-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Functional Specialization of the Plant miR396 Regulatory Network through Distinct MicroRNA–Target Interactions
MicroRNAs (miRNAs) are ∼21 nt small RNAs that regulate gene expression in animals and plants. They can be grouped into families comprising different genes encoding similar or identical mature miRNAs. Several miRNA families are deeply conserved in plant lineages and regulate key aspects of plant development, hormone signaling, and stress response. The ancient miRNA miR396 regulates conserved targets belonging to the GROWTH-REGULATING FACTOR (GRF) family of transcription factors, which are known to control cell proliferation in Arabidopsis leaves. In this work, we characterized the regulation of an additional target for miR396, the transcription factor bHLH74, that is necessary for Arabidopsis normal development. bHLH74 homologs with a miR396 target site could only be detected in the sister families Brassicaceae and Cleomaceae. Still, bHLH74 repression by miR396 is required for margin and vein pattern formation of Arabidopsis leaves. MiR396 contributes to the spatio-temporal regulation of GRF and bHLH74 expression during leaf development. Furthermore, a survey of miR396 sequences in different species showed variations in the 5′ portion of the miRNA, a region known to be important for miRNA activity. Analysis of different miR396 variants in Arabidopsis thaliana revealed that they have an enhanced activity toward GRF transcription factors. The interaction between the GRF target site and miR396 has a bulge between positions 7 and 8 of the miRNA. Our data indicate that such bulge modulates the strength of the miR396-mediated repression and that this modulation is essential to shape the precise spatio-temporal pattern of GRF2 expression. The results show that ancient miRNAs can regulate conserved targets with varied efficiency in different species, and we further propose that they could acquire new targets whose control might also be biologically relevant.
Published in the journal: . PLoS Genet 8(1): e32767. doi:10.1371/journal.pgen.1002419
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1002419Summary
MicroRNAs (miRNAs) are ∼21 nt small RNAs that regulate gene expression in animals and plants. They can be grouped into families comprising different genes encoding similar or identical mature miRNAs. Several miRNA families are deeply conserved in plant lineages and regulate key aspects of plant development, hormone signaling, and stress response. The ancient miRNA miR396 regulates conserved targets belonging to the GROWTH-REGULATING FACTOR (GRF) family of transcription factors, which are known to control cell proliferation in Arabidopsis leaves. In this work, we characterized the regulation of an additional target for miR396, the transcription factor bHLH74, that is necessary for Arabidopsis normal development. bHLH74 homologs with a miR396 target site could only be detected in the sister families Brassicaceae and Cleomaceae. Still, bHLH74 repression by miR396 is required for margin and vein pattern formation of Arabidopsis leaves. MiR396 contributes to the spatio-temporal regulation of GRF and bHLH74 expression during leaf development. Furthermore, a survey of miR396 sequences in different species showed variations in the 5′ portion of the miRNA, a region known to be important for miRNA activity. Analysis of different miR396 variants in Arabidopsis thaliana revealed that they have an enhanced activity toward GRF transcription factors. The interaction between the GRF target site and miR396 has a bulge between positions 7 and 8 of the miRNA. Our data indicate that such bulge modulates the strength of the miR396-mediated repression and that this modulation is essential to shape the precise spatio-temporal pattern of GRF2 expression. The results show that ancient miRNAs can regulate conserved targets with varied efficiency in different species, and we further propose that they could acquire new targets whose control might also be biologically relevant.
Introduction
MicroRNAs (miRNAs) are small RNA molecules, ∼21 nt in length, that are widespread regulators of gene expression in animals and plants [reviewed in [1], [2]]. They recognize target RNAs by base complementarity and guide them to cleavage or translational arrest [1]. MiRNA-encoding genes are transcribed as primary transcripts harboring a fold-back structure with the miRNA embedded in one of its arms. In plants, these precursors are processed in the nucleus by the ribonuclease III DICER-LIKE1 (DCL1) together with accessory components [reviewed in [3]]. The outcome of this processing activity is a miRNA/miRNA* duplex, which is 2′-O-methylated by HEN1 and incorporated into an AGO complex [reviewed in [1]].
The current version of the miRNA database (miRBase 17.0) states the existence of more than 200 MIRNAs in Arabidopsis thaliana [4]. In many cases, MIRNA-genes can be grouped into families comprising different loci encoding similar or identical mature miRNAs [5]. However, the majority of the miRNAs in Arabidopsis are single young molecules indicating that their generation is a frequent process [6], [7], [8], [9], [10]. It is unclear whether these recently evolved miRNAs have a relevant biological contribution [11], [12]. One exception might be the regulation of AGL16 by the young miR824, which participates in stomatal development in Arabidopsis [13]. On the other hand, twenty-one miRNAs families are conserved in angiosperms, with some of them even present in lycopods and bryophytes [9], [11], [14]. In contrast to the younger ones, conserved miRNAs regulate key aspects of plant development, hormone signaling and stress response [11].
While plant miRNAs have extensive base-pairing to their targets, the interaction along the 5′ portion of the miRNA is the most relevant feature for its activity [15], [16]. Interestingly, variations in the sequence of the small RNA among family members can have consequences on miRNA specificity [17]. In Arabidopsis, the miR159/miR319 family of miRNAs comprises six small RNAs that share 17 out of their 21 nt and regulate transcription factors of the TCP and MYB classes [16], [17], [18], [19], [20]. While miR319 can guide both types of targets to cleavage [17], miR159 can only affect the MYBs [16], [17], [19].
MiRNA miR396 regulates GROWTH-REGULATING FACTORs (GRFs) [21], [22], [23], [24], a plant specific family of transcription factors known to be involved in the control of cell proliferation during leaf development [22], [24], [25], [26], [27], [28]. The interaction between miR396 and the GRFs is unusual in plants as it contains a bulge in the 5′ region [22], [23]. MiR396 accumulates with leaf age and restricts the pattern of expression of the GRFs to the proliferative region of the organ [22]. Overexpression of the miRNA causes a drastic reduction in cell number, while abolishing the regulation of GRF2 by miR396 promotes a moderate increase in organ size [22]. Interestingly, variations in the miR396 sequence can be found in different species (miRBase 17.0), such as a base insertion in the 5′ region of the miRNA, which is found only in rice and other monocotyledons [29], [30].
It has been observed since the discovery of plant miRNAs that ancient miRNAs usually recognize similarly conserved target genes [16], [23], [31], [32]. The occurrence of non-conserved targets recently incorporated during evolution to pre-existing miRNA networks has not been systematically studied so far, and more importantly, it is unknown whether the regulation of newly acquired targets has biological significance. Furthermore, miRNAs can have variations in their mature sequences in different species, which could potentially lead to neo-functionalization of the small RNA regulatory networks. Here, we characterize the miR396 regulatory network. We demonstrate its expansion to regulate a new target in species related to Arabidopsis thaliana and provide evidence pointing to the importance of this regulation in Arabidopsis development. We also show that monocot-specific variants of miR396 display an enhanced activity towards the conserved GRF transcription factors, while the sub-optimal regulation of the GRFs by miR396 in Arabidopsis might be important to quantitatively control GRF levels.
Results
Analysis of miR396 targets
GRF regulation by miR396 is conserved at least in angiosperms and gymnosperms based on the presence of the small RNA [14], [33] and GRF transcription factors harboring the miR396 target site (Figure 1A). We began analyzing the existence of additional miR396 targets by searching the rice, poplar and Arabidopsis genomes using empirically-derived miRNA-target rules [16]. Transcription factors of the GRF class are the only conserved targets among these species (see Tables S1, S2, S3) [23], and their regulation by miR396 is known to be relevant for Arabidopsis development [22].
Fig. 1. Analysis of potential miR396 targets in Arabidopsis thaliana. 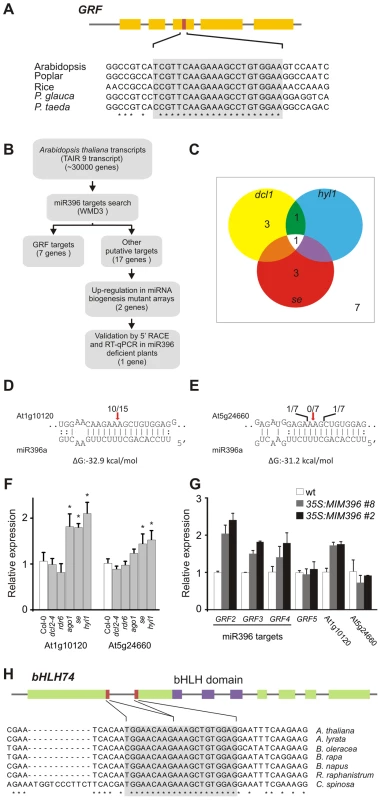
(A) Scheme representing a typical GRF gene and the conservation of the target site in selected angiosperm and gymnosperm species. Conserved positions across all species are indicated by asterisks. Note that the number of exons might vary among GRF genes. See Table S4 for accession numbers of sequences used. (B) Scheme representing the strategy used to identify new miR396 targets of potential biological significance. Target search was performed over the TAIR9 database using the WMD3 target search tool (http://wmd3.weigelworld.org/), allowing 5 mismatches and gaps in the miRNA-target pairs. Predicted targets are shown in Table S1. (C) Diagram showing putative miR396 targets that are up-regulated at least 30% in miRNA mutants [32], [35]. Expression levels were obtained from Genevestigator (www.genevestigator.com). (D,E) Modified RACE-PCR mapping of At1g10120 and At5g24660 mRNA cleavage sites. Red arrows indicate predicted miR396 cleavage sites. (F) At1g10120 and At5g24660 transcript levels in different small RNA mutant plants estimated by RT-qPCR. Data shown are mean ± SEM of 3 biological replicates. See Table S7 for a list of mutant alleles used. (G) At1g10120 and At5g24660 transcript levels in plants expressing an artificial target-mimic against miR396 (MIM396) estimated by RT-qPCR. Data shown are mean ± SEM of 3 biological replicates. (H) Scheme representing the At1g10120 locus. The miR396 target site is formed after the splicing of the first two exons. Target site conservation in several species is indicated (see Table S4 for accession numbers of sequences used). Conserved positions across all species are indicated by asterisks. We also observed that 17, 26 and 12 additional potential target genes that do not encode GRF transcription factors are predicted in Arabidopsis, poplar and rice, respectively (see Tables S1, S2, S3). Detection of RNAs cleaved at positions 10–11 with respect to cognate small RNAs is a hallmark of miRNA activity [34], however, these products do not necessarily indicate a biologically relevant process. Therefore, we studied additional and potential miR396 targets from several points of view to identify those targets whose regulation would be more likely to have biological importance (Figure 1B). Similar integrated strategies have previously allowed the identification of ta-siRNA targets [32].
First, we analyzed the expression of the 17 predicted targets in mutants of the miRNA biogenesis pathway in Arabidopsis using published ATH1 microarray data [32], [35]. We found that two genes, At5g24660 and At1g10120 were up-regulated at least 30% in the miRNA biogenesis mutants hyl1, serrate (se) and dcl1 (Figure 1C; see Table S1). This increase was similar to that observed for the miR396-regulated GRFs (see Table S1). At1g10120 analysis by a modified 5′ RACE-PCR revealed mRNA fragments consistent with a miR396-guided cleavage (Figure 1D) in agreement with previous results obtained for this gene by genome-wide analysis of miRNA targets [36], [37]. In contrast, we did not find any pattern of miR396 activity on At5g24660 (Figure 1E).
We then performed a RT-qPCR with oligos spanning miR396 cognate sites in several siRNA and miRNA biogenesis mutants (Figure 1F). At1g10120 was up-regulated two-fold in ago1, se and hyl1 mutants, while no change was detected in dcl2-4 or rdr6 mutants (Figure 1F). At5g24660 showed a moderate increase only in se and hyl1 mutants (Figure 1F), albeit to a lower extent than that observed for At1g10120.
Finally, we prepared transgenic plants expressing an artificial target mimic directed against miR396 (MIM396) to decrease the endogenous miRNA activity. These plants did not have any obvious phenotypic defects, similar to a previously described MIM396 prepared along a collection of target-mimics [12], probably due to remaining miR396 activity. However, at the molecular level, we found that At1g10120 was up-regulated two-fold in 35S:MIM396 plants, which was analogous to the increase observed in miR396-regulated GRFs (Figure 1G). In contrast, transcript levels of At5g24660 and GRF5, which is not regulated by miR396, were unaffected by the expression of MIM396 (Figure 1G). Taken together, these results indicate that miR396 has a meaningful impact on the RNA regulation of this new target and that this regulation is quantitatively similar to the one observed in conserved GRFs.
At1g10120 encodes a basic Helix-Loop-Helix (bHLH) transcription factor, namely bHLH74 [38], [39], [40]. Interestingly, we observed that the miR396-binding site of bHLH74 is formed after the splicing of the first two exons (Figure 1H). We searched for bHLH74 homologs with a miR396 target site in EST and genome sequence databases of species related to Arabidopsis thaliana. Our observations indicated that the miR396-bHLH74 regulatory module is present in Brassicaceae species. We also found that conservation extends to Cleome spinosa which belongs to the sister family Cleomaceae, separated 40–50 million years from Arabidopsis thaliana [41], [42] (Figure 1H; see Figure S1 and Table S4).
We did not find evidence of bHLH74 homologs with a miR396 target site in more distant species of Arabidopsis thaliana, either looking at syntenic regions of sequenced genomes such as poplar or by BLAST search against EST databases. The search was also performed trying to identify relaxed target sites, for example, looking only at the 5′ region which is completely located in the second exon of bHLH74 without intron interruption. In neither case did we find an additional bHLH74 homologue that could be potentially regulated by miR396.
Regulation of bHLH74 by miR396
The up-regulation of bHLH74 in miR396-deficient plants and its conservation in a group of related species suggested that miR396 regulation might have a biological significance. To study the importance of bHLH74 regulation by miR396 in more detail, we prepared a version of the gene with mutations that impaired its interaction with the miRNA (rbHLH74). We introduced silent mutations to avoid changing the encoded amino acids and did not modify the region next to the intron-exon junction (Figure 2A).
Fig. 2. Characterization of transgenic plants expressing a miR396-resistant bHLH74. 
(A) Schematic representation of bHLH74 and rbHLH74 constructs. (B) bHLH74 transcript levels estimated by RT-qPCR in mature leaves of plants expressing a wild-type (bHLH74:wtbHLH74) or miR396-resistant (bHLH74:rbHLH74) form of the gene encoding the transcription factor. Data shown are mean ± SEM of 3 biological replicates. Asterisks indicate significant differences between transgenic and wild-type plants, as determined by ANOVA (P<0.05). (C) Angle determination at the distal tip of leaf #5 in transgenics depicted on panel (B). Asterisks indicate significant differences between transgenics and wild-type plants, as determined by ANOVA (P<0.05). (D) Morphology of fully-expanded leaf #5. The different angles at the distal edge of the leaf are highlighted in yellow for wild-type and bHLH74:rbHLH74 leaves. An inset on the right shows the difference in venation (secondary veins are highlighted in red), with the number of branching points (NBP) indicated below. Data shown are mean ± SEM of 8 biological replicates. Scale Bar: 1 cm. (E) Scheme highlighting differences in leaf edges of wild-type and miR396-resistant bHLH74 plants. First, we prepared transgenic plants expressing the wild-type and miR396 resistant gene from the viral CaMV 35S promoter. We observed that both transgenes were able to cause developmental defects, however the effects caused by the overexpression of the miRNA resistant gene (35S:rbHLH74) were stronger and in the most extreme cases led to the formation of chlorotic seedlings, which failed to develop shoot apical meristems (see Figure S2). These results demonstrate that high levels of bHLH74 can be toxic for normal plant development and that miR396 can down-regulate bHLH74 expression levels in vivo.
Next, two vectors expressing a genomic version of the transcription factor with different sensitivities to miR396, including the endogenous upstream regulatory regions, were constructed. For an initial characterization of the resulting transgenic plants, we analyzed bHLH74 transcript levels in mature leaves. As miR396 accumulates with leaf age [22], we expected large differences in bHLH74 mRNA abundance in these samples. The genomic version of the transcription factor containing silent mutations that impaired its regulation by miR396 (bHLH74:rbHLH74) accumulated varied levels of mRNA reaching levels eighty-fold higher than those for the endogenous transcript in the most extreme cases (Figure 2B). On the other hand, the wild-type version (bHLH74:wtbHLH74) accumulated at most eight-fold more (Figure 2B). These results are consistent with high levels of endogenous miR396 guiding the cleavage of the wild-type bHLH74 transcript. Note that the differences in mRNA accumulation between bHLH74:rbHLH74 and bHLH74:bHLH74 are smaller in younger developing tissues, where miR396 levels are low (see below).
These transgenic plants also displayed alterations in leaf development, especially in the vein pattern and organ shape, which had sharper edges than those of wild-type leaves (Figure 2D–2E). The angle formed at the distal part of the leaf was significantly reduced in most bHLH74:rbHLH74 transgenics (Figure 2C–2D). Furthermore, there was also a reduction in the number of branching points (NBP) in the vasculature [43] in plants with high bHLH74 levels (Figure 2D, inset). These results show that bHLH74 regulation by miR396 might be biologically important for Arabidopsis development.
A bhlh74 mutant has leaf developmental changes opposite to rbHLH74
The analysis of transgenic plants harboring a rbHLH74 transgene suggested that bHLH74 might play a role during Arabidopsis leaf development. To further explore this possibility, we identified a loss-of-function mutant for transcription factor bhlh74-1 (Figure 3A). Determination of bHLH74 mRNA levels indicated that they were severely reduced in this mutant (Figure 3B).
Fig. 3. Analysis of bhlh74 mutants. 
(A) Scheme of the bHLH74 locus showing the T-DNA insertion corresponding to the GABI-Kat 720G11 line. Arrows indicate the pairs of primers (1–2 or 3–4, see also Table S7) used to quantify bHLH74 transcript levels by RT-qPCR. (B) bHLH74 transcript levels in wt and bhlh74-1 (GABI-Kat 720G11) seedlings (12–day old), using pairs of primers shown in (A). Data shown are mean ± SEM of 3 biological replicates; n.d.: not detected. Asterisks indicate significant differences between mutant and wild-type plants, as determined by ANOVA (P<0.05). (C) Number of branching points (NBP) in fully-expanded first leaves of wt, bhlh74-1 and bHLH74:rbHLH74 (lines #18 and #8) plants. Bars marked with different letters are significantly different as determined by ANOVA and Duncan's multiple range test (P<0.05). (D) Fully-expanded leaves (#1) from wt, bhlh74-1 and bHLH74:rbHLH74 (lines #18 and #8) plants. Red dots highlight branching points. As rbHLH74 caused a reduction in the number of branching points in the leaf vasculature, we analyzed NBP values for bhlh74 mutants. Interestingly, we found that the NBP was increased approximately 24% in bhlh74-1 with respect to the wild type (Figure 3C–3D). This phenotype was opposite to that found in plants harboring a rbHLH74 transgene. Actually, we observed a quantitative response between the increase of rbHLH74 mRNA levels and the reduction of NBP (Figure 2B and Figure 3C–3D). Together with previous reports [22], these results show that both types of miR396 targets, namely GRFs and bHLH74, have biological roles during leaf development.
MiR396 contributes to the spatio-temporal expression of bHLH74 and GRF2
We then compared the regulation of the new bHLH74 target by miR396 to the regulation of an ancient GRF target, such as GRF2. First, we prepared a reporter to follow miR396 expression by fusing the 2 Kb upstream regulatory sequences of MIR396b to a GUS reporter. The miRNA reporter increased its expression during leaf development (Figure 4A). In young leaves MIR396b:GUS was expressed in a gradient along the longitudinal axis of the leaf, with higher expression at the distal part (Figure 4A). At later developing stages, MIR396b:GUS was detected in whole organs (Figure 4A). The profile of the reporter matched previous small RNA blots performed for this miRNA [22].
Fig. 4. MiR396 coordinates the spatio-temporal expression of bHLH74 and GRF2. 
(A) GUS staining of MIR396b:GUS plants of different age. Arrowheads show leaf #1. Numerals indicate plant age expressed in DAS (Days After Sowing). The inset shows a closer look at a developing leaf displaying a miR396 expression gradient. (B) GUS staining of wtGRF2-GUS (right) and rGRF2-GUS lines (15-day old). rGRF2-GUS has synonymous mutations in the miR396-target site. Left, scheme representing the reporters. The upstream regulatory regions and the first 4 exons were fused to GUS. The miRNA-target site is indicated in red. (C) GUS staining of wtbHLH74-GUS (right) and rbHLH74-GUS lines (15-day old). rbHLH74-GUS has synonymous mutations in the miR396 target site. Left, scheme representing the reporters. The upstream regulatory regions and the first 2 exons were fused to GUS. The miRNA target site is indicated in red. (D) miR396, GRF2 and bHLH74 RNA levels estimated by RT-qPCR in wild-type leaf #5 (DAE: Days After leaf #5 Emergence). Data shown are mean ± SEM of 3 biological replicates. Asterisks indicate significant differences between leaves, as determined by ANOVA (P<0.05). (E) bHLH74 expression levels at different ages of leaf #5 in bHLH74:wtbHLH74 and bHLH74:rbHLH74 plants. Expression levels were normalized to 3 DAE of the bHLH74:wtbHLH74 line #3. Data are mean ± SEM of 3 biological replicates. Asterisks indicate significant differences between leaves, as determined by ANOVA (P<0.05). Scale Bars: 2 mm. A wild-type reporter for GRF2 containing the upstream regulatory region as well as the first four exons harboring the miR396 target site was expressed in young leaves and in proximo-distal gradient along the longitudinal axis of the organ (Figure 4B) [22]. Interestingly, the pattern of MIR396b and GRF2 expression was complementary during leaf development. This was especially noticeable in 15-day old seedlings where MIR396b was expressed in older organs and in the distal part of young leaves, while GRF2 was expressed in the proximal part of young organs (Figure 4A–4B). A miR396-resistant GRF2 reporter, with mutations in the target site, was expressed in a broader domain, highlighting the activity of the miRNA in shaping GRF2 expression (Figure 4B) [22].
We then prepared two reporters to follow the regulation of bHLH74 by miR396. We fused its promoter and the first two exons, which generate the miRNA target site, to the GUS gene (Figure 4C). This first sensor, which has a functional miR396-binding site (wtbHLH74-GUS) was strongly expressed in young leaves, especially in the veins, while its expression decreased in older leaves (10 out of 16 independent transgenic plants) (Figure 4C). The second sensor contained silent mutations in the miRNA binding site, which impaired its regulation by miR396 (rbHLH74-GUS). This reporter was expressed in organs much older than those of the wild-type version highlighting the role of miR396 in restricting its activity to younger organs (12 out of 16 independent transgenic plants) (Figure 4C). Although we cannot disregard the possibility of the existence of other regulatory levels affecting bHLH74 expression such as post-translational modifications, which are not detected by our sensors, the results show that miR396 contributes to the spatio-temporal regulation of bHLH74. Furthermore, the expression of the bHLH74 sensors in the veins is in agreement with the biological role of the transcription factor in the control of leaf vasculature development.
We then analyzed miR396, GRF2 and bHLH74 transcript levels by RT-qPCR in young and fully-expanded leaves (Figure 4D). We observed that while miR396 was induced several times during leaf development, both GRF2 and bHLH74 decreased significantly. These quantitative measurements are in accordance with the whole-mount GUS stainings, supporting the function of miR396 in the regulation of both types of targets during organ growth (Figure 4A–4D).
Finally, we measured the accumulation of bHLH74 mRNA in transgenic plants expressing the wild-type and miR396 resistant version of bHLH74 at two leaf developmental stages. At young stages when miR396 levels are low, bHLH74 was only slightly higher in bHLH74:rbHLH74 than in bHLH74:wtbHLH74 transgenic plants (Figure 4E). However, the wild-type bHLH74 was significantly down-regulated more than ten times in older organs, in agreement with the activation of miR396 expression (Figure 4E). These results further support the role of miR396 in the regulation of bHLH74 expression.
Variations among miR396 family members in plants
The fact that bHLH74 regulation by miR396 is important for Arabidopsis development suggests that the miR396 regulatory network could be relatively dynamic at least with respect to the acquisition of new targets. We then explored whether there could be biologically relevant variations in the miRNA.
Analysis of miR396 variants in different species using the miRNA database (miRBase 17.0) indicates that there are, indeed, several variants of miR396 (Figure 5). In Selaginella, miR396 has a G at position 7, while both genes in Arabidopsis encode small RNAs with an A at that position (Figure 5A). Pine and poplar have precursors for both types of mature miR396 species (Figure 5A). Interestingly, there is a miR396 variant with an insertion of a G at position 7–8 of the miRNA (Figure 5A). This variant was first detected in rice [29], and further studies indicated that it is found only in monocotyledons (Figure 5B) [29], [30], [44].
Fig. 5. Variations among miR396 family members in plants. 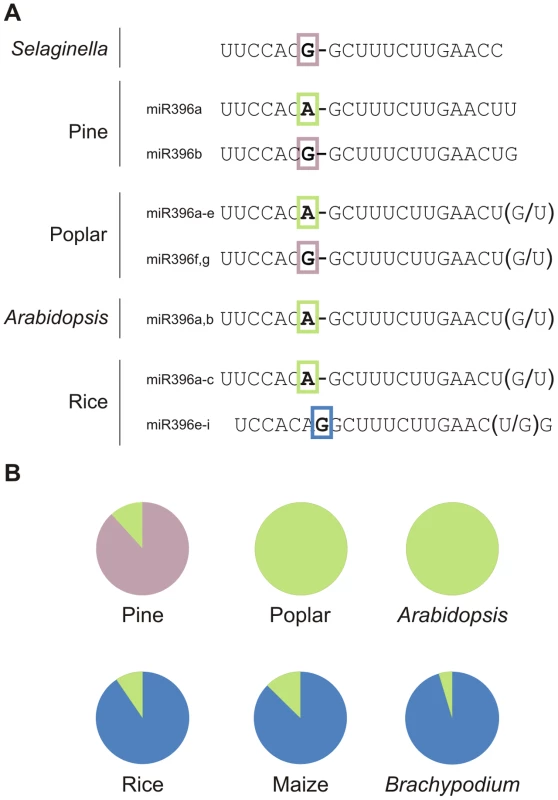
(A) MiR396 family composition of selected species. Differences in the 5′ region are highlighted with colored boxes, while variations at the 3′ end for each case are indicated in parentheses. (B) Diagrams showing the relative abundance of miR396 variants in pine, poplar, Arabidopsis and monocot libraries according to deep sequencing data (see Table S5). Sequence differences among related miRNAs could be important in plants as it has been shown for miR319 and miR159, which have very similar sequences but regulate different genes [17]. Interestingly, the changes observed for the miR396 sequence at position 7–8 are predicted to have an important effect on miRNA activity based on previous biochemical data and mutant analysis [15], [16], [17]. We also searched for potential variations in other ancient miRNAs annotated in the miRNA database (miRBase 17.0), and found that changes in the 5′ region of the miRNA might also exist in other cases (see Figure S3).
It should be considered that some of the polymorphisms observed could arise from non-expressed paralogs that have been annotated due to their homology to other known miRNAs. To confirm the expression of the miR396 sequences, we analyzed publicly available deep-sequencing small RNA libraries from several species [29], [33], [45], [46], [47], [48], [49], [50]. We found that most small RNAs were detected in vivo, confirming that a complex spectrum of miR396 sequences co-exists in nature (Figure 5B; see Table S5). Interestingly, we observed that the monocot-specific variant was the most abundant miR396 variant in rice, maize and Brachypodium distachyon as judged by deep-sequencing (Figure 5B).
Differential activity of miR396 variants
Recognition of the GRF target site by miR396 generates a bulge between positions 7 and 8 of the miRNA (Figure 6A). The insertion of one nucleotide in the monocot-specific version of miR396 eliminates this bulge, strengthening the interaction of the miRNA-target pair by 7 kcal/mol (Figure 6A–6B). Since the contribution of a bulge to the activity of a miRNA has not yet been assayed in plants, we decided to explore it in more detail.
Fig. 6. The monocot-version of miR396 is hyperactive towards the GRFs. 
(A,B) Scheme showing the interaction between Arabidopsis GRF2 and either miR396a (A) or rice miR396e,f (miR396_7-8insG) (B), which is a monocot-specific variant. (C–E) Phenotype frequency in independent transgenic plants (T1) expressing an empty vector (C), Arabidopsis miR396a (D), rice miR396e,f (miR396_7-8insG) (E). MiR396a and miR396_7-8insG were expressed from the viral 35S (left) and MIR396b (right) promoters. At least 100 independent transgenic plants were scored for each vector. (F) Phenotypes of transgenic plants (12-day old seedlings) harboring the different vectors. Phenotypes were classified according to the area reduction in leaves #1 and #2. Scale Bar: 2 mm. (G) to (I) miR396, GRF2 and bHLH74 levels in control plants (transformed with an empty vector, gray) and transgenic plants overexpressing Arabidopsis miR396a (light green) or miR396_7-8insG (blue) displaying an intermediate phenotype. RNA levels were determined by RT-qPCR using pools of 20 independent T1 seedlings. Data shown are mean ± SEM of 3 biological replicates. Bars marked with different letters are significantly different as determined by ANOVA and Duncan's multiple range test (P<0.05). For this purpose, we expressed the two versions of miR396 from the MIR319a precursor in Arabidopsis, which has already been shown as an efficient driver of artificial miRNA sequences [51]. Ectopic expression of the Arabidopsis miR396 mature sequence from the MIR319a precursor caused smaller and lanceolated leaves (Figure 6C–6F), in a similar way to the overexpression of the endogenous MIR396 precursor [21], [22]. Expression of the monocot-version of miR396 (miR396_7-8insG) caused stronger effects on the leaf lamina (Figure 6C–6F). We further expressed both miR396 variants from the endogenous MIR396b promoter. While an additional copy of the endogenous miR396 sequence caused a minor impact on leaf development, expression of the monocot miR396 version from the MIR396b promoter affected leaf development in more than 50% of the independent transgenic plants (Figure 6C–6F). These results show that the miR396 monocot variant is more active in vivo.
We then set up an assay to quantify both miR396 variants by RT-qPCR in the same reaction (for details see Figure S4). We focused on transgenic plants with a moderate reduction in leaf lamina (∼60%), which was observed in 52% or 38% of the primary transgenic plants expressing the endogenous or the monocot-specific miR396 sequence from the 35S viral promoter, respectively (Figure 6D–6F). We measured the levels of miR396 in the transgenics over-accumulating the endogenous Arabidopsis miRNA and found that it was eight-fold higher compared to wild-type levels (Figure 6G, for a small RNA blot see Figure S5). In contrast, transgenic seedlings with the same phenotype but expressing the monocot-specific miR396 variant displayed only a two-fold increase (Figure 6G).
Analysis of the miR396-regulated gene GRF2 in seedlings with moderated phenotypes revealed a decrease of its mRNA levels to 40% in both types of transgenic plants (Figure 6H). We also tested the activity of the two miRNA variants on bHLH74. While the overexpression of the endogenous miR396 significantly down-regulated bHLH74 mRNA (approximately 90%), the monocot-specific version had only a minor effect (Figure 6I). Furthermore, bHLH74 transcript levels were reduced only 25% in plants expressing the highest levels of the monocot-specific version (data not shown). Therefore, this monocot-specific version of miR396 is selectively more efficient towards the GRFs. The addition of an extra nucleotide to this variant causes a bulge to be formed on the miRNA side of the bHLH74/miR396 pair (see Figure S6). Altogether, these results show that asymmetric bulges located either in the miRNA or in the target dampen the miRNA-guided cleavage reaction.
We also analyzed the activity of the miR396 variant found in Selaginella, pine and poplar (Figure 4A) and determined that it caused slightly stronger developmental defects than the wild-type precursor (see Figure S7). A possible explanation to this is that the miR396_7A>G version replaces an interaction between the A-U pair with a stronger G-C pair, causing a concomitant change of more than two kcal/mol in the interaction energy of the miR396/GRF pair (see Figure S7).
Suboptimal regulation of miR396 targets in Arabidopsis thaliana
The previous results show that a single miR396 gradient generates opposing gradients of expression for its targets (Figure 4), and that a perfect match between miR396 and the GRFs increase the in vivo efficiency of the miRNA (Figure 6). Next, we decided to analyze whether the bulge present in the miR396-GRF pair plays a role in patterning the expression of GRF2 during leaf development in Arabidopsis.
To test this, we designed another GRF2-GUS reporter where the bulge was eliminated from the interaction with the endogenous miR396, thus generating a nearly perfect pairing (pGRF2-GUS) (Figure 7A; see Table S6). Whole-mount stainings of wtGRF2-GUS revealed its expression in young developing leaves of Arabidopsis in 17 out of 20 primary transgenics (Figure 7A). The pGRF2-GUS construct had a more limited expression and was restricted towards the proximal part of the leaf. This typical expression pattern was observed in 15 out of 20 transgenic plants for pGRF2-GUS (Figure 7A). The remaining reporter lines displayed even weaker levels of GUS staining.
Fig. 7. Activity of endogenous miR396 towards different substrates in Arabidopsis thaliana. 
(A) GUS stainings of typical transgenic plants harboring wtGRF2 and pGRF2 reporters (15-day old seedlings). Sensors were built by fusing the upstream regulatory regions of GRF2 and its first 4 exons to GUS. The miR396 target site was modified as indicated below the pictures. Interaction energy values for miR396b are indicated below each miRNA-target pair. Scale Bar: 2 mm. (B) Expression levels of GRF2-GUS RNA in leaves #1 and #2 (15-day old seedlings) of the different sensors. Two representative lines for each vector out of a total of 20 independent lines were selected. Expression levels were normalized to wtGRF2-GUS line #3. Data shown are mean ± SEM of 4 biological replicates. Asterisks indicate significant differences between plants harboring different transgenes, as determined by ANOVA (P<0.05). (C) GUS staining in developing leaves #4 (right) and #5 (left) of transgenic plants harboring miR396, rGRF2, wtGRF2, pGRF2 and CYCLIN B1;1 reporters (14-day old seedlings). Scale Bar: 1 mm. We then measured the GUS mRNA in two representative lines for each vector and observed that bulge removal from the GRF2 reporter caused approximately a two-fold reduction in GUS RNA, confirming its higher sensitivity to miR396 (Figure 7B). These results further support our previous findings, i.e. the monocot-specific version of miR396, which does not have a bulge in the miRNA-target pair, has a higher activity towards the GRFs than the one from Arabidopsis.
It has been shown that GRF2 is expressed in the proximal part of the leaf, which contains proliferating cells [22]. We then compared the expression pattern of several reporters in developing leaves of Arabidopsis thaliana. We observed that MIR396b has an expression gradient opposite to that of wtGRF2-GUS and pGRF2-GUS expression (Figure 7C). However, the shape of the latter two gradients is different, being pGRF2-GUS tapered off faster than wtGRF2-GUS. As expected, rGRF2-GUS was expressed throughout the leaf (Figure 7C).
We also stained a CYCLINB1;1 reporter to identify proliferating cells in similar organs. Comparison of the expression patterns for GRF2 reporters revealed that only the one harboring the wild-type target sequence was co-expressed with the proliferating cells (Figure 7C). Altogether, these results suggest that particular miRNA-target interactions, which are not a perfect match and therefore likely operate at sub-optimal activity, might have biological implications.
Discussion
Acquisition of new targets by ancient miRNAs
Most of the conserved miRNAs regulate transcription factors involved in development and hormone signaling. These target genes generally contain similarly conserved miRNA binding-sites. Studies performed in Arabidopsis thaliana and other species have shown that interfering with the regulation of conserved targets by changes in the recognition sites, mutations in the miRNAs [reviewed in [1], [2]] or expressing miRNA-target mimics [12] usually lead to important developmental defects.
Genome-wide analyses of miRNA targets have revealed that ancient miRNAs can regulate targets that are not broadly conserved [36], [37]. However, detection of miRNA-guided cleavage is not necessarily indicative of a relevant biological function. This has already been pointed out for young miRNAs, whose biological functions remain unclear, even though their activities can be detected in vivo [9], [11]. The results presented here show that the regulation of bHLH74 by miR396 has a meaningful impact on its RNA levels, in a similar way to that observed for the widely distributed GRF transcription factors. Interestingly, both targets are involved in leaf development. While the GRFs control cell number [21], [22], [24], [26], [28], we found that bHLH74 regulates the vein patterning of the leaf. Furthermore, GRF2 [22] and bHLH74 transgenes harboring silent mutations in their miRNA target sites affect leaf development, suggesting that the regulation of both type of targets by miR396 is important for Arabidopsis development.
GRF regulation by miR396 can be traced back to at least the gymnosperms based on the existence of GRF transcription factors with a miRNA target site. In contrast, we could only find bHLH74 homologues with miR396-binding sites in species within the sister families Cleomaceae and Brassicaceae. However, it is tempting to speculate that the conservation of a miRNA-target sequence in related species might still be significant and could serve as a tool to identify additional miRNA targets whose regulation might be of biological relevance.
Subfunctionalization of miRNA family members
Ancient miRNAs are usually found as small gene families, encoding small RNAs of similar or identical sequences. One of the advantages of having families with multiple miRNA members is to provide flexibility in the way miRNAs are themselves regulated [52]. Additionally, differences in the miRNA sequences could cause related miRNAs to regulate different sets of targets. This has been previously shown for the miRNAs miR319 and miR159, which are similar in sequence but still regulate different genes [16], [17], [18], [19]. While miR319 can guide TCP and MYB genes to cleavage, specific base differences prevent miR159 activity on the TCPs [17].
The results reported here suggest that similar sequences could have also acquired specialized functions to regulate the same set of targets with different efficiency. The conserved interaction between miR396 and the GRFs has a bulge at position 7–8 of the target site. Removal of this bulge by either the addition of one base to the miRNA, as seen in the monocot-specific miRNA variant, or by the removal of a base from the GRF target site, results in a higher miRNA activity. Interestingly, the addition of a base in the Arabidopsis miR396 selectively improves its efficiency towards the GRFs at the expense of losing activity towards bHLH74, suggesting that bulges in miRNA/target pairs could be used for differential target regulation in miRNA networks.
Our systematic analysis of evolutionary conserved plant miRNAs has shown that additional variations exist in other miRNA sequences, including changes in the 5′ sensitive region. It might be interesting to further explore the occurrence in nature of additional examples of miRNA specialization, and whether they cause changes in target specificity and/or efficiency.
It has been proposed that Arabidopsis miR396 contributes to the fine-tuning of GRF expression [22]. Here, we have further shown that only a GRF2 reporter under suboptimal regulation by the endogenous miR396 can overlap the proliferative region of a developing leaf in Arabidopsis thaliana. It is plausible, however, that a gross down-regulation of the GRFs under specific conditions or in specific cells, such as the one caused by the monocot miR396 variant, could also be advantageous.
Multiple target regulation by miR396 in Arabidopsis thaliana
MiR396 is expressed at low levels in the meristem and leaf primordia, and then it steadily accumulates as leaves develop [22]. When considering a single developing organ, miR396 accumulates in the more mature and distal part, with a miRNA gradient proceeding towards the base of the organ.
Analysis of bHLH74 and GRF2 expression patterns revealed that they both shared a temporal component, which is imposed by the accumulation of miR396 during leaf development. Both genes are expressed in young organs as a consequence of miR396 activity. Still, bHLH74 and GRF2 reporters do not have identical expression patterns, as the bHLH is more restricted to the vasculature while the GRF is more widely distributed throughout the leaf mesophyll cells. The exact tissue of expression might be governed by cis regulatory sequences in the promoters of the different targets. Therefore, several layers of regulation can ultimately generate unique and coordinated expression patterns on target genes belonging to the same miRNA regulatory network.
Another potential level of complexity in the regulation of different targets by miR396 might arise from the miRNA expression gradient in a developing leaf, which extends from the distal part of the organ towards its base. In principle, a single miRNA gradient can generate different expression gradients of its targets, depending at least partially on the exact nature of the miRNA-target pair.
Materials and Methods
Plant material and leaf analysis
Arabidopsis ecotype Col-0 was used for all experiments, with the exception of the GRF2-GUS and bHLH74-GUS reporters, which were analyzed in rdr6 background. Plants were grown in long (16 h light/8 h dark) or short photoperiods (8 h light/16 h dark) at 23°C.
To analyze the vein pattern, leaves were fixed with FAA and cleared with a chloral hydrate solution. Pictures were then taken under dark field illumination in a dissecting microscope. The number of branching points (NBP) [43] was measured per leaf half in Figure 2D, and in the whole leaf in Figure 3D.
Transgenes
See Table S6 for a list of binary plasmids used in this study. The miRNA target motif in GRF2 and bHLH74 was altered introducing synonymous mutations in a cloned wild-type genomic fragment using the QuikChange Site Directed Mutagenesis Kit (Stratagene). Artificial miRNAs [51] were generated by PCR, and MIM396 was generated by gene synthesis (Mr. Gene GmbH).
Expression analysis
RNA was extracted using TRIzol reagent (Invitrogen) and 1,0 µg of total RNA was treated with RQ1 RNase-free Dnase (Promega). Next, first-strand cDNA synthesis was carried out using SuperScriptTM III Reverse Transcriptase (Invitrogen) with the appropriate primers. PCR reactions were performed in a Mastercycler ep realplex thermal cycler (Eppendorf) using SYBRGreen I (Roche) to monitor dsDNA synthesis. MiR396 and miR396_7-8insG levels were concurrently determined in each sample by stem-loop RT-qPCR [53]. A scheme of the strategy used for the simultaneous quantification of miR396 and miR396_7-8insG is provided in Figure S4. Relative transcript level was determined for each sample, normalized using PROTEIN PHOSPHATASE 2A (AT1G13320) cDNA levels [54]. MiR396 levels were also estimated by small RNA blots as described previously [22]. Primer sequences are given in Table S7.
To visualize reporter activity, transgenic plants were subjected to GUS staining, as described previously [55]. RNA adaptor ligation, reverse transcription and 5′RACE were performed according to the procedure described previously in order to determine RNA degradation products [56].
Sequence analysis
Small RNA sequences obtained from miRBase (17.0) [4] were used for analyses of miRNA sequence variations in conserved miRNA families in angiosperms [9]. A consensus sequence was identified for each family and deviations from the consensus at each position were quantified. The number of variations was normalized to the total number of miRNA family members, so that each family contributed equally.
Accession numbers
A list of relevant AGI locus identifiers is provided in Table S7.
Supporting Information
Zdroje
1. VoinnetO 2009 Origin, biogenesis, and activity of plant microRNAs. Cell 136 669 687
2. Jones-RhoadesMWBartelDPBartelB 2006 MicroRNAS and their regulatory roles in plants. Annu Rev Plant Biol 57 19 53
3. ChenX 2008 MicroRNA metabolism in plants. Curr Top Microbiol Immunol 320 117 136
4. Griffiths-JonesSSainiHKvan DongenSEnrightAJ 2008 miRBase: tools for microRNA genomics. Nucleic Acids Res 36 D154 158
5. MeyersBCAxtellMJBartelBBartelDPBaulcombeD 2008 Criteria for Annotation of Plant MicroRNAs. Plant Cell
6. FahlgrenNJogdeoSKasschauKDSullivanCMChapmanEJ 2010 MicroRNA gene evolution in Arabidopsis lyrata and Arabidopsis thaliana. Plant Cell 22 1074 1089
7. FahlgrenNHowellMDKasschauKDChapmanEJSullivanCM 2007 High-throughput sequencing of Arabidopsis microRNAs: evidence for frequent birth and death of MIRNA genes. PLoS ONE 2 e219 doi:10.1371/journal.pone.0000219
8. MaZCoruhCAxtellMJ 2010 Arabidopsis lyrata small RNAs: transient MIRNA and small interfering RNA loci within the Arabidopsis genus. Plant Cell 22 1090 1103
9. AxtellMJBowmanJL 2008 Evolution of plant microRNAs and their targets. Trends Plant Sci 13 343 349
10. RajagopalanRVaucheretHTrejoJBartelDP 2006 A diverse and evolutionarily fluid set of microRNAs in Arabidopsis thaliana. Genes Dev 20 3407 3425
11. AxtellMJ 2008 Evolution of microRNAs and their targets: are all microRNAs biologically relevant? Biochim Biophys Acta 1779 725 734
12. TodescoMRubio-SomozaIPaz-AresJWeigelD 2010 A collection of target mimics for comprehensive analysis of microRNA function in Arabidopsis thaliana. PLoS Genet 6 e1001031 doi:10.1371/journal.pgen.1001031
13. KutterCSchobHStadlerMMeinsFJrSi-AmmourA 2007 MicroRNA-mediated regulation of stomatal development in Arabidopsis. Plant Cell 19 2417 2429
14. AxtellMJBartelDP 2005 Antiquity of microRNAs and their targets in land plants. Plant Cell 17 1658 1673
15. MalloryACReinhartBJJones-RhoadesMWTangGZamorePD 2004 MicroRNA control of PHABULOSA in leaf development: importance of pairing to the microRNA 5′ region. Embo J 23 3356 3364
16. SchwabRPalatnikJFRiesterMSchommerCSchmidM 2005 Specific effects of microRNAs on the plant transcriptome. Dev Cell 8 517 527
17. PalatnikJFWollmannHSchommerCSchwabRBoisbouvierJ 2007 Sequence and expression differences underlie functional specialization of Arabidopsis microRNAs miR159 and miR319. Dev Cell 13 115 125
18. PalatnikJFAllenEWuXSchommerCSchwabR 2003 Control of leaf morphogenesis by microRNAs. Nature 425 257 263
19. AchardPHerrABaulcombeDCHarberdNP 2004 Modulation of floral development by a gibberellin-regulated microRNA. Development 131 3357 3365
20. OriNCohenAREtzioniABrandAYanaiO 2007 Regulation of LANCEOLATE by miR319 is required for compound-leaf development in tomato. Nat Genet 39 787 791
21. LiuDSongYChenZYuD 2009 Ectopic expression of miR396 suppresses GRF target gene expression and alters leaf growth in Arabidopsis. Physiol Plant 136 223 236
22. RodriguezREMecchiaMADebernardiJMSchommerCWeigelD 2010 Control of cell proliferation in Arabidopsis thaliana by microRNA miR396. Development 137 103 112
23. Jones-RhoadesMWBartelDP 2004 Computational identification of plant microRNAs and their targets, including a stress-induced miRNA. Mol Cell 14 787 799
24. WangLGuXXuDWangWWangH 2011 miR396-targeted AtGRF transcription factors are required for coordination of cell division and differentiation during leaf development in Arabidopsis. J Exp Bot 62 761 773
25. HoriguchiGFerjaniAFujikuraUTsukayaH 2006 Coordination of cell proliferation and cell expansion in the control of leaf size in Arabidopsis thaliana. J Plant Res 119 37 42
26. HoriguchiGKimGTTsukayaH 2005 The transcription factor AtGRF5 and the transcription coactivator AN3 regulate cell proliferation in leaf primordia of Arabidopsis thaliana. Plant J 43 68 78
27. KimJHKendeH 2004 A transcriptional coactivator, AtGIF1, is involved in regulating leaf growth and morphology in Arabidopsis. Proc Natl Acad Sci U S A 101 13374 13379
28. KimJHChoiDKendeH 2003 The AtGRF family of putative transcription factors is involved in leaf and cotyledon growth in Arabidopsis. Plant J 36 94 104
29. SunkarRGirkeTJainPKZhuJK 2005 Cloning and characterization of microRNAs from rice. Plant Cell 17 1397 1411
30. SunkarRJagadeeswaranG 2008 In silico identification of conserved microRNAs in large number of diverse plant species. BMC Plant Biol 8 37
31. RhoadesMWReinhartBJLimLPBurgeCBBartelB 2002 Prediction of plant microRNA targets. Cell 110 513 520
32. AllenEXieZGustafsonAMCarringtonJC 2005 microRNA-directed phasing during trans-acting siRNA biogenesis in plants. Cell 121 207 221
33. AxtellMJSnyderJABartelDP 2007 Common functions for diverse small RNAs of land plants. Plant Cell 19 1750 1769
34. LlaveCXieZKasschauKDCarringtonJC 2002 Cleavage of Scarecrow-like mRNA targets directed by a class of Arabidopsis miRNA. Science 297 2053 2056
35. LobbesDRallapalliGSchmidtDDMartinCClarkeJ 2006 SERRATE: a new player on the plant microRNA scene. EMBO Rep 7 1052 1058
36. Addo-QuayeCEshooTWBartelDPAxtellMJ 2008 Endogenous siRNA and miRNA targets identified by sequencing of the Arabidopsis degradome. Curr Biol 18 758 762
37. GermanMAPillayMJeongDHHetawalALuoS 2008 Global identification of microRNA-target RNA pairs by parallel analysis of RNA ends. Nat Biotechnol 26 941 946
38. Toledo-OrtizGHuqEQuailPH 2003 The Arabidopsis basic/helix-loop-helix transcription factor family. Plant Cell 15 1749 1770
39. HeimMAJakobyMWerberMMartinCWeisshaarB 2003 The basic helix-loop-helix transcription factor family in plants: a genome-wide study of protein structure and functional diversity. Mol Biol Evol 20 735 747
40. BaileyPCMartinCToledo-OrtizGQuailPHHuqE 2003 Update on the basic helix-loop-helix transcription factor gene family in Arabidopsis thaliana. Plant Cell 15 2497 2502
41. SchranzMEMitchell-OldsT 2006 Independent ancient polyploidy events in the sister families Brassicaceae and Cleomaceae. Plant Cell 18 1152 1165
42. KochMHauboldBMitchell-OldsT 2001 Molecular systematics of the Brassicaceae: evidence from coding plastidic matK and nuclear Chs sequences. Am J Bot 88 534 544
43. Rolland-LaganAGAminMPakulskaM 2009 Quantifying leaf venation patterns: two-dimensional maps. Plant J 57 195 205
44. ZhangLChiaJMKumariSSteinJCLiuZ 2009 A genome-wide characterization of microRNA genes in maize. PLoS Genet 5 e1000716 doi:10.1371/journal.pgen.1000716
45. LuSSunYHAmersonHChiangVL 2007 MicroRNAs in loblolly pine (Pinus taeda L.) and their association with fusiform rust gall development. Plant J 51 1077 1098
46. OhTJWartellRMCairneyJPullmanGS 2008 Evidence for stage-specific modulation of specific microRNAs (miRNAs) and miRNA processing components in zygotic embryo and female gametophyte of loblolly pine (Pinus taeda). New Phytol 179 67 80
47. KlevebringDStreetNRFahlgrenNKasschauKDCarringtonJC 2009 Genome-wide profiling of populus small RNAs. BMC Genomics 10 620
48. YaoYGuoGNiZSunkarRDuJ 2007 Cloning and characterization of microRNAs from wheat (Triticum aestivum L.). Genome Biol 8 R96
49. BarakatAWallPKDiloretoSDepamphilisCWCarlsonJE 2007 Conservation and divergence of microRNAs in Populus. BMC Genomics 8 481
50. MorinRDAksayGDolgosheinaEEbhardtHAMagriniV 2008 Comparative analysis of the small RNA transcriptomes of Pinus contorta and Oryza sativa. Genome Res 18 571 584
51. SchwabROssowskiSRiesterMWarthmannNWeigelD 2006 Highly specific gene silencing by artificial microRNAs in Arabidopsis. Plant Cell 18 1121 1133
52. Rubio-SomozaICuperusJTWeigelDCarringtonJC 2009 Regulation and functional specialization of small RNA-target nodes during plant development. Curr Opin Plant Biol
53. ChenCRidzonDABroomerAJZhouZLeeDH 2005 Real-time quantification of microRNAs by stem-loop RT-PCR. Nucleic Acids Res 33 e179
54. CzechowskiTStittMAltmannTUdvardiMKScheibleWR 2005 Genome-wide identification and testing of superior reference genes for transcript normalization in Arabidopsis. Plant Physiol 139 5 17
55. DonnellyPMBonettaDTsukayaHDenglerREDenglerNG 1999 Cell cycling and cell enlargement in developing leaves of Arabidopsis. Dev Biol 215 407 419
56. KasschauKDXieZAllenELlaveCChapmanEJ 2003 P1/HC-Pro, a viral suppressor of RNA silencing, interferes with Arabidopsis development and miRNA unction. Dev Cell 4 205 217
Štítky
Genetika Reprodukční medicína
Článek USF-1 Is Critical for Maintaining Genome Integrity in Response to UV-Induced DNA PhotolesionsČlánek Checkpoints in a Yeast Differentiation Pathway Coordinate Signaling during Hyperosmotic StressČlánek Two-Component Elements Mediate Interactions between Cytokinin and Salicylic Acid in Plant ImmunityČlánek Adaptive Evolution of the Lactose Utilization Network in Experimentally Evolved Populations of
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2012 Číslo 1- Akutní intermitentní porfyrie
- Farmakogenetické testování pomáhá předcházet nežádoucím efektům léčiv
- Růst a vývoj dětí narozených pomocí IVF
- Pilotní studie: stres a úzkost v průběhu IVF cyklu
- Intrauterinní inseminace a její úspěšnost
-
Všechny články tohoto čísla
- DNA Methylation and Gene Expression Changes in Monozygotic Twins Discordant for Psoriasis: Identification of Epigenetically Dysregulated Genes
- An siRNA Screen in Pancreatic Beta Cells Reveals a Role for in Insulin Production
- Parallel Mapping and Simultaneous Sequencing Reveals Deletions in and Associated with Discrete Inherited Disorders in a Domestic Dog Breed
- Cytoplasmic Polyadenylation Element Binding Protein Deficiency Stimulates PTEN and Stat3 mRNA Translation and Induces Hepatic Insulin Resistance
- Nucleolar Association and Transcriptional Inhibition through 5S rDNA in Mammals
- USF-1 Is Critical for Maintaining Genome Integrity in Response to UV-Induced DNA Photolesions
- Heterochromatin Formation Promotes Longevity and Represses Ribosomal RNA Synthesis
- Genetic Evidence for an Indispensable Role of Somatic Embryogenesis Receptor Kinases in Brassinosteroid Signaling
- Poly(ADP-Ribose) Polymerase 1 (PARP-1) Regulates Ribosomal Biogenesis in Nucleoli
- Genome Engineering in : A Feasible Approach to Address Biological Issues
- RIC-7 Promotes Neuropeptide Secretion
- Adaptation and Preadaptation of to Bile
- Checkpoints in a Yeast Differentiation Pathway Coordinate Signaling during Hyperosmotic Stress
- Progressive Polycomb Assembly on H3K27me3 Compartments Generates Polycomb Bodies with Developmentally Regulated Motion
- A High Density SNP Array for the Domestic Horse and Extant Perissodactyla: Utility for Association Mapping, Genetic Diversity, and Phylogeny Studies
- Two-Component Elements Mediate Interactions between Cytokinin and Salicylic Acid in Plant Immunity
- Cdc5-Dependent Asymmetric Localization of Bfa1 Fine-Tunes Timely Mitotic Exit
- A Genome-Wide Analysis of Promoter-Mediated Phenotypic Noise in
- Contribution of Intragenic DNA Methylation in Mouse Gametic DNA Methylomes to Establish Oocyte-Specific Heritable Marks
- Adaptive Evolution of the Lactose Utilization Network in Experimentally Evolved Populations of
- Microenvironmental Regulation by Fibrillin-1
- Unraveling the Regulatory Mechanisms Underlying Tissue-Dependent Genetic Variation of Gene Expression
- A Half-Century of Inspiration: An Interview with Hamilton Smith
- Reduced Lentivirus Susceptibility in Sheep with Mutations
- High-Density SNP Mapping of the HLA Region Identifies Multiple Independent Susceptibility Loci Associated with Selective IgA Deficiency
- Calpains Mediate Integrin Attachment Complex Maintenance of Adult Muscle in
- Genomic Ancestry of North Africans Supports Back-to-Africa Migrations
- Functional Specialization of the Plant miR396 Regulatory Network through Distinct MicroRNA–Target Interactions
- The Seminal Fluid Protease “Seminase” Regulates Proteolytic and Post-Mating Reproductive Processes
- Insulin Signaling Regulates Fatty Acid Catabolism at the Level of CoA Activation
- A Genome-Wide Association Study Identified as a Susceptibility Locus for Systemic Lupus Eyrthematosus in Japanese
- A Spontaneous Mutation of the Rat Gene Leads to Impaired Function of Regulatory T Cells Linked to Inflammatory Bowel Disease
- The Yeast Complex I Equivalent NADH Dehydrogenase Rescues Mutants
- A Flexible Bayesian Model for Studying Gene–Environment Interaction
- Sex Pheromone Evolution Is Associated with Differential Regulation of the Same Desaturase Gene in Two Genera of Leafroller Moths
- Genome-Wide Assessment of AU-Rich Elements by the ARE Algorithm
- Inference of Population Structure using Dense Haplotype Data
- A Gene Regulatory Network for Root Epidermis Cell Differentiation in Arabidopsis
- Sequencing of Pooled DNA Samples (Pool-Seq) Uncovers Complex Dynamics of Transposable Element Insertions in
- A Genome-Wide Association Scan on the Levels of Markers of Inflammation in Sardinians Reveals Associations That Underpin Its Complex Regulation
- Tempo and Mode in Evolution of Transcriptional Regulation
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Poly(ADP-Ribose) Polymerase 1 (PARP-1) Regulates Ribosomal Biogenesis in Nucleoli
- Microenvironmental Regulation by Fibrillin-1
- Parallel Mapping and Simultaneous Sequencing Reveals Deletions in and Associated with Discrete Inherited Disorders in a Domestic Dog Breed
- Two-Component Elements Mediate Interactions between Cytokinin and Salicylic Acid in Plant Immunity
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



