-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
A Survey of Genomic Traces Reveals a Common Sequencing Error, RNA Editing, and DNA Editing
While it is widely held that an organism's genomic information should remain constant, several protein families are known to modify it. Members of the AID/APOBEC protein family can deaminate DNA. Similarly, members of the ADAR family can deaminate RNA. Characterizing the scope of these events is challenging. Here we use large genomic data sets, such as the two billion sequences in the NCBI Trace Archive, to look for clusters of mismatches of the same type, which are a hallmark of editing events caused by APOBEC3 and ADAR. We align 603,249,815 traces from the NCBI trace archive to their reference genomes. In clusters of mismatches of increasing size, at least one systematic sequencing error dominates the results (G-to-A). It is still present in mismatches with 99% accuracy and only vanishes in mismatches at 99.99% accuracy or higher. The error appears to have entered into about 1% of the HapMap, possibly affecting other users that rely on this resource. Further investigation, using stringent quality thresholds, uncovers thousands of mismatch clusters with no apparent defects in their chromatograms. These traces provide the first reported candidates of endogenous DNA editing in human, further elucidating RNA editing in human and mouse and also revealing, for the first time, extensive RNA editing in Xenopus tropicalis. We show that the NCBI Trace Archive provides a valuable resource for the investigation of the phenomena of DNA and RNA editing, as well as setting the stage for a comprehensive mapping of editing events in large-scale genomic datasets.
Published in the journal: . PLoS Genet 6(5): e32767. doi:10.1371/journal.pgen.1000954
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1000954Summary
While it is widely held that an organism's genomic information should remain constant, several protein families are known to modify it. Members of the AID/APOBEC protein family can deaminate DNA. Similarly, members of the ADAR family can deaminate RNA. Characterizing the scope of these events is challenging. Here we use large genomic data sets, such as the two billion sequences in the NCBI Trace Archive, to look for clusters of mismatches of the same type, which are a hallmark of editing events caused by APOBEC3 and ADAR. We align 603,249,815 traces from the NCBI trace archive to their reference genomes. In clusters of mismatches of increasing size, at least one systematic sequencing error dominates the results (G-to-A). It is still present in mismatches with 99% accuracy and only vanishes in mismatches at 99.99% accuracy or higher. The error appears to have entered into about 1% of the HapMap, possibly affecting other users that rely on this resource. Further investigation, using stringent quality thresholds, uncovers thousands of mismatch clusters with no apparent defects in their chromatograms. These traces provide the first reported candidates of endogenous DNA editing in human, further elucidating RNA editing in human and mouse and also revealing, for the first time, extensive RNA editing in Xenopus tropicalis. We show that the NCBI Trace Archive provides a valuable resource for the investigation of the phenomena of DNA and RNA editing, as well as setting the stage for a comprehensive mapping of editing events in large-scale genomic datasets.
Introduction
With the exception of infrequent random somatic mutations, it is widely believed that the same genomic content should be fixed in an organism throughout its lifetime. This information will also serve as a template for exact RNA copies. Proteins that can modify genomic content, nevertheless, have been identified in humans and in many other organisms.
RNA editing involves alteration of particular RNA nucleotides by specifically changing Adenosine (A) into Inosine (I), which in turn is read as Guanosine (G) [1]. It is performed by the adenosine deaminase that acts on RNA (ADAR) family of deaminases [2]–[5] and this process has been implicated in several vital neurological functions [6]. A-to-I editing is known to target only RNA molecules [7] with numerous instances of editing events in the human transcriptome [8]–[12]. A different family of proteins, the AID/APOBEC family of deaminases, can edit both DNA and RNA nucleotides, specifically changing Cytosine (C) into Uracil (U) [13]. The first family member to be found and studied was the apolipoprotein B editing complex 1 (APOBEC1). This protein edits the apolipoprotein B (ApoB) RNA, which is involved in lipid transport [14], [15] but APOBEC1 can also deaminate cytidine in DNA [16]. Additional members of the family were found to target DNA. Activation-induced deaminase (AID) was discovered to be vital for the antigen-driven diversification of immunoglobulin genes in the vertebrate adaptive immune system [17]–[19] and the APOBEC3s were shown to be involved in the restriction of retrovirus proliferation in primates [20], [21].
For many years, the only known human endogenous target of the APOBEC protein family was the apoB RNA transcript. In this case, editing in position 6,666 by APOBEC1 leads to a stop codon and eventually results in two functionally distinct isoforms of apolipoprotein B (ApoB) [15], [22]. This editing reaction is mediated by the APOBEC complementation factor (ACF) [23], [24] which guides APOBEC1 to the target locus.
Deamination of cytosines to uracils in DNA (DNA editing) by various APOBEC protein families is characterized, in many cases, by clusters of G-to-A mismatches between the reference genome and the edited sequence. These mismatches are the end product of deamination of “C” into “U” in the other DNA strand. Recently, it was found that APOBEC3G can serve as a potent inhibitor of a wide range of retroviruses, including endogenous retrotransposons. This protein introduces large numbers of C-to-U mutations in the minus-strand of the viral DNA, eventually leading to G-to-A mutations after plus-strand synthesis [25]–[29]. Also, it has been demonstrated that APOBEC3G is capable of editing the mouse IAP retrotransposon [30]. Little is known, however, about the frequency or localization of editing in vivo.
Although editing of retrotransposons and their integration back into the genome is expected to be rare, very deep DNA sequencing can be used to identify these events. In this paper we report initial results of a novel bioinformatic approach for detection of endogenous RNA and DNA candidate sites in various organisms. We obtained 600 million sequence traces from the NCBI Trace archive. This data repository contains DNA sequence chromatograms (traces) from various large-scale capillary electrophoresis sequencing projects, base calls, and quality estimates. Next, we aligned these traces to their consensus reference genomes and searched for clusters of mismatches. Interestingly, we have found not only evidence of genuine RNA and DNA editing events but have also isolated a very common technical sequencing artifact that leads to such clusters.
Results
One hallmark of editing enzymes is a cluster of mismatches of the same type in the edited substrate. While the results of the RNA editing ADARs are clusters of A-to-G mismatches, the hallmark of members of the APOBEC3s protein family is a cluster of G-to-A mismatches in the newly formed DNA strand after reverse transcription. In order to find new endogenous editing events we looked for such mismatch clusters in the largest available repository of “raw” sequencing data, before they have been processed and assembled. We aligned “raw” sequencing reads from the NCBI trace archive to their consensus, reference genome. We repeated this procedure, in parallel, for each of ten organisms (in total more than 600 million reads - see Materials and Methods). In order to reduce noise caused by low sequencing quality or from misalignment to the genome, only long alignments (400bp or more) with 97% identity to the reference were considered. In addition, no insertions or deletions and no ambiguity in the location of the alignment were accepted (see Materials and Methods). Applying these strict criteria we do not expect results from current ABI SOLiD, Roche 454, Illumina GA, or Helicos sequencing reads.
In sum, we curated more than 56 gigabases of aligned sequence in human, about 62 gigabases of aligned sequence in mouse and much lower numbers for other organisms reflecting smaller genomes and/or lower coverage. In human, 85,181,171 traces aligned uniquely to the reference genome, 4,626,984 traces aligned to multiple locations, and 123,110,314 traces had no alignment under our strict cutoffs. For all organisms combined, approximately 300 million, out of 603,249,815 traces in total, were analyzed further (See Table 1).
Tab. 1. Summary of computation. 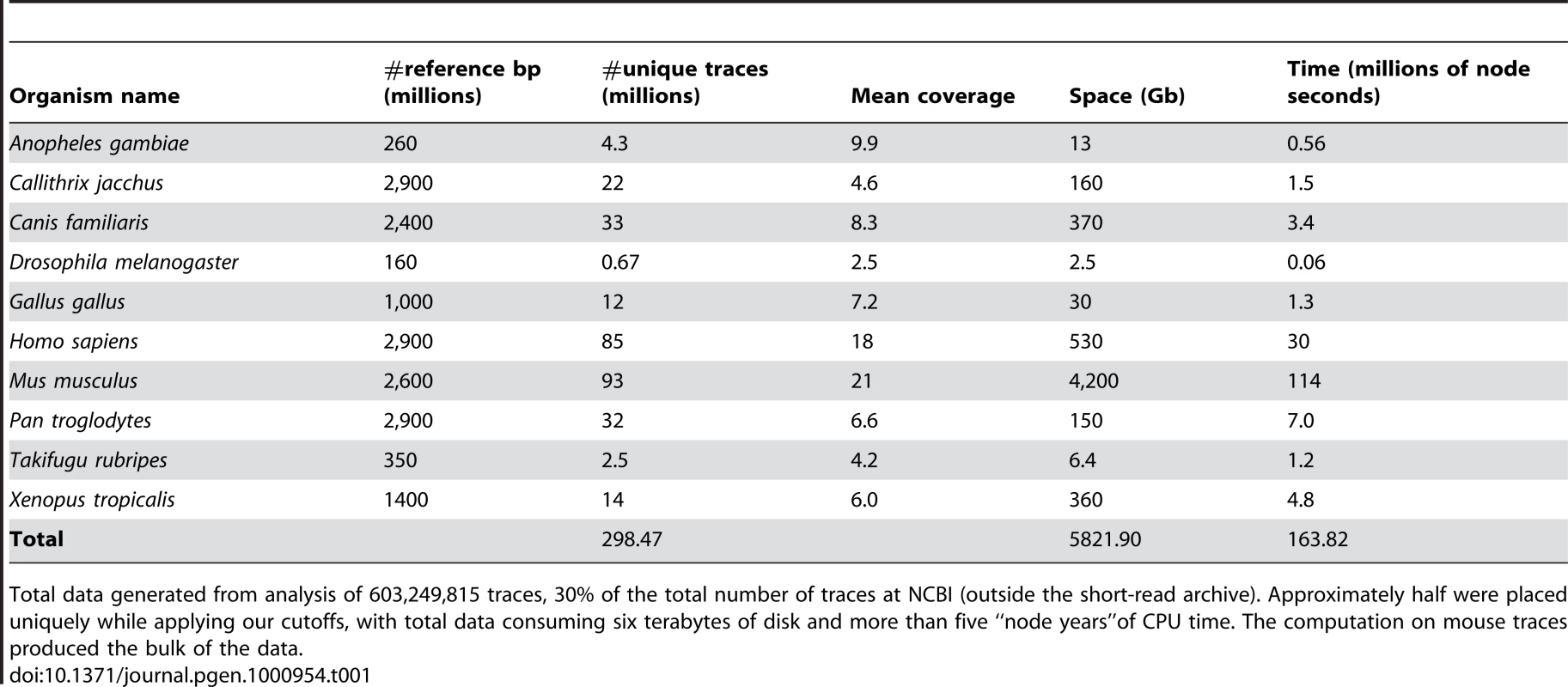
Total data generated from analysis of 603,249,815 traces, 30% of the total number of traces at NCBI (outside the short-read archive). Approximately half were placed uniquely while applying our cutoffs, with total data consuming six terabytes of disk and more than five “node years”of CPU time. The computation on mouse traces produced the bulk of the data. Clusters of consecutive mismatches of the same type (C-to-T or G-to-A) are common in APOBEC targets, such as IAP mouse retroelements edited by APOBEC3 [30], thus we focused on such “runs” in the aligned traces. In human, we found G-to-A mismatches to be over-represented compared to other types of mismatch, with longer runs. There were 657,826 human traces with runs of five or more mismatches of the same type. Of these, 218,595 (33%) human traces had runs of five or more G-to-A mismatches, much more than any other mismatch type.
Since editing enzymes have a preferred sequence context, the large data set allows us to restrict our search to traces with the same three base-pair motif centered at each mismatch site in the trace [31]. Moreover, as sequencing errors tend to cluster in certain regions, especially in low complexity areas, hence forming relatively short mismatch-dense regions, we applied another filter and discarded runs that span less than 100 base-pairs (the distance between the first and last consecutive mismatch). We also discarded traces in which the reference or the trace nucleotides around or at the mismatch site were not called (“N”).
Out of the 53,639 total examples conforming to the above criteria, we found 46,483 (82%) examples of G-to-A traces in human. Thus, the restrictions above reduced the total number of traces more than 12-fold while only reducing the number of G-to-A examples by less than 5-fold. Moreover, we found a striking preference for either an “AGA-to-AAA” mismatch motif (26,694/53,639 traces) or an “AGG-to-AAG” motif (21,274/53,639 traces). This tendency was observed in traces from all sequencing centers tested but one (Celera) (see Figure 1B). Since most of the sequencing for the human genome project was done in eight centers, results from only these centers are shown.
Fig. 1. Evidence for editing events emerges by enrichment for clusters of mismatches. 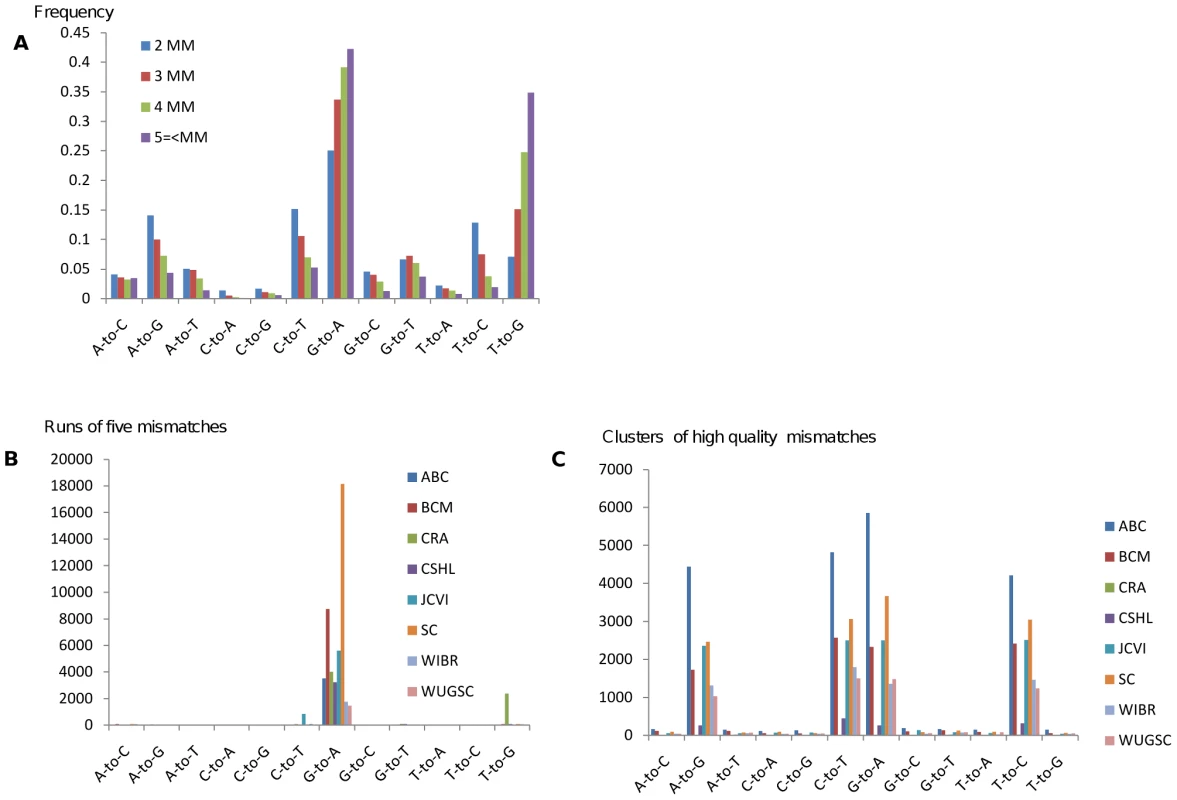
(A) Human traces are mined for clusters of mismatches of the same type. Shown is the percent frequency of clusters by type. The G-to-A mismatch type becomes more dominant with increasing numbers of mismatches (as does T-to-G). (B) Runs of five (or more) mismatches by type and sequencing center with an identical 3bp motif centered on each mismatch. Data from eight sequencing centers is shown. All of these centers had at least 1000 examples that meet the above criteria. (C) Clusters with three (or more) mismatches with at least two very high quality mismatches (Phred 40). A mismatch spectrum consistent with editing can be observed. Sequence traces are derived from both DNA strands, thus one would expect to observe a symmetric over representation of C-to-T mismatch clusters. Lack of similar numbers of complementary mismatches led us to the conclusion that most of these mismatches are not caused by a biological source but rather are sequencing artifacts.
In order to understand the origin of the artifact, we analyzed sample traces, and noticed that traces with “runs” of mismatches, with identical three base-pair motifs, centered on the mismatch, often had a peculiar defect in their chromatograms. Such defects can arise when the florescent dyes used in DNA sequencing have sequence specific incorporation differences which lead to unevenly spaced or shaped peaks in the electronic trace chromatogram after capillary electrophoresis. Figure 2 shows a comparison of representative chromatograms: one with the “AGA-to-AAA” motif (Figure 2A) and one that matches the consensus genome (Figure 2B). A mismatch is highlighted at position 244 and matches position 90 in the control. We can see that every peak is preceded by a small, identical sub-peak. There is also another “AAA” motif at position 253 which corresponds to an “AGA” motif at position 99 in the control. Independently, we noticed that “AGA” sequences are prone to form a pattern of high, low, high intensity peaks, hence the “G” has a low peak while the preceding and the subsequent “A” peaks are much taller (see control). The combination of these two common effects, in one trace, leads to occurrences where the sub-peak from the high “A” can dominate the “G” resulting in a G-to-A mismatch in an “AGA” context.
Fig. 2. G-to-A sequencing artifact. 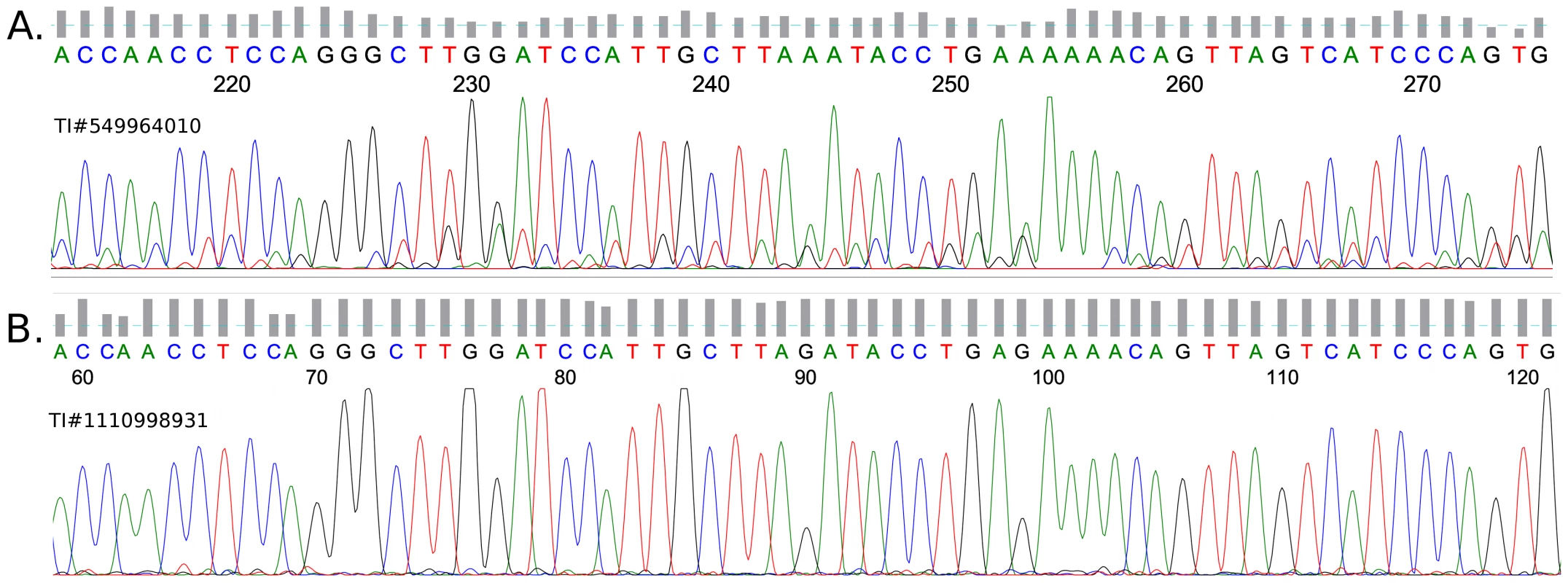
(A) A chromatogram, from a trace matching the criteria in Figure 1B. An AAA motif is centered at position 244 and corresponds with position 90 in the control; another AAA motif occurs at position 253 which corresponds to position 99 in the control. It can be seen that each peak in this chromatogram is preceded by a smaller, identical sub-peak. This has the effect of making it likely that a normally small peak (see control) will be overwhelmed by the sub-peak of the adjacent, normally tall peak (see control). (B) A chromatogram from a control trace that matches the reference—position 90 is the center of an AGA motif. We used strict criteria to construct the artifact set, thus the actual number of those errors is probably much larger than the 260K we found and may disrupt the accuracy of genomic assemblies. Indeed, we found evidence that these common errors influence the consensus sequence of a few genomes. The number of runs of G-to-A mismatches with the AGA motif was much higher in genomes with high coverage, where each position in the reference genome has many traces to support each call. In these cases, the reference is determined according to the “majority voting” of all the supporting traces. Since the reported type of mismatch is much less abundant than the correct call, the reference will have the correct “G” in virtually all cases. In genomic projects with lower coverage, however, such events can become part of the reference genome and therefore could not have been detected by our method. Indeed, we found that genomes with lower coverage tended to be free of G-to-A mismatches. This effect is most striking in drosophila where mean coverage of the reference by aligned traces is only 2.5 (See Table 1 and Table 2). This finding suggests the integration of these sequencing errors into the reference genome in many cases.
Tab. 2. Editing enriched traces—higher quality. 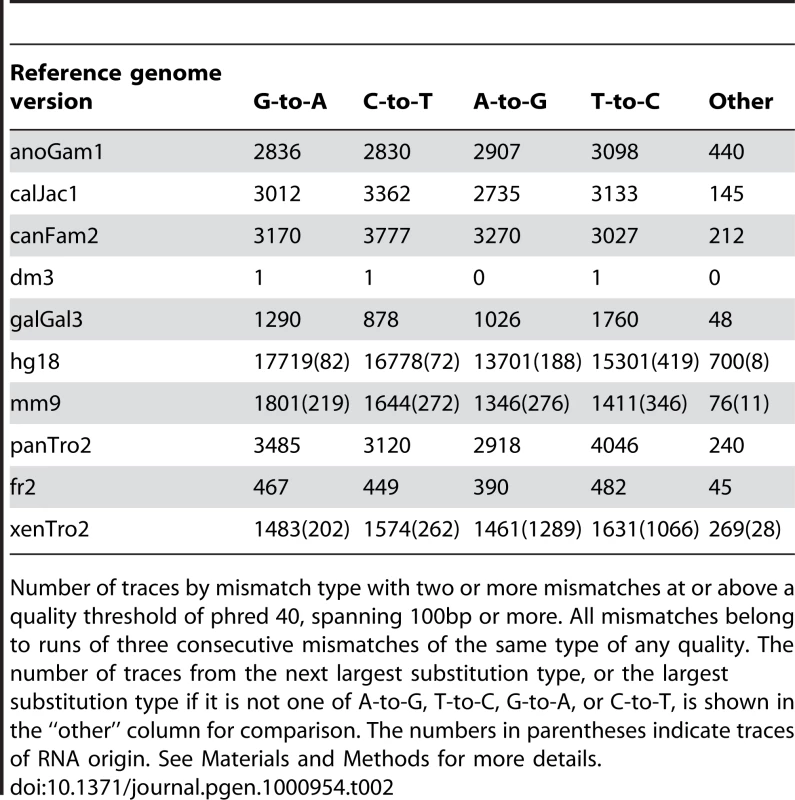
Number of traces by mismatch type with two or more mismatches at or above a quality threshold of phred 40, spanning 100bp or more. All mismatches belong to runs of three consecutive mismatches of the same type of any quality. The number of traces from the next largest substitution type, or the largest substitution type if it is not one of A-to-G, T-to-C, G-to-A, or C-to-T, is shown in the “other” column for comparison. The numbers in parentheses indicate traces of RNA origin. See Materials and Methods for more details. Another effect of this error was found in the assignment of single nucleotide polymorphisms (SNPs). A sequencing error in one genomic trace will not usually lead to the determination of a SNP at this position. However, since many of the “AGA” mismatches have a quality score of phred 20 or higher, which is considered an acceptable quality with an estimated error probability of only 1% [32] we suspected that some of them might be classified as SNPs. Indeed, we found 46,483 traces with 3bp G-to-A motif in runs of five or more. Of ∼260K G-to-A mismatches with this motif, we found that 28,722 appear as SNPs in dbSNP (The Single Nucleotide Polymorphism database) and 11,145 even appear in the HapMap dataset and were genotyped in four populations. Strong support that the vast majority are actually errors and not real SNPs comes from the observation that 10,532 (94.5%) of the mismatches that appear in HapMap are homozygous for the reference allele (G) with no representation of the other SNP allele in any of the 90 individuals that were genotyped in the Yoruba population, a population that is typically the most diverse. By contrast, only 521,405 out of the 3,782,819 (13.8%) of SNPs that appear in the HapMap show a similar lack of variability (p-value≪e-200, Fisher's Exact Test). Over-representation of G as the observed allele in A/G SNPs (or C in C/T SNPs) in the group of SNPs that have only one observed allele, when comparing it to the SNPs with two observed alleles, suggests that up to 1.8% of the HapMap SNPs are a result of the artifact and are not real SNPs (data not shown) the ratio is probably larger in dbSNP which is less curated.
Once we realized that the majority of “AGA” and “AGG” mismatch motifs were caused by a sequencing error, we endeavored to eliminate such errors from our dataset. To do so, we incorporated phred quality scores, also available from the trace archive. We obtained quality scores for all traces with a run of three or more substitutions of the same type. This set contains 20.7 million traces out of the 300 million that aligned uniquely. We then applied various quality score thresholds on to the data (see Materials and Methods). At quality scores above phred 40, where the chances of incorrect calls are just 1 in 10,000 [33], the number of G-to-A substitutions becomes roughly equivalent to C-to-T, A-to-G and T-to-C substitutions, in agreement with the current knowledge of mutations and the expected distribution of SNPs. This suggests that the systematic sequencing error we detected is diminished at such high phred values and traces are further enriched for genuine editing sites (Figure 1C).
DNA editing
Recently, DNA editing has been reported to be a powerful defense mechanism against the threat of genomic instability imposed by viruses and retrotransposons. However, the full magnitude of the phenomenon in vivo is not yet elucidated. We wanted to investigate whether our curated dataset of G-to-A mismatch clusters may actually include some examples of DNA editing. To test this assumption we looked at mismatch clusters in the mouse genome. We found that the total number of A-to-G and T-to-C mismatches was similar to the number of C-to-T and G-to-A mismatches (7,860 vs. 9,799). However, in genomic regions of IAP (intracisternal A-particle) elements, for which a few members are still active, there was a significant dominance of the G-to-A / T-to-C mismatches (114 compared to 49 A-to-G / T-to-C) (p-value of 0.00018, Fisher's Exact Test). This supports the idea that the origin of the mismatches is a result of editing by APOBEC after reverse transcription of the retrotransposons. An example of a DNA editing candidate, in a mouse retrotransposon, is given in Figure S1.
Active retrotransposons exist in human. For example, two edited HERVK elements have been recently discovered [34]. Thus, we applied our approach to human genomic sequences. Indeed we found evidence for DNA editing. We detected 247 events of G-to-A / C-to-T mismatch clusters versus 129 A-to-G / T-to-C events (while overall in the genome the ratio is 91,120 to 79,401 respectively) (p-value of 0.0000017, Fisher's Exact Test). One such candidate of editing by APOBEC in human retrotransposon HERVL-A1 is shown in Figure 3. An additional example for a probable editing event in a human retrotransposon is present in Figure 4 where clusters of G-to-A mismatches are found in the most active SINE family in human, AluY. All of these mismatches have high sequencing quality (Phred 40 or greater). Moreover, previously it was demonstrated that APOBEC3 can inhibit retrotransposition of Alu [35].
Fig. 3. DNA editing in human HERVL-A1. 
Trace 1735626615 aligns uniquely to chromosome 2 where the known retrotransposon HERVL-A1 is located (chr2: 100697697–100700125). A cluster of 15 G-to-A mismatches (worst mismatch phred 35; best mismatch phred 49) suggests that the trace originates from an edited version of the element. Support for the APOBEC source of the editing comes from the preferred GG-to-AG motif (11 out of the 15 cases) and GA-to-AA (remaining 4 cases) which is the dinucleotide context (in the same order) in an HIV hypermutated genome, and is the sequence motif of APOBEC3G and APOBEC3F [31]. Fig. 4. DNA editing in human AluY. 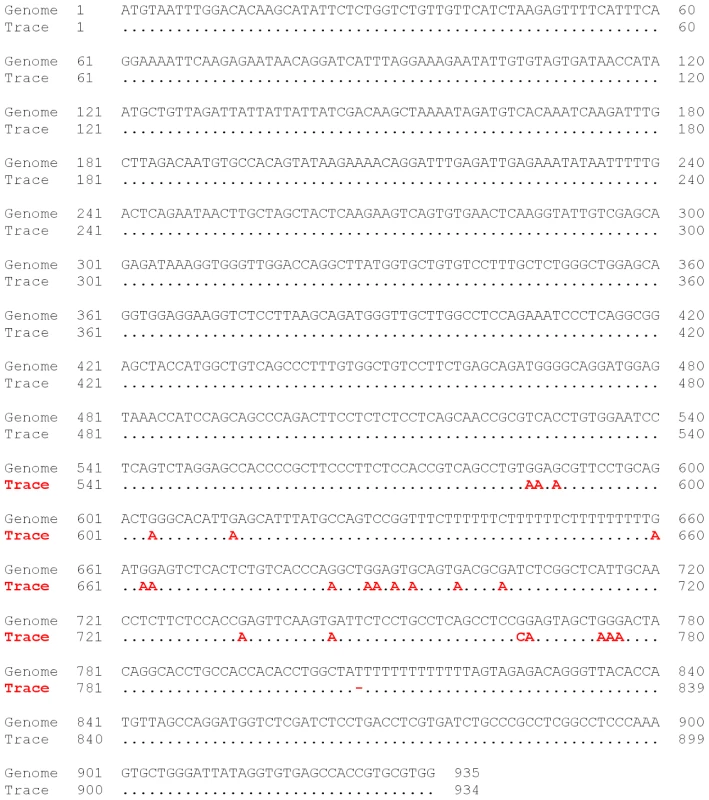
Example of possible DNA editing in human chr21:40977741–40978045. Alignment of trace 1745107496 to the human reference genome lead to large number of G-to-A mismatches which are indications for possible DNA editing in this retrotransposon. All the mismatches are located in high quality sequence positions, reducing the possibility of sequence errors. The actual number of edited traces in the trace archive is most probably much higher than we have found, for several reasons: More than half of all traces were rejected with our alignment parameters, at least partially due to the fact that DNA editing tends to lead to hyper-mutation in its target sequences [31]. Furthermore, we expect that a significant number of traces from retrotransposons, which are known targets for the APOBEC in their cDNA stage, are too redundant to align uniquely. Indeed, we found that in many cases the second best alignment of a putatively edited trace almost qualified for the 97% cut-off criteria, meaning that the trace was close to being rejected for having multiple possible genomic alignments. Thus, future work should find ways to curate the data in a less stringent manner so that editing, in traces with multiple hits to the genome or that do not meet our identity cut-offs, can still be detected. This would foster the development of a more complete picture of the occurrence of DNA editing in mammalian genomes.
RNA editing
RNA editing is a general term for the modification of RNA after it is transcribed from DNA. The most common modification in mammals is A-to-I editing by the ADAR protein family. As I (Inosine) is read as a G (Guanosine) after sequencing, this editing type manifests itself as an A-to-G substitutions after cDNA sequencing and alignment to the original genomic locus. Recently it was found that the human genome harbors large numbers of editing events that are located in clusters, mainly in Alu repeats [9], [10], [11], [12]. The origin of mismatch clusters in some of our traces, therefore, can be the result of ADAR activity.
A fraction of the human, mouse and Xenopus tropicalis sequences obtained from the trace archive are labeled as derived from RNA, rather than DNA. In total, after passing the stringent alignment criteria, 250K, 513K and 454K traces, respectively, of those genomes have RNA origin, thus A-to-G or T-to-C mismatches in these traces could be the result of RNA editing. No over-representation (38% of the total MM clusters) of A-to-G or T-to-C clusters appear in the RNA trace set (Figure 5A), but as demonstrated above, the vast majority of mismatches are probably derived from a sequencing artifact. To overcome this issue we filtered those RNA traces and generated a higher quality, enriched set which required 3 consecutive mismatches of any quality and two mismatches separated by at least 100bp of phred 40 or greater. When we consider our higher quality, editing enriched set (See Figure 5B), we find, in human, over-representation of mismatches that can be the result of RNA editing (A-to-G and T-to-C), a total of 79% of the mismatch clusters are now of this type (p-value 1.5e-119; Fisher's Exact Test.) These observations suggest that RNA editing is the cause of the mismatches in the higher quality RNA sets.
Fig. 5. Evidence for RNA editing in the cDNA traces. 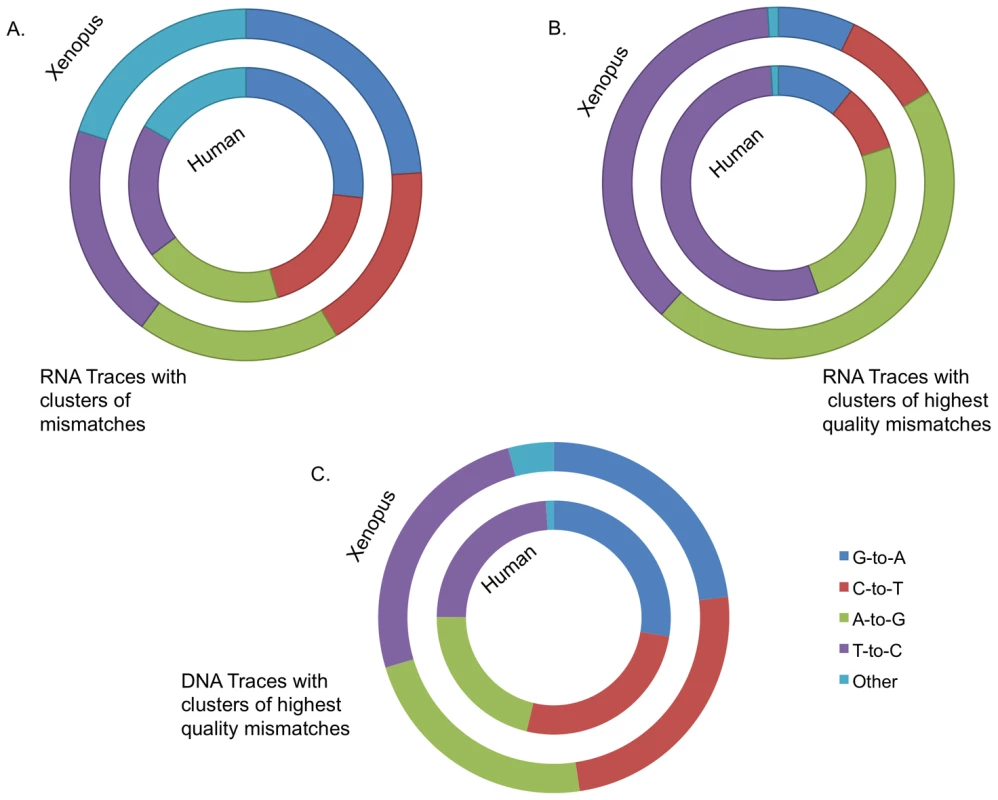
(A) While no over-representation of the RNA derived mismatches (A-to-G and its complimentary T-to-C) clusters are observed in the full set of RNA traces in human (n = 238,370) and Xenopus tropicalis (n = 444,526), (B) significant over-representation of RNA editing type is observed in high quality cDNA sequencing set of human (n = 769; p-value 1.5e-119; Fisher's Exact Test.) and Xenopus (n = 2,847; p-value≪e-200). (C) No such over-representation was observed in the set of high quality DNA traces (human: n = 64,191; Xenopus: n = 3,471). These observations support that RNA editing is the cause of the mismatches in the sets of higher quality cDNA. Further evidence that the higher quality set is indeed a result of RNA editing comes from two additional observations. First, a significant under-representation of “G” immediately upstream to the editing sites which is in agreement with the known sequence motif of the ADAR proteins [36]. In the enriched, higher quality set there was a G upstream of the mismatch in only 7.85% (265 out of 3,374) of the cases versus 30.3% (41,661 out of 137,313) in the non-enriched set (p-value 1.9e-143) [36],[37](See Figure 6). Second, most known editing events in human are located in Alu repeats and indeed 72% of the mismatches in the higher quality set are located in Alu repeats while Alu represents only about 10% of human DNA (p-value of 1.7e-110).
Fig. 6. ADAR signature in the cDNA edited traces. 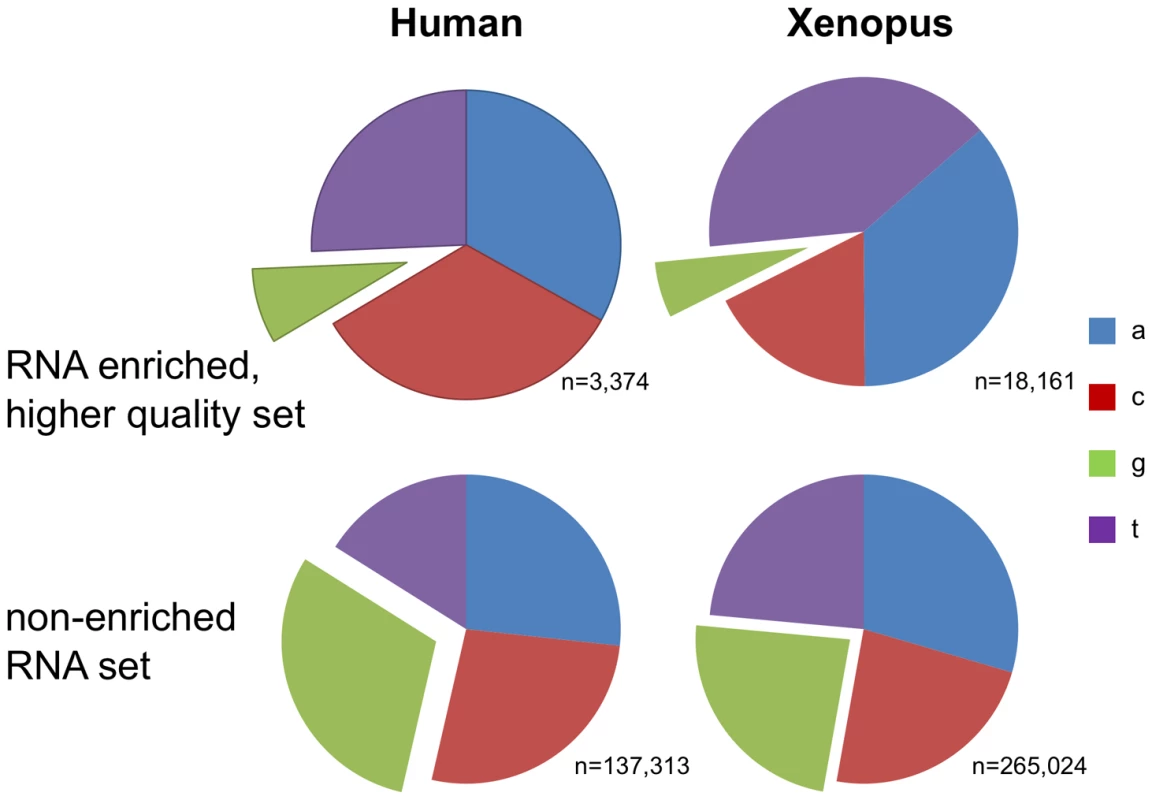
Significant under-representation of “G” immediately upstream to the editing sites which is in agreement with the known sequence motif of the ADAR proteins. Detection of RNA editing from short EST sequences has proven to be challenging, due to their relatively low sequence quality [38] and indeed, almost all A-to-I sites found until now were detected from alignment of a small set (<200,000) of full length RNAs [9], [10], [11], [39]. In the present work we used the human EST data deposited in the trace archive (currently including 2M ESTs which are mostly derived from poly-A mRNA) and found thousands of potential editing sites. Only 156 sites out of the 3374 sites in the higher quality, enriched set overlap with the known set of about 20,000 editing sites reported by alignment of RNA to the genome (total of 3,218 new sites). This suggests that ESTs, after accounting for sequence quality, can serve as a rich source for RNA editing site predictions.
Of the organisms we studied, only human, mouse and Xenopus tropicalis had significant numbers of RNA traces. If we use our enriched, higher quality set as a proxy for the total number of editing events, our data shows that in mouse, editing occurs at an estimated rate of 1 mismatch per 100,000 unique, expressed base-pairs. In human, in agreement with previous publications [11], [39], [40], our figures show ten-fold higher frequency. A striking picture emerges in Xenopus tropicalis. A closely related species, Xenopus laevis, is a principal model organism for the study of RNA editing as ADAR activity was first described in Xenopus laevis oocytes [41] and recently, research on hyper edited sequences in Xenopus laevis lead to the suggestion that editing can down-regulate gene expression in trans [42]. Only one endogenous hyper editing target is known in Xenopus - basic fibroblast growth factor (bFGF) [43], [44]. Using our approach for detection of RNA editing we have observed significant over-representation of A-to-I derived mismatch in Xenopus tropicalis. In the enriched set 83% of the mismatch clusters are of the A-to-G and T-to-C type, while these types contribute only 39% of the mismatch clusters in the non-enriched set (p-value≪e-200) (Figure 5). This strongly suggests that the mismatches in the enriched set are caused by RNA editing.
The Xenopus tropicalis genome has not been completed yet and the annotation is still partial. Thus, we cannot determine if the editing sites are located in one type or a small number of genomic repetitive regions. Interestingly, we found that 10001 out of the total 18161 mismatches in our editing-enriched, higher quality set occur in clusters of ten sites or more, larger than the common clusters detected in human RNAs sequences which have a typical size of less than 6 mismatches. By further examining a few mismatch clusters, we found that they tend to occur in palindromic regions that can form tight double stranded RNA. These structures are known to be required for ADAR editing (See Figure 7). As in human, we observed the ADAR signature of low abundance of “G” upstream of editing sites (5.8% for the higher quality enriched set versus 24% in the non-enriched set) (Figure 6, Tables S1, S2, S3, S4.). A full list with genomic coordinates of RNA editing sites in human and Xenopus is given in Datasets S1, S2.
Fig. 7. RNA editing in Xenopus tropicalis. 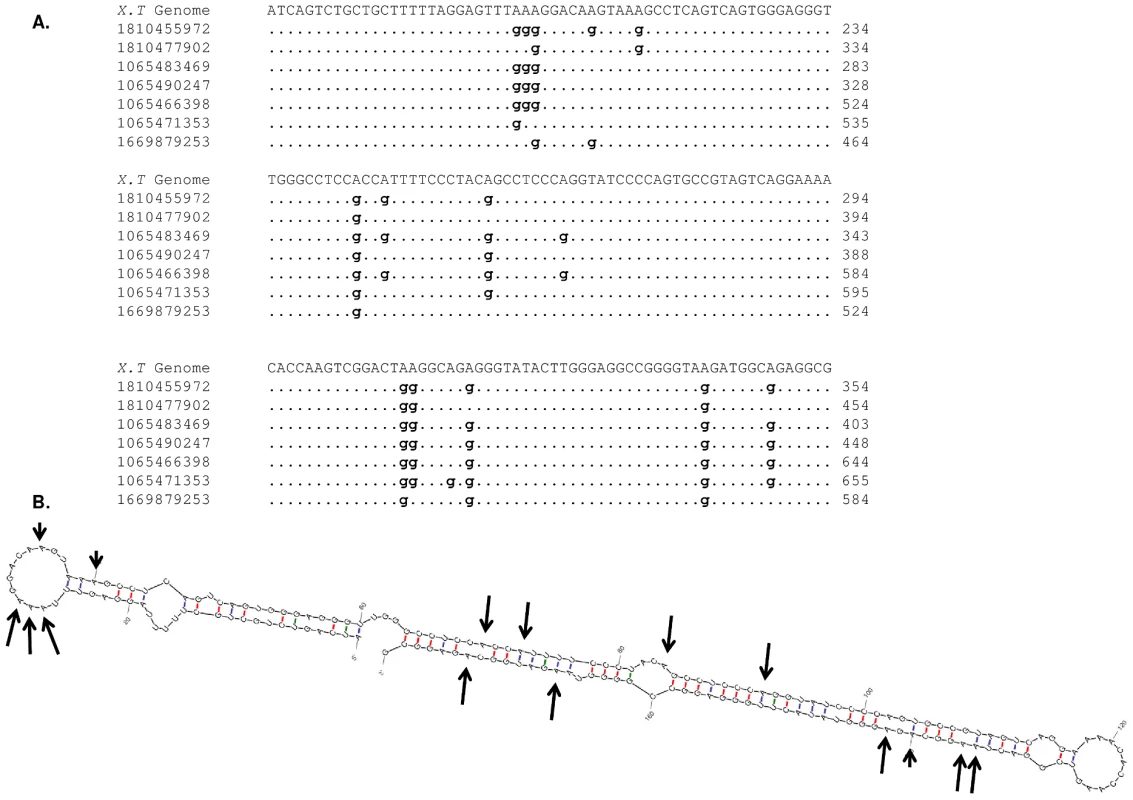
(A) Evidence for RNA editing can be seen in this locus as multiple traces of RNA origin align to it with numerous A-to-G mismatches. The trace accession numbers and their coordinates are given in the multiple alignment. (B) Predicted RNA structure of the genomic locus indicates a long and stable dsRNA structure which is a favorite target for editing by ADARs. Each editing site from the multiple alignment is marked by an arrow. The length of the arrow corresponds to the editing level. Discussion
The NCBI trace archive serves as a repository of raw data for the assembly of consensus genomes. Recently, it was utilized for a different purpose in the search for structural variation in the human genome [45]. Here, we show that it can also be used in the search for DNA and RNA editing. In the future, sequencing results deposited in the NCBI short-read archive might shed more light on these phenomena. Shorter reads, however, will pose a more challenging analysis problem.
Recently, we did an initial analysis of Illumina's human resequencing reads and the SOLiD reads from the same individual. These reads are available at the NCBI short-read archive and are the basis for the first individual African consensus genome [46], [47]. Given the importance of read-length and quality scores on the outcome of our current work, the current SOLiD and Illumina reads represent interesting trade-offs for the detection of editing. While Illumina's current read lengths are generally longer than SOLiD, the latter has much higher per-base quality. Adapting the techniques presented here to this new data presents an interesting opportunity for future research.
The availability of computational resources for carrying out our analyses was essential to this project, as large computational effort was needed, six terabytes of disk for intermediate data and more than five “node years” of CPU time. With further computational effort, combining existing data in the trace archive with next generation sequencing data sets from multiple sequencing platforms and chemistries, it should be possible to greatly improve genomic databases and eliminate the sequencing errors reported here.
By using well-calibrated quality scores and selecting traces with clusters of consecutive mismatches, we are able to investigate the scope of RNA editing sites in human and other genomes. The application of this technique in the search for editing events will make many large EST datasets more accessible for other organisms where quality scores are available. Currently, only a very small number of organisms, with large sets of full length RNA sequences, have been the subject of large-scale editing studies. Using quality scores, many additional genomes can be surveyed for editing with the opportunity for new discoveries in this emerging field.
As a demonstration of the value of using quality data for ESTs, we are able to find a large number of candidate RNA editing events in Xenopus tropicalis. This discovery makes X. tropicalis the non human organism with the largest number of known editing sites so far. Since Xenopus is already an important model organism for the research of RNA editing, this new data-set could help foster new discoveries in this field.
Despite the identification of thousands of newly discovered RNA editing sites in the current work, it is reasonable to believe that the actual number of editing sites is still significantly under-estimated. Support for this assertion comes from the stringency of our parameters: including length of alignment, percentage of identity and exclusion of insertions or deletions. These choices most likely limited the subset of EST data that we analyzed. Refinement of these criteria could lead to more comprehensive detection of RNA editing levels and, due to the breadth of EST data, even permit the comparison of editing levels in different tissues and disease conditions.
In this work we also found evidence for recent or active events of DNA editing. While the true scope of these phenomena must be explored in future work, our approach, including the use of strict alignment criteria and quality scores, has proved effective at finding many intriguing examples. Using different parameters, mainly lower cutoffs and relaxation of the requirement for unique alignments, more DNA editing sites could be detected in the trace archive. Careful investigation, most likely combined with next-generation sequencing experiments, will help unravel the mechanisms of retroelement defenses in a variety of organisms. Moreover, DNA editing is known not to be limited to retrotransposons and can take place in other genomic loci. The most recognized example is the AID protein, which is a member of the AID/APOBEC protein family, and targets single stranded DNA in the immunoglobulin locus in B-cells. Similar approaches to the ones used here provide an exciting opportunity to survey how leakage of DNA editing events, outside retroelements, or immunoglobulins could cause many simultaneous mutations in the genome, a process that can eventually lead to cancer.
Materials and Methods
We obtained all traces for 10 organisms (600M traces in total), in FASTA format, at the NCBI Trace Archive [48] (http://www.ncbi.nlm.nih.gov/Traces/home/, May 2008) and aligned them with their reference genomes obtained from the UCSC Genome Browser [49]. We did not attempt to filter the initial set of traces by type which would have required the combination of FASTA format sequences with auxiliary information that provides the trace type. Instead we used strict placement criteria, described further below, to obtain the initial dataset summarized in Table 1. We inspected chromatograms for individual traces using the tools provided at the trace archive. We further downloaded SCF raw binary data from the archive, by hand, and analyzed them using Phred version “0.071220.b” [32]. This Phred version can generate an alternate base call for every position in the trace. This results in two sets of sequences for any given trace. By aligning the two sequences from the same trace separately, and looking for a large alignment with a single base-pair offset, we can identify the sequencing error from Figure 1. This might be the basis for an automated test to eliminate this particular sequencing error.
We augmented the above data by downloading auxiliary information and quality scores for a subset of about 20.7 million traces which were, potentially, enriched for editing events. We used runs of three consecutive mismatches of the same type as the enrichment criteria. The number of high quality traces for each editing type (G-to-A, C-to-T, A-to-G, and T-to-C) - is listed in Table 2. For all organisms, except for mosquito and fly, there are more than ten times the number of examples from these four types than the next most frequent type. Furthermore, we extracted the lowest quality subset of these traces enriched for editing to be used for comparison purposes. The number of traces of each editing type from this set, G-to-A, C-to-T, A-to-G, T-to-C, as well as the most frequent or next most frequent type, is listed in Table S5. For Mouse, Human, and Xenopus tropicalis these tables also provide (in brackets) the number of traces that likely originated from RNA.
The complete set of mismatches found in these two sets of traces is available to the community as two files, “all.c2.t100.q40+.bed.gz” (5.95MB) and “all.c2.t100.q0-9.bed.gz” (122MB), respectively. The first set is included on the journal's web-site while the second file is available, on request, from the authors. The files contain: the genomic coordinate of the mismatch, the mismatch type, the position on the trace, the quality of the mismatch, the length of the run in which the mismatch was found, the sequencing center, the trace id, the organism, and the likely origin of the trace, DNA or RNA. In order to be counted, each trace must have at least two mismatches with phred 40 or greater that are separated by 100bp or more. Only mismatches with phred scores of 40 or greater are included in the high quality set (see Figures S2, S3, S4 for more data). In the lower quality set, at least two mismatches with phred less than 10 separated by 100bp or more are required. Only mismatches with phred scores of less than 10 are included in the low quality set.
For sequence alignment, we used MegaBlast [50] version 2.2.13 from NCBI. The parameters used were: -W60 (a 60bp seed was selected as a good compromise between computational efficiency and sensitivity, given our requirement of high identity to the reference), -s 400 -p 97 (at least 400bp with 97% identity) -F F (no filtering) -G25 -E10 (these gap and extension penalties preclude insertions and/or deletions in matches). In addition, only unique alignments matching the above criteria were retained. These parameters were chosen for simplicity of subsequent analysis and to reduce the already onerous computational requirements.
Two computational clusters were used to perform the analysis. These clusters were built to assist in deploying data intensive web services [51]. In total, the clusters use a variety of older and newer hardware and consist of 96 nodes w/ (predominantly) 4×1.8GHZ Opteron cores, 4–16GB of RAM per node, and 0–3750GB disk per node. The workflows to generate the initial analysis of the data are written in Perl. The human analysis consumed 347 node days and 530GB of space which was reduced to 22GB of compressed data after parsing the MegaBlast output and discarding redundant matches. A summary of the traces and space/time used by the computation can be found in Table 1. The startling amount of intermediate space required by the mouse analysis, greater than 4.2 terabytes, suggests that many traces in mouse did not place uniquely and consumed large amounts of space, even with our strict chosen cut-offs and using gzip compression on the output of MegaBlast.
Supporting Information
Zdroje
1. BassBL
2002 RNA editing by adenosine deaminases that act on RNA. Annu Rev Biochem 71 817 846
2. HurstSR
HoughRF
AruscavagePJ
BassBL
1995 Deamination of mammalian glutamate receptor RNA by Xenopus dsRNA adenosine deaminase: similarities to in vivo RNA editing. Rna 1 1051 1060
3. KimU
WangY
SanfordT
ZengY
NishikuraK
1994 Molecular cloning of cDNA for double-stranded RNA adenosine deaminase, a candidate enzyme for nuclear RNA editing. Proc Natl Acad Sci U S A 91 11457 11461
4. MelcherT
MaasS
HerbA
SprengelR
SeeburgPH
1996 A mammalian RNA editing enzyme. Nature 379 460 464
5. O'ConnellMA
KrauseS
HiguchiM
HsuanJJ
TottyNF
1995 Cloning of cDNAs encoding mammalian double-stranded RNA-specific adenosine deaminase. Mol Cell Biol 15 1389 1397
6. MaasS
KawaharaY
TamburroKM
NishikuraK
2006 A-to-I RNA editing and human disease. RNA Biol 3 1 9
7. KeeganLP
LeroyA
SproulD
O'ConnellMA
2004 Adenosine deaminases acting on RNA (ADARs): RNA-editing enzymes. Genome Biol 5 209
8. LiJB
LevanonEY
YoonJK
AachJ
XieB
2009 Genome-wide identification of human RNA editing sites by parallel DNA capturing and sequencing. Science 324 1210 1213
9. AthanasiadisA
RichA
MaasS
2004 Widespread A-to-I RNA Editing of Alu-Containing mRNAs in the Human Transcriptome. PLoS Biol 2 e391 10.1371/journal.pbio.0020391
10. BlowM
FutrealPA
WoosterR
StrattonMR
2004 A survey of RNA editing in human brain. Genome Res 14 2379 2387
11. KimDD
KimTT
WalshT
KobayashiY
MatiseTC
2004 Widespread RNA editing of embedded alu elements in the human transcriptome. Genome Res 14 1719 1725
12. LevanonEY
EisenbergE
YelinR
NemzerS
HalleggerM
2004 Systematic identification of abundant A-to-I editing sites in the human transcriptome. Nat Biotechnol 22 1001 1005
13. ConticelloSG
2008 The AID/APOBEC family of nucleic acid mutators. Genome Biol 9 229
14. NavaratnamN
MorrisonJR
BhattacharyaS
PatelD
FunahashiT
1993 The p27 catalytic subunit of the apolipoprotein B mRNA editing enzyme is a cytidine deaminase. J Biol Chem 268 20709 20712
15. TengB
BurantCF
DavidsonNO
1993 Molecular cloning of an apolipoprotein B messenger RNA editing protein. Science 260 1816 1819
16. HarrisRS
Petersen-MahrtSK
NeubergerMS
2002 RNA editing enzyme APOBEC1 and some of its homologs can act as DNA mutators. Mol Cell 10 1247 1253
17. MuramatsuM
SankaranandVS
AnantS
SugaiM
KinoshitaK
1999 Specific expression of activation-induced cytidine deaminase (AID), a novel member of the RNA-editing deaminase family in germinal center B cells. J Biol Chem 274 18470 18476
18. MuramatsuM
KinoshitaK
FagarasanS
YamadaS
ShinkaiY
2000 Class switch recombination and hypermutation require activation-induced cytidine deaminase (AID), a potential RNA editing enzyme. Cell 102 553 563
19. RevyP
MutoT
LevyY
GeissmannF
PlebaniA
2000 Activation-induced cytidine deaminase (AID) deficiency causes the autosomal recessive form of the Hyper-IgM syndrome (HIGM2). Cell 102 565 575
20. JarmuzA
ChesterA
BaylissJ
GisbourneJ
DunhamI
2002 An anthropoid-specific locus of orphan C to U RNA-editing enzymes on chromosome 22. Genomics 79 285 296
21. SheehyAM
GaddisNC
ChoiJD
MalimMH
2002 Isolation of a human gene that inhibits HIV-1 infection and is suppressed by the viral Vif protein. Nature 418 646 650
22. WedekindJE
DanceGS
SowdenMP
SmithHC
2003 Messenger RNA editing in mammals: new members of the APOBEC family seeking roles in the family business. Trends Genet 19 207 216
23. MehtaA
KinterMT
ShermanNE
DriscollDM
2000 Molecular cloning of apobec-1 complementation factor, a novel RNA-binding protein involved in the editing of apolipoprotein B mRNA. Mol Cell Biol 20 1846 1854
24. LellekH
KirstenR
DiehlI
ApostelF
BuckF
2000 Purification and molecular cloning of a novel essential component of the apolipoprotein B mRNA editing enzyme-complex. J Biol Chem 275 19848 19856
25. HarrisRS
BishopKN
SheehyAM
CraigHM
Petersen-MahrtSK
2003 DNA deamination mediates innate immunity to retroviral infection. Cell 113 803 809
26. MangeatB
TurelliP
CaronG
FriedliM
PerrinL
2003 Broad antiretroviral defence by human APOBEC3G through lethal editing of nascent reverse transcripts. Nature 424 99 103
27. MarianiR
ChenD
SchrofelbauerB
NavarroF
KonigR
2003 Species-specific exclusion of APOBEC3G from HIV-1 virions by Vif. Cell 114 21 31
28. VartanianJP
GuetardD
HenryM
Wain-HobsonS
2008 Evidence for editing of human papillomavirus DNA by APOBEC3 in benign and precancerous lesions. Science 320 230 233
29. YuQ
KonigR
PillaiS
ChilesK
KearneyM
2004 Single-strand specificity of APOBEC3G accounts for minus-strand deamination of the HIV genome. Nat Struct Mol Biol 11 435 442
30. EsnaultC
HeidmannO
DelebecqueF
DewannieuxM
RibetD
2005 APOBEC3G cytidine deaminase inhibits retrotransposition of endogenous retroviruses. Nature 433 430 433
31. ChiuYL
GreeneWC
2008 The APOBEC3 cytidine deaminases: an innate defensive network opposing exogenous retroviruses and endogenous retroelements. Annu Rev Immunol 26 317 353
32. EwingB
GreenP
1998 Base-calling of automated sequencer traces using phred. II. Error probabilities. Genome Res 8 186 194
33. EwingB
HillierL
WendlMC
GreenP
1998 Base-calling of automated sequencer traces using phred. I. Accuracy assessment. Genome Res 8 175 185
34. LeeYN
MalimMH
BieniaszPD
2008 Hypermutation of an ancient human retrovirus by APOBEC3G. J Virol 82 8762 8770
35. ChiuYL
WitkowskaHE
HallSC
SantiagoM
SorosVB
2006 High-molecular-mass APOBEC3G complexes restrict Alu retrotransposition. Proc Natl Acad Sci U S A 103 15588 15593
36. LehmannKA
BassBL
2000 Double-stranded RNA adenosine deaminases ADAR1 and ADAR2 have overlapping specificities. Biochemistry 39 12875 12884
37. WongSK
SatoS
LazinskiDW
2001 Substrate recognition by ADAR1 and ADAR2. Rna 7 846 858
38. HillierLD
LennonG
BeckerM
BonaldoMF
ChiapelliB
1996 Generation and analysis of 280,000 human expressed sequence tags. Genome Res 6 807 828
39. EisenbergE
NemzerS
KinarY
SorekR
RechaviG
2005 Is abundant A-to-I RNA editing primate-specific? Trends Genet 21 77 81
40. NeemanY
LevanonEY
JantschMF
EisenbergE
2006 RNA editing level in the mouse is determined by the genomic repeat repertoire. Rna 12 1802 1809
41. BassBL
WeintraubH
1987 A developmentally regulated activity that unwinds RNA duplexes. Cell 48 607 613
42. ScaddenAD
2007 Inosine-containing dsRNA binds a stress-granule-like complex and downregulates gene expression in trans. Mol Cell 28 491 500
43. KimelmanD
KirschnerMW
1989 An antisense mRNA directs the covalent modification of the transcript encoding fibroblast growth factor in Xenopus oocytes. Cell 59 687 696
44. SaccomannoL
BassBL
1999 A minor fraction of basic fibroblast growth factor mRNA is deaminated in Xenopus stage VI and matured oocytes. Rna 5 39 48
45. TuzunE
SharpAJ
BaileyJA
KaulR
MorrisonVA
2005 Fine-scale structural variation of the human genome. Nat Genet 37 727 732
46. McKernanKJ
PeckhamHE
CostaGL
McLaughlinSF
FuY
2009 Sequence and structural variation in a human genome uncovered by short-read, massively parallel ligation sequencing using two-base encoding. Genome Res
47. BentleyDR
BalasubramanianS
SwerdlowHP
SmithGP
MiltonJ
2008 Accurate whole human genome sequencing using reversible terminator chemistry. Nature 456 53 59
48. WheelerDL
BarrettT
BensonDA
BryantSH
CaneseK
2008 Database resources of the National Center for Biotechnology Information. Nucleic Acids Res 36 D13 21
49. KentWJ
SugnetCW
FureyTS
RoskinKM
PringleTH
2002 The human genome browser at UCSC. Genome Res 12 996 1006
50. ZhangZ
SchwartzS
WagnerL
MillerW
2000 A greedy algorithm for aligning DNA sequences. J Comput Biol 7 203 214
51. ZaranekA
CleggT
VandewegeW
ChurchG
Free Factories: Unified Infrastructure for Data Intensive Web Services; 2008 Boston, MA 391 404
Štítky
Genetika Reprodukční medicína
Článek Epistasis of Transcriptomes Reveals Synergism between Transcriptional Activators Hnf1α and Hnf4αČlánek SMA-10/LRIG Is a Conserved Transmembrane Protein that Enhances Bone Morphogenetic Protein SignalingČlánek B1 SOX Coordinate Cell Specification with Patterning and Morphogenesis in the Early Zebrafish EmbryoČlánek Bulk Segregant Analysis by High-Throughput Sequencing Reveals a Novel Xylose Utilization Gene fromČlánek Abundant Quantitative Trait Loci Exist for DNA Methylation and Gene Expression in Human Brain
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2010 Číslo 5- Akutní intermitentní porfyrie
- Růst a vývoj dětí narozených pomocí IVF
- Farmakogenetické testování pomáhá předcházet nežádoucím efektům léčiv
- Pilotní studie: stres a úzkost v průběhu IVF cyklu
- Vliv melatoninu a cirkadiálního rytmu na ženskou reprodukci
-
Všechny články tohoto čísla
- Digital Quantification of Human Eye Color Highlights Genetic Association of Three New Loci
- Common Genetic Variants near the Brittle Cornea Syndrome Locus Influence the Blinding Disease Risk Factor Central Corneal Thickness
- Age- and Temperature-Dependent Somatic Mutation Accumulation in
- All About Mitochondrial Eve: An Interview with Rebecca Cann
- Aging and Chronic Sun Exposure Cause Distinct Epigenetic Changes in Human Skin
- Transposed Genes in Arabidopsis Are Often Associated with Flanking Repeats
- A Survey of Genomic Traces Reveals a Common Sequencing Error, RNA Editing, and DNA Editing
- Gene Transposition Causing Natural Variation for Growth in
- The Polyproline Site in Hinge 2 Influences the Functional Capacity of Truncated Dystrophins
- Epistasis of Transcriptomes Reveals Synergism between Transcriptional Activators Hnf1α and Hnf4α
- Integration of Light Signals by the Retinoblastoma Pathway in the Control of S Phase Entry in the Picophytoplanktonic Cell
- The Proprotein Convertase Encoded by () Is Required in Corpora Cardiaca Endocrine Cells Producing the Glucose Regulatory Hormone AKH
- Sgs1 and Exo1 Redundantly Inhibit Break-Induced Replication and Telomere Addition at Broken Chromosome Ends
- A MATE-Family Efflux Pump Rescues the 8-Oxoguanine-Repair-Deficient Mutator Phenotype and Protects Against HO Killing
- The Relationship among Gene Expression, the Evolution of Gene Dosage, and the Rate of Protein Evolution
- SMA-10/LRIG Is a Conserved Transmembrane Protein that Enhances Bone Morphogenetic Protein Signaling
- The Nuclear Receptor DHR3 Modulates dS6 Kinase–Dependent Growth in
- Involvement of Global Genome Repair, Transcription Coupled Repair, and Chromatin Remodeling in UV DNA Damage Response Changes during Development
- B1 SOX Coordinate Cell Specification with Patterning and Morphogenesis in the Early Zebrafish Embryo
- Linkage and Association Mapping of Flowering Time in Nature
- Bulk Segregant Analysis by High-Throughput Sequencing Reveals a Novel Xylose Utilization Gene from
- Abundant Quantitative Trait Loci Exist for DNA Methylation and Gene Expression in Human Brain
- Ablation of Whirlin Long Isoform Disrupts the USH2 Protein Complex and Causes Vision and Hearing Loss
- Characterization of Oxidative Guanine Damage and Repair in Mammalian Telomeres
- DNA Adenine Methylation Is Required to Replicate Both Chromosomes Once per Cell Cycle
- Genome-Wide Copy Number Variation in Epilepsy: Novel Susceptibility Loci in Idiopathic Generalized and Focal Epilepsies
- FACT Prevents the Accumulation of Free Histones Evicted from Transcribed Chromatin and a Subsequent Cell Cycle Delay in G1
- GC-Biased Evolution Near Human Accelerated Regions
- Liver and Adipose Expression Associated SNPs Are Enriched for Association to Type 2 Diabetes
- Myeloid Cell-Restricted Insulin Receptor Deficiency Protects Against Obesity-Induced Inflammation and Systemic Insulin Resistance
- The Mating Type Locus () and Sexual Reproduction of : Insights into the Evolution of Sex and Sex-Determining Chromosomal Regions in Fungi
- B-Cyclin/CDKs Regulate Mitotic Spindle Assembly by Phosphorylating Kinesins-5 in Budding Yeast
- Post-Replication Repair Suppresses Duplication-Mediated Genome Instability
- Genome Sequence of the Plant Growth Promoting Endophytic Bacterium sp. 638
- Transcription Factors Mat2 and Znf2 Operate Cellular Circuits Orchestrating Opposite- and Same-Sex Mating in
- The Use of Orthologous Sequences to Predict the Impact of Amino Acid Substitutions on Protein Function
- Mutation in Archain 1, a Subunit of COPI Coatomer Complex, Causes Diluted Coat Color and Purkinje Cell Degeneration
- Shelterin-Like Proteins and Yku Inhibit Nucleolytic Processing of Telomeres
- Affecting Function Causes a Dilated Heart in Adult
- Manipulation of Behavioral Decline in with the Rag GTPase
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Common Genetic Variants near the Brittle Cornea Syndrome Locus Influence the Blinding Disease Risk Factor Central Corneal Thickness
- All About Mitochondrial Eve: An Interview with Rebecca Cann
- The Relationship among Gene Expression, the Evolution of Gene Dosage, and the Rate of Protein Evolution
- SMA-10/LRIG Is a Conserved Transmembrane Protein that Enhances Bone Morphogenetic Protein Signaling
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



