-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Five Questions about Mycoviruses
article has not abstract
Published in the journal: . PLoS Pathog 11(11): e32767. doi:10.1371/journal.ppat.1005172
Category: Pearls
doi: https://doi.org/10.1371/journal.ppat.1005172Summary
article has not abstract
What Are Mycoviruses?
Mycoviruses are viruses that infect fungi. The first mycovirus was reported in 1962 from the cultivated mushroom, Agaricus bisporus; the infected mushrooms developed malformed fruiting bodies, grew slowly, and matured early, resulting in serious yield losses [1]. Like viruses that infect animals and plants, mycoviruses require the living cells of other organisms to replicate. While sharing some characteristics with animal and plant viruses, mycoviruses also have the following unique characteristics: (1) most mycoviruses lack an extracellular route for infection; (2) mycoviruses are transmitted intercellularly only through cell division, sporulation, and cell fusion; and (3) mycoviruses apparently lack a movement protein, which is essential for the life cycle of animal and plant viruses.
According to the most recent report concerning virus taxonomy, the genome of most mycoviruses consists of double-stranded RNA (dsRNA), while the genome of about 30% of mycoviruses is composed of a positive, single-stranded RNA (+ssRNA) [2]. A geminivirus-related DNA mycovirus was recently reported for the first time [3]. Mycoviruses have been detected in all of the major phyla of fungi, including the Chytridiomycota, Zygomycota, Ascomycota, Deuteromycota, and Basidiomycota [1]. Although many mycoviruses and their host fungi have been identified, many mycoviruses undoubtedly remain unknown. Recently developed metagenomic approaches will be useful for detecting and identifying new mycoviruses.
How Did Mycoviruses Arise?
Mycoviruses have been classified into seven linear dsRNA families (Chrysoviridae, Endornaviridae, Megabirnaviridae, Quadriviridae, Partitiviridae, Reoviridae, and Totiviridae), five linear positive-sense ssRNA families (Alphaflexiviridae, Barnaviridae, Gammaflexiviridae, Hypoviridae, and Narnaviridae), unclassified linear negative-sense ssRNA, and circular ssDNA virus [1]. Mycoviruses have usually been detected by the purification of dsRNA molecules because many mycoviruses produce dsRNA or dsRNA replicative intermediates in their fungal hosts [4]. The profiling of purified dsRNA has demonstrated the diversity of mycoviruses. Several dsRNA-containing fungal isolates showed multiple dsRNA patterns that might represent segmented viral genomes, mixed infections of more than two viruses, or defective RNAs [4,5]. The levels of dsRNA and/or ssRNA mycoviruses may be overestimated, however, by the use of dsRNA-enriching protocols.
Phylogenetic studies have demonstrated that viruses in the same taxonomic families can infect diverse hosts, including fungi, plants, animals, and protozoa [1,6]. For example, a recent taxonomic review indicated that the family Partitiviridae contained dsRNA viruses that infect plants, fungi, or protozoa [7]. In addition, the positive-strand RNA mycoviruses, which include Cryphonectria parasitica hypovirus 1–4 (CHV1–4), Fusarium graminearum virus 1 (FgV1), and Botrytis virus X, are phylogenetically related to plant viruses. Their genomic organization and expression strategy resemble those of plant potyviruses or potex-like viruses [1,8]. Moreover, Sclerotinia scerotiorum RNA virus L is closely related to the human pathogen hepatitis E virus and rubi-like viruses [3].
Two major hypotheses have been proposed to explain the origin of mycoviruses [4]. The “ancient coevolution hypothesis” states that although the origin of mycoviruses is unknown, the association between mycoviruses and fungi is ancient and reflects long-term coevolution. The “plant virus hypothesis,” in contrast, suggests that mycoviruses originated relatively recently from plant viruses; i.e., the original mycovirus was a plant virus that moved from plant to fungus within the same host plant. Similar scenarios might also explain the origin of plant viruses; i.e., some plant viruses may have originated from mycoviruses that moved from fungus to plant [7]. Because convincing data are lacking, however, the origin of mycoviruses remains a mystery.
What Do Mycoviruses Do in Their Fungal Hosts?
Although mycoviruses are common among fungi, they usually remain latent and seldom induce symptoms [4]. Some mycoviruses, however, cause dramatic changes in their hosts, including irregular growth, abnormal pigmentation, and altered sexual reproduction [1,3,4,9]. Perhaps the most important effect is the reduced virulence—i.e., hypovirulence—of plant-pathogenic fungi. Hypovirulence has attracted much attention because it has the potential to reduce the losses of crops and forests caused by plant-pathogenic fungi [9,10].
Over the last 50 years, research on mycoviruses that induce hypovirulence has greatly increased our understanding of mycoviruses and their interactions with their plant-pathogenic fungal hosts [1]. Much of the early research on mycoviruses concerned the interaction between hypovirus CHV1 and the chestnut blight fungus Cryphonectria parasitica. Infection by CHV1 resulted in reduced growth and abnormal pigmentation in C. parasitica. Most importantly, CHV1 induced hypovirulence in C. parasitica [9,11]. Along with the CHV1, the mycoviruses that infect the important plant-pathogenic fungus F. graminearum have also been detected and studied [12]. Among them, FgV1 confers hypovirulence to F. graminearum just as CHV1 confers hypovirulence to C. parasitica [12]. When infected by FgV1, F. graminearum shows decreased vegetative growth, abnormal pigmentation, and reduced mycotoxin production [12]. Interestingly, FgV1 can also be transmitted to C. parasitica and to other Fusarium species, and FgV1 induces more severe hypovirulence than CHV1 in C. parasitica [13].
In addition to the mycoviruses mentioned in previous paragraph, several hypovirulence-associated mycoviruses, such as Sclerotinia sclerotiorum hypoviruses, Helminthosporium victoriae viruses, and Rosellinia necatrix viruses, have been detected and studied using reverse genetic approaches [4,10]. Because these viruses lack an extracellular phase, researchers have investigated transfection methods using purified virus particles, full-length viral cDNA clones, and in vitro RNA transcripts [14–16]. These infection assays will facilitate identification of viral and/or host factor(s) involved in symptom induction or virus replication for many mycovirus–host systems. These methods can also be used to expand the host ranges of some mycoviruses.
As mentioned earlier, mycoviruses are transmitted intercellularly only through hyphal anastomosis or spores. Virus transmission between different strains is restricted by fungal vegetative incompatibility (vic). Vegetative incompatibility is an obstacle in the use of hypovirulent mycoviruses as biological control agents. Recent research has demonstrated that the seven vic genes associated with five of six vic loci in C. parasitica contribute to incompatibility and affect virus transmission [17].
Although much of the research concerning mycoviruses has dealt with hypovirulence of pathogenic fungal hosts, mycoviruses of yeasts and nonpathogenic fungi are also important. Several dsRNA and ssRNA viruses have been found in the yeast Saccharomyces cerevisiae [18]. Among them, Saccharomyces cerevisiae virus L-A (ScV-L-A) and its killer toxin-encoding satellites have been well characterized.
How Do Mycoviruses Change the Expression of Host Genes?
Recently developed analytical techniques have enabled research on mycoviruses to enter a new phase. Using genome-wide linkage analysis, for example, researchers have begun to answer the question, “How do mycoviruses affect their fungal hosts?” RNA-Seq–based, genome-wide expression analysis revealed totally distinct expression patterns of F. graminearum transcriptomes in response to infections by four phylogenetically different mycoviruses (FgV1–4) [19]. Among these viruses, FgV3 and FgV4 did not cause any visible changes in the phenotypes of the host fungus even though they caused equal or greater changes in transcriptome expression levels than FgV1 and FgV2, which did cause visible changes in the host phenotype [19]. Further detailed study will undoubtedly increase our understanding of the interactions between mycoviruses and their hosts.
As obligate intracellular parasites, mycoviruses reprogram host cell metabolism in order to replicate within host cells and avoid antiviral responses. Identifying the crucial determinants in all steps of the viral life cycle is important for understanding the pathology caused by mycoviruses [20]. To identify host factors important in the interaction between mycovirus and fungus, researchers have used genome-wide approaches in their studies of Cryphonectria hypoviruses, Fusarium graminearum viruses, and Sclerotinia sclerotiorum debilitation-associated RNA virus [19–22]. The results revealed that the expression level of specific host genes differed not only between virus-free and virus-infected fungus isolates but also among viruses belonging to different groups and among virus strains that differed in the degree of hypovirulence that they induced.
These data indicate that mycoviruses depend on various host factors as well as cellular processes and pathways, including those related to metabolism, cellular transport, RNA processing, and signaling. The host genes that have been analyzed for biological function in mycovirus–host interactions are listed in Table 1. Among these host genes in C. parasitica, the transcription factors cpst12 and pro1 are down-regulated by CHV1 infection and are related to female sterility and viral replication, respectively [23]. In F. graminearum, the host gene hex1 is up-regulated by FgV1 infection and is associated with the accumulation of FgV1 RNA in host cells [24]. Viral RNA accumulation is decreased in Δhex1 and increased in the overexpression mutant compared to the wild type [24]. In yeast, the host protein Mak3p, which is an N-acetyl transferase, is required for acetylation of the major coat protein of ScV-L-A, and such acetylation is necessary for virus assembly. In addition, superkiller (Ski) proteins of yeast have anti-RNA virus activities [18]. Taken together, the combination of genome-wide and single-gene investigation will provide a more comprehensive understanding of the interactions between mycoviruses and fungal hosts.
Tab. 1. Host genes involved in interactions between mycoviruses and host fungi. 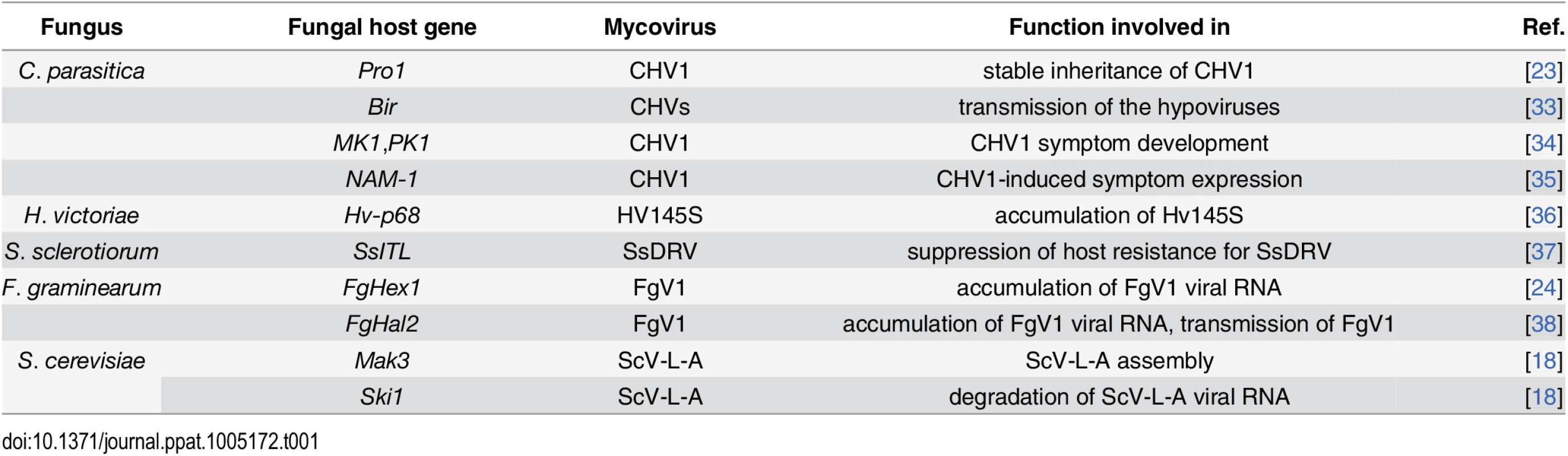
RNA silencing, which is termed quelling in fungi [25], has been studied in depth in the model fungus Neurospora crassa, and virus-induced quelling has been reported in C. parasitica and Aspergillus nidulans [25]. Because mycovirus genomes consist of RNA, quelling has obvious potential for fungal defense against mycovirus infection. In C. parasitica, dcl2 and agl2 genes, which are required for RNA-silencing antiviral defense responses, are induced by CHV1 infection [26]. Significant changes of expression level of some silencing-related genes (rdr1, dcl1, dcl2, and agl2) upon virus infection were also observed in Fusarium gramineraum viruses-infected F. graminearum [19]. Transcript accumulation levels of dcl2 and agl2 were decreased only in FgV1-infected F. graminearum, whereas accumulation levels of these transcripts were increased by FgV2–4 infections [19]. Therefore, it seems possible that RNA silencing pathways can be induced by mycoviruses. The key RNAi components responsible for the regulation of this antiviral mechanism in F. graminearum remain unclear. To reduce a host’s virus defense responses, many viruses, including mycoviruses, produce silencing suppressors that incapacitate RNA silencing. For example, the p29 protein of mycovirus CHV1 and the S10 gene product of Rosellinia necatrix mycoreovirus 3 function as silencing suppressors [11,27].
Should Mycoviruses Be Regarded As Harmful or Beneficial?
Most of the initial research on mycoviruses concerned their identification and their effects on commercial mushrooms and other valued fungi. Although mycoviruses are considered undesirable when they attack commercial mushrooms, they are considered beneficial when they act as biological control agents of fungal pathogens in economically important plants. Several approaches using hypovirulent strains have been attempted to manage fungal diseases in plants [3,9,10]. In the 1980s, spores of C. parasitica containing hypovirus were artificially introduced into fungal populations to control chestnut blight. This approach completely failed in forests but was successful in orchards in eastern North America and in Europe [9]. These differences in efficacy resulted from differences in vegetative compatibility among fungal isolates or from the properties of the hypoviruses [17]. These results suggest that development of mycoviruses as effective biological control agents may require consideration of multiple factors, including both host and virus properties. Sclerotinia sclerotiorum hypovirulence-associated DNA virus 1 was recently applied to control rapeseed stem rot caused by Sclerotinia sclerotiorum. This hypovirulent strain suppressed the disease, whether it was applied as a suspension of virus-infected hyphal fragments or virus particles [3,10]. Several challenges must be overcome in the use of hypovirulent strains to control plant-pathogenic fungi. As noted previously, one challenge is vegetative incompatibility, which prevents mycovirus transmission from a hypovirulent strain to a target strain. Another challenge is the potential lack of fitness of the hypovirulent strain. These barriers to achieving biological control with mycoviruses are likely to be overcome as research increases our understanding of mycoviruses and their interactions with host fungi and the environment [17].
As mentioned earlier, our understanding of the interactions between mycoviruses and their fungal hosts has been enriched by research on those mycoviruses that induce hypovirulence [9,10]. However, many mycoviruses do not significantly affect their fungal hosts. This suggests that these viruses may be adapted to living with their hosts for long periods and that the association may even benefit both mycovirus and fungus [28,29]. In yeast, “the killer phenomenon” is caused by the combined presence of cytoplasmically inherited dsRNA virus and satellites or DNA virus-like elements (VLEs) [18]. Although these viruses do not induce symptoms in their hosts, they do substantially affect host biology. A recent report suggested that killer systems are so beneficial to their hosts that they have resulted in the loss of host RNAi systems [30]. In another recent report, Kast et al. [31] found that the auto-selection of cytoplasmic virus-like elements encoding toxin/antitoxin systems in the yeasts Pichia acaciae and Kluyveromyces lactis involves a nuclear barrier for immunity gene expression. These results indicate that symptomless or latent mycoviruses may have unknown functions in their hosts. Researchers have also described a three-way symbiotic relationship among a mycovirus, an endophytic fungus, and tropical panic grass; in the absence of the mycovirus, the endophytic fungus and grass cannot survive high soil temperatures [32]. Other mycoviruses that benefit their hosts probably remain to be discovered.
Although 50 years of research has enriched our understanding of mycoviruses, researchers still do not know how to initiate infection so as to determine cause and effect with respect to mycoviruses. As noted earlier, transfection methods and reverse genetic systems have been developed for several mycoviruses [14–16]. The future development of reverse genetics systems for many other mycoviruses should contribute to our understanding of mycovirus molecular biology and should facilitate the stable application of mycoviruses as biological control agents or as virus-based expression vectors. The reverse genetics system should overcome restrictions to horizontal virus transmission caused by fungal vegetative incompatibility and nonself recognition systems and should thus increase the use of hypoviruses in biological control. In addition, future studies are likely to continue to reveal important clues regarding novel role(s) of mycoviruses in host biology. Supported by continued advances in scientific technology, research on mycoviruses and their fungal hosts will provide new insights into the largely unknown world of mycoviruses.
Zdroje
1. Ghabrial SA, Castón JR, Jiang D, Nibert ML, Suzuki N (2015) 50-plus years of fungal viruses. Virology 479 : 356–368. doi: 10.1016/j.virol.2015.02.034 25771805
2. King AM, Adams MJ, Lefkowitz EJ (2011) Virus taxonomy: classification and nomenclature of viruses: Ninth Report of the International Committee on Taxonomy of Viruses: Elsevier.
3. Jiang D, Fu Y, Ghabrial SA (2013) Mycoviruses: Chapter eight—Viruses of the plant pathogenic fungus Sclerotinia sclerotiorum. Adv Virus Res 86 : 215–248. doi: 10.1016/B978-0-12-394315-6.00008-8 23498908
4. Pearson MN, Beever RE, Boine B, Arthur K (2009) Mycoviruses of filamentous fungi and their relevance to plant pathology. Mol Plant Pathol 10 : 115–128. doi: 10.1111/j.1364-3703.2008.00503.x 19161358
5. Herrero N, Dueñas E, Quesada-Moraga E, Zabalgogeazcoa I (2012) Prevalence and diversity of viruses in the entomopathogenic fungus Beauveria bassiana. Appl Environ Microbiol 78 : 8523–8530. doi: 10.1128/AEM.01954-12 23001673
6. Roossinck MJ (2014) Metagenomics of plant and fungal viruses reveals an abundance of persistent lifestyles. Front Microbiol 5 : 767–769. doi: 10.3389/fmicb.2014.00767 25628611
7. Nibert ML, Ghabrial SA, Maiss E, Lesker T, Vainio EJ, et al. (2014) Taxonomic reorganization of family Partitiviridae and other recent progress in partitivirus research. Virus Res 188 : 128–141. doi: 10.1016/j.virusres.2014.04.007 24768846
8. Kwon S-J, Lim W-S, Park S-H, Park M-R (2007) Molecular characterization of a dsRNA mycovirus, Fusarium graminearum virus-DK21, which is phylogenetically related to hypoviruses but has a genome organization and gene expression strategy resembling those of plant potex-like viruses. Mol Cell 23 : 304–315.
9. Nuss DL (2005) Hypovirulence: mycoviruses at the fungal–plant interface. Nat Rev Microbiol 3 : 632–642. 16064055
10. Xie J, Jiang D (2014) New Insights into mycoviruses and exploration for the biological control of crop fungal diseases. Annu Rev Phytopathol 52 : 45–68. doi: 10.1146/annurev-phyto-102313-050222 25001452
11. Craven M, Pawlyk D, Choi GH, Nuss DL (1993) Papain-like protease p29 as a symptom determinant encoded by a hypovirulence-associated virus of the chestnut blight fungus. J Virol 67 : 6513–6521. 8411354
12. Chu Y-M, Jeon J-J, Yea S-J, Kim Y-H, Yun S-H, et al. (2002) Double-stranded RNA mycovirus from Fusarium graminearum. Appl Environ Microbiol 68 : 2529–2534. 11976130
13. Lee K-M, Yu J, Son M, Lee Y-W, Kim K-H (2011) Transmission of Fusarium boothii mycovirus via protoplast fusion causes hypovirulence in other phytopathogenic fungi. PLoS One 6: e21629. doi: 10.1371/journal.pone.0021629 21738738
14. Marzano S-YL, Hobbs HA, Nelson BD, Hartman GL, Eastburn DM, et al. (2015) Transfection of Sclerotinia sclerotiorum with in vitro transcripts of a naturally occurring interspecific recombinant of Sclerotinia sclerotiorum hypovirus 2 significantly reduces virulence of the fungus. J Virol 89 : 5060–5071. doi: 10.1128/JVI.03199-14 25694604
15. Chen B, Choi GH, Nuss DL (1994) Attenuation of fungal virulence by synthetic infectious hypovirus transcripts. Science 264 : 1762–1764. 8209256
16. Choi GH, Nuss DL (1992) Hypovirulence of chestnut blight fungus conferred by an infectious viral cDNA. Science 257 : 800–803. 1496400
17. Choi GH, Dawe AL, Churbanov A, Smith ML, Milgroom MG, et al. (2012) Molecular characterization of vegetative incompatibility genes that restrict hypovirus transmission in the chestnut blight fungus Cryphonectria parasitica. Genetics 190 : 113–127. doi: 10.1534/genetics.111.133983 22021387
18. Wickner RB, Fujimura T, Esteban R (2013) Viruses and prion of Saccharomyces cerevisiae. Adv Virus Res 86 : 1–36. doi: 10.1016/B978-0-12-394315-6.00001-5 23498901
19. Lee K-M, Cho WK, Yu J, Son M, Choi H, et al. (2014) A Comparison of transcriptional patterns and mycological phenotypes following infection of Fusarium graminearum by four mycoviruses. PloS One 9: e100989. doi: 10.1371/journal.pone.0100989 24964178
20. Allen TD, Dawe AL, Nuss DL (2003) Use of cDNA microarrays to monitor transcriptional responses of the chestnut blight fungus Cryphonectria parasitica to infection by virulence-attenuating hypoviruses. Eukaryot Cell 2 : 1253–1265. 14665460
21. Li H, Fu Y, Jiang D, Li G, Ghabrial SA, et al. (2008) Down-regulation of Sclerotinia sclerotiorum gene expression in response to infection with Sclerotinia sclerotiorum debilitation-associated RNA virus. Virus Res 135 : 95–106. doi: 10.1016/j.virusres.2008.02.011 18384901
22. Cho WK, Yu J, Lee K - M, Son M, Min K, et al. (2012) Genome-wide expression profiling shows transcriptional reprogramming in Fusarium graminearum by Fusarium graminearum virus 1-DK21 infection. BMC Genomics 13 : 173–188. doi: 10.1186/1471-2164-13-173 22559730
23. Sun Q, Choi GH, Nuss DL (2009) Hypovirus-responsive transcription factor gene pro1 of the chestnut blight fungus Cryphonectria parasitica is required for female fertility, asexual spore development, and stable maintenance of hypovirus infection. Eukaryot Cell 8 : 262–270. doi: 10.1128/EC.00338-08 19114501
24. Son M, Lee K-M, Yu J, Kang M, Park JM, et al. (2013) The hex1 gene of Fusarium graminearum is required for fungal asexual reproduction and pathogenesis and for efficient viral RNA accumulation of Fusarium graminearum virus 1. J Virol 87 : 10356–10367. doi: 10.1128/JVI.01026-13 23864619
25. Chang S-S, Zhang Z, Liu Y (2012) RNA interference pathways in fungi: mechanisms and functions. Annu Rev Microbiol 66 : 305–323. doi: 10.1146/annurev-micro-092611-150138 22746336
26. Zhnag DX, Spiering Ml, Nuss DL (2014) Charaterizing the roles of Cryphonectria parasitica RNA-dependent RNA polymerase-like genes in antiviral defense, viral recombination and transposon transcript accumulation. PLoS One 9: e108653. doi: 10.1371/journal.pone.0108653 25268858
27. Yaegashi H, Yoshikawa N, Ito T, Kanematsu S (2013) A mycoreovirus suppresses RNA silencing in the white root rot fungus, Rosellinia necatrix. Virology 444 : 409–416. doi: 10.1016/j.virol.2013.07.010 23896640
28. Roossinck MJ (2011) The good viruses: viral mutualistic symbioses. Nat Rev Microbiol 9 : 99–108. doi: 10.1038/nrmicro2491 21200397
29. Márquez LM, Roossinck MJ (2012) Do persistent RNA viruses fit the trade-off hypothesis of virulence evolution? Curr Opin Virol 2 : 556–560. doi: 10.1016/j.coviro.2012.06.010 22819020
30. Drinnenberg IA, Fink GR, Bartel DP (2011) Compatibility with killer explains the rise of RNAi-deficient fungi. Science 333 : 1592. doi: 10.1126/science.1209575 21921191
31. Kast A, Voges R, Schroth M, Schaffrath R, Klassen, et al. (2015) Autoselection of cytoplasmic yeast virus like elements encoding toxin/antitoxin system involvoes a nuclear barrier for immunity gene expression. PLoS Genet 11: e1005005. doi: 10.1371/journal.pgen.1005005 25973601
32. Márquez LM, Redman RS, Rodriguez RJ, Roossinck MJ (2007) A virus in a fungus in a plant: three-way symbiosis required for thermal tolerance. Science 315 : 513–515. 17255511
33. Gao K, Xiong Q, Xu J, Wang K, Wang K (2013) CpBir1 is required for conidiation, virulence and anti-apoptotic effects and influences hypovirus transmission in Cryphonectria parasitica. Fungal Genet Biol 51 : 60–71. doi: 10.1016/j.fgb.2012.09.011 23084963
34. Park S-M, Choi E-S, Kim M-J, Cha B-J, Yang M-s, et al (2004) Charaterization of HOG1 homologue, CpMK1, from Cryphonectria parasitica and evidence for hypovirus mediated perturbation of its phosphorylation in response to hypertonic stress. Mol Microbiol 51 : 1267–1277. 14982623
35. Faruk MI, Eusebio-Cope A, Suzuki N (2008) A host factor involved in hypovirus symptom expression in the chestnut blight fungus, Cryphonectria parasitica. J Virol 82 : 740–754. 17977965
36. Soldevila AI, Havens WM, Ghabrial SA (2000) A cellular protein with ana RNA-binding activity co-purifies with viral dsRNA from mucovirus-infect Helminthosporium victoriae. Virol 272 : 183–190.
37. Zue W, Wei W, Fu Y, Cheng J, Xie J, et al (2013) A secretory protein of necrotrophic fungus Sclerotinia sclerotiorum that suppresses host resistance. PLoS One 8: e53901. doi: 10.1371/journal.pone.0053901 23342034
38. Yu J, Lee K-M, Son M, Kim K-H (2014) Effects of the deletion and over-expression of Fusarium graminearum gene FgHal2 on host response to mycovirus Fusarium graminearum virus 1. Mol Plant Pathol 16 : 641–652.
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek Increased Susceptibility of Humanized NSG Mice to Panton-Valentine Leukocidin and Skin InfectionČlánek Phosphorylation of a Myosin Motor by TgCDPK3 Facilitates Rapid Initiation of Motility during egress
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2015 Číslo 11- Stillova choroba: vzácné a závažné systémové onemocnění
- Diagnostika virových hepatitid v kostce – zorientujte se (nejen) v sérologii
- Perorální antivirotika jako vysoce efektivní nástroj prevence hospitalizací kvůli COVID-19 − otázky a odpovědi pro praxi
- Choroby jater v ordinaci praktického lékaře – význam jaterních testů
- Diagnostický algoritmus při podezření na syndrom periodické horečky
-
Všechny články tohoto čísla
- Parasite Glycobiology: A Bittersweet Symphony
- On the Discovery of TOR As the Target of Rapamycin
- Broadening of Virus-Specific CD8 T-Cell Responses Is Indicative of Residual Viral Replication in Aviremic SIV Controllers
- PML/TRIM19-Dependent Inhibition of Retroviral Reverse-Transcription by Daxx
- Cleavage of a Neuroinvasive Human Respiratory Virus Spike Glycoprotein by Proprotein Convertases Modulates Neurovirulence and Virus Spread within the Central Nervous System
- Kaposi’s Sarcoma-Associated Herpesvirus (KSHV) Induces the Oncogenic miR-17-92 Cluster and Down-Regulates TGF-β Signaling
- Interferon-α Subtypes in an Model of Acute HIV-1 Infection: Expression, Potency and Effector Mechanisms
- Perivascular Arrest of CD8 T Cells Is a Signature of Experimental Cerebral Malaria
- Targeting HIV Reservoir in Infected CD4 T Cells by Dual-Affinity Re-targeting Molecules (DARTs) that Bind HIV Envelope and Recruit Cytotoxic T Cells
- Evolution and Emergence of Enteroviruses through Intra- and Inter-species Recombination: Plasticity and Phenotypic Impact of Modular Genetic Exchanges in the 5’ Untranslated Region
- Interferon-γ Inhibits Ebola Virus Infection
- Dengue Virus Non-structural Protein 1 Modulates Infectious Particle Production via Interaction with the Structural Proteins
- P-Type Cyclin CYC3 Modulates Endomitotic Growth during Oocyst Development in Mosquitoes
- Diversity of across Evolutionary Scales
- 50 Years of Disease in Humans: The Dramatic Emergence of a Cluster of Novel Fungal Pathogens
- Worse Comes to Worst: Bananas and Panama Disease—When Plant and Pathogen Clones Meet
- Arenavirus Glycan Shield Promotes Neutralizing Antibody Evasion and Protracted Infection
- Infection-Induced Retrotransposon-Derived Noncoding RNAs Enhance Herpesviral Gene Expression via the NF-κB Pathway
- Structural Insight into Archaic and Alternative Chaperone-Usher Pathways Reveals a Novel Mechanism of Pilus Biogenesis
- Increased Susceptibility of Humanized NSG Mice to Panton-Valentine Leukocidin and Skin Infection
- Global Analysis of the Fungal Microbiome in Cystic Fibrosis Patients Reveals Loss of Function of the Transcriptional Repressor Nrg1 as a Mechanism of Pathogen Adaptation
- The Transcription and Translation Landscapes during Human Cytomegalovirus Infection Reveal Novel Host-Pathogen Interactions
- The N-terminal Helical Region of the Hepatitis C Virus p7 Ion Channel Protein Is Critical for Infectious Virus Production
- Activation of Type I and III Interferon Response by Mitochondrial and Peroxisomal MAVS and Inhibition by Hepatitis C Virus
- Hsp70 Isoforms Are Essential for the Formation of Kaposi’s Sarcoma-Associated Herpesvirus Replication and Transcription Compartments
- Distinct Upstream Role of Type I IFN Signaling in Hematopoietic Stem Cell-Derived and Epithelial Resident Cells for Concerted Recruitment of Ly-6C Monocytes and NK Cells via CCL2-CCL3 Cascade
- and Bats: Story of an Emerging Friendship
- Emergence of Pathogenicity in Lagoviruses: Evolution from Pre-existing Nonpathogenic Strains or through a Species Jump?
- Ebolavirus Evolution: Past and Present
- Host and Symbiont Jointly Control Gut Microbiota during Complete Metamorphosis
- Non-Human Primates Harbor Diverse Mammalian and Avian Astroviruses Including Those Associated with Human Infections
- Lactate Dehydrogenase Is Associated with the Parasitophorous Vacuole Membrane and Is a Potential Target for Developing Therapeutics
- Five Questions about Mycoviruses
- Phosphorylation of a Myosin Motor by TgCDPK3 Facilitates Rapid Initiation of Motility during egress
- Ethanolamine Signaling Promotes Niche Recognition and Adaptation during Infection
- Cross-Species Transmission and Differential Fate of an Endogenous Retrovirus in Three Mammal Lineages
- Memory Th1 Cells Are Protective in Invasive Infection
- Transcription Factor SomA Is Required for Adhesion, Development and Virulence of the Human Pathogen
- An -Methyltransferase Is Required for Infection of Tick Cells by
- RNA-seq Brings New Insights to the Intra-Macrophage Transcriptome of Typhimurium
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Dengue Virus Non-structural Protein 1 Modulates Infectious Particle Production via Interaction with the Structural Proteins
- On the Discovery of TOR As the Target of Rapamycin
- Parasite Glycobiology: A Bittersweet Symphony
- Broadening of Virus-Specific CD8 T-Cell Responses Is Indicative of Residual Viral Replication in Aviremic SIV Controllers
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



