruiting bodies (see Materials and Methods). An rfb = 0 indicates random mixing of cells, while rfb = 1 is associated with complete kin discrimination favoring fruiting body formation of clonemates. We measured rfb in mixing experiments between strains of different levels of nucleotide divergence, with SNP differences ranging from 41 to 74 SNPs. We found evidence for between-strain discrimination in chimeric fruiting bodies of D. discoideum, with various levels of relatedness in strain mixing experiments, from rfb = 0.03 to 0.38 with a mean of 0.14±0.03. The strain pair that shows the greatest relatedness is QS49 versus QS150 (both from Virginia).
A plot of the rfb values among strain pairs of different SNP divergence levels is shown in Figure 5. For this measure, there was no significant correlation with SNP divergence and the fit to the data was low (R2 = 0.04). We also employed the Levene's (LS) statistic as well as the variance in the arcsine square-root transform [15] of the proportion of strains in the fruiting body as measures of kin discrimination. No correlation between the extent of discrimination and SNP divergence was observed using these two measures (see Figure S3 and Figure S4).
Fig. 5. Relationship between social parameters and SNP divergence. 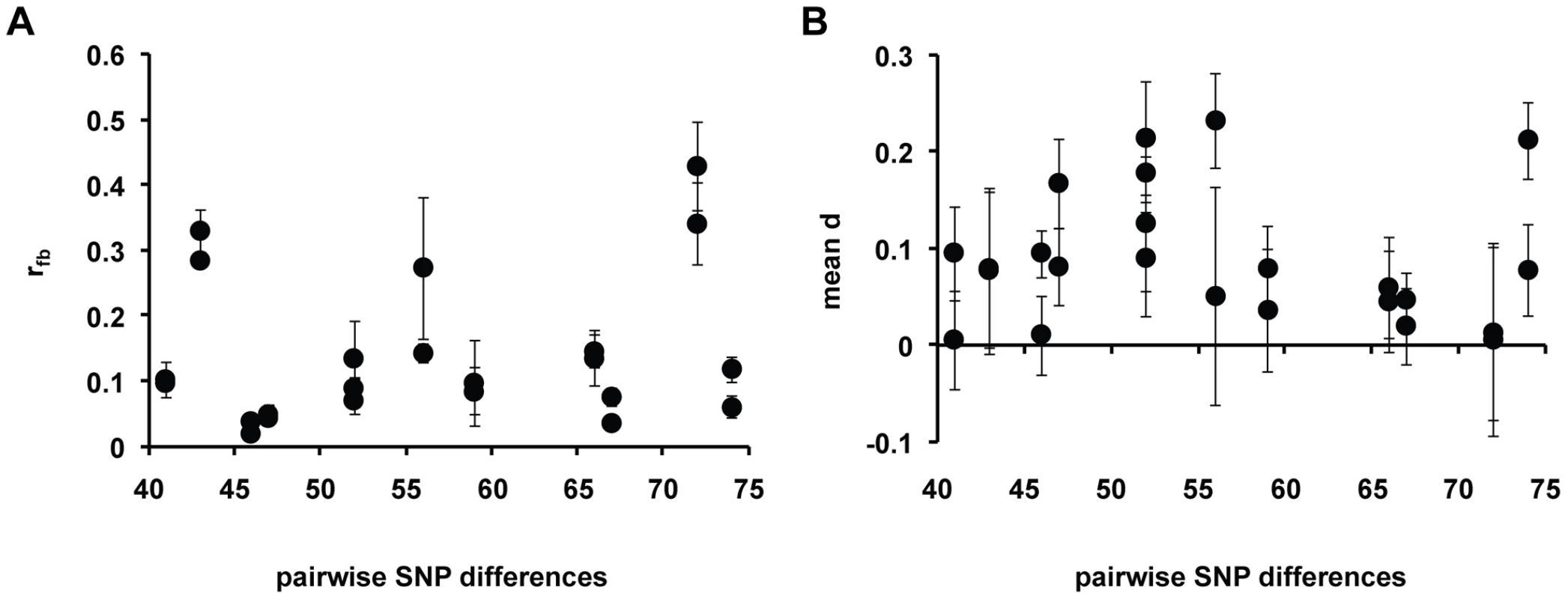
(A) Plot of relatedness rfb versus SNP divergence between strain pair. Mean and standard error of rfb values were calculated for each SNP used in the strain quantification and shown on the plot. (B) Plot of the means of absolute dominance value d versus SNP divergence between strain pairs. Mean and standard error of d values were calculated for each SNP used in the strain quantification and shown on the plot. We also examined the dominance of a given participant strain in various chimeric fruiting bodies using the d value (see Materials and Methods). A positive d value means a specified participant strain occupies a larger proportion in the sorus than would be expected given the strain frequency of the mixed cells in the experiment. We found that dominance occurred between strains in chimeric fruiting bodies; the absolute d values range from 0.008 to 0.195 with a mean of 0.087±0.016 among the various chimeric mixing experiments (see Figure 5). The strain pair that shows the greatest dominance is QS132 (Virginia) when in a chimera with QS83 (Missouri); this pair has an absolute d of 0.195±0.035. Like rfb, there is no correlation between mean d and the number of SNP differences between strains (R2 = 0.019).
One problem in this assay is that the differences in the SNP divergence levels between strain pairs are small and this compromises the resolution of genetic distances. We decided to further compare strain pairs in our experiments by partitioning them into pairs with either low or high divergence. Among all strains in our sample, the median pairwise divergence was 52 SNPs, and we designated strain pairs below and above this median pairwise SNP divergence level as low - and high-divergence pairs, respectively. We found no difference in either kin discrimination or dominance metrics between these two divergence categories (see Table S1).
Although we did not find a relationship between discrimination and SNP divergence, we observed greater discrimination between strains from the same location versus strains collected from different locations. Using the fruiting body relatedness as our discrimination metric, the level of within-site discrimination is rfb = 0.23±0.08, while between-site rfb = 0.12±0.03 (see Figure 6). This increase in fruiting body relatedness in experiments with sympatric strains compared to mixing experiments with allopatric strains is significant (P<0.042). We do not, however, observe a significant increase in dominance in sympatric versus allopatric strains (P<0.49). To examine this further, we also tested for a correlation between kin discrimination, rfb, and geographic distance. There is a negative relationship between kin discrimination and geographic distance (r2 = 0.22), but this correlation is not statistically significant (see Figure 6).
Fig. 6. Relationship between kin discrimination and sympatry. 
(A) The mean relatedness rfb is plotted against geographic distance of strain locations used in chimeric mixing experiments. Mean and standard error of rfb values among the three experimental replicates were calculated for each strain pair and shown on the plot. Strains within the Mountain Lake Biological Station site in Virginia are given a distance of 1 km. The line through the data is based on the fit with a linear regression model, and shows a negative relationship of rfb with geographic distance [R2 = 0.22], but this relationship is not significant [P<0.62]. (B) The mean rfb for strain pairs from the Mountain Lake Biological Station site (left) is compared with those for strain pairs between sites (right)[see Table S5]. Standard errors for the mean rfb is shown by the bars. The difference in mean rfb is significant (P<0.038). Rapid decay of linkage disequilibrium and evidence of recombination
The sexual phase in D. discoideum has been difficult to observe, and it is possible that sex is rare in this social amoeba. If sex were indeed rare, then we would expect low levels of recombination and high levels of linkage disequilibrium across the genome.
First, we assayed for mating types of our natural strains to determine their geographic distribution. Mating types were assigned by observing macrocyst formation when incubated against tester strains of known mating types A1 and A2. Strains that mate with both testers are designated as mating type A3. Of our 24 tested strains, we were able to assign mating types to 18 strains, with 6 failing to form macrocysts with either tester strain A1 or A2 (see Table 2). We also found that all three mating types were present in the Virginia site, which would indicate that individual amoeba can readily find mating partners within localized areas and thus undertake sexual reproduction.
Tab. 2. Mating types of <i>D. discoideum</i> strains. 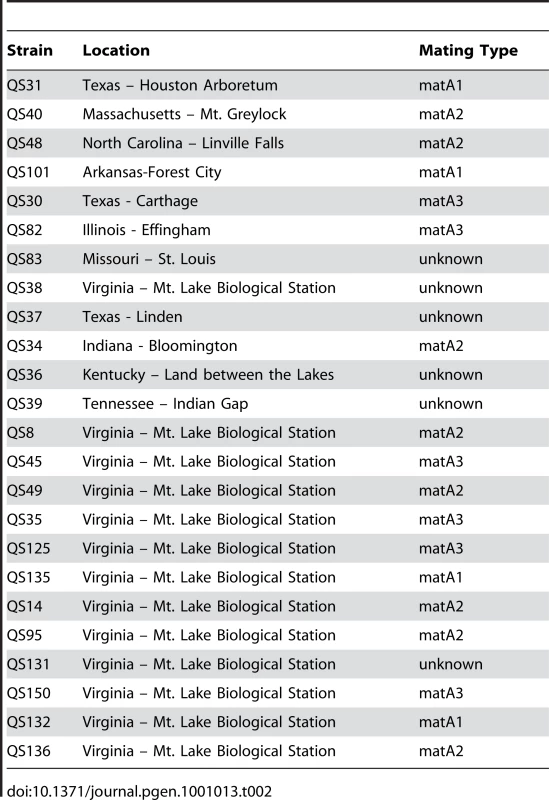
The extent to which sex and recombination occurs in D. discoideum can be ascertained by examining the population genetic data for evidence of recombination and measuring the extent of linkage disequilibrium and its rate of decay. We thus characterized linkage disequilibrium among SNPs on chromosome 4, which was more densely sampled for gene fragment re-sequencing than the other chromosomes.
We found little evidence of high levels of linkage disequilibrium among segregating sites across this D. discoideum chromosome (see Figure 7). A plot of linkage disequilibrium with physical distance across chromosome 4 indicates that LD decays substantially at short distances (see Figure 8). The baseline level of LD, measured as the mean r2 for SNPs on separate chromosomes, is 0.136 and the 80th percentile is 0.209. Linkage disequilibrium decays to twice the baseline at less than 10 kb, and reaches the baseline level (as well as the 80th percentile) at between 10 and 25 kb (see Figure 8). More than one-fifth of the SNP pairs that were perfectly correlated (r2 = 1) are found within gene fragments at distances less than 300 bps, although high r2 values are observed even between SNPs separated by ∼5 Mb. Using a permutation test, we found that the decay in r2 with distance was significant (P<0.03). This test of r2 with distance was significant regardless of whether we included or excluded low frequency variants.
Fig. 7. Pairwise linkage disequilibrium in chromosome 4. 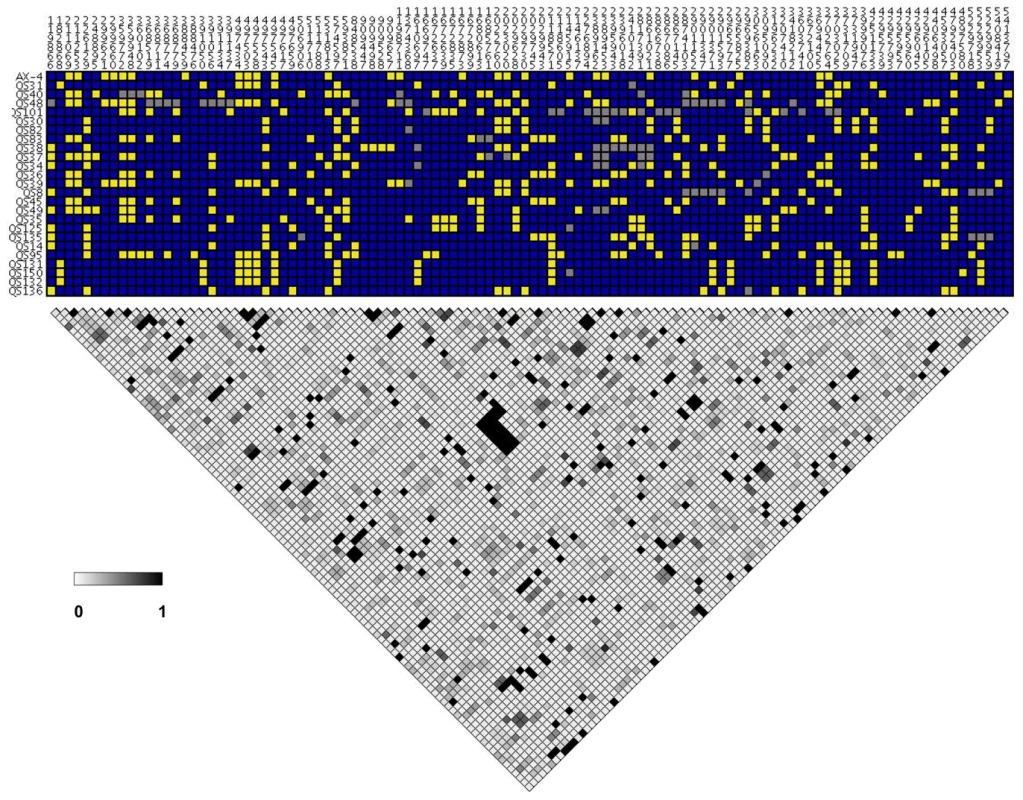
The segregating SNPs for each strain are shown on top, going from the proximal to distal positions along the chromosome from left to right. Each horizontal row on top is a separate strain. The blue and yellow indicate the major and minor SNP allele, based on frequency in the strains. The gray is missing SNP data. The heat map at the bottom is based on the linkage disequilibrium measure r2 between SNPs, with the grayscale legend for the level shown. Fig. 8. Decay of linkage disequilibrium with distance in chromosome 4. 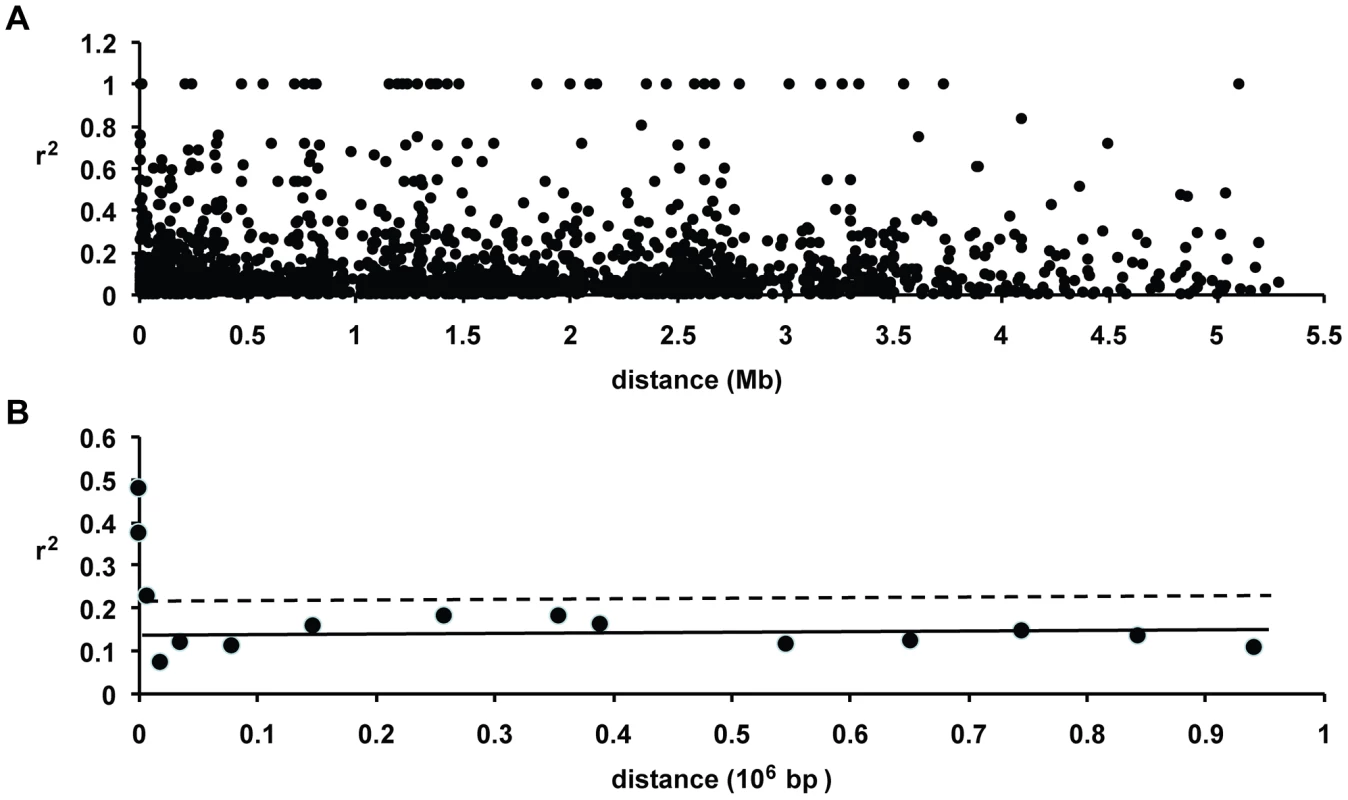
(A) Pairwise r2 plotted against distance. Linkage disequilibrium is calculated only for the SNPs that have at least 10% frequency in the sample. A permutation analysis indicates that the decay of LD with distance is significant (P<0.031). (B) Plot of mean r2 in various distance bins. The solid horizontal line gives the mean r2 and the dashed line is the 80th percentile for unlinked markers. The baseline linkage disequilibrium in D. discoideum is achieved between ∼10–25 kb. The other five chromosomes had too few SNPs in our study to obtain a meaningful estimate of LD decay with distance for each chromosome separately. When we combined all the within-chromosome data from these five other chromosomes, however, we observed a similar pattern of short-distance LD decay (see Figure S5). The LD decay over short distances on chromosome 4 is also observed if we consider only the Virginia versus geographically widespread sample, except that the latter had higher levels of LD with a baseline r2 of 0.182.
Application of the 4-gamete test provided direct evidence of recombination in the Dictyostelium genome. We observed a minimum number of recombinants (RM) of 14 across chromosome 4 in the 25 strains (see Figure 9). Most of these recombination events occur between gene fragments, although at least one intragenic recombination was observed. Further insight into the history of recombination in Dictyostelium can be gleaned from the population recombination parameter ρ, a compound parameter equal to 2Nρr(1−F), where Nρ is the effective population size estimated from recombinational diversity, r is the recombination rate and F is the inbreeding coefficient. Based on the SNP data across chromosome 4, we obtained a moments-based estimate of ρ = 82.46 using the method described by Wakeley [25]. To obtain a more precise estimate of ρ, however, we used an extension of the parametric method of Hudson [26] that is robust to different mutation models. The likelihood plot for the estimate shows a steep increase in likelihood from 0 to ∼20, with a maximum likelihood estimate for ρ at 37.75 (see Figure 9). A likelihood permutation test indicates that this estimate is significantly different from zero (P = 0.000); this test was significant whether we included or excluded low frequency variants. While the spacing of our markers may cause us to underestimate ρ across chromosome 4 using this methodology, it is clear from our analysis that sexual (meiotic) recombination has occurred frequently in the history of the chromosomes surveyed.
Fig. 9. Recombination in the D. discoideum genome. 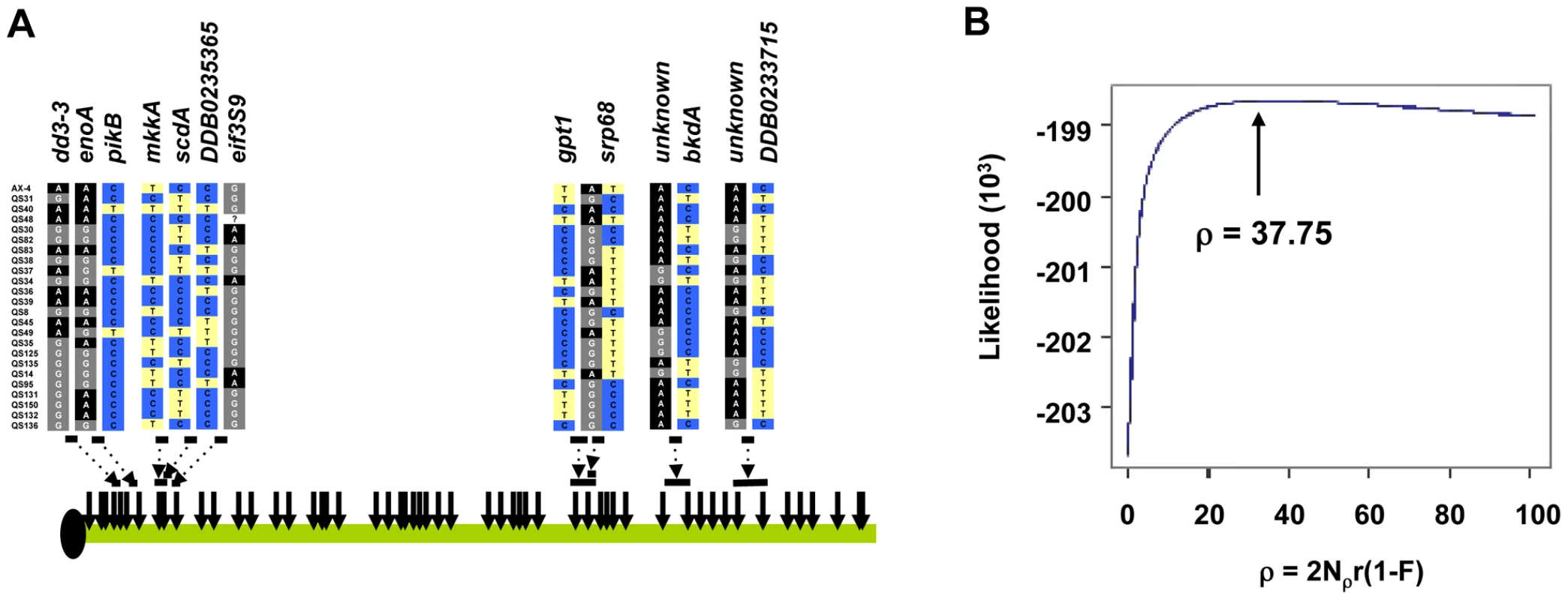
(A) The locations of several recombination events between SNPs are indicated by the horizontal bars and the sequence at those SNPs are shown. The SNPs and the genes they are found in are shown and color-coded. Each row of SNPs represents an individual D. discoideum strain. The dotted arrows indicate the SNPs flanking the recombination events, which are inferred by the presence of all 4 possible genotypes between SNP pairs. (B) Composite likelihood plot of estimates of ρ. The plot shows a steep decrease in likelihood at approximately ρ<10, and the maximum is at ρ = 37.75. The likelihood surface is nearly flat at approximately ρ>20. Discussion
D. discoideum has one of the lowest levels of nucleotide variation observed in any eukaryotic species (see Figure 3), which indicates that very few mutations differentiate even genetically distinct strains. The estimated levels of nucleotide variation suggest that, on average, two D. discoideum strains should differ by only ∼27,000 SNPs over the length of its 34 Mb genome. The source of this low level of variation is unclear, but could be attributed to a population at equilibrium with small effective size, a recent bottleneck event or a low mutation rate. Little is known about the mutation rate in this species, although a low rate has been observed at microsatellite loci in mutation accumulation experiments [27].
D. discoideum strains do not appear to form geographically distinct subgroups in the eastern United States. There is no clear pattern of geographic structuring of nucleotide variation and no evidence of isolation-by-distance in our sample set. The formation of the fruiting body is believed to facilitate dispersal of spores [28], and these social amoebae are thought to be moved by water, small arthropods, nematodes and birds [29]–[32]. Our results suggest that these vectors lead to a high rate of dispersal, which may also explain the relative diversity in genotypes found within small population patches (e.g. the Virginia population, also see refs. 13 and 14). We should note that although we do not observe any large-scale geographic pattern among the strains, a separate study sampling from several localities has shown that there nevertheless is differentiation between D. discoideum populations [33].
The transition from solitary cells to a multicellular fruiting body is one of the quintessential examples of social cooperation, and the death of stalk cells for the survival of spores provides an example of altruistic behavior [7]–[12]. Although social cooperation in D. discoideum strains has been largely studied in cells with the same genetic background, clones of different genetic backgrounds are known to co-occur in the field [13]. It has been demonstrated, for example, that cheating behavior is commonly seen in some chimeric fruiting bodies comprised of disparate strains, in which selfish individuals (cheaters) preferentially occupy a larger proportion of spores. Moreover, kin discrimination between distinct genotypes is positively correlated with genetic distance between strains [15]. Genetic relatedness is thus a key component of the dynamics of social interaction.
We find that many of our D. discoideum strain pairs show evidence of kin discrimination between strains that results in assortative association in the sorus during fruiting body formation, as well as dominance of one strain over another. This indicates that social dynamics in D. discoideum may differ genetically between strain pairs. Our results, however, also suggest that there is no correlation between the observed SNP divergence and kin discrimination between strains in the fruiting body.
This result differs from a relatedness-based prediction of kin selection theory [1] as well as previous work that suggests kin discrimination in this species [15]. Several reasons might explain this discrepancy. First, the range of SNP differences between strains in our sample is small, and may therefore lead to poor resolution of genomic differentiation between strains. This is a direct consequence, in part, of the very low variation in this species, which limits the number of SNPs in our analysis. We obviate this somewhat by partitioning our data into those strain pairs that had low - or high-divergence with respect to the median pairwise SNP divergence in our sample. The pattern of no correlation in measures of kin discrimination with SNP divergence still holds in this analysis (see Table S1).
Second, previous studies [15] examined chimera formation with one fixed axenic line and employed highly polymorphic microsatellite loci to estimate genetic distances between pairs. Genome-wide SNP differences may not be a relevant measure of genetic relatedness in D. discoideum during social interactions; it is possible that what is relevant are the genetic states of specific set of genes responsible for certain social behavior, and this may differ from overall genome-wide genetic relatedness. An extreme scenario invokes greenbeard genes, which leads to cooperation specifically directed towards other individuals carrying the same allele at a particular locus [1], [34], [35]. Notably, it also appears that polymorphic lagB1 and lagC1 (now called tgrB1 and tgrC1) genes, which encode transmembrane proteins that participate in cell adhesion and signaling, are associated with kin discrimination in D. discoideum [16]. This observation suggests the possibility that only a small group of genes condition social interaction during chimeric fruiting body formation.
The possibility that variation at specific loci is responsible for determining self/no-self recognition in D. discoideum is buttressed by our results that there is an effect of geographic distance between strain origins on kin discrimination. Our observation of greater kin discrimination among strains within a site versus between sites suggests the evolution of mechanisms to prevent chimera formation among strains that co-occur in a particular area. This is consistent with very high levels of relatedness (from 0.86 to 0.98) found among fruiting bodies in the wild [14]. If our result of greater kin discrimination in sympatry is true, then a consequence would be that strains that encounter each other in local populations preferentially form fruiting bodies with clonemates, and that this preference is diminished if strains do not encounter each other because of allopatry. Kin discrimination may thus evolve between D. discoideum strains in close proximity to maintain multicellular cooperation within fruiting bodies and control against invasion by cheater mutants in the wild [14].
The specific patterns of both the social behavior and population structure of D. discoideum may also contribute to this geographic effect on kin discrimination. Contrast our results with recent work, for example, in the social bacterium Myxococcus xanthus, which displays greater antagonisms among strains from different locations versus those from short spatial scales [36]. Fitness antagonisms are also observed between local strains in M. xanthus, but divergence among allopatric strains appears to reinforce the ability to discriminate between self and non-self [36]. This bacterium, however, appears to have strong geographic structuring of genetic variation; in comparison, our D. discoideum samples are not strongly structured, with a low Fst between Virginia versus non-Virginia samples, and the observation that genetically divergent strains co-exist in one site (see Figure 6). Moreover, M. xanthus exists as swarms during their predatory phase, which results in social cohesion even before fruiting body formation in this species. In contrast, D. discoideum cells have a distinct predatory single-cell stage, which increases the likelihood of chimera formation during periods of starvation. Both these factors – the potential presence of highly divergent strains in a given geographic area coupled with the higher probability of chimera formation – may select for stronger self/non-self recognition mechanisms for sympatric strains in D. discoideum.
The sexual phase of D. discoideum also represents another distinct but poorly understood mechanism for social cooperation in this species. Unlike fruiting bodies, which are readily induced in the laboratory, the sexual phase (see Figure 1) has been difficult to study. The extent of sex in the life cycle of D. discoideum in nature is unclear, but molecular population genetic analysis provides an independent indicator of the importance of the sexual phase in this social species. The rapid decay of LD, the presence of recombinant strains, and the high levels of the population recombination parameter across chromosome 4 all suggest that recombination is widespread within D. discoideum. The pattern of LD decay is in line with those observed in other sexual species (see Figure 10). The only laboratory estimate of recombination in D. discoideum is from one study, which reported a high level of recombination (r = 0.001 morgans kb−1) [20]. If this high estimate is correct and representative of genome-wide recombination rates, we would intuitively expect a more rapid decay of LD with distance than what we observe, of the same order of magnitude as outcrossing species such as D. melanogaster or C. remanei. Our observed pattern of LD decay in D. discoideum suggests either that this previous estimate of recombination rate is too high, or possibly that there is a high level of inbreeding in this species.
Fig. 10. Comparison of LD distance decay between various species. 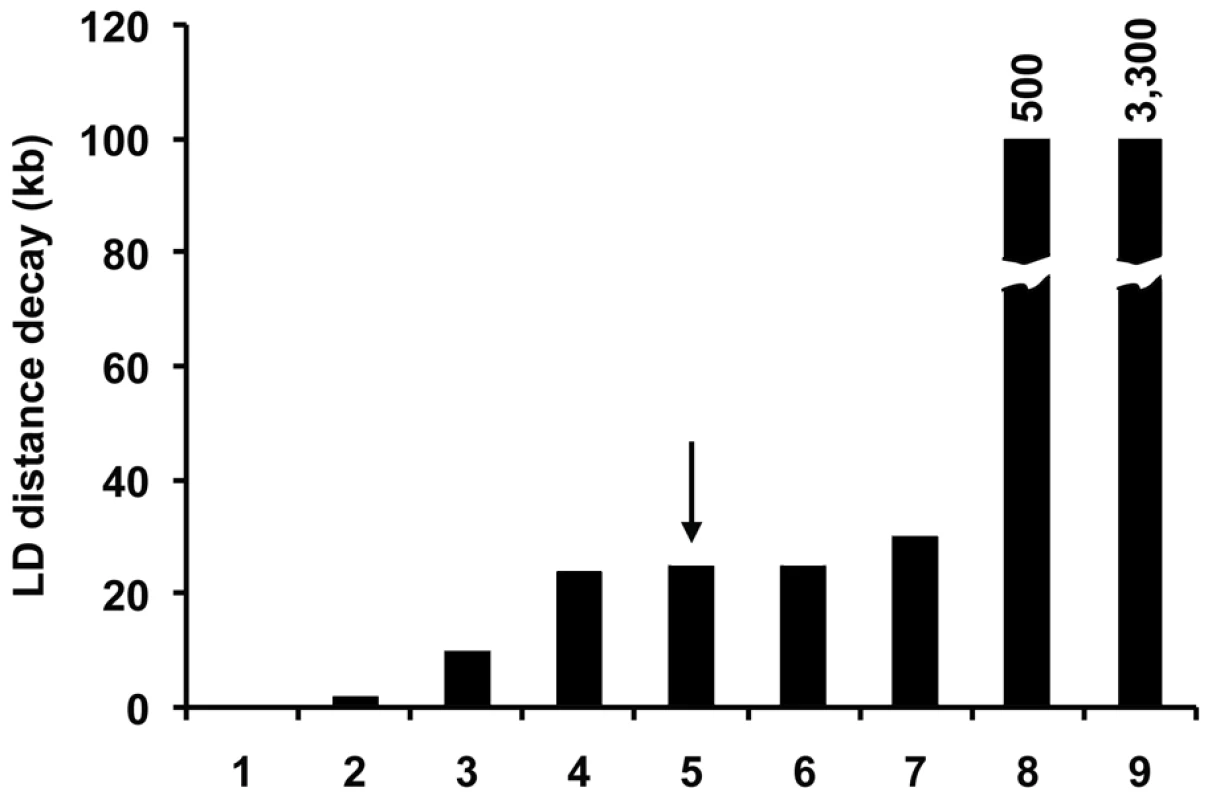
For species in which the LD distance decay is reported as a range, we plotted the maximum distance. The species are 1 - D. melanogaster, 2 - C. remanei, 3 - A. thaliana, 4 – S. cerevisiae, 5 – D. discoideum, 6 – S. paradoxus, 7 – H. sapiens, 8 - O. sativa, 9 – C. elegans. For both O. sativa and C. elegans, the reported LD distance decay is off the scale, and the actual numbers are indicated on top of the bars. An arrow highlights the D. discoideum estimate. Data for this slide from other species are from previous work [21], [22], [54]–[59]. Our results suggest that sex as a form of social interaction may be an important aspect of this organism's life cycle. Given that we find different mating types within a local population (e.g. the Mountain Lake Biological Station population in Virginia), there clearly is ample opportunity for strains to form macrocysts in nature, which support our finding of a history of recombination in the D. discoideum genome. One intriguing aspect of the sexual phase is that the developing zygote, upon cell fusion, signals and attracts hundreds of neighboring solitary cells and proceeds to consume them for nutrition in the process of macrocyst formation [17]–[19]. The same cAMP signal that leads to fruiting body formation appears to play a similar role in the attraction and cannibalistic consumption of free-living cells by the developing zygote, and this may represent a different mode of social cooperation. A social evolution perspective would probably consider the cannibalized cells to be altruistic rather than pure victims since they construct a cellulose prison for themselves before they are eaten [37]. It is unclear, however, what ecological conditions favor the asexual fruiting body or the sexual diploid macrocyst in nature. The evidence for sexual recombination in the wild, however, demonstrates that while fruiting bodies are readily formed in laboratory conditions, the sexual macrocyst phase may be an important aspect of the biology of D. discoideum in the field that needs to be closely studied on order to understand the full spectrum of phenomena associated with the social biology of this species.
Our molecular population genetic data also has wide implications for the use of this species as a model system. D. discoideum is already a key model organism, with an extensive community of researchers, a whole-genome sequence, an active stock center and community database. It has already provided key insights into the nature of development and differentiation, the mechanisms of cell-cell signaling, the evolution of social cooperation and the rise of multicellularity. Nevertheless, this species has yet to be used as a genetic model organism because sex has been observed infrequently in the laboratory that has hampered mutant analysis.
Our results open the possibility that Dictyostelium genetic studies using controlled matings may be possible once we fully understand how to bring sex and recombination from the wild into the laboratory environment. Moreover, our study of the patterns of molecular diversity and linkage disequilibrium can aid workers in designing association genetic approaches to identify genes involved in natural variation (including social interaction). These approaches can expand the potential scope of Dictyostelium genetic studies, and allows researchers to harness this unique and fascinating social microbe for fundamental studies in development and evolution.
Materials and Methods
Samples and sequencing
A panel of 24 D. discoideum wild strains was chosen to represent the diversity found within the species in North America (see Table S2 and Figure 2). Thirteen of these strains were collected at a single site at Mountain Lake Biological Station, Virginia while the other 11 were collected throughout the range of D. discoideum in North America. For our analysis, we also used the complete genome sequence from the AX-4 strain [6]. DNA was extracted from single cell cultures. Gene fragments, approximately 400–600 bp in length, from across the Dictyostelium genome were chosen for sequencing (see Figure 2 and Table S3). Ninety-four of these fragments were in chromosome 4, and were selected with adjacent gene fragments separated by various lengths (∼2–223 kb); this design was chosen to allow a better estimation of linkage disequilibrium. The other 44 gene fragments were chosen randomly from the other 5 chromosomes.
Primers were designed from the D. discoideum genomic sequence available from Dictybase (www.dictybase.org) using Primer3 [38] (see Table S4). Primers were designed in exons and flanked intron sequences within each fragment. DNA sequencing was carried out at the Cogenics sequencing facilities (New Haven, CT) as described in [39]. Base pair calls, quality score assignment and construction of contigs were carried out using the Phred and Phrap programs (Codon Code, Dedham, MA). Sequence alignment and editing were carried out with BioLign Version 2.09.1 (Tom Hall, NC State Univ.). For all analyses, the published sequence of the AX-4 strain was included [6]. From previous studies, we find that this procedure has a sequencing error rate of <0.01% [22]. All sequences are deposited in Genbank.
Diversity and linkage disequilibrium (LD) analyses
We estimated the levels of nucleotide variation (θW) [40], nucleotide diversity (π) [41] and Tajima's D [23], and Fst [42] across all STS fragments, and determined the frequency distributions of SNPs across the genome [43].
For the analysis of LD, only biallelic SNPs of at least 10% frequency were considered, as rare alleles can have large variances in LD estimates. We calculated the LD as the correlation coefficient r2 between each SNP pair [44]. LD heatmaps and illustration of SNP configurations were made using the Seattle SNPs Genome Variation Server (http://gversusgs.washington.edu/GVS/). Due to the large amount of variance in the estimates of LD for any particular SNP pair, we combined SNP pairs into distance intervals to reduce the influence of outliers and to obtain a better visual description of the LD decay with distance. For the estimate of genome-wide LD using the chromosome 4 dataset, the distance classes are <0.1 kb, 0.1–0.5 kb, 0.5–10 kb, 10–25 kb, 25–50 kb, 50–100 kb, then every 100 kb until 1Mb, and 1 Mb distance windows for SNP pairs >1Mb in distance. The use of smaller intervals at short distance scales was determined in part by the observation that LD seemed to decay substantially at these distances.
We plotted the mean of r2 for each distance window. We consider a particular intermarker distance interval to have LD elevated above the background level if it contains more than 10 SNP pairs and the interval mean r2 exceeds the 80th percentile of the unlinked pairs (which we chose from the r2 values between all unlinked pairwise SNPs between chromosomes). The significance of r2 decay with distance was tested using a permutation test with 1,000 permutations of distance with r2 in the data [26].
Population stratification analyses
Population structure among D. discoideum strains was evaluated with STRUCTURE 2.1 using an admixture model with linkage [45]. All analyses had a burn-in length of 200,000 iterations and a run length of 200,000 iterations. Ten replicates at each value of K (population number) were carried out. Simulations were run with a model of linkage and uncorrelated allele frequencies. The appropriate K value was determined using the method of Evanno et al. 2005 [46] (see Figure S2). To further assess relationships among strains, all STS fragment alignments were concatenated to form a single dataset. Clustering relationships of strains based on genetic similarity were estimated with a neighbor-joining analysis as implemented in PHYLIP 3.68 [47], with distances calculated using the Kimura 2-parameter model [48]. Branch bootstrap estimates were obtained from 1000 replicates and visualization of the consensus tree was realized in FigTree v1.2.2 (http://tree.bio.ed.ac.uk/software/figtree/). Finally, based on the complete SNP dataset multiple correspondence analysis (MCA) was also carried out to investigate the pattern of population relationships using R package FactoMineR [24].
Recombination analysis
A composite likelihood method [26] as implemented in the LDhat software was used to estimate the population recombination parameter ρ for chromosome 4. For the analysis of LD, only biallelic SNPs of at least 10% frequency were considered, as rare alleles can have large variances in LD estimates and inference of recombination has been found to be sensitive to the frequencies of alleles included in the analysis [26]. This method can also estimate recombination across the chromosome, and a likelihood permutation test with 1,000 permutations of the data was performed to test whether this recombination rate was significantly higher than zero. We used the 4-gamete test to identify recombination breakpoint intervals and estimate the minimum number of recombination events [49].
Social assays among chimeric fruiting bodies
Twelve strain pairs that are diverged at various SNP levels were chosen for social mixing experiments [15], to test for kin discrimination and dominance (see Table S5). All strains were first grown on SM-agar plates to the mid - exponential phase, with cell densities measured by a hematocytometer. Solitary cells from each strain were then harvested, washed, and resuspended to a density of 6×107 cells/ml in KK2 buffer. Two strains were mixed at 50∶50 proportions and approximately 1.5×107 cells were deposited on a nitrocellulose filter. These cells were allowed to develop into fruiting bodies in a dark, humid environment at 21°C for 24 hours. The development conditions were as described [15], and a minimum of three independent cell mixing experiments was performed for each pair.
To quantify the relative abundance of individuals of each strain in chimeric mixtures, genomic DNA was extracted before and after fruiting body formation as described [15], and used for allele quantification by pyrosequencing [50]. Two SNP sites with distinct polymorphisms in separate genes from the two strains in each pair were used as genetic markers to differentiate individuals. Although 50∶50 of each strain was the targeted ratio, we expected deviations from this proportion; to control for experimental variation in mixture proportions, genomic DNA was extracted from 20 uL of cell mixtures before plating to determine the actual proportions in the input cell mixtures. Sixteen fruiting bodies from each of the three replicate plates were also picked and DNA extracted from each fruiting body.
SNP pyrosequencing primers were designed by PSQ Assay Design Software V1.0.6 and pyrosequencing reactions were carried out on PSQ 96MA (Biotage, Stockholm, Sweden). To construct standard curves for each SNP, genomic DNA from strains carrying distinct alleles at the marker SNP sites were mixed at specific proportions ranging from 0 to 1 for a final concentration of 10 ng/ul (see Figure S6). Three independent mixtures were made at each proportion to serve as replicates. Each replicate was PCR amplified and pyrosequencing carried out separately as described [50]. Peak heights measured from the three replicates were plotted against known allele proportions, and a least-squares linear regression model was used to estimate relative levels of alleles in the experiment. The two alternate SNPs serve as technical replicates, and were measured on the same genomic DNA from each fruiting body.
We developed a metric for the dominance status of one strain over a second in a chimeric mixing experiment. We define dominance as a general term to mean the difference in proportion of a strain in the fruiting body relative to the starting proportions in the single cell mixtures. The dominance value d is given bywhereand pi represents the frequency of one strain in a fruiting body, while po represents the frequency of the same strain in the initial cell mix.
Discrimination manifests itself when strains preferentially form fruiting bodies with genetically identical cells (clonemates). To examine kin discrimination in the formation of chimeric fruiting bodies, we employed three approaches. We estimated the relatedness (r) [1] within fruiting bodies relative to other fruiting bodies resulting from the cell mixture [51]. If a strain has frequency pi in the ith fruiting body, and the average over fruiting bodies in the sample is p, its relatedness in that fruiting body can be estimated as
This measure, ri, is the observed deviation in frequency of a strain relative to the mean frequency, scaled by the maximum possible deviation. For the other strain, the frequency in the cell mix is 1 − and in the ith fruiting body is 1−pi, so relatedness for this strain isWe sum over replicate fruiting bodies to obtain a measure of global change in relatedness for a pair of strains [51]. In each fruiting body, there is a fraction, pi, of individuals that have a relative change in relatedness, ri, and 1−pi individuals with ri′. Summing over all fruiting bodies and including both strains we obtain a global relatednessthat represents how strongly strains are clustered in different fruiting bodies. It can range from zero for random clustering to one for complete segregation.
We also used two alternate measures to infer the extent of kin discrimination. Levene's statistic (LS) [52] is a measure of individual deviation from the mean, and is given asThe mean of Levene's statistic was calculated for dominant strains in each mixing experiment and reduces any possible confounding effects of the variance with the mean. Finally, we estimated the variance of the arcsine square-root transform of xj and utilized it as a measure of kin discrimination as described in a previous study of D. discoideum [15].
Mating type assay
Mating type tests were carried out as previously described [53]. Tests were performed in 0.5 ml LP agar, where 0.5 ml Bonner's salt solution and 5 µl K. aerogenes were loaded before spores from D. dictyostelium strains were added. Either NC4 (mating type A1) or V12 (mating type A2) was used as standard strain in a single test. Every strain of interest was assessed versus NC4, V12 and itself with three replicates each. Twenty-four well plates containing strains being tested were wrapped in aluminum foil and incubated for seven days at 21°C without shaking. Examination for formation of macrocysts was performed under 10× magnification. If we observed macrocysts in two or more replicates, the reaction was considered to be positive. If macrocysts formed with only V12, the mating type of the strain was matA1. If macrocysts formed with only NC4, the mating type of the strain was matA2. If macrocysts formed with both, the strain was ambivalent, and designated as matA3.
Supporting Information
Zdroje
1. HamiltonWD
1964 The genetical evolution of social behavior. J Theor Biol 7 1 52
2. MichodR
2007 Evolution of individuality during the transition from unicellular to multicellular life. Proc Natl Acad Sci USA 104 8613 8618
3. CrespiBJ
2001 The evolution of social behavior in microorganisms. Trends Ecol Evol 16 178 183
4. GriffinA
WestSA
2002 Kin selection: fact or fiction. Trends Ecol Evol 17 15 21
5. KessinRH
2001 Dictyostelium - Evolution, cell biology, and the development of multicellularity Cambridge, UK Cambridge Univ. Press
6. EichingerL
2005 The genome of the social amoeba Dictyostelium discoideum. Nature 435 43 57
7. ChisholmRL
FirtelRA
2004 Insights into morphogenesis from a simple developmental system. Nature Rev Mol, Cell Biol 5 531 541
8. FosterKR
FortunatoA
StrassmannJE
QuellerDC
2002 The costs and benefits of being a chimera. Proc Roy Soc London B 269 2357 2362
9. FosterKR
ShaulskyG
StrassmannJE
QuellerDC
ThompsonCRL
2004 Pleiotropy as a mechanism to stabilize cooperation. Nature 431 693 696
10. QuellerDC
PonteE
BozzaroS
StrassmannJE
2003 Single-gene greenbeard effects in the social amoeba, Dictyostelium discoideum. Science 299 105 106
11. SantorelliL
ThompsonC
VillegasE
SvetzJ
DinhC
2008 Facultative cheater mutants reveal the genetic complexity of cooperation in social amoebae. Nature 451 1107 1110
12. StrassmannJE
ZhuY
QuellerDC
2000 Altruism and social cheating in the social amoeba Dictyostelium discoideum. Nature 408 965 967
13. FortunatoA
StrassmannJE
Santorelli, LL
QuellerDC
2003 Co-occurrence in nature of different clones of the social amoeba, Dictyostelium discoideum. Mol Ecol 12 1031 1038
14. GilbertOM
FosterKR
MehdiabadiNJ
StrassmannJE
QuellerDC
2007 High relatedness maintains multicellular cooperation in a social amoeba by controlling cheater mutants. Proc Natl Acad Sci USA 104 8913 8917
15. OstrowskiEA
KatohM
ShaulskyG
QuellerDC
StrassmannJE
2008 Kin discrimination increases with genetic distance in a social amoeba. PLOS Biol 6 e287 doi:10.1371/journal.pbio.0060287
16. BenabentosR
HiroseS
SucgangR
CurkT
KatohM
2009 Polymorphic members of the lag gene family mediate kin discrimination in Dictyostelium. Curr Biol 19 567 572
17. RaperKB
1984 The Dictyostelids Princeton, NJ Princeton University Press
18. WallaceMA
RaperKB
1979 Genetic exchanges in the macrocysts of Dictyostelium discoideum. J Gen Microbiol 113 327 337
19. ErdosGW
RaperKB
VogenLK
1973 Mating types and macrocyst formation in Dictyostelium discoideum. Proc Nat Acad Sci USA 70 1828 1830
20. FrancisD
1998 High frequency recombination during the sexual cycle of Dictyostelium discoideum. Genetics 148 1829 1832
21. International SNP Map Working Group 2001 A map of human genome sequence variation containing 1.42 million single nucleotide polymorphisms. Nature 409 928 933
22. CaicedoAL
WilliamsonSH
HernandezRD
BoykoA
Fledel-AlonA
2007 Genome-wide patterns of nucleotide polymorphism in domesticated rice. PLoS Genet 3 e163 doi:10.1371/journal.pgen.0030163
23. TajimaF
1989 Statistical method for testing the neutral mutation hypothesis by DNA polymorphism. Genetics 123 585 595
24. LêS
JosseJ
HussonF
2008 FactoMineR: An R package for multivariate analysis. J Stat Soft 25 5
25. WakeleyJ
1997 Using the variance of pairwise differences to estimate the recombination rate. Genet Res 69 45 48
26. McVeanG
AwadallaP
FearnheadP
2002 A coalescent-based method for detecting and estimating recombination from gene sequences. Genetics 160 1231 1241
27. McConnellR
MiddlemistS
ScalaC
StrassmannJE
QuellerDC
2007 An unusually low microsatellite mutation rate in Dictyostelium discoideum, an organism with unusually abundant microsatellites. Genetics 177 1499 1507
28. BonnerJT
1982 Evolutionary strategies and developmental constraints in the cellular slime molds. Am Nat 119 530 552
29. KessinRH
GundersenGG
ZaydfudimV
GrimsonM
1996 How cellular slime molds evade nematodes. Proc Natl Acad Sci USA 93 4857 4861
30. SuthersHB
1985 Ground-feeding migratory songbirds as cellular slime mold distribution vectors. Oecologia 65 526 530
31. GilbertOM
QuellerDC
StrassmannJE
2009 Discovery of a large clonal patch of a social amoeba: Implications for social evolution. Mol Ecol 18 1273 1281
32. HussMJ
1989 Dispersal of cellular slime molds by two soil invertebrates. Mycologia 81 677 682
33. SmithMH
2004 Genotypic diversity and population structure in Dictyostelium discoideum. M.A. Thesis, Rice University, Houston, Texas
34. DawkinsR
1976 The Selfish Gene Oxford Oxford University Press
35. GardnerA
WestSA
2010 Greenbeards. Evolution 64 25 38
36. VosM
VelicerG
2009 Social conflict in centimeter - and global-scale populations of the bacterium Myxococcus xanthus. Curr Biol 19 1763 1767
37. LewisKE
O'DayDH
1994 Cannibalistic sexual phagocytosis in Dictyostelium discoideum is modulated by adenosine via an A2-like receptor. Cellular Signalling 6 217 222
38. RozenS
SkaletskyHJ
2000 Primer3 on the WWW for general users and for biologist programmers.
KrawetzS
MisenerS
Bioinformatics Methods and Protocols: Methods in Molecular Biology Totowa, NJ Humana Press
39. OlsenKM
CaicedoAL
PolatoN
McClungA
McCouchS
2006 Selection under domestication: Evidence for a sweep in the rice waxy genomic region. Genetics 173 975 983
40. WattersonGA
1975 On the number of segregating sites in genetical models without recombination. Theor Popul Biol 7 256 276
41. TajimaF
1983 Evolutionary relationship of DNA sequences in finite populations. Genetics 105 437 460
42. HudsonRR
SlatkinM
MaddisonWP
1992 Estimation of levels of gene flow from DNA sequence data. Genetics 132 583 589
43. ThorntonK
2003 Libsequence: A C++ class library for evolutionary genetic analysis. Bionformatics 19 2325 2327
44. HillWG
RobertsonA
1968 Linkage disequilibrium in finite populations. Theor Appl Genet 38 226 231
45. FalushD
StephensM
PritchardJK
2003 Inference of population structure using multilocus genotype data: linked loci and correlated allele frequencies. Genetics 164 1567 1587
46. EvannoG
RegnautS
GoudetJ
2005 Detecting the number of clusters of individuals using the software STRUCTURE: A simulation study. Mol Ecol 14 2611 2620
47. FelsensteinJ
1989 PHYLIP – Phylogeny Inference Package (Version 3.2). Cladistics 5 164 166
48. KimuraM
1980 A simple method for estimating evolutionary rate of base substitutions through comparative studies of nucleotide sequences. J Mol Evol 16 111 120
49. HudsonRR
KaplanN
1985 Statistical properties of the number of recombination events in the history of a sample. Genetics 111 147 164
50. RonaghiM
UhlenM
MyrenP
1998 A sequencing method based on real-time pyrophosphate. Science 281 363 365
51. QuellerDC
GoodnightKF
1989 Estimation of genetic relatedness using allozyme data. Evolution 43 258 275
52. SchultzBB
1985 Levene's test for relative variation. Sys Zool 34 449 456
53. RobsonG
WilliamsK
1980 The mating system of the cellular slime mould Dictyostelium discoideum. Curr Genetics 1 229 0232
54. CutterAD
2006 Nucleotide polymorphism and linkage disequilibrium in wild populations of the partial selfer Caenorhabditis elegans. Genetics 172 171 184
55. CutterAD
BairdSE
CharlesworthD
2006 High nucleotide polymorphism and rapid decay of linkage disequilibrium in wild populations of Caenorhabditis remanei. Genetics 174 901 913
56. KimS
PlagnolV
HuTT
ToomajianC
ClarkRM
2007 Recombination and linkage disequilibrium in Arabidopsis thaliana. Nat Genet 39 1151 1155
57. MatherKA
CaicedoAL
PolatoN
OlsenKM
McCouchSR
2007 The extent of linkage disequilibrium in rice (Oryza sativa L.). Genetics 177 2223 2232
58. SchachererJ
ShapiroJA
RuderferDM
KruglyakL
2009 Comprehensive polymorphism survey elucidates population structure of Saccharomyces cerevisiae. Nature 458 342 346
59. TsaiIJ
BensassonD
BurtA
KoufopanouV
2008 Population genomics of the wild yeast Saccharomyces paradoxus: Quantifying the life cycle. Proc Natl Acad Sci USA 105 4957 4962
Štítky
Genetika Reprodukční medicína
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2010 Číslo 7- Růst a vývoj dětí narozených pomocí IVF
- Délka menstruačního cyklu jako marker ženské plodnosti
- Vztah užívání alkoholu a mužské fertility
- Šanci na úspěšný průběh těhotenství snižují nevhodné hladiny progesteronu vznikající při umělém oplodnění
- Vliv melatoninu a cirkadiálního rytmu na ženskou reprodukci
-
Všechny články tohoto čísla
- Question and Answer: An Anniversary Interview with Jane Gitschier
- Multi-Variant Pathway Association Analysis Reveals the Importance of Genetic Determinants of Estrogen Metabolism in Breast and Endometrial Cancer Susceptibility
- Tinkering Evolution of Post-Transcriptional RNA Regulons: Puf3p in Fungi as an Example
- The Importance of Imprinting in the Human Placenta
- Regulator of G Protein Signaling 3 Modulates Wnt5b Calcium Dynamics and Somite Patterning
- Lysosomal Dysfunction Promotes Cleavage and Neurotoxicity of Tau
- Combinatorial Binding Leads to Diverse Regulatory Responses: Lmd Is a Tissue-Specific Modulator of Mef2 Activity
- Variation, Sex, and Social Cooperation: Molecular Population Genetics of the Social Amoeba
- Comparative Analysis of DNA Replication Timing Reveals Conserved Large-Scale Chromosomal Architecture
- The Fitness Landscapes of -Acting Binding Sites in Different Promoter and Environmental Contexts
- Cohesin Is Limiting for the Suppression of DNA Damage–Induced Recombination between Homologous Chromosomes
- Genome-Wide Analysis Reveals Novel Genes Essential for Heme Homeostasis in
- Genome-Wide Meta-Analysis for Serum Calcium Identifies Significantly Associated SNPs near the Calcium-Sensing Receptor () Gene
- Rad3 Decorates Critical Chromosomal Domains with γH2A to Protect Genome Integrity during S-Phase in Fission Yeast
- Quantitative and Molecular Genetic Analyses of Mutations Increasing Life Span
- Association of Variants at with Chronic Kidney Disease and Kidney Stones—Role of Age and Comorbid Diseases
- Breast Cancer DNA Methylation Profiles Are Associated with Tumor Size and Alcohol and Folate Intake
- Calpain 8/nCL-2 and Calpain 9/nCL-4 Constitute an Active Protease Complex, G-Calpain, Involved in Gastric Mucosal Defense
- A Collection of Target Mimics for Comprehensive Analysis of MicroRNA Function in
- A Genome-Wide Analysis Reveals No Nuclear Dobzhansky-Muller Pairs of Determinants of Speciation between and , but Suggests More Complex Incompatibilities
- Microevolution of during Prolonged Infection of Single Hosts and within Families
- Id4, a New Candidate Gene for Senile Osteoporosis, Acts as a Molecular Switch Promoting Osteoblast Differentiation
- CHD7 Targets Active Gene Enhancer Elements to Modulate ES Cell-Specific Gene Expression
- Chromatin Remodeling in Development and Disease: Focus on CHD7
- Extensive DNA End Processing by Exo1 and Sgs1 Inhibits Break-Induced Replication
- Requirement of Male-Specific Dosage Compensation in Females—Implications of Early X Chromosome Gene Expression
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- CHD7 Targets Active Gene Enhancer Elements to Modulate ES Cell-Specific Gene Expression
- Extensive DNA End Processing by Exo1 and Sgs1 Inhibits Break-Induced Replication
- Question and Answer: An Anniversary Interview with Jane Gitschier
- Multi-Variant Pathway Association Analysis Reveals the Importance of Genetic Determinants of Estrogen Metabolism in Breast and Endometrial Cancer Susceptibility
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



