-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
A Ukrainian multicenter prospective study of the value of PET/ CT prognostic role in primary patients with Hodgkin’s lymphoma in a real-life cohort
Ukrajinská multicentrická prospektivní studie hodnoty prognostické role PET/ CT u primárních pacientů s Hodgkinovým lymfomem v kohortě z reálného života
Úvod: V posledních letech se pozitronová emisní tomografie v kombinaci s výpočetní tomografií (PET/CT) změnila a zcela se zlepšily léčebné postupy u pacientů s Hodgkinovým lymfomem (HL). Hlavní myšlenkou několika studií je použití PET/CT a protokolů Mezinárodního prognostického skóre (International Prognostic Score – IPS) při identifikaci pacientů s vysokým rizikem a potenciálního časného relapsu/refrakterního onemocnění. Materiál a metody: Tato studie vychází z hodnocení PET/CT a léčebných strategií u pacientů z osmi hematologických center na Ukrajině. Pacientům ve věku 67 let nebo mladším, kteří byli zařazeni do studie, byl nově diagnostikován HL. Byla jim podávána léčba v režimu ABVD nebo BEACOPP-14/esc nebo „switched“ režimech (ABVD + BEACOPP-esc/14, BEACOPP-esc/14 + ABVD). Primárními endpointy bylo stanovení korelace mezi nálezem na PET/CT v době diagnózy, odpovědí na léčbu a klinické výsledky (relaps/úmrtí) u pacienta s HL v časných nebo pokročilých stadiích. Mezi sekundární endpointy patřilo stanovení vztahu mezi IPS a nálezy z PET/CT. Výsledky: Ve studijní skupině bylo 106 pacientů. Celková míra odpovědí (overall response rate – ORR) byla 90,5 %. ORR u pacientů ve stadiu I–II byla 96,5 % (55/57) vs. 91 % (41/45) u pacientů ve stadiu III–IV. K progresi onemocnění došlo u 58,3 % (7/12) PET2+ pacientů a u 13,3 % (12/90) PET2 − pacientů (p < 0,05). U pacientů PET2+ vs. PET2 − nebyly zjištěny významné rozdíly mezi mírou přežitím bez příhody (EFS) a IPS (log-rank test; p = 0.4). Status PET3 − byl zjištěn u 88,8 % (79/89) pacientů studijní skupiny a 1,2 % (10/89) mělo status PET3+ (p < 0.05). Pomocí Coxovy regrese jsme potvrdili významnou korelaci mezi EFS s PET3 podle Deauville stupnice (DS) a IPS. U pacientů s DS 1–2, DS 3 a DS 4–5 bylo jednoleté EFS 94,4 %, 100 % a 33 % (HR 0.56; 95% CI 1.07–2.8; p < 0,02). Naše multivariační analýza neprokázala statisticky významnou korelaci mezi PET2+ a PET3+ statusem a extranodálním postižením nebo velkou zátěží tumorem. Závěr: Výsledky využití PET/CT u pacienta s primárním HL ukázaly vysokou prognostickou hodnotu PET na konci léčby. Navíc jsme potvrdili prediktivní roli prognostického modelu IPS ve smyslu výsledku léčby v závislosti na PET statusu.
Klíčová slova:
Hodgkinův lymfom – pozitronová emisní tomografie – prognóza – přežití
Authors: Olga Novosad 1; Tetiana Skrypets 1; Ian Pastushenko 1; Tetiana Kadnikova 1; Oleksandr Gorbach 1; Viktor Kozlov 2; Larisa Mykhalska 3; Viktoria Kosinova 4; Nina Kostiukova 5; Oksana Karnabeda 6; Viktoria Stratienko 7; Mykola Novikov 4; Olena Oliinichenko 8; Iaroslav Kmetyuk 9; Olga Karpova 9; Andrii Ashykhmin 10; Olena Lukjanec 11; Irina Kriachok 1
Authors place of work: Oncology and Hematology, National Cancer Institute, Kyiv, Ukraine 1; Regional Clinical Hospital, Odessa, Ukraine 2; Center of Hematology, Feofaniya Clinical Hospital, Kyiv, Ukraine 3; Oncology Hospital “LISOD”, Kyiv, Ukraine 4; Kyiv Centre of Bone Marrow Transplantation, Kyiv, Ukraine 5; Hospital of Oncology “Innovacia”, Kyiv, Ukraine 6; Regional Clinical Hospital, Kherson, Ukraine 7; Center of Nuclear Medicine, Kyiv, Ukraine 8; Feofaniya Clinical Hospital, Kyiv, Ukraine 9; Radiology, National Cancer Institute, Kyiv, Ukraine 10; Cherkassy Regional Oncology Center, Cherkasy, Ukraine 11
Published in the journal: Klin Onkol 2020; 33(6): 450-457
Category: original article
Summary
Introduction: In recent years, the positron emission tomography combined with computed tomography (PET/CT) has changed and the treatment approaches in Hodgkin’s lymphoma (HL) patients have entirely improved. The main idea in several studies is the use of PET/CT and the International Prognostic Score (IPS) protocols in identification of patients within a high-risk group and potential early relapse/refractory disease. Materials and methods: This study was based on PET/CT evaluation and treatment strategies of patients from eight Centers of Hematology in Ukraine. The patients included were newly diagnosed with HL and were aged 67 years or younger. They received a treatment with ABVD or BEACOPP-14/esc or “switched-regimens” (ABVD + BEACOPP-esc/14, BEACOPP-esc/14 + ABVD). The primary endpoints were to assess a correlation between PET/CT findings at the time of diagnosis, response to the therapy and clinical outcome (relapse/death) for patients with early and advanced stages of HL. The secondary endpoints were to evaluate the relationship between IPS and PET/CT findings. Results: The study group included 106 patients. The overall response rate (ORR) was 90.5%. The ORR for patients with stages I–II was 96.5% (55/57) vs. 91% (41/45) for stage III–IV patients. In total, the disease progression occurred in 58.3% (7/12) of PET2+ patients and in 13.3% (12/90) of PET2 − patients (P < 0.05). No significant difference was found between the event free survival (EFS) rate and IPS for patients with PET2+ vs. PET2−, (log-rank test; P = 0.4). The PET3 − status was found in 88.8% (79/89) of the study group patients and 1.2% (10/89) had a PET3+ status (P < 0.05). Using the Cox regression, we confirmed a significant correlation between EFS with PET3 Deauville scale (DS) and IPS. Patients with DS 1–2, DS 3 and DS 4–5 had a 1-year event-free survival of 94.4%, 100% and 33%, respectively (HR 0.56; 95% CI 1.07–2.8; P < 0.02). Our multivariable analysis showed no statistically significant correlation between PET2+ and PET3+ status and extranodal involvement or large tumor burden. Conclusion: The results of using PET/CT in patients with primary HL demonstrated a high prognostic value of PET at the end of the treatment. In addition, we confirmed the predictive role of IPS prognostic model in the treatment outcome depending on PET status.
Keywords:
Hodgkin’s lymphoma – positron emission tomography – prognosis – survival
Introduction
Hodgkin’s lymphoma (HL) has become a potentially curable disease with an ultimately good prognosis. Currently, an individualized treatment approach to patient assessment requires more accurate staging on our end. There have been continuous historical debates as for the escalated therapy regimen use for primary advanced HL patients, or treatment intensification only for poor-risk individuals. Similar questions arise for early stage patients: should a chemo and radiotherapy treatment combination apply for all patients, or are there cases where we can skip radiation? The main goal of these discussions is to reduce the excessive toxicity related to HL patients’ treatment.
We need new reliable markers to predict tumor response to the therapy of each individual case, as the mortality rate induced by HL patients’ treatment may be higher than the one caused by a disease relapse or refractory, secondary cancers or other complications [1,2]. Therefore, developing an individualized treatment approach is important due to the chemoresistance risk and chemotherapy complications for a large number of patients.
Considering the heterogeneity of patients with different HL stages, we need a rapid and objective tumor response assessment, using imaging in particular.
The 18-fluoro-2-deoxyglucose positron emission tomography (FDG-PET), one of the biggest advanced functional imaging methods, promises a great development in new successful treatment approaches [3,4]. FDG-PET is now a standard procedure for staging and therapy response evaluation of HL patients and some other non-Hodgkin’s lymphoma subtypes [5,6]. The PET scan high prognostic value is commonly associated with HL high chemosensitivity manifested by a rapidly reduced metabolic activity of responders [7–10].
Big randomized studies are being currently finalized; they can potentially prove the PET main factor role for primary HL patients. This is particularly important for intermediate risk group patients when opting for or against the switched-regimen [11–13].
Materials and methods
Our study is based on a group of patients‘ PET evaluations and treatment regimens from eight Hematological Centers of Ukraine. Patients of up to 67 years of age with newly diagnosed HL were treated with ABVD or BEACOPP-14/esc or “switched-regimens” (ABVD + BEACOPP-esc/14, BEACOPP-esc/14 + ABVD), based on their stage and risk group (Fig. 1). The response to therapy was determined according to Chesson 2008 criteria.
Fig. 1. Patients flow chart.
HL – Hodgkin’s lymphoma, IPS – International Prognostic Score, PET – positron emission tomography, PET0 – baseline PET, PET2 – PET after two chemotherapy cycles, PET3 – PET after 3 weeks from the last chemotherapy cycle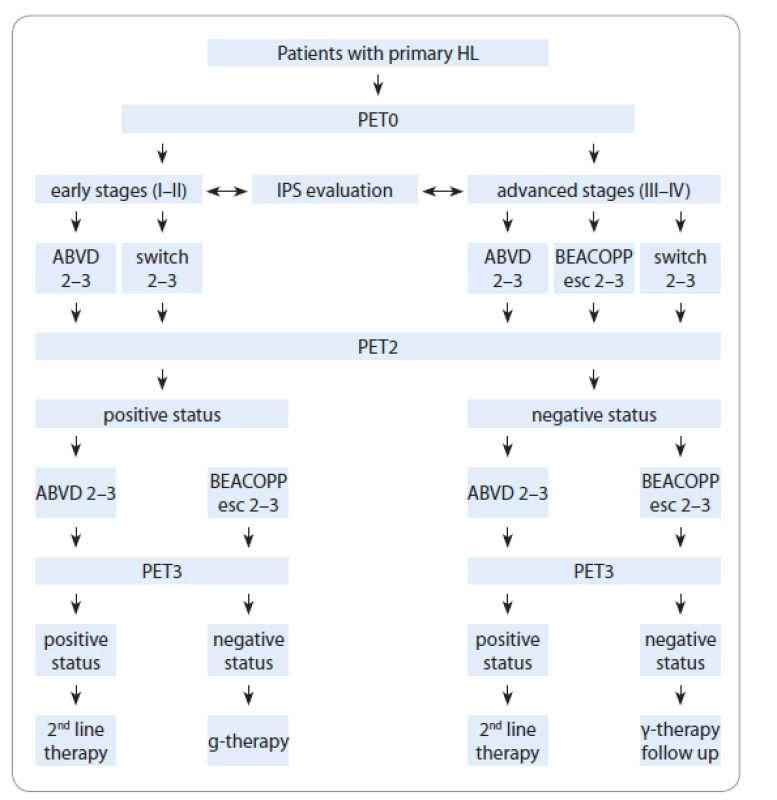
We marked three steps of positron emission tomography combined with computed tomography (PET/CT) as following:
PET0 – step 1: PET/CT performed at baseline for all patients before the treatment;
PET2 – step 2: PET/CT performed in interim analysis after two chemotherapy cycles or later;
PET3 – step 3: PET/CT performed within 3 weeks from the last chemotherapy cycle in order to monitor the treatment response.
The study primary endpoint was to assess a correlation between PET/CT findings at the time of diagnosis, response to the therapy and clinical outcome (relapse/death) of early and advanced stage HL patients.
The secondary endpoint was to evaluate the correlation between the IPS and PET/CT findings and the type of chemotherapy.
PET/CT procedure
PET/CT studies were performed at the Ukrainian Center of Radiosurgery of Feofaniya Clinical Hospital, Radiology Department of LISOD Oncologic Hospital, and Center of Nuclear Medicine. The patients fasted 4–6 hours prior to the procedure. Positive oral contrast material was administered before the procedure to ensure an adequate bowel and pelvic depiction.
18F-fluorodeoxyglucose was used as a radiopharmaceutical. The administered activity was adjusted to patient’s body weight; the mean administered activity comprised 370–420 MBq. The blood glucose level was recorded prior to the radiopharmaceutical administration. The patients with blood glucose exceeding a 180 mg/dL-threshold were rescheduled. These patients were advised to avoid consumption of liquids prior to image acquisition in order to minimize urine activity.
The radiopharmaceutical was administered via an intravenous route in a separate ward; the distribution or uptake time varied from 45 to 50 min. The image acquisition was performed on Siemens Biograph 64 or Philips Gemini 16 scanners, including CT part with intravenous contrast administration from the skull base to the middle thighs (95–111 mAs, 130 kVp, slice reconstruction thickness: 2 mm), followed by a consecutive 3D PET data acquisition. The total scan time varied from 20 to 35 min. The scanning technique was adjusted according to the European Association of Nuclear Medicine (EANM) Procedure Guidelines for tumor imaging.
The PET/CT data analysis was performed on Siemens Multimodality Workplace Syngo TrueD software. The PET/CT data were interpreted per Deauville 5-PS and International Harmonized Protocol (IHP) criteria. The interim and end-of--treatment scans were correlated to the baseline scans data.
Statistical analysis
The event-free survival (EFS) was defined as the time from study entry to any treatment failure and defined as disease progression or discontinuation of the therapy for any reason. The overall survival (OS) was defined as a death from any cause from the time of diagnosis. The survival curves were calculated using Kaplan and Meier curves; the statistical significance of parameter difference was determined using the log-rank test and the Chi-square test. The Cox proportional hazards regression model was performed in order to investigate the contribution of individual prognostic factors. The risk ratio (RR) with the 95% confidence intervals (CI) was calculated for the factors identified by the above mentioned regression model. The data was processed statistically on Statistica 10 and MedClac 12.6.1.0 software.
Results
The study group included 106 primary HL patients, with 66% (70) of females and 34% (36) of males; age range: 17–67 years (median 30.5 years).
According to the risk factors prognostic model, 67% (71) of patients had ≤ 2 risk factors at the time of diagnosis (Tab. 1). The majority of patients from the study group had a stage ІІА and 1–2 unfavorable prognoses of risk factors.
Tab. 1. Patient groups based on disease stage and number of risk factors according to International Prognostic Score. 
We diagnosed 52.8% (56/106) and 47.1% (50/106) of early and advanced stage patients, respectively. Both groups of early and advanced stage patients had a bulky disease (> 10 cm in any dimension), represented in a similar proportion: 32% (18/56) vs. 38% (19/50), respectively. However, stage III–IV patients’ B-symptoms were diagnosed in a larger number compared to stage I–II patients: 58% (29/50) vs. 28.5% (17/56); P < 0.05.
The patients received 2–6 cycles of chemotherapy (average rate 5 ± 1.2 cycles), depending on the stage and IPS. Both chemotherapy and radiation were used more frequently compared to chemotherapy treatment only (62.2 vs. 37.7% of patients; P < 0.05) (Tab. 2).
Tab. 2. Types of chemotherapy regimens for primary patients with Hodgkin’s lymphoma. 
The total of 80.4% patients with early stages were treated with ABVD compared to one-half of the study group patients with advanced stages (52%), P < 0.05.
The ORR of 106 patients (complete response – CR, partial response – PR) was 90.5%. The ORR of stage I–II patients was 96.5% (55/57) vs. 91% (41/45) of stage III–IV patients (P < 0.05).
We have diagnosed 10.4% (11/106) of patients with an early relapse, 5.7% (6/106) of patients with a late relapse and 1.9% (2/106) of patients with a refractory disease (P < 0.05).
We found a similar number of relapses among early and advanced stage patients (16 and 20%, respectively). However, stage III–IV patients had more occurrences of early relapse and relapse/refractory disease (70% (7/10) and 20% (2/10), respectively) compared to stage I–II patients who had 50% of cases in each category.
The maximum follow-up period was 62.9 months (median 18.3 months) for all stages. Furthermore, 88.6% (94/106) of patients are continuously followed-up.
The 3-year EFS and OS rates for all patients were 85 and 97%, respectively; the 5-year EFS and OS rates were 82 and 97%, respectively (log-rank test; P = 0.004) (Fig. 2).
Fig. 2. Overall event-free survival and overall survival for 106 primary Hodgkin’s lymphoma patients in this study.
The 3-year to 5-year EFS was 85–82% and OS was 97–97%. EFS – event-free survival, OS – overall survival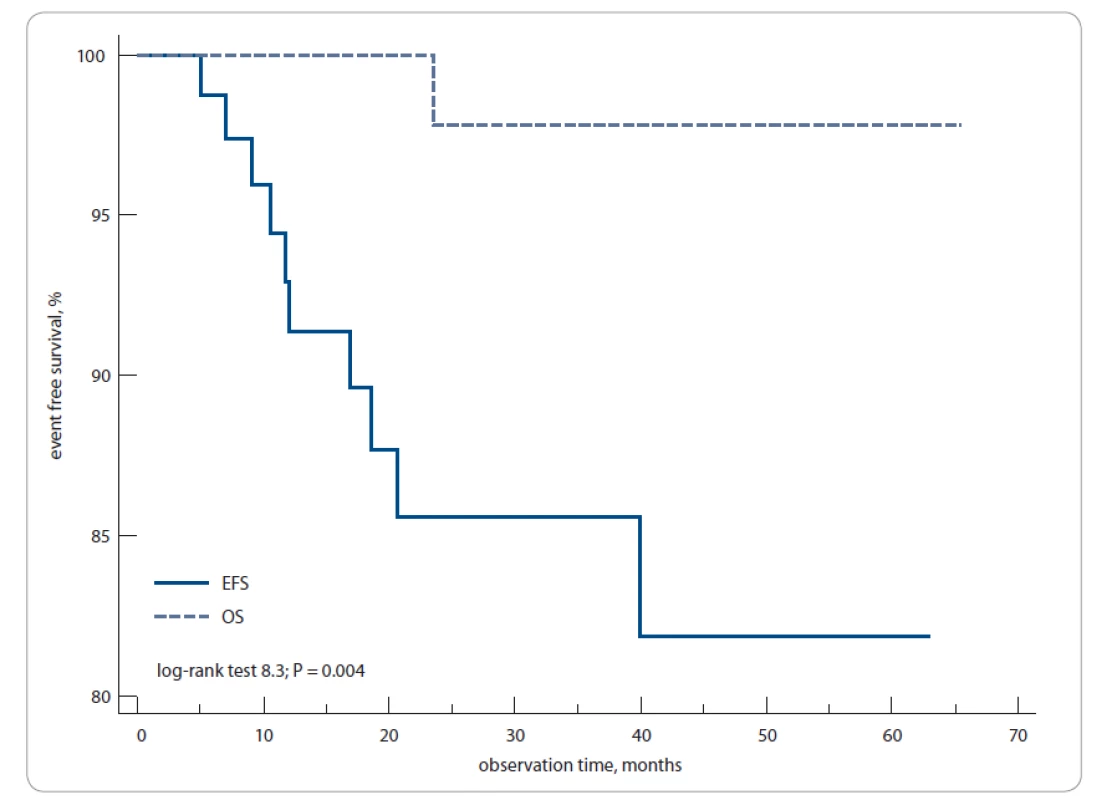
During the follow-up period, one death of HL progression (24 months) was registered in the group of stage III–IV patients.
PET/CT (PET0) at the time of diagnosis
The PET0 scan was performed for 103/106 patients prior to the treatment. We did not find a significant relationship between the prognosis, pre-treatment PET values and clinical outcome.
Interim PET/CT (PET2) at the time of the response to therapy
The PET2 scan was performed in 102/106 patients prior to the treatment and was performed on the 15.5 ± 3 days (range 5–26) after the 2–3rd and 4–6th cycles of treatment in 79.4 and 20.6% of cases, respectively (P < 0.05).
A total of 88.2% (90/102) of patients from the study group were reported scores 1–3 of PET2 according to Deauville 5-PS (prognostic score), and 11.8% (12/102) of patients had a score 4–5, respectively (P < 0.05). The PET2 with Deauville scores 1–2 or 3 was considered as a status-negative result (PET2−), while scores 4–5 were a status-positive (PET2+) result.
In total, the disease progression was documented in 58.3% (7/12) of PET2+ patients and in 13.3% (12/90) of PET2 − patients (P < 0.05).
We diagnosed 14% (8/57) of early stage patients as status PET2+ compared to 9% (4/45) of advanced disease patients. The disease progression was verified more often in PET2+ patients with stages I–II vs. PET2 − (37.5% (3/8) vs. 12.2% (6/49), respectively, P > 0.05). Similar tendency was observed with stage III–IV patients (100 % (4/4) and 15% (6/41), respectively, p > 0.05) (Fig. 3).
Fig. 3. PET2 status according to Deauville scale and stages in study group.
DS – Deauville scale, PET2 – positron emission tomography after two chemotherapy cycles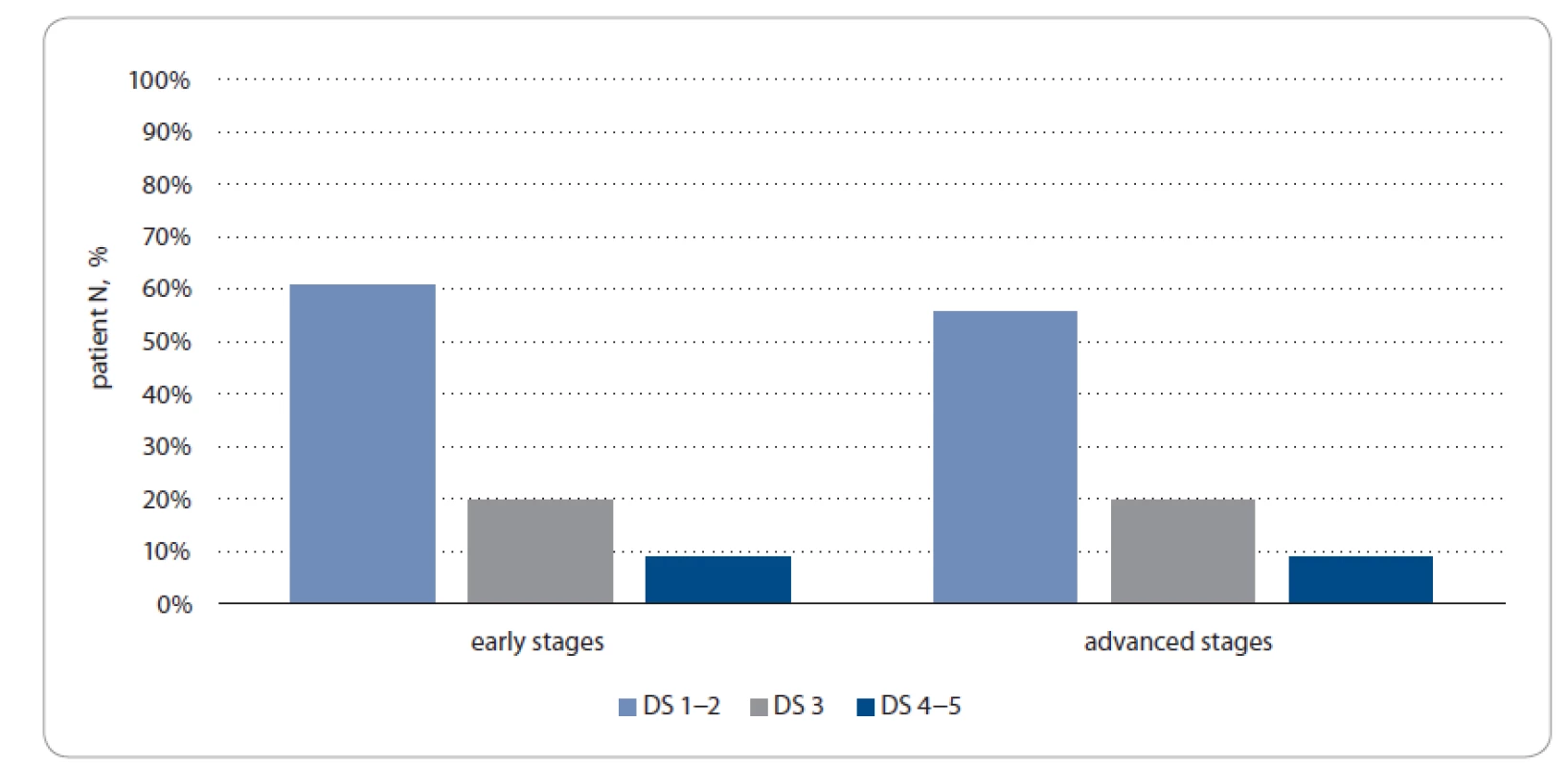
However, no significant difference was found between the EFS rate and IPS for patients with PET2+ vs. PET2 − (long-rank test; P = 0.4).
End of treatment PET/CT (PET3)
Most of the cases of our cohort had a PET3 performed on them (89/106 patients). A total of 88.8% (79/89) and 11.2% (10/89) of patients in the study group had a PET3 − and PET3+ status, respectively (P < 0.05) (Fig. 4).
Fig. 4. PET3 status according to Deauville scale in study group.
PET3 – PET after 3 weeks from the last chemotherapy cycle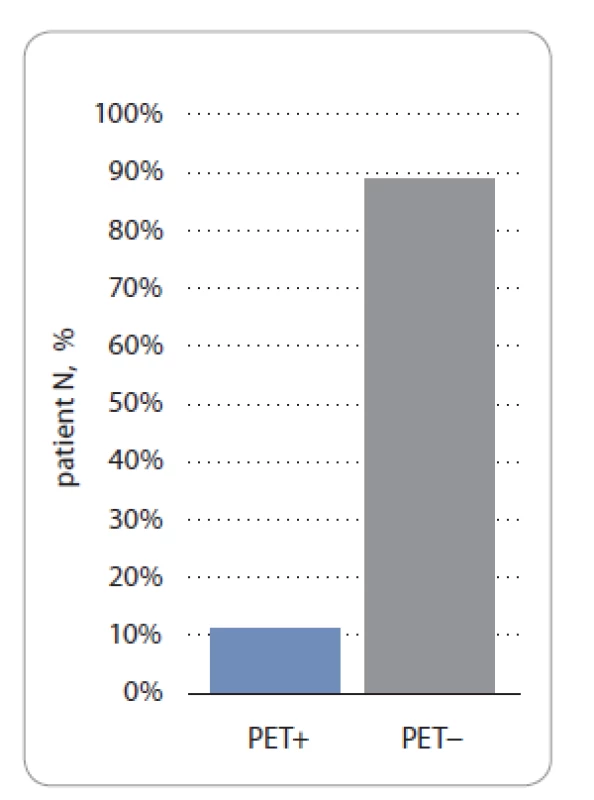
Most of the patients with an early and advanced disease achieved CR having a PET3 − status (95.7 and 80%, respectively). However, we identified 20 vs. 4.2% of patients who had a PET3+ status with stage III–IV vs. stage I–II, respectively (P < 0.05).
The 1-year EFS rate was 88.8% compared to 30% of patients whose PET3 was negative and positive, respectively (log-rank test; P = 0.0001) (Fig. 5).
Fig. 5. Event-free survival, PET3− vs. PET3+ patients.
PET3 – PET after 3 weeks from the last chemotherapy cycle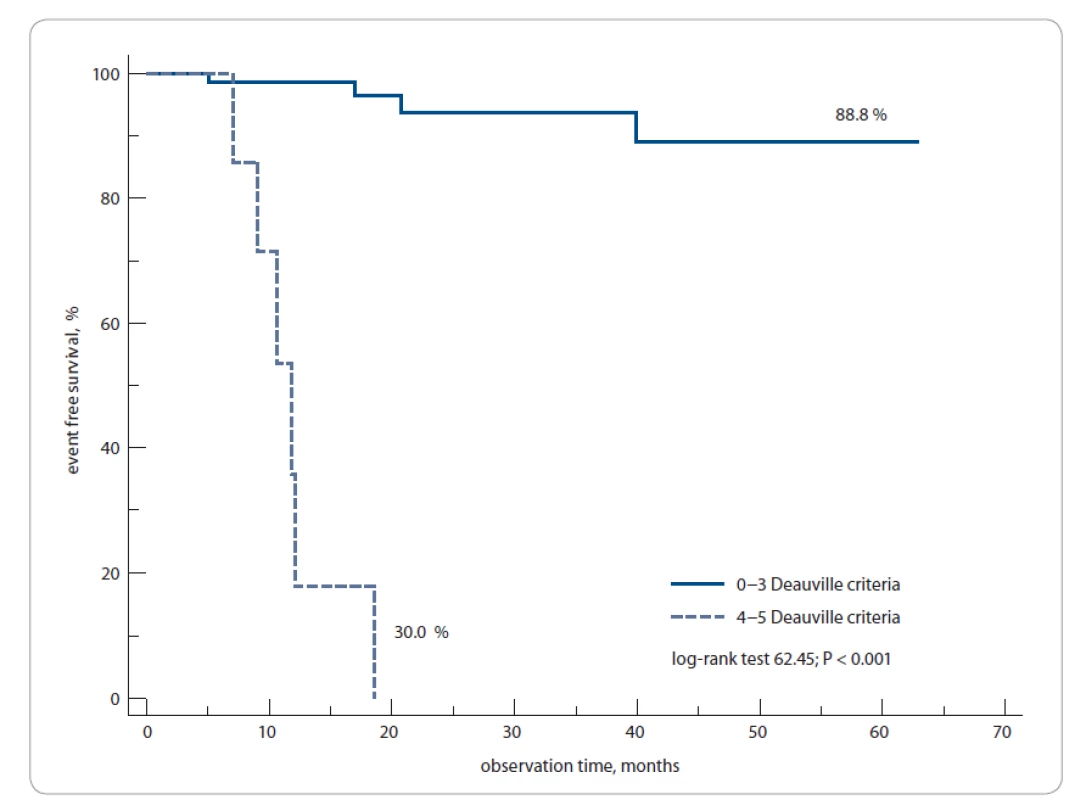
We have computed historic prognostic models using available clinical and lab data. The score distribution of these models, although smaller in overall sample size, was similar to our cohort compared to previously published studies [14,15]. The 1-year EFS rates were 100 vs. 50% of patients with PET3 − (IPS 0–2) vs. PET3+ (IPS 0–2), as well as 87 vs. 30% of PET3 − (IPS 3) vs. PET3+ (IPS 3), respectively (log-rank test; P < 0.0001) (Fig. 6).
Fig. 6. The 1-year event-free survival of Hodgkin’s lymphoma patients according to International Prognostic Score and PET3 status (positive/negative).
PET3 – PET after 3 weeks from the last chemotherapy cycle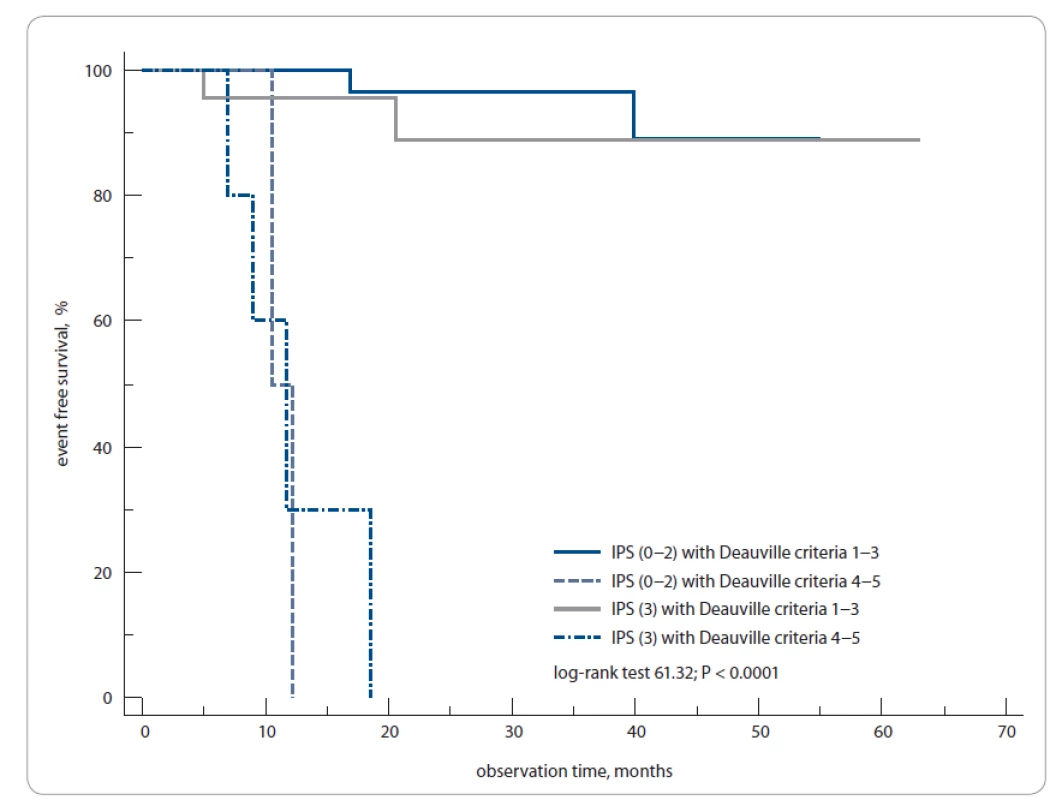
Using Cox regression, we confirmed a significant correlation between the EFS with PET3 DS and IPS. With this, the patients with DS 1–2, DS 3 and DS 4–5 had a 1-year EFS of 94.4, 100 and 33% respectively (HR 0.56; 95% CI 1.07–2.8; P < 0.02). The RR was calculated along with the treatment-stage-based prognostic models. The PET3 was found to be significant in predicting the EFS with an RR of 2.2 (CI 1.54–3.13; P = 0.000012) for early stage patients who had an ABVD regimen treatment. We confirmed the PET3 predicting role in EFS with an RR of 2.5 (CI 1.4–4.6; P = 0.002) for advanced stage patients as well.
The multivariable analysis has not demonstrated any statistically significant correlation between PET2+ or PET3+ status and extranodal involvement site of lymphoma or large tumor burden.
Discussion
Hodgkin’s lymphoma usually affects people of different age groups but mostly these are young adults [16]. In our study most patients were of a young age; the median age was 30.5 years. HL patients‘ ORR worldwide is quite high and varies between 80–95%. We received a similar rate during our studies [17]. According to Subset Analysis of the North American Intergroup E2496 Trial, where HL patients with stages I –II were treated with ABVD, the overall response rate was 83%. When assessing our patients with HL stage I–II and stage III–IV, the ORR was 96.5 and 91%, respectively. In addition, most patients were treated with an ABVD regimen (80.4% with early stages and 52% with advanced stages; P < 0.05). The data we obtained agree with the data from international trials [18].
It is known that HL is a highly curable disease, with the OS and EFS rate reaching almost 90%. Our studies showed the 5-year OS and EFS of 82 and 97%, respectively. The results of LY09 Trial showed the 3-year EFS and OS of 75% and 90% for patients with advanced stages who had an ABVD regimen treatment [19]. Just as in our study group, the advanced stage patients achieved a lower level of a 3-year EFS compared to early stages (75 vs. 87%, respectively).
Although HL is a highly curable disease, there are still 10–20% of patients who will have a relapse during the first 2 years after the treatment [20]. In our study, only 10% of patients had a relapse. Early relapses were verified more often in stage III–IV HL patients.
The PET/CT in HL diagnosis has already become an international standard in the diagnostics algorithm. However, disputes still take place as to the interim or end-of-treatment PET/CT application depending on a risk group. In Ukraine, there is another issue of the relatively high cost of PET/CT that can be considered as an additional risk factor, being an out-of-pocket expense for 90% of patients. This may well explain the low number of patient groups and the frequency of PET/CT procedures per one patient. Generally, patients in Ukraine have only one end-of-treatment PET/CT and do not qualify for this study, as at least two procedures are required for comparison. Although the low number of patients and stage heterogeneity may have affected the achievement of exact and accurate results, such as in RAPID, HD 10 and HD18 studies [21–23], this has nonetheless been the first PET/CT study ever performed in Ukraine, and its retrospective analysis and data evaluation do not contradict the world data.
Several studies showed a significant connection between risk factors at the time of diagnosis and PET/CT. Gallamini et al showed that PET2+ statistically decreases the 3-year EFS compared to PET2 − (28 vs. 95%, respectively; P < 0.0001) [24]. Those studies, however, had their challenges [24,25]. In particular, when a disease progression was suspected, the histological confirmation of its relapse was rarely performed in most cases. It was generally determined by further visualization. Considering a high false-positive PET monitoring frequency in HL, the PET2+ prognostic value may have been significantly over-estimated by these studies [26]. Therefore, the PET+ prognostic value after two chemotherapy treatment cycles is still to be proved.
In our study, we did not detect a PET2+ statistically significant influence on patients‘ EFS and OS when they were treated with ABVD and “switched-regimens”. A biopsy and histological verification were performed on all patients with a relapse.
However, when analyzing PET3 findings, we did detect its statistically significant influence: the 1-year EFS was 94.4, 100 and 33% in patients with DS 1–2, DS 3 and DS 4–5, respectively (P < 0.02).
The patients were grouped according to the IPS scale: IPS 0–2, IPS 3 based on the maximum number of prognostic factors equaling to 3. The patients with PET3 − and IPS 0–2 or IPS 3 had a high and similar level of EFS of 87%, while patients with PET3+ and IPS 3 had the worst prognosis; their EFS rate was 30%.
The interim and end-of-treatment PET prognostic role determination in primary HL patients has important implications for specific treatment. The refractory/relapse diagnostic verification may play an important role in personalized treatment [27].
Conclusion
Our results of PET/CT use with primary HL patients have demonstrated a high prognostic value of the end-of-treatment PET. In addition, we confirmed that the IPS prognostic model has a predictive role for the treatment outcome depending on the PET status. However, standardization of interpretation and reproducibility of PET/CT (i.e. Deauville) requires further studies.
This research did not receive any funds from
private, public or non-profi t sector.
We thank all our colleagues who treated patients
from eight Ukrainian Centers: Irina Tytorenko,
Olena Aleksik, Yana Stepanishina,
Katerina Filonenko, Arina Martynchyk, Kate Ulianchenko,
Evgeniy Kushchevyy and Oksana
Tkachenko.
The authors declare they have no potential
conflicts of interest concerning drugs, products,
or services used in the study.
Autoři deklarují, že v souvislosti s předmětem
studie nemají žádné komerční zájmy.
The Editorial Board declares that the manuscript
met the ICMJE recommendation for
biomedical papers.
Redakční rada potvrzuje, že rukopis práce
splnil ICMJE kritéria pro publikace zasílané do
bi omedicínských časopisů.
Olga Novosad
Department of Oncohematology
National Cancer Institute
33/43 Lomonosova street
03022 Kiev
Ukraine
e-mail: novosad.o.ua@gmail.com
Submitted/Obdrženo: 17. 2. 2020
Accepted/Přijato: 15. 6. 2020
Zdroje
http://novosad.o.ua@gmail.com1. Armitage JO. Current concepts – early stage Hodgkin’s lymphoma. Boston: Massachusetts Medical Society 2010.
2. Novosad О, Kriachok I, Grabovy OM et al. Prognostic risk factors for newly diagnosed patients with advanced stages classical Hodgkin’s lymphoma. Lik. Sprava 2014; 9–10 : 88–94.
3. Cheson BD, Pfistner B, Juweid ME et al. Revised response criteria for malignant lymphoma. J Clin Oncol 2007; 25 (5): 579–586. doi: 10.1200/JCO.2006.09.2403.
4. Seam P, Juweid ME, Cheson BD. The role of FDG-PET scans in patients with lymphoma. Blood 2007; 110 (10): 3507–3516. doi: 10.1182/blood-2007-06-097238.
5. Gallamini A, Rigacci L, Merli F et al. The predictive value of positron emission tomography scanning performed after two courses of standard therapy on treatment outcome in advanced stage Hodgkin’s disease. Haematologica 2006; 91 (4): 475–481.
6. Gallamini A, Hutchings M, Rigacci L et al. Early interim 2-[18F]fluoro-2-deoxy-D-glucose positron emission tomography is prognostically superior to international prognostic score in advanced-stage Hodgkin’s lymphoma: A report from a joint Italian-Danish study. J Clin Oncol 2007; 25 (24): 3746–3752. doi: 10.1200/JCO.2007.11.6525.
7. Mounier N, on behalf of the Lymphoma Study Association (LYSA), Brice P et al. ABVD (8 cycles) versus BEACOPP (4 escalated cycles ≥4 baseline): final results in stage III–IV low-risk Hodgkin’s lymphoma (IPS 0–2) of the LYSA H34 randomized trial. Ann Oncol 2014; 25 (8): 1622–1628. doi: 10.1093/annonc/mdu189.
8. Meignan M, Gallamini A, Meignan M et al. Report on the First International Workshop on interim-PET scan in lymphoma. Leuk Lymphoma 2009; 50 (8): 1257–1260. doi: 10.1080/10428190903040048.
9. Meignan M. Interim PET in lymphoma: a step towards standardization. Eur J Nucl Med Mol Imaging 2010; 37 (10): 1821–1823. doi: 10.1007/s00259-010-1546-6.
10. Engert A, Plütschow A, Eich HT et al. Reduced treatment intensity in patients with early-stage Hodgkin’s lymphoma. N Engl J Med 2010; 363 (7): 640–652. doi: 10.1056/NEJMoa1000067.
11. Hutchings M, Loft A, Hansen M et al. FDG-PET after two cycles of chemotherapy predicts treatment failure and progression-free survival in Hodgkin’s lymphoma. Blood 2006; 107 (1): 52–59. doi: 10.1182/blood-2005-06-2252.
12. Hutchings M, Mikhaeel NG, Fields PA et al. Prognostic value of interim FDG-PET after two or three cycles of chemotherapy in Hodgkin’s lymphoma. Ann Oncol 2005; 16 (7): 1160–1168. doi: 10.1093/annonc/mdi200.
13. Meignan M. Interim PET in lymphoma: a step towards standardization. Eur J Nucl Med Mol Imaging 2010; 37 (10): 1821–1823. doi: 10.1007/s00259-010-1546-6.
14. Coyle M, Kostakoglu L, Evens AM. The evolving role of response-adapted PET imaging in Hodgkin’s lymphoma. Ther Adv Hematol 2016; 7 (2): 108–125. doi: 10.1177/2040620715625615.
15. Kriachok I, Novosad O, Pastushenko IV et al. Prognostic role of positron emission tomography in patients with primary Hodgkin’s lymphoma. Lik. Sprava 2017; 8 : 63–70.
16. Howlader N, Noone AM, Krapcho M (eds). SEER Cancer Statistics Review, 1975–2014, National Cancer Institute. [online]. Avaiable from: https: //seer.cancer.gov/csr/ 1975_2014/.
17. Kelly KM. Hodgkin’s lymphoma in children and adolescents: improving the therapeutic index. Blood 2015; 126 (22): 2452–2458. doi: 10.1182/blood-2015-07-641035
18. Advani RH, Hong F, Fisher RI et al. Randomized phase III trial comparing ABVD plus radiotherapy with the Stanford V regimen in patients with stages I or II locally extensive, bulky mediastinal Hodgkin’s lymphoma: A subset analysis of the North American Intergroup E2496 trial. J Clin Oncol 2015; 33 (17): 1936–1942. doi: 10.1200/JCO.2014.57.8138.
19. Johnson PWM, Radford JA, Cullen MH et al. Comparison of ABVD and alternating or hybrid multidrug regimens for the treatment of advanced Hodgkin’s lymphoma: Results of the United Kingdom Lymphoma Group LY09 Trial (ISRCTN97144519). J Clin Oncol 2005; 23 (36): 9208–9218. doi: 10.1200/JCO.2005.03.2151.
20. von Tresckow B, Moskowitz CH. Treatment of relapsed and refractory Hodgkin’s Lymphoma. Semin Hematol 2016; 53 (3): 180–185. doi: 10.1053/j.seminhematol.2016.05.010.
21. Radford J, Illidge T, Counsell N et al. Results of a trial of PET-directed therapy for early-stage Hodgkin’s lymphoma. N Engl J Med 2015; 372 (17): 1598–1607. doi: 10.1056/NEJMoa1408648.
22. Raemaekers JM, André MP, Federico M et al. Omitting radiotherapy in early positron emission tomography–negative stage I/II Hodgkin’s lymphoma is associated with an increased risk of early relapse: clinical results of the preplanned interim analysis of the randomized EORTC/LYSA/FIL H10 trial. J Clin Oncol 2014; 32 (12): 1188–1194. doi: 10.1200/JCO.2013.51.9298
23. Kobe C, Goergen H, Baues C et al. Outcome-based interpretation of early interim PET in advanced-stage Hodgkin’s lymphoma. Blood 2018; 132 (21): 2273–2279. doi: 10.1182/blood-2018-05-852129.
24. Gallamini A, Barrington SF, Biggi A, et al. The predictive role of interim positron emission tomography for Hodgkin’s lymphoma treatment outcome is confirmed using the interpretation criteria of the Deauville five-point scale. Haematologica 2014; 99 (6): 1107–1113. doi: 10.3324/haematol.2013.103218.
25. Agostinelli C, Gallamini A, Stracqualursi L et al. The combined role of biomarkers and interim PET scan in prediction of treatment outcome in classical Hodgkin’s lymphoma: a retrospective, European, multicentre cohort study. Lancet Haematol 2016; 3 (10): e467–e479. doi: 10.1016/S2352-3026 (16) 30108-9.
26. El-Galaly TC, d’Amore F, Mylam KJ et al. Routine bone marrow biopsy has little or no therapeutic consequence for positron emission tomography / computed tomography-staged treatment-naive patients with Hodgkin’s lymphoma. J Clin Oncol 2012; 30 (36): 4508–4514. doi: 10.1200/JCO.2012.42.4036.
27. Adams HJ, Kwee TC. Reply: Interim PET in Hodgkin’s lymphoma: is it so useless? J Nucl Med 2017; 58 (7): 1180–1182. doi: 10.2967/jnumed.117.192294.
Štítky
Dětská onkologie Chirurgie všeobecná Onkologie
Článek Chemie jménem CRISPRČlánek Aktuality z odborného tisku
Článek vyšel v časopiseKlinická onkologie
Nejčtenější tento týden
- Metamizol jako analgetikum první volby: kdy, pro koho, jak a proč?
- Nejasný stín na plicích – kazuistika
- Nejlepší kůže je zdravá kůže: 3 úrovně ochrany v moderní péči o stomii
- Hojení análních fisur urychlí čípky a gel
-
Všechny články tohoto čísla
- Chemie jménem CRISPR
- Practical instructions for testing and targeted therapy in adult patients with solid tumours with NTRK gene fusion in common clinical practice
- Treatment opinion of rehabilitation in sarcopenia and cachexia for oncological patients
- Immunostimulatory and anticancer effect of Reishi and Coriol extracts at the level of clinical studies and their implementation in practice
- Stomatitis in mTOR inhibitors treatment and other targeted cancer therapy, possibilities of infl uencing it, and the use of local corticotherapy
- First experience in the Czech Republic with perirectal hydrogel injection before radiotherapy for prostate cancer
- SNHG7 and FAIM2 are up-regulated and co-expressed in colorectal adenocarcinoma tissues
- A Ukrainian multicenter prospective study of the value of PET/ CT prognostic role in primary patients with Hodgkin’s lymphoma in a real-life cohort
- Dabrafenib monotherapy in BRAF+ non-small cell lung cancer – our experience
- Multiresistent opportunistic talaromycosis in a patient with ovarian cancer
- Atezolizumab a bevacizumab v léčbě hepatocelulárního karcinomu
- Aktuality z odborného tisku
- Low dose exposure evaluation for hypofractionated breast intensity modulated radiation therapy – taking into account the fraction-size effect with the linear quadratic model
- Profesor Pavel Šlampa slaví 60 let
- Klinická onkologie
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Immunostimulatory and anticancer effect of Reishi and Coriol extracts at the level of clinical studies and their implementation in practice
- First experience in the Czech Republic with perirectal hydrogel injection before radiotherapy for prostate cancer
- Stomatitis in mTOR inhibitors treatment and other targeted cancer therapy, possibilities of infl uencing it, and the use of local corticotherapy
- Treatment opinion of rehabilitation in sarcopenia and cachexia for oncological patients
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



