-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Point Mutations in Centromeric Histone Induce Post-zygotic Incompatibility and Uniparental Inheritance
The centromeric histone protein, CENH3, plays an important role in chromosome segregation during mitosis and meiosis. Here we show that single amino acid changes in CENH3, while producing no obvious effect on mitosis or meiosis, affect segregation postzygotically upon outcrossing to plants carrying wild-type centromeres. This results in uniparental inheritance among some progeny, and seed death in a larger fraction of progeny. Interestingly, changes competent to induce haploid in Arabidopsis existed in a TILLING population and in unrelated plant species. Our findings have two major consequences. First, uniparental inheritance facilitates the production of haploid plants that can easily be doubled to produce completely homozygous lines in a single generation. Secondly, our findings suggest that natural variation in CENH3 may result in partial reproductive isolation, because chromosomes of the mutant parent from F1 hybrid progeny are culled during embryonic development, while no reproductive defects are observed in self-pollinated plants. We do not know if the same mutations are haploid-inducing in other species, but uniparental chromosome loss, and the seed abortion that accompanies it results in an outcrossing-specific penalty that could potentially be involved in reproductive isolation.
Published in the journal: . PLoS Genet 11(9): e32767. doi:10.1371/journal.pgen.1005494
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1005494Summary
The centromeric histone protein, CENH3, plays an important role in chromosome segregation during mitosis and meiosis. Here we show that single amino acid changes in CENH3, while producing no obvious effect on mitosis or meiosis, affect segregation postzygotically upon outcrossing to plants carrying wild-type centromeres. This results in uniparental inheritance among some progeny, and seed death in a larger fraction of progeny. Interestingly, changes competent to induce haploid in Arabidopsis existed in a TILLING population and in unrelated plant species. Our findings have two major consequences. First, uniparental inheritance facilitates the production of haploid plants that can easily be doubled to produce completely homozygous lines in a single generation. Secondly, our findings suggest that natural variation in CENH3 may result in partial reproductive isolation, because chromosomes of the mutant parent from F1 hybrid progeny are culled during embryonic development, while no reproductive defects are observed in self-pollinated plants. We do not know if the same mutations are haploid-inducing in other species, but uniparental chromosome loss, and the seed abortion that accompanies it results in an outcrossing-specific penalty that could potentially be involved in reproductive isolation.
Introduction
Accurate segregation of eukaryotic chromosomes into daughter cells requires the presence of a centromere. Centromeres are, in most species, a region on each chromosome that directs the assembly of the kinetochore during mitosis and meiosis. The kinetochore is a substantial molecular motor, consisting of hundreds of proteins, which regulates and drives the migration of sister chromatids (in mitosis) or homologous chromosomes (in meiosis I) to opposite poles of the cell [1–4]. Centromeres are largely thought to be determined epigenetically by the presence of nucleosomes containing the centromere-specific histone H3 variant CENH3 (aka, CENP-A) [5–9].
In stark contrast to conventional histones, which are among the most conserved proteins in eukaryotes, CENH3 is rapidly evolving [10]. CENH3 structure is divided into two domains, a highly variable (in length and sequence) N-terminal tail and the more conserved C-terminal Histone Fold Domain (HFD). Although a handful of amino acids are highly conserved at the N-terminus of the N-terminal tail domain, the rest of the tail is so rapidly evolving that it cannot be aligned even among fairly related clades. For example, among the eudicots known CENH3 N-terminal tails range in length from 23 to 194 amino acids [11]. The HFD is in contrast, relatively well conserved, although it displays signatures of adaptive evolution in some residues [10, 12]. Given the proven role of CENH3 in the specification of the centromere, it is of no surprise that null alleles, though transmissible, are lethal as homozygotes [13–16]. Similarly, defects in the localization of CENH3 - either a failure to reload or promiscuous loading to more than one site per chromosome - would be expected to lead to severe genetic abnormalities. Defects in CENH3 loading have been shown to cause chromosomes instability in several organisms, including budding yeast, humans and Arabidopsis [17–19].
Manipulation of CENH3 itself also has dramatic effects on chromosome segregation, an outcome with both basic and applied significance [11, 20–22]. Swapping the CENH3 hypervariable N-terminal tail with that of histone H3.3-like and concurrent fusion to GFP (“GFP-tailswap”) produces in a partially sterile plants showing meiotic defects. Interestingly, when the GFP-tailswap line is crossed to the wild type, the chromosomes derived from the parent expressing this chimeric protein misseggregate during embryogenesis, resulting in elimination of the corresponding parental genome, producing haploid plants whose chromosomes were derived from only the wild-type CENH3 parent. Maheshwari et al [11], recently demonstrated that transgenic CENH3 genes derived from progressively distant relatives (through the monocot Z. mays), can complement the lethality of a cenh3 -/ - null mutant of Arabidopsis, and the transgenic plants were fertile. However, when crossed with plants expressing wild-type CENH3, the progeny displayed various degrees of embryonic lethality, aneuploidy and haploidy. Missegregation affected only chromosomes from the parent expressing the distant CENH3.
Translation of these discoveries to haploid production in crops would accelerate trait mapping and plant breeding [23–25]. Implementing the GFP-tailswap or transgenic-complementation approach, however, requires two steps. First, a CENH3 knockout (KO) must be obtained, as the haploid induction trait conferred by the variant CENH3 is suppressed by the wild-type CENH3 protein. Second, this KO mutant must be complemented with the chimeric or trans-species transgene, a genetic modification likely to require expensive regulatory approval, which in some cases is unacceptable to the public.
These findings pose a basic question. Could a single amino acid change in CENH3 result in the missegregation syndrome, i.e. in a plant which is fertile on self-pollination, but whose centromeres malfunction when confronted zygotically with centromeres determined by wild-type CENH3? To address this, we decided to explore how single amino acid substitutions in CENH3 affect centromere function and chromosome segregation. Here, we show that changes in CENH3 sequence that could be derived naturally (or through simple chemical mutagenesis) can result in haploid induction upon hybridization, apparently without secondary effects on growth and fertility. This finding indicates that single amino acid changes at this rapidly evolving centromeric protein have dramatic consequences on the mutant ability to hybridize. At the same time, it provides a simple, non-transgenic tool for developing haploid inducers in crops.
Results
AtCENH3 consists of an N-terminal tail region and a C-terminal histone fold domain (HFD). To identify the conserved domains of CENH3 (and so identify particularly critical amino acids) we aligned the CENH3 protein sequences of over 50 plant species. The tail region is highly variable whereas the HFD is relatively conserved across species (S1 Fig), and for this reason we focused our attention on the HFD. We identified amino acids in Arabidopsis thaliana, as well as in cultivated dicot species Brassica rapa, Solanum lycopersicum and the monocot Zea mays that were conserved and could be mutated to produce the same amino acid change in all four species by G to A or C to T transition (reflecting the mutation spectrum of alkylating chemical mutagens). We identified 47 possible mutations in 30 amino acids in the HFD that fit these criteria (S1 Table). A comparison of CENH3s from these four plant species to CENH3s from yeast and human shows that some of these amino acids are conserved across kingdom (Fig 1).
Fig. 1. Multiple sequence alignment of CENH3 Histone Fold Domain (HFD) of Arabidopsis thaliana, Brassica rapa, Solanum lycopersicum, Zea mays, Saccharomyces cerevisiae and Homo sapiens. 
The annotations in the boxes above the alignment blocks indicate the single amino acid substitutions that can be mutated by G to A or C to T transition in four plant species (A. thaliana, B. rapa, S. lycopersicum, Z. mays). Green boxes indicate the point mutations that result in the induction of haploids and magenta boxes indicate point mutations that did not result in induction of haploids (at the scale measured here, Table 1) in Arabidopsis thaliana. The brown boxes are other EMS-inducible missense mutations identified in this study. Amino acid residue numbers within the green and magenta boxes correspond to positions of Arabidopsis thaliana CENH3. Scoring matrix: Blosum. Inset red box shows the similarity index. To identify potentially important amino acid changes, we used SIFT [26, 27] to predict whether a substitution of one amino acid for another would be functionally tolerated. SIFT predicted that 38 of our candidates would not be tolerated while 9 were more benign (S2 Table). We selected six mutant alleles (Table 1) and tested their ability to transgenically complement a cenh3-1 null mutation (the null allele is zygotic lethal), support fertility, and produce haploids upon crossing with wild-type Arabidopsis.
Tab. 1. Haploid induction and seed abortion frequency of transgenic and TILLING lines used in this study. 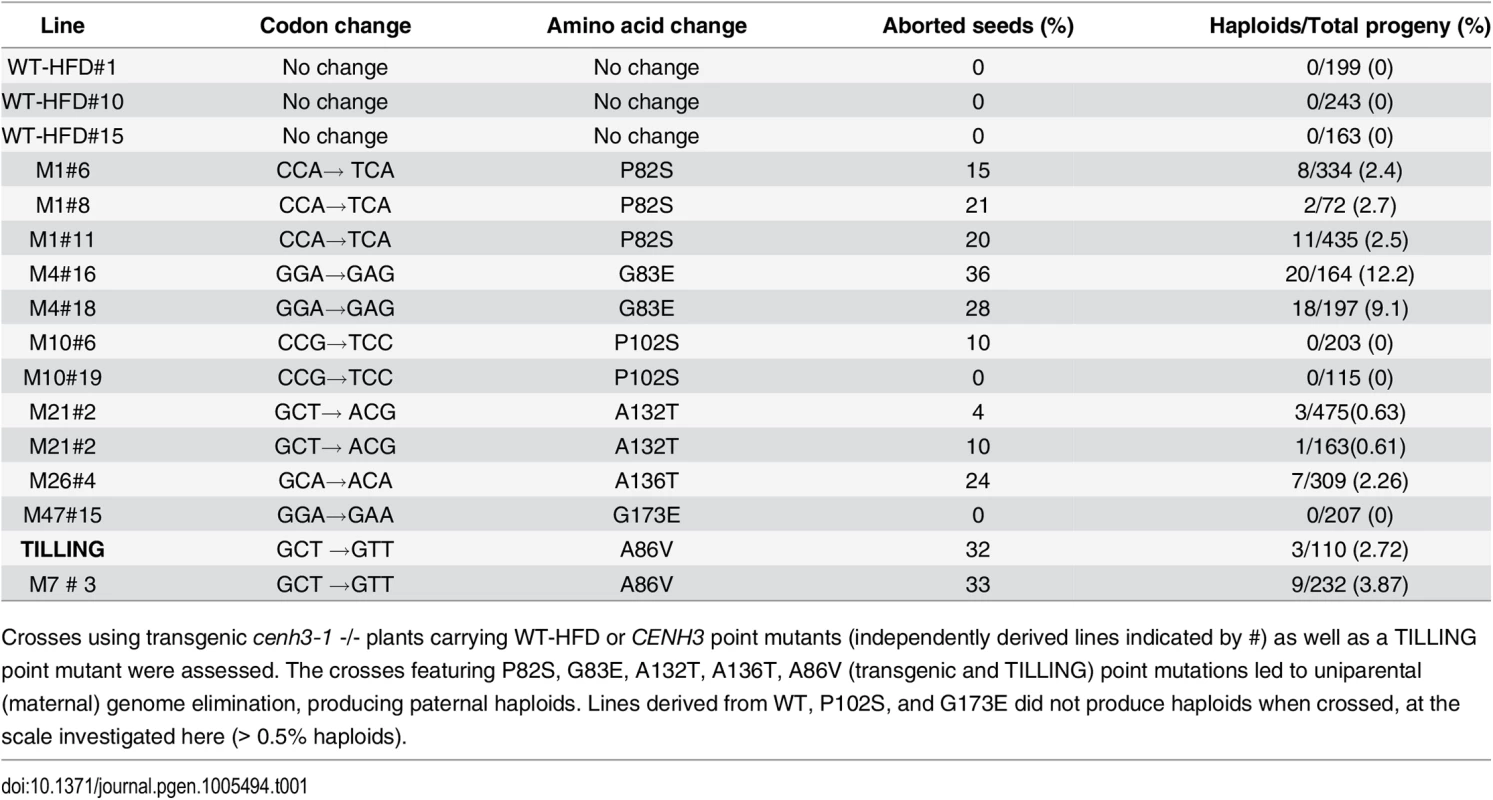
Crosses using transgenic cenh3-1 -/- plants carrying WT-HFD or CENH3 point mutants (independently derived lines indicated by #) as well as a TILLING point mutant were assessed. The crosses featuring P82S, G83E, A132T, A136T, A86V (transgenic and TILLING) point mutations led to uniparental (maternal) genome elimination, producing paternal haploids. Lines derived from WT, P102S, and G173E did not produce haploids when crossed, at the scale investigated here (> 0.5% haploids). In order to avoid lethality[16], our constructs were transformed into CENH3/cenh3-1 plants and their offspring were screened for both the presence of the transgene and native CENH3 genotype (Fig 2A and S2 Fig). To determine whether alteration in the level of expression of CENH3 (caused by variable levels of expression of the transgene in independently derived transformants) leads to a haploid inducing effect, we generated a wild-type version of our transgene, using the same vector backbone. This transgene (WT-HFD) has the native CENH3 promoter, native 5’ UTR and CENH3 tail domain with a synthetic wild-type histone fold domain. Three independent insertion lines carrying WT-HFD were analyzed. In all three WT-HFD lines (cenh3-1/cenh3-1 expressing WT-HFD CENH3) were able to complement the nullimorphic cenh3-1 mutation without any obvious phenotypic effects. These WT-HFD plants were fully fertile, and produced 100% normal seeds upon self-pollination.
Fig. 2. Schematic representation of transgenic CENH3 point mutant transformation and crossing. 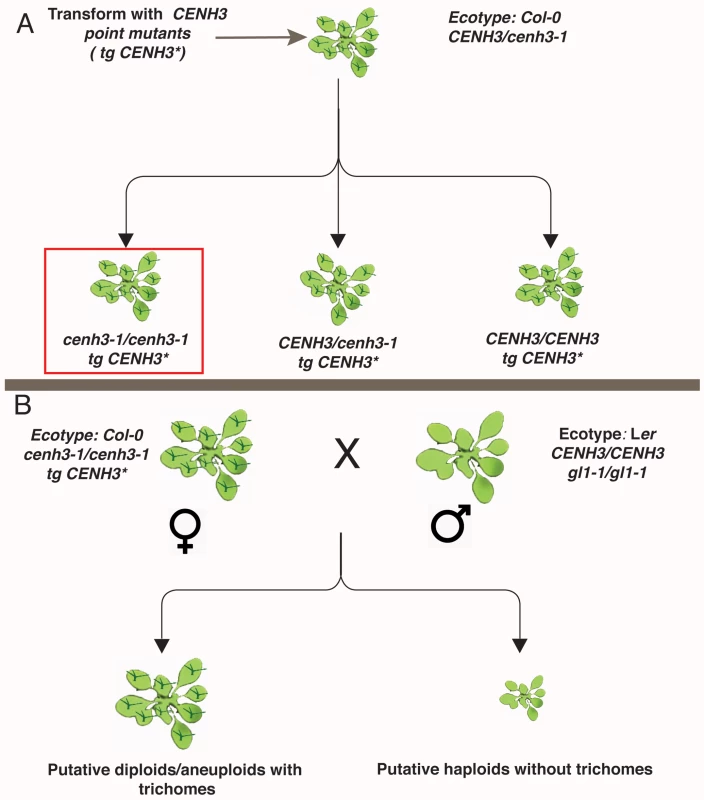
(A) Steps involved in generation of transgenic CENH3 point mutants in cenh3-1/cenh3-1 background indicated in the red box. (B) CENH3 point mutants in cenh3-1/cenh3-1 background in Col-0 ecotype with trichomes (GL1/GL1) were used as female parent and crossed by gl1-1/gl1-1 plants that carry wild-type allele for CENH3 (CENH3/CENH3) in Landsberg erecta ecotype. The possible outcomes and their phenotype are represented below. Trichomes are represented in dark green. Uniparental paternal haploids do not have trichomes a feature that was used for identification of haploids. tg CENH3* stands for transgenic CENH3 with point mutations. Transgenic plants expressing the single-amino acid substitutions P82S, G83E, P102S, A132T, A136T and G173E were also viable and fully fertile on self-pollination. These plants did not show any phenotypic difference compared to wild-type plants (S3 Fig). Analyses of pollen viability in these lines also showed that pollen from these transgenic point mutants appear normal (S4 Fig). Thus, the mutant transgenes were able to complement the cenh3-1 mutation both mitotically and meiotically. To determine whether the complemented lines were haploid inducers, we crossed them by Landsberg erecta glabrous1 (Ler gl1-1/gl1-1 CENH3/CENH3) (Fig 2B). These recessive er (compact growth habit) and gl1 (hairless leaves) mutations are on chromosome 2 and 3, respectively. Based on earlier research [21] we hypothesized that elimination of maternal chromosomes, might lead to the production of paternal haploids, which would then exhibit both the erecta and glabrous phenotypes. Crosses of our WT-HFD transgenics (cenh3-1/cenh3-1 expressing WT-HFD CENH3) with tester line (Ler gl1-1/gl1-1 CENH3/CENH3), produced 100% normal seeds without obvious induction of seed death, a trait associated with haploid induction, and 100% of the F1 progeny displayed wild-type phenotype, indicating that they were diploids carrying both maternal and paternal chromosomes.
The mutant P82S lines (cenh3-1/cenh3-1 expressing P82S-CENH3), when crossed with the same tester pollen (Ler gl1-1/gl1-1 CENH3/CENH3), produced 15–20% dead seeds, and of the viable offspring 2–3% were both erecta and glabrous, consistent with loss of the dominant maternal markers. These putative haploid plants were smaller than corresponding diploids (Fig 3A), trichomeless (Fig 3B and 3C) and sterile (Fig 3D), also consistent with haploidy. Analysis of putative haploids from the point mutant line by flow cytometry against the diploid control confirmed their haploid status. A sample plot of diploid control and haploid from mutant P82S is shown (Fig 3E and 3F). Cytogenetic analyses confirmed haploid content, corresponding to 5 chromosomes vs. 10 in diploids (Fig 3H and 3I). Similarly, G83E (cenh3-1/cenh3-1 expressing G83E-CENH3), A132T (cenh3-1/cenh3-1 expressing A132T-CENH3) and A136T (cenh3-1/cenh3-1 expressing A136T-CENH3) point mutants, while somatically normal and fully fertile on self-pollination, produced both aborted seeds and flow cytometry-confirmed haploid progeny, on crossing with tester pollen (Ler gl1-1/gl1-1 CENH3/CENH3) (Table 1). Notwithstanding the conservation of these amino acids among angiosperms (S2 Table) and the “not tolerated” prediction by SIFT, the phenotype of plants expressing the altered CENH3 in lieu of the wild-type CENH3 was indistinguishable from wild-type unless crossed with pollen carrying centromeres determined by wild-type CENH3. G173E (cenh3-1/cenh3-1 expressing G173E-CENH3), another mutation predicted “not tolerated”, appeared to be wild-type even on crossing by wild-type pollen. Similarly, a 6th mutation, P102S (cenh 3-1/cenh3-1 expressing P102S-CENH3), was predicted to be tolerated and indeed displayed no effect on CENH3 function.
Fig. 3. Haploid plants produced by genome elimination in crosses of CENH3 point mutants by Ler gl1-1. 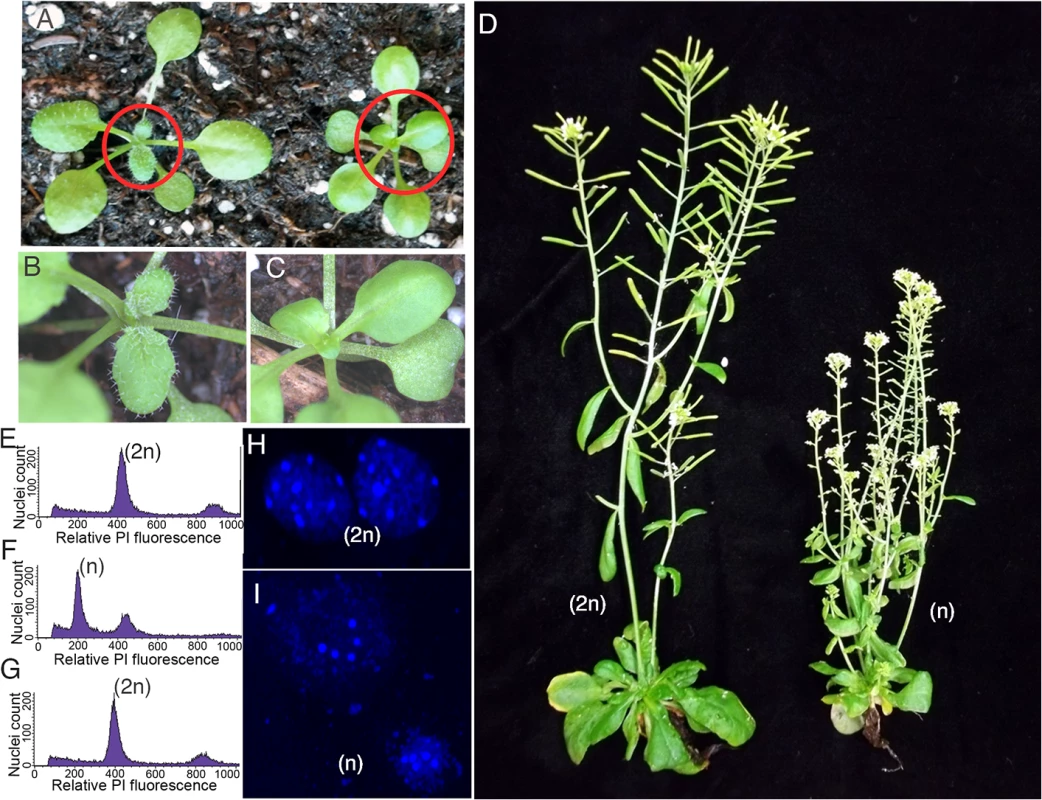
(A) Comparison of diploid hybrid with trichomes on the left and smaller haploid plant without trichomes on the right from haploid inducing cross. (B-C) Inset enlargements (region circled in red) show a diploid plant with trichomes on the left and a trichomeless haploid on the right. (D) Phenotype of a Ler gl1-1 haploid with undeveloped siliques on the right and a diploid Ler gl1-1 showing healthy siliques on the left. (E-G) Analysis of nuclei stained with propidium iodide (PI) by flow cytometry for a diploid control diploid (E), Ler gl1-1 glabrous haploid (F) offspring and a Ler gl1-1 doubled haploid (G). (H-I) DAPI stained nuclei of a diploid plant showing 10 chromocenters (H) and a haploid plant showing 5 chromocenters (I). Scale bars = 5 μm. Next, we performed whole genome sequencing on the resulting haploids to determine their genome contributions. A total of 41 glabrous plants (putative haploids based on phenotyping or flow cytometry) from haploid induction crosses were analyzed(S3 Table). On a genomic dosage plot [20, 28], true paternal haploids will appear euploid with no change in the relative copy number of each chromosome. These chromosomes, however, will carry only paternal sequences (Ler SNPs), in contrast to a true Col-0/Ler diploid from the cross that carries 50% Col-0 SNPs (Fig 4A). Of the 17 putative haploids from P82S crosses, 14 were euhaploids (Fig 4B). The remainders of the haploids were Ler plants carrying, in addition, parts of the Col-0 genome: one was disomic for Chr4 (Fig 4C), one contained a Chr4 minichromosome (Fig 4D) and one was disomic Chr4 and also had a Chr5-derived minichromosome. Analyses of 18 putative haploids from G83E showed that 17 were true Ler haploids except for one, which was a Chr4 disomic. Lastly, all 7 glabrous plants from the A136T cross were true Ler haploids.
Fig. 4. Characterization of haploid genotypes using whole-genome sequencing. 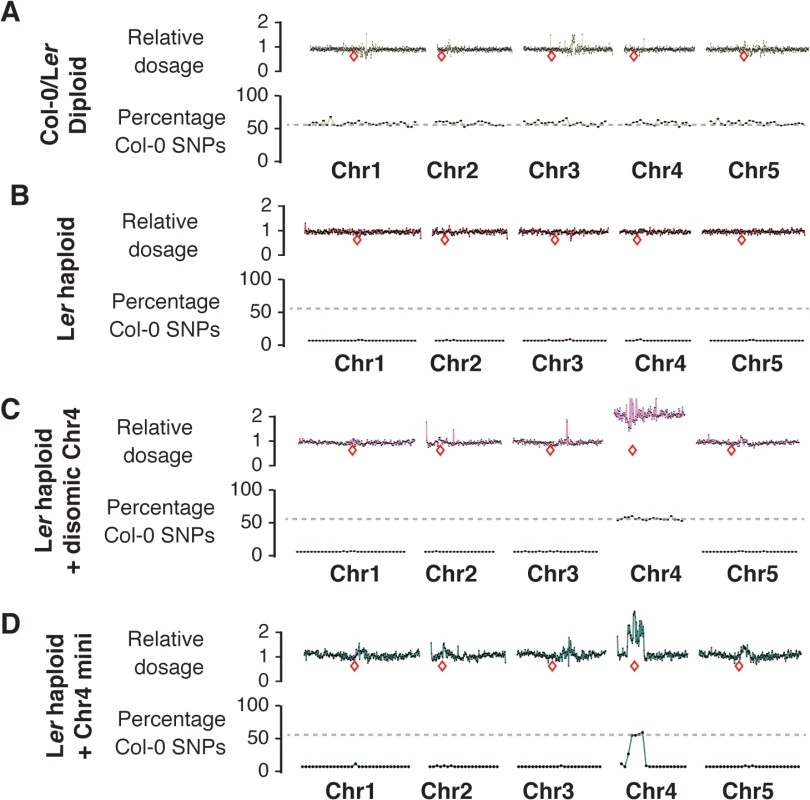
(A-D): Top panels show the dosage plots for non-overlapping 100 kb bins across all five Arabidopsis chromosomes with the relative dosage indicated on the y-axis. The bottom panels in each section show SNP analysis based on 1 Mb bins with the percentage of Col-0 SNPs plotted. Regions with 100% Ler SNPs will have 0% Col-0 SNPs. Relative locations of centromeres are indicated by a red box. A diploid Col/Ler hybrid control (A) is shown along with a Ler haploid (B). Aneuploid haploids such as a haploid with disomic Chr4 (C) and a Chr4 minichromosome (D) are shown here as well. To determine whether these putative haploids would spontaneously double to produce diploids, we allowed these (nearly sterile) plants to self-pollinate. All haploids plants from the mutants P82S and G83E produced seeds albeit at very low level (20–30 seeds/plant vs. several thousand for wild-type). The seeds were normal in appearance, germinated well and produced glabrous, erecta and fully fertile offspring. Analysis of ploidy by flow cytometry revealed that the 2C peak of these plants indeed matched the position of the 2C peak of Ler gl1-1 (Fig 3G). These diploid progeny of haploid plants might have arisen via the fortuitous fusion of gametes that were carrying a complete set of five chromosomes each, as has been previously observed in mutants of Arabidopsis in which the gametes segregate without pairing [29].
Our transgenic experiments suggest that a variety of mutations in conserved residues of the CENH3 histone fold domain may result in haploid-inducers that are normal in appearance and fully fertile on self-pollination, while inducing haploids on out-crossing. Thus, haploid inducers may exist among mutagenized populations, or even among natural variants. To test this hypothesis, we analyzed a TILLING (Targeting Induced Local Lesions IN Genomes) population generated by Henikoff and Comai [30]. The mutation density of this EMS(Ethylmethane sulfonate)-treated population was about 3.89 mutations per megabase [30] per plant. In a previous screen [30] of approximately 3000 plants from this population, 4 point mutations were found in the histone fold domain. Among these, one was a silent mutation. The remaining three were A86V, R176K and W178* (W178 to stop codon). Using SIFT, A86V and W178* were predicted to be “not tolerated” and R176K to be tolerated. However, W178 is the last amino acid of CENH3 and this residue is not conserved (Fig 1). We tested the haploid inducing potential of homozygous A86V plants by pollinating them with Ler gl1-1. The F1 seeds displayed 32% seed death, a trait found when other CENH3-based haploid inducers are crossed with wild type [11, 21, 31]. We found that 3/110 (2.7%) of the surviving F1 offspring were trichomeless, consistent with these being paternal haploids. Subsequently we created the same A86V mutation synthetically and transformed into CENH3/cenh3-1 plants. cenh3-1/cenh3-1 segregants expressing A86V-CENH3 when crossed by pollen from Ler gl-1/gl1-1 CENH3/CENH3 produced 3.87% haploids. This demonstrates the equivalency of the transgenic and mutational approach. Importantly, haploid inducing lines can be derived from existing populations of plants without transgenic manipulation, simply by screening for mutations in conserved residues of the histone fold domain.
In order to determine whether our simple four-species comparison (A. thaliana, B. rapa. S. lycopersicum, and Z. mays) was somehow unrepresentative of the diversity of CENH3 in angiosperms at these 7 residues, we searched additional published plant genomes to determine whether any of these species carry the amino acid substitutions described here. We found no changes in the HFD residues in 60 published Arabidopsis ecotypes [32], but found one amino change in the hypervariable N-terminal tail (S4 Table). Our comparison of 53 angiosperm sequences from 50 different species (S1 Fig, S5 Table), revealed that 5 of our 7 investigated amino acids displayed no variation at all, while 2 (equivalent positions P82 and G173) did exhibit some diversity (S5 Fig, S6 Table). One of these substitutions (P82S) confers a haploid inducer phenotype in Arabidopsis, displaying approx. 19% seed death on outcrossing. Evidently, this same amino acid change arose and persisted in 4 different clades of angiosperms, ranging from dicots to monocots (S5 Fig, S6 Table) [33].
Discussion
Our results on the effects of CENH3 single amino acid variation have two major implications, one basic, the other applied. On the basic side, our results reveal that some single amino acid substitutions can be as efficient as large-scale changes in producing haploid inducers. We found that altering single highly conserved amino acid residues in the histone fold domain results in fit and fertile plants that display postzygotic incompatibility and produce haploids when crossed to the wild type. Centromeres determined by point mutations in CENH3 specify efficient chromosome inheritance in self-crosses, but lead to missegregation in an F1 hybrid when confronted with centromeres determined by the wild-type CENH3. As a result, the hybrid embryo undergoes genome elimination, producing frequent abortion (which may be due to aneuploidy-induced failure of the embryo or endosperm), and aneuploidy or haploidy among the surviving seeds.
Using human cell lines, Tachiwana et al., 2011[34] have shown that mutations in CENP-A (human CENH3) HFD loop 1 residues R80 and G81 lead to reduced CENP-A retention in the centromere. CENP-A residues L111, L128 and I132 are involved in CENH3/CENH3 interaction [34, 35]. In addition, mutation in CID (Drosophila CENH3) D211 also results reduced dimerization and mislocalization of the protein [36]. Although we do not have complementation data on the corresponding residues in Arabidopsis CENH3, three of our point mutant haploid inducers, P82S, G83E and A86V, are located immediately before the α-N-helix (Fig 1). Based on the crystal structure of CENP-A, Tachiwana et al. [34] proposed that decreased length of the CENP-A α-N-helix compared the homologous region of H3 confers loose conformation to DNA at the entrance and exit of the CENH3 nucleosomes and that the residues corresponding to the At-CENH3 P82, G83, A86 interact with DNA. The loose connection of DNA to CENP-A nucleosome may be important for centromeric function [34]. These mutations may thus alter the fundamental properties of CENH3 nucleosome thus disrupting the normal behavior of centromeric chromatin.
Further in the HFD, two of our haploid inducing mutations, A132T and A136T, reside in the CATD domain, which in human CENP-A was shown to interact with HJURP [37], a factor necessary for efficient loading of CENP-A into nucleosomes. Even though the HJURP homolog has not been identified in plants, the KNL2 protein of A. thaliana is related to the factor that recruits HJURP to the centromere [19], suggesting some conservation in CENH3 recruitment to centromeric chromatin. The deleterious post-zygotic defects observed in hybrids of these mutants to wild-type CENH3, are consistent with the possibility of defective loading.
Plants carrying the haploid-inducing point mutations described above are fully fertile, thus the gametes produced by these plants obviously carry functional centromeres. However, when encountering centromeres from wild-type plants, the mutant-derived chromosomes missegregate frequently while the wild-type derived chromosomes segregate normally [11, 20, 21]. The striking difference between embryos that inherited the mutant CENH3 from both parents, and those that inherited a mutant and a wild-type allele implies that the mutant-determined centromeres are defective in the context of the wild-type ones. A competition may be set up for some as-of-yet unidentified aspect of centromere specification, kinetochore building, or spindle attachment. Zygotic reloading of CENH3 has been suggested by observation of GFP-tagged CENH3 by Ingouff et al [38]. Defective reloading of CENH3 has been detected in developing embryos in Hordeum crosses leading to natural genome elimination [39]. Accordingly, differential loading rate or density of CENH3 or other centromeric factor between wild-type and “mutant” centromeres could explain why centromeres determined by mutant CENH3 function well in self crosses but fail in out crosses.
Our choice of highly conserved amino acids as targets for mutagenesis was largely motivated by our desire to be able to translate our results to crop species. Given the fact that our plants are viable and fully self - fertile, our results raise the question of why these particular amino acids are conserved and what, if at all, is the evolutionary significance of the outcrossing incompatibility determined by the observed changes.
Survey of natural variation found that five of the seven changes are conserved. Although no deleterious effect is apparent in our analyses, it is possible that these changes may have hidden or conditional fitness consequences. Four out of the five amino acid substitutions tested result in a penalty on outcrossing, as a large fraction (up to approximately 30%) of outcross progeny spontaneously abort, and the mutant genome is lost from among a smaller fraction of the survivors. It is possible that this outcrossing penalty alone is sufficient to explain the purifying selection of the residues at these particular positions (G83, A86, A132, A136).
Two of the residues we tested were not conserved among angiosperms. One of these substitutions (P82S) is a haploid inducer in Arabidopsis, displaying approx. 19% seed death on outcrossing. Nevertheless, this same amino acid change apparently arose and persisted in 4 different clades of angiosperms, ranging from dicots to monocots (S5 Fig, S6 Table) [33]. While we have yet to determine whether this mutation has a reproductively isolating effect in any species other than Arabidopsis, this result suggests that the mutation is well tolerated (as are P82A and P82V). In conclusion, alleles found to be HI-inducing in Arabidopsis have been evolutionarily successful in other plant species.
On the applied side, our findings are relevant to plant breeding. Haploids, which can be doubled to produce perfect homozygotes [21, 40–43] greatly accelerate plant breeding [40], genome assembly from sequence reads in heterozygous species[44], the production of recombinant inbred lines [45] and genetic analysis [31], but are not available for many crop species. Haploid induction through the chimeric version of CENH3 (GFP-tailswap) has been demonstrated in reverse breeding[46], synthetic clonal reproduction [47] and rapid QTL mapping [45] in the model plant Arabidopsis thaliana, underscoring the potential of this method [48]. The delayed application in crop plants, however, indicates the difficulties in engineering a system that requires combining a chimeric transgene with a knockout of the endogenous gene. A single-step, non-transgenic haploid inducer system such as described here overcomes this shortcoming (Fig 5). To extend its applicability across plant species, this study focused on amino acid residues conserved in angiosperms. It is plausible that single amino acid changes in variable or less conserved residues may have similar effects. The point mutants of CENH3 that can produce uniparental haploids, all G:C to A:T transitions, are readily identified in existing TILLING [49] populations and so can be immediately applied to crop species, or could be induced in a single step by CRISPR-Cas9 mediated changes. Our analysis suggests that there are 47 highly conserved, EMS-mutable targets in the CENH3 histone fold domain, of which 38 are predicted by SIFT to be “not tolerated”. Given the frequency at which we identified haploid inducers among the mutations predicted “not tolerated” by SIFT (4 out of 5 tested), our results suggest that our list of 38 mutable sites, if found to be able to complement the cenh3-1 null (Fig 1 and S1 Table), would be excellent candidates for haploid induction.
Fig. 5. Schematic comparison of transgenic two-step vs. non-transgenic one step haploid inducers. 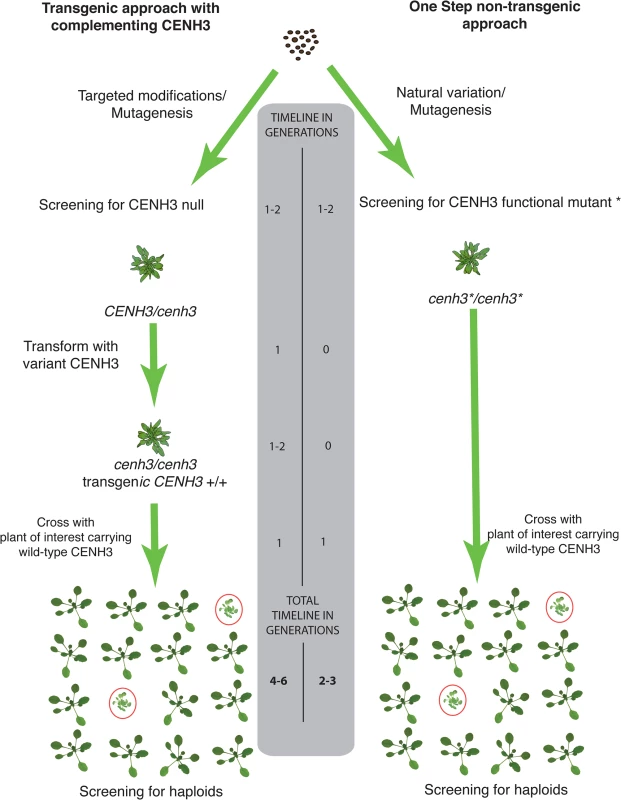
In the first approach represented on the left, a CENH3 knockout can be generated by CRISPR-CAS9 or identified from an EMS mutagenized population and complemented with an altered version of CENH3. On the right, the one step non-transgenic approach functional point mutants are identified by TILLING or from natural variation and used directly as haploid inducers. A comparison of the estimated generation times for each approach is shown in the center. Materials and Methods
Cloning and transformation: Binary vector pCAMBIA-1300 (GenBank: AF234296.1) was used for cloning. The native CENH3 promoter, 5’ UTR and 3’ UTR were cloned into this vector for earlier studies[21, 50]. This clone was used as a starting vector for our study. Cloning was done in three steps. Step 1: CENH3 tail region with introns until first half of intron before HFD was cloned into the KpnI, XbaI site between 5’ and 3’ UTR. Step 2: fragment containing attR1 and attR2 site with CcdB resistance gene was cloned between the CENH3 tail and 3’ UTR into BglI and XbaI site. Step 3: WT-HFD and the point mutants flanked by attL1 and attL2 were synthesized without introns through Genewiz Inc. LR recombination was done to obtain the complete CENH3 and transformed into E. coli strain DH5α. The destination vectors were sequenced and transformed into Agrobacterium tumefaciens GV3101 strain and used for Arabidopsis transformation by conventional floral dip method [51].
Plant growth conditions
Plants were grown in Sunshine professional growing mix # 1 (Sun Gro Horticulture) at 16h/8h light/dark cycles at 20°C in Conviron walk in chamber with a relative humidity of 50–55%.
DNA extraction and genotyping
The plants were screened on hygromycin selection for T-DNA integration. The antibiotic resistant lines were analyzed for native CENH3 loci by two-step genotyping method. Genomic DNA was extracted from two-week-old seedling using standard methods using CTAB buffer. First a 3 KB region specific to the native CENH3 was amplified. The first round amplification was targeted with primers flanking 2 kb upstream of the start codon of the native CENH3 and within intron number 1 of the histone fold domain (HFD). Second round of PCR was performed with specific dCAPS primers to amplify a specific 210bp fragment flanking the site of mutation. The second round PCR product was digested with XbaI for overnight and resolved on 2.5% agarose gel.
Crossing and analysis of F1 offspring
Lines carrying transgene with that were homozygous for cenh3-1/cenh3-1 at the native CENH3 loci were used as female parent in the crossing. Young flower buds were emasculated and crossed by pollen from Ler gl-1/gl1-1 CENH3/CENH3. The seeds were harvested after three weeks. Seed death was assessed under dissection microscope. Offspring were phenotyped for glabrous (as 2 week old seedlings, under a dissecting microscope) and erecta (as 2 month old plants) traits and subsequently analyzed by flow cytometry and chromosome count
Flow cytometry
Flow cytometric determination of genome content of the wild-type, putative haploids and double haploids were done as described [52]. Young unopened flower buds were used for nuclei isolation. The samples were chopped vigorously in chopping buffer (15mM HEPES, 1mM EDTA, 80mM KCL, 20mM NaCL, 300mM sucrose, 0.20% triton-X (for nuclei stability), 0.5mM spermine)using new razor blade (VWR Cat. #55411–050) and filtered through Falcon Blue Nylon Mesh Cell Strainer, 40 Micron (BD 352340). The flow through was then centrifuged at 500g for 7 minutes at 4°C. The nuclear pellet was then washed twice in 0.5 ml of chopping buffer. The samples were then resuspended in 0.5 ml of ice-cold-staining solution containing propidium iodide. Flowcytometric analysis was done on Becton Dickinson FACScan Flow Cytometer equipped with dual laser of 488 and 635nm wavelength and five photodetectors. The data was acquired and analyzed using the Cellquest software.
Chromosome count
Chromosome count of the wild-type, haploids and double haploids were performed as described in[53]. Young flower buds were fixed in Carnoy’s fixative overnight and washed three times in 70% ethanol. Mature flower buds were dissected out and young flower buds at the apex of the inflorescence were selected for further treatment. The young flower buds were then digested with 3% cellulase and 3% pectinase for three hours. Following digestion, the flower buds were dissected under a microscope to isolate anthers. After removing the debris, the anthers were teased to release the cells followed by addition of 60% acetic acid. The cells were spread using 60 ul of ice-cold 3 : 1 Ethanol acetic acid. The slides were allowed to dry and DAPI (1 um) stain was added. The slides were visualized under Zeiss LSM 710 confocal microscope and the image were acquired using Zeiss image processing software.
Imaging of haploids and control
Seedlings were allowed to germinate on MS plates for two weeks. The presence/absence of trichomes on the first true leaves were observed and imaged using a dissection microscope equipped with Carl Zeiss Axiocam color HRc camera for imaging. The image was acquired with Zeiss software. To observe the fertility phenotype, plants were allowed to grow for 6–7 weeks. Wild-type control and haploid plant of same age grown under same conditions were imaged using Kodak easyshare 14MP camera equipped with AF3x optical Aspheric lens 32mm-96 mm.
Whole genome sequencing
DNA extraction was performed using Nucleon PhytoPure DNA extraction kit (GE Healthcare Life Sciences Inc.). DNA was sheared to 300–400 bp fragments using Covaris E220 sonicator under following settings: Peak incident power 175, duty factor 5%, cycle per burst 200, treatment time 60s at 7°C. Library prep for illumina sequencing was done using standard NEB next DNA Library prep and BIOO Scientific NEXTFlex-96 adapters were used. Samples were pooled and sequenced on MiSeq 2500 for 50bp single reads. The resulting reads were further analyzed as described in[28].
Pollen fertility assay
Pollen fertility assay was performed using standard staining protocol as described in[54]. Anthers from the unopened flower bud were dissected and stained with the staining solution containing 1% each of Malchite green, Acid fuchsin and Orange G. Images were acquired using Nikon eclipse E600 microscope equipped with Nikon Digital sight DS5C camera under 10X magnification using NIS Elements software version F3.0.
Alignment of protein sequences
Protein sequences were downloaded from GeneBank (http://www.ncbi.nlm.nih.gov/genbank/). The CENH3/CENP-A annotated tail regions were removed from the sequences and the histone fold domains were aligned using Geneious software version 6.0.5[55] using the ClustalW alignment method with the following conditions: Cost matrix: Blosum, Gap open cost: 10 and Gap extend cost: 0.1.
Supporting Information
Zdroje
1. Steiner FA, Henikoff S. Diversity in the organization of centromeric chromatin. Current Opinion in Genetics & Development. 2015;31(0):28–35. doi: http://dx.doi.org/10.1016/j.gde.2015.03.010.
2. Fukagawa T, Earnshaw William C. The Centromere: Chromatin Foundation for the Kinetochore Machinery. Developmental Cell. 30(5):496–508. doi: 10.1016/j.devcel.2014.08.016 25203206
3. Cheeseman IM. The Kinetochore. Cold Spring Harbor Perspectives in Biology. 2014;6(7). doi: 10.1101/cshperspect.a015826
4. Duro E, Marston AL. From equator to pole: splitting chromosomes in mitosis and meiosis. Genes & Development. 2015;29(2):109–22. doi: 10.1101/gad.255554.114
5. Allshire RC, Karpen GH. Epigenetic regulation of centromeric chromatin: old dogs, new tricks? Nat Rev Genet. 2008;9(12):923–37. doi: 10.1038/nrg2466 19002142
6. Sekulic N, Black BE. Molecular underpinnings of centromere identity and maintenance. Trends in Biochemical Sciences. 37(6):220–9. doi: 10.1016/j.tibs.2012.01.003 22410197
7. Palmer DK, O'Day K, Trong HL, Charbonneau H, Margolis RL. Purification of the centromere-specific protein CENP-A and demonstration that it is a distinctive histone. Proceedings of the National Academy of Sciences. 1991;88(9):3734–8. doi: 10.1073/pnas.88.9.3734
8. Earnshaw W, Migeon B. Three related centromere proteins are absent from the inactive centromere of a stable isodicentric chromosome. Chromosoma. 1985;92(4):290–6. doi: 10.1007/BF00329812 2994966
9. Talbert PB, Masuelli R, Tyagi AP, Comai L, Henikoff S. Centromeric Localization and Adaptive Evolution of an Arabidopsis Histone H3 Variant. The Plant Cell Online. 2002;14(5):1053–66. doi: 10.1105/tpc.010425
10. Dalal Y, Furuyama T, Vermaak D, Henikoff S. Structure, dynamics, and evolution of centromeric nucleosomes. Proceedings of the National Academy of Sciences. 2007;104(41):15974–81. doi: 10.1073/pnas.0707648104
11. Maheshwari S, Tan EH, West A, Franklin FCH, Comai L, Chan SWL. Naturally Occurring Differences in CENH3 Affect Chromosome Segregation in Zygotic Mitosis of Hybrids. Plos Genet. 2015;11(1):e1004970. doi: 10.1371/journal.pgen.1004970 25622028
12. Malik HS, Henikoff S. Phylogenomics of the nucleosome. Nat Struct Mol Biol. 2003;10(11):882–91.
13. Stoler S, Keith KC, Curnick KE, Fitzgerald-Hayes M. A mutation in CSE4, an essential gene encoding a novel chromatin-associated protein in yeast, causes chromosome nondisjunction and cell cycle arrest at mitosis. Genes & Development. 1995;9(5):573–86. doi: 10.1101/gad.9.5.573
14. Buchwitz BJ, Ahmad K, Moore LL, Roth MB, Henikoff S. Cell division: A histone-H3-like protein in C. elegans. Nature. 1999;401(6753):547–8. 10524621
15. Howman EV, Fowler KJ, Newson AJ, Redward S, MacDonald AC, Kalitsis P, et al. Early disruption of centromeric chromatin organization in centromere protein A (Cenpa) null mice. Proceedings of the National Academy of Sciences. 2000;97(3):1148–53. doi: 10.1073/pnas.97.3.1148
16. Ravi M, Kwong PN, Menorca RMG, Valencia JT, Ramahi JS, Stewart JL, et al. The Rapidly Evolving Centromere-Specific Histone Has Stringent Functional Requirements in Arabidopsis thaliana. Genetics. 2010;186(2):461–71. doi: 10.1534/genetics.110.120337 20628040
17. Dunleavy EM, Roche D, Tagami H, Lacoste N, Ray-Gallet D, Nakamura Y, et al. HJURP Is a Cell-Cycle-Dependent Maintenance and Deposition Factor of CENP-A at Centromeres. Cell. 2009;137(3):485–97. doi: http://dx.doi.org/10.1016/j.cell.2009.02.040. doi: 10.1016/j.cell.2009.02.040 19410545
18. Mishra PK, Au W-C, Choy JS, Kuich PH, Baker RE, Foltz DR, et al. Misregulation of Scm3p/HJURP Causes Chromosome Instability in Saccharomyces cerevisiae and Human Cells. Plos Genet. 2011;7(9):e1002303. doi: 10.1371/journal.pgen.1002303 PMC3183075. 21980305
19. Lermontova I, Kuhlmann M, Friedel S, Rutten T, Heckmann S, Sandmann M, et al. Arabidopsis KINETOCHORE NULL2 is an upstream component for centromeric histone H3 variant cenH3 deposition at centromeres. The Plant Cell. 2013;25(9):3389–404. doi: 10.1105/tpc.113.114736 24014547
20. Tan EH, Henry IM, Ravi M, Bradnam KR, Mandakova T, Marimuthu MP, et al. Catastrophic chromosomal restructuring during genome elimination in plants. eLife. 2015:e06516.
21. Ravi M, Chan SWL. Haploid plants produced by centromere-mediated genome elimination. Nature. 2010;464(7288):615–8. doi: http://www.nature.com/nature/journal/v464/n7288/suppinfo/nature08842_S1.html. doi: 10.1038/nature08842 20336146
22. Lermontova I, Koroleva O, Rutten T, Fuchs J, Schubert V, Moraes I, et al. Knockdown of CENH3 in Arabidopsis reduces mitotic divisions and causes sterility by disturbed meiotic chromosome segregation. The Plant Journal. 2011;68(1):40–50. doi: 10.1111/j.1365-313X.2011.04664.x 21635586
23. Forster BP, Thomas WTB. Doubled Haploids in Genetics and Plant Breeding. Plant Breeding Reviews: John Wiley & Sons, Inc.; 2010. p. 57–88.
24. Wędzony M, Forster BP, Żur I, Golemiec E, Szechyńska-Hebda M, Dubas E, et al. Progress in Doubled Haploid Technology in Higher Plants. In: Touraev A, Forster B, Jain SM, editors. Advances in Haploid Production in Higher Plants: Springer Netherlands; 2009. p. 1–33.
25. Tester M, Langridge P. Breeding Technologies to Increase Crop Production in a Changing World. Science. 2010;327(5967):818–22. doi: 10.1126/science.1183700 20150489
26. Ng PC, Henikoff S. Predicting the Effects of Amino Acid Substitutions on Protein Function. Annual Review of Genomics and Human Genetics. 2006;7(1):61–80. doi: 10.1146/annurev.genom.7.080505.115630 16824020.
27. Kumar P, Henikoff S, Ng PC. Predicting the effects of coding non-synonymous variants on protein function using the SIFT algorithm. Nat Protocols. 2009;4(8):1073–81.
28. Henry IM, Dilkes BP, Miller ES, Burkart-Waco D, Comai L. Phenotypic Consequences of Aneuploidy in Arabidopsis thaliana. Genetics. 2010;186(4):1231–45. doi: 10.1534/genetics.110.121079 20876566
29. Cifuentes M, Rivard M, Pereira L, Chelysheva L, Mercier R. Haploid Meiosis in Arabidopsis: Double-Strand Breaks Are Formed and Repaired but Without Synapsis and Crossovers. PLoS ONE. 2013;8(8):e72431. doi: 10.1371/journal.pone.0072431 23951324
30. Till BJ, Reynolds SH, Greene EA, Codomo CA, Enns LC, Johnson JE, et al. Large-scale discovery of induced point mutations with high-throughput TILLING. Genome research. 2003;13(3):524–30. 12618384
31. Ravi M, Marimuthu MPA, Tan EH, Maheshwari S, Henry IM, Marin-Rodriguez B, et al. A haploid genetics toolbox for Arabidopsis thaliana. Nat Commun. 2014;5. doi: 10.1038/ncomms6334
32. Cao J, Schneeberger K, Ossowski S, Gunther T, Bender S, Fitz J, et al. Whole-genome sequencing of multiple Arabidopsis thaliana populations. Nat Genet. 2011;43(10):956–63. doi: http://www.nature.com/ng/journal/v43/n10/abs/ng.911.html—supplementary-information. doi: 10.1038/ng.911 21874002
33. Hirsch CD, Wu Y, Yan H, Jiang J. Lineage-Specific Adaptive Evolution of the Centromeric Protein CENH3 in Diploid and Allotetraploid Oryza Species. Molecular Biology and Evolution. 2009;26(12):2877–85. doi: 10.1093/molbev/msp208 19741004
34. Tachiwana H, Kagawa W, Shiga T, Osakabe A, Miya Y, Saito K, et al. Crystal structure of the human centromeric nucleosome containing CENP-A. Nature. 2011;476(7359):232–5. doi: http://www.nature.com/nature/journal/v476/n7359/abs/nature10258.html—supplementary-information. doi: 10.1038/nature10258 21743476
35. Sekulic N, Bassett EA, Rogers DJ, Black BE. The structure of (CENP-A-H4)2 reveals physical features that mark centromeres. Nature. 2010;467(7313):347–51. doi: http://www.nature.com/nature/journal/v467/n7313/abs/nature09323.html—supplementary-information. doi: 10.1038/nature09323 20739937
36. Zhang W, Colmenares Serafin U, Karpen Gary H. Assembly of Drosophila Centromeric Nucleosomes Requires CID Dimerization. Molecular Cell. 2012;45(2):263–9. doi: http://dx.doi.org/10.1016/j.molcel.2011.12.010. doi: 10.1016/j.molcel.2011.12.010 22209075
37. Bassett Emily A, DeNizio J, Barnhart-Dailey Meghan C, Panchenko T, Sekulic N, Rogers Danielle J, et al. HJURP Uses Distinct CENP-A Surfaces to Recognize and to Stabilize CENP-A/Histone H4 for Centromere Assembly. Developmental Cell. 2012;22(4):749–62. doi: http://dx.doi.org/10.1016/j.devcel.2012.02.001. doi: 10.1016/j.devcel.2012.02.001 22406139
38. Ingouff M, Rademacher S, Holec S, Šoljić L, Xin N, Readshaw A, et al. Zygotic Resetting of the HISTONE 3 Variant Repertoire Participates in Epigenetic Reprogramming in Arabidopsis. Current Biology. 2010;20(23):2137–43. doi: http://dx.doi.org/10.1016/j.cub.2010.11.012. doi: 10.1016/j.cub.2010.11.012 21093266
39. Sanei M, Pickering R, Kumke K, Nasuda S, Houben A. Loss of centromeric histone H3 (CENH3) from centromeres precedes uniparental chromosome elimination in interspecific barley hybrids. Proceedings of the National Academy of Sciences. 2011;108(33):E498–E505. doi: 10.1073/pnas.1103190108
40. Forster BP, Heberle-Bors E, Kasha KJ, Touraev A. The resurgence of haploids in higher plants. Trends in Plant Science. 2007;12(8):368–75. doi: http://dx.doi.org/10.1016/j.tplants.2007.06.007. 17629539
41. Chan SWL. Chromosome engineering: power tools for plant genetics. Trends in Biotechnology. 28(12):605–10. doi: 10.1016/j.tibtech.2010.09.002 20933291
42. Dunwell JM. Haploids in flowering plants: Origins and exploitation. Plant Biotechnol Journal. 2010; 8 : 377–424.
43. Murovec J, Bohanec B. Haploids and Doubled Haploids in Plant Breeding. In: Abdurakhmonov I, editor. Plant Breeding2012.
44. Feuillet C, Leach JE, Rogers J, Schnable PS, Eversole K. Crop genome sequencing: lessons and rationales. Trends in Plant Science. 2011;16(2):77–88. doi: http://dx.doi.org/10.1016/j.tplants.2010.10.005. doi: 10.1016/j.tplants.2010.10.005 21081278
45. Seymour DK, Filiault DL, Henry IM, Monson-Miller J, Ravi M, Pang A, et al. Rapid creation of Arabidopsis doubled haploid lines for quantitative trait locus mapping. Proceedings of the National Academy of Sciences. 2012 : 4227–32. doi: 10.1073/pnas.1117277109
46. Wijnker E, van Dun K, de Snoo CB, Lelivelt CLC, Keurentjes JJB, Naharudin NS, et al. Reverse breeding in Arabidopsis thaliana generates homozygous parental lines from a heterozygous plant. Nat Genet. 2012;44(4):467–70. doi: http://www.nature.com/ng/journal/v44/n4/abs/ng.2203.html—supplementary-information. doi: 10.1038/ng.2203 22406643
47. Marimuthu MPA, Jolivet S, Ravi M, Pereira L, Davda JN, Cromer L, et al. Synthetic Clonal Reproduction Through Seeds. Science. 2011;331(6019):876. doi: 10.1126/science.1199682 21330535
48. Ravi M, Marimuthu MPA, Tan EH, Maheshwari S. A haploid genetics toolbox for Arabidopsis thaliana. Nature Communications. In Press.
49. Comai L, Henikoff S. TILLING: practical single-nucleotide mutation discovery. The Plant Journal. 2006;45(4):684–94. doi: 10.1111/j.1365-313X.2006.02670.x 16441355
50. Ravi M, Shibata F, Ramahi JS, Nagaki K, Chen CB, Murata M, et al. Meiosis-Specific Loading of the Centromere-Specific Histone CENH3 in Arabidopsis thaliana. Plos Genet. 2011;7(6). doi: Artn E1002121 doi: 10.1371/Journal.Pgen.1002121 WOS:000292386300037.
51. Clough SJ, Bent AF. Floral dip: a simplified method forAgrobacterium‐mediated transformation ofArabidopsis thaliana. The plant journal. 1998;16(6):735–43. 10069079
52. Henry IM, Dilkes BP, Young K, Watson B, Wu H, Comai L. Aneuploidy and Genetic Variation in the Arabidopsis thaliana Triploid Response. Genetics. 2005;170(4):1979–88. doi: 10.1534/genetics.104.037788 15944363
53. Armstrong SJ, Franklin FCH, Jones GH. Nucleolus-associated telomere clustering and pairing precede meiotic chromosome synapsis in Arabidopsis thaliana. Journal of Cell Science. 2001;114(23):4207–17.
54. Peterson R, Slovin JP, Chen C. A simplified method for differential staining of aborted and non-aborted pollen grains. International Journal of Plant Biology. 2010;1(2):13.
55. Kearse M, Moir R, Wilson A, Stones-Havas S, Cheung M, Sturrock S, et al. Geneious Basic: an integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics. 2012;28(12):1647–9. doi: 10.1093/bioinformatics/bts199 22543367
Štítky
Genetika Reprodukční medicína
Článek The Chromatin Protein DUET/MMD1 Controls Expression of the Meiotic Gene during Male Meiosis inČlánek Tissue-Specific Gain of RTK Signalling Uncovers Selective Cell Vulnerability during Embryogenesis
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2015 Číslo 9- Akutní intermitentní porfyrie
- Farmakogenetické testování pomáhá předcházet nežádoucím efektům léčiv
- Růst a vývoj dětí narozených pomocí IVF
- Hypogonadotropní hypogonadismus u žen a vliv na výsledky reprodukce po IVF
- Molekulární vyšetření pro stanovení prognózy pacientů s chronickou lymfocytární leukémií
-
Všechny články tohoto čísla
- Retraction: RNAi-Dependent and Independent Control of LINE1 Accumulation and Mobility in Mouse Embryonic Stem Cells
- Signaling from Within: Endocytic Trafficking of the Robo Receptor Is Required for Midline Axon Repulsion
- A Splice Region Variant in Lowers Non-high Density Lipoprotein Cholesterol and Protects against Coronary Artery Disease
- The Chromatin Protein DUET/MMD1 Controls Expression of the Meiotic Gene during Male Meiosis in
- A NIMA-Related Kinase Suppresses the Flagellar Instability Associated with the Loss of Multiple Axonemal Structures
- Slit-Dependent Endocytic Trafficking of the Robo Receptor Is Required for Son of Sevenless Recruitment and Midline Axon Repulsion
- Expression of Concern: Protein Under-Wrapping Causes Dosage Sensitivity and Decreases Gene Duplicability
- Mutagenesis by AID: Being in the Right Place at the Right Time
- Identification of as a Genetic Modifier That Regulates the Global Orientation of Mammalian Hair Follicles
- Bridges Meristem and Organ Primordia Boundaries through , , and during Flower Development in
- Evaluating the Performance of Fine-Mapping Strategies at Common Variant GWAS Loci
- KLK5 Inactivation Reverses Cutaneous Hallmarks of Netherton Syndrome
- Differential Expression of Ecdysone Receptor Leads to Variation in Phenotypic Plasticity across Serial Homologs
- Receptor Polymorphism and Genomic Structure Interact to Shape Bitter Taste Perception
- Cognitive Function Related to the Gene Acquired from an LTR Retrotransposon in Eutherians
- Critical Function of γH2A in S-Phase
- Arabidopsis AtPLC2 Is a Primary Phosphoinositide-Specific Phospholipase C in Phosphoinositide Metabolism and the Endoplasmic Reticulum Stress Response
- XBP1-Independent UPR Pathways Suppress C/EBP-β Mediated Chondrocyte Differentiation in ER-Stress Related Skeletal Disease
- Integration of Genome-Wide SNP Data and Gene-Expression Profiles Reveals Six Novel Loci and Regulatory Mechanisms for Amino Acids and Acylcarnitines in Whole Blood
- A Genome-Wide Association Study of a Biomarker of Nicotine Metabolism
- Cell Cycle Regulates Nuclear Stability of AID and Determines the Cellular Response to AID
- A Genome-Wide Association Analysis Reveals Epistatic Cancellation of Additive Genetic Variance for Root Length in
- Tissue-Specific Gain of RTK Signalling Uncovers Selective Cell Vulnerability during Embryogenesis
- RAB-10-Dependent Membrane Transport Is Required for Dendrite Arborization
- Basolateral Endocytic Recycling Requires RAB-10 and AMPH-1 Mediated Recruitment of RAB-5 GAP TBC-2 to Endosomes
- Dynamic Contacts of U2, RES, Cwc25, Prp8 and Prp45 Proteins with the Pre-mRNA Branch-Site and 3' Splice Site during Catalytic Activation and Step 1 Catalysis in Yeast Spliceosomes
- ARID1A Is Essential for Endometrial Function during Early Pregnancy
- Predicting Carriers of Ongoing Selective Sweeps without Knowledge of the Favored Allele
- An Interaction between RRP6 and SU(VAR)3-9 Targets RRP6 to Heterochromatin and Contributes to Heterochromatin Maintenance in
- Photoreceptor Specificity in the Light-Induced and COP1-Mediated Rapid Degradation of the Repressor of Photomorphogenesis SPA2 in Arabidopsis
- Autophosphorylation of the Bacterial Tyrosine-Kinase CpsD Connects Capsule Synthesis with the Cell Cycle in
- Multimer Formation Explains Allelic Suppression of PRDM9 Recombination Hotspots
- Rescheduling Behavioral Subunits of a Fixed Action Pattern by Genetic Manipulation of Peptidergic Signaling
- A Gene Regulatory Program for Meiotic Prophase in the Fetal Ovary
- Cell-Autonomous Gβ Signaling Defines Neuron-Specific Steady State Serotonin Synthesis in
- Discovering Genetic Interactions in Large-Scale Association Studies by Stage-wise Likelihood Ratio Tests
- The RCC1 Family Protein TCF1 Regulates Freezing Tolerance and Cold Acclimation through Modulating Lignin Biosynthesis
- The AMPK, Snf1, Negatively Regulates the Hog1 MAPK Pathway in ER Stress Response
- The Parkinson’s Disease-Associated Protein Kinase LRRK2 Modulates Notch Signaling through the Endosomal Pathway
- Multicopy Single-Stranded DNA Directs Intestinal Colonization of Enteric Pathogens
- Recurrent Domestication by Lepidoptera of Genes from Their Parasites Mediated by Bracoviruses
- Three Different Pathways Prevent Chromosome Segregation in the Presence of DNA Damage or Replication Stress in Budding Yeast
- Identification of Four Mouse Diabetes Candidate Genes Altering β-Cell Proliferation
- The Intolerance of Regulatory Sequence to Genetic Variation Predicts Gene Dosage Sensitivity
- Synergistic and Dose-Controlled Regulation of Cellulase Gene Expression in
- Genome Sequence and Transcriptome Analyses of : Metabolic Tools for Enhanced Algal Fitness in the Prominent Order Prymnesiales (Haptophyceae)
- Ty3 Retrotransposon Hijacks Mating Yeast RNA Processing Bodies to Infect New Genomes
- FUS Interacts with HSP60 to Promote Mitochondrial Damage
- Point Mutations in Centromeric Histone Induce Post-zygotic Incompatibility and Uniparental Inheritance
- Genome-Wide Association Study with Targeted and Non-targeted NMR Metabolomics Identifies 15 Novel Loci of Urinary Human Metabolic Individuality
- Outer Hair Cell Lateral Wall Structure Constrains the Mobility of Plasma Membrane Proteins
- A Large-Scale Functional Analysis of Putative Target Genes of Mating-Type Loci Provides Insight into the Regulation of Sexual Development of the Cereal Pathogen
- A Genetic Selection for Mutants Reveals an Interaction between DNA Polymerase IV and the Replicative Polymerase That Is Required for Translesion Synthesis
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Arabidopsis AtPLC2 Is a Primary Phosphoinositide-Specific Phospholipase C in Phosphoinositide Metabolism and the Endoplasmic Reticulum Stress Response
- Bridges Meristem and Organ Primordia Boundaries through , , and during Flower Development in
- KLK5 Inactivation Reverses Cutaneous Hallmarks of Netherton Syndrome
- XBP1-Independent UPR Pathways Suppress C/EBP-β Mediated Chondrocyte Differentiation in ER-Stress Related Skeletal Disease
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



