-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Evidence for Retromutagenesis as a Mechanism for Adaptive Mutation in
The basic principle of neo-Darwinian genetics is that mutations occurring during growth enable the subsequent survival of the mutants under selective environmental conditions. However, new mutants can arise from a non-growing bacterial population during selection in an apparently Lamarckian way. The phenomenon is called adaptive mutation. In one suggested pathway, retromutagenesis, a damaged gene produces a mutant protein that enables enough growth for a mutant gene to be copied onto daughter chromosomes. This hypothesis is supported by evidence that, in several experimental systems, a damaged gene can produce a mutant protein rather than no protein at all, and that both RNA and DNA polymerase will pair the same base with a lesion. Because this model requires gene expression before DNA synthesis, a third feature is predicted: in a non-growing population, adaptive mutations will occur preferentially on the transcribed strand of a gene. In this paper, we describe a bacterial genetic system that can distinguish between mutations occurring on either DNA strand, and we use it to confirm this prediction. The findings enhance our general understanding of evolution in all organisms, the majority of which are in a non-growing state most of the time.
Published in the journal: . PLoS Genet 11(8): e32767. doi:10.1371/journal.pgen.1005477
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1005477Summary
The basic principle of neo-Darwinian genetics is that mutations occurring during growth enable the subsequent survival of the mutants under selective environmental conditions. However, new mutants can arise from a non-growing bacterial population during selection in an apparently Lamarckian way. The phenomenon is called adaptive mutation. In one suggested pathway, retromutagenesis, a damaged gene produces a mutant protein that enables enough growth for a mutant gene to be copied onto daughter chromosomes. This hypothesis is supported by evidence that, in several experimental systems, a damaged gene can produce a mutant protein rather than no protein at all, and that both RNA and DNA polymerase will pair the same base with a lesion. Because this model requires gene expression before DNA synthesis, a third feature is predicted: in a non-growing population, adaptive mutations will occur preferentially on the transcribed strand of a gene. In this paper, we describe a bacterial genetic system that can distinguish between mutations occurring on either DNA strand, and we use it to confirm this prediction. The findings enhance our general understanding of evolution in all organisms, the majority of which are in a non-growing state most of the time.
Introduction
A central tenet of genetics is that mutation precedes selection rather than the reverse. This principle was verified by isolation, through sibling selection, of mutants from cultures that had never been exposed to the growth pressure of a selective agent [1,2]. In apparent contradiction to this principle, it was later observed that new mutants would arise continuously from an ostensibly non-growing population of cells for many days after being plated on a selective medium [3–5]. The phenomenon has been termed “adaptive mutation” or “stationary-phase mutagenesis.” Much of our knowledge of the underlying mechanisms has come from studies of reversions of frameshift mutations in the lacZ (β-galactosidase) gene of Escherichia coli in a system refined by Cairns and Foster [6]. As reviewed by Roth [7], the mechanisms include: occult growth of a sub-population aided by the residual activity of the defective gene product and amplification of the gene by homologous recombination, both of which increase the number of mutational targets, and stress-related amplification, and possibly induction of dinB, which encodes an error-prone DNA polymerase that may produce mutations during gene amplification. Thus, the apparent Lamarckian adaptations are actually due to occult mechanisms that still conform to the neo-Darwinian principle of mutation preceding selection. Controversy still exists around the contributions of residual growth and enhanced mutation rates, and so adaptive mutagenesis is best defined simply as the process by which new mutations arise under selective conditions regardless of the mechanism [7].
Davis [8] proposed a unique mechanism to explain reversion of lacZ mutants in a non-growing population. The lactose in the selection medium induces transcription of the lac operon, and the non-transcribed strand (NTS) is displaced by mRNA in a transcription bubble. Because the NTS is now single-stranded, it is unusually susceptible to environmental damage. Before the reversion mutation specified by the DNA damage can be expressed, however, a round of DNA replication is required to copy the defect onto the transcribed strand (TS). The energy for this DNA synthesis was proposed to come from the breakdown of cellular constituents, particularly ribosomes, which is expected to occur after three days of incubation on growth media. Davis’s model predicts that the new mutations will arise from the NTS.
An alternative mechanism, which we explore in this paper, was proposed by Bridges [3] and has come to be known as “retromutagenesis” [9]. It differs from most others in that expression of a damaged gene precedes its replication. Therefore, it will only yield mutants from damage to the TS of a gene. If the DNA damage escapes repair before DNA replication, the altered gene may be transcribed and translated to produce a mutant protein that enables the cell to undergo at least one round of DNA replication during selection (Fig 1). If the DNA polymerase then misreads the lesion in the same way that the RNA polymerase did, the mutation will be permanently established in a daughter cell. In contrast, lesions on the NTS will not enable the outgrowth of mutants under stringent selection because, before the mutant gene can be expressed, the mutation specified by the lesion must be copied onto the TS by a round of DNA replication.
Fig. 1. Example of retromutagenesis. 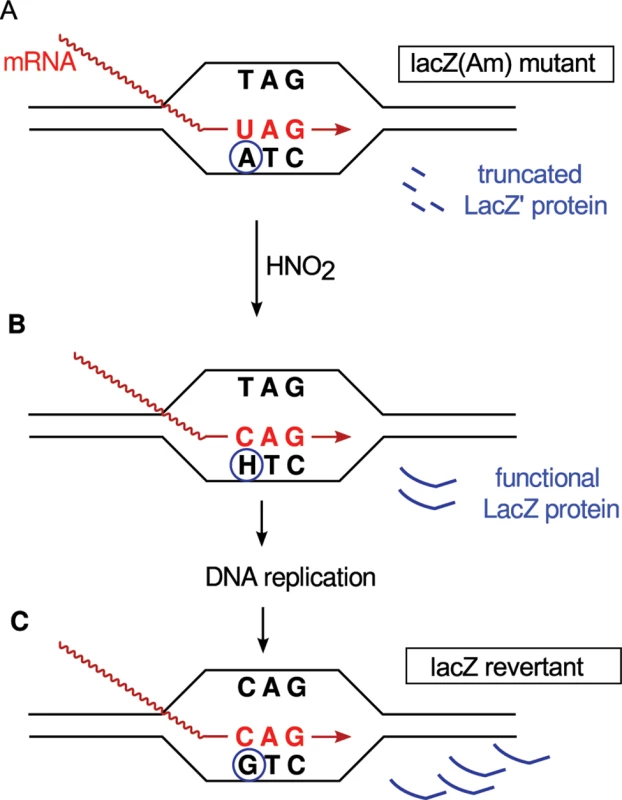
Shown are schematic representations of a transcription bubble that contains an amber stop codon from which is produced inactive, truncated LacZ' polypeptide. (A) An adenine in the TS is deaminated by HNO2 to hypoxanthine (H). (B) RNA polymerase pairs the hypoxanthine with cytidine instead of the original uracil, leading to the translation of a full-length functional β-galactosidase (LacZ), which allows the cell to grow on selective media. (C) This gene expression enables DNA replication, which establishes the A:T → G:C mutation producing the Lac+ phenotype. The first requirement for the retromutagenesis model is that a damaged gene must produce an altered protein in the absence of DNA synthesis, a process called transcriptional mutagenesis. A second requirement for retromutagenesis is that the RNA and DNA polymerases of a cell incorporate analogous nucleoside triphosphates opposite a lesion. As reviewed by Bregeon and Doetsch [10], these requirements have been fulfilled for several types of DNA lesions in both prokaryotes and eukaryotes. However, for the model to be verified, it must be demonstrated that adaptive mutation favors the TS of a gene. Two reported findings are consistent with this prediction. During selection for tryptophan prototrophy in strains with the trp5-A149C missense mutation, an ogg1 mutant of Saccharomyces cerevisiae, defective in the repair 8-oxoguanine oxidative lesions in DNA, displayed a high frequency of late-arising Trp+ revertants resulting from G:C → T:A transversions [11]. The results were consistent with an implied mispairing of an 8-oxoguanine in the TS. In another experiment, 8-oxo-dGTP that was electroporated into a trp5 mutant could cause reversions when incorporated into the NTS, but such revertants were only found after replication, and not after immediate selection under non-growth conditions [12]. Unfortunately, these studies could not test both TS and NTS strand mutations in the same experimental system.
In this paper, we describe an experimental system in Escherichia coli that can distinguish between mutations arising from damage to either DNA strand. We use it to test the hypothesis that retromutagenesis plays a role in the appearance of new bacterial mutants during stationary phase, and we discuss its implications for higher organisms.
Results
Rationale and experimental design
We have constructed an experimental system by which mutations on both strands can be separately measured. We used nitrous acid (HNO2), a deaminating agent that attacks adenine in an A:T base pair. The resulting hypoxanthine [13] pairs with cytosine and produces A:T→G:C transition mutations after replication. Our target was a premature TAG (amber) stop codon that replaced codon 17 (TGG) of the lacZ (ß-galactosidase) gene (GenBank accession number V00296.1; http://www.ncbi.nlm.nih.gov/nuccore/NC_000913.3). The amber mutants were Lac-, that is, unable to grow on lactose as the sole carbon source, but they could undergo HNO2-induced reversion to Lac+. The amber codon contains adenines on both the transcribed and the non-transcribed strands. From the DNA sequence of each Lac+ revertant, we can determine which strand was mutated (Fig 2).
Fig. 2. Different sequences produced by nitrosative deamination of adenine on the (a) TS or (b) NTS. 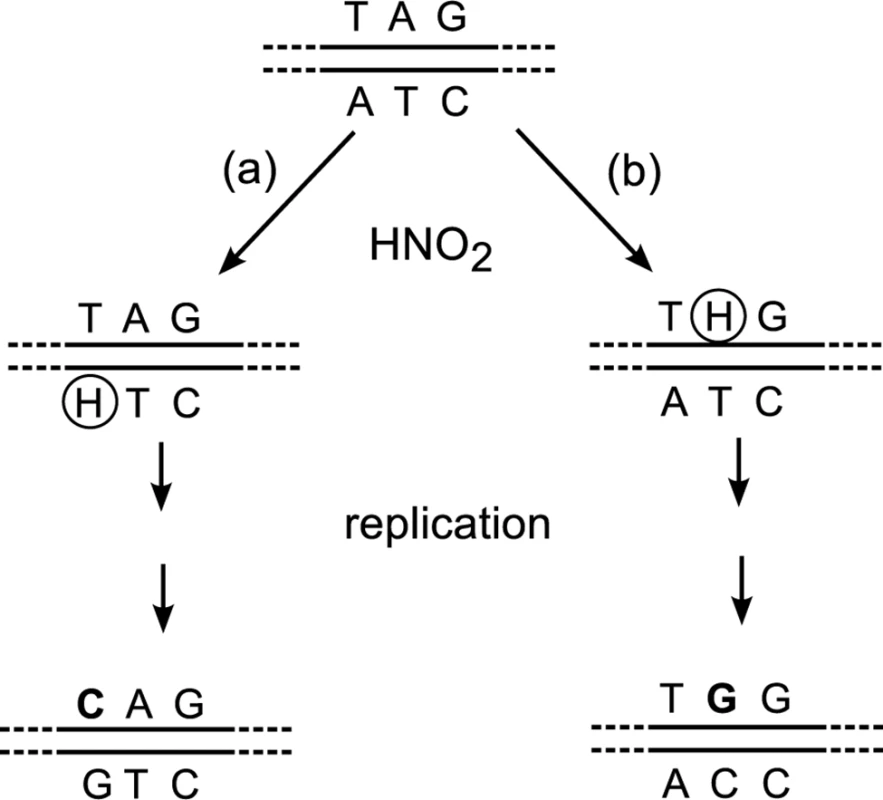
Although TGG (Trp) is the wild type codon and CAG (Gln) is a missense codon, they both restore functionality (see Results), and can be distinguished through DNA sequencing of Lac+ revertants. H, hypoxanthine. The tester strains contained a disrupted allele of nfi, which encodes endonuclease V [14,15]. Endonuclease V catalyzes the first step in the excision repair of hypoxanthine-containing DNA [16], and nfi mutants have an enhanced frequency of HNO2-induced A:T→G:C transition mutations [17]. The tester strains also contained a chromosomal deletion for the lac operon. The lacZ amber mutant alleles were on an attλ element [18], which is a non-replicating DNA segment inserted at the bacterial attachment site for bacteriophage λ. Because gene orientation affects strand bias for mutagenesis, two tester strains were constructed such that the attλ elements (and therefore the lacZ genes) were in opposite orientations: the transcribed strand of lacZ was either the leading or the lagging strand during DNA replication.
Steps were taken to minimize DNA replication during selection because it would reduce any strand bias. The mutagenesis was performed on stationary phase cells that were starved by being washed and incubated in a nutrient-free solution, and the agar selection medium was purged of carbon sources (other than lactose) by prior growth of a Δlac mutant (BW5660) on its surface. In addition, we eliminated the possibility of a sampling bias based on colony size. After 48 h of growth on lactose-minimal media the mean colony diameters (± 1 SD) were the same [1.1 (± 0.1) mm] for the both the TGG and CAG revertants, based on random samples of fifty colonies each.
HNO2 treatment and outgrowth of mutants
A preliminary experiment was performed to determine whether the transcribed and non-transcribed strands were equally susceptible to damage by HNO2. A culture that was grown to saturation without added glucose was exposed to HNO2. Before being plated on lactose-minimal media, the cells were grown overnight in LB broth to allow the propagation of mutants containing lesions in either strand. Lac reversion was induced greater than 20-fold among cells treated with HNO2. Thirty Lac+ revertants were picked, all of which had NTS mutations. However, for our planned experiment to work, we needed conditions under which both strands were equally damaged at the outset so that we would see if selection produced a strand bias in the mutations. The skewed result might be explained by the presence of lacZ transcription bubbles at the time of HNO2 treatment, coupled with the suggestion by Davis [8] that the single-stranded region of an NTS in a transcription bubble is highly sensitive to DNA-damaging agents. If so, we should be able to eliminate this effect by reducing lac transcription through catabolite repression [19]. The lac operon is positively regulated by a cAMP/CAP (catabolite activator protein) complex that binds near the lac promoter. In the presence of glucose, the preferred carbon source, cAMP levels fall, and lac transcription is drastically reduced even in the presence of an inducer [19]. As predicted by our hypothesis, when we grew the cells to saturation in glucose-containing medium before mutagenesis, we no longer observed a preponderance of NTS mutations, but rather distribution of mutations balanced between the two strands, as will be shown below. This result confirmed that the strong bias toward NTS mutations that we had previously observed (when cells were grown without gluscose) was indeed due to transcription-associated mutagenesis, and it implied that DNA-bound transcripts persist in stationary phase (see Discussion). Therefore, in all subsequent experiments we grew the cells in the presence of glucose before mutagenesis to enable catabolite repression of lac transcription.
The strand specificity for mutagenesis was determined as detailed under Methods and outlined in Fig 3. Briefly, the amber mutant tester strains were grown to saturation with glucose, washed, and starved. Multiple samples were treated with NaNO2 in an acidic buffer, with a survival of 29 to 33%. After the treatment was stopped with a neutral buffer, each sample was divided in two. One portion was spread immediately on the selective medium; the other was grown in LB broth before selection (Fig 3). If reversion occurred entirely by retromutagenesis, direct plating would reveal that only TS mutations were selected, whereas after intermediate growth, both TS and NTS mutations would appear among the revertants.
Fig. 3. Experimental scheme. 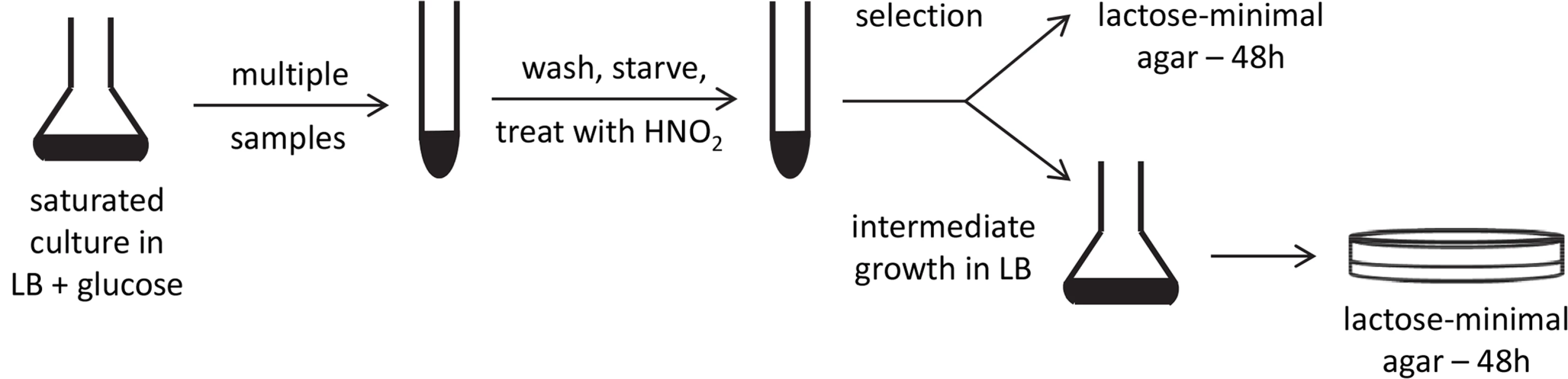
A stationary phase, saturated culture of lac mutants in LB broth + glucose was divided into many samples, washed by centrifigation, starved by incubation in 10 mM MgSO4, resuspended in acidic buffer, and incubated with or without added NaNO2. Each sample was divided in half. One part was grown in LB broth before being plated on lactose-minimal agar. The other was plated directly on the lactose-minimal agar. After 48 h, Lac+ revertant colonies were picked for DNA sequencing. Exposure to HNO2 increased the frequency of Lac+ revertants by 18 - to 51-fold (Table 1). Because of the relatively low frequency of spontaneous mutants and their statistically small sample size, the sequencing data for the HNO2-induced revertants (Table 2) were not corrected for those of the untreated samples. When the mutagenized cultures were allowed to grow in LB broth before selection, they yielded CAG and TGG Lac+ revertants in almost equal number (Table 2), indicating that both strands were about equally mutagenized (p = 0.7, Fisher exact test). However, when the mutagenized cells were plated directly on selective medium, without intermediate growth, CAG mutants predominated, indicating a preference for TS mutations (p < 0.01, Fisher's exact test) in strains with either gene orientation. Therefore, during selection, most mutations of the NTS were not replicated, allowing those of the TS to prevail. The results were not significantly affected by gene orientation (Table 2) (p < 0.01, Cochran-Mantel-Haenszel test), which is consistent with our expectation that at the time of treatment with HNO2, stationary phase cells did not contain a significant number of replication forks. These findings support the transcription-mediated mechanism of retromutagenesis for the inheritance of new mutations under selective conditions.
Tab. 1. Reversion frequencies after exposure to nitrous acid. 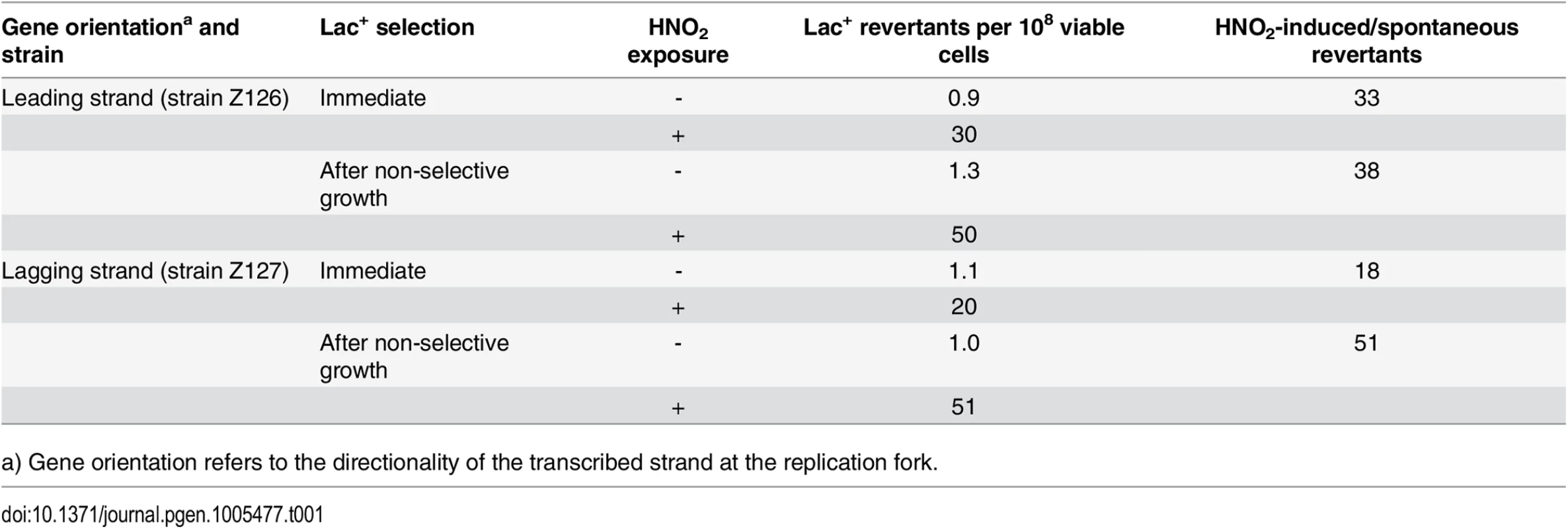
a) Gene orientation refers to the directionality of the transcribed strand at the replication fork. Tab. 2. Spectrum of mutations in a lacZ amber codon after HNO2 mutagenesis. 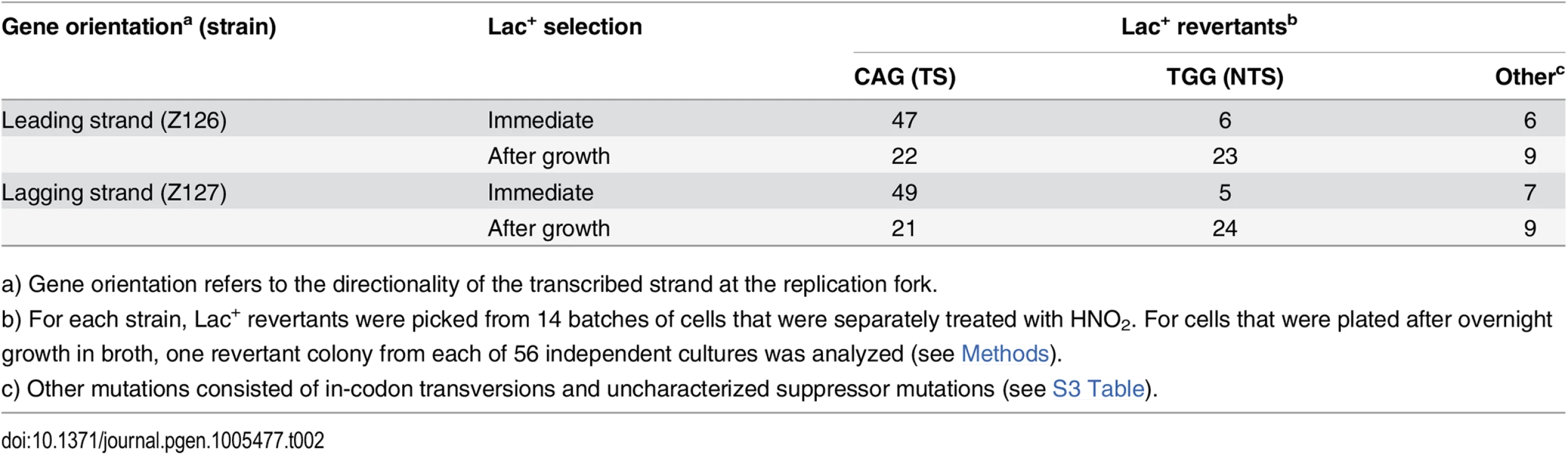
a) Gene orientation refers to the directionality of the transcribed strand at the replication fork. Discussion
Retromutagenesis provides a simple, direct explanation for adaptive mutation. However, it is not as obvious as it first seems because it relies on several tacit assumptions. Retromutagenesis requires that RNA polymerases bypass lesions in DNA and incorporate mutations analogous to those caused by DNA polymerase bypass (described in Introduction). Our experimental demonstration relied on a further assumption, which we verified. During selection, the starved cells had to be able to harvest enough energy to make functional protein (β-galactosidase) from the damaged gene before they have enough energy for DNA synthesis. In addition, our results provide evidence, albeit indirect, that hypoxanthine can be added to the list of abnormal bases [10] that undergo similar base mispairing when bypassed by either DNA or RNA polymerase in E. coli.
For many years, adaptive mutation has been operationally defined by the observation of mutagenesis during selection as evidenced by the continuous outgrowth of new mutants many days after selective plating, during which there is no observable background growth of the parental cells. In our experiments, however, DNA damage was induced before selection, and the mutant colonies appeared by 48 h of growth. The rapid, exclusive growth of revertants under selection enabled us to avoid interference from growth-dependent mechanisms that may contribute to adaptive mutation. Most of the previous experiments on adaptive mutation have attempted to rule out growth of the parent cells during selection, which is almost impossible to do with the necessary rigor. Although there may be no observable overall growth, there might still be growth of a sub-population that gives rise to new mutants. Consequently, it is generally acknowledged that much apparent adaptive mutation stems from occult growth of at least some parental cells, or at least of parts of their chromosomes through gene amplification [7]. In our approach, however, there is no need to rule out DNA replication occurring before selection, because it would only have reduced the observed strand bias. Instead of measuring overall DNA replication via cellular growth in the parental population during selection as in previously published experiments, we are able to gauge DNA replication specifically in the revertants via NTS mutations. Low frequency of NTS mutations found after direct selection indicates there was little DNA synthesis before gene expression and strongly supports the model of retromutagenesis.
Our studies employed an amber mutation in the tester strains. The expected low residual activity of the protein containing the amber mutation minimized growth of the parental cells during selection. Its position in a dispensable part of the lacZ gene [20] meant that unlike most other mutation indicators, it should be able to revert by more than one type of amino acid substitution, enabling detection of mutations at different positions in the codon. However, there is a caveat to using this method as a general approach. Although the early region of the gene can be deleted without significant loss of function, it is possible that some amino acid substitutions may cause a deleterious alteration of protein structure. In a preliminary experiment, we found that we were unable to use an amber mutation in codon 3 of lacZ. Following mutagenesis and intermediate growth, rapidly growing revertants displayed AT → GC mutations at only the first position of the codon, which restored the wild type sequence. Mutants with transitions at the second position of the codon grew too slowly on the selection plates.
When the cells were grown without added glucose, exposed to HNO2, and then grown before selection, all of the revertants studied were NTS mutants. Although transcription-coupled repair [21] could create such a strand bias by selectively repairing the TS, it acts only on lesions that obstruct RNA polymerase and that are repaired by the UvrABC system, both of which are not features expected of hypoxanthine in DNA. Our subsequent demonstration of a high frequency of TS mutations during both direct and delayed selection after catabolite repression (Table 2) provides direct experimental evidence that transcription-coupled repair is not functioning at a high level on these lesions. NTS mutations in the absence of catabolite repression are best explained by “transcription-associated mutagenesis” (not to be confused with “transcriptional mutagenesis”), reviewed by Jinks-Robertson and Bhagwat [22]. This mechanism provides the basis for an alternative model of adaptive mutation, proposed by Davis [8], which was prompted by the widespread observation that single-stranded DNA, such as that in a transcription bubble, is unusually sensitive to many types of DNA damage. This susceptibility has been specifically confirmed for nitrosative deamination in vitro, which is ten times faster in single-stranded than in double-stranded DNA [23]. In contrast to retromutagenesis, the Davis model states that selectable mutations result from damage to the NTS, and a round of DNA replication is required to encode a mutation in the TS for gene expression to begin. Our results indicated that our experimental system can detect mutational bias toward the NTS as well as toward the TS, although we did not specifically test the contribution of this transcription-associated mutagenesis to adaptive mutation. However, we may have gained some insight into its significance. The extent of this NTS bias surprised us for several reasons. First, previous reports had indicated only a fourfold to tenfold increase in spontaneous mutations of the NTS during transcription, although mutations on the TS were not studied at the same time [24,25]. Second, we did not expect persistent transcripts of the lac operon in our starved, stationary phase cells at the time of mutagenic treatment. Our strains had been constructed with a tested, repressible lac operon and they had been grown without an added inducer. The explanation may lie in the spontaneous cAMP-dependent derepression of the lac operon observed in cells entering the stationary phase [26] and in the possible contamination, by lactose, of tryptone (digested casein) in the medium [27]. Third, it seemed unlikely that DNA-bound RNAs should persist in stationary phase cells. They should be digested by ribonuclease HI, or else they could serve as primers for aberrant (“constitutive stable”) DNA replication [28]. It appears that stationary-phase cells may be primed for transcription-associated mutagenesis and adaptive mutation by the induction of cAMP-regulated genes and the persistence of transcription bubbles.
How widespread is retromutagenesis in nature? Although it does not apply to growing cells, most organisms in nature and most cells in our body exist in a state of limited growth. Retromutagenesis applies only to dominant mutations, but this should be true of most adaptive mutations, which are usually due to a gain of function. Theoretically, retromutagenesis cannot provide resistance to inhibitors of transcription or of protein synthesis because it relies on gene expression to enable the initial DNA synthesis that establishes heritability. It could, however, mediate resistance to DNA synthesis inhibitors or to tumor suppressors. For example, adaptive mutation to ciprofloxacin resistance was observed in E. coli; new mutants arose continuously days after plating in the presence of the antibiotic [29]. During this time there was no measurable growth of the parental cells; ciprofloxacin is an inhibitor of gyrase and therefore of DNA synthesis. Remarkably, in a parallel experiment, stationary phase cells did not become resistant to rifampin, an inhibitor of RNA polymerase. Although it was not appreciated at the time, these combined findings point to retromutagenesis as the adaptive mechanism. Similarly, retromutagenesis could be involved in resistance to the antimicrobial agent trimethoprim or the antitumor compound methotrexate, both based on dominant mutations that block the binding of the drugs to dihydrofolate reductase [30,31], which would otherwise ultimately inhibit DNA synthesis. Other possible examples in eukaryotes are the common mutations in p53 that turn this tumor suppressor gene into a dominant transforming oncogene [32] and dominant gain-of-function mutations in JAK2 that result in myeloproliferative disorders [33]. Similarly, TM can induce single-base changes in Ras transcripts that cause pro-growth increases in phosphorylated ERK protein [34].
Retromutagenesis is a transcription-mediated process of adaptive mutation that requires a damaged gene to be expressed before it can be replicated. In light of previous evidence, it is probably one of several mechanisms by which cells can undergo directed selection in the absence of apparent growth.
Materials and Methods
Strain construction
The E. coli K-12 F-λ- strains used in this study and the details of their construction are listed in S1 Table. We constructed two tester strains that had amber mutations in lacZ genes in opposite orientations on the chromosome. The amber mutation in codon 17 of lacZ was previously generated by oligonucleotide-mediated transformation. Subsequent gene transfers were by generalized transduction with phage P1 dam rev6 [35]. To orient the genes differently, we used attλ elements, which were plasmid-derived DNA segments that had integrated into the bacterial chromosome in opposite directions at the attachment site of phage λ. They contained a selectable ampicillin resistance marker and a lac operon with a lacZ missense mutation. They were transduced into an amber suppressor strain, BW5660, to create strains Z122 and Z123. Then the lacZ amber allele was transduced into attλ elements of these strains, crossing out the recipient's lac missense mutation. (The chromosomal lac deletions encompassed a genomic region too large to undergo P1-mediated transduction.) Selection was for the Lac+ phenotype specified by the suppressed lacZ amber allele. The lacI (constitutive) mutations in the recipients were crossed out at the same time, as determined by scoring with X-Gal and IPTG [36]. Finally, the attλ elements, which specified ampicillin resistance, were transduced into BW1181, a non-suppressing strain that had an nfi mutation and a lac deletion, thereby creating strains Z126 and Z127, which had the following relevant genotype: Δlac nfi-1::cat attλ::[lacZ(Am)Y+Z+]. In strain Z126, the lacZ gene on the attλ element is codirectional with the leading strand during DNA synthesis, and in strain Z127, it is in the opposite orientation. The lac point mutations were confirmed by DNA sequencing. The nfi-1::cat insertion and the orientations of the attλ elements were confirmed by PCR with primers listed in S2 Table.
Bacteriological media
LB media were the rich media used used for routine growth. Lactose-minimal agar was Medium E [37] supplemented with 0.1% lactose (Sigma Aldrich, > 99% pure by gas chromatography) and thiamine at 1 μg/ml. It was pretreated with a Δlac scavenger strain, BW5660 (S1 Table), to remove traces of carbon sources other than lactose [38]: a saturated culture of strain BW5660 grown in LB broth was centrifuged and resuspended in one-tenth its original volume of 10 mM MgSO4, and 0.1 ml was spread on each plate and incubated for 48 h at 37°C.
Mutagenesis
Strains Z126 and Z127 were grown overnight with aeration at 37°C in LB broth containing 0.4% glucose. The inocula contained less than 104 cells so that they would be unlikely to contain spontaneous revertants. The saturated cultures were centrifuged, and the cells were washed and resuspended in 10 mM MgSO4, then starved by incubation with shaking for 30 min at 37°C. They were separated into twenty-eight 1-ml aliquots and centrifuged. The cells were resuspended in 0.5 ml of 0.1 M sodium acetate buffer (pH 4.6) with or without 80 mM NaNO2 and incubated for 9 min at 37°C. The reactions were stopped by the addition of 5.0 ml of cold Medium A buffer [36]. The remaining cells were centrifuged and resuspended in 0.4 ml of 10 mM MgSO4. From each treated sample, 0.2 ml were spread on lactose-minimal agar (14 plates), and 0.05 ml were added to each of 4 tubes (56 total) containing 1.8 ml of LB broth and incubated overnight with shaking at 37°C. The untreated samples were handled similarly, except that the intermediate growth was performed in two tubes each. The saturated cultures were centrifuged, and the cells were resuspended in 10 mM MgSO4 and plated on lactose-minimal media. Before and after overnight growth, two samples each of the treated and untreated cells were diluted and plated on LB agar to determine cell survival and cell concentration. After 48 h of growth at 37°C, random, uniformly large Lac+ colonies were picked for DNA sequencing.
Other methods
Colony diameters were measured with a loupe containing a reticle (Edmund Scientific Co.). The 5'-terminal lacZ region of each Lac+ colony to be sequenced was amplified by colony PCR [39] with primers lacZ-249F and lacZ+178R (S2 Table). Sequencing was carried out by Beckman-Coulter Genomics (Danvers, MA) via dye termination capillary electrophoresis on an ABI 3730XL (Applied Biosystems, Inc.) instrument, using the lacZ-249F primer. Statistical significances were determined by the two-tailed Fisher's exact test [40] using an online interface (http://www.langsrud.com/fisher.htm), and the Cochran–Mantel–Haenszel test [41] using a published spreadsheet (http://www.biostathandbook.com/cmh.xls).
Supporting Information
Zdroje
1. Lederberg J, Lederberg EM (1952) Replica plating and indirect selection of bacterial mutants. J Bacteriol 63 : 399–406. 14927572
2. Luria SE, Delbruck M (1943) Mutations of Bacteria from Virus Sensitivity to Virus Resistance. Genetics 28 : 491–511. 17247100
3. Bridges BA (1994) Starvation-associated mutation in Escherichia coli: a spontaneous lesion hypothesis for "directed" mutation. Mutat Res 307 : 149–156. 7513791
4. Cairns J, Overbaugh J, Miller S (1988) The origin of mutants. Nature 335 : 142–145. 3045565
5. Ryan FJ, Okada T, Nagata T (1963) Spontaneous mutation in spheroplasts of Escherichia coli. J Gen Microbiol 30 : 193–199. 13975747
6. Cairns J, Foster PL (1991) Adaptive reversion of a frameshift mutation in Escherichia coli. Genetics 128 : 695–701. 1916241
7. Roth JR, Kugelberg E, Reams AB, Kofoid E, Andersson DI (2006) Origin of mutations under selection: the adaptive mutation controversy. Annu Rev Microbiol 60 : 477–501. 16761951
8. Davis BD (1989) Transcriptional bias: a non-Lamarckian mechanism for substrate-induced mutations. Proc Natl Acad Sci U S A 86 : 5005–5009. 2740338
9. Doetsch PW (2002) Translesion synthesis by RNA polymerases: occurrence and biological implications for transcriptional mutagenesis. Mutation research 510 : 131–140. 12459449
10. Bregeon D, Doetsch PW (2011) Transcriptional mutagenesis: causes and involvement in tumour development. Nat Rev Cancer 11 : 218–227. 21346784 doi: 10.1038/nrc3006
11. Shockley AH, Doo DW, Rodriguez GP, Crouse GF (2013) Oxidative damage and mutagenesis in Saccharomyces cerevisiae: genetic studies of pathways affecting replication fidelity of 8-oxoguanine. Genetics 195 : 359–367. 23893481 doi: 10.1534/genetics.113.153874
12. Rodriguez GP, Romanova NV, Bao G, Rouf NC, Kow YW, et al. (2012) Mismatch repair-dependent mutagenesis in nondividing cells. Proc Natl Acad Sci U S A 109 : 6153–6158. 22474380 doi: 10.1073/pnas.1115361109
13. Shapiro R, Pohl SH (1968) The reaction of ribonucleosides with nitrous acid. Side products and kinetics. Biochemistry 7 : 448–455. 5758560
14. Guo G, Ding Y, Weiss B (1997) nfi, the gene for endonuclease V in Escherichia coli K-12. J Bacteriol 179 : 310–316. 8990280
15. Yao M, Kow YW (1996) Cleavage of insertion/deletion mismatches, flap and pseudo-Y DNA structures by deoxyinosine 3'-endonuclease from Escherichia coli. J Biol Chem 271 : 30672–30676. 8940043
16. Yao M, Hatahet Z, Melamede RJ, Kow YW (1994) Purification and characterization of a novel deoxyinosine-specific enzyme, deoxyinosine 3' endonuclease, from Escherichia coli. J Biol Chem 269 : 16260–16268. 8206931
17. Schouten KA, Weiss B (1999) Endonuclease V protects Escherichia coli against specific mutations caused by nitrous acid. Mutat Res 435 : 245–254. 10606815
18. Diederich L, Rasmussen LJ, Messer W (1992) New cloning vectors for integration in the lambda attachment site attB of the Escherichia coli chromosome. Plasmid 28 : 14–24. 1387714
19. Pastan I, Perlman RL (1969) Repression of beta-galactosidase synthesis by glucose in phosphotransferase mutants of Escherichia coli. Repression in the absence of glucose phosphorylation. J Biol Chem 244 : 5836–5842. 4310826
20. Miller JH, Albertini AM (1983) Effects of surrounding sequence on the suppression of nonsense codons. J Mol Biol 164 : 59–71. 6188840
21. Hanawalt PC, Spivak G (2008) Transcription-coupled DNA repair: two decades of progress and surprises. Nat Rev Mol Cell Biol 9 : 958–970. 19023283 doi: 10.1038/nrm2549
22. Jinks-Robertson S, Bhagwat AS (2014) Transcription-associated mutagenesis. Annu Rev Genet 48 : 341–359. 25251854 doi: 10.1146/annurev-genet-120213-092015
23. Caulfield JL, Wishnok JS, Tannenbaum SR (1998) Nitric oxide-induced deamination of cytosine and guanine in deoxynucleosides and oligonucleotides. J Biol Chem 273 : 12689–12695. 9582291
24. Beletskii A, Bhagwat AS (1996) Transcription-induced mutations: increase in C to T mutations in the nontranscribed strand during transcription in Escherichia coli. Proc Natl Acad Sci U S A 93 : 13919–13924. 8943036
25. Beletskii A, Bhagwat AS (1998) Correlation between transcription and C to T mutations in the non-transcribed DNA strand. Biol Chem 379 : 549–551. 9628351
26. Grossman TH, Kawasaki ES, Punreddy SR, Osburne MS (1998) Spontaneous cAMP-dependent derepression of gene expression in stationary phase plays a role in recombinant expression instability. Gene 209 : 95–103. 9524234
27. Studier FW (2005) Protein production by auto-induction in high density shaking cultures. Protein Expr Purif 41 : 207–234. 15915565
28. Kogoma T (1997) Stable DNA replication: interplay between DNA replication, homologous recombination, and transcription. Microbiol Mol Biol Rev 61 : 212–238. 9184011
29. Riesenfeld C, Everett M, Piddock LJ, Hall BG (1997) Adaptive mutations produce resistance to ciprofloxacin. Antimicrob Agents Chemother 41 : 2059–2060. 9303418
30. Dale GE, Broger C, D'Arcy A, Hartman PG, DeHoogt R, et al. (1997) A single amino acid substitution in Staphylococcus aureus dihydrofolate reductase determines trimethoprim resistance. J Mol Biol 266 : 23–30. 9054967
31. Simonsen CC, Levinson AD (1983) Isolation and expression of an altered mouse dihydrofolate reductase cDNA. Proc Natl Acad Sci U S A 80 : 2495–2499. 6573667
32. Gannon JV, Greaves R, Iggo R, Lane DP (1990) Activating mutations in p53 produce a common conformational effect. A monoclonal antibody specific for the mutant form. EMBO J 9 : 1595–1602. 1691710
33. Kralovics R, Passamonti F, Buser AS, Teo SS, Tiedt R, et al. (2005) A gain-of-function mutation of JAK2 in myeloproliferative disorders. N Engl J Med 352 : 1779–1790. 15858187
34. Saxowsky TT, Meadows KL, Klungland A, Doetsch PW (2008) 8-Oxoguanine-mediated transcriptional mutagenesis causes Ras activation in mammalian cells. Proc Natl Acad Sci U S A 105 : 18877–18882. 19020090 doi: 10.1073/pnas.0806464105
35. Sternberg NL, Maurer R (1991) Bacteriophage-mediated generalized transduction in Escherichia coli and Salmonella typhimurium. Methods Enzymol 204 : 18–43. 1943777
36. Miller J (1993) A short course in bacterial genetics: a laboratory manual and handbook for Escherichia coli and related bacteria. Trends in Biochemical Sciences-Library Compendium 18 : 193.
37. Vogel HJ, Bonner DM (1956) Acetylornithinase of Escherichia coli: partial purification and some properties. J Biol Chem 218 : 97–106. 13278318
38. Smith TF, Sadler JR (1971) The nature of lactose operator constitive mutations. J Mol Biol 59 : 273–305. 4935786
39. Sheu DS, Wang YT, Lee CY (2000) Rapid detection of polyhydroxyalkanoate-accumulating bacteria isolated from the environment by colony PCR. Microbiology 146 (Pt 8): 2019–2025. 10931906
40. Agresti A (1992) A Survey of Exact Inference for Contingency Tables. Statistical Science 7 : 131–153.
41. McDonald JH (2014) Handbook of Biological Statistics (3rd ed.). Baltimore, Maryland: Sparky House Publishing.
Štítky
Genetika Reprodukční medicína
Článek Loss and Gain of Natural Killer Cell Receptor Function in an African Hunter-Gatherer PopulationČlánek Let-7 Represses Carcinogenesis and a Stem Cell Phenotype in the Intestine via Regulation of Hmga2Článek Binding of Multiple Rap1 Proteins Stimulates Chromosome Breakage Induction during DNA ReplicationČlánek SLIRP Regulates the Rate of Mitochondrial Protein Synthesis and Protects LRPPRC from DegradationČlánek Protein Composition of Infectious Spores Reveals Novel Sexual Development and Germination Factors inČlánek The Formin Diaphanous Regulates Myoblast Fusion through Actin Polymerization and Arp2/3 RegulationČlánek Runx1 Transcription Factor Is Required for Myoblasts Proliferation during Muscle Regeneration
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2015 Číslo 8- Akutní intermitentní porfyrie
- Růst a vývoj dětí narozených pomocí IVF
- Vliv melatoninu a cirkadiálního rytmu na ženskou reprodukci
- Intrauterinní inseminace a její úspěšnost
- Délka menstruačního cyklu jako marker ženské plodnosti
-
Všechny články tohoto čísla
- Putting the Brakes on Huntington Disease in a Mouse Experimental Model
- Identification of Driving Fusion Genes and Genomic Landscape of Medullary Thyroid Cancer
- Evidence for Retromutagenesis as a Mechanism for Adaptive Mutation in
- TSPO, a Mitochondrial Outer Membrane Protein, Controls Ethanol-Related Behaviors in
- Evidence for Lysosome Depletion and Impaired Autophagic Clearance in Hereditary Spastic Paraplegia Type SPG11
- Loss and Gain of Natural Killer Cell Receptor Function in an African Hunter-Gatherer Population
- Trans-Reactivation: A New Epigenetic Phenomenon Underlying Transcriptional Reactivation of Silenced Genes
- Early Developmental and Evolutionary Origins of Gene Body DNA Methylation Patterns in Mammalian Placentas
- Strong Selective Sweeps on the X Chromosome in the Human-Chimpanzee Ancestor Explain Its Low Divergence
- Dominance of Deleterious Alleles Controls the Response to a Population Bottleneck
- Transient 1a Induction Defines the Wound Epidermis during Zebrafish Fin Regeneration
- Systems Genetics Reveals the Functional Context of PCOS Loci and Identifies Genetic and Molecular Mechanisms of Disease Heterogeneity
- A Genome Scale Screen for Mutants with Delayed Exit from Mitosis: Ire1-Independent Induction of Autophagy Integrates ER Homeostasis into Mitotic Lifespan
- Non-synonymous FGD3 Variant as Positional Candidate for Disproportional Tall Stature Accounting for a Carcass Weight QTL () and Skeletal Dysplasia in Japanese Black Cattle
- The Relationship between Gene Network Structure and Expression Variation among Individuals and Species
- Calmodulin Methyltransferase Is Required for Growth, Muscle Strength, Somatosensory Development and Brain Function
- The Wnt Frizzled Receptor MOM-5 Regulates the UNC-5 Netrin Receptor through Small GTPase-Dependent Signaling to Determine the Polarity of Migrating Cells
- Nbs1 ChIP-Seq Identifies Off-Target DNA Double-Strand Breaks Induced by AID in Activated Splenic B Cells
- CCNYL1, but Not CCNY, Cooperates with CDK16 to Regulate Spermatogenesis in Mouse
- Evidence for a Common Origin of Blacksmiths and Cultivators in the Ethiopian Ari within the Last 4500 Years: Lessons for Clustering-Based Inference
- Of Fighting Flies, Mice, and Men: Are Some of the Molecular and Neuronal Mechanisms of Aggression Universal in the Animal Kingdom?
- Hypoxia and Temperature Regulated Morphogenesis in
- The Homeodomain Iroquois Proteins Control Cell Cycle Progression and Regulate the Size of Developmental Fields
- Evolution and Design Governing Signal Precision and Amplification in a Bacterial Chemosensory Pathway
- Rac1 Regulates Endometrial Secretory Function to Control Placental Development
- Let-7 Represses Carcinogenesis and a Stem Cell Phenotype in the Intestine via Regulation of Hmga2
- Functions as a Positive Regulator of Growth and Metabolism in
- The Nucleosome Acidic Patch Regulates the H2B K123 Monoubiquitylation Cascade and Transcription Elongation in
- Rhoptry Proteins ROP5 and ROP18 Are Major Murine Virulence Factors in Genetically Divergent South American Strains of
- Exon 7 Contributes to the Stable Localization of Xist RNA on the Inactive X-Chromosome
- Regulates Refractive Error and Myopia Development in Mice and Humans
- mTORC1 Prevents Preosteoblast Differentiation through the Notch Signaling Pathway
- Regulation of Gene Expression Patterns in Mosquito Reproduction
- Molecular Basis of Gene-Gene Interaction: Cyclic Cross-Regulation of Gene Expression and Post-GWAS Gene-Gene Interaction Involved in Atrial Fibrillation
- The Spalt Transcription Factors Generate the Transcriptional Landscape of the Wing Pouch Central Region
- Binding of Multiple Rap1 Proteins Stimulates Chromosome Breakage Induction during DNA Replication
- Functional Divergence in the Role of N-Linked Glycosylation in Smoothened Signaling
- YAP1 Exerts Its Transcriptional Control via TEAD-Mediated Activation of Enhancers
- Coordinated Evolution of Influenza A Surface Proteins
- The Evolutionary Potential of Phenotypic Mutations
- Genome-Wide Association and Trans-ethnic Meta-Analysis for Advanced Diabetic Kidney Disease: Family Investigation of Nephropathy and Diabetes (FIND)
- New Routes to Phylogeography: A Bayesian Structured Coalescent Approximation
- SLIRP Regulates the Rate of Mitochondrial Protein Synthesis and Protects LRPPRC from Degradation
- Satellite DNA Modulates Gene Expression in the Beetle after Heat Stress
- SHOEBOX Modulates Root Meristem Size in Rice through Dose-Dependent Effects of Gibberellins on Cell Elongation and Proliferation
- Reduced Crossover Interference and Increased ZMM-Independent Recombination in the Absence of Tel1/ATM
- Suppression of Somatic Expansion Delays the Onset of Pathophysiology in a Mouse Model of Huntington’s Disease
- Protein Composition of Infectious Spores Reveals Novel Sexual Development and Germination Factors in
- The Evolutionarily Conserved LIM Homeodomain Protein LIM-4/LHX6 Specifies the Terminal Identity of a Cholinergic and Peptidergic . Sensory/Inter/Motor Neuron-Type
- SmD1 Modulates the miRNA Pathway Independently of Its Pre-mRNA Splicing Function
- piRNAs Are Associated with Diverse Transgenerational Effects on Gene and Transposon Expression in a Hybrid Dysgenic Syndrome of .
- Retinoic Acid Signaling Regulates Differential Expression of the Tandemly-Duplicated Long Wavelength-Sensitive Cone Opsin Genes in Zebrafish
- The Formin Diaphanous Regulates Myoblast Fusion through Actin Polymerization and Arp2/3 Regulation
- Genome-Wide Analysis of PAPS1-Dependent Polyadenylation Identifies Novel Roles for Functionally Specialized Poly(A) Polymerases in
- Runx1 Transcription Factor Is Required for Myoblasts Proliferation during Muscle Regeneration
- Regulation of Mutagenic DNA Polymerase V Activation in Space and Time
- Variability of Gene Expression Identifies Transcriptional Regulators of Early Human Embryonic Development
- The Drosophila Gene Interacts Genetically with and Shows Female-Specific Effects of Divergence
- Functional Activation of the Flagellar Type III Secretion Export Apparatus
- Retrohoming of a Mobile Group II Intron in Human Cells Suggests How Eukaryotes Limit Group II Intron Proliferation
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Exon 7 Contributes to the Stable Localization of Xist RNA on the Inactive X-Chromosome
- YAP1 Exerts Its Transcriptional Control via TEAD-Mediated Activation of Enhancers
- SmD1 Modulates the miRNA Pathway Independently of Its Pre-mRNA Splicing Function
- Molecular Basis of Gene-Gene Interaction: Cyclic Cross-Regulation of Gene Expression and Post-GWAS Gene-Gene Interaction Involved in Atrial Fibrillation
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



