-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
A Dual Role for SOX10 in the Maintenance of the Postnatal Melanocyte Lineage and the Differentiation of Melanocyte Stem Cell Progenitors
During embryogenesis, the transcription factor, Sox10, drives the survival and differentiation of the melanocyte lineage. However, the role that Sox10 plays in postnatal melanocytes is not established. We show in vivo that melanocyte stem cells (McSCs) and more differentiated melanocytes express SOX10 but that McSCs remain undifferentiated. Sox10 knockout (Sox10fl; Tg(Tyr::CreER)) results in loss of both McSCs and differentiated melanocytes, while overexpression of Sox10 (Tg(DctSox10)) causes premature differentiation and loss of McSCs, leading to hair graying. This suggests that levels of SOX10 are key to normal McSC function and Sox10 must be downregulated for McSC establishment and maintenance. We examined whether the mechanism of Tg(DctSox10) hair graying is through increased expression of Mitf, a target of SOX10, by asking if haploinsufficiency for Mitf (Mitfvga9) can rescue hair graying in Tg(DctSox10) animals. Surprisingly, Mitfvga9 does not mitigate but exacerbates Tg(DctSox10) hair graying suggesting that MITF participates in the negative regulation of Sox10 in McSCs. These observations demonstrate that while SOX10 is necessary to maintain the postnatal melanocyte lineage it is simultaneously prevented from driving differentiation in the McSCs. This data illustrates how tissue-specific stem cells can arise from lineage-specified precursors through the regulation of the very transcription factors important in defining that lineage.
Published in the journal: . PLoS Genet 9(7): e32767. doi:10.1371/journal.pgen.1003644
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1003644Summary
During embryogenesis, the transcription factor, Sox10, drives the survival and differentiation of the melanocyte lineage. However, the role that Sox10 plays in postnatal melanocytes is not established. We show in vivo that melanocyte stem cells (McSCs) and more differentiated melanocytes express SOX10 but that McSCs remain undifferentiated. Sox10 knockout (Sox10fl; Tg(Tyr::CreER)) results in loss of both McSCs and differentiated melanocytes, while overexpression of Sox10 (Tg(DctSox10)) causes premature differentiation and loss of McSCs, leading to hair graying. This suggests that levels of SOX10 are key to normal McSC function and Sox10 must be downregulated for McSC establishment and maintenance. We examined whether the mechanism of Tg(DctSox10) hair graying is through increased expression of Mitf, a target of SOX10, by asking if haploinsufficiency for Mitf (Mitfvga9) can rescue hair graying in Tg(DctSox10) animals. Surprisingly, Mitfvga9 does not mitigate but exacerbates Tg(DctSox10) hair graying suggesting that MITF participates in the negative regulation of Sox10 in McSCs. These observations demonstrate that while SOX10 is necessary to maintain the postnatal melanocyte lineage it is simultaneously prevented from driving differentiation in the McSCs. This data illustrates how tissue-specific stem cells can arise from lineage-specified precursors through the regulation of the very transcription factors important in defining that lineage.
Introduction
In the adult animal, tissue-specific stem cells exist in a number of organs and function to sustain these tissues during normal homeostasis. However, our understanding of the origin and establishment of tissue-specific stem cells during organogenesis is incomplete. Using melanocytes as a model, we investigated the process of lineage-specific stem cell fate acquisition by examining the role of the transcription factor SOX10 in the formation of the melanocyte stem cell (McSC) within the mouse hair follicle.
Melanocytes of the hair follicle have gained increasing attention for studying cell-specific contributions to organ development and maintenance. Individual hair follicles act as ‘mini-organs’ [1], and each contains melanocytes that provide pigment to the hair shaft concomitantly with hair cycling. Two primary subpopulations of follicular melanocytes exist and are defined by their anatomical location—McSCs remain in hair bulge, whereas the terminally-differentiated and pigmented melanocytes reside in the transient hair bulb region [2], [3]. Identification of each of these subpopulations has been defined molecularly, in part through the use of immunohistochemistry [4]–[7]. Disruption of McSC function results in hair graying, a non-lethal and visible phenotype, and gray-haired mouse models have been used successfully to study adult stem cell establishment and maintenance [7]–[11].
The most critical time point for establishing McSCs appears to be during hair morphogenesis. Studies using the KIT-blocking antibody, ACK, to deplete melanocyte populations perinatally show that McSCs inhabit hair follicles around P4, demonstrated by the fact that they survive independent of KIT-signaling and are sufficient to restore coat color pigmentation [2], [6]. Many of the melanogenic genes expressed by melanoblasts or bulb melanocytes exist at low/absent levels in McSCs. This distinction arises between stages 6 and 8 of hair follicle morphogenesis (∼P4–8) and is indicated by the loss of ki67 expression and the downregulation of MITF, TRP1, TYR, and SOX10 within presumptive McSCs [4], [7], [12]. Although McSCs are not responsible for pigmenting the first morphogenetic hair [3], they are retained within the hair bulge while melanocytes of the hair bulb undergo apoptosis during hair regression [13], [14]. These McSCs then function to regenerate bulb melanocytes during subsequent hair cycles [8].
The subpopulation-specific expression of the transcription factor Sox10, where it is expressed in melanoblasts of the skin and melanocytes of the hair bulb but absent from McSCs, suggests that transcriptionally downregulating Sox10 is the mechanism by which melanoblasts acquire a McSC fate. This hypothesis fits well with the known function of Sox10 as a transcription factor that participates in melanocyte differentiation by upregulating Mitf, the master regulatory gene for melanogenesis. The loss of melanin synthesis proteins, TRP1 and TYR, within presumptive McSCs further supports this idea since SOX10 transcriptionally activates these genes, and that TYR is required by mouse melanocytes to generate pigment [15]–[18]. In the mouse, Sox10 is expressed during neural crest development and its loss embryonically results in several neurocristopathies, including congenital hypopigmentation [19]–[24]. However, perinatal lethality in Sox10 null mice has precluded functional analysis of Sox10 in adult melanocytes. Using conditional transgenics we can now explore the role of Sox10 postnatally in the melanocytes of the mouse hair follicle.
Here we report that postnatal mouse melanocytes both express and require Sox10 for normal hair pigmentation. However, constitutive expression of Sox10 by McSCs disrupts their maintenance by driving their premature differentiation. We also demonstrate that Mitf contributes to this regulation, likely through a negative feedback mechanism. Together, these data support the theory that transcription factors responsible for the specification of lineage-defined precursors can later participate in the specification and maintenance of stem cells derived from those precursors.
Results
SOX10 is retained postnatally by McSCs and differentiated melanocytes
The expression of SOX10 within the postnatal McSCs and differentiated melanocytes of the hair bulb was compared to the expression of the melanocyte marker, dopachrome tautomerase (DCT). In this study, we define the McSC population by several characteristics: cells that exist within the hair bulge, are capable of self-renewal, and can give rise to melanocyte progenitors that colonize the newly developing hair bulb. Previously, we have shown that the transgenic line, Tyr::CreERT2, can target cells with these properties when induced either during postnatal development or within adults [25]. To specifically demonstrate that DCT marks this McSC population, we performed a similar lineage mapping analysis here (Fig. S1). Tyr::CreERT2; Rosa26tm1sor pups were given a pulse of tamoxifen (TAM) on postnatal days 2 and 3 (P2–3), and assessed for recombined cells by β-galactosidase staining. Melanocytes in Tyr::CreERT2 mice express CRE at P2, and within the same hair cycle as TAM treatment (P14), we observed LacZ+ cells in the hair bulge and bulb (Fig. S1A–B). This suggests by anatomical position that we have targeted both McSCs and differentiated melanocytes. We further confirmed that these LacZ+ bulge cells are McSCs by challenging them to repopulate new hairs after hair plucking. Hair plucking eliminates all differentiated melanocytes leaving only the McSCs to replenish newly generated hairs. Indeed, seven days after initiating a new hair cycle (7 days post plucking, 7dpp) we observed the retention of LacZ+ cells in the hair bulge and LacZ+ progeny in the hair bulb (Fig. S1C). This confirms that these LacZ+ bulge cells are indeed McSCs. We further analyzed these LacZ+ McSCs with immunolabeling (Fig. S1D) and confirm that nearly all (97%) express DCT. Thus for the remainder of our analysis we refer to DCT+ cells within the hair bulge as the McSC population.
Next we examined SOX10 expression in postnatal melanocytes during several key stages of hair morphogenesis and hair cycling (Fig. 1A); P2, P6, P14, adult anagen III/IV (7dpp) and adult catagen VII (21 days post plucking, 21dpp). Despite the availability of a number of useful fluorescent Sox10 reporter mice [26]–[28], we opted to characterize protein expression with immunolabeling as this method is applicable to non-transgenic mouse studies. Using antibodies, we observed that nearly all DCT+ cells within hairs co-express SOX10 regardless of time point, location or differentiation status (Fig. S2).
Fig. 1. Differentiation status of LPP and UTP melanocytes varies with hair cycling. 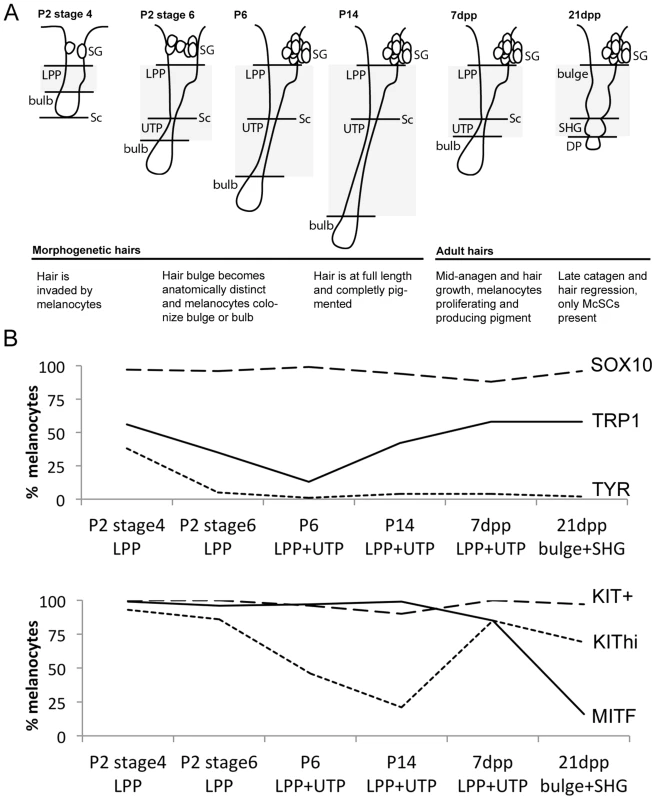
(A) Schematic of hair follicles during hair morphogenesis (P2 stage 4; P2 stage 6, P6, and P14) and adult hair cycling (7dpp, and 21dpp). Grayed area represents anatomical regions quantified in B. (B) Percent of melanocytes that double-label with DCT and the indicated marker. Counts include melanocytes located within the LPP plus UTP at P2, P6, P14, and 7dpp or bulge and secondary hair germ at 21dpp (complete data available in Table S1 and Fig. S2, S3, S4, S5, S6). KIT expression is reported as KIThi being melanocytes displaying high intensity fluorescence and KIT+ being melanocytes positive for KIT independent of fluorescence intensity. LPP, lower permanent portion of the hair; UTP, upper transitory portion of the hair; dpp, days post plucking; SHG, secondary hair germ of the hair; DP, dermal papilla; SG, sebaceous gland; Sc, subcutis. We expanded our results for the McSC population by quantifying the percentage of DCT+/SOX10+ cells within the lower permanent portion (LPP) of the hair (hair bulge) and the upper transitory portion (UTP) of the hair since these regions contained the majority of DCT+ melanocytes that exist along the hair shaft (Fig. 1B, Table S1). As defined previously, the LPP extends from the opening of the sebaceous gland to the junction between the dermis and subcutis, and the UTP sits between the same junction and the hair bulb (Fig. 1A, [29]). In late catagen hairs (21dpp), the entire follicle exists within the dermis and is divided into the hair bulge and the secondary hair germ (SHG), with the SHG visible as a small cluster of cells between the hair bulge and dermal papilla (Fig. 1A, [30]). Between P2 and P14, SOX10 is detected in 94–99% of LPP+UTP melanocytes (Fig. 1B). SOX10+/DCT+ cells comprise 87% of the LPP+UTP melanocytes at anagen (7dpp), and 96% of bulge+SHG melanocytes at catagen (21dpp, Fig. 1B). This result contradicts previous reports showing that Sox10 mRNA and protein are downregulated by the melanocytes that have colonized the hair bulge beginning at P2, and is absent in melanocytes found in catagen stage hairs [4], [31]. However, we suspect that higher sensitivity of our SOX10 antibody may limit our ability to distinguish melanocytes with variable levels of SOX10 expression, thus explaining our observation that DCT and SOX10 co-label more bulge melanocytes than previously reported.
In contrast to SOX10, the expression patterns of other melanocyte markers, MITF, KIT, TRP1 and TYR, within postnatal hairs is more variable (Fig. S3, S4, S5, S6). Beginning at P2, the majority of DCT+ LPP melanocytes in stage 4 hairs double-label with MITF and KIT whereas only 56% and 38% express the melanogenic enzymes TRP1 and TYR, respectively (Fig. 1B, Table S1). As hairs progress to stage 6 of their morphogenesis, nearly all LPP+UTP melanocytes continue to express MITF while the percentage of LPP+UTP melanocytes expressing TRP1 and TYR decreases. Previously, the intensity of KIT expression within the McSC niche was observed to be bipolar, either KIThigh/+ or KITlow/−, with the KITlow/− melanocytes corresponding to the McSC population [3], [32]. Although we rarely saw KIT− melanocytes at any stage, we did observe an emergence of DCT+/KITlow LPP+UTP cells in stage 6 hairs of P2 skins. By P14, LPP+UTP melanocytes have downregulated their differentiation markers and exist predominately in a SOX10+/MITF+/KITlow state. Strikingly, the initiation of anagen (7dpp) corresponds with a dramatic escalation of the percentage KIThigh cells and a moderate increase of TRP1+ cells amongst LPP+UTP melanocytes. In contrast, entry into catagen (21dpp) is associated with LPP+UTP melanocytes downregulating MITF while still retaining TRP1, KIT and SOX10.
Across all hairs that contain bulbs (excluding catagen hairs), DCT+ melanocytes within the hair bulb also double-label with SOX10, MITF, TRP1 and TYR (Fig. S2, S3, S4, S5). KIT, on the other hand is robustly detected amongst melanocytes colonizing the bulbs of stage 4 morphogenetic hairs, but expressed with varying intensity in the matrix of older hairs (Fig. S6).
This analysis demonstrates that while the differentiation status of melanocytes that exists within the hair bulge fluctuates in concert with hair morphogenesis and adult hair cycling, SOX10 expression remains static amongst LPP and UTP melanocytes. We next assessed how perturbation of Sox10 influences these expression patterns within follicular melanocytes.
Sox10 is required for the retention of McSCs and differentiated melanocytes and for pigment production
In light of our discovery that SOX10 is retained by both McSCs and differentiated bulb melanocytes within the hair follicle, paired with previous in vitro experiments indicating that TYR expression in mouse is Sox10-dependent [15], we anticipate Sox10 plays an important role in postnatal melanocyte biology. We tested this by generating Sox10fl/fl; Tyr::CreERT2 mice to conditionally knockout Sox10 in mouse melanocytes postnatally [33], [34]. Previously, we confirmed that Tyr::CreERT2 is effective at inducing recombination of floxed alleles in a significant number of McSCs as well as the more differentiated melanocytes when TAM is administered transiently during perinatal growth or during adult anagen [25]. Using the same approach, we administered TAM in a pulse-like fashion to both pups and adult animals. Pups were given TAM just prior to their initial hair growth by receiving breastmilk from lactating mothers injected intraperitoneally (IP) with TAM on P0–P3. Adults, at approximately eight weeks of age, were plucked on their lower backs to induce anagen, and TAM was administered by IP injection on the same day as plucking and for three additional days (0–3dpp). In both cases, we observed hypopigmentation in a subset of the hairs in the Sox10fl/fl; Tyr::CreERT2 mice in regions of the skin where newly grown hairs were emerging. This loss of pigmentation was not observed in similarly-treated Sox10fl/fl and Sox10fl/+;Tyr::CreERT2 mice or Sox10fl/fl; Tyr::CreERT2 mice that were not treated with tamoxifen (Fig. 2A–B, D–E; Fig. S7A–B). Using PAX3 as a marker for melanocytes, we found that this lack of pigmentation is associated with an overall reduction in the number of differentiated melanocytes per hair bulb, with a significant percentage of hair bulbs lacking melanocytes altogether (Fig. 2C, F). This indicates that Sox10 is required for the retention of differentiated melanocytes in the hair. In Sox10fl/fl; Tyr::CreERT2 animals, we also observed a population of PAX3+/SOX10− cells within the melanocytic region of the hair matrix whose presence correlated with hair bulbs that contained little or no pigmentation (Fig. 2G–I). This was particularly noticeable in TAM-treated Sox10fl/fl; Tyr::CreERT2 adults (Fig. 2I). This indicates that Sox10 is also required by bulb melanocytes to differentiate, or produce pigment. These PAX3+/SOX10− cells also do not express MITF, a SOX10 target gene, suggesting that the reduced pigment seen in SOX10− bulb melanocytes is likely a result of aberrant melanocytic transcriptional regulation (Fig. S7C–D). The fate of melanocytes lacking Sox10 remains unclear as positive staining for the apoptosis markers CC3 and/or TUNEL is not correlated with PAX3+/SOX10− bulb cells or non-pigmented hairs in tamoxifen-treated Sox10fl/fl; Tyr::CreERT2 pups or adult animals (not shown). Nevertheless, these data demonstrate that the hypopigmentation observed with Sox10 knockout is due to an overall loss of bulb melanocytes and a deficiency in their ability to produce pigment.
Fig. 2. Sox10 is required by bulb melanocytes postnatally. 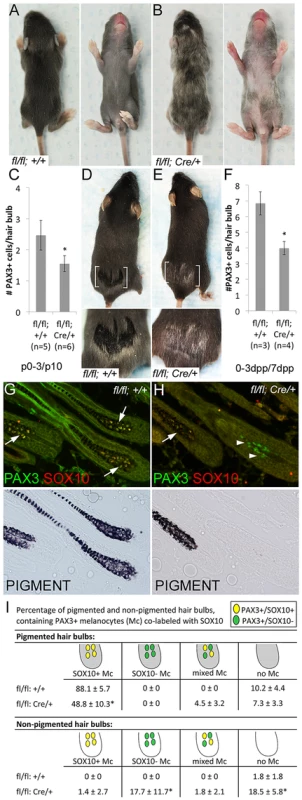
(A–B) Sox10fl/fl (fl/fl; +/+) and Sox10fl/fl; Tyr::CreERT2 (fl/fl; Cre/+) pups treated with TAM by IP injection to the lactating mother on P0–3 display variegated hypopigmentation on the belly and back and exhibit a white head spot upon the emergence of the morphogenetic coat (P10 shown here, n>5). (C) Number of PAX3+ melanocytes per hair bulb in skins harvested from these mice at P10 are significantly decreased in Sox10fl/fl; Tyr::CreERT2 animals compared to similarly-treated Sox10fl/fl animals (*p = 0.002). (D–E) Adult Sox10fl/fl; Tyr::CreERT2 mice treated with TAM by IP injection on 0–3dpp exhibit white hairs within the plucked region upon hair regrowth that is not visible in similarly treated Sox10fl/fl mice (brackets indicate plucked region, lower image is a magnification of plucked region). (F) Number of PAX3+ melanocytes per hair bulb in skins harvested from similarly-treated mice at 7dpp are significantly decreased in Sox10fl/fl; Tyr::CreERT2 animals compared to Sox10fl/fl animals (*p = 0.001). (G–H) Fluorescent and corresponding brightfield images of hair bulbs from mice described in D–E. Arrows and arrowheads indicate PAX3+/SOX10+ and PAX3+/SOX10− melanocytes, respectively. (I) Distribution of melanocytes double-labeled for PAX3 and SOX10 within pigmented (gray) and non-pigmented (white) hair bulbs in skins from Sox10fl/fl (n = 3) and Sox10fl/fl; Tyr::CreERT2 (n = 4) harvested on 7dpp from mice treated with TAM on 0–3dpp (*p<0.006). We have shown previously that the Tyr::CreERT2 transgene is effective at inducing recombination in McSCs [25], and thus we also analyzed the effects of Sox10 knockout on LPP (bulge) melanocytes. Using KIT as our marker for melanocytes, we discovered that LPP melanocytes are decreased in Sox10fl/fl; Tyr::CreERT2 mice when induced perinatally or as adults (Fig. 3A). Corroborating the idea that we are affecting the McSC population, we asked whether the white hairs and reduced LPP cells observed in TAM treated Sox10fl/fl; Tyr::CreERT2 adults are retained with hair cycling. To test this, we plucked adult animals on their lower back, administered TAM on 0–3dpp, allowed these hairs to regrow (similar to Fig. 2E), then replucked in the same region, and assessed this subsequent round of hair growth for pigmentation and melanocytes. The Sox10fl/fl; Tyr::CreERT2 mice treated in this manner still exhibit white hairs and reduced bulb melanocytes (Fig. 3B–C). However, the PAX3+/SOX10− bulb cells that were observed in Sox10fl/fl; Tyr::CreERT2 mice after the initial adult treatment period (Fig. 2I) are rarely visible after replucking (Fig. 3D). Instead, the non-pigmented hairs in these replucked animals almost completely lack bulb melanocytes (Fig. 3D). This suggests that the PAX3+/SOX10− bulb cells observed after the initial TAM treatment of Sox10fl/fl; Tyr::CreERT2 adults were likely a consequence of recombining the Sox10fl allele within a partially differentiated melanocyte rather than progeny from a PAX3+/SOX10− McSC. Hypopigmentation observed in Sox10fl/fl; Tyr::CreERT2 animals treated with TAM at five weeks persists for at least two years of natural hair cycling (Fig. 3E), and together this data demonstrates that loss of Sox10 leads to a permanent reduction in the number of McSCs and an inability of remaining McSCs to fully replenish the bulb melanocyte population in newly generated hairs.
Fig. 3. Sox10 is required by LPP melanocytes postnatally. 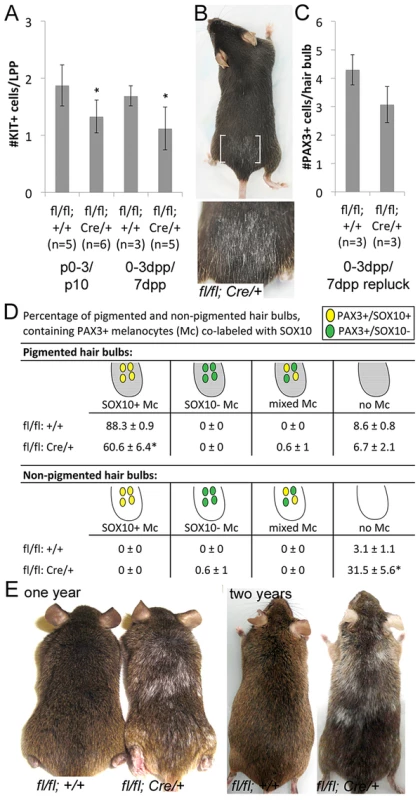
(A) Number of KIT+ LPP melanocytes within hairs from Sox10fl/fl (fl/fl; +/+) and Sox10fl/fl; Tyr::CreERT2 (fl/fl; Cre/+) mice. P0–3/P10 indicates skins harvested from pups on P10 that were maintained by lactating mothers that were IP injected with TAM on P0–3. 0–3dpp/7dpp indicates skins harvested from adult mice on 7dpp after IP injections of TAM on 0–3dpp. (B) White hairs remain visible in adult Sox10fl/fl; Tyr::CreERT2 mice that were treated with TAM by IP injection on 0–3dpp, allowed for complete hair regeneration, replucked and allowed for a second round of hair regrowth (brackets indicate plucked/replucked region, lower image is a magnification of plucked region; mouse in 2E and 3B are the same, imaged prior to and after replucking). (C) Number of PAX3+ bulb melanocytes within hairs from Sox10fl/fl and Sox10fl/fl; Tyr::CreERT2 mice treated as described in B but harvested on 7dpp after replucking (0–3dpp/7dpp repluck). (D) Distribution of melanocytes double-labeled for PAX3 and SOX10 within pigmented (gray) and non-pigmented (white) hair bulbs in skins from Sox10fl/fl (n = 3) and Sox10fl/fl; Tyr::CreERT2 (n = 3) mice treated as described in B but harvested on 7dpp after replucking (*p<0.002). (E) Persistent hair graying is visible in Sox10fl/fl; Tyr::CreERT2 mice treated with IP TAM for pulse of five days beginning at five weeks old and imaged at one and two years old. Together these observations reveal a postnatal requirement for Sox10 in mouse melanocytes. This extends to both the McSC and differentiated melanocyte populations and demonstrates that Sox10 is necessary during the establishment of melanocytes within the hair follicle during hair morphogenesis as well as during the regeneration of melanocytes during adult hair cycling.
Overexpression of Sox10 induces McSC loss and premature hair graying
Expression of SOX10 by McSCs of the hair, a subpopulation that by their nature is inhibited from differentiation, suggests that McSCs uniquely regulate Sox10 in order to maintain their stem cell properties. To determine whether changing the threshold of Sox10 levels in the melanocyte lineage affects the ability of melanocytes to become established in the hair or maintained as McSCs, we examined mice that overexpress Sox10 in melanocytes under the control of the Dct promoter (Tg(DctSox10, line CF1-10; [35]). This transgene exhibits a 2.4-fold increase in Sox10 expression in skins obtained from Tg(DctSox10)/+ animals compared to wild type (Fig. S8A).
The increase in Sox10 expression manifests in two ways: congenital hypopigmentation (white spotting) and hair graying (Fig. 4A, B). At P8, Tg(DctSox10)/+ mice exhibit hypopigmentation that is evident as small, ventral belly spots that are highly penetrant (97% in adults, n = 29/30 with belly spots; Fig. S8B). Tg(DctSox10)/Tg(DctSox10) mice at P8 have more extensive hypopigmentation with large white ventral spots that encompass the majority of the belly, dorsal spotting and occasional head spots. The white spotting observed with Tg(DctSox10) suggests that overexpression of Sox10 affects the embryonic melanoblast population. Tg(DctSox10)/Tg(DctSox10) mice also display variable loss of hair pigmentation (premature hair graying) after the onset of the first adult hair cycle (first adult anagen is ∼P28, Fig. 4B).
Fig. 4. Tg(DctSox10) results in congenital white spotting and premature hair graying. 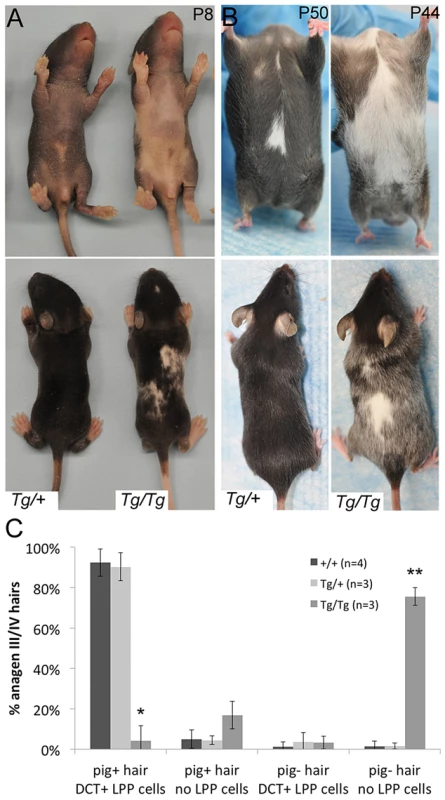
(A, B) Ventral and dorsal views demonstrating variable hypopigmentation in Tg(DctSox10)/+ and Tg(DctSox10)/Tg(DctSox10) mice during hair morphogenesis and adult hair cycling. (C) Frequency of pigmented (pig+) and non-pigmented (pig−) anagen III/IV (7dpp) hairs that contain (DCT+ LPP cells) or do not contain (no LPP cells) LPP melanocytes within Tg(DctSox10) or +/+ mice. The ages of mice analyzed ranged between 9–22 weeks at harvest. Significance determined by chi-square analysis (p<<0.0001) and evaluation of standardized residuals (*, z = −8.84; **, z = 12.24). Hair graying in Tg(DctSox10)/Tg(DctSox10) mice continues to increase progressively as these animals age (not shown). Tg(DctSox10)/+ mice also exhibit hair graying but with a reduction in severity and with a later onset, beginning after the second adult hair cycle (second adult anagen is ∼12 weeks, 4/11 animals exhibit sparse gray hairs at ∼16 weeks). Hair graying in the Tg(DctSox10) line was first examined histologically in mice after the first adult hair cycle (between 9–22 weeks in age) and after hair cycle synchronization by plucking (Fig. 4C). Analysis at anagen (7dpp) demonstrated that in wild type and Tg(DctSox10)/+ mice, the majority of hairs were both pigmented and contained LPP melanocytes (92.4±6.8% and 90.3±6.9%, respectively). In contrast, Tg(DctSox10)/Tg(DctSox10) mice exhibited primarily non-pigmented hairs that lacked LPP melanocytes (75.6±4.3%). From these observations we conclude that Tg(DctSox10)-induced hair graying is a direct consequence of McSC deficiency.
Overexpression of Sox10 disrupts McSC establishment
The fact that Tg(DctSox10)/Tg(DctSox10) animals exhibit premature hair graying at the first adult hair cycle suggests that the loss of McSCs observed in these animals occurs during hair morphogenesis when melanocytes colonize the hair. Melanocytes within the morphogenetic hair bulge and bulb are thought to become molecularly and anatomically distinct around P4 [4]. If Sox10 overexpression affects McSC establishment then we would expect that the LPP melanocytes in Tg(DctSox10)/Tg(DctSox10) animals will decrease with age after P4. First, as indicated by the postnatal coat color, we confirmed in Tg(DctSox10) animals at P2 that regions of the coat unaffected by congenital white spotting contained hairs that were similarly pigmented in the hair bulb and shaft in comparison to +/+ littermates (Fig. 5A). Counts of DCT+ cells within stage 6 hairs of P2 skins (the time point when the hair bulge is anatomically recognizable) show that while LPP melanocytes are detected in Tg(DctSox10)/Tg(DctSox10) mice, their numbers are moderately reduced. By P7/8, the number of LPP melanocytes in hairs of Tg(DctSox10)/Tg(DctSox10) mice decreases further to less than half of those observed in Tg(DctSox10)/+ and +/+ animals (Fig. 5B). The presence of a reduced number of LPP melanocytes in Tg(DctSox10) homozygotes confirms that while there may be fewer overall melanocytes in these animals due to the embryonic effects of Sox10 overexpression, their ability to colonize the hair bulge at P2 is maintained. However, in Tg(DctSox10)/Tg(DctSox10) mice, the decrease in LPP melanocytes over time, and their absence in adults, suggests that high Sox10 levels during melanocyte colonization of the hair follicle disrupts the establishment of McSCs.
Fig. 5. LPP melanocytes are reduced in Tg(DctSox10) homozygotes during hair morphogenesis. 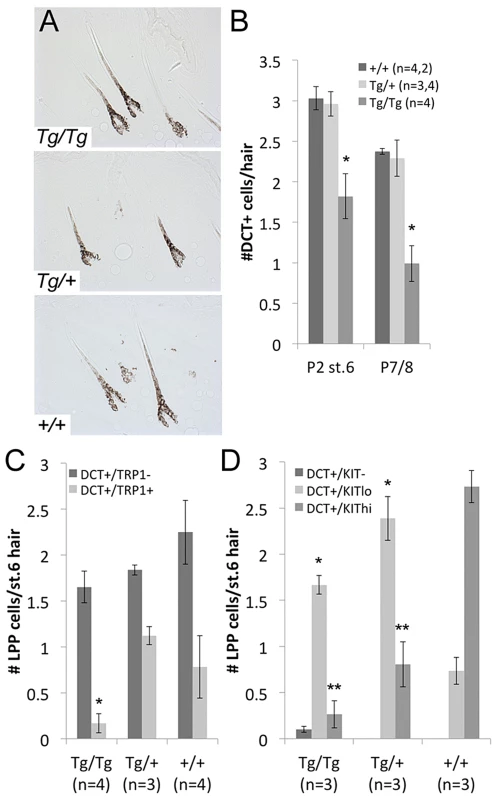
(A) Brightfield images of hairs in Tg(DctSox10) and +/+ littermates at P2. (B) Number of DCT+ melanocytes within the LPP of hairs at P2 (stage 6 hairs) and P7/8. At both time points, LPP melanocytes per hair are reduced in Tg(DctSox10)/Tg(DctSox10) compared to Tg(DctSox10)/+ and +/+ mice (*p<0.017). (C, D) Quantitative immunohistochemical analysis of stage 6 hairs from P2 skins for DCT and TRP1, or DCT and KIT. The population of DCT+/TRP1+ cells is significantly reduced in Tg(DctSox10)/Tg(DctSox10) in comparison to Tg(DctSox10)/+ and +/+ mice (*p<0.008). Tg(DctSox10) also causes a switch in KIT intensity from KIThi in wild type to KITlow in Tg(DctSox10) animals (*KITlo and **KIThi comparisons made between +/+ and Tg(DctSox10)/+ or +/+ and Tg(DctSox10)/Tg(DctSox10); p<0.005). To examine whether Tg(DctSox10) affects other melanogenic proteins within McSCs at P2, we evaluated the LPP expression patterns of TRP1, a known target of SOX10, and KIT receptor, and a protein not known to be downstream of SOX10. We find a noticeable depletion of DCT+/TRP1+ LPP melanocytes in Tg(DctSox10)/Tg(DctSox10) in comparison to Tg(DctSox10)/+ or +/+ mice (Fig. 5C). We also find that Tg(DctSox10) dramatically changes the KIT expression profile; while the majority of LPP melanocytes are KIThigh in wild type, this switches to KITlow in Tg(DctSox10) mice (Fig. 5D). Thus altered expression of TRP1 and KIT in LPP melanocytes precedes the loss of McSCs and consequent hair graying observed in Tg(DctSox10)/Tg(DctSox10) mice. Together these experiments demonstrate that although increased Sox10 expression does not affect the ability of melanocytes to produce normally pigmented morphogenetic hairs, it does result in changes in the expression status of the LPP melanocytes that may affect their establishment as McSCs.
Overexpression of Sox10 disrupts McSC maintenance
The absence of McSCs in the adult hairs of Tg(DctSox10) homozygotes precludes our ability to phenotypically assess them at this age, however, the fact that Tg(DctSox10)/+ mice exhibit changes in LPP expression profiles at P2 suggests that a closer look at Tg(DctSox10)/+ skins is warranted. First as expected, immunolabeling validates the presence of SOX10 in both LPP and bulb melanocytes of anagen (7dpp) hairs in Tg(DctSox10)/+ and wild type adult mice (Fig. S8C). Second, in contrast to the dramatic loss of LPP melanocytes observed in adult Tg(DctSox10) homozygotes, no change in the total number of melanocytes per LPP was detected within anagen (7dpp) hairs of Tg(DctSox10)/+ animals in comparison to wild type (Fig. 6A) when assayed in mice ranging from 9–22 weeks of age.
Fig. 6. Overexpression of Sox10 results in premature differentiation of LPP melanocytes in anagen hairs. 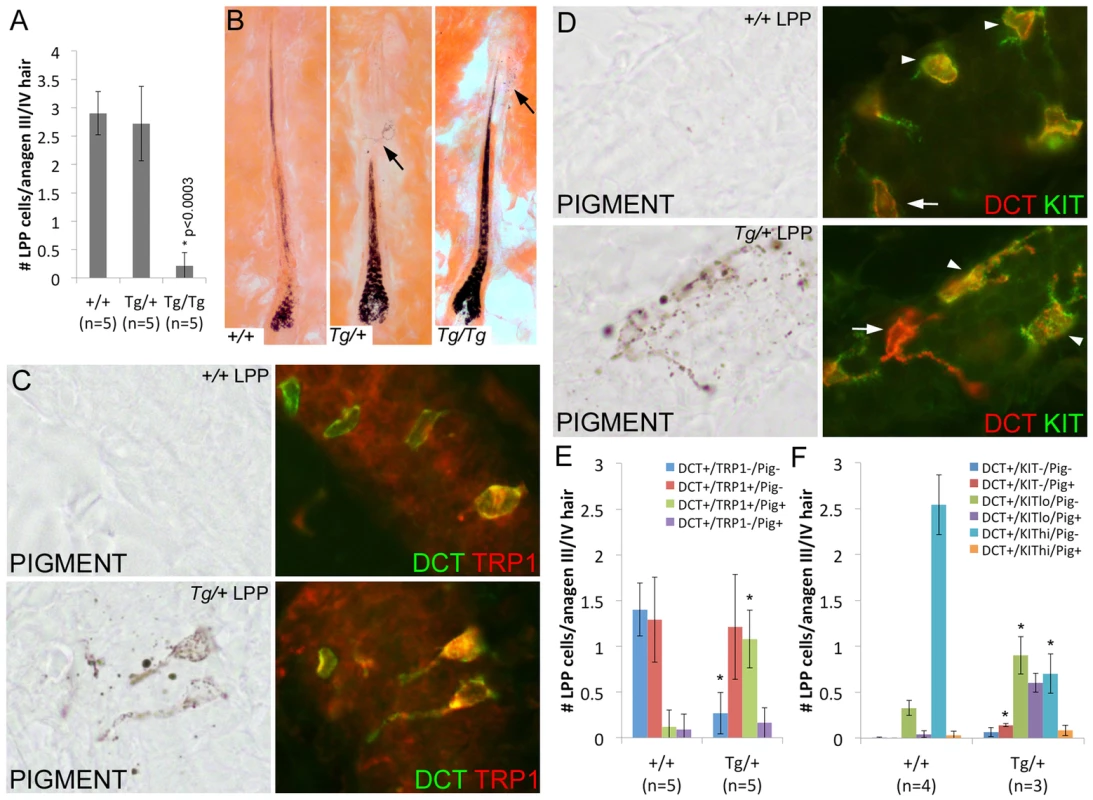
(A) Number of DCT+ LPP melanocytes per anagen III/IV hair follicle (independent of the presence or absence of hair pigmentation) is significantly reduced in Tg(DctSox10)/Tg(DctSox10) mice when compared to wild type and Tg(DctSox10)/+ mice (*p<0.0003). The ages of mice analyzed ranged between 9–22 weeks at harvest. (B) Eosin-stained skin sections of these hairs demonstrate the presence of ectopic pigmentation in the LPP of Tg(DctSox10)/+ and Tg(DctSox10)/Tg(DctSox10) hairs (arrows) that is not see in wild type hairs. In Tg(DctSox10)/+ LPP regions, this pigmentation often appeared in cells that were highly dendritic. (C, D) Brightfield and corresponding fluorescent images of anagen III/IV hair follicles double labeled for DCT and TRP1 (C) or KIT (D) in wild type and Tg(DctSox10)/+ animals. The intensity of KIT fluorescence expression was variable, and categorized as KITlo (arrows) or KIThi (arrowheads), and did not appear to correlate with the presence or absence of pigmentation. (E,F) Comparison of the number LPP melanocytes per anagen III/IV hair follicle in +/+ and Tg(DctSox10)/+ animals that express DCT, and TRP1 or KIT, and produce ectopic pigmentation (*p<0.008). Closer inspection of Tg(DctSox10)/+ hairs revealed the presence of pigmented, often dendritic, cells within the McSC compartment. Ectopic LPP pigmentation was also detected in Tg(DctSox10)/Tg(DctSox10) hairs that remained pigmented into the adult hair cycle, but was rarely present in wild type hairs (Fig. 6B). LPP melanocytes of Tg(DctSox10)/+ adult mice also exhibit changes in the expression pattern of TRP1 and KIT at anagen (Fig. 6C–F). In wild type animals, LPP melanocytes are mostly unpigmented and fall evenly into two categories, either DCT+-only or DCT+/TRP1+. In contrast, Tg(DctSox10)/+ hairs contain considerably more pigmented DCT+/TRP1+ LPP melanocytes with an accompanying decrease in the number of DCT+-only LPP melanocytes (Fig. 6E). Tg(DctSox10) also affects the DCT/KIT expression profile in adult mice with Tg(DctSox10)/+ hairs showing an increase in KITlo/pigment− and KITlo/pigment+ LPP melanocytes at the expense of those that are KIThi/pigment− (Fig. 6F). These data indicate that increasing Sox10 expression drives the inappropriate differentiation of LPP melanocytes into mature pigmented melanocytes. Together with the observation that Tg(DctSox10)/+ animals also exhibit early hair graying we conclude that the Sox10 levels must be tightly regulated to maintain the integrity of the McSC population.
Changing levels of Mitf differentially affects congenital white spotting and hair graying in Tg(DctSox10) mice
Sox10 is well documented as a transcription factor that binds directly to and regulates Mitf. In order to ascertain whether overexpression of Sox10, through an increase in Mitf, upregulates downstream pigment-producing genes, we asked whether reduction of Mitf could suppress pigmentation phenotypes in Tg(DctSox10). Based on our observation that MITF is retained in the majority of LPP melanocytes at adult anagen (Fig. 1B), we first confirmed that a similar percentage of LPP cells in Tg(DctSox10)/+ animals display normal MITF expression with approximately half exhibiting ectopic pigmentation (Fig. S8D–E).
When mice that are Tg(DctSox10)/Tg(DctSox10) are combined with a hypomorphic Mitf mutant allele, Mitfvga9 [36], they display a partial rescue of congenital hypopigmentation (normal dorsal pigmentation and a reduction in the size of ventral belly spotting). However, after the first adult hair cycle, these same Tg(DctSox10)/Tg(DctSox10); Mitfvga9/+ mice proceed to gray prematurely like their Tg(DctSox10) homozygote counterparts (compare Fig. 7A, B). In contrast, we independently confirmed that both the congenital and graying phenotypes of Tg(DctSox10) are ameliorated by reducing endogenous Sox10 with heterozygosity for a Sox10 null allele (Sox10tm1Weg, here called Sox10lacZ; Fig. 7C). These data suggest that reduction of Mitf differentially affects the defects in embryonic melanoblasts and McSCs that are a result of increased Sox10 expression.
Fig. 7. Alteration of the Tg(DctSox10) phenotype through the reduction of Mitf. 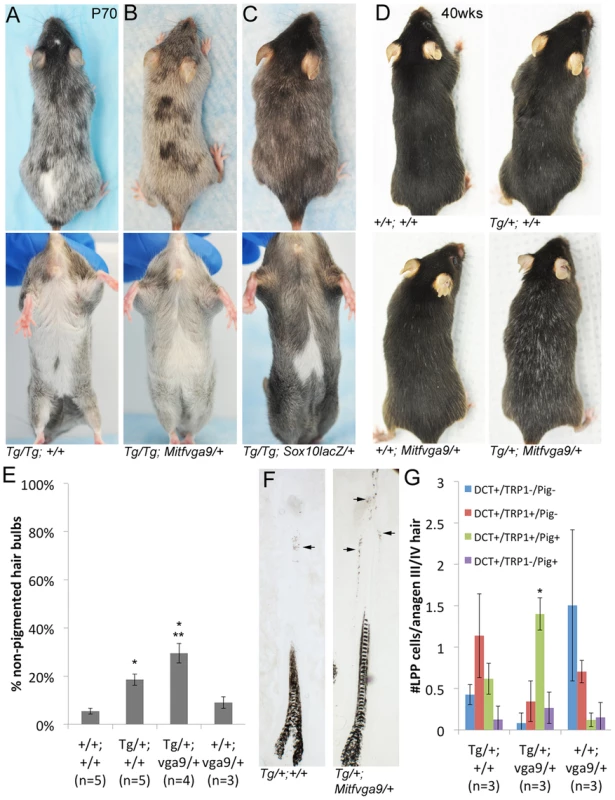
(A–B) Comparison of Tg(DctSox10)/Tg(DctSox10) and Tg(DctSox10)/Tg(DctSox10); Mitfvga9/+ animals at P70. Addition of the Mitfvga9/+ allele reduces the congenital hypopigmentation seen in Tg(DctSox10)/Tg(DctSox10) animals, and is evident in dorsal views (loss of back spotting) and in ventral views (reduction in belly spot size). Premature hair graying of Tg(DctSox10) homozygotes seen at p70 is retained with Mitfvga9 (n = 6). (C) Introduction of Sox10lacZ into Tg(DctSox10)/Tg(DctSox10) homozygotes partially rescues both congenital white spotting and premature hair graying (n = 2). (D) At 40 weeks of age, Tg(DctSox10)/+; Mitfvga9/+ double heterozygotes exhibit visibly increased hair graying in comparison to Tg(DctSox10)/+. (E) Hair graying severity was determined in animals 6–10 weeks of age by quantitating the number of non-pigmented anagen III/IV hair bulbs in +/+, Tg(DctSox10)/+, Tg(DctSox10)/+; Mitfvga9/+, and Mitfvga9/+ skins after plucking and harvesting at 7dpp. Tg(DctSox10)/+; Mitfvga9/+ mice exhibit a significant increase in non-pigmented hair bulbs in comparison to the single heterozygotes or +/+ animals (**p<0.0015). Tg(DctSox10)/+ animals also produce more non-pigmented hair bulbs in comparison to +/+ and Mitfvga9/+ animals (*p<0.002). (F) Tg(DctSox10)/+; Mitfvga9/+ animals (from E) display extensive ectopic pigmentation within the LPP of their hair follicles beyond what is normally observed in Tg(DctSox10)/+ animals (arrows, n = 4). (G) Number of LPP melanocytes per anagen III/IV hair follicle in Tg(DctSox10)/+, Tg(DctSox10)/+; Mitfvga9/+, Mitfvga9/+ animals (from E) that double label for DCT, TRP1, and produce ectopic pigmentation. Hairs from Tg(DctSox10)/+; Mitfvga9/+ animals exhibit significantly more TRP1+/PIG+ LPP melanocytes than in either single heterozygote (*p<0.0125). Since LPP melanocytes of Tg(DctSox10) heterozygotes exhibit demonstrable changes in expression of differentiation markers, we evaluated whether Mitfvga9 also affects this aspect of the Sox10 overexpression phenotype. Upon visual inspection, an increased severity of hair graying in Tg(DctSox10)/+; Mitfvga9/+ over Tg(DctSox10)/+ mice is particularly noticeable as these animals approach one year of age (Fig. 7D, imaged at 40 weeks). Even by the second to third adult anagen (induced by plucking at 6–10 weeks of age), we observed significantly more non-pigmented hair bulbs in Tg(DctSox10)/+; Mitfvga9/+ mice in comparison to either single heterozygote or +/+ animals (Fig. 7E). Hairs from Tg(DctSox10)/+; Mitfvga9/+ mice also display a noticeable expansion in the amount of ectopic pigmentation within the LPP in comparison to Tg/+ animals (Fig. 7F). By immunofluorescent analysis we discovered that at this time point (6–10 weeks of age), nearly all melanocytes present in the LPPs of Tg(DctSox10)/+; Mitfvga9/+ mice express TRP1 and are pigmented. This phenomena is less pronounced in Tg(DctSox10)/+ mice and is not observed in Mitfvga9/+ or +/+ mice (Fig. 7G, 6E). The fact that loss of Mitf exacerbates rather than alleviates the premature differentiation of LPP melanocytes and hair graying seen in this Tg(DctSox10) line, suggests that MITF participates in the negative regulation of Sox10 or Sox10-dependent processes within the McSC.
Discussion
In this study we show that Sox10 is critical postnatally for the establishment and maintenance of cells in the melanocyte lineage. The role of Sox10 is twofold—first, it is necessary for the retention of mature bulb melanocytes and undifferentiated McSCs, and second, it is required for the production of normal follicular pigmentation. The apparently contradictory requirements for Sox10 by undifferentiated McSCs and in the differentiation of melanocyte progenitors can be explained through our evidence that the McSC is maintained through the modulation of Sox10 levels itself. Accordingly, while SOX10 is expressed at all stages of the melanocyte lineage in mouse, increased Sox10 levels results in premature differentiation of the McSC population eliminating their capacity for self-renewal. This observation supports the idea that Sox10 activity within the McSC is normally decreased, and we provide evidence suggesting that this may occur through a MITF-mediated negative feedback loop. From these observations we propose a model for McSC establishment during early postnatal development whereby melanocytes migrating into the morphogenetic hair assume either a stem or differentiated cell fate depending on the environment they colonize. In this model, the hair follicle bulge would activate unique stem cell-specific signaling pathways in resident melanocytes, including one involving MITF-mediated negative feedback. Subsequent downregulation of Sox10 would establish the McSC. Melanocytes that colonize the hair bulb would not be subject to these repressive signals, Sox10 activity would remain high, and these melanocytes would undergo differentiation. This mechanism is also applicable to the maintenance of the McSC and production of pigmented melanocytes during adult hair cycling.
The idea that Sox10 can contribute to the preservation of the undifferentiated McSC population while also driving melanogenesis is in agreement with current views on the ability of SOX proteins to confer different states of cellular maturity. Sox10, in particular, is credited for defining successive stages of neural crest cell development during embryogenesis; beginning with the maintenance of multipotency within the neural crest stem cell [37], [38], and later for its participation in cell fate specification and survival of non-skeletogenic neural crest sublineages, particularly the melanocytes and glial cells (reviewed in [39]).
The mechanism by which Sox10 levels are so precisely regulated within the postnatal melanocyte remains unclear. Previously, Sox10 expression was reported to decrease as melanocytes colonized the hair bulge leading to the speculation that establishment of the McSC is dependent on downregulation of Sox10 [4]. Despite the fact that our SOX10 immunolabeling does not exhibit the same temporal pattern, our loss and gain of function results do not contradict this theory. Basal levels of Sox10 may provide survival of the postnatal melanocyte lineage, McSCs included, while a higher threshold of Sox10 expression is required to drive melanocyte progenitor differentiation and pigment production. This idea is similar to the Mitf rheostat model proposed by Carreira et al. [40] to explain how varying levels of Mitf expression can produce a range of melanoma phenotypes from stem cell-like to proliferative to terminally differentiated. While the precise mechanisms regarding Sox10 regulation are not fully known, conserved regulatory regions have been identified for Sox10 and encompass binding sites for transcriptional activators including SOX9B, NOTCH, β-catenin, LEF1, MED1(PBP), ATF2, and TFAP2 [41]–[44]. WNT/β-catenin signaling, in particular, is a candidate for controlling the switch in Sox10 expression—β-catenin remains in the cytoplasm of McSCs during telogen, but shuttles to the nucleus during anagen where it is sufficient to drive the melanocyte differentiation program [9]. Interestingly, constitutive activation of WNT signaling also results in ectopic pigmentation of McSCs and premature hair graying after several hair cycles.
The above observations do not discount the possibility that stem and progenitor fates in the melanocyte lineage may also be explained by a combinatorial mechanism where the availability of SOX10, regulatory regions of its targets, or partner transcription factors influence the cell state. For instance, SOX10 functions synergistically with a number of cofactors, namely PAX3, MITF, and CREB, during the activation of downstream genes [17], [45]–[48]. In particular, SOX10 and MITF cooperate to promote the transcription of DCT [17], [47], an interaction that is repressed by PAX3 and the corepressor GRG4. Displacement of PAX3 by activated β-catenin releases repression allowing SOX10/MITF-mediated upregulation of Dct expression [12]. Similar negative regulation of SOX10 function is observed in melanoblasts; SOX5 can both compete with SOX10 for binding while also recruit HDAC1 and CtBP2 corepressors to melanocyte gene promoters [20]. In other neural crest-derived cell types, the repression of SOX10 can also be achieved by direct sequestration. For example, in oligodendrocyte progenitors, an effector of Notch signaling, HES5, can bind SOX10 affecting its bioavailability [49]. This observation is intriguing in that Hes1 and Hes5 are expressed by melanoblasts, and that Notch signaling, is critical in their survival [50]. In particular, loss of Notch signaling in adult mice results in premature hair graying characterized by ectopically pigmented McSCs [29], [51], [52]. This suggests a possible link between the Notch pathway, Sox10 and McSC maintenance.
A number of observations support the idea that MITF may repress McSC differentiation. First, the possibility of a negative feedback mechanism for the regulation of Sox10 by MITF was shown using mathematical modeling to explain the dynamics of melanocyte differentiation within zebrafish [53]. Second, hypomorphic, Mitfvit/vit mice exhibit similar ectopic pigmentation and hair graying defects as we observed with Tg(DctSox10)/+; Mitfvga/+ mice [8]. Lastly, the fact that Mitfvga9 reduces congenital white spotting but exacerbates hair graying in with Tg(DctSox10) suggests a role for MITF within the McSC that is unique from its role within the melanoblast. In regards to the latter, we believe the white spotting phenotypes observed in Tg(DctSox10) mice may be explained by increased Mitf expression. MITF directly binds and upregulates genes required for melanin synthesis and melanosome biogenesis, including Tyr, Trp1, Dct, SILV, MLANA, and GPR143 [12], [54]–[57]. MITF is also widely implicated in cell cycle regulation. In particular, MITF can positively control transcription of the cell cycle inhibitor genes, CDKN1A (p21) and CDKN2A (p16) [58], [59]. Fittingly, loss of MITF results in increased proliferation of melanoblasts in vivo [60]. Studies of the Chx10 mutant mouse reveal that inappropriate maintenance of Mitf within the retinal progenitor cells leads to their reduced proliferation, transdifferentiation into pigmented cells, and consequent micropthalmia [61], [62]. Anecdotally, we observe that Tg(DctSox10)/Tg(DctSox10) mice have small eyes that are rescued by haploinsuffiency for Mitf (Hakami, RM, Arnheiter, H, and Pavan WJ; unpublished observation). Together these observations indirectly support the idea that the hypopigmentation observed in this Tg(DctSox10) line may be attributed to increased levels of MITF within melanoblasts inhibiting their proliferation and/or causing their inappropriate temporal differentiation.
The presence of ectopically pigmented cells within the hair bulges of Tg(DctSox10) mice fits with the assertion that overexpression of Sox10 drives the premature differentiation of McSCs. The increase in the percentage of LPP melanocytes that are TRP1+/pigment+ in hairs of adult Tg(DctSox10) heterozygotes compared to wild type animals confirms this. However, we also observed an unexpected change in KIT receptor expression in LPP melanocytes with Sox10 overexpression. At adult anagen, the majority of bulge melanocytes in wild type mice exhibit high KIT immunofluorescence intensity (KIThi) and those in Tg(DctSox10) mice appear KITlow. Previous reports show that McSC progenitors rely on KIT signaling for their appropriate proliferation and pigmentation during hair growth, and bulge melanocytes that retain a KITlow/− status represent the McSC population. [2], [6], [32]. Together with our data, showing that overexpression of Sox10 produces numerous pigmented, Kitlow bulge melanocytes, suggests that regulation of melanocyte lineage differentiation can also occur independent of high KIT expression. This idea is supported by the observation that Kit mutants, when treated with ionizing radiation, produce ectopic pigmentation within the hair bulge and exhibit hair graying [63]. No evidence to date has identified a role for SOX10 in the transcriptional control of Kit, and this is exemplified in recent microarray studies showing that Sox10 knockdown in melanoma cells results in no significant change in Kit expression (data analysis by GEO2R for datasets GSE37059, GSE25501; [64], [65]). Further investigation into Kit regulation and how KIT signaling contributes to McSC maintenance during aging is warranted.
The translational importance of Sox10 in melanocytic disease is highlighted in recent studies linking Sox10 with cell cycle regulation and reduction of SOX10 expression correlating with reduced tumor cell burden in a mouse melanoma model [64]. Our study on the role of Sox10 in the postnatal follicular melanocytes suggests a mechanism where SOX10 supports the maintenance of the melanocyte lineage while being inhibited from driving McSC differentiation. Our illustration of how tissue-specific stem cells might arise from lineage-specified precursors, and how this can occur through the regulation of the transcription factors critical in specifying this lineage may lead to further insights into how these processes can be disrupted or manipulated within disease.
Materials and Methods
Ethics statement
Animal care and experimental animal procedures were performed in accordance with the NIH IACUC.
Animals
TYR::CreERT2 and Sox10LacZ (Sox10tm1Weg) mice were rederived on and maintained by outcross to C57BL/6J [34], [66]. Rosa26tm1sor mice were obtained as homozygotes, maintained by intercross and bred together with TYR::CreERT2 mice to generate compound heterozygotes [67]. Sox10fl and Mitfvga9 mice were rederived on C57BL/6J and maintained by intercross [33], [68]. The Tg(DctSox10) line (CF1-10, [35]) was maintained through a combination of outcrossing to C57BL/6J and by intercross.
Genotyping
Mice were genotyped using DNA isolated from tail tips and PCR analysis. Primers for the TYR::CreERT2 allele, 5′-TCCGCCGCATAACCAGTGAA-3′ and 5′ - CGGAAATGGTTTCCCGCAGA, were used to amplify the Cre recombinase sequence under standard PCR conditions (30 cycles of 45 s at 94°C, 45 s at 65°C and 60 s at 72°C). Mitfvga9 and Sox10LacZ alleles were detected using PCR primers for β-galactosidase, 5′-GATCCGCGCTGGCTACCGGC-3′ and 5′-GGATACTGACGAAACGCCTGCC-3′, using the same PCR conditions described above. Primers and cycling conditions for the Sox10fl allele was described previously [33]. Zygosity for the Tg(DctSox10) transgene was determined by TaqMan analysis for two SNPs flanking the transgene on chromosome 1 that distinguish the original FVB donor strain from the C57BL/6 background strain (rs13475895 and rs13475987).
Induction of CRE activity
TAM (T5648, Sigma) was dissolved in corn oil or a combination of ethanol and sunflower oil. TAM treatment was performed by IP injection of lactating mothers or adults with 2 mg/animal for the number of days indicated.
Hair cycle staging and synchronization
Morphogenetic and adult hairs were staged according to [69], [70]. Plucking was performed to synchronize adult hairs. Briefly, mice were anesthetized and hairs were removed by hand over a 1.5 cm×2 cm region on the lower back. Hairs within this region were allowed to regenerate for 7 (7days post plucking, 7dpp) or 21 days (21 days post plucking, 21dpp). At each stage, the regions of the hair follicle were strictly defined based on visible anatomical landmarks (as described in [29]).
Immunohistochemistry
After shaving, skin from the lower back was immersed in 2% formaldehyde, and irradiated in a 540W variable wattage microwave (BioWave, Pelco) three times in intervals of 30 s irradiation followed by 60 s on ice. After microwaving, samples remained in fixative for an additional 25 minutes on ice. Skins were cryoprotected in 10% sucrose overnight, embedded in NEG-50 (Thermo Scientific), frozen and sectioned with a cryostat (10 µm). For brightfield imaging, eosin-Y was sometimes used as a counterstain.
Sections for immunolabeling were first rinsed in PBS with 0.1% Tween 20. For nuclear antigens sections were subjected to antigen retrieval by boiling for 20 minutes in a Tris-EDTA solution and then permeabilized by treating with 1% Triton X-100 for 15 minutes. Sections were blocked for two hours in 1% bovine serum albumin (Sigma) and incubated with primary antibody overnight at 4°C. Primary antibodies include those against DCT (1∶300; TRP2, Santa Cruz Bio, sc-10451), SOX10 (1∶75; Santa Cruz Bio, sc-17342), PAX3 (1∶75, Developmental Studies Hybridoma Bank), MITF (1∶1000; rabbit polyclonal, gift from Heinz Arnheiter, NINDS-NIH), c-KIT (1∶100; ACK4, Cedarlane, CL8936AP), TRP1 and TYR (1∶300; PEP-1 and PEP-7, rabbit polyclonals, gift from Vince Hearing, NCI-NIH), Cre recombinase (1∶1000; Novagen, #69050-3), β-galactosidase (1∶32,000; MP Bio, #08559761), and cleaved Caspase-3 (1∶100; Cell Signaling, #9661). After washing, sections were incubated in the appropriate secondary antibodies (1∶5000; Alexafluor488 or 568, Invitrogen) for two hours at room temperature.
Sequential immunolabeling was performed for co-detection of DCT and SOX10 as these antibodies were both generated in goat. After labeling for SOX10 using the protocol described above, sections were blocked with rabbit α-goat IgG FAB (1∶10; Jackson Immuno, #305-007-003) for two hours, washed and then labeled for DCT as described above.
Brightfield and fluorescence microscopy was performed on a Zeiss Observer.D1 compound microscope. Images were obtained with an Axiocam Hrc camera using the Axiovision 4.8.2 software and processed with Adobe Photoshop. Quantitation of hair and cell phenotypes of immunolabeled tissue was performed on every fourth section of sequentially obtained skin sections. Data is presented as the mean ± standard deviation. Student's T-test with Bonferroni correction was used to determine statistical significance, unless stated otherwise.
β-galactosidase staining
Skin samples were fixed in 2% formaldehyde/0.2% glutaraldehyde for 30 min at room temperature. Samples were then washed with rinse buffer (2 mM MgCl2/0.1% NP40/PBS) and stained overnight in X-Gal solution consisting of 0.32 mg/ml X-Gal, 5 mM ferrothiocyanide, and 5 mM ferrithiocyanide in rinse buffer.
Quantitative PCR
RNA from E17.5 skins from wild type and Tg(DctSox10)/+ mice was reverse transcribed using the High Capacity cDNA Reverse Transcription Kit (ABI). Quantitative PCR was performed using Taqman Fast Universal PCR Master Mix (ABI) and the following Taqman gene expression assays: Sox10 (Mm01300162_m1) and Pax3 (Mm00435493_m1). All experiments were performed with technical and biological replicates of ≥3.
Supporting Information
Zdroje
1. TobinDJ, PausR (2001) Graying: gerontobiology of the hair follicle pigmentary unit. Exp Gerontol 36 : 29–54.
2. NishimuraEK, JordanSA, OshimaH, YoshidaH, OsawaM, et al. (2002) Dominant role of the niche in melanocyte stem-cell fate determination. Nature 416 : 854–860 doi:10.1038/416854a
3. MakS-S, MoriyamaM, NishiokaE, OsawaM, NishikawaS-I (2006) Indispensable role of Bcl2 in the development of the melanocyte stem cell. Dev Biol 291 : 144–153 doi:10.1016/j.ydbio.2005.12.025
4. OsawaM, EgawaG, MakS-S, MoriyamaM, FreterR, et al. (2005) Molecular characterization of melanocyte stem cells in their niche. Development 132 : 5589–5599 doi:10.1242/dev.02161
5. BotchkarevaNV, BotchkarevVA, GilchrestBA (2003) Fate of melanocytes during development of the hair follicle pigmentary unit. J Investig Dermatol Symp Proc 8 : 76–79 doi:10.1046/j.1523-1747.2003.12176.x
6. BotchkarevaNV, KhlgatianM, LongleyBJ, BotchkarevVA, GilchrestBA (2001) SCF/c-kit signaling is required for cyclic regeneration of the hair pigmentation unit. FASEB J 15 : 645–658 doi:10.1096/fj.00-0368com
7. NishimuraEK, SuzukiM, IgrasV, DuJ, LonningS, et al. (2010) Key roles for transforming growth factor beta in melanocyte stem cell maintenance. Cell Stem Cell 6 : 130–140 doi:10.1016/j.stem.2009.12.010
8. NishimuraEK (2005) Mechanisms of Hair Graying: Incomplete Melanocyte Stem Cell Maintenance in the Niche. Science 307 : 720–724 doi:10.1126/science.1099593
9. RabbaniP, TakeoM, ChouW, MyungP, BosenbergM, et al. (2011) Coordinated activation of Wnt in epithelial and melanocyte stem cells initiates pigmented hair regeneration. Cell 145 : 941–955 doi:10.1016/j.cell.2011.05.004
10. InomataK, AotoT, BinhNT, OkamotoN, TanimuraS, et al. (2009) Genotoxic stress abrogates renewal of melanocyte stem cells by triggering their differentiation. Cell 137 : 1088–1099 doi:10.1016/j.cell.2009.03.037
11. MoriyamaM, DurhamA-D, MoriyamaH, HasegawaK, NishikawaS-I, et al. (2008) Multiple roles of Notch signaling in the regulation of epidermal development. Dev Cell 14 : 594–604 doi:10.1016/j.devcel.2008.01.017
12. LangD, LuMM, HuangL, EnglekaKA, ZhangM, et al. (2005) Pax3 functions at a nodal point in melanocyte stem cell differentiation. Nature 433 : 884–887 doi:10.1038/nature03292
13. TobinD, SlominskiA, BotchkarevV, PausR (1999) The fate of hair follicle melanocytes during the hair growth cycle. J Investig Dermatol Symp Proc 4 : 323–332.
14. TobinD, HagenE, BotchkarevV, PausR (1998) Do hair bulb melanocytes undergo apotosis during hair follicle regression (catagen)? J Invest Dermatol 111 : 941–947.
15. HouL, ArnheiterH, PavanWJ (2006) Interspecies difference in the regulation of melanocyte development by SOX10 and MITF. Proc Natl Acad Sci USA 103 : 9081–9085 doi:10.1073/pnas.0603114103
16. MurisierF, GuichardS, BeermannF (2007) The tyrosinase enhancer is activated by Sox10 and Mitf in mouse melanocytes. Pigment Cell Res 20 : 173–184 doi:10.1111/j.1600-0749.2007.00368.x
17. JiaoZ, MollaaghababaR, PavanWJ, AntonellisA, GreenED, et al. (2004) Direct interaction of Sox10 with the promoter of murine Dopachrome Tautomerase (Dct) and synergistic activation of Dct expression with Mitf. Pigment Cell Res 17 : 352–362 doi:10.1111/j.1600-0749.2004.00154.x
18. MurisierF, GuichardS, BeermannF (2006) A conserved transcriptional enhancer that specifies Tyrp1 expression to melanocytes. Dev Biol 298 : 644–655 doi:10.1016/j.ydbio.2006.05.011
19. MollaaghababaR, PavanWJ (2003) The importance of having your SOX on: role of SOX10 in the development of neural crest-derived melanocytes and glia. Oncogene 22 : 3024–3034 doi:10.1038/sj.onc.1206442
20. StoltCC, LommesP, HillgärtnerS, WegnerM (2008) The transcription factor Sox5 modulates Sox10 function during melanocyte development. Nucleic Acids Res 36 : 5427–5440 doi:10.1093/nar/gkn527
21. Southard-SmithEM, KosL, PavanWJ (1998) SOX10 mutation disrupts neural crest development in Dom Hirschsprung mouse model. Nat Genet 18 : 60–64 doi:10.1038/ng0198-60
22. LanePW, LiuHM (1984) Association of megacolon with a new dominant spotting gene (Dom) in the mouse. J Hered 75 : 435–439.
23. InoueK, TanabeY, LupskiJR (1999) Myelin deficiencies in both the central and the peripheral nervous systems associated with a SOX10 mutation. Ann Neurol 46 : 313–318.
24. HerbarthB, PingaultV, BondurandN, KuhlbrodtK, Hermans-BorgmeyerI, et al. (1998) Mutation of the Sry-related Sox10 gene in Dominant megacolon, a mouse model for human Hirschsprung disease. Proc Natl Acad Sci USA 95 : 5161–5165.
25. HarrisML, PavanWJ (2013) CreER(T) (2) transgene. Pigment Cell Melanoma Res 26 : 269–274 doi:10.1111/pcmr.12048
26. MotohashiT, YamanakaK, ChibaK, MiyajimaK, AokiH, et al. (2011) Neural crest cells retain their capability for multipotential differentiation even after lineage-restricted stages. Dev Dyn 240 : 1681–1693 doi:10.1002/dvdy.22658
27. CorpeningJC, DealKK, CantrellVA, SkeltonSB, BuehlerDP, et al. (2011) Isolation and live imaging of enteric progenitors based on Sox10-Histone2BVenus transgene expression. Genesis 49 : 599–618 doi:10.1002/dvg.20748
28. ShibataS, YasudaA, Renault-MiharaF, SuyamaS, KatohH, et al. (2010) Sox10-Venus mice: a new tool for real-time labeling of neural crest lineage cells and oligodendrocytes. Mol Brain 3 : 31 doi:10.1186/1756-6606-3-31
29. Aubin-HouzelsteinG, Djian-ZaoucheJ, BernexF, GadinS, DelmasV, et al. (2008) Melanoblasts' proper location and timed differentiation depend on Notch/RBP-J signaling in postnatal hair follicles. J Invest Dermatol 128 : 2686–2695 doi:10.1038/jid.2008.120
30. GrecoV, ChenT, RendlM, SchoberM, PasolliHA, et al. (2009) A two-step mechanism for stem cell activation during hair regeneration. Cell Stem Cell 4 : 155–169 doi:10.1016/j.stem.2008.12.009
31. SharovA, TobinDJ, SharovaTY, AtoyanR, BotchkarevVA (2005) Changes in different melanocyte populations during hair follicle involution (catagen). J Invest Dermatol 125 : 1259–1267 doi:10.1111/j.0022-202X.2005.23959.x
32. Nishikawa-TorikaiS, OsawaM, NishikawaS-I (2011) Functional Characterization of Melanocyte Stem Cells in Hair Follicles. J Invest Dermatol 131 : 2358–67 doi:10.1038/jid.2011.195
33. FinzschM, SchreinerS, KichkoT, ReehP, TammER, et al. (2010) Sox10 is required for Schwann cell identity and progression beyond the immature Schwann cell stage. J Cell Biol 189 : 701–712 doi:10.1083/jcb.200912142
34. BosenbergM, MuthusamyV, CurleyDP, WangZ, HobbsC, et al. (2006) Characterization of melanocyte-specific inducible Cre recombinase transgenic mice. Genesis 44 : 262–267 doi:10.1002/dvg.20205
35. HakamiR, HouL, BaxterL, LoftusS, Southard-SmithE, et al. (2006) Genetic evidence does not support direct regulation of EDNRB by SOX10 in migratory neural crest and the melanocyte lineage. Mech Dev 123 : 124–134.
36. HodgkinsonCA, MooreKJ, NakayamaA, SteingrímssonE, CopelandNG, et al. (1993) Mutations at the mouse microphthalmia locus are associated with defects in a gene encoding a novel basic-helix-loop-helix-zipper protein. Cell 74 : 395–404.
37. KimJ, LoL, DormandE, AndersonDJ (2003) SOX10 maintains multipotency and inhibits neuronal differentiation of neural crest stem cells. Neuron 38 : 17–31.
38. McKeownS, LeeV, Bronner-FraserM, NewgreenD, FarlieP (2005) Sox10 overexpression induces neural crest-like cells from all dorsoventral levels of the neural tube but inhibits differentiation. Dev Dyn 233 : 430–44.
39. KelshRN (2006) Sorting outSox10 functions in neural crest development. Bioessays 28 : 788–798 doi:10.1002/bies.20445
40. CarreiraS, GoodallJ, DenatL, RodriguezM, NuciforoP, et al. (2006) Mitf regulation of Dia1 controls melanoma proliferation and invasiveness. Genes Dev 20 : 3426–3439 doi:10.1101/gad.406406
41. DuttonJR, AntonellisA, CarneyTJ, RodriguesFSLM, PavanWJ, et al. (2008) An evolutionarily conserved intronic region controls the spatiotemporal expression of the transcription factor Sox10. BMC Dev Biol 8 : 105 doi:10.1186/1471-213X-8-105
42. ZhuYT, JiaY, HuL, QiC, PrasadMK, et al. (2010) Peroxisome-proliferator-activated receptor-binding protein (PBP) is essential for the growth of active Notch4-immortalized mammary epithelial cells by activating SOX10 expression. Biochem J 425 : 435–444 doi:10.1042/BJ20091237
43. ShahM, BhoumikA, GoelV, DewingA, BreitwieserW, et al. (2010) A role for ATF2 in regulating MITF and melanoma development. PLoS Genet 6: e1001258 doi:10.1371/journal.pgen.1001258
44. Van OtterlooE, LiW, GarnettA, CattellM, MedeirosDM, et al. (2012) Novel Tfap2-mediated control of soxE expression facilitated the evolutionary emergence of the neural crest. Development 139 : 720–730 doi:10.1242/dev.071308
45. PotterfSB, FurumuraM, DunnKJ, ArnheiterH, PavanWJ (2000) Transcription factor hierarchy in Waardenburg syndrome: regulation of MITF expression by SOX10 and PAX3. Hum Genet 107 : 1–6.
46. BondurandN, PingaultV, GoerichDE, LemortN, SockE, et al. (2000) Interaction among SOX10, PAX3 and MITF, three genes altered in Waardenburg syndrome. Hum Mol Genet 9 : 1907–1917.
47. LudwigA, RehbergS, WegnerM (2004) Melanocyte-specific expression of dopachrome tautomerase is dependent on synergistic gene activation by the Sox10 and Mitf transcription factors. FEBS Lett 556 : 236–244.
48. HuberWE, PriceER, WidlundHR, DuJ, DavisIJ, et al. (2003) A tissue-restricted cAMP transcriptional response: SOX10 modulates alpha-melanocyte-stimulating hormone-triggered expression of microphthalmia-associated transcription factor in melanocytes. J Biol Chem 278 : 45224–45230 doi:10.1074/jbc.M309036200
49. LiuA, LiJ, Marin-HusstegeM, KageyamaR, FanY, et al. (2006) A molecular insight of Hes5-dependent inhibition of myelin gene expression: old partners and new players. EMBO J 25 : 4833–4842 doi:10.1038/sj.emboj.7601352
50. MoriyamaM, OsawaM, MakS-S, OhtsukaT, YamamotoN, et al. (2006) Notch signaling via Hes1 transcription factor maintains survival of melanoblasts and melanocyte stem cells. J Cell Biol 173 : 333–339 doi:10.1083/jcb.200509084
51. KumanoK, MasudaS, SataM, SaitoT, LeeS-Y, et al. (2008) Both Notch1 and Notch2 contribute to the regulation of melanocyte homeostasis. Pigment Cell Melanoma Res 21 : 70–78 doi:10.1111/j.1755-148X.2007.00423.x
52. SchouweyK, DelmasV, LarueL, Zimber-StroblU, StroblLJ, et al. (2007) Notch1 and Notch2 receptors influence progressive hair graying in a dose-dependent manner. Dev Dyn 236 : 282–289 doi:10.1002/dvdy.21000
53. GreenhillER, RoccoA, VibertL, NikaidoM, KelshRN (2011) An iterative genetic and dynamical modelling approach identifies novel features of the gene regulatory network underlying melanocyte development. PLoS Genet 7: e1002265 doi:10.1371/journal.pgen.1002265
54. GalibertMD, YavuzerU, DexterTJ, GodingCR (1999) Pax3 and regulation of the melanocyte-specific tyrosinase-related protein-1 promoter. J Biol Chem 274 : 26894–26900.
55. la Serna deIL, OhkawaY, HigashiC, DuttaC, OsiasJ, et al. (2006) The microphthalmia-associated transcription factor requires SWI/SNF enzymes to activate melanocyte-specific genes. J Biol Chem 281 : 20233–20241 doi:10.1074/jbc.M512052200
56. DuJ, MillerAJ, WidlundHR, HorstmannMA, RamaswamyS, et al. (2003) MLANA/MART1 and SILV/PMEL17/GP100 are transcriptionally regulated by MITF in melanocytes and melanoma. Am J Pathol 163 : 333–343 doi:10.1016/S0002-9440(10)63657-7
57. VetriniF, AuricchioA, DuJ, AngelettiB, FisherDE, et al. (2004) The microphthalmia transcription factor (Mitf) controls expression of the ocular albinism type 1 gene: link between melanin synthesis and melanosome biogenesis. Mol Cell Biol 24 : 6550–6559 doi:10.1128/MCB.24.15.6550-6559.2004
58. LoercherAE, TankEMH, DelstonRB, HarbourJW (2005) MITF links differentiation with cell cycle arrest in melanocytes by transcriptional activation of INK4A. J Cell Biol 168 : 35–40 doi:10.1083/jcb.200410115
59. CarreiraS, GoodallJ, AksanI, La RoccaSA, GalibertM-D, et al. (2005) Mitf cooperates with Rb1 and activates p21Cip1 expression to regulate cell cycle progression. Nature 433 : 764–769 doi:10.1038/nature03269
60. HornyakTJ, HayesDJ, ChiuLY, ZiffEB (2001) Transcription factors in melanocyte development: distinct roles for Pax-3 and Mitf. Mech Dev 101 : 47–59.
61. RowanS, ChenC-MA, YoungTL, FisherDE, CepkoCL (2004) Transdifferentiation of the retina into pigmented cells in ocular retardation mice defines a new function of the homeodomain gene Chx10. Development 131 : 5139–5152 doi:10.1242/dev.01300
62. BurmeisterM, NovakJ, LiangMY, BasuS, PloderL, et al. (1996) Ocular retardation mouse caused by Chx10 homeobox null allele: impaired retinal progenitor proliferation and bipolar cell differentiation. Nat Genet 12 : 376–384 doi:10.1038/ng0496-376
63. AokiH, HaraA, MotohashiT, TakahiroKunisada (2011) Protective effect of Kit signaling for melanocyte stem cells against radiation-induced genotoxic stress. J Invest Dermatol 131 : 1906–1915 doi:10.1038/jid.2011.148
64. ShakhovaO, ZinggD, SchaeferSM, HariL, CivenniG, et al. (2012) Sox10 promotes the formation and maintenance of giant congenital naevi and melanoma. Nat Cell Biol 14 : 882–90 doi:10.1038/ncb2535
65. SeongI, MinHJ, LeeJ-H, YeoC-Y, KangDM, et al. (2012) Sox10 controls migration of B16F10 melanoma cells through multiple regulatory target genes. PLoS ONE 7: e31477 doi:10.1371/journal.pone.0031477
66. BritschS, GoerichDE, RiethmacherD, PeiranoRI, RossnerM, et al. (2001) The transcription factor Sox10 is a key regulator of peripheral glial development. Genes Dev 15 : 66–78.
67. SorianoP (1999) Generalized lacZ expression with the ROSA26 Cre reporter strain. Nat Genet 21 : 70–71 doi:10.1038/5007
68. TachibanaM, HaraY, VyasD, HodgkinsonC, FexJ, et al. (1992) Cochlear disorder associated with melanocyte anomaly in mice with a transgenic insertional mutation. Mol Cell Neurosci 3 : 433–445.
69. Müller-RöverS, HandjiskiB, Van Der VeenC, EichmüllerS, FoitzikK, et al. (2001) A comprehensive guide for the accurate classification of murine hair follicles in distinct hair cycle stages. J Invest Dermatol 117 : 3–15 doi:10.1046/j.0022-202x.2001.01377.x
70. PausR, Müller-RöverS, Van Der VeenC, MaurerM, EichmüllerS, et al. (1999) A comprehensive guide for the recognition and classification of distinct stages of hair follicle morphogenesis. J Invest Dermatol 113 : 523–532 doi:10.1046/j.1523-1747.1999.00740.x
71. PetersEMJ, MaurerM, BotchkarevVA, JensenKD, WelkerP, et al. (2003) Kit is expressed by epithelial cells in vivo. J Invest Dermatol 121 : 976–984 doi:10.1046/j.1523-1747.2003.12478.x
Štítky
Genetika Reprodukční medicína
Článek Independent Evolution of Transcriptional Inactivation on Sex Chromosomes in Birds and MammalsČlánek The bHLH Subgroup IIId Factors Negatively Regulate Jasmonate-Mediated Plant Defense and DevelopmentČlánek Selective Pressures to Maintain Attachment Site Specificity of Integrative and Conjugative ElementsČlánek Reassembly of Nucleosomes at the Promoter Initiates Resilencing Following Decitabine ExposureČlánek Hepatocyte Growth Factor Signaling in Intrapancreatic Ductal Cells Drives Pancreatic Morphogenesis
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2013 Číslo 7- Růst a vývoj dětí narozených pomocí IVF
- Délka menstruačního cyklu jako marker ženské plodnosti
- Vztah užívání alkoholu a mužské fertility
- Šanci na úspěšný průběh těhotenství snižují nevhodné hladiny progesteronu vznikající při umělém oplodnění
- Vliv melatoninu a cirkadiálního rytmu na ženskou reprodukci
-
Všechny články tohoto čísla
- An Solution for Crossover Formation
- Genome-Wide Association Mapping in Dogs Enables Identification of the Homeobox Gene, , as a Genetic Component of Neural Tube Defects in Humans
- Independent Evolution of Transcriptional Inactivation on Sex Chromosomes in Birds and Mammals
- Stepwise Activation of the ATR Signaling Pathway upon Increasing Replication Stress Impacts Fragile Site Integrity
- Genomic Analysis of Natural Selection and Phenotypic Variation in High-Altitude Mongolians
- Modification of tRNA by Elongator Is Essential for Efficient Translation of Stress mRNAs
- Role of CTCF Protein in Regulating Locus Transcription
- Gene Set Signature of Reversal Reaction Type I in Leprosy Patients
- Mapping of PARK2 and PACRG Overlapping Regulatory Region Reveals LD Structure and Functional Variants in Association with Leprosy in Unrelated Indian Population Groups
- Is Required for Formation of the Genital Ridge in Mice
- Monopolin Subunit Csm1 Associates with MIND Complex to Establish Monopolar Attachment of Sister Kinetochores at Meiosis I
- Recombination Dynamics of a Human Y-Chromosomal Palindrome: Rapid GC-Biased Gene Conversion, Multi-kilobase Conversion Tracts, and Rare Inversions
- Mechanisms of Protein Sequence Divergence and Incompatibility
- Histone Methyltransferase DOT1L Drives Recovery of Gene Expression after a Genotoxic Attack
- Female Behaviour Drives Expression and Evolution of Gustatory Receptors in Butterflies
- Combinatorial Regulation of Meiotic Holliday Junction Resolution in by HIM-6 (BLM) Helicase, SLX-4, and the SLX-1, MUS-81 and XPF-1 Nucleases
- The bHLH Subgroup IIId Factors Negatively Regulate Jasmonate-Mediated Plant Defense and Development
- The Role of Interruptions in polyQ in the Pathology of SCA1
- Dietary Restriction Induced Longevity Is Mediated by Nuclear Receptor NHR-62 in
- Fine Time Course Expression Analysis Identifies Cascades of Activation and Repression and Maps a Putative Regulator of Mammalian Sex Determination
- Genome-scale Co-evolutionary Inference Identifies Functions and Clients of Bacterial Hsp90
- Oxidative Stress and Replication-Independent DNA Breakage Induced by Arsenic in
- A Moonlighting Enzyme Links Cell Size with Central Metabolism
- Budding Yeast Greatwall and Endosulfines Control Activity and Spatial Regulation of PP2A for Timely Mitotic Progression
- The Conserved Intronic Cleavage and Polyadenylation Site of CstF-77 Gene Imparts Control of 3′ End Processing Activity through Feedback Autoregulation and by U1 snRNP
- The BTB-zinc Finger Transcription Factor Abrupt Acts as an Epithelial Oncogene in through Maintaining a Progenitor-like Cell State
- The Cohesion Protein SOLO Associates with SMC1 and Is Required for Synapsis, Recombination, Homolog Bias and Cohesion and Pairing of Centromeres in Drosophila Meiosis
- The RNA-binding Proteins FMR1, Rasputin and Caprin Act Together with the UBA Protein Lingerer to Restrict Tissue Growth in
- Pattern Dynamics in Adaxial-Abaxial Specific Gene Expression Are Modulated by a Plastid Retrograde Signal during Leaf Development
- A Network of HMG-box Transcription Factors Regulates Sexual Cycle in the Fungus
- Bacterial Adaptation through Loss of Function
- ENU-induced Mutation in the DNA-binding Domain of KLF3 Reveals Important Roles for KLF3 in Cardiovascular Development and Function in Mice
- Interplay between Structure-Specific Endonucleases for Crossover Control during Meiosis
- FGF Signalling Regulates Chromatin Organisation during Neural Differentiation via Mechanisms that Can Be Uncoupled from Transcription
- The Arabidopsis RNA Binding Protein with K Homology Motifs, SHINY1, Interacts with the C-terminal Domain Phosphatase-like 1 (CPL1) to Repress Stress-Inducible Gene Expression
- Selective Pressures to Maintain Attachment Site Specificity of Integrative and Conjugative Elements
- The Conserved ADAMTS-like Protein Lonely heart Mediates Matrix Formation and Cardiac Tissue Integrity
- The cGMP-Dependent Protein Kinase EGL-4 Regulates Nociceptive Behavioral Sensitivity
- RBM5 Is a Male Germ Cell Splicing Factor and Is Required for Spermatid Differentiation and Male Fertility
- Disease-Related Growth Factor and Embryonic Signaling Pathways Modulate an Enhancer of Expression at the 6q23.2 Coronary Heart Disease Locus
- Yeast Pol4 Promotes Tel1-Regulated Chromosomal Translocations
- A Dual Role for SOX10 in the Maintenance of the Postnatal Melanocyte Lineage and the Differentiation of Melanocyte Stem Cell Progenitors
- SLC26A4 Targeted to the Endolymphatic Sac Rescues Hearing and Balance in Mutant Mice
- Odoriferous Defensive Stink Gland Transcriptome to Identify Novel Genes Necessary for Quinone Synthesis in the Red Flour Beetle,
- Prediction of Complex Human Traits Using the Genomic Best Linear Unbiased Predictor
- Gene × Physical Activity Interactions in Obesity: Combined Analysis of 111,421 Individuals of European Ancestry
- Reassembly of Nucleosomes at the Promoter Initiates Resilencing Following Decitabine Exposure
- Exquisite Light Sensitivity of Cryptochrome
- miR-133a Regulates Adipocyte Browning In Vivo
- Strabismus Promotes Recruitment and Degradation of Farnesylated Prickle in Planar Polarity Specification
- Hepatocyte Growth Factor Signaling in Intrapancreatic Ductal Cells Drives Pancreatic Morphogenesis
- Is a Potential Tumor Suppressor Gene Commonly Inactivated by Epigenetic Mechanisms in Colorectal Cancer
- Joint Molecule Resolution Requires the Redundant Activities of MUS-81 and XPF-1 during Meiosis
- The Mating Competence of Geographically Diverse Strains in Their Natural and Unnatural Sand Fly Vectors
- Defective Repair of Oxidative Base Lesions by the DNA Glycosylase Nth1 Associates with Multiple Telomere Defects
- Effective Blocking of the Enhancer Requires Cooperation between Two Main Mechanisms Suggested for the Insulator Function
- Trans-Ancestral Studies Fine Map the SLE-Susceptibility Locus
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- SLC26A4 Targeted to the Endolymphatic Sac Rescues Hearing and Balance in Mutant Mice
- Bacterial Adaptation through Loss of Function
- The Cohesion Protein SOLO Associates with SMC1 and Is Required for Synapsis, Recombination, Homolog Bias and Cohesion and Pairing of Centromeres in Drosophila Meiosis
- Gene × Physical Activity Interactions in Obesity: Combined Analysis of 111,421 Individuals of European Ancestry
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



