-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Integrating Global and National Knowledge to Select Medicines for Children: The Ghana National Drugs Programme
article has not abstract
Published in the journal: . PLoS Med 10(5): e32767. doi:10.1371/journal.pmed.1001449
Category: Health in Action
doi: https://doi.org/10.1371/journal.pmed.1001449Summary
article has not abstract
Summary Points
-
This paper reports the experience of the Ghana National Drugs Programme as they reviewed the international evidence base for five priority paediatric medicines.
-
Applying the global recommendations to Ghana was not straightforward for any of the five medicines, regardless of the presence of high quality evidence of important clinical benefits.
-
Four main factors generated debate and uncertainty in the committee: (1) effect unproven in African settings; (2) control group in trials not consistent with current practice; (3) little evidence on cost and cost effectiveness; and (4) limited supply chain.
-
This project demonstrates why global recommendations should be presented alongside transparent descriptions of the evidence base, allowing policy groups to identify where, when, and how the interventions have been evaluated, and any factors limiting applicability.
-
As many policy questions are relevant across sub-Saharan Africa, and policy makers are likely to encounter similar problems, we encourage regional collaboration on health technology assessment, and sharing of information and resources.
Context
In 2011, the World Health Organization (WHO) Essential Medicines Programme published a list of “priority medicines” they considered essential for countries to achieve the Millennium Development Goals in child and maternal health [1]. This list was a subset of the model Essential Medicines List (EML), produced every 2 years by the WHO, which selects medicines on the basis of public health relevance, comparative effectiveness, safety, cost, and regulatory status [2].
The WHO model EML is adapted for use in Ghana by the Ghana National Drugs Programme of the Ministry of Health (MOH), and access to essential medicines is now largely financed through the National Health Insurance Scheme (NHIS). This scheme was established by the government of Ghana in 2003, and covers over 60% of the population [3]. Membership of the NHIS is through annual subscription, but free of charge to those under 18, over 70, pregnant, or the very poor, and members may access care through accredited public and private health care providers [4].
Following publication of the 2011 model EML it was noted that five of the priority paediatric medicines were not included in the 2010 Ghana EML: oral zinc sulphate for acute diarrhoea, injectable artesunate for severe malaria, topical chlorhexidine for preventing neonatal cord sepsis, dispersible oral amoxicillin for community acquired pneumonia, and oral and injectable caffeine citrate for neonatal apnoea [5].
Before adopting these medicines, the Ghana National Drugs Programme (GNDP) wanted to review the evidence base and how it applied to Ghana, using a transparent and evidence-informed approach, which further considered the local priorities, feasibility, and resource implications. In this paper we report on how the GNDP did this, and the difficulties experienced when interpreting and applying global recommendations to a national context.
About This Project
The National Drugs Programme first prepared concise evidence summaries for each of the five WHO “priority” medicines. These five summaries were then used by the Ghana “Standard Treatment Guidelines” expert review committee in November 2011 (with representation from the NHIS and GNDP), to facilitate an open and informed discussion.
Training in the retrieval, appraisal, and interpretation of systematic reviews was provided for a selected team of Ministry of Health staff by specialists from the Liverpool School of Tropical Medicine. These staff then wrote the summaries, following a structure based on the work of the SUPPORT collaboration, summarising existing systematic reviews, rather than conducting new reviews [6]. The summaries addressed four main questions:
-
What are the benefits and harms of [drug name]?
-
What would be the public health impact of introducing [drug name] in Ghana?
-
What are the resource implications to the country of introducing it?
-
Is introduction currently feasible and acceptable in Ghana?
For evidence of benefits and harms participants searched the Cochrane Library and PubMed for existing systematic reviews. When more than one systematic review was found, the most reliable review was chosen on the basis of an evaluation of the search strategy and methods. When the most recent review was more than 2 years old an additional PubMed search was conducted for recently published randomized controlled trials. Confidence in the methods of systematic reviews was appraised using the AMSTAR checklist [7], and confidence in the results was appraised using the GRADE approach for assessing the quality of evidence [8]. For evidence on cost-effectiveness, participants reviewed the NHS Economics Database for economic evaluations relevant to each medicine, and appraised the methods using the CHEC-list [9]. The applicability of the systematic reviews and economic evaluations to Ghana was assessed following the guidance of the SUPPORT collaboration [10].
The potential public health impact of introducing each medicine was estimated by applying the relative mortality reductions from trial data to the best available national statistics for disease burden. A commentary on the costs and feasibility of introduction was prepared by reviewing international price guides for potential suppliers [11],[12], comparing the new drug price to the current alternative, and identifying any additional system or educational requirements for successful introduction [13].
A brief summary of the findings of each evidence summary is presented in Table 1. The full summaries are available as on-line supplements to this paper, and may provide useful templates for other countries.
Tab. 1. Summary of Ghana evidence summaries. 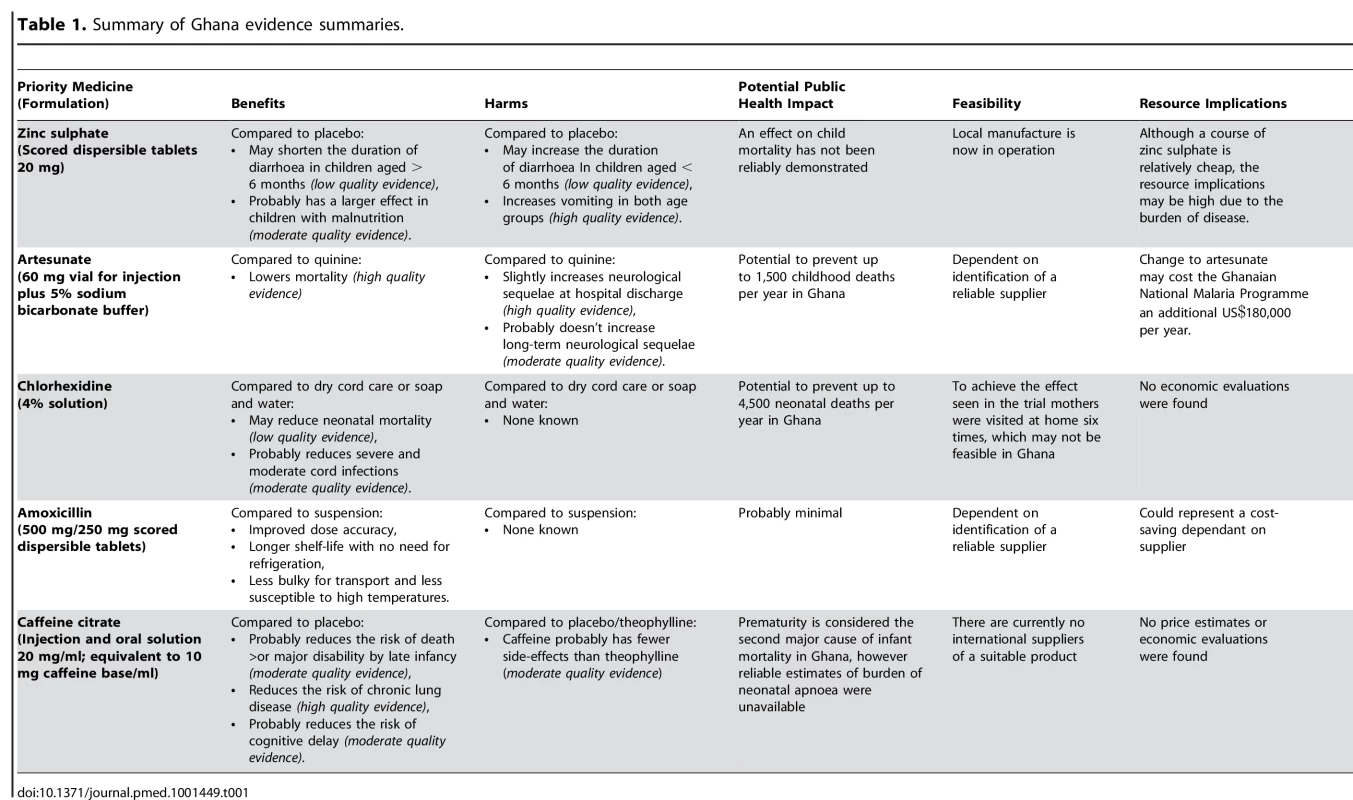
Our Experience
Applying global recommendations to Ghana was not straightforward for any of the five medicines, regardless of the presence of high quality evidence of important clinical benefits (Table 2). We have summarised the four key factors that generated debate and uncertainty in the committee, and tempered automatic adoption of the five medicines.
Tab. 2. Problems applying the global recommendations to Ghana. 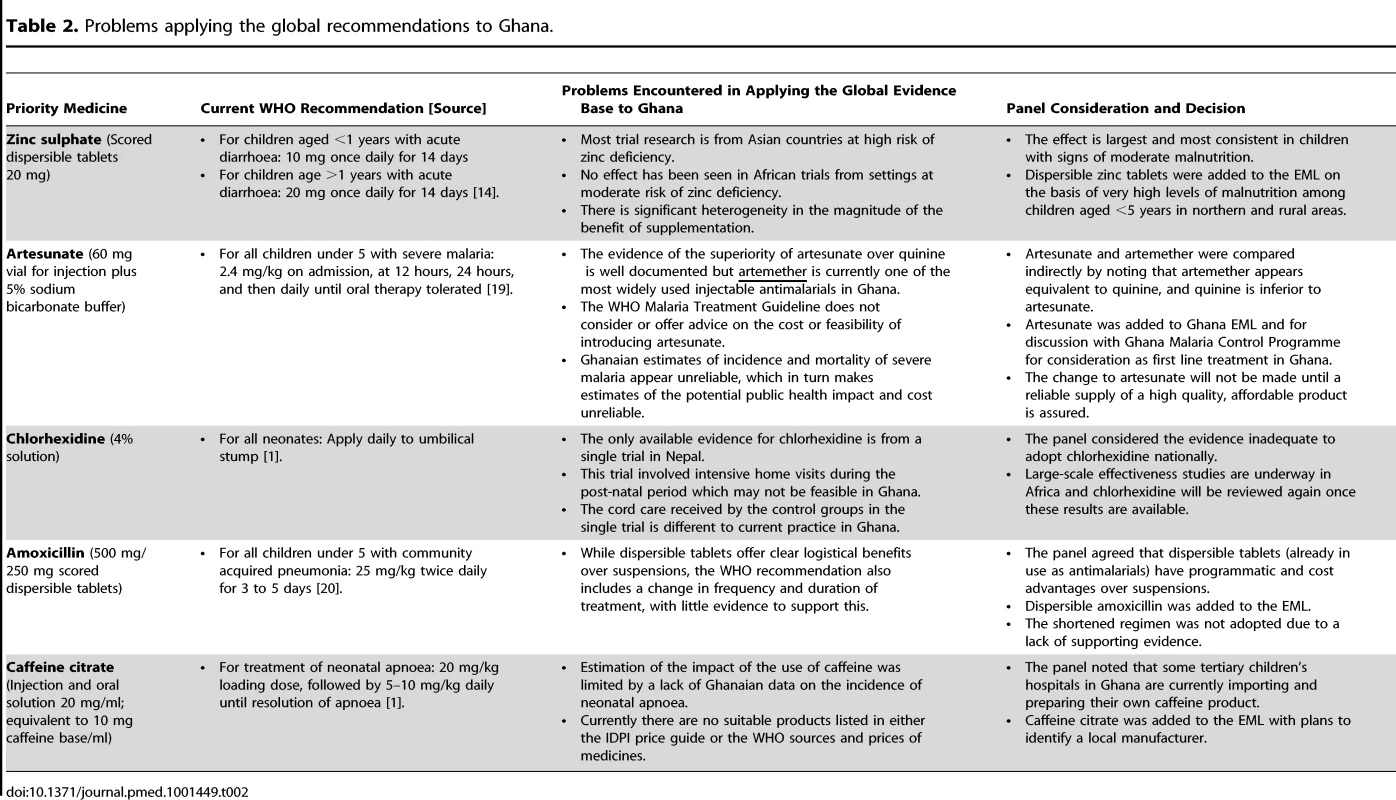
1. Applicability: Few of the Trials Were Conducted in Africa
The applicability of the evidence base for both zinc sulphate (for diarrhoea) and chlorhexidine 4% solution (for cord care) to Ghana was limited, as the majority of available data came from Asian countries, where the effects could reasonably be expected to be different.
Zinc sulphate is recommended by the WHO as an adjunct to oral rehydration therapy for children with acute diarrhoea [14]. However, of the 44 trials included in the Cochrane review of zinc therapy, only two were conducted in Africa, neither of which demonstrated a clinical benefit [15]. In Asia, zinc appeared to shorten the duration of diarrhoea but with significant heterogeneity in the size of this effect. Sub-group analyses suggests that the effect is largest in children aged greater than 6 months with signs of moderate malnutrition, and as a nutritional intervention this has a logical consistency. It was on this basis that the committee decided to introduce Zinc sulphate in Ghana, where malnutrition in some rural areas is above 30% [16].
2. Applicability: The Control Groups Used in Trials Differ from Current Practice
The applicability of the global evidence base for chlorhexidine, and artesunate (for severe malaria), was limited because the control groups used in the primary research were different to current practice in Ghana.
Chlorhexidine was included on the WHO list of priority medicines for application to the umbilical stump to prevent neonatal sepsis [1]. The published evidence evaluating chlorhexidine (available in 2011), was limited to a single large trial from Nepal [17]. This trial had two control interventions: soap and water and dry cord care, whereas current practice in Ghana most commonly involves the application of alcohol: an intervention that has itself never been evaluated. In addition, the intervention in the Nepalese efficacy trial was delivered via an intensive regimen of post-natal home visits, which would be neither feasible nor affordable in Ghana. As a consequence, the committee considered the evidence insufficient to introduce chlorhexidine nationwide at that time.
Similarly, most trials evaluating artesunate have compared it to quinine, but in Ghana, as in many sub-Saharan countries, injectable artemether has become widely popular due to the ease of intra-muscular administration. Only a single trial in adults from Asia has directly compared artesunate with artemether and the result did not reach statistical significance [18].
3. Cost and Cost-Effectiveness: Data Not Available
The cost of introducing each medicine was important for the committee, but none of the WHO documents recommending these five medicines included advice or guidance on costs [1],[14],[19]–[21].
Searches of the NHS Economics Evaluations Database found two economic evaluations of artesunate versus quinine [22],[23]. One of these presented a cost-benefit analysis from three African study sites involved in a large efficacy trial [24], and found artesunate to be highly cost-effective in comparison to quinine (an estimated US$123 per additional life saved) [22].
Only one further cost-effectiveness evaluation was available, for zinc sulphate, which had limited applicability to Ghana [25]. This evaluation concluded that the additional costs of zinc sulphate were offset by gains in the mothers' time and productivity through reduced illness duration. From a health providers' perspective, although a course of zinc is relatively cheap (US$0.28), the burden of diarrhoeal disease is such that drug costs may be substantial.
4. Feasibility: Limitations with the Current Supply Chain
The committee was concerned about the harmful effects of changing national policy before a reliable supply of a new drug was assured.
Artesunate was recommended as the first-line antimalarial for severe malaria in Africa in a 2011 update to the WHO Malaria Treatment Guidelines [19]. The committee accepted the evidence base provided, and the cost implications of introducing artesunate described above, but were concerned that if they authorised a switch from quinine and artemether (which are widely available and of good manufacturing quality), to artesunate (where the supply may be less reliable and with added threat of fake drugs), this may actually increase mortality from malaria. The committee therefore added artesunate to the Ghana EML but deferred changes to the national malaria guidelines until a reliable supply was established. These feasibility concerns were discussed at a global stakeholder meeting in November 2011 [26].
Reliable international or national suppliers were also limited for dispersible zinc tablets, dispersible amoxicillin tablets, and caffeine citrate [11],[12].
Project Outcome
Four of the five priority medicines were approved by the expert committee for addition to the Ghana EML: zinc sulphate, artesunate, dispersible amoxicillin, and caffeine citrate. The fifth, chlorhexidine, will be re-considered once the ongoing African effectiveness studies have been published.
Learning Points
Confident national decisions require understanding and debate of the evidence-base underlying global recommendations, plus additional consideration of national conditions and resources.
To facilitate this, global recommendations should be presented alongside transparent descriptions of the evidence base, allowing policy groups to identify where, when, and how the interventions have been evaluated, and any factors limiting wider applicability.
In addition, for interventions where feasibility and affordability are likely to vary from setting to setting, the WHO could further assist national decision-makers by providing implementation guidance on the assessment of health system implications, training and education requirements, and country level cost analyses.
As many policy questions are relevant across sub-Saharan Africa, and national policy makers are likely to encounter similar problems, we strongly encourage regional collaboration on evidence evaluation, and sharing of information and resources. We hope this paper will encourage further capacity building initiatives, which facilitate and empower countries to make more informed decisions, choosing the interventions that are right for their context, and not implementing unproven interventions.
Supporting Information
Zdroje
1. World Health Organization (2011) Priority medicines for women and children. Available: http://www.who.int/medicines/publications/emp_mar2011.1/en/index.html. Accessed 12 January 2013.
2. World Health Organization (2011) Model essential medicines List. Available: http://www.who.int/selection_medicines/list/en/. Accessed 12 January 2013.
3. Ghana Ministry of Health (2010) Ghana essential medicines list, sixth edition. Ghana National Drugs Programme. Available: http://ghndp.org. Accessed 12 January 2013.
4. BlanchetNJ, FinkG, Osei-AkotoI (2012) The effect of Ghana's National Health Insurance Scheme on health care utilisation. Ghana Med J 46 : 76–84.
5. Ghana National Health Insurance Authority (2009) Annual report. Available: http://www.nhis.gov.gh/?CategoryID=158. Accessed 12 January 2013.
6. LavisJN, OxmanAD, LewinS, FretheimA (2009) SUPPORT tools for evidence-informed health Policymaking (STP). Health Res Policy Syst 7 Suppl 1: L1.
7. SheaB, GrimshawJ, WellsG, BoersM, AnderssonN, et al. (2007) Development of AMSTAR: a measurement tool to assess the methodological quality of systematic reviews. BMC Med Res Methodol 7 : 10.
8. EversS, GoossensM, de VetH, van TulderM, AmentA (2005) Criteria list for assessment of methodological quality of economic evaluations: consensus on health economic criteria. Int J Technol Assess Health Care 21 : 240–245.
9. GuyattG, OxmanAD, AklE, KunzR, VistG, et al. (2011) GRADE guidelines 1. Introduction-GRADE evidence profiles and summary of findings tables. J Clin Epidemiol 64 : 383–394.
10. LavisJN, OxmanAD, SouzaNM, LewinS, GruenRL, et al. (2009) SUPPORT Tools for evidence-informed health Policymaking (STP) 9: assessing the applicability of the findings of a systematic review. Health Res Policy Syst 7 Suppl 1: S9.
11. World Health Organization, UNICEF (2010) Sources and prices of selected medicines for children including therapeutic food, dietary vitamin and mineral supplementation, second edition. Available: http://www.who.int/medicines/publications/essentialmedicines/Sources_Prices2010.pdf. Accessed 12 January 2013.
12. Frye JE (2010) International drug price indicator guide. Available: http://erc.msh.org/mainpage.cfm?file=1.0.htm&id=1&temptitle=Introduction&module=DMP&language=English. Accessed 12 January 2013.
13. LewinS, OxmanAD, LavisJN, FretheimA, MartiS, et al. (2009) SUPPORT Tools for evidence-informed health Policymaking (STP) 11: finding and using evidence about local conditions. Health Res Policy Syst 7 Suppl 1: S11.
14. World Health Organization, UNICEF (2004) Clinical management of acute diarrhoea: WHO/UNICEF joint statement. Available: http://www.unicef.org/nutrition/files/ENAcute_Diarrhoea_reprint.pdf. Accessed 12 January 2013.
15. LazzeriniM, RonfaniL (2012) Oral zinc for treating diarrhoea in children. Cochrane Database Syst Rev 6: CD005436.
16. Ghana Health Service (2009) Facts and figures. Available: http://www.ghanahealthservice.org/includes/upload/publications/Facts%20and%20Figures%202009.pdf. Accessed 20 October 2011.
17. MullanyLC, DarmstadtGL, KhatrySK, KatzJ, LeClerqSC, et al. (2006) Topical applications of chlorhexidine to the umbilical cord for prevention of omphalitis and neonatal mortality in southern Nepal: a community-based, cluster-randomised trial. Lancet 367 : 910–918.
18. PhuNH, TuanPQ, DayN, MaiNTH, ChauTTH, et al. (2010) Randomized controlled trial of artesunate or artemether in Vietnamese adults with severe falciparum malaria. Malar J 9 : 97.
19. World Health Organization (2010) Guidelines for the treatment of malaria, second edition. Geneva: World Health Organization.
20. GrantGB, CampbellH, DowellSF, GrahamSM, KlugmanKP, et al. (2009) Recommendations for treatment of childhood non-severe pneumonia. Lancet Infect Dis 9 : 185–196.
21. World Health Organization (2003) Consultative meeting to review evidence and research priorities in the management of acute respiratory infections (ARI). Geneva, September 29–October 1, 2003. Meeting report [WHO/FCH/CAH/04.2]. Available at: http://whqlibdoc.who.int/hq/2004/WHO_FCH_CAH_04.2.pdf. Accessed 11 October 2013.
22. LubellY, RiewpaiboonA, DondorpAM, von SeidleinL, MokuoluOA, et al. (2011) Cost-effectiveness of parenteral artesunate for treating children with severe malaria in sub-Saharan Africa. Bull World Health Organ 89 : 504–512.
23. LubellY, YeungS, DondorpAM, DayNP, NostenF, et al. (2009) Cost-effectiveness of artesunate for the treatment of severe malaria. Trop Med Int Health 14 : 332–37.
24. DondorpAM, FanelloCI, HendriksenIC, GomesE, SeniA, et al. (2010) Artesunate versus quinine in the treatment of severe falciparum malaria in African children (AQUAMAT): an open-label, randomised trial. Lancet 376 : 1647–1657.
25. GregorioGV, DandLF, CorderoCP, PaneloCA (2007) Zinc supplementation reduced cost and duration of acute diarrhea in children. J Clin Epidemiol 60 : 560–566.
26. Medicines for Malaria Venture (2011) Saving more lives with artesunate injection: injectable artesunate stakeholder's meeting report. Available at: http://www.mmv.org/sites/default/files/uploads/docs/publications/Injectable%20Artesunate%20Stakeholders%20Meeting%20Report.pdf. Accessed 11 November 2012.
Štítky
Interní lékařství
Článek vyšel v časopisePLOS Medicine
Nejčtenější tento týden
2013 Číslo 5- Není statin jako statin aneb praktický přehled rozdílů jednotlivých molekul
- Magnosolv a jeho využití v neurologii
- Moje zkušenosti s Magnosolvem podávaným pacientům jako profylaxe migrény a u pacientů s diagnostikovanou spazmofilní tetanií i při normomagnezémii - MUDr. Dana Pecharová, neurolog
- Biomarker NT-proBNP má v praxi široké využití. Usnadněte si jeho vyšetření POCT analyzátorem Afias 1
- S prof. Vladimírem Paličkou o racionální suplementaci kalcia a vitaminu D v každodenní praxi
-
Všechny články tohoto čísla
- Measuring Coverage in MNCH: Challenges and Opportunities in the Selection of Coverage Indicators for Global Monitoring
- Measuring Coverage in MNCH: Tracking Progress in Health for Women and Children Using DHS and MICS Household Surveys
- Measuring Coverage in MNCH: Population HIV-Free Survival among Children under Two Years of Age in Four African Countries
- Tobacco Company Efforts to Influence the Food and Drug Administration-Commissioned Institute of Medicine Report An Analysis of Documents Released through Litigation
- Irreconcilable Conflict: The Tobacco Industry and the Public Health Challenge of Tobacco Use
- Providing Impetus, Tools, and Guidance to Strengthen National Capacity for Antimicrobial Stewardship in Viet Nam
- Measuring Coverage in MNCH: New Findings, New Strategies, and Recommendations for Action
- Grand Challenges: Integrating Maternal Mental Health into Maternal and Child Health Programmes
- Measuring Coverage in MNCH: Challenges in Monitoring the Proportion of Young Children with Pneumonia Who Receive Antibiotic Treatment
- Measuring Coverage in MNCH: Current Indicators for Measuring Coverage of Diarrhea Treatment Interventions and Opportunities for Improvement
- Measuring Coverage in MNCH: Evaluation of Community-Based Treatment of Childhood Illnesses through Household Surveys
- Measuring Coverage in MNCH: Accuracy of Measuring Diagnosis and Treatment of Childhood Malaria from Household Surveys in Zambia
- Grand Challenges: Integrating Mental Health Care into the Non-Communicable Disease Agenda
- Integrating Global and National Knowledge to Select Medicines for Children: The Ghana National Drugs Programme
- Grand Challenges: Integrating Mental Health Services into Priority Health Care Platforms
- Disability Transitions and Health Expectancies among Adults 45 Years and Older in Malawi: A Cohort-Based Model
- Comparative Efficacy of Seven Psychotherapeutic Interventions for Patients with Depression: A Network Meta-Analysis
- Measuring Coverage in MNCH: Design, Implementation, and Interpretation Challenges Associated with Tracking Vaccination Coverage Using Household Surveys
- Measuring Coverage in MNCH: A Prospective Validation Study in Pakistan and Bangladesh on Measuring Correct Treatment of Childhood Pneumonia
- Contribution of and Smoking Trends to US Incidence of Intestinal-Type Noncardia Gastric Adenocarcinoma: A Microsimulation Model
- Carriage of in the Upper Respiratory Tract of Symptomatic and Asymptomatic Children: An Observational Study
- Setting Research Priorities to Reduce Mortality and Morbidity of Childhood Diarrhoeal Disease in the Next 15 Years
- Grand Challenges: Improving HIV Treatment Outcomes by Integrating Interventions for Co-Morbid Mental Illness
- Gene Expression Classification of Colon Cancer into Molecular Subtypes: Characterization, Validation, and Prognostic Value
- Domestic Violence and Perinatal Mental Disorders: A Systematic Review and Meta-Analysis
- Intimate Partner Violence and Incident Depressive Symptoms and Suicide Attempts: A Systematic Review of Longitudinal Studies
- Effect of Facilitation of Local Maternal-and-Newborn Stakeholder Groups on Neonatal Mortality: Cluster-Randomized Controlled Trial
- Intimate Partner Violence and Population Mental Health: Why Poverty and Gender Inequities Matter
- Assessing Population Aging and Disability in Sub-Saharan Africa: Lessons from Malawi?
- The Paradox of Mental Health: Over-Treatment and Under-Recognition
- Measuring Coverage in MNCH: Determining and Interpreting Inequalities in Coverage of Maternal, Newborn, and Child Health Interventions
- Measuring Coverage in MNCH: Total Survey Error and the Interpretation of Intervention Coverage Estimates from Household Surveys
- Measuring Coverage in MNCH: Indicators for Global Tracking of Newborn Care
- PLOS Medicine
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Gene Expression Classification of Colon Cancer into Molecular Subtypes: Characterization, Validation, and Prognostic Value
- Domestic Violence and Perinatal Mental Disorders: A Systematic Review and Meta-Analysis
- Intimate Partner Violence and Incident Depressive Symptoms and Suicide Attempts: A Systematic Review of Longitudinal Studies
- Measuring Coverage in MNCH: Challenges in Monitoring the Proportion of Young Children with Pneumonia Who Receive Antibiotic Treatment
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



