The Length of Vesicular Stomatitis Virus Particles Dictates a Need for Actin Assembly during Clathrin-Dependent Endocytosis
Microbial pathogens exploit the clathrin endocytic machinery to enter host cells. Vesicular stomatitis virus (VSV), an enveloped virus with bullet-shaped virions that measure 70×200 nm, enters cells by clathrin-dependent endocytosis. We showed previously that VSV particles exceed the capacity of typical clathrin-coated vesicles and instead enter through endocytic carriers that acquire a partial clathrin coat and require local actin filament assembly to complete vesicle budding and internalization. To understand why the actin system is required for VSV uptake, we compared the internalization mechanisms of VSV and its shorter (75 nm long) defective interfering particle, DI-T. By imaging the uptake of individual particles into live cells, we found that, as with parental virions, DI-T enters via the clathrin endocytic pathway. Unlike VSV, DI-T internalization occurs through complete clathrin-coated vesicles and does not require actin polymerization. Since VSV and DI-T particles display similar surface densities of the same attachment glycoprotein, we conclude that the physical properties of the particle dictate whether a virus-containing clathrin pit engages the actin system. We suggest that the elongated shape of a VSV particle prevents full enclosure by the clathrin coat and that stalling of coat assembly triggers recruitment of the actin machinery to finish the internalization process. Since some enveloped viruses have pleomorphic particle shapes and sizes, our work suggests that they may use altered modes of endocytic uptake. More generally, our findings show the importance of cargo geometry for specifying cellular entry modes, even when the receptor recognition properties of a ligand are maintained.
Published in the journal:
. PLoS Pathog 6(9): e32767. doi:10.1371/journal.ppat.1001127
Category:
Research Article
doi:
https://doi.org/10.1371/journal.ppat.1001127
Summary
Microbial pathogens exploit the clathrin endocytic machinery to enter host cells. Vesicular stomatitis virus (VSV), an enveloped virus with bullet-shaped virions that measure 70×200 nm, enters cells by clathrin-dependent endocytosis. We showed previously that VSV particles exceed the capacity of typical clathrin-coated vesicles and instead enter through endocytic carriers that acquire a partial clathrin coat and require local actin filament assembly to complete vesicle budding and internalization. To understand why the actin system is required for VSV uptake, we compared the internalization mechanisms of VSV and its shorter (75 nm long) defective interfering particle, DI-T. By imaging the uptake of individual particles into live cells, we found that, as with parental virions, DI-T enters via the clathrin endocytic pathway. Unlike VSV, DI-T internalization occurs through complete clathrin-coated vesicles and does not require actin polymerization. Since VSV and DI-T particles display similar surface densities of the same attachment glycoprotein, we conclude that the physical properties of the particle dictate whether a virus-containing clathrin pit engages the actin system. We suggest that the elongated shape of a VSV particle prevents full enclosure by the clathrin coat and that stalling of coat assembly triggers recruitment of the actin machinery to finish the internalization process. Since some enveloped viruses have pleomorphic particle shapes and sizes, our work suggests that they may use altered modes of endocytic uptake. More generally, our findings show the importance of cargo geometry for specifying cellular entry modes, even when the receptor recognition properties of a ligand are maintained.
Introduction
Eukaryotic cells internalize constituents of the plasma membrane and extracellular cargos by entrapping them in membrane-bound carriers. The most prominent and well-characterized endocytic carriers are clathrin-coated vesicles (reviewed in [1]–[3]). Coated vesicles transport lipids, proteins, and other essential macromolecules from the cell surface to endosomal organelles. Extensive biochemical and cell biological research supports the following model for conventional coated vesicle formation in higher eukaryotes. The AP-2 clathrin adaptor complex recruits clathrin to the cytosolic leaflet of the plasma membrane and sequesters cargos at the endocytic site [4], [5]. The continued assembly of clathrin into a lattice-like configuration helps deform the underlying membrane and ultimately creates an invagination, or ‘pit’ [2]. Recruitment of the GTPase, dynamin, then facilitates scission of the coated pit from the plasma membrane [6], and clathrin is rapidly removed from the cargo-loaded vesicle by the combined action of the heat shock cognate protein 70 (Hsc70) and its co-chaperone auxilin [7], [8]. The entire process is typically complete within 30–60 s [9], [10].
Coated pits incorporate and internalize soluble cargos of various sizes, such as transferrin (5 nm) [9], [11] and low density lipoproteins (25 nm) [9], [12]. Many viruses and intracellular bacteria are also internalized by the clathrin machinery [9], [13]–[16]. We previously evaluated how cells internalize the 70×200 nm bullet-shaped vesicular stomatitis virus (VSV). We found that VSV internalization occurs through elongated, partially clathrin-coated structures that have longer lifetimes (∼2 min.) than typical endocytic clathrin-coated vesicles and require local actin polymerization for uptake [15]. During VSV internalization, the clathrin coat first assembles as a partially closed dome at one end of the virion [15], [17], and growth of the coat stalls when it encounters the long particle axis. Actin assembly then drives one or more late stage(s) of the internalization process, as recruitment of the actin machinery peaks during completion of clathrin assembly, and pharmacological inhibition of actin polymerization blocks VSV internalization without interfering with clathrin coat assembly [15]. Relatively small, spherical viruses like dengue virus (50 nm) [13] and some influenza A viruses (X-31 strain, ∼120 nm) [14], [18] also enter using a clathrin-dependent route, but it is unclear whether actin function is required for their uptake. Our observations with VSV led us to hypothesize that the physical dimensions of the virion block the ongoing polymerization of clathrin during its uptake, and that the stalled structure recruits regulators of actin assembly whose activity is required to complete the internalization process.
Defective interfering (DI) particles arise spontaneously during virus replication. Such particles depend upon coinfecting helper virus to support their replication but contain all the essential cis-acting regulatory elements for genome replication and assembly. One such well- characterized DI particle of VSV is termed DI-T, which lacks 82% of the viral genome [19], [20]. Since the length of a VSV particle is dictated by the genome size [21], DI-T particles are 75 nm long and appear as truncated bullets by electron microscopy [22]. DI-T particles contain normal proportions of the viral structural proteins [23], including the viral surface glycoprotein (G), which mediates VSV attachment and entry into host cells.
Here we took advantage of significant differences in the physical dimensions of VSV and DI-T to investigate how the geometry of a viral cargo influences the actin-dependency of clathrin internalization. Using live cell fluorescence microscopic imaging, we compared the uptake mechanisms of VSV and DI-T at the single particle level. We report that in contrast to the clathrin- and actin-dependent uptake of VSV, the shorter DI-T particles enter cells through fully coated clathrin carriers that do not require actin dynamics for vesicle budding. These observations highlight the plasticity of the clathrin endocytic system, where clathrin coats serve as a scaffold to direct actin assembly when the clathrin machinery alone is not sufficient to mediate internalization.
Results
Biological properties of DI-T particles
To generate a clonal population of VSV DI-T particles, we recovered DI-T from cDNA (Figure 1A) [24] and amplified the particles by co-infection of cells with VSV [25]. We separated DI-T particles from VSV by rate zonal centrifugation in a sucrose density gradient. Electron microscopic analysis (Figure 1B, C) confirmed that VSV virions measure 70+/−8 nm by 204+/−14 nm (n = 114) [21], while the shorter DI-T particles have a length of 76+/−8 nm (n = 81) [22]. DI-T particles, like VSV, are covered with spike-like projections that correspond to homotrimers of G protein (Figure 1B), and SDS-PAGE analysis of purified particles confirmed that VSV and DI-T particles contain similar ratios of G protein to core virion components (Figure 1D) [23]. The purified stocks of DI-T lacked full-length virions (Figure 1B), with only a single VSV virion observed amongst more than 3,000 DI-T particles. Limited dilutions of the purified DI-T stock contained ∼1x106 plaque forming units of virus per microgram of total viral protein, or 10-5 times fewer infectious particles than for an equivalent protein quantity of VSV particles (not shown). Thus, we have successfully purified relatively homogeneous populations of VSV and DI-T particles, which differ only in their physical dimensions.
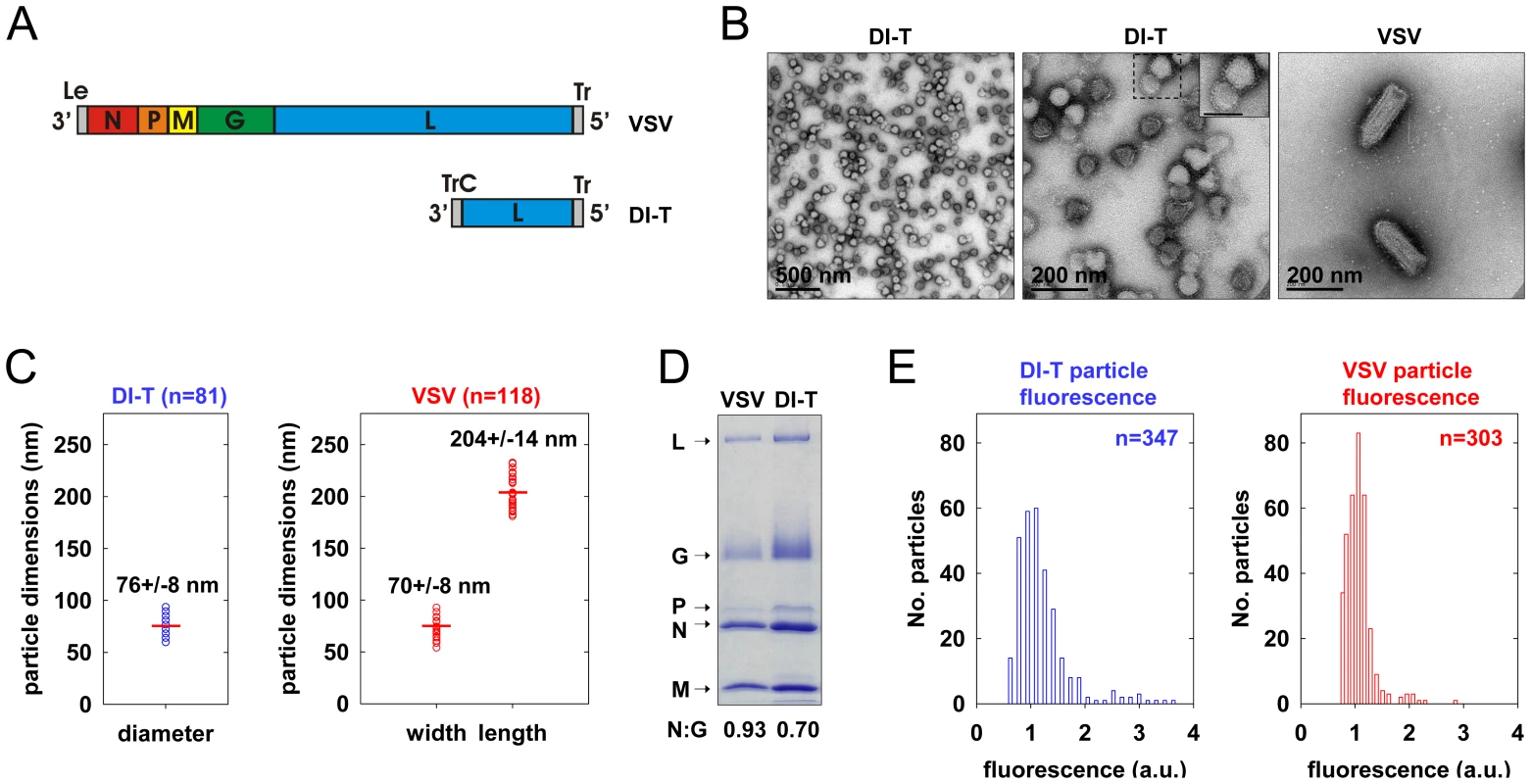
DI-T particles enter cells by clathrin-dependent endocytosis
To visualize VSV and DI-T by fluorescence microscopy, we covalently labeled the G proteins with spectrally separable fluorescent dye molecules (Alexa Fluor 568 and 647, respectively) using conditions that do not reduce viral infectivity [15]. Spinning disk confocal images of labeled particles adsorbed onto glass coverslips showed diffraction-limited objects with single-peaked distributions of fluorescence intensity values (Figure 1E), indicating that the DI-T and VSV populations primarily consist of individual particles [15]. We tracked the entry of DI-T particles into BSC1 cells stably expressing an eGFP-tagged σ2 subunit of the AP-2 adaptor complex (σ2-eGFP), which incorporates into all clathrin-coated structures that form on the plasma membrane [9], [15], [26]. Single DI-T particles readily attached to cells and progressed through the following set of defined events (see Figures 2A, B; Video S1 for examples): (1) membrane-bound DI-T particles diffused slowly (D = 5×10−11–5×10−12 cm2 s−1) and with the random directionality characteristic of Brownian motion; (2) shortly after DI-T attachment, a dim spot of AP-2 signal arose and remained colocalized with the particle, signifying incorporation of DI-T into an assembling clathrin-coated pit; (3) the AP-2 signal steadily increased over time until it peaked as coat assembly completed, and the DI-T particle then underwent an abrupt movement into the cell, after which the AP-2 signal disappeared due to clathrin uncoating. This sequence of events is identical to what we previously observed for VSV entering cells by clathrin-dependent endocytosis [15]. Moreover, the efficiency of DI-T uptake via the clathrin pathway is similar to that of the full-length VSV particles, as 89% (55/62) of DI-T particles that attached to 3 individual cells during imaging entered by clathrin-dependent endocytosis. These data show that DI-T efficiently enters cells through the clathrin pathway and validate the use of DI-T and VSV as comparative endocytic cargos.
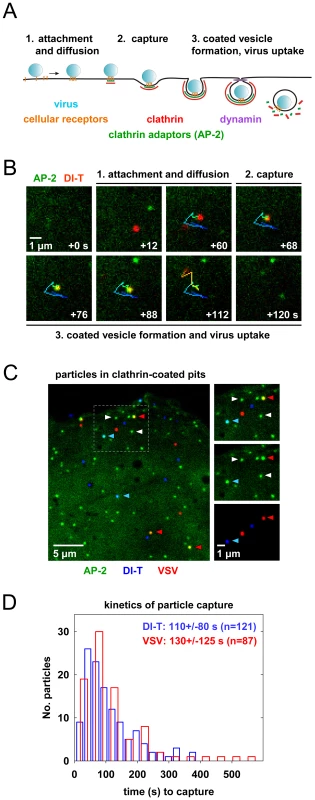
Clathrin structures capture VSV and DI-T particles with similar kinetics
To directly compare how DI-T and VSV particles engage the clathrin machinery, we simultaneously inoculated BSC1 cells with the two spectrally distinct particle forms and then analyzed their mode of incorporation into AP-2 containing clathrin structures on a single cell basis (Figure 2C). For each complete virus uptake event, we quantified the kinetics of particle capture by measuring the elapsed time between virion attachment and the appearance of an AP-2 signal that colocalized with the bound particle. The capture time for DI-T particles was 110+/−80 s (n = 121), which is statistically indistinguishable (Student's t-test, p = 0.2) to that measured for VSV (130+/−125 s (n = 87)) and agrees well with our prior measurements (Figure 2D) [15]. The AP-2 structures that captured DI-T or VSV initiated within a ∼250 nm zone (the resolution limit of the optical system) of the attached particle. We therefore conclude that DI-T and VSV particles engage the clathrin system in an indistinguishable manner, which likely reflects a shared mechanism triggered by the same viral glycoprotein-receptor interactions.
Cells internalize DI-T particles using conventional clathrin-coated vesicles
To investigate the characteristics of the clathrin coat responsible for DI-T internalization, we imaged the uptake of both particle forms by the same BSC1 cell. We found that DI-T particles are internalized through AP-2 containing structures significantly faster than full-length virions (Figure 3A, B; Video S2). Quantitative analysis of data compiled from 4 cells showed that pits incorporating DI-T (n = 36) form in 43+/−14 s, which is similar to the assembly kinetics of pits that lack virus particles (n = 212, 35+/−10 s) (Figure 3C, Table 1). As expected, AP-2 structures that capture VSV (n = 29) require longer (75+/−22 s) to complete (Figure 3C, Table 1). A similar analysis conducted in cells transiently expressing eGFP-tagged clathrin light chain A1 (eGFP-LCa) yielded analogous results (Figure 3D, E, Table 1, Videos S3, S4). The interaction of full-length VSV with a cell had no impact on the uptake kinetics of DI-T into the same cell (Table 1).
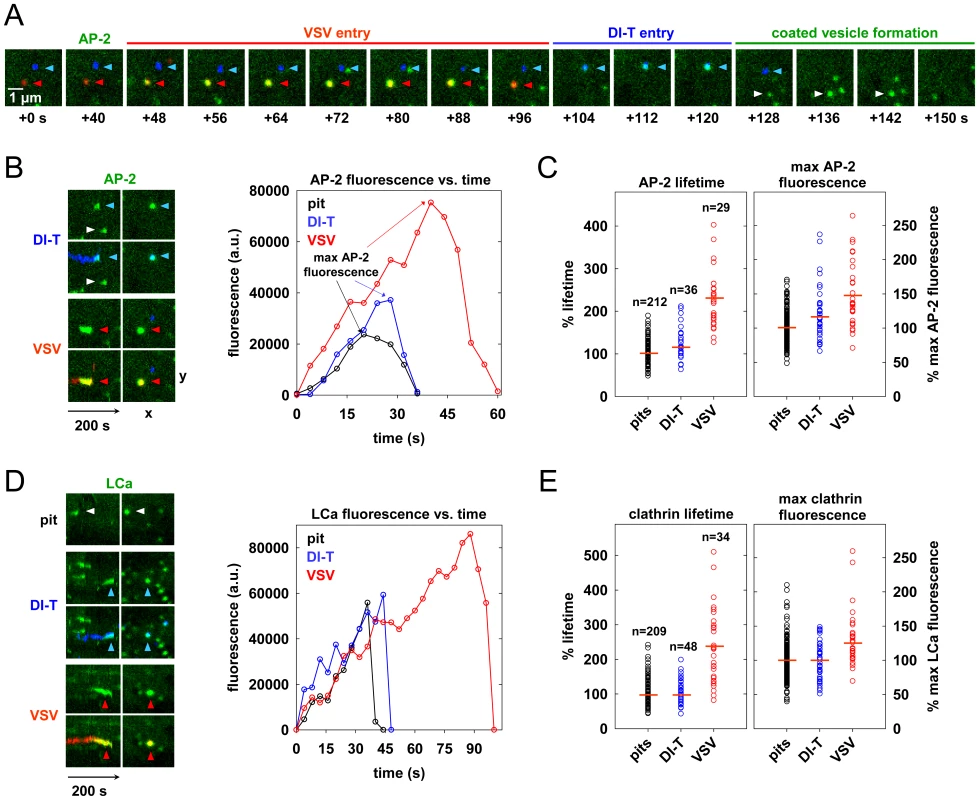
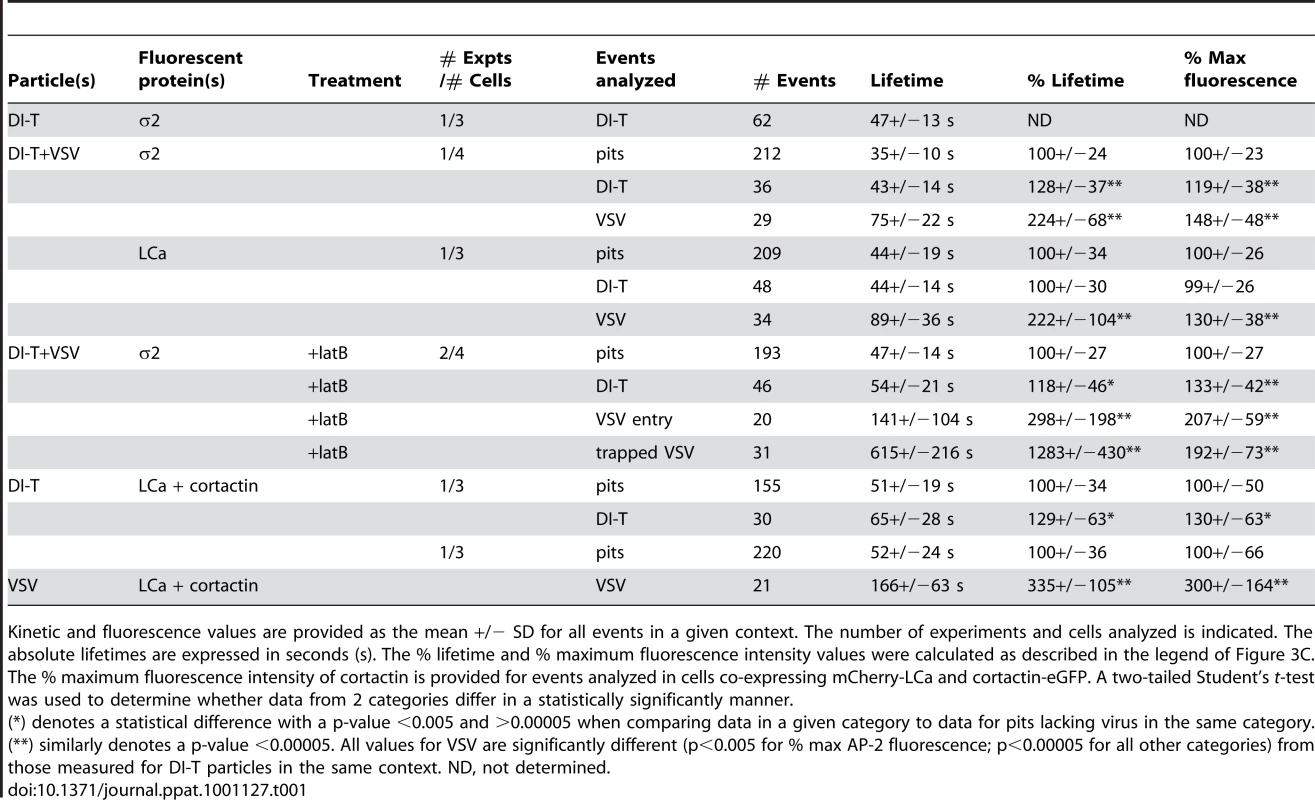
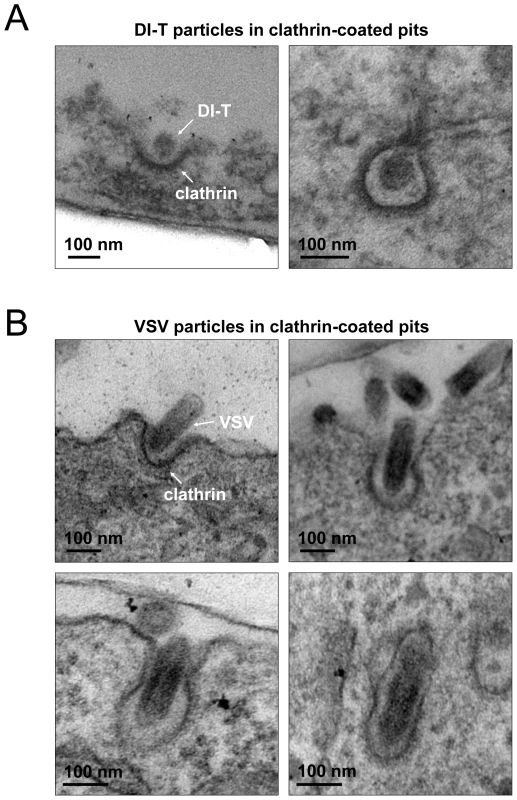
The different kinetics of DI-T versus VSV internalization suggests that DI-T enters cells through conventional fully coated clathrin structures and not the partially coated vesicles responsible for VSV uptake. To further investigate this possibility, we measured the maximum fluorescent signal of eGFP-tagged AP-2 or clathrin molecules, as this peak signal is known to be proportional to the overall size of a clathrin coat [9], [15], [26]. We compared this value for structures that contained DI-T with those that contained VSV or lacked either particle. Similar quantities of coat components were present in structures associated with DI-T to those lacking viral particles (Figure 3C, E, Table 1). As expected, VSV-containing structures accumulate more AP-2 and LCa molecules than structures lacking virus (Figure 3C, E, Table 1). Taken together, the above experiments suggest that DI-T enters cells through pits that acquire a full clathrin coat. Consistent with this, electron micrographs of DI-T particles captured during cell entry show particles present in circular pits entirely surrounded by a clathrin coat (Figure 4A). This is in marked contrast to the partial clathrin coat found at one end of the endocytic carriers that internalize VSV (Figure 4B) [15].
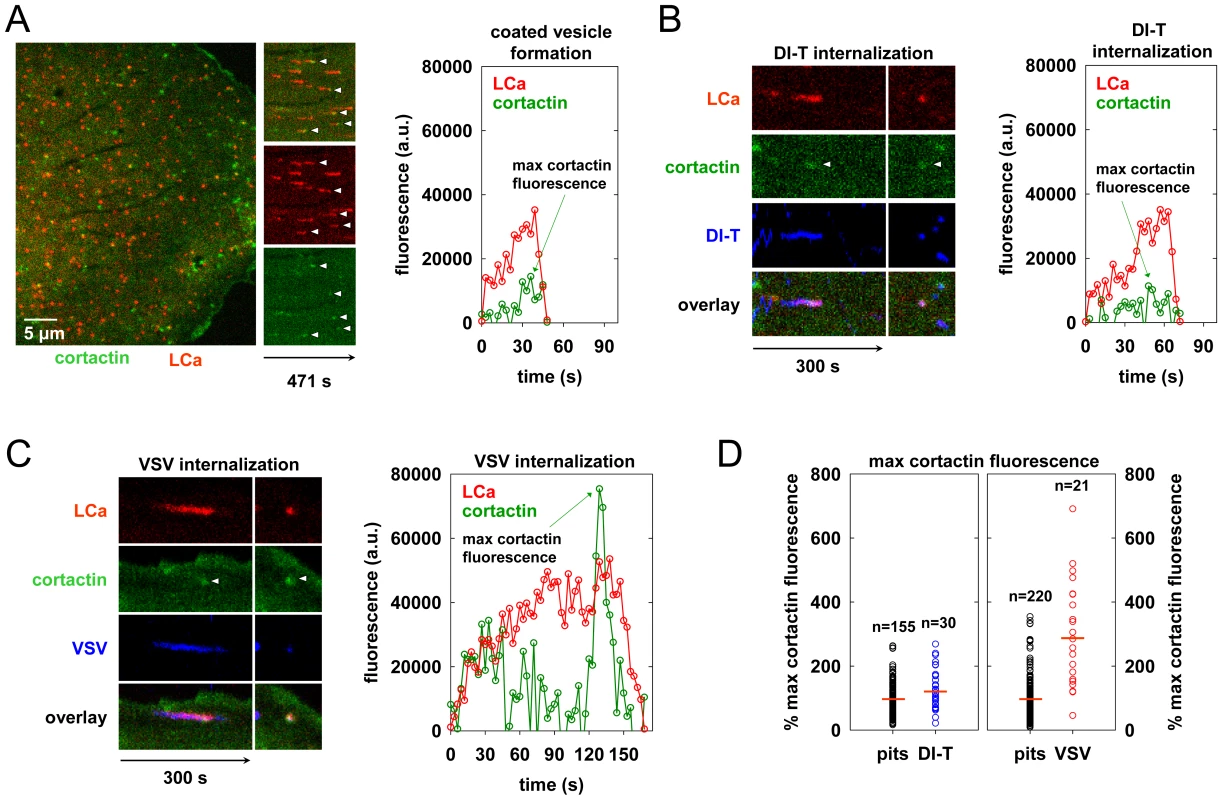
Clathrin structures containing VSV recruit more cortactin that pits that internalize DI-T
During the final phase of coat assembly, endocytic clathrin structures associated with VSV show a strong recruitment of cortactin, an F-actin and dynamin binding protein that activates Arp2/3-mediated assembly of branched actin filaments [15], [27], [28]. To determine whether DI-T uptake is associated with an acute recruitment of cortactin, we monitored internalization into BSC1 cells transiently co-expressing monomeric Cherry-LCa (mCherry-LCa) and low levels of cortactin-eGFP. As previously shown [15], [26], [29], conventional clathrin-coated pits exhibit minimal cortactin recruitment that typically peaks just before completion of clathrin assembly (Figure 5A, Video S5). Cortactin recruitment is similarly sparse during the uptake of DI-T particles (Figure 5B, Video S6). In marked contrast, and as expected [15], large bursts of cortactin accompany the internalization of VSV (Figure 5C, Video S7). Quantitative analysis revealed that the peak fluorescence intensity of cortactin detected in the late phase of VSV uptake averaged 3-fold higher than the signal associated with pits containing DI-T or pits that did not capture a virus particle (Figure 5D, Table 1). These data suggest that while formation of short-branched actin filaments is required during the late stages of clathrin-mediated VSV entry, this need is obviated during clathrin-dependent DI-T entry.
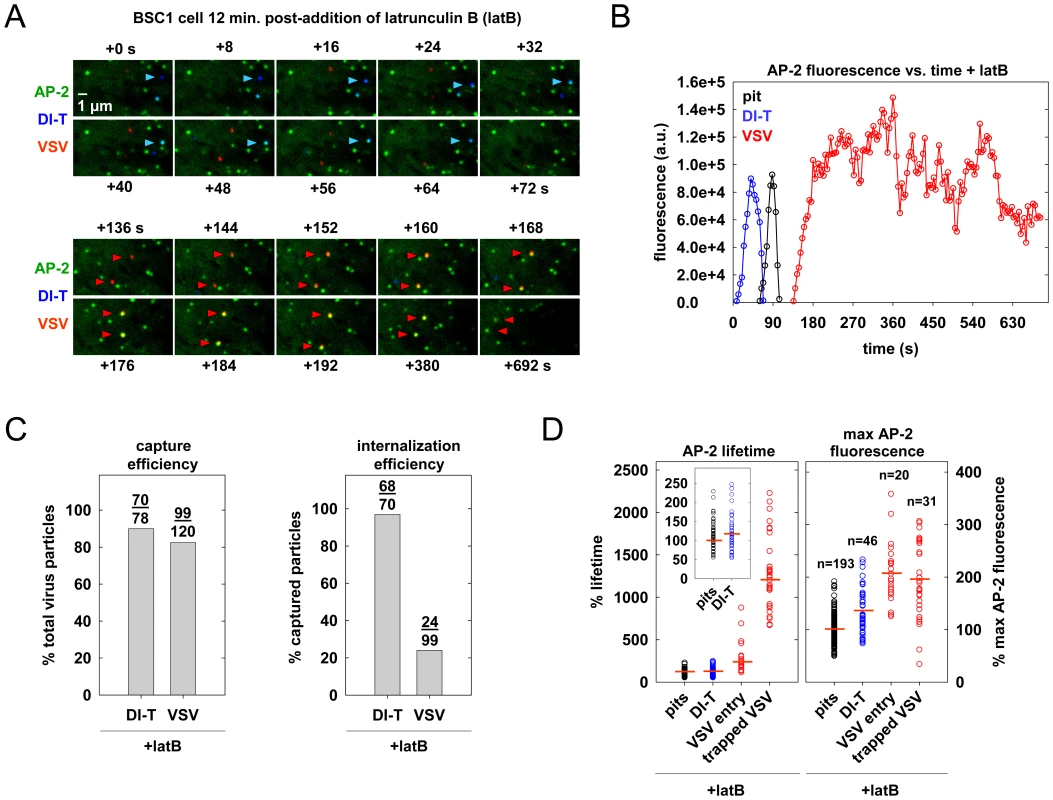
Actin polymerization is not required for DI-T internalization
To directly test whether actin assembly is required for DI-T entry, we treated BSC1 cells with latrunculin B (latB), a chemical inhibitor of actin filament assembly [30], and tracked the endocytic fate of DI-T and VSV in the same cells. Treatment of cells with 6.3 µM latB did not change the efficiency of DI-T entry (Figure 6A–C, Video S8), but it reduced the internalization of VSV by >75% (Figure 6A–C). As expected [15], latB treatment did not affect the capture efficiency of either particle type by clathrin (Figure 6C). The lifetimes and AP-2 content of pits lacking particles or containing DI-T was similarly unaffected by latB (Figure 6D). We conclude that the shorter DI-T particles bypass the actin requirement displayed by the larger VSV for efficient clathrin-based uptake.
Discussion
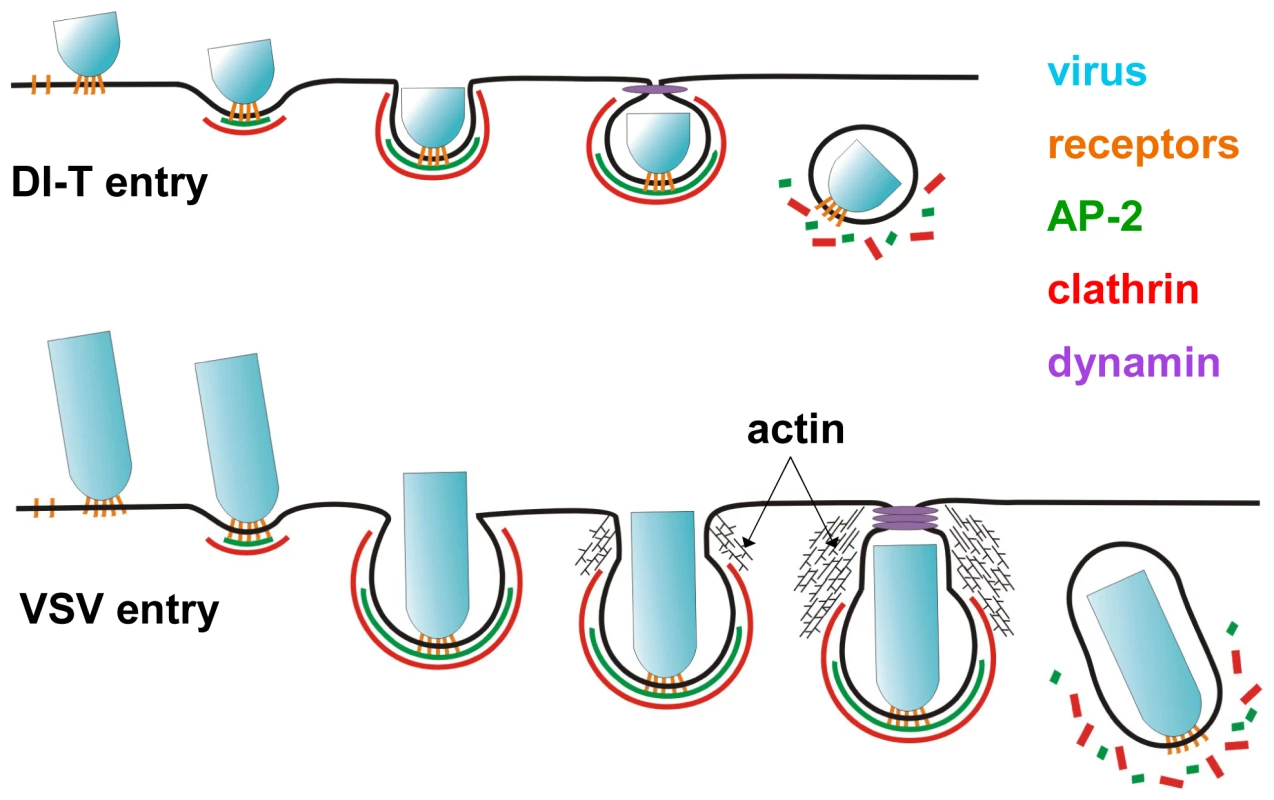
The major conclusion of our study is that the physical properties of a virus particle dictate the need for engagement of the actin system during its clathrin-dependent uptake into cells. We formulate this conclusion based on tracking the clathrin-dependent internalization of VSV and its shorter DI-T particle into live cells. Internalization of VSV is accompanied by the recruitment of cortactin at a late step of the endocytic process, and chemical inhibition of actin polymerization blocks virus uptake (this study and [15]). By contrast, internalization of the shorter DI-T particles is insensitive to chemical inhibition of actin polymerization and is not accompanied by the same spike of cortactin recruitment observed for the full-length VSV particles. VSV and DI-T particles differ in their length and not the density of the glycoprotein spike that dictates their entry. We suggest that the shape of the full-length VSV particles presents a physical barrier that frustrates completion of the clathrin-coated pit. The stalled clathrin structure then recruits the actin machinery required to finalize internalization. The shorter DI-T particles no longer provide such a physical barrier during engulfment, which results in their actin-independent internalization through coated vesicles that acquire a full complement of clathrin.
The importance of particle length for the clathrin-dependent uptake of VSV
Receptor-dependent signaling events lead to actin filament assembly during the clathrin-independent uptake of other viruses, including poliovirus and coxsackie B virus [31], [32]. Here we show that the initial interactions of DI-T with the cell are indistinguishable to those of VSV, as both particle types diffuse slowly on the cell surface and are captured by coated pits with similar kinetics (Figure 2). Such similar behavior suggests that DI-T and VSV engage an as yet unknown viral receptor in an analogous manner. Since initial receptor interactions appear indistinguishable, it seems remote that VSV G-receptor interactions induce a signaling cascade that leads to actin polymerization at sites of VSV but not DI-T uptake. We therefore conclude that the glycoprotein is not the primary trigger of actin assembly during clathrin-dependent VSV internalization.
We previously showed that endocytic structures containing VSV do not acquire a full clathrin coat [15], which agreed with earlier electron micrographs depicting virus particles in tubular invaginations with clathrin at the cytosolic tip [17]. The morphology of those structures suggests that cells initially capture one tip of the virus particle, and clathrin assembly stalls when the constricting coat encounters the long axis of the virion (Figure 7). Here we found that DI-T particles do not alter the process of clathrin-coated vesicle formation (Figures 3, 4). This finding implies that clathrin can fully enclose cargos displaying VSV G provided that the particle shape does not physically prohibit clathrin assembly or closure of the plasma membrane (Figure 7). Consequently, the physical properties of the VSV particle captured by a clathrin-coated pit are what dictate the altered mode of actin-dependent uptake. We therefore propose the model (Figure 7) that it is the incomplete clathrin structure formed during VSV uptake that elicits the actin-based response to rescue the endocytic process and leads to the successful engulfment of the trapped virus particle.
Actin-dependent clathrin-mediated endocytosis
The importance of actin function during clathrin-mediated endocytosis varies. In yeast cells, actin polymerization drives invagination and endocytosis of long-lived (>2 min.) clathrin assemblies through tubular intermediates with clathrin at the cytosolic tip [33]–[35]. Mammalian cells also form large (0.2–1 µm), relatively flat arrays of clathrin, or ‘plaques,’ on the adherent cell surface [26], [36]. These structures also exhibit long lifetimes (2-15+ min.) and require local actin assembly for internalization [26]. Coated pit internalization from the apical (but not the basolateral) surface of polarized mammalian cells [37], [38] and lamprey neuronal synapses [39], [40] is also actin-dependent. By contrast, actin polymerization is only required for the uptake of clathrin plaques and not conventional coated pits in several types of nonpolarized mammalian cells [26], [41]. Thus, while actin dynamics play an evolutionarily conserved role in clathrin-dependent endocytosis, mammalian cells regulate the interplay between the clathrin and actin systems.
Our analyses of VSV and coated plaque internalization reveal two properties that correlate with their actin-dependent uptake mechanisms. First, plaques and VSV-containing structures remain on the plasma membrane for >2-fold longer than standard coated pits [15], [26] (Figure 3C, E). The prolonged presence of a clathrin lattice might promote interactions between clathrin-associated proteins and regulators of actin polymerization. Second, clathrin plaques and VSV-containing structures physically differ from standard coated pits. Plaques fail to constrict their outer boundaries during the final phase of clathrin assembly [26], and pits containing VSV lack a complete clathrin coat [15], [17]. Such unusual structural features might attract proteins that associate with exposed lipids, such as dynamin. Indeed, significantly more dynamin molecules accumulate during the final stages of VSV uptake [15]. This localized increase in dynamin may enhance the recruitment of dynamin-interacting proteins with the capacity to bind lipids and activate the Arp2/3 complex through N-WASP, including endophilin, syndapin, and SNX9 [42]–[46]. Although these proteins may link the clathrin and actin systems and facilitate localized membrane remodeling during endocytosis (reviewed in [47]), further studies are needed to determine whether they play a role in clathrin-dependent VSV endocytosis. Comparative studies of VSV and DI-T uptake may provide a useful tool to dissect the mechanisms that regulate actin assembly during clathrin-mediated endocytosis.
Implications for the internalization mechanisms of other viruses
The dimensions of the actin-dependent VSV particle and the actin-independent DI-T particle fall within the range of shapes present in many pleomorphic viruses. Our work suggests that this may lead to important distinctions in their mode of uptake. For example, some influenza A virus strains, such as X-31, produce spherical particles that measure 80–120 nm in diameter [18]. By contrast, the Udorn strain forms filamentous particles that measure 80–100 nm wide and up to 30 microns in length [48], [49]. Although influenza A virus can enter cells by clathrin-dependent and -independent mechanisms [14], the impact of particle shape on the entry pathway remains unknown. It seems likely that remodeling of the cortical actin cytoskeleton will be important for uptake of filamentous influenza particles, and clathrin may facilitate local membrane deformation during the endocytic process. The Arenaviridae generate roughly spherical particles that range in diameter from 40–300 nm [50], [51], and it is known that clathrin function is important for efficient infection of cells by some New World arenaviruses [52]. It will now be of interest to determine whether spherical particles of different diameter employ altered modes of clathrin-based endocytosis.
Pseudotyping is often used to study the entry pathway of highly pathogenic viruses, including the long filamentous filoviruses, Ebola and Marburg, as well as several arenaviruses. Such pseudotypes are frequently based on VSV or retroviral virions in which the endogenous entry proteins have been replaced with the surface glycoproteins of the pathogenic virus [53]–[56]. Although viral pseudotypes are useful for studying the entry process, VSV and the spherical virions of retroviruses (∼100 nm in diameter) do not accurately recapitulate the sizes or shapes of the pleomorphic viruses. Our studies of VSV and DI-T clearly show that virion geometry can fundamentally alter aspects of the viral internalization process. Therefore, it is critically important to study viral endocytosis using pseudotyped or virus-like particles that closely approximate the physical properties of a virus in question.
Materials and Methods
Cells and viruses
African green monkey kidney BS-C-1 cells (herein BSC1, American Type Culture Collection (ATCC) CCL-26; Manassas, VA) and Vero cells (ATCC) were maintained at 37°C and 5% CO2 in Dulbecco's Modified Eagle Medium (DMEM, Invitrogen Corporation; Carlsbad, CA) supplemented with 10% fetal bovine serum (Tissue Culture Biologicals; Tulare, CA). BSC1 cells stably expressing rat σ2 adaptin-eGFP (σ2-eGFP) [9] were maintained as above in the presence of 0.4 mg mL−1 geneticin (G418, Invitrogen).
Recombinant VSV (rVSV) [25] was amplified and purified as before [15]. Defective interfering T (DI-T) particles of VSV were recovered from a cDNA clone of the DI-T genome [24]. The DI-T particles were amplified by co-infecting baby hamster kidney cells (BHK-21, ATCC C-13) with rVSV (multiplicity of infection (MOI) 50). A subsequent passage was performed by inoculating cells with filtered, undiluted supernatant from the primary amplification and rVSV (MOI of 50). Viruses were concentrated by centrifugation at 44,000× g, and the virus pellet was resuspended in NTE (10 mM Tris pH 7.4, 100 mM NaCl, 1 mM EDTA). The two particle forms were separated on a 15–45% sucrose gradient prepared in NTE by centrifugation at 77,000× g for 5 h. The DI-T particles were extracted from the upper virus band, concentrated as before, and resuspended to 1 mg mL−1 of total protein in PBS.
Dye conjugation to virus particles
Purified DI-T and VSV particles were labeled with Alexa Fluor dye molecules (Molecular Probes, Invitrogen) as previously described [15] except that the final dye concentration in the labeling reaction was reduced to 25 µg ml−1. Plaque assays of virus preps before and after labeling showed that dye conjugation did not affect the infectivity of VSV particles or the capacity of DI-T virions to inhibit plaque formation by VSV. The surface density of G protein on VSV or DI-T particles was estimated by measuring the ratio of G protein to N protein in each particle population. To separate and visualize the viral proteins, purified virions were subjected to SDS-PAGE using 10% polyacrylamide and 0.13% bis-acrylamide and stained with Coomassie blue. The relative amounts of N or G protein were established using ImageJ (U. S. National Institutes of Health, Bethesda, Maryland; http://rsb.info.nih.gov/ij/).
Nucleic acid transfection
BSC1 cells were seeded into 6-well plates at ∼60,000 cells per well 16–20 h prior to transfection. Plasmid DNA was introduced into the cells using FuGENE 6 (Roche Diagnostics; Indianapolis, IN) according to the manufacturer's instructions. Prior to transfection, media on the cells was replaced with 1 ml OPTIMEM (Invitrogen). After addition of the transfection mixture, cells were incubated at 37°C for 5 h, and the existing media was supplemented with 2 ml DMEM containing 10% FBS. To ensure optimal replacement of endogenous clathrin light chain molecules with rat eGFP-clathrin light chain A1 (eGFP-LCa) [9], cells were transfected with 0.75 µg of plasmid DNA encoding eGFP-LCa. The cells were cultured for ∼36 h and seeded onto glass coverslips ∼14 h prior to image acquisition. Co-expression of mCherry-LCa (constructed as described for tomato-LCa [8]) and mouse cortactin-eGFP [57], [58] was achieved by transfection of cells on glass coverslips with 1 µg of each plasmid, and the cells were imaged ∼18 h later.
Live cell imaging
Cells on 25 mm coverslips (No. 1.5, Electron Microscopy Sciences; Hatfield, PA) were placed into a perfusion chamber and overlaid with α-MEM lacking phenol red (Invitrogen) and supplemented with 20 mM HEPES pH 7.4 and 2% FBS. The chamber was placed in a heated sample holder (20/20 Technology Inc.; Wilmington, NC) mounted on the stage of a Mariana imaging system (Intelligent Imaging Innovations, Denver, CO) based on an Axiovert 200M inverted microscope (Carl Zeiss, Inc.; Thornwood, NY). The microscope stage and objective lenses were maintained at 37°C within an environmental chamber, and the air above the cells was supplied with 5% CO2. The position of the sample holder with respect to the objective lens was manipulated using a PZ-2000 automated stage (Applied Scientific Instrumentation; Eugene, OR). Samples were illuminated using 40–50 mW solid state lasers (λ = 488, Coherent, Inc.; Santa Clara, CA, λ = 561, Cobolt AB; Solna, Sweden, λ = 640, 40 mW; Coherent) directed through an acousto-optic tunable filter (AOTF; Gooch and Housego L.L.C.; Melbourne, FL). Laser illumination was imparted on the sample through a CSU-X1 spinning disk confocal unit (Yokogawa Electric Corporation; Tokyo, Japan) and a 63X objective lens (Plan-Apochromat, NA 1.4, Carl Zeiss). Emission spectra were selected using single band width emission filters (LF405/488/561/635-A, Semrock; Rochester, NY), and after transmission through a spherical aberration correction device (Infinity Photo-Optical; Boulder, CO), the emission photons were collected using a Cascade II:512B back-illuminated EMCCD camera (Roper Scientific, Photometrics; Tuscon, AZ). Under this configuration, a single pixel corresponds to 0.07×0.07 µm2. Slidebook 4.2.13 (Intelligent Imaging Innovations, Inc. (III); Denver, CO) was used to command the hardware devices and visualize the acquired data.
To image virus internalization, Alexa-labeled virus was centrifuged briefly to remove aggregates, and cells were inoculated with virus to result in attachment of <50 particles per cell within 20 min. (∼1×107 p.f.u. of VSV, ∼MOI 100, 0.005–0.05 particles per µm2 of cell surface area). Time-lapse acquisitions were typically carried out for 8–10 min. per cell, and images were captured at 3–4 s intervals after sequentially illuminating the sample with the appropriate laser for 50–100 ms per wavelength. To assess the effect of latrunculin B (latB) (Sigma-Aldrich, Inc.; St. Louis, MO) on coated vesicle formation and virus entry, cells were treated with 6.3 µM of latB for ∼10 min. at 37°C prior to the addition of virus particles.
Image analysis
Image analysis was performed as previously described [15] with the following modifications. Slidebook 4.2.13 (III) was used to view, scale, and export images for publication. Movies were generated by exporting a series of continuous TIFF files from a Slidebook time-lapse acquisition and compiling the images into a single AVI file using ImageJ (NIH). SigmaPlot 8.0 (SYSTAT; Point Richmond, CA) was used to plot data. Microsoft Excel was used to determine whether data from 2 categories differ in a statistically significant manner using two-tailed Student's t-tests. An automated image analysis application (IMAB) [8] developed within MATLAB (Mathworks; Natick, MA) was used to track the formation of clathrin-coated structures and the internalization of single virus particles. Images were processed for analysis as previously described, and established criteria were used to exclude incomplete endocytic events and events in which pixels assigned to one clathrin structure merged with or split from an adjacent structure [8]. For each cell of interest, the first ∼50 pits lacking virus particles were analyzed in detail to measure the coat lifetime and protein composition (see below). All complete virus uptake events that occurred in these cells were also analyzed in a similar manner. With the exception of internalization events blocked by latB, incomplete internalization events or events truncated by the start/end of image acquisition were not analyzed. Aggregates of virus were identified as objects with fluorescence intensities greater than that of single particles and were excluded from all analyses.
IMAB was used to measure the fluorescence intensity of coat components or virus particles in the following manner. A roughly spherical mask encompassing 69 pixels was centered on the peak fluorescence intensity of the object in all frames that the object was detectable above the local background fluorescence. The contribution of the local background fluorescence was estimated by measuring the average intensity of pixels within a ring of single pixel width that extended from the outer boundary of the object mask. The intensity of the pixels within the object mask was then summed, and the average intensity value of pixels in the local background was subtracted from each pixel within the object mask to estimate the fluorescence contributed by proteins (or dye molecules) within the object of interest.
Electron microscopy
Purified virus particles were deposited onto carbon-coated copper grids and stained with 2% phosphotungstic acid (PTA) in H2O (pH 7.5). To visualize DI-T particles in clathrin-coated pits, BSC1 cells were inoculated with unlabeled DI-T particles using a dose that yielded ∼1000 attached particles per cell after 10 min. Samples were then processed for ultra-thin sectioning as previously described [15], [59]. Electron micrographs of VSV particles in clathrin endocytic structures were obtained from cells infected with VSV for 6 h, where the entry of newly released particles could readily be visualized. Virus particles and ultra-thin sections of cells were viewed using a Tecnai G2 Spirit BioTWIN transmission electron microscope (FEI, Hillsboro, OR).
Supporting Information
Zdroje
1. ConnerSD
SchmidSL
2003 Regulated portals of entry into the cell. Nature 422 37 44
2. KirchhausenT
2000 Clathrin. Annu Rev Biochem 69 699 727
3. KirchhausenT
2009 Imaging endocytic clathrin structures in living cells. Trends Cell Biol 19 596 605
4. OwenDJ
EvansPR
1998 A structural explanation for the recognition of tyrosine-based endocytotic signals. Science 282 1327 1332
5. HoningS
RicottaD
KraussM
SpateK
SpolaoreB
2005 Phosphatidylinositol-(4,5)-bisphosphate regulates sorting signal recognition by the clathrin-associated adaptor complex AP2. Mol Cell 18 519 531
6. DamkeH
BabaT
WarnockDE
SchmidSL
1994 Induction of mutant dynamin specifically blocks endocytic coated vesicle formation. J Cell Biol 127 915 934
7. LeeDW
ZhaoX
ZhangF
EisenbergE
GreeneLE
2005 Depletion of GAK/auxilin 2 inhibits receptor-mediated endocytosis and recruitment of both clathrin and clathrin adaptors. J Cell Sci 118 4311 4321
8. MassolRH
BollW
GriffinAM
KirchhausenT
2006 A burst of auxilin recruitment determines the onset of clathrin-coated vesicle uncoating. Proc Natl Acad Sci U S A 103 10265 10270
9. EhrlichM
BollW
Van OijenA
HariharanR
ChandranK
2004 Endocytosis by random initiation and stabilization of clathrin-coated pits. Cell 118 591 605
10. LoerkeD
MettlenM
YararD
JaqamanK
JaqamanH
2009 Cargo and dynamin regulate clathrin-coated pit maturation. PLoS Biol 7 e57
11. HanoverJA
BeguinotL
WillinghamMC
PastanIH
1985 Transit of receptors for epidermal growth factor and transferrin through clathrin-coated pits. Analysis of the kinetics of receptor entry. J Biol Chem 260 15938 15945
12. AndersonRG
BrownMS
GoldsteinJL
1977 Role of the coated endocytic vesicle in the uptake of receptor-bound low density lipoprotein in human fibroblasts. Cell 10 351 364
13. van der SchaarHM
RustMJ
ChenC
van der Ende-MetselaarH
WilschutJ
2008 Dissecting the cell entry pathway of dengue virus by single-particle tracking in living cells. PLoS Pathog 4 e1000244
14. RustMJ
LakadamyaliM
ZhangF
ZhuangX
2004 Assembly of endocytic machinery around individual influenza viruses during viral entry. Nat Struct Mol Biol 11 567 573
15. CuretonDK
MassolRH
SaffarianS
KirchhausenTL
WhelanSP
2009 Vesicular stomatitis virus enters cells through vesicles incompletely coated with clathrin that depend upon actin for internalization. PLoS Pathog 5 e1000394
16. VeigaE
GuttmanJA
BonazziM
BoucrotE
Toledo-AranaA
2007 Invasive and adherent bacterial pathogens co-Opt host clathrin for infection. Cell Host Microbe 2 340 351
17. SimpsonRW
HauserRE
DalesS
1969 Viropexis of vesicular stomatitis virus by L cells. Virology 37 285 290
18. HarrisA
CardoneG
WinklerDC
HeymannJB
BrecherM
2006 Influenza virus pleiomorphy characterized by cryoelectron tomography. Proc Natl Acad Sci U S A 103 19123 19127
19. MeierE
HarmisonGG
KeeneJD
SchubertM
1984 Sites of copy choice replication involved in generation of vesicular stomatitis virus defective-interfering particle RNAs. J Virol 51 515 521
20. HuangAS
WagnerRR
1966 Defective T particles of vesicular stomatitis virus. II. Biologic role in homologous interference. Virology 30 173 181
21. PengG
TsaoJ
ScheinS
GreenTJ
LuoM
2010 Cryo-EM model of the bullet-shaped vesicular stomatitis virus. Science 327 689 693
22. HuangAS
GreenawaltJW
WagnerRR
1966 Defective T particles of vesicular stomatitis virus. I. Preparation, morphology, and some biologic properties. Virology 30 161 172
23. WagnerRR
SchnaitmanTA
SnyderRM
1969 Structural proteins of vesicular stomatitis viruses. J Virol 3 395 403
24. PattnaikAK
BallLA
LeGroneAW
WertzGW
1992 Infectious defective interfering particles of VSV from transcripts of a cDNA clone. Cell 69 1011 1020
25. WhelanSP
BallLA
BarrJN
WertzGT
1995 Efficient recovery of infectious vesicular stomatitis virus entirely from cDNA clones. Proc Natl Acad Sci U S A 92 8388 8392
26. SaffarianS
CocucciE
KirchhausenT
2009 Distinct dynamics of endocytic clathrin-coated pits and coated plaques. PLoS Biol 7 e1000191
27. UrunoT
LiuJ
ZhangP
FanY
EgileC
2001 Activation of Arp2/3 complex-mediated actin polymerization by cortactin. Nat Cell Biol 3 259 266
28. McNivenMA
KimL
KruegerEW
OrthJD
CaoH
2000 Regulated interactions between dynamin and the actin-binding protein cortactin modulate cell shape. J Cell Biol 151 187 198
29. MerrifieldCJ
PerraisD
ZenisekD
2005 Coupling between clathrin-coated-pit invagination, cortactin recruitment, and membrane scission observed in live cells. Cell 121 593 606
30. CoueM
BrennerSL
SpectorI
KornED
1987 Inhibition of actin polymerization by latrunculin A. FEBS Lett 213 316 318
31. CoyneCB
BergelsonJM
2006 Virus-induced Abl and Fyn kinase signals permit coxsackievirus entry through epithelial tight junctions. Cell 124 119 131
32. CoyneCB
KimKS
BergelsonJM
2007 Poliovirus entry into human brain microvascular cells requires receptor-induced activation of SHP-2. Embo J 26 4016 4028
33. KaksonenM
ToretCP
DrubinDG
2006 Harnessing actin dynamics for clathrin-mediated endocytosis. Nat Rev Mol Cell Biol 7 404 414
34. KaksonenM
SunY
DrubinDG
2003 A pathway for association of receptors, adaptors, and actin during endocytic internalization. Cell 115 475 487
35. IdrissiFZ
GrotschH
Fernandez-GolbanoIM
Presciatto-BaschongC
RiezmanH
2008 Distinct acto/myosin-I structures associate with endocytic profiles at the plasma membrane. J Cell Biol 180 1219 1232
36. HeuserJ
1980 Three-dimensional visualization of coated vesicle formation in fibroblasts. J Cell Biol 84 560 583
37. GottliebTA
IvanovIE
AdesnikM
SabatiniDD
1993 Actin microfilaments play a critical role in endocytosis at the apical but not the basolateral surface of polarized epithelial cells. J Cell Biol 120 695 710
38. Da CostaSR
SouE
XieJ
YarberFA
OkamotoCT
2003 Impairing actin filament or syndapin functions promotes accumulation of clathrin-coated vesicles at the apical plasma membrane of acinar epithelial cells. Mol Biol Cell 14 4397 4413
39. ShupliakovO
BloomO
GustafssonJS
KjaerulffO
LowP
2002 Impaired recycling of synaptic vesicles after acute perturbation of the presynaptic actin cytoskeleton. Proc Natl Acad Sci U S A 99 14476 14481
40. BourneJ
MorganJR
PieriboneVA
2006 Actin polymerization regulates clathrin coat maturation during early stages of synaptic vesicle recycling at lamprey synapses. J Comp Neurol 497 600 609
41. BoucrotE
SaffarianS
MassolR
KirchhausenT
EhrlichM
2006 Role of lipids and actin in the formation of clathrin-coated pits. Exp Cell Res 312 4036 4048
42. QualmannB
RoosJ
DiGregorioPJ
KellyRB
1999 Syndapin I, a synaptic dynamin-binding protein that associates with the neural Wiskott-Aldrich syndrome protein. Mol Biol Cell 10 501 513
43. SouletF
YararD
LeonardM
SchmidSL
2005 SNX9 regulates dynamin assembly and is required for efficient clathrin-mediated endocytosis. Mol Biol Cell 16 2058 2067
44. YararD
Waterman-StorerCM
SchmidSL
2007 SNX9 couples actin assembly to phosphoinositide signals and is required for membrane remodeling during endocytosis. Dev Cell 13 43 56
45. RingstadN
NemotoY
De CamilliP
1997 The SH3p4/Sh3p8/SH3p13 protein family: binding partners for synaptojanin and dynamin via a Grb2-like Src homology 3 domain. Proc Natl Acad Sci U S A 94 8569 8574
46. OtsukiM
ItohT
TakenawaT
2003 Neural Wiskott-Aldrich syndrome protein is recruited to rafts and associates with endophilin A in response to epidermal growth factor. J Biol Chem 278 6461 6469
47. DawsonJC
LeggJA
MacheskyLM
2006 Bar domain proteins: a role in tubulation, scission and actin assembly in clathrin-mediated endocytosis. Trends Cell Biol 16 493 498
48. RobertsPC
CompansRW
1998 Host cell dependence of viral morphology. Proc Natl Acad Sci U S A 95 5746 5751
49. RobertsPC
LambRA
CompansRW
1998 The M1 and M2 proteins of influenza A virus are important determinants in filamentous particle formation. Virology 240 127 137
50. NeumanBW
AdairBD
BurnsJW
MilliganRA
BuchmeierMJ
2005 Complementarity in the supramolecular design of arenaviruses and retroviruses revealed by electron cryomicroscopy and image analysis. J Virol 79 3822 3830
51. MurphyFA
WebbPA
JohnsonKM
WhitfieldSG
ChappellWA
1970 Arenoviruses in Vero cells: ultrastructural studies. J Virol 6 507 518
52. RojekJM
SanchezAB
NguyenNT
de la TorreJC
KunzS
2008 Different mechanisms of cell entry by human-pathogenic Old World and New World arenaviruses. J Virol 82 7677 7687
53. ChandranK
SullivanNJ
FelborU
WhelanSP
CunninghamJM
2005 Endosomal proteolysis of the Ebola virus glycoprotein is necessary for infection. Science 308 1643 1645
54. NegreteOA
LevroneyEL
AguilarHC
Bertolotti-CiarletA
NazarianR
2005 EphrinB2 is the entry receptor for Nipah virus, an emergent deadly paramyxovirus. Nature 436 401 405
55. ClementeR
de la TorreJC
2009 Cell entry of Borna disease virus follows a clathrin-mediated endocytosis pathway that requires Rab5 and microtubules. J Virol 83 10406 10416
56. BhattacharyyaS
WarfieldKL
RuthelG
BavariS
AmanMJ
2010 Ebola virus uses clathrin-mediated endocytosis as an entry pathway. Virology 401 18 28
57. Le ClaincheC
PaulyBS
ZhangCX
Engqvist-GoldsteinAE
CunninghamK
2007 A Hip1R-cortactin complex negatively regulates actin assembly associated with endocytosis. Embo J 26 1199 1210
58. KaksonenM
PengHB
RauvalaH
2000 Association of cortactin with dynamic actin in lamellipodia and on endosomal vesicles. J Cell Sci 113 Pt 24 4421 4426
59. MaupinP
PollardTD
1983 Improved preservation and staining of HeLa cell actin filaments, clathrin-coated membranes, and other cytoplasmic structures by tannic acid-glutaraldehyde-saponin fixation. J Cell Biol 96 51 62
Štítky
Hygiena a epidemiologie Infekční lékařství LaboratořČlánek vyšel v časopise
PLOS Pathogens
2010 Číslo 9
- Diagnostický algoritmus při podezření na syndrom periodické horečky
- Stillova choroba: vzácné a závažné systémové onemocnění
- Perorální antivirotika jako vysoce efektivní nástroj prevence hospitalizací kvůli COVID-19 − otázky a odpovědi pro praxi
- Choroby jater v ordinaci praktického lékaře – význam jaterních testů
- Jak souvisí postcovidový syndrom s poškozením mozku?
Nejčtenější v tomto čísle
- Structure of the Extracellular Portion of CD46 Provides Insights into Its Interactions with Complement Proteins and Pathogens
- The Length of Vesicular Stomatitis Virus Particles Dictates a Need for Actin Assembly during Clathrin-Dependent Endocytosis
- Inhibition of TIR Domain Signaling by TcpC: MyD88-Dependent and Independent Effects on Virulence
- Cellular Entry of Ebola Virus Involves Uptake by a Macropinocytosis-Like Mechanism and Subsequent Trafficking through Early and Late Endosomes
