-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Mutations in Conserved Residues of the microRNA Argonaute ALG-1 Identify Separable Functions in ALG-1 miRISC Loading and Target Repression
microRNAs are small non-coding RNAs that function in diverse processes by post-transcriptionally regulating gene expression. Argonautes form the core of the microRNA Induced Silencing Complex (miRISC) and are required for microRNA biogenesis and function. Here we describe the identification and characterization of a novel set of mutations in alg-1, a Caenorhabditis elegans microRNA specific Argonaute. This new class of alg-1 mutations causes phenotypes more severe than the complete loss of alg-1. Interestingly, the mutant ALG-1 proteins are able to promote microRNA biogenesis, but are defective in mediating microRNA target gene repression. We found that mutant ALG-1 associates more with Dicer, but less with miRISC effector AIN-1, compared to wild type ALG-1. We propose that these mutant ALG-1 proteins assemble nonfunctional complexes that effectively compete with the paralogous ALG-2 for critical miRISC components, including mature microRNAs. This new class of Argonaute mutants highlights the role of Argonaute in mediating a functional transition for miRISC from microRNA processing phase to target repression phase.
Published in the journal: . PLoS Genet 10(4): e32767. doi:10.1371/journal.pgen.1004286
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1004286Summary
microRNAs are small non-coding RNAs that function in diverse processes by post-transcriptionally regulating gene expression. Argonautes form the core of the microRNA Induced Silencing Complex (miRISC) and are required for microRNA biogenesis and function. Here we describe the identification and characterization of a novel set of mutations in alg-1, a Caenorhabditis elegans microRNA specific Argonaute. This new class of alg-1 mutations causes phenotypes more severe than the complete loss of alg-1. Interestingly, the mutant ALG-1 proteins are able to promote microRNA biogenesis, but are defective in mediating microRNA target gene repression. We found that mutant ALG-1 associates more with Dicer, but less with miRISC effector AIN-1, compared to wild type ALG-1. We propose that these mutant ALG-1 proteins assemble nonfunctional complexes that effectively compete with the paralogous ALG-2 for critical miRISC components, including mature microRNAs. This new class of Argonaute mutants highlights the role of Argonaute in mediating a functional transition for miRISC from microRNA processing phase to target repression phase.
Introduction
Development of complex organisms requires execution of molecular and cellular events with precise temporal control. Impaired developmental timing can result in severe morphological abnormalities. In C. elegans, the heterochronic gene network controls the stage-specific execution of larval developmental events. C. elegans heterochronic mutants display either precocious or retarded development, due to skipping or reiteration of certain stage-specific cell fate programs. The heterochronic regulatory network consists of transcriptional regulatory proteins, RNA binding proteins, and microRNAs that regulate the developmental expression of those proteins (reviewed in [1]). In particular, the let-7-Family microRNAs, let-7, mir-241, mir-48, and mir-84, are key players in the heterochronic genetic network that controls progression through the C. elegans larval development [1]–[3]. Deletion of the let-7 microRNA or its sisters miR-48, miR-241 and miR-84 results in reiteration of larval stage-specific cell fates, delaying the adoption of adult cell fates [1]–[3].
microRNAs bind to target mRNAs via imperfect base pairing, bringing along the microRNA Induced Silencing Complex (miRISC), which in turn acts to destabilize the target mRNA and/or repress its translation (reviewed in [4]–[7]). microRNAs associate with Argonaute proteins (in C. elegans, ALG-1 or ALG-2), forming the core of the miRISC. Argonautes are not only critical for the activity of mature microRNAs in regulating their targets, but also function in conjunction with the ribonuclease Dicer (DCR-1 in C. elegans) in processing of microRNA hairpin precursors into mature microRNAs [8], [9] (reviewed in [10]). Loss of alg-1 or depletion of alg-1/alg-2 or dcr-1 via RNA interference (RNAi) results in accumulation of microRNA precursors, consistent with ALG-1/2 and Dicer acting together at the microRNA biogenesis step of miRISC maturation [8], [9]. Upon miRISC maturation, ALG-1/ALG-2 form miRISC complexes with the effector proteins, including the GW182 homologs, AIN-1/AIN-2 [11], [12]. AIN-1/2 proteins are critical components of the distinct effector miRISC that functions on the mRNA targets to mediate mRNA degradation and/or translational repression [11]–[17]. However, while some recent data brought new insights about the contribution of Argonautes in the miRISC maturation process [8], little is known about the transition from the DCR-1-containing microRNA-processing complex to the GW182-containing effector complex.
LIN-28 is an evolutionarily conserved RNA-binding protein that acts in opposition to let-7-Family microRNAs. C. elegans lin-28(lf) mutants skip developmental programs of the second (L2) and occasionally the third (L3) larval stages. Consequently, lin-28(lf) mutants execute adult-specific terminal differentiation one to two stages earlier than normal [18], [19]. By contrast, loss of let-7-sisters (mir-241, mir-48, and mir-84) results in the reiteration of L2 fates [1], [2], and loss of let-7 causes the reiteration of L3 fates [1]–[3], [20]. lin-28 promotes early cell fates via two distinct mechanisms (Figure 1). First, LIN-28 controls let-7 microRNA levels by binding to the let-7 pre-microRNA and facilitating its active turnover, a role first described in mammals [1]–[3], [21]–[28]. Second, lin-28 positively regulates expression of hbl-1 (Figure 1), [4]–[7], [20]. HBL-1 is a zinc-finger transcription factor that normally functions to repress the cell fates associated with the third larval stage (L3) [8], [9], [29], [30], and has been shown to inhibit transcription of let-7 [8], [9], [31]. In turn, hbl-1 is down regulated by the let-7-Family microRNAs prior to the third larval stage to allow the progression from the L2 to L3 to occur (Figure 1), [2], [11], [12], [29], [30]. In addition, lin-28 itself is a predicted target for let-7-Family microRNAs [2], [11]–[17], [20], [32]. Thus, lin-28, hbl-1 and let-7-Family microRNAs function in a complex regulatory network that controls the progression of C. elegans larval development (Figure 1), (reviewed by [1], [8], [33]).
Fig. 1. Genetic network of heterochronic genes regulating the developmental timing of cell divisions associated with the larval stages of C. elegans development. 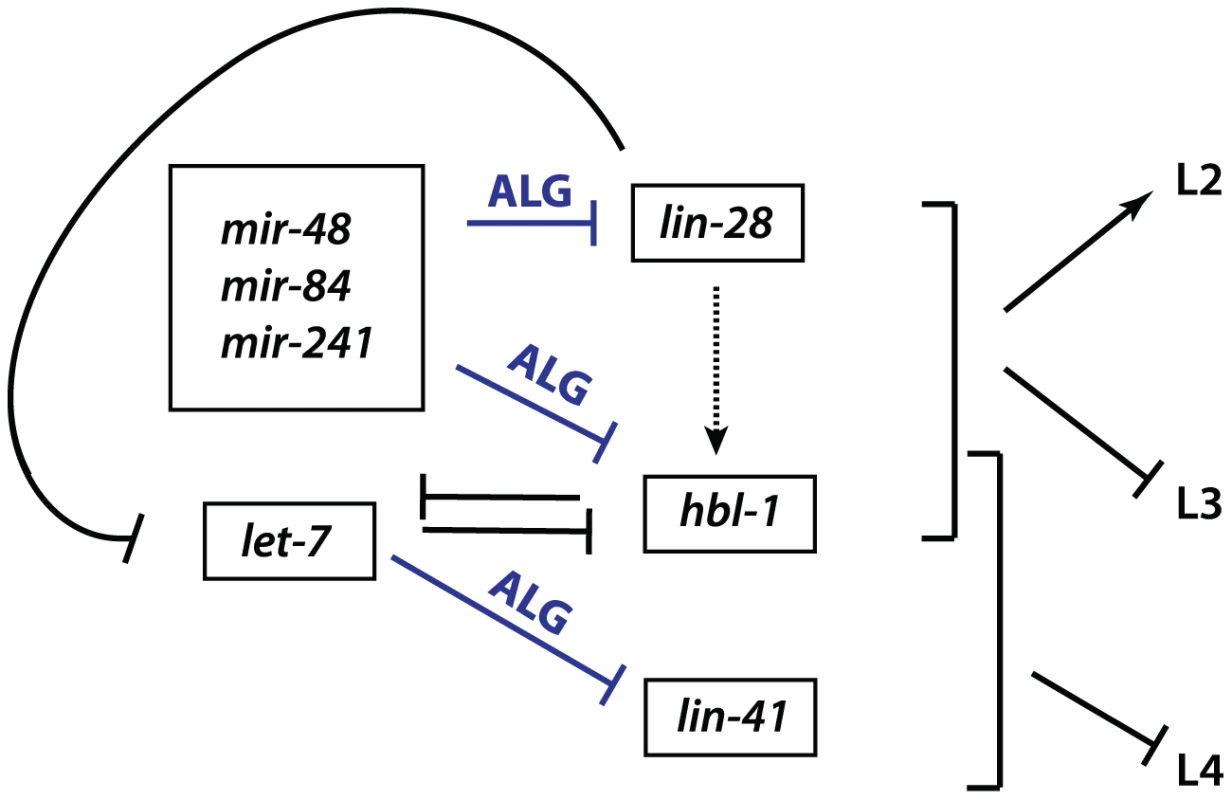
Based on [1]. With the goal of identifying microRNA co-factors that normally positively influence microRNA function, we took advantage of the opposing relationship between lin-28 and let-7-Family microRNAs and performed a forward genetic screen for suppressors of a hypomorphic allele of lin-28(lf). From this screen, we identified novel alleles in one of the two C. elegans microRNA Argonautes, ALG-1. Here, we report the unusual antimorphic properties of these mutations, which highlight the importance of the ALG-1 in miRISC maturation from a microRNA-processing complex to an effector complex. We interpret the nature of these alleles to reflect distinct and separable ALG-1 functions in microRNA biogenesis versus mRNA target repression.
Results
A lin-28(lf) suppressor screen yields mutations in the C. elegans microRNA specific Argonaute ALG-1
The highly penetrant developmental defects of lin-28(lf) mutants are in large part the consequence of elevated activity of certain microRNAs, principally let-7 and other developmental timing microRNAs [20], [32]. Therefore, to identify factors that function in conjunction with let-7-Family microRNAs, we performed a forward genetic screen (estimated 39,000 EMS mutagenized genomes) for suppressors of lin-28(lf) phenotypes. lin-28(lf) animals are 100% egg laying defective, owing to an abnormal vulval morphology that results from their precocious cell lineage defects [18], [34]. Genetic suppressors of lin-28(lf) were identified by restoration of egg laying. Six of the eleven suppressor mutations were alleles of lin-46, a heterochronic gene previously shown to function downstream of lin-28 [35]. The other five suppressor mutations mapped to the X chromosome and displayed a range of suppression of the lin-28(lf) heterochronic phenotypes. All five of these X linked suppressor mutations were determined to be alleles of alg-1, a gene that encodes one of two C. elegans microRNA specific Argonautes.
Four of five alg-1 novel alleles are missense mutations that permit accumulation of full length ALG-1 protein
Four of the five alg-1 alleles isolated as lin-28(lf) suppressors (ma192, ma195, ma202, and ma203) are missense mutations (Figure 2A). The fifth mutation, alg-1(ma198) is a predicted null allele of alg-1 (Figure 2A) and genetically behaves similarly to previously isolated null allele alg-1(tm492) (Figure 2A, Table 1, and data not shown). The alg-1 locus is predicted to produce two protein isoforms (Figure 2B). However, the expected molecular weights of these annotated isoforms do not fully account for the apparent difference in electrophoretic mobility of the two major isoforms detected in our Western blot experiments (Figure 2C) suggesting a possible contribution of proteolytic processing, and/or other hypothetical post-translational modifications of ALG-1. Each missense allele produces two isoforms of apparently full-length ALG-1 proteins similar to wild type (Figure 2C). The abundance of the mutant ALG-1 proteins is reduced compared to the wild type (Figure 2C), suggesting that those particular mutations may affect ALG-1 stability to various degrees. The abundance of the higher molecular weight isoform seems to be more affected by the alg-1(anti) alleles; however this apparent differential isoform accumulation does not appear to correlate with the severity of the alg-1(anti) mutant phenotypes (Table 1).
Fig. 2. Four of five newly isolated alg-1 alleles carry missense mutations. 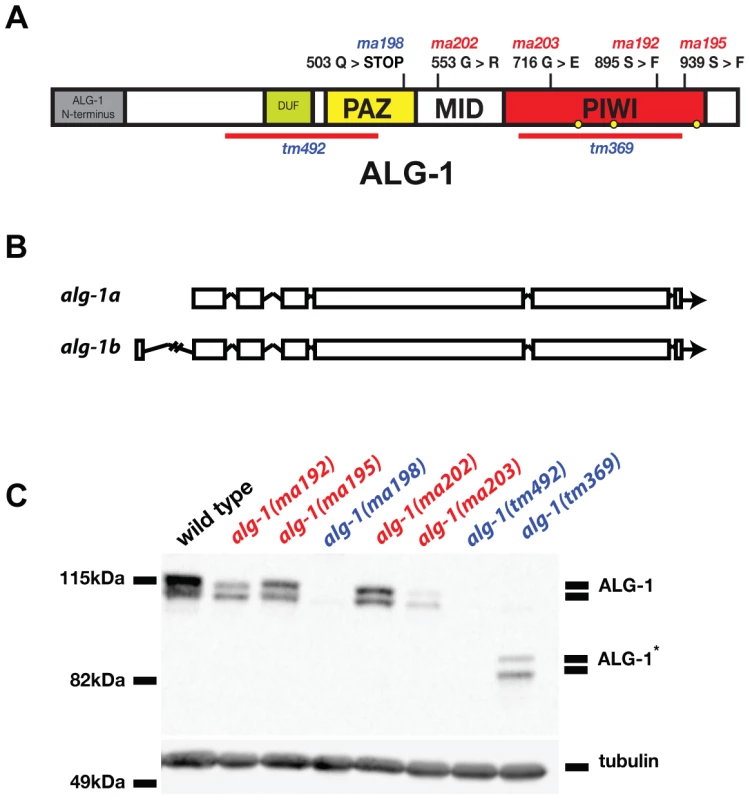
(A) A schematic showing the positions of the newly identified alg-1 mutations within the ALG-1 protein (black tics) and the existing deletion alleles (red lines). Positions of catalytic sites are indicated by yellow circles. (B) Exon/intron schematic of the two alg-1 isoforms predicted and supported by cDNA evidence (Wormbase.org). Boxes represent exonic regions. (C). Western blot analysis on total protein lysate from wild type and alg-1 mutant animals. All non-null alg-1 alleles, like the wild type, produce 2 isoforms of ALG-1. Newly identified missense alleles of alg-1 are marked in red and null alleles are in blue. alg-1(tm369) is a loss of function allele that deletes most of the PIWI domain and produces 2 truncated isoforms of ALG-1(*). All strains with the exception of alg-1(tm492) and alg-1(tm369) carry lin-31(lf) and col-19::gfp in the background. Tab. 1. alg-1 mutations cause retarded development. 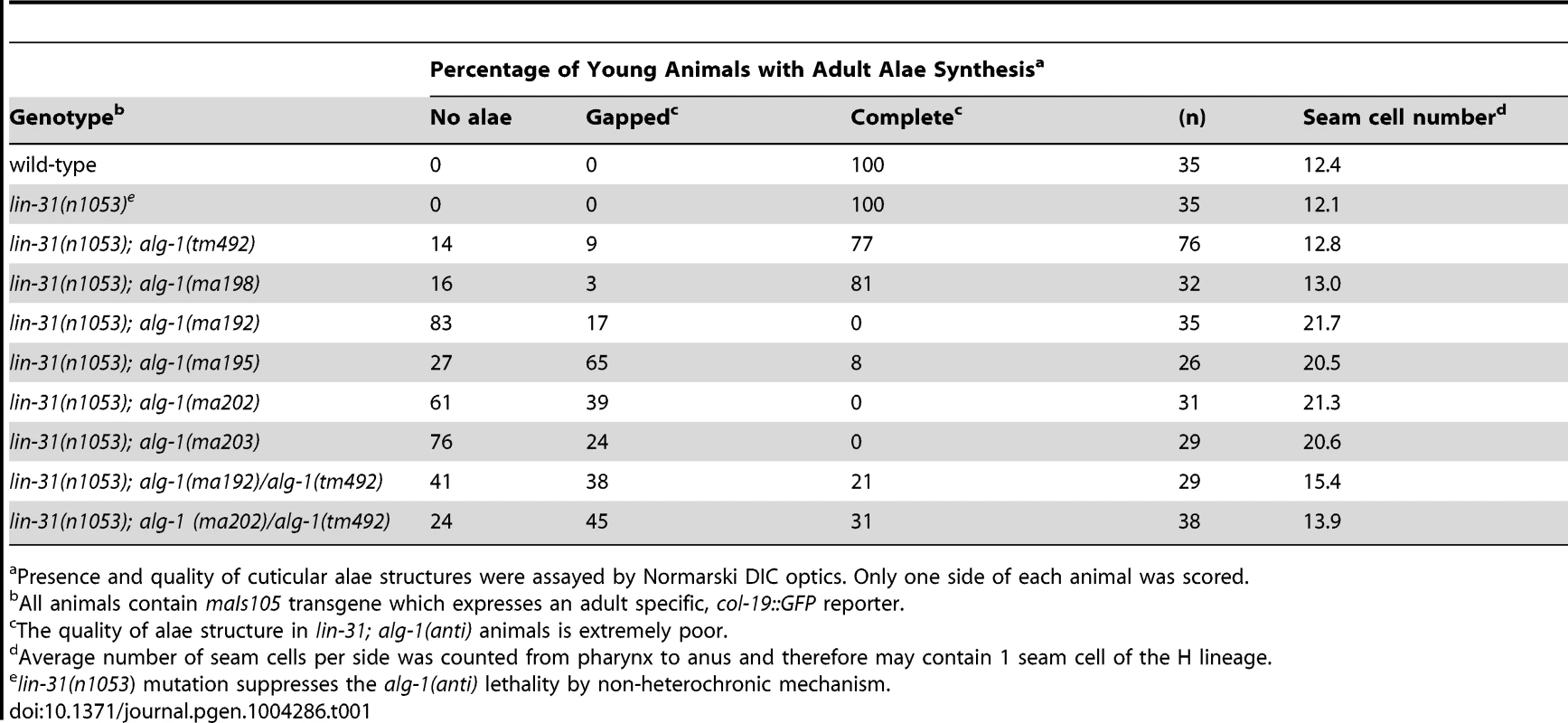
Presence and quality of cuticular alae structures were assayed by Normarski DIC optics. Only one side of each animal was scored. The novel alg-1 mutations suppress multiple cell lineage and gene expression phenotypes of lin-28 mutants
lin-28(lf) mutants skip certain developmental events characteristic of the second larval stage (L2), which results in precocious execution of cell divisions in the vulval and lateral hypodermal cell lineages. These alterations in temporal cell fates have dramatic consequences that include defects in egg laying, precocious expression of an adult-specific collagen gene, col-19, precocious production of adult cuticle, and precocious cessation of molting after the third (sometimes second) larval molt–one to two stages earlier than normal (Figure 3 and [18], [19]). We found that mutations in alg-1 suppressed all of these cell-lineage and gene expression phenotypes of lin-28(lf) mutants (Table 2, Figure 3).
Fig. 3. Mutations in alg-1 suppress precocious development of lin-28(lf) (lin-28(n947)) animals. 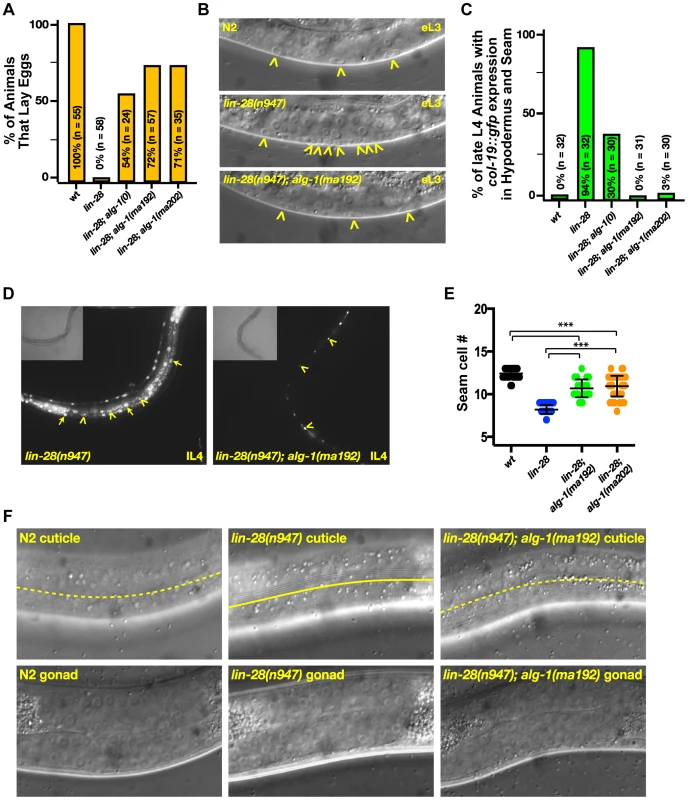
(A, B) alg-1 mutations suppress the egg-laying defect of lin-28(lf) animals by suppressing the precocious divisions of the vulval precursor cells; (B) Early third larval (eL3) stage animals. Arrowheads indicate vulval cell nuclei. Three vulval precursor cells (P5.p, P6.p, and P7.p) are undivided in the top (N2) and bottom (lin-28(n947); alg-1(ma192)) panels, but in the middle panel (lin-28(n947), P6.p and P7.p have divided twice (one P7.p granddaughter is out of the plane of focus). (C, D) alg-1 mutations suppress the lin-28(lf) precocious expression of the adult cell fate marker col-19::gfp. lL4-late fourth larval stage. (E) alg-1 mutations also increase the seam cell number (#) produced lin-28(lf) mutant animals (***p<0.0001), and (F) suppress precocious alae formation of L4 animals; dotted line represents absence of the alae, solid line underlines the alae structure. All strains col-19::gfp transgene in the background. n = number of animals scored. Tab. 2. lin-28(lf) phenotypes are suppressed by mutations in alg-1. 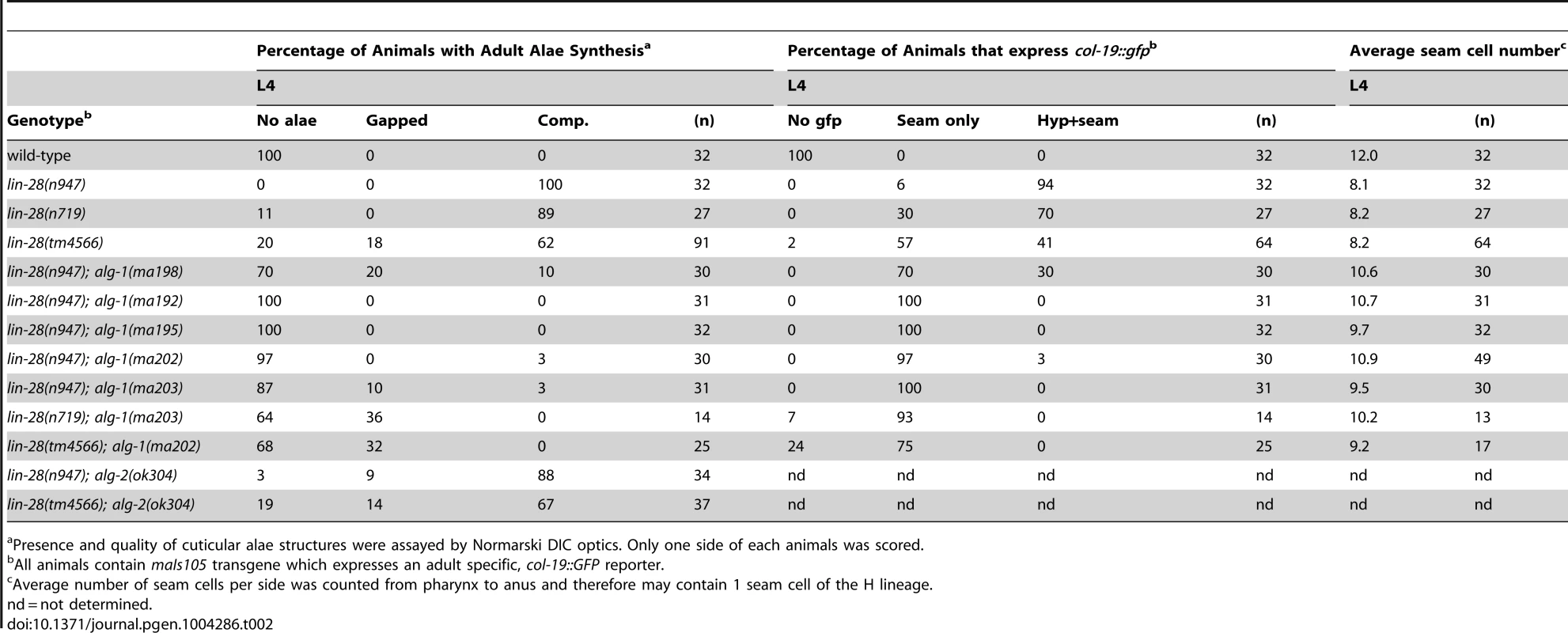
Presence and quality of cuticular alae structures were assayed by Normarski DIC optics. Only one side of each animals was scored. A striking property of the missense alg-1 alleles isolated as suppressors of lin-28(lf) is that their suppression is much stronger than that exerted by alg-1(ma198) null allele (Table 2, Figure 3A, C). This is the case for almost all heterochronic phenotypes assessed: the premature vulval precursor cell (VPC) division and egg laying defects (Figure 3A), precocious expression of the adult-specific seam hypodermal marker, col-19::gfp (Table 2, Figure 3C), and precocious formation of adult-specific lateral alae on the cuticle (Table 2). These results suggest that the alg-1 missense mutations differ in their fundamental properties from a complete loss of alg-1.
The alg-1 missense mutations also efficiently suppressed the precocious seam cell division patterns of lin-28(lf), thereby restoring the seam cell numbers to nearly wild type (Table 2, Figure 3E). This is noteworthy in light of previous work showing that simultaneous loss of four of seven let-7-Family genes (mir-84, mir-48, mir-241, and let-7) was not sufficient to suppress the reduced seam cell number of lin-28(lf) [20]. Therefore the potent suppression that we observe in lin-28(lf) carrying alg-1 mutations suggests that the lin-28(lf) phenotype results from hyperactivity of microRNAs in addition to mir-84, mir-48, mir-241, and let-7. Although the newly isolated missense alleles of alg-1 do not all appear to affect the same domain of ALG-1 (Figure 2A), all four mutants displayed similar phenotypic and genetic characteristics (Table 1, Table 2). We therefore focused our further characterizations on two of the four missense alleles, alg-1(ma192) and alg-1(ma202).
alg-1 missense mutations cause heterochronic phenotypes more severe than those of alg-1(0) animals
To further understand the newly isolated missense alleles of alg-1, we phenotypically characterized the novel alg-1 mutant animals in the absence of the lin-28(lf) mutation. Loss of alg-1 is known to result in mild retarded developmental phenotypes primarily from decreased levels and/or activity of heterochronic microRNAs (Table 1, Figure 4A, B), [9]. The alg-1(null)(referred to as alg-1(0)) heterochronic phenotypes are relatively weak most likely because of the redundancy of ALG-1 with its paralog, ALG-2 and include a failure of individual lateral seam cells to terminally differentiate or to deposit an adult-specific cuticle during the larval-to-adult molt (Table 1, Figure 4A, B), [9], [32]. We found that the novel missense alg-1 alleles also display retarded heterochronic development, but these phenotypes are much more severe than alg-1(0) mutants (Table 1, Figure 4A–D). Specifically, alg-1(0) animals display only modest retarded defects, with 77% of the young adult animals producing alae, and 89% of the young adult animals expressing a col-19::gfp pattern similar to the wild type (Table 1, Figure 4A, B). By contrast, alg-1(ma202) and alg-1(ma192) homozygous mutant animals completely fail to produce alae as young adults (Table 1, Figure 4A) and do not display the wild type hypodermal and seam col-19::gfp expression at that stage (Figure 4B). Similarly, seam cells of alg-1(0) animals rarely reiterate L2 cell divisions, while this phenotype is much more penetrant and expressive for the novel alg-1 missense mutants (Table 1, Figure 4C, D). Young adult animals carrying alg-1(ma202) or alg-1(ma192) on average had 8–10 more seam cells than wild type (Table 1, Figure 4D), a phenotype similar to let-7-Family microRNA mutants that fail to down-regulate hbl-1 expression [2].
Fig. 4. Newly isolated alg-1 alleles are antimorphic, exhibit retarded development, and display phenotypes more severe than those of alg-1(0). 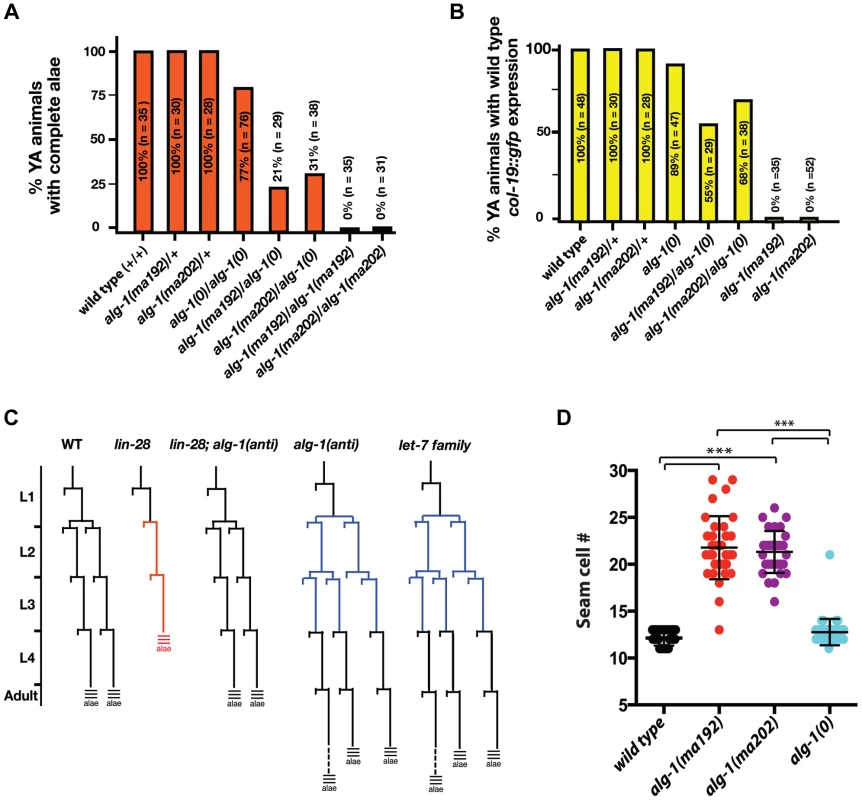
(A, B) Bar graphs showing the percent of young adult (YA) animals with wild type alae formation (A) and col-19::gfp adult marker expression (B), where alg-1(anti) alleles have a dosage dependent effect on both phenotypes. (C) Schematic of representative V1–V4 and V6 lineage cell divisions in the wild type, alg-1(anti), and other heterochronic mutants. (D) alg-1(anti) mutations display increased numbers of seam cells as young adults. ***p<0.001. All strains carry lin-31(lf) and col-19::gfp in the background. The lin-31 mutation is present in order to suppress alg-1(anti) vulval bursting phenotypes by non-heterochronic methods. n = number of animals scored. alg-1(anti) mutations are antimorphic
To characterize the dosage-dependence of the alg-1(anti) phenotypes, we examined the phenotypes of heterozygous and hemizygous alg-1(anti) animals. 100% of alg-1(ma202)/+ or alg-1(ma192)/+ animals display wild type phenotypes with respect to alae formation and col-19::gfp expression (Table 1, Figure 4A, B), suggesting that these alleles are either recessive or so weakly semi-dominant that we are unable to detect the dominant allelic behavior in our assays. In contrast, animals carrying one alg-1 missense alleles in trans to an alg-1(0) allele are strongly retarded; only 21–31% of alg-1(ma192)/alg-(0) or alg-1(ma202)/alg-1(0) young adults produce complete alae (Figure 4A); similarly, animals of those genotypes exhibited retarded phenotypes with respect to adult-specific col-19::gfp expression (Figure 4B). These results suggest that one copy of alg-1(ma202) or alg-1(ma192) is more detrimental than the complete lack of ALG-1 protein. Because these missense alg-1 mutations can cause defects more severe than those of complete loss of alg-1, we conclude that these mutations are antimorphs (anti) and hypothesize that they do not simply reduce ALG-1 activity, but may also interfere (directly or indirectly) with the function of the semi-redundant Argonaute ALG-2.
alg-1(anti) heterochronic phenotypes involve de-repression of the let-7-Family microRNA target, hbl-1
The hbl-1 gene encodes for a transcription factor that is a direct target of the let-7-Family microRNAs [2], [29], [30] and is also involved in feedback regulation of let-7 transcription [31]. Down regulation of hbl-1 by the let-7-Family microRNAs is required for proper progression of cell division programs from the second to third larval stages of development (Figure 1), [2]. In wild type animals, hbl-1 is down-regulated by let-7-sisters (mir-48, mir-84, and mir-241) between the L2 and L3 stages of larval development [2]. We observed that in the alg-1(anti) L3 larvae, hbl-1::gfp::hbl-1 3′UTR is not down regulated as it is in wild type (Figure 5A, B). Consistent with the hypothesis that increased hbl-1 activity contributes to alg-1(anti) heterochronic phenotypes, knock down of hbl-1 by RNAi in the alg-1(anti) background results in efficient suppression of the retarded phenotype as measured by expression of the adult-specific col-19::gfp reporter transgene (Figure 5C).
Fig. 5. alg-1(anti) mutations affect hbl-1 expression. 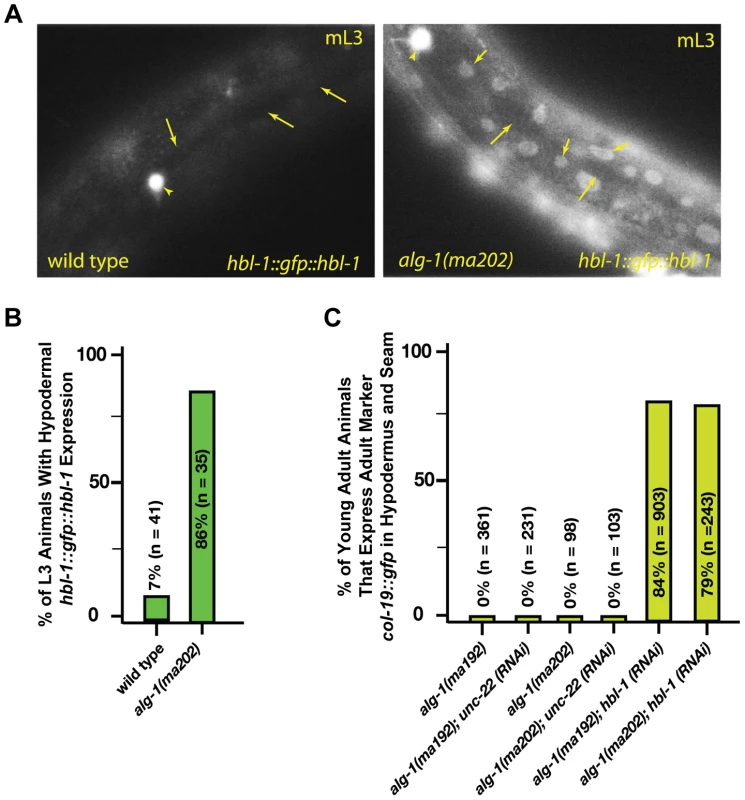
(A) hbl-1::gfp::hbl-1 3′UTR expression in wild type and alg-1(anti) mutant animals. mL3-mid third larval stage. Short arrows-hypodermal nuclei; long arrows-seam cell nuclei. HSN neuron (arrowhead) is shown as a point of reference. Images were captured at identical exposure, and processed identically. (B) Quantification of hypodermal hbl-1::gfp::hbl-1 3′UTR expression in wild type and alg-1(ma202) animals. (C) hbl-1 RNAi rescues the retarded expression of the adult marker col-19::gfp in alg-1(anti) mutant animals. All strains carry lin-31(lf) and col-19::gfp in the background. The lin-31 mutation is present in order to suppress alg-1(anti) vulval bursting phenotypes by non-heterochronic methods. n = number of animals scored. alg-1(anti) mutations impair functions of non-heterochronic microRNAs
Since alg-1(anti) mutants display strong heterochronic phenotypes and were isolated in a screen for suppression of heterochronic phenotypes, we tested whether alg-1(anti) mutations affect functions of microRNAs involved in other processes. lsy-6 is a microRNA that regulates cell fate specification of two bilaterally symmetric neurons, ASEL and ASER [36]. lsy-6 microRNA expression in the ASEL cell down-regulates cog-1, the primary determinant of the ASER cell fate, thereby allowing specification of the ASEL fate [36]. Loss of lsy-6 function results in transformation of the ASEL cell fate to that of ASER [36]. lsy-6(ot150) is a hypomorphic allele that alters a conserved cis-regulatory element in the lsy-6 promoter and reduces but does not eliminate lsy-6 microRNA function [37]. lsy-6(ot150) animals display an ASEL to ASER cell fate transformation of approximately 18% whereas lsy-6(0) animals display an essentially 100% penetrant phenotype ([37], (Figure 6A)). Using the ASEL-specific transgenic reporter, Plim-6::gfp [36], [38], (Figure 6A), and the lsy-6(ot150) sensitized background, we tested for effects of the alg-1 mutations on the lsy-6(ot150) phenotype. We observed that complete removal of alg-1 in lsy-6(ot150) mutant background using a homozygous alg-1(0) allele increases the penetrance of the ASEL to ASER fate transformation phenotype to 53% of animals (Figure 6A). However, combining the alg-1(anti) alleles with lsy-6(ot150) leads to a more dramatic enhancement, with 90% of animals displaying the ASEL to ASER transformation phenotype (Figure 6A). These data show that an alg-1(anti) mutation reduces the function of lsy-6 microRNA, and does so more severely than alg-1(0) (Figure 6A), similar to the effect of alg-1(anti) on the heterochronic phenotypes.
Fig. 6. alg-1(anti) mutations affect functions of many microRNAs and exhibit phenotypes more severe than those of alg-1(0). 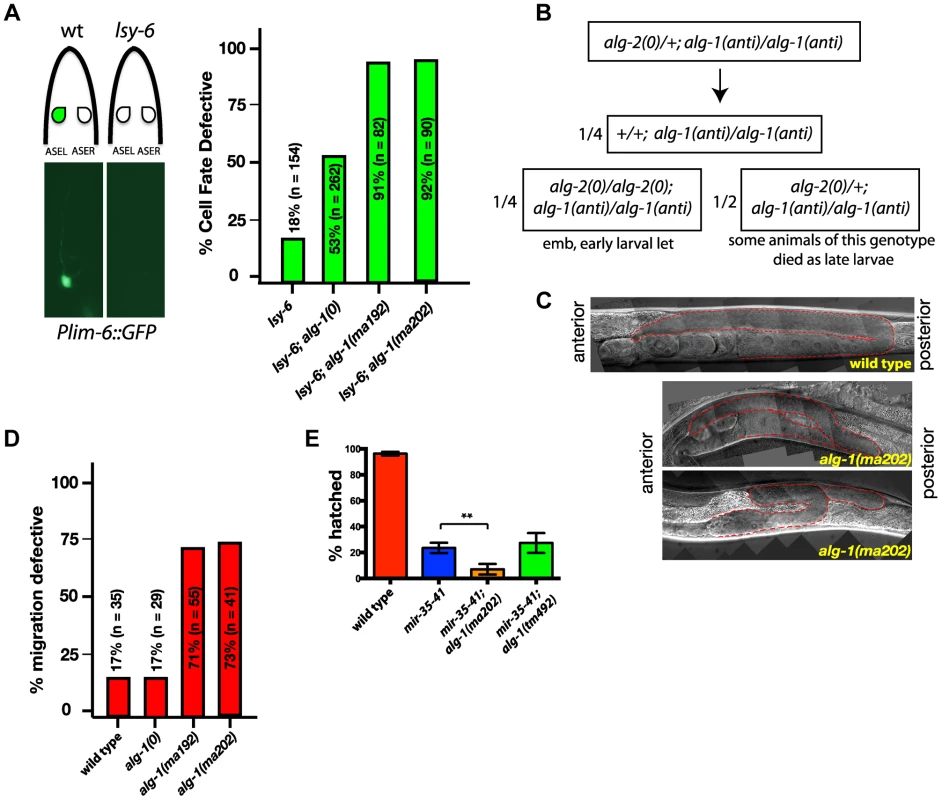
(A) Plim-6::gfp expression marks ASEL neuronal cell fate in wild type and mutant animals. lsy-6(ot150) mutants lack the Plim-6::gfp expression in the ASEL neurons some of the time. This phenotype is enhanced by the loss of alg-1 and even more so in the presence of either of the two alg-1(anti) mutations. All strains carry lin-31 mutation in order to suppress alg-1(anti) vulval bursting phenotypes by non-heterochronic methods. (B) Combination of alg-1(anti) and alg-2(0) mutations results in embryonic lethality (emb) and early larval lethality (let), with some late larval lethality present in alg-2(0)/+; alg-1(anti)/alg-1(anti) mutant animals. (C, D) Distal tip cell migration phenotypes of wild type and mutant animals. alg-1(anti) mutant animals display defects affecting all three phases of gonad migration. In (C) gonads are marked by a dashed line. (E) alg-1(anti), but not alg-1(0) mutation enhances the embryonic lethality of mir-35–41 mutants. (**p<0.001). All strains carry lin-31(lf) and col-19::gfp in the background. The lin-31 mutation is present in all strains in order to suppress alg-1(anti) vulval bursting phenotypes by non-heterochronic methods. n = number of animals scored. ALG-1 and ALG-2 Argonautes function semi-redundantly, and together are essential for viability: loss of both alg-1 and alg-2 results in embryonic lethality [9], [39]. Similarly, combining alg-1(anti) with a null mutation in alg-2 results in lethality, as determined by the absence of viable alg-1(anti); alg-2(0) doubly homozygous mutant animals among the progeny of heterozygous mothers (Figure 6B). alg-1(anti); alg-2(0) animals appeared to arrest as embryos or early stage larvae indicating that alg-1(anti) alleles affect functions of microRNAs normally required for viability and embryonic development [39]–[41]. In addition, we observed that alg-1(anti)/alg-1(anti); alg-2(0)/+ animals frequently arrested as L3/L4 larvae (Figure 6B), indicating that two copies of alg-1(anti) can interfere with the function of a single alg-2(+) allele.
alg-1(0) mutant hermaphrodites exhibit occasional gonad migration phenotypes that can be enhanced in combination with mutations in certain microRNA genes [42], indicating that one or more microRNAs function to ensure proper gonad morphogenesis. Consistent with alg-1(anti) alleles affecting microRNA activity more severely than alg-1(0), the distal tip cell (DTC) migration defects of alg-1(anti) animals were far more penetrant and more expressive than those of alg-1(0) (Figure 6C, D). It should be noted that the lin-31 mutation, present in the background of all alg-1 mutations in order to suppress alg-1(anti) vulval bursting, causes a mild migration phenotype on its own (Figure 6C and data not shown). Specifically, the distal tip cells of lin-31 mutant animals migrate beyond the primary vulva site approximately 17% of the time (Figure 6D). However, alg-1(anti) animals display a wide range of DTC migration phenotypes during all phases of migration at a much higher penetrance than lin-31 worms (Figure 6C, D).
Deletion of all mir-35 family members (mir-34–42) results in early embryonic lethality, while deletion of mir-35–41 (leaving mir-42 functional) results in an incompletely penetrant temperature sensitive lethality [40], [41]. Combining the alg-1(anti) mutation, but not the alg-1(0) deletion, with the mir-35–41 deletion enhances the mir-35–41 temperature sensitive embryonic lethality phenotype (Figure 6E), suggesting that the alg-1(anti) mutations affect the functions of the mir-35 family microRNAs in embryonic development.
let-7-Family microRNA biogenesis is largely unaffected in alg-1(anti) mutants
The suppression of lin-28(lf) phenotypes by alg-1(anti) mutations and the retarded phenotypes that alg-1(anti) mutants have on their own suggest that alg-1(anti) mutations could result in decreased levels and/or activity of the let-7-Family microRNAs. We therefore assayed the abundance of the let-7-Family microRNAs in RNA samples from L2 larvae (the stage where the alg-1(anti) heterochronic defects are induced). Vadla and colleagues previously showed that let-7 microRNA levels increase dramatically in lin-28(lf) animals at the L2 stage [20]. Similarly, we see a dramatic increase in let-7 microRNA levels in lin-28(lf) L2 larvae (Figure 7A). Interestingly, let-7 microRNA levels were decreased approximately 2-fold in lin-28(lf); alg-1(anti) L2 larvae compared to lin-28(lf); alg-1(+), but still elevated compared to wild type levels (Figure 7A). Importantly, alg-1(0) and alg-1(anti) mutations reduced let-7-Family microRNA levels similarly in the lin-28(lf) background (Figure 7A). This result suggests that the more efficient suppression of the lin-28(lf) phenotypes by alg-1(anti) alleles compared to alg-1(0) is not entirely due to reduced let-7-Family microRNA levels, and that the ALG-1(anti) mutant proteins may be impaired in an activity or activities downstream of microRNA biogenesis. In addition, alg-1(anti); lin-28(+) mutants exhibit less severe reductions in let-7-Family microRNA than do alg-1(0) animals (Figure 7B), even though the alg-1(anti) heterochronic phenotypes are far more severe than those of alg-1(0) (Figure 4). This is also consistent with a potent effect of alg-1(anti) mutations on ALG-1 function(s) other than microRNA biogenesis. The absence of lin-28 dampens the effects of alg-1 mutations on the levels of let-7 and miR-48 microRNAs. Both let-7 and miR-48 levels are reduced at best two-fold in the absence of lin-28 (Figure 7A), but 5–10 fold when lin-28 is intact (Figure 7B). This would indicate that in the presence of lin-28 these microRNAs are more vulnerable to the absence of functional ALG-1, suggesting that alg-1 opposes the destabilizing activity of lin-28 upon let-7 and mir-48. These data might suggest that LIN-28 may function to inhibit processing of not only let-7 (as previously shown [22]), but also miR-48.
Fig. 7. The effect of alg-1 mutation on the levels of mature microRNAs in total RNA from L2 larvae using FirePlex miRSelect assay. 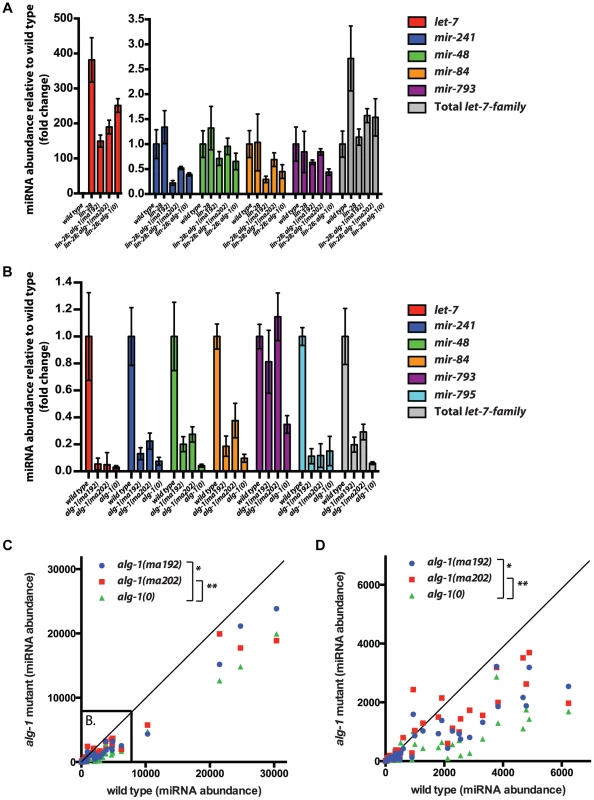
(A, B) Quantification of let-7-Family microRNA abundance (normalized to wild type), in the context of suppression of lin-28(0) mutants by alg-1 mutations (A), and for alg-1 mutations in the lin-28(+) genetic background (B). Only detectable let-7-Family microRNAs are shown. (A) let-7 microRNA levels increase dramatically in lin-28(lf) mutants and are reduced by the addition of the alg-1 mutations. Levels of other let-7 family members are not increased to statistically significant levels in lin-28 mutants, and are either not affected or are decreased by the alg-1 mutations to varying degrees. All strains carry the col-19::gfp transgene. (B) let-7-Family microRNA levels are decreased in all alg-1 mutants compared to wild type, but alg-1(0) decreases microRNA abundance more than alg-1(anti) mutations do. (C, D) Scatterplots comparing abundance of microRNAs in total RNA from L2 larvae of wild type (X-axis, arbitrary units) and alg-1 mutants (Y-axis, arbitrary units) using FirePlex miRSelect assays for 53 microRNAs. Complete loss of ALG-1 in alg-1(0) and compromised ALG-1 function in alg-1(ma202) and alg-1(ma192) results in under accumulation of microRNAs. alg-1(ma202) and alg-1(ma192) mutants have higher levels of microRNAs than alg-1(0) animals, *p = 0.01, **p = 0.003. (D) Subset of data in (C) zoomed in to show the lower abundance microRNAs. All strains in (B–D) carry lin-31(lf) and col-19::gfp in the background. The lin-31 mutation is present in order to suppress alg-1(anti) vulval bursting phenotypes by non-heterochronic methods. To assess whether the relatively mild effect of alg-1(anti) mutations on microRNA levels (compared to alg-1(0)) extends to other microRNAs, we determined the levels of 53 microRNAs in wild type, alg-1(0), and alg-1(anti) L2 larvae using the FirePlex miRSelect (Firefly) method. We found that microRNA levels were consistently and statistically significantly more reduced in alg-1(0) animals than in alg-1(anti) animals (Figure 7C, D). These data further support the idea that the more severe phenotypes of alg-1(anti) mutants compared to alg-1(0) result from impaired microRNA functions and cannot be attributed solely to reduced microRNA levels.
Unlike alg-1 null mutations, alg-1(anti) mutations do not affect pre-microRNA processing
The four antimorphic alg-1 alleles affect amino acids that are evolutionarily conserved in AGO family proteins and are located in various domains of the Argonaute protein (Figure 2A, Figure 8A, B, Figure S1A). In addition, the affected amino acids are conserved in many of the non-microRNA Argonautes as well (Figure S1B). Based on published high resolution Argonaute structures [43]–[49], it appears that none of the amino acids affected by alg-1(anti) mutations are predicted to make direct contact with the microRNA (Figure 8A). Mapping the four amino acids affected by the alg-1(anti) mutations onto the crystal structure of hAGO2 [50] confirms that (Figure 8B). Interestingly, this analysis shows that the two serines affected by alg-1(ma192) and alg-1(ma195) mutations are located in the PIWI domain and directly face each other (Figure 8B).
Fig. 8. alg-1(anti) alleles affect conserved amino acids within the ALG-1 protein, do not affect pre-microRNA processing, and associate with microRNAs to levels comparable to wild type. 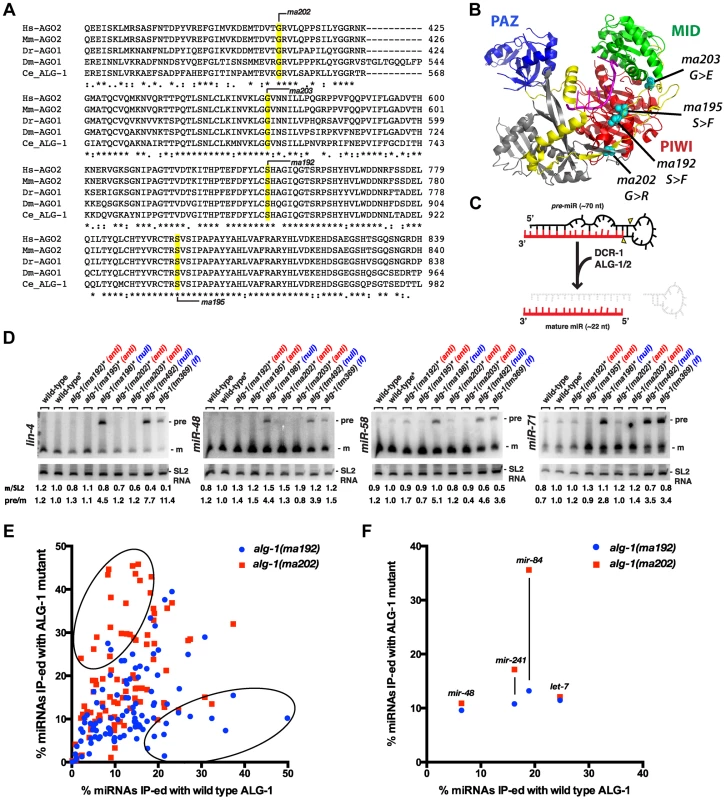
(A) Alignments of Argonaute sequences from multiple species shows conservation of the amino acids affected by alg-1(anti) mutations. (B) Locations of conserved amino acids affected by alg-1(anti) mutations are mapped onto the crystal structure of hAGO2 (PDB ID 4EI1 [51]) using PYMOL. PAZ domain colored in blue, Linker 2 in yellow, MID domain in green, and PIWI domain in red. Amino acids affected by each mutation are highlighted in teal. (C) A schematic showing precursor microRNA processing into the mature guide microRNA. (D) Northern blot analysis of total RNA extracted from mixed population wild type and alg-1 mutant animals using probes to specific microRNAs. Only alg-1(0) null and alg-1(tm369) loss of function mutants accumulate the precursor species of microRNAs. *Strains carry lin-31, col-19::gfp in the background. m/SL2, ratio of mature microRNA to SL2 loading control normalized to wild type lin-31; col-19::gfp. pre/m, ratio of precursor to mature microRNA normalized to wild type lin-31; col-19::gfp. (E) Scatterplot comparing the efficiency with which microRNAs co- immunoprecipitated wild type (X-axis) or mutant (Y-axis) ALG-1. microRNA extracted from ALG-1 immunoprecipitations was quantified using Taqman qRT-PCR. microRNA abundance in each IP was normalized to a synthetic spike-in, and also to the amount of microRNA in the starting material and to the amount of ALG-1 immunoprecipitated (IP-ed). The graph shows average % IP-ed from 3 biological replicates. (F) Subset of data in (E), showing % of let-7-Family microRNAs IP-ed with ALG-1. All strains in (E, F) carry lin-31(lf) and col-19::gfp in the background. The lin-31 mutation is present in order to (non-heterochronically) suppress alg-1(anti) vulval bursting phenotypes. Mature ∼22 nt microRNAs are produced from longer precursor molecules through a series of steps involving the Dicer ribonuclease (dcr-1) and Argonautes alg-1 and alg-2 (Figure 8C), [8], [9]. Previous work showed that reduction of alg-1 by mutation or by RNAi results in accumulation of unprocessed microRNA precursors, as is the case for reduction of dcr-1 function [8], [9]. To determine if alg-1(anti) mutations affect the processing of pre-microRNAs, we performed a Northern blot analysis of total RNA from wild type, alg-1(anti), and alg-1(0) animals (Figure 8D). The results indicate that alg-1(anti) animals, unlike alg-1(0) mutants, do not accumulate unprocessed microRNA precursors (Figure 8D). This suggests that the processing function of the ALG-1(anti) Argonautes proteins is essentially normal, and that the severe microRNA loss-of-function phenotypes of alg-1(anti) animals reflect defect(s) in miRISC maturation or activity downstream of microRNA precursor processing. These data also suggest that the modest reductions in microRNA levels in alg-1(anti) mutants (Figure 7) are not due to pre-microRNA processing defects, but rather might result from indirect effects of ALG-1(anti) proteins on microRNA stability. Interestingly, we did not observe a decrease in mature miR-48 microRNA levels by Northern blot analysis of RNA from mixed stage populations (Figure 8D), whereas Firefly assays of RNA from synchronized L2 larvae did detect a reduction of the miR-48 microRNA (Figure 7B). While there could be many reasons for this discrepancy, including the inherent differences between Northern blot and Firefly assays, it is also possible that mir-48 may be particularly vulnerable to the loss of alg-1 during the second larval stage.
ALG-1(anti) mutant proteins associate with microRNAs in vivo
To determine whether microRNAs form stable complexes with ALG-1(anti) Argonaute proteins we used an anti-ALG-1 antibody to immunoprecipitate ALG-1 protein from wild type and alg-1 mutant animals. A portion of the resulting immunoprecipitation (IP) was assessed for efficiency by Western blotting, while the remainder of the IP was used for RNA isolation. We quantified the association of 106 microRNAs with the wild type and mutant ALG-1 proteins using a multiplexed Taqman qRT assay platform. microRNA levels in each IP were normalized to: 1) a spike-in synthetic microRNA to control for differences in RNA preparation, 2) microRNA levels in the input material of the IP, and 3) the quantity of ALG-1 protein immunoprecipitated (as determined by quantitative Western blot analysis). This analysis did not indicate a pattern of overall decreased association of microRNAs with ALG-1(anti) compared to wild type ALG-1 (Figure 8E, F). The majority of the 106 microRNAs assayed fell into a class of microRNAs whose levels correlated between IP of ALG-1(anti) compared to wild type (and whose level of association changed less than two-fold between wild type and mutant). However, a subset of microRNAs appeared to be more efficiently recovered in IP of ALG-1(anti) (Figure 8E, upper oval; Figure S2A) and another subset of microRNAs appeared to be less efficiently recovered in IP of ALG-1(anti) (Figure 8E, Figure S2B). It should be noted that appreciable variability in the efficiency with which certain microRNAs were co-immunoprecipitated with ALG-1 was observed among biological replicates (Figure S2C). We do not understand the basis of this variation, but it is possible that the same, or similar causes could underlie the observed wide range of efficiencies with which various microRNAs were recovered in ALG-1(anti) immunoprecipitates. Importantly, it should be emphasized that our results do not indicated a general depletion of microRNAs in ALG-1(anti) complexes, suggesting that the functional defect of ALG-1(anti) lies downstream of the association of mature microRNA with miRISC.
ALG-1(anti) proteins co-immunoprecipitate more DCR-1 but less AIN-1 than wild type ALG-1
To determine if ALG-1(anti) engages in stable complexes with known protein partners, we performed ALG-1 immunoprecipitations followed by Western blotting for the critical microRNA biogenesis factor DCR-1 (Dicer) and the essential miRISC effector protein AIN-1 (GW182). As expected, IP of ALG-1 from wild type animals resulted in co-precipitation of detectable quantities of DCR-1 and AIN-1 (Figure 9). In contrast, IP of ALG-1(anti) from alg-1(anti) animals yielded an approximately four-fold increase in the quantity of co-precipitated DCR-1 and an approximately three-fold decrease in co-precipitated AIN-1 (Figure 9). It should be noted that the ALG-1/AIN-1 ratio tended to be variable among biological replicates (not shown), although on average we observed an approximate two-fold decrease in the amount of AIN-1 co-precipitated with ALG-1(anti), compared with the wild type. Perhaps ALG-1(anti)/AIN-1 complexes are relatively unstable under the conditions of our IP experiments, which could render the yield of co-precipitation of AIN-1 with ALG-1(anti) particularly sensitive to experimental perturbations. These data are consistent with the hypothesis that the functional defect of ALG-1(anti) containing miRISC is at a step subsequent to Dicer processing of pre-miRs and microRNA loading, and prior to transition of miRISC to effector activity.
Fig. 9. Western blot analysis of ALG-1 immunoprecipitated complexes from extracts of alg-1(anti) and wild type animals. 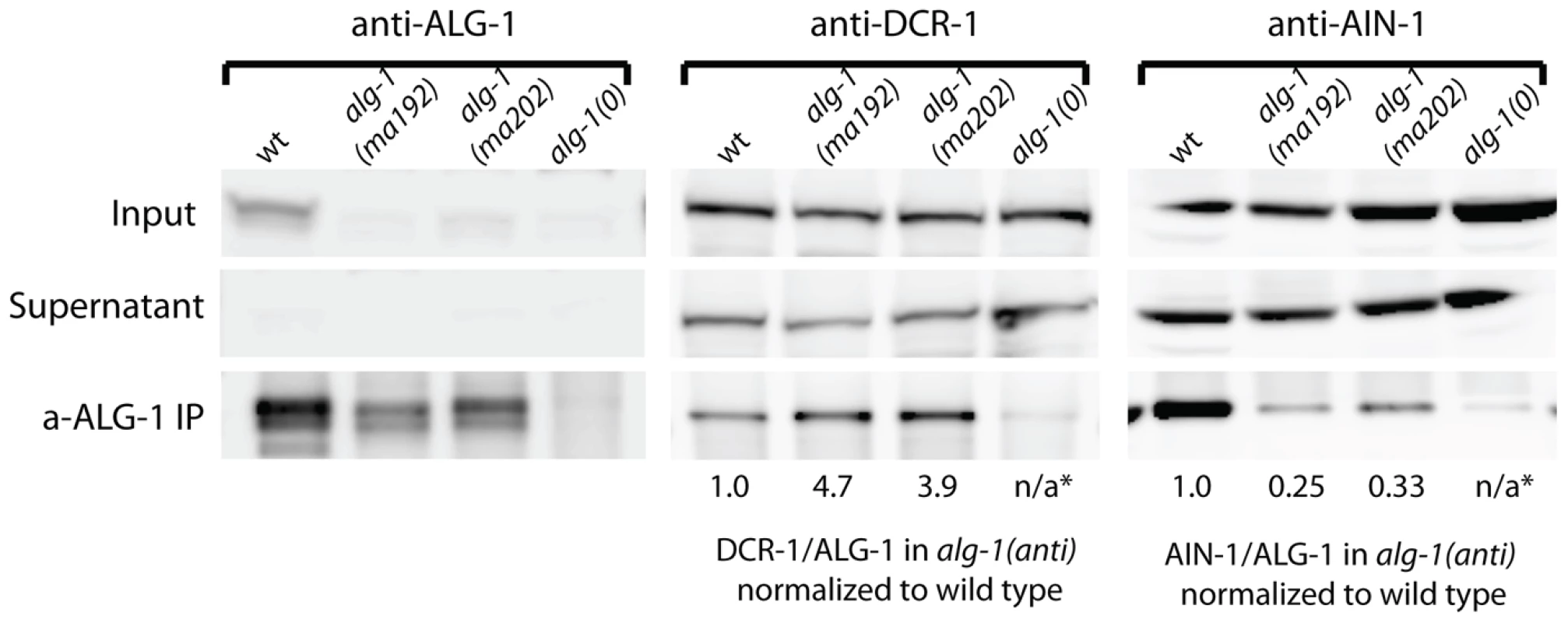
Immunoprecipitated ALG-1(anti) shows an increased association with DCR-1, and a decreased association with AIN-1, compared to wild type ALG-1. The ratio of DCR-1 to ALG-1 and AIN-1 to ALG-1 were determined by quantitation of the Western blot signals, and each of those ratios for the alg-1(anti) mutants is normalized to that of the wild type. * means not applicable (n/a). ALG-1(anti) proteins interact with RNA duplexes in vitro and retain target cleavage activity similar to wild type ALG-1
ALG-1 has been previously shown to possess slicing activity in vitro, an activity which contributes to miRISC maturation [8]. We examined if the ALG-1(anti) proteins retained their ability to cleave an RNA target in vitro. Proteins encoded by both alg-1(ma192) and alg-1(ma202) alleles were GST-tagged, expressed in E. coli, and purified to obtain rALG-1(S895F) and rALG-1(G553R), respectively (Figure 10A). The ability of each rALG-1 protein to cleave a perfect target was assessed by pre-loading each protein with a single stranded siRNA and then subsequently presenting a perfectly complementary target (Figure 10B). Both rALG-1(S895F) and rALG-1(G553R) were able to cleave a target at an efficiency similar to wild type rALG-1 (Figure 10B). We next tested whether rALG-1(S895F) and rALG-1(G553R) proteins could cleave the passenger strand of an siRNA duplex (Figure 10C). We found that rALG-1(S895F) and rALG-1(G553R) were able to both load the duplex and cleave the passenger-like strand of the duplex (Figure 10C). Finally, we took advantage of the in vitro assay cleavage to indirectly assess the ability of the ALG-1(anti) to interact with the mRNA targets. rALG-1, rALG-1(S895F), and rALG-1(G553R) were pre-incubated with a microRNA-like duplex containing two mismatches, and then challenged with an RNA target (Figure 10D). Both mutant rALG-1 proteins were able to induce cleavage of the target RNA with efficiency similar to wild type (Figure 10D), suggesting that both rALG-1(S895F) and rALG-1(G553R) could release the passenger strand of the duplex microRNA in order to interact with the target, at least under the in vitro conditions of this assay. While the cleavage reactions were found to be inefficient under the in vitro conditions of the assay (possibly due to the fact that Argonaute proteins can purify with small RNA already occupying the protein, [50], [51]), the data nonetheless suggest that the rALG-1(anti) proteins retain their catalytic activity to levels similar to wild type.
Fig. 10. Purified recombinant ALG-1(anti) retain the slicing ability similar to the wild type ALG-1. 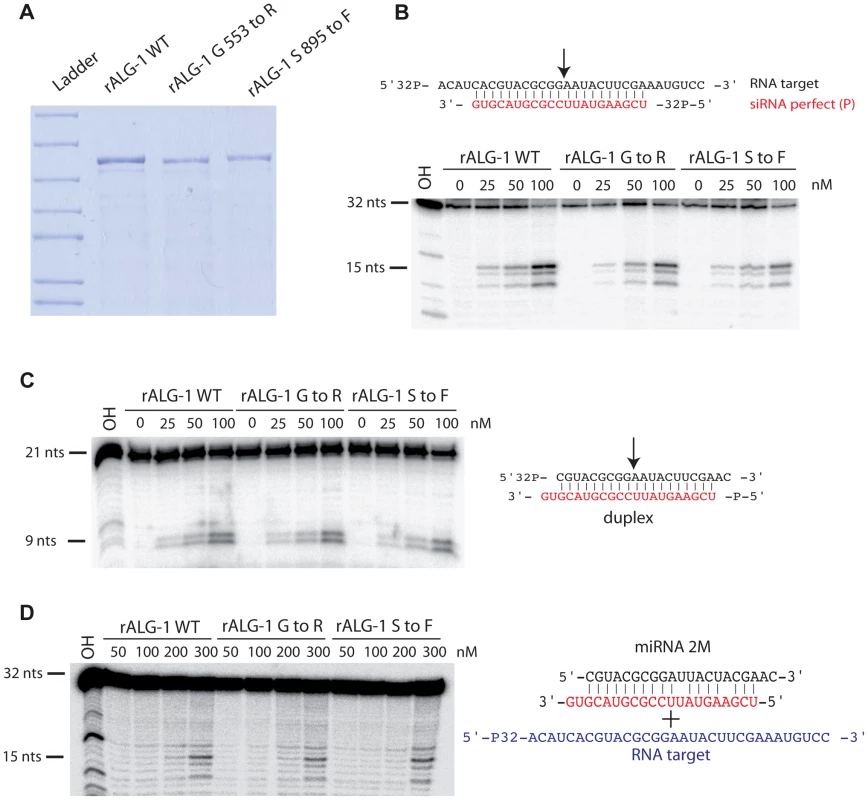
(A) SDS-PAGE gel of recombinant ALG-1 proteins. Wild type ALG-1, ALG-1 G553R and ALG-1 S895F were bacterially expressed and purified. (B) Radiolabeled target RNA is cleaved by rALG-1 proteins preloaded with a siRNA (red) to produce a major 15 nt cleavage species as well as two minor cleavage species. (C) rALG-1 proteins, including rALG-1(anti), bind and cleave a perfectly base-paired duplex. (D) rALG-1 proteins bind a microRNA duplex containing containing two mismatches. Pre-bound rALG-1/duplex complexes are able to cleave an RNA target, producing a 15-nt major cleavage RNA species. The results that bacterially expressed ALG-1(anti) proteins can be catalytically active, taken together with our findings that ALG-1(anti) proteins support Dicer processing and associate with mature microRNAs in worms, lend strong support to a model wherein the ALG-1(anti) proteins are defective in a previously-unrecognized, post-processing Argonaute miRISC maturation activity.
Discussion
Novel Argonaute mutations globally disrupt microRNA activity
Here we report the identification of point mutations in conserved amino acids of a microRNA Argonaute ALG-1 that confer novel and informative biochemical and genetic properties. These mutations cause stronger phenotypes than alg-1 null alleles, and hence are referred to as “antimorphic” (“anti”). Normally, the two orthologous proteins ALG-1 and ALG-2 exhibit nearly redundant function, such that genetic knockout of one ortholog causes only weak or undetectable phenotypes [9], [39]. By contrast, the alg-1(anti) mutations we describe here cause severe developmental defects consistent with dramatic impairment of let-7-Family microRNA activity, impair functions of other microRNAs including lsy-6 and mir-35-Family, and result in lethality in combination with alg-2(0), consistent with broad defects in the activity of additional microRNAs. We interpret these results to indicate that the alg-1(anti) mutations not only impair ALG-1 function, but they also cause the mutant ALG-1 protein to (directly or indirectly) compete with another component of miRISC, presumably the semi-redundant ALG-2. Our observation that the alg-1(anti) alleles are recessive to alg-1(+) suggests that the mutant ALG-1(anti) proteins may be more effective in competing with wild type ALG-2 than they are with wild type ALG-1.
How might ALG-1(anti) proteins interfere with ALG-2 activity? Although it is possible that the mutant ALG-1(anti) may inhibit ALG-2 by direct ALG-1/ALG-2 physical interaction, Argonaute proteins are not known to form heterodimers. It is also possible that alg-1(anti) may affect regulation of alg-2 by decreasing the amount of ALG-2 protein produced. However, we found that alg-1(anti) mutations did not significantly reduce the amount of alg-2 mRNA (Figure S3). While it is possible that ALG-2 protein accumulation may still be affected by the alg-1(anti) mutations, such effects would not appear to be via regulation of the alg-2 transcript.
We propose that ALG-1(anti) mutant proteins essentially compete with ALG-2 in trans by sequestering microRNAs and possibly other limiting miRISC components (such as Dicer) into nonfunctional complexes (Figure 11). While alg-1(anti) mutants show reduced amounts of ALG-1 protein, our IP experiments show that ALG-1(anti) protein associates with microRNAs to levels similar to wild type ALG-1, when normalized to the amount of immunoprecipitated ALG-1. Therefore, unlike genetic contexts where alg-1 is deleted and all the microRNAs are free to associate with ALG-2 (Figure 11B), the presence of ALG-1(anti) protein may result in some of the microRNAs being partitioned into defective ALG-1(anti) complexes and sequestered from ALG-2 (Figure 11C).
Fig. 11. Model representing a proposed mode of action of antimorphic mutant ALG-1. 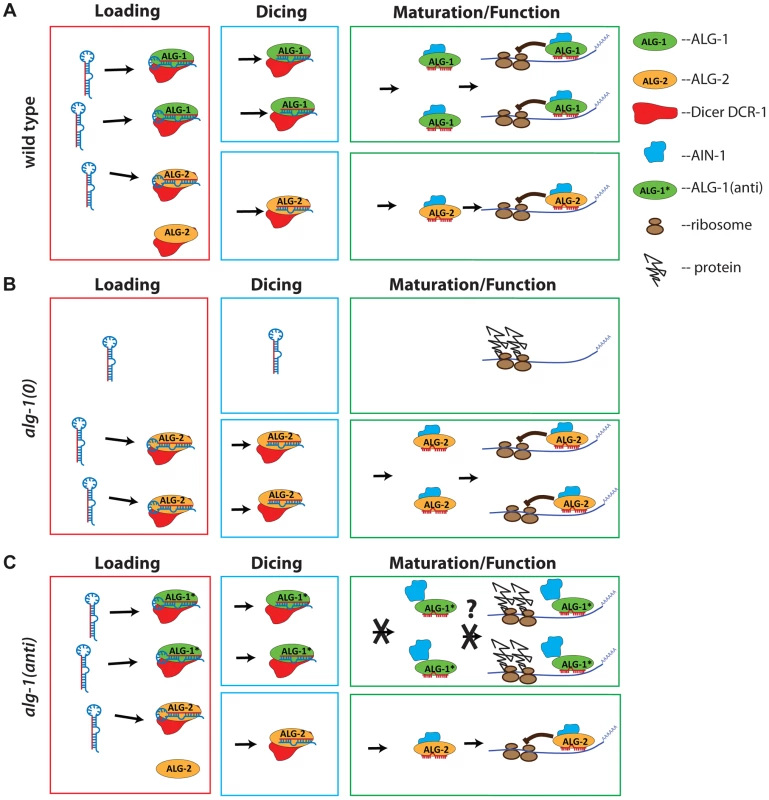
(A) In wild type animals, ALG-1 and ALG-2 associate with microRNA precursors (Loading), act in conjunction with Dicer to facilitate cleavage of the precursor to yield mature microRNA (Dicing), and through a series of miRISC maturation steps, associate with effector partners including AIN-1/GW182, and repress mRNA targets (Maturation/Function). According to the model, ALG-1 and ALG-2 are partially redundant, and function asymmetrically; ALG-1 carries a majority share of microRNA function. (B) In the absence of ALG-1, ALG-2 is able to partially cover for reduced overall ALG activity, and so alg-1(0) animals exhibit weak microRNA loss-of-function phenotypes, and accumulate abnormal levels of microRNA precursors. (C) In alg-1(anti) animals, microRNA activity is globally poisoned by the ALG-1(anti) protein. This is because ALG-1(anti) protein is competent for Loading and Dicing, but is blocked in one or more steps of miRISC maturation wherein ALG-1 would normally transition from Dicer associated microRNA processing to AIN-1/GW182 associated target repression. This hypothetical miRISC maturation defect results in sequestration of miRISC components (including microRNAs) in inactive complexes. Jagged lines attached to ribosomes (brown ovals) represent un-repressed protein synthesis from target mRNA. What could be defective about ALG-1(anti) miRISC? Our in vitro biochemical analysis of ALG-1(anti) proteins indicates that the mutant Argonautes can load a guide RNA and are capable (at least under our assay conditions) of eliciting slicer activity to a level that is indistinguishable from wild type ALG-1. It was previously shown that ALG-1 slicer activity is important for function in vivo, likely due to passenger strand cleavage [8]. In addition, the ability of ALG-1(anti) proteins to bind microRNAs suggests that ALG-1(anti) mutants are likely defective in other step(s) of miRISC maturation, turnover and/or effector phases of microRNA-mediated repression. The alg-1(anti) mutations could affect the structure of the ALG-1 protein, and/or affect essential protein-protein interactions. Indeed, we find that a substantially larger fraction of ALG-1 population associates with DCR-1 in alg-1(anti) mutants. These findings, considered together with our observation of a reduced association of ALG-1(anti) with the miRISC effector protein AIN-1, are consistent with the model that ALG-1(anti) miRISC is impaired in maturation from biogenesis to effector activity, thereby sequestering a critical fraction of microRNAs and perhaps other essential miRISC components in nonfunctional complexes (Figure 11). It should be noted that it might not be necessary for this hypothetical microRNA sequestration to be quantitative in order to reduce the amounts of functional miRISC below phenotypic thresholds.
According to this model it is also possible that titration of DCR-1 activity could occur in alg-1(anti) animals, since DCR-1 associated with ALG-1(anti) complexes would presumably be unavailable for processing of additional microRNA precursors. In that event, we might have expected to observe precursor accumulation, and a corresponding reduction of mature microRNA levels. While our data do show a modest reduction in mature microRNA levels in alg-1(anti) mutants, precursor accumulation was not detectable, at least not within the sensitivity of our Northern blot assay. This suggests that the fraction of DCR-1 associated with ALG-1(anti) may not be sufficient to significantly deplete the pool of DCR-1 available for precursor processing.
ALG-1 microRNA biogenesis and effector functions
alg-1(0) mutants accumulate unprocessed microRNA precursor hairpins, similar to Dicer (dcr-1) loss of function [8], [9] (see also Figure 8), suggesting that ALG-1 directly or indirectly functions as a cofactor for Dicer cleavage of pre-microRNA hairpins. This implies that ALG-1 is a multifunctional protein with roles in microRNA biogenesis as well as in miRISC effector activity. A role for ALG-1 as a functional co-factor that supports the pre-microRNA processing by Dicer is also supported by both co-IP and proteomic data [52], [53]. Moreover, properties of the alg-1(anti) mutations described here also support a multifunctional role for ALG-1 in the microRNA pathway. Two striking properties of alg-1(anti) mutations are that ALG-1(anti) proteins appear to associate with mature microRNAs to normal levels, and moreover, unlike alg-1(0) or alg-1(lf) animals, alg-1(anti) mutants do not accumulate unprocessed microRNA precursors. Thus, the ALG-1(anti) mutant proteins seem to retain the capacity to promote Dicer cleavage of microRNA precursors and to associate with mature microRNAs. However, our observation that ALG-1(anti) co-immunoprecipitates with more DCR-1 and less AIN-1 than wild type ALG-1 suggests that ALG-1(anti) may be impaired in a maturation process involving DCR-1 release and AIN-1 association. It should be noted that, although all four existing alg-1(anti) alleles display similar phenotypes, they may not necessarily affect precisely the same step(s) of miRISC maturation downstream of microRNA biogenesis and Argonaute loading.
alg-1(anti) developmental timing phenotypes
We recovered mutations affecting general microRNA function as suppressors of the developmental timing mutant, lin-28(lf). This indicates that lin-28(lf) phenotypes for the most part involve hyperactivity of microRNAs. What microRNA(s) in particular? Since lin-28(lf) mutants over-express the let-7 microRNA in the L2 stage (Figure 7A and [21]), let-7 (perhaps in combination with other let-7-Family microRNAs) was a plausible candidate. However, previous findings showed that the precocious L3 fate phenotype of lin-28(lf) larvae cannot be entirely attributed to let-7-Family hyperactivity: mutations that simultaneously remove three (mir-48, mir-84, mir-241), or even all four (mir-48, mir-84, mir-241, let-7) of the major let-7-Family microRNAs did not suppress the precocious L3 fates of lin-28(lf) [2], [20]. These data, in combination with our observation that lin-28(lf) seam cell lineage defects are suppressed by alg-1(anti) are consistent with the idea that other microRNAs in addition to mir-48, mir-84, mir-241, and let-7 may be hyperactive in lin-28(lf) and may contribute to controlling the L2-L3 transition.
The particular alg-1(anti) mutations identified here were isolated as viable suppressors of lin-28(lf). More severely antimorphic ALG-1 mutant proteins that more effectively compete with ALG-2 might have resulted in lethality, and hence would not have been recovered in our lin-28(lf) suppressor screen. Nevertheless, this screen did yield mutations that generally affect microRNA functions and therefore supports the possibility that further screening may lead to isolation of animals carrying mutations in other miRISC components, provided such mutations do not result in lethality. An alg-1(he210) missense mutation was also previously isolated in a screen for disorganized seam cells [54]. The strength of the phenotypes reported [54], as well as the occurrence of similar seam cell disorganization phenotypes in our antimorphic mutants (data not shown) is consistent with the alg-1(he210) allele also being antimorphic in nature.
Genetic dissection of Argonautes
Redundant genes can be notoriously resistant to genetic analysis by straightforward loss-of-function genetics, since all or most of the set of redundant genes may need to be simultaneously eliminated to cause a phenotype. However, our results illustrate how antimorphic mutations that partially disable one member of a redundant family of proteins can poison the collective functions of the family, and thereby produce phenotypes that reveal activities not otherwise accessible by conventional forward genetic screens. In principle, different antimorphic alleles could affect different aspects of ALG-1 function. Therefore the genetic suppressor screen used here to recover alg-1(anti) mutations offers the potential for genetic dissection of Argonaute functionality.
ALG-1 and ALG-2 proteins are 77% identical, and ALG-2 contains the same conserved residues as those affected in the alg-1(anti) [39]. The genetic redundancy and conservation of molecular function raises interesting questions including: why did we recover antimorphic alleles of alg-1 but not alg-2? We do not believe that the screen was saturated, and it is possible that further screening may result in recovery of hypothetical alg-2(anti) mutations. Alternatively, perhaps ALG-1 and ALG-2 are not entirely equivalent functionally or biochemically, such that ALG-2 antimorphic mutations might be either too potent (and hence lethal) or too weak to be recovered in our screen. There is evidence that ALG-1 and ALG-2 are not strictly functionally equivalent. We observed that loss of alg-1 can partially suppress lin-28(lf) mutant phenotypes, but loss of alg-2 Argonaute does not (Table 1). Loss of alg-1 results in overt, albeit mild, heterochronic developmental abnormalities, while alg-2(0) mutants display no detectable heterochronic phenotypes (Table 2 and data not shown). This suggests that ALG-1 plays a greater role in heterochronic microRNA function than does ALG-2. Other distinctions have been reported between ALG-1 and ALG-2 in their expression patterns and profiles of associated microRNAs [39], and in their relative roles in microRNA processing [8]. Other genetic evidence also points to additional functional non-redundancy between ALG-1 and ALG-2 [55].
Our data indicate that the alg-1(anti) mutations are synthetic lethal in combination with alg-2(0), which suggests that recovery of viable antimorphic mutations in an Argonaute could only occur in animals where there are multiple microRNA Argonaute genes, as is the case for C. elegans, or mammals, where there are four Argonaute family proteins that function with microRNAs [56]–[58]. The nematode alg-1(anti) mutations described here are all substitutions in amino acids that are broadly conserved in Argonaute family proteins, including the four mammalian miRISC Argonautes, AGO1 – AGO4 (Figure S1). It remains to be determined if the detrimental effects of the alg-1(anti) mutations are a peculiarity of C. elegans or whether the corresponding amino acid substitutions in other Argonautes-in C. elegans or in other organisms-can cause antimorphic effects on microRNA activity. Such hypothetical antimorphic mutations in mammalian Argonautes could contribute to diseases that involve microRNAs, such as cancer. For example, global reduction of microRNA activity has been shown to be oncogenic in certain circumstances [59]. Wilms tumors often carry a deletion of three AGO genes [60], [61], and mutations of AGO2 were frequently found in colorectal and gastric cancers [62]. In addition, Dicer1 mutations are associated with many tumors (reviewed in [63]), and Dicer1 functions as a haploinsufficient tumor suppressor [64], and so mammalian Ago(anti) mutations could predispose cells to tumor formation.
Methods
Strains and C. elegans culture
C. elegans culture was performed using standard nematode growth conditions [18], [19], [65]. All strains were grown at 20°C unless otherwise noted. To obtain synchronized nematode populations, gravid adults were treated with hypochlorite, the resulting eggs were hatched overnight in M9 and then placed onto E. coli lawns for recovery and growth. Animals were collected as young L2 larvae following the first-molt lethargus. Data shown for alg-1 null (alg-1(0)) was generated using the alg-1(tm492), except in Figure 7, where alg-1(ma198) null allele was used. Because of their strong heterochronic phenotypes, alg-1(anti) mutant animals often burst through the vulva during the L4-adult molt. Therefore alg-1(anti) mutations were maintained in a lin-31(n1053) genetic background that impairs vulva development and thereby suppresses the bursting phenotype of alg-1 mutants while leaving their heterochronic phenotypes intact. The col-19::gfp adult marker is also present in all the strains unless otherwise noted. Therefore, unless otherwise noted, all strains contain lin-31; col-19::gfp and their phenotypes are compared to their wild type equivalent lin-31; col-19::gfp.
Screen for lin-28 suppressors; mutation mapping and cloning
lin-28(n947) animals were mutagenized for four hours using 0.05 M ethylmethylsulfonate (EMS), and their F2 progeny were screened for suppression of the severe morphological and egg-laying defects characteristic of lin-28(lf) mutants. Suppressor mutations were backcrossed to CB4856 Hawaii strain and mapped using the snip-SNP protocol as previously described [66]. The region containing the mutation was scanned for candidate genes, which were subsequently sequenced.
Phenotypic characterizations
Heterochronic phenotypes were assessed by scoring for the stage-specificity of adult lateral alae on the cuticle surface and the expression of an adult-specific hypodermal reporter transgene col-19::gfp [19], [67]. Differential interference (DIC) microscopy was used to score seam cell number in L4 stage larvae or young adult animals, the presence of lateral alae on L4 or young adult animals, and gonad morphology in young adult stage animals. The col-19::gfp expression was scored using a fluorescence equipped dissecting or a compound microscope. ASEL/ASER fates were determined by assessing the Plim-6::gfp transgene expression that is specific to ASEL in the wild type. Expression of the Plim-6::gfp reporter was observed using a fluorescence equipped Zeiss dissecting scope. Enhancement of mir-35–41 embryonic lethality phenotype was assessed by determining the percentage of embryos capable of hatching for mir-35–41 embryos in combination with alg-1 mutations (the lin-31 mutation was present in the background of all strains to keep the alg-1 animals from bursting through the vulva). L4 animals were placed at 25°C for 24 hours, after which the animals were cut open to release the eggs. The eggs were placed at 25°C and assessed for their ability to hatch following a 16 hours incubation, and reassessed 12 hours later. It should be noted that many animals with the mir-35–41 deletion arrested as larvae after hatching.
microRNA quantification by FirePlex miRSelect
Total RNA was extracted from worms using Trizol (Invitrogen). For samples yielding greater or equal to 1 micrograms of total RNA, microRNA abundance was determined by using Firefly Bioworks FirePlex miRSelect method [68] with a custom miRSelect panel using standard kit protocol. In short, RNA samples were hybridized to the custom microRNA-specific probes coated on the FirePlex hydrogel beads. A universal biotinylated adapter was then ligated onto the captured microRNAs and labeled with a fluorescent reporter. The level of fluorescence for a given particle (and therefore a given microRNA) was detected using a Guava easyCyte 8HT flow cytometer. Assays for U18 RNA were included as normalization controls for RNA input. Data was analyzed using FireCode software (Firefly Bioworks), and microRNA abundance was normalized to U18 RNA levels.
Northern blot analysis
Total RNA was isolated from a mixed population of animals, and Northern blots were performed as previously described [69], [70]. Density of the signal was quantified using ImageJ software.
RNAi
hbl-1(RNAi), unc-22(RNAi), and empty vector control (L4440) RNAi was performed by feeding the worms E. coli producing dsRNA as described [71]. Animals were fed RNAi food starting as starved L1s, and throughout development. Phenotypes were scored when the animals reached the young adult stage.
Immunoprecipitations and western blot analysis
To assess ALG-1 protein levels, total protein was extracted from mixed stage animals as previously described [72], except RNAse inhibitor (Invitrogen) was added to the lysis buffer. Western blotting was performed as previously described [72]. ALG-1 custom polyclonal rabbit antibody (Anaspec) was used for ALG-1 protein detection as described [72]. ALG-1 immunoprecipitations were performed as previously described using a custom ALG-1 specific polyclonal antibody (Anaspec) [72]. In short, whole protein lysis sample was divided into two equal parts: one was used for subsequent ALG-1 immunoprecipitation(IP), one for RNA preparation in order to determine the amount of microRNAs present in the starting material. Half of the immunoprecipitated sample was used to assess the efficiency of immunoprecipitation by western blotting for ALG-1 protein, and the other half was used for RNA preparation. For protein co-IP, the precipitated material was probed with a custom rabbit polyclonal ALG-1 antibody [69], with a rabbit anti-DCR-1 antibody [52], or a rat anti-AIN-1 antibody [11].
microRNA quantification by qRT-PCR (Taqman)
Total RNA was extracted from worms using Trizol (Invitrogen). For samples with total RNA yields less than 1 microgram (such as ALG-1 associated RNAs recovered by immunoprecipitation) and the corresponding starting material samples, microRNA levels were determined using a panel of Taqman-based real time PCR assays (Applied Biosystems). RNA was extracted in the presence of a synthetic Arabidopsis mir-159 spike-in; all Ct values were first normalized to the synthetic mir-159 spike-in in order to control for variation in RNA sample preparation. Ct values resulting from the qRT-PCR performed on the immunoprecipitated RNA were also normalized to the amount of ALG-1 protein recovered by the immunoprecipitation. The Ct values resulting from the qRT-PCR performed on the input RNA were normalized to the mass of starting RNA material. Percent of microRNA immunoprecipitated with ALG-1 was determined from the ratio of microRNA in the IP sample to the starting material sample.
Recombinant protein purification and rALG-1/RNA binding and slicing assays
Recombinant C. elegans ALG-1 protein purification and ALG-1/RNA binding and slicing assays were performed as previously described [8].
Supporting Information
Zdroje
1. ResnickTD, McCullochKA, RougvieAE (2010) miRNAs give worms the time of their lives: Small RNAs and temporal control in Caenorhabditis elegans. Dev Dyn 239 : 1477–1489 doi:10.1002/dvdy.22260
2. AbbottAL, Alvarez-SaavedraE, MiskaEA, LauNC, BartelDP, et al. (2005) The let-7 MicroRNA Family Members mir-48, mir-84, and mir-241 Function Together to Regulate Developmental Timing in Caenorhabditis elegans. Developmental Cell 9 : 403–414 doi:10.1016/j.devcel.2005.07.009
3. ReinhartBJ, SlackFJ, BassonM, PasquinelliAE, BettingerJC, et al. (2000) The 21-nucleotide let-7 RNA regulates developmental timing in Caenorhabditis elegans. Nature 403 : 901–906.
4. FabianMR, SonenbergN (2012) The mechanics of miRNA-mediated gene silencing: a look under the hood of miRISC. Nat Struct Mol Biol 19 : 586–593 doi:10.1038/nsmb.2296
5. FabianMR, SonenbergN, FilipowiczW (2010) Regulation of mRNA Translation and Stability by microRNAs. Annu Rev Biochem 79 : 351–379 doi:10.1146/annurev-biochem-060308-103103
6. DingXC, WeilerJ, GroßhansH (2009) Regulating the regulators: mechanisms controlling the maturation of microRNAs. Trends in Biotechnology 27 : 27–36 doi:10.1016/j.tibtech.2008.09.006
7. FilipowiczW, BhattacharyyaSN, SonenbergN (2008) Mechanisms of post-transcriptional regulation by microRNAs: are the answers in sight? Nature Publishing Group 2008 : 102–114 doi:10.1038/nrg2290
8. BouaskerS, SimardMJ (2012) The slicing activity of miRNA-specific Argonautes is essential for the miRNA pathway in C. elegans. Nucleic Acids Research 40 : 10452–10462 doi:10.1093/nar/gks748
9. GrishokA, PasquinelliAE, ConteD, LiN, ParrishS, et al. (2001) Genes and Mechanisms Related to RNA Interference Regulate Expression of the Small Temporal RNAs that Control C. elegans Developmental Timing. Cell 106 : 23–34.
10. MeisterG (2013) Argonaute proteins: functional insights and emerging roles. Nature Publishing Group 14 : 447–459 doi:10.1038/nrg3462
11. ZhangL, DingL, CheungTH, DongM-Q, ChenJ, et al. (2007) Systematic Identification of C. elegans miRISC Proteins, miRNAs, and mRNA Targets by Their Interactions with GW182 Proteins AIN-1 and AIN-2. Molecular Cell 28 : 598–613 doi:10.1016/j.molcel.2007.09.014
12. DingL, SpencerA, MoritaK, HanM (2005) The Developmental Timing Regulator AIN-1 Interacts with miRISCs and May Target the Argonaute Protein ALG-1 to Cytoplasmic P Bodies in C. elegans. Molecular Cell 19 : 437–447 doi:10.1016/j.molcel.2005.07.013
13. EulalioA, HuntzingerE, IzaurraldeE (2008) GW182 interaction with Argonaute is essential for miRNA-mediated translational repression and mRNA decay. Nat Struct Mol Biol 15 : 346–353 doi:10.1038/nsmb.1405
14. REHWINKELJ (2005) A crucial role for GW182 and the DCP1:DCP2 decapping complex in miRNA-mediated gene silencing. RNA 11 : 1640–1647 doi:10.1261/rna.2191905
15. LiuJ, RivasFV, WohlschlegelJ, YatesJR, ParkerR, et al. (2005) A role for the P-body component GW182 in microRNA function. Nature Cell Biology 7 : 1261–1266 doi:10.1038/ncb1333
16. JakymiwA, LianS, EystathioyT, LiS, SatohM, et al. (2005) Disruption of GW bodies impairs mammalian RNA interference. Nature Cell Biology 7 : 1267–1274 doi:10.1038/ncb1334
17. MeisterG, LandthalerM, PetersL, ChenPY, UrlaubH, et al. (2005) Identification of Novel Argonaute-Associated Proteins. Current Biology 15 : 2149–2155 doi:10.1016/j.cub.2005.10.048
18. MossEG, LeeRC, AmbrosV (1997) The Cold Shock Domain Protein LIN-28 Controls Developmental Timing in C. elegans and Is Regulated by the lin-4 RNA. Cell 88 : 637–646.
19. AmbrosV, HorvitzHR (1984) Heterochronic mutants of the nematode Caenorhabditis elegans. Science 226 : 409–416.
20. VadlaB, KemperK, AlaimoJ, HeineC, MossEG (2012) lin-28 controls the succession of cell fate choices via two distinct activities. 8 : 11 doi:10.1371/journal.pgen.1002588
21. Van WynsberghePM, KaiZS, MassirerKB, BurtonVH, YeoGW, et al. (2011) LIN-28 co-transcriptionally binds primary let-7 to regulate miRNA maturation in Caenorhabditis elegans. Nat Struct Mol Biol 18 : 302–308 doi:10.1038/nsmb.1986
22. LehrbachNJ, ArmisenJ, LightfootHL, MurfittKJ, BugautA, et al. (2009) LIN-28 and the poly(U) polymerase PUP-2 regulate let-7 microRNA processing in Caenorhabditis elegans. Nat Struct Mol Biol 16 : 1016–1020 doi:10.1038/nsmb.1675
23. HaganJP, PiskounovaE, GregoryRI (2009) Lin28 recruits the TUTase Zcchc11 to inhibit let-7 maturation in mouse embryonic stem cells. Nat Struct Mol Biol 16 : 1021–1025 doi:10.1038/nsmb.1676
24. HeoI, JooC, KimY-K, HaM, YoonM-J, et al. (2009) TUT4 in Concert with Lin28 Suppresses MicroRNA Biogenesis through Pre-MicroRNA Uridylation. Cell 138 : 696–708 doi:10.1016/j.cell.2009.08.002
25. HeoI, JooC, ChoJ, HaM, HanJ, et al. (2008) Lin28 Mediates the Terminal Uridylation of let-7 Precursor MicroRNA. Molecular Cell 32 : 276–284 doi:10.1016/j.molcel.2008.09.014
26. NewmanMA, ThomsonJM, HammondSM (2008) Lin-28 interaction with the Let-7 precursor loop mediates regulated microRNA processing. RNA 14 : 1539–1549 doi:10.1261/rna.1155108
27. PiskounovaE, ViswanathanSR, JanasM, LaPierreRJ, DaleyGQ, et al. (2008) Determinants of MicroRNA Processing Inhibition by the Developmentally Regulated RNA-binding Protein Lin28. Journal of Biological Chemistry 283 : 21310–21314 doi:10.1074/jbc.C800108200
28. ViswanathanSR, DaleyGQ, GregoryRI (2008) Selective Blockade of MicroRNA Processing by Lin28. Science 320 : 97–100 doi:10.1126/science.1154040
29. AbrahanteJE, DaulAL, LiM, VolkML, TennessenJM, et al. (2003) The Caenorhabditis elegans hunchback-like Gene lin-57/hbl-1 Controls Developmental Time and Is Regulated by MicroRNAs. Developmental Cell 4 : 625–637.
30. LinS-Y, JohnsonSM, AbrahamM, VellaMC, PasquinelliA, et al. (2003) The C elegans hunchback homolog, hbl-1, controls temporal patterning and is a probable microRNA target. Developmental Cell 4 : 639–650.
31. RoushSF, SlackFJ (2009) Transcription of the C. elegans let-7 microRNA is temporally regulated by one of its targets, hbl-1. Dev Biol 334 : 523–534 doi:10.1016/j.ydbio.2009.07.012
32. MoritaKK, HanMM (2006) Multiple mechanisms are involved in regulating the expression of the developmental timing regulator lin-28 in Caenorhabditis elegans. EMBO J 25 : 5794–5804 doi:10.1038/sj.emboj.7601451
33. MossEG (2007) Heterochronic Genes and the Nature of Developmental Time. Current Biology 17: R425–R434 Available: http://www.sciencedirect.com/science/article/pii/S0960982207011475.
34. EulingS, AmbrosV (1996) Heterochronic genes control cell cycle progress and developmental competence of C. elegans vulva precursor cells. Cell 84 : 667–676 doi:10.1016/S0092-8674(00)81045-4
35. PepperASR (2004) The C. elegans heterochronic gene lin-46 affects developmental timing at two larval stages and encodes a relative of the scaffolding protein gephyrin. Development 131 : 2049–2059 doi:10.1242/dev.01098
36. JohnstonRJ, HobertO (2003) A microRNA controlling left/right neuronal asymmetry in Caenorhabditis elegans. Nature 426 : 845–849 doi:10.1038/nature02255
37. SarinS, O'MearaMM, FlowersEB, AntonioC, PooleRJ, et al. (2007) Genetic Screens for Caenorhabditis elegans Mutants Defective in Left/Right Asymmetric Neuronal Fate Specification. Genetics 176 : 2109–2130 doi:10.1534/genetics.107.075648
38. ChangSS, JohnstonRJR, HobertOO (2003) A transcriptional regulatory cascade that controls left/right asymmetry in chemosensory neurons of C. elegans. Genes & Development 17 : 2123–2137 doi:10.1101/gad.1117903
39. Vasquez-RifoA, JannotG, ArmisenJ, LabouesseM, BukhariSIA, et al. (2012) Developmental Characterization of the MicroRNA-Specific C. elegans Argonautes alg-1 and alg-2. PLoS ONE 7: e33750 doi:10.1371/journal.pone.0033750.s005
40. Alvarez-SaavedraE, HorvitzHR (2010) Many Families of C. elegans MicroRNAs Are Not Essential for Development or Viability. Current Biology 20 : 367–373 doi:10.1016/j.cub.2009.12.051
41. MiskaEA, Alvarez-SaavedraE, AbbottAL, LauNC, HellmanAB, et al. (2007) Most Caenorhabditis elegans microRNAs Are Individually Not Essential for Development or Viability. PLoS Genet 3: e215 doi:10.1371/journal.pgen.0030215.st003
42. BrennerJL, JasiewiczKL, FahleyAF, KempBJ, AbbottAL (2010) Loss of Individual MicroRNAs Causes Mutant Phenotypes in Sensitized Genetic Backgrounds in C. elegans. Current Biology 20 : 1321–1325 doi:10.1016/j.cub.2010.05.062
43. FrankF, SonenbergN, NagarB (2010) Structural basis for 5′-nucleotide base-specific recognition of guide RNA by human AGO2. Nature 465 : 818–822 doi:10.1038/nature09039
44. WangY, JuranekS, LiH, ShengG, WardleGS, et al. (2009) Nucleation, propagation and cleavage of target RNAs in Ago silencing complexes. Nature 461 : 754–761 doi:10.1038/nature08434
45. MaJ-B, YuanY-R, MeisterG, PeiY, TuschlT, et al. (2005) Structural basis for 5′-end-specific recognition of guide RNA by the A. fulgidus Piwi protein. Nature Cell Biology 434 : 666–670 doi:10.1038/nature03514
46. ParkerJS, RoeSM, BarfordD (2005) Structural insights into mRNA recognition from a PIWI domain-siRNA guide complex. Nature 434 : 663–666 doi:10.1038/nature03462
47. WangY, JuranekS, LiH, ShengG, TuschlT, et al. (2008) Structure of an argonaute silencing complex with a seed-containing guide DNA and target RNA duplex. Nature 456 : 921–926 doi:10.1038/nature07666
48. WangY, ShengG, JuranekS, TuschlT, PatelDJ (2008) Structure of the guide-strand-containing argonaute silencing complex. Nature 456 : 209–213 doi:10.1038/nature07315
49. MaJ-B, YeK, PatelDJ (2004) Structural basis for overhang-specific small interfering RNA recognition by the PAZ domain. Nature 429 : 318–322 doi:10.1038/nature02519
50. SchirleNT, MacRaeIJ (2012) The Crystal Structure of Human Argonaute2. Science 336 : 1037–1040 doi:10.1126/science.1221551
51. NakanishiK, WeinbergDE, BartelDP, PatelDJ (2012) Structure of yeast Argonaute with guide RNA. Nature 486 : 368–374 doi:10.1038/nature11211
52. DuchaineTF, WohlschlegelJA, KennedyS, BeiY, ConteDJr, et al. (2006) Functional Proteomics Reveals the Biochemical Niche of C. elegans DCR-1 in Multiple Small-RNA-Mediated Pathways. Cell 124 : 343–354 doi:10.1016/j.cell.2005.11.036
53. SawhAN, DuchaineTF (2013) A Truncated Form of Dicer Tilts the Balance of RNA Interference Pathways. Cell Reports 4 : 454–463 doi:10.1016/j.celrep.2013.07.013
54. WildwaterM, SanderN, de VreedeG, van den HeuvelS (2011) Cell shape and Wnt signaling redundantly control the division axis of C. elegans epithelial stem cells. Development 138 : 4375–4385 doi:10.1242/dev.066431
55. TOPSBBJ, PLASTERKRHA, KETTINGRF (2006) The Caenorhabditis elegans Argonautes ALG-1 and ALG-2: Almost Identical yet Different. Cold Spring Harbor Symposia on Quantitative Biology 71 : 189–194 doi:10.1101/sqb.2006.71.035
56. LiuJ (2004) Argonaute2 Is the Catalytic Engine of Mammalian RNAi. Science 305 : 1437–1441 doi:10.1126/science.1102513
57. MeisterG, LandthalerM, PatkaniowskaA, DorsettY, TengG, et al. (2004) Human Argonaute2 Mediates RNA Cleavage Targeted by miRNAs and siRNAs. Molecular Cell 15 : 185–197 doi:10.1016/j.molcel.2004.07.007
58. PILLAIRS (2004) Tethering of human Ago proteins to mRNA mimics the miRNA-mediated repression of protein synthesis. RNA 10 : 1518–1525 doi:10.1261/rna.7131604
59. KumarMS, LuJ, MercerKL, GolubTR, JacksT (2007) Impaired microRNA processing enhances cellular transformation and tumorigenesis. Nat Genet 39 : 673–677 doi:10.1038/ng2003
60. KoestersR, AdamsV, BettsD, MoosR, SchmidM, et al. (1999) Human eukaryotic initiation factor EIF2C1 gene: cDNA sequence, genomic organization, localization to chromosomal bands 1p34-p35, and expression. Genomics 61 : 210–218 doi:10.1006/geno.1999.5951
61. DomeJS, CoppesMJ (2002) Recent advances in Wilms tumor genetics. Curr Opin Pediatr 14 : 5–11.
62. KimMS, OhJE, KimYR, ParkSW, KangMR, et al. (2010) Somatic mutations and losses of expression of microRNA regulationrelated genes AGO2 and TNRC6A in gastric and colorectal cancers. J Pathol 221 : 139–146 doi:10.1002/path.2683
63. BahubeshiAA, TischkowitzMM, FoulkesWDW (2011) miRNA processing and human cancer: DICER1 cuts the mustard. Sci Transl Med 3 : 111ps46–111ps46 doi:10.1126/scitranslmed.3002493
64. KumarMS, PesterRE, ChenCY, LaneK, ChinC, et al. (2009) Dicer1 functions as a haploinsufficient tumor suppressor. Genes & Development 23 : 2700–2704 doi:10.1101/gad.1848209
65. BrennerS (1974) The Genetics of CAENORHABDITIS ELEGANS. Genetics 77 : 71–94.
66. WicksSR, YehRT, GishWR, WaterstonRH, PlasterkRH (2001) Rapid gene mapping in Caenorhabditis elegans using a high density polymorphism map. Nat Genet 28 : 160–164 doi:10.1038/88878
67. AbrahanteJE, MillerEA, RougvieAE (1998) Identification of heterochronic mutants in Caenorhabditis elegans: temporal misexpression of a collagen:: green fluorescent protein fusion gene. Genetics 149 : 1335–1351.
68. ChapinSC, AppleyardDC, PregibonDC, DoylePS (2011) Rapid microRNA Profiling on Encoded Gel Microparticles. Angew Chem Int Ed 50 : 2289–2293 doi:10.1002/anie.201006523
69. LEERC (2006) Interacting endogenous and exogenous RNAi pathways in Caenorhabditis elegans. RNA 12 : 589–597 doi:10.1261/rna.2231506
70. LEERC (2001) An Extensive Class of Small RNAs in Caenorhabditis elegans. Science 294 : 862–864 doi:10.1126/science.1065329
71. KamathRS, Martinez-CamposM, ZipperlenP, FraserAG, AhringerJ (2001) Effectiveness of specific RNA-mediated interference through ingested double-stranded RNA in Caenorhabditis elegans. Genome Biology 2: RESEARCH0002 doi:10.1186/gb-2000-2-1-research0002
72. ZouY, ChiuH, ZinovyevaA, AmbrosV, ChuangC-F, et al. (2013) Developmental decline in neuronal regeneration by the progressive change of two intrinsic timers. Science 340 : 372–376 doi:10.1126/science.1231321
Štítky
Genetika Reprodukční medicína
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2014 Číslo 4- Růst a vývoj dětí narozených pomocí IVF
- Intrauterinní inseminace a její úspěšnost
- Mateřský haplotyp KIR ovlivňuje porodnost živých dětí po transferu dvou embryí v rámci fertilizace in vitro u pacientek s opakujícími se samovolnými potraty nebo poruchami implantace
- Akutní intermitentní porfyrie
- Souvislost haplotypu M2 genu pro annexin A5 s opakovanými reprodukčními ztrátami
-
Všechny články tohoto čísla
- The Challenges of Mitochondrial Replacement
- Concocting Cholinergy
- Genome-Wide Diet-Gene Interaction Analyses for Risk of Colorectal Cancer
- Statistical Power to Detect Genetic (Co)Variance of Complex Traits Using SNP Data in Unrelated Samples
- Mouse Pulmonary Adenoma Susceptibility 1 Locus Is an Expression QTL Modulating -4A
- Transcription-Associated R-Loop Formation across the Human CGG-Repeat Region
- Epigenetic Regulation by Heritable RNA
- Protein Quantitative Trait Loci Identify Novel Candidates Modulating Cellular Response to Chemotherapy
- Genome-Wide Profiling of Yeast DNA:RNA Hybrid Prone Sites with DRIP-Chip
- The Mechanism of Gene Targeting in Human Somatic Cells
- A LINE-1 Insertion in DLX6 Is Responsible for Cleft Palate and Mandibular Abnormalities in a Canine Model of Pierre Robin Sequence
- Interaction between Two Timing MicroRNAs Controls Trichome Distribution in
- DNA Glycosylases Involved in Base Excision Repair May Be Associated with Cancer Risk in and Mutation Carriers
- The Myc-Mondo/Mad Complexes Integrate Diverse Longevity Signals
- Evolutionarily Diverged Regulation of X-chromosomal Genes as a Primal Event in Mouse Reproductive Isolation
- Mutations in Conserved Residues of the microRNA Argonaute ALG-1 Identify Separable Functions in ALG-1 miRISC Loading and Target Repression
- Genetic Predisposition to In Situ and Invasive Lobular Carcinoma of the Breast
- Isl1 Directly Controls a Cholinergic Neuronal Identity in the Developing Forebrain and Spinal Cord by Forming Cell Type-Specific Complexes
- A Synthetic Community Approach Reveals Plant Genotypes Affecting the Phyllosphere Microbiota
- The Sequence-Specific Transcription Factor c-Jun Targets Cockayne Syndrome Protein B to Regulate Transcription and Chromatin Structure
- Determining the Control Circuitry of Redox Metabolism at the Genome-Scale
- DNA Repair Pathway Selection Caused by Defects in , , and Telomere Addition Generates Specific Chromosomal Rearrangement Signatures
- Methylome Diversification through Changes in DNA Methyltransferase Sequence Specificity
- Folliculin Regulates Ampk-Dependent Autophagy and Metabolic Stress Survival
- Fine Mapping of Dominant -Linked Incompatibility Alleles in Hybrids
- Unexpected Role of the Steroid-Deficiency Protein Ecdysoneless in Pre-mRNA Splicing
- Three Groups of Transposable Elements with Contrasting Copy Number Dynamics and Host Responses in the Maize ( ssp. ) Genome
- Sox5 Functions as a Fate Switch in Medaka Pigment Cell Development
- Synergistic Interactions between the Molecular and Neuronal Circadian Networks Drive Robust Behavioral Circadian Rhythms in
- Chromatin Landscapes of Retroviral and Transposon Integration Profiles
- Widespread Use of Non-productive Alternative Splice Sites in
- Ras GTPase-Like Protein MglA, a Controller of Bacterial Social-Motility in Myxobacteria, Has Evolved to Control Bacterial Predation by
- Cell Type-Specific Functions of Genes Revealed by Novel Adipocyte and Hepatocyte Circadian Clock Models
- Phenotype Ontologies and Cross-Species Analysis for Translational Research
- Embryogenesis Scales Uniformly across Temperature in Developmentally Diverse Species
- In Pursuit of the Gene: An Interview with James Schwartz
- Molecular Mechanisms of Hypoxic Responses via Unique Roles of Ras1, Cdc24 and Ptp3 in a Human Fungal Pathogen
- Analysis of the Genome and Transcriptome of var. Reveals Complex RNA Expression and Microevolution Leading to Virulence Attenuation
- Genotypic and Functional Impact of HIV-1 Adaptation to Its Host Population during the North American Epidemic
- RNA Editome in Rhesus Macaque Shaped by Purifying Selection
- Proper Actin Ring Formation and Septum Constriction Requires Coordinated Regulation of SIN and MOR Pathways through the Germinal Centre Kinase MST-1
- Interplay of the Serine/Threonine-Kinase StkP and the Paralogs DivIVA and GpsB in Pneumococcal Cell Elongation and Division
- A Quality Control Mechanism Coordinates Meiotic Prophase Events to Promote Crossover Assurance
- CNNM2 Mutations Cause Impaired Brain Development and Seizures in Patients with Hypomagnesemia
- The RNA-Binding Protein QKI Suppresses Cancer-Associated Aberrant Splicing
- Uncoupling Transcription from Covalent Histone Modification
- Rad51–Rad52 Mediated Maintenance of Centromeric Chromatin in
- FRA2A Is a CGG Repeat Expansion Associated with Silencing of
- A General Approach for Haplotype Phasing across the Full Spectrum of Relatedness
- A Novel Highly Divergent Protein Family Identified from a Viviparous Insect by RNA-seq Analysis: A Potential Target for Tsetse Fly-Specific Abortifacients
- A Central Role for in Regulation of Islet Function in Man
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- The Sequence-Specific Transcription Factor c-Jun Targets Cockayne Syndrome Protein B to Regulate Transcription and Chromatin Structure
- The Mechanism of Gene Targeting in Human Somatic Cells
- Genetic Predisposition to In Situ and Invasive Lobular Carcinoma of the Breast
- Widespread Use of Non-productive Alternative Splice Sites in
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Revma Focus: Spondyloartritidy
nový kurz
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



