-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
FliO Regulation of FliP in the Formation of the Flagellum
The type III secretion system of the Salmonella flagellum consists of 6 integral membrane proteins: FlhA, FlhB, FliO, FliP, FliQ, and FliR. However, in some other type III secretion systems, a homologue of FliO is apparently absent, suggesting it has a specialized role. Deleting the fliO gene from the chromosome of a motile strain of Salmonella resulted in a drastic decrease of motility. Incubation of the ΔfliO mutant strain in motility agar, gave rise to pseudorevertants containing extragenic bypass mutations in FliP at positions R143H or F190L. Using membrane topology prediction programs, and alkaline phosphatase or GFPuv chimeric protein fusions into the FliO protein, we demonstrated that FliO is bitopic with its N-terminus in the periplasm and C-terminus in the cytoplasm. Truncation analysis of FliO demonstrated that overexpression of FliO43–125 or FliO1–95 was able to rescue motility of the ΔfliO mutant. Further, residue leucine 91 in the cytoplasmic domain was identified to be important for function. Based on secondary structure prediction, the cytoplasmic domain, FliO43–125, should contain beta-structure and alpha-helices. FliO43–125-Ala was purified and studied using circular dichroism spectroscopy; however, this domain was disordered, and its structure was a mixture of beta-sheet and random coil. Coexpression of full-length FliO with FliP increased expression levels of FliP, but coexpression with the cytoplasmic domain of FliO did not enhance FliP expression levels. Overexpression of the cytoplasmic domain of FliO further rescued motility of strains deleted for the fliO gene expressing bypass mutations in FliP. These results suggest FliO maintains FliP stability through transmembrane domain interaction. The results also demonstrate that the cytoplasmic domain of FliO has functionality, and it presumably becomes structured while interacting with its binding partners.
Published in the journal: . PLoS Genet 6(9): e32767. doi:10.1371/journal.pgen.1001143
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1001143Summary
The type III secretion system of the Salmonella flagellum consists of 6 integral membrane proteins: FlhA, FlhB, FliO, FliP, FliQ, and FliR. However, in some other type III secretion systems, a homologue of FliO is apparently absent, suggesting it has a specialized role. Deleting the fliO gene from the chromosome of a motile strain of Salmonella resulted in a drastic decrease of motility. Incubation of the ΔfliO mutant strain in motility agar, gave rise to pseudorevertants containing extragenic bypass mutations in FliP at positions R143H or F190L. Using membrane topology prediction programs, and alkaline phosphatase or GFPuv chimeric protein fusions into the FliO protein, we demonstrated that FliO is bitopic with its N-terminus in the periplasm and C-terminus in the cytoplasm. Truncation analysis of FliO demonstrated that overexpression of FliO43–125 or FliO1–95 was able to rescue motility of the ΔfliO mutant. Further, residue leucine 91 in the cytoplasmic domain was identified to be important for function. Based on secondary structure prediction, the cytoplasmic domain, FliO43–125, should contain beta-structure and alpha-helices. FliO43–125-Ala was purified and studied using circular dichroism spectroscopy; however, this domain was disordered, and its structure was a mixture of beta-sheet and random coil. Coexpression of full-length FliO with FliP increased expression levels of FliP, but coexpression with the cytoplasmic domain of FliO did not enhance FliP expression levels. Overexpression of the cytoplasmic domain of FliO further rescued motility of strains deleted for the fliO gene expressing bypass mutations in FliP. These results suggest FliO maintains FliP stability through transmembrane domain interaction. The results also demonstrate that the cytoplasmic domain of FliO has functionality, and it presumably becomes structured while interacting with its binding partners.
Introduction
For many bacteria, locomotion is possible using the flagellum, which functions like a helical propeller. It is a highly complex nanomachine consisting of about 30 different proteins, and it is organized into three substructures: the basal body, the hook, and the filament. Export of the components of the flagellum across the cytoplasmic membrane requires a specialized secretion apparatus at its base, which shares homology to the type III secretion apparatus of the bacterial needle used by some Gram-negative bacteria in pathogenesis [1]. For the flagella systems of Escherichia coli and Salmonella enterica serovar Typhimurium, the secretion apparatus is postulated to consist of six integral membrane proteins: FlhA, FlhB, FliO, FliP, FliQ, and FliR; and three cytoplasmic proteins: FliH, FliI and FliJ [2], [3]. Biochemical and genetic studies have determined the location of the membrane proteins FlhA, FlhB, FliP, and FliR to be within the flagellar basal body [4]–[6]. It has been demonstrated that the hook-capping protein (FlgD) and hook protein (FlgE) required all of the proteins of the secretion apparatus for their export [7].
A detailed picture of the workings of the secretion apparatus is gradually being elucidated [8], [9]. It has been demonstrated that the secretion apparatus harnesses the proton motive force to drive export of the external flagellar components [10], [11]. FlhA and FlhB, the two largest membrane proteins of the flagellar secretion apparatus, which both have predominant C-terminal cytoplasmic domains have been most characterized. The crystal structures of the cytoplasmic domains of FlhA from Salmonella and Helicobacter pylori, and the crystal structure of the cytoplasmic domain of the type III secretion system apparatus paralogue InvA from Salmonella have recently been solved [12]–[14]. Also, several crystal structures of the cytoplasmic domains of paralogues of FlhB found in virulence-associated needles from enteric bacteria have recently been described [15]–[17].
The cytoplasmic domains of FlhA and FlhB form a docking platform for FliH, FliI, and FliJ. The cytoplasmic domain of FlhA has been shown to bind the FliH, FliI, and FliJ proteins, and it is also thought to be directly involved in the translocation of the export substrates into the central channel of the growing flagellar structure [18]–[22]. The cytoplasmic domain of FlhB has been shown to undergo autocleavage associated with interaction with FliK, which switches specificity of export of rod/hook-like substrates to filament-type substrates [23]–[29]. The N-terminal transmembrane region of FlhA has been implicated to interact with the surrounding MS ring, from studies involving the isolation of extragenic suppressor mutations [5].
Less is known about the functional role of the FliO, FliP, FliQ, and FliR proteins in the secretion complex, though the protein products of the corresponding genes have been determined [30]–[33]. FliP, FliQ, and FliR are very hydrophobic and were predicted to be predominantly located within the cytoplasmic membrane. However, FliO was predicted to be a bitopic membrane protein with a predominant soluble domain. Intriguingly, FliO shows the least conservation among the secretion system apparatus membrane proteins, even being absent from some systems [3], [34], [35]. Notably, the type III secretion system apparatus of the virulence-associated needles of Salmonella serovar Typhimurium lack a FliO homologue, as does the flagellum of the ancient hyper-thermophilic bacterium Aquifex aeolicus [3], [35]. Since the FliO protein is not necessary for some systems it suggests that the FliO protein might function as an accessory protein and have a specialized role where it is present. We have undertaken a mutational analysis of FliO described herein, to determine whether or not it is essential for flagellar assembly, which parts of the protein are important, its orientation in the membrane, and possible interaction partners to help understand its function.
Results
Incubation of a ΔfliO mutant in motility agar gave rise to pseudorevertants containing bypass mutations in fliP
We first sought to characterize the FliO protein through mutagenesis to investigate its function. To provide a genetic background for complementation studies, a ΔfliO mutant was engineered from the wild-type strain SJW1103. We found that the ΔfliO mutant, CB186, was not completely non-motile, and displayed a weakly motile phenotype after several hours in motility agar. Surprisingly, pseudorevertants arose from this strain with enhanced motility after about 42 hours extended incubation (Figure 1A). In comparison strain CB184, which encodes a non-polar Δ(fliO-fliP) deletion was completely non-motile (Figure 1B). Four motile pseudorevertants from CB186 were purified, and the two most motile were characterized further. These two strains were designated CB191 and CB227.
Fig. 1. Isolation of pseudorevertants containing bypass mutations in fliP from a poorly motile ΔfliO mutant. 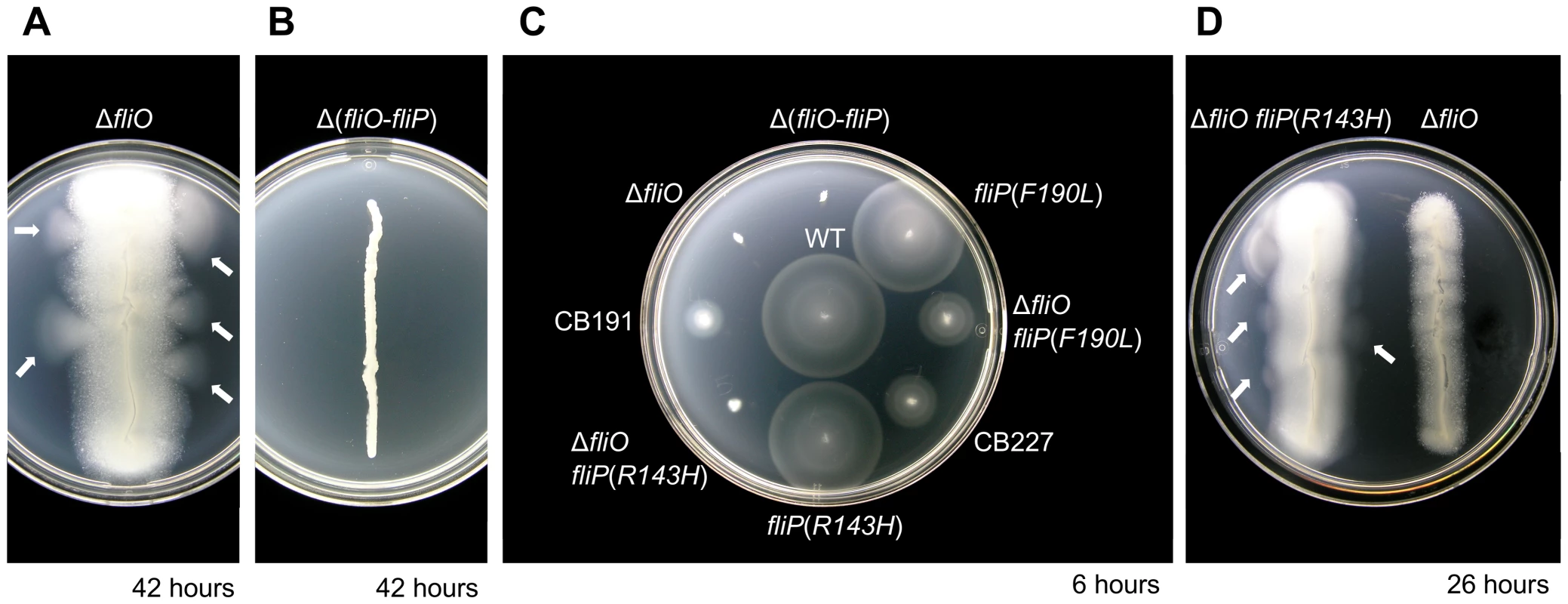
(A) Incubation of a ΔfliO strain, CB186, in soft-tryptone motility agar after 42 hours. The strain is extremely poorly motile, but outgrowths of motile pseudorevertants can be observed as motility halos, indicated by white arrows. (B) Incubation of a non-motile Δ(fliO-fliP) strain, CB184, in motility agar after 42 hours. (C) Comparison of motility of pseudorevertants, purified from CB186, to engineered strains after 6 hours in motility agar: WT, wild-type is strain SJW1103; CB191 is a pseudorevertant strain and encodes ΔfliO fliP(R143H); CB227 is a pseudorevertant strain and encodes ΔfliO fliP(F190L). Other strains were engineered mutants constructed from SJW1103 by λ-Red genetic engineering. (D) Formation of motile pseudorevertants by the ΔfliO fliP(R143H ) engineered mutant after only 26 hours. All plates were incubated at 30°C. To screen for the bypass mutations the genomic DNA of CB191 and CB227 was isolated and the flhA, flhB, fliO, fliP, fliQ, and fliR genes were sequenced along with the fliF gene encoding the MS ring, which surrounds the secretion apparatus in the membrane. These genes were considered the most likely to contain suppressor mutations. Each pseudorevertant contained a point mutation in the fliP gene only. CB191 encoded an R143H mutation in FliP, and CB227 encoded an F190L mutation in FliP.
To investigate the physiological effects of the extragenic bypass mutations in fliP, engineered strains were constructed from SJW1103 using λ-Red genetic engineering (Figure 1C). Engineered mutants encoding fliP(R143H) or fliP(F190L) mutations only showed the same motility phenotype as SJW1103. Moreover, an engineered mutant encoding ΔfliO fliP(F190L) was equally as motile as CB227, which confirmed that the fliP(F190L) mutation was responsible for the extragenic suppression of the fliO deletion in strain CB227. However, an engineered strain encoding ΔfliO fliP(R143H) was not as motile as CB191. This appeared to suggest that the fliP(R143H) mutation was not the suppressor mutation responsible for the improved motility in pseudorevertant strain CB191. However, the engineered ΔfliO fliP(R143H) mutant gave rise to pseudorevertants with enhanced motility after a much shorter incubation time of 26 hours in motility agar in comparison to a ΔfliO mutant, which took at least 42 hours (Figure 1D). Therefore, in strain CB191 an additional bypass mutation must be encoded along with fliP(R143H) to overcome the ΔfliO deletion.
The FliO N-terminus is in the periplasm and its C-terminus is in the cytoplasm
The above results suggested that FliO is regulating FliP so we decided to characterize the FliO protein further to determine how this might occur. Prior to this study the membrane topology of FliO was unknown, so we started by determining the topology of FliO. We used various prediction programs for the determination of the topology of transmembrane proteins, and the majority suggested that the 125-residue FliO protein is bitopic with a short N-terminal periplasmic domain from residues 1 to 16–22, a transmembrane region between residues 17–23 to 39–43, and a large C-terminal cytoplasmic domain from residues 40–44 to 125 (Table S1).
To experimentally determine the membrane topology of FliO we constructed plasmids to express chimeric gene fusions of the phoA gene encoding the mature form of alkaline phosphatase, at three specific points into the fliO gene. The mature form of alkaline phosphatase is only active when found in the periplasm, and its use is well established to determine membrane topology of transmembrane proteins [36]–[38]. The entire amino acid sequence of FliO was present in fusion product; and alkaline phosphatase was encoded between residues 6 and 7, 100 and 101, or 115 and 116 of FliO (Figure 2A). These fusion sites were permissive for motility (data not shown). Immunoblotting using anti-alkaline phosphatase antibody against whole cell lysates of cells expressing the fusions, and the lysates fractioned into insoluble membrane pellet fractions and soluble supernatant fractions demonstrated that the three FliO/PhoA chimeras were expressed stably and mainly partitioned with the insoluble membrane pellet fraction (Figure 2B). To detect expression of alkaline phosphatase, cells expressing the plasmid encoded fusions were inoculated onto L-agar plates containing 40 µg ml−1 5-Bromo-4-chloro-3-indoyl phosphate (Figure 2C). The phoN gene was deleted from the chromosome of the strains used in this experiment, to reduce background phosphatase expression. Alkaline phosphatase activity was detectable only with the FliO1–6::PhoA22–471::FliO7–125 fusion. These fusions were next genetically engineered onto the chromosome of Salmonella. Alkaline phosphatase assays were performed, and higher phosphatase activity was measured for the FliO1–6::PhoA22–471::FliO7–125 fusion, compared to the other chimeras, whether it was expressed from the chromosome only or also expressed from plasmid pTrc99A-FF4 (Table S2). This revealed that the N-terminus of FliO is in the periplasm.
Fig. 2. The FliO N-terminus is in the periplasm and its C-terminus is in the cytoplasm. 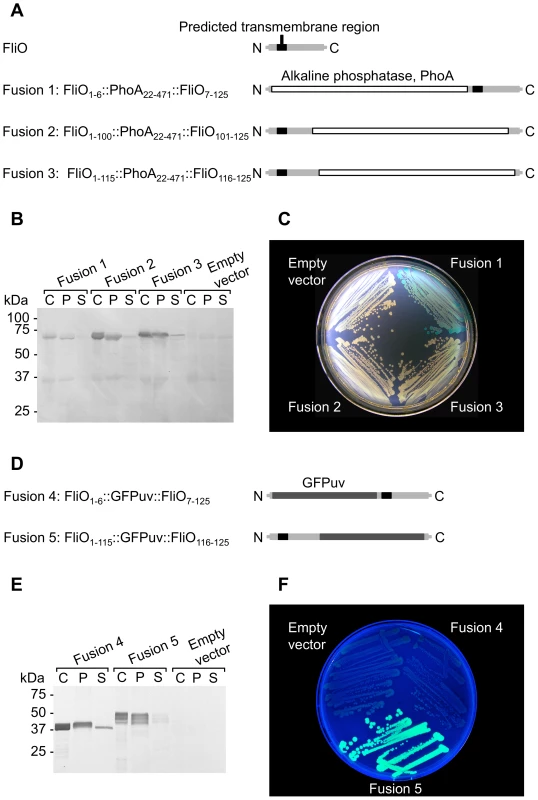
(A) Schematic overview of chimeric fusions of alkaline phosphatase within FliO. (B) Immunoblotting using anti-alkaline phosphatase antibody against cell fractions of strains expressing FliO/alkaline phosphatase chimeras (60.3 kDa): C, whole cells; P, insoluble membrane pellet fraction; S, soluble supernatant fraction. (C) Growth of strains expressing FliO/alkaline phosphatase chimeras on L-agar plates containing 40 µg ml−1 5-Bromo-4-chloro-3-indoyl phosphate at 37°C. All strains contained a ΔphoN301 deletion. (D) Schematic overview of chimeric fusions of GFPuv within FliO. (E) Immunoblotting using anti-GFP antibody against cell fractions of strains expressing FliO/GFPuv chimeras (39.9 kDa). (F) Growth of strains expressing FliO/GFPuv chimeras on L-agar plates at 30°C. All fusions were expressed from plasmid pTrc99A-FF4 without IPTG induction. GFPuv has been reported to be useful as a reporter for membrane protein topology in bacteria and it is fluorescent when it is located in the cytoplasm [39]. We constructed plasmids to encode chimeric gene fusions of the GFPuv gene between residues 6 and 7, or 115 and 116 of FliO (Figure 2D). These fusions were permissive for motility (data not shown). Immunoblotting using anti-GFP antibody against whole cell lysates of cells expressing the fusions, and the lysates fractioned into insoluble membrane pellet fractions and soluble supernatant fractions demonstrated that the two FliO/GFPuv chimeras were expressed stably and mainly partitioned with the insoluble membrane pellet fraction. However, a difference in migration between the two fusions is apparent, and the FliO1–115::GFPuv::FliO116–125 fusion produced multiple bands (Figure 2E). The reason for this is not clear. In previous studies, multiple bands of FliO were detected by immunoblotting [32], [33]. The authors could not conclusively identify the source of the multiple bands, but speculated that the N-terminus of FliO underwent proteolytic cleavage, which did not appear to be physiologically important. We presume that the chimeric fusions we have constructed are somehow affecting this N-terminal modification of FliO. However, fluorescence was detectable only with the FliO1–115::GFPuv::FliO116–125 fusion (Figure 2F). This revealed that the C-terminus of FliO is in the cytoplasm.
Mutational analysis of FliO reveals the cytoplasmic domain of FliO is functionally important
Having defined the membrane topology of FliO, we next sought to determine the functionally important regions of the FliO protein by examining the ability of FliO proteins truncated from the N-terminus or the C-terminus to rescue the motility of a ΔfliO strain in motility agar. The truncated proteins were expressed from plasmid pTrc99A-FF4 without IPTG induction (Figure 3A). Immunoblotting using polyclonal anti-FliO43–125-6xHis antibodies was performed first to confirm expression of the proteins. Full-length FliO1–125, FliO22–125, FliO43–125, FliO1–95, FliO1–105, and FliO1–115 were detected by immunoblotting (Figure 3B). FliO1–125, FliO1–95, FliO1–105, and FliO1–115 produced multiple bands of FliO, while FliO22–125 and FliO43–125 did not. As mentioned in the previous section, it was suggested in a previous study that FliO is subject to N-terminal cleavage or modification by an unknown mechanism and the multiple bands presumably reflect this [33]. It was not possible to detect FliO at physiological levels from whole-cell lysates of SJW1103, therefore, the proteins produced from pTrc99A-FF4 were detectable by immunoblotting, because they were being over-expressed from this vector. It was not possible to detect FliO1–65, FliO1–75, and FliO1–85 by immunoblotting, which could be due to the specificity of the antibody or because these proteins were not expressed. A FLAG-tag (N-MDYKDDDDK-C) was engineered at the N-terminus of FliO1–125, FliO1–65, FliO1–75, and FliO1–85 to attempt to detect protein expression by immunoblotting using ANTI-FLAG antibody. However, only FLAG-tagged FliO1–125 was detectable by immunoblotting, so if FLAG-tagged FliO1–65, FliO1–75 and FliO1–85 were produced expression was much lower (data not shown).
Fig. 3. Complementation of a ΔfliO strain by FliO truncated at the N-terminus or C-terminus. 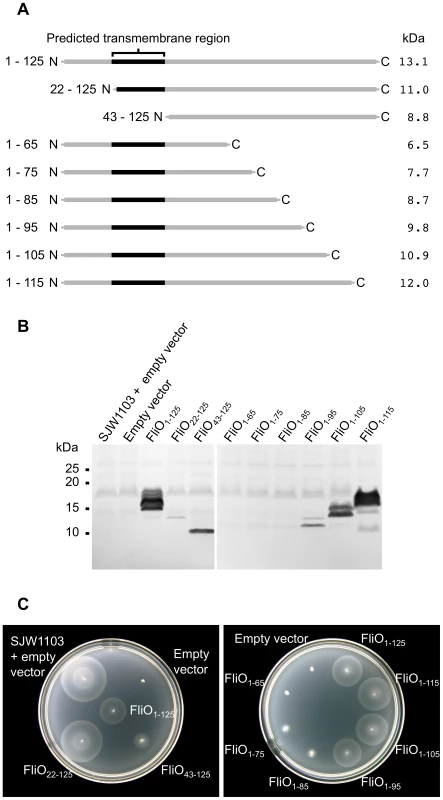
(A) Schematic overview of FliO deletion mutants with the corresponding molecular weight of the protein. (B) Immunoblotting using polyclonal anti-FliO43-125-6xHis antibody against 10–20% gradient SDS-PAGE separated whole cell lysates of the wild-type strain, SJW1103, containing empty pTrc99A-FF4 vector, or a ΔfliO mutant (strain CB186) containing empty vector, or strain CB186 expressing the truncated FliO proteins from plasmid pTrc99A-FF4 (without IPTG induction). (C) Complementation of strain CB186 by the truncated FliO proteins expressed from plasmid pTrc99A-FF4. SJW1103, containing empty pTrc99A-FF4 vector, was included for comparison. Soft-tryptone motility agar inoculated with the strains was incubated for 6 hours at 30°C. Expression of FliO22–125 or FliO43–125, which are truncated at the N-terminus, was able to complement the ΔfliO strain CB186 (Figure 3C). FliO22–125 encoded a methionine at position 22 in place of a natural valine and this truncation was included since it has been shown to be functional in previous studies [31], . Surprisingly, FliO43–125 could complement, as this corresponds to the cytoplasmic domain of FliO, and is without the transmembrane domain. Overexpression was necessary for the cytoplasmic domain to rescue motility of CB186, since FliO43–125 was not able to complement when expressed from the T7 promoter of plasmid pET-22b(+), without IPTG induction, while full-length FliO1–125 was able to rescue motility when expressed from this vector (data not shown). FliO1–95, FliO1–105, and FliO1–115, which are truncated from the C-terminus were able to restore almost full motility to CB186, so the C-terminal 30 amino acids of FliO are not essential. In comparison, FliO1–75 and FliO1–85 could only weakly restore motility to CB186, while FliO1–65 could not (Figure 3C).
Residue leucine 91 of the cytoplasmic domain of FliO is important for function
N-terminal and C-terminal truncation analysis of FliO have defined residues 22–95 as the most important. Moreover, overexpression of the cytoplasmic domain of FliO could rescue motility of a ΔfliO mutant, suggesting that this domain is functionally important. We next undertook a site-directed mutagenesis study of the cytoplasmic domain to identify important residues to confirm it is functionally important. We aligned the FliO proteins from closely related Gammaproteobacteria, to identify conserved residues. There was considerable sequence variation, but several conserved amino acids between residues 43–95 of the cytoplasmic domain of FliO could be identified (Figure S1). To screen for the important residues, we performed site-directed mutagenesis for full-length FliO creating point substitutions for alanine or point deletions at the following positions: G64, R68, V74, G82, T84, L91, and L94. Plasmids encoding these mutations were then examined for their ability to rescue motility of the ΔfliO mutant, CB186, in soft-tryptone motility agar. FliO(Δ91) was identified to cause a reduction in motility (data not shown). Immunoblotting using polyclonal anti-FliO43–125-6xHis antibodies demonstrated that FliO(L91A) and FliO(Δ91) could be expressed stably (Figure 4A). Strains containing the fliO(L91A) or fliO(ΔL91) mutant alleles on the chromosome were engineered, and both strains were dramatically less motile (Figure 4B). Residue leucine 91 of the cytoplasmic domain is therefore very important for the function of FliO.
Fig. 4. Residue leucine 91 is important for FliO function. 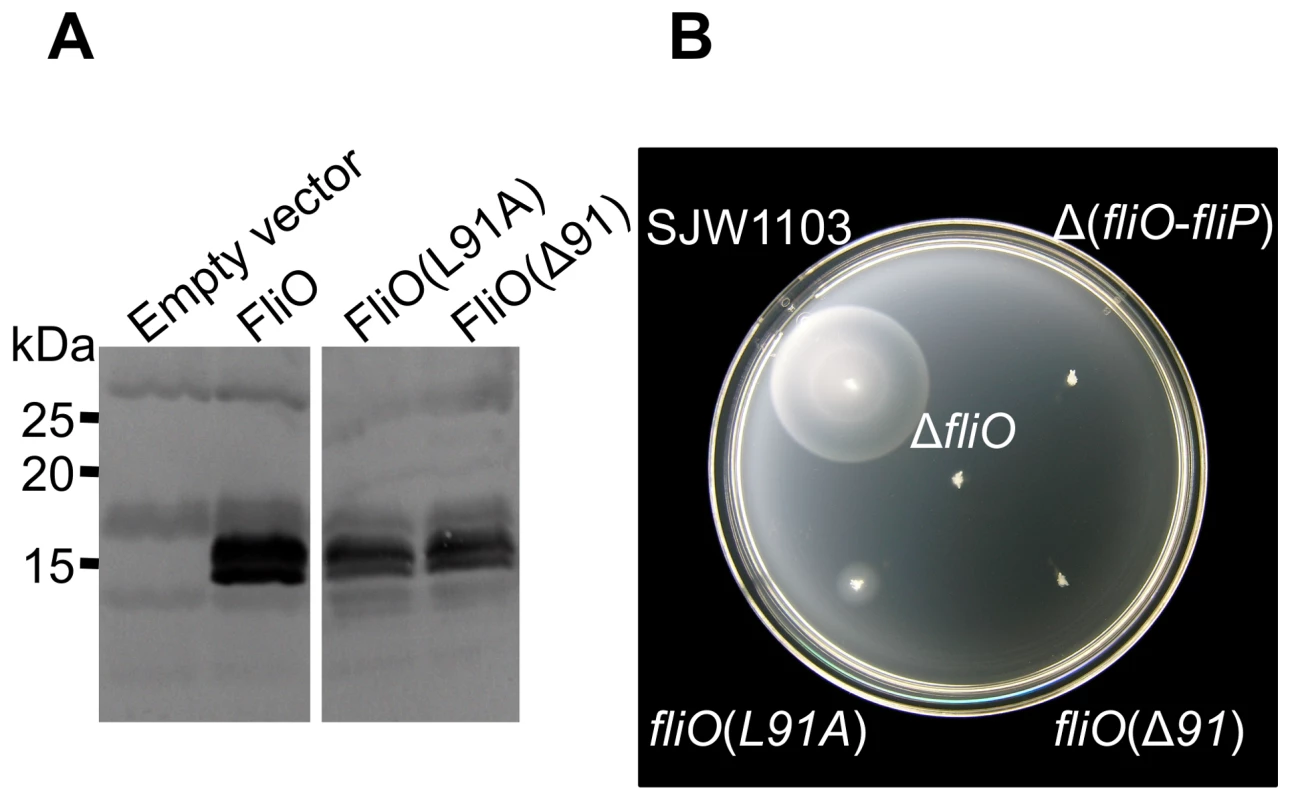
(A) Immunoblotting using polyclonal anti-FliO43–125-6xHis antibody against 10–20% gradient SDS-PAGE separated whole cell lysates of a ΔfliO mutant (strain CB186) containing empty vector, or strain CB186 expressing FliO or mutated FliO proteins (approximately 13 kDa) from plasmid pTrc99A-FF4 without IPTG induction. (B) Incubation of the wild-type strain SJW1103 or genetically engineered mutants in soft-tryptone motility agar for 6 hours at 30°C. Circular dichroism (CD) spectroscopy analysis of the cytoplasmic domain of FliO
We have revealed that the cytoplasmic domain of FliO is functionally important, so we next studied this domain by circular dichroism (CD) spectroscopy to see whether it is structured. Based on secondary structure prediction the cytoplasmic domain of FliO should contain four beta-strands and one alpha-helix (Figure 5A). FliO43–125-Ala was purified using a C-terminal intein fusion tag. An additional alanine residue at the C-terminus was added to facilitate removal of the intein-tag. After cleavage of the intein fusion tag and purification FliO43–125-Ala was studied using CD spectroscopy. From the shape of the spectra, the structure of FliO43–125-Ala apparently consists mostly of random coil and some β-structure (Figure 5B). Melting of FliO43–125-Ala was irreversible due to aggregation, this often happens with β-structural proteins [40]. Then FliO43–125-Ala was titrated with urea and no two-state transition was observed. From these data we conclude that this peptide is disordered and its structure is a mixture of random coil and beta-sheet.
Fig. 5. Circular Dichroism (CD) Spectroscopy analysis of the cytoplasmic domain of FliO. 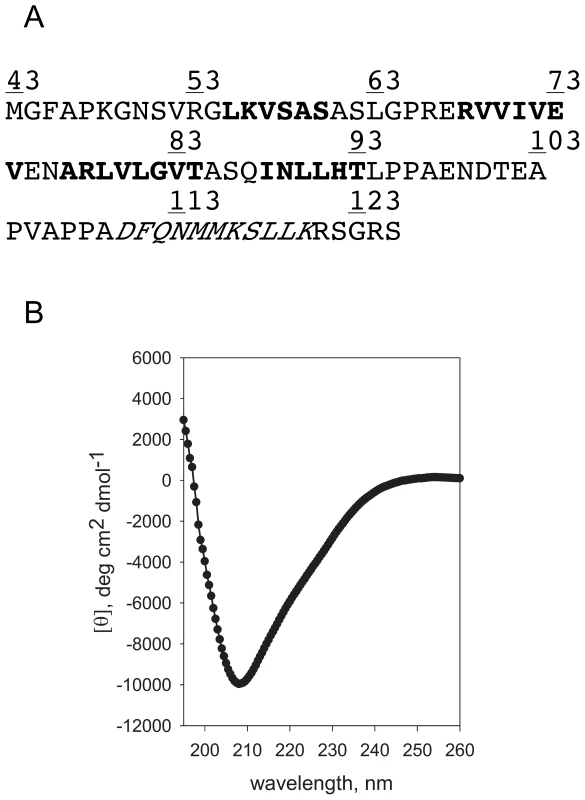
(A) Predicted regions of secondary structure of FliO43–125: Bold, regions with predicted β-structure; Italic, regions with predicted α-helix. (B) CD spectrum (mean residue ellipticity vs wavelength) of FliO43–125-Ala in 20 mM Na/K phosphate, pH 6.2; 100 mM NaCl. Full-length FliO increases FliP expression
We have shown that bypass mutations in fliP can rescue motility for cells deleted for the fliO gene. We have also shown through mutagenesis that the most important residues of FliO are between amino acids 22 to 95, and the cytoplasmic domain alone has functionality. To further characterize the apparent regulation of FliP by FliO, we investigated the effect of FliO co-expression on the synthesis of plasmid expressed FLAG-tagged FliP in the Δ(fliO-fliP) strain CB184. A FLAG-tag epitope (N-DYKDDDDK-C) was encoded between codons 22 and 23 of fliP. The FLAG-tag was added to enable detection of FliP expression by immunoblotting with ANTI-FLAG antibody. After cleavage of the N-terminal signal peptide of FliP, which occurs between residues 21 and 22, the FLAG-tag would be encoded immediately after the glutamine residue. FLAG-tagged FliP was expressed alone, or co-expressed with full-length FliO, or co-expressed with the cytoplasmic domain of FliO. FLAG-tagged FliP expression levels were not improved if the R143H or F190L bypass mutations were encoded (data not shown). Expression of full-length FliO or the cytoplasmic domain FliO43–125, was detected from the co-expression vectors by immunoblotting with polyclonal anti-FliO43–125-6xHis antibody (Figure 6A). Expression of FLAG-tagged FliP was dramatically improved when full-length FliO was co-expressed. However, expression levels of FLAG-tagged FliP were not improved when the cytoplasmic domain of FliO was co-expressed (Figure 6B).
Fig. 6. FliO increases expression of FliP. 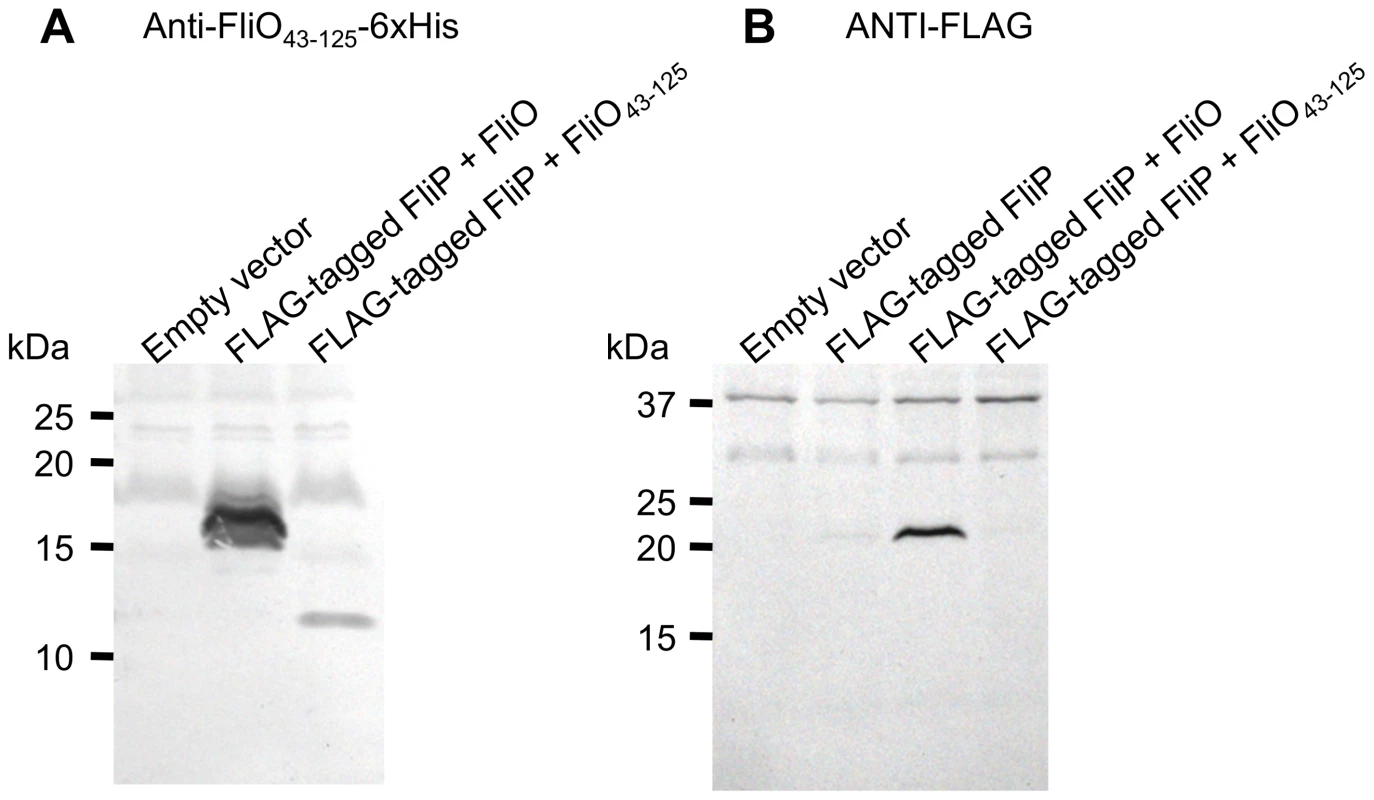
FLAG-tagged FliP (25.4 kDa) was expressed alone, or co-expressed with FliO (13.1 kDa), or co-expressed with the cytoplasmic domain of FliO, FliO43–125 (8.8 kDa), from plasmid pTrc99A-FF4 without IPTG induction. Whole cell lysates were separated by 10–20% gradient SDS-PAGE, prior to immunoblotting. (A) Immunoblotting with polyclonal anti-FliO43–125-6xHis antibody. (B) Immunoblotting with ANTI-FLAG antibody. Results are representative of the experiment performed in triplicate. Overexpression of the cytoplasmic domain of FliO in strains deleted for fliO and expressing bypass mutations in fliP further rescues motility
We have revealed that full-length FliO can stabilize FliP expression, while the cytoplasmic domain of FliO cannot. We have also shown that overexpression of the cytoplasmic domain of FliO, FliO43–125, can rescue motility of cells with the fliO gene deleted. We have further shown that extragenic bypass mutations within the fliP gene can also partially restore motility to the ΔfliO mutant. We next demonstrated that expression of FliO43–125 from pTrc99A-FF4 could further rescue motility to near wild-type levels for engineered strains encoding the ΔfliO fliP(R143H) or ΔfliO fliP(F190L) mutations (Figure 7).
Fig. 7. Rescue of motility by overexpression of the cytoplasmic domain of FliO. 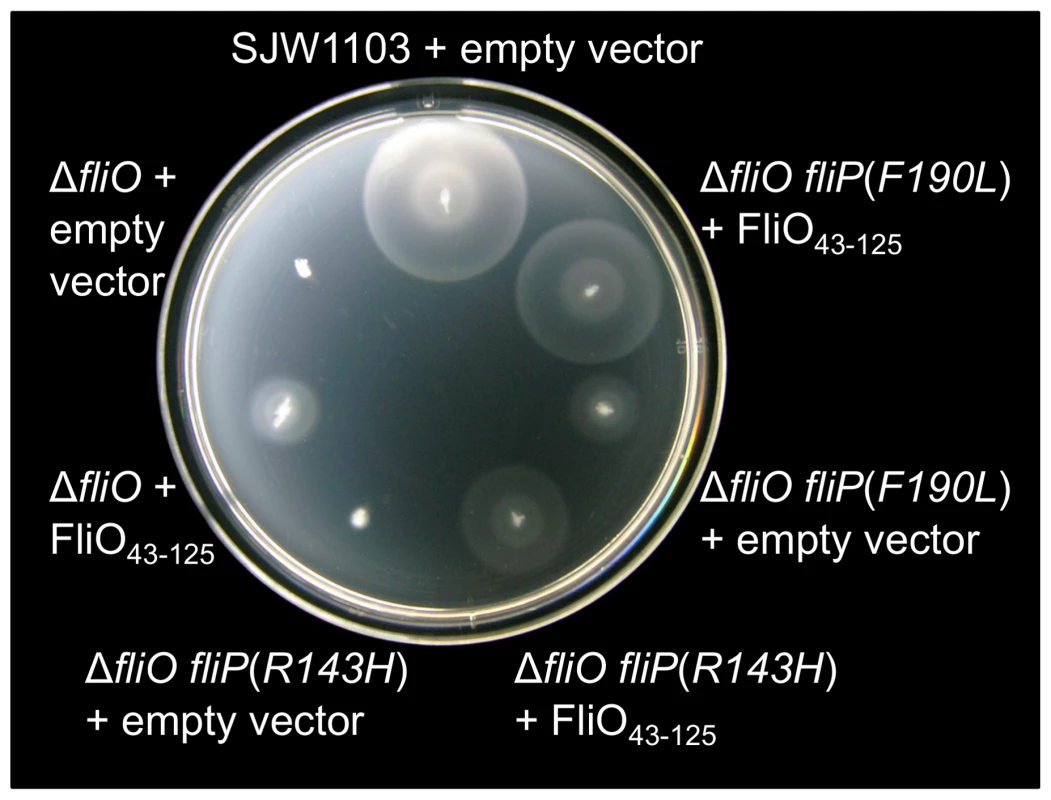
SJW1103 is a wild-type strain. Strains genetically engineered from SJW1103 contained a fliO gene deletion with or without bypass mutations in the gene for fliP. The cytoplasmic domain of FliO, FliO43–125, was expressed from plasmid pTrc99A-FF4 without IPTG induction. Soft-tryptone motility agar inoculated with the strains was incubated for 6 hours at 30°C. Discussion
In this study, we have undertaken a detailed molecular analysis of the FliO secretion apparatus protein and its role in flagella assembly. FliO was previously considered to be necessary for secretion in Salmonella [7]. However, we have demonstrated using an engineered non-polar ΔfliO mutant that while deletion of the fliO gene leads to a dramatic reduction in motility, cells are not completely non-motile. Moreover, it was possible to readily isolate pseudorevertants containing bypass mutations in fliP, which help to rescue motility. The FliO protein is not conserved in all type III secretion systems, yet in this study we have shown that it has an important functional role in regulating FliP stability, which is a highly conserved member of the secretion system membrane proteins.
We have also shown that FliO has a predominant C-terminal cytoplasmic domain, which is in contrast to the previously predicted membrane topology of FliO, which suggested that the C-terminus is in the periplasm [32]. Coexpression of the cytoplasmic domain of FliO with FliP did not increase FliP expression levels, while coexpression of full-length FliO with FliP improved FliP expression levels. This suggests that the transmembrane region of FliO stabilizes FliP, since the periplasmic domain of FliO appears to be non-essential from our truncation analyses. However, we have shown that the cytoplasmic domain represents a functional unit beyond merely being required for FliO membrane insertion, since overexpression of the cytoplasmic domain only of FliO could improve motility of cells encoding a fliO gene deletion, with or without additional bypass mutations in fliP.
In Buchnera sp. APS a gene fusion of fliO and fliP exists [41]. This suggests along with the results presented here that FliO and FliP probably also interact in Salmonella. This is important because FliP has been demonstrated to be located within the flagellar basal body [4]. FliP is predicted to contain 4 transmembrane loops with the 2nd and 3rd loops connected by a large (approximately 80-residue) periplasmic domain (Figure S2). The nature of the bypass mutations described here in the primary sequence of FliP, appear to be remarkably subtle. The bypass mutation F190L is located in predicted transmembrane loop 3, and was physiologically relevant by itself at improving motility in a fliO deletion background. However, the F190L mutation did not improve levels of FliP suggesting that the mutation is a gain-of-function mutation that overcomes the supporting role of FliO through a different mechanism. The FliP(R143H) mutation alone was not sufficient to greatly improve motility, rather it somehow increases the spectrum of bypass mutations permissible for rescuing motility of a fliO deletion mutant. Since arginine 143 is located in the periplasm, mutations might occur in other proteins, which interact with this domain. We are presently mapping the additional bypass mutation in strain CB191, which exists together with the fliP(R143H) bypass mutation. FliP(R143H) and FliP(F190L) do not correspond to residues found at the same position for the A. aeolicus FliP orthologue or the Salmonella serovar Typhimurium FliP needle paralogues, SsaR and SpaP, which are all found in type III secretion systems without a FliO homologue (Figure S2). So these FliP homologues might consist of amino acid residues that increase their stability, or they are part of secretion systems, which do not require the supporting role(s) that FliO plays in the Salmonella system.
The most highly conserved part of the FliO protein is between residues 22 to 95. This is consistent with the results of the truncation analysis, which showed that the periplasmic domain and C-terminal 30 amino acid residues are non-essential. However, mutating residue leucine 91 of the cytoplasmic domain severely disrupted FliO function. Furthermore, overexpression of the cytoplasmic domain of FliO can partially rescue motility of a ΔfliO mutant, demonstrating functionality of this domain. Presumably, overexpression of the cytoplasmic domain of FliO overcomes the localization defect of not producing the transmembrane domain, and so overexpression enables the cytoplasmic domain to find its interaction partner(s). However, it is intriguing to know how this domain can function without being anchored to the membrane, since this suggests it might function as bridging domain to maintain protein complex stability, rather than having a catalytic role in protein translocation? Using circular dichroism spectroscopy we showed that the structure of the cytoplasmic domain FliO43–125-Ala is a mixture of beta-sheet and random coil. We assume that the FliO cytoplasmic domain becomes structured while interacting with its binding partners similar to binding domains of many other proteins that acquire tertiary structure upon binding to their partners, such as in flagellin/flagellin or tropomodulin/tropomyosin interactions [42], [43].
Materials and Methods
Bacterial strains and plasmids
The bacterial strains and plasmids used in this study are listed (Table S3 and Table S4, respectively). Soft-tryptone motility agar, 0.35% (w/v), was used in motility assays [44]. Motility agar plates were maintained at 30°C, and inoculated from colonies from fresh overnight transformations. Ampicillin was used in media at 100 µg ml−1 for Salmonella strains and 50 µg ml−1 for E. coli strains. Kanamycin was used at 50 µg ml−1, tetracycline at 15 µg ml−1, chloramphenicol at 34 µg ml−1, and 5-Bromo-4-chloro-3-indoyl phosphate at 40 µg ml−1, where applicable. All chemicals were obtained from Sigma-Aldrich or Wako, Japan.
Genetic engineering procedures
The oligonucleotides used in the strain and plasmid constructions are listed (Table S5 and Table S6, respectively). To construct chromosomal gene deletions and replacements λ-Red-based recombination was used employing plasmid pKD46 [45], [46]. To construct chromosomal gene deletions a kanamycin-resistance cassette was obtained from plasmid pKD13 by PCR, flanked with approximately 40-bp ends homologous to the target site. After chromosomal integration the kanamycin resistance cassette was excised using plasmid pCP20 so that the ‘scar’, which remains afterwards within the target gene(s) was encoded as an in-frame short polypeptide for the non-polar deletions, or the ‘scar’ was in the reverse orientation for the ΔphoN301 allele. The ΔfliO22252 deletion encodes the first 5 residues, and the final 8 residues of FliO, linked by 27-amino acid residues from an internal ‘scar’ sequence within the fliO gene. The Δ(fliO-fliP)22251 deletion encodes the first 5 residues of FliO and the last 5 residues of FliP, joined by 28 residues encoded by a scar sequence.
To construct chromosomal gene replacements a tetRA tetracycline-resistance cassette was obtained by PCR from SGSC3718 genomic DNA flanked by approximately 40-bp ends homologous to the target site. After chromosomal integration, it was possible to counter-select against the tetracycline-resistance cassette on medium containing fusaric acid. Then the targeted region of the chromosome was replaced using PCR-amplified DNA containing homologous-ends. To create fliO::phoA or fliO::gfpuv chimeric gene fusions in plasmid pTSO17, which carries the fliO gene, it was first linearized at the desired point by non-strand displacing PCR. Then either a phoA gene PCR product obtained from E. coli K-12 MG1655 genomic DNA or a GFPuv gene PCR product obtained from plasmid pGFPuv (Clontech), which was flanked by 15-bp fliO homologous ends, was inserted by the homologous recombination-based In-fusion PCR cloning procedure (Clontech). Site-directed mutagenesis was performed using the QuickChange Lightning Site-Directed Mutagenesis Kit (Stratagene).
Purification of FliO43–125-Ala and FliO43–125-6xHis
FliO43–125-Ala was purified using a C-terminal intein tag encoded by a pTXB1-based expression plasmid, according to manufacturer's instructions (New England Biolabs). BL21 Star containing plasmid pTSO133 was cultivated. The cell pellet from 5-L culture was suspended in 200-ml buffer consisting of 40 mM Tris-HCl (pH 8.0), 1 M NaCl, 2 mM EDTA, and 20% glycerol, and lysed by sonication. Insoluble proteins and cell debris were collected by low-speed centrifugation and suspended in 100-ml buffer containing 40 mM Tris-HCl (pH 8.0), 0.5 M NaCl, and 8 M urea. The suspension was incubated with stirring at room temperature for 3 hours. Insoluble material was removed by centrifugation. After centrifugation, the supernatant was diluted four times with washing buffer consisting of 40 mM Tris-HCl (pH 8.0), 0.5 M NaCl, and 1 M urea, and loaded onto a chitin bead column equilibrated with washing buffer (the column volume was 30-ml). The column was washed with 200-ml of washing buffer, and then with 100-ml of washing buffer containing 0.1 M DTT. After that the column was incubated at room temperature for about 20 hours. FliO43–125-Ala was eluted from the chitin column with 50-ml of washing buffer and further purified by gel-filtration on a HiLoad Superdex-75 gel filtration column (GE healthcare) equilibrated with the same buffer. Fractions containing pure FliO43–125-Ala were pooled together, and the protein was transferred by dialysis in 20 mM Na/K phosphate (pH 6.2), and 100 mM NaCl. FliO43–125-6xHis was purified according to standard procedures, using nickel affinity chromatography under denaturing conditions after expression from plasmid pESO221.
Immunoblotting
Immunoblotting was performed similar to a previously described method [25]. For immunoblotting of whole cell lysates, colonies of Salmonella serovar Typhimurium containing the desired plasmid, from an overnight transformation, were inoculated into 5-ml LB with antibiotic and grown with shaking for 6 hours at 37°C. Cells were harvested by centrifugation at 16,100-g for 5 minutes, and the pellets were re-suspended in SDS-PAGE loading buffer (100 mM Tris-HCl, pH 6.8, 2% SDS, 1 mM β-mercaptoethanol, 7 M urea, and 0.1% bromophenol blue) and incubated at 95°C for 15 minutes. An equivalent amount of each whole cell lysate was separated by SDS PAGE and proteins were either detected by staining with Coomassie brilliant blue or transferred to PVDF membranes. Immunoblotting was performed using the anti-rabbit WesternBreeze Chromogenic Kit (Invitrogen), according to manufacturer's instructions. The following antibodies and dilutions were used: anti-alkaline phosphatase (Rockland, PA, USA), 1∶20,000 dilution; anti-GFP (Clontech), 1∶5,000 dilution; ANTI-FLAG (Sigma-Aldrich), 1∶5,000 dilution; and polyclonal anti-FliO43–125-6xHis antibody, 1∶10,000 dilution.
Cell fractionation
Cells were separated into the insoluble membrane pellet fraction and soluble supernatant fraction after lysis similar to a previously described method [47]. Briefly, from overnight transformations, colonies of Salmonella serovar Typhimurium containing the desired plasmid were inoculated into 50-ml LB with antibiotic and grown with shaking for 7 hours at 37°C. Cells were harvested by centrifugation at 6000-g for 10 minutes, and the pellets were re-suspended in 11-ml 50 mM Tris-HCl (pH 8.0), 10 mM MgCl2, and 440-µl 25× EDTA-free protease inhibitor cocktail (Roche). Cell pellets were lysed by sonication at 25% power for 5 cycles of one minute each. After incubation at room temperature for 30 minutes unlysed cell debris was removed by low-speed centrifugation at 10,000-g for 10 minutes. The insoluble membrane pellet was obtained by high-speed centrifugation at 100,000-g for 1 hour and resuspended in 1-ml 50 mM Tris-HCl (pH 8.0), and 10 mM MgCl2. One-µl was added to 5-µl SDS-PAGE loading buffer for electrophoresis. The supernatant was further clarified by centrifugation at 100,000-g for 1 hour and 10-µl was added to 5-µl SDS-PAGE loading buffer for electrophoresis.
Observation of GFPuv fluorescence and measurement of alkaline phosphatase activity
Expression of FliO/GFPuv chimeras was detected for strains inoculated onto L-agar plates containing the appropriate antibiotic, after 4 days incubation at 30°C, using a transilluminator set at 365 nm UV. Alkaline phosphatase assays are detailed in Text S1.
Circular Dichroism (CD) Spectroscopy
CD spectra of FliO43–125-Ala was measured using an Aviv model 400 spectropolarimeter (Lakewood, NJ) in 0.1 cm cuvettes at 0°C in 20 mM Na/K phosphate, pH 6.2; 100 mM NaCl. Urea titration ellipticity at 220 nm was measured for samples in 0, 1, 2, 3, 4, 5, 6, 7 and 8 M urea at 10°C.
Bioinformatics
Protein secondary structure prediction was performed using Jpred 3 [48]. Programs used to align protein sequences, and programs used to predict membrane protein-membrane topology are detailed in Text S1.
Supporting Information
Zdroje
1. BlockerA
KomoriyaK
AizawaS-I
2003 Type III secretion systems and bacterial flagella: Insights into their function from structural similarities. Proc Natl Acad Sci U S A 100 3027 3030
2. MacnabRM
2003 How bacteria assemble flagella. Annu Rev Microbiol 57 77 100
3. MacnabRM
2004 Type III flagellar protein export and flagellar assembly. Biochim Biophys Acta 1694 207 217
4. FanF
OhnishiK
FrancisNR
MacnabRM
1997 The FliP and FliR proteins of Salmonella typhimurium, putative components of the type III flagellar export apparatus, are located in the flagellar basal body. Mol Microbiol 26 1035 1046
5. KiharaM
MinaminoT
YamaguchiS
MacnabRM
2001 Intergenic suppression between the flagellar MS ring protein FliF of Salmonella and FlhA, a membrane component of its export apparatus. J Bacteriol 183 1655 1662
6. Van ArnamJS
McMurryJL
KiharaM
MacnabRM
2004 Analysis of an engineered Salmonella flagellar fusion protein, FliR-FlhB. J Bacteriol 186 2495 2498
7. MinaminoT
MacnabRM
1999 Components of the Salmonella flagellar export apparatus and classification of export substrates. J Bacteriol 181 1388 1394
8. ChevanceFFV
HughesKT
2008 Coordinating assembly of a bacterial macromolecular machine. Nat Rev Microbiol 6 455 465
9. MinaminoT
ImadaK
NambaK
2008 Mechanisms of type III protein export for bacterial flagellar assembly. Mol BioSyst 4 1105 1115
10. MinaminoT
NambaK
2008 Distinct roles of the FliI ATPase and proton motive force in bacterial flagellar protein export. Nature 451 485 488
11. PaulK
ErhardtM
HiranoT
BlairDF
HughesKT
2008 Energy source of flagellar type III secretion. Nature 451 489 492
12. Saijo-HamanoY
ImadaK
MinaminoT
KiharaM
ShimadaM
2010 Structure of the cytoplasmic domain of FlhA and implication for flagellar type III protein export. Mol Microbiol 76 260 268
13. MooreSA
JiaY
2010 Structure of the cytoplasmic domain of the flagellar secretion apparatus component FlhA from Helicobacter pylori. J Biol Chem 285 21060 21069
14. WorrallLJ
VuckovicM
StrynadkaNC
2010 Crystal structure of the C-terminal domain of the Salmonella type III secretion system export apparatus protein InvA. Protein Sci 19 1091 1096
15. ZarivachR
DengW
VuckovicM
FeliseHB
NguyenHV
2008 Structural analysis of the essential self-cleaving type III secretion proteins EscU and SpaS. Nature 453 124 127
16. DeaneJE
GrahamSC
MitchellEP
FlotD
JohnsonS
2008 Crystal structure of Spa40, the specificity switch for the Shigella flexneri type III secretion system. Mol Microbiol 69 267 276
17. LountosGT
AustinBP
NallamsettyS
WaughDS
2009 Atomic resolution structure of the cytoplasmic domain of Yersinia pestis YscU, a regulatory switch involved in type III secretion. Prot Sci 18 467 474
18. MinaminoT
MacnabRM
2000 Interactions among components of the Salmonella flagellar export apparatus and its substrates. Mol Microbiol 35 1052 1064
19. FraserGM
González-PedrajoB
TameJRH
MacnabRM
2003 Interactions of FliJ with the Salmonella type III flagellar export apparatus. J Bacteriol 185 5546 5554
20. McMurryJL
Van ArnamJS
KiharaM
MacnabRM
2004 Analysis of the cytoplasmic domains of Salmonella FlhA and interactions with components of the flagellar export machinery. J Bacteriol 186 7586 7592
21. Saijo-HamanoY
MinaminoT
MacnabRM
NambaK
2004 Structural and functional analysis of the C-terminal cytoplasmic domain of FlhA, an integral membrane component of the type III flagellar protein export apparatus in Salmonella. J Mol Biol 343 457 466
22. MinaminoT
ShimadaM
OkabeM
Saijo-HamanoY
ImadaK
2010 Role of the C-terminal cytoplasmic domain of FlhA in bacterial flagellar type III protein export. J Bacteriol 192 1929 1936
23. MinaminoT
González-PedrajoB
YamaguchiK
AizawaS-I
MacnabRM
1999 FliK, the protein responsible for flagellar hook length control in Salmonella, is exported during hook assembly. Mol Microbiol 34 295 304
24. MinaminoT
MacnabRM
2000 Domain structure of Salmonella FlhB, a flagellar export component responsible for substrate specificity switching. J Bacteriol 182 4906 4914
25. FraserGM
HiranoT
FerrisHU
DevganLL
KiharaM
2003 Substrate specificity of type III flagellar protein export in Salmonella is controlled by subdomain interactions in FlhB. Mol Microbiol 48 1043 1057
26. FerrisHU
FurukawaY
MinaminoT
KroetzMB
KiharaM
2005 FlhB regulates ordered export of flagellar components via autocleavage mechanism. J Biol Chem 280 41236 41242
27. MinaminoT
FerrisHU
MoriyaN
KiharaM
NambaK
2006 Two parts of the T3S4 domain of the hook-length control protein FliK are essential for the substrate specificity switching of the flagellar type III export apparatus. J Mol Biol 362 1148 1158
28. MoriyaN
MinaminoT
HughesKT
MacnabRM
NambaK
2006 The type III flagellar export specificity switch is dependent on FliK ruler and a molecular clock. J Mol Biol 359 466 477
29. ErhardtM
HiranoT
SuY
PaulK
WeeDH
2010 The role of the FliK molecular ruler in hook-length control in Salmonella enterica. Mol Micro 75 1272 1284
30. MalakootiJ
KomedaY
MatsumuraP
1989 DNA sequence analysis, gene product identification, and localization of flagellar motor components of Escherichia coli. J Bacteriol 171 2728 2734
31. MalakootiJ
ElyB
MatsumuraP
1994 Molecular characterization, nucleotide sequence, and expression of the fliO, fliP, fliQ, and fliR genes of Escherichia coli. J Bacteriol 176 189 197
32. OhnishiK
FanF
SchoenhalsGJ
KiharaM
MacnabRM
1997 The FliO, FliP, FliQ, and FliR proteins of Salmonella typhimurium: putative components for flagellar assembly. J Bacteriol 179 6092 6099
33. SchoenhalsGJ
KiharaM
MacnabRM
1998 Translation of the flagellar gene fliO of Salmonella typhimurium from putative tandem starts. J Bacteriol 180 2936 2942
34. LiuR
OchmanH
2007 Stepwise formation of the bacterial flagellar system. Proc Natl Acad Sci U S A 104 7116 7121
35. PallenMJ
PennCW
ChaudhuriRR
2005 Bacterial flagellar diversity in the post-genomic era. Trends Microbiol 13 143 149
36. ManoilC
BeckwithJ
1986 A genetic approach to analyzing membrane protein topology. Science 233 1403 1408
37. EhrmannM
BoydD
BeckwithJ
1990 Genetic analysis of membrane protein topology by a sandwich gene fusion approach. Proc Natl Acad Sci U S A 87 7574 7578
38. BoydD
TraxlerB
BeckwithJ
1993 Analysis of the topology of a membrane protein by using a minimum number of alkaline phosphatase fusions. J Bacteriol 175 553 556
39. FeilmeierBJ
IsemingerG
SchroederD
WebberH
PhillipsGJ
2000 Green fluorescent protein functions as a reporter for protein localization in Escherichia coli. J Bacteriol 182 4068 4076
40. RichardsonJS
RichardsonDC
2002 Natural β-sheet proteins use negative design to avoid edge-to-edge aggregation. Proc Natl Acad Sci U S A 99 2754 2759
41. ShigenobuS
WatanabeH
HattoriM
SakakiY
IshikawaH
2000 Genome sequence of the endocellular bacterial symbiont of aphids Buchnera sp. APS. Nature 407 81 86
42. KostyukovaAS
PyatibratovMG
FilimonovVV
FederovOV
1988 Flagellin parts acquiring a regular structure during polymerization are disposed on the molecule ends. FEBS Lett 241 141 144
43. KostyukovaAS
TiktopuloEI
MaédaY
2001 Folding properties of functional domains of tropomodulin. Biophys J 81 345 351
44. TokerAS
KiharaM
MacnabRM
1996 Deletion analysis of the FliM flagellar switch protein of Salmonella typhimurium. J Bacteriol 178 7069 7079
45. DatsenkoKA
WannerBL
2000 One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc Natl Acad Sci USA 97 6640 6645
46. KarlinseyJE
2007 λ-Red genetic engineering in Salmonella enterica serovar Typhimurium. Methods Enzymol 421 199 209
47. EthierJ
BoydJM
2000 Topological analysis and role of the transmembrane domain in polar targeting of PilS, a Pseudomonas aeruginosa sensor kinase. Mol Microbiol 38 891 903
48. ColeC
BarberJD
BartonGJ
2008 The Jpred 3 secondary structure prediction server. Nucleic Acids Res 36 W197 W201
Štítky
Genetika Reprodukční medicína
Článek Allelic Variation at the 8q23.3 Colorectal Cancer Risk Locus Functions as a Cis-Acting Regulator ofČlánek Allelic Selection of Amplicons in Glioblastoma Revealed by Combining Somatic and Germline AnalysisČlánek Lactic Acidosis Triggers Starvation Response with Paradoxical Induction of TXNIP through MondoAČlánek Rice a Cinnamoyl-CoA Reductase-Like Gene Family Member, Is Required for NH1-Mediated Immunity to pv.Článek Differentiation of Zebrafish Melanophores Depends on Transcription Factors AP2 Alpha and AP2 Epsilon
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2010 Číslo 9- Akutní intermitentní porfyrie
- Růst a vývoj dětí narozených pomocí IVF
- Vliv melatoninu a cirkadiálního rytmu na ženskou reprodukci
- Intrauterinní inseminace a její úspěšnost
- Délka menstruačního cyklu jako marker ženské plodnosti
-
Všechny články tohoto čísla
- Optimal Strategy for Competence Differentiation in Bacteria
- Mutational Patterns Cannot Explain Genome Composition: Are There Any Neutral Sites in the Genomes of Bacteria?
- Frail Hypotheses in Evolutionary Biology
- Genetic Architecture of Complex Traits and Accuracy of Genomic Prediction: Coat Colour, Milk-Fat Percentage, and Type in Holstein Cattle as Contrasting Model Traits
- Allelic Variation at the 8q23.3 Colorectal Cancer Risk Locus Functions as a Cis-Acting Regulator of
- Allelic Selection of Amplicons in Glioblastoma Revealed by Combining Somatic and Germline Analysis
- Germline Variation Controls the Architecture of Somatic Alterations in Tumors
- Mice Doubly-Deficient in Lysosomal Hexosaminidase A and Neuraminidase 4 Show Epileptic Crises and Rapid Neuronal Loss
- Analysis of Population Structure: A Unifying Framework and Novel Methods Based on Sparse Factor Analysis
- FliO Regulation of FliP in the Formation of the Flagellum
- Cdc20 Is Critical for Meiosis I and Fertility of Female Mice
- dMyc Functions Downstream of Yorkie to Promote the Supercompetitive Behavior of Hippo Pathway Mutant Cells
- DCAF26, an Adaptor Protein of Cul4-Based E3, Is Essential for DNA Methylation in
- Genome-Wide Double-Stranded RNA Sequencing Reveals the Functional Significance of Base-Paired RNAs in
- An Immune Response Network Associated with Blood Lipid Levels
- Genetic Variants and Their Interactions in the Prediction of Increased Pre-Clinical Carotid Atherosclerosis: The Cardiovascular Risk in Young Finns Study
- The Histone H3K36 Methyltransferase MES-4 Acts Epigenetically to Transmit the Memory of Germline Gene Expression to Progeny
- Long- and Short-Term Selective Forces on Malaria Parasite Genomes
- Lactic Acidosis Triggers Starvation Response with Paradoxical Induction of TXNIP through MondoA
- Identification of Early Requirements for Preplacodal Ectoderm and Sensory Organ Development
- Orphan CpG Islands Identify Numerous Conserved Promoters in the Mammalian Genome
- Analysis of the Basidiomycete Reveals Conservation of the Core Meiotic Expression Program over Half a Billion Years of Evolution
- ETS-4 Is a Transcriptional Regulator of Life Span in
- The SR Protein B52/SRp55 Is Required for DNA Topoisomerase I Recruitment to Chromatin, mRNA Release and Transcription Shutdown
- The Baker's Yeast Diploid Genome Is Remarkably Stable in Vegetative Growth and Meiosis
- Chromatin Landscape Dictates HSF Binding to Target DNA Elements
- The APETALA-2-Like Transcription Factor OsAP2-39 Controls Key Interactions between Abscisic Acid and Gibberellin in Rice
- Accurately Assessing the Risk of Schizophrenia Conferred by Rare Copy-Number Variation Affecting Genes with Brain Function
- Widespread Over-Expression of the X Chromosome in Sterile F Hybrid Mice
- The Characterization of Twenty Sequenced Human Genomes
- The Genome of a Pathogenic : Cooptive Virulence Underpinned by Key Gene Acquisitions
- A Single Element Maintains Repression of the Key Developmental Regulator
- Identification of New Genetic Risk Variants for Type 2 Diabetes
- Effect of Correlated tRNA Abundances on Translation Errors and Evolution of Codon Usage Bias
- Evidence of Selection upon Genomic GC-Content in Bacteria
- Proteomic Changes Resulting from Gene Copy Number Variations in Cancer Cells
- Rice a Cinnamoyl-CoA Reductase-Like Gene Family Member, Is Required for NH1-Mediated Immunity to pv.
- Longitudinal Genome-Wide Association of Cardiovascular Disease Risk Factors in the Bogalusa Heart Study
- Response to Mechanical Stress Is Mediated by the TRPA Channel Painless in the Heart
- DNMT3L Modulates Significant and Distinct Flanking Sequence Preference for DNA Methylation by DNMT3A and DNMT3B
- Identifying Signatures of Natural Selection in Tibetan and Andean Populations Using Dense Genome Scan Data
- Incremental Genetic Perturbations to MCM2-7 Expression and Subcellular Distribution Reveal Exquisite Sensitivity of Mice to DNA Replication Stress
- Loss of Maternal ATRX Results in Centromere Instability and Aneuploidy in the Mammalian Oocyte and Pre-Implantation Embryo
- Comparative Genomic Hybridization (CGH) Reveals a Neo-X Chromosome and Biased Gene Movement in Stalk-Eyed Flies (Genus )
- Differentiation of Zebrafish Melanophores Depends on Transcription Factors AP2 Alpha and AP2 Epsilon
- Gene–Environment Interactions at Nucleotide Resolution
- Dementia Revealed: Novel Chromosome 6 Locus for Late-Onset Alzheimer Disease Provides Genetic Evidence for Folate-Pathway Abnormalities
- Critical Functions of Rpa3/Ssb3 in S-Phase DNA Damage Responses in Fission Yeast
- Preferential Re-Replication of Heterochromatin in the Absence of Geminin
- The Potential for Enhancing the Power of Genetic Association Studies in African Americans through the Reuse of Existing Genotype Data
- Evidence That Mutation Is Universally Biased towards AT in Bacteria
- Perturbation Analysis of Heterochromatin-Mediated Gene Silencing and Somatic Inheritance
- Diversity of Eukaryotic DNA Replication Origins Revealed by Genome-Wide Analysis of Chromatin Structure
- Genetic Deletion of the Desmosomal Component Promotes Tumor Microinvasion in a Mouse Model of Pancreatic Neuroendocrine Carcinogenesis
- The Metabolic Enzyme ManA Reveals a Link between Cell Wall Integrity and Chromosome Morphology
- SNPs Associated with Cerebrospinal Fluid Phospho-Tau Levels Influence Rate of Decline in Alzheimer's Disease
- Synthesizing and Salvaging NAD: Lessons Learned from
- A Central Regulatory System Largely Controls Transcriptional Activation and Repression Responses to Phosphate Starvation in Arabidopsis
- An Insect Herbivore Microbiome with High Plant Biomass-Degrading Capacity
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Synthesizing and Salvaging NAD: Lessons Learned from
- Optimal Strategy for Competence Differentiation in Bacteria
- Long- and Short-Term Selective Forces on Malaria Parasite Genomes
- Identifying Signatures of Natural Selection in Tibetan and Andean Populations Using Dense Genome Scan Data
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



