-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Global Mortality Estimates for the 2009 Influenza Pandemic from the GLaMOR Project: A Modeling Study
Background:
Assessing the mortality impact of the 2009 influenza A H1N1 virus (H1N1pdm09) is essential for optimizing public health responses to future pandemics. The World Health Organization reported 18,631 laboratory-confirmed pandemic deaths, but the total pandemic mortality burden was substantially higher. We estimated the 2009 pandemic mortality burden through statistical modeling of mortality data from multiple countries.Methods and Findings:
We obtained weekly virology and underlying cause-of-death mortality time series for 2005–2009 for 20 countries covering ∼35% of the world population. We applied a multivariate linear regression model to estimate pandemic respiratory mortality in each collaborating country. We then used these results plus ten country indicators in a multiple imputation model to project the mortality burden in all world countries. Between 123,000 and 203,000 pandemic respiratory deaths were estimated globally for the last 9 mo of 2009. The majority (62%–85%) were attributed to persons under 65 y of age. We observed a striking regional heterogeneity, with almost 20-fold higher mortality in some countries in the Americas than in Europe. The model attributed 148,000–249,000 respiratory deaths to influenza in an average pre-pandemic season, with only 19% in persons <65 y. Limitations include lack of representation of low-income countries among single-country estimates and an inability to study subsequent pandemic waves (2010–2012).Conclusions:
We estimate that 2009 global pandemic respiratory mortality was ∼10-fold higher than the World Health Organization's laboratory-confirmed mortality count. Although the pandemic mortality estimate was similar in magnitude to that of seasonal influenza, a marked shift toward mortality among persons <65 y of age occurred, so that many more life-years were lost. The burden varied greatly among countries, corroborating early reports of far greater pandemic severity in the Americas than in Australia, New Zealand, and Europe. A collaborative network to collect and analyze mortality and hospitalization surveillance data is needed to rapidly establish the severity of future pandemics.
Please see later in the article for the Editors' Summary
Published in the journal: . PLoS Med 10(11): e32767. doi:10.1371/journal.pmed.1001558
Category: Research Article
doi: https://doi.org/10.1371/journal.pmed.1001558Summary
Background:
Assessing the mortality impact of the 2009 influenza A H1N1 virus (H1N1pdm09) is essential for optimizing public health responses to future pandemics. The World Health Organization reported 18,631 laboratory-confirmed pandemic deaths, but the total pandemic mortality burden was substantially higher. We estimated the 2009 pandemic mortality burden through statistical modeling of mortality data from multiple countries.Methods and Findings:
We obtained weekly virology and underlying cause-of-death mortality time series for 2005–2009 for 20 countries covering ∼35% of the world population. We applied a multivariate linear regression model to estimate pandemic respiratory mortality in each collaborating country. We then used these results plus ten country indicators in a multiple imputation model to project the mortality burden in all world countries. Between 123,000 and 203,000 pandemic respiratory deaths were estimated globally for the last 9 mo of 2009. The majority (62%–85%) were attributed to persons under 65 y of age. We observed a striking regional heterogeneity, with almost 20-fold higher mortality in some countries in the Americas than in Europe. The model attributed 148,000–249,000 respiratory deaths to influenza in an average pre-pandemic season, with only 19% in persons <65 y. Limitations include lack of representation of low-income countries among single-country estimates and an inability to study subsequent pandemic waves (2010–2012).Conclusions:
We estimate that 2009 global pandemic respiratory mortality was ∼10-fold higher than the World Health Organization's laboratory-confirmed mortality count. Although the pandemic mortality estimate was similar in magnitude to that of seasonal influenza, a marked shift toward mortality among persons <65 y of age occurred, so that many more life-years were lost. The burden varied greatly among countries, corroborating early reports of far greater pandemic severity in the Americas than in Australia, New Zealand, and Europe. A collaborative network to collect and analyze mortality and hospitalization surveillance data is needed to rapidly establish the severity of future pandemics.
Please see later in the article for the Editors' SummaryIntroduction
Recurring seasonal influenza epidemics impose a moderate, if variable, mortality burden every year. But when a new human-transmissible influenza virus emerges, the ensuing pandemic can be catastrophic; the 1918 Spanish influenza pandemic, for example, killed approximately 1%–2% of the global population. Understanding the global mortality impact of pandemic influenza—who died, where, and when—is fundamental to understanding how pandemics emerge and evolve, and will help to guide responses to future pandemics. And because so few pandemics have occurred in the modern era, it is essential that each one be studied thoroughly—even if, as was the case with the 2009 influenza A H1N1 pandemic (H1N1pdm09), the catastrophe failed to appear.
As of 31 August 2010 the World Health Organization (WHO) received reports of 18,449 laboratory-confirmed deaths from H1N1pdm09 infection [1]. This modest number has caused many to wonder what all the excitement was about, and some to question whether the pandemic response was excessive [2],[3]. But what is not widely appreciated is that the laboratory-confirmed total greatly underestimates the mortality burden, because only a minority of influenza-related deaths are ever definitively diagnosed as such. Additional influenza deaths result from secondary bacterial infections and exacerbation of preexisting chronic conditions, but are not recorded as being in any way related to influenza infection.
Statistical methods are therefore used to separate the influenza-attributable fraction of deaths from the background [4],[5]. These methods involve modeling seasonal cyclical patterns in mortality time series compiled from vital statistics, often coupled with viral surveillance data to provide information on the timing of influenza circulation. Vital statistics data usually contain information about cause of death, allowing researchers to estimate influenza-attributable “excess” deaths in broad categories such as pneumonia, respiratory, or cardiorespiratory deaths during influenza periods. The influenza-related excess in respiratory deaths can be measured with higher precision than the less specific all-cause and cardiorespiratory categories. However, because some deaths triggered by influenza are recorded as having been caused by underlying non-respiratory causes such as heart attack, stroke, diabetes, or chronic kidney conditions, analysis of the broader cardiorespiratory and all-cause categories typically captures more completely the influenza-related burden.
The majority of deaths from seasonal influenza occur among people aged 65 y or older, but in a pandemic the proportion of deaths among the young increases [4],[6]–[8]. Single-country studies of H1N1pdm09 mortality, using various cause-of-death outcomes and modeling techniques [9]–[14], have repeatedly documented such an age shift. For example, a comprehensive hospital-based sentinel surveillance study by Liang and colleagues found that in China only 4% of cases, 8% of hospitalizations, and 23% of pandemic deaths occurred in persons over 50 y of age [15]. On a global level, Van Kerkhove et al. reported a median age of 46 y among fatal laboratory-confirmed cases [16]. McCallum et al. reported that in the Western Pacific region during 2009, only 1% of laboratory-confirmed cases and 13% of laboratory-confirmed deaths were among persons 65 y of age or older [17].
The Global Pandemic Mortality (GLaMOR) project aimed to make a conservative estimate of the global H1N1pdm09 mortality burden in 2009 using statistical models applied to mortality, virology, and other available data. The project was funded by WHO, which requested global and regional estimates of H1N1pdm09 influenza deaths for the year 2009; thus, all mentions of pandemic flu mortality refer specifically to deaths that occurred in the last 9 mo of 2009. We invited global collaborators to contribute national mortality data detailed by week, age, and cause of death for 2005 through 2009, at minimum.
Our novel method was inspired by a study that estimated the 1918–1920 global pandemic mortality burden using a two-stage statistical approach [18]. In Stage 1, we used detailed time series of national mortality and virology data from collaborating teams to estimate the 2009 pandemic respiratory mortality in each collaborating country/administrative region. Each participating GLaMOR team included influenza experts with whom the core GLaMOR team discussed Stage 1 mortality estimates in detail. The Stage 1 model and results were made available to each collaborating team to encourage individual country publications of national burden estimates. In Stage 2, we used a hierarchical multiple imputation model that used geographical, economic, and health country indicators to project the Stage 1 single-country estimates to all world countries, and summed to obtain regional and world estimates (Figure S2) .
A previous study of global H1N1pdm09 mortality, published in 2012 by Dawood et al. [19], was conducted before 2009 mortality data became available. To overcome that problem, these authors implemented a probability model that took into account symptomatic attack rates and case fatality ratios measured in a set of wealthier countries, then used a “respiratory mortality multiplier” based on pre-2009 data to adjust for differences in respiratory disease fatality rates in different parts of the world. In contrast, we used national vital statistics data for 2009 to measure the actual pandemic mortality and its age patterns in countries representing ∼35% of the global population, then estimated the burden in the remaining countries using a novel projection method. As a result, while our global burden estimate is comparable to that of Dawood et al. [19], the regional pattern we found, with the Americas hit hard and Europe largely spared, corresponds more closely to what was reported as the pandemic unfolded. In addition, our method simultaneously generates pre-pandemic global seasonal influenza mortality estimates that we have presented here for comparison.
Methods
Data Sources and Preparation
We obtained weekly virology data from the WHO FluNet [20] to identify influenza active periods (some collaborators provided more detailed virology data). We created 3-wk moving averages of these data with a lag of 1 wk, to achieve the clinically observed lag of 1 wk from disease onset to death. We inspected the data for weeks in which the reported count dropped dramatically coincident with a major holiday; in three cases (Spain, Mexico, and Japan) we replaced the reported number with the average of positive counts in the two surrounding weeks. In most cases we encountered insufficient subtyping of influenza A viruses. Age-specific virology data were not available.
We requested weekly national mortality time series based on the “underlying” cause-of-death determination from 1 January 2005 to 31 December 2009, stratified by at least two age groups (<65 and ≥65 y) and by four International Classification of Diseases–10 (ICD-10)–coded outcomes: all causes, cardiorespiratory (J and I codes), respiratory (J codes), and pneumonia and influenza (codes J10–J18) (Table 1). Contributing countries/administrative zones represented ∼35% of the global population. To prepare the time series mortality data for modeling, we created 3-wk moving averages after removing data in affected countries from summer weeks with a documented heat wave or armed conflict with significant mortality. We de-trended the time series using a spline factor modeled from summer periods. The exclusions and summer periods are given in Table 2. We obtained age distribution data for H1N1pdm09 laboratory-confirmed deaths from collaborators in a subset of GLaMOR countries. Because of concerns about sharing data, collaborators in the UK and China ran the GLaMOR SAS code on their own data. As only aggregate (de-identified) national summary mortality data were used in this study, it was exempt from human subjects regulations.
Tab. 1. Participating countries for which GLaMOR estimated all-cause, cardiorespiratory, or respiratory pandemic-associated mortality (Stage 1) or which were used to evaluate performance of global projection methods (Stage 2). 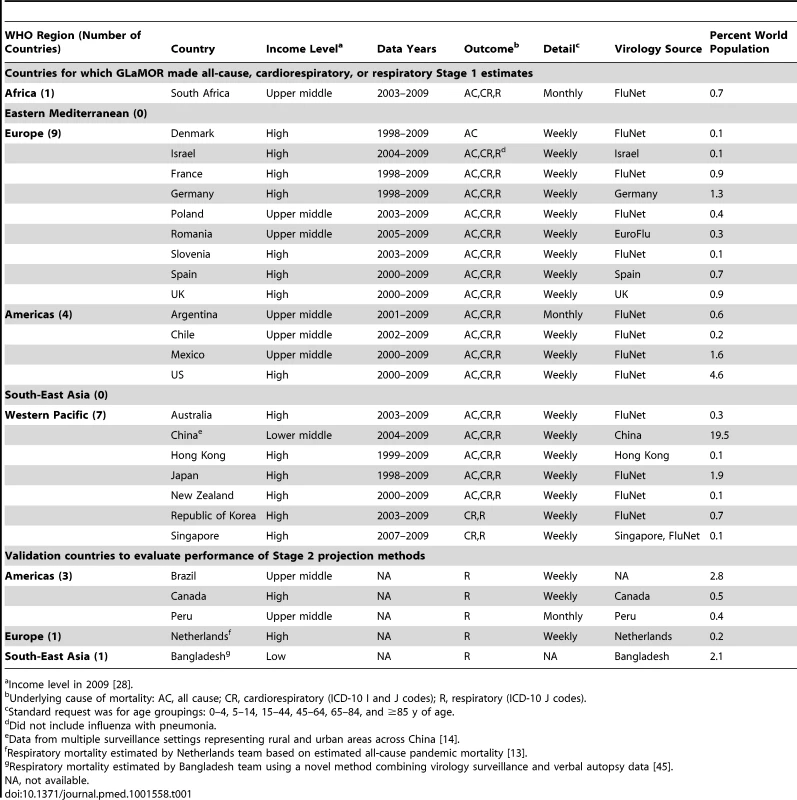
Income level in 2009 [28]. Tab. 2. Stage 1 customizations and all-ages model fit for 20 GLaMOR participating countries. 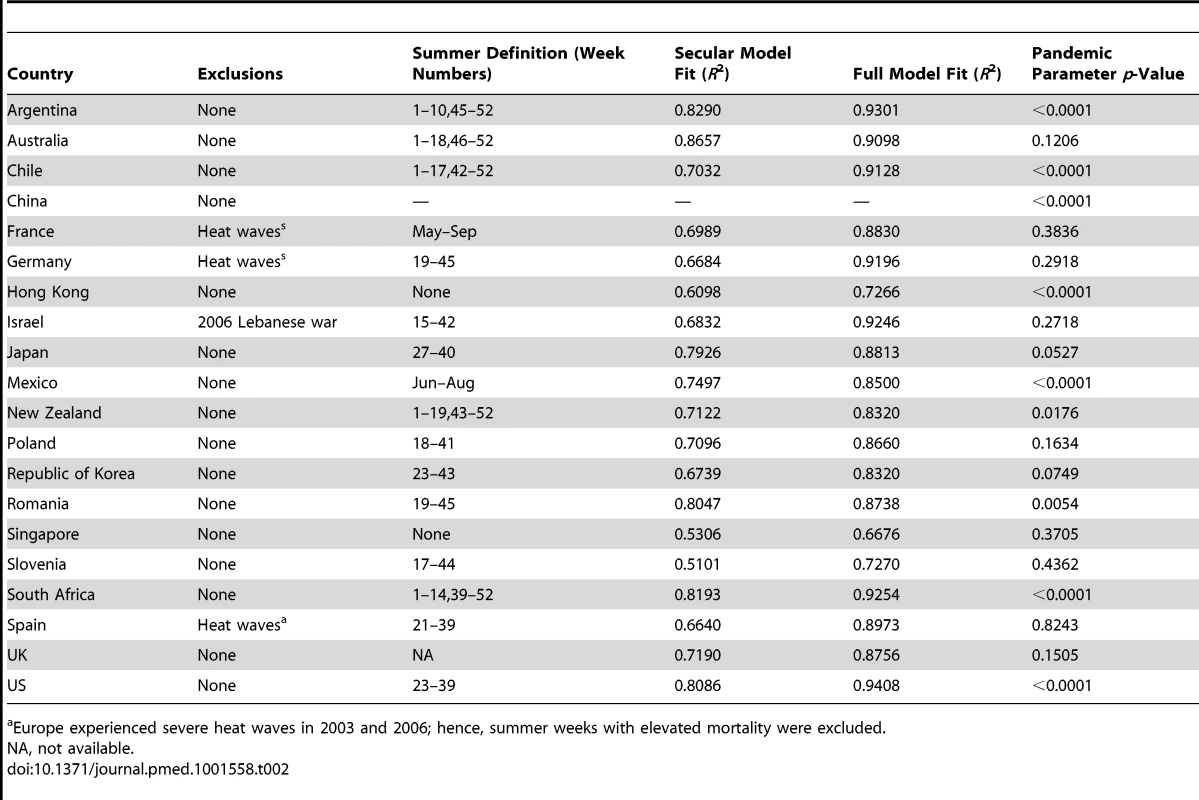
Europe experienced severe heat waves in 2003 and 2006; hence, summer weeks with elevated mortality were excluded. When choosing which outcome to use as our primary estimate of mortality, we had to make a trade-off between sensitivity and specificity while maintaining sufficient precision. Modeling all-cause mortality data would by definition ensure that all deaths are captured (100% sensitivity), but would sacrifice specificity and therefore precision. At the other extreme, pneumonia and influenza (P&I) is a specific influenza outcome but captures only a fraction of total pandemic deaths.
After much deliberation and advice from the Ad Hoc Advisory Committee on H1N1pdm09 Mortality Estimates [21], we focused on respiratory deaths in order to provide a minimum estimate of pandemic mortality burden with reasonable confidence limits. This was necessary as we had found that it was difficult to tease out any influenza-attributable increase in all-cause mortality in most countries.
Stage 1: Single-Country Pandemic Mortality Estimates
We developed a multivariate linear regression model of influenza burden based on correlations between laboratory surveillance and national mortality data [22]–[25]. The model included virology surveillance time series data, as well as terms for linear, square, cubic, and cyclical secular trends. We applied the same model form to each country. Where possible, the analysis was performed for four different mortality outcomes: all-cause deaths, respiratory and cardiovascular deaths, respiratory deaths, and deaths due to pneumonia and influenza. Data collection was carried out between 1 July 2011 and 31 March 2012. Stage 1 estimates were comparable in that we used standard ICD-10 definitions of mortality outcomes for each cause and applied the same multivariable linear regression model. We made estimates of respiratory and cardiorespiratory mortality for 20 countries, and estimates of all-cause mortality for 19 countries. Only estimates made with the GLaMOR model were used in the Stage 2 projections.
The GLaMOR Stage 1 model form was:
where t is the running week variable, and the linear and polynomial terms track remaining secular trends. The cyclical terms (as whole - and half-year cycles) tracked seasonal mortality patterns from other respiratory pathogens and factors such as temperature and relative humidity. Each influenza A season had its own variable, which we set to 0 in all other seasons. This way, case fatality in individual seasons is allowed to vary. Models of this type usually use separate terms for influenza A subtypes because the mortality burden of H3N2 is known to be far greater than that of seasonal H1N1; however, because adequate subtyped laboratory surveillance data were not available from many Stage 1 countries, we instead introduced into the model separate influenza A terms for each season. Collaborators supplied data for a variable number of seasons (s1 to sn), with β(s1) to β(sn) the respective coefficients. Most collaborators supplied five seasons; thus, the model had ∼14 explanatory factors and ∼250 observed data points for most countries. The H1N1pdm09 season was defined as the weeks beginning 5 April through 27 December 2009, inclusive. For Hong Kong, Japan, and South Africa, where H3N2 co-circulated with the pandemic virus, we included separate time series of H1N1pdm09 and H3N2 samples during the pandemic period. We did not remove nonsignificant terms from the model. Because of a lack of respiratory syncytial virus virology data, we did not control for respiratory syncytial virus co-circulation.We computed the pandemic attributions as the sums of the products of the pandemic model parameter β9 multiplied by the H1N1pdm09 positive count. When negative parameter values were obtained, mortality burden estimates were set to zero (as negative burden is not biologically meaningful). The confidence intervals were derived from uncertainty on the pandemic model parameter estimate β9. We determined the Stage 1 95% confidence intervals from the standard error on the H1N1pdm09 parameter estimate. We did not address autocorrelation in the residuals, and therefore the confidence intervals are narrow. We evaluated the model fit by three criteria: (1) the adjusted R2 for the full model, (2) the “lift” achieved by adding the virology terms to a base model of only secular and cyclical terms, and (3) the significance of the pandemic term β9 in the model. We used SAS 9.2 for all Stage 1 analyses.
Choosing among Four Stage 2 Global Projection Strategies
We explored four strategies to project our single-country estimates to the rest of the world before settling on multiple imputation, a Monte Carlo method that imputes values to missing data points and is often used to supply missing values in survey and census data [26].
1. Survey method
The survey method was a direct extrapolation of the average Stage 1 pandemic mortality rates using bootstrapping, in which the average excess mortality rate and upper and lower 95% confidence levels were used to calculate the global numbers and rates of pandemic deaths by age group and WHO region.
The limitation of this method is that it assumes that the average pandemic mortality experience in the Stage 1 countries is representative of the experience in all countries, and gives only a global estimate (no country - or region-specific estimates).
2. Gross national income/latitude method
The gross national income (GNI)/latitude method was derived from the method Murray et al. employed to estimate the 1918 global pandemic burden [18]. Influenza-attributable mortality was based on the relationship between measured pandemic mortality and per capita GNI and geographical latitude. Ordinary least squares regression models were used to relate the Stage 1 estimates to GNI and absolute latitude after natural logarithmic transformation of GNI and the dependent variable. The relationship was then used to estimate mortality in all world countries.
The limitation of this method is that the model assumes that GNI and latitude are sufficient proxies for the many variables that influence influenza mortality, and that mortality and the reasons for its variability in Stage 1 countries are representative for all countries. Importantly, there was an assumption that the relationship between GNI and excess mortality is exponential, so that the method yields very high mortality rates for low-income countries (e.g., countries in Africa).
3. Matching method
The matching method was devised by the GLaMOR team. It obtains the missing data points by matching (as closely as possible) Stage 1 countries to non–Stage 1 countries based on a set of country indicators. It involves two steps: (1) a data creation step using the matching approach, and (2) a data analysis step where a hierarchical linear random effects model is used to provide a single estimate for each country.
The data creation step involves calculation of multiple estimates per country based on the indicators listed in Table 3. We chose these indicators because they could reasonably be expected to affect pandemic mortality and were available for all countries in the world from public domain sources [27]–[30]. WHO region and latitude reflect the differing epidemiology of seasonal influenza in the differing regions of the world. Age group all-cause mortality rates, population density, physician density, and rural population percent reflect both the access to health care and the likelihood of influenza transmission. Population age structure reflects the documented age-related impact of influenza virus infections. The prevalence of comorbidities (i.e., obesity, HIV, and tuberculosis) is likely to be correlated with a greater probability of a severe outcome of influenza infection. The mortality estimates in the Stage 1 countries were examined and matched, for each indicator, to countries in which the mortality was not known.
Tab. 3. Country indicators used as factors in the Stage 2 model that projects the measured Stage 1 pandemic and seasonal influenza mortality estimates to global and regional estimates. 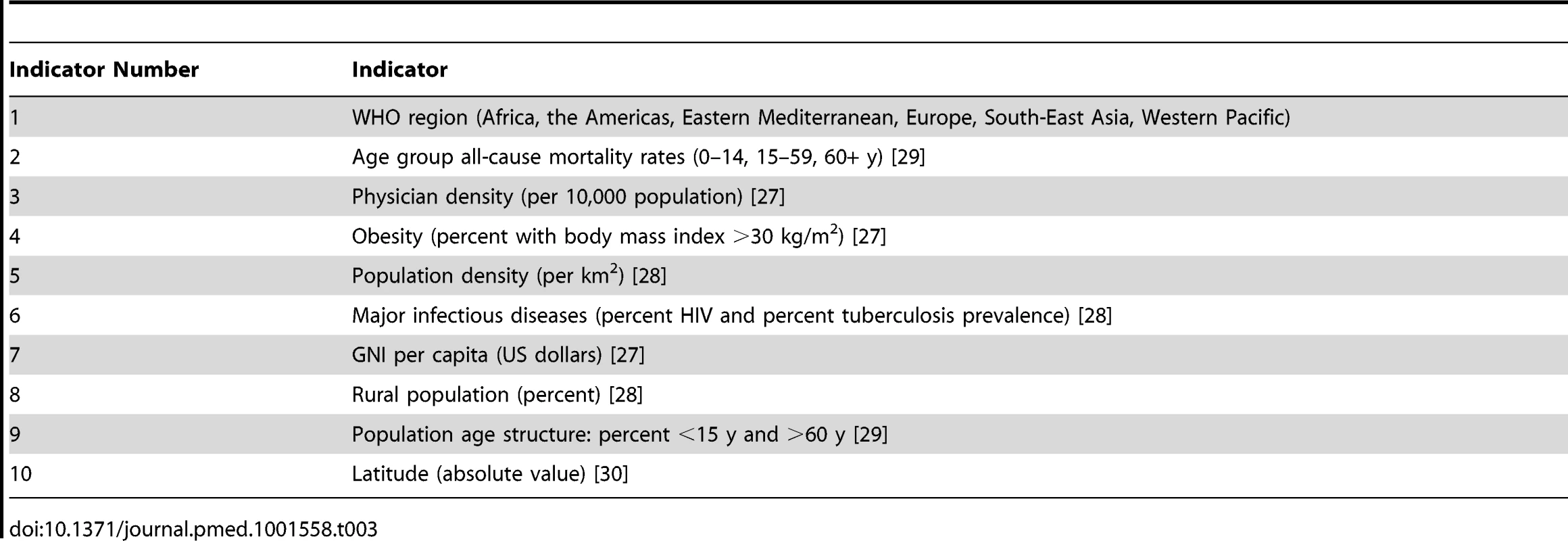
Figure 1 illustrates the matching procedure for an example country (Country Y) and three example indicators.
Fig. 1. Schematic illustration of the matching method based on country indicators. 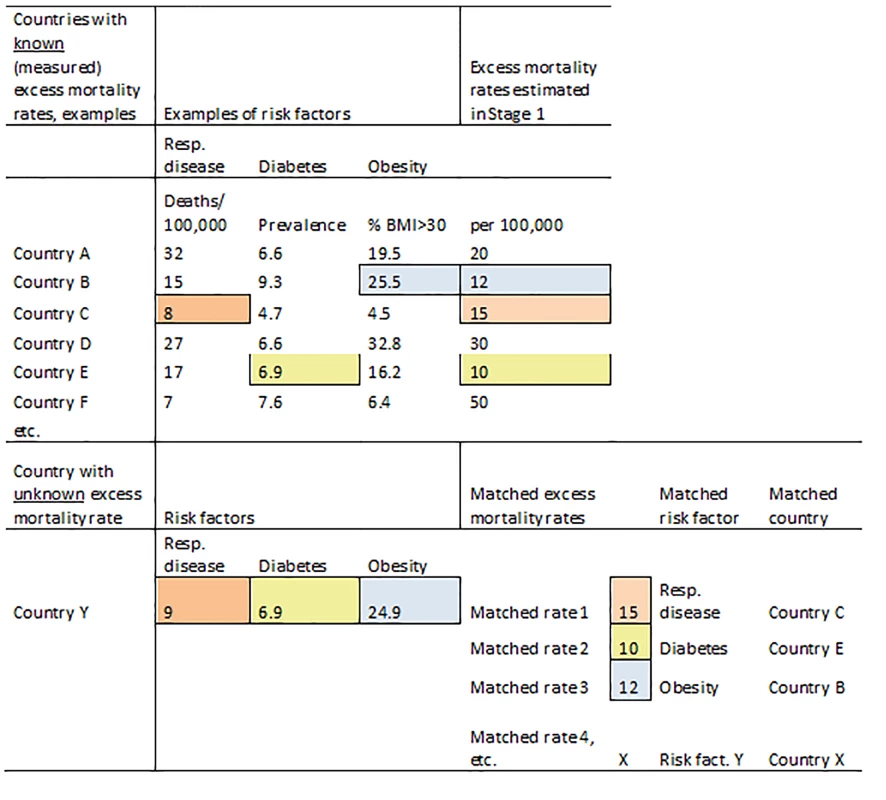
BMI, body mass index; Resp. disease, respiratory disease. The data analysis step of the matching method is similar to that of the multiple imputation method (below), with the same hierarchical linear random effects model but with the imputed datasets replaced by the matched datasets.
4. Multiple imputation method
We chose this method to make our Stage 2 extrapolations. Like the matching method, the multiple imputation method involves two steps, a data creation step followed by a hierarchical regression modeling step to project the burden in all world countries.
In the data creation step, we used statistical correlations between the same set of country indicators that we used in the matching method (Table 3) and all the Stage 1 mortality estimates to create a distribution of possible mortality values for each unknown country in the world. We then chose a random sample of 20 possible values from each country's distribution for further analysis. Because each Stage 1 point estimate was associated with uncertainty, we repeated the data creation step for each of the Stage 1 lower and upper 95% CI bounds. Thus, the final analysis dataset contained 60 estimates per country.
In the analysis step, we applied a hierarchical linear random effects regression model [31] to the final dataset to generate a point estimate (with standard error) for each country [32],[33]. We calculated the Stage 2 mortality rates (and their confidence intervals) simultaneously (i.e., in one model) for each country, each region, and the entire globe. The procedure was undertaken for persons of all ages and separately for persons <65 y. The model used was
whereY = imputed individual rate
i = individual measurement
j = country (1 … 197)
μj = between-country variance; μj∼N(0, )
εf = error variance for factor/imputed dataset, normally distributed; μf∼N(0, )
r = WHO region (1 … 7); coding (r = 1…6), ([0,1]−1)/6; r = 7, countries not belonging to a region (1 if r = 7, else 0)
f = imputed datasets (1 … 20); coding ([0,1]−1)/n; n = number of factors or datasets
Estimated rates were then given by:
World = β0
WHO region = β0+βr
Country = β0+βr+μj
Statistical 95% confidence intervals for these estimates were calculated using standard methods. We performed the imputation procedures with the Amelia II software package [34], and used the MLwiN version 2.1 package for the analysis model [35].
We evaluated the performance of each of the four candidate Stage 2 methods. We rejected the survey method because the results could not show regional variation, a major disadvantage given that the Stage 1 results showed considerable variation among the regions. We rejected the GNI/latitude method for two reasons. First, the estimates for our validation countries did not match the validation estimates the method produced (Table 4). Second, GNI and latitude did not result in statistically significant coefficients.
Tab. 4. External validation of GLaMOR Stage 2 projections for pandemic respiratory deaths, comparing GLaMOR Stage 2 projections for each projection method to five single-entity estimates made by collaborators using methods other than the GLaMOR regression model. 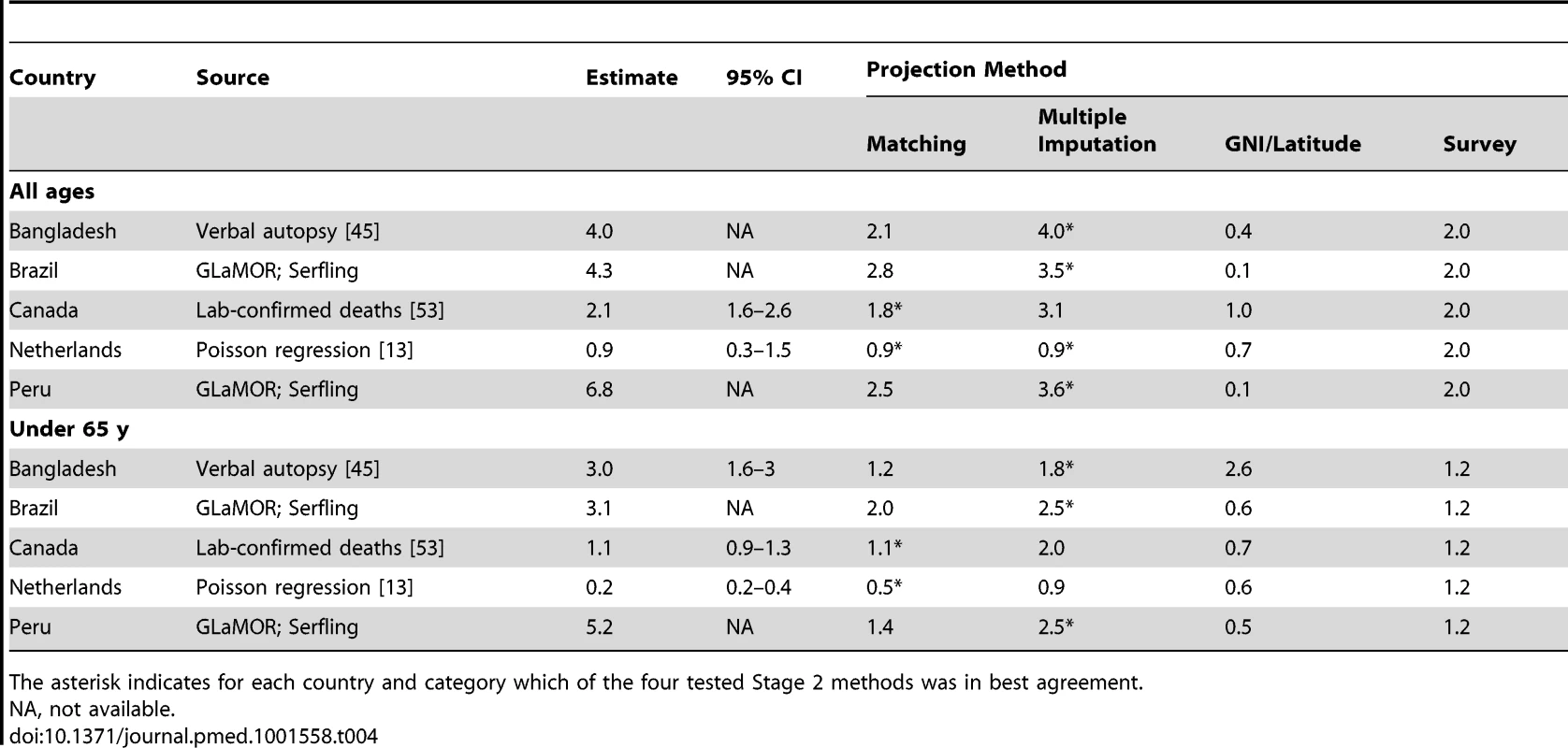
The asterisk indicates for each country and category which of the four tested Stage 2 methods was in best agreement. Choosing between Matching and Multiple Imputation
After eliminating the first two methods, only the matching and the multiple imputation methods remained. We first compared the multiple imputation and matching data creation steps for a single country in each WHO region. The results (Figure 2) demonstrate that each method produces a range of values and provides an indication of the uncertainty of the actual value. We then applied the following validation criteria to choose between the two.
Fig. 2. Output of the data creation phase for multiple imputation and matching for one randomly selected country per region. 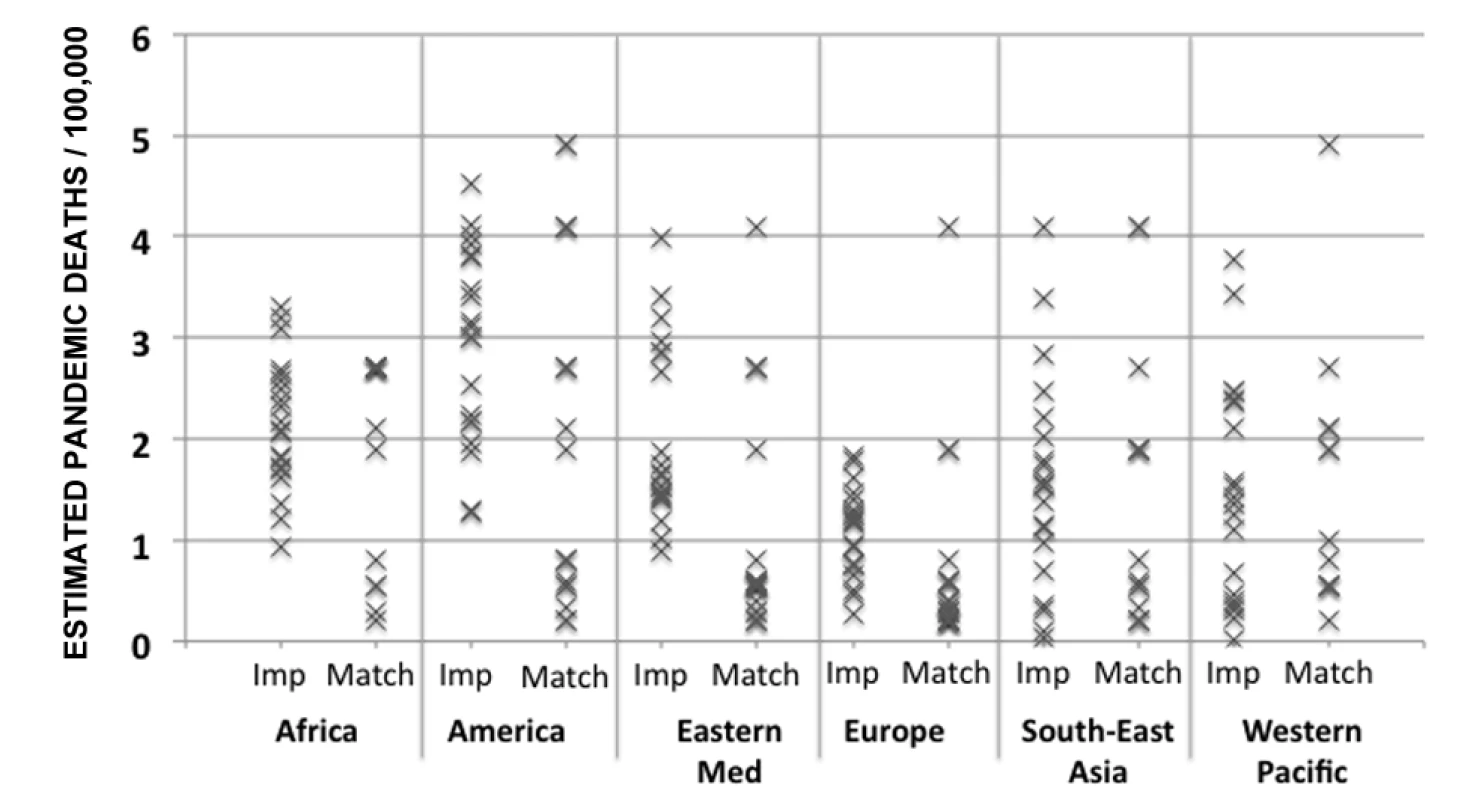
Eastern Med, Eastern Mediterranean; Imp, multiple imputation method; Match, matching method. 1. Reliability coefficients
Reliability, or internal consistency, is an important consideration. The reliability coefficient ranges from 0 to 1 (zero indicates no systematic effect) [36]. A value of 0.8 or higher indicates good (high) reliability. For the multiple imputation method the reliability coefficient for respiratory mortality was 0.78 for all ages and 0.87 for people <65 y. For the matching method the corresponding values were 0.94 and 0.96, respectively. It is important to realize, however, that this indicates only that both models had sufficient information to capture the country differences in a statistically reliable way, with no indication that one method performed better than the other.
2. Stage 1 versus Stage 2 estimates
We assessed the difference between the Stage 2 single-country estimates obtained with the matching and multiple imputation methods and the original Stage 1 estimates. The differences, expressed as standard deviations, were smaller for the multiple imputation method.
3. Lab-confirmed deaths as a minimum ground truth
We compared the Stage 2 estimates to country reports of the number of laboratory-confirmed H1N1pdm09 deaths (Table S1; data available for 67 countries until 31 December 2009). The GLaMOR estimates should logically be higher than the total number of laboratory-confirmed deaths in each country. Both models performed well, and the test indicated that both models had sufficient information to capture differences between countries in a statistically reliable way.
4. Distribution of the predicted Stage 2 estimates
We compared the highest and lowest 20% of national estimates (both for all ages and for persons <65 y of age) derived from the multiple imputation and matching methods. For all ages, the matching method distributes the highest burden over Africa and the Americas, with 88% of the countries in the highest quintile in the Americas and 18% in Africa. The multiple imputation method distributes the highest burden not only over the Americas and Africa, but also over South-East Asia. As we have no Stage 1 estimates for South-East-Asia and only one for Africa, we cannot be sure which method is better, and this test was inconclusive.
5. Comparison to five GLaMOR validation estimates
Five of the 26 Stage 1 countries (Bangladesh, Brazil, Canada, Peru, and the Netherlands) could not be analyzed with the GLaMOR Stage 1 model for various reasons. Country collaborators in Bangladesh and Canada had each generated estimates using their own methods, as national vital statistics could not be provided. For Brazil and Peru the virological data did not align with pneumonia pandemic mortality spikes (see also Schuck-Paim et al. [12]); instead we applied a classical Serfling model to regional data, which does not require virology data (similar to the approach used by Charu et al. [9]). For the Netherlands, the GLaMOR H1N1pdm09 estimates were substantially lower than those generated by the country collaborators [13]. We ran both their and our model on respiratory mortality data and found that the GLaMOR estimates were negative for the elderly, and that the upper 95% confidence interval on the all-age estimate excluded the number of laboratory-confirmed deaths. However, the Dutch model placed most H1N1pdm09 deaths in seniors (≥65 y), which was in disagreement with the age distribution in the Dutch laboratory-confirmed mortality data. Unable to reconcile these discrepancies, we accepted the country collaborators' preference for their own model results. These five country estimates (based on alternative modeling strategies) were used only to validate the Stage 2 projections. The multiple imputation method estimates were more often closer to the independent national estimates than the matching method estimates were (Table 4).
Multiple Imputation Method Chosen
The validation tests for the most part yielded only small differences between the multiple imputation and matching methods. We selected the multiple imputation method because it produced estimates that were more consistent with those generated by country collaborators in the five GLaMOR validation countries than the estimates produced by the matching method, and because multiple imputation is an established method while the matching method was one we developed. The choice of multiple imputation had two important consequences: it resulted in systematically higher country mortality rates compared to the matching method, and it placed a higher burden in people aged 65 y and over, especially in Asia and Africa.
Global Seasonal Burden Estimates
We computed the average global seasonal influenza burden (type A plus type B) for the years immediately prior to the pandemic. Specifically, we calculated the average pre-pandemic seasonal influenza mortality for each Stage 1 country using model parameter values from each pre-pandemic season, then projected these estimates to global and regional values using our Stage 2 multiple imputation procedure (Table S4). The number of pre-pandemic seasons available to us from each country was variable, and we made no attempt to control for differences in influenza type or subtype dominance.
Calculating All-Age Mortality
Because of large background mortality in the elderly, it was difficult to measure all-age influenza-related mortality with precision in lower-burden countries. For example, in some European countries the H1N1pdm09 mortality impact was so subtle that the model applied to all-age time series produced a negative point estimate for the H1N1pdm09 burden, with confidence intervals that at times excluded the “ground truth” minimum of the reported number of laboratory-confirmed H1N1pdm09 deaths from that country. Modeling the data for the <65-y age group, however, almost always resulted in estimated H1N1pdm09 mortality rates that were comparable to, or far higher than, the laboratory-confirmed mortality count.
We therefore elected to generate Stage 2 all-age burden projections in two ways: one based on Stage 2 all-age estimates, and the other based on the <65-y Stage 2 estimates, which we proportionally projected to all ages using data from laboratory surveillance indicating that 85% of confirmed H1N1pdm09 deaths occurred in the younger group (Table S1).
Sensitivity Analysis
We investigated the sensitivity of our global Stage 2 burden estimates to changes in the Stage 1 sample by successively removing one Stage 1 country from the Stage 2 input dataset and rerunning the Stage 2 model. Because the range from this analysis was always wider than the 95% confidence intervals derived from the Stage 1 and 2 statistical procedures, we chose to report this range as a more realistic view of the uncertainty (see Table 5; the statistical 95% CIs can be found in Tables S2 and S3).
Tab. 5. Global and regional GLaMOR Stage 2 projections of pandemic respiratory mortality, where all age estimates were derived both from Stage 1 all-age estimates and from the <65-y age group results adjust to 100% using the laboratory-confirmed mortality surveillance age distribution. 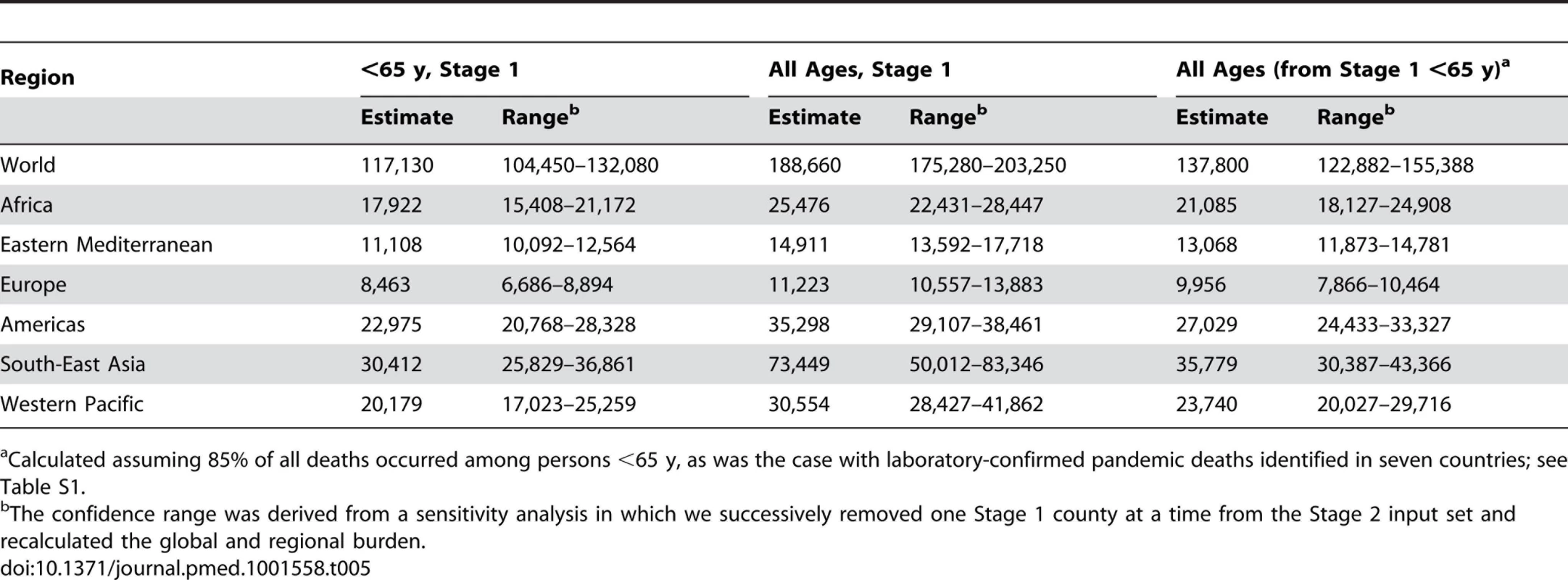
Calculated assuming 85% of all deaths occurred among persons <65 y, as was the case with laboratory-confirmed pandemic deaths identified in seven countries; see Table S1. Results
Stage 1 Findings
The GLaMOR Stage 1 countries experienced one to three pandemic waves during 2009. Most H1N1pdm09 deaths occurred in winter months: November–December in the northern hemisphere and July–August in the southern hemisphere. Several Asian countries experienced an H3N2 epidemic in the months immediately before their major H1N1pdm09 wave.
Figure 3 shows Stage 1 country H1N1pdm09 mortality rates per 100,000 population with 95% confidence intervals. The model fit (adjusted R2) for Stage 1 respiratory mortality estimates among people <65 y was generally excellent (80%–90%), and the “lift” upon introducing the influenza virus explanatory components into the base secular model was substantial (Table 2). The pandemic burden varied considerably between countries. For countries where the burden was high, the Stage 1 model could easily separate the pandemic signal from the background noise. But where the burden was low, the H1N1pdm09 mortality point estimates became unreliable.
Fig. 3. Pandemic excess mortality estimates for Stage 1 countries, by age and outcome (respiratory, cardiorespiratory, and all cause). 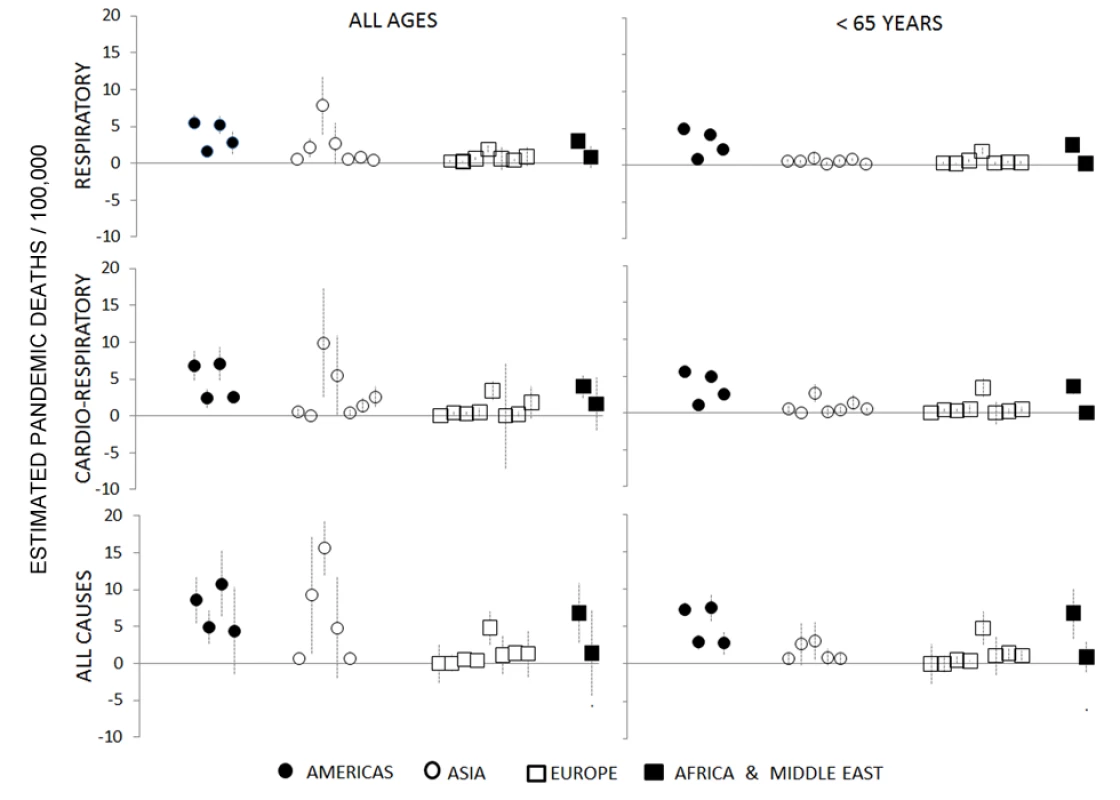
Data are grouped into four geographical regions. The various outcomes and age stratifications each provided a different balance between sensitivity and specificity. In high-burden countries such as Mexico and Argentina, the pandemic impact could be modeled with precision even for all-age time series of all-cause and cardiorespiratory mortality outcomes, as evidenced by tight 95% confidence intervals and agreement with a published Mexico study using a different Serfling regression modeling approach [9]. But in lower-burden countries, such as France and Germany, we could estimate a significant H1N1pdm09 mortality attribution only in persons <65 y and for respiratory deaths; Figure 4 illustrates the burdens in Mexico and France. Furthermore, for multiple countries we could not obtain a good model fit for finer age groups (e.g., <5, 5–14, 15–44, and 45–64 y) because of the small and fluctuating numbers of weekly deaths. We therefore chose to focus the global analysis on respiratory mortality among persons <65 y and ≥65 y, summing these point estimates to arrive at the all-age estimates.
Fig. 4. Examples of regional heterogeneity in pandemic mortality impact: Mexico (high burden) and France (low burden). 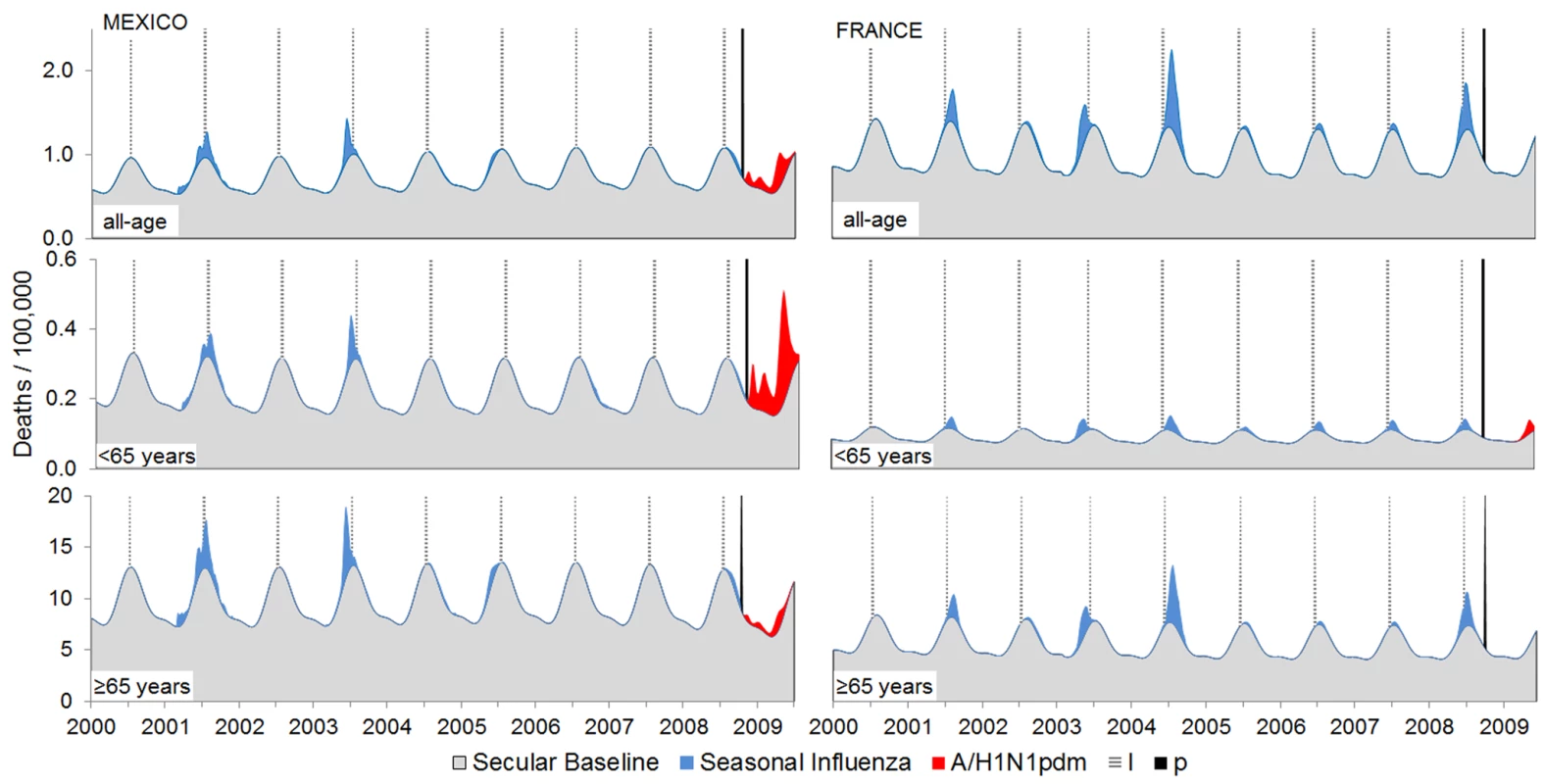
In Mexico, a substantial H1N1pdm09 respiratory mortality burden (red areas above gray background mortality) occurred among children, young adults, and middle-aged persons (<65 y) of age but not among seniors (≥65 y). In France, however, there was a far less dramatic pandemic impact that, despite the similar population size, was captured only in the <65-y age group model. Seasonal influenza burden (blue areas) was also generated by the Stage 1 model. The vertical black line represents the start of the pandemic. Overall, Stage 1 respiratory mortality rates were consistently higher in the Americas, with the highest measurements in Central and South American countries. South Africa's pandemic burden was moderate and on par with that of the US and China, suggesting that Africa may have experienced a lower pandemic burden than Central and South America. In Europe, the pandemic burden was generally low and on par with national numbers of laboratory-confirmed H1N1pdm09 deaths. Spain, France, and Germany averaged a rate of just 0.3/100,000, while Romania, the lowest-income European country included in this study, felt an approximately 6-fold greater impact. In South and Central America, however, we did not find a consistent relationship between H1N1pdm09 mortality and country income group: Argentina and Mexico had particularly high pandemic death rates (∼5/100,000), whereas Chile, a country with a similar economy, had a pandemic death rate that was more than 3-fold lower.
Across all Stage 1 countries, the model placed an average of 66% of respiratory pandemic deaths in persons <65 y. However, that proportion varied widely, from 100% in several European countries, to 70%–90% in Argentina and Mexico, to less than 10% in Hong Kong and Japan. Our Stage 1 estimates for seniors (≥65 y) were considerably more uncertain than those for the <65-y age group; this was due in part to the difficulty in precisely measuring the small H1N1pdm09 burden in seniors against their high background mortality, and partly because Hong Kong and Japan were outliers with a far higher burden among seniors than all the other countries.
In the few high-burden countries where we could measure Stage 1 all-cause mortality with confidence (e.g., Argentina and Mexico), the ratio of all-cause to respiratory mortality ranged from 1.6 to 2.3.
Stage 2 Global Projections
Using the Stage 1 model results for all ages (sum of <65-y and ≥65-y age group estimates) as input, the Stage 2 model projected a global pandemic respiratory mortality during 2009 of 189,000 (range, 175,000–203,000) deaths. These estimates correspond to an incidence rate of 2.77 (range, 2.57–2.98) per 100,000 population. Globally, 62% of these deaths were estimated to occur in persons <65 y, varying from 41% to 75% among WHO regions.
Because the pandemic virus caused considerable excess mortality in the <65-y age group, Stage 1 estimates for this age group were more reliable. To take advantage of that greater reliability, we computed the Stage 2 global and regional projections based on these estimates alone, by adjusting the <65-y estimates to all ages. To accomplish this we compiled data on the age distribution of laboratory-confirmed H1N1pdm09 deaths from mortality surveillance efforts in seven countries; these data indicated that an average of 85% of all pandemic deaths occurred in persons <65 y (Table S1). This alternative approach yielded a lower global pandemic respiratory mortality of 138,000 deaths (range, 123,000–155,000). Table 5 shows the estimated numbers of global and regional deaths obtained by both methods; mortality rates and 95% confidence intervals are given in Tables S2 and S3.
We found substantial regional heterogeneity in H1N1pdm09 mortality rates (Figure 5). The regional patterns observed among Stage 1 countries were borne out in the Stage 2 estimates, with high rates in the Americas and low rates in Europe.
Fig. 5. Pandemic respiratory mortality rates projected to all world countries with the Stage 2 multiple imputation model, stratified by age. 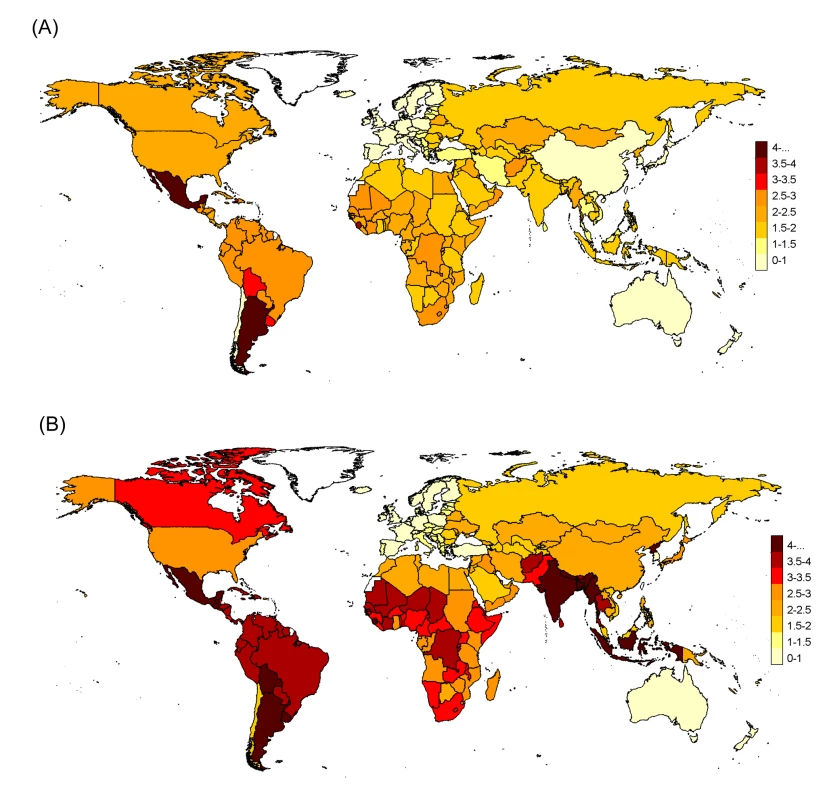
(A) Under 65 y and (B) all ages. Numbers in map legend are pandemic mortality rates per 100,000 persons. In the sensitivity analysis in which one Stage 1 country at a time was removed from the Stage 2 analysis, the global H1N1pdm09 mortality estimates were stable, with all-age point estimates ranging from 2.6 to 3.0 per 100,000 population from all-ages data (Figure 6A) and 1.7 to 2.1 per 100,000 from <65-y age group data (Figure 6B). However, regional estimates were more sensitive to the removal of individual countries, reflecting the importance of inclusion or exclusion of countries with “outlier” Stage 1 measurements such as Mexico and Chile in the Americas and Hong Kong in the South-East Asia region.
Fig. 6. Sensitivity analysis of global and regional pandemic respiratory mortality rates. 
The Stage 2 model was run multiple times, each time removing one Stage 1 country, for (A) all ages and (B) <65 y. The global estimates (black diamonds) were relatively stable, but some regions were sensitive to the removal of individual countries. Figure S1 depicts the corresponding sensitivity analysis results for seasonal estimates. Eastern Med, Eastern Mediterranean; SEAR, South-East Asia; Western Pac, Western Pacific. Seasonal Influenza Mortality Estimates
We generated an estimate of the average global seasonal influenza mortality burden, based on Stage 1 average seasonal influenza attributions across the pre-pandemic period. The Stage 2 multiple imputation method projected a global seasonal influenza burden of 210,000 influenza-related respiratory deaths per influenza season; of these only 19% occurred in persons <65 y of age. The range of seasonal influenza mortality estimates in the sensitivity analysis (148,000 to 249,000 deaths per year) was again wider than the 95% confidence intervals; thus we believe the range to be a better measure of uncertainty. The effect of removing individual Stage 1 countries on the seasonal influenza mortality estimates was more pronounced than for the pandemic estimates (Figure S1). Figure 7 shows the marked age shift in global and regional pandemic mortality burden toward persons aged <65 y.
Fig. 7. Age distribution of projected global and regional respiratory mortality, for both pandemic and seasonal influenza mortality estimates. 
East.Med, Eastern Mediterranean. Discussion
Our analysis suggests that between 123,000 and 203,000 H1N1pdm09 respiratory deaths occurred globally in 2009. This range is derived from the all-age burden computed in two different ways: the higher figure is the upper bound of the all-age Stage 2 projection as given by the sensitivity analysis, while the lower estimate is the lower sensitivity analysis bound of the more reliable <65-y age group estimate projected to all ages.
That range places H1N1pdm09 mortality below that of previous influenza pandemics, which varied from ∼1 million deaths in 1968 to ∼50 million deaths in 1918 [37]. But the majority (62%–85%) of the H1N1pdm09 deaths occurred among persons under 65 y of age, compared to only 19% of the 148,000 to 249,000 seasonal influenza respiratory deaths per season. It is this “signature age shift” that sets pandemic influenza apart from seasonal influenza [4],[6],[38],[39]. The severity of the 2009 pandemic would therefore be better measured with more complex metrics, such as life-years lost [8], when the necessary age - and risk-stratified mortality data become available.
When the H1N1pdm09 virus first emerged, early assessments of the threat level were mixed. Events in Mexico and Argentina in April and May 2009 suggested a severe pandemic on par with the 1957 pandemic or worse [40],[41], while data from New Zealand during June through August (their winter season) revealed a mild mortality impact [42]. By applying the same Stage 1 model form to comparable mortality data across countries, we documented that large regional variability did in fact occur: the GLaMOR Stage 1 estimates revealed an almost 20-fold higher mortality impact for several countries in the Americas than for New Zealand, Australia, and most countries in Europe.
Many factors might have contributed to the regional differences in H1N1pdm09 mortality impact, including the previous influenza exposure history of the population, use of antiviral drugs, the number and duration of pandemic waves during 2009, influenza vaccination coverage in preceding seasons, access to intensive care, and use of public health mitigation strategies. Use of pandemic vaccine is not on the list as it became available too late to play a role in 2009 [43]. One intriguing possibility is that H1N1pdm09 severity might have been exacerbated by recent circulation of H1N1 viruses distantly related to the pandemic virus. This is consistent with H1N1 having predominated in the Americas and H3N2 in Europe in the 2008–2009 season. It is also supported by a recent study demonstrating enhanced H1N1pdm09 disease in piglets recently immunized with a genetically distant seasonal H1N1 vaccine [44].
Our global estimates were in reasonable agreement with those of Dawood et al. [19], to our knowledge the only other published study of global mortality from the 2009 pandemic. The GLaMOR respiratory estimate range fell within Dawood's ranges of global respiratory deaths (25th to 75th percentile, 105,700 to 395,600; 5th to 95th percentile, 39,000 to 1,315,800). However, the two studies used very different modeling strategies. Dawood et al. collected symptomatic attack rates and case fatality rates from a set of high-income countries, then applied a country-specific “respiratory mortality multiplier” proportional to the underlying risk of dying from respiratory diseases in pre-pandemic years.
But at the regional level, the two approaches produced entirely different patterns (Figure 8). For example, we measured the highest H1N1pdm09 mortality rates in the Americas, while Dawood et al. projected low mortality rates there, having set the “respiratory mortality multiplier” to 1 for that region. Furthermore, Dawood et al. projected the highest mortality in Africa, while our Stage 1 pandemic respiratory estimate for South Africa—based on actual mortality data from South Africa—was manyfold lower than Dawood et al.'s. Their projection for India and South Asia was also high, although a Bangladesh study team measured a pandemic burden that was not particularly high [45]. Finally, surveillance data indicate that H1N1pdm09 virus activity was delayed in African countries, so that most H1N1pdm09 deaths occurred in subsequent years in those countries [46].
Fig. 8. Comparison of GLaMOR mortality estimates to those of Dawood et al. 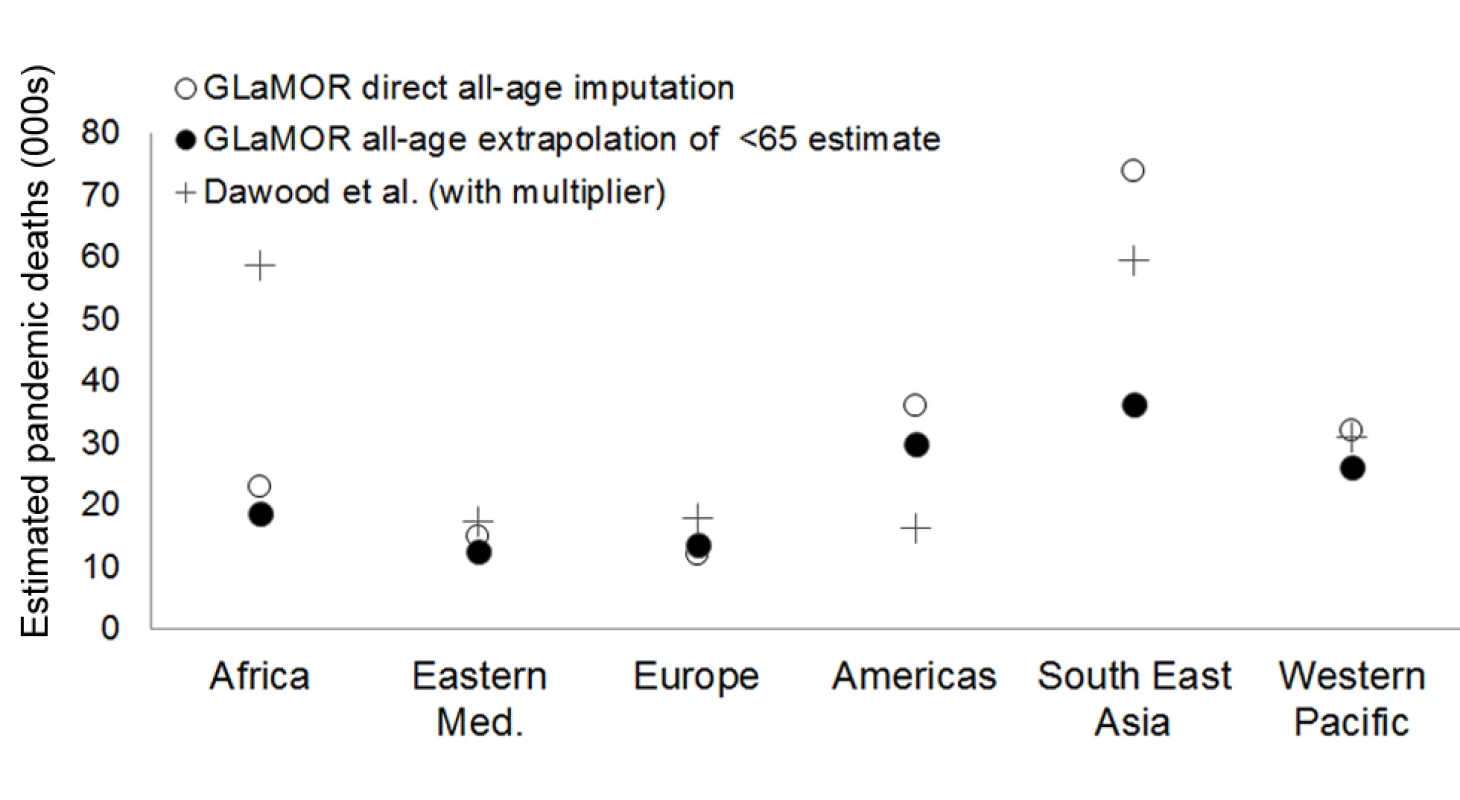
GLaMOR all-age respiratory mortality estimated directly from all-age multiple imputation (open circles) and by proportional extrapolation of the <65-y age group estimate to all ages using the age distribution of laboratory-confirmed mortality surveillance (black circles), compared to estimates by Dawood et al. [19] (black plus signs). Eastern Med, Eastern Mediterranean. Neither Dawood et al. nor our study had many data from Africa and South-East Asia, however, so what actually happened in these largely tropical regions remains unclear. However, a recent study demonstrated that the H1N1pdm09 mortality impact was far greater in temperate and wealthy southern regions of Brazil than in tropical and less-wealthy northern regions [12]. This unique insight from one large country that straddles climate zones suggests that the burden in the tropics was not necessarily higher than in temperate climates.
The H1N1pdm09 burden estimates from a few GLaMOR participating countries have been published. As shown in Figures 9 and 10, the GLaMOR Stage 2 projections for persons <65 y are in good agreement with published estimates for Mexico, China, Australia, US, and France, despite substantial differences in modeling approaches. In contrast, the senior (≥65 y) estimates are quite variable, as were the GLaMOR all-age results.
Fig. 9. Comparison of Stage 1 modeled pandemic respiratory mortality rates, by age, to published estimates for Mexico, Australia, US, China, and France by authors using various modeling strategies. 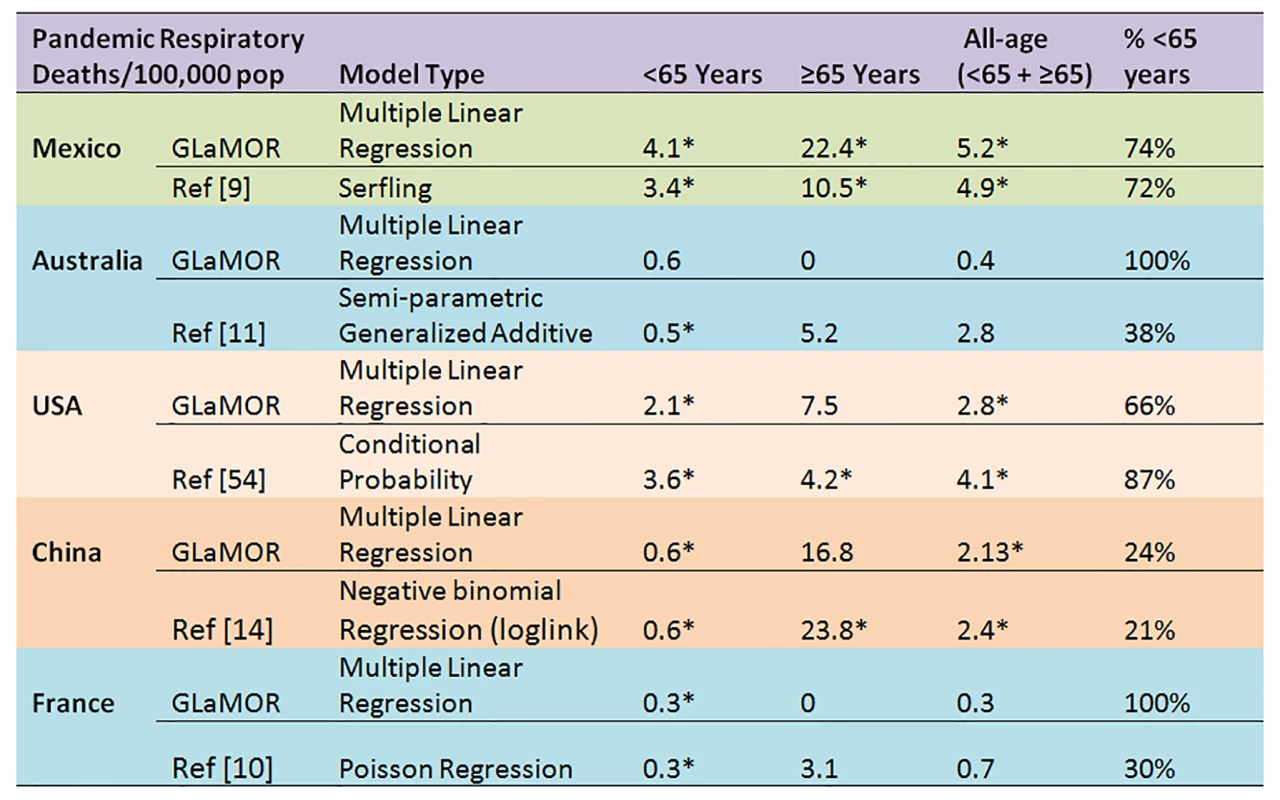
Asterisks indicate significance at the p<0.05 level. Fig. 10. Comparison of Stage 1 modeled pandemic all-age mortality rates, by cause, to published estimates for Mexico, Australia, US, China, and France by authors using various modeling strategies. 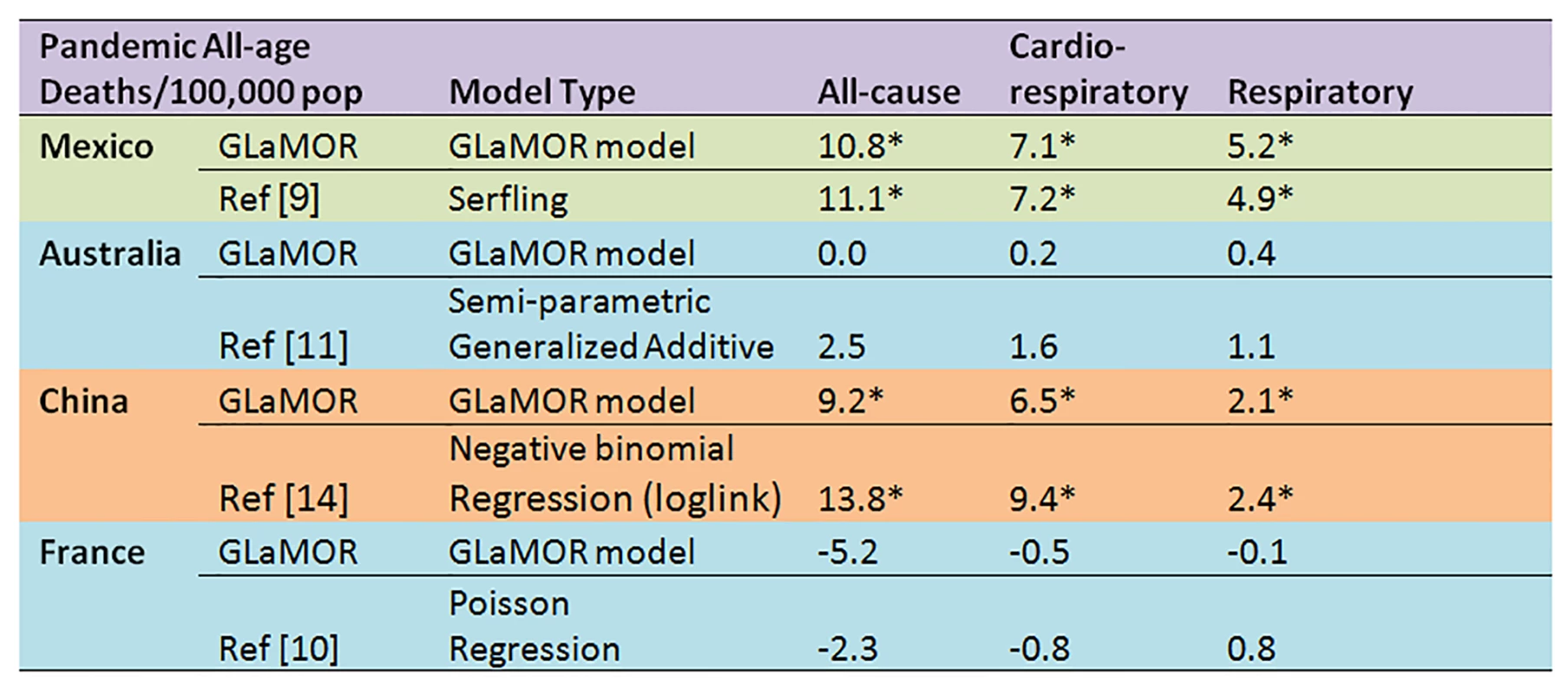
Asterisks indicate significance at the p<0.05 level. Although our conservative range of global respiratory mortality estimates is an order of magnitude greater than the reported global number of WHO laboratory-confirmed deaths, it likely substantially understated the total H1N1pdm09 mortality burden. First, our study missed deaths that occurred late in the 2009–2010 winter as well as those occurring in later pandemic waves. For example, one-third of Germany's laboratory-confirmed first-wave deaths occurred in early 2010, while substantial waves of H1N1pmd09 mortality were observed later in the UK in 2010–2011 [47] and in Mexico in 2011–2012 [48],[49]. Second, many H1N1pdm09 deaths may not have been recorded as respiratory deaths. Furthermore, we were forced to model “underlying cause of death” data, which may be biased towards underlying chronic disease and thus undercount respiratory deaths. Moreover, we refrained from generating a global estimate based on all-cause or cardiorespiratory deaths (the latter a preferred outcome for measuring seasonal influenza burden), since we were unable to detect significant pandemic excess mortality for these outcomes in most Stage 1 countries. We agree with Lemaitre et al. [10] that all-cause mortality is not a useful outcome for assessing H1N1pdm09 mortality in mild impact countries. However, in the few high-burden countries where we could measure all-cause mortality with confidence (e.g., Argentina and Mexico), the ratio of all-cause to respiratory mortality was ∼2∶1; Charu et al. reported a similar ratio for Mexico [9]. If this 2∶1 ratio pertained globally, the global pandemic all-cause mortality burden would have been about 300,000–400,000 deaths, approximately double our range of respiratory estimates.
Our approach had several strengths. Because the analysis was based on the pandemic “excess” mortality that actually occurred in 20 Stage 1 countries in 2009, we were able to map large and important regional differences in the H1N1pdm09 mortality burden that had not been captured in a previous study [19]. Because our collaborators contributed several years of data, we also were able to generate a global estimate of average seasonal influenza mortality, to which we could compare the pandemic burden. And because our single-country Stage 1 estimates were based on widely used analytical methods, our H1N1pdm09 mortality burden estimates are comparable to estimates made for historic influenza pandemics.
We recognize several caveats of our study, however. First, we lacked good representation of low-income countries and countries in South-East Asia, the Eastern Mediterranean, and Africa. Second, we were unable to explain the substantial pandemic mortality attributions among seniors that we and others measured in Hong Kong [50] and Japan, a pattern very unlike that documented by laboratory-confirmed mortality surveillance efforts in multiple countries globally (Table S1). Our Hong Kong collaborators maintained that seniors could have been missed in the laboratory-confirmed surveillance effort, while our Japanese collaborators argued that their pandemic mortality surveillance system was not age biased. Thus, the high measured burden among seniors may be real, or a mis-measurement due to an inability of statistical models to fully control for H3N2 co-circulation in 2009 in some Asian settings. In any event, we could not resolve the issue. We therefore also generated global and regional estimates based on the less contentious <65-y age group results. Third, we might not have accounted sufficiently for spatial dependency between countries and the likely spillover effect of influenza. And finally, the seasonal estimates were not ideal because Stage 1 countries contributed data from a variable number of seasons, and not all modeling issues were fully resolved (e.g., we noted some degree of misalignment between influenza virology and seasonal pneumonia mortality peaks in some countries).
Health care policy decisions depend on reliable and timely data whereby the risks and cost-effectiveness of interventions can be evaluated. In the GLaMOR study we developed methods whereby we can make robust and comparable mortality estimates in any future pandemic. But the lack of timeliness of such reports must be remedied. Ideally, a set of sentinel countries with timely hospitalization and/or mortality data could form a global sentinel system measuring severity, provided a common protocol was in place to allow comparisons across settings. EuroMOMO (European Monitoring of Excess Mortality for Public Health Action), which collects timely age-specific all-cause mortality data in European countries, is a big step in the right direction [51]. Also Mexico, Hong Kong, and New Zealand should be lauded for timely surveillance systems that captured hospitalization, case fatality, and mortality impact in a large segment of their populations in near real time [42],[49],[52] and Canada for its timely investigations into unexpected effects of seasonal vaccines on the pandemic [55]. Going forward, these and other countries and existing networks should partner to collaboratively and rapidly assess the severity of future pandemic threats.
Supporting Information
Zdroje
1. World Health Organization (2010) Pandemic (H1N1) 2009—update 112. Available: http://www.who.int/csr/don/2010_08_06/en/index.html. Accessed 12 October 2012.
2. EnserinkM, CohenJ (2009) Virus of the year. The novel H1N1 influenza. Science 326 : 1607.
3. FlynnP (2010) The handling of the H1N1 pandemic: more transparency needed. Council of Europe Parliamentary Assembly
4. SimonsenL, ClarkeMJ, SchonbergerLB, ArdenNH, CoxNJ, et al. (1998) Pandemic versus epidemic influenza mortality: a pattern of changing age distribution. J Infect Dis 178 : 53–60.
5. ViboudC, GraisRF, LafontBA, MillerMA, SimonsenL (2005) Multinational impact of the 1968 Hong Kong influenza pandemic: evidence for a smoldering pandemic. J Infect Dis 192 : 233–248.
6. MillerMA, ViboudC, BalinskaM, SimonsenL (2009) The signature features of influenza pandemics—implications for policy. N Engl J Med 360 : 2595–2598.
7. MillerMA, ViboudC, OlsonDR, GraisRF, RabaaMA, et al. (2008) Prioritization of influenza pandemic vaccination to minimize years of life lost. J Infect Dis 198 : 305–311.
8. ViboudC, MillerM, OlsonD, OsterholmM, SimonsenL (2010) Preliminary estimates of mortality and years of life lost associated with the 2009 A/H1N1 pandemic in the US and comparison with past influenza seasons. PLoS Curr 2: RRN1153 doi:10.1371/currents.RRN1153
9. CharuV, ChowellG, Palacio MejiaLS, Echevarria-ZunoS, Borja-AburtoVH, et al. (2011) Mortality burden of the A/H1N1 pandemic in Mexico: a comparison of deaths and years of life lost to seasonal influenza. Clin Infect Dis 53 : 985–993.
10. LemaitreM, CarratF, ReyG, MillerM, SimonsenL, et al. (2012) Mortality burden of the 2009 A/H1N1 influenza pandemic in France: comparison to seasonal influenza and the A/H3N2 pandemic. PLoS ONE 7: e45051 doi:10.1371/journal.pone.0045051
11. MuscatelloDJ, CretikosMA, MacintyreCR (2010) All-cause mortality during first wave of pandemic (H1N1) 2009, New South Wales, Australia, 2009. Emerg Infect Dis 16 : 1396–1402.
12. Schuck-PaimC, ViboudC, SimonsenL, MillerMA, MouraFE, et al. (2012) Were equatorial regions less affected by the 2009 influenza pandemic? The Brazilian experience. PLoS ONE 7: e41918 doi:10.1371/journal.pone.0041918
13. WijngaardCC, AstenL, KoopmansMP, PeltW, NagelkerkeNJ, et al. (2012) Comparing pandemic to seasonal influenza mortality: moderate impact overall but high mortality in young children. PLoS ONE 7: e31197 doi:10.1371/journal.pone.0031197
14. YuH, FengL, ViboudCG, ShayDK, JiangY, et al. (2013) Regional variation in mortality impact of the 2009 A(H1N1) influenza pandemic in China. Influenza Other Respi Viruses E-pub ahead of print. doi: 10.1111/irv.12121
15. LiangW, FengL, XuC, XiangN, ZhangY, et al. (2012) Response to the first wave of pandemic (H1N1) 2009: experiences and lessons learnt from China. Public Health 126 : 427–436.
16. Van KerkhoveMD, VandemaeleKA, ShindeV, Jaramillo-GutierrezG, KoukounariA, et al. (2011) Risk factors for severe outcomes following 2009 influenza A (H1N1) infection: a global pooled analysis. PLoS Med 8: e1001053 doi:10.1371/journal.pmed.1001053
17. McCallumL (2010) Epidemiological characteristics of the influenza A(H1N1) 2009 pandemic in the Western Pacific Region. Western Pac Surveill Response J 1 : 5–11.
18. MurrayCJ, LopezAD, ChinB, FeehanD, HillKH (2006) Estimation of potential global pandemic influenza mortality on the basis of vital registry data from the 1918–20 pandemic: a quantitative analysis. Lancet 368 : 2211–2218.
19. DawoodFS, IulianoAD, ReedC, MeltzerMI, ShayDK, et al. (2012) Estimated global mortality associated with the first 12 months of 2009 pandemic influenza A H1N1 virus circulation: a modelling study. Lancet Infect Dis 12 : 687–695.
20. World Health Organization (2013) FluNet [database]. Available: http://www.who.int/influenza/gisrs_laboratory/flunet/en/. Accessed 22 October 2013.
21. World Health Organization (2011) Summary of WHO technical consultation: H1N1pdm mortality estimates. Available: http://www.who.int/influenza/surveillance_monitoring/updates/MortalityEstimates/en/. Accessed 26 October 2013.
22. PitmanRJ, MelegaroA, GelbD, SiddiquiMR, GayNJ, et al. (2007) Assessing the burden of influenza and other respiratory infections in England and Wales. J Infect 54 : 530–538.
23. ThompsonWW, ShayDK, WeintraubE, BrammerL, CoxN, et al. (2003) Mortality associated with influenza and respiratory syncytial virus in the United States. JAMA 289 : 179–186.
24. ThompsonWW, WeintraubE, DhankharP, ChengPY, BrammerL, et al. (2009) Estimates of US influenza-associated deaths made using four different methods. Influenza Other Respi Viruses 3 : 37–49.
25. ZhouH, ThompsonWW, ViboudCG, RingholzCM, ChengPY, et al. (2012) Hospitalizations associated with influenza and respiratory syncytial virus in the United States, 1993–2008. Clin Infect Dis 54 : 1427–1436.
26. Buuren S (2012) Flexible imputation of missing data. Boca Raton (Florida): CRC Press.
27. World Health Organization (2013) Global health observatory data repository [database]. Available: http://apps.who.int/gho/data/view.main. Accessed 11 November 2011.
28. World Bank (2013) Data: indicators [database]. Available: http://data.worldbank.org/indicator/. Accessed 1 November 2011.
29. United Nations Statistics Division (2013) UNdata: explorer [database]. Available: http://data.un.org/Explorer.aspx?d=POP. Accessed 1 November 2011.
30. Thematic Mapping (2013) World borders database [database]. Available: http://thematicmapping.org/downloads/world_borders.php. Accessed 1 November 2011.
31. Raudenbush SW, Bryk AS (2002) Hierarchical linear models, applications and data analysis methods. Thousand Oaks (California): Sage Publications.
32. GoldsteinH, SpiegelhalterDJ (1996) League tables and their limitations: statistical issues in comparisons of institutional performance. J R Stat Soc Ser A Stat Soc 159 : 385–443.
33. NormandS, GlickmanM, GastonisCA (1997) Statistical methods for profiling providers of medical care: issues and applications. J Am Stat Assoc 92 : 803–814.
34. HonakerJ, KingG, BlackwellM (2011) Amelia II: a program for missing data. J Stat Softw 45 : 1–47 Available: http://www.jstatsoft.org/v45/i07/. Accessed 29 October 2013.
35. Hasbash J, Charlton C, Browne WJ, Healy M, Cameron B (2009) MLwiN, version 2.1. Bristol: Centre for Multilevel Modelling, University of Bristol.
36. CookDA, BeckmanTJ (2006) Current concepts in validity and reliability for psychometric instruments: theory and application. Am J Med 119 : 166.e7–16.
37. World Health Organization (2005) WHO global influenza preparedness plan: the role of WHO and recommendations for national measures before and during pandemics. WHO/CDS/CSR/GIP/20055. Geneva: World Health Organization.
38. AndreasenV, ViboudC, SimonsenL (2008) Epidemiologic characterization of the 1918 influenza pandemic summer wave in Copenhagen: implications for pandemic control strategies. J Infect Dis 197 : 270–278.
39. OlsonDR, SimonsenL, EdelsonPJ, MorseSS (2005) Epidemiological evidence of an early wave of the 1918 influenza pandemic in New York City. Proc Natl Acad Sci U S A 102 : 11059–11063.
40. ChowellG, BertozziSM, ColcheroMA, Lopez-GatellH, Alpuche-ArandaC, et al. (2009) Severe respiratory disease concurrent with the circulation of H1N1 influenza. N Engl J Med 361 : 674–679.
41. FraserC, DonnellyCA, CauchemezS, HanageWP, Van KerkhoveMD, et al. (2009) Pandemic potential of a strain of influenza A (H1N1): early findings. Science 324 : 1557–1561.
42. BakerM, KellyH, WilsonN (2009) Pandemic H1N1 influenza lessons from the southern hemisphere. Euro Surveill 14.
43. BorseRH, ShresthaSS, FioreAE, AtkinsCY, SingletonJA, et al. (2013) Effects of vaccine program against pandemic influenza A(H1N1) virus, United States, 2009–2010. Emerg Infect Dis 19 : 439–448.
44. GaugerPC, VincentAL, LovingCL, LagerKM, JankeBH, et al. (2011) Enhanced pneumonia and disease in pigs vaccinated with an inactivated human-like (delta-cluster) H1N2 vaccine and challenged with pandemic 2009 H1N1 influenza virus. Vaccine 29 : 2712–2719.
45. HomairaN, LubySP, AlamgirAS, IslamK, PaulR, et al. (2012) Influenza-associated mortality in 2009 in four sentinel sites in Bangladesh. Bull World Health Organ 90 : 272–278.
46. KatzMA, LeboE, EmukuleG, NjugunaHN, AuraB, et al. (2012) Epidemiology, seasonality, and burden of influenza and influenza-like illness in urban and rural Kenya, 2007–2010. J Infect Dis 206 (Suppl 1) S53–S60.
47. PebodyRG, AndrewsN, FlemingDM, McMenaminJ, CottrellS, et al. (2012) Age-specific vaccine effectiveness of seasonal 2010/2011 and pandemic influenza A(H1N1) 2009 vaccines in preventing influenza in the United Kingdom. Epidemiol Infect 13 : 1–11.
48. ChowellG, Echevarría-ZunoS, ViboudC, SimonsenL, Grajales MunizC, et al. (2012) Recrudescent wave of pandemic A/H1N1 influenza in Mexico, winter 2011–2012: age shift and severity. PLoS Curr 4: RRN1306 doi:10.1371/currents.RRN1306
49. Borja-AburtoVH, ChowellG, ViboudC, SimonsenL, MillerMA, et al. (2012) Epidemiological characterization of a fourth wave of pandemic A/H1N1 influenza in Mexico, winter 2011–2012: age shift and severity. Arch Med Res 43 : 563–570.
50. YangL, ChanKP, CowlingBJ, ChiuSS, ChanKH, et al. (2012) Excess mortality associated with the 2009 pandemic of influenza A(H1N1) in Hong Kong. Epidemiol Infect 140 : 1542–1550.
51. EuroMOMO (2013) European mortality bulletin, week 23, 2013. Copenhagen: EuroMOMO.
52. WuJT, MaES, LeeCK, ChuDK, HoPL, et al. (2010) The infection attack rate and severity of 2009 pandemic H1N1 influenza in Hong Kong. Clin Infect Dis 51 : 1184–1191.
53. SchanzerDL, SchwartzB (2013) Impact of seasonal and pandemic influenza on emergency department visits, 2003–2010, Ontario, Canada. Acad Emerg Med 20 : 388–397.
54. ShresthaSS, SwerdlowDL, BorseRH, PrabhuVS, FinelliL, et al. (2011) Estimating the burden of 2009 pandemic influenza A (H1N1) in the United States (April 2009–April 2010). Clin Infect Dis 52 (Suppl 1) S75–S82.
55. SkowronskiDM, De SerresG, CrowcroftNS, JanjuaNZ, BoulianneN, et al. (2010) Association between the 2008?09 seasonal influenza vaccine and pandemic H1N1 illness during spring?summer 2009: four observational studies from Canada. PLoS Med 7 (4) e1000258doi:10.1371/journal.pmed.1000258
Štítky
Interní lékařství
Článek vyšel v časopisePLOS Medicine
Nejčtenější tento týden
2013 Číslo 11- Není statin jako statin aneb praktický přehled rozdílů jednotlivých molekul
- Moje zkušenosti s Magnosolvem podávaným pacientům jako profylaxe migrény a u pacientů s diagnostikovanou spazmofilní tetanií i při normomagnezémii - MUDr. Dana Pecharová, neurolog
- Magnosolv a jeho využití v neurologii
- S prof. Vladimírem Paličkou o racionální suplementaci kalcia a vitaminu D v každodenní praxi
- Biomarker NT-proBNP má v praxi široké využití. Usnadněte si jeho vyšetření POCT analyzátorem Afias 1
-
Všechny články tohoto čísla
- Antenatal Syphilis Screening Using Point-of-Care Testing in Sub-Saharan African Countries: A Cost-Effectiveness Analysis
- From Ideals to Tools: Applying Human Rights to Maternal Health
- Interactions between Non-Physician Clinicians and Industry: A Systematic Review
- Harnessing Poverty Alleviation to Reduce the Stigma of HIV in Sub-Saharan Africa
- Integrating Health Care to Meet the Needs of the Mother–Infant Pair: A Call for Papers for Year 3 of the Maternal Health Task Force–PLOS Collection
- Changes in Chinese Policies to Promote the Rational Use of Antibiotics
- Same Song, Different Audience: Pharmaceutical Promotion Targeting Non-Physician Health Care Providers
- A Gene Expression Signature for RSV: Clinical Implications and Limitations
- Global Research Priorities to Better Understand the Burden of Iatrogenic Harm in Primary Care: An International Delphi Exercise
- Measles Outbreak Response Immunization Is Context-Specific: Insight from the Recent Experience of Médecins Sans Frontières
- Global Mortality Estimates for the 2009 Influenza Pandemic from the GLaMOR Project: A Modeling Study
- When to Start Antiretroviral Therapy in Children Aged 2–5 Years: A Collaborative Causal Modelling Analysis of Cohort Studies from Southern Africa
- Burden of Depressive Disorders by Country, Sex, Age, and Year: Findings from the Global Burden of Disease Study 2010
- Role of DNA Methylation and Epigenetic Silencing of in Endometrial Cancer Development
- Characterization of Regional Influenza Seasonality Patterns in China and Implications for Vaccination Strategies: Spatio-Temporal Modeling of Surveillance Data
- Whole Blood Gene Expression Profiles to Assess Pathogenesis and Disease Severity in Infants with Respiratory Syncytial Virus Infection
- Complex Disease Dynamics and the Design of Influenza Vaccination Programs
- A Brief Patient-Reported Outcomes Quality of Life (PROQOL) Instrument to Improve Patient Care
- PLOS Medicine
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- A Brief Patient-Reported Outcomes Quality of Life (PROQOL) Instrument to Improve Patient Care
- Burden of Depressive Disorders by Country, Sex, Age, and Year: Findings from the Global Burden of Disease Study 2010
- From Ideals to Tools: Applying Human Rights to Maternal Health
- Role of DNA Methylation and Epigenetic Silencing of in Endometrial Cancer Development
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání





