-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Narrow Bottlenecks Affect Populations during Vertical Seed Transmission but not during Leaf Colonization
The effective size of populations (Ne) determines whether selection or genetic drift is the predominant force shaping their genetic structure and evolution. Populations having high Ne adapt faster, as selection acts more intensely, than populations having low Ne, where random effects of genetic drift dominate. Estimating Ne for various steps of plant virus life cycle has been the focus of several studies in the last decade, but no estimates are available for the vertical transmission of plant viruses, although virus seed transmission is economically significant in at least 18% of plant viruses in at least one plant species. Here we study the co-dynamics of two variants of Pea seedborne mosaic virus (PSbMV) colonizing leaves of pea plants (Pisum sativum L.) during the whole flowering period, and their subsequent transmission to plant progeny through seeds. Whereas classical estimators of Ne could be used for leaf infection at the systemic level, as virus variants were equally competitive, dedicated stochastic models were needed to estimate Ne during vertical transmission. Very little genetic drift was observed during the infection of apical leaves, with Ne values ranging from 59 to 216. In contrast, a very drastic genetic drift was observed during vertical transmission, with an average number of infectious virus particles contributing to the infection of a seedling from an infected mother plant close to one. A simple model of vertical transmission, assuming a cumulative action of virus infectious particles and a virus density threshold required for vertical transmission to occur fitted the experimental data very satisfactorily. This study reveals that vertically-transmitted viruses endure bottlenecks as narrow as those imposed by horizontal transmission. These bottlenecks are likely to slow down virus adaptation and could decrease virus fitness and virulence.
Published in the journal: . PLoS Pathog 10(1): e32767. doi:10.1371/journal.ppat.1003833
Category: Research Article
doi: https://doi.org/10.1371/journal.ppat.1003833Summary
The effective size of populations (Ne) determines whether selection or genetic drift is the predominant force shaping their genetic structure and evolution. Populations having high Ne adapt faster, as selection acts more intensely, than populations having low Ne, where random effects of genetic drift dominate. Estimating Ne for various steps of plant virus life cycle has been the focus of several studies in the last decade, but no estimates are available for the vertical transmission of plant viruses, although virus seed transmission is economically significant in at least 18% of plant viruses in at least one plant species. Here we study the co-dynamics of two variants of Pea seedborne mosaic virus (PSbMV) colonizing leaves of pea plants (Pisum sativum L.) during the whole flowering period, and their subsequent transmission to plant progeny through seeds. Whereas classical estimators of Ne could be used for leaf infection at the systemic level, as virus variants were equally competitive, dedicated stochastic models were needed to estimate Ne during vertical transmission. Very little genetic drift was observed during the infection of apical leaves, with Ne values ranging from 59 to 216. In contrast, a very drastic genetic drift was observed during vertical transmission, with an average number of infectious virus particles contributing to the infection of a seedling from an infected mother plant close to one. A simple model of vertical transmission, assuming a cumulative action of virus infectious particles and a virus density threshold required for vertical transmission to occur fitted the experimental data very satisfactorily. This study reveals that vertically-transmitted viruses endure bottlenecks as narrow as those imposed by horizontal transmission. These bottlenecks are likely to slow down virus adaptation and could decrease virus fitness and virulence.
Introduction
Evolution of virus populations depends on several forces including mutation, recombination, genetic drift, selection and migration, acting concomitantly but exerting pressures that vary widely in direction and intensity. It makes therefore difficult to predict viral emergences or the durability of control strategies. The relative intensity of these forces will determine whether evolution follows predominantly stochastic or deterministic patterns. The concept of effective size of populations, Ne, plays a core role since it determines the rate of random fluctuations of the frequency of virus variants caused by genetic drift across generations in a model population. Ne estimates the number of individuals that pass on their genes through generations. It is usually much smaller than the total size of populations: although the total size of virus populations in their host plants can be tremendous and reach 107 to 109 virus particles [1], [2], estimates of Ne are below 500 and most of them are actually close to one [3], [4]. Importantly, for populations affected by periodic size changes like bottlenecks or founder effects, Ne is given by the harmonic mean of population sizes over generations [5]. As a consequence, even short periods of small population size during the life cycle or history of populations can have disproportionately strong influences on Ne. Ne helps to predict the loss and distribution of neutral genetic variation [6], the fixation probabilities of beneficial or deleterious alleles [7], and the fitness and survival of small populations [8]. Therefore, knowledge of Ne is of major interest for modeling disease emergence and can be an important issue in agriculture as illustrated by the breakdown of plant resistance genes by adapted virus variants [9], [10].
It has been shown recently that plant virus populations undergo transient and recurrent bottlenecks at different steps of their life cycle, like during horizontal transmission, i.e. plant inoculation by vectors [9], [11], by contact with an infected plant [12] or by artificial inoculation [13], or during the colonization of plant cells [14], [15], [16] and tissues [4], [13], [15], [17]. By contrast, no estimates of bottleneck sizes during vertical transmission of plant viruses, i.e. infection of plant progenies by the parental plant(s), are available yet.
There are three major ways of vertical transmission of plant viruses via the contamination of true seeds. In only a few examples, particularly stable viruses such as tobamoviruses can be retained in the seed coat and then transmitted to the seedling after germination [18]. In that case, there is no contamination of the embryo and the process of seedling infection resembles horizontal transmission through contact with an infected plant. The two other ways of contamination correspond to invasion of the embryo by the virus, either from infected maternal tissues or, more rarely, via infected pollen. Although seed embryos are usually protected against invasion by viruses that affect the mother plant, many viruses have the capacity to circumvent this barrier. Even low rates of seed transmission can be epidemiologically important because secondary spread of viruses can begin as soon as the germination stage [19] and virus seed transmission can be economically significant for at least 18% of plant viruses [20].
The goal of this work was to compare the size of bottlenecks affecting populations of Pea seedborne mosaic virus (PSbMV) (genus Potyvirus, family Potyviridae) in pea plants during vertical seed transmission and during the colonization of leaves.
Materials and Methods
Plant and virus material
The PSbMV isolate DPD1 and the variant DPD1-R only differ at codon position 116 in the VPg (Virus protein genome-linked)-coding region were used. Codon 116 is GTG (valine) and CGA (arginine) in DPD1 and DPD1-R, respectively [21], and these three adjacent nucleotide differences allowed identification and quantification of the two PSbMV variants in mixed-infected plants (see below).
The pea (Pisum sativum L.) cultivar ‘Vedette’ that transmits PSbMV through seeds at high frequencies [22] was used for all experiments. No pollen transmission of PSbMV was observed in this genotype [23]. Plants were grown under greenhouse conditions from November 2011 to April 2012.
Quantification of PSbMV variants in inocula and pea leaves
DPD1 and DPD1-R isolates were multiplied separately in Vedette plants and mixed at two different ratios, corresponding to 1∶1 and 1∶4 weights of infected leaf material, to create inocula 1 and 2, respectively. For each inoculum, 25 Vedette plants were inoculated 28 days after sowing (7 to 8 expanded leaf stage) on the two upper expanded leaves (Fig. 1A). All plants were mechanically inoculated. The Vedette plants were then split into three sets corresponding to three different leaf and seed sampling designs, and randomized. For inoculum 2, one plant died before leaf sampling. For plants numbered 1 to 19, leaves were collected at two different dates (Fig. 1B). At 22 days post inoculation (dpi), corresponding to the anthesis of the first flower in the plant population, the three leaves immediately above the inoculated ones were collected separately (leaves L1 to L3, Fig. 1A) and at 61 dpi (end of flowering), the three leaves immediately above leaf L3 were collected separately (leaves L4 to L6, Fig. 1A). For plants 20 to 39, only leaf L5 was collected (at 61 dpi). Finally, no leaves at all were collected on plants 40 to 49.
Fig. 1. Virus sampling design for pea plants inoculated with PSbMV. 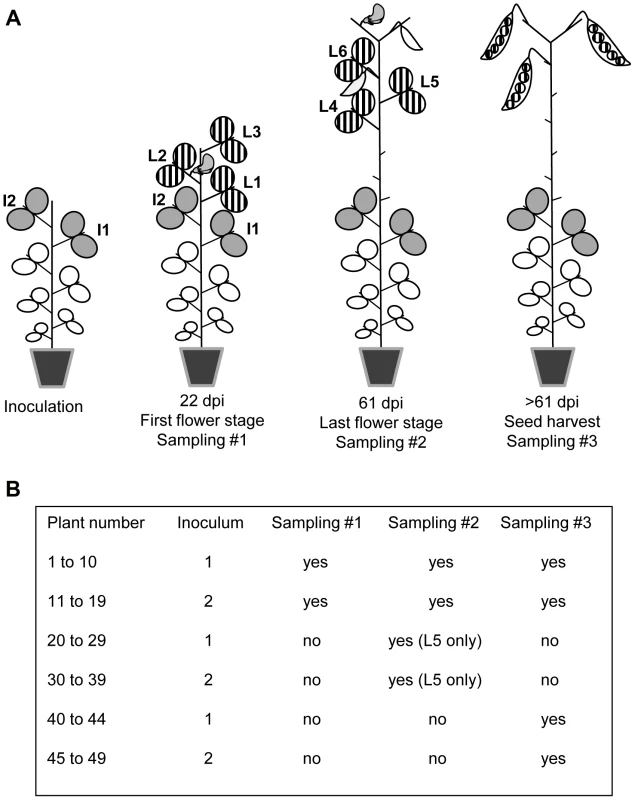
(A) Plants of the pea cultivar Vedette were mechanically inoculated with mixtures of two PSbMV variants 28 days after sowing on the two leaves I1 and I2. Twenty-two days post inoculation (dpi), corresponding to the anthesis of the first flower in the plant population, the three leaves L1 to L3 immediately above I2 were collected separately and analyzed. Sixty-one dpi, corresponding to the end of anthesis, the three leaves L4 to L6 immediately above L3 were collected separately and analyzed. Finally, all pods produced by the main stem of the plants were harvested at desiccation step, seeds were sown and seedlings were analyzed 22 days after sowing. (B) Different sets of plants were subjected to different sampling schemes. For plants numbered 20 to 49, samplings at 22 dpi and/or at 61 dpi were omitted. For inoculation, RNA extraction and enzyme-linked immunosorbent assay (ELISA), leaf tissue was homogenized in four volumes (wt/vol) of 0.03 M phosphate buffer (pH 7.0) supplemented with 2% (wt/vol) diethyldithiocarbamate. For RT-PCR, total RNA was extracted from a 150 µL aliquot using the Tri Reagent kit (Molecular Research Center Inc., Cincinnati, OH, USA). To amplify the VPg coding region that contained the polymorphic codon between DPD1 and DPD1-R, reverse transcription (RT) was performed on 2 µL of each RNA extract using Avian myeloblastosis virus reverse transcriptase (Promega Corp., Madison, WI, USA) followed by polymerase chain reaction (PCR) with Thermus aquaticus DNA polymerase (Promega Corp.). Primer DPD1-VPGR (5′-AAACTGACCAAATCCGATGCC complementary to nucleotides 6690 to 6710 of DPD1 genome, accession number D10930) was used for RT and primers DPD1-VPGR and DPD1-VPGF (5′-AAAACACTGCAGCTTAAGGG corresponding to nucleotides 5868 to 5887) were used for PCR. The PCR program started with 3 min at 95°C followed by 35 cycles (45 s at 95°C, 30 s at 55°C, 50 s at 72°C) and a final extension at 72°C for 10 min. Amplification products were sequenced directly with primer DPD1-VPGF by Genoscreen (Lille, France). We estimated the relative proportions of the two PSbMV variants in inocula and leaves from the height of peaks corresponding to the three polymorphic codon positions in the chromatograms. The reliability of this quantification method was evaluated with artificial mixtures of known quantities of the two PSbMV variants obtained after virus purification. As illustrated in Fig. S1, a linear regression allowed a very accurate prediction of the percentage of each variant in mixed-infected leaves (slope = 1.01, R2 = 0.99). This chromatogram-based quantification method was also compared to another method based on the cloning of RT-PCR products obtained with primers DPD1-VPGR and DPD1-VPGF into an Escherichia coli plasmid vector. For this, 5 pea leaves with contrasted frequencies of variant DPD1-R (from 24% to 69% based on the “chromatogram” method) were chosen and, for each of them, the RT-PCR products were cloned into the pGEM-T Easy vector (Promega Corp., Madison, WI, USA) and the number of clones corresponding to DPD1 and DPD1-R among a total of 40 clones per leaf was determined using the specific primers DPD1-VPgF-116V and DPD1-VPgF-116R described below. Again, the “chromatogram” and “cloning” methods provided highly similar frequency estimates (slope = 1.07, R2 = 0.96), hence further validating the “chromatogram” quantification method.
Determination of seed transmission rates of PSbMV variants
Pods produced by the main stem (Fig. 1) of plants 1 to 19 and 40 to 49 were harvested at desiccation time. Harvested seeds were then sown and all leaves from each seedling were collected 22 days later. Seedling extracts were tested for PSbMV infection by antigen coated plate-ELISA (ACP-ELISA) using an antiserum specific for the PSbMV coat protein. To detect the presence of either the DPD1 or the DPD1-R PSbMV variants, total RNA was extracted from seedlings of mother plants with a minimum of nine ELISA-positive seedlings. The generic DPD1-VPGR primer was used for RT and for PCR in combination with either the primer DPD1-VPgF-116V (5′-CTCGATAAACAATTGTTTGTG) or the primer DPD1-VPgF-116R (5′-CTCGATAAACAATTGTTTCGA) corresponding to nucleotides 6336–6356 of DPD1 andDPD1-R, respectively. The PCR programs started with 3 min at 95°C followed by 40 cycles (45 s at 95°C, 30 s at 63°C, 30 s at 72°C) and a final extension at 72°C for 10 min. Artificial mixtures of known proportions of RNAs of the two PSbMV variants obtained after virus purification [9] were used to evaluate the sensitivity of the RT-PCR method. In these artificial mixtures, each variant could be detected up to a 0.1% relative concentration.
Estimation of effective population size during leaf colonization
To estimate Ne during PSbMV colonization of upper uninoculated leaves (L1 to L6 in Fig. 1A), we used the “variance method” based on the differences in the variance of the viral genotype frequencies between the two sampling dates at 22 and 61 dpi, and the “FST method” based on the difference between these 2 dates of Wright's FST statistics [24] calculated on within - and between-plant viral genetic diversities [25]. These methods are based on the assumption that the PSbMV variants within the viral population under consideration are equally competitive.
According to the variance method, Ne = E(P)×(1−E(P))/[Var(P′)−Var(P)], where P and P′ are the random variables of the frequencies of the viral marker for each plant at the first and second sampling dates, respectively, E(P) is the expected value of P in the plant population and Var(P) its variance. In practice, E(P) and Var(P) were estimated by the sample mean and variance of the frequencies of the viral marker measured on a set of plants (Table 1). Because Var(P) was negligible compared to E(P) in our datasets, the Ne estimates provided by this equation were almost identical to those obtained with equation (14) of [26]: Ne = [E(P)×(1−E(P))−Var(P)]/[Var(P′)−Var(P)]. According to the FST method, Ne = (1−FST)/(FST′−FST), where FST and FST′ are values of the FST statistics of the viral populations at the first and second sampling dates, respectively (see [25] for details). For both methods, Ne confidence intervals were obtained by bootstrapping 10,000 times among plants.
Tab. 1. Ne estimates for the systemic colonization of pea leaves by PSbMV between 22 and 61 days post inoculation (dpi). 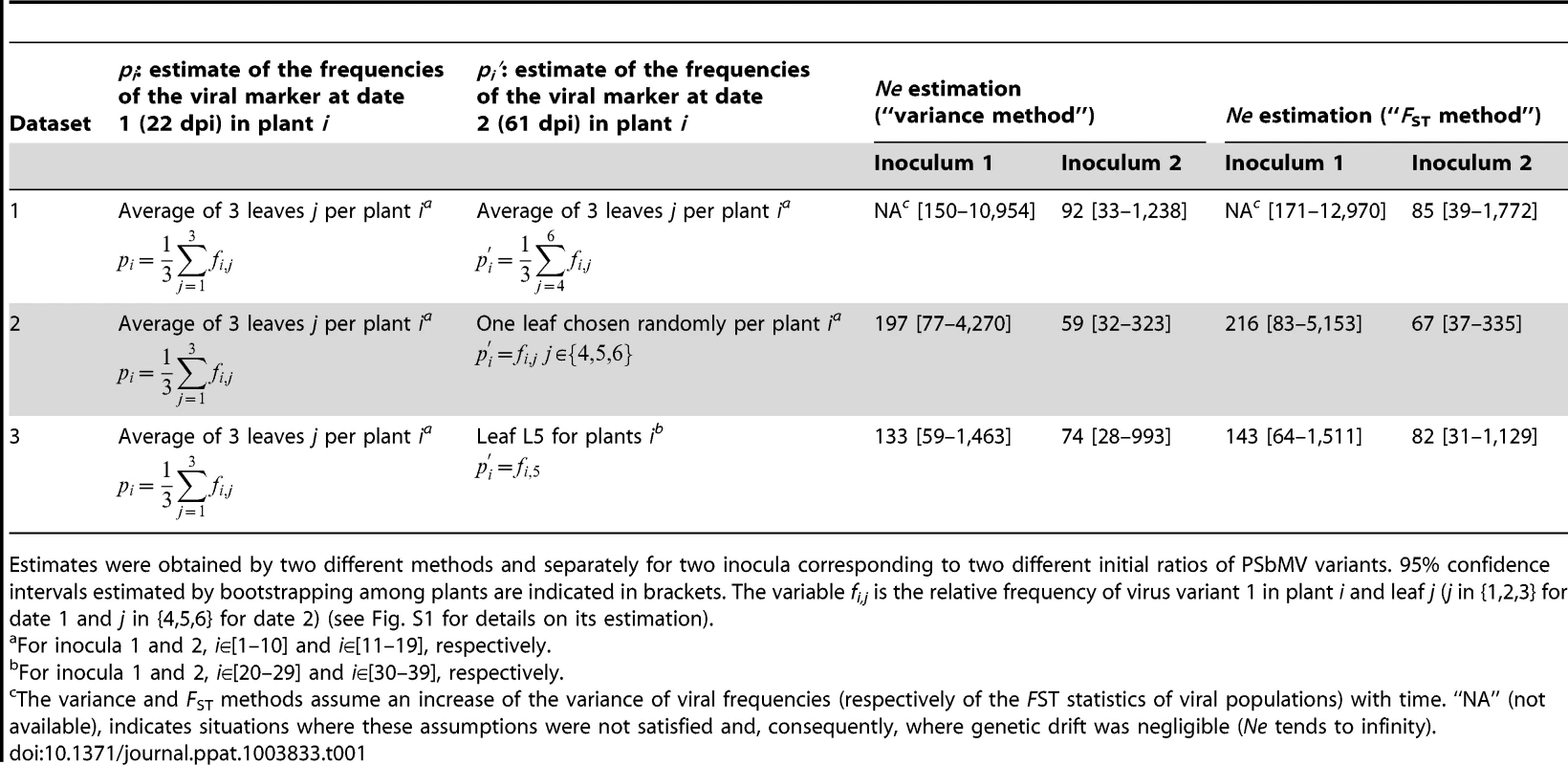
Estimates were obtained by two different methods and separately for two inocula corresponding to two different initial ratios of PSbMV variants. 95% confidence intervals estimated by bootstrapping among plants are indicated in brackets. The variable fi,j is the relative frequency of virus variant 1 in plant i and leaf j (j in {1,2,3} for date 1 and j in {4,5,6} for date 2) (see Fig. S1 for details on its estimation). With the nested sampling design used (several leaves being analyzed for each plant) and with the different plants sets available (plants 1 to 19, analyzed at 22 and 61 dpi and plants 20 to 39 analyzed at 61 dpi only, Fig. 1B), several datasets can be used to estimate Ne (Table 1). All leaves can be considered to estimate the variant frequencies at date 2 and Ne reflects the overall genetic drift process in the whole plant (dataset 1) or a single leaf per plant can be considered at date 2 (as in [25]) and, in that case, Ne can be viewed as the number of founding virus particles contributing to the colonization of an individual leaf (datasets 2 and 3). In addition, different sets of plants can be considered for each date (dataset 3) to test the influence of sampling leaves at date 1 on Ne estimates (by comparing dataset 2 and dataset 3).
Estimation of the size of population bottlenecks during PSbMV seed transmission
In order to estimate the size of bottlenecks undergone by PSbMV populations during seed transmission and to explore the mechanisms underlying seed transmission, we developed dedicated models. These models describe the two sequential processes leading to seedling infection: (1) virus entry into the seed (or more precisely into seed embryos, see the Discussion section) and (2) seedling infection from the contaminated seed. Concerning the first step, we assumed that the two virus variants act independently and, for a given variant, virus particles also act independently (i.e. there is no variant-variant nor virus-virus interactions). Concerning the second step, both types of interactions were considered (Table 2).
Tab. 2. Models for virus vertical transmission. 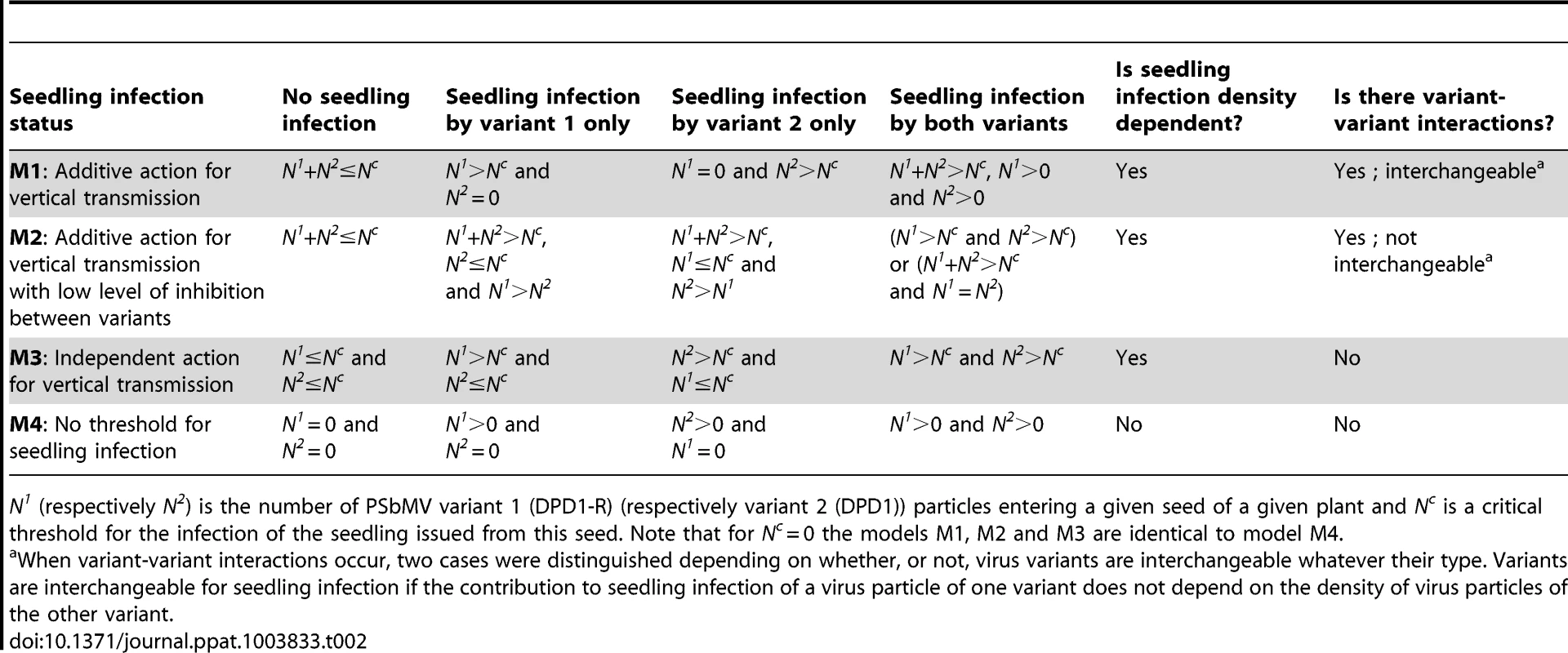
N1 (respectively N2) is the number of PSbMV variant 1 (DPD1-R) (respectively variant 2 (DPD1)) particles entering a given seed of a given plant and Nc is a critical threshold for the infection of the seedling issued from this seed. Note that for Nc = 0 the models M1, M2 and M3 are identical to model M4. For the first step (virus entry into the seed), we assumed that the proportions of PSbMV variants DPD1-R (variant 1) and DPD1 (variant 2) in coinfected plants can fluctuate in time during the period of seed infection (i.e. from 22 to 61 dpi) and within the plant because of spatial heterogeneity of distribution of virus variants. We considered that the relative frequencies f1 of variant 1 and f2 = (1−f1) of variant 2 in the vicinity of a given seed at infection time were realization of random variables that followed Beta distributions of parameters (α,β) and (β,α), respectively, α and β varying from plant to plant. We assumed that the numbers of viral particles of each variant entering a given seed, N1 and N2, were described by independent Poisson processes of parameters λ1×f1 and λ2×(1−f1), respectively, where λ1 and λ2 are the efficiencies of seed infection by variants 1 and 2. This hypothesis implies that all virus particles of a given variant have the same probability of entering a seed, and that they enter into the seeds independently of each other (i.e. there is no virus-virus interactions). Moreover, assuming that these Poisson processes are independent implies that there is no interaction between DPD1 and DPD1-R variants for entering a seed (however they can enter with different efficiencies).
For the second step of PSbMV seed transmission (seedling infection), we hypothesized that vertical transmission occurs if a minimal number Nc+1 of viral particles entered into a seed. Nc was chosen randomly and independently for each seed (and plant) from a Poisson distribution of parameter λc. Four alternative models were considered to describe the mechanism of seedling infection (Table 2, Fig. 2). Models M1, M2 and M3 assume virus-virus interactions, seedling infection being a virus density-dependent process. In models M1 and M2, variant-variant interactions occur, as seedlings become infected if the total number of particles of virus variants 1 and 2 entering into a seed (i.e. N1+N2) strictly exceeds Nc. In model M1, a variant is transmitted vertically if at least one particle of this variant has entered into the seed, meaning that the contribution to seedling infection of a virus particle of one variant does not depend on the density of the other variant: virus particles are interchangeable, whatever their type. In contrast, in model M2, a variant is transmitted vertically if its density is higher than Nc or higher than the density of the other variant (when N1 = N2 the seedling becomes infected by both variants). Here, the contribution of a virus particle of one variant to seedling infection depends on the density of the other variant: virus particles are not interchangeable and model M2 assumed some inhibition between variants when one variant outnumbers the other. In model M3, there is no variant-variant interaction: the virus variants initiate seedling infection independently. A variant is transmitted vertically if the number of particles of this variant entering into the seed strictly exceeds Nc. Finally, in model M4 there is no virus-virus, nor variant-variant interaction. Nc is indeed set to zero: a virus variant is transmitted vertically if at least one particle of this virus variant has entered the seed.
Fig. 2. The 4 models of PSbMV vertical transmission. 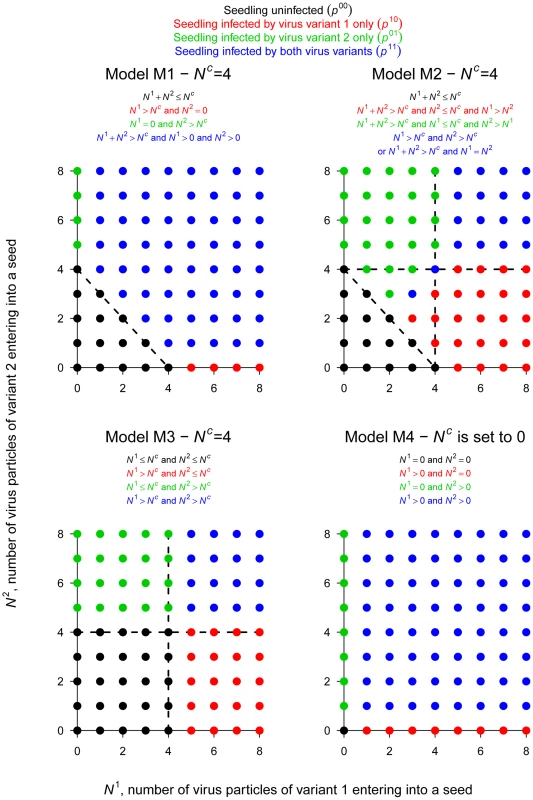
This figure illustrates the 4 sets of infection rules governing vertical transmission (i.e. seedling infection) and corresponding to the 4 models considered here (models M1, M2, M3 and M4). For each model, the rules leading to the 4 possible categories of seedling infection ((i) healthy, (ii) infected only by variant 2 (DPD1), (iii) infected only by variant 1 (DPD1-R) and (iv) infected by both PSbMV variants) are indicated and illustrated for values of N1 and N2 ranging from 0 to 8 and Nc = 4. Let remember that where N1 (resp. N2) is the number of particles of type 1 (resp. 2) entering into the seed and Nc is a threshold for efficient seedling infection. The R plants of the experimental design, indexed by r, were assumed to be independent. For a given plant, the variables describing the infection status of the seedlings, indexed by s (1≤s≤Sr), were supposed to be independent and identically distributed, but potentially with different distributions, for distinct plants. The variable with , describes the infection status of seedling s issued from mother plant r. This seedling is either not infected (), infected only by variant 1 (), infected only by variant 2 (), or infected by both variants (). defines a categorical (or 1-trial multinomial) variable. Let for model M1, M2 and M3 or for model M4 and be the probability density function (pdf) of Poisson distribution, and let be the beta pdf of the random variable standing for the proportion of variant 1 circulating into the phloem.
For seedling s of plant r, if the proportion of variant 1 present in the circulating viral population is known, the conditional probabilities of the different seedling infection statuses are denoted .
For model M1, we have:
For model M2, we have: The formula for given for model M1 is the same for model M2.For model M3, we have:
Finally, for model M4, we have: Since only the variables are observed in the experiments but neither nor , the likelihood of observing is obtained by integrating over the values of variable Φ. In our case, the plant specific parameters (αr, βr) were considered as known parameters and have been estimated for a given plant using the proportions of the two virus variants in leaves L1, L2 and L3 at 22 dpi and in leaves L4, L5 and L6 at 61 dpi (Fig. 1). Since the realized frequencies f were not observed, the probability for a seedling of a given plant r to be in the infectious status ij is obtained by integrating over all possible realizations of , that is .The likelihood of a given model is obtained as the product of R multinomial distributions as
where with since the Xrs are independent for the different plants.After checking that the four models were practically identifiable in our experimental conditions (Text S1), model parameter inferences were performed by minimizing the log of the likelihood function for each model Mj using the “bbmle” package with the “nlminb” optimization routines of the R software environment (http://cran.r-project.org/). 95% confidence intervals for model parameters were estimated using the function “profile” of the “bbmle” package.
Results
To analyze genetic drift and bottlenecks affecting virus populations during leaf infection and seed transmission, we inoculated plants of Pisum sativum cv. Vedette with two mixtures of the PSbMV variants DPD1 and DPD1-R (inocula 1 contained 38% of variant DPD1-R and inocula 2 contained 66% of variant DPD1-R) and we examined the composition of the viral populations at two time points in apical leaves of the inoculated plants, and in the plant progeny issued from the seeds collected on these mother plants. Changes in frequency of the PSbMV variants during the infection process and in the seedling progeny could be due either to genetic drift, selection or both. Since many models used to estimate Ne assume that changes of genotype frequencies in populations are due to genetic drift only, and not to selection, we tested whether the marker that allows distinguishing DPD1 from DPD1-R was neutral, i.e. if the two variants were equally competitive.
The two PSbMV variants are equally competitive for systemic movement in leaves
To estimate the relative competitiveness of PSbMV variants DPD1 and DPD1-R for infection of leaves at the systemic level, we compared their relative frequencies in apical leaves sampled at 22 and 61 dpi (Fig. 1). Analysis a posteriori based on the sequence chromatograms (Fig. S1) indicated that the DPD1-R variant represented 37.8% and 65.9% of inocula 1 and 2, respectively (the method used to estimate the relative frequency of the two PSbMV variants in inocula and mixed-infected leaves is described in details in Fig. S1). Sequence chromatograms of the VPg coding region showed also clearly that both PSbMV variants were present in each of the 134 leaves examined at 22 and 61 dpi (for plants 1 to 19) or at 61 dpi only (for plants 20 to 39) (Fig. 1B). Indeed, at the sequence region polymorphic between DPD1 and DPD1-R, the lowest of the six peaks (two different nucleotides for each of the three polymorphic codon positions) was 3.0 to 9.5 times (5.0 times on average) higher than the highest peak of background noise. The minimum and maximum percentages of DPD1-R among the 134 leaves were 21.3 and 70.6%, respectively. At 22 dpi, the mean proportion of DPD1-R observed in three sampled leaves was 32.3% for inoculum 1 and 55.7% for inoculum 2 (Table 3). In the same plants at 61 dpi, these average proportions were 31.0% and 51.7%, respectively, indicating almost no change in average frequency between the two dates and equal competitiveness of the two viral variants during leaf colonization. Confirming this, the difference of variant proportions in the plants between the two dates was 2.5% on average (with a 5.4% standard deviation). It was lower than 5% for 16 of the 19 analyzed plants. Twelve plants showed a decrease and seven an increase of DPD1-R frequency, which is not significantly different from random fluctuations (P = 0.25; Wilcoxon matched pairs signed ranks test). In addition, the sampling at 22 dpi had no influence on the average composition of the viral populations. Indeed, the average proportions of DPD1-R frequency in plants sampled only at 61 dpi (plants 20 to 39) were 31.4% and 58.2% for inocula 1 and 2, respectively, which is not significantly different from the DPD1-R frequencies at 22 or 61 dpi in plants that were sampled twice (plants 1 to 19) (P>0.20; Mann-Whitney tests) (Table 3).
Tab. 3. Frequency of two PSbMV variants in pea leaves and seedlings in three sets of plants corresponding to three sampling designs. 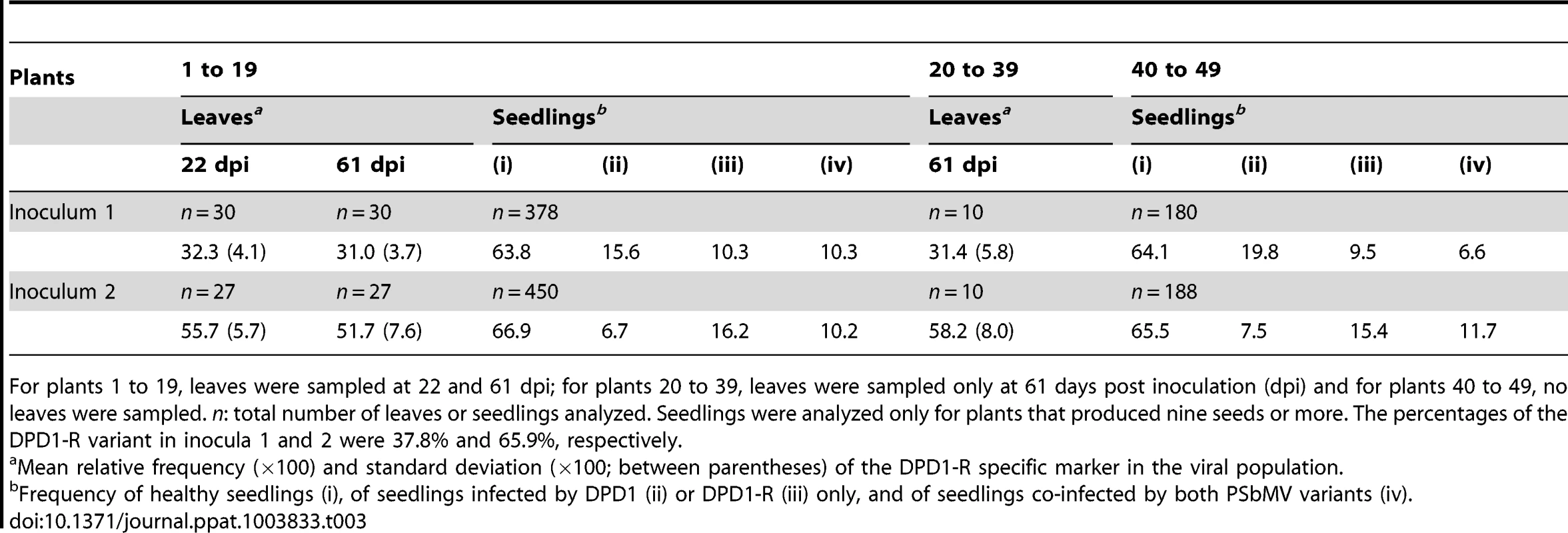
For plants 1 to 19, leaves were sampled at 22 and 61 dpi; for plants 20 to 39, leaves were sampled only at 61 days post inoculation (dpi) and for plants 40 to 49, no leaves were sampled. n: total number of leaves or seedlings analyzed. Seedlings were analyzed only for plants that produced nine seeds or more. The percentages of the DPD1-R variant in inocula 1 and 2 were 37.8% and 65.9%, respectively. Consequently, the two PSbMV variants DPD1 and DPD1-R can be considered as equally competitive with regard to the colonization of leaves at the systemic level between 22 and 61 dpi.
Evidence for slightly higher seed transmissibility for DPD1-R over DPD1
To estimate the relative competitiveness of PSbMV variants DPD1 and DPD1-R for seed transmission, we compared their relative frequencies in seedlings derived from inoculated mother plants and in leaves of these mother plants sampled at 22 and 61 dpi.
The number of harvested pea seeds in the different PSbMV-infected plants varied from seven to 95, with an average of 54. All harvested seeds germinated and the infection status of each seedling was analyzed by ELISA. From a total of 1022 seedlings derived from mother plants 1 to 19, the average seedling infection rate was 33.4% (33.1% and 33.7% for inocula 1 and 2 respectively). The average seedling infection rate was also similar to the infection rates observed in the seedlings of control plants inoculated by DPD1-R only (36% for a total of n = 206 seedlings) or DPD1 only (31%; n = 195), (P = 0.27; Khi2 tests). Accordingly, the fact that similar percentages of seed transmission were observed in single-infected or mixed-infected plants suggests independence between PSbMV variants for seed infection and justifies the Poissonian assumptions made for modeling virus entry into seeds. Finally, the seedling infection rates were similar to those observed for mixed-infected plants for which no leaves were sampled (plants 40 to 49) (34%; n = 584; P>0.30 for both inocula; Khi2 tests). All these values are in the range of seed transmission rates obtained independently with PSbMV DPD1 and Vedette pea plants (25 to 53% seed transmission [27]).
For mother plants having nine or more infected seedlings, the proportion of seedlings corresponding to categories (ii) seedling infected only by variant DPD1, (iii) seedling infected only by variant DPD1-R and (iv) seedling infected by both PSbMV variants was determined (Table 3). Accordingly, among plants 1 to 19, the seedlings obtained from 12 plants (six plants initially inoculated with 38% of variant DPD1-R (inoculum 1) and six plants initially inoculated with 66% of variant DPD1-R (inoculum 2)) were analyzed. In contrast to plant leaves, the two PSbMV variants were detected simultaneously in a minority of infected seedlings, i.e. 28.5% of seedlings for inoculum 1 and 30.9% of seedlings for inoculum 2. The DPD1-R variant was observed in 39.8% and 70.9% of seedlings infected by a single virus variant (considering only seedling categories (ii) and (iii)) for inocula 1 and 2, respectively. Compared to the PSbMV variant frequencies in the leaves of the mother plants, DPD1-R seemed to be somewhat better seed-transmitted than DPD1, a difference which is significant only for inoculum 2 (P = 0.01; Khi2 test). Examining seed transmission results for each mother plant individually did not reveal any significant difference between the distributions of variants among the seedlings and the average proportion of PSbMV variants in leaves.
The percentages of seedlings infected simultaneously by the two PSbMV variants were similar for mother plants which leaves were sampled twice (numbers 1 to 19) and for mother plants for which no leaves were sampled (plants 40 to 49) (P>0.2; Khi2 tests) (Table 3). The distributions of the two PSbMV variants among the seedlings were also similar for these two sets of mother plants (P>0.2; Khi2 tests) (Table 3). Consequently, the sampling procedure did not affect the seed transmission of the PSbMV variants and will not bias the estimates of bottleneck sizes during PSbMV seed transmission.
Small genetic drift effects during the systemic colonization of pea leaves by PSbMV
Since the two inoculated PSbMV variants DPD1 and DPD1-R were equally competitive during the colonization of plant leaves from 22 to 61 dpi, we used the methods described in [25] to estimate Ne. These methods are based on the differences in variance of the viral variant frequencies (“variance method”) or on the difference of Wright's FST statistics (“FST method”) between two sampling dates. For these methods, an underlying assumption is that the variance of the viral variant frequencies (or the FST statistics) increases with time. Indeed, considering that all variants are equally fit in the population, variant frequency fluctuations are due only to genetic drift, which affects both the amount and distribution of neutral genetic diversity over time (i.e. across generations) and space (i.e. between subpopulations at a given time).
Whatever the datasets used, we observed very small differences of variance of virus variant frequencies or FST statistics for the PSbMV populations at 22 and 61 dpi, suggesting very limited effect, if any, of genetic drift on viral populations during the systemic invasion of apical leaves (Table 1). Accordingly, Ne estimates ranged from 59 to 216, with a mean of all Ne estimates of 111 and 119 for the variance and FST methods, respectively, and of 172 and 77 for inocula 1 and 2, respectively. In some cases, no Ne estimates could be obtained because the variance of viral frequencies and FST statistics decreased between 22 and 61 dpi (no drift was observed). Overall, little difference was observed between the “variance” and “FST” methods and between the different datasets used to estimate viral frequencies at the two dates of observations (Table 1). Notably, leaf sampling at date 1 did not affect significantly the results: Ne estimates were comprised between 74 and 143 for dataset 3 (independent sets of plants were sampled at each date) and between 59 and 216 for dataset 2 (the same set of plants was sampled at both dates) (Table 1). Dataset 3 provided the most homogeneous Ne estimates and smallest confidence intervals.
Bootstrapping among plants allowed obtaining confidence intervals for Ne estimates. The 95% confidence intervals were large because of the small number of plants and because the small differences in virus frequency variances or population FST between dates 1 and 2 had large impacts on Ne estimates (Table 1). All these results demonstrated the lack of narrow population bottlenecks during the leaf colonization at the systemic level, and provided Ne estimates similar to those obtained for CaMV [25].
Narrow bottlenecks affect PSbMV populations during vertical seed transmission
The “variance” and “FST” methods provide unbiased estimates of Ne only if the variants analyzed are equally competitive. This is not the case for our vertical transmission dataset, as variant DPD1-R was somewhat better transmitted to seedlings than DPD1. Thus, we developed stochastic models to estimate the size of bottlenecks undergone by PSbMV populations during seed transmission that (i) take into account the difference in seed transmissibility between variants and (ii) that allow to disentangle different seedling infection processes (see the Materials, methods and models section). These models showed that the mean number of PSbMV particles contributing to the infection of an individual seedling was close to one. We first checked whether our experimental design (number and nature of the data) was sufficiently informative to estimate accurately the model parameters using practical identifiability tests (Text S1). Numerical simulations indicated clearly that all four models had a very good practical identifiability. Indeed, whatever the parameters considered, the coefficient of correlation between their true and estimated values were ≥0.94 (Table 4). Moreover, the four models of virus seed transmission could be very efficiently discriminated using Akaike Information Criterion (AIC) [28]. When the data were simulated under model 1, the AIC selected model 1 (respectively models 2, 3 and 4) in 92% (respectively 2%, 6% and 0%) of simulations. Similarly, when the data were generated under models 2, 3 or 4, the AIC identified the correct model in 94%, 100% and 88% of the simulations.
Tab. 4. Practical identifiability of virus seed transmission models. 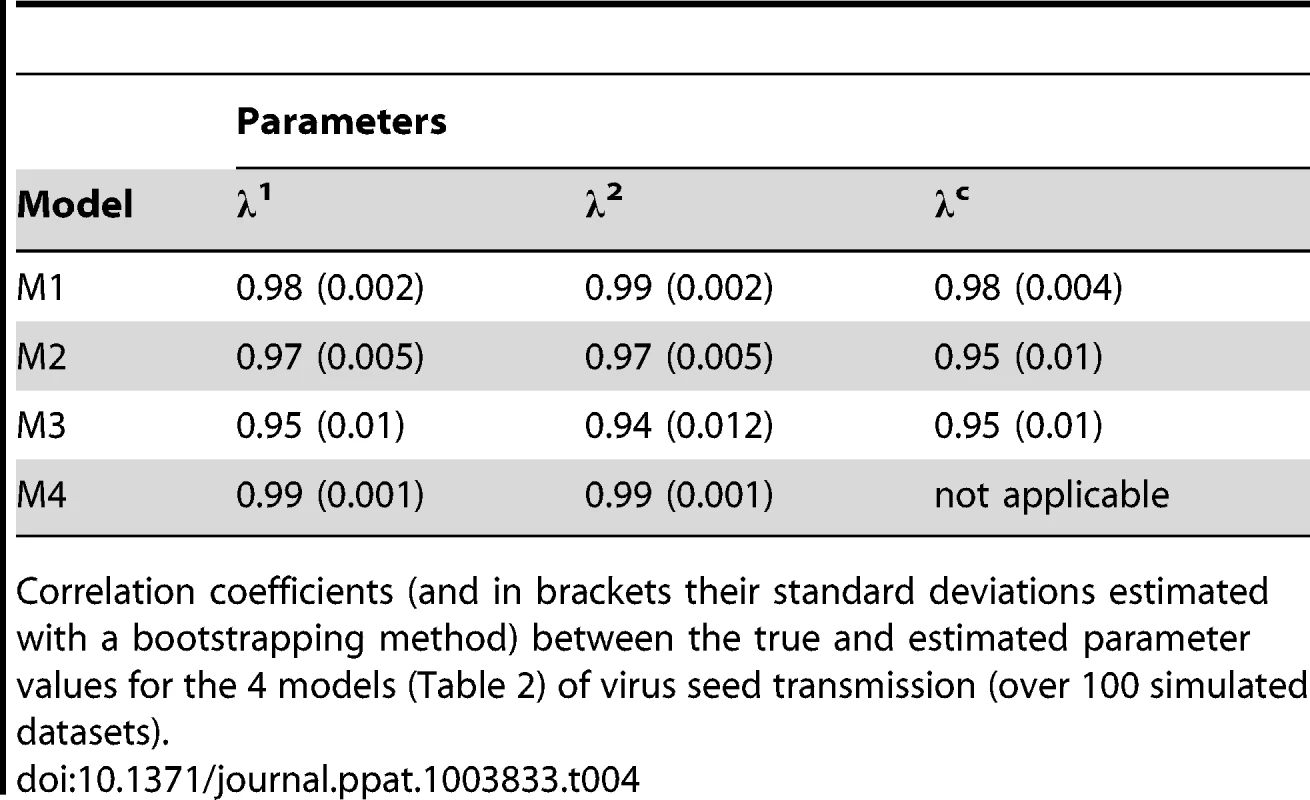
Correlation coefficients (and in brackets their standard deviations estimated with a bootstrapping method) between the true and estimated parameter values for the 4 models (Table 2) of virus seed transmission (over 100 simulated datasets). The model selection procedure applied to the experimental data set (Table S1) indicates that the AIC values of models M1 to M4 were 195, 196, 203 and 207, respectively. The corresponding Akaike weights, which provide the relative support for each model, were 0.59, 0.40, 0.01 and nearly zero (10−17). Thus, although model M1 is supported best by the data, model M2 has also a substantial support [29]. Assuming that λ1 = λ2, the AIC of the models increased to 207, 210, 219 and 279 for M1, M2, M3 and M4, respectively, indicating that the mean number of viruses contributing to the infection of a seedling was significantly different for virus variants 1 and 2. Under model M1, parameter inference indicated that the mean number of DPD1-R variant infectious particles contributing to the infection of a pea seedling was 1.08 (with a 95% confidence interval, CI95%, ranging from 0.9 to 1.29) and 0.74 for virus variant DPD1 (with a CI95% ranging from 0.61 to 0.88), while the mean number of virus particles required to infect a pea seedling was 0.84 (CI95% = [0.63, 1.05]). Parameter inferences under model M2 (which is almost as likely as model M1 with an Akaike weight of 0.4) were close to those obtained with M1, although always slightly higher: 1.52 for λ1 with a CI95% ranging from 1.08 to 2.74, 1.06 for λ2 with a CI95% ranging from 0.75 to 1.95 and 1.36 for λc with a CI95% ranging from 0.85 to 2.63.
Importantly, models M1 and M2 fitted very satisfactorily the experimental data. First, the observed and predicted mean numbers of seedlings corresponding to the four categories of seedling infection (i.e. (i) healthy, (ii) infected only by variant 2 (DPD1), (iii) infected only by variant 1 (DPD1-R) and (iv) infected by both PSbMV variants) were highly correlated (R2 = 0.88) for both models. Second, between-plant variability was very well represented, as an 80% (resp. 90%) confidence interval predicted by model M1 contained 78% (resp. 83%) of the observed data. For model M2, an 80% (resp. 90%) predicted confidence interval contained 80% (resp. 89%) of the observed data.
Discussion
We used the PSbMV-pea pathosystem to estimate the size of bottlenecks affecting a plant virus population during vertical transmission through seed embryo. We observed a very drastic genetic drift during vertical transmission, with an average number of infectious virus particles contributing to the infection of a seedling from an infected mother plant close to one. On the opposite, almost no genetic drift was observed during the infection of apical leaves of the mother plants during the same time-frame corresponding to the flowering period.
Estimation of Ne during the infection cycle of plant virus populations is quite complicated because of (i) the lack of estimates of generation times for viruses [30], which is due to the difficulties inherent to the definition of a viral generation (different lengths of time may be required for the production of the different components of progeny virus particles, like structural proteins and genome components), to the overlap between replication of virus entities within populations, and to the complex kinetics of virus replication [31] and (ii) the succession of different steps in the virus infection cycle that potentially follow different growth dynamics (intracellular accumulation, cell-to-cell movement, systemic translocation and plant-to-plant transmission). In spite of these limitations, several estimates of Ne or of the bottleneck size corresponding to particular steps of the virus life cycle have been obtained.
Concerning the colonization of plant leaves by viruses, estimates obtained for Ne are quite contrasted [3], [4]. The low genetic drift (large Ne) observed during the systemic colonization of pea plants by PSbMV corroborates previous results obtained with Cauliflower mosaic virus (genus Caulimovirus) [3], [25] or Tobacco etch virus (genus Potyvirus) [4], where the composition of virus populations were compared between inoculated and apical leaves [4] or between apical leaves sampled at two different dates [25]. In contrast, small Ne values were obtained by comparing the virus populations between the inoculum and apical leaves [13], [17]. These observations were reconciled by showing that most genetic drift occurs at the inoculation step whereas little genetic drift is subsequently observed during the systemic colonization of plants [4]. However, genetic drift during the systemic colonization of plants by viruses is not necessarily low. For example, 13 years after inoculation, each leaf of a peach tree was colonized by a single viral variant of Plum pox virus (PPV, genus Potyvirus) whereas a total of 33 viral variants were observed in the whole set of leaves analyzed, indicating that narrow bottlenecks acted on PPV populations during the infection of individual leaves [32]. Clearly, additional studies are needed to unravel the plant, virus and environmental factors which determine the patterns and intensity of genetic drift during plant colonization by viruses. Recently, the number of virus colonizing leaves was shown to increase with the concentration of viruses circulating within the plant sap [33]. This suggests that the low level of genetic drift observed during the systemic colonization of pea plants by PSbMV during the flowering period could result from high concentrations of virus circulating into the plant vasculature.
On the opposite, during the same time-frame, we showed that a single infectious PSbMV particle contributed on average to the infection of an individual seedling derived from an inoculated mother plant. To our knowledge, this is the first estimate of the bottleneck size imposed by vertical transmission to a virus population. Strong bottlenecks were also observed during vertical mother-to-child transmission of Human immunodeficiency virus-1 (HIV-1) [34], [35], [36]. For the majority of in utero or intrapartum transmission cases examined in these three studies (65%; 22/49) the infants harbored a single viral variant, which suggested the occurrence of narrow population bottlenecks at transmission. Note, that this percentage is very close to our own estimates for PSbMV (we observed from 66% to 82% single-infected pea seedlings among the infected ones, depending on inocula and plant sets; Table 3). A recent study conducted on seed transmission of ZYMV (Zucchini yellow mosaic virus, genus Potyvirus) in Cucurbita pepo showed that 16 of 24 ZYMV variants present in the mother plant were also present in vertically-transmitted virus populations, either of the first or second plant generation [37]. These figures suggest that bottleneck sizes during vertical transmission could be larger in that case. However, in none of these studies was the transmissibility of the different virus variants or their abundance in the mother's plasma (or in the mother plant) taken into account, which hampers the derivation of unbiased estimates for the bottleneck size.
Vertical transmission of PSbMV occurs through the infection of the pea seed embryos [38]. Usually, viruses are excluded from plant reproductive tissues. Because pathogens must cross several barriers intended to protect the developing embryo, the occurrence of narrow population bottlenecks during pathogen vertical transmission could be a quite general rule. The capacity of viruses to invade plant embryos and withstand seed maturation and desiccation depends both on virus and host genotypes, as demonstrated for PSbMV [23], [27], [39]. Seed transmission of PSbMV in pea occurs exclusively by direct invasion of immature embryos from virus-infected maternal tissues. It occurs only during a precise temporal window and from virus accumulated at a precise location in the developing seed. Such conditions are therefore favorable to the occurrence of strong virus population bottlenecks. Early infection of the mother plant is necessary for PSbMV vertical transmission to occur [23]. PSbMV invasion of pea embryos occurs from virus infection spreading from the maternal cells in the micropylar region of the embryo to the endosperm cytoplasm, then to the embryonic suspensor and finally to the embryo. Since the embryonic suspensor undergoes a programmed cell death, it acts for the virus as a “transient conduit” for embryo invasion [39]. The ability of the virus to invade the micropylar region before the suspensor programmed cell death therefore explains why early PSbMV infection of the mother plant is required for seed transmission, and could also explain why some pea cultivars are resistant to PSbMV seed transmission and why some PSbMV isolates are not seed transmitted in pea. In addition, no PSbMV replication could be detected in the endosperm cytoplasm [38], suggesting that only a small amount of virus is able to accumulate into the endosperm cytoplasm and further enter the suspensor. Based on these observations, Roberts et al [38] suggested that seed transmission of PSbMV was largely based on the chance of the virus to be in the right place at the right time. In these conditions, even a small degree of heterogeneity in the distribution of virus variants in the cells of infected plants, as observed for some potyviruses [16], [31], [40], could contribute to the genetic drift that occurs during PSbMV seed transmission. In agreement with these observations, the models that we used to estimate the bottleneck size during seed transmission considered that the virus variant frequency could fluctuate randomly at the time and place of virus entry into seed embryos. Consequently, the biological processes involved in PSbMV seed transmission are in accordance with, and provide plausible mechanisms for the narrow bottlenecks endured by virus populations during vertical seed transmission. To go further, it would be worth investigating whether the virus load in plants is linked to the intensity of genetic drift during the colonization of leaves, as evidenced by [33], and whether it affects also genetic drift during vertical transmission, at least at some critical time points during embryo infection.
From a methodological point of view, the mathematical framework introduced here allowed disentangling the relative importance of selection and genetic drift in shaping the genetic composition of viral populations during seed transmission. It could be of broad interest to estimate Ne when the effect of deterministic evolutionary forces, typically selection, cannot be excluded. Indeed, the temporal methods classically used to estimate Ne assume that the observed changes in allele frequency are due to genetic drift only and thus require the use of neutral genetic markers for the population of interest [6]. Such markers could be difficult to identify or to generate, especially for viruses, which typically possess highly constrained genomes and are impacted by strongly negative average mutational effects on fitness [41]. From a biological point of view, model selection analysis indicated that seedling infection was a virus density-dependent process, where particles of the two virus variants sum up their action to exceed an infection threshold, rather than a process where each variant acts independently (models M1 and M2 were preferred to model M3). These results echo the study of Lafforgue et al. [42], who showed that the delay of systemic infection of a plant was determined by the cumulative effect of independently-acting foci of primary infection. Results also showed that one or a small number of viral particle(s) is (are) enough for virus seed transmission (as λc was low), indicating that each virus particle has a quite high probability of causing efficient seed transmission. However, rejection of model M4 indicates that one viral particle is not always sufficient to initiate efficient seedling infection. Model M1 being only slightly preferable to model M2, it remained unclear whether virus particles belonging to the two variants are interchangeable or not in the cumulative infection process, interchangeability meaning that the contribution to seedling infection of a virus particle of one variant does not depend on the density of virus particles of the other variant. Consequently, more data should be gathered to clearly distinguish whether frequency-dependent selection of PSbMV variants occurred (as in model M2) or not (as in model M1) during seed transmission.
The small Ne values observed for PSbMV vertical transmission are expected to impact more deeply virus evolution than bottlenecks of the same size that would be experienced during horizontal transmission [9], [11], at least for large host populations. This is suggested by theoretical work on the evolution of parasites virulence (defined as the harm that they inflict to their host) according to their mode of transmission. The classical mechanism to explain why vertically-transmitted parasites evolve reduced virulence is through an indirect selection to improve host survival and/or reproduction [43]. Our study suggests that such reduced virulence could also be the consequence of narrow bottlenecks during vertical transmission. Indeed, using a model that assumed a tight association between parasites fitness and virulence, Bergstrom et al. [44] suggested that a direct effect of narrow bottlenecks is to select much lower levels of virulence in vertically-transmitted than in horizontally-transmitted pathogens. This was mainly due to the decrease of intra-host competition between virus variants in case of vertical transmission. Said another way, the strength of selection is reduced in case of vertical transmission as virus particles are separated into many distinct evolutionary host lineages. In their study, this difference between vertical and horizontal transmission was particularly strong when only one or two virus particles initiate the infection of a new host. In agreement with these theoretical results, repeated vertical transmission events were shown to affect drastically the evolution of PSbMV populations. As soon as the second generation of pea plants contaminated by PSbMV through seed transmission, PSbMV populations derived from four different isolates were shown to differ largely from the initial inocula: in contrast to the initially inoculated plants (generation 0), or plants of the first generation issued from contaminated seeds, the infected plants of the second generation did not express any symptom and PSbMV was not detectable in their vegetative parts [45]. Such a rapid evolution could be, at least in part, a consequence of the severe bottlenecks experienced by PSbMV populations during vertical transmission. Similar declines in virulence [46] or symptom induction [37], [47], [48] have been observed for other seed-transmitted plant viruses.
Exploring to which extent such decrease in virulence or symptomatology (two life history traits that are not necessarily correlated in plant viruses) can be explained by bottleneck sizes is an important issue in parasite evolution. From an applied perspective, many vertically-transmitted plant viruses are also transmitted horizontally by vectors. For example, PSbMV is transmitted by a large number of aphid species. In the field, ecological (e.g. host density, aphid population dynamics) and agronomic factors (e.g use of virus-free seeds) determine which mode of transmission is prevailing. Undoubtedly, a deeper understanding of the balance between the relative importance of these transmission modes during the course of epidemics, coupled with a deeper knowledge of the bottleneck sizes associated with these transmission modes is needed to better understand the evolution of important pathogen life history traits such as virulence, symptom severity and yield losses. Ultimately, this research could help designing more efficient strategies of plant protection relying on the knowledge and manipulation of evolutionary changes in parasites populations.
Supporting Information
Zdroje
1. HarrisonBD (1956) The infectivity of extracts made from leaves at intervals after inoculation with viruses. J Gen Microbiol 15 : 210–220.
2. MalpicaJ, FraileA, MorenoI, ObiesCI, DrakeJW, et al. (2002) The rate and character of spontaneous mutation in an RNA virus. Genetics 162 : 1505–1511.
3. GutiérrezS, MichalakisY, BlancS (2012) Virus population bottlenecks during within-host progression and host-to-host transmission. Curr Opin Virol 2 : 1–10.
4. ZwartMP, DaròsJA, ElenaSF (2011) One is enough: In vivo effective population size is dose-dependent for a plant RNA virus. PLoS Pathog 7: e1002122.
5. Wright S (1969) Evolution and the genetics of populations. II. The theory of gene frequencies. Chicago: University of Chicago Press. 520 p.
6. WangJ (2005) Estimation of effective population sizes from data on genetic markers. Phil Trans R Soc B 360 : 1395–1409.
7. RobertsonA (1961) Inbreeding in artificial selection programmes. Genet Res 2 : 189–194.
8. LynchM, ConeryJ, BurgerR (1995) Mutation accumulation and the extinction of small populations. Am Nat 146 : 489–518.
9. MouryB, FabreF, SenoussiR (2007) Estimation of the number of virus particles transmitted by an insect vector. Proc Natl Acad Sci U S A 104 : 17891–17896.
10. FabreF, MontarryJ, CovilleJ, SenoussiR, SimonV, et al. (2012) Modelling the evolutionary dynamics of viruses within their hosts: A case study using high-throughput sequencing. PLoS Pathog 8: e1002654.
11. BetancourtM, FereresA, FraileA, García-ArenalF (2008) Estimation of the effective number of founders that initiate an infection after aphid transmission of a multipartite plant virus. J Virol 82 : 12416–12421.
12. SacristánS, DíazM, FraileA, García-ArenalF (2011) Contact transmission of Tobacco mosaic virus: a quantitative analysis of parameters relevant for virus evolution. J Virol 85 : 4974–4981.
13. SacristánS, MalpicaJM, FraileA, García-ArenalF (2003) Estimation of population bottlenecks during systemic movement of Tobacco mosaic virus in tobacco plants. J Virol 77 : 9906–9911.
14. González-JaraP, FraileA, CantoT, García-ArenalF (2009) The multiplicity of infection of a plant virus varies during colonization of its eukaryotic host. J Virol 83 : 7487–7494.
15. MiyashitaS, KishinoH (2010) Estimation of the size of genetic bottlenecks in cell-to-cell movement of soil-borne Wheat mosaic virus and the possible role of the bottlenecks in speeding up selection of variations in trans-acting genes or elements. J Virol 84 : 1828–1837.
16. GutiérrezS, YvonM, ThébaudG, MonsionB, MichalakisY, et al. (2010) Dynamics of the multiplicity of cellular infection in a plant virus. PLoS Pathog 6: e1001113.
17. FrenchR, StengerDC (2005) Population structure within lineages of Wheat streak mosaic virus derived from a common founding event exhibits stochastic variation inconsistent with the deterministic quasi-species model. Virology 343 : 179–189.
18. BroadbentL (1965) The epidemiology of tomato mosaic XI: Seed-transmission of TMV. Ann Appl Biol 56 : 177–205.
19. CouttsBA, PrinceRT, JonesRAC (2009) Quantifying effects of seedborne inoculum on virus spread, yield losses, and seed infection in the Pea seedborne mosaic virus-field pea pathosystem. Phytopathology 99 : 1156–1167.
20. JohansenE, EdwardsMC, HamptonRO (1994) Seed transmission of viruses: Current perspectives. Annu Rev Phytopathol 32 : 363–386.
21. BorgstrømB, JohansenIE (2001) Mutations in Pea seedborne mosaic virus genome-linked protein VPg alter pathotype-specific virulence in Pisum sativum. Mol Plant-Microbe Interact 14 : 707–714.
22. WangD, WoodsRD, CockbainAJ, MauleAJ, BiddleAJ (1993) The susceptibility of pea cultivars to Pea seed-borne mosaic virus infection and seed transmission in the UK. Plant Pathol 42 : 42–47.
23. WangD, MauleAJ (1992) Early embryo invasion as a determinant in pea of the seed transmission of Pea seed-borne mosaic virus. J Gen Virol 73 : 1615–1620.
24. WrightS (1931) Evolution in Mendelian populations. Genetics 16 : 97–159.
25. MonsionB, FroissartR, MichalakisY, BlancS (2008) Large bottleneck size in Cauliflower mosaic virus populations during host plant colonization. PLoS Pathog 4: e1000174.
26. FelsensteinJ (1971) Inbreeding and variance effective numbers in populations with overlapping generations. Genetics 68 : 581–597.
27. JohansenIE, DoughertyWG, KellerKE, WangD, HamptonRO (1996) Multiple viral determinants affect seed transmission of Pea seedborne mosaic virus in Pisum sativum. J Gen Virol 77 : 3149–3154.
28. JohnsonJB, OmlandKS (2004) Model selection in ecology and evolution. Trends Ecol Evol 19 : 101–108.
29. BurnhamKP, AndersonDR (2004) Multimodel Inference: Understanding AIC and BIC Model Selection. Sociol Method Res 33 : 261–304.
30. KhelifaM, MasseD, BlancS, DruckerM (2010) Evaluation of the minimal replication time of Cauliflower mosaic virus in different hosts. Virology 396 : 238–245.
31. MartinezF, SardanyesJ, ElenaSF (2011) Dynamics of a Plant RNA Virus Intracellular Accumulation: Stamping Machine vs. Geometric Replication. Genetics 188 : 637–646.
32. JridiC, MartinJF, Marie-JeanneV, LabonneG, BlancS (2006) Distinct viral populations differentiate and evolve independently in a single perennial host plant. J Virol 80 : 2349–2357.
33. GutiérrezS, YvonM, PirollesE, GarzoE, FereresA, et al. (2012) Circulating Virus Load Determines the Size of Bottlenecks in Viral Populations Progressing within a Host. PLoS Pathog 8 (11) e1003009.
34. VerhofstedeC, DemecheleerE, De CabooterN, GaillardP, MwanyumbaF, et al. (2003) Diversity of the human immunodeficiency virus type 1 (HIV-1) env sequence after vertical transmission in mother-child pairs infected with HIV-1 subtype A. J Virol 77 : 3050–3057.
35. SamleeratT, BraibantM, JourdainG, MoreauA, Ngo-Giang-HuongN, et al. (2008) Characteristics of HIV type 1 (HIV-1) glycoprotein 120 env sequences in mother-infant pairs infected with HIV-1 subtype CRF01_AE. J Infect Dis 198 : 868–876.
36. RussellES, KwiekJJ, KeysJ, BartonK, MwapasaV, et al. (2011) The genetic bottleneck in vertical transmission of subtype C HIV-1 is not driven by selection of especially neutralization-resistant virus from the maternal viral population. J Virol 85 : 8253–8262.
37. SimmonsHE, DunhamJP, ZinnKE, MunkvoldGP, HolmesEC (2013) Zucchini yellow mosaic virus (ZYMV, Potyvirus): Vertical transmission, seed infection and cryptic infections. Virus Res 176 : 259–264.
38. RobertsIM, WangD, ThomasCL, MauleAJ (2003) Pea seed-borne mosaic virus seed transmission exploits novel symplastic pathways to infect the pea embryo and is, in part, dependent upon chance. Protoplasma 222 : 31–43.
39. WangD, MauleAJ (1994) A model for seed transmission of a plant virus: Genetic and structural analyses of pea embryo invasion by Pea seed-borne mosaic virus. Plant Cell 6 : 777–787.
40. DietrichC, MaissE (2003) Fluorescent labelling reveals spatial separation of potyvirus populations in mixed infected Nicotiana benthamiana plants. J Gen Virol 84 : 2871–2876.
41. CarrascoP, de la IglesiaF, ElenaSF (2007) Distribution of fitness and virulence effects caused by single-nucleotide substitutions in tobacco etch virus. J Virol 81 : 12979–12984.
42. LafforgueG, TromasN, ElenaSF, ZwartMP (2012) Dynamics of the Establishment of Systemic Potyvirus Infection: Independent yet Cumulative Action of Primary Infection Sites. J Virol 86 : 12912–12922.
43. EwaldPW (1987) Transmission Modes and Evolution of the Parasitism-Mutualism Continuum. Ann N Y Acad Sci 503 : 295–306.
44. BergstromCT, McElhanyP, RealLA (1999) Transmission bottlenecks as determinants of virulence in rapidly evolving pathogens. Proc Natl Acad Sci U S A 96 : 5095–5100.
45. LigatLS, RandlesJW (1993) An eclipse of Pea seed-borne mosaic virus in vegetative tissue of pea following repeated transmission through the seed. Ann Appl Biol 122 : 39–47.
46. StewartAD, LogsdonJM, KelleyS (2005) An empirical study of the evolution of virulence under both horizontal and vertical transmission. Ecology 59 : 730–739.
47. GallitelliD (2000) The ecology of Cucumber mosaic virus and sustainable agriculture. Virus Res 71 : 9–21.
48. AliA, KobayashiM (2010) Seed transmission of Cucumber mosaic virus in pepper. J Virol Methods 163 : 234–237.
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek Serotonin Signaling in : A Serotonin–Activated G Protein-Coupled Receptor Controls Parasite MovementČlánek Regulators of Cell Cycle Progression and Differentiation Identified Using a Kinome-Wide RNAi ScreenČlánek IFNγ/IL-10 Co-producing Cells Dominate the CD4 Response to Malaria in Highly Exposed ChildrenČlánek Functions of CPSF6 for HIV-1 as Revealed by HIV-1 Capsid Evolution in HLA-B27-Positive SubjectsČlánek Decreases in Colonic and Systemic Inflammation in Chronic HIV Infection after IL-7 Administration
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2014 Číslo 1- Stillova choroba: vzácné a závažné systémové onemocnění
- Diagnostika virových hepatitid v kostce – zorientujte se (nejen) v sérologii
- Perorální antivirotika jako vysoce efektivní nástroj prevence hospitalizací kvůli COVID-19 − otázky a odpovědi pro praxi
- Choroby jater v ordinaci praktického lékaře – význam jaterních testů
- Diagnostický algoritmus při podezření na syndrom periodické horečky
-
Všechny články tohoto čísla
- HIV-1 Accessory Proteins Adapt Cellular Adaptors to Facilitate Immune Evasion
- Ranaviruses: Not Just for Frogs
- Effectors and Effector Delivery in
- Plasmacytoid Dendritic Cell Dynamics Tune Interferon-Alfa Production in SIV-Infected Cynomolgus Macaques
- Lu/BCAM Adhesion Glycoprotein Is a Receptor for Cytotoxic Necrotizing Factor 1 (CNF1)
- A Substrate-Fusion Protein Is Trapped inside the Type III Secretion System Channel in
- Parvovirus-Induced Depletion of Cyclin B1 Prevents Mitotic Entry of Infected Cells
- Red Blood Cell Invasion by : Structural Basis for DBP Engagement of DARC
- NsrR, GadE, and GadX Interplay in Repressing Expression of the O157:H7 LEE Pathogenicity Island in Response to Nitric Oxide
- Loss of Circulating CD4 T Cells with B Cell Helper Function during Chronic HIV Infection
- TREM-1 Deficiency Can Attenuate Disease Severity without Affecting Pathogen Clearance
- Origin, Migration Routes and Worldwide Population Genetic Structure of the Wheat Yellow Rust Pathogen f.sp.
- Glutamate Utilization Couples Oxidative Stress Defense and the Tricarboxylic Acid Cycle in Phagosomal Escape
- Serotonin Signaling in : A Serotonin–Activated G Protein-Coupled Receptor Controls Parasite Movement
- Recovery of an Antiviral Antibody Response following Attrition Caused by Unrelated Infection
- Regulators of Cell Cycle Progression and Differentiation Identified Using a Kinome-Wide RNAi Screen
- Absence of Intestinal PPARγ Aggravates Acute Infectious Colitis in Mice through a Lipocalin-2–Dependent Pathway
- Induction of a Stringent Metabolic Response in Intracellular Stages of Leads to Increased Dependence on Mitochondrial Metabolism
- CTCF and Rad21 Act as Host Cell Restriction Factors for Kaposi's Sarcoma-Associated Herpesvirus (KSHV) Lytic Replication by Modulating Viral Gene Transcription
- Gammaherpesviral Gene Expression and Virion Composition Are Broadly Controlled by Accelerated mRNA Degradation
- The Arabidopsis Silencing Pathway Modulates PAMP- and Effector-Triggered Immunity through the Post-transcriptional Control of Disease Resistance Genes
- Inflammatory Stimuli Reprogram Macrophage Phagocytosis to Macropinocytosis for the Rapid Elimination of Pathogens
- Alphavirus Mutator Variants Present Host-Specific Defects and Attenuation in Mammalian and Insect Models
- Phosphopyruvate Carboxylase Identified as a Key Enzyme in Erythrocytic Carbon Metabolism
- IFNγ/IL-10 Co-producing Cells Dominate the CD4 Response to Malaria in Highly Exposed Children
- Electron Tomography of HIV-1 Infection in Gut-Associated Lymphoid Tissue
- Characterisation of a Multi-ligand Binding Chemoreceptor CcmL (Tlp3) of
- Single Cell Stochastic Regulation of Pilus Phase Variation by an Attenuation-like Mechanism
- Cell Tropism Predicts Long-term Nucleotide Substitution Rates of Mammalian RNA Viruses
- Functions of CPSF6 for HIV-1 as Revealed by HIV-1 Capsid Evolution in HLA-B27-Positive Subjects
- RNA-seq Analysis of Host and Viral Gene Expression Highlights Interaction between Varicella Zoster Virus and Keratinocyte Differentiation
- Kaposi's Sarcoma Associated Herpesvirus Tegument Protein ORF75 Is Essential for Viral Lytic Replication and Plays a Critical Role in the Antagonization of ND10-Instituted Intrinsic Immunity
- DAMP Molecule S100A9 Acts as a Molecular Pattern to Enhance Inflammation during Influenza A Virus Infection: Role of DDX21-TRIF-TLR4-MyD88 Pathway
- Variable Suites of Non-effector Genes Are Co-regulated in the Type III Secretion Virulence Regulon across the Phylogeny
- Reengineering Redox Sensitive GFP to Measure Mycothiol Redox Potential of during Infection
- Preservation of Tetherin and CD4 Counter-Activities in Circulating Alleles despite Extensive Sequence Variation within HIV-1 Infected Individuals
- KSHV 2.0: A Comprehensive Annotation of the Kaposi's Sarcoma-Associated Herpesvirus Genome Using Next-Generation Sequencing Reveals Novel Genomic and Functional Features
- Nutrient Limitation Governs Metabolism and Niche Adaptation in the Human Nose
- Decreases in Colonic and Systemic Inflammation in Chronic HIV Infection after IL-7 Administration
- Investigation of Acetylcholine Receptor Diversity in a Nematode Parasite Leads to Characterization of Tribendimidine- and Derquantel-Sensitive nAChRs
- Intranasal Vaccination Promotes Detrimental Th17-Mediated Immunity against Influenza Infection
- -Mediated Inhibition of Iron Export Promotes Parasite Replication in Macrophages
- Variation in RNA Virus Mutation Rates across Host Cells
- A Single Amino Acid in the Stalk Region of the H1N1pdm Influenza Virus HA Protein Affects Viral Fusion, Stability and Infectivity
- Group B Engages an Inhibitory Siglec through Sialic Acid Mimicry to Blunt Innate Immune and Inflammatory Responses
- Synthesis and Biological Properties of Fungal Glucosylceramide
- HIV Protective KIR3DL1/S1-HLA-B Genotypes Influence NK Cell-Mediated Inhibition of HIV Replication in Autologous CD4 Targets
- Recruitment of PfSET2 by RNA Polymerase II to Variant Antigen Encoding Loci Contributes to Antigenic Variation in
- Human and Plant Fungal Pathogens: The Role of Secondary Metabolites
- Lyme Disease: Call for a “Manhattan Project” to Combat the Epidemic
- Enhancing Virus-Specific Immunity by Combining Therapeutic Vaccination and PD-L1 Blockade in Chronic Hepadnaviral Infection
- Suppression of Interferon Lambda Signaling by SOCS-1 Results in Their Excessive Production during Influenza Virus Infection
- Inflammation Fuels Colicin Ib-Dependent Competition of Serovar Typhimurium and in Blooms
- Host-Specific Enzyme-Substrate Interactions in SPM-1 Metallo-β-Lactamase Are Modulated by Second Sphere Residues
- STING-Dependent Type I IFN Production Inhibits Cell-Mediated Immunity to
- From Scourge to Cure: Tumour-Selective Viral Pathogenesis as a New Strategy against Cancer
- Lysine Acetyltransferase GCN5b Interacts with AP2 Factors and Is Required for Proliferation
- Narrow Bottlenecks Affect Populations during Vertical Seed Transmission but not during Leaf Colonization
- Targeted Cytotoxic Therapy Kills Persisting HIV Infected Cells During ART
- Murine Gammaherpesvirus M2 Protein Induction of IRF4 via the NFAT Pathway Leads to IL-10 Expression in B Cells
- iNKT Cell Production of GM-CSF Controls
- Malaria-Induced NLRP12/NLRP3-Dependent Caspase-1 Activation Mediates Inflammation and Hypersensitivity to Bacterial Superinfection
- Detection of Host-Derived Sphingosine by Is Important for Survival in the Murine Lung
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Lyme Disease: Call for a “Manhattan Project” to Combat the Epidemic
- Origin, Migration Routes and Worldwide Population Genetic Structure of the Wheat Yellow Rust Pathogen f.sp.
- IFNγ/IL-10 Co-producing Cells Dominate the CD4 Response to Malaria in Highly Exposed Children
- Human and Plant Fungal Pathogens: The Role of Secondary Metabolites
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání















