-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
A Permeable Cuticle Is Associated with the Release of Reactive Oxygen Species and Induction of Innate Immunity
Wounded leaves of Arabidopsis thaliana show transient immunity to Botrytis cinerea, the causal agent of grey mould. Using a fluorescent probe, histological staining and a luminol assay, we now show that reactive oxygen species (ROS), including H2O2 and O2−, are produced within minutes after wounding. ROS are formed in the absence of the enzymes Atrboh D and F and can be prevented by diphenylene iodonium (DPI) or catalase. H2O2 was shown to protect plants upon exogenous application. ROS accumulation and resistance to B. cinerea were abolished when wounded leaves were incubated under dry conditions, an effect that was found to depend on abscisic acid (ABA). Accordingly, ABA biosynthesis mutants (aba2 and aba3) were still fully resistant under dry conditions even without wounding. Under dry conditions, wounded plants contained higher ABA levels and displayed enhanced expression of ABA-dependent and ABA-reporter genes. Mutants impaired in cutin synthesis such as bdg and lacs2.3 are already known to display a high level of resistance to B. cinerea and were found to produce ROS even when leaves were not wounded. An increased permeability of the cuticle and enhanced ROS production were detected in aba2 and aba3 mutants as described for bdg and lacs2.3. Moreover, leaf surfaces treated with cutinase produced ROS and became more protected to B. cinerea. Thus, increased permeability of the cuticle is strongly linked with ROS formation and resistance to B. cinerea. The amount of oxalic acid, an inhibitor of ROS secreted by B. cinerea could be reduced using plants over expressing a fungal oxalate decarboxylase of Trametes versicolor. Infection of such plants resulted in a faster ROS accumulation and resistance to B. cinerea than that observed in untransformed controls, demonstrating the importance of fungal suppression of ROS formation by oxalic acid. Thus, changes in the diffusive properties of the cuticle are linked with the induction ROS and attending innate defenses.
Published in the journal: . PLoS Pathog 7(7): e32767. doi:10.1371/journal.ppat.1002148
Category: Research Article
doi: https://doi.org/10.1371/journal.ppat.1002148Summary
Wounded leaves of Arabidopsis thaliana show transient immunity to Botrytis cinerea, the causal agent of grey mould. Using a fluorescent probe, histological staining and a luminol assay, we now show that reactive oxygen species (ROS), including H2O2 and O2−, are produced within minutes after wounding. ROS are formed in the absence of the enzymes Atrboh D and F and can be prevented by diphenylene iodonium (DPI) or catalase. H2O2 was shown to protect plants upon exogenous application. ROS accumulation and resistance to B. cinerea were abolished when wounded leaves were incubated under dry conditions, an effect that was found to depend on abscisic acid (ABA). Accordingly, ABA biosynthesis mutants (aba2 and aba3) were still fully resistant under dry conditions even without wounding. Under dry conditions, wounded plants contained higher ABA levels and displayed enhanced expression of ABA-dependent and ABA-reporter genes. Mutants impaired in cutin synthesis such as bdg and lacs2.3 are already known to display a high level of resistance to B. cinerea and were found to produce ROS even when leaves were not wounded. An increased permeability of the cuticle and enhanced ROS production were detected in aba2 and aba3 mutants as described for bdg and lacs2.3. Moreover, leaf surfaces treated with cutinase produced ROS and became more protected to B. cinerea. Thus, increased permeability of the cuticle is strongly linked with ROS formation and resistance to B. cinerea. The amount of oxalic acid, an inhibitor of ROS secreted by B. cinerea could be reduced using plants over expressing a fungal oxalate decarboxylase of Trametes versicolor. Infection of such plants resulted in a faster ROS accumulation and resistance to B. cinerea than that observed in untransformed controls, demonstrating the importance of fungal suppression of ROS formation by oxalic acid. Thus, changes in the diffusive properties of the cuticle are linked with the induction ROS and attending innate defenses.
Introduction
The cuticle is mainly considered as a constitutive barrier against water loss, irradiation, xenobiotics or pathogens [1], [2]. The structure of this lipid boundary layer covering aerial parts of plants is made of waxes covering and interspersed in cutin, a polymer layer formed by a network of esterified Ω-hydroxylated fatty acids that are produced and secreted by the epidermis cells [3]. Waxes comprise a mixture of very long-chain fatty acids (24–36 carbon atoms) that seem ubiquitously present in most plant species. In addition, triterpenes, β-diketones as well as phenylpropanoids are associated with the wax fraction [4], [5]. The enzymatic machinery for wax biosynthesis is in the endoplasmic reticulum and members of a subfamily of ATP binding cassette (ABC) transporters export the resulting products through the membrane to the cell wall [5]. In many plant species the cutin polyester contains C16 or C18, fatty acids as well as glycerol [6], [7]. The fatty acids in the cutin can be hydroxylated at midchains (C8, C9, or C10) or in Ω-positions and are linked together or to glycerol by ester bonds. It is still unclear if the cutin polymers exist as free polymers or if they are anchored in some ways to the cell wall [8]. The polymerization and the transport of the cutin precursors are likely to occur in the cell and conveyed to the cell wall via oleophilic droplets, secretion vesicles, lipid transfer proteins (LTP) or ABC transporters [7], [8]. While considerable knowledge is available on single components, the detailed chemical structure of the entire cuticle is still not known and the relation between the structure and the biological function of the individual components remains to be defined.
The cuticle has been proposed to be a physical barrier to the penetration by pathogens. This intuitive view is supported by the fact that most pathogens that penetrate directly through the cell wall produce cutinase. However, the biological relevance of cutinase or cutinolytic lipase could not be documented in all cases. Studies using antibodies directed against cutinase support a role for this enzyme in pathogenicity [9], [10]. Moreover, the effects of cutinase disruption were studied in various fungal pathogens, but only few studies found supportive evidence [11], [12], while most other results question the function of cutinase as a breaching enzyme [13]–[16]. These contradictory results might be the consequence of the functional redundancy of these enzymes, making gene disruption experiments difficult to interpret.
Components of the cuticle might function as important developmental cues perceived by invading microorganisms [6]. For instance, cutin monomers can induce the expression of cutinase [17], [18] or act as a plant signal for the induction of germination and appressorium in fungal pathogens [17]–[20]. Similarly, surface waxes can also affect the development of fungi at the plant surface [21].
The plant itself might also perceive degradation products of its own cuticle. Synthetic cutin monomers of the C18 fatty acid family applied on leaves of barley or rice increased resistance to Erysiphe graminis and Magnaporthe grisea respectively, without detectable direct fungicidal activity [22], [23]. Cell cultures of Solanum tuberosum respond to cutin monomers by medium alkalinization, ethylene (ET) production and accumulation of defense-related genes [23]. Abraded cucumber hypocotyls respond to cutin hydrolysates of cucumber, apple and tomato by producing H2O2 [24] that has been repeatedly associated with defense either as a signal, as an executer of cell death or as cofactor in the strengthening of the cell wall [25]–[27]. A decrease in the lesion size caused by Rhizoctonia solani was observed when bean leaves were inoculated with spore droplets amended with a fully active cutinase compared to droplets with an inactive cutinase or without cutinase [28]. These observations support the notion that plants have the potential to recognize breakdown products of the cuticle and activate defense-related mechanisms. Experiments with transgenic plants over expressing an active fungal cutinase in the apoplasm (CUTE plants) also support these observations [29].
An alternative explanation was provided by observations in CUTE plants as well as a series of mutants with defects in the formation of the cuticle such as lacerata (lcr; affected in the cytochrome P450-dependent enzyme CYP86A8, likely to be involved in fatty acid hydroxylation of cutin monomers) [30], bodyguard (bdg; impaired in a member of the a/b hydrolase family associated with the organization of the cutin polyester) [31] or bre1/lacs1 (a mutant of the long-chain acyl-CoA synthetase2, LACS2, involved in the development of the cuticle and essential for its biosynthesis) [32]. All those mutants display a strong resistance to B. cinerea; this was always associated with an increased cuticular permeability and production of a diffusate endowed with growth-inhibiting activity against B. cinerea [32]. The increased cuticular permeability was proposed to allow the diffusion of toxic compound(s) from the cell to the surface or facilitate the passage of MAMPs or DAMPs (microbe or damage-associated molecular patterns [33]) from the surface to the inside of the cell, resulting in increased resistance [32], [34].
Since biochemical alterations of the cuticle were found to increase the resistance of plants to pathogens, physical alterations of the cuticle, such as wounding were tested to see if they could also increase the defense potential of the plant to virulent necrotrophic pathogens. Wounding of Arabidopsis thaliana leaves leads to strong and transient immunity to the virulent pathogen B. cinerea. This resistance is strictly limited to the wound site and is independent of the major plant defense signaling pathways involving salicylic acid (SA), jasmonic acid (JA), and ET [34].
In this work, we have attempted to further our understanding of the molecular events taking place after wounding. A rapid formation of ROS has been observed after wounding and ROS can act as a signal for innate immunity but can also serve as an oxidant for lignification [35], [36]. This prompted us to carry out observations on the formation of ROS under our conditions of wounding. A strong correlation was observed between ROS formation and resistance to B. cinerea. We discovered that ABA is involved in the regulation of ROS production most likely causing changes in the permeability of the cellular envelope. This led to the finding that an increase in ROS also takes place in plants where cuticular permeability was affected by mutations or simply by a digestive treatment with cutinase. We propose that the cuticle acts as a sensor for pathogens that invade directly through the cell wall, leading to ROS formation whenever the cuticle is degraded. B. cinerea is known to form oxalic acid that can potentially prevent ROS formation. In line with this observation we have also shown that transgenic plants constitutively expressing an oxalic acid-degrading enzyme recovered their ability to produce ROS in response to B. cinerea infection and were resistant to this fungus.
Results
Wounding induces an oxidative burst that is accompanied by resistance to B. cinerea
Wounding A. thaliana leaves with forceps as previously described [34] lead to an increase of fluorescence when leaves infiltrated with the 5-(and-6)-carboxy-2,7-dichlorodihydrofluorescein diacetate (DCF-DA) probe were viewed under the microscope (Fig. 1B). This dye detects a broad range of oxidizing reagents including H2O2 and O2− [37]. A detailed time-course of ROS production was determined in a fluorimeter using wounded leaf discs. Fluorescence appeared within the first minutes after wounding and increased steadily thereafter (Fig. 1A); unwounded controls also showed a detectable increase, likely the result of wounding inflicted during the preparation of leaf discs. In whole excised leaves, fluorescence started to be detected 2 min after wounding (Fig. S1). After inoculation with B. cinerea, fluorescence started to accumulate 12 h after inoculation (Fig. 1B) and wounded leaves inoculated with B. cinerea showed the same behavior as mock-treated wounded leaves (Fig. 1B). Staining of wounded leaves with diaminobenzidine (DAB) or with nitroblue tetrazolium (NBT) identified production of H2O2 and O2− respectively, at the wounding site (Fig. 1C). ROS production was seriously diminished with a concomitant increase in fungal growth on leaves that were treated with DPI, an inhibitor of superoxide formation (Fig. 1D). Infiltration of leaves with catalase prevented the coloration of wounding sites with DAB confirming the production of H2O2 upon wounding (Fig. 1E). The luminol assay [38] was used to detect the formation of H2O2. A strong luminescence became visible at the wound sites (Fig. 1F). Wound-induced ROS and wound-induced resistance (WIR) to B. cinerea were still detected in mutants of NADPH oxidase D (atrboh D) and F (atrboh F) as well as in the double mutant (atrboh D/F) meaning that other genes are possibly implicated in ROS production (Fig. S2). ROS accumulation was still detected after wounding of the triple mutant (nia1nia2noa1-2) that is impaired in the biosynthesis of NO [39] (Fig. 1G). Laser confocal microscopy (LCM) was used to determine the localization of ROS in leaves treated with DCF-DA. In wounded leaves the fluorescence mainly localized at chloroplasts in mesophyll cells and to some extent to membranes in mesophyll and epidermal cells (Fig. 2). We have also tested the effects of H2O2 applied on leaves together with B. cinerea. H2O2 at concentrations as low as 1 nM caused enhanced resistance to fungal infection (Fig. S3A). This concentration is lower than the IC50 value of H2O2 (determined at 8.3 mM) for inhibition of hyphal growth in vitro (Fig. S3B). Taken together, these results show that wounding leads to a rapid burst of ROS, including H2O2, which could potentially take part in the resistance of A. thaliana to B. cinerea.
Fig. 1. A: ROS production in response to wounding in leaves of A. thaliana. 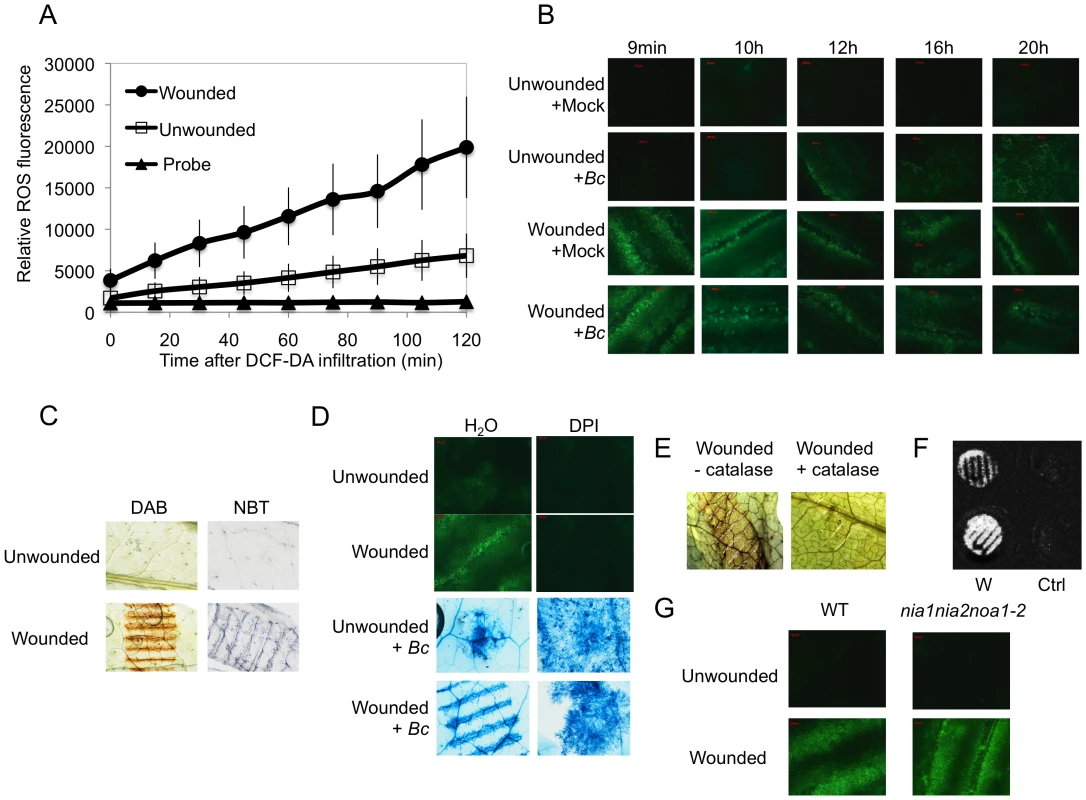
(A) Fluorescence after DCF-DA staining was measured on leaf discs using fluorescence spectrophotometry. The unwounded and wounded leaf discs were infiltrated with the DCF-DA probe and the fluorescence was directly measured at intervals of 15 min during 120 min (n = 12; ±SD). The experiment was carried out 3 times with similar results. (B) Time-course of ROS production observed as DCF-DA fluorescence by fluorescence microscopy in unwounded or wounded B. cinerea (Bc)-inoculated leaves compared to mock controls. After treatment, all plants were kept under humid conditions. The experiment was carried out 5 times with similar results. (C) H2O2 formation observed as DAB staining and superoxide (O2−) formation observed as NBT staining in unwounded or wounded leaves. Plants were stained immediately after wounding. The experiment was carried out 3 times with similar results. (D) Effect of DPI on ROS formation (measured as DCF-DA fluorescence) and growth of B. cinerea (determined by Trypan blue staining) in unwounded or wounded leaves. Leaves were treated for 24 h with DPI, then rinsed and either wounded, immediately stained and examined for fluorescence or inoculated with B. cinerea (Bc) and incubated in humid condition (symptoms were observed at 3 dpi). (E) The effect of catalase on ROS formation was tested by infiltration of catalase (1100 U ml−1) immediately prior to wounding. Plants were stained with DAB immediately after wounding. The experiments in Fig 1D and E were carried out 5 times with similar results. (F) Production of H2O2 in unwounded control (Ctrl) or wounded (W) leaf discs detected by the chemiluminescence reaction with luminol immediately after wounding. The experiment was carried out 3 times with similar results. (G) Production of ROS in unwounded and wounded WT plants and in nia1nia2noa1-2, a triple mutant deficient in nitrate reductase (NIA/NR)- and Nitric Oxide-Associated1 (AtNOA1)-mediated NO biosynthetic pathways. ROS were detected as DCF-DA fluorescence immediately after wounding. The experiment was carried out 3 times with similar results. Fig. 2. Subcellular localization of ROS at wounded sites in A. thaliana leaves. 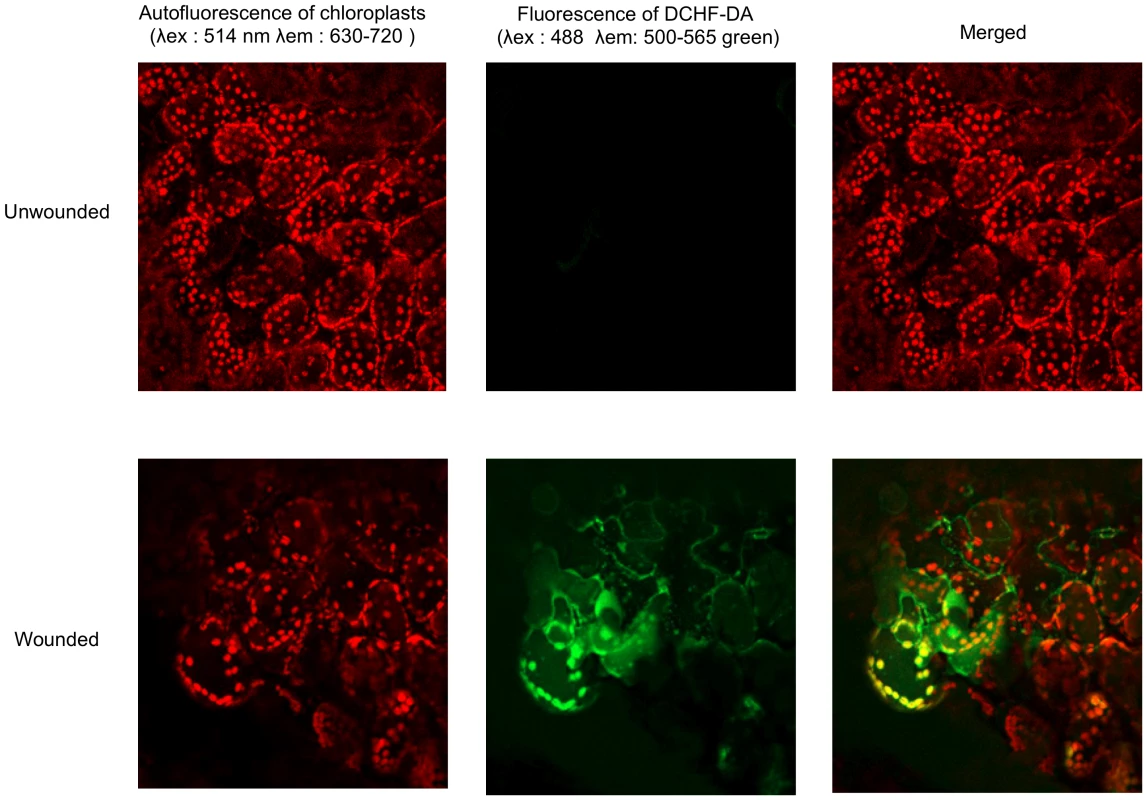
Leaves were infiltrated with DCF-DA, then wounded and ROS accumulation was observed by laser confocal microscopy immediately after wounding. The experiment was carried out 3 times with similar results. Wounding can be disconnected from ROS and resistance in the presence of ABA
We observed that both ROS production and WIR could be strongly impaired when the plants were maintained uncovered for 1.5 h at ambient humidity after wounding before the measurements of ROS and resistance (Fig. 3A and B). Thereafter, we will refer to these conditions as dry in contrast to the humid incubation environment in covered trays. In contrast, ROS production and WIR to B. cinerea remained unaffected when, after wounding, plants were kept for 1.5 h under high humidity in covered plastic trays (Fig. 3A and B). Maintaining plants under dry conditions after wounding strongly reduced wound-induced callose formation, a typical defense reaction to wounding (Fig. 3C). The strong effects of a dry environment on the suppression of H2O2 production and WIR to B. cinerea made us suspect a possible involvement of ABA in the suppression of ROS. Indeed, mutants blocked in the late steps of ABA biosynthesis, such as aba2 and aba3, were not blocked in ROS production after wounding under dry conditions and induced WIR in response to B. cinerea (Fig. 4). Both unwounded aba2 and aba3 mutants showed a marked resistance to B. cinerea accompanied by a faster and more intense ROS production after B. cinerea inoculation compared to WT plants (Figs. 4B and 5). The kinetics of ROS production was then also tested in unwounded aba mutants and showed that ROS were already released 3 h after exposure to water, PDB medium (mock treatment) or B. cinerea infection without any wounding (Fig. 5). The level of ABA was increased in wounded plants incubated in dry conditions (Fig. 6A). To confirm an increase in the level of ABA, the expression of ABA-dependent genes RAB18, RDB29 and NCED23 was tested. These genes showed an increase in expression after wounding and mock treatment as well as wounding and B. cinerea in dry conditions that was clearly detectable at 15 min after treatment (except NCED23 in mock-treated plants) (Fig. S4). Changes in ABA levels were further tested using transgenic plants containing a LUC reporter gene under the control of the ABA-specific promoters LTI23 or HB6. The activity of the reporter gene was mainly observed at the wound site and it was stronger in wounded plants incubated under dry compared to humid conditions (Fig. 6B). Furthermore, exogenous applications of ABA at 100 mM led to a suppression of ROS and WIR in response to B. cinerea (Fig. 6C). Thus, ABA is likely to be involved in the suppression of wound-induced ROS when plants are kept under dry conditions [25].
Fig. 3. The effect of humidity on resistance to B. cinerea, ROS and callose accumulation after wounding. 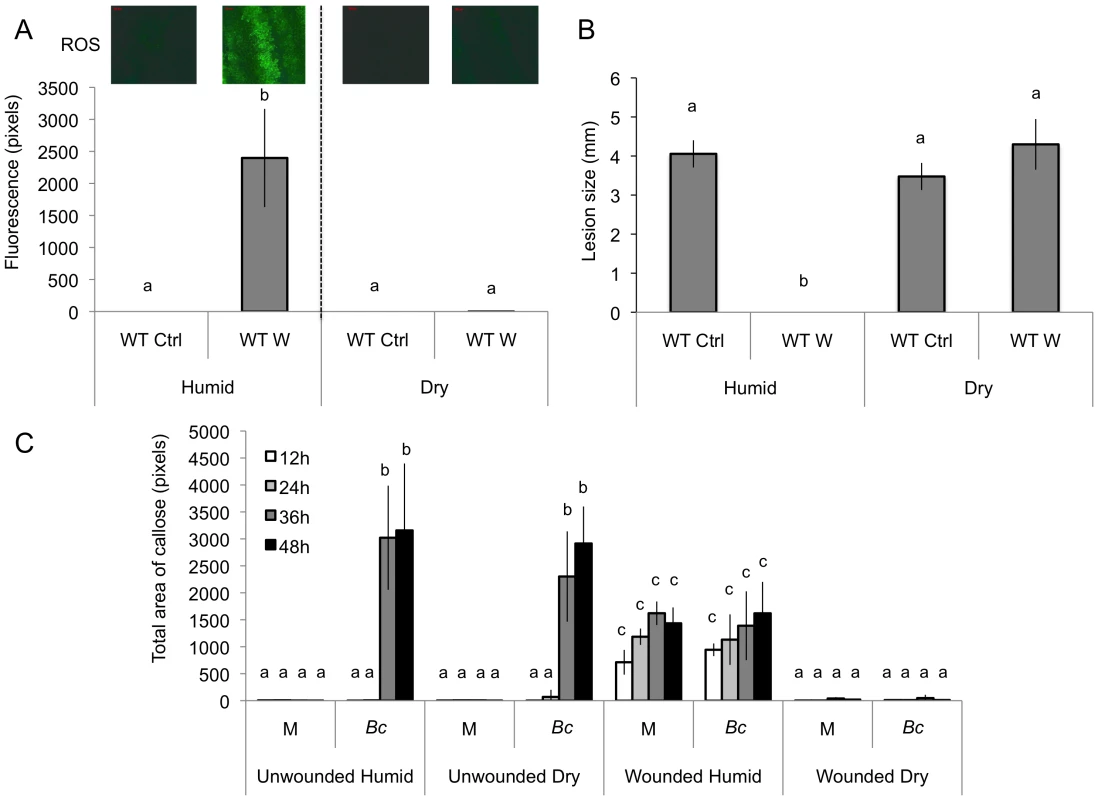
Wild type (WT) leaves were wounded and maintained for 1.5 h under high humidity in tightly covered well-watered trays (humid) or in uncovered trays at room conditions (dry) prior to ROS or infection with B. cinerea or callose detection. (A) Densitometric quantification of ROS production, (B) resistance to B. cinerea. W: wounded; Ctrl: unwounded control plants. (C) Callose formation. M: mock; Bc: B. cinerea-inoculated. For ROS production (n = 4; ±SD), one representative image of the fluorescent leaf surface was placed above each histogram as a visual illustration. For resistance (n = 48; ±SE) and callose formation (n = 4; ±SD), all plants were kept under humid conditions after treatment and the experiment was carried out twice with similar results. Different letters above each bar represent statistically significant differences (Dunn's test; P<0.05). Fig. 4. Wounding in mutants of ABA biosynthesis. 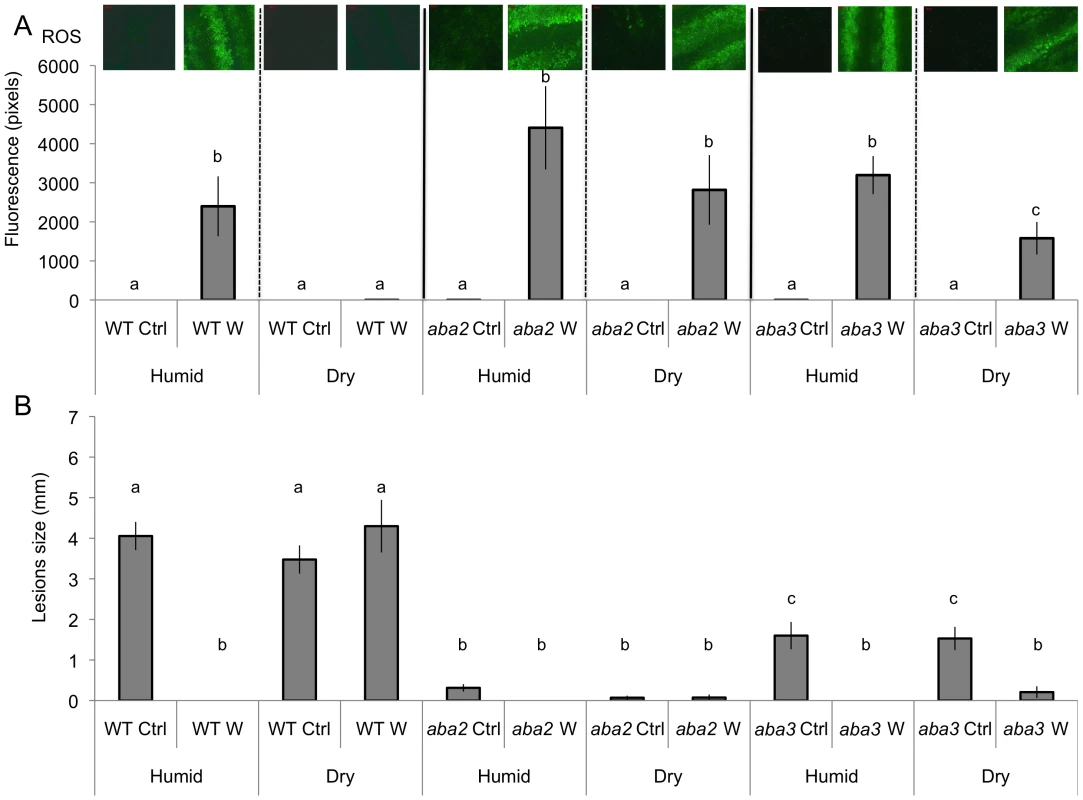
Leaves were wounded and maintained for 1.5 h under high humidity in tightly covered well-watered trays (humid) or in uncovered trays at room conditions (dry) prior to ROS detection or infection with B. cinerea. W: wounded; Ctrl: unwounded control plants. (A) Densitometric quantification of ROS production in unwounded and wounded aba2, aba3 mutants and in WT plants. For ROS production (n = 4; ±SD), one representative image of the fluorescent leaf surface was placed above each histogram as a visual illustration. Different letters above each bar represent statistically significant differences (Dunn's test; P<0.05). (B) Effects of wounding on resistance to B. cinerea in aba2 and aba3 mutants and WT plants. After B. cinerea inoculation, all plants were kept under humid conditions (n = 32; ±SE); the experiment was repeated twice with similar results. Different letters above each bar represent statistically significant differences (Dunn's test; P<0.05). Fig. 5. Densitometric quantification of ROS production in ABA and cuticle mutants. 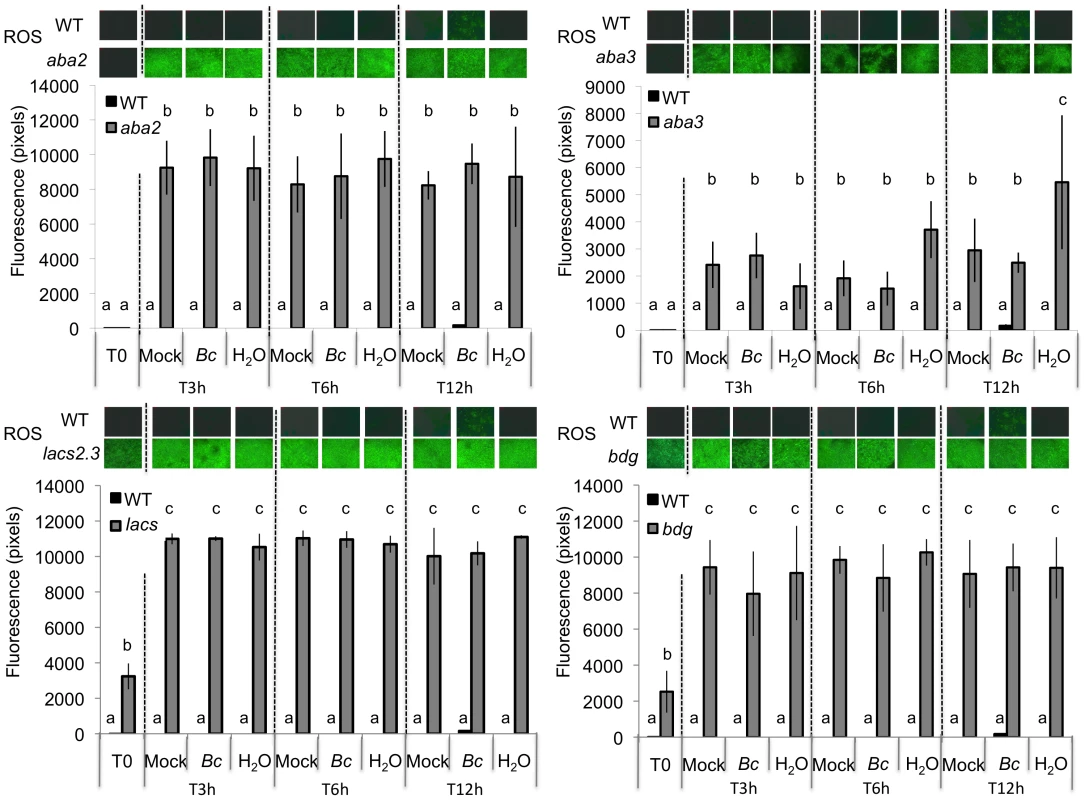
ROS production at 3, 6, 12 h post inoculation with B. cinerea (Bc), mock or H2O treatments in aba2 and aba3 as well as in bdg and lacs2.3 mutants compared to the WT. After treatment, all plants were kept under humid conditions. Low level of fluorescence density in the WT were detected only after B. cinerea at 12 h post inoculation and were not detected in response to H2O or mock treatment. For ROS production (n = 4; ±SD), one representative image of the fluorescent leaf surface was placed above each histogram as a visual illustration. Different letters above each bar represent statistically significant differences (Dunn's test; P<0.05). Fig. 6. ABA accumulates after wounding under dry conditions and affects resistance to B. cinerea. 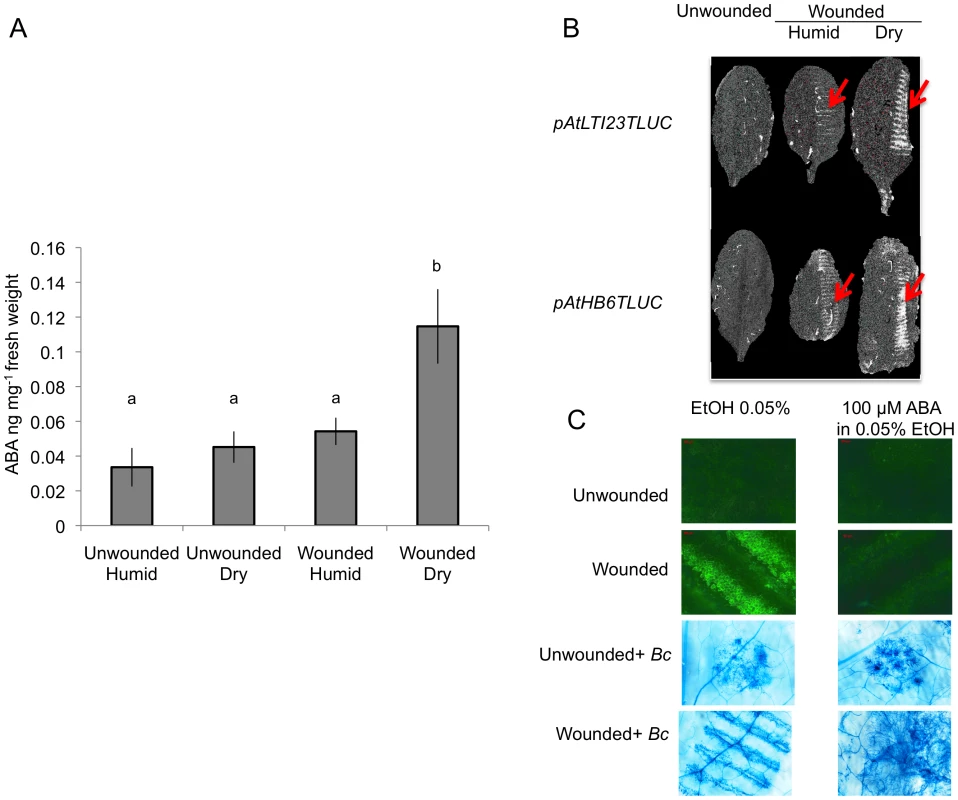
Leaves were wounded and maintained for 1.5 h under high humidity in tightly covered well-watered trays (humid) or in uncovered trays at room conditions (dry) prior to measurement of ABA or luciferase activity. (A) Measurement of ABA in ng mg−1 fresh weight of plant tissue in unwounded or wounded plants, incubated under humid or dry conditions (n = 6; ±SD). Different letters above each bar represent statistically significant differences (Dunn's test; P<0.05). (B) Expression of pAtLTI23T::LUC or pAtHB6T::LUC in wounded leaves incubated either under humid or dry conditions compared to unwounded plants. The wounds inflicted by the forceps (arrows) show a stronger expression of the LUC gene in plants incubated under dry conditions. The experiment was repeated 3 times, one typical result is represented. (C) Effect of exogenous ABA treatment (leaf discs were floated on 100 mM ABA, 1 d prior to wounding). After wounding, ROS (measured as DCF-DA fluorescence) and growth of B. cinerea (Bc) (leaf discs were floated on water and inoculated; Trypan blue staining was carried out 2 d after inoculation) were determined (the experiment was carried out 3 times, one typical result is represented). Common phenotypes between aba mutants and cuticle mutants
The resistance to B. cinerea displayed in aba mutants was comparable to that observed in mutants with permeable cuticles such as bdg or lacs2.3 [32]. We therefore tested and confirmed that bdg and lacs2.3 also produced ROS after wounding (data not shown). Furthermore, both bdg and lacs2.3 strongly displayed DCF-DA fluorescence after treatment with water, mock or B. cinerea compared to WT plants (Fig. 5). The cuticle of bdg and lacs2.3 was previously shown to be more permeable and it was postulated that this feature would allow an easier diffusion of elicitors into the cell or an improved passage of antibiotic substances towards the surface [32]. Consequently, we tested if the aba mutants also display alterations in cuticle permeability. This was tested using the cell wall stain toluidine blue applied as droplets on the adaxial side of leaves as previously described [32]. Results showed that aba2 and aba3 mutants strongly stained in blue, as did bdg and lacs2.3 compared to WT plants, indicating altered cuticular properties (Fig. 7A). We have ascertained that stomatal density did not interfere with the toluidine blue tests. The density of stomata in WT Col-0, aba2, aba3, bdg and lacs2.3 mutants showed some differences that could however not account for the toluidine blue staining observed only in the mutants compared to WT plants (Fig. S5). Calcofluor staining was also used to visualize permeable cuticles [32] and marked differences were obtained between WT Col-0 and aba2, aba3, bdg and lacs2.3 mutants (Fig. 7A). Increased efflux of chlorophyll is another measure of cuticular permeability [31], [40]. When dipped in ethanol, aba2 and aba3 as well as lacs2.3 and to a lesser extent bdg mutants released chlorophyll faster than WT Col-0 plants (Fig. 7B) thus corroborating the results of the toluidine blue and Calcofluor tests. Cuticular permeability was also compared when wounded plants were incubated under humid or dry conditions. Calcofluor staining was more intense at wounded sites in plants incubated under humid conditions compared to dry conditions indicating a better access of Calcofluor to the cell wall glucans (Fig. 7C). Similarly, chlorophyll leaching proceeded more rapidly when tested on wounded leaves incubated under humid compared to dry conditions (Fig. 7D). Furthermore, the expression of the BDG and LACS genes involved in cuticular biosynthesis were compared in wounded plants incubated under humid or dry conditions. Wounding under dry conditions enhanced the expression of BDG or LACS genes, compared to wounded plants incubated under humid conditions. While BDG was already enhanced within 15 min after wounding and dry conditions, the expression of LACS2.3 was clearly higher 30 min after treatment under the same condition (Fig. 8). In all experiments, expression of both genes after wounding followed by B. cinerea inoculation was not as extensive as the expression after wounding and mock treatment. The composition of the cuticle was altered in wounded plants after incubation in dry conditions compared to humid conditions as was the composition of the cuticle in aba2 and aba3 mutants compared to WT plants (Fig. S6). Furthermore, exogenous treatment of A. thaliana with ABA decreased the cuticular permeability (Fig. S7). ABA might therefore be involved in the control of a wound repair mechanism that decreases the permeability of the cuticle.
Fig. 7. Cuticle permeability is impaired in ABA mutants and after wounding under humid conditions. 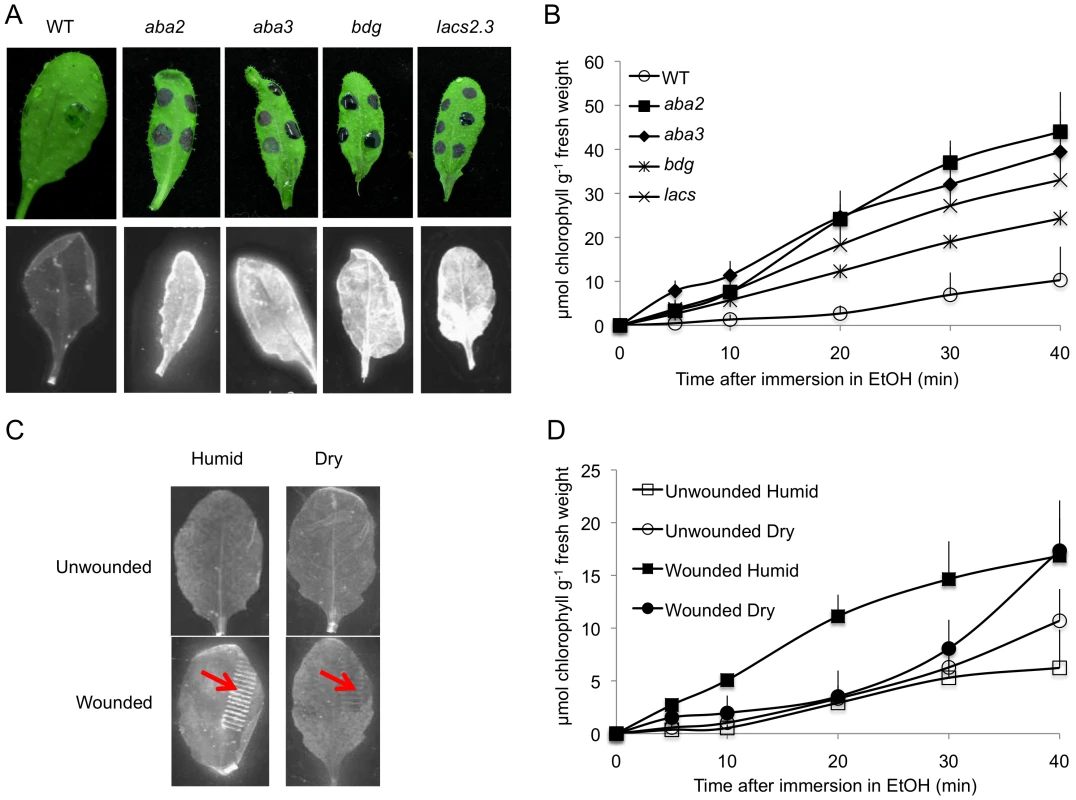
Permeability of the cuticle in ABA and cuticle mutants (positive controls) or WT plants (A) and (B). (A) Upper panels: a droplet of toluidine blue was placed on the leaf surface for 2 h in high humidity then the leaf surface was rinsed with water. The blue stain that remains attached to the cell wall is indicative of a permeable cuticle. Lower panels: leaves were bleached overnight in ethanol then stained with Calcofluor white that binds to cellulose, and viewed under UV light. Calcofluor staining to the leaf is indicative of a permeabilized cuticle (all experiments were carried out 12 times, one typical result is represented). (B) Leaves were placed in ethanol and the release of chlorophyll was followed over time. Chlorophyll leached out more rapidly in all mutants compared to WT indicating a higher cuticle permeability (n = 6; ±SD). Permeability of the cuticle in unwounded and wounded plants incubated under humid or dry conditions (C) and (D). (C) Leaves were wounded and maintained for 1.5 h under high humidity in tightly covered well-watered trays (humid) or in uncovered trays at room conditions (dry). The permeability of the cuticle was assessed using Calcofluor white. Wounding (arrow) followed by incubation under humid conditions lead to white staining visible at the wound sites and to a lesser extent in other parts of the leaf. Wounding followed by incubation in dry conditions showed no staining (the experiment was carried out 12 times, one typical result is represented). (D) Chlorophyll leached out more rapidly in plants incubated under humid conditions compared to plants incubated under dry conditions (n = 5; ±SD). Fig. 8. Expression of genes involved in cuticle formation in wounded and unwounded plants. 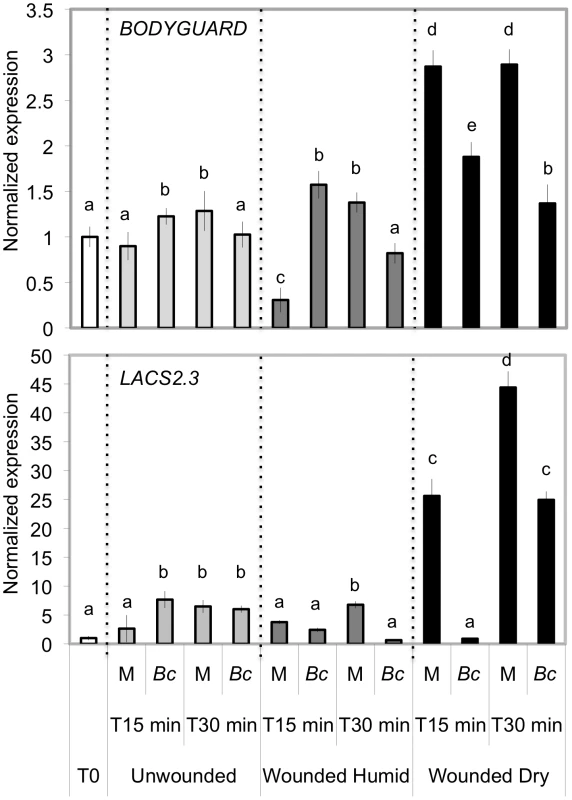
Leaves were wounded and maintained for 1.5 h under high humidity in tightly covered well-watered trays (humid) or in uncovered trays at room conditions (dry) prior to expression of BDG and LACS2.3 genes. Gene expression was determined 0, 15 or 30 min after wounding in plants incubated under humid or dry conditions and either mock-inoculated (M) or inoculated with B. cinerea (Bc) (n = 3; ±SD). The experiment was carried out twice with similar results. The expression levels of unwounded control plants behaved similarly under dry or humid conditions. Different letters above each bar represent statistically significant differences (Dunn's test; P<0.05). Cutinase treatment leads to ROS and enhanced resistance to B. cinerea
We have further tested the relation between cuticle, permeability, ROS and resistance to B. cinerea after a localized treatment with cutinase (from Fusarium solani, prepared using heterologous expression in S. cerevisiae). Localized application of cutinase led to ROS production visualized with DAB and DCF-DA staining (Fig. 9A) as well as to an increase in resistance to B. cinerea (Fig. 9B). These experiments support the hypothesis that plants can perceive and react to the degradation of the cuticle.
Fig. 9. ROS production and resistance to B. cinerea in response to exogenous treatments with cutinase. 
A droplet containing cutinase (5 mg l−1, a non-toxic concentration; see [47]) was applied to the surface of an A. thaliana leaf. After 72 h in high humidity, the drop was removed, and (A) the leaf was stained with DAB or DCF-DA (insert) to detect ROS production or (B) replaced by B. cinerea spores (6 µL; 5×104 spores ml−1) applied at the same location. After 72 h, lesion sizes were determined (n = 27; ±SE). The experiment was carried out twice with similar results. Different letters above each bar represent statistically significant differences (Dunn's test; P<0.05). Removal of oxalic acid, a pathogenicity factor of B. cinerea leads to increased ROS and resistance to B. cinerea
The cutinase produced by B. cinerea during infection [16] might potentially cause ROS production during the early stages of infection. But the data presented in Figs. 1B and 5 show an increase in fluorescence beyond 12 h after inoculation. B. cinerea was reported to release oxalic acid during infection [41]. Oxalic acid inhibits ROS production in tobacco and soybean cells [42] and might potentially slow down ROS production during the infection. The importance of oxalic acid as a suppressor of ROS was tested using transformed A. thaliana over-expressing an oxalate decarboxylase gene from the basidiomycete Trametes versicolor [43]. By removing oxalic acid released by B. cinerea, we would predict an increase in both ROS formation and resistance. In line with our expectations, transgenic T3 lines that over expressed the OXALATE DECARBOXYLASE gene and exhibited increased oxalate decarboxylase activity showed an increase in resistance to B. cinerea (Fig. 10A). ROS appeared as early as 3 hours post inoculation at sites inoculated with B. cinerea (Fig. 10B) in transgenic plants compared to controls. Thus, oxalic acid produced by B. cinerea might help the fungus to avoid the effects of ROS produced during the early steps of infection.
Fig. 10. Effect of OXALATE DECRABOXYLASE over expression in A. thaliana on resistance to B. cinerea and ROS production. 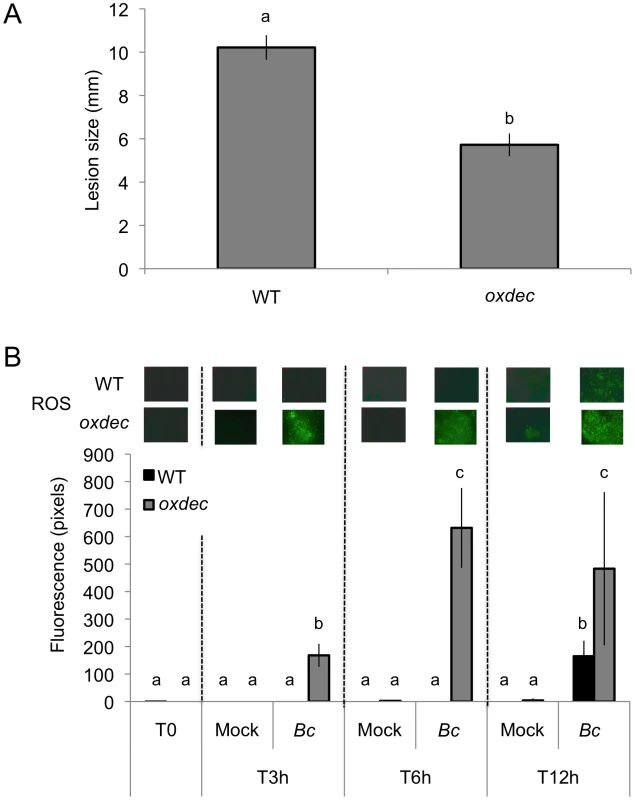
(A) Resistance displayed by T3 A. thaliana lines over expressing the OXALATE DECARBOXLYLASE gene from T. versicolor (oxdec plants); all plants were previously checked for the over expression of the oxalate decarboxylase activity (n = 130; ±SE). Different letters above each bar represent statistically significant differences (Dunn's test; P<0.05). (B) Densitometric quantification of ROS production at 3, 6, 12 h post inoculation with B. cinerea (Bc) and mock in oxdec plants compared to the WT. After treatment, all plants were kept under humid conditions. Low level of fluorescence density in the WT is detected only after B. cinerea at 12 h post inoculation and not in the mock treatment. For ROS production (n = 4; ±SD), one representative image of the fluorescent leaf surface was placed above each histogram as a visual illustration. Different letters above each bar represent statistically significant differences (Dunn's test; P<0.05). Discussion
We have previously reported a very marked resistance in A. thaliana to B. cinerea in response to localized wounding. This resistance is based on priming of camalexin synthesis, of the expression GLUTATHIONE S TRANSFERASE 1 (GST1) gene, and MAPK kinase activity [34]. Here, we have followed up these observations and described early events associated with WIR. We have described the production of ROS within 2 minutes at the site of wounding using the fluorescent dye DCF-DA [44]. To check the validity of the DCF-DA dye for ROS detection under our experimental conditions, we have also monitored ROS production using luminol, a method that mainly detects H2O2 [38] and could confirm ROS production after wounding (Fig. 1F). The wound sites also reacted to DAB and NBT staining confirming the formation of H2O2 and O2− (Fig. 1C). Treatment with the NADPH oxidoreductase inhibitor DPI (Fig. 1D) or infiltration of leaves with catalase (Fig. 1E) before wounding inhibited ROS development, as measured by DCF-DA fluorescence or DAB accumulation, respectively indicating that a substantial part of ROS is O2− and H2O2. Mutants unable to produce NO still produced ROS after wounding (Fig. 1G), making a contribution of NO to the initial wound-induced burst of ROS unlikely. Observations of plants with the LCM indicated strong fluorescence at the chloroplasts and a weaker one at the cell border after wounding (Fig. 2). The plastidic origin of some of the ROS might explain in part why ROS were still observed in atrbohD or atrbohF mutants since AtRBOHD or AtRBOHF are localized at the plasma membrane (Fig. S2). Taken together, our observations indicate a rapid (within 2 min) production of ROS after wounding (Fig. S1). Since we cannot exclude the presence of other ROS besides superoxide and H2O2, we will use the term ROS to collectively refer to the oxidative species that can be detected after wounding. Our experiments with exogenous applications of H2O2 show that ROS can have both a direct effect against B. cinerea and an indirect effect possibly by activation of defenses (Fig. S3). These observations are in line with previous studies showing ROS production after wounding [24], [36], [45], or in response to pathogens [26] including B. cinerea [46].
What is the biological importance of ROS production for WIR to B. cinerea?
Firstly, exogenously applied H2O2 can inhibit growth of B. cinerea (Fig. S3). Secondly treatments with DPI and catalase or incubation under dry conditions abolished ROS, WIR to B. cinerea as well as callose accumulation (Figs. 1D, E and 3). Thirdly, the absence of ROS formation and resistance to B. cinerea under dry conditions could be rescued in mutants impaired in ABA biosynthesis (aba2 and aba3) and exogenous application of ABA suppressed both ROS and resistance to B. cinerea (Figs. 4 and 6C). Finally, localized treatments with cutinase resulted both in ROS production and increased resistance to B. cinerea on treated sites (Fig. 9) [47]. Taken together, these results support the biological importance of ROS produced in response to wounding for WIR to B. cinerea. Several reports have proposed that ROS and subsequent cell death formed in response to B. cinerea or other necrotrophs might facilitate the infection by the pathogen (reviewed in [26]). This is clearly different from the situation described here where ROS produced after wounding are strongly induced prior to an inoculation and lead to an early induction of defenses such as rapid callose formation (Figs. 1 and 3). In a previous article, we have shown wound-induced priming of camalexin synthesis, expression of GLUTATHIONE S TRANSFERASE 1 (GST1) and MAPK kinase activity [34].
How do our data agree with the conventional observation that wounding is associated with susceptibility? Unless wounded plants are maintained under humid conditions WIR is lost. This resolves the apparent paradox, since most of the time plants wounded under natural conditions may not be under conditions of saturating humidity.
What is exactly the contribution of ABA? Our experiments have shown that ABA is implicated in the control of ROS formation in wounded plants that are incubated under dry conditions. Wounding followed by incubation in dry conditions lead to an increase in ABA levels (Fig. 6A). Furthermore, both the expression of ABA-dependent genes (RAB18, RDB29 and NCED23) [48] and the expression of the ABA reporter gene constructs ATH6: LUC and ATLTI23:LUC [49] were induced (Figs. S4 and 6B). ABA applied on leaves suppressed wound-induced ROS and subsequent resistance to B. cinerea (Fig. 6C). Our data are in agreement with observations made on ABA-deficient sitiens mutants of tomato, where the accumulation of H2O2 was both earlier and stronger than in WT plants after inoculation with B. cinerea [50]. Our results and those of Asselbergh et al. (2007)[50] suggest a negative control of ABA on ROS formation and resistance. Several reports show a link between ABA and increased susceptibility to pathogens that was mostly explained by antagonistic interactions of ABA with defense signaling controlled by SA, JA or ET [51]. How ABA prevents wound-induced ROS accumulation in A. thaliana remains a study to be carried out on its own. This will be interesting, since ABA controls stomatal closure via ROS production [52]. But the action of ABA further unveiled when we observed that aba mutants had a resistant phenotype reminiscent of plants affected in cuticle integrity such as CUTE or the cuticle mutants bdg and lacs2.3 that are immune to B. cinerea [32], [47]. For those reasons, ROS production was followed in bdg or lacs2.3. Indeed, these mutants displayed a strong DCF-DA fluorescence even after water or mock treatments or inoculation with B. cinerea (Fig. 5). The bdg or lacs2.3 mutants were previously shown to have an increased cuticular permeability compared to WT plants (Fig. 7A, B and [32], [53]). Accordingly, we have characterized cuticular properties in aba mutants and observed a higher permeability in aba2 and aba3 mutants than in WT plants (Fig. 7A and B). Cuticular permeability measured by Calcofluor white or chlorophyll efflux was also decreased after incubation of wounded plants under dry conditions compared to plants left at high humidity despite the opening caused by the wound (Fig. 7C and D). In addition, a change was observed in the composition of aliphatic cuticle monomers [30], [31] and in the expression of the LACS and BDG genes involved in cuticle biosynthesis in wounded plants incubated under dry compared to humid conditions (Fig. 8). Incubation of wounded plants for 1.5 h under dry conditions was sufficient to increase detectable changes in of 16 : 0 and 18 : 3 cuticle monomers (Fig. S6). Thus, the changes cuticle properties in response to the environmental conditions are accompanied by changes in aliphatic monomers of the cuticle. The aba2 and aba3 mutants also displayed a different composition in aliphatic cuticle monomers compared to Col-0 WT plants (Fig. S6). However, it is not yet feasible to link changes in structural components of the cuticle to functions such as cuticular permeability.
Exogenous application of ABA to WT or aba2, aba 3 lacs2.3 and bdg mutants decreased the cuticle permeability, further supporting a role for ABA in this process (Fig. S7). Curvers et al. (2010)[54] reported recently that tomato mutants impaired in ABA biosynthesis show enhanced cuticular permeability and resistance to B. cinerea. Our results in A. thaliana are in full agreement with an effect of ABA on the cuticle in tomato. It becomes now interesting to determine how ABA exerts its effects on the structure of the cuticle.
How could an increase in cuticular permeability affect the formation of ROS? The strong resistance of cuticular mutants to B. cinerea was explained by the facilitated diffusion of potentially antibiotic compounds produced by the plant and/or of elicitors from the medium/pathogen through the permeable cuticle surface [32], [53]. The perception of elicitors, including breakdown products of plant cuticles, has been previously described to produce ROS [22]–[24], [26]. The fact that cuticular mutants lacs2.3, bdg and aba2, aba3 produced ROS even when exposed to water or mock solution alone (Fig. 5) might be explained by the perception of elicitors present at the surface of the non-sterile leaves that dissolve in the water or in the mock solution and diffuse through the cuticle to the cell where they are perceived. The hypothesis that diffusion is facilitated through a permeabilized cuticle is further supported by the effect of cutinase treatments. This enzyme was already shown to degrade cutin and increase the permeability of the cuticle and resistance to B. cinerea when expressed constitutively in A. thaliana [29], [47]. By digesting the cuticle, this enzyme generates cutin monomers, increases the permeability of the cuticle and therefore improves diffusion of breakdown products that, together with other possible elicitors present at the leaf surface, would subsequently be recognized and lead to ROS formation and resistance (Fig. 9). Recognition of cutin monomers as well as wax components has also been described to induce the production of H2O2 in abraded epicotyls of cucumber [24].
How can these results be tied into a comprehensive model? When the cuticle is intact, it functions in protection against water loss, irradiation and xenobiotics. When it is permeabilized upon degradation by either enzymes secreted during pathogenesis or acted upon by mechanical action, elicitors might have a facilitated access to the cell, will be recognized and eventually lead to a rapid release of ROS and subsequent defense reactions. The model in Fig. 11 explains why plants are not continuously in an induced state, despite the existence of an extensive microbial flora at the leaf surface. As long as the cuticle prevents passage of elicitors, no induction of defenses takes place, illustrating economic energy management by the plant.
Fig. 11. Model illustrating the role of the cuticle at the interface between B. cinerea and A. thaliana. 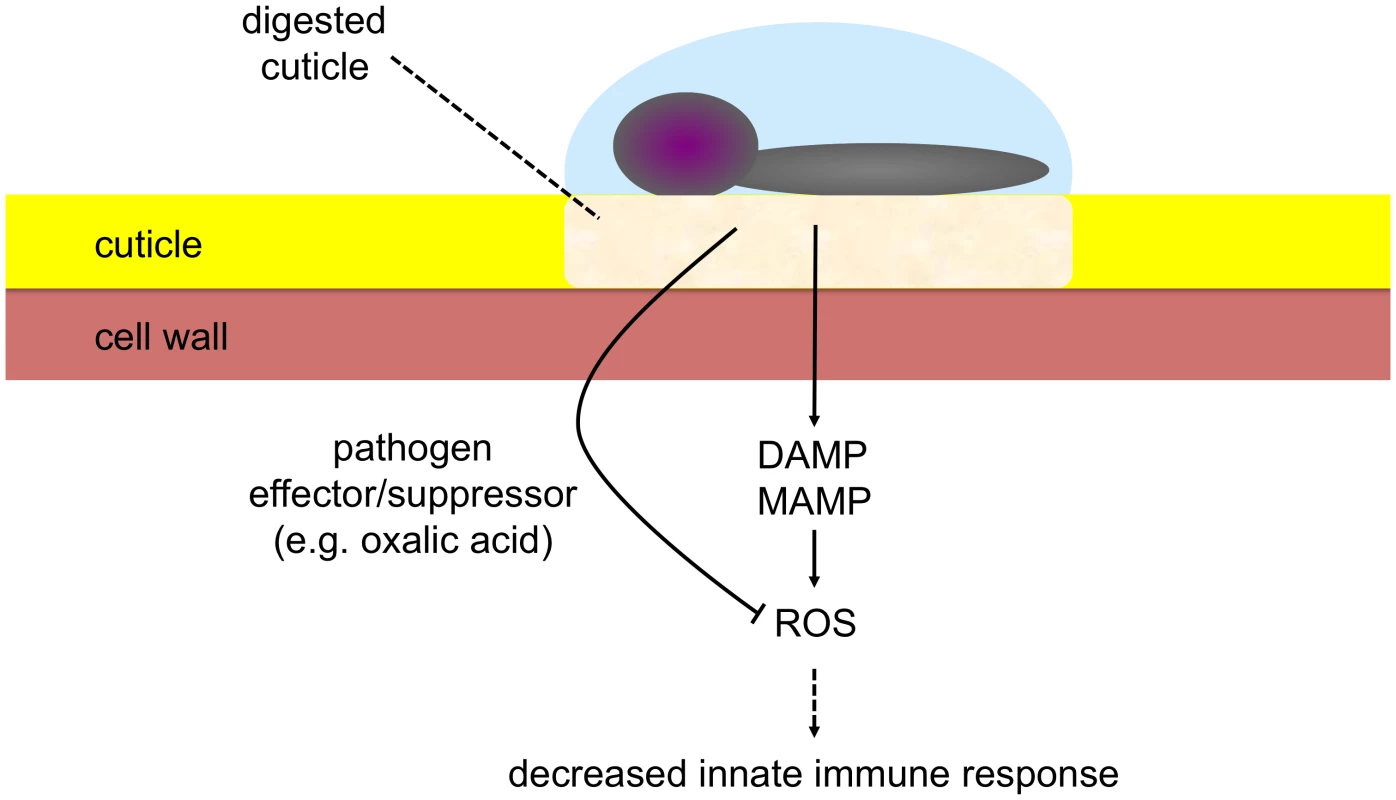
Under the action of digestive enzymes, permeabilization of the cuticle increases, allowing for early sensing and perception of elicitors (MAMPs/DAMPs) with subsequent induction of ROS and potential activation of innate immune responses. The virulent pathogen produces effector(s)/suppressor(s) (e.g. oxalic acid) that interfere with ROS build-up leading to decreased defenses and allowing colonization. Why does a virulent necrotrophic pathogen like B. cinerea that degrades the cuticle not lead to a rapid burst of ROS? Virulent pathogens produce suppressors or effectors that can interfere with plant defenses [55]. Oxalic acid produced by B. cinerea [41] can suppress the formation of ROS [42] and might thus protect the fungus from their effects. We have previously shown that removal of oxalic acid produced by B. cinerea enhances the protection of A. thaliana [56]. Results obtained here with plants over expressing an oxalate decarboxylase extend these findings. In oxdec plants ROS appeared early during infection and growth of B. cinerea was decreased (Fig. 10). Similar observations were published for the virulent oxalate-producing fungus Sclerotinia sclerotiorum and B. cinerea [43], [57]. In addition, B. cinerea was shown to produce ABA [58], [59] that might also be possibly involved in repressing ROS production during infection. B. cinerea can also induce ABA production in the plant [60]. The AP-1 transcription factor Bap1 plays a pivotal role in ROS detoxification of B. cinerea in vitro. But Bap1 was not found to be essential for pathogenesis and the role of an oxidative burst was questioned [61]. Here we show that removal of oxalic acid can indeed restore early ROS production by the plant and impair pathogenicity of B. cinerea, thus confirming previous results on the importance of oxalic acid for B. cinerea [56], [57].
In conclusion, we propose a model whereby the cuticle is part of a sensing device besides its passive protective role of aerial plant surfaces. Without modification of the diffusive properties of the cuticle, defenses are not induced. Modifications of the surface with subsequent increased permeability will allow for a better passage for molecular determinants that can be recognized by the plant and lead to the activation of defenses. This mechanism is doubled up by another mechanism provided by the wall itself, that, when exposed to the appropriate enzymes will breakdown to damage associated determinants (DAMPs) that are also recognized and initiate defenses. Virulent pathogens have evolved mechanisms to interfere with such mechanisms and data presented here support these findings. Future work should now be directed at the molecular mechanisms that lead to a rapid generation of ROS. It will be interesting to determine if they overlap with a similar responses observed when plant react to other elicitors that lead to ROS formation such as flagellin.
Materials and Methods
Plant maintenance
Arabidopsis thaliana seeds were grown on a pasteurized soil mix of humus and Perlite (3∶1). Seeds were kept at 4°C for two days and then transferred to the growth chamber. Plants were grown in a 12 h light/12 h dark cycle with 60–70% of relative humidity, with a day temperature of 20–22°C and a night temperature of 16–18°C. WT plants were obtained from the Nottingham Arabidopsis Stock Center (Nottingham, UK). The Arabidopsis mutant referred to as aba2 was aba2–1 and aba3 was aba3–1 [62]. The lacs2–3, bdg2 and the nia1nia2noa1–2 mutants were previously described [31], [32], [39].
Culture of B. cinerea, inoculation, staining of hyphae and wounding procedure
B. cinerea strains BMM, provided by Brigitte Mauch-Mani (University of Neuchâtel, Switzerland), were grown on Difco (Becton Dickinson, http://www.bd.com) potato dextrose agar 39 g l−1. Spores were harvested in water and filtered through glass wool to remove hyphae. Spores were diluted in ¼ strength Difco potato dextrose broth (PDB) at 6 g l−1 for inoculation. Droplets of 6 µl spore suspension (5×104 spores ml−1) were deposited on leaves of 4-week-old plants for quantification of lesions size (mm) after 3 days. Spores (2×105 spores ml−1) were also sprayed on whole plants for RT-PCR experiments. The inoculated plants were kept under high humidity in covered trays. Control plants were mock inoculated with ¼ strength PDB solution. Leaves were wounded by gently pressing the lamina with a laboratory forceps. For wounding of entire leaves, the pressing was carried out on both sides of the main vein. Wounded leaves were incubated in covered trays at high humidity (referred to as humid conditions); in some cases the trays were left uncovered after wounding (referred to as dry conditions) under the same laboratory conditions. Inoculation with B. cinerea was performed within 10 min after wounding, by placing a droplet of spores on the wound site. Fungal structures and dead plant cells were stained by boiling inoculated leaves for 5 min in a solution of alcoholic lactophenol trypan blue. Stained leaves were extensively cleared in chloral hydrate (2.5 g ml−1) at room temperature by gentle shaking, and then observed using a Leica DMR microscope with bright-field settings.
Detection of ROS
ROS were detected using the fluorescent probe 5-(and 6)-carboxy-2′,7′-dichloro dihydrofluorescein diacetate (DCF-DA) (Sigma-Aldrich, www.sigmaaldrich.com). Wounded or unwounded leaves were vacuum-infiltrated (3×3 min) in 60 µM of DCF-DA in a standard medium (1 mM KCl, 1 mM MgCl2, 1 mM CaCl2, 5 mM 2-morpholinoethanesulfonic acid adjusted to pH 6.1 with NaOH) [44]. Leaves were then rapidly rinsed in DCF-DA medium and observed using a Leica DMR epifluorescence microscope with a GFP filter set (excitation 480/40 nm, emission 527/30 nm) (Leica, www.leica.com). Microscope images were saved as TIFF files and processed for densitometric quantification with Image J version 1.44 (NIH). Software settings were kept the same for every image analyzed; the surface of each analyzed picture was the same (2.278 mm2). One representative image of the fluorescent leaf surface was placed above each histogram as a visual illustration. Quantification of ROS using DCF-DA was also performed on wounded or unwounded leaf discs of 5 mm incubated in 60 µM of DCF-DA in a 96-well plate (Sarstedt, www.sarstedt.com). After vacuum-infiltration (3×3 min), ROS were determined using a FL×800 microplate fluorescence reader with a excitation filter 485/20 nm and an emission filter 528/40 nm (Bio-Tek instruments, www.biotek.com). Accumulation of O2− and H2O2 in leaves was determined using nitroblue tetrazolium (NBT) staining [63] and 3,3′-diaminobenzidine (DAB) staining [64] respectively. The destained leaves were observed using a Leica DMR microscope with bright-field settings. The H2O2 accumulation was also determined using the luminol test [65]. A solution containing 50 µl of 0.5 mM luminol (3-aminophtalhydrazide, Sigma-Aldrich) in 0.2 N NH3, pH 9.5 added to 0.8 ml of 0.2 N NH3, pH 9.5 and 100 µl of 0.5 mM K3Fe(CN)6 in 0.2 N NH3, pH 9.5 was added to leaf discs of 8 mm in 24-well plates (Corning incorporated, www.corning.com) and luminescence was measured immediately using CCD camera (Princeton Instrument Versarray system, www.princetoninstruments.com) equipped with a Sigma Aspherical objective (www.sigma-foto.de) in a dark box. The pictures were analyzed with an Imaging System.
Luciferase activity
Arabidopsis reporter lines consisting of either a pAtHB6 or pLTI65 promoter fragment fused to the LUC gene were generously given by Prof. Erwin Grill [66]. For imaging of LUC activity, plants were sprayed with a solution of 1 mM luciferin (Applichem, www.applichem.com) in 10 mM MES, pH 7.0, 0.01% Tween 80. Ten min after luciferin spraying, light emission was detected using an intensified CCD camera (Princeton Instrument Versarray system, www.princetoninstruments.com) equipped with a Sigma Aspherical objective (www.sigma-foto.de) in a dark box. The pictures were analyzed with the MetaVue Imaging System (www.biovis.com/metavue.htm).
Callose staining
Twenty-four hours post infiltration, leaves were harvested and distained in 3∶1 ethanol: lactic acid, previously diluted in 1 : 2 ethanol. The solution was changed several times until the chlorophyll had totally disappeared. Translucent leaves were progressively re-hydrated in 70% ethanol for about 2 hours and in 50% ethanol for 2 hours. Leaves were left in water and gently shaken overnight. Leaves were then incubated for 24 hours in 150 mM K2HPO4 (pH 9.5) containing 0.01% aniline blue. Stained material was mounted on glass slides in 50% glycerol and examined under UV light with a LEICA DMR fluorescence microscope. The callose deposition was determined by counting the pixels using the Image J 1.44 software (NIH).
RNA extraction and real time RT-PCR
RNA was prepared using the Trizol reagent containing 38% saturated phenol, 0.8 M guanidine thiocyanate, 0.4 M ammonium thiocyanate, 0.1 M sodium acetate and 5% glycerol. RNA (1 µg) was then retrotranscribed into cDNA (Omniscript RT kit, Qiagen, www.qiagen.com). RT-PCR was performed using Sensimix SYBR Green Kit (Bioline, www.bioline.com). Gene expression values were normalized to expression of the plant gene At4g26410, previously described as a stable reference gene [67]. The primers used were rab18fw 5′ - AACATGGCGTCTTACCAGAA; rab18rev 5′-AGTTCCAAAGCCTTCAGTCC; rd29bfw 5′-GAATCAAAAGCTGGGATGGA; rd29brev 5′-TGCTCTGTGTAGGTGCTTGG; nced23fw 5′-ATTGGCTATGTCGGAGGATG; nced23rev 5′-CGACGTCCGGTGATTTAGTT; lacs2-3fw 5′-GTGCCGAGAGGAGAGATTTG; lacs2-3rev 5′-CGAGGTTTTCAACAGCAACA; bdgfw 5′-TTCTTGGCTTTCCTCTTCCA; bdgrev 5′ - CCATAACCCAACAGGTCCAC.
Treatments with ABA, DPI, catalase and cutinase
Leaf discs of 8 mm were floated in 24-well plates (Corning incorporated, www.corning.com) filled with 1.5 ml in each well of either 50 µM DPI (Sigma-Aldrich), 100 µM ABA (Sigma-Aldrich) in 0.05% EtOH and distilled water or EtOH 0.05% as controls for 24 h before wounding. Catalase (300 U ml−1 catalase; Sigma-Aldrich) was infiltrated into the leaves prior to wounding. After wounding, either ROS were visualized on the discs with DCF-DA or DAB or discs were floated on distilled water for 24 h and inoculated with B. cinerea and infection was determined after 3 days. The effect of ABA on cuticle permeability was determined after spraying leaves with 100 µM ABA in 0.05% EtOH followed by a 24 h incubation period under humid conditions. Eight mL droplets of purified preparation of cutinase (5 mg l−1) from F. oxysporum [68] or 10 mM Na-acetate pH 5.2 in controls were applied on the leaf surface. After 72 h incubation under moist conditions, the droplets were removed and leaves were stained with DCF-DA or DAB to detect ROS. Alternatively, the droplet was rinsed off the leaf and replaced by a droplet of spore suspension of B. cinerea and incubated as described above.
Quantification of abscisic acid
Leaf material (ca 500 mg) was frozen in liquid nitrogen and collected in 2 ml Eppendorf tubes, and then homogenized (twice) with 1000 µl of 70°C-warm extraction buffer (water/propane-1-ol/HCl : 1/2/0.005, v/v) without thawing. The sample was transferred to a glass tube and 200 ng of the internal standard abscisic acid-d6 (Santa Cruz Biotechnology, www.scbt.com) and 2 ml of dichloromethane were added. The sample was then mixed 15 s with a vortex and centrifuged 1 min at 14,000 g. The lower organic phase was transferred to a new glass tube and dried by the addition of anhydrous Na2SO4. Then, carboxylic acids including ABA were methylated to their corresponding methyl esters at room temperature for 30 min after the addition of 10 µl of 2 M bis-trimethylsilyldiazomethane (Sigma-Aldrich) and 100 µl of MeOH. Methylation was stopped by the addition of 10 µl of 2 M acetic acid during 30 min at room temperature. Extraction of the vapor phase was performed using a VOC column conditioned with 3×1 ml of dichlororomethane. The VOC column and a nitrogen needle were fixed on the screw cap of the tube. The solvent was evaporated under a nitrogen stream at 70°C and heated for 2 min at 200°C. The VOC column was eluted with 1 ml of dichloromethane in a new glass tube. The eluate was evaporated and then dissolved in 20 µl of hexane before injecting 3 µl on a capillary column HP1 (25 m×0.25 mm) GC column (Agilent, www.agilent.com) fitted to a Hewlett Packard 5980 GC coupled to a 5970 mass specific detector. The methyl esters of ABA and ABA-d6 were detected and quantified by selective ion monitoring at m/z 190 and 194 respectively. The amount of ABA (measured as methyl ABA) was calculated by reference to the amount of internal standard. The results are expressed in ng mg−1 fresh weight of plant tissue.
Chemical composition of cuticle
The surface of 15 to 20 leaves of 4 weeks-old A. thaliana Col-0 was first determined as described previously [69]. Then the leaves were extracted with chloroform:methanol (1∶1; v/v) and dried before depolymerization. The samples were depolymerized using transesterification with 2 ml BF3 (Fluka, Sigma-Aldrich) for 12 h at 75°C. After addition of 2 ml saturated NaCl/H2O and 20 µg dotriacontane as internal standard, aliphatic monomers were extracted 3 times with 1 ml of chloroform. The combined organic phase was evaporated in a stream of nitrogen to a volume of ∼100 µl. All samples were treated with bis-(N,N,-trimethylsilyl)-tri-fluoroacetamide (BSTFA; Macherey-Nagel, www.mn-net.com) for 40 min at 70°C to convert free hydroxyl and carboxyl groups into their corresponding trimethylsilyl (TMS) derivatives. Remaining solvent and derivatization reagents were removed under a stream of N2 and the samples were resolubilized in 100 ml dichloromethane prior to vapour phase extraction. Monomers were identified on the basis of their electron-impact MS spectra (70 eV, m/z 50–700) on a HP 6890 GC system coupled to an HP 5973 mass-selective detector (USA). The depolymerisation products were separated by on a capillary column (ZB-AAA, 10 m, 0.25 mm, Zebron, Phenomenex, www.phenomenex.com) by injection at 50°C, 2 min at 50°C, 5 °C min−1 to 225°C, 1 min at 225°C, 20°C min−1 to 310°C, 10 min at 310°C. The results are expressed in g cm−2 leaf tissue.
Tests of cuticle permeability
Chlorophyll extraction and quantification was performed according to the protocol of Sieber et al. 2009 [29]. Leaves were cut at the petiole, weighed and immersed in 30 ml of 80% ethanol. Chlorophyll was extracted in the dark at room temperature with gentle agitation. Aliquots were removed at 2, 5, 10, 20, 30 and 40 min after immersion. After ABA treatment, aliquots were removed at 40 and 60 min after immersion in ethanol. The chlorophyll content was determined by measuring absorbance at 664 and 647 nm and the micromolar concentration of total chlorophyll per gram of fresh weight of tissue was calculated from the following equation: (7.93×(A664 nm) + 19.53×(A647 nm)) g−1 fresh weight. The toluidine blue test was carried out by placing 6 ml droplets of a 0.025% toluidine blue solution in ¼ PDB placed on the leaf surface. After 2 h incubation leaves were washed gently with distilled water to remove excess of the toluidine blue solution from leaves. For staining with Calcofluor white, leaves were bleached in absolute ethanol overnight, equilibrated in 0.2 M NaPO4 (pH 9) for 1 h, and incubated for 1 min in 0.5% Calcofluor white in 0.2 M NaPO4 (pH 9). Leaves were rinsed in NaPO4 buffer to remove excess of Calcofluor white and viewed under UV light on a GelDoc 2000 system (Biorad, www.biorad.com).
Production of oxdec lines and detection of the oxalic acid decarboxylase activity
The gene used to transform A. thaliana plants is the OXALATE DECARBOXYLASE from Trametes versicolor (TOXDEC, Genbank accession number AY370675). The gene was kindly provided by Andreas Walz (Institute for Phytomedicine, University of Hohenheim) as cDNA cloned into the transformation vector pBI 101 (p221-TOXDC). A. thaliana Col-0 plants were transformed using Agrobacterium tumefaciens and the flower dip method [70]. Resulting seeds were collected and grown on selective medium; the expression of the gene was determined respectively by crude PCR and Northern Blot. The activity of oxalate decarboxylase was measured in the T3 generation along with resistance to B. cinerea strain BMM. Oxalate decarboxylase activity was measured as described [71] with the following modifications. Leaves (100 mg) were homogenized using a Polytron (Kinematica, www.kinematica-inc.com) in 1 ml extraction buffer containing 50 mM potassium phosphate buffer pH 7.5, 1 mM EDTA, 1 mM phenylmethylsulphonyl fluoride and 5 mM sodium ascorbate; 200 µl of this extract was added to 10 µl oxalic acid (1 M, pH 6.2) and incubated 3 h at 37°C. After centrifugation during 5 min, 12 µl of a 30% sodium acetate solution (w/v) and 300 µl reagent solution containing 0.5 g citric acid monohydrate, 10 g acetic acetamide in 100 ml isopropanol were added to 150 µl extract. The solution added to 1 ml of acetic acid anhydride was incubated 40 min at 50°C; the intensity of the resulting pink color reflects oxalate decarboxylase activity.
Statistical analyses
Kruskal-Wallis one way analysis of variance (ANOVA) on ranks followed by a Dunn's test was performed using SigmaPlot version 11.1 software (Systat Software, San Jose, CA). Different letters above each bar represent statistically significant differences (Dunn's test; P<0.05).
Supporting Information
Zdroje
1. RiedererMSchreiberL 2001 Protecting against water loss: analysis of the barrier properties of plant cuticles. J Exp Bot 52 2023 2032
2. NawrathC 2006 Unraveling the complex network of cuticular structure and function. Curr Opin Pl Biol 9 281 287
3. GoodwinSMJenksMA 2005 Plant cuticle function as a barrier to water loss. Plant cuticle function as a barrier to water loss. JenksMAHasegawaPM Oxford, UK Blackwell Publishing Inc 14 36
4. KunstLSamuelsL 2009 Plant cuticles shine: advances in wax biosynthesis and export. Cur Opin Plant Biol 12 721 727
5. SamuelsLKunstLJetterR 2008 Sealing plant surfaces: Cuticular wax formation by epidermal cells. Annu Rev Plant Biol 59 683 707
6. KolattukudyPERogersLMLiDXHwangCSFlaishmanMA 1995 Surface signaling in pathogenesis. Proc Natl Acad Sci USA 92 4080 4087
7. HerediaA 2003 Biophysical and biochemical characteristics of cutin, a plant barrier biopolymer. Bioch Biophys Acta 1620 1 7
8. PollardMBeissonFLiYOhlroggeJB 2008 Building lipid barriers: biosynthesis of cutin and suberin. Trends Plant Sci 13 236 246
9. CommenilPBelingheriLDehorterB 1998 Antilipase antibodies prevent infection of tomato leaves by Botrytis cinerea. Phys Mol Plant Pathol 52 1 14
10. MaitiIBKolattukudyPE 1979 Prevention of fungal infection of plants by specific-inhibition of cutinase. Science 205 507 508
11. LiDAshbyAMJohnstoneK 2003 Molecular evidence that the extracellular cutinase Pbc1 is required for pathogenicity of Pyrenopeziza brassicae on oilseed rape. Mol Plant Microbe Interact 16 : 16 545 552
12. RogersLMFlaishmanMAKolattukudyPE 1994 Cutinase gene disruption in Fusarium solani f sp pisi decreases its virulence on pea. Plant Cell 6 935 945
13. ReisHPfiffiSHahnM 2005 Molecular and functional characterization of a secreted lipase from Botrytis cinerea. Mol Plant Pathol 6 257 267
14. StahlDJTheuerkaufAHeitefussRSchaferW 1994 Cutinase of Nectria haematococca (Fusarium solani f sp pisi) is not required for fungal virulence or organ specificity on pea. Mol Plant Microbe Interact 7 713 725
15. SweigardJAChumleyFGValentB 1992 Disruption of a Magnaporthe grisea cutinase gene. Mol Gen Genetics 232 183 190
16. van KanJALvant KloosterJWWagemakersCAMDeesDCTvan der Vlugt BergmansCJB 1997 Cutinase A of Botrytis cinerea is expressed, but not essential, during penetration of gerbera and tomato. Mol Plant Microbe Interact 10 30 38
17. LinTSKolattukudyPE 1978 Induction of a bio-polyester hydrolase (cutinase) by low-levels of cutin monomers in Fusarium solani f sp pisi. J Bacteriol 133 942 951
18. WoloshukCPKolattukudyPE 1986 Mechanism by which contact with plant cuticle triggers cutinase gene-expression in the spores of Fusarium solani f sp pisi. Proc Natl Acad Sci USA 83 1704 1708
19. FrancisSADeweyFMGurrSJ 1996 The role of cutinase in germling development and infection by Erysiphe graminis f sp hordei. Physiol Mol Plant Pathol 49 201 211
20. GilbertRDJohnsonAMDeanRA 1996 Chemical signals responsible for appressorium formation in the rice blast fungus Magnaporthe grisea. Physiol Mol Plant Pathol 48 335 346
21. PodilaGKRogersLMKolattukudyPE 1993 Chemical signals from avocado surface wax trigger germination and appressorium formation in Colletotrichum gloeosporioides. Plant Physiol 103 267 272
22. SchweizerPJeanguénatAMétrauxJPMösingerE 1994 Plant protection by free cutin monomers in two cereal pathosystems. DanielsMJDownieJAOsbournAE Advances Mol Genet Plant-Microbe Interact Dordrecht Kluwer 371 374
23. SchweizerPFelixGBuchalaAMullerCMétrauxJP 1996 Perception of free cutin monomers by plant cells. Plant J 10 331 341
24. FauthMSchweizerPBuchalaAMarkstadterCRiedererM 1998 Cutin monomers and surface wax constituents elicit H2O2 in conditioned cucumber hypocotyl segments and enhance the activity of other H2O2 elicitors. Plant Physiol 117 1373 1380
25. ApelKHirtH 2004 Reactive oxygen species: Metabolism, oxidative stress, and signal transduction. Annu Rev Plant Biol 55 373 399
26. TorresMAJonesJDDanglJL 2006 Reactive oxygen species signaling in response to pathogens. Plant Physiol 141 373 378
27. TorresMA 2010 ROS in biotic interactions. Physiol Plant 138 414 429
28. ParkerDMKollerW 1998 Cutinase and other lipolytic esterases protect bean leaves from infection by Rhizoctonia solani. Mol Plant Microbe Interact 11 514 522
29. SieberPSchorderetMRyserUBuchalaAKolattukudyP 2000 Transgenic Arabidopsis plants expressing a fungal cutinase show alterations in the structure and properties of the cuticle and postgenital organ fusions. Plant Cell 12 721 737
30. WellesenKDurstFPinotFBenvenisteINettesheimK 2001 Functional analysis of the LACERATA gene of Arabidopsis provides evidence for different robes of fatty acid omega-hydroxylation in development. Proc Natl Acad Sci USA 98 9704 9699
31. KurdyukovSFaustANawrathCBarSVoisinD 2006 The epidermis-specific extracellular BODYGUARD controls cuticle development and morphogenesis in Arabidopsis. Plant Cell 18 321 339
32. BessireMChassotCJacquatACHumphryMBorelS 2007 A permeable cuticle in Arabidopsis leads to a strong resistance to Botrytis cinerea. EMBO J 26 2158 2168
33. BollerTFelixG 2009 A renaissance of elicitors: perception of microbe-associated molecular patterns and danger signals by pattern-recognition receptors. Annu Rev Plant Biol 60 379 406
34. ChassotCBuchalaASchoonbeekHMétrauxJPLamotteO 2008 Wounding of Arabidopsis leaves causes a powerful but transient protection against Botrytis infection. Plant J 55 555 567
35. LeonJRojoESanchez-SerranoJJ 2001 Wound signalling in plants. J Exp Bot 52 1 9
36. Orozco-CardenasMLNarvaez-VasquezJRyanCA 2001 Hydrogen peroxide acts as a second messenger for the induction of defense genes in tomato plants in response to wounding, systemin, and methyl jasmonate. Plant Cell 13 179 191
37. HempelSLBuettnerGRO'MalleyYQWesselsDAFlahertyDM 1999 Dihydrofluorescein diacetate is superior for detecting intracellular oxidants: Comparison with 2 ',7 '-dichlorodihydrofluorescein diacetate, 5(and 6)-carboxy-2', 7 '-dichlorodihydrofluorescein diacetate, and dihydrorhodamine 123. Free Radical Biol Medicine 27 146 159
38. WarmELatiesGG 1982 Quantification of hydrogen peroxide in plant extracts by the chemiluminescence reaction with luminol. Phytochemistry 21 827 831
39. Lozano-JusteJLeonJ 2010 Enhanced abscisic acid-mediated responses in nia1nia2noa1-2 triple mutant impaired in NIA/NR - and AtNOA1-dependent nitric oxide biosynthesis in Arabidopsis. Plant Physiol 152 891 903
40. VoisinDNawrathCKurdyukovSFrankeRBReina-PintoJJ 2009 Dissection of the complex phenotype in cuticular mutants of Arabidopsis reveals a role of SERRATE as a mediator. Plos Genetics 5 1 19
41. PrinsTWTudzynskiPvon TiedemannATudzynskiBTen HaveA 2000 Infection strategies of Botrytis cinerea and related necrotrophic pathogens. KronstadJW Fungal Pathology Dordrecht Kluwer Academic Publishers 33 64
42. CessnaSGSearsVEDickmanMBLowPS 2000 Oxalic acid, a pathogenicity factor for Sclerotinia sclerotiorum, suppresses the oxidative burst of the host plant. Plant Cell 12 2191 2200
43. WalzAZingen-SellITheisenSKortekampA 2008 Reactive oxygen intermediates and oxalic acid in the pathogenesis of the necrotrophic fungus Sclerotinia sclerotiorum. Europ J Plant Pathol 120 317 330
44. HoffmannAHammesEPliethCDeselCSattelmacherB 2005 Effect of CO2 supply on formation of reactive oxygen species in Arabidopsis thaliana. Protoplasma 227 3 9
45. KesslerABaldwinIT 2002 Plant responses to insect herbivory: The emerging molecular analysis. Annu Rev Plant Biol 53 299 328
46. Van BaarlenPWolteringEJStaatsMvan KanJAL 2007 Histochemical and genetic analysis of host and non-host interactions of Arabidopsis with three Botrytis species: an important role for cell death control. Mol Plant Pathol 8 41 54
47. ChassotCNawrathCMétrauxJP 2007 Cuticular defects lead to full immunity to a major plant pathogen. Plant J 49 972 980
48. WuYSanchezJPLopez-MolinaLHimmelbachAGrillE 2003 The abi1-1 mutation blocks ABA signaling downstream of cADPR action. Plant J 34 307 315
49. HimmelbachAHoffmannTLeubeMHohenerBGrillE 2002 Homeodomain protein ATHB6 is a target of the protein phosphatase ABI1 and regulates hormone responses in Arabidopsis. EMBO J 21 3029 3038
50. AsselberghBCurversKFrancaSCAudenaertKVuylstekeM 2007 Resistance to Botrytis cinerea in sitiens, an abscisic acid-deficient tomato mutant, involves timely production of hydrogen peroxide and cell wall modifications in the epidermis. Plant Physiol 144 1863 1877
51. Mauch-ManiBMauchF 2005 The role of abscisic acid in plant-pathogen interactions. Curr Opin Plant Biol 8 409 414
52. RaghavendraASGonuguntaVKChristmannAGrillE 2010 ABA perception and signalling. Trends Plant Sci 15 395 401
53. ChassotCNawrathCMétrauxJP 2008 The cuticle: not only a barrier for defence. Plant Signal Behav 3 142 144
54. CurversKSeifiHMouilleGDe RyckeRAsselberghB 2010 ABA-deficiency causes changes in cuticle permeability and pectin composition that influence tomato resistance to Botrytis cinerea. Plant Physiol 154 847 860
55. MétrauxJ-PJacksonRWEstherSGoldbachRW 2009 Plant pathogens as suppressors of host defense. Van LoonLC Advances in Botanical Research Academic Press 39 89
56. SchoonbeekHJacquat-BovetACMascherFMétrauxJP 2007 Oxalate-degrading bacteria can protect Arabidopsis thaliana and crop plants against Botrytis cinerea. Mol Plant Microbe Interact 20 1535 1544
57. WalzAZingen-SellILoefflerMSauerM 2008 Expression of an oxalate oxidase gene in tomato and severity of disease caused by Botrytis cinerea and Sclerotinia sclerotiorum. Plant Pathol 57 453 458
58. SiewersVKokkelinkLSmedsgaardJTudzynskiP 2006 Identification of an abscisic acid gene cluster in the grey mold Botrytis cinerea. Appl Environ Microbiol 72 4619 4626
59. SiewersVSmedsgaardJTudzynskiP 2004 The p450 monooxygenase BcABA1 is essential for abscisic acid biosynthesis in Botrytis cinerea. Appl Environ Microbiol 70 3868 3876
60. KettnerJDörfflingK 1995 Biosynthesis and metabolism of abscisic acid in tomato leaves infected with Botrytis cinerea. Planta 196 627 634
61. TemmeNTudzynskiP 2009 Does Botrytis cinerea ignore H2O2-induced oxidative stress during infection? Characterization of BOTRYTIS ACTIVATOR PROTEIN 1. Mol Plant Microbe Interact 22 987 998
62. Léon-KloosterzielKMGilMARuijsGJJacobsenSEOlszewskiNE 1996 Isolation and characterization of abscisic acid-deficient Arabidopsis mutants at two new loci. Plant J 10 655 661
63. MengisteTChenXSalmeronJDietrichR 2003 The BOTRYTIS SUSCEPTIBLE1 gene encodes an R2R3MYB transcription factor protein that is required for biotic and abiotic stress responses in Arabidopsis. Plant Cell 15 2551 2565
64. Thordal-ChristensenHZhangZWeiYCollingeDB 1997 Subcellular localization of H2O2 in plants. H2O2 accumulation in papillae and hypersensitive response during the barley powdery mildew interaction. Plant J 11 1187 1194
65. ChenZXSilvaHKlessigDF 1993 Active oxygen species in the induction of plant systemic acquired resistance by salicylic acid. Science 262 1883 1886
66. ChristmannAHoffmannTTeplovaIGrillEMullerA 2005 Generation of active pools of abscisic acid revealed by in vivo imaging of water-stressed Arabidopsis. Plant Physiol 137 209 219
67. CzechowskiTStittMAltmannTUdvardiMKScheibleWR 2005 Genome-wide identification and testing of superior reference genes for transcript normalization in Arabidopsis. Plant Physiol 139 5 17
68. Van GemerenIAMustersWVan Den HondelCAMJJVerripsCT 1995 Construction and heterologous expression of a synthetic copy of the cutinase cDNA from Fusarium solani pisi. J Biotech 40 155 162
79. FrankeRBriesenIWojciechowskiTFaustAYephremovA 2005 Apoplastic polyesters in Arabidopsis surface tissues - A typical suberin and a particular cutin. Phytochemistry 66 2643 2658
70. CloughSJBentAF 1998 Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J 16 735 743
71. LangELangH 1972 Specific color-reaction for direct identification of formic-acid. Fresenius Zeitschr Anal Chemie 260 8 10
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek A Typhimurium-Typhi Genomic Chimera: A Model to Study Vi Polysaccharide Capsule Function In VivoČlánek SUMO Pathway Dependent Recruitment of Cellular Repressors to Herpes Simplex Virus Type 1 GenomesČlánek A Structural Model for Binding of the Serine-Rich Repeat Adhesin GspB to Host Carbohydrate ReceptorsČlánek Dynamic Evolution of Pathogenicity Revealed by Sequencing and Comparative Genomics of 19 IsolatesČlánek Widespread Endogenization of Genome Sequences of Non-Retroviral RNA Viruses into Plant GenomesČlánek The Cost of Virulence: Retarded Growth of Typhimurium Cells Expressing Type III Secretion System 1Článek A Role for the Chemokine RANTES in Regulating CD8 T Cell Responses during Chronic Viral Infection
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2011 Číslo 7- Stillova choroba: vzácné a závažné systémové onemocnění
- Perorální antivirotika jako vysoce efektivní nástroj prevence hospitalizací kvůli COVID-19 − otázky a odpovědi pro praxi
- Jak souvisí postcovidový syndrom s poškozením mozku?
- Diagnostika virových hepatitid v kostce – zorientujte se (nejen) v sérologii
- Infekční komplikace virových respiračních infekcí – sekundární bakteriální a aspergilové pneumonie
-
Všechny články tohoto čísla
- What Do We Really Know about How CD4 T Cells Control ?
- “Persisters”: Survival at the Cellular Level
- E6 and E7 from Beta Hpv38 Cooperate with Ultraviolet Light in the Development of Actinic Keratosis-Like Lesions and Squamous Cell Carcinoma in Mice
- Selection of Resistant Bacteria at Very Low Antibiotic Concentrations
- The Extracytoplasmic Domain of the Ser/Thr Kinase PknB Binds Specific Muropeptides and Is Required for PknB Localization
- CD39/Adenosine Pathway Is Involved in AIDS Progression
- Hypoxia and a Fungal Alcohol Dehydrogenase Influence the Pathogenesis of Invasive Pulmonary Aspergillosis
- One Is Enough: Effective Population Size Is Dose-Dependent for a Plant RNA Virus
- Effects of Interferon-α/β on HBV Replication Determined by Viral Load
- A Typhimurium-Typhi Genomic Chimera: A Model to Study Vi Polysaccharide Capsule Function In Vivo
- Dual Chaperone Role of the C-Terminal Propeptide in Folding and Oligomerization of the Pore-Forming Toxin Aerolysin
- Rotavirus Stimulates Release of Serotonin (5-HT) from Human Enterochromaffin Cells and Activates Brain Structures Involved in Nausea and Vomiting
- Dissociation of Infectivity from Seeding Ability in Prions with Alternate Docking Mechanism
- The Impact of Recombination on dN/dS within Recently Emerged Bacterial Clones
- The Regulation of Sulfur Metabolism in
- Illumination of Parainfluenza Virus Infection and Transmission in Living Animals Reveals a Tissue-Specific Dichotomy
- A Permeable Cuticle Is Associated with the Release of Reactive Oxygen Species and Induction of Innate Immunity
- A Concerted Action of Hepatitis C Virus P7 and Nonstructural Protein 2 Regulates Core Localization at the Endoplasmic Reticulum and Virus Assembly
- SUMO Pathway Dependent Recruitment of Cellular Repressors to Herpes Simplex Virus Type 1 Genomes
- Re-localization of Cellular Protein SRp20 during Poliovirus Infection: Bridging a Viral IRES to the Host Cell Translation Apparatus
- Divergent Effects of Human Cytomegalovirus and Herpes Simplex Virus-1 on Cellular Metabolism
- A Structural Model for Binding of the Serine-Rich Repeat Adhesin GspB to Host Carbohydrate Receptors
- Transformation of Natural Genetic Variation into Genomes
- EBV Latency Types Adopt Alternative Chromatin Conformations
- Global mRNA Degradation during Lytic Gammaherpesvirus Infection Contributes to Establishment of Viral Latency
- Dynamic Evolution of Pathogenicity Revealed by Sequencing and Comparative Genomics of 19 Isolates
- Microbial Virulence as an Emergent Property: Consequences and Opportunities
- Widespread Endogenization of Genome Sequences of Non-Retroviral RNA Viruses into Plant Genomes
- Structural Basis of Chemokine Sequestration by CrmD, a Poxvirus-Encoded Tumor Necrosis Factor Receptor
- Cross-Species Transmission of a Novel Adenovirus Associated with a Fulminant Pneumonia Outbreak in a New World Monkey Colony
- An Interaction between KSHV ORF57 and UIF Provides mRNA-Adaptor Redundancy in Herpesvirus Intronless mRNA Export
- Elevated 17β-Estradiol Protects Females from Influenza A Virus Pathogenesis by Suppressing Inflammatory Responses
- The Role of IL-15 Deficiency in the Pathogenesis of Virus-Induced Asthma Exacerbations
- Fluorescence Lifetime Imaging Unravels Metabolism and Its Crosstalk with the Host Cell
- Programmed Death (PD)-1-Deficient Mice Are Extremely Sensitive to Murine Hepatitis Virus Strain-3 (MHV-3) Infection
- Hemoglobin Promotes Nasal Colonization
- Crystallography of a Lewis-Binding Norovirus, Elucidation of Strain-Specificity to the Polymorphic Human Histo-Blood Group Antigens
- The Cost of Virulence: Retarded Growth of Typhimurium Cells Expressing Type III Secretion System 1
- A Genome-Wide Approach to Discovery of Small RNAs Involved in Regulation of Virulence in
- Requires Glycerol for Maximum Fitness During The Tick Phase of the Enzootic Cycle
- C Metabolic Flux Analysis Identifies an Unusual Route for Pyruvate Dissimilation in Mycobacteria which Requires Isocitrate Lyase and Carbon Dioxide Fixation
- A Role for the Chemokine RANTES in Regulating CD8 T Cell Responses during Chronic Viral Infection
- Glycosaminoglycans and Sialylated Glycans Sequentially Facilitate Merkel Cell Polyomavirus Infectious Entry
- Regulation of Stomatal Tropism and Infection by Light in : Evidence for Coordinated Host/Pathogen Responses to Photoperiod?
- Multiple Translocation of the Effector Gene among Chromosomes of the Rice Blast Fungus and Related Species
- Comparative Genomics Yields Insights into Niche Adaptation of Plant Vascular Wilt Pathogens
- Unique Cell Adhesion and Invasion Properties of O:3, the Most Frequent Cause of Human Yersiniosis
- C-Terminal Region of EBNA-2 Determines the Superior Transforming Ability of Type 1 Epstein-Barr Virus by Enhanced Gene Regulation of LMP-1 and CXCR7
- Novel Chikungunya Vaccine Candidate with an IRES-Based Attenuation and Host Range Alteration Mechanism
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Requires Glycerol for Maximum Fitness During The Tick Phase of the Enzootic Cycle
- Comparative Genomics Yields Insights into Niche Adaptation of Plant Vascular Wilt Pathogens
- The Role of IL-15 Deficiency in the Pathogenesis of Virus-Induced Asthma Exacerbations
- “Persisters”: Survival at the Cellular Level
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



