-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Host and Symbiont Jointly Control Gut Microbiota during Complete Metamorphosis
The majority of animals are holometabolous insects and change dramatically through development. They undergo a dramatic transformation from a larval stage, adapted to feed, to an adult separated by a pupal stage. During this pupal stage the majority of the organs are renewed including the gut. This creates a risky situation that we study here: when the gut is renewed insects risk losing beneficial microbiota while simultaneously being at risk of opportunistic infection. Here, by manipulating host and symbiont we show how host and symbiont succeed in jointly controlling opportunistic pathogens. If one or both of the partners are compromised, opportunistic pathogens dominate the gut microbiota resulting in increased mortality. These findings may be broadly applicable to insects with complete metamorphosis, including many disease vectors.
Published in the journal: . PLoS Pathog 11(11): e32767. doi:10.1371/journal.ppat.1005246
Category: Research Article
doi: https://doi.org/10.1371/journal.ppat.1005246Summary
The majority of animals are holometabolous insects and change dramatically through development. They undergo a dramatic transformation from a larval stage, adapted to feed, to an adult separated by a pupal stage. During this pupal stage the majority of the organs are renewed including the gut. This creates a risky situation that we study here: when the gut is renewed insects risk losing beneficial microbiota while simultaneously being at risk of opportunistic infection. Here, by manipulating host and symbiont we show how host and symbiont succeed in jointly controlling opportunistic pathogens. If one or both of the partners are compromised, opportunistic pathogens dominate the gut microbiota resulting in increased mortality. These findings may be broadly applicable to insects with complete metamorphosis, including many disease vectors.
Introduction
The vast majority of animal species are insects [1,2]. Most species of insects are holometabolous, including important vectors of infectious disesases (sandflies, mosquitoes) and model organisms (Drosophila melanogaster, Galleria mellonella) with distinct larval and adult stages separated by metamorphosis, which entails dramatic remodeling of external and internal anatomy [3,4]. The evolutionary advantage of metamorphosis is usually explained by the adaptive decoupling hypothesis [5]: traits in larvae and adults are genetically decoupled, facilitating adaptation to life-stage specific selection [6]. Anatomical re-organization of the body, however, poses a significant problem during the replacement of the gut, as the gut hosts a microbiota. Either the organism must eradicate and subsequently re-establish the microbiota from the environment, or it must maintain its microbiota while preventing opportunistic microbes from entering the hemocoel and causing infections.
Early studies clearly demonstrated the presence and maintenance of bacteria in the gut during metamorphosis in Lepidoptera and Diptera [7–9], and more recent work has described the same phenomenon in Coleoptera [10], Diptera [11], Lepidoptera [12], and Hymenoptera [13]. Two competing mechanisms have been proposed to explain the composition of the retained gut microbiota. One explanation holds that bacterial competition drives the composition of the adult gut microbiota [14,15]. Alternatively, the host immune system plays an important role in shaping the gut microbiota [16,17]. However despite continued interest in insect gut immunity [18], the interaction between host immunity and bacterial competition during complete metamorphosis has remained unstudied.
Here we exploit an ancient [19], facultative and prevalent [20–26] symbiosis between Lepidoptera and enterococci to reconcile these approaches by studying the role of host immunity and bacterial competition during metamorphosis within a single system. The gut microbiota of Lepidoptera is limited to a handful of bacterial species that varies with habitat and diet but often is dominated by enterococci that persist through metamorphosis [12]. In the lepidopteran gut, enterococci interact with pathogens through (i) competitive exclusion (ii) attenuation by direct antagonism or (iii) eliciting protective host immune responses and provide lepidopterans including Galleria mellonella with protection against one of the most virulent entomopathogens, Bacillus thuringiensis [21,27–29] (reviewed in [30]).
In Lepidoptera, the contents of the gut lumen and the peritrophic matrix are purged at the onset of metamorphosis. Basal midgut stem cells proliferate and differentiate to form a continuous layer beneath the larval gut epithelium where lysozyme accumulates in apical vacuoles [16]. Following ecdysis of the larval epithelium, the vacuole contents are released into the gut lumen producing a burst of antibacterial activity that is presumed to prevent septicemic infection [16]. The detached larval epithelium undergoes autophagy and apoptosis and degenerates as the 'yellow body' [31]. Bacteria that resist mechanical and immunological exclusion by the host during pupation, compete intensely for colonization of the pupal gut as has been demonstrated in flies [14].
In the model lepidopteran Galleria mellonella, Enterococcus mundtii (syn. Streptococcus faecalis Andrewes and Horder) is passed from female to offspring via the surface of the egg [25]. In vitro observations of lepidopteran gut microbes imply that E. mundtii has the highest abundance, in adults it is often the only detectable microbe in the gut, and that this is mediated by synergy between lysozyme and a broad-spectrum bacteriocin [28]. As is common in many Lepidoptera [32], G. mellonella adults lack functional mouthparts and therefore additional microbes cannot be acquired during adult life.
Using the Galleria-Enterococcus symbiosis we tested the hypothesis that host and symbiont interact to determine the adult gut microbiota. We manipulated host gut immunity and bacterial competitive ability during metamorphosis in a full factorial fashion. Based on these findings we studied fitness consequences of altered microbiotas for adult hosts.
Results and Discussion
Previous studies show that lysozyme is important in host-microbe interactions in the pre-pupae of another lepidopteran [16], and in G. mellonella a synergistic interaction between C-type lysozyme and antimicrobial peptides was recently demonstrated [33]. Based on the expression of a C-type lysozyme (Swiss-Prot accession P82174) in the gut during metamorphosis (S1 Fig) and the reported synergism of G. mellonella lysozyme we hypothesized that lysozyme mediates changes in the microbiota during metamorphosis. We knocked down lysozyme expression using RNAi in insect hosts that were colonized by either E. mundtii strain G2-mun+, which produces the broad-spectrum bacteriocin mundticin [34], or by E. mundtii strain G2-mun-, which is unable to express the mundticin-encoding gene munA.
To test the impact of these manipulations we cured the gut microbiota from final-instar larvae using antibiotics and re-inoculated these individuals with either E. mundtii G2-mun+ or G2-mun-, and reared them on conventional non-sterile diet until pupation.
Using a combination of 16S rRNA gene amplicon sequencing, qPCR, and conventional bacterial culturing we monitored the composition of the gut microbiota during the larval-pupal molt as well as after adult eclosion.
The microbiota of the wild-type host during pupation was increasingly dominated by Enterococcus, whereas Serratia and Staphylococcus were undetectable in the adult stage by culturing, 16S rRNA gene amplicon sequencing (S2 Fig, S1 Table), and 16S rRNA gene qPCR (Fig 1). Host lysozyme-knockdown resulted in a significant increase in Enterobacteriaceae and persistence into the adult stage (T = -25.145, df = 456, p = <0.0001), which appear to be entirely composed of a Serratia sp. When the host is instead associated with E. mundtii G2-mun - (which does not produce the bacteriocin munditicin), Staphylococcus becomes highly abundant after pupation (T = -96.48, df = 456, p <0.0001, Fig 1) and Enterococcus are reduced by two orders of magnitude. When both host and symbiont are disenabled, Enterobacteriaceae (Serratia sp.) dominates (T = -28.655, df = 456, p < 0.0001) and again Enterococcus are strongly reduced on reaching the adult stage (T = 10.290, df = 448, p < 0.0001, Fig 1).
Fig. 1. Abundance of bacteria during host metamorphosis. 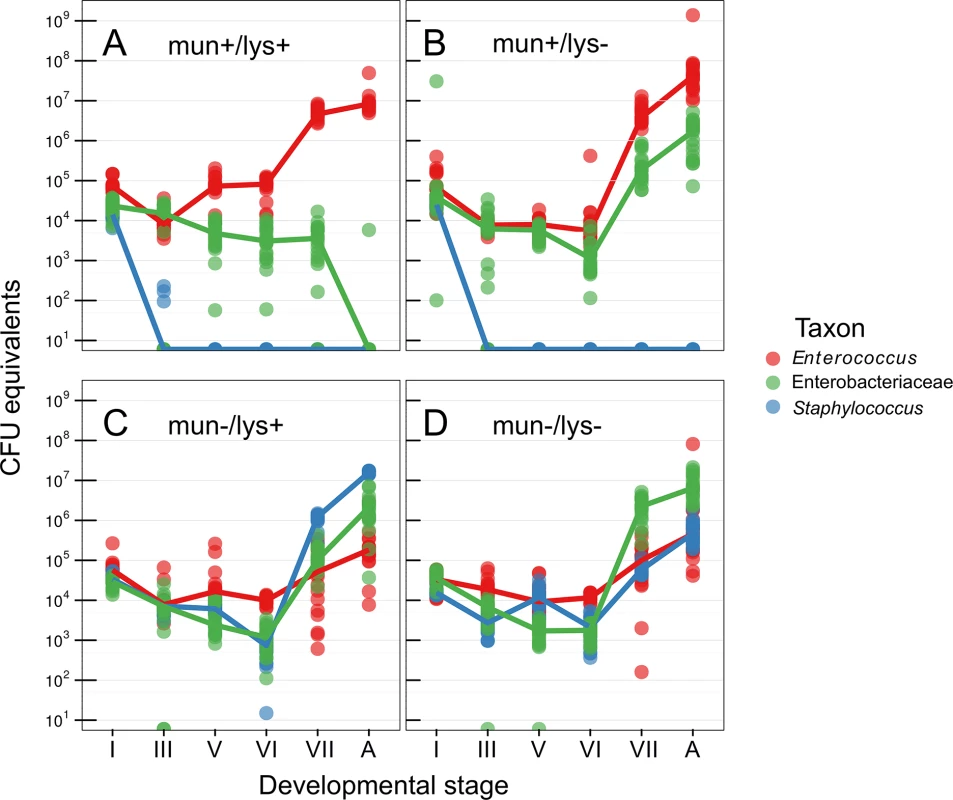
Using 16S rRNA gene qPCR the microbiota was sampled across metamorphosis in (A) wild-type hosts with bacteriocin-producing Entercoccus mundtii G2 symbiont, (B) immunocompromised hosts where RNAi was used to knock down lysozyme gene expression, (C) wild-type hosts with mutant Entercoccus mundtii symbionts that do not produce bacteriocin, and (D) both immunocompromised hosts and mutant symbionts. Roman numerals correspond to precise stages of the larval-pupal molt (see materials and methods). A, adult. We found that the gut microbiota composition significantly changes when either the symbiont and/or host and symbiont were both disenabled (Fig 1). Based on these results we therefore inoculated mature larvae, that were first cleared of their microbiota using antibiotics (‘re-inoculated larvae’), with a defined microbiota comprising either wild-type Enterococcus mundtii, Staphylococcus sp. (reflecting the results when the symbiont is disenabled) or Serratia sp. (as found when both host and symbiont are disenabled), which were isolated from G. mellonella larvae. This made it possible to investigate fitness costs of a defined microbiota without the confounding effects of the manipulation of the host immune system by RNAi or changes to symbiont competitive ability by manipulating mundticin expression. Survival of the resulting adults was monitored after eclosion. Independent of host sex, G. mellonella individuals with E. mundtii survived significantly longer than those with a Staphylococcus - (Z = -4.72, p = <0.0001), or Serratia - (Z = -2.97, p = 0.003) dominated microbiota (Fig 2, S2 Table). There was no difference in survival between G. mellonella adults derived from larvae that were either cured of their microbiota and maintained axenically, or which were re-inoculated with E. mundtii (S3 Fig). This supports the conclusion that the main benefit of E. mundtii mutualism is the interaction with other members of the gut microbiota.
Fig. 2. Survival of adults with experimentally defined gut microbiota. 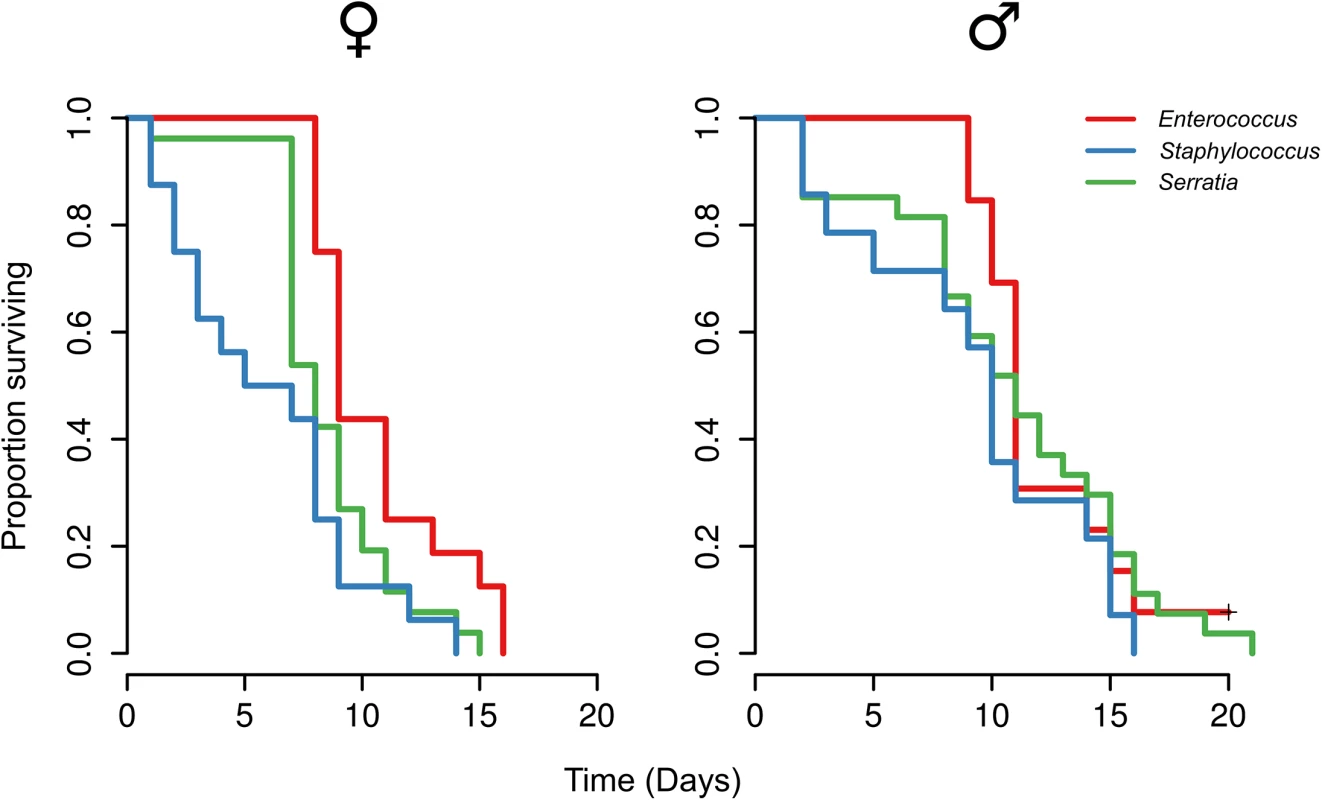
Larvae were re-inoculated with a defined microbiota comprising either wild-type Enterococcus mundtii, Staphylococcus sp. or Serratia sp. based on previous results (Fig 1). Our study shows that host and symbiont interact to maintain a ‘healthy’ gut microbiota through complete metamorphosis. Given the protection that E. mundtii confers to the lepidopteran host [28,30] the selective advantage for the host is clear. The transmission of the gut microbiota between individuals is usually considered as mixed-mode transmission, combining vertical and horizontal transmission [35]. For the bacterial symbiont, passage through metamorphosis constitutes an important component of vertical transmission. Complete sterilization of the gut by the host would spell disaster for the symbiont. The transition of the symbiont through metamorphosis is crucial to guarantee vertical transmission to the host offspring. In social species, this can be overcome through exposure to feces of nest mates [36]. But most holometabolous insects are solitary and therefore this mechanism of re-acquisition of symbionts from conspecifics after metamorphosis is impractical. Some species of ants and weevils have resolved the transition of the microbiota through metamorphosis with bacteriocytes, crypts, and other specialized structures that harbour symbionts throughout the life cycle [37,38], this is not the case in Lepidoptera.
Our study also sheds light on an important aspect of the evolution of complete metamorphosis, a key innovation of holometabolous insects [39] that resulted in their extraordinary diversity. An understanding of the benefits and costs of complete metamorphosis is essential to explain the evolutionary success of holometabolous insects. Ecological and evolutionary models of metamorphosis are sufficient to explain the evolution of complex life cycles [5], but not of the pupal stage that defines complete metamorphosis. One adaptive explanation that has been proposed, but barely tested, is the decoupling of growth and differentiation into consecutive stages of the larva and the pupa [40]. The evolution of such a novel complex trait as the pupal stage also creates significant problems. The pupa is a sessile and hence vulnerable stage in the insect life cycle. Some parasitoids specialize on pupal hosts and display adaptations that exploit host endocrinology during metamorphosis [41]. Here, by contrast, we have identified and described a ubiquitous problem encountered by most holometabolous insect during ontogeny: the passage of symbiontic bacteria through pupation. Our data show how the host and symbiont interact to achieve this passage. Moreover, the data also suggest that the risk of opportunistic infection during the destruction of the larval gut is countered by the host through up-regulation of immune function. The destruction of the gut, and hence the abundance of danger signals, in combination with microbe-derived immune elicitors result in a strong immune response [42] as found here.
Materials and Methods
Bacterial strains and growth conditions
Enterococcus mundtii G2 was isolated from a long-term laboratory population of Galleria mellonella where insects were reared on a natural diet of honeycomb [43]. To cure E. mundtii G2 of its munditicin-encoding plasmid, a single colony was picked and grown in BHI broth at 42°C for 5 24-h serial passages. The resultant strain was transformed by the method of Dunny et al. [44] with either pRK1 or pRK62 which both contain the entire mun locus with and without a munA promoter, respectively [34]. Both strains express the mundticin ABC transporter protein and mundticin immunity protein (MunB and MunC respectively) [34], and enterococci are intrinsically resistant to lysozyme [45]. The resultant strains are referred to as mun+ and mun-. The wild-type mun locus comprises munA, which encodes the bacteriocin mundticin; munB encoding a mundticin-translocating ABC transporter; and munC encoding a mundticin immunity protein. munBC expression is under independent transcriptional control from munA and is driven by a promoter located between munA and munB and downstream of the munA terminator [34]. To enumerate gut bacteria, insects were dissected and their guts were homogenized with 5-mm steel bead using a TissueLyser (Qiagen) at 20 Hz for 10 s. Homogenates were serially diluted in sterile saline and plated onto 1/10 strength TSA (Oxoid).
Amplification and sequencing of 16S rRNA genes
Bacterial colonies were categorized by morphotype and representatives were subjected to colony PCR with universal primers 27F and 1492r. Sanger sequencing of PCR products was performed by MWG Biotech (Ebersberg, Germany) or GATC (Konstanz, Germany). For high-throughput amplicon sequencing, total DNA was recovered from gut homogenates by bead milling and CTAB extraction [46] and 24 pools of DNA were constructed representing each combination of treatment and developmental stage using 100 ng of purified DNA from each individual. Pools were subjected to PCR with barcoded versions of the universal primers 27f and 519r. Roche multiplex identifiers were incorporated between the sequences of adaptor A and 519r to give the structure: 5'-Adaptor_A-sequencing_key-multiplex_identifier-519r-3'. PCR consisted of an initial denaturation step of 2 min at 94°C and 25 cycles of of 30 s at 94°C, 20 s at 52°C, and 60 s at 65°C. PCR products were checked by gel electrophoresis, purified with AMPure beads, and sequenced on a 454 titanium GS FLX at 24-plex per quarter pico-titer plate. Amplicon sequence data were processed using QIIME version 1.6 [47]. Sequences were assigned to operational taxonomic units according to a 97% identity threshold using uclust [48]. Data were deposited in the NCBI SRA under accession PRJNA268795.
16S rRNA gene qPCR
Based on the high-throughput 16S rRNA gene amplicon sequencing, taxon-specific 16S rRNA gene primers were used to quantify the three dominant taxa for the genera Enterococcus [49], Staphylococcus [50], and the family Enterobacteriaceae [49] in each individual. Dilution plating and high-throughput 16S rRNA amplicon sequencing showed the presence of three bacterial genera: Enterococcus, Staphylococcus, and Serratia however since Serratia-specific 16S rRNA gene primers could not be designed, family-specific Enterobacteriaceae primers were used to generate individual-based qPCR measurements. Standard curves were prepared using samples derived from axenic guts spiked with known quantities of either Enterococcus mundtii G2, Staphylococcus sp, or Serratia sp. CFU. qPCR was performed on an ABI StepOne using KAPA SYBR FAST ABI Prism mastermix (Peqlab). The resulting data were log transformed and analysed using linear models in R 3.1.3.
Insect rearing
G. mellonella larvae were reared in the dark at 30°C on a grain-honey diet described previously [29]. Hoffman's tobacco hornworm diet was used to manipulate the G. mellonella gut microbiota. Sterile artificial diet was produced using an autoclave and heat-labile components such as Vanderzants vitamin mixture (Sigma-Aldrich) and antibiotics were dissolved in water and filter-sterilized before combing with molten diet at 60°C. To remove the gut microbiota, diet was amended with 100 μg ml-1 streptomycin and tetracycline (Sigma-Aldrich). Mature final-instar larvae were starved for 4 h before being transferred to sterile antibiotic-amended diet for 24 h. Removal of the microbiota was confirmed by dissecting and plating the guts of 30 randomly-chosen larvae onto TSA plates.
To associate larvae with a specific bacterial strain, sterile diet (without antibiotics) was amended with an aliquot of an overnight culture to a final concentration of 103 CFU ml-1. Larvae were removed from sterile antibiotic-amended diet, starved for 4 h, and transferred to bacteria-amended diet for 16 h. As is common in many Lepidoptera [32], G. mellonella adults do not possess functional mouthparts therefore oral infection of adults is not possible. Larvae were subsequently starved for 4h before being returned to a conventional grain-honey diet.
Plasmid segregational stability
The segregational stability of the plasmids pRK1 and pRK62 in E. mundtii G2 was determined according to Simon and Chopin [51] in both non-selective MRS broth (Oxoid) as well as in insect hosts. To quantify stability in broth, an overnight culture was diluted in non-selective MRS broth, grown to late exponential phase and plated onto non-selective MRS agar. 384 colonies were arrayed in duplicate onto erythromycin-selective and non-selective MRS agar. To quantify stability in insects, mature larvae were mono-associated as described above with E. mundtii G2 carrying either pRK1 or pRK62, and returned to conventional grain-honey diet to complete larval and pupal development. The frequency of vector loss was < 2.8 x 10−3 both in broth culture and insect hosts. In the case of broth culture, this stability is comparable to the parent vector pIL253 [51]. Upon eclosion, 10 adults carrying either mun+ or mun - strains were dissected and their guts were plated onto non-selective MRS agar (S4 Fig). 384 colonies were tested for erythromycin-sensitivity as described above. 46 randomly selected EmR colonies from each larva were screened for the presence of the plasmid by colony PCR using T7 promoter-specific primers.
Determination of the stages during metamorphosis
Complete detachment of the larval gut epithelium occurs prior to ecdysis of the larval cuticle. Therefore the staging system of Kühn and Piepho [52], as adapted by Uwo et al. [31], was used to specify the stages of midgut metamorphosis in larvae and pupae (see Uwo et al. [31] and Ellis et al. [53] for illustration). Stage I is a wandering larva that has ceased feeding and started spinning. Stemmatal pigments have not started to migrate and the midgut is empty. Stage II, is the spinning larva and pigments have started to migrate from the stemmata. Stage III, a l ater spinning larva, where the pigments have left the stemmata but are still in contact with the cuticle. The larval gut epithelium has completely detached and floats freely in the lumen. Stage IV defines a mature spinning larva and the pigments have sunk beneath the cuticle but are still visible. Stage V is the prepupa that has ceased spinning and stemmatal pigments are not visible. The midgut is laterally flattened and the detached larval gut has formed the yellow body which undergoes apoptosis. The new pupa is classed as stage VI; the cuticle has not sclerotized and is completely white. Stage VII describes a sclerotized pupa approximately 24 h after the larval-pupal molt. The midgut is cylindrical and surrounded by an extra-epithelial layer.
The migration of stemmatal pigments was monitored under a stereo microscope.
RNAi
An internal region of the cDNA sequence encoding a C-type lysozyme, previously designated lysozyme GALME (Swiss-Prot accession P82174) [54], was amplified with T7-tailed primers Gm_Lys_T7_F1 (5'-TAATACGACTCACTATAGGGAGAGCAAGCCGAATAAAAATGGA-3') and Gm_Lys_T7_R1 (5'-TAATACGACTCACTATAGGGAGATATCTGGCAGCGGCTTATTT-3') and used as template to synthesize dsRNA using a MEGAscript T7 Kit (Ambion). In order to knockdown lysozyme GALME expression, 500 ng of purified dsRNA was injected into the hemocoel of mature final-instar larvae. RNAi efficacy was monitored throughout the larval-pupal molt by performing relative qPCR on cDNA derived from dissected guts using the primers Gm_Lys_qPCR_F1 (5'-ACTTTTACGAGATGCGGACTG-3') and Gm_Lys_qPCR_R1 (5'-TCTCATTCTCAACAAGGCACAC-3'), which target a region upstream of the region chosen as template for dsRNA synthesis, as well as S7e_forward and S7e_reverse which target the gene encoding ribosomal protein S7e [55], which was analyzed as a reference. Relative expression was calculated using the relative Ct method. cDNA was synthesized using a cDNA-Synthesis Kit H Plus (Peqlab) from 100 ng of total RNA from a pool of RNA from 3 individual insects. qPCR was performed using a peqGOLD Hot Start-Mix kit (Peqlab) and a StepOne real-time thermocycler (Applied Biosystems) according to the manufacturer’s instructions.
Survival analysis
Mature pupae were weighed and segregated individually in plastic cups covered with muslin cloth at 30°C. Newly eclosed adults were sexed according to the forewing margin [53] and survival was recorded every 24 h. The data were analysed in R with an accelerated failure time model using the survival package [56]. The Bayesian information criterion was used to select the final model.
Supporting Information
Zdroje
1. Misof B, Liu S, Meusemann K, Peters RS, Donath a., Mayer C, et al. Phylogenomics resolves the timing and pattern of insect evolution. Science. 2014; 346 : 763–767. doi: 10.1126/science.1257570 25378627
2. Mora C, Tittensor DP, Adl S, Simpson AGB, Worm B. How many species are there on Earth and in the ocean? PLoS Biol. 2011; 9: e1001127. doi: 10.1371/journal.pbio.1001127 21886479
3. Truman JW, Riddiford LM. The origins of insect metamorphosis. Nature. 1999; 401 : 447–452. doi: 10.1038/46737 10519548
4. Grimaldi D, Engel M. Evolution of the Insects. Cambridge University Press; 2005.
5. Moran N. Adaptation and constraint in the complex life cycles of animals. Annu Rev Ecol Syst. 1994; 25 : 573–600. Available: http://www.jstor.org/stable/2097325
6. Stansbury M, Moczek A. The evolvability of arthropods. Arthropod Biology and Evolution. Springer Berlin Heidelberg; 2013. pp. 479–493.
7. Leach JG. The method of survival of bacteria in the puparia of the seed-corn maggot (Hylernyia Cilicrura Rond.). Zeitschrift für Angew Entomol. 1934; 20 : 150–161.
8. Bacot AW. On the Persistence of Bacilli in the Gut of an Insect during Metamorphosis. Trans Entomol Soc London. 1911; 497–500.
9. Bakula M. The persistence of a microbial flora during postembryogenesis of Drosophila melanogaster. J Invertebr Pathol. 1969;14 : 365–374. doi: 10.1016/0022-2011(69)90163-3 4904970
10. Delalibera I, Vasanthakumar A, Burwitz B, Schloss PD, Klepzig KD, Handelsman J, et al. Composition of the bacterial community in the gut of the pine engraver, Ips pini (Say) (Coleoptera) colonizing red pine. Symbiosis. 2007; 43 : 97–104.
11. Wong CNA, Ng P, Douglas AE. Low-diversity bacterial community in the gut of the fruitfly Drosophila melanogaster. Environ Microbiol. 2011; 13 : 1889–1900. doi: 10.1111/j.1462-2920.2011.02511.x 21631690
12. Hammer TJ, McMillan WO, Fierer N. Metamorphosis of a butterfly-associated bacterial community. PLoS One. 2014;9: e86995. doi: 10.1371/journal.pone.0086995 24466308
13. Brucker RM, Bordenstein SR. The roles of host evolutionary relationships (genus: Nasonia) and development in structuring microbial communities. Evolution. 2012; 66 : 349–362. doi: 10.1111/j.1558-5646.2011.01454.x 22276533
14. Greenberg B. Salmonella suppression by known populations of bacteria in flies. J Bacteriol. 1969; 99 : 629–35. Available: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=250072&tool=pmcentrez&rendertype=abstract 4984172
15. Greenberg B, Klowden M. Enteric Bacterial Interactions in Insects. Am J Clin Nutr. 1972; 25 : 1459–1466. 4629542
16. Russell V, Dunn PE. Antibacterial proteins in the midgut of Manduca sexta during metamorphosis. J Insect Physiol. 1996;42 : 65–71.
17. Tebbutt H. On the influence of metamorphosis of Musca domestica upon bacteria administered in the larval Stage. J Hyg (Lond). 1912;12 : 516–526.
18. Buchon N, Broderick NA, Lemaitre B. Gut homeostasis in a microbial world: insights from Drosophila melanogaster. Nat Rev Microbiol. 2013; 11 : 615–626. doi: 10.1038/nrmicro3074 23893105
19. Wheeler D, Redding AJ, Werren JH. Characterization of an ancient lepidopteran lateral gene transfer. PLoS One. 2013; 8: e59262. doi: 10.1371/journal.pone.0059262 23533610
20. Van Frankenhuyzen K, Liu YH, Tonon A. Interactions between Bacillus thuringiensis subsp kurstaki HD-1 and midgut bacteria in larvae of gypsy moth and spruce budworm. J Invertebr Pathol. 2010;1 03 : 124–131.
21. Hernández-Martínez P, Naseri B, Navarro-Cerrillo G, Escriche B, Ferré J, Herrero S. Increase in midgut microbiota load induces an apparent immune priming and increases tolerance to Bacillus thuringiensis. Environ Microbiol. 2010; 12 : 2730–2737. doi: 10.1111/j.1462-2920.2010.02241.x 20482744
22. Broderick NA, Raffa KF, Goodman RM, Handelsman J. Census of the bacterial community of the gypsy moth larval midgut by using culturing and culture-independent methods. Appl Environ Microbiol. 2004; 70 : 293–300. doi: 10.1128/AEM.70.1.293 14711655
23. Brooks MA. The Microorganisms of Healthy Insects. In: Steinhaus EA, editor. Insect Pathology: An Advanced Treatise vol 1. London: Academic Press; 1963. pp. 215–250.
24. Martin JD, Mundt JO. Enterococci in insects. Appl Microbiol. 1972; 24 : 575–80. Available: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=380616&tool=pmcentrez&rendertype=abstract 4628796
25. Bucher GE. Survival of populations of Streptococcus faecalis Andrewes and Horder in the gut of Galleria mellonella (Linnaeus) during metamorphosis, and transmission of the bacteria to the filial generation of the host. J Insect Pathol. 1963; 5 : 336–343.
26. Xiang H, Wei G, Jia S, Huang J, Miao X, Zhou Z, et al. Microbial communities in the larval midgut of laboratory and field populations of cotton bollworm (Helicoverpa armigera). Can J Microbiol. 2006; 52 : 1085–1092. doi: 10.1139/W06-064 17215900
27. Raymond B, Johnston PR, Wright DJ, Ellis RJ, Crickmore N, Bonsall MB. A mid-gut microbiota is not required for the pathogenicity of Bacillus thuringiensis to diamondback moth larvae. Environ Microbiol. 2009; 11 : 2556–2563. doi: 10.1111/j.1462-2920.2009.01980.x 19555371
28. Jarosz J. Gut flora of Galleria mellonella suppressing ingested bacteria. J Invertebr Pathol. 1979;34 : 192–198. 119813
29. Johnston PR, Crickmore N. Gut bacteria are not required for the insecticidal activity of Bacillus thuringiensis toward the tobacco hornworm, Manduca sexta. Appl Environ Microbiol. 2009; 75 : 5094–5099. doi: 10.1128/AEM.00966-09 19525273
30. Raymond B, Johnston PR, Nielsen-LeRoux C, Lereclus D, Crickmore N. Bacillus thuringiensis: an impotent pathogen? Trends Microbiol. 2010;18 : 189–94. doi: 10.1016/j.tim.2010.02.006 20338765
31. Uwo MF, Ui-Tei K, Park P, Takeda M. Replacement of midgut epithelium in the greater wax moth, Galleria mellonela, during larval-pupal moult. Cell Tissue Res. 2002;308 : 319–31. doi: 10.1007/s00441-002-0515-1 12037588
32. Tammaru T, Haukioja E. Capital breeders and income breeders among Lepidoptera: consequences to population dynamics. Oikos. 1996;77 : 561–564. doi: 10.2307/3545946
33. Zdybicka-Barabas A, Stączek S, Mak P, Skrzypiec K, Mendyk E, Cytryńska M. Synergistic action of Galleria mellonella apolipophorin III and lysozyme against Gram-negative bacteria. Biochim Biophys Acta. 2013;1828 : 1449–56. doi: 10.1016/j.bbamem.2013.02.004 23419829
34. Kawamoto S, Shima J, Sato R, Eguchi T, Ohmomo S, Shibato J, et al. Biochemical and Genetic Characterization of Mundticin KS, an Antilisterial Peptide Produced by Enterococcus mundtii NFRI 7393. Appl Env Microbiol. 2002; 68 : 3830–3840. doi: 10.1128/AEM.68.8.3830
35. Ebert D. The Epidemiology and Evolution of Symbionts with Mixed-Mode Transmission. Annu Rev Ecol Evol Syst. 2013; 44 : 623–643. doi: 10.1146/annurev-ecolsys-032513-100555
36. Koch H, Schmid-Hempel P. Socially transmitted gut microbiota protect bumble bees against an intestinal parasite. Proc Natl Acad Sci U S A. 2011; 108 : 19288–92. doi: 10.1073/pnas.1110474108 22084077
37. Vigneron A, Masson F, Vallier A, Balmand S, Rey M, Vincent-Monégat C, et al. Insects recycle Endosymbionts when the benefit is over. Curr Biol. 2014; 24 : 2267–2273. doi: 10.1016/j.cub.2014.07.065 25242028
38. Stoll S, Feldhaar H, Fraunholz MJ, Gross R. Bacteriocyte dynamics during development of a holometabolous insect, the carpenter ant Camponotus floridanus. BMC Microbiol. 2010; 10 : 308. doi: 10.1186/1471-2180-10-308 21122115
39. Nicholson D, Ross A, Mayhew P. Fossil evidence for key innovations in the evolution of insect diversity. Proc R Soc B Biol Sci. 2014; 281 : 1783. Available: http://rspb.royalsocietypublishing.org/content/281/1793/20141823.short
40. Arendt J. Adaptive intrinsic growth rates: an integration across taxa. Q Rev Biol. 1997; 72 : 149–177.
41. Godfray H. Parasitoids: behavioral and evolutionary ecology. Princeton University Press; 1994.
42. Lazzaro BP, Rolff J. Danger, microbes, and homeostasis. Science. 2011; 332 : 43–44. doi: 10.1126/science.1200486 21454776
43. Fedhila S, Buisson C, Dussurget O, Serror P, Glomski IJ, Liehl P, et al. Comparative analysis of the virulence of invertebrate and mammalian pathogenic bacteria in the oral insect infection model Galleria mellonella. J Invertebr Pathol. 2010; 103 : 24–29. doi: 10.1016/j.jip.2009.09.005 19800349
44. Dunny GM, Lee LN, LeBlanc DJ. Improved electroporation and cloning vector system for gram-positive bacteria. Appl Environ Microbiol. 1991; 57 : 1194–201. Available: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=182867&tool=pmcentrez&rendertype=abstract 1905518
45. Hébert L, Courtin P, Torelli R, Sanguinetti M, Chapot-Chartier MP, Auffray Y, et al. Enterococcus faecalis constitutes an unusual bacterial model in lysozyme resistance. Infect Immun. 2007; 75 : 5390–5398. doi: 10.1128/IAI.00571-07 17785473
46. Ausubel FM, Brent R, Kingston RE, Moore DD, Seidman JG, Smith J a, et al. Current Protocols in Molecular Biology. Molecular Biology. 2003.
47. Caporaso JG, Kuczynski J, Stombaugh J, Bittinger K, Bushman FD, Costello EK, et al. QIIME allows analysis of high-throughput community sequencing data. Nature methods. 2010; 7 : 335–336. doi: 10.1038/nmeth.f.303 20383131
48. Edgar RC. Search and clustering orders of magnitude faster than BLAST. Bioinformatics. 2010; 26 : 2460–2461. doi: 10.1093/bioinformatics/btq461 20709691
49. Hermann-Bank ML, Skovgaard K, Stockmarr A, Larsen N, Mølbak L. The gut microbiotassay: a high-throughput qPCR approach combinable with next generation sequencing to study gut microbial diversity. BMC Genomics. 2013;14 : 788. doi: 10.1186/1471-2164-14-788 24225361
50. Arboleya S, Binetti A, Salazar N, Fernández N, Solís G, Hernández-Barranco A, et al. Establishment and development of intestinal microbiota in preterm neonates. FEMS Microbiol Ecol. 2012; 79 : 763–772. doi: 10.1111/j.1574-6941.2011.01261.x 22126419
51. Simon D, Chopin A. Construction of a vector plasmid family and its use for molecular cloning in Streptococcus lactis. Biochimie. 1988; 0 : 559–66. Available: http://www.ncbi.nlm.nih.gov/pubmed/2844302 2844302
52. Kühn A, Piepho H. Über hormonale Wirkungen bei der Verpuppung der Schmetterlinge. Nachrichten von der Gesellschaft der Wissenschaften zu Göttingen. 1936; 2 : 141–154.
53. Ellis JD, Graham JR, Mortensen A. Standard methods for wax moth research. J Api Res. 2013; 52 : 1–18. doi: 10.3896/IBRA.1.52.1.10
54. Vogel H, Altincicek B, Glöckner G, Vilcinskas A. A comprehensive transcriptome and immune-gene repertoire of the lepidopteran model host Galleria mellonella. BMC Genomics. 2011; 12 : 308. doi: 10.1186/1471-2164-12-308 21663692
55. Wojda I, Jakubowicz T. Humoral immune response upon mild heat-shock conditions in Galleria mellonella larvae. J Insect Physiol. 2007; 53 : 1134–1144. doi: 10.1016/j.jinsphys.2007.06.003 17631308
56. Therneau T. A Package for Survival Analysis in S. R package version 2.37–7 [Internet]. Survival. 2014. Available: http://cran.r-project.org/package=survival
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek Increased Susceptibility of Humanized NSG Mice to Panton-Valentine Leukocidin and Skin InfectionČlánek Phosphorylation of a Myosin Motor by TgCDPK3 Facilitates Rapid Initiation of Motility during egress
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2015 Číslo 11- Stillova choroba: vzácné a závažné systémové onemocnění
- Perorální antivirotika jako vysoce efektivní nástroj prevence hospitalizací kvůli COVID-19 − otázky a odpovědi pro praxi
- Diagnostika virových hepatitid v kostce – zorientujte se (nejen) v sérologii
- Jak souvisí postcovidový syndrom s poškozením mozku?
- Infekční komplikace virových respiračních infekcí – sekundární bakteriální a aspergilové pneumonie
-
Všechny články tohoto čísla
- Parasite Glycobiology: A Bittersweet Symphony
- On the Discovery of TOR As the Target of Rapamycin
- Broadening of Virus-Specific CD8 T-Cell Responses Is Indicative of Residual Viral Replication in Aviremic SIV Controllers
- PML/TRIM19-Dependent Inhibition of Retroviral Reverse-Transcription by Daxx
- Cleavage of a Neuroinvasive Human Respiratory Virus Spike Glycoprotein by Proprotein Convertases Modulates Neurovirulence and Virus Spread within the Central Nervous System
- Kaposi’s Sarcoma-Associated Herpesvirus (KSHV) Induces the Oncogenic miR-17-92 Cluster and Down-Regulates TGF-β Signaling
- Interferon-α Subtypes in an Model of Acute HIV-1 Infection: Expression, Potency and Effector Mechanisms
- Perivascular Arrest of CD8 T Cells Is a Signature of Experimental Cerebral Malaria
- Targeting HIV Reservoir in Infected CD4 T Cells by Dual-Affinity Re-targeting Molecules (DARTs) that Bind HIV Envelope and Recruit Cytotoxic T Cells
- Evolution and Emergence of Enteroviruses through Intra- and Inter-species Recombination: Plasticity and Phenotypic Impact of Modular Genetic Exchanges in the 5’ Untranslated Region
- Interferon-γ Inhibits Ebola Virus Infection
- Dengue Virus Non-structural Protein 1 Modulates Infectious Particle Production via Interaction with the Structural Proteins
- P-Type Cyclin CYC3 Modulates Endomitotic Growth during Oocyst Development in Mosquitoes
- Diversity of across Evolutionary Scales
- 50 Years of Disease in Humans: The Dramatic Emergence of a Cluster of Novel Fungal Pathogens
- Worse Comes to Worst: Bananas and Panama Disease—When Plant and Pathogen Clones Meet
- Arenavirus Glycan Shield Promotes Neutralizing Antibody Evasion and Protracted Infection
- Infection-Induced Retrotransposon-Derived Noncoding RNAs Enhance Herpesviral Gene Expression via the NF-κB Pathway
- Structural Insight into Archaic and Alternative Chaperone-Usher Pathways Reveals a Novel Mechanism of Pilus Biogenesis
- Increased Susceptibility of Humanized NSG Mice to Panton-Valentine Leukocidin and Skin Infection
- Global Analysis of the Fungal Microbiome in Cystic Fibrosis Patients Reveals Loss of Function of the Transcriptional Repressor Nrg1 as a Mechanism of Pathogen Adaptation
- The Transcription and Translation Landscapes during Human Cytomegalovirus Infection Reveal Novel Host-Pathogen Interactions
- The N-terminal Helical Region of the Hepatitis C Virus p7 Ion Channel Protein Is Critical for Infectious Virus Production
- Activation of Type I and III Interferon Response by Mitochondrial and Peroxisomal MAVS and Inhibition by Hepatitis C Virus
- Hsp70 Isoforms Are Essential for the Formation of Kaposi’s Sarcoma-Associated Herpesvirus Replication and Transcription Compartments
- Distinct Upstream Role of Type I IFN Signaling in Hematopoietic Stem Cell-Derived and Epithelial Resident Cells for Concerted Recruitment of Ly-6C Monocytes and NK Cells via CCL2-CCL3 Cascade
- and Bats: Story of an Emerging Friendship
- Emergence of Pathogenicity in Lagoviruses: Evolution from Pre-existing Nonpathogenic Strains or through a Species Jump?
- Ebolavirus Evolution: Past and Present
- Host and Symbiont Jointly Control Gut Microbiota during Complete Metamorphosis
- Non-Human Primates Harbor Diverse Mammalian and Avian Astroviruses Including Those Associated with Human Infections
- Lactate Dehydrogenase Is Associated with the Parasitophorous Vacuole Membrane and Is a Potential Target for Developing Therapeutics
- Five Questions about Mycoviruses
- Phosphorylation of a Myosin Motor by TgCDPK3 Facilitates Rapid Initiation of Motility during egress
- Ethanolamine Signaling Promotes Niche Recognition and Adaptation during Infection
- Cross-Species Transmission and Differential Fate of an Endogenous Retrovirus in Three Mammal Lineages
- Memory Th1 Cells Are Protective in Invasive Infection
- Transcription Factor SomA Is Required for Adhesion, Development and Virulence of the Human Pathogen
- An -Methyltransferase Is Required for Infection of Tick Cells by
- RNA-seq Brings New Insights to the Intra-Macrophage Transcriptome of Typhimurium
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Dengue Virus Non-structural Protein 1 Modulates Infectious Particle Production via Interaction with the Structural Proteins
- On the Discovery of TOR As the Target of Rapamycin
- Parasite Glycobiology: A Bittersweet Symphony
- Broadening of Virus-Specific CD8 T-Cell Responses Is Indicative of Residual Viral Replication in Aviremic SIV Controllers
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



