-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Phylogenetic Approach Reveals That Virus Genotype Largely Determines HIV Set-Point Viral Load
HIV virulence, i.e. the time of progression to AIDS, varies greatly among patients. As for other rapidly evolving pathogens of humans, it is difficult to know if this variance is controlled by the genotype of the host or that of the virus because the transmission chain is usually unknown. We apply the phylogenetic comparative approach (PCA) to estimate the heritability of a trait from one infection to the next, which indicates the control of the virus genotype over this trait. The idea is to use viral RNA sequences obtained from patients infected by HIV-1 subtype B to build a phylogeny, which approximately reflects the transmission chain. Heritability is measured statistically as the propensity for patients close in the phylogeny to exhibit similar infection trait values. The approach reveals that up to half of the variance in set-point viral load, a trait associated with virulence, can be heritable. Our estimate is significant and robust to noise in the phylogeny. We also check for the consistency of our approach by showing that a trait related to drug resistance is almost entirely heritable. Finally, we show the importance of taking into account the transmission chain when estimating correlations between infection traits. The fact that HIV virulence is, at least partially, heritable from one infection to the next has clinical and epidemiological implications. The difference between earlier studies and ours comes from the quality of our dataset and from the power of the PCA, which can be applied to large datasets and accounts for within-host evolution. The PCA opens new perspectives for approaches linking clinical data and evolutionary biology because it can be extended to study other traits or other infectious diseases.
Published in the journal: . PLoS Pathog 6(9): e32767. doi:10.1371/journal.ppat.1001123
Category: Research Article
doi: https://doi.org/10.1371/journal.ppat.1001123Summary
HIV virulence, i.e. the time of progression to AIDS, varies greatly among patients. As for other rapidly evolving pathogens of humans, it is difficult to know if this variance is controlled by the genotype of the host or that of the virus because the transmission chain is usually unknown. We apply the phylogenetic comparative approach (PCA) to estimate the heritability of a trait from one infection to the next, which indicates the control of the virus genotype over this trait. The idea is to use viral RNA sequences obtained from patients infected by HIV-1 subtype B to build a phylogeny, which approximately reflects the transmission chain. Heritability is measured statistically as the propensity for patients close in the phylogeny to exhibit similar infection trait values. The approach reveals that up to half of the variance in set-point viral load, a trait associated with virulence, can be heritable. Our estimate is significant and robust to noise in the phylogeny. We also check for the consistency of our approach by showing that a trait related to drug resistance is almost entirely heritable. Finally, we show the importance of taking into account the transmission chain when estimating correlations between infection traits. The fact that HIV virulence is, at least partially, heritable from one infection to the next has clinical and epidemiological implications. The difference between earlier studies and ours comes from the quality of our dataset and from the power of the PCA, which can be applied to large datasets and accounts for within-host evolution. The PCA opens new perspectives for approaches linking clinical data and evolutionary biology because it can be extended to study other traits or other infectious diseases.
Introduction
One of the hallmarks of HIV infection is the enormous variation in disease progression. While some untreated patients survive for more than 25 years, others die within a year of infection [1]. Delineating what fraction of this variation is attributable to host versus virus genotype has important clinical and epidemiological applications. Whole genome association studies showed that at least 15% of the variations in traits associated with disease progression in HIV can be explained by common host genetic variants [2], [3]. In this study, we adopt a new approach to quantify the contribution of the viral genotype to disease progression.
The natural course of an HIV infection is divided into three clinical stages: the acute phase, which lasts for several weeks; the asymptomatic phase, which can last several years; and the AIDS phase, which can last several months to a few years and ends with the death of the patient. The effect of the HIV viral genotype on disease progression is debated. Arguably, this is due to the fact that, as for most infectious diseases of humans, it is difficult to determine the transmission chain (i.e. ‘Who infected whom?’). Early studies showed that long times to AIDS are associated with the receipt of blood from donors who developed AIDS late [4], [5]. A study found a strong correlation between maternal and infant viral load [6], but mother-to-child transmission introduces confounding factors through host genetic relatedness. In some cases, transmission pairs are known and one can then measure the heritability of traits from one infection to the next. This heritability corresponds to the fraction of the variance among patients that is explained by the virus genotype [7], [8]. Studies found that 21% [9], 23% [8], 25% [10] and 55% [11] of the variance in set-point viral load (spVL) can be explained by the virus genotype. These studies suggest that infection traits can be heritable but the accuracy of their estimates is likely to be limited by the sample size of the data and the accuracy of their spVL measurement.
The phylogenetic comparative approach (PCA) allows to estimate the phylogenetic signal for a trait measured in several species if the species' phylogeny is known. This signal indicates the extent to which the phylogeny explains observed trait values (see the Methods). Several studies show that this signal is very similar to heritability [12], [13], which we also verify in this study. Here, we use this classical evolutionary biology method [14] to address epidemiological questions.
We consider the case of HIV infection traits using the data from the Swiss HIV Cohort Study (SHCS [15]). As the majority of previous studies [2], [3], [6], [8], [9], [16], [17], we focus on the set-point viral load (spVL, see the Methods) measured during the asymptomatic phase of an HIV infection because it has been shown to be associated with the time to AIDS, i.e. virulence [16], [18]–[20]. The quality of the SHCS patient data allows us to have a better estimation of spVL than many studies that often rely on single viral load measurements to estimate spVL (see the Materials and Methods). Further, we consider the decline slope of the CD4 T-cells (dsCD4), which also predicts virulence [21], but to a smaller extent than spVL [20].
We use sequences of the HIV pol gene isolated from infected patients to build a phylogenetic tree, where each leaf corresponds to a patient whose trait values are known (Figure 1). Proximity in the resulting tree reflects proximity in the transmission chain [22], [23] but we emphasise that the phylogeny is only an approximation of the true transmission history. As we will show later on, our approach has the advantage of being robust to this noise in the phylogeny. We then quantify phylogenetic signal for each of the traits on the tree [24]. In addition to log(spVL) and dsCD4, we study a third trait as a control: the probability for resistance to zidovudine (AZT), denoted prAZT. We expect the latter trait to be strongly heritable because it is evaluated from the virus pol sequence. Note that there is no overlap between the information used to build the phylogeny and that used to evaluate prAZT (see the Methods).
Fig. 1. Combining phylogenies and trait values in the MSM strict dataset. 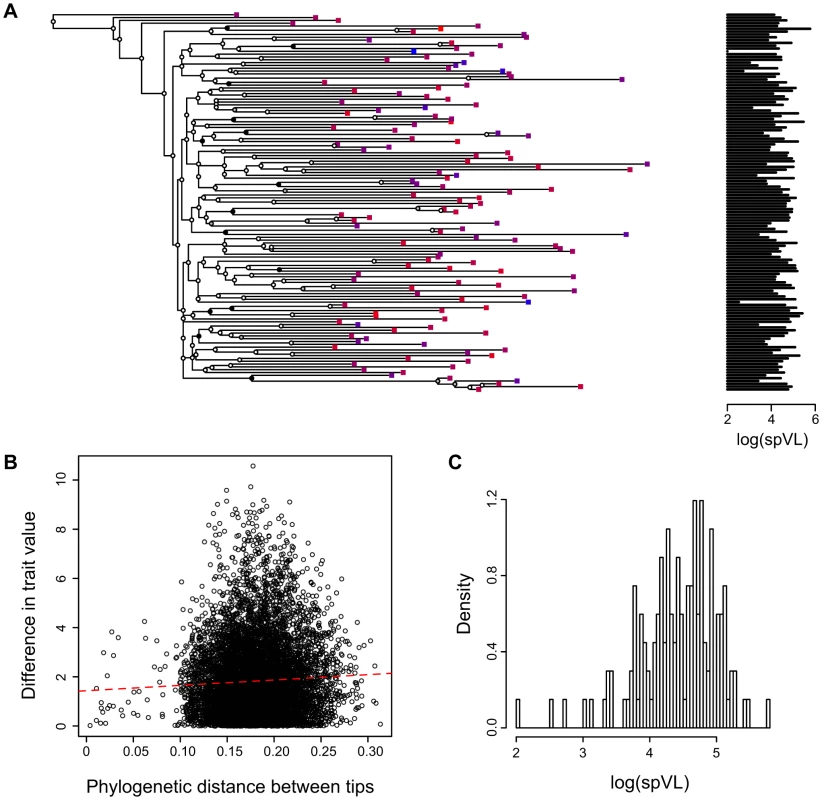
A) A phylogeny based on HIV sequences obtained from patients with known set-point viral loads, B) Phylogenetic distance between two tips versus difference in trait value between these two tips (slope and p-value) and C) Distribution of log(spVL) values in the MSM strict dataset. Panel A shows the maximum likelihood phylogenetic tree built with the MSM strict dataset. Squares on the tips of the tree correspond to infected patients. The colour of the squares and the graph on the right indicate the set-point viral load (colours range from blue to red for increasing log(spVL)). The PCA tests the correlation between proximity in the phylogeny and trait values (log(spVL)). The circles on the tree nodes indicate bootstrap values: black is greater than 90%, grey is between 50 and 90% and white is lower than 50%. We split the data into 4 datasets using 2 criteria. First, in addition to the ‘strict’ definition used in previous studies to define spVL from multiple viral load measurements [2], [3], we also consider a ‘liberal’ definition (see the Methods). The strict definition is known to improve the accuracy of the spVL measure and we want to assess the importance of the quality of this estimate on our ability to detect heritability. Second, we study one of the transmission groups (‘men having sex with men’, or MSM) separately. This group is likely to yield a more accurate phylogeny because of denser sampling in the SHCS [25]. We show in Supplementary Results (Text S1) that focusing on these datasets also removes some of the confounding factors, such as patient sex, transmission group or age, on infection trait values.
We quantify phylogenetic signal using two estimators (denoted [12] and [26]) that are based on two different methods to better detect potential artifactual values (see the Methods). A signal value of 0 implies that the phylogeny does not contain any information to explain the variance of the trait in the population. Conversely, a signal value of 1 means that the distance between tips in the phylogeny used best explains the tip data assuming a Brownian model of evolution of the trait on the tree.
Results
Trait heritability
We first present the results obtained with the ‘MSM strict’ dataset. This dataset is the smallest we study. It has the advantage of relying on the criterion for spVL already used by earlier studies and of minimising noise in the data linked with transmission groups. The estimator for phylogenetic signal reveals a significantly high signal for log(spVL) (, Table 1). The standard deviation estimated from the bootstrapped trees is .
Tab. 1. Phylogenetic signal in the 4 datasets for the three different traits. 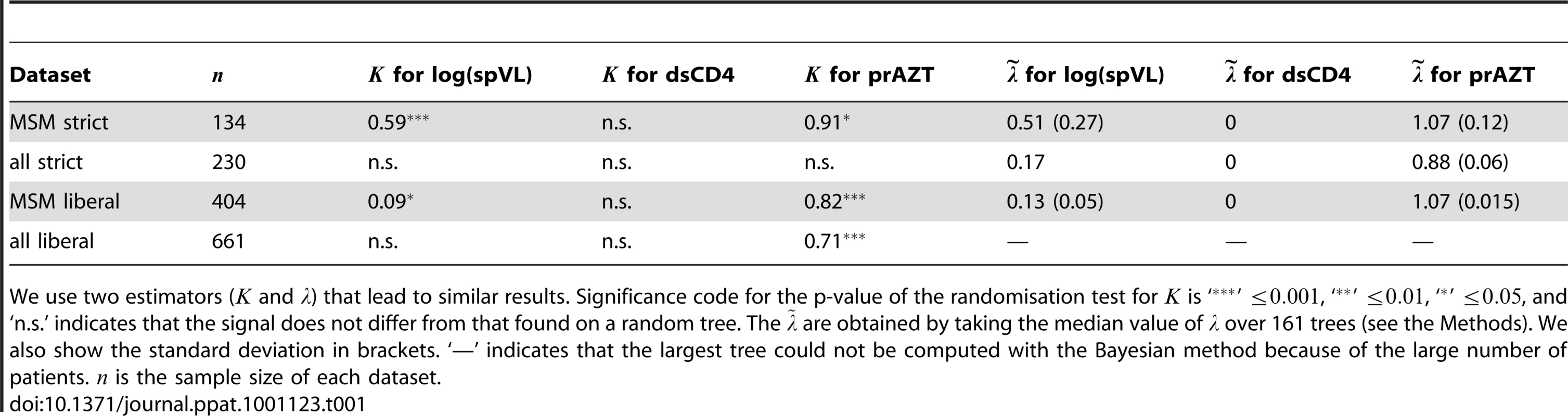
We use two estimators ( and ) that lead to similar results. Significance code for the p-value of the randomisation test for is ‘’ , ‘’ , ‘’ , and ‘n.s.’ indicates that the signal does not differ from that found on a random tree. The are obtained by taking the median value of over 161 trees (see the Methods). We also show the standard deviation in brackets. ‘—’ indicates that the largest tree could not be computed with the Bayesian method because of the large number of patients. is the sample size of each dataset. As explained above, uncertainty is inherent to any HIV phylogeny. Therefore, one might argue that the value we find is underestimated because of the noise in the phylogeny. Improving a phylogeny is a difficult task but making it worse is easy. We thus check for the robustness of the value of by introducing errors in the phylogeny in two ways. The first way consists in measuring on the bootstrapped trees, which are by definition less accurate than the consensus tree. The second way consists in swapping 3, 10 or 20% of the tip values at random. As shown in the Supplementary Results, on average considering the trees from the bootstrap or trees with errors does not significantly affect the signal intensity (i.e. the value of ): with more noise, the value of remains constant but the significance level decreases.
The intensity of the signal strongly decreases when we use the liberal criterion for spVL ( in the ‘MSM liberal’ dataset), which highlights the importance of the accuracy of the spVL estimate. The signal even becomes non-significant when we consider all the transmission groups. This can be explained by the fact that, in the SHCS, patients from the MSM transmission group tend to cluster on a phylogeny and are more densely sampled than patients from other transmission groups (see the Methods). As a consequence, a phylogeny built only on sequences from MSM patients is likely to be closer to the transmission chain. Also, using the liberal criterion or considering all the transmission groups means that log(spVL) is affected by confounding factors.
Results show no phylogenetic signal for dsCD4 in all datasets (Table 1). A possibility is that CD4 densities vary strongly within patients on short time scales. However, even a less variable estimate, the decline slope of the ratio of CD4 to CD8 T-cells, did not exhibit significant phylogenetic signal. This suggests that this trait is not directly affected by the viral genotype or that the effect is too weak to be detected.
For prAZT in the MSM strict dataset, we find (with a standard deviation on bootstrapped trees of 0.084). As for the log(spVL), this result is robust to noise in the phylogeny (see Supplementary Results). Using the liberal criterion has little effect on this value ( in the MSM lib dataset), which is consistent with the fact that drug resistance is genetically determined. The drop in heritability is likely to be a consequence of the increase in tree size, which decreases the accuracy of (see Supplementary Results). When we consider all the transmission groups, we find a non-significant value for the strict dataset and in the liberal dataset. This could be due to the fact that average prAZT is higher in one of the transmission groups (injection drug users) and to the fact that a phylogeny built with MSM patients better reflects the transmission chain.
We also perform these measures using a different estimator for phylogenetic signal, [26]. Results are similar to those found with (Table 1).
Many studies argue that phylogenetic signal can be interpreted as a phylogenetic heritability [12], [13]. At first, this may seem problematic because phylogenetic signal is measured for different species, whereas heritability is defined in population genetics as the ratio between the genetic and the phenotypic variance observed for a trait in a given population [7]. Working on traits of infectious diseases bridges phylogenetics and population genetics because it involves a population of infected individuals instead of a collection of species. This would also be the case for any phylogenetic approach based on a phylogeny of individuals.
In order to check that there indeed is an equivalency between the concepts of heritability and phylogenetic signal, we simulate phylogenetic trees reflecting the evolution of a trait under an evolutionary process with known heritability (see the Methods). The values of (black) and (red) estimated on these simulated trees strongly correlate with known heritability (Figure 2). Note that performs better than when the heritability is low. Furthermore, the variance in is much greater, which is why we only estimate it as a median value over many phylogenies (the posterior tree distribution). The simulations confirm that estimating phylogenetic signal for a trait yields useful quantitative estimates of trait heritability.
Fig. 2. Phylogenetic signal estimated for evolutionary processes with known heritability. 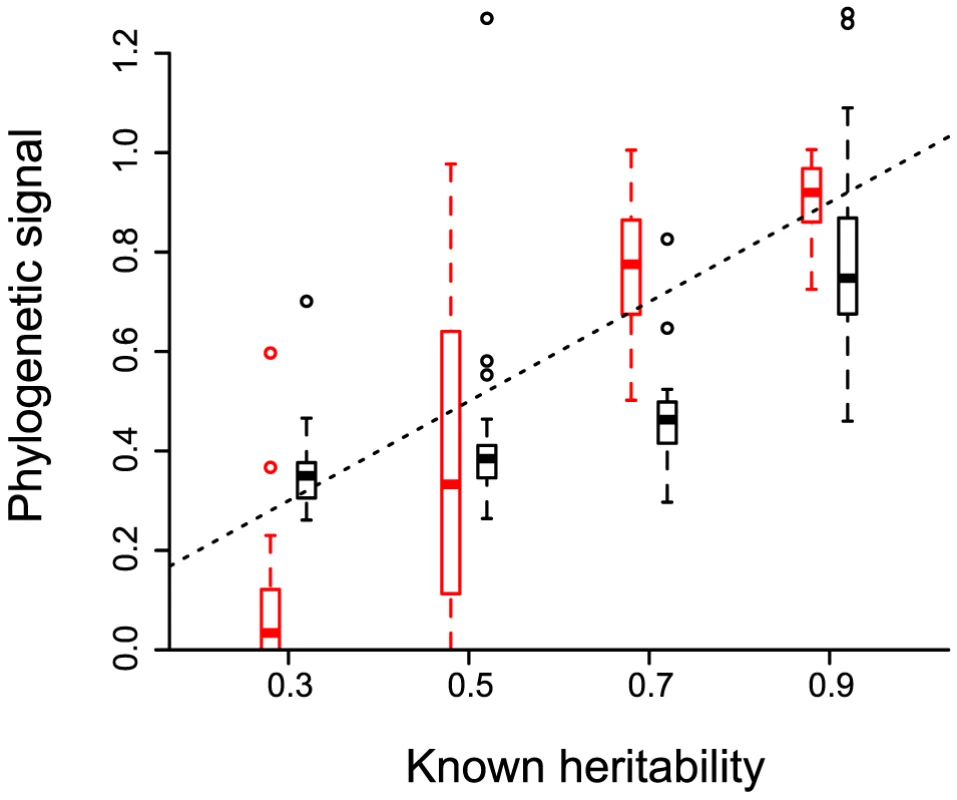
20 phylogenies are simulated to model the evolution of an infection trait in a case where heritability is set to a given value. Phylogenetic signal ( in black and in red) is then estimated on each tree using only 128 leaves to account for incomplete sampling. The box plot shows the median values, the three quartiles and the outliers. The dashed line shows . The slope is (p-value and adjusted ) for and of (p-value and adjusted ) for . Correlation among traits
The phylogenetic comparative approach (PCA) is widely used to study correlations among traits because taxa are not independent and comparisons between traits should be weighted according to the distance between taxa in the phylogeny [12]–[14], [24], [26], [27]. These ideas can be applied to infection trait values.
In Table 2, we show the difference between a classical regression between two traits and a regression that incorporates the non-independence of the data for HIV infection traits. The slope of the regression between spVL and dsCD4 decreases by approximately 60% when the phylogeny is taken into account. Furthermore, spVL does not explain much of the variance in dsCD4 (a linear model yields an adjusted of 0.047). We also find that accounting for the non-independence of the data can alter the significance of a correlation: the dsCD4 varies among transmission groups with a classical test but not with a test including the phylogeny. This provides new data to the recent debate on the link between these two predictors of virulence (see [28] and the resulting correspondence).
Tab. 2. Regressions between life-history traits with and without correction for phylogenetic signal. 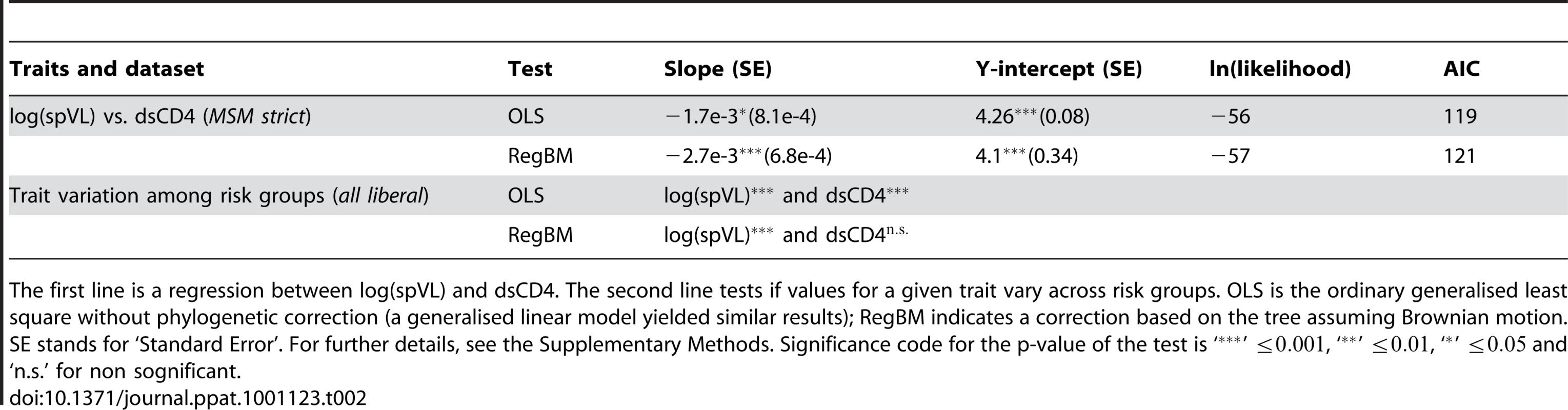
The first line is a regression between log(spVL) and dsCD4. The second line tests if values for a given trait vary across risk groups. OLS is the ordinary generalised least square without phylogenetic correction (a generalised linear model yielded similar results); RegBM indicates a correction based on the tree assuming Brownian motion. SE stands for ‘Standard Error’. For further details, see the Supplementary Methods. Significance code for the p-value of the test is ‘’ , ‘’ , ‘’ and ‘n.s.’ for non sognificant. These results can appear as contradictory with our earlier result that dsCD4 does not exhibit phylogenetic signal. The reason for the effect of the phylogeny on regressions involving dsCD4 is that when the PCA is used to study trait correlations, the correction applied to the phylogeny (i.e. the value of or ) is calculated using the value of both traits (the model finds the covariance between trait values of pairs of tips that best explains the data assuming a Brownian model of evolution of the trait on the tree [26]).
Discussion
The control of the virus genome on the virulence of an HIV infection is a controversial issue. HIV-1 is known to be more virulent than HIV-2. Within HIV-1 group M, there is also evidence that some subtypes are less virulent than others, e.g. viruses from subtype A seem to be associated with slower disease progression than viruses from subtype D [29]–[31]. Also, there is experimental evidence for differences in virulence among SIV viruses [32]. However, there is much less evidence supporting the existence of a control of the virus genotype over the duration of the infection within a given subtype of HIV-1. Here, we show that even within subtype B, the set-point viral load (spVL), which predicts HIV virulence, can be inherited from one infection to the next thus indicating that this trait depends strongly on the virus genotype. The validity of our approach is supported by the fact that we find heritability values close to 1 for a trait associated with drug resistance. Our results are statistically significant and robust to noise in the phylogeny. This robustness is important because HIV phylogenies tend to be inaccurate [22] due to processes such as recombination and co-infections.
The heritability value we find is high compared to previous studies [8]–[10], especially considering the fact that most (if not all) of these studies include hosts infected by different subtypes of HIV-1, which is likely to increase the genetical component of the variance observed in the population and hence the heritability. The magnitude of our estimate only compares with that of Hecht et al. [11]. However, one should be careful with comparing the two studies. First, they do not use spVL but the earliest measure of viral load. It is therefore possible that their measure is not linked to disease progression. Second, it is not clear if all the patients they consider are infected by the same subtype of HIV, which could increase the heritability, as explained above. Third, they include both treated and untreated patients, whereas we only include untreated patients. Fourth, and perhaps most important, they have only 49 patients in their largest dataset.
Implications of virulence heritability
An increasing number of studies attempt to estimate heritability of HIV set-point viral load, which is a measure of virulence [6], [8]–[11]. The magnitude of the heritability value for spVL we find is of clinical and epidemiological importance.
The clinical importance stems from the fact that for a trait to be heritable from one infection to the next, there needs to be a control from the virus genotype over the trait. In the case of HIV, the course of the natural history of the infection is still largely not understood. For instance, the exact role of within-host evolution in the onset of AIDS is still unclear [33]. Here, we show that the duration of the infection is at least partially controlled by the genotype of the virus that infects the host. An implication is that some of the answers to AIDS pathogenesis might be found in the genome of the virus.
Trait heritability from one infection to the next also has implications for evolutionary epidemiology. For natural selection to act on a trait, three conditions must be fulfilled: the trait must be variable in the population, it must affect the fitness of individuals bearing it and it must be heritable (at least partially). This reasoning can be extended to evolutionary epidemiology by viewing an infection as an individual, and defining fitness as the number of new infections caused [34]. In the case of HIV, spVL is known to vary among patients [35] and to affect the infection fitness, because viral load correlates with the transmission rate [16], [36] and the virulence [16], [18], [21]. Our finding that spVL is highly heritable implies that this trait is subject to natural selection at the between-host level, which supports the possibility that a trade-off between virulence and transmission rate can drive the evolution of HIV [16]. This also sheds a new light on the ongoing debate concerning why studies have failed to provide a clear picture of how HIV virulence evolved over the last decade (some find that it decreased [37], [38], others find that it increased [17], [39]–[42] and some find no significant trend [43]–[49]). These conflicting trends could be explained by different dates of origin of the pandemics and different initial values of virulence [17]. Another possibility is that the host genotype control over virulence is greater than that of the virus (see next paragraph). Finally, the structure of sexual transmission networks is likely to have a strong effect on the fitness of an infection and hence on viral evolution.
Host effects
Set-point viral load and disease progression have been shown to be affected by host factors, especially human leucocyte antigen (HLA) alleles [2], [3], [50], [51]. This could introduce a bias in our results if hosts with similar HLA alleles tend to be close in the transmission chain. Note that this bias also occurs in studies based on transmission pairs. Unfortunately, in the subset of the SHCS data we used, there was not enough information about the patients to correct for this bias. More generally, we cannot exclude correlations between the environments in which the patients live but these effects are likely to be smaller in our study than in studies based on known couples because the PCA does not require patient couples.
Another potential concern is that other diseases could be transmitted sexually with HIV that could affect trait values. The information of the SHCS allows us to show that there is no correlation between spVL and infection by hepatitis C, hepatitis B or syphilis (see Supplementary Results).
Furthermore, viruses evolving in similar within-host environment (either because of similar HLA alleles or drug treatments) might tend to evolve in a similar fashion. As a result, the proximity in the phylogeny could indicate proximity in within-host environment rather than proximity in the transmission chain. In order to limit the effect of host*virus interactions, we built a phylogeny using mutations on third codon positions only. As shown in the Supplementary Results, the outcome of the test is similar: is equal to 0.52 for spVL and to 0.82 for prAZT in the MSM strict dataset. That a phylogeny built on synonymous mutations only leads to similar results allows us to rule out convergent evolution as a potential bias.
We do not know which proportion of the virus genotype effect is due to the interaction between virus and host genotypes (i.e. that some host genotypes are more sensitive to some virus genotypes). This implies that, in theory, the host control on spVL could also be high. A way to test this effect so that it could be compared to the present result would be to conduct a similar approach but with a host phylogeny instead of a virus phylogeny. The tip data would be the same and the results would tell us what the host genotype control over the trait is. This analysis could not be performed with the subset of the SHCS data we used in this study because we lacked information for many hosts. However, it might be achievable with another subset of the SHCS data.
More speculatively, we find that, in agreement with an earlier study [28], the correlation between spVL and dsCD4 is weak (low ). However, both these traits are known to be early predictors of virulence [18], [21]. A way to reconcile these two apparently contradictory facts would be to show that spVL and dsCD4 are both correlated with processes that add up to determine virulence. Another way to write this is that if , both and are correlated with but needs not to be correlated with . In our case, would be virulence and and pathogenic processes linked with dsCD4 and spVL. We show that the virus has a strong control over spVL but not over dsCD4. If dsCD4 was shown to be strongly controlled by the host genotype, the and processes that contribute to virulence could be interpreted as a virus and a host contribution. Our results suggest that a way to better understand the progression to AIDS could be to disentangle host and viral contributions to virulence, which are highlighted here by the different control of the virus over dsCD4 and spVL.
Advantages of the phylogenetic comparative approach
Some studies have used phylogenies of infections to understand the epidemiology of infectious diseases (see [52] and references therein). However, these studies are based on qualitative traits, usually the presence/absence at a given location (geographically or within a type of cells). Also, these studies use estimators to link the phylogeny and the qualitative traits that are different from phylogenetic signal and thus not directly associable with heritability.
To our knowledge, our study is the first application of the phylogenetic comparative approach (PCA) to infection quantitative traits. As such, it makes it relevant to discuss the prospects of its application to infectious diseases. The main advantage of the PCA is that it does not require any prior knowledge about the transmission pairs. First, this greatly increases the amount of data accessible. Indeed, in many cohort studies, such as the SHCS, there is no information about transmission pairs. Previous studies estimating the heritability of spVL have 155 [9], 194 [8], 112 [10] and 48 patients [11] in their largest dataset. Here, we have 661 patients in our largest dataset and 134 in the smallest. Having more patient data allows us to improve the phylogeny by focusing on MSM only. It also allows us to improve the estimation of spVL by selecting patients for whom we have several measures of viral load, which could explain why we detect higher signal than earlier studies in which many spVL had to be estimated from a single measurement [8]. Second, working without pairs reduces some of the biases inherent to studies based on known couples (e.g. living in the same environment). Third, it incorporates within-host evolution through the branch length. Even though all the genetic data we use originates from after the acute phase, the sampling time is indirectly taken into account because a virus sampled during chronic infection should generate longer branches in the phylogeny than a virus transmitted early in the infection.
The PCA can also be used to study correlations among infection traits. To our knowledge, no other study has raised the problem that, for rapidly evolving diseases, data from different patients are not independent and that regressions should be weighted by distance in the transmission chain.
Finally, for most non-human diseases, standard population genetics settings can be conducted to estimate experimentally trait heritability. In the case of human diseases, we work with epidemiological data. The PCA is thus especially suited to study rapidly evolving diseases of humans.
Limitations
We were unable to detect phylogenetic signal for the log(spVL) when we used a liberal spVL definition or when we considered all the transmission groups. This is consistent with earlier studies that find that restricting the dataset to improve the phylogeny [8] or the spVL measure [10], [11] greatly increases the heritability estimate. Our interpretation is that the strict criterion we use allows us to improve the spVL measurement (thus decreasing measurement error) and that restricting the dataset to MSM patients yields a more accurate phylogeny (because of a better estimation of the transmission chain in the SHCS). Moreover, both restrictions remove the effect of confounding factors, such as patient sex or age, on trait values.
For completeness, we mention two alternative hypotheses to account for the low signal value in the ‘MSM liberal’ dataset. First, it is possible that high within-host variability in viral load measurements is positively correlated with the amount of within-host evolution, which itself has been shown to be linked with disease progression [53]. In short, our strict selection criterion might be selecting for infections with little within-host evolution. However, this is unlikely because, as we show in the Supplementary Results, the log(spVL) is slightly higher in the strict than in the liberal dataset. If variability in spVL was correlated with within-host evolution, we would expect patients from the strict dataset to have a slow disease progression (i.e. a low spVL). A possibility to test this effect would be to vary the level of strictness of the spVL definition. Second, it is possible that the method we use is less efficient at detecting phylogenetic signal in trees that are too large. In the Supplementary Results, we show that tree size could have a slight effect on the accuracy of , but this effect is less important than the intensity of the signal.
As shown in Figure 2, the correlation between heritability and phylogenetic signal is not 1 to 1. This means that, even if there is a strong control of the virus genotype on the spVL, it is not yet possible to say if this value is closer to 40 or 60%. Further analyses are required to understand analytically the exact mapping between heritability and phylogenetic signal in the case of infectious diseases.
Finally, we built the phylogeny using the pol gene, which is the gene routinely sequenced in the SHCS. However, using a different gene is unlikely to affect our results: a recent study shows that phylogenies built on clonal env sequences were almost identical to phylogenies built on pol sequences from known transmission pairs [54].
Perspectives
The PCA argues that HIV set-point viral load is strongly controlled by the virus genotype. Our study can be extended by considering other infection life-history traits (e.g. the duration of the infection) or other infectious diseases that evolve rapidly enough in their host for a phylogeny to be inferred. Further studies are also needed to identify the specificities of the application of the comparative method to phylogenies of infectious diseases. Nevertheless, this approach opens new perspectives for evolutionary epidemiology by allowing a better understanding of how natural selection acts on infection traits. It also has the practical advantage to be applicable to many datasets of infectious diseases of humans because it does not require any prior knowledge of the transmission chain.
Materials and Methods
The data
The Swiss HIV Cohort Study (SHCS) is a nationwide prospective study based on voluntary participation of persons infected with HIV-1. The rationale, organisation and baseline characteristics of the study [15], [55] and the drug resistance database [56] we use have been described elsewhere in detail. Data of 1100 patients could be incorporated in this study. Each patient is represented only once in the data. We selected SHCS participants infected by HIV-1 subtype B (which is the majority in Switzerland) with a genotypic drug resistance test while still ART-naive and with at least three HIV RNA measurements. We only included measurements that were collected after the acute phase of HIV infection (as described in [25]), but prior to start of ART, or the first CDC C event, or the time when the CD4 count first drops below 200 cells. Many HIV-infected patients in Switzerland, as in other countries, receive their HIV diagnosis at a late disease stage and almost immediately start HIV treatment. However, these late presenting patients do not differ from other patients infected by HIV-1 subtype B [57], and this is not likely to introduce a bias on the 1100 patients we consider.
The three main transmission groups in the SHCS are heterosexuals (HET), injection drug users (IDU) and men having sex with men (MSM). We focused on MSM because, on the basis of all newly reported positive HIV Tests in Switzerland between 2000 and 2006 (Federal Office of Public Health, Switzerland), we estimate that the liberal dataset includes 25% of all newly diagnosed MSM, as opposed to 10% for IDU and 4% for HET. Also, a previous study has shown that patients from this transmission group tend to cluster in phylogenetic trees [25]. In our case, it means that the phylogeny we obtain using this transmission group only is likely to be closer to the actual transmission chain.
The phylogenies
For each patient, we know the RNA sequence generated by bulk sequencing of the HIV polymerase (the pol gene). The sequence isolated from 49 patients infected by HIV-1 subtype C was used as an outgroup. We removed all major amino acid positions that are strongly correlated with antiretroviral drug resistance and built a different tree for each of the four subsets of the dataset. In all the trees we built, the ingroup and the outgroup were monophyletic. All phylogenies were built both with a maximum likelihood approach and with a Bayesian approach.
The spVL
Measuring spVL often generates passionate debates. The notion of spVL originates from the realisation that during the asymptomatic phase of an HIV infection, the viral load remains generally stable. The problem is that fluctuations can occur in some patients [58]. Also, there seems to be a tendency for the viral load to increase during the asymptomatic phase [59], which means that defining spVL as a line (with a slope and an intercept) could provide us with more information. Overall, the most appropriate measurement for spVL largely depends on the data available and on the question asked.
Having multiple viral load measures in each patient allows to improve the quality of the spVL estimate. Some use the median value of viral load measurements [20]. Other studies on the host genetic control over spVL variations define spVL as the mean log10 virus load per mL in patients and only consider cases where all the viral load measurement fluctuate within a 0.5-log band around the patient specific mean [2], [3]. Our ‘strict’ definition of spVL is similar except for the fact that we allow measures to fluctuate in a 1-log band (). We also consider a ‘liberal’ definition, where spVL is the mean viral load taken over at least three consecutive viral loads, fluctuating within a 1-log band (). Note that spVL was a continuous (and normally distributed) trait in all our datasets so that the variation we observed are not likely to be linked to a specific allele of a gene.
The effects of patient age, sex and transmission group on the traits we study (spVL, dsCD4 and prAZT) are described in Supplementary Results (see also [19] for a review).
The prAZT
This trait is linked to the probability that a virus is resistant to zidovudine (AZT) without ever having been exposed to this drug. It is estimated from the pol sequence using the geno2pheno system [60]. In order to remove potential correlations between this trait and the phylogeny, we only used the positions associated with drug resistance that we removed to build the phylogeny to estimate prAZT. These relevant positions were inserted in a neutral background sequence (for which there was no drug resistance).
Phylogenetic signal
Phylogenetic signal measures the extent to which the fact that some species tend to have similar trait values can be explained statistically by their close evolutionary history (i.e. the fact that they share a recent common ancestor). Estimators are usually based on Felsenstein's method of independent contrasts [14], [24]. A contrast is the difference between two trait values of two tips of the phylogeny, which is weighted by the distance between the tips in the phylogeny. Mathematically, the contrast between two tips and is given by:(1)where and are the trait values of and and is the distance between and in the phylogeny.
If the variance in all the independent contrasts of a phylogeny is low (resp. high), it means that related species tend to have similar (resp. different) trait values. This simple approach is not entirely satisfactory because, for instance, it does consider the fact that specific shapes of the phylogeny can be more prone to exhibit higher or lower values in variance of contrasts. To solve this problem, we use two recently developed estimators.
The first estimator, [12], is based on the mean squared error (MSE) of the contrasts or of the terms of Pagel's covariance matrix, which is described below. The ratio between the MSE obtained after transformation of the tree by a factor (denoted MSE) and the MSE obtained with the real tree (denoted MSE) indicates how well the transformed tree fits the data. is a normalisation of this quantity. It is obtained by dividing the ratio MSE/MSE by the expected ratio MSE/MSE given the shape and size of the phylogeny. has the advantage of being normalised, i.e. that its value accounts for the fact that phylogenetic signal depends on the shape of the phylogeny. The significance of the estimate is assessed through a randomisation test based on a shuffling of the tip values.
The second estimator, , was introduced by Pagel [26], [61]. The idea is to formalise the phylogeny using a single variance-covariance matrix (denoted ), which predicts the covariance between the traits of two tips based on their distance in the phylogeny (i.e. how old their most recent common ancestor is) and assuming Brownian motion. From this covariance matrix , the model can generate a predicted distribution of traits (a vector ) in the population (i.e. tip values). is used to multiply the off-diagonal terms of the matrix. It is then possible to generate a predicted distribution of tip values that depends on (). The phylogenetic signal is the value of that generates the tip data () the closest to the observed data (). The fit is estimated with a Maximum Likelihood approach. is less robust than (see e.g. the variance in Figure 2), which is why we apply it to the set of trees resulting from a Bayesian estimation of the phylogeny and present the median value we obtain (). For further details about these estimators and about their high level of robustness, see the Supplementary Results.
The model
We simulate an evolutionary process of a trait with known heritability values () on a tree to compare the performance of our two estimators (Figure 2). We initiate the system with an ancestor that has a trait value () drawn from the empirical distribution of traits (spVL) in the population (note however that this model is more general and can be applied to any trait of any infectious disease). Every new infection is modelled as a branching in the tree: one of the new branches corresponds to the infecting individuals and is given the trait value of the ancestor branch (), whereas the other branch corresponds to the infected individual and is given a new trait value (). This new trait is obtained with the following rule:(2)where is the heritability of the trait and is a random variable drawn from the empirical trait distribution in the population (in the ‘liberal’ dataset). Another possibility would have been to model explicitly the environmental and the genetical component of the trait (as in [13]). The problem with such an approach is that it would require the introduction of a stabilising selection model, instead of the Brownian motion model, to describe trait evolution. Here, the random variable is drawn in a trait distribution, which allows us to obtain a final set of trait values that has a mean and a variance close to what is observed empirically.
We introduce host death events as well as incomplete sampling of the hosts. The probability of dying was taken to be 1/3 of the probability of transmitting (thus approximating an of 3 for the disease). We model the evolution over 13 generations and then sample uniformly at random 128 tips for each tree. There were 20 replicates for each of the heritability values we model. and were estimated for each of the replicates. The resulting trees before sampling did not have the same size (because of the stochastic death process) but we found no significant effect of the intensity of sampling on the value of the estimator.
Further details about the Materials and Methods are available in the Supplementary Methods (Text S2).
Ethics statement
The Swiss HIV Cohort Study (SHCS) has been approved by ethical committees of all participating institutions and written informed consent has been obtained from the participants. This project has been approved by the Scientific Board of the SHCS as project 606.
Gene accession numbers
For scientific and ethical reasons explained in details in [25], only a fraction (approximately 10%) of the sequences of the SHCS is accessible via GenBank (accession numbers, GU344102GU344671). However, all data in the SHCS can be used for well-defined projects that are in accordance with the guidelines of the SHCS, if a corresponding project proposal is approved by the SHCS scientific board.
Supporting Information
Zdroje
1. BuchbinderSP
KatzMH
HessolNA
O'MalleyPM
HolmbergSD
1994 Long-term HIV-1 infection without immunologic progression. AIDS 8 1123 8
2. FellayJ
ShiannaKV
GeD
ColomboS
LedergerberB
2007 A whole-genome association study of major determinants for host control of HIV-1. Science 317 944 947
3. FellayJ
GeD
ShiannaKV
ColomboS
LedergerberB
2009 Common genetic variation and the control of HIV-1 in humans. PLoS Genet 5 e1000791
4. WardJW
BushTJ
PerkinsHA
LiebLE
AllenJR
1989 The natural history of transfusion-associated infection with human immunodeficiency virus. Factors influencing the rate of progression to disease. N Engl J Med 321 947 52
5. AshtonLJ
LearmontJ
LuoK
WylieB
StewartG
1994 HIV infection in recipients of blood products from donors with known duration of infection. Lancet 344 718 20
6. IoannidisJPA
TatsioniA
AbramsEJ
BulterysM
CoombsRW
2004 Maternal viral load and rate of disease progression among vertically HIV-1-infected children: an international meta-analysis. AIDS 18 99 108
7. LynchM
WalshB
1998 Genetics and Analysis of Quantitative Traits Sunderland, MA, USA Sinauer Associates, Inc
8. HollingsworthTD
LaeyendeckerO
ShirreffG
DonnellyCA
SerwaddaD
2010 HIV-1 transmitting couples have similar viral load set-points in Rakai, Uganda. PLoS Pathog 6 e1000876
9. TangJ
TangS
LobashevskyE
ZuluI
AldrovandiG
2004 HLA allele sharing and HIV type 1 viremia in seroconverting Zambians with known transmitting partners. AIDS Res Hum Retroviruses 20 19 25
10. van der KuylAC
JurriaansS
PollakisG
BakkerM
CornelissenM
2010 HIV RNA levels in transmission sources only weakly predict plasma viral load in recipients. AIDS 24 1607 8
11. HechtFM
HartogensisW
BraggL
BacchettiP
AtchisonR
2010 HIV RNA level in early infection is predicted by viral load in the transmission source. AIDS 24 941 5
12. BlombergSP
GarlandTJ
IvesAR
2003 Testing for phylogenetic signal in comparative data: behavioral traits are more labile. Evolution 57 717 745
13. HousworthEA
MartinsEP
LynchM
2004 The phylogenetic mixed model. Am Nat 163 84 96
14. FelsensteinJ
2004 Inferring phylogenies Sunderland, MA, USA Sinauer Associates, Inc
15. The Swiss HIV Cohort Study 2010 Cohort Profile: The Swiss HIV Cohort Study. Int J Epidemiol in press doi:10.1093/ije/dyp321
16. FraserC
HollingsworthTD
ChapmanR
de WolfF
HanageWP
2007 Variation in HIV-1 set-point viral load: epidemiological analysis and an evolutionary hypothesis. Proc Natl Acad Sci USA 104 17441 17446
17. MüllerV
MaggioloF
SuterF
LadisaN
De LucaA
2009 Increasing clinical virulence in two decades of the Italian HIV epidemic. PLoS Pathog 5 e1000454
18. MellorsJW
RinaldoCRJ
GuptaP
WhiteRM
ToddJA
1996 Prognosis in HIV-1 infection predicted by the quantity of virus in plasma. Science 272 1167 1170
19. LangfordSE
AnanworanichJ
CooperDA
2007 Predictors of disease progression in HIV infection: a review. AIDS Res Ther 4 11
20. MellorsJW
MargolickJB
PhairJP
RinaldoCR
DetelsR
2007 Prognostic value of HIV-1 RNA, CD4 cell count, and CD4 Cell count slope for progression to AIDS and death in untreated HIV-1 infection. JAMA 297 2349 50
21. MellorsJW
MuñozA
GiorgiJV
MargolickJB
TassoniCJ
1997 Plasma viral load and CD4+ lymphocytes as prognostic markers of HIV-1 infection. Ann Intern Med 126 946 54
22. LeitnerT
EscanillaD
FranzenC
UhlenM
AlbertJ
1996 Accurate reconstruction of a known HIV-1 transmission history by phylogenetic tree analysis. Proc Natl Acad Sci USA 93 10864 10869
23. HuéS
ClewleyJP
CanePA
PillayD
2004 HIV-1 pol gene variation is sufficient for reconstruction of transmissions in the era of antiretroviral therapy. AIDS 18 719 728
24. FelsensteinJ
1985 Phylogenies and the Comparative Method. Am Nat 125 1 15
25. KouyosRD
von WylV
YerlyS
BöniJ
TafféP
2010 Molecular epidemiology reveals long-term changes in HIV type 1 subtype B transmission in Switzerland. J Infect Dis 201 1488 97
26. FreckletonRP
HarveyPH
PagelM
2002 Phylogenetic analysis and comparative data: a test and review of evidence. Am Nat 160 712 726
27. FreckletonRP
2009 The seven deadly sins of comparative analysis. J Evol Biol 22 1367 75
28. RodríguezB
SethiAK
CheruvuVK
MackayW
BoschRJ
2006 Predictive value of plasma HIV RNA level on rate of CD4 T-cell decline in untreated HIV infection. JAMA 296 1498 1506
29. KankiPJ
HamelDJ
SankaléJL
HsiehCc
ThiorI
1999 Human immunodeficiency virus type 1 subtypes differ in disease progression. J Infect Dis 179 68 73
30. AriënKK
VanhamG
ArtsEJ
2007 Is HIV-1 evolving to a less virulent form in humans? Nat Rev Microbiol 5 141 151
31. TaylorBS
SobieszczykME
McCutchanFE
HammerSM
2008 The challenge of HIV-1 subtype diversity. N Engl J Med 358 1590 602
32. GoldsteinS
OurmanovI
BrownCR
PlishkaR
Buckler-WhiteA
2005 Plateau levels of viremia correlate with the degree of CD4+-T-cell loss in simian immunodeficiency virus SIVagm-infected pigtailed macaques: variable pathogenicity of natural SIVagm isolates. J Virol 79 5153 5162
33. RambautA
PosadaD
CrandallKA
HolmesEC
2004 The causes and consequences of HIV evolution. Nat Rev Genet 5 52 61
34. AndersonRM
MayRM
1982 Coevolution of hosts and parasites. Parasitology 85 411 426
35. BonhoefferS
FunkGA
GunthardHF
FischerM
MullerV
2003 Glancing behind virus load variation in HIV-1 infection. Trends Microbiol 11 499 504
36. HollingsworthTD
AndersonRM
FraserC
2008 HIV-1 transmission, by stage of infection. J Infect Dis 198 687 693
37. KeetIP
VeugelersPJ
KootM
de WeerdMH
RoosMT
1996 Temporal trends of the natural history of HIV-1 infection following seroconversion between 1984 and 1993. AIDS 10 1601 2
38. AriënKK
TroyerRM
GaliY
ColebundersRL
ArtsEJ
2005 Replicative fitness of historical and recent HIV-1 isolates suggests HIV-1 attenuation over time. AIDS 19 1555 64
39. SiniccoA
ForaR
RaiteriR
SciandraM
BechisG
1997 Is the clinical course of HIV-1 changing? Cohort study. Brit Med J 314 1232 7
40. VanhemsP
LambertJ
GuerraM
HirschelB
AllardR
1999 Association between the rate of CD4+ T cell decrease and the year of human immunodeficiency virus (HIV) type 1 seroconversion among persons enrolled in the Swiss HIV cohort study. J Infect Dis 180 1803 8
41. DorrucciM
RezzaG
PorterK
PhillipsA
Concerted Action on Seroconversion to AIDS and Death in Europe Collaboration 2007 Temporal trends in postseroconversion CD4 cell count and HIV load: the Concerted Action on Seroconversion to AIDS and Death in Europe Collaboration, 1985-2002. J Infect Dis 195 525 34
42. GrasL
JurriaansS
BakkerM
van SighemA
BezemerD
2009 Viral load levels measured at set-point have risen over the last decade of the HIV epidemic in the Netherlands. PLoS One 4 e7365
43. HolmbergSD
ConleyLJ
LubySP
CohnS
WongLC
1995 Recent infection with human immunodeficiency virus and possible rapid loss of CD4 T lymphocytes. J Acquir Immune Defic Syndr Hum Retrovirol 9 291 6
44. O'BrienTR
HooverDR
RosenbergPS
ChenB
DetelsR
1995 Evaluation of secular trends in CD4+ lymphocyte loss among human immunodeficiency virus type 1 (HIV-1)-infected men with known dates of seroconversion. Am J Epidemiol 142 636 42
45. CASCADE Collaboration 2000 Time from HIV-1 seroconversion to AIDS and death before widespread use of highly-active antiretroviral therapy: a collaborative re-analysis. Collaborative Group on AIDS Incubation and HIV Survival including the CASCADE EU Concerted Action. Concerted Action on SeroConversion to AIDS and Death in Europe. Lancet 355 1131 7
46. CASCADE Collaboration 2003 Differences in CD4 cell counts at seroconversion and decline among 5739 HIV-1-infected individuals with well-estimated dates of seroconversion. J Acquir Immune Defic Syndr 34 76 83
47. MüllerV
LedergerberB
PerrinL
KlimkaitT
FurrerH
2006 Stable virulence levels in the HIV epidemic of Switzerland over two decades. AIDS 20 889 894
48. HerbeckJT
GottliebGS
LiX
HuZ
DetelsR
2008 Lack of evidence for changing virulence of HIV-1 in North America. PLoS ONE 3 e1525
49. TroudeP
ChaixML
TranL
DeveauC
SengR
2009 No evidence of a change in HIV-1 virulence since 1996 in France. AIDS 23 1261 7
50. TrachtenbergE
KorberB
SollarsC
KeplerTB
HraberPT
2003 Advantage of rare HLA supertype in HIV disease progression. Nat Med 9 928 35
51. GoulderPJR
WatkinsDI
2008 Impact of MHC class I diversity on immune control of immunodeficiency virus replication. Nat Rev Immunol 8 619 30
52. ParkerJ
RambautA
PybusOG
2008 Correlating viral phenotypes with phylogeny: Accounting for phylogenetic uncertainty. Infect Genet Evol 8 239 246
53. LemeyP
Kosakovsky PondSL
DrummondAJ
PybusOG
ShapiroB
2007 Synonymous substitution rates predict HIV disease progression as a result of underlying replication dynamics. PLoS Comput Biol 3 e29
54. RiederP
JoosB
von WylV
KusterH
GrubeC
2010 HIV-1 transmission after cessation of early antiretroviral therapy among men having sex with men. AIDS 24 1177 1183
55. LedergerberB
von OverbeckJ
EggerM
LuthyR
1994 The Swiss HIV Cohort Study: rationale, organization and selected baseline characteristics. Soz Praventivmed 39 387 394
56. von WylV
YerlyS
BöniJ
BürgisserP
KlimkaitT
2007 Emergence of HIV-1 drug resistance in previously untreated patients initiating combination antiretroviral treatment: a comparison of different regimen types. Arch Intern Med 167 1782 90
57. WolbersM
BucherHC
FurrerH
RickenbachM
CavassiniM
2008 Delayed diagnosis of HIV infection and late initiation of antiretroviral therapy in the Swiss HIV Cohort Study. HIV Med 9 397 405
58. RaboudJM
MontanerJS
ConwayB
HaleyL
SherlockC
1996 Variation in plasma RNA levels, CD4 cell counts, and p24 antigen levels in clinically stable men with human immunodeficiency virus infection. J Infect Dis 174 191 4
59. LylesRH
MuñozA
YamashitaTE
BazmiH
DetelsR
2000 Natural history of human immunodeficiency virus type 1 viremia after seroconversion and proximal to AIDS in a large cohort of homosexual men. Multicenter AIDS Cohort Study. J Infect Dis 181 872 80
60. BeerenwinkelN
DäumerM
OetteM
KornK
HoffmannD
2003 Geno2pheno: Estimating phenotypic drug resistance from HIV-1 genotypes. Nucleic Acids Res 31 3850 5
61. PagelM
1994 Detecting correlated evolution on phylogenies: a general method for the comparative analysis of discrete characters. Proc R Soc Lond B 255 37 45
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek SRFR1 Negatively Regulates Plant NB-LRR Resistance Protein Accumulation to Prevent AutoimmunityČlánek Inhibition of TIR Domain Signaling by TcpC: MyD88-Dependent and Independent Effects on VirulenceČlánek A Family of Plasmodesmal Proteins with Receptor-Like Properties for Plant Viral Movement Proteins
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2010 Číslo 9- Stillova choroba: vzácné a závažné systémové onemocnění
- Perorální antivirotika jako vysoce efektivní nástroj prevence hospitalizací kvůli COVID-19 − otázky a odpovědi pro praxi
- Diagnostika virových hepatitid v kostce – zorientujte se (nejen) v sérologii
- Jak souvisí postcovidový syndrom s poškozením mozku?
- Familiární středomořská horečka
-
Všechny články tohoto čísla
- Azole Drugs Are Imported By Facilitated Diffusion in and Other Pathogenic Fungi
- Two Genes on A/J Chromosome 18 Are Associated with Susceptibility to Infection by Combined Microarray and QTL Analyses
- Impact of Simian Immunodeficiency Virus Infection on Chimpanzee Population Dynamics
- Breaking the Stereotype: Virulence Factor–Mediated Protection of Host Cells in Bacterial Pathogenesis
- The Canine Papillomavirus and Gamma HPV E7 Proteins Use an Alternative Domain to Bind and Destabilize the Retinoblastoma Protein
- Rescue of HIV-1 Release by Targeting Widely Divergent NEDD4-Type Ubiquitin Ligases and Isolated Catalytic HECT Domains to Gag
- Steric Shielding of Surface Epitopes and Impaired Immune Recognition Induced by the Ebola Virus Glycoprotein
- Dynamics of the Multiplicity of Cellular Infection in a Plant Virus
- HLA Class I Binding of HBZ Determines Outcome in HTLV-1 Infection
- Pathogenic Bacteria Target NEDD8-Conjugated Cullins to Hijack Host-Cell Signaling Pathways
- The HA and NS Genes of Human H5N1 Influenza A Virus Contribute to High Virulence in Ferrets
- SRFR1 Negatively Regulates Plant NB-LRR Resistance Protein Accumulation to Prevent Autoimmunity
- Cyclin-Dependent Kinase Activity Controls the Onset of the HCMV Lytic Cycle
- The N-Terminal Domain of the Arenavirus L Protein Is an RNA Endonuclease Essential in mRNA Transcription
- Generation of Neutralizing Antibodies and Divergence of SIVmac239 in Cynomolgus Macaques Following Short-Term Early Antiretroviral Therapy
- Inhibition of TIR Domain Signaling by TcpC: MyD88-Dependent and Independent Effects on Virulence
- Intracellular Proton Conductance of the Hepatitis C Virus p7 Protein and Its Contribution to Infectious Virus Production
- The Transcriptome of the Human Pathogen at Single-Nucleotide Resolution
- The Epidermal Growth Factor Receptor (EGFR) Promotes Uptake of Influenza A Viruses (IAV) into Host Cells
- Surface Co-Expression of Two Different PfEMP1 Antigens on Single -Infected Erythrocytes Facilitates Binding to ICAM1 and PECAM1
- Sequestration and Tissue Accumulation of Human Malaria Parasites: Can We Learn Anything from Rodent Models of Malaria?
- Phylogenomics of Ligand-Gated Ion Channels Predicts Monepantel Effect
- Generation of Covalently Closed Circular DNA of Hepatitis B Viruses via Intracellular Recycling Is Regulated in a Virus Specific Manner
- CpG-Methylation Regulates a Class of Epstein-Barr Virus Promoters
- Molecular and Evolutionary Bases of Within-Patient Genotypic and Phenotypic Diversity in Extraintestinal Infections
- A Bistable Switch and Anatomical Site Control Virulence Gene Expression in the Intestine
- Are Members of the Fungal Genus (a) Commensals; (b) Opportunists; (c) Pathogens; or (d) All of the Above?
- Structures of Receptor Complexes of a North American H7N2 Influenza Hemagglutinin with a Loop Deletion in the Receptor Binding Site
- Phylogenetic Approach Reveals That Virus Genotype Largely Determines HIV Set-Point Viral Load
- The Coevolution of Virulence: Tolerance in Perspective
- Involvement of the Cytokine MIF in the Snail Host Immune Response to the Parasite
- Structure of the Extracellular Portion of CD46 Provides Insights into Its Interactions with Complement Proteins and Pathogens
- A Family of Plasmodesmal Proteins with Receptor-Like Properties for Plant Viral Movement Proteins
- High Content Phenotypic Cell-Based Visual Screen Identifies Acyltrehalose-Containing Glycolipids Involved in Phagosome Remodeling
- A Novel Small Molecule Inhibitor of Hepatitis C Virus Entry
- The Microbiota Mediates Pathogen Clearance from the Gut Lumen after Non-Typhoidal Diarrhea
- RNA Polymerases (L-Protein) Have an N-Terminal, Influenza-Like Endonuclease Domain, Essential for Viral Cap-Dependent Transcription
- Pathogen Specific, IRF3-Dependent Signaling and Innate Resistance to Human Kidney Infection
- Cellular Entry of Ebola Virus Involves Uptake by a Macropinocytosis-Like Mechanism and Subsequent Trafficking through Early and Late Endosomes
- The Length of Vesicular Stomatitis Virus Particles Dictates a Need for Actin Assembly during Clathrin-Dependent Endocytosis
- Formation of Mobile Chromatin-Associated Nuclear Foci Containing HIV-1 Vpr and VPRBP Is Critical for the Induction of G2 Cell Cycle Arrest
- Association of Tat with Promoters of PTEN and PP2A Subunits Is Key to Transcriptional Activation of Apoptotic Pathways in HIV-Infected CD4+ T Cells
- Metal Hyperaccumulation Armors Plants against Disease
- Cyclin-Dependent Kinase-Like Function Is Shared by the Beta- and Gamma- Subset of the Conserved Herpesvirus Protein Kinases
- Role of Acetyl-Phosphate in Activation of the Rrp2-RpoN-RpoS Pathway in
- Ebolavirus Is Internalized into Host Cells Macropinocytosis in a Viral Glycoprotein-Dependent Manner
- A Novel Family of IMC Proteins Displays a Hierarchical Organization and Functions in Coordinating Parasite Division
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Structure of the Extracellular Portion of CD46 Provides Insights into Its Interactions with Complement Proteins and Pathogens
- The Length of Vesicular Stomatitis Virus Particles Dictates a Need for Actin Assembly during Clathrin-Dependent Endocytosis
- Inhibition of TIR Domain Signaling by TcpC: MyD88-Dependent and Independent Effects on Virulence
- The Coevolution of Virulence: Tolerance in Perspective
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



