-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Memory B lymphocytes in peripheral blood in coeliac disease – a pilot study
Paměťové B lymfocyty v periferní krvi u celiakie – pilotní studie
Funkční hyposplenizmus je stav provázející řadu chorob, vč. autoimunitních onemocnění a lymfomů. Hyposplenizmus se běžně vyskytuje také u celiakie (až ve 20 % nekomplikované a 80 % komplikované nemoci). Hyposplenizmus je spojen se zvýšeným rizikem závažných infekcí (Streptococcus pneumoniae, Neisseria meningitidis and Haemophilus influenzae). Cílem této prospektivní pilotní studie bylo vyšetřit paměťové B lymfocyty coby nepřímý biomarker funkčního hyposplenizmu. Bylo vyšetřeno 42 pacientů s celiakií (11 mužů, 31 žen, průměrný věk 49 ± 14 let) a 10 kontrolních osob, dárců krve (2 muži, 8 žen, průměrný věk 39 ± 7 let). Pomocí panelu DuraClone IM byly průtokovou cytometrií (Navios, Beckman Coulter) vyšetřeny jednotlivé subpopulace B lymfocytů v periferní krvi. Imunoglobulin (Ig) M a IgD paměťové B lymfocyty byly u nemocných s celiakií signifikantně nižší ve srovnání s kontrolními osobami. Dysfunkce paměťových B lymfocytů může být zodpovědná za zvýšené riziko bakteriálních infekcí u celiakie. Pacienti s celiakií s prokázanou dysfunkcí paměťových B lymfocytů jsou indikováni k pneumokokové vakcinaci.
Doručeno: 4. 7. 2019
Přijato: 9. 8. 2019
Konflikt zájmů: Autoři deklarují, že text článku odpovídá etickým standardům, byla dodržena anonymita pa cientů a prohlašují, že v souvislosti s předmětem článku nemají finanční, poradenské ani jiné komerční zájmy.
Publikační etika: Příspěvek nebyl dosud publikován ani není v současnosti zaslán do jiného časopisu pro posouzení. Autoři souhlasí s uveřejněním svého jména a e-mailového kontaktu v publikovaném textu.
Dedikace: Tato práce byla podpořena projektem PERSONMED – Centrum pro rozvoj personalizované medicíny u nemocí souvisejících s věkem, reg. zn. CZ.02.1.01/ 0.0/ 0.0/ 17_048/ 0007441.
Redakční rada potvrzuje, že rukopis práce splnil ICMJE kritéria pro publikace zasílané do bio medicínských časopisů.
Klíčová slova:
celiakie – funkční hyposplenizmus – průtoková cytometrie – paměťové B lymfocyty
Authors: Douda L. 1; Vokurková D. 2; Douda T. 1; Ilja Tachecí 1
; Jirkovský V. 1; Tomáš Fejfar 1
; D. Kohoutová 1,3
; Vašátko T. 1; Peterová E. 1; S. Rejchrt 1
; M. Kopáčová 1
; Bureš J. 1
Authors place of work: 2nd Department of Internal Medicine – Gastroenterology, Faculty of Medicine in Hradec Králové, Charles University and University Hospital Hradec Králové, Czech Republic 1; Institute of Clinical Immunology and Allergology, Faculty of Medicine in Hradec Králové, Charles University and University Hospital Hradec Králové, Czech Republic 2; The Royal Marsden Hospital NHS Foundation Trust, London, United Kingdom 3
Published in the journal: Gastroent Hepatol 2019; 73(4): 296-302
Category: Klinická a experimentální gastroenterologie: původní práce
doi: https://doi.org/10.14735/amgh2019296Summary
Functional hyposplenism is a condition accompanying many diseases including autoimmune disorders and lymphomas. Hyposplenism is also commonly found in adult coeliac disease (up to 20% of non-complicated and up to 80% of complicated disease). Hyposplenism is associated with an increased risk of severe infections (Streptococcus pneumoniae, Neisseria meningitidis and Haemophilus influenzae). The aim of this prospective study was to investigate memory B lymphocytes as an indirect biomarker of functional hyposplenism. A total of 42 patients with coeliac disease (11 men, 31 women; mean age 49 ± 14 years) and 10 healthy controls, blood donors (2 men, 8 women; mean age 39 ± 7 years) were enrolled into the study. None of the patients had a history of splenectomy and none of them suffered. from immunodeficiency. The DuraClone IM panel was used to identify B lymphocytes subpopulations in peripheral blood samples by flow cytometry Navios (Beckman Coulter) with software analysis using Kaluza version 1.2. Patients with coeliac disease and controls did not differ in basic parameters of leukocyte and total lymphocyte blood count. Switched memory B lymphocytes (CD19+CD27+IgD-), non-switched memory/marginal-zone-like B lymphocytes (CD19+CD27+IgD+) and immunoglobulin (Ig) M memory B lymphocytes (CD19+CD27+IgM++) were significantly lower in patients with coeliac compared to controls. Follicular (naïve) B lymphocytes were not significantly different between coeliac disease and controls. In conclusion, dysfunction of memory B lymphocytes can be responsible for an increased risk of severe bacterial infections in coeliac disease. Patients with coeliac disease with dysfunction of memory B lymphocytes are clearly indicated for pneumococcal vaccination.
Keywords:
celiakie – funkční hyposplenizmus – průtoková cytometrie – paměťové B lymfocyty
Introduction
Coeliac disease is a chronic multiorgan autoimmune disease, affecting the small bowel and other organs and systems, in genetically predisposed individuals, precipitated by the ingestion of gluten [1,2]. Several gastrointestinal infections in-crease the risk of coeliac disease autoimmunity in children with genetic susceptibility to this autoimmune disorder. The risk is modified by the HLA genotype, infant gluten consumption, breastfeeding, and rotavirus vaccination, indicating complex interactions among infections, genetic factors, and diet in the aetiology of coeliac disease in early childhood [3]. Chronic Helicobacter pylori infection is a controversial issue, however, a current study does not support a „protective“ role of H. pylori against coeliac disease [4], as previously reported [5]. Therefore, there are no reasons to avoid correctly indicated eradication of H. pylori also in subjects genetically susceptible to coeliac disease [4]. On the other hand, coeliac disease is associated with a higher risk of several infections, all viral, bacterial and parasitic [6–12]. There are serious concerns about pneumococcal infection in particular due to an impaired immunity to encapsulated bacteria [13–16]. Meta-analysis of a total of 156 articles (published between 1980–2017) and three large databases (the Swedish National Inpatient Register, the Oxford Record Linkage Study and the English National Hospital Episode Statistics) found increased incidence of pneumococcal infection among hospitalised celiac patients compared with controls (odds ratio 1.66) [17]. Preventive pneumococcal vaccination should be considered for those with coeliac disease, with special attention to those aged 15–64 years who have not received the scheduled pneumococcal vaccination series in the infant age [17]. Functional hyposplenism is a condition accompanying many diseases, including coeliac disease, other autoimmune disorders and lymphomas [13,14,18]. Hyposplenism is considered to be present if the size of the spleen is small on imaging, or in the presence of circulating Howell-Jolly bodies, mild degree of thrombocytosis and leukocytosis [18–20]. Association of hyposplenism with coeliac disease was recognized as early as in 1980, where almost half of patients had impaired splenic function [21]. Nowadays hyposplenism is commonly found in adult coeliac disease (up to 20% of non-complicated and up to 80% complicated disease) [13,22]. Hyposplenism is associated with an increased risk of severe infections (Streptococcus pneumoniae, Neisseria meningitidis and Haemophilus influenzae) [13,22,23]. The aim of this prospective pilot study was to investigate memory B lymphocytes as an indirect biomarker of functional hyposplenism.
Material and methods
A total of 42 patients with coeliac disease (11 men, 31 women; mean age 49 ± 14 years) and 10 healthy controls (blood donors; 2 men, 8 women; mean age 39 ± 7 years) were enrolled into the study. All patients but one were on long-term gluten-free diet. Coeliac disease was recently diagnosed just in one subject. None of the patients had a history of splenectomy and none of them suffered from immunodeficiency.
The DuraClone IM panel was used to identify B lymphocytes subpopulations in peripheral blood samples by flow cytometry Navios (Beckman Coulter Inc., Brea, CA, USA) with software analysis using Kaluza version 1.2. The DuraClone IM B cells are 8-colour, 8-monoclonal antibody reagents that allow to identify B lymphocyte subpopulations which are present in whole blood samples. The cocktail of monoclonal antibodies included: anti-IgD-FITC, anti-CD21-PE, anti-CD19-ECD, anti-CD27-PC7, anti-CD24-APC, anti-CD38-APC-A750, anti-IgM-PB and anti-CD45-KO. This test is based on the ability of specific monoclonal antibodies to bind to the antigen determinants which are expressed on B lymphocytes. Specific staining of leukocytes is performed by incubating the sample with IM B cells Panel. Subsequently, erythrocytes are removed by lysis. Five thousand CD19+ cells (B lymphocytes) are acquired and analyzed by flow cytometry.
The obtained data was tested statistically by means of descriptive statistics. Data with normal distribution were further analysed by parametric unpaired t-test and data with non-normal distribution were tested by non-parametric Mann-Whitney test. The Pearson test was used for correlation analysis (SigmaStat software, version 3.1, Jandel Corp., Erkrath, Germany).
The study was approved by the Joint University Ethics Committee. All procedures were in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki declaration and its latter amendments.
All patients signed written consent. For all data obtained, all personal identification information was deleted in compliance with the laws for the protection of confidentiality of the Czech Republic.
Results
Patients with coeliac disease and controls did not differ in basic parameters of leukocyte and total lymphocyte values (Tab. 1). Switched memory B lymphocytes (CD19+CD27+IgD-), non-switched memory/marginal-zone-like B lymphocytes (CD19+CD27+IgD+) and IgM memory B lymphocytes (CD19+CD27+IgM++) were significantly lower in coeliac disease compared to controls (Graph 1–6, Fig. 1 and 2). Follicular (naïve) B lymphocytes were not significantly different between coeliac disease (median 8.0; interquartile range 3.2–20.9) and controls (median 10.3; interquartile range 9.0–20.6; p = 0.530).
Tab. 1. Leukocyte and lymphocyte blood count in 42 patients with coeliac disease and 10 controls (healthy blood donors).
Tab. 1. Krevní obraz leukocytů a lymfocytů u 42 pacientů s celiakií a 10 kontrol (zdravých dárců krve).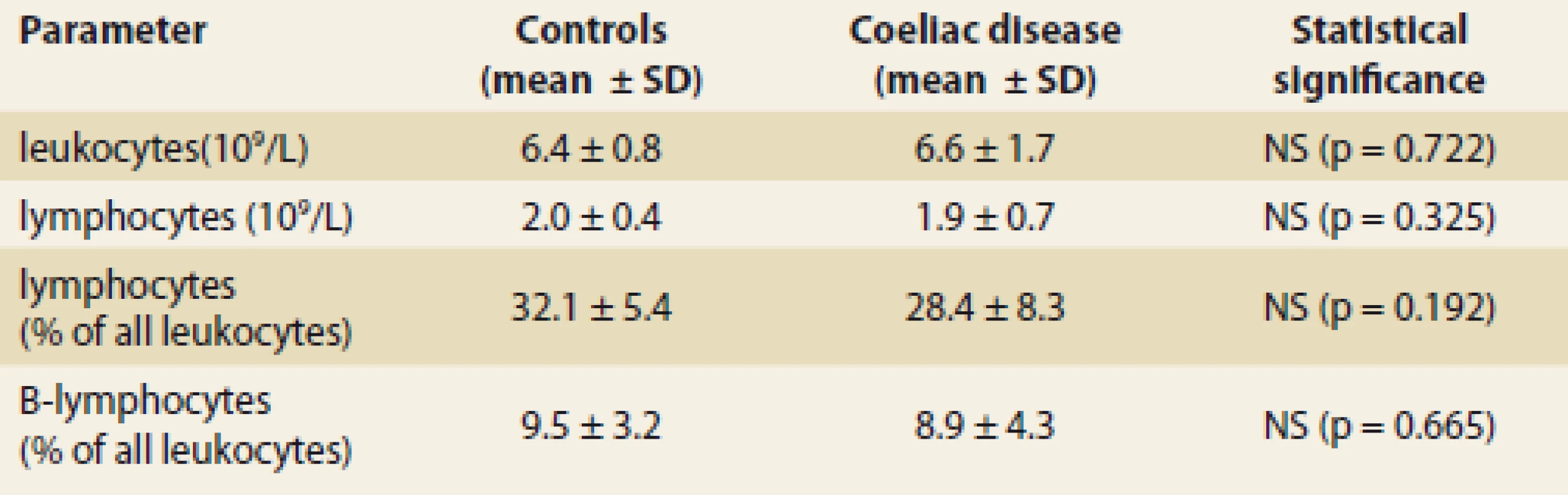
SD – standard deviation, NS – not signifi cant, L – litre Graph 1. Switched memory B lymphocytes (CD19+CD27+IgD-) in coeliac disease and controls; relative values in % of all B lymphocytes (mean + SD; p < 0.001).
Graf 1. Paměťové B lymfocyty (CD19+CD27+IgD-) u celiakie a u kontrolních osob; relativní hodnoty v % všech B lymfocytů (průměr + SD; p <0,001).
Graph 2. Switched memory B lymphocytes (CD19+CD27+IgD-) in coeliac disease and controls; absolute values (mean + SD; p = 0.010).
Graf 2. Paměťové B lymfocyty (CD19+CD27+IgD-) u celiakie a u kontrolních osob; absolutní hodnoty (průměr + SD; p = 0,010).
Graph 3. IgM memory B lymphocytes (CD19+CD27+IgM++) in coeliac disease and controls; relative values in % of all B lymphocytes (mean + SD; p = 0.007).
Graf 3. IgM paměťové B lymfocyty (CD19+CD27+IgM++) u celiakie a u kontrolních osob; relativní hodnoty v % všech B lymfocytů (průměr + SD; p = 0,007).
Graph 4. IgM memory B lymphocytes (CD19+CD27+IgM++) in coeliac disease and controls; absolute values (mean + SD; p = 0.004).
Graf 4. IgM paměťové B lymfocyty (CD19+CD27+IgM++) u celiakie a u kontrolních osob; absolutní hodnoty (průměr + SD; p = 0,004).
Graph 5. Non-switched memory/ marginal-zone-like B lymphocytes (CD19+CD27+IgD+) in coeliac disease and controls; relative values in % of all B lymphocytes (mean + SD; p = 0.040).
Graf 5. Paměťové B-lymfocyty (CD19+CD27+IgD+) u celiakie a u kontrolních osob; relativní hodnoty v % všech B lymfocytů (průměr + SD; p = 0,040).
Graph 6. Non-switched memory/marginal- zone-like B lymphocytes (CD19+ CD27+IgD+) in coeliac disease and controls; absolute values (mean + SD; trend to signifi cant difference: p = 0.059).
Graf 6. Paměťové B-lymfocyty (CD19 + CD27 + IgD +) u celiakie a u kontrolních osob; absolutní hodnoty (průměr + SD; trend k statisticky významnému rozdílu: p = 0,059).
Fig. 1. Flow cytometry. IgM+ memory B lymphocytes (CD19+CD27+IgM++), displayed as green dots. On the left: a healthy blood donor; IgM+ memory B lymphocytes comprise 2% of all B lymphocytes. On the right: a patient with coeliac disease; IgM+ memory B lymphocytes comprise 0.5% of all B lymphocytes.
Obr. 1. Průtoková cytometrie. IgM+ paměťové B lymfocyty (CD19+CD27+IgM++), zobrazeny jako zelené tečky. Vlevo: zdravý dárce krve; IgM+ paměťové B lymfocyty tvoří 3 % všech B lymfocytů. Vpravo: pacient s celiakií; IgM+ paměťové B lymfocyty tvoří 0,5 % všech B lymfocytů.
Fig. 2. Flow cytometry. Switched memory B lymphocytes (CD19+CD27+IgD-), displayed as red dots. On the left: a healthy blood donor; Switched memory B lymphocytes (CD19+CD27+IgD-) comprise 3% of all B lymphocytes. On the right: a patient with coeliac disease; Switched memory B lymphocytes (CD19+CD27+IgD-) comprise 1.5% of all B lymphocytes.
Obr. 2. Průtoková cytometrie. Paměťové B lymfocyty (CD19+CD27+IgD-), zobrazeny jako červené tečky. Vlevo: zdravý dárce krve; paměťové B lymfocyty (CD19+CD27+IgD-) tvoří 3 % všech B lymfocytů. Vpravo: pacient s celiakií;paměťové B lymfocyty (CD19+CD27+IgD-) tvoří 1,5 % všech B lymfocytů.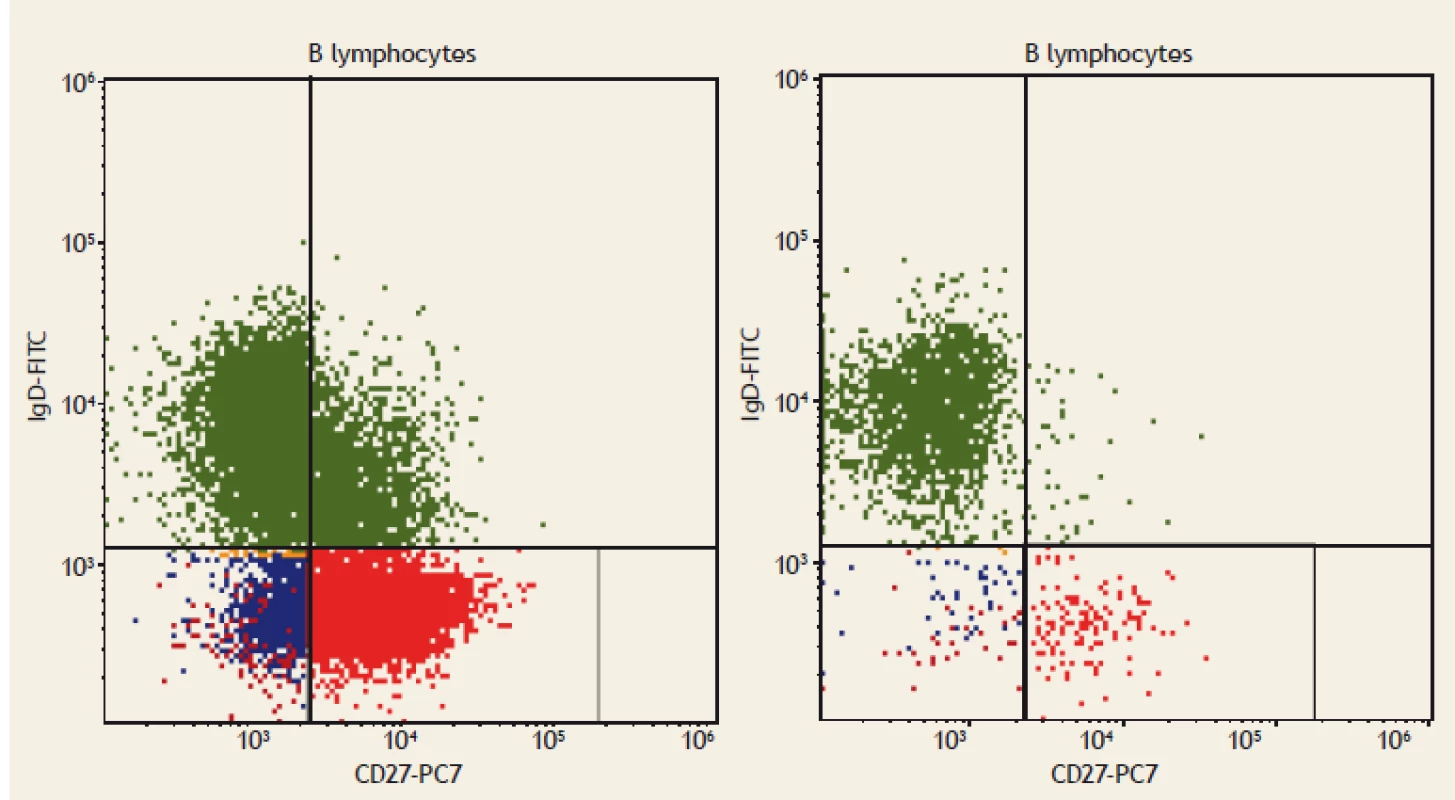
Discussion
Our current prospective pilot study has shown important dysfunction of memory B lymphocytes in patients with coeliac compared to controls. Switched memory B lymphocytes (CD19+CD27+IgD-), non-switched memory/marginal-zone-like B lymphocytes (CD19+CD27+IgD+) and IgM memory B lymphocytes (CD19+CD27+IgM++) were significantly lower in coeliac disease compared to controls. In humans, 30–60% of the B lymphocytes express CD27 and are considered to be memory B cells. Memory B lymphocytes are described as highly specific and long-lived cells, and are usually generated in response to infectious agents or vaccines. They persist in the organism and produce antibodies rapidly upon a second challenge with the same pathogen. Half of them are IgM memory B cells and the rest are switched memory B cells. In the peripheral blood as well as in the bone marrow, memory B lymphocytes have been identified in populations of B cells expressing either class-switched immunoglobulin isotypes: IgM and IgD, or IgM only. CD19+CD27+IgD-cells are called „switched“ memory B lymphocytes and are indicators of normal B-cell activation and development in the germinal centres in the lymph nodes or other secondary lymphoid tissues. Switched memory B cells do not express IgD, they have undergone class-switching and express IgG, IgA or IgE [24,25]. It was important for an appropriate evaluation of our results that basic parameters of leukocyte and total lymphocyte blood count were within normal values [26] and did not differ between coeliac disease and controls. Thus, dysfunction of memory B cells could be clearly seen.
Etiology and pathogenesis of functional hyposplenism has not been fully clarified yet. Even more, there is no specific marker which would be fully reliable. Hrncir et al. [27] demonstrated persistent character of marginal-zone-like B lymphocytes deficiency in the peripheral blood, suggesting that this might be a possible biomarker of functional hyposplenism in systemic lupus erythematosus. Decreased values of peripheral IgM memory B lymphocytes were also found up in up to 80% patients with inflammatory bowel disease [28], however, there was no correlation with activity and/or clinical course of the disease. Splenic function improved with the anti-tumour necrosis factor-alpha therapy [29]. Di Sabatino et al. [13] found decreased peripheral IgM memory B lymphocytes in up to 20% of non-complicated and up to 80% complicated coeliac disease. Nevertheless, these results have not yet been supported by any subsequent studies from other centres. Long-term, hyposplenism could represent a risk of severe complications of coeliac disease, including type II refractory sprue and lymphoma [13]. Nowadays, B cell panels for flow cytometry have been improved significantly, so that more exact evaluation of B lymphocyte subpopulations is possible, e. g. transient B cells, plasmablasts, class switched and class non-switched B lymphocytes etc. We are aware of possible limits of our pilot study. Our patients with coeliac disease were older compared to control subjects (10 years on the average). The ability to internalize and to present antigens decreases with aging. In case of specific immunity, age-induced changes include decreasing number of naïve B lymphocytes, resulting in a reduced ability to respond to new antigens and to create immune memory. In contrast, specific memory B lymphocytes and plasma cells accumulate and may produce monoclonal IgG with a weaker affinity to antigens. The pool of naïve and memory T lymphocytes is reduced, the receptors of which show similarly impaired reactivity when meeting new antigens [30].
Further studies are needed to prove our pilot data. Nevertheless, our current results support the recommendation that patients with coeliac disease with dysfunction of memory B lymphocytes are clearly indicated to receive pneumococcal vaccination (13-valent pneumococcal conjugate vaccine followed by the 23-valent pneumococcal polysaccharide vaccine). Other vaccines recommended in patients with impaired splenic function should be considered individually (Haemophilus influenzae type b vaccine, monovalent meningococcal serogroup B vaccine) [31].
Conclusions
Dysfunction of switched memory B lymphocytes can be responsible for an increased risk of severe bacterial infections in coeliac disease.
Submitted: 4. 7. 2019
Accepted: 9. 8. 2019
Prof. MUDr. Jan Bureš, CSc., FCMA
2nd Department of Internal Medicine – Gastroenterology
Faculty of Medicine in Hradec Králové
Charles University and University Hospital Hradec Králové
Sokolská 581
500 05 Hradec Králové
Czech Republic
Conflict of Interest: The authors declare that the article/manuscript complies with ethical standards and patient anonymity has been respected and they state that they have no financial, advisory or other commercial interests in relation to the subject matter.
Publication Ethics: This article/manuscript has not been published or is cur rently be ing submitted for another review.
The authors agree to publish their name and e-mail in the published article/manuscript.
Dedication: This work was supported by the project PERSONMED – Centre for the Development of Personalised Medicine in Age-Related Dis eases, Reg. No. CZ.02.1.01/ 0.0/ 0.0/ 17_048/ 0007441.
The Editorial Board declares that the manuscript met the ICMJE „uniform requirements“ for bio medical papers.
Zdroje
1. Bai JC, Ciacci C, Corazza GR et al. WGO practice guideline – celiac disease. [online]. Available from: http: //www.worldgastroenterology.org/guidelines/global-guidelines/celiac-disease. 2019.
2. Al-Toma A, Volta U, Auricchio R et al. European Society for the Study of Coeliac Disease (ESsCD) guideline for coeliac disease and other gluten-related disorders. United European Gastroenterol J 2019; 7 (5): 583-613. doi: 10.1177/2050640619844125.
3. Kemppainen KM, Lynch KF, Liu E et al. Factors that increase risk of celiac disease autoimmunity after a gastrointestinal infection in early life. Clin Gastroenterol Hepatol 2017; 15 (5): 694–702. doi: 10.1016/j.cgh.2016.10.033.
4. Dore MP, Salis R, Loria MF et al. Helicobacter pylori infection and occurrence of celiac disease in subjects HLA-DQ2/DQ8 positive: a prospective study. Helicobacter 2018; 23 (2): e12465. doi: 10.1111/hel.12465.
5. Rostami-Nejad M, Javad Ehsani-Ardakani M, Assadzadeh H et al. Pathological and clinical correlation between celiac disease and Helicobacter pylori infection: a review of controversial reports. Middle East J Dig Dis 2016; 8 (2): 85–92. doi: 10.15171/mejdd.2016.12.
6. Ludvigsson JF, Sanders DS, Maeurer M et al. Risk of tuberculosis in a large sample of patients with coeliac disease – a nationwide cohort study. Aliment Pharmacol Ther 2011; 33 (6): 689–696. doi: 10.1111/j.1365-2036.2010.04572.x.
7. Rostami Nejad M, Rostami K, Cheraghipour K et al. Celiac disease increases the risk of Toxoplasma gondii infection in a large cohort of pregnant women. Am J Gastroenterol 2011; 106 (3): 548–549. doi: 10.1038/ajg.2010. 425.
8. Mårild K, Kahrs CR, Tapia G et al. Infections and risk of celiac disease in childhood: a prospective nationwide cohort study. Am J Gastroenterol 2015; 110 (10): 1475–1484. doi: 10.1038/ajg.2015.287.
9. Perfetti V, Baldanti F, Lenti MV et al. Detection of active epstein-barr virus infection in duodenal mucosa of patients with refractory celiac disease. Clin Gastroenterol Hepatol 2016; 14 (8): 1216–1220. doi: 10.1016/j.cgh.2016.03. 022.
10. Giacomin P, Zakrzewski M, Jenkins TP et al. Changes in duodenal tissue-associated microbiota following hookworm infection and consecutive gluten challenges in humans with coeliac disease. Sci Rep 2016; 6 : 36797. doi: 10.1038/srep36797.
11. Lebwohl B, Nobel YR, Green PH et al. Risk of clostridium difficile infection in patients with celiac disease: a population-based study. Am J Gastroenterol 2017; 112 (12): 1878–1884. doi: 10.1038/ajg.2017.400.
12. Urganci N, Kalyoncu D. Tuberculosis and tuberculin skin test reactivity in pediatric patients with celiac disease. Minerva Pediatr 2017; 69 (1): 30–35. doi: 10.23736/S0026-4946.16.04210-9.
13. Di Sabatino A, Rosado MM, Cazzola P et al. Splenic hypofunction and the spectrum of autoimmune and malignant complications in celiac disease. Clin Gastroenterol Hepatol 2006; 4 (2): 179–186.
14. Mårild K, Fredlund H, Ludvigsson JF. Increased risk of hospital admission for influenza in patients with celiac disease: a nationwide cohort study in Sweden. Am J Gastroenterol 2010; 105 (11): 2465–2473. doi: 10.1038/ajg.2010. 352.
15. Zingone F, Abdul Sultan A, Crooks CJ et al. The risk of community-acquired pneumonia among 9803 patients with coeliac disease compared to the general population: a cohort study. Aliment Pharmacol Ther 2016; 44 (1): 57–67. doi: 10.1111/apt.13652.
16. Röckert Tjernberg A, Bonnedahl J, Inghammar M et al. Coeliac disease and invasive pneumococcal disease: a population-based cohort study. Epidemiol Infect 2017; 145 (6): 1203–1209. doi: 10.1017/S0950268816003204.
17. Simons M, Scott-Sheldon LA, Risech-Neyman Y et al. Celiac disease and increased risk of pneumococcal infection: a systematic review and meta-analysis. Am J Med 2018; 131 (1): 83–89. doi: 10.1016/j.amjmed.2017.07. 021.
18. Ludvigsson JF, Olén O, Bell M et al. Coeliac disease and risk of sepsis. Gut 2008; 57 (8): 1074–1080. doi: 10.1136/gut.2007.133868.
19. Kirkineska L, Perifanis V, Vasiliadis T. Functional hyposplenism. Hippokratia 2014; 18 (1): 7–11.
20. van Gils T, Nijeboer P, van Waesberghe JH et al. Splenic volume differentiates complicated and non-complicated celiac disease. United European Gastroenterol J 2017; 5 (3): 374–379. doi: 10.1177/2050640616663571.
21. Robinson PJ, Bullen AW, Hall R et al. Splenic size and function in adult coeliac disease. Br J Radiol 1980; 53 (630): 532–537. doi: 10.1259/0007-1285-53-630-532.
22. Di Sabatino A, Rosado MM, Ciccocioppo R et al. Depletion of immunoglobulin M memory B cells is associated with splenic hypofunction in inflammatory bowel disease. Am J Gastroenterol 2005; 100 (8): 1788–1795. doi: 10.1111/j.1572-0241.2005.41939.x.
23. Di Sabatino A, Brunetti L, Carnevale Maffè G et al. Is it worth investigating splenic function in patients with celiac disease? World J Gastroenterol 2013; 19 (15): 2313–2318. doi: 10.3748/wjg.v19.i15.2313.
24. Kruetzmann S, Rosado MM, Weber H et al. Human immunoglobulin M memory B cells controlling Streptococcus pneumoniae infections are generated in the spleen. J Exp Med 2003; 197 (7): 939–945. doi: 10.1084/jem.200220 20.
25. LeBien TW, Tedder TF. B lymphocytes: how they develop and function. Blood 2008; 112 (5): 1570–1580. doi: 10.1182/blood-2008-02-078071.
26. Morbach H, Eichhorn EM, Liese JG et al. Reference values for B cell subpopulations from infancy to adulthood. Clin Exp Immunol 2010; 162 (2): 271–279. doi: 10.1111/j.1365-2249.2010.04206.x.
27. Hrncir Z, Vokurkova D, Drahosova M et al. Marginal-zone-like b cells deficiency repeatedly detected in peripheral blood as a possible biomarker of hyposplenism/asplenia in SLE. Lupus 2018; 5 (Suppl 1): A83.
28. Di Sabatino A, Rosado MM, Ciccocioppo R et al. Depletion of immunoglobulin M memory B cells is associated with splenic hypofunction in inflammatory bowel disease. Depletion of immunoglobulin M memory B cells is associated with splenic hypofunction in inflammatory bowel disease. Am J Gastroenterol 2005; 100 (8): 1788–1795. doi: 10.1111/j.1572-0241.2005.41939.x.
29. Di Sabatino A, Rosado MM, Cazzola P et al. Splenic function and IgM-memory B cells in Crohn’s disease patients treated with infliximab. Inflamm Bowel Dis 2008; 14 (5): 591–596. doi: 10.1002/ibd.20374.
30. Siegrist CA, Aspinall R. B-cell responses to vaccination at the extremes of age. Nat Rev Immunol 2009; 9 (3): 185–194. doi: 10.1038/nri2508.
31. Pasternack MS. Prevention of infection in patients with impaired splenic function. [online]. Available from: https: //www.uptodate.com/contents/prevention-of-infection-in-patients-with-impaired-splenic-function.
Štítky
Dětská gastroenterologie Gastroenterologie a hepatologie Chirurgie všeobecná
Článek vyšel v časopiseGastroenterologie a hepatologie
Nejčtenější tento týden
2019 Číslo 4- Horní limit denní dávky vitaminu D: Jaké množství je ještě bezpečné?
- Metamizol jako analgetikum první volby: kdy, pro koho, jak a proč?
- Nejlepší kůže je zdravá kůže: 3 úrovně ochrany v moderní péči o stomii
-
Všechny články tohoto čísla
- Clinical and experimental gastroenterology
- Kvíz z klinické praxe
- Evolution of insulin resistance after liver transplantation – a prospective study
- Memory B lymphocytes in peripheral blood in coeliac disease – a pilot study
- Cystic fibrosis and exocrine pancreatic insufficiency
- The role of pH impedance in patients with excessive supragastric belching
- Imaging activity in nerves that mediate pain from gastrointestinal organs
- Does proton pump inhibitors treatment affect the risk of gastrointestinal bleeding in patients on novel antithrombotic therapy?
- New oral anticoagulants and upper gastrointestinal bleeding
- Insulinoma as a cause of severe hypoglycemia in a patient with neurological symptoms
- Dysphagia as a symptom of an adverse effect of anticoagulant therapy
- Unusual manifestation of tuberculosis in an ulcerative colitis patient treated with infliximab
- Part 4 – Mortality and life expectancy in IBD patients
- OLOMOUC LIVE ENDOSCOPY 2019
- Dvě dekády endoskopické léčby časného karcinomu žaludku v České republice perspektivou kongresu IGCC 2019
- The selection from international journals
- Správná odpověď na kvíz
- Kreditovaný autodidaktický test: klinická a experimentální gastroenterologie
- Tofacitinib – the fi rst JAK inhibitor in the treatment of ulcerative colitis. Beginning of the therapeutic era of „small molecules“ in infl ammatory bowel disease?
- Gastroenterologie a hepatologie
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Insulinoma as a cause of severe hypoglycemia in a patient with neurological symptoms
- Part 4 – Mortality and life expectancy in IBD patients
- New oral anticoagulants and upper gastrointestinal bleeding
- Dysphagia as a symptom of an adverse effect of anticoagulant therapy
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



