-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
ChIP-seq and In Vivo Transcriptome Analyses of the SREBP SrbA Reveals a New Regulator of the Fungal Hypoxia Response and Virulence
Despite improvements in diagnostics and antifungal drug treatments, mortality rates from invasive mold infections remain high. Defining the fungal adaptation and growth mechanisms at the infection site microenvironment is one research focus that is expected to improve treatment of established invasive fungal infections. The Aspergillus fumigatus transcription factor SrbA is a major regulator of the fungal response to hypoxia found at sites of invasive fungal growth in vivo. In this study, new insights into how SrbA mediates hypoxia adaptation and virulence were revealed through identification of direct transcriptional targets of SrbA under hypoxic conditions. A major novel finding from these studies is the identification of a critical role in fungal hypoxia adaptation and virulence of an SrbA target gene, srbB, which is also in the SREBP family. SrbB plays a major role in regulation of heme biosynthesis and carbohydrate metabolism early in the response to hypoxia. The discovery of SrbA-dependent regulation of srbB gene expression, and the target genes they regulate opens new avenues to understand how SREBPs and their target genes mediate adaptation to the in vivo infection site microenvironment and responses to current antifungal therapies.
Published in the journal: . PLoS Pathog 10(11): e32767. doi:10.1371/journal.ppat.1004487
Category: Research Article
doi: https://doi.org/10.1371/journal.ppat.1004487Summary
Despite improvements in diagnostics and antifungal drug treatments, mortality rates from invasive mold infections remain high. Defining the fungal adaptation and growth mechanisms at the infection site microenvironment is one research focus that is expected to improve treatment of established invasive fungal infections. The Aspergillus fumigatus transcription factor SrbA is a major regulator of the fungal response to hypoxia found at sites of invasive fungal growth in vivo. In this study, new insights into how SrbA mediates hypoxia adaptation and virulence were revealed through identification of direct transcriptional targets of SrbA under hypoxic conditions. A major novel finding from these studies is the identification of a critical role in fungal hypoxia adaptation and virulence of an SrbA target gene, srbB, which is also in the SREBP family. SrbB plays a major role in regulation of heme biosynthesis and carbohydrate metabolism early in the response to hypoxia. The discovery of SrbA-dependent regulation of srbB gene expression, and the target genes they regulate opens new avenues to understand how SREBPs and their target genes mediate adaptation to the in vivo infection site microenvironment and responses to current antifungal therapies.
Introduction
Invasive fungal infections have increased in frequency due to a substantial rise in the number of immune compromised patients [1]–[3]. In particular, the filamentous fungal pathogen Aspergillus fumigatus is a major cause of morbidity and mortality in patients undergoing immunosuppressive therapy for organ transplants and/or cancer treatment [2], [4], [5]. Treatment options for invasive aspergillosis (IA) remain limited and associated mortality rates are high [6]. Epidemiological studies suggest that over 200,000 cases of aspergillosis occur worldwide on an annual basis, and there is general consensus that disease caused by A. fumigatus is under diagnosed [2]. It is clear that new insights into the pathophysiology of this too often lethal disease are needed to develop new diagnostic and treatment strategies to improve patient outcomes. Along these lines, investigating fungal growth in vivo at infection site microenvironments is an important area of research with significant therapeutic potential.
Observations from human IA cases and recent discoveries in murine models of invasive pulmonary aspergillosis (IPA) indicate that the infection microenvironment is characterized in part by low oxygen availability (hypoxia) [7]–[10]. Hypoxia is associated with poor clinical outcomes for many human diseases, but its impact on invasive fungal infections remains understudied [11]–[16]. One hypothesis is that hypoxia promotes fungal virulence through induction of a fungal metabolic, or bioenergetics, program that contributes to host damage. In support of this hypothesis, fungal genes required for hypoxic growth are generally critical for fungal virulence in murine models, and airway ischemia was recently shown to promote A. fumigatus invasion in an orthotropic tracheal transplant model of Aspergillus infection [15]–[20].
One fungal gene family required for hypoxia adaptation and growth is the sterol regulatory element binding protein family (SREBP) [21]. First identified in mammals, two distinct mammalian SREBP genes exist, SREBP-1 and SREBP-2. SREBP-1 produces two isoforms, SREBP-1a and SREBP 1-c, derived from alternative splicing of the first exon [22]. These SREBP-1 isoforms share the same DNA-binding domain, the sterol regulatory element (SRE), and have been shown to mainly regulate lipid metabolism, whereas SREBP-2 predominantly regulates cholesterol metabolism [23]. Recent genomic approaches have yielded new insights into mammalian SREBP functions through identification of novel target genes. In mice, ChIP-seq analyses revealed that SREBP-1c binds upstream of genes associated with lipid biosynthesis, insulin dependent pathways, carbohydrate metabolism, and additional novel gene ontology (GO) categories including intracellular protein trafficking, cell proliferation and differentiation, and apoptosis [24]. ChIP-seq analysis of SREBP-2 target genes in murine hepatic chromatin revealed new roles for this protein in apoptosis and autophagy [25]. Thus, it has been suggested that SREBPs are necessary for coordination of the cellular nutritional state and transcriptional activation by interacting with different cofactors in response to dynamic nutritional microenvironments [26].
With regard to SREBP function in fungi, a seminal study in the fission yeast Schizosaccharomyces pombe identified a molecular link between SREBP function and fungal hypoxia adaptation and growth [27]. In this organism, the SREBP bHLH domain containing protein, Sre1, has been identified as a primary regulator of anaerobic gene expression [28]. Accordingly, an sre1 deletion mutant is unable to grow in anaerobic conditions confirming an indirect molecular mechanism of oxygen sensing through Sre1 mediated monitoring of ergosterol levels [27], [29]. Sre1 proteolytic cleavage requires an E3 ligase, which is distinct from the mammalian SREBP cleavage mechanism utilizing site-1 and site-2 proteases [27]–[35]. Subsequent studies in the human pathogenic yeast Cryptococcus neoformans and filamentous fungus A. fumigatus identified a critical role for fungal SREBPs and associated regulatory factors in hypoxia adaptation, iron homeostasis, azole drug responses, and importantly fungal virulence [18], [19], [36]–[39].
The molecular basis for fungal SREBPs' role in virulence remains to be fully elucidated. Identification of putative SREBP target genes through genome-wide gene expression analyses indicates that fungal SREBPs are important regulators of ergosterol metabolism and iron uptake [18], [21], [38], [39]. In A. fumigatus, microarray analyses of gene expression in the presence and absence of SrbA in hypoxic environments revealed significant changes in transcript levels of approximately 12% of the genes in the genome [36]–[38]. Many of the gene transcript levels that are affected by SrbA are associated with biological processes induced by hypoxia in wild type A. fumigatus including: ergosterol biosynthesis, iron homeostasis, cell wall biosynthesis, amino acid biosynthesis, general carbon metabolism, and the GABA shunt [40]. However it remains unclear which hypoxia induced genes are directly SrbA dependent. Understanding fungal SREBP function is further complicated by the presence of at least two SREBPs in many of the fungal genomes queried to date [21], [27], [36], [41], [42]. Data also suggest that additional SREBP interacting partners are required for proper modulation of sterol levels in several organisms [21], [26], [43].
Given the little we know of direct fungal SREBP target genes, and the differing transcriptional networks that organisms utilize to respond to similar microenvironments, we sought to precisely define the SrbA-mediated transcriptome (regulon) of A. fumigatus in response to hypoxia. In order to definitively delineate the SrbA hypoxia regulon, we utilized a multi-faceted approach to validate not only direct transcriptional targets of SrbA, but also the putative significance of these targets during an invasive pulmonary infection. In addition to identifying a role for A. fumigatus SrbA in direct transcriptional regulation of novel genes involved in the fungal hypoxia response, we identified and functionally characterized a second SREBP family member, designated SrbB, which is a direct transcriptional target of SrbA in hypoxia. Together, SrbA and SrbB regulate and co-regulate genes critical for fungal metabolism, virulence, and responses to antifungal drugs. These results place SrbA and SrbB as central transcriptional regulators of fungal metabolic responses required for in vivo fungal growth and host damage. Consequently, further characterization of the pathways and networks regulated by SrbA-SrbB is expected to promote a novel research direction aimed at inhibiting the function of this in vivo associated fungal genetic network to improve IPA prognosis.
Results
ChIP-seq Analysis Identifies a Core Set of Novel SrbA Target Genes in Response to Hypoxia
The workflow for this study was informed by previous research that assessed the significance of SrbA in hypoxia growth and fungal pathogenesis [36] and subsequent studies looking at transcriptional changes associated with the A. fumigatus response to hypoxia [38], [40], [44]. To gain new mechanistic insights into how SrbA mediates these important phenotypes, ChIP-seq analysis using an SrbA specific antibody after 4 hours (two biological samples) and 12 hours (one biological sample) exposure to hypoxia was conducted [37], [38]. The overall number of Illumina 76 base-pair paired end reads used for peak calling among the three sets of ChIP-seq samples were 2992021 and 3345809 for ChIP and input control respectively (Table S1). Reads were aligned to the A. fumigatus A1163 genome (Figure 1A) and used for peak calling with the Macs2 program either in aggregate, or one sample at a time [45]. By including input controls (samples with no SrbA antibody) in the analysis with an FDR of 0.05, a core set of 111 peaks corresponding to 97 genes were identified (Table S2). A subset of 30 genes of biological interest associated with SrbA binding events are listed in Table 1. 25 of 30 (83%) of these peaks were located within 1 kb upstream of translational start sites (Table 1). Figure 1B shows examples of peak regions from eight target genes. Using additional independent biological replicates, ChIP-qPCR confirmed SrbA binding to the promoter regions of the selected genes in response to hypoxia (Figure 1C).
Fig. 1. Genome wide ChIP-seq Analysis Identifies Direct Transcriptional Targets of SrbA in Hypoxic Conditions. 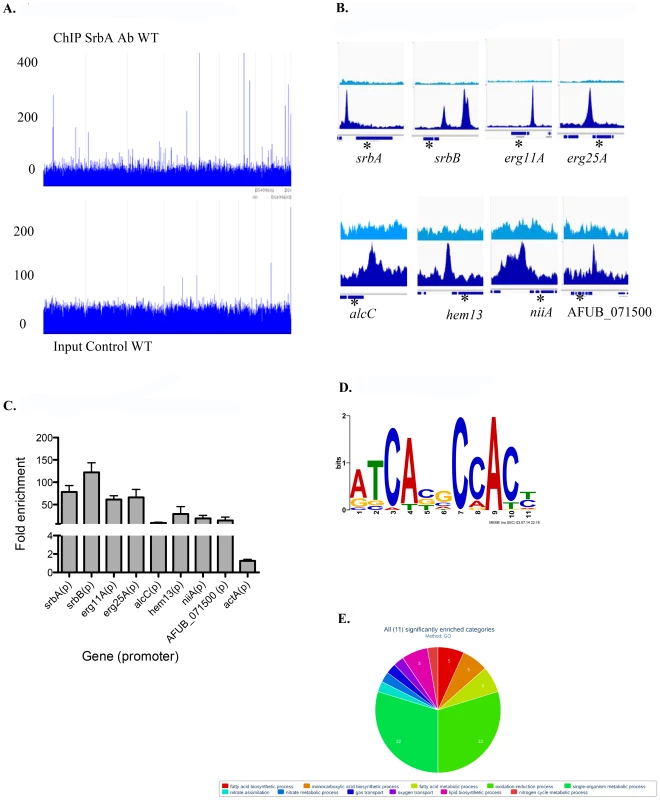
(A). Genome scale view of ChIP-seq data for one 4-hour ChIP sample. Blue lines rising above background are peaks identified as an excess of sequence fragments aligning to the genome of A. fumigatus sequenced strain A1163. The genome-wide view of the ChIP-seq reads aligned to the A1163 genome reveals strong visible peaks in the SrbA antibody ChIP sample, with few strong peaks observed in the A1163 wild type input control sample. Grey lines demarcate genome scaffolds. (B). ChIP-seq peaks for selected genes: srbA, srbB, erg 11A and erg 25A were among the highest ChIP-seq peaks and have relatively high enrichment (lower panels) vs. input control (upper panels). alcC, hem13, niiA and AFUB_071500 show significant, though lower enrichment vs. input control. In each case the peaks are upstream of the translational start site of the indicated genes. Asterisks mark the relevant gene model within each 3 kb region. (C). ChIP qPCR was conducted to validate ChIP-seq results for select targets. Data are presented as the mean and standard error of two biological replicates. The actin promoter (actA(p)) was included as a non-specific target. SrbA was enriched on the promoters of all tested genes. (D). SrbA binding motif as identified using MEME. (E). GO pie chart. Tab. 1. Selected SrbA ChIP Peaks with flanking gene information. 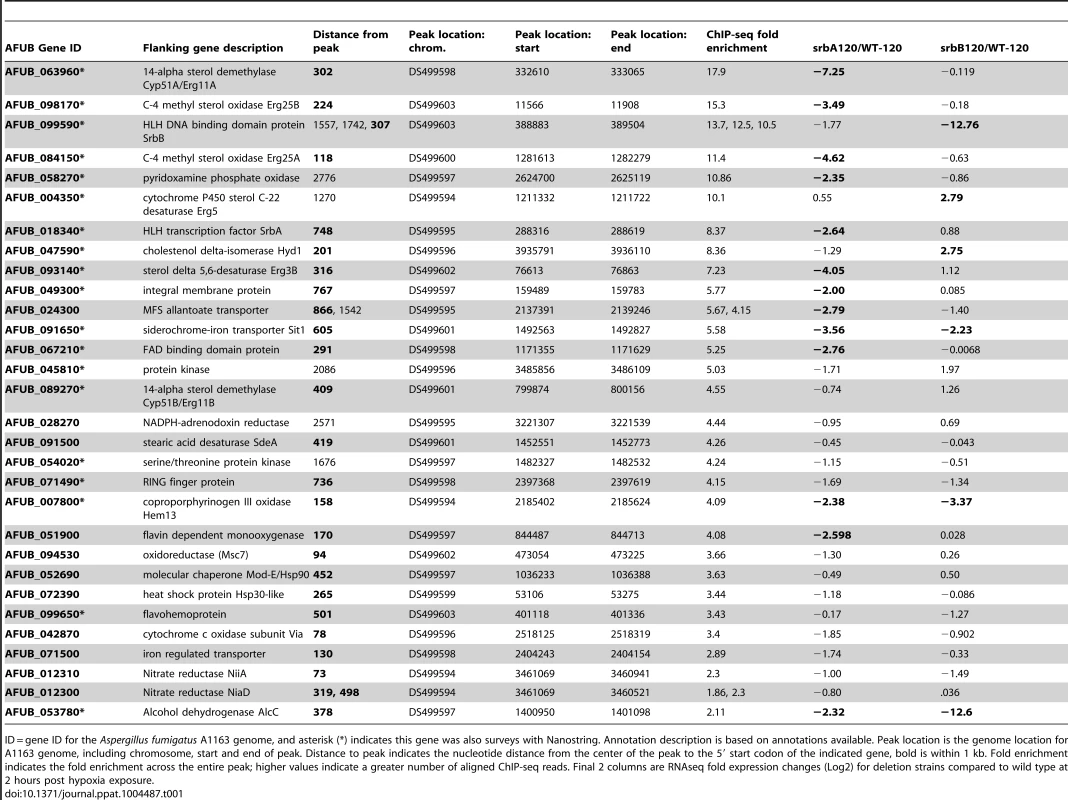
ID = gene ID for the Aspergillus fumigatus A1163 genome, and asterisk (*) indicates this gene was also surveys with Nanostring. Annotation description is based on annotations available. Peak location is the genome location for A1163 genome, including chromosome, start and end of peak. Distance to peak indicates the nucleotide distance from the center of the peak to the 5′ start codon of the indicated gene, bold is within 1 kb. Fold enrichment indicates the fold enrichment across the entire peak; higher values indicate a greater number of aligned ChIP-seq reads. Final 2 columns are RNAseq fold expression changes (Log2) for deletion strains compared to wild type at 2 hours post hypoxia exposure. An SrbA-bound DNA motif (Figure 1D) was discovered using multiple EM for motif elicitation (MEME) of the identified peaks [46]. The SrbA DNA binding motif was identified as an 11-bp binding region predominated by ATCA in positions 1–4, a cytosine in position 7, and an adenine in position 9. Other positions were more variable, with a predominance of cytosine residues. This DNA motif (5′ (A/G)TCA(T/C/G)(C/G)CCAC(T/C)-3′) is similar to a previously identified SrbA DNA binding motif that was discovered using bioinformatic tools, 5′-ATC(G/A)(T/G)(A/G)(C/T)(G/C)AT-3′ [47]. The sequence TCACNCCAC has been identified in humans as the SRE binding motif [48], [49]. Additionally in S. pombe, a motif was defined using MEME to be (A/G)(C/T)C(A/G/T)NN(C/T)(C/T/G)A(C/T), which contains similar conserved residues as our sequence [28]. Recently, the bHLH transcription factor Hms1 in C. albicans was found to bind the consensus sequence ATCACCCCAC, which is strikingly similar to the identified motif for SrbA [50]. Using this motif, we analyzed a previously published ΔsrbA microarray dataset, which revealed that this SrbA DNA binding motif is overrepresented among the differentially expressed genes when comparing wild type to ΔsrbA further validating the SRE motif [38]. Five of the identified peaks had two-to-three occurrences of the motif, including SrbA itself. The binding of SrbA to its own promoter reveals an autoregulatory positive feedback loop for modulation of srbA mRNA levels.
To further determine the biological processes and molecular function of SrbA direct target genes, SrbA target genes were analyzed for gene ontology (GO) and FunCat enrichment with FungiFun2 (Figure 1E, Table S3) [51]. Of the 97 unique SrbA target genes, only 19 were significantly enriched in FunCat categories (P≤0.05) and 33 in GO categories (P≤0.05). Overall, approximately 35% of the SrbA target genes are not currently annotated. Consistent with our previous microarray-based transcriptome analysis, and known functions of yeast and mammalian SREBPs, SrbA is a direct regulator of genes involved in lipid, fatty acid and isoprenoid biosynthesis, which includes ergosterol biosynthesis (Table S3). In addition, A. fumigatus SrbA target genes were associated with heme and oxygen binding and nitrate metabolic processes. Taken together, annotated SrbA direct target genes are associated with biological processes impacted by both oxygen and iron limitation that are critical for fungal virulence. These results thus indicate that SrbA is a major transcriptional regulator of fungal metabolism (bioenergetics) in response to hypoxia.
RNA-seq and Transcript Analysis of ΔsrbA in Hypoxia Confirms SrbA Target Genes and Associated Biological Functions
To further define SrbA target genes and confirm SrbA regulation of genes with associated SrbA DNA binding events in their promoter regions, RNA-seq analysis of the hypoxia transcriptome of ΔsrbA was conducted pooling total RNA samples from independent biological cultures in triplicate. A 30 and 120 minute response to hypoxia was examined by comparing ΔsrbA samples to the same time point as the wild type. Consistent with the previously published microarray and RNA-seq analyses of the A. fumigatus hypoxia response, RNA-seq analyses here revealed substantial changes to the transcriptome of wild type and ΔsrbA in response to hypoxia [38], [40], [44].
Somewhat surprisingly, loss of SrbA had minimal effect on the transcriptome of A. fumigatus after 30 minutes exposure to hypoxia, as only 48 genes had transcript levels decrease 4-fold or greater in ΔsrbA compared to wild type (Table S4) during this time period. Perhaps of interest, these 48 genes were enriched for functions involving post-translational modification of amino acids. Notably, genes involved in ergosterol biosynthesis are generally unaffected at this time point in the absence of SrbA. However, at 30 minutes post-hypoxia exposure, loss of SrbA resulted in transcript level increases for 383 genes compared to wild type. Interestingly, these genes were enriched for degradation of the amino acids tyrosine and phenylalanine. Previously, an analysis of the free amino acid pool in ΔsrbA in response to iron replete and limiting conditions revealed substantial changes in the amino acid pools of ΔsrbA [38].
SrbA's role in hypoxic adaptation becomes evident at 120 minutes post-exposure to hypoxia with 520 genes having a 4-fold or greater decrease in transcript levels in the absence of SrbA compared to wild type. These genes are enriched for fatty acid and lipid biosynthesis, iron ion binding, carbohydrate metabolism, virulence, isoprenoid metabolism, and cellular oxidoreductase activity (Figure 2A). Importantly, the majority of SrbA direct target genes identified in our ChIP-seq analysis fall into this group of genes with reduced mRNA levels strongly suggesting that SrbA directly positively regulates their mRNA abundance in response to hypoxia. Conversely, 467 genes had a 4-fold or greater increase in transcript levels in the absence of SrbA compared to wild type. These genes were enriched in part for transcription factor activity, and as no enrichment of the SRE motif could be found in this dataset (P-value - 0.96 Fisher Exact test), we interpret the increase in transcript levels of these genes as indirect responses to the loss of SrbA activity in the cell, rather than SrbA acting as a transcriptional repressor of these genes. Alternatively, SrbA may positively regulate an unidentified transcriptional repressor(s). Taken together, these results strongly suggest that SrbA is a direct positive transcriptional regulator of genes required for adaptation to hypoxia. Moreover, these results strongly suggest that loss of SrbA fundamentally alters fungal cellular bioenergetics in response to hypoxia.
Fig. 2. RNA-seq and nCounter Analyses of ΔsrbA Confirms SrbA Regulation of ChIP-seq Target Genes in vitro and in vivo during Invasive Pulmonary Aspergillosis. 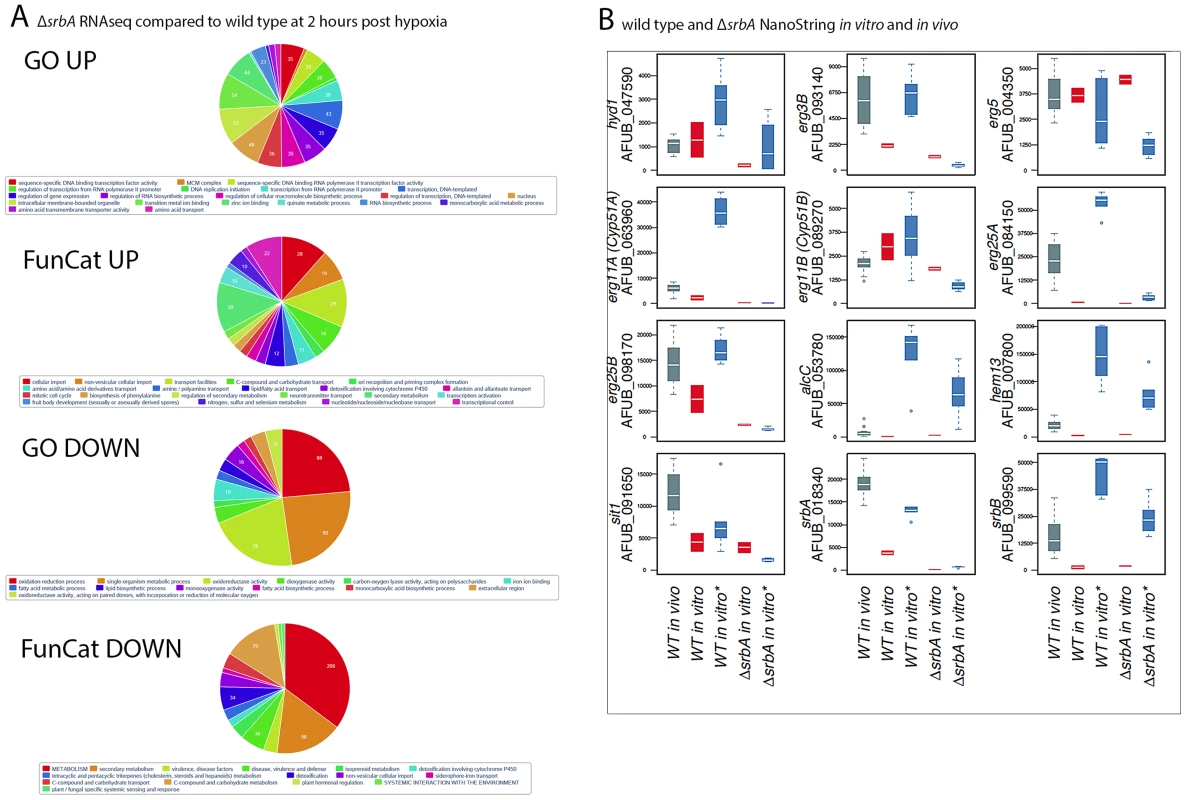
(A). Enrichment of RNA-seq differentially expressed genes in GO/Funcat categories of up- and down-regulated genes in srbA cells under hypoxia 120 minutes versus WT (B). Analysis of transcript levels of 12 of the ChiP-seq target genes in vivo in a murine model of invasive pulmonary aspergillosis for wild type (CEA10) and in vitro under normoxic/hypoxic conditions for ΔsrbA and wild type in vivo samples were at 48–96 hours post-infection (grey, n = 16). in vitro samples were wild type normoxia (red, n = 2) and hypoxia (blue, n = 6) followed by ΔsrbA under normoxia (red, n = 2) and hypoxia (blue, n = 6). Time under hypoxia for both wild type and ΔsrbA ranged from 30 to 120 minutes. Expression values are represented as total number of normalized counts per transcript. Quantitation and normalization was as follows: Digital counts for 60 genes (ChIP targets, housekeeping genes and other genes of interest) were adjusted for binding efficiency with background subtraction using the included positive and negative controls from the manufacturer as per NanoString nCounter data analysis guidelines. Data sets were normalized to facilitate across sample comparisons using the geometric mean of 20 stably expressed genes. Nanostring nCounter Transcript Abundance Analysis Reveals High Transcript Levels for SrbA Target Genes in a Murine Model of Invasive Pulmonary Aspergillosis
To gain insights into SrbA target genes critical for pathogenesis, we interrogated the mRNA abundance of a sub-set of SrbA target genes in an in vivo murine model of IPA, and in vitro in response to normoxia and hypoxia utilizing Nanostring nCounter technology [52], [53]. Consistent with the RNA-seq data, nCounter analyses confirmed that transcript abundance of sterol biosynthesis genes in hypoxia decreased in vitro with loss of srbA (Figure 2B). Moreover, erg3B, erg11A, erg25A, erg25B, all displayed high transcript levels in vivo in lung parenchyma tissue consistent with their SrbA-dependent hypoxia induction in vitro and the occurrence of hypoxia in vivo (Figure 2B, Table S5). Additional genes that showed strong SrbA dependency in vitro and high transcript levels in vivo included AFUB_091650 (sit1), a putative siderophore-iron transporter, that is critical for response to low iron and previously shown to be directly transcriptionally regulated by SrbA using ChIP-qPCR [38], [54]. Sit1 may be critical for virulence as mRNA abundance is higher in vivo than in either of the tested in vitro conditions. The high transcript levels of sit1 early in infection confirm the iron-limited environment of the murine lung. These data support direct regulation of iron uptake in vivo by SrbA, and further support the link between SrbA, hypoxia adaptation, and iron homeostasis in A. fumigatus.
In addition to sit1, two other metabolic genes, hem13 and alcC, show less SrbA dependency in vitro than the ergosterol biosynthesis genes, but significant transcript induction in vivo. Hem13 is a coproporphyrinogen III oxidase that involved in heme biosynthesis, and heme has been characterized as a major oxygen-sensing molecule in S. cerevisiae [55]. It has been reported that expression of genes encoding the main heme biosynthesis enzymes are induced in response to hypoxia in S. pombe, C. neoformans, and A. fumigatus [19], [27], [38]. In yeast, heme and oxygen negatively regulate HEM13 expression, and the repression of HEM13 involves a negative transcriptional regulator of hypoxic genes, ROX1 [56], [57]. AlcC is the alcohol dehydrogenase critical for ethanol fermentation in A. fumigatus and important for in vivo fungal growth as previously observed by Grahl et al. 2011. While SrbA is involved in regulation of these two metabolic genes, their dependency on SrbA is not as significant as genes involved in ergosterol biosynthesis.
SrbA itself intriguingly displays higher transcript levels in vivo than in vitro and 2 additional putative transcriptional regulators, AFUB_090280 (Table S5) and AFUB_099590 (Figure 2B), display strong in vitro hypoxia induction that is partially SrbA dependent with strong expression in vivo. Previous informatics based analyses of AFUB_099590 [21], [58], suggested an important role for this DNA binding protein in both metabolism and virulence. Transcript levels for AFUB_099590, which we designate srbB, are highly induced in response to hypoxia in vitro in a partially SrbA dependent manner as our microarray based analysis of ΔsrbA also previously suggested. Intriguingly, srbB transcript is one of the highest in vivo abundant genes relative to in vitro normoxic conditions. Moreover, a temporal analysis of srbA and srbB transcript levels in response to hypoxia revealed that srbB mRNA levels increase before srbA transcript levels and rise five minutes after exposure to hypoxia (Figure 3). Consequently, functional characterization of SrbB was undertaken with genetic and phenotypic analyses.
Fig. 3. Temporal transcriptional induction of SrbA and SrbB in response to Hypoxia Reveals SrbB transcript levels are induced prior to SrbA. 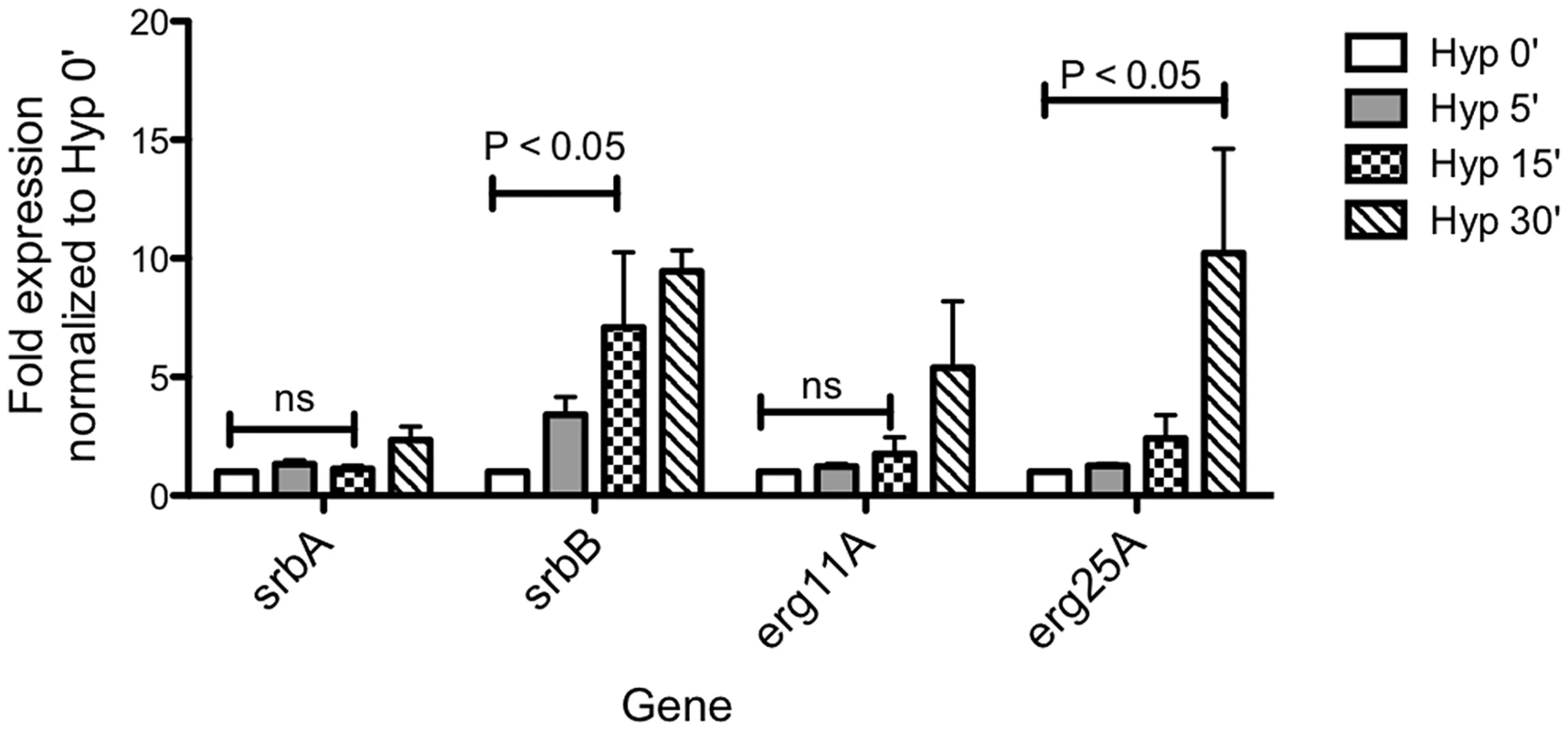
Aspergillus fumigatus wild type was cultured in normoxia at 37°C for 18 hours and shifted to hypoxia for additional incubation for 5, 15, and 30 minutes. Data are presented as the mean and standard error of three biological replicates. Compared to normoxia (Hyp 0′), srbB expression was significantly induced in hypoxia 15 min, which was earlier than the initial srbA induction. The data were analyzed by two-way ANOVA followed by Bonferroni posttests. Characterization of SrbB Reveals a New Hypoxia Transcriptional Regulator with a Role in A. fumigatus Virulence
Amino acid sequence alignment of SrbB (HLH domain, Figure S1) revealed that this protein is an SREBP homolog, as previously suggested [21]. Characteristic of SREBP family members, SrbB contains a tyrosine substitution in the basic portion of the bHLH domain. Intriguingly, SrbB does not contain any predicted transmembrane domains, suggesting this SREBP family member may not be regulated via proteolytic cleavage like SrbA.
Functional analysis of srbB was initiated through generation of a genetic null mutant and reconstituted strain (Figure S2). Loss of SrbB results in ∼50% reduction in colony radial growth on solid glucose minimal media (GMM) in hypoxia (Figure 4A). In addition to the decrease in growth rate on solid medium, ΔsrbB colonies were noticeably less dense and often contained fluffy mycelia in hypoxic conditions. Though not statistically significant, there was a trend toward increased growth and mycelial density in normoxic conditions with ΔsrbB. The impact of SrbB loss in response to hypoxia was pronounced in liquid GMM culture conditions where a significant reduction of biomass was observed compared to normoxia cultures (Figure 4B). These results strongly suggest that SrbB is a major transcriptional regulator of the fungal response to hypoxia. Unlike our previous reports with ΔsrbA, a minimal increase in tolerance to the triazole voriconazole was observed in ΔsrbB in normoxia and hypoxia suggesting a limited role for SrbB in regulating responses to triazoles (Figure 4C). Of significant note, upon culture in liquid GMM, ΔsrbB exhibited a strong red pigmentation of the mycelia (Figure 4D). This coloration was limited to the mycelia, and only occurred in hypoxic conditions.
Fig. 4. Loss of the Hypoxia Induced Transcriptional Regulator SrbB Results in a Significant Growth Defect and Red Pigmented Mycelia in Hypoxia. 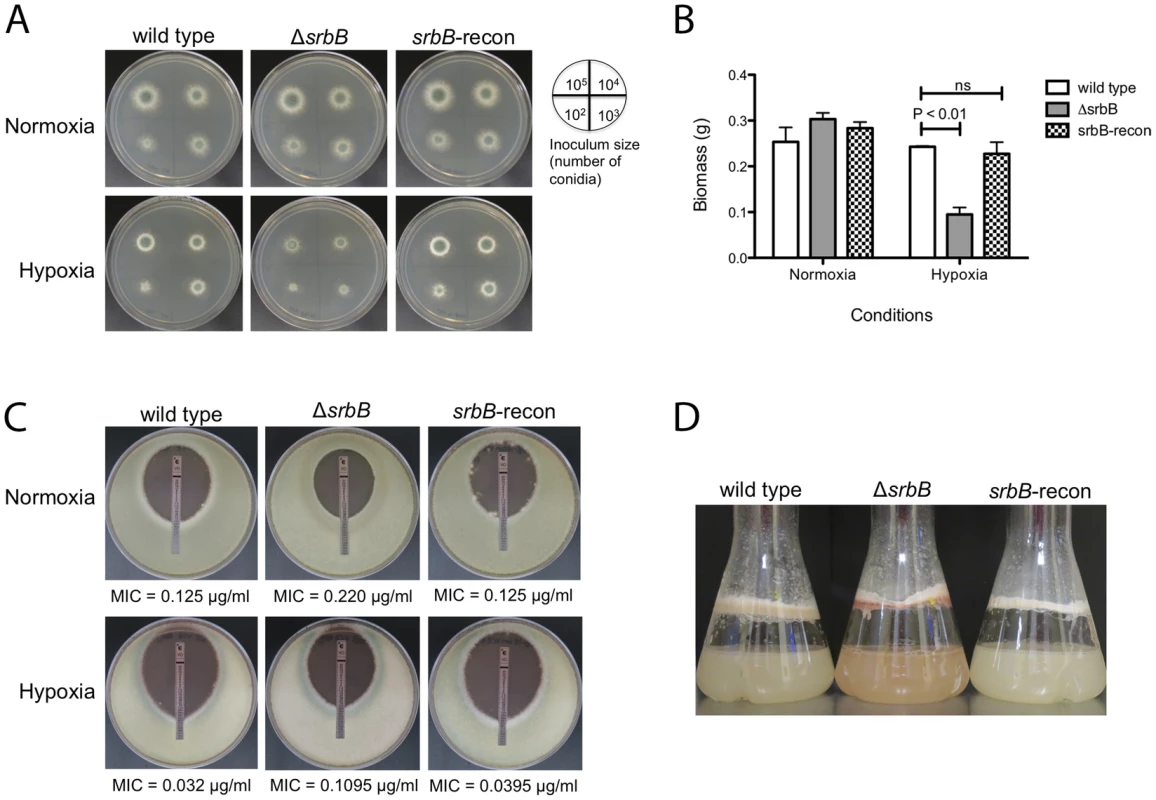
(A). Growth of ΔsrbB in normoxia and hypoxia on solid media. Wild type, ΔsrbB, and srbB-reconstituted strains were incubated on GMM at 37°C for 3 days in normoxia or hypoxia. The number of conidia used for inoculation is illustrated by the plate image. Compared to wild type and the reconstituted strain, growth of ΔsrbB is restricted in hypoxia. (B). A biomass test with wild type, an srbB null mutant, and an srbB reconstituted strain in liquid cultures in normoxia or hypoxia. Mycelia of wild type, ΔsrbB and srbB-reconstituted (srbB-recon) strains in liquid cultures were harvested, dried and weighed for the biomass study. Data are presented as the mean and standard error of three biological replicates. No significant differences between wild type and srbB-recon biomass were observed in all conditions tested. When analyzed by two-way ANOVA followed by Bonferroni posttest, biomass of ΔsrbB was not different from wild type or srbB-recon in normoxia. However, biomass of ΔsrbB significantly decreases in hypoxia compared to wild type (p<0.001). (C). E-test strips were utilized to test susceptibility to VCZ. 105 conidia were overlaid on RPMI media, cultured at 37°C for 2 days. Minimal inhibitory concentrations (MIC, marked as an arrow) were measured. In both normoxia and hypoxia, ΔsrbB is slightly more tolerant to VCZ compared to wild type and the srbB reconstituted strain. MIC ratios of ΔsrbB to wild type are 1.76 and 3.42 in normoxia and hypoxia, respectively. (D). Conidia of each strain were cultured in LGMM at 37°C, 200 rpm for 2 days in hypoxia. ΔsrbB produces reddish mycelia compared to the wild type and reconstituted strain. SrbB is a Major Hypoxia Transcriptional Regulator of Carbohydrate, Heme, and Lipid Metabolism
To better understand SrbB's function in hypoxia, the origin of the red pigment produced in ΔsrbB hypoxic mycelia, and SrbB's genetic relationship to SrbA, RNA-seq analysis was conducted with ΔsrbB and wild type under the same conditions and time points as ΔsrbA (Figure 2, Figure 5). In contrast to ΔsrbA, but consistent with SrbB's early induction in response to hypoxia (Figure 3), the transcriptome of ΔsrbB was significantly changed at 30 minutes post-exposure to hypoxia. 490 genes had 4 fold or greater reductions in transcript levels in ΔsrbB compared to wild type under hypoxic conditions (Table S4 and S6). These genes were enriched (P≤0.05) in FunCat categories of carbohydrate metabolism, virulence, secondary metabolism, and detoxification (Figure 5). In contrast, 135 genes had transcript levels increase 4-fold or greater in ΔsrbB compared to wild type at this early hypoxia time point. Genes with increased transcript levels in ΔsrbB were enriched in transport of toxic products, which could suggest a global dysregulation of metabolism in ΔsrbB. At 120 minutes post-exposure to hypoxia, the impact of SrbB loss on transcript levels was particularly strong, with 1026 transcripts decreasing 4-fold or greater. Over half of these genes are not currently annotated (522), but those annotated are enriched in carbohydrate and nitrogen metabolism, virulence, secondary metabolism, and transport of various molecules. Moreover, at 120 minutes 491 genes had transcript levels increased 4-fold or greater in ΔsrbB compared to wild type. Perhaps associated with the enrichment of toxic product detoxification in the genes with reduced transcript levels, genes with increased transcript levels in ΔsrbB were associated strongly with amino acid metabolism and degradation. Overall, these data suggest a significant metabolic or bioenergetics dysregulation in A. fumigatus cultured under hypoxic conditions when SrbB function is absent. Consequently, loss of SrbB results in major alterations of the A. fumigatus hypoxia transcriptome that impact hypoxia growth.
Fig. 5. SrbB is a transcriptional regulator of genes involved in carbon metabolism, lipid metabolism, and heme biosynthesis. 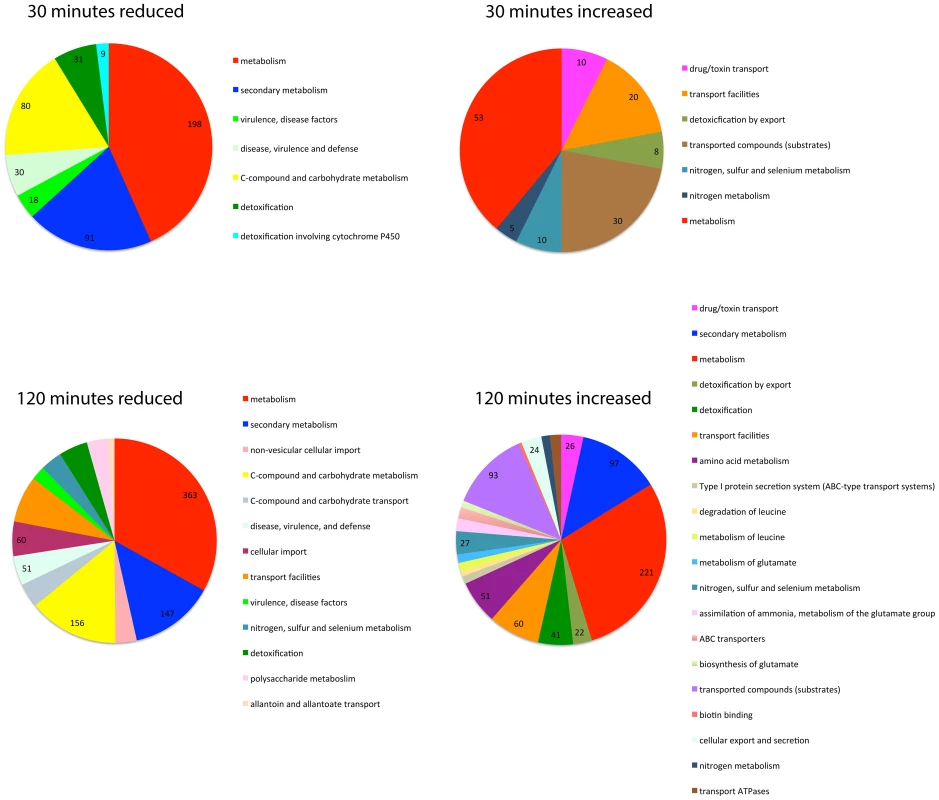
The FungiFun2 web server was utilized to assign FunCat and gene ontology enrichment in genes with transcript levels increased or decreased 4 fold in ΔsrbB compared to the wild type strain. Statistically significant (P≤0.05) FunCat categories are presented at the respective time points in hypoxia. Interestingly, hem13 transcript levels were markedly reduced in ΔsrbB mycelia exposed to hypoxia, providing a potential cause of the red pigment previously observed in this strain. To test the hypothesis that the red pigmentation of ΔsrbB is associated with a defect in heme metabolism, we quantified the accumulation of heme biosynthesis intermediates in ΔsrbB in normoxia and hypoxia (Figure 6A–B). Consistent with the red pigmentation and RNA-seq analysis of ΔsrbB, a striking accumulation of heme biosynthesis intermediates including protoporphyrin IX were observed in ΔsrbB (Figure 6A–B). Intriguingly, when 5 µM hemin was exogenously added into the culture media, ΔsrbB growth was significantly improved in hypoxia compared to GMM alone (Figure 6C). In contrast, addition of hemin to ΔsrbA did not restore this strain's growth under the conditions examined. Taken together, these data suggest that SrbB is a critical regulator of heme biosynthesis in A. fumigatus and that accumulation of toxic heme intermediates may contribute to the hypoxia growth defect observed in ΔsrbB.
Fig. 6. Loss of srbB impairs heme biosynthesis and results in accumulation of heme intermediates. 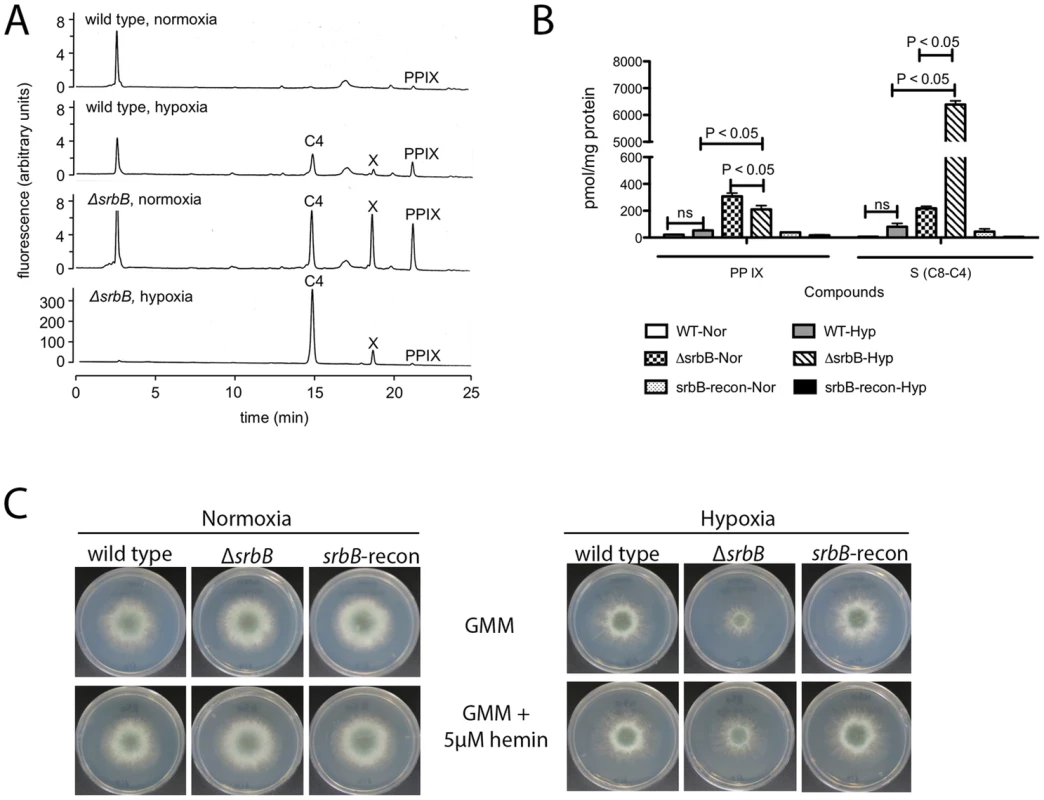
(A–B). Amount of Protoporphyrin IX (PP IX) and intermediate compounds including uroporphyrin (C8), heptacarboxylporphyrin (C7), hexacarboxylporphyrin (C6), pentacarboxylporphyrin (C5), and coproporphyrin (C4) were analyzed using HPLC. Mycelia used for HPLC analysis were harvested from cultures in LGMM at 37°C for 2 days in hypoxia. Compared to wild type, ΔsrbB produces more PP IX and other intermediates in hypoxia. (A) is a chromatogram from HPLC analysis, and (B) is a graph to present the HPLC result with statistical analysis. Data are presented as the mean and standard error of three biological replicates, and analyzed by one-way ANOVA followed by a Tukey's multiple comparison test. (C). A thousand conidia were inoculated on GMM or GMM containing 5 µM hemin. In 2 days, radial growth of each strain in normoxia or hypoxia was observed. Addition of hemin improved ΔsrbB growth in hypoxia. SrbA and SrbB have Dependent and Independent Functions in Hypoxic Gene Regulation
Inspection of genes regulated by SrbA and SrbB identified using ChIP-seq and RNA-seq analyses suggested a close functional relationship between these two SREBP family members. To compare expression patterns of strains lacking each respective transcription factor, a hierarchical clustered heat map was generated for SrbA direct annotated target genes (Figure 7). Examination of the heat map reveals that a sub-set of SrbA target genes also depends on SrbB for wild-type transcript levels in hypoxia. Examples of these target genes include hem13, niiA, and erg25A. In contrast, a sub-set of SrbA target genes had increased or wild type transcript levels in the absence of SrbB. These include srbA itself and its target genes erg11A/cyp51A, erg11B/cyp51B, and erg3B. These latter genes appear to be dependent on SrbA and not SrbB under the examined conditions.
Fig. 7. A sub-set of SrbA ChIP Target Genes are co-regulated by SrbB. 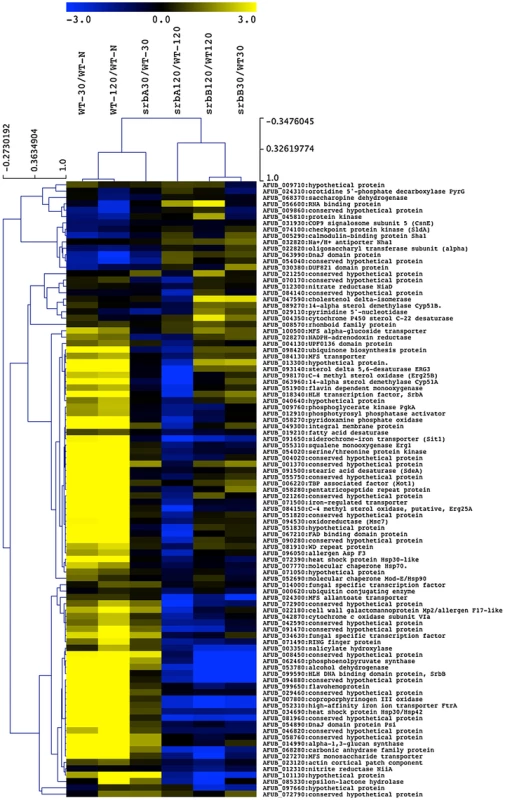
The RNA-seq data for annotated genes corresponding to SrbA ChIP-seq peaks are shown as ratios of 30 and 120-minute wild-type hypoxia vs. wild-type normoxia, and gene deletion strains are shown as the deletion strain vs. the equivalent wild type hypoxia time point. Genes discussed and/or examined in detail in this manuscript are noted with asterisks. MeV analysis was performed using hierarchical clustering. Optimized gene and leaf ordering groups the wild type 30- and 120-minute hypoxic conditions together, with the 30-minute ΔsrbA sample more similar to wild type for the SrbA targets, using Pearson correlation with complete linkage clustering. Next, to further define the genetic relationship between SrbA and SrbB, we generated 3 additional strains: (1) ΔsrbAΔsrbB (2) srbB over-expression in ΔsrbA (srbB-ove;ΔsrbA) and (3) srbA over-expression in ΔsrbAΔsrbB (srbA-ove;ΔsrbB). In order to over-express srbB in ΔsrbA, the A. fumigatus flavohemoprotein (flavA(p), AFUB_099650) or A. nidulans glyceraldehyde 3-phosphate dehydrogenase (gpdA(p), AN8041) promoters were utilized. These promoters were chosen because their respective genes are highly expressed in hypoxia in glucose minimal media with nitrate as a sole nitrogen source. The flavA(p) was chosen from the RNA-seq conditions where AFUB_099650 was one of the highest expressed genes in response to hypoxia [59]. Previously, in A. oryzae, it was reported that the FlavA homolog responds strongly to nitric oxide stress, which could occur in our media conditions with NO3 as a nitrogen source [60]. The resulting strains were designated TDC43.18 (flavA(p):srbB;ΔsrbA) and TDC44.2 (gpdA(p):srbB;ΔsrbB), respectively, and TDC43.18 was selected to study gene expression in the srbB-ove;ΔsrbA strain. qRT-PCR analysis of select SREBP targets was conducted on cultures exposed to hypoxia for four hours, similar to ChIP-seq conditions. Consistent with the RNA-seq data, loss of SrbB resulted in a modest increase in srbA transcript levels (Figure 8). Conversely, loss of SrbA results in a strong decrease in srbB transcript levels suggesting that SrbA positively regulates srbB mRNA levels in hypoxia.
Fig. 8. Co-Regulation of SrbA target genes by SrbB. 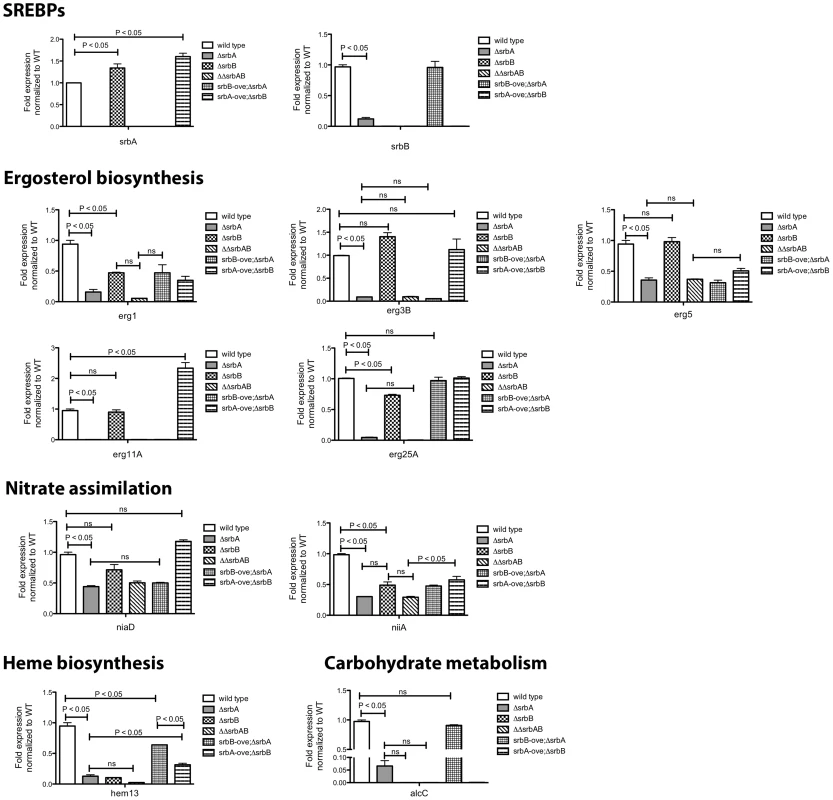
Conidia of each strain were cultured in normoxia at 37°C, 250 rpm for 18 hours and shifted to hypoxia for additional incubation for 4 hours. Expression of SrbA target genes involved in ergosterol biosynthesis, nitrate assimilation, heme biosynthesis, and carbohydrate metabolism in the strains was studied using qRT-PCR. Data are presented as the mean and standard error of two biological replicates, and analyzed by one-way ANOVA followed by Bonferroni's posttests. Expression of erg1, erg25A, niiA, hem13, and alcC requires both SrbA and SrbB. In contrast, expression of erg3B, erg11A, and niaD are regulated by only SrbA. SrbB appears to have a dominant role over SrbA in regulation of hem13 and alcC expression. ΔΔsrbAsrbB: a double knock-out mutant of srbA and srbB, srbB-ove;ΔsrbA: ΔsrbA with restored srbB expression, srbA-ove;ΔsrbB: a srbA overexpression strain in ΔsrbAΔsrbB. Transcript levels of nine genes, plus srbA and srbB, representing ergosterol biosynthesis, heme biosynthesis, carbohydrate metabolism, and nitrate assimilation from ChIP-seq data were investigated in ΔsrbA, ΔsrbB, ΔsrbAΔsrbB, srbB-ove;ΔsrbA, and srbA-ove;ΔsrbB compared to wild type in hypoxia for four hours. Genes that require both SrbA and SrbB for full abundance include erg1, erg25A, niiA, and hem13 as suggested by the RNA-seq analysis. In contrast, expression of erg3B, erg5, erg11A, and niaD requires SrbA but not SrbB (Figure 8). However, interpretation of this data is complicated by the fact that SrbA positively regulates srbB transcript levels. This is exemplified with mRNA levels of the ethanol fermentation and virulence factor alcC. Loss of SrbA significantly reduces alcC transcript levels, but not to the extent as loss of SrbB (Figure 8). SrbA is modestly enriched on the alcC promoter (Table 1, Table S2, Figure 1). However, over-expression of SrbB in ΔsrbA essentially fully restores alcC transcript levels. Taken together, these results strongly suggests that alcC transcript levels are primarily regulated by SrbB, although SrbA can bind the SRE site found in the alcC promoter and contribute to its regulation.
To seek further insights into regulation of a sub-set of these target genes by SrbA and SrbB, we performed ChIP-qPCR with a SrbB:GFP strain using a GFP antibody. SrbB tagged with GFP was ectopically expressed in the A. fumigatus wild type background. Transcript levels of srbB in the SrbB:GFP strain under the same conditions used for ChIP-qPCR was similar to wild type, and SrbB:GFP is localized to the nucleus in this strain (Figure S3). Cultures for ChIP were prepared in hypoxia for 4 hours and enrichment of SrbB on the SrbA binding sites of srbA, srbB, erg11A, erg25A, hem13 and alcC was examined compared to wild type (Figure 9A). The negligible SrbB enrichment on the actin promoter supports binding specificity of the GFP antibody used for ChIP. ChIP-qPCR results show that SrbB binds to the SrbA binding sites of srbA, erg25A, and hem13 whose transcript levels require both SrbA and SrbB as described above.
Fig. 9. Binding of SrbB to the promoter of specific SrbA target genes and binding of SrbA in ΔsrbB. 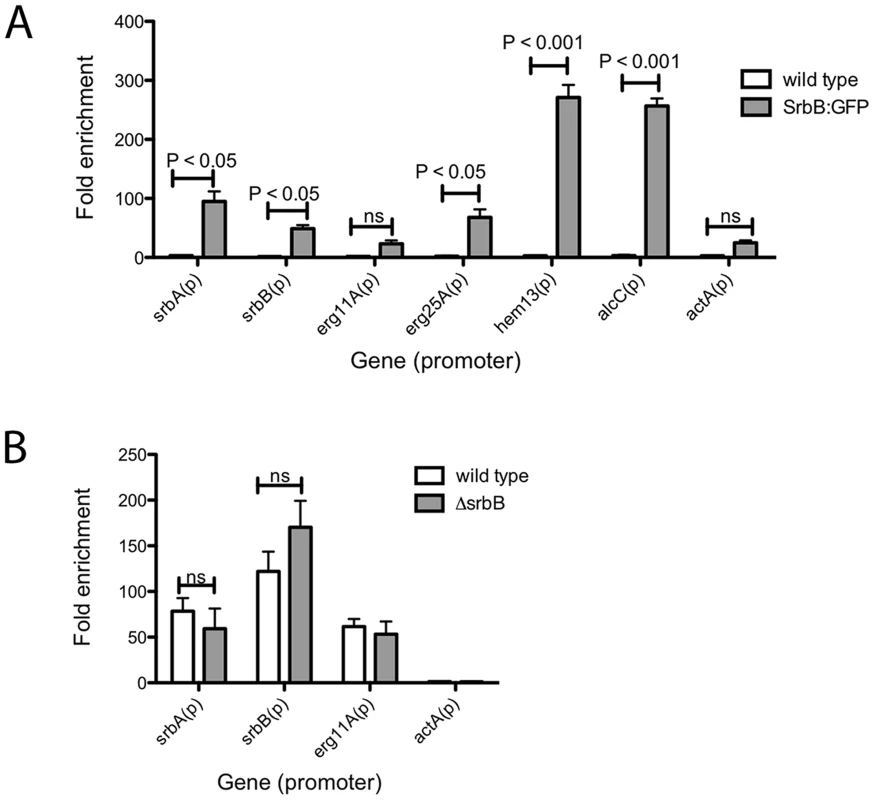
(A) SrbB tagged with GFP was expressed in A. fumigatus wild type. The resulting strain was cultured in normoxia at 37°C, 250 rpm for 18 hours and shifted to hypoxia for additional incubation for 4 hours. ChIP was conducted using GFP antibody followed by ChIP-qPCR to study SrbB enrichment on the promoters of SrbA target genes. Compared to wild type control, SrbB enrichment was significant in SrbB:GFP for srbA, srbB, erg25A, hem13, and alcC, which suggest SrbB directly binds on the promoter of these genes for transcriptional regulation. In contrast, SrbB enrichment on the promoters of erg11A and actA were not significant. Data are presented as the mean and standard error of two biological replicates, and analyzed by two-way ANOVA followed by Bonferroni posttest. (B) SrbA binding to the promoter of srbA, srbB, and erg11A in ΔsrbB was examined by ChIP-qPCR. Compared to wild type, SrbA enrichment on the gene promoters was not altered by disruption of SrbB. Data are presented as the mean and standard error of two biological replicates and analyzed by two-way ANOVA followed by Bonferroni posttests. In contrast, SrbB enrichment on the SrbA binding site of erg11A, whose transcript level solely relies on SrbA, was not significant compared to wild type. Interestingly, similar to SrbA, SrbB binds to its own promoter (Figure 9A). We next tested whether loss of SrbB would affect binding of SrbA to its direct target genes (Figure 9B). Compared to wild type, SrbA binding on the promoters of srbA, srbB, and erg11A did not change in ΔsrbB. Considering that both SrbA and SrbB are able to bind promoters of hypoxic genes including srbA, srbB, and erg11A (Figure 9A), these two transcription factors may form either homodimers or heterodimers to regulate gene expression. Thus, similar SrbA enrichment between wild type and ΔsrbB observed in Figure 9B is likely because the SrbA homodimer (or monomer) is the major form bound to the promoters of these genes. Overall, our data suggest that SrbA and SrbB are critical for hypoxia adaptation in A. fumigatus with both dependent and independent functions in regulation of genes critical for hypoxia adaptation and growth.
Restoration of Full srbB Transcript Levels in the ΔsrbA Background Partially Restores Hypoxic Growth
These data suggest that SrbB co-regulates a sub-set of SrbA target genes and that loss of SrbA also markedly reduces SrbB levels. Consequently, we hypothesized that restoration of full SrbB levels in ΔsrbA may ameliorate the severe hypoxic growth defect of ΔsrbA. As described above, we generated two strains that have restored srbB expression in ΔsrbA using two promoters, flavA(p) and gpdA(p) (TDC43.18 and TDC44.2, respectively). Quantitative real-time PCR was conducted to verify if these promoters induced transcript level increases of srbB. Compared to ΔsrbA, srbB transcript levels increased by 5.1 - and 3.7-fold in normoxia and 2.7 - and 1.4-fold in hypoxia in TDC43.18 (Figure 10A). Restoration of srbB transcript levels strongly promotes growth of ΔsrbA in hypoxia, however growth is not fully restored to wild type levels and was dependent on srbB transcript levels (Figure 10B). In addition, as predicted, restoration of srbB transcript levels in ΔsrbA did not rescue the triazole drug susceptibility of ΔsrbA (Figure 10C). This result is consistent with cyp51A/erg11A mRNA levels primarily regulated directly by SrbA.
Fig. 10. Restoration of srbB transcript levels in ΔsrbA promotes growth in hypoxia. 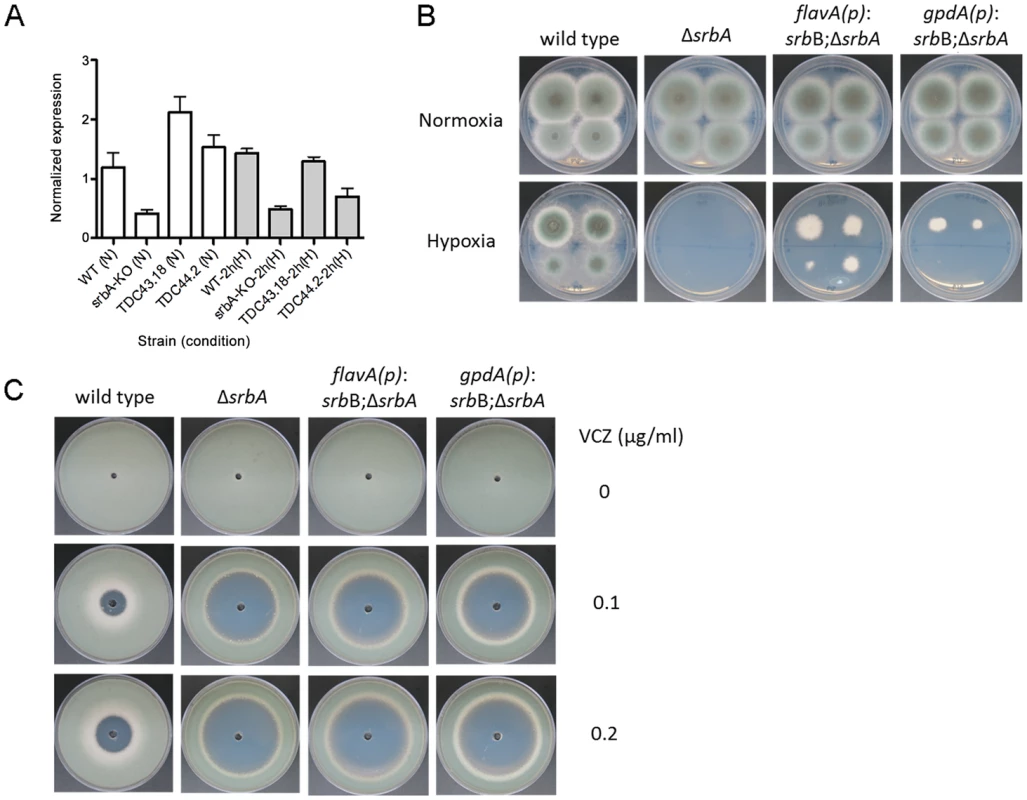
(A). Using two promoters, flavA(p) and gpdA(p), srbB was expressed in ΔsrbA. Expression levels of srbB in TDC43.18 (flavA(p)) and TDC44.2 (gpdA(p)) in normoxia or hypoxia 2 h were verified using quantitative PCR. Data are presented as the mean and standard error. Compared to ΔsrbA, srbB transcript in TDC43.18 and TDC44.2 was more abundant by 5- and 3.7-fold in normoxia and 2.7- and 1.4-fold in hypoxia 2 h, respectively. This indicates these promoters function properly. (B). Sequentially diluted conidia (102–105) were inoculated on GMM plates and culture in normoxia or hypoxia at 37°C for 3 days. Increased expression of srbB using flavA(p) or gpdA(p) partially restores defective hypoxia growth in ΔsrbA. (C). Susceptibility of TDC43.18 and TDC44.2 to the triazole drug, voriconazole (VCZ) was tested. A million conidia were overlaid on GMM, and VCZ in DMSO was applied to the center of the plate. Cleared areas represent inhibited fungal growth in response to VCZ. Although growth in hypoxia was partially rescued in both strains as shown (B), increased susceptibility to VCZ in ΔsrbA compared to wild type was not affected by restoration of srbB expression. The ΔsrbAΔsrbB strain generated to study gene transcript levels of SrbA target genes was further characterized to study the genetic relationship between SrbA and SrbB. ΔsrbAΔsrbB showed similar phenotypes to ΔsrbA, with a complete lack of growth in hypoxia and marked increased in azole drug susceptibility compared to wild type (Figure S4A–B). Moreover, when srbA was over-expressed in ΔsrbAΔsrbB, the resulting strain grew similar to wild type in hypoxia (Figure S4C). Taken together, these data are consistent with a model where SrbA positively regulates srbB gene expression in response to hypoxia. It also further supports that both SrbA and SrbB are involved in hypoxic gene regulation but SrbA plays a dominant role over SrbB with regard to genes essential for hypoxia growth in the tested conditions.
SrbB is Required for Full Virulence of Aspergillus fumigatus
Of particular importance for understanding A. fumigatus pathogenesis, loss of SrbB results in a significant virulence attenuation in a steroid murine model of IPA (Figure 11A). Kaplan-Meier curves with ΔsrbB show a significant increase in survival for animals inoculated with ΔsrbB compared to wild type and reconstituted strains (Log rank test, p = 0.0027). To gain insights into the relative contributions of SrbA and SrbB to virulence, we examined total fungal growth in vivo utilizing qRT-PCR based quantitation of fungal burden as we have previously described (Figure 11B) [7], [61]. As expected, ΔsrbA displayed a significant decrease in fungal burden compared to wild type consistent with its attenuated virulence in murine models of IPA (P = 0.008) [36], [37]. Surprisingly, ΔsrbB fungal burden was as low as ΔsrbA despite it having a higher level of virulence than ΔsrbA as measured by murine survival. Consequently, ΔsrbAΔsrbB exhibited an even a greater reduction in virulence than either single mutant alone (P = 0.016 between ΔsrbB and ΔsrbAΔsrbB), though the difference with ΔsrbA did not achieve statistical significance (P = 0.31), Figure 11B). Taken together, these data suggest that SrbB is an important SrbA-dependent regulator of the fungal response to hypoxia and required for full fungal virulence, and that both SrbA and SrbB make novel contributions to A. fumigatus virulence.
Fig. 11. Loss of SrbB attenuates Aspergillus fumigatus virulence through reductions in pulmonary fungal burden. 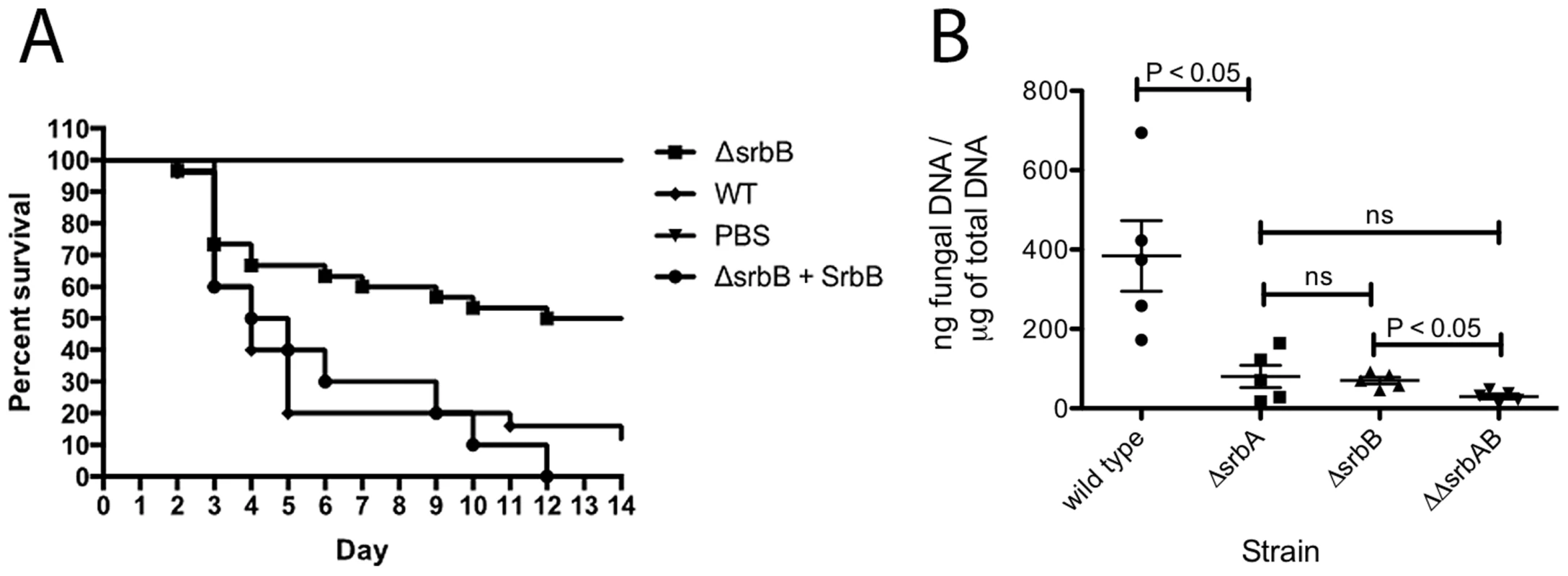
(A). 6–8 week old immunosuppressed CD-1 mice (each group N = 20) inoculated via the intranasal route with 2×106 conidia of wild type, ΔsrbB and srbB-reconstituted strains. Comparing wild type and reconstituted strain Kaplan-Meier curves with the ΔsrbB strain shows a significant increase in survival for the animals inoculated with the ΔsrbB strain (Log rank test, p = 0.0027). All control PBS inoculated animals survived. (B) Triamcinolone mouse model was used for fungal burden analysis. Mice were infected with 106 conidia of each strain and lungs were collected on day +3. Data represented are the mean and standard error of 3–5 mice per group. Discussion
Previously, a link between the transcription factor SrbA, and the ability of A. fumigatus to cause disease in murine models of IPA has been observed [36]–[38]. However, the mechanism(s) by which SrbA mediates in vivo fungal growth and virulence are not fully defined. Given the conservation of SrbA with mammalian SREBPs, uncovering the fungal specific functions of SrbA is important in order to yield potential mechanisms that can be targeted for therapeutic development. Support for this rationale comes from several elegant studies showing that targets of conserved transcription factors are often not shared between distantly related organisms and even closely related species [62]–[65]. Thus, in order to fully maximize the attenuated virulence of fungal pathogens lacking SREBP homologs for therapeutic benefit, in-depth investigation into their regulons and mechanisms of regulation and activity are needed.
Here, the A. fumigatus SrbA transcriptional regulon in response to hypoxia was interrogated utilizing ChIP-seq, RNA-seq and Nanostring nCounter technologies. Ninety-seven genes whose promoters were strongly bound by SrbA were identified in the ChIP-seq analysis. This number appears low considering the large number of genes differentially expressed in the absence of SrbA as detected here via RNA-seq and previously with microarray analysis [38]. However, a low overlap between transcript levels of genes affected by transcription factor loss and target genes identified in ChIP experiments appears to be the norm rather than the exception [66]. Consequently, our results suggest that we may have herein underestimated our direct SrbA targets or, more likely, there is an extensive network of genes involved with SrbA that are indirectly regulated though other transcription factors, small molecules, and proteins. The identification of ergosterol biosynthesis genes as SrbA direct targets and the sequence conservation of the ChIP identified SRE DNA binding motif strongly support the robustness of our analyses.
A major novel finding from our study of SrbA target genes was the identification of SrbB and its genetic relationship with SrbA. Based on sequence similarity, Bien and Espenshade hypothesized that SrbB was an SREBP-like transcription factor [21]. While our amino acid sequence analysis of SrbB did not reveal the presence of transmembrane domains that are found in the prototypical SREBPs, SrbB contains the hallmark tyrosine residue in the bHLH domain characteristic of SREBP family members. Moreover, a srbB::GFP fusion protein was observed to localize solely to the nucleus on preliminary analyses (Figure S3) further suggesting that SrbB is not membrane bound like SrbA and other SREBP family members (though constitutive cleavage cannot currently be ruled out). Transcript levels of srbB were strongly induced in response to hypoxia, earlier and to a greater magnitude than srbA. Thus, transcriptional regulation of srbB mRNA levels appears to play a major role in regulation of its function. In hypoxia, SrbA was found to bind to the promoter region of srbB at three locations. Loss of SrbA, however, did not completely eliminate srbB transcript levels in hypoxia suggesting the presence of additional srbB transcriptional regulators that remain to be defined.
Consistent with increased transcript in vitro in response to hypoxia and strong induction of transcript levels in vivo in a murine model of IPA, loss of SrbB markedly attenuated hypoxia growth and virulence of A. fumigatus. Unlike loss of SrbA, loss of SrbB had a minor effect on susceptibility to triazole antifungal drugs. The lack of an azole drug phenotype is consistent with the observation that SrbB does not appear to regulate cyp51A/erg11A mRNA levels, at least under the conditions tested here. However, full expression of the ergosterol biosynthesis genes erg1 and erg25A does require the presence of SrbB. In a similar manner, a major finding with regard to SrbB function is its clear importance for regulation of heme biosynthesis in normoxia and hypoxia. Heme plays a critical role in oxygen sensing and gene expression regulation in many organisms, and here our results link SrbB mediated regulation of heme biosynthesis with A. fumigatus hypoxia growth and virulence [67]–[70]. While SrbA binds to the promoter of hem13, and hem13 mRNA levels are reduced in ΔsrbA, SrbB appears to be the major transcriptional regulator required for hem13 in hypoxia and consequently heme biosynthesis (Figure 8). Further investigations are needed into the role of heme in oxygen sensing and virulence in A. fumigatus.
SrbB displays significant sequence similarity with the recently characterized Aspergillus oryzae transcription factor sclR that is critical for hyphal morphology and sclerotial formation [71]. Intriguingly, the authors noted that loss of sclR negatively affected pellet formation in liquid culture. ΔsclR pellets exhibited hollow interiors and fluffy exteriors that is consistent with SclR having a role in hypoxic adaptation. In addition to sclR, SrbB has sequence similarity with the Candida albicans bHLH transcription factor Cph2, though reciprocal BLAST analyses do not confirm orthology. Like srbB, CPH2 is expressed in vivo in models of candidiasis and is required for colonization of the murine gastrointestinal tract [72]–[75]. Its role in hypoxia adaptation in C. albicans is unclear; though also similar to SrbB it is induced early in the Candida hypoxic response [76].
While further analyses are needed to define the genetic and potentially physical relationships between SrbA and SrbB in A. fumigatus, our data hint at likely mechanisms that are modeled in Figure 12. First, data suggest that SrbA and SrbB have both mutually exclusive and co-regulated target genes in their regulons, and that they are involved in reciprocally regulating transcript levels. The increase in srbA transcript levels in the absence of srbB suggests that at some level SrbB could act as a transcriptional repressor. It is unclear if the effect on srbA transcript levels is direct and experiments to uncover direct SrbB target genes are ongoing in our laboratory. Analysis of SrbB DNA binding utilizing a SrbB:GFP fusion protein strongly suggests that SrbB can bind the SRE motif found in the srbA promoter region. It is likely, however, that the increase in transcript levels of several SrbA target genes in ΔsrbB is driven by increases in SrbA levels and activity. In this model, besides its role as a transcriptional activator of genes important for the hypoxia response, SrbB also functions in a negative feedback loop to modulate SrbA activity. Accordingly, persistence of high SrbA levels would at some point become detrimental to cellular homeostasis perhaps through increases in an SrbA dependent molecule(s) that become toxic to the cell. We note that genes associated with export of toxic molecules were enriched in the upregulated gene set in ΔsrbB.
Fig. 12. Working models for transcriptional regulation of the hypoxia response by SrbA and SrbB in A. fumigatus. 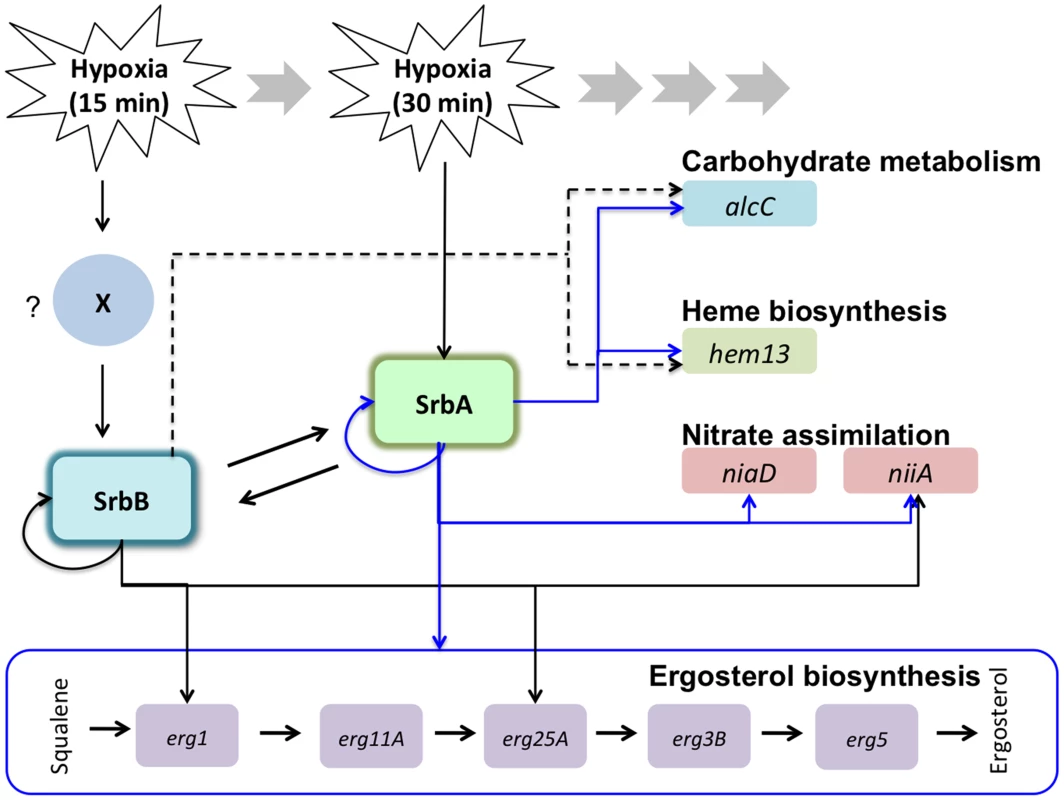
A. fumigatus SREBPs, SrbA and SrbB have dependent and independent functions in regulation of hypoxic genes involved in ergosterol biosynthesis, carbohydrate metabolism, heme biosynthesis, and nitrate assimilation. In response to hypoxia, srbB transcription is induced earlier than srbA (15 minutes in hypoxia) possibly by an unknown factor (marked as ‘X’). SrbA regulates srbB and its own transcript abundance. Similarly, SrbB regulates srbA and its own transcript abundance. qRT-PCR and ChIP-qPCR data presented in this study show that abundance of erg1, erg25A, hem13, niiA, and alcC is regulated by both SrbA and SrbB. In contrast, abundance of erg11A, erg3B, erg5, and niaD is dependent on SrbA. SrbB appears to have a dominant function over SrbA in regulation of hem13 and alcC transcript abundance (indicated by dotted lines). Of course there are several possible mechanisms through which SrbA and SrbB could co-regulate gene transcription, however in mammals it is known that SREBPs can physically interact to regulate gene expression through formation of homo and heterodimers [77], [78]. It may be possible that genes co-regulated by SrbA and SrbB in response to hypoxia are regulated through heterodimer formation, while genes exclusive to the respective transcription factor are regulated in part by homo-dimer formation. Moreover, in mammals, SREBP homo-and heterodimers have different levels of transcriptional activity [77]. Thus, it is plausible that in ΔsrbB, SrbA homodimers are more potent activators of SrbA target genes such as srbA itself than an SrbA-SrbB heterodimer that is present in the wild type. It is well established that the ability of bHLH proteins to form multiple dimer combination based on availability of binding partners and environmental conditions is a critical and elegant form of gene regulation [79]. Along these lines, genetic null mutants of fungal SREBPs alter the stoichiometric ratios of potential binding partners, which is further complicated by the fact that SrbA and SrbB are involved in regulating the other's expression. We also note that a third SREBP family member, SrbC, exists in A. fumigatus and awaits further investigation. Whether a preferred dimer form exists for regulation of specific SREBP regulated genes has not been extensively studied. Together, our data suggest that transcriptional activity of SrbA homodimer is efficient to activate erg3B, erg5, erg11A, and niaD, and an SrbA homodimer might be a preferred or dominant dimerization form for regulation of these genes over SrbA-SrbB heterodimers. In contrast, erg1, erg25A, niiA, alcC, and hem13 might be favorably regulated by SrbA-SrbB heterodimers rather than SrbA - or SrbB - homodimers. Another possibility is that the promoter of erg1, erg25A, niiA, alcC, and hem13 might contain an SrbB-specific binding site(s) in addition to the identified SrbA SRE motif. Consequently, both SrbA and SrbB could simultaneously regulate these genes by binding at the separate promoter sites.
However, the utilization of genetic null mutants and over-expression strains in null mutant backgrounds has allowed us to pinpoint the contribution of SrbA and SrbB to expression of specific target genes (Figure 8). These target genes yield new additional insights into how A. fumigatus adapts and grows in the mammalian lung environment. Of particular interest is the strong reduction in ΔsrbB growth in the murine model that was similar to the reduction in growth observed in mice inoculated with ΔsrbA. The difference in murine survival between mice inoculated with the two strains could be due to increases in host damage caused by ΔsrbB. For example, as SrbB is the major regulator of the ethanol fermentation alcohol dehydrogenase AlcC, one would predict an increase in inflammation in ΔsrbB inoculated mice due to the immune suppressive effects of ethanol previously reported in our murine model [7]. Alternatively or in conjunction with increased immunopathogenesis, it may be plausible that host heme is utilized by ΔsrbB to promote growth and host death later in infection when host damage is more severe and free heme may be available as addition of hemin to ΔsrbB partially restored in vitro hypoxia growth. In addition, the further decrease in fungal burden observed in ΔsrbAΔsrbB strain inoculated strongly suggests that the combination of decreased iron uptake and ergosterol biosynthesis, regulated by SrbA, and defects in carbon metabolism and heme biosynthesis, regulated by primarily by SrbB, consequently severely inhibit in vivo fungal growth. As all of these biological processes related to fungal metabolism/bioenergetics are impacted by oxygen availability, manipulation of in vivo oxygen levels may be a viable therapeutic strategy to reduce A. fumigatus growth in vivo.
In conclusion, while it is well established that fungal SREBPs are critical regulators of ergosterol biosynthesis and iron homeostasis, our analyses of SrbA and SrbB expand the known functions of these fungal virulence and antifungal drug-associated transcription factors (Figure 12). The major enrichment of genes involved in oxidoreductase activity, carbohydrate and nitrogen metabolism, and heme biosynthesis in the regulons of SrbA and SrbB presents an exciting and important area for further investigation into how these processes affect hypoxia adaptation, fungal virulence, and responses to antifungal drugs. From the big picture of the SrbA-SrbB regulons, we propose that the SrbA-SrbB genetic network allows A. fumigatus to “reprogram” its bioenergetics to allow invasive growth to cause disease in the mammalian lung in the face of oxygen and iron limitation. Consequently, these fungal SREBPs are much more than regulators of sterol biosynthesis, rather they are global regulators of fungal bioenergetics potential/metabolism; also an emerging theme with mammalian SREBPs [80]. Finding a mean(s) to unplug this fungal genetic network is an ongoing research goal that is expected to yield a significant therapeutic breakthrough for IPA.
Materials and Methods
Strains and Media
Aspergillus fumigatus strain CEA17 was used to construct the ΔsrbA mutant [36]. The wild type strain referred to in this article is strain CBS 144.89, also called CEA10. All strains are routinely grown on glucose minimal media (GMM) that contains 1% glucose, salt solution and trace minerals, at 37°C [81]. The recipe for liquid glucose minimal media is identical to that for GMM, except without agar. Liquid cultures for RNA analysis were grown under agitation (200 RPM) in baffle flasks.
Construct Design for an srbB Null Mutant and srbA or srbB Over-Expression Strains
To generate an srbB null mutant, a 1.2 kb up - and downstream sequences were PCR-amplified from A. fumigatus genomic DNA (gDNA). As a selectable marker, a 3.2 kb pyrG from A. parasiticus was PCR-amplified from the plasmid pJW24. The three DNA fragments were used as a template to generate a final construct via double-joint PCR, and the PCR product was transformed to A. fumigatus wild type CEA17 [30]. Southern blot analysis was conducted to confirm homologous gene replacement (Figure S2). To regain srbB expression in the srbB deletion strain, a 4.1 kb DNA fragment including a srbB promoter and a coding sequence was PCR-amplified. As a selectable marker, a 3.0 kb hygB fragment was PCR-amplified from pBC-hyg plasmid DNA. These two PCR products were used as a template to generate a final reconstituted construct via a double-joint PCR [82]. The final PCR product was transformed to ΔsrbB and transformants were screened using PCR.
A double null mutant of srbA and srbB was generated by deletion of srbB in the ΔsrbA (pyrG-) strain. The srbB deletion construct designed to generate ΔsrbB above was transformed to ΔsrbA. Gene replacement in the resulting transformants were screened by PCR and verified by Southern bot analysis. To over-express srbB in ΔsrbA background, either A. fumigatus flavohemoprotein (flavA(p), AFUB_099650) or A. nidulans glyceraldehyde-3-phosphate dehydrogenase (gpdA(p), AN8041) promoter was utilized. A 1 kb flavA(p) and a 2 kb gpdA(p) DNA fragment was PCR-amplified from A. fumigatus and A. nidulans gDNA. A 2.5 kb DNA fragment including the srbB coding region and downstream sequence was PCR-amplified from A. fumigatus gDNA. As a selectable marker, a 3.2 kb pyrG from A. parasiticus was PCR-amplified from the plasmid pJW24. These three PCR products were used to generate a 6.5 (flavA(p)) and 7.5 kb (gpdA(p)) final construct via double-joint PCR [82]. The final constructs were transformed into ΔsrbA resulting in TDC43.18 (flavA(p)) or TDC44.2 (gpdA(p)) strains. Single copy integration of the srbB-overexpression construct in ΔsrbA was confirmed by Southern blot analysis.
To over-express srbA in ΔsrbAΔsrbB, srbA was amplified along with 1.2 kb 5′ upstream sequence from A. fumigatus wild type gDNA. Purified PCR product was transformed in the ΔsrbAΔsrbB strain. Transformants were selected in hypoxia using the inability of hypoxia growth of ΔsrbA. Over-expression of srbA in the resulting transformants was confirmed by qRT-PCR (Figure 8).
Chromatin Immunoprecipitation (ChIP): Growth Conditions
For ChIP experiments, 1×106 spores/mL of Aspergillus fumigatus strain CBS144.89 and ΔsrbA were grown in 200 mL of liquid glucose minimal media (LGMM) in 500 mL shaking flask cultures for 24 hours. Samples were centrifuged, and 200 mg of mycelia were transferred to 100 mL pre-conditioned fresh LGMM in 250 mL Erlenmeyer flasks, and then placed in hypoxia chamber on platform shaker at 200 rpm for 4 and 12 hours hypoxia exposure. Samples were collected by vacuum filtration and transferred to cross-linking solution for ChIP experiments, or flash frozen and lyophilized for RNA extraction.
Cross-linking
A sample of mycelia for later RNA isolation was frozen on liquid nitrogen immediately before crosslinking, and stored at −80°C. Remaining filtered samples were added to 20 mL of buffer for crosslinking (0.4 M Sucrose, 10 mM Tris-HCl, pH 8.0, 1 mM EDTA, adding 1 mM PMSF and 1% formaldehyde just before use) in a 125 mL flask for 20 min under shaking (100 rpm) at 30°C. Crosslinking was stopped by adding 1 mL of 2 M glycine, and continued shaking incubation for 10 minutes. Mycelia were collected and dried using vacuum filtration and rinsed with sterile ddH2O and transferred sample to 2 mL screw cap tube, and frozen immediately with liquid nitrogen and stored at −80°C.
DNA Sonication
Approximately 200 mg of frozen mycelia were ground to a fine powder in a chilled mortar and pestle with liquid nitrogen added. Powder was transferred to 10 mL of ChIP lysis buffer (CLB: 50 mM HEPES pH 7.5, 150 mM NaCl, 1 mM EDTA, 1% Triton X-100, 0.1% Deoxycholate (Sigma D6750), 0.1% SDS, 1 mM PMSF, 1× fungal proteinase inhibitor cocktail (Sigma, USA)). Each sample was vortexed and then split into multiple 300 µl volume (maximum volume for sonication) 1.5 mL microfuge tubes appropriate for sonication. Samples were sonicated with Biorupter UCD-200 (Diagenode, USA) with the following condition: 30 sec ON and 30 sec OFF at power level High for a total of 30 minutes in a cold room. Ice was added every 10 minutes to ensure samples remain at 4°C. Tubes were centrifuged at 10,000 g for 5 minutes at 4°C. Supernatant was transferred into new tube. 30 µL was reserved as input control (IC) fraction for reverse crosslinking to verify sonication and control for ChIP and qPCR.
Chromatin Immunoprecipitation
30 µL of Protein A Dynabeads (Dynal, Invitrogen) were used for each sample. Beads were washed twice on magnetic stand with 500 µL of CLB, with 5 minutes of slow rotation at 4°C for each. 100 µL of blocking buffer (0.1 mg BSA, 200 µg Yeast tRNA, in 1 mL of CLB) with 1 µg of antibody/100 µL (IgG (Invitrogen, Rabbit) or SrbA (Willger et al. 2008, anti-rabbit antibody) was added to the washed Dynabeads on a magnetic stand. The beads and blocking buffer were incubated 16 hours at 4°C with slow rotation to coat Dynabeads. After incubation, Dynabeads were washed twice by 500 µL of CLB as above. Immediately after removing last wash, 100 µL of sonicated sample was added to beads. Samples were incubated 16 h at 4°C with rotation. In cold room, samples were washed twice by 500 µL of CLB as above, then washed as above with 0.5 ml of LNDET (0.25 M LiCl; 1% NP40 (Nonidet P40 Substitute, USB); 1% Deoxycholate; 1 mM EDTA), and finally washed twice with 0.5 ml of Tris-EDTA (TE: 10 mM Tris-HCl, pH 8.0; 1 mM EDTA, pH 8.0). After removal of last TE wash, DNA was eluted from antibody with 50 µl of fresh elution buffer (EB: 1% SDS; 0.1 M NaHCO3; 0.2 mg/ml proteinase K; 1 mM DTT) and incubated at 65°C for 10 minutes. On a magnetic stand, supernatant was transferred to a new tube. A second elution with 50 µl of EB was performed so that the final elution volume was 100 µl. All samples were incubated for 16 hours at 65°C for reverse crosslinking.
DNA Purification for ChIP Samples
After reverse crosslinking, samples were treated with 2.5 µg of RNase A and incubated for 30 minutes at room temperature. DNA was extracted either by using PCR purification kit (Qiagen) following the high pH protocol, or EtOH precipitation, with 50 µL as final volume. 5 µL of sample was assayed on Qubit using high sensitivity dsDNA kit (Invitrogen). To check sonication, 10 µL of IC was run on E-gel EX 2% agarose gel (Invitrogen) and fragment size ranged from 1 kb to 100 bp.
ChIP Sequencing
ChIP-seq libraries were created following a published Illumina ChIP-seq library preparation protocol [83]. Briefly, fragmented ends were repaired, an adenine molecule was added to the repaired end, PAGE-purified adapters were added to the overhang, and the mixture amplified with primer 1.0 and primer 2.0 with an individual index tag to allow for multiplex of samples in a single lane. Samples were gel purified to obtain a size range between 200–400 bp. Libraries were validated by real time PCR, concentration was determined with Qubit (Invitrogen) and integrity was checked with an Agilent Bioanalyzer. Samples were sent to the Ohio State University sequencing facility for 76 bp paired end sequencing and four ChIP samples were indexed per lane on the Illumina HiSeq 2000. Full read data are deposited at the NCBI GEO repository under accession GSE61974.
ChIP Sequencing Alignment, Peak Finding
Paired end reads from three independent ChIP-seq experiments (multiplexed in 1 lane each) were quality checked with fastqc (http://www.bioinformatics.babraham.ac.uk/projects/fastqc/). Reads were trimmed and cleaned of contaminating Illumina adaptors using trimmomatic [84] and aligned to the A. fumigatus A1163 CADRE genome from ensembl fungi, version 18 using bowtie2-2.1.0 [85]. Reads that aligned concordantly as paired ends were retained and used for peak calling. The resulting bam files were used as input for peak calling and for plotting. Peaks were called using Model-based Analysis for ChIP-Sequencing (MACS2) version 2.0.10.20131216 [45], with the subpeak calling option enabled. Peak calling was done with the wild type ChIP-seq samples and wild type input control samples using an fdr cutoff of 0.05. Results reported herein are for the combined reads from all 3 samples. Similar sets of peaks and genes were identified when each independent biological replicate was assessed separately.
Motif Discovery and Enrichment in SrbA Regulated Genes
To find sequence motifs within ChIP-seq peaks, 100 bp centered on each of the peaks (Table S2) were selected. Multiple Em for motif elicitation (Meme) version 4.9.1 [86] was run with the minimum and maximum motif widths of 4 and 12, the zoops model and the x_branch option. The resulting 11 bp motif was reported for 54 sites. Similar motifs were found using the meme oops and anr models or using other motif discovery tools [87], [88].
ChIP qRT-PCR
Prior to sequencing, all ChIP samples were diluted 10-fold for PCR. 1 µl of template was used in a 10 µl total volume reaction using Promega 2× GoTaq qPCR master mix and 0.4 µM of each primer. Realtime PCR was performed with 40 cycles of 95°C for 15 s and 60°C for 30 s on Mastercycler ep realplex PCR machine. PCR was performed in triplicate for each separate ChIP experiment using primers designed for regions identified as enriched in preliminary analysis. Three genes were chosen from this analysis as positive for enrichment in all ChIP conditions (srbA, erg11A and erg25A) based on previous microarray experiments [40]. Percent input method was used to calculate the signal of enrichment of the promoter region for each gene (http://cshprotocols.cshlp.org/cgi/content/full/2009/9/pdb.prot5279 and Invitrogen website). Briefly, 100*(2(InputCt-ChIPCt)) was calculated for each reaction and the average and standard deviation calculated from these values. No correction for adjusted input was necessary as both templates were diluted equally prior to PCR. Lack of enrichment for at least two of the three genes, or non-amplifying PCR, was evidence for poor ChIP, and these samples were not sequenced. ChIP-qPCRs with SrbB:GFP were performed for the genes that showed significant SrbA enrichment on the promoters. ChIP samples were prepared from cultures grown under the same conditions used for ChIP-seq. All ChIP samples were diluted 10-fold for PCR. 2 µl of template was used in a 20 µl total volume reaction using SYBR green master mix (BioRad) and 0.4 µM of each primer. Fold enrichment method was used to calculate the signal of the promoter region for each gene (http://www.lifetechnologies.com/us/en/home/life-science/epigenetics-noncoding-rna-research/chromatin-remodeling/chromatin-immunoprecipitation-chip/chip-analysis.html).
RNA Extraction for Nanostring and RNA-seq
For RNA experiments, 1×106 conidia of Aspergillus fumigatus strain CBS144.89, ΔsrbA and ΔsrbB strains were grown in 50 mL of LGMM in 250 mL shaking baffle flasks for 16 hours at 300 RPM. Samples were transferred to a hypoxia chamber on platform shaker at 200 RPM for variable hypoxia exposure. Samples were collected by vacuum filtration, flash frozen in liquid nitrogen and lyophilized for RNA extraction. Lyophilized tissue was mixed with 0.2 mL of 0.5 mm glass beads (BioSpec, USA) and mixed for 30 seconds on Mini-beadbeater-16 (BioSpec, USA). Ground mycelia were suspended in 1 mL of TriSure (BioLine, USA), and incubated for 5 minutes. 200 µl of chloroform was added and mixed well, incubated two minutes. Samples were centrifuged at maximum speed for 15 minutes at 4°C. Upper layer was collected and mixed with an equal volume of 80% EtOH, and pipetted immediately to RNeasy spin column (Qiagen, USA). Columns were centrifuged one minute, flow through discarded and washed twice with kit supplied RPE buffer, and column completely dried after last wash. Filter column was transferred to RNase free 1.5 mL tube, and 200 uL of RNase free water was added an incubated for 1 minute, and then centrifuged to obtain nucleic acid. Samples were analyzed with NanoDrop ND-1000 (Thermo-Fisher).
RNA Sample Preparation and Illumina Sequencing (RNA-Seq)
To identify transcriptionally active genes, cDNAs obtained from the fungal mycelia incubated at stated conditions were sequenced with the Illumina platform to determine transcript abundance. Samples were DNased using the RNeasy kit (Qiagen), following the protocol for DNase Digestion before RNA Cleanup. Sequencing libraries were generated using the ScriptSeq kit v2 (Epicenter) following manufacturer's directions. After each preparation step sample quality and quantity was assessed using Bioanalyzer and the Agilent RNA 6000 Nano Kit (Agilent). All cDNA libraries were sequenced (4 samples per lane) using the Illumina HiSeq2000 instrument (www.illumina.com) at the Oregon Health Sciences University Massively Parallel Sequencing Shared Resource (http://www.ohsu.edu/xd/research/research-cores/mpssr/). RNA-seq analysis was performed using the bowtie-tophat-cufflinks pipeline [85], [89], [90]. RNA-seq data is deposited at NCBI SRA under BioProject ID PRJNA240563: accession numbers SAMN02677488, SAMN02677489, SAMN02677490.
FunCat and GO Enrichment Analysis
The FungiFun2 2.27 beta web-based server https://elbe.hki-jena.de/fungifun/fungifun.php was utilized to interrogate the functional enrichment of FunCat and gene ontologies in the respective datasets. For RNA-seq analysis, genes whose mRNA levels changed 4 fold or greater were included in the analysis. The A1163 genome selection was utilized for all analyses. Settings used for statistical significance include: significance level 0.05, significance test Hypergeometric distribution, and the Benjamini-Hochberg adjustment method.
cDNA Synthesis and Quantitative Real-Time-PCR
Aspergillus fumigatus strains were cultured in liquid media (glucose-minimal-media or induction/repression minimal media) for sixteen hours, then shifted to hypoxia for the indicated times. Mycelia were harvested via vacuum filtration and lyophilized overnight prior to homogenization with 0.1 mm glass beads. Total RNA was extracted using TRisure (Bioline) according to the manufacturer's instruction and purified via RNeasy column protocol (Qiagen). Genomic DNA purification was completed with Turbo DNAse I (Ambion). A secondary genomic DNA purification was done with the Qiagen QuantiTect Reverse Transcription Kit (Qiagen), as well as oligo-DT-primed cDNA synthesis. qRT-PCR was conducted in technical duplicates except where noted. The normalized fold expression graphed in each figure represents the mean and percent error of two-to-three biological replicates as normalized to the housekeeping gene tefA. A no-template mRNA control was used to ensure no gDNA contamination in each analysis.
Murine Model of IPA and In Vivo Analysis of Transcript Abundance
Virulence study for ΔsrbB, wild type and reconstitution strains (20 animals per strain, 2 experiments) was conducted in CD1 mice (Charles Rivers, USA) using the triamcinolone infection model for A. fumigatus as previously described [7]. For in vivo RNA analysis, mouse lungs were harvested on day 2, 3 and 4 post-infection. Lungs were flash frozen in liquid nitrogen and lyophilized for 24–48 hours until lung tissue was completely dry. Tissue was processed in same manner as above for fungal RNA. Final RNA samples were combined as whole lung, after verifying fungal RNA was present in each sample by qRT-PCR.
Fungal Burden Assay
For the determination of fungal burden, triamcinolone mouse model of IPA was utilized as described previously [7]. Briefly, mice were immunosuppressed with a single sub-cutaneous injection of steroid triamcinolone (Kenalog −10) (40 mg/kg) on day −1. Mice were inoculated with 106 conidia of the respective strains in 40 µl PBS intranasally on day zero. Control mice were infected with PBS only. Lungs were collected on day +3 and were immediately frozen in liquid nitrogen. DNA was isolated from lyophilized lungs, and treated with RNAse overnight. qRT-PCR was done to determine the amount of fungal DNA in each sample by comparing with a standard curve of known concentrations as described previously [7], [61]. Data represented are the mean and standard error of 3–5 mice per group and analyzed by t-tests between 2 experimental groups.
Ethics Statement
We carried out our animal studies in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Research Council (Council, 1996). The animal experimental protocol was approved by the Institutional Animal Care and Use Program (IACUC) at Montana State University Federal-Wide Assurance Number: A3637-01) and by the Institutional Animal Care and Use Committee (IACUC) at Dartmouth College (Federal-Wide Assurance Number: A3259-01).
Nanostring Quantitation of In Vitro and In Vivo RNA
Digital counts for 60 genes (ChIP targets, housekeeping genes and other genes of interest) were adjusted for binding efficiency with background subtraction using the included positive and negative controls from the manufacturer (Nanostring Technologies, Seattle, WA, USA), as per Nanostring nCounter data analysis guidelines. Data sets were normalized to facilitate across sample comparisons using the geometric mean of 20 stably expressed genes. A subset of 12 of these genes were examined and presented herein with complete data in Table S5. Boxplots were generated in R [91].
Growth and Biomass Production Tests
Sequentially diluted spore suspensions in 5 µl sterile water (102–105 conidia) were dropped on GMM plates and cultured at 37°C for 3 days in normoxia or hypoxia (1% O2, 5% CO2). In order to study biomass production, 108 conidia were incubated in 200 mL liquid GMM at 37°C, 200 rpm for 2 days. Mycelia were harvested, lyophilized, and weighed. Biomass test was performed in triplicate.
Susceptibility Test to Voriconazole (VCZ)
Susceptibility of fungal strains to VCZ was tested using either commercially available E-test strips (Biomerieux, Inc. Durham, NC) or a semi-quantitative method directly using VCZ (Sigma) solutions with appropriate concentrations. Five mL of RPMI media or GMM containing 105 conidia were overlaid onto a 25 mL RPMI/GMM plate. Then, E-test strips were placed on the plate, or VCZ (Sigma) solutions were added in the center of the plate. After 2 days incubation at 37°C, clearance of fungal growth was observed and susceptibility to VCZ was decided.
Analysis of Porphyrins
Porphyrins were extracted from powdered dried mycelia by homogenization in phosphate buffered saline and the protein content determined in an aliquot by the Bradford method. The extract was then mixed with an equal volume of acetone/concentrated HCl (97.5/2.5 v/v), centrifuged and the supernatant analyzed for porphyrins by reversed phase HPLC with fluorescence and UV detection [92] using porphyrin standards from Frontier Scientific (Logan, UT, USA)
Supporting Information
Zdroje
1. BarronMA, MadingerNE (2008) Opportunistic Fungal Infections, Part 2: Candida and Aspergillus. Infections in Medicine 25 : 498–505.
2. BrownGD, DenningDW, GowNA, LevitzSM, NeteaMG, et al. (2012) Hidden killers: human fungal infections. Science translational medicine 4 : 165rv113.
3. BrownGD, DenningDW, LevitzSM (2012) Tackling human fungal infections. Science 336 : 647.
4. Ben-AmiR, LewisRE, KontoyiannisDP (2010) Enemy of the (immunosuppressed) state: an update on the pathogenesis of Aspergillus fumigatus infection. British Journal of Haematology 150 : 406–417.
5. SteinbachWJ (2013) Are We There Yet? Recent Progress in the Molecular Diagnosis and Novel Antifungal Targeting of Aspergillus fumigatus and Invasive Aspergillosis. PLoS Pathogens 9: e1003642.
6. WillgerS, GrahlN, CramerR (2009) Aspergillus fumigatus metabolism: Clues to mechanisms of in vivo fungal growth and virulence. Medical Mycology 47: S72–S79.
7. GrahlN, PuttikamonkulS, MacdonaldJM, GamcsikMP, NgoLY, et al. (2011) In vivo hypoxia and a fungal alcohol dehydrogenase influence the pathogenesis of invasive pulmonary aspergillosis. PLoS Pathogens 7: e1002145.
8. TarrandJJ, HanXY, KontoyiannisDP, MayGS (2005) Aspergillus hyphae in infected tissue: Evidence of physiologic adaptation and effect on culture recovery. Journal of Clinical Microbiology 43 : 382–386.
9. HallLA, DenningDW (1994) Oxygen Requirements of Aspergillus Species. Journal of Medical Microbiology 41 : 311–315.
10. BrockM, JouvionG, Droin-BergereS, DussurgetO, NicolaMA, et al. (2008) Bioluminescent Aspergillus fumigatus, a new tool for drug eficiency testing and in vivo monitoring of invasive aspergillosis. Applied and Environmental Microbiology 74 : 7023–7035.
11. BrownJM, WilsonWR (2004) Exploiting tumour hypoxia in cancer treatment. Nature reviews Cancer 4 : 437–447.
12. KoeppenM, EckleT, EltzschigHK (2011) The hypoxia-inflammation link and potential drug targets. Current opinion in anaesthesiology 24 : 363–369.
13. EltzschigHK, CarmelietP (2011) Hypoxia and inflammation. The New England Journal of Medicine 364 : 656–665.
14. MoellerBJ, RichardsonRA, DewhirstMW (2007) Hypoxia and radiotherapy: opportunities for improved outcomes in cancer treatment. Cancer Metastasis Reviews 26 : 241–248.
15. GrahlN, CramerRAJr (2010) Regulation of hypoxia adaptation: an overlooked virulence attribute of pathogenic fungi? Medical Mycology: Official Publication of the International Society for Human and Animal Mycology 48 : 1–15.
16. ErnstJF, TielkerD (2009) Responses to hypoxia in fungal pathogens. Cellular Microbiology 11 : 183–190.
17. HsuJL, KhanMA, SobelRA, JiangX, ClemonsKV, et al. (2013) Aspergillus fumigatus invasion increases with progressive airway ischemia. PloS One 8: e77136.
18. ChunCD, LiuOW, MadhaniHD (2007) A link between virulence and homeostatic responses to hypoxia during infection by the human fungal pathogen Cryptococcus neoformans. PLoS Pathogens 3: e22.
19. ChangYC, BienCM, LeeH, EspenshadePJ, Kwon-ChungKJ (2007) Sre1p, a regulator of oxygen sensing and sterol homeostasis, is required for virulence in Cryptococcus neoformans. Molecular microbiology 64 : 614–629.
20. GrahlN, ShepardsonKM, ChungD, CramerRA (2012) Hypoxia and fungal pathogenesis: to air or not to air? Eukaryotic cell 11 : 560–570.
21. BienCM, EspenshadePJ (2010) Sterol regulatory element binding proteins in fungi: hypoxic transcription factors linked to pathogenesis. Eukaryotic Cell 9 : 352–359.
22. HuaX, WuJ, GoldsteinJL, BrownMS, HobbsHH (1995) Structure of the human gene encoding sterol regulatory element binding protein-1 (SREBF1) and localization of SREBF1 and SREBF2 to chromosomes 17p11.2 and 22q13. Genomics 25 : 667–673.
23. HortonJD (2002) Sterol regulatory element-binding proteins: transcriptional activators of lipid synthesis. Biochemical Society transactions 30 : 1091–1095.
24. SeoYK, ChongHK, InfanteAM, ImSS, XieX, et al. (2009) Genome-wide analysis of SREBP-1 binding in mouse liver chromatin reveals a preference for promoter proximal binding to a new motif. Proceedings of the National Academy of Sciences of the United States of America 106 : 13765–13769.
25. SeoYK, JeonTI, ChongHK, BiesingerJ, XieX, et al. (2011) Genome-wide localization of SREBP-2 in hepatic chromatin predicts a role in autophagy. Cell Metabolism 13 : 367–375.
26. ReedBD, CharosAE, SzekelyAM, WeissmanSM, SnyderM (2008) Genome-wide occupancy of SREBP1 and its partners NFY and SP1 reveals novel functional roles and combinatorial regulation of distinct classes of genes. PLoS Genetics 4: e1000133.
27. HughesAL, ToddBL, EspenshadePJ (2005) SREBP pathway responds to sterols and functions as an oxygen sensor in fission yeast. Cell 120 : 831–842.
28. ToddBL, StewartEV, BurgJS, HughesAL, EspenshadePJ (2006) Sterol regulatory element binding protein is a principal regulator of anaerobic gene expression in fission yeast. Molecular and Cellular Biology 26 : 2817–2831.
29. PorterJR, BurgJS, EspenshadePJ, IglesiasPA (2010) Ergosterol regulates sterol regulatory element binding protein (SREBP) cleavage in fission yeast. The Journal of Biological Chemistry 285 : 41051–41061.
30. LloydSJ, RaychaudhuriS, EspenshadePJ (2013) Subunit architecture of the Golgi Dsc E3 ligase required for sterol regulatory element-binding protein (SREBP) cleavage in fission yeast. The Journal of Biological Chemistry 288 : 21043–21054.
31. CheungR, EspenshadePJ (2013) Structural requirements for sterol regulatory element-binding protein (SREBP) cleavage in fission yeast. The Journal of Biological Chemistry 288 : 20351–20360.
32. PorterJR, LeeCY, EspenshadePJ, IglesiasPA (2012) Regulation of SREBP during hypoxia requires Ofd1-mediated control of both DNA binding and degradation. Molecular Biology of the Cell 23 : 3764–3774.
33. StewartEV, LloydSJ, BurgJS, NwosuCC, LintnerRE, et al. (2012) Yeast sterol regulatory element-binding protein (SREBP) cleavage requires Cdc48 and Dsc5, a ubiquitin regulatory X domain-containing subunit of the Golgi Dsc E3 ligase. The Journal of Biological Chemistry 287 : 672–681.
34. HughesBT, NwosuCC, EspenshadePJ (2009) Degradation of sterol regulatory element-binding protein precursor requires the endoplasmic reticulum-associated degradation components Ubc7 and Hrd1 in fission yeast. The Journal of Biological Chemistry 284 : 20512–20521.
35. HughesBT, EspenshadePJ (2008) Oxygen-regulated degradation of fission yeast SREBP by Ofd1, a prolyl hydroxylase family member. The EMBO Journal 27 : 1491–1501.
36. WillgerSD, PuttikamonkulS, KimKH, BurrittJB, GrahlN, et al. (2008) A sterol-regulatory element binding protein is required for cell polarity, hypoxia adaptation, azole drug resistance, and virulence in Aspergillus fumigatus. PLoS Pathogens 4: e1000200.
37. WillgerSD, CornishEJ, ChungD, FlemingBA, LehmannMM, et al. (2012) Dsc orthologs are required for hypoxia adaptation, triazole drug responses, and fungal virulence in Aspergillus fumigatus. Eukaryotic Cell 11 : 1557–1567.
38. BlatzerM, BarkerBM, WillgerSD, BeckmannN, BlosserSJ, et al. (2011) SREBP coordinates iron and ergosterol homeostasis to mediate triazole drug and hypoxia responses in the human fungal pathogen Aspergillus fumigatus. PLoS Genetics 7: e1002374.
39. ChangYC, IngavaleSS, BienC, EspenshadeP, Kwon-ChungKJ (2009) Conservation of the sterol regulatory element-binding protein pathway and its pathobiological importance in Cryptococcus neoformans. Eukaryotic Cell 8 : 1770–1779.
40. BarkerBM, KrollK, VodischM, MazurieA, KniemeyerO, et al. (2012) Transcriptomic and proteomic analyses of the Aspergillus fumigatus hypoxia response using an oxygen-controlled fermenter. BMC genomics 13 : 62.
41. ButlerG (2013) Hypoxia and gene expression in eukaryotic microbes. Annual Review of Microbiology 67 : 291–312.
42. SailsberyJK, AtchleyWR, DeanRA (2012) Phylogenetic analysis and classification of the fungal bHLH domain. Molecular Biology and Evolution 29 : 1301–1318.
43. DaviesBS, RineJ (2006) A role for sterol levels in oxygen sensing in Saccharomyces cerevisiae. Genetics 174 : 191–201.
44. LosadaL, BarkerBM, PakalaS, JoardarV, ZafarN, et al. (2014) Large-scale transcriptional response to hypoxia in Aspergillus fumigatus observed using RNAseq identifies a novel hypoxia regulated ncRNA. Mycopathologia [Epub ahead of print].
45. ZhangY, LiuT, MeyerCA, EeckhouteJ, JohnsonDS, et al. (2008) Model-based analysis of ChIP-Seq (MACS). Genome Biology 9: R137.
46. GrantCE, BaileyTL, NobleWS (2011) FIMO: scanning for occurrences of a given motif. Bioinformatics 27 : 1017–1018.
47. LindeJ, HortschanskyP, FaziusE, BrakhageAA, GuthkeR, et al. (2012) Regulatory interactions for iron homeostasis in Aspergillus fumigatus inferred by a Systems Biology approach. BMC Systems Biology 6 : 6.
48. YokoyamaC, WangX, BriggsMR, AdmonA, WuJ, et al. (1993) SREBP-1, a basic-helix-loop-helix-leucine zipper protein that controls transcription of the low density lipoprotein receptor gene. Cell 75 : 187–197.
49. KimJB, SpottsGD, HalvorsenYD, ShihHM, EllenbergerT, et al. (1995) Dual DNA binding specificity of ADD1/SREBP1 controlled by a single amino acid in the basic helix-loop-helix domain. Molecular and Cellular Biology 15 : 2582–2588.
50. PerezJC, KumamotoCA, JohnsonAD (2013) Candida albicans commensalism and pathogenicity are intertwined traits directed by a tightly knit transcriptional regulatory circuit. PLoS Biology 11: e1001510.
51. PriebeS, LindeJ, AlbrechtD, GuthkeR, BrakhageAA (2011) FungiFun: a web-based application for functional categorization of fungal genes and proteins. Fungal Genetics and Biology 48 : 353–358.
52. GeissGK, BumgarnerRE, BirdittB, DahlT, DowidarN, et al. (2008) Direct multiplexed measurement of gene expression with color-coded probe pairs. Nature Biotechnology 26 : 317–325.
53. MalkovVA, SerikawaKA, BalantacN, WattersJ, GeissG, et al. (2009) Multiplexed measurements of gene signatures in different analytes using the Nanostring nCounter Assay System. BMC Research Notes 2 : 80.
54. SchrettlM, KimHS, EisendleM, KraglC, NiermanWC, et al. (2008) SreA-mediated iron regulation in Aspergillus fumigatus. Molecular Microbiology 70 : 27–43.
55. ZnaidiS, NesseirA, ChauvelM, RossignolT, d'EnfertC (2013) A comprehensive functional portrait of two heat shock factor-type transcriptional regulators involved in Candida albicans morphogenesis and virulence. PLoS Pathogens 9: e1003519.
56. ZagorecM, BuhlerJM, TreichI, KengT, GuarenteL, et al. (1988) Isolation, sequence, and regulation by oxygen of the yeast HEM13 gene coding for coproporphyrinogen oxidase. The Journal of Biological Chemistry 263 : 9718–9724.
57. KengT (1992) HAP1 and ROX1 form a regulatory pathway in the repression of HEM13 transcription in Saccharomyces cerevisiae. Molecular and Cellular Biology 12 : 2616–2623.
58. SorianiFM, MalavaziI, FerreiraMED, SavoldiM, KressMRV, et al. (2008) Functional characterization of the Aspergillus fumigatus CRZ1 homologue, CrzA. Molecular Microbiology 67 : 1274–1291.
59. BlosserSJ, MerrimanB, GrahlN, ChungD, CramerRA (2014) Two C4-sterol methyl oxidases (Erg25) catalyze ergosterol intermediate demethylation and impact environmental stress adaptation in Aspergillus fumigatus. Microbiology DOI: 10.1099/mic.0.080440-0
60. ZhouS, FushinobuS, KimSW, NakanishiY, MaruyamaJ, et al. (2011) Functional analysis and subcellular location of two flavohemoglobins from Aspergillus oryzae. Fungal Genetics and Biology 48 : 200–207.
61. LiH, BarkerBM, GrahlN, PuttikamonkulS, BellJD, et al. (2011) The small GTPase RacA mediates intracellular reactive oxygen species production, polarized growth, and virulence in the human fungal pathogen Aspergillus fumigatus. Eukaryotic Cell 10 : 174–186.
62. BornemanAR, GianoulisTA, ZhangZD, YuH, RozowskyJ, et al. (2007) Divergence of transcription factor binding sites across related yeast species. Science 317 : 815–819.
63. OdomDT, DowellRD, JacobsenES, GordonW, DanfordTW, et al. (2007) Tissue-specific transcriptional regulation has diverged significantly between human and mouse. Nature Genetics 39 : 730–732.
64. SchmidtD, WilsonMD, BallesterB, SchwaliePC, BrownGD, et al. (2010) Five-vertebrate ChIP-seq reveals the evolutionary dynamics of transcription factor binding. Science 328 : 1036–1040.
65. TuchBB, GalgoczyDJ, HerndayAD, LiH, JohnsonAD (2008) The evolution of combinatorial gene regulation in fungi. PLoS Biology 6: e38.
66. HughesTR, de BoerCG (2013) Mapping yeast transcriptional networks. Genetics 195 : 9–36.
67. ZitomerRS, LowryCV (1992) Regulation of gene expression by oxygen in Saccharomyces cerevisiae. Microbiological Reviews 56 : 1–11.
68. HonT, DoddA, DirmeierR, GormanN, SinclairPR, et al. (2003) A mechanism of oxygen sensing in yeast. Multiple oxygen-responsive steps in the heme biosynthetic pathway affect Hap1 activity. The Journal of Biological Chemistry 278 : 50771–50780.
69. HickmanMJ, WinstonF (2007) Heme levels switch the function of Hap1 of Saccharomyces cerevisiae between transcriptional activator and transcriptional repressor. Molecular and Cellular Biology 27 : 7414–7424.
70. ThorsnessM, SchaferW, D'AriL, RineJ (1989) Positive and negative transcriptional control by heme of genes encoding 3-hydroxy-3-methylglutaryl coenzyme A reductase in Saccharomyces cerevisiae. Molecular and Cellular Biology 9 : 5702–5712.
71. JinFJ, TakahashiT, MatsushimaK, HaraS, ShinoharaY, et al. (2011) SclR, a basic helix-loop-helix transcription factor, regulates hyphal morphology and promotes sclerotial formation in Aspergillus oryzae. Eukaryotic cell 10 : 945–955.
72. RosenbachA, DignardD, PierceJV, WhitewayM, KumamotoCA (2010) Adaptations of Candida albicans for growth in the mammalian intestinal tract. Eukaryotic Cell 9 : 1075–1086.
73. NobileCJ, FoxEP, NettJE, SorrellsTR, MitrovichQM, et al. (2012) A recently evolved transcriptional network controls biofilm development in Candida albicans. Cell 148 : 126–138.
74. NettJE, LepakAJ, MarchilloK, AndesDR (2009) Time course global gene expression analysis of an in vivo Candida biofilm. The Journal of Infectious Diseases 200 : 307–313.
75. LaneS, ZhouS, PanT, DaiQ, LiuH (2001) The basic helix-loop-helix transcription factor Cph2 regulates hyphal development in Candida albicans partly via TEC1. Molecular and Biology 21 : 6418–6428.
76. SellamA, van het HoogM, TebbjiF, BeaurepaireC, WhitewayM, et al. (2014) Modeling the transcriptional regulatory network that controls the early hypoxic response in Candida albicans. Eukaryot Cell 13 : 675–690.
77. DattaS, OsborneTF (2005) Activation domains from both monomers contribute to transcriptional stimulation by sterol regulatory element-binding protein dimers. The Journal of Biological Chemistry 280 : 3338–3345.
78. ZoumiA, DattaS, LiawLH, WuCJ, ManthripragadaG, et al. (2005) Spatial distribution and function of sterol regulatory element-binding protein 1a and 2 homo - and heterodimers by in vivo two-photon imaging and spectroscopy fluorescence resonance energy transfer. Molecular and Cellular Biology 25 : 2946–2956.
79. RobinsonKA, LopesJM (2000) SURVEY AND SUMMARY: Saccharomyces cerevisiae basic helix-loop-helix proteins regulate diverse biological processes. Nucleic Acids Research 28 : 1499–1505.
80. ShaoW, EspenshadePJ (2012) Expanding roles for SREBP in metabolism. Cell Metabolism 16 : 414–419.
81. ShimizuK, KellerNP (2001) Genetic involvement of a cAMP-dependent protein kinase in a g protein signaling pathway regulating morphological and chemical transitions in Aspergillus nidulans. Genetics 157 : 591–600.
82. YuJH, HamariZ, HanKH, SeoJA, Reyes-DominguezY, et al. (2004) Double-joint PCR: a PCR-based molecular tool for gene manipulations in filamentous fungi. Fungal genetics and biology 41 : 973–981.
83. QuailMA, KozarewaI, SmithF, ScallyA, StephensPJ, et al. (2008) A large genome center's improvements to the Illumina sequencing system. Nature Methods 5 : 1005–1010.
84. LohseM, BolgerAM, NagelA, FernieAR, LunnJE, et al. (2012) RobiNA: a user-friendly, integrated software solution for RNA-Seq-based transcriptomics. Nucleic Acids Research 40: W622–627.
85. LangmeadB, TrapnellC, PopM, SalzbergSL (2009) Ultrafast and memory-efficient alignment of short DNA sequences to the human genome. Genome Biology 10: R25.
86. BaileyTL, ElkanC (1994) Fitting a mixture model by expectation maximization to discover motifs in biopolymers. Proceedings/International Conference on Intelligent Systems for Molecular Biology; ISMB International Conference on Intelligent Systems for Molecular Biology 2 : 28–36.
87. CarlsonJM, ChakravartyA, DeZielCE, GrossRH (2007) SCOPE: a web server for practical de novo motif discovery. Nucleic Acids Research 35: W259–264.
88. PavesiG, MereghettiP, MauriG, PesoleG (2004) Weeder Web: discovery of transcription factor binding sites in a set of sequences from co-regulated genes. Nucleic Acids Research 32: W199–203.
89. RobertsA, TrapnellC, DonagheyJ, RinnJL, PachterL (2011) Improving RNA-Seq expression estimates by correcting for fragment bias. Genome Biology 12: R22.
90. KimD, PerteaG, TrapnellC, PimentelH, KelleyR, et al. (2013) TopHat2: accurate alignment of transcriptomes in the presence of insertions, deletions and gene fusions. Genome Biology 14: R36.
91. RDevelopmentCoreTeam (2011) R: A language and environment for statistical computing. Vienna: R Foundation for Statistical Computing.
92. BonkovskyHL, WoodSG, HowellSK, SinclairPR, LincolnB, et al. (1986) High-performance liquid chromatographic separation and quantitation of tetrapyrroles from biological materials. Anal Biochem 155 : 56–64.
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek Acidification Activates Motility and Egress by Enhancing Protein Secretion and Cytolytic ActivityČlánek Plasticity between MyoC- and MyoA-Glideosomes: An Example of Functional Compensation in InvasionČlánek Coronavirus Cell Entry Occurs through the Endo-/Lysosomal Pathway in a Proteolysis-Dependent MannerČlánek NK Cell Activation in Human Hantavirus Infection Explained by Virus-Induced IL-15/IL15Rα Expression
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2014 Číslo 11- Stillova choroba: vzácné a závažné systémové onemocnění
- Diagnostika virových hepatitid v kostce – zorientujte se (nejen) v sérologii
- Perorální antivirotika jako vysoce efektivní nástroj prevence hospitalizací kvůli COVID-19 − otázky a odpovědi pro praxi
- Jak souvisí postcovidový syndrom s poškozením mozku?
- Infekční komplikace virových respiračních infekcí – sekundární bakteriální a aspergilové pneumonie
-
Všechny články tohoto čísla
- Peculiarities of Prion Diseases
- Inhibitors of Peptidyl Proline Isomerases As Antivirals in Hepatitis C and Other Viruses
- War and Infectious Diseases: Challenges of the Syrian Civil War
- Microbial Contamination in Next Generation Sequencing: Implications for Sequence-Based Analysis of Clinical Samples
- Acidification Activates Motility and Egress by Enhancing Protein Secretion and Cytolytic Activity
- Co-dependence of HTLV-1 p12 and p8 Functions in Virus Persistence
- Shed GP of Ebola Virus Triggers Immune Activation and Increased Vascular Permeability
- Plasticity between MyoC- and MyoA-Glideosomes: An Example of Functional Compensation in Invasion
- The Type III Translocon Is Required for Biofilm Formation at the Epithelial Barrier
- Retromer Regulates HIV-1 Envelope Glycoprotein Trafficking and Incorporation into Virions
- IFI16 Restricts HSV-1 Replication by Accumulating on the HSV-1 Genome, Repressing HSV-1 Gene Expression, and Directly or Indirectly Modulating Histone Modifications
- Coronavirus Cell Entry Occurs through the Endo-/Lysosomal Pathway in a Proteolysis-Dependent Manner
- Silencing by H-NS Potentiated the Evolution of
- Crystal Structure of Cytomegalovirus IE1 Protein Reveals Targeting of TRIM Family Member PML via Coiled-Coil Interactions
- GAPDH-A Recruits a Plant Virus Movement Protein to Cortical Virus Replication Complexes to Facilitate Viral Cell-to-Cell Movement
- Genomic Insights into the Fungal Pathogens of the Genus : Obligate Biotrophs of Humans and Other Mammals
- Unravelling Human Trypanotolerance: IL8 is Associated with Infection Control whereas IL10 and TNFα Are Associated with Subsequent Disease Development
- The Skin Microbiome: A Focus on Pathogens and Their Association with Skin Disease
- Human Cytomegalovirus Vaccine Based on the Envelope gH/gL Pentamer Complex
- IL-37 Inhibits Inflammasome Activation and Disease Severity in Murine Aspergillosis
- Host-Specific Parvovirus Evolution in Nature Is Recapitulated by Adaptation to Different Carnivore Species
- Activation of HIV Transcription with Short-Course Vorinostat in HIV-Infected Patients on Suppressive Antiretroviral Therapy
- PUL21a-Cyclin A2 Interaction is Required to Protect Human Cytomegalovirus-Infected Cells from the Deleterious Consequences of Mitotic Entry
- Programmed Ribosomal Frameshift Alters Expression of West Nile Virus Genes and Facilitates Virus Replication in Birds and Mosquitoes
- Aminoterminal Amphipathic α-Helix AH1 of Hepatitis C Virus Nonstructural Protein 4B Possesses a Dual Role in RNA Replication and Virus Production
- NK Cell Activation in Human Hantavirus Infection Explained by Virus-Induced IL-15/IL15Rα Expression
- Structure and Specificity of the Bacterial Cysteine Methyltransferase Effector NleE Suggests a Novel Substrate in Human DNA Repair Pathway
- Genetics, Receptor Binding Property, and Transmissibility in Mammals of Naturally Isolated H9N2 Avian Influenza Viruses
- A Gatekeeper Chaperone Complex Directs Translocator Secretion during Type Three Secretion
- A Conserved Peptide Pattern from a Widespread Microbial Virulence Factor Triggers Pattern-Induced Immunity in
- Succinate Dehydrogenase is the Regulator of Respiration in
- The Plasmodesmal Protein PDLP1 Localises to Haustoria-Associated Membranes during Downy Mildew Infection and Regulates Callose Deposition
- Dysregulated B Cell Expression of RANKL and OPG Correlates with Loss of Bone Mineral Density in HIV Infection
- Restriction of Genetic Diversity during Infection of the Vector Midgut
- The Epithelial αvβ3-Integrin Boosts the MYD88-Dependent TLR2 Signaling in Response to Viral and Bacterial Components
- The Relationship between Host Lifespan and Pathogen Reservoir Potential: An Analysis in the System
- Multiple Roles of the Cytoskeleton in Bacterial Autophagy
- The Evolution and Genetics of Virus Host Shifts
- ChIP-seq and In Vivo Transcriptome Analyses of the SREBP SrbA Reveals a New Regulator of the Fungal Hypoxia Response and Virulence
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Coronavirus Cell Entry Occurs through the Endo-/Lysosomal Pathway in a Proteolysis-Dependent Manner
- Peculiarities of Prion Diseases
- Host-Specific Parvovirus Evolution in Nature Is Recapitulated by Adaptation to Different Carnivore Species
- War and Infectious Diseases: Challenges of the Syrian Civil War
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



