-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Renal allograft biopsies: a guide of ins and outs for best results
Biopsie renálních štěpů: průvodce spletitými detaily pro získání nejlepších výsledků
Nejlepším diagnostickým nástrojem pro vyšetření typu a stupně poškození štěpu ledviny zůstává biopsie. Pokud je vyhodnocena dobře, poskytuje terapeutické a prognostické informace zhodnocením rozsahu nevratného chronického poškození tkáně. Tento přehled se soustředí na odpovídající aspekty ve vztahu k tomu, jak získat optimální bioptické vzorky, a také jak interpretovat diagnózu. Patologům a klinikům jsou k dispozici shrnující informace a praktické typy, jak co udělat a naopak čeho se vyvarovat a co mít na paměti.
Klíčová slova:
štěp ledviny – biopsie štěpu ledviny – diagnostický přístup
Authors: Volker Nickeleit
Published in the journal: Čes.-slov. Patol., 51, 2015, No. 4, p. 181-186
Category: Přehledový článek
Summary
Renal allograft biopsies remain the best diagnostic tool to investigate the type and degree of graft injury, provide therapeutic and prognostic information and to assess the extent of irreversible chronic organ damage – if done right. This review highlights pertinent aspects relevant not only for collecting optimal tissue samples but also for rendering diagnoses. Pathologists and clinicians are provided with “take home messages” and practical tips what to do, what to avoid and what to keep in mind.
Keywords:
kidney allograft – kidney allograft biopsy – diagnostic approach
Biopsies remain the gold standard to determine the cause of graft dysfunction. Biopsies best distinguish acute rejection, acute tubular necrosis, infections such as polyomavirus nephropathy, thrombotic microangiopathy, recurrence of original disease, calcineurin inhibitor toxicity and chronic rejection (1-4). Thus biopsies give unique opportunities to “inspect” the condition of renal allografts, determine the degree of acute and chronic tissue injury including the potential reversibility of lesions, guide therapeutic interventions, and provide prognostic information. The overall “power” of information a biopsy can give on concurring disease processes, such as acute rejection superimposed on chronic pre-existing tissue injury, is unsurpassed (5). Neither blood nor urine based assays nor molecular testing rival the diagnostic yield of histologic studies. Biopsy findings change the clinical diagnosis in an average of 36 % of patients (27 – 46 %) and therapy in 59 %, with no obvious diminishing value in the last years (2,3,6-11). Biopsy results change therapy in both the early and late (> 1 year) post-transplant periods (9,10). Most importantly, biopsy findings lead to reduced immunosuppression in 22 % (19 – 39 %) of patients.
Ideally allograft biopsies should be first obtained at time of implantation, i.e. so-called “zero-hour biopsies”, to evaluate the condition of the donor organ and to assess the degree of pre-existing chronic parenchymal damage for subsequent comparative analyses (5,12). Subsequently early episodes of delayed or suboptimal graft function as well as unexplained graft failure with a sudden rise of serum creatinine by 15 % above baseline require a histologic diagnosis. The detection of proteinuria and/or hematuria raises suspicion of glomerular injury that might be rejection related, e.g. transplant glomerulitis or glomerulopathy often with subnephrotic range proteinuria, or it might be due to a de-novo or recurrent glomerulonephritis; a graft biopsy will provide accurate diagnostic information. Since serum creatinine or BUN levels only poorly reflect “intra graft” events, such as early phases of polyomavirus nephropathies, protocol biopsies performed on grafts with stable function can reveal unexpected lesions benefitting from therapeutic intervention (13,14).
TECHNICAL CONSIDERATIONS
Since renal allografts are typically placed into the iliac fossa, technical aspects of ultrasound guided biopsy procedures are usually not challenging. However, all biopsies are invasive and special efforts should be made to obtain optimal tissue (also see below: adequacy of a biopsy sample). The biopsy should provide proper diagnostic insight into the status of the transplant. Some pertinent aspects are important to consider.
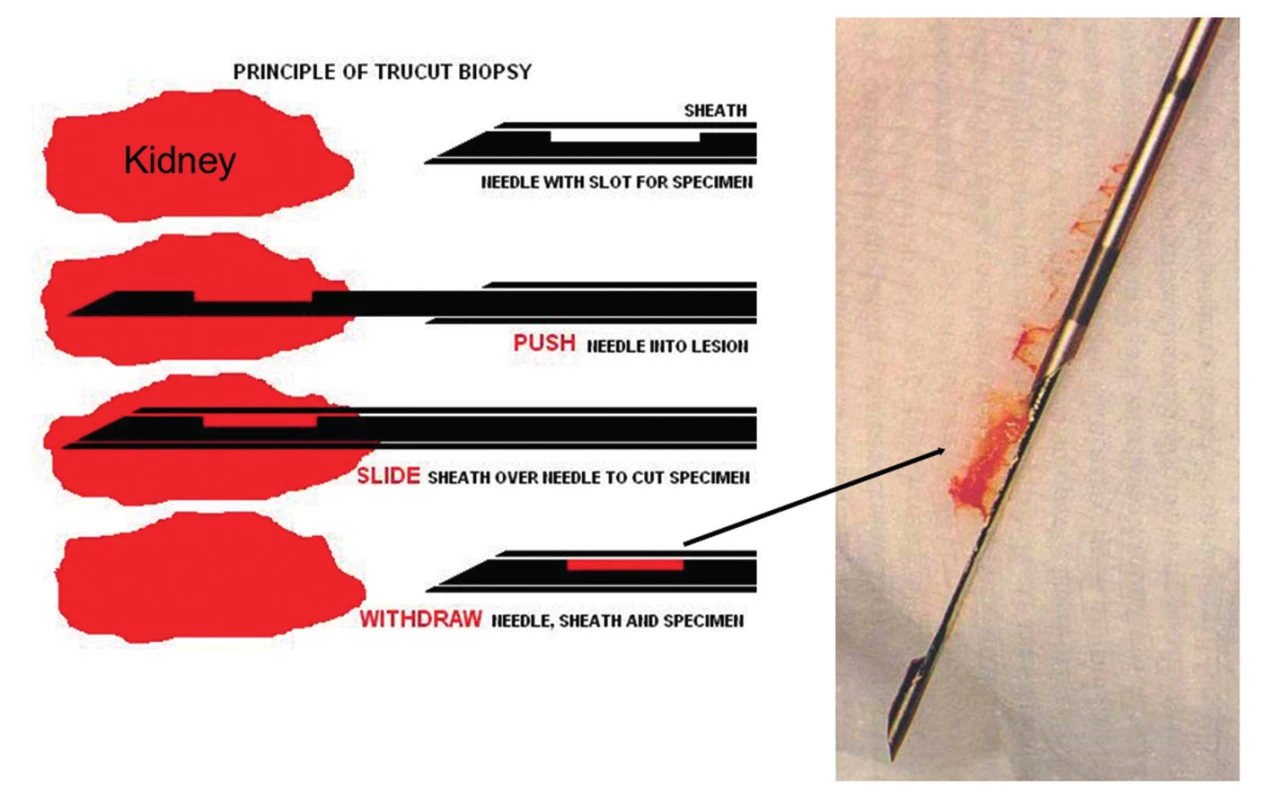
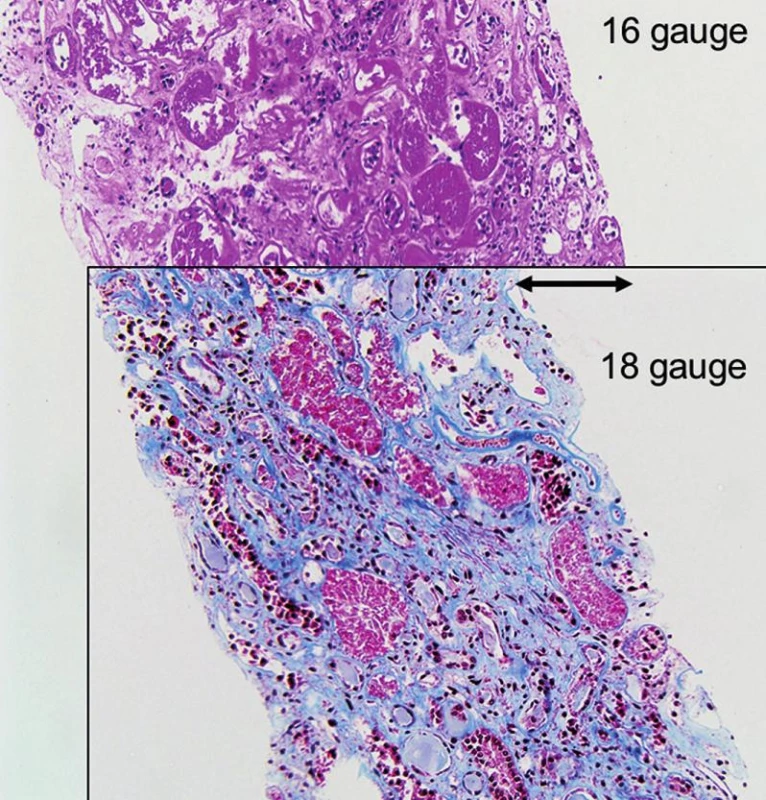
- All biopsies should be obtained with 15 or 16 gauge spring loaded so-called biopsy guns (15,16). The use of smaller needles, i.e. 18 or higher gauges or fine needle aspiration, is strongly discouraged (Fig. 1, 2 and 3). Two or better 3 biopsy cores of approximately 1.5 cm in length containing cortex and medulla should be collected.
- Tissue should be immediately examined in the ultrasound suite for adequacy under a dissecting scope (or alternatively with a hand held 15x magnifying lens) by a specialty trained pathology assistant (Fig. 4). If the first passages do not render adequate tissue, i.e. a total of greater than 1.5 cm cortex in the sampled cores with multiple clearly discernible glomeruli, then additional cores should be obtained (Fig. 5 and 6). Note: subcapsular parenchyma in renal allografts is commonly of no diagnostic value due to non-specific scarring secondary to truncated trans-capsular blood supply.
- The pathology assistant “is in charge in the ultrasound suite”; only he or she can decide in uncomplicated cases on the adequacy of a biopsy following careful tissue inspection. Of course complications such as bleeding might prevent nephrologists or radiologists from obtaining additional tissue.
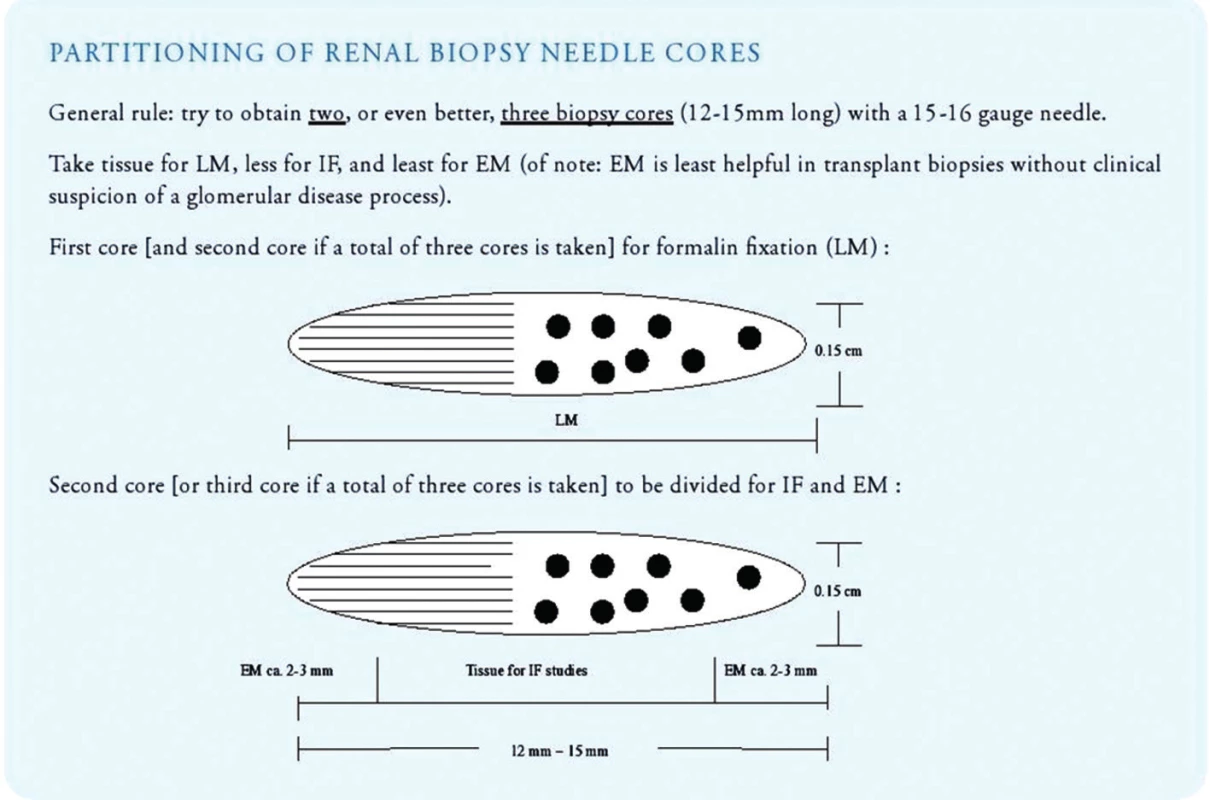
- Once sufficient biopsy cores have been collected, the pathology assistant should triage the tissue immediately for light microscopy, immunofluorescence microscopy and electron microscopy (Fig. 7). All three lines of histologic investigation should ideally be performed on all transplant biopsies (with the exception of zero-hour implantation biopsies where electron microscopy can be omitted).
- Tissue fixed in fresh 4% neutral buffered formalin can be used for paraffin embedding/light microscopy as well as for electron microscopic work-up.
- Tissue prepared for light microscopy should be sectioned at 3 micrometer with 3 sections per glass slide and 11 slides for investigation (= total of 33 step/tissue sections on 11 glass slides). Sections should be stained with H&E (level 1, 4 and 9), PAS (level 2, 5 and 10), trichrome (level 3, 6 and 11), Jones’ silver (level 7) and elastic tissue stains (level 8; to detect inflammation induced arterial intimal scarring as a sign of chronic rejection). Additional step sections into the paraffin block should be obtained in all cases lacking a morphologic correlate for clinical symptoms.
- Immunofluorescence studies (or alternatively immunohistochemistry on formalin fixed and paraffin embedded tissue sections) should be routinely performed on ALL biopsies with antibodies directed against IgG, IgA, IgM, complement factors C3 and C1q, kappa and lambda light chains, fibrinogen and the complement degradation product C4d. Other stains might be obtained additionally in certain cases, such as with antibodies directed against the SV40-T antigen (to detect evidence of polyomavirus replication/polyomavirus nephropathy), CD3, CD20, CD68, CMV immediate early antigen, adenovirus etc. In-situ hybridization is best suited to detect EBER (Epstein-Barr Virus encoded RNA) in cases of PTLD (post transplant lymphoproliferative disorder).
- Rush tissue processing can be made available with slides ready for review within 4 - 5 hours.
- Tissue prepared for immunofluorescence microscopy should be transported to the laboratory on moist gauze (physiological saline solutions). The tissue should not be immersed in saline, and it should not dry out prior to freezing. Best results are obtained with tissue embedded into so-called OCT (= Optimal Cutting Temperature compound used to embed tissue samples prior to frozen sectioning on a microtome-cryostat) and freezing protocols using pre-cooled isopentane baths (see footnote 1); freezing of renal biopsies directly in liquid nitrogen or alternatively directly in a cryostat causes very severe artifacts. Immunofluorescence staining results are typically available within 3 hours. We also recommend performing H&E and PAS stains on all frozen tissue cores to search for structural abnormalities (such as transplant endarteritis) possibly unsampled in the tissue cores examined by standard light microscopy.
- Electron microscopy is recommended on all allograft biopsies (particularly post year-1) to search not only for early signs of recurrent glomerulopathies but additionally also for early chronic rejection, such as transplant glomerulopathy (Banff 2013 cg1a) or multilamination of peritubular capillary basement membranes (17,18). Toluidine blue stained so-called semi thin sections should be prepared to carefully examine tissue collected for EM also by light microscopy.
- Proper diagnostic evaluation requires in-depth clinical information, such as information on underlying native kidney disease, failed previous grafts, baseline serum creatinine levels, peak levels, type of immunosuppression, evidence of hematuria, proteinuria, evidence of polyomavirus activation with urinary decoy cell shedding/viruria/viremia, possible anti-rejection therapy immediately prior to biopsy, status of donor specific antibodies (DSA), other clinical signs of a more generalized illness or infection including suspicion of a pyelonephritis etc (see footnote 2 for link to an example of a downloadable renal allograft biopsy requisition form).
Footnote 1: Freezing OCT embedded tissue in isopentane (2-methylbutane) baths. Advantage: superb preservation of tissue and lack of ice crystal artifact. Tissues should be kept moist and cool until snap freezing procedure is initiated. Protocol: A. Gently blot excess liquid off tissue. Place tissue in plastic mold and embed/cover with OCT. Note: avoid air bubbles, and make sure needle core specimen is oriented evenly in the mold in order to provide you with a full length tissue section post freezing! B. Fill an appropriate container with liquid nitrogen. C. Partially immerse appropriate cup filled with isopentane into the liquid nitrogen (do NOT fully immerse cup!!). The levels of the two solutions in the two containers should be the same for even freezing of your specimen. D. After approximately 4 to 5 minutes the isopentane will look opaque (milky) white and will have a rim of frozen isopentane when it is chilled enough (-150 degrees C ) to snap freeze your specimen. Add more liquid nitrogen to your container to keep the level of the two liquids equal if needed. E. While holding the plastic embedding mold containing your OCT embedded biopsy core between a pair of long handled forceps, carefully lower the entire mold into the metal cup containing the chilled isopentane. Do not let the mold go but hold it with your forceps for 25 seconds until the needle core biopsy is frozen. Timing is important! F. After freezing in isopentane quickly place frozen tissue on dry ice and wrap in aluminium foil or box for -80 freezer storage or let the mold sit in a cryostat ( - 20 degrees) for 15 minutes before cutting specimen.
Footnote 2: Link to a downloadable example of a renal allograft biopsy requisition form. www.uncnephropathology.org/downloadable forms (specimen referral form for transplant kidneys)
All afore mentioned aspects are guidelines and can, of course, not always be considered during routine work-up of biopsy specimens. However, careful and dedicated tissue collection, preparation and proper communication of clinical information guarantees optimal diagnostic interpretations with best results for patient management.
BIOPSY ADEQUACY
Our requirements for tissue adequacy are (Table 1): a) at least two biopsy cores for standard light microscopic studies; b) fifteen or more glomeruli (deep to the subcapsular region that often shows non-specific sclerosis); c) three or more “large” interlobular arteries/branches of arcuate caliber vessels (i.e. vessels with at least two to three layers of medial smooth muscle cells); d) a portion of medulla (to rule out polyomavirus nephropathy, pyelonephritis). These requirements are higher than the previously reported Banff ’97 adequacy criteria and better suited to render diagnoses; so-called “minimal adequacy criteria” as suggested by “Banff” are misleading and should not serve as standard (19,20). If a biopsy does not meet “our” criteria of adequacy we note this fact in the diagnosis. The use of larger needles, i.e. commonly 16 gauge or in some centers 15 gauge, increases the rate of “adequate” tissue sampling (also see Fig. 3).
Tab. 1. “Chapel Hill” criteria for assessing the diagnostic adequacy of renal allograft biopsies 
SAFETY
Practically all biopsies are done with so-called ultrasound guided biopsy guns that have a superb safety record. In the adult renal allograft patient population no deaths due to biopsy have been reported (0/5026) and only exceptionally rare cases of graft loss (1/3996, 0.03%) (7,15,16,21-23). Similar observations were made in pediatric patients (24). The forms of complication secondary to renal allograft biopsy are the same as from biopsies of native kidneys: hematuria, arteriovenous fistulas, ureteral obstruction from clots, and perirenal hemorrhage (25). Three quarters of the arteriovenous fistulas closed spontaneously and none required further therapeutic intervention in some series (26,27). Few transplant centers use 15 gauge needles with good success. Of note: the “larger” the biopsy needle size the better the tissue yield (Fig. 2 and 3)(25)! The overall complication rate does not seem to significantly increase by using a larger needle size, i.e. 16 gauge versus 18 gauge, if the biopsy procedure is performed by an experienced physician under ultrasound guidance following general guidelines and considering potential contraindications for biopsy (25).
Diagnoses of rejection are made “in the cortex” and adequate tissue collection is of uttermost importance. Ultrasound guidance increases the probability of obtaining cortex from 75 to 91 %; further guidance on site by a pathology assistant (Fig. 4) as outlined above increases adequacy to 100 % (28). Transfemoral vein renal biopsies offer an alternative method for some patients, although adequate graft tissue is only obtained in approximately 60 % of cases.
SENSITIVITY AND SPECIFICITY
The sensitivity of a renal allograft biopsy depends on the size, number and content of the cores. Acute rejection was only found in one of two cores in 10 % of cases in one study (20). Thus, the sensitivity of a single core is approximately 90 %. Similarly, 10 % of paired biopsy cores had one core that was insufficient for the diagnosis of rejection (29). Accordingly, if one core has a sensitivity of 90 %, two cores have a predicted sensitivity of 99 %.
The specificity of a biopsy is impossible to measure because no higher standard for comparison is available. In this context the short-term clinical course or response to therapy is not the final arbiter, because rejection may be subclinical without elevated serum creatinine levels or anti rejection therapy may be unsuccessful and not leading to improved graft function.
DIAGNOSTIC APPROACH TO BIOPSIES
Each stained histologic section (see above) is carefully examined for 1) the nature and degree of the interstitial cellular infiltrate, 2) arterial and arteriolar lesions (e.g., endarteritis, myocyte necrosis, thrombi, nodular hyaline), 3) tubular injury, inflammation (tubulitis) and viral inclusion bodies, 4) glomerular lesions including glomerulitis and transplant glomerulopathy and 5) degree of chronic changes/fibrosis. Further levels into the blocks are obtained if “changes are unclear” since much of the paraffin embedded tissue awaits “further exploration”. Rejection can be acute/active, chronic active, or chronic inactive and caused by a T-cell response, antibodies or a combination of both factors (Schema 1 and 2).
A diagnosis of rejection is made in the renal cortex. Of note: normal medulla does not rule out rejection (30), because the medulla has a lower sensitivity for rejection than cortical parenchyma (31). However, when a prominent mononuclear infiltrate and tubulitis is found in the medulla, rejection is likely, provided infection including polyomavirus nephropathy, obstruction and drug allergy are excluded (31). The complement degradation product C4d can be detected along peritubular capillaries in the cortex and/or in the medulla in viable parenchymal zones lacking fibrosis or marked edema; glomeruli are not needed. This means that if tissue is sparse, the portion with medulla can be used for C4d staining.
Banff Criteria and Scoring System
Several grading systems have been proposed over the years to codify renal allograft rejection. At the present time the most widely used system is called the “Banff schema” (or simply “Banff”). Banff started as a collaborative effort of investigators to achieve a consensus that would be acceptable to the FDA for drug trials and also useful for routine diagnoses (19). This system has gone through a number of significant revisions and modifications over the years since it was first published in 1993 (latest update from 2013) (17). Banff scores the individual elements of the biopsy by light microscopy (considering C4d staining results and some EM findings as well) and uses combinations of individual scores to define various categories of acute and chronic rejection. While many details in the Banff scheme are simply arbitrarily set and undergo further refinement, Banff has had, nevertheless, a beneficial effect on the overall standardization of rejection, on comparative data analysis and publications and to a certain degree also on the management of individual patients.
The criteria for diagnosis of rejection are not absolute, but based on clinico-pathologic correlations in patients on standard immunosuppressive therapy presenting with deterioration of graft function and responding to anti rejection therapy. Minimal criteria to establish a diagnosis of rejection, in particular in stable grafts not presenting with deterioration of serum creatinine levels, remain undetermined. This fact is reflected in the highly controversial Banff category 3 of so-called “borderline’ changes. Drugs have the ability to modify the histologic phenotype of rejection. For example, calcineurin inhibitors can decrease the intensity of inflammatory cell infiltrates (caveat: impression of “borderline Banff changes”!) or steroids may reduce the number of eosinophils and the degree of edema. Novel drugs entering the market in future years will undoubtedly result in novel histologic changes further challenging our diagnostic decision making process.
DONOR BIOPSIES: PROCUREMENT AND ZERO-HOUR IMPLANTATION
Grafts typically had a previous “life”, and they often show signs of hypertension induced so-called arterionephrosclerosis and occasionally even other renal diseases. Both procurement and zero-hour implantation biopsies designate tissue samples obtained at time of grafting to assess donor disease. Procurement biopsies are collected at time of cadaveric organ harvest to give information on the organ suitability for transplantation, while zero-hour implantation biopsies are obtained post-reperfusion in the operating room to provide insight into pre-existing diseases relevant for comparative analyses post-transplantation and overall prognosis.
Procurement biopsies are often evaluated on a rush basis by general surgical pathologists during off-hours in the frozen section laboratory, and the analysis/scoring is commonly rudimentary. In this context it is important to remember that chronic injury does not provide universal guidelines for the suitability of donor kidneys for transplantation, and thus far no study has established an absolute, validated threshold of glomerular sclerosis, interstitial fibrosis or arteriosclerosis beyond which a donor kidney must not be used (5,12,32,33).
Zero-hour implantation biopsies are typically evaluated as “allograft biopsies” according to criteria outlined above (in Chapel Hill including immunofluorescence studies but skipping electron microscopy). These specimens provide a unique view not only into the degree of chronic donor derived tissue injury but also into subclinical renal lesions in healthy individuals. The most common subclinical glomerular finding is IgA deposition, which was noted in 11 % to 24 % of donor organs (34-36); in Chapel Hill the incidence is 2 %. Donor derived IgA disappears within weeks to few months on follow-up biopsies (37-40), and graft survival is no different from those grafts without IgA (36).
In conclusion, kidney transplantation has evolved into a very effective method to treat patients with end-stage renal diseases and to not only improve morbidity and mortality but also quality of life. When evaluating renal allografts, a “look through the microscope” gives many important pieces of information on “primary” and “secondary” disease processes as well as insights into disease “activity” and “chronicity”, thereby making pathology a very powerful tool with an unsurpassed degree of information. However, we must be prepared for changes since much remains to be learned and discovered in the future.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest regarding the publication of this paper.
Correspondence address:
Volker Nickeleit, MD
Professor of Pathology
Dr. med. habil., Privatdozent
Director: UNC Division of Nephropathology
Department of Pathology and Laboratory Medicine
The University of North Carolina at Chapel Hill
Chapel Hill, NC, 27599-7525 (USA)
tel: ++ 919-966-2421
fax: ++ 919-966-4542
e-mail: volker_nickeleit@med.unc.edu
Zdroje
1. Nickeleit V, Singh HK. Polyomaviruses and disease: is there more to know than viremia and viruria? Current opinion in organ transplantation 2015; 20(3): 348-358.
2. Waltzer WC, Miller F, Arnold A, et al. Value of percutaneous core needle biopsy in the differential diagnosis of renal transplant dysfunction. J Urol 1987; 137(6): 1117-1121.
3. Matas AJ, Tellis VA, Sablay L, et al. The value of needle renal allograft biopsy. III. A prospective study. Surgery 1985; 98(5): 922-926.
4. Nickeleit V, Mihatsch MJ. Polyomavirus nephropathy in native kidneys and renal allografts: an update on an escalating threat. Transpl Int 2006; 19(12): 960-73.
5. Nickeleit V. Pathology: donor biopsy evaluation at time of renal grafting. Nature reviews. Nephrology 2009; 5(5): 249-251.
6. Parfrey PS, Kuo YL, Hanley JA, et al. The diagnostic and prognostic value of kidney transplant biopsy. Transplantation 1984; 38 : 586-593.
7. Kiss D, Landman J, Mihatsch M, et al. Risks and benefits of graft biopsy in renal transplantation under cyclosporin-A. Clinical Nephrol 1992; 38(3): 132-134.
8. Manfro RC, Lee JY, Lewgoy J, et al. The role of percutaneous renal biopsy in kidney transplant. Revista Da Associacao Medica Brasileira 1994; 40(2): 108-112.
9. Kon SP, Templar J, Dodd SM, et al. Diagnostic contribution of renal allograft biopsies at various intervals after transplantation. Transplantation 1997; 63(4): 547-550.
10. Pascual M, Vallhonrat H, Cosimi AB, et al. The clinical usefulness of the renal allograft biopsy in the cyclosporine era: a prospective study. Transplantation 1999; 67(5): 737-741.
11. Al-Awwa IA, Hariharan S, First MR. Importance of allograft biopsy in renal transplant recipients: correlation between clinical and histological diagnosis. Am J Kidney Dis 1998; 31(6 Suppl 1): S15-18.
12. Nickeleit V. Foretelling the future: predicting graft outcome by evaluating kidney baseline transplant biopsies. J Am Soc Nephrol 2013; 24(11): 1716-1719.
13. Buehrig CK, Lager DJ, Stegall MD, et al. Influence of surveillance renal allograft biopsy on diagnosis and prognosis of polyomavirus-associated nephropathy. Kidney Int 2003; 64(2): 665-673.
14. Haas M, Montgomery RA, Segev DL, et al. Subclinical acute antibody-mediated rejection in positive crossmatch renal allografts. Am J Transplant 2007; 7(3): 576-585.
15. Nast CC, Blifeld C, Danovitch GM, et al. Needle biopsy of renal allografts: comparison of two techniques. Radiology 1990; 174(1): 273-275.
16. Mahoney MC, Racadio JM, Merhar GL, et al. Safety and efficacy of kidney transplant biopsy: Tru-Cut needle vs sonographically guided Biopty gun. Am J Roentgenol 1993; 160(2): 325-326.
17. Haas M, Sis B, Racusen LC, et al. Banff 2013 meeting report: inclusion of c4d-negative antibody-mediated rejection and antibody-associated arterial lesions. Am J Transplant 2014; 14(2): 272-283.
18. Liapis H, Singh HK, Derebail VK, et al. Diagnostic significance of peritubular capillary basement membrane multilaminations in kidney allografts: old concepts revisited. Transplantation 2012; 94(6): 620-629.
19. Solez K, Axelsen RA, Benediktsson H, et al. International standardization of criteria for the histologic diagnosis of renal allograft rejection: the Banff working classification of kidney transplant pathology. Kidney Int 1993; 44(2): 411-422.
20. Colvin RB, Cohen AH, Saiontz C, et al. Evaluation of pathologic criteria for acute renal allograft rejection: reproducibility, sensitivity, and clinical correlation. J Am Soc Nephrol 1997; 8(12): 1930-1941.
21. Hanas E, Larsson E, Fellström B, et al. Safety aspects and diagnostic findings of serial renal allograft biopsies, obtained by an automatic technique with a midsize needle. Scand J Urol Nephrol 1992; 26(4): 413-420.
22. Wilczek HE. Percutaneous needle biopsy of the renal allograft. A clinical safety evaluation of 1129 biopsies. Transplantation 1990; 50(5): 790-797.
23. Furness PN, Philpott CM, Chorbadjian MT, et al. Protocol biopsy of the stable renal transplant: a multicenter study of methods and complication rates. Transplantation 2003; 76(6): 969-973.
24. Benfield MR, Herrin J, Feld L, et al. Safety of kidney biopsy in pediatric transplantation: a report of the Controlled Clinical Trials in Pediatric Transplantation Trial of Induction Therapy Study Group. Transplantation 1999; 67(4): 544-547.
25. Schwarz A, Gwinner W, Hiss M, et al. Safety and adequacy of renal transplant protocol biopsies. Am J Transplant 2005; 5(8): 1992-1996.
26. Merkus JW, Zeebregts CJ, Hoitsma AJ, et al. High incidence of arteriovenous fistula after biopsy of kidney allografts. Br J Surg 1993; 80(3): 310-312.
27. Schwarz A, Hiss M, Gwinner W, et al. Course and relevance of arteriovenous fistulas after renal transplant biopsies. Am J Transplant 2008; 8(4): 826-831.
28. Beckingham IJ, Nicholson ML, Kirk G, et al. Comparison of three methods to obtain percutaneous needle core biopsies a renal allograft. Br J Surg 1994; 81(6): 898-899.
29. Sorof JM, Vartanian RK, Olson JL, et al. Histopathological concordance of paired renal allograft biopsy cores. Effect on the diagnosis and management of acute rejection. Transplantation 1995; 60(11): 1215-1219.
30. Wilczek HE, Groth CG, Bohman SO. Effect of reduced cyclosporin dosage on long-term renal allograft histology. Transplant Int 1992; 5(2): 65-70.
31. Wang H, Nanra RS, Carney SL, et al. The renal medulla in acute renal allograft rejection: comparison with renal cortex. Nephrol Dial Transplant 1995; 10(8): 1428-1431.
32. Munivenkatappa RB, Schweitzer EJ, Papadimitriou JC, et al. The Maryland aggregate pathology index: a deceased donor kidney biopsy scoring system for predicting graft failure. Am J Transplant 2008; 8(11): 2316-2324.
33. De Vusser K, Lerut E, Kuypers D, et al. The predictive value of kidney allograft baseline biopsies for long-term graft survival. J Am Soc Nephrol 2013; 24(11): 1913-1923.
34. Rosenberg HG, Martinez PS, Vaccarezza AS, et al. Morphological findings in 70 kidneys of living donors for renal transplant. Pathol Res Pract 1990; 186(5): 619-624.
35. Cosyns JP, Malaise J, Hanique G, et al. Lesions in donor kidneys: nature, incidence, and influence on graft function. Transplant Int 1998; 11(1): 22-27.
36. Ji S, Liu M, Chen J, et al. The fate of glomerular mesangial IgA deposition in the donated kidney after allograft transplantation. Clin Transplant 2004; 18(5): 536-540.
37. Silva FG, Chandler P, Pirani CL, et al. Disappearance of glomerular mesangial IgA deposits after renal allograft transplantation. Transplantation 1982; 33(214): 214-216.
38. Tolkoff-Rubin NE, Cosimi AB, Fuller T, et al. IgA nephropathy in HLA-identical siblings. Transplantation 1978; 26(6): 430-433.
39. Sanfilippo F, Croker BP, Bollinger RR. Fate of four cadaveric donor renal allografts with mesangial IgA deposits. Transplantation 1982; 33(370): 370-372.
40. Curschellas E, Landmann J, Durig M, et al. Morphologic findings in “zero-hour” biopsies of renal transplants. Clin Nephrol 1991; 36(5): 215-222.
41. Wieczorek G, Bigaud M, Menninger K, et al. Acute and chronic vascular rejection in nonhuman primate kidney transplantation. Am J Transplant 2006; 6(6): 1285-1296.
Štítky
Patologie Soudní lékařství Toxikologie
Článek vyšel v časopiseČesko-slovenská patologie

2015 Číslo 4-
Všechny články tohoto čísla
- Transplantační patologie - II. díl
- Veškerá mizérie je v tom, že hlupákům je všechno jasné, zatímco moudří jsou samá pochybnost (Bertrand Russell)
- MONITOR aneb nemělo by vám uniknout, že ...
- MONITOR aneb nemělo by vám uniknout, že ...
- Transplantace jater z pohledu hepatologa
- Role patologa v programu transplantací jater
- MONITOR aneb nemělo by vám uniknout, že ...
- Morphology of surgical complications in liver biopsies early after transplantation
- Karcinomy dutiny ústní a hltanu
- Diagnosis of rejection in a transplanted liver
- Recurrence of primary diseases after liver transplantation
- MONITOR aneb nemělo by vám uniknout, že ...
- Transplantations of lungs in the Czech Republic – from the perspective of the pathologist
- MONITOR aneb nemělo by vám uniknout, že ...
- Renal allograft biopsies: a guide of ins and outs for best results
- Surgical techniques of organ transplants
- MONITOR aneb nemělo by vám uniknout, že ...
- Periosteal osteosarcoma - personal experience with five cases
- MONITOR aneb nemělo by vám uniknout, že ...
- The autopsy of the brain and spinal cord in the diagnosis of neurodegenerative diseases - a practical approach to optimize the examination
- Česko-slovenská patologie
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Periosteal osteosarcoma - personal experience with five cases
- Transplantations of lungs in the Czech Republic – from the perspective of the pathologist
- Diagnosis of rejection in a transplanted liver
- Surgical techniques of organ transplants
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání