-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Genetic Evidence for an Indispensable Role of Somatic Embryogenesis Receptor Kinases in Brassinosteroid Signaling
The Arabidopsis thaliana Somatic Embryogenesis Receptor Kinases (SERKs) consist of five members, SERK1 to SERK5, of the leucine-rich repeat receptor-like kinase subfamily II (LRR-RLK II). SERK3 was named BRI1-Associated Receptor Kinase 1 (BAK1) due to its direct interaction with the brassinosteroid (BR) receptor BRI1 in vivo, while SERK4 has also been designated as BAK1-Like 1 (BKK1) for its functionally redundant role with BAK1. Here we provide genetic and biochemical evidence to demonstrate that SERKs are absolutely required for early steps in BR signaling. Overexpression of four of the five SERKs—SERK1, SERK2, SERK3/BAK1, and SERK4/BKK1—suppressed the phenotypes of an intermediate BRI1 mutant, bri1-5. Overexpression of the kinase-dead versions of these four genes in the bri1-5 background, on the other hand, resulted in typical dominant negative phenotypes, resembling those of null BRI1 mutants. We isolated and generated single, double, triple, and quadruple mutants and analyzed their phenotypes in detail. While the quadruple mutant is embryo-lethal, the serk1 bak1 bkk1 triple null mutant exhibits an extreme de-etiolated phenotype similar to a null bri1 mutant. While overexpression of BRI1 can drastically increase hypocotyl growth of wild-type plants, overexpression of BRI1 does not alter hypocotyl growth of the serk1 bak1 bkk1 triple mutant. Biochemical analysis indicated that the phosphorylation level of BRI1 in serk1 bak1 bkk1 is incapable of sensing exogenously applied BR. As a result, the unphosphorylated level of BES1 has lost its sensitivity to the BR treatment in the triple mutant, indicating that the BR signaling pathway has been completely abolished in the triple mutant. These data clearly demonstrate that SERKs are essential to the early events of BR signaling.
Published in the journal: . PLoS Genet 8(1): e32767. doi:10.1371/journal.pgen.1002452
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1002452Summary
The Arabidopsis thaliana Somatic Embryogenesis Receptor Kinases (SERKs) consist of five members, SERK1 to SERK5, of the leucine-rich repeat receptor-like kinase subfamily II (LRR-RLK II). SERK3 was named BRI1-Associated Receptor Kinase 1 (BAK1) due to its direct interaction with the brassinosteroid (BR) receptor BRI1 in vivo, while SERK4 has also been designated as BAK1-Like 1 (BKK1) for its functionally redundant role with BAK1. Here we provide genetic and biochemical evidence to demonstrate that SERKs are absolutely required for early steps in BR signaling. Overexpression of four of the five SERKs—SERK1, SERK2, SERK3/BAK1, and SERK4/BKK1—suppressed the phenotypes of an intermediate BRI1 mutant, bri1-5. Overexpression of the kinase-dead versions of these four genes in the bri1-5 background, on the other hand, resulted in typical dominant negative phenotypes, resembling those of null BRI1 mutants. We isolated and generated single, double, triple, and quadruple mutants and analyzed their phenotypes in detail. While the quadruple mutant is embryo-lethal, the serk1 bak1 bkk1 triple null mutant exhibits an extreme de-etiolated phenotype similar to a null bri1 mutant. While overexpression of BRI1 can drastically increase hypocotyl growth of wild-type plants, overexpression of BRI1 does not alter hypocotyl growth of the serk1 bak1 bkk1 triple mutant. Biochemical analysis indicated that the phosphorylation level of BRI1 in serk1 bak1 bkk1 is incapable of sensing exogenously applied BR. As a result, the unphosphorylated level of BES1 has lost its sensitivity to the BR treatment in the triple mutant, indicating that the BR signaling pathway has been completely abolished in the triple mutant. These data clearly demonstrate that SERKs are essential to the early events of BR signaling.
Introduction
Brassinosteroids (BRs) are naturally produced plant hormones regulating many developmental processes from seed germination to flowering and senescence [1]. BR deficiency or response mutants show typical phenotypic defects including decreased rate of seed germination, rounded and epinastic rosette leaves, extremely dwarfed stature, delayed flowering time, reduced male fertility, postponed leaf senescence, and extremely de-etiolated phenotypes grown under dark conditions [2]–[5]. Although both plants and animals use steroids as growth regulators, the signaling pathways in the two kingdoms are divergent [6]. While animal steroids are known to be perceived by nuclear receptors which can directly regulate gene transcription upon ligand binding, BRs are sensed by a single-pass transmembrane leucine-rich repeat receptor-like protein kinase (LRR-RLK) named Brassinosteroid Insensitive 1 (BRI1) and two BRI1 paralogs, BRI1-Like 1 (BRL1) and BRL3 [3], [7]–[9]. When BR is absent, BRI1 was found to exist as a homodimer whose cytoplasmic domain interacts with a membrane-anchored protein termed BRI1 Kinase Inhibitor 1 (BKI1), blocking the interaction between the kinase domains of BRI1 and its co-receptor BRI1-Associated Receptor Kinase 1 (BAK1) [10]–[13]. Recent crystal structure analyses demonstrated that the LRRs of BRI1 form an extremely twisted helical solenoid structure and the hydrophobic pocket formed by LRRs and the “island” domain provides a direct binding site for BRs [14], [15]. The ligand-receptor interaction initiates the BR signaling cascade, mostly via reversible phosphorylation and dephosphorylation [16].
It was proposed that during early events of BR signaling, the BR receptor BRI1 and its co-receptor BAK1 follow a reciprocal and sequential phosphorylation process before downstream components can be activated [17]. Interaction of BR with the extracellular domain of BRI1 triggers a conformational change of the cytoplasmic domain of BRI1, causing phosphorylation of BKI1 on a conserved tyrosine residue, resulting in the dissociation of phosphorylated BKI1 from BRI1 [12], [18]. It is likely that the BRI1 kinase domain is autophosphorylated and activated via an intermolecular mechanism, and the activated BRI1 then recruits BAK1, via a kinase-to-kinase and extracellular domain-to-extracellular domain double lock mechanism, in close proximity and phosphorylates several Thr residues within the activation loop of BAK1, activating the co-receptor [19], [20]. The active BAK1 then phosphorylates multiple residues within the juxtamembrane and carboxyl terminus regions of BRI1, fully activating BRI1 and creating proper docking sites for association of other BRI1 downstream components such as BR-Signaling Kinases (BSKs) [21]. The activated BSKs can inhibit kinase activity of a negative regulator Brassinosteroid Insensitive 2 (BIN2) by activating a protein phosphatase named bri1 Suppressor 1 (BSU1) to dephosphorylate a phosphotyrosine residue (pTyr200) on BIN2 [22]. Inactivation of BIN2 causes the accumulation of two unphosphorylated transcription factors Brassinazole-Resistant 1 (BZR1) and bri1-Ems-Suppressor 1 (BES1) in nuclei, directly mediating the expression of BR responsive genes [23]–[28]. Phosphorylated forms of BES1 and BZR1, on the other hand, are trapped in cytoplasm by interacting with 14-3-3 proteins and eventually degraded via a 26S proteasome-mediated pathway [29]–[32].
The role of BAK1 has been mainly defined by various gain-of-function genetic and biochemical analyses [10], [11], [17], [20]. The significance of the function of BAK1, however, has so far not been demonstrated by a loss-of-function genetic analysis which is considered as the most reliable approach to reveal biological functions of a given gene. A BAK1 null mutant only exhibits a subtle bri1-like defective phenotype suggesting either additional homologues of BAK1 play redundant roles with BAK1, or BAK1 and its homologues only provide an enhancing but not an essential role to BR signaling. The significance of BAK1 and its homologues in mediating BR signaling should be determined in a mutant plant with lesions of BAK1 and all its functionally redundant genes. Recent studies indicated that BAK1 and its homologues also play important roles in regulating several BR-independent signaling pathways such as anther development, cell-death control, and disease resistance [33]. For example, the serk1 serk2 double mutant shows an anther defective phenotype [34], [35]; the bak1 bkk1 double null mutant displays light-dependent spontaneous cell death at the seedling stage [36], [37]; and the bak1 single mutant exhibits uncontrolled cell death and reduced innate immunity responses to a variety of pathogens [38]–[42]. These findings add additional complexity to our efforts towards understanding the significance of BAK1 in BR signaling pathway.
In this study, we show that four out of five members of the SERK subfamily (SERK1 to SERK4), in the wild-type Arabidopsis Columbia background (Col-0), may play functionally redundant roles in BR signaling. In Col-0, SERK5 contains a mutation in an important amino acid residue which likely abolishes the kinase activity of SERK5 [36]. We subsequently isolated null mutants for all four kinase active SERKs, and generated double, triple, and quadruple mutants using two sets of independent null mutants. Our detailed analysis indicates that dark grown serk1 bak1 bkk1 triple mutants show a typical de-etiolated phenotype resembling a null bri1 mutant. Physiological and biochemical analyses indicate that the triple mutant is insensitive to BR treatment similar to a null bri1 mutant. Furthermore, the phosphorylation level of BRI1 in the triple mutant is completely unresponsive to BR treatment, suggesting that BRI1 cannot initiate BR signaling without BAK1 and its homologues. These results provide clear genetic and biochemical evidence that BAK1 plays an essential role in the BR signal transduction pathway.
Results
SERK1, SERK2, BAK1, and BKK1 are the only members in the LRR-RLK II subfamily which can suppress bri1-5 when overexpressed
BAK1 was previously identified as a coreceptor of BRI1 in mediating BR signaling [10], [11]. Genetic data demonstrating BAK1 is essential to BR signal transduction is still lacking. If BAK1 plays a role which is as critical as BRI1, a mutant plant with loss-of-function mutation of BAK1 and all its functionally redundant genes should exhibit a typical bri1 null mutant phenotype (Figure 1A). A bak1 null mutant, however, shows a subtle bri1-like phenotype suggesting that there may be the LRR-RLK II subfamily genes that are functionally redundant with BAK1. The five SERKs are grouped in a single clade of the LRR-RLK II subfamily according to phylogenetic analyses [43], [44]. Based on sequence similarity, it is logical to hypothesize that any BAK1 functionally redundant proteins would be members of the LRR-RLK II subfamily. To test this hypothesis, all 14 LRR-RLK II members were overexpressed in the intermediate bri1-5 mutant in the WS2 background [45]. Our results indicated that only SERK1, SERK2, BAK1, and BKK1 could partially suppress bri1-5 phenotypes when overexpressed (Figure 1B). These overexpressed transgenic plants showed elongated petioles and wild-type like bolting and flowering time.
Fig. 1. SERK1, SERK2, BAK1, BKK1 are the only four genes in the LRR-RLK II subfamily playing redundant roles in mediating BR signal transduction. 
A. Phenotypes of wild-type Arabidopsis (WS2), an intermediate BRI1 mutant, bri1-5, and a null BRI1 mutant, bri1-4. B. Overexpression of SERK1, SERK2, BAK1, and BKK1 can partially suppress the defective phenotypes of bri1-5. Overexpression of SERK5 and other members in LRR-RLK II subfamily can not suppress the bri1-5 phenotypes. C. Overexpression of kinase-inactive mutants, mSERK1 (K330E), mSERK2 (K333E), mBAK1 (K317E), and mBKK1 (K322E) dramatically enhances bri1-5 defective phenotypes. Overexpression of mSERK5 (K303E) and other kinase-inactive mutants in LRR-RLK II subfamily does not enhance the bri1-5 phenotypes. Representative 18-day-old plants were photographed. Previous studies already indicated in vivo physical interactions of BRI1 with SERK1, BAK1, and BKK1, respectively [10], [11], [36], [46]. In this study, we tested the in vivo interaction between BRI1 and SERK2 using transgenic plants overexpressing BRI1-FLAG and SERK2-GFP. Our result indicated that BRI1-FLAG can weakly interact with SERK2-GFP in vivo. Unlike the interactions of BRI1 with BAK1 and BKK1, the interaction between BRI1 and SERK2 cannot be significantly enhanced with exogenously applied BR (Figure S1), but the phosphorylation of SERK2 can be dramatically enhanced by the supplementation of BR (Figure S1). These results suggest that SERK2 may have fewer roles in BR signaling compared to the other three SERKs, but unnatural manipulation such as overexpression of SERK2 and exogenous application of BR can reveal its functions in BR signaling.
To further confirm the function of SERKs in BR signaling, kinase-inactive versions of all 14 LRR-RLK II members were generated by mutating a conserved lysine residue in the ATP binding site of each of these genes. These mutated genes were named mSERK1, mSERK2, mBAK1, mBKK1, etc. All 14 mutated genes were overexpressed in bri1-5. If mutated mSERKs still can interact with bri1-5, the resulting transgenic plants should show a dominant negative phenotype. Our results indicated that most of the transgenic plants obtained for all the four constructs of mSERKs showed phenotypes resembling that of a null bri1 mutant, such as bri1-4 [45] (Figure 1C). These data suggest that SERK1, SERK2, BAK1, and BKK1 are the only 4 LRR-RLK II genes that are involved in BR signaling.
serk1 bak1 bkk1 triple null mutant shows null bri1-like phenotypes in the dark
To better understand the genetic significance of SERKs in BR signaling, single, double, triple, and quadruple mutants for all four SERKs were generated. Two independent sets of T-DNA insertion lines for all the four SERKs were obtained from the Arabidopsis Biological Resource Center (ABRC) (Figure 2). RT-PCR reactions were performed to confirm that all the lines used do not express full-length wild-type mRNAs (Figure S2). Therefore, these lines are likely null mutants. A novel BRI1 T-DNA insertion line named bri1-701 was also obtained from ABRC. bri1-701 not only showed no full-length mRNA expression but also exhibited a phenotype identical to a typical null bri1 mutant. bri1-701 was therefore used as a null bri1 control throughout the entire studies. Double serk mutants were generated by crossing two different serk mutants. Because serk1 serk2, serk1 bak1, and bak1 bkk1 showed male sterility, reduced male fertility, and cell death phenotypes respectively, we had to be strategic about generating triple mutants, as discussed in materials and methods.
Fig. 2. Two independent sets of T-DNA insertion null mutants used in this study. 
Exons are indicated with filled black boxes. Lines between boxes represent introns. The insertion sites are shown with triangles and the T-DNA orientations are indicated with arrows. The T-DNA insertion mutants labeled with blue were the 1st set of single null mutants used to create double, triple and quadruple mutants. The ones labeled with green were the 2nd set of mutants used to generate double, triple, and quadruple mutants. We first phenotypically analyzed the first set of single mutants of the four SERKs, serk1-8, serk2-1, bak1-4, and bkk1-1 (Figure 2). Only bak1-4 showed subtle bri1-like phenotypes such as shortened petioles, reduced rosette size, and reduced height when grown in the light. When grown in the dark, bak1-4 showed slightly shortened hypocotyls compared to wild-type seedlings as reported previously (Figure 3; [10], [11]). None of the other SERK mutants showed any visible defective phenotypes. We then generated double mutants with the four single mutants. Among all 6 possible double mutants generated, only serk1-8 bak1-4 showed a weak bri1-like phenotype which is more severe than the bak1-4 single mutant, including more compact rosette leaves and more shortened hypocotyls (Figure 3). As a matter of fact, the hypocotyls of serk1-8 bak1-4 are only about half of the length of wild-type (Figure 3B, 3C), which is still significantly taller than the bri1-701 null mutant, suggesting that BR signaling is severely but not completely disrupted in serk1-8 bak1-4. We confirmed that serk1-8 serk2-1 shows a defective male gametogenesis phenotype; and bak1-4 bkk1-1 shows seedling lethality phenotypes about two weeks after germination as previously reported [34]–[36].
Fig. 3. Representative loss-of-function mutant phenotypes of SERKs. 
A. Representative loss-of-function phenotypes of 20-day-old SERK mutants in the light. Only bak1-4 shows a subtle bri1-like phenotype among the single knock-out mutants. The double knock-out mutant serk1-8 bak1-4 shows phenotypes similar to the bri1 weak allele, bri1-301; and bak1-4 bkk1-1 shows a seedling-lethality phenotype at the early developmental stage [36]. The triple knock-out mutant serk1-8 bak1-4 bkk1-1 shows phenotypes similar to the bak1-4 bkk1-1 mutant plants. B. Representative loss-of-function phenotypes of SERK mutants grown in the dark for 5 days. The mutant bri1-701, a T-DNA insertion mutant of BRI1, shows a typical null bri1-like phenotype in the dark with opened cotyledons, short and swollen hypocotyls. The double knock-out mutant serk1-8 bak1-4 shows partially de-etiolated phenotypes with opened cotyledons and semi-dwarfed hypocotyls. The triple knock-out mutant serk1-8 bak1-4 bkk1-1 shows a de-etiolated phenotype almost identical to that of the BRI1 null mutant, bri1-701. C. Measurements of the dark-grown seedlings shown in B. Error bars represent standard deviation (SD). Our priority in these genetic studies was to investigate whether knocking-out BAK1 and its functionally redundant genes in a single plant can completely abolish BR signaling pathway and recapitulate a bri1-like phenotype. Because bak1-4 bkk1-1 shows a seedling lethality phenotype due to failure of a light-dependent cell death control [36], we did not anticipate observing a light-grown null bri1-like phenotype for the triple or quadruple mutants. Therefore, the phenotypes of the triple and quadruple mutants were mainly analyzed within the dark-grown conditions. To our surprise, some of the serk1-8 serk2-1 bak1-4 triple mutant displayed an embryo-defective phenotype, and the serk1-8 serk2-1 bak1-4 bkk1-1 quadruple mutant are most likely embryo-lethal (data not shown). Our attention was drawn to one of the triple mutants, serk1-8 bak1-4 bkk1-1, because this mutant showed an extreme de-etiolated phenotype similar to that of bri1-701 including shortened hypocotyls and opened cotyledons (Figure 3B, 3C).
To further confirm that the triple mutant phenotypes result from the null mutations of the corresponding SERKs, we generated double, triple, and quadruple mutants using a different set of single null mutants, serk1-1, serk2-2, bak1-6, and bkk1-2 (Figure 2). All the defective phenotypes observed are similar to the mutants generated by the first set of null alleles (Figure S3). Most importantly, serk1-1 bak1-6 bkk1-2 also showed a severe bri1-like de-etiolated phenotype (Figure S3). The phenotypic resemblance suggests that the BR signaling pathway is abolished in the serk triple mutant similar to that in the bri1 null mutant.
The phenotypes of SERK mutants can be complemented by corresponding SERK genes
To verify that the observed phenotypes are caused by the loss-of-function of the SERK genes, we performed complementation experiments using native SERK promoters. Because the double, triple, and quadruple mutants generated from 2 sets of independent mutants look very similar, we performed the complementation and all other biochemical experiments using the mutants generated from the 1st set of single null mutant. We were never able to clone the BKK1 promoter into these constructs; therefore, the BAK1 promoter was used to drive the expression of BKK1. When native promoter-driven SERK1 and BAK1 were transformed into serk1-8 bak1-4, the double mutant phenotypes were largely rescued (Figure 4A). When BAK1 promoter driven BAK1 and BKK1 were transformed into serk1-8 bak1-4 bkk1-1 triple mutant background, the lethality phenotype of the triple mutant was also rescued (Figure 4A). When SERK1 promoter driven SERK1 was introduced into the serk1-8 bak1-4 bkk1-1 background, no rescued plants were ever obtained because of cell death resulted from knock-out of BAK1 and BKK1 (data not shown). The transgenic plants were also grown in the dark to examine whether they restored the de-etiolated phenotype of the mutant plants. Indeed, SERK1 and BAK1 restored the mutant phenotypes of serk1-8 bak1-4 in the dark. BAK1 and BKK1 completely rescued the mutant phenotypes of serk1-8 bak1-4 bkk1-1 in the dark. The complementation of SERK1 driven by its own promoter only partially restored the mutant phenotypes, producing semi-dwarf hypocotyls (Figure 4B, 4C). The reason why pS1::SERK1 only partially rescued the hypocotyl phenotype of serk1-8 bak1-4 bkk1-1 is not known. Results from two sets of independent null mutants and from the complementation experiments clearly indicated that the defective mutant phenotypes were caused by the null mutations in the SERKs.
Fig. 4. The phenotypes of SERK mutants can be complemented by each of the SERKs. 
A. Rescued mutant phenotypes of 21-day-old plants in the light. The pS1::SERK1-GFP construct restored the serk1-8 bak1-4 mutant phenotype to a typical bak1-4 like mutant phenotype, and the pS3::BAK1-GFP construct restored the serk1-8 bak1-4 mutant phenotype to a wild-type like plant. The pS3::BAK1-GFP and pS3::BKK1-GFP constructs restored the serk1-8 bak1-4 bkk1-1 mutant phenotype to almost a wild-type like plant. However, pS1::SERK1-GFP can not rescue the serk1-8 bak1-4 bkk1-1 phenotype in the light. B. Rescued mutant phenotypes of 5-day-old seedlings in the dark. The pS1::SERK1-GFP and pS3::BAK1-GFP constructs restored the serk1-8 bak1-4 mutant phenotypes. The pS1::SERK1-GFP construct partially restored the serk1-8 bak1-4 bkk1-1 mutant phenotypes to the bak1-4 bkk1-1 double mutant phenotypes with opened cotyledons and taller hypocotyls than the triple mutant. The pS3::BAK1-GFP and pS3::BKK1-GFP constructs restored the serk1-8 bak1-4 bkk1-1 mutant phenotype to a wild-type like seedling. C. Measurements of the dark-grown seedlings shown in B. Error bars represent SD. The serk1 bak1 bkk1 triple null mutant is insensitive to BR treatment
To examine whether the null bri1-like phenotype seen in the dark grown serk1 bak1 bkk1 triple null mutant was caused by the disruption of BR signaling, a classic root growth inhibition assay for BR sensitivity was performed [2]. If SERKs play an essential role in BR signaling, SERK mutants should show a reduced response to exogenously applied BR. Plants were grown for 7 days on half strength MS medium agar plates supplied with or without different concentrations of BR. Wild-type and bak1-4 are sensitive to the root growth inhibition of different concentrations of 24-epiBL ranging from 1 to 1000 nM, with bak1 showing slightly reduced sensitivity (Figure 5A). The root growth of serk1-8 bak1-4 was insensitive to 24-epiBL from 1 to 100 nM, but showed some sensitivity at 1000 nM. Analyses of hypocotyls also showed reduced sensitivity of serk1-8 bak1-4 to exogenously applied 24-epiBL (Figure 5B). The root and hypocotyl growth of bri1-701 and serk1-8 bak1-4 bkk1-1, however, was completely insensitive to 24-epiBL at concentrations from 1 to 1000 nM. These results indicate that the triple mutant plants are insensitive to exogenously applied BR (Figure 5A, 5B). To confirm that the de-etiolated phenotype seen in the triple mutant is caused by the disruption of the BR signaling pathway instead of the general photomorphogenesis pathways, we used two constitutive photomorphogenesis (COP1) mutants, cop1-4 and cop1-6, as controls to determine whether they also show insensitivity to exogenous BR treatment (Figure S4A, S4B). Our results indicated that although COP1 mutants exhibit a de-etiolated phenotype, they are sensitive to exogenous BR treatment.
Fig. 5. serk1-8 bak1-4 bkk1-1 triple null mutant is insensitive to exogenous BR treatment. 
A, B. Root and hypocotyl growth analyses of wild-type and mutant plant seedlings grown on medium containing different 24-epiBL concentrations. A. Seven-day-old seedlings grown in the light for root growth analysis. B. Five-day-old seedlings grown in the dark for hypocotyl growth analysis. The double mutant serk1-8 bak1-4 shows reduced sensitivity to BR treatment and the triple mutant serk1-8 bak1-4 bkk1-1 is completely insensitive to BR treatment. Error bars represent SD. C, D. Expression of CPD and DWF4 in serk1-8 bak1-4 bkk1-1 shows responses to exogenously applied BR similarly to the null bri1-701 mutant but differently to wild-type seedlings. Seven-day-old seedlings were treated with or without 1 µM 24-epiBL. Relative expression level of CPD and DWF4 was measured by quantitative RT-PCR. The feedback inhibition of CPD in serk1-8 bak1-4 is also reduced. ACTIN2 was used as the reference gene. The used primers are listed in Table S1. Error bars represent SD (n = 3). E, F. The phosphorylation level of BES1 is not responsive to BR treatment in the serk1-8 bak1-4 bkk1-1 triple null mutant. E. Nine-day-old seedlings of wild-type and mutants grown in the light were treated with 0 or 1 µM 24-epiBL for 4 h. Total proteins were analyzed by an immuo-blotting assay with a specific anti-BES1 antibody. F. Coomassie blue staining showing equally loaded proteins between each pair of samples. −, without 24-epiBL treatment; +, with 24-epiBL treatment. BES1-P, phosphorylated BES1; BES1, unphosphorylated BES1. In Col-0, the expression of CPD is down-regulated by exogenously applied BR via a negative feedback mechanism. When BR signaling is disrupted as in bri1 null mutants, however, the expression of CPD is not responsive to BR treatment [47]. To further confirm that the BR signaling is blocked in serk1 bak1 bkk1 triple mutant, quantitative RT-PCR experiments were performed to detect the expression levels of CPD and DWF4 in wild-type and mutant plants treated with or without BR (Figure 5C, 5D). Similar to previous reports, the expression level of CPD was decreased to about 20% of wild-type plants when treated with BR. In bri1-701 null mutant, the expression of CPD was not significantly down-regulated when treated with BR (Figure 5C). Consistent with its weak bri1-like phenotype, the expression level of CPD in bak1-4 was decreased dramatically similar to wild-type upon BR treatment. However, the double knock-out mutant serk1-8 bak1-4 showed drastically reduced sensitivity to BR treatment with slightly decreased CPD expression upon BR treatment. The expression level of CPD in the BR treated serk1-8 bak1-4 bkk1-1 triple null mutant was not decreased (Figure 5C). Similar to the CPD response, the expression level of DWF4 was down-regulated to about 20% in wild-type plants when BR was applied exogenously. However, BR treated serk1-8 bak1-4 bkk1-1 mutant showed dramatically reduced sensitivity to exogenous BR application similar to the bri1-701 mutant plant (Figure 5D). These data indicate that the BR signaling pathway in the triple mutant of SERKs is blocked to the same extent as bri1 null mutants.
Phosphorylation level of BES1 is not responsive to BR in the serk1 bak1 bkk1 triple null mutant
To biochemically test whether the serk1 bak1 bkk1 triple null mutant is insensitive to exogenously applied BR, the phosphorylation status of BES1 was investigated with a specific anti-BES1 antibody after the wild-type and triple mutant were treated with or without 1 µM 24-epiBL (Figure 5E, 5F; Figure S5) [48]. In Col-0 wild-type seedlings without BR treatment, two BES1 bands with almost equal signal intensity were observed, indicating that both phosphorylated and unphosphorylated BES1 were present. Upon BR treatment, unphosphorylated BES1 in wild-type was increased dramatically and phosphorylated BES1 disappeared, suggesting that the BR signaling pathway was activated. In the untreated seedlings of the weak allele of bri1-301, the amount of phosphorylated BES1 was slightly higher than that of unphosphorylated BES1. In the BR-treated bri1-301 seedlings, the amount of unphosphorylated BES1 became greater than phosphorylated BES1. However, the untreated null mutant bri1-701 showed higher levels of phosphorylated than unphosphorylated BES1, and the ratio between the two types of BES1 was not changed when treated with BR. All single SERK null mutants showed response to BR in a way similar to that of wild-type plants. Although serk1-8 bak1-4 showed an enhanced bri1-like phenotype compared to bak1-4 single mutant (Figure 3), BES1 phosphorylation levels in the double mutant still show a wild-type like response (Figure 5E). A previous report showed that BAK1 and BKK1 function redundantly in BR signaling [36], which is also supported by the result from this study that untreated bak1-4 bkk1-1 seedlings showed predominantly phosphorylated BES1 protein, suggesting that BR signaling is dramatically impaired in the double mutant. Upon BR treatment, both phosphorylated and unphosphorylated BES1 were detected although the phosphorylated BES1 was still dominant, indicating that the BR signaling pathway was not disrupted completely in bak1-4 bkk1-1. Interestingly, the serk1-8 bak1-4 bkk1-1 triple null mutant only showed the phosphorylated BES1 band with or without the exogenous BR application, suggesting that BR signal transduction was entirely blocked in the triple mutant. serk2-1 bak1-4 bkk1-1 exhibited a BES1 phosphorylation response similar to bak1-4 bkk1-1. When serk1-8 serk2-1 bak1-4 was treated with BR, the amount of unphosphorylated BES1 increased dramatically (Figure 5E). These results indicate that serk2 has an undetectable effect on BR signaling in Arabidopsis seedlings; and BR signaling appears to be entirely blocked in the serk1 bak1 bkk1 triple null mutant. Similar results were obtained from the second set of knockouts (Figure S5). Although BR insensitive mutants showed a de-etiolated phenotype, de-etiolated mutants do not always show BR insensitivity. For example, cop1-4 and cop1-6 showed wild-type like BES1 response to BR treatment (Figure S4C).
Overexpression of BRI1 cannot rescue the de-etiolated phenotype of the serk1 bak1 bkk1 triple mutant, and the phosphorylation level of BRI1 in the triple mutant is unresponsive to BR treatment
It was previously reported that overexpression of BRI1 can drastically increase hypocotyl growth of wild-type plants [7], [20]. To test whether BRI1 is still able to promote hypocotyl growth of the serk1 bak1 bkk1 triple null mutant, we overexpressed BRI1-GFP in wild-type and the serk1-8 bak1-4 bkk1-1 respectively. Our results indicated that overexpression of BRI1-GFP can increase hypocotyl growth of wild-type plants but has no effect on the triple null mutants (Figure 6A, 6B, 6C). To understand why BRI1 has lost its physiological roles in the triple mutant, we next analyzed whether the phosphorylation levels of BRI1 can respond to exogenously applied BR. In the wild-type plants, exogenous BR treatment could significantly increase the phosphorylation levels of BRI1 as reported (Figure 6D; [17], [20]); whereas in the serk1 bak1 bkk1 triple null mutant, phosphorylation of BRI1 remains at an extremely low level regardless of BR treatment (Figure 6D). These results suggest that BR signaling is blocked in the triple mutant most likely because BRI1 has lost its responsiveness to internal bioactive BRs.
Fig. 6. Overexpression of BRI1 dramatically increases hypocotyl growth of wild type but does not promote hypocotyl growth of serk1-8 bak1-4 bkk1-1. 
A. 35S::BRI1-GFP can not suppress the mutant phenotype of serk1-8 bak1-4 bkk1-1. Five-day-old dark grown seedlings were photographed. B. Measurements of the dark-grown seedlings shown in A. Error bars represent SD. C. Western hybridization indicates the expressed BRI-GFP fusion protein in transgenic plants. D. Phosphorylation of BRI1 does not respond to exogenously applied BR in the mutant serk1-8 bak1-4 bkk1-1. The membrane proteins were extracted from seven-day-old light-grown seedlings and immunoprecipitated with an anti-GFP antibody. The threonine phosphorylation levels of BRI1 were detected with a phosphoThr antibody by immuno-blotting analysis. The immunoprecipitated BRI1-GFP was immuno-blotted with an anti-GFP antibody to show equal loading. −BL, samples not treated with 24-epiBL; +BL, samples treated with 100 nM 24-epiBL for 90 min. Discussion
The function of BAK1 in mediating BR signaling was independently identified by using a yeast-two hybrid screen for BRI1 interacting proteins and activation tagging for genetic suppressors of an intermediate BRI1 mutant, bri1-5 [10], [11]. Since then, numerous data supported the biochemical roles of BAK1 in regulating early events of BR signal transduction [17], [20], [49]. The biological significance of BAK1 in BR signaling, however, has never been convincingly substantiated due to lack of loss-of-function genetic evidence. If BAK1 is a key component in BR signaling, a plant lacking BAK1 and all its functionally redundant genes should exhibit a phenotype identical to a bri1 null mutant. In addition, the mutant plant should show no response to BR treatment in a way similar to what has been revealed for null bri1 mutants. To resolve these questions, we set to identify all genes in the Arabidopsis genome which might play redundant roles with BAK1. We overexpressed all 14 genes from the LRR-RLK II subfamily, to which the 5 SERKs belong, in the bri1-5 background to evaluate their possible roles in BR signaling. Interestingly, only SERK1, SERK2, BAK1, and BKK1 can suppress the defective phenotypes of bri1-5 when overexpressed. None of the other 10 genes showed any bri1-5 suppression phenotypes (Figure 1A, 1B).
We also overexpressed kinase-inactive mutants of all these 14 genes in bri1-5. Consistent with the SERK overexpression results, only mSERK1, mSERK2, mBAK1, and mBKK1 give a dominant negative phenotype (Figure 1C). We therefore focused on generating single, double, tripe, and quadruple mutants of these four genes in order to estimate the genetic contribution of these four SERKs to BR signaling. Early studies indicated that bak1 bkk1 double nulls are lethal due to failure of a BR-unrelated light-dependent cell death control pathway [36], [37], therefore we did not expect to observe that a multiple null mutant plant would recapitulate the bri1 null mutant phenotype shown under light grown conditions, such as dark green, round and compact leaves, and extreme dwarfism [2]. Rather, we anticipated seeing that a multiple null mutant would express a de-etiolated phenotype resembling that of a bri1 null mutant if SERKs play a key role in regulating BR signal transduction.
From our current results, we conclude that BAK1 and its homologues play an indispensable role in initiating BR signaling (Figure 7). This conclusion is mainly supported by the following key observations described in this study. First, the serk1 bak1 bkk1 triple null mutant showed a characteristic de-etiolated phenotype similar to that of a null bri1 mutant under dark grown conditions (Figure 3B, Figure S3B). Second, the triple null mutant is insensitive to BR according to the root growth inhibition analysis and the hypocotyl growth analysis (Figure 5A, 5B), CPD, DWF4 feedback inhibition analysis (Figure 5C, 5D), and accumulation of unphosphorylated BES1 assay (Figure 5E, Figure S5). In the triple null mutant, no unphosphorylated forms of BES1 can be detected, indicating that the BR signaling pathway has been blocked, at least under a normal physiological setting; and the unphosphorylated levels of BES1 cannot be induced by exogenously applied BR, as shown in wild-type plants and other SERK single mutants (Figure 5E; Figure S5). Finally, overexpression of BRI1 cannot alter the growth of the triple null mutant most likely because the phosphorylation of BRI1 is unresponsive to the fluctuation of the amount of internal biologically active BR (Figure 6). Together, these results suggest that the biological function of BRI1 in regulating plant growth is entirely dependent upon the action of SERKs.
Fig. 7. A current model showing SERKs are indispensable to BR signaling. 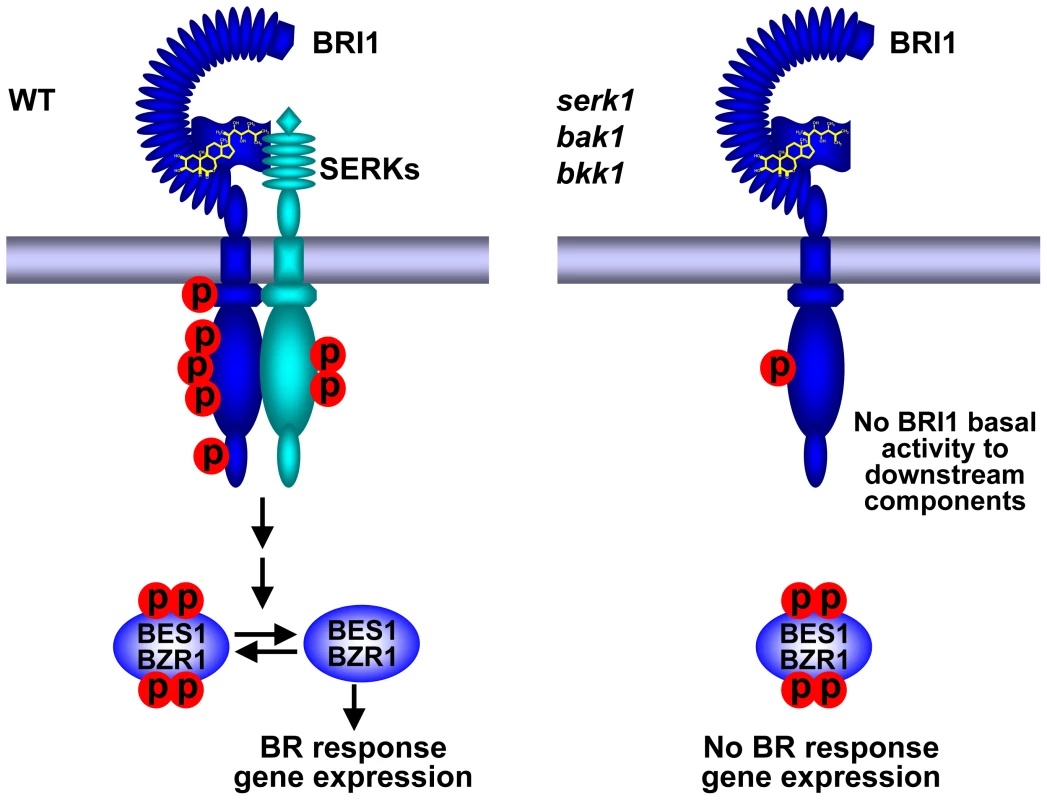
Left: In wild-type plants, upon perception of BR by the receptor BRI1 and coreceptor BAK1 or its functionally redundant proteins, the phosphorylation levels of BRI1 respond to BR, triggering downstream BR signaling cascade, resulting the accumulation of unphosphorylated BES1, and ultimately leading to the expression of BR responsive genes. Right: In serk1 bak1 bkk1 mutant plants, the phosphorylation levels of BRI1 remain at an almost undetectable basal level and do not alter regardless of elevated concentrations of BR. As a result, most of the BES1 protein exists as an inactive phosphorylated form, which cannot enter to the nuclei and is incapable to regulate the expression of BR responsive genes. SERK2 is apparently critical for the earlier development of embryos, as the development of the serk1 serk2 bak1 bkk1 quadruple null mutant is completely arrested at an early embryo stage (unpublished data). No viable quadruple mutant seeds were ever recovered from these genetic analyses. Therefore, analysis of BR response in quadruple mutant was impracticable. Evidently, SERK2 plays no significant role at an early postembryonic seedling developmental stage; and the interaction between SERK2 and BRI1 is extremely weak compared to that of other SERK members and BRI1 (Figure S1) [10], [36], [46]. Exogenous application of BR cannot increase BRI1/SERK2 interactions (Figure S1). Under an unnatural condition, SERK2 may show BAK1-like biochemical properties. For example, seedlings treated with exogenously applied BR can drastically induce SERK2 phosphorylation (Figure S1); and overexpression of SERK2 can partially suppress the defective phenotypes of bri1-5 (Figure 1B). Our genetic and biochemical results, however, clearly indicate that SERK1, BAK1, and BKK1 are the major players for BR signaling at the Arabidopsis seedling stage.
It is worth noting that phenotypes of single, double, triple, and quadruple mutants of SERK1, SERK2, BAK1, and BKK1 were discussed in a previous research article [50]. In that report, the authors claimed that serk3 serk4 double mutant showed an early senescence but not a lethality phenotype. It was also reported that the serk1 serk3 serk4 triple or serk1 serk2 serk3 serk4 quadruple mutants did not show any enhanced phenotype over the serk1 serk3 double mutant phenotype. The main cause for the discrepancy of our observations and the results from that report is that the mutant allele of BAK1/SERK3 used in their genetic analyses, serk3-1/bak1-3/SALK_ 034523, is actually a leaky mutant. bak1-3 contains a T-DNA insertion in the 4th intron of the BAK1 genomic DNA (Figure S6A). RT-PCR analysis and DNA sequence analysis indicated that bak1-3 can express a reduced amount of wild-type BAK1 mRNA in bak1-3 bkk1-1 background, although the transcription level of BAK1 in bak1-3 single mutant is not detectable (Figure S6B). Consistent with these observations, bak1-3 single mutant does exhibit a typical null bak1 mutant phenotype which is indistinguishable from bak1-4 (Figure S6C). bak1-3 bkk1-1 double mutant, however, shows a much reduced early senescence phenotype than bak1-4 bkk1-1 (Figure S6C). Therefore, bak1-3 is not a true null mutant of BAK1, especially in the situation when BKK1 is also knocked out (Figure S6B, [33]). In this study, we performed the genetic analysis by using two independent sets of confirmed transcriptional nulls (Figure S2B, S2C). Identical results were obtained from these two sets of mutant analyses (Figure 3A, 3B; Figure 5E; Figure S3A, S3B; Figure S5). In addition, the null bri1-like phenotype seen in the dark-grown serk1 bak1 bkk1 triple mutant can be complemented with BAK1 or BKK1 driven by the BAK1 promoter, but cannot be rescued by SERK1 with its native promoter (Figure 4A, 4B). The later result is consistent with our previous discovery that the bak1 bkk1 double null mutant is a seedling lethal mutant [36].
Our current observations suggest that SERKs play a critical role in the early events of BR signaling likely via a reciprocal and sequential phosphorylation model as proposed previously, with some modifications [20]. In wild-type plants, BR interacting with its receptor BRI1 in the extracellular domain triggers a conformational change within the cytoplasmic domain, leading to the basal activation of cytoplasmic BRI1 kinase, releasing of the BKI1 inhibitor, and inducing interaction between BRI1 and BAK1 or its homologues. The initially activated BRI1 evidently only can activate SERKs but not other downstream components such as BSKs. Activation of downstream components by BRI1 requires full activation of BRI1 by BAK1 and its functionally redundant homologues, most likely as a consequence of transphosphorylation. As a result, unphosphorylated forms of BES1 are accumulated which can directly regulate BR responsive gene expression (Figure 7). In a serk1 bak1 bkk1 triple null mutant plant, on the other hand, BR signaling apparently is entirely blocked from BRI1 to BES1 due to lack of the involvement of BAK1 and its functionally redundant proteins (Figure 7). As a consequence, BES1 accumulates as the phosphorylated form, which is incapable of mediating the expression of BR-responsive genes. Therefore the triple null mutant plant exhibits a phenotype similar to a bri1 null mutant. Both are the outcome of disrupted BR signaling. Although unphosphorylated BES1 level in both the bri1 and serk1 bak1 bkk1 triple null mutants showed almost an undetectable response to exogenously applied BR, the bril null mutant showed both phosphorylated and unphosphorylated BES1 (Figure 5E; Figure S5), suggesting a BR signal leakage in the bri1 null mutant. This could be contributed by BRL1 and BRL3, both of which play partially redundant roles with BRI1 [9], [51]. In a previous report, it was hypothesized that without SERKs, BRI1 has basal kinase activity towards downstream components [20]. The hypothesis was based upon several observations from the bak1 bkk1 double null mutant including that the de-etiolation of the bak1 bkk1 is not as severe as the bri1 null mutant; overexpression of BRI1 can significantly increase hypocotyl growth of the bak1 bkk1 double null mutant; and the phosphorylation levels of BRI1 can still respond to exogenous BR treatment. From this study, we now propose that without SERKs, BRI1 does not appear to have basal activities towards downstream components because the serk1 bak1 bkk1 triple null shows a bri1 null mutant phenotype; overexpression of BRI1 cannot increase the hypocotyl growth of the triple mutant; and in the triple null mutant the phosphorylation of BRI1 is completely unresponsive to BR treatment, hinting that BRI1 also cannot respond to internal BR levels. The BR signaling appears to be entirely blocked due to a blocked full BRI1 activation event by SERKs. In the future, analysis will be conducted to determine why phosphorylation of BRI1 cannot respond to BR in the absence of SERKs. The ultimate understanding of the roles of SERKs in BRI1-mediated signaling may rely on a combination of genetics and structural biology.
Materials and Methods
Plant materials, growth conditions, and crossing experiments
Arabidopsis accessions WS2 and Columbia-0 (Col-0) were grown at 22°C in a long-day condition (16 h of light and 8 h of dark) in a greenhouse except those for phenotypic analysis in the dark which were grown in half strength MS agar plates with 1% (w/v) sucrose. To create knock-out mutants used in this study, T-DNA insertion lines were obtained from The Arabidopsis Biological Resource center (ABRC) for serk1-1 (SALK_044330), serk1-8 (SALK_071511), serk2-1 (SALK_058020), serk2-2 (SAIL_119_G03), bak1-3 (SALK_ 034523), bak1-4 (SALK_116202), bak1-6 (SAIL_513_A11), bkk1-1 (SALK_057955), bkk1-2 (SALK_105409), serk5-1 (SALK_089460), and bri1-701 (SALK_003371).
Reverse transcription-polymerase chain reactions (RT-PCR) were performed to examine the expression of BRI1 and SERKs in the used mutants. To amplify the full-length CDS and the flanking mRNA sequence of the T-DNA insertion site of BRI1 and SERKs, primer pairs BRI1FL-F/BRI1FL-R, BRI1M-F/BRI1M-R, SERK1F-F/SERK1F-R, SERK1M-F/SERK1M-R, SERK2F-F/SERK2F-R, SERK2M-F/SERK2M-R, BAK1F/BAK1R, SERK3M-F/SERK3M-R, SERK4F-F/SERK4F-R, and SERK4M-F/SERK4M-R were used, respectively. ACTIN2 was amplified as a control by primers RT-actin2F and RT-actin2R. All the used primers are listed in Table S1.
The T-DNA mutant plants were genotyped by PCR. Homozygous single knock-out lines were used to generate double knock-out mutants. Double knock-out mutants of serk1 serk2, and bak1 bkk1 were obtained by segregating from corresponding mutant plants with homozygous insertion for one gene and heterozygous for the second gene. Triple knock-out mutants were obtained by crossing fertile pairs serk1+/− serk2 and serk2 bak1, serk1+/− serk2 and serk2 bkk1, serk1 bak1 and bak1+/− bkk1, serk2 bak1 and bak1+/− bkk1. The triple knock-out mutants were segregated from self-pollinated mutant plants serk1+/− serk2 bak1, serk1+/− serk2 bkk1, serk1 bak1+/− bkk1 and serk2 bak1+/− bkk1 which contain heterozygous insertion for one gene and homozygous insertions for two other genes.
DNA cloning and plant transformation
Gateway technology was employed to clone all the coding sequences of SERK cDNA sequences for overexpression in bri1-5 and complementation experiments in mutants as previously described [44] using the primers listed in Table S1. The amplified CDS sequences of SERK genes were introduced into the destination vector pB35GWF with the help of Gateway technology. Site-directed mutagenesis was carried out according to the manual of the QuickChange Site-directed Mutagenesis Kit (Stratagene, La Jolla, CA). Entry clones of cloned SERK genes were used as templates for chain extension with primers listed in Table S1. The mutated entry clones of SERK genes were used for in vitro DNA recombination with the destination vector pB35GWF to create expression constructs for Arabidopsis transformation.
To generate constructs for native promoter-driven expression of SERKs, promoter sequences of SERKs were PCR amplified from Arabidopsis genomic DNA and inserted into the pBIB-BASTA-GWR-GFP vector [44] before the gateway cassette by using the primers listed in Table S1. The resulting constructs were named as pSERK1-GWR-GFP, pSERK2-GWR-GFP and pSERK3-GWR-GFP, respectively. Then the cloned coding sequences of SERK genes were transferred into these destination vectors by in vitro DNA recombination to create expression constructs of pS1::SERK1-GFP, pS2::SERK2-GFP, pS3::SERK3-GFP and pS3::SERK4-GFP, respectively.
All the cloned sequences were confirmed by sequencing analysis and the expression constructs were transferred into appropriate Arabidopsis plants by the floral dip method [52].
Root and hypocotyl growth analyses
Seeds were surface sterilized and placed on half strength MS plates with 0.8% (w/v) agar, 1% (w/v) sucrose and different concentrations of 24-epiBL (Sigma, St. Louis, MO). The plates were cold treated at 4°C for 2 days to ensure uniform germination. Seeds were considered to begin germination after the plates were kept at 22°C for 24 hr. The root length was measured seven days after germination in the light and the hypocotyl length was measured five days after germination in the dark.
Membrane protein isolation, coimmunoprecipitation, and Western analysis
Seven-day-old seedlings of transgenic plants with 35S::BRI1-GFP and 11-day-old liquid-cultured seedlings of transgenic plants harboring 35S::SERK2-GFP and 35S::BRI1-FLAG were treated with or without 24-epiBL for 90 min, respectively, and ground to fine powder in liquid N2 [17]. Membrane protein isolation was performed as previously described [10]. BRI1-FLAG was immunoprecipitated from solubilized membrane protein with agarose-linked α-FLAG antibody (Sigma, St. Louis, MO). SERK2-GFP and BRI1-GFP were immunoprecipitated with α-GFP antibody (Invitrogen, Carlsbad, CA) and protein G beads (Roche, Indianapolis, IN). The immunoprecipitated proteins were separated on 7.5% SDS polyacrylamide gel for Western analyses with α-GFP, α-FLAG, α-phosphothreonine antibodies as previously described [10]. Arabidopsis seedlings grown on half strength MS plates were harvested and treated with or without 1 µM 24-epiBL for 4 hr and the total protein was extracted and separated on 12% SDS polyacrylamide gel to detect the phosphorylation status of BES1 with an anti-BES1 antibody. Horseradish peroxidase-linked anti-rabbit or anti-mouse antibodies were used as secondary antibodies and the signal was detected by Western Lightning Chemiluminescence Reagent Plus (Perkin-Elmer, Waltham, MA).
Supporting Information
Zdroje
1. ClouseSDSasseJM 1998 BRASSINOSTEROIDS: Essential regulators of plant growth and development. Annu Rev Plant Physiol Plant Mol Biol 49 427 451
2. ClouseSDLangfordMMcMorrisTC 1996 A brassinosteroid-insensitive mutant in Arabidopsis thaliana exhibits multiple defects in growth and development. Plant Physiol 111 671 678
3. LiJChoryJ 1997 A putative leucine-rich repeat receptor kinase involved in brassinosteroid signal transduction. Cell 90 929 938
4. KauschmannAJessopAKonczCSzekeresMWillmitzerL 1996 Genetic evidence for an essential role of brassinosteroids in plant development. Plant J 9 701 713
5. SzekeresMNemethKKoncz-KalmanZMathurJKauschmannA 1996 Brassinosteroids rescue the deficiency of CYP90, a cytochrome P450, controlling cell elongation and de-etiolation in Arabidopsis. Cell 85 171 182
6. ThummelCSChoryJ 2002 Steroid signaling in plants and insects–common themes, different pathways. Genes Dev 16 3113 3129
7. WangZYSetoHFujiokaSYoshidaSChoryJ 2001 BRI1 is a critical component of a plasma-membrane receptor for plant steroids. Nature 410 380 383
8. KinoshitaTCano-DelgadoASetoHHiranumaSFujiokaS 2005 Binding of brassinosteroids to the extracellular domain of plant receptor kinase BRI1. Nature 433 167 171
9. Cano-DelgadoAYinYYuCVafeadosDMora-GarciaS 2004 BRL1 and BRL3 are novel brassinosteroid receptors that function in vascular differentiation in Arabidopsis. Development 131 5341 5351
10. LiJWenJLeaseKADokeJTTaxFE 2002 BAK1, an Arabidopsis LRR receptor-like protein kinase, interacts with BRI1 and modulates brassinosteroid signaling. Cell 110 213 222
11. NamKHLiJ 2002 BRI1/BAK1, a receptor kinase pair mediating brassinosteroid signaling. Cell 110 203 212
12. WangXChoryJ 2006 Brassinosteroids regulate dissociation of BKI1, a negative regulator of BRI1 signaling, from the plasma membrane. Science 313 1118 1122
13. WangXLiXMeisenhelderJHunterTYoshidaS 2005 Autoregulation and homodimerization are involved in the activation of the plant steroid receptor BRI1. Dev Cell 8 855 865
14. HothornMBelkhadirYDreuxMDabiTNoelJP 2011 Structural basis of steroid hormone perception by the receptor kinase BRI1. Nature 474 467 471
15. SheJHanZKimTWWangJChengW 2011 Structural insight into brassinosteroid perception by BRI1. Nature 474 472 476
16. KimTWWangZY 2010 Brassinosteroid signal transduction from receptor kinases to transcription factors. Annu Rev Plant Biol 61 681 704
17. WangXGosheMBSoderblomEJPhinneyBSKucharJA 2005 Identification and functional analysis of in vivo phosphorylation sites of the Arabidopsis BRASSINOSTEROID-INSENSITIVE1 receptor kinase. Plant Cell 17 1685 1703
18. JaillaisYHothornMBelkhadirYDabiTNimchukZL 2011 Tyrosine phosphorylation controls brassinosteroid receptor activation by triggering membrane release of its kinase inhibitor. Genes Dev 25 232 237
19. LiJ 2011 Direct involvement of leucine-rich repeats in assembling ligand-triggered receptor-coreceptor complexes. Proc Natl Acad Sci U S A 108 8073 8074
20. WangXKotaUHeKBlackburnKLiJ 2008 Sequential transphosphorylation of the BRI1/BAK1 receptor kinase complex impacts early events in brassinosteroid signaling. Dev Cell 15 220 235
21. TangWKimTWOses-PrietoJASunYDengZ 2008 BSKs mediate signal transduction from the receptor kinase BRI1 in Arabidopsis. Science 321 557 560
22. KimTWGuanSSunYDengZTangW 2009 Brassinosteroid signal transduction from cell-surface receptor kinases to nuclear transcription factors. Nat Cell Biol 11 1254 1260
23. SunYFanXYCaoDMTangWHeK 2010 Integration of brassinosteroid signal transduction with the transcription network for plant growth regulation in Arabidopsis. Dev Cell 19 765 777
24. LuoXMLinWHZhuSZhuJYSunY 2010 Integration of light - and brassinosteroid-signaling pathways by a GATA transcription factor in Arabidopsis. Dev Cell 19 872 883
25. HeJXGendronJMSunYGampalaSSGendronN 2005 BZR1 is a transcriptional repressor with dual roles in brassinosteroid homeostasis and growth responses. Science 307 1634 1638
26. WangZYNakanoTGendronJHeJChenM 2002 Nuclear-localized BZR1 mediates brassinosteroid-induced growth and feedback suppression of brassinosteroid biosynthesis. Dev Cell 2 505 513
27. YinYVafeadosDTaoYYoshidaSAsamiT 2005 A new class of transcription factors mediates brassinosteroid-regulated gene expression in Arabidopsis. Cell 120 249 259
28. YuXLiLZolaJAluruMYeH 2011 A brassinosteroid transcriptional network revealed by genome-wide identification of BESI target genes in Arabidopsis thaliana. Plant J 65 634 646
29. BaiMYZhangLYGampalaSSZhuSWSongWY 2007 Functions of OsBZR1 and 14-3-3 proteins in brassinosteroid signaling in rice. Proc Natl Acad Sci U S A 104 13839 13844
30. GampalaSSKimTWHeJXTangWDengZ 2007 An essential role for 14-3-3 proteins in brassinosteroid signal transduction in Arabidopsis. Dev Cell 13 177 189
31. RyuHChoHKimKHwangI 2010 Phosphorylation dependent nucleocytoplasmic shuttling of BES1 is a key regulatory event in brassinosteroid signaling. Mol Cells 29 283 290
32. RyuHKimKChoHParkJChoeS 2007 Nucleocytoplasmic shuttling of BZR1 mediated by phosphorylation is essential in Arabidopsis brassinosteroid signaling. Plant Cell 19 2749 2762
33. LiJ 2010 Multi-tasking of somatic embryogenesis receptor-like protein kinases. Curr Opin Plant Biol 13 509 514
34. AlbrechtCRussinovaEHechtVBaaijensEde VriesS 2005 The Arabidopsis thaliana SOMATIC EMBRYOGENESIS RECEPTOR-LIKE KINASES1 and 2 control male sporogenesis. Plant Cell 17 3337 3349
35. ColcombetJBoisson-DernierARos-PalauRVeraCESchroederJI 2005 Arabidopsis SOMATIC EMBRYOGENESIS RECEPTOR KINASES1 and 2 are essential for tapetum development and microspore maturation. Plant Cell 17 3350 3361
36. HeKGouXYuanTLinHAsamiT 2007 BAK1 and BKK1 regulate brassinosteroid-dependent growth and brassinosteroid-independent cell-death pathways. Curr Biol 17 1109 1115
37. HeKGouXPowellRAYangHYuanT 2008 Receptor-like protein kinases, BAK1 and BKK1, regulate a light-dependent cell-death control pathway. Plant Signal Behav 3 813 815
38. KemmerlingBSchwedtARodriguezPMazzottaSFrankM 2007 The BRI1-Associated Kinase 1, BAK1, has a brassinolide-independent role in plant cell-death control. Curr Biol 17 1116 1122
39. ChinchillaDZipfelCRobatzekSKemmerlingBNurnbergerT 2007 A flagellin-induced complex of the receptor FLS2 and BAK1 initiates plant defence. Nature 448 497 500
40. HeeseAHannDRGimenez-IbanezSJonesAMHeK 2007 The receptor-like kinase SERK3/BAK1 is a central regulator of innate immunity in plants. Proc Natl Acad Sci U S A 104 12217 12222
41. BollerTFelixG 2009 A renaissance of elicitors: perception of microbe-associated molecular patterns and danger signals by pattern-recognition receptors. Annu Rev Plant Biol 60 379 406
42. RouxMSchwessingerBAlbrechtCChinchillaDJonesA 2011 The Arabidopsis leucine-rich repeat receptor-like kinases BAK1/SERK3 and BKK1/SERK4 are required for innate immunity to hemibiotrophic and biotrophic pathogens. Plant Cell 23 2440 2455
43. ShiuSHBleeckerAB 2001 Receptor-like kinases from Arabidopsis form a monophyletic gene family related to animal receptor kinases. Proc Natl Acad Sci U S A 98 10763 10768
44. GouXHeKYangHYuanTLinH 2010 Genome-wide cloning and sequence analysis of leucine-rich repeat receptor-like protein kinase genes in Arabidopsis thaliana. BMC Genomics 11 19
45. NoguchiTFujiokaSChoeSTakatsutoSYoshidaS 1999 Brassinosteroid-insensitive dwarf mutants of Arabidopsis accumulate brassinosteroids. Plant Physiol 121 743 752
46. KarlovaRBoerenSRussinovaEAkerJVervoortJ 2006 The Arabidopsis SOMATIC EMBRYOGENESIS RECEPTOR-LIKE KINASE1 protein complex includes BRASSINOSTEROID-INSENSITIVE1. Plant Cell 18 626 638
47. LiJNamKHVafeadosDChoryJ 2001 BIN2, a new brassinosteroid-insensitive locus in Arabidopsis. Plant Physiol 127 14 22
48. YinYWangZYMora-GarciaSLiJYoshidaS 2002 BES1 accumulates in the nucleus in response to brassinosteroids to regulate gene expression and promote stem elongation. Cell 109 181 191
49. RussinovaEBorstJWKwaaitaalMCano-DelgadoAYinY 2004 Heterodimerization and endocytosis of Arabidopsis brassinosteroid receptors BRI1 and AtSERK3 (BAK1). Plant Cell 16 3216 3229
50. AlbrechtCRussinovaEKemmerlingBKwaaitaalMde VriesSC 2008 Arabidopsis SOMATIC EMBRYOGENESIS RECEPTOR KINASE proteins serve brassinosteroid-dependent and -independent signaling pathways. Plant Physiol 148 611 619
51. ZhouAWangHWalkerJCLiJ 2004 BRL1, a leucine-rich repeat receptor-like protein kinase, is functionally redundant with BRI1 in regulating Arabidopsis brassinosteroid signaling. Plant J 40 399 409
52. CloughSJBentAF 1998 Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J 16 735 743
Štítky
Genetika Reprodukční medicína
Článek USF-1 Is Critical for Maintaining Genome Integrity in Response to UV-Induced DNA PhotolesionsČlánek Checkpoints in a Yeast Differentiation Pathway Coordinate Signaling during Hyperosmotic StressČlánek Two-Component Elements Mediate Interactions between Cytokinin and Salicylic Acid in Plant ImmunityČlánek Adaptive Evolution of the Lactose Utilization Network in Experimentally Evolved Populations of
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2012 Číslo 1- Růst a vývoj dětí narozených pomocí IVF
- Délka menstruačního cyklu jako marker ženské plodnosti
- Vztah užívání alkoholu a mužské fertility
- Šanci na úspěšný průběh těhotenství snižují nevhodné hladiny progesteronu vznikající při umělém oplodnění
- Vliv melatoninu a cirkadiálního rytmu na ženskou reprodukci
-
Všechny články tohoto čísla
- DNA Methylation and Gene Expression Changes in Monozygotic Twins Discordant for Psoriasis: Identification of Epigenetically Dysregulated Genes
- An siRNA Screen in Pancreatic Beta Cells Reveals a Role for in Insulin Production
- Parallel Mapping and Simultaneous Sequencing Reveals Deletions in and Associated with Discrete Inherited Disorders in a Domestic Dog Breed
- Cytoplasmic Polyadenylation Element Binding Protein Deficiency Stimulates PTEN and Stat3 mRNA Translation and Induces Hepatic Insulin Resistance
- Nucleolar Association and Transcriptional Inhibition through 5S rDNA in Mammals
- USF-1 Is Critical for Maintaining Genome Integrity in Response to UV-Induced DNA Photolesions
- Heterochromatin Formation Promotes Longevity and Represses Ribosomal RNA Synthesis
- Genetic Evidence for an Indispensable Role of Somatic Embryogenesis Receptor Kinases in Brassinosteroid Signaling
- Poly(ADP-Ribose) Polymerase 1 (PARP-1) Regulates Ribosomal Biogenesis in Nucleoli
- Genome Engineering in : A Feasible Approach to Address Biological Issues
- RIC-7 Promotes Neuropeptide Secretion
- Adaptation and Preadaptation of to Bile
- Checkpoints in a Yeast Differentiation Pathway Coordinate Signaling during Hyperosmotic Stress
- Progressive Polycomb Assembly on H3K27me3 Compartments Generates Polycomb Bodies with Developmentally Regulated Motion
- A High Density SNP Array for the Domestic Horse and Extant Perissodactyla: Utility for Association Mapping, Genetic Diversity, and Phylogeny Studies
- Two-Component Elements Mediate Interactions between Cytokinin and Salicylic Acid in Plant Immunity
- Cdc5-Dependent Asymmetric Localization of Bfa1 Fine-Tunes Timely Mitotic Exit
- A Genome-Wide Analysis of Promoter-Mediated Phenotypic Noise in
- Contribution of Intragenic DNA Methylation in Mouse Gametic DNA Methylomes to Establish Oocyte-Specific Heritable Marks
- Adaptive Evolution of the Lactose Utilization Network in Experimentally Evolved Populations of
- Microenvironmental Regulation by Fibrillin-1
- Unraveling the Regulatory Mechanisms Underlying Tissue-Dependent Genetic Variation of Gene Expression
- A Half-Century of Inspiration: An Interview with Hamilton Smith
- Reduced Lentivirus Susceptibility in Sheep with Mutations
- High-Density SNP Mapping of the HLA Region Identifies Multiple Independent Susceptibility Loci Associated with Selective IgA Deficiency
- Calpains Mediate Integrin Attachment Complex Maintenance of Adult Muscle in
- Genomic Ancestry of North Africans Supports Back-to-Africa Migrations
- Functional Specialization of the Plant miR396 Regulatory Network through Distinct MicroRNA–Target Interactions
- The Seminal Fluid Protease “Seminase” Regulates Proteolytic and Post-Mating Reproductive Processes
- Insulin Signaling Regulates Fatty Acid Catabolism at the Level of CoA Activation
- A Genome-Wide Association Study Identified as a Susceptibility Locus for Systemic Lupus Eyrthematosus in Japanese
- A Spontaneous Mutation of the Rat Gene Leads to Impaired Function of Regulatory T Cells Linked to Inflammatory Bowel Disease
- The Yeast Complex I Equivalent NADH Dehydrogenase Rescues Mutants
- A Flexible Bayesian Model for Studying Gene–Environment Interaction
- Sex Pheromone Evolution Is Associated with Differential Regulation of the Same Desaturase Gene in Two Genera of Leafroller Moths
- Genome-Wide Assessment of AU-Rich Elements by the ARE Algorithm
- Inference of Population Structure using Dense Haplotype Data
- A Gene Regulatory Network for Root Epidermis Cell Differentiation in Arabidopsis
- Sequencing of Pooled DNA Samples (Pool-Seq) Uncovers Complex Dynamics of Transposable Element Insertions in
- A Genome-Wide Association Scan on the Levels of Markers of Inflammation in Sardinians Reveals Associations That Underpin Its Complex Regulation
- Tempo and Mode in Evolution of Transcriptional Regulation
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Poly(ADP-Ribose) Polymerase 1 (PARP-1) Regulates Ribosomal Biogenesis in Nucleoli
- Microenvironmental Regulation by Fibrillin-1
- Parallel Mapping and Simultaneous Sequencing Reveals Deletions in and Associated with Discrete Inherited Disorders in a Domestic Dog Breed
- Two-Component Elements Mediate Interactions between Cytokinin and Salicylic Acid in Plant Immunity
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



