-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
High Content Phenotypic Cell-Based Visual Screen Identifies Acyltrehalose-Containing Glycolipids Involved in Phagosome Remodeling
The ability of the tubercle bacillus to arrest phagosome maturation is considered one major mechanism that allows its survival within host macrophages. To identify mycobacterial genes involved in this process, we developed a high throughput phenotypic cell-based assay enabling individual sub-cellular analysis of over 11,000 Mycobacterium tuberculosis mutants. This very stringent assay makes use of fluorescent staining for intracellular acidic compartments, and automated confocal microscopy to quantitatively determine the intracellular localization of M. tuberculosis. We characterised the ten mutants that traffic most frequently into acidified compartments early after phagocytosis, suggesting that they had lost their ability to arrest phagosomal maturation. Molecular analysis of these mutants revealed mainly disruptions in genes involved in cell envelope biogenesis (fadD28), the ESX-1 secretion system (espL/Rv3880), molybdopterin biosynthesis (moaC1 and moaD1), as well as in genes from a novel locus, Rv1503c-Rv1506c. Most interestingly, the mutants in Rv1503c and Rv1506c were perturbed in the biosynthesis of acyltrehalose-containing glycolipids. Our results suggest that such glycolipids indeed play a critical role in the early intracellular fate of the tubercle bacillus. The unbiased approach developed here can be easily adapted for functional genomics study of intracellular pathogens, together with focused discovery of new anti-microbials.
Published in the journal: . PLoS Pathog 6(9): e32767. doi:10.1371/journal.ppat.1001100
Category: Research Article
doi: https://doi.org/10.1371/journal.ppat.1001100Summary
The ability of the tubercle bacillus to arrest phagosome maturation is considered one major mechanism that allows its survival within host macrophages. To identify mycobacterial genes involved in this process, we developed a high throughput phenotypic cell-based assay enabling individual sub-cellular analysis of over 11,000 Mycobacterium tuberculosis mutants. This very stringent assay makes use of fluorescent staining for intracellular acidic compartments, and automated confocal microscopy to quantitatively determine the intracellular localization of M. tuberculosis. We characterised the ten mutants that traffic most frequently into acidified compartments early after phagocytosis, suggesting that they had lost their ability to arrest phagosomal maturation. Molecular analysis of these mutants revealed mainly disruptions in genes involved in cell envelope biogenesis (fadD28), the ESX-1 secretion system (espL/Rv3880), molybdopterin biosynthesis (moaC1 and moaD1), as well as in genes from a novel locus, Rv1503c-Rv1506c. Most interestingly, the mutants in Rv1503c and Rv1506c were perturbed in the biosynthesis of acyltrehalose-containing glycolipids. Our results suggest that such glycolipids indeed play a critical role in the early intracellular fate of the tubercle bacillus. The unbiased approach developed here can be easily adapted for functional genomics study of intracellular pathogens, together with focused discovery of new anti-microbials.
Introduction
Upon engulfment by host macrophages, M. tuberculosis, the main etiological agent of tuberculosis in humans, localizes in vacuoles or phagosomes that fail to fuse with host cell lysosomes and to acidify [1], [2]. This process, called phagosome maturation arrest, may contribute to mycobacterial pathogenicity and to mycobacterial evasion from host immune surveillance. Molecular determinants for the specific features of this host-pathogen interaction are starting to be elucidated, though no clear picture has yet been established [1], [2], [3], [4], [5]. Several mycobacterial products affecting phagosome maturation, such as cell wall lipoglycans and glycolipids [6], or allowing resistance to the acidic milieu of the phagosome [7] have been reported. Apart from focused, candidate gene-based studies [8], [9], large scale approaches have been performed using mutant libraries to obtain a comprehensive picture of the process. Pethe et al. used a M. tuberculosis CDC1551 transposon mutant library to identify mutants that fail to prevent phagosome-lysosome fusion [10]. With the same aim, by use of flow cytometry, Stewart et al. identified a series of Mycobacterium bovis BCG mutants that fail to prevent phagosome acidification [11]. Both studies generated comprehensive lists of novel mycobacterial genes possibly involved in phagosome maturation arrest. However they were performed by infecting cells with large pools of mutants and necessitated several rounds of amplification, thereby introducing a mutant selection bias. Indeed mutants that traffic into late endosomal compartments are likely to be impaired in growth and could be lost during the amplification process. Moreover, such competitive infections may miss detection of mutants that can be trans-complemented by other clones within the mixed infection. This may occur when the interrupted gene encodes a secreted virulence factor or a factor that interferes with processes involving secretion of host factors.
This led us to develop a new type of screening system whereby mutants would be individually investigated, in the absence of other competitive strains. We took advantage of automated confocal fluorescence microscopy and dedicated image analysis to monitor sub-cellular mycobacterial localization of a large number of samples. A transposon mutant library made in a virulent clinical isolate of M. tuberculosis of the W/Beijing family and containing over 11,000 individual mutants was used to infect macrophages in vitro. Ten mutants with an impaired ability to prevent phagosome acidification were isolated. Strikingly, out of these ten mutants, two carried independent insertions in the adjacent moaC1 and moaD1 genes, and two further mutants carried independent insertions in Rv1503c and Rv1506c located within the so-called losA locus that is involved in the synthesis of lipooligosaccharides (LOS) in another mycobacterial species, Mycobacterium marinum [12], [13]. This prompted us to further investigate the lipid content of the concerned transposon mutants, which allowed us to identify novel lipids that are involved in the acidification of phagosomes. The results of this study thus strenghten the hypothesis that M. tuberculosis modulate the biosynthesis of specific glycolipids to manipulate phagosome maturation and shed new light on the genetic locus and the synthesis pathways involved.
Results
Positive LysoTracker staining of macrophages correlates with presence of M. tuberculosis in acidified phagosomes
In order to set-up the optimal conditions of M. tuberculosis infection, mouse bone marrow-derived macrophages were infected with mycobacteria that had previously been covalently labeled with the red fluorescent dye CypHer5. M. tuberculosis reference strain H37Rv, the W-Beijing strain GC1237 [14] and a GC1237 ΔphoP/R attenuated mutant [15] as well as heat-killed bacteria were used. After 2 h of infection, macrophages were pulsed with the acidotropic green fluorescent dye LysoTracker DND-26 to label the acidified compartments. After fixation and nuclei labeling, sample images from four fields per well were acquired using an automated confocal microscope. For each field, three images were recorded: one for the cell nuclei (blue-channel), one for the CypHer5 labeled mycobacterium-positive compartment (red-channel) and one for the LysoTracker-DND-26 positive compartment (green-channel). The acquisition parameters were set so that the LysoTracker signal was minimal in resting non-infected cells. In these settings, cells infected with heat-killed bacteria exhibited a strong LysoTracker signal, whereas cells infected with live bacilli showed a weak signal (Figure 1a). This first set of experiments led us to investigate whether the sole LysoTracker signal could be used as a positive indirect readout for the presence of mycobacteria in acidified compartments. An image analysis script was then developed to determine the parameters that best describe the images and that would be a correlate of infected cells, bacterial load, and LysoTracker-positive compartments. First, the nuclear stain DAPI that was used enabled accurate macrophages number quantification and their spatial positioning. Next, spots in either the red-channel or the green-channel images were labeled as bacterium - or LysoTracker-positive objects, respectively, if their size surface was at least 3 pixels with intensity above a defined threshold. Because the nuclear labeling of the macrophage did not allow us to delineate the exact contour of the cell, a macrophage was considered infected if labeled bacterium objects were distant of less than 5 pixels from the DAPI signal surface. Similarly, LysoTracker-positive objects were labeled as acidified compartments if they were found distant of less than 5 pixels from the cell nucleus in our acquisition conditions. Nearly 100% of the cells were found to be infected after 2 h (Figure 1b). The fraction of bacteria co-localizing with LysoTracker-positive staining (Figure 1c) was found to be less than 10% in cells infected with M. tuberculosis H37Rv (2±1%) or GC1237 (7±1%). In contrast, upon infection with heat-killed H37Rv or GC1237, more than 50% of the bacteria were found to co-localize with acidified compartments (61±3% and 56±4%, respectively). In addition, 20±3% of the live attenuated ΔphoP/R mutant showed a marked acidification of their vacuoles. Opposite patterns were observed when infected cells were stained for the early endosomal marker Rab5A (Figure S1a). Of particular interest, quantification of the percentage of cells containing detectable acidified compartments led to similar results between strains and conditions (Figure 1d). In addition to this latter, another parameter deduced from the images is the surface of acidified compartments proximal to cell nuclei that refers to the total surface area of all the Lysotracker positive objects that are distant of less than 5 pixels from the DAPI signal surface in each field (Figure 2a).
Fig. 1. Quantification of M. tuberculosis intracellular trafficking into acidified compartments by automated confocal imaging. 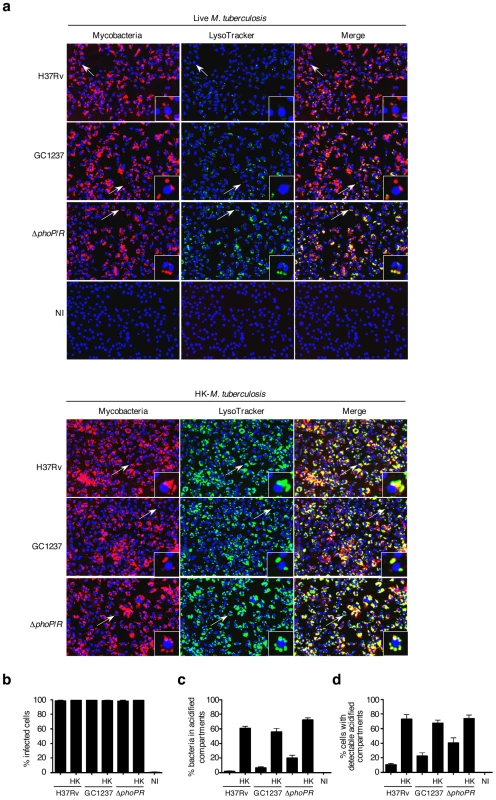
(a) Representative pictures of mouse bone-marrow-derived macrophages infected with CypHer5 (red)-labeled M. tuberculosis H37Rv, GC1237, or GC1237-derived ΔphoP/R mutant, or heat-killed (HK) bacteria. After 2 hours of infection, cells were stained with LysoTracker DND-26 (green) and DAPI (blue) for acidic compartments and cell nuclei labeling, respectively. Non-infected macrophages (NI) are shown as control. Images span 0.450×0.340 mm2. Arrows indicate cells shown at higher magnification in the insets. (b-d) Percentage of infected cells, bacteria inside LysoTracker-positive acidified compartments, and cells with LysoTracker-positive compartments. Values for each condition are the average ± s.d. determined for seven different infections for which four fields were recorded and analyzed, and are representative of two independent experiments. Fig. 2. Phenotypic cell-based genetic screen. 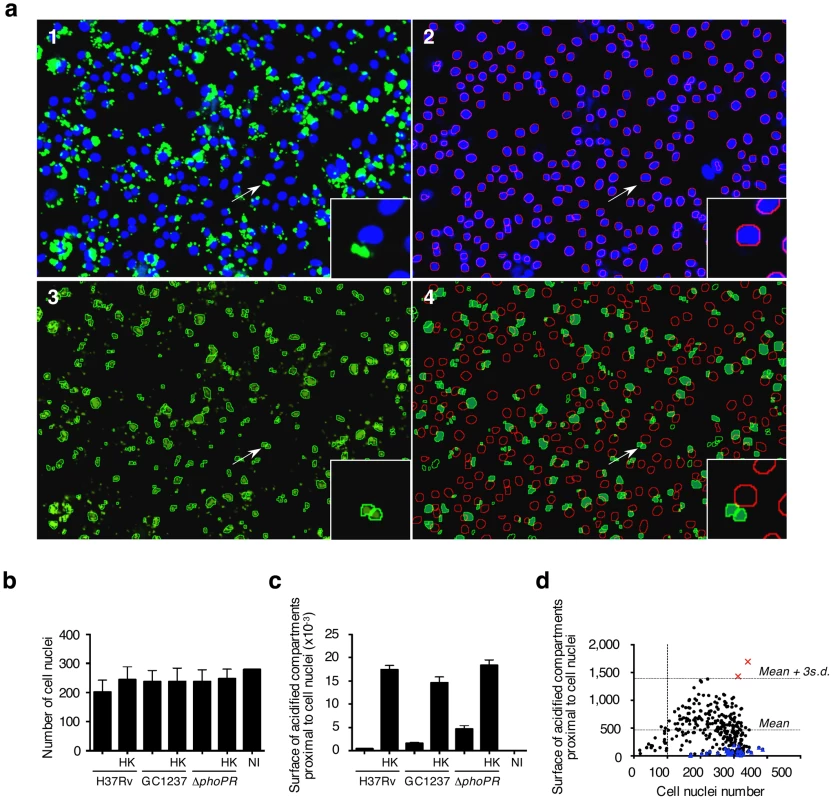
(a) Mouse bone marrow-derived macrophages were infected with HK-M. tuberculosis H3Rv. Image analysis. 1: Typical 2-color image of LysoTracker (green)- and DAPI (blue)-stained infected cells; 2: Red-circled blue objects correspond to detected cell nuclei from the blue channel image; 3: Green-circled green objects correspond to detected acidic compartments from the green channel image; 4: Green-filled surfaces correspond to acidic compartments detected as being proximal to red-circled cell nuclei. Images span 0.450×0.340 mm2. (b,c) Image-based quantification of the cell nuclei number and the total acidic compartment surface proximal to cell nuclei. Values for each condition are the average ± s.d. determined for seven different infections for which four fields were recorded and analyzed, and are representative of three independent experiments and of controls from each 384-well screening microplates. Abbreviations are as in Figure 1. (d) Library screening. Automated confocal imaging-based analysis of a selected microplate containing mouse bone marrow-derived macrophages infected for 2 h with a subset of the M. tuberculosis GC1237-derived mutant library (black dots & red squares), or left uninfected (blue triangles), and subsequently labeled with LysoTracker. Red crosses correspond to two hit mutants selected from this particular microplate. The dynamic range of the assay was confirmed by monitoring the dose-response of Zymosan A, a substance that is known to rapidly traffic into lysosomes. With increasing concentrations of Zymosan A, there was a subsequent linear increase in the intracellular surface positive for LysoTracker staining (Figure S1b,c). This further demonstrates that the acidified compartment surface parameter is directly proportional to the amount of particles within acidic vacuoles and can be used as a correlate of the localization of mycobacteria in acidified vacuoles without additional staining and detection of the bacilli.
To simplify the protocol for high throughput screening (HTS), macrophages were distributed into 384-well plates that had previously been inoculated with mycobacteria. Infection was carried out at 37°C for 2 h, a time-lapse sufficient for macrophage adherence. The monolayer was then subjected to LysoTracker and nuclei staining as described above. The day-to-day as well as plate-to-plate reproducibility was ascertained by including live and heat-killed M. tuberculosis H37Rv, GC1237 and ΔphoP/R mutant in each plate. All samples corresponding to the various reference strains contained a similar number of cell nuclei (Figure 2b). The surface of acidified compartments proximal to cell nuclei in live samples was significantly lower than in heat-killed samples with a Z' score value (live vs. heat-killed) above 0.2 (Figure 2c). Thus this parameter was further chosen as a read-out for HTS.
Our screening assay therefore consisted in the search for bacterial mutants that induce a positive LysoTracker staining phenotype upon macrophage infection.
Identification of transposon mutants that fail to induce phagosome maturation arrest
An 11,180-member mutant library was constructed by transposition of the IS1096-derived Tn5367 transposon in the M. tuberculosis W-Beijing strain GC1237 as previously described [16]. Transposon site hybridization (TraSH) analysis of a 500-member pool subset of the library showed that insertions occurred uniformly in the chromosome (Figure S2). Given that the M. tuberculosis genome spans around 4,000 open reading frame [17], our library represents a 3X-coverage of the whole genome. The mutant library was formated into fourty-four 384-well microplates, which included 5 control wells for each of M. tuberculosis GC1237, the ΔphoP/R mutant, or heat killed GC1237. In addition, all plates included a set of wells with non-infected cells.
The validation of the screen (Z' score value for live vs. heat-killed >0.2) was first ascertained by control analysis on each microplate. Mutants were selected using a stringent threshold, i.e. for values of acidified compartment surface of 3 s.d. above the average, and for nuclei numbers above 100, which corresponds to low cytotoxicity. A typical plate is shown in Figure 2d, whereby two mutants with aberrant trafficking are highlighted. From this analysis, a series of 10 mutants was selected for further characterization. The trafficking phenotype of all selected mutants was confirmed on the individual level in mouse and human primary macrophages (Figure 3). The ability of the ten mutants to survive and replicate inside mouse macrophages was investigated to determine whether enhanced trafficking into acidified compartments correlated with impaired intracellular growth (Figure 3c). Strikingly, intracellular trafficking to acidified vacuoles did not necessarily correlate with growth attenuation; indeed the mutant in lppM (mutant P65B12) for instance, that largely trafficked to an acidified vacuole, could actively multiply inside host cells on the longer term.
Fig. 3. Phenotypic cell-based assay identifies 10 M. tuberculosis mutants that rapidly traffic into acidified phagosomes. 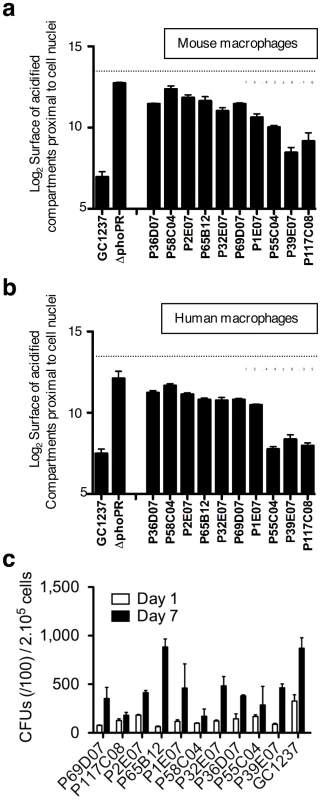
Mouse bone marrow- (a) and human monocyte- (b) derived macrophages were infected for 2 h with the various mutants at a multiplicity of infection (MOI) of 10 bacteria per cell, stained with LysoTracker and subsequently analyzed by confocal imaging. Cells infected with M. tuberculosis GC1237, heat-killed (HK)-GC1237 or with the ΔphoPR mutant are shown as controls. Values for each condition are the average ± s.d. determined for three different infections for which four fields were recorded and analyzed, and are representative of two independent experiments. The dotted lines and the numbers below the lines indicate values for HK-bacteria. (c) Intracellular survival ability of the mutants. Mouse bone marrow-derived macrophages were infected with the mutant and wild type strains at a MOI of 1 bacterium per cell. CFUs were scored 1 and 7 days after infection. The bars for each condition are the mean ± s.d of a triplicate experiment. As selected examples of individual confirmation of altered trafficking, two mutants are displayed in Figure 4, where mouse macrophages were infected with GFP-expressing strains, subsequently stained with LysoTracker red, and examined by confocal microscopy (Figure 4a). Image analysis confirmed that the percentages of acidified compartment-containing cells were higher for the selected mutants than for the GC1237 parental wild-type strain (Figure 4b), which correlated with higher percentages of bacteria in acidic vacuoles (Figure 4c). Indeed more than 25% of the mutant bacilli were found in acidic vacuoles, compared to less than 10% for the control strain.
Fig. 4. M. tuberculosis mutants #1 and #6 traffic rapidly to acidified vacuoles. 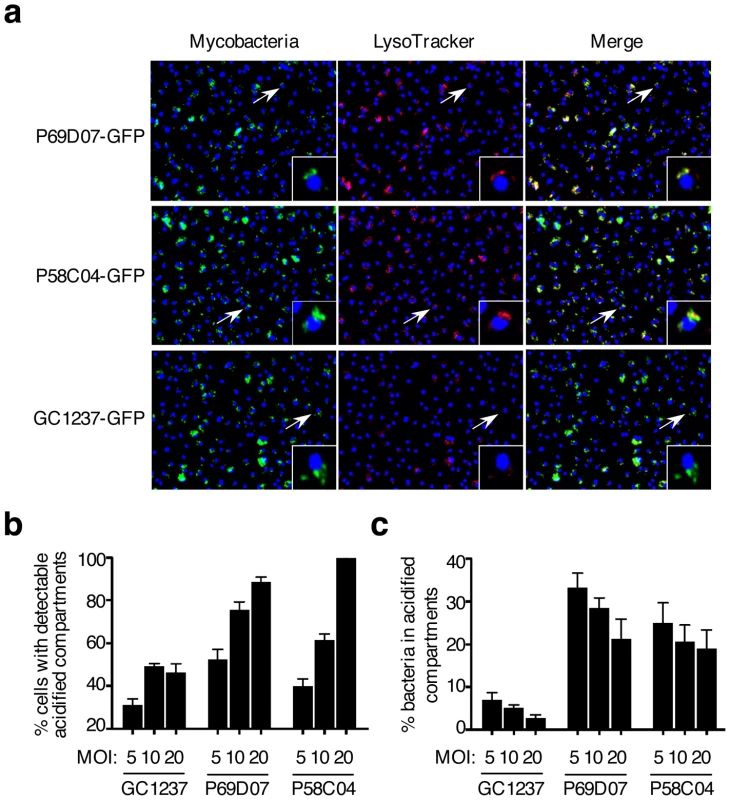
(a) Confocal microscopy analysis of LysoTracker (red)-stained mouse bone marrow-derived macrophages infected for 2 h with GFP-expressing M. tuberculosis #1 and #6 mutants and GC1237. Images span 0.450×0.340 mm2. (b,c) Image-based quantification of bacteria inside LysoTracker-positive acidic compartments, and cells with LysoTracker-positive acidic compartments for different multiplicities of infection (MOI). Values for each condition are the average ± s.d. determined for three different infections for which four fields were recorded and analyzed, and are representative of two independent experiments. Genes disrupted by transposon insertion in the 10 selected mutants were identified by ligation-mediated PCR and sequencing (Table 1) [16]. Among others, genes involved in cell envelope biogenesis (fadD28, ppe54, lppM), phosphate uptake (pstS3), ESX-1-mediated secretion (espL/Rv3880) and molybdopterin biosynthesis (moaC1/moaD1) were found. The maturation stage of the mycobacterial phagosome was further characterized for two mutants (in Rv1503c and Rv1506c) by immunofluorescence and confocal microscopy observation. Similarly to the phagosomes of wild type M. tuberculosis, the phagosomes of mutant strains were mostly negative for the lysosomal marker CD63 (Figure S3a). The fraction of v-ATPase-positive (Figure S3b) and LAMP-1-positive (data not shown) phagosomes was increased in cells infected with the mutants, as compared to the wild-type strain, which is in agreement with our results using LysoTracker. Genetic complementation of the ten mutants was achieved using pYUB412-derived integrating cosmids [18]. The different cosmids carry large size DNA fragments from M. tuberculosis Erdman, which encompass the corresponding region in the genome of M. tuberculosis H37Rv (Table 2). Trafficking of all the complemented mutant strains was either fully or at least partially restored, as assessed by LysoTracker staining of infected macrophages (Table 1). Our results confirm that phagosome maturation arrest and intracellular survival are multifactorial processes involving a combination of mycobacterial genes [9], [10]. In addition, we show that increased phagosome maturation does not necessarily correlate with mycobacterial stasis or killing in the infected cells. As none of the 10 trafficking mutants showed a growth defect in culture broth (Figure S4), these processes seem to have evolved during adaptation of tubercle bacilli to the mammalian host.
Tab. 1. List of confirmed mutants that rapidly traffic into acidified compartments. 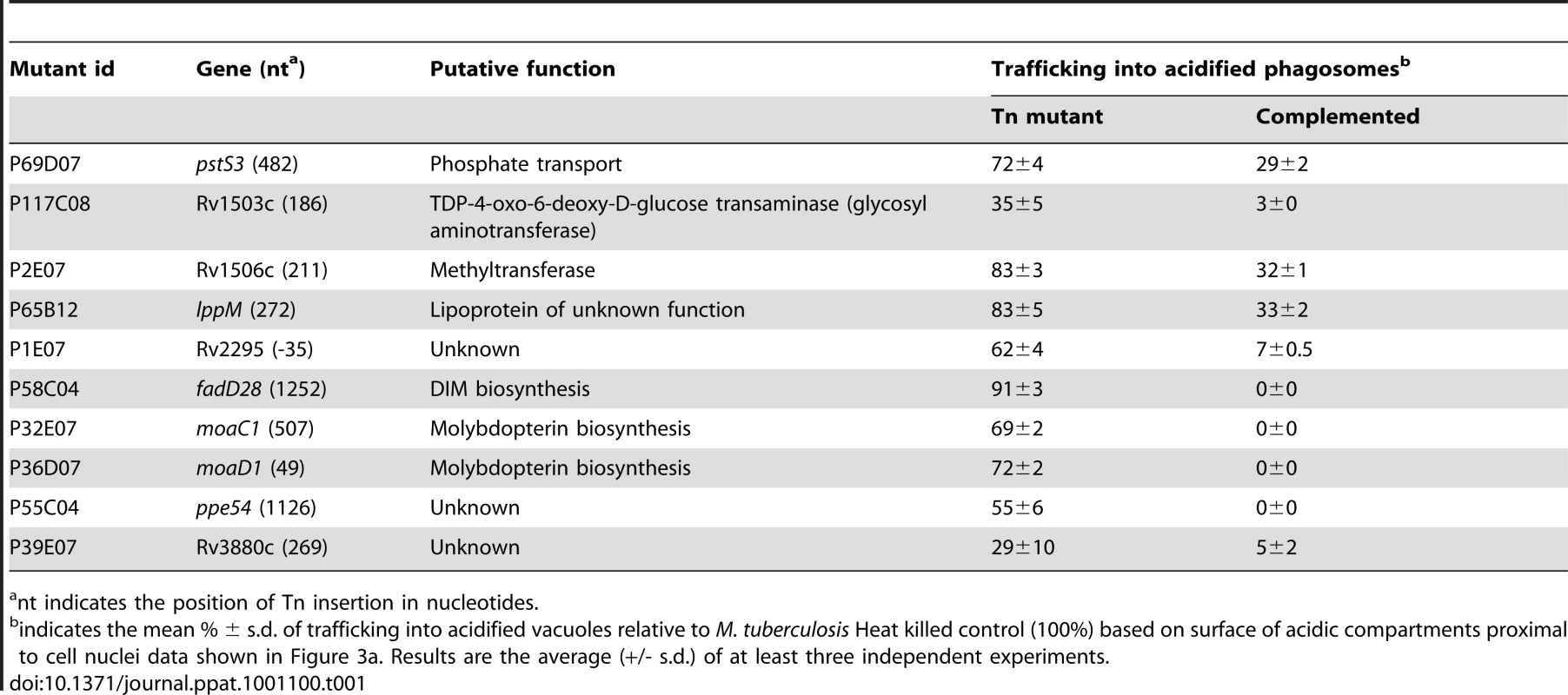
nt indicates the position of Tn insertion in nucleotides. The finding that two genomic loci (moaC1/D1 and Rv1503/06c) were each represented by two independent transposon mutants suggests that the identified regions indeed play key roles in the phagosome maturation arrest induced by M. tuberculosis. These results highlight the power and stringency of our screening method and prompted us to investigate one of the identified loci (Rv1503c/06c) in more detail. Indeed this genomic region is similar to a locus in the closely related fish pathogen Mycobacterium marinum that is encoding enzymes involved in LOS synthesis [12], [13], which might play important roles in host-pathogen interaction.
Acyltrehalose-containing glycolipids are involved in early phagosome maturation arrest
We further characterized the mutants in Rv1503c and Rv1506c. These genes encode a putative TDP-4-oxo-6-deoxy-D-glucose transaminase and a putative methyltransferase, respectively, as evaluated by comparative bioinformatic analyses. They are located in a locus that resembles the lipooligosaccharides (LOS) locus of M. marinum [12], [13], which is partially conserved between the two species. Interestingly, the Rv1503c-to-Rv1507c genes belong to a single operon, as assessed by RT-PCR amplification of the gene junctions (data not shown). Rv1503c and Rv1506c share 89% amino acid similarity with their M. marinum orthologs MMAR2320/wecE and MMAR2322, respectively. We first hypothesized that this locus may be involved in the synthesis of LOS or LOS-related glycolipids in M. tuberculosis. However with the exception of particular strains of the M. canetti family [19], [20] which represents rare and more distantly related tubercle bacilli [21], LOS have not been identified in tubercle bacilli. In agreement with these data, we did not detect any LOS production in M. tuberculosis GC1237 (data not shown). Thus, we next hypothesized that the gene cluster under study may be involved in the synthesis of other forms of acyltrehaloses. Lipid extracts from bacterial cells were examined by TLC upon anthrone staining (Figure 5a) or metabolic labeling of methyl-branched fatty acid-containing lipids with [1-14C]propionate (Figure 5b,c). These analyses revealed that the Rv1503c::Tn and Rv1506c::Tn mutants markedly overproduced a molecule (named “II” in Figure 5a-d), whereas they produce lower amounts of another molecule (named “III” in Figure 5a–d), as compared to the GC1237 strain. Genetic complementation of the two mutants with the IE586 cosmid encompassing the region 1653–1697 kb in the genome of M. tuberculosis H37Rv, which spans the Rv1503c-6c cluster, restored production of lipids II and III to wild-type levels (Figure 5d). Importantly, genetic complementation of the mutant strains restored significantly their trafficking phenotype, as assessed by LysoTracker staining of infected macrophages (Figure 5e,f). Lipids named “I” to “IV” were purified (Figure 6a for lipid III, Figure 6d for lipid II, not shown for lipids I and IV), and their structure was analyzed by mass spectrometry and NMR. The I to IV lipids were identified as trehalose dimycolates (TDM, data not shown), tetracylated sulfoglycolipid (Ac4SGL, Figure 6d–f), 2,3-di-O-acyltrehaloses (DAT, Figure 6a–c), and triacylated sulfoglycolipid (Ac3SGL, data not shown), respectively. Identification of molecule I as TDM thus explained the very low labelling with [1-14C]propionate (Figure 5b,c), as propionate cannot be readily incorporated into this molecule. Lipid quantification using [1-14C]acetate did not reveal differences in the amount of TDM produced by the wild-type and the two mutant strains (data not shown). These results show that DAT synthesis is impaired and sulfolipid (mostly Ac4SGL) synthesis is increased in the Rv1503c and Rv1506c transposon mutants, as compared to in the wild-type strain. Additional lipids, previously reported to interfere with intracellular trafficking, such as lipoarabinomannan (LAM; [22]) and phosphatidyl-myo-inositol mannosides (PIMs; [2]) were analyzed, and we found no difference in the amount and structure of these molecules in the mutant strains, as compared to the wild type strain (Figure S5). These data indicate that deletion of Rv1503c or Rv1506c does not affect the structure and the biosynthesis of M. tuberculosis lipoglycans.
Fig. 5. Lipid content and phenotype of the M. tuberculosis GC1237 wild-type, Rv1503c::Tn and Rv1506c::Tn mutants, and complemented strains. 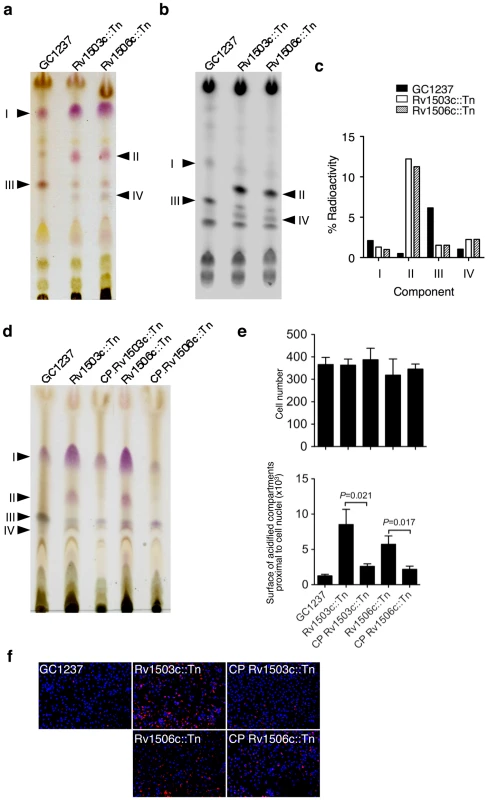
(a,b) anthrone-stained (a) and autoradiogram (b) of thin-layer chromatography of [1-14C]propionate labelled lipids (20000 cpm/lane). (c) Quantification of the lipids from (b). One representative out of four experiments is shown. (d) TLC analysis of the lipids of cosmid-complemented strains vs wild-type and mutant strains. (e,f) Trafficking phenotype of the wild-type, mutant and complemented strains. Representative pictures of mouse bone-marrow-derived macrophages infected with M. tuberculosis GC1237, Rv1503c::Tn and Rv1506c::Tn mutants, and complemented strains. After 2 hours of infection, cells were stained with LysoTracker DND-99 (red) and DAPI (blue) for acidic compartments and cell nuclei labeling, respectively. In (a), lipids were subjected to TLC with CHCl3/CH3OH/H2O 30/8/1 (v/v/v) as the solvent; in (b) and (d), lipids were subjected to TLC with CHCl3/CH3OH/H2O 35/8/1 (v/v/v) as the solvent. Fig. 6. Identification of lipids II and III as Ac4SGL and DAT respectively. 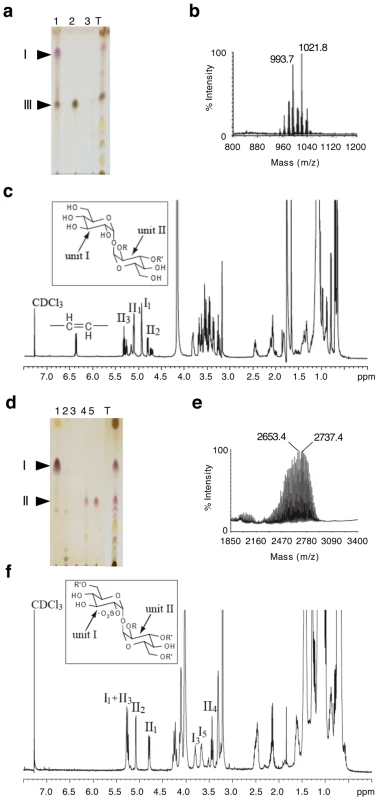
(a,d) TLC analysis of fractions from an anion exchange column loaded with total chloroform extract (T) eluted with CHCl3 (1), CHCl3/CH3OH 9/1 (2), CHCl3/CH3OH/H2O 60/35/8 (3), chloroform/methanol 1/2 containing 0.1 M ammonium acetate (4), chloroform/methanol 1/2 containing 0.3 M ammonium acetate (5). In (a), lipids were extracted from the GC1237 strain. In (d), lipids were extracted from the Rv1506c mutant strain. (b,e) MALDI-MS spectra of DAT and Ac4SGL, respectively. The spectrum of DAT was recorded in positive mode while the spectrum of Ac4SGL was recorded in negative mode. (c,f) 1D 1H NMR spectra (∂ 1H: 0-7.5) of DAT and Ac4SGL, respectively. I1 stands for proton 1 of unit I. The characteristic protons are interpreted on the figure. Insets in (c) and (f) show the structure of DAT and Ac4SGL, respectively. R corresponds to palmitic or stearic acids while R' corresponds to polymethyl-branched fatty acid. The two glucose units are labeled I and II. The two mutants, in particular in Rv1503c (P117C08) grew poorly inside host macrophages (Figure 3c). Interestingly, their ability to colonize the lungs was strongly impaired as assessed in a murine model of M. tuberculosis infection, and again the virulence phenotype could be restored by cosmid-mediated complementation (Figure 7).
Fig. 7. Virulence of the Rv1503c::Tn, Rv1506c::Tn mutant and complemented (CP) strains in vivo. 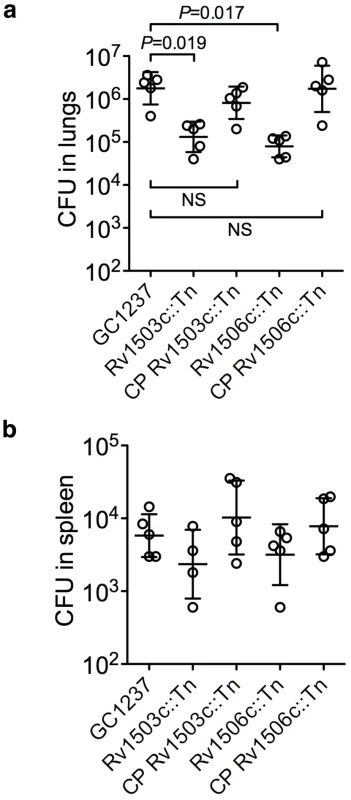
Balb/c mice were infected with 102 CFUs of the different strains via the intranasal route. After 42 days of infection, mice were sacrificed, and the lungs (a) and spleen (b) homogenized and plated onto agar medium for CFU determination. Each circle represents one animal, and the bars indicate means ± s.d. Differences in the spleen samples were not significant. No differences in the lungs 20 days after infection were observed (not shown). To further dissect the altered trafficking phenotype of the Rv1503c/06c::Tn mutants we evaluated the potential of the DAT and Ac4SGL (the major form of sulfolipid overproduced in the mutants) in influencing phagosomal acidification. Using lipid-coated silica beads and LysoTracker staining, we found that phagosomes loaded with Ac4SGL-coated beads acidified more than phagosomes containing non-coated beads. In contrast, DAT coating did not influence intracellular trafficking of the particles as compared to non-coated controls (Figure 8a,b). In addition, the pH of the vacuoles containing the different particles was measured using a flow cytometry-based technique [23]. Whereas the pH of the DAT-coated beads and the control beads reached a value of ∼5.2 after 60 min phagocytosis, the pH of the Ac4SGL-coated beads was significantly lower (∼4.7) at the same time-point (Figure 8c). These results suggest that overproduction of Ac4SGL in the Rv1503c::Tn and Rv1506c::Tn mutant strains may account, at least in part, for their increased intracellular trafficking phenotype.
Fig. 8. Effect of purified DAT and Ac4SGL on intracellular trafficking of silica beads in human macrophages. 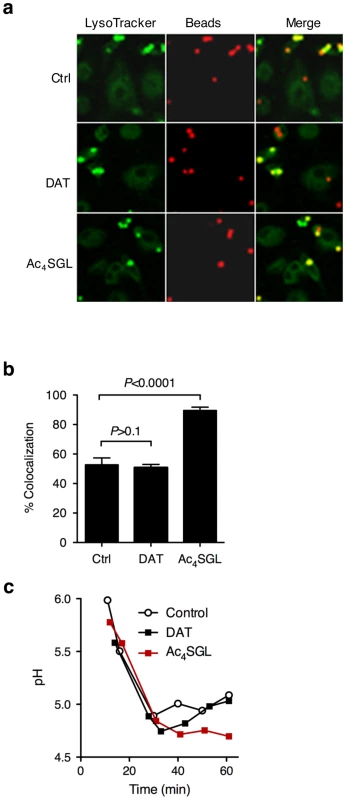
(a) Human monocyte-derived macrophages were pulsed for 15 min with 3-µm silica beads (3-5 beads per cell) coated with BSA-alexa Fluor 647 conjugate (red) and with Ac4SGL or DAT. Control beads (ctrl) are not coated with lipids. Cells were chased for 70 min in bead-free medium containing 250 nM LysoTracker Green DND-26 to follow phagosome acidification (green). Cells were fixed and immediately examined under the confocal microscope. (b) Phagosome acidification was measured by recording colocalization between the red and the green signals in about 100 phagosomes in 5 independent fields. Data are expressed as mean % of colocalization (+/- s.d.) and are representative of two independent experiments. Data were analyzed using the Student's t-test. (c) Dynamic pH measurement of phagosomes coated with DAT, Ac4SGL, and control phagosomes in human macrophages over a 1 h phagocytosis-period. Beads were coated with BSA-FITC and -Alexa 647, and the different lipids, and incubated with human monocyte-derived macrophages for various periods of time (up to one hour). Dual fluorescence was recorded, and the ratio FLFITC/FL647 was used to calculate the pH values of the phagosomes. A standard curve was constructed after recording the fluorescence values of cells permeabilized and incubated in buffers of defined pH values. Discussion
Here we report the high throughput visual screen of a high density M. tuberculosis mutant library for the search of microbial virulence genes involved in phagosome maturation arrest. The power of our approach lies both in: i/the analysis of a single labelling, i.e. “acidic compartments”, to quantify mycobacterial phagosome acidification, which allowed us to exclude any bias due to false co-localizations; ii/the possibility to distinguish between tens of thousands of mutant strains at the single strain level; iii/the possibility of a very stringent selection of the hits.
Many of the genes identified in our study are predicted to be located within operons (pstS3, Rv1503c, Rv1506c, fadD28, moaC1, moaD1, Rv3880c) [24]. Using RT-PCR amplification of gene junctions we have confirmed this to be true for the genes Rv1503c-to-Rv1507c on which we focus our detailed analysis (data not shown). Although we could restore the lipid profile in the Rv1506c::Tn mutant by complementing with a plasmid encoding Rv1506c (data not shown), we have chosen as a general strategy to complement our mutants with integrating cosmids. These constructs integrate as single copy into the chromosome and encode the target gene as well as surrounding genes and regulatory elements. This strategy provides for a more realistic reproduction of wild-type gene expression levels of the target gene and has been successfully used in a number of previous studies (e.g. [25]). However, cosmid complementation may complement polar effects of transposon insertion on neighboring genes and does not formally prove the involvement of a single mutated gene in an observed phenotype. The discussion below should be put into this context. The putative involvement of genomic loci is discussed rather than the exact function of given genes.
Here we found only one gene (fadD28) that was already shown to play a part in phagosome maturation arrest and has been isolated in a previous similar global approach [10]. In addition, Pethe et al. have also identified Rv1522c/mmpL12, encoding a putative lipid transporter, as involved in phagosome maturation arrest [10]. Interestingly, this gene belongs to the same genomic locus as the Rv1503c and Rv1506c genes identified here. These three genes may be functionally related. The only modest overlap with datasets from other global screening studies examining related phenotypes [10], [11] may be due to the differences in bacterial strains, host cells and selection techniques employed in the different studies. Indeed, our study is the first to screen for intracellular phenotypes in the GC1237 W-Beijing clinical isolate of M. tuberculosis and the selection used was highly stringent, isolating and sequencing only the 10 mutants that gave the strongest LysoTracker signals, i.e. 3 s.d. above the mean. It is likely that we have missed some borderline mutants in genes previously reported.
The fatty acid-CoA ligase-encoding gene fadD28 is required for the synthesis of phtiocerol dimycocerosates (PDIM). These complex lipids have already been shown to be involved in mycobacterial virulence [10], [11], [16], [26], [27], and a recent report has suggested that PDIM may directly prevent phagosome maturation through insertion into the phagosomal membrane [28]. The lppM gene was identified as involved in phagosome arrest and encodes a putative lipoprotein that may be anchored in the bacterial membrane. In favour of a membrane modification, the lppM::Tn mutant grows in clumps relative to wild type (data not shown). The ppe54 gene encodes a protein belonging to the large PE/PPE family whose members have mostly been shown to be membrane bound and effective immunogens. Such features have not been reported so far for PPE54. Strikingly, its expression was found specifically upregulated in IFN-γ-activated macrophages [29] suggesting a role in mycobacterial intracellular persistence in agreement with our findings. We also isolated a mutant in Rv3880c, recently named espL as part of the ESX-1 secretion system [30]. The exact function of EspL is unknown, however studies in M. marinum suggest that proteins encoded by neighboring genes might interfere with phagosome maturation [31]. Other components of the ESX-1 secretome might be involved in phagosome maturation arrest as well [9]. More generally, the ESX-1 secretion system has been involved in mycobacterial virulence in various in vivo and in vitro systems, including in macrophages [32], [33], [34], [35]. Secreted or membrane-anchored products such as PDIM, lipoproteins, PE/PPE proteins and the ESX-1 secretome might interact with host cell endocytic machinery components yet to be identified.
Additional genes identified here include pstS3 and genes involved in molybdenum metabolism, namely moaC1 and moaD1. The periplasmic phosphate binding lipoprotein-encoding gene pstS3 belongs to a larger genetic locus, the pho regulon, involved in inorganic phosphate uptake, and previously reported to play a part in mycobacterial virulence [36]. The pstS3 gene itself has been implicated in mycobacterial intracellular survival, possibly through phagosome maturation arrest [37]. How phosphate uptake is linked to buffering phagosome acidification is unclear and requires further investigation.
The ability of some mutants for intracellular growth despite vacuole acidification is striking and may be explained by several reasons. The exact pH values of the mutants' phagosomes have not been precisely measured here, and it is possible that some mutants reside in more acidic vacuoles than others. As M. tuberculosis is highly resistant to very low pH [38], phagosomes of mutants in which acidity is the only altered factor may still be permissive for mycobacterial growth. In addition, as can be seen in Figure 4, a fraction of the phagocytosed mycobacteria does not localize in acidic compartments and thus can be the sub-population that indeed replicates at later time points. It is important to explicitly determine whether the bacterium being observed in a specific sub-cellular compartment is really the one that will replicate, and latest development of life time automated confocal imaging suggest that this may become foreseeable in a near future.
One of the most striking findings of our study is the independent isolation of two pairs of mutants in genes located within the same genetic loci, namely Rv1503c/Rv1506c, and moaC1/moaD1. Given the size and coverage of our library, it is unlikely that such isolations happened by chance. The adjacent genes moaC1 and moaD1 are part of a chromosomal region (Rv3108-20) presumably involved in molybdopterin biosynthesis. We have recently shown that this gene cluster actually forms a genomic island that arose in the ancestor of the tubercle bacilli through horizontal transfer from environmental proteobacteria [39]. Molybdopterin is a precursor of the so-called molybdenum cofactor (MoCo), a coenzyme for various oxidoreductases, including nitrate reductase and sulfite oxidase for instance. Our results are reminiscent of a recent study in which lesion in moeB1, another gene possibly involved in MoCo biosynthesis and distant from the Rv3108-20 locus, was shown to affect mycobacterial phagosome biogenesis [9]. The genome of the tubercle bacillus contains several loci potentially involved in MoCo biosynthesis [17], and a few gene products in M. tuberculosis harbour molybdopterin/MoCo-binding sites and might use MoCo as a cofactor. This is the case of the nitrate reductase subunit-encoding genes, narG and narX, of the formate and aldehyde dehydrogenase-encoding genes fdhF and nuoG, respectively, as well as of two other gene products, Rv0197, an uncharacterized oxidoreductase, and Rv0218, a possible sulfite oxidase. Although we can only speculate at this stage, we can anticipate that MoCo-dependent redox reactions play a crucial part in early intracellular fate of the tubercle bacillus, which should deserve further attention in future studies.
We also isolated two independent mutants carrying genetic disruptions in Rv1503c and Rv1506c. The homologues of these genes in M. marinum belong to a locus involved in the synthesis of LOS [13]. Here we showed that, like other M. tuberculosis strains [19], [20], our W-Beijing mother strain does not synthesize LOS. However we showed that the Rv1503c::Tn and Rv1506c::Tn mutants are impaired in the synthesis of other acyltrehalose-containing lipids, namely DAT, and that they overproduce sulfoglycolipids (SGL), Ac3SGL and more importantly Ac4SGL. DAT and SGL are glycolipids based on trehaloses [40]. Although some of the genes involved in DAT [40], [41] and SGL [42], [43], [44] synthesis have been identified, knowledge of the enzymes, transporter and regulators involved in these pathways is still incomplete. Transcriptional regulation of the genes involved in synthesis of these molecules has been shown to be coordinated by the PhoP regulator [15]. This suggests that Ac4SGL/Ac3SGL overproduction in the mutant strains may be due to a compensation mechanism associated with impairment of DAT production. However, the Rv1503c and Rv1506c proteins do not carry the signature of enzymes such as acyltransferases and polyketide synthases (Pks), and it is thus unclear what their exact involvement in DAT synthesis could be. The Rv1505c gene encodes a putative acyltransferase and the Rv1505c and Rv1506c genes are predicted to form an operon; the possibility thus remains that impairment of DAT synthesis in the Rv1506c mutants, and eventually in the Rv1503c mutants, is actually due to inactivation of Rv1505c because of operon disruption and/or of polar effects. Interestingly, transcription of Rv1505c, as well as that of other genes located in the same genomic region, namely Rv1517, Rv1518, Rv1522c/mmpL12, Rv1525/wbbL2, Rv1527c/pks5 and Rv1528c/papA4, has been proposed to be regulated by PhoP [45]. Whether and how these genes are involved in DAT synthesis will require further investigation and is beyond the scope of the present study.
Most importantly, our results suggest that Ac4SGL, but not DAT, increase phagosome acidification. This is at variance with conclusions from an early study suggesting that mycobacterial sulfolipids (SL) tend to impair phagosome maturation [46]. However these authors incubated purified sulfolipids directly with the cells, and the model systems used in this study and in ours are barely comparable. Nevertheless, our results obtained with beads suggest that SL increase phagosome maturation, which may account, at least in part, for the increased trafficking of the Rv1503c and Rv1506c mutants, although the exact mechanism of increased acidification of these two mutants has not been formally identified here. Furthermore, analysis of the production of cytokines and chemokines using protein arrays upon infection with Rv1503c and Rv1506c mutants did not reveal any significant differences compared to the wild type strain (data not shown). This suggests that the mutants altered trafficking phenotypes were not caused by changes in their ability to induce different inflammatory cytokine production [47], [48] and indicates that other mechanisms might account for the observed trafficking behaviour. For instance, other possible explanations include an overall altered cell wall, or a more generally reduced intracellular fitness of the mutants, which both may impact intracellular trafficking. In this regard, it is interesting to notice that the phoP mutant shows a significant, though partial, relocation to an acidic phagosome (Figure 1). The PhoP transcriptional regulator controls the expression of many genes, some of which have been involved in phagosome remodelling; this is the case of fadD28, fadD26 and mmpL12 for instance [10], [45]. It also regulates the synthesis of important lipids, such as diacyltrehaloses and sulfolipids [15], which may be involved in intracellular trafficking according to our data. The partial inability of the phoP mutant to arrest phagosome maturation may thus be due to a defect in production of these factors. It may also be due to a broader defect in fitness [49]. Further study of our mutants and other mutants isolated in previous screens will help understand the general relationship that may exist between mycobacterial fitness and intracellular trafficking.
In summary, the unbiased approach developed here allowed us to identify novel mycobacterial genes involved in M. tuberculosis intracellular parasitism, and suggests the involvement of important M. tuberculosis-specific glycolipids in this process. This approach can easily be adapted for the comprehensive quantitative analysis of mycobacterial vacuole sorting, as well as for the study of intracellular parasitism by pathogenic microorganisms, and for phenotypic drug screening [50].
Methods
Bacteria and mutant library
M. tuberculosis H37Rv, GC1237[14] and GC1237 ΔphoP/R [15] strains were grown in Middlebrook 7H9 culture medium (Difco, Sparks MD) supplemented with 10% oleic acid-albumin-dextrose-catalase (OADC, Difco), glycerol, 0.05% Tween 80, and 25 µg/ml kanamycin in the case of the ΔphoP/R mutant. A library of 11,180 members was constructed using M. tuberculosis Beijing GC1237 as a host strain and the pCG113 plasmid that contains the IS1096-derived Tn5367 as previously described [16]. After electroporation, transformants were amplified in Middlebrook 7H9 culture medium supplemented with 10% OADC, glycerol, 0.05% Tween 80 and 25 µg/ml kanamycin at 32°C, a temperature that allows replication of circular pCG113. The mutants were selected on Middlebrook 7H10-OADC agar medium supplemented with 25 µg/ml kanamycin and 2% sucrose at 39°C. This temperature, and the presence of sucrose, allows to select mutants in which the plasmid has been eliminated and the double cross over has occurred. Eleven thousand one hundred and eighty individual mutant clones were isolated and seeded into 96-well plates containing 180 µl of Middlebrook 7H9 culture medium supplemented with 10% OADC, glycerol, 0.05% Tween 80 and 25 µg/ml kanamycin. The plates were incubated at 37°C for three weeks and then kept at 4°C. Three weeks before screening, a 10-fold dilution of suspension from each of the M. tuberculosis transposon mutants was performed. Selected strains were transformed with a GFP-expressing integrative plasmid kindgly provided by Dr. Winter (Institut Pasteur, Paris). Transformants were selected on hygromycin.
TraSH analysis
Mapping of transposon insertion sites by microarray was carried out essentially as described previously [11]. Genomic DNA was prepared from a pool of 500 Tn5367 transposon mutants and digested with BssHII and MluI. Approximately 100 ng of digested genomic DNA was ligated to 100 pmol of Y-linker [11], and then 10 ng of ligated DNA was used as template for PCR amplification of transposon-flanking regions using the transposon-specific primer IS1 (5′-GCACGTCGAGGTCTTTCAGATGGATGGCG-3′) and the Y-linker-specific primer TA4 (5′-ACGCACGCGACGAGACGTAGC-3′) in the presence of 8% DMSO. Following an initial denaturation step for 2 minutes at 95°C, the reaction was hot-started and cycled between 94.5°C (30 s) and 72°C (90 s) for 22 cycles. Amplification products were gel purified and used in a second round of PCR amplification between the Y-linker-specific primer TA4 and a nested transposon-specific primer IS2Nest (3′-TGGATGGCGTAGGAACCTCCATCATCGGA-5′) to further enrich for transposon-flanking products and to incorporate Cy3-dCTP (Amersham). The reaction mix included 20 µM Cy3-dCTP, 180 µM dCTP and 200 µM dGTP/dATP/dTTP and the cycling conditions were as before, but for 14 cycles. The labelled PCR products were cleaned up using a Qiagen MinElute kit, eluting in water.
The fluorescently labelled transposon insertion sites were hybridised to whole genome microarrays, prepared by spotting the M. tuberculosis 70-mer oligonucleotide set (Operon, Qiagen) onto Corning GAPS Coated Slides. Signal intensities of hybridisation were collected using Genepix Pro 3.0 and an Axon 4000B microarray scanner.
Controls preparation and mycobacteria staining
Bacteria were harvested, washed three times and resuspended in phosphate buffer saline (PBS). The bacteria were then sonicated and allowed to stand for 30 minutes to allow residual aggregates to settle. The bacterial suspensions were then aliquoted and frozen at −80°C. A single defrosted aliquot was used to quantify the CFUs prior to inoculation and typical stock concentrations ranged between 2 and 5×108 CFUs/ml. Dead bacteria were prepared by heating one aliquot at 95°C for 20 minutes. Where appropriate, mycobacteria were covalently labeled with CypHer5 mono ester dye (Sigma Aldrich, Saint-Louis, MO) in 0.1 M sodium carbonate buffer (pH 9) and washed three times before use [51]. Three µm size-Zymosan A from Saccharomyces cerevisiae (Sigma Aldrich) was titrated at 1.5×106 particules/ml.
Macrophages, infection, and fluorescence assay set up
Mouse bone-marrow-derived macrophages were obtained by seeding 107 bone marrow cells from C57BL/6 mice in 75 cm2 dishes in RPMI 1640 supplemented with 10% heat-inactivated fetal calf serum (FCS) and 10% L-cell conditioned medium (all from Gibco at Invitrogen, Carlsbad, CA). Peripheral Blood Mononuclear Cells (PBMC) were isolated from buffy coat from healthy volunteers. 15 ml of Ficoll-Paque Plus (Amersham Biosciences, Sweden) were added to PBS diluted buffy coat diluted and centrifuged at 2500×g for 20 min. PBMC were obtained by CD14+ beads separation (Miltenyi Biotec, Germany), washed 3-times with PBS containing 1% FCS and transferred to 75 cm2 culture flask containing RPMI 1640 media, 10% FCS and 50 ng/ml of recombinant-human macrophage colony stimulating factor (rh-MCSF, R & D systems, Minneapolis). After 6 days, murine or human macrophages were harvested with Versene (Gibco) and seeded at a density of 1.5×105 cells per well in 384-well plates (Evotec, Hambourg, Germany) in 50 µl RPMI 1640 supplemented with 10% heat-inactivated fetal calf serum and 10% L-cell conditioned medium. Adherent cells were then infected with bacterial suspensions at a MOI varying from 20 to 1 bacteria per cell and incubated for 2 h. Cells were then washed three times with PBS supplemented with 1% FCS and further incubated with 2 µM LysoTracker green DND-26 (Invitrogen) for 1.5 h. Finally, cells were fixed with 1.5% formaldehyde BD Lyse solution (BD Biosciences, San Jose, CA) for 10 min, washed twice and stained with 5 µg/ml DAPI dilactate (Sigma) in 0.1% Triton X-100 (Sigma) in PBS. In some preparations, fixed cells were labelled with an anti-Rab5A (Abcam, Ab13253, 1/500 dilution), -LAMP-1 (Cell Signaling, C54H11, 1/200), -v-ATPase (Synaptic Systems, 109002, 1/100 dilution), and -CD63 (Caltag Laboratories, 18-7300, dilution 1/100) antibodies, subsequently detected using a FITC-, Alexa 555-, or rhodamine-conjugated rabbit anti-mouse IgG or mouse anti-rabbit antibody (Sigma or Invitrogen).
Image acquisition by automated confocal microscopy and data analysis for Lysotracker assay - set-up
Confocal images were recorded on an automated fluorescent confocal microscope Opera (Evotec) using a 20X water objective (NA 0.70), 405-nm, 488-nm and 635-nm lasers and a 510 primary dichroic mirror with two sequential exposures. Each image was then processed using dedicated in-house image analysis software (IM). Each recorded field contained three images (three colors): one for the cell nuclei (blue-channel), one for the LysoTracker-DND-26 positive compartment (green-channel) and one for the CypHer5 labeled mycobacterium-positive compartment (red-channel). Cell nuclei, mycobacteria and LysoTracker-DND-26 positive compartment are then segmented in the “nuclei” band, “bacteria” and the “acidic compartment” band respectively using in-house method [52]. Briefly, this method relies on a succession of i) thresholding the histogram of the original image, the threshold being taken as the maximum value between a manual threshold and an automatic one (lowest value of a 3 classes K-means); ii) Gaussian filtering the original image with a standard deviation that is set equal to the nuclei (/mycobacterium and acidic compartments) average radius; iii) searching for local maxima of the filtered image that provides nuclei (/mycobacterium and acidic compartments) centers as seeds for iv) region growing that defines the individual surface of each nucleus (/mycobacterium and acidic compartments) and finally v) removing extremely small nuclei (/mycobacterium and acidic compartments) as potential artifacts or noise. Once mycobacterium and acidic compartments and nuclei are individually identified, final results are i) the nuclei number, ii) the number of nuclei that are proximal to at least one mycobacterium or acidic object, iii) the surface covered by acidic or mycobacterium compartments in the vicinity of the nuclei. Proximity is a parameter manually set-up by the user. Given the fact that one cell has only one nucleus, these results can be extrapolated to i) the number of cells, ii) the number of cells containing at least one acidic object/one mycobacterium, and iii) the surface of acidic compartments or bacterial load proximal to cell nuclei. Final results are expressed as the average over all the images of the well.
Mutant library screening
Frozen bone-marrow progenitors (6×108 cells) were seeded in three 500 cm2 dishes in RPMI 1640 supplemented with 10% FCS and 10% L-cell conditioned medium. At day 5, 50 µl of M. tuberculosis Beijing GC1237 mutants were two-fold diluted in PBS and seeded into a 96-well plate (Nunc), and 20 µl of the diluted suspension were subsequently transferred into a 384-well assay plate using the BioTek Precision XS sample processor. Before infecting cells, the concentration of each mutant was determined by OD600 measurement and the mean titer was within the range of 0.2 to 0.6 showing that the mycobacteria were in exponential growth phase (data not shown). Plate titration was performed on randomly selected mutants and the mean titer was 2×107 colony forming units (CFUs)/ml. 20 µl of heat-killed M. tuberculosis Beijing GC1237 ΔphoP/R, M. tuberculosis Beijing GC1237 ΔphoP/R and wild type strains were added manually in column 2, 3 and 22 respectively. The next day, after harvesting, 30 µl of the cell suspension at 5×105 cells/ml was distributed into the 384-well assay plate that had been priory seeded with the mutants using the AquaMax DW4 liquid handling device (Molecular Devices). After a 2 h incubation at 37°C under 5% CO2, extracellular mycobacteria were removed by 3 washes in PBS and macrophages were labeled with 2 µM LysoTracker red DND-99 (Invitrogen) for 1.5 h. Finally, cells were fixed with 1.5% formaldehyde BD Lyse solution for 10 min, stained with 5 µg/ml DAPI in 0.1% Triton X-100 (Sigma) in PBS for 2 min and kept in 1% FCS in PBS at 4°C until image acquisition.
Image acquisition by automated confocal microscopy and data analysis for high throughput screening (HTS)
Confocal images were recorded on an automated fluorescent confocal microscope Opera (Evotec) using a 20X water objective (NA 0.70), 405-nm and 561-nm lasers and a 580 primary dichroic mirror. Each image was then processed using dedicated in-house image analysis software (IM). Images contained two bands (two colors): one for the LysoTracker-positive compartment (red-channel) and one for the cell nuclei (blue-channel). The image dynamics in both bands (both colors) was first checked through a series of statistical tests including average intensity and standard deviation designed to remove black or out of focus images. Cell nuclei and acidic compartments are then segmented in the “nuclei” band, and the “acidic compartment” band respectively using in-house method [52]. Briefly, this method relies on a succession of i) thresholding the histogram of the original image, the threshold being taken as the maximum value between a manual threshold and an automatic one (lowest value of a 3 classes K-means); ii) Gaussian filtering the original image with a standard deviation that is set equal to the nuclei (/acidic compartments) average radius; iii) searching for local maxima of the filtered image that provides nuclei (/acidic compartments) centers as seeds for iv) region growing that defines the individual surface of each nucleus (/acidic compartments) and finally v) removing extremely small nuclei (/acidic compartments) as potential artifacts or noise. Once acidic compartments and nuclei are individually identified, final results are i) the nuclei number ii) the number of nuclei that are proximal to at least one acidic object iii) the surface covered by acidic compartments in the vicinity of the nuclei. Proximity is a parameter manually set-up by the user. Given the fact that one cell has only one nucleus, these results can be extrapolated to i) the number of cells) ii) the number of cells containing at least one acidic object and iii) the surface of acidic compartments proximal to cell nuclei. Final results are expressed as the average over four fields recorded per 384-plate well.
Statistical analysis
The screen statistical data (Z', CV etc. for the control plates) was calculated using an in-house software. Additional analyses with regards to quality control were performed with the Spotfire software.
Genetic analysis of the mutants and complementation
The transposon insertion sites were identified by ligation mediated PCR following a slightly modified version of a previously described protocol [53]. Briefly, genomic DNA was digested with BamH1, XhoI or BglII. DNA was then ligated to BamHI-linkers, and amplified as described. The Rv1503c and Rv1506c mutants were complemented as previously described [27], with the pYUB412-derived cosmid [18] MTCI586, which carries a 38.7 - and 20.8-kb DNA fragment covering the 1653 - to 1697-kb region of the M. tuberculosis chromosome. This cosmid encompasses the Rv1503c-6c gene cluster (1694.5 to 1696.4 kb, genomic coordinates). A description of the other cosmids used in the study is provided in Table 2.
Tab. 2. List of the cosmids used to complement the mutants. 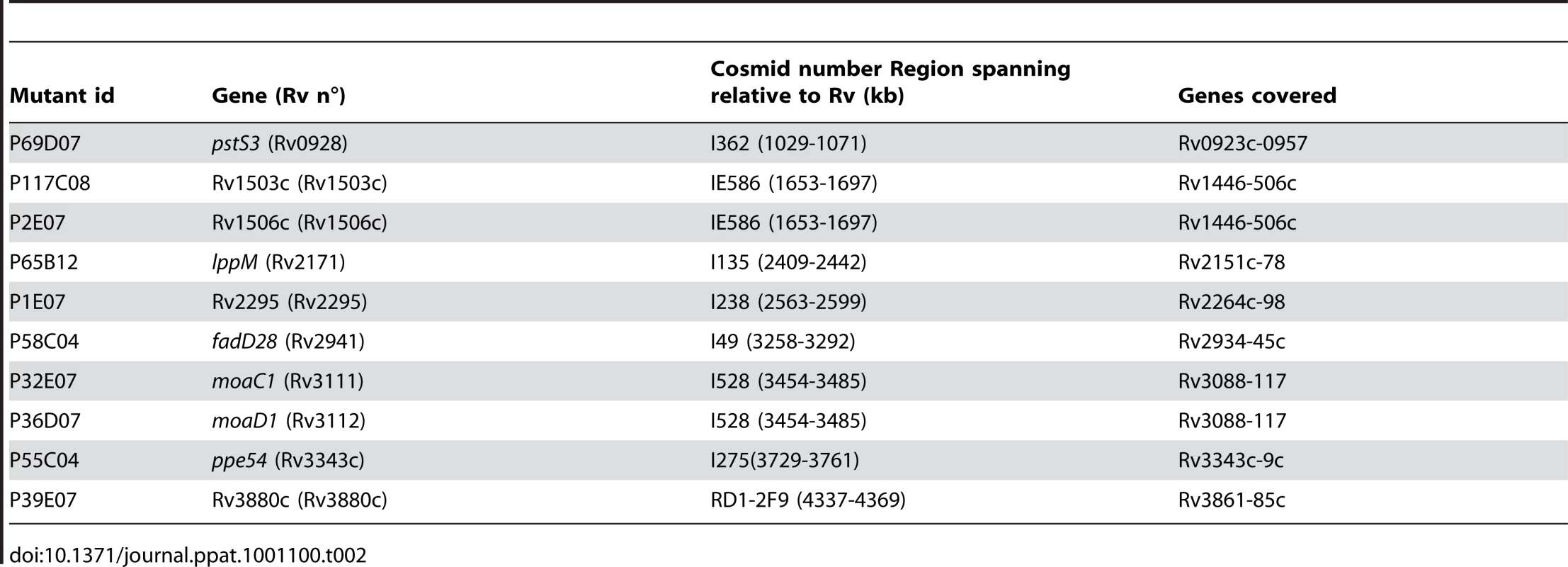
Preparation, purification and analysis of lipids
Lipids were extracted from 21-day old M. tuberculosis cultures first by adding 2 volumes of CH3OH and 1 volume of CHCl3 for 2 days, then in 2∶1 CHCl3/CH3OH (v/v). Pooled extracts were concentrated, washed with water and evaporated to dryness. For specific radiolabeling, each strain was cultured to exponential phase and labeled by incubation with [1-14C]propionate (54 Ci.mol−1, ARC Radiochemical, St Louis, MO) for 2 days. Lipids were first analyzed on silica gel 60 TLC plates (E. Merck, Darmstadt, Germany) in various solvent systems (see the legend to figures). Radiolabeled lipids were visualized with a Typhoon PhosphorImager (Amersham, Biosciences). For lipid purification, the chloroform phase was applied to an anion exchange Sep-pak cartridge (Waters Accell Plus QMA; Waters Corporation) eluted successively by 10 ml CHCl3, 10 ml of CHCl3/CH3OH 95/5 (v/v), 10 ml of CHCl3/CH3OH 9/1 (v/v) to elute DAT, 10 ml of CHCl3/CH3OH/H2O 60/35/8 (v/v) to elute residual neutral compounds, 10 ml of chloroform/methanol 1/2 (v/v) containing 0.1 M ammonium acetate to elute negatively charged compounds (phospholipids) and 10 ml of chloroform/methanol 1/2 (v/v) containing 0.3 M ammonium acetate for the elution of sulfolipids. PIMs were extracted from bacterial cells and subjected to MALDI-MS analysis in the negative ion mode as described previously [54].
MALDI-Tof-MS
MALDI-Tof-MS analysis were performed on a 4700 Proteomics Analyser (with Tof-Tof Optics, Applied Biosystems) using the reflectron mode. Ionization was effected by irradiation with pulsed UV light (355 nm) from an Nd:YAG laser. Samples were analyzed by the instrument operating at 20 kV in the positive ion mode using an extraction delay time set at 20 ns. Typically, spectra from 1,000 to 2,500 laser shots were summed to obtain the final spectrum. The HABA (2-[4-hydroxy-phenylazo]-benzoic acid) matrix was used at a concentration of ∼10 mg/ml in ethanol/water (1 : 1, v/v). Then, 0.5 µl sample solution and 0.5 µl of the matrix solution were deposited on the target, mixed with a micropipette and dried under a gentle stream of warm air. The measurements were externally calibrated at two points with mycobacterial PIM.
Chemical analysis of the mannose cap on LAM
The presence of mannose caps in M. tuberculosis strains GC1237, Rv1503c::Tn and Rv1506c::Tn was analyzed by capillary electrophoresis (CE) as described earlier [55]. In short, partially purified ManLAM is partially degradated by controlled acid hydolysis (0.1 M HCl for 20 min. at 110°C), and the oligosaccharides liberated tagged with the fluorescent label 8-aminopyrene-1,3,6-trisulfonate (APTS). During CE, the labeled oligosaccharides are separated and peaks are detected by laser-induced fluorescence and elution times compared with the appropriate standards.
NMR analysis
NMR spectra were recorded with an Avance DMX500 spectrometer (Bruker GmbH, Karlsruhe, Germany) equipped with an Origin 200 SGI using Xwinnmr 2.6. Native molecules were dissolved in CDCl3-CD3OD, 9 : 1, v/v and analyzed in 200×5 mm 535-PP NMR tubes at 295 K. Proton chemical shifts are expressed in ppm downfield from the signal of the chloroform (dH/TMS 7.27). All the details concerning used COSY, HOHAHA and 1H-13C HMQC sequences and experimental procedures were as previously reported [56].
Trafficking study with silica beads
Silica beads (3 µM diameter; Kisker Biotech, Germany) were washed two times with sterile PBS before incubation overnight at 4°C with 5% (w/v) BSA-Alexa Fluor 647 (Invitrogen, Eugene, OR). The beads were then washed two times with PBS to remove unbound BSA. BSA coated beads were added in a glass tube containing 100 µg of DAT or Ac4SGL and sonicated for 10 minutes. All coated beads were washed once again two times with PBS before trafficking experiments. The efficiency of lipid coating was evaluated by re-extracting the lipids from the coated beads followed by TLC analysis, and was found to be nearly 100% (not shown).
Lipid-coated beads suspended in RPMI (+10% human serum) were added at a ratio of 3-5 beads/cell on human macrophages differentiated from adherent mononuclear cells from healthy donor in the presence of 5 UI/ml of hMCSF (Miltenyi Biotech, Germany) for 6 days in 48-well plates. Plates were centrifuged for 1 minute at 1,000 rpm and incubated at 37°C under 5% CO2. Fifteen minutes later, cells were washed with PBS and 200 µl RPMI containing 250 nM of LysoTracker green DND-26 (Invitrogen) was added before incubation at 37°C under 5%CO2. Cells were fixed 70 minutes later with 4% PFA and the number of LysoTracker-positive phagosomes was immediately estimated using a Leica confocal fluorescence microscope (SP2) equipped with a Plan Apo 4061.4 Ph 6 objective (Olympus Optical) and CoolSNAP-Pro CF digital camera in conjunction with Image-Pro Plus version 4.5.1.3 software (Media Cybernetics). At least 500 beads from at least 5 independent fields were counted for each experiment. For phagosomal pH quantification, a flow cytometry-based method of dual fluorescence measurement was employed [23]. Briefly, beads were coated with 5% FITC - and Alexa Fluor 647-conjugated BSA in PBS overnight at 4°C. Beads were washed twice in PBS, and sonicated in PBS in the presence of the different purified lipids (100 µg lipids/2.106 beads) at room temperature for 15 min. Beads were washed twice in PBS, and used to pulse the cells (2 beads/cell). Fluorescence recording (LSR II apparatus, Becton Dickinson, San Jose, CA) and pH calculation were done as previously and extensively detailed in [23].
In vivo experiments
6 week-old female Balb/C mice (ORIENTBIO Inc., South Korea) were challenged with M. tuberculosis GC1237, Rv1503c::Tn, Rv1506c::Tn and their corresponding complemented counterparts via the intranasal route with 10 µl of a suspension containing 5.105 organisms/ml to obtain an inhaled dose of 100 CFU in lungs. After 42 days, organs from killed mice were homogenized by use of an MM300 apparatus (Qiagen) and 2.5-mm diameter glass beads. Serial 10-fold dilutions in medium were plated on 7H11 agar and colony forming unit counts were ascertained at 37°C after 3 weeks of growth.
Ethics statement
Animal studies were carried out in strict accordance with the recommendations from the Animal Protection Law in Korea. The protocol was approved by the Institutional Animal Care and Use Committee of Institut Pasteur Korea (Permit Number: IPK-10003). All efforts were made to minimize suffering of the animals. Human monocytes were purified from blood samples obtained from healthy blood donors under strict anonymity (Etablissement Français du Sang, EFS, Toulouse). Written informed consents were obtained from the donors under EFS contract n°21/PVNT/TOU/IPBS01/2009-0052. With respect to Decree n°2007-1220 (articles L1243-4, R1243-61 and following) dated August 10th 2007 of the French Public Health Code (published in the Official Journal of the French Republic of August 14th 2007), the contract received formal approval by the French Ministry of Science and Technology (decision n°AC 2009-921). As a “collection” is defined by Decree 2007-1220 as “a collection, for scientific use, of biological samples from a group of persons who have been identified and selected on the basis of clinical or biological characteristics of one or several members of the group”, and as the blood samples used in our study were not obtained from individuals that have been “identified or selected on the basis of clinical or biological characteristics”, these samples do not constitute a so-called “collection”, which waives the need for ethical approval by the ethical committee “Comité de Protection des Personnes”, in agreement with Decree 2007-1220, article R1243-63.
Supporting Information
Zdroje
1. RussellDG
2001 Mycobacterium tuberculosis: here today, and here tomorrow. Nat Rev Mol Cell Biol 2 569 577
2. VergneI
ChuaJ
SinghSB
DereticV
2004 Cell biology of mycobacterium tuberculosis phagosome. Annu Rev Cell Dev Biol 20 367 394
3. RussellDG
2003 Phagosomes, fatty acids and tuberculosis. Nat Cell Biol 5 776 778
4. HestvikAL
HmamaZ
Av-GayY
2005 Mycobacterial manipulation of the host cell. FEMS Microbiol Rev 29 1041 1050
5. NguyenL
PietersJ
2005 The Trojan horse: survival tactics of pathogenic mycobacteria in macrophages. Trends Cell Biol 15 269 276
6. VergneI
FrattiRA
HillPJ
ChuaJ
BelisleJ
2004 Mycobacterium tuberculosis phagosome maturation arrest: mycobacterial phosphatidylinositol analog phosphatidylinositol mannoside stimulates early endosomal fusion. Mol Biol Cell 15 751 760
7. VandalOH
PieriniLM
SchnappingerD
NathanCF
EhrtS
2008 A membrane protein preserves intrabacterial pH in intraphagosomal Mycobacterium tuberculosis. Nat Med 14 849 854
8. WalburgerA
KoulA
FerrariG
NguyenL
Prescianotto-BaschongC
2004 Protein kinase G from pathogenic mycobacteria promotes survival within macrophages. Science 304 1800 1804
9. MacGurnJA
CoxJS
2007 A genetic screen for Mycobacterium tuberculosis mutants defective for phagosome maturation arrest identifies components of the ESX-1 secretion system. Infect Immun 75 2668 2678
10. PetheK
SwensonDL
AlonsoS
AndersonJ
WangC
2004 Isolation of Mycobacterium tuberculosis mutants defective in the arrest of phagosome maturation. Proc Natl Acad Sci U S A 101 13642 13647
11. StewartGR
PatelJ
RobertsonBD
RaeA
YoungDB
2005 Mycobacterial mutants with defective control of phagosomal acidification. PLoS Pathog 1 269 278
12. BurguiereA
HitchenPG
DoverLG
KremerL
RidellM
2005 LosA, a key glycosyltransferase involved in the biosynthesis of a novel family of glycosylated acyltrehalose lipooligosaccharides from Mycobacterium marinum. J Biol Chem 280 42124 42133
13. RenH
DoverLG
IslamST
AlexanderDC
ChenJM
2007 Identification of the lipooligosaccharide biosynthetic gene cluster from Mycobacterium marinum. Mol Microbiol 63 1345 1359
14. CamineroJA
PenaMJ
Campos-HerreroMI
RodriguezJC
GarciaI
2001 Epidemiological evidence of the spread of a Mycobacterium tuberculosis strain of the Beijing genotype on Gran Canaria Island. Am J Respir Crit Care Med 164 1165 1170
15. Gonzalo AsensioJ
MaiaC
FerrerNL
BariloneN
LavalF
2006 The virulence-associated two-component PhoP-PhoR system controls the biosynthesis of polyketide-derived lipids in Mycobacterium tuberculosis. J Biol Chem 281 1313 1316
16. CamachoLR
EnsergueixD
PerezE
GicquelB
GuilhotC
1999 Identification of a virulence gene cluster of Mycobacterium tuberculosis by signature-tagged transposon mutagenesis. Mol Microbiol 34 257 267
17. ColeST
BroschR
ParkhillJ
GarnierT
ChurcherC
1998 Deciphering the biology of Mycobacterium tuberculosis from the complete genome sequence. Nature 393 537 544
18. BangeFC
CollinsFM
JacobsWRJr
1999 Survival of mice infected with Mycobacterium smegmatis containing large DNA fragments from Mycobacterium tuberculosis. Tuber Lung Dis 79 171 180
19. DaffeM
McNeilM
BrennanPJ
1991 Novel type-specific lipooligosaccharides from Mycobacterium tuberculosis. Biochemistry 30 378 388
20. van SoolingenD
HoogenboezemT
de HaasPE
HermansPW
KoedamMA
1997 A novel pathogenic taxon of the Mycobacterium tuberculosis complex, Canetti: characterization of an exceptional isolate from Africa. Int J Syst Bacteriol 47 1236 1245
21. GutierrezMC
BrisseS
BroschR
FabreM
OmaisB
2005 Ancient origin and gene mosaicism of the progenitor of Mycobacterium tuberculosis. PLoS Pathog 1 e5
22. VergneI
ChuaJ
DereticV
2003 Tuberculosis toxin blocking phagosome maturation inhibits a novel Ca2+/calmodulin-PI3K hVPS34 cascade. J Exp Med 198 653 659
23. SavinaA
VargasP
GuermonprezP
LennonAM
AmigorenaS
Measuring pH, ROS production, maturation, and degradation in dendritic cell phagosomes using cytofluorometry-based assays. Methods Mol Biol 595 383 402
24. RobackP
BeardJ
BaumannD
GilleC
HenryK
2007 A predicted operon map for Mycobacterium tuberculosis. Nucleic Acids Res 35 5085 5095
25. PymAS
BrodinP
BroschR
HuerreM
ColeST
2002 Loss of RD1 contributed to the attenuation of the live tuberculosis vaccines Mycobacterium bovis BCG and Mycobacterium microti. Mol Microbiol 46 709 717
26. CoxJS
ChenB
McNeilM
JacobsWRJr
1999 Complex lipid determines tissue-specific replication of Mycobacterium tuberculosis in mice. Nature 402 79 83
27. Rosas-MagallanesV
Stadthagen-GomezG
RauzierJ
BarreiroLB
TailleuxL
2007 Signature-tagged transposon mutagenesis identifies novel Mycobacterium tuberculosis genes involved in the parasitism of human macrophages. Infect Immun 75 504 507
28. Astarie-DequekerC
Le GuyaderL
MalagaW
SeaphanhFK
ChalutC
2009 Phthiocerol dimycocerosates of M. tuberculosis participate in macrophage invasion by inducing changes in the organization of plasma membrane lipids. PLoS Pathog 5 e1000289
29. VoskuilMI
SchnappingerD
RutherfordR
LiuY
Schoolnik GK 2004 Regulation of the Mycobacterium tuberculosis PE/PPE genes. Tuberculosis (Edinb) 84 256 262
30. BitterW
HoubenEN
BottaiD
BrodinP
BrownEJ
2009 Systematic genetic nomenclature for type VII secretion systems. PLoS Pathog 5 e1000507
31. McLaughlinB
ChonJS
MacGurnJA
CarlssonF
ChengTL
2007 A mycobacterium ESX-1-secreted virulence factor with unique requirements for export. PLoS Pathog 3 e105
32. BrodinP
MajlessiL
MarsollierL
de JongeMI
BottaiD
2006 Dissection of ESAT-6 system 1 of Mycobacterium tuberculosis and impact on immunogenicity and virulence. Infect Immun 74 88 98
33. GuinnKM
HickeyMJ
MathurSK
ZakelKL
GrotzkeJE
2004 Individual RD1-region genes are required for export of ESAT-6/CFP-10 and for virulence of Mycobacterium tuberculosis. Mol Microbiol 51 359 370
34. HsuT
Hingley-WilsonSM
ChenB
ChenM
DaiAZ
2003 The primary mechanism of attenuation of bacillus Calmette-Guerin is a loss of secreted lytic function required for invasion of lung interstitial tissue. Proc Natl Acad Sci U S A 100 12420 12425
35. StanleySA
RaghavanS
HwangWW
CoxJS
2003 Acute infection and macrophage subversion by Mycobacterium tuberculosis require a specialized secretion system. Proc Natl Acad Sci U S A 100 13001 13006
36. PeirsP
LefevreP
BoarbiS
WangXM
DenisO
2005 Mycobacterium tuberculosis with disruption in genes encoding the phosphate binding proteins PstS1 and PstS2 is deficient in phosphate uptake and demonstrates reduced in vivo virulence. Infect Immun 73 1898 1902
37. RengarajanJ
BloomBR
RubinEJ
2005 Genome-wide requirements for Mycobacterium tuberculosis adaptation and survival in macrophages. Proc Natl Acad Sci U S A 102 8327 8332
38. VandalOH
RobertsJA
OdairaT
SchnappingerD
NathanCF
2009 Acid-susceptible mutants of Mycobacterium tuberculosis share hypersusceptibility to cell wall and oxidative stress and to the host environment. J Bacteriol 191 625 631
39. BecqJ
GutierrezMC
Rosas-MagallanesV
RauzierJ
GicquelB
2007 Contribution of horizontally acquired genomic islands to the evolution of the tubercle bacilli. Mol Biol Evol 24 1861 1871
40. HatziosSK
SchelleMW
HolsclawCM
BehrensCR
BotyanszkiZ
2009 PapA3 is an acyltransferase required for polyacyltrehalose biosynthesis in Mycobacterium tuberculosis. J Biol Chem 284 12745 12751
41. DubeyVS
SirakovaTD
KolattukudyPE
2002 Disruption of msl3 abolishes the synthesis of mycolipanoic and mycolipenic acids required for polyacyltrehalose synthesis in Mycobacterium tuberculosis H37Rv and causes cell aggregation. Mol Microbiol 45 1451 1459
42. SirakovaTD
ThirumalaAK
DubeyVS
SprecherH
KolattukudyPE
2001 The Mycobacterium tuberculosis pks2 gene encodes the synthase for the hepta - and octamethyl-branched fatty acids required for sulfolipid synthesis. J Biol Chem 276 16833 16839
43. SchelleMW
BertozziCR
2006 Sulfate metabolism in mycobacteria. Chembiochem 7 1516 1524
44. KumarP
SchelleMW
JainM
LinFL
PetzoldCJ
2007 PapA1 and PapA2 are acyltransferases essential for the biosynthesis of the Mycobacterium tuberculosis virulence factor sulfolipid-1. Proc Natl Acad Sci U S A 104 11221 11226
45. WaltersSB
DubnauE
KolesnikovaI
LavalF
DaffeM
2006 The Mycobacterium tuberculosis PhoPR two-component system regulates genes essential for virulence and complex lipid biosynthesis. Mol Microbiol 60 312 330
46. GorenMB
D'Arcy HartP
YoungMR
ArmstrongJA
1976 Prevention of phagosome-lysosome fusion in cultured macrophages by sulfatides of Mycobacterium tuberculosis. Proc Natl Acad Sci U S A 73 2510 2514
47. SchaibleUE
Sturgill-KoszyckiS
SchlesingerPH
RussellDG
1998 Cytokine activation leads to acidification and increases maturation of Mycobacterium avium-containing phagosomes in murine macrophages. J Immunol 160 1290 1296
48. ViaLE
FrattiRA
McFaloneM
Pagan-RamosE
DereticD
1998 Effects of cytokines on mycobacterial phagosome maturation. J Cell Sci 111 Pt7 897 905
49. PerezE
SamperS
BordasY
GuilhotC
GicquelB
2001 An essential role for phoP in Mycobacterium tuberculosis virulence. Mol Microbiol 41 179 187
50. ChristopheT
JacksonM
JeonHK
FenisteinD
Contreras-DominguezM
2009 High content screening identifies decaprenyl-phosphoribose 2′ epimerase as a target for intracellular antimycobacterial inhibitors. PLoS Pathog 5 e1000645
51. BeletskiiA
CooperM
SriramanP
ChiriacC
ZhaoL
2005 High-throughput phagocytosis assay utilizing a pH-sensitive fluorescent dye. Biotechniques 39 894 897
52. FenisteinD
LenseigneB
ChristopheT
BrodinP
GenovesioA
2008 A fast fully automated cell segmentation algorithm for high throughput and high content screening. Cytometry 73 958 964
53. Prod'homG
LagierB
PelicicV
HanceAJ
GicquelB
1998 A reliable amplification technique for the characterization of genomic DNA sequences flanking insertion sequences. FEMS Microbiol Lett 158 75 81
54. KordulakovaJ
GilleronM
MikusovaK
PuzoG
BrennanPJ
2002 Definition of the first mannosylation step in phosphatidylinositol mannoside synthesis. PimA is essential for growth of mycobacteria. J Biol Chem 277 31335 31344
55. NigouJ
VercelloneA
PuzoG
2000 New structural insights into the molecular deciphering of mycobacterial lipoglycan binding to C-type lectins: lipoarabinomannan glycoform characterization and quantification by capillary electrophoresis at the subnanomole level. J Mol Biol 299 1353 1362
56. GilleronM
RonetC
MempelM
MonsarratB
GachelinG
2001 Acylation state of the phosphatidylinositol mannosides from Mycobacterium bovis bacillus Calmette Guerin and ability to induce granuloma and recruit natural killer T cells. J Biol Chem 276 34896 34904
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek SRFR1 Negatively Regulates Plant NB-LRR Resistance Protein Accumulation to Prevent AutoimmunityČlánek Inhibition of TIR Domain Signaling by TcpC: MyD88-Dependent and Independent Effects on VirulenceČlánek Phylogenetic Approach Reveals That Virus Genotype Largely Determines HIV Set-Point Viral LoadČlánek A Family of Plasmodesmal Proteins with Receptor-Like Properties for Plant Viral Movement Proteins
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2010 Číslo 9- Stillova choroba: vzácné a závažné systémové onemocnění
- Diagnostika virových hepatitid v kostce – zorientujte se (nejen) v sérologii
- Perorální antivirotika jako vysoce efektivní nástroj prevence hospitalizací kvůli COVID-19 − otázky a odpovědi pro praxi
- Choroby jater v ordinaci praktického lékaře – význam jaterních testů
- Diagnostický algoritmus při podezření na syndrom periodické horečky
-
Všechny články tohoto čísla
- Azole Drugs Are Imported By Facilitated Diffusion in and Other Pathogenic Fungi
- Two Genes on A/J Chromosome 18 Are Associated with Susceptibility to Infection by Combined Microarray and QTL Analyses
- Impact of Simian Immunodeficiency Virus Infection on Chimpanzee Population Dynamics
- Breaking the Stereotype: Virulence Factor–Mediated Protection of Host Cells in Bacterial Pathogenesis
- The Canine Papillomavirus and Gamma HPV E7 Proteins Use an Alternative Domain to Bind and Destabilize the Retinoblastoma Protein
- Rescue of HIV-1 Release by Targeting Widely Divergent NEDD4-Type Ubiquitin Ligases and Isolated Catalytic HECT Domains to Gag
- Steric Shielding of Surface Epitopes and Impaired Immune Recognition Induced by the Ebola Virus Glycoprotein
- Dynamics of the Multiplicity of Cellular Infection in a Plant Virus
- HLA Class I Binding of HBZ Determines Outcome in HTLV-1 Infection
- Pathogenic Bacteria Target NEDD8-Conjugated Cullins to Hijack Host-Cell Signaling Pathways
- The HA and NS Genes of Human H5N1 Influenza A Virus Contribute to High Virulence in Ferrets
- SRFR1 Negatively Regulates Plant NB-LRR Resistance Protein Accumulation to Prevent Autoimmunity
- Cyclin-Dependent Kinase Activity Controls the Onset of the HCMV Lytic Cycle
- The N-Terminal Domain of the Arenavirus L Protein Is an RNA Endonuclease Essential in mRNA Transcription
- Generation of Neutralizing Antibodies and Divergence of SIVmac239 in Cynomolgus Macaques Following Short-Term Early Antiretroviral Therapy
- Inhibition of TIR Domain Signaling by TcpC: MyD88-Dependent and Independent Effects on Virulence
- Intracellular Proton Conductance of the Hepatitis C Virus p7 Protein and Its Contribution to Infectious Virus Production
- The Transcriptome of the Human Pathogen at Single-Nucleotide Resolution
- The Epidermal Growth Factor Receptor (EGFR) Promotes Uptake of Influenza A Viruses (IAV) into Host Cells
- Surface Co-Expression of Two Different PfEMP1 Antigens on Single -Infected Erythrocytes Facilitates Binding to ICAM1 and PECAM1
- Sequestration and Tissue Accumulation of Human Malaria Parasites: Can We Learn Anything from Rodent Models of Malaria?
- Phylogenomics of Ligand-Gated Ion Channels Predicts Monepantel Effect
- Generation of Covalently Closed Circular DNA of Hepatitis B Viruses via Intracellular Recycling Is Regulated in a Virus Specific Manner
- CpG-Methylation Regulates a Class of Epstein-Barr Virus Promoters
- Molecular and Evolutionary Bases of Within-Patient Genotypic and Phenotypic Diversity in Extraintestinal Infections
- A Bistable Switch and Anatomical Site Control Virulence Gene Expression in the Intestine
- Are Members of the Fungal Genus (a) Commensals; (b) Opportunists; (c) Pathogens; or (d) All of the Above?
- Structures of Receptor Complexes of a North American H7N2 Influenza Hemagglutinin with a Loop Deletion in the Receptor Binding Site
- Phylogenetic Approach Reveals That Virus Genotype Largely Determines HIV Set-Point Viral Load
- The Coevolution of Virulence: Tolerance in Perspective
- Involvement of the Cytokine MIF in the Snail Host Immune Response to the Parasite
- Structure of the Extracellular Portion of CD46 Provides Insights into Its Interactions with Complement Proteins and Pathogens
- A Family of Plasmodesmal Proteins with Receptor-Like Properties for Plant Viral Movement Proteins
- High Content Phenotypic Cell-Based Visual Screen Identifies Acyltrehalose-Containing Glycolipids Involved in Phagosome Remodeling
- A Novel Small Molecule Inhibitor of Hepatitis C Virus Entry
- The Microbiota Mediates Pathogen Clearance from the Gut Lumen after Non-Typhoidal Diarrhea
- RNA Polymerases (L-Protein) Have an N-Terminal, Influenza-Like Endonuclease Domain, Essential for Viral Cap-Dependent Transcription
- Pathogen Specific, IRF3-Dependent Signaling and Innate Resistance to Human Kidney Infection
- Cellular Entry of Ebola Virus Involves Uptake by a Macropinocytosis-Like Mechanism and Subsequent Trafficking through Early and Late Endosomes
- The Length of Vesicular Stomatitis Virus Particles Dictates a Need for Actin Assembly during Clathrin-Dependent Endocytosis
- Formation of Mobile Chromatin-Associated Nuclear Foci Containing HIV-1 Vpr and VPRBP Is Critical for the Induction of G2 Cell Cycle Arrest
- Association of Tat with Promoters of PTEN and PP2A Subunits Is Key to Transcriptional Activation of Apoptotic Pathways in HIV-Infected CD4+ T Cells
- Metal Hyperaccumulation Armors Plants against Disease
- Cyclin-Dependent Kinase-Like Function Is Shared by the Beta- and Gamma- Subset of the Conserved Herpesvirus Protein Kinases
- Role of Acetyl-Phosphate in Activation of the Rrp2-RpoN-RpoS Pathway in
- Ebolavirus Is Internalized into Host Cells Macropinocytosis in a Viral Glycoprotein-Dependent Manner
- A Novel Family of IMC Proteins Displays a Hierarchical Organization and Functions in Coordinating Parasite Division
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Structure of the Extracellular Portion of CD46 Provides Insights into Its Interactions with Complement Proteins and Pathogens
- The Length of Vesicular Stomatitis Virus Particles Dictates a Need for Actin Assembly during Clathrin-Dependent Endocytosis
- Inhibition of TIR Domain Signaling by TcpC: MyD88-Dependent and Independent Effects on Virulence
- The Coevolution of Virulence: Tolerance in Perspective
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



