-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaAlphavirus Mutator Variants Present Host-Specific Defects and Attenuation in Mammalian and Insect Models
Arboviruses cycle through both vertebrates and invertebrates, which requires them to adapt to disparate hosts while maintaining genetic integrity during genome replication. To study the genetic mechanisms and determinants of these processes, we use chikungunya virus (CHIKV), a re-emerging human pathogen transmitted by the Aedes mosquito. We previously isolated a high fidelity (or antimutator) polymerase variant, C483Y, which had decreased fitness in both mammalian and mosquito hosts, suggesting this residue may be a key molecular determinant. To further investigate effects of position 483 on RNA-dependent RNA-polymerase (RdRp) fidelity, we substituted every amino acid at this position. We isolated novel mutators with decreased replication fidelity and higher mutation frequencies, allowing us to examine the fitness of error-prone arbovirus variants. Although CHIKV mutators displayed no major replication defects in mammalian cell culture, they had reduced specific infectivity and were attenuated in vivo. Unexpectedly, mutator phenotypes were suppressed in mosquito cells and the variants exhibited significant defects in RNA synthesis. Consequently, these replication defects resulted in strong selection for reversion during infection of mosquitoes. Since residue 483 is conserved among alphaviruses, we examined the analogous mutations in Sindbis virus (SINV), which also reduced polymerase fidelity and generated replication defects in mosquito cells. However, replication defects were mosquito cell-specific and were not observed in Drosophila S2 cells, allowing us to evaluate the potential attenuation of mutators in insect models where pressure for reversion was absent. Indeed, the SINV mutator variant was attenuated in fruit flies. These findings confirm that residue 483 is a determinant regulating alphavirus polymerase fidelity and demonstrate proof of principle that arboviruses can be attenuated in mammalian and insect hosts by reducing fidelity.
Published in the journal: . PLoS Pathog 10(1): e32767. doi:10.1371/journal.ppat.1003877
Category: Research Article
doi: https://doi.org/10.1371/journal.ppat.1003877Summary
Arboviruses cycle through both vertebrates and invertebrates, which requires them to adapt to disparate hosts while maintaining genetic integrity during genome replication. To study the genetic mechanisms and determinants of these processes, we use chikungunya virus (CHIKV), a re-emerging human pathogen transmitted by the Aedes mosquito. We previously isolated a high fidelity (or antimutator) polymerase variant, C483Y, which had decreased fitness in both mammalian and mosquito hosts, suggesting this residue may be a key molecular determinant. To further investigate effects of position 483 on RNA-dependent RNA-polymerase (RdRp) fidelity, we substituted every amino acid at this position. We isolated novel mutators with decreased replication fidelity and higher mutation frequencies, allowing us to examine the fitness of error-prone arbovirus variants. Although CHIKV mutators displayed no major replication defects in mammalian cell culture, they had reduced specific infectivity and were attenuated in vivo. Unexpectedly, mutator phenotypes were suppressed in mosquito cells and the variants exhibited significant defects in RNA synthesis. Consequently, these replication defects resulted in strong selection for reversion during infection of mosquitoes. Since residue 483 is conserved among alphaviruses, we examined the analogous mutations in Sindbis virus (SINV), which also reduced polymerase fidelity and generated replication defects in mosquito cells. However, replication defects were mosquito cell-specific and were not observed in Drosophila S2 cells, allowing us to evaluate the potential attenuation of mutators in insect models where pressure for reversion was absent. Indeed, the SINV mutator variant was attenuated in fruit flies. These findings confirm that residue 483 is a determinant regulating alphavirus polymerase fidelity and demonstrate proof of principle that arboviruses can be attenuated in mammalian and insect hosts by reducing fidelity.
Introduction
During replication, RNA viruses generate approximately 1 error per 104 nucleotides copied, giving rise to an immense population of genetically distinct but closely related variants [1], [2], [3], [4]. The genetic diversity of these “mutant swarms” is not detected by consensus sequencing, which to-date has been the basis for most studies of viral infection. However, this lack of information on genetic diversity has obscured crucial aspects of virus biology. Although RNA-dependent RNA polymerases (RdRp) have a high intrinsic error rate, their mutation rates can be altered to generate both higher and lower fidelity variants (antimutators and mutators, respectively) [5], [6], [7], [8], [9], [10], [11], [12], [13], [14]. Thus far, antimutator variants are thought to replicate more slowly, making fewer genomes with greater accuracy; in contrast, mutator variants have been shown to replicate more quickly, synthesizing more viral genomes but introducing many errors during the replication process [8], [15], [16], [17], [18]. Despite this, overall growth and titers of polymerase fidelity variants are not significantly different when grown in isolation in cell culture; for mutators the negative effects of accumulating deleterious mutations are only noticeable after several rounds of replication [6], [19]. In recent works, these variants have been useful in exploring how the course of viral infection is affected by either restricted or expanded population diversity [4], [10].
Current evidence indicates that mutation frequencies of RNA viruses have been optimized over time to be neither too accurate nor too erroneous [4], [6], [16], [20], [21], [22], [23]. It is thought that error-prone replication allows the virus to explore sequence space to gain adaptability and accumulate potentially advantageous mutations. For several RNA viruses, limiting viral population diversity has fitness costs in vivo. Despite similar in vitro growth phenotypes, variants that make fewer errors have reduced titers and exhibit restricted tropism in animal models [19], . This restriction in tropism may be due to cooperative inter-variant interactions or beneficial minority variants that are missing in a situation with restricted population diversity [19]. It is also proposed that high mutation rates of influenza A may contribute to altered tropism, allowing infection of new hosts [26]. Therefore, it seems that the relatively high error rates of RNA viruses generate a level of diversity that facilitates adaptive fitness advantages.
In contrast, there is also an upper threshold to mutation frequencies; if crossed, extreme error rates lead to the accumulation of deleterious mutations and loss of genetic integrity. Evidence for this is demonstrated by treatment of numerous RNA viruses with nucleoside analog mutagens, which increase mutation frequencies and result in extinction by lethal mutagenesis [27], [28], [29], [30], [31]. Although thus far RdRp mutators have not exhibited growth defects in isolation in vitro, a recent paper showed that HIV mutator and antimutator strains were less fit than wildtype in competition assays [32]. In addition, several studies recently report in vivo attenuation of mutator strains: Coxsackie virus B3 mutator strains present reduced viral titers in key organs and fail to establish persistent infections in mice [6], and a severe acute respiratory syndrome (SARS) coronavirus mutator strain exhibits reduced pathogenesis in several mouse models [7].
Antimutator and mutator variants are valuable tools to study where the threshold of advantageous polymerase error exists for viruses facing different selective pressures. In this respect, arboviruses represent a special evolutionary position due to their need to replicate in disparate hosts, which is accompanied by distinct selective pressures. Arbovirus fitness is not necessarily reduced due to obligate host-cycling (alternating passages of CHIKV did not limit viral fitness), yet it has been shown that evolvability may be reduced due to these evolutionary constraints [33], [34], [35], [36], [37], [38]. For alphaviruses, evidence suggests that viral diversity is most restricted in the insect host, due to more stringent population bottlenecks and selective pressures [33], [34], [35], [37], [39], [40]. Since minority variants are thought to play important roles in arbovirus pathogenesis, transmission, and emergence [25], [41], [42], [43], [44], [45], the implications of altered polymerase fidelity and mutation rates merit further study. Recently, this question was partially addressed using a chikungunya virus antimutator variant [25].
Chikungunya virus (CHIKV) is a re-emerging arbovirus, transmitted by Aedes species mosquitoes. This positive-stranded RNA virus (family Togaviridae, genus Alphavirus) has an 11.8 kB genome, of which the first 7.5 kb encode four nonstructural proteins (nsP1-4) involved in diverse processes including RNA synthesis, immune evasion, and host tropism [46], [47], [48], [49], [50], [51], [52]. In most cases, functions of these proteins are putative in CHIKV and have only been shown in related model viruses, such as Semliki forest virus and Sindbis virus [53], [54], [55], [56]. Nsp4 is the RdRp, responsible for nucleotide incorporation during replication [57]. Previously, we isolated an antimutator strain of CHIKV by passaging virus in ribavirin, an RNA nucleoside analog. Ribavirin causes nucleotide misincorporation by the RdRp, adding selective pressure for an intrinsically more faithful polymerase [14], [58], [59]. This antimutator strain harbored a single amino acid change (483Y) in nsp4. Although 483Y showed no growth defects in vitro, the variant was moderately attenuated in vivo in both mammalian and mosquito hosts [25]. However, no arbovirus mutators have been isolated thus far. To this end, we mutated the conserved cysteine residue at position 483 to obtain several mutators in the arboviruses CHIKV and SINV, confirming this position's importance in determining alphavirus fidelity. We used these novel mutator strains to examine how increased polymerase error affects arbovirus fitness in vitro and in vivo; interestingly, mutator strains presented distinct cell - and host-specific phenotypes.
Materials and Methods
Cells and viruses
Mammalian cell lines Vero, HeLa, and BHK-21 were maintained in DMEM (Gibco) supplemented with 10% newborn calf serum (NCS, Gibco) and 1% penicillin-streptomycin (P/S, Sigma), at 37°C with 5% CO2. Mosquito cell lines C6/36 and U4.4 (Aedes albopictus) and Aag2 (Aedes aegypti) were grown in L-15 media, supplemented with 10% fetal bovine serum (FBS, Gibco), 1% P/S, 1% tryptose phosphate, and 1% non-essential amino acids (NEAA), at 28°C with 5% CO2. Drosophila melanogaster S2 cells were grown in Schneider's Drosophila media (Gibco), supplemented with 10% FBS, 1% L-Glutamine, and 1% P/S at 25°C.
Wildtype CHIKV was generated from the La Reunion strain 06-049 infectious clone, previously described [33]. Nsp4 position 483 mutants were generated by site-directed mutagenesis of the infectious clone using the QuikChange II XL Site-Directed Mutagenesis kit (Stratagene). All newly generated DNA plasmids were Sanger sequenced in full (GATC Biotech) to confirm mutagenesis of position 483 and to ensure no second-site mutations were introduced. Select SINV mutants were constructed in the same fashion from the pTR339 wildtype infectious clone [60]. CHIKV and SINV expression plasmids were linearized with NotI or XhoI respectively, purified by phenol-chloroform extraction and ethanol precipitation, and subsequently used for in vitro transcription of viral RNAs using the SP6 mMESSAGE mMACHINE kit (Ambion). RNAs were then purified by phenol:chloroform extraction and ethanol precipitation, quantified, diluted to 1 µg/µl and stored at −80°C.
For RNA transfections, BHK-21 cells were trypsinized, washed twice with ice-cold PBS, and resuspended at a concentration of 2×107 cells/ml in ice-cold PBS. Cells (0.390 ml) were mixed with 10 µg of in vitro transcribed viral RNA, placed in 2 mm cuvette and electroporated at 1.2 kV, 25 µF with infinite Ω in a XCell Gene Pulser (BioRad). Cells were allowed to recover for 10 minutes at room temperature then mixed with 6 ml of pre-warmed media and placed into a T-25 flask. After 48 hours incubation at 37°C, viral titers were determined by standard plaque assay. In brief, 10-fold serial dilutions of each virus in DMEM were incubated on a confluent monolayer of Vero cells for 1 hour at 37°C. Following incubation, cells were overlaid with 0.8% agarose dissolved in DMEM and 2% NCS and incubated at 37°C for 72 hours. The cells were then fixed with 4% formalin for 1 hour, the agarose plugs were removed, and plaques were visualized by the addition of crystal violet. Plaque size was quantified by scanning the crystal violet-stained cell monolayer, then quantifying the size of each individual plaque in square millimeters using ImageJ (http://rsbweb.nih.gov/ij).
Each virus was then passaged once over a 70–80% confluent monolayer of BHK-21 cells, titered as described above, aliquoted, and stored at −80°C until use. To analyze each virus for reversion at position 483, viral RNA was extracted for each electroporation and BHK-21 passage using TRIzol reagent (Invitrogen). For CHIKV, this RNA was used to amplify a 3184 bp region corresponding to nucleotides (4522–7706), which included position 483, using the forward primer (5′-GATGAGCACATCTCCATAG-3′) and the reverse primer (5′-GTTTGGGTTGGGATGAACT-3′) and the Titan One Tube RT-PCR Kit (Roche). For SINV, a 2225 bp region (nucleotides 6556–8781) was amplified in the same fashion using forward primer (5′-ACCAGGCACGAAACACACAGAA-3′) and reverse primer (5′-ACTGGGCGGAAGTCTGTATGCG -3′). Each PCR product was cleaned using the Nucleospin PCR and Gel Extraction Kit (Macherey-Nagel) and Sanger sequenced at position 483/482 to confirm genetic stability. At passage 3, all viruses used were fully sequenced to ensure no second site mutations.
Ribavirin sensitivity assay
HeLa cells (250,000 cells/well in 12-well tissue culture plates) were pre-treated for two hours with either media containing no mutagen, or media containing 200 µM or 400 µM ribavirin (Sigma). Post-treatment, media was removed and the cells were inoculated with virus in DMEM at an MOI 0.1 for one hour at 37°C. Following incubation, mutagen-containing media was replaced and cells were incubated for 72 hours at 37°C. Virus was harvested at 72 hours and mean titers were obtained by TCID50. In brief, a 96-well tissue culture plates was plated for each virus with 1×104 Vero cells/well. Viruses were serially diluted in 8 ten-fold dilutions in DMEM. Each dilution was distributed in a row of the 96-well plate, with each well receiving 100 µl of diluted virus. Viruses and cells were incubated 5–7 days at 37°C with 5% CO2. Following incubation, cells were fixed with 50 µl of 4% formalin for 30 minutes. All media were removed, and 50 µl of crystal violet was added to each well. Viruses that exhibited significant sensitivity or resistance compared to wildtype at P<0.05 or greater at either 200 µM or 400 µM ribavirin were considered potential fidelity variants, and mutation frequencies were estimated (Table 1).
Tab. 1. Characterization of CHIKV 483 polymerase variants. 
a Hydrophobicities from Eisenberg et al., 1982 [94]. Mutation frequencies by molecular cloning
To determine mutation frequencies, all mutants were electroporated in tandem into BHK-21 cells. Supernatants were collected 48 hours later and viral RNA was extracted. For CHIKV, an approximately 800 bp region corresponding to nucleotides 9943–10726 was amplified of the E1 region of the genome using forward primer 5′-TACGAACACGTAACAGTGATCC-3′ and reverse primer 5′-CGCTCTTACCGGGTTTGTTG-3′. For SINV, the analogous region was amplified using forward primer 5′-TACGAACATGCGACCACTGTTC-3′ and reverse primer 5′-CGCTCGGAGCGGATTTACTG-3′, and approximately 500 bases of this fragment was included in the analysis. Amplified fragments were purified as described above, and 3 µl of each product was modified by a 3′ A-overhang addition reaction (1 µl AmpliTaq Gold 10× buffer, 1 µl 10 mM dATP). Modified products were cloned using the TopoTA cloning kit (Invitrogen), and single colonies were picked for sequencing. Mutation frequencies were determined as previously described [61]. Mutation frequencies in mosquito cells were obtained in the same fashion using the samples obtained from C6/36 growth curves (we determined mutation frequency for a wildtype sample electroporated into C6/36 cells, and there was no difference between samples generated by infection or electroporation; the nonviability of mutators transfected into mosquito cells made it impossible to estimate mutation frequencies in C6/36 by electroporation). We sequenced approximately 75 clones per viral population in C6/36 cells. Mutation frequencies from mouse muscle were determined using RNA extracted from homogenized muscle samples from mice that most closely represented the median titer for that variants. For estimating in vivo mutation frequencies, a minimum of 50 clones were sampled per population. To confirm that the presence of RNA or aberrant viral particles in supernatants/homogenates did not affect mutation frequencies, we purified virus on 20% sucrose cushion and re-estimated mutation frequencies; no differences were observed.
Population diversity by deep sequencing
To estimate the population diversity of variants by deep sequencing, cDNA libraries were prepared by Superscript III from RNA extracted from virus generated in BHK-21 or C6/36 cells, and the viral genome was amplified using a high fidelity polymerase (Phusion) to generate 5 overlapping amplicons 2–3 kb in length. PCRs were fragmented (Fragmentase), multiplexed, clustered, sequenced in the same lane with Illumina cBot and GAIIX technology and analyzed with established deep sequencing data analysis tools and in house scripts. Briefly, per-base Phred quality scores were utilized to trim bases with error probabilities higher than 0.001, and sequences with less than 16 bases after trimming were discarded. For this purpose we used the fastq-mcf tool from the ea-utils toolkit at http://code.google.com/p/ea-utils [62]. The alignment step is performed using Burrows Wheeler Aligner [63] and Pileup is performed using SAMtools [64]. Once the pileup is done, an in-house script collects the data per-position and calculates the variance at each nucleotide position by root mean square deviation (RMSD) and determines the mean variance and standard error across the whole genome [65].
Neutralization assays
To estimate population diversity in a phenotypic assay, we performed neutralization assays using viruses which had been passaged 3 times on BHK-21 cells, using the n Neutralizing antibody CHK-102 (a kind gift from Dr. M.S. Diamond [66]). 100 pfu of wildtype and mutator CHIKV strains were incubated for 1 hour at 37°C with serial dilutions of antibody, ranging from 2 µg/ml to .0001 µg/mL, or left untreated. Virus-antibody complexes were added to pre-seeded confluent monolayers of Vero cells, and allowed to bind at 37°C for 1 hour. Assays were then overlayed with agarose and developed as described above for a plaque assay. Plaques were counted and normalized to the untreated control for each virus.
Viral replication and extracellular RNA synthesis
Virus growth was evaluated for WT and all mutant viruses in BHK-21, C6/36, U4.4, Aag2, and S2 cells and titers were determined by TCID50 on Vero cells as described above. Using the 24 hour time point from the C6/36 growth curve, we also performed a cytopathic effect (CPE) assay on C6/36 cells on all viruses (CellTiter 96 AQueuos One Solution Cell Proliferation Assay (MTS) kit; Promega). We obtained similar titers by standard TCID50 and CPE assay, indicating that viruses amplified on mosquito cells were still equally infectious when titered on Vero cells. For CHIKV, genome copy number was determined by extracting viral RNA from the supernatant at each time point using the TRIzol reagent and performing quantitative RT-PCR (qRT-PCR) using the TaqMan RNA-to-Ct kit (Applied Biosystems). Ct values were determined in duplicate based on amplification of nsp4 transcripts using forward (5′-TCACTCCCTGCTGGACTTGATAGA-3′) and reverse (5′-TGACGAACAGAGTTAGGAACATACC-3′) primers and probe 5′ - [6-FAM] AGGTACGCGCTTCAAGTTCGGCG-3′ as previously published [33], [67]. To determine genome copy number for SINV, viral RNA was extracted in the same manner and quantitative PCR was performed based on amplification of nsp3 transcripts using forward (5′-AAAACGCCTACCATGCAGTG-3′) and reverse (5′-TTTTCCGGCTGCGTAAATGC-3′) primers and the SYBR green PCR master mix (Applied Biosystems). Standard curves were performed in each run using samples of in vitro transcribed CHIKV or SINV RNA.
Northern blot analysis
In vitro transcribed RNA was transfected in BHK-21 cells in duplicate, as described above or at 28°C, including RNA from a construct in which the polymerase active site (GDD) was replaced with GNN by site-directed mutagenesis to abrogate replication and alongside a mock transfection where no RNA was added. Transfections in C6/36 and U4.4 cells were modified by pulsing with 250 V, 50 µF, and 550 Ω. Forty-eight hours post-transfection, supernatant containing progeny virus was collected. Cells were washed twice in PBS and RNA was TRIzol (Invitrogen) extracted, quantified and diluted to the same concentration. Samples were prepared in NorthernMax formaldehyde loading dye (Ambion) with 1 µl of ethidium bromide, heated to 65°C for 10 minutes, then separated on a 1.2% LE agarose (Lonza) gel containing 1× morpholinepropanesulfonic acid (MOPS) running buffer (Ambion) and 6.7% formaldehyde. RNA was transferred onto nitrocellulose membrane, cross-linked by ultraviolet irradiation (UVP), and prehybridized at 68°C for 1 hour in ULTRAhyb ultrasensitive hybridization buffer (Ambion). A plasmid used for the expression of CHIKV RNA probes corresponding to the 3′ portion of the E2 glycoprotein was generated by first amplifying the region of the CHIKV genome from 8703 (5′-GAAGCGACAGACGGGACG-3′) to 9266 (5′-GTTACATTTGCCAGCGGAA-3′) by PCR and subsequently TOPO-TA cloning the PCR product into the pCRTOPO-II vector. RNA probes complementary to positive strand RNA were labeled with 32P using the MAXIscript SP6 In Vitro Transcription Kit (Ambion), unincorporated nucleotides were removed using illustra MicroSpin S200 HR columns (GE healthcare), and probe was hybridized to the membrane overnight at 68°C. Membranes were washed several times at 68°C with 0.1× SSC with 0.1% SDS, then imaged using Amersham Hyperfilm MP autoradiography film (GE Healthcare). Quantification was done using ImageJ (http://rsbweb.nih.gov/ij).
Mouse infections
C57BL/6 mice (Janvier) or CD-1 mice (Charles River) were housed according to Institut Pasteur guidelines in biosafety level 3 isolators, with the approved experimental protocol #10.620, reviewed by the Institut Pasteur ethics committee under dossier #CETEA 2013-0021. At 8-days old, litters of C57BL/6 were inoculated with 200 pfu of wildtype or mutant CHIKV viruses subcutaneously (n = 4/variant). Eight-day old CD-1 litters were inoculated with 100 pfu of wildtype or mutant SINV strains in the same fashion, and monitored for symptoms of hind limb paralysis and survival. In addition, seven days post-infection, CHIKV and SINV-infected mice were sacrificed and brains, thigh muscles, livers and blood were harvested and homogenized in 300 µl of PBS at 30 shakes/second for 2 min (MM300 Retsch). RNA was extracted and viral genome copies were determined by qRT-PCR as described.
Mosquito infections
Principal CHIKV vectors Ae. albopictus Providence (ALPROV, F8 generation) from La Reunion and Ae. aegypti Paea (PAE, a lab colony at Institut Pasteur since 1994) from Tahiti, in French Polynesia were fed on artificial bloodmeals containing 106 pfu/ml of virus in PBS-washed rabbit blood [68]. CHIKV wildtype and mutators were fed to both Ae. albopictus and Ae. aegypti, and SINV wildtype and mutator 482G were fed to Ae. aegypti. The blood meals were warmed to 37°C and presented to 10 day-old females in membrane feeders, and engorged mosquitoes were incubated for 7 days. Seven days post infection, mosquitoes were dissected to obtain legs and wings, and saliva was obtained by in vitro transmission assay; in brief, mosquitoes were salivated for 30–45 min by placing the proboscis in a pipette tip containing FBS. Following salivation, bodies were frozen. To confirm ingestion, a sample of engorged mosquitoes was immediately homogenized at time 0. Samples were homogenized as described for mouse tissues, RNA was extracted, and qRT-PCR was performed. A standard curve was generated using serial dilutions of a CHIKV bloodmeal of known titer.
Fruit fly infections
Drosophila melanogaster flies (strain w1118) were reared on standard medium at 25°C. Three - to four-day-old female flies were injected with 50 nL of a virus dilution containing 400 pfu in 10 mM Tris-HCl (pH 7.5) using a Drummond nanoject injector as previously described [69]. Fly mortality at day 1 was attributed to damage produced by the injection, and these flies were excluded from further analyses. Mortality was monitored daily for 10 days, and every 3–4 days flies were transferred to fresh vials. In all experiments, 30–60 flies per genotype group were injected. Homogenates of individual flies were titrated on by plaque assay on Vero cells, as described above.
Statistical tests
All experiments were performed in triplicate unless noted otherwise. Statistics, noted where applied, were performed in Microsoft Excel and GraphPad Prism. P-values>0.05 were considered non-significant (ns).
Results
Characterization of CHIKV polymerase variants
We previously described a CHIKV antimutator variant that possessed a single amino acid change from a cysteine to a tyrosine at position 483 (C483Y) of the RNA-dependent RNA polymerase nsp4 [25]. Since Coxsackie virus B3 mutator strains are situated in a structurally analogous area, we hypothesized that this position plays important roles in modulating intrinsic CHIKV RdRp fidelity [6]. To address this, we substituted each amino acid at position 483 of the CHIKV full-length infectious clone (Table 1). After three passages in BHK-21 cells, viruses were Sanger sequenced to determine genetic stability. Of the 19 substitutions, 12 were viable and genetically stable (Table 1). This high number of viable variants indicates that position 483 has structural plasticity and can tolerate a wider range of substitutions than in previous attempts at generating fidelity variants of other RNA viruses [6], [19]. Interestingly, unstable viruses did not readily revert to wildtype, but mutated to other variants, including the antimutator form of the protein, 483Y (Table 1). The only strict biochemical requirement we observed was a necessity for uncharged residues, as all variants with charged residues (483D, E, H, K, or R) were unstable or not recoverable. In addition, we observed a general correlation between hydrophobicity of the substituted amino acid and stability or viability of the variant, where hydrophobic amino acids were preferred. Finally, as a first characterization of virus fitness, we measured the mean size of plaques. Variants 483A, G, L, N, Q, T, and W had significantly smaller plaques than wildtype (Table 1).
Identification of ribavirin sensitive variants
Because polymerase fidelity variants have altered intrinsic rates of (in)correct nucleotide incorporation, they have often been identified by their relative resistance or sensitivity to nucleoside analog RNA mutagens [14], [19], [25]. Therefore, we addressed the sensitivity of all 12 genetically stable variants to ribavirin (Table 1 and Figure 1A). Viruses were grown in the presence of either 200 µM or 400 µM ribavirin, or left untreated. We expect antimutator variants (such as 483Y) to demonstrate resistance, and mutator variants to demonstrate sensitivity when compared to wildtype. As previously described, the antimutator 483Y demonstrated significantly higher survival than wildtype (P<0.001, two-way ANOVA) as did 483M and 483N (P<0.001 for both, two-way ANOVA). Additionally, we identified several mutator candidates that were significantly more sensitive to ribavirin (483A, G, W, T, Q; P<0.05 for all, two-way ANOVA). All ribavirin-sensitive variants presented small-plaque phentoypes, as well as variant 483N (P<0.01 for all, Student's t-test). Though these variants presented small plaque phenotypes, virus stocks reached wildtype-like titers, with the exception of 483N and 483Q (Table 1).
Fig. 1. Mutagenizing position 483 variants allows isolation of mutator variants. 
(A) HeLa cells, treated with 200 µM or 400 µM ribavirin, or left untreated, were infected at an MOI of 0.1. The percentage of infectious progeny virus surviving treatment at both concentrations of ribavirin relative to the untreated control is shown (mean values ± SEM, n = 3, *P at least<0.05, two-way ANOVA with Bonferroni posttest). ND, not determined. (B) Average mutation frequencies of WT CHIKV and variants with significantly altered fidelity. Mutation frequency is shown as the mean number of mutations per 10,000 nucleotides sequenced by molecular cloning. Variants 483A, G and W made significantly more errors than WT virus (*P<0.05, **P<0.01, ***P<0.001, χ2 test). (C) Average diversity of confirmed fidelity variants at each position across the genome. The root-mean-square-deviation (RMSD) is shown (mean values ± SEM, ***P<0.001, Mann-Whitney u test). (D) Neutralization assay showing enhanced escape of mutators due to greater population diversity (mean values ± SEM, *P<0.05, **P<0.01, ***P<0.001, two-way ANOVA with Bonferroni posttest). Confirmation of mutator phenotypes by molecular clone sequencing
As observed previously for picornaviruses [5], [6], [14], the ribavirin-resistant and -sensitive phenotypes of these CHIKV variants suggested altered polymerase fidelity. To address this further in a genetic assay, we estimated the mutation frequencies of each variant that demonstrated significantly altered ribavirin sensitivity at either concentration of ribavirin. Viral RNA from the supernatants of BHK-21 cells was extracted, and an approximately 800 nucleotide fragment of the E1 genome was amplified by RT-PCR and TOPO cloned as previously described [61]. We sequenced approximately 150 individual clones per viral population (corresponding to an average of 122,200 nucleotides) to calculate the mutation frequencies (Figure 1B and Table 1). Since previous studies with 483Y required >350 clones per population to distinguish more subtle differences in mutation frequencies [25], we could only statistically confirm the altered fidelities of three mutator strains (483A, G and W; P<0.05, P<0.001, P<0.01, respectively, χ2 test) (Figure 1B). We excluded variants that did not exhibit significant fidelity differences compared to wildtype (483M, N, and Q). As a complementary approach, we performed deep sequencing on these same virus populations to characterize the relative diversity in these virus populations. In accordance with the mutation frequency data, the mean variance across the whole genome was significantly lower for the antimutator 483Y variant (P = 0.0006, Mann-Whitney u test) and significantly higher for the 483A, G and W mutator variants, compared to wildtype virus (P<0.0001 for all, Mann-Whitney u test; Figure 1C).
Chikungunya mutator strains have normal replication and reduced specific infectivity in mammalian cells
Next, we examined growth of these variants in mammalian cells. As seen previously, the antimutator 483Y presented no significant difference in amount of progeny virus (Figure 2A) or number of genome copies (Figure 2B). As observed with Coxsackie virus mutators, CHIKV mutator strains (483A, G, and W) generated the same or more genomes than wildtype virus (Figure 2B), but slightly fewer infectious progeny (Figure 2A). Consequently, these mutator variants have a lower specific infectivity than wildtype in mammalian cells (Figure 1C). This is consistent with previously published results showing that mutator variants make more lethally mutagenized RNA [6], [22], [28], [70].
Fig. 2. Mutators produce more RNA genomes and have lower specific infectivity than wildtype. 
Production of infectious particles determined by TCID50 (A) and genome copies determined by qRT-PCR (B) were measured in BHK-21 cells. No significant difference compared to WT was found for any variant in terms of titer. Mutators 483A, G, W tended to have greater amounts of extracellular RNA genomes at 24 hours (mean values ± SEM, n = 3, *P<0.05, **P<0.01, two-way ANOVA with Bonferroni posttest). (C) Specific infectivity (PFU/RNA genomes from A and B) of mutator strains were lower than that of WT virus (mean values ± SEM, n = 3, *P<0.05, two-way ANOVA with Bonferroni posttest). Mutator strains of chikungunya virus are attenuated in mice
Recently, low fidelity polymerase mutators of Coxsackie virus and exonuclease activity deficient mutators of coronaviruses were shown to be attenuated in mice [6], [7]. To determine whether this holds true for alphaviruses, we administered a sublethal infection of either wildtype or 483A, G and W viruses to 8-day old C57BL/6 mice. At 7 days of infection, when titers peak and virus is rapidly cleared thereafter, viral loads were determined in different compartments (muscle, blood, brain, liver). Viral loads were significantly lower for all three mutator strains in each tissue (Figure 3A). Since the in vivo mutation frequencies of mutator strains had not been previously reported, we examined the virus populations in the muscle of the wildtype - or the mutator-infected mouse that presented the median viral load. Although we cannot predict whether selection will act differently on these variants in mice to potentially skew the mutation frequencies, they remained elevated to varying degrees for the mutator strains. Interestingly, higher mutation frequencies in vivo correlated with increased attenuation (Figure 3B).
Fig. 3. Mutator variants 483A, G, and W are attenuated in mice. 
(A) Seven days post-infection, mutators have significantly fewer RNA genomes present in the muscle, blood, brain, and liver of newborn mice compared to WT (median values ± IQR are shown, n = 4, *P<0.05, **P<0.01, two-way ANOVA with Bonferroni posttest). (B) Average mutation frequencies of WT CHIKV and mutators 483A, G and W from the muscle of a mouse representing the median titer of each group. All mutators retain an elevated mutation frequency, with the most attenuated variant (C483W) making significantly more errors than WT virus (**P<0.01, χ2 test). Chikungunya mutator variants present a host-specific replication defect in mosquito cells
Because arboviruses must cycle through both vertebrate and arthropod hosts, and since mutator strains of other RNA viruses were only examined in mammalian systems [6], [7], [19], [24], we addressed viral replication in three mosquito cell lines: Ae. albopictus C6/36 cells, Ae. aegypti Aag2 cells and Ae. albopictus U4.4 cells. The replication profile for the antimutator 483Y was indistinguishable from wildtype in all conditions. On the other hand, the mutator strains 483A, G, and W presented significantly lower infectious progeny in C6/36 (P<0.001 for all, two-way ANOVA; Figure 4A), Aag2 (P<0.05 for 483A and G, two-way ANOVA; Figure 4B) and U4.4 (P<0.05 for 483A and W, P<0.01 for 483G, two-way ANOVA; Figure 4C) cells. Unexpectedly, we observed unprecedented reduction in genomic RNA released into the supernatant in all three mosquito cell types (Figure 4D–F). These results are discordant with the existing literature that found mutator polymerases synthesize RNA at faster rates than wildtype [6], in which case decreases in virus titer resulted directly from the increased mutational burden.
Fig. 4. Mutators have replication defects in Ae. albopictus- and Ae. aegypti- derived cell culture. 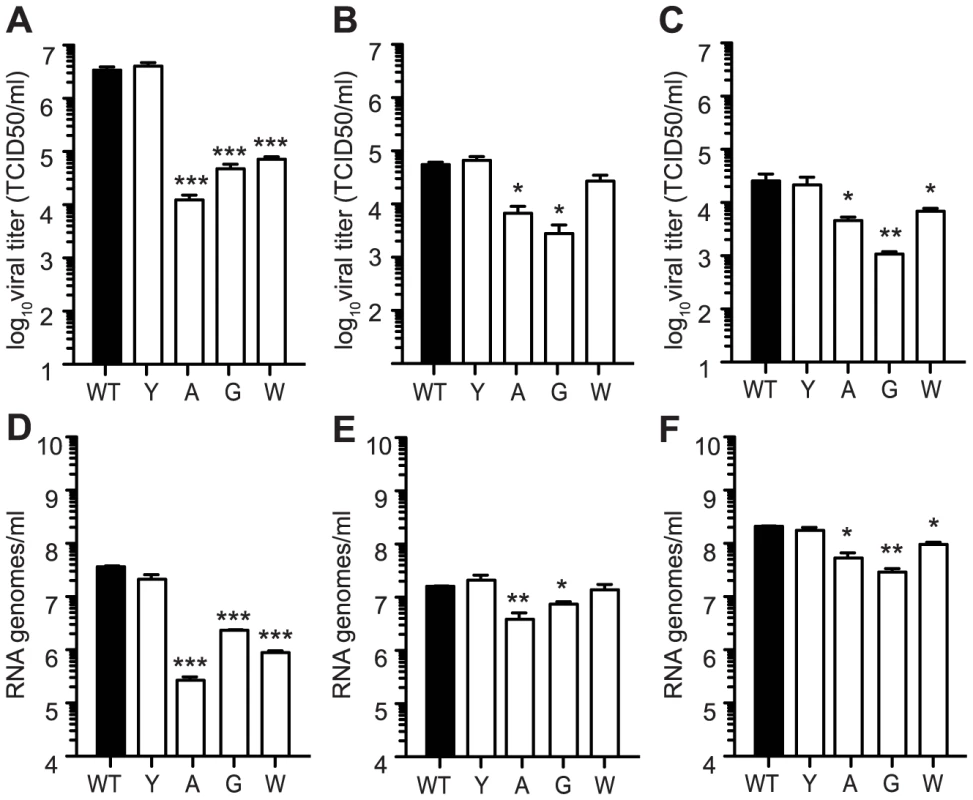
Production of infectious particles determined by TCID50 (A, B, and C) and genome copies determined by qRT-PCR (D, E, and F) were measured at 24 hours by one-step growth kinetics in C6/36 (A and D), Aag2 (B and E), and U4.4 (C and F) cells. While antimutator 483Y is similar to WT CHIKV, all mutators exhibit attenuation in terms of both titer and RNA genomes (mean values ± SEM, n = 3, *P<0.05, **P<0.01, two-way ANOVA with Bonferroni posttest). Here, the reduced viral titers obtained in mosquito cells seem to result from a host-specific replication defect, rather than the effect of lethal mutation. To further distinguish between these two effects, we examined whether mutation frequencies differed in mosquito versus mammalian cells, comparing wildtype CHIKV to the mutator strains. It is important to note that because mutator strains replicate so poorly in mosquito cells, these strains may present artificially low mutation frequencies. Unfortunately, it is not possible to uncouple replication from mutation frequency in this model. Nevertheless, the mutation frequencies of all viruses, including wildtype), were lower in C6/36 cells (Figure 5) than in BHK-21 cells (Figure 1B). Furthermore, the significant differences that existed between mutators and wildtype in mammalian cells were negated in mosquito cells, as evidenced by molecular clone sequencing (Figure 5A) and whole-genome deep sequencing (Figure 5B). We thus hypothesized that the negative fitness cost of mutator polymerases in mosquito cells is more closely linked to replication defects. To further confirm this, we generated genetically homogenous in vitro transcribed RNA corresponding to each variant, which do not present the differences in mutation frequencies of virus stocks generated in cell culture. Following transfection of mammalian BHK-21 cells, there were no significant differences in RNA synthesis (Figure 6A) or production of infectious virus (Figure 6C); however, in mosquito C6/36 cells, there was a very marked defect in replication for the mutator variants, compared to wildtype virus or the antimutator 483Y strain (Figure 6B), that correlated with the significant reduction in progeny (P<0.01 for all mutators, one-way ANOVA; Figure 6D). Similarly, no detectable infectious progeny was produced following transfection of U4.4 cells with the mutator variants (Figure 6E), further confirming the replication defect observed during infection of cells with virus stocks.
Fig. 5. Mutation frequencies of all variants are reduced in Ae. albopictus cells. 
(A) Average mutation frequencies determined by molecular clone sequencing of WT and mutator strains in C6/36. Approximately 75 clones were sequenced per variant. No significant differences were found in mutation frequencies of mutators compared to WT (χ2 test). (B) Average diversity of WT and mutator 483G at each position across the genome. There were no significant differences found between WT and 483G. The root-mean-square-deviation (RMSD) is shown (mean values ± SEM, *P<0.05, Mann-Whitney u test). Fig. 6. Mutator attenuation in mosquito cells is due to a replication defect. 
(A) BHK-21 cells or (B) C6/36 cells were transfected, and 48 hours later intracellular RNA was collected for northern blot analysis. (A) In BHK-21 cells, all mutator variants were able to synthesize subgenomic RNA (sgRNA). (B) In C6/36 cells, mutator sgRNA was undetectable or greatly reduced. Loading control shows ribosomal RNA (rRNA). Fold-difference compared to WT was calculated in Image J (n = 2; ND, not detected). Titers of virus progeny recovered from the supernatant from the transfections in (C) BHK-21 cells from A or (D) C6/36 cells from B were determined by plaque assay (mean values ± SEM, n = 2, **P<0.01, one-way ANOVA with Dunn's multiple comparison test; dotted line = limit of detection). (E) U4.4 cells were transfected and 48 hours later viral progeny from the supernatant was titered by plaque assay (mean values ± SEM, n = 2, dotted line = limit of detection). (F) Production of infectious particles determined by TCID50 was measured by one-step growth kinetics in BHK-21 cells at 28°C. No significant difference compared to WT was found for any variant at 24 hours (mean values ± SEM, n = 3, Student's t test). (G) BHK-21 cells were transfected, virus was grown at 28°C, and 48 hour later intracellular RNA was analyzed by northern blot. Fold-difference compared to WT was calculated in ImageJ (n = 2; ND, not detected). (H) Mutation frequencies were compared between WT and mutator 483G in both C6/36 and BHK-21 cells grown at 28°C. To exclude the possibility that this replication defect is the result of temperature-sensitivity rather than host-specificity, we performed infections in mammalian BHK-21 cells at 28°C (mosquito cell temperature). We observed no difference in the growth of any variant compared to wildtype (Figure 6F). In addition, we transfected mammalian cells grown at 28°C, and saw no difference in subgenomic RNA synthesis, indicating that the reduced polymerase processivity of mutators in mosquito cells is not due to reduced temperature (Figure 6B and 6G). Finally, we determined whether lower temperature could be responsible for the reduced mutation frequencies we observed in mosquito cells. In mammalian cells at 28°C, mutator 483G makes significantly more mutations than in mosquito cells at 28°C (P<0.05, χ2 test; Figure 6H). In contrast to what we observed in mosquito cells, mutator 483G also made significantly more mutations than WT (P<0.05, χ2 test; Figure 6H). These data indicate that lower temperature is responsible for neither the replication defects nor the reductions in mutation frequencies we observed in mosquito cells.
In the mosquito host, selective pressure against the replication defects of mutator strains causes reversion
Since host-specific replication defects were observed in mosquito cell culture, we hypothesized that these variants would be even more attenuated in mosquitoes than in mice. We orally infected both Aedes species CHIKV hosts (Ae. albopictus and Ae. aegypti) with a blood meal containing either wildtype or the 483A, G and W mutators. Seven days after infection, when CHIKV has reached peak titers, we quantified viral loads in bodies (infection), legs and wings (dissemination) and saliva (transmission) of individual mosquitoes (Figure 7). Surprisingly, no significant defect was observed in either Aedes species for any of the variants. To address the possibility that the fitness cost of defective replication, observed in mosquito cell culture, would favor the reversion of these mutant polymerases to wildtype, we deep sequenced virus from the body of an individual mosquito that presented the median titer from each group. Indeed, reversion to wildtype (or other replication competent variants, such as 483T or 483V) occurred in 483A (81%), 482G (93%) and 483W (39%). Whether position 483 changed to wildtype depended on the genetic distance of the mutated codon from wildtype: for example, W (TGG) reverted completely to WT (TGT), while A (GCT) reverted predominantly to a combination of V (66%; GTT) and T (ACT; 13%). Interestingly, when we examined higher passages (passage 3) of mutators in C6/36 cell culture, we also observed varying levels of reversion (ranging from less than 1% to as much as 50%), highlighting the strong selective pressure acting against this replication defect.
Fig. 7. Mutators are under strong pressure to revert in mosquito models. 
(A–C) Ae. aegypti or (D–F) Ae. albopictus were infected with WT and mutator strains of CHIKV, and mosquitoes were collected at 1, 3, 7 and 14 days post-infection. Ae. aegypti mosquitoes exhibited no significant differences in RNA genomes in (A) bodies, (B) legs and wings, or (C) saliva at 7 days post-infection. Seven days post-infection Ae. albopictus mosquitoes exhibited no differences in RNA genomes in (D) bodies with the exception of a significant but slight decrease for 483A. There were no significant differences observed in the (E) legs and wings or (F) saliva (median values ± IQR, n = 10, *P<0.001, two-way ANOVA with Bonferroni posttest). Confirmation of the role of residue 482 in Sindbis virus in altering fidelity and affecting virus fitness in vivo
After confirming that polymerase position 483 plays an important role in modulating fidelity in CHIKV, we examined if this residue is a universal fidelity determinant among the alphaviruses. Indeed, this region of the nsp4 gene containing a cysteine is conserved across the alphavirus family (Figure 8A). Thus, we generated the analogous fidelity variants (482A, G, W) in the well-studied, distantly related alphavirus Sindbis virus (SINV). Genetically stable mutants (482A and G) were screened for changes in ribavirin sensitivity. Both showed significantly higher sensitivity than wildtype SINV (for 482A, at least P<0.01, for 482G, P<0.05, two-way ANOVA; Figure 8B). Moreover, the mutation frequencies determined by molecular clone sequencing confirmed the mutator phenotypes suggested by ribavirin screening (Figure 8C): in comparison to wildtype that presented 3.3 mutations per 10,000 nucleotides, 482A presented 6.0, and 482G presented 6.9 (P<0.05, χ2 test). This confirms that this conserved residue is a general fidelity determinant for the alphaviruses.
Fig. 8. Mutagenizing SINV position C482 generates mutator strains that exhibit replication defects in mosquito cells. 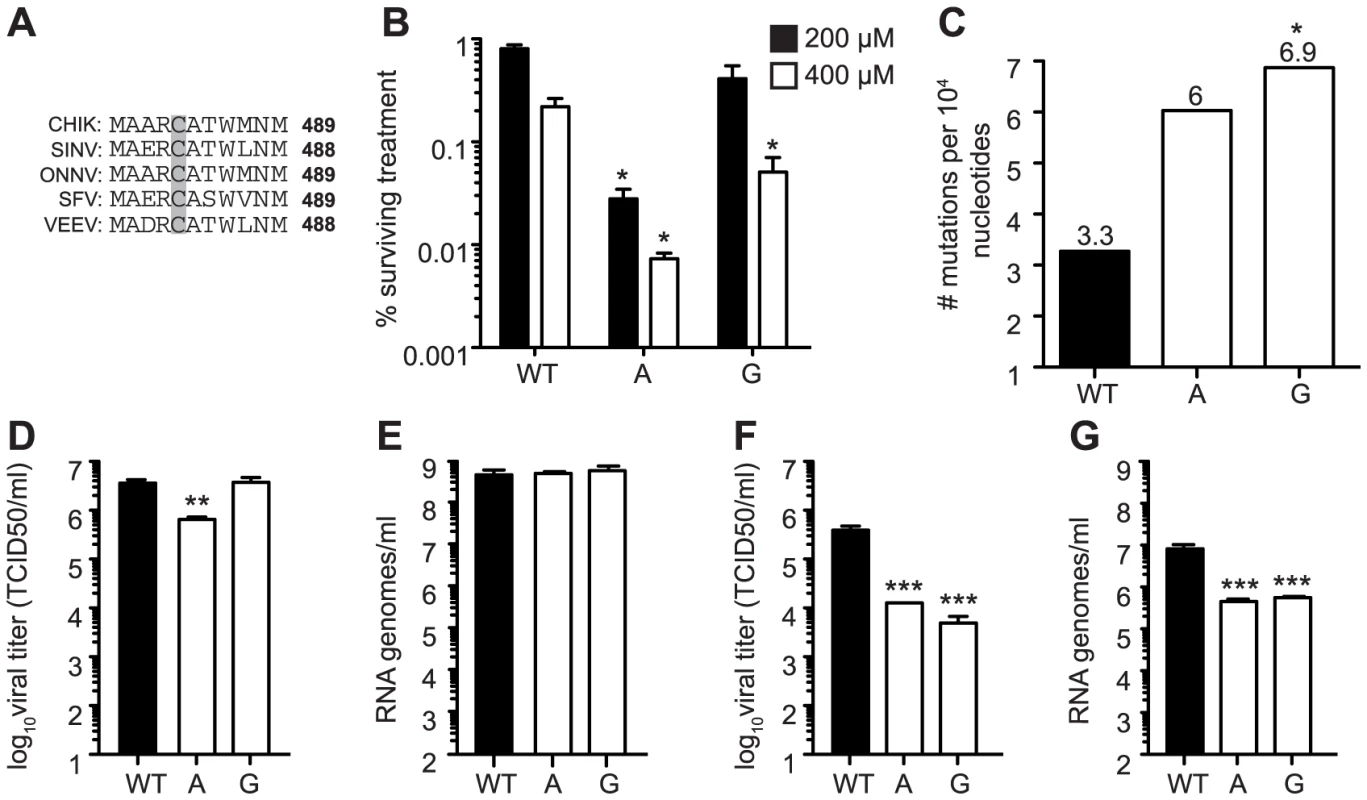
(A) Alignment of five alphaviruses at the conserved cysteine residue at position 482/483 (SINV/CHIKV) in CHIKV, SINV, Semliki forest virus (SFV), O'nyong nyong virus (ONNV), and Venezuelan equine encephalitis virus (VEEV). (B) Ribavirin sensitivity of potential SINV mutators. HeLa cells, treated with 200 µM or 400 µM ribavirin, or left untreated, were infected at an MOI of 0.1. The percentage of infectious progeny virus surviving treatment at both concentrations of ribavirin relative to the untreated control is shown (mean values ± SEM, n = 3, *P at least<0.05, two-way ANOVA with Bonferroni posttest). (C) Average mutation frequencies determined by molecular clone sequencing of WT SINV and variants 482A and G. Approximately 75 clones were sequenced per variant (*P<0.05, χ2 test). (D–G) Viral titers determined by TCID50 (D and F) and RNA genomes determined by qRT-PCR (E and G) of SINV mutators in (D and E) BHK-21 cells and (F and G) Ae. albopictus C6/36 cells. Mutator variants exhibit significant defects in C6/36 but these defects are reduced in BHK-21 cells (mean values ± SEM, n = 3, **P<0.01, ***P<0.001, two-way ANOVA with Bonferroni posttest). We next addressed whether replication defects also existed for these SINV mutators. In mammalian BHK-21 cells, mutator variants produce near wildtype-like titers of infectious particles (Figure 8D), and the same amounts of extracellular RNA genomes (Figure 8E). Importantly, as was observed for CHIKV strains, the SINV mutators presented more significant drops in virus titers in mosquito C6/36 cells (P<0.001, two-way ANOVA; Figure 8F), that correlated with a significant decrease in extracellular RNA genomes (P<0.001, two-way ANOVA; Figure 8G). Given the similarity of in vitro, host-specific phenotypes of CHIKV and SINV mutators, we hypothesized that SINV mutators would behave as CHIKV mutators in vivo (exhibiting attenuation in a mouse model and reversion in mosquitoes). We inoculated 8-day old mice with wildtype and 482G SINV strains, and observed significantly higher survival in mice infected with the mutator (91% compared to 50% for the wildtype, P = 0.0474; Figure 9A). In addition, only 36% of mice inoculated with 482G exhibited complete hind limb paralysis, compared to 100% of mice infected with wildtype SINV (P<0.0001, χ2 test; Figure 9A). Interestingly, this reduced paralysis correlated with significantly lower titers in the brain at day 7 post-infection (P<0.05, Student's t-test), confirming the attenuation of mutator strains in mammalian models in yet another virus (Figure 9B). We next examined the in vivo phenotype of SINV mutator 482G in Ae. aegypti mosquitoes. As expected, we observed reversion of position 482G to wildtype, and therefore, no differences in titers in the mosquito host (Figure 9C–E).
Fig. 9. SINV mutator 482G is attenuated in mice but reverts in mosquitoes. 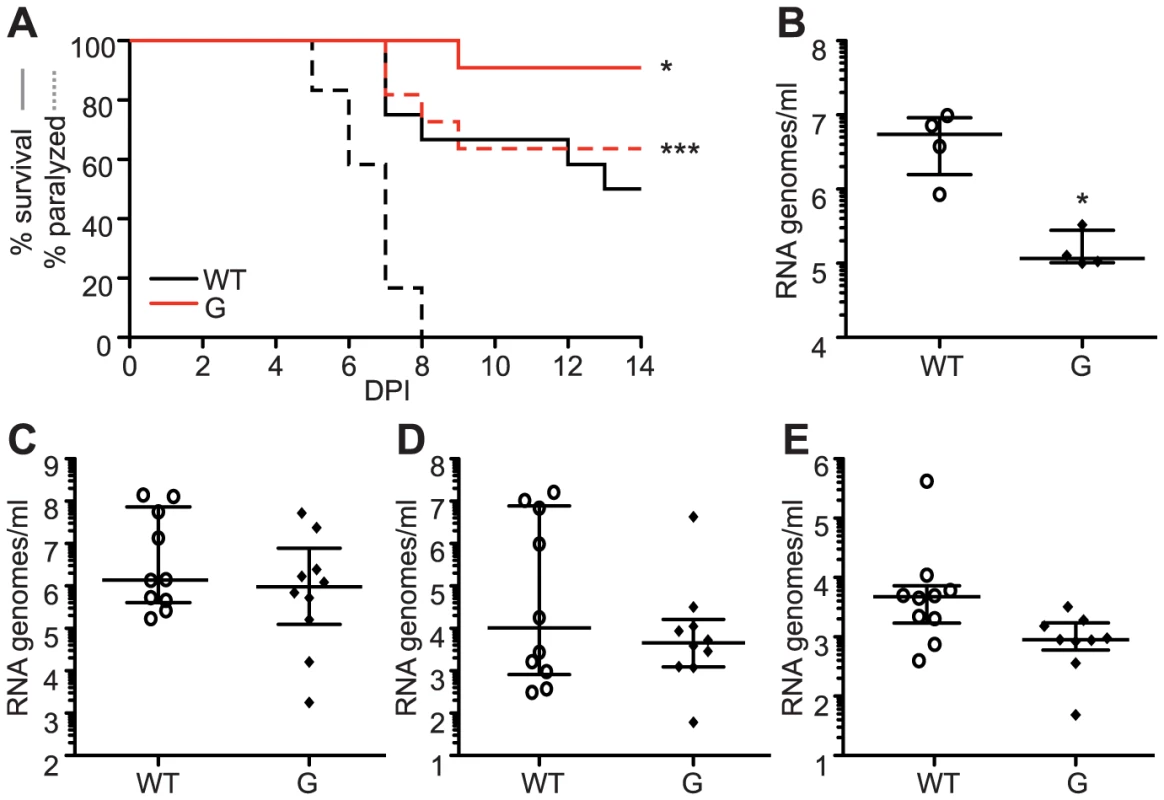
(A) Percent survival (solid line) is significantly increased and paralysis (dashed line) is significantly reduced in mice inoculated with mutator 482G compared to WT (*P<0.05, ***P<0.0001, χ2 test). (B) Seven days post-infection, mutator 482G has significantly fewer RNA genomes present in the brain of mice than WT (median values ± IQR are shown, n = 4, *P<0.05, Student's t-test). DPI, days post-nfection. (C–E) Mutator 482G reverts in mosquitoes, exhibiting similar amounts of viral genomes as WT in the (C) bodies, (D) legs and wings, and (E) saliva (median values ± IQR, n = 10, Student's t-test). Since SINV has a broader host range than CHIKV, we examined whether the replication defect was mosquito cell-specific, or more general to insects, by infecting Drosophila S2 cells. Interestingly, the mutator strains were replication competent, generating virus titers (Figure 10A) and RNA genome copies (Figure 10B) at levels comparable to wildtype virus. Finally, we injected Drosophila with SINV wildtype and 482G mutator and followed the kinetics of infection by titering virus in flies for seven days post-infection. In contrast to CHIKV and SINV mutators in mosquitoes, when Drosophila flies were infected with wildtype and mutator strains of SINV, mutator 482G presented significantly lower titers than wildtype on day 3 and 5 (P<0.01, Student's t-test; Figure 10C). Sequencing of virus from 482G-infected flies at day 3 and 5 confirmed that no reversion had occurred. These results indicate that in principle, mutators can be attenuated in insects.
Fig. 10. SINV mutator 482G is attenuated in fruit flies. 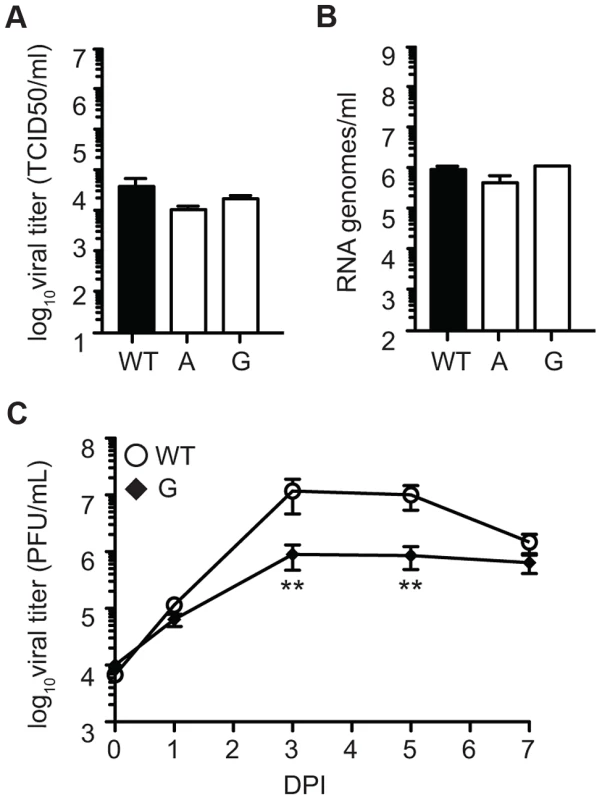
Viral titers determined by TCID50 (A) and RNA genomes determined by qRT-PCR (B) in D. melanogaster S2 cells. No significant differences are found in replication of mutator 482G compared to WT (mean values ± SEM, n = 3, two-way ANOVA with Bonferroni posttest). (C) Fruit flies were injected with either WT or mutator 482G and titers were monitored by plaque assay over the course of seven days. At days three and five, mutator 482G had significantly lower titers than WT (mean values ± SEM, n = 9, *P<0.05, Student's t test). DPI, days post-infection. Discussion
Previous work on antimutator CHIKV 483Y suggested this residue could be important for determining intrinsic RdRp fidelity [25]. Although there is no crystal structure available for an alphavirus RdRp, structural models predict that position 483 is located in the same area of the RdRp that generated Coxsackie virus RdRp fidelity variants (Figure S1) [6]. By substituting all other amino acids at this position, more mutator variants were isolated than antimutators, consistent with variants obtained for Coxsackie virus and with variants identified by characterizing the mutation frequencies of previously published reverse transcriptase variants for HIV [6], [32]. To date, all viable RdRp fidelity variants present error rates that remain within the same order of magnitude as their wildtype counterpart [5], [6], [14], [25], [71], [72]. Interestingly, although biochemical assays using purified RdRp of picornaviruses indicate that altering fidelity beyond an order of magnitude is enzymatically possible, these viruses are not viable [73]. Together, these studies suggest that within this viable range, wildtype fidelity sits closer to higher fidelity than lower fidelity. This may be further reflection of how RNA viruses are considered to exist close to a maximum threshold of error [74]. In support of this, in conditions where this reversion does not occur, mutator CHIKV variants present more significant fitness defects in vivo (Figure 3) than antimutator virus [25].
A review of the antimutator and mutator RdRp variant literature in virology reveals the following trends: antimutator strains tend to generate less RNA in vitro, but have higher specific infectivity, and have only been reported to lose fitness in vivo or in competition assays (Figures 2 and 4, and [16], [19], [25]); while mutators generate more RNA in vitro, but of lower specific infectivity, with more prominent fitness defects in mice [6]. Accordingly, CHIKV mutators showed congruous trends, exhibiting no significant replication defects in BHK-21 cells, but showing marked attenuation in the mouse model. Importantly, we showed that the mutator status of these variants (higher mutation frequencies) was maintained in the mouse model at the primary site of CHIKV replication and was likely responsible for the observed attenuation (Figure 3).
However, when we examined CHIKV mutators in the invertebrate host, the previous trends for how mutators behave was reversed. First, the differences in mutation frequencies between wildtype and mutator strains became virtually indistinguishable in mosquito cells (Figure 5), although it is difficult to draw clear-cut conclusions given the reduced replication rate of mutators. For all viruses, mutation frequency was lower in mosquito cells compared to mammalian cells (Figure 1). The role that these differences may play in arbovirus evolvability and fitness remain contradictory. Our observations corroborate previous observations in alphaviruses that inter-host cycling slows adaptation [33], [35], [75]; while flavivirus studies report that diversity is maintained in the mosquito host [38], [41], [76], [77], [78], [79], [80]. Second, and contrary to expectations, we observed a severe replication defect in three different mosquito cell cultures (Figure 4), which had never been observed for RdRp fidelity variants in mammalian cell culture. The lower titers of infectious progeny were not the result of accumulation of detrimental mutations as was observed for mutators in mammalian hosts; rather, there was a direct defect in genomic RNA synthesis in mosquito cells (Figure 4 and 6). Interestingly, similar host-specific replication defects were observed for RdRp mutants of West Nile virus (although it is unclear if these variants have altered fidelity). While differences in host temperature do not seem to be the cause, the cellular host factors implicated or missing in these host cell lines remain to be elucidated. Finally, we could not address whether mutators were attenuated in vivo in mosquitoes; sequencing of virus populations from mosquitoes revealed partial or total reversion of the fidelity-altering residues at position 483. Although one could expect a variant with severe replication defects to be highly attenuated, it is possible that when coupled to a mutator phenotype, reversion would more quickly and favorably occur when the pressure to increase replication remains, as is the case in mosquitoes that are persistently infected. Whether this defect is general to all mutators in mosquitoes, or whether only amino acids A, G, and W at position 483 bear this curiously coupled mutator/replication effect remains to be seen (since not all variants at this position were defective, as 483Y has no replication defects in mosquito cells [25]). Isolation of additional arbovirus mutators mapping to other residues in the polymerase should resolve this issue.
Since the cysteine at position 483 is conserved in the alphavirus genus, we obtained additional arbovirus mutators in Sindbis virus. SINV mutators also showed severe replication defects in mosquito cells, and SINV mutator 482G exhibited the same phenotypes we previously observed in CHIKV mutators in both mice and mosquitoes. However, the wider host range of SINV allowed us to test whether these replication defects occur across all insects or if they were mosquito-specific [81], [82]. In S2 cells, mutators did not present replication defects, allowing us to test, in principle, whether mutators could be attenuated in an insect model (Drosophila flies). Indeed, in the absence of any in vitro replication defect and resulting pressure to revert, the mutator strain was attenuated in fruit flies. Thus, our results confirm that arbovirus mutators can, in principle, be attenuated in insects.
Since the isolation of the first antimutator variant of a RNA virus, the growing body of literature shows that either increasing or decreasing replication fidelity has detrimental effects to virus fitness [6], [7], [16], [19], [24], [25]. However, how mutation rates and replication capacity are coupled will require more study, and the degree of attenuation resulting from altering these biochemical properties needs to be more carefully evaluated. A future challenge will be to quantitatively link the measurements of mutation frequencies (average mutations per nucleotide sequenced) performed in this work to actual mutation rates (average mutations per nucleotide site per replication) [2], [83] and to in vitro biochemical fidelity (rates of incorporation of correct and incorrect nucleotides in absence of selection)[6], [8], [73], [84], [85], [86], [87]. It is possible that the higher mutation frequencies measured for these alphavirus mutator strains are partly skewed by their producing more RNA genomes in shorter replication cycles and thus accumulating mutations more rapidly, rather than incorporating more errors per genome during each replication cycle. Indeed, biochemical studies of single-nucleotide incorporation by other mutator polymerases confirm that mutators are both faster enzymes and have higher frequency of mis-incorporation events per replication. In absence of a biochemical assay for alphaviruses, new technologies using microfluidic single-cell analysis of virus strains during single replication cycles should help correlate mutation frequencies, mutation rates, and enzyme fidelity with more confidence.
Recent studies have proposed both antimutator and mutator strains as candidates for rationally designed live attenuated vaccines [6], [7], [19], [25], [71]. Overall, fidelity variants present attenuated titers in vivo that range from one to several orders of magnitude lower than wildtype virus. Whether this degree of attenuation is sufficient to elicit protective immunity without causing disease will require more careful evaluation in more relevant animal models, as virtually all work has been performed in mice using viruses that are often not natural mouse pathogens. In vitro systems and artificial hosts may alter many of the selective pressures to which a virus would be subjected in a natural host [88], [89], [90], [91]. The present study and other work highlight that intrinsic fidelity and the mutant spectrum are labile and subject to stringent and disparate selective pressures in different hosts [34], [35], [75], [76], [79], [82], [92], [93]. A more comprehensive understanding of the selective pressures in natural hosts is crucial to predicting how viruses will behave in vivo, and essential to evaluating the feasibility of using fidelity variants as vaccines, whether stand-alone or coupled with other, conventional attenuating mutations. Despite the necessity for further research, from a vaccine development perspective these data support that in principle, mutators can be attenuated in a wider range of hosts and may be viable candidates for live-attenuated vaccines.
Supporting Information
Zdroje
1. EigenM (1993) Viral quasispecies. Sci Am 269 : 42–49.
2. DrakeJW, HollandJJ (1999) Mutation rates among RNA viruses. Proc Natl Acad Sci U S A 96 : 13910–13913.
3. HollandJJ, De La TorreJC, SteinhauerDA (1992) RNA virus populations as quasispecies. Curr Top Microbiol Immunol 176 : 1–20.
4. LauringAS, AndinoR (2010) Quasispecies theory and the behavior of RNA viruses. PLoS Pathog 6: e1001005.
5. PfeifferJK, KirkegaardK (2003) A single mutation in poliovirus RNA-dependent RNA polymerase confers resistance to mutagenic nucleotide analogs via increased fidelity. Proc Natl Acad Sci U S A 100 : 7289–7294.
6. GnadigNF, BeaucourtS, CampagnolaG, BorderiaAV, Sanz-RamosM, et al. (2012) Coxsackievirus B3 mutator strains are attenuated in vivo. Proc Natl Acad Sci U S A 109: E2294–2303.
7. GrahamRL, BeckerMM, EckerleLD, BollesM, DenisonMR, et al. (2012) A live, impaired-fidelity coronavirus vaccine protects in an aged, immunocompromised mouse model of lethal disease. Nat Med 18 : 1820–1826.
8. ArnoldJJ, VignuzziM, StoneJK, AndinoR, CameronCE (2005) Remote site control of an active site fidelity checkpoint in a viral RNA-dependent RNA polymerase. J Biol Chem 280 : 25706–25716.
9. Ferrer-OrtaC, SierraM, AgudoR, de la HigueraI, AriasA, et al. (2010) Structure of foot-and-mouth disease virus mutant polymerases with reduced sensitivity to ribavirin. J Virol 84 : 6188–6199.
10. ManskyLM, CunninghamKS (2000) Virus mutators and antimutators: roles in evolution, pathogenesis and emergence. Trends Genet 16 : 512–517.
11. SpeyerJF (1965) Mutagenic DNA polymerase. Biochem Biophys Res Commun 21 : 6–8.
12. DrakeJW, AllenEF, ForsbergSA, PreparataRM, GreeningEO (1969) Genetic control of mutation rates in bacteriophageT4. Nature 221 : 1128–1132.
13. SuarezP, ValcarcelJ, OrtinJ (1992) Heterogeneity of the mutation rates of influenza A viruses: isolation of mutator mutants. J Virol 66 : 2491–2494.
14. SadeghipourS, BekEJ, McMinnPC (2013) Ribavirin-resistant mutants of human enterovirus 71 express a high replication fidelity phenotype during growth in cell culture. J Virol 87 : 1759–1769.
15. FurioV, MoyaA, SanjuanR (2007) The cost of replication fidelity in human immunodeficiency virus type 1. Proc Biol Sci 274 : 225–230.
16. FurioV, MoyaA, SanjuanR (2005) The cost of replication fidelity in an RNA virus. Proc Natl Acad Sci U S A 102 : 10233–10237.
17. WeeksSA, LeeCA, ZhaoY, SmidanskyED, AugustA, et al. (2012) A Polymerase mechanism-based strategy for viral attenuation and vaccine development. J Biol Chem 287 : 31618–31622.
18. GongP, PeersenOB (2010) Structural basis for active site closure by the poliovirus RNA-dependent RNA polymerase. Proc Natl Acad Sci U S A 107 : 22505–22510.
19. VignuzziM, StoneJK, ArnoldJJ, CameronCE, AndinoR (2006) Quasispecies diversity determines pathogenesis through cooperative interactions in a viral population. Nature 439 : 344–348.
20. HolmesEC (2003) Error thresholds and the constraints to RNA virus evolution. Trends Microbiol 11 : 543–546.
21. Holland EDaJJ (1994) Mutation rates and rapid evolution of RNA viruses. In: Morse SS, editor. The Evolutionary Biology of Viruses. New York: Raven Press. pp. 161–184.
22. HollandJJ, DomingoE, de la TorreJC, SteinhauerDA (1990) Mutation frequencies at defined single codon sites in vesicular stomatitis virus and poliovirus can be increased only slightly by chemical mutagenesis. J Virol 64 : 3960–3962.
23. LoebLA, EssigmannJM, KazaziF, ZhangJ, RoseKD, et al. (1999) Lethal mutagenesis of HIV with mutagenic nucleoside analogs. Proc Natl Acad Sci U S A 96 : 1492–1497.
24. PfeifferJK, KirkegaardK (2005) Increased fidelity reduces poliovirus fitness and virulence under selective pressure in mice. PLoS Pathog 1: e11.
25. CoffeyLL, BeeharryY, BorderiaAV, BlancH, VignuzziM (2011) Arbovirus high fidelity variant loses fitness in mosquitoes and mice. Proc Natl Acad Sci U S A 108 : 16038–16043.
26. SchultzU, FitchWM, LudwigS, MandlerJ, ScholtissekC (1991) Evolution of pig influenza viruses. Virology 183 : 61–73.
27. VignuzziM, StoneJK, AndinoR (2005) Ribavirin and lethal mutagenesis of poliovirus: molecular mechanisms, resistance and biological implications. Virus Res 107 : 173–181.
28. AndersonJP, DaifukuR, LoebLA (2004) Viral error catastrophe by mutagenic nucleosides. Annu Rev Microbiol 58 : 183–205.
29. BullJJ, SanjuanR, WilkeCO (2007) Theory of lethal mutagenesis for viruses. J Virol 81 : 2930–2939.
30. GraciJD, CameronCE (2008) Therapeutically targeting RNA viruses via lethal mutagenesis. Future Virol 3 : 553–566.
31. DappMJ, PattersonSE, ManskyLM (2012) Back to the future: revisiting HIV-1 lethal mutagenesis. Trends Microbiol 21 : 56–62.
32. DappMJ, HeinemanRH, ManskyLM (2013) Interrelationship between HIV-1 fitness and mutation rate. J Mol Biol 425 : 41–53.
33. CoffeyLL, VignuzziM (2011) Host alternation of chikungunya virus increases fitness while restricting population diversity and adaptability to novel selective pressures. J Virol 85 : 1025–1035.
34. CoffeyLL, ForresterN, TsetsarkinK, VasilakisN, WeaverSC (2013) Factors shaping the adaptive landscape for arboviruses: implications for the emergence of disease. Future Microbiol 8 : 155–176.
35. CoffeyLL, VasilakisN, BraultAC, PowersAM, TripetF, et al. (2008) Arbovirus evolution in vivo is constrained by host alternation. Proc Natl Acad Sci U S A 105 : 6970–6975.
36. CiotaAT, LovelaceAO, JiaY, DavisLJ, YoungDS, et al. (2008) Characterization of mosquito-adapted West Nile virus. J Gen Virol 89 : 1633–1642.
37. GreeneIP, WangE, DeardorffER, MilleronR, DomingoE, et al. (2005) Effect of alternating passage on adaptation of sindbis virus to vertebrate and invertebrate cells. J Virol 79 : 14253–14260.
38. DeardorffER, FitzpatrickKA, JerzakGV, ShiPY, KramerLD, et al. (2011) West Nile virus experimental evolution in vivo and the trade-off hypothesis. PLoS Pathog 7: e1002335.
39. ForresterNL, GuerboisM, SeymourRL, SprattH, WeaverSC (2012) Vector-borne transmission imposes a severe bottleneck on an RNA virus population. PLoS Pathog 8: e1002897.
40. WeaverSC, Rico-HesseR, ScottTW (1992) Genetic diversity and slow rates of evolution in New World alphaviruses. Curr Top Microbiol Immunol 176 : 99–117.
41. CiotaAT, NgoKA, LovelaceAO, PayneAF, ZhouY, et al. (2007) Role of the mutant spectrum in adaptation and replication of West Nile virus. J Gen Virol 88 : 865–874.
42. LinSR, HsiehSC, YuehYY, LinTH, ChaoDY, et al. (2004) Study of sequence variation of dengue type 3 virus in naturally infected mosquitoes and human hosts: implications for transmission and evolution. J Virol 78 : 12717–12721.
43. JerzakG, BernardKA, KramerLD, EbelGD (2005) Genetic variation in West Nile virus from naturally infected mosquitoes and birds suggests quasispecies structure and strong purifying selection. J Gen Virol 86 : 2175–2183.
44. KurosuT (2011) Quasispecies of dengue virus. Trop Med Health 39 : 29–36.
45. CiotaAT, EhrbarDJ, Van SlykeGA, WillseyGG, KramerLD (2012) Cooperative interactions in the West Nile virus mutant swarm. BMC Evol Biol 12 : 58.
46. KarpeYA, AherPP, LoleKS (2011) NTPase and 5′-RNA triphosphatase activities of Chikungunya virus nsP2 protein. PLoS One 6: e22336.
47. RathoreAP, NgML, VasudevanSG (2013) Differential unfolded protein response during Chikungunya and Sindbis virus infection: CHIKV nsP4 suppresses eIF2alpha phosphorylation. Virol J 10 : 36.
48. MaletH, CoutardB, JamalS, DutartreH, PapageorgiouN, et al. (2009) The crystal structures of Chikungunya and Venezuelan equine encephalitis virus nsP3 macro domains define a conserved adenosine binding pocket. J Virol 83 : 6534–6545.
49. FrosJJ, DomeradzkaNE, BaggenJ, GeertsemaC, FlipseJ, et al. (2012) Chikungunya virus nsP3 blocks stress granule assembly by recruitment of G3BP into cytoplasmic foci. J Virol 86 : 10873–10879.
50. JonesPH, MaricM, MadisonMN, MauryW, RollerRJ, et al. (2013) BST-2/tetherin-mediated restriction of chikungunya (CHIKV) VLP budding is counteracted by CHIKV non-structural protein 1 (nsP1). Virology 438 : 37–49.
51. SchwartzO, AlbertML (2010) Biology and pathogenesis of chikungunya virus. Nat Rev Microbiol 8 : 491–500.
52. SolignatM, GayB, HiggsS, BriantL, DevauxC (2009) Replication cycle of chikungunya: a re-emerging arbovirus. Virology 393 : 183–197.
53. Mayuri, GedersTW, SmithJL, KuhnRJ (2008) Role for conserved residues of sindbis virus nonstructural protein 2 methyltransferase-like domain in regulation of minus-strand synthesis and development of cytopathic infection. J Virol 82 : 7284–7297.
54. LemmJA, RiceCM (1993) Roles of nonstructural polyproteins and cleavage products in regulating Sindbis virus RNA replication and transcription. J Virol 67 : 1916–1926.
55. Saxton-ShawKD, LedermannJP, BorlandEM, StovallJL, MosselEC, et al. (2013) O'nyong nyong Virus Molecular Determinants of Unique Vector Specificity Reside in Non-Structural Protein 3. PLoS Negl Trop Dis 7: e1931.
56. KimDY, FirthAE, AtashevaS, FrolovaEI, FrolovI (2011) Conservation of a packaging signal and the viral genome RNA packaging mechanism in alphavirus evolution. J Virol 85 : 8022–8036.
57. TomarS, HardyRW, SmithJL, KuhnRJ (2006) Catalytic core of alphavirus nonstructural protein nsP4 possesses terminal adenylyltransferase activity. J Virol 80 : 9962–9969.
58. CrottyS, MaagD, ArnoldJJ, ZhongW, LauJY, et al. (2000) The broad-spectrum antiviral ribonucleoside ribavirin is an RNA virus mutagen. Nat Med 6 : 1375–1379.
59. CameronCE, CastroC (2001) The mechanism of action of ribavirin: lethal mutagenesis of RNA virus genomes mediated by the viral RNA-dependent RNA polymerase. Curr Opin Infect Dis 14 : 757–764.
60. McKnightKL, SimpsonDA, LinSC, KnottTA, PoloJM, et al. (1996) Deduced consensus sequence of Sindbis virus strain AR339: mutations contained in laboratory strains which affect cell culture and in vivo phenotypes. J Virol 70 : 1981–1989.
61. BeaucourtS, BorderiaAV, CoffeyLL, GnadigNF, Sanz-RamosM, et al. (2011) Isolation of fidelity variants of RNA viruses and characterization of virus mutation frequency. J Vis Exp (52) e2953.
62. Aronesty E (2011) Command-line tools for processing biological sequencing data. Available: http://code.google.com/p/ea-utils/. Accessed 13 December 2013.
63. LiH, DurbinR (2009) Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics 25 : 1754–1760.
64. LiH, HandsakerB, WysokerA, FennellT, RuanJ, et al. (2009) The Sequence Alignment/Map format and SAMtools. Bioinformatics 25 : 2078–2079.
65. LiK, VenterE, YoosephS, StockwellTB, EckerleLD, et al. (2010) ANDES: Statistical tools for the ANalyses of DEep Sequencing. BMC Res Notes 3 : 199.
66. PalP, DowdKA, BrienJD, EdelingMA, GorlatovS, et al. (2013) Development of a highly protective combination monoclonal antibody therapy against Chikungunya virus. PLoS Pathog 9: e1003312.
67. LanciottiRS, KosoyOL, LavenJJ, PanellaAJ, VelezJO, et al. (2007) Chikungunya virus in US travelers returning from India, 2006. Emerg Infect Dis 13 : 764–767.
68. PowersAM, LogueCH (2007) Changing patterns of chikungunya virus: re-emergence of a zoonotic arbovirus. J Gen Virol 88 : 2363–2377.
69. CherryS, PerrimonN (2004) Entry is a rate-limiting step for viral infection in a Drosophila melanogaster model of pathogenesis. Nat Immunol 5 : 81–87.
70. DenisonMR, GrahamRL, DonaldsonEF, EckerleLD, BaricRS (2011) Coronaviruses: an RNA proofreading machine regulates replication fidelity and diversity. RNA Biol 8 : 270–279.
71. VignuzziM, WendtE, AndinoR (2008) Engineering attenuated virus vaccines by controlling replication fidelity. Nat Med 14 : 154–161.
72. AriasA, ArnoldJJ, SierraM, SmidanskyED, DomingoE, et al. (2008) Determinants of RNA-dependent RNA polymerase (in)fidelity revealed by kinetic analysis of the polymerase encoded by a foot-and-mouth disease virus mutant with reduced sensitivity to ribavirin. J Virol 82 : 12346–12355.
73. KorneevaVS, CameronCE (2007) Structure-function relationships of the viral RNA-dependent RNA polymerase: fidelity, replication speed, and initiation mechanism determined by a residue in the ribose-binding pocket. J Biol Chem 282 : 16135–16145.
74. CrottyS, CameronCE, AndinoR (2001) RNA virus error catastrophe: direct molecular test by using ribavirin. Proc Natl Acad Sci U S A 98 : 6895–6900.
75. VasilakisN, DeardorffER, KenneyJL, RossiSL, HanleyKA, et al. (2009) Mosquitoes put the brake on arbovirus evolution: experimental evolution reveals slower mutation accumulation in mosquito than vertebrate cells. PLoS Pathog 5: e1000467.
76. JerzakGV, BernardK, KramerLD, ShiPY, EbelGD (2007) The West Nile virus mutant spectrum is host-dependant and a determinant of mortality in mice. Virology 360 : 469–476.
77. JerzakGV, BrownI, ShiPY, KramerLD, EbelGD (2008) Genetic diversity and purifying selection in West Nile virus populations are maintained during host switching. Virology 374 : 256–260.
78. BertolottiL, KitronUD, WalkerED, RuizMO, BrawnJD, et al. (2008) Fine-scale genetic variation and evolution of West Nile Virus in a transmission “hot spot” in suburban Chicago, USA. Virology 374 : 381–389.
79. CiotaAT, LovelaceAO, JonesSA, PayneA, KramerLD (2007) Adaptation of two flaviviruses results in differences in genetic heterogeneity and virus adaptability. J Gen Virol 88 : 2398–2406.
80. BrackneyDE, PeskoKN, BrownIK, DeardorffER, KawatachiJ, et al. (2011) West Nile virus genetic diversity is maintained during transmission by Culex pipiens quinquefasciatus mosquitoes. PLoS One 6: e24466.
81. RosePP, HannaSL, SpiridigliozziA, WannissornN, BeitingDP, et al. (2011) Natural resistance-associated macrophage protein is a cellular receptor for sindbis virus in both insect and mammalian hosts. Cell Host Microbe 10 : 97–104.
82. MerklingSH, van RijRP (2013) Beyond RNAi: antiviral defense strategies in Drosophila and mosquito. J Insect Physiol 59 : 159–170.
83. SanjuanR, NebotMR, ChiricoN, ManskyLM, BelshawR (2010) Viral mutation rates. J Virol 84 : 9733–9748.
84. CastroC, ArnoldJJ, CameronCE (2005) Incorporation fidelity of the viral RNA-dependent RNA polymerase: a kinetic, thermodynamic and structural perspective. Virus Res 107 : 141–149.
85. ArnoldJJ, GhoshSK, CameronCE (1999) Poliovirus RNA-dependent RNA polymerase (3D(pol)). Divalent cation modulation of primer, template, and nucleotide selection. J Biol Chem 274 : 37060–37069.
86. FreistadtMS, VaccaroJA, EberleKE (2007) Biochemical characterization of the fidelity of poliovirus RNA-dependent RNA polymerase. Virol J 4 : 44.
87. LeviLI, GnadigNF, BeaucourtS, McPhersonMJ, BaronB, et al. (2010) Fidelity variants of RNA dependent RNA polymerases uncover an indirect, mutagenic activity of amiloride compounds. PLoS Pathog 6: e1001163.
88. KlimstraWB, RymanKD, JohnstonRE (1998) Adaptation of Sindbis virus to BHK cells selects for use of heparan sulfate as an attachment receptor. J Virol 72 : 7357–7366.
89. BernardKA, KlimstraWB, JohnstonRE (2000) Mutations in the E2 glycoprotein of Venezuelan equine encephalitis virus confer heparan sulfate interaction, low morbidity, and rapid clearance from blood of mice. Virology 276 : 93–103.
90. BrackneyDE, BeaneJE, EbelGD (2009) RNAi targeting of West Nile virus in mosquito midguts promotes virus diversification. PLoS Pathog 5: e1000502.
91. TeoTH, LumFM, LeeWW, NgLF (2012) Mouse models for Chikungunya virus: deciphering immune mechanisms responsible for disease and pathology. Immunol Res 53 : 136–147.
92. CiotaAT, JiaY, PayneAF, JerzakG, DavisLJ, et al. (2009) Experimental passage of St. Louis encephalitis virus in vivo in mosquitoes and chickens reveals evolutionarily significant virus characteristics. PLoS One 4: e7876.
93. PeskoKN, EbelGD (2012) West Nile virus population genetics and evolution. Infect Genet Evol 12 : 181–190.
94. EisenbergD, WeissRM, TerwilligerTC, WilcoxW (1982) Hydrophobic Moments and Protein-Structure. Faraday Symposia of the Chemical Society 17 : 109–120.
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek Serotonin Signaling in : A Serotonin–Activated G Protein-Coupled Receptor Controls Parasite MovementČlánek Regulators of Cell Cycle Progression and Differentiation Identified Using a Kinome-Wide RNAi ScreenČlánek IFNγ/IL-10 Co-producing Cells Dominate the CD4 Response to Malaria in Highly Exposed ChildrenČlánek Functions of CPSF6 for HIV-1 as Revealed by HIV-1 Capsid Evolution in HLA-B27-Positive SubjectsČlánek Decreases in Colonic and Systemic Inflammation in Chronic HIV Infection after IL-7 Administration
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2014 Číslo 1- Stillova choroba: vzácné a závažné systémové onemocnění
- Perorální antivirotika jako vysoce efektivní nástroj prevence hospitalizací kvůli COVID-19 − otázky a odpovědi pro praxi
- Diagnostika virových hepatitid v kostce – zorientujte se (nejen) v sérologii
- Jak souvisí postcovidový syndrom s poškozením mozku?
- Parazitičtí červi v terapii Crohnovy choroby a dalších zánětlivých autoimunitních onemocnění
-
Všechny články tohoto čísla
- HIV-1 Accessory Proteins Adapt Cellular Adaptors to Facilitate Immune Evasion
- Ranaviruses: Not Just for Frogs
- Effectors and Effector Delivery in
- Plasmacytoid Dendritic Cell Dynamics Tune Interferon-Alfa Production in SIV-Infected Cynomolgus Macaques
- Lu/BCAM Adhesion Glycoprotein Is a Receptor for Cytotoxic Necrotizing Factor 1 (CNF1)
- A Substrate-Fusion Protein Is Trapped inside the Type III Secretion System Channel in
- Parvovirus-Induced Depletion of Cyclin B1 Prevents Mitotic Entry of Infected Cells
- Red Blood Cell Invasion by : Structural Basis for DBP Engagement of DARC
- NsrR, GadE, and GadX Interplay in Repressing Expression of the O157:H7 LEE Pathogenicity Island in Response to Nitric Oxide
- Loss of Circulating CD4 T Cells with B Cell Helper Function during Chronic HIV Infection
- TREM-1 Deficiency Can Attenuate Disease Severity without Affecting Pathogen Clearance
- Origin, Migration Routes and Worldwide Population Genetic Structure of the Wheat Yellow Rust Pathogen f.sp.
- Glutamate Utilization Couples Oxidative Stress Defense and the Tricarboxylic Acid Cycle in Phagosomal Escape
- Serotonin Signaling in : A Serotonin–Activated G Protein-Coupled Receptor Controls Parasite Movement
- Recovery of an Antiviral Antibody Response following Attrition Caused by Unrelated Infection
- Regulators of Cell Cycle Progression and Differentiation Identified Using a Kinome-Wide RNAi Screen
- Absence of Intestinal PPARγ Aggravates Acute Infectious Colitis in Mice through a Lipocalin-2–Dependent Pathway
- Induction of a Stringent Metabolic Response in Intracellular Stages of Leads to Increased Dependence on Mitochondrial Metabolism
- CTCF and Rad21 Act as Host Cell Restriction Factors for Kaposi's Sarcoma-Associated Herpesvirus (KSHV) Lytic Replication by Modulating Viral Gene Transcription
- Gammaherpesviral Gene Expression and Virion Composition Are Broadly Controlled by Accelerated mRNA Degradation
- The Arabidopsis Silencing Pathway Modulates PAMP- and Effector-Triggered Immunity through the Post-transcriptional Control of Disease Resistance Genes
- Inflammatory Stimuli Reprogram Macrophage Phagocytosis to Macropinocytosis for the Rapid Elimination of Pathogens
- Alphavirus Mutator Variants Present Host-Specific Defects and Attenuation in Mammalian and Insect Models
- Phosphopyruvate Carboxylase Identified as a Key Enzyme in Erythrocytic Carbon Metabolism
- IFNγ/IL-10 Co-producing Cells Dominate the CD4 Response to Malaria in Highly Exposed Children
- Electron Tomography of HIV-1 Infection in Gut-Associated Lymphoid Tissue
- Characterisation of a Multi-ligand Binding Chemoreceptor CcmL (Tlp3) of
- Single Cell Stochastic Regulation of Pilus Phase Variation by an Attenuation-like Mechanism
- Cell Tropism Predicts Long-term Nucleotide Substitution Rates of Mammalian RNA Viruses
- Functions of CPSF6 for HIV-1 as Revealed by HIV-1 Capsid Evolution in HLA-B27-Positive Subjects
- RNA-seq Analysis of Host and Viral Gene Expression Highlights Interaction between Varicella Zoster Virus and Keratinocyte Differentiation
- Kaposi's Sarcoma Associated Herpesvirus Tegument Protein ORF75 Is Essential for Viral Lytic Replication and Plays a Critical Role in the Antagonization of ND10-Instituted Intrinsic Immunity
- DAMP Molecule S100A9 Acts as a Molecular Pattern to Enhance Inflammation during Influenza A Virus Infection: Role of DDX21-TRIF-TLR4-MyD88 Pathway
- Variable Suites of Non-effector Genes Are Co-regulated in the Type III Secretion Virulence Regulon across the Phylogeny
- Reengineering Redox Sensitive GFP to Measure Mycothiol Redox Potential of during Infection
- Preservation of Tetherin and CD4 Counter-Activities in Circulating Alleles despite Extensive Sequence Variation within HIV-1 Infected Individuals
- KSHV 2.0: A Comprehensive Annotation of the Kaposi's Sarcoma-Associated Herpesvirus Genome Using Next-Generation Sequencing Reveals Novel Genomic and Functional Features
- Nutrient Limitation Governs Metabolism and Niche Adaptation in the Human Nose
- Decreases in Colonic and Systemic Inflammation in Chronic HIV Infection after IL-7 Administration
- Investigation of Acetylcholine Receptor Diversity in a Nematode Parasite Leads to Characterization of Tribendimidine- and Derquantel-Sensitive nAChRs
- Intranasal Vaccination Promotes Detrimental Th17-Mediated Immunity against Influenza Infection
- -Mediated Inhibition of Iron Export Promotes Parasite Replication in Macrophages
- Variation in RNA Virus Mutation Rates across Host Cells
- A Single Amino Acid in the Stalk Region of the H1N1pdm Influenza Virus HA Protein Affects Viral Fusion, Stability and Infectivity
- Group B Engages an Inhibitory Siglec through Sialic Acid Mimicry to Blunt Innate Immune and Inflammatory Responses
- Synthesis and Biological Properties of Fungal Glucosylceramide
- HIV Protective KIR3DL1/S1-HLA-B Genotypes Influence NK Cell-Mediated Inhibition of HIV Replication in Autologous CD4 Targets
- Recruitment of PfSET2 by RNA Polymerase II to Variant Antigen Encoding Loci Contributes to Antigenic Variation in
- Human and Plant Fungal Pathogens: The Role of Secondary Metabolites
- Lyme Disease: Call for a “Manhattan Project” to Combat the Epidemic
- Enhancing Virus-Specific Immunity by Combining Therapeutic Vaccination and PD-L1 Blockade in Chronic Hepadnaviral Infection
- Suppression of Interferon Lambda Signaling by SOCS-1 Results in Their Excessive Production during Influenza Virus Infection
- Inflammation Fuels Colicin Ib-Dependent Competition of Serovar Typhimurium and in Blooms
- Host-Specific Enzyme-Substrate Interactions in SPM-1 Metallo-β-Lactamase Are Modulated by Second Sphere Residues
- STING-Dependent Type I IFN Production Inhibits Cell-Mediated Immunity to
- From Scourge to Cure: Tumour-Selective Viral Pathogenesis as a New Strategy against Cancer
- Lysine Acetyltransferase GCN5b Interacts with AP2 Factors and Is Required for Proliferation
- Narrow Bottlenecks Affect Populations during Vertical Seed Transmission but not during Leaf Colonization
- Targeted Cytotoxic Therapy Kills Persisting HIV Infected Cells During ART
- Murine Gammaherpesvirus M2 Protein Induction of IRF4 via the NFAT Pathway Leads to IL-10 Expression in B Cells
- iNKT Cell Production of GM-CSF Controls
- Malaria-Induced NLRP12/NLRP3-Dependent Caspase-1 Activation Mediates Inflammation and Hypersensitivity to Bacterial Superinfection
- Detection of Host-Derived Sphingosine by Is Important for Survival in the Murine Lung
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Lyme Disease: Call for a “Manhattan Project” to Combat the Epidemic
- Origin, Migration Routes and Worldwide Population Genetic Structure of the Wheat Yellow Rust Pathogen f.sp.
- IFNγ/IL-10 Co-producing Cells Dominate the CD4 Response to Malaria in Highly Exposed Children
- Human and Plant Fungal Pathogens: The Role of Secondary Metabolites
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



