-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
The lncRNA Controls Cryptococcal Morphological Transition
The involvement of non-protein regulators in developmental processes in higher eukaryotes is an area that has come to light more recently. Earlier known as the dark matter of the genome, the non-protein coding genes are now recognized for their important regulatory roles in the life of eukaryotes. Using forward genetic screen, we identified RZE1 as a lncRNA with key function in regulating morphogenesis in Cryptococcus. We further discovered that RZE1 regulates the transcription and transcript export of ZNF2, which encodes the key morphogenesis transcription factor. RZE1 is the first functionally characterized lncRNA in a human fungal pathogen. Given the potential large number of lncRNAs in Cryptococcus and other fungal pathogens, the RZE1-ZNF2 regulatory system could serve as a paradigm for the investigation of lncRNAs in development and virulence in eukaryotic pathogens.
Published in the journal: . PLoS Genet 11(11): e32767. doi:10.1371/journal.pgen.1005692
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1005692Summary
The involvement of non-protein regulators in developmental processes in higher eukaryotes is an area that has come to light more recently. Earlier known as the dark matter of the genome, the non-protein coding genes are now recognized for their important regulatory roles in the life of eukaryotes. Using forward genetic screen, we identified RZE1 as a lncRNA with key function in regulating morphogenesis in Cryptococcus. We further discovered that RZE1 regulates the transcription and transcript export of ZNF2, which encodes the key morphogenesis transcription factor. RZE1 is the first functionally characterized lncRNA in a human fungal pathogen. Given the potential large number of lncRNAs in Cryptococcus and other fungal pathogens, the RZE1-ZNF2 regulatory system could serve as a paradigm for the investigation of lncRNAs in development and virulence in eukaryotic pathogens.
Introduction
In many human fungal pathogens, the morphological transition from yeast to hypha plays a central role in pathogenesis [1, 2], as demonstrated in the ascomycetes Candida albicans, Penicillium marneffei, Histoplasma capsulatum, Coccidioides immitis, and Paracoccidiodides brasiliensis [3–6]. Different morphotypes also display different levels of pathogenicity in the basidiomycetous fungus Cryptococcus neoformans [1, 7], the causative agent of the deadly cryptococcal meningitis [8]. Although primarily considered as yeasts, Cryptococcus undergoes yeast-to-hypha transition during unisexual mating (self-fruiting) or bisexual a-α mating [9–11].
The zinc finger transcription factor Znf2 ultimately controls this morphotype transition. During mating, Znf2 is activated by the pheromone MAPK pathway controlled by the HMG domain transcription factor Mat2 [12–15] (Fig 1A). Mat2 is essential for pheromone sensing and response, which leads to the cell fusion event. Hyphal growth commences after cell fusion and eventually gives rise to fruiting structures and meiotic spores [9, 16]. However, Mat2 does not control hyphal morphogenesis per se [12]. By contrast, Znf2 governs hypha generation and it is dispensable for the early mating events like cell fusion [12, 17] (Fig 1A). Under non-mating inducing conditions, Znf2 could be activated by the matri-cellular signal protein Cfl1 through a positive feedback regulation [18, 19]. It is unknown whether other host or environmental factors can also regulate Znf2 activity.
Fig. 1. The phenotypes caused by the loss or disruption of RZE1 resemble the ones caused by the deletion of ZNF2. 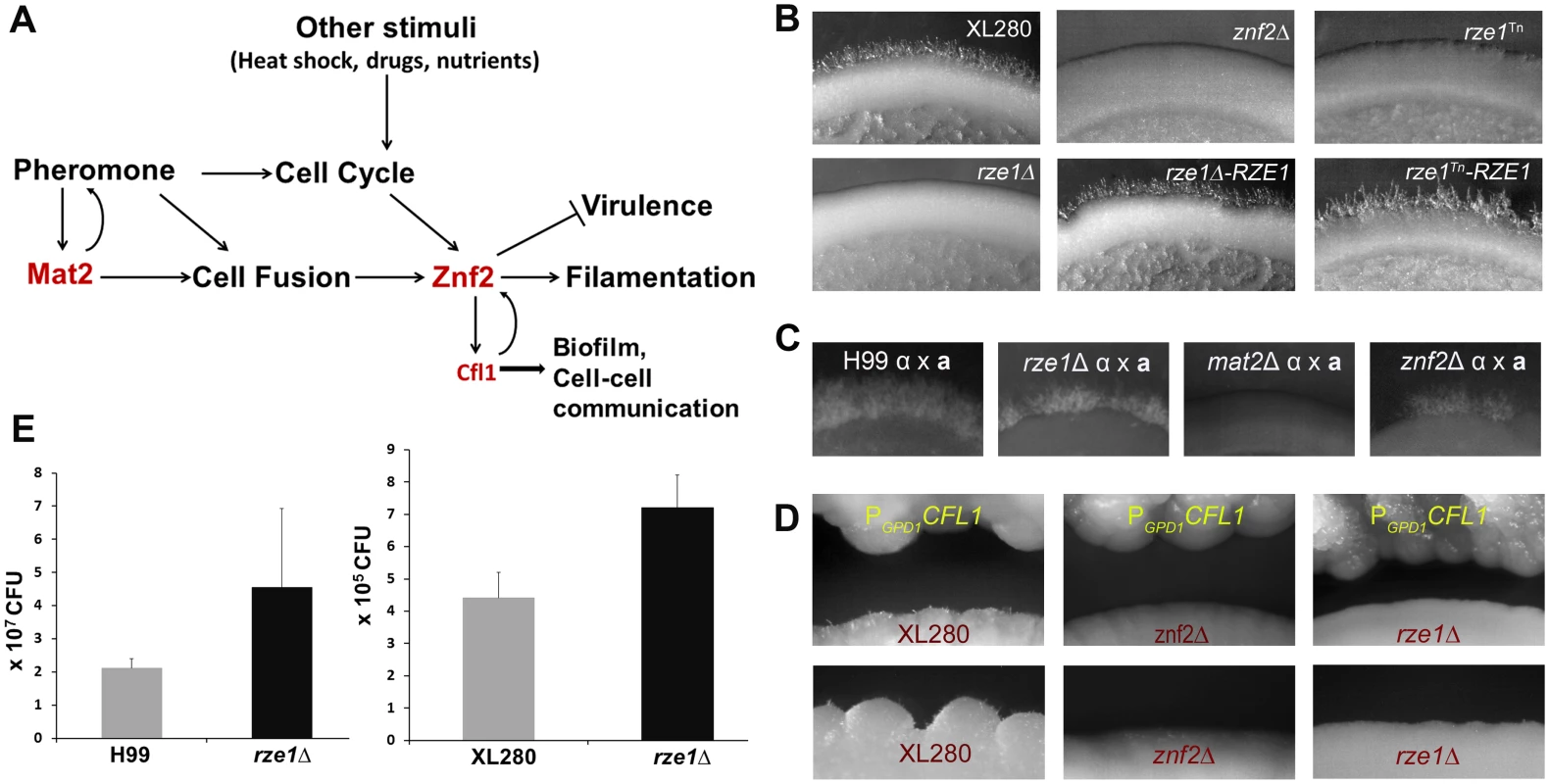
(A) A diagram of various signals (pheromone and other stimuli) that lead to activation of ZNF2 and consequently filamentation in Cryptococcus. (B) The self-filamentation assay of the wild type XL280, the znf2Δ mutant, the rze1Tn mutant, the rze1Δ mutant, and the complemented strains rze1Tn-pRZE1 and rze1Δ-pRZE1 with the wild type allele of RZE1 integrated at the native locus. Strains were cultured on V8 medium for 4 days. (C) Filamentation assay of unilateral crosses during bisexual mating involving a mutant strain and a wild-type mating partner. The α partner is a mutant in the non-self-filamentous H99 background (the rze1Δ mutant, the mat2Δ mutant, and the znf2Δ mutant). The a partner is the wild type KN99a that is congenic with H99. Cells were cultured on V8 medium for about 2 weeks. (D) Confrontation assay with the PGPD1-CFL1 strain as the donor and the wild type XL280, the rze1Δ mutant, or the znf2Δ mutant as the recipient. (E) Fungal burden analysis from lungs of mice inoculated with the rze1Δ mutants and the corresponding wild-type strains. p = 0.0429 for H99 background and 0.0716 for XL280 background. Znf2 is an anti-virulence factor in the mouse model of cryptococcosis [12, 20]. The deletion of the ZNF2 gene locks the fungal cells in the yeast form, making them more virulent [12]. Conversely, the activation of ZNF2 drives filamentation and attenuates Cryptococcus virulence [21, 22]. The ZNF2 overexpression cells, either in the live or heat-killed form, can protect the hosts from a subsequent challenge with otherwise lethal wild-type cells [22]. Thus manipulation of ZNF2 activity could be a potential means to alleviate cryptococcosis. Besides its anti-virulence effect during cryptococcal infection in a mammalian host, Znf2 also shapes cryptococcal interaction with other heterologous hosts, such as the soil amoeba Acanthamoeba castellanii and the insect Galleria mellonella [23]. The essential role of Znf2 in Cryptococcus sexual cycle and its pivotal role in regulating cryptococcal interaction with various host species make this transcription factor a potential target for multi-layered regulation in response to various stimuli.
To identify the upstream regulators of ZNF2, we conducted a forward genetic screen to find mutations that cause similar phenotypes as those caused by the disruption of ZNF2. The screen led to the discovery of RZE1 that functions upstream of ZNF2. Further investigation revealed that RZE1 functions primarily as a cis-acting, and less efficiently a trans-acting, lncRNA. Furthermore, this lncRNA is functionally restricted to its native nuclei based on heterokaryon assay. We found that RZE1 exerts its impact on cryptococcal morphogenesis by regulating ZNF2 transcription and by influencing the nuclear versus cytoplasmic distribution of ZNF2 transcripts, which consequently affects ZNF2’s ability to get translated into protein.
Although genomic and transcriptomic data suggest the presence of lncRNAs showing infection - or tissue-specific expression in fungal species pathogenic to plants and animals [24], it is unclear if lncRNAs have any biological relevance in the life cycle or the development of human fungal pathogens. RZE1 is the first lncRNA that is functionally characterized in a human fungal pathogen. The importance of RZE1 in cryptococcal morphogenesis raises the possibility that lncRNAs may be important regulators that contribute to the complexity in genetic regulation in these eukaryotic pathogens.
Results
Identification of the RZE1 gene for its importance in filamentation via forward genetic screen
Cryptococcus undergoes filamentation in response to the mating signal and other environmental cues. Znf2 is the essential regulator of this morphological switch and it bridges morphogenesis and virulence in this fungal pathogen [12, 17–19]. To identify the regulatory network of the Znf2-controlled filamentation pathway, we conducted a random insertional mutagenesis screen for znf2Δ-like phenotype in the self-filamentous strain XL280 [25]. XL280 has been commonly used in morphogenesis studies [12, 20, 25]. It has a publically released genome sequence (~19 Mb, ~7000 genes) [26] and a well characterized congenic pair [21]. We generated 63,000 insertional mutants via Agrobacterium-mediated transformation in XL280 and screened these mutants on filamentation-inducing V8 medium for non-filamentous phenotypes. Among a set of selected non-filamentous mutants, 15 had their insertion site identified by inverse PCR and sequencing (Table 1). Of the 15 insertion sites identified, one T-DNA insertion (Tn) in mutant X261 was found to be in the genetic locus that we named RZE1. The RZE1 gene encodes a 1,268 nt long transcript in XL280 based on our primer walking and RACE PCR results (S1 Fig). Only one transcription start site and one transcription stop site were identified for RZE1 under the tested condition.
Tab. 1. Insertion mutants that show lack of filamentation and their insertion sites. 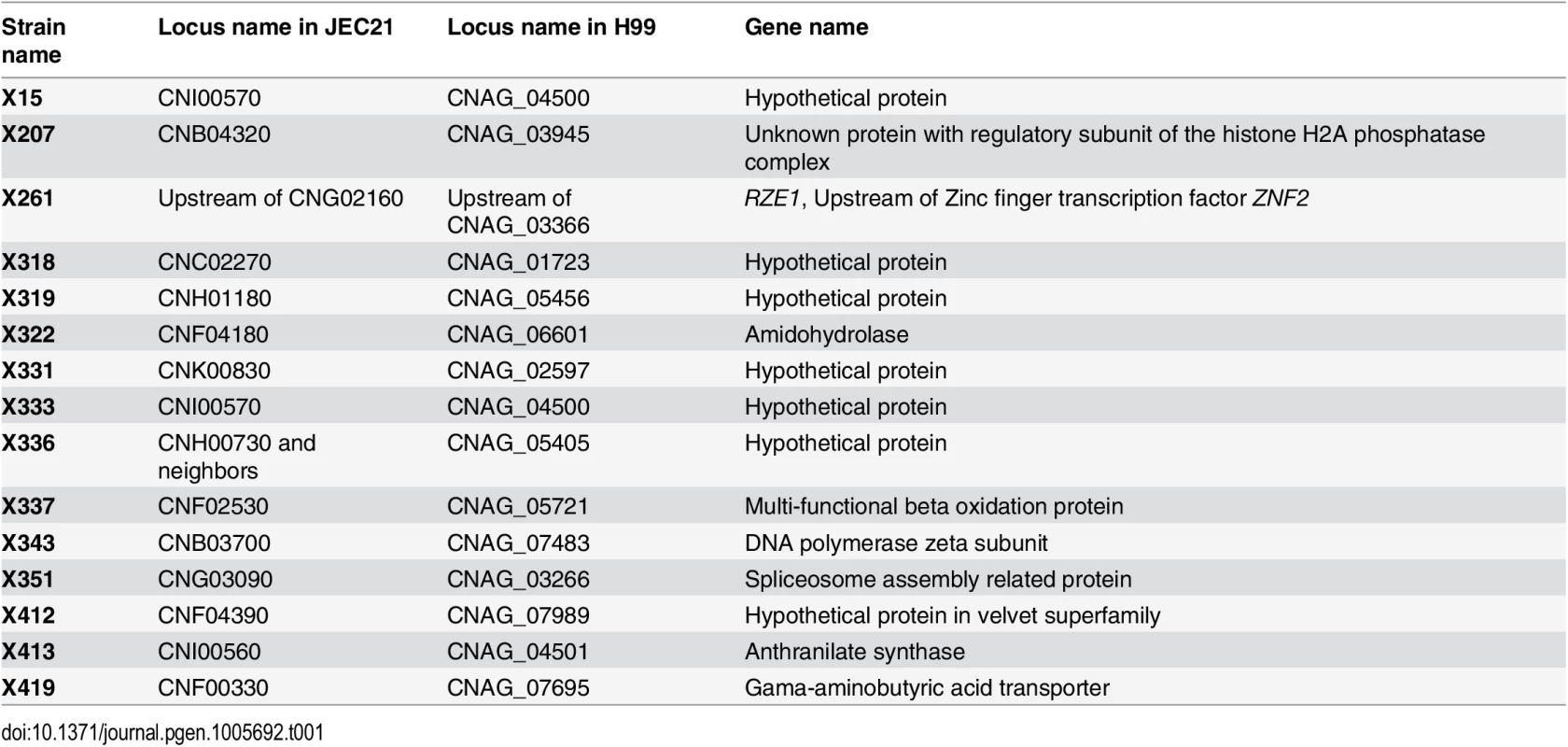
Disruption of RZE1 recapitulates the phenotypes caused by the deletion of ZNF2
The rze1Tn mutant, like the znf2Δ mutant, is non-filamentous under mating-inducing conditions such as on V8 media (Fig 1B). To confirm the role of RZE1 in hyphal growth, we deleted the RZE1 gene in the XL280 background. The targeted deletion of RZE1 also abolished self-filamentation (Fig 1B). To ensure that the non-filamentous phenotype of the rze1Tn and the rze1Δ mutants was attributable to the disruption of RZE1 and not due to other cryptic mutations, a wild-type copy of the RZE1 gene was re-introduced ectopically into the rze1Tn and the rze1Δ mutants. The ectopically integrated RZE1 partially restored the filamentation defects in these rze1 mutants (S2 Fig). We also introduced the wild-type RZE1 back to its native locus in the rze1Tn and the rze1Δ mutants. The introduced RZE1 gene at its native locus effectively restored the ability of both mutants to filament (Fig 1B), indicating that RZE1 indeed is required for filamentation.
The pheromone sensing pathway is critical for filamentation under mating-inducing conditions. Disruption of the pheromone sensing pathway (e.g. the deletion of HMG domain transcription factor Mat2 or the MAPKK Ste7), even in one mating partner, will abolish filamentation produced by bisexual mating between a and α partners [12, 14]. The lack of the bisexual mating hyphae is due to lack of cell fusion. It is previously established that the disruption of ZNF2 does not impair pheromone pathway or abolish cell fusion [12]. Thus a unilateral cross involving the znf2Δ mutant and a wild-type partner still produces mating filaments, in contrast to the unilateral cross involving one mat2Δ mating partner [12]. We then decided to assess if this also holds true for RZE1. To avoid the complication due to self-filamentation in the XL280 background, we chose to delete RZE1 in the non-self-filamentous strain H99. In the H99 background, hyphae can only result from bisexual mating following cell fusion between a and α cells activated by the pheromone pathway. We found that the unilateral crosses between the rze1Δ (or the rze1Tn) mutant with a wild-type mating partner produced mating filaments (Fig 1C), as in the znf2Δ mutant and unlike the mat2Δ mutant (Fig 1C). Consistently, the pheromone production in the rze1Δ mutant was at par with the wild type, indicating that RZE1, like ZNF2, is not required for pheromone sensing pathway.
Under non-mating inducing conditions like on the YPD medium, ZNF2 and thereby filamentation can be activated by the matricellular signaling protein Cfl1 through a positive feedback loop [18]. Znf2 is absolutely necessary for Cryptococcus to respond to exogenous Cfl1 signal to promote filamentation [20]. Similarly, we found that RZE1 is also required for the recipient strain to filament in response to the CFL1 protein released from nearby donor cells under mating-suppressing conditions (Fig 1D).
Morphological switch from the yeast state to the filamentous state is inversely correlated with virulence in Cryptococcus. Consequently, the loss of ZNF2 modestly increases fungal virulence [20]. Thus we hypothesize that the upstream regulator of ZNF2 might also influence cryptococcal virulence potential. Since mice are highly susceptible to XL280 (C. neoformans var. neoformans, serotype D) [21, 27] and H99 (C. neoformans var. grubii, serotype A) [28, 29], we decided to compare the virulence between the wild-type strain and the rze1Δ mutant made in both XL280 and H99 backgrounds using the fungal burden assays. We inoculated the mice with the rze1Δ mutants and their corresponding wild-type strains through inhalation and measured the fungal burdens in the lungs at day 10 post inoculation. The fungal burdens in the lungs infected by the rze1Δ mutants were close to 2 fold higher than those infected by the corresponding wild-type controls (Fig 1E), indicating enhanced virulence of the rze1Δ mutants. Given that an increase in virulence with gene disruption is rare, these results are consistent with the observed enhanced virulence of the znf2Δ mutant, which showed about 2.4 fold increase in lung fungal burden compared to the wild type at the same time point during infection [12, 20]. In line with our previous observations for the znf2Δ mutant [12, 20], the rze1Δ mutant did not show any apparent difference from the wild type in classic virulence traits such as melanization, capsule production, or thermo-tolerance (S3 Fig). We also did not observe any apparent alteration in the susceptibility of the rze1Δ mutant to stressors like SDS, caspofungin (inhibitor of β -1,3-glucan synthase), calcoflour white (inhibitor of chitin), iron chelator, UV radiation, or oxidative stress (H2O2) when compared to the wild-type control (S3 Fig). Taken together, these observations indicate that the disruption of RZE1 recapitulates in vitro and in vivo phenotypes caused by the deletion of ZNF2. Thus RZE1 might be a highly selective regulator of ZNF2 or a major target of ZNF2.
RZE1 is physically and functionally upstream of ZNF2
RZE1 is located ~2.5 kb upstream of ZNF2. Although the average intergenic space is less than 800 bp in Cryptococcus [30], the physical location of RZE1 raises a concern that the RZE1 transcript could be part of the ZNF2 transcript and the disruption of RZE1 would cause the disruption of ZNF2 itself. To address this concern, we amplified and compared four regions (I—IV in Fig 2A) that cover RZE1, the beginning of the ZNF2 coding region, and the region between RZE1 and ZNF2, using templates of total cDNA or genomic DNA derived from the wild-type XL280 strain. Region II that lies between RZE1 and the 5’ region upstream of the ZNF2 ORF failed to yield any detectable amplicon when cDNA was used as the template (Fig 2A). This suggests that RZE1 is likely produced as a separate transcript independent from ZNF2. Furthermore, the ability of the ectopically introduced RZE1 to partially complement the rze1Tn and the rze1Δ mutants (S2A Fig) reinforces the idea that RZE1 is a functionally independent transcript from that of ZNF2. In addition, since the phenotype of the znf2Δ mutant can be complemented effectively using an ectopic copy of ZNF2 with 1kb sequence upstream of the ZNF2 ORF that does not include RZE1 (S4 Fig), it is reasonable to conclude that RZE1 and ZNF2 encode separate transcripts that can function independently.
Fig. 2. RZE1 is an independent gene and it functions upstream of ZNF. 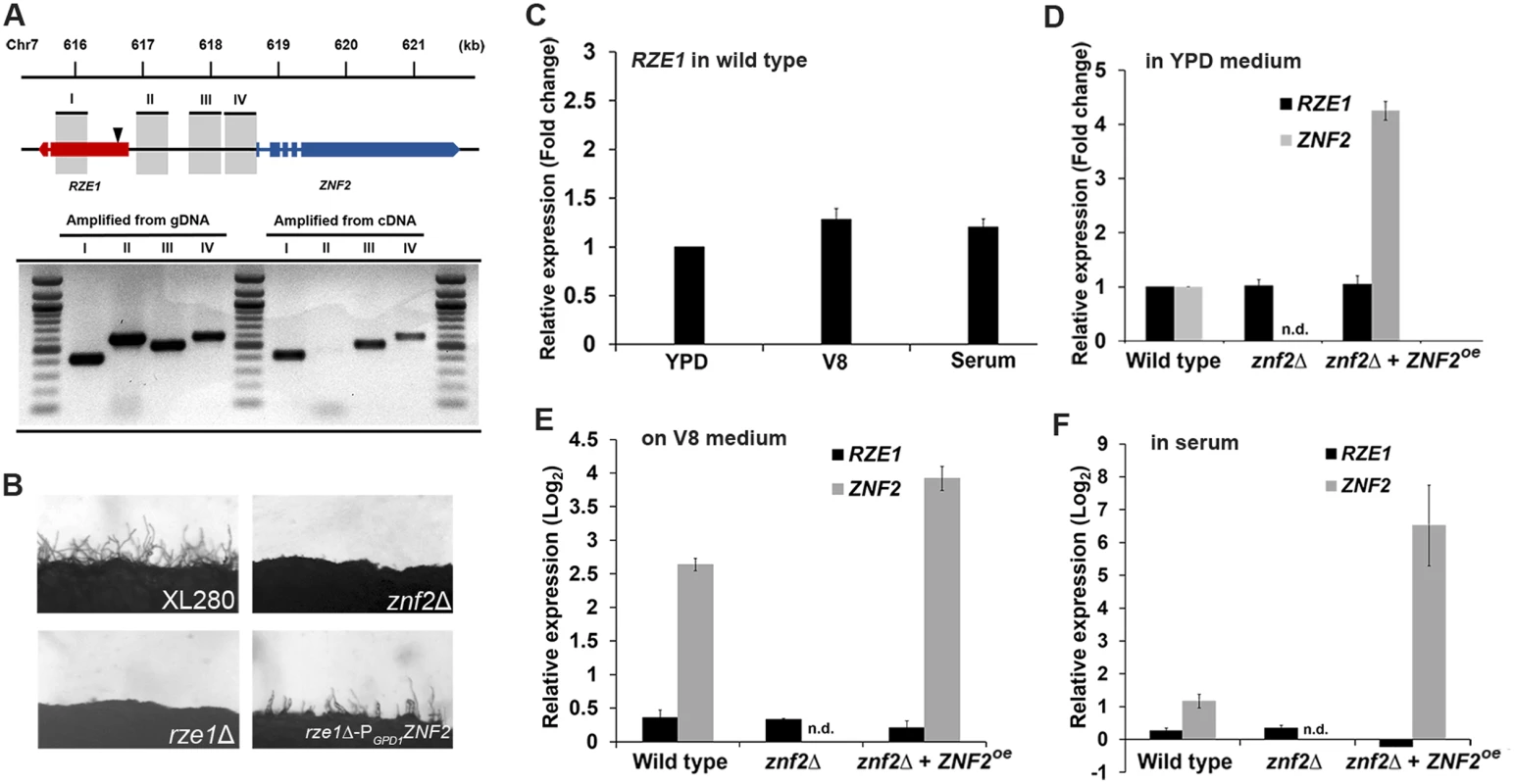
(A) The diagram of the RZE1-ZNF2 genomic region in XL280. The location of the insertion site in the rze1Tn mutant identified by inverse PCR is shown by the arrow. The products amplified from different regions between RZE1 and ZNF2 using either cDNA or genomic DNA as the template were separated on a gel by electrophoresis. The introns of the ZNF2 gene and one intron of the RZE1 gene are indicated as spaces. (B) The constitutive expression of ZNF2 (PGPD1-ZNF2) in the rze1Δ mutant partially restores filamentation in the mutant. The znf2Δ mutant and the rze1Δ mutant in XL280 background are blocked in filamentation. (C) The relative transcript levels of RZE1 in wild-type H99 cultured in YPD medium, on V8 agar medium during mating, or in fetal bovine serum for 24 hours as measured by qRT-PCR. The transcript level of RZE1 in YPD medium was set as 1 for comparison. (D-F) The relative transcript levels of RZE1 and ZNF2 in the wild-type H99, the znf2Δ mutant, and the ZNF2oe strain when cultured in YPD medium (D), on V8 agar medium during mating (E), or in fetal bovine serum for 24 hours (F) as measured by qRT-PCR. n.d.: not detectable. For panels D-F, the transcript level of RZE1 or ZNF2 of the wild type in YPD medium was set arbitrarily as 1 for comparison (1 in fold change or 0 in Log2 value). To investigate the relationship between RZE1 and ZNF2, we examined the RZE1 transcript level in the wild type, the znf2Δ mutant, and the ZNF2oe strain under three different growth conditions (YPD/rich medium, serum/host relevant, and V8/mating-inducing). The transcript level of RZE1 in the wild-type H99 strain was relatively stable, and there was no dramatic difference when cells were cultured in YPD medium, on V8 medium, or in serum (Fig 2C). Under the same culture conditions, the RZE1 transcript level remained constant among the different strains (black bars in Fig 2D–2F), even though the ZNF2 transcript level was drastically different among these strains (grey bars in Fig 2D–2F). This indicates that RZE1 is not responsive to changes in the ZNF2 transcript level. This result supports the earlier conclusion that RZE1 is a transcript independent of ZNF2 and likely functions in the filamentation pathway upstream of ZNF2. Consistently, the constitutive expression of ZNF2 driven by the GPD1 promoter led to the partial restoration of filamentation to these rze1 mutants (Fig 2B), indicating that ZNF2 indeed functions downstream of RZE1. These observations are in accordance with the idea that RZE1 functions in the filamentation pathway upstream of ZNF2.
RZE1 likely functions as a lncRNA
Based on the Cryptococcus EST databases, the RZE1 transcript is present in all subspecies of the Cryptococcus neoformans species complex, namely C. neoformans var. grubii (serotype A), C. neoformans var. neoformans (serotype D), and the subspecies C. gatii (serotype B and C). An analysis of the RZE1 transcript sequence suggests that the RZE1 gene is not a typical protein-coding gene. Rather than encoding one open reading frame (ORF), as expected for most mRNAs in fungi, RZE1 contains 5 short potential ORFs (Fig 3A), with three potential translation start codons (ATG) in a poor translation context (S1 Fig). BLAST searches using these potential ORFs against fungal genome databases or GenBank did not yield any significant hits. Thus, these potential ORFs do not encode conserved protein products.
Fig. 3. RZE1 is a likely lncRNA. 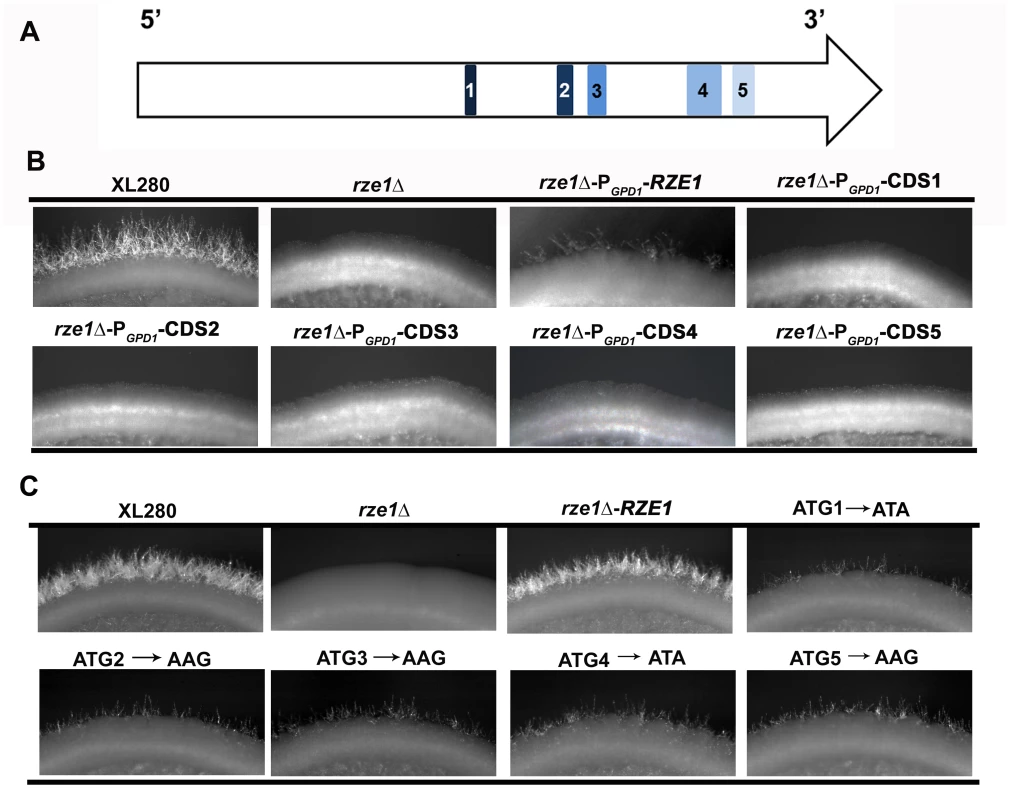
(A) The diagram of the five putative small open reading frames in RZE1 is shown in the top row. (B) The constitutive (PGPD1) or overexpression (PCTR4-2, see S5 Fig) of each putative open reading frame of RZE1 does not restore filamentation in the rze1Δ mutant. Cells were cultured on V8 medium for 4 days. (C) Partial complementation of the rze1Δ mutant with RZE1 modified for each potential translation start codon. All the start codon mutated alleles generated by single nucleotide mutagenesis and the wild-type allele of RZE1 were integrated at its native genetic locus in the rze1Δ mutant background. The mutated alleles showed partial complementation. Better complementation was achieved by the wild-type allele of RZE1. Cells were cultured on V8 medium for 4 days. To test if products from any of the small ORFs confer RZE1 function in filamentation, we inserted the constitutive active GPD1 promoter [31] (Fig 3B) or the CTR4 inducible promoter [32] (S5 Fig) upstream of each of the five ORFs contained within the RZE1 transcript. We introduced these constructs into the rze1Δ mutant and then tested the transformants for their ability to filament. None of the PGPD1-ORF transformants were able to produce filaments (Fig 3B). Similarly, none of the PCTR4-2-ORF transformants were able to filament under inducing conditions (S5 Fig). The results suggest that the potential ORFs carried within the RZE1 transcript are unlikely to produce protein products that function in the filamentation pathway. We postulate that RZE1 may function as a lncRNA instead.
To further determine whether RZE1 functions as a protein or a transcript, we did site-directed single nucleotide mutagenesis to alter the translation start codon of each of the five potential ORFs in RZE1 (Fig 3C). We mutated ATG to ATA or AAG as such changes are known to almost abolish translation initiation in other fungi [33]. These codon changes are expected to prevent or considerably reduce the translation of these potential ORFs. We then introduced each of these mutated RZE1 alleles into the rze1Δ mutant and selected transformants with the mutated RZE1 allele integrated into the RZE1’s native locus. We then examined the ability of these transformants to produce hyphae. To our amazement, all the mutated RZE1 alleles were able to restore the filamentation defect of the rze1Δ mutant (Fig 3C), although to a lesser degree compared to the wild-type RZE1 allele integrated at the native locus (Fig 3C). This finding indicates that these RZE1 alleles are at least partly functional in promoting filamentation, even though the specific nucleotides that are potential start-codons are mutated. Collectively, our results strongly suggest that RZE1 is a long non-coding RNA regulating morphogenesis in Cryptococcus.
RZE1 is functionally restricted to the nucleus
As a lncRNA, it is possible to be functionally restricted to its target within its native nucleus, as XIST or MALAT-1 [34–37], or to be functional in the cytoplasm [38]. This is in contrast to protein-coding mRNAs, which need to be exported from the nucleus to the cytoplasm for translation to make the functional products. Proteins produced by mRNAs would be able to function with its targets produced by any nucleus that shares the same cytoplasm. To narrow down the potential mode of action of RZE1, we decided to first examine if RZE1 is functionally restricted to its native nucleus. For this purpose, we designed a heterokaryon assay where two cells conjugate and thus share the cytoplasm, but their nuclei do not fuse. Heterokaryon assay was previously used to show that the lncRNA MALAT1 is functionally restricted to the nucleus [39]. Forming heterokaryons is a natural process during the a-α bisexual mating in Cryptococcus [9, 40, 41] (Fig 4A). The formation of α-a dikaryon after mating conjugation brings the homeodomain proteins Sxi1α and Sxi2a together [42], which triggers the expression of ZNF2 and the production of dikaryotic mating hyphae [9, 12, 43]. It is important to note that RZE1 controls filamentation but not conjugation (cell fusion), as established previously for Znf2 (Fig 1C) [12, 17]. Furthermore, the dikaryon will be blocked from filamentation only when ZNF2 is disrupted in both mating partners [12, 43]. For this assay, we used cryptococcal strains in H99 background because H99 does not self-filament. As a result, filaments produced from the α-a bisexual mating are all derived from conjugated dikaryons. This allows us to examine if RZE1 produced by one nucleus could compensate the loss of RZE1 in the other nucleus that shares the same cytoplasm. The idea is that if the RZE1 from one nucleus was able to function in the cytoplasm or in another nucleus in the same conjugated cell, then it would have been able to regulate ZNF2 activity even if ZNF2 is produced by the other nucleus (Fig 4A).
Fig. 4. RZE1 is functionally restricted to its native nucleus. 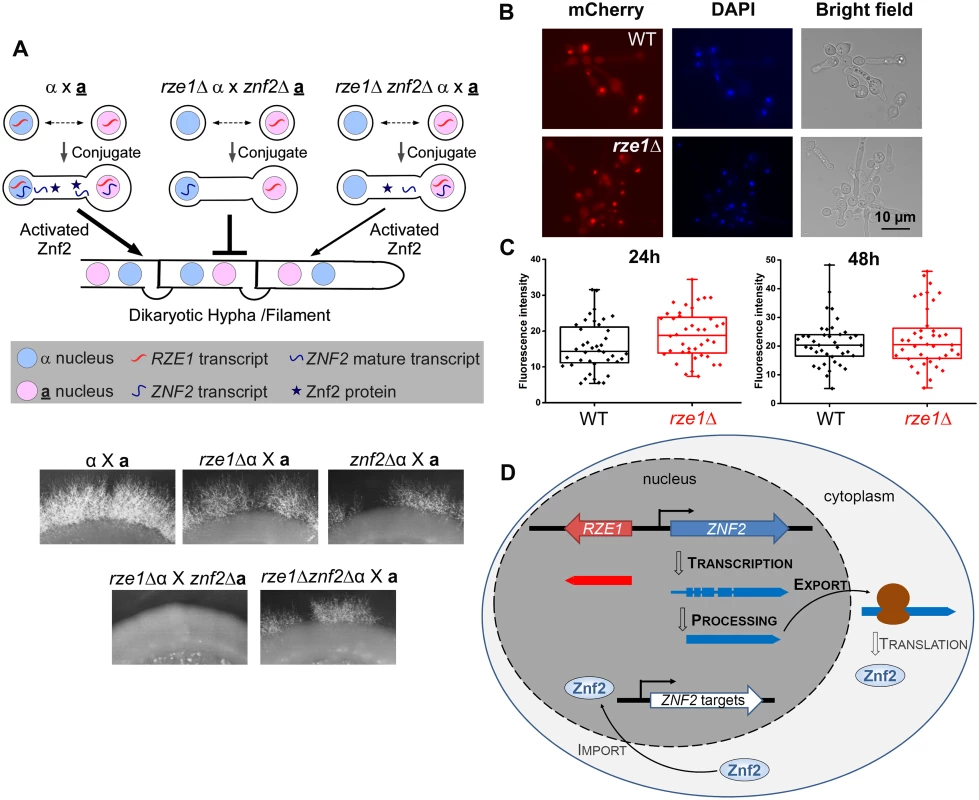
(A) Heterokaryon assay showing the functional restriction of RZE1 to the nucleus of its origin. The diagram above shows the rationale behind this heterokaryon assay. The strains used for this assay are all in the non-self-filamentous H99 background. The complete lack of filamentation in the rze1Δ α x znf2Δ a cross compared to the reduced filamentation from the rze1Δznf2Δ α x a cross (gene dosage consideration) suggests that the RZE1 from the second mating partner is unable to compensate the loss of RZE1 from the first mating partner. Bisexual mating was assayed on V8 medium for about 3 weeks. The localization (B) and intensity (C) of the fluorescence signal from the PCTR4-2-Znf2-mCherry strain cultured in YPD+BCS with or without RZE1. p = 0.89 for 24h and 0.59 for 48h. (D) Working model for RZE1’s nuclear function in Cryptococcus. As expected, the unilateral crosses rze1Δ α x a, znf2Δ α x a, and rze1Δznf2Δ α x a all filamented at a reduced level compared to the WT cross α x a (Fig 4A). However, the dikaryons generated from the cross rze1Δ α x znf2Δ a or the cross rze1Δ a x znf2Δ α failed to filament (Fig 4A). These dikaryons contain one nucleus with RZE1 but no ZNF2, and the other nucleus with ZNF2 but no RZE1. The inability of these dikaryons to filament indicates that RZE1 produced by one nucleus failed to support the activity of ZNF2 transcribed by the other nucleus even though the two nuclei shared the same cytoplasm. Because the diploid α/a wild-type cells in H99 background did not filament under this condition, it precludes us from further assessing whether the diploid cells derived from nuclear fusion of the rze1Δa x znf2Δα heterokaryon could filament. The decreased ability of the heterozygous diploid cells (α/a) compared to the corresponding dikaryons (α-a) to continue through the mating program appears to be a common phenomenon in basidiomycetes. Nonetheless, these findings indicate that RZE1 is functionally restricted to its native nucleus and as such it is highly unlikely for RZE1 to directly affect the ZNF2 translation or other post-translational processes that occur in the cytosol.
To further test the hypothesis that RZE1 does not play a direct role in translation or Znf2 protein localization to the nucleus, we used a system where the expression of an ectopically introduced ZNF2-mCherry is controlled by the CTR4 promoter. We compared the fluorescent intensity (indicative of protein level) and the subcellular localization of Znf2 in the presence or absence of RZE1. We found that the loss of RZE1 does not affect either the intensity or the localization of Znf2-mCherry (Fig 4B and 4C). This is consistent with the idea that RZE1 does not directly regulate ZNF2 at the level of translation or protein translocation. Taken together, these results strongly suggest that RZE1 is functionally restricted in its native nucleus, and is not directly involved in the Znf2 protein processing or translocation. In the nucleus, however, it is possible that RZE1 could directly or indirectly regulate ZNF2 at different levels (Fig 4D): (i) promote ZNF2 transcription or transcript stability, (ii) support the production of the functional ZNF2 transcript isoform through its influence on alternative start, alternative termination, or alternative splicing [28], or (iii) assist the export of the ZNF2 transcript from nucleus to cytosol. Regulatory activity of RZE1 in any of the above processes could result in an effect on the Znf2 protein level or activity in the wild type strain.
RZE1 regulates the ZNF2 transcript level
The evidence presented so far suggests that RZE1 functions in its native nucleus and it functions in a regulatory capacity upstream of ZNF2 (Fig 4D). We decided to examine the first hypothesis of transcriptional control. To obtain a holistic view of the effect of the RZE1 deletion on cryptococcal transcriptome, we first conducted a pilot comparative transcriptome analysis between the wild-type XL280 and the rze1Δ mutant undergoing self-filamentation by RNA sequencing (RNA-seq). A total of 265 genes with the 1.5 fold change as the cut-off threshold (P<0.05) were differentially expressed in the rze1Δ mutant relative to the wild type at 24h post inoculation on filamentation-inducing V8 media (S1 Table). These genes are classified into eight functional categories (Fig 5A). Among the enriched GO terms, genes involved in the septin complex, cell cycle, and DNA replication are known to affect morphogenesis [44–46], which is consistent with the regulatory function of RZE1 in morphogenesis. Based on the RNA-seq data, the transcript level of ZNF2 in the rze1Δ mutant was lower compared to the wild type at 24 hours post inoculation (Fig 5B). The reduction of the transcript level of ZNF2 in the rze1Δ mutant was less obvious at 72 hours post inoculation compared to the 24h and 28h time points (S6B Fig). The lower ZNF2 transcript level in the rze1Δ mutant was further confirmed by quantitative real-time PCR (Fig 5C). These results suggest that RZE1, directly or indirectly, up-regulates ZNF2 at the transcriptional level.
Fig. 5. RZE1 regulates the transcription of ZNF2. 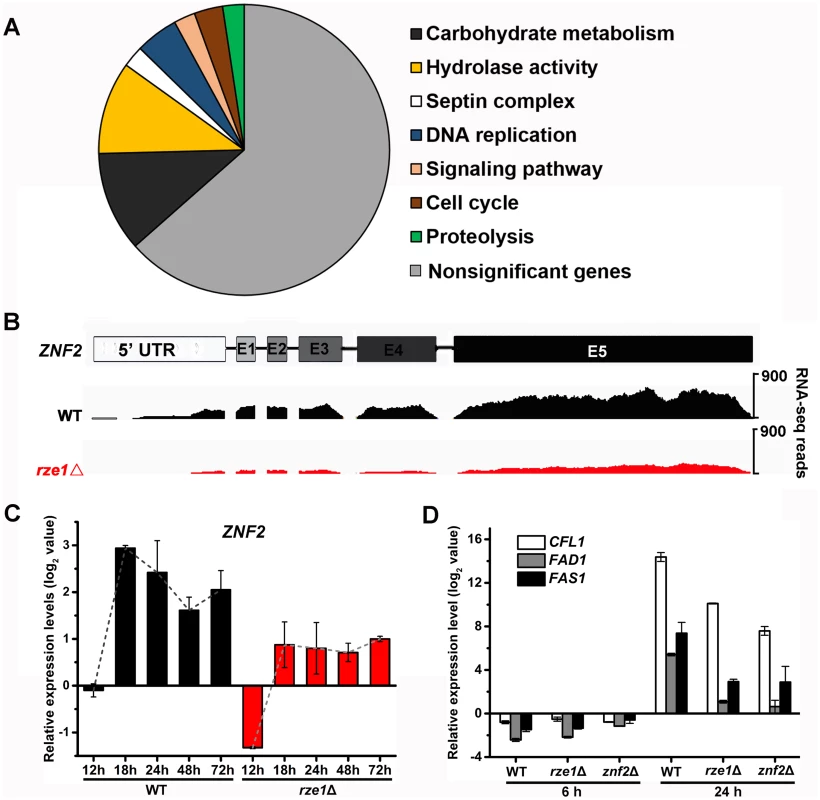
(A) The diagrammatic representation of enriched GO term classification of differentially expressed genes in the rze1Δ mutant compared to the wild-type strain. (B) The RNA sequence reads over the ZNF2 locus is reduced ~2.6 fold in the rze1Δ mutant during self-filamentation at 24h on V8 medium (RNA-seq). (C) The transcript level of ZNF2 is reduced in the rze1Δ mutant during self-filamentation at 12h, 18h, 24h, 48h, and 72h on V8 medium measured by qPCR. The transcript levels were compared to the transcript level of ZNF2 in the wild type strain at the 12h time point. (D) The transcript levels of the downstream targets of ZNF2, namely CFL1, FAD1, and FAS1, were much reduced in the rze1Δ mutant and the znf2Δ mutant compared to those in the wild type. Since filamentation is obvious at 24h in XL280 under inducing condition, we suspect that the ZNF2 gene expression might be induced earlier. We therefore expanded our examination of the ZNF2 transcript level in the rze1Δ mutant and the wild type to include more time points (12h, 18h, 24h, 48h, and 72h post inoculation on V8 medium) by qPCR. As expected [12], the ZNF2 transcript level in the wild-type strain of all the later time points examined was increased compared to the reference level at 12h (Fig 5C). To our surprise, the ZNF2 transcript level in the rze1Δ mutant is also increased, suggesting that the signal transduction to promote filamentation still occurs in the mutant. However, the level of ZNF2 transcripts in the rze1Δ mutant is lower than that in the wild type, with the biggest difference observed at 18h (~4 fold) and smaller differences at 48h and 72h (Fig 5C). This might be consistent with the observation that the number of differentially expressed genes in the rze1Δ mutant is much higher at the 24h time point than the later 48h and 72h time points based on RNA-seq (S1 Table). These observations suggest an intriguing possibility that RZE1 might play an important regulatory role in driving filamentation at the early stage of morphogenesis.
Given the modest effect on the ZNF2 transcript level by the disruption of RZE1, we were surprised by the blocked filamentation in the rze1Δ mutant. Other mutants with reduced levels of ZNF2 typically show reduced but not abolished filamentation. Thus it is enigmatic that the disruption of RZE1 could completely abolish self-filamentation. The paradox between the observed reduction in the transcript level of ZNF2 and the abolished filamentation in the rze1Δ mutant thus prompted us to examine the effect of RZE1 deletion on the previously characterized downstream targets of Znf2 that are known to be important for filamentation: CFL1, FAD1, and FAS1 [17, 18]. Both qPCR and RNA-seq results indicated that the transcript levels of these filamentation markers were considerably reduced in the rze1Δ mutant (Fig 5D and S7 Fig), often more comparable to those observed in the znf2Δ mutant (Fig 5D). The drastic reduction in the transcript level of CFL1, an auto-inducer working in positive feedback with ZNF2 [18], is also in accordance with the results of the confrontation assay (Fig 1D). Thus it appears that although the deletion of RZE1 only exerts a modest impact on the transcript level of ZNF2, it exerts a drastic impact on factors downstream of ZNF2 and may inactivate at least part of the ZNF2 regulon that controls filamentation. This raises the possibility that RZE1 may affect additional processes between the ZNF2 transcription and translation.
The deletion of RZE1 shows no obvious effect on ZNF2 transcript intron splicing
As transcript isoforms different from those in the wild type could be non-functional, we decided to examine the impact of RZE1’s disruption on ZNF2 isoforms (Fig 4D). In C. neoformans 99.5% of all expressed genes have introns and an average gene contains multiple introns [30]. Thus alternative splicing could be one major way of generating different transcript isoforms. Indeed, multiple introns were found in ZNF2 wild type allele in the serotype D strain XL280 (Fig 5B and S6A Fig) as well as in the serotype A strain H99 [28]. The RNA-seq data showed that introns of the ZNF2 transcripts were spliced in both the wild-type strain XL280 and in the rze1Δ mutant (Fig 5B). Consistently, amplicons of the transcript that covered the whole ZNF2 ORF region in the wild type and in the rze1Δ mutant by reverse transcription PCR showed no apparent difference in size, again supporting the idea that introns of the ZNF2 transcripts could be spliced in the absence of RZE1. To examine if there was any quantitative difference in splicing of the ZNF2 transcript at different regions, we analyzed the transcript level of five different regions of the ZNF2 transcripts in the wild type and in the rze1Δ mutant during self-filamentation. These five regions included two regions in the 5’UTR, the junction between exon 1 and 2, the junction between exon 3 and 4, and the exon 5 region (Fig 6A). The levels of all five tested regions of the ZNF2 transcripts were lower in the rze1Δ mutant relative to those in the wild type, again with the biggest difference observed at 18h and smaller differences observed at 48h and 72h (Fig 6A). The pattern was similar to the earlier observation based on the conserved exon 5 region using the P5 primer set (Fig 5C). Collectively, the results suggest that RZE1 does not affect intron splicing of the ZNF2 transcripts.
Fig. 6. ZNF2 transcript appears to be processed correctly in the absence of RZE1. 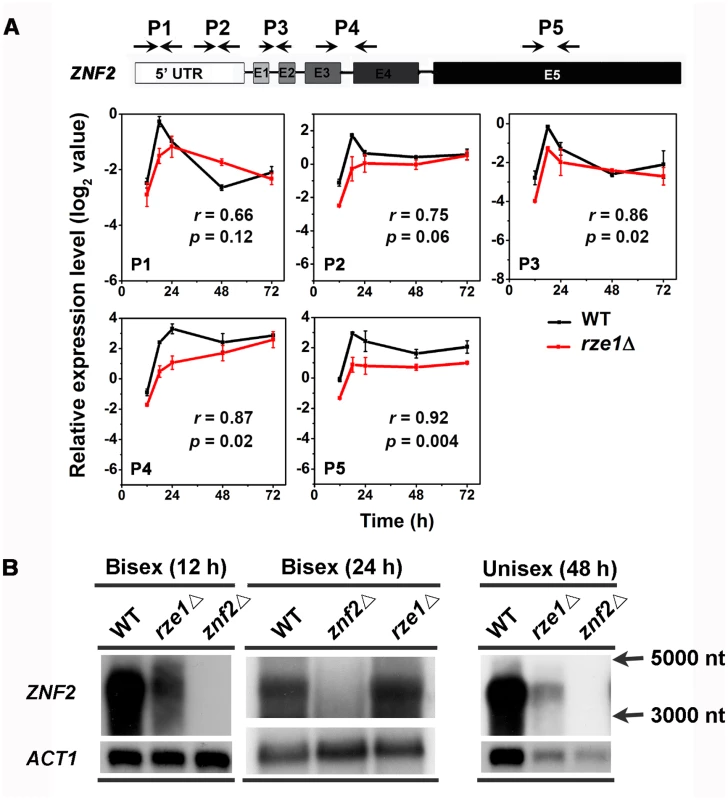
(A) There was no apparent difference in the processed ZNF2 transcripts in the rze1Δ mutant compared to those produced in the wild-type XL280 under the conditions analyzed. The level of different regions of ZNF2 transcripts in the wild type and in the rze1Δ mutant cultured on V8 medium at various time points were analyzed by qPCR. The transcript level of ZNF2 in the rze1Δ mutant shows a similar pattern in all regions. (B) Northern blot of Poly(A) RNAs probed with the DNA sequence of the ZNF2 ORF showed the ZNF2 transcripts of the same size in both the wild type and the rze1Δ mutant during bisexual mating (12h and 24h time points) and unisexual mating (48h time point). The ACT1 transcripts serve as a control. The single stranded RNA markers of 5,000 nt and 3,000 nt were indicated to the right. To test if RZE1 plays a role in other aspects of ZNF2 transcript processing, we performed northern blot and probed with the ZNF2 ORF. As ZNF2 basal expression is low and is induced during bisexual mating [12], we first examined purified poly(A)-RNAs extracted from wild type, the znf2Δ mutant, and the rze1Δ mutant at the 12h and 24h time points during bisexual mating. One band that was larger than 3,000 nt and close to 4,000 nt was detected in both wild type and the rze1Δ mutant, but not in the znf2Δ mutant (Fig 6B). The size is consistent with the predicted approximately 3,600 nt-long mature transcript for ZNF2. The reduction in ZNF2 transcript level in the rze1Δ mutant during bisexual mating was apparent at the 12h time point, but not at the 24h time point (Fig 5C and S6B Fig). This might be because the impact of RZE1 deletion on the ZNF2 transcript level becomes more modest at later time points, as we observed in the unisexual mating cells (S6B Fig). We also compared the ZNF2 transcript size by northern hybridization using purified poly (A) RNAs extracted from wild type, the znf2Δ mutant, and the rze1Δ mutant at 48h during self-filamentation. We found that the size of the ZNF2 transcript remained ~3.6kb in wild type and the rze1Δ mutant (Fig 6B).
Taken together, these results indicate that ZNF2 only has one transcript isoform under the conditions we tested. Furthermore, the loss of RZE1 does not appear to affect cryptococcal ability to process introns of the ZNF2 transcripts.
Disruption of RZE1 reduces the number of ZNF2 transcripts and altered their subcellular distribution
We decided to test the third hypothesis: the effect of RZE1 on the export of the ZNF2 transcripts from the nucleus to the cytosol (Fig 4D). For this purpose, we used the technique called single molecule Fluorescent In Situ Hybridization (smFISH), which helps visualize and quantify transcripts in the cell [47]. Unlike qPCR or RNA-seq that measure the total transcript level from the whole population, smFISH can measure the level of a particular transcript in a single cell and can be used to examine the heterogeneity in gene expression in that population. This microscopy based technique can also reveal the subcellular localization of the examined transcript. Although this technique has been used in research in other organisms (e.g. mammalian cells and model yeasts) [47–49], it has not been applied to Cryptococcus because of the unique challenge of making spheroplast/protoplast in this organism due to the presence of cell wall and polysaccharide capsule. Thus, we did pilot studies to optimize the experimental conditions for Cryptococcus using the U2 spliceosomal RNA as the positive control. We chose the U2 snRNA as the control because of its abundance in intron-rich eukaryotic cells and its exclusive nuclear localization [50]. Consistently, we found abundant U2 snRNA in Cryptococcus nuclei (Fig 7A).
Fig. 7. RZE1 regulates the subcellular distribution of ZNF2 transcripts. 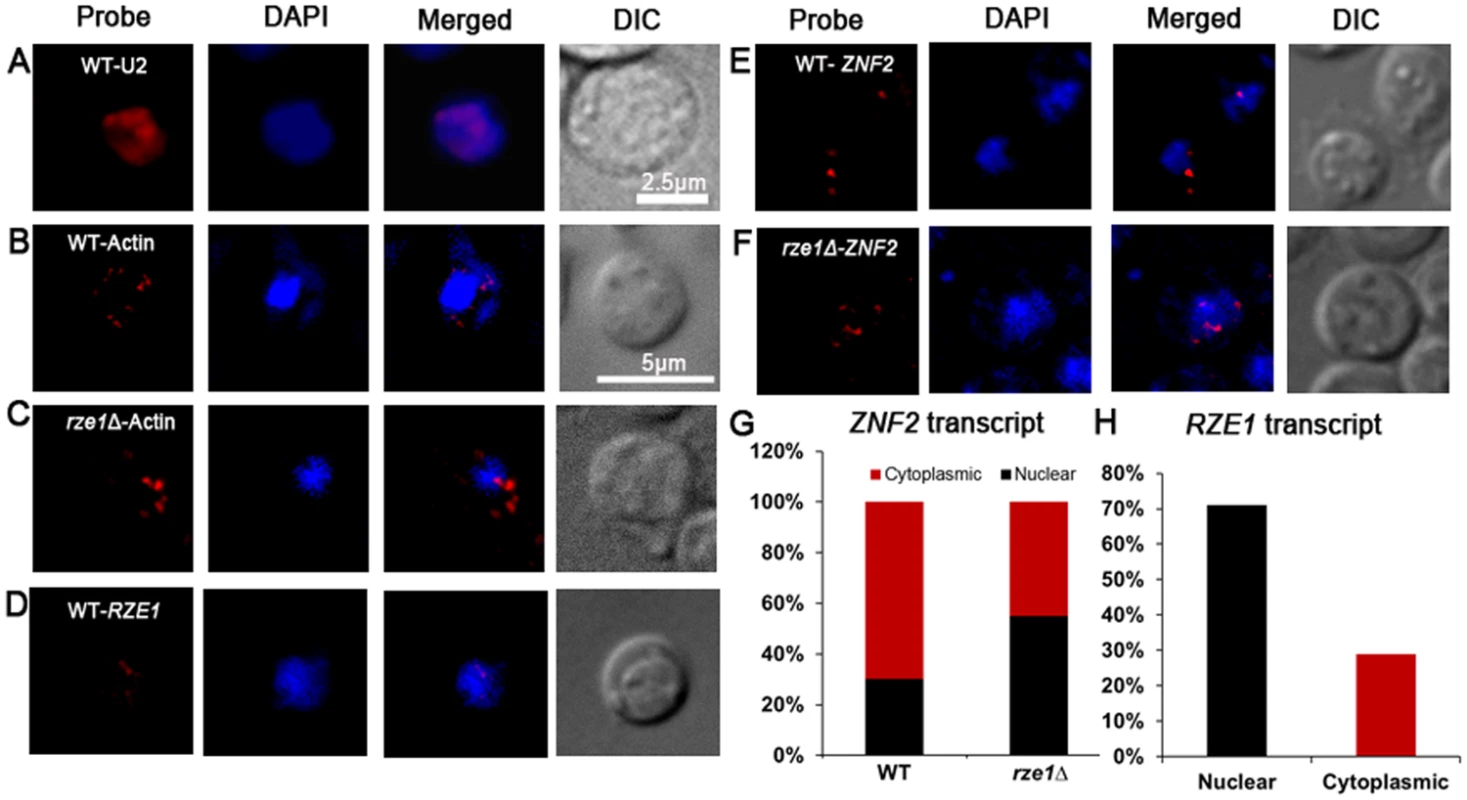
(A) The subcellular localization of U2 snRNA by smFISH in the wild-type strain XL280 cultured in YPD overnight. The image of the wild-type XL280 cells: bright field, Cy3 (probe of U2 snRNA or actin mRNAs), DAPI (nuclei), and Cy3 and DAPI merged. The subcellular localization of ACT1 mRNAs in the wild type (B) and the rze1Δ mutant (C) cells cultured on V8 medium for 18 hours. The DIC and maximum intensity projection of z-stack images for DAPI and Cy3/ACT1 probe channels. (D) The DIC and maximum intensity projection of z-stack images (merged for DAPI and Cy3/RZE1 probe channels) showing the distribution of RZE1 transcripts in the cytoplasm and the nuclei of the wild type cultured on V8 medium for 18 hours. The DIC and maximum intensity projection of z-stack images (merged for DAPI and Cy3/ZNF2 probe channels) showing the distribution of ZNF2 transcripts in the cytoplasm and the nuclei of the wild type (E) and the rze1Δ mutant (F) cultured on V8 medium for 18 hours. The znf2Δ mutant processed under the same conditions was used as negative control to calculate base level of signal. The nuclei were stained with DAPI. (G) The graphic comparison of ZNF2 subcellular localization in the wild type or in the rze1Δ mutant. (H) The graphic representation of the subcellular distribution of RZE1 in wild type. To further establish a control of messenger RNAs that will be localized to the nucleus (for transcription) and cytosol (for translation), we chose to examine the actin transcripts (ACT1) in cells plated on mating media (V8 medium, 18h) (Fig 7B). We found that 62.7% of cells in wild type (n = 884) and 59.3% of cells in the rze1Δ mutant (n = 899) were positive for the actin probe, likely due to incomplete cell wall digestion and the consequent occlusion of the probe from the remaining cells (S2 Table). However, digestion for longer period led to visible damage in some cells and was thus undesirable for the purpose of assessment of the transcripts’ subcellular localization. Thus we decided not to extend enzymatic digestion to prevent cellular damage. We therefore assumed ~50–60% efficiency in probe penetration of cells prepared under such conditions. Among the actin transcripts examined, 70–80% of them were found in the cytosol and 20–30% in the nucleus (S2 Table). Such predominant distribution in the cytosol is expected for messenger RNAs as they primarily serve as templates for translation. Interestingly but not surprisingly, the deletion of RZE1 did not affect the subcellular distribution of ACT1, with still 71% found in the cytosol in the rze1Δ mutant (Fig 7C and S2 Table).
We then proceeded to visualize the ZNF2 transcripts in cryptococcal cells in the absence or the presence of RZE1 under the filamentation-inducing condition that induces the expression of ZNF2 (Fig 7E and 7F). The znf2Δ mutant cells processed under the same conditions were used as the negative control. We found that 49.18% of cells in wild type (n = 612) and 43.9% of cells in the rze1Δ mutant (n = 664) expressed ZNF2 (S2 and S3 Tables.). We believe that the percentage of wild-type cells expressing the ZNF2 gene was an under-estimation. This is because some of the wild type cells expressing higher levels of ZNF2 were turning into hyphae under this condition. These hyphal cells were more likely to be damaged during cell collection from solid agar plates and during the fixation and digestion process. Thus, wild-type cells that likely expressed higher levels of ZNF2 were also more likely to be excluded from the analysis. Nonetheless, as expected for an mRNA, we observed ZNF2 transcripts in both the nucleus and the cytosol (Fig 7E and 7F). Interestingly, we observed a higher percentile of ZNF2 transcripts localized in the nuclei in the rze1Δ mutant (44.7% cytoplasmic: 55.2% nuclear distribution compared to that in the wild type (69.6% cytoplasmic: 30.4% nuclear distribution) (Fig 7G and S2 and S3 Tables). The increased ratio of nucleus versus cytosol distribution of ZNF2 transcripts in the rze1Δ mutant could be caused by a defect in exporting ZNF2 transcripts to the cytoplasm, or alternatively increased cytoplasmic degradation of ZNF2 transcripts. Improperly processed transcripts are known to be subject to degradation in the cytoplasm by RNA surveillance mechanisms [51]. However, we did not detect any apparent difference in intron splicing of the ZNF2 transcripts generated in the rze1Δ mutant (Fig 5B). If ZNF2 transcripts undergo increased degradation in the cytoplasm, the involvement of RZE1 in that process must be, if any, indirect. This is because RZE1 is functionally restricted to its native nucleus (Fig 4A). Thus, we favor the other possibility of increased nuclear retention of ZNF2 transcripts (or reduced ZNF2 transport) in the absence of RZE1. That said, although the direct effect of RZE1 may be in the nucleus, we cannot exclude any indirect effect happening in the cytoplasm. Future investigation is warranted to further distinguish these hypotheses.
It was previously shown that functionally nuclear restricted lncRNAs could be physically confined to nuclei (as exemplified by lncRNAs MALATI and XIST [36, 37]). LncRNAs with nuclear function could also be distributed both in the cytoplasm and nuclei, although the cytoplasmic transcripts are considered non-functional as deductible from their mode of action (e.g. FLO11 lncRNAs in S. cerevisiae [52]). We therefore decided to examine the subcellular localization of RZE1 transcripts in the wild-type cells under a mating-inducing condition. We found that RZE1 was predominantly localized in the nucleus (29.3% cytoplasmic: 70.6% nuclear distribution; n = 514) (Fig 7H and S2 Table). The predominant nuclear localization of RZE1 again is consistent with the evidence presented earlier supporting RZE1 acting as a transcript rather than as an mRNA.
Discussion
Non-coding RNAs constitute a large part of genomes in higher eukaryotes [53, 54]. Among ncRNAs, lncRNAs are typically regulatory ncRNAs arbitrarily defined as transcripts of over 200 nt with minimum protein-coding potential [55]. The first discovered lncRNA H19 [56] was classified as a lncRNA in 1990 after the analysis of cloned gene sequence revealed its poor coding potential and its lack of observed sedimentation with the ribosomes [57]. The famous Xist RNA was identified by virtue of its exclusive expression by the inactivated X chromosome and its association with the X-Inactivation Center in 1991 [58–61]. Xist was classified as a lncRNA a year later due to lack of conserved open reading frames in its sequence and its exclusive nuclear localization [62]. Among the functionally characterized lncRNAs in higher eukaryotes, many are involved in growth, development, and differentiation [63–68]. Consequently, mis-regulation of these lncRNAs could result in tumerogenesis [69, 70], cardiovascular disorders [71, 72], or neurological diseases like Alzheimer’s and Parkinson’s [73]. Some of these lncRNAs are being exploited for the detection and treatment of cancers and lung diseases [74–76]. The mode of action by Xist in silencing the whole duplicated chromosome is being explored to treat diseases caused by trisomies [77]. Thus the investigation into the function of lncRNAs contributes significantly to our understanding of eukaryotic biology and diseases.
In lower eukaryotes such as fungi, however, few lncRNAs involved in the development and stress response have been characterized, mostly in the model systems such as Saccharomyces cerevisiae and Schizosaccharomyces pombe [52, 78, 79]. In Neurospora crassa and Aspergillus flavus, natural antisense transcripts (NATs) and lncRNAs are found to be differentially expressed in response to specific stimuli that induce developmental changes [80, 81]. One N. crassa lncRNA named qrf is experimentally shown to be important in maintaining circadian clock rhythmicity and clock resetting [82]. Although lncRNAs have been identified in phytopathogenic oomycete Phytophthora, and NATs in Ustilago are implicated in its virulence [83], none have been functionally characterized [84, 85]. Unfortunately, the prevalence or the potential functions of lncRNAs is unknown for human fungal pathogens.
Here we discovered and functionally characterized the lncRNA RZE1, which controls cryptococcal morphogenesis through its regulation of ZNF2. An ortholog of RZE1 could not be identified outside of the Cryptococcus species complex by sequence similarity searches. Even within the Cryptococcus species complex, the level of sequence conservation of the RZE1 gene is lower than protein-coding genes such as ZNF2, GPD1, or ACT1. This is not unexpected as lncRNAs are known to evolve at a faster rate and are sometimes species-specific. This, however, does raise the possibilities that RZE1 either is confined to the Cryptococcus species, or it retains evolutionary conservation across different fungal species but does so without sequence conservation. In higher eukaryotes, instances of functional conservation of lncRNAs without sequence conservation have been observed [85–87]. Thus, a functional equivalent of RZE1 in related and distant relatives of Cryptococcus is a possibility worth exploring. Another intriguing possibility is that in Cryptococcus, RZE1 might have evolved to be a regulator dedicated to ZNF2 and/or some of Znf2’s downstream targets. Consistently, the loss of RZE1 does not affect the transcript level of genes CNAG_03365 and CNAG_03367 that are adjacent to ZNF2, reflective of RZE1’s selectivity towards ZNF2 and/or its regulon. This makes RZE1 different from some other lncRNAs that can act globally (e.g. Xist for the entire X chromosome [86–88]).
RZE1 appears to act on ZNF2 primarily through transcription regulation. There are several instances of lncRNAs in the model yeast Saccharomyces that regulate by turning on or off their target transcription directly or indirectly [48, 89]. In this case, the amplitude of ZNF2 transcription induction was reduced about 2–3 folds in general in the rze1Δ mutant. Thus the role of RZE1 in ZNF2 transcriptional regulation appears to be more of modulating role than that of a switch. Given that other mutants with reduced ZNF2 expression level only shows decreased but not blocked filamentation, it stands to reason that the regulatory function of RZE1 goes beyond its effect on ZNF2 transcription. Intriguingly, RZE1 directly or indirectly regulates the distribution of ZNF2 transcripts, although the exact mechanism of RZE1 in regulating Znf2 activity is yet to be established.
Another enigmatic aspect of RZE1 is its position effect. The genetic position of RZE1 seems to have more drastic effect on Znf2 activity than RZE1’s expression level per se. For instance, the introduction of RZE1 in the ‘cis’ position seems to restore filamentation to the rze1Δ mutant much more effectively than an ectopic copy of RZE1 or the multi-copy RZE1 in episomally maintained vector (Fig 1B and S2 and S5 Figs). When comparing the level of RZE1 transcripts in the ‘cis’ complemented strain to the strains complemented with the site-directed mutated RZE1 alleles in the cis position, there were wide variations in the transcript level (S8 Fig). Most of these strains expressed RZE1 at a level higher than that in the wild type, yet their transcript level does not correlate with their robustness in filamentation (Fig 3C and S8 Fig). Similarly, ectopically expressing RZE1 using the strong CTR4 or the GPD1 promoter in the rze1Δ mutant yielded poorer filamentation than the RZE1 integrated at its native locus (S5 Fig). Collectively, these observations indicate that the physical distance between RZE1and ZNF2, and consequently their newly made transcripts, matters greatly for their functional efficiency.
While we speculate that RZE1 specifically regulates ZNF2, we did not find any specific repeats or regions in RZE1 that share sequence similarity with ZNF2. This suggests that their direct interaction, if it exists, is unlikely to be based on simple sequence pairing. The identification of molecules directly interacting with this lncRNA might help solve that mystery. Nonetheless, RZE1 is the first of its kind identified in Cryptococcus. The RZE1-ZNF2 can serve as a paradigm and a stimulus for the investigation of other regulatory lncRNAs in fungal genomes. Our preliminary transcriptional analysis of XL280 and H99 strains suggests that there are more than one thousand conserved lncRNAs in serotype D and serotype A, an equivalent of 1 lncRNA gene for every 6 protein-coding genes in Cryptococcus. Thus, exploring the function of these lncRNAs may give novel insights into the regulatory networks that control the morphogenesis and virulence of this organism. Such an additional layer of genetic regulation might explain the complexity of life cycle and pathogenic strategies of these “simple” eukaryotes.
Materials and Methods
Ethics statement
All the animal experiments were performed according to the guidelines of NIH and Texas A&M University Institutional Animal Care and Use Committee (protocol numbers: 2011–22 and 2014–0049).
Strains and growth conditions
The strains and plasmids used in this study are listed in S4 Table. Yeast cells were grown routinely on YPD media unless otherwise specified. Mating assays were conducted on V8 agar medium in the dark at 22°C as previously described [12]. Cells collected for qPCR were grown on V8 media at 22°C in ambient air or in serum at 37°C in presence of 5% CO2. Transformed strains were obtained by electroporation [90] or biolistic methods [91] and they were selected on YPD with 100 μg/ml of nourseothricin (NAT) or neomycin (NEO). Strains carrying genes driven by the inducible promoter of the CTR4 gene [32] were grown in media supplemented with 25 μM of CuSO4 for suppression or 200 μM of bathocuproine disulphonate (BCS) for induction [32].
Insertional mutagenesis via agrobacterium-mediated transformation and mutant screen
Agrobacterium tumefaciencs strain EHA105 containing the Ti plasmid pPZP-NATcc was used for the insertional mutagenesis as described previously [12, 92]. Briefly, the bacterium was grown overnight at 22°C, washed twice with sterile water, and transferred to an induction medium containing 100μM acetosyringone and incubated for 6h. Overnight culture of Cryptococcus grown in liquid YPD was washed in induction medium and re-suspended to get 107 cells/ml. Fungal and bacterial aliquots (200μl each) were mixed and plated on induction medium and co-cultured for 3 days at 22°C. The co-cultured cells were scraped and plated onto selective medium of YPD containing the antibiotic cefotaxime to remove Agrobacterium and the selective drug NAT 100μg/ml for fungal transformants. A total of 63,000 transformants were generated and screened for filamentation defect on V8 juice agar media using an Olympus SZX16 stereoscope. The colonies showing filamentation defect were further tested for intact pheromone sensing pathway by using unilateral crosses with the mating type a reference strain JEC20. The insertion site in selected 15 transformants with znf2Δ phenotype was identified using inverse PCR and sequencing as described below.
Inverse PCR and sequencing
Genomic DNA from the selected insertion mutants having znf2Δ like phenotype was extracted, digested with restriction enzymes, and then self-ligated as described previously [12, 92]. Primers AI076 and AI077 were used for inverse PCR. The PCR amplicons were sequenced and the sequence was BLAST searched against the C. neoformans (JEC21) serotype D genome database at GenBank to identify the insertion site.
In vitro phenotypic assays
Phenotypic assays were performed as described previously [93]. The strains to be tested were grown overnight in YPD. The cells were washed, adjusted to the same cell density (OD600 = 1.0), and serially diluted. The dilutions were plated onto appropriate media for various tests. To analyze the capacity of the strain to melanize, the original culture and the dilutions were plated onto media containing L-dihydroxyphenylalanine (L-DOPA) and incubated at 37°C in the dark. To observe capsule production, cells were plated on Dulbecco’s Modified Eagle’s medium (DMEM) (Invitrogen) and grown at 37°C under 5% CO2. Capsule was visualized by India ink exclusion and examined under a light microscope. For testing the ability of the fungus to grow at high temperatures and their sensitivity to UV radiation, equal number of cells were plated onto YNB agar media and grown at 37°C or exposed to 300 J/m2 of UV for 1, 5, and 10s. Cells were then cultured at 22°C for additional 2 days. To test the tolerance of fungal cells to osmotic and other stressors, cells were grown at 22°C for 2 days on YNB medium that was supplemented with H2O2 (21mM), calcoflour white (200 μg/ml), iron chelator-bathophenanthroline disulfonic acid (300 μg/ml), SDS (0.1%, 0.01% and 0.001%/shown in SF3), or caspofungin (16 μg/ml).
Confrontation assay
Confrontation assays were performed as described earlier [18]. Donor cells (OD600 = 3) were dropped onto YPD plates 2.5 days before dropping the recipient. The recipient cells (3μl) of OD600 0.8 were dropped in close proximity without touching. The colonies were observed 60h after dropping of the recipient and photographed with Olympus SZX16 stereoscope.
Rapid Amplification of cDNA Ends (RACE) cloning
Gene Racer kit (Invitrogen, CA, USA) was used to amplify 5’ and 3’ ends of RZE1 and ZNF2 RNA. Full length transcript of RZE1 was amplified using the primers indicated in S5 Table from RNA extracted from cells grown on V8 for 24h following the manufacturer’s instructions. All the 3 sets of 5’ and 3’ RACE primers yielded the same start and stop termini. The amplified bands were separated on 0.8% agarose gel and extracted using the gel purification kit (Invitrogen), cloned in TOPO 2.1 (Invitrogen) and sequenced.
Gene manipulations
The targeted deletion of RZE1 (excluding the first 100 bp after the transcription start) was conducted by introducing the deletion construct with the 1 kb flanks of the gene and split part of the dominant marker NAT, by electroporation or biolistic transformation as described earlier. Mutants generated were confirmed by PCR and by the genetic linkage assay using meiotic progeny dissected from genetic crosses [94]. The deletion mutant in the MATa background was obtained by crossing the MATα rze1Δ strains with the corresponding congenic wild type a strains. For complementation, RZE1 transcript with 1kb of promoter region was amplified by PCR, digested, and inserted into the plasmid pXL1 [20]. Overexpression construct was created by amplifying the entire ORF by PCR and similarly inserting into pXL1 behind GPD1 or CTR4 promoters as described before [31, 32]. The RZE1 multi-copy expression strains were generated by cloning the RZE1 with 1kb of its own promoter into the pPM8 vector [95]. The linearized vector was introduced into the auxotrophic rze1Δ ura5 strain by electroporation. The primers used for the generation of the mutants and for their analysis are listed in S5 Table.
RNA purification, qPCR, and northern blot
RNA extraction and real-time qPCR were carried out as we previously described [20]. Briefly, total RNA was extracted using PureLink RNA mini kit (Life Technologies). DNase (Ambion) treated RNA samples were analyzed on a denaturing formaldehyde agarose gel for assessing quality and concentration. Superscript III cDNA synthesis kit (Life Technology) was used for the first strand cDNA synthesis following the manufacturer’s instructions. Constitutively expressing housekeeping gene TEF1 was used as endogenous control for the normalization of expression of the other genes studied.
For Northern blot, total RNA was extracted from cultures on V8 medium (pH = 7.0) from the indicated strains under the conditions as described in the texts. Poly(A) tailed RNAs were purified using the PolyATtract mRNA Isolation System IV (Promega) according to the manufacture’s instruction. PolyA tailed RNAs were then separated on denaturing formaldehyde agarose gels and then transferred to nylon membrane. The Random Primers DNA Labeling System (Life technologies) was used to generate probes. Primer sequences used to make gene-specific templates are listed in S5 Table.
RNA-seq analysis
Wild-type strain XL280 and the rze1Δ mutant were cultured on YPD and V8 (pH = 7) media. Cells were collected at 24, 48 and 72h from mating media and used for isolation of total RNA. Strand-specific RNA-seq was performed at the TAMU genomic facility according to the standard protocol for Illumina Genome Analyzer IIx. Sequenced reads were aligned to the XL280 reference sequence [26] using Tophat [96]. Reads that aligned uniquely to the reference sequence were considered for gene expression quantification with Cufflinks [97]. Gene expression was normalized using the DESeq package [98] in R [99]. Differential expression analysis comparing mutant to wild type was performed with using a 5% false discovery rate. RNA-Seq data is deposited at NCBI (BioProject ID PRGNA278291, accession 278291).
Site-directed mutagenesis
To introduce single nucleotide mutations in the potential translation start sites of RZE1, Quick Change II XL site directed mutagenesis kit from (Agilent Technologies) was used following manufacturer’s instructions. Primers for introducing the mutations (S5 Table) were designed using the QuickChange primer design program from Agilent Technologies. RZE1 along with 1kb of its 5’ flank were amplified by PCR and cloned into pGEMT easy vector (Promega). The entire plasmid with insert was then amplified using specific primers to change each of the ATGs to AAGs or ATAs. The modified inserts were then amplified from the plasmid using PCR with high fidelity Phusion enzyme (New England Biolabs) and used as template for overlap PCR with part of NEO marker gene to obtain a merged construct of RZE1 with 5’ flank and part of the marker. Similar overlap PCR with 3’ flank of RZE1 and part of NEO was used to obtain the other half of insertion construct. The two parts of insertion construct were then mixed and introduced biolistically into Cryptococcus rze1Δ mutant strain to replace the rze1Δ deletion construct. The stable transformants that showed resistance to NEO (indicative of the integration of the mutant RZE1 allele) and sensitivity to NAT (indicative of the loss of the rze1Δ construct) were selected and further analyzed by PCR to confirm the replacement.
Virulence assay using the inhalation infection model of murine cryptococcosis
Female A/J mice of 6–8 week were intranasally inoculated with 5x104 cells/mouse of serotype A strains or 5x105 cells/mouse of serotype D strains with 5 mice each group as we previously described [100–102]. The mice were sacrificed 10 days post inoculation. The organs from sacrificed animals were dissected and homogenized in phosphate buffered saline, serially diluted, and plated on YNB plates. After 2 days of incubation at 30°C, the CFUs were counted. One way ANOVA was used to analyze the fungal burden. All statistical analyses were performed using the Graphpad Prism 5 program and and P values lower than 0.05 was considered statistically significant.
Heterokaryon assay
All strains in H99 background were grown overnight in YPD liquid medium. Cells were washed and suspended in PBS with the same cell density (OD600 = 1.0). Equal number of cells of each pair of strains as indicated in the figure and the text were mixed and spotted onto V8 agar pH 5.0 and incubated at 22°C in the dark for 2.5 weeks. The colony morphology was observed and photographed using an Olympus SZX16 stereo microscope.
Measurement of fluorescence intensity
The fluorescent intensity of Znf2 tagged with mCherry was measured using the Zen Lite software from Carl Zeiss. The microscopic images taken on a Zeiss Axioplan 2 microscope were analyzed using the interactive fluorescence measurement feature of Zen. Fluorescence intensity of Znf2-mCherry from forty cells in either the wild-type background or the rze1Δ mutant background was measured using the software and statistically analyzed using Prism V from Graphpad.
Single molecule FISH experiments
The smFISH experiments were performed as described before [103–105] with modifications for Cryptococcus. Strains either grown to exponential growth phase in YPD or the co-culture of the congenic mating pairs on V8 media were collected and fixed in 4% paraformaldehyde for 30 minutes at 22°C. The cells were digested in sphaeroplasting buffer (1M sorbitol, 10 mM EDTA, and 100 mM sodium citrate) using 100mg/ml of lyzing enzyme (Sigma) for 45 min. Sphaeroplasted cells were permeabilized after being incubated in 70% ethanol at 4°C overnight. Cells were then hybridized with TAMRA labeled ZNF2 specific probes or ALEXA FLOUR labelled RZE1 probes (Stellaris, Biosearch Technologies) in buffer containing 10% formamide for 16h at 37°C. Counter staining to visualize nuclei was done using 10 mg/ml of DAPI. The cells were visualized on a Zeiss Imager M2 microscope and the z-stack images were then subjected to 3D deconvolution by using AutoQuant software (Media Cybernetics). After deconvolution, the images were imported in Zen Blue software and the channels were merged and analyzed.
Supporting Information
Zdroje
1. Wang L, Lin X. Morphogenesis in fungal pathogenicity: shape, size, and surface. PLoS Pathog. 2012;8(12):e1003027. doi: 10.1371/journal.ppat.1003027 23236274
2. Sil A, Andrianopoulos A. Thermally dimorphic human fungal pathogens-polyphyletic pathogens with a convergent pathogenicity trait. Cold Spring Harbor perspectives in medicine. 2014.
3. Andrianopoulos A. Control of morphogenesis in the human fungal pathogen Penicillium marneffei. Int J Med Microbiol. 2002;292(5–6):331–47. 12452280
4. Klein BS, Tebbets B. Dimorphism and virulence in fungi. Curr Opin Microbiol. 2007;10(4):314–9. 17719267
5. Liu H. Co-regulation of pathogenesis with dimorphism and phenotypic switching in Candida albicans, a commensal and a pathogen. Int J Med Microbiol. 2002;292(5–6):299–311. 12452278
6. Stoldt VR, Sonneborn A, Leuker CE, Ernst JF. Efg1p, an essential regulator of morphogenesis of the human pathogen Candida albicans, is a member of a conserved class of bHLH proteins regulating morphogenetic processes in fungi. EMBO J1997 1997-04-15 00 : 00 : 00. 1982–91 p. 9155024
7. Lin X. Cryptococcus neoformans: Morphogenesis, infection, and evolution. Infect Genet Evol. 2009;9(4):401–16. doi: 10.1016/j.meegid.2009.01.013 19460306
8. Park BJ, Wannemuehler KA, Marston BJ, Govender N, Pappas PG, Chiller TM. Estimation of the current global burden of cryptococcal meningitis among persons living with HIV/AIDS. AIDS. 2009;23(4):525–30. doi: 10.1097/QAD.0b013e328322ffac 19182676
9. Kwon-Chung KJ. Morphogenesis of Filobasidiella neoformans, the sexual state of Cryptococcus neoformans. Mycologia. 1976;68(4):821–33. 790172
10. Heitman J. Evolution of eukaryotic microbial pathogens via covert sexual reproduction. Cell Host Microbe. 2010;8(1):86–99. doi: 10.1016/j.chom.2010.06.011 20638645
11. Lin X, Hull CM, Heitman J. Sexual reproduction between partners of the same mating type in Cryptococcus neoformans. Nature. 2005;434(7036):1017–21. 15846346
12. Lin X, Jackson JC, Feretzaki M, Xue C, Heitman J. Transcription factors Mat2 and Znf2 operate cellular circuits orchestrating opposite - and same-sex mating in Cryptococcus neoformans. PLoS Genet. 2010;6(5):e1000953. doi: 10.1371/journal.pgen.1000953 20485569
13. Nichols CB, Fraser JA, Heitman J. PAK kinases Ste20 and Pak1 govern cell polarity at different stages of mating in Cryptococcus neoformans. Mol Biol Cell. 2004;15(10):4476–89. 15282344
14. Davidson RC, Nichols CB, Cox GM, Perfect JR, Heitman J. A MAP kinase cascade composed of cell type specific and non-specific elements controls mating and differentiation of the fungal pathogen Cryptococcus neoformans. Mol Microbiol. 2003;49(2):469–85. 12828643
15. Kruzel EK, Giles SS, Hull CM. Analysis of Cryptococcus neoformans sexual development reveals rewiring of the pheromone-response network by a change in transcription factor identity. Genetics. 2012;191(2):435–49. doi: 10.1534/genetics.112.138958 22466042
16. Kwon-Chung KJ. A new genus, filobasidiella, the perfect state of Cryptococcus neoformans. Mycologia. 1975;67(6):1197–200. Epub 1975/11/01. 765816
17. Wang L, Tian X, Gyawali R, Upadhyay S, Foyle D, Wang G, et al. Morphotype Transition and Sexual Reproduction Are Genetically Associated in a Ubiquitous Environmental Pathogen. PLoS pathogens. 2014;10(6):e1004185. doi: 10.1371/journal.ppat.1004185 24901238
18. Wang L, Tian X, Gyawali R, Lin X. Fungal adhesion protein guides community behaviors and autoinduction in a paracrine manner. Proc Natnl Acad Sci USA. 2013;110(28):11571–6.
19. Tian X, Lin X. Matricellular protein Cfl1 regulates cell differentiation. Comm Integ Biol. 2013;6(6):e26444.
20. Wang L, Zhai B, Lin X. The link between morphotype transition and virulence in Cryptococcus neoformans. PLoS Pathog. 2012;8(6):e1002765. Epub 2012/06/28. doi: 10.1371/journal.ppat.1002765 22737071
21. Zhai B, Zhu P, Foyle D, Upadhyay S, Idnurm A, Lin X. Congenic strains of the filamentous form of Cryptococcus neoformans for studies of fungal morphogenesis and virulence. Infect Immun. 2013;81(7):2626–37. doi: 10.1128/IAI.00259-13 23670559
22. Zhai B WK, Masso - Silva J, Upadhyay S, Hole C, Rivera A, Wormley FL, and Lin X,. Development of protective inflammation and cell-mediated immunity against Cryptococcus neoformans after exposure to hyphal mutants. mBio. 2015;6(5):e01433–15. doi: 10.1128/mBio.01433-15 26443458
23. Lin J, Idnurm A, Lin X. Morphology and its underlying genetic regulation impact the interaction between Cryptococcus neoformans and its hosts. Medical Mycol. 2015;53(5):493–504.
24. Chacko N, Lin X. Non-coding RNAs in the development and pathogenesis of eukaryotic microbes. Appl Microbiol Biot. 2013;97(18):7989–97.
25. Lin X, Huang JC, Mitchell TG, Heitman J. Virulence attributes and hyphal growth of C. neoformans are quantitative traits and the MATα allele enhances filamentation. PLoS Genet. 2006;2(11):e187. 17112316
26. Ni M, Feretzaki M, Li W, Floyd-Averette A, Mieczkowski P, Dietrich FS, et al. Unisexual and heterosexual meiotic reproduction generate aneuploidy and phenotypic diversity de novo in the yeast Cryptococcus neoformans. PLoS Biol. 2013;11(9):e1001653. doi: 10.1371/journal.pbio.1001653 24058295
27. Feretzaki M, Heitman J. Genetic circuits that govern bisexual and unisexual reproduction in Cryptococcus neoformans. PLoS Genet. 2013;9(8):e1003688. doi: 10.1371/journal.pgen.1003688 23966871
28. Janbon G, Ormerod KL, Paulet D, Byrnes EJ 3rd, Yadav V, Chatterjee G, et al. Analysis of the genome and transcriptome of Cryptococcus neoformans var. grubii reveals complex RNA expression and microevolution leading to virulence attenuation. PLoS Genet. 2014;10(4):e1004261. Epub 2014/04/20. doi: 10.1371/journal.pgen.1004261 24743168
29. Nielsen K, Cox GM, Wang P, Toffaletti DL, Perfect JR, Heitman J. Sexual cycle of Cryptococcus neoformans var. grubii and virulence of congenic a and α Isolates. Infect Immun. 2003;71(9):4831–41. 12933823
30. Janbon G, Ormerod KL, Paulet D, Byrnes EJ III, Yadav V, Chatterjee G, et al. Analysis of the genome and transcriptome of Cryptococcus neoformans var. grubii reveals complex RNA expression and microevolution leading to virulence attenuation. PLoS Genet. 2014;10(4):e1004261. doi: 10.1371/journal.pgen.1004261 24743168
31. Xue C, Bahn Y-S, Cox GM, Heitman J. G Protein-coupled receptor Gpr4 senses amino acids and activates the cAMP-PKA pathway in Cryptococcus neoformans. Mol Biol Cell. 2006;17(2):667–79. 16291861
32. Ory JJ, Griffith CL, Doering TL. An efficiently regulated promoter system for Cryptococcus neoformans utilizing the CTR4 promoter. Yeast. 2004;21(11):919–26. 15334556
33. Wei J, Zhang Y, Ivanov IP, Sachs MS. The stringency of start codon selection in the filamentous fungus Neurospora crassa. J Biol Chem. 2013;288(13):9549–62. doi: 10.1074/jbc.M112.447177 23396971
34. van Heesch S, van Iterson M, Jacobi J, Boymans S, Essers PB, de Bruijn E, et al. Extensive localization of long noncoding RNAs to the cytosol and mono - and polyribosomal complexes. Genome Biol. 2014;15(1):R6–R. doi: 10.1186/gb-2014-15-1-r6 24393600
35. Zhang K, Shi ZM, Chang YN, Hu ZM, Qi HX, Hong W. The ways of action of long non-coding RNAs in cytoplasm and nucleus. Gene. 2014;547(1):1–9. Epub 2014/06/27. doi: 10.1016/j.gene.2014.06.043 24967943
36. Miyagawa R, Tano K, Mizuno R, Nakamura Y, Ijiri K, Rakwal R, et al. Identification of cis - and trans-acting factors involved in the localization of MALAT-1 noncoding RNA to nuclear speckles. RNA. 2012;18(4):738–51. Epub 2012/02/23. doi: 10.1261/rna.028639.111 22355166
37. Cohen HR, Panning B. XIST RNA exhibits nuclear retention and exhibits reduced association with the export factor TAP/NXF1. Chromosoma. 2007;116(4):373–83. Epub 2007/03/03. 17333237
38. Cabili MN, Dunagin MC, McClanahan PD, Biaesch A, Padovan-Merhar O, Regev A, et al. Localization and abundance analysis of human lncRNAs at single-cell and single-molecule resolution. Genome Biol. 2015;16(1):20. Epub 2015/01/30.
39. Miyagawa R, Tano K, Mizuno R, Nakamura Y, Ijiri K, Rakwal R, et al. Identification of cis - and trans-acting factors involved in the localization of MALAT-1 noncoding RNA to nuclear speckles. RNA. 2012;18(4):738–51. doi: 10.1261/rna.028639.111 22355166
40. Lin X, Hull CM, Heitman J. Sexual reproduction between partners of the same mating type in Cryptococcus neoformans. Nature. 2005;434(7036):1017–21. 15846346
41. Hull CM, Heitman J. Genetics of Cryptococcus neoformans. Annu Rev Genet. 2002;36 : 557–615. 12429703
42. Hull CM, Boily M-J, Heitman J. Sex-specific homeodomain proteins Sxi1α and Sxi2a coordinately regulate sexual development in Cryptococcus neoformans. Eukaryot Cell. 2005;4(3):526–35. 15755915
43. Gyawali R, Lin X. Prezygotic and postzygotic control of uniparental mitochondrial DNA inheritance in Cryptococcus neoformans. mBio. 2013;4(2). Epub 2013/04/25.
44. Kozubowski L, Heitman J. Septins enforce morphogenetic events during sexual reproduction and contribute to virulence of Cryptococcus neoformans. Mol Microbiol. 2010;75(3):658–75. doi: 10.1111/j.1365-2958.2009.06983.x 19943902
45. Ballou ER, Kozubowski L, Nichols CB, Alspaugh JA. Ras1 acts through duplicated Cdc42 and Rac proteins to regulate morphogenesis and pathogenesis in the human fungal pathogen Cryptococcus neoformans. PLoS Genet. 2013;9(8):e1003687. doi: 10.1371/journal.pgen.1003687 23950731
46. Fu J, Morris IR, Wickes BL. The production of monokaryotic hyphae by Cryptococcus neoformans can be induced by high temperature arrest of the cell cycle and Is independent of same-sex mating. PLoS Pathog. 2013;9(5):e1003335. doi: 10.1371/journal.ppat.1003335 23658522
47. McIsaac RS, Silverman SJ, Parsons L, Xu P, Briehof R, McClean MN, et al. Visualization and analysis of mRNA molecules using Fluorescence In Situ Hybridization in Saccharomyces cerevisiae. J Visual Exp. 2013;(76):50382.
48. Bumgarner SL, Neuert G, Voight BF, Symbor-Nagrabska A, Grisafi P, van Oudenaarden A, et al. Single-cell analysis reveals that noncoding RNAs contribute to clonal heterogeneity by modulating transcription factor recruitment. Mol Cell. 2012;45(4):470–82. doi: 10.1016/j.molcel.2011.11.029 22264825
49. Tripathi V, Ellis JD, Shen Z, Song DY, Pan Q, Watt AT, et al. The nuclear-retained noncoding RNA MALAT1 regulates alternative splicing by modulating SR splicing factor phosphorylation. Mol Cell. 2010;39(6):925–38. doi: 10.1016/j.molcel.2010.08.011 20797886
50. Kohler A, Hurt E. Exporting RNA from the nucleus to the cytoplasm. Nat Rev Mol Cell Biol. 2007;8(10):761–73. 17786152
51. Garneau NL, Wilusz J, Wilusz CJ. The highways and byways of mRNA decay. Nat Rev Mol Cell Biol. 2007;8(2):113–26. 17245413
52. Bumgarner SL, Dowell RD, Grisafi P, Gifford DK, Fink GR. Toggle involving cis-interfering noncoding RNAs controls variegated gene expression in yeast. Proc Natl Acad Sci USA. 2009;106(43):18321–6. doi: 10.1073/pnas.0909641106 19805129
53. Mattick JS. The functional genomics of noncoding RNA. Science. 2005;309(5740):1527–8. 16141063
54. Prasanth KV, Spector DL. Eukaryotic regulatory RNAs: an answer to the 'genome complexity' conundrum. Genes & development. 2007;21(1):11–42. Epub 2007/01/11.
55. Mercer TR, Dinger ME, Mattick JS. Long non-coding RNAs: insights into functions. Nat Rev Genet. 2009;10(3):155–9. doi: 10.1038/nrg2521 19188922
56. Pachnis V, Belayew A, Tilghman SM. Locus unlinked to alpha-fetoprotein under the control of the murine raf and Rif genes. Proc Natl Acad Sci USA. 1984;81(17):5523–7. 6206499
57. Brannan CI, Dees EC, Ingram RS, Tilghman SM. The product of the H19 gene may function as an RNA. Mol Cell Biol. 1990;10(1):28–36. 1688465
58. Brown CJ, Ballabio A, Rupert JL, Lafreniere RG, Grompe M, Tonlorenzi R, et al. A gene from the region of the human X inactivation centre is expressed exclusively from the inactive X chromosome. Nature. 1991;349(6304):38–44. Epub 1991/01/03. 1985261
59. Brockdorff N, Ashworth A, Kay GF, Cooper P, Smith S, McCabe VM, et al. Conservation of position and exclusive expression of mouse Xist from the inactive X chromosome. Nature. 1991;351(6324):329–31. 2034279
60. Borsani G, Tonlorenzi R, Simmler MC, Dandolo L, Arnaud D, Capra V, et al. Characterization of a murine gene expressed from the inactive X chromosome. Nature. 1991;351(6324):325–9. 2034278
61. Brown CJ, Lafreniere RG, Powers VE, Sebastio G, Ballabio A, Pettigrew AL, et al. Localization of the X inactivation centre on the human X chromosome in Xq13. Nature. 1991;349(6304):82–4. 1985270
62. Brockdorff N, Ashworth A, Kay GF, McCabe VM, Norris DP, Cooper PJ, et al. The product of the mouse Xist gene is a 15 kb inactive X-specific transcript containing no conserved ORF and located in the nucleus. Cell. 1992;71(3):515–26. 1423610
63. Loewer S, Cabili MN, Guttman M, Loh Y-H, Thomas K, Park IH, et al. Large intergenic non-coding RNA-RoR modulates reprogramming of human induced pluripotent stem cells. Nat Genet. 2010;42(12):1113–7. doi: 10.1038/ng.710 21057500
64. Ng SY, Johnson R, Stanton LW. Human long non-coding RNAs promote pluripotency and neuronal differentiation by association with chromatin modifiers and transcription factors. EMBO J. 2012;31(3):522–33. doi: 10.1038/emboj.2011.459 22193719
65. Cesana M, Cacchiarelli D, Legnini I, Santini T, Sthandier O, Chinappi M, et al. A long noncoding RNA controls muscle differentiation by functioning as a competing endogenous RNA. Cell. 2011;147(2):358–69. Epub 2011/10/18. doi: 10.1016/j.cell.2011.09.028 22000014
66. Klattenhoff Carla A, Scheuermann Johanna C, Surface Lauren E, Bradley Robert K, Fields Paul A, Steinhauser Matthew L, et al. Braveheart, a long noncoding RNA required for cardiovascular lineage commitment. Cell. 2013;152(3):570–83. doi: 10.1016/j.cell.2013.01.003 23352431
67. Grote P, Wittler L, Hendrix D, Koch F, Währisch S, Beisaw A, et al. The tissue-specific lncRNA Fendrr is an essential regulator of heart and body wall development in the mouse. Dev Cell. 2013;24(2):206–14. doi: 10.1016/j.devcel.2012.12.012 23369715
68. Kretz M, Siprashvili Z, Chu C, Webster DE, Zehnder A, Qu K, et al. Control of somatic tissue differentiation by the long non-coding RNA TINCR. Nature. 2013;493(7431):231–5. doi: 10.1038/nature11661 23201690
69. Huang JF, Guo YJ, Zhao CX, Yuan SX, Wang Y, Tang GN, et al. Hepatitis B virus X protein (HBx)-related long noncoding RNA (lncRNA) down-regulated expression by HBx (Dreh) inhibits hepatocellular carcinoma metastasis by targeting the intermediate filament protein vimentin. Hepatology. 2013;57(5):1882–92. doi: 10.1002/hep.26195 23239537
70. Liu Q, Huang J, Zhou N, Zhang Z, Zhang A, Lu Z, et al. LncRNA loc285194 is a p53-regulated tumor suppressor. Nucleic Acids Res. 2013;41(9):4976–87. doi: 10.1093/nar/gkt182 23558749
71. Han P, Li W, Lin C-H, Yang J, Shang C, Nurnberg ST, et al. A long noncoding RNA protects the heart from pathological hypertrophy. Nature. 2014;514(7520):102–6. doi: 10.1038/nature13596 25119045
72. Greco S, Gorospe M, Martelli F. Noncoding RNA in age-related cardiovascular diseases. J Mol Cell Cardiol. 2015;(0). Epub Jan 29
73. Manel E. Non-coding RNAs in human disease. Nat Rev Genet. 2011;12(12):861–74. doi: 10.1038/nrg3074 22094949
74. Castanotto D, Rossi JJ. The promises and pitfalls of RNA-interference-based therapeutics. Nature. 2009;457(7228):426–33. doi: 10.1038/nature07758 19158789
75. Prensner JR, Chinnaiyan AM. The emergence of lncRNAs in cancer biology. Cancer disc. 2011;1(5):391–407.
76. Comer BS, Ba M, Singer CA, Gerthoffer WT. Epigenetic targets for novel therapies of lung diseases. Pharmacol Therapeut. 2015;147 : 91–110.
77. Jiang J, Jing Y, Cost GJ, Chiang J-C, Kolpa HJ, Cotton AM, et al. Translating Dosage Compensation to Trisomy 21. Nature. 2013;500(7462): doi: 10.1038/nature12394
78. Gelfand B, Mead J, Bruning A, Apostolopoulos N, Tadigotla V, Nagaraj V, et al. Regulated antisense transcription controls expression of cell-type-specific genes in yeast. Mol Cell Biol. 2011;31(8):1701–9. doi: 10.1128/MCB.01071-10 21300780
79. Ding D-Q, Okamasa K, Yamane M, Tsutsumi C, Haraguchi T, Yamamoto M, et al. Meiosis-specific noncoding RNA mediates robust pairing of homologous chromosomes in meiosis. Science. 2012;336(6082):732–6. doi: 10.1126/science.1219518 22582262
80. Arthanari Y, Heintzen C, Griffiths-Jones S, Crosthwaite SK. Natural antisense transcripts and long non-coding RNA in Neurospora crassa. PLoS ONE. 2014;9(3):e91353. doi: 10.1371/journal.pone.0091353 24621812
81. Smith C, Robertson D, Yates B, Nielsen D, Brown D, Dean R, et al. The effect of temperature on Natural Antisense Transcript (NAT) expression in Aspergillus flavus. Curr Genet. 2008;54(5):241–69. doi: 10.1007/s00294-008-0215-9 18813928
82. Xue Z, Ye Q, Anson SR, Yang J, Xiao G, Kowbel D, et al. Transcriptional interference by antisense RNA is required for circadian clock function. Nature. 2014;514(7524):650–3. doi: 10.1038/nature13671 25132551
83. Donaldson ME, Saville BJ. Ustilago maydis natural antisense transcript expression alters mRNA stability and pathogenesis. Mol Microbiol. 2013;89(1):29–51. doi: 10.1111/mmi.12254 23650872
84. Avrova AO, Whisson SC, Pritchard L, Venter E, De Luca S, Hein I, et al. A novel non-protein-coding infection-specific gene family is clustered throughout the genome of Phytophthora infestans. Microbiology. 2007;153(Pt 3):747–59. 17322195
85. Morrison EN, Donaldson ME, Saville BJ. Identification and analysis of genes expressed in the Ustilago maydis dikaryon: uncovering a novel class of pathogenesis genes. Can J Plant Pathol. 2012;34(3):417–35.
86. Clemson CM, McNeil JA, Willard HF, Lawrence JB. XIST RNA paints the inactive X chromosome at interphase: evidence for a novel RNA involved in nuclear/chromosome structure. J Cell Biol. 1996;132(3):259–75. 8636206
87. Zhao J, Sun BK, Erwin JA, Song J-J, Lee JT. Polycomb proteins targeted by a short repeat RNA to the mouse X chromosome. Science. 2008;322(5902):750–6. doi: 10.1126/science.1163045 18974356
88. Jeon Y, Lee Jeannie T. YY1 tethers Xist RNA to the inactive X nucleation center. Cell. 2011;146(1):119–33. doi: 10.1016/j.cell.2011.06.026 21729784
89. Bird AJ, Gordon M, Eide DJ, Winge DR. Repression of ADH1 and ADH3 during zinc deficiency by Zap1—induced intergenic RNA transcripts2006 2006-12-13 00 : 00 : 00. 5726–34 p.
90. Lin X, Chacko N, Wang L, Pavuluri Y. Generation of stable mutants and targeted gene deletion strains in Cryptococcus neoformans through electroporation. Medical Mycol. 2014. Epub Dec 24.
91. Toffaletti DL, Rude TH, Johnston SA, Durack DT, Perfect JR. Gene transfer in Cryptococcus neoformans by use of biolistic delivery of DNA. J Bacteriol. 1993;175(5):1405–11. 8444802
92. Idnurm A, Reedy JL, Nussbaum JC, Heitman J. Cryptococcus neoformans virulence gene discovery through insertional mutagenesis. Eukaryot Cell. 2004;3(2):420–9. 15075272
93. Lin X, Nielsen K, Patel S, Heitman J. Impact of mating type, serotype, and ploidy on the virulence of Cryptococcus neoformans. Infect Immun. 2008;76(7):2923–38. doi: 10.1128/IAI.00168-08 18426889
94. Idnurm A. A tetrad analysis of the basidiomycete fungus Cryptococcus neoformans. Genetics. 2010;185(1):153–63. doi: 10.1534/genetics.109.113027 20157004
95. Mondon P, Chang YC, Varma A, Kwon-Chung KJ. A novel episomal shuttle vector for transformation of Cryptococcus neoformans with the ccdB gene as a positive selection marker in bacteria2000 2000-06-01 00 : 00 : 00. 41–5 p.
96. Trapnell C, Pachter L, Salzberg SL. TopHat: discovering splice junctions with RNA-Seq. Bioinformatics. 2009;25(9):1105–11. doi: 10.1093/bioinformatics/btp120 19289445
97. Trapnell C, Williams BA, Pertea G, Mortazavi A, Kwan G, van Baren MJ, et al. Transcript assembly and quantification by RNA-Seq reveals unannotated transcripts and isoform switching during cell differentiation. Nature biotechnology. 2010;28(5):511–5. doi: 10.1038/nbt.1621 20436464
98. Anders S, Huber W. Differential expression analysis for sequence count data. Genome Biol. 2010;11(10):R106. doi: 10.1186/gb-2010-11-10-r106 20979621
99. Team RDC. R: A language and environment for statistical computing Vienna, Austria2008. http://www.R-project.org.
100. Zhai B, Zhu P, Foyle D, Upadhyay S, Idnurm A, Lin X. Congenic strains of the filamentous form of Cryptococcus neoformans for studies of fungal morphogenesis and virulence. Infection and immunity. 2013;81(7):2626–37. Epub 2013/05/15. doi: 10.1128/IAI.00259-13 23670559
101. Zhai B, Lin X. Evaluation of the anticryptococcal activity of the antibiotic polymyxin B in vitro and in vivo. International journal of antimicrobial agents. 2013;41(3):250–4. Epub 2013/01/15. doi: 10.1016/j.ijantimicag.2012.11.006 23313397
102. Zhai B, Wu C, Wang L, Sachs MS, Lin X. The antidepressant sertraline provides a promising therapeutic option for neurotropic cryptococcal infections. Antimicrob Agents Chemother. 2012;56(7):3758–66. Epub 2012/04/18. doi: 10.1128/AAC.00212-12 22508310
103. Raj A, van den Bogaard P, Rifkin SA, van Oudenaarden A, Tyagi S. Imaging individual mRNA molecules using multiple singly labeled probes. Nat Meth. 2008;5(10):877–9.
104. Trcek T, Chao JA, Larson DR, Park HY, Zenklusen D, Shenoy SM, et al. Single-mRNA counting using fluorescent in situ hybridization in budding yeast. Nat Protocols. 2012;7(2):408–19. doi: 10.1038/nprot.2011.451 22301778
105. Lee C, Occhipinti P, Gladfelter AS. PolyQ-dependent RNA–protein assemblies control symmetry breaking. J Cell Biol. 2015;208(5):533–44. doi: 10.1083/jcb.201407105 25713414
Štítky
Genetika Reprodukční medicína
Článek A Hereditary Enteropathy Caused by Mutations in the Gene, Encoding a Prostaglandin TransporterČlánek Exocyst-Dependent Membrane Addition Is Required for Anaphase Cell Elongation and Cytokinesis inČlánek Spindle-F Is the Central Mediator of Ik2 Kinase-Dependent Dendrite Pruning in Sensory Neurons
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2015 Číslo 11- Akutní intermitentní porfyrie
- Farmakogenetické testování pomáhá předcházet nežádoucím efektům léčiv
- Růst a vývoj dětí narozených pomocí IVF
- Hypogonadotropní hypogonadismus u žen a vliv na výsledky reprodukce po IVF
- Molekulární vyšetření pro stanovení prognózy pacientů s chronickou lymfocytární leukémií
-
Všechny články tohoto čísla
- Agricultural Genomics: Commercial Applications Bring Increased Basic Research Power
- Ernst Rüdin’s Unpublished 1922-1925 Study “Inheritance of Manic-Depressive Insanity”: Genetic Research Findings Subordinated to Eugenic Ideology
- Convergent Evolution During Local Adaptation to Patchy Landscapes
- The Locus Controls Age at Maturity in Wild and Domesticated Atlantic Salmon ( L.) Males
- A Hereditary Enteropathy Caused by Mutations in the Gene, Encoding a Prostaglandin Transporter
- Absence of Maternal Methylation in Biparental Hydatidiform Moles from Women with Maternal-Effect Mutations Reveals Widespread Placenta-Specific Imprinting
- Calibrating the Human Mutation Rate via Ancestral Recombination Density in Diploid Genomes
- Anaplastic Lymphoma Kinase Acts in the Mushroom Body to Negatively Regulate Sleep
- Connecting Replication and Repair: YoaA, a Helicase-Related Protein, Promotes Azidothymidine Tolerance through Association with Chi, an Accessory Clamp Loader Protein
- UFBP1, a Key Component of the Ufm1 Conjugation System, Is Essential for Ufmylation-Mediated Regulation of Erythroid Development
- Mosaic and Intronic Mutations in Explain the Majority of TSC Patients with No Mutation Identified by Conventional Testing
- Members of the Epistasis Group Contribute to Mitochondrial Homologous Recombination and Double-Strand Break Repair in
- QTL Mapping of Sex Determination Loci Supports an Ancient Pathway in Ants and Honey Bees
- Genetic Interactions Implicating Postreplicative Repair in Okazaki Fragment Processing
- Genomics of Cancer and a New Era for Cancer Prevention
- Adaptation to High Ethanol Reveals Complex Evolutionary Pathways
- Dynamics of Transcription Factor Binding Site Evolution
- Exocyst-Dependent Membrane Addition Is Required for Anaphase Cell Elongation and Cytokinesis in
- Enhancer Runaway and the Evolution of Diploid Gene Expression
- Cattle Sex-Specific Recombination and Genetic Control from a Large Pedigree Analysis
- Drosophila Mutants Model Cornelia de Lange Syndrome in Growth and Behavior
- Pleiotropic Effects of Immune Responses Explain Variation in the Prevalence of Fibroproliferative Diseases
- Leaderless Transcripts and Small Proteins Are Common Features of the Mycobacterial Translational Landscape
- Tissue-Specific Effects of Reduced β-catenin Expression on Mutation-Instigated Tumorigenesis in Mouse Colon and Ovarian Epithelium
- Genus-Wide Comparative Genomics of Delineates Its Phylogeny, Physiology, and Niche Adaptation on Human Skin
- Mapping of Craniofacial Traits in Outbred Mice Identifies Major Developmental Genes Involved in Shape Determination
- Conserved Genetic Interactions between Ciliopathy Complexes Cooperatively Support Ciliogenesis and Ciliary Signaling
- Metabolomic Quantitative Trait Loci (mQTL) Mapping Implicates the Ubiquitin Proteasome System in Cardiovascular Disease Pathogenesis
- DNA Repair Cofactors ATMIN and NBS1 Are Required to Suppress T Cell Activation
- Spindle-F Is the Central Mediator of Ik2 Kinase-Dependent Dendrite Pruning in Sensory Neurons
- Ernst Rüdin and the State of Science
- ABCs of Insect Resistance to Bt
- Epigenetic Control of O-Antigen Chain Length: A Tradeoff between Virulence and Bacteriophage Resistance
- Encodes Dual Oxidase, Which Acts with Heme Peroxidase Curly Su to Shape the Adult Wing
- The Fanconi Anemia Pathway Protects Genome Integrity from R-loops
- Controls Quantitative Variation in Maize Kernel Row Number
- Genome-Wide Association Study of Golden Retrievers Identifies Germ-Line Risk Factors Predisposing to Mast Cell Tumours
- Insect Resistance to Toxin Cry2Ab Is Conferred by Mutations in an ABC Transporter Subfamily A Protein
- A Cytosine Methytransferase Modulates the Cell Envelope Stress Response in the Cholera Pathogen
- Conserved piRNA Expression from a Distinct Set of piRNA Cluster Loci in Eutherian Mammals
- The Multi-allelic Genetic Architecture of a Variance-Heterogeneity Locus for Molybdenum Concentration in Leaves Acts as a Source of Unexplained Additive Genetic Variance
- The lncRNA Controls Cryptococcal Morphological Transition
- Sae2 Function at DNA Double-Strand Breaks Is Bypassed by Dampening Tel1 or Rad53 Activity
- A Tandem Duplicate of Anti-Müllerian Hormone with a Missense SNP on the Y Chromosome Is Essential for Male Sex Determination in Nile Tilapia,
- Ectodysplasin/NF-κB Promotes Mammary Cell Fate via Wnt/β-catenin Pathway
- The QTL within the Complex Involved in the Control of Tuberculosis Infection in Mice Is the Classical Class II Gene
- Identifying Loci Contributing to Natural Variation in Xenobiotic Resistance in
- Variation in Rural African Gut Microbiota Is Strongly Correlated with Colonization by and Subsistence
- A Flexible, Efficient Binomial Mixed Model for Identifying Differential DNA Methylation in Bisulfite Sequencing Data
- Competition between Heterochromatic Loci Allows the Abundance of the Silencing Protein, Sir4, to Regulate Assembly of Heterochromatin
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- UFBP1, a Key Component of the Ufm1 Conjugation System, Is Essential for Ufmylation-Mediated Regulation of Erythroid Development
- Metabolomic Quantitative Trait Loci (mQTL) Mapping Implicates the Ubiquitin Proteasome System in Cardiovascular Disease Pathogenesis
- Genus-Wide Comparative Genomics of Delineates Its Phylogeny, Physiology, and Niche Adaptation on Human Skin
- Encodes Dual Oxidase, Which Acts with Heme Peroxidase Curly Su to Shape the Adult Wing
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



