-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Elevated 17β-Estradiol Protects Females from Influenza A Virus Pathogenesis by Suppressing Inflammatory Responses
Studies of the 1918 H1N1 influenza pandemic, the H5N1 avian influenza outbreak, and the 2009 H1N1 pandemic illustrate that sex and pregnancy contribute to severe outcome from infection, suggesting a role for sex steroids. To test the hypothesis that the sexes respond differently to influenza, the pathogenesis of influenza A virus infection was investigated in adult male and female C57BL/6 mice. Influenza infection reduced reproductive function in females and resulted in greater body mass loss, hypothermia, and mortality in females than males. Whereas lung virus titers were similar between the sexes, females had higher induction of proinflammatory cytokines and chemokines, including TNF-α, IFN-γ, IL-6, and CCL2, in their lungs than males. Removal of the gonads in both sexes eliminated the sex difference in influenza pathogenesis. Manipulation of testosterone or dihydrotestosterone concentrations in males did not significantly impact virus pathogenesis. Conversely, females administered high doses of estradiol had a ≥10-fold lower induction of TNF-α and CCL2 in the lungs and increased rates of survival as compared with females that had either low or no estradiol. The protective effects of estradiol on proinflammatory cytokines and chemokines, morbidity, and mortality were primarily mediated by signaling through estrogen receptor α (ERα). In summary, females suffer a worse outcome from influenza A virus infection than males, which can be reversed by administration of high doses of estradiol to females and reflects differences in the induction of proinflammatory responses and not in virus load.
Published in the journal: . PLoS Pathog 7(7): e32767. doi:10.1371/journal.ppat.1002149
Category: Research Article
doi: https://doi.org/10.1371/journal.ppat.1002149Summary
Studies of the 1918 H1N1 influenza pandemic, the H5N1 avian influenza outbreak, and the 2009 H1N1 pandemic illustrate that sex and pregnancy contribute to severe outcome from infection, suggesting a role for sex steroids. To test the hypothesis that the sexes respond differently to influenza, the pathogenesis of influenza A virus infection was investigated in adult male and female C57BL/6 mice. Influenza infection reduced reproductive function in females and resulted in greater body mass loss, hypothermia, and mortality in females than males. Whereas lung virus titers were similar between the sexes, females had higher induction of proinflammatory cytokines and chemokines, including TNF-α, IFN-γ, IL-6, and CCL2, in their lungs than males. Removal of the gonads in both sexes eliminated the sex difference in influenza pathogenesis. Manipulation of testosterone or dihydrotestosterone concentrations in males did not significantly impact virus pathogenesis. Conversely, females administered high doses of estradiol had a ≥10-fold lower induction of TNF-α and CCL2 in the lungs and increased rates of survival as compared with females that had either low or no estradiol. The protective effects of estradiol on proinflammatory cytokines and chemokines, morbidity, and mortality were primarily mediated by signaling through estrogen receptor α (ERα). In summary, females suffer a worse outcome from influenza A virus infection than males, which can be reversed by administration of high doses of estradiol to females and reflects differences in the induction of proinflammatory responses and not in virus load.
Introduction
Males and females differ in their responses to infection with many viral pathogens, including human immunodeficiency virus (HIV), herpes simplex viruses, and hantaviruses [1]. Although societal and behavioral factors can influence exposure to viruses and access to vaccines and treatments for infection [2], genetic and physiological differences between the sexes can cause differential immune responses to viruses [3]. Because females tend to mount higher innate [4], [5], cell-mediated [5], [6], [7], and humoral [8] immune responses than males, viral loads are often reduced among females [1]. Heightened immunity in females also can lead to the development of immunopathology following viral infection [5]. Elevated immunity in females represents a balance between immune responses conferring protection and causing pathology.
Growing evidence links sex differences in immune function with circulating sex steroid hormones [9], [10]. Receptors for sex steroids are expressed in a variety of lymphoid cells [11], [12]. Androgens, including dihydrotestosterone (DHT) and testosterone (T), suppress the activity of immune cells [9], [13], [14]. Estradiol (E2) can have divergent effects, with low doses enhancing proinflammatory cytokine production (e.g., IL-1, IL-6, and TNF-α) and T helper cell type 1 (Th1) responses and high or sustained concentrations reducing production of proinflammatory cytokines and augmenting Th2 responses and humoral immunity [15]. Elevated E2 also attenuates production of CXC chemokine ligand (CXCL)-8, CXCL10, chemokine (C-C motif) ligand 2 (CCL2), and CCL20 and recruitment of leukocytes and monocytes into several tissues, including the lungs [16], [17], [18], [19]. The anti-inflammatory effects of high E2 are mediated by signaling through estrogen receptors (ERs), which inhibits activation of NF-κB-mediated inflammatory responses [20].
Observational data for influenza reveal that the outcome of pandemic influenza as well as avian H5N1 is generally worse for young adult females [21]. In the United States, during the 1957 H2N2 pandemic, mortality was higher among females than males 1–44 years of age [22]. Worldwide as of 2008, females were 1.6 times less likely to survive H5N1 infection than males [23]. During the 2009 H1N1 pandemic, a significant majority of patients hospitalized with severe 2009 H1N1 disease were young adult females (15–49 years of age) [21], [24], [25], [26], [27], [28]. Pregnancy and other risk factors (e.g., asthma and chronic obstructive pulmonary disorder) contribute to the severity of disease in females [21].
The mechanisms mediating how the sexes differ in response to influenza virus infection as well as the effects of sex steroids on influenza pathogenesis remain largely undefined. We hypothesize that biological differences in immune responses may explain variation between the sexes during influenza virus infection. Several studies reveal that excessive proinflammatory responses (i.e., the cytokine storm) contribute significantly to morbidity and mortality from influenza virus infection [29], [30], [31], [32], [33]. Our data reveal that females experience greater morbidity and mortality than males, which can be reversed by administration of exogenous E2 or an ERα agonist to females. Our data further indicate that sex differences and the effects of E2 on influenza pathogenesis reflect differences in the production of proinflammatory cytokines and chemokines as opposed to differences in virus load.
Results
Morbidity and mortality from H1N1 influenza is greater in females than males
To examine whether the sexes respond differently to influenza A virus infection, adult male and female C57BL/6 mice were inoculated with 102 TCID50 of influenza A/Puerto Rico/8/1934 (PR8; H1N1) and monitored daily for changes in morbidity and mortality for 21 days. Females showed a greater percent reduction in body mass (Fig. 1A) and body temperature (Fig. 1B) than males, with these differences being most pronounced 7–13 days post-inoculation (p.i.) (MANOVA sex x day P<0.0001 in each case). Survival following influenza infection was significantly reduced in females compared with males (log rank P<0.001), in which no females survived infection with PR8, whereas 47% of the males survived through 21 days p.i. (Fig. 1C; Χ2 P<0.05). The average day of death was 2 days earlier for females (9.4±0.6 days) than males (11.5±0.7 days) (t-test P<0.05).
Fig. 1. Influenza virus infection causes greater morbidity and mortality in females than males. 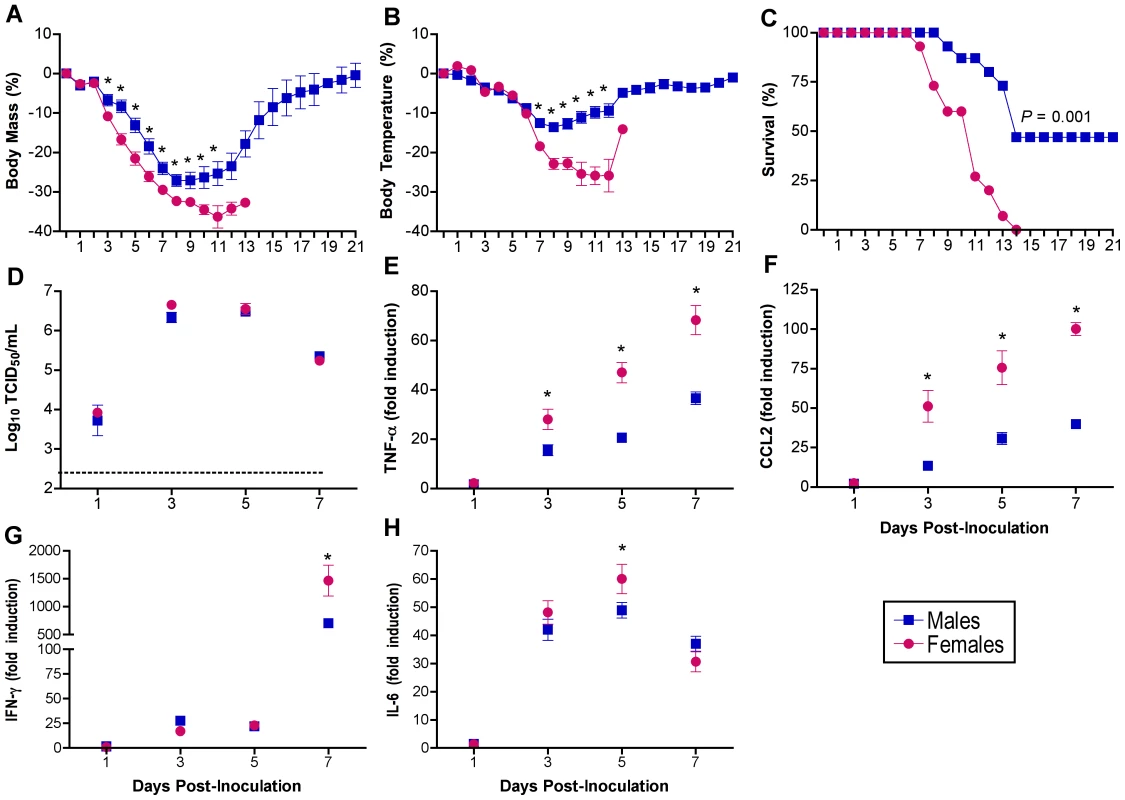
Male and female mice were inoculated with PR8 and monitored for changes in body mass (A), rectal temperature (B), and survival (C) for up to 21 days (n = 15/sex). Infectious virus titers (D) as well as concentrations of TNF-α (E), CCL2 (F), IFN-γ (G), and IL-6 (H) were measured in homogenates of lungs removed 0, 1, 3, 5, or 7 days p.i. (n = 10–15/sex/time-point). Fold change represents concentrations of proteins at Days 1–7 p.i. relative to concentrations at Day 0. Data represent the mean ± SEM. The dotted line in panel D represents the limit of detection for the assay. Significant differences between the sexes as determined by post hoc analyses at each time-point are represented by an asterisk (*), P<0.05. Titers of infectious virus peaked 3–5 days after infection, but sex differences were not observed (Fig. 1D), suggesting that changes in virus load alone were not responsible for the observed sex differences in morbidity and mortality. Highly pathogenic influenza viruses cause severe disease by initiating profound proinflammatory cytokine and chemokine responses [29], [33]. Inflammatory cytokine responses increased in both sexes, in a time-dependent manner as documented previously [30], [34], [35], [36]. Interleukin-1β, IL-12p70, IL-10, and TGF-β were induced within 24 h p.i.; IFN-β, IL-6, TNF-α, CCL2, and CCL3 were induced within 72 h p.i.; and IFN-γ and IL-10 were induced 7 days p.i. in both sexes (Table S1; 2-way ANOVAs, main effect of day P<0.05). Females showed a greater induction of CCL2, TNF-α, IFN-γ, and IL-6 than males (Fig. 1E-H, 2-way ANOVAs sex x day P<0.05).
Influenza virus infection suppresses reproductive function in both sexes
Inflammatory immune responses induced by fatal infection with pathogens (e.g., HIV) affect the brain to reduce reproductive function, appetite, and thermoregulation [37], [38], which in mice can result in greater suppression of reproductive activity in females than males [39]. To evaluate the effects of PR8 infection on reproductive physiology, T concentrations in males and E2 concentrations in females were evaluated in plasma samples collected from separate mice at several time-points during the first week p.i. Infection of males reduced circulating T concentrations on days 3–7 p.i. as compared with uninfected males (Fig. 2A, 1-way ANOVA P<0.05).
Fig. 2. Influenza virus infection alters sex steroid concentrations and reproductive function in males and females. 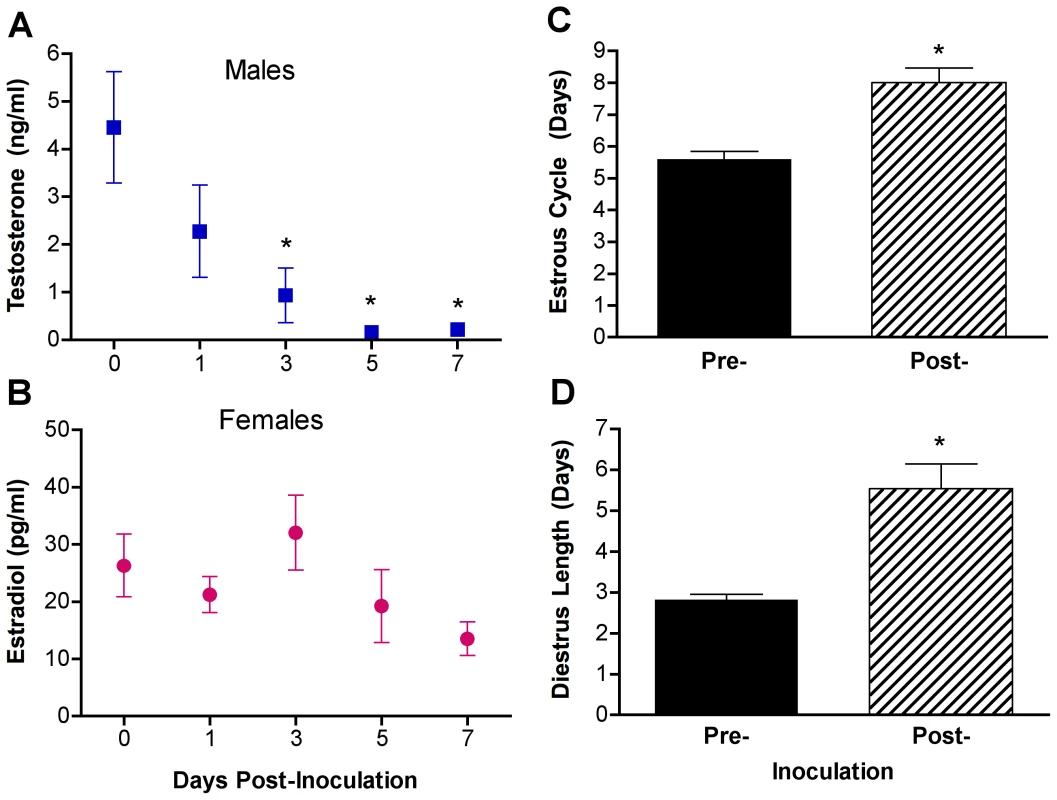
Male and female mice (n = 10/sex/time-point) were inoculated with PR8, serum was collected, and T concentrations in males (A) and E2 concentrations in females (B) were analyzed Days 0, 1, 3, 5, or 7 p.i. To assess estrous cycles, females (n = 16) were sampled daily by vaginal lavage before and after inoculation with PR8 and the duration of the estrous cycle (C) and diestrus (D) was quantified. For sex steroid hormone measurements, plasma from uninfected animals were collected at the same time as other samples and designated as Day 0 p.i. Data represent the mean ± SEM. Significant differences between Day 0 and other time-points p.i. based on post hoc analyses are represented by an asterisk (*), P<0.05. In females, infection with PR8 appeared to cause persistently low E2 concentrations (Fig. 2B); single time-point sampling of cyclical hormones, however, is difficult to accurately evaluate in females [40]. To better characterize the hormonal milieu of females during influenza virus infection, we monitored the estrous cycles of female mice before and after infection with PR8. The average duration of the estrous cycle increased significantly following influenza virus infection (Fig. 2C; paired t-test P<0.05) and this increase in estrous cycle length was attributed to an increase in the duration of diestrus (Fig. 2D; paired t-test P<0.05). As diestrus is the stage of the estrus cycle that corresponds with the follicular phase, when both estrogens and progesterone are at their nadir [40], these data suggest that influenza virus infection suppresses ovarian function in females and results in persistently low circulating E2.
Removal of the gonads reduces sex differences in influenza pathogenesis
To establish whether gonadal secretions modulate sex differences in influenza pathogenesis, we compared morbidity and mortality following PR8 infection in gonadally-intact (sham) and gonadectomized (gdx) male and female mice. Consistent with previous data (Fig. 1), hypothermia (MANOVA sex x day P<0.0001) and mortality (log rank P<0.001) following influenza virus infection were greater among gonadally-intact (sham) females than gonadally-intact (sham) males (Fig. 3A and B). Gonadectomy of males resulted in more pronounced hypothermia (MANOVA treatment x day P<0.003) and death (log rank P = 0.04) as compared with gonadally-intact males (Fig. 3A and B). Mortality (log rank P = 0.046), but not morbidity, was lower among gdx than gonadally-intact females during influenza virus infection. Among gdx animals, removal of the gonads in male and female mice eliminated the dimorphism in hypothermia and survival (Fig. 3A and B). In summary, sex differences in influenza pathogenesis are partially mediated by the presence of gonadal secretions.
Fig. 3. Removal of the gonads reduces the sex difference in influenza pathogenesis. 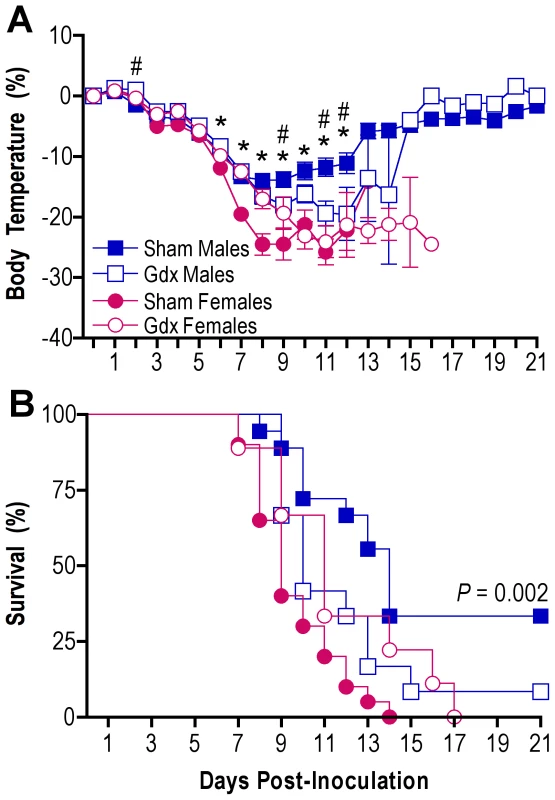
Rectal temperature (A) and survival (B) were monitored for 21 days after inoculation of intact (sham) male (n = 18), sham female (n = 20), gonadectomized (gdx) male (n = 12), or gdx female (n = 9) mice with PR8. Data represent the mean ± SEM. Significant differences between sham males and females as determined by post hoc analyses at each time-point are represented by an asterisk (*), P<0.05. Significant differences between intact and gdx males as determined by post hoc analyses at each time-point are represented by a number symbol (#), P<0.05. Estradiol treatment protects females against lethal influenza infection by suppressing proinflammatory responses
To establish whether androgens in males and estrogens in females affect responses to influenza, we examined the effects of removal and replacement of sex steroids on influenza pathogenesis. Among males, gdx animals that received exogenous T had greater concentrations of circulating T (14.74±0.65 ng/ml) than gdx males (1.00±0.01 ng/ml) (t-test P<0.05). Treatment with either T or DHT did not significantly reverse the effects of gdx on either hypothermia (Fig. 4A) or mortality (Fig. 4C). Titers of PR8 also did not differ among hormonally-manipulated and gonadally-intact males (Fig. 4D). Manipulation of androgens in males affected concentrations of CCL3, IFN-γ, and IL-10, but not in a discernable pattern associated with morbidity and mortality (Table S2; 2-way ANOVAs treatment x day P<0.05).
Fig. 4. Androgen replacement does not significantly affect morbidity or mortality from influenza virus infection in males. 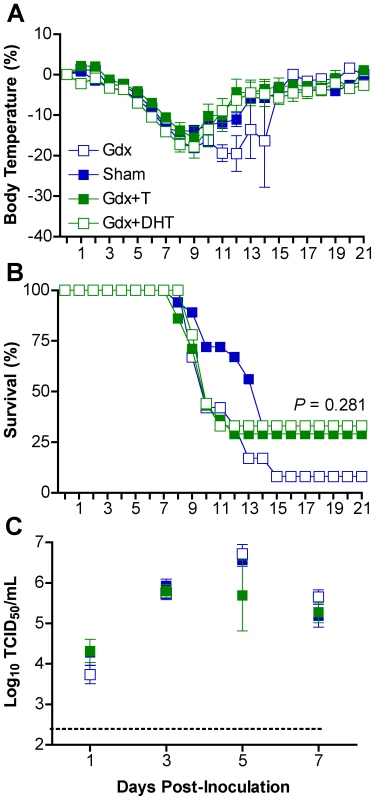
Males were left intact (sham; n = 10–18/time-point/experiment) or gonadectomized (gdx) and implanted with silastic capsules that were empty (n = 9–12/time-point/experiment) or filled with testosterone (T; n = 8–14/time-point/experiment) or dihydrotestosterone (DHT; n = 10). Males were inoculated with PR8 and monitored daily for changes in body temperature (A) and survival (B). Infectious virus titers (C) were measured by TCID50 in lungs removed Days 1, 3, 5, or 7 p.i. The dotted line in panel C represents the limit of detection for the assay. Data represent the mean ± SEM. Among females, those that received exogenous E2 had significantly higher serum concentrations of E2 (978±39 pg/ml) than gdx females (1±26 pg/ml) (t-test P<0.05). Administration of exogenous E2 mitigated hypothermia (Fig. 5A; MANOVA treatment x day P<0.005) and mortality (Fig. 5B; log rank P<0.001) following PR8 infection as compared with gonadally-intact and gdx female mice. Females that received E2 were more likely to survive PR8 infection and those that died had a later day of death (12.8±1.2 days) than did sham (8.6±0.4 days) or gdx (10.7±1.1 days) female mice (1-way ANOVA P<0.05). Administration of E2 did not affect virus replication kinetics (Fig. 5C), but diminished the rise in TNF-α and CCL2 in the lungs that was apparent among gonadally-intact and gdx female mice (Fig. 5D and E; 2-way ANOVA treatment x sex P<0.001). Although hormone manipulation in females altered other cytokines, including IFN-γ, IL-10, IL-12(p70), and CCL3 (Table S3; 2-way ANOVAs treatment x sex P<0.05), the patterns were not associated with changes in morbidity or mortality. In summary, females with low (sham) or no (gdx) circulating E2 suffer a worse outcome from infection and have higher proinflammatory responses than females with high E2.
Fig. 5. Exogenous E2 protects females against influenza virus infection. 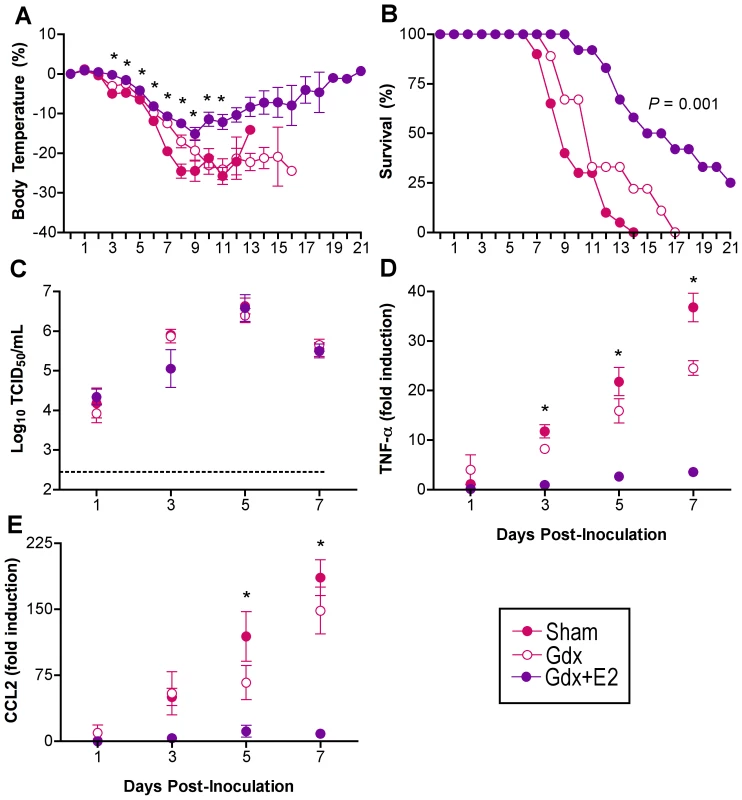
Females were intact (sham; n = 9–20/time-point/experiment), gonadectomized (gdx; n = 7–10/time-point/experiment), or gdx and treated with E2 (n = 8–12/time-point/experiment). Females were inoculated with PR8 and monitored daily for changes in rectal temperature (A) and survival (B). Infectious virus titers (C) as well as concentrations of TNF-α (D) and CCL2 (E) were measured in lungs removed Days 1, 3, 5, or 7 p.i. Fold change represents concentrations of proteins at Days 1–7 p.i. relative to concentrations at Day 0. Data represent the mean ± SEM. The dotted line in panel C represents the limit of detection for the assay. Significant differences between E2-treated and sham or gdx females, as determined by post hoc analyses, are represented by an asterisk (*), P<0.05. Estradiol protects against lethal influenza by signaling through ERα
The anti-inflammatory effects of high E2 are mediated by signaling through two nuclear receptors, ERα and ERβ [41], which antagonizes nuclear factor kappa B (NF-κB) activity [20]. To determine which ER was mediating the effects of E2 on influenza pathogenesis, gdx females were administered E2, vehicle, or vehicle containing agonists specific to ERα (Propylpyrazole-triol; PPT) or ERβ (diarylpropionitrile; DPN). Treatment with the ERα agonist, but not vehicle or the ERβ agonist, reduced hypothermia (Fig. 6A; MANOVA treatment x day P<0.0001) and increased rates of survival (Fig. 6B; log rank P<0.01) to levels that were similar to females treated with E2. Titers of PR8 in the lungs peaked for all females at Day 3 p.i., but were not affected by ER manipulation (data not shown). Treatment with the ERα agonist reduced TNF-α and CCL2 (Fig. 6C and D; 1-way ANOVA P<0.001) in the lungs to levels that were similar to those of females treated with E2. Treatment with the ERβ agonist reduced TNF-α (Fig. 6C; 1-way ANOVA P<0.001), but not CCL2 (Fig. 6D) as compared with vehicle-treated females. Although administration of the ERα and ERβ agonists altered IL-6 and IL-10, these patterns were not correlated with morbidity and mortality (Table S4; 1-way ANOVAs P<0.05). The effects of E2 on proinflammatory responses to infection, in particular CCL2 responses, and disease outcome are primarily mediated by signaling through ERα.
Fig. 6. Estradiol protects females against influenza through ERα signaling. 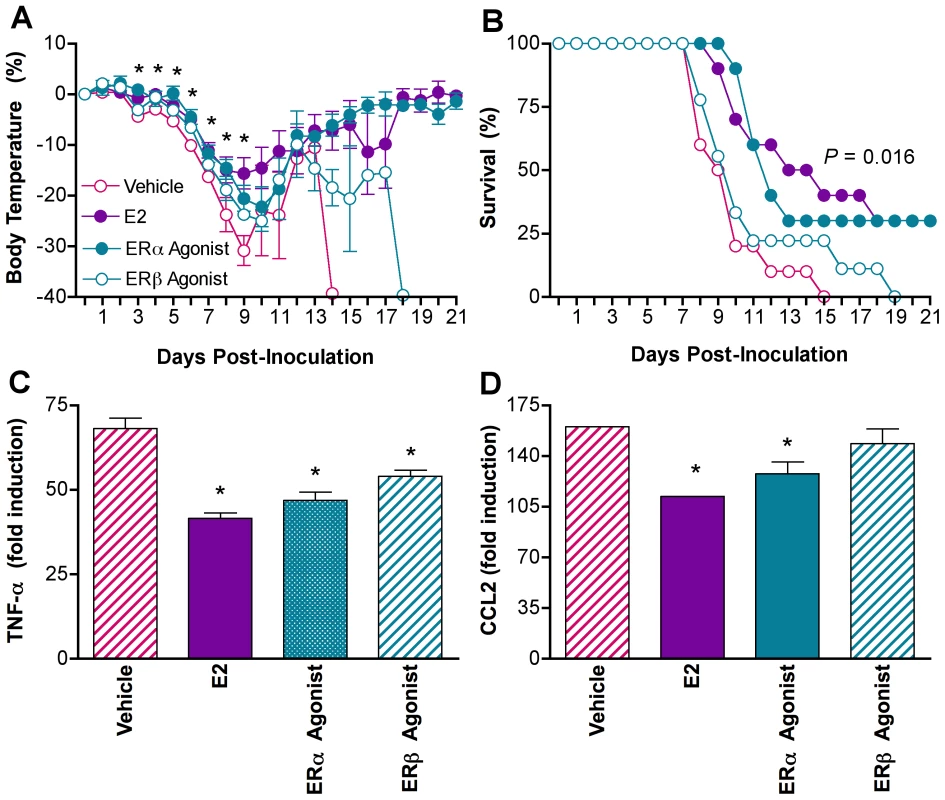
Females were gonadectomized (gdx) and assigned to received vehicle (n = 9–12/time-point/experiment), E2 (n = 9–10/time-point/experiment), the ERα agonist (PPT; n = 9–10/time-point/treatment), or the ERβ agonist (DPN; n = 8–9/time-point/experiment). Females were inoculated with PR8 and monitored daily for changes in rectal temperature (A) and survival (B). Concentrations of TNF-α (C) and CCL2 (D) were quantified in lung homogenates collected from females at Days 0 and 7 p.i. Fold change represents concentrations of proteins at Day 7 p.i. relative to concentrations at Day 0. Data represent the mean ± SEM. Significant differences between treatment groups and vehicle controls, as determined by post hoc analyses, are represented by an asterisk (*), P<0.05. Discussion
Although epidemiological data suggest that females experience more severe disease and suffer a worse outcome from influenza virus infection than males [21], whether these differences reflect sex or gender is difficult to assess as both factors can affect exposure and vulnerability to influenza A viruses [21]. Using a small animal model, our data and data from others [42] illustrate that there are distinct biological differences in how males and females respond to influenza.
Disease associated with highly pathogenic influenza viruses and the clinical manifestations that ensue in humans can be mediated by the proinflammatory response (e.g., TNF-α, IL-6, CCL2, CCL3, and CXCL10) initiated by the host in response to infection [29], [30], [31], [32], [33]. Studies of patients infected with avian influenza viruses further reveal that higher proinflammatory responses are correlated with mortality during infection [33]. Elevated production of CCL3 and CCL2 and expression of CCR2 recruit monocytes and neutrophils into the lungs and regulate inflammation and influenza A virus replication [43]. The data from the present study illustrate that inflammatory immune responses, including induction of CCL2, IFN-γ, IL-6, and TNF-α, are elevated in the lungs of females compared with males. Infectious virus titers, however, do not differ between the sexes and are not altered by hormones. Similarly, infection of adult BALB/c mice with a mouse-adapted H3N1 influenza A virus results in greater lung hyperresponsiveness to methacholine challenge and production of CCL2, but not virus titers, in females compared with males [42]. These data support and extend the hypothesis that host-mediated immunopathology rather than virus replication underlies influenza pathogenesis.
Sex differences in disease outcome are likely mediated by multiple factors, including sex steroids, glucocorticoids, and the direct activity of sex chromosomal genes [44]. In the present study, removal of gonadal secretions in both males and females reduced the sex difference in morbidity and mortality, illustrating that the sex difference in influenza pathogenesis is reversible and that activational sex steroids in adulthood affect the outcome of infection. Furthermore, sex differences in response to influenza A virus infection are not observed among pre-pubertal mice [42]. Within males, however, manipulation of androgens did not significantly affect influenza pathogenesis, suggesting that some androgenic effects may be organized early during sexual differentiation. The extent to which sex differences in immunity are hard-wired early during development must be considered [44].
Our data reveal that estrogens are one mechanism mediating influenza pathogenesis in females. Infection with influenza virus disrupted reproductive function in gonadally-intact females, resulting in a prolonged state of diestrus, which is the stage of the reproductive cycle when E2 and progesterone concentrations are at their lowest [40]. Gonadectomized females (i.e., females with no circulating E2) and gonadally-intact females (i.e., females with low circulating E2 as a result of infection) produced higher inflammatory responses and suffered a worse outcome from infection than gdx females administered exogenous E2. These data support the hypothesis that low concentrations of E2 in females promote excessive inflammatory responses that contribute to disease pathogenesis [15].
In the present study, exogenous administration of E2 reduced the induction of pulmonary inflammatory responses and protected females against influenza. High doses of estrogens also are protective in animal models of multiple sclerosis (MS), in which supraphysiological doses of estrogens reduce inflammatory responses and progression of this autoimmune disease [45], [46]. In contrast, low cyclical levels of estrogens in gonadally-intact females have little effect on the outcome of MS. High E2 has potent anti-inflammatory actions, including repression of proinflammatory gene transcription and cytokine production [11], [47], which is partially mediated by inhibition of NF-κB transcriptional activity [48]. The anti-inflammatory effects of estrogens have been observed in several models for diseases, including autoimmunity, atherosclerosis, arthritis, inflammatory bowel disease, and asthma [15]; our data reveal that influenza is another disease of public health importance that is influenced by estrogens.
The proinflammatory effects of low or no E2 and the anti-inflammatory effects of high E2 in females are mediated by signaling through the ER, which regulates the activity of NF-κB [20]. Administration of an ERα, but not an ERβ, agonist protected females against influenza infection. ERα has been identified in several immune cells, including DCs, macrophages, and T cells, whereas ERβ is expressed in epithelial cells, macrophages, and B cells [11], [41]. The differential effects of ERα and ERβ agonists in vivo provide insight into the cell types that may be responsible for the exacerbated inflammatory responses observed in influenza-infected females with low or no E2.
Our data suggest a number of important avenues of research that require further investigation. Mechanisms in addition to low circulating E2 likely influence sex differences in influenza pathogenesis and we are actively investigating the effect of other sex-specific factors on viral disease. In addition to the mouse-adapted PR8 (H1N1), sex differences are reported in response to mouse-adapted H3N1 [42] and H3N2 (Lorenzo et al. unpublished data); mouse models utilizing mouse-adapted influenza A viruses, however, may not completely reflect virus pathogenesis in humans. Because clinical isolates of influenza A viruses cause limited pathology in mice [49], [50], [51], examination of sex differences and the effects of sex steroids in response to non-adapted strains of influenza in mice would need to be limited to highly pathogenic viruses such as the 1918 virus strain and avian H5N1 viruses. Alternatively, sex differences in response to infection with clinical isolates of influenza A viruses could be evaluated in alternative animal models, such as ferrets [49]. The use of mouse-adapted strains of influenza to demonstrate sex differences and effects of sex steroids on influenza pathogenesis in mice reveal significant differences and suggest that these differences should be considered in evaluations of epidemiological and clinical human data. The observation that elevated E2 reduces, rather than elevates, the severity of influenza A virus infection does not explain why pregnancy is associated with worse outcome after infection. Elevation of E2 concentrations in non-pregnant females does not completely recapitulate pregnancy as several other hormones, including progesterone, estriol, and glucocorticoids also dramatically change during pregnancy and can impact immune function [52], [53]. While sex steroid-modulation of influenza pathogenesis likely contributes to the increased severity of disease during pregnancy, the data from the current study suggest that E2 is not the mechanism mediating severe outcome of infection during pregnancy.
There is clinical relevance to uncovering the mechanisms mediating how sex and sex steroids affect responses to influenza viruses as this may result in preventative measures and treatments that are optimized for each sex. Most epidemiological and clinical studies of influenza in humans do not partition or analyze data by sex and a majority of animal studies of influenza either use only females or do not report the sex of their animals [21]. The data from the present study provide evidence that the pathogenesis of influenza virus infection differs between the sexes and is influenced by the effects of sex hormones on inflammatory immune responses.
Materials and Methods
Animals
Adult (6–8 weeks old) male (total n = 308) and female (total n = 356) C57BL/6 mice were purchased from NCI Frederick, housed 5/microisolater cage with food and water available ad libitum, and handled using Biosafety Level (BSL)-2 practices.
Ethics statement
All experiments were performed in compliance with the standards outlined in the National Research Council's Guide to the Care and Use of Laboratory Animals. The animal protocol (MO09H26) was reviewed and approved by the Johns Hopkins University Animal Care and Use Committee. All efforts were made to minimize animal suffering.
Surgical procedures
Male and female mice were anesthetized with 2.5% isoflurane (Baxter Healthcare Corporation, Deerfield, IL) mixed with oxygen and bilaterally gonadectomized as previously described [54], [55], [56]. All animals were given two weeks to recover prior to infection.
Vaginal cell cytology
Vaginal cell samples were collected at 1600–1700 h, smeared onto clean glass slides, fixed, stained with Diff-Quick Staining kit (Andwin Scientific, Addison, IL), and diagnosed for stage of estrus based on the cellular profile of each sample: proestrus (80–100% intact, healthy epithelial cells), estrus (100% cornified epithelial cells), diestrus I (∼50% cornified epithelial cells and 50% leukocytes), and diestrus II (80–100% leukocytes) [57], [58]. Only females that exhibited at least 3 regular estrous cycles (16/20) prior to infection were included.
Sex hormone capsules
Hormone and placebo capsules were made with 10 mm of silastic tubing (inner diameter = 0.04 in; outer diameter = 0.085 in; VWR International, Bridgeport, NJ) and sealed with silastic medical adhesive (Dow Corning, Midland, MI). Hormone capsules were left empty (placebo) or filled with 5 mm of testosterone (T), dihydrotestosterone (DHT), or 17β-estradiol (E2) purchased from Sigma Aldrich (St. Louis, MO) and sealed with 2.5 mm of adhesive at either end [56]. Capsules were incubated in 0.9% saline at 37°C overnight prior to implantation.
Estrogen receptor agonists
Propylpyrazole-triol (PPT) and diarylpropionitrile (DPN) were purchased from Tocris Bioscience (Ellisville, MO), suspended in Miglyol 812N oil (kindly provided by Sasol, Hamburg, Germany), and administered at 10 mg/kg and 8 mg/kg, respectively [59]. Animals received daily subcutaneous injections of either vehicle (90% Miglyol, 10% EtOH) or vehicle containing PPT or DPN.
Sample collection
For experiments in which morbidity and mortality were monitored for up to 21 days post-inoculation, animals were not euthanized as death was an approved endpoint in our IACUC protocol. Body mass and rectal temperature were measured daily between 0800 and 1000 h. Animals were weighed to the nearest hundredth of a gram and rectal temperature was monitored with a Thermalert TH-5 monitor (25°C–45°C) and RET-3 rectal microprobe for mice (Physitemp Instruments, Inc., NJ), which stably evaluates body temperature to the nearest 0.1°C in 3–5 seconds. For time course experiments, animals were randomly assigned to be euthanized at 0, 1, 3, 5, or 7 days p.i., at which time they were anesthetized with isoflurane and terminally bled from the retro-orbital sinus into heparinized tubes and plasma was stored at −80°C and used to measure hormones. Whole lungs were collected, snap-frozen, and stored at −80°C until homogenized in Dulbecco's Modified Eagles Medium (DMEM) supplemented with 1% Penicillin/Streptomycin and 1% L-glutamine (Invitrogen, Carlsbad, CA). Homogenates were centrifuged and the supernatants were stored at −80°C and used to measure virus titers and cytokine concentrations.
Virus infection and quantification
Mouse-adapted influenza A virus, A/Puerto Rico/8/34 (PR8) was provided by Dr. Maryna Eichelberger at the Food and Drug Administration. Mice were anesthetized with Ketamine/Xylazine (80 mg/kg and 6 mg/kg, respectively) and intranasally inoculated with 30 µl of vehicle (DMEM) or 102 50% tissue culture infective dose (TCID50) units of PR8 diluted in DMEM (which corresponds to 1.24 50% mouse lethal dose (MLD50) for males and 0.78 MLD50 for females, based on the Reed and Meunch method) between 0800 and 1000 h. The challenge dose was selected based on previous experiments that quantified the lethal dose that killed 50% of the animals (LD50). Virus quantification was performed using the TCID50 method measuring cytopathic effects (CPE) of influenza A virus on a monolayer of Madin Darby Canine Kidney (MDCK) cells [60].
Sex hormone auantification
Hormones were concentrated from plasma by ether extraction and hormone quantification was performed using testosterone and estradiol enzyme immunosorbant assays (EIA) purchased from Cayman Chemicals (Ann Arbor, MI).
Cytokine quantification
Supernatants from lung homogenates were used to measure CCL3, IFN-β, IL-1β, and TGF-β by ELISAs (R&D Systems, BD Biosciences, PBL Biomedical Laboratories) and CCL2, IL-12(p70), TNF-α, INF-γ, IL-10, and IL-6 with the mouse inflammation cytometric bead array (BD Biosciences).
Statistical analysis
Kaplan Meier survival curves were compared using log rank analyses. The proportion of animals that survived influenza A virus infection was compared among experimental groups using χ2 analyses. Morbidity data were analyzed with multivariate ANOVAs (MANOVAs) with one within-subjects variable (days) and one between-subjects variable (sex or treatment) and significant interactions were further analyzed using planned comparisons. Virus titers and protein concentrations were analyzed with 2-way ANOVAs with day p.i. and sex/treatment as the independent variables and significant interactions were further analyzed using the Bonferroni method for pairwise multiple comparisons. Hormone concentrations were analyzed by t-tests or 1-way ANOVAs followed by Bonferroni post hoc tests. Changes in estrous cycle length were evaluated using paired t-tests. Mean differences were considered statistically significant if P<0.05 (SYSTAT 13, Systat Software, Chicago, IL).
Supporting Information
Zdroje
1. KleinSLHuberS 2010 Sex differences in susceptibility to viral infection. KleinSLRobertsCW Sex hormones and immunity to infection Berlin Springer-Verlag 93 122
2. TheilerRNRasmussenSATreadwellTAJamiesonDJ 2008 Emerging and zoonotic infections in women. Infect Dis Clin North Am 22 755 772, vii–viii
3. FishEN 2008 The X-files in immunity: sex-based differences predispose immune responses. Nat Rev Immunol 8 737 744
4. MarriottIHuet-HudsonYM 2006 Sexual dimorphism in innate immune responses to infectious organisms. Immunol Res 34 177 192
5. MeierAChangJJChanESPollardRBSidhuHK 2009 Sex differences in the Toll-like receptor-mediated response of plasmacytoid dendritic cells to HIV-1. Nat Med 15 955 959
6. VillacresMCLongmateJAugeCDiamondDJ 2004 Predominant type 1 CMV-specific memory T-helper response in humans: evidence for gender differences in cytokine secretion. Hum Immunol 65 476 485
7. HewagamaAPatelDYarlagaddaSStricklandFMRichardsonBC 2009 Stronger inflammatory/cytotoxic T-cell response in women identified by microarray analysis. Genes Immun 10 509 516
8. CookIF 2008 Sexual dimorphism of humoral immunity with human vaccines. Vaccine 26 3551 3555
9. RobertsCWWalkerWAlexanderJ 2001 Sex-associated hormones and immunity to protozoan parasites. Clin Microbiol Rev 14 476 488
10. AhmedSAKarpuzogluEKhanD 2010 Effects of sex steroids on innate and adaptive immunity. KleinSLRobertsCW Sex hormones and immunity to infection Berlin Springer-Verlag 19 52
11. GilliverSC 2010 Sex steroids as inflammatory regulators. J Steroid Biochem Mol Biol 120 105 115
12. KovatsSCarrerasEAgrawalH 2010 Sex steroid receptors in immune cells. KleinSLRobertsCW Sex hormones and immunity to infection Berlin Springer-Verlag 53 92
13. D'AgostinoPMilanoSBarberaCDi BellaGLa RosaM 1999 Sex hormones modulate inflammatory mediators produced by macrophages. Ann N Y Acad Sci 876 426 429
14. RettewJAHuet-HudsonYMMarriottI 2008 Testosterone reduces macrophage expression in the mouse of toll-like receptor 4, a trigger for inflammation and innate immunity. Biol Reprod 78 432 437
15. StraubRH 2007 The complex role of estrogens in inflammation. Endocr Rev 28 521 574
16. GiraudSNCaronCMPham-DinhDKitabgiPNicotAB 2010 Estradiol inhibits ongoing autoimmune neuroinflammation and NFkappaB-dependent CCL2 expression in reactive astrocytes. Proc Natl Acad Sci U S A 107 8416 8421
17. WiraCRFaheyJVGhoshMPatelMVHickeyDK 2010 Sex hormone regulation of innate immunity in the female reproductive tract: the role of epithelial cells in balancing reproductive potential with protection against sexually transmitted pathogens. Am J Reprod Immunol 63 544 565
18. SpeyerCLRancilioNJMcClintockSDCrawfordJDGaoH 2005 Regulatory effects of estrogen on acute lung inflammation in mice. Am J Physiol Cell Physiol 288 C881 890
19. ChotirmallSHGreeneCMOglesbyIKThomasWO'NeillSJ 2010 17Beta-estradiol inhibits IL-8 in cystic fibrosis by up-regulating secretory leucoprotease inhibitor. Am J Respir Crit Care Med 182 62 72
20. BiswasDKSinghSShiQPardeeABIglehartJD 2005 Crossroads of estrogen receptor and NF-kappaB signaling. Sci STKE 2005 pe27 pe27
21. KleinSLPekoszAPassarettiCAnkerMOlukoyaP 2010 Sex, gender and influenza. Geneva World Health Organization 1 58
22. SerflingREShermanILHouseworthWJ 1967 Excess pneumonia-influenza mortality by age and sex in three major influenza A2 epidemics, United States, 1957-58, 1960 and 1963. Am J Epidemiol 86 433 441
23. WHO 2008 Update: WHO-confirmed human cases of avian influenza A (H5N1) infection, November 2003-May 2008. Wkly Epidemiol Rec 83 415 420
24. KumarAZarychanskiRPintoRCookDJMarshallJ 2009 Critically ill patients with 2009 influenza A(H1N1) infection in Canada. Jama 302 1872 1879
25. CampbellARodinRKroppRMaoYHongZ 2010 Risk of severe outcomes among patients admitted to hospital with pandemic (H1N1) influenza. CMAJ 182 349 355
26. FieldingJHigginsNGregoryJGrantKCattonM 2009 Pandemic H1N1 influenza surveillance in Victoria, Australia, April - September, 2009. Euro Surveill 14 pii:19368
27. OliveiraWCarmoEPennaGKuchenbeckerRSantosH 2009 Pandemic H1N1 influenza in Brazil: analysis of the first 34,506 notified cases of influenza-like illness with severe acute respiratory infection (SARI). Euro Surveill 14 pii:19362
28. DenholmJTGordonCLJohnsonPDHewagamaSSStuartRL 2010 Hospitalised adult patients with pandemic (H1N1) 2009 influenza in Melbourne, Australia. Med J Aust 192 84 86
29. GuanYPoonLLCheungCYEllisTMLimW 2004 H5N1 influenza: a protean pandemic threat. Proc Natl Acad Sci U S A 101 8156 8161
30. SzretterKJGangappaSLuXSmithCShiehWJ 2007 Role of host cytokine responses in the pathogenesis of avian H5N1 influenza viruses in mice. J Virol 81 2736 2744
31. KobasaDJonesSMShinyaKKashJCCoppsJ 2007 Aberrant innate immune response in lethal infection of macaques with the 1918 influenza virus. Nature 445 319 323
32. KashJCTumpeyTMProllSCCarterVPerwitasariO 2006 Genomic analysis of increased host immune and cell death responses induced by 1918 influenza virus. Nature 443 578 581
33. de JongMDSimmonsCPThanhTTHienVMSmithGJ 2006 Fatal outcome of human influenza A (H5N1) is associated with high viral load and hypercytokinemia. Nat Med 12 1203 1207
34. TumpeyTMLuXMorkenTZakiSRKatzJM 2000 Depletion of lymphocytes and diminished cytokine production in mice infected with a highly virulent influenza A (H5N1) virus isolated from humans. J Virol 74 6105 6116
35. BuchweitzJPHarkemaJRKaminskiNE 2007 Time-dependent airway epithelial and inflammatory cell responses induced by influenza virus A/PR/8/34 in C57BL/6 mice. Toxicol Pathol 35 424 435
36. XuTQiaoJZhaoLWangGHeG 2006 Acute respiratory distress syndrome induced by avian influenza A (H5N1) virus in mice. Am J Respir Crit Care Med 174 1011 1017
37. GrinspoonS 2005 Androgen deficiency and HIV infection. Clin Infect Dis 41 1804 1805
38. DantzerRO'ConnorJCFreundGGJohnsonRWKelleyKW 2008 From inflammation to sickness and depression: when the immune system subjugates the brain. Nat Rev Neurosci 9 46 56
39. AvitsurRYirmiyaR 1999 The immunobiology of sexual behavior: gender differences in the suppression of sexual activity during illness. Pharmacol Biochem Behav 64 787 796
40. BeckerJBArnoldAPBerkleyKJBlausteinJDEckelLA 2005 Strategies and methods for research on sex differences in brain and behavior. Endocrinology 146 1650 1673
41. MoraniAWarnerMGustafssonJA 2008 Biological functions and clinical implications of oestrogen receptors alfa and beta in epithelial tissues. J Intern Med 264 128 142
42. LarcombeANFoongREBozanichEMBerryLJGarrattLW 2011 Sexual dimorphism in lung function responses to acute influenza A infection. Influenza and Other Respiratory Viruses DOI:10.1111/j.1750-2659.2011.00236.x
43. LinKLSuzukiYNakanoHRamsburgEGunnMD 2008 CCR2+ monocyte-derived dendritic cells and exudate macrophages produce influenza-induced pulmonary immune pathology and mortality. J Immunol 180 2562 2572
44. ArnoldAP 2009 The organizational-activational hypothesis as the foundation for a unified theory of sexual differentiation of all mammalian tissues. Horm Behav 55 570 578
45. JanssonLOlssonTHolmdahlR 1994 Estrogen induces a potent suppression of experimental autoimmune encephalomyelitis and collagen-induced arthritis in mice. J Neuroimmunol 53 203 207
46. KimSLivaSMDalalMAVerityMAVoskuhlRR 1999 Estriol ameliorates autoimmune demyelinating disease: implications for multiple sclerosis. Neurology 52 1230 1238
47. CvoroATatomerDTeeMKZogovicTHarrisHA 2008 Selective estrogen receptor-beta agonists repress transcription of proinflammatory genes. J Immunol 180 630 636
48. ChadwickCCChippariSMatelanEBorges-MarcucciLEckertAM 2005 Identification of pathway-selective estrogen receptor ligands that inhibit NF-kappaB transcriptional activity. Proc Natl Acad Sci U S A 102 2543 2548
49. MainesTRJayaramanABelserJAWadfordDAPappasC 2009 Transmission and Pathogenesis of Swine-Origin 2009 A(H1N1) Influenza Viruses in Ferrets and Mice. Science 325 484 487
50. BelserJAWadfordDAPappasCGustinKMMainesTR 2010 Pathogenesis of pandemic influenza A (H1N1) and triple-reassortant swine influenza A (H1) viruses in mice. J Virol 84 4194 4203
51. WardAC 1997 Virulence of influenza A virus for mouse lung. Virus Genes 14 187 194
52. TulchinskyDHobelCJYeagerEMarshallJR 1972 Plama estradiol, estriol, and progesterone in human pregnancy. II. Clinical applications in Rh-isoimmunization disease. Am J Obstet Gynecol 113 766 770
53. Szekeres-BarthoJPolgarB 2010 Progesterone, Pregnancy, and Innate Immunity. KleinSLaRCW Sex Hormones and Immunity to Infection Berlin Springer-Verlag 205 226
54. CernetichAGarverLSJedlickaAEKleinPWKumarN 2006 Involvement of gonadal steroids and gamma interferon in sex differences in response to blood-stage malaria infection. Infect Immun 74 3190 3203
55. KleinSLBirdBHGlassGE 2000 Sex differences in Seoul virus infection are not related to adult sex steroid concentrations in Norway rats. J Virol 74 8213 8217
56. SiracusaMCOverstreetMGHousseauFScottALKleinSL 2008 17{beta}-Estradiol Alters the Activity of Conventional and IFN-Producing Killer Dendritic Cells. J Immunol 180 1423 1431
57. HuberSA 2008 Coxsackievirus B3-induced myocarditis: infection of females during the estrus phase of the ovarian cycle leads to activation of T regulatory cells. Virology 378 292 298
58. CohenPEZhuLNishimuraKPollardJW 2002 Colony-stimulating factor 1 regulation of neuroendocrine pathways that control gonadal function in mice. Endocrinology 143 1413 1422
59. Tiwari-WoodruffSMoralesLBLeeRVoskuhlRR 2007 Differential neuroprotective and antiinflammatory effects of estrogen receptor (ER)alpha and ERbeta ligand treatment. Proc Natl Acad Sci U S A 104 14813 14818
60. McCownMFPekoszA 2005 The influenza A virus M2 cytoplasmic tail is required for infectious virus production and efficient genome packaging. J Virol 79 3595 3605
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek A Typhimurium-Typhi Genomic Chimera: A Model to Study Vi Polysaccharide Capsule Function In VivoČlánek SUMO Pathway Dependent Recruitment of Cellular Repressors to Herpes Simplex Virus Type 1 GenomesČlánek A Structural Model for Binding of the Serine-Rich Repeat Adhesin GspB to Host Carbohydrate ReceptorsČlánek Dynamic Evolution of Pathogenicity Revealed by Sequencing and Comparative Genomics of 19 IsolatesČlánek Widespread Endogenization of Genome Sequences of Non-Retroviral RNA Viruses into Plant GenomesČlánek The Cost of Virulence: Retarded Growth of Typhimurium Cells Expressing Type III Secretion System 1Článek A Role for the Chemokine RANTES in Regulating CD8 T Cell Responses during Chronic Viral Infection
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2011 Číslo 7- Stillova choroba: vzácné a závažné systémové onemocnění
- Perorální antivirotika jako vysoce efektivní nástroj prevence hospitalizací kvůli COVID-19 − otázky a odpovědi pro praxi
- Diagnostika virových hepatitid v kostce – zorientujte se (nejen) v sérologii
- Jak souvisí postcovidový syndrom s poškozením mozku?
- Familiární středomořská horečka
-
Všechny články tohoto čísla
- What Do We Really Know about How CD4 T Cells Control ?
- “Persisters”: Survival at the Cellular Level
- E6 and E7 from Beta Hpv38 Cooperate with Ultraviolet Light in the Development of Actinic Keratosis-Like Lesions and Squamous Cell Carcinoma in Mice
- Selection of Resistant Bacteria at Very Low Antibiotic Concentrations
- The Extracytoplasmic Domain of the Ser/Thr Kinase PknB Binds Specific Muropeptides and Is Required for PknB Localization
- CD39/Adenosine Pathway Is Involved in AIDS Progression
- Hypoxia and a Fungal Alcohol Dehydrogenase Influence the Pathogenesis of Invasive Pulmonary Aspergillosis
- One Is Enough: Effective Population Size Is Dose-Dependent for a Plant RNA Virus
- Effects of Interferon-α/β on HBV Replication Determined by Viral Load
- A Typhimurium-Typhi Genomic Chimera: A Model to Study Vi Polysaccharide Capsule Function In Vivo
- Dual Chaperone Role of the C-Terminal Propeptide in Folding and Oligomerization of the Pore-Forming Toxin Aerolysin
- Rotavirus Stimulates Release of Serotonin (5-HT) from Human Enterochromaffin Cells and Activates Brain Structures Involved in Nausea and Vomiting
- Dissociation of Infectivity from Seeding Ability in Prions with Alternate Docking Mechanism
- The Impact of Recombination on dN/dS within Recently Emerged Bacterial Clones
- The Regulation of Sulfur Metabolism in
- Illumination of Parainfluenza Virus Infection and Transmission in Living Animals Reveals a Tissue-Specific Dichotomy
- A Permeable Cuticle Is Associated with the Release of Reactive Oxygen Species and Induction of Innate Immunity
- A Concerted Action of Hepatitis C Virus P7 and Nonstructural Protein 2 Regulates Core Localization at the Endoplasmic Reticulum and Virus Assembly
- SUMO Pathway Dependent Recruitment of Cellular Repressors to Herpes Simplex Virus Type 1 Genomes
- Re-localization of Cellular Protein SRp20 during Poliovirus Infection: Bridging a Viral IRES to the Host Cell Translation Apparatus
- Divergent Effects of Human Cytomegalovirus and Herpes Simplex Virus-1 on Cellular Metabolism
- A Structural Model for Binding of the Serine-Rich Repeat Adhesin GspB to Host Carbohydrate Receptors
- Transformation of Natural Genetic Variation into Genomes
- EBV Latency Types Adopt Alternative Chromatin Conformations
- Global mRNA Degradation during Lytic Gammaherpesvirus Infection Contributes to Establishment of Viral Latency
- Dynamic Evolution of Pathogenicity Revealed by Sequencing and Comparative Genomics of 19 Isolates
- Microbial Virulence as an Emergent Property: Consequences and Opportunities
- Widespread Endogenization of Genome Sequences of Non-Retroviral RNA Viruses into Plant Genomes
- Structural Basis of Chemokine Sequestration by CrmD, a Poxvirus-Encoded Tumor Necrosis Factor Receptor
- Cross-Species Transmission of a Novel Adenovirus Associated with a Fulminant Pneumonia Outbreak in a New World Monkey Colony
- An Interaction between KSHV ORF57 and UIF Provides mRNA-Adaptor Redundancy in Herpesvirus Intronless mRNA Export
- Elevated 17β-Estradiol Protects Females from Influenza A Virus Pathogenesis by Suppressing Inflammatory Responses
- The Role of IL-15 Deficiency in the Pathogenesis of Virus-Induced Asthma Exacerbations
- Fluorescence Lifetime Imaging Unravels Metabolism and Its Crosstalk with the Host Cell
- Programmed Death (PD)-1-Deficient Mice Are Extremely Sensitive to Murine Hepatitis Virus Strain-3 (MHV-3) Infection
- Hemoglobin Promotes Nasal Colonization
- Crystallography of a Lewis-Binding Norovirus, Elucidation of Strain-Specificity to the Polymorphic Human Histo-Blood Group Antigens
- The Cost of Virulence: Retarded Growth of Typhimurium Cells Expressing Type III Secretion System 1
- A Genome-Wide Approach to Discovery of Small RNAs Involved in Regulation of Virulence in
- Requires Glycerol for Maximum Fitness During The Tick Phase of the Enzootic Cycle
- C Metabolic Flux Analysis Identifies an Unusual Route for Pyruvate Dissimilation in Mycobacteria which Requires Isocitrate Lyase and Carbon Dioxide Fixation
- A Role for the Chemokine RANTES in Regulating CD8 T Cell Responses during Chronic Viral Infection
- Glycosaminoglycans and Sialylated Glycans Sequentially Facilitate Merkel Cell Polyomavirus Infectious Entry
- Regulation of Stomatal Tropism and Infection by Light in : Evidence for Coordinated Host/Pathogen Responses to Photoperiod?
- Multiple Translocation of the Effector Gene among Chromosomes of the Rice Blast Fungus and Related Species
- Comparative Genomics Yields Insights into Niche Adaptation of Plant Vascular Wilt Pathogens
- Unique Cell Adhesion and Invasion Properties of O:3, the Most Frequent Cause of Human Yersiniosis
- C-Terminal Region of EBNA-2 Determines the Superior Transforming Ability of Type 1 Epstein-Barr Virus by Enhanced Gene Regulation of LMP-1 and CXCR7
- Novel Chikungunya Vaccine Candidate with an IRES-Based Attenuation and Host Range Alteration Mechanism
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Requires Glycerol for Maximum Fitness During The Tick Phase of the Enzootic Cycle
- Comparative Genomics Yields Insights into Niche Adaptation of Plant Vascular Wilt Pathogens
- The Role of IL-15 Deficiency in the Pathogenesis of Virus-Induced Asthma Exacerbations
- “Persisters”: Survival at the Cellular Level
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



