-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Parasite Glycobiology: A Bittersweet Symphony
article has not abstract
Published in the journal: . PLoS Pathog 11(11): e32767. doi:10.1371/journal.ppat.1005169
Category: Pearls
doi: https://doi.org/10.1371/journal.ppat.1005169Summary
article has not abstract
Human infections caused by parasitic protozoans and helminths are among the world's leading causes of death. More than a million people die each year from diseases like malaria and neglected tropical diseases like leishmaniasis, trypanosomiasis, and schistosomiasis. Patients also endure disabilities that cause lifelong suffering and that affect productivity and development [1]. More insidiously, parasites generate important economic losses, since they often also infect commercially valuable animals. Worldwide, exposure to parasites is increasing due to growing international travel and migrations, as well as climate changes, which affect the geographic distribution of the parasite vectors. The parasitic threat is also aggravated by the rise of the immunocompromised population, which is particularly sensitive to parasite infections (e.g., individuals with AIDS and other immunodeficiencies).
A common feature of protozoan parasites and helminths is the synthesis of glycoconjugates and glycan-binding proteins for protection and to interact and respond to changes in their environment. To address the many challenges associated with the study of the structure, the biosynthesis, and the biology of parasitic glycans, the authors of this article have established GlycoPar, a European Marie Curie training program steered by some of the world's academic leaders in the field of parasite glycobiology, in close association with European industrial enterprises. The main scientific goal of this network is the description of novel paradigms and models by which parasite glycoconjugates play a role in the successful colonization of the different hosts. By means of a training-through-research program, the aim of the network is to contribute to the training of a generation of young scientists capable of tackling the challenges posed by parasite glycobiology.
Parasites Are Covered by a Protective Glycocalyx
Due to the complexity of their life cycles, parasites need to sequentially exploit various host species to complete the different stages involved in their survival and development. The interactions with their different hosts are critical for the completion of each life stage and are often based on carbohydrate recognition. In particular, parasites have developed different strategies to escape the immune and defense systems of the different infected organisms. Their surfaces are covered by glycoconjugates of varied natures, often of types absent from mammals. This so-called glycocalyx is protective against the host defense systems but may also be implicated in "hijacking" proteins involved in host innate immunity (Fig 1) [2,3]. Thus, through a "glycan gimmickry" designated process, helminths express host-like glycans that interact with host lectins to modulate the immune response [4]. Furthermore, the walls that protect different parasitic cysts from harsh environments are also rich in polysaccharides and polysaccharide-binding lectins [5]. Thereby, glycans are crucial for parasite virulence and survival.
Fig. 1. The surfaces of parasites, such as Trypanosoma brucei brucei, are covered by glycoconjugates forming a protective glycocalyx against the host defense systems. 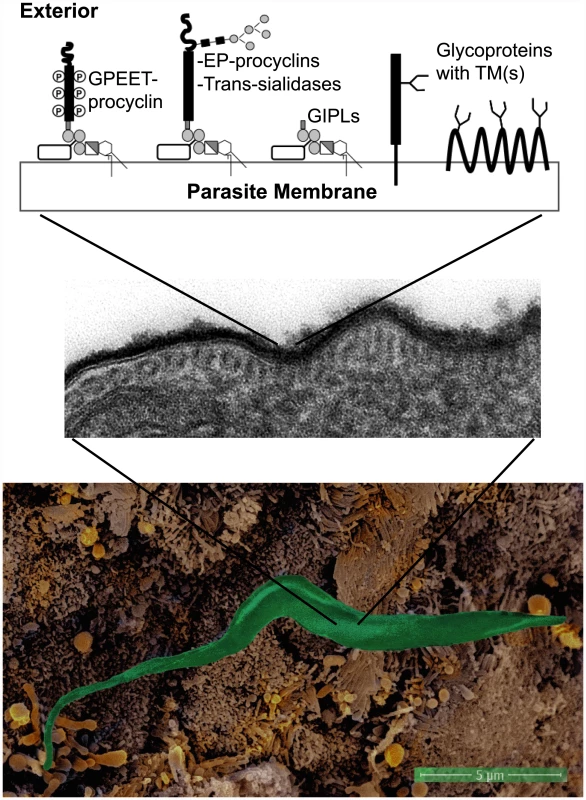
False-color scanning electron microscopy (EM) of a T. b. brucei procyclic interacting with cell microvilli in the tsetse fly proventriculus (bottom panel). Transmission EM of ruthenium-red stained ultrathin sections showing the surface glycocalyx of T. b. brucei procyclic cells (middle panel). Scheme summarizing the main surface glycosylphosphatidylinositol (GPI)-anchored (EP- and GPEET-procyclins and trans-sialidases) and transmembrane (including polytopic) glycoproteins and glycolipids expressed by T. b. brucei procyclics (top panel) [2,24]. Open rectangles linked to GPI molecules represent side chains characteristic of surface glycoconjugates from procyclic T. b. brucei. GIPLs: glycoinositolphospholipids, or free GPIs. EM images obtained by C. Rose, A. Beckett, L. Tetley, I. Prior, and A. Acosta-Serrano. Since glycans are central to host–parasite interactions, their study constitutes a fertile, but currently largely unexploited, area for therapeutic applications. Research in parasite glycosylation provides new opportunities for the discovery of vaccine candidates and for the development of novel chemotherapy approaches and diagnostic tools. Thus, for instance, besides its effect modulating the host immune response against the infection, glycans from Schistosoma mansoni are currently being explored as targets for vaccination and/or serodiagnosis of human schistosomiasis [6]. Nevertheless, there are many challenges associated with working with parasites, including problems in obtaining sufficient amounts of biological material for analytical purposes, difficulties of culturing the different life stages, and, on occasion, the lack of tools for functional genomics and molecular biology approaches. Glycans add another level of difficulty to these studies, due to their extensive diversity and exquisite complexity. In contrast to nucleic acid and proteins, their biosynthesis is only indirectly template-driven and generates an important amount of structural variability in biological systems. This complexity is critical in molecular recognition events including cell–cell, cell–matrix, and cell–molecule interactions during essential steps of pathogenesis. Thus, the thorough characterization of parasite glycobiology requires systematic approaches that focus on the description of the glycosylation precursors, the glycan-processing enzymes, and the structure and functional significance of parasitic glycans. In addition, most of the medically and veterinarially important parasites are phylogenetically ancient organisms and represent good models for studying evolutionary aspects of eukaryotic glycosylation. Thus, the study of parasite glycans may unravel novel mechanisms also present in higher eukaryotes. Excellent examples are the description of the structure of glycosylphosphatidylinositol (GPI) membrane anchors in African trypanosomes [7] or the discovery of the glycoprotein quality control cycle, thanks to seminal studies on the N-glycosylation pathway of trypanosomatid parasites [8]. Interestingly enough, different parasitic protists present variable lengths in their N-glycan precursors that directly affect this N-glycan-dependent quality control system [9].
The Metabolic Precursors of Parasite Glycosylation
Glycan synthesis requires activated monosaccharides, mainly in the form of nucleotide sugars that will be used by glycosyltransferase enzymes as glycosyl donor substrates in glycosylation reactions. Therefore, the presence of activated sugars is a prerequisite for glycan biosynthesis, and their availability influences the glycan structures that may be synthesized by a parasite (the glycome). Thus, valuable information about the glycome can be gained from the identification and quantification of the sugar nucleotide pools maintained during the life stages of different parasites. For example, the capping of Leishmania major surface lipophosphoglycan with arabinose side chains, which is required for detachment of the infectious parasites from the sand fly midgut, correlates with a strong increase of the GDP-α-D-arabinopyranose pool [10].
Sugar nucleotides are formed by de novo pathways requiring the bioconversion of an existing sugar or sugar nucleotide or by salvage pathways involving the activation of the sugar using a kinase and a pyrophosphorylase. The conservation of specific biosynthetic pathways in the parasite genomes are strong hints of the presence of nucleotide sugar pools [11,12]. Monosaccharide activation usually takes place in the cytoplasm, although in Trypanosoma brucei brucei and possibly other kinetoplastid parasites, these biosynthetic reactions occur in a specific organelle called glycosome [13]. Since sugar nucleotides are mostly used by glycosyltransferases in the endoplasmic reticulum and/or the Golgi apparatus, they must be translocated to these cellular compartments by specific transporters (Fig 2). This metabolic compartmentalization and the study of the transporters involved also offer new opportunities for the selective inhibition of crucial glycosylation reactions in parasites.
Fig. 2. Glycosylation processes involve different cellular compartments. 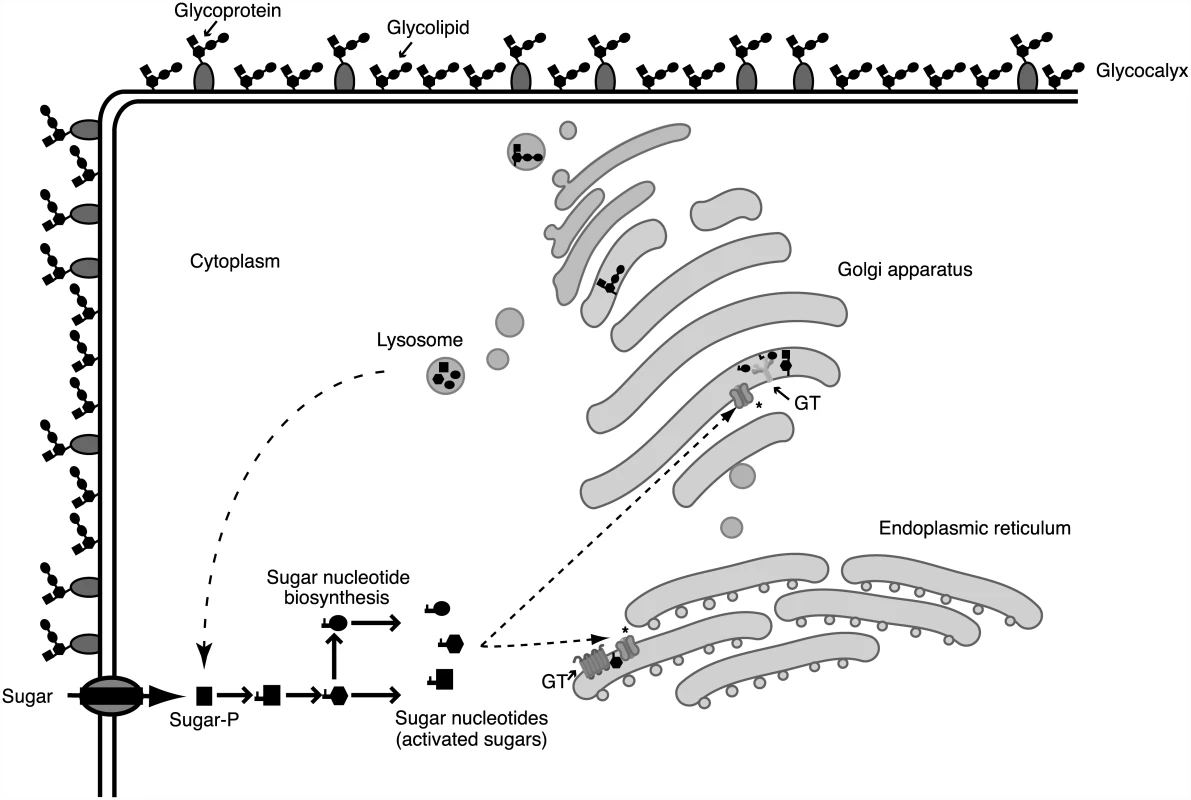
Glycan biosynthesis and cellular compartments involved in the glycosylation process. Sugars are carried across the plasma membrane into cells or are salvaged from degraded glycoconjugates at lysosomes. Through biosynthetic and interconversion reactions, monosaccharides are activated into different nucleotide sugars. Sugar activation generally takes place in the cytoplasm, although several enzymes involved in sugar nucleotide biosynthesis in T. b. brucei are localized in the glycosome. After being activated, sugar nucleotides are transported into the endoplasmic reticulum/Golgi apparatus and used by different glycosyltransferases (GT). Glycosyltransferases and other glycan-processing enzymes define the assembly and final structure of glycans that are secreted or located in the cell surface, forming a protective glycocalyx. Sugar nucleotide transporters are marked with an asterisk (*). Parasitic Glycan-Processing Enzymes and Glycan-Binding Proteins
Glycosyltransferases transfer sugar moieties from activated donors to specific acceptor molecules, generating glycosidic linkages between carbohydrates or between a carbohydrate and a noncarbohydrate moiety. Therefore, they define the assembly and final structure of glycan chains, which can be linear or branched and of various lengths. Glycoside hydrolases, the enzymes that hydrolyze glycosidic bonds, form another main group of carbohydrate-active enzymes that also play important roles in determining the final structure of mature glycans. The combined action of several of these enzymes in the secretory pathway leads to a vast and diverse array of glycan structures. Additionally, parasitic glycan-binding proteins interact with specific parasite and host glycan structures present in the surface of cells.
Sequence-based families of glycosyltransferases, glycoside hydrolases, and carbohydrate-binding proteins group together according to their function, indicating that the acquisition of the specificities of these enzymes evolved from common progenitors. Therefore, despite the huge diversity of glycans, the activities and molecular mechanism of the enzymes involved in their biosynthesis can often be inferred from their sequences [14]. Nevertheless, because of the substantial evolutionary distance between protozoan parasites and higher eukaryotes, it can be challenging to define the precise function of specific parasitic glycosyltransferases from sequence similarity [15,16] or by inference from the final structures determined by a particular glycosylation pathway [17,18]. Glycosyltransferases and other glycan-processing enzymes involved in the biosynthesis of glycans essential for the survival and infectivity of parasites might be exploited as drug targets. Therefore, increasing our knowledge of the different parasitic glycosylation pathways and their biological relevance will contribute to uncovering the therapeutic potential therein.
Parasite Glycomics and the Biological Function of Glycoconjugates
The characterization and quantification of the complete set of glycans and glycoconjugates made by a cell or organism at a given time is defined as glycomics. Since glycosylation is the most structurally diverse, and one of the most abundant, protein and lipid modifications, the description of the spectrum of all glycan structures—the glycome—of even just a single cell type is a huge challenge. Nevertheless, to shed light on the structure–function relationship of parasite glycans at the molecular level, a detailed knowledge of their structures is an important prerequisite that can only be achieved through the use of different analytical methodologies and glycoproteomics and glycolipidomics strategies. Currently, mass spectrometry is a key tool in glycomics and has revealed highly unusual glycans from a number of unicellular and metazoan parasites [19].
The assessment of the functional significance of the different glycosylation states will only be achieved by employing adequate screening and/or genetic tools that, in the case of particular parasites, are still in the development stage [20]. Host receptor molecules can specifically recognize glycans, and these glycan–receptor interactions are related to migration, invasion, adhesion, toxin production, and other essential processes during the course of parasitic infections. By a thorough exploration of the glycomic capacity of parasites and its influence on the interactions with their hosts, the code defined by the different glycan structures can be gradually characterized. In addition, glycomic approaches can be illuminating in the discovery of novel antigenic glycans for the development of diagnostic tools or glycovaccines. An important step in this respect would be the development of glycan microarrays reflecting parasite glycomes in order to identify binding partners in the human proteome, such as components of the innate immune system. Similarly, identifying host glycan structures recognized by parasite proteins with lectin-like properties will be fundamental for describing host–parasite interactions in parasitic diseases.
Future Perspectives: The Translation of Parasitic Glycobiology
Glycobiology has become a well-established area of study in recent decades and is currently providing drug targets against several pathogens and diseases. Ethambutol, Caspofungin, Zanamivir, and Oseltamivir are well-known examples of commercial drugs in use—as therapies against tuberculosis, candidiasis, aspergillosis, and influenza—that target glycosylation and carbohydrate processing. In this regard, echinocandins, antifungal drugs that target β-1,3-glucan synthesis, also inhibit oocyst wall biosynthesis in Eimeria [21]. Similarly, bacterial polysaccharide–protein conjugate vaccines have recently revolutionized vaccination strategies. This approach may be applied to prevent or treat parasitic diseases, using parasite-derived xeno-glycans absent in the human glycome [6,22]. Furthermore, the identification of parasitic glycan antigen structures and monoclonal antibodies to these epitopes holds unprecedented promise for the development of novel diagnostic procedures for various parasitic infections [23]. Thus, through profound and systematic approaches to this important but frequently neglected area of pathogenic parasite research, knowledge about the biology of these organisms will be extended, and novel methods to tackle them will likely be uncovered.
Zdroje
1. Hotez PJ, Alvarado M, Basáñez M-G, Bolliger I, Bourne R, Boussinesq M, et al. The global burden of disease study 2010: interpretation and implications for the neglected tropical diseases. PLoS Negl Trop Dis. 2014;8: e2865. doi: 10.1371/journal.pntd.0002865 25058013
2. Ferguson MA. The surface glycoconjugates of trypanosomatid parasites. Philos Trans R Soc Lond B Biol Sci. 1997;352 : 1295–302. 9355120
3. Van Die I, Cummings RD. Glycan gimmickry by parasitic helminths: a strategy for modulating the host immune response? Glycobiology. 2010;20 : 2–12. doi: 10.1093/glycob/cwp140 19748975
4. Kuijk LM, Klaver EJ, Kooij G, van der Pol SMA, Heijnen P, Bruijns SCM, et al. Soluble helminth products suppress clinical signs in murine experimental autoimmune encephalomyelitis and differentially modulate human dendritic cell activation. Mol Immunol. 2012;51 : 210–8. doi: 10.1016/j.molimm.2012.03.020 22482518
5. Samuelson J, Robbins P. A simple fibril and lectin model for cyst walls of Entamoeba and perhaps Giardia. Trends Parasitol. 2011;27 : 17–22. doi: 10.1016/j.pt.2010.09.002 20934911
6. Nyame AK, Kawar ZS, Cummings RD. Antigenic glycans in parasitic infections: implications for vaccines and diagnostics. Arch Biochem Biophys. 2004/05/26 ed. 2004;426 : 182–200. 15158669
7. Ferguson MA. The structure, biosynthesis and functions of glycosylphosphatidylinositol anchors, and the contributions of trypanosome research. J Cell Sci. 1999;112 (Pt 1 : 2799–2809.
8. Parodi AJ. N-glycosylation in trypanosomatid protozoa. Glycobiology. 1993;3 : 193–199. 8358146
9. Samuelson J, Robbins PW. Effects of N-glycan precursor length diversity on quality control of protein folding and on protein glycosylation. Semin Cell Dev Biol. 2014;41 : 121–128. doi: 10.1016/j.semcdb.2014.11.008 25475176
10. McConville MJ, Thomas-Oates JE, Ferguson MA, Homans SW. Structure of the lipophosphoglycan from Leishmania major. J Biol Chem. 1990;265 : 19611–19623. http://www.jbc.org/content/265/32/19611.short 2246247
11. Turnock DC, Ferguson MAJ. Sugar nucleotide pools of Trypanosoma brucei, Trypanosoma cruzi, and Leishmania major. Eukaryot Cell. 2007;6 : 1450–63. 17557881
12. Sanz S, Bandini G, Ospina D, Bernabeu M, Mariño K, Fernández-Becerra C, et al. Biosynthesis of GDP-fucose and other sugar nucleotides in the blood stages of Plasmodium falciparum. J Biol Chem. 2013;288 : 16506–17. doi: 10.1074/jbc.M112.439828 23615908
13. Güther MLS, Urbaniak MD, Tavendale A, Prescott A, Ferguson MAJ. High-confidence glycosome proteome for procyclic form Trypanosoma brucei by epitope-tag organelle enrichment and SILAC proteomics. J Proteome Res. American Chemical Society; 2014;13 : 2796–806.
14. Cantarel BL, Coutinho PM, Rancurel C, Bernard T, Lombard V, Henrissat B. The Carbohydrate-Active EnZymes database (CAZy): an expert resource for Glycogenomics. Nucleic Acids Res. 2009;37: D233–8. doi: 10.1093/nar/gkn663 18838391
15. Damerow M, Rodrigues JA, Wu D, Güther MLS, Mehlert A, Ferguson MAJ. Identification and functional characterization of a highly divergent N-acetylglucosaminyltransferase I (TbGnTI) in Trypanosoma brucei. J Biol Chem. 2014;289 : 9328–39. d doi: 10.1074/jbc.M114.555029 24550396
16. Titz A, Butschi A, Henrissat B, Fan Y-Y, Hennet T, Razzazi-Fazeli E, et al. Molecular basis for galactosylation of core fucose residues in invertebrates: identification of caenorhabditis elegans N-glycan core alpha1,6-fucoside beta1,4-galactosyltransferase GALT-1 as a member of a novel glycosyltransferase family. J Biol Chem. 2009;284 : 36223–33. doi: 10.1074/jbc.M109.058354 19858195
17. Izquierdo L, Schulz BL, Rodrigues JA, Guther ML, Procter JB, Barton GJ, et al. Distinct donor and acceptor specificities of Trypanosoma brucei oligosaccharyltransferases. Embo J. 2009/07/25 ed. 2009;28 : 2650–2661. doi: 10.1038/emboj.2009.203 19629045
18. Paschinger K, Wilson IBH. Glycoscience: Biology and Medicine [Internet]. Taniguchi N, Endo T, Hart GW, Seeberger PH, Wong C-H, editors. Tokyo: Springer Japan; 2015.
19. Schiller B, Hykollari A, Yan S, Paschinger K, Wilson IBH. Complicated N-linked glycans in simple organisms. Biol Chem. 2012;393 : 661–73. doi: 10.1515/hsz-2012-0150 22944671
20. Izquierdo L, Güther MLS, Ferguson MAJ. Creation and characterization of glycosyltransferase mutants of Trypanosoma brucei. Methods Mol Biol. 2013;1022 : 249–75. doi: 10.1007/978-1-62703-465-4_19 23765667
21. Bushkin GG, Ratner DM, Cui J, Banerjee S, Duraisingh MT, Jennings C V, et al. Suggestive evidence for Darwinian Selection against asparagine-linked glycans of Plasmodium falciparum and Toxoplasma gondii. Eukaryot Cell. 2010;9 : 228–41. doi: 10.1128/EC.00197-09 19783771
22. Yilmaz B, Portugal S, Tran TM, Gozzelino R, Ramos S, Gomes J, et al. Gut Microbiota Elicits a Protective Immune Response against Malaria Transmission. Cell. 2014;159 : 1277–1289. doi: 10.1016/j.cell.2014.10.053 25480293
23. De Andrade AL, Zicker F, de Oliveira RM, Almeida Silva S, Luquetti A, Travassos LR, et al. Randomised trial of efficacy of benznidazole in treatment of early Trypanosoma cruzi infection. Lancet. 1996/11/23 ed. 1996;348 : 1407–1413. 8937280
24. Acosta-Serrano A, Cole RN, Mehlert A, Lee MG, Ferguson MA, Englund PT. The procyclin repertoire of Trypanosoma brucei. Identification and structural characterization of the Glu-Pro-rich polypeptides. J Biol Chem. 1999 Oct 15;274(42): 29763–71. 10514452
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek Increased Susceptibility of Humanized NSG Mice to Panton-Valentine Leukocidin and Skin InfectionČlánek Phosphorylation of a Myosin Motor by TgCDPK3 Facilitates Rapid Initiation of Motility during egress
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2015 Číslo 11- Stillova choroba: vzácné a závažné systémové onemocnění
- Diagnostika virových hepatitid v kostce – zorientujte se (nejen) v sérologii
- Perorální antivirotika jako vysoce efektivní nástroj prevence hospitalizací kvůli COVID-19 − otázky a odpovědi pro praxi
- Choroby jater v ordinaci praktického lékaře – význam jaterních testů
- Diagnostický algoritmus při podezření na syndrom periodické horečky
-
Všechny články tohoto čísla
- Parasite Glycobiology: A Bittersweet Symphony
- On the Discovery of TOR As the Target of Rapamycin
- Broadening of Virus-Specific CD8 T-Cell Responses Is Indicative of Residual Viral Replication in Aviremic SIV Controllers
- PML/TRIM19-Dependent Inhibition of Retroviral Reverse-Transcription by Daxx
- Cleavage of a Neuroinvasive Human Respiratory Virus Spike Glycoprotein by Proprotein Convertases Modulates Neurovirulence and Virus Spread within the Central Nervous System
- Kaposi’s Sarcoma-Associated Herpesvirus (KSHV) Induces the Oncogenic miR-17-92 Cluster and Down-Regulates TGF-β Signaling
- Interferon-α Subtypes in an Model of Acute HIV-1 Infection: Expression, Potency and Effector Mechanisms
- Perivascular Arrest of CD8 T Cells Is a Signature of Experimental Cerebral Malaria
- Targeting HIV Reservoir in Infected CD4 T Cells by Dual-Affinity Re-targeting Molecules (DARTs) that Bind HIV Envelope and Recruit Cytotoxic T Cells
- Evolution and Emergence of Enteroviruses through Intra- and Inter-species Recombination: Plasticity and Phenotypic Impact of Modular Genetic Exchanges in the 5’ Untranslated Region
- Interferon-γ Inhibits Ebola Virus Infection
- Dengue Virus Non-structural Protein 1 Modulates Infectious Particle Production via Interaction with the Structural Proteins
- P-Type Cyclin CYC3 Modulates Endomitotic Growth during Oocyst Development in Mosquitoes
- Diversity of across Evolutionary Scales
- 50 Years of Disease in Humans: The Dramatic Emergence of a Cluster of Novel Fungal Pathogens
- Worse Comes to Worst: Bananas and Panama Disease—When Plant and Pathogen Clones Meet
- Arenavirus Glycan Shield Promotes Neutralizing Antibody Evasion and Protracted Infection
- Infection-Induced Retrotransposon-Derived Noncoding RNAs Enhance Herpesviral Gene Expression via the NF-κB Pathway
- Structural Insight into Archaic and Alternative Chaperone-Usher Pathways Reveals a Novel Mechanism of Pilus Biogenesis
- Increased Susceptibility of Humanized NSG Mice to Panton-Valentine Leukocidin and Skin Infection
- Global Analysis of the Fungal Microbiome in Cystic Fibrosis Patients Reveals Loss of Function of the Transcriptional Repressor Nrg1 as a Mechanism of Pathogen Adaptation
- The Transcription and Translation Landscapes during Human Cytomegalovirus Infection Reveal Novel Host-Pathogen Interactions
- The N-terminal Helical Region of the Hepatitis C Virus p7 Ion Channel Protein Is Critical for Infectious Virus Production
- Activation of Type I and III Interferon Response by Mitochondrial and Peroxisomal MAVS and Inhibition by Hepatitis C Virus
- Hsp70 Isoforms Are Essential for the Formation of Kaposi’s Sarcoma-Associated Herpesvirus Replication and Transcription Compartments
- Distinct Upstream Role of Type I IFN Signaling in Hematopoietic Stem Cell-Derived and Epithelial Resident Cells for Concerted Recruitment of Ly-6C Monocytes and NK Cells via CCL2-CCL3 Cascade
- and Bats: Story of an Emerging Friendship
- Emergence of Pathogenicity in Lagoviruses: Evolution from Pre-existing Nonpathogenic Strains or through a Species Jump?
- Ebolavirus Evolution: Past and Present
- Host and Symbiont Jointly Control Gut Microbiota during Complete Metamorphosis
- Non-Human Primates Harbor Diverse Mammalian and Avian Astroviruses Including Those Associated with Human Infections
- Lactate Dehydrogenase Is Associated with the Parasitophorous Vacuole Membrane and Is a Potential Target for Developing Therapeutics
- Five Questions about Mycoviruses
- Phosphorylation of a Myosin Motor by TgCDPK3 Facilitates Rapid Initiation of Motility during egress
- Ethanolamine Signaling Promotes Niche Recognition and Adaptation during Infection
- Cross-Species Transmission and Differential Fate of an Endogenous Retrovirus in Three Mammal Lineages
- Memory Th1 Cells Are Protective in Invasive Infection
- Transcription Factor SomA Is Required for Adhesion, Development and Virulence of the Human Pathogen
- An -Methyltransferase Is Required for Infection of Tick Cells by
- RNA-seq Brings New Insights to the Intra-Macrophage Transcriptome of Typhimurium
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Dengue Virus Non-structural Protein 1 Modulates Infectious Particle Production via Interaction with the Structural Proteins
- On the Discovery of TOR As the Target of Rapamycin
- Parasite Glycobiology: A Bittersweet Symphony
- Broadening of Virus-Specific CD8 T-Cell Responses Is Indicative of Residual Viral Replication in Aviremic SIV Controllers
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



