-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Genetics, Receptor Binding Property, and Transmissibility in Mammals of Naturally Isolated H9N2 Avian Influenza Viruses
Avian influenza viruses continue to present challenges to human health. Recently the H7N9 and H10N8 viruses that are of low pathogenicity for poultry have caused human infections and deaths in China. H9N2 influenza virus have been isolated worldwide from wild and domestic avian species for several decades, and their low pathogenic nature to poultry made them a low priority for animal disease control, which has allowed them to continue to evolve and spread. Here, we investigated a series of H9N2 influenza viruses that were detected in live poultry markets in southern China. We found that these viruses are able to preferentially bind to the human-type receptor, and some of them can cause disease and transmit between ferrets by respiratory droplet. All the transmissible H9N2 viruses have a similar internal gene constellation, which was also present in the H7N9 and H10N8 viruses. Our study indicates that the widespread dissemination of H9N2 viruses poses a threat to human health not only because of the potential of these viruses to cause an influenza pandemic, but also because they can function as “vehicles” to deliver different subtypes of influenza viruses from avian species to humans.
Published in the journal: . PLoS Pathog 10(11): e32767. doi:10.1371/journal.ppat.1004508
Category: Research Article
doi: https://doi.org/10.1371/journal.ppat.1004508Summary
Avian influenza viruses continue to present challenges to human health. Recently the H7N9 and H10N8 viruses that are of low pathogenicity for poultry have caused human infections and deaths in China. H9N2 influenza virus have been isolated worldwide from wild and domestic avian species for several decades, and their low pathogenic nature to poultry made them a low priority for animal disease control, which has allowed them to continue to evolve and spread. Here, we investigated a series of H9N2 influenza viruses that were detected in live poultry markets in southern China. We found that these viruses are able to preferentially bind to the human-type receptor, and some of them can cause disease and transmit between ferrets by respiratory droplet. All the transmissible H9N2 viruses have a similar internal gene constellation, which was also present in the H7N9 and H10N8 viruses. Our study indicates that the widespread dissemination of H9N2 viruses poses a threat to human health not only because of the potential of these viruses to cause an influenza pandemic, but also because they can function as “vehicles” to deliver different subtypes of influenza viruses from avian species to humans.
Introduction
Avian influenza viruses of several subtypes continue to present challenges to human health. The H5N1 influenza viruses have caused 380 fatal cases among the 641 documented human infections in 16 countries [1], and several studies have documented the transmission potential of H5N1 mutants or reassortants [2]–[4]. Eighty-seven human cases of H7N7 influenza virus infection were confirmed in the Netherlands in 2003, one of which was fatal [5], [6]. H6 viruses can infect and cause illness in mice and ferrets, and are transmissible in guinea pigs [7]–[9]; an H6N1 virus was isolated from a human with influenza-like symptoms in Taiwan in 2013 [10]. An approximately 30% mortality rate is associated with the 400 human infections with the newly emerged H7N9 viruses in China reported by the end of March, 2014 [11]. H10N8 virus caused three human infections in China in 2013, two of which were fatal [12]. These facts emphasize that it is not only the highly pathogenic H5N1 and H7N7 influenza viruses that pose a severe threat to human health, but also that the nonlethal influenza viruses circulating in avian species can cause disease and even death in humans.
During the last several decades, H9N2 influenza viruses have been isolated worldwide from wild and domestic avian species [13], [14]. These viruses have also been detected in pigs [15]–[17]. Many studies have been performed to evaluate the pandemic potential of the H9N2 influenza viruses. The viruses have been shown to replicate in mice without pre-adaptation [18]–[21], and some strains from poultry in Asia have human virus-like receptor specificity [22]. Sorrell et al. reported that following adaptation in the ferret, a reassortant carrying the surface proteins of an avian H9N2 in a human H3N2 backbone could transmit efficiently via respiratory droplet [23]. Other studies have reported that H9N2 reassortants bearing genes from the 2009 H1N1 pandemic virus exhibited increased virulence in mice [24] or transmissibility in ferrets [25]. Wan et al. found that two of five wild-type H9N2 viruses isolated from different avian species between 1988 and 2003 transmitted to direct contact ferrets [26]. However, none of the naturally isolated H9N2 viruses has been reported to transmit to ferrets via respiratory droplet.
Although the H9N2 viruses have been detected in chickens and ducks in many provinces in China since 1993 [18], [27], their low pathogenic nature to poultry has made them a low priority for animal disease control. However, the H9N2 viruses caused human infections in China in 1999, 2003, and 2013 [28]–[30], and some poultry workers in China, India, Cambodia, Romania, America, Nigeria, and Vietnam were reportedly serologically positive for H9N2 viruses [31]–[38], implying a substantial threat to public health. Recent studies indicated that the H9N2 viruses contributed the six internal genes to the newly emerged H7N9 virus in southern China and to the H10N8 virus that caused three human infections in Jiangxi province, China [12], [39], [40]. These facts prompted us to assess the biologic properties and pandemic potential of H9N2 influenza viruses circulating in poultry.
Results
Genetic characterization of H9N2 influenza viruses isolated in poultry between 2009 and 2013
To investigate the genetic relationship of the viruses from different times and places, we sequenced the genomes of 35 viruses that were collected from 2009 to 2013 from 12 provinces in southern China (Figure S1). The amino acid motif at the cleavage site of the hemagglutinin (HA) of these isolates is –RSSR-, which is a characteristic of viruses of low pathogenicity in chickens. The HA gene of the 35 viruses shared 87.8%–99.7% identity at the nucleotide level, and they formed five phylogenetic groups (Figure 1A). The neuraminidase (NA) genes of these viruses shared 85.4%–99.6% identity at the nucleotide level and formed four phylogenetic groups (Figure S2A). The 31 viruses in groups 1, 2 and 3 have a 3-amino acid deletion in the NA stalk (residues 62–64), whereas the four isolates in group 4 have no such deletion.
Fig. 1. Genetic relationships among the HA genes and genotype evolution of H9N2 viruses. 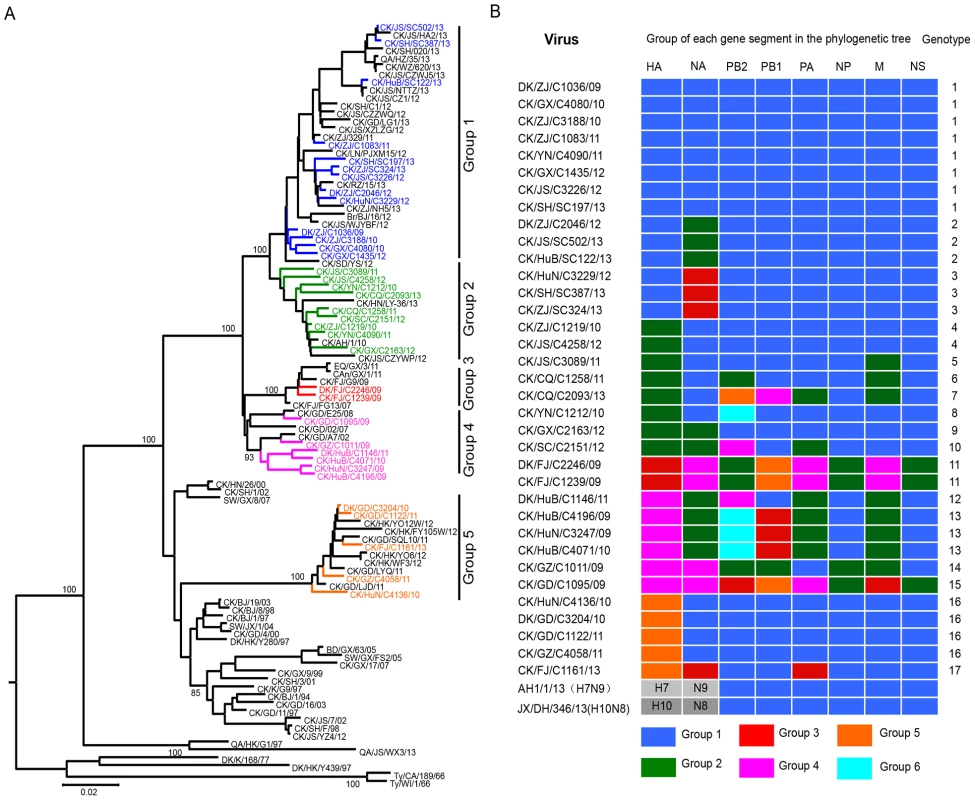
(A) Phylogenetic tree of HA. The tree was based on nucleotides (nt) 128 to 1540 and rooted to A/Duck/Alberta/60/76(H12N5). Sequences of viruses with names in black were downloaded from available databases; viruses with names in other colors were sequenced in this study. Abbreviations are as follows: Br, Brambling; CAn, Canine; CK, Chicken; DK, Duck; Env, Environment; GS, Goose; Pg, Pigeon; QA, Quail; SW, Swine; Ty, Turkey; AH, Anhui; BJ, Beijing; CA, California; CQ, Chongqing; DH, Donghu; FJ, Fujian; GD, Guangdong; GX, Guangxi; GZ, Guangzhou; HZ, Hangzhou; HN, Henan; HK, Hong Kong; HuB, Hubei; HuN, Hunan; JS, Jiangsu; SD, Shandong; SH, Shanghai; TZ, Taizhou; WI, Wisconsin; XZ, Xuzhou; YZ, Yangzhou; YN, Yunnan; ZJ, Zhejiang. (B) Genotypes of the H9N2 viruses. The eight gene segments are indicated at the top of each bar. Several amino acid changes related to the increased replication or virulence of avian influenza viruses in mammals [41] were detected in these H9N2 viruses (Table S1). The amino acid changes R207K, H436Y, and M677T in basic polymerase 1 (PB1) [42], [43], A515T in acidic polymerase (PA) [42], N30D and T215A in matrix protein (M1) [44], and P42S in nonstructural protein 1 (NS1) were conserved in all strains [45], whereas the virulence-related mutation I368V in PB1[3], T139A in M1[46], and the amantadine and rimantadine resistance-conferring mutation S31N/G in M2 were detected in some of the strains (Table S1) [47]. Two amino acid changes in basic polymerase 2 (PB2), glutamic acid to lysine at position 627(E627K) and aspartic acid to asparagine at position 701 (D701N), are important for the virulence and transmission of H5N1 viruses in mammals [48]–[50], and are also frequently presented in the H7N9 viruses isolated from humans [40], [51]. A detailed comparison of the amino acid differences among the H9N2 viruses showed that all 35 of the H9N2 viruses have the amino acid combination of 627E/701D in their PB2.
The six internal genes of the H9N2 viruses showed distinct diversity, with PB2, PB1, PA, nucleoprotein (NP), M, NS genes of the 35 viruses sharing 84.8%–99.4%, 86.4%–99.6%, 87.9%–99.5%, 92.5%–99.6%, 94.1%–99.9%, and 91.3%–99.6% identity, respectively, at the nucleotide level. The PB2 genes formed six groups in their polygenic trees (Figure S2B), and the PB1 genes formed five groups in their polygenic trees (Figure S2C). The PA and M genes each formed four groups in their polygenic trees (Figure S2D and Figure S2F), whereas the NP and NS genes each formed two groups in their polygenic trees (Figure S2E and Figure S2G).
On the basis of this genomic diversity, the viruses examined in this study were divided into 17 genotypes (Figure 1B). Of note, 19 viruses in genotypes 1, 2, 3, 4, and 16 have a similar combination of their six internal genes (the DK/ZJ/C1036/09-like combination).
Receptor-binding preference of the H9N2 influenza viruses
Receptor-binding preference has important implications for influenza virus replication and transmission [3], [4]. The change of receptor-binding preference from α-2, 3-linked sialic acids (Sias) (avian-type receptors) to α-2, 6-linked Sias (human-type receptors) is thought to be a prerequisite for an avian influenza virus to transmit from human to human. By using a solid-phase binding assay as described previously [4], [7], we tested the receptor-binding specificity of 41 H9N2 viruses, the 35 viruses described above and six "early" viruses that were isolated from poultry in China between 1996 and 2001 [18] (Figure 2, Figure S3), to two different glycopolymers: the α-2, 3-siaylglycopolymer [Neu5Acα2-3Galβ1-4GlcNAcβ1-pAP (para-aminophenyl)-alpha-polyglutamic acid (α-PGA)] and the α-2, 6-sialylglycopolymer [Neu5Acα2-6Galβ1-4GlcNAcβ1-pAP (para-aminophenyl)-alpha-polyglutamic acid (α-PGA)]. All 41 viruses were able to bind to the α-2, 6-siaylglycopolymer, although eight, including the six "early" viruses, also bound to the α-2, 3-siaylglycopolymer with moderate to high affinity (Figure 2, Figure S3, and Table S2). These results indicate that H9N2 viruses isolated naturally from poultry have acquired the ability to preferentially bind to the human-type receptor, similar to the widely circulating human influenza viruses.
Fig. 2. Characterization of the receptor-binding properties of H9N2 viruses. 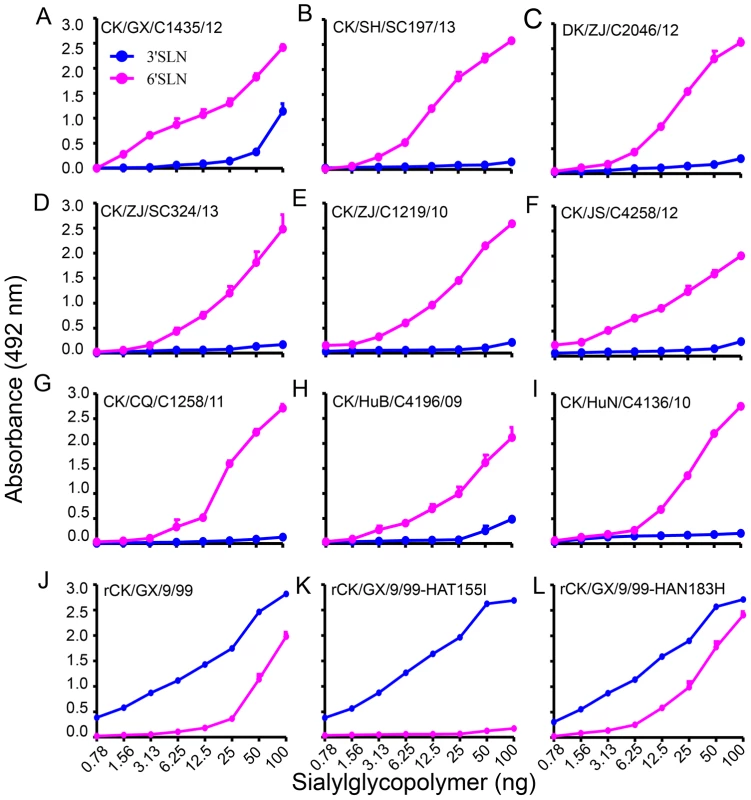
The binding of the viruses to two different biotinylated glycans (α-2, 3 glycan, blue; α-2, 6 glycan, pink) was tested. The data shown are the means of three repeats; the error bars indicate the standard deviations. (A) CK/GX/C1435/12. (B) CK/SH/SC197/13. (C) DK/ZJ/C2046/12. (D) CK/ZJ/SC324/13. (E) CK/ZJ/C1219/10. (F) CK/JS/C4258/12. (G) CK/CQ/C1258/11. (H) CK/HuB/C4196/09. (I) CK/HuN/C4136/10. (J) rCK/GX/9/99. (K) rCK/GX/9/99-HAT155I. (L) rCK/GX/9/99-HAN183H. The amino acid mutation I155T (H3 numbering used throughout) in HA favors the binding of H9N2 virus to the α-2, 6-siaylglycopolymer
The amino acid change Q226L is reported to contribute to the human-type receptor binding of H9N2 virus [26], but this mutation was not detected in the six "early" isolates in our study (Table S1), which were able to bind to the α-2, 6-siaylglycopolymer (Figure S3), suggesting that some other amino acid(s) may contribute to this phenotype. As shown in Table S1, three more amino acid changes (I155T, H183N, and A190V) that have been reported to affect the receptor binding preference of other subtypes of influenza viruses were also detected in these H9N2 viruses. Since the amino acid at position 190 of HA was not conserved, and this amino acid has not been linked to the receptor-binding phenotype observed, we investigated the contributions of only I155T and H183N to the human-type receptor binding of H9N2 virus.
By using plasmid-based reverse genetics, we constructed a reassortant virus containing the HA and NA genes of the "early" H9N2 isolate A/chicken/Guangxi/9/99 (CK/GX/9/99) and the six internal genes of the A/Puerto Rico/8/1934 (H1N1) (PR8) virus and designated it as rCK/GX/9/99. Then, we introduced the avian influenza virus-like amino acids I and H into the HA gene at positions 155 and 183, respectively, to create the mutants we designated as rCK/GX/9/99-HAT155I and rCK/GX/9/99-HAN183H, respectively. Receptor binding analysis indicated that, similar to the wild-type CK/GX/9/99 virus (Figure S3), the rCK/GX/9/99 and rCK/GX/9/99-HAN183H viruses bound to both the α-2, 3-siaylglycopolymer and α-2, 6-siaylglycopolymer (Figure 2J and L); however, the rCK/GX/9/99-HAT155I variant only maintained the ability to bind to the α-2, 3-siaylglycopolymer and lost its ability to bind to the α-2, 6-siaylglycopolymer (Figure 2K). These results indicate that, in addition to the Q226L mutation, the I155T mutation in HA also plays an important role in the binding of H9N2 virus to the human-type receptor.
Replication and virulence of the H9N2 viruses in mice
We selected 26 H9N2 influenza viruses, to include one virus from each genotype from each year, and evaluated their replication and virulence in BALB/c mice. All 26 viruses replicated in lungs of mice, with titers ranging from 1.8 to 6.8log10TCID50, 22 viruses were also detected in the nasal turbinates of mice, with titers ranging from 0.7 to 5.3log10TCID50 (Figure 3). Virus was not detected in the spleen, kidneys, or brain of any mice. Mice infected with these viruses showed diverse body weight changes during the observation period: fourteen viruses caused 1.5% to 17.5% body weight loss in mice, whereas mice gained body weight despite inoculation with the other twelve viruses (Figure 3). All mice survived during the observation period. These results indicate that, unlike the H7N9 viruses isolated from poultry, which did not cause any disease in mice [40], the H9N2 viruses isolated from poultry show a range of virulence in mice.
Fig. 3. Replication and virulence of H9N2 viruses in mice. 
Virus titers in organs of mice on day 3 p.i. with 106 EID50 of test virus. Data shown are the mean titers from three mice; the error bars indicate the standard deviations. *, mice inoculated with CK/ZJ/C1219/10-PB2/627K virus died before day 8 p.i. The dashed line indicates the lower limit of detection. Replication of H9N2 viruses in ferrets
As shown in Figure 1B and Table S3, the viruses of genotypes 1–4, 13, and 16 were detected from multiple provinces and in different years, and we therefore selected one or two viruses from these genotypes and tested their replication and transmission in ferrets. However, the viruses of genotypes 5–12, 14, 15, and 17 were only isolated from individual provinces; therefore, we speculated that those strains were unlikely to be widespread and, accordingly, only one strain from genotype 6 was selected and tested in ferrets (Table S3).
Two ferrets were inoculated i.n. with 106 EID50 of each virus, and the nasal turbinates, tonsils, trachea, various lung lobes, brain, spleen, kidneys, and liver from each ferret were collected on day 4 p.i. for virus titration in MDCK cells. All of the nine viruses replicated in the nasal turbinates of ferrets, with titers ranging from 2.8–7.3log10TCID50 (Figure 4). Virus replication in trachea was detected in ferrets inoculated by eight viruses, but not in the CK/ZJ/C1219/10 virus-infected ferrets (Figure 4E). Virus was detected in all lobes of lungs of ferrets inoculated with CK/ZJ/SC324/13 and CK/HuB/C4196/09 (Figure 4D and H), but was not detectable in some of lobes of the lungs of ferrets inoculated with the other seven viruses (Figure 4). Virus was detected in the spleen of one ferret inoculated with CK/JS/C4258/12 and two ferrets inoculated with CK/CQ/C1258/11(Figure 4F and G), but was not detected in the brain, kidney, or liver of any ferret.
Fig. 4. Replication of H9N2 viruses in ferrets. 
(A) CK/GX/C1435/12. (B) CK/SH/SC197/13. (C) DK/ZJ/C2046/12. (D) CK/ZJ/SC324/13. (E) CK/ZJ/C1219/10. (F) CK/JS/C4258/12. (G) CK/CQ/C1258/11. (H) CK/HuB/C4196/09. (I) CK/HuN/C4136/10. (J) CK/ZJ/C1219/10-PB2/627K. (K) CK/ZJ/C1219/10-PB2/701N. Each color bar represents the virus titer from an individual animal. The dashed black lines indicate the lower limit of detection. Pathological studies were performed on lung samples from the virus-infected ferrets. Most of the lungs showed mild damage after infection with CK/ZJ/C1219/10 or DK/ZJ/C2046/12 (Figure 5A, Figure S4A). By contrast, the lungs of the other seven virus-infected ferrets showed severe bronchopneumonia (Figure 5 B and C, and Figure S4B–F).
Fig. 5. Histological lesions caused by H9N2 viruses in the lungs of ferrets. 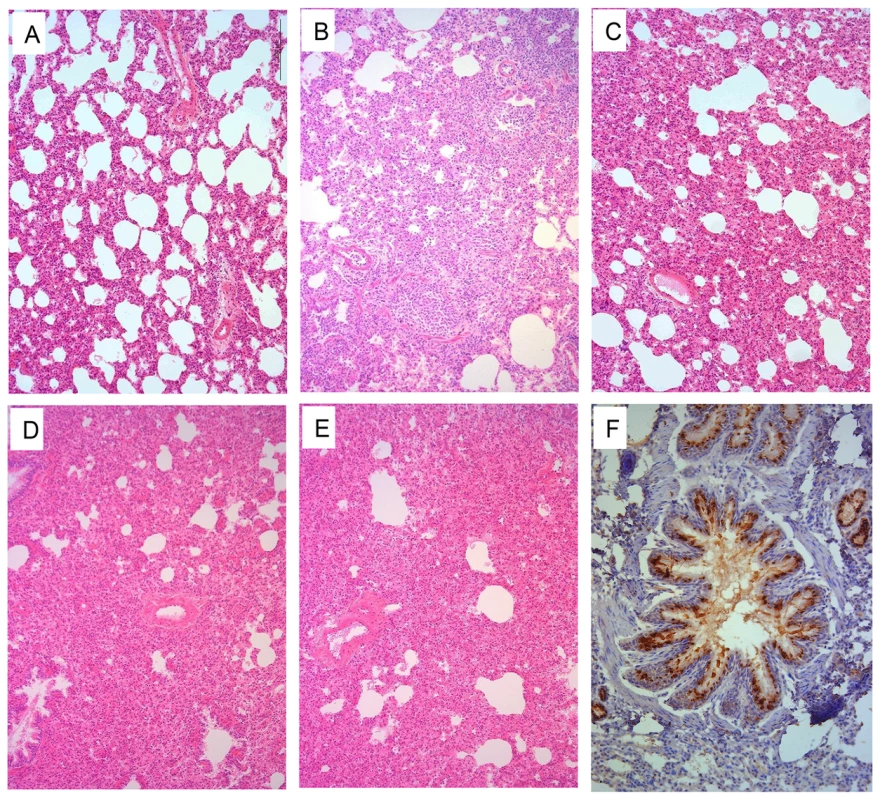
Ferrets were euthanized on day 4 p.i. with 106EID50 of test virus, and the lungs were collected for pathological study. The lungs of CK/ZJ/C1219/10 virus-inoculated animal showed only mild histopathological changes (H&E staining,) (A), whereas the lungs of CK/GX/C1435/12 (B), CK/JS/C4258/12 (C), CK/ZJ/C1219/10-PB2/627K (D), and CK/ZJ/C1219/10-PB2/701N (E) virus-inoculated ferrets showed severe pathological lesions (H&E staining) Viral antigen was detected in the epithelial cells of bronchus and alveoli by means of immunohistochemical (IHC) staining (F, from the lung samples of a ferret inoculated with CK/JS/C4258/12 virus). Images A–E were taken at ×100 magnification; Image F was taken at ×200 magnification. Transmission of H9N2 influenza viruses between ferrets
To investigate respiratory droplet transmission, we inoculated three ferrets i.n. with 106.0 EID50 of test virus and then housed them separately in solid stainless-steel cages within an isolator. Twenty-four hours later, three naïve ferrets were placed in adjacent cages. Each pair of animals was separated by a double-layered net divider as described previously [2], [40]. Nasal washes were collected every 2 days from all of the animals beginning 2 days p.i. [1 day post-exposure (p.e.)] for the detection of virus shedding. Sera were collected from all animals on day 21 p.i. for hemagglutinin inhibition (HI) antibody detection. Respiratory droplet transmission was confirmed when virus was detected in the nasal washes or by seroconversion of the naïve exposed animals at the end of the 3-week observation period.
Virus was detected in all of the directly infected animals (Figure 6A–L). However, virus was not detected in any of the animals exposed to the CK/CQ/C1258/11-, CK/HuB/C4196/09-, and CK/HuN/C4136/10-inoculated ferrets (Figure 6G, H, and I). Virus was detected in one ferret exposed to the ferrets that had been inoculated with the CK/SH/SC197/13, DK/JS/C2046/12, CK/ZJ/SC324/13, and CK/ZJ/C1219/10 viruses (Figure 6B, C, D, and E). Virus was detected in all three ferrets exposed to the ferrets that had been inoculated with CK/GX/C1435/12 and CK/JS/C4258/12 (Figure 6A and F). Because the efficient transmission of naturally isolated H9N2 influenza viruses has never been reported before, we repeated this respiratory droplet transmission study with the CK/JS/C4258/12 virus in ferrets and found the results to be reproducible (Figure 6J). The ferrets that were inoculated with these nine viruses experienced a 1.4% to 7.9% body weight loss, and the body weight loss of the exposed ferrets was up to 8.8% (Table 1 and Table S4). Seroconversion occurred in all of the virus-inoculated animals and in all exposed animals that were virus-positive (Table 1). These results indicate that six of the nine H9N2 viruses tested can transmit between ferrets, and two of them transmit highly efficiently via respiratory droplet.
Fig. 6. Respiratory droplet transmission of H9N2 viruses in ferrets. 
(A) CK/GX/C1435/12. (B) CK/SH/SC197/13. (C) DK/ZJ/C2046/12. (D) CK/ZJ/SC324/13. (E) CK/ZJ/C1219/10. (F) CK/JS/C4258/12. (G) CK/CQ/C1258/11. (H) CK/HuB/C4196/09. (I) CK/HuN/C4136/10. (J) CK/JS/C4258/12 (replicate). (K) CK/ZJ/C1219/10-PB2/627K. (L) CK/ZJ/C1219/10-PB2/701N. Each color bar represents the virus titer from an individual animal. The dashed black lines indicate the lower limit of detection. Tab. 1. Virus replication in and seroconversion of ferrets inoculated with or exposed to different H9N2 influenza viruses. 
Data shown are from the animal in that group with the maximum body temperature increase or maximum body weight loss. The 627K and 701N mutations in PB2 increase the virulence and transmission of H9N2 viruses in mammals
Previous studies showed that the H7N9 viruses easily acquire the 627K or 701N mutations in PB2 during their replication in humans [40], [52], [53]. To investigate whether the H9N2 viruses similarly acquire such mutations during replication in ferrets, we sequenced the PB2 gene of ten randomly selected clones of each sample recovered at different time points from each ferret. The 627K or 701N mutations in PB2 were detected from the samples recovered from the ferrets that were inoculated or exposed to CK/GX/C1435/12, CK/ZJ/C1219/10, CK/CQ/C1258/11, CK/JS/C4258/12, CK/HuB/C4196/09, and CK/HuN/C4136/10, but were not detected in the samples recovered from the ferrets that were inoculated with or exposed to CK/SH/SC197/13, DK/ZJ/C2046/12, and CK/ZJ/SC324/13 viruses (Table 2). To investigate whether these mutations existed in the inoculums, the PB2 gene of all nine viruses tested in ferrets was checked by using a deep sequencing approach; the 627K and 701N mutations in PB2 were not detected in any of the viral stocks (Table 2, Table S5). We also deep sequenced the PB2 of nine selected samples that were recovered from the virus-inoculated ferrets, and found that the ratios of the PB2 627K and 701N mutations were comparable to our previous sequencing results presented in Table 2 (Table S5).
Tab. 2. Assessment of the presence or absence of the 627K and 701N mutations in the PB2 segment of viruses recovered from the nasal washes of H9N2 influenza virus infected or exposed ferrets. 
Results were obtained by using a deep sequencing approach. To investigate whether the 627K or 701N mutations in PB2 could increase the virulence and transmissibility of the H9N2 viruses in mammals, we plaque-purified two mutants, CK/ZJ/C1219/10-PB2/627K and CK/ZJ/C1219/10-PB2/701N, and tested them in mice and ferrets. The viral titers in the nasal turbinates and lungs of the mutant-infected mice were significantly higher than that of the CK/ZJ/C1219/10 virus-inoculated mice (Figure 3). The CK/ZJ/C1219/10-PB2/627K virus killed all five mice by day 6 p.i., whereas the mice inoculated with the CK/ZJ/C1219/10-PB2/701N virus experienced a 9.2% body weight loss and survived the infection for the observation period (Figure 3). Viral titers in the nasal turbinates and lungs of the two mutant-inoculated ferrets were notably higher than that of the CK/ZJ/C1219/10-inoculated ferrets, and virus was also detected in the spleen of one ferret inoculated with the CK/ZJ/C1219/10-PB2/627K virus (Figure 4, J and K). The lung damage of the two mutant-inoculated ferrets was much more severe than that of the CK/ZJ/C1219/10-inoculated ferrets (Figure 5, D and E). Both mutants transmitted to two of three ferrets via respiratory droplet (Figure 6, K and L, Table 1). The CK/ZJ/C1219/10-PB2/627K-inoculated and -exposed ferrets experienced 6.4% and 4.5% weight loss, respectively, and the body weight loss of the CK/ZJ/C1219/10-PB2/701N-inoculated and -exposed ferrets was 7.2% and 4.4%, respectively (Table 1, Table S4). These results indicate that the 627K and 701N mutations in PB2 increase the virulence and further promote the transmissibility of H9N2 viruses in mammals.
Discussion
Receptor-binding preference has important implications for influenza virus replication and transmission [3], [4], [54], [55]. Generally, it is believed that the HA of human infective influenza subtypes preferentially recognizes α-2, 6 - linked Sias, whereas the HA of avian influenza subtypes preferentially recognizes α-2, 3-linked Sias [3], [56]. Some naturally isolated avian influenza viruses of the H5 and H6 subtypes have been reported to bind to α-2, 6-linked Sias [2], [7], [50], but their affinity to the α-2, 3-linked Sias was much higher than that to α-2, 6-linked Sias. The newly emerged H7N9 viruses isolated from both avian species and humans bind to α-2, 6-linked Sias with high affinity and to α-2, 3-linked Sias with affinity that varies among strains [40], [57]. However, most of the H9N2 viruses circulating in China bind exclusively to α-2, 6-linked Sias, as is observed with human influenza viruses. Thus influenza viruses can acquire the ability to bind human-type receptors during their circulation in avian species, and mammalian intermediate hosts, for example pigs, are not necessarily needed for this process.
The molecular determinants of the receptor binding preference of influenza viruses are not fully understood. Several amino acid changes in HA, including I155T, H183N, A190V, Q226L, and G228S, have been reported to promote the affinity of avian influenza viruses for human-type receptors [22], [50], [54], [58], [59]. Although 155T and 183N were conserved in all of the H9N2 viruses (Table S1), our mutagenesis study indicated that 155T, but not 183N, is necessary for the H9N2 virus to bind to the human-type receptor. The amino acid at 190 was not conserved in these strains, and the avian influenza virus-like 190A was present in several viruses that exclusively bound to the α-2,6-linked Sias; the amino acid change G228S was not detected in any of our H9N2 viruses. Therefore, the H183N, A190V, and G228S mutations in HA are not necessary for an H9N2 virus to bind to the human-type receptor, whereas the mutations I155T and Q226L play important roles in H9N2 virus binding to the human-type receptor.
Avian influenza viruses can acquire different mutations that confer increased receptor-binding ability, virulence, or transmissibility during their replication in mammalian hosts, and some of these mutations have been detected in the H9N2 viruses (Table S1), although we were not able to find a strong relationship between these changes and the observed virulence or transmissibility in mammals of our H9N2 viruses. The 627K and 701N mutations in PB2 were detected in some viruses recovered from both inoculated and exposed ferrets, indicating that certain H9N2 viruses are predisposed to acquiring the 627K or 701N mutation in their PB2 gene when they replicate in mammals. However, the absence of the 627K and 701N mutations in the PB2 of some of the viruses recovered from the exposed animals (Table 2, Table S5) suggests that transmission of H9N2 viruses in ferrets may be independent of these changes, although such changes in PB2 could further increase their virulence and transmission, as was seen with the H5N1 and H7N9 viruses [48]–[50], [52], [60].
In addition to the receptor-binding preference conferred by HA, internal gene combinations also play a determinative role in virus transmissibility in mammals [2]. Similar to other avian influenza viruses circulating in poultry in Southern China [7], [61]–[63], the H9N2 viruses formed multiple genotypes. The DK/ZJ/C1036/09-like internal gene combination was detected in the H9N2 viruses with different groups of HA and NA genes that were isolated between 2009 and 2013 in nine of the 12 provinces investigated, and was also detected in the H7N9 and H10N8 viruses that have infected humans [12], [40] (Figure 1B), suggesting that this predominant internal gene combination is more stable and compatible with different surface genes. This internal gene combination also functions in transmission because it was present in all six of the transmissible viruses. Therefore, the H9N2 viruses pose a threat to human health not only because they will likely cause new influenza pandemic, but also because they can transfer different subtypes of influenza viruses from avian species to humans.
Materials and Methods
Ethics statements
This study was carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the Ministry of Science and Technology of the People's Republic of China. The protocols for animal studies were approved by the Committee on the Ethics of Animal Experiments of the Harbin Veterinary Research Institute (HVRI) of the Chinese Academy of Agricultural Sciences (CAAS) (approval numbers BRDW-XBS–12 for mice and BRDW-XD–12 for ferrets).
Facility
All experiments with live H9N2 viruses were conducted within the enhanced animal biosafety level 2+ (ABSL2+) facility in the HVRI of the CAAS. The animal isolators in the facility are hyper-filtered. The researchers who work with mice and ferrets wear N95 masks and disposable overalls; they shower on exiting the facility.
Viruses and cells
The H9N2 viruses used in this study were isolated from poultry in different regions of China between 2009 and 2013. Virus stocks were grown in specific pathogen-free (SPF) chicken eggs. Madin-Darby canine kidney (MDCK) cells used for virus titration were cultured in DMEM (CORNING, Cellgro) medium with 4% fetal bovine serum (FBS). 293T cells were cultured in DMEM medium with 10% FBS. All cells were incubated at 37°C with 5% CO2.
Genetic and phylogenetic analyses
Viral gene amplification and sequencing was carried out as described previously [18], [61]. Sequence data were compiled with the SEQMAN program (DNASTAR, Madison, WI), and phylogenetic analyses were carried out with the PHYLIP program of MEGA 5.0 software using the neighbor-joining algorithm. Bootstrap values of 1,000 were used, and 95% sequence identity cutoffs were used to categorize each gene segment in the phylogenetic trees.
Receptor-binding analysis
Receptor specificity was analyzed by use of a solid-phase direct binding assay as described previously with modified using two different glycopolymers: α-2, 3-siaylglycopolymer [Neu5Acα2-3Galβ1-4GlcNAcβ1-pAP (para-aminophenyl)-alpha-polyglutamic acid (α-PGA)] and the α-2, 6-sialylglycopolymer [Neu5Acα2-6Galβ1-4GlcNAcβ1-pAP (para-aminophenyl)-alpha-polyglutamic acid (α-PGA)] [4], [7]. Briefly, viruses were grown in eggs, clarified by low-speed centrifugation, laid over a cushion of 30% sucrose in phosphate buffered saline (PBS), and ultracentrifuged at 28,000 r.p.m for 2 h at 4°C. Virus stocks were aliquoted and stored at −80°C until use. Virus concentrations were determined by using haemagglutination assays with 0.5% cRBCs. Microtitre plates (Nunc) were incubated with serial two-fold dilutions of sodium salts of sialyglycopolymers in PBS at 4°C for 30 min. Then the plates were exposed to UV light (254 nm) for 10 min. After the glycopolymer solution was removed, the plates were washed three times with PBS. Then 50 µl of virus suspensions diluted with PBST (PBS containing 0.1% Twen-20) was added to the wells and the plate was incubated at 4°C for 2 to 3 h. After being washed five times with 250 µl of PBST, the plates were fixed with 10% formalin in PBST for 30 min. After the plates were again washed five times with PBST, 50 µl of chicken antiserum against the CK/JS/C4258/12 (H9N2) virus diluted with PBST was added to the wells and incubated at 37°C for 1 h. After being washed for a further five times with PBST, the plates were incubated with a horseradish peroxidase (HRP)-conjugated goat-anti-chicken antibody (Sigma-Aldrich, St. Louis, MO, USA) for 1 h at 37°C.The plates were then washed again and incubated with O-phenylenediamine (Sigma-Aldrich, St. Louis, MO, USA) in PBS containing 0.01% H2O2 for 10 min at room temperature. The reaction was stopped with 0.05 ml of 0.5 M H2SO4. The optical density at 490 nm was determined in a microplate reader (BIO-RAD). Dose-response curves of virus binding to the glycopolymers were analyzed by using a single site binding algorithm and curve fitting by GraphPad Prism to determine the association constant values (Ka). Each value presented is the mean ± SD of three experiments, which were each performed in triplicate.
Site-directed mutagenesis and virus generation
The HA and NA segments of CK/GX/9/99 were inserted into the bidirectional transcription vector pBD as described previously [49]. The QuikChange Lighting Site-Directed Mutagenesis Kit (Stratagene, http://www.agilent.com) was used to create specific mutations in the HA gene by using the following primers: forward: 5′AGCATGAGATGGTTGATTCAA AAGGACAAC, reverse: 5′AGCGTTGTCCTTTTGAATCAACCATCTCAT (for HAT155I mutation); and forward: 5′ TTCATGTGGGGCATACATCACCCACCCACC, reverse: 5′TCGGTGGGTGGGTGA TGTATGCCCCACATG (for HAN183H mutation). The HA (with or without the mutations) and NA genes of the CK/GX/9/99 virus and the six internal genes of the PR8 virus were used to generate the viruses as previously described [49]. All HA and NA genes of the constructs and rescued viruses were completely sequenced to ensure the absence of unwanted mutations. The gene sequences of the CK/GX/9/99 virus used in this study have been reported previously [18].
Mouse experiments
Groups of six-week-old female BALB/c mice (Beijing Vital River Laboratories, Beijing, China) were anesthetized with CO2 and inoculated intranasally (i.n.) with 106.0 EID50 of test viruses in a volume of 50 µl. Three mice were euthanized on day 3 p.i., and the nasal turbinates, lungs, kidneys, spleens, and brains were collected for virus titration in MDCK cells. The remaining five mice in each group were monitored daily for 14 days for weight loss and survival.
Ferret studies
Four-month-old female ferrets (Wuxi Cay Ferret Farm, Jiangsu, China) that were serologically negative for influenza viruses were used in these studies. The animals were anesthetized via intramuscular injection of ketamine (20 mg/kg) and xylazine (1 mg/kg). To examine virus replication, groups of two ferrets were anesthetized and inoculated i.n. with 106.0 EID50 of test viruses in a 500 µl volume (250 µl per nostril). The ferrets were euthanized on day 4 p.i. and the nasal turbinates, tonsils, trachea, lung, spleen, kidneys, liver, and brain were collected for virus titration in MDCK cells. The lung tissue was also collected for histologic study as described previously [64].
For the respiratory droplet transmission studies, groups of three ferrets were inoculated i.n. with 106.0 EID50 of test virus and housed in specially designed cages inside an isolator as described previously [40]. Twenty-four hours later, three naïve animals were placed in an adjacent cage. Nasal washes were collected at 2-day intervals, beginning on day 2 p.i. (1 day post-exposure) and titrated in MDCK cells. The ambient conditions for these studies were set at 20–22°C and 30%–40% relative humidity. The airflow in the isolator was horizontal with a speed of 0.1 m/s; the airflow direction was from the inoculated animals to the exposed animals.
Deep sequencing
Viral RNA was extracted and converted to cDNA by use primer 5′AGC RAA AGC AGG. Specific amplification of a 1000-nucleotide PB2 fragment covering codons 627 to 701 was applied by use a pair of specific primers (Forward: 5′GCAACRGCTATYYTRAGGAAAGC; Reverse 5′ AGTAGAAACAAGGTCGTTTTTAAA). PCR fragments of each virus were pooled in equal concentrations, and libraries were created for each virus by using the Ion Xpress Plus Fragment Library Kit (Life Technologies). Sequencing runs were performed by using the Ion Torrent personal genome machine (PGM, Life Technologies). Sequence reads were sorted by the Ion Xpress Barcode Adaptors 1–18 (Life Technologies). Reads were aligned to the PB2 reference sequence of each virus by using CLC Genomics Workbench 5.0.1. The threshold for mutation detection was manually set at 1%.
The genome sequences of the 35 viruses reported in this study are available in GenBank with the access numbers of KM113042 – KM113321.
Supporting Information
Zdroje
1. WHO (2014) Cumulative number of confirmed human cases for avian influenza A (H5N1) reported to WHO, 2003–2014. Available: http://www.who.int/influenza/human_animal_interface/EN_GIP_20140124CumulativeNumberH5N1cases.pdf. Accessed 24 January 2014.
2. ZhangY, ZhangQ, KongH, JiangY, GaoY, et al. (2013) H5N1 hybrid viruses bearing 2009/H1N1 virus genes transmit in guinea pigs by respiratory droplet. Science 340 : 1459–1463.
3. HerfstS, SchrauwenEJ, LinsterM, ChutinimitkulS, de WitE, et al. (2012) Airborne transmission of influenza A/H5N1 virus between ferrets. Science 336 : 1534–1541.
4. ImaiM, WatanabeT, HattaM, DasSC, OzawaM, et al. (2012) Experimental adaptation of an influenza H5 HA confers respiratory droplet transmission to a reassortant H5 HA/H1N1 virus in ferrets. Nature 486 : 420–428.
5. FouchierRA, SchneebergerPM, RozendaalFW, BroekmanJM, KeminkSA, et al. (2004) Avian influenza A virus (H7N7) associated with human conjunctivitis and a fatal case of acute respiratory distress syndrome. Proc Natl Acad Sci U S A 101 : 1356–1361.
6. KoopmansM, WilbrinkB, ConynM, NatropG, van der NatH, et al. (2004) Transmission of H7N7 avian influenza A virus to human beings during a large outbreak in commercial poultry farms in the Netherlands. Lancet 363 : 587–593.
7. WangG, DengG, ShiJ, LuoW, ZhangG, et al. (2014) H6 influenza viruses pose a potential threat to human health. J Virol 88 : 3953–3964.
8. NamJH, KimEH, SongD, ChoiYK, KimJK, et al. (2011) Emergence of Mammalian Species-Infectious and -Pathogenic Avian Influenza H6N5 Virus with No Evidence of Adaptation. Journal of Virology 85 : 13271–13277.
9. Gillim-RossL, SantosC, ChenZ, AspelundA, YangCF, et al. (2008) Avian influenza h6 viruses productively infect and cause illness in mice and ferrets. J Virol 82 : 10854–10863.
10. YuanJ, ZhangL, KanX, JiangL, YangJ, et al. (2013) Origin and molecular characteristics of a novel 2013 avian influenza A (H6N1) virus causing human infection in Taiwan. Clin Infect Dis 57 : 1367–1368.
11. WHO (2014) Confirmed human cases of avian influenza A(H7N9) reported to WHO. Available: http://www.who.int/influenza/human_animal_interface/influenza_h7n9/16_ReportWebH7N9Number_20140325.pdf?ua=1. Accessed 25 March 2014.
12. ChenH, YuanH, GaoR, ZhangJ, WangD, et al. (2014) Clinical and epidemiological characteristics of a fatal case of avian influenza A H10N8 virus infection: a descriptive study. Lancet 383 : 714–721.
13. HommePJ, EasterdayBC, AndersonDP (1970) Avian influenza virus infections. II. Experimental epizootiology of influenza A-turkey-Wisconsin-1966 virus in turkeys. Avian Dis 14 : 240–247.
14. KawaokaY, ChambersTM, SladenWL, WebsterRG (1988) Is the gene pool of influenza viruses in shorebirds and gulls different from that in wild ducks? Virology 163 : 247–250.
15. PeirisJS, GuanY, MarkwellD, GhoseP, WebsterRG, et al. (2001) Cocirculation of avian H9N2 and contemporary "human" H3N2 influenza A viruses in pigs in southeastern China: potential for genetic reassortment? J Virol 75 : 9679–9686.
16. XuC, FanW, WeiR, ZhaoH (2004) Isolation and identification of swine influenza recombinant A/Swine/Shandong/1/2003(H9N2) virus. Microbes Infect 6 : 919–925.
17. YuH, HuaRH, WeiTC, ZhouYJ, TianZJ, et al. (2008) Isolation and genetic characterization of avian origin H9N2 influenza viruses from pigs in China. Vet Microbiol 131 : 82–92.
18. LiC, YuK, TianG, YuD, LiuL, et al. (2005) Evolution of H9N2 influenza viruses from domestic poultry in Mainland China. Virology 340 : 70–83.
19. Lin Z, Xu C, Liu B, Ji Y, Fu Y, et al.. (2014) Analysis of the phylogeny of Chinese H9N2 avian influenza viruses and their pathogenicity in mice. Arch Virol. 2575–86.
20. LuX, RenshawM, TumpeyTM, KellyGD, Hu-PrimmerJ, et al. (2001) Immunity to influenza A H9N2 viruses induced by infection and vaccination. J Virol 75 : 4896–4901.
21. GovorkovaEA, LenevaIA, GoloubevaOG, BushK, WebsterRG (2001) Comparison of efficacies of RWJ-270201, zanamivir, and oseltamivir against H5N1, H9N2, and other avian influenza viruses. Antimicrob Agents Chemother 45 : 2723–2732.
22. MatrosovichMN, KraussS, WebsterRG (2001) H9N2 influenza A viruses from poultry in Asia have human virus-like receptor specificity. Virology 281 : 156–162.
23. SorrellEM, WanH, ArayaY, SongH, PerezDR (2009) Minimal molecular constraints for respiratory droplet transmission of an avian-human H9N2 influenza A virus. Proc Natl Acad Sci U S A 106 : 7565–7570.
24. SunY, QinK, WangJ, PuJ, TangQ, et al. (2011) High genetic compatibility and increased pathogenicity of reassortants derived from avian H9N2 and pandemic H1N1/2009 influenza viruses. Proc Natl Acad Sci U S A 108 : 4164–4169.
25. KimbleJB, SorrellE, ShaoH, MartinPL, PerezDR (2011) Compatibility of H9N2 avian influenza surface genes and 2009 pandemic H1N1 internal genes for transmission in the ferret model. Proc Natl Acad Sci U S A 108 : 12084–12088.
26. WanH, SorrellEM, SongH, HossainMJ, Ramirez-NietoG, et al. (2008) Replication and transmission of H9N2 influenza viruses in ferrets: evaluation of pandemic potential. PLoS One 3: e2923.
27. ZhangP, TangY, LiuX, LiuW, ZhangX, et al. (2009) A novel genotype H9N2 influenza virus possessing human H5N1 internal genomes has been circulating in poultry in eastern China since 1998. J Virol 83 : 8428–8438.
28. PeirisM, YuenKY, LeungCW, ChanKH, IpPL, et al. (1999) Human infection with influenza H9N2. Lancet 354 : 916–917.
29. ButtKM, SmithGJ, ChenH, ZhangLJ, LeungYH, et al. (2005) Human infection with an avian H9N2 influenza A virus in Hong Kong in 2003. J Clin Microbiol 43 : 5760–5767.
30. WHO (2014) Influenza at the human-animal interface. Available: http://www.who.int/influenza/human_animal_interface/Influenza_Summary_IRA_HA_interface_24January14.pdf. Accessed 24 January 2014.
31. WangM, FuCX, ZhengBJ (2009) Antibodies against H5 and H9 avian influenza among poultry workers in China. N Engl J Med 360 : 2583–2584.
32. PawarSD, TandaleBV, RautCG, ParkhiSS, BardeTD, et al. (2012) Avian influenza H9N2 seroprevalence among poultry workers in Pune, India, 2010. PLoS One 7: e36374.
33. BlairPJ, PutnamSD, KruegerWS, ChumC, WierzbaTF, et al. (2013) Evidence for avian H9N2 influenza virus infections among rural villagers in Cambodia. J Infect Public Health 6 : 69–79.
34. ComanA, MafteiDN, KruegerWS, HeilGL, FriaryJA, et al. (2013) Serological evidence for avian H9N2 influenza virus infections among Romanian agriculture workers. J Infect Public Health 6 : 438–447.
35. GrayGC, FergusonDD, LowtherPE, HeilGL, FriaryJA (2011) A national study of US bird banders for evidence of avian influenza virus infections. J Clin Virol 51 : 132–135.
36. OkoyeJ, EzeD, KruegerWS, HeilGL, FriaryJA, et al. (2013) Serologic evidence of avian influenza virus infections among Nigerian agricultural workers. J Med Virol 85 : 670–676.
37. UyekiTM, NguyenDC, RoweT, LuX, Hu-PrimmerJ, et al. (2012) Seroprevalence of antibodies to avian influenza A (H5) and A (H9) viruses among market poultry workers, Hanoi, Vietnam, 2001. PLoS One 7: e43948.
38. WangQ, JuL, LiuP, ZhouJ, LvX, et al. (2014) Serological and Virological Surveillance of Avian Influenza A Virus H9N2 Subtype in Humans and Poultry in Shanghai, China, Between 2008 and 2010. Zoonoses Public Health
39. GaoR, CaoB, HuY, FengZ, WangD, et al. (2013) Human infection with a novel avian-origin influenza A (H7N9) virus. N Engl J Med 368 : 1888–1897.
40. ZhangQ, ShiJ, DengG, GuoJ, ZengX, et al. (2013) H7N9 influenza viruses are transmissible in ferrets by respiratory droplet. Science 341 : 410–414.
41. CDC (2012) H5N1 Genetic Changes Inventory: A Tool for Influenza Surveillance and Preparedness. Available: http://www.cdc.gov/flu/pdf/avianflu/h5n1-inventory.pdf. Accessed 26 June 2012.
42. Hulse-PostDJ, FranksJ, BoydK, SalomonR, HoffmannE, et al. (2007) Molecular changes in the polymerase genes (PA and PB1) associated with high pathogenicity of H5N1 influenza virus in mallard ducks. J Virol 81 : 8515–8524.
43. LiJ, LiY, HuY, ChangG, SunW, et al. (2011) PB1-mediated virulence attenuation of H5N1 influenza virus in mice is associated with PB2. J Gen Virol 92 : 1435–1444.
44. FanS, DengG, SongJ, TianG, SuoY, et al. (2009) Two amino acid residues in the matrix protein M1 contribute to the virulence difference of H5N1 avian influenza viruses in mice. Virology 384 : 28–32.
45. JiaoP, TianG, LiY, DengG, JiangY, et al. (2008) A single-amino-acid substitution in the NS1 protein changes the pathogenicity of H5N1 avian influenza viruses in mice. J Virol 82 : 1146–1154.
46. SmeenkCA, WrightKE, BurnsBF, ThakerAJ, BrownEG (1996) Mutations in the hemagglutinin and matrix genes of a virulent influenza virus variant, A/FM/1/47-MA, control different stages in pathogenesis. Virus Res 44 : 79–95.
47. BeanWJ, ThrelkeldSC, WebsterRG (1989) Biologic potential of amantadine-resistant influenza A virus in an avian model. J Infect Dis 159 : 1050–1056.
48. HattaM, GaoP, HalfmannP, KawaokaY (2001) Molecular basis for high virulence of Hong Kong H5N1 influenza A viruses. Science 293 : 1840–1842.
49. LiZ, ChenH, JiaoP, DengG, TianG, et al. (2005) Molecular basis of replication of duck H5N1 influenza viruses in a mammalian mouse model. J Virol 79 : 12058–12064.
50. GaoY, ZhangY, ShinyaK, DengG, JiangY, et al. (2009) Identification of amino acids in HA and PB2 critical for the transmission of H5N1 avian influenza viruses in a mammalian host. PLoS Pathog 5: e1000709.
51. ChenY, LiangW, YangS, WuN, GaoH, et al. (2013) Human infections with the emerging avian influenza A H7N9 virus from wet market poultry: clinical analysis and characterisation of viral genome. Lancet 381 : 1916–1925.
52. ZhangH, LiX, GuoJ, LiL, ChangC, et al. (2014) The PB2 E627K mutation contributes to the high polymerase activity and enhanced replication of H7N9 influenza virus. J Gen Virol 95 : 779–786.
53. MokCK, LeeHH, LestraM, NichollsJM, ChanMC, et al. (2014) Amino Acid Substitutions in Polymerase Basic Protein 2 Gene Contribute to the Pathogenicity of the Novel A/H7N9 Influenza Virus in Mammalian Hosts. J Virol 88 : 3568–3576.
54. VinesA, WellsK, MatrosovichM, CastrucciMR, ItoT, et al. (1998) The role of influenza A virus hemagglutinin residues 226 and 228 in receptor specificity and host range restriction. J Virol 72 : 7626–7631.
55. TumpeyTM, MainesTR, Van HoevenN, GlaserL, SolorzanoA, et al. (2007) A two-amino acid change in the hemagglutinin of the 1918 influenza virus abolishes transmission. Science 315 : 655–659.
56. RogersGN, PaulsonJC (1983) Receptor determinants of human and animal influenza virus isolates: differences in receptor specificity of the H3 hemagglutinin based on species of origin. Virology 127 : 361–373.
57. WatanabeT, KisoM, FukuyamaS, NakajimaN, ImaiM, et al. (2013) Characterization of H7N9 influenza A viruses isolated from humans. Nature 501 : 551–555.
58. WatanabeY, IbrahimMS, EllakanyHF, KawashitaN, MizuikeR, et al. (2011) Acquisition of human-type receptor binding specificity by new H5N1 influenza virus sublineages during their emergence in birds in Egypt. PLoS Pathog 7: e1002068.
59. LinsterM, van BoheemenS, de GraafM, SchrauwenEJ, LexmondP, et al. (2014) Identification, characterization, and natural selection of mutations driving airborne transmission of A/H5N1 virus. Cell 157 : 329–339.
60. SteelJ, LowenAC, MubarekaS, PaleseP (2009) Transmission of influenza virus in a mammalian host is increased by PB2 amino acids 627K or 627E/701N. PLoS Pathog 5: e1000252.
61. ChenH, DengG, LiZ, TianG, LiY, et al. (2004) The evolution of H5N1 influenza viruses in ducks in southern China. Proc Natl Acad Sci U S A 101 : 10452–10457.
62. LiY, ShiJ, ZhongG, DengG, TianG, et al. (2010) Continued evolution of H5N1 influenza viruses in wild birds, domestic poultry, and humans in China from 2004 to 2009. J Virol 84 : 8389–8397.
63. DengG, TanD, ShiJ, CuiP, JiangY, et al. (2013) Complex reassortment of multiple subtypes of avian influenza viruses in domestic ducks at the Dongting Lake Region of China. J Virol 87 : 9452–9462.
64. ZhangY, ZhangQ, GaoY, HeX, KongH, et al. (2012) Key molecular factors in hemagglutinin and PB2 contribute to efficient transmission of the 2009 H1N1 pandemic influenza virus. J Virol 86 : 9666–9674.
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek Acidification Activates Motility and Egress by Enhancing Protein Secretion and Cytolytic ActivityČlánek Plasticity between MyoC- and MyoA-Glideosomes: An Example of Functional Compensation in InvasionČlánek Coronavirus Cell Entry Occurs through the Endo-/Lysosomal Pathway in a Proteolysis-Dependent MannerČlánek NK Cell Activation in Human Hantavirus Infection Explained by Virus-Induced IL-15/IL15Rα Expression
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2014 Číslo 11- Stillova choroba: vzácné a závažné systémové onemocnění
- Perorální antivirotika jako vysoce efektivní nástroj prevence hospitalizací kvůli COVID-19 − otázky a odpovědi pro praxi
- Jak souvisí postcovidový syndrom s poškozením mozku?
- Diagnostika virových hepatitid v kostce – zorientujte se (nejen) v sérologii
- Infekční komplikace virových respiračních infekcí – sekundární bakteriální a aspergilové pneumonie
-
Všechny články tohoto čísla
- Peculiarities of Prion Diseases
- Inhibitors of Peptidyl Proline Isomerases As Antivirals in Hepatitis C and Other Viruses
- War and Infectious Diseases: Challenges of the Syrian Civil War
- Microbial Contamination in Next Generation Sequencing: Implications for Sequence-Based Analysis of Clinical Samples
- Acidification Activates Motility and Egress by Enhancing Protein Secretion and Cytolytic Activity
- Co-dependence of HTLV-1 p12 and p8 Functions in Virus Persistence
- Shed GP of Ebola Virus Triggers Immune Activation and Increased Vascular Permeability
- Plasticity between MyoC- and MyoA-Glideosomes: An Example of Functional Compensation in Invasion
- The Type III Translocon Is Required for Biofilm Formation at the Epithelial Barrier
- Retromer Regulates HIV-1 Envelope Glycoprotein Trafficking and Incorporation into Virions
- IFI16 Restricts HSV-1 Replication by Accumulating on the HSV-1 Genome, Repressing HSV-1 Gene Expression, and Directly or Indirectly Modulating Histone Modifications
- Coronavirus Cell Entry Occurs through the Endo-/Lysosomal Pathway in a Proteolysis-Dependent Manner
- Silencing by H-NS Potentiated the Evolution of
- Crystal Structure of Cytomegalovirus IE1 Protein Reveals Targeting of TRIM Family Member PML via Coiled-Coil Interactions
- GAPDH-A Recruits a Plant Virus Movement Protein to Cortical Virus Replication Complexes to Facilitate Viral Cell-to-Cell Movement
- Genomic Insights into the Fungal Pathogens of the Genus : Obligate Biotrophs of Humans and Other Mammals
- Unravelling Human Trypanotolerance: IL8 is Associated with Infection Control whereas IL10 and TNFα Are Associated with Subsequent Disease Development
- The Skin Microbiome: A Focus on Pathogens and Their Association with Skin Disease
- Human Cytomegalovirus Vaccine Based on the Envelope gH/gL Pentamer Complex
- IL-37 Inhibits Inflammasome Activation and Disease Severity in Murine Aspergillosis
- Host-Specific Parvovirus Evolution in Nature Is Recapitulated by Adaptation to Different Carnivore Species
- Activation of HIV Transcription with Short-Course Vorinostat in HIV-Infected Patients on Suppressive Antiretroviral Therapy
- PUL21a-Cyclin A2 Interaction is Required to Protect Human Cytomegalovirus-Infected Cells from the Deleterious Consequences of Mitotic Entry
- Programmed Ribosomal Frameshift Alters Expression of West Nile Virus Genes and Facilitates Virus Replication in Birds and Mosquitoes
- Aminoterminal Amphipathic α-Helix AH1 of Hepatitis C Virus Nonstructural Protein 4B Possesses a Dual Role in RNA Replication and Virus Production
- NK Cell Activation in Human Hantavirus Infection Explained by Virus-Induced IL-15/IL15Rα Expression
- Structure and Specificity of the Bacterial Cysteine Methyltransferase Effector NleE Suggests a Novel Substrate in Human DNA Repair Pathway
- Genetics, Receptor Binding Property, and Transmissibility in Mammals of Naturally Isolated H9N2 Avian Influenza Viruses
- A Gatekeeper Chaperone Complex Directs Translocator Secretion during Type Three Secretion
- A Conserved Peptide Pattern from a Widespread Microbial Virulence Factor Triggers Pattern-Induced Immunity in
- Succinate Dehydrogenase is the Regulator of Respiration in
- The Plasmodesmal Protein PDLP1 Localises to Haustoria-Associated Membranes during Downy Mildew Infection and Regulates Callose Deposition
- Dysregulated B Cell Expression of RANKL and OPG Correlates with Loss of Bone Mineral Density in HIV Infection
- Restriction of Genetic Diversity during Infection of the Vector Midgut
- The Epithelial αvβ3-Integrin Boosts the MYD88-Dependent TLR2 Signaling in Response to Viral and Bacterial Components
- The Relationship between Host Lifespan and Pathogen Reservoir Potential: An Analysis in the System
- Multiple Roles of the Cytoskeleton in Bacterial Autophagy
- The Evolution and Genetics of Virus Host Shifts
- ChIP-seq and In Vivo Transcriptome Analyses of the SREBP SrbA Reveals a New Regulator of the Fungal Hypoxia Response and Virulence
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Coronavirus Cell Entry Occurs through the Endo-/Lysosomal Pathway in a Proteolysis-Dependent Manner
- Peculiarities of Prion Diseases
- Host-Specific Parvovirus Evolution in Nature Is Recapitulated by Adaptation to Different Carnivore Species
- War and Infectious Diseases: Challenges of the Syrian Civil War
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



