-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Mutation in Archain 1, a Subunit of COPI Coatomer Complex, Causes Diluted Coat Color and Purkinje Cell Degeneration
Intracellular trafficking is critical for delivering molecules and organelles to their proper destinations to carry out normal cellular functions. Disruption of intracellular trafficking has been implicated in the pathogenesis of various neurodegenerative disorders. In addition, a number of genes involved in vesicle/organelle trafficking are also essential for pigmentation, and loss of those genes is often associated with mouse coat-color dilution and human hypopigmentary disorders. Hence, we postulated that screening for mouse mutants with both neurological defects and coat-color dilution will help identify additional factors associated with intracellular trafficking in neuronal cells. In this study, we characterized a mouse mutant with a unique N-ethyl-N-nitrosourea (ENU)–induced mutation, named nur17. nur17 mutant mice exhibit both coat-color dilution and ataxia due to Purkinje cell degeneration in the cerebellum. By positional cloning, we identified that the nur17 mouse carries a T-to-C missense mutation in archain 1 (Arcn1) gene which encodes the δ subunit of the coat protein I (COPI) complex required for intracellular trafficking. Consistent with this function, we found that intracellular trafficking is disrupted in nur17 melanocytes. Moreover, the nur17 mutation leads to common characteristics of neurodegenerative disorders such as abnormal protein accumulation, ER stress, and neurofibrillary tangles. Our study documents for the first time the physiological consequences of the impairment of the ARCN1 function in the whole animal and demonstrates a direct association between ARCN1 and neurodegeneration.
Published in the journal: . PLoS Genet 6(5): e32767. doi:10.1371/journal.pgen.1000956
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1000956Summary
Intracellular trafficking is critical for delivering molecules and organelles to their proper destinations to carry out normal cellular functions. Disruption of intracellular trafficking has been implicated in the pathogenesis of various neurodegenerative disorders. In addition, a number of genes involved in vesicle/organelle trafficking are also essential for pigmentation, and loss of those genes is often associated with mouse coat-color dilution and human hypopigmentary disorders. Hence, we postulated that screening for mouse mutants with both neurological defects and coat-color dilution will help identify additional factors associated with intracellular trafficking in neuronal cells. In this study, we characterized a mouse mutant with a unique N-ethyl-N-nitrosourea (ENU)–induced mutation, named nur17. nur17 mutant mice exhibit both coat-color dilution and ataxia due to Purkinje cell degeneration in the cerebellum. By positional cloning, we identified that the nur17 mouse carries a T-to-C missense mutation in archain 1 (Arcn1) gene which encodes the δ subunit of the coat protein I (COPI) complex required for intracellular trafficking. Consistent with this function, we found that intracellular trafficking is disrupted in nur17 melanocytes. Moreover, the nur17 mutation leads to common characteristics of neurodegenerative disorders such as abnormal protein accumulation, ER stress, and neurofibrillary tangles. Our study documents for the first time the physiological consequences of the impairment of the ARCN1 function in the whole animal and demonstrates a direct association between ARCN1 and neurodegeneration.
Introduction
Intracellular trafficking is critical for delivering molecules and organelles to their proper destinations to perform normal cellular functions (reviewed in [1]). Proteins and lipids are transported to the target cellular compartments through vesicular trafficking pathways, and organelles are trafficked along microtubules and actin cytoskeleton. Impairment of intracellular trafficking has been implicated in the pathogenesis of various neurodegenerative disorders, such as Alzheimer's disease (reviewed in [2], [3]), Huntington disease [4]–[6] and Parkinson's disease [7], indicating the importance of intracellular trafficking for proper function and maintenance of neuronal cells. However, factors that are involved in this process and their associations with the mechanisms causing neurodegeneration are not yet fully understood.
The molecular mechanisms of intracellular trafficking are shared by different cell types and organ systems. This is evident from the fact that multiple organ systems are affected in a number of human diseases caused by defects in intracellular trafficking. For example, Hermansky-Pudlak Syndrome (HPS), a collection of heterogeneous genetic disorders caused by defects in intracellular vesicle trafficking, is characterized by oculocutaneous albinism and defective platelet storage [8], suggesting that the affected protein trafficking pathways are shared between skin and blood cells. Fundamental intracellular trafficking mechanisms are also shared by the skin and nervous systems. For example, Griscelli syndrome (GS) type 1 which is caused by mutations in the myosin 5a (MYO5A) gene is characterized by pigmentary dilution of the skin and hair and neurological defects with severe ataxic movement [9]. The Myo5a mouse mutant, dilute, also exhibits coat color dilution and severe ataxic movement [10]. Studies on dilute mice demonstrated that MYO5A, an actin-based motor protein, participates in organelle trafficking in both melanocytes and neuronal cells [11]–[13]. Intracellular melanosome trafficking is disrupted in melanocytes of dilute mice, leading to coat color dilution [13]. Moreover, endoplasmic reticulum (ER) transport in cerebellar Purkinje cells (PCs) is disrupted in dilute mutants, which may result in neurological defects [11]. Mutations in Fig4 and Vac14, affecting the conversion of phosphatidylinositol-3-phosphate (PI3P) to the signaling lipid phosphatidylinositol-3,5-bisphosphate (Pl(3,5)P2), also lead to neurodegeneration and diluted pigmentation in mice [14], [15]. Pl(3,5)P2 regulates vesicle trafficking in the endosome-lysosome axis in yeast [16], and abnormalities indicative of defects in the regulation of endosomal vesicles are observed in these mutant mice [14], [15]. Pigment containing hair follicles are greatly reduced in the skin of Fig4 mutant mice, suggesting that lysosome-melanosome biogenesis may be affected [14]. Another mouse mutant with both pigmentary and neurological defects is the sandy mouse with a mutation in a component of biogenesis of lysosome-related organelles complex 1 (BLOC-1), which regulates trafficking to lysosome-related organelles including melanosomes [17].
Based on the findings that the vesicle/organelle trafficking pathways are shared among multiple organs, we postulated that screening for mouse mutants exhibiting phenotypes indicative of defects in intracellular trafficking in multiple tissues may yield molecules important for this process. Among defects in different organs, coat color dilution is a good indicator for defective intracellular trafficking because the defect can be readily observed in the whole animal. A number of mouse mutants that show coat color dilution have been isolated and defective vesicle and organelle trafficking pathways are the major causes of coat color dilution in these mice [8], [18]. For example, all the sixteen mouse mutants with dilute coat color that were identified as models to study HPS carry mutations in genes that are involved in vesicle trafficking to melanosomes [8], [19], [20]. Mouse models of Griscelli syndrome also exhibit dilute coat color and bear mutations in components of the RAB27A-MLPH-MYO5A complex that are necessary for proper intracellular trafficking of melanosomes [10], [21], [22].
In this study, we screened for mice with both a neurological defect (ataxia) and coat color dilution, and identified a novel mutant, neurological 17 (nur17). We performed positional cloning of the nur17 mutation and identified a single nucleotide substitution in archain 1 (Arcn1), a highly conserved gene that encodes the δ subunit of the coat protein I (COPI) complex [23]. Further characterization of the coat color and neuronal phenotypes indicated that the nur17 mutation perturbs intracellular protein trafficking and ER function in the affected tissues. Our study is the first demonstration of the physiological consequences of the impairment of the ARCN1 function in mammalian tissues in vivo, and provides a direct link between the ARCN1 functions and neurodegeneration.
Results
Coat-color dilution and progressive Purkinje cell degeneration in nur17 mice
The autosomal recessive nur17 mutation was generated by ENU mutagenesis at Mouse Mutagenesis Center for Developmental Defects at Baylor College of Medicine [24], [25]. Initial phenotypic characterization showed that nur17 mice exhibit both coat color dilution (Figure 1A) and ataxic movements. The coat color dilution phenotype of nur17 mice was noted to be milder compared with that of dilute-lethal (Myo5ad-l) mice [10], and to become more notable with age. The ataxic phenotype, which is also milder in nur17 mice than that of Myo5ad-l mice, was observed around the age of 2 months and developed progressively as the mice age. The size of nur17 mice was smaller compared to unaffected control littermates (+/+ or nur17/+) (Figure 1A and 1B) (control: n = 5, nur17 mice: n = 7; p<0.01 by student's t-test).
Fig. 1. Coat color dilution and Purkinje cell degeneration in nur17 mice. 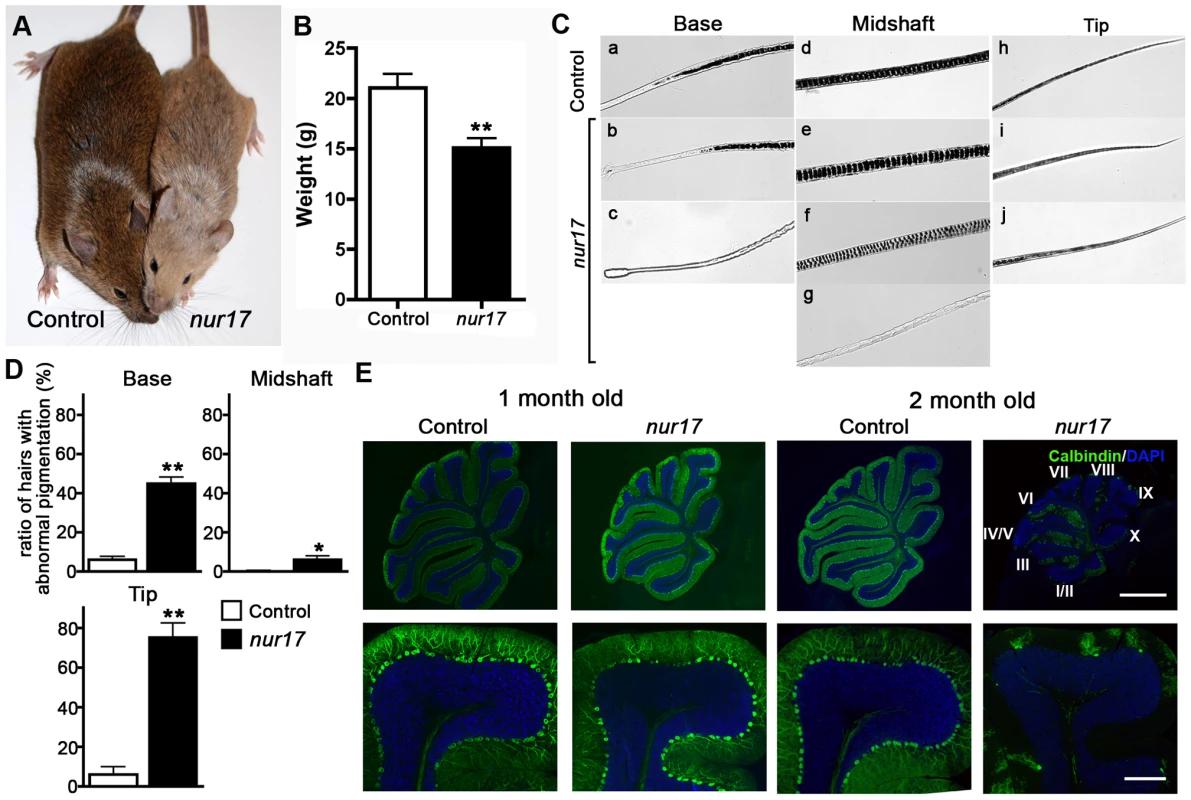
(A) Diluted coat color in nur17 mice (right) compared with control mice (left). (B) Body weight of 2-month old nur17 (n = 7, female) and control (n = 5, female) mice. Error bars represent standard error. **p<0.01 (C) Pigment distribution in the hair of control and nur17 mice. Base, midshaft and tips of the hair of control (top) and nur17 (bottom) mice are shown. Representative pictures from 30 hairs of each genotype are shown. (D) Ratio of hairs with abnormal pigmentation in the base (left), midshaft (center) and tip (right). Total of 150 hairs from 5 nur17 and 5 control mice (30 hairs each) were examined. Error bar represent standard error. *p<0.05; **p<0.01 (E) Degenerative loss of the Purkinje cells in nur17 mice. Purkinje cells are labeled with anti-calbindin (green) and nuclei are stained with DAPI (blue). Note that by 2 months of age, nur17 mice lose their Purkinje cells in Lobule IX. Upper scale bar: 1mm; Bottom scale bar: 0.1mm. To further characterize the coat color dilution, we examined the distribution of pigment in hair samples from nur17 and littermate control (+/+, nur17/+) mice. In the hair of control mice, the pigment was incorporated all the way to the tip in a regularly repeated pattern (Figure 1C, top). In the hair of nur17 mice, we observed increased spacing between pigment bands and an occasional absence of pigment in the midshaft (Figure 1C, bottom). The ratio of hairs with abnormal pigmentation at the base, midshaft and tip was significantly higher in nur17 mice compared to control mice (control: n = 5, nur17 mice: n = 5, 30 hairs from each mouse, Student's t-test, Figure 1D). These hair phenotypes suggest that the nur17 gene product is required for the proper transport/incorporation of pigment into the hair.
nur17 mice also exhibit ataxic movements beginning around 2 months of age. Because the cerebellum is the center for motor coordination, we examined the morphology of the cerebellum in nur17 mice. Purkinje cells (PCs) were visualized using a PC marker, anti-calbindin [26]. In littermate control (+/+, nur17/+) mice, PCs were aligned in a single cell layer adjacent to each other (Figure 1E). At 1 month of age before the appearance of ataxic movements, nur17 mice showed normal cerebellar morphology with an intact PC layer despite showing a few signs of degeneration (Figure 1E). However, in 2-month-old nur17 mice, we observed extensive loss of PCs compared to control mice (Figure 1E, right panels). We also found a specific regional pattern for PC degeneration in nur17 mice. By 2 months of age, most of the PCs in a part of lobule VI and lobules VII to X of nur17 mice have degenerated (Figure 1E). In contrast, PCs in lobules I to V remained at this age (Figure 1E).
Positional cloning of the nur17 gene
We genetically mapped the nur17 gene on mouse chromosome 9 using an F2 intercross (nur17 × AKR). All F1 animals (14 females and 13 males) were phenotypically normal and did not show any coat color dilution or ataxic phenotypes. To map the nur17 locus, we employed microsatellite markers and single-nucleotide polymorphisms (SNPs) to distinguish between the alleles of nur17 (mixed background of C57BL/6J, 129S6/SvEvTac and 129S1/SvImJ) and AKR. For initial mapping, we tested genomic DNA from F2 mice including 8 affected mice with 80 microsatellite markers across the whole genome. We observed co-segregation of a marker D9Mit69 with the nur17 phenotypes and found no significant linkages with other chromosomal loci. Additional F2 animals were tested to further narrow down the nur17 locus. We collected a total of 822 meioses (411 F2 mice) (Figure 2A). Of note, the coat color dilution and ataxic phenotypes always co-segregated in this mapping cross, indicating that a single gene mutation likely accounts for both phenotypes. After progeny testing of F2 mice carrying critical recombination (Figure 2B), the minimal genetic region of nur17 was determined to be between D9SNP25 and D9Mit69 (Figure 2C). A total of 36 genes are localized within this 1.06 Mb interval. We selected 4 genes, dolichyl-phosphate N-acetylglucosamine phosphotransferase (Dpagt1), vacuolar protein sorting 11 (Vps11), trafficking protein particle complex 4 (Trappc4) and archain 1 (Arcn1), as candidates for nur17, since they are known to be associated with vesicle trafficking. We sequenced the coding regions of these candidate genes in nur17 mice and did not observe any nucleotide change in Dpagt1, Vps11 and Trappc4. However, we identified a single nucleotide change in the Arcn1 gene (Figure 2D). This T to C conversion in the 10th exon of the Arcn1 gene causes an amino acid change from isoleucine (Ile) to threonine (Thr) at amino acid 422 (Figure 2D and 2E). The mutation is in the vicinity of the cargo-binding site of ARCN1 which recognizes arginine-based ER localization signals [27].
Fig. 2. Positional cloning of the nur17 gene. 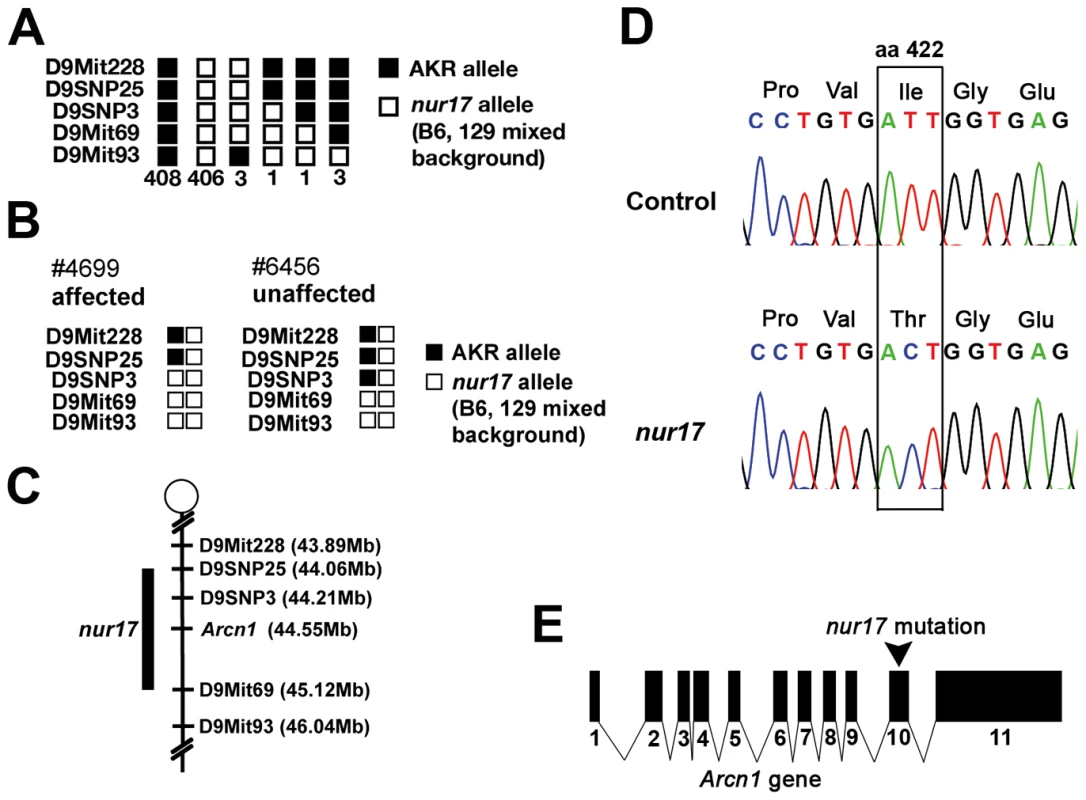
(A) The haplotypes for 822 chromosomes from 411 F2 mice are indicated. (B) Genotypes and phenotypes of two recombinant mice that were critical for determining the minimal region for the nur17 locus. (C) Minimal genetic region of the nur17 locus. (D) Point mutation in the Arcn1 gene from nur17 mice. T to C nucleotide transition causes an amino-acid change from Ile to Thr at amino acid (aa) 422. (E) Point mutation is located in the 10th exon of the Arcn1 gene in nur17 mice. Transgenic expression of wild-type Arcn1 rescues nur17 phenotypes
To confirm that the Arcn1 mutation is responsible for the phenotypes in nur17 mice, we tested whether transgenic (Tg) expression of wild-type Arcn1 can rescue the nur17 phenotypes. We generated a Tg construct (Figure 3A), in which expression of the Arcn1 cDNA is driven by the chicken β-actin promoter and is followed by the rabbit β-globin poly-A sequence (Figure 3A) [28]. We obtained 2 founder mice (2361 and 2368) that carried the transgene and named the mice generated from these 2 founder mice as line 2361 and line 2368 (Figure S1A). Phenotypes of nur17 mice were rescued in line 2361, in which expression of the transgene was confirmed (Figure 3B–3D, Figure S1B). The coat color dilution and lower body weight in nur17 mice were completely rescued in mice expressing the wild-type Arcn1 transgene (Figure 3B and 3C). We also analyzed the ataxic phenotype using rotarod (Figure 3D). While this analysis revealed significantly decreased latency in nur17 mice compared to control mice (p = 0.0046 by student's t-test), this phenotype was rescued by the expression of the Arcn1 transgene (Figure 3D). In addition, we examined the PC degeneration phenotype in 2 month-old mice and observed no PC degeneration in nur17 mice carrying the transgene (Figure 3D, right). We tested a total of 7 nur17/nur17;Tg/+ mice and the phenotypes were rescued in all of these mice. In line 2368, the phenotypes of nur17 mice were not rescued (data not shown). However, we found that the transgene is not expressed in this line (Figure S1B). These results demonstrated that the single nucleotide change in Arcn1 is the causal mutation in nur17 mice.
Fig. 3. Phenotypes of nur17 mice are rescued by wild-type Arcn1. 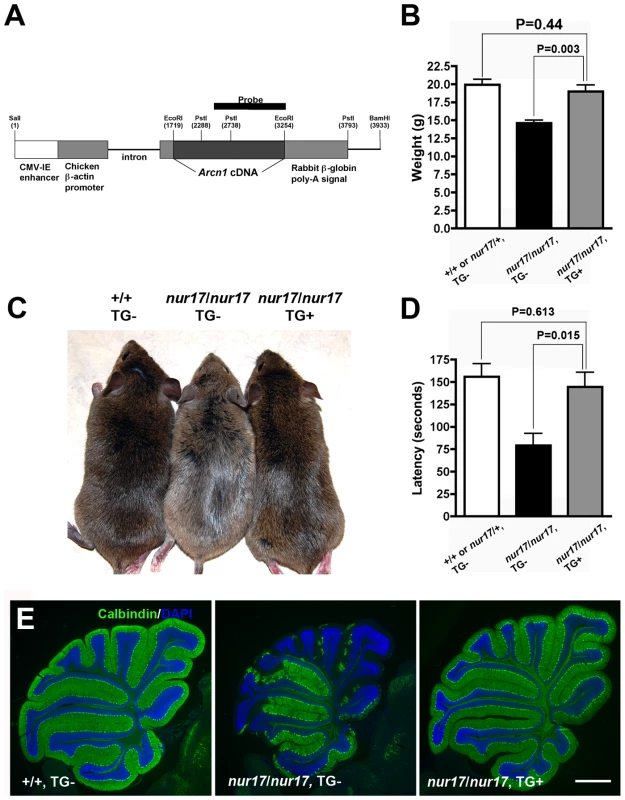
(A) Transgenic construct for generating the transgenic mice expressing Arcn1 gene. Restriction enzyme (PstI) sites to digest DNA for southern blotting were labeled. The black bar represents the region used for the probe for southern blot. (B) Rescue of smaller body weight by transgene in nur17 mice. Smaller size in nur17 mice (middle, n = 13, female) is rescued by the introduction of transgene, Arcn1 (right, n = 6, female) (p = 0.003). There is no significant difference between the weight of rescued mice (right) and the control mice (left, n = 7, female) (p = 0.44). (C) Rescue of coat color dilution. Coat color dilution phenotype in nur17 mice (middle) is rescued by the introduction of transgene, Arcn1 (right). (D) Rescue of the motor coordination defect. Latency to fall on the accelerating rotatod was recorded for control mice (n = 5), nur17 mice (n = 5) and nur17 mice with the Arcn1 transgene (n = 5). The rotarod performance was significantly improved by the transgene (p = 0.015), and was comparable to that in control mice (p = 0.613). (E) Rescue of Purkinje cell degeneration. Purkinje Cell degeneration phenotype in 2-month old nur17 mice (middle) is completely rescued by the introduction of transgene, Arcn1 (right). Purkinje cells are labeled with anti-calbindin (green) and nuclei are stained with DAPI (blue). Scale bar: 1mm. Expression and cellular localization of ARCN1
ARCN1, also known as δ-COP, is a subunit of the coat protein I (COPI) complex [29]. The COPI complex was first isolated from mammalian sources as a cytosolic protein complex and components of Golgi derived vesicles [30], [31]. It was originally found to be involved in retrograde trafficking from the cis-Golgi to the rough ER (reviewed in [32]) and in intra-Golgi trafficking (reviewed in [33], [34]) mostly based on studies in yeast. Studies using mammalian cells with a mutation or depletion of a COPI subunit have shown that COPI is essential for compartmentalization of secretory compartments, proper Golgi structure, ER-Golgi transport and endosome functions [35]–[38]. Although the phenotypes of a mutant for the yeast homolog of ARCN1 suggests its involvement in both retro and antero-grade ER-Golgi trafficking [39], the role of this particular COPI subunit (δ-COP) in mammalian cells and the physiological consequences of its impairment in the whole animal have not been examined. In order to understand the function of ARCN1 in mammalian cells, we first examined sub-cellular localization of ARCN1 using anti-ARCN1 antibody (green) in primary cultured mouse melanocytes (Figure 4). ARCN1 was co-localized with Golgi (Figure 4Ai) and ER (Figure 4Aii) markers (red) and showed a punctate staining pattern in the cytoplasm suggesting its localization in the vesicular compartment (Figure 4Ai and Aii). In mouse melanocytes, ARCN1 was co-localized with β-COP (red, Figure 4Aiii), which is a known binding partner of ARCN1 in yeast [29], mainly in ER and Golgi. This result indicated that ARCN1 (δ-COP) is present in a complex with β-COP in mammalian cells. We also noted that there are vesicles positive for only ARCN1 (green, arrowheads) and not β-COP (red) (Figure 4Aiii). This observation was confirmed by co-expressing GFP-tagged ARCN1 and V5-tagged β-COP (COPB1) in Neuro-2a cells (Figure 4B). The ARCN1 signal does not always co-localize with β-COP signal. These results indicate that ARCN1 may also exist unbound to β-COP in mammalian cells, suggesting the possibility that ARCN1 may also have a function independent of β-COP or the COPI complex. Further biochemical studies are required to test these possibilities.
Fig. 4. Subcellular localization of ARCN1. 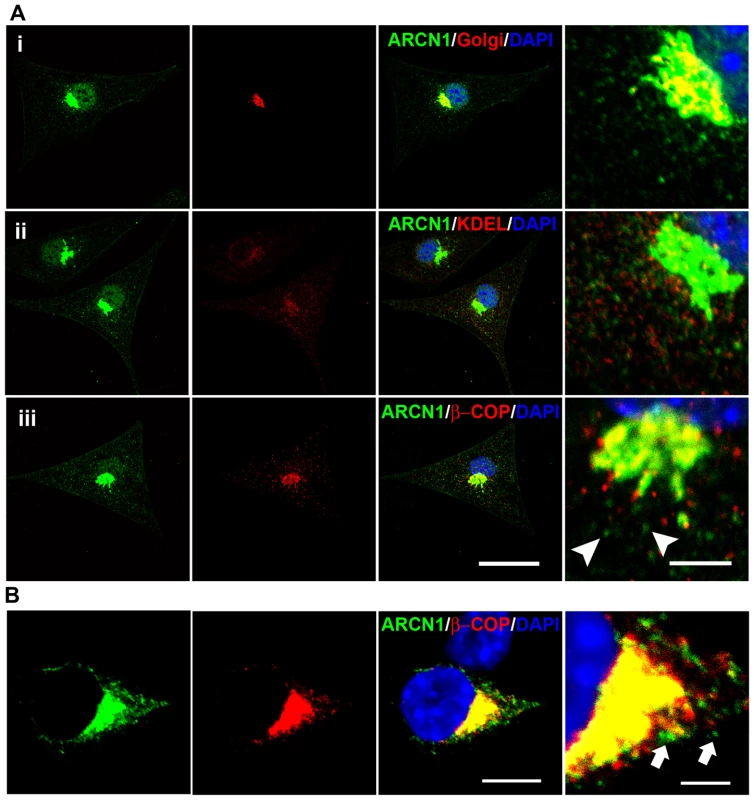
(A) Primary culture of wild-type mouse melanocytes are labeled with anti-ARCN1 (green) indicating the localization of ARCN1 and anti-Golgi protein (red) (i); anti-KDEL (red) (ii) and anti-βCOP (red) (iii). (B) Neuro-2a cells transfected with constructs for GFP-tagged ARCN1 (green) and V5-tagged βCOP (red). Nuclei are stained with DAPI (blue). Merged pictures are shown and high magnification pictures are shown in the right panel. Scale bar for low magnification: 10µm. Scale bar for high magnification: 5µm. ARCN1 is involved in ER–Golgi and/or intra-Golgi trafficking in melanocytes
In order to examine protein trafficking through ER and Golgi in nur17 cells, we performed pulse-chase analysis in nur17 melanocytes using the melanosomal membrane protein Tyrp1 as an indicator for intracellular trafficking [40], [41]. We monitored ER-Golgi transport using endoglycosidase H (endo H), which cleaves N-linked sugar chain moieties on newly synthesized proteins that are localized in the ER or intermediate compartments but not those that have been modified in the Golgi. Without endo H treatment [endo H (−)], newly synthesized Tyrp1 molecules that have been glycosylated during biosynthetic transport through ER and Golgi are represented by bands with higher molecular weight compared to the band in the endo H (−) lane at 0 min. With endo H treatment [endo H(+)], sugar chains on Tyrp1 molecules that have been processed in Golgi and thus become resistant to endo H digestion are represented by bands with higher molecular weight compared to the band in the endo H (+) lane at 0 min. In control melanocytes (from +/+, nur17/+ littermates), after 35 minutes of chase, higher molecular weight bands were observed with endo H and without endo H (black arrowheads in Figure 5A), suggesting that some of the Tyrp1 molecules have been trafficked into Golgi and undergone processing that confers endo H resistance. In contrast, little or no higher molecular weight bands were observed in the nur17 melanocytes at this timepoint (red arrowheads in Figure 5A). At 45 min, the bulk of Tyrp1 protein was shifted to a higher molecular weight form in control melanocytes, whereas a band of lower molecular weight still remained in nur17 melanocytes (white arrowheads). We quantified this shift by measuring the intensity of radioactive signals in these bands as shown in Figure 5B. The proportion of protein without complex N-linked glycans (lower band) was significantly higher in melanocytes of nur17 mice compared to cells from control mice (P<0.0001, 2 way ANOVA). These results suggest that the efficiency of protein trafficking through ER and Golgi may be affected in nur17 mice. Alternatively, it is possible that these results indicate pertubation of the glycosylation process in the Golgi of nur17 mice. If retrograde transport of oligosaccharide transferases within the Golgi apparatus is defective due to the mutation, deprivation of these enzymes in appropriate Golgi cisternae could occur, causing delay in glycosylation. This scenario would be consistent with the proposed function of COPI in intra-Golgi retrograde transport of resident proteins in the cisternal maturation model of protein transport through Golgi [34].
Fig. 5. Kinetic analysis of biosynthesis and transport of Tyrp1 in the melanocyte. 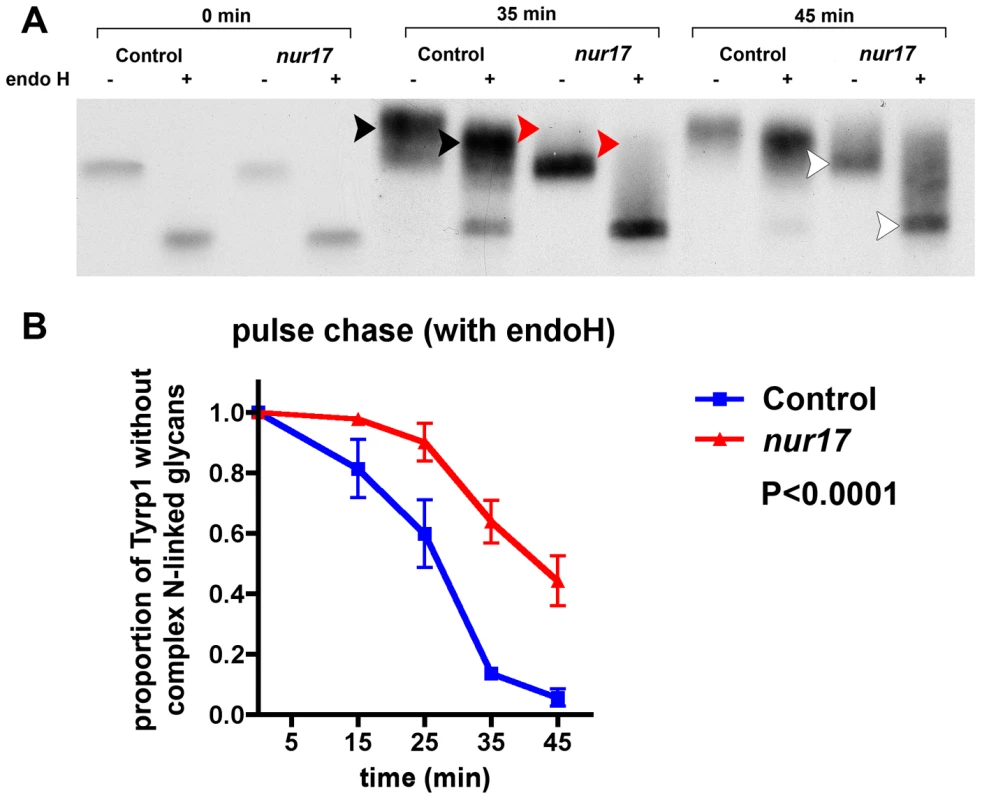
(A) Pulse chase analysis of control and nur17 melanocytes. Control and nur17 melanocytes were pulse labeled for 10 min with [35S] methionine followed by a chase as the indicated times. The size of Tryp1 is monitored after pulse labeling with [35S] and analyzed by 9% SDS-PAGE. (Molecular weight markers are shown on the left.) (B) Quantification of the proportion of Tyrp1 without complex N-linked glycans at 5 different chasing time points (0min, 15min, 25min, 35min and 45min). Error bar represents standard error. ER stress and abnormal protein aggregation in nur17 mice
Based on the possible trafficking defects observed in melanocytes of nur17 mice, we hypothesized that such intracellular trafficking mechanism is also impaired by the nur17 mutation in the PC. Electron microscopic analysis of the cerebellum of nur17 mice showed abnormal accumulation of proteins in the dendrites of PCs (red arrow, Figure 6A) as well as in perinuclear areas (red arrow, Figure 6B, PC nucleus is marked by red star) [42]. Since accumulation of misfolded protein has been associated with defects in vesicle trafficking followed by ER stress [7], we then examined whether ER stress occurs in the degenerating PCs of nur17 mice using an ER stress marker, anti-CCAAT/enhancer-binding protein homologous protein (CHOP) [42]. We observed high levels of CHOP signals in the cell bodies of some PCs in nur17 mice (Figure 6D, white arrowhead), while we never observed CHOP signals in PCs of littermate control (+/+, nur17/+) mice (Figure 6C). The mean ratio of CHOP positive PCs against total PCs was statistically higher in nur17 mice (0.84%; n = 4) compared to control mice (0%; n = 3) (p = 0.016 by student's t-test). The fact that not all PCs were positive for CHOP staining is consistent with the idea that PC degeneration does not occur simultaneously in all PCs but rather occurs progressively. We also performed immunohistochemistry with an anti-KDEL antibody, which recognizes the ER stress markers, BiP/Grp78 and Grp94. Although KDEL signals were detected in PCs of both control and nur17 mice, no difference was observed between them (Figure S2).
Fig. 6. Abnormal protein aggregation and upregulated ER stress marker in the cerebellum of nur17 mice. 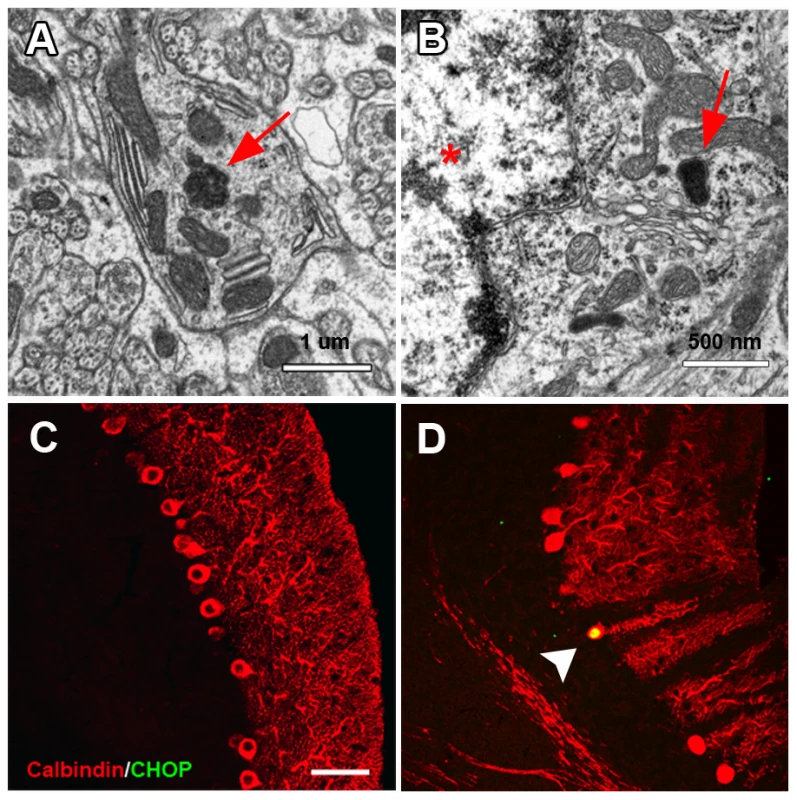
(A) Ultrastructural analysis showed protein inclusion (red arrow) in the dendrite of PC. (B) Protein inclusion (red arrow) also exists in the perinuclear region of PC. PC nucleus is marked by a red star. (C, D) PC labeled with anti-Calbindin (red) is negative for anti-CHOP antibody staining (green) in control mice (C) but positive in nur17 mice (D, white arrowhead). Scale bar: 30um. Neurofibrillary tangles in the cerebellum of nur17 mice
Through electron microscopic analysis, we also observed abnormal filamentous lesions in the cerebellum of all 30-day-old nur17 mice examined (n = 3) (Figure 7A), but not in the littermate control (+/+, nur17/+) (n = 3) mice. We examined the distribution of abnormal accumulation of filaments by Gallyas staining, which is known to stain protein aggregates of neurofilaments [43]. Central sagittal sections of the cerebellum from 4 control and 5 nur17 mice at 4 weeks of age were subjected to Gallyas staining (Figure 7B–7D), and the number of positively-stained cells was counted. While we observed no positively-stained cells in control mice (n = 4), an average of 83±28.8 PCs in the central sagittal section of cerebellum were positively stained in nur17 mice (n = 5, p<0.05, Student's t-test). High magnification images (Figure 7D) showed that staining is localized to the PC body and dendrites. Although hyperphosphorylated tau is known as the major component of NFTs in Alzheimer's disease, we did not observe signals when immunohistochemistry was performed on the cerebellum of nur17 mice using anti-hyperphosphorylated tau antibodies (data not shown), indicating that NFTs in nur17 mice may not contain hyperphosphorylated tau. It has been suggested that hyperphosphorylation of tau is not obligatory in the formation of neurofibrillary tangles [44], which may be the case in this mutant.
Fig. 7. Neurofibrilary tangles in the cerebellum of nur17 mice. 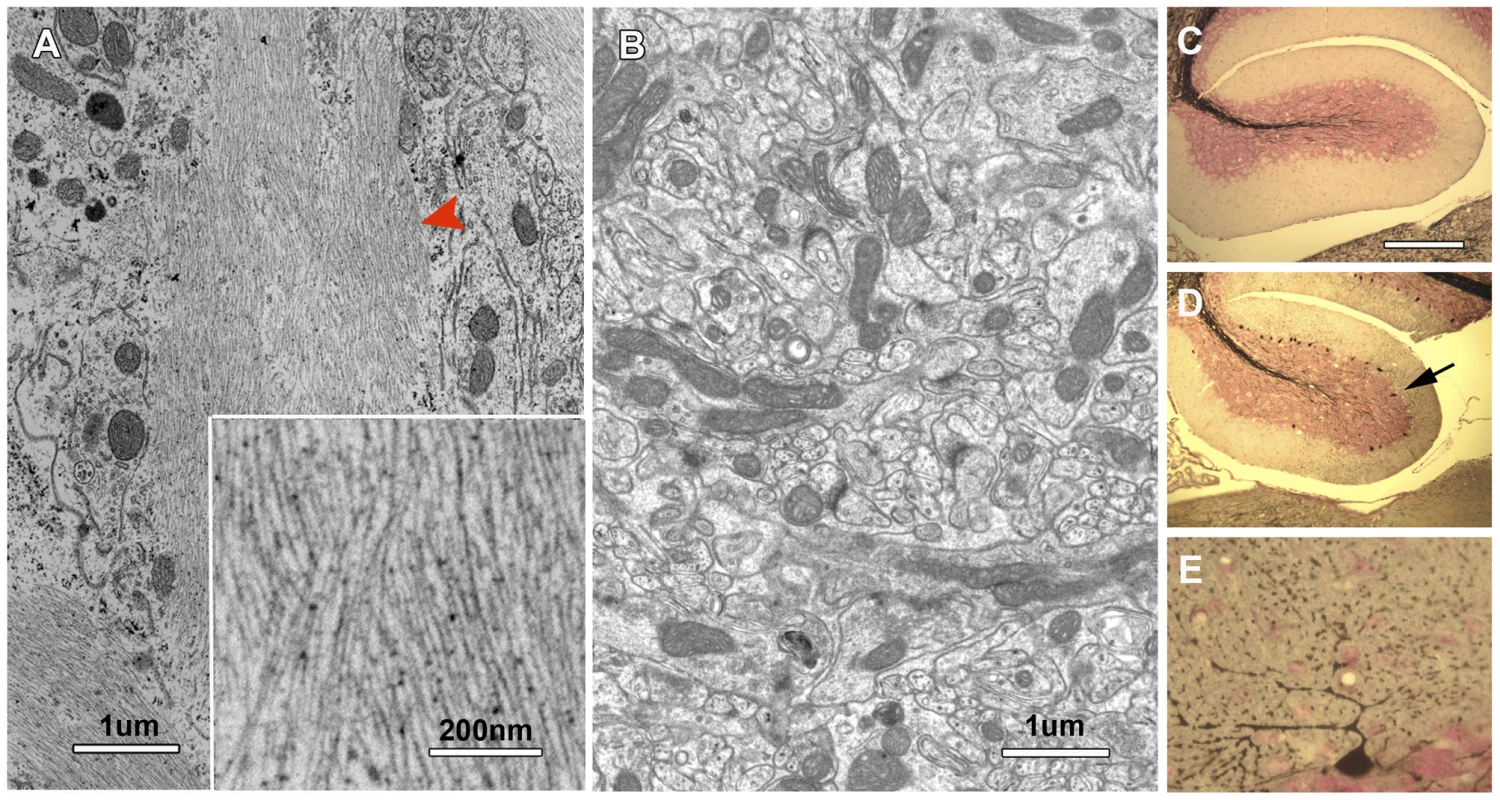
(A, B) Ultrastructural analysis showed abnormal filamentous lesions (red arrowhead) in the cerebellum of nur17 mice (A), while such lesions are not observed in control mice (B). The inset in (A) shows the higher magnification of filamentous lesion. (C–E) Gallyas staining for control (C) and nur17 mice (D, E). Arrow denotes the PC that is positive for Gallyas staining. Scale bar for (C, D): 1mm. Scale bar for (E): 0.1mm. Discussion
Here we show that a mutation in Arcn1 is responsible for the phenotypes in an ENU-induced mouse mutant, nur17, which was identified by screening for mice with both diluted coat color and ataxia. We identified a missense mutation in Arcn1 in nur17 mice and confirmed that it is responsible for coat color dilution and PC degeneration by transgenic rescue. ARCN1, also known as δ-COP, is a sub-unit of the coat protein I (COPI) complex, which comprises one of the protein coats that mediate vesicle budding from the membrane (reviewed in [32], [45]–[47]). Thus, nur17 mice provide a unique model to study the intracellular trafficking and its association with neuronal function and pigmentation in mammals.
Our results suggest that the single nucleotide substitution causing an amino acid change from isoleucine to threonine results in abnormal function of the ARCN1 protein. Since the mode of inheritance for the nur17 mutation is recessive, the mutation was expected to cause loss of the protein function rather than gain of function. Our transgenic rescue experiment showed that the wild-type allele of Arcn1 completely rescues the phenotypes of nur17 mice, providing evidence for this notion. We postulate that nur17 causes partial rather than complete loss of ARCN1 function based on the following reasons. First, Arcn1 encodes a protein that is highly conserved from yeast to human, and mutations of Arcn1 (δ-COP) orthologs in other species such as yeast [29], Drosophila (http://flybase.org, Flybase ID: FBal0096846) and C. elegans (http://www.wormbase.org/db, Wormbase RNAi ID: WBRNAi00033328 and WBRNAi00076118, [48]) all result in lethality, suggesting that this molecule is fundamental to cellular integrity and function. Secondly, mutants of other subunits of the COPI complex in Drosophila and zebrafish also result in early embryonic lethality [49], [50]. Compared to the phenotypes in other organisms, the phenotypes of nur17 mice are rather mild, supporting the possibility that the nur17 mutation causes partial loss of ARCN1 function. Thirdly, the mutation does not cause RNA instability or improper processing of the protein, which could cause complete loss of function, as the expression and localization of ARCN1 are not altered in the nur17 melanocytes (Figure S3). Alternatively, it is possible that other molecules with similar functions compensate for the loss of ARCN1 function to some extent. However, there are no obvious or known molecules that could play such a role. Thus, it is likely that this missense mutation uncovers roles for COPI that would not be discovered in a complete loss of function allele.
The COPI complex was originally found to be involved in retrograde vesicle trafficking from the cis-Golgi to the rough ER (reviewed in [32]). However, studies in yeast suggested that δ-COP may be involved in both anterograde and retrograde ER-Golgi trafficking [39]. Other studies also suggested expanded roles of COPI complex in anterograde ER-Golgi trafficking and trafficking between other compartments such as early endosomes and the secretory pathway [35], [38], [51]. Consistent with these findings, our results support the possibility that δ-COP has multiple roles in vesicle trafficking. First, the in vitro pulse-chase assay indicated that the nur17 mutation causes either delays in anterograde ER-Golgi protein trafficking or defects in intra-Golgi retrograde transport of resident proteins, suggesting the involvement of δ-COP in these processes in mammalian cells. Secondly, the localization study showed that ARCN1 is not only localized in ER-Golgi, but also distributed in the cytosolic space, which may represent localization of ARCN1 to other vesicular structures such as endosomes or lysosomes. Further analysis of nur17 mice should reveal whether ARCN1 functions in these organelles and whether the mutation affects their functions. Our pulse-chase study suggested a potential link between the Arcn1 mutation and coat color dilution. Tyrp1 is a tyrosinase related protein (TRP) and has been implicated in the biogenesis of melanosomes [52], [53]. The retardation of ER-Golgi transport of Tyrp1 from ER to Golgi in nur17 melanocytes or impaired glycosylation of this protein could potentially delay or inhibit melanosome biogenesis resulting in coat color dilution. It is also possible that ER-Golgi trafficking of other proteins required for melanosome synthesis is affected as well. However, impaired ER-Golgi and/or intra-Golgi trafficking may not be the only cause of coat color dilution. As discussed above, the potential site of action of ARCN1 is not restricted to ER-Golgi. Since biogenesis of melanosomes includes multiple aspects of vesicle regulation such as endocytosis and recycling of vesicles (reviewed in [54]), these processes could be also affected in nur17 melanocytes. Further analysis is needed to test these possibilities.
Defects in ER-Golgi or intra-Golgi trafficking similar to those observed in melanocytes of nur17 mice could also be responsible for PC degeneration in the cerebellum. For example, defective ER-Golgi trafficking could be the direct cause of the formation of abnormal protein accumulation in the ER, which could in turn result in ER-stress. On the other hand, ER-Golgi trafficking is required for the unfolded protein response (UPR), which is activated by ER-stress [55]. UPR helps to restore the normal ER function by halting protein synthesis and upregulating molecular chaperones involved in protein folding and ER associated degradation (ERAD) to clear the protein accumulation (reviewed in [56]). Therefore, defective ER-Golgi trafficking could be the indirect cause of abnormal protein accumulation as well. Our findings that abnormal protein accumulation and CHOP expression, which is a sign of ER stress, are observed in nur17 mice support both hypotheses. Since ER-stress is known to trigger signal transduction events that could induce cell death (reviewed in [57]), it may cause degeneration of PCs in nur17 mice. However, we did not observe upregulation of another ER stress marker, KDEL, which is the protein sequence found in both BiP/Grp78 and Grp94. This result may indicate that abnormalities in nur17 PCs do not affect the typical ER stress pathway. Alternatively, we may not be able to detect the increase in these proteins, because only small numbers of PCs may be experiencing it at a time, which would be consistent with the low number of CHOP-positive cells we observed in nur17 mice. Nevertheless, our results provide evidence that the defect in the COPI complex leads to neurodegeneration, and suggest that ER abnormalities may be involved in the neurodegenerative mechanism. Recent discovery of Scy1-like1 (Scyl1) as a gene responsible for motor neuron and PC degeneration in mice also suggests a link between the COPI pathway and neurodegeneration [58], [59]. SCYL1 binds to and co-localizes with β-COP and regulates COPI-mediated retrograde ER-Golgi trafficking [59]. Together with our results, these findings support the notion that compromised COPI-mediated vesicle trafficking causes neurodegeneration.
In addition to abnormal protein accumulation and possible signs of ER-stress, nur17 mice show neurofibrillary tangles (NFT) in the cerebellum that are commonly observed in a number of neurodegenerative disorders such as Alzheimer's Disease, Amyotrophic lateral sclerosis and Down's Syndrome (reviewed in [60]). Although ER stress and NFT are often observed together in many human neurodegenerative diseases [61]–[63], the relationship between these conditions is largely unknown. Our findings that a mutation in a component of the COPI complex, which regulates ER-Golgi vesicle trafficking, results in formation of NFT suggests a possible new mechanism underlying NFT formation. Impaired ER-Golgi vesicle trafficking could be the cause for NFT formation in this model. Further analysis of nur17 mice should advance our understanding of the molecular mechanism causing the formation of NFT, which may be shared by a variety of neurodegenerative disorders.
Provided that the Arcn1 gene is likely expressed ubiquitously in all cell types, it is intriguing that gross abnormalities have been detected only in the skin/hair and the cerebellum. It is possible that there is another protein that can compensate for the loss of ARCN1 in other cell types and tissues, although such protein has not been biochemically discovered to date. Alternatively, the affected tissues and cell types may be extraordinarily susceptible to minor changes in efficiency of COPI function. A mutation in another ubiquitously expressed protein known to function in ER-Golgi and intra-Golgi trafficking, TRAPPC6A, also leads to a tissue-restricted phenotype, pigment dilution, in mice [64]. Skin cells may be particularly sensitive to defects in these trafficking mechanisms. On the other hand, skin may be a unique tissue where subtle defects in intracellular trafficking can be visibly identified without the integrity of the skin being grossly affected, making it a great in vivo model system to investigate the mechanisms of intracellular trafficking.
Materials and Methods
Animals
All experiments were performed in accordance with the National Institute of Health Guide for the Care and Use of Laboratory Animals and were approved by the Animal Care and Use Committee at the University of Wisconsin-Madison.
At Baylor College of Medicine, ENU mutagenesis was performed using C57BL/6J males [25]. nur17 mice were isolated, crossed to 129S6/SvEvTac three times and maintained in a mixed background of C57BL/6J and 129S6/SvEvTac.
After importing nur17 mice to the University of Wisconsin-Madison, we maintained the nur17 mutant allele and obtained the affected animals for characterization by repeating this mating scheme: nur17/nur17 founder females were mated with wild-type 129S1/SvImJ mice. nur17/+ F1 mice were intercrossed to obtain affected nur17/nur17 mice. nur17/nur17 female mice were again mated with 129S1/SvImJ.nur17/+ or +/+ littermate mice were used as control mice in the experiments.
129S1/SvImJ and AKR/J mice were obtained from The Jackson Laboratory.
Both 129S6/SvEvTac and 129S1/SvImJ strains are homozygous for the Aw allele and display white-bellied agouti coat color. All nur17 and control mice that were phenotyped in this study also displayed agouti coat color.
We replaced the EGFP sequence in the pCX-EGFP vector (kindly provided by Dr. Junichi Miyazaki; [65]) with the full length Arcn1 cDNA and named it pCX-Arcn1. We used pCX-Arcn1 for the transgene construct for the rescue experiment after linearization with BamHI and SalI (New England Biolabs). The construct was micro-injected into pronuclei of FVB/NJ embryos to generate transgenic mice by the transgenic animal facility of the Biotechnology Center at the University of Wisconsin-Madison. We obtained two transgene-positive founders (2361 and 2368) and crossed them to nur17 mice, and then intercrossed to obtain Tg-positive nur17/nur17 mice.
Southern blotting genotyping procedures
We identified Transgene (Tg)-positive clones by Southern-blot hybridization using PstI (New England Biolabs) digestion and probes specific to Arcn1. The southern blot differentiated the endogenous Arcn1 and the transgene based on size of bands (1.05kb and 0.45kb for the transgene).
Tail DNA was prepared as described in [66]. ∼15µg of DNA was digested with the restriction enzyme PstI (New England Biolabs) and resolved by 0.8% agarose gel electrophoresis. DNA was transferred to Hybond N membrane (Amersham, GE Healthcare) using alkaline capillary transfer, crosslinked with UV (Spectroline). The membrane was prehybridized with prehybridization buffer (4xSSC, 1% milk powder, 1% SDS, 10× Denharts Reagent) and hybridized with probes labeled with [P32] dCTP (Perkin Elmer) by Rediprime II Random Prime Labeling System (Amersham, GE Healthcare) at 65°C using hybridization buffer [4xSSC, 1% milk powder, 1% SDS and 37µg/ml salmon sperm DNA (Sigma)]. The probe used for hybridization was PCR amplified from mouse brain cDNA using primers, mArcn1-F3 (5′ AAGCACCAGGATTTGGCGG 3′) and mArcn1-R16 (5′ TTACAGGATTTCGTATTTGTC 3′). Membranes were washed three times with wash buffer I (2xSSC and 0.1% SDS) and twice with wash buffer II (0.5xSSC and 0.1% SDS) for 15 min at 65°C for each wash. CL-X Posure Film (Thermo Scientific) was used to detect the hybridization signal.
Northern blotting
The expression of the transgene was tested by a northern blot that differentiates the endogenously expressed Arcn1 (4.4kb) and expressed transgene (2.2kb). Total RNA was extracted from mouse brains by Trizol (Invitrogen). mRNA was purified from total RNA by Oligotex mRNA Midi Kit (Qiagen). mRNA was resolved by formaldehyde agarose electrophoresis and transferred to Hybond N membrane (Amersham, GE Healthcare) using alkaline capillary transfer, crosslinked with UV (Spectroline). The membrane was prehybridized with Rapid-Hyb Buffer (Amersham) with 37µg/ml salmon sperm DNA (Sigma) and hybridized with probes labeled with [P32] dCTP (Perkin Elmer) by Rediprime II Random Prime Labeling System (Amersham, GE Healthcare) at 65°C using Rapid-Hyb Buffer (Amersham) with 37µg/ml salmon sperm DNA (Sigma). The probe used was the same described above for the Southern-blot; similarly, the membranes were washed and hybridization signal was detected as described above.
Genetic mapping
To map the nur17 gene, we performed a whole genome scan using F2 animals from mating (nur17×AKR/J) F1 mice. We initially used 80 microsatellite markers, which distinguish AKR alleles from both 129S1/SvImJTac and C57BL/6J alleles across the whole genome. All F2 animals were phenotyped by both coat color dilution and ataxia movement. Once the chromosomal locus on chromosome 9 was identified for the nur17 mutation, additional F2 mice were collected and additional markers were used to further narrow the genetic region. We used two SNPs, D9SNP3 (SNP ID on NCBI: NES11330700) and D9SNP25 (SNP ID on NCBI: NES11338581), to differentiate AKR and nur17 alleles.
Genotyping
All genotyping was carried out by polymerase chain reaction (PCR). For nur17 genotyping, PCR primers, mArcn1-F18 (5′ CCTCAAACTCAGAAATCCGC 3′) and mArcn1-R19 (5′ TTGGCATCAATCACTGGC 3′), were used for amplification of the wild type (WT) allele and nur17 allele (470bp). BsrI (New England Biolabs) was used to digest the nur17 allele specifically to generate two bands (392bp and 78bp).
Immunohistochemistry
Littermate control (+/+, nur17/+) and nur17 mice were deeply anesthetized with a mixture of ketamine and xylazine and perfused with 4% paraformaldehyde (PFA). The heads were immersed in 4% PFA overnight at 4°C, and the brain was dissected out. For paraffin sections, the brain was dehydrated in a graded ethanol series, cleared in xylene, and embedded in paraffin. Sections were cut at 6 µm, mounted on slides pretreated with Vectabond (Vector Laboratories). For cryostat sections, the brain was cryoprotected at 4°C in a series of sucrose gradients after dissection. Brains were embedded in optimal cutting temperature compound (OCT) (Sakura Finetek) and sectioned at 12µm thickness.
For immunohistochemistry, sections were blocked with 2% donkey serum and were incubated overnight with the primary antibody against Calbindin-D (Swant), Calbindin (Abcam), phosphorylated tau (abcam), hyperphosphorylated tau (Thermo Scientific) and CHOP (Santa Cruz Biotechnology). Sections were rinsed in PBS, and incubated with a 1∶200 diluted Alexa 488 conjugated secondary antibody (Invitrogen) and/or Cy3 conjugated secondary antibody (Jackson Immunoresearch) for 45 minutes at room temperature. All sections were imaged on an Eclipse E600 microscope (Nikon) using a SPOT camera (Spot Diagnostics).
Electron microscopy
Littermate control (+/+, nur17/+) and nur17 mice were anesthetized with ketamine/xylazine and perfused with 2% PFA and 2.5% glutaraldehyde in 0.1M phosphate buffer for 11 min. Cerebella were removed from the head and immersion fixed for 30 min. Then, cerebella were sectioned sagittally at 100µm intervals with a Vibratome. Sections of the cerebella were osmicated (1% osmium tetroxide) for 1 hr and washed in 0.1M phosphate buffer, dehydrated through an ascending series of ethanol and propylene oxide and embedded in Epon [25g Epon 812, 13g Dodecenyl Succinic Anhydride (DDSA), 12g Nadic Methyl Anhydride (NMA) and 1ml 2,4,6-tris(dimethylaminomethyl)phenol (DMP-30), Electron Microscopy Sciences]. Ultra-thin sections (70nm) were cut and stained with uranyl acetate and lead citrate. Sections were imaged on a Philips CM120 Scanning Transmission Electron Microscope.
Gallyas-silver staining
The Gallyas-silver staining of the littermate control (+/+, nur17/+) and nur17 cerebellum was performed as described in [67] in order to examine the existence of neurofibrillary tangles.
Melanocyte culture
Mouse melanocytes were isolated from the skin on the back of the Day2 littermate control (+/+, nur17/+) and nur17 mice and cultured. Fresh skin specimens were incubated in foreskin media (Dulbecco's modified minimal essential medium (DMEM) (Gibco) supplemented with 2% fetal bovine serum (Sigma-Aldrich), penicillin (100 units/ml, Sigma) and gentamicin (100 ug/ml) (Gibco) at 4°C overnight. The next day, the skin was washed three times with Hanks' balanced salt solution and excess fat was removed. The samples were cut into small pieces and incubated in 0.25% trypsin solution (Hyclone) supplemented with 3.86 mg/ml trypsin, from porcine pancreas (Sigma-Aldrich) at 4°C overnight. Epidermis was separated from the dermis and epidermal cells were suspended and cultured in Ham's F10 nutrient medium supplemented with 10% fetal bovine serum, 85 nmol/L 12-O-tetradecanoylphorbol-13-acetate (TPA) (Sigma-Aldrich), 0.1 mmol/L 3-isobutyl-1-methylxanthine (IBMX) (Sigma-Aldrich), 2.5 nmol/L cholera toxin (CT) (ICN Biochemicals) and penicillin (100 units/ml) (Gibco).
Expression constructs and transfection of Neuro-2a cells
Ultimate ORF Human Clones for ARCN1 and coatomer protein complex, subunit beta 1 (COPB1 or beta-COP) in Gateway entry vector (Invitrogen; ARCN1: IOH43520, COPB: IOH27122) were purified using a QIAprep Spin Miniprep Kit (Qiagen) after culturing on the LB agar plate containing 10µg/mL of kanamycin and in the LB liquid medium. pcDNA-DEST53 (GFP-attR1-CmR-ccdB-attR2; Invitrogen) was used as the destination vector for ARCN1, and pcDNA™/V5-DEST (attR1-ccdB-CmR-attR2-V5 epitope; Invitrogen) was used as the destination vector for COPB1. The LR recombination reaction between the entry clone and a destination vector was carried out using LR Clonase Enzyme (Invitrogen) according to the protocols recommended in the product manual. The expression constructs were then purified using a QIAfilter Plasmid Midi Kit (Qiagen).
Mouse Neuro-2a cells (ATCC) were cultured in DMEM with high glucose (Gibco), supplemented with 10% FBS (Sigma). The ARCN1 and COP1B expression constructs were transfected into Neuro2A cells using SuperFect Transfection Reagent (Qiagen) following the manufacture's protocol and cultured for 24 hrs.
Immunocytochemistry
Cells were cultured on coverslips and were fixed by 4% PFA for 10 min at 4°C. Cells were permeabilized using 0.5% Triton-X in PBS for 30 min followed by blocking in 2% normal donkey serum for 30 min. Then the cells were incubated with 1∶200 diluted primary antibody against ARCN1 (Novus Biologicals), 58K Golgi protein (58K-9) (Abcam), KDEL (Abcam), β-COP (Sigma), GFP (Synaptic System) and V5 (Invitrogen) in PBS with 0.1% Triton-X for 1 hour at room temperature. Cells were washed in PBS and then incubated with 1∶200 diluted Alexa 488 conjugated secondary antibody (Invitrogen) and Cy3 conjugated secondary antibody (Jackson Immunoresearch) for 45 min at room temperature. All immunocytochemistry slides were imaged on a Zeiss 510 confocal laser scanning system and Axio Imager microscope using LSM 510 software (release 4.2) (Carl Zeiss MicroImaging, Inc).
Pulse-chase labeling
Melanocytes grown in a 5cm culture dish until 90–100% confluency were starved in DMEM without methionine (Cellgro) supplemented with 10% fetal bovine serum (Sigma-Aldrich), penicillin (100 units/ml) (Sigma) for 2 hours. Each plate of cells was labeled with 50 uCi [35S] methionine (EXPRE35S35S protein labeling mix; New England Nuclear) for 10 min, washed twice, and chased in normal growth medium for different periods of time. Cells were harvested, washed twice with ice-cold PBS, and lysed in lysis buffer (PBS with 0.1% triton X). The lysates were cleared by centrifugation at 15,000 g for 10 min at 4°C, and incubated with mAb TA99 (1∶10 dilution, homemade) at 4°C for overnight followed by rabbit anti-mouse IgG coupled to protein A Sepharose (1∶10 dilution) for 2 hours at 4°C. Immunoprecipitates were washed with 10 mM Tris/HCl, pH 7.5, 0.15 M NaC1, 5 mM EDTA, and 1% NP-40 (TNEN), followed by TNEN containing 0.5 M NaCI, and finally with distilled water. For Endo H digestion, the immunoprecipitates were dissociated by suspending in 0.2% SDS, heated for 5 min at 100°C, and diluted with 25 ul 0.1 M sodium citrate buffer, pH 5.5, Endo H, 50 mU/ml. The reaction mixture was layered over with 50ul toluene and digestion was carried out at 37°C for 18–24 h. Littermate control (+/+, nur17/+) tubes were treated similarly, except that equal volume of 0.1 M sodium citrate buffer, pH 5.5, was added instead of Endo H. Proteins were analyzed by 9% SDS-PAGE. Radioactive protein bands were visualized by fluorography. The proportion of Tyrp1 without complex N-linked glycans against the total protein was determined by scanning densitometry using the gel analysis function of the ImageJ software (http://rsb.info.nih.gov/ij). The signals at the lower band position were quantified for Tyrp1 without complex N-linked glycans, and all the signals spanning the lower and higher band positions were quantified for the total protein.
Statistical analysis
Student's t-test was performed for statistical comparison of the weight of mice, the number of cells positive for Gallyas staining or the CHOP1 staining between littermate control (+/+, nur17/+) and nur17 mice and the latency to fall in the rotarod analysis. Two-way analysis of variance (ANOVA) was performed for the statistical comparison of the proportion of protein without complex N-linked glycans in the pulse-chase experiment. GraphPad Prism software (GraphPad software, Inc.) was used for statistical analysis and to create all graphs reporting numerical values. * p<0.05, ** p<0.01.
Rotarod analysis
Mice that were of the genotype of +/+ or nur17/+ TG − (n = 5), nur17/nur17 TG − (n = 5) and nur17/nur17 TG+ (n = 5) at 5–8 months of age were subjected to rotarod analysis. Mice were first exposed to a training period at constant speed to familiarize them with the rotarod apparatus (Ugo Basile). For the testing, the rotorod was gradually accelerated (1 rpm/s) from 4 rpm over the course of 3 min. The time was recorded when a mouse fell from the device (latency to fall). After all mice fell from the rotorod, the group was given a rest period and then reloaded on the device. The testing was performed a total of 3 times for each mouse. Values from the three trials for each mouse were averaged to give a single score.
Supporting Information
Zdroje
1. SchwartzAL
1990 Cell biology of intracellular protein trafficking. Annu Rev Immunol 8 195 229
2. AnnaertW
De StrooperB
2002 A cell biological perspective on Alzheimer's disease. Annu Rev Cell Dev Biol 18 25 51
3. UemuraK
KuzuyaA
ShimohamaS
2004 Protein trafficking and Alzheimer's disease. Curr Alzheimer Res 1 1 10
4. DiFigliaM
SappE
ChaseK
SchwarzC
MeloniA
1995 Huntingtin is a cytoplasmic protein associated with vesicles in human and rat brain neurons. Neuron 14 1075 1081
5. GilJM
RegoAC
2008 Mechanisms of neurodegeneration in Huntington's disease. Eur J Neurosci 27 2803 2820
6. StrehlowAN
LiJZ
MyersRM
2007 Wild-type huntingtin participates in protein trafficking between the Golgi and the extracellular space. Hum Mol Genet 16 391 409
7. CooperAA
GitlerAD
CashikarA
HaynesCM
HillKJ
2006 Alpha-synuclein blocks ER-Golgi traffic and Rab1 rescues neuron loss in Parkinson's models. Science 313 324 328
8. LiW
RusiniakME
ChintalaS
GautamR
NovakEK
2004 Murine Hermansky-Pudlak syndrome genes: regulators of lysosome-related organelles. Bioessays 26 616 628
9. PasturalE
BarratFJ
Dufourcq-LagelouseR
CertainS
SanalO
1997 Griscelli disease maps to chromosome 15q21 and is associated with mutations in the myosin-Va gene. Nat Genet 16 289 292
10. MercerJA
SeperackPK
StrobelMC
CopelandNG
JenkinsNA
1991 Novel myosin heavy chain encoded by murine dilute coat colour locus. Nature 349 709 713
11. TakagishiY
OdaS
HayasakaS
Dekker-OhnoK
ShikataT
1996 The dilute-lethal (dl) gene attacks a Ca2+ store in the dendritic spine of Purkinje cells in mice. Neurosci Lett 215 169 172
12. WuX
BowersB
WeiQ
KocherB
HammerJA
3rd
1997 Myosin V associates with melanosomes in mouse melanocytes: evidence that myosin V is an organelle motor. J Cell Sci 110 ( Pt 7) 847 859
13. ProvanceDWJr
WeiM
IpeV
MercerJA
1996 Cultured melanocytes from dilute mutant mice exhibit dendritic morphology and altered melanosome distribution. Proc Natl Acad Sci U S A 93 14554 14558
14. ChowCY
ZhangY
DowlingJJ
JinN
AdamskaM
2007 Mutation of FIG4 causes neurodegeneration in the pale tremor mouse and patients with CMT4J. Nature 448 68 72
15. JinN
ChowCY
LiuL
ZolovSN
BronsonR
2008 VAC14 nucleates a protein complex essential for the acute interconversion of PI3P and PI(3,5)P(2) in yeast and mouse. Embo J 27 3221 3234
16. MichellRH
HeathVL
LemmonMA
DoveSK
2006 Phosphatidylinositol 3,5-bisphosphate: metabolism and cellular functions. Trends Biochem Sci 31 52 63
17. LiW
ZhangQ
OisoN
NovakEK
GautamR
2003 Hermansky-Pudlak syndrome type 7 (HPS-7) results from mutant dysbindin, a member of the biogenesis of lysosome-related organelles complex 1 (BLOC-1). Nat Genet 35 84 89
18. SteingrimssonE
CopelandNG
JenkinsNA
2006 Mouse coat color mutations: from fancy mice to functional genomics. Dev Dyn 235 2401 2411
19. ChintalaS
LiW
LamoreuxML
ItoS
WakamatsuK
2005 Slc7a11 gene controls production of pheomelanin pigment and proliferation of cultured cells. Proc Natl Acad Sci U S A 102 10964 10969
20. GwynnB
MartinaJA
BonifacinoJS
SviderskayaEV
LamoreuxML
2004 Reduced pigmentation (rp), a mouse model of Hermansky-Pudlak syndrome, encodes a novel component of the BLOC-1 complex. Blood 104 3181 3189
21. MatesicLE
YipR
ReussAE
SwingDA
O'SullivanTN
2001 Mutations in Mlph, encoding a member of the Rab effector family, cause the melanosome transport defects observed in leaden mice. Proc Natl Acad Sci U S A 98 10238 10243
22. WilsonSM
YipR
SwingDA
O'SullivanTN
ZhangY
2000 A mutation in Rab27a causes the vesicle transport defects observed in ashen mice. Proc Natl Acad Sci U S A 97 7933 7938
23. TunnacliffeA
van de VrugtH
PensottiV
RadiceP
1996 The coatomer protein delta-COP, encoded by the archain gene, is conserved across diverse eukaryotes. Mamm Genome 7 784 786
24. HerronBJ
LuW
RaoC
LiuS
PetersH
2002 Efficient generation and mapping of recessive developmental mutations using ENU mutagenesis. Nat Genet 30 185 189
25. KileBT
HentgesKE
ClarkAT
NakamuraH
SalingerAP
2003 Functional genetic analysis of mouse chromosome 11. Nature 425 81 86
26. HirasawaM
XuX
TraskRB
MaddatuTP
JohnsonBA
2007 Carbonic anhydrase related protein 8 mutation results in aberrant synaptic morphology and excitatory synaptic function in the cerebellum. Mol Cell Neurosci 35 161 170
27. MichelsenK
SchmidV
MetzJ
HeusserK
LiebelU
2007 Novel cargo-binding site in the beta and delta subunits of coatomer. J Cell Biol 179 209 217
28. IkeguchiY
WangX
McCloskeyDE
ColemanCS
NelsonP
2004 Characterization of transgenic mice with widespread overexpression of spermine synthase. Biochem J 381 701 707
29. FaulstichD
AuerbachS
OrciL
RavazzolaM
WegchingelS
1996 Architecture of coatomer: molecular characterization of delta-COP and protein interactions within the complex. J Cell Biol 135 53 61
30. SerafiniT
StenbeckG
BrechtA
LottspeichF
OrciL
1991 A coat subunit of Golgi-derived non-clathrin-coated vesicles with homology to the clathrin-coated vesicle coat protein beta-adaptin. Nature 349 215 220
31. WatersMG
SerafiniT
RothmanJE
1991 ‘Coatomer’: a cytosolic protein complex containing subunits of non-clathrin-coated Golgi transport vesicles. Nature 349 248 251
32. LeeMC
MillerEA
GoldbergJ
OrciL
SchekmanR
2004 Bi-directional protein transport between the ER and Golgi. Annu Rev Cell Dev Biol 20 87 123
33. BethuneJ
WielandF
MoellekenJ
2006 COPI-mediated transport. J Membr Biol 211 65 79
34. RabouilleC
KlumpermanJ
2005 Opinion: The maturing role of COPI vesicles in intra-Golgi transport. Nat Rev Mol Cell Biol 6 812 817
35. DaroE
SheffD
GomezM
KreisT
MellmanI
1997 Inhibition of endosome function in CHO cells bearing a temperature-sensitive defect in the coatomer (COPI) component epsilon-COP. J Cell Biol 139 1747 1759
36. GuoQ
PenmanM
TrigattiBL
KriegerM
1996 A single point mutation in epsilon-COP results in temperature-sensitive, lethal defects in membrane transport in a Chinese hamster ovary cell mutant. J Biol Chem 271 11191 11196
37. GuoQ
VasileE
KriegerM
1994 Disruptions in Golgi structure and membrane traffic in a conditional lethal mammalian cell mutant are corrected by epsilon-COP. J Cell Biol 125 1213 1224
38. StyersML
O'ConnorAK
GrabskiR
Cormet-BoyakaE
SztulE
2008 Depletion of beta-COP reveals a role for COP-I in compartmentalization of secretory compartments and in biosynthetic transport of caveolin-1. Am J Physiol Cell Physiol 294 C1485 1498
39. CossonP
DemolliereC
HenneckeS
DudenR
LetourneurF
1996 Delta - and zeta-COP, two coatomer subunits homologous to clathrin-associated proteins, are involved in ER retrieval. Embo J 15 1792 1798
40. XuY
VijayasaradhiS
HoughtonAN
1998 The cytoplasmic tail of the mouse brown locus product determines intracellular stability and export from the endoplasmic reticulum. J Invest Dermatol 110 324 331
41. VijayasaradhiS
XuY
BouchardB
HoughtonAN
1995 Intracellular sorting and targeting of melanosomal membrane proteins: identification of signals for sorting of the human brown locus protein, gp75. J Cell Biol 130 807 820
42. ZhaoL
Longo-GuessC
HarrisBS
LeeJW
AckermanSL
2005 Protein accumulation and neurodegeneration in the woozy mutant mouse is caused by disruption of SIL1, a cochaperone of BiP. Nat Genet 37 974 979
43. LewisJ
McGowanE
RockwoodJ
MelroseH
NacharajuP
2000 Neurofibrillary tangles, amyotrophy and progressive motor disturbance in mice expressing mutant (P301L) tau protein. Nat Genet 25 402 405
44. BondareffW
HarringtonCR
WischikCM
HauserDL
RothM
1995 Absence of abnormal hyperphosphorylation of tau in intracellular tangles in Alzheimer's disease. J Neuropathol Exp Neurol 54 657 663
45. KirchhausenT
2000 Three ways to make a vesicle. Nat Rev Mol Cell Biol 1 187 198
46. BonifacinoJS
GlickBS
2004 The mechanisms of vesicle budding and fusion. Cell 116 153 166
47. McMahonHT
MillsIG
2004 COP and clathrin-coated vesicle budding: different pathways, common approaches. Curr Opin Cell Biol 16 379 391
48. HamamichiS
RivasRN
KnightAL
CaoS
CaldwellKA
2008 Hypothesis-based RNAi screening identifies neuroprotective genes in a Parkinson's disease model. Proc Natl Acad Sci U S A 105 728 733
49. CoutinhoP
ParsonsMJ
ThomasKA
HirstEM
SaudeL
2004 Differential requirements for COPI transport during vertebrate early development. Dev Cell 7 547 558
50. JayaramSA
SentiKA
TiklovaK
TsarouhasV
HemphalaJ
2008 COPI vesicle transport is a common requirement for tube expansion in Drosophila. PLoS ONE 3 e1964 doi:10.1371/journal.pone.0001964
51. GuF
AnientoF
PartonRG
GruenbergJ
1997 Functional dissection of COP-I subunits in the biogenesis of multivesicular endosomes. J Cell Biol 139 1183 1195
52. HalabanR
MoellmannG
1990 Murine and human b locus pigmentation genes encode a glycoprotein (gp75) with catalase activity. Proc Natl Acad Sci U S A 87 4809 4813
53. VijayasaradhiS
BouchardB
HoughtonAN
1990 The melanoma antigen gp75 is the human homologue of the mouse b (brown) locus gene product. J Exp Med 171 1375 1380
54. MarksMS
SeabraMC
2001 The melanosome: membrane dynamics in black and white. Nat Rev Mol Cell Biol 2 738 748
55. SpearED
NgDT
2003 Stress tolerance of misfolded carboxypeptidase Y requires maintenance of protein trafficking and degradative pathways. Mol Biol Cell 14 2756 2767
56. PaschenW
MengesdorfT
2005 Endoplasmic reticulum stress response and neurodegeneration. Cell Calcium 38 409 415
57. KimI
XuW
ReedJC
2008 Cell death and endoplasmic reticulum stress: disease relevance and therapeutic opportunities. Nat Rev Drug Discov 7 1013 1030
58. SchmidtWM
KrausC
HogerH
HochmeisterS
OberndorferF
2007 Mutation in the Scyl1 gene encoding amino-terminal kinase-like protein causes a recessive form of spinocerebellar neurodegeneration. EMBO Rep 8 691 697
59. BurmanJL
BourbonniereL
PhilieJ
StrohT
DejgaardSY
2008 Scyl1, mutated in a recessive form of spinocerebellar neurodegeneration, regulates COPI-mediated retrograde traffic. J Biol Chem 283 22774 22786
60. LeeVM
GoedertM
TrojanowskiJQ
2001 Neurodegenerative tauopathies. Annu Rev Neurosci 24 1121 1159
61. HoozemansJJ
van HaastertES
NijholtDA
RozemullerAJ
EikelenboomP
2009 The unfolded protein response is activated in pretangle neurons in Alzheimer's disease hippocampus. Am J Pathol 174 1241 1251
62. HetzC
Russelakis-CarneiroM
MaundrellK
CastillaJ
SotoC
2003 Caspase-12 and endoplasmic reticulum stress mediate neurotoxicity of pathological prion protein. Embo J 22 5435 5445
63. AtkinJD
FargMA
WalkerAK
McLeanC
TomasD
2008 Endoplasmic reticulum stress and induction of the unfolded protein response in human sporadic amyotrophic lateral sclerosis. Neurobiol Dis 30 400 407
64. GwynnB
SmithRS
RoweLB
TaylorBA
PetersLL
2006 A mouse TRAPP-related protein is involved in pigmentation. Genomics 88 196 203
65. NiwaH
YamamuraK
MiyazakiJ
1991 Efficient selection for high-expression transfectants with a novel eukaryotic vector. Gene 108 193 199
66. BuffoneGJ
DarlingtonGJ
1985 Isolation of DNA from biological specimens without extraction with phenol. Clin Chem 31 164 165
67. GallyasF
1971 Silver staining of Alzheimer's neurofibrillary changes by means of physical development. Acta Morphol Acad Sci Hung 19 1 8
Štítky
Genetika Reprodukční medicína
Článek Epistasis of Transcriptomes Reveals Synergism between Transcriptional Activators Hnf1α and Hnf4αČlánek SMA-10/LRIG Is a Conserved Transmembrane Protein that Enhances Bone Morphogenetic Protein SignalingČlánek B1 SOX Coordinate Cell Specification with Patterning and Morphogenesis in the Early Zebrafish EmbryoČlánek Bulk Segregant Analysis by High-Throughput Sequencing Reveals a Novel Xylose Utilization Gene fromČlánek Abundant Quantitative Trait Loci Exist for DNA Methylation and Gene Expression in Human Brain
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2010 Číslo 5- Akutní intermitentní porfyrie
- Růst a vývoj dětí narozených pomocí IVF
- Vliv melatoninu a cirkadiálního rytmu na ženskou reprodukci
- Intrauterinní inseminace a její úspěšnost
- Délka menstruačního cyklu jako marker ženské plodnosti
-
Všechny články tohoto čísla
- Digital Quantification of Human Eye Color Highlights Genetic Association of Three New Loci
- Common Genetic Variants near the Brittle Cornea Syndrome Locus Influence the Blinding Disease Risk Factor Central Corneal Thickness
- Age- and Temperature-Dependent Somatic Mutation Accumulation in
- All About Mitochondrial Eve: An Interview with Rebecca Cann
- Aging and Chronic Sun Exposure Cause Distinct Epigenetic Changes in Human Skin
- Transposed Genes in Arabidopsis Are Often Associated with Flanking Repeats
- A Survey of Genomic Traces Reveals a Common Sequencing Error, RNA Editing, and DNA Editing
- Gene Transposition Causing Natural Variation for Growth in
- The Polyproline Site in Hinge 2 Influences the Functional Capacity of Truncated Dystrophins
- Epistasis of Transcriptomes Reveals Synergism between Transcriptional Activators Hnf1α and Hnf4α
- Integration of Light Signals by the Retinoblastoma Pathway in the Control of S Phase Entry in the Picophytoplanktonic Cell
- The Proprotein Convertase Encoded by () Is Required in Corpora Cardiaca Endocrine Cells Producing the Glucose Regulatory Hormone AKH
- Sgs1 and Exo1 Redundantly Inhibit Break-Induced Replication and Telomere Addition at Broken Chromosome Ends
- A MATE-Family Efflux Pump Rescues the 8-Oxoguanine-Repair-Deficient Mutator Phenotype and Protects Against HO Killing
- The Relationship among Gene Expression, the Evolution of Gene Dosage, and the Rate of Protein Evolution
- SMA-10/LRIG Is a Conserved Transmembrane Protein that Enhances Bone Morphogenetic Protein Signaling
- The Nuclear Receptor DHR3 Modulates dS6 Kinase–Dependent Growth in
- Involvement of Global Genome Repair, Transcription Coupled Repair, and Chromatin Remodeling in UV DNA Damage Response Changes during Development
- B1 SOX Coordinate Cell Specification with Patterning and Morphogenesis in the Early Zebrafish Embryo
- Linkage and Association Mapping of Flowering Time in Nature
- Bulk Segregant Analysis by High-Throughput Sequencing Reveals a Novel Xylose Utilization Gene from
- Abundant Quantitative Trait Loci Exist for DNA Methylation and Gene Expression in Human Brain
- Ablation of Whirlin Long Isoform Disrupts the USH2 Protein Complex and Causes Vision and Hearing Loss
- Characterization of Oxidative Guanine Damage and Repair in Mammalian Telomeres
- DNA Adenine Methylation Is Required to Replicate Both Chromosomes Once per Cell Cycle
- Genome-Wide Copy Number Variation in Epilepsy: Novel Susceptibility Loci in Idiopathic Generalized and Focal Epilepsies
- FACT Prevents the Accumulation of Free Histones Evicted from Transcribed Chromatin and a Subsequent Cell Cycle Delay in G1
- GC-Biased Evolution Near Human Accelerated Regions
- Liver and Adipose Expression Associated SNPs Are Enriched for Association to Type 2 Diabetes
- Myeloid Cell-Restricted Insulin Receptor Deficiency Protects Against Obesity-Induced Inflammation and Systemic Insulin Resistance
- The Mating Type Locus () and Sexual Reproduction of : Insights into the Evolution of Sex and Sex-Determining Chromosomal Regions in Fungi
- B-Cyclin/CDKs Regulate Mitotic Spindle Assembly by Phosphorylating Kinesins-5 in Budding Yeast
- Post-Replication Repair Suppresses Duplication-Mediated Genome Instability
- Genome Sequence of the Plant Growth Promoting Endophytic Bacterium sp. 638
- Transcription Factors Mat2 and Znf2 Operate Cellular Circuits Orchestrating Opposite- and Same-Sex Mating in
- The Use of Orthologous Sequences to Predict the Impact of Amino Acid Substitutions on Protein Function
- Mutation in Archain 1, a Subunit of COPI Coatomer Complex, Causes Diluted Coat Color and Purkinje Cell Degeneration
- Shelterin-Like Proteins and Yku Inhibit Nucleolytic Processing of Telomeres
- Affecting Function Causes a Dilated Heart in Adult
- Manipulation of Behavioral Decline in with the Rag GTPase
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Common Genetic Variants near the Brittle Cornea Syndrome Locus Influence the Blinding Disease Risk Factor Central Corneal Thickness
- All About Mitochondrial Eve: An Interview with Rebecca Cann
- The Relationship among Gene Expression, the Evolution of Gene Dosage, and the Rate of Protein Evolution
- SMA-10/LRIG Is a Conserved Transmembrane Protein that Enhances Bone Morphogenetic Protein Signaling
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



