-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Dissociation of Infectivity from Seeding Ability in Prions with Alternate Docking Mechanism
Previous studies identified two mammalian prion protein (PrP) polybasic domains that bind the disease-associated conformer PrPSc, suggesting that these domains of cellular prion protein (PrPC) serve as docking sites for PrPSc during prion propagation. To examine the role of polybasic domains in the context of full-length PrPC, we used prion proteins lacking one or both polybasic domains expressed from Chinese hamster ovary (CHO) cells as substrates in serial protein misfolding cyclic amplification (sPMCA) reactions. After ∼5 rounds of sPMCA, PrPSc molecules lacking the central polybasic domain (ΔC) were formed. Surprisingly, in contrast to wild-type prions, ΔC-PrPSc prions could bind to and induce quantitative conversion of all the polybasic domain mutant substrates into PrPSc molecules. Remarkably, ΔC-PrPSc and other polybasic domain PrPSc molecules displayed diminished or absent biological infectivity relative to wild-type PrPSc, despite their ability to seed sPMCA reactions of normal mouse brain homogenate. Thus, ΔC-PrPSc prions interact with PrPC molecules through a novel interaction mechanism, yielding an expanded substrate range and highly efficient PrPSc propagation. Furthermore, polybasic domain deficient PrPSc molecules provide the first example of dissociation between normal brain homogenate sPMCA seeding ability from biological prion infectivity. These results suggest that the propagation of PrPSc molecules may not depend on a single stereotypic mechanism, but that normal PrPC/PrPSc interaction through polybasic domains may be required to generate prion infectivity.
Published in the journal: . PLoS Pathog 7(7): e32767. doi:10.1371/journal.ppat.1002128
Category: Research Article
doi: https://doi.org/10.1371/journal.ppat.1002128Summary
Previous studies identified two mammalian prion protein (PrP) polybasic domains that bind the disease-associated conformer PrPSc, suggesting that these domains of cellular prion protein (PrPC) serve as docking sites for PrPSc during prion propagation. To examine the role of polybasic domains in the context of full-length PrPC, we used prion proteins lacking one or both polybasic domains expressed from Chinese hamster ovary (CHO) cells as substrates in serial protein misfolding cyclic amplification (sPMCA) reactions. After ∼5 rounds of sPMCA, PrPSc molecules lacking the central polybasic domain (ΔC) were formed. Surprisingly, in contrast to wild-type prions, ΔC-PrPSc prions could bind to and induce quantitative conversion of all the polybasic domain mutant substrates into PrPSc molecules. Remarkably, ΔC-PrPSc and other polybasic domain PrPSc molecules displayed diminished or absent biological infectivity relative to wild-type PrPSc, despite their ability to seed sPMCA reactions of normal mouse brain homogenate. Thus, ΔC-PrPSc prions interact with PrPC molecules through a novel interaction mechanism, yielding an expanded substrate range and highly efficient PrPSc propagation. Furthermore, polybasic domain deficient PrPSc molecules provide the first example of dissociation between normal brain homogenate sPMCA seeding ability from biological prion infectivity. These results suggest that the propagation of PrPSc molecules may not depend on a single stereotypic mechanism, but that normal PrPC/PrPSc interaction through polybasic domains may be required to generate prion infectivity.
Introduction
Prions are infectious proteinaceous particles that cause fatal neurodegenerative diseases, including Creutzfeldt-Jakob disease (CJD), bovine spongiform encephalopathy (BSE), and chronic wasting disease (CWD). Prions contain PrPSc, a protease-resistant detergent-insoluble β-sheet-rich conformer of the normal cellular protein PrPC [1], [2]. PrPSc is an essential and possibly the sole component of infectious prions. Prion propagation and disease require the presence of PrPC, encoded by the host Prnp gene [3], [4], [5], [6].
Cell-free in vitro propagation systems have emerged as valuable tools to investigate PrPSc and prion propagation [7]. By serial protein misfolding cyclic amplification (sPMCA), prion infectivity can be propagated in vitro [8]. More rapid and less costly than the gold standard inoculation bioassay, sPMCA has been proposed as an in vitro method to detect prion infectivity [9]. Indeed, samples which seed robust sPMCA propagation have been previously associated with biological infectivity [8], [10]. However, it is unknown if PrPSc molecules that robustly seed PMCA propagation in wild type brain homogenate are always associated with appropriate levels of specific infectivity.
Recent studies have reconstituted infectious PrPSc propagation using purified PrPC substrate and supplementary conversion cofactors, a set of minimal components that appear necessary for prion propagation [11], [12]. PrPSc appears to propagate by autocatalysis, binding PrPC to induce conversion into a new PrPSc molecule [13]. However, the mechanisms of binding and conversion remain unclear.
Studies using motif-grafted antibodies or PrP-derived peptides identified two polybasic regions that bind strongly to PrPSc [14], [15], [16], suggesting that these PrPC domains may serve as docking sites for PrPSc. The N-terminal (N-PBD, 23–28) and central (C-PBD, 100–109) polybasic domains both fall in the N-terminal flexible region of PrPC, which is less ordered than the C-terminus [17], [18]. Antibodies directed at C-PBD can impede prion propagation in cultured cells and in vivo [19], [20], possibly by blocking a PrPSc-binding site on PrPC. The polybasic domains may also be involved in interaction with lipid molecules during the conversion process [21] and may affect the structure of misfolded prion protein [22].
Mice overexpressing PrP transgenes lacking amino acids 23–88 show reduced prion susceptibility [23], but these studies did not examine N-PBD in isolation. Transgenic mice with deletions that include C-PBD display lethal neurologic illness, but this phenotype precluded inoculation experiments assessing prion susceptibility [24], [25], [26]. The specific role of these domains in the context of the entire PrPC molecule has been studied in N2a cells co-expressing wild-type and mutant PrPC [27], but biochemical examinations of binding interactions and of propagation with pure substrates are lacking.
In full-length PrPC, the relative importance of the N-terminal and central polybasic domains in binding PrPSc is unclear. Moreover, while much evidence points to a significant role for PrPC polybasic domains, it is not known if PrPSc-PrPC interactions are universally stereotypic – whether all PrPSc molecules use the same PrPC epitopes for interaction.
Using a combination of Chinese hamster ovary (CHO) cell expression, protein purification, and reconstitution sPMCA techniques, we engineered PrPSc molecules lacking polybasic domains. These novel PrPSc molecules, which propagate robustly in vitro, enabled us to assess whether PrPC polybasic domains are universally required for PrPSc docking and propagation. PBD-deficient PrPSc molecules also provided us with a unique opportunity to dissect the relationship between the ability to seed formation of wild type PrPSc molecules in vitro and prion infectivity in vivo.
Materials and Methods
Mutagenesis and expression of recPrP-myc for binding
Sequence encoding mouse PrP-A 23–230 was amplified, using the following primers to add an N-terminal start codon and a C-terminal tag encoding amino acids 410–419 of human c-myc (NCBI accession number NP_002458.2): N: 5′ - aaaaaacatatgaaaaagcggccaaagcctggagggt-3′, C: 5′-aaaactcgagtcattacagatcctcttctgagatgagtttttgttcggatcttctcccgtcgtaatag-3′. This fusion gene was inserted into pET22b(+) for bacterial expression. N-terminal (ΔN, 23–28) and central (ΔC, 100–109) polybasic domains were deleted by site-directed mutagenesis (GeneTailor, Invitrogen, Carlsbad, CA). From transfected Escherichia coli Rosetta cells, recombinant PrP was purified in a manner similar to that described by Wang et al. [12]. Cells from 500 mL induced (Overnight Express Autoinduction System, Novagen, EMD Chemicals, Gibbstown, NJ) overnight culture were pelleted at 8,000×g for 10 min.; lysed with 40 mL BugBuster, Lysonase (Novagen), and intermittent sonication over 20 min.; and inclusion bodies were prepared by two cycles of centrifugation (16,000×g, 15 min.) and 0.1× Bugbuster resuspension, followed by an additional 16,000×g 15 min. spin. Inclusion body pellets were solubilized in 8 mL 8 M guanidine hydrochloride, insoluble material removed by centrifugation (8,000×g, 10 min.), and protein added to 3.6 g Ni-NTA Hisbind Superflow resin (Novagen) pre-equilibrated in denaturing buffer (100 mM sodium phosphate, 10 mM Tris, 6 M Guanidine, 10 mM β-mercaptoethanol, pH 8.0). After 30 min. binding, resin was transferred to column support, and protein was refolded by a linear 12 hr. 125 mL gradient of denaturing to refolding buffer (100 mM sodium phosphate, 10 mM Tris, pH 8.0), followed by 60 mL wash at 1 mL/min. in refolding buffer. Protein was eluted from nickel affinity resin by 1 mL/min. 500 mM imidazole, 100 mM sodium phosphate pH 6.5; then dialyzed into 20 mM sodium phosphate pH 6.5 (3×2 L for 30 min.) and into water (3×2 L for 30 min., 1×4 L overnight). If observed, precipitate was removed by centrifugation at 100,000×g for 60 min. The nickel eluate was loaded on 2 mL pre-equilibrated (30 mL 10 mM sodium phosphate pH 6.5, 1 mL/min.) CM sepharose (Sigma, St. Louis, MO) at 0.5 mL/min., washed with 50 mL NaCl at 1 mL/min., and eluted with a gradient (300–650 mM NaCl, 0.5 mL/min.). Fractions containing >0.1 OD280 (>0.037 mg/mL PrP) [28] were pooled and dialyzed into water (2×4 L for 30 min., 1×4 L overnight), then stored at −70°C. Protein concentration was determined by bicinchoninic acid (BCA) assay.
PrPSc-PrP binding assays
RML scrapie-infected brain homogenate (5% BH in tris-buffered saline) or CHO ΔC-PrPSc (sPMCA round 16 product, therefore 1016-fold dilution of original wild-type scrapie seed) were vortexed for 15 sec., sonicated for 1 min. at 70% power (Misonix 4000 with Microplate Horn, Qsonica, Newtown, CT), and centrifuged at 500×g for 15 min. Normalized amounts of this clarified preparation (12.4 µL brain homogenate PrPSc, 16.5 µL PrPSc) were incubated with 3.5 µg purified E. coli recPrP-myc (wild-type or lacking polybasic domain) in 250 µL binding buffer (50 mM tris, 200 mM NaCl, 1% Triton X-100, 1% Tween-20, pH 7.5) for 1 hr. at 4°C with 10 r.p.m. end-over-end rotation. Concurrently, 16 µL 30 mg/mL Dynabeads Protein A (Invitrogen) was washed with 2×500 µL phosphate-buffered saline (PBS), collected by a Magnetic Particle Separator (PureBiotech, Middlesex, NJ), and incubated with 3.6 µg 9E10 anti-myc antibody (Santa Cruz Biotechnology, Santa Cruz, CA) in 250 µL binding buffer for 30 min. at room temp. with 10 r.p.m. end-over-end rotation. Next, the solution of PrPSc and PrP-myc was added to the bead-anti-myc complexes, and rotated for 1 hr. at 4°C. Following this incubation, beads were collected, and supernatant was aspirated. Beads were rinsed in 4×500 µL wash buffer (50 mM tris, 200 mM NaCl, 0.05% Tween 20), and analyzed for bound PrPSc. Signal intensities were quantified by densitometry with ImageGauge V4.22 (Fujifilm) in quant mode.
Expression and preparation of PrP from Chinese hamster ovary (CHO) cells
Sequences encoding wild-type and polybasic domain deletion mutant (ΔN = Δ23–28; ΔC-PBD = Δ100–109; ΔΔ-PBD = Δ23–28 Δ100–109) PrP were inserted in pcDNA5/FRT plasmids. These were used to express PrP from CHO cells, which was prepared as described previously [29]. For hamster experiments, homologous deletion mutants were used (Δ23–28, Δ101–110, or ΔΔ).
Reconstituted serial protein misfolding cyclic amplification (sPMCA) with CHO-expressed PrP
Reactions were prepared and carried out as described by Geoghegan et al. [29]. Briefly, CHO-expressed mouse PrP (wild-type or polybasic domain deletion mutant) was mixed with Prnp0/0 (Zurich) brain homogenate (2.5% final concentration). Ninety microliters of this reconstituted substrate was mixed with 10 µL seed. Reactions were initially seeded with 0.1% scrapie-infected brain homogenate (mouse strain RML) in PBS, with 10 µL of product used to seed the subsequent round. ΔC-PrPSc seed was produced by 16 rounds of sPMCA (1016-fold dilution of original wild-type scrapie seed), containing <1 original PrPSc molecule [8]. Unseeded reactions were given 10 µL PBS as initial seed. For reactions lacking cofactor, buffer (PBS 1% Triton X-100) replaced Prnp0/0 brain homogenate. Each PMCA round consisted of incubation at 37°C for 24 hr. with 20 sec. microplate horn sonication at 85% power every 30 min. One set of seeded samples was not subjected to PMCA (round 0), while another set was not subjected to protease digestion (−PK) to observe input PrP. For hamster sPMCA experiments, or Sc237 strain was used, CHO HaPrP was supplemented with 20 µg/mL synthetic poly(A) RNA (Sigma, St. Louis, MO), and PrPSc was detected by immunoblot after 50 µg/mL digestion for 60 min.
sPMCA with brain homogenate
Brain homogenate sPMCA experiments were adapted from Castilla et al. [30]. 90 µL 10% CD-1 mouse brain (Biochemed, Winchester, VA) homogenate was prepared in PBS, 1% Triton X-100, 5 mM EDTA, Roche Complete mini protease inhibitor, then seeded with PrPSc (10 µL of round 16 product). One round of PMCA consisted of 30 sec. 90% power microplate horn sonication pulses every 30 min. for 24 hr. at 37°C. 10 µL of each reaction product was transferred to fresh brain homogenate for the following round.
PrPSc detection
Mouse PrPSc was detected by digestion with 25 µg/mL proteinase K (Roche, Indianapolis, IN) for 30 min. at 37°C and 750 r.p.m. shaking, polyacrylamide gel electrophoresis (PAGE), transfer to polyvinylidene fluoride (PVDF), and Western detection with anti-PrP and horseradish peroxidase-conjugated anti-mouse sheep IgG (GE Healthcare, Piscataway, NJ). Signals were detected by enhanced chemiluminescence (ECL) (SuperSignal West Femto Substrate, Pierce, Rockford, IL) and visualized by a Fuji (Fujifilm) LAS-3000 chemiluminescence documentation system. Hamster PrPSc was detected with 50 µg/mL proteinase K for 60 min. Experiments detecting only wild-type PrP used 6D11 anti-PrP antibody. Because the central polybasic domain (C-PBD) forms part of the 6D11 epitope, and therefore ΔC PrP is not detected by 6D11 (data not shown), reconstituted sPMCA experiments utilized anti-PrP mAb 27/33.
Biological infectivity assay
RML PrPSc, propagated with CHO wild-type or ΔC - PrPC in vitro by sPMCA (Figures 1B, S4) for at least 14 rounds, was diluted 1∶10 in diluent (PBS+1 mg/mL BSA). ΔN - and ΔΔ-PrPSc molecules, propagated from ΔC-PrPSc (Figure 2B), were prepared in the same manner. By serial dilution, each inoculum contained 10−15 of the original seed, equivalent to less than one original PrPSc molecule [8]. PrPSc preparations (30 µL) were injected intracerebrally into female CD-1 mice aged 6 weeks (Charles River Laboratories, Wilmington, MA). For two samples (ΔC-PrPSc and ΔΔ-PrPSc), end-point dilution bioassays were performed, in which 10-fold serial dilutions were inoculated to measure the amount of prion infectivity present in the original sample. As described previously [11], animals were monitored daily for clinical signs of neurological dysfunction over the standard one-year observation period. Animals showing terminal scrapie were sacrificed, and their brains were analyzed for PrPSc (by 25 µg/mL protease digestion and immunoblot) and for spongiform degeneration (by hematoxylin & eosin histology) [11]. Random asymptomatic animals were sacrificed after one year, and their brains were likewise analyzed for PrPSc and spongiform degeneration.
Fig. 1. Interaction of wild-type PrPSc with mutant PrP molecules. 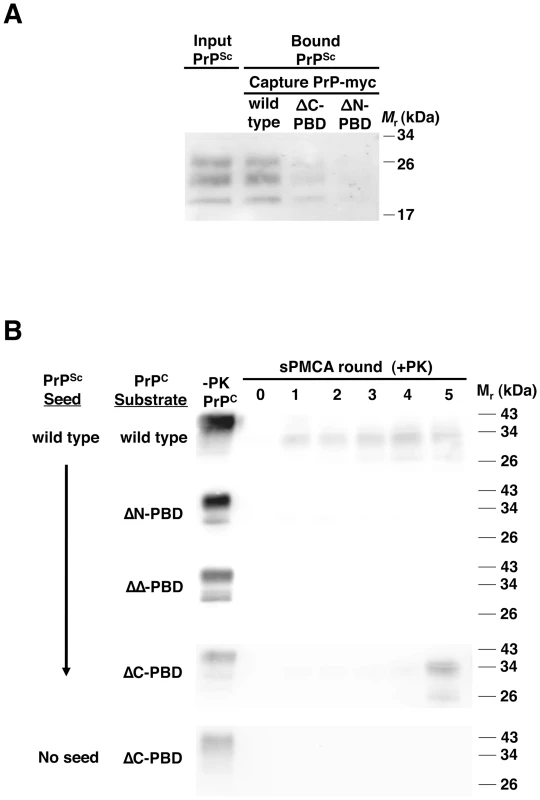
(A) Binding of PrPSc to PrP. RML scrapie-infected mouse brain homogenate was incubated with myc-tagged PrP of wild-type sequence or lacking the central (ΔC-PBD: Δ100–109) or N-terminal (ΔN-PBD: Δ23–28) polybasic domain. Bound PrPSc was captured with 9E10 anti-myc antibody on magnetic protein A Dynabeads, and detected by 25 µg/mL proteinase K digestion and anti-PrP (6D11) immunoblot. (B) Propagation of PrPSc. RML scrapie-infected mouse brain homogenate was propagated by serial protein misfolding cyclic amplification (sPMCA) for five rounds with wild-type or polybasic deletion mutant PrPC prepared from Chinese hamster ovary (CHO) cells. Reactions were supplemented with Prnp0/0 mouse brain homogenate. In addition to scrapie-seeded reactions, an unseeded reaction was performed with ΔC-PBD PrP substrate. One sample of each reaction was not subjected to protease digestion (−PK). All others were subjected to limited proteolysis with 25 µg/mL proteinase K. PrP was detected by immunoblot (anti-PrP 27/33). Fig. 2. Interaction of ΔC-PBD PrPSc with mutant PrP molecules. 
(A) Binding of ΔC-PBD PrPSc to PrP. ΔC-PBD PrPSc was incubated with wild-type or polybasic mutant (ΔC-PBD, ΔN-PBD) myc-tagged PrP. Bound PrPSc was captured with 9E10 anti-myc antibody on magnetic protein A Dynabeads, and detected by 25 µg/mL proteinase K digestion and anti-PrP (27/33) immunoblot. (B) Propagation of ΔC-PBD PrPSc. ΔC-PBD PrPSc was propagated by sPMCA with polybasic deletion mutant PrPC prepared from Chinese hamster ovary (CHO) cells. Reactions were supplemented with Prnp0/0 mouse brain homogenate. One sample of each reaction was not subjected to protease digestion (−PK), loading ¼ of volume. All others were subjected to limited proteolysis by 25 µg/mL proteinase K digestion. PrP was detected by immunoblot (anti-PrP 27/33). Ethics statement
All animals were handled in strict accordance with good animal practice, as defined by the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. The Dartmouth College Institutional Animal Care and Use Committee approved the animal work (assurance number A3259-01). Inoculations were performed under isoflurane anesthesia, and all efforts were made to minimize suffering.
Results
Interaction and propagation of wild-type PrPSc with PrP molecules lacking polybasic domains
Studies using fragments of PrP identified N-terminal (23–28) and central (100–109 in mouse) polybasic domains as potential binding sites for PrPSc [14], [16]. To investigate the role of these domains in the context of full-length PrPC, we developed a novel magnet-based assay to examine direct interaction between PrPSc and PrPC molecules with or without deletions of these polybasic regions. In this assay, PrPSc was incubated with purified recombinant myc-tagged PrP, and then adherent molecules were pulled down by α-myc antibodies attached to Protein A magnetic beads. Substitution of a short myc epitope in place of the C-terminal glycophosphatidylinositol anchor of PrPC permitted specific capture. The assay specifically assesses PrPSc binding to PrP (Figure S1 in Supporting Information S1), and indicates that purified PrPSc binds to purified PrP (Figure S2 in Supporting Information S1). Next, we compared binding of PrPSc to wild-type and mutant PrP substrates. In contrast to quantitative RML PrPSc binding by wild-type PrP (∼100% by densitometry), PrP lacking the central polybasic domain (ΔC) bound significantly less PrPSc (∼50%) and N-terminal polybasic deletion mutant PrP (ΔN) bound still less PrPSc (∼20%) (Figure 1A). Purified PrPSc also adhered to wild-type PrP more strongly than to polybasic mutant PrP (Figure S2 in Supporting Information S1). These results suggest that both PrP polybasic domains contribute to PrPSc binding.
To test the function of PrPC polybasic domains in prion propagation, we performed sPMCA experiments with wild type and mutant PrPC substrate. These PrPC molecules, prepared from CHO cells transfected with wild-type or mutant Prnp DNA, were detergent-soluble (Figure S3 in Supporting Information S1) and membrane-anchored (Figure S4 in Supporting Information S1), indicative of proper folding and intracellular trafficking. In reconstituted, three-round sPMCA experiments with PrPC and Prnp0/0 brain homogenate, PrPSc propagated efficiently, converting the PrPC to protease-resistant autocatalytic PrPSc molecules (Figure 1B and S5 in Supporting Information S1). In contrast, PrPC molecules missing one (ΔN, ΔC) or both (ΔΔ) polybasic domains did not support efficient propagation, indicating that PrPC requires these domains to facilitate PrPSc propagation. This result was also found with Sc237 hamster prions, as we observed no propagation of protease-resistant PrP with polybasic mutant hamster PrPC (Figure S5 in Supporting Information S1).
When we examined propagation in polybasic mutant substrates beyond three rounds, surprisingly, one mutant (ΔC) reproducibly produced PrPSc molecules, typically between rounds 3–5 (Figure 1B). Further, these ΔC-PrPSc molecules propagated robustly and indefinitely (Figure S6 in Supporting Information S1), indicating capability of PrPC to support propagation despite absence of the central polybasic domain. Indeed, ΔC-PrP conversion was extremely efficient, with ∼100% of substrate converted to PrPSc, outpacing even wild-type PrP conversion ratios (∼20% of substrate). ΔC-PrPSc molecules appear to derive from the original wild-type PrPSc seed, as unseeded reactions did not generate PrPSc (Figure 1B).
Interaction range of ΔC-PrPSc molecules
The unexpected generation of ΔC-PrPSc molecules led us to conduct a series of experiments to assess the role of polybasic domains in ΔC-PrPSc propagation. Using the magnetic myc-capture assay, we tested binding to various PrP substrates. In contrast to wild-type PrPSc, ΔC-PrPSc reproducibly bound more strongly to ΔC-PrP (∼50% of input) than to wild type PrP (∼35% of input) (Figure 2A). Furthermore, ΔC-PrPSc bound to ΔN-PrP (∼30% of input), suggesting a significantly different interaction mechanism than wild-type PrPSc. Interestingly, wild-type PrPSc-PrPC binding appeared stronger than ΔC-PBD mutant PrPSc-PrPC interactions.
We next examined ΔC-PrPSc propagation with other polybasic mutant substrates. Fitting with its binding behavior, ΔC-PrPSc propagated successfully in ΔN-PrPC (Figure 2B). Moreover, the ΔC-PrPSc seed also propagated in ΔΔ-PrPC with robust conversion, indicating that presence of a polybasic domain is not an absolute requirement for PrPC to convert to a protease-resistant autocatalytic form. Indeed, all three types of ΔPBD-PrPSc molecules propagated robustly, with stringent proteinase K digestion (25 µg/mL) revealing a protease-resistant core with a ∼7 kDa molecular weight shift.
Cofactor dependence and seeding specificity of PrPSc molecules lacking polybasic domains
Infectious wild-type PrPSc molecules depend on accessory cofactor molecules for propagation [11], [12], [31]. We tested whether polybasic domain mutant PrPSc molecules exhibit this characteristic by performing parallel sPMCA reactions, omitting the supplemental Prnp0/0 brain homogenate from one set (Figure 3). All three PrPSc mutants (ΔC, ΔN, and ΔΔ) failed to propagate in the absence of Prnp0/0 brain homogenate, pointing to a cofactor-dependent propagation mechanism. The signals seen in initial rounds of cofactor-negative propagation are likely due to the presence of residual Prnp0/0 brain homogenate carried over from the PrPSc seed mixture. By the third round of sPMCA in cofactor-free substrate, no PrPSc molecules were detected.
Fig. 3. Effect of accessory cofactors on the propagation of ΔPBD PrPSc molecules. 
In vitro-generated ΔC-PBD, ΔN-PBD, and ΔΔ-PBD PrPSc molecules were propagated by sPMCA with autologous PrPC prepared from Chinese hamster ovary (CHO) cells. One set of reactions was supplemented with Prnp0/0 mouse brain homogenate (+cofactor), while a second set received only buffer (−cofactor). One sample of each reaction was not subjected to protease digestion (−PK), while all others were digested with 25 µg/mL proteinase K. –PK reactions for ΔC-PBD and ΔΔ-PBD were loaded with ¼ volume. PrP was detected by immunoblot (anti-PrP 27/33). Infectious PrPSc seeds propagation in wild-type brain homogenate [32], [33] to the extent that successful sPMCA propagation is considered potentially diagnostic for prion infectivity [9]. We tested if ΔPBD-PrPSc molecules could seed propagation in wild-type brain homogenate (Figure 4). Like wild-type PrPSc, all three PrPSc polybasic mutants (ΔC, ΔN, and ΔΔ) seeded serial propagation with success. Each of these reactions displayed positive conversion signals in round 1, suggesting a high amount of prion infectivity [34]. Interestingly, the PrPSc molecules that formed were predominantly diglycosylated, whereas PrPSc molecules formed from wild-type seeds displayed the three glycoforms in equivalent amounts. It has been shown that the ability to produce different PrPSc glycoforms is not due to preferential interaction, but rather inherent conformation [35]. This study found that mouse RML prions only require unglycosylated PrPC for propagation, and that glycosylated forms of PrPSc can be made in its presence. This suggests that the observed glycosylation pattern described here may be caused by a different fold in polybasic mutant PrPSc.
Fig. 4. ΔPBD PrPSc molecules seeding wild-type brain homogenate sPMCA reactions. 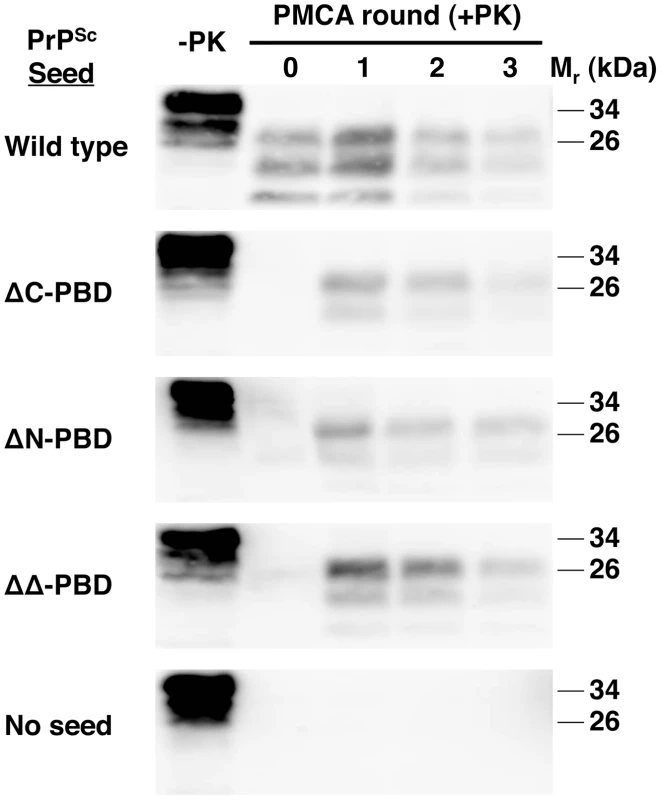
In vitro-generated ΔC-PBD, ΔN-PBD, and ΔΔ-PBD PrPSc molecules were propagated by sPMCA for three rounds with wild-type mouse brain homogenate, containing wild-type PrPC substrate. Control reactions seeded with wild-type native RML prions and unseeded reactions were also tested. One sample of each reaction was not subjected to protease digestion (−PK), while all others were digested with 25 µg/mL proteinase K. PrP was detected by immunoblot (anti-PrP 6D11). Thus, all three PBD mutant PrPSc molecules share the hallmark biochemical characteristics of infectious wild-type PrPSc molecules [11]: (1) a highly protease-resistant core, (2) cofactor-dependent propagation, and (3) the ability to seed wild-type brain homogenate sPMCA reactions.
Infectivity of polybasic mutant PrPSc molecules
We tested whether mutant PrPSc molecules, generated in vitro from CHO-expressed PrP, are infectious to animals. Following sPMCA propagation sufficient to dilute out original seeds, wild-type and mutant (ΔC, ΔN, and ΔΔ) PrPSc molecules were inoculated intracerebrally into wild-type mice. Wild-type CHO PrPSc contains significant infectivity, causing scrapie disease in all animals with an incubation period comparable to RML prions propagated in vitro in brain homogenate [36] (Table 1). Affected animals displayed accumulation of protease-resistant prion protein and significant histopathological spongiform degeneration (Figure 5), as we previously reported for hamster prions propagated in vitro in wild-type CHO-expressed hamster PrP [29]. Animals inoculated with wild-type CHO PrPSc also showed robust levels of protease-resistant PrPSc on Western blot.
Fig. 5. Biochemical and neuropathological analysis of mice inoculated with in vitro-generated PrPSc molecules. 
Brains were dissected from wild-type mice showing terminal scrapie signs (PrPSc and ΔC-PBD PrPSc inocula) or similarly aged mice not displaying scrapie signs (mock-propagated, ΔN-PBD PrPSc, and ΔΔ-PBD PrPSc inocula). (A) Equivalent amounts of 10% brain homogenate were treated with buffer (−PK) or 25 µg/mL proteinase K (+PK to show PrPSc) and detected by anti-PrP (6D11) immunoblot. A greater exposure of the same immunoblot is displayed below, to illustrate samples containing low amounts of PrPSc. (B) Neuropathology of cerebellum and hippocampus. Brain sections were stained with hematoxylin and eosin (H&E). The black bar denotes 100 µm. Tab. 1. Biological infectivity assay of in vitro-generated autocatalytic PrPSc molecules. 
RML prions were propagated in vitro by sPMCA with purified CHO-expressed PrPC+Prnp0/0 brain homogenate. In contrast to wild-type, polybasic mutant PrPSc molecules show diminished in vivo infectivity. ΔC-PrPSc caused scrapie disease only in a fraction of animals, including those receiving the most concentrated inocula, with an incubation time 2.5-fold greater than animals inoculated with wild-type PrPSc. ΔC-PrPSc-inoculated diseased animals showed mild vacuolation (Figures 5B and S7 in Supporting Information S1) and displayed low levels of PrPSc on Western blot, with diglycosylated molecules most abundant. Such a glycoform ratio contrasts with the even distribution of glycoforms in animals inoculated with wild-type PrPSc (Figures 5A and S8 in Supporting Information S1), also seen with in vitro propagation (Figure 4). Both wild-type and ΔC-PrPSc induced scrapie illness characterized neurologically by lethargy and ataxia. ΔN-PrPSc molecules induced scrapie illness in a single animal, following a 564-day incubation period (Figure S9 in Supporting Information S1), with other such inoculated animals showing no scrapie illness (Figure 5). Due to the sudden nature of some deaths, we did not obtain tissue from all animals, but brain samples from many non-scrapie deaths were obtained as indicated in Table 1. ΔΔ-PrPSc molecules did not induce scrapie illness, as gauged by clinical observation and lack of protease-resistant PrP or histopathological change in animals sacrificed over one year after inoculation (Figure 5). Thus, while polybasic deletion mutant PrPSc molecules recapitulated in vitro many characteristics of infectious PrPSc molecules, they displayed low specific infectivity in wild-type mice.
Discussion
In this study, we have engineered polybasic domain deficient PrPSc molecules that efficiently interact with and catalyze the conversion of an expanded range of PrPC substrates, including native PrPC in wild-type mouse brain homogenate. Remarkably, despite possessing the biochemical characteristics and in vitro seeding activity of wild-type prions, PBD-deficient PrPSc molecules possessed little or no in vivo infectivity.
Requirement of PrPC polybasic domains for propagation of wild-type PrPSc
Studies of PrP fragments have indicated that polybasic domains can interact with PrPSc [14], [16]. To investigate both prion binding and conversion in the context of the entire PrP molecule, we designed mutant prion proteins lacking one or both polybasic domains, which were detergent-soluble and properly trafficked, as determined by PI-PLC release. Using a novel magnetic capture assay, we found that both PrPC polybasic domains were required for optimal PrPSc binding. In reconstituted sPMCA experiments with PrP expressed and prepared from CHO cells, deletion of either or both polybasic domains of substrate PrPC prevented efficient propagation of wild-type PrPSc. As mutants lacking either domain did not efficiently bind or propagate wild-type PrPSc, both PrPC polybasic domains appear required to contact PrPSc for efficient propagation of wild-type prions.
Work using N2a cells co-expressing wild-type and mutant PrP suggested that the N-terminal polybasic domain may not play a role in conversion [27]. However, the presence of endogenous wild-type PrPC may have provided conditions that facilitated conversion of the weaker-binding mutant PrP substrate. The results presented in this paper, using mutant substrate alone to test binding and propagation, suggest that the PrPC N-terminal polybasic domain indeed participates in wild-type PrPSc binding and propagation.
Delayed conversion of PrP lacking polybasic domains
When sPMCA reactions seeded with wild-type prions were carried out to 3–5 rounds, ΔC-PrPC substrate molecules converted to a form resistant to protease digestion under stringent conditions (25 µg/mL proteinase K at 37°C). After protease digestion, this form displayed a classical shift in molecular weight of ∼7 kDa. This form was also autocatalytic, continuing to propagate robustly (∼100% conversion) and indefinitely in ΔC-PrPC substrate. In seeking to understand the surprising kinetics of the initial conversion event, which occurred after four rounds and 10−4 dilution of the original seed, we found that unseeded control reactions did not generate the mutant PrPSc product. Thus, ΔC-PrPSc did not arise de novo [11], but may have emerged from amplification of a few molecules of a minority conformation present in the original PrPSc seed. Such an event is consistent with recent reports of multiple prion strains emerging from a single “pure” source [37], [38], and 4–5 serial amplification rounds are required to detect PrPSc conformers at very low concentrations [34].
Expanded substrate range and increased converting efficiency of ΔC-PrPSc
Once formed, the novel ΔC-PrPSc molecules bound to and propagated in ΔN-PrPC and ΔΔ-PrPC substrates, forming new PrPSc molecules lacking either one or both polybasic domains. Thus, in contrast to wild-type PrPSc, ΔC-PrPSc is universally catalytic in polybasic mutant PrPC, exhibiting an expanded substrate range that points to an alternative docking mechanism during propagation. Each of the ΔPBD PrPSc species propagated robustly, including even the mutant lacking both polybasic domains (ΔΔ). While wild-type PrPSc propagates by binding to substrate polybasic domains, ΔPBD mutant PrPSc molecules appear to utilize a different mechanism to bind PrPC, as outlined by the model in Figure 6. This indicates that prion docking, and perhaps other events in conversion, do not necessarily follow a rigidly conserved mechanism. In contrast, self-replicating fungal prions appear to utilize a stereotypic mechanism requiring glutamine/asparagine repeats [39].
Fig. 6. Model of PrP replicative interaction mechanisms. 
This diagram summarizes a proposed model for the interaction, propagation, and infectivity behavior of wild-type and polybasic deletion mutant PrP molecules. Wild-type PrPSc seed binds and propagates efficiently with autologous PrPC substrate, using PrPC polybasic domains (represented by rectangular protrusion) for docking. However, if PrPC lacks one or both polybasic domains, PrPSc binds less well and exhibits impaired propagation. If PrPSc molecules lacking polybasic domains can be formed, they can bind and propagate efficiently with an expanded range of PrPC substrates. ΔPBD-PrPSc may propagate by a different mechanism than wild-type PrPSc, utilizing different residues (symbolized by round protrusion) of PrPC for binding. A neoepitope (round depression) may be exposed in the polybasic mutant PrPSc molecules. Legend: Sc = PrPSc; C = PrPC; WT = wild-type. ΔPBD = deletion in polybasic domain; ++ = polybasic domain. The expanded interaction range of mutant PrPSc molecules could be explained by the exposure of a neoepitope on mutant PrPSc (Figure 6). If ΔPBD mutant PrPSc molecules propagate from a minority constituent conformer within wild-type PrPSc, the putative neoepitope may also be present on these minority seed molecules. Mechanisms of PrPSc docking may also vary between different natural prion strains. Further studies are required to assess binding-site heterogeneity of PrPSc-PrPC interactions, including identification of specific docking sites used by ΔΔ-PBD molecules.
We also observed that ΔC-PrPSc molecules, once formed, catalyzed nearly complete conversion of autologous substrate, ∼5-fold greater than wild-type PrPSc autocatalysis. Thus, ΔC-PrPSc molecules show robust catalytic activity in terms of both substrate range and prion protein converting efficiency.
Polybasic deficient PrPSc molecules resemble wild-type PrPSc in vitro
Our biochemical analysis revealed that ΔPBD PrPSc mutants possess all of the biochemical hallmarks that characterize wild-type infectious prions. All three ΔPBD PrPSc mutants (ΔC, ΔN, and ΔΔ) drive conversion of PrPC in wild-type brain homogenate sPMCA experiments, a model of the conversion event in prion pathogenesis [8]. Furthermore, ΔPBD PrPSc molecules are resistant to stringent protease digestion, demonstrating a post-digestion molecular weight shift that is characteristic of PrPSc. ΔPBD PrPSc molecules are also autocatalytic, propagating indefinitely in autologous PrPC substrate. The propagation of ΔPBD PrPSc requires an accessory cofactor provided by Prnp0/0 brain homogenate. Thus, unlike some fungal protein conformations, which have been shown to propagate in vitro with only the alternatively folded substrate protein [40], [41], [42], [43], ΔPBD PrPSc behaves like infectious mammalian prions in requiring a supplementary cofactor for propagation [11], [12], [31], [44], [45].
Dissociation of brain homogenate sPMCA seeding activity from in vivo infectivity
PrPSc table-1-captionmolecules generated from CHO-expressed wild-type PrPC induced scrapie illness in wild-type mice with a 100% attack rate (scrapie incubation time = 180±11 days). In contrast, despite exhibiting the in vitro hallmarks of wild-type infectious PrPSc as described above, mutant ΔC-PrPSc and ΔN-PrPSc molecules showed low infectivity in vivo (≤3000 LD50 units/mL and ∼300 LD50 units/mL, respectively), and ΔΔ-PrPSc showed no infectivity. Some inoculum-host PrP sequence N-terminal differences can mildly prolong scrapie incubation time, but these did not alter attack rates [46], [47], and much larger N-terminal PrPSc deletions than the ΔN-PrPSc that we report here did not significantly affect incubation time or attack rate in wild-type animals [23]. For example, protease-digested PrPSc yields PrP27-30, which is highly infectious to wild-type animals despite lacking amino acids 23–88 [48], [49]. Given that ΔPBD PrPSc molecules drive robust propagation in vitro in wild-type brain homogenate sPMCA, their diminished biological infectivity was highly unexpected, and represents dissociation between in vitro catalysis and in vivo infectivity, most notably for ΔN-PBD and ΔΔ-PBD PrPSc.
To our knowledge, this report is the first demonstration of absent or minimal infectivity in samples that successfully seed propagation of wild-type brain homogenate during sPMCA, suggesting that the normal route of PrPC/PrPSc interaction through polybasic domains may be required for generating infectious prions. Why might appropriate PBD-mediated interaction be required for infectivity? One possibility is that PBD-deficient PrPSc molecules may be more susceptible to existing host mechanisms for prion clearance [50], [51], [52], [53], perhaps by exposure of a neoepitope on PrPSc that serves as a clearance signal. If this explanation were correct, then all three PBD-deficient PrPSc molecules must be preferential targets for the clearance mechanism.
A more plausible explanation is that the non-PBD mediated interaction mechanisms used by ΔPBD-PrPSc molecules to propagate in vitro lead to the production of alternative PrPSc conformations that are intrinsically non-infectious or have reduced infectivity. Consistent with this explanation, we observed different PrPSc glycosylation patterns in animals inoculated with ΔC-PrPSc compared to animals inoculated with wild-type PrPSc. Such a distinction could be caused by an altered ability of mutant PrPSc to interact with non-PrP host molecules during in vivo propagation. For example, the in vitro detergent micelle environment differs from the membrane environments where propagation occurs in vivo. On the other hand, high titers of strain-preserved prion infectivity are propagated in vitro in detergent micelles [8], [11], [32], suggesting that in vitro propagation recapitulates native events fairly well.
Finally, it should be emphasized that although mutant PrPSc molecules were used in this study to uncover the dissociation between seeding ability and infectivity, the results are directly relevant to the mechanisms responsible for forming naturally occurring prions because normal wild type brain homogenates and normal wild type mice were used in sPMCA seeding assays and in vivo bioassays, respectively. The contrast between highly infectious wild type PrPSc and minimally infectious ΔPBD-PrPSc molecules provides a novel paradigm that can be used to determine the specific structural basis of prion infectivity.
Supporting Information
Zdroje
1. PrusinerSB 1982 Novel proteinaceous infectious particles cause scrapie. Science 216 136 144
2. PrusinerSB 1998 Prions. Proc Natl Acad Sci U S A 95 13363 13383
3. BrandnerSIsenmannSRaeberAFischerMSailerA 1996 Normal host prion protein necessary for scrapie-induced neurotoxicity. Nature 379 339 343
4. BuelerHAguzziASailerAGreinerRAAutenriedP 1993 Mice devoid of PrP are resistant to scrapie. Cell 73 1339 1347
5. OeschBWestawayDWalchliMMcKinleyMPKentSB 1985 A cellular gene encodes scrapie PrP 27–30 protein. Cell 40 735 746
6. MansonJCClarkeARMcBridePAMcConnellIHopeJ 1994 PrP gene dosage determines the timing but not the final intensity or distribution of lesions in scrapie pathology. Neurodegeneration 3 331 340
7. KociskoDAComeJHPriolaSAChesebroBRaymondGJ 1994 Cell-free formation of protease-resistant prion protein. Nature 370 471 474
8. CastillaJSaaPHetzCSotoC 2005 In vitro generation of infectious scrapie prions. Cell 121 195 206
9. SaaPCastillaJSotoC 2006 Ultra-efficient replication of infectious prions by automated protein misfolding cyclic amplification. J Biol Chem 281 35245 35252
10. MurayamaYYoshiokaMHoriiHTakataMYokoyamaT 2006 Protein misfolding cyclic amplification as a rapid test for assessment of prion inactivation. Biochem Biophys Res Commun 348 758 762
11. DeleaultNRHarrisBTReesJRSupattaponeS 2007 Formation of native prions from minimal componenets in vitro. Proc Natl Acad Sci U S A 104 9741 9746
12. WangFWangXYuanCGMaJ 2010 Generating a Prion with Bacterially Expressed Recombinant Prion Protein. Science 327 1132 1135
13. HoriuchiMPriolaSAChabryJCaugheyB 2000 Interactions between heterologous forms of prion protein: binding, inhibition of conversion, and species barriers. Proc Natl Acad Sci U S A 97 5836 5841
14. LauALYamAYMichelitschMMWangXGaoC 2007 Characterization of prion protein (PrP)-derived peptides that discriminate full-length PrPSc from PrPC. Proc Natl Acad Sci U S A 104 11551 11556
15. MoronciniGKanuNSolforosiLAbalosGTellingGC 2004 Motif-grafted antibodies containing the replicative interface of cellular PrP are specific for PrPSc. Proc Natl Acad Sci U S A 101 10404 10409
16. SolforosiLBellonASchallerMCruiteJTAbalosGC 2007 Toward molecular dissection of PrPC-PrPSc interactions. J Biol Chem 282 7465 7471
17. RiekRHornemannSWiderGGlockshuberRWuthrichK 1997 NMR characterization of the full-length recombinant murine prion protein, mPrP(23–231). FEBS Lett 413 282 288
18. ZahnR 2003 The octapeptide repeats in mammalian prion protein constitute a pH-dependent folding and aggregation site. J Mol Biol 334 477 488
19. PeretzDWilliamsonRAKanekoKVergaraJLeclercE 2001 Antibodies inhibit prion propagation and clear cell cultures of prion infectivity. Nature 412 739 743
20. WhiteAREneverPTayebiMMushensRLinehanJ 2003 Monoclonal antibodies inhibit prion replication and delay the development of prion disease. Nature 422 80 83
21. WangFYinSWangXZhaLSyMS 2010 Role of the highly conserved middle region of prion protein (PrP) in PrP-lipid interaction. Biochemistry 49 8169 8176
22. OstapchenkoVGMakaravaNSavtchenkoRBaskakovIV 2008 The polybasic N-terminal region of the prion protein controls the physical properties of both the cellular and fibrillar forms of PrP. J Mol Biol 383 1210 1224
23. SupattaponeSMuramotoTLegnameGMehlhornICohenFE 2001 Identification of two prion protein regions that modify scrapie incubation time. J Virol 75 1408 1413
24. BaumannFTolnayMBrabeckCPahnkeJKlozU 2007 Lethal recessive myelin toxicity of prion protein lacking its central domain. EMBO J 26 538 547
25. LiAChristensenHMStewartLRRothKAChiesaR 2007 Neonatal lethality in transgenic mice expressing prion protein with a deletion of residues 105–125. EMBO J 26 548 558
26. ShmerlingDHegyiIFischerMBlattlerTBrandnerS 1998 Expression of amino-terminally truncated PrP in the mouse leading to ataxia and specific cerebellar lesions. Cell 93 203 214
27. AbalosGCCruiteJTBellonAHemmersSAkagiJ 2008 Identifying key components of the PrPC-PrPSc replicative interface. J Biol Chem 283 34021 34028
28. MakaravaNBaskakovIV 2008 Expression and purification of full-length recombinant PrP of high purity. Methods Mol Biol 459 131 143
29. GeogheganJCMillerMBKwakAHHarrisBTSupattaponeS 2009 Trans-dominant inhibition of prion propagation in vitro is not mediated by an accessory cofactor. PLoS Pathog 5 e1000535
30. CastillaJSaaPMoralesRAbidKMaundrellK 2006 Protein misfolding cyclic amplification for diagnosis and prion propagation studies. Methods Enzymol 412 3 21
31. DeleaultNRKascsakRGeogheganJCSupattaponeS 2010 Species-dependent differences in cofactor utilization for formation of the protease-resistant prion protein in vitro. Biochemistry 49 3928 3934
32. CastillaJMoralesRSaaPBarriaMGambettiP 2008 Cell-free propagation of prion strains. Embo J 27 2557 2566
33. SaborioGPPermanneBSotoC 2001 Sensitive detection of pathological prion protein by cyclic amplification of protein misfolding. Nature 411 810 813
34. ChenBMoralesRBarriaMASotoC 2010 Estimating prion concentration in fluids and tissues by quantitative PMCA. Nat Methods 7 519 520
35. NishinaKADeleaultNRMahalSPBaskakovILuhrsT 2006 The stoichiometry of host PrPC glycoforms modulates the efficiency of PrPSc formation in vitro. Biochemistry 45 14129 14139
36. CastillaJGonzalez-RomeroDSaaPMoralesRDe CastroJ 2008 Crossing the species barrier by PrP(Sc) replication in vitro generates unique infectious prions. Cell 134 757 768
37. AngersRCKangHENapierDBrowningSSewardT 2010 Prion strain mutation determined by prion protein conformational compatibility and primary structure. Science 328 1154 1158
38. LiJBrowningSMahalSPOelschlegelAMWeissmannC 2009 Darwinian Evolution of Prions in Cell Culture. Science 327 869 872
39. DePaceAHSantosoAHillnerPWeissmanJS 1998 A critical role for amino-terminal glutamine/asparagine repeats in the formation and propagation of a yeast prion. Cell 93 1241 1252
40. BrachmannABaxaUWicknerRB 2005 Prion generation in vitro: amyloid of Ure2p is infectious. Embo J 24 3082 3092
41. GloverJRKowalASSchirmerECPatinoMMLiuJJ 1997 Self-seeded fibers formed by Sup35, the protein determinant of [PSI+], a heritable prion-like factor of S. cerevisiae. Cell 89 811 819
42. KingCYDiaz-AvalosR 2004 Protein-only transmission of three yeast prion strains. Nature 428 319 323
43. TanakaMChienPNaberNCookeRWeissmanJS 2004 Conformational variations in an infectious protein determine prion strain differences. Nature 428 323 328
44. AbidKMoralesRSotoC 2010 Cellular factors implicated in prion replication. FEBS Lett 584 2409 2414
45. GrahamJFAgarwalSKurianDKirbyLPinheiroTJ 2010 Low density subcellular fractions enhance disease-specific prion protein misfolding. J Biol Chem 285 9868 9880
46. FischerMRulickeTRaeberASailerAMoserM 1996 Prion protein (PrP) with amino-proximal deletions restoring susceptibility of PrP knockout mice to scrapie. Embo J 15 1255 1264
47. SupattaponeSBosquePMuramotoTWilleHAagaardC 1999 Prion protein of 106 residues creates an artifical transmission barrier for prion replication in transgenic mice. Cell 96 869 878
48. DeleaultAMDeleaultNRHarrisBTReesJRSupattaponeS 2008 The effects of prion protein proteolysis and disaggregation on the strain properties of hamster scrapie. J Gen Virol 89 2642 2650
49. McKinleyMPBoltonDCPrusinerSB 1983 A protease-resistant protein is a structural component of the scrapie prion. Cell 35 57 62
50. AguibYHeisekeAGilchSRiemerCBaierM 2009 Autophagy induction by trehalose counteracts cellular prion infection. Autophagy 5 361 369
51. EnariMFlechsigEWeissmannC 2001 Scrapie prion protein accumulation by scrapie-infected neuroblastoma cells abrogated by exposure to a prion protein antibody. Proc Natl Acad Sci U S A 98 9295 9299
52. LuhrKMNordstromEKLowPLjunggrenHGTaraboulosA 2004 Scrapie protein degradation by cysteine proteases in CD11c+ dendritic cells and GT1-1 neuronal cells. J Virol 78 4776 4782
53. MohanJHopkinsJMabbottNA 2005 Skin-derived dendritic cells acquire and degrade the scrapie agent following in vitro exposure. Immunology 116 122 133
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek A Typhimurium-Typhi Genomic Chimera: A Model to Study Vi Polysaccharide Capsule Function In VivoČlánek SUMO Pathway Dependent Recruitment of Cellular Repressors to Herpes Simplex Virus Type 1 GenomesČlánek A Structural Model for Binding of the Serine-Rich Repeat Adhesin GspB to Host Carbohydrate ReceptorsČlánek Dynamic Evolution of Pathogenicity Revealed by Sequencing and Comparative Genomics of 19 IsolatesČlánek Widespread Endogenization of Genome Sequences of Non-Retroviral RNA Viruses into Plant GenomesČlánek The Cost of Virulence: Retarded Growth of Typhimurium Cells Expressing Type III Secretion System 1Článek A Role for the Chemokine RANTES in Regulating CD8 T Cell Responses during Chronic Viral Infection
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2011 Číslo 7- Stillova choroba: vzácné a závažné systémové onemocnění
- Jak souvisí postcovidový syndrom s poškozením mozku?
- Perorální antivirotika jako vysoce efektivní nástroj prevence hospitalizací kvůli COVID-19 − otázky a odpovědi pro praxi
- Diagnostika virových hepatitid v kostce – zorientujte se (nejen) v sérologii
- Infekční komplikace virových respiračních infekcí – sekundární bakteriální a aspergilové pneumonie
-
Všechny články tohoto čísla
- What Do We Really Know about How CD4 T Cells Control ?
- “Persisters”: Survival at the Cellular Level
- E6 and E7 from Beta Hpv38 Cooperate with Ultraviolet Light in the Development of Actinic Keratosis-Like Lesions and Squamous Cell Carcinoma in Mice
- Selection of Resistant Bacteria at Very Low Antibiotic Concentrations
- The Extracytoplasmic Domain of the Ser/Thr Kinase PknB Binds Specific Muropeptides and Is Required for PknB Localization
- CD39/Adenosine Pathway Is Involved in AIDS Progression
- Hypoxia and a Fungal Alcohol Dehydrogenase Influence the Pathogenesis of Invasive Pulmonary Aspergillosis
- One Is Enough: Effective Population Size Is Dose-Dependent for a Plant RNA Virus
- Effects of Interferon-α/β on HBV Replication Determined by Viral Load
- A Typhimurium-Typhi Genomic Chimera: A Model to Study Vi Polysaccharide Capsule Function In Vivo
- Dual Chaperone Role of the C-Terminal Propeptide in Folding and Oligomerization of the Pore-Forming Toxin Aerolysin
- Rotavirus Stimulates Release of Serotonin (5-HT) from Human Enterochromaffin Cells and Activates Brain Structures Involved in Nausea and Vomiting
- Dissociation of Infectivity from Seeding Ability in Prions with Alternate Docking Mechanism
- The Impact of Recombination on dN/dS within Recently Emerged Bacterial Clones
- The Regulation of Sulfur Metabolism in
- Illumination of Parainfluenza Virus Infection and Transmission in Living Animals Reveals a Tissue-Specific Dichotomy
- A Permeable Cuticle Is Associated with the Release of Reactive Oxygen Species and Induction of Innate Immunity
- A Concerted Action of Hepatitis C Virus P7 and Nonstructural Protein 2 Regulates Core Localization at the Endoplasmic Reticulum and Virus Assembly
- SUMO Pathway Dependent Recruitment of Cellular Repressors to Herpes Simplex Virus Type 1 Genomes
- Re-localization of Cellular Protein SRp20 during Poliovirus Infection: Bridging a Viral IRES to the Host Cell Translation Apparatus
- Divergent Effects of Human Cytomegalovirus and Herpes Simplex Virus-1 on Cellular Metabolism
- A Structural Model for Binding of the Serine-Rich Repeat Adhesin GspB to Host Carbohydrate Receptors
- Transformation of Natural Genetic Variation into Genomes
- EBV Latency Types Adopt Alternative Chromatin Conformations
- Global mRNA Degradation during Lytic Gammaherpesvirus Infection Contributes to Establishment of Viral Latency
- Dynamic Evolution of Pathogenicity Revealed by Sequencing and Comparative Genomics of 19 Isolates
- Microbial Virulence as an Emergent Property: Consequences and Opportunities
- Widespread Endogenization of Genome Sequences of Non-Retroviral RNA Viruses into Plant Genomes
- Structural Basis of Chemokine Sequestration by CrmD, a Poxvirus-Encoded Tumor Necrosis Factor Receptor
- Cross-Species Transmission of a Novel Adenovirus Associated with a Fulminant Pneumonia Outbreak in a New World Monkey Colony
- An Interaction between KSHV ORF57 and UIF Provides mRNA-Adaptor Redundancy in Herpesvirus Intronless mRNA Export
- Elevated 17β-Estradiol Protects Females from Influenza A Virus Pathogenesis by Suppressing Inflammatory Responses
- The Role of IL-15 Deficiency in the Pathogenesis of Virus-Induced Asthma Exacerbations
- Fluorescence Lifetime Imaging Unravels Metabolism and Its Crosstalk with the Host Cell
- Programmed Death (PD)-1-Deficient Mice Are Extremely Sensitive to Murine Hepatitis Virus Strain-3 (MHV-3) Infection
- Hemoglobin Promotes Nasal Colonization
- Crystallography of a Lewis-Binding Norovirus, Elucidation of Strain-Specificity to the Polymorphic Human Histo-Blood Group Antigens
- The Cost of Virulence: Retarded Growth of Typhimurium Cells Expressing Type III Secretion System 1
- A Genome-Wide Approach to Discovery of Small RNAs Involved in Regulation of Virulence in
- Requires Glycerol for Maximum Fitness During The Tick Phase of the Enzootic Cycle
- C Metabolic Flux Analysis Identifies an Unusual Route for Pyruvate Dissimilation in Mycobacteria which Requires Isocitrate Lyase and Carbon Dioxide Fixation
- A Role for the Chemokine RANTES in Regulating CD8 T Cell Responses during Chronic Viral Infection
- Glycosaminoglycans and Sialylated Glycans Sequentially Facilitate Merkel Cell Polyomavirus Infectious Entry
- Regulation of Stomatal Tropism and Infection by Light in : Evidence for Coordinated Host/Pathogen Responses to Photoperiod?
- Multiple Translocation of the Effector Gene among Chromosomes of the Rice Blast Fungus and Related Species
- Comparative Genomics Yields Insights into Niche Adaptation of Plant Vascular Wilt Pathogens
- Unique Cell Adhesion and Invasion Properties of O:3, the Most Frequent Cause of Human Yersiniosis
- C-Terminal Region of EBNA-2 Determines the Superior Transforming Ability of Type 1 Epstein-Barr Virus by Enhanced Gene Regulation of LMP-1 and CXCR7
- Novel Chikungunya Vaccine Candidate with an IRES-Based Attenuation and Host Range Alteration Mechanism
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Requires Glycerol for Maximum Fitness During The Tick Phase of the Enzootic Cycle
- Comparative Genomics Yields Insights into Niche Adaptation of Plant Vascular Wilt Pathogens
- The Role of IL-15 Deficiency in the Pathogenesis of Virus-Induced Asthma Exacerbations
- “Persisters”: Survival at the Cellular Level
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



