-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
and Deficiency Cooperate in the Progression of Mouse Prostate Tumourigenesis
Epidemiological studies have shown that one of the strongest risk factors for prostate cancer is a family history of the disease, suggesting that inherited factors play a major role in prostate cancer susceptibility. Germline mutations in BRCA2 predispose to breast and ovarian cancer with its predominant tumour suppressor function thought to be the repair of DNA double-strand breaks. BRCA2 has also been implicated in prostate cancer etiology, but it is unclear the impact that mutations in this gene have on prostate tumourigenesis. Here we have undertaken a genetic analysis in the mouse to determine the role of Brca2 in the adult prostate. We show that deletion of Brca2 specifically in prostate epithelia results in focal hyperplasia and low-grade prostate intraepithelial neoplasia (PIN) in animals over 12 months of age. Simultaneous deletion of Brca2 and the tumour suppressor Trp53 in prostate epithelia gave rise to focal hyperplasia and atypical cells at 6 months, leading to high-grade PIN in animals from 12 months. Epithelial cells in these lesions show an increase in DNA damage and have higher levels of proliferation, but also elevated apoptosis. Castration of Brca2;Trp53 mutant animals led to regression of PIN lesions, but atypical cells persisted that continued to proliferate and express nuclear androgen receptor. This study provides evidence that Brca2 can act as a tumour suppressor in the prostate, and the model we describe should prove useful in the development of new therapeutic approaches.
Published in the journal: . PLoS Genet 6(6): e32767. doi:10.1371/journal.pgen.1000995
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1000995Summary
Epidemiological studies have shown that one of the strongest risk factors for prostate cancer is a family history of the disease, suggesting that inherited factors play a major role in prostate cancer susceptibility. Germline mutations in BRCA2 predispose to breast and ovarian cancer with its predominant tumour suppressor function thought to be the repair of DNA double-strand breaks. BRCA2 has also been implicated in prostate cancer etiology, but it is unclear the impact that mutations in this gene have on prostate tumourigenesis. Here we have undertaken a genetic analysis in the mouse to determine the role of Brca2 in the adult prostate. We show that deletion of Brca2 specifically in prostate epithelia results in focal hyperplasia and low-grade prostate intraepithelial neoplasia (PIN) in animals over 12 months of age. Simultaneous deletion of Brca2 and the tumour suppressor Trp53 in prostate epithelia gave rise to focal hyperplasia and atypical cells at 6 months, leading to high-grade PIN in animals from 12 months. Epithelial cells in these lesions show an increase in DNA damage and have higher levels of proliferation, but also elevated apoptosis. Castration of Brca2;Trp53 mutant animals led to regression of PIN lesions, but atypical cells persisted that continued to proliferate and express nuclear androgen receptor. This study provides evidence that Brca2 can act as a tumour suppressor in the prostate, and the model we describe should prove useful in the development of new therapeutic approaches.
Introduction
Prostate cancer is the most common cancer in men in developed countries, with a rising incidence of the disease. However, the etiology of this malignancy is still unclear. Prostate cancer progresses through a pathologically defined series of steps involving increasing grades of PIN, invasive adenocarcinoma and metastatic cancer [1]. Androgens are crucial for normal prostate function, and act as pro-survival and proliferation factors in cancer cells. As such, prostate cancer is sensitive to androgen levels and androgen depletion therapy via chemical or surgical castration is an initial step in treatment, typically resulting in tumour regression. However, the cancer normally re-grows and develops as a castration-independent tumour.
Epidemiological studies have shown that one of the strongest risk factors for prostate cancer is a family history of the disease, suggesting that inherited factors play a major role in prostate cancer susceptibility [2], [3]. Approximately 10% of prostate cancers are thought to be hereditary, and this number increases with early on-set disease. In spite of this, little is known about the mechanisms of tumourigenesis of inherited prostate cancer. Prostate cancer frequently clusters in families that have breast cancer, indicating a genetic link between these two diseases [4]–[6]. Germline mutations in BRCA2 predispose to both breast and ovarian cancer making it a good candidate gene for prostate cancer etiology. There is an increased risk of prostate cancer in individuals carrying a mutation in BRCA2, particularly early-onset disease [7]–[10]. The Breast Cancer Linkage Consortium found a significant relative risk of 4.65 for prostate cancer in male carriers of a deleterious BRCA2 mutation that rose to 7.33 in men under 65 years of age [7]. Consistent with this, analysis of men with early-onset disease indicates that BRCA2 carriers account for between 0.8–2% of prostate cancer cases, compared with the prevalence of 0.1% BRCA2 mutations in the general population [11], [12]. In addition, BRCA2 mutation carriers have been associated with aggressive prostate cancer [13]–[16].
BRCA2 is thought to act as a tumour suppressor, with tumours arising from BRCA2 mutations frequently demonstrating loss-of-heterozygosity with loss of the wild-type allele. BRCA2 plays an important role in the repair of DNA double-strand breaks (DSB) through homologous recombination (HR) [17]. When there is a second identical DNA copy (i.e. the sister chromatid after replication) HR is the primary method of repair and is a relatively error-free DNA repair pathway. After DNA damage, BRCA2 directly interacts with the recombinase RAD51, a process that is essential for HR-mediated repair of DSBs [18]. When HR is defective or no sister chromatid is available the error-prone methods of single-strand annealing and non-homologous end joining are used for DNA repair [19]. BRCA2-deficient cells form chromosomal aberrations spontaneously in culture and are more sensitive to certain DNA damaging agents [19]–[21]. Hence, loss of BRCA2 is thought to principally lead to tumour progression by the failure to repair DNA by HR, leading to genomic instability.
Mouse models have shown a direct in vivo tumour suppressor role for Brca2 in the mammary gland and have demonstrated a synergistic tumour suppressor activity with Trp53. However, Brca2 heterozygous animals do not show a predisposition to tumour formation and Brca2 null mice result in embryonic lethality [18], [22], [23]. To circumvent this prenatal lethality, the Cre-LoxP system has been used to conditionally delete Brca2 in a tissue-specific manner. Deletion of Brca2 from the mouse mammary epithelium either fails to produce mammary-gland tumours or results in mammary-gland tumour formation with long latency (1.4–1.6 years) [24]–[26]. Tumour latency was reduced in Brca2 mutant mice that were Trp53 heterozygous [24]. In addition, mice with conditional inactivation of Brca2 and Trp53 developed mammary tumours with high penetrance at 6 months [25].
To understand the role of Brca2 in prostate cancer we have used a prostate–specific Cre line and a conditional Brca2 allele to delete Brca2 in adult mouse prostate epithelia. We show that loss of Brca2 in the prostate results in focal hyperplasia and low-grade (LG) PIN. Mice with conditional deletion of Brca2 and Trp53 have a high incidence of high-grade (HG) PIN, which contain cells with elevated DNA damage. PIN lesions in Brca2;Trp53 homozygous mutant prostates persist and continue to proliferate after androgen depletion. This work confirms the role of Brca2 as a tumour suppressor in the prostate and provides a model to test potential therapeutics in Brca2-deficient prostate neoplasia.
Results
Deletion of Brca2 from prostate epithelia results in hyperplasia and low-grade PIN
To investigate the role of Brca2 in the prostate we deleted Brca2 from the adult mouse prostate epithelia. To achieve this we mated mice carrying a Brca2 allele that has exon 11 flanked by loxP sites (Brca2F/F) to transgenic mice carrying Cre recombinase under the control of a prostate-specific composite rat probasin promoter, PBCre4 [25], [27]. This Cre line has been used successfully to delete tumour suppressor genes and activate oncogenes to drive prostate neoplasia and tumour progression [28]–[30]. Deletion of this Brca2 conditional allele results in the loss of a Rad51-interacting domain, and consequently, homozygous germline deletion leads to embryonic lethality [25].
Cohorts of male control (Brca2F/F), Brca2 heterozygous (Brca2F/+;PBCre4) and Brca2 mutant (Brca2F/F;PBCre4) animals were generated and analysed for tumour progression at 6 months, 10–14 months and 15–20 months of age. None of the Brca2F/+;PBCre4 prostates had any observable morphological differences compared to control prostates at any time point analysed (Figure 1 and Table 1). Focal hyperplasia that contained atypical cells was first observed in Brca2F/F;PBCre4 prostates at 10–14 months and was also present in these animals at 15–20 months (Table 1). In addition, at 15–20 months a significant number of Brca2 homozygous mutant prostates had focal LG PIN in their lumen compared to control animals (6/17 compared to 0/28; Z-test p = 0.0001) (Figure 1A and Table 1). LG PIN lesions formed characteristic tufting and cribiform patterns (Figure 1B). These focal lesions contained multiple atypical cells that had prominent nucleoli and hyperchromasia. Hyperplasia and LG PIN lesions were present in all four prostatic lobes of Brca2 mutants.
Fig. 1. Deletion of Brca2 from prostate epithelia results in hyperplasia and LG PIN. 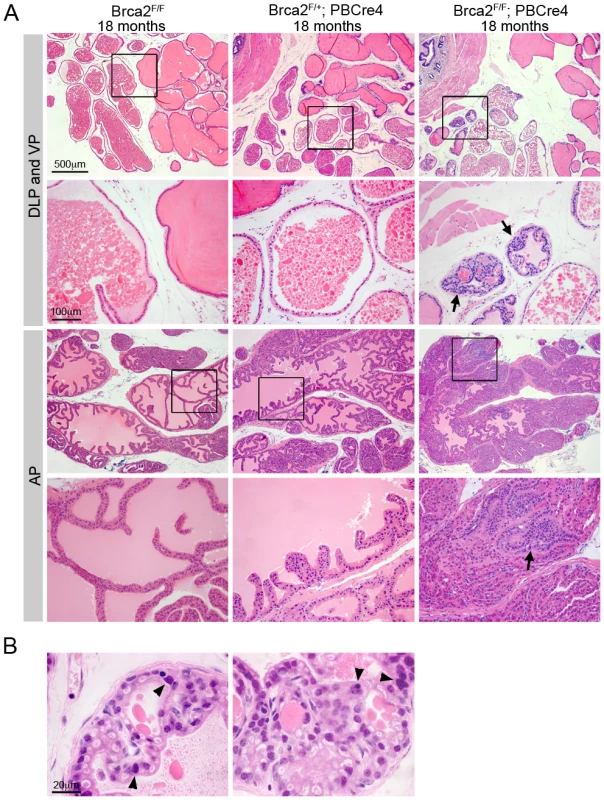
(A) Haematoxylin and eosin stained sections of dorsolateral prostate (DLP), ventral prostate (VP) and anterior prostate (AP) of control and Brca2 mutants. Control (Brca2F/F) and Brca2 heterozygous (Brca2F/+;PBCre4) prostates do not have PIN. Prostate-specific homozygous deletion of Brca2 (Brca2F/F;PBCre4) results in focal hyperplasia and LG PIN (indicated with arrows). Black box shows the area shown in higher magnification in the panel below. (B) Detail of LG PIN in the DLP of Brca2 mutant prostates. Left panel shows a lumen with tufting pattern and right panel shows cribiform pattern. Arrowheads indicate atypical cells. Tab. 1. The number of control, Brca2 mutant and Brca2;Trp53 mutant prostates analysed and their phenotype. 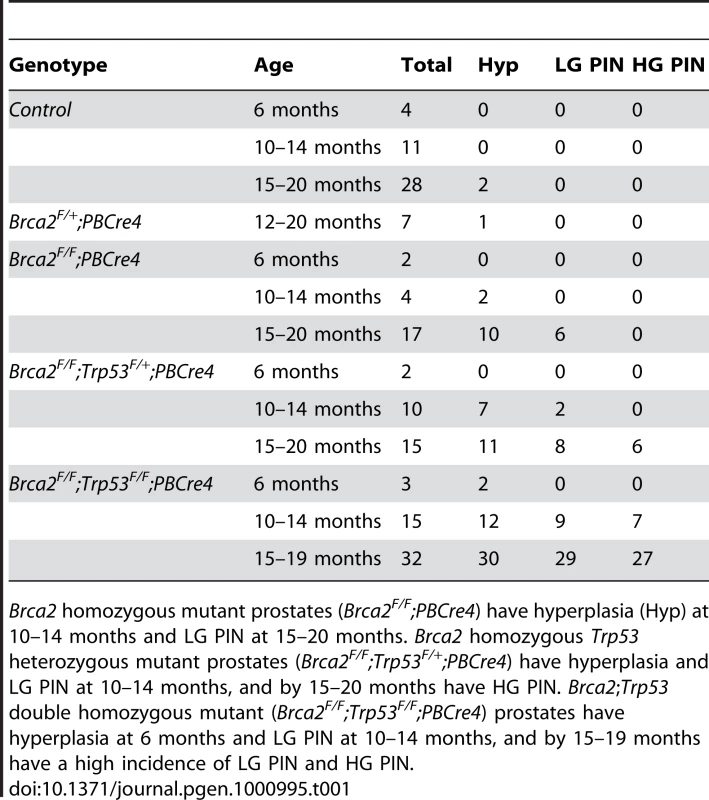
Brca2 homozygous mutant prostates (Brca2F/F;PBCre4) have hyperplasia (Hyp) at 10–14 months and LG PIN at 15–20 months. Brca2 homozygous Trp53 heterozygous mutant prostates (Brca2F/F;Trp53F/+;PBCre4) have hyperplasia and LG PIN at 10–14 months, and by 15–20 months have HG PIN. Brca2;Trp53 double homozygous mutant (Brca2F/F;Trp53F/F;PBCre4) prostates have hyperplasia at 6 months and LG PIN at 10–14 months, and by 15–19 months have a high incidence of LG PIN and HG PIN. Combined deletion of Brca2 and Trp53 leads to frequent high-grade PIN
The tumour suppressor TP53 is frequently mutated in BRCA2 cancers and studies in the mouse have shown a genetic interaction between Brca2 and Trp53 [24], [31], [32]. To test if Brca2 and Trp53 cooperate in the prostate we deleted both of these genes in the prostate epithelia using the PBCre4 transgene. Cohorts of male control (Brca2F/F;Trp53F/F), Brca2 homozygous and Trp53 heterozygous (Brca2F/F;Trp53F/+;PBCre4) and Brca2 and Trp53 double homozygous (Brca2F/F;Trp53F/F;PBCre4) animals were generated and analysed for tumour progression.
In addition to hyperplasia and LG PIN observed in Brca2 mutants, deletion of Brca2 and Trp53 resulted in the formation of HG PIN lesions. At 10–14 months, Brca2F/F;Trp53F/+;PBCre4 animals had focal LG PIN and hyperplasia (Figure 2A and Table 1). By 15–20 months, LG PIN was still present and a significant number of animals had focal HG PIN compared to control animals (6/15 compared to 0/28; Z-test p = 0.0017). However, the frequency of HG PIN was significantly higher in Brca2F/F;Trp53F/F;PBCre4 animals compared to Brca2F/F;Trp53F/+;PBCre4 animals at this age (27/32 compared to 6/15; Z-test p = 0.0058) (Figure 2A and Table 1). In these animals, focal areas of hyperplasia consisting of atypical cells were present as early as 6 months. At 10–14 months, LG PIN was present and a significant number of Brca2F/F;Trp53F/F;PBCre4 animals had HG PIN compared to control animals (7/15 compared to 0/11; Z-test p = 0.0276). Hyperplasia and LG PIN lesions were similar to those found in Brca2F/F;PBCre4 mutants. Frequently multiple ducts of each lobe had HG PIN, which were present in proximal and distal regions of the prostate and consisted of many atypical cells filling the lumen. Atypical cells were unorganised with poor orientation, severe nuclear pleomorphism and abnormal nuclear to cytoplasm ratios (Figure 2B). Mitotic figures, apoptotic bodies and areas of necrosis were also present within HG PIN lesions (Figure 2B). In some cases, epithelial cells of the lumen protrude into the adjacent stroma and the smooth muscle surrounding the ducts was no longer continuous but was broken up (Figure 2B). These areas contained atypical smooth muscle cells and desmoplasia in the surrounding stroma. HG PIN lesions were predominantly seen in the anterior prostate (AP) and dorsal prostate (DP) of Brca2;Trp53 homozygous mutant animals, with a small number observed in lateral and ventral lobes. Deletion of LoxP flanked Brca2 and Trp53 alleles by the PBCre4 transgene in the prostate was confirmed by PCR analysis on micro dissected tissue (Figure S1).
Fig. 2. Combined deletion of Brca2 and Trp53 leads to HG PIN. 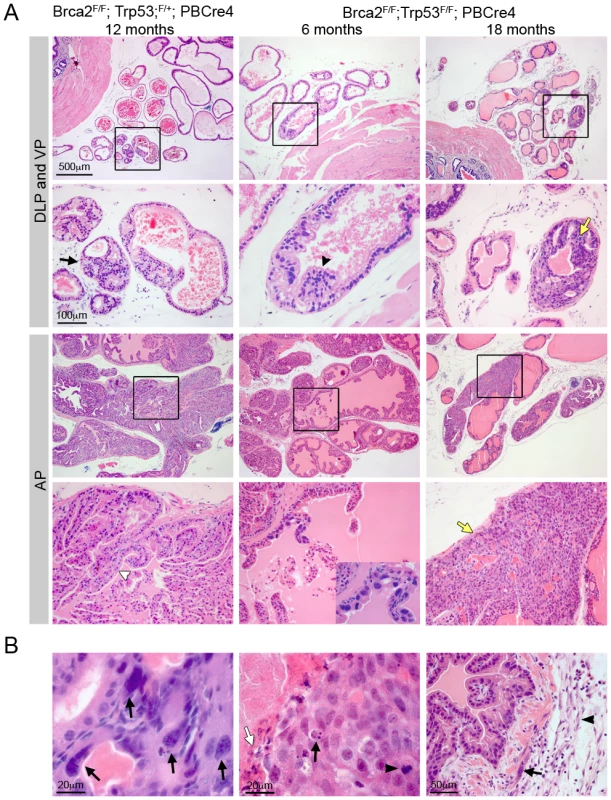
(A) Haematoxylin and eosin stained sections of dorsolateral prostate (DLP), ventral prostate (VP) and anterior prostate (AP) of Brca2;Trp53 mutants. Homozygous deletion of Brca2 and heterozygous loss of Trp53 (Brca2F/F;Trp53F/+;PBCre4) in prostate epithelia results in hyperplasia (indicated with white arrowhead) and PIN (indicated with black arrow). Prostate-specific homozygous deletion of Brca2 and Trp53 (Brca2F/F;Trp53F/F;PBCre4) results in hyperplasia (indicated with black arrowhead) and atypical cells at 6 months of age. Close-up in bottom panel shows cluster of atypical cells. Brca2;Trp53 homozygous mutants have focal HG PIN (indicated with yellow arrows). Black box shows the area that is shown in higher magnification in the panel below. (B) Detail of HG PIN in the AP of Brca2;Trp53 mutants. Left panel shows atypical cells with arrows indicating severe pleomorphic nuclei. Middle panel shows a mitotic figure (black arrowhead), apoptotic body (black arrow) and an area of necrosis (white arrow). Right panel shows epithelial cells protruding into broken-up atypical smooth muscle cells (black arrow) and desmoplastic stroma (black arrowhead). Brca2;Trp53 HG PIN lesions display increased DNA damage and apoptosis
As the predominant tumour suppressor function of BRCA2 is thought to be the repair of DNA DSBs, we assessed the level of spontaneous DNA damage in Brca2 and Brca2;Trp53 mutant prostates. An early response to DNA damage is the phosphorylation of histone H2AX (γH2AX) [33]. Areas of hyperplasia and PIN in Brca2 mutant and Brca2;Trp53 mutant prostates contained cells that were positive for γH2AX, which were not present in control prostates (Figure 3A). While Brca2F/F;PBCre4 and Brca2F/F;Trp53F/+;PBCre4 LG PIN lesions had individual or small groups of γH2AX positive cells, Brca2;Trp53 homozygous mutant prostates had large focal areas with many positive cells. These areas of γH2AX correlated with focal HG PIN lesions and were predominantly present in the AP and DP.
Fig. 3. Brca2;Trp53 mutants have increased DNA damage and apoptosis. 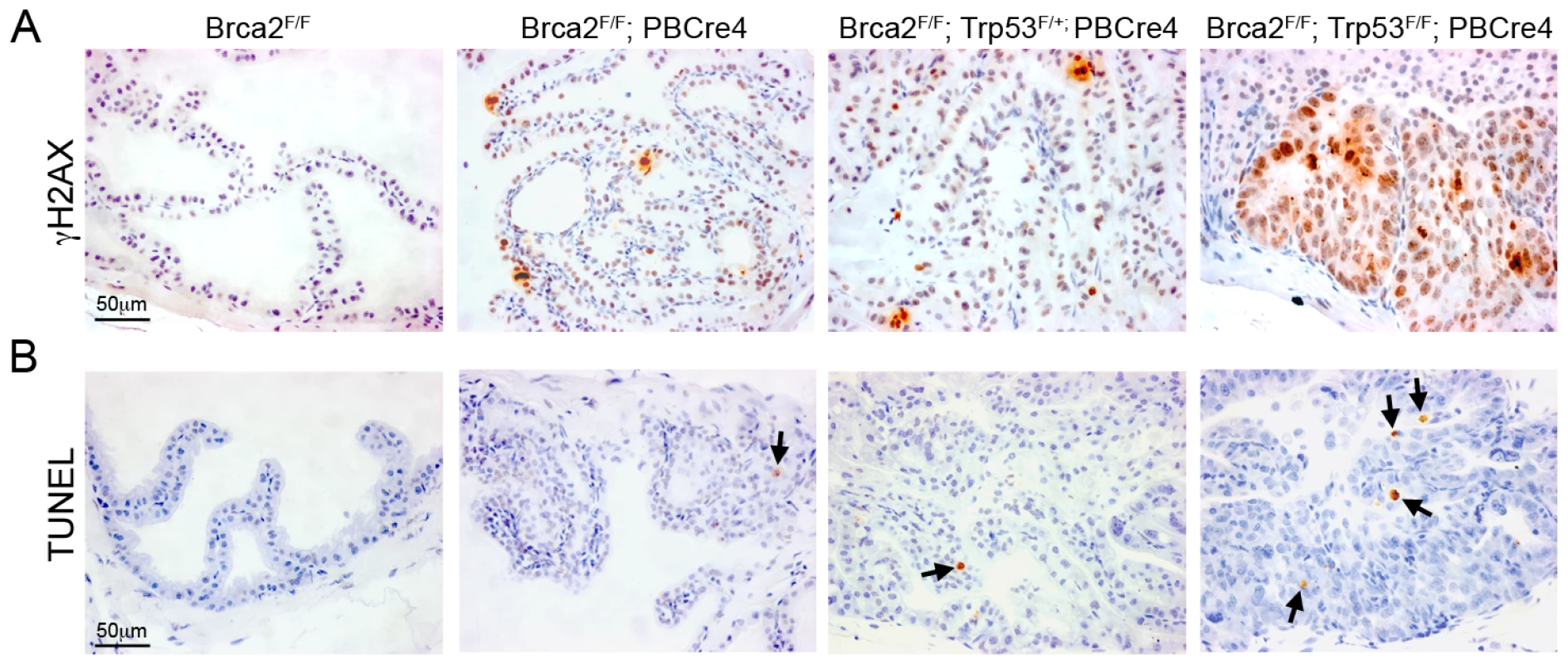
(A) Immunohistochemistry for γH2AX shows several cells have DNA damage in LG PIN areas in Brca2F/F;PBCre4 and Brca2F/F;Trp53F/+;PBCre4 mutant prostates, whereas control (Brca2F/F) prostates have undetectable levels of DNA damage. Brca2F/F;Trp53F/F;PBCre4 mutants have multi-focal areas with a large number of cells with DNA damage in regions of HG PIN. (B) TUNEL assay on sections of prostates demonstrates Brca2F/F;PBCre4 and Brca2F/F;Trp53F/+;PBCre4 mutant prostates have an increased level of apoptosis, compared to control (Brca2F/F) prostates. The level of apoptotic cells increases further in HG PIN lesions of Brca2F/F;Trp53F/F;PBCre4 mutants. Arrows indicate apoptotic cells. The anterior prostates of 16-month-old animals are shown. Deletion of Brca2 frequently results in increased levels of cellular apoptosis, presumably as a result of increased constitutive DNA damage. To determine the level of apoptosis in prostate epithelia after deletion of Brca2 and Trp53 we used the TUNEL assay, which has been used to identify apoptosis in Brca2 null neural tissue [32]. Brca2 mutant prostates showed a 3 fold increase in TUNEL positive cells in areas of hyperplasia and LG PIN, compared to control prostates that had few apoptotic cells (0.3% TUNEL positive cells vs 0.1% in control) (Figure 3B). A 4 fold increase in apoptosis was observed in Brca2F/F;Trp53F/+;PBCre4 PIN foci (0.4% TUNEL positive cells vs 0.1% in control). Notably, there was a 20 fold increase in apoptotic cells in areas of HG PIN in Brca2;Trp53 homozygous mutant prostates (2% TUNEL positive cells vs 0.1% in control) (Figure 3B). An anti-Caspase-3 antibody and histological analysis confirmed that TUNEL positive cells were apoptotic and not the result of labelling damaged DNA (data not shown).
Brca2;Trp53 mutant prostates have hallmarks of cancer
Ki-67 is a marker of proliferating cells and a prognostic indicator in prostate cancer [34]. Analysis of Ki-67 showed a low number of proliferating cells in control prostates that increased 6 fold in areas of hyperplasia and LG PIN in Brca2F/F;PBCre4 mutant prostates (2.2% Ki-67 positive cells vs 0.4% in control) (Figure 4A). Levels of proliferation were 9 fold higher in Brca2F/F;Trp53F/+;PBCre4 PIN lesions (3.5% Ki-67 positive cells vs 0.4% in control), and dramatically increased by 30 fold in Brca2F/F;Trp53F/F;PBCre4 HG PIN lesions (12% Ki-67 positive cells vs 0.4% in control) (Figure 4A).
Fig. 4. Brca2;Trp53 mutant prostates have hallmarks of cancer. 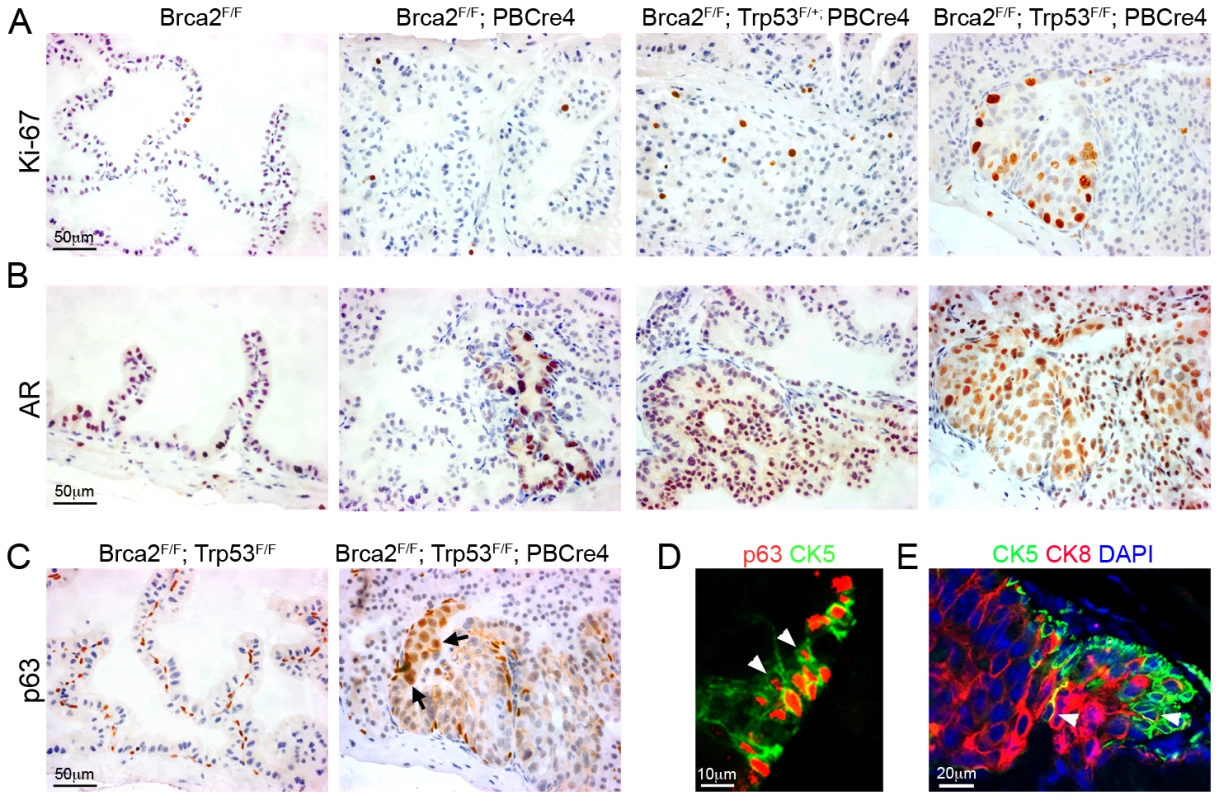
(A) Ki-67 immunohistochemistry shows increased proliferation in areas of LG PIN in Brca2F/F;PBCre4 and Brca2F/F;Trp53F/+;PBCre4 mutant prostates, a low number of proliferating cells are present in control (Brca2F/F) prostates. Brca2F/F;Trp53F/F;PBCre4 mutants have a large number of proliferating cells in areas of HG PIN. (B) AR immunohistochemistry demonstrating increased levels of expression throughout the nucleus and cytoplasm in areas of LG PIN in Brca2F/F;PBCre4 and Brca2F/F;Trp53F/+;PBCre4 mutant prostates, compared to control prostates (Brca2F/F). Brca2F/F;Trp53F/F;PBCre4 mutants have increased AR expression predominantly in the nucleus of cells in areas of HG PIN. (C) p63 immunohistochemistry shows an increase in p63-expressing cells in HG PIN lesions in Brca2F/F;Trp53F/F;PBCre4 mutant prostates and normal expression in the basal cells of control (Brca2F/F;Trp53F/F) prostates. Arrows indicate a cluster of abnormal p63-expressing cells that are rounder and nearer the lumen. (D) Left panel shows p63 (red) and CK5 (green) fluorescent immunohistochemistry analysis with labelled cells protruding into the lumen (white arrowheads) in a region of PIN in Brca2F/F;Trp53F/F;PBCre4 mutants. Right panel shows CK5 (green, basal cells) and CK8 (red, luminal cells) fluorescent immunohistochemistry with PIN lesions in Brca2F/F;Trp53F/F;PBCre4 mutants displaying an increase in luminal cells next to clusters of basal cells. White arrowhead marks CK5 and CK8 double-labelled cells. DAPI nuclear stain is blue. The anterior prostates of 16-month-old animals are shown. Levels of the androgen receptor (AR) expressed in the luminal epithelium usually increase in the nucleus during human prostate carcinoma progression [35]. Brca2F/F;PBCre4 and Brca2F/F;Trp53F/+;PBCre4 mutant prostates had increased AR expression in the cytoplasm and nucleus of epithelial cells that correlated with regions of LG PIN (Figure 4B). The level of AR increased significantly more in Brca2F/F;Trp53F/F;PBCre4 HG PIN lesions where it was found predominantly in the nucleus of luminal epithelial cells (Figure 4B).
The human and mouse prostate comprise of basal, luminal and rare neuroendocrine epithelial cells. In addition, intermediate or transit amplifying (TA) cells are observed in human prostates [36]. Brca2;Trp53 homozygous mutant HG PIN lesions frequently contained large groups of p63-expressing cells, a marker of basal cells (Figure 4C). Instead of their normal flat shape and position basal to the luminal cells, some p63-expressing cells were rounder and in a position near the lumen of the prostate. Sections fluorescently double labelled with p63 and the basal cell cytokeratin CK5 confirmed the presence of clusters of aberrant basal cells that protrude into the lumen (Figure 4D). Brca2;Trp53 homozygous mutant HG PIN lesions were then double labelled with CK5 and CK8, a marker of differentiated luminal cells. Areas of neoplasia showed an increase in CK8-expressing luminal cells that were often adjacent to a population of expanded CK5-expressing basal cells (Figure 4D). These regions occasionally had cells that were labelled with both CK8 and CK5, similar to human TA cells that co-express basal and luminal markers, which are not seen in control mouse prostates (Figure 4D) [36].
Brca2;Trp53 PIN lesions proliferate post-castration
Androgen ablation is the standard treatment for human prostate cancer. To assess the response of neoplasias formed after deletion of Brca2 and Trp53 to androgen ablation, we surgically castrated animals at 16 months when HG PIN lesions have already formed and analysed them 4 days post-castration. Castration of control animals resulted in normal prostate regression, with a reduction in lumen size (Figure 5A). Brca2F/F;Trp53F/F;PBCre4 animals still contained focal areas of neoplasia with atypical cells following androgen-depletion (8/8 mutant animals) (Figure 5A). However, we did not observe ducts with filled lumens as seen in areas of PIN in non-castrated mutant animals.
Fig. 5. Brca2;Trp53 PIN lesions proliferate post-castration. 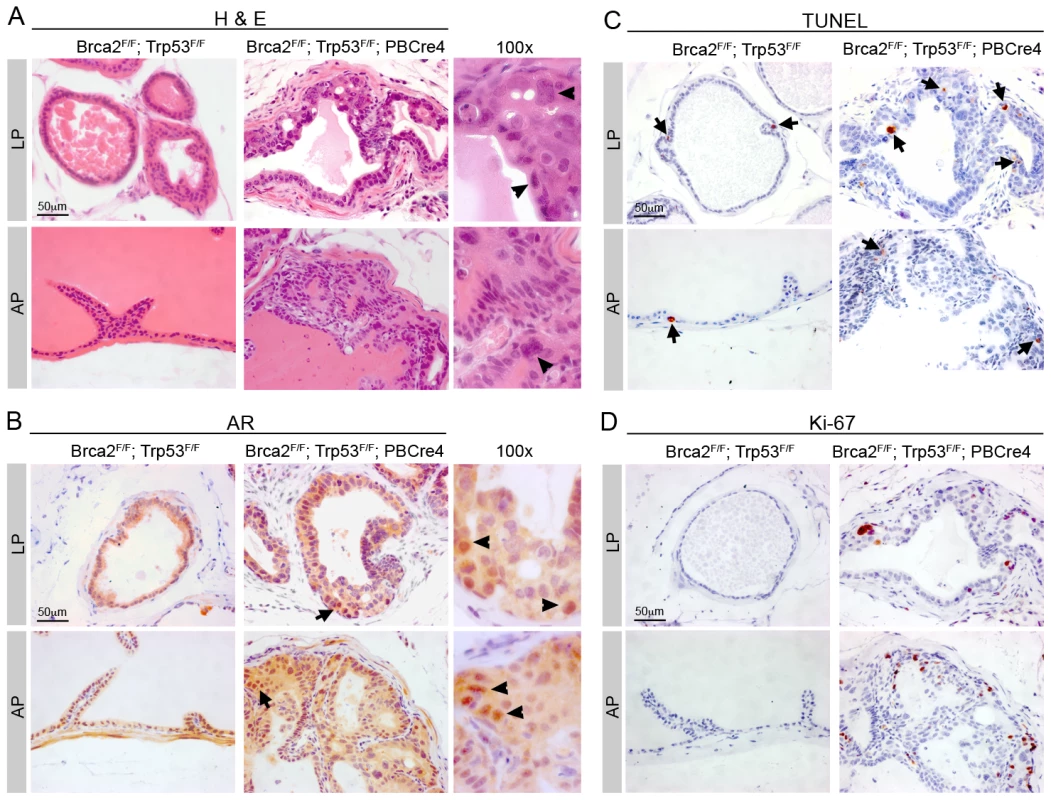
Control (Brca2F/F;Trp53F/F) and mutant Brca2F/F;Trp53F/F;PBCre4 prostates were surgically castrated at 16 months and culled 4 days later. (A) Haematoxylin and eosin stain shows atypical cells are still present in Brca2F/F;Trp53F/F;PBCre4 mutant prostates 4 days after castration, but there is a reduction in cells present in the lumen. Right panels show detail of neoplasia, atypical cells are indicated with arrowheads. (B) AR immunohistochemistry shows expression 4 days post-castration is predominantly throughout the cytoplasm of control and Brca2F/F;Trp53F/F;PBCre4 prostates. Arrows indicate cells that have higher nuclear AR expression. Right panels show detail of cells with nuclear AR expression, indicated with arrowheads. (C) TUNEL assay analysis demonstrates an increase in apoptotic cells in control and mutant prostates after castration. Arrows indicate some apoptotic cells. (D) Ki-67 immunohistochemistry shows there are proliferating cells in castrated Brca2F/F;Trp53F/F;PBCre4 mutants. LP is lateral prostate, AP is anterior prostate. Following castration of control male mice, AR is expressed at low levels predominantly throughout the cytoplasm of luminal and stromal cells (Figure 5B). Castration of Brca2F/F;Trp53F/F;PBCre4 animals resulted in AR expression in a diffuse pattern throughout most prostate luminal and stromal cells (Figure 5B). However, AR is expressed at higher levels in the nucleus of a small number of cells in the PIN lesions of mutant animals post-castration (Figure 5B).
A reduction in the level of circulating androgens results in apoptosis of the AR-expressing luminal epithelial cells, normally leading to initial prostate tumour regression. As expected, luminal cells of control castrated animals contained apoptotic cells throughout the prostate post-castration (Figure 5C). Castrated Brca2;Trp53 homozygous mutants contained cells in all prostatic lobes undergoing programmed cell death, including apoptotic cells in PIN lesions (2.2% TUNEL positive cells vs 1.8% in castrated control, p = 0.49) (Figure 5C).
Interestingly, Ki-67 staining showed an 18 fold increase in proliferating cells in areas of neoplasia in Brca2;Trp53 mutants that persisted after castration, compared to control castrated animals (5.5% Ki-67 positive cells vs 0.3% in castrated control) (Figure 5D).
Discussion
Studies on human carriers of deleterious BRCA2 mutations have implicated this gene in prostate cancer etiology, but its function in this malignancy is unclear. We have undertaken a genetic analysis of Brca2 function in the adult mouse prostate to define its role in prostate cancer and to create an in vivo model of Brca2-dependent prostate disease progression. Our study has demonstrated that loss of Brca2 in the mouse prostate epithelium results in hyperplasia and LG PIN. These lesions have an increase in the number of cells with DNA damage and apoptotic cells, which could be the result of the impairment of DNA repair pathways. This demonstrates not only that Brca2 can play a role in the initiation of prostate neoplasia but also that other factors are required for prostate tumour progression.
Deletion of Brca2 and Trp53 in mouse prostate epithelia resulted in a shorter latency and increased frequency of prostate neoplasia compared to deletion of Brca2 alone (Figure 6). Moreover, the severity of neoplasia increased in Brca2;Trp53 mutants, with the formation of hyperplasia and LG PIN at initial stages followed by a high incidence of multi-focal, proliferative HG PIN lesions with progressive cellular atypia (Figure 6). HG PIN lesions that form after deletion of Brca2 and Trp53 contained many cells with DNA damage, indicating increased genomic instability [17]. Multi-focal lesions are a common feature of human prostate cancer and may be due to defects in the DNA damage response [37]. The formation of HG PIN lesions in Brca2;Trp53 mutant prostates may reflect the loss of key regulatory p53-dependent functions in response to DNA damage controlling cell-cycle checkpoints, apoptosis and senescence [38]. This demonstrates a co-operative tumour suppressor function of Brca2 and Trp53 in the prostate similar to the mammary gland [24]–[26].
Fig. 6. Schematic showing the progression of prostate neoplasia in Brca2 and Brca2;Trp53 mutant animals. 
Note that prostate neoplasia onset is earlier and the severity of PIN increases in Brca2 mutants as Trp53 is lost. A recent study investigating whether TP53 and BRCA2 are frequently mutated together in human prostate cancer found that TP53 overexpression could not distinguish BRCA2 carriers with prostate cancer from a control group of prostate cancer cases [39]. However, this study was limited by the small number of BRCA2 cases and inability to detect TP53 mutations that do not stabilize the protein, which are frequently detected in tumours with impaired homologous recombination [40].
Deletion of Brca2 in other tissues frequently leads to an increase in apoptosis, which is partially or fully rescued upon loss of Trp53 [31], [32], [41]. However, we see more apoptotic cells in Brca2;Trp53 mutant HG PIN lesions than in Brca2 mutant LG PIN lesions. The increase in apoptosis in HG lesions could be due to the rapid accumulation of additional mutations by proliferating cells, which causes catastrophic amounts of DNA damage. This suggests that while some Brca2-null cells may be rescued from apoptosis after loss of Trp53, other cells in Brca2;Trp53 deficient HG neoplastic lesions undergo p53-independent cell death. Several different DNA damage-induced p53-independent mechanisms of apoptosis have been reported in different cell types [42].
AR expression is increased in the nucleus of cells in Brca2;Trp53 HG PIN lesions suggesting they are androgen sensitive. Consistent with this, castration of Brca2F/F;Trp53F/F;PBCre4 animals led to regression of PIN and a reduction of cells within the lumen. This suggests that BRCA2-driven prostate cancer would initially respond to conventional androgen ablation. However, atypical cells persist in Brca2;Trp53 HG PIN lesions that continue to proliferate, indicating these lesions may be able to re-grow and become castration-resistant. Interestingly, some cells in Brca2;Trp53 mutant PIN lesions expressed AR at higher levels in the nucleus after castration, indicative of active AR signalling. Castration-resistant human prostate cancer growth commonly remains AR-dependent and is thought to occur through several mechanisms including AR amplification, AR mutation, changes in AR co-regulators and growth factor activation [43]. The presence of nuclear AR in castrated Brca2;Trp53 mutant prostates may indicate the regulation of proliferation by this factor after androgen depletion.
We often observed an increase in p63 positive basal cells, the presence of TA-like cells and an adjacent expansion of luminal cells in Brca2;Trp53 mutant HG PIN lesions. This suggests increased proliferation of the basal cell population, with maintenance of differentiation into luminal cells. Similarly, deletion of the tumour suppressor Pten with the PBCre4 transgene results in tumour formation with an increase in basal cells that contain a progenitor cell sub-population, the presence of TA-like cells and luminal cell differentiation [44]. The increase in basal progenitor cell population could represent an expansion of cancer-initiating cells, indicating that HG PIN lesions in Brca2;Trp53 mutant prostates may originate from these cells. Several other murine models of prostate cancer that utilize the PBCre4 transgene display increased and aberrant p63 expression during early stages of cancer progression [29], [30]. This transgene is expressed in both the basal and luminal cells of the mouse prostate [44]. In contrast, deletion of Pten using a PSA-driven Cre only expressed in luminal cells results in cancer without an expansion in p63 cells [45]. However, this model has slower kinetics than the Pten; PBCre4 model and tumours are initiated from cells in the luminal epithelial compartment. Frequently during human prostate tumour progression there is an increase in TA/progenitor cells, which has led to the proposal that these cells could be tumour-initiating cells [36], [44], [46]. Taken together, these data suggest that an increase in the basal progenitor cells could be a common early event in prostate neoplasia but may be dependent on the origin of the cancer-initiating cell.
Although we frequently observed HG PIN lesions in Brca2;Trp53 mutant prostates no invasive carcinoma formed. In contrast, deletion of Brca2 and Trp53 in the mouse mammary gland results in invasive carcinoma at 6 months, and consistent with this, human carriers of BRCA2 mutations have a high-risk of breast cancer. The lack of prostate carcinoma in our mutants may reflect the relatively low penetrance of prostate cancer in human BRCA2 mutation carriers and suggests there is only a subset of BRCA2 carriers that develop aggressive forms of the disease [13]–[16]. This subset may be dependent on additional genetic modifiers or environmental factors that influence the risk of individuals carrying a BRCA2 mutation forming prostate tumours [47]. It is possible that human carriers of deleterious BRCA2 mutations frequently form HG PIN lesions similar to our Brca2;Trp53 model but that never progress to carcinoma and therefore go undetected. Ongoing work into genetic modifiers of BRCA2 may identify which subgroups of patients with BRCA2 mutations are more at risk of developing aggressive forms of the disease.
Variations in genetic background can have a modifying effect on prostate tumour development in mice with tumour suppressor deletions [48]. Due to the complex nature of mouse breeding we were not able to investigate the effects of genetic background on the prostate lesions observed in Brca2;Trp53 mutant mice in this study. Although a change in genetic background may alter the frequency of tumour phenotype, we only observed PIN in mutant animals suggesting these lesions are an effect of Brca2 and Trp53 loss.
This murine study has demonstrated that deletion of the tumour suppressor Brca2 results in LG PIN, with the additional loss of a second tumour suppressor Trp53 leading to HG PIN. Other mouse models of tumour suppressor gene loss result in varying degrees of prostate tumour progression. Mice with prostate-specific homozygous Pten deletion progress to invasive carcinoma and metastasis [28]. The further loss of Trp53 in this model results in a shorter latency to invasive carcinoma [49]. Deletion of Nkx3.1, a gene involved in prostate epithelial cell differentiation, leads to mice that develop epithelial hyperplasia and dysplastic lesions that resemble human PIN, but do not progress to invasive carcinoma [50], [51].
Our pre-invasive model could be used in the future to test the response to potential therapeutic agents and combination therapies. For example, a recent synthetic lethal approach using PARP inhibitors has been used successfully to specifically induce cytotoxicity in HR-deficient cells [52]. Promising phase I clinical data in BRCA2 carriers with a PARP inhibitor has shown antitumour activity, including resolution of bone metastases in one patient with prostate cancer [53]. These Brca2 mutant mice may provide a useful model to examine cellular responses, such as apoptosis, to combinations of therapies for optimisation of treatment. In addition, this study demonstrates that Brca2 acts as a tumour suppressor and can interact genetically with Trp53 deficiency in the prostate preventing DNA damage accumulation and neoplasia progression.
Materials and Methods
Ethics statement
Animals were handled in strict accordance with UK Home Office regulations.
Generation of prostate-specific Brca2 and Trp53 deletion mice
Brca2F/F (targeting exon 11) mice and Trp53F/F (targeting exons 2–10) mice [25] and ARR2PBCre transgenic mice, PBCre4 [27], have been previously described. The animals were bred on a mixed genetic background.
Mouse prostate histology and statistical analysis
Histological phenotype of samples was assessed on haematoxylin and eosin stained sections. Serial sections were then stained for immunohistochemical analysis. Histological assessment was based on published guidelines and assisted by a pathologist [54], [55]. PIN lesions noted as LG were equivalent to PIN I-II and those noted as HG were equivalent to PIN III-IV in Park et al [54]. The two-sample Z-test was performed to test if there is a significant difference between groups of animals.
Quantification of proliferation and cell death
Ki-67 or TUNEL staining was performed by immunohistochemistry on sections and cells stained with nuclear brown DAB chromogen were counted as positive. Cells from at least 4 high power fields were counted per animal, which totalled more than 900 cells per animal. Five animals of each genotype were analysed. Randomly selected fields were counted for control analysis and sections corresponding to histologically identified areas of hyperplasia and PIN were counted for mutant animals. All values are significant with p<0.05 using Student t-test unless otherwise stated.
Immunohistochemistry analysis
Antibody stains were done on paraffin sections as previously described [56]. The following antibodies were used: Ki-67 (TEC-3, Dako, 1∶200 with amplification), AR (PG-21, Upstate, 1∶250 with amplification), p63 (4A4, Santa Cruz Biotechnology, 1∶200 with amplification, 1∶50), γH2AX (JBW301, Upstate, 1∶800 with amplification), CK5 (PRB-160P, Covance, 1∶50), CK8 (MMS-162P, Covance, 1∶200). The ABC elite vector kit was used for amplification using biotinlyated secondary antibodies (Vector Laboratories) and the DAB substrate (Dako). Secondary fluorescent antibodies were obtained from Molecular Probes and were used at a 1∶1000 dilution. TUNEL analysis was carried out using the ApopTag apoptosis detection kit (Chemicon).
Supporting Information
Zdroje
1. BostwickDG
LiuL
BrawerMK
QianJ
2004
High-grade prostatic intraepithelial neoplasia.
Rev Urol
6
171
179
2. EelesRA
1999
Genetic predisposition to prostate cancer.
Prostate Cancer Prostatic Dis
2
9
15
3. OstranderEA
StanfordJL
2000
Genetics of prostate cancer: too many loci, too few genes.
Am J Hum Genet
67
1367
1375
4. AndersonDE
BadziochMD
1993
Familial effects of prostate and other cancers on lifetime breast cancer risk.
Breast Cancer Res Treat
28
107
113
5. ThiessenEU
1974
Concerning a familial association between breast cancer and both prostatic and uterine malignancies.
Cancer
34
1102
1107
6. TuliniusH
EgilssonV
OlafsdottirGH
SigvaldasonH
1992
Risk of prostate, ovarian, and endometrial cancer among relatives of women with breast cancer.
BMJ
305
855
857
7. 1999
Cancer risks in BRCA2 mutation carriers.The Breast Cancer Linkage Consortium.
J Natl Cancer Inst
91
1310
1316
8. JohannssonO
LomanN
MollerT
KristofferssonU
BorgA
1999
Incidence of malignant tumours in relatives of BRCA1 and BRCA2 germline mutation carriers.
Eur J Cancer
35
1248
1257
9. TuliniusH
OlafsdottirGH
SigvaldasonH
ArasonA
BarkardottirRB
2002
The effect of a single BRCA2 mutation on cancer in Iceland.
J Med Genet
39
457
462
10. van AsperenCJ
BrohetRM
Meijers-HeijboerEJ
HoogerbruggeN
VerhoefS
2005
Cancer risks in BRCA2 families: estimates for sites other than breast and ovary.
J Med Genet
42
711
719
11. AgalliuI
KarlinsE
KwonEM
IwasakiLM
DiamondA
2007
Rare germline mutations in the BRCA2 gene are associated with early-onset prostate cancer.
Br J Cancer
97
826
831
12. EdwardsSM
Kote-JaraiZ
MeitzJ
HamoudiR
HopeQ
2003
Two percent of men with early-onset prostate cancer harbor germline mutations in the BRCA2 gene.
Am J Hum Genet
72
1
12
13. AgalliuI
GernR
LeanzaS
BurkRD
2009
Associations of high-grade prostate cancer with BRCA1 and BRCA2 founder mutations.
Clin Cancer Res
15
1112
1120
14. MitraA
FisherC
FosterCS
JamesonC
BarbachannoY
2008
Prostate cancer in male BRCA1 and BRCA2 mutation carriers has a more aggressive phenotype.
Br J Cancer
98
502
507
15. NarodSA
NeuhausenS
VichodezG
ArmelS
LynchHT
2008
Rapid progression of prostate cancer in men with a BRCA2 mutation.
Br J Cancer
99
371
374
16. TryggvadottirL
VidarsdottirL
ThorgeirssonT
JonassonJG
OlafsdottirEJ
2007
Prostate cancer progression and survival in BRCA2 mutation carriers.
J Natl Cancer Inst
99
929
935
17. GudmundsdottirK
AshworthA
2006
The roles of BRCA1 and BRCA2 and associated proteins in the maintenance of genomic stability.
Oncogene
25
5864
5874
18. SharanSK
MorimatsuM
AlbrechtU
LimDS
RegelE
1997
Embryonic lethality and radiation hypersensitivity mediated by Rad51 in mice lacking Brca2.
Nature
386
804
810
19. TuttA
BertwistleD
ValentineJ
GabrielA
SwiftS
2001
Mutation in Brca2 stimulates error-prone homology-directed repair of DNA double-strand breaks occurring between repeated sequences.
EMBO J
20
4704
4716
20. Kraakman-van der ZwetM
OverkampWJ
van LangeRE
EssersJ
van Duijn-GoedhartA
2002
Brca2 (XRCC11) deficiency results in radioresistant DNA synthesis and a higher frequency of spontaneous deletions.
Mol Cell Biol
22
669
679
21. PatelKJ
YuVP
LeeH
CorcoranA
ThistlethwaiteFC
1998
Involvement of Brca2 in DNA repair.
Mol Cell
1
347
357
22. LudwigT
ChapmanDL
PapaioannouVE
EfstratiadisA
1997
Targeted mutations of breast cancer susceptibility gene homologs in mice: lethal phenotypes of Brca1, Brca2, Brca1/Brca2, Brca1/p53, and Brca2/p53 nullizygous embryos.
Genes Dev
11
1226
1241
23. SuzukiA
de la PompaJL
HakemR
EliaA
YoshidaR
1997
Brca2 is required for embryonic cellular proliferation in the mouse.
Genes Dev
11
1242
1252
24. CheungAM
EliaA
TsaoMS
DoneS
WagnerKU
2004
Brca2 deficiency does not impair mammary epithelium development but promotes mammary adenocarcinoma formation in p53(+/-) mutant mice.
Cancer Res
64
1959
1965
25. JonkersJ
MeuwissenR
van der GuldenH
PeterseH
van der ValkM
2001
Synergistic tumor suppressor activity of BRCA2 and p53 in a conditional mouse model for breast cancer.
Nat Genet
29
418
425
26. LudwigT
FisherP
MurtyV
EfstratiadisA
2001
Development of mammary adenocarcinomas by tissue-specific knockout of Brca2 in mice.
Oncogene
20
3937
3948
27. WuX
WuJ
HuangJ
PowellWC
ZhangJ
2001
Generation of a prostate epithelial cell-specific Cre transgenic mouse model for tissue-specific gene ablation.
Mech Dev
101
61
69
28. WangS
GaoJ
LeiQ
RozengurtN
PritchardC
2003
Prostate-specific deletion of the murine Pten tumor suppressor gene leads to metastatic prostate cancer.
Cancer Cell
4
209
221
29. BruxvoortKJ
CharbonneauHM
GiambernardiTA
GoolsbyJC
QianCN
2007
Inactivation of Apc in the mouse prostate causes prostate carcinoma.
Cancer Res
67
2490
2496
30. PearsonHB
PhesseTJ
ClarkeAR
2009
K-ras and Wnt signaling synergize to accelerate prostate tumorigenesis in the mouse.
Cancer Res
69
94
101
31. HayT
PatrickT
WintonD
SansomOJ
ClarkeAR
2005
Brca2 deficiency in the murine small intestine sensitizes to p53-dependent apoptosis and leads to the spontaneous deletion of stem cells.
Oncogene
24
3842
3846
32. FrappartPO
LeeY
LamontJ
McKinnonPJ
2007
BRCA2 is required for neurogenesis and suppression of medulloblastoma.
EMBO J
26
2732
2742
33. RogakouEP
PilchDR
OrrAH
IvanovaVS
BonnerWM
1998
DNA double-stranded breaks induce histone H2AX phosphorylation on serine 139.
J Biol Chem
273
5858
5868
34. BerneyDM
GopalanA
KudahettiS
FisherG
AmbroisineL
2009
Ki-67 and outcome in clinically localised prostate cancer: analysis of conservatively treated prostate cancer patients from the Trans-Atlantic Prostate Group study.
Br J Cancer
100
888
893
35. ScherHI
SawyersCL
2005
Biology of progressive, castration-resistant prostate cancer: directed therapies targeting the androgen-receptor signaling axis.
J Clin Oncol
23
8253
8261
36. HudsonDL
2004
Epithelial stem cells in human prostate growth and disease.
Prostate Cancer Prostatic Dis
7
188
194
37. HallstromTM
LaihoM
2008
Genetic changes and DNA damage responses in the prostate.
Prostate
68
902
918
38. VogelsteinB
LaneD
LevineAJ
2000
Surfing the p53 network.
Nature
408
307
310
39. MitraA
JamesonC
BarbachanoY
SodhaZ
Kote-JaraiN
2009
Overexpression of TP53 is Associated with Aggressive Prostate Cancer but does not Distinguish Disease in BRCA1 or BRCA2 Mutation Carriers from a Control Group.
The Open Prostate Cancer Journal
2
38
45
40. HolstegeH
JoosseSA
van OostromCT
NederlofPM
de VriesA
2009
High incidence of protein-truncating TP53 mutations in BRCA1-related breast cancer.
Cancer Res
69
3625
3633
41. CheungAM
HandeMP
JalaliF
TsaoMS
SkinniderB
2002
Loss of Brca2 and p53 synergistically promotes genomic instability and deregulation of T-cell apoptosis.
Cancer Res
62
6194
6204
42. NorburyCJ
ZhivotovskyB
2004
DNA damage-induced apoptosis.
Oncogene
23
2797
2808
43. HeinleinCA
ChangC
2004
Androgen receptor in prostate cancer.
Endocr Rev
25
276
308
44. WangS
GarciaAJ
WuM
LawsonDA
WitteON
2006
Pten deletion leads to the expansion of a prostatic stem/progenitor cell subpopulation and tumor initiation.
Proc Natl Acad Sci U S A
103
1480
1485
45. KorstenH
Ziel-van der MadeA
MaX
van der KwastT
TrapmanJ
2009
Accumulating progenitor cells in the luminal epithelial cell layer are candidate tumor initiating cells in a Pten knockout mouse prostate cancer model.
PLoS ONE
4
e5662
doi:10.1371/journal.pone.0005662
46. SchalkenJA
van LeendersG
2003
Cellular and molecular biology of the prostate: stem cell biology.
Urology
62
11
20
47. IsaacsW
De MarzoA
NelsonWG
2002
Focus on prostate cancer.
Cancer Cell
2
113
116
48. FreemanD
LescheR
KerteszN
WangS
LiG
2006
Genetic background controls tumor development in PTEN-deficient mice.
Cancer Res
66
6492
6496
49. ChenZ
TrotmanLC
ShafferD
LinHK
DotanZA
2005
Crucial role of p53-dependent cellular senescence in suppression of Pten-deficient tumorigenesis.
Nature
436
725
730
50. KimMJ
Bhatia-GaurR
Banach-PetroskyWA
DesaiN
WangY
2002
Nkx3.1 mutant mice recapitulate early stages of prostate carcinogenesis.
Cancer Res
62
2999
3004
51. AbdulkadirSA
MageeJA
PetersTJ
KaleemZ
NaughtonCK
2002
Conditional loss of Nkx3.1 in adult mice induces prostatic intraepithelial neoplasia.
Mol Cell Biol
22
1495
1503
52. FarmerH
McCabeN
LordCJ
TuttAN
JohnsonDA
2005
Targeting the DNA repair defect in BRCA mutant cells as a therapeutic strategy.
Nature
434
917
921
53. FongPC
BossDS
YapTA
TuttA
WuP
2009
Inhibition of poly(ADP-ribose) polymerase in tumors from BRCA mutation carriers.
N Engl J Med
361
123
134
54. ParkJH
WallsJE
GalvezJJ
KimM
Abate-ShenC
2002
Prostatic intraepithelial neoplasia in genetically engineered mice.
Am J Pathol
161
727
735
55. ShappellSB
ThomasGV
RobertsRL
HerbertR
IttmannMM
2004
Prostate pathology of genetically engineered mice: definitions and classification. The consensus report from the Bar Harbor meeting of the Mouse Models of Human Cancer Consortium Prostate Pathology Committee.
Cancer Res
64
2270
2305
56. ThomsenMK
ButlerCM
ShenMM
SwainA
2008
Sox9 is required for prostate development.
Dev Biol
316
302
311
Štítky
Genetika Reprodukční medicína
Článek The Met 470 Allele Is Associated with Lower Birth Rates in Fertile Men from a Population IsolateČlánek Copy Number Variation and Transposable Elements Feature in Recent, Ongoing Adaptation at the Locus
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2010 Číslo 6- Akutní intermitentní porfyrie
- Růst a vývoj dětí narozených pomocí IVF
- Vliv melatoninu a cirkadiálního rytmu na ženskou reprodukci
- Délka menstruačního cyklu jako marker ženské plodnosti
- Intrauterinní inseminace a její úspěšnost
-
Všechny články tohoto čísla
- Translational Selection Is Ubiquitous in Prokaryotes
- Whole-Genome Sequencing of a Single Proband Together with Linkage Analysis Identifies a Mendelian Disease Gene
- Web-Based, Participant-Driven Studies Yield Novel Genetic Associations for Common Traits
- The Met 470 Allele Is Associated with Lower Birth Rates in Fertile Men from a Population Isolate
- Contributions of Status and Allele Expression, But Not Copy Number Variation, to the Control of SIVmac251 Replication in Indian-Origin Rhesus Monkeys
- A Genome-Wide Association Study of Optic Disc Parameters
- Amplification of a Cytochrome P450 Gene Is Associated with Resistance to Neonicotinoid Insecticides in the Aphid
- The Transcription Factor REST Is Lost in Aggressive Breast Cancer
- 14-3-3 Mediates Histone Cross-Talk during Transcription Elongation in
- Copy Number Variation and Transposable Elements Feature in Recent, Ongoing Adaptation at the Locus
- Use of Genome-Wide Expression Data to Mine the “Gray Zone” of GWA Studies Leads to Novel Candidate Obesity Genes
- Genome-Wide RNAi Screen Identifies Multiple Regulators of HIF–Dependent Transcription in Hypoxia
- The CYCLIN-A CYCA1;2/TAM Is Required for the Meiosis I to Meiosis II Transition and Cooperates with OSD1 for the Prophase to First Meiotic Division Transition
- Inactivation of hnRNP K by Expanded Intronic AUUCU Repeat Induces Apoptosis Via Translocation of PKCδ to Mitochondria in Spinocerebellar Ataxia 10
- Mice with Alopecia, Osteoporosis, and Systemic Amyloidosis Due to Mutation in , a Gene Coding for Palmitoyl Acyltransferase
- siRNA–Mediated Methylation of Telomeres
- Chromosome 4 Replicates in Two Phases That Correlate with Chromatin State
- Dynamic Switch of Negative Feedback Regulation in Akt–TOR Signaling
- On the Use of Variance per Genotype as a Tool to Identify Quantitative Trait Interaction Effects: A Report from the Women's Genome Health Study
- and Deficiency Cooperate in the Progression of Mouse Prostate Tumourigenesis
- An Integration of Genome-Wide Association Study and Gene Expression Profiling to Prioritize the Discovery of Novel Susceptibility Loci for Osteoporosis-Related Traits
- Consent and Internet-Enabled Human Genomics
- Understanding Adaptation in Large Populations
- Identification of a Functional Genetic Variant at 16q12.1 for Breast Cancer Risk: Results from the Asia Breast Cancer Consortium
- Evidence that Adaptation in Is Not Limited by Mutation at Single Sites
- The IG-DMR and the -DMR at Human Chromosome 14q32.2: Hierarchical Interaction and Distinct Functional Properties as Imprinting Control Centers
- Cushing's Syndrome and Fetal Features Resurgence in Adrenal Cortex–Specific Knockout Mice
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Web-Based, Participant-Driven Studies Yield Novel Genetic Associations for Common Traits
- The IG-DMR and the -DMR at Human Chromosome 14q32.2: Hierarchical Interaction and Distinct Functional Properties as Imprinting Control Centers
- Amplification of a Cytochrome P450 Gene Is Associated with Resistance to Neonicotinoid Insecticides in the Aphid
- Copy Number Variation and Transposable Elements Feature in Recent, Ongoing Adaptation at the Locus
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



