-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Emergence of Pathogenicity in Lagoviruses: Evolution from Pre-existing Nonpathogenic Strains or through a Species Jump?
article has not abstract
Published in the journal: . PLoS Pathog 11(11): e32767. doi:10.1371/journal.ppat.1005087
Category: Opinion
doi: https://doi.org/10.1371/journal.ppat.1005087Summary
article has not abstract
Introduction
Emergence of pathogenic viruses is of great concern, although the underlying mechanisms for emergence remain often poorly understood. RNA viruses are frequently implicated and recent examples include viruses within Orthomyxoviridae, Flaviviridae, or Coronaviridae [1–3]. Within Caliciviridae, the Lagovirus genus is particularly intriguing because it has generated viruses of exceptional pathogenicity on several occasions within the past 40 years. The genus Lagovirus encompasses two pathogenic viruses, Rabbit hemorrhagic disease virus (RHDV) affecting European rabbit (Oryctolagus cuniculus), and European brown hare syndrome virus (EBHSV) affecting Brown, Mountain, and Italian hare (Lepus europaeus, L. timidus, and L. corsicanus) [4]. These two viruses show a similar structure and ~70% homology [5–15]. They cause two distinct diseases, RHD (rabbit hemorrhagic disease) and EBHS (European brown hare syndrome), that emerged in the late 1970s to early 1980s [16–17]. Both cause high mortalities and impose a heavy economic burden on the rabbit industry and game animal management. They have also contributed to declines of wild leporid populations throughout Europe, resulting in major ecological impact in natural ecosystems where leporids are key species [18–25].
RHD was first detected in China in 1984, apparently in rabbits imported from Germany [16], suggesting that RHDV originated in Europe. Rabbit lagoviruses consist of pathogenic viruses (RHDV) and related, but genetically divergent, nonpathogenic viruses [26–34]. Phylogenetic analyses of pathogenic RHDV strains show three distinct groups: the classic RHDV with the genogroups G1–G5 [27,35–55], the antigenic variant RHDVa/G6 [35,56–61], and RHDV2/RHDVb [62–77]. The RHDV and RHDVa are phylogenetically related and differ from RHDV2 by more than 15% in nucleotide diversity. The RHDV strains have emerged at different times: RHDV was first isolated in 1984 [16] and RHDV2 in 2010 [62]. RHDV2 was identified in France and has since spread throughout other Western European countries, replacing the circulating strains in France and the Iberian Peninsula [64,67,71,76], while in Italy, it currently cocirculates with the “original” strains [64].
EBHS was first reported in Sweden in 1980 [17] and later found in other European countries [11,78–85]. It may have emerged earlier, as suggested by descriptions of hares with lesions consistent with the disease in 1976 in England [86]. Otherwise, antibodies against the virus have also been found in archived sera [87], and the virus was detected by RT-PCR in samples collected in Sweden before 1980 [88].
Two competing hypotheses can be put forward to explain RHDV and EBHSV origin and the emergence of RHDV2 : 1) the evolution from pre-existing nonpathogenic viruses circulating in European leporids; 2) a species jump. The first hypothesis is shared by several authors and originates from the detection of anti-RHDV antibodies in rabbit blood samples collected before the first documented outbreak in Europe and Australia [89–92] and later in the characterization of different nonpathogenic viruses in European rabbits [26–34]. However, this hypothesis has not been confirmed and fails to explain the abrupt emergence of high pathogenicity on several occasions in a short period of time. Notably, the pathogenic and nonpathogenic viruses are phylogenetically separated and display ~20% of nucleotide divergence in the capsid gene [26–34], suggesting that the pathogenic forms did not directly originate from the nonpathogenic ones. Nevertheless, nonpathogenic strains have not been exhaustively characterized in European leporids, which may explain why the ancestors of pathogenic strains have not yet been found. The second hypothesis involves a species jump from species sympatric with European leporids, either native or previously introduced. Among these species, Eastern cottontail (Sylvilagus floridanus), a leporid native to North America, would constitute a worthwhile species. Although no data is available on the presence of original lagoviruses, they likely would have caused asymptomatic infection in its natural host, with a course of infections similar to what occurs with myxoma virus, benign in Sylvilagus species, but lethal in the European rabbit following a species jump [93]. Indeed, it is intriguing that both RHDV and EBHSV emerged at around the same time, coinciding with the introduction of the Eastern cottontail in Europe. Large numbers of introductions of Eastern cottontails by hunters occurred in Europe from the 1960s, but because they were illegal, these introductions are poorly documented. The first known introduction attempt from the United States was in 1966 in Italy. This was followed by massive introductions involving thousands of animals, especially in Italy and France. It is highly likely that localized introductions still occur, as suggested by the existence of cottontail breeders in France.
In the Po valley in Italy, where Eastern cottontails are invasive and widespread, a serological study showed that 18% and 33% of them carry antibodies detected by both anti-EBHSV and anti-RHDV serological tests, proving the susceptibility of the species to lagoviruses [94]. Moreover, recent documentation of RHDV strains in Iberian hares (L. granatensis) with lesions compatible with RHD [95], demonstration of the capacity of RHDV2 to infect Sardinian Cape and Italian hares (L. capensis mediterraneus and L. corsicanus, respectively) causing RHDV-like disease [65,70], and the experimental infection of cottontails by EBHSV [94], show the feasibility of species jumps of lagoviruses between leporid species.
We therefore suggest that European leporids carry lagoviruses of two distinct origins (Fig 1): nonpathogenic strains that have evolved with these species for a long time and a second group including pathogenic strains that possibly emerged following species jumps from S. floridanus and that have then evolved in European leporids. Pathogenic strains may be pure cottontail viruses or recombinants of cottontail viruses and nonpathogenic viruses of European leporid species. Indeed, recombination in RHDV is reported [96,97], and the recent documentation of recombination events between genome regions encoding the capsid and VP10 structural proteins of RHDV2 and the nonstructural proteins from nonpathogenic or pathogenic G1 lagoviruses suggests that recombination could have had an important role in the lagovirus evolution [74].
Fig. 1. Possible origin of European rabbit (O. cuniculus) lagoviruses according to the hypothesis of a species jump. 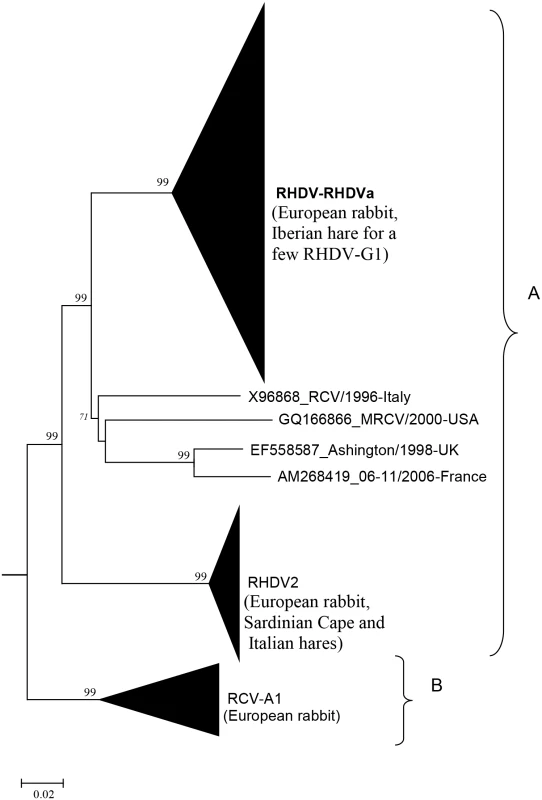
A) Lagoviruses that may share common ancestors following several species jump(s), B) Nonpathogenic viruses that have evolved in European rabbit for a long time. Phylogenetic tree (Neighbor-joining method) derived from 303 rabbit lagovirus sequences of the VP60 gene available on public databases (May 2015). The pathogenic RHDV, RHDVa, RHDV2, and the nonpathogenic RCV-A1 branches are collapsed; the name of the leporid species where these strains were isolated is given in brackets. X96868_RCV/1996-Italy, GQ166866_MRCV/2000-USA, EF558587_Ashington/1998-UK, and AM268419_06-11/2006-France are nonpathogenic strains isolated in the European rabbit. Percentage greater than 70% of replicate trees in which the associated taxa clustered together in the bootstrap test (500 replicates) are given at major branch nodes. The EBHSV strain GD (Z69620) was used as an outgroup to root the tree. Similar clustering was observed in several recent works [63,64,66,70,74]. Evaluation of potential emergence of new pathogenic lagoviruses from the nonpathogenic strains circulating among native European leporid species and Sylvilagus species, as well as characterization of the genetic determinisms of pathogenicity, are key to identify the mechanisms of disease emergence and may help to evaluate the possibility of emergence of other highly pathogenic lagoviruses.
Zdroje
1. Bengis RG, Leighton FA, Fischer JR, Artois M, Mörner T, Tate CM. The roleof wildlife in emerging and re-emerging zoonoses. Rev Sci Tech. 2004;23 : 497–511. 15702716
2. Gautret P, Gray GC, Charrel RN, Odezulu NG, Al-Tawfiq JA, Zumla A, Memish ZA. Emerging viral respiratory tract infections—environmental risk factors and transmission. Lancet Infect Dis. 2014;14 : 1113–1122. doi: 10.1016/S1473-3099(14)70831-X 25189350
3. Mandl JN, Ahmed R, Barreiro LB, Daszak P, Epstein JH, Virgin HW, Feinberg MB. Reservoir host immune responses to emerging zoonotic viruses. Cell. 2015;160 : 20–35. doi: 10.1016/j.cell.2014.12.003 25533784
4. Abrantes J, van der Loo W, Le Pendu J, Esteves PJ. Rabbit haemorrhagic disease (RHD) and rabbit haemorrhagic disease virus (RHDV): a review. Veterinary Research. 2011;43 : 12.
5. Capucci L, Scicluna MT, Lavazza A. Diagnosis of viral haemorrhagic disease of rabbits and the European brown hare syndrome. Rev Sci Tech. 1991;10 : 347–370. 1662098
6. Marcato PS, Benazzi C, Vecchi G, Galeotti M, Della Salda L, Sarli G, Lucidi P. Clinical and pathological features of viral haemorrhagic disease of rabbits and the European brown hare syndrome. Rev Sci Tech. 1991;10 : 371–392. 1760582
7. Chasey D, Lucas M, Westcott D, Williams M. European brown hare syndrome in the U.K.; a calicivirus related to but distinct from that of viral haemorrhagic disease in rabbits. Arch Virol. 1992;124 : 363–370. 1318711
8. Fuchs A, Weissenbock H. Comparative histopathological study of rabbit haemorrhagic disease (RHD) and European brown hare syndrome (EBHS). J Comp Pathol 1992;107 : 103–113. 1430343
9. Wirblich C, Meyers G, Ohlinger VF, Capucci L, Eskens U, Haas B, Thiel HJ. European brown hare syndrome virus: relationship to rabbit hemorrhagic disease virus and other caliciviruses. J Virol. 1994;68 : 5164–5173. 7518531
10. Lavazza A, Scicluna MT, Capucci L. Susceptibility of hares and rabbits to the European brown hare syndrome virus (EBHSV) and rabbit haemorrhagic disease virus (RHDV) under experimental conditions. Zentralbl Veterinarmed B. 1996;43 : 401–410. 8885705
11. Le Gall G, Huguet S, Vende P, Vautherot J-F, Rasschaert D. European brown hare syndrome virus: molecular cloning and sequencing of the genome. J Gen Virol. 1996;77 : 1693–1697. 8760416
12. Nowotny N, Bascunana CR, Ballagi-Pordany A, Gavier-Widen D, Uhlen M, Belak S. Phylogenetic analysis of rabbit haemorrhagic disease and European brown hare syndrome viruses by comparison of sequences from the capsid protein gene. Arch. Virol. 1997;142 : 657–673. 9170495
13. Le Gall-Reculé G, Zwingelstein F, Laurent S, Portejoie Y, Rasschaert D. Molecular epidemiology of European brown hare syndrome virus in France between 1989 and 2003. Arch. Virol. 2006;151 : 1713–1721. 16596329
14. Lopes AM, Gavier-Widén D, Le Gall-Reculé G, Esteves PJ, Abrantes J. Complete coding sequences of European brown hare syndrome virus (EBHSV) strains isolated in 1982 in Sweden. Archives of Virology 2013;158 : 2193–2196. doi: 10.1007/s00705-013-1714-7 23640583
15. Lopes AM, Capucci L, Gavier-Widén D, Le Gall-Reculé G, Brocchi E, Barbieri I, Quéméner A, Le Pendu J, Geoghegan JL, Holmes EC, Esteves PJ, Abrantes J. Molecular evolution and antigenic variation of European brown hare syndrome virus (EBHSV). Virology 2014;468–470 : 104–12. doi: 10.1016/j.virol.2014.08.002 25155199
16. Liu SJ, Xue HP, Pu BQ, Qian NH. A new viral disease in rabbit. Anim Husb Vet Med. 1984;16 : 253–255.
17. Gavier-Widén D, Mörner T. Epidemiology and diagnosis of the European brown hare syndrome in Scandinavian countries: a review. Rev Sci Tech. 1991;10 : 453–458. 1760585
18. Xu WY. Viral haemorrhagic disease of rabbits in the People’s Republic ofChina: epidemiology and virus characterisation. Rev Sci Tech 1991;10 : 393–408. 1760583
19. Gregg DA, House C, Meyer R, Berninger M. Viral haemorrhagic disease of rabbits in Mexico: epidemiology and viral characterization. Rev Sci Tech 1991;10 : 435–451. 1760584
20. Mitro S, Krauss H. Rabbit hemorrhagic disease: a review with special reference to its epizootiology. Eur J Epidemiol. 1993;9 : 70–78. 8386104
21. Villafuerte R, Calvete C, Blanco JC, Lucientes J. Incidence of viral hemorrhagic disease in wild rabbit populations in Spain. Mammalia. 1995;59 : 651–660.
22. Marchandeau S, Chantal J, Portejoie Y, Barraud S, Chaval Y. Impact of viral haemorrhagic disease on a wild population of European rabbits in France. Journal of Wildlife Diseases. 1998;34 : 429–435. 9706551
23. Marchandeau S, Chaval Y, Le Goff E. Prolonged decline in the abundance of wild European rabbits Oryctolagus cuniculus and high immunity level over three years following the arrival of rabbit haemorrhagic disease. Wildlife Biology. 2000;6 : 141–147.
24. Delibes-Mateos M, Delibes M, Ferreras P, Villafuerte R. Key role of European rabbits in the conservation of the Western Mediterranean basin hotspot. Conserv Biol. 2008;22 : 1106–17. doi: 10.1111/j.1523-1739.2008.00993.x 18680504
25. Delibes-Mateos M, Ferreira C, Carro F, Escudero MA, Gortázar C. Ecosystem effects of variant rabbit hemorrhagic disease virus, Iberian Peninsula. Emerg Infect Dis. 2014;20 : 2166–2168. doi: 10.3201/eid2012.140517 25417710
26. Capucci L, Fusi P, Lavazza A, Pacciarini ML, Rossi C. Detection and preliminary characterization of a new rabbit calicivirus related to rabbit hemorrhagic disease virus but nonpathogenic. J Virol. 1996;70 : 8614–8623. 8970986
27. Moss SR, Turner SL, Trout RC, White PJ, Hudson PJ, Desai A, Armesto M, Forrester NL, Gould EA. Molecular epidemiology of Rabbit haemorrhagic disease virus. J Gen Virol. 2002; 83 : 2461–2467. 12237428
28. Forrester NL, Trout RC, Gould EA. Benign circulation of rabbit haemorrhagic disease virus on Lambay Island, Eire. Virology. 2007;358 : 18–22. 17049958
29. Forrester NL, Boag B, Buckley A, Moureau G, Gould EA. Co-circulation of widely disparate strains of Rabbit haemorrhagic disease virus could explain localized epidemicity in the United Kingdom. Virology. 2009;393 : 42–48. doi: 10.1016/j.virol.2009.07.008 19692104
30. Strive T, Wright JD, Robinson AJ. Identification and partial characterization of a new Lagovirus in Australian wild rabbits. Virology. 2009;384 : 97–105. doi: 10.1016/j.virol.2008.11.004 19049842
31. Bergin IL, Wise AG, Bolin SR, Mullaney TP, Kiupel M, Maes RK. Novel calicivirus identified in rabbits, Michigan, USA. Emerg. Infect. Dis. 2009;15 : 1955–1962. doi: 10.3201/eid1512.090839 19961675
32. Abrantes J, Esteves PJ. Not-so-novel Michigan rabbit calicivirus. Emerg Infect Dis. 2010;16 : 1331–1332. doi: 10.3201/eid1608.091803 20678344
33. Jahnke M, Holmes EC, Kerr PJ, Wright JD, Strive T. Evolution and phylogeography of the nonpathogenic calicivirus RCV-A1 in wild rabbits in Australia. 2010;84 : 12397–404.
34. Le Gall-Recule G, Zwingelstein F, Fages MP, Bertagnoli S, Gelfi J, Aubineau J, Roobrouck A, Botti G, Lavazza A, Marchandeau S. Characterisation of a non-pathogenic and non-protective infectious rabbit lagovirus related toRHDV. Virology. 2011;410 : 395–402. doi: 10.1016/j.virol.2010.12.001 21195443
35. Le Gall-Recule G, Zwingelstein F, Laurent S, de Boisseson C, Portejoie Y, Rasschaert D. Phylogenetic analysis of rabbit haemorrhagic disease virus in France between 1993 and 2000, and the characterisation of RHDV antigenic variants. Arch Virol; 2003;148 : 65–81. 12536296
36. Gould AR, Kattenbelt JA, Lenghaus C, Morrissy C, Chamberlain T, Collins BJ, Westbury HA. The complete nucleotide sequence of rabbit haemorrhagic disease virus (Czech strain V351): use of the polymerase chain reaction to detect replication in Australian vertebrates and analysis of viral population sequence variation. Virus Res. 1997;47 : 7–17. 9037732
37. Muller A, Freitas J, Silva E, Le Gall-Recule G, Zwingelstein F, Abrantes J, Esteves PJ, Alves PC, van der Loo W, Kolodziejek J, Nowotny N, Thompson G. Evolution of rabbit haemorrhagic disease virus (RHDV) in the European rabbit (Oryctolagus cuniculus) from the Iberian Peninsula. Vet Microbiol. 2009;135 : 368–373. doi: 10.1016/j.vetmic.2008.09.057 18977620
38. Milton ID, Vlasak R, Nowotny N, Rodak L, Carter MJ. Genomic 3’ terminalsequence comparison of three isolates of rabbit haemorrhagic disease virus. FEMS Microbiol Lett. 1992;72 : 37–42. 1497750
39. Boga JA, Casais R, Marin MS, Martin-Alonso JM, Carmenes RS, Prieto M, Parra F. Molecular cloning, sequencing and expression in Escherichia coliof the capsid protein gene from rabbit haemorrhagic disease virus (Spanish isolate AST/89). J Gen Virol. 1994;75 : 2409–2413. 8077941
40. Parra F, Boga JA, Marin MS, Casais R. The amino terminal sequence of VP60 from rabbit hemorrhagic disease virus supports its putative subgenomic origin. Virus Res. 1993, 27 : 219–228. 8488721
41. Le Gall G, Arnauld C, Boilletot E, Morisse JP, Rasschaert D. Molecular epidemiology of rabbit haemorrhagic disease virus outbreaks in France during 1988 to 1995. J Gen Virol. 1998;79 : 11–16. 9460916
42. Matiz K, Ursu K, Kecskemeti S, Bajmocy E, Kiss I. Phylogenetic analysis of rabbit haemorrhagic disease virus (RHDV) strains isolated between 1988 and 2003 in eastern Hungary. Arch. Virol. 2006;151 : 1659–1666. 16521047
43. Tian L, Liao J, Li JW, Zhou WR, Zhang XL, Wang HN. Isolation and identification of a non-haemagglutinating strain of rabbit hemorrhagic disease virus from China and sequence analysis for the VP60 Gene. Virus Genes. 2007;35 : 745–752. 17705093
44. Guittre C, Baginski I, Le Gall G, Prave M, Trepo C, Cova L. Detection of rabbit haemorrhagic disease virus isolates and sequence comparison of the N-terminus of the capsid protein gene by the polymerase chain reaction. Res Vet Sci. 1995;58 : 128–132. 7761690
45. Rasschaert D, Huguet S, Madelaine MF, Vautherot JF. Sequence and genomic organization of a rabbit hemorrhagic disease virus isolated from a wild rabbit. Virus Genes. 1995;9 : 121–132. 7732658
46. Viaplana E, Villaverde A. Microheterogeneity of p60 capsid protein andthe encoding gene among contemporary isolates of rabbit hemorrhagic disease virus. Virus Genes. 1996;12 : 189–192. 8879136
47. Asgari S, Hardy JR, Cooke BD. Sequence analysis of rabbit haemorrhagic disease virus (RHDV) in Australia: alterations after its release. Arch. Virol. 1999;144 : 135–145. 10076514
48. van de Bildt MW, van Bolhuis GH, van Zijderveld F, van Riel D, Drees JM, Osterhaus AD, Kuiken T. Confirmation and phylogenetic analysis of rabbit hemorrhagic disease virus in free-living rabbits from the Netherlands. J Wildl Dis. 2006;42 : 808–812. 17255447
49. Yang L, Wang F, Hu B, Xue J, Hu Y, Zhou B, Wang D, Xu W. Development of an RT-PCR for rabbit hemorrhagic disease virus (RHDV) and the epidemiology of RHDV in three eastern provinces of China. J. Virol. Methods 2008;151 : 24–29. doi: 10.1016/j.jviromet.2008.04.003 18499276
50. Hukowska-Szematowicz B, Pawlikowska M, Deptula W. Genetic variability of Czech and German RHD virus strains. Pol. J. Microbiol. 2009;58 : 237–245. 19899617
51. Kerr PJ, Kitchen A, Holmes EC. Origin and phylodynamics of rabbit hemorrhagic disease virus. J Virol. 2009;83 : 12129–12138. doi: 10.1128/JVI.01523-09 19759153
52. Alda F, Gaitero T, Suárez M, Merchán T, Rocha G, Doadrio I. Evolutionary history and molecular epidemiology of rabbit haemorrhagic disease virus in the Iberian Peninsula and Western Europe. BMC Evol Biol. 2010;10 : 347. doi: 10.1186/1471-2148-10-347 21067589
53. Abrantes J, Lopes AM, Esteves PJ. Complete genomic sequences of rabbit hemorrhagic disease virus G1 strains isolated in the European rabbit original range. J Virol. 2012;86 : 13886. doi: 10.1128/JVI.02683-12 23166278
54. Esteves PJ, Lopes AM, Magalhães MJ, Pinheiro A, Gonçalves D, Abrantes J. Rabbit hemorrhagic disease virus detected in Pico, Azores, Portugal, revealed a unique endemic strain with more than 17 years of independent evolution. Viruses. 2014; 6 : 2698–2707. doi: 10.3390/v6072698 25025834
55. Kovaliski J, Sinclair R, Mutze G, Peacock D, Strive T, Abrantes J, Esteves PJ, Holmes EC. Molecular epidemiology of Rabbit Haemorrhagic Disease Virus in Australia: when one became many. Mol Ecol. 2014;23 : 408–20 doi: 10.1111/mec.12596 24251353
56. Capucci L, Fallacara F, Grazioli S, Lavazza A, Pacciarini ML, Brocchi E. A further step in the evolution of rabbit hemorrhagic disease virus: the appearance of the first consistent antigenic variant. Virus Res. 1998; 58 : 115–126. 9879768
57. Farnos O, Rodriguez D, Valdes O, Chiong M, Parra F, Toledo JR, Fernandez E,Lleonart R, Suarez M. Molecular and antigenic characterization of rabbit hemorrhagic disease virus isolated in Cuba indicates a distinct antigenic subtype. Arch Virol. 2007;152 : 1215–1221. 17334949
58. Schirrmeier H, Reimann I, Kollner B, Granzow H. Pathogenic, antigenic and molecular properties of rabbit haemorrhagic disease virus (RHDV) isolated from vaccinated rabbits: detection and characterization of antigenic variants. Arch Virol. 1999;144 : 719–735. 10365163
59. McIntosh MT, Behan SC, Mohamed FM, Lu Z, Moran KE, Burrage TG, Neilan JG, Ward GB, Botti G, Capucci L, Metwally SA. A pandemic strain of calicivirus threatens rabbit industries in the Americas. Virol J. 2007;4 : 96. 17910765
60. Oem JK, Lee KN, Roh IS, Lee KK, Kim SH, Kim HR, Park CK, Joo YS. Identification and characterization of rabbit hemorrhagic disease virus genetic variants isolated in Korea. J Vet Med Sci 2009;71 : 1519–1523. 19959905
61. Abrantes J, Lopes AM, Dalton KP, Parra F, Esteves PJ. Detection of RHDVa on the Iberian Peninsula: isolation of an RHDVa strain from a Spanish rabbitry. Arch Virol. 2014;159 : 321–326. doi: 10.1007/s00705-013-1808-2 23942953
62. Le Gall-Reculé G, Zwingelstein F, Boucher S, Le Normand B, Plassiart G, Portejoie Y, Decors A, Bertagnoli S, Guérin JL, Marchandeau S. Detection of a new variant of rabbit haemorrhagic disease virus in France. Vet Rec. 2011;168 : 137–138.
63. Dalton KP, Nicieza I, Balseiro A, Muguerza MA, Rosell JM, Casais R, Álvarez ÁL, Parra F. Variant rabbit hemorrhagic disease virus in young rabbits, Spain. Emerg Infect Dis. 2012;18 : 2009–2012. doi: 10.3201/eid1812.120341 23171812
64. Le Gall-Reculé G, Lavazza A, Marchandeau S, Bertagnoli S, Zwingelstein F, Cavadini P, Martinelli N, Lombardi G, Guérin JL, Lemaitre E, Decors A, Boucher S, Le Normand B, Capucci L. Emergence of a new lagovirus related to Rabbit Haemorrhagic Disease Virus. Vet Res. 2013;44 : 81. doi: 10.1186/1297-9716-44-81 24011218
65. Puggioni G, Cavadini P, Maestrale C, Scivoli R, Botti G, Ligios C, Le Gall-Reculé G, Lavazza A, Capucci L. The new French 2010 Rabbit Hemorrhagic Disease Virus causes an RHD-like disease in the Sardinian Cape hare (Lepus capensis mediterraneus).Vet Res. 2013;44 : 96. doi: 10.1186/1297-9716-44-96 24099575
66. Abrantes J, Lopes AM, Dalton KP, Melo P, Correia JJ, Ramada M, Alves PC, Parra F, Esteves PJ. New variant of rabbit hemorrhagic disease virus, Portugal, 2012–2013. Emerg Infect Dis. 2013;19 : 1900–1902. doi: 10.3201/eid1911.130908 24206671
67. Dalton KP, Nicieza I, Abrantes J, Esteves PJ, Parra F. Spread of new variant RHDV in domestic rabbits on the Iberian Peninsula. Vet Microbiol. 2014;169 : 67–73. doi: 10.1016/j.vetmic.2013.12.015 24461551
68. Baily JL, Dagleish MP, Graham M, Maley M, Rocchi MS. RHDV variant 2 presence detected in Scotland. Vet Rec. 2014;174 : 411.
69. Simpson V, Everest D, Westcott D. RHDV variant 2 and Capillaria hepatica infection in rabbits. Vet Rec. 2014;174 : 486.
70. Camarda A, Pugliese N, Cavadini P, Circella E, Capucci L, Caroli A, Legretto M, Mallia E, Lavazza A. Detection of the new emerging rabbit haemorrhagic disease type 2 virus (RHDV2) in Sicily from rabbit (Oryctolagus cuniculus) and Italian hare (Lepus corsicanus). Res Vet Sci. 2014;97 : 642–645. doi: 10.1016/j.rvsc.2014.10.008 25458493
71. Lopes AM, Correia J, Abrantes J, Melo P, Ramada M, Magalhães MJ, Alves PC, Esteves PJ. Is the new variant RHDV replacing genogroup 1 in Portuguese wild rabbit populations? Viruses. 2014 : 7: 27–36. doi: 10.3390/v7010027 25559218
72. Dalton KP, Abrantes J, Lopes AM, Nicieza I, Álvarez ÁL, Esteves PJ, Parra F Complete genome sequence of two rabbit hemorrhagic disease virus variant b isolates detected on the Iberian Peninsula. Arch. Virol. 2015; 160 : 877–881. doi: 10.1007/s00705-014-2329-3 25577166
73. Westcott DG, Choudhury B. Rabbit haemorrhagic disease virus 2-like variant in Great Britain. Vet Rec. 2015;176 : 74. doi: 10.1136/vr.102830 25477331
74. Lopes AM, Dalton KP, Magalhães MJ, Parra F, Esteves PJ, Holmes EC, Abrantes J. Full genomic analysis of new variant Rabbit Hemorrhagic Disease Virus (RHDVb) revealed multiple recombination events. J Gen Virol. 2015;96 : 1309–1319. doi: 10.1099/vir.0.000070 25626685
75. Duarte M, Henriques M, Barros SC, Fagulha T, Ramos F, Luís T, Fevereiro M, Benevides S, Flor L, Barros SV, Bernardo S. Detection of RHDV variant 2 in the Azores. Vet Rec. 2015;176 : 130. doi: 10.1136/vr.h497 25634926
76. Calvete C, Sarto P, Calvo AJ, Monroy F, Calvo JH. Could the new Rabbit haemorrhagic disease virus variant (RHDVb) be fully replacing classical RHD strains in the Iberian Peninsula? World Rabbit Sci. 2014;22 : 91.
77. Almeida T, Lopes AM, Magalhães MJ, Neves F, Pinheiro A, Gonçalves D, Leitão M, Esteves PJ, Abrantes J. Tracking the evolution of the G1/RHDVb recombinant strains introduced from the Iberian Peninsula to the Azores islands, Portugal. Infection, Genetics and Evolution 2015;34 : 307–313. doi: 10.1016/j.meegid.2015.07.010 26165506
78. Poli A, Nigro M, Gallazzi D, Siroli G, Lavazza A, Gelmetti D. Acute hepatosis in the European Brown Hare (Lepus europaeus) in Italy. J Wild Dis. 1991;27 : 621–629.
79. Zanni ML, Benassi ML, Scicluna MT, Lavazza A, Capucci L. Clinical evolution and diagnosis of an episode of European Brown Hare Syndrome (EBHS) in hares reared in captivity. Rev. sci. tech. Off. int. Epiz. 1993;12 : 931–940.
80. Scicluna MT, Capucci L, Lavazza A. European brown hare syndrome in northern Italy: results of a virological and serological survey. Rev. sci. tech. Off. int. Epiz. 1994;13 : 893–904.
81. Billinis C, Psychas V, Tontis DK, Spyrou V, Birtsas PK, Sofia M, Likotrafitis F, Maslarinou OM, Kanteres D. European brown hare syndrome in wild European brown hares from Greece. J. Wildl. Dis. 2005;41 : 783–786. 16456168
82. Chasey D, Duff P. European brown hare syndrome and associated virus particles in the UK. Vet. Rec. 1990;126 : 623–624. 2165701
83. Frölich K, Fickel J, Ludwig A, Lieckfeldt D, Streich WJ, Jurčík R, Slamečka J, Wibbelt G. New variants of European brown hare syndrome virus strains in free ranging European brown hares (Lepus europaeus) from Slovakia. J. Wildl. Dis. 2007;43 : 89–96. 17347397
84. Frölich K, Meyer HHD, Pielowski Z, Ronsholt L, Seck-Lanzendorf Sv, Stolte M. European brown hare syndrome in free-ranging hares in Poland. J. Wildl. Dis. 1996;32 : 280–285. 8722266
85. Lavazza A, Vecchi G. Osservazioni su alcuni episodi di mortalità nelle lepri. Evidenziazione al microscopio elettronico di una particella virale. Nota preliminare. Selezione Veterinaria. 1989;30 : 461–467.
86. Duff JP, Chasey D, Munro R, Wooldridge M. European brown hare syndrome in England. Vet. Rec. 1994; 134 : 669–673. 7941275
87. Duff JP, Whitwell K, Chasey D. The emergence and epidemiology of European brown hare syndrome in the UK, Proceedings of the First International Symposium on Caliciviruses, United Kingdom, 1997;pp. 176–181.
88. Ros Bascuñana C, Nowotny N, Belák S. Detection and differentiation of rabbit hemorrhagic disease and European brown hare syndrome viruses by amplification of VP60 genomic sequences from fresh and fixed tissue specimens. J. Clin. Microbiol. 1997;35 : 2492–2495. 9316895
89. Rodak L, Smid B, Valicek L, Vesely T, Stepanek J, Hampl J, Jurak E. Enzyme-linked immunosorbent assay of antibodies to rabbit haemorrhagic disease virus and determination of its major structural proteins. J. Gen. Virol. 1990;71 : 1075–1080. 2161044
90. O'Keefe JS, Tempero JE, Motha MXJ, Hansen MF, Atkinsona PH. Serology of rabbit haemorrhagic disease virus in wild rabbits before and after relase of the virus in New Zealand. Vet Microbiol. 1999;66 : 29–40. 10223320
91. Cooke BD, Robinson AJ, Merchant JC, Nardin A, Capucci L. Use of ELISAs in field studies of rabbit haemorrhagic disease (RHD) in Australia. Epidemiol Infect. 2000;124 : 563–576. 10982081
92. Robinson AJ, Kirkland PD, Forrester RI, Capucci L, Cooke BD, Philbey AW. Serological evidence for the presence of a calicivirus in Australian wild rabbits, Oryctolagus cuniculus, before the introduction of rabbit haemorrhagic disease virus (RHDV): its potential influence on thespecificity of a competitive ELISA for RHDV. Wildl Res. 2002;29 : 655–662.
93. Fenner F, Ratcliffe FN. Myxomatosis; Cambridge University Press. 1965; Cambridge, UK.
94. Lavazza A, Cavadini P, Barbieri I, Tizzani P, Pinheiro A, Abrantes J, Esteves PJ, Grilli G, Gioia E, Zanoni M, Meneguz P, Guitton JS, Marchandeau S, Chiari M, Capucci L. Field and experimental data indicate that the eastern cottontail (Sylvilagus floridanus) is susceptible to infection with European brown hare syndrome (EBHS) virus and not with rabbit haemorrhagic disease (RHD) virus. Vet Res. 2015; 46 : 13. doi: 10.1186/s13567-015-0149-4 25828691
95. Lopes AM, Marques S, Silva E, Magalhaes MJ, Pinheiro A, Alves PC, Le Pendu J, Esteves PJ, Thompson G, Abrantes J. Detection of RHDV strains in the Iberian hare (Lepus granatensis): earliest evidence of rabbit lagovirus cross-species infection. Vet Res. 2014;45 : 94. doi: 10.1186/s13567-014-0094-7 25248407
96. Abrantes J, Esteves PJ, van der Loo W. Evidence for recombination in the major capsid gene VP60 of the rabbit haemorrhagic disease virus (RHDV). Archives of Virology 2008;153 : 329–335. doi: 10.1007/s00705-007-1084-0 18193156
97. Forrester NL, Moss SR, Turner SL, Schirrmeier H, Gould EA. Recombination in Rabbit haemorrhagic disease virus: possible impact on evolution and epidemiology. Virology 2008;376 : 390–396. doi: 10.1016/j.virol.2008.03.023 18455748
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek Increased Susceptibility of Humanized NSG Mice to Panton-Valentine Leukocidin and Skin InfectionČlánek Phosphorylation of a Myosin Motor by TgCDPK3 Facilitates Rapid Initiation of Motility during egress
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2015 Číslo 11- Stillova choroba: vzácné a závažné systémové onemocnění
- Perorální antivirotika jako vysoce efektivní nástroj prevence hospitalizací kvůli COVID-19 − otázky a odpovědi pro praxi
- Diagnostika virových hepatitid v kostce – zorientujte se (nejen) v sérologii
- Jak souvisí postcovidový syndrom s poškozením mozku?
- Familiární středomořská horečka
-
Všechny články tohoto čísla
- Parasite Glycobiology: A Bittersweet Symphony
- On the Discovery of TOR As the Target of Rapamycin
- Broadening of Virus-Specific CD8 T-Cell Responses Is Indicative of Residual Viral Replication in Aviremic SIV Controllers
- PML/TRIM19-Dependent Inhibition of Retroviral Reverse-Transcription by Daxx
- Cleavage of a Neuroinvasive Human Respiratory Virus Spike Glycoprotein by Proprotein Convertases Modulates Neurovirulence and Virus Spread within the Central Nervous System
- Kaposi’s Sarcoma-Associated Herpesvirus (KSHV) Induces the Oncogenic miR-17-92 Cluster and Down-Regulates TGF-β Signaling
- Interferon-α Subtypes in an Model of Acute HIV-1 Infection: Expression, Potency and Effector Mechanisms
- Perivascular Arrest of CD8 T Cells Is a Signature of Experimental Cerebral Malaria
- Targeting HIV Reservoir in Infected CD4 T Cells by Dual-Affinity Re-targeting Molecules (DARTs) that Bind HIV Envelope and Recruit Cytotoxic T Cells
- Evolution and Emergence of Enteroviruses through Intra- and Inter-species Recombination: Plasticity and Phenotypic Impact of Modular Genetic Exchanges in the 5’ Untranslated Region
- Interferon-γ Inhibits Ebola Virus Infection
- Dengue Virus Non-structural Protein 1 Modulates Infectious Particle Production via Interaction with the Structural Proteins
- P-Type Cyclin CYC3 Modulates Endomitotic Growth during Oocyst Development in Mosquitoes
- Diversity of across Evolutionary Scales
- 50 Years of Disease in Humans: The Dramatic Emergence of a Cluster of Novel Fungal Pathogens
- Worse Comes to Worst: Bananas and Panama Disease—When Plant and Pathogen Clones Meet
- Arenavirus Glycan Shield Promotes Neutralizing Antibody Evasion and Protracted Infection
- Infection-Induced Retrotransposon-Derived Noncoding RNAs Enhance Herpesviral Gene Expression via the NF-κB Pathway
- Structural Insight into Archaic and Alternative Chaperone-Usher Pathways Reveals a Novel Mechanism of Pilus Biogenesis
- Increased Susceptibility of Humanized NSG Mice to Panton-Valentine Leukocidin and Skin Infection
- Global Analysis of the Fungal Microbiome in Cystic Fibrosis Patients Reveals Loss of Function of the Transcriptional Repressor Nrg1 as a Mechanism of Pathogen Adaptation
- The Transcription and Translation Landscapes during Human Cytomegalovirus Infection Reveal Novel Host-Pathogen Interactions
- The N-terminal Helical Region of the Hepatitis C Virus p7 Ion Channel Protein Is Critical for Infectious Virus Production
- Activation of Type I and III Interferon Response by Mitochondrial and Peroxisomal MAVS and Inhibition by Hepatitis C Virus
- Hsp70 Isoforms Are Essential for the Formation of Kaposi’s Sarcoma-Associated Herpesvirus Replication and Transcription Compartments
- Distinct Upstream Role of Type I IFN Signaling in Hematopoietic Stem Cell-Derived and Epithelial Resident Cells for Concerted Recruitment of Ly-6C Monocytes and NK Cells via CCL2-CCL3 Cascade
- and Bats: Story of an Emerging Friendship
- Emergence of Pathogenicity in Lagoviruses: Evolution from Pre-existing Nonpathogenic Strains or through a Species Jump?
- Ebolavirus Evolution: Past and Present
- Host and Symbiont Jointly Control Gut Microbiota during Complete Metamorphosis
- Non-Human Primates Harbor Diverse Mammalian and Avian Astroviruses Including Those Associated with Human Infections
- Lactate Dehydrogenase Is Associated with the Parasitophorous Vacuole Membrane and Is a Potential Target for Developing Therapeutics
- Five Questions about Mycoviruses
- Phosphorylation of a Myosin Motor by TgCDPK3 Facilitates Rapid Initiation of Motility during egress
- Ethanolamine Signaling Promotes Niche Recognition and Adaptation during Infection
- Cross-Species Transmission and Differential Fate of an Endogenous Retrovirus in Three Mammal Lineages
- Memory Th1 Cells Are Protective in Invasive Infection
- Transcription Factor SomA Is Required for Adhesion, Development and Virulence of the Human Pathogen
- An -Methyltransferase Is Required for Infection of Tick Cells by
- RNA-seq Brings New Insights to the Intra-Macrophage Transcriptome of Typhimurium
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Dengue Virus Non-structural Protein 1 Modulates Infectious Particle Production via Interaction with the Structural Proteins
- On the Discovery of TOR As the Target of Rapamycin
- Parasite Glycobiology: A Bittersweet Symphony
- Broadening of Virus-Specific CD8 T-Cell Responses Is Indicative of Residual Viral Replication in Aviremic SIV Controllers
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



