-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
What Do We Really Know about How CD4 T Cells Control ?
article has not abstract
Published in the journal: . PLoS Pathog 7(7): e32767. doi:10.1371/journal.ppat.1002196
Category: Opinion
doi: https://doi.org/10.1371/journal.ppat.1002196Summary
article has not abstract
Two recent papers in PLoS Pathogens have investigated the activity of antigen-specific cells within the lung of mice infected with Mycobacterium tuberculosis (Mtb) [1], [2]. To the uninitiated this may seem to be redundant, as “we all know” that antigen-specific cells make interferon gamma (IFNγ) and tumor necrosis factor (TNF), which activate infected phagocytes to kill the bacteria. However, what we really know is that IFNγ and TNF are essential for controlling both bacterial growth and immunopathology and that the acquired immune response is critical to orchestrating immunity [3]. What we also know is that cessation of bacterial growth in the lungs correlates temporally with the accumulation of IFNγ-producing antigen-specific T cells in the infected lung [3]. But several questions have been circulating in the field for some time, including, What are effector T cells doing during tuberculosis to mediate protection, and How does the environment within the granuloma affect this activity? [3].
In the first of these stimulating PLoS Pathogens papers, Gallegos et al. provided compelling evidence that CD4 T cells can induce Mtb growth arrest, even when unable to secrete IFNγ, TNF, or both cytokines [1]. In the second paper, Bold et al. showed that CD4 T cell activation (as measured by production of IFNγ) is suboptimal in the lungs of infected animals, and they suggest that this contributes to the inability of the host to eliminate the infection; they also link this low frequency of T cell activation to the level of cognate antigen in the lung [2].
In the paper by Gallegos et al., they investigated the relevance of cytokine-producing CD4 T cells during experimental Mtb infection by the transfer of T cell receptor transgenic (TCR Tg) cells into host mice. They found that growth arrest over the first 21 days after aerosol challenge occurred even when these cells were unable to express the Th1-promoting transcription factor T-bet or to secrete IFNγ, TNF, or both cytokines [1]. Of equal importance was the fact that there was no need for host IFNγ, TNF, the inducible nitric oxide synthase (iNOS) gene, or superoxide-generating machinery to mediate this control [1]. This antigen-specific effect could be seen both with in vitro expanded and polarized T cells as well as, to a lesser degree, naive T cells [1]. In addition, the authors showed that this was not a property of all in vitro–generated cells, since Th2 differentiated cells could not induce Mtb growth arrest as efficiently as Th1 or even Th17 differentiated cells. While the mechanism was not identified, previously published data have shown that in vitro–generated memory CD4 T cells enhance protection in the flu model by induction of multiple innate cytokines and chemokines in the lung in an antigen-dependent, but IFNγ and TNF independent, manner [4]. Another possibility is that elevated precursor frequency may dampen the activation of antigen-specific regulatory T cells, and indeed, the authors report that the adoptively transferred cells delayed the priming of the endogenous response [1]. Recent data have shown that regulatory T cells are induced very early and can regulate effector function in the aerosol Mtb models [5]. Bold et al. considered the importance of competition in their study but were concerned that the endogenous response was limiting the transferred response as a result of competition or regulatory activity—they found, however, that depletion of half of the endogenous cells within the lung did not result in increased activation of the transferred effector cells [2].
The “take home” message of the Bold paper is that the frequency of cells that produce IFNγ is low, even at the peak of the response, and that it decreases during the chronic phase [2], supporting previous work that suggested this pattern [6]. In the Bold paper, just as in the Gallegos paper, the authors transferred pre-activated antigen-specific TCR Tg CD4 T cells and used the expression of IFNγ (assessed directly ex vivo) as a marker of antigen recognition. They showed that the frequency of IFNγ-producing cells correlated with the availability of cognate antigen and that delivery of the cognate peptide resulted in greatly increased frequency of cytokine expression [2]. They also saw a modest decrease in bacterial numbers when the antigen was either forcibly expressed by the Mtb or if the antigen was delivered exogenously to the infected mice. Initiation of CD4 T cell responses during tuberculosis occurs in the lung-draining lymph nodes rather than in the lung; however, the data by Bold et al. support the hypothesis that CD4 T cells need to see antigen once again within the infection site to express their effector function. Another recent paper, wherein intravital multiphoton imaging was used to compare the movement patterns and effector function of pre-activated and control TCR Tg CD4 T cells (from the p25 mouse specific for Ag85 [7] also used in [2]), has also shown that effector function is poorly expressed in the granuloma [8]. The authors made the surprising observations that both mycobacteria-specific and non-specific CD4 T cell migrated vigorously through the granuloma, with very few antigen-specific T cells showing migration arrest, a hallmark of potent antigen recognition and presentation. Despite the observed rapid migration, the antigen-specific cells reacted differently to the antigen in the environment as they upregulated CD69, whilst the control non-antigen-specific T cells did not. As was seen in the Bold paper, there was very little real-time expression of IFNγ within the granuloma in this model. These data were taken to reflect the failure of the available antigen to signal both migration arrest and cytokine production by the effector cell, and this was supported by the fact that delivery of exogenous peptide resulted in expression of these functions by the antigen-specific cells within the granuloma [8]. One exciting observation of this work was that the cells that did exhibit migration arrest could (but not always) produce a targeted release of cytokine to a closely adjacent infected cell; these data suggest that the accepted protective mechanism of T cell–derived IFNγ-mediation of infected phagocyte activation does occur in the granuloma.
However, putting these recent observations together, it is clear that the accepted mechanism may not be all there is to the control of Mtb. It would seem that expression of full effector activity by antigen-specific CD4 T cells within the granuloma is constrained by antigen availability and that there is the potential for antigen-specific T cells to mediate their effector function without the use of cytokine. As we cannot currently measure this effector function, other than by bacterial arrest, we cannot discount the possibility that the appropriate effector function is being expressed, but that it does not require significant migration arrest or cytokine production (Figure 1).
Fig. 1. Effector T cells do not find the granuloma to be a stimulating environment. 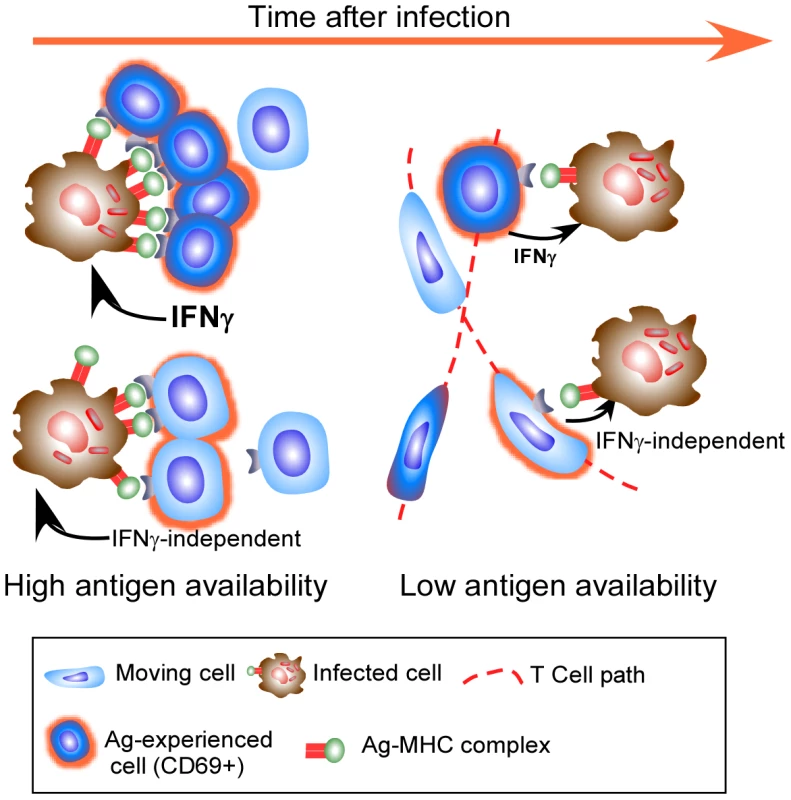
Effector T cells enter the granuloma and only a few exhibit significant migration arrest (dark blue cells) and targeted release of IFNγ, likely when they encounter a high level of cognate antigen on infected phagocytes. As their cognate antigen is reduced, even fewer cells undergo migration arrest, with many more cells continuing to move throughout the granuloma (light blue motile cells). Although these cells do not stop migrating, they do up regulate CD69 in an antigen-specific manner. Cells entering the granuloma may mediate their effector function without the release of IFNγ, and while this activity does require recognition of antigen, it may not need migration arrest. Other factors to take into account when thinking about the above papers is the artificial nature of transferred TCR Tg T cells, which may allow them to act differently to endogenous responses. It is also important to remember that specific protective immune mechanisms have different levels of importance depending on the potency of the bacterial challenge (discussed in [9]). Most importantly, the relative levels of specific mycobacterial antigens, particularly Ag85, which is the target of the TCR Tg cells used to assess effector function in the granuloma [2], [8], change over time as a function of bacterial physiology in the face of host immunity [10], [11]. These changes in bacterial activity will substantially impact the readout to any one antigen, and it is therefore important to investigate the activity of cells specific for other antigen as well as to measure activities other than IFNγ production. Despite the caveats, these papers remind us that although bacterial growth ceases within the resistant mouse model, we still do not know quite how this occurs. These recent excellent papers prompt us to continue to investigate the microanatomy of T cell function within the granuloma and to not be content with “what we know”.
Finally, the key question is, how can this information improve control of tuberculosis? It is certainly critical to define the protective effector functions of antigen-specific T cells as well as to determine the significance of suboptimal T cell activation. In this way we will be better able to design more effective vaccines and to determine whether the limited T cell activation in the granuloma is a host mechanism to cope with chronic infection or a mechanism Mtb evolved to prevent elimination by the host [12]. By continuing to pursue the above goals, we will be able to manipulate T cell responses in the infection site to enhance their effector function and to tip the balance of disease in favor of the host with minimal immunopathological consequences.
Zdroje
1. GallegosAMvan HeijstJWJSamsteinMSuXPamerEG 2011 A gamma interferon independent mechanism of CD4 T cell mediated control of M. tuberculosis infection in vivo. PLoS Pathog 7 e1002052 doi:10.1371/journal.ppat.1002052
2. BoldTDBanaeiNWolfAJErnstJD 2011 Suboptimal activation of antigen-specific CD4+ effector cells enables persistence of M. tuberculosis in vivo. PLoS Pathog 7 e1002063 doi:10.1371/journal.ppat.1002063
3. CooperAM 2009 Cell mediated immune responses in tuberculosis. Annu Rev Immunol 27 393 422
4. StruttTMMcKinstryKKDibbleJPWinchellCKuangY 2010 Memory CD4+ T cells induce innate responses independently of pathogen. Nat Med 16 558 564
5. ShafianiSTucker-HeardGKariyoneATakatsuKUrdahlKB 2010 Pathogen-specific regulatory T cells delay the arrival of effector T cells in the lung during early tuberculosis. J Exp Med 207 1409 1420
6. WinslowGMRobertsADBlackmanMAWoodlandDL 2003 Persistence and turnover of antigen-specific CD4 T cells during chronic tuberculosis infection in the mouse. J Immunol 170 2046 2052
7. TamuraTArigaHKinashiTUeharaSKikuchiT 2004 The role of antigenic peptide in CD4+ T helper phenotype development in a T cell receptor transgenic model. Int Immunol 16 1691 1699
8. EgenJGRothfuchsAGFengCGHorwitzMASherA 2011 Intravital imaging reveals limited antigen presentation and T cell effector function in mycobacterial granulomas. Immunity 34 807 819
9. CooperAMayer-BarberKSherA 2011 Role of innate cytokines in mycobacterial infection. Mucosal Immunol 4 252 260
10. ShiLJungYTyagiSGennaroMNorthR 2003 Expression of Th1-mediated immunity in mouse lungs induces a Mycobacterium tuberculosis transcription pattern characteristic of nonreplicating persistence. Proc Natl Acad Sci U S A 100 241 246
11. AagaardCHoangTDietrichJCardonaP-JIzzoA 2011 A multistage tuberculosis vaccine that confers efficient protection before and after exposure. Nat Med 17 189 194
12. TorradoERobinsonRTCooperAM 2011 Cellular response to mycobacteria: balancing protection and pathology. Trends Immunol 32 66 72
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek A Typhimurium-Typhi Genomic Chimera: A Model to Study Vi Polysaccharide Capsule Function In VivoČlánek SUMO Pathway Dependent Recruitment of Cellular Repressors to Herpes Simplex Virus Type 1 GenomesČlánek A Structural Model for Binding of the Serine-Rich Repeat Adhesin GspB to Host Carbohydrate ReceptorsČlánek Dynamic Evolution of Pathogenicity Revealed by Sequencing and Comparative Genomics of 19 IsolatesČlánek Widespread Endogenization of Genome Sequences of Non-Retroviral RNA Viruses into Plant GenomesČlánek The Cost of Virulence: Retarded Growth of Typhimurium Cells Expressing Type III Secretion System 1Článek A Role for the Chemokine RANTES in Regulating CD8 T Cell Responses during Chronic Viral Infection
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2011 Číslo 7- Stillova choroba: vzácné a závažné systémové onemocnění
- Diagnostika virových hepatitid v kostce – zorientujte se (nejen) v sérologii
- Perorální antivirotika jako vysoce efektivní nástroj prevence hospitalizací kvůli COVID-19 − otázky a odpovědi pro praxi
- Choroby jater v ordinaci praktického lékaře – význam jaterních testů
- Diagnostický algoritmus při podezření na syndrom periodické horečky
-
Všechny články tohoto čísla
- What Do We Really Know about How CD4 T Cells Control ?
- “Persisters”: Survival at the Cellular Level
- E6 and E7 from Beta Hpv38 Cooperate with Ultraviolet Light in the Development of Actinic Keratosis-Like Lesions and Squamous Cell Carcinoma in Mice
- Selection of Resistant Bacteria at Very Low Antibiotic Concentrations
- The Extracytoplasmic Domain of the Ser/Thr Kinase PknB Binds Specific Muropeptides and Is Required for PknB Localization
- CD39/Adenosine Pathway Is Involved in AIDS Progression
- Hypoxia and a Fungal Alcohol Dehydrogenase Influence the Pathogenesis of Invasive Pulmonary Aspergillosis
- One Is Enough: Effective Population Size Is Dose-Dependent for a Plant RNA Virus
- Effects of Interferon-α/β on HBV Replication Determined by Viral Load
- A Typhimurium-Typhi Genomic Chimera: A Model to Study Vi Polysaccharide Capsule Function In Vivo
- Dual Chaperone Role of the C-Terminal Propeptide in Folding and Oligomerization of the Pore-Forming Toxin Aerolysin
- Rotavirus Stimulates Release of Serotonin (5-HT) from Human Enterochromaffin Cells and Activates Brain Structures Involved in Nausea and Vomiting
- Dissociation of Infectivity from Seeding Ability in Prions with Alternate Docking Mechanism
- The Impact of Recombination on dN/dS within Recently Emerged Bacterial Clones
- The Regulation of Sulfur Metabolism in
- Illumination of Parainfluenza Virus Infection and Transmission in Living Animals Reveals a Tissue-Specific Dichotomy
- A Permeable Cuticle Is Associated with the Release of Reactive Oxygen Species and Induction of Innate Immunity
- A Concerted Action of Hepatitis C Virus P7 and Nonstructural Protein 2 Regulates Core Localization at the Endoplasmic Reticulum and Virus Assembly
- SUMO Pathway Dependent Recruitment of Cellular Repressors to Herpes Simplex Virus Type 1 Genomes
- Re-localization of Cellular Protein SRp20 during Poliovirus Infection: Bridging a Viral IRES to the Host Cell Translation Apparatus
- Divergent Effects of Human Cytomegalovirus and Herpes Simplex Virus-1 on Cellular Metabolism
- A Structural Model for Binding of the Serine-Rich Repeat Adhesin GspB to Host Carbohydrate Receptors
- Transformation of Natural Genetic Variation into Genomes
- EBV Latency Types Adopt Alternative Chromatin Conformations
- Global mRNA Degradation during Lytic Gammaherpesvirus Infection Contributes to Establishment of Viral Latency
- Dynamic Evolution of Pathogenicity Revealed by Sequencing and Comparative Genomics of 19 Isolates
- Microbial Virulence as an Emergent Property: Consequences and Opportunities
- Widespread Endogenization of Genome Sequences of Non-Retroviral RNA Viruses into Plant Genomes
- Structural Basis of Chemokine Sequestration by CrmD, a Poxvirus-Encoded Tumor Necrosis Factor Receptor
- Cross-Species Transmission of a Novel Adenovirus Associated with a Fulminant Pneumonia Outbreak in a New World Monkey Colony
- An Interaction between KSHV ORF57 and UIF Provides mRNA-Adaptor Redundancy in Herpesvirus Intronless mRNA Export
- Elevated 17β-Estradiol Protects Females from Influenza A Virus Pathogenesis by Suppressing Inflammatory Responses
- The Role of IL-15 Deficiency in the Pathogenesis of Virus-Induced Asthma Exacerbations
- Fluorescence Lifetime Imaging Unravels Metabolism and Its Crosstalk with the Host Cell
- Programmed Death (PD)-1-Deficient Mice Are Extremely Sensitive to Murine Hepatitis Virus Strain-3 (MHV-3) Infection
- Hemoglobin Promotes Nasal Colonization
- Crystallography of a Lewis-Binding Norovirus, Elucidation of Strain-Specificity to the Polymorphic Human Histo-Blood Group Antigens
- The Cost of Virulence: Retarded Growth of Typhimurium Cells Expressing Type III Secretion System 1
- A Genome-Wide Approach to Discovery of Small RNAs Involved in Regulation of Virulence in
- Requires Glycerol for Maximum Fitness During The Tick Phase of the Enzootic Cycle
- C Metabolic Flux Analysis Identifies an Unusual Route for Pyruvate Dissimilation in Mycobacteria which Requires Isocitrate Lyase and Carbon Dioxide Fixation
- A Role for the Chemokine RANTES in Regulating CD8 T Cell Responses during Chronic Viral Infection
- Glycosaminoglycans and Sialylated Glycans Sequentially Facilitate Merkel Cell Polyomavirus Infectious Entry
- Regulation of Stomatal Tropism and Infection by Light in : Evidence for Coordinated Host/Pathogen Responses to Photoperiod?
- Multiple Translocation of the Effector Gene among Chromosomes of the Rice Blast Fungus and Related Species
- Comparative Genomics Yields Insights into Niche Adaptation of Plant Vascular Wilt Pathogens
- Unique Cell Adhesion and Invasion Properties of O:3, the Most Frequent Cause of Human Yersiniosis
- C-Terminal Region of EBNA-2 Determines the Superior Transforming Ability of Type 1 Epstein-Barr Virus by Enhanced Gene Regulation of LMP-1 and CXCR7
- Novel Chikungunya Vaccine Candidate with an IRES-Based Attenuation and Host Range Alteration Mechanism
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Requires Glycerol for Maximum Fitness During The Tick Phase of the Enzootic Cycle
- Comparative Genomics Yields Insights into Niche Adaptation of Plant Vascular Wilt Pathogens
- The Role of IL-15 Deficiency in the Pathogenesis of Virus-Induced Asthma Exacerbations
- “Persisters”: Survival at the Cellular Level
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



