-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Measuring Coverage in MNCH: Population HIV-Free Survival among Children under Two Years of Age in Four African Countries
Background:
Population-based evaluations of programs for prevention of mother-to-child HIV transmission (PMTCT) are scarce. We measured PMTCT service coverage, regimen use, and HIV-free survival among children ≤24 mo of age in Cameroon, Côte D'Ivoire, South Africa, and Zambia.Methods and Findings:
We randomly sampled households in 26 communities and offered participation if a child had been born to a woman living there during the prior 24 mo. We tested consenting mothers with rapid HIV antibody tests and tested the children of seropositive mothers with HIV DNA PCR or rapid antibody tests. Our primary outcome was 24-mo HIV-free survival, estimated with survival analysis. In an individual-level analysis, we evaluated the effectiveness of various PMTCT regimens. In a community-level analysis, we evaluated the relationship between HIV-free survival and community PMTCT coverage (the proportion of HIV-exposed infants in each community that received any PMTCT intervention during gestation or breastfeeding). We also compared our community coverage results to those of a contemporaneous study conducted in the facilities serving each sampled community. Of 7,985 surveyed children under 2 y of age, 1,014 (12.7%) were HIV-exposed. Of these, 110 (10.9%) were HIV-infected, 851 (83.9%) were HIV-uninfected, and 53 (5.2%) were dead. HIV-free survival at 24 mo of age among all HIV-exposed children was 79.7% (95% CI: 76.4, 82.6) overall, with the following country-level estimates: Cameroon (72.6%; 95% CI: 62.3, 80.5), South Africa (77.7%; 95% CI: 72.5, 82.1), Zambia (83.1%; 95% CI: 78.4, 86.8), and Côte D'Ivoire (84.4%; 95% CI: 70.0, 92.2). In adjusted analyses, the risk of death or HIV infection was non-significantly lower in children whose mothers received a more complex regimen of either two or three antiretroviral drugs compared to those receiving no prophylaxis (adjusted hazard ratio: 0.60; 95% CI: 0.34, 1.06). Risk of death was not different for children whose mothers received a more complex regimen compared to those given single-dose nevirapine (adjusted hazard ratio: 0.88; 95% CI: 0.45, 1.72). Community PMTCT coverage was highest in Cameroon, where 75 of 114 HIV-exposed infants met criteria for coverage (66%; 95% CI: 56, 74), followed by Zambia (219 of 444, 49%; 95% CI: 45, 54), then South Africa (152 of 365, 42%; 95% CI: 37, 47), and then Côte D'Ivoire (3 of 53, 5.7%; 95% CI: 1.2, 16). In a cluster-level analysis, community PMTCT coverage was highly correlated with facility PMTCT coverage (Pearson's r = 0.85), and moderately correlated with 24-mo HIV-free survival (Pearson's r = 0.29). In 14 of 16 instances where both the facility and community samples were large enough for comparison, the facility-based coverage measure exceeded that observed in the community.Conclusions:
HIV-free survival can be estimated with community surveys and should be incorporated into ongoing country monitoring. Facility-based coverage measures correlate with those derived from community sampling, but may overestimate population coverage. The more complex regimens recommended by the World Health Organization seem to have measurable public health benefit at the population level, but power was limited and additional field validation is needed.
Please see later in the article for the Editors' Summary
Published in the journal: . PLoS Med 10(5): e32767. doi:10.1371/journal.pmed.1001424
Category: Research Article
doi: https://doi.org/10.1371/journal.pmed.1001424Summary
Background:
Population-based evaluations of programs for prevention of mother-to-child HIV transmission (PMTCT) are scarce. We measured PMTCT service coverage, regimen use, and HIV-free survival among children ≤24 mo of age in Cameroon, Côte D'Ivoire, South Africa, and Zambia.Methods and Findings:
We randomly sampled households in 26 communities and offered participation if a child had been born to a woman living there during the prior 24 mo. We tested consenting mothers with rapid HIV antibody tests and tested the children of seropositive mothers with HIV DNA PCR or rapid antibody tests. Our primary outcome was 24-mo HIV-free survival, estimated with survival analysis. In an individual-level analysis, we evaluated the effectiveness of various PMTCT regimens. In a community-level analysis, we evaluated the relationship between HIV-free survival and community PMTCT coverage (the proportion of HIV-exposed infants in each community that received any PMTCT intervention during gestation or breastfeeding). We also compared our community coverage results to those of a contemporaneous study conducted in the facilities serving each sampled community. Of 7,985 surveyed children under 2 y of age, 1,014 (12.7%) were HIV-exposed. Of these, 110 (10.9%) were HIV-infected, 851 (83.9%) were HIV-uninfected, and 53 (5.2%) were dead. HIV-free survival at 24 mo of age among all HIV-exposed children was 79.7% (95% CI: 76.4, 82.6) overall, with the following country-level estimates: Cameroon (72.6%; 95% CI: 62.3, 80.5), South Africa (77.7%; 95% CI: 72.5, 82.1), Zambia (83.1%; 95% CI: 78.4, 86.8), and Côte D'Ivoire (84.4%; 95% CI: 70.0, 92.2). In adjusted analyses, the risk of death or HIV infection was non-significantly lower in children whose mothers received a more complex regimen of either two or three antiretroviral drugs compared to those receiving no prophylaxis (adjusted hazard ratio: 0.60; 95% CI: 0.34, 1.06). Risk of death was not different for children whose mothers received a more complex regimen compared to those given single-dose nevirapine (adjusted hazard ratio: 0.88; 95% CI: 0.45, 1.72). Community PMTCT coverage was highest in Cameroon, where 75 of 114 HIV-exposed infants met criteria for coverage (66%; 95% CI: 56, 74), followed by Zambia (219 of 444, 49%; 95% CI: 45, 54), then South Africa (152 of 365, 42%; 95% CI: 37, 47), and then Côte D'Ivoire (3 of 53, 5.7%; 95% CI: 1.2, 16). In a cluster-level analysis, community PMTCT coverage was highly correlated with facility PMTCT coverage (Pearson's r = 0.85), and moderately correlated with 24-mo HIV-free survival (Pearson's r = 0.29). In 14 of 16 instances where both the facility and community samples were large enough for comparison, the facility-based coverage measure exceeded that observed in the community.Conclusions:
HIV-free survival can be estimated with community surveys and should be incorporated into ongoing country monitoring. Facility-based coverage measures correlate with those derived from community sampling, but may overestimate population coverage. The more complex regimens recommended by the World Health Organization seem to have measurable public health benefit at the population level, but power was limited and additional field validation is needed.
Please see later in the article for the Editors' SummaryThis paper is part of the PLOS Medicine “Measuring Coverage in MNCH” Collection.
Introduction
While transmission of HIV from mother to infant has been virtually eliminated in high-income countries, substantial challenges remain in low - and middle-income settings, where almost 1,000 children are thought to acquire HIV each day [1]–[3]. The efficacy of antiretroviral (ARV) regimens during pregnancy and postpartum to prevent mother-to-child transmission is well established, and prevention of mother-to-child transmission (PMTCT) programs have been implemented in most countries in Africa [4],[5]. However, objective measures of program effectiveness and public health impact are largely absent from the refereed literature [6],[7]. Despite calls for the virtual elimination of pediatric HIV, global coverage of PMTCT services remains far below what will be required to meet such an aggressive goal [8]. Current ARV guidelines for resource-limited settings recommend earlier and more effective regimens at higher CD4+ T-lymphocyte thresholds in pregnancy, as well as postpartum treatment or prophylaxis to the mother or child to prevent transmission through breastfeeding. More recently, the initiation of lifelong highly active antiretroviral therapy (HAART) has been proposed for all pregnant, HIV-infected women [9]–[11].
The performance of national PMTCT programs is commonly assessed by aggregating routinely collected service coverage indicators, which are often incomplete and inaccurate, and typically lack the site-level nuance needed to drive meaningful quality improvement efforts [12]–[14]. Recent service-based evaluations have used umbilical cord blood surveillance [15],[16] and infant PCR testing at immunization clinics [17],[18] to improve data quality, but since these methods are facility-based they may not represent the entire community [6].
Methods
The PMTCT Effectiveness in Africa: Research and Linkages to Care Study (PEARL Study) was a multi-country evaluation of the effectiveness of PMTCT services at the community and facility level. The study's methodology and facility-based components have been reported elsewhere [6],[15],[19]–[21]. The community survey component of the study was conducted in 26 communities in Cameroon, Côte D'Ivoire, South Africa, and Zambia between April 2007 and May 2009.
Household Selection
Each country employed a two-stage sampling technique. The first stage involved random selection of facilities providing PMTCT services and has been described in detail elsewhere [15]. Once the health facilities were selected in the first stage of sampling, the catchment area of each facility was defined. In all countries, this was done at the local level by reviewing available district data and consulting with local staff. In South Africa and Zambia, we used publically available satellite images (http://www.google.com/earth); in Cameroon and Côte D'Ivoire, we relied upon local maps. Once the catchment boundaries were defined, we subdivided each evaluation area by overlaying a grid of 1 km by 1 km squares. We then estimated the approximate number of households in each square. If the number of households exceeded 50, the square was further subdivided into four smaller squares. All squares of at least five households were then ranked in random order using a “rank probability proportional to size” technique and were then visited in sequence, with all households in each successive grid approached until at least 250 households had been surveyed. In cases where no one was home to participate in the interview, we made two additional attempts to return, after which we counted the household as unavailable.
Each country team employed different ways to educate the communities prior to the surveys, but in each case we met with local community leaders to introduce the survey. Drama performances at public markets and targeted radio announcement were also used. Households were eligible to participate if a child was born in the home during the 24 mo prior to the study visit (whether the mother or infant was currently alive or dead). If the child's mother was not available for interview, we made up to two additional attempts to find her. Failing that, or in the case of a deceased mother, a caregiver was interviewed.
Study Questionnaire
The survey instrument was adapted from the Zambia Demographic and Health Surveys (DHS) [22] and comprised three parts. Team members used an eligibility form to enumerate all living and deceased household members, their sex, and their age. If there was a child born in the household within the 2 y prior to the survey, the household was deemed eligible for participation in the study and informed consent was obtained. The primary survey respondent was the mother of the eligible child. If the mother was not available, the child's primary caregiver was asked to participate. The maternal questionnaire focused on household characteristics; demographic information; HIV knowledge, attitudes, and perceptions; and previous obstetrical history. The infant module covered specific information about the index pregnancy, including access to and utilization of PMTCT, delivery and postnatal care, and infant feeding. Teams of at least two members, including at least one registered nurse, were trained to administer the survey in local languages at each site.
Specimen Collection and Testing
In all countries we tested all participating HIV-exposed infants under 24 mo of age for HIV infection. Our procedures for household testing and results disclosure varied by country. In Cameroon, mothers and children over 1 y of age were antibody tested in the household, where pre - and post-test counseling was performed, and results were provided during the visit. Infant dried blood spots for HIV PCR testing were not collected from children whose mothers were seronegative or from those whose mothers were seropositive, but who were over 12 mo of age, no longer breastfeeding, and themselves HIV antibody negative. In the other countries, specimens were obtained from all consenting mothers and children, with HIV testing performed off-site. In Zambia, we provided a card with a linked identification number that allowed participants to access their results (and/or those of their child) at the local health facility. In South Africa and Côte D'Ivoire, test results were not provided to participants. This was a requirement of local ethics review boards, since we were unable to do confirmatory testing in the home. Women were encouraged to access routine counseling and testing services if they so desired, and the study team provided information about where free services could be accessed. In Zambia, we also offered infant hemoglobin testing with referral for children found to be anemic.
All countries used the Determine HIV-1 rapid antibody test (Abbott Laboratories) for initial testing. Confirmatory tests differed by country. We tested infant dried blood spots for HIV DNA PCR using Roche Amplicor version 1.5 with manual extraction.
Informed Consent and Ethical Approvals
In each country setting, informed consent was obtained prior to administering the questionnaire and blood draws. The informed consent information was customized to suit the procedure and research ethics requirements in the respective countries. In cases of participant illiteracy, a non-biased literate witness joined the informed consent procedure to ensure that objective information was given about the study and that the patient understood. Ethical approval was obtained from the institutional review boards at the University of Alabama at Birmingham (Birmingham, Alabama, US) and the US Centers for Disease Control and Prevention (Atlanta, Georgia, US), as well as the local research ethics review bodies in each of the participating countries.
Analysis
Our primary outcome was HIV-free survival among HIV-exposed children. Our sample size was chosen to deliver a half-confidence interval of 5% around the primary outcome in each country; we assumed a binomial distribution. The actual number of houses approached per community also took into account country-specific estimates of maternal HIV seroprevalence and baseline child survival. Statistical analyses were performed using SAS statistical software version 9.1.4 (SAS Institute) and R software version 2.4.1 (http://www.r-project.org). Background characteristics were described using medians and interquartile ranges (IQRs) for continuous variables and percentages for categorical variables. Children were stratified according to their HIV-exposure status, HIV-status, and mortality. The present analysis is limited to those children who were known to be exposed to HIV, either through the detection of HIV antibodies in the infant him - or herself, or through a positive maternal HIV diagnosis. HIV-exposed children who were alive and tested HIV negative by DNA PCR were right-censored at the time of testing in time-to-event analyses. HIV-infected children were left-censored at the time of testing, because transmission was known to have occurred prior to the test date. Twenty-four-month HIV-free survival was estimated using a Weibull regression model and a parametric accelerated failure time model with corresponding 95% confidence intervals. Hazard ratios (HRs) for various covariates of interest were fitted in multivariate Weibull regression models and were restricted to children with responses for each of the covariates of interest. Children born in a particular community or a particular country may be similar to each other. We accounted for this potential correlation by including a separate fixed effect variable for both community and country in our multivariable model. In cases where one mother contributed more than one child to the analysis (e.g., twin gestation or short birth interval), we accounted for this correlation by including a “mother” variable as a random effect. To understand the potential for variable co-linearity, we calculated the variance inflation factors of all variables included in the multivariate model. The variance inflation factor for each variable was less than 1.5, which indicates variable multi-co-linearity is not an issue in our model. The effect of infant feeding on HIV-free survival was limited to those children who were at least 6 mo of age.
In a planned secondary analysis, we compared coverage of PMTCT services at the facility level (“facility PMTCT coverage”) to coverage of PMTCT services at the community level (“community PMTCT coverage”). We also compared facility PMTCT coverage to HIV-free survival among HIV-exposed infants. Facility PMTCT coverage was ascertained through an anonymous cord blood surveillance exercise that has been described elsewhere [15]. In the present report, we compare this facility-based coverage measure to the results of our community surveys. Community PMTCT coverage is defined as the proportion of children in our survey born to HIV-positive mothers in whom any ARV drug is reported to have been used during pregnancy. We weighted our estimates according to the number of HIV-exposed infants in a given service cluster and restricted the analysis to clusters with at least 15 exposed infants.
Results
The household survey was conducted in six areas in Cameroon, six in Côte D'Ivoire, six in South Africa, and eight in Zambia. Collectively, these areas represent the entire catchment population of the 43 facilities included in the sampling scheme [15]. Of 28,942 households visited between 16 May 2008 and 20 May 2009, 9,348 (32%) were eligible (Figure 1). In total, 10,236 children had been born to 9,606 mothers in the previous 2 y. Some children could not be analyzed: 1,465 (14%) mothers or caregivers refused to participate in the survey, while 588 (6%) agreed to be interviewed but refused to provide specimens. In addition, we lost or could not analyze 198 specimens (2%).
Fig. 1. Description of the cohort of children born in the previous 2 y (<i>n</i> = 10,236) from all eligible households (<i>n</i> = 9,348) visited from May 2008 to May 2009. 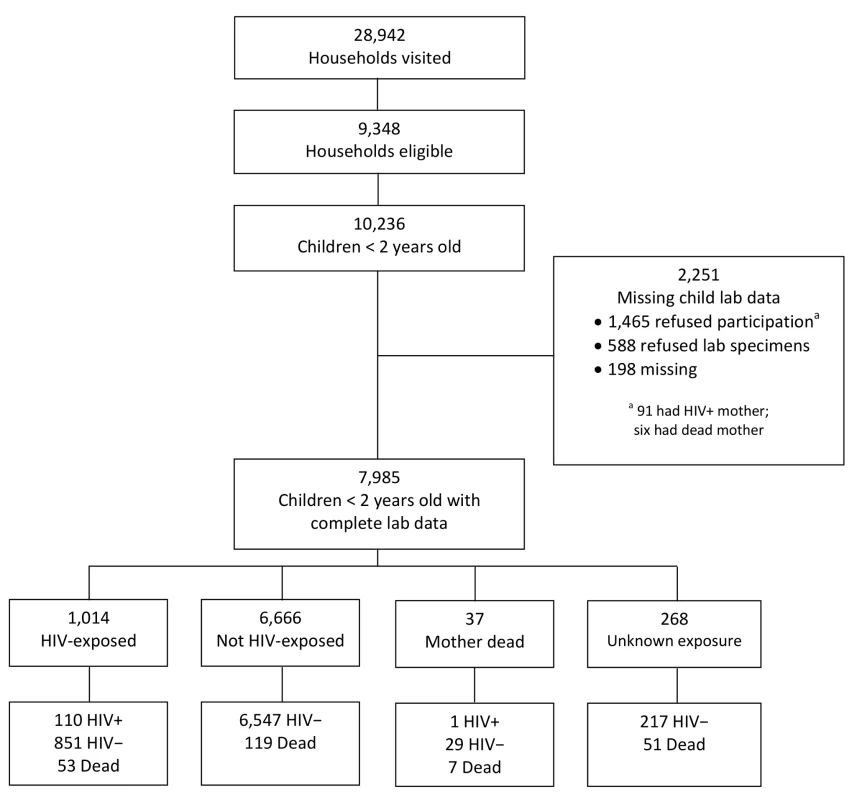
Of 7,985 surveyed and analyzable children under 2 y of age, 1,014 (12.7%) were HIV-exposed. Of these, 110 (10.9%) were HIV-infected, 851 (83.9%) were HIV-uninfected, and 53 (5.2%) were dead (Figure 1). Overall response rates—defined as complete child data available—varied by country, with South Africa having the lowest (64%), followed by Côte D'Ivoire (73%), Zambia (88%), and Cameroon (90%).
Participant Characteristics
Household characteristics are shown in Table 1. The proportion of households reporting access to a flush toilet or pit latrine ranged from 41% in Côte D'Ivoire to 98% in Cameroon. More than 60% of all households in each of the four countries had finished floors (e.g., cement, tile, or wood). Access to electricity was highest in South Africa and Côte D'Ivoire (95%), and lower in Cameroon (66%) and Zambia (33%). South Africa had the highest proportion of households with a refrigerator (75%) and television (81%). The proportion of homes with mobile phones ranged from 55% in Zambia to 87% in South Africa.
Tab. 1. Characteristics of households surveyed with a child under 2 y of age in the PEARL Study. 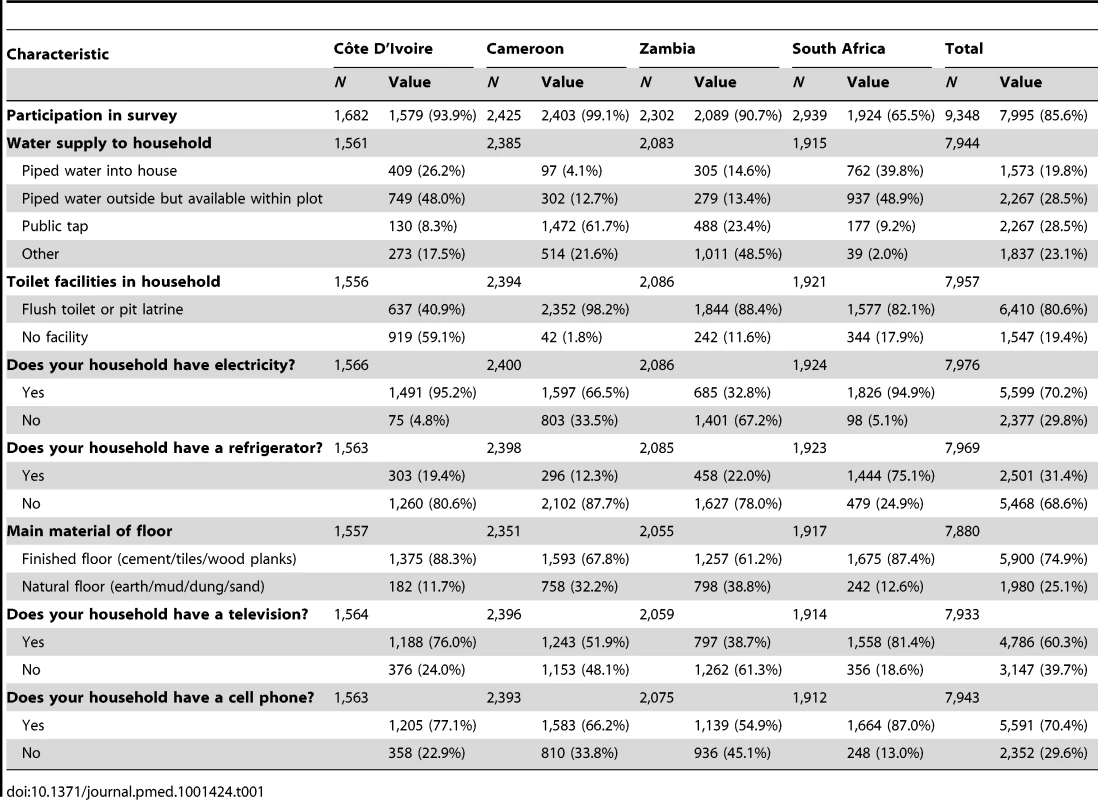
The median age of women participating in the survey was 25 y (IQR: 21–30) and the median parity was 2 (IQR: 1–3). With the exception of South African mothers (43%), the majority of women were married or cohabiting. Employment was highest in Cameroon (67%) and lowest in South Africa (20%). In South Africa 83% of mothers had completed at least secondary level school, but only 13% had done so in Cameroon (Table 2).
Tab. 2. Characteristics of mothers surveyed with a child under 2 y of age in the PEARL Study. 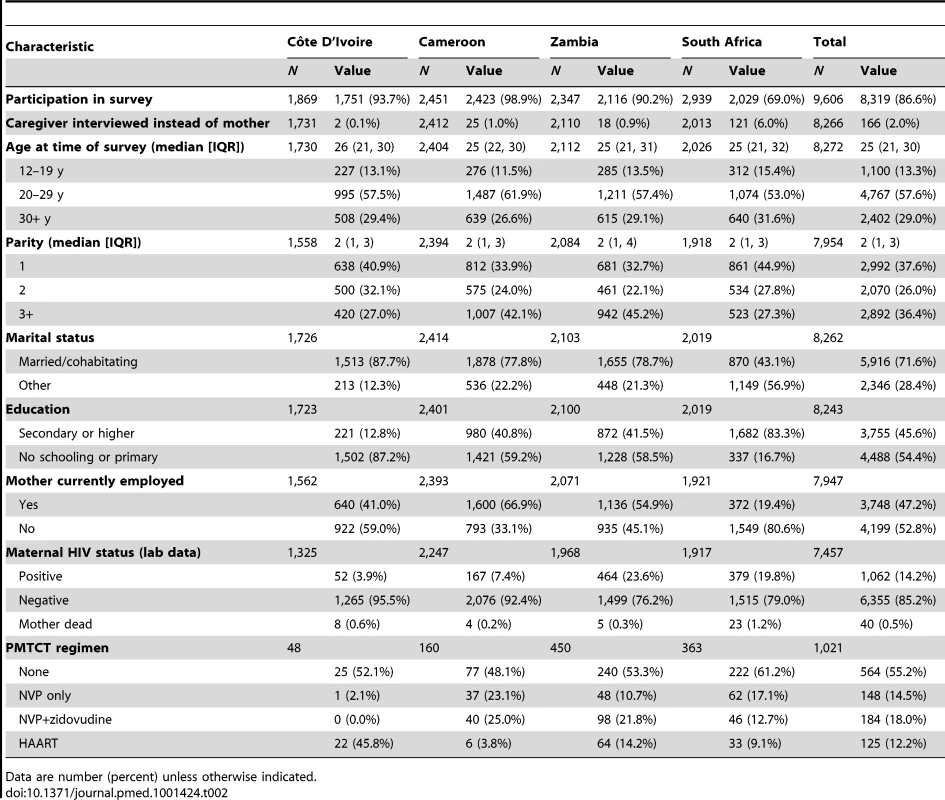
Data are number (percent) unless otherwise indicated. Reported utilization of antenatal and delivery services was high across all countries (Table 3). More than 90% of women had attended at least one antenatal visit during their pregnancy with the index child, and the proportion reporting a facility delivery ranged from 87% in Côte D'Ivoire to 96% in Cameroon. The median gestational age at which women initiated antenatal care was 4 mo (IQR: 3–6) in Côte D'Ivoire and South Africa and 5 mo (IQR: 4–6) in Cameroon and Zambia. The proportion of respondents who reported having undergone HIV testing during the index pregnancy was high for Zambia (91%), Cameroon (93%), and South Africa (94%), but much lower in Côte D'Ivoire (39%). Among women who reported having been tested for HIV during the index pregnancy, the rates of self-reported HIV-positivity were significantly lower than the actual HIV seroprevalence of mothers from blood collected at the time of the survey (Côte D'Ivoire [0.3% versus 3.9%; p<0.01], Cameroon [4.9% versus 7.4%; p<0.01], South Africa [9.1% versus 19.8%; p<0.01], and Zambia [12.6% versus 23.6%; p<0.01]).
Tab. 3. Characteristics of antenatal and postnatal care for children under 2 y of age in the PEARL Study. 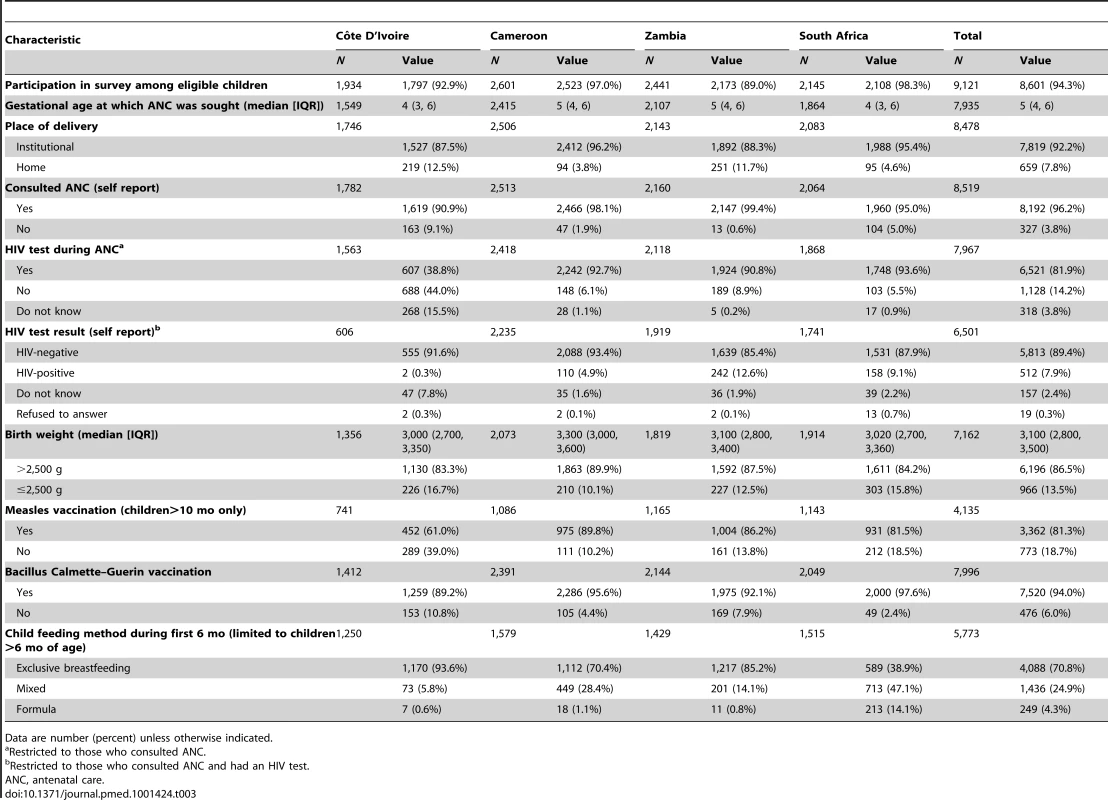
Data are number (percent) unless otherwise indicated. The median birth weight of the index pregnancy was similar across countries (total cohort median 3,100 g; IQR: 2,800–3,500). Measles vaccination coverage by 10 mo of age ranged between 61% (Côte D'Ivoire) and 90% (Cameroon). In Côte D'Ivoire, Cameroon, and Zambia, more than 75% of children more than 6 mo of age were reported to have been exclusively breastfed for 6 mo, while in South Africa, only 40% of children were reported to have been exclusively breastfed, and mixed feeding was common (47%). The proportion of HIV-exposed infants in each country who received no PMTCT prophylaxis at all varied from 48% in Cameroon to 61% in South Africa. The highest proportion of infants whose mothers received HAART during pregnancy was found in Côte D'Ivoire (46%), followed by 14% in Zambia, 9% in South Africa, and 4% in Cameroon (Table 2).
HIV-Free Survival
HIV-free survival at 24 mo of age among HIV-exposed children was 79.7% (95% CI: 76.4, 82.6). These rates differed by country, but not significantly so: Cameroon (72.6%; 95% CI: 62.3, 80.5), South Africa (77.7%; 95% CI: 72.5, 82.1), Zambia (83.1%; 95% CI: 78.4, 86.8), and Côte D'Ivoire (84.4%; 95% CI: 70.0, 92.2) (Figure 2A). HIV-free survival was highest in children born to mothers who received dual ARV prophylaxis or HAART (88.5%; 95% CI: 82.4, 92.6), followed by children whose mothers received single-dose nevirapine (NVP) (82.2%; 95% CI: 73.7, 88.2), followed by children who received no PMTCT intervention (78.3%; 95% CI: 73.9, 82.0), although these results did not differ significantly (Figure 2B). In adjusted analyses, risk of death or HIV infection among children receiving a more complex regimen of either dual ARV prophylaxis or HAART was lower (adjusted HR: 0.60; 95% CI: 0.34, 1.06; p = 0.067), compared to those receiving no prophylaxis, but not significantly so. In a pairwise comparison of children receiving a more complex regimen of either dual ARV prophylaxis or HAART versus those receiving only single-dose NVP prophylaxis, we did not observe additional benefit (adjusted HR: 0.88; 95% CI: 0.45, 1.44), but power to detect a difference was limited. No other parameters studied were associated with improved HIV-free survival (Table 4).
Fig. 2. HIV-free survival by country and by PMTCT regimen in the PEARL Study. 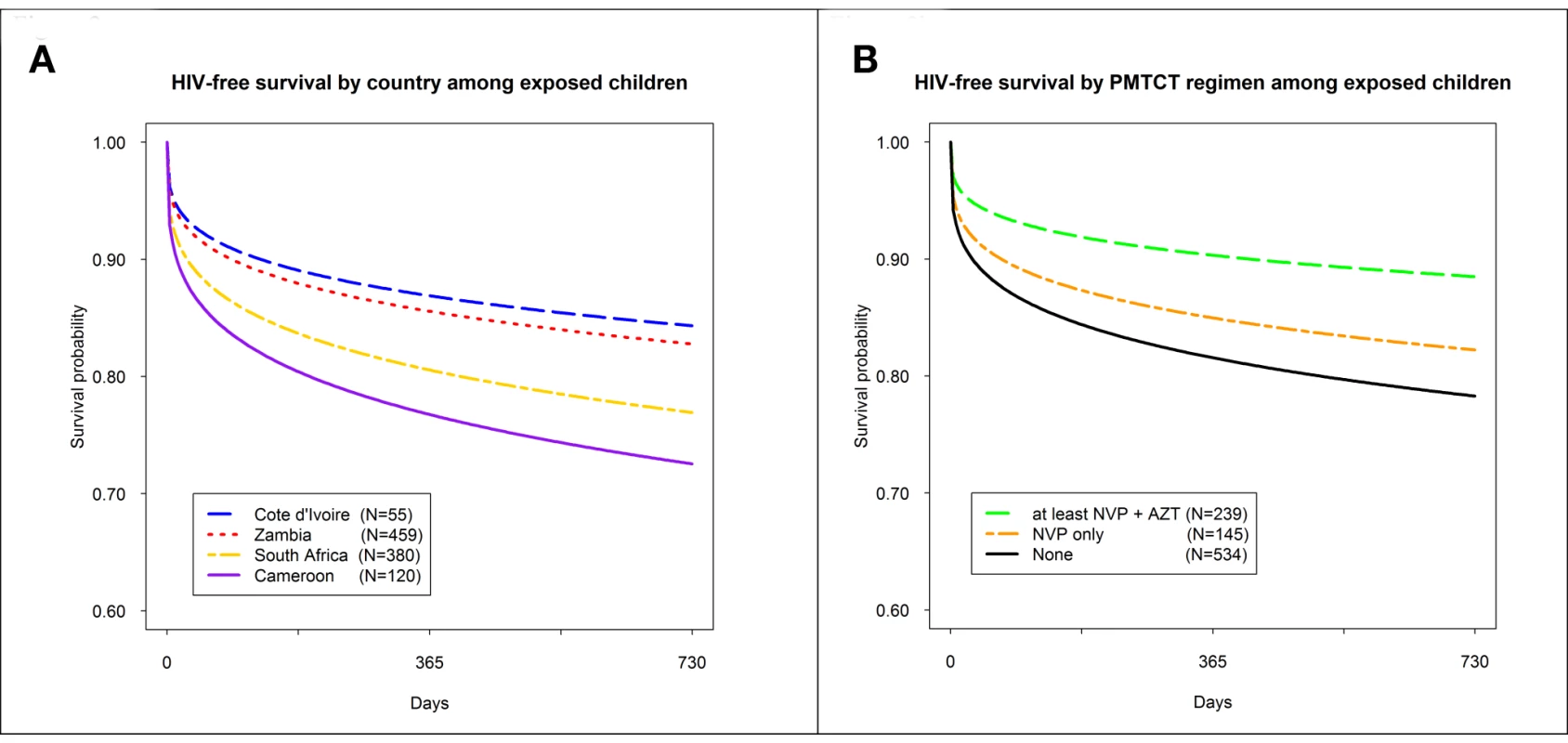
(A) HIV-free survival by country among exposes children; (B) HIV-free survival by PMTCT regimen among exposed children. AZT, zidovudine. Tab. 4. Adjusted hazard of HIV infection or death among HIV-exposed children under 2 y of age in the PEARL Study (n = 893). 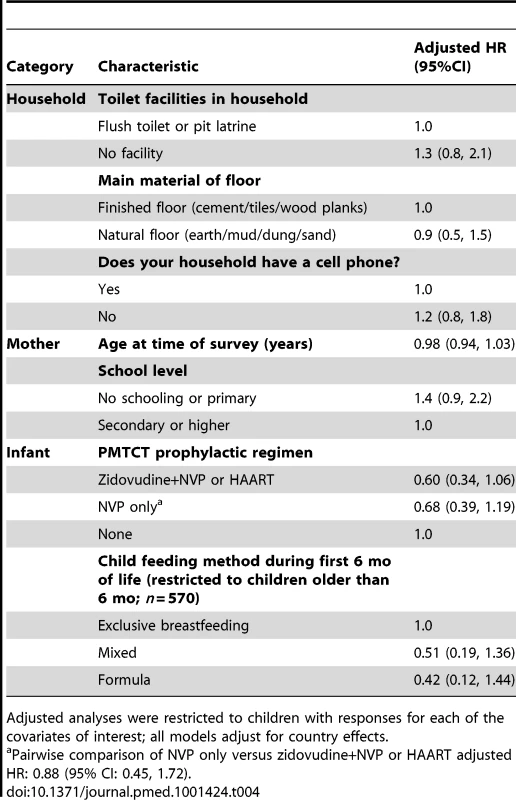
Adjusted analyses were restricted to children with responses for each of the covariates of interest; all models adjust for country effects. PMTCT Intervention Coverage
Of 976 HIV-exposed infants in the study for whom complete laboratory and survey data are available, 449 reportedly received at least some ARV drug prophylaxis during pregnancy or the postnatal period. This represents an unadjusted community PMTCT coverage of 46% (95% CI: 43, 49). Community PMTCT coverage differed significantly by country. It was highest in Cameroon, where 75 of 114 HIV-exposed infants met criteria for coverage (66%; 95% CI: 56, 74), followed by Zambia (219 of 444, 49%; 95% CI: 45, 54), then South Africa (152 of 365, 42%; 95% CI: 37, 47), and then Côte D'Ivoire (3 of 53, 5.7%; 95% CI: 1.2, 16).
In a cluster-level analysis limited to the 16 facility–community pairs that had at least 15 HIV-exposed infants, we found community PMTCT coverage to be highly correlated with facility PMTCT coverage [15] (Pearson's correlation coefficient r = 0.85). In every instance except for two, the facility-based coverage measure exceeded that observed in the community (Table 5; Figure 3A). While community PMTCT coverage was moderately correlated with HIV-free survival (r = 0.29; Figure 3B), we did not observe an association between facility PMTCT coverage and community HIV-free survival (r = 0.02; Figure 3C).
Fig. 3. Relationship among service coverage at the facility level, service coverage at the community level, and HIV-free survival in children under 2 y of age in the PEARL Study. 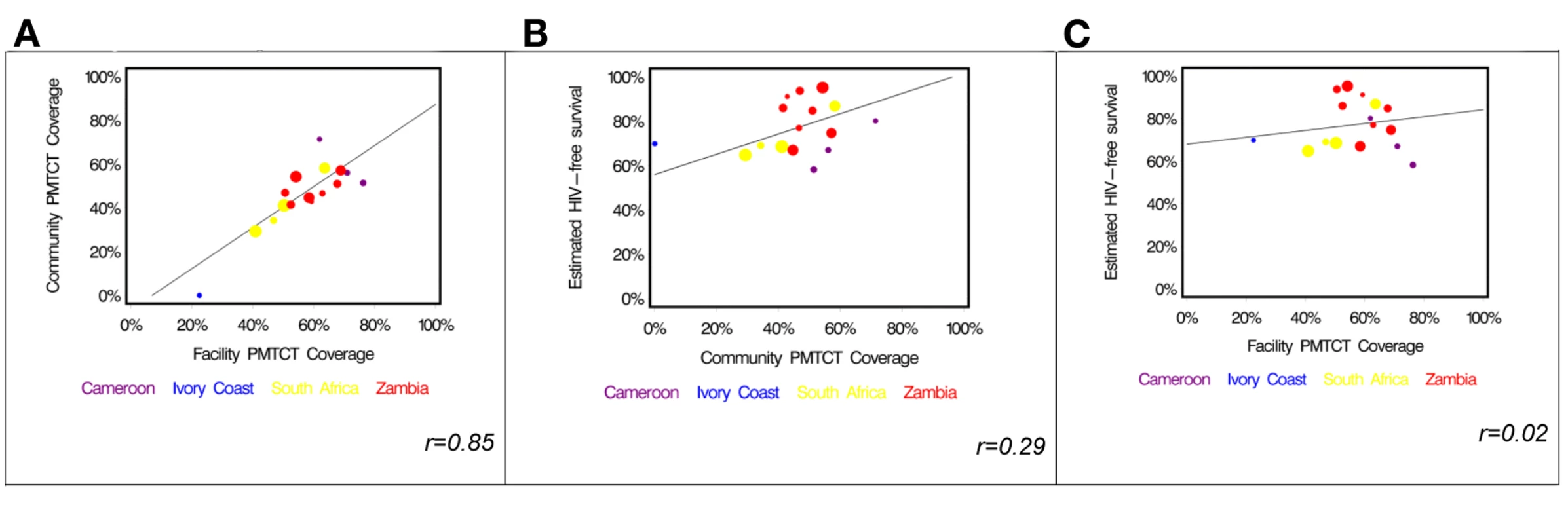
(A) Facility-based coverage versus community-based coverage; (B) community-based coverage versus HIV-free survival; (C) facility-based coverage versus HIV-free survival. Tab. 5. Comparison of PMTCT service coverage in the community survey compared to results of a simultaneous survey in corresponding facilities [15]. ![Comparison of PMTCT service coverage in the community survey compared to results of a simultaneous survey in corresponding facilities <em class="ref">[15]</em>.](https://www.prolekare.cz/media/cache/resolve/media_object_image_small/media/image/d61f28e28350b93b0f67dd90d5089843.png)
Coverage defined as the proportion of children born to HIV-positive mothers in whom any ARV drug is reported to have been used during pregnancy. Discussion
The PEARL Study community survey sampled representative households in four African countries to estimate 24-mo HIV-free survival among HIV-exposed children. Despite high reported HIV testing rates and utilization of delivery services, overall community PMTCT coverage was low, with less than half of HIV-exposed infants having received any ARV prophylaxis during gestation or the postnatal period. We think there is an important lesson to be learned in comparing the previously reported facility-based coverage estimates [15] to the community-based estimates reported here. While the estimates correlate highly (r = 0.85), we found the facility-based coverage estimate to be higher than the community-based coverage estimate in every facility–community pair except for two (Figure 3A). This suggests to us that the facility population can be thought of as a subset of the community, and a coverage estimate derived from it will be valid only in settings where most patients come to the facility for services. There are mother–infant pairs in the community who are simply not sampled by a facility-based estimate, and, not surprisingly, the PMTCT coverage rates among these women were generally lower. This finding is consistent with the observed associations between the two PMTCT coverage estimates and HIV-free survival (Figure 3B and 3C). While there is a moderate association between community PMTCT coverage and HIV-free survival, the association weakens considerably when the coverage estimate is derived from the facility population only.
The risk of death was lower among children using a potent ARV regime for PMTCT, but due to limited power, the association was not significant. An effect of drug potency is suggested by a dose response in the adjusted analysis and in Figure 2B. While this phenomenon has been known for some time in the controlled environment of clinical trials, its demonstration at the population level provides support for World Health Organization recommendations to implement more complex PMTCT ARV regimens on a wide scale.
In aggregate, we think our findings suggest a critical role for both facility - and community-based monitoring of PMTCT programs. The facility-based estimates—particularly those capable of recreating the PMTCT “cascade” [15],[17]—can provide important, practical information about how to best improve service delivery at the site level. Facility-based estimates are also easier to obtain, and are thus suited for more routine performance monitoring. Since some patients do not interact with the healthcare system at the level of the facility, however, periodic evaluation of community PMTCT coverage must also be considered. This aspect of monitoring is most useful when utilization of facility-based services is low, but is also subject to bias if sampling is not random. In our study, for instance, we used random sampling, but excluded those sites and communities known not to have any PMTCT services at all. Thus, both our facility and community samples are optimistic representations.
We observed an overall rate of HIV-free survival that was higher than anticipated by our original modeling [6]. This could be explained by at least three sources of bias. First, all infant deaths in the communities may not have been identified in the survey. While every attempt was made to ensure complete ascertainment, including use of census enumerators from the local community and study instruments that queried child deaths in multiple ways, we cannot be certain that all deaths were captured. Second, because we chose to sample only those communities that had at least some access to PMTCT services, the included clusters may have not been representative of the population as a whole. Since better-resourced facilities will be the first to implement PMTCT services, our sampled patients may have had access to better services than others in their respective countries. This suspicion is supported by the finding that over 85% of respondents reported having delivered at a facility, which is higher than we expected from national figures [23]–[26] but which is perhaps explained by our selection of households based on their proximity to particular health facilities. A third potential bias of our study lies in its imperfect participant response rates. Although a response rate of 78% is typical for a community-based survey, households where occupants were not found or refused to participate may have been systematically different from those that were included. If a household with a death under 24 mo of age was more likely to refuse entry to study staff, this could have resulted in overestimation of HIV-free survival, especially if that refusal was more likely among households affected by HIV.
The questionnaire instruments and sampling methodology for this study drew heavily on the widely used DHS surveys [22]. The successful conduct of the PEARL Study, and others of similar design [27], demonstrates that with relatively minor modification, the DHS surveys could serve as a platform for widespread estimation of PMTCT program effectiveness. The most critical modification would require testing mothers for HIV antibodies (to ascertain infant HIV exposure) and, for those who are seropositive, testing their infants for infection. Some countries already perform HIV testing as part of their DHS surveys, but others do not, and this modification would have to be planned and resources for its implementation identified.
Monitoring PMTCT programs remains a formidable challenge. Given the continually increasing emphasis on program scale-up, the unprecedented donor investment in affected countries, and the recent call by the World Health Organization and others for the virtual elimination of HIV in children by 2015, it is imperative that the international research and policy communities agree upon standard methodologies to measure the effectiveness of programs over time. Population-based evaluations of PMTCT effectiveness placed within periodic national health surveys could represent an important program monitoring resource.
Zdroje
1. Joint United Nations Programme on HIV/AIDS (2010) Report on the global AIDS epidemic 2010. New York: Joint United Nations Programme on HIV/AIDS.
2. Joint United Nations Programme on HIV/AIDS (2011) Global plan towards the elimination of new HIV infections among children by 2015 and keeping their mothers alive. New York: Joint United Nations Programme on HIV/AIDS.
3. LallemantM, ChangS, CohenR, PecoulB (2011) Pediatric HIV—A neglected disease? N Engl J Med 365 : 581–583.
4. CooperER, CharuratM, MofensonL, HansonC, PittJ, et al. (2002) Combination antiretroviral strategies for the treatment of pregnant HIV-1-infected women and prevention of perinatal HIV-1 transmission. J Acquir Immune Defic Syndr 29 : 484–494.
5. SpensleyA, SripipatanaT, TurnerA, HoblitzelleC, RobinsonJ, et al. (2009) Preventing mother-to-child transmission of HIV in resource-limited settings: The Elizabeth Glaser Pediatric AIDS Foundation experience. Am J Public Health 99 : 631–337.
6. StringerEM, ChiBH, ChintuN, CreekTL, EkoueviDK, et al. (2008) Monitoring effectiveness of programmes to prevent mother-to-child HIV transmission in lower-income countries. Bull World Health Organ 86 : 57–62.
7. ReithingerR, MegazziniK, DurakoSJ, HarrisDR, VermundSH (2007) Monitoring and evaluation of programmes to prevent mother to child transmission of HIV in Africa. BMJ 334 : 1143–1146.
8. World Health Organization (2011) Towards the elimination of mother-to-child transmission of HIV: Report of a WHO technical consultation. Available: http://www.who.int/hiv/pub/mtct/elimination_report/en/index.html. Accessed 20 November 2012.
9. SchoutenEJ, JahnA, MidianiD, MakombeSD, MnthambalaA, et al. (2001) Prevention of mother-to-child transmission of HIV and the health-related Millennium Development Goals: Time for a public health approach. Lancet 378 : 282–284.
10. World Health Organization (2010) Antiretroviral drugs for treating pregnant women and preventing HIV infection in infants: recommendations for a public health approach. Geneva: World Health Organization.
11. World Health Organization (2012) Programmatic update: use of antiretroviral drugs for treating pregnant women and preventing HIV infection in infants. Geneva: World Health Organization.
12. MateKS, BennettB, MphatsweW, BarkerP, RollinsN (2009) Challenges for routine health system data management in a large public programme to prevent mother-to-child HIV transmission in South Africa. PLoS ONE 4: e5483 doi:10.1371/journal.pone.0005483.
13. EkoueviDK, KarcherS, CoffiePA (2011) Strengthening health systems through HIV monitoring and evaluation in sub-Saharan Africa. Curr Opin HIV AIDS 6 : 245–250.
14. Hayashi C (2009) International guidance on methods to measure PMTCT impact [abstract]. 6th International AIDS Society Conference on HIV Pathogenesis, Treatment and Prevention; 17–20 July 2011; Rome, Italy.
15. StringerEM, EkoueviDK, CoetzeeD, TihP, CreekTL, et al. (2010) Coverage of nevirapine-based services to prevent mother-to-child transmission in four African countries. JAMA 304 : 293–302.
16. StringerJSA, SinkalaM, GoldenbergR, VermundS, AcostaE (2003) Monitoring nevirapine-based programmes for prevention of mother-to-child transmission of HIV-1. Lancet 362 : 667–667.
17. RollinsN, MzoloS, MoodleyT, EsterhuizenT, van RooyenH (2009) Universal HIV testing of infants at immunization clinics: An acceptable and feasible approach for early infant diagnosis in high HIV prevalence settings. AIDS 23 : 1851–1857.
18. Goga A, Dinh T-H, Jackson D, The SAPMTCTE Study Group (2010) Evaluation of the effectiveness of the national prevention of mother-to-child transmission (PMTCT) programme on infant HIV measured at six weeks postpartum in South Africa, 2010. Cape Town: South African Medical Research Council. Available: http://www.doh.gov.za/docs/reports/2012/pmtcteffectiveness.pdf. Accessed 16 November 2012.
19. CoffiePA, KanhonSK, TourH, HapsatouT, Ettiegne-TraoréV, et al. (2011) Nevirapine for the prevention of mother-to-child transmission of HIV: A nation-wide coverage survey in Côte d'Ivoire. J Acquir Immune Defic Syndr 57 (Suppl 1)
S3–S8.
20. EkoueviDK, StringerE, CoetzeeD, TihP, CreekT, et al. (2012) Health facility characteristics and their relationship to coverage of PMTCT of HIV services across four African countries: The PEARL Study. PLoS ONE 7: e29823 doi:10.1371/journal.pone.0029823.
21. StinsonK, BoulleA, SmithPJ, StringerEM, StringerJSA, et al. (2012) Coverage of the prevention of mother-to-child transmission programme in the Western Cape, South Africa using cord blood surveillance. J Acquir Immune Defic Syndr 60 : 199–204.
22. Central Statistical Office, Ministry of Health in Zambia, Tropical Diseases Research Centre, University of Zambia, Macro International (2009) Zambia Demographic and Health Survey 2007. Calverton (Maryland): Central Statistical Office and Macro International. Available: http://www.measuredhs.com/pubs/pdf/FR211/FR211[revised-05-12-2009].pdf. Accessed 20 November 2012.
23. United Nations Children's Fund (2013) Statistics and monitoring: South Africa—delivery care coverage (%), institutional delivery (2006–2010). New York: United Nations Children's Fund.
24. United Nations Children's Fund (2013) Statistics and monitoring: Zambia—delivery care coverage (%), institutional delivery (2006–2010). New York: United Nations Children's Fund.
25. United Nations Children's Fund (2013) Statistics and monitoring: Cameroon—delivery care coverage (%), institutional delivery (2006–2010). New York: United Nations Children's Fund.
26. United Nations Children's Fund (2013) Statistics and monitoring: Côte d'Ivoire—delivery care coverage (%), institutional delivery (2006–2010). New York: United Nations Children's Fund.
27. RutonH, MugwanezaP, ShemaN, LyambabajeA, de Dieu BizimanaJ, et al. (2012) HIV-free survival among nine - to 24-mo-old children born to HIV-positive mothers in the Rwandan national PMTCT programme: a community-based household survey. J Int AIDS Soc 15 : 4.
Štítky
Interní lékařství
Článek vyšel v časopisePLOS Medicine
Nejčtenější tento týden
2013 Číslo 5- Není statin jako statin aneb praktický přehled rozdílů jednotlivých molekul
- Magnosolv a jeho využití v neurologii
- Moje zkušenosti s Magnosolvem podávaným pacientům jako profylaxe migrény a u pacientů s diagnostikovanou spazmofilní tetanií i při normomagnezémii - MUDr. Dana Pecharová, neurolog
- Biomarker NT-proBNP má v praxi široké využití. Usnadněte si jeho vyšetření POCT analyzátorem Afias 1
- S prof. Vladimírem Paličkou o racionální suplementaci kalcia a vitaminu D v každodenní praxi
-
Všechny články tohoto čísla
- Measuring Coverage in MNCH: Challenges and Opportunities in the Selection of Coverage Indicators for Global Monitoring
- Measuring Coverage in MNCH: Tracking Progress in Health for Women and Children Using DHS and MICS Household Surveys
- Measuring Coverage in MNCH: Population HIV-Free Survival among Children under Two Years of Age in Four African Countries
- Tobacco Company Efforts to Influence the Food and Drug Administration-Commissioned Institute of Medicine Report An Analysis of Documents Released through Litigation
- Irreconcilable Conflict: The Tobacco Industry and the Public Health Challenge of Tobacco Use
- Providing Impetus, Tools, and Guidance to Strengthen National Capacity for Antimicrobial Stewardship in Viet Nam
- Measuring Coverage in MNCH: New Findings, New Strategies, and Recommendations for Action
- Grand Challenges: Integrating Maternal Mental Health into Maternal and Child Health Programmes
- Measuring Coverage in MNCH: Challenges in Monitoring the Proportion of Young Children with Pneumonia Who Receive Antibiotic Treatment
- Measuring Coverage in MNCH: Current Indicators for Measuring Coverage of Diarrhea Treatment Interventions and Opportunities for Improvement
- Measuring Coverage in MNCH: Evaluation of Community-Based Treatment of Childhood Illnesses through Household Surveys
- Measuring Coverage in MNCH: Accuracy of Measuring Diagnosis and Treatment of Childhood Malaria from Household Surveys in Zambia
- Grand Challenges: Integrating Mental Health Care into the Non-Communicable Disease Agenda
- Integrating Global and National Knowledge to Select Medicines for Children: The Ghana National Drugs Programme
- Grand Challenges: Integrating Mental Health Services into Priority Health Care Platforms
- Disability Transitions and Health Expectancies among Adults 45 Years and Older in Malawi: A Cohort-Based Model
- Comparative Efficacy of Seven Psychotherapeutic Interventions for Patients with Depression: A Network Meta-Analysis
- Measuring Coverage in MNCH: Design, Implementation, and Interpretation Challenges Associated with Tracking Vaccination Coverage Using Household Surveys
- Measuring Coverage in MNCH: A Prospective Validation Study in Pakistan and Bangladesh on Measuring Correct Treatment of Childhood Pneumonia
- Contribution of and Smoking Trends to US Incidence of Intestinal-Type Noncardia Gastric Adenocarcinoma: A Microsimulation Model
- Carriage of in the Upper Respiratory Tract of Symptomatic and Asymptomatic Children: An Observational Study
- Setting Research Priorities to Reduce Mortality and Morbidity of Childhood Diarrhoeal Disease in the Next 15 Years
- Grand Challenges: Improving HIV Treatment Outcomes by Integrating Interventions for Co-Morbid Mental Illness
- Gene Expression Classification of Colon Cancer into Molecular Subtypes: Characterization, Validation, and Prognostic Value
- Domestic Violence and Perinatal Mental Disorders: A Systematic Review and Meta-Analysis
- Intimate Partner Violence and Incident Depressive Symptoms and Suicide Attempts: A Systematic Review of Longitudinal Studies
- Effect of Facilitation of Local Maternal-and-Newborn Stakeholder Groups on Neonatal Mortality: Cluster-Randomized Controlled Trial
- Intimate Partner Violence and Population Mental Health: Why Poverty and Gender Inequities Matter
- Assessing Population Aging and Disability in Sub-Saharan Africa: Lessons from Malawi?
- The Paradox of Mental Health: Over-Treatment and Under-Recognition
- Measuring Coverage in MNCH: Determining and Interpreting Inequalities in Coverage of Maternal, Newborn, and Child Health Interventions
- Measuring Coverage in MNCH: Total Survey Error and the Interpretation of Intervention Coverage Estimates from Household Surveys
- Measuring Coverage in MNCH: Indicators for Global Tracking of Newborn Care
- PLOS Medicine
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Gene Expression Classification of Colon Cancer into Molecular Subtypes: Characterization, Validation, and Prognostic Value
- Domestic Violence and Perinatal Mental Disorders: A Systematic Review and Meta-Analysis
- Intimate Partner Violence and Incident Depressive Symptoms and Suicide Attempts: A Systematic Review of Longitudinal Studies
- Measuring Coverage in MNCH: Challenges in Monitoring the Proportion of Young Children with Pneumonia Who Receive Antibiotic Treatment
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



