-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Rhoptry Proteins ROP5 and ROP18 Are Major Murine Virulence Factors in Genetically Divergent South American Strains of
Parasites and the hosts they infect are in constant struggle with each other for survival. On the one hand, the host needs to control parasite growth, while the parasite needs to evade the host response long enough to allow for efficient transmission. The parasite Toxoplasma gondii has evolved virulence factors ROP5 and ROP18 to evade innate immune mechanisms of its natural intermediate host, small rodents. These genes were initially identified in clonal parasite types isolated from Europe and North America, but the factors that contribute to virulence in genetically divergent South American strains have not been tested. Here we used forward and reverse genetic analyses to show that ROP5 and ROP18 are also major virulence factors in genetically distinct virulent South American strains. Given that ROP5 and ROP18 function as virulence factors in strains from North America, Europe, and South America they likely acquired their functions before Toxoplasma gondii radiated into its present global population structure.
Published in the journal: . PLoS Genet 11(8): e32767. doi:10.1371/journal.pgen.1005434
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1005434Summary
Parasites and the hosts they infect are in constant struggle with each other for survival. On the one hand, the host needs to control parasite growth, while the parasite needs to evade the host response long enough to allow for efficient transmission. The parasite Toxoplasma gondii has evolved virulence factors ROP5 and ROP18 to evade innate immune mechanisms of its natural intermediate host, small rodents. These genes were initially identified in clonal parasite types isolated from Europe and North America, but the factors that contribute to virulence in genetically divergent South American strains have not been tested. Here we used forward and reverse genetic analyses to show that ROP5 and ROP18 are also major virulence factors in genetically distinct virulent South American strains. Given that ROP5 and ROP18 function as virulence factors in strains from North America, Europe, and South America they likely acquired their functions before Toxoplasma gondii radiated into its present global population structure.
Introduction
Intracellular parasites have to contend with immune responses mounted by the hosts they infect if they are to survive long enough to effectively transmit. The parasite Toxoplasma gondii is one of the more successful parasites in terms of transmission as it can infect all mammals and many birds [1], both of which serve as intermediate hosts where the parasite propagates asexually. The parasite undergoes sexual recombination only within the intestinal tract of members of the Felidae family when these predators ingest chronically infected intermediate hosts [2]. Transmission by this predator-prey life cycle has shaped the co-evolution of parasite virulence factors and host responses.
T. gondii readily infects humans but mainly causes disease in situations where the immune system has become compromised, although associations between parasite genotype and disease severity may also occur in healthy individuals [3]. The association of virulence with parasite genotypes has been more fully investigated in laboratory mice, where this trait can be more easily assessed and where the parasite has likely had more opportunities to evolve within a host that is common prey of cats. Initially it was shown that parasite isolates from Europe and North America differed in their ability to cause disease in laboratory mice. Type 1 strains lead to lethal infection in all conventional strains of laboratory mice including outbred animals (LD100 = 1) during the first two weeks of infection (i.e. the acute phase), and any survivors observed at low inoculum are invariably not infected (i.e. they remain serologically negative) [4,5,6]. In contrast, type 2 strains show intermediate virulence (LD50 varies with mouse strain, although these strain generally do not cause mortality in outbred lines), and type 3 strains are avirulent (they are not lethal in inbred mice at any dose), respectively [4,5,6]. These phenotypic differences prompted the generation of genetic crosses between these three strain types, resulting in the identification of the rhoptry kinase ROP18 as a virulence factor in genetic crosses between type 1 and type 3 or between type 2 and type 3 strains [7,8], and the rhoptry pseudokinase ROP5 as a virulence factor in genetic crosses between type 2 and 3 or between type 1 and 2 strains [9,10]. Together these proteins are involved in resisting innate immune mechanisms that are induced by IFNg stimulation [11]. The parasite secretes ROP5 and ROP18 into the host cell during invasion where they co-localize to the surface of the parasitophorous vacuole [12]. ROP18 is tethered to the vacuole membrane by an N-terminal low complexity region [13] and this association is required for its virulence enhancing properties [14].
At the vacuole surface, ROP18 phosphorylates host immune-related GTPase (IRGs) and prevents them from accumulating on the membrane and destroying the parasite vacuole [12,15]. The association of ROP18 with ROP5 increases the phosphorylation activity of ROP18 [16], and may also make substrates available to the kinase by binding to members of the IRG family [17,18,19]. Biochemical studies subsequently showed that ROP5 also associates with ROP17, and that this kinase works synergistically with ROP18 to phosphorylate and block IRGs [20]. Deletion of either ROP17 or ROP18 alone leads to a modest attenuation in the type I RH strain, evident as a delay in time until death; however, mice still succumb to low challenge doses (i.e. 100 parasites). In contrast, deletion of both ROP17 and ROP18 leads a marked attenuation of virulence where mice survive high challenge does (≥ 105 parasites) [20], similar to the deletion of ROP5 (≥106 parasites) [9,10].
South American stains are much more genetically diverse than those in the North and our current estimate of the population structure consists of 6 major clades that contain a total of 16 distinct haplogroups [21,22]. Notably, most common haplotypes from South America are also virulent in mice [23], similar to the type I lineage of North America where this phenotype is otherwise rare. Importantly, South American strains also exhibit the trait that a single infectious organism is lethal in all conventional laboratory mice (i.e. LD100 = 1) and they do not give rise to serologically positive surviving animals [23]. Previous studies have shown that ROP5 alleles in North and South American strains are correlated with resistance to IRG coating of the parasitophorous vacuole [18] and that expression of ROP18 from the South American strain RUB (type 5) confers virulence to an avirulent type 3 parasite [24]. However, the precise contributions of ROP5, ROP18, or other genetic factors, to acute virulence in these strains are unknown. For that reason, we mapped the genetic basis for differences in acute virulence between the intermediate virulence type 2 ME49 strain and the highly virulent type 10 VAND strain using a recently conducted genetic cross [25]. The type 2 ME49 parental strain was isolated from a sheep in the USA [26], whereas the type 10 VAND parental strain was originally isolated from an immunocompetent human adult in French Guiana [27]. Using quantitative trait locus (QTL) mapping and CRISPR-Cas9 generated knockout (KO) lines we were able to identify ROP5 as the single major virulence factor underlying the virulence differences in laboratory mice between type 2 and type 10 parasites. Additionally assessment of the contribution of ROP18 to virulence in several South American strains revealed a stronger phenotype than has been previously described for North American type 1 strains. These findings demonstrate that the genetic basis of mouse virulence is conserved across diverse lineages of T. gondii.
Results
Virulence maps to the ROP5 locus in the ME49-FUDRr X VAND-SNFr cross
We utilized a previously described genetic cross between type 2 ME49-FUDRr and type 10 VAND-SNFr strains [25,28]. Whole-genome sequencing of 24 progeny and mapping based on genome-wide SNP analysis was previously used to identify the molecular basis of sinefungin resistance [29]. Here we used the existing genetic map from this cross to analyze virulence of 24 recombinant progeny in CD-1 outbred mice. The parental ME49-FUDRr strain generally does not cause lethal infection in CD-1 mice and 7 of the progeny inherited this trait, where most or all of the mice infected with these progeny survived the 30 day course of the experiment (Fig 1A). There were 14 progeny that acquired the virulent trait from the VAND-SNFr parent, exhibiting ≥ 75% mortality in CD-1 mice (Fig 1A). Only three progeny showed an intermediate phenotype, with 50% of the mice succumbing to infection when infected with these progeny (Fig 1A).
Fig. 1. Genetic mapping of the virulence phenotype identified the ROP5 locus as the single major QTL. 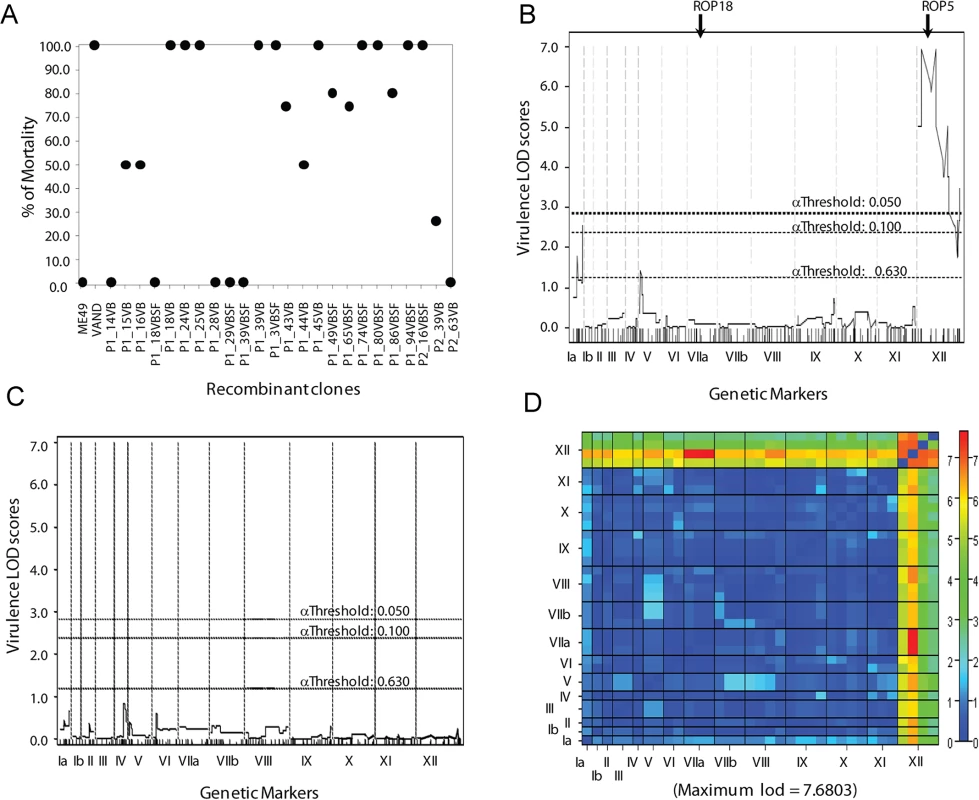
A) The parents and progeny of the genetic cross between type 2 ME49-FUDRr and type 10 VAND-SNFr strains were assessed for virulence in 5 outbred CD-1 mice per strain. (B) A QTL scan of the virulence phenotype generates one significant peak on the left end of chromosome XII with a LOD score of 6.95 and effect size 71%. Genome-wide LOD significance thresholds were obtained from 10,000 permutation tests, α .05 ≥ LOD 2.7. (C) A secondary scan of the virulence phenotype with the locus on chromosome XII run as an additive covariate. (D) A two-dimensional genome scan with a full model testing for interacting QTLs (top half) and an additive model testing for multiple QTLs without interaction (bottom half). Genome-wide LOD significance thresholds were obtained from 1,000 permutation tests. A genome-wide primary QTL scan of virulence (% mortality) run as a continuous phenotype generated one significant peak between the physical locations of 0.48 MB and 2.94 MB on chromosome XII with a log10 of odds (LOD) score of 6.95 (Fig 1B). A second QTL was detected in the primary scan with a LOD score of 2.54 on chromosome Ia; however, this peak did not reach significance (Fig 1B). The single peak on chromosome XII accounted for 71% of the effect-size, or variance in the virulence phenotype. A secondary scan with the primary QTL on XII run as an additive covariate failed to produce additional significant peaks (Fig 1C). Although no secondary peaks were identified, analysis of the virulence phenotype using a two-locus model identified a significant interaction between the primary peak on XII and chromosome VIIa, with a LOD score of 7.68. This finding is of interest as the known virulence factors ROP5 and ROP18 are located on chromosomes XII and VIIa, respectively.
Deletion of ROP5 in the VAND strain reduced virulence in mice
Because the peak on XII includes the tandem repeat of the ROP5 gene that has been shown to be a virulence factor in other strains, we chose to investigate ROP5 further. To delete the large locus spanning all copies of ROP5 on chromosome XII, we modified the T. gondii CRISPR-Cas9 plasmid [30] to express two separate single-guide RNAs (sgRNAs). The two sgRNAs target unique sites upstream and downstream of the ROP5 locus (Fig 2A). When expressed in T. gondii, the sgRNAs should generate two double-strand breaks in the genome and allow for replacement of the intervening region with a selection cassette containing homologous regions outside the sgRNA cut sites. This strategy is expected to generate a knockout of all ROP5 alleles including the expanded copy number ROP5 genes represented by TGVAND_308090, and two adjacent single copy genes that encode predicted pseudokinases that are paralogs of ROP5: TGVAND_308093 and TGVAND_308096 (Fig 2A). We co-electroporated the double-CRISPR plasmid with a loxP-DHFR*-mCherry-loxP selection cassette into VAND and acquired stable parasites after selection with pyrimethamine. A PCR screen for integration of the loxP-DHFR*-mCherry-loxP selection cassette at the ROP5 locus identified parasite clones that were positive for homologous integration (Fig 2B) and lacked all ROP5 coding regions (S1A Fig). Western blot analysis with rabbit α-ROP5 confirmed that no protein expression was detectable (Fig 2C). Wild type VAND and two different clones of VANDΔrop5 parasites were injected i.p. into CD-1 mice to test virulence. Only mice infected with wild type parasites succumbed to infection. Mice infected with VANDΔrop5 survived the course of the experiment, even at a high dose of 105 parasites (Fig 2D). Surviving mice from these experiments (Fig 2D) were tested for the generation of T. gondii antibodies via ELISA to ensure they were initially infected (S2 Fig). To confirm that ROP5 is sufficient for virulence we complemented the VANDΔrop5 parasite with the TOXOM52 cosmid that spans the _308090 tandem ROP5 locus, but does not include the adjacent _308093 or _308096 genes. TOXOM52 transgenic parasites were positive by PCR for the ROP5 coding sequence (S1A Fig) and showed wild type levels of ROP5 protein expression (Fig 2C). Mice infected with VANDΔrop5::TOXOM52 succumbed to infection in a similar timeframe as those infected with wild type parasites, demonstrating that the tandemly repeated _308090 ROP5 alleles are sufficient to restore virulence to wild type levels (Fig 2D). These results confirm that ROP5 is the major locus responsible for virulence differences between type 2 ME49 and type 10 VAND.
Fig. 2. ROP5 accounts for virulence phenotype in the type 10 VAND strain. 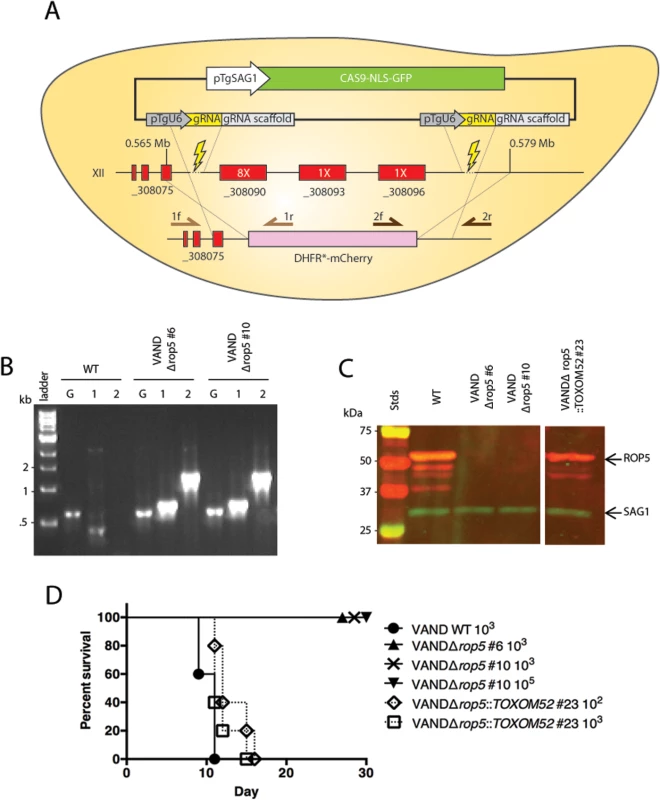
(A) Schematic diagram for knocking out the ROP5 locus on chromosome XII. Representation of the CRISPR-Cas9 plasmid (green box) and double CRISPR sgRNAs (yellow boxes), the sgRNA cut sites (lightning bolts), genes at the locus (red boxes) with copy number estimates of the ROP5 genes (white text in red box), the integration of the DHFR*-mCherry cassette (pink), and primers used in diagnostic PCR (arrows). (B) Diagnostic PCR showing integration of the DHFR*-mCherry cassette in two VANDΔrop5 clones, G: GRA1 promoter positive control—primer 1: 5’ integration—primer 2: 3’ integration—primer Set5-2. (C) Western blot of the VANDΔrop5 mutant and a complemented clone expressing the ToxoM52 cosmid containing ROP5. Probed with α-rabbit ROP5 (IRDye 680: red) and mAb DG52 to SAG1 (IRDye 800: green–control), Stds: protein standards. The separate panels of the image come from the same gel where the intervening lanes have been removed as they were not related. (D) CD-1 mice were injected i.p. with indicated number of parasites, five mice per clone. The percentage of surviving mice was adjusted for those that were not infected, based on serological testing. ROP5 is a major virulence factor in Clade B South American strains TgCtBr5 and TgCtBr18
Genetic crosses have now implicated ROP5 as a major virulence factor using type 1 GT1 [9] (Clade A), type 2 ME49 (Clade D), type 3 VEG (Clade C) [7,10] and type 10 VAND (Clade F) parasites as parental strains. These strain types represent a broad, yet incomplete, spectrum of the global T. gondii genetic diversity (Fig 3A) [21,22]. Given this, we were interested to test the contribution of ROP5 to virulence in strains from Clade B, a genetically distinct group not represented by the currently available genetic crosses. Using the double-CRISPR strategy applied to generate the VANDΔrop5 strain, we created Δrop5 parasites for virulent South American type 8 TgCtBr5 and type 4 TgCtBr18 strains. Knockouts were confirmed by PCR screening of clones for integration of the selection cassette (S3A and S3B Fig), loss of the ROP5 coding region (S1B and S1C Fig), and loss of ROP5 protein expression by Western blotting (Fig 3B and 3D). Mice succumbed to infection with wild type strains of TgCtBr5 or TgCtBr18, yet when mice were infected with either TgCtBr5Δrop5 or TgCtBr18Δrop5 they survived the course of the experiment (Fig 3C and 3E). These data demonstrate that ROP5 is also a major contributor to virulence in Clade B strains of T. gondii.
Fig. 3. ROP5 is a virulence factor in South American strains TgCtBr5 and TgCtBr18. 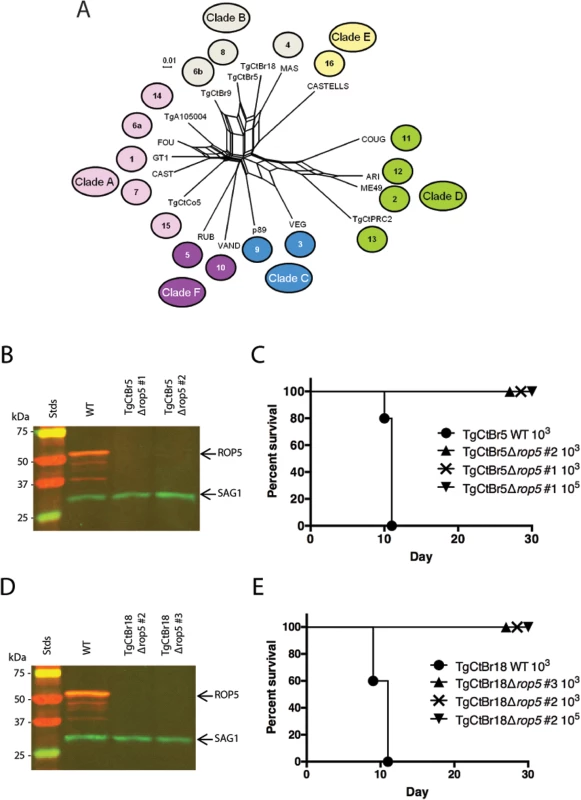
(A) Population genetic network analysis using genome-wide SNPs from 16 Toxoplasma gondii reference strains plus strain TgCtBr18. Numbers represent the haplogroup of the strain and colors represent the Clade: Clade A (pink), Clade B (gray), Clade C (blue), Clade D (green), Clade E (yellow), Clade F (purple). (B) Western blot of the TgCtBr5Δrop5 clones probing with rabbit α-ROP5 (IRDye 680: red) and mAb DG52 against SAG1 (IRDye 800: green–control), Stds: protein standards. (C) CD-1 mice were i.p. injected with indicated number of parasites, five mice per clone. (D) Western blot of the TgCtBr18Δrop5 clones probing with rabbit α-ROP5 (IRDye 680: red) and mAb DG52 against SAG1 (IRDye 800: green–control), Stds: protein standards. (E) CD-1 mice were i.p. injected with indicated number of parasites, five mice per clone. The percentage of surviving mice was adjusted for those that were not infected, based on serological testing. Loss of ROP18 renders South American strains VAND, TgCtBr5, and TgCtBr18 avirulent
ROP5 and ROP18 have previously been shown to function together to resist host IRG-mediated parasite killing [16,18]. Although ROP5 plays a dominant role in many strains, the effect of deleting ROP18 varies with different backgrounds. For example, deletion of ROP18 results in a delay in the time until death in the RHΔrop18 strain [12] and a modest shift in the LD50 in GT1Δrop18 strain [30] parasites. Consequently, we assessed the role of ROP18 in the virulence of South American VAND strains. Using the sgRNA-ROP18 CRISPR disruption strategy described previously [30], we deleted ROP18 from the VAND-SNFr parental strain. PCR screening for integration of the DHFR* selection cassette at the ROP18 locus was confirmed in stable clones (S4 Fig), resulting in the loss of ROP18 protein expression, as determined by Western blotting (Fig 4A). Unlike the delayed death phenotype seen previously for type 1 RHΔrop18 parasites, we observed 100% survival rates for CD-1 mice infected with two different clones of VANDΔrop18 parasites (Fig 4B), even at a dose of 105 parasites. Virulence was restored in VANDΔrop18 (Fig 4B) when it was complemented with a ROP18-Ty tagged version of the protein (Fig 4A).
Fig. 4. ROP18 is essential for virulence in South American strains VAND, TgCtBr5, and TgCtBr18. 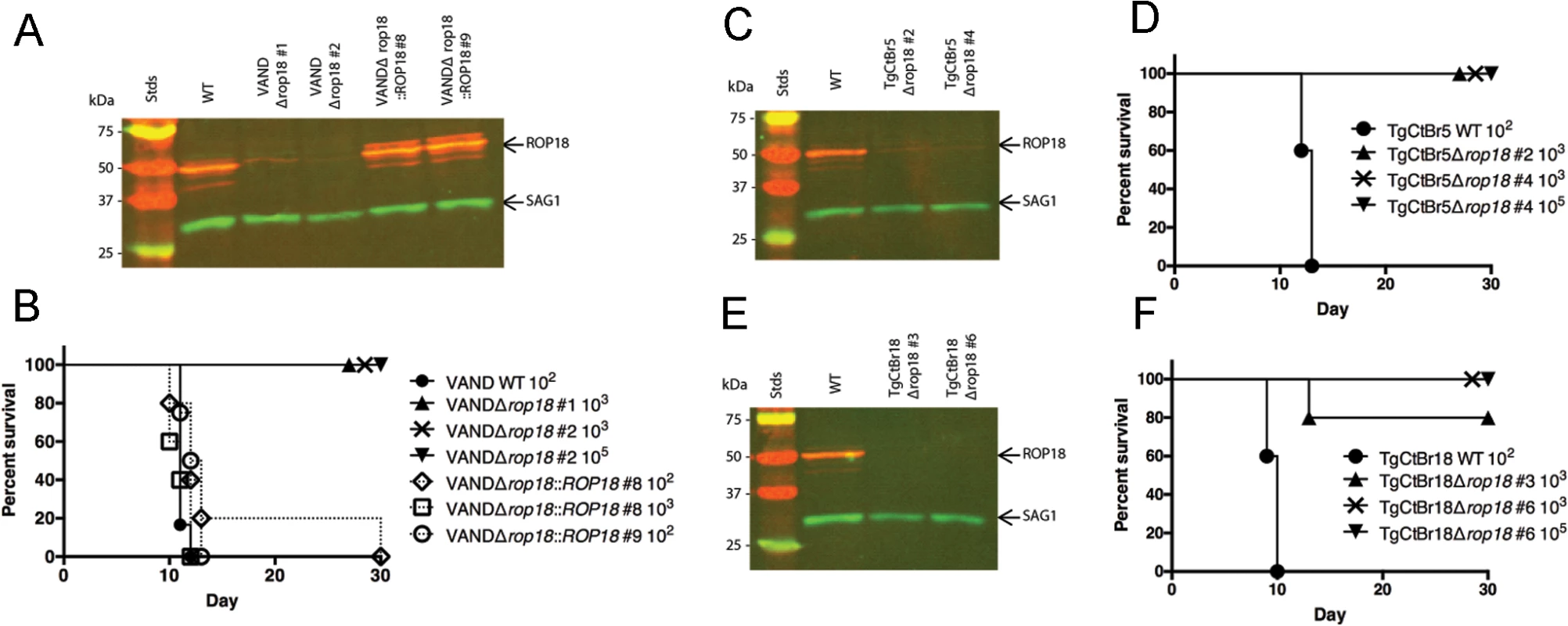
(A) Western blot of the VANDΔrop18 and complemented lines probed with rabbit α-ROP18 (IRDye 680: red) and mAb DG52 against SAG1 (IRDye 800: green–control), Stds: protein standards. (B) CD-1 mice were injected i.p. with indicated number of parasites, five mice per clone. (C) Western blot of the TgCtBr5Δrop18 clones probing with rabbit α-ROP18 (IRDye 680: red) and mAb DG52 against SAG1 (IRDye 800: green–control), Stds: protein standards. (D) CD-1 mice were injected i.p. with indicated number of parasites, five mice per clone. (E) Western blot of the TgCtBr18Δrop18 clones probing with rabbit α-ROP18 (IRDye 680: red) and mAb DG52 against SAG1 (IRDye 800: green–control), Stds: protein standards. (F) CD-1 mice were injected i.p. with indicated number of parasites, five mice per clone. The percentage of surviving mice was adjusted for those that were not infected, based on serological testing. To determine how the loss of ROP18 would affect virulence in Clade B strains, we knocked out ROP18 in TgCtBr5 and TgCtBr18 using the same strategy used in VAND. After pyrimethamine selection we obtained parasite clones that integrated the DHFR* selection cassette at the ROP18 locus (S4B and S4C Fig), resulting in the loss of expression of ROP18 as determined by Western blotting (Fig 4C and 4E). Similar to type 10 VAND parasites lacking ROP18, TgCtBr5Δrop18 and TgCtBr18Δrop18 parasites were completely avirulent in CD-1 mice, even at a dose of 105 (Fig 4D and 4F)
CNV and allelic structure of ROP5 in South American strains
The locus encoding ROP5 shows evidence of expansion of tandem copies of the gene in different strains of T. gondii [10,16]. Alignments of genome-wide sequence to the assembled ME49 chromosomes revealed that the copy number for the ROP5 gene (_308090) varies between strains, whereas the more divergent _308093 and _308096 ROP5 paralogs that lie adjacent in the genome are each single copy (Fig 5A) [31]. The number of copies of ROP5 (_308090) (i.e. copy number variation (CNV)) for each of the strains was estimated by comparing the trace reads to the CDS, yielding the following estimates: GT1 (5), ME49 (10), VEG (4), TgCtBr18 (6), TgCtBr5 (7), and VAND (8) (Fig 5A and 5B). Previous studies have shown that each strain contains a dominant allele that is expressed at higher levels based on CNV. Dominant alleles have been referred to as “C”, while less common alleles were labelled as “A” or “B”, in work reported by Resse et al., [10], while these two categories were called M for major (C alleles) and m for minor alleles (B and A alleles) by Behnke et al., [9]. ROP5 alleles in VAND and TgCtBr5 were also previously named using A and B alleles, based on how they grouped phylogenetically with a collection of various strains including the type 2 strain ME49 [18]. We were able to estimate the CNV for the individual ROP5 alleles from these alignments for the strains VAND and TgCtBr5 (Fig 5C). These genes can also be defined as major and minor alleles based on copy number: TgCtBr5-B1 corresponds to the major allele (M), while TgCtBr5-B2 is a minor allele (m1); VAND-B2 is the major allele (M); VAND-B1, VAND-B3, and VAND-A are minor alleles (m1-3, respectively).
Fig. 5. CNV and phylogenetic analysis of ROP5 copies in VAND, TgCtBr5, TgCtBr18. 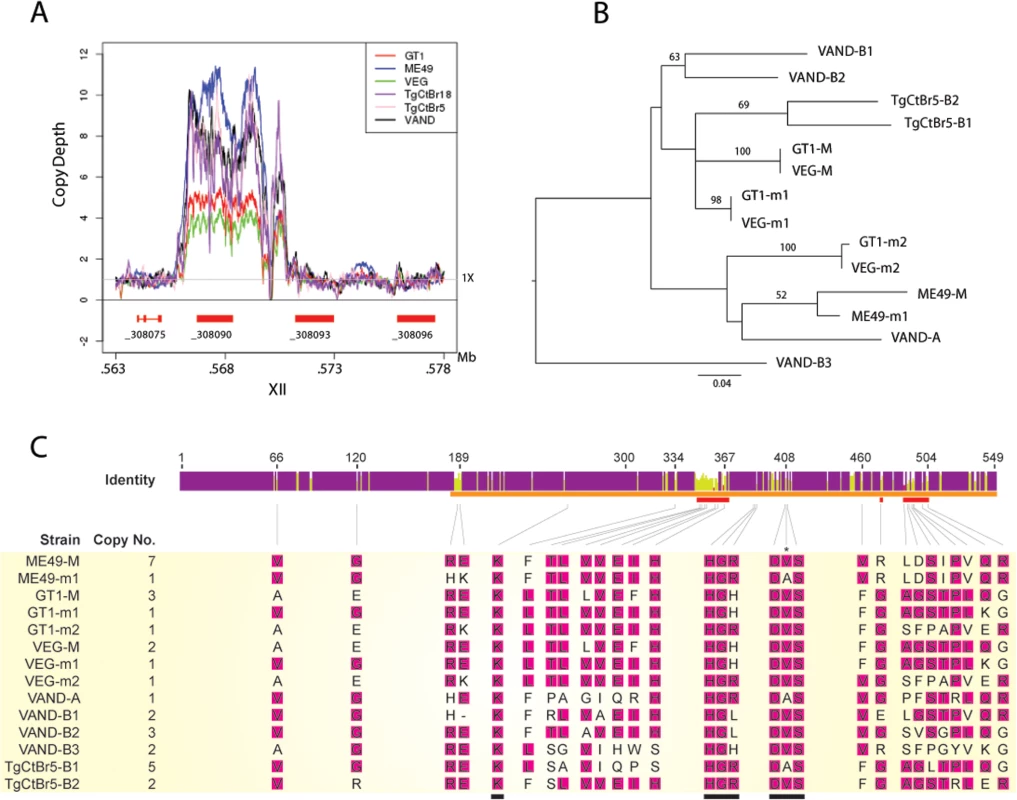
(A) Copy number variation depth for T, gondii strains across the ROP5 locus on chromosome XII. Copy number estimates are based on the read depth per base pair normalized to 1X across the respective genome. (B) A Maximum Likelihood tree for T. gondii strains based on ROP5 amino acid sequences. Only maximum likelihood bootstrap values above 50 are shown. (C) The sequence identity across all sequences at each position is shown for the ROP5 amino acid sequence alignment. Purple indicates residues at positions with 100% sequence identity. Yellow indicates less than complete sequence identity and red indicates very low sequence identity at a given position. The ROP5 pseudokinase domain, catalytic triad, and residues involved in IRGa6 interactions are indicated by orange, black, and red bars, respectively. The predicted ROP5 allele copy numbers are shown beneath the sequence identity graph along with the amino acid alignments at positions where the respective codons are evolving under positive selection. The most frequent amino acid residue in a given position of the alignment is highlighted in pink. Amino acid positions/alignments of the catalytic triad are also shown; only a single codon position within the catalytic triad is evolving under positive selection (indicated by an asterisk). Allele names for the respective strains used in B and C are based on NCBI entries. Further discussion of the relationship among the alleles is provided in the results. Although the number of ROP5 copies found in a strain doesn’t correlate with virulence, amplification of specific alleles types is associated with virulence in the murine model [10,16]. For example, the major alleles shared by type 1 and 3 strains (GT1-M, VEG-M corresponding to ROP5CI/III) are associated with virulence, while those from type 2 (ME49-M or ROP5CII) are not [9,10]. To expand on this relationship, we conducted phylogenetic analysis using the previous sequences for ME49, GT1, VEG [16] and VAND, TgCtBr5 [18] ROP5 alleles that are available from NCBI. Phylogenetic analysis of the ROP5 protein sequences revealed a lack of monophyly among the three strain types (Fig 5B). However, most alleles from the virulent strains group together, with VAND-B1 (m1), VAND-B2 (M), TgCtBr5-B1 (M), and TgCtBr5-B2 (m1) being closer to GT1-M. This cluster also included the major type 3 allele in VEG (VEG-M), which is functionally similar to the major allele in type 1 strains (the avirulence of VEG is due to hypo-expression of ROP18 not a defect in ROP5). In contrast, GT1-m2, VEG-m2, and VAND-A from virulent parasites group with the avirulent ME49-M and ME49-m1 alleles (Fig 5B). VAND-B3 appeared as a more divergent allele occurring on a long branch at the base of the tree (Fig 5B).
The function of ROP5 alleles in virulence may be attributed to differences the polymorphic surface of ROP5, which interacts with host IRGs and likely contributes to IRG specificity [19]. Indeed, when we analyzed the ratio of non-synonymous to synonymous mutations we detected 23 codon sites evolving under significant positive diversifying selection, with the majority of codon sites (74% of positive codons) localized within the regions encoding the polymorphic surface of ROP5 (S1 Table and Fig 5C). Interestingly, one codon site that evolves under positive diversifying selection was found within the DFG motif that is normally part of the activation loop in active kinases (S1 Table and Fig 5C). To provide greater insight into the region of ROP5 that is under diversifying selection, we mapped these residues on the recently described binding site for Irga6 as revealed by a X-ray co-crystal structure [19]. A majority of the residues in ROP5 that are under positive selection cluster tightly to a region that interacts directly with Irga6 (Fig 6A). A total of 13 residues (56.5%) under positive selection are directly at the interface between ROP5 and Irga6 (purple in Fig 6B, S1 Table), as identified by PDBePISA [32,33]. An additional 3 residues (13%) under positive selection are close to the interface and may play a supporting role in stabilizing the primary interface residues (S1 Table and Fig 6).
Fig. 6. Codons under positive diversifying selection encode amino acid residues at the ROP5BI /IRGa6 interface. 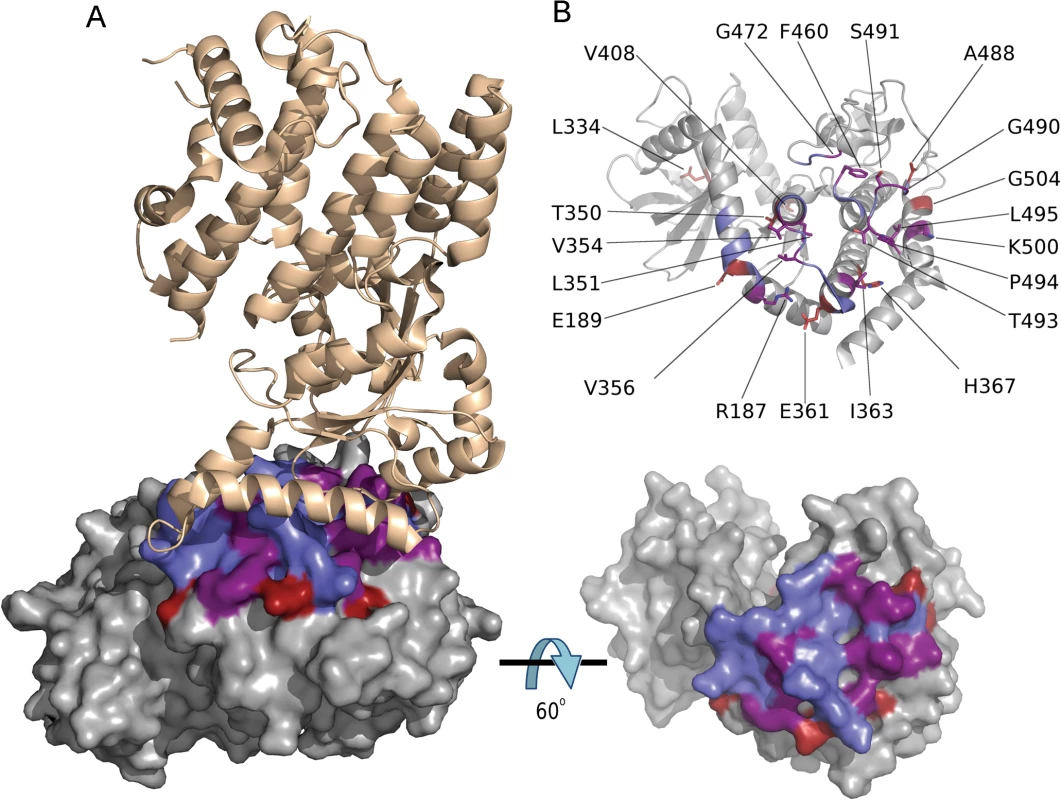
(A) Structure of T. gondii ROP5BI (grey) bound to murine Irga6 (tan) showing amino acid residues under positive diversifying selection (red), ROP5BI residues at the ROP5BI /Irga6 interface (blue), and residues that are under positive diversifying selection and at the interface (purple). (B) Both panels are a rotation of 60° about the x-axis with respect to A, showing ROP5BI with Irga6 removed for clarity. The color scheme is as in A. Bottom panel is a surface representation showing the overlap in surface residues. The top panel shows the position of all amino acid residues under positive diversifying selection. Discussion
Our studies extend the application of forward genetic crosses and reverse genetic engineering using CRISPR-Cas9 to South American strains to provide an expanded framework for examining the basis of acute virulence in the mouse model. These studies reveal a conserved role for ROP5 and ROP18 in acute virulence in the mouse, likely due to their previously demonstrated role in protecting the parasite from host innate immune responses [12,15]. The identification of only a single significant QTL in analyzing phenotypic differences among progeny from the genetic cross between type 2 ME49 and type 10 VAND led to the identification of ROP5 as the major locus in controlling acute virulence in the mouse. Although ROP18 was not detected in a primary scan, a two-locus interaction occurred between regions containing ROP5 (chromosome XII) and ROP18 (chromosome VIIa), suggesting that these two genes collectively explain the major virulence differences among diverse strains of T. gondii in the mouse. This prediction was borne out by reverse genetic engineering of ROP5 and ROP18 disruptant mutants in other South American strains, the latter of which showed more severe defects in acute virulence than previously observed in the type 1 background. The widespread importance of these two virulence factors suggests that they evolved before the global spread and divergence of the parasite, consistent with recent reports that functional alleles of ROP5 and ROP18 also exist in the close relative Hammondia hammondi [34], even though this parasite is not virulent in mice. Collectively, these data suggest that ROP5 and ROP18 have been important in the evolution of these parasites within their rodent hosts, likely due to their interaction with the IRG family.
Despite extensive polymorphism between alleles, the major difference in the contribution of ROP18 to mouse virulence is due to different expression levels, being high in types 1 and 2, and very low in type 3 [7,8]. Complementation of a type 3 strain by over-expression of either ROP18I [8] or ROP18II [7] was sufficient to restore virulence. Additionally previous studies have shown that complementation of an avirulent type 3 strain with type 1-like alleles of ROP18 from South American strains results in a similar gain of virulence [24]. Combined with the fact that ROP18 was not seen as a major QTL in the cross between type 1 and 2, where ROP5 was mapped [9], these data suggest that different alleles of ROP18 function similarly, and their differential contribution to virulence is primary due to expression level. Given these previous findings, it is perhaps not surprising that ROP18 was not identified as a major QTL in the present cross. However, it was somewhat surprising that ROP5 was identified as the gene responsible for the majority of difference in acute virulence between type 2 ME49 and type 10 VAND, given the extensive genetic differences between these strains [21,22]. The mechanism by which ROP18 and ROP5 thwart innate immunity has been well established in North American strain types, where the combination of alleles present in type 1 strains leads to resistance to IRG loading relative to type 2 or 3 strains [11]. Although we did not test the recruitment of IRGs directly in this study, the role of ROP5 and ROP18 in the virulence of South American strains is likely due to their function in resisting host IRG recruitment to the parasitophorous vacuole. Consistent with this prediction, previous studies have shown that in IFNg-activated host cells, vacuoles containing virulent VAND (type 10) and TgCtBr5 (type 8) parasites recruit less Irgb6 than susceptible type 2 and 3 parasites [18].
The only other single peak observed on chromosome Ia was below the threshold of significance. In a previous genetic cross between type 1 and 3, a minor QTL was detected on chromosome Ia from markers M48 (~330 kbp)–AK4 (~485 kbp) with a LOD score of 2.38 [8]. However, in the present study, the QTL peak on chromosome Ia lies at marker MV24 near the right end of the chromosome (~1,500 kbp) with a LOD score of 2.54, and a marginal level of significance. There is also a second non-significant peak in the center of the chromosome from MV2 (~570 kbp) to MV9 (~830 kbp). These findings suggest that genes on this chromosome play a minor role in acute virulence in the mouse, although the resolution of the present data is insufficient to precisely map these or to identify candidate genes.
Although forward genetic mapping allowed us to identify a locus containing ROP5 as important in the virulence of South American strains, the newly adapted CRISPR-Cas9 technology was critical for testing its function by reverse genetics. The default DNA damage repair pathway in T. gondii is nonhomologous DNA end joining, and for this reason the use of a Δku80 background is important to efficiently generate knockout parasites [35,36]. Several attempts were made to generate a VANDΔku80 strain for use in this study but all were unsuccessful, perhaps because VAND is a low passage isolate that has not been adapted to in vitro culture conditions for long periods of time. However, gene disruption using CRISPR-Cas9 is highly efficient even in wild type KU80 proficient parasites, as shown previously [30], and this technique was also successfully applied here to diverse strains. We also extended this strategy by creating a CRISPR-Cas9 plasmid that expressed two sgRNAs targeting sites surrounding a large genomic locus. This strategy was efficient at deleting the ~30 kb ROP5 locus in three different low-passage South American strains. The acute virulence phenotype of VANDΔrop5 mutant was restored using a cosmid that only contains the ROP5 cluster, but not the adjacent pseudokinase paralogs, indicating that these adjacent genes contribute little to the phenotype and that the ROP5 alleles are the primary determinants of acute virulence.
Phylogenetic analysis revealed that VAND and TgCtBr5 contain ROP5 alleles that are similar to the major alleles in the virulent type 1 strain GT1, with only minor alleles being similar to the type 2 strain ME49. Based on this similarity, we decided to complement the VAND ROP5 locus knockout with a cosmid containing the cluster from the type I RH strain. We have not tested the function of individual alleles in restoring this phenotype, nor complemented directly with individual VAND alleles, and further testing would be needed to establish if they have conserved functions. Interestingly, all of the ROP5 alleles contain a functional change in HRD motif, that forms part of the catalytic triad, where major alleles in the virulent strains (those related to GT1-M corresponding to ROP5CI) and some minor alleles such as GT1-m1 (ROP5BI) contain “H”, while the major allele in the type 2 strain ME49 (ME49-M, ROP5CII), and minor alleles such as VAND-A, GT1-m2 (ROP5AI), and VEG-m2 contain a “R”. In contrast, VAND-B1 and VAND-B2 alleles show a new residue in the catalytic triad of “L”, which again is predicted to lack kinase activity. None of these residues is predicted to be active as this residue is normally a D in active kinases [37]. The functional significance of these changes in the catalytic triad of ROP5 is unclear as the interface that interacts with IRGs is on the opposite face of the molecule [19]. However these different alleles also correlate with differences in function in laboratory mice: the former group, including GT1-M, GT1-m1, and related alleles, inhibit recruitment of IRGs to the parasitophorous vacuole in vivo, while the later group, including ME49-M and related alleles, do not [16,17]. The diversity of alleles found in ROP5 suggests that alleles that lack activity in the mouse may be active in others hosts, for example other rodents or birds.
Previous studies have emphasized that this binding interface is polymorphic in ROP5 [19], and our analysis of residues that are under strong positive selection in ROP5 further defines this interface as a cluster of residues that directly interacts with Irga6. Additional nearby polymorphic residues in ROP5 may act to influence the Irga6 interaction through allosteric interactions, or they may be important in binding to other polymorphic alleles of Irga6. Importantly, IRGs also show polymorphism in this region of the molecule [19], suggesting that ROP5 is under selective pressure for enhanced binding while IRGs have evolved to avoid recognition. In support of this model, binding of ROP5 to Irga6 has been shown to directly limit polymerization in vitro [19], as well as to enhance phosphorylation of IRGs by ROP18 in vitro and in vivo [16]. Collectively, these findings provide evidence for the hypothesis that the polymorphic regions of ROP5 are evolving under positive diversifying selection driven by interaction with host innate immunity factors.
We were also able to efficiently delete ROP18 in different genetic backgrounds, demonstrating the potential of CRISPR-Cas9 for genetic manipulation of diverse strains of T. gondii. Unlike ROP5 knockouts where all Δrop5 strains tested are equally avirulent despite different genetic backgrounds, there are large differences in the phenotype of Δrop18 disruptants between strains. For example, previous studies have reported that deletion of ROP18 in the type 1 RH strain led to delayed time until death, but not a change in the lethal dose [12] vs. a several log increase in the lethal dose using the type 1 GT1 strain [30]. In contrast, deletion of ROP18 in type 4, 8, and 10 strains led to a complete loss of virulence with no animals dying at a challenge dose of up to 105 (present study). The reasons for these differences are uncertain but are unlikely to be due to experimental variation in the mouse model since they were obtained in the same laboratory using a standard protocol that is highly reproducible. Type 1 strains normally result in lethal infection in all strains of conventional laboratory mice with an inoculum of a single parasite [4,5,6]. In contrast animals infected here with Δrop18 mutants in type 4, 8, and 10 did not succumb to infection, yet had very high antibody titers, reflecting an active infection. Previous studies have stressed that the major contribution of ROP18 in acute virulence appears to be due to expression level differences, and yet all the strains tested here show high levels of expression [24]. Hence, it is not immediately clear why the contribution of ROP18 to acute virulence would vary so extensively among the strains tested here. Once possibility is that the small number of polymorphisms in ROP18 alleles found between type 1 strains and the isolates studies here may account for these differences. Consistent with this, previous studies have also shown that virulent alleles of ROP18 are also under diversifying selection [24]. The marked differences in attenuation with the deletion of ROP18 might also be due to the relative importance of other genes that contribute to acute virulence. For example, the active rhoptry kinase ROP17 [20] or the secreted dense granule GRA7 [38], which have been shown to be important in the type I RH strain, may partially compensate for loss of ROP18 in some backgrounds. The fact that ROP18 plays a less important role in the RH strain, may also be due to its passage history, which has been propagated extensively in mice and in vitro since its original isolation more than 75 years ago [39], resulting in numerous changes that favor growth. Further studies of the precise function of various ROP18 alleles in different genetic backgrounds would be necessary to decipher among these alternatives.
Despite their conserved functions in the murine model, ROP5 and ROP18 do not appear to mediate resistance to IFNg-activated control of replication in human cells [18]. Because humans lack the expanded IRG family [40], other mechanisms of control likely contribute to cell autonomous control of replication. However, ROP18 has also been shown to phosphorylate host cellular protein ATF6-β targeting it for degradation, thus hindering antigen presentation [41]. This pathway may contribute to disruption of adaptive immunity in hosts other than murine, including humans. Consistent with this possibility, among human patients with ocular toxoplasmosis in Columbia, mouse-virulent alleles of ROP18 were correlated with more severe disease [42]. Additionally, certain South American strains appear to be more virulent in humans in that they are associated with higher rates of ocular disease in the case of Clade B [43], and they are associated with severe disease in healthy individuals in the case of Clade F [44,45,46]. The parasite factors responsible for increased acute disease severity in humans are unknown, but these traits could be suitable for genetic mapping if appropriate screens were available for assessing these phenotypes in vitro. Although it is unlikely that resistance in human cells is a driving factor for evolution of T. gondii virulence, given the fact that humans are rarely involved in transmission, such traits could represent conserved functions that are important in multiple vertebrate hosts.
Using forward genetic mapping and reverse genetic engineering with CRISPR-Cas9, we demonstrate the conserved nature of the ROP5 and ROP18 virulence factors in South American strains of T. gondii. We also found a more dramatic role for ROP18 in the virulence of these strains than had been appreciated using North American isolates of the parasite. Recent genetic studies in the house mouse reveal novel alleles of IRGs that are responsible for their enhanced resistance to otherwise virulent type 1 lineages of T. gondii [47]. The predator-prey relationship between the house mouse and domestic cat has likely existed for at least 10,000 years in association with human settlement [48]. In contrast, strains harboring similar ROP18 and ROP5 alleles have been estimated to have diverged several million years ago [22]. Collectively these studies suggest that parasite virulence determinants are co-evolving against the pressure of murine immunity factors in a backdrop of more ancient history that is conserved among diverse strains of T. gondii.
Materials and Methods
Ethics statement
All animal experiments were conducted according to the U.S.A. Public Health Service Policy on Humane Care and Use of Laboratory Animals. Animals were maintained in an Association for Assessment and Accreditation of Laboratory Animal Care International-approved facility. All protocols were approved by the Institutional Care Committee at the School of Medicine, Washington University in St. Louis.
Parasite culture
The parents and progeny of the genetic cross between type 2 ME49-FUDRr and type 10 VAND-SNFr strains [25], and parasite strains TgCtBr5-SNFr and TgCtBr18-SNFr were grown as tachyzoites in human foreskin fibroblast (HFF) monolayers. The TgCtBr5 and TgCtBr18 strains were made SNF resistant using N-ethyl-N-nitrosourea as described previously [49]. Cultures were maintained in D10 media composed of Dulbecoc’s modified Eagle medium (DMEM), 10% fetal bovine serum (FBS), gentamycin, and glutamine at 37°C with 5% CO2. Infected monolayers were scrapped and force passaged through 21 gauge needles to harvest parasites.
Acute infection and ELISA
CD-1 mice were acquired from commercial vendors (Jackson Labs) and housed under SPF conditions at Washington University School of Medicine. Acute virulence was determined by i.p. injection of indicated number of tachyzoites into groups of five 8–12 week old female outbred CD-1 mice per experiment. Survival was monitored for 30 days, after which surviving mice were bled to collect serum for serological testing by enzyme-linked immunosorbent assay (ELISA), as described previously [50]. The percentage of surviving animals was determined by the number of animals that succumbed / total number of animals that were infected x 100. Survival values were corrected for animals that failed to become infected (i.e. those that remained serologically negative).
Quantitative trait locus analysis for virulence
The virulence phenotype was analyzed by QTL mapping using J/qtl [51] to establish the genome-wide association of phenotypes with genotypes of progeny from the genetic cross between type 2 ME49-FUDRr and type 10 VAND-SNFr strains described previously [25]. Virulence QTLs were confirmed by interval mapping based on a one-dimensional genome scan with 1,000 permutation to generate a log likelihood plot (P < 0.001). Covariant analysis and a two-dimensional genome scan were conducted using J/qtl to identify minor QTLs and epistatic interactions, respectively using criteria above.
Double-CRISPR and pUC19-ROP5HR-DHFR*mCherry plasmids
In order to make the double-CRISPR plasmid used to knockout the ROP5 locus, two single sgRNA CRISPR plasmids were constructed. The pSAG1::CAS9-U6::sgUPRT plasmid (Addgene #54467) was used as a template to amplify with Set1-1 or Set1-2 primers (S2 Table) and PCR products were processed using the Q5 Site-Directed Mutagenesis Kit (NEB), as described previously [30] to create the pSAG1::CAS9-U6::sg5’ROP5 and pSAG1::CAS9-U6::sg3’ROP5 plasmids. The sgRNA cassette (containing the U6 promoter, sgRNA, and scaffold) was PCR amplified with Q5 High-Fidelity DNA polymerase (NEB) from the pSAG1::CAS9-U6::sg3’ROP5 plasmid using Set2-1 primers (S2 Table) that contain terminal KpnI and XhoI restriction enzyme sites. The sg3’ROP5 cassette PCR product and the pSAG1::CAS9-U6::sg5’ROP5 plasmid were cut with KpnI/XhoI (NEB), buffer 4 at 37°C overnight, gel purified on a 1% agarose gel, and purified with the Qiagen Gel Purification Kit (Qiagen). The digested and purified PCR products and plasmid were ligated using T4 ligase (NEB) at 16°C over-night, ligations were transformed into OmniMAX chemically competent cells (Invitrogen), and a correct clone was identified with KpnI/XhoI restriction fragment mapping. The plasmid named pSAG1::CAS9-U6::sg5’ROP5-sg3’ROP5 plasmid contains two sgRNAs targeting the 5’ and 3’ side of the ROP5 locus.
To select for the excision of the ROP5 locus in pSAG1::CAS9-U6::sg5’ROP5-sg3’ROP5 transfected parasites, we constructed a selection cassette flanked by homologous regions (HR) adjacent to the 5’ and 3’ sgROP5 CRISPR cut sites using Gibson Assembly (NEB). Four PCR fragments were amplified using Q5 High-Fidelity DNA polymerase (NEB) from the following template/primer pairs: (1) the p-loxP-DHFR*-mCherry-loxP plasmid with Set3-1 primers, (2) VAND genomic lysate with Set 3-2primers, (3) VAND genomic lysate with Set3-3 primers, (4) the pUC19 plasmid with Set3-4 primers (S2 Table). PCR products were gel purified as above and combined in a Gibson Assembly reaction (NEB) using manufactures protocol. The reaction was transformed into NEB 5-alpha cells and a correct clone was identified using three different restriction fragment mappings (BamHI/NotI, BamHI/ScaI, and ScaI/NotI) and named pUC19-ROP5HRs-loxP-DHFR*-mCherry. This plasmid was used as a template in a Q5 High-Fidelity DNA polymerase PCR to amplify the 5’ROP5HR-loxP-DHFR*mCherry-loxP-3’ROP5HR cassette using the Set4-1 primers (S2 Table). This amplified cassette was gel purified as above for use as a selection cassette in creating ROP5 KO parasites.
ROP5 KOs and complementation
The VAND-SNFr, TgCtBr5-SNFr, and TgCtBr18-SNFr strains were individually electroporated with the double-CRISPR pSAG1::CAS9-U6::sg5’ROP5-sg3’ROP5 plasmid and a PCR amplified 5’ROP5HR-loxP-DHFR*mCherry-loxP-3’ROP5HR selection cassette. Parasites were selected in 2 μM pyrimethamine (Sigma-Aldrich), cloned, and screened by PCR for integration of the DHFR*-mCherry cassette at the ROP5 locus using Set5-1 primers for 5’ integration, Set5-2 primers for 3’ integration, Set5-3 for the ROP5 coding sequence and Set5-4 primers to the GRA1 promoter as a positive control for the PCR (S2 Table). Knockout parasites were identified, protein harvested, and Western blotted to confirm the loss of ROP5 protein.
In order to complement with a cosmid containing the DHFR* pyrimethamine selection cassette, we excised loxP-DHFR*-mCherry-loxP from a VAND ROP5 knockout clone using Cre recombinase. The VANDΔrop5 #10 strain was electroporated with the pmin-Cre-eGFP plasmid [52], cloned without selection, and mCherry negative parasites were screened by immune-fluorescence assay (IFA) using primary rat aντι-mCherry mAb (Life Technologies) and secondary goat aντι-rat Alexa Fluor 594 (Life Technologies), to identify a VANDΔrop5Δdhfr*mCherry parasite. Sensitivity to 2 μM pyrimethamine was confirmed for VANDΔrop5Δdhfr*mCherry. To generate the complement, the VANDΔrop5Δdhfr*mCherry parasite was electroporated with the TOXOM52 cosmid, derived from the type I RH strain (http://toxomap.wustl.edu/cosmid.html) [53]. The parasites were selected with 2 μM pyrimethamine (Sigma-Aldrich), and clones were screened for expression of ROP5 by IFA using primary rabbit anti-ROP5 and secondary goat anti-rabbit Alexa-Fluor 594 (Life Technologies). ROP5 expressing clones were identified, protein harvested, and Western blotted to confirm wild type levels of expression in the complement, creating VANDΔrop5::ROP5.
ROP18 KOs and complementation
The ROP18 KOs and complement were created as described previously [30]. Briefly, the VAND-SNFr, TgCtBr5-SNFr, and TgCtBr18-SNFr strains were individually electroporated with the pSAG1::CAS9-U6::sgROP18 CRISPR plasmid and a PCR amplified selection cassette containing ROP18 homologous regions flanking a DHFR* pyrimethamine resistance marker. Parasites were selected with 2 μM pyrimethamine (Sigma-Aldrich), cloned by limiting dilution, and PCR screened for integration of the selection cassette at the ROP18 CRISPR cut site. Knockout clones were identified, proteins harvested, and Western blots were run to confirm the loss of ROP18 protein. To create a complemented strain, the VANDΔrop18 #1 clone was electroporated with the pSAG1::CAS9-U6::sgUPRT plasmid and a PCR amplified selection cassette containing UPRT homologous regions flanking an IMC1p-ROP18Ty expression construct. Parasites were selected with 10 μM FUDR (Sigma-Aldrich), cloned and screened for expression of Ty by IFA: using primary α-Ty-BB2 mAb and secondary goat-α-mouse Alexa Fluor 488 (Life Technologies). ROP18-Ty expressing clones were identified and protein harvested, and Western blots were run to confirm wild type expression levels of ROP18 in the complement, VANDΔrop18::ROP18.
Genomic lysate/PCR screens
Genomic lysates were harvested from various parasite strains for use as templates in PCR screens. Parasites were grown in HFF monolayers, harvest by scrape/needle pass, spun at 400 rcf and supernatant was aspirated from the parasite pellet. The pellet was suspended in genomic lysate buffer (1X PBS with 10% Taq polymerase buffer (Invitrogen) and 400 ng/μl proteinase K (Life Technologies)), the sample was incubated at 37°C for 1hr, 50°C for 30 min, and 95°C for 5–10 min, and spun to pellet debris.
Genomic lysates were used in PCR screens to detect the integration or removal of selection cassettes. For the ROP5 knockouts and complementation the primer sets outlined in S2 Table were used, and for the ROP18 disruptants and complementation the primer sets described previously [30] were used in a Taq polymerase (NEB) PCR. PCR products were run on a 1% agarose gel and imaged on a UV light box.
Protein harvest/western blotting
Parasites were grown and harvested as above, counted on a hemocytometer, the pellet was resuspended in protein lysate buffer (1X PBS, 1% Triton-X, 1% DNase, 1% protease inhibitors (Sigma-Aldrich)) to 1 X 106 parasites/μl, vortexed, and spun to pellet debris. Each sample contained protein from 5 X 106 parasites in 1X sample buffer with 10% β-Me (Sigma-Aldrich) and was incubated for 5–10 min at 95°C. Samples were resolved on a 10% SDS-polyacrylamide (Bio-Rad) gel at 100V for 1 hr. Gels were transferred to nitrocellulose, blocked in 5% milk, and probed with (all diluted to 1 : 10,000) primary—rabbit α-ROP5 or rabbit α-ROP18 and mouse mAb DG52 α-SAG1 antibodies, and secondary antibodies consisting of goat α-rabbit IgG IRDye 680 (LI-COR Biosciences) and goat α-mouse IgG IRDye 800 (LI-COR Biosciences). All Blots were imaged on a LI-COR Odyessy CLx infrared imaging system (LI-COR Biosciences).
Network analysis and CNV
Network analysis using genome wide SNP data from the 16 Toxoplasma gondii reference genomes and the TgCtBr18 genome (obtained from ToxoDB) was conducted using SplitsTree v4.4 [54] to generate unrooted phylogenetic networks using a neighbor-net method and 1,000 bootstrap replicates, as previously described [31].
ROP5 alleles used in this study were defined in previous studies [9,18] and were obtained from NCBI accession records for the following strains: GT1, VEG, and ME49 alleles (BK008043.1-BK008057.1), VAND alleles (JQ743716.1, JQ743730.1, JQ743760.1, JQ743772.1, JQ743778.1, JQ743783.1), TgCtBr5 alleles (JQ743734.1, JQ743742.1, JQ743743.1). Briefly, ROP5 alleles for GT1, VEG and ME49 were previously reconstructed in Behnke et al. [9] by aligning Sanger sequences of each strain to a reference ROP5 CDS allowing for the reconstruction of SNPs patterns. In this case, the Sanger sequences are long enough to combine overlapping reads containing the same SNP patterns together to define novel alleles, as detailed in Figure S6 of Behnke et al., [9]. Alleles for VAND and TgCtBr5 were previously defined by allele-specific amplification and direct sequencing as described in Niedelman et al. [18].
Copy-number variation (CNV) for the ROP5 locus was determined as previously described [31]. Briefly, NextGen sequence reads of the respective genomes were aligned to the ME49 assembled chromosomes using bowtie2 –end-to-end [55] the average 1X read bases for each genome and the average read bases for TGME49_308090, TGME49_308093, and TGME49_308096 were determined using samtools mpileup exported data [56]. The reads surrounding the ROP5 locus for each genome were exported with samtools mpileup and data were graphed in R. To determine CNV for individual ROP5 alleles, the reads aligning to the region surrounding TGME49_308090 were exported from the bowtie2 alignment using mpileup and realigned to the ME49 assembled chromosomes using CLC Genomics Workbench. Reads that corresponded to a particular allele were identified based on SNP pattern and the allele CNV was estimating by calculating the ratio of allele reads as compared to the total CNV for the ROP5 locus [9].
Phylogenetic analysis and diversifying selection
DNA sequences were translated and aligned using MUSCLE with Geneious v7.0.4. Phylogenetic analysis of the protein sequences was implemented in RAxML [57] using maximum likelihood and the JTT+I+G+F model. For maximum likelihood, we used the ModelGenerator v85 [58] to determine the most appropriate amino acid substitution model based on the Akaike Information Criterion. Positively selected codons were estimated by the Fast Unconstrained Bayesian AppRoximation method using HyPhy package [59,60]. Posterior probability values above 0.95 were chosen for codon sites where the distribution of non-synonymous substitution rates (β) are significantly greater than the synonymous (α) substitution rate.
Molecular modeling studies
Amino acid residues at the ROP5/IRGa6 interface were identified by PDBePISA [32,33] based on the crystal structure of ROP5BI (corresponding to ROP5 GT1-m1 in the present study) bound to murine IRGa6 (PDB code 4LV5) [19]. Residues close to the interface were identified by visual inspection. Figures were generated in PyMOL.
Supporting Information
Zdroje
1. Dubey JP, Jones JL (2008) Toxoplasma gondii infection in humans and animals in the United States. Int J Parasitol 38 : 1257–1278. doi: 10.1016/j.ijpara.2008.03.007 18508057
2. Dubey JP (2009) History of the discovery of the life cycle of Toxoplasma gondii. Int J Parasitol 39 : 877–882. 19630138
3. Xiao J, Yolken RH (2015) Strain hypothesis of Toxoplasma gondii infection on the outcome of human diseases. Acta Physiol (Oxf).
4. Howe DK, Sibley LD (1995) Toxoplasma gondii comprises three clonal lineages: correlation of parasite genotype with human disease. J Infect Dis 172 : 1561–1566. 7594717
5. Sibley LD, Boothroyd JC (1992) Virulent strains of Toxoplasma gondii comprise a single clonal lineage. Nature 359 : 82–85. 1355855
6. Su C, Howe DK, Dubey JP, Ajioka JW, Sibley LD (2002) Identification of quantitative trait loci controlling acute virulence in Toxoplasma gondii. Proc Natl Acad Sci (USA) 99 : 10753–10758.
7. Saeij JP, Boyle JP, Coller S, Taylor S, Sibley LD, et al. (2006) Polymorphic secreted kinases are key virulence factors in toxoplasmosis. Science 314 : 1780–1783. 17170306
8. Taylor S, Barragan A, Su C, Fux B, Fentress SJ, et al. (2006) A secreted serine-threonine kinase determines virulence in the eukaryotic pathogen Toxoplasma gondii. Science 314 : 1776–1780. 17170305
9. Behnke MS, Khan A, Wootton JC, Dubey JP, Tang K, et al. (2011) Virulence differences in Toxoplasma mediated by amplification of a family of polymorphic pseudokinases. Proceedings of the National Academy of Sciences of the United States of America 108 : 9631–9636. doi: 10.1073/pnas.1015338108 21586633
10. Reese ML, Zeiner GM, Saeij JP, Boothroyd JC, Boyle JP (2011) Polymorphic family of injected pseudokinases is paramount in Toxoplasma virulence. Proceedings of the National Academy of Sciences of the United States of America 108 : 9625–9630. doi: 10.1073/pnas.1015980108 21436047
11. Hunter CA, Sibley LD (2012) Modulation of innate immunity by Toxoplasma gondii virulence effectors. Nat Rev Microbiol 10 : 766–778. doi: 10.1038/nrmicro2858 23070557
12. Fentress SJ, Behnke MS, Dunay IR, Mashayekhi M, Rommereim LM, et al. (2010) Phosphorylation of immunity-related GTPases by a Toxoplasma gondii-secreted kinase promotes macrophage survival and virulence. Cell Host Microbe 8 : 484–495. doi: 10.1016/j.chom.2010.11.005 21147463
13. Reese ML, Boothroyd JC (2009) A helical membrane-binding domain targets the Toxoplasma ROP2 family to the parasitophorous vacuole. Traffic 10 : 1458–1470. doi: 10.1111/j.1600-0854.2009.00958.x 19682324
14. Fentress SJ, Sibley LD (2012) An arginine-rich domain of ROP18 is necessary for vacuole targeting and virulence in Toxopalsma gondii. Cell Microbiol 14 : 1921–1933. doi: 10.1111/cmi.12022 22906355
15. Steinfeldt T, Konen-Waisman S, Tong L, Pawlowski N, Lamkemeyer T, et al. (2010) Phosphorylation of mouse immunity-related GTPase (IRG) resistance proteins is an evasion strategy for virulent Toxoplasma gondii. PLoS Biol 8: e1000576. doi: 10.1371/journal.pbio.1000576 21203588
16. Behnke MS, Fentress SJ, Mashayekhi M, Li LX, Taylor GA, et al. (2012) The polymorphic pseudokinase ROP5 controls virulence in Toxoplasma gondii by regulating the active kinase ROP18. PLoS Pathog 8: e1002992. doi: 10.1371/journal.ppat.1002992 23144612
17. Fleckenstein MC, Reese ML, Konen-Waisman S, Boothroyd JC, Howard JC, et al. (2012) A Toxoplasma gondii pseudokinase inhibits host IRG resistance proteins. PLoS Biol 10: e1001358. doi: 10.1371/journal.pbio.1001358 22802726
18. Niedelman W, Gold DA, Rosowski EE, Sprokholt JK, Lim D, et al. (2012) The rhoptry proteins ROP18 and ROP5 mediate Toxoplasma gondii evasion of the murine, but not the human, interferon-gamma response. PLoS Pathog 8: e1002784. doi: 10.1371/journal.ppat.1002784 22761577
19. Reese ML, Shah N, Boothroyd JC (2014) The Toxoplasma pseudokinase ROP5 is an allosteric inhibitor of the immunity-related GTPases. The Journal of biological chemistry 289 : 27849–27858. doi: 10.1074/jbc.M114.567057 25118287
20. Etheridge RD, Alaganan A, Tang K, Lou HJ, Turk BE, et al. (2014) The Toxoplasma pseudokinase ROP5 forms complexes with ROP18 and ROP17 kinases that synergize to control acute virulence in mice. Cell Host Microbe 15 : 537–550. doi: 10.1016/j.chom.2014.04.002 24832449
21. Khan A, Fux B, Su C, Dubey JP, Darde ML, et al. (2007) Recent transcontinental sweep of Toxoplasma gondii driven by a single monomorphic chromosome. Proc Natl Acad Sci (USA) 104 : 14872–14877.
22. Su C, Khan A, Zhou P, Majumdar D, Ajzenberg D, et al. (2012) Globally diverse Toxoplasma gondii isolates comprise six major clades originating from a small number of distinct ancestral lineages. Proceedings of the National Academy of Sciences of the United States of America 109 : 5844–5849. doi: 10.1073/pnas.1203190109 22431627
23. Fux B, Nawas J, Khan A, Gill DB, Su C, et al. (2007) Toxoplasma gondii strains defective in oral transmission are also defective in developmental stage differentiation. Infect Immun 75 : 2580–2590. 17339346
24. Khan A, Taylor S, Ajioka JW, Rosenthal BM, Sibley LD (2009) Selection at a single locus leads to widespread expansion of Toxoplasma gondii lineages that are virulent in mice. PLoS Genet 5: e1000404. doi: 10.1371/journal.pgen.1000404 19266027
25. Khan A, Shaik JS, Behnke M, Wang Q, Dubey JP, et al. (2014) NextGen sequencing reveals short double crossovers contribute disproportionately to genetic diversity in Toxoplasma gondii. BMC Genomics 15 : 1168. doi: 10.1186/1471-2164-15-1168 25532601
26. Lunde MN, Jacobs L (1983) Antigenic differences between endozoites and cystozoites of Toxoplasma gondii. J Parasitol 69 : 806–808. 6200590
27. Carme B, Bissuel F, Ajzenberg D, Bouyne R, Aznar C, et al. (2002) Severe acquired toxoplasmosis in immunocompetent adult patients in French Guiana. J Clin Microbiol 40 : 4037–4044. 12409371
28. Shaik JS, Khan A, Beverley SM, Sibley L (2015) REDHORSE-REcombination and Double crossover detection in Haploid Organisms using next-geneRation SEquencing data. BMC Genomics 16 : 133. doi: 10.1186/s12864-015-1309-7 25766039
29. Behnke MS, Khan A, Sibley LD (2015) Genetic Mapping Reveals that Sinefungin Resistance in Toxoplasma gondii Is Controlled by a Putative Amino Acid Transporter Locus That Can Be Used as a Negative Selectable Marker. Eukaryotic cell 14 : 140–148. doi: 10.1128/EC.00229-14 25480939
30. Shen B, Brown KM, Lee TD, Sibley LD (2014) Efficient gene disruption in diverse strains of Toxoplasma gondii using CRISPR/CAS9. MBio 5: e01114–01114. doi: 10.1128/mBio.01114-14 24825012
31. Lorenzi H, Khan A, Behnke MS, Namasivayam S, Seshadri S, et al. (2015) Local admixture of amplified and diversified secreted pathogenesis determinants shapes mosaic Toxoplasma gondii genomes. Nature Communications Under Review.
32. Krissinel E (2010) Crystal contacts as nature's docking solutions. Journal of computational chemistry 31 : 133–143. doi: 10.1002/jcc.21303 19421996
33. Krissinel E, Henrick K (2007) Inference of macromolecular assemblies from crystalline state. Journal of molecular biology 372 : 774–797. 17681537
34. Walzer KA, Adomako-Ankomah Y, Dam RA, Herrmann DC, Schares G, et al. (2013) Hammondia hammondi, an avirulent relative of Toxoplasma gondii, has functional orthologs of known T. gondii virulence genes. Proceedings of the National Academy of Sciences of the United States of America 110 : 7446–7451. doi: 10.1073/pnas.1304322110 23589877
35. Fox BA, Ristuccia JG, Gigley JP, Bzik DJ (2009) Efficient gene replacements in Toxoplasma gondii strains deficient for nonhomologous end joining. Eukaryotic cell 8 : 520–529. doi: 10.1128/EC.00357-08 19218423
36. Huynh MH, Carruthers VB (2009) Tagging of endogenous genes in a Toxoplasma gondii strain lacking Ku80. Eukaryotic cell 8 : 530–539. doi: 10.1128/EC.00358-08 19218426
37. Hanks SK, Hunter T (1995) Protein kinases 6. The eukaryotic protein kinase superfamily: kinase (catalytic) domain structure and classification. FASEB Journal 9 : 576–596. 7768349
38. Alaganan A, Fentress SJ, Tang K, Wang Q, Sibley LD (2014) Toxoplasma GRA7 effector increases turnover of immunity-related GTPases and contributes to acute virulence in the mouse. Proceedings of the National Academy of Sciences of the United States of America 111 : 1126–1131. doi: 10.1073/pnas.1313501111 24390541
39. Khan A, Behnke MS, Dunay IR, White MW, Sibley LD (2009) Phenotypic and gene expression changes among clonal type I strains of Toxoplasma gondii. Eukaryotic cell 8 : 1828–1836. doi: 10.1128/EC.00150-09 19801420
40. Gazzinelli RT, Mendonca-Neto R, Lilue J, Howard J, Sher A (2014) Innate resistance against Toxoplasma gondii: an evolutionary tale of mice, cats, and men. Cell Host Microbe 15 : 132–138. doi: 10.1016/j.chom.2014.01.004 24528860
41. Yamamoto M, Ma JS, Mueller C, Kamiyama N, Saiga H, et al. (2011) ATF6beta is a host cellular target of the Toxoplasma gondii virulence factor ROP18. J Exp Med 208 : 1533–1546. doi: 10.1084/jem.20101660 21670204
42. Sanchez V, de-la-Torre A, Gomez-Marin JE (2014) Characterization of ROP18 alleles in human toxoplasmosis. Parasitol Int 63 : 463–469. doi: 10.1016/j.parint.2013.10.012 24177250
43. Khan A, Jordan C, Muccioli C, Vallochi AL, Rizzo LV, et al. (2006) Genetic divergence of Toxoplasma gondii strains associated with ocular toxoplasmosis, Brazil. Emerg Infect Dis 12 : 942–949. 16707050
44. Carme B, Bissuel F, Ajzenberg D, Bouyne R, Aznar C, et al. (2002) Severe acquired toxoplasmsis in immunocompetent adult patients in French Guiana. J Clin Microbiol 40 : 4037–4044. 12409371
45. Dardé ML, Villena I, Pinon JM, Beguinot I (1998) Severe toxoplasmosis caused by a Toxoplasma gondii strain with a new isotype acquired in French Guyana. J Clin Microbiol 36 : 324. 9431981
46. Demar M, Hommel D, Djossou F, Peneau C, Boukhari R, et al. (2012) Acute toxoplasmoses in immunocompetent patients hospitalized in an intensive care unit in French Guiana. Clin Microbiol Infect 18: E221–231. doi: 10.1111/j.1469-0691.2011.03648.x 21958195
47. Lilue J, Muller UB, Steinfeldt T, Howard JC (2013) Reciprocal virulence and resistance polymorphism in the relationship between Toxoplasma gondii and the house mouse. Elife 2: e01298. doi: 10.7554/eLife.01298 24175088
48. Vigne JD, Guilaine J, Debue K, Haye L, Gerard P (2004) Early taming of the cat in Cyprus. Science 304 : 259. 15073370
49. Pfefferkorn ER, Kasper LH (1983) Toxoplasma gondii: genetic crosses reveal phenotypic suppression of hydroxyurea resistance by fluorodeoxyuridine resistance. Exp Parasitol 55 : 207–218. 6219892
50. Khan A, Ajzenberg D, Mercier A, Demar M, Simon S, et al. (2014) Geographic separation of domestic and wild strains of Toxoplasma gondii in French Guiana correlates with a monomorphic version of chromosome1a. PLoS Negl Trop Dis 8: e3182. doi: 10.1371/journal.pntd.0003182 25233228
51. Smith R, Sheppard K, DiPetrillo K, Churchill G (2009) Quantitative trait locus analysis using J/qtl. Methods Mol Biol 573 : 175–188. doi: 10.1007/978-1-60761-247-6_10 19763928
52. Heaslip AT, Nishi M, Stein B, Hu K (2011) The motility of a human parasite, Toxoplasma gondii, is regulated by a novel lysine methyltransferase. PLoS Pathog 7: e1002201. doi: 10.1371/journal.ppat.1002201 21909263
53. Gajria B, Bahl A, Brestelli J, Dommer J, Fischer S, et al. (2008) ToxoDB: an integrated Toxoplasma gondii database resource. Nucleic Acids Res 36: D553–556. 18003657
54. Huson DH, Bryant D (2006) Application of phylogenetic networks in evolutionary studies. Mol Biol Evol 23 : 254–267. 16221896
55. Langmead B, Salzberg SL (2012) Fast gapped-read alignment with Bowtie 2. Nat Methods 9 : 357–359. doi: 10.1038/nmeth.1923 22388286
56. Li H, Handsaker B, Wysoker A, Fennell T, Ruan J, et al. (2009) The Sequence Alignment/Map format and SAMtools. Bioinformatics 25 : 2078–2079. doi: 10.1093/bioinformatics/btp352 19505943
57. Stamatakis A (2006) RAxML-VI-HPC: maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics 22 : 2688–2690. 16928733
58. Keane TM, Creevey CJ, Pentony MM, Naughton TJ, McLnerney JO (2006) Assessment of methods for amino acid matrix selection and their use on empirical data shows that ad hoc assumptions for choice of matrix are not justified. BMC Evol Biol 6 : 29. 16563161
59. Murrell B, Moola S, Mabona A, Weighill T, Sheward D, et al. (2013) FUBAR: a fast, unconstrained bayesian approximation for inferring selection. Mol Biol Evol 30 : 1196–1205. doi: 10.1093/molbev/mst030 23420840
60. Pond SL, Frost SD, Muse SV (2005) HyPhy: hypothesis testing using phylogenies. Bioinformatics 21 : 676–679. 15509596
Štítky
Genetika Reprodukční medicína
Článek Loss and Gain of Natural Killer Cell Receptor Function in an African Hunter-Gatherer PopulationČlánek Let-7 Represses Carcinogenesis and a Stem Cell Phenotype in the Intestine via Regulation of Hmga2Článek Binding of Multiple Rap1 Proteins Stimulates Chromosome Breakage Induction during DNA ReplicationČlánek SLIRP Regulates the Rate of Mitochondrial Protein Synthesis and Protects LRPPRC from DegradationČlánek Protein Composition of Infectious Spores Reveals Novel Sexual Development and Germination Factors inČlánek The Formin Diaphanous Regulates Myoblast Fusion through Actin Polymerization and Arp2/3 RegulationČlánek Runx1 Transcription Factor Is Required for Myoblasts Proliferation during Muscle Regeneration
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2015 Číslo 8- Růst a vývoj dětí narozených pomocí IVF
- Délka menstruačního cyklu jako marker ženské plodnosti
- Vztah užívání alkoholu a mužské fertility
- Intrauterinní inseminace a její úspěšnost
- Šanci na úspěšný průběh těhotenství snižují nevhodné hladiny progesteronu vznikající při umělém oplodnění
-
Všechny články tohoto čísla
- Putting the Brakes on Huntington Disease in a Mouse Experimental Model
- Identification of Driving Fusion Genes and Genomic Landscape of Medullary Thyroid Cancer
- Evidence for Retromutagenesis as a Mechanism for Adaptive Mutation in
- TSPO, a Mitochondrial Outer Membrane Protein, Controls Ethanol-Related Behaviors in
- Evidence for Lysosome Depletion and Impaired Autophagic Clearance in Hereditary Spastic Paraplegia Type SPG11
- Loss and Gain of Natural Killer Cell Receptor Function in an African Hunter-Gatherer Population
- Trans-Reactivation: A New Epigenetic Phenomenon Underlying Transcriptional Reactivation of Silenced Genes
- Early Developmental and Evolutionary Origins of Gene Body DNA Methylation Patterns in Mammalian Placentas
- Strong Selective Sweeps on the X Chromosome in the Human-Chimpanzee Ancestor Explain Its Low Divergence
- Dominance of Deleterious Alleles Controls the Response to a Population Bottleneck
- Transient 1a Induction Defines the Wound Epidermis during Zebrafish Fin Regeneration
- Systems Genetics Reveals the Functional Context of PCOS Loci and Identifies Genetic and Molecular Mechanisms of Disease Heterogeneity
- A Genome Scale Screen for Mutants with Delayed Exit from Mitosis: Ire1-Independent Induction of Autophagy Integrates ER Homeostasis into Mitotic Lifespan
- Non-synonymous FGD3 Variant as Positional Candidate for Disproportional Tall Stature Accounting for a Carcass Weight QTL () and Skeletal Dysplasia in Japanese Black Cattle
- The Relationship between Gene Network Structure and Expression Variation among Individuals and Species
- Calmodulin Methyltransferase Is Required for Growth, Muscle Strength, Somatosensory Development and Brain Function
- The Wnt Frizzled Receptor MOM-5 Regulates the UNC-5 Netrin Receptor through Small GTPase-Dependent Signaling to Determine the Polarity of Migrating Cells
- Nbs1 ChIP-Seq Identifies Off-Target DNA Double-Strand Breaks Induced by AID in Activated Splenic B Cells
- CCNYL1, but Not CCNY, Cooperates with CDK16 to Regulate Spermatogenesis in Mouse
- Evidence for a Common Origin of Blacksmiths and Cultivators in the Ethiopian Ari within the Last 4500 Years: Lessons for Clustering-Based Inference
- Of Fighting Flies, Mice, and Men: Are Some of the Molecular and Neuronal Mechanisms of Aggression Universal in the Animal Kingdom?
- Hypoxia and Temperature Regulated Morphogenesis in
- The Homeodomain Iroquois Proteins Control Cell Cycle Progression and Regulate the Size of Developmental Fields
- Evolution and Design Governing Signal Precision and Amplification in a Bacterial Chemosensory Pathway
- Rac1 Regulates Endometrial Secretory Function to Control Placental Development
- Let-7 Represses Carcinogenesis and a Stem Cell Phenotype in the Intestine via Regulation of Hmga2
- Functions as a Positive Regulator of Growth and Metabolism in
- The Nucleosome Acidic Patch Regulates the H2B K123 Monoubiquitylation Cascade and Transcription Elongation in
- Rhoptry Proteins ROP5 and ROP18 Are Major Murine Virulence Factors in Genetically Divergent South American Strains of
- Exon 7 Contributes to the Stable Localization of Xist RNA on the Inactive X-Chromosome
- Regulates Refractive Error and Myopia Development in Mice and Humans
- mTORC1 Prevents Preosteoblast Differentiation through the Notch Signaling Pathway
- Regulation of Gene Expression Patterns in Mosquito Reproduction
- Molecular Basis of Gene-Gene Interaction: Cyclic Cross-Regulation of Gene Expression and Post-GWAS Gene-Gene Interaction Involved in Atrial Fibrillation
- The Spalt Transcription Factors Generate the Transcriptional Landscape of the Wing Pouch Central Region
- Binding of Multiple Rap1 Proteins Stimulates Chromosome Breakage Induction during DNA Replication
- Functional Divergence in the Role of N-Linked Glycosylation in Smoothened Signaling
- YAP1 Exerts Its Transcriptional Control via TEAD-Mediated Activation of Enhancers
- Coordinated Evolution of Influenza A Surface Proteins
- The Evolutionary Potential of Phenotypic Mutations
- Genome-Wide Association and Trans-ethnic Meta-Analysis for Advanced Diabetic Kidney Disease: Family Investigation of Nephropathy and Diabetes (FIND)
- New Routes to Phylogeography: A Bayesian Structured Coalescent Approximation
- SLIRP Regulates the Rate of Mitochondrial Protein Synthesis and Protects LRPPRC from Degradation
- Satellite DNA Modulates Gene Expression in the Beetle after Heat Stress
- SHOEBOX Modulates Root Meristem Size in Rice through Dose-Dependent Effects of Gibberellins on Cell Elongation and Proliferation
- Reduced Crossover Interference and Increased ZMM-Independent Recombination in the Absence of Tel1/ATM
- Suppression of Somatic Expansion Delays the Onset of Pathophysiology in a Mouse Model of Huntington’s Disease
- Protein Composition of Infectious Spores Reveals Novel Sexual Development and Germination Factors in
- The Evolutionarily Conserved LIM Homeodomain Protein LIM-4/LHX6 Specifies the Terminal Identity of a Cholinergic and Peptidergic . Sensory/Inter/Motor Neuron-Type
- SmD1 Modulates the miRNA Pathway Independently of Its Pre-mRNA Splicing Function
- piRNAs Are Associated with Diverse Transgenerational Effects on Gene and Transposon Expression in a Hybrid Dysgenic Syndrome of .
- Retinoic Acid Signaling Regulates Differential Expression of the Tandemly-Duplicated Long Wavelength-Sensitive Cone Opsin Genes in Zebrafish
- The Formin Diaphanous Regulates Myoblast Fusion through Actin Polymerization and Arp2/3 Regulation
- Genome-Wide Analysis of PAPS1-Dependent Polyadenylation Identifies Novel Roles for Functionally Specialized Poly(A) Polymerases in
- Runx1 Transcription Factor Is Required for Myoblasts Proliferation during Muscle Regeneration
- Regulation of Mutagenic DNA Polymerase V Activation in Space and Time
- Variability of Gene Expression Identifies Transcriptional Regulators of Early Human Embryonic Development
- The Drosophila Gene Interacts Genetically with and Shows Female-Specific Effects of Divergence
- Functional Activation of the Flagellar Type III Secretion Export Apparatus
- Retrohoming of a Mobile Group II Intron in Human Cells Suggests How Eukaryotes Limit Group II Intron Proliferation
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Exon 7 Contributes to the Stable Localization of Xist RNA on the Inactive X-Chromosome
- YAP1 Exerts Its Transcriptional Control via TEAD-Mediated Activation of Enhancers
- SmD1 Modulates the miRNA Pathway Independently of Its Pre-mRNA Splicing Function
- Molecular Basis of Gene-Gene Interaction: Cyclic Cross-Regulation of Gene Expression and Post-GWAS Gene-Gene Interaction Involved in Atrial Fibrillation
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



