-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Human and Plant Fungal Pathogens: The Role of Secondary Metabolites
article has not abstract
Published in the journal: . PLoS Pathog 10(1): e32767. doi:10.1371/journal.ppat.1003859
Category: Pearls
doi: https://doi.org/10.1371/journal.ppat.1003859Summary
article has not abstract
What Are Secondary Metabolites?
Secondary metabolites (SM) are small organic molecules produced by various microorganisms (mainly fungi and actinobacteria, among others) through the action of large enzymes like nonribosomal peptide synthetases (NRPS) and polyketide synthases (PKS) or by enzymes like dimethylallyl transferases and prenyltransferases. These special metabolites are in general not essential for growth but are believed to be advantageous for the producing organism under certain conditions and in distinct habitats. In fungi, the genes responsible for SM biosynthesis are usually arranged in clusters [1], which are often found at the end of the chromosomes in subtelomeric regions [2], [3].
Are Secondary Metabolites Essential for Fungal Pathogenicity?
Yes, a few of these molecules have been shown to contribute to pathogenicity of several fungi (Figure 1). An excellent example of secondary metabolites that are essential for fungal pathogenicity is given by melanins [4]. What are melanins? Globally, they are defined as dark pigments of high molecular mass derived by oxidative polymerisation from phenolic precursors [5].
Fig. 1. SM with influence on virulence of fungi in the respective host system. 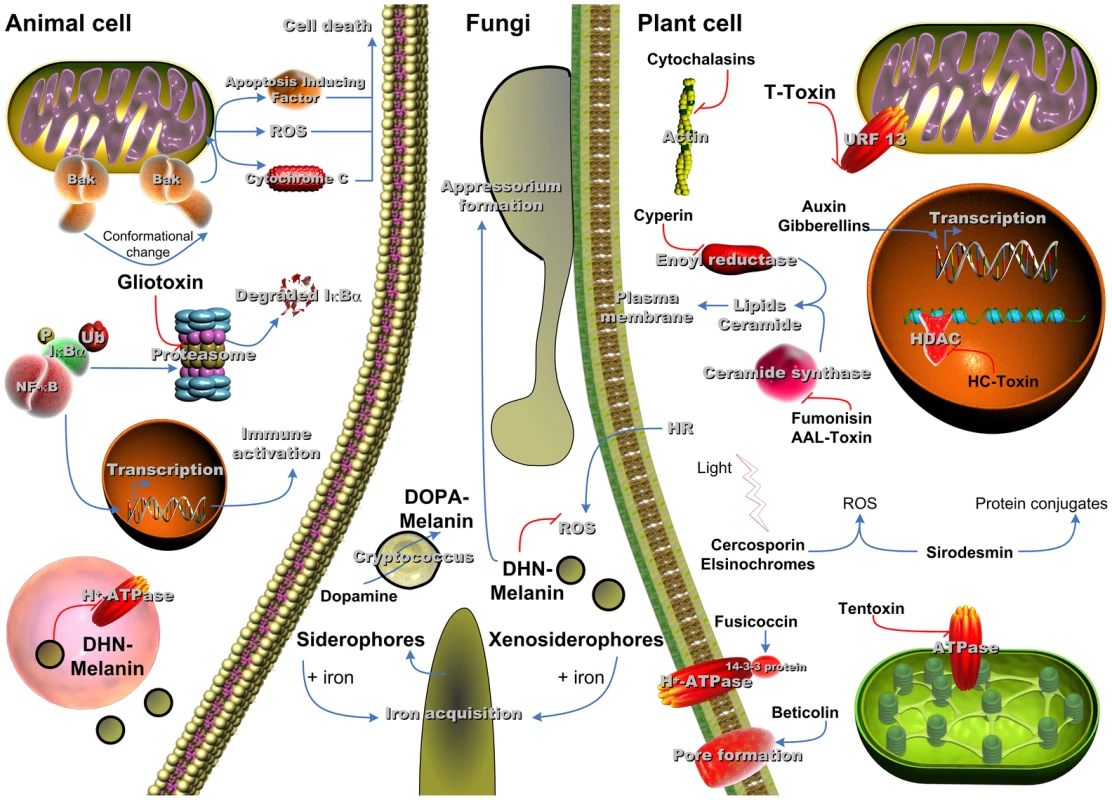
Animal cell: Gliotoxin acts on the mitochondrial protein Bak, which leads to apoptosis and inhibits the activation of NFκB through blocking proteasomal activity; DHN-melanin from A. fumigatus inhibits vH+-ATPase activity and therefore acidification of the phagolysosome, which counteracts killing of the conidia. Fungi: Siderophores are released by the fungus to ensure iron availability; DHN-melanin from plant pathogens is crucial for appressorium formation and counteracting reactive oxygen species (ROS) produced by the plant through hypersensitive response (HR) to prevent the spread of fungal infection; DOPA-melanin produced by C. neoformans from external precursors is an important part of the fungal capsule. Plant cell: Cytochalasins block cytokinesis; T-Toxin inhibits mitochondrial energy production; Fumonisin, AAL-Toxin, and Cyperin affect the membrane integrity; Auxin and Gibberellins act as phytohormones and alter transcription activity; HC-Toxin inhibits histone deacetylase; after light-driven activation both Cercosporin and Elsinochromes produce ROS, which damage the cell; Sirodesmin induces ROS and the formation of protein-conjugates; Tentoxin inhibits chloroplastidial energy production; Fusicoccin mediates irreversible stomata opening; Beticolin forms pores in the membrane and leads to leakage of the cell. In fungi, two main types of melanin, dihydroxynaphthalene (DHN) melanin and dihydroxy phenylalanine (DOPA) melanin, and derivatives thereof have been reported to contribute to virulence. The ability to produce melanin via polymerisation of di - or tetrahydroxynaphthalene is widespread among fungi, and for some fungi the production of DOPA from L-tyrosine via dihydroxyphenylalanine has been shown. The function of melanins is extraordinarily wide. They exert beneficial functions to the producing organism as they mediate general protection against a wide variety of exogenous stresses. Melanins are often associated with the cell wall and thereby also contribute to fungal structures, such as ornamentation of the spore surface or pressure-stabilisation. Not surprisingly, melanins contribute to pathogenicity in several plant and human pathogenic fungi.
The two most prominent fungal organisms for which DHN-melanin is a prerequisite for virulence are the human pathogen Aspergillus fumigatus and the plant pathogen Magnaporthe oryzae. In A. fumigatus, DHN-melanin is mainly produced during conidiogenesis and accounts for the characteristic grey-green colour of the spores. These spores are ubiquitous in nature, with an estimation that every human inhales hundreds to thousands of conidia each day. This represents an important issue especially for patients suffering from an impaired immune system, making A. fumigatus the most important airborne fungal pathogen of humans [6].
In the lung, A. fumigatus conidia are confronted with the host's immune system from which phagocytes represent the first line of defense. Pigmentless white conidia are significantly reduced in virulence when tested in murine infection models. Fully pigmented conidia exert a dual effect on phagocytes: first, they prevent killing after phagocytosis by interfering with phagolysosomal acidification, and second, they inhibit apoptosis of macrophages. Thereby, A. fumigatus melanin is decisively pivotal in establishing a niche within phagocytes in which the fungus is protected from further immune reactions, providing the opportunity to grow out and hindering clearance of hyphae [3].
In the plant pathogenic fungus M. oryzae that causes the most important disease of rice worldwide, namely rice blast, the underlying mechanism by which DHN-melanin impacts virulence differs fundamentally from that of A. fumigatus [7]. Infection of rice plant leaves requires fungal penetration of the plant cell wall. The high turgor that is needed for this process is produced by a specialized DHN-melanin–containing fungal structure, the appressorium, from which the melanin layer hinders the efflux of solute [8]. Consequently, melanin-deficient M. oryzae mutants produce non-pigmented appressoria that are unable to penetrate epidermal cells of the leaf cuticle [9]. Targeting enzymes of the M. oryzae DHN-melanin pathway by chemicals such as carpropamid has been shown to efficiently prevent an infection.
Cryptococcus neoformans, the main opportunistic fungal infectious agent of patients suffering from AIDS, produces melanin exclusively via the L-dopa pathway from a variety of exogenously recruited precursers (e.g., L-DOPA, D-DOPA, dopamine, norepinephrine). Melanization occurs during infection, and mutants of C. neoformans lacking melanin pigment showed a reduced virulence in a murine infection model [4], [10].
Another good example of SM essential for fungal virulence is siderophores. As iron is an indispensable nutrient for all eukaryotic organisms, the ability to overcome iron limitation also represents a prerequisite for pathogenicity. Although iron is highly abundant in the oxidized form in the environment, the availability within a host, both animal and plant, is extremely low. For example, humans utilize carrier proteins like lactoferrin, transferrin, ferritin, and hemoglobin to bind iron, resulting in a final concentration of free iron of ∼10−24 M that is far from sufficient to meet a fungal pathogen's need to maintain its iron homeostasis during infection. As a countermove, fungal pathogens have evolved several strategies, amongst which is the production of siderophores for iron acquisition. Siderophores are small secreted molecules chelating iron with a complex formation constant that can reach >1030 M and thereby enable the fungal pathogen to capture iron from the host organism and even from stainless steel. Siderophores represent SM because their synthesis involves an NRPS, which also constitutes a promising drug target, as such enzymes are lacking in humans.
This struggle for iron obviously suggests a correlation of siderophore production and virulence of pathogenic fungi [11]. Again, A. fumigatus might serve as a good example of a human pathogenic fungus producing siderophores to cope with low iron availability in the host. For extracellular iron acquisition, A. fumigatus produces mainly the hydroxamate type siderophore triacetylfusarinine C (TAFC) by the NRPS SidD. Intracellular iron storage within A. fumigatus hyphae is mediated by the ferrichrome ferricrocin for which biosynthesis SidC is the core NRPS [12]. In conidia, hydroxyferricrocin is employed to guarantee iron supply during germination. Dissecting the pathways for intra - and extracellular siderophore biosynthesis pathways revealed their distinct cellular and disease-related roles during infection. For example, conidial hydroxyferricrocin is crucial, not only for developmental processes such as germ tube formation, sporulation, and stress resistance, but also for initiation of infection. By contrast, elimination of extracellular siderophores attenuates virulence but has no effect on germ tube formation.
With regard to animal–pathogen interaction, siderophore biosynthesis genes were found to be transcriptionally activated by confrontation with immune cells and during infection. Not surprisingly, A. fumigatus mutants in the siderophore biosynthesis gene sidA, which is essential for biosynthesis of a precursor for all secreted and intracellular siderophores, were even unable to initiate an infection in a murine model of pulmonary aspergillosis. Deletion of sidC or sidD caused at least partial attenuation of virulence [11].
The essential need to cope with low iron concentrations in the host is pertinent also for phytopathogenic fungi. Depending on the host-plant/pathogen combination, several secreted siderophores were identified as virulence determinants (Fusarium graminearum/rice, or Alternaria brassicicola/Arabidopsis thaliana). Notably, M. oryzae only produces an intracellular siderophore, ferricrocin, that also contributes to pathogenicity on rice by interfering with turgor generation by the appressorium essential for plant cell infection, as described above for melanin [13].
Besides producing their own iron chelators, some fungi, such as the pathogenic yeasts Candida albicans and C. neoformans, are also able to utilize xenosiderophores, iron-loaded siderophores originally produced by other microorganisms in the same habitat [14].
Taken together, melanins and siderophores are important pathogenicity factors in both plant and human pathogenic fungi because they are crucial for certain steps of pathogenesis like infiltration or survival.
Can Secondary Metabolites Increase the Virulence of Fungal Pathogens?
There are several examples showing that SM contribute to the outcome of an infection but not to the onset of an infection. For some of these molecules, the mechanism of action is known (Figure 1). Such metabolites may naturally act as agents of competition or as communication signals in the environment and thus gain importance in certain pathogen–host interactions. Two SM produced by Beauveria bassiana provide excellent examples [15]. This fungus produces the SM bassinolide and beauvericin and is a facultative pathogen of insects with a broad host range. The abrogation of production of one of these molecules resulted in a decreased virulence in different insect infection models. The mechanism behind the action of bassinolide and beauvericin as virulence factors is unknown.
By contrast, the mechanism of host-selective T-toxin in the Cochliobolus heterostrophus/maize pathosystem is well established. T-toxin selectively affects mitochondria through the binding to the URF13 protein. This causes a conformational change in the polypeptide, leading to the formation of a pore in the mitochondrial membrane, which significantly damages the plant cells [16], [17].
Another example is gliotoxin (GT) produced by A. fumigatus, which was shown to attribute to virulence in mice immunosuppressed by exclusive administration of cortisone acetate [18], [19]. The GT cluster is up-regulated after confrontation with immune cells [2], [20]. The toxin can kill immune cells that normally clear the fungus from the lung epithelium. The toxicity of the molecule depends on its disulphide bridge, which is reduced after uptake in the host cell [21], [22]. Reduced GT can then inactivate essential proteins by the formation of protein-GT disulphides and initiate a redox cycle that permanently produces reactive oxygen intermediates, thereby harming the cell. Thus, the production of GT constitutes an advantage as soon as the fungus is confronted by immune cells or other enemies in the environment (Figure 1).
In conclusion, a few SM are essential for manifestation of an infection in animal or plant hosts. The two most important examples are melanins and siderophores, and the deletion of their key biosynthetic genes leads to attenuated and apathogenic strains, respectively. Other SM are, at least from the point of view of the pathogen, advantageous for the infection process and contribute in modulating the progress of a disease. Fungal SM act in different ways and increase the pathogen's ability to counteract adverse conditions in the host environment, irrespective of whether it is an animal or plant host. Since filamentous fungi encode between 30 to 70 secondary metabolism gene clusters (the products of most of these clusters are unknown) and there even exists cross-talk between clusters, resulting in the formation of hybrid molecules, it can be expected that up to 100 SM are produced by a single filamentous fungus. All of these compounds have the potential to contribute to pathogenicity. Thus, it will be very important to elucidate the nature and impact of these compounds on pathogenicity.
Zdroje
1. BrakhageAA (2013) Regulation of fungal secondary metabolism. Nat Rev Microbiol 11 : 21–32.
2. McDonaghA, FedorovaND, CrabtreeJ, YuY, KimS, et al. (2008) Sub-telomere directed gene expression during initiation of invasive aspergillosis. PLoS Pathog 4: e1000154 doi:10.1371/journal.ppat.1000154
3. PalmerJM, KellerNP (2010) Secondary metabolism in fungi: does chromosomal location matter? Curr Opin Microbiol 13 : 431–436.
4. HeinekampT, ThywissenA, MacheleidtJ, KellerS, ValianteV, et al. (2013) Aspergillus fumigatus melanins: interference with the host endocytosis pathway and impact on virulence. Front Microbiol 3 : 440.
5. EisenmanHC, CasadevallA (2012) Synthesis and assembly of fungal melanin. Appl Microbiol and Biotechnol 93 : 931–940.
6. BrakhageAA, BrunsS, ThywissenA, ZipfelPF, BehnsenJ (2010) Interaction of phagocytes with filamentous fungi. Curr Opin Microbiol 13 : 409–415.
7. WilsonRA, TalbotNJ (2009) Under pressure: investigating the biology of plant infection by Magnaporthe oryzae. Nat Rev Microbiol 7 : 185–195.
8. SoanesDM, ChakrabartiA, PaszkiewiczKH, DaweAL, TalbotNJ (2012) Genome-wide transcriptional profiling of appressorium development by the rice blast fungus Magnaporthe oryzae. PLoS Pathog 8: e1002514 doi:10.1371/journal.ppat.1002514
9. HowardRJ, ValentB (1996) Breaking and entering: host penetration by the fungal rice blast pathogen Magnaporthe grisea. Annu Rev of Microbiol 50 : 491–512.
10. LiuGY, NizetV (2009) Color me bad: microbial pigments as virulence factors. Trends Microbiol 17 : 406–413.
11. SchrettlM, HaasH (2011) Iron homeostasis–Achilles' heel of Aspergillus fumigatus? Curr Opin Microbiol 14 : 400–405.
12. SchrettlM, BignellE, KraglC, SabihaY, LossO, et al. (2007) Distinct roles for intra - and extracellular siderophores during Aspergillus fumigatus infection. PLoS Pathog 3 : 1195–1207 doi:10.1371/journal.ppat.0030128
13. HofC, EisfeldK, WelzelK, AnteloL, FosterAJ, et al. (2007) Ferricrocin synthesis in Magnaporthe grisea and its role in pathogenicity in rice. Mol Plant Pathol 8 : 163–172.
14. HowardDH (1999) Acquisition, transport, and storage of iron by pathogenic fungi. Clin Microbiol Rev 12 : 394–404.
15. XuY, OrozcoR, Kithsiri WijeratneEM, Espinosa-ArtilesP, Leslie GunatilakaAA, et al. (2009) Biosynthesis of the cyclooligomer depsipeptide bassianolide, an insecticidal virulence factor of Beauveria bassiana. Fungal Genet Biol 46 : 353–364.
16. MobiusN, HertweckC (2009) Fungal phytotoxins as mediators of virulence. Curr Opin in Plant Biol 12 : 390–398.
17. WolpertTJ, DunkleLD, CiuffettiLM (2002) Host-selective toxins and avirulence determinants: what's in a name? Annu Rev of Phytopathol 40 : 251–285.
18. SpikesS, XuR, NguyenCK, ChamilosG, KontoyiannisDP, et al. (2008) Gliotoxin production in Aspergillus fumigatus contributes to host-specific differences in virulence. J Infect Dis 197 : 479–486.
19. SuguiJA, PardoJ, ChangYC, ZaremberKA, NardoneG, et al. (2007) Gliotoxin is a virulence factor of Aspergillus fumigatus: gliP deletion attenuates virulence in mice immunosuppressed with hydrocortisone. Eukaryot Cell 6 : 1562–1569.
20. SuguiJA, KimHS, ZaremberKA, ChangYC, GallinJI, et al. (2008) Genes differentially expressed in conidia and hyphae of Aspergillus fumigatus upon exposure to human neutrophils. PLoS One 3: e2655 doi:10.1371/journal.pone.0002655
21. ScharfDH, HeinekampT, RemmeN, HortschanskyP, BrakhageAA, et al. (2012) Biosynthesis and function of gliotoxin in Aspergillus fumigatus. Appl Microbiol Biotechnol 93 : 467–472.
22. ScharfDH, RemmeN, HeinekampT, HortschanskyP, BrakhageAA, et al. (2010) Transannular disulfide formation in gliotoxin biosynthesis and its role in self-resistance of the human pathogen Aspergillus fumigatus. J Am Chem Soc 132 : 10136–10141.
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek Serotonin Signaling in : A Serotonin–Activated G Protein-Coupled Receptor Controls Parasite MovementČlánek Regulators of Cell Cycle Progression and Differentiation Identified Using a Kinome-Wide RNAi ScreenČlánek IFNγ/IL-10 Co-producing Cells Dominate the CD4 Response to Malaria in Highly Exposed ChildrenČlánek Functions of CPSF6 for HIV-1 as Revealed by HIV-1 Capsid Evolution in HLA-B27-Positive SubjectsČlánek Decreases in Colonic and Systemic Inflammation in Chronic HIV Infection after IL-7 Administration
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2014 Číslo 1- Stillova choroba: vzácné a závažné systémové onemocnění
- Diagnostika virových hepatitid v kostce – zorientujte se (nejen) v sérologii
- Perorální antivirotika jako vysoce efektivní nástroj prevence hospitalizací kvůli COVID-19 − otázky a odpovědi pro praxi
- Jak souvisí postcovidový syndrom s poškozením mozku?
- Infekční komplikace virových respiračních infekcí – sekundární bakteriální a aspergilové pneumonie
-
Všechny články tohoto čísla
- HIV-1 Accessory Proteins Adapt Cellular Adaptors to Facilitate Immune Evasion
- Ranaviruses: Not Just for Frogs
- Effectors and Effector Delivery in
- Plasmacytoid Dendritic Cell Dynamics Tune Interferon-Alfa Production in SIV-Infected Cynomolgus Macaques
- Lu/BCAM Adhesion Glycoprotein Is a Receptor for Cytotoxic Necrotizing Factor 1 (CNF1)
- A Substrate-Fusion Protein Is Trapped inside the Type III Secretion System Channel in
- Parvovirus-Induced Depletion of Cyclin B1 Prevents Mitotic Entry of Infected Cells
- Red Blood Cell Invasion by : Structural Basis for DBP Engagement of DARC
- NsrR, GadE, and GadX Interplay in Repressing Expression of the O157:H7 LEE Pathogenicity Island in Response to Nitric Oxide
- Loss of Circulating CD4 T Cells with B Cell Helper Function during Chronic HIV Infection
- TREM-1 Deficiency Can Attenuate Disease Severity without Affecting Pathogen Clearance
- Origin, Migration Routes and Worldwide Population Genetic Structure of the Wheat Yellow Rust Pathogen f.sp.
- Glutamate Utilization Couples Oxidative Stress Defense and the Tricarboxylic Acid Cycle in Phagosomal Escape
- Serotonin Signaling in : A Serotonin–Activated G Protein-Coupled Receptor Controls Parasite Movement
- Recovery of an Antiviral Antibody Response following Attrition Caused by Unrelated Infection
- Regulators of Cell Cycle Progression and Differentiation Identified Using a Kinome-Wide RNAi Screen
- Absence of Intestinal PPARγ Aggravates Acute Infectious Colitis in Mice through a Lipocalin-2–Dependent Pathway
- Induction of a Stringent Metabolic Response in Intracellular Stages of Leads to Increased Dependence on Mitochondrial Metabolism
- CTCF and Rad21 Act as Host Cell Restriction Factors for Kaposi's Sarcoma-Associated Herpesvirus (KSHV) Lytic Replication by Modulating Viral Gene Transcription
- Gammaherpesviral Gene Expression and Virion Composition Are Broadly Controlled by Accelerated mRNA Degradation
- The Arabidopsis Silencing Pathway Modulates PAMP- and Effector-Triggered Immunity through the Post-transcriptional Control of Disease Resistance Genes
- Inflammatory Stimuli Reprogram Macrophage Phagocytosis to Macropinocytosis for the Rapid Elimination of Pathogens
- Alphavirus Mutator Variants Present Host-Specific Defects and Attenuation in Mammalian and Insect Models
- Phosphopyruvate Carboxylase Identified as a Key Enzyme in Erythrocytic Carbon Metabolism
- IFNγ/IL-10 Co-producing Cells Dominate the CD4 Response to Malaria in Highly Exposed Children
- Electron Tomography of HIV-1 Infection in Gut-Associated Lymphoid Tissue
- Characterisation of a Multi-ligand Binding Chemoreceptor CcmL (Tlp3) of
- Single Cell Stochastic Regulation of Pilus Phase Variation by an Attenuation-like Mechanism
- Cell Tropism Predicts Long-term Nucleotide Substitution Rates of Mammalian RNA Viruses
- Functions of CPSF6 for HIV-1 as Revealed by HIV-1 Capsid Evolution in HLA-B27-Positive Subjects
- RNA-seq Analysis of Host and Viral Gene Expression Highlights Interaction between Varicella Zoster Virus and Keratinocyte Differentiation
- Kaposi's Sarcoma Associated Herpesvirus Tegument Protein ORF75 Is Essential for Viral Lytic Replication and Plays a Critical Role in the Antagonization of ND10-Instituted Intrinsic Immunity
- DAMP Molecule S100A9 Acts as a Molecular Pattern to Enhance Inflammation during Influenza A Virus Infection: Role of DDX21-TRIF-TLR4-MyD88 Pathway
- Variable Suites of Non-effector Genes Are Co-regulated in the Type III Secretion Virulence Regulon across the Phylogeny
- Reengineering Redox Sensitive GFP to Measure Mycothiol Redox Potential of during Infection
- Preservation of Tetherin and CD4 Counter-Activities in Circulating Alleles despite Extensive Sequence Variation within HIV-1 Infected Individuals
- KSHV 2.0: A Comprehensive Annotation of the Kaposi's Sarcoma-Associated Herpesvirus Genome Using Next-Generation Sequencing Reveals Novel Genomic and Functional Features
- Nutrient Limitation Governs Metabolism and Niche Adaptation in the Human Nose
- Decreases in Colonic and Systemic Inflammation in Chronic HIV Infection after IL-7 Administration
- Investigation of Acetylcholine Receptor Diversity in a Nematode Parasite Leads to Characterization of Tribendimidine- and Derquantel-Sensitive nAChRs
- Intranasal Vaccination Promotes Detrimental Th17-Mediated Immunity against Influenza Infection
- -Mediated Inhibition of Iron Export Promotes Parasite Replication in Macrophages
- Variation in RNA Virus Mutation Rates across Host Cells
- A Single Amino Acid in the Stalk Region of the H1N1pdm Influenza Virus HA Protein Affects Viral Fusion, Stability and Infectivity
- Group B Engages an Inhibitory Siglec through Sialic Acid Mimicry to Blunt Innate Immune and Inflammatory Responses
- Synthesis and Biological Properties of Fungal Glucosylceramide
- HIV Protective KIR3DL1/S1-HLA-B Genotypes Influence NK Cell-Mediated Inhibition of HIV Replication in Autologous CD4 Targets
- Recruitment of PfSET2 by RNA Polymerase II to Variant Antigen Encoding Loci Contributes to Antigenic Variation in
- Human and Plant Fungal Pathogens: The Role of Secondary Metabolites
- Lyme Disease: Call for a “Manhattan Project” to Combat the Epidemic
- Enhancing Virus-Specific Immunity by Combining Therapeutic Vaccination and PD-L1 Blockade in Chronic Hepadnaviral Infection
- Suppression of Interferon Lambda Signaling by SOCS-1 Results in Their Excessive Production during Influenza Virus Infection
- Inflammation Fuels Colicin Ib-Dependent Competition of Serovar Typhimurium and in Blooms
- Host-Specific Enzyme-Substrate Interactions in SPM-1 Metallo-β-Lactamase Are Modulated by Second Sphere Residues
- STING-Dependent Type I IFN Production Inhibits Cell-Mediated Immunity to
- From Scourge to Cure: Tumour-Selective Viral Pathogenesis as a New Strategy against Cancer
- Lysine Acetyltransferase GCN5b Interacts with AP2 Factors and Is Required for Proliferation
- Narrow Bottlenecks Affect Populations during Vertical Seed Transmission but not during Leaf Colonization
- Targeted Cytotoxic Therapy Kills Persisting HIV Infected Cells During ART
- Murine Gammaherpesvirus M2 Protein Induction of IRF4 via the NFAT Pathway Leads to IL-10 Expression in B Cells
- iNKT Cell Production of GM-CSF Controls
- Malaria-Induced NLRP12/NLRP3-Dependent Caspase-1 Activation Mediates Inflammation and Hypersensitivity to Bacterial Superinfection
- Detection of Host-Derived Sphingosine by Is Important for Survival in the Murine Lung
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Lyme Disease: Call for a “Manhattan Project” to Combat the Epidemic
- Origin, Migration Routes and Worldwide Population Genetic Structure of the Wheat Yellow Rust Pathogen f.sp.
- IFNγ/IL-10 Co-producing Cells Dominate the CD4 Response to Malaria in Highly Exposed Children
- Human and Plant Fungal Pathogens: The Role of Secondary Metabolites
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



