-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
IFNγ/IL-10 Co-producing Cells Dominate the CD4 Response to Malaria in Highly Exposed Children
Although evidence suggests that T cells are critical for immunity to malaria, reliable T cell correlates of exposure to and protection from malaria among children living in endemic areas are lacking. We used multiparameter flow cytometry to perform a detailed functional characterization of malaria-specific T cells in 78 four-year-old children enrolled in a longitudinal cohort study in Tororo, Uganda, a highly malaria-endemic region. More than 1800 episodes of malaria were observed in this cohort, with no cases of severe malaria. We quantified production of IFNγ, TNFα, and IL-10 (alone or in combination) by malaria-specific T cells, and analyzed the relationship of this response to past and future malaria incidence. CD4+ T cell responses were measurable in nearly all children, with the majority of children having CD4+ T cells producing both IFNγ and IL-10 in response to malaria-infected red blood cells. Frequencies of IFNγ/IL10 co-producing CD4+ T cells, which express the Th1 transcription factor T-bet, were significantly higher in children with ≥2 prior episodes/year compared to children with <2 episodes/year (P<0.001) and inversely correlated with duration since malaria (Rho = −0.39, P<0.001). Notably, frequencies of IFNγ/IL10 co-producing cells were not associated with protection from future malaria after controlling for prior malaria incidence. In contrast, children with <2 prior episodes/year were significantly more likely to exhibit antigen-specific production of TNFα without IL-10 (P = 0.003). While TNFα-producing CD4+ T cells were not independently associated with future protection, the absence of cells producing this inflammatory cytokine was associated with the phenotype of asymptomatic infection. Together these data indicate that the functional phenotype of the malaria-specific T cell response is heavily influenced by malaria exposure intensity, with IFNγ/IL10 co-producing CD4+ T cells dominating this response among highly exposed children. These CD4+ T cells may play important modulatory roles in the development of antimalarial immunity.
Published in the journal: . PLoS Pathog 10(1): e32767. doi:10.1371/journal.ppat.1003864
Category: Research Article
doi: https://doi.org/10.1371/journal.ppat.1003864Summary
Although evidence suggests that T cells are critical for immunity to malaria, reliable T cell correlates of exposure to and protection from malaria among children living in endemic areas are lacking. We used multiparameter flow cytometry to perform a detailed functional characterization of malaria-specific T cells in 78 four-year-old children enrolled in a longitudinal cohort study in Tororo, Uganda, a highly malaria-endemic region. More than 1800 episodes of malaria were observed in this cohort, with no cases of severe malaria. We quantified production of IFNγ, TNFα, and IL-10 (alone or in combination) by malaria-specific T cells, and analyzed the relationship of this response to past and future malaria incidence. CD4+ T cell responses were measurable in nearly all children, with the majority of children having CD4+ T cells producing both IFNγ and IL-10 in response to malaria-infected red blood cells. Frequencies of IFNγ/IL10 co-producing CD4+ T cells, which express the Th1 transcription factor T-bet, were significantly higher in children with ≥2 prior episodes/year compared to children with <2 episodes/year (P<0.001) and inversely correlated with duration since malaria (Rho = −0.39, P<0.001). Notably, frequencies of IFNγ/IL10 co-producing cells were not associated with protection from future malaria after controlling for prior malaria incidence. In contrast, children with <2 prior episodes/year were significantly more likely to exhibit antigen-specific production of TNFα without IL-10 (P = 0.003). While TNFα-producing CD4+ T cells were not independently associated with future protection, the absence of cells producing this inflammatory cytokine was associated with the phenotype of asymptomatic infection. Together these data indicate that the functional phenotype of the malaria-specific T cell response is heavily influenced by malaria exposure intensity, with IFNγ/IL10 co-producing CD4+ T cells dominating this response among highly exposed children. These CD4+ T cells may play important modulatory roles in the development of antimalarial immunity.
Introduction
Clinical immunity to malaria eventually develops in endemic populations, but only after repeated infections with significant morbidity to both individuals and their communities [1]. Studies in regions of high malaria transmission intensity have consistently shown that the incidence of severe disease decreases considerably after the first years of life, but sterile immunity (i.e. protection against parasitemia) develops rarely if ever [2], [3]. Moreover, previously immune individuals may lose protection against symptomatic infection in the absence of continuous exposure [4], [5]. The reasons underlying the slow acquisition of clinical immunity and the failure to develop sterilizing immunity are unclear, but may include parasite diversity and evasion [6], age-related differences in immune responses [7]–[12], and/or host immunoregulatory mechanisms induced by the parasite [13]–[19]. As the incidence of malaria continues to be high in many parts of Africa despite insecticide-treated bednets and artemisinin-based combination therapy [20]–[22], there is a tremendous need to better understand mechanisms of immunity to malaria in naturally exposed populations. The identification of immunologic correlates of exposure and protection in naturally exposed children would significantly help with the rational design of vaccines and other malaria control interventions.
Both CD4+ and CD8+ T cells have been demonstrated to play an important role in protective antimalarial immunity in mouse models [23]–[30], and experimental challenge models in humans and mice strongly suggest that malaria-specific T cells contribute to protective immunity [31]–[36]. However, the identification of T cell correlates of immunity in field-based studies of naturally exposed humans has proven to be quite challenging. Prior studies employing cross-sectional or prospective cohort designs have found associations between cellular immune responses and protection from future malaria, including IFNγ responses to liver stage [37]–[40] and/or merozoite stage malaria antigens [41]–[44]. However, such studies may be confounded by the level of exposure to malaria-infected mosquitoes, which varies greatly within populations, leading subjects with lower exposure to be miscategorized as “protected” [45], [46]. Because naturally acquired immunity confers relative rather than absolute protection – manifested by a gradual decline in the incidence of clinical disease - careful quantitative outcome measures are essential, but few population-based studies of natural immunity have included careful measurement of malaria incidence over time.
Pathogen-specific T cells exhibit notable functional heterogeneity, largely dependent on the antigen and cytokine microenvironment encountered during activation, and measurement of a single parameter of T cell function (i.e. IFNγ production) may overlook others that are more critical for protection [47]. In other parasitic infections such as leishmania [48], [49] and toxoplasma [50], the functional phenotype of the CD4+ T cell response correlates with the success or failure to clear the pathogen. Recent observations in individuals naturally exposed to malaria suggest an important role for CD4+ T cell production of TNFα, with or without IFNγ, as a potential immunologic correlate of protection [51]. Conversely, CD4+ T cell production of the regulatory cytokine IL-10 has been implicated in modulating the severity of disease [18], [52] and may interfere with the development of protective immunity [14], [42], [53]. The role of these inflammatory and regulatory cytokines in mediating protective immunity in naturally exposed children, and in determining the balance between immunopathology and chronic repeated infection, remains unknown.
In this study we performed a detailed functional characterization of malaria-specific T cell responses among four-year-old children residing in a highly malaria-endemic region to determine whether naturally acquired T cell responses correlate with exposure to and/or protection from malaria. We hypothesized that CD4+ T cells producing the pro-inflammatory cytokines IFNγ and/or TNFα are associated with protection from malaria, and that T cell production of the regulatory cytokine IL-10 may interfere with the acquisition of protection. Our results suggest that the functional phenotype of the malaria-specific T cell response was heavily influenced by prior malaria exposure intensity, with CD4+ T cells co-producing IFNγ and IL10 dominating this response among highly exposed children. However, these IFNγ/IL-10 co-producing cells were not independently associated with protection from future malaria, and may be associated with increased risk.
Results
Study population and clinical outcomes
The study cohort consisted of 78 HIV-uninfected children followed from infancy through 5 years of age (Table 1). Blood for this study was drawn at four years of age (range 49–51 months), and 92% of children continued to be followed through 5 years of age. A total of 1855 incident cases of malaria were observed in this cohort through 5 years of age. All children were treated promptly with artemisinin-based combination therapy, and despite the strikingly high numbers of malaria episodes, only 4 cases of malaria were deemed “complicated” (all based on a single convulsion). No cases of severe malaria (including severe anemia) were observed. Among children with a lower prior incidence of malaria (<2 episodes per person year (ppy) between 1 and 4 years of age, n = 10), 90% lived in town; whereas among children with higher prior malaria incidence (> = 2 episodes ppy, n = 68), only 7% of children lived in town. This suggests that children with the lowest prior incidence had less exposure to malaria-infected mosquitoes. Episodes of asymptomatic parasitemia were rare in this cohort (median 1 episode per subject over the entire study period, IQR 0–4, Table 1) and the incidence of malaria declined only slightly in the year following the blood draw (from 5.7 to 5.1 episodes ppy), suggesting that effective clinical immunity had not yet emerged in most children. One child had symptomatic malaria (parasitemia with a fever requiring treatment) at the time of the blood draw, and 17 (22%) had blood smears demonstrating parasitemia.
Tab. 1. Descriptive statistics of study cohort. 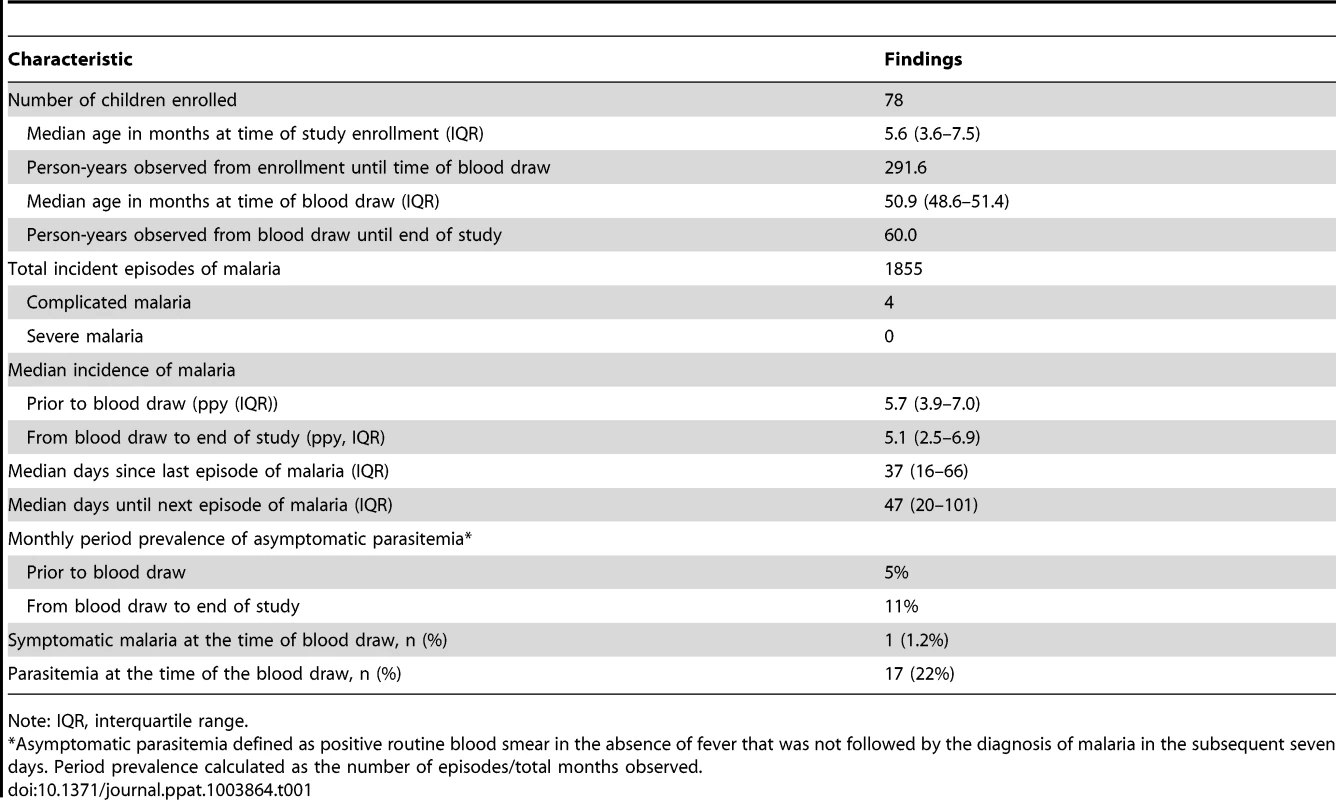
Note: IQR, interquartile range. The functional phenotype of malaria-specific CD4+ T cells is influenced by prior malaria incidence
To define the frequency and function of malaria-specific T cell responses, PBMC were stimulated with malaria-infected red blood cells (iRBC) and analyzed by flow cytometry for production of IFNγ, IL-10, and TNFα (Fig. 1a). The median frequency of malaria-specific CD4+ T cell responses producing any of these cytokines, alone or in combination, was 0.20% (IQR 0.12%–0.35%). Among all children, frequencies of CD4+ T cells producing IFNγ (median 0.16%) and IL-10 (median 0.14%) were significantly higher than those producing TNFα (median 0.04%, P<0.001, Fig. 1b). Production of these two cytokines largely overlapped, with a median of 83% of IL-10-producing cells also making IFNγ, and a median of 71% of IFNγ-producing cells also making IL-10. Malaria-specific production of IL-2 was tested in a subset of children (n = 44), but responses were consistently of low magnitude (median frequency 0.02%, data not shown). At the time of the assay 17 of the 78 children had positive blood smears; however there was no significant difference in the overall frequency of malaria-specific IFNγ+ (P = 0.20), TNFα+ (P = 0.29), or IL-10+ (P = 0.21) CD4+ T cells between children with or without parasitemia. Malaria-specific CD8 T cell responses were not observed in the peripheral blood of any of the 78 children, although this does not exclude their presence in the liver and other tissues as demonstrated by non-human primate studies [54].
Fig. 1. T cell responses to malaria-infected red blood cells using multiparameter flow cytometry. 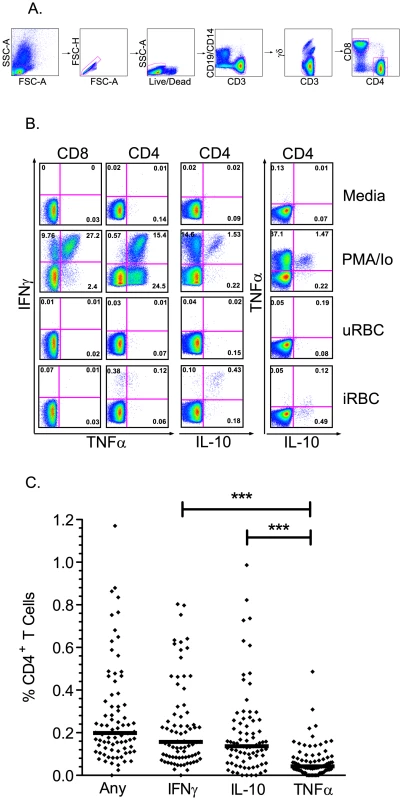
A. Gating strategy to identify live CD3+ γδ− T cells. B. Intracellular cytokine assay demonstrating the T cell response of one representative malaria-exposed child to Pf-infected RBC (iRBC; bottom row), with negative controls (uRBC and media) and positive control (PMA/Io) shown in rows above. Shown are CD8 (first column) and CD4 (right 3 columns) production of IFNγ (y-axis, columns 1–3), TNFα (x-axis, columns 1–2; y-axis, column 4), and IL-10 (x-axis, column 3–4). C. The overall malaria-specific CD4+ T cell response (left column) is followed by the overall frequency of CD4+ T cells producing IFNγ, IL-10, and TNFα in all participants (n = 78, horizontal black lines indicate the median response for each group, *** P<0.001, Wilcoxon Rank-Sum). The pattern of cytokine production by malaria-specific CD4+ T cells was noted to differ markedly based on children's prior incidence of malaria (Fig. 2a–c). Both IL-10-producing CD4+ T cells and IFNγ-producing CD4+ T cells were present at higher frequencies among children with a higher prior incidence of malaria (≥2 episodes ppy) than among those with a lower prior incidence (<2 episodes ppy, P<0.001 and P = 0.02, respectively, Fig. 2a). Most strikingly, CD4+ T cells co-producing IFNγ and IL-10 dominated the response among children with higher prior incidence, but were virtually absent among lower incidence children (P<0.001, Fig. 2b). Production of TNFα followed the opposite pattern, with higher frequencies of TNFα+/IL10− CD4+ T cells observed among children with lower prior incidence than among those with a higher prior incidence (P = 0.003, Fig. 2b). Interestingly, despite these differences in cytokine production profiles, the overall frequency of malaria-specific CD4+ T cells (i.e. those producing any cytokine) did not statistically differ between the higher and lower incidence groups (P = 0.13).
Fig. 2. Prior malaria incidence influences function of malaria-specific CD4+ T cell response. 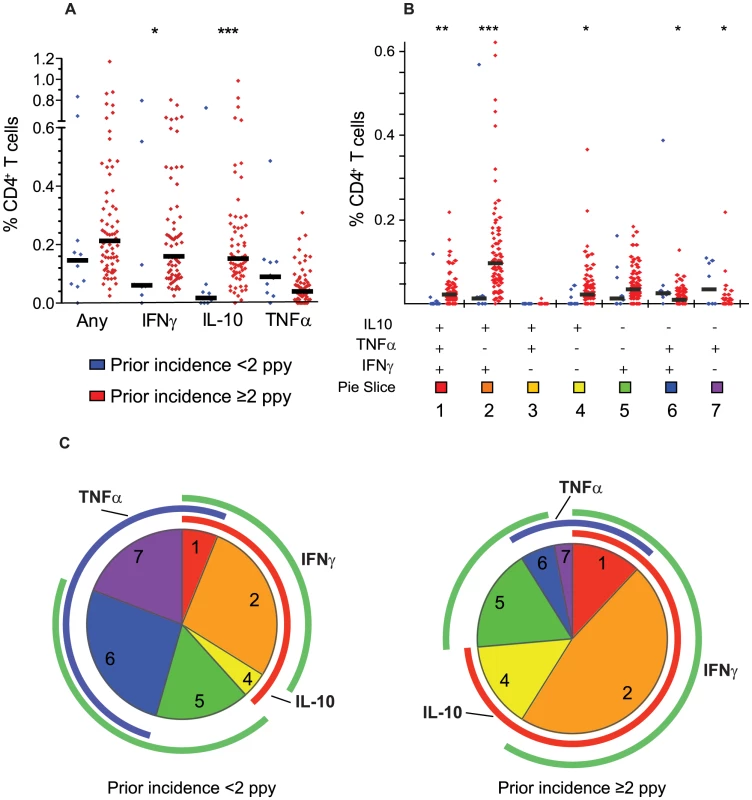
A. The overall malaria-specific CD4+ T cell response (left column) is followed by the overall frequency of CD4+ T cells producing IFNγ, IL-10, and TNFα stratified by prior malaria incidence. Blue dots represent responses from children with lower prior malaria incidence (<2 episodes ppy, n = 10) and red dots represent responses from children with higher prior malaria incidence (≥2 episodes ppy, n = 68,* P<0.05, *** P<0.001, Wilcoxon Rank-Sum. Horizontal black lines indicate the median response for each group). Median frequencies of cytokine producing cells were similar in children with ≥2–5 and >5 episodes ppy (data not shown). B–C. Boolean gating of malaria-specific CD4+ T cells reveals 7 distinct cytokine-producing populations. Shown are the absolute frequency (B) and the relative proportion (C) of each individual combination of IFNγ, IL-10, or TNF-producing cells. Blue dots again represent responses from children with <2 prior episodes ppy, and red dots represent responses from children with ≥2 episodes ppy (* P<0.05, ** P<0.01, *** P<0.001, Wilcoxon Rank-Sum. Horizontal black lines indicate the median response for each group). For pie charts, blue arcs represent total proportion of CD4+ T cells producing TNFα; red arcs represent total proportion of CD4+ T cells producing IL-10; and green arcs represent total proportion of CD4+ T cells producing IFNγ. The proportion of IFNγ−/IL-10+/TNFα− (population 3) producing cells is <0.01% of the total malaria-specific response, and thus does not have a visible corresponding pie slice. We also analyzed the relationship of prior malaria incidence with the “composition” of the malaria-specific response (i.e. the proportion of each cytokine combination amongst the total malaria-specific CD4+ T cell population), and found similar results. Among children with <2 episodes ppy, TNFα-producing CD4+ T cells (including TNFα single-producers and IFNγ/TNFα double producers) comprised a greater proportion of the malaria-specific response than among children with ≥2 prior episodes ppy, whereas in children with a higher prior malaria incidence, IL-10-producing CD4+ T cells (including IL-10 single-producers and IFNγ/IL-10 double producers) comprised a far greater fraction of the malaria-specific response (P<0.001, Fig. 2c). There was no significant difference in the proportion of IFNγ-producing CD4+ T cells between children with higher and lower incidence. These findings suggest that the functional phenotype of the malaria-specific CD4+ T cell response differs according to prior exposure, and that with more prior episodes, the overall response is more regulatory (IL-10 producing) and less inflammatory (TNFα producing).
IFNγ/IL-10 co-producing CD4+ T cells correlate with recent malaria exposure
While the data above demonstrate that there is a strong relationship between the functional phenotype of malaria-specific CD4+ T cells and prior malaria history, we wished to determine whether this phenotype was influenced by the time elapsed since the most recent malaria episode, the cumulative number of prior malaria episodes, or both, as these parameters are both logically and statistically related (Spearman's Rho = −0.46, P<0.001). We observed a strong inverse correlation between the frequency of IFNγ+/IL-10+/TNFα− CD4+ T cells and the duration since the last episode of malaria (Spearman's Rho = −0.39, P<0.001, Fig. 3d), with more recent malaria associated with a higher frequency of these co-producing cells, as well as a positive correlation with the total cumulative number of prior episodes (Spearman's Rho = 0.23, P = 0.04, Fig. 3e). However, when assessed in a multivariate model, the frequency of malaria-specific IFNγ/IL-10 co-producing CD4+ T cells remained strongly associated with the duration since malaria, whereas the total prior incidence was no longer significant. Similar results were observed for total IL-10 (Fig. 3a) and total IFNγ-producing (Fig. 3b) populations, and when assessing the duration since last episode of parasitemia (data not shown). Interestingly, the opposite relationship was observed between total TNFα+ producing cells and the duration since last episode of malaria, with more recent malaria associated with a lower frequency of TNFα -producing cells (Spearman's Rho = 0.23, P = 0.041, Fig. 3c). Further, there was no significant correlation between the number of cumulative prior malaria episodes and TNFα+ producing cells. Together these data suggest that recency of malaria infection, rather than the total number of past episodes, exerts a dominant influence on the functional phenotype of malaria-specific CD4+ T cells. Similar findings were obtained when analyzing the “composition” (i.e. the proportion of responding cells producing IFNγ, TNFα, and/or IL10) of the malaria-specific response and duration since last malaria infection.
Fig. 3. CD4+ T cell functions and relationship with recent and cumulative malaria infection. 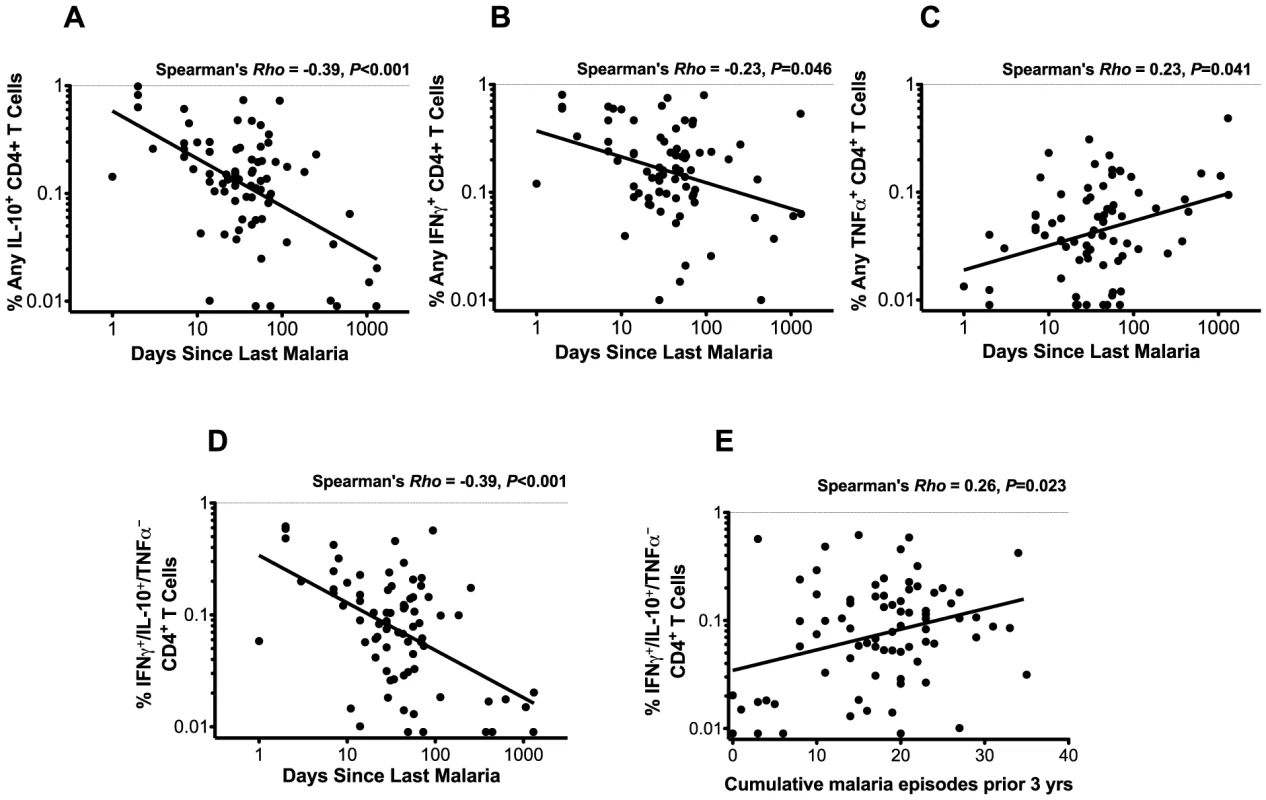
The frequencies of CD4+ T cells producing any IL-10 (A) and any IFNγ (B) are inversely associated with days since last malaria episode (Spearman's Rho = −0.39, P<0.001; Rho = −0.23, P = 0.046, respectively). Frequencies of CD4+ T cells producing any TNFα (C) are positively correlated with days since last malaria episode infection (Spearman's Rho = 0.23, P = 0.041). Frequencies of IFNγ+/IL-10+/TNFα− CD4+ T cells are inversely associated with days since last malaria episode (D, Spearman's Rho = −0.39, P<0.001) and positively associated with the cumulative number of episodes in the prior 3 years (E, Spearman's Rho = 0.26, P = 0.023). Malaria-specific CD4+ T cells are not independently associated with protection from malaria
Protection from clinical malaria in naturally exposed individuals can be defined using a number of outcomes, including a delayed time to reinfection [37], [38], [41]–[43], [51], a decreased incidence of malaria over time [53], and/or a decreased probability of clinical disease once parasitemic [46]. In all cases, identification of immune correlates of protection is challenging due to the difficulty of distinguishing protection from a lack of exposure to malaria-infected mosquitos [45], [46]. To address this, we assessed the relationship between malaria-specific T cell functional subsets and protection from malaria, while adjusting for prior malaria (duration since last episode and/or cumulative number of prior episodes) as a surrogate measure of exposure intensity. We also evaluated potential associations with the overall prevalence of asymptomatic parasitemia, as clinical immunity to malaria is normally characterized by a transition from symptomatic to asymptomatic disease [3].
In univariate Cox proportional hazards analysis evaluating time to next episode of malaria, a higher frequency of CD4+ T cells producing any IFNγ or IL10, or the combinations IFNγ+/IL-10+/TNFα− and IFNγ−/IL-10+/TNFα− was associated with a significantly increased hazard of malaria (Table 2, left columns). However following adjustment for surrogates of exposure intensity (duration since last episode of malaria and/or cumulative prior malaria episodes) in a multivariate model, none of these associations remained significant. Similar relationships were observed when we analyzed the total malaria incidence in the year following the assay in a multivariate regression model (Table 2, middle columns). However, in this analysis both IFNγ+/IL-10+/TNFα− (IRR 1.40 per 10 fold increase, P = 0.038) and any IL-10-producing CD4+ T cells (IRR 1.41 per 10 fold increase, P = 0.039) remained independently associated with an increased risk of malaria after controlling for duration since last malaria infection. Nearly identical results were obtained when analyzing the total composition of cytokine producing cells: both the fraction of IFNγ+/IL-10+/TNFα− and any IL10+ cells among all cytokine-producing cells were associated with increased malaria risk (IRR 1.47, P = 0.038 and 1.40, P = 0.039 per 50% increase in fraction of responding cells, respectively). Together, these data suggest that the dominant population of malaria-specific CD4+ cells, which co-produce IFNγ and IL-10, are not associated with protection from future malaria, and may in fact be associated with an increased risk of malaria.
Tab. 2. Magnitude of malaria-specific CD4+ T cell responses and protection from symptomatic malaria. 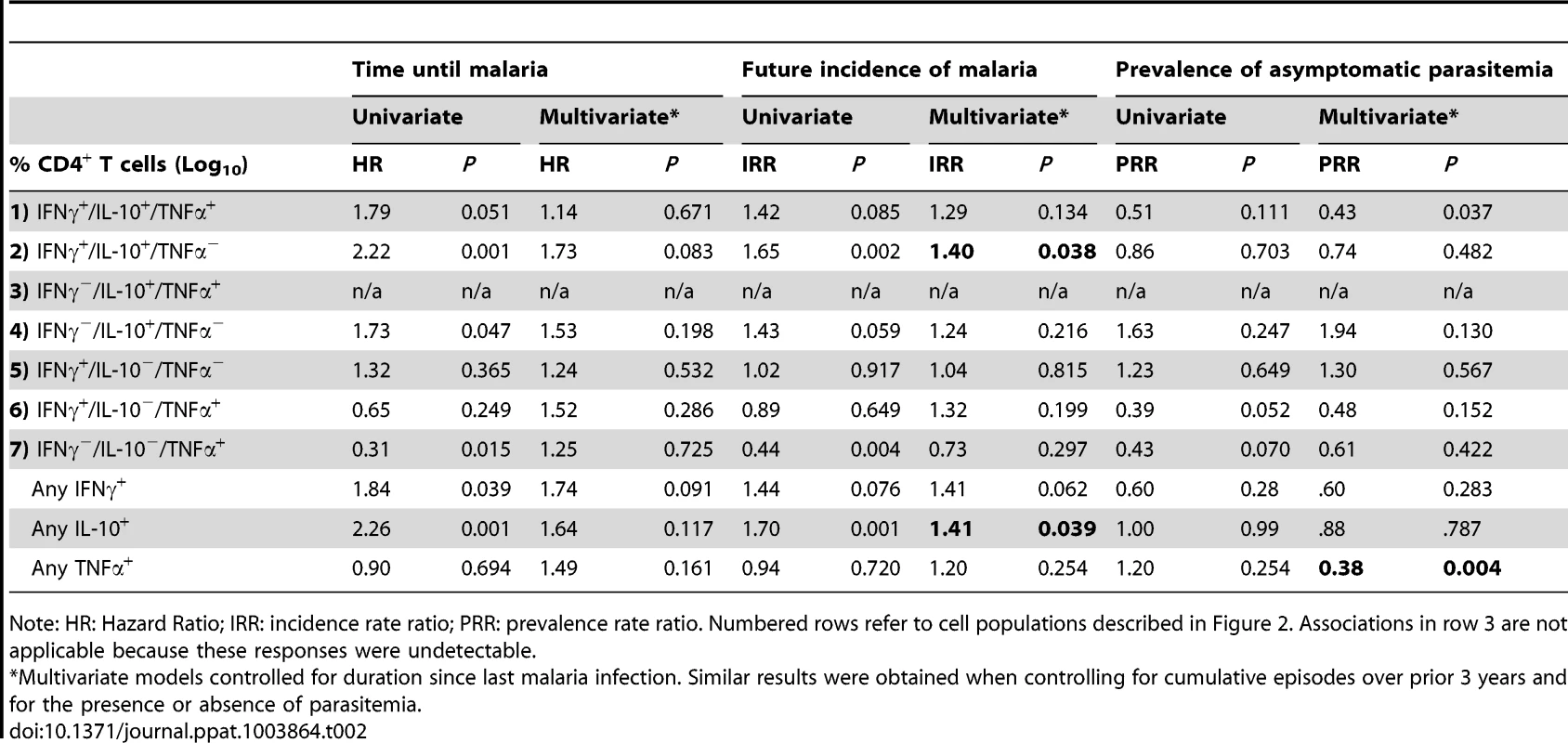
Note: HR: Hazard Ratio; IRR: incidence rate ratio; PRR: prevalence rate ratio. Numbered rows refer to cell populations described in Figure 2. Associations in row 3 are not applicable because these responses were undetectable. We next assessed the relationship of TNFα− producing CD4+ T cells with protection. In RTS/S vaccine recipients, malaria-specific CD4+ T cells producing TNFα in the absence of IFNγ or IL-2 have recently been shown to correlate with protection from malaria infection [55]. In our cohort, a greater frequency of malaria-specific CD4+ T cells producing TNFα alone (IFNγ−/IL-10−/TNFα+) was associated with a significantly reduced hazard of developing malaria (HR 0.31, P = 0.015 per 10 fold increase) and lower prospective incidence (IRR 0.44, P = 0.004 per 10 fold increase) in univariate analysis, but in multivariate models controlling for duration since malaria and/or cumulative prior malaria episodes, these associations were no longer significant (Table 2). Interestingly, however, the frequency of malaria-specific CD4+ T cells producing any TNFα was inversely associated with the monthly prevalence of asymptomatic parasitemia, even after controlling for duration since last episode of malaria and/or cumulative prior malaria episodes (PRR 0.41 per 10 fold increase, P = 0.011). Thus, the absence of malaria-specific CD4+ T cells producing TNFα may be associated with the phenotype of asymptomatic infection.
IFNγ/IL-10 co-producing cells express T-bet and are of an effector memory phenotype
Although IL10 production by T cells was initially believed to occur predominantly within Th2 and FoxP3+ Treg CD4+ T cell subsets, it is now known that additional subsets, including cells expressing the Th1 master regulator T-bet, produce IL-10 under conditions of continuous antigen exposure [56], [57]. We assessed transcription factor expression within the dominant population of malaria-specific IFNγ/IL-10 co-producing cells (Fig. 4a) and found that these cells uniformly were TBet+ and FoxP3− (Fig. 4b–c). These IFNγ/IL-10 co-producing CD4+ T cells were predominantly of an early effector memory phenotype (CD45RA−, CCR7 − CD27+; Fig. 4d–e).
Fig. 4. CD4+ T cells co-producing IFNγ and IL-10 express T-bet and are of an early effector memory phenotype. 
CD4+ T cells were analyzed for transcription factor expression and maturational phenotype. Panel 4A shows the proportion of CD4+ T cells co-producing IFNγ/IL-10 in response to iRBC stimulation for one representative child (upper right quadrant)) and panel 4B shows intranuclear transcription factor staining with T-bet and FoxP3 of all CD4+ T cells (grey) with IFNγ/IL-10 CD4+ T cells overlayed (4B, blue dots). Panel 4C shows the percentage of iRBC-stimulated IFNγ+, IL-10+, and IFNγ/IL-10 co-producing CD4+ T cells staining for intranuclear T-bet (n = 10). CD4+ T cells were also analyzed for cell surface maturation markers CD45RA (x axis, panels 4D–E), CCR7 (4D), and CD27 (4E). The total CD4+ T cell population is shown in grey, with IFNγ/IL-10 co-producing CD4+ T cells overlayed as blue dots. CD4+ T cell IFNγ/IL-10 responses to the polyclonal mitogen PMA/Io have previously been shown to correlate with relative protection against severe malaria [52]. We therefore compared the response to iRBC and PMA/Io stimulation, and found a strong correlation between the frequency of IFNγ/IL-10 double producing CD4+ T cells following iRBC or PMA stimulation (Spearman's Rho = 0.88, P<0.001, Supplemental Fig. S1). As PMA/Io stimulation is thought to induce cytokine production by recently activated cells, these data suggest that this mitogen stimulates cytokine production by malaria-specific T cells that have recently seen their cognate antigen.
Plasma IL-10 levels are elevated during malaria infection but do not correlate with the frequency of IL-10 producing CD4+ T cells
IL-10 levels measured concurrently in plasma were significantly higher among children with parasitemia at the time of the blood draw compared with children with no parasitemia (median 30.4 pg/ml vs 11.4 pg/ml, P = 0.0035), consistent with prior reports [58]–[61]. Similar to IL-10 producing CD4+ T cells, plasma IL-10 strongly correlated with recent malaria (Spearman's Rho = 0.30, P = 0.009, Supplemental Fig. S2a). However plasma IL-10 levels did not correlate with the frequency of total IL-10 producing CD4+ T cells (Spearman's Rho = 0.11, P = 0.35, Supplemental Fig. S2b), suggesting that additional cell types, including cells of the myeloid lineage, may contribute to plasma IL-10 levels during malaria infection [19].
Impaired malaria-specific CD4+ T cell proliferation in heavily exposed children is partially reversed by IL-10 blockade
Immunomodulation through downregulation of antigen-specific CD4+ T cell proliferative responses has been described in the context of several chronic parasitic infections [62]–[65], as well as chronic viral infections that result in persistent antigenemia [66], [67]. We assessed proliferation of malaria-specific CD4+ T cells by measuring CFSE dilution following stimulation with schizont extract (PfSE) in a subset of children (n = 42). A significant inverse correlation was observed between malaria-specific CD4+ T cell proliferation and cumulative prior incidence (Spearman's Rho = −0.39, P = 0.011; Fig. 5a), suggesting that heavy antigen exposure may result in a proliferative defect in malaria-specific CD4+ T cells. We also observed an inverse correlation between CD4+ T cell proliferation following PfSE stimulation and the frequency of IFNγ/IL-10 co-producing CD4+ T cells (Spearman's Rho = −0.31, P = 0.049). It has previously been suggested that IFNγ/IL-10 co-producing CD4+ T cells may play an autoregulatory role through suppression of proliferative responses in an IL-10 mediated manner [68]. We therefore assessed whether in vitro IL10 blockade would reverse the observed proliferative defect. The ability of CD4+ T cells to proliferate in response to PfSE was partially restored in 8 of 9 subjects upon blockade of IL-10 receptor alpha (fold change 1.7, P = 0.01, Fig. 5b–c), suggesting that the CD4+ T cell proliferative defect observed in heavily exposed children may be in part due to IL-10 mediated suppression.
Fig. 5. CD4+ T cell proliferation impaired in setting of heavy prior exposure. 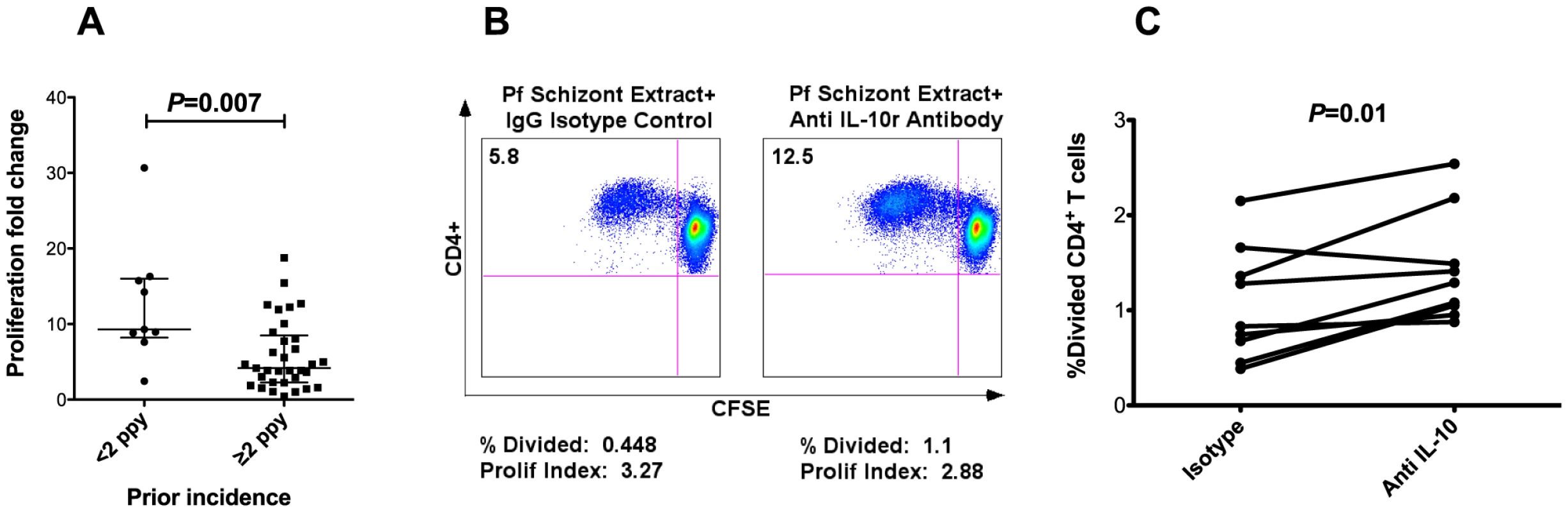
A. The proliferation fold change (fraction of CFSE-lo cells following PfSE stimulation vs uRBC stimulation) is significantly reduced in children with higher prior malaria exposure (≥2 episodes ppy, n = 33) vs children with low malaria exposure (<2 episodes ppy, n = 9, P = 0.007, Wilcoxon Rank Sum. Horizontal lines show medians for each group with 95% CI). B. Impact of IL-10 blockade on CD4+ T cell proliferation following PfSE stimulation in one representative subject. The left panel shows CFSE dilution following PfSE stimulation with addition of isotype control, and the right panel shows CFSE dilution following PfSE stimulation with addition of anti IL-10 receptor α blocking antibody. C. Change in the percent of CD4+ T cells divided following isotype control vs anti IL-10 receptor α blocking antibody in a subset of 9 children from whom additional cells were available (fold change 1.7, P = 0.01). Discussion
In this cohort of young children living in an area of very high transmission intensity in Uganda, very little evidence of clinical immunity had emerged by five years of age. In this setting, the functional phenotype of the malaria-specific CD4+ T cell response was significantly influenced by prior malaria exposure; with less prior malaria, the overall malaria-specific CD4+ T cell response was more inflammatory (TNFα-producing), but with heavier exposure, the overall malaria-specific response was more regulatory (IL-10 producing). To our knowledge, this is the first study to show that Th1 IFNγ/IL-10 co-producing cells constitute the dominant population of CD4+ T cells responding to malaria in heavily exposed children. Moreover, we found no evidence that these IFNγ/IL-10 co-producing cells were associated with protection from future malaria.
Interest in IFNγ/IL-10 co-producing Th1 cells has increased in recent years as these cells have been found to be important regulators of the immune response to several infectious, allergic, and autoimmune diseases [18], [49], [50], [52], [56], [69], [70]. In a murine model of Toxoplasma gondii, IFNγ produced by these cells was shown to be required for pathogen eradication, and concomitant production of IL-10 was vital for the resolution of the inflammatory response and to prevent tissue pathology [50]. However, in a murine model of Leishmania major, co-production of IL-10 by Th1 cells prevented pathogen eradication, contributing to chronic infection [49]. These data suggest that IL-10 co-production by Th1 T cells may help prevent immunopathology, but this may come at the cost of chronic pathogen persistence [71].
IL-10 levels are increased during malaria infection [58], [59], [61] and this regulatory cytokine is thought to play a key role in dampening proinflammatory responses and preventing the development of severe malarial anemia and cerebral malaria [72]. In mice, Th1 cells were elegantly shown to be the major producer of IL-10 and were critical for limiting the pathology associated with malaria infection [18]. T cell production of IL-10 has also been described in reports of human malaria infection [14], [52], [73]–[77]. Plebanski and colleagues described a switch in production from IFNγ to IL-10 in CD4+ T cells from Gambian adults stimulated with altered peptide ligands of the circumsporozoite protein, with an associated suppression of proliferative responses in vitro [14]. T cells co-producing IFNγ/IL-10 following nonspecific PMA/ionomycin stimulation were described in the context of acute malaria infection [73], and were also more abundant among children with uncomplicated rather than severe malaria [52], consistent with a role in modulating inflammation. More recently, Gitau and colleagues described malaria-specific co-production of IFNγ and IL-10 following stimulation of CD4+ T cells with a variety of expressed PfEMP variants, although these co-producing cells represented a minor fraction of the total antigen-specific CD4+ T cell response [75]. The potential role that malaria-specific IFNγ/IL-10 co-producing CD4+ T cell cells play in mediating or inhibiting protective immunity in humans has not thus far been investigated [77].
We observed that CD4+ T cells co-producing IFNγ/IL-10 dominate the T cell response to malaria in heavily exposed children, and that the overall frequency and proportion of these cells among malaria-specific T cells was strongly correlated with recent exposure to malaria, more so than cumulative prior exposure. These IFNγ/IL-10 co-producing cells express T-bet, indicating that they have differentiated along the Th1 pathway. The dominance of this functional phenotype among malaria-specific T cells has not previously been reported, and may be related to the unusually high malaria exposure intensity of our cohort, as this cell population was of much lower frequency among children with <2 malaria episodes per year. Further, frequencies of IL-10–producing and IFNγ/IL10 co-producing cells were not associated with protection from future malaria after controlling for recent and/or cumulative prior malaria, but were instead associated with an increased risk of cumulative malaria in the year following the assay, although this may be due to the inability to fully adjust for the level of environmental exposure to malaria using clinical surrogates such as prior malaria incidence.
We further observed that heavy malaria exposure was associated with a decreased ability of CD4+ T cells to proliferate in response to malaria antigens, and that this impaired proliferation is partially reversed by IL-10 blockade. These data are consistent with in vitro studies of recently activated IL7R−, CD25−, CD4+ T cells which co-produce IFNγ and IL-10 and limit CD4+ T cell proliferation through IL-10 dependent mechanisms [68]. In addition, prior studies have shown that IL-10 blockade increases malaria-specific IFNγ cytokine production in filaria-coinfected individuals [78] and in cord blood mononuclear cells from neonates born to mothers exposed to malaria [79]. A similar IL10-dependent functional impairment of CD4+ T cells has been described in other infections such as HIV that are characterized by chronic high-level antigen stimulation [80], [81].
Together, these data are consistent with the hypothesis that IFNγ/IL-10 co-producing CD4+ T cells primarily function to limit the immunopathology associated with malaria infection – including cerebral malaria, anemia, and death - through autoregulation of CD4+ T cell proliferation and cytokine production. A similar role has been attributed to IL-10-producing Th1 cells in other parasitic diseases characterized by heavy continuous antigen exposure [49], [50], with evidence that IL-10 produced by Th1 effector cells acts through a negative feedback loop to regulate CD4+ T cell responsiveness, limiting inflammation and tissue pathology at the cost of impaired pathogen clearance [56], [71]. It is possible that unmeasured confounders, such as helminthic co-infections, may have been unequally represented in the high and low-incidence groups, particularly as the lower incidence children were more likely to reside in town. However routine deworming was performed in all study subjects every 3–6 months, lessening the likelihood that co-infection with helminths explains our findings. Further studies are needed to determine if IL-10-producing Th1 cells contribute to pathogen persistence, and to the failure of humans to develop sterile protective immunity to malaria.
In addition, we found that children with the fewest prior episodes of malaria were significantly more likely to have malaria-specific production of TNFα without IL-10, and that the absence of this inflammatory cytokine was associated with the phenotype of asymptomatic infection. Studies in murine models have shown that TNFα plays an important role in inhibiting the development of hepatic stages of malaria [82], [83]. Importantly, a recent study of RTS/S vaccine recipients identified antigen-specific CD4+ T cell production of TNFα as a correlate of protection in vaccinees [55]. In contrast to that study, we found no evidence of protection after controlling for prior malaria, though we did observe that asymptomatic infection was inversely associated with the frequency of TNFα producing CD4+ T cells, independent of prior malaria. Together our data suggest that production of this inflammatory cytokine may decrease with increasing cumulative malaria exposure, enabling a transition to asymptomatic infections.
A notable strength of this study was the availability of comprehensive malaria clinical histories spanning from early infancy to the time of the immunologic assessment, plus one additional year thereafter, which enabled us to assess for T cell correlates of both exposure to and protection from malaria. Several prior studies have reported correlations between T cell responses or IL-10 production and protection from malaria in naturally exposed children [37], [42], [53], but such studies have generally been unable to adequately account for prior malaria exposure. While we did observe associations, both positive and negative, between malaria-specific CD4+ T cells of varying functional phenotypes and the risk of future malaria, most of these associations were not significant after adjusting for recent or cumulative prior episodes of malaria, surrogates for the level of ongoing exposure to malaria-infected mosquitos. Hence the failure to account for malaria exposure intensity may lead to spurious associations with protection. Although we did not identify T cell phenotypes that were associated with protection from future malaria, this may be related to the young age of children in this cohort, as there was little evidence that clinical immunity had developed prior to 5 years of age. Future longitudinal studies examining responses in older children and adults, incorporating more precise entomological measurements of malaria exposure, are underway.
In conclusion, among naturally exposed children living in a high endemicity setting, malaria-specific CD4+ T cells were present in the vast majority of children, and their functional phenotype differed greatly based on the level of prior exposure to malaria, in particular the duration of time since last infection. IFNγ/IL-10 co-producing Th1 cells dominated the CD4+ T cell response to malaria in these heavily exposed children, but were not associated with protection from future infection. These CD4+ T cells may play important immunomodulatory roles in the pathogenesis of malaria in childhood.
Methods
Study site, participants, and follow-up procedures
Samples for this study were obtained from children enrolled in the Tororo Child Cohort (TCC) in Tororo, Uganda, a rural district in south-eastern Uganda with an entomological inoculation rate (EIR) estimated at 379 infective bites per person year (PPY) in 2012 [20]. Details of this cohort have been described elsewhere, and the sub-study described in this report includes only HIV-uninfected children born to HIV-uninfected mothers [20], [84]–[87]. Briefly, children in the TCC were enrolled at infancy (median 5.5 months of age) and followed for all medical problems at a dedicated study clinic open seven days a week. Monthly assessments were done to ensure compliance with study protocols and perform routine blood smears. All children were prophylactically dewormed with mebendazole every 3–6 months per Ugandan Ministry of Health guidelines [88]. Children who presented with a documented fever (tympanic temperature ≥38.0°C) or history of fever in the previous 24 hours had blood obtained by finger prick for a thick smear. If the thick smear was positive for malaria parasites, the patient was diagnosed with malaria regardless of parasite density, and given artemisinin-based combination therapy for treatment of uncomplicated malaria. Children were followed until 5 years of age unless prematurely withdrawn.
Incident episodes of malaria were defined as all febrile episodes accompanied by any parasitemia requiring treatment, but not preceded by another treatment in the prior 14 days [20]. The incidence of malaria was calculated as the number of episodes per person years (ppy) at risk. Asymptomatic parasitemia was defined as a positive routine blood smear in the absence of fever that was not followed by the diagnosis of malaria in the subsequent seven days, and was reported as a count outcome as it was measured via monthly surveillance. The period prevalence of asymptomatic parasitemia was calculated as the number of episodes/total months observed.
Ethical approval
Written informed consent was obtained from the parent or guardian of all study participants. The study protocol was approved by the Uganda National Council of Science and Technology and the institutional review boards of the University of California, San Francisco, Makerere University and the Centers for Disease Control and Prevention.
Sample collection and processing
At approximately 4 years of age, ∼6–10 mls of whole blood was obtained from each subject in acid citrate dextrose tubes. Plasma was collected, and peripheral blood mononuclear cells (PBMC) were isolated by density gradient centrifugation (Ficoll-Histopaque; GE Life Sciences). PBMC were cryopreserved in liquid nitrogen and shipped to our laboratory in San Francisco for additional studies.
Malaria antigens
Plasmodium falciparum blood-stage 3D7 parasites were grown by standard methods and harvested at 5–10% parasitemia. Red blood cells infected with mature asexual stages were purified magnetically, washed, and cryopreserved in glycerolyte prior to use (iRBC). Uninfected RBCs (uRBC) were used as controls. To assess the impact of parasite diversity on T cell responses, responses to iRBCs prepared from 4 distinct Tororo field strains were compared to iRBC prepared from 3D7. Responses to the 4 field strains were very similar, indicating that parasite diversity does not significantly influence the T cell response magnitude (Supplemental Fig. S3). Schizont extracts (PfSE) for use in proliferation assays [89] were prepared by 3 freeze-thaw cycles of iRBC in liquid N2 for freezing and 37°C water bath for thawing, then resuspended in R10 media and stored at −20°C until use.
Intracellular cytokine staining
Thawed PBMC were rested overnight in 10% fetal bovine serum (Gibco) and counted prior to stimulation with uRBC, iRBC, media, or phorbol miristate acetate/calcium ionophore (PMA/Io) at 1×106 cells/condition. An E:T ratio of 1∶3 was used with uRBC and iRBC [90]. Anti-CD28 and –CD49d were added for costimulation (0.5 µg/ml, BD Pharmingen). Brefeldin-A and Monensin (BD Pharmingen) were added at 6 hours of incubation at a final concentration of 10 µg/ml to inhibit cytokine secretion. At 24 hours of incubation, cells were washed, fixed and permeabilized per standard protocols (Invitrogen/Caltag; Ebioscience fix/perm reagents used for nuclear transcription factor analysis).
Surface and/or intracellular staining of PBMC was done with standard protocols [91], [92] using the following antibodies for the primary analysis: Brilliant violet 650-conjugated CD4 (Biolegend), PerCP–conjugated anti-CD3, APC-H7-conjugated CD8, PE-Cy7-conjugated IFNγ, PE-conjugated anti-IL-10, and FITC-conjugated TNFα (BD Pharmingen). Alexa 700-conjugated CD14 and CD19, APC-conjugated anti-γδ (Biolegend), and Live/dead aqua amine (Invitrogen) were included as exclusion gates to reduce unwanted nonspecific antibody binding when measuring antigen-specific T cell populations [93]. Additional experiments utilized Brilliant violet 421-conjugated anti-IL-2, Brilliant violet 605-conjugated CD45RA, Brilliant violet 710-conjugated CD27 (Biolegend), APC-conjugated CCR7 (R&D Systems); eFluor 660-conjugated T-bet and FITC-conjugated FoxP3 (Ebioscience).
CFSE proliferation assay
Thawed PBMC were rested for one hour, washed in 10% Human AB media (Gemini), and 3–6×106 PBMC were labeled with 1 ml of 1.25 µM 5,6-carboxyfluorescein diacetate succinimidyl ester (CFSE; Molecular Probes) for seven minutes. CFSE-labeled PBMC were incubated in 96-well, deep-well culture plates (Nunc, Roskilde, Denmark) at a density of 106 PBMC per well at a final volume of 1 ml for 7 days. In a subset of patients, CFSE-labeled PBMC were incubated with antigen in the presence of IL-10 receptor alpha chain (IL-10Rα) blocking antibody (clone 37607; R&D Systems) or IgG1 isotype control antibody at 10 µg/mL. Antigens tested included media, phytohemagglutinin (PHA; 5 µg/mL; Sigma-Aldrich), uRBC, or PfSE at an E:T ratio of 1∶3 schizont equivalents. At day 7 cells were treated with 100 units DNase I (Invitrogen) in culture medium at 37°C for 10 min, washed, and stained with surface antibodies (PerCP–conjugated anti-CD3, APC-H7-conjugated CD8 (BD Pharmingen), Brilliant violet 650-conjugated CD4, Alexa 700-conjugated CD14 and CD19, and APC-conjugated anti-γδ (Biolegend)) before acquisition.
Flow cytometry data analysis
Flow cytometry profiles were gated on CD3+, γδ-negative lymphocytes, and 200,000 to 300,000 events were collected. Samples were analyzed on an LSR2 three laser flow cytometer (Becton Dickinson) with FACSDiva software. Color compensations were performed for each patient's PBMC using beads or samples single stained for each of the fluorochromes used. Data were analyzed using FlowJo (Tree Star, San Carlos, CA) and Pestle (version 1.7)/SPICE (version 5.3; M. Roederer, Vaccine Research Center, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, MD). In experiments with CFSE-labeled cells, the ratio of CFSE-lo cells following PfSE stimulation vs uRBC stimulation was calculated and reported as the proliferation fold change. The FlowJo Proliferation Platform provided additional information about the division characteristics of CD4+ T cells. To examine the effect of IL-10 blockade on proliferation, these parameters for CD4+ T cells were generated in samples that had been stimulated with PfSE plus anti-IL-10Rα and compared to the values obtained from samples stimulated with PfSE plus isotype control.
Plasma IL-10
Plasma levels of IL-10 were measured by dual Ab sandwich-ELISA kits, according to manufacturer's instruction (R&D Systems, Minneapolis, MN). Each sample was tested in duplicate, and cytokine concentrations were calculated using a standard curve generated from recombinant cytokines. Cytokine values were expressed as picograms (pg) per milliliter.
Statistical methods
All statistical analyses were performed using Prism 4.0 (GraphPad), STATA version 12 (College Station), or SPICE v.5.3 (NIAID). Frequencies of malaria-specific cytokine producing T cells (alone or in combination) are reported after background subtraction of the frequency of the identically gated population of cells from the same sample stimulated with control. Background-subtracted responses were consider positive if >0.01% parent population [94]. Comparisons of cytokine frequencies between prior malaria incidence groups were done using the Wilcoxon rank sum test, and the Wilcoxon signed-rank test was used to compare paired data. Statistical analyses of global cytokine profiles (pie charts) were performed by partial permutation tests using the SPICE software [94] . Continuous variables were compared using Spearman correlation. For multivariate regression models, non-normal variables were log-transformed. To allow for nonlinear relationships between clinical exposure variables and immunologic outcomes, we fit linear splines with knots chosen to best represent observed relationships. Associations between immune parameters and time to next malaria episode were evaluated using the Kaplan-Meier product limit formula, and a multivariate cox proportional hazards model was used to adjust for surrogates of malaria exposure found to be associated with these parameters (duration since last episode of malaria and/or cumulative episodes in the prior 3 years). Negative binomial regression was used to estimate associations between immune parameters and the prospective incidence of malaria in the following year (incidence rate ratios, IRR) and prevalence of asymptomatic parasitemia during the entire study period (prevalence rate ratios, PRR), adjusting for prior malaria as above. Two-sided p-values were calculated for all test statistics and P<0.05 was considered significant.
Supporting Information
Zdroje
1. LanghorneJ, NdunguFM, SponaasAM, MarshK (2008) Immunity to malaria: more questions than answers. Nat Immunol 9 : 725–732.
2. MarshK, KinyanjuiS (2006) Immune effector mechanisms in malaria. Parasite Immunol 28 : 51–60.
3. TranTM, LiS, DoumboS, DoumtabeD, HuangCY, et al. (2013) An intensive longitudinal cohort study of Malian children and adults reveals no evidence of acquired immunity to Plasmodium falciparum infection. Clin Infect Dis 57 : 40–47.
4. DeloronP, ChougnetC (1992) Is immunity to malaria really short-lived? Parasitol Today 8 : 375–378.
5. Di PerriG, SolbiatiM, VentoS, De ChecchiG, LuzzatiR, et al. (1994) West African Immigrants and New Patterns of Malaria Imported to North Eastern Italy. Journal of Travel Medicine 1 : 147–151.
6. BarryAE, SchultzL, BuckeeCO, ReederJC (2009) Contrasting population structures of the genes encoding ten leading vaccine-candidate antigens of the human malaria parasite, Plasmodium falciparum. PLoS ONE 4: e8497.
7. AdkinsB (1999) T-cell function in newborn mice and humans. Immunol Today 20 : 330–335.
8. PassRF, StagnoS, BrittWJ, AlfordCA (1983) Specific cell-mediated immunity and the natural history of congenital infection with cytomegalovirus. J Infect Dis 148 : 953–961.
9. AdkinsB, LeclercC, Marshall-ClarkeS (2004) Neonatal adaptive immunity comes of age. Nat Rev Immunol 4 : 553–564.
10. HoltPG (2003) Functionally mature virus-specific CD8(+) T memory cells in congenitally infected newborns: proof of principle for neonatal vaccination? J Clin Invest 111 : 1645–1647.
11. WilsonCB, LewisDB (1990) Basis and implications of selectively diminished cytokine production in neonatal susceptibility to infection. Rev Infect Dis 12 (Suppl 4) S410–420.
12. WilsonCB, LewisDB, EnglishBK (1991) T cell development in the fetus and neonate. Adv Exp Med Biol 310 : 17–27.
13. HoM, WebsterHK, LooareesuwanS, SupanaranondW, PhillipsRE, et al. (1986) Antigen-specific immunosuppression in human malaria due to Plasmodium falciparum. J Infect Dis 153 : 763–771.
14. PlebanskiM, FlanaganKL, LeeEA, ReeceWH, HartK, et al. (1999) Interleukin 10-mediated immunosuppression by a variant CD4 T cell epitope of Plasmodium falciparum. Immunity 10 : 651–660.
15. WaltherM, TongrenJE, AndrewsL, KorbelD, KingE, et al. (2005) Upregulation of TGF-beta, FOXP3, and CD4+CD25+ regulatory T cells correlates with more rapid parasite growth in human malaria infection. Immunity 23 : 287–296.
16. BejonP, MwacharoJ, KaiO, TodrykS, KeatingS, et al. (2007) The induction and persistence of T cell IFN-gamma responses after vaccination or natural exposure is suppressed by Plasmodium falciparum. J Immunol 179 : 4193–4201.
17. ButlerNS, MoebiusJ, PeweLL, TraoreB, DoumboOK, et al. (2012) Therapeutic blockade of PD-L1 and LAG-3 rapidly clears established blood-stage Plasmodium infection. Nat Immunol 13 : 188–195.
18. Freitas do RosarioAP, LambT, SpenceP, StephensR, LangA, et al. (2012) IL-27 promotes IL-10 production by effector Th1 CD4+ T cells: a critical mechanism for protection from severe immunopathology during malaria infection. J Immunol 188 : 1178–1190.
19. Freitas do RosarioAP, LanghorneJ (2012) T cell-derived IL-10 and its impact on the regulation of host responses during malaria. Int J Parasitol 42 : 549–555.
20. JagannathanP, MuhindoMK, KakuruA, ArinaitweE, GreenhouseB, et al. (2012) Increasing incidence of malaria in children despite insecticide-treated bed nets and prompt anti-malarial therapy in Tororo, Uganda. Malar J 11 : 435.
21. Roca-FeltrerA, KwizombeCJ, SanjoaquinMA, SesaySS, FaragherB, et al. (2012) Lack of decline in childhood malaria, Malawi, 2001–2010. Emerging infectious diseases 18 : 272–278.
22. TrapeJF, TallA, DiagneN, NdiathO, LyAB, et al. (2011) Malaria morbidity and pyrethroid resistance after the introduction of insecticide-treated bednets and artemisinin-based combination therapies: a longitudinal study. Lancet Infectious Diseases 11 : 925–932.
23. SchofieldL, VillaquiranJ, FerreiraA, SchellekensH, NussenzweigR, et al. (1987) Gamma interferon, CD8+ T cells and antibodies required for immunity to malaria sporozoites. Nature 330 : 664–666.
24. RomeroP, MaryanskiJL, CorradinG, NussenzweigRS, NussenzweigV, et al. (1989) Cloned cytotoxic T cells recognize an epitope in the circumsporozoite protein and protect against malaria. Nature 341 : 323–326.
25. RodriguesM, NussenzweigRS, ZavalaF (1993) The relative contribution of antibodies, CD4+ and CD8+ T cells to sporozoite-induced protection against malaria. Immunology 80 : 1–5.
26. TsujiM, BergmannCC, Takita-SonodaY, MurataK, RodriguesEG, et al. (1998) Recombinant Sindbis viruses expressing a cytotoxic T-lymphocyte epitope of a malaria parasite or of influenza virus elicit protection against the corresponding pathogen in mice. J Virol 72 : 6907–6910.
27. StephensR, AlbanoFR, QuinS, PascalBJ, HarrisonV, et al. (2005) Malaria-specific transgenic CD4(+) T cells protect immunodeficient mice from lethal infection and demonstrate requirement for a protective threshold of antibody production for parasite clearance. Blood 106 : 1676–1684.
28. SchmidtNW, PodyminoginRL, ButlerNS, BadovinacVP, TuckerBJ, et al. (2008) Memory CD8 T cell responses exceeding a large but definable threshold provide long-term immunity to malaria. Proc Natl Acad Sci U S A 105 : 14017–14022.
29. OverstreetMG, CockburnIA, ChenYC, ZavalaF (2008) Protective CD8 T cells against Plasmodium liver stages: immunobiology of an ‘unnatural’ immune response. Immunol Rev 225 : 272–283.
30. StephensR, LanghorneJ (2010) Effector memory Th1 CD4 T cells are maintained in a mouse model of chronic malaria. PLoS Pathog 6: e1001208.
31. NussenzweigRS, VanderbergJ, MostH, OrtonC (1967) Protective immunity produced by the injection of x-irradiated sporozoites of plasmodium berghei. Nature 216 : 160–162.
32. ClydeDF, MostH, McCarthyVC, VanderbergJP (1973) Immunization of man against sporozite-induced falciparum malaria. Am J Med Sci 266 : 169–177.
33. HoffmanSL, DoolanDL (2000) Malaria vaccines-targeting infected hepatocytes. Nat Med 6 : 1218–1219.
34. RoestenbergM, McCallM, HopmanJ, WiersmaJ, LutyAJ, et al. (2009) Protection against a malaria challenge by sporozoite inoculation. N Engl J Med 361 : 468–477.
35. FriesenJ, SilvieO, PutriantiED, HafallaJC, MatuschewskiK, et al. (2010) Natural immunization against malaria: causal prophylaxis with antibiotics. Sci Transl Med 2 : 40ra49.
36. RoestenbergM, TeirlinckAC, McCallMB, TeelenK, MakamdopKN, et al. (2011) Long-term protection against malaria after experimental sporozoite inoculation: an open-label follow-up study. Lancet 377 : 1770–1776.
37. ReeceWH, PinderM, GothardPK, MilliganP, BojangK, et al. (2004) A CD4(+) T-cell immune response to a conserved epitope in the circumsporozoite protein correlates with protection from natural Plasmodium falciparum infection and disease. Nat Med 10 : 406–410.
38. TodrykSM, BejonP, MwangiT, PlebanskiM, UrbanB, et al. (2008) Correlation of memory T cell responses against TRAP with protection from clinical malaria, and CD4 CD25 high T cells with susceptibility in Kenyans. PLoS ONE 3: e2027.
39. KurtisJD, HollingdaleMR, LutyAJ, LanarDE, KrzychU, et al. (2001) Pre-erythrocytic immunity to Plasmodium falciparum: the case for an LSA-1 vaccine. Trends Parasitol 17 : 219–223.
40. HoffmanSL, OsterCN, MasonC, BeierJC, SherwoodJA, et al. (1989) Human lymphocyte proliferative response to a sporozoite T cell epitope correlates with resistance to falciparum malaria. J Immunol 142 : 1299–1303.
41. LutyAJ, LellB, Schmidt-OttR, LehmanLG, LucknerD, et al. (1999) Interferon-gamma responses are associated with resistance to reinfection with Plasmodium falciparum in young African children. J Infect Dis 179 : 980–988.
42. MoormannAM, SumbaPO, ChelimoK, FangH, TischDJ, et al. (2013) Humoral and Cellular Immunity to Plasmodium falciparum Merozoite Surface Protein 1 and Protection From Infection With Blood-Stage Parasites. J Infect Dis 208 : 149–158.
43. D'OmbrainMC, RobinsonLJ, StanisicDI, TaraikaJ, BernardN, et al. (2008) Association of early interferon-gamma production with immunity to clinical malaria: a longitudinal study among Papua New Guinean children. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America 47 : 1380–1387.
44. McCallMB, HopmanJ, DaouM, MaigaB, DaraV, et al. (2010) Early interferon-gamma response against Plasmodium falciparum correlates with interethnic differences in susceptibility to parasitemia between sympatric Fulani and Dogon in Mali. J Infect Dis 201 : 142–152.
45. BejonP, WarimweG, MackintoshCL, MackinnonMJ, KinyanjuiSM, et al. (2009) Analysis of immunity to febrile malaria in children that distinguishes immunity from lack of exposure. Infect Immun 77 : 1917–1923.
46. GreenhouseB, HoB, HubbardA, Njama-MeyaD, NarumDL, et al. (2011) Antibodies to Plasmodium falciparum antigens predict a higher risk of malaria but protection from symptoms once parasitemic. J Infect Dis 204 : 19–26.
47. DouekDC, RoedererM, KoupRA (2009) Emerging concepts in the immunopathogenesis of AIDS. Annual Review of Medicine 60 : 471–484.
48. DarrahPA, PatelDT, De LucaPM, LindsayRW, DaveyDF, et al. (2007) Multifunctional TH1 cells define a correlate of vaccine-mediated protection against Leishmania major. Nature medicine 13 : 843–850.
49. AndersonCF, OukkaM, KuchrooVJ, SacksD (2007) CD4(+)CD25(−)Foxp3(−) Th1 cells are the source of IL-10-mediated immune suppression in chronic cutaneous leishmaniasis. J Exp Med 204 : 285–297.
50. JankovicD, KullbergMC, FengCG, GoldszmidRS, CollazoCM, et al. (2007) Conventional T-bet(+)Foxp3(−) Th1 cells are the major source of host-protective regulatory IL-10 during intracellular protozoan infection. J Exp Med 204 : 273–283.
51. OlotuA, MorisP, MwacharoJ, VekemansJ, KimaniD, et al. (2011) Circumsporozoite-Specific T Cell Responses in Children Vaccinated with RTS,S/AS01(E) and Protection against P falciparum Clinical Malaria. PLoS ONE 6: e25786.
52. WaltherM, JeffriesD, FinneyOC, NjieM, EbonyiA, et al. (2009) Distinct roles for FOXP3 and FOXP3 CD4 T cells in regulating cellular immunity to uncomplicated and severe Plasmodium falciparum malaria. PLoS Pathog 5: e1000364.
53. ZhangG, ManacaMN, McNamara-SmithM, MayorA, NhabombaA, et al. (2012) Interleukin-10 (IL-10) polymorphisms are associated with IL-10 production and clinical malaria in young children. Infect Immun 80 : 2316–2322.
54. Epstein JE, Tewari K, Lyke KE, Sim BK, Billingsley PF, et al. (2011) Live Attenuated Malaria Vaccine Designed to Protect through Hepatic CD8+ T Cell Immunity. Science.
55. OlotuA, MorisP, MwacharoJ, VekemansJ, KimaniD, et al. (2011) Circumsporozoite-specific T cell responses in children vaccinated with RTS,S/AS01E and protection against P falciparum clinical malaria. PLoS ONE 6: e25786.
56. CopeA, Le FriecG, CardoneJ, KemperC (2011) The Th1 life cycle: molecular control of IFN-gamma to IL-10 switching. Trends in Immunology 32 : 278–286.
57. O'GarraA, VieiraP (2007) T(H)1 cells control themselves by producing interleukin-10. Nature reviews Immunology 7 : 425–428.
58. WenischC, ParschalkB, NarztE, LooareesuwanS, GraningerW (1995) Elevated serum levels of IL-10 and IFN-gamma in patients with acute Plasmodium falciparum malaria. Clinical Immunology and Immunopathology 74 : 115–117.
59. PeyronF, BurdinN, RingwaldP, VuillezJP, RoussetF, et al. (1994) High levels of circulating IL-10 in human malaria. Clinical and experimental immunology 95 : 300–303.
60. WilsonNO, BythwoodT, SolomonW, JollyP, YatichN, et al. (2010) Elevated levels of IL-10 and G-CSF associated with asymptomatic malaria in pregnant women. Infectious Diseases in Obstetrics and Gynecology 2010: pii: 317430.
61. Pinzon-CharryA, WoodberryT, KienzleV, McPhunV, MinigoG, et al. (2013) Apoptosis and dysfunction of blood dendritic cells in patients with falciparum and vivax malaria. J Exp Med 210 : 1635–1646.
62. O'ConnorRA, JensonJS, OsborneJ, DevaneyE (2003) An enduring association? Microfilariae and immunosuppression [correction of immunosupression] in lymphatic filariasis. Trends Parasitol 19 : 565–570.
63. SteelC, GuineaA, McCarthyJS, OttesenEA (1994) Long-term effect of prenatal exposure to maternal microfilaraemia on immune responsiveness to filarial parasite antigens. Lancet 343 : 890–893.
64. WammesLJ, HamidF, WiriaAE, WibowoH, SartonoE, et al. (2012) Regulatory T cells in human lymphatic filariasis: stronger functional activity in microfilaremics. PLoS Neglected Tropical Diseases 6: e1655.
65. MaizelsRM, YazdanbakhshM (2003) Immune regulation by helminth parasites: cellular and molecular mechanisms. Nature reviews Immunology 3 : 733–744.
66. McNeilAC, ShupertWL, IyasereCA, HallahanCW, MicanJA, et al. (2001) High-level HIV-1 viremia suppresses viral antigen-specific CD4(+) T cell proliferation. Proc Natl Acad Sci U S A 98 : 13878–13883.
67. ThimmeR, OldachD, ChangKM, SteigerC, RaySC, et al. (2001) Determinants of viral clearance and persistence during acute hepatitis C virus infection. J Exp Med 194 : 1395–1406.
68. HaringerB, LozzaL, SteckelB, GeginatJ (2009) Identification and characterization of IL-10/IFN-gamma-producing effector-like T cells with regulatory function in human blood. J Exp Med 206 : 1009–1017.
69. BoussiotisVA, TsaiEY, YunisEJ, ThimS, DelgadoJC, et al. (2000) IL-10-producing T cells suppress immune responses in anergic tuberculosis patients. J Clin Invest 105 : 1317–1325.
70. MeilerF, ZumkehrJ, KlunkerS, RuckertB, AkdisCA, et al. (2008) In vivo switch to IL-10-secreting T regulatory cells in high dose allergen exposure. J Exp Med 205 : 2887–2898.
71. O'GarraA, VieiraP (2007) T(H)1 cells control themselves by producing interleukin-10. Nat Rev Immunol 7 : 425–428.
72. LiC, CorralizaI, LanghorneJ (1999) A defect in interleukin-10 leads to enhanced malarial disease in Plasmodium chabaudi chabaudi infection in mice. Infect Immun 67 : 4435–4442.
73. WinklerS, WillheimM, BaierK, SchmidD, AichelburgA, et al. (1998) Reciprocal regulation of Th1 - and Th2-cytokine-producing T cells during clearance of parasitemia in Plasmodium falciparum malaria. Infect Immun 66 : 6040–6044.
74. FlanaganKL, PlebanskiM, OdhiamboK, SheuE, MwangiT, et al. (2006) Cellular reactivity to the p. Falciparum protein trap in adult kenyans: novel epitopes, complex cytokine patterns, and the impact of natural antigenic variation. Am J Trop Med Hyg 74 : 367–375.
75. GitauEN, TujuJ, StevensonL, KimaniE, KaranjaH, et al. (2012) T-cell responses to the DBLalpha-tag, a short semi-conserved region of the Plasmodium falciparum membrane erythrocyte protein 1. PLoS ONE 7: e30095.
76. RoetynckS, OlotuA, SimamJ, MarshK, StockingerB, et al. (2013) Phenotypic and functional profiling of CD4 T cell compartment in distinct populations of healthy adults with different antigenic exposure. PLoS ONE 8: e55195.
77. Freitas do RosarioAP, LanghorneJ (2012) T cell-derived IL-10 and its impact on the regulation of host responses during malaria. International Journal for Parasitology 42 : 549–555.
78. MetenouS, DembeleB, KonateS, DoloH, CoulibalyYI, et al. (2011) Filarial infection suppresses malaria-specific multifunctional Th1 and Th17 responses in malaria and filarial coinfections. Journal of immunology 186 : 4725–4733.
79. BrustoskiK, MollerU, KramerM, PetelskiA, BrennerS, et al. (2005) IFN-gamma and IL-10 mediate parasite-specific immune responses of cord blood cells induced by pregnancy-associated Plasmodium falciparum malaria. Journal of immunology 174 : 1738–1745.
80. BrockmanMA, KwonDS, TigheDP, PavlikDF, RosatoPC, et al. (2009) IL-10 is up-regulated in multiple cell types during viremic HIV infection and reversibly inhibits virus-specific T cells. Blood 114 : 346–356.
81. BrooksDG, TrifiloMJ, EdelmannKH, TeytonL, McGavernDB, et al. (2006) Interleukin-10 determines viral clearance or persistence in vivo. Nat Med 12 : 1301–1309.
82. DepinayN, FranetichJF, GrunerAC, MauduitM, ChavatteJM, et al. (2011) Inhibitory effect of TNF-alpha on malaria pre-erythrocytic stage development: influence of host hepatocyte/parasite combinations. PLoS ONE 6: e17464.
83. NusslerA, PiedS, GomaJ, ReniaL, MiltgenF, et al. (1991) TNF inhibits malaria hepatic stages in vitro via synthesis of IL-6. International Immunology 3 : 317–321.
84. KatrakS, GasasiraA, ArinaitweE, KakuruA, WanziraH, et al. (2009) Safety and tolerability of artemether-lumefantrine versus dihydroartemisinin-piperaquine for malaria in young HIV-infected and uninfected children. Malaria Journal 8 : 272.
85. SandisonTG, HomsyJ, ArinaitweE, WanziraH, KakuruA, et al. (2011) Protective efficacy of co-trimoxazole prophylaxis against malaria in HIV exposed children in rural Uganda: a randomised clinical trial. BMJ 342: d1617.
86. VoraN, HomsyJ, KakuruA, ArinaitweE, WanziraH, et al. (2010) Breastfeeding and the risk of malaria in children born to HIV-infected and uninfected mothers in rural Uganda. Journal of Acquired Immune Deficiency Syndromes 55 : 253–261.
87. ArinaitweE, SandisonTG, WanziraH, KakuruA, HomsyJ, et al. (2009) Artemether-lumefantrine versus dihydroartemisinin-piperaquine for falciparum malaria: a longitudinal, randomized trial in young Ugandan children. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America 49 : 1629–1637.
88. (2010) Uganda Clinical Guidelines 2010. National Guidelines on Management of Common Conditions. Kampala, Uganda: Uganda Ministry of Health.
89. WipasaJ, OkellL, SakkhachornphopS, SuphavilaiC, ChawansuntatiK, et al. (2011) Short-lived IFN-gamma effector responses, but long-lived IL-10 memory responses, to malaria in an area of low malaria endemicity. PLoS pathogens 7: e1001281.
90. HorowitzA, NewmanKC, EvansJH, KorbelDS, DavisDM, et al. (2010) Cross-talk between T cells and NK cells generates rapid effector responses to Plasmodium falciparum-infected erythrocytes. J Immunol 184 : 6043–6052.
91. MaeckerHT, TrotterJ (2006) Flow cytometry controls, instrument setup, and the determination of positivity. Cytometry Part A 69 : 1037–1042.
92. LamoreauxL, RoedererM, KoupR (2006) Intracellular cytokine optimization and standard operating procedure. Nat Protoc 1 : 1507–1516.
93. McLaughlinBE, BaumgarthN, BigosM, RoedererM, De RosaSC, et al. (2008) Nine-color flow cytometry for accurate measurement of T cell subsets and cytokine responses. Part I: Panel design by an empiric approach. Cytometry Part A 73 : 400–410.
94. RoedererM, NozziJL, NasonMX (2011) SPICE: Exploration and analysis of post-cytometric complex multivariate datasets. Cytometry Part A [Epub ahead of print].
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek Serotonin Signaling in : A Serotonin–Activated G Protein-Coupled Receptor Controls Parasite MovementČlánek Regulators of Cell Cycle Progression and Differentiation Identified Using a Kinome-Wide RNAi ScreenČlánek Functions of CPSF6 for HIV-1 as Revealed by HIV-1 Capsid Evolution in HLA-B27-Positive SubjectsČlánek Decreases in Colonic and Systemic Inflammation in Chronic HIV Infection after IL-7 Administration
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2014 Číslo 1- Stillova choroba: vzácné a závažné systémové onemocnění
- Jak souvisí postcovidový syndrom s poškozením mozku?
- Perorální antivirotika jako vysoce efektivní nástroj prevence hospitalizací kvůli COVID-19 − otázky a odpovědi pro praxi
- Diagnostika virových hepatitid v kostce – zorientujte se (nejen) v sérologii
- Infekční komplikace virových respiračních infekcí – sekundární bakteriální a aspergilové pneumonie
-
Všechny články tohoto čísla
- HIV-1 Accessory Proteins Adapt Cellular Adaptors to Facilitate Immune Evasion
- Ranaviruses: Not Just for Frogs
- Effectors and Effector Delivery in
- Plasmacytoid Dendritic Cell Dynamics Tune Interferon-Alfa Production in SIV-Infected Cynomolgus Macaques
- Lu/BCAM Adhesion Glycoprotein Is a Receptor for Cytotoxic Necrotizing Factor 1 (CNF1)
- A Substrate-Fusion Protein Is Trapped inside the Type III Secretion System Channel in
- Parvovirus-Induced Depletion of Cyclin B1 Prevents Mitotic Entry of Infected Cells
- Red Blood Cell Invasion by : Structural Basis for DBP Engagement of DARC
- NsrR, GadE, and GadX Interplay in Repressing Expression of the O157:H7 LEE Pathogenicity Island in Response to Nitric Oxide
- Loss of Circulating CD4 T Cells with B Cell Helper Function during Chronic HIV Infection
- TREM-1 Deficiency Can Attenuate Disease Severity without Affecting Pathogen Clearance
- Origin, Migration Routes and Worldwide Population Genetic Structure of the Wheat Yellow Rust Pathogen f.sp.
- Glutamate Utilization Couples Oxidative Stress Defense and the Tricarboxylic Acid Cycle in Phagosomal Escape
- Serotonin Signaling in : A Serotonin–Activated G Protein-Coupled Receptor Controls Parasite Movement
- Recovery of an Antiviral Antibody Response following Attrition Caused by Unrelated Infection
- Regulators of Cell Cycle Progression and Differentiation Identified Using a Kinome-Wide RNAi Screen
- Absence of Intestinal PPARγ Aggravates Acute Infectious Colitis in Mice through a Lipocalin-2–Dependent Pathway
- Induction of a Stringent Metabolic Response in Intracellular Stages of Leads to Increased Dependence on Mitochondrial Metabolism
- CTCF and Rad21 Act as Host Cell Restriction Factors for Kaposi's Sarcoma-Associated Herpesvirus (KSHV) Lytic Replication by Modulating Viral Gene Transcription
- Gammaherpesviral Gene Expression and Virion Composition Are Broadly Controlled by Accelerated mRNA Degradation
- The Arabidopsis Silencing Pathway Modulates PAMP- and Effector-Triggered Immunity through the Post-transcriptional Control of Disease Resistance Genes
- Inflammatory Stimuli Reprogram Macrophage Phagocytosis to Macropinocytosis for the Rapid Elimination of Pathogens
- Alphavirus Mutator Variants Present Host-Specific Defects and Attenuation in Mammalian and Insect Models
- Phosphopyruvate Carboxylase Identified as a Key Enzyme in Erythrocytic Carbon Metabolism
- IFNγ/IL-10 Co-producing Cells Dominate the CD4 Response to Malaria in Highly Exposed Children
- Electron Tomography of HIV-1 Infection in Gut-Associated Lymphoid Tissue
- Characterisation of a Multi-ligand Binding Chemoreceptor CcmL (Tlp3) of
- Single Cell Stochastic Regulation of Pilus Phase Variation by an Attenuation-like Mechanism
- Cell Tropism Predicts Long-term Nucleotide Substitution Rates of Mammalian RNA Viruses
- Functions of CPSF6 for HIV-1 as Revealed by HIV-1 Capsid Evolution in HLA-B27-Positive Subjects
- RNA-seq Analysis of Host and Viral Gene Expression Highlights Interaction between Varicella Zoster Virus and Keratinocyte Differentiation
- Kaposi's Sarcoma Associated Herpesvirus Tegument Protein ORF75 Is Essential for Viral Lytic Replication and Plays a Critical Role in the Antagonization of ND10-Instituted Intrinsic Immunity
- DAMP Molecule S100A9 Acts as a Molecular Pattern to Enhance Inflammation during Influenza A Virus Infection: Role of DDX21-TRIF-TLR4-MyD88 Pathway
- Variable Suites of Non-effector Genes Are Co-regulated in the Type III Secretion Virulence Regulon across the Phylogeny
- Reengineering Redox Sensitive GFP to Measure Mycothiol Redox Potential of during Infection
- Preservation of Tetherin and CD4 Counter-Activities in Circulating Alleles despite Extensive Sequence Variation within HIV-1 Infected Individuals
- KSHV 2.0: A Comprehensive Annotation of the Kaposi's Sarcoma-Associated Herpesvirus Genome Using Next-Generation Sequencing Reveals Novel Genomic and Functional Features
- Nutrient Limitation Governs Metabolism and Niche Adaptation in the Human Nose
- Decreases in Colonic and Systemic Inflammation in Chronic HIV Infection after IL-7 Administration
- Investigation of Acetylcholine Receptor Diversity in a Nematode Parasite Leads to Characterization of Tribendimidine- and Derquantel-Sensitive nAChRs
- Intranasal Vaccination Promotes Detrimental Th17-Mediated Immunity against Influenza Infection
- -Mediated Inhibition of Iron Export Promotes Parasite Replication in Macrophages
- Variation in RNA Virus Mutation Rates across Host Cells
- A Single Amino Acid in the Stalk Region of the H1N1pdm Influenza Virus HA Protein Affects Viral Fusion, Stability and Infectivity
- Group B Engages an Inhibitory Siglec through Sialic Acid Mimicry to Blunt Innate Immune and Inflammatory Responses
- Synthesis and Biological Properties of Fungal Glucosylceramide
- HIV Protective KIR3DL1/S1-HLA-B Genotypes Influence NK Cell-Mediated Inhibition of HIV Replication in Autologous CD4 Targets
- Recruitment of PfSET2 by RNA Polymerase II to Variant Antigen Encoding Loci Contributes to Antigenic Variation in
- Human and Plant Fungal Pathogens: The Role of Secondary Metabolites
- Lyme Disease: Call for a “Manhattan Project” to Combat the Epidemic
- Enhancing Virus-Specific Immunity by Combining Therapeutic Vaccination and PD-L1 Blockade in Chronic Hepadnaviral Infection
- Suppression of Interferon Lambda Signaling by SOCS-1 Results in Their Excessive Production during Influenza Virus Infection
- Inflammation Fuels Colicin Ib-Dependent Competition of Serovar Typhimurium and in Blooms
- Host-Specific Enzyme-Substrate Interactions in SPM-1 Metallo-β-Lactamase Are Modulated by Second Sphere Residues
- STING-Dependent Type I IFN Production Inhibits Cell-Mediated Immunity to
- From Scourge to Cure: Tumour-Selective Viral Pathogenesis as a New Strategy against Cancer
- Lysine Acetyltransferase GCN5b Interacts with AP2 Factors and Is Required for Proliferation
- Narrow Bottlenecks Affect Populations during Vertical Seed Transmission but not during Leaf Colonization
- Targeted Cytotoxic Therapy Kills Persisting HIV Infected Cells During ART
- Murine Gammaherpesvirus M2 Protein Induction of IRF4 via the NFAT Pathway Leads to IL-10 Expression in B Cells
- iNKT Cell Production of GM-CSF Controls
- Malaria-Induced NLRP12/NLRP3-Dependent Caspase-1 Activation Mediates Inflammation and Hypersensitivity to Bacterial Superinfection
- Detection of Host-Derived Sphingosine by Is Important for Survival in the Murine Lung
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Lyme Disease: Call for a “Manhattan Project” to Combat the Epidemic
- Origin, Migration Routes and Worldwide Population Genetic Structure of the Wheat Yellow Rust Pathogen f.sp.
- IFNγ/IL-10 Co-producing Cells Dominate the CD4 Response to Malaria in Highly Exposed Children
- Human and Plant Fungal Pathogens: The Role of Secondary Metabolites
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



