-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Is Required for Formation of the Genital Ridge in Mice
In mammals, both testis and ovary arise from a sexually undifferentiated precursor, the genital ridge, which first appears during mid-gestation as a thickening of the coelomic epithelium on the ventromedial surface of the mesonephros. At least four genes (Lhx9, Sf1, Wt1, and Emx2) have been demonstrated to be required for subsequent growth and maintenance of the genital ridge. However, no gene has been shown to be required for the initial thickening of the coelomic epithelium during genital ridge formation. We report that the transcription factor GATA4 is expressed in the coelomic epithelium of the genital ridge, progressing in an anterior-to-posterior (A-P) direction, immediately preceding an A-P wave of epithelial thickening. Mouse embryos conditionally deficient in Gata4 show no signs of gonadal initiation, as their coelomic epithelium remains a morphologically undifferentiated monolayer. The failure of genital ridge formation in Gata4-deficient embryos is corroborated by the absence of the early gonadal markers LHX9 and SF1. Our data indicate that GATA4 is required to initiate formation of the genital ridge in both XX and XY fetuses, prior to its previously reported role in testicular differentiation of the XY gonad.
Published in the journal: . PLoS Genet 9(7): e32767. doi:10.1371/journal.pgen.1003629
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1003629Summary
In mammals, both testis and ovary arise from a sexually undifferentiated precursor, the genital ridge, which first appears during mid-gestation as a thickening of the coelomic epithelium on the ventromedial surface of the mesonephros. At least four genes (Lhx9, Sf1, Wt1, and Emx2) have been demonstrated to be required for subsequent growth and maintenance of the genital ridge. However, no gene has been shown to be required for the initial thickening of the coelomic epithelium during genital ridge formation. We report that the transcription factor GATA4 is expressed in the coelomic epithelium of the genital ridge, progressing in an anterior-to-posterior (A-P) direction, immediately preceding an A-P wave of epithelial thickening. Mouse embryos conditionally deficient in Gata4 show no signs of gonadal initiation, as their coelomic epithelium remains a morphologically undifferentiated monolayer. The failure of genital ridge formation in Gata4-deficient embryos is corroborated by the absence of the early gonadal markers LHX9 and SF1. Our data indicate that GATA4 is required to initiate formation of the genital ridge in both XX and XY fetuses, prior to its previously reported role in testicular differentiation of the XY gonad.
Introduction
In male and female mammals alike, the embryonic gonad initially forms as a sexually bipotential structure called the genital ridge. The genital ridge subsequently differentiates to become a testis or an ovary.
Formation of the genital ridge begins with increasing proliferation of coelomic epithelial cells to establish a dense and pseudostratified layer on the ventromedial surface of the mesonephros [1]–[4]. At about the same time, the underlying basement membrane becomes fragmented, allowing the epithelial cells to migrate inward and form a thickened, multilayered structure [5]–[7]. These morphological changes, which first appear anteriorly and gradually extend in a posterior direction, create the genital ridge [1], [3], [4], [8].
In mouse embryos, formation of the genital ridge starts at about embryonic day 10.5 (E10.5) and continues until E11.5-E12.0, when sexual differentiation of the gonad becomes evident [9], [10]. Full development of the genital ridge requires a set of genes that includes Steroidogenic factor 1 (Sf1) [11], Lim homeobox protein 9 (Lhx9) [12], Wilms tumor 1 (Wt1) [13], and Empty spiracles homeobox 2 (Emx2) [7], [14]. In mouse embryos homozygous for a null mutation in any one of these genes, the coelomic epithelial layer shows initial thickening but regresses before the genital ridge is fully formed. Deficiency of either Sf1 or Wt1 results in the death of somatic cells within the developing genital ridge, whereas loss of Lhx9 disrupts proliferation of these cells [11], [12], [15], [16]. Emx2 deletion impairs the migration of epithelial cells through the basement membrane to form a multilayered structure [7]. Each of these genes is thus required for growth and maintenance of the genital ridge, but not for its initial formation.
How the coelomic epithelium begins to differentiate into the genital ridge remains unknown. Previously reported mouse mutants that lack gonads during fetal development show signs of epithelial thickening or various levels of gonadal development before its regression. Examples include those mentioned above and Osr1-null embryos [17] (Y.C. Hu and D.C. Page, unpublished data). Mouse mutants lacking the epithelial thickening that heralds the genital ridge have not yet been reported.
GATA4 is an evolutionarily conserved transcription factor that is essential for early development of multiple organs, including heart, foregut, liver, and ventral pancreas [18]–[20]. Interestingly, Gata4 is also expressed in the genital ridge, and this expression pattern is conserved across many organisms, including mammals [21]–[23], chicken [24], fish [25], and turtles [26]. Gata4's expression in the genital ridge has been linked to its role in testis differentiation. Specifically, GATA4, together with WT1, synergistically activates transcription of Sry [27], which triggers testicular differentiation of the genital ridge. In XY mouse embryos homozygous for a Gata4 knock-in allele (Gata4ki) that abrogates GATA4 binding to the cofactor FOG2 (or FOG1), genital ridges form, but further differentiation into testes is blocked, and the transcriptional program downstream of Sry is greatly attenuated [28]. Mouse embryos heterozygous for Gata4ki on specific genetic backgrounds also show sex reversal of genetic males to phenotypic females [29]. In another study where Gata4 was removed conditionally from XY genital ridges after E10.5, subsequent testis differentiation was disrupted and ovarian somatic markers were upregulated [30]. These studies clearly indicate a requirement for Gata4 in testis determination and differentiation.
In this study, we investigated whether Gata4 plays a role in formation of the genital ridge, prior to the point of testis determination. Gata4-null embryos die before the genital ridges form [19], [20], so we utilized the tamoxifen-inducible Cre/loxP system to conditionally delete Gata4 in mouse embryos after E8.75. Our approach allows mutant embryos to survive to ∼E11.5, thus providing us an opportunity to investigate Gata4's role in early gonadal development. Here we report that embryos conditionally deficient for Gata4 show neither coelomic epithelial thickening nor expression of the early gonadal differentiation factors LHX9 and SF1. Therefore, our study indicates that Gata4 is required for formation of the genital ridge, prior to its previously reported role in testis determination.
Results
GATA4 expression precedes thickening of genital ridge epithelium and progresses in an A-P direction
The earliest stage of gonadogenesis is characterized by epithelial thickening, which begins in mouse embryos at ∼E10.5 (8 tail somite [ts] stage) [5], [7]. According to morphological studies, formation of the genital ridge progresses in an A-P direction along the axis of the mesonephros [1]. If GATA4 is involved in the signaling pathway that drives genital ridge formation, it should be expressed in the coelomic epithelium before initial thickening and ideally in a similar A-P direction. In order to investigate the timing and location of GATA4 expression, we performed whole-mount immunofluorescence on C57BL/6 embryos at E10.0, E10.2 and E10.4.
The nascent gonads first appear as a paired coelomic epithelial layer lying along the surface of the mesonephroi, lateral to the dorsal mesentery, and extending between the front and hind limbs. After dissection to expose the entire length of the genital ridges, we were able to image them longitudinally by confocal laser scanning microscopy (Figure 1A). We found that GATA4 protein was present in the anterior half of the genital ridge as early as ∼E10.0 (26–27 total somites) and extended to the posterior half by ∼E10.2 (2 ts) (Figure 1B). Thus, expression of GATA4 protein progressed in an A-P direction. This expression did not extend into the metanephric region (Figure S1), suggesting that GATA4 is restricted to the region that will later form the genital ridge.
Fig. 1. GATA4 expression precedes coelomic epithelial thickening and progresses from anterior to posterior. 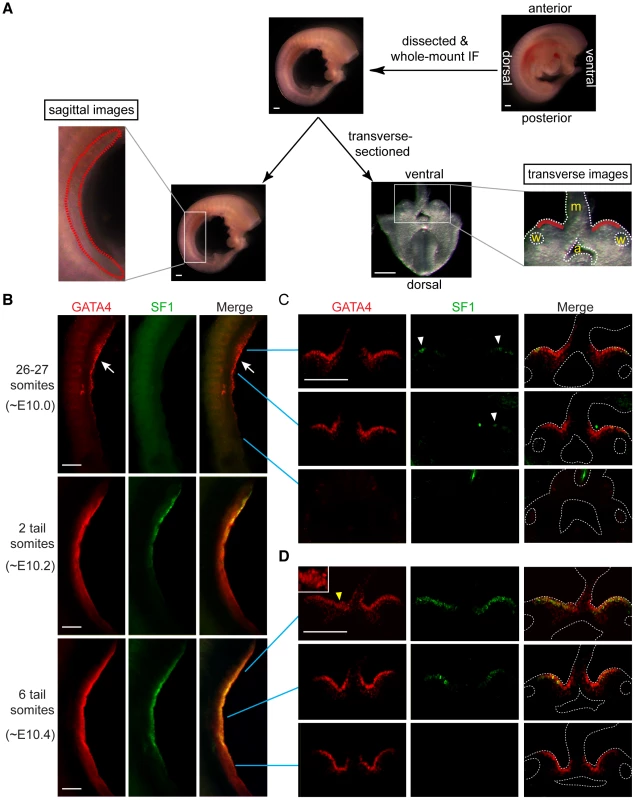
(A) Schematic representation of experiment. Mouse embryos were dissected to remove limbs, body walls and internal organs, and then subjected to whole-mount immunofluorescence (IF) staining with GATA4 and SF1 antibodies. Stained embryos were imaged sagittally by confocal microscopy, and then transversely (following transverse section), again by confocal microscopy. Red dashed and solid lines in, respectively, sagittal and transverse images indicate location of developing gonads. a, dorsal aorta; m, mesentery; w, Wolffian duct. (B–D) Expression analysis of GATA4 (red) and SF1 (green) protein during early gonadogenesis. GATA4 expression in coelomic epithelia of genital ridges begins in anterior (arrow) and then spreads posteriorly. Epithelial thickening is observed in anterior region of genital ridge at 6-tail-somite stage (yellow arrowhead and inset). SF1 (white arrowheads) is expressed only sporadically in anterior half at 26–27 somite stage. Scale bars: 50 µm. Sf1 has been suggested as one of the earliest markers of gonadal cells [31]. Therefore, we compared the expression of GATA4 and SF1 proteins in the same tissues. In the sagittal images, SF1 was clearly detected at the anterior half of the genital ridge at ∼E10.2 (2 ts) and later extended into the posterior half (Figure 1B). Similar to GATA4, SF1 was also expressed in an A-P direction. However, expression of GATA4 preceded expression of SF1.
To confirm and extend these observations, we transversely sectioned the stained embryos with a vibratome and took sections from anterior, middle, and posterior parts of the genital ridges for confocal imaging (Figure 1A). Consistent with the results from the sagittal images, at ∼E10.0 GATA4 was expressed homogeneously in the coelomic epithelial layer of the genital ridge from the anterior to the middle portions, while SF1 was expressed only sporadically in the same areas (Figure 1C, white arrowheads). At ∼E10.4 (6 ts), GATA4 expression had already spread to the posterior end of the genital ridge, while SF1 expression had spread only to the middle region (Figure 1D). Therefore, the A-P expression of GATA4 was earlier than that of SF1, suggesting the possibility that GATA4 might function upstream of SF1. In addition, we consistently observed that the coelomic epithelium at the anterior of the ridge was developmentally more advanced than that at the posterior. For instance, at ∼E10.4, the coelomic epithelium already showed more than one layer of cells in the anterior region (Figure 1D, yellow arrowhead), while it remained a monolayer in the middle-to-posterior areas. Taken together, these findings demonstrate that GATA4 is expressed in the genital ridge epithelium in an A-P direction and just before thickening occurs, thus fitting the profile of a candidate gene that regulates the formation of the genital ridge.
Gata4 is required for genital ridge formation
Having observed that expression of GATA4 correlates with genital ridge formation, we wanted to know whether GATA4 is required for the earliest event, the initial thickening of the coelomic epithelium. Gata4-null embryos die between E7.5 and E9.5 [19], [20], so we used the tamoxifen-inducible Cre/loxP system to inactivate Gata4 gene activity in a temporally specific manner. We first intercrossed CAG-CreER; Gata4+/Δ male to Gata4flox/flox female mice to produce embryos carrying CAG-CreER; Gata4flox/Δ, where the CAG promoter drives ubiquitous expression of CreER [32]. We then injected the dams with tamoxifen at E8.75 days to generate Gata4 conditional knockout embryos, hereafter referred to as Gata4 cKO (CAG-CreER). Gata4flox/+ embryos from the same litters served as controls.
We first examined whether GATA4 expression was ablated by our conditional deletion strategy, and whether the epithelial thickening was affected in mutant embryos. Following tamoxifen treatment, Gata4 cKO (CAG-CreER) embryos died between E11.0 and E11.5, likely due to the requirement for Gata4 in early development of multiple organs, including the heart, gut, and liver. We therefore collected embryos at ∼E10.7 (10 ts). In control embryos, GATA4 was clearly detected in the thickened coelomic epithelium of the developing gonad (Figure 2A, arrows) as well as in cells of the genital mesenchyme, mesentery, and gut endoderm. In Gata4 cKO (CAG-CreER) embryos, GATA4 was almost undetectable in the corresponding coelomic epithelium and in the rest of the embryo section, indicating that the tamoxifen-induced Gata4 deletion was nearly complete (Figure 2B). We found that the coelomic epithelium of mutant embryos remained as a single layer of cells (Figure 2B), suggesting that initial thickening was disrupted by the loss of Gata4.
Fig. 2. Gata4 is required for thickening of coelomic epithelium that gives rise to genital ridge. 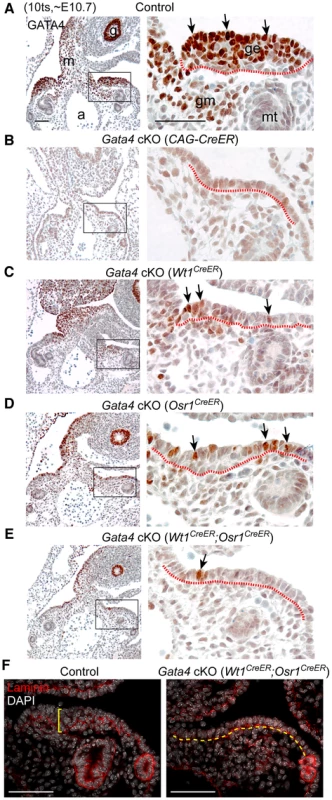
(A–E) IHC staining for GATA4 protein in transverse sections of control and Gata4 cKO embryos. Sections were chosen to represent similar anterior positions in genital ridges. Right panel shows higher magnification of boxed area in left panel. Arrows indicate examples of positive GATA4 staining. Red dashed lines mark boundary between coelomic epithelium and underlying mesenchyme. Control and conditional mutant embryos, except for Gata4 cKO (CAG-CreER), were from the same litter. a, dorsal aorta; g, gut endoderm; ge, genital ridge epithelium; gm, genital mesenchyme; m, mesentery; mt, mesonephric tubule. (F) Immunofluorescent staining for laminin protein in sections of control and Gata4 cKO embryos. Laminin marks basement membrane. Control genital ridge shows thickened epithelial layer (yellow bracket), whereas the coelomic epithelium in Rb cKO embryo remains a single-cell layer (yellow dashed line). Scale bars: 50 µm. Given that Gata4 has a widespread function in organogenesis, we wanted to confirm that the lack of epithelial thickening is a direct consequence of Gata4 removal in the genital ridges instead of a secondary effect caused by ubiquitous deletion of Gata4 via CAG-CreER. We used two tissue-specific CreER systems, Osr1-eGFP-CreERt2 [33] and Wt1-CreERt2 [34], to replace the CAG-CreER in the experiment described above. In these two systems, eGFP-CreERt2 and CreERt2 cassettes were targeted into the endogenous Osr1 and Wt1 loci, respectively, to create knock-in alleles. Osr1 is expressed in the intermediate mesoderm and its derivatives, including genital ridges, with peak expression between E8.5 and E9.5 [35]. The gene Wt1 is thought to act downstream of Osr1 and is detected in the urogenital ridges starting at ∼E9.5 [17], [36]. Wt1 and Osr1 are also expressed in the embryonic heart and some other tissues during the time frame of the study. The temporal and spatial activity of the CreERt2 cassettes in Osr1eGFP-CreERt2/+ and Wt1CreERt2/+ embryos correlated well with that of the respective endogenous genes, as evaluated by the CAG-LSL-tdTomato reporter using Gt(ROSA)26SorCAG-tdTomato mice (data not shown). We then introduced these knock-in alleles into Gata4+/Δ males, which were bred with Gata4flox/flox females to produce Gata4 conditional mutant embryos. Gata4 deletion efficiency in the genital ridge was assayed by immunohistochemical staining for GATA4 protein.
Following the previously described tamoxifen injection scheme, we assessed GATA4 expression and gonadal phenotype of the mutant embryos carrying Wt1CreERt2/+; Gata4flox/Δ or Osr1eGFP-CreERt2/+; Gata4flox/Δ, hereafter referred to as Gata4 cKO (Wt1CreER) and Gata4 cKO (Osr1CreER), respectively. Compared to the controls at E10.7 (10 ts), the number of GATA4-expressing cells was greatly reduced in the mutant coelomic epithelium, while GATA4 expression in the mesentery and gut endoderm remained unaffected (Figure 2C, 2D). Thickening of the coelomic epithelium was much less prominent in both mutants (Figure 2C, 2D). To improve the efficiency of tamoxifen-induced Gata4 deletion, we introduced both knock-in alleles into the same animals, hereafter referred to as Gata4 cKO (Wt1CreER;Osr1CreER). As expected, we obtained a greater degree of reduction in the number of GATA4-expressing cells in the coelomic epithelium of Gata4 cKO (Wt1CreER;Osr1CreER) embryos, and the coelomic epithelium remained as a single cell layer of cells (Figure 2E). However, it should be noted that the efficiency of Wt1CreER - and/or Osr1CreER-mediated Gata4 deletion varied among mutants. Only mutants that displayed a high degree of Gata4 deletion were chosen for detailed study, and these embryos died between E11.0 and E11.5. Mutant embryos that survived beyond E12.0 did not show sufficient deletion of Gata4 to block gonadal initiation.
To assess the degree of epithelial thickening in control and mutant embryos, we determined the number of cell layers by staining for the basement membrane with an antibody against laminin. While control genital ridges had grown to a multilayered epithelial structure, the mutant coelomic epithelium remained a single cell layer without clear signs of thickening (Figure 2F). Thus, we conclude that Gata4 is essential for the initial thickening of the coelomic epithelium that gives rise to the genital ridge. Note that the absence of this thickening was seen in both XX and XY Gata4 cKO mutants, which is consistent with the fact that genital ridge formation precedes gonadal sex differentiation.
Gata4 deficiency impairs epithelial proliferation and basement membrane fragmentation
Epithelial thickening is first observed around E10.3–E10.4 (5–6 ts) at the anterior genital ridge. It has been reported that an increase in proliferation of the coelomic epithelium immediately precedes the thickening event [1], [3], [4], [37]. Therefore, we tested whether the lack of epithelial thickening in Gata4 cKO embryos is caused by lack of epithelial cell proliferation. We collected BrdU-treated control and Gata4 cKO (Wt1CreER;Osr1CreER) embryos at two different time points: ∼E10.3 (3–5 ts) and ∼E10.7 (9–11 ts), representing just before and after the occurrence of epithelial thickening. After immunostaining sections with antibodies against BrdU and laminin (Figure 3A), we analyzed the fraction of gonadal somatic cells that had incorporated BrdU. We found that the control epithelium exhibited a significantly higher proliferation rate, compared to the Gata4 cKO (Wt1CreER;Osr1CreER) epithelium, just before thickening occurred (Figure 3B). After thickening had taken place, the control epithelium proliferated at a similar rate as the mutant epithelium (Figure 3B). These data suggest that Gata4 is required to increase the proliferation of the coelomic epithelium, which seemingly contributes to the subsequent thickening event.
Fig. 3. Gata4 deficiency impairs epithelial proliferation and basement membrane breakdown. 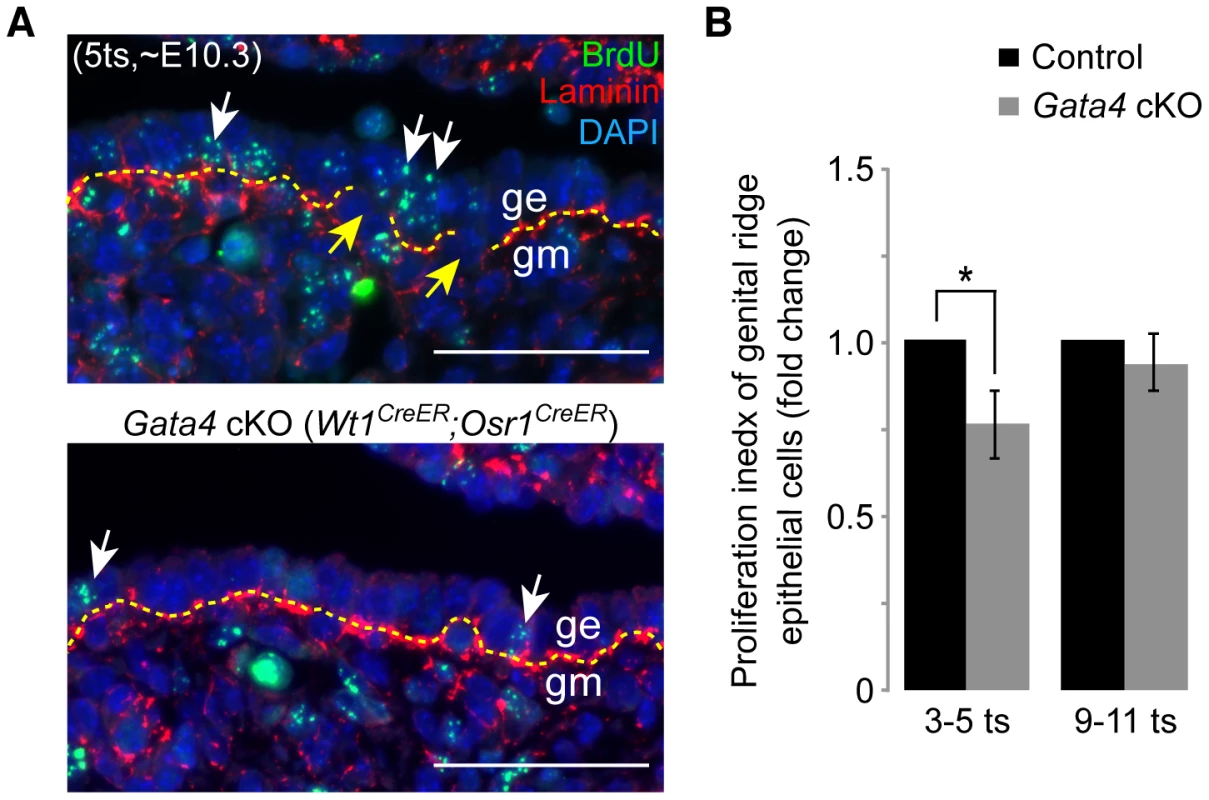
(A) Immunofluorescent staining for BrdU (green) and laminin (red) in sections of control and Gata4 cKO (Wt1CreER;Osr1CreER) embryos where tamoxifen and BrdU were injected at, respectively, E8.75 and ∼E10.0 (6 hours before sacrifice). Yellow dashed lines mark basement membrane. Yellow arrows mark discontinuities in basement membrane in control genital ridge. White arrows indicate representative BrdU-positive epithelial cells. Nuclei stained with DAPI (blue). ge, genital ridge epithelium; gm, genital mesenchyme. Scale bars: 50 µm. (B) Relative proliferation index, comparing the fractions of coelomic epithelial cells positive for BrdU in control and Gata4 cKO (Wt1CreER;Osr1CreER) embryos of the same sex, from the same litter. The index in controls was set at 1. Germ cells were excluded from the counting. At each of the two stages shown, three pairs of control and Gata4 cKO embryos were studied. Plotted here are means ± standard deviation. *, P<0.05 (two-tailed Student's t-test). For thickening to occur, the basement membrane underneath the coelomic epithelium has to lose its continuity, permitting epithelial cells to migrate inward to form additional layers. To determine the integrity of the basement membrane underneath control and Gata4 cKO (Wt1CreER;Osr1CreER) coelomic epithelia, we labeled embryo sections with laminin antibody. We observed that the basement membrane in the controls had become fragmented by ∼E10.3 (5 ts) (Figure 3A, yellow arrows). In contrast, the basement membrane in the mutants remained continuous, thus hindering the thickening process. Therefore, Gata4 cKO coelomic epithelium does not show any features of gonadal differentiation.
To determine whether increased cell death also contributed to the lack of epithelial thickening in Gata4 cKO embryos, we measured cell apoptosis by TUNEL staining. We found that mutant coelomic epithelial layers showed slightly higher TUNEL labeling indices compared to controls, but the difference did not reach statistical significance (data not shown).
Expression of LHX9 and SF1 is dependent on Gata4
To substantiate the finding that Gata4 deficiency disrupts formation of the genital ridge, we determined whether expression of early gonadal regulators was affected in Gata4 cKO mutants. Each of the genes Lhx9, Sf1, Wt1, and Emx2 is known to control the growth and maintenance of the genital ridge [11]–[14]. Lhx9 and Sf1 are expressed specifically in the coelomic epithelium of the developing genital ridge, whereas Wt1 and Emx2 are expressed more broadly. Having found that GATA4 is primarily expressed in the coelomic epithelial layer of the genital ridge during the initiation period (Figure 1), we first tested whether LHX9 and SF1 are regulated by Gata4, using whole-mount immunofluorescence. We examined embryos with near complete deletion of Gata4 at ∼E10.7–E10.8 for the experiment.
In control embryos, both GATA4 and LHX9 were co-expressed and co-localized in the epithelial cells along the length of the genital ridges (Figure 4A, 4B). By contrast, the Gata4 cKO (Wt1CreER) coelomic epithelium showed minimal expression of GATA4 and LHX9 (Figure 4A, 4B). There was some residual expression at the epithelium of the dorsal mesentery. Likewise, in control genital ridges, SF1 was restricted to the thickened epithelial layer, and all SF1-positive cells were also GATA4-positive (Figure 4C). SF1 did not extend to the dorsal mesentery, where many cells expressed GATA4 alone. By contrast, SF1 was absent in the coelomic epithelium of both Gata4 cKO (Wt1CreER) and Gata4 cKO (CAG-CreER) embryos (Figure 4C and Figure S2). These results suggest that Gata4 is required for expression of LHX9 and SF1, and that these two factors act downstream of GATA4. Notably, Gata4 cKO mutants that failed to express LHX9 or SF1 also did not exhibit thickening of the coelomic epithelium (Figure 4B, 4C, yellow brackets).
Fig. 4. Gata4 is required for expression of early gonadal differentiation regulators LHX9 and SF1. 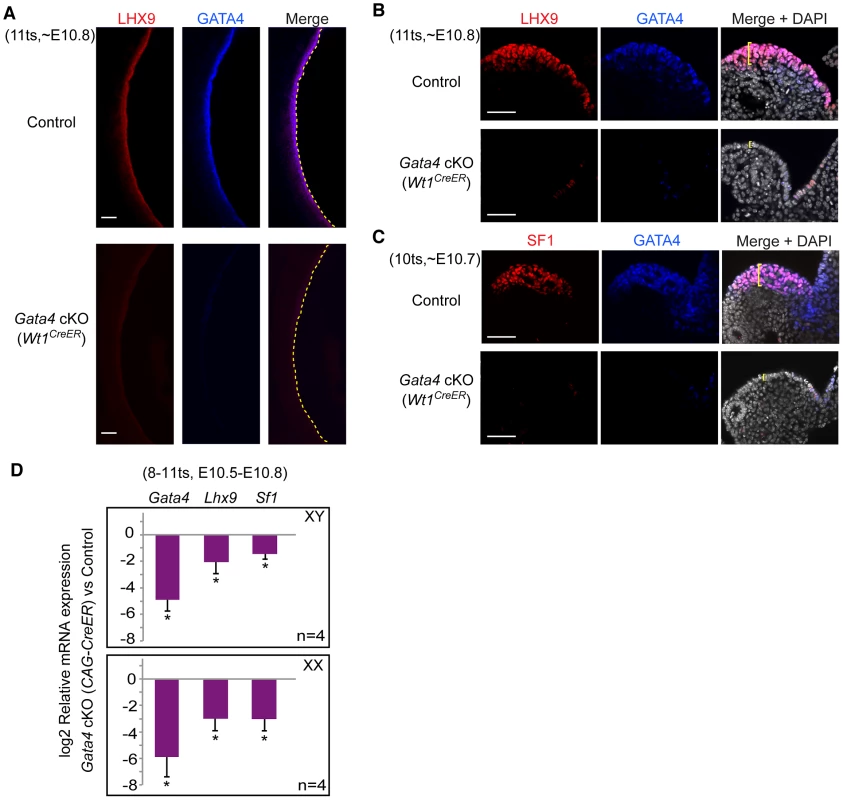
(A–C) Whole-mount immunofluorescent staining for LHX9, SF1, and GATA4 protein in control and Gata4 cKO (Wt1CreER) embryos. Confocal images were taken either sagittally (A) or transversely (B and C). Yellow dashed lines outline coelomic epithelial surface. Yellow brackets indicate thickness of epithelial layer. Scale bars: 50 µm. (D) Quantitative analysis of Gata4, Lhx9, and Sf1 mRNA levels in control and Gata4 cKO (CAG-CreER) urogenital ridges. Plotted here are means ± standard deviation from biological replicates (all values normalized to beta-actin). *, P<0.001 (two-tailed Student's t-test). To further confirm the loss of Lhx9 and Sf1 expression in the absence of Gata4, we measured the mRNA expression levels of these genes in XY and XX urogenital ridges that contained gonad/mesonephros complexes and the dorsal part of the mesentery. In this experiment, we used Gata4 cKO embryos carrying CAG-CreER, instead of Wt1CreER or Osr1CreER, to avoid any potential influence of Osr1 or Wt1 haploinsufficiency on gonadal gene expression. As expected, at E10.5–E10.8 (8–11 ts), mRNA for Lhx9 and Sf1 was significantly reduced in the Gata4 cKO samples of both sexes (Figure 4D). These findings indicate that Gata4 cKO coelomic epithelial cells fail to express the early gonadal differentiation regulators Lhx9 and Sf1, supporting the hypothesis that Gata4 is required for initiation of genital ridge formation.
In contrast to the dependence of LHX9 and SF1 expression on Gata4, both WT1 and EMX2 were expressed in the coelomic epithelium of Gata4 cKO (Wt1CreER;Osr1CreER) and Gata4 cKO (CAG-CreER) embryos (Figure 5 and Figure S3). In addition, the overall expression patterns of WT1 and EMX2 in the mesonephric region were indistinguishable between control and Gata4 cKO embryos. Therefore, WT1 and EMX2 are not downstream of GATA4. Although both Wt1 and Emx2 play an essential role in genital ridge development, they are not required to initiate the process 13,14. Likewise, Gata4, which is required for initiation, is present in both Wt1 - and Emx2-deficient genital ridges [7], [38]. Thus, regulation of Wt1 and Emx2 is independent of Gata4 in gonadogenesis, and vice versa.
Fig. 5. Gata4 is not required for expression of WT1 and EMX2 in genital ridge epithelium. 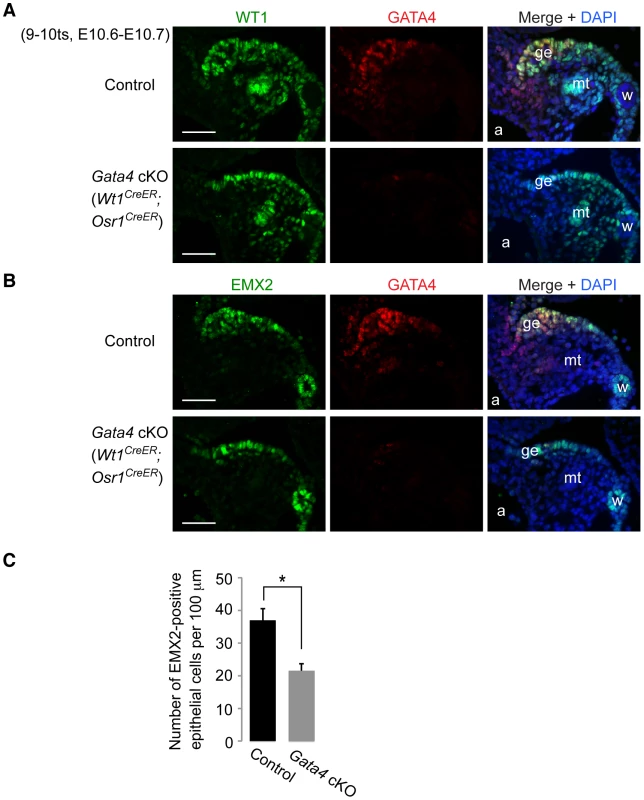
(A and B) Immunofluorescent staining for WT1, EMX2, and GATA4 protein in transverse sections of control and Gata4 cKO (Wt1CreER;Osr1CreER) embryos. a, dorsal aorta; ge, genital ridge epithelium; mt, mesonephric tubule; w, Wolffian duct. Scale bars: 50 µm. (C) Comparison of numbers of EMX2-positive cells per 100 µm (along length of epithelial surface) in control and Gata4 cKO (Wt1CreER;Osr1CreER) embryos. Cells were counted on transverse sections of embryos taken at similar A-P positions. Plotted here are means ± standard deviation from biological replicates (n = 3 for each genotype). *, P = 0.003 (two-tailed Student's t-test). The presence of EMX2 staining in the coelomic epithelium of Gata4 cKO embryos allowed us to quantitate the reduction in epithelial cell number in Gata4 cKO embryos, compared to controls. We counted the number of EMX2-positive coelomic epithelial cells per unit length. By E10.6–10.7, the EMX2-positive epithelial layer in Gata4 cKO (Wt1CreER;Osr1CreER) embryos contained about half the number of cells seen in controls (Figure 5C), reflecting the absence of epithelial thickening in Gata4 cKO embryos.
Gata4 deficiency does not interfere with primordial germ cell migration
Primordial germ cells (PGCs) migrate from the base of the allantois to the developing genital ridge, arriving there between E10.0 and E11.5 [39]–[41]. A defect in genital ridge growth does not block PGC migration in Lhx9−/−, Sf1−/−, Wt1−/−, and Emx2−/− mutants [11]–[14]. One possible explanation is that the initial genital ridge formation seen in these mutants is sufficient to attract PGCs. Having shown that the genital ridge does not begin to form in the absence of Gata4, we tested whether migration of PGCs was affected in Gata4 cKO embryos. We performed whole-mount immunofluorescence on embryos at E10.3 (3 ts), when PGCs are arriving at the genital ridges. In control embryos (Gata4flox/+;Oct4-EGFP), a longitudinal view showed that GATA4-expressing cells were present along the entire length of the genital ridge, and PGCs marked by an Oct4-EGFP transgene [42] had arrived in the coelomic epithelium of the genital ridge at this time point (Figure 6). In Gata4 cKO (Wt1CreER);Oct4-EGFP embryos, GATA4 was absent yet PGCs still migrated to the corresponding coelomic epithelium despite the absence of genital ridge formation (Figure 6). We next looked at transverse sections of mesonephric regions from additional lines, Gata4 cKO (CAG-CreER) and Gata4 cKO (Wt1CreER;Osr1CreER). We found that PGCs, labeled with germ cell marker SSEA1, had migrated to the coelomic epithelium on the ventromedial side of the mesonephros despite the absence there of the epithelial thickening that would normally mark the beginning of genital ridge formation (Figure S4). These results suggest that neither Gata4 nor gonadal development is required for migration of PGCs during embryogenesis.
Fig. 6. Migration of primordial germ cells is unaffected in Gata4 cKO embryos. 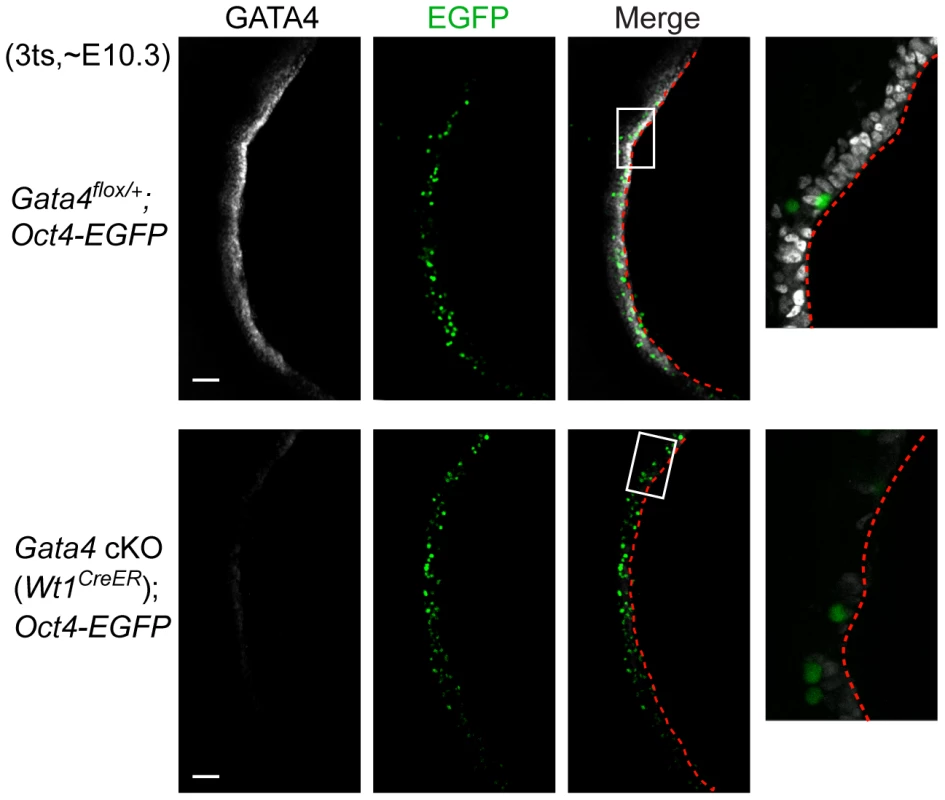
Whole-mount immunofluorescent staining for GATA4 protein (grey) in control and Gata4 cKO (Wt1CreER) embryos. Confocal images were taken sagittally for a longitudinal view of the genital ridge. Oct4-EGFP transgene marks germ cells (green). Panels on far right provide higher magnification views of boxed areas. Red dashed lines outline the coelomic epithelial surface. Scale bars: 50 µm. Discussion
The genital ridge is the sexually undifferentiated, or bipotential, precursor of testis or ovary. We explored the question of how formation of the genital ridge is initiated. More specifically, we investigated the genetic regulation of the thickening of the coelomic epithelium, which constitutes the first step in gonadogenesis. Our data show that GATA4 expression in the coelomic epithelium precedes thickening and progresses in an A-P direction, correlating well with the A-P progression of genital ridge formation [1]. When we conditionally deleted Gata4 from the E8.75 embryos, we found that formation of the genital ridge was Gata4-dependent. Our results were consistent using a number of different tamoxifen-inducible Cre drivers. Collectively, in the absence of Gata4, we did not observe the cellular changes critical for thickening of the coelomic epithelium, such as increasing proliferation and basement membrane fragmentation. Therefore, the coelomic epithelium shows no features of gonadal differentiation in the absence of Gata4. Failure of genital ridge formation is further confirmed by the lack of LHX9 and SF1 expression in Gata4 cKO embryos. Thus, Gata4 is the first genetic regulator shown to be required for the initiation of gonadogenesis (Figure 7).
Fig. 7. A proposed model for formation of the genital ridge. 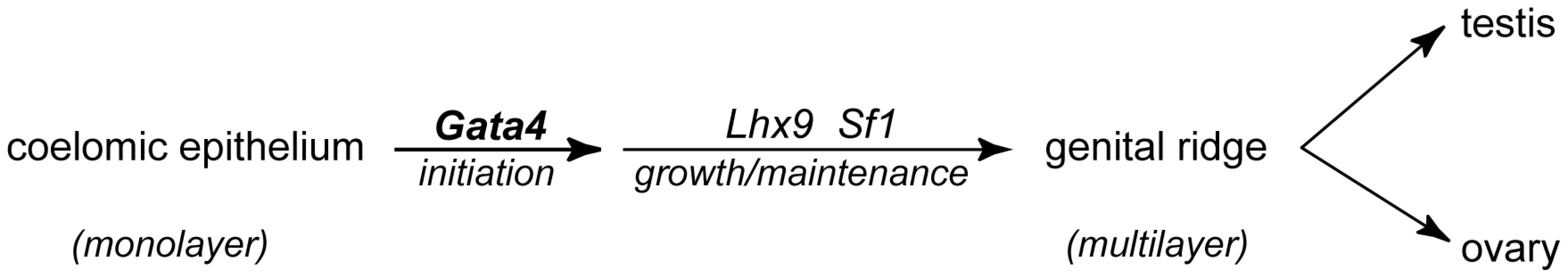
Formation of the testis or ovary begins when Gata4-dependent thickening of the coelomic epithelium gives rise to the genital ridge. LHX9 and SF1, acting downstream of GATA4, are subsequently induced, promoting growth and maintenance of the genital ridge. WT1 and EMX2 (not shown), though widely expressed in the genital ridge, are not dependent on Gata4 and likely function in parallel to support growth and maintenance of the genital ridge. The genital ridge then develops as either an ovary or a testis, depending on the sex chromosome constitution of the embryo. Gata4 has been previously studied as an important regulator of the testis determination pathway [27]–[30]. Correct dosage of fully functional Gata4 is critical for properly initiating the testis differentiation program in the genital ridge. Manuylov et al. showed that when Gata4 deletion was induced by tamoxifen injection at E10.5 via Wt1CreER, testis differentiation was blocked and sex reversal was observed [30]. When Gata4 deletion was induced later, at E11.5, testis formation occurred although the testicular cords were somewhat disorganized [30]. In the present study, we induced Gata4 deletion by tamoxifen injection at an earlier time point, E8.75, and noted the complete absence of genital ridge formation in both XX and XY embryos. These results suggest that Gata4 plays at least two crucial roles in early gonadal development: initiation of genital ridge formation, followed by testis differentiation of the genital ridge. Therefore, the specific timing of Gata4 loss leads to divergent phenotypes in mouse embryonic gonads. Moreover, previous studies of the Gata4ki allele have indicated the importance of GATA4-FOG interaction in mouse testis differentiation [28], [29]. Because the genital ridge is still formed in Gata4ki animals, GATA4 likely exerts its specific function in gonadal initiation independent of its interaction with FOG cofactors.
Our Gata4 cKO embryos died before ∼E11.5, due to broad Gata4 deletion induced by the CreER or CreERt2 drivers used in the present study. This early death prevented us from studying sexual differentiation and later development of embryos that lack a gonad. We did not observe any signs of coelomic epithelial thickening, or expression there of LHX9 or SF1, in Gata4 cKO embryos. However, we cannot rule out the possibility that the onset of gonadogenesis may simply be delayed in these mutants. Therefore, generation of a genital ridge-specific Cre mouse line will be necessary for future studies.
We have shown that Lhx9 and Sf1 are not expressed in the coelomic epithelium of Gata4 cKO embryos, indicating that expression of these genes depends on Gata4. Given that the promoters of both genes contain consensus GATA4 binding sites [43], [44], it is plausible that GATA4 may directly activate Lhx9 and Sf1 transcription in the coelomic epithelium. However, GATA4 is known to function as both a transcriptional activator and a transcriptional repressor. For instance, in the epicardial mesothelium, GATA4, together with its cofactor FOG2, suppresses Lhx9 expression at E11.5 by binding directly to conserved binding sites in the promoter [43]. Furthermore, an in vitro biochemical study suggests that GATA4 can either activate or inhibit the activity of the Sf1 promoter through the consensus binding site depending on the cellular environment [44]. In the cellular context of the coelomic epithelium, it is possible that GATA4 directly stimulates the Lhx9 and Sf1 promoters by cooperating with a specific set of cofactors. Another possibility is that GATA4 indirectly activates transcription of Lhx9 and Sf1 through repression of their repressors.
The regulation of Lhx9 expression is rather specific to Gata4, as Lhx9 expression is affected in Gata4 cKO but not in Sf1−/−, Wt1−/−, and Emx2−/− mutants [7], [12], [45]. In contrast, Sf1 expression is inactivated in the coelomic epithelia of all known mutants defective in genital ridge development, including Lhx9−/−, Wt1−/−, Emx2−/−, and Gata4 cKO embryos [7], [12], [45], implying that Sf1 may be a common downstream target of multiple signaling cascades that control the formation and growth of the genital ridge. Indeed, the Sf1 promoter contains functional binding sites for multiple factors, including GATA4, WT1, and LHX9 [44], [45]. We also found that expression of SF1 is restricted to the genital ridge epithelium, while expression of GATA4 and LHX9 extends to the dorsal end of the mesentery area (Figure 1D and Figure 4B, 4C) [7]. Therefore, these data imply that SF1 marks the identity of true gonadal somatic precursor cells.
GATA4 is a transcription factor whose functions appear to be conserved among vertebrates. For instance, Gata4−/− ES cell-derived mouse embryos and Gata4-deficient zebrafish embryos have strikingly similar phenotypes, including defects in heart tube looping, ventricle expansion, liver bud expansion, and derivation of the pancreas from the foregut [20], [46]–[48]. Xenopus embryos also require Gata4 for heart and liver development [49]. Considering the conserved expression of Gata4 in the genital ridge across vertebrates [21]–[26], we propose that the gonad is initiated through an evolutionarily conserved mechanism in which differentiation of the coelomic epithelium into the genital ridge is dependent on a common regulator, Gata4. In the current study, we established that Lhx9 and Sf1 act downstream of Gata4 during the initiation of gonadogenesis in mouse embryos (Figure 4). Conserved expression of Lhx9 and Sf1 with Gata4 in the genital ridges of different species would support a conserved role of Gata4 in gonadal initiation. Indeed, Gata4, Lhx9, and Sf1 are all expressed in the genital ridges, in both sexes, of chicken embryos [24]. Sf1 was also detected in the genital ridges of dogs and turtles [50], [51].
PGCs migrate from the base of the allantois through the hindgut and mesentery to their final destination, the gonads, where they ultimately give rise to gametes. Our data show that PGCs were able to migrate to the coelomic epithelium on the ventromedial side of the mesonephros in Gata4 cKO embryos (Figure 6 and Figure S4), suggesting that neither Gata4 nor genital ridge formation is required for PGC migration. In agreement with this finding, it has been shown that PGCs actively migrate out of the hindgut toward the presumptive gonadal region at ∼E9.5 before the genital ridges are formed [41]. In addition, somatic factors that guide PGC migration, such as the chemokine SDF1/CXCL2 and the transcription factor FOXC1, are expressed not only in genital ridges, but also in the mesentery and mesonephros, starting as early as E9.0 [52]–[54]. We conclude that these somatic factors, expressed independently of Gata4, are sufficient to direct PGCs to their final destination, or to its proximity, even in the absence of the genital ridges.
Gata4 cKO mutants show no signs of gonadal initiation or differentiation, unlike the previously reported mutants (Lhx9−/−, Sf1−/−, Wt1−/−, and Emx2−/−) where the genital ridge is formed but then degenerates. Gata4 exhibits a functional role in gonadogenesis earlier than Lhx9, Sf1, Wt1, and Emx2. Notably, GATA4 is a transcription factor that lies in the middle of signaling cascades. Identification of upstream regulators and additional downstream targets of Gata4 will provide insights into the regulation of gonadal initiation. Thus, our findings open to study the earliest steps in the formation of testes and ovaries.
Materials and Methods
Ethics statement
All experiments involving mice were approved by the Committee on Animal Care at the Massachusetts Institute of Technology.
Mice
Gata4flox/+ [47], CAG-CreER [32], Osr1eGFP-CreERt2/+ [33], Wt1CreERt2/+ [34], and Gt(ROSA)26SorCAG-tdTomato mice were obtained from Jackson Laboratory (Stock Numbers 008194, 004682, 009061, 010912, and 007908, respectively) and then intercrossed for the experiment. Mice carrying an Oct4-EGFP transgene were also obtained from Jackson Laboratory (Stock Number 004654) and then backcrossed to the C57BL/6 strain (Taconic Farms) for at least 11 generations. Gata4-conditional-mutant embryos were generated by mating males carrying Gata4+/Δ and the indicated CreER to Gata4flox/flox females. Tamoxifen (Sigma) was dissolved in corn oil (Sigma) at a concentration of 20 mg/ml. Dams were injected intraperitoneally at 8.75 days postcoitum with a single shot of tamoxifen (4–5 mg/40 g body weight) to induce excision of the floxed Gata4 allele. The injection scheme was optimized for maximum embryo survival and Gata4 excision efficiency. Embryos were collected between E10.0 and E11.5; tail somites were counted to determine precise age. Genotypes were assayed by PCR according to protocols from the Jackson Laboratory website.
Whole-mount immunofluorescent staining
Mouse embryos were either left whole or dissected to remove heads, limbs, body walls, and internal organs. Embryos were fixed at 4°C overnight in 4% paraformaldehyde and then blocked with 3% BSA/5% donkey serum/0.1% Triton X-100/PBS for another night. After washing with 0.1% Triton X-100/PBS, embryos were incubated at 4°C overnight with antibodies against GATA4 (sc-25310 or sc-1237, Santa Cruz Biotechnology), SF1 (PP-N1665-00, R&D Systems) and/or LHX9 (sc-19348, Santa Cruz Biotechnology). After washing for at least 8 hours, embryos were then incubated at 4°C overnight with donkey secondary antibodies conjugated with FITC, Rhodamine Red X, or DyLight 649 (Jackson ImmunoResearch). All antibodies were diluted 1∶100 in 1% BSA/0.1% Triton X-100/PBS solution. After washing for at least 8 hours, embryos were preserved in SlowFade Gold Antifade reagent (Life Technologies). Images were taken using an LSM710 confocal microscope (Zeiss). For transverse views, embryos were embedded in 7.5% low-melting point agarose, cut into 300 µm-thick transverse sections with a vibratome, and imaged via confocal microscopy.
Immunohistochemistry/immunofluorescence
Immunohistochemical staining of embryonic sections was carried out as described previously [55]. Briefly, whole embryos were fixed at 4°C overnight in 4% paraformaldehyde, paraffin embedded, and sectioned. Sections representing the anterior portion of the genital ridges were used for all experiments. Slides were then dewaxed, rehydrated, and antigen-retrieved by microwaving in citrate buffer. After blocking, slides were incubated with primary antibodies. For colorimetric staining, slides were incubated with rabbit or mouse ImmPress reagent (Vector Labs), developed using DAB substrate kit (Vector Labs), and counterstained with hematoxylin. For fluorescent staining, slides were incubated with donkey secondary antibodies conjugated with FITC, Rhodamine Red X or DyLight 649 (Jackson ImmunoResearch) and mounted with ProLong Gold Antifade reagent with DAPI (Life Technologies).
For BrdU incorporation experiments, pregnant mice were injected intraperitoneally with BrdU (100 mg/kg body weight) six hours before sacrifice. Embryos were removed and processed for immunofluorescent staining, following the procedure described above, except that slides were denatured with 3.5N HCl for 30 seconds before blocking. Germ cells, characterized by their large, round, clear nuclei with clumps of chromatin scattered around the nuclear periphery, were excluded from counting. TUNEL staining was carried out with In Situ Cell Death Detection Kit (Roche Applied Science) according to the manufacturer's instructions.
Primary antibodies against GATA4 (sc-25310, Santa Cruz Biotechnology), SF1 (PP-N1665-00, R&D Systems), laminin (L9393, Sigma), BrdU (OBT0030, Accurate Chemical and Scientific), WT1 (RB-9267, Thermo Scientific), and EMX2 (a kind gift of Ken-ichirou Morohashi, Kyushu University, Fukuoka, Japan) [7], [56] were used in the study.
Quantitative RT-PCR
Urogenital ridges were collected, submerged in TRIzol (Life Technologies), and then stored at −80°C until genotyping was completed. Total RNA was prepared according to the manufacturer's instructions and DNase-treated using DNA Free Turbo (Ambion). Three hundred ng of total RNA was reverse transcribed, and qPCR was performed with SYBR Green dye, as previously described [55]. qPCR primer pairs employed were as follows:
5′ - CGGAAGCCCAAGAACCTGAATAAATC-3′ and
5′ - GCTGCTGTGCCCATAGTGAGATGAC-3′ for Gata4,
5′ - GAGTTCGTCTGTCTCAAGTTCCTCATCC-3′ and
5′ - ACCTCCACCAGGCACAATAGCAAC-3′ for Sf1,
5′ - ACCAGCAGCCTTATCCACCTTCACAG-3′ and
5′ - TGTAATGCCCCAAGATTTGTTCTCCC-3′ for Lhx9, and
5′ - GAGAGCCAGCCTACCATCC-3′ and
5′ - GGGTCCTCGTGTTTGAAGGAA-3′ for Wt1.
Results were analyzed using the delta-delta Ct method with β-actin as a normalization control.
Supporting Information
Zdroje
1. BrambellFWR (1927) The development and morphology of the gonads of the mouse - Part I The morphogenesis of the indifferent gonad and of the ovary. Proc R Soc Lond B Biol Sci 101 : 391–409.
2. GroppA, OhnoS (1966) The presence of a common embryonic blastema for ovarian and testicular parenchymal (follicular, interstitial and tubular) cells in cattle Bos taurus. Z Zellforsch Mikrosk Anat 74 : 505–528.
3. PelliniemiLJ (1975) Ultrastructure of gonadal ridge in male and female pig embryos. Anat Embryol (Berl) 147 : 20–34.
4. WartenbergH, KinskyI, ViebahnC, SchmolkeC (1991) Fine structural characteristics of testicular cord formation in the developing rabbit gonad. J Electron Microsc Tech 19 : 133–157.
5. KarlJ, CapelB (1998) Sertoli cells of the mouse testis originate from the coelomic epithelium. Dev Biol 203 : 323–333.
6. ParankoJ (1987) Expression of type I and III collagen during morphogenesis of fetal rat testis and ovary. Anat Rec 219 : 91–101.
7. KusakaM, Katoh-FukuiY, OgawaH, MiyabayashiK, BabaT, et al. (2010) Abnormal epithelial cell polarity and ectopic epidermal growth factor receptor (EGFR) expression induced in Emx2 KO embryonic gonads. Endocrinology 151 : 5893–5904.
8. YoshinagaK, HessDL, HendrickxAG, ZamboniL (1988) The development of the sexually indifferent gonad in the prosimian, Galago crassicaudatus crassicaudatus. Am J Anat 181 : 89–105.
9. HackerA, CapelB, GoodfellowP, Lovell-BadgeR (1995) Expression of Sry, the mouse sex determining gene. Development 121 : 1603–1614.
10. SchmahlJ, EicherEM, WashburnLL, CapelB (2000) Sry induces cell proliferation in the mouse gonad. Development 127 : 65–73.
11. LuoX, IkedaY, ParkerKL (1994) A cell-specific nuclear receptor is essential for adrenal and gonadal development and sexual differentiation. Cell 77 : 481–490.
12. BirkOS, CasianoDE, WassifCA, CogliatiT, ZhaoL, et al. (2000) The LIM homeobox gene Lhx9 is essential for mouse gonad formation. Nature 403 : 909–913.
13. KreidbergJA, SariolaH, LoringJM, MaedaM, PelletierJ, et al. (1993) WT-1 is required for early kidney development. Cell 74 : 679–691.
14. MiyamotoN, YoshidaM, KurataniS, MatsuoI, AizawaS (1997) Defects of urogenital development in mice lacking Emx2. Development 124 : 1653–1664.
15. HammesA, GuoJK, LutschG, LehesteJR, LandrockD, et al. (2001) Two splice variants of the Wilms' tumor 1 gene have distinct functions during sex determination and nephron formation. Cell 106 : 319–329.
16. BlandML, FowkesRC, IngrahamHA (2004) Differential requirement for steroidogenic factor-1 gene dosage in adrenal development versus endocrine function. Mol Endocrinol 18 : 941–952.
17. WangQ, LanY, ChoES, MaltbyKM, JiangR (2005) Odd-skipped related 1 (Odd 1) is an essential regulator of heart and urogenital development. Dev Biol 288 : 582–594.
18. WattAJ, ZhaoR, LiJ, DuncanSA (2007) Development of the mammalian liver and ventral pancreas is dependent on GATA4. BMC Dev Biol 7 : 37.
19. MolkentinJD, LinQ, DuncanSA, OlsonEN (1997) Requirement of the transcription factor GATA4 for heart tube formation and ventral morphogenesis. Genes Dev 11 : 1061–1072.
20. KuoCT, MorriseyEE, AnandappaR, SigristK, LuMM, et al. (1997) GATA4 transcription factor is required for ventral morphogenesis and heart tube formation. Genes Dev 11 : 1048–1060.
21. McCoardSA, WiseTH, FahrenkrugSC, FordJJ (2001) Temporal and spatial localization patterns of Gata4 during porcine gonadogenesis. Biol Reprod 65 : 366–374.
22. VigerRS, MertineitC, TraslerJM, NemerM (1998) Transcription factor GATA-4 is expressed in a sexually dimorphic pattern during mouse gonadal development and is a potent activator of the Mullerian inhibiting substance promoter. Development 125 : 2665–2675.
23. AnttonenM, KetolaI, ParviainenH, PusaAK, HeikinheimoM (2003) FOG-2 and GATA-4 Are coexpressed in the mouse ovary and can modulate mullerian-inhibiting substance expression. Biol Reprod 68 : 1333–1340.
24. OrealE, MazaudS, PicardJY, MagreS, Carre-EusebeD (2002) Different patterns of anti-Mullerian hormone expression, as related to DMRT1, SF-1, WT1, GATA-4, Wnt-4, and Lhx9 expression, in the chick differentiating gonads. Dev Dyn 225 : 221–232.
25. LiJ, ChenW, WangD, ZhouL, SakaiF, et al. (2012) GATA4 is involved in the gonadal development and maturation of the teleost fish tilapia, Oreochromis niloticus. J Reprod Dev 58 : 237–242.
26. BarskeLA, CapelB (2010) Estrogen represses SOX9 during sex determination in the red-eared slider turtle Trachemys scripta. Dev Biol 341 : 305–314.
27. MiyamotoY, TaniguchiH, HamelF, SilversidesDW, VigerRS (2008) A GATA4/WT1 cooperation regulates transcription of genes required for mammalian sex determination and differentiation. BMC Mol Biol 9 : 44.
28. TevosianSG, AlbrechtKH, CrispinoJD, FujiwaraY, EicherEM, et al. (2002) Gonadal differentiation, sex determination and normal Sry expression in mice require direct interaction between transcription partners GATA4 and FOG2. Development 129 : 4627–4634.
29. BoumaGJ, WashburnLL, AlbrechtKH, EicherEM (2007) Correct dosage of Fog2 and Gata4 transcription factors is critical for fetal testis development in mice. Proc Natl Acad Sci U S A 104 : 14994–14999.
30. ManuylovNL, ZhouB, MaQ, FoxSC, PuWT, et al. (2011) Conditional ablation of Gata4 and Fog2 genes in mice reveals their distinct roles in mammalian sexual differentiation. Dev Biol 353 : 229–241.
31. IkedaY, ShenWH, IngrahamHA, ParkerKL (1994) Developmental expression of mouse steroidogenic factor-1, an essential regulator of the steroid hydroxylases. Mol Endocrinol 8 : 654–662.
32. HayashiS, McMahonAP (2002) Efficient recombination in diverse tissues by a tamoxifen-inducible form of Cre: a tool for temporally regulated gene activation/inactivation in the mouse. Dev Biol 244 : 305–318.
33. MugfordJW, SipilaP, McMahonJA, McMahonAP (2008) Osr1 expression demarcates a multi-potent population of intermediate mesoderm that undergoes progressive restriction to an Osr1-dependent nephron progenitor compartment within the mammalian kidney. Dev Biol 324 : 88–98.
34. ZhouB, MaQ, RajagopalS, WuSM, DomianI, et al. (2008) Epicardial progenitors contribute to the cardiomyocyte lineage in the developing heart. Nature 454 : 109–113.
35. SoPL, DanielianPS (1999) Cloning and expression analysis of a mouse gene related to Drosophila odd-skipped. Mech Dev 84 : 157–160.
36. ArmstrongJF, Pritchard-JonesK, BickmoreWA, HastieND, BardJB (1993) The expression of the Wilms' tumour gene, WT1, in the developing mammalian embryo. Mech Dev 40 : 85–97.
37. PetersH (1976) Intrauterine gonadal development. Fertil Steril 27 : 493–500.
38. KlattigJ, SierigR, KruspeD, MakkiMS, EnglertC (2007) WT1-mediated gene regulation in early urogenital ridge development. Sex Dev 1 : 238–254.
39. GinsburgM, SnowMH, McLarenA (1990) Primordial germ cells in the mouse embryo during gastrulation. Development 110 : 521–528.
40. GompertsM, WylieC, HeasmanJ (1994) Primordial germ cell migration. Ciba Found Symp 182 : 121–134 discussion 134-129.
41. MolyneauxKA, StallockJ, SchaibleK, WylieC (2001) Time-lapse analysis of living mouse germ cell migration. Dev Biol 240 : 488–498.
42. SzaboPE, HubnerK, ScholerH, MannJR (2002) Allele-specific expression of imprinted genes in mouse migratory primordial germ cells. Mech Dev 115 : 157–160.
43. SmagulovaFO, ManuylovNL, LeachLL, TevosianSG (2008) GATA4/FOG2 transcriptional complex regulates Lhx9 gene expression in murine heart development. BMC Dev Biol 8 : 67.
44. TremblayJJ, VigerRS (2001) GATA factors differentially activate multiple gonadal promoters through conserved GATA regulatory elements. Endocrinology 142 : 977–986.
45. WilhelmD, EnglertC (2002) The Wilms tumor suppressor WT1 regulates early gonad development by activation of Sf1. Genes Dev 16 : 1839–1851.
46. ZeisbergEM, MaQ, JuraszekAL, MosesK, SchwartzRJ, et al. (2005) Morphogenesis of the right ventricle requires myocardial expression of Gata4. J Clin Invest 115 : 1522–1531.
47. WattAJ, BattleMA, LiJ, DuncanSA (2004) GATA4 is essential for formation of the proepicardium and regulates cardiogenesis. Proc Natl Acad Sci U S A 101 : 12573–12578.
48. HoltzingerA, EvansT (2005) Gata4 regulates the formation of multiple organs. Development 132 : 4005–4014.
49. HaworthKE, KotechaS, MohunTJ, LatinkicBV (2008) GATA4 and GATA5 are essential for heart and liver development in Xenopus embryos. BMC Dev Biol 8 : 74.
50. FlemingA, WibbelsT, SkipperJK, CrewsD (1999) Developmental expression of steroidogenic factor 1 in a turtle with temperature-dependent sex determination. Gen Comp Endocrinol 116 : 336–346.
51. Meyers-WallenVN (2005) Sf1 and Mis expression: molecular milestones in the canine sex determination pathway. Mol Reprod Dev 70 : 383–389.
52. MattiskeD, KumeT, HoganBL (2006) The mouse forkhead gene Foxc1 is required for primordial germ cell migration and antral follicle development. Dev Biol 290 : 447–458.
53. MolyneauxKA, ZinsznerH, KunwarPS, SchaibleK, SteblerJ, et al. (2003) The chemokine SDF1/CXCL12 and its receptor CXCR4 regulate mouse germ cell migration and survival. Development 130 : 4279–4286.
54. AraT, NakamuraY, EgawaT, SugiyamaT, AbeK, et al. (2003) Impaired colonization of the gonads by primordial germ cells in mice lacking a chemokine, stromal cell-derived factor-1 (SDF-1). Proc Natl Acad Sci U S A 100 : 5319–5323.
55. GillME, HuYC, LinY, PageDC (2011) Licensing of gametogenesis, dependent on RNA binding protein DAZL, as a gateway to sexual differentiation of fetal germ cells. Proc Natl Acad Sci U S A 108 : 7443–7448.
56. NomuraM, KawabeK, MatsushitaS, OkaS, HatanoO, et al. (1998) Adrenocortical and gonadal expression of the mammalian Ftz-F1 gene encoding Ad4BP/SF-1 is independent of pituitary control. J Biochem 124 : 217–224.
Štítky
Genetika Reprodukční medicína
Článek Independent Evolution of Transcriptional Inactivation on Sex Chromosomes in Birds and MammalsČlánek The bHLH Subgroup IIId Factors Negatively Regulate Jasmonate-Mediated Plant Defense and DevelopmentČlánek Selective Pressures to Maintain Attachment Site Specificity of Integrative and Conjugative ElementsČlánek Reassembly of Nucleosomes at the Promoter Initiates Resilencing Following Decitabine ExposureČlánek Hepatocyte Growth Factor Signaling in Intrapancreatic Ductal Cells Drives Pancreatic Morphogenesis
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2013 Číslo 7- Akutní intermitentní porfyrie
- Růst a vývoj dětí narozených pomocí IVF
- Farmakogenetické testování pomáhá předcházet nežádoucím efektům léčiv
- Pilotní studie: stres a úzkost v průběhu IVF cyklu
- Vliv melatoninu a cirkadiálního rytmu na ženskou reprodukci
-
Všechny články tohoto čísla
- An Solution for Crossover Formation
- Genome-Wide Association Mapping in Dogs Enables Identification of the Homeobox Gene, , as a Genetic Component of Neural Tube Defects in Humans
- Independent Evolution of Transcriptional Inactivation on Sex Chromosomes in Birds and Mammals
- Stepwise Activation of the ATR Signaling Pathway upon Increasing Replication Stress Impacts Fragile Site Integrity
- Genomic Analysis of Natural Selection and Phenotypic Variation in High-Altitude Mongolians
- Modification of tRNA by Elongator Is Essential for Efficient Translation of Stress mRNAs
- Role of CTCF Protein in Regulating Locus Transcription
- Gene Set Signature of Reversal Reaction Type I in Leprosy Patients
- Mapping of PARK2 and PACRG Overlapping Regulatory Region Reveals LD Structure and Functional Variants in Association with Leprosy in Unrelated Indian Population Groups
- Is Required for Formation of the Genital Ridge in Mice
- Monopolin Subunit Csm1 Associates with MIND Complex to Establish Monopolar Attachment of Sister Kinetochores at Meiosis I
- Recombination Dynamics of a Human Y-Chromosomal Palindrome: Rapid GC-Biased Gene Conversion, Multi-kilobase Conversion Tracts, and Rare Inversions
- Mechanisms of Protein Sequence Divergence and Incompatibility
- Histone Methyltransferase DOT1L Drives Recovery of Gene Expression after a Genotoxic Attack
- Female Behaviour Drives Expression and Evolution of Gustatory Receptors in Butterflies
- Combinatorial Regulation of Meiotic Holliday Junction Resolution in by HIM-6 (BLM) Helicase, SLX-4, and the SLX-1, MUS-81 and XPF-1 Nucleases
- The bHLH Subgroup IIId Factors Negatively Regulate Jasmonate-Mediated Plant Defense and Development
- The Role of Interruptions in polyQ in the Pathology of SCA1
- Dietary Restriction Induced Longevity Is Mediated by Nuclear Receptor NHR-62 in
- Fine Time Course Expression Analysis Identifies Cascades of Activation and Repression and Maps a Putative Regulator of Mammalian Sex Determination
- Genome-scale Co-evolutionary Inference Identifies Functions and Clients of Bacterial Hsp90
- Oxidative Stress and Replication-Independent DNA Breakage Induced by Arsenic in
- A Moonlighting Enzyme Links Cell Size with Central Metabolism
- Budding Yeast Greatwall and Endosulfines Control Activity and Spatial Regulation of PP2A for Timely Mitotic Progression
- The Conserved Intronic Cleavage and Polyadenylation Site of CstF-77 Gene Imparts Control of 3′ End Processing Activity through Feedback Autoregulation and by U1 snRNP
- The BTB-zinc Finger Transcription Factor Abrupt Acts as an Epithelial Oncogene in through Maintaining a Progenitor-like Cell State
- The Cohesion Protein SOLO Associates with SMC1 and Is Required for Synapsis, Recombination, Homolog Bias and Cohesion and Pairing of Centromeres in Drosophila Meiosis
- The RNA-binding Proteins FMR1, Rasputin and Caprin Act Together with the UBA Protein Lingerer to Restrict Tissue Growth in
- Pattern Dynamics in Adaxial-Abaxial Specific Gene Expression Are Modulated by a Plastid Retrograde Signal during Leaf Development
- A Network of HMG-box Transcription Factors Regulates Sexual Cycle in the Fungus
- Bacterial Adaptation through Loss of Function
- ENU-induced Mutation in the DNA-binding Domain of KLF3 Reveals Important Roles for KLF3 in Cardiovascular Development and Function in Mice
- Interplay between Structure-Specific Endonucleases for Crossover Control during Meiosis
- FGF Signalling Regulates Chromatin Organisation during Neural Differentiation via Mechanisms that Can Be Uncoupled from Transcription
- The Arabidopsis RNA Binding Protein with K Homology Motifs, SHINY1, Interacts with the C-terminal Domain Phosphatase-like 1 (CPL1) to Repress Stress-Inducible Gene Expression
- Selective Pressures to Maintain Attachment Site Specificity of Integrative and Conjugative Elements
- The Conserved ADAMTS-like Protein Lonely heart Mediates Matrix Formation and Cardiac Tissue Integrity
- The cGMP-Dependent Protein Kinase EGL-4 Regulates Nociceptive Behavioral Sensitivity
- RBM5 Is a Male Germ Cell Splicing Factor and Is Required for Spermatid Differentiation and Male Fertility
- Disease-Related Growth Factor and Embryonic Signaling Pathways Modulate an Enhancer of Expression at the 6q23.2 Coronary Heart Disease Locus
- Yeast Pol4 Promotes Tel1-Regulated Chromosomal Translocations
- A Dual Role for SOX10 in the Maintenance of the Postnatal Melanocyte Lineage and the Differentiation of Melanocyte Stem Cell Progenitors
- SLC26A4 Targeted to the Endolymphatic Sac Rescues Hearing and Balance in Mutant Mice
- Odoriferous Defensive Stink Gland Transcriptome to Identify Novel Genes Necessary for Quinone Synthesis in the Red Flour Beetle,
- Prediction of Complex Human Traits Using the Genomic Best Linear Unbiased Predictor
- Gene × Physical Activity Interactions in Obesity: Combined Analysis of 111,421 Individuals of European Ancestry
- Reassembly of Nucleosomes at the Promoter Initiates Resilencing Following Decitabine Exposure
- Exquisite Light Sensitivity of Cryptochrome
- miR-133a Regulates Adipocyte Browning In Vivo
- Strabismus Promotes Recruitment and Degradation of Farnesylated Prickle in Planar Polarity Specification
- Hepatocyte Growth Factor Signaling in Intrapancreatic Ductal Cells Drives Pancreatic Morphogenesis
- Is a Potential Tumor Suppressor Gene Commonly Inactivated by Epigenetic Mechanisms in Colorectal Cancer
- Joint Molecule Resolution Requires the Redundant Activities of MUS-81 and XPF-1 during Meiosis
- The Mating Competence of Geographically Diverse Strains in Their Natural and Unnatural Sand Fly Vectors
- Defective Repair of Oxidative Base Lesions by the DNA Glycosylase Nth1 Associates with Multiple Telomere Defects
- Effective Blocking of the Enhancer Requires Cooperation between Two Main Mechanisms Suggested for the Insulator Function
- Trans-Ancestral Studies Fine Map the SLE-Susceptibility Locus
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- SLC26A4 Targeted to the Endolymphatic Sac Rescues Hearing and Balance in Mutant Mice
- Bacterial Adaptation through Loss of Function
- The Cohesion Protein SOLO Associates with SMC1 and Is Required for Synapsis, Recombination, Homolog Bias and Cohesion and Pairing of Centromeres in Drosophila Meiosis
- Gene × Physical Activity Interactions in Obesity: Combined Analysis of 111,421 Individuals of European Ancestry
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



