-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Requires Glycerol for Maximum Fitness During The Tick Phase of the Enzootic Cycle
Borrelia burgdorferi, the spirochetal agent of Lyme disease, is a vector-borne pathogen that cycles between a mammalian host and tick vector. This complex life cycle requires that the spirochete modulate its gene expression program to facilitate growth and maintenance in these diverse milieus. B. burgdorferi contains an operon that is predicted to encode proteins that would mediate the uptake and conversion of glycerol to dihydroxyacetone phosphate. Previous studies indicated that expression of the operon is elevated at 23°C and is repressed in the presence of the alternative sigma factor RpoS, suggesting that glycerol utilization may play an important role during the tick phase. This possibility was further explored in the current study by expression analysis and mutagenesis of glpD, a gene predicted to encode glycerol 3-phosphate dehydrogenase. Transcript levels for glpD were significantly lower in mouse joints relative to their levels in ticks. Expression of GlpD protein was repressed in an RpoS-dependent manner during growth of spirochetes within dialysis membrane chambers implanted in rat peritoneal cavities. In medium supplemented with glycerol as the principal carbohydrate, wild-type B. burgdorferi grew to a significantly higher cell density than glpD mutant spirochetes during growth in vitro at 25°C. glpD mutant spirochetes were fully infectious in mice by either needle or tick inoculation. In contrast, glpD mutants grew to significantly lower densities than wild-type B. burgdorferi in nymphal ticks and displayed a replication defect in feeding nymphs. The findings suggest that B. burgdorferi undergoes a switch in carbohydrate utilization during the mammal to tick transition. Further, the results demonstrate that the ability to utilize glycerol as a carbohydrate source for glycolysis during the tick phase of the infectious cycle is critical for maximal B. burgdorferi fitness.
Published in the journal: . PLoS Pathog 7(7): e32767. doi:10.1371/journal.ppat.1002102
Category: Research Article
doi: https://doi.org/10.1371/journal.ppat.1002102Summary
Borrelia burgdorferi, the spirochetal agent of Lyme disease, is a vector-borne pathogen that cycles between a mammalian host and tick vector. This complex life cycle requires that the spirochete modulate its gene expression program to facilitate growth and maintenance in these diverse milieus. B. burgdorferi contains an operon that is predicted to encode proteins that would mediate the uptake and conversion of glycerol to dihydroxyacetone phosphate. Previous studies indicated that expression of the operon is elevated at 23°C and is repressed in the presence of the alternative sigma factor RpoS, suggesting that glycerol utilization may play an important role during the tick phase. This possibility was further explored in the current study by expression analysis and mutagenesis of glpD, a gene predicted to encode glycerol 3-phosphate dehydrogenase. Transcript levels for glpD were significantly lower in mouse joints relative to their levels in ticks. Expression of GlpD protein was repressed in an RpoS-dependent manner during growth of spirochetes within dialysis membrane chambers implanted in rat peritoneal cavities. In medium supplemented with glycerol as the principal carbohydrate, wild-type B. burgdorferi grew to a significantly higher cell density than glpD mutant spirochetes during growth in vitro at 25°C. glpD mutant spirochetes were fully infectious in mice by either needle or tick inoculation. In contrast, glpD mutants grew to significantly lower densities than wild-type B. burgdorferi in nymphal ticks and displayed a replication defect in feeding nymphs. The findings suggest that B. burgdorferi undergoes a switch in carbohydrate utilization during the mammal to tick transition. Further, the results demonstrate that the ability to utilize glycerol as a carbohydrate source for glycolysis during the tick phase of the infectious cycle is critical for maximal B. burgdorferi fitness.
Introduction
Borrelia burgdorferi is the spirochetal agent of Lyme disease, the most frequently reported vector-borne disease in the United States [1]. In the Northeastern United States, B. burgdorferi is transmitted between mammalian hosts by the bite of the black legged deer tick, Ixodes scapularis, with the white-footed mouse (Peromyscus leucopus) serving as the primary reservoir host [2], [3]. The transmission cycle is as intricate as the life of the tick itself. B. burgdorferi are acquired by uninfected larvae feeding on an infected small mammal [4]. This is essential for the continued maintenance of B. burgdorferi in nature, since there is no transovarial transmission in Ixodes spp. [5], [6]. The bacteria remain in the midgut of engorged larval ticks through the molt. The infected nymph will take a blood meal on a mammal, at which point B. burgdorferi multiply and begin their migration from the tick midgut to the salivary glands from which they are transmitted to a mammalian host [7]–[9], thereby completing the enzootic cycle.
B. burgdorferi must adjust its gene expression program in response to the different physiological cues encountered during the natural enzootic cycle. In bacteria, regulation of gene expression in response to environmental cues is often mediated by two-component systems (TCS) and/or alternative sigma factors [10], [11]. The B. burgdorferi genome encodes only two alternative sigma factors and two TCS [12], [13]. Thus, B. burgdorferi must orchestrate its complex expression programs with a limited repertoire of known transcriptional regulators. Studies by Norgard and co-workers demonstrated a link between one TCS, Hk2-Rrp2, and the alternative sigma factors RpoN and RpoS [14], [15]. The expression of several virulence genes, including ospC, dbpA and bbk32, are dependent on RpoS [14], [16], [17]. RpoS is also essential for repression of genes whose expression is required during the tick phase, but not in the mammalian host [17], [18]. BB0647 (BosR, Fur) has also been shown to play a role in RpoN-dependent expression of rpoS [19]–[21]. Less is currently known regarding the second TCS, consisting of Hk1 and Rrp1, but recent studies have begun to elucidate the processes that are regulated by this TCS [22]–[24]. In particular, Rrp1 has been shown to be responsible for production of bis-(3′-5′)-cyclic dimeric guanosine monophosphate (c-di-GMP) and mutagenesis of Rrp1 results in alteration of expression for a substantial number of genes, including those involved in uptake and dissimilation of glycerol [22], [23], [25].
Different carbohydrates are selectively available to B. burgdorferi during its enzootic cycle. Glucose is the primary carbohydrate constituent in mammalian blood [26], [27] and B. burgdorferi can use glucose to support growth [28]. Ticks rely on a high concentration of carbohydrates and other nutrients available in the blood meal for molting and successful oogenesis [29], [30]. During feeding, ticks create a peritrophic matrix above the epithelial cell layer, which serves both as a compartment to trap the blood meal and as a barrier to prevent invasion by microorganisms that accompany the blood meal. However, the peritrophic matrix is permeable to hexose sugars [29], [31]. Once hexoses permeate across the matrix, they are sequestered by midgut epithelial cells during larval feeding [29], [31]. Consequently, nutrients present in the blood meal are rapidly depleted during larval feeding and are likely non-existent in an unfed nymphal midgut. Therefore, spirochetes resident in the midgut must identify and utilize alternative carbohydrates until the unfed nymph takes its next blood meal.
Glycerol, a diffusible carbohydrate, is a readily available nutrient in the tick. Glycerol is produced by Ixodes spp. and serves as a colligative antifreeze for tick survival during the winter [32]–[34]. B. burgdorferi encodes a putative glycerol utilization operon consisting of three genes. glpF (bb0240) encodes a putative transmembrane facilitator protein that mediates the entry of free glycerol into the cell. glpK (bb0241) encodes a putative kinase that would produce glycerol 3-phosphate (G3P) which would be the substrate for glycerol 3-phosphate dehydrogenase (G3PDH), an enzyme putatively encoded by the third gene in the operon, bb0243. The resulting product, dihydroxyacetone phosphate, can enter glycolysis through the action of triose phosphate isomerase and ultimately result in the net production of one ATP molecule per original glycerol molecule [12], [28]. Alternatively, G3P may be converted to phosphatidic acid through the action of two enzymes, BB0327 (G3P acyltransferase) and BB0037 (Lysophosphatidic acid acyltransferase); this pathway is required for phospholipid biosynthesis and production of new cell membrane [12] (Figure 1).
Fig. 1. Predicted carbohydrate utilization pathways in B. burgdorferi. 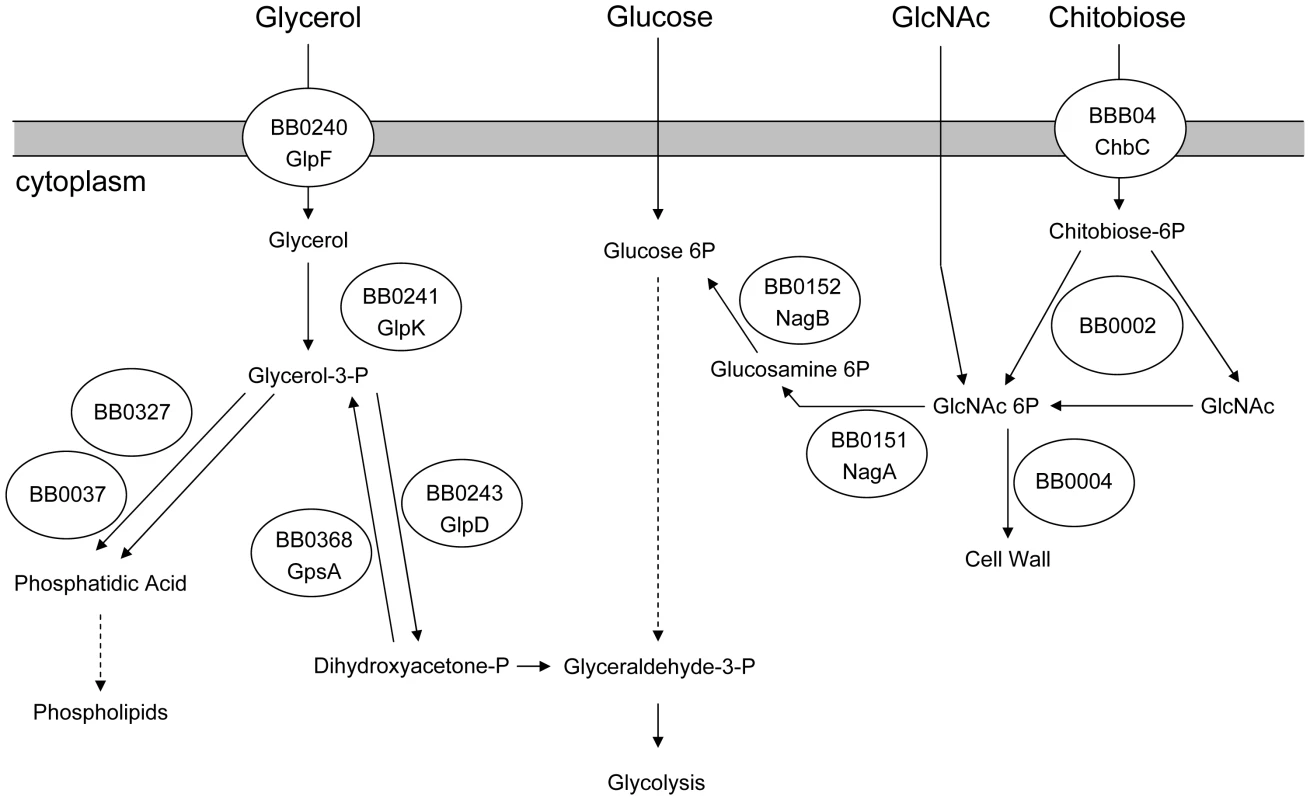
Based on Fraser et al. [12] and von Lackum and Stevenson [28]. Three lines of evidence suggest that glycerol utilization may be important during the vector phase of the enzootic cycle. Ojaimi et al. reported that all genes of the glycerol utilization operon are more highly expressed during in vitro growth in BSK-II medium at 23°C as compared to growth at 35°C [35]. Caimano et al. demonstrated that repression of glp operon expression is dependent on RpoS within the mammalian host [17]. Moreover, constitutive expression of the glp operon partially restores the ability of Rrp1-deficient B. burgdorferi to survive within feeding ticks [23].
In order to elucidate the role of glycerol uptake and utilization by B. burgdorferi during its natural life cycle and the regulatory events that govern glp operon expression, the gene predicted to encode G3PDH (bb0243) was disrupted and the effects of mutagenesis were evaluated in vitro and during infection of ticks or mice. The results demonstrate that the ability to utilize glycerol as a carbohydrate for use in the glycolytic pathway during the tick phase of the infectious cycle is critical for maximal B. burgdorferi fitness.
Results
Bioinformatic analysis of B. burgdorferi glycerol 3-phosphate dehydrogenase
The B. burgdorferi G3PDH genomic sequence has been annotated to putatively encode the anaerobic form of the enzyme based on its similarity to the anaerobic G3PDH ortholog of Haemophilus influenzae strain Rd (glpA) [12]. Other organisms containing an anaerobic GlpA (such as E. coli) contain two additional subunits as part of the functional G3PDH enzyme; GlpB, a subunit involved in FMN binding [36] and GlpC, a small membrane anchoring subunit [37]. Together, the individual protein molecules form a functional GlpABC heterotrimer [36]. Whole genome sequencing of B. burgdorferi failed to reveal putative genes with homology to any known glpB or glpC orthologs [12]. Further, BLASTP analysis revealed no orthologs in B. burgdorferi with similarity to either E. coli strain K12 or H. influenzae strain Rd GlpB or GlpC.
The tertiary structure of B. burgdorferi G3PDH was predicted using the SWISS-MODEL server [38]–[40] by comparison to E. coli K12 aerobic GlpD and anaerobic GlpA, as well as H. influenzae strain Rd anaerobic GlpA. B. burgdorferi G3PDH autoaligned with E. coli aerobic GlpD (PDB 2QCU), but not with E. coli GlpA [41] (Figure 2). E. coli anaerobic GlpA and H. influenzae GlpA auto-aligned to the anaerobic GlpA of Bacillus halodurans (PDB 3DA1) [42] (Figure 2). The modeling predicts that B. burgdorferi G3PDH and E. coli aerobic GlpD share similar tertiary structures. Yeh et al. have described 14 amino acid residues that participate in the E. coli GlpD active site based on a 1.75 Å structural model [41]. B. burgdorferi G3PDH contains conserved residues at 12/14 positions, in contrast to the E. coli and H. influenzae GlpA proteins (9/14). Taken together, the bioinformatic analyses suggest that B. burgdorferi G3PDH has greater similarity to aerobic forms of the enzyme. We propose that annotation of bb0243 should be changed to indicate that it putatively encodes an aerobic GlpD and B. burgdorferi G3PDH is referred to as GlpD in the remainder of this report.
Fig. 2. Predicted tertiary structure of B. burgdorferi G3PDH. 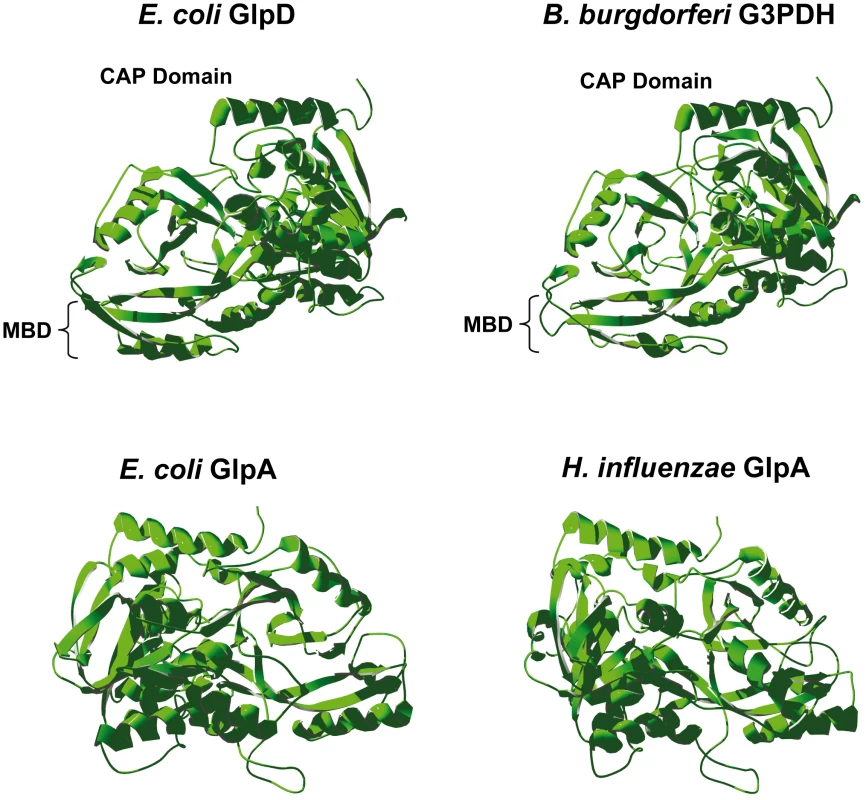
The B. burgdorferi G3PDH sequence was mapped onto known bacterial G3PDH three-dimensional structures using SWISS-MODEL software. Structures were rotated so they are aligned in an identical orientation, with the C-terminal α-helix (red) at the top in all structures. MBD, putative membrane binding domain [41]. B. burgdorferi glpD has elevated expression in ticks
The physical linkage of bb0240, bb0241, and bb0243 in the B. burgdorferi chromosome suggests that these genes comprise an operon [35]. RT-PCR analysis using RNA extracted from B. burgdorferi strain B31-A3 revealed that these genes are transcribed as a single operon (Figure 3). Ojaimi et al. reported that the B. burgdorferi glycerol operon is more highly transcribed at 23°C relative to transcript levels in cells grown at 35°C [35]. In order to explore if this increased transcript level is reflected in protein, strain B31-A3 whole cell lysate was tested to determine the protein expression levels at these two temperatures. Immunoblot analysis revealed that when B. burgdorferi strain B31-A3 was grown at 25°C, 7-fold more GlpD was generated compared to the level in cells grown at 37°C (Figure 4).
Fig. 3. The B. burgdorferi glp region constitutes an operon. 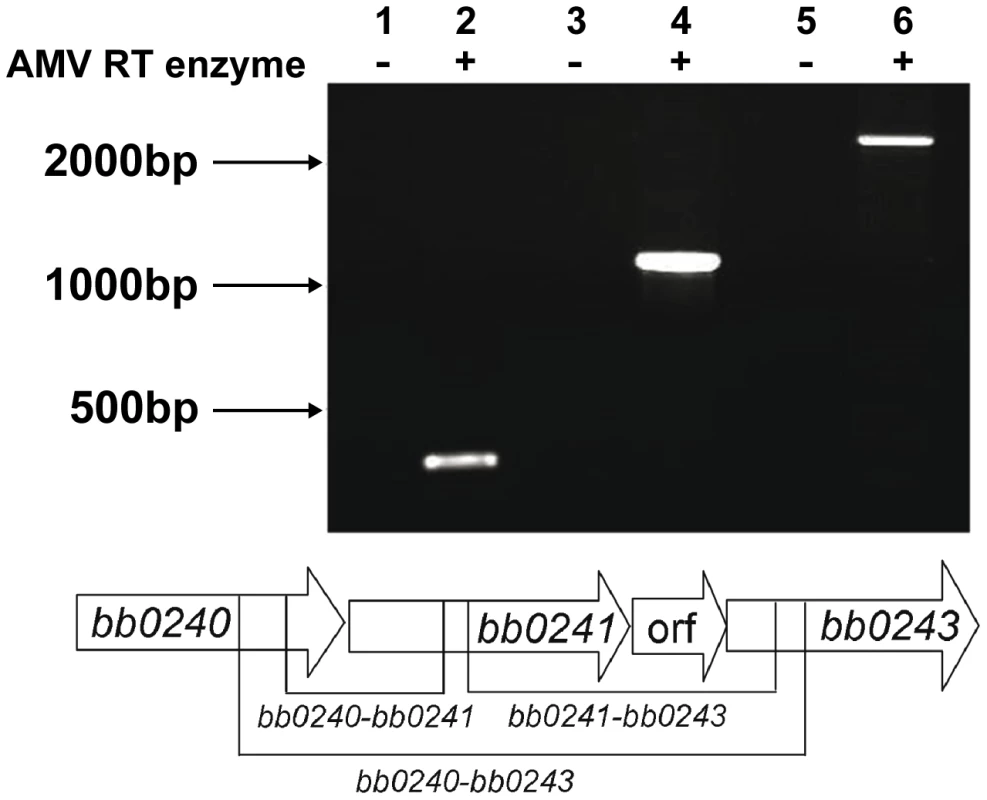
RT-PCR of RNA extracted from B31-A3 grown at 37°C in BSK-II medium was performed using primers listed in table 4. Lanes 1, 2: bb0240-bb0241 (expected size 349 bp); lanes 3, 4: bb0241–bb0243 (expected size 1141 bp); lanes 5, 6: bb0240–bb0243 (expected size 2383 bp). Migration positions of DNA size markers are indicated on left. A schematic diagram showing the operon structure and sizes of expected amplification products is shown below the gel picture. Fig. 4. B. burgdorferi GlpD expression is temperature dependent. 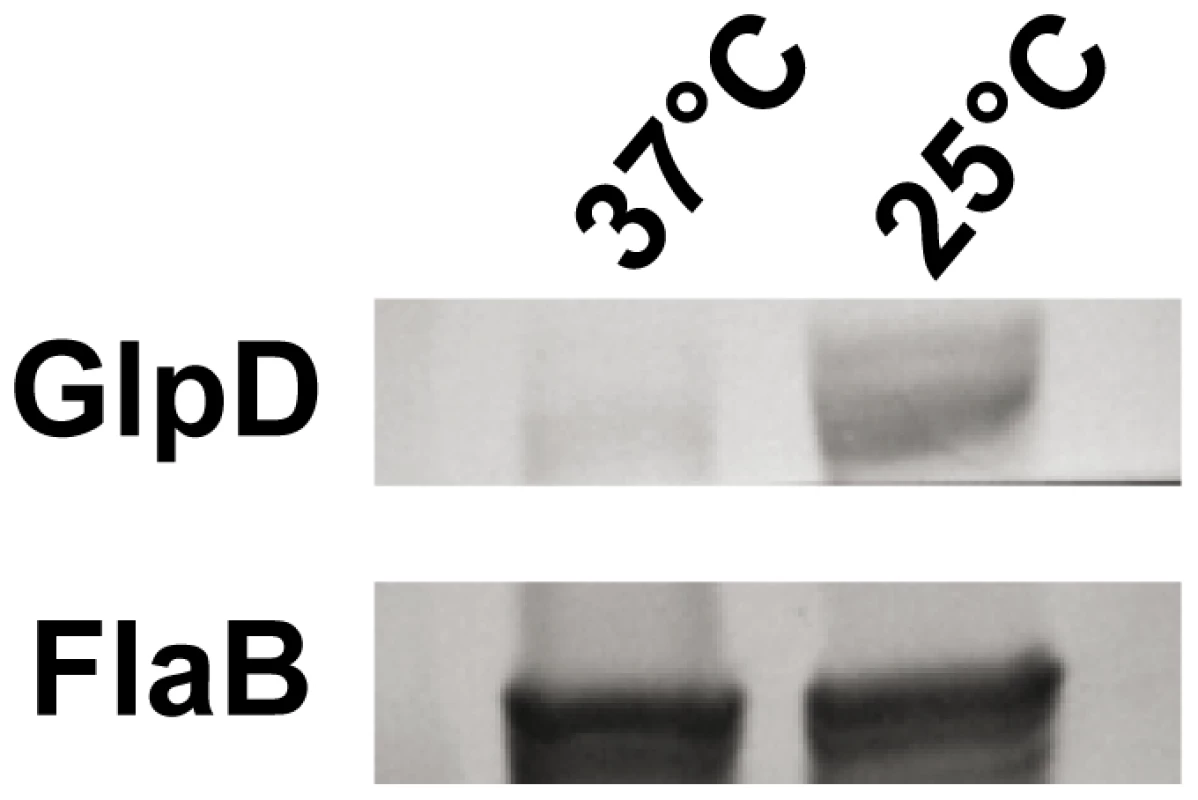
Whole cell lysates of B. burgdorferi B31-A3 grown at 37°C and 25°C were analyzed by immunoblotting. FlaB levels were determined as a control. To explore the possibility that expression may be differentially regulated in vivo, glp transcript levels were measured in infected ticks or mouse joints by real time RT-PCR; transcription of ospA and ospC was monitored as a control. Expression of the latter genes followed the expected pattern; ospA was expressed exclusively in ticks and ospC transcript was detected only in feeding nymphs and mouse joints (Figure 5). glpD expression was substantially higher during all tick stages (fed larvae, 3.68±2.72 copies/10 copies of flaB; unfed nymphs, 5.31±4.42; fed nymphs, 4.97±0.74) than in mouse joints (1.45±1.98 copies/10 copies of flaB). A similar expression pattern was observed for glpF, the first gene in the operon (fed larvae, 4.42±1.07 copies/10 copies of flaB; unfed nymphs, 1.48±0.61; fed nymphs, 3.70±1.89; mouse joint, 0.13±0.23) (Figure 5). Caimano et al. showed that transcription of glp operon genes is subject to RpoS-dependent repression [17]. This was confirmed at the protein level for GlpD as shown in Figure 6. Wild-type or RpoS mutant cells were grown in vitro at either 23°C or 37°C or in dialysis membrane chambers (DMCs) implanted in rat peritoneal cavities. Induction of OspC expression and repression of OspA expression in DMCs confirmed that B. burgdorferi attained the host-adapted state and abrogation of these changes in expression in the RpoS mutant showed that these alterations were dependent on RpoS, as expected. GlpD expression was virtually abolished in wild-type B. burgdorferi grown in DMC and this effect was not observed in the RpoS mutant cells (figure 6).
Fig. 5. Transcriptional analysis of selected genes at different stages of the enzootic cycle. 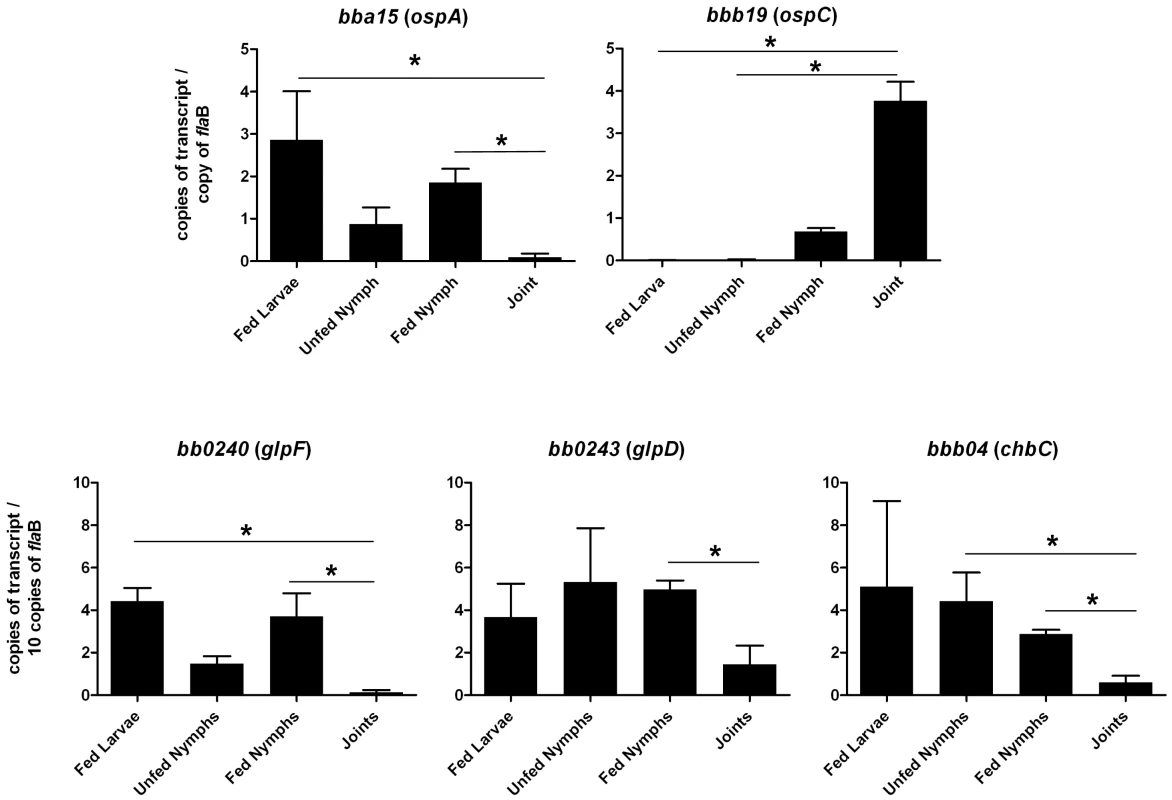
qRT-PCR was performed with RNA isolated from strain B31-A3-infected ticks and mouse joints as described in Methods. Statistical analysis was determined by Kruskal-Wallis multiple comparison Z- value test; * significant difference (P≤.05) between samples. Error bars indicate standard error of the mean (SEM). Fig. 6. Repression of GlpD expression requires RpoS. 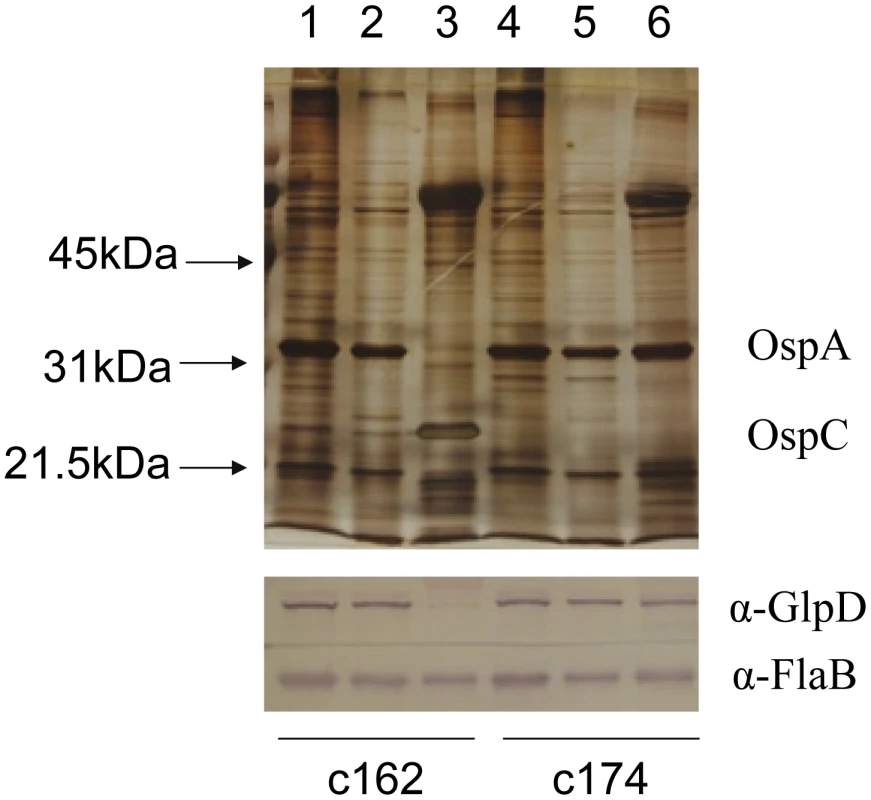
Wild type (c162) and RpoS mutant (c174) B. burgdorferi were cultivated in vitro at either 23°C or 37°C or in DMC. Cell lysates were subjected to SDS-PAGE and gels were either silver stained (top panel) or blotted to PVDF membranes and developed with antibodies to GlpD or FlaB (bottom panel). Lanes 1 and 4, 23°C; lanes 2 and 5, 37°C; lanes 3 and 6, DMC. Migration positions of protein molecular mass markers are indicated on left in top panel. Construction of a B. burgdorferi glpD mutant
To study the role of glycerol utilization in B. burgdorferi, glpD, the distal ORF in the glycerol operon was inactivated in strain B31-A3 by disruption with a flgB-aadA cassette inserted at residue K149 (Figure 7). Three mutants, two with the flgB-aadA cassette in the same orientation as the operon (CP176, CP177) and one with the insert in the opposite orientation (CP257), were isolated (Figure 8A). Southern blot analysis confirmed a disruption in glpD and showed that recombination occurred by a double crossover event in all three mutants (Figure 8B). Western blot analysis revealed that GlpD was absent in the mutants (Figure 9). Analysis of plasmid content of the wild type by PCR revealed that it lacked lp5 and cp9 and contained all other B31 linear plasmids, including those essential for murine infectivity. GlpD mutants had the same plasmid profile as the parental strain (data not shown).
Fig. 7. Strategy for generation of glpD disruption mutants. 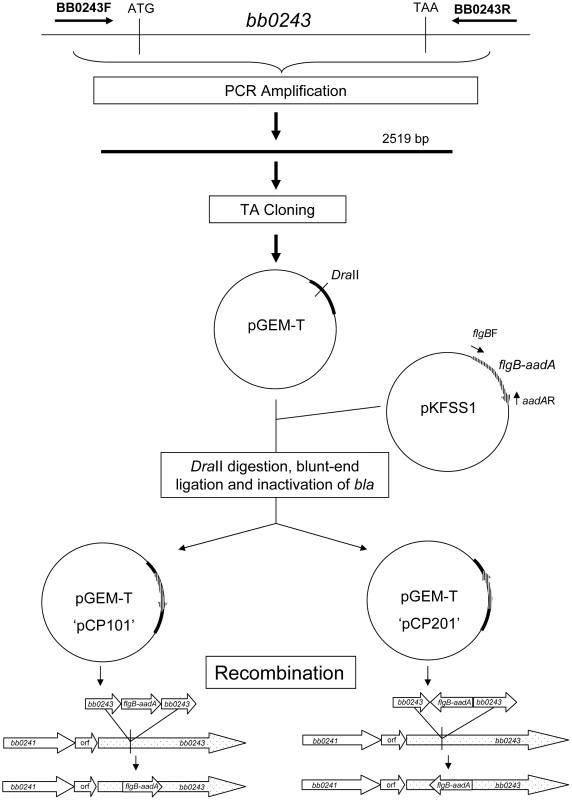
glpD mutant clones CP176 and CP177 were generated by recombination with pCP101; glpD mutant clone CP257 was generated by recombination with pCP201. Fig. 8. Confirmation of glpD disruption. 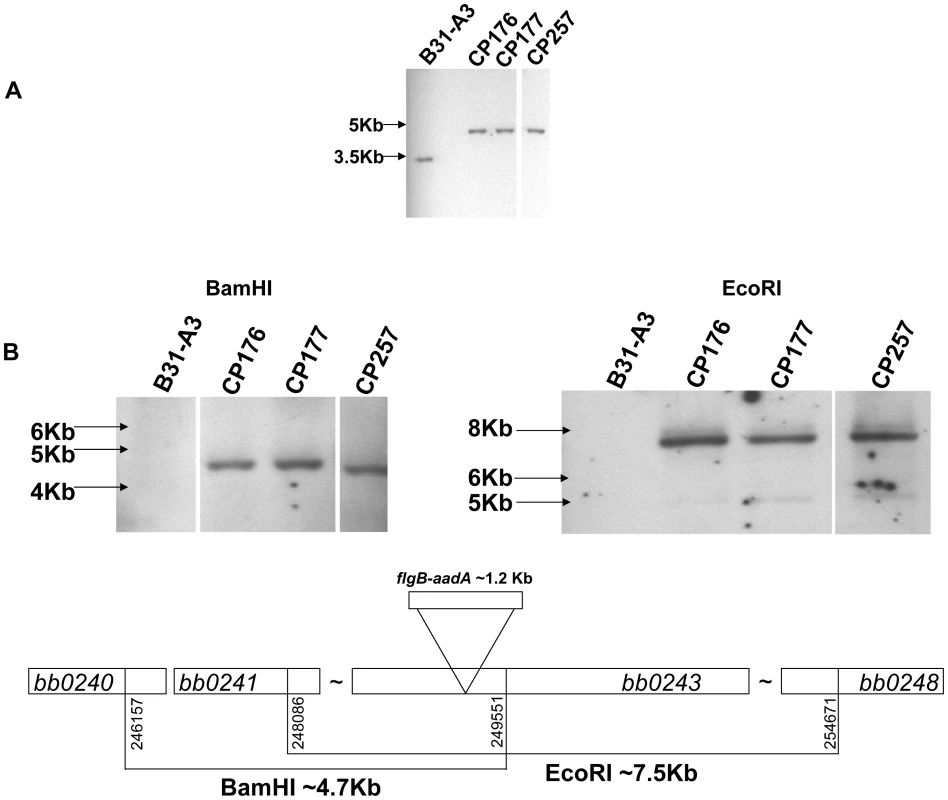
A) Southern blot analysis confirms the disruption of glpD. Whole genomic DNA from B31-A3 and glpD disruption mutants CP176, CP177, CP257 were digested with BamHI and blotted on a nylon membrane. Digoxygenein-11-dUTP labeled bb0243 probe was utilized to visualize glpD. Migration positions of DNA molecular size markers are indicated on the left. B) glpD disruption occurred via by a double crossover insertion of flgB-aadA. Whole genomic DNA from B31-A3 and glpD disruption mutants CP176, CP177, CP257 were digested with BamHI or EcoRI, which would be specific for the proximal or distal bb0243 flanking chromosomal regions, respectively, and blotted to a nylon membrane. Blots were developed with a digoxygenein-11-dUTP-labeled aadA probe. A schematic diagram indicates the sizes of the fragments expected to contain flgB-aadA. Migration positions of DNA molecular size markers are indicated on the left of each panel. Fig. 9. GlpD is absent in disruption mutants. 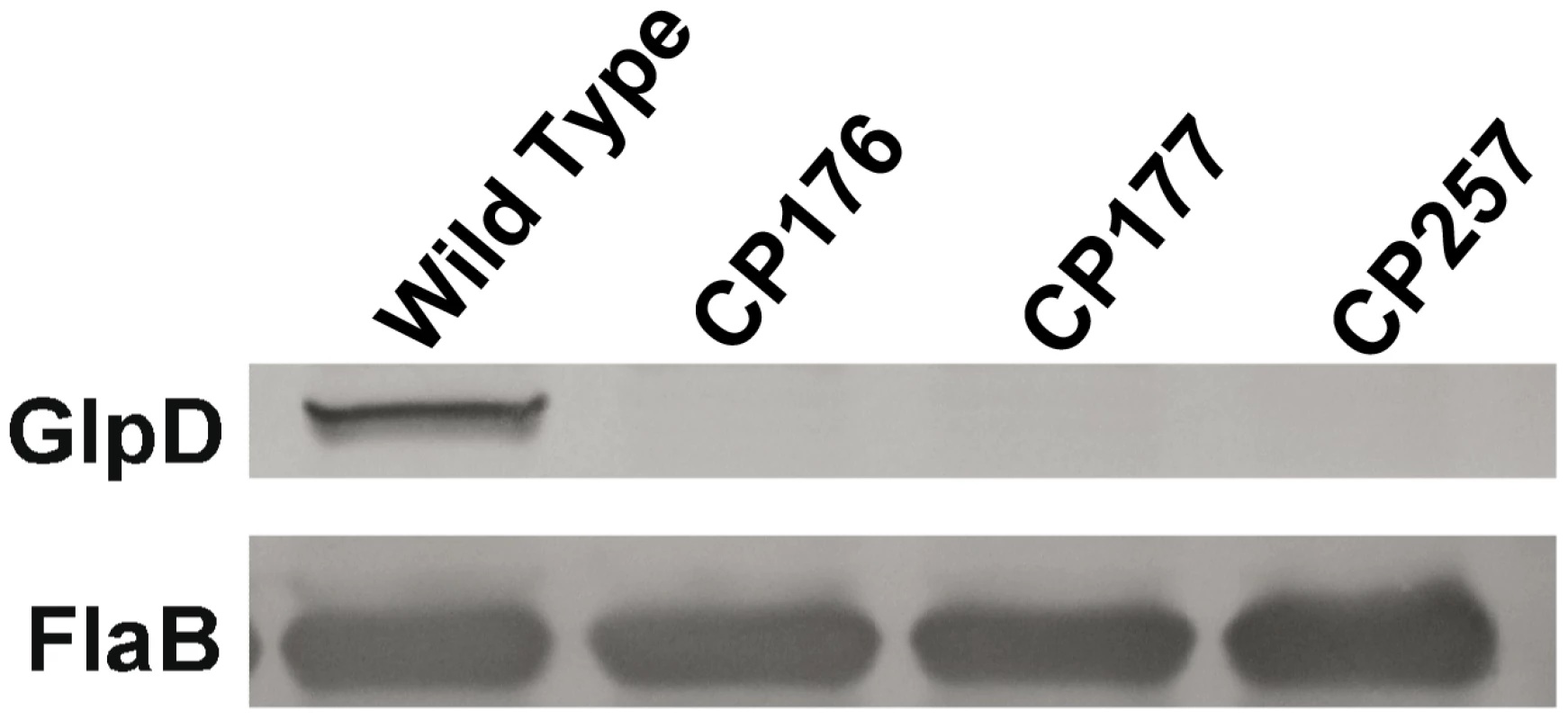
Whole cell lysates from B31-A3, CP176, CP177, and CP257 were separated by SDS-PAGE, followed by immunoblot analysis using GlpD-specific antiserum. Presence of FlaB was also measured as a control. Repeated attempts to isolate a complemented mutant strain were unsuccessful. Most experiments described below were carried out with all three isolated glpD mutants. Mutants CP176 and CP177 were isolated from one transformation and CP257 was obtained independently, thereby mitigating the concern that the observed mutant phenotypes were the result of a second site mutation.
GlpD is required for maximal in vitro growth when glycerol is the principal carbohydrate source
To begin to characterize the role of GlpD in B. burgdorferi physiology, growth of glpD mutants was compared to that of wild-type B31-A3 in BSK-II, an undefined, enriched medium that contains glucose as the principal carbohydrate source [43]. No differences in final cell density were detected between wild-type and glpD mutants at either 25°C (1.3×109 and 1.1×109, respectively) or 37°C (1.4×109 and 1.3×109, respectively). In addition, no difference in growth characteristics was observed (data not shown).
Previous studies have shown that N-acetyl glucosamine (GlcNAc) is required for growth of B. burgdorferi in vitro [44]–[46]. There were no differences in growth characteristics between B31-A3 and CP176 grown in a modified BSK-II medium that did not contain glucose but had GlcNAc as the carbohydrate source (BSK-lite [28]) (Figure 10A). In contrast to growth in BSK-II with GlcNAc only or medium supplemented with glucose (data not shown), there was a significant difference in growth between wild type and glpD mutants when glycerol was supplied as the principal carbohydrate source. B31-A3 reached a significantly higher cell density in BSK-glycerol medium compared to CP176 when grown at 25°C (6.4×108 and 1.1×108, respectively; P<0.001) (Figure 10B). Interestingly, this effect was observed only at 25°C; when cultivated at 37°C, the growth characteristics of wild type and CP176 were indistinguishable. Indeed, B31-A3 cultures achieved significantly higher cell densities at 25°C as compared to 37°C in BSK-glycerol medium (6.4×108 and 9.1×107, respectively; P<0.001). These experiments were repeated with the other two independent glpD mutants (CP177, CP257) with essentially identical results (data not shown). These findings suggest that B. burgdorferi can utilize glycerol to support enhanced growth at the lower temperature. This observation would be consistent with the elevated expression of GlpD at 25°C (Figure 4).
Fig. 10. Growth of B31-A3 and CP176 in modified enriched medium. 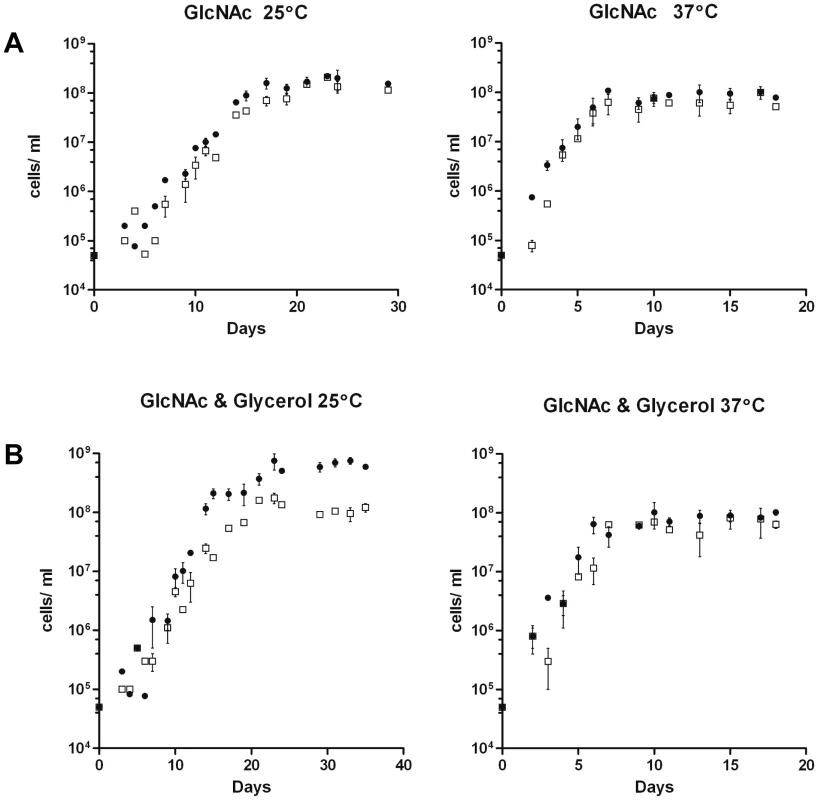
A) BSK-lite containing GlcNAc as the sole carbohydrate. •, B31-A3; □, CP176. Left panel, 25°C: B31-A3 achieved a stationary phase cell concentration of 1.8×108 and CP176 achieved a stationary phase cell concentration of 1.5×108. Right panel, 37°C: B31-A3 achieved a stationary phase cell concentration of 8.5×107 and CP176 achieved a stationary phase cell concentration of 6.3×107. All data points were determined in quadruplicate. Results are representative of two independent experiments. B) BSK-lite containing GlcNAc and glycerol as the principal carbohydrate. •, B31-A3; □, CP176. Left panel, 25°C: B31-A3 achieved a stationary phase cell concentration of 6.4×108 and CP176 achieved a stationary phase cell concentration of 1.1×108. The difference in stationary phase cell concentration between B31-A3 and CP176 at 25°C was significant (P<.001). Right panel, 37°C: B31-A3 achieved a stationary phase cell concentration of 9.1×107 and CP176 achieved a stationary phase cell concentration of 6.4×107. All data points were measured in quadruplicate. Results are representative of three independent experiments. Error bars indicate SEM. GlpD is not required for murine infection by B. burgdorferi
In order to determine whether the absence of GlpD affects the pathogenic properties of B. burgdorferi, C3H/HeJ mice were needle inoculated with 1×104 cells of either wild type or glpD mutant. All mice in both the wild type and glpD mutant groups were infected and were seropositive by four weeks post-inoculation (Table 1). Unfed larvae were allowed to feed to repletion on these infected mice to allow spirochete acquisition by ticks. Infected fed larvae that molted to nymphs were fed to repletion on naïve C3H/HeJ mice. Viable spirochetes were recovered from all mice that were fed on by either wild type - or glpD mutant-infected ticks and seroconverted by 4 weeks post-feeding (Table 1). These results demonstrate that GlpD is not required for murine infection by B. burgdorferi. Further, GlpD-deficient spirochetes were acquired by larvae fed on infected mice, persisted through the molt and were transmitted to naïve mice by infected nymphs.
Tab. 1. Infectivity of B. burgdorferi wild type and GlpD mutants in mice. 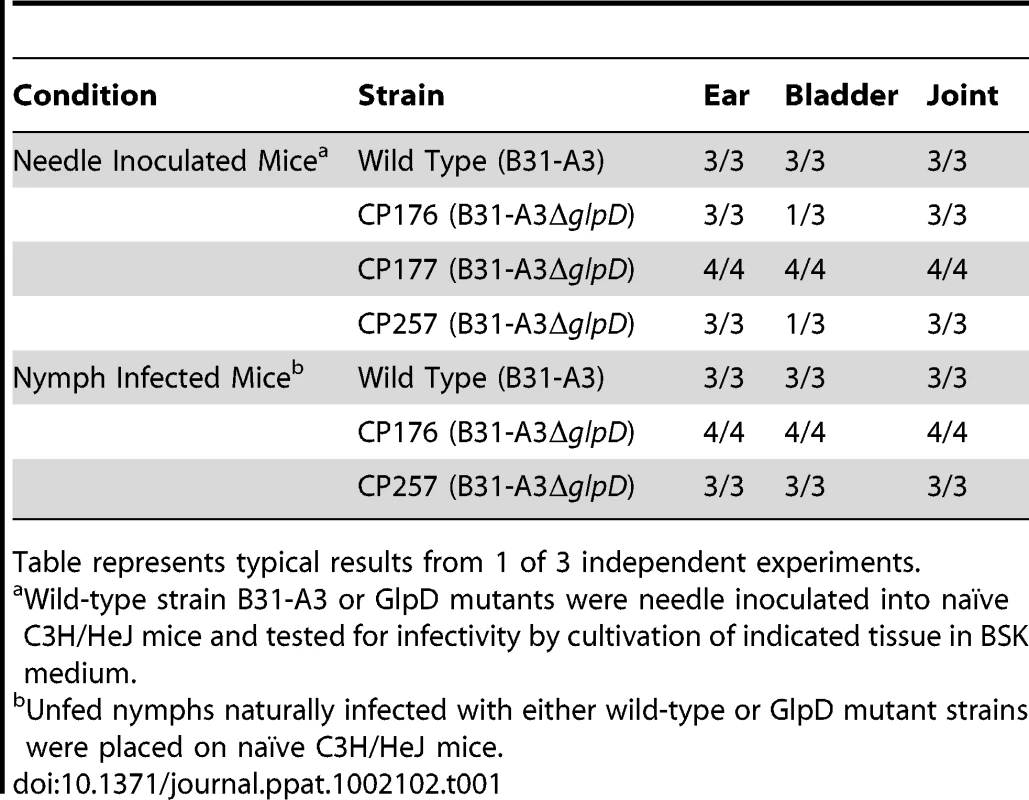
Table represents typical results from 1 of 3 independent experiments. glpD mutants have a lower spirochete density in unfed nymphs
The enhanced growth of B. burgdorferi at lower ambient temperature in vitro when glycerol is the principal carbohydrate source, elevated expression of GlpD at the lower temperature and its RpoS-dependent repression in DMCs suggested that glycerol may be an important nutrient for B. burgdorferi during the tick phase of its life cycle. Therefore, the effect of glpD disruption was explored more extensively in infected ticks. Naïve, unfed larvae were placed on mice infected with either B31-A3 or CP176, allowed to feed until repletion and molt to the nymphal stage. Spirochete loads in infected ticks were measured by qPCR (Table 2). Larvae infected with either B31-A3 or CP176 had similar spirochete loads (approximately 700 spirochetes/larvae).
Tab. 2. Spirochete density at different tick phases. 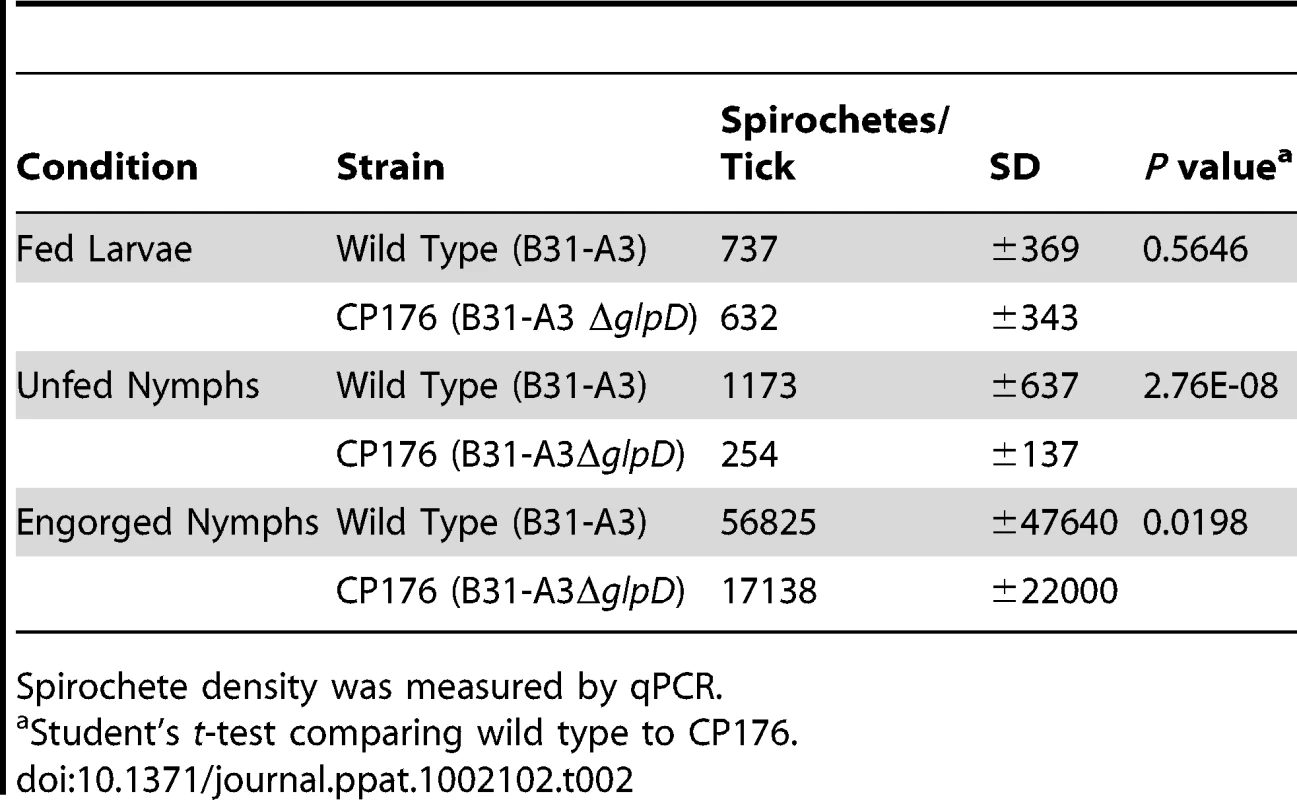
Spirochete density was measured by qPCR. Spirochete numbers in wild type-infected ticks increased slightly after larval molting to nymphs but decreased in nymphs infected with any of the glpD mutant strains. This resulted in a significant five-fold decrease in CP176 density in unfed nymphs compared to the wild-type (Table 2). Further, in independent experiments, CP177 and CP257 had an identical phenotype to that observed for CP176, i.e. spirochete densities were significantly lower after molting as compared to spirochete loads in B31-A3-infected ticks (data not shown).
Infected nymphs were fed on naïve mice and spirochete loads were measured in the resulting fed nymphs. As expected, spirochete numbers increased substantially during nymphal feeding in both wild type - and CP176-infected nymphs, although the spirochete burdens in the glpD-infected engorged nymphs were significantly lower than in nymphs infected with the parental strain (P<.02) (Table 2). These results suggest a role for glycerol utilization by B. burgdorferi as an important factor for spirochete maintenance during transtadial transition.
Absence of GlpD results in impaired B. burgdorferi replication in feeding nymphs
As previously described, wild type - and glpD mutant-infected nymphs are equally capable of transmitting B. burgdorferi to, and causing infection in, mice (Table 1). However, those studies were conducted by allowing nymphs to feed to repletion. A feeding nymph will attach and feed on a host for 72 hours or longer [47]. During this time, replicating B. burgdorferi surround midgut epithelial cells, penetrate the midgut basement membrane, and enter the hemocoel and salivary glands from which they are ultimately transmitted to the mammal [8], [9]. Therefore, replication is a critical step in spirochete transmission from the vector to the mammalian host. Since the density of glpD mutant spirochetes decreases as a result of molting and was five-fold lower than in wild type-infected unfed nymphs, we reasoned that nymphs harboring glpD mutant spirochetes would require a longer feeding period before transmission to the host due to its delayed exit from the tick midgut.
To explore this possibility, B31-A3 - and CP176-infected nymphs were placed on naïve mice, allowed to begin feeding, but forcibly removed at different time points post-attachment. Mice were then monitored for evidence of infection. In a pilot experiment, nymphs were fed on naïve mice for 65 hours; 2/2 mice fed on by B31-A3-infected nymphs became infected, whereas 0/3 mice fed on by CP176-infected nymphs acquired infection. Based on this pilot study, B31-A3 - or CP176-infected unfed nymphs were placed on the outer ear of naïve C3H/HeJ mice and allowed to feed for either 24, 48, 55, 62 or 72 hours or collected at drop off (>72 hours). Ticks were removed at each time point and spirochete load was determined by qPCR. Results presented in Figure 11 demonstrate that CP176 experienced a lag in replication and achieved significantly lower spirochete loads at times beyond 48 hours of feeding (P<0.001). At these points, spirochete loads per tick were 3.5–5 fold lower in CP176-infected ticks than in those infected with B31-A3 (e.g., at 55 hours of feeding spirochete loads were 42,633 and 8,581 for B31-A3 and CP176-infected nymphs, respectively) (Figure 11). Mice were also monitored for infection. Results demonstrate that mice fed on by wild type-infected nymphs were infected by 62 hours of feeding. In contrast, CP176-infected nymphs produced infection in mice only after at least 72 hours of feeding (Table 3). These data suggest that wild type-infected nymphs are more readily able to infect naïve mice due to a more rapid increase in spirochete density induced on commencement of tick feeding. Further, disruption of glycerol utilization results in reduced fitness of the spirochete during the tick phase and spirochetes that are unable to utilize glycerol are at a disadvantage for transmission to a mammalian host.
Fig. 11. Replication of B31-A3 or CP176 in feeding nymphs. 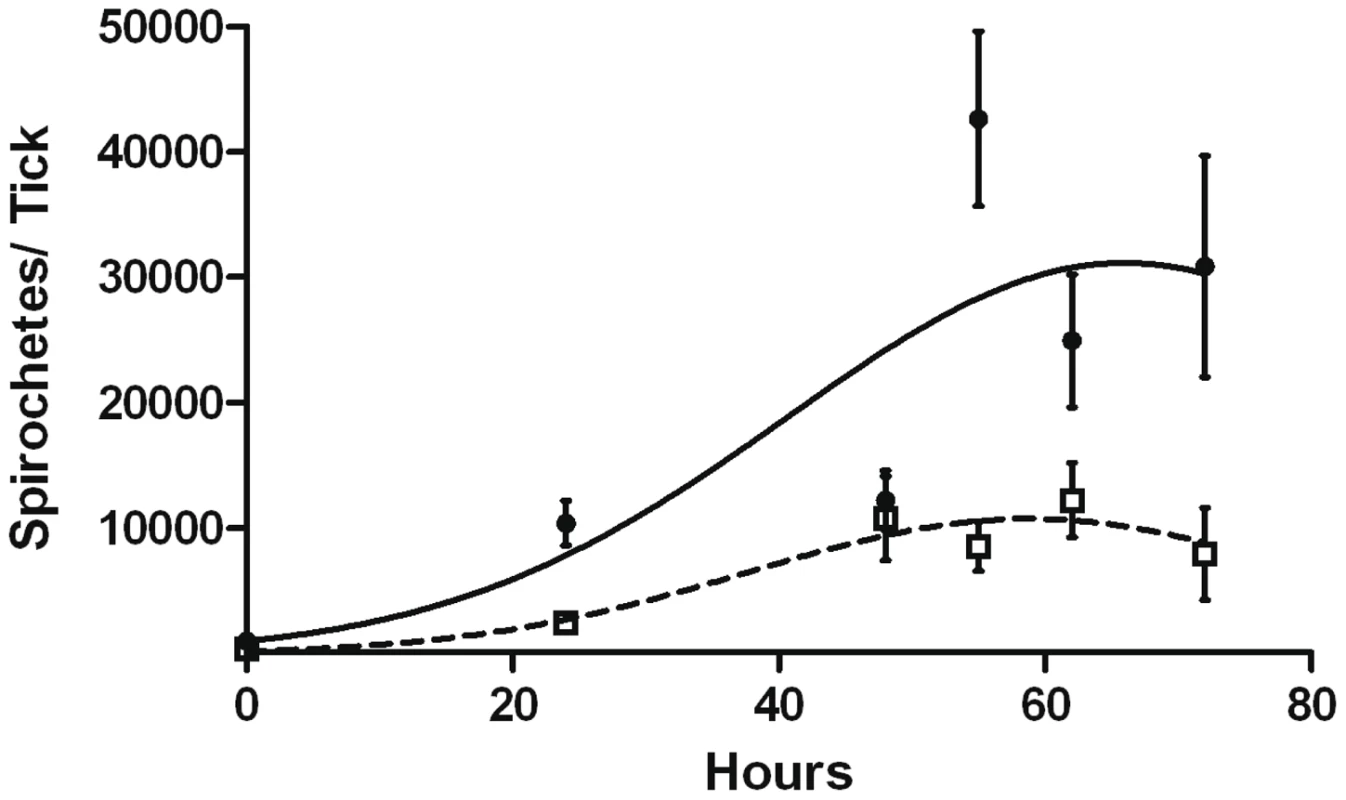
B31-A3- or CP176- infected nymphs were placed on naïve mice, removed at the indicated times and spirochete density was determined by qPCR. •, B31-A3; □, CP176. Spirochete densities for B31-A3 and CP176 were significantly different from each other (P<.05) at all time points except 48 hours. Error bars indicate SEM. Tab. 3. Kinetics of Mouse Infectivity by Tick Feeding. 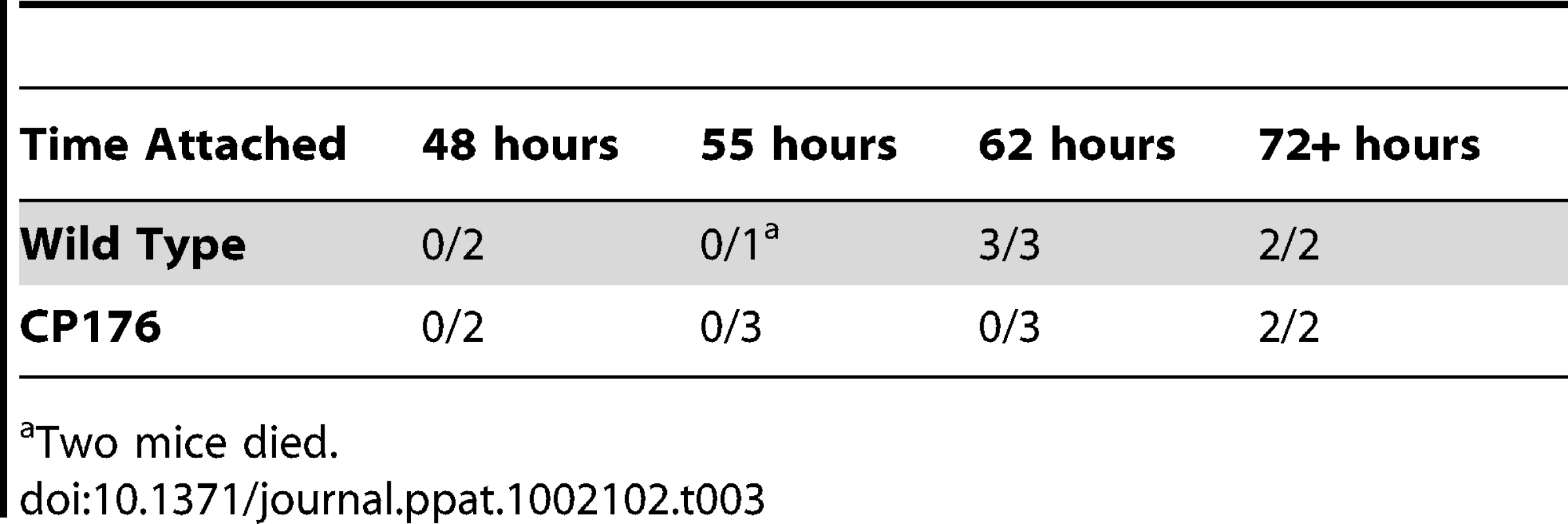
Two mice died. Expression of chbC, encoding a chitobiose transporter subunit, is elevated in ticks
Chitobiose is a di-GlcNAc molecule that is a component of the peritrophic matrix and tick chitin [45], [46], [48], [49]. The B. burgdorferi genome contains open reading frames that encode gene products that can mediate chitobiose transport and metabolism [12], [46], [48], [49]. These include the three subunits of a chitobiose transporter (BBB04-BBB06), a putative chitobiase (BB0002) and NagA and NagB (BB0151 and BB0152). In combination, the actions of these gene products would result in production of glucose 6-phosphate that could enter the glycolytic pathway (Figure 1). Both chitobiose and chitin can support B. burgdorferi growth in vitro [45], [46], [48], [49]. Therefore, the transcript levels for chbC (bbb04), which encodes subunit C of the chitobiose transporter, were also measured to determine whether chitobiose utilization by B. burgdorferi may be important during the tick phase of the enzootic cycle. Substantially higher expression of chbC occurred during the various tick stages (fed larvae, 5.10±7.00 copies/10 copies of flaB; unfed nymphs, 4.42±2.33; fed nymphs, 2.87±0.36) as compared to expression in mouse joints (0.59±0.71 copies/10 copies of flaB) (figure 5).
Discussion
On acquisition by feeding larvae from an infected mammal, B. burgdorferi must initially adapt to the new host (i.e. tick) environment. The spirochete must then survive the tick molting process and endure a substantial period in a nutrient-poor milieu (unfed nymph). This is not a period of metabolic dormancy since several studies, including our own, demonstrate that B. burgdorferi gene expression is modulated in different tick developmental stages and that expression of some genes is higher in unfed nymphs than in fed nymphs [17], [50], [51]. During the subsequent nymphal blood meal, B. burgdorferi enter a rapid replication phase, experiencing a significant increase in density within a 48 hr period [8], [9] and must prepare for transmission back to a mammal. How does B. burgdorferi generate the energy required to withstand this harsh environment?
Glycerol and its metabolites play important roles in cellular biochemistry [52] and glycerol is a readily available carbohydrate in Ixodes ticks [32]–[34]. Most bacteria have the ability to acquire glycerol from the surrounding milieu or to re-utilize it from its own metabolites [52], [53]. G3P is a crucial intermediate for energy metabolism (via its conversion to dihydoxyacetone phosphate and entry into the glycolytic pathway) and for phospholipid biosynthesis (via its conversion to phosphatidic acid [Figure 1]). The B. burgdorferi genome putatively encodes all the enzymes required for both processes [12], [54]. The possibility that glycerol uptake and utilization may play an important role during the tick phase of the enzootic cycle was suggested by previous studies showing that genes comprising the glp operon had elevated expression during growth in vitro at 23°C and were subject to RpoS-dependent repression within the mammalian host [17], [35]. A number of findings from the current study confirm that this is the case. First, in medium supplemented with glycerol as the principal carbohydrate source, wild-type B. burgdorferi grew to a significantly higher cell density compared to a glpD mutant during growth at 25°C (Figure 10B). This difference was not observed during growth at 37°C or when glucose was employed as the principal carbohydrate source. Second, transcript levels for glpF and glpD were significantly lower in mouse joints relative to their levels in ticks (Figure 5). Third, GlpD protein was not produced during growth in DMCs and its repression was dependent on the presence of RpoS (Figure 6). Finally, the glpD mutant was fully infectious in mice when introduced by either needle or tick inoculation (Table 1), but had a replication defect in ticks (Table 3 and Figure 11).
Absence of GlpD results in reduced spirochete fitness in the tick. This defect is manifested at two distinct points during this phase of the enzootic cycle. Spirochete loads are reduced five-fold after the larval molt in the mutant relative to the wild type. Spirochete loads were measured in larvae that had fed to repletion and in unfed nymphs two weeks after the molt. Therefore, it is not clear whether the reduction in B. burgdorferi density occurred during the molt or during the initial period in the unfed nymph. We favor the latter possibility. At the onset of nymphal feeding, the glpD mutants display a lag prior to beginning replication; as a result, they replicate more slowly than wild-type spirochetes and fail to achieve the same final spirochete densities (Figure 11). As a consequence, there is delayed transmission of glpD mutant spirochetes to mice during feeding. Whereas ticks infected with wild-type B. burgdorferi caused infection by 62 hours of feeding, those infected with mutant spirochetes required at least 72 hours of feeding before productive transmission occurred. Dunham-Ems et al. have demonstrated that B. burgdorferi migration from the midgut to the salivary glands for transmission to a mammal proceeds in two phases. In the initial step, replicating spirochetes form non-motile networks that advance toward the basolateral surface of the gut epithelium. The non-motile spirochetes then transition to motile organisms that penetrate the basement membrane into the hemocoel and migrate to the salivary gland [9]. This model of B. burgdorferi dissemination provides an explanation for the delayed transmission phenotype of the glpD mutant. As dissemination of B. burgdorferi in the tick during the first phase of feeding does not depend on motility, but instead is replication driven, the reduced replication rate of the mutant would result in delayed dissemination to the hemocoel. As a result, the mutant would require additional time for successful tick to mammal transmission.
Spirochete loads of wild type and glpD mutant were identical in fed larvae whereas those of the mutant were reduced approximately five-fold after the larval molt; the reduced level of mutant persisted throughout the subsequent stages of the tick cycle (Table 2). In a very recent study, He et al. showed that a glpF polar deletion mutant, which does not express any of the glp operon genes, had a phenotype both in vitro and in vivo very similar to that described here for a glpD mutant (i.e. the mutant failed to reach the same cell density as the wild type when cultured in medium with glycerol as the principal carbohydrate and had reduced spirochete loads in infected nymphs) [23]. Interestingly, the reduction in mutant spirochete levels in nymphs was much more severe (>2 logs) in their study than was observed here for the glpD mutant. Presumably, this difference is due to the fact that the glpD mutant will only have an effect on glycerol utilization for glycolysis, whereas the glp operon mutant will also affect phospholipid biosynthesis (Figure 1). Thus, study of the glpD mutant is important in allowing evaluation of the contribution of glycerol utilization for energy metabolism without any confounding from effects on other metabolic pathways.
Why isn't the glpD mutation lethal rather than being simply growth inhibitory? Clearly, there must be an alternative carbohydrate source that can be metabolized via glycolysis to produce the required ATP. We propose that this alternative energy source is chitobiose. Tilly et al. reported that chbC transcript is elevated at 23°C relative to 34°C [45] and we have found that chbC expression is significantly higher in ticks than in mouse joints (Figure 5). Taken together, these data suggest that chitobiose utilization by B. burgdorferi is important during the tick phase of the cycle. Chitobiose would be available to the spirochete during tick feeding, when it is shed from the forming peritrophic matrix, as well as during molting when the tick cuticle is being re-modeled for growth [31], [49]. It has been suggested that chitobiose utilization would be essential for B. burgdorferi during the tick phase of the enzootic cycle for spirochete glycolysis and cell wall synthesis. However, chbC mutants, which cannot take up exogenous chitobiose or utilize chitin to support growth, successfully complete the mouse-tick-mouse infectious cycle [48]. It is possible that glycerol availability may be partially responsible for rescue of the chbC mutant. This would suggest that B. burgdorferi maintains spirochete fitness in the nutrient deplete environment of the tick midgut by utilizing either glycerol and/or chitobiose as glycolytic precursors. It would be of interest to determine whether a glpD-chbC double mutant would be capable of completing the natural infectious cycle.
As described earlier, signals that lead to phosphorylation of Rrp2 result in activation of RpoN which, in turn, initiates transcription of rpoS [14], [15]. RpoS is expressed only in feeding nymphs and mammals and therefore, is thought to be responsible for the regulon that is required for mammalian infection [17], [55]. This is consistent with the fact that Rrp2, RpoN and RpoS mutants cannot establish infection in mice [16], [56], [57]. The RpoS regulon includes genes that are absolutely dependent on RpoS for their transcription (e.g. ospC), as well as genes subject to RpoS-dependent repression [17], [18]. The glp operon is in the latter category. Several recent studies have begun to reveal the role of the Hk1/Rrp1 TCS in B. burgdorferi [22]–[24]. Lack of Hk1 and Rrp1 has no effect on infectivity in mice. However, Hk1 and Rrp1 mutants are killed within the tick midgut during feeding [23], [24]. Hk1 and Rrp1 appear to be expressed during all stages of the B. burgdorferi life cycle [22], [24], but the important consideration is whether Rrp1 is phosphorylated leading to the production of c-di-GMP. Interestingly, Caimano et al. have recently shown that Hk1 mutants are killed during the larval and nymphal blood meals, indicating that c-di-GMP is required during both tick feeding stages [24]. It is reasonable to conclude that the Rrp2/RpoN/RpoS pathway governs the expression of B. burgdorferi genes required in the mammalian host, whereas Hk1/Rrp1 controls a subset of borrelial gene products that is critical for survival in the tick vector. A number of genes that are induced by Rrp1 (i.e. c-di-GMP) are subject to RpoS-dependent repression; these include the glp operon genes and bba74 [22], [23].
The current study demonstrates that glp operon expression is modulated by nutrient availability. Several reports have established that this operon is regulated in a reciprocal manner by RpoS and Rrp1 [17], [22], [23]. The glp genes are the first borrelial gene products linked to spirochete metabolism whose expression is subject to regulation by both B. burgdorferi TCSs. As such, these genes represent a valuable paradigm for elucidating the interplay between these two regulatory pathways. A model that integrates both carbohydrate availability and presence/absence of transcriptional regulators is presented in Figure 12. It is presumed that early in larval feeding RpoS is still present, but must be degraded in order to allow for expression of tick phase genes; the precise timing of RpoS disappearance is not currently known. Glucose should be available at this stage in quantities sufficient to support B. burgdorferi growth. Later in the blood meal hexose sugars and other serum constituents crossing the peritrophic matrix are sequestered by tick midgut epithelial cells. This creates a nutrient-poor environment in which B. burgdorferi must rely on glycerol and rapidly depleting chitobiose to support glycolysis. The turnover of RpoS during larval feeding would result in the de-repression of glycerol pathway enzymes and presence of c-di-GMP will activate their expression, ensuring that spirochetes can rapidly switch from a glucose-based to a glycerol-based metabolism. Once spirochete infection is established in the midgut and the larva molts to an unfed nymph, B. burgdorferi remains a metabolically active spirochete that must rely on glycolysis for maintenance of cellular integrity. Glycerol is presumed to be the primary carbohydrate at this stage and absence of RpoS would allow expression of the glp operon. Early in nymphal feeding while blood constituents are scarce, B. burgdorferi must actively replicate and migrate through the epithelial midgut lumen to begin its migration to the salivary glands. At this point glycerol would be a primary energy source for support of cellular replication, as chitobiose will not be available until the eventual breakdown of the peritrophic matrix. Inability to utilize glycerol, as in the glpD mutant, would result in delayed and reduced spirochete replication that could impact transmission of B. burgdorferi to the mammalian host. Presence of c-di-GMP would result in sustained expression of glp operon genes. As RpoS levels increase during the nymphal blood meal, presence of c-di-GMP will counteract the repressive effects of RpoS on glp gene expression, ensuring that glycerol utilization can continue until the spirochetes are transmitted to a mammalian host.
Fig. 12. Carbohydrate availability and transcriptional regulators at different stages of the B. burgdorferi enzootic cycle. 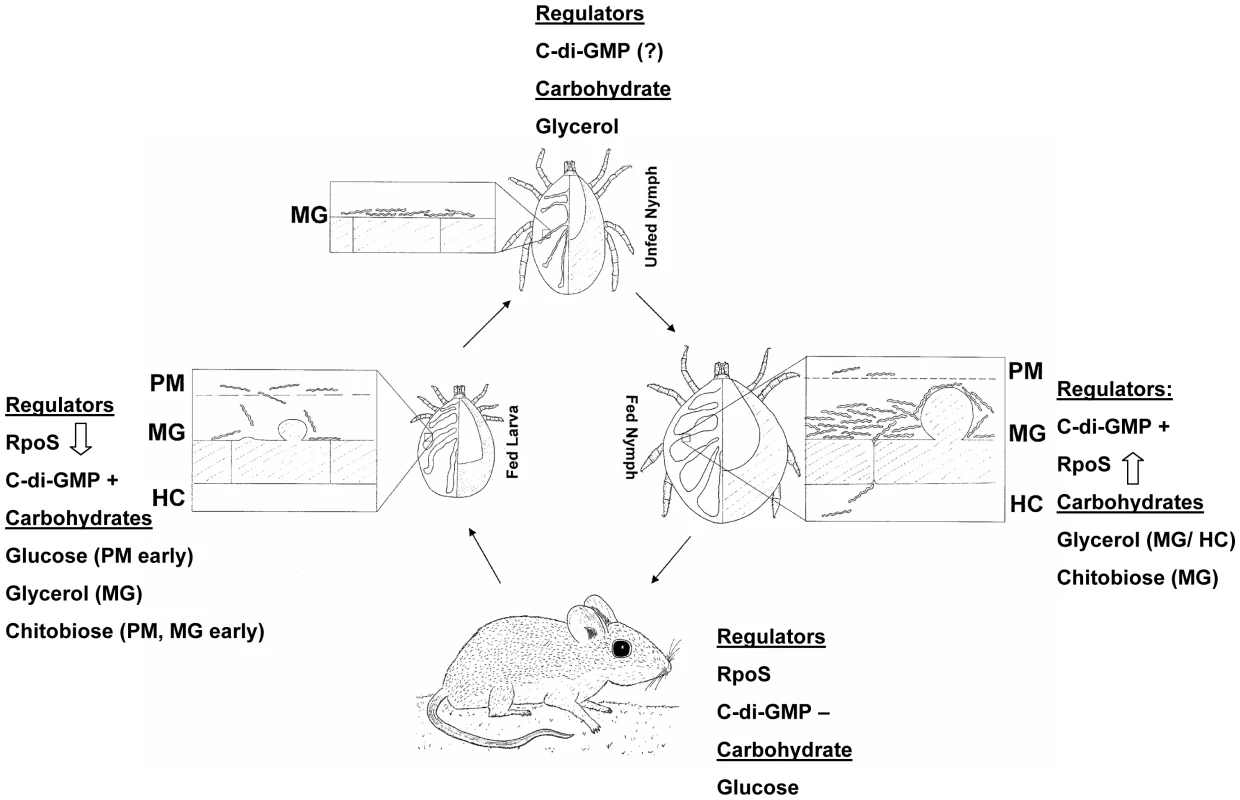
Up and down arrows indicate increasing or decreasing RpoS levels; c-di-GMP+ and c-di-GMP− indicate presence or absence of c-di-GMP. PM, peritrophic matrix; MG, midgut; HC, hemocoel. The model presented in Figure 12 accounts for carbohydrate source availability and presence of regulatory molecules throughout the tick-mouse enzootic cycle and highlights the interdependence of these two parameters. Concentrations of glucose and glycerol in mouse plasma are approximately 150 and 2.8 mg/100 mL, respectively [58]. Glycerol is abundantly present during all tick stages. On this basis, the model assumes that B. burgdorferi utilizes glucose as the preferred nutrient source when it is available in the mammal or at certain stages during the tick blood meal, but switches to utilizing glycerol, especially in the unfed nymph when glucose is not present. This may represent the B. burgdorferi version of carbon catabolite repression (CCR), which is defined as a regulatory mechanism by which the expression and enzymatic activities of enzymes involved in the use of secondary carbohydrates are reduced in the presence of sufficient levels of the preferred carbohydrate [59]. The mechanisms underlying CCR in most bacteria involve a glucose-specific phosphotranferase subunit (EIIA) that can be reversibly phosphorylated based on the phosphoenolpyruvate to pyruvate ratios. B. burgdorferi encodes a putative EIIA subunit (BB0559), but does not appear to contain other major components that modulate CCR in other bacteria [12]. It is reasonable to assume that B. burgdorferi possesses sensing mechanisms that monitor the relative levels of glucose and glycerol in the environment. It is tempting to speculate that modulation of Hk1 kinase activity is one outcome of the fluctuating nutrient ratios. When the glycerol/glucose ratio increases Hk1 would phosphorylate Rrp1 leading to the production of c-di-GMP. The specific molecular signal that is recognized by Hk1 is not currently known. Likewise, the precise timing of the transcriptional activation/repression of RpoS and the possible reciprocal modulation of c-di-GMP levels is not known. Studies designed to elucidate the molecular events underlying the proposed model are warranted.
Materials and Methods
Ethics statement
All animal experimentation was conducted in strict accordance with the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. The protocols were approved by the Institutional Animal Care and Use Committee of New York Medical College (Approval number 31-1-0310H).
Strains and growth conditions
B. burgdorferi strains B31-A3 [60], 297 (c162) and a strain 297-based RpoS mutant (c174) [17] were employed in this study. Spirochetes were grown in modified Barbour-Stoenner-Kelley-II medium [43], [61] supplemented with 6% heat inactivated rabbit serum (Sigma, St. Louis, MO) (BSK-II). BSK-lite medium was based on the formulation of Barbour [43] with modifications as previously described [28].
B. burgdorferi were grown to late log phase (5–10×107 cells/ml) in BSK-II medium at 25°C. For BSK-lite experiments, spirochetes were diluted 100 fold in BSK-lite medium to remove BSK-II medium constituents. 5×104 spirochetes in 40 ml of BSK-lite medium with a specific carbohydrate (either glucose or glycerol) were aliquoted into eight 5 ml tubes. Four tubes of each sample were placed at either 25°C or 37°C and observed for up to 60 days. Individual tubes were counted daily for cultures grown at 37°C and every two days for those incubated at 25°C. Spirochete density was enumerated by dark field microscopy as previously described [62]. Student's two-tailed, unpaired t-tests were performed on data collected during exponential phase and stationary phases of cell growth. Significance was defined as a P<0.01.
Cultivation of c162 and c174 in DMCs was carried out as described [63].
Construction of a B. burgdorferi glpD mutant
The strategy for disruption of B. burgdorferi is presented in figure 7. A 2519 bp region of B. burgdorferi chromosomal DNA containing bb0243 was amplified by PCR using primers bb0243F/R (Table 4) ligated into the pGEM-T vector (Promega, Madison, WI), transformed into E. coli DH5α and cells containing recombinant plasmids were selected by blue-white screening. Selected transformants were purified to single colonies and plasmids were confirmed to contain bb0243 by DNA sequence analysis (Davis Sequencing, Davis, CA). The pGEM-T-bb0243 construct was digested with DraII (corresponding to position 248,983 in the B. burgdorferi chromosome). A DNA fragment containing flgB-aadA (a spectinomycin/streptomycin resistance cassette driven by the B. burgdorferi flgB promoter) was amplified from vector pKFSS1 as previously described [64] and inserted into the DraII site within PGEM-T-bb0243 by blunt-end ligation. The construct was transformed into E. coli DH5α and selected by growth on LB agar plates supplemented with 100 µg/ml of spectinomycin. Transformants harboring plasmids containing bb0243 disrupted by the flgB-aadA cassette were isolated, purified to single colonies and plasmid inserts were confirmed by PCR and DNA sequence analysis. flgB-aadA cassette orientation was determined by restriction enzyme digestion. A plasmid construct designated pCP100 had the flgB-aadA cassette in the same orientation as bb0243 and a plasmid construct designated pCP200 had the flgB-aadA cassette in the reverse orientation. The ampicillin resistance cassette (bla) located in pGEM-T was disrupted in both constructs as previously described [65] yielding a plasmid designated pCP101 from pCP100 and pCP201 from pCP200. Spectinomycin-resistant, ampicillin-sensitive colonies of each construct were selected by growth on LB agar containing either 100 µg/ml ampicillin or 100 µg/ml spectinomycin.
Tab. 4. Oligonucleotide primers used in this study. 
For each primer pair, F refers to forward and R to reverse primer. pCP101 and pCP201 were isolated and transformed into B. burgdorferi B31-A3 competent cells by electroporation as described [66]. Transformants were screened by growth in a 96 well plate in the presence of streptomycin (100 µg/ml). Selected transformants were cloned by limiting dilution in BSK-II medium containing streptomycin (100 µg/ml). The glpD disruption in selected transformants was confirmed by Southern blot and Western blot analyses (Figures 8 and 9). Plasmid content for selected mutants was determined as previously described [67] to ensure that all plasmids essential for murine infectivity were present.
Southern hybridization
Southern blot analysis and generation of a digoxygenin-labeled bb0243 probe were performed as previously described [67] with the following modifications. A 196 base pair fragment of bb0243 was generated from strain B31-A3 by PCR using primers 243probeF/R (Table 4). B. burgdorferi DNA was fragmented by incubation with 4.5 units of BamHI in 1× buffer B (Fermentas, Glen Burnie, MD) or EcoRI in buffer EcoRI (Fermentas) overnight at 37°C.
Acquisition of B. burgdorferi by I. scapularis and transmission to mice
C3H/HeJ mice (Jackson Laboratories, Bar Harbor, ME) were infected with either wild-type or glpD mutant B. burgdorferi by needle inoculation as previously described [68], [69]. Once infection was established as determined by culture of ear biopsy, mice were anesthetized with ketamine and 100–300 naïve, unfed larvae were placed in and around the ear canal. Mice were placed individually into cages with approximately 1 cm water at the bottom. A metal grid of the same length and width as the cage, and standing 1.5 cm high, was placed in the cage. Larvae were allowed to feed until repletion. Following drop off, larvae were collected, rinsed in water, pooled into groups of 30 in 5 ml tubes with a porous cover and maintained in a desiccator at 21°C, >95% relative humidity with a 16 hour∶8 hour light: dark cycle. Larvae molted to unfed nymphs in approximately 5–6 weeks. At four weeks post molt, three unfed nymphs were placed on three-week old uninfected C3H/HeJ mice (Jackson) and allowed to feed until repletion. Fed nymphs were collected as described above.
For interrupted feeding experiments, 3 unfed nymphs were allowed to feed on naïve C3H/HeJ mice for 24, 48, 55, 62, and 72 hours or to repletion. Ticks were carefully removed from mice by forceps at the indicated time point. Ticks were processed for DNA isolation as described below. Mice were tested for infection as previously described [68], [69].
DNA isolation
DNA isolation from 5×108 B. burgdorferi was performed using the Puregene DNA isolation kit as per manufacturer's instructions (Qiagen, Valencia CA). DNA pellets were resuspended in 30 µl of nuclease free water. DNA concentration was measured by spectrophotometric analysis at 260 nm.
DNA was isolated from pools of 10 fed larvae. DNA was obtained from unfed nymphs that were processed either individually, in groups of 5 or in groups of 10. DNA was isolated from individually processed fed nymphs. Ticks were surface sterilized by washing successively with 800 µl of sterile H2O, 0.5% sodium hypochlorite, 3% hydrogen peroxide (Sigma), 70% ethanol (Fischer Scientific, Pittsburgh, PA) and sterile H2O each for 1 minute. DNA extraction was performed as adapted from the Qiagen DNeasy blood and tissue kit as described by Beati et al. [70] with the following modifications. Ticks were homogenized with an 18.5 gauge needle. Samples were lysed with 220 µl animal lysis buffer and 0.45 mg recombinant proteinase K (Roche, Mannheim, Germany) per reaction overnight in a 56°C incubator. Following all wash steps, mixtures were centrifuged at 10,000 rpm. DNA was eluted twice with 25 µl of PCR-grade H2O pre-warmed to 72°C.
Quantitative PCR
qPCR reaction mixtures (25 µl total volume) contained 2 µl of sample DNA, 3 µl of nuclease free water, 20 pmol each of primers FL-571F/FL-677R, 5 pmol of flaB - specific Taqman probe (flaBFAM) (table 4), and 12.5 µl Taqman PCR mastermix (Roche). DNA copy number was determined on an ABI prism 7900HT thermocycler with an amplification profile of 50°C for 2 minutes, 95°C for 10 minutes, followed by 40 cycles of 95°C for 15 seconds and 60°C for 1 minute. Samples were run in duplicate and each plate contained two samples lacking DNA as negative controls. Ct values were obtained using the SDS2.1 software program (Applied Biosystems, Carlsbad, CA). To assess spirochete density per sample, standard curves were generated for flaB, a constitutively expressed gene, in log increments (10–104).
Copy numbers were compared by a two-tailed, unpaired t-test for each condition (fed larvae, unfed nymph, fed nymph), where significance was defined as P≤0.05.
RNA extraction
Ticks infected with B. burgdorferi were processed in pools of 50 for fed larvae, 100 for unfed nymphs and 35 for fed nymphs. Ticks were homogenized in 1 ml TRIzol reagent (Invitrogen, Carlsbad, CA) for 5 minutes. For in vitro experiments, 50 ml of cell culture (approximately 2.5×109 cells) was centrifuged at 12,000 rpm for 10 minutes and 1 ml TRIzol was added to the cell pellet. RNA was recovered as per manufacturer's instructions (Invitrogen). The RNA pellet was resuspended in 30 µl nuclease-free water and DNase treated twice with the Ambion DNA free kit per manufacturer's instructions (Ambion, Austin, TX).
Mammalian hind limb joints were surgically removed from euthanized C3H/HeJ mice, snap frozen in liquid nitrogen and pulverized with mortar and pestle. The powdered tissue was transferred to a glass homogenizer and homogenized with 0.5 ml denaturation solution and the supernatant containing total RNA was isolated from samples as per manufacturer's instructions (ToTALLY RNA, Ambion). RNA was rehydrated in 30 µl of nuclease free water and DNase treated as described above. Mouse RNA was removed by MICROBEnrich as per manufacturer's instructions (Ambion). The recovered RNA pellet was resuspended in 15 µl of sodium citrate buffer (Ambion) and 15 µl of nuclease free water.
cDNA was generated from RNA samples by addition of 2 µg of purified RNA to a mixture containing 4 µl of 5× reverse transcriptase buffer (Promega), 0.02 mM dNTPs (Roche), 0.5 µg random hexamer (Promega), 2 units of RNase inhibitor (Ambion), 5 units of AMV reverse transcriptase enzyme (Promega) and nuclease free water in 20 µl total volume. The reaction mixture was incubated at 42°C for 2 hours. Reverse transcriptase enzyme was heat inactivated at 95°C for 5 minutes and cDNA was stored at −20°C until further use.
Quantitative RT-PCR
For generation of standard curves, specific gene fragments for bb0240, bb0243 and bbb04 were amplified by PCR using primers pairs bb0240qRTPCRF/bb0240qRTPCRR, bb0243qRTPCRF/bb0243qRTPCRR, bbb04qRT-PCRF/bbb04qRT-PCRR, ospA-288F/ospA-369R and ospC-B31FTq/ospC-B31RTq, respectively (table 4). PCR reaction mixtures contained 100 ng of B31-A3 DNA, 0.25 µl Taq polymerase (Roche), 0.5 µl dNTPs (Roche), and 1× Taq polymerase buffer (Roche) in a total volume of 25 µl. Amplification conditions were 95°C for 5 minutes, followed by 36 cycles of 95°C for 30 seconds, 55°C for 30 seconds and 72°C for 30 seconds and a final incubation at 72°C for 10 minutes. Production of the expected product was confirmed by gel electrophoresis and the PCR products were ligated into the TOPO 2.1 cloning vector, the vector was transformed into E. coli Mach1 cells and recombinant clones were selected as per manufacturer's instructions (Invitrogen). Clonal isolates were grown in 10 ml of LB broth supplemented with 100 µg/ml ampicillin and the plasmids were extracted as described above. PCR confirmed the presence of the desired gene fragments. Plasmid concentration was determined by spectrophotometric analysis at 260 nm, followed by mathematical computation of copy number (http://www.uri.edu/research/gsc/resources/cndna.html).
Transcript levels for bb0240 (glpF), bb0243 (glpD), bbb04 (chbC), ospA, ospC and flaB were determined by performing qRT-PCR as previously described [65] using the primer pairs listed in table 4 on an ABI Prism 7900HT thermocycler followed by analysis using the SDS2.1 software program (Applied Biosystems). For each experimental run, standard curves for these genes were generated using known quantities (10–104 in log increments) of gene specific plasmids for calculation of absolute copy number.
One-way analysis of variance was performed on qRT-PCR results. To determine significance, a Kruskal-Wallis multiple comparison Z-value test (Dunn's test) was performed, where significance was defined as P≤0.05.
Production of recombinant GlpD (rGlpD) and GlpD antibodies
bb0243 was amplified using primers NdeI243F/XhoI243R (Table 4), cloned into the TOPO 2.1 vector, transformed into E. coli Top10 cells and recombinant clones were selected per manufacturer's instructions (Invitrogen). The bb0243 insert was excised from the TOPO 2.1 plasmid by double digestion with NdeI and XhoI (Fermentas) and the insert was purified after separation by gel electrophoresis using the Wizard SV genomic gel purification kit according to manufacturer's instructions (Promega). pET-15b (Novagen, Gibbstown, NJ) was digested with NdeI and XhoI and the gel-purified bb0243 insert was ligated with the NdeI/XhoI-cut pET-15b at a 2∶1 ratio with 10 units of T4 ligase (New England Biolabs, Ipswich, MA). The recombinant plasmid was transformed into E. coli DH5α and clones were selected on LB agar plates containing 100 µg/ml ampicillin. Recombinant pET-15b carrying bb0243 was transformed into E. coli BL21-DE3 and grown on LB agar plates containing 100 µg/ml ampicillin. A clone containing bb0243 was selected and subjected to DNA sequencing. This sequence contained two single nucleotide changes relative to the reported sequence in strain B31-MI [12]. Nucleotide 107 had a T to C change that would result in a predicted amino acid change of I359T and nucleotide 591 had an A to T substitution that would result in an amino acid change of E197D.
The selected clone was grown at 37°C in 250 ml Luria broth containing 100 µg/ml ampicillin and 1 mM IPTG with agitation for 4 hours. Cells were recovered by centrifugation at 8000 RPM for 10 minutes and rGlpD was isolated from the cells using Ni-NTA His Bind Resin (Novagen) according to the manufacturer's instructions. Fractions containing rGlpD, as determined by SDS-PAGE, were pooled and loaded into an Amicon Ultra 50 kDa molecular weight cut off spin column (Millipore, Billerica, MA). The protein sample was centrifuged at 7500× g for approximately 8 minutes to a volume of 800 µl. The protein solution was dialyzed against 2 liters of 1× PBS, 6 M urea (pH 7.4) with stirring overnight at 4°C. Identity of the protein as B. burgdorferi rGlpD was confirmed by LC-MS/MS analysis (Keck Biotechnology Resource Laboratory,New Haven, CT). The yield of purified rGlpD was 1.3 mg.
100 µg of purified rGlpD in 1×PBS, 6 M urea (pH 7.4) was inoculated along with Freund's adjuvant into two Sprague-Dawley rats by Harlan Laboratories (Madison, WI). The rats received a boost at day 28 and day 56 post-inoculation and were sacrificed and bled on day 70 post inoculation. GlpD antiserum was tested by ELISA and confirmed to be specific for GlpD by immunoblot analysis.
SDS-PAGE and immunoblot analysis
B. burgdorferi cells grown in vitro or in DMCs were lysed with Bugbuster HT (Novagen) and 1 µg/ml of lysozyme (Sigma) according to manufacturer's instructions. 2 µg of whole cell lysate was subjected to 12.5% SDS-PAGE and separated proteins were visualized by silver staining as described [71]. For immunoblotting, separated proteins were transferred to PVDF membrane. Membranes were exposed to protein-specific primary rat antiserum (GlpD, 1∶400 dilution; FlaB, 1∶2500 dilution) followed by alkaline phosphatase-linked anti-rat secondary antibody (1∶500) (KPL, Gaithersburg, MD). The membrane was washed three times for 10 minutes with 1× TBS/0.05% Tween 20 and developed with BCIP/NBT phosphatase substrate (KPL) until band development (approximately 2–4 minutes).
To determine seroconversion in mouse infection studies, mouse serum was added to 1× TBS with 0.5% dry milk at 1∶200 dilution and incubated with whole B. burgdorferi lysate Marblot strips (MarDX, Jamestown, NY) for 1 hour at room temperature. The remaining procedure is as described above, with anti-mouse secondary antibody (1∶5000 dilution).
Zdroje
1. BaconRMKugelerKJMeadPS 2008 Surveillance for Lyme disease–United States, 1992–2006. MMWR Surveill Summ 57 1 9
2. LevineJFWilsonMLSpielmanA 1985 Mice as reservoirs of the Lyme disease spirochete. Am J Trop Med Hyg 34 355 360
3. TsaoJI 2009 Reviewing molecular adaptations of Lyme borreliosis spirochetes in the context of reproductive fitness in natural transmission cycles. Vet Res 40 36
4. MannelliAFishDDanielsTJKharitonenkovITunH 1997 Detection of Borrelia burgdorferi OspA in Ixodes scapularis larvae by an antigen-capture enzyme-linked immunosorbent assay. New Microbiol 20 355 359
5. SteereACGrodzickiRLKornblattANCraftJEBarbourAG 1983 The spirochetal etiology of Lyme disease. N Engl J Med 308 733 740
6. MagnarelliLAAndersonJFFishD 1987 Transovarial transmission of Borrelia burgdorferi in Ixodes dammini (Acari:Ixodidae). J Infect Dis 156 234 236
7. de SilvaAMFikrigE 1995 Growth and migration of Borrelia burgdorferi in Ixodes ticks during blood feeding. Am J Trop Med Hyg 53 397 404
8. PiesmanJSchneiderBSZeidnerNS 2001 Use of quantitative PCR to measure density of Borrelia burgdorferi in the midgut and salivary glands of feeding tick vectors. J Clin Microbiol 39 4145 4148
9. Dunham-EmsSMCaimanoMJPalUWolgemuthCWEggersCH 2009 Live imaging reveals a biphasic mode of dissemination of Borrelia burgdorferi within ticks. J Clin Invest 119 3652 3665
10. KazmierczakMJWiedmannMBoorKJ 2005 Alternative sigma factors and their roles in bacterial virulence. Microbiol Mol Biol Rev 69 527 543
11. BeierDGrossR 2006 Regulation of bacterial virulence by two-component systems. Curr Opin Microbiol 9 143 152
12. FraserCMCasjensSHuangWMSuttonGGClaytonR 1997 Genomic sequence of a Lyme disease spirochaete, Borrelia burgdorferi. Nature 390 580 586
13. CasjensSPalmerNvanVRHuangWMStevensonB 2000 A bacterial genome in flux: the twelve linear and nine circular extrachromosomal DNAs in an infectious isolate of the Lyme disease spirochete Borrelia burgdorferi. Mol Microbiol 35 490 516
14. HubnerAYangXNolenDMPopovaTGCabelloFC 2001 Expression of Borrelia burgdorferi OspC and DbpA is controlled by a RpoN-RpoS regulatory pathway. Proc Natl Acad Sci U S A 98 12724 12729
15. YangXFAlaniSMNorgardMV 2003 The response regulator Rrp2 is essential for the expression of major membrane lipoproteins in Borrelia burgdorferi. Proc Natl Acad Sci U S A 100 11001 11006
16. CaimanoMJEggersCHHazlettKRRadolfJD 2004 RpoS is not central to the general stress response in Borrelia burgdorferi but does control expression of one or more essential virulence determinants. Infect Immun 72 6433 6445
17. CaimanoMJIyerREggersCHGonzalezCMortonEA 2007 Analysis of the RpoS regulon in Borrelia burgdorferi in response to mammalian host signals provides insight into RpoS function during the enzootic cycle. Mol Microbiol 65 1193 1217
18. CaimanoMJEggersCHGonzalezCARadolfJD 2005 Alternate sigma factor RpoS is required for the in vivo-specific repression of Borrelia burgdorferi plasmid lp54-borne ospA and lp6.6 genes. J Bacteriol 187 7845 7852
19. OuyangZKumarMKariuTHaqSGoldbergM 2009 BosR (BB0647) governs virulence expression in Borrelia burgdorferi. Mol Microbiol 74 1331 1343
20. HydeJAShawDKSmithIRTrzeciakowskiJPSkareJT 2009 The BosR regulatory protein of Borrelia burgdorferi interfaces with the RpoS regulatory pathway and modulates both the oxidative stress response and pathogenic properties of the Lyme disease spirochete. Mol Microbiol 74 1344 1355
21. HydeJAShawDKSmithRIIITrzeciakowskiJPSkareJT 2010 Characterization of a conditional bosR mutant in Borrelia burgdorferi. Infect Immun 78 265 274
22. RogersEATerekhovaDZhangHMHovisKMSchwartzI 2009 Rrp1, a cyclic-di-GMP-producing response regulator, is an important regulator of Borrelia burgdorferi core cellular functions. Mol Microbiol 71 1551 1573
23. HeMOuyangZTroxellBXuHMohA 2011 Cyclic di-GMP is essential for the survival of the Lyme disease spirochete in ticks. PLoS Pathogens In Press
24. CaimanoMJKenedyMRKairuTDesrosiersDDHarmanM 2011 The hybrid histidine kinase Hk1 is part of a two-component system that is essential for survival of Borrelia burgdorferi in feeding Ixodes scapularis ticks. Infect Immun In Press
25. RyjenkovDATarutinaMMoskvinOVGomelskyM 2005 Cyclic diguanylate is a ubiquitous signaling molecule in bacteria: insights into biochemistry of the GGDEF protein domain. J Bacteriol 187 1792 1798
26. YoungDSHarrisEKCotloveE 1971 Biological and analytic components of variation in long-term studies of serum constituents in normal subjects. IV. Results of a study designed to eliminate long-term analytic deviations. Clin Chem 17 403 410
27. WeltonRFMartinRJBaumgardtBR 1973 Effects of feeding and exercise regimens on adipose tissue glycerokinase activity and body composition of lean and obese mice. J Nutr 103 1212 1219
28. von LackumKStevensonB 2005 Carbohydrate utilization by the Lyme borreliosis spirochete, Borrelia burgdorferi. FEMS Microbiol Lett 243 173 179
29. BalashovYS 1968 Bloodsucking Ticks (Ixododidea) - Vectors of Diseases of Man and Animals Leningrad Nauka Publishers Translation 500 (T500): Cairo, Egypt: Medical Zoology Department, United States Naval Medical Research Unit Number Three 319
30. JamesAMZhuXXOliverJHJr 1997 Vitellogenin and ecdysteroid titers in Ixodes scapularis during vitellogenesis. J Parasitol 83 559 563
31. ShaoLDevenportMJacobs-LorenaM 2001 The peritrophic matrix of hematophagous insects. Arch Insect Biochem Physiol 47 119 125
32. LeeRJBaustJG 1987 Cold-hardiness in the Antarctic tick, Ixodes uriae. Physiol Zool 60 499 506
33. BurksCSStewartRJNeedhamGLeeRE 1996 Cold hardiness in the Ixodid ticks (Ixodidae). MitchellRHornDJNeedhamGRWelbournWC Acarology IX: vol. 1, Proceedings Columbus, OH Ohio Biological Survey 85 87
34. VandykJKBartholomewDMRowleyWAPlattKB 1996 Survival of Ixodes scapularis (Acari: Ixodidae) exposed to cold. J Med Entomol 33 6 10
35. OjaimiCBrooksCCasjensSRosaPEliasA 2003 Profiling of temperature-induced changes in Borrelia burgdorferi gene expression by using whole genome arrays. Infect Immun 71 1689 1705
36. ColeSTEiglmeierKAhmedSHonoreNElmesL 1988 Nucleotide sequence and gene-polypeptide relationships of the glpABC operon encoding the anaerobic sn-glycerol-3-phosphate dehydrogenase of Escherichia coli K-12. J Bacteriol 170 2448 2456
37. ShimizuKHayashiSKakoTSuzukiMTsukagoshiN 2005 Discovery of glpC, an organic solvent tolerance-related gene in Escherichia coli, using gene expression profiles from DNA microarrays. Appl Environ Microbiol 71 1093 1096
38. GuexNPeitschMC 1997 SWISS-MODEL and the Swiss-PdbViewer: an environment for comparative protein modeling. Electrophoresis 18 2714 2723
39. SchwedeTKoppJGuexNPeitschMC 2003 SWISS-MODEL: An automated protein homology-modeling server. Nucleic Acids Res 31 3381 3385
40. ArnoldKBordoliLKoppJSchwedeT 2006 The SWISS-MODEL workspace: a web-based environment for protein structure homology modelling. Bioinformatics 22 195 201
41. YehJIChinteUDuS 2008 Structure of glycerol-3-phosphate dehydrogenase, an essential monotopic membrane enzyme involved in respiration and metabolism. Proc Natl Acad Sci U S A 105 3280 3285
42. KuzinAPAbashidzeMSeetharamanJWangDAnjuaH 2008 X-ray structure of the glycerol-3-phosphate dehydrogenase from Bacillus halodurans complexed with FAD. Northeast Structural Genomics Consortium target BhR167. Available: http://pdbwiki.org/index.php/3da1. Accessed 25 May 2011
43. BarbourAG 1984 Isolation and cultivation of Lyme disease spirochetes. Yale J Biol Med 57 521 525
44. KellyR 1971 Cultivation of Borrelia hermsi. Science 173 443 444
45. TillyKEliasAFErrettJFischerEIyerR 2001 Genetics and regulation of chitobiose utilization in Borrelia burgdorferi. J Bacteriol 183 5544 5553
46. RhodesRGCoyWNelsonDR 2009 Chitobiose utilization in Borrelia burgdorferi is dually regulated by RpoD and RpoS. BMC Microbiol 9 108
47. PiesmanJMatherTNSinskyRJSpielmanA 1987 Duration of tick attachment and Borrelia burgdorferi transmission. J Clin Microbiol 25 557 558
48. TillyKGrimmDBueschelDMKrumJGRosaP 2004 Infectious cycle analysis of a Borrelia burgdorferi mutant defective in transport of chitobiose, a tick cuticle component. Vector Borne Zoonotic Dis 4 159 168
49. RhodesRGAtoyanJANelsonDR 2010 The chitobiose transporter, chbC, is required for chitin utilization in Borrelia burgdorferi. BMC Microbiol 10 21
50. GilmoreRDJrMbowMLStevensonB 2001 Analysis of Borrelia burgdorferi gene expression during life cycle phases of the tick vector Ixodes scapularis. Microbes Infect 3 799 808
51. BykowskiTWoodmanMECooleyAEBrissetteCABradeV 2007 Coordinated expression of Borrelia burgdorferi complement regulator-acquiring surface proteins during the Lyme disease spirochete's mammal-tick infection cycle. Infect Immun 75 4227 4236
52. BrissonDVohlMCSt-PierreJHudsonTJGaudetD 2001 Glycerol: a neglected variable in metabolic processes? Bioessays 23 534 542
53. LinEC 1976 Glycerol dissimilation and its regulation in bacteria. Annu Rev Microbiol 30 535 578
54. SchwanTGBattistiJMPorcellaSFRaffelSJSchrumpfME 2003 Glycerol-3-phosphate acquisition in spirochetes: distribution and biological activity of glycerophosphodiester phosphodiesterase (GlpQ) among Borrelia species. J Bacteriol 185 1346 1356
55. MulayVBCaimanoMJIyerRDunham-EmsSLiverisD 2009 Borrelia burgdorferi bba74 is expressed exclusively during tick feeding and is regulated by both arthropod - and mammalian host-specific signals. J Bacteriol 191 2783 2794
56. FisherMAGrimmDHenionAKEliasAFStewartPE 2005 Borrelia burgdorferi sigma54 is required for mammalian infection and vector transmission but not for tick colonization. Proc Natl Acad Sci U S A 102 5162 5167
57. BoardmanBKHeMOuyangZXuHPangX 2008 Essential role of the response regulator Rrp2 in the infectious cycle of Borrelia burgdorferi. Infect Immun 76 3844 3853
58. MaedaNFunahashiTHibuseTNagasawaAKishidaK 2004 Adaptation to fasting by glycerol transport through aquaporin 7 in adipose tissue. Proc Natl Acad Sci U S A 101 17801 17806
59. GorkeBStulkeJ 2008 Carbon catabolite repression in bacteria: many ways to make the most out of nutrients. Nat Rev Microbiol 6 613 624
60. EliasAFStewartPEGrimmDCaimanoMJEggersCH 2002 Clonal polymorphism of Borrelia burgdorferi strain B31 MI: implications for mutagenesis in an infectious strain background. Infect Immun 70 2139 2150
61. WangGIyerRBittkerSCooperDSmallJ 2004 Variations in Barbour-Stoenner-Kelly culture medium modulate infectivity and pathogenicity of Borrelia burgdorferi clinical isolates. Infect Immun 72 6702 6706
62. SchwartzIWormserGPSchwartzJJCooperDWeissenseeP 1992 Diagnosis of early Lyme disease by polymerase chain reaction amplification and culture of skin biopsies from erythema migrans lesions. J Clin Microbiol 30 3082 3088
63. AkinsDRBourellKWCaimanoMJNorgardMVRadolfJD 1998 A new animal model for studying Lyme disease spirochetes in a mammalian host-adapted state. J Clin Invest 101 2240 2250
64. FrankKLBundleSFKresgeMEEggersCHSamuelsDS 2003 aadA confers streptomycin resistance in Borrelia burgdorferi. J Bacteriol 185 6723 6727
65. OjaimiCMulayVLiverisDIyerRSchwartzI 2005 Comparative transcriptional profiling of Borrelia burgdorferi clinical isolates differing in capacities for hematogenous dissemination. Infect Immun 73 6791 6802
66. SamuelsDS 1995 Electrotransformation of the spirochete Borrelia burgdorferi. Methods Mol Biol 47 253 259
67. IyerRKaluOPurserJNorrisSStevensonB 2003 Linear and circular plasmid content in Borrelia burgdorferi clinical isolates. Infect Immun 71 3699 3706
68. WangGOjaimiCIyerRSaksenbergVMcClainSA 2001 Impact of genotypic variation of Borrelia burgdorferi sensu stricto on kinetics of dissemination and severity of disease in C3H/HeJ mice. Infect Immun 69 4303 4312
69. WangGOjaimiCWuHSaksenbergVIyerR 2002 Disease severity in a murine model of Lyme borreliosis is associated with the genotype of the infecting Borrelia burgdorferi sensu stricto strain. J Infect Dis 186 782 791
70. BeatiLKeiransJE 2001 Analysis of the systematic relationships among ticks of the genera Rhipicephalus and Boophilus (Acari: Ixodidae) based on mitochondrial 12S ribosomal DNA gene sequences and morphological characters. J Parasitol 87 32 48
71. MorrisseyJH 1981 Silver stain for proteins in polyacrylamide gels: a modified procedure with enhanced uniform sensitivity. Anal Biochem 117 307 310
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek A Typhimurium-Typhi Genomic Chimera: A Model to Study Vi Polysaccharide Capsule Function In VivoČlánek SUMO Pathway Dependent Recruitment of Cellular Repressors to Herpes Simplex Virus Type 1 GenomesČlánek A Structural Model for Binding of the Serine-Rich Repeat Adhesin GspB to Host Carbohydrate ReceptorsČlánek Dynamic Evolution of Pathogenicity Revealed by Sequencing and Comparative Genomics of 19 IsolatesČlánek Widespread Endogenization of Genome Sequences of Non-Retroviral RNA Viruses into Plant GenomesČlánek The Cost of Virulence: Retarded Growth of Typhimurium Cells Expressing Type III Secretion System 1Článek A Role for the Chemokine RANTES in Regulating CD8 T Cell Responses during Chronic Viral Infection
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2011 Číslo 7- Stillova choroba: vzácné a závažné systémové onemocnění
- Perorální antivirotika jako vysoce efektivní nástroj prevence hospitalizací kvůli COVID-19 − otázky a odpovědi pro praxi
- Jak souvisí postcovidový syndrom s poškozením mozku?
- Diagnostika virových hepatitid v kostce – zorientujte se (nejen) v sérologii
- Infekční komplikace virových respiračních infekcí – sekundární bakteriální a aspergilové pneumonie
-
Všechny články tohoto čísla
- What Do We Really Know about How CD4 T Cells Control ?
- “Persisters”: Survival at the Cellular Level
- E6 and E7 from Beta Hpv38 Cooperate with Ultraviolet Light in the Development of Actinic Keratosis-Like Lesions and Squamous Cell Carcinoma in Mice
- Selection of Resistant Bacteria at Very Low Antibiotic Concentrations
- The Extracytoplasmic Domain of the Ser/Thr Kinase PknB Binds Specific Muropeptides and Is Required for PknB Localization
- CD39/Adenosine Pathway Is Involved in AIDS Progression
- Hypoxia and a Fungal Alcohol Dehydrogenase Influence the Pathogenesis of Invasive Pulmonary Aspergillosis
- One Is Enough: Effective Population Size Is Dose-Dependent for a Plant RNA Virus
- Effects of Interferon-α/β on HBV Replication Determined by Viral Load
- A Typhimurium-Typhi Genomic Chimera: A Model to Study Vi Polysaccharide Capsule Function In Vivo
- Dual Chaperone Role of the C-Terminal Propeptide in Folding and Oligomerization of the Pore-Forming Toxin Aerolysin
- Rotavirus Stimulates Release of Serotonin (5-HT) from Human Enterochromaffin Cells and Activates Brain Structures Involved in Nausea and Vomiting
- Dissociation of Infectivity from Seeding Ability in Prions with Alternate Docking Mechanism
- The Impact of Recombination on dN/dS within Recently Emerged Bacterial Clones
- The Regulation of Sulfur Metabolism in
- Illumination of Parainfluenza Virus Infection and Transmission in Living Animals Reveals a Tissue-Specific Dichotomy
- A Permeable Cuticle Is Associated with the Release of Reactive Oxygen Species and Induction of Innate Immunity
- A Concerted Action of Hepatitis C Virus P7 and Nonstructural Protein 2 Regulates Core Localization at the Endoplasmic Reticulum and Virus Assembly
- SUMO Pathway Dependent Recruitment of Cellular Repressors to Herpes Simplex Virus Type 1 Genomes
- Re-localization of Cellular Protein SRp20 during Poliovirus Infection: Bridging a Viral IRES to the Host Cell Translation Apparatus
- Divergent Effects of Human Cytomegalovirus and Herpes Simplex Virus-1 on Cellular Metabolism
- A Structural Model for Binding of the Serine-Rich Repeat Adhesin GspB to Host Carbohydrate Receptors
- Transformation of Natural Genetic Variation into Genomes
- EBV Latency Types Adopt Alternative Chromatin Conformations
- Global mRNA Degradation during Lytic Gammaherpesvirus Infection Contributes to Establishment of Viral Latency
- Dynamic Evolution of Pathogenicity Revealed by Sequencing and Comparative Genomics of 19 Isolates
- Microbial Virulence as an Emergent Property: Consequences and Opportunities
- Widespread Endogenization of Genome Sequences of Non-Retroviral RNA Viruses into Plant Genomes
- Structural Basis of Chemokine Sequestration by CrmD, a Poxvirus-Encoded Tumor Necrosis Factor Receptor
- Cross-Species Transmission of a Novel Adenovirus Associated with a Fulminant Pneumonia Outbreak in a New World Monkey Colony
- An Interaction between KSHV ORF57 and UIF Provides mRNA-Adaptor Redundancy in Herpesvirus Intronless mRNA Export
- Elevated 17β-Estradiol Protects Females from Influenza A Virus Pathogenesis by Suppressing Inflammatory Responses
- The Role of IL-15 Deficiency in the Pathogenesis of Virus-Induced Asthma Exacerbations
- Fluorescence Lifetime Imaging Unravels Metabolism and Its Crosstalk with the Host Cell
- Programmed Death (PD)-1-Deficient Mice Are Extremely Sensitive to Murine Hepatitis Virus Strain-3 (MHV-3) Infection
- Hemoglobin Promotes Nasal Colonization
- Crystallography of a Lewis-Binding Norovirus, Elucidation of Strain-Specificity to the Polymorphic Human Histo-Blood Group Antigens
- The Cost of Virulence: Retarded Growth of Typhimurium Cells Expressing Type III Secretion System 1
- A Genome-Wide Approach to Discovery of Small RNAs Involved in Regulation of Virulence in
- Requires Glycerol for Maximum Fitness During The Tick Phase of the Enzootic Cycle
- C Metabolic Flux Analysis Identifies an Unusual Route for Pyruvate Dissimilation in Mycobacteria which Requires Isocitrate Lyase and Carbon Dioxide Fixation
- A Role for the Chemokine RANTES in Regulating CD8 T Cell Responses during Chronic Viral Infection
- Glycosaminoglycans and Sialylated Glycans Sequentially Facilitate Merkel Cell Polyomavirus Infectious Entry
- Regulation of Stomatal Tropism and Infection by Light in : Evidence for Coordinated Host/Pathogen Responses to Photoperiod?
- Multiple Translocation of the Effector Gene among Chromosomes of the Rice Blast Fungus and Related Species
- Comparative Genomics Yields Insights into Niche Adaptation of Plant Vascular Wilt Pathogens
- Unique Cell Adhesion and Invasion Properties of O:3, the Most Frequent Cause of Human Yersiniosis
- C-Terminal Region of EBNA-2 Determines the Superior Transforming Ability of Type 1 Epstein-Barr Virus by Enhanced Gene Regulation of LMP-1 and CXCR7
- Novel Chikungunya Vaccine Candidate with an IRES-Based Attenuation and Host Range Alteration Mechanism
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Requires Glycerol for Maximum Fitness During The Tick Phase of the Enzootic Cycle
- Comparative Genomics Yields Insights into Niche Adaptation of Plant Vascular Wilt Pathogens
- The Role of IL-15 Deficiency in the Pathogenesis of Virus-Induced Asthma Exacerbations
- “Persisters”: Survival at the Cellular Level
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



