-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Exquisite Light Sensitivity of Cryptochrome
Drosophila melanogaster shows exquisite light sensitivity for modulation of circadian functions in vivo, yet the activities of the Drosophila circadian photopigment cryptochrome (CRY) have only been observed at high light levels. We studied intensity/duration parameters for light pulse induced circadian phase shifts under dim light conditions in vivo. Flies show far greater light sensitivity than previously appreciated, and show a surprising sensitivity increase with pulse duration, implying a process of photic integration active up to at least 6 hours. The CRY target timeless (TIM) shows dim light dependent degradation in circadian pacemaker neurons that parallels phase shift amplitude, indicating that integration occurs at this step, with the strongest effect in a single identified pacemaker neuron. Our findings indicate that CRY compensates for limited light sensitivity in vivo by photon integration over extraordinarily long times, and point to select circadian pacemaker neurons as having important roles.
Published in the journal: . PLoS Genet 9(7): e32767. doi:10.1371/journal.pgen.1003615
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1003615Summary
Drosophila melanogaster shows exquisite light sensitivity for modulation of circadian functions in vivo, yet the activities of the Drosophila circadian photopigment cryptochrome (CRY) have only been observed at high light levels. We studied intensity/duration parameters for light pulse induced circadian phase shifts under dim light conditions in vivo. Flies show far greater light sensitivity than previously appreciated, and show a surprising sensitivity increase with pulse duration, implying a process of photic integration active up to at least 6 hours. The CRY target timeless (TIM) shows dim light dependent degradation in circadian pacemaker neurons that parallels phase shift amplitude, indicating that integration occurs at this step, with the strongest effect in a single identified pacemaker neuron. Our findings indicate that CRY compensates for limited light sensitivity in vivo by photon integration over extraordinarily long times, and point to select circadian pacemaker neurons as having important roles.
Introduction
Nearly all plants and animals use daily patterns of day and night to entrain their endogenous circadian oscillators. These responses utilize photic input from both visual photoreceptors, as well as from non-visual circadian photopigments (reviewed in [1]–[3]). Both visual photopigments and the circadian blue light photopigment cryptochrome (CRY) are required in Drosophila for normal entrainment to a light/dark cycle, but CRY is the sole photopigment required to shift circadian phase after a light pulse given in subjective night, and flies are circadian blind when both cryptochrome and visual photopigments are absent [4]–[7]. Additionally, CRY is the photopigment leading to behavioral arrhythmicity in response to constant light [8]. In addition to these light input pathways, another less well defined pathway involves the developmental gene glass [4], [9], [10], with a role in light/dark entrainment.
CRY has two circadian roles in Drosophila. In the core circadian oscillator, it functions to trigger light dependent ubiquitinylation and degradation of its target timeless (TIM), a core circadian factor, as well as itself [11]. In cells peripheral to the central oscillator, CRY functions as a transcriptional repressor of CLK/CYC, binding to the PER protein, a role similar to its role in mammals [12] in addition to its role in making most fly tissues inherently light sensitive [13]. A pathway has been worked out for the light signaling of CRY in Drosophila through a large series of studies (reviewed in [2], [3]). To summarize briefly, CRY binds TIM following a light dependent conformational change. This then triggers the Jetlag dependent degradation of both CRY and TIM, with more rapid degradation of TIM vs CRY based on enhanced affinity of JET for TIM [14].
Flies are extremely light sensitive for circadian clock entrainment [15], responding to less than 0.03 nw/cm2 12 hr ‘days’ of blue light in an LD cycle. Half-maximal shifts of circadian phase resulting from a light pulse during late subjective night can result from a 20–30 µw/cm2,10 minute white light pulse [16], [17] Half-maximal shifts in phase of eclosion timing occur at blue light intensities of 100 nw/cm2 (3×1011 photons/cm2/sec) in a related Drosophilid [18]. However, physiological and biochemical responses of CRY to light are observed at far higher intensities, at or above 1 mw/cm2. These include a light induced conformational change [19], light induced ubiquitinylation of the TIM protein and subsequent CRY degradation [11], and light induced stimulation of neuronal firing rate, either in normal CRY containing neurons, or in neurons with ectopic CRY expression [20]. This discrepancy indicates that some process must be operative in vivo to increase the effective light sensitivity of CRY, or that the high light intensity responses of CRY may not be relevant to its in vivo function.
Here we investigate the discrepancy between the low light sensitivity of CRY for its measured activities, relative to the extreme light sensitivity for its in vivo phase shifting effects. We measure the half-maximal responses of flies to a late subjective night light pulse, varying both light intensity and duration, and find far greater light sensitivity than previously appreciated. We find a surprising intensity vs duration relationship, with increasing phase shift amplitude as photon number is held constant with increased light pulse duration. This implies an ability to integrate photon information over durations of hours that is almost exclusively dependent on CRY photic input. We then show that these photon-limited responses lead to TIM degradation, with significantly more TIM degraded by an equal-photon-number long duration light pulse. This indicates that temporal integration increases efficacy of TIM degradation. These observations provide a general means by which a low-sensitivity photopigment can achieve extraordinarily high effective light sensitivity.
Results
The basic light pulse paradigm used in this manuscript is illustrated in Figure 1A. This figure shows a median actogram derived from 12 individual flies. Flies were entrained to a 12 hr∶12 hr light/dark schedule (green box), then given a 6 hr exposure to a pulse of blue light (blue box) late in subjective night at ZT18-24. This light pulse stimulates activity within the flies subjective night and leads to a phase advance, as shown by the double arrow line. This line compares circadian phase in LD, taken as the lights off point, versus the extrapolated activity off points in constant darkness, with the blue line derived from RMS match to the red activity off points. Light pulses late in subjective night result in phase advances, whereas light pulses early in subjective night result in phase delays (Figure 1B). In this manuscript we restrict our analyses to the phase advance region of the phase response curve, centering light pulses around the maximum phase advance at ZT20-21.
Fig. 1. A double-plotted median actogram illustrating the basic paradigm used in this manuscript (A), and a Phase Response Curve (B). 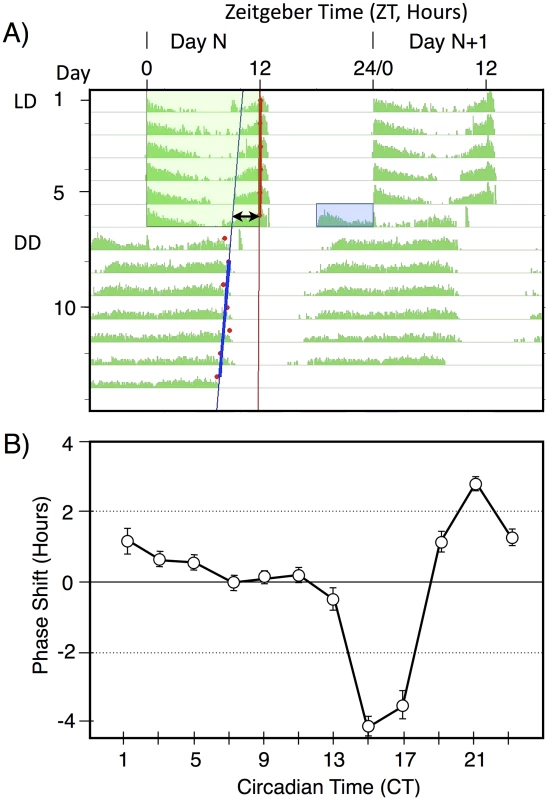
A) A median actogram showing the results of a light pulse during late subjective night on circadian phase in constant darkness (DD). Flies were entrained to a 12 hr;12 hr light dark schedule with green light for 6 days, then given a 6 hr blue light pulse of intensity 33 nw/cm2 at ZT18-24. The resulting phase advance (double arrow line) is shown by extrapolating from the computer called RMS line (blue) marking the activity off times (red dots) in DD. Phase shift data in this manuscript is computed from individual flies, relative to the phase shift of a control set of flies not receiving a light pulse. Data in this figure is double plotted for ease of viewing, but annotations are only shown for one of the two occurrences. B) A phase response curve, showing the averaged phase shifts resulting from a 1 hr light pulse at the indicated circadian times. Light pulses were of white light of intensity ∼150 µw/cm2. Averaged data reprinted from [24]. To determine light sensitivity for circadian phase shifting by light pulses in subjective night, we determined half-maximal light sensitivity for blue light pulses centered at ZT20 for two time intervals, 10 min (Figure 2A), and 120 min (Figure 2B). To account for the extra time of illumination in the 120 min pulses, levels were adjusted 12× lower than for the 10 min pulses. Flies responded with graded phase advances to the 10 min pulses, with a significant phase advances at or above 600 nw/cm2. The 2.4 hr phase advance resulting from the 7,000 nw/cm2 pulse is saturating, because higher intensities do not result in a larger phase advance [16], [17]. Our half-maximal light intensity values are significantly lower, i.e., more light sensitive, than published data for ZT21 light pulses [16], [17], but the published data uses white light, whereas our monochromatic blue light is better matched to the light sensitivity of CRY for its phase shifting activity [21].
Fig. 2. Phase shift magnitude as a function of intensity and duration of blue light pulses. 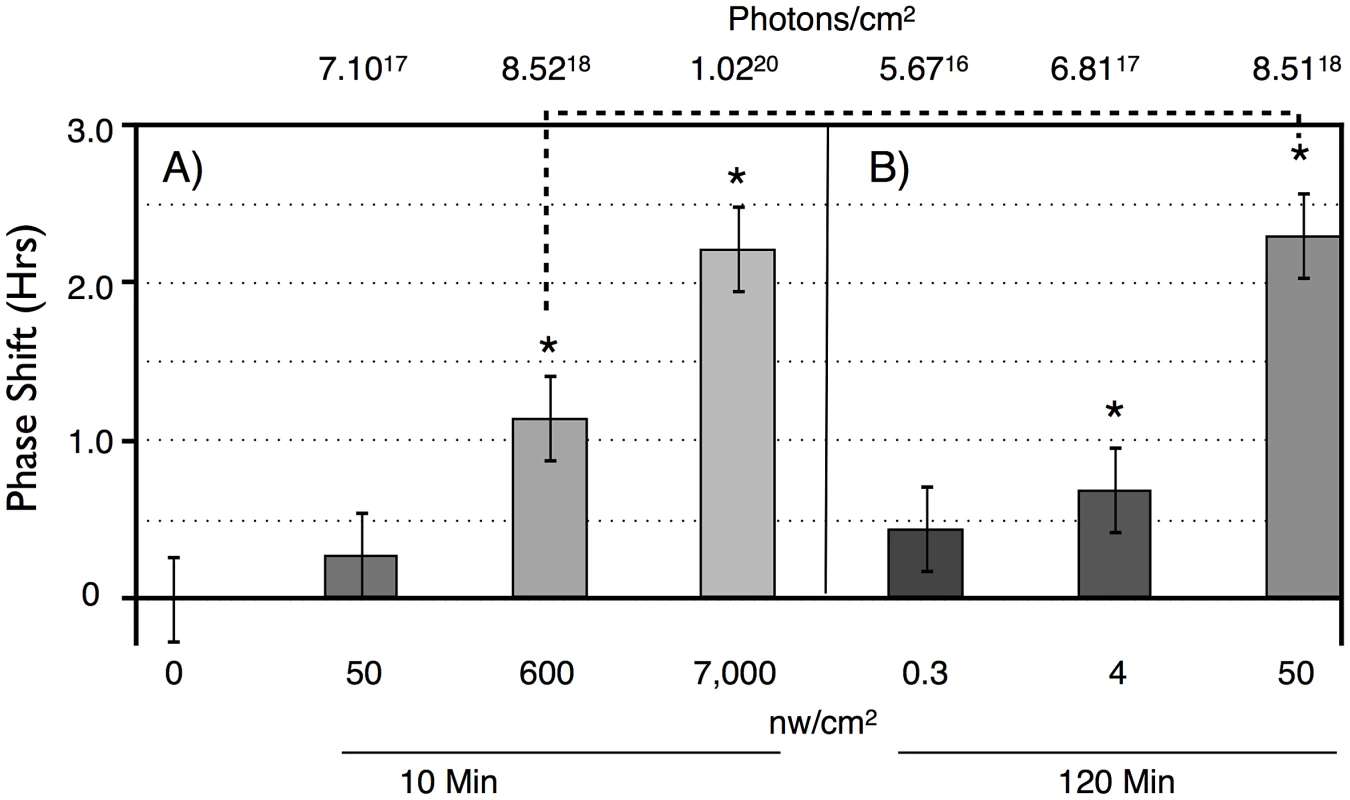
LD-entrained flies were given light pulses of either: A) 10 min, or B) 120 min, at different intensities at times centered at ZT20. n = 10 per condition. For both duration light pulses, there are graded phase shift responses as a function of increasing light intensity. 10 min: 7,000 vs 50 nw/cm2, P = 0.02; 120 min: 50 vs 4 nw/cm2: P = 0.04; 4 vs 0.3 nw/cm2: P = 0.03 (ANOVA). Asterisks indicate significantly different values from the no pulse control. 10 min: 600 nw/cm2 vs no pulse: P = 0.02; 7,000 nw/cm2 vs no pulse: P = 0.007. 120 min: 0.3, 4 nw/cm2 vs no pulse: not significant; 50 nw/cm2 vs no pulse: P = 0.0.006. Error bars = SEM. The dashed line indicates two light pulses with equal numbers of photons: not significantly different. Flies show greater light sensitivity in response to the 120 min light pulses, with 4 nw/cm2 showing slightly less than a half-maximal advance. The enhanced light sensitivity in response to the 120 min vs 10 min light pulses can also be seen in a comparison of two datapoints in which flies received equal numbers of photons, the 600 nw/cm2 10 min light pulse, versus the 50 nw/cm2 120 min light pulse (dashed line in Figure 2). Though these phase advances are not significantly different (P = 0.11, ANOVA), the latter is certainly not smaller than the former. This shows the possibility of a surprising intensity vs duration relationship, suggesting that flies might utilize a mechanism that allows them to integrate photon information over long time intervals.
To explicitly investigate temporal integration, we performed an experiment in which photon number was held constant as light intensity and time of a ZT20-centered blue light pulse was varied reciprocally in log increments over 3 log units (Figure 3A). This results in a graded increase in phase advance amplitude as light pulse duration increases. This indicates that phase advances in response to light pulses with equal numbers of photons are larger when light pulses are administered over 100 min as compared to 0.1 or 1 min (P<0.001; P = 0.027, respectively, ANOVA), which would imply an ability to integrate and stably store photon information for up to 100 min. As we will show later in Figure 4, light sensitivity is further enhanced for 360 min light pulses, indicating that this process occurs efficiently over times of several hours.
Fig. 3. Phase shift magnitude as a function of blue light pulses with equal numbers of photons, reciprocally varying time and intensity. 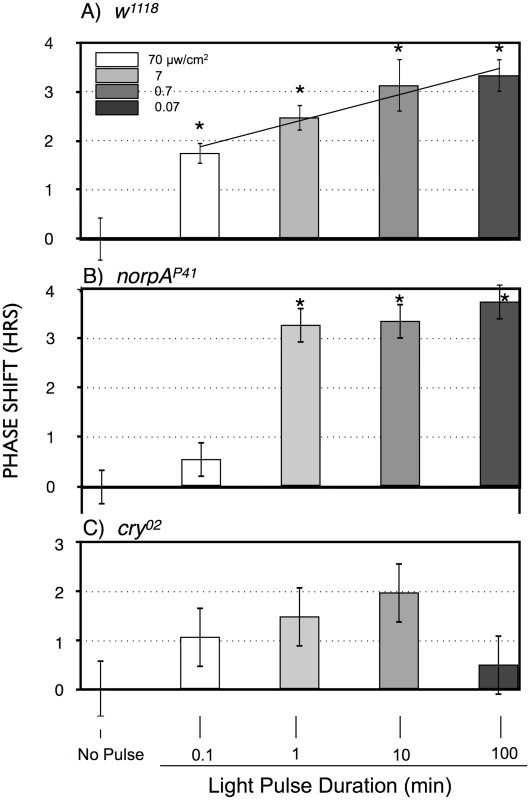
A) w1118; B) norpAP41; C) cry02 LD-entrained flies were given light pulses of indicated duration centered at ZT20. Light intensities were controlled such that all flies received the same number of photons. Statistics, ANOVA, with post-hoc correction for multiple comparisons; n = 16–20 for each light pulse. A) All phase shifts were significant relative to the no pulse control (alpha <0.0125). The line through the light pulse points is a linear regression through the light pulse values (R2 = 0.14), showing a significant positive slope of 0.55±0.13 (P = 4×10−5), using an X scale of log10 light pulse duration. B) The 1, 10 and 100 min light pulses are all highly significantly different relative to the no pulse control or to the 0.1 min light pulse (P<10−8). C) Only the 100 min point approaches significance relative to the no pulse control (P = 0.018, alpha = 0.0125). Asterisks indicate significantly different values from the no pulse controls. Error bars = SEM. Fig. 4. Large amplitude phase advance responses to 6 hr ZT21-centered light pulses depend primarily on CRY. 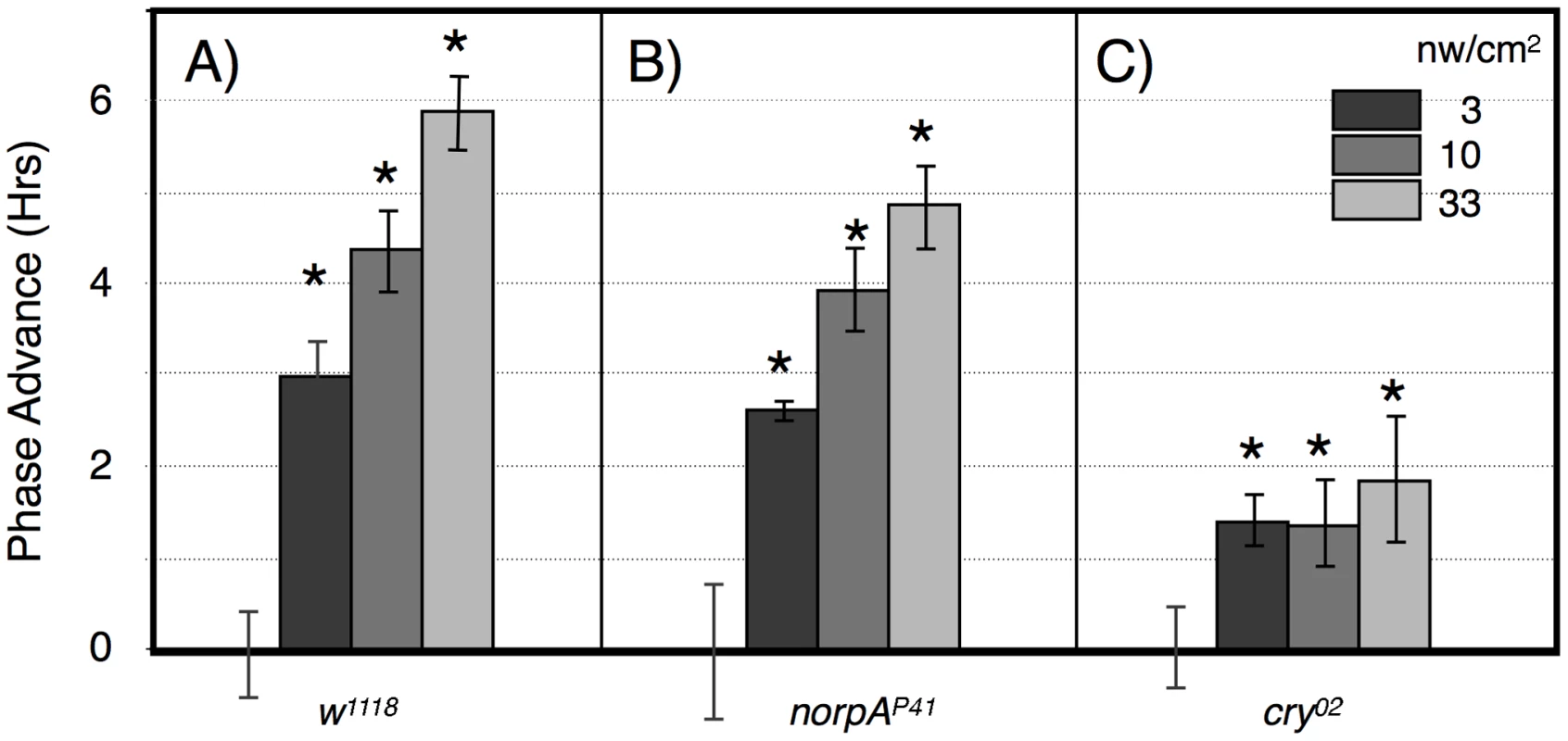
A) w1118 n = 15–18 per condition. B) norpAP41; n = 6–8 per condition. C) cry02 n = 7–12 per condition. Asterisks: significant phase advances relative to the no pulse control (ANOVA, significance set at alpha = 0.017). Error bars = SEM. The two possible photic input pathways to the Drosophila circadian clock are the circadian photoreceptor cryptochrome, CRY, and the opsin visual photoreceptors in the eye. In Figure 3B we examined phase advances in response to equal-photon-number ZT20 centered light pulses in norpA flies, with severely compromised vision due to a norpAP41 null mutation in the visual specific phospholipase C [22], [23]. The responses are strikingly similar to wild type flies at the three longer time intervals, indicating that the long duration photic integration is independent of the visual photoreceptors, and must be due to CRY mediated photoreception, and/or PLC-ß independent opsin signaling from eyelet [23]. The one difference between the two strains occurs at the shortest duration/highest intensity time point, where w1118 but not norpA shows a significant phase advance (P = 0.006). This indicates that CRY photoreception functions more efficiently with longer durations and lower intensities of light than visual photoreception. To confirm this, in Figure 3C we tested cry null flies, cry02, in the same paradigm. As expected, these flies show highly muted phase advance amplitudes [24], [25], with none of the light pulses resulting in a statistically significant phase advance relative to the no pulse control. Comparing the data in Figure 3 for each light pulse time as a function of genotype, only the 100 min light pulse for cry is significantly different from w1118 or norpA (P<0.001, ANOVA). Thus, cry null flies retain at most a minimal capability for temporal integration for a duration of no more than 10 min, which must be due to visual photoreceptors. Repetition of this experiment utilizing cry02 at 10× or 100× (Figure S2) the intensities used in Figure 3C similarly resulted in no statistically significant phase shifts relative to the no pulse controls, indicating that light sensitivity of cry02 for circadian phase shifts is at least 100× reduced relative to wild type flies (Figure 3A). As such, we turned to another assay to investigate the role of CRY vs visual photoreception in light pulse induced phase shifts.
The alternative assay examines phase advances in response to graded intensities of 360 min intervals of blue light centered at ZT21, using this time point instead of ZT20 to avoid going into the phase delay region of the phase response curve [24], [25]. Wild type flies show a graded response to increasing blue light illumination, with a half-maximal advance at 3 nw/cm2, and large amplitude 6 hr phase advances at 33 nw/cm2 (Figure 4A). This amplitude is saturating as shown using a similar though not identically timed 360 min light pulse [24]. We performed similar studies using norpA (Figure 4B) and cry02 (Figure 4C). NorpA flies show phase advance responses that are indistinguishable from w1118 except possibly at the highest intensity, indicating that visual phototransduction plays virtually no role in these responses. In contrast, the responses in cry02 flies are severely muted, showing a ∼1.5 hr phase advance at all intensities. Similarly muted phase shift responses have long been seen for cry mutants [21], [24], [25], with [24] showing significant phase shifts at ZT21 and 23.
The results from this light pulse phase advance study indicate that phase advances use both CRY and visual photoreceptors, but that the major contributor to phase advance amplitude is from CRY. The lack of significant variation in phase advance amplitude as a function of intensity in cry02 is most readily interpreted as showing a high sensitivity but low amplitude response, i.e., that the light intensity would need to be reduced further in order to show a half-maximal response, due to visual photoreceptors. This is consistent with the function of the opsin visual photoreceptors as extremely efficient light gathering pigments, responding to single photons by virtue of dense packaging in specialized visual structures and coupling to highly efficient downstream signaling systems [26]. In contrast, the contribution from CRY photoreception, as observed in the visually compromised norpA flies, has inherently low light sensitivity, but this pathway is far more effective in promoting large amplitude phase advances.
The phase shifting effect of a light pulse during subjective night signals via CRY dependent degradation of the core circadian gene product TIM (reviewed in [2]). To determine whether TIM degradation parallels phase shift amplitude at limiting light levels, we measured TIM levels following dim light pulses, covering the critical intensities/durations as determined in Figure 2. Since we measure TIM levels in whole mount brain preparations, we examined responses in particular clusters of brain circadian neurons, as shown in Figure 5, with quantitation by immunofluorescence in Figure 6. In this experiment, we subjected flies to ZT20 light pulses at a half-maximal level of 10 min, 600 nw/cm2, an equal photon dose of 50 nw/cm2 120 min, and a saturating phase shifting pulse of 7000 nw/cm2 for 10 min, measuring levels of TIM IR at ZT22.
Fig. 5. TIM and PDF immunostaining in various LNv neurons. 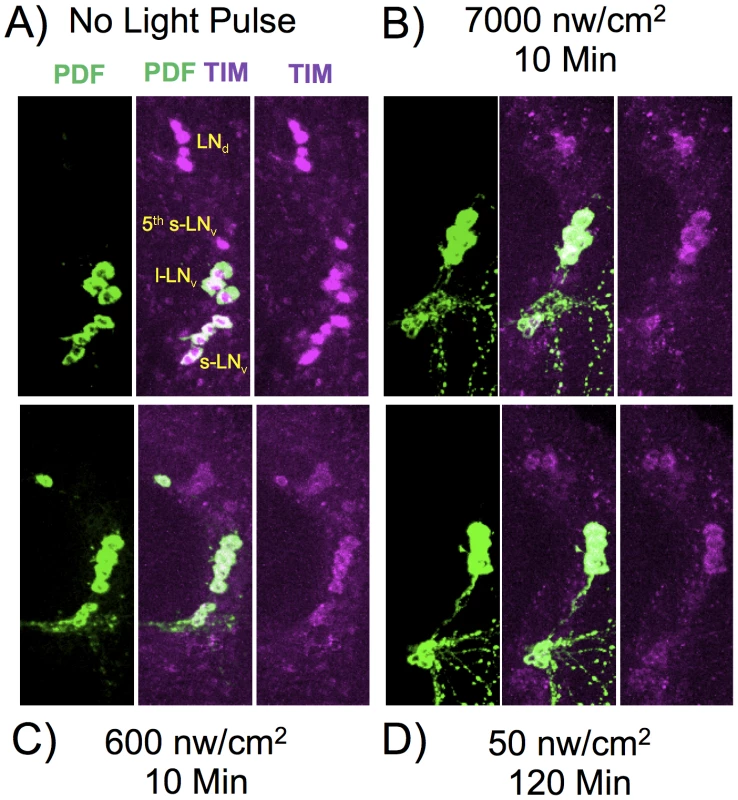
Flies were subject to light pulses at initiating at ZT20 and assayed at ZT22. Blue light pulse conditions: A) No light pulse control. B) 7000 nw/cm2, 10 min. C) 600 nw/cm2 10 min. D) 50 nw/cm2 120 min. Four of the five s-LNv neurons are labeled with PDF as well as TIM. The 5th-sLNv is identified by TIM immunoreactivity and by position and morphology. Fig. 6. Quantitation of TIM levels in the specific LN subsets as shown in Figure 5. 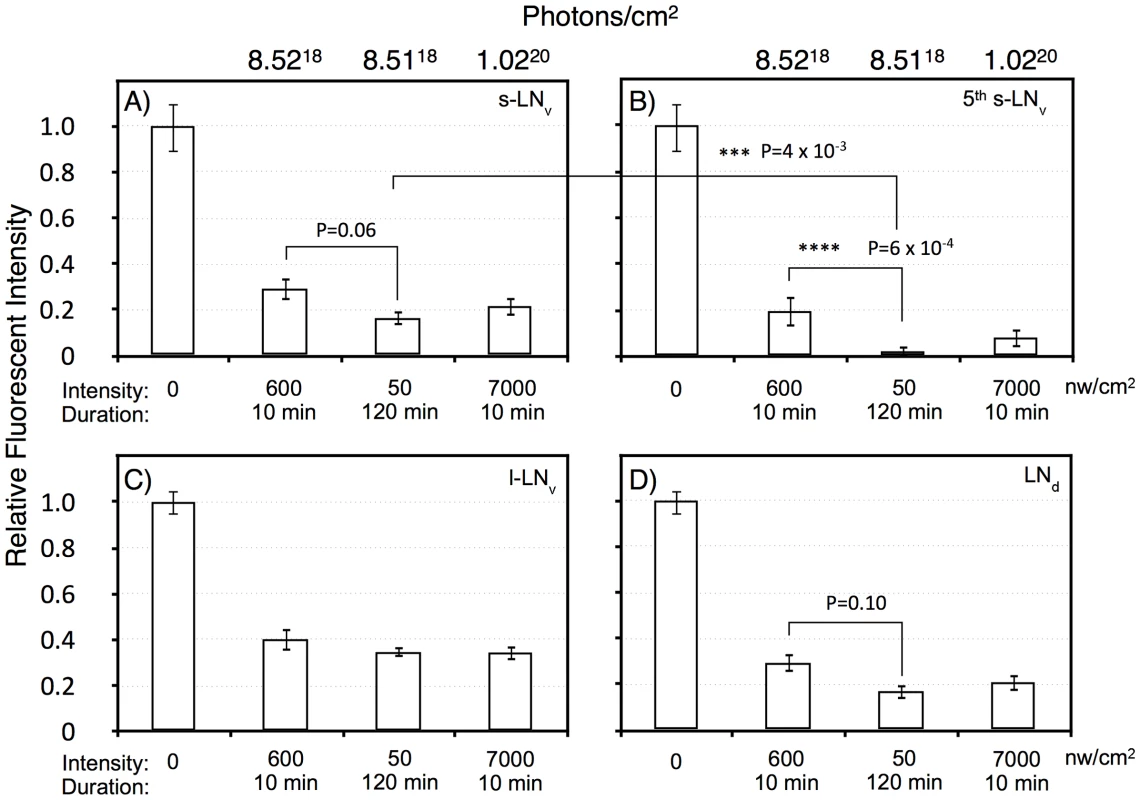
A) s-LNv. B) 5th s-LNv (PDF negative). C) l-LNv. D) LNd. Intensity/durations were designed based on the data in Figure 2. For each neuronal subset, we show the no light pulse control (0), 600 nw/cm2 10 min, representing a roughly half-maximal intensity-duration; 50 nw/cm2 120 min, an equal-photon exposure to the former, and 7,000 nw/cm2, 10 min, yielding a stronger phase shift. All neuronal classes show highly significant TIM degradation in response to light pulses relative to the no pulse control (P<10−6). The PDF negative 5th s-LNv was visible in 10 of 11 LNv clusters in the no pulse control, and the absolute intensity of TIM IR in this neuron was indistinguishable from the s-LNv or any of the other neuronal clusters: 209±21 (SEM, arbitrary fluorescence units), vs 192±19 for the other s-LNv. Thus, the near disappearance of TIM immunoreactivity in the 5th s-LNv in response to the 50 nw/cm2 120 min light pulse cannot be explained by differential detection sensitivity. This neuron showed TIM immunoreactivity in 6 of 11 LNv clusters following the 600 nw/cm2 10 min light pulse, but only 1 of 11 following the 50 nw/cm2 120 min light pulse. Statistics were performed using two way ANOVA on square root transformed data to better approximate normality (Stat Plus, Analystsoft). Two way ANOVA values for the interaction of specific light pulse x cell types are shown in Table S1. The six LNd neurons are heterogeneous, with only three showing detectable CRY immunoreactivity [34], but our analyses do not detect a subset showing differential TIM degradation. All the light pulse conditions show a major effect on TIM levels versus the no pulse control (two way ANOVA, F(3,160) = 104, P = 1×10−13), with all light pulses resulting in at least 60% degradation of TIM. There is a weaker effect of increased intensity of the 10 min light pulses, 600 vs 7,000 nw/cm2, on TIM levels (two way ANOVA, F(1,80) = 4, P = 0.039). This could be due to a kinetic limitation from assaying TIM two hours subsequent to the pulses, or to a non-linearity between TIM degradation and phase shift amplitude.
Temporal integration in response to dim light phase shifts could act at the level of the CRY target TIM, or at a downstream step. Thus, we determined whether any of the neuronal classes showed a signature indicating that temporal integration is acting at the level of TIM degradation. For the equal photon light pulses, 600 nw/cm2 for 10 min vs 50 nw/cm2 for 120 min, there is a significant variation across all the neuronal classes (two way ANOVA, F(1,79) = 14, P = 3×10−4). Similar to the phase shift amplitudes shown in Figure 2, the longer yet dimmer light pulse shows a more severe effect on TIM degradation. This effect is enhanced in the core circadian neurons, the s-LNv's, 5th s-LNv, and the LNd's, as compared to the l-LNv's (two way ANOVA, F(1,84) = 11, P = 0.001). The l-LNv's are not core circadian clock neurons, but instead mediate arousal and the direct effects of light on locomotor activity [27]–[29]. Within the core circadian neurons, the s-LNv class (including the 5th s-LNv) shows significantly enhanced TIM degradation (two way ANOVA, F(1,62) = 12, P = 1×10−3), with a major effect occurring in the 5th s-LNv(two way ANOVA, P = 6×10−4). This unexpected observation indicates that the 5th s-LNv neuron may be particularly important for the process of photic temporal integration.
Discussion
We find that the Drosophila CRY circadian photopigment is extraordinarily efficient at integrating photon information over long time periods extending to at least six hours. This finding aids understanding of the extraordinary light sensitivity of flies to 12∶12 hr LD entrainment [15], and provides a general means by which an inherently low-light sensitivity photopigment can achieve extraordinarily high effective light sensitivity.
Our results indicate that circadian photoreception via Drosophila CRY has unexpected similarities to the structurally distinct vertebrate circadian photoreceptor, melanopsin. Mouse melanopsin generates prolonged electrophysiological responses to single photons in the intrinsically photosensitive retinal ganglion cells (ipRGC's), yet the half-saturating response of the intrinsically photosensitive retinal ganglion cells requires 104–106 fold more photons than cones or rods, respectively [30]. This is largely due to the low pigment density of melanopsin in the ipRGC's relative to the opsin based photopigments in rods and cones, resulting in a low probability of photon capture. Thus, high effective light sensitivity must be generated via temporal integration over a time course of at least minutes [31], [32]. The same process holds for Drosophila CRY, though as we show above, efficient temporal integration can occur over time periods of hours. As with vertebrate melanopsin, dCRY is expressed in neurons lacking any specializations that would serve to concentrate the CRY photopigment. At ‘normal’ non-image forming protein concentrations, probability of photon capture per CRY molecule is low, potentially explaining the extremely high light levels required to visualize short term physiological responses [11], [20]. Thus, temporal integration appears to be a universal principle extending from arthropods to higher vertebrates as a mechanism to enhance physiological light sensitivity for non image forming light responses.
TIM degradation in the circadian pacemaker neurons shows a dose response relationship following limiting light intensity ZT20 light pulse induced phase shifts. This indicates that temporal integration is acting directly at the step of TIM degradation rather than at a downstream signaling step. Among the core circadian lateral neurons, the single 5th-s-LNv neuron shows both the strongest effect of light, and the strongest effect of intensity duration reciprocity. The 5th-s-LNv, characterized by lack of PDF immunoreactivity, is distinguished from the other s-LNv 's as a neuron that is part of a cluster of neurons more involved in evening rather than morning activity, and is thus more related to the ‘evening’ LNd neurons [33]. The enhanced light sensitivity in the 5th-s-LNv implies an important role in dim light detection for this neuron. Since CRY levels in the 5th-s-LNv are not enhanced relative to other pacemaker neurons [34], some other component of the TIM degradation machinery must be more efficient in this neuron.
The described activities of Drosophila CRY have been observed at very high light intensities, ≥1 mw/cm2, ∼6 logs higher than the responses studied here, and ∼8 logs higher than required for dim light LD entrainment [15]. A particularly interesting activity of CRY is a light induced conformational change that persists in the dark with half-times of minutes to tens of minutes depending on oxygen concentration [19], [35]. Bright light exposure of CRY also leads to degradation of both TIM [36] and CRY itself [11], with CRY degradation occurring more slowly than TIM [11], [21] due to enhanced affinity of JET for TIM [14]. The CRY response to light is thus normally self-limiting, in that CRY itself can be degraded in a light dependent fashion. These pathways are at least partly distinct, as evidenced by dependence on distinct factors detected in a forward genetic screen [37].
The above studies in Drosophila could be misleading in several respects with regards to the dim light activity of CRY that we report. First, the high light intensities in which these studies were performed could hide differential light sensitivity of individual reactions. Second, many of the aforementioned studies were performed in cultured cells or in extracts from whole fly heads and thus could be misleading relative to CRY light dependent degradation reactions in the central circadian oscillator neurons of the fly. When examining whole brain extracts, the central oscillator neurons are so few in number that they are effectively hidden by the large number of peripheral CRY-containing neurons [17], and cultured cells that don't themselves have oscillatory capability may lack other factors found in central oscillatory neurons. Finally, the half-maximal light sensitivity of phase advancing light pulses that we measure is far higher than has been observed previously [16], [17], [38]. Part of the explanation is due to our use of monochromatic 466 nm blue light that is well matched to the action spectra of CRY [6], [21]. An additional explanation likely comes from our protocol for light/dark entrainment, in which we use relatively dim green light that is off the spectral maximum for CRY, yet is still able to efficiently entrain flies, presumably via contributions from visual photoreceptors. Bright light during entrainment can lead to destruction of CRY [34], with a half-time for recovery of 12–24 hrs. Thus, brain CRY levels during our light pulses are likely far higher than in previous studies. One could imagine that light-induced destruction of CRY could account for enhanced light sensitivity as durations increase and intensity decreases. However, we find no CRY degradation under the light pulse conditions used in this study (data not shown).
Light intensity is an under-appreciated variable in behavioral studies, with many studies neglecting to measure or mention these values. Our studies, performed at limiting light levels, are showing the importance of being aware of intensity as a critical variable. That Drosophila CRY and vertebrate melanopsin use a similar dim light integration mechanism indicates a striking example of an evolutionarily convergent solution to the problem of dim light detection in non image forming neurons.
Materials and Methods
The dim light source is 5050 RGB Waterproof SMD Flexible LED Strips, 30 LEDs/meter, currently available from several suppliers on eBay. Each LED consists of separately addressable RGB LEDs, with sets of three LED connected in series with internal resistors, & groups of three then connected in parallel. Maximal output of the blue LED is at 466 nm, and the green LED is at 521 nm (Figure S1) as measured with a spectrophotometer (Ocean Optics, USB4000). LED strips were cut to ∼18″ lengths and mounted behind a light diffusing opaque plexiglass plate. Intensity was controlled by varying the voltage supplied to the LEDs, and light intensities measured as detailed in [15].
Light pulses were given to flies entrained for several days to 2 µw/cm2 green light (5.2×106 photons/cm2/sec) from the LED strips, using green light to limit possible photopigment degradation [34]. Pulses were given on the evening of the last day of light.
Fly strains
The null alleles cry02 [39] and norpAP41 [23] were obtained from the laboratory of Herman Wijnen. Since both strains are in a w mutant background, the w1118 strain was used as a wild type control. Sequence analyses (data not shown) show that each of these strains contains the s-tim allele [40].
Flies were housed in Trikinetics (Waltham, MA) 5 mm activity monitor tubes in light tight coffins in a room controlled to ∼21C and 55–60% relative humidity. Phase changes following light pulses were assayed by detecting computer called activity off times using Clocklab software (Actimetrics Corp, Wilmette, IL). Phase shift data was accumulated on individual flies. Statistics were determined by ANOVA with post-hoc adjustment for multiple comparisons.
Immunofluorescence
Whole flies were fixed for 2.5 hrs at room temperature in 4% PFA with 0.1% Triton X-100, followed by four 10 min washes in PBS (130 mM NaCl, 7 mM Na2HPO4, 3 mM KH2PO4), then stored in fresh PBS+0.01% NaN3.
Brains were dissected and washed in PBT (PBS with 0.1% BSA and 0.3% Triton X-100) 2×5 min, then incubated overnight with primary antibodies diluted in PBT. This was followed by three 5 min and two 30 min washes in PBT with 1% goat serum, 6 hr incubation in secondary antibodies diluted in the same solution, and then brains are washed in PBT four times over a 24 hr period, followed by a final wash in PBS prior to mounting in Vectashield mounting medium (Vector laboratories).
Antibodies
Here we used rat anti-Tim (1∶1000; kindly provided by Jadwiga Giebultowicz) & mouse anti-PDF antibody (1∶1000; Developmental Studies Hybridoma Bank, University of Iowa), secondaries are goat anti-mouse (PDF) IgG conjugated to AlexaFluor 488 (Molecular Probes) and goat anti-rat (Tim) IgG conjugated to Cy3 (Jackson ImmunoResearch).
Confocal microscopy was performed with a Fluoview300 (Olympus).
Quantitation
The quantification of the TIM staining intensity was performed on single confocal images with ImageJ (http://rsbweb.nih.gov/ij/) as previously described [41]. PDF immunostaining was used to identify LNv's. First we chose the appropriate slice within stack for each neuron, then selected ROI based on PDF staining (except for LNd's & the 5th s-LNv), and measured mean pixel intensity of TIM staining. To compensate for background, the mean pixel intensity surrounding the neuron was subtracted. The compensated values were averaged for all neurons of a given class. We used one hemisphere per brain for the quantification and calculated mean ± SEM from 11 hemispheres of 11 brains. We could not measure any bleed through from intense PDF positive neuronal projections into the TIM channel.
Supporting Information
Zdroje
1. GolombekDA, RosensteinRE (2010) Physiology of circadian entrainment. Physiol Rev 90 : 1063–1102.
2. HardinPE (2011) Molecular genetic analysis of circadian timekeeping in Drosophila. Adv Genet 74 : 141–173.
3. PeschelN, Helfrich-ForsterC (2011) Setting the clock–by nature: circadian rhythm in the fruitfly Drosophila melanogaster. FEBS Lett 585 : 1435–1442.
4. Helfrich-ForsterC, WinterC, HofbauerA, HallJC, StanewskyR (2001) The circadian clock of fruit flies is blind after elimination of all known photoreceptors. Neuron 30 : 249–261.
5. WheelerDA, Hamblen-CoyleMJ, DushayMS, HallJC (1993) Behavior in light-dark cycles of Drosophila mutants that are arrhythmic, blind, or both. J Biol Rhythms 8 : 67–94.
6. SuriV, QianZ, HallJC, RosbashM (1998) Evidence that the TIM light response is relevant to light-induced phase shifts in Drosophila melanogaster. Neuron 21 : 225–234.
7. YangZ, EmersonM, SuHS, SehgalA (1998) Response of the timeless protein to light correlates with behavioral entrainment and suggests a nonvisual pathway for circadian photoreception. Neuron 21 : 215–223.
8. EmeryP, StanewskyR, HallJC, RosbashM (2000) A unique circadian-rhythm photoreceptor. Nature 404 : 456–457.
9. KlarsfeldA, MalpelS, Michard-VanheeC, PicotM, ChelotE, et al. (2004) Novel features of cryptochrome-mediated photoreception in the brain circadian clock of Drosophila. J Neurosci 24 : 1468–1477.
10. VeleriS, BrandesC, Helfrich-ForsterC, HallJC, StanewskyR (2003) A self-sustaining, light-entrainable circadian oscillator in the Drosophila brain. Curr Biol 13 : 1758–1767.
11. LinFJ, SongW, Meyer-BernsteinE, NaidooN, SehgalA (2001) Photic signaling by cryptochrome in the Drosophila circadian system. Mol Cell Biol 21 : 7287–7294.
12. CollinsB, MazzoniEO, StanewskyR, BlauJ (2006) Drosophila CRYPTOCHROME is a circadian transcriptional repressor. Curr Biol 16 : 441–449.
13. IvanchenkoM, StanewskyR, GiebultowiczJM (2001) Circadian photoreception in Drosophila: functions of cryptochrome in peripheral and central clocks. J Biol Rhythms 16 : 205–215.
14. PeschelN, ChenKF, SzaboG, StanewskyR (2009) Light-dependent interactions between the Drosophila circadian clock factors cryptochrome, jetlag, and timeless. Curr Biol 19 : 241–247.
15. HirshJ, RiemenspergerT, CoulomH, IcheM, CouparJ, et al. (2010) Roles of dopamine in circadian rhythmicity and extreme light sensitivity of circadian entrainment. Curr Biol 20 : 209–214.
16. YuanQ, LinF, ZhengX, SehgalA (2005) Serotonin modulates circadian entrainment in Drosophila. Neuron 47 : 115–127.
17. EmeryP, StanewskyR, Helfrich-ForsterC, Emery-LeM, HallJC, et al. (2000) Drosophila CRY is a deep brain circadian photoreceptor. Neuron 26 : 493–504.
18. FrankKD, ZimmermanWF (1969) Action spectra for phase shifts of a circadian rhythm in Drosophila. Science 163 : 688–689.
19. OzturkN, SelbyCP, AnnayevY, ZhongD, SancarA (2011) Reaction mechanism of Drosophila cryptochrome. Proc Natl Acad Sci U S A 108 : 516–521.
20. FogleKJ, ParsonKG, DahmNA, HolmesTC (2011) CRYPTOCHROME is a blue-light sensor that regulates neuronal firing rate. Science 331 : 1409–1413.
21. BuszaA, Emery-LeM, RosbashM, EmeryP (2004) Roles of the two Drosophila CRYPTOCHROME structural domains in circadian photoreception. Science 304 : 1503–1506.
22. BloomquistBT, ShortridgeRD, SchneuwlyS, PerdewM, MontellC, et al. (1988) Isolation of a putative phospholipase C gene of Drosophila, norpA, and its role in phototransduction. Cell 54 : 723–733.
23. SzularJ, SehadovaH, GentileC, SzaboG, ChouWH, et al. (2012) Rhodopsin 5 - and Rhodopsin 6-mediated clock synchronization in Drosophila melanogaster is independent of retinal phospholipase C-beta signaling. J Biol Rhythms 27 : 25–36.
24. KistenpfennigC, HirshJ, YoshiiT, Helfrich-ForsterC (2012) Phase-Shifting the Fruit Fly Clock without Cryptochrome. J Biol Rhythms 27 : 117–125.
25. StanewskyR, KanekoM, EmeryP, BerettaB, Wager-SmithK, et al. (1998) The cryb mutation identifies cryptochrome as a circadian photoreceptor in Drosophila. Cell 95 : 681–692.
26. HardieRC (2001) Phototransduction in Drosophila melanogaster. J Exp Biol 204 : 3403–3409.
27. PariskyKM, AgostoJ, PulverSR, ShangY, KuklinE, et al. (2008) PDF cells are a GABA-responsive wake-promoting component of the Drosophila sleep circuit. Neuron 60 : 672–682.
28. SheebaV, FogleKJ, KanekoM, RashidS, ChouYT, et al. (2008) Large ventral lateral neurons modulate arousal and sleep in Drosophila. Curr Biol 18 : 1537–1545.
29. ShangY, GriffithLC, RosbashM (2008) Light-arousal and circadian photoreception circuits intersect at the large PDF cells of the Drosophila brain. Proc Natl Acad Sci U S A 105 : 19587–19594.
30. DoMT, KangSH, XueT, ZhongH, LiaoHW, et al. (2009) Photon capture and signalling by melanopsin retinal ganglion cells. Nature 457 : 281–287.
31. NelsonDE, TakahashiJS (1991) Sensitivity and integration in a visual pathway for circadian entrainment in the hamster (Mesocricetus auratus). J Physiol 439 : 115–145.
32. Van Den PolAN, CaoV, HellerHC (1998) Circadian system of mice integrates brief light stimuli. Am J Physiol 275: R654–657.
33. RiegerD, ShaferOT, TomiokaK, Helfrich-ForsterC (2006) Functional analysis of circadian pacemaker neurons in Drosophila melanogaster. J Neurosci 26 : 2531–2543.
34. YoshiiT, TodoT, WulbeckC, StanewskyR, Helfrich-ForsterC (2008) Cryptochrome is present in the compound eyes and a subset of Drosophila's clock neurons. J Comp Neurol 508 : 952–966.
35. BerndtA, KottkeT, BreitkreuzH, DvorskyR, HennigS, et al. (2007) A novel photoreaction mechanism for the circadian blue light photoreceptor Drosophila cryptochrome. J Biol Chem 282 : 13011–13021.
36. MyersMP, Wager-SmithK, Rothenfluh-HilfikerA, YoungMW (1996) Light-induced degradation of TIMELESS and entrainment of the Drosophila circadian clock. Science 271 : 1736–1740.
37. SathyanarayananS, ZhengX, KumarS, ChenCH, ChenD, et al. (2008) Identification of novel genes involved in light-dependent CRY degradation through a genome-wide RNAi screen. Genes Dev 22 : 1522–1533.
38. IshikawaT, MatsumotoA, KatoTJr, TogashiS, RyoH, et al. (1999) DCRY is a Drosophila photoreceptor protein implicated in light entrainment of circadian rhythm. Genes Cells 4 : 57–65.
39. DolezelovaE, DolezelD, HallJC (2007) Rhythm defects caused by newly engineered null mutations in Drosophila's cryptochrome gene. Genetics 177 : 329–345.
40. KohK, ZhengX, SehgalA (2006) JETLAG resets the Drosophila circadian clock by promoting light-induced degradation of TIMELESS. Science 312 : 1809–1812.
41. YoshiiT, VaninS, CostaR, Helfrich-ForsterC (2009) Synergic entrainment of Drosophila's circadian clock by light and temperature. J Biol Rhythms 24 : 452–464.
Štítky
Genetika Reprodukční medicína
Článek Independent Evolution of Transcriptional Inactivation on Sex Chromosomes in Birds and MammalsČlánek The bHLH Subgroup IIId Factors Negatively Regulate Jasmonate-Mediated Plant Defense and DevelopmentČlánek Selective Pressures to Maintain Attachment Site Specificity of Integrative and Conjugative ElementsČlánek Reassembly of Nucleosomes at the Promoter Initiates Resilencing Following Decitabine ExposureČlánek Hepatocyte Growth Factor Signaling in Intrapancreatic Ductal Cells Drives Pancreatic Morphogenesis
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2013 Číslo 7- Akutní intermitentní porfyrie
- Farmakogenetické testování pomáhá předcházet nežádoucím efektům léčiv
- Růst a vývoj dětí narozených pomocí IVF
- Pilotní studie: stres a úzkost v průběhu IVF cyklu
- Vliv melatoninu a cirkadiálního rytmu na ženskou reprodukci
-
Všechny články tohoto čísla
- An Solution for Crossover Formation
- Genome-Wide Association Mapping in Dogs Enables Identification of the Homeobox Gene, , as a Genetic Component of Neural Tube Defects in Humans
- Independent Evolution of Transcriptional Inactivation on Sex Chromosomes in Birds and Mammals
- Stepwise Activation of the ATR Signaling Pathway upon Increasing Replication Stress Impacts Fragile Site Integrity
- Genomic Analysis of Natural Selection and Phenotypic Variation in High-Altitude Mongolians
- Modification of tRNA by Elongator Is Essential for Efficient Translation of Stress mRNAs
- Role of CTCF Protein in Regulating Locus Transcription
- Gene Set Signature of Reversal Reaction Type I in Leprosy Patients
- Mapping of PARK2 and PACRG Overlapping Regulatory Region Reveals LD Structure and Functional Variants in Association with Leprosy in Unrelated Indian Population Groups
- Is Required for Formation of the Genital Ridge in Mice
- Monopolin Subunit Csm1 Associates with MIND Complex to Establish Monopolar Attachment of Sister Kinetochores at Meiosis I
- Recombination Dynamics of a Human Y-Chromosomal Palindrome: Rapid GC-Biased Gene Conversion, Multi-kilobase Conversion Tracts, and Rare Inversions
- Mechanisms of Protein Sequence Divergence and Incompatibility
- Histone Methyltransferase DOT1L Drives Recovery of Gene Expression after a Genotoxic Attack
- Female Behaviour Drives Expression and Evolution of Gustatory Receptors in Butterflies
- Combinatorial Regulation of Meiotic Holliday Junction Resolution in by HIM-6 (BLM) Helicase, SLX-4, and the SLX-1, MUS-81 and XPF-1 Nucleases
- The bHLH Subgroup IIId Factors Negatively Regulate Jasmonate-Mediated Plant Defense and Development
- The Role of Interruptions in polyQ in the Pathology of SCA1
- Dietary Restriction Induced Longevity Is Mediated by Nuclear Receptor NHR-62 in
- Fine Time Course Expression Analysis Identifies Cascades of Activation and Repression and Maps a Putative Regulator of Mammalian Sex Determination
- Genome-scale Co-evolutionary Inference Identifies Functions and Clients of Bacterial Hsp90
- Oxidative Stress and Replication-Independent DNA Breakage Induced by Arsenic in
- A Moonlighting Enzyme Links Cell Size with Central Metabolism
- Budding Yeast Greatwall and Endosulfines Control Activity and Spatial Regulation of PP2A for Timely Mitotic Progression
- The Conserved Intronic Cleavage and Polyadenylation Site of CstF-77 Gene Imparts Control of 3′ End Processing Activity through Feedback Autoregulation and by U1 snRNP
- The BTB-zinc Finger Transcription Factor Abrupt Acts as an Epithelial Oncogene in through Maintaining a Progenitor-like Cell State
- The Cohesion Protein SOLO Associates with SMC1 and Is Required for Synapsis, Recombination, Homolog Bias and Cohesion and Pairing of Centromeres in Drosophila Meiosis
- The RNA-binding Proteins FMR1, Rasputin and Caprin Act Together with the UBA Protein Lingerer to Restrict Tissue Growth in
- Pattern Dynamics in Adaxial-Abaxial Specific Gene Expression Are Modulated by a Plastid Retrograde Signal during Leaf Development
- A Network of HMG-box Transcription Factors Regulates Sexual Cycle in the Fungus
- Bacterial Adaptation through Loss of Function
- ENU-induced Mutation in the DNA-binding Domain of KLF3 Reveals Important Roles for KLF3 in Cardiovascular Development and Function in Mice
- Interplay between Structure-Specific Endonucleases for Crossover Control during Meiosis
- FGF Signalling Regulates Chromatin Organisation during Neural Differentiation via Mechanisms that Can Be Uncoupled from Transcription
- The Arabidopsis RNA Binding Protein with K Homology Motifs, SHINY1, Interacts with the C-terminal Domain Phosphatase-like 1 (CPL1) to Repress Stress-Inducible Gene Expression
- Selective Pressures to Maintain Attachment Site Specificity of Integrative and Conjugative Elements
- The Conserved ADAMTS-like Protein Lonely heart Mediates Matrix Formation and Cardiac Tissue Integrity
- The cGMP-Dependent Protein Kinase EGL-4 Regulates Nociceptive Behavioral Sensitivity
- RBM5 Is a Male Germ Cell Splicing Factor and Is Required for Spermatid Differentiation and Male Fertility
- Disease-Related Growth Factor and Embryonic Signaling Pathways Modulate an Enhancer of Expression at the 6q23.2 Coronary Heart Disease Locus
- Yeast Pol4 Promotes Tel1-Regulated Chromosomal Translocations
- A Dual Role for SOX10 in the Maintenance of the Postnatal Melanocyte Lineage and the Differentiation of Melanocyte Stem Cell Progenitors
- SLC26A4 Targeted to the Endolymphatic Sac Rescues Hearing and Balance in Mutant Mice
- Odoriferous Defensive Stink Gland Transcriptome to Identify Novel Genes Necessary for Quinone Synthesis in the Red Flour Beetle,
- Prediction of Complex Human Traits Using the Genomic Best Linear Unbiased Predictor
- Gene × Physical Activity Interactions in Obesity: Combined Analysis of 111,421 Individuals of European Ancestry
- Reassembly of Nucleosomes at the Promoter Initiates Resilencing Following Decitabine Exposure
- Exquisite Light Sensitivity of Cryptochrome
- miR-133a Regulates Adipocyte Browning In Vivo
- Strabismus Promotes Recruitment and Degradation of Farnesylated Prickle in Planar Polarity Specification
- Hepatocyte Growth Factor Signaling in Intrapancreatic Ductal Cells Drives Pancreatic Morphogenesis
- Is a Potential Tumor Suppressor Gene Commonly Inactivated by Epigenetic Mechanisms in Colorectal Cancer
- Joint Molecule Resolution Requires the Redundant Activities of MUS-81 and XPF-1 during Meiosis
- The Mating Competence of Geographically Diverse Strains in Their Natural and Unnatural Sand Fly Vectors
- Defective Repair of Oxidative Base Lesions by the DNA Glycosylase Nth1 Associates with Multiple Telomere Defects
- Effective Blocking of the Enhancer Requires Cooperation between Two Main Mechanisms Suggested for the Insulator Function
- Trans-Ancestral Studies Fine Map the SLE-Susceptibility Locus
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- SLC26A4 Targeted to the Endolymphatic Sac Rescues Hearing and Balance in Mutant Mice
- Bacterial Adaptation through Loss of Function
- The Cohesion Protein SOLO Associates with SMC1 and Is Required for Synapsis, Recombination, Homolog Bias and Cohesion and Pairing of Centromeres in Drosophila Meiosis
- Gene × Physical Activity Interactions in Obesity: Combined Analysis of 111,421 Individuals of European Ancestry
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



