-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Of Fighting Flies, Mice, and Men: Are Some of the Molecular and Neuronal Mechanisms of Aggression Universal in the Animal Kingdom?
Aggressive behavior is widespread in the animal kingdom, but the degree of molecular conservation between distantly related species is still unclear. Recent reports suggest that at least some of the molecular mechanisms underlying this complex behavior in flies show remarkable similarities with such mechanisms in mice and even humans. Surprisingly, some aspects of neuronal control of aggression also show remarkable similarity between these distantly related species. We will review these recent findings, address the evolutionary implications, and discuss the potential impact for our understanding of human diseases characterized by excessive aggression.
Published in the journal: . PLoS Genet 11(8): e32767. doi:10.1371/journal.pgen.1005416
Category: Review
doi: https://doi.org/10.1371/journal.pgen.1005416Summary
Aggressive behavior is widespread in the animal kingdom, but the degree of molecular conservation between distantly related species is still unclear. Recent reports suggest that at least some of the molecular mechanisms underlying this complex behavior in flies show remarkable similarities with such mechanisms in mice and even humans. Surprisingly, some aspects of neuronal control of aggression also show remarkable similarity between these distantly related species. We will review these recent findings, address the evolutionary implications, and discuss the potential impact for our understanding of human diseases characterized by excessive aggression.
Introduction
Aggression is a complex social behavior present in most, perhaps even all, animal species. Being aggressive benefits animals to compete for valuable resources but is energetically costly and carries the risk of injury, loss of resources, and even death [1–4]. In humans, symptoms of excessive aggressive behavior can be a complicating factor in the treatment of neurological syndromes and diseases [5–8]. Despite the fact that aggression is vital for organismal fitness and is important in human society and disease, much remains to be discovered about the underlying molecular and neuronal mechanisms that drive this behavior. Decades of research in many model systems have identified several signaling molecules implicated in aggressive behavior, including classical neurotransmitters and neuropeptides, and much has been learned about the brain regions involved in aggression (for review, see [9–11]). However, the degree to which mechanisms in distantly related species are conserved remains largely unexplored.
In this review, we will focus exclusively on recent findings on transcriptional regulation and neuropeptide signaling [12,13] in aggressive behavior in Drosophila melanogaster, which suggest an unexpected degree of conservation with mammals. We will speculate on the evolutionary implications of these findings in the vinegar fly as well as their potential implications for human health and disease. For a more in-depth review of the different mechanisms that have been described in different species, several recent reviews provide excellent background that both complement and contrast with the viewpoint that we will discuss here [9–11,14–18].
Conserved Transcriptional Control of Aggressive Behavior?
Conserved transcriptional modules that consist of conserved transcription factors, their co-activators or repressors, DNA binding sites, and targets (Fig 1A) regulate related complex biological programs in organisms throughout the evolutionary tree. The field of developmental biology is replete with examples of conserved transcriptional regulatory mechanisms, although there is also enormous flexibility in the evolutionary deployment of these signaling modules [19–26]. Fewer examples exist in the control of behavior, but the classical example is the conserved transcriptional double feedback loop that regulates circadian rhythm across a broad range of species [27–29]. Konopka isolated several mutations in one of the key transcription factors in this feedback loop in the now-classic screen for altered eclosion rhythm in Drosophila [30,31]. This single experiment transformed the field of circadian biology [32], but the idea that a mutation in a single gene could significantly impact “complex” behavior was not without skeptics [33].
Fig. 1. Evolutionary conservation of molecular and neuronal aggression mechanisms. 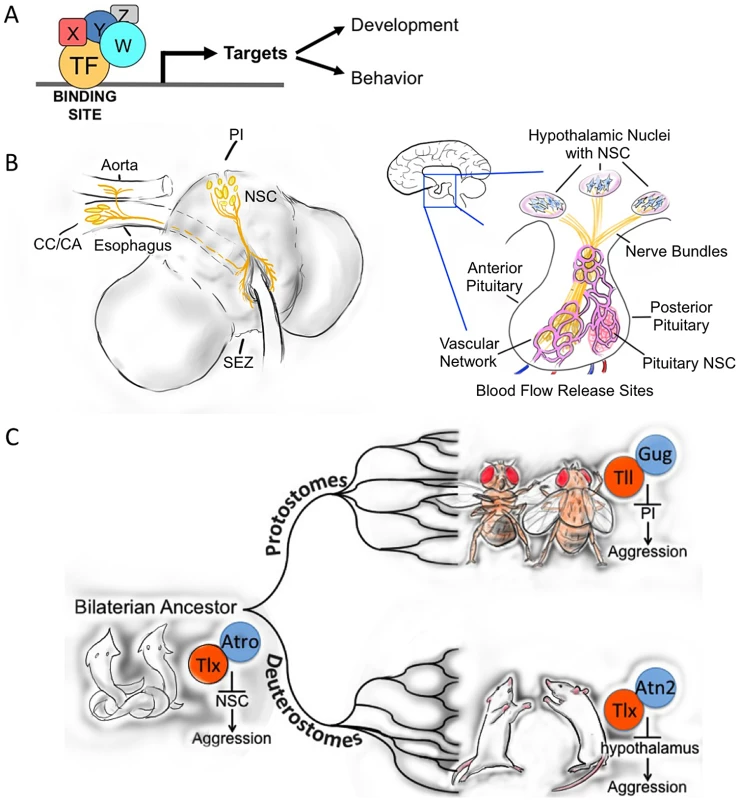
A. Transcriptional control module, consisting of conserved transcription factors, co-factors, DNA binding sites, and activated or repressed target genes, regulating development and/or behavior. B. Comparative diagram of the structural relationship of the Drosophila PI and mammalian hypothalamus. C. Simplified evolutionary tree showing the putative conserved transcriptional control mechanism that regulates the release of neuropeptides from the neurosecretory cells in the brain in the regulation of aggression. We postulate that this control module was already present in the bilaterian ancestor (more than 600 million years ago [MYA]) and operates today in extant protostome and deuterostome species (Illustrations courtesy of Josh Rivera). Abbreviations: PI, pars intercerebralis; NSC, neurosecretory cells; SEZ, subesophageal zone; CC, corpora cardiaca; CA, corpora allata. Does a conserved transcriptional module that controls aggression in all animals exist? Twenty years ago, a transcriptional repressor orthologous to Drosophila Tailless (Tll) was identified in the mouse and shown to be expressed in the developing brain [34]. The mouse knock-out of tailless (tlx, now called Nr2e1) displayed abnormal brain development and behavior, including extreme aggression [35,36]. An independently isolated Nr2e1 null mutant was called fierce because of its extreme pathological aggression [37]. The fierce mutant was fully rescued with a genomic clone that covered the human NR2E1 gene, demonstrating conservation of gene function and regulation between mouse and human [38]. How Nr2e1 regulates aggression, however, has remained unsolved.
Because Tll is a Drosophila ortholog of Nr2e1 with conserved co-repressors [39–42], binding sites [34], and targets [43], we explored whether Tll might also affect aggression in Drosophila. We found that knock-down of tll affects aggression, similarly to its effect in mice. Interestingly, we showed Tll functions through the neurosecretory cells of the adult pars intercerebralis (PI) [13], a brain region similar to the mammalian hypothalamus [44], which is known for its critical role in aggression [18,45–48].
In mice, Nr2e1 is expressed in numerous regions throughout the brain, and Nr2e1 - / - mice have brain abnormalities including reduced brain size, smaller olfactory bulbs, incomplete extension of the cortex, and reduced cortical layers II and III [35,36,49]. It is not clear whether these morphological brain abnormalities cause the behavioral phenotypes, or whether the developmental and behavioral abnormalities can be uncoupled, as is the case in the fly. A conditional mutant was generated using a CamKIIα - Cre line expressing Cre recombinase in the hippocampus, cortex, amygdala, striatum, thalamus, and hypothalamus [50]. Morphologically, these conditional null mutants (CamKIIα-Cre; Nr2e1Flox/Flox) have structural brain abnormalities similar to mice derived from a germline mutation in Nr2e1, and although they have normal contextual, associative, and spatial learning, they are hyperaggressive, suggesting that a role of Nr2e1 in the regulation of aggressive behavior can be mapped to CamKIIα positive neurons [51]. Whether the effect of Nr2e1 on aggression involves the hypothalamus specifically and whether its effect is developmental or adult specific is currently not known.
Additional evidence for the role of Tll as part of a conserved transcriptional module controlling aggression in Drosophila comes from Atrophin, a conserved co-repressor of Tll in flies and mice [40]. Although Atrophin is pan-neuronally expressed, knock-down in the PI alone mimicked Tll knock-down in these cells. Moreover, Tll and Atro genetically and physically interact in the PI, suggesting that they function together to control aggression through these neurosecretory cells [13].
In mice, two Atrophin paralogs exist, Atrophin 1 (Atn1) and Atrophin 2 (Atn2), each having features in common with the fly ortholog [40]. Drosophila Atrophin (also known as Grunge, Gug) shares the most sequence similarity with Atn2 (also known as Rere), which is the longer of the two mammalian Atrophins, but also harbors a polyQ stretch unique to Atn1 [40]. Intriguingly, a polyQ expansion of human ATN1 causes dentatorubral pallidoluysian atrophy (DRPLA) [52,53], a neurodegenerative disease similar to Huntington disease. Patients with this devastating progressive neurodegenerative disorder also have psychiatric symptoms, including abnormal aggressive behavior [54]. In some cases, abnormal behavior precedes the onset of neurodegenerative symptoms [55]. While this could be explained by abnormal neuronal function preceding the actual degeneration of the neurons, it is also possible that the mechanisms that cause neurodegeneration may be uncoupled from the behavioral effects. Interestingly, polyQ expanded Atn1 binds more strongly to Atn2 [56], raising the possibility that the mechanism underlying abnormal aggression in DRPLA patients may depend on diminished ATN2 function and may be part of a putative transcriptional module controlling the function of the hypothalamus, similar to what we found in Drosophila. This hypothesis is consistent with the fact that some of the phenotypes associated with polyQ expanded repeats are due to gain-of-function effects, while others are caused by loss-of-function effects [57].
What is the neuronal mechanism of this transcription factor complex in the control of aggression? Given that Tll and Atro function in the PI of the adult fly, the neurosecretory function of this brain region is a logical candidate. Blocking neuropeptide processing or release fully suppressed the aggression phenotype induced by knock-down of tll [13], showing that neuropeptide signaling is required to mediate the transcriptional regulation of Tll in its control of aggression through the PI.
Conserved Neuropeptide Signaling in the Control of Aggressive Behavior
Neuropeptides are typically involved in the regulation of a specific subset of neurons, as opposed to globally across the entire brain [58]. They play important roles in the control of a range of physiological processes [59] as well as innate behaviors [60]. Several neuropeptides have been implicated in mammalian aggression [61,62], and vasopressin may be the most notable of these [63].
In Drosophila, direct evidence for a specific neuropeptide affecting aggression was lacking until recently. Asahina et al. found this evidence by exploring the role of neuropeptides in aggression in a somewhat unusual manner [12]. They cloned parts of the promoters of 18 of the 40 or so known neuropeptide encoding genes in Drosophila and used them to transgenically activate the neurons defined by these promoter fragments via a thermosensitive ion channel, TrpA1 [64]. This is one of the many tools developed in flies to manipulate neuronal activity [65], in this case, in a temperature-sensitive manner. They found that the promoter fragment of Tachykinin, a Drosophila ortholog of Substance P, caused a strong aggression response when combined with TrpA1 [12]. Loss of the Tachykinin neuropeptide partially suppressed this response, while its overexpression enhanced it, showing directly that Tachykinin plays a pivotal, albeit not exclusive, role in this response. Interestingly, loss of one of two known Drosophila Tachykinin receptor-encoding genes suppressed the response more strongly than loss of the peptide gene itself, indicating that this neuropeptide circuit is indeed important for aggression in flies but may receive other inputs as well. In addition, they found that a few of these Tachykinin-producing neurons are unique to Drosophila males, and that activating just this very small number of male-specific peptidergic neurons was necessary and sufficient to elicit the strong behavioral response. As is the case in many species, male flies show dramatically more aggression than females, and these male-specific Tachykinin neurons appear to be critical to this sex difference in aggressive behavior in flies. Although sex determination shows remarkable evolutionary plasticity, even in relatively related species [66,67], it is nevertheless intriguing that Drosophila Tachykinin plays a role in aggression because it is related to mammalian Substance P, which has also been implicated in aggression in mammals [68–70] but is probably better known for its role in pain transmission [59]. Even in humans, the levels of Substance P are correlated with aggression levels in certain patient groups [71]. While the evidence in humans for a role of substance P in aggression is limited, together, these results do suggest that another molecular pathway regulating aggression in flies shows conservation with mechanisms in mammals.
The peptidergic neurons defined by the Tachykinin promoter are distinct from the neurosecretory cells in the PI that are controlled by the action of the Tll/Atro transcriptional repressor complex [12,13]. Activation of the PI neurons also showed a significant increase in aggression, and this response was also completely suppressed by blocking neuropeptide processing, showing that activation of these neurons affects their peptidergic function [13]. Multiple neuropeptides are expressed in this complex group of neurons, and it is not clear at present what the culprit neuropeptide is for the aggression response caused by genetic electrical activation of the PI neurons or knock-down of tll or Atro. However, given that Tachykinin is expressed in a separate set of neurons, it suggests that different neuropeptides are involved in the regulation of aggression in flies (and perhaps converging onto the same output system). This complexity also mimics the complex neuropeptidergic control of aggression in mammals [63,69,72]. Such complex neuromodulatory control is also observed in other behaviors in flies and mice, including sleep, feeding, reproduction, and learning and memory [73–78].
Small Subsets of Neurons with Big Impact on Aggression: Conserved Neuronal Control of Aggression
As is clear from the data presented above, the very few male-specific Tachykinin-producing neurons and the small number of neurosecretory cells in the PI have a big impact on aggressive behavior in flies [12,13]. Intriguingly, the PI is thought to be very similar to the mammalian hypothalamus [44,79], a structure that is also important in aggression in many different mammals, including humans [18]. The interesting parallelism between the neuroendocrine systems of the pars intercerebralis–corpora cardiaca (and allata) in insects and hypothalamo-pituitary axis in mammals was noted long ago [80]. More recently, it has been argued that the fundamental elements of a primordial neuroendocrine system must have been present in the last common ancestor of flies and mammals, the first bilaterally symmetrical animals, or so-called bilaterian ancestor. This argument is based on structural, developmental, and functional similarities between the PI and the mammalian hypothalamus (reviewed in [44]). Other brain structures in insects have been found to show remarkable similarities with structures in the mammalian brain [81–83]. Structural, developmental, and functional similarities in central complex and basal ganglia have been suggested to reflect true homology rather than convergence [82].
Structurally, both the PI and hypothalamus contain neurosecretory cells that innervate endocrine centers important in the regulation of growth, metabolism, and reproduction and fertility of the animal (Fig 1B) [84,85]. While the mammalian pituitary combines a posterior neural portion with an anterior glandular portion, these “neuroglandular” parts are separated in insects in the corpora cardiaca and allata, although these structures are tightly connected in the ring gland of the fly [44,60]. Developmentally, the key signaling molecules that specify the precursor structures that will form the adult neuroendocrine systems in flies and mice are also remarkably conserved [44]. Functionally, the neurosecretory cells of the PI and hypothalamus produce a range of neuropeptides and regulate metabolism, growth, water homeostasis, sleep, and feeding behavior [86–94]. We have now shown that the PI also affects aggressive behavior in flies [13]. Moreover, in crickets, increased levels of the immediate early marker c-Fos have been detected after crickets engaged in aggressive encounters and after electrical stimulation of the antennae [95].
The evidence for the role of the mammalian hypothalamus in aggression is abundant and has been documented in many species. Studies in small mammals show that activation of specific regions of the hypothalamus is sufficient to initiate aggressive behavior [18,96–98]. In cats and rats, regions of the brain in and around the hypothalamus that control offensive and defensive aggression are known as the hypothalamic attack area [18,48,97]. In non-human primates, electrical stimulation and lesion studies have shown that the hypothalamus plays a role in aggressive vocalizations and physical aggressiveness [99,100]. In humans, abnormal hypothalamic oscillations have been linked to pathologic aggressive behavior [101]. Surgical interventions for hyperaggressiveness have targeted the hypothalamus and successfully reduced aggression in a subset of treated individuals [102–104]. Deep brain stimulation of the posterior hypothalamus has been used to treat hyperaggressive behavior [105,106]. Intraoperative electrical stimulation of a small region in the hypothalamus caused severe transient aggression in previously non-aggressive patients [107]. And a subset of patients with hypothalamic hamartomas display excessive aggressive behavior [108] that is resolved with removal of the tumor [109,110]. These data demonstrate that disruptions in hypothalamic function greatly alter aggressive behavior.
Evolutionary Implications: Is the Molecular and Neuronal Control of Aggression Deeply Conserved?
The evidence presented above for conserved molecular components that play a role in the control of aggression in flies and mice suggests a deep molecular root for these control mechanisms. The fact that the major neurosecretory regions of the adult brain in flies and mammals are also important in the control of aggression would at first seem quite remarkable, but given the already firm functional connection between the pars intercerebralis and the hypothalamus, it should not be that surprising. In Drosophila, we connected some of the pieces of the complex puzzle of the regulation of aggression and showed that Tll is critical for the transcriptional control of aggression by regulating neuropeptide release from these neurosecretory centers of the adult fly brain [13]. We propose that Nr2e1 may play the same role in the control of mammalian aggression by regulating hypothalamic release of specific neuropeptides. We speculate that these molecular and neuronal control mechanisms evolved in both protostomes and deuterstomes because the fundamental elements were already present in their last common precursor, the eubilaterian ancestor (Fig 1C). If this were indeed the case, we would expect to find in a very large part of the animal kingdom this unified mechanism of transcriptional control of neuropeptide release from neurosecretory cells in the control of aggression (Fig 1C).
What is the evidence that all these fundamental elements were already present in the last common ancestor of protostomes and deuterostomes? As has been alluded to above and detailed elsewhere [44], neurosecretory cells were already present in these early ancestors, but what about behavior? The urbilaterian and the later eubilaterian ancestors are thought to have been a flatworm-like animal [111–113]. While flatworms occupy a range of phyla [112], there is strong evidence that worm species do indeed show aggressive behavior [114,115]. The marine flatworm, Pseudoceros bifurcus, shows a remarkable mating behavior that has been described as “penis fencing” [114] that can last for several hours. When two of these hermaphrodites meet in shallow marine waters they will literally stab each other with their array of penises to inseminate the opponent. The inseminated animal then bears the cost of wound healing and fertilization [114]. Additional evidence that worms fight, despite their simple anatomy and lack of weaponry, was recently shown in the roundworm, Steinernema longicaudum (although they belong to the Ecdysozoa-like flies). Under certain conditions, males of this nematode species kill each other by what looks like strangulation [115]. Clearly, worm species are capable of fierce forms of aggression. The urbilaterian ancestor can be believed to have been a fighter.
What is known about the role for their Tailless ortholog? Both roundworms and flatworms have a Tll/Nr2e1 ortholog. In C. elegans the Tll ortholog is called Nhr67 and plays a number of developmental roles including gustatory neuron specification [116], although nothing is known about its role in behavior. The sequenced genome of the freshwater planaria, Schmidtea mediterranea, clearly shows a Tll and Atro ortholog [117]. Knock-down of its tll/Nr2e1 ortholog caused morphological and behavioral defects—shrinking of the brain region and slower movement [118]. Neuropeptide signaling also has deep molecular roots [119]. The idea that a universal transcriptional control module regulates neuropeptide release from adult neurosecretory cells in the control of aggression in all animals is at least plausible and certainly testable. The shared role in aggression of Substance P and a Drosophila ortholog, Tachykinin, further support conservation of molecular mechanisms between members of these distantly related phyla.
Do Any of These Mechanisms Apply to Humans and Human Disease?
As mentioned before, there is a large body of evidence for a role of the hypothalamus in human aggression. Some neuropeptides have also been implicated in human aggression, including Substance P [71]. Human NR2E1 has been associated with bipolar disease, schizophrenia, and psychopathy, diseases known for their association with abnormal aggression, but the evidence for NR2E1 directly affecting aggression in humans is certainly limited. Abnormal aggression has been more strongly connected to DRPLA, the previously mentioned neurodegenerative disease caused by a polyQ expanded repeat in ATN1 [52,53], an ortholog of Drosophila Atrophin [40]. A mouse model for this disease recapitulates both neurodegenerative phenotypes and excessive aggression [120,121] but, as mentioned above, the mechanism is not currently known.
To take a closer look at the connection between human disease and aggression, we compiled a list of all known Mendelian disorders with excessive aggressive behavior as part of their clinical picture. This search in Online Mendelian Inheritance in Man (OMIM) [122] produces a list of approximately 90 diseases and their causal genes. Although many of these diseases are developmental disorders characterized by mental retardation, intellectual disability alone is not likely to account for the increased aggression symptoms since there are more than 400 genes that cause intellectual disability [123] and relatively few are associated with increased aggression and some are associated with pleasant behavior [124–126]. Almost half of the proteins encoded by these disease genes are connected into a single network based on STRING analysis (Fig 2 and S1 Table) [127]. Not all of the interactions represent direct protein—protein interactions, but many of them do. The small number of disease genes associated with aggression and the highly connected nature of their network suggests specific and finite mechanisms. This observation stands in stark contrast with the suggestion that one-third of the genome may play a significant role in aggressive behavior [16]. One simple explanation for this discrepancy may be the low specificity of their assay used to assess aggression in flies. Although we still know very little about the regulation of aggression in humans and although it is no doubt more complex than in mice, let alone flies, investigating the role of this protein network in the control of the peptidergic function of the hypothalamus would be a good place to start.
Fig. 2. Human aggression disease interactome. 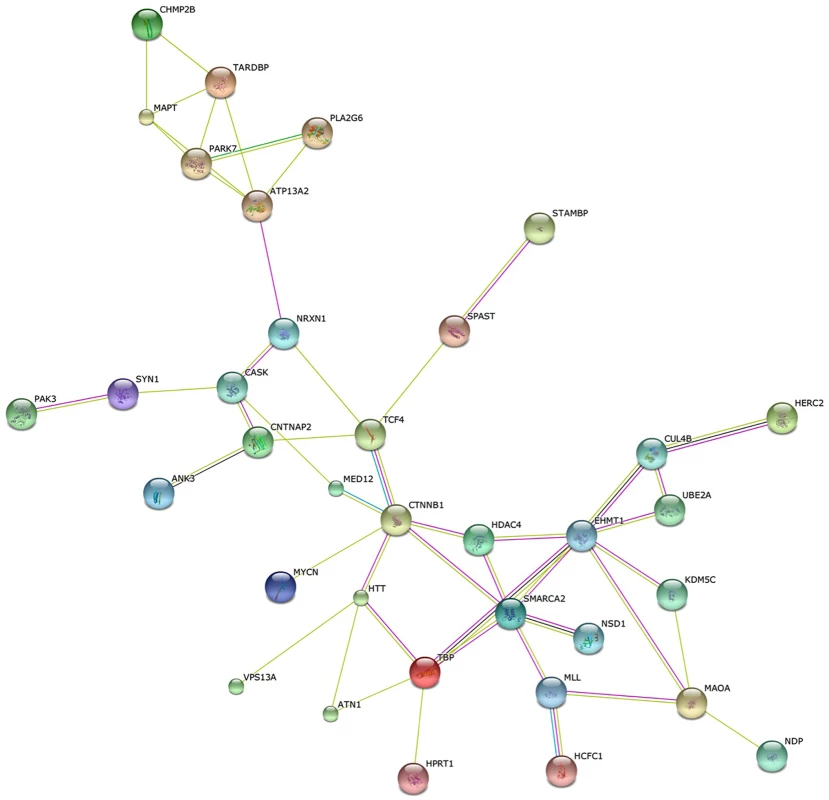
String analysis of a subset of proteins encoded by disease genes that are characterized by excessive aggression as part of their clinical picture. Roughly 40% of the approximately 90 genes in this category in OMIM form a single network (p = 4.7–10, S1 Table), suggesting that there are mechanistic connections between these components. Conclusion
Aggression is pervasive throughout the animal kingdom and is both a beneficial and costly behavior. Recent evidence suggests that both molecular and neuronal mechanisms underlying aggressive behavior may be conserved throughout evolutionary history. The orphan nuclear receptor Nr2e1 and its associated co-repressors affect aggressive behavior in both flies and mice, and evidence in flies shows that its effect on aggressive behavior depends on the neurosecretory function of a very small number of neurons in the adult brain. In humans, NR2E1 is part of a large protein interactome of disease genes with a clinical picture marked by aggression, suggesting that this transcriptional module may be part of a larger network of proteins that also regulate aggression in humans. Further examination of the role of this conserved transcriptional module should be carried out to better understand its role in aggression in animal models, as well as to pursue a putative explanation for some of the heterogeneity observed in human populations and disease.
Supporting Information
Zdroje
1. Miczek K a, Maxson SC, Fish EW, Faccidomo S (2001) Aggressive behavioral phenotypes in mice. Behav Brain Res 125 : 167–181. 11682108
2. Watts DP, Muller M, Amsler SJ, Mbabazi G, Mitani JC (2006) Lethal intergroup aggression by chimpanzees in Kibale National Park, Uganda. Am J Primatol 68 : 161–180. 16429415
3. Stevenson PA, Rillich J (2012) The decision to fight or flee—insights into underlying mechanism in crickets. Front Neurosci 6 : 118. doi: 10.3389/fnins.2012.00118 22936896
4. Williams JM, Oehlert GW, Carlis J V., Pusey AE (2004) Why do male chimpanzees defend a group range? Anim Behav 68 : 523–532.
5. Herrmann N, Cappell J, Eryavec GM, Lanctôt KL (2011) Changes in nursing burden following memantine for agitation and aggression in long-term care residents with moderate to severe Alzheimer’s disease: an open-label pilot study. CNS Drugs 25 : 425–433. doi: 10.2165/11588160-000000000-00000 21476613
6. Langthorne P, McGill P (2012) An indirect examination of the function of problem behavior associated with fragile X syndrome and Smith-Magenis syndrome. J Autism Dev Disord 42 : 201–209. doi: 10.1007/s10803-011-1229-6 21442360
7. Moskowitz LJ, Carr EG, Durand VM (2011) Behavioral intervention for problem behavior in children with fragile X syndrome. Am J Intellect Dev Disabil 116 : 457–478. doi: 10.1352/1944-7558-116.6.457 22126659
8. Powell A, Flynn P, Rischbieth S, McKellar D (2014) Managing severe aggression in frontotemporal dementia. Australas Psychiatry 22 : 86–89. doi: 10.1177/1039856213510576 24176944
9. Adams DB (2006) Brain mechanisms of aggressive behavior: an updated review. Neurosci Biobehav Rev 30 : 304–318. 16289283
10. Comai S, Tau M, Gobbi G (2012) The psychopharmacology of aggressive behavior: a translational approach: part 1: neurobiology. J Clin Psychopharmacol 32 : 83–94. 22198449
11. Nelson RJ, Trainor BC (2007) Neural mechanisms of aggression. Nat Rev Neurosci 8 : 536–546. 17585306
12. Asahina K, Watanabe K, Duistermars BJ, Hoopfer E, González CR, et al. (2014) Tachykinin-Expressing Neurons Control Male-Specific Aggressive Arousal in Drosophila. Cell 156 : 221–235. doi: 10.1016/j.cell.2013.11.045 24439378
13. Davis SM, Thomas AL, Nomie KJ, Huang L, Dierick HA (2014) Tailless and Atrophin control Drosophila aggression by regulating neuropeptide signalling in the pars intercerebralis. Nat Commun 5 : 3177. doi: 10.1038/ncomms4177 24495972
14. Anderson DJ (2012) Optogenetics, sex, and violence in the brain: implications for psychiatry. Biol Psychiatry 71 : 1081–1089. doi: 10.1016/j.biopsych.2011.11.012 22209636
15. Zwarts L, Versteven M, Callaerts P (2012) Genetics and neurobiology of aggression in Drosophila. Fly 6 : 35–48. doi: 10.4161/fly.19249 22513455
16. Anholt RRH, Mackay TFC (2012) Genetics of aggression. Annu Rev Genet 46 : 145–164. doi: 10.1146/annurev-genet-110711-155514 22934647
17. Fernández MP, Kravitz E a (2013) Aggression and courtship in Drosophila: pheromonal communication and sex recognition. J Comp Physiol A Neuroethol Sens Neural Behav Physiol 199 : 1065–1076. doi: 10.1007/s00359-013-0851-5 24043358
18. Haller J (2013) The neurobiology of abnormal manifestations of aggression—a review of hypothalamic mechanisms in cats, rodents, and humans. Brain Res Bull 93 : 97–109. doi: 10.1016/j.brainresbull.2012.10.003 23085544
19. Gehring WJ, Ikeo K (1999) Pax 6: mastering eye morphogenesis and eye evolution. Trends Genet 15 : 371–377. 10461206
20. Kumar JP (2001) Signalling pathways in Drosophila and vertebrate retinal development. Nat Rev Genet 2 : 846–857. 11715040
21. Fisher a. L, Caudy M (1998) Groucho proteins: transcriptional corepressors for specific subsets of DNA-binding transcription factors in vertebrates and invertebrates. Genes Dev 12 : 1931–1940. 9649497
22. Fritzsch B, Beisel KW (2001) Evolution of the Nervous System. Evolution and development of the vertebrate ear. Brain Res Bull 55 : 711–721.
23. Hobert O, Westphal H (2000) Functions of LIM-homeobox genes. Trends Genet 16 : 75–83. 10652534
24. Nieto MA (2002) The snail superfamily of zinc-finger transcription factors. Nat Rev Mol Cell Biol 3 : 155–166. 11994736
25. Patient RK, McGhee JD (2002) The GATA family (vertebrates and invertebrates). Curr Opin Genet Dev 12 : 416–422. 12100886
26. Weinmaster G (1997) The ins and outs of notch signaling. Mol Cell Neurosci 9 : 91–102. 9245493
27. Hall JC (2005) Systems approaches to biological rhythms in Drosophila. Methods Enzymol 393 : 61–185. 15817287
28. Hardin PE (2005) The circadian timekeeping system of Drosophila. Curr Biol 15: R714–22. 16139204
29. Rosato E, Kyriacou CP (2001) Flies, clocks and evolution. Philos Trans R Soc Lond B Biol Sci 356 : 1769–1778. 11710984
30. Konopka RJ, Benzer S (1971) Clock mutants of Drosophila melanogaster. Proc Natl Acad Sci U S A 68 : 2112–2116. 5002428
31. Takahashi JS (2004) Finding new clock components: past and future. J Biol Rhythms 19 : 339–347. 15536063
32. Rosbash M (2015) Ronald J. Konopka (1947–2015). Cell 161 : 187–188. 26042238
33. Greenspan RJ (1990) The emergence of neurogenetics. Semin Neurosci 2 : 145–157.
34. Yu RT, McKeown M, Evans RM, Umesono K (1994) Relationship between Drosophila gap gene tailless and a vertebrate nuclear receptor Tlx. Nature 370 : 375–379. 8047143
35. Monaghan AP, Bock D, Gass P, Schwäger A, Wolfer DP, et al. (1997) Defective limbic system in mice lacking the tailless gene. Nature 390 : 515–517. 9394001
36. Roy K, Thiels E, Monaghan AP (2002) Loss of the tailless gene affects forebrain development and emotional behavior. Physiol Behav 77 : 595–600. 12527005
37. Young KA, Berry ML, Mahaffey CL, Saionz JR, Hawes NL, et al. (2002) Fierce: a new mouse deletion of Nr2e1; violent behaviour and ocular abnormalities are background-dependent. Behav Brain Res 132 : 145–158. 11997145
38. Abrahams BS, Kwok MCH, Trinh E, Budaghzadeh S, Hossain SM, et al. (2005) Pathological aggression in “fierce” mice corrected by human nuclear receptor 2E1. J Neurosci 25 : 6263–6270. 16000615
39. Wang L, Rajan H, Pitman JL, McKeown M, Tsai C-C (2006) Histone deacetylase - associating Atrophin proteins are nuclear receptor corepressors. Genes Dev 20 : 525–530. 16481466
40. Wang L, Tsai C-C (2008) Atrophin proteins: an overview of a new class of nuclear receptor corepressors. Nucl Recept Signal 6: e009. doi: 10.1621/nrs.06009 19043594
41. Zhang C-L, Zou Y, Yu RT, Gage FH, Evans RM (2006) Nuclear receptor TLX prevents retinal dystrophy and recruits the corepressor atrophin1. Genes Dev 20 : 1308–1320. 16702404
42. Zhang S, Xu L, Lee J, Xu T (2002) Drosophila atrophin homolog functions as a transcriptional corepressor in multiple developmental processes. Cell 108 : 45–56. 11792320
43. Yu RT, Chiang MY, Tanabe T, Kobayashi M, Yasuda K, et al. (2000) The orphan nuclear receptor Tlx regulates Pax2 and is essential for vision. Proc Natl Acad Sci U S A 97 : 2621–2625. 10706625
44. Hartenstein V (2006) The neuroendocrine system of invertebrates: a developmental and evolutionary perspective. J Endocrinol 190 : 555–570. 17003257
45. Kruk MR (1991) Ethology and pharmacology of hypothalamic aggression in the rat. Neurosci Biobehav Rev 15 : 527–538. 1792015
46. Lin D, Boyle MP, Dollar P, Lee H, Lein ES, et al. (2011) Functional identification of an aggression locus in the mouse hypothalamus. Nature 470 : 221–226. doi: 10.1038/nature09736 21307935
47. Siegel a, Roeling T a, Gregg TR, Kruk MR (1999) Neuropharmacology of brain - stimulation-evoked aggression. Neurosci Biobehav Rev 23 : 359–389. 9989425
48. Toth M, Fuzesi T, Halasz J, Tulogdi A, Haller J (2010) Neural inputs of the hypothalamic “aggression area” in the rat. Behav Brain Res 215 : 7–20. doi: 10.1016/j.bbr.2010.05.050 20685366
49. Land PW, Monaghan AP (2003) Expression of the transcription factor, tailless, is required for formation of superficial cortical layers. Cereb Cortex 13 : 921–931. 12902391
50. Casanova E, Fehsenfeld S, Mantamadiotis T, Lemberger T, Greiner E, et al. (2001) A CamKIIalpha iCre BAC allows brain-specific gene inactivation. Genesis 31 : 37–42. 11668676
51. Belz T, Liu H-K, Bock D, Takacs A, Vogt M, et al. (2007) Inactivation of the gene for the nuclear receptor tailless in the brain preserving its function in the eye. Eur J Neurosci 26 : 2222–2227. 17953618
52. Koide R, Ikeuchi T, Onodera O, Tanaka H, Igarashi S, et al. (1994) Unstable expansion of CAG repeat in hereditary dentatorubral-pallidoluysian atrophy (DRPLA). Nat Genet 6 : 9–13. 8136840
53. Nagafuchi S, Yanagisawa H, Ohsaki E, Shirayama T, Tadokoro K, et al. (1994) Structure and expression of the gene responsible for the triplet repeat disorder, dentatorubral and pallidoluysian atrophy (DRPLA). Nat Genet 8 : 177–182. 7842016
54. Adachi N, Arima K, Asada T, Kato M, Minami N, et al. (2001) Dentatorubral - pallidoluysian atrophy (DRPLA) presenting with psychosis. J Neuropsychiatry Clin Neurosci 13 : 258–260. 11449034
55. Licht DJ, Lynch DR (2002) Juvenile dentatorubral-pallidoluysian atrophy: new clinical features. Pediatr Neurol 26 : 51–54. 11814736
56. Yanagisawa H, Bundo M, Miyashita T, Okamura-Oho Y, Tadokoro K, et al. (2000) Protein binding of a DRPLA family through arginine-glutamic acid dipeptide repeats is enhanced by extended polyglutamine. Hum Mol Genet 9 : 1433–1442. 10814707
57. Crespo-Barreto J, Fryer JD, Shaw C, Orr HT, Zoghbi HY (2010) Partial loss of ataxin-1 function contributes to transcriptional dysregulation in spinocerebellar ataxia type 1 pathogenesis. PLoS Genet 6: e1001021. doi: 10.1371/journal.pgen.1001021 20628574
58. Kandel E, Schwartz J, Jessell T (2000) Principles of Neural Science. New Jersey: McGraw-Hill Medical. 10634775
59. Strand F (1999) Neuropeptides. Cambridge, MA: MIT Press.
60. Nässel DR, Winther AME (2010) Drosophila neuropeptides in regulation of physiology and behavior. Prog Neurobiol 92 : 42–104. doi: 10.1016/j.pneurobio.2010.04.010 20447440
61. Li Q, Deng X, Singh P (2007) Significant increase in the aggressive behavior of transgenic mice overexpressing peripheral progastrin peptides: associated changes in CCK2 and serotonin receptors in the CNS. Neuropsychopharmacology 32 : 1813–1821. 17228339
62. Rutkoski NJ, Lerant AA, Nolte CM, Westberry J, Levenson CW (2002) Regulation of neuropeptide Y in the rat amygdala following unilateral olfactory bulbectomy. Brain Res 951 : 69–76. 12231458
63. Caldwell HK, Lee H-J, Macbeth AH, Young WS (2008) Vasopressin: behavioral roles of an “original” neuropeptide. Prog Neurobiol 84 : 1–24. 18053631
64. Hamada FN, Rosenzweig M, Kang K, Pulver SR, Ghezzi A, et al. (2008) An internal thermal sensor controlling temperature preference in Drosophila. Nature 454 : 217–220. doi: 10.1038/nature07001 18548007
65. Venken KJT, Simpson JH, Bellen HJ (2011) Genetic manipulation of genes and cells in the nervous system of the fruit fly. Neuron 72 : 202–230. doi: 10.1016/j.neuron.2011.09.021 22017985
66. Haag ES, Doty AV (2005) Sex determination across evolution: connecting the dots. PLoS Biol 3: e21. 15660158
67. Salz HK (2011) Sex determination in insects: a binary decision based on alternative splicing. Curr Opin Genet Dev 21 : 395–400. doi: 10.1016/j.gde.2011.03.001 21474300
68. Bhatt S, Gregg TR, Siegel A (2003) NK1 receptors in the medial hypothalamus potentiate defensive rage behavior elicited from the midbrain periaqueductal gray of the cat. Brain Res 966 : 54–64. 12646308
69. Katsouni E, Sakkas P, Zarros A, Skandali N, Liapi C (2009) The involvement of substance P in the induction of aggressive behavior. Peptides 30 : 1586–1591. doi: 10.1016/j.peptides.2009.05.001 19442694
70. Shaikh MB, Steinberg A, Siegel A (1993) Evidence that substance P is utilized in medial amygdaloid facilitation of defensive rage behavior in the cat. Brain Res 625 : 283–294. 7506110
71. Coccaro EF, Lee R, Owens MJ, Kinkead B, Nemeroff CB (2012) Cerebrospinal fluid substance P-like immunoreactivity correlates with aggression in personality disordered subjects. Biol Psychiatry 72 : 238–243. doi: 10.1016/j.biopsych.2012.02.023 22449753
72. Karl T, Herzog H (2007) Behavioral profiling of NPY in aggression and neuropsychiatric diseases. Peptides 28 : 326–333. 17196302
73. Cansell C, Denis RGP, Joly-Amado A, Castel J, Luquet S (2012) Arcuate AgRP neurons and the regulation of energy balance. Front Endocrinol (Lausanne) 3 : 169.
74. Foltenyi K, Andretic R, Newport JW, Greenspan RJ (2007) Neurohormonal and Neuromodulatory Control of Sleep in Drosophila. Cold Spring Harb Symp Quant Biol 72 : 565–72. doi: 10.1101/sqb.2007.72.045 18419316
75. Griffith LC (2013) Neuromodulatory control of sleep in Drosophila melanogaster: integration of competing and complementary behaviors. Curr Opin Neurobiol 23 : 819–823. doi: 10.1016/j.conb.2013.05.003 23743247
76. Kahsai L, Zars T (2011) Learning and memory in Drosophila: behavior, genetics, and neural systems. Int Rev Neurobiol 99 : 139–167. doi: 10.1016/B978-0-12-387003-2.00006-9 21906539
77. Taghert PH, Nitabach MN (2012) Peptide neuromodulation in invertebrate model systems. Neuron 76 : 82–97. doi: 10.1016/j.neuron.2012.08.035 23040808
78. Dembrow N, Johnston D (2014) Subcircuit-specific neuromodulation in the prefrontal cortex. Front Neural Circuits 8 : 54. doi: 10.3389/fncir.2014.00054 24926234
79. De Velasco B, Erclik T, Shy D, Sclafani J, Lipshitz H, et al. (2007) Specification and development of the pars intercerebralis and pars lateralis, neuroendocrine command centers in the Drosophila brain. Dev Biol 302 : 309–323. 17070515
80. Scharrer B, Scharrer E (1944) Neurosecretion VI. A comparison between the Intercerebralis-Cardiacum-Allatum system of insects and the Hypothalamo - Hypophyseal system of Vertebrates. Biol Bull 87 : 242–251.
81. Lin C-Y, Chuang C-C, Hua T-E, Chen C-C, Dickson BJ, et al. (2013) A comprehensive wiring diagram of the protocerebral bridge for visual information processing in the Drosophila brain. Cell Rep 3 : 1739–1753. doi: 10.1016/j.celrep.2013.04.022 23707064
82. Strausfeld NJ, Hirth F (2013) Homology versus convergence in resolving transphyletic correspondences of brain organization. Brain Behav Evol 82 : 215–219. doi: 10.1159/000356102 24296550
83. Strausfeld NJ, Hirth F, Origin BF (2013) Deep Homology of Arthropod Central Complex and Vertebrate Basal Ganglia. 340 : 157–162.
84. Rak-Mardyla A (2013) Ghrelin role in hypothalamus-pituitary-ovarian axis. J Physiol Pharmacol 64 : 695–704. 24388883
85. Siga S (2003) Anatomy and functions of brain neurosecretory cells in diptera. Microsc Res Tech 62 : 114–131. 12966498
86. Al-Anzi B, Armand E, Nagamei P, Olszewski M, Sapin V, et al. (2010) The leucokinin pathway and its neurons regulate meal size in Drosophila. Curr Biol 20 : 969–978. doi: 10.1016/j.cub.2010.04.039 20493701
87. Crocker A, Shahidullah M, Levitan IB, Sehgal A (2010) Identification of a neural circuit that underlies the effects of octopamine on sleep:wake behavior. Neuron 65 : 670–681. doi: 10.1016/j.neuron.2010.01.032 20223202
88. Foltenyi K, Greenspan RJ, Newport JW (2007) Activation of EGFR and ERK by rhomboid signaling regulates the consolidation and maintenance of sleep in Drosophila. Nat Neurosci 10 : 1160–1167. 17694052
89. Rajan A, Perrimon N (2012) Drosophila cytokine unpaired 2 regulates physiological homeostasis by remotely controlling insulin secretion. Cell 151 : 123–137. doi: 10.1016/j.cell.2012.08.019 23021220
90. Rulifson EJ, Kim SK, Nusse R (2002) Ablation of insulin-producing neurons in flies: growth and diabetic phenotypes. Science 296 : 1118–1120. 12004130
91. Schwartz MW, Seeley RJ, Campfield LA, Burn P, Baskin DG (1996) Identification of targets of leptin action in rat hypothalamus. J Clin Invest 98 : 1101–1106. 8787671
92. Stanley BG, Leibowitz SF (1985) Neuropeptide Y injected in the paraventricular hypothalamus: a powerful stimulant of feeding behavior. Proc Natl Acad Sci U S A 82 : 3940–3943. 3858854
93. Teubner BJW, Bartness TJ (2013) PYY(3–36) into the arcuate nucleus inhibits food deprivation-induced increases in food hoarding and intake. Peptides 47 : 20–28. doi: 10.1016/j.peptides.2013.05.005 23816798
94. Zandawala M (2012) Calcitonin-like diuretic hormones in insects. Insect Biochem Mol Biol 42 : 816–825. doi: 10.1016/j.ibmb.2012.06.006 22820711
95. Ghosal K, Naples SP, Rabe AR, Killian KA (2010) Agonistic behavior and electrical stimulation of the antennae induces Fos-like protein expression in the male cricket brain. Arch Insect Biochem Physiol 74 : 38–51. doi: 10.1002/arch.20360 20422717
96. Chi CC, Flynn JP (1971) Neuroanatomic projections related to biting attack elicited from hypothalamus in cats. Brain Res 35 : 49–66. 5167403
97. Kruk MR, Van der Laan CE, Mos J, Van der Poel AM, Meelis W, et al. (1984) Comparison of aggressive behaviour induced by electrical stimulation in the hypothalamus of male and female rats. Prog Brain Res 61 : 303–314. 6543251
98. Woodworth CH (1971) Attack elicited in rats by electrical stimulation of the lateral hypothalamus. Physiol Behav 6 : 345–353. 4948213
99. Lipp HP, Hunsperger RW (1978) Threat, attack and flight elicited by electrical stimulation of the ventromedial hypothalamus of the marmoset monkey Callithrix jacchus. Brain Behav Evol 15 : 260–293. 100172
100. Perachio AA, Alexander M, Marr LD (1973) Hormonal and social factors affecting evoked sexual behavior in Rhesus monkeys. Am J Phys Anthropol 38 : 227–232. 4632072
101. Rosa M, Franzini A, Giannicola G, Messina G, Altamura AC, et al. (2012) Hypothalamic oscillations in human pathological aggressiveness. Biol Psychiatry 72: e33–5. doi: 10.1016/j.biopsych.2012.06.007 22789687
102. Dieckmann G, Schneider-Jonietz B, Schneider H (1988) Psychiatric and neuropsychological findings after stereotactic hypothalamotomy, in cases of extreme sexual aggressivity. Acta Neurochir Suppl (Wien) 44 : 163–166.
103. Ramamurthi B (1988) Stereotactic operation in behaviour disorders. Amygdalotomy and hypothalamotomy. Acta Neurochir Suppl (Wien) 44 : 152–157.
104. Sano K, Mayanagi Y, Sekino H, Ogashiwa M, Ishijima B (1970) Results of stimulation and destruction of the posterior hypothalamus in man. J Neurosurg 33 : 689–707. 5488801
105. Franzini A, Messina G, Cordella R, Marras C, Broggi G (2010) Deep brain stimulation of the posteromedial hypothalamus: indications, long-term results, and neurophysiological considerations. Neurosurg Focus 29: E13.
106. Pedrosa-Sánchez M, Sola RG (2003) [Modern day psychosurgery: a new approach to neurosurgery in psychiatric disease]. Rev Neurol 36 : 887–897. 12717678
107. Bejjani BP, Houeto JL, Hariz M, Yelnik J, Mesnage V, et al. (2002) Aggressive behavior induced by intraoperative stimulation in the triangle of Sano. Neurology 59 : 1425–1427. 12427896
108. Reeves AG, Plum F (1969) Hyperphagia, rage, and dementia accompanying a ventromedial hypothalamic neoplasm. Arch Neurol 20 : 616–624. 5769839
109. De Almeida AN, Fonoff ET, Ballester G, Teixeira MJ, Marino R (2008) Stereotactic disconnection of hypothalamic hamartoma to control seizure and behavior disturbance: case report and literature review. Neurosurg Rev 31 : 343–349. doi: 10.1007/s10143-008-0142-8 18443834
110. Weissenberger AA, Dell ML, Liow K, Theodore W, Frattali CM, et al. (2001) Aggression and psychiatric comorbidity in children with hypothalamic hamartomas and their unaffected siblings. J Am Acad Child Adolesc Psychiatry 40 : 696–703. 11392348
111. Baguñà J, Riutort M (2004) The dawn of bilaterian animals: the case of acoelomorph flatworms. Bioessays 26 : 1046–1057. 15382134
112. Bailly X, Reichert H, Hartenstein V (2013) The urbilaterian brain revisited: novel insights into old questions from new flatworm clades. Dev Genes Evol 223 : 149–157. doi: 10.1007/s00427-012-0423-7 23143292
113. Miller DJ, Ball EE (2009) The gene complement of the ancestral bilaterian—was Urbilateria a monster? J Biol 8 : 89. doi: 10.1186/jbiol192 19939290
114. Michiels N, Newman L (1998) Sex and violence in hermaphrodites. Nature 266 : 20560.
115. Zenner ANRL, O’Callaghan KM, Griffin CT (2014) Lethal Fighting in Nematodes Is Dependent on Developmental Pathway: Male-Male Fighting in the Entomopathogenic Nematode Steinernema longicaudum. PLoS One 9: e89385. doi: 10.1371/journal.pone.0089385 24586738
116. Sarin S, Antonio C, Tursun B, Hobert O (2009) The C. elegans Tailless/TLX transcription factor nhr-67 controls neuronal identity and left/right asymmetric fate diversification. Development 136 : 2933–2944. doi: 10.1242/dev.040204 19641012
117. Adamidi C, Wang Y, Gruen D, Mastrobuoni G, You X, et al. (2011) De novo assembly and validation of planaria transcriptome by massive parallel sequencing and shotgun proteomics. Genome Res 21 : 1193–1200. doi: 10.1101/gr.113779.110 21536722
118. Raška O, Kostrouchová V, Behenský F, Yilma P, Saudek V, et al. (2011) SMED - TLX-1 (NR2E1) is critical for tissue and body plan maintenance in Schmidtea mediterranea in fasting/feeding cycles. Folia Biol (Praha) 57 : 223–231.
119. Mirabeau O, Joly J (2013) Molecular evolution of peptidergic signaling systems in bilaterians. Proc Natl Acad Sci U S A 110(22):E2028–37 doi: 10.1073/pnas.1219956110 23671109
120. Schilling G, Wood JD, Duan K, Slunt HH, Gonzales V, et al. (1999) Nuclear accumulation of truncated atrophin-1 fragments in a transgenic mouse model of DRPLA. Neuron 24 : 275–286. 10677044
121. Schilling G, Jinnah HA, Gonzales V, Coonfield ML, Kim Y, et al. (2001) Distinct behavioral and neuropathological abnormalities in transgenic mouse models of HD and DRPLA. Neurobiol Dis 8 : 405–418. 11442350
122. Amberger J, Bocchini C, Scott AF, Hamosh A (2009) McKusick’s Online Mendelian Inheritance in Man (OMIM). Nucleic Acids Res 37: D793–6. doi: 10.1093/nar/gkn665 18842627
123. Oortveld MAW, Keerthikumar S, Oti M, Nijhof B, Fernandes AC, et al. (2013) Human intellectual disability genes form conserved functional modules in Drosophila. PLoS Genet 9: e1003911. doi: 10.1371/journal.pgen.1003911 24204314
124. Egger JI, Wingbermühle E, Verhoeven WM, Dijkman M, Radke S, et al. (2013) Hypersociability in the behavioral phenotype of 17q21.31 microdeletion syndrome. Am J Med Genet A 161A: 21–26. doi: 10.1002/ajmg.a.35652 23169757
125. Evans E, Einfeld S, Mowat D, Taffe J, Tonge B, et al. (2012) The behavioral phenotype of Mowat-Wilson syndrome. Am J Med Genet A 158A: 358–366. doi: 10.1002/ajmg.a.34405 22246645
126. Williams CA (2010) The behavioral phenotype of the Angelman syndrome. Am J Med Genet C Semin Med Genet 154C: 432–437. doi: 10.1002/ajmg.c.30278 20981772
127. Franceschini A, Szklarczyk D, Frankild S, Kuhn M, Simonovic M, et al. (2013) STRING v9.1: protein-protein interaction networks, with increased coverage and integration. Nucleic Acids Res 41: D808–15. doi: 10.1093/nar/gks1094 23203871
Štítky
Genetika Reprodukční medicína
Článek Loss and Gain of Natural Killer Cell Receptor Function in an African Hunter-Gatherer PopulationČlánek Let-7 Represses Carcinogenesis and a Stem Cell Phenotype in the Intestine via Regulation of Hmga2Článek Binding of Multiple Rap1 Proteins Stimulates Chromosome Breakage Induction during DNA ReplicationČlánek SLIRP Regulates the Rate of Mitochondrial Protein Synthesis and Protects LRPPRC from DegradationČlánek Protein Composition of Infectious Spores Reveals Novel Sexual Development and Germination Factors inČlánek The Formin Diaphanous Regulates Myoblast Fusion through Actin Polymerization and Arp2/3 RegulationČlánek Runx1 Transcription Factor Is Required for Myoblasts Proliferation during Muscle Regeneration
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2015 Číslo 8- Růst a vývoj dětí narozených pomocí IVF
- Délka menstruačního cyklu jako marker ženské plodnosti
- Vztah užívání alkoholu a mužské fertility
- Šanci na úspěšný průběh těhotenství snižují nevhodné hladiny progesteronu vznikající při umělém oplodnění
- Vliv melatoninu a cirkadiálního rytmu na ženskou reprodukci
-
Všechny články tohoto čísla
- Putting the Brakes on Huntington Disease in a Mouse Experimental Model
- Identification of Driving Fusion Genes and Genomic Landscape of Medullary Thyroid Cancer
- Evidence for Retromutagenesis as a Mechanism for Adaptive Mutation in
- TSPO, a Mitochondrial Outer Membrane Protein, Controls Ethanol-Related Behaviors in
- Evidence for Lysosome Depletion and Impaired Autophagic Clearance in Hereditary Spastic Paraplegia Type SPG11
- Loss and Gain of Natural Killer Cell Receptor Function in an African Hunter-Gatherer Population
- Trans-Reactivation: A New Epigenetic Phenomenon Underlying Transcriptional Reactivation of Silenced Genes
- Early Developmental and Evolutionary Origins of Gene Body DNA Methylation Patterns in Mammalian Placentas
- Strong Selective Sweeps on the X Chromosome in the Human-Chimpanzee Ancestor Explain Its Low Divergence
- Dominance of Deleterious Alleles Controls the Response to a Population Bottleneck
- Transient 1a Induction Defines the Wound Epidermis during Zebrafish Fin Regeneration
- Systems Genetics Reveals the Functional Context of PCOS Loci and Identifies Genetic and Molecular Mechanisms of Disease Heterogeneity
- A Genome Scale Screen for Mutants with Delayed Exit from Mitosis: Ire1-Independent Induction of Autophagy Integrates ER Homeostasis into Mitotic Lifespan
- Non-synonymous FGD3 Variant as Positional Candidate for Disproportional Tall Stature Accounting for a Carcass Weight QTL () and Skeletal Dysplasia in Japanese Black Cattle
- The Relationship between Gene Network Structure and Expression Variation among Individuals and Species
- Calmodulin Methyltransferase Is Required for Growth, Muscle Strength, Somatosensory Development and Brain Function
- The Wnt Frizzled Receptor MOM-5 Regulates the UNC-5 Netrin Receptor through Small GTPase-Dependent Signaling to Determine the Polarity of Migrating Cells
- Nbs1 ChIP-Seq Identifies Off-Target DNA Double-Strand Breaks Induced by AID in Activated Splenic B Cells
- CCNYL1, but Not CCNY, Cooperates with CDK16 to Regulate Spermatogenesis in Mouse
- Evidence for a Common Origin of Blacksmiths and Cultivators in the Ethiopian Ari within the Last 4500 Years: Lessons for Clustering-Based Inference
- Of Fighting Flies, Mice, and Men: Are Some of the Molecular and Neuronal Mechanisms of Aggression Universal in the Animal Kingdom?
- Hypoxia and Temperature Regulated Morphogenesis in
- The Homeodomain Iroquois Proteins Control Cell Cycle Progression and Regulate the Size of Developmental Fields
- Evolution and Design Governing Signal Precision and Amplification in a Bacterial Chemosensory Pathway
- Rac1 Regulates Endometrial Secretory Function to Control Placental Development
- Let-7 Represses Carcinogenesis and a Stem Cell Phenotype in the Intestine via Regulation of Hmga2
- Functions as a Positive Regulator of Growth and Metabolism in
- The Nucleosome Acidic Patch Regulates the H2B K123 Monoubiquitylation Cascade and Transcription Elongation in
- Rhoptry Proteins ROP5 and ROP18 Are Major Murine Virulence Factors in Genetically Divergent South American Strains of
- Exon 7 Contributes to the Stable Localization of Xist RNA on the Inactive X-Chromosome
- Regulates Refractive Error and Myopia Development in Mice and Humans
- mTORC1 Prevents Preosteoblast Differentiation through the Notch Signaling Pathway
- Regulation of Gene Expression Patterns in Mosquito Reproduction
- Molecular Basis of Gene-Gene Interaction: Cyclic Cross-Regulation of Gene Expression and Post-GWAS Gene-Gene Interaction Involved in Atrial Fibrillation
- The Spalt Transcription Factors Generate the Transcriptional Landscape of the Wing Pouch Central Region
- Binding of Multiple Rap1 Proteins Stimulates Chromosome Breakage Induction during DNA Replication
- Functional Divergence in the Role of N-Linked Glycosylation in Smoothened Signaling
- YAP1 Exerts Its Transcriptional Control via TEAD-Mediated Activation of Enhancers
- Coordinated Evolution of Influenza A Surface Proteins
- The Evolutionary Potential of Phenotypic Mutations
- Genome-Wide Association and Trans-ethnic Meta-Analysis for Advanced Diabetic Kidney Disease: Family Investigation of Nephropathy and Diabetes (FIND)
- New Routes to Phylogeography: A Bayesian Structured Coalescent Approximation
- SLIRP Regulates the Rate of Mitochondrial Protein Synthesis and Protects LRPPRC from Degradation
- Satellite DNA Modulates Gene Expression in the Beetle after Heat Stress
- SHOEBOX Modulates Root Meristem Size in Rice through Dose-Dependent Effects of Gibberellins on Cell Elongation and Proliferation
- Reduced Crossover Interference and Increased ZMM-Independent Recombination in the Absence of Tel1/ATM
- Suppression of Somatic Expansion Delays the Onset of Pathophysiology in a Mouse Model of Huntington’s Disease
- Protein Composition of Infectious Spores Reveals Novel Sexual Development and Germination Factors in
- The Evolutionarily Conserved LIM Homeodomain Protein LIM-4/LHX6 Specifies the Terminal Identity of a Cholinergic and Peptidergic . Sensory/Inter/Motor Neuron-Type
- SmD1 Modulates the miRNA Pathway Independently of Its Pre-mRNA Splicing Function
- piRNAs Are Associated with Diverse Transgenerational Effects on Gene and Transposon Expression in a Hybrid Dysgenic Syndrome of .
- Retinoic Acid Signaling Regulates Differential Expression of the Tandemly-Duplicated Long Wavelength-Sensitive Cone Opsin Genes in Zebrafish
- The Formin Diaphanous Regulates Myoblast Fusion through Actin Polymerization and Arp2/3 Regulation
- Genome-Wide Analysis of PAPS1-Dependent Polyadenylation Identifies Novel Roles for Functionally Specialized Poly(A) Polymerases in
- Runx1 Transcription Factor Is Required for Myoblasts Proliferation during Muscle Regeneration
- Regulation of Mutagenic DNA Polymerase V Activation in Space and Time
- Variability of Gene Expression Identifies Transcriptional Regulators of Early Human Embryonic Development
- The Drosophila Gene Interacts Genetically with and Shows Female-Specific Effects of Divergence
- Functional Activation of the Flagellar Type III Secretion Export Apparatus
- Retrohoming of a Mobile Group II Intron in Human Cells Suggests How Eukaryotes Limit Group II Intron Proliferation
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Exon 7 Contributes to the Stable Localization of Xist RNA on the Inactive X-Chromosome
- YAP1 Exerts Its Transcriptional Control via TEAD-Mediated Activation of Enhancers
- SmD1 Modulates the miRNA Pathway Independently of Its Pre-mRNA Splicing Function
- Molecular Basis of Gene-Gene Interaction: Cyclic Cross-Regulation of Gene Expression and Post-GWAS Gene-Gene Interaction Involved in Atrial Fibrillation
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



