-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Contributions of Status and Allele Expression, But Not Copy Number Variation, to the Control of SIVmac251 Replication in Indian-Origin Rhesus Monkeys
CCL3 is a ligand for the HIV-1 co-receptor CCR5. There have recently been conflicting reports in the literature concerning whether CCL3-like gene (CCL3L) copy number variation (CNV) is associated with resistance to HIV-1 acquisition and with both viral load and disease progression following infection with HIV-1. An association has also been reported between CCL3L CNV and clinical sequelae of the simian immunodeficiency virus (SIV) infection in vivo in rhesus monkeys. The present study was initiated to explore the possibility of an association of CCL3L CNV with the control of virus replication and AIDS progression in a carefully defined cohort of SIVmac251-infected, Indian-origin rhesus monkeys. Although we demonstrated extensive variation in copy number of CCL3L in this cohort of monkeys, CCL3L CNV was not significantly associated with either peak or set-point plasma SIV RNA levels in these monkeys when MHC class I allele Mamu-A*01 was included in the models or progression to AIDS in these monkeys. With 66 monkeys in the study, there was adequate power for these tests if the correlation of CCL3L and either peak or set-point plasma SIV RNA levels was 0.34 or 0.36, respectively. These findings call into question the premise that CCL3L CNV is important in HIV/SIV pathogenesis.
Published in the journal: . PLoS Genet 6(6): e32767. doi:10.1371/journal.pgen.1000997
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1000997Summary
CCL3 is a ligand for the HIV-1 co-receptor CCR5. There have recently been conflicting reports in the literature concerning whether CCL3-like gene (CCL3L) copy number variation (CNV) is associated with resistance to HIV-1 acquisition and with both viral load and disease progression following infection with HIV-1. An association has also been reported between CCL3L CNV and clinical sequelae of the simian immunodeficiency virus (SIV) infection in vivo in rhesus monkeys. The present study was initiated to explore the possibility of an association of CCL3L CNV with the control of virus replication and AIDS progression in a carefully defined cohort of SIVmac251-infected, Indian-origin rhesus monkeys. Although we demonstrated extensive variation in copy number of CCL3L in this cohort of monkeys, CCL3L CNV was not significantly associated with either peak or set-point plasma SIV RNA levels in these monkeys when MHC class I allele Mamu-A*01 was included in the models or progression to AIDS in these monkeys. With 66 monkeys in the study, there was adequate power for these tests if the correlation of CCL3L and either peak or set-point plasma SIV RNA levels was 0.34 or 0.36, respectively. These findings call into question the premise that CCL3L CNV is important in HIV/SIV pathogenesis.
Introduction
Host genetic factors influence susceptibility to HIV-1 replication and progression to AIDS. A number of genes in humans have been shown to impact the development of classical immune effector responses against HIV-1 infection and HIV-1 entry into cells. The genes associated with immune control of HIV-1 include selected MHC class I alleles, including HLA B*5701, HLA B*27 [1], HLA C alleles [2], and HLA Bw4-8OI in association with KIR3DS1 [3]. Expression of the delta 32 allelic form of the HIV-1 co-receptor CCR5 is associated with an inhibition of HIV-1 entry into cells [4]–[6].
CCL3, formerly known as macrophage inflammatory factor 1α (MIP-1α), a ligand for CCR5, is a chemokine with profound HIV-1 inhibitory activity [7]–[9]. The two functional genes coding for CCL3, CCL3 and CCL3-like 1 (CCL3L1), map to a narrow region on human chromosome 17. While CCL3 exists as two copies per diploid genome (pdg), CCL3L1 arose from CCL3 through segmental duplication, and the copy number of CCL3L1 varies among individuals as a consequence of unequal duplications [10], [11]. It has been suggested that more copies of a gene should translate into more protein expression, and higher CCL3L1 copy number has been correlated with enhanced CCL3 production [11]. Several groups reported that a low CCL3L1 copy number is associated with an increased risk of acquiring HIV-1 as well as both high viral loads and rapid progression to AIDS following infection [7], [9]. However, a recent series of reports show no effect of CCL3L1 CNV on HIV-1 infection, viral loads, or progression to AIDS following infection [12]–[14].
CNV at the CCL3L loci exists in both chimpanzees and rhesus monkeys [7], [15]. Indeed, a link between CCL3L CNV and AIDS susceptibility has recently been reported in rhesus monkeys infected with SIV [15]. The present study was initiated to explore the possibility of an association between CCL3L CNV and AIDS in rhesus monkeys.
Results
This study was done to assess the effect of CCL3L CNV on viral load and disease progression following SIVmac251 infection in Indian-origin rhesus monkeys. We first conducted experiments to standardize the technical approach for determining CCL3L copy number for this study. We assessed whether CCL3L copy number calculated from the CCL3L-derived signal in our qPCR assay systematically increased or decreased according to the quantity of input DNA as described by Urban et al. [12]. We evaluated the copy number of CCL3L using 18 ng of input DNA by the qPCR method described by Degenhardt et al. [15] and concurrently evaluated CCL3L copy number using either 9 ng or 36 ng of input DNA. We explored the reproducibility of this qPCR assay using duplicates for each of 69 DNA samples in each of two separate experiments and evaluating the percent difference (defined as the difference between CCL3L copy numbers in the duplicates divided by their average, called Vi) as described by Urban et al. [12]. Higher quantities of input DNA tended to be associated with larger ranges of Vi (Figure 1A); the averages of Vi over all 69 samples and the two experiments were 5.591 (9 ng), 9.681 (18 ng), and 7.725 (36 ng). Assessing the CCL3L copy number averaged over the two duplicates, the copy numbers obtained by the two separate experiments had greater Pearson correlations using 9 ng than either 18 ng or 36 ng of input DNA (Figure 1B for related regressions). Thus, the use of more concentrated DNA samples was associated with greater variability between replicates.
Fig. 1. Intra-experiment reproducibility and inter-experiment variability in CCL3L copy-number determination. 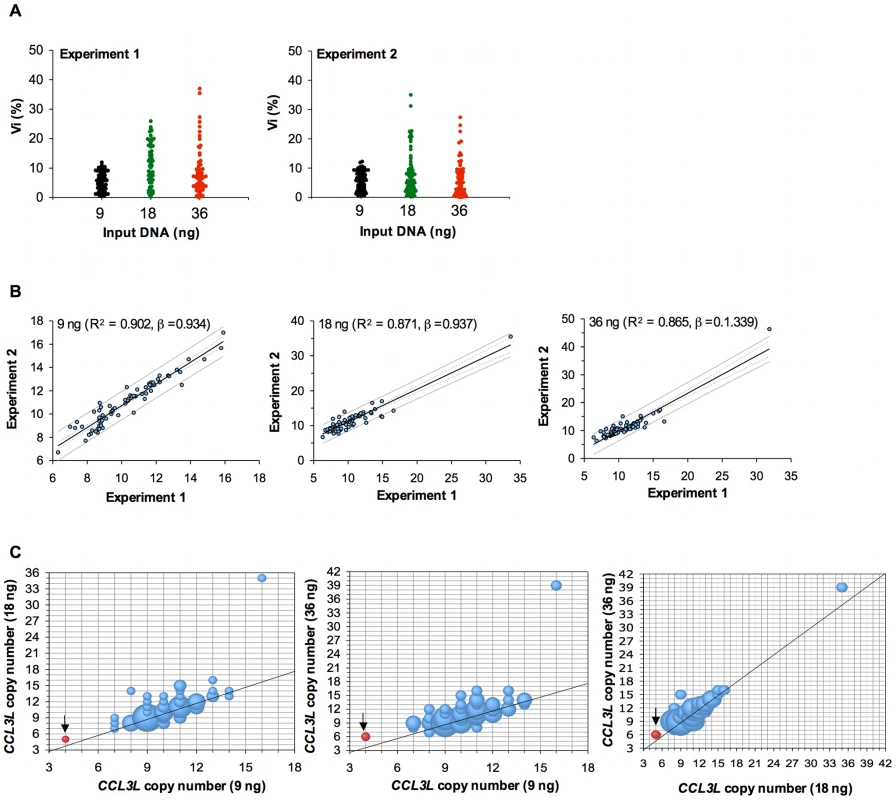
(A) Intra-experiment reproducibility as determined using duplicates for each DNA sample from 84 rhesus monkeys. The results of two separate experiments are shown. (B) Inter-experiment variability. CCL3L copy numbers were determined by real-time qPCR using different amounts of input DNA. Correlations between the mean of the unrounded CCL3L copy number estimates from two separate experiments are shown. (C) Bubble plots showing the concordance between rounded CCL3L copy number estimates determined using different amounts of input DNA. The precision of each assay was determined by the rounded CCL3L copy number estimate of from reference sample, the A431 human cell line (black arrow). We then looked at the average (of 4 numbers – duplicates from the two experiments) of the CCL3L copy numbers and regressed the experiments using 18 ng and 36 ng of input DNA on the experiment using 9 ng of input DNA. We found that 18 ng of input DNA underestimated in 14 (20.3%) and overestimated in 20 (29.0%) samples, and also found that 36 ng of input DNA underestimated in 19 (27.5%), and overestimated in 28 (40.6%) samples (Figure 1C). Therefore, we only used determinations of CCL3L copy number generated from DNA samples diluted to 9 ng in this study.
Then, to determine the accuracy of our qPCR assay, we assessed the copy number of CCL3L in the reference A431 human cell line. The A431 cell line has previously been shown to have two copies of CCL3L1 and two copies of CCL3 pdg, for a total copy number of four CCL3L [11]. We found that the A431 human cell line had 4 CCL3L copies when 9 ng of input DNA was assessed. However, CCL3L copy number appeared to be higher than 4 when 18 or 36 ng of input DNA input were assayed (Figure 1C).
Since we have previously shown that Indian-origin and Chinese-origin rhesus monkeys differ substantially in their control of SIVmac251 replication [16], we reasoned that studying genetic determinants of SIVmac251 control in a combined population of both Indian-origin and Chinese-origin rhesus monkeys might introduce a bias into the results. We therefore chose to pursue the present study to evaluate the association of CCL3L CNV with the control of SIV replication and AIDS progression in a cohort of SIVmac251-infected Indian-origin rhesus monkeys.
The expression of particular MHC class I alleles in Indian-origin rhesus monkeys is associated with the efficient control of SIV replication and the rate of disease progression following SIV infection [17]–[19]. We also recently demonstrated a central role of TRIM5 polymorphism in limiting the replication of SIVmac251 in a primate host [20]. Although MHC class I, TRIM5 and CCL3L are located on different chromosomes, it is possible that the alleles of these genes are correlated with each other. If that were the case, a CCL3L copy number effect could be confounded or masked by either MHC class I or TRIM5 alleles associated with SIV control. In order to address this issue, we included the expression of both the MHC class I allele Mamu-A*01 and specific TRIM5 alleles in our analysis to assess the effect of CCL3L CNV on the control of SIVmac251 replication in Indian-origin rhesus monkeys.
We evaluated CCL3L CNV in 84 Indian-origin rhesus monkeys. The distribution of CCL3L copy number in this cohort of rhesus monkeys is shown in Figure 2A. We observed extensive variation in CCL3L copy number in these animals, with a range of 7 to 19 copies pdg (median 10.539). Data are displayed three ways in Figure 2A: combining all monkeys, dividing the monkeys into 2 cohorts, one Mamu-A*01− and the other Mamu-A*01+, and dividing the monkeys into 2 cohorts, one of animals expressing only TRIM5 alleles 1–5 and the other of animals expressing at least 1 TRIM5 allele of the groups 6–11 as previously described [20]. CCL3L copy number did not appear to be equally distributed in subgroups defined by Mamu-A*01 and by TRIM5. Animals with high CCL3L copy number were overpresented among the Mamu-A*01+ monkeys (shift to the right in Figure 2A). Importantly, we found that Mamu-A*01+ rhesus monkeys possessed a significantly greater number of CCL3L gene copies than the Mamu-A*01 - rhesus monkeys (Mann-Whitney P = 0.003). However, the expression of particular TRIM5 alleles was not significantly associated with the CCL3L copy number in the same cohort of rhesus monkeys (Figure 2B).
Fig. 2. Distribution of CCL3L copy number in Indian-origin rhesus monkeys. 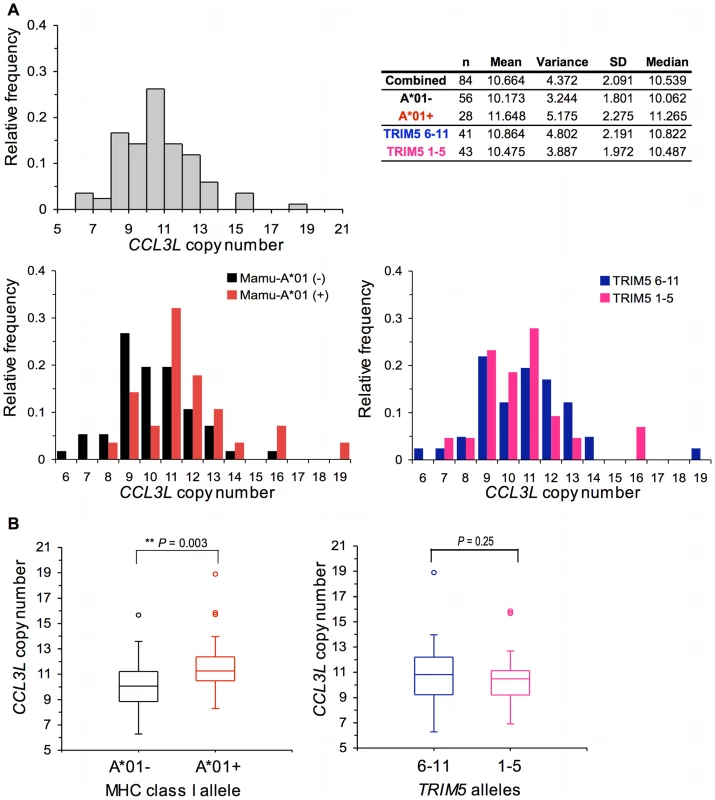
Copy numbers of CCL3L genes were estimated using real-time PCR in 84 Indian-origin rhesus monkeys. (A) Frequency distribution of CCL3L copy number. Data are displayed three ways; combining all monkeys, dividing the monkeys into 2 cohorts: one Mamu-A*01− and the other Mamu-A*01+, or dividing the monkeys into 2 cohorts: one of animlas expressing only TRIM5 alleles 1–5 and the other of animals expressing at least 1 TRIM5 allele of the groups 6–11. The mean, variance, standard deviation (SD) and median of the copy number are shown. (B) Boxplots of CCL3L copy number in Mamu-A*01− and Mamu-A*01+ animals, and, in one of animlas expressing only TRIM5 alleles 1–5 and the other of animals expressing at least 1 TRIM5 allele of the groups 6–11. The comparisons were analyzed using the Mann-Whitney U test (two-tailed). Using all of these findings to inform our experimental approach, we evaluated associations of CCL3L CNV with the in vivo control of SIVmac251 replication during the period of acute infection in a cohort of Indian-origin rhesus monkeys following intravenous infection with SIVmac251. Plasma virus RNA levels were assessed on day 14, at the time of peak virus replication, and on day 70, at the time of set-point virus replication following SIVmac251 infection. The peak and set-point plasma SIV RNA levels have been shown to be predictors of disease progression in SIVmac251-infected monkeys [21], [22].
To evaluate the effects of CCL3L copy number (used as a continuous variable), Mamu-A*0l, and TRIM5 alleles (both evaluated as binary variables) separately, we first used single covariate linear regressions of log plasma SIV RNA levels at both peak and set-point. When considered individually, we found that each of the three variables were associated with log peak plasma SIV RNA levels, but Mamu-A*01 was by far the most significant (P<0.0001 vs. 0.04 for each of the other two covariates; Table 1). In the regressions for log set-point plasma SIV RNA levels, the expression of specific TRIM5 alleles was the most significant single covariate (P = 0.001) and Mamu-A*01 was significant (P = 0.04) but CCL3L copy number was not significant (P = 0.53).
Tab. 1. Regressions with single covariates. 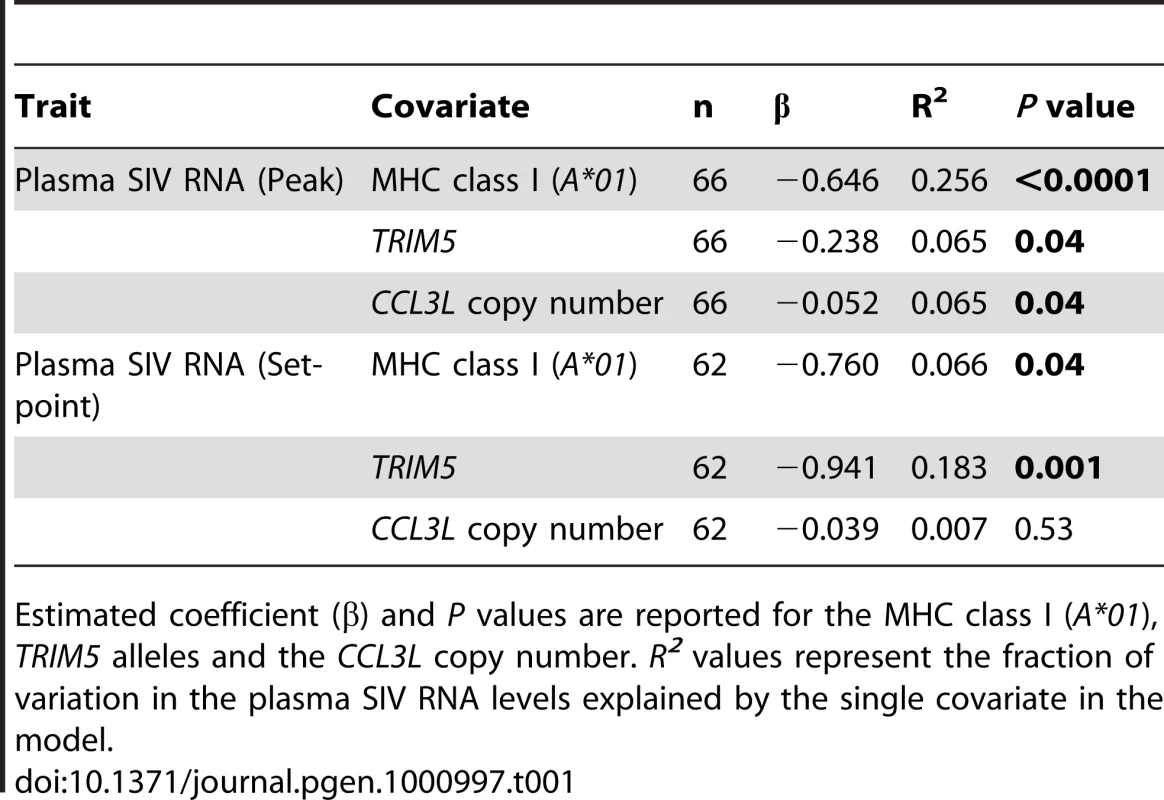
Estimated coefficient (β) and P values are reported for the MHC class I (A*01), TRIM5 alleles and the CCL3L copy number. R2 values represent the fraction of variation in the plasma SIV RNA levels explained by the single covariate in the model. When considering several covariates together in the linear regressions, the effect of CCL3L copy number was not significantly associated with log peak plasma SIV RNA levels in any model containing Mamu-A*01 (Table 2). CCL3L copy number was significant (P = 0.02) in the model containing only TRIM5 alleles, but that model had a much smaller R2 than any model involving Mamu-A*01. The effect of CCL3L copy number was not significant in any of the models for log set-point plasma SIV RNA levels (with any combination of the other two covariates, Table 2).
Tab. 2. Effect of CCL3L copy number in regressions with several covariates. 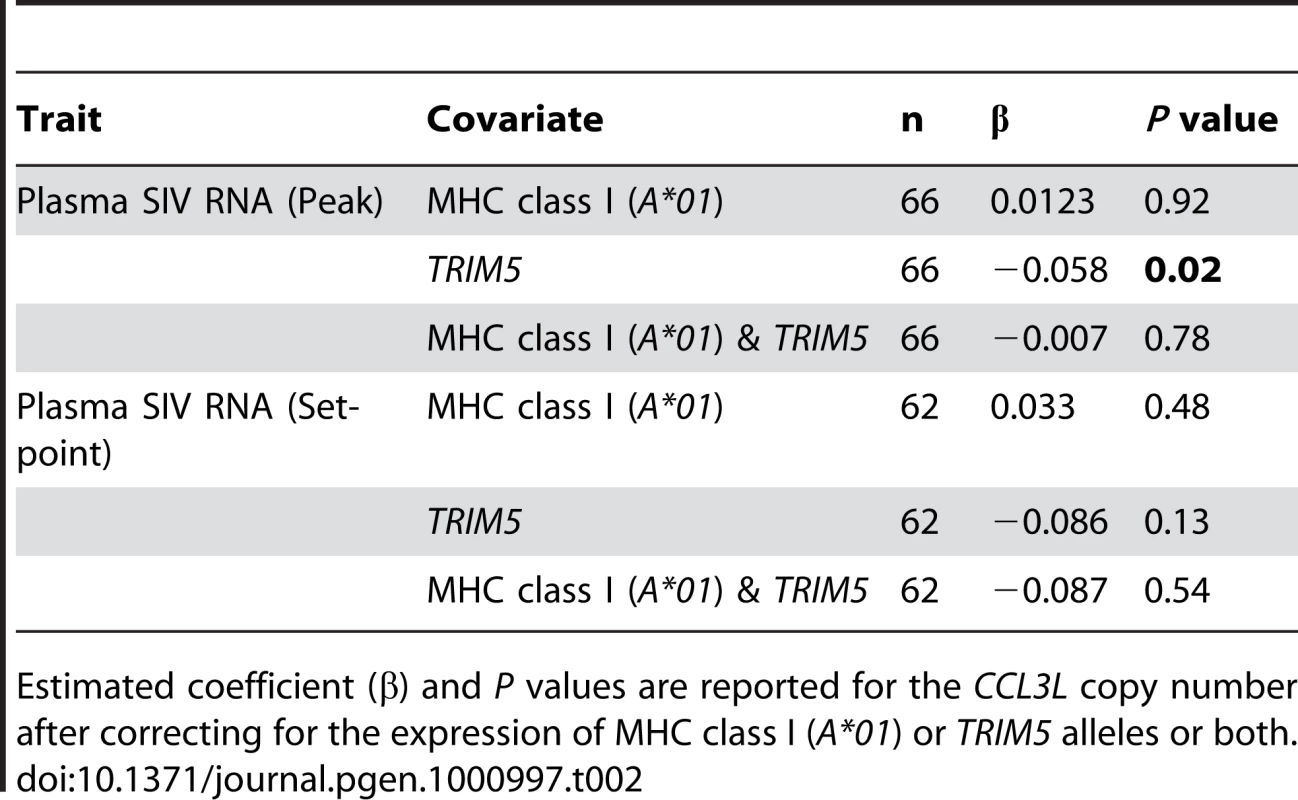
Estimated coefficient (β) and P values are reported for the CCL3L copy number after correcting for the expression of MHC class I (A*01) or TRIM5 alleles or both. This analysis suggests that CCL3L copy number could simply serve as a surrogate for the expression of the MHC class I allele Mamu-A*01 and the early virus control associated with high or low CCL3L copy number might be consequence of that MHC class I allele. Therefore, our results show that it is important to control for MHC class I alleles or other factors that might be associated with CCL3L copy number in the study.
For power calculations, we considered the residual (Res) log plasma SIV RNA levels when Mamu-A*01 was in the model (i.e., for each animal, the difference between the observed viral load and that predicted by Mamu-A*01). For the peak viral load regressions, with 66 animals there is 80% power to detect as significant the contribution of CCL3L to the model if the true correlation between Res and CCL3L is at least 0.34. (For comparison, the observed value of the Pearson correlation was 0.01). For the set-point viral load regressions, there is 80% power to detect as significant the contribution of CCL3L copy number to the model if the true correlation between Res and CCL3L copy number is at least 0.36 (the observed value of the Pearson correlation was 0.13).
We also divided the rhesus monkeys used in this study into four separate groups according to the expression of Mamu-A*01 and TRIM5 alleles by the monkeys as shown in Figure 3. There was a significant difference in CCL3L copy number between these 4 groups (Kruskal-Wallis P = 0.0017), primarily between Mamu-A*01+ and Mamu-A*01−. The effect of CCL3L copy number was not significant (using a linear regression) for either log peak plasma SIV RNA levels or log set-point plasma SIV RNA levels for most of these subgroups. Only for the group of monkeys that expressed Mamu-A*01 and one of TRIM5 alleles from the group of allele 6–11 was the CCL3L copy number coefficient significant, but the number of monkeys in that group was very small (n = 3) (Table 3).
Fig. 3. Distribution of CCL3L copy number in a cohort of Indian-rhesus monkeys. 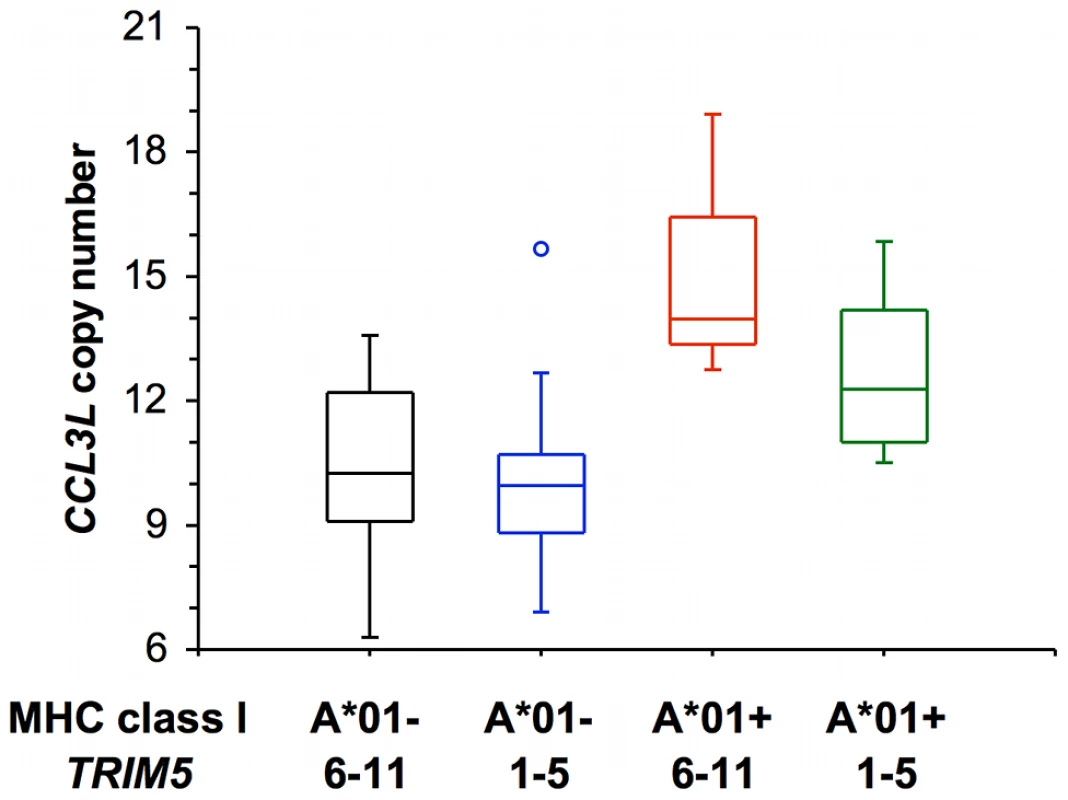
Rhesus monkeys are divided into 4 groups according to their expression of Mamu-A*01 allele (A*01− and A*01+) and selected TRIM5 alleles (TRIM5 1–5: one expressing only TRIM5 alleles 1–5, TRIM5 6–11: the other expressing only TRIM5 alleles 6–11). Tab. 3. Comparison of the regression coefficient (β) values and the significance of CCL3L copy number from rhesus monkeys expressing various combinations of both Mamu-A*01 and TRIM5 alleles. 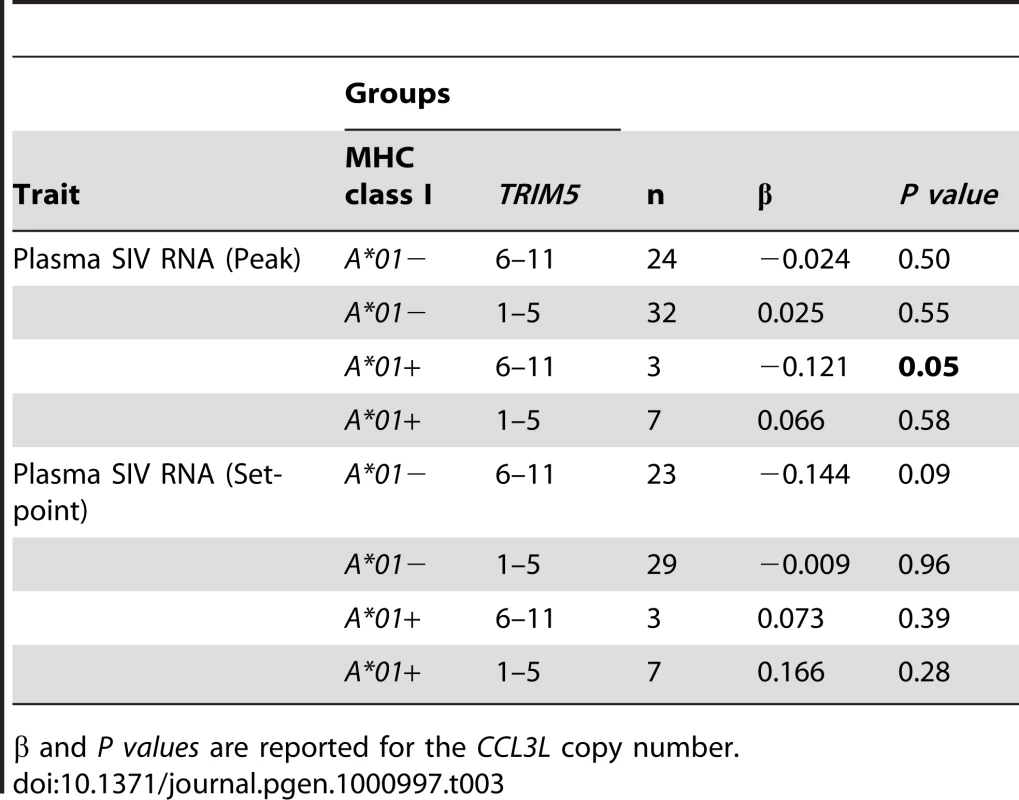
β and P values are reported for the CCL3L copy number. We assessed whether the quantitation of the copy number of CCL3L using a primer/probe set that does not differentiate between CCL3 and CCL3L gene paralogs as described by Degenhardt et al. could be subject to systematic biases. To address this possibility, we designed an alternative primer set and probe specific for CCL3-like gene 1 (CCL3L1) using the known rhesus monkey genome sequence (NCBI Reference Sequence: NW_001160084) and found that this primer set and probe are unique for CCL3L1 by “blastn” analysis (http://www.ncbi.nlm.nih.gov/BLAST/). We again observed extensive variation in CCL3L1 copy number in a cohort of animals, with a range of 3 to 13 copies pdg (median 8.04) (Supplementary Figure 3 in Text S1). Consistent with the results of CCL3L1 CNV, these results showed that there was a significant difference (Mann-Whitney P = 0.04) in the CCL3L1 copy numbers between Mamu-A*01 − and Mamu-A*01+ rhesus monkeys. However, the association between CCL3L1 copy number and either log peak or log set-point plasma SIV RNA levels was not significant in linear regression analysis (Supplementary Figure 4 in Text S1).
Fig. 4. Lack of association of CCL3L CNV with virus replication in Indian-origin rhesus monkeys following SIVmac251 infection. 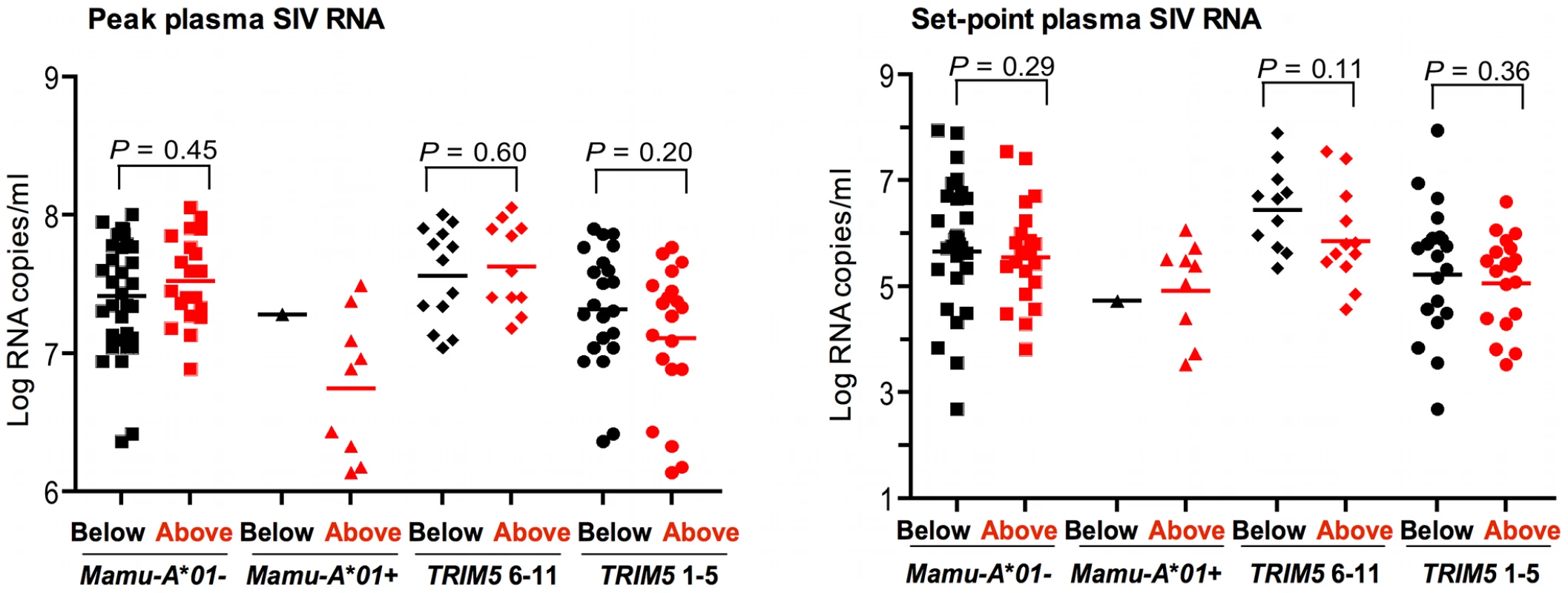
The plasma SIV RNA levels were assessed on days 14 and 70 following challenge, representing peak and set-point plasma SIV RNA levels, respectively. The monkeys were divided into 2 groups, one having CCL3L copy numbers below the median in this cohort of monkeys (black), and the other having CCL3L copy numbers above the median (red). These two groups of rhesus monkeys were subdivided into four separate groups according to their expression of Mamu-A*01 (− or +) and TRIM5 alleles (6–11 or 1–5). The association of CCL3L groups with plasma SIV RNA levels was assessed. The comparisons were analyzed using the Mann-Whitney U test (two-tailed). We also divided the monkeys into 2 groups according to whether the monkey's CCL3L copy number was “below” and “above” the median copy number (10.54) for the entire population of 66 monkeys. To be sure that this comparison was not affected by the expression of Mamu-A*01 or TRIM5 alleles in the two CCL3L groups, we divided each group of monkeys into four separate groups according to their expression of Mamu-A*01 ( − or +) and TRIM5 alleles (6–11 or 1–5) as shown in Figure 4. In this analysis we again found no evidence for an association of CCL3L copy number with either peak or set-point plasma SIV RNA levels (Figure 4).
Finally, we assessed whether CCL3L copy number groups (Below, < median of 10.54, or Above, ≥ median) are associated with the percent of monkeys dying by day 235 (the follow-up time was chosen ahead of time; after 235 days some monkeys were euthanized and some were used in other experiments) in 45 monkeys from 7 experiments. These percents were compared by Fisher exact test using each covariate (Mamu-A*01, TRIM5 alleles and CCL3L) separately. CCL3L groups were not significant (P = 0.33); however, Mamu-A*01 − status was associated with significantly more frequent death (P = 0.04) as was TRIM5 alleles 6–11 (P = 0.005 (Table 4). To be sure that the CCL3L comparison was not affected by an imbalance of Mamu-A*01 − status or TRIM5 alleles 6–11 in the two CCL3L groups, we also did an exact stratified Fisher test for CCL3L groups, stratified separately on Mamu-A*01 − status (P = 0.99) and on TRIM5 alleles (P = 0.83). There were too few death (especially among Mamu-A*01+ monkeys) to stratify on both Mamu-A*01 and on TRIM5 alleles (Table 5).
Tab. 4. Analysis of the survival status on day 235 following SIVmac251 infection. 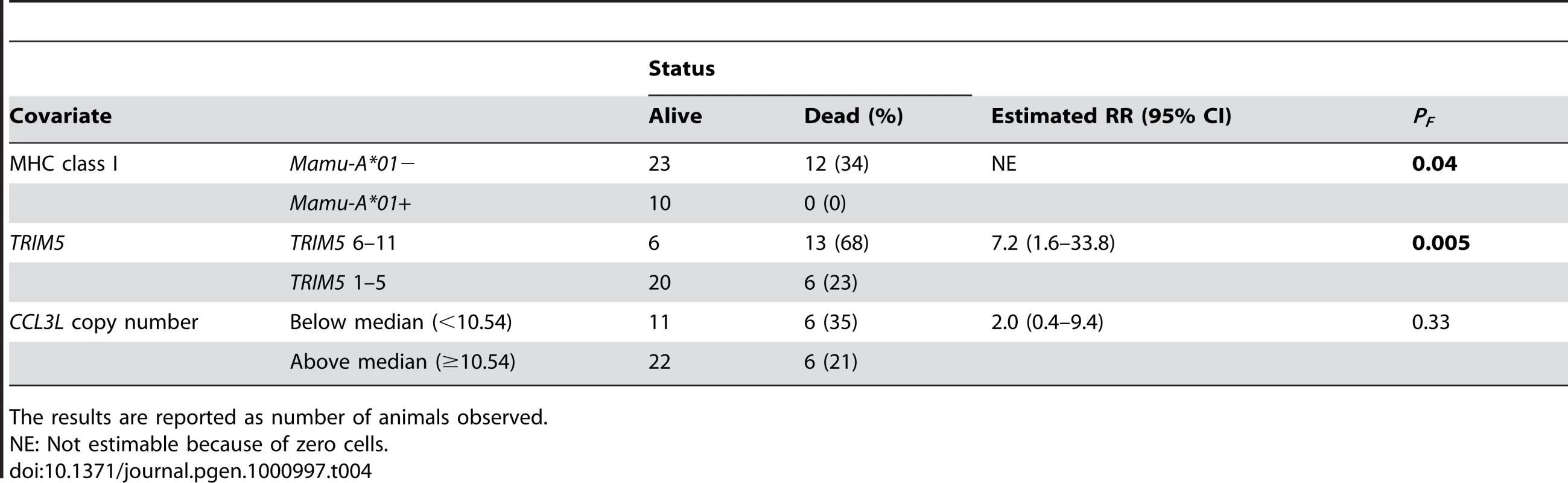
The results are reported as number of animals observed. Tab. 5. Test of <i>CCL3L</i> copy number group stratified on other covariates. 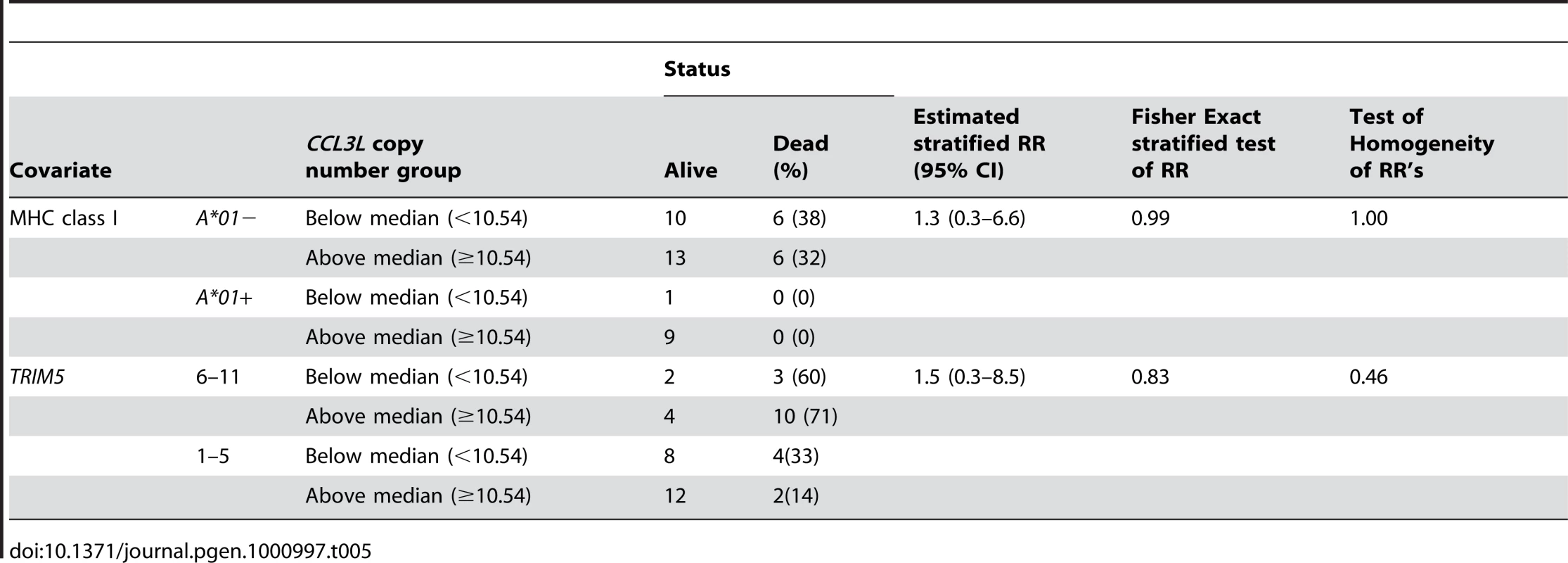
Discussion
We demonstrated extensive variation in CCL3L copy number in a cohort of SIVmac251-infected Indian-origin rhesus monkeys using real-time qPCR. However, we also showed that CCL3L copy number co-stratified in this cohort of monkeys with Mamu-A*01, an MHC class I allele associated with SIVmac251 control. Thus, Mamu-A*01+ rhesus monkeys possess a significantly greater number of CCL3L gene copies than Mamu-A*01 − rhesus monkeys. These findings suggest that CCL3L copy number might simply serve as a surrogate for the Mamu-A*01 allele and the association of control of SIV/AIDS pathogenesis associated with high or low CCL3L copy number reported by Degenhardt et al. might actually be a consequence of the expression of that MHC class I allele.
Degenhardt et al. also suggested that CCL3L CNV underlies not only a significant inter-individual variation in the rate of progression to AIDS in rhesus monkeys, but also the differences in the pathogenicity of SIVmac251 between Indian-origin and Chinese-origin rhesus monkeys. Indian-origin and Chinese-origin rhesus monkeys certainly differ substantially in their control of SIVmac251 replication [20], [23]. However, these differences in control may be a consequence of other genetic factors. We therefore chose to evaluate the association of CCL3L CNV with the control of SIV replication and AIDS progression in a cohort of SIVmac251-infected Indian-origin rhesus monkeys. Exploring a possible association of CCL3L CNV with SIVmac251 control in such a defined cohort of rhesus monkeys would eliminate a bias that might be introduced into the results by the diverse genetic factors that may exist in a combined population of rhesus monkey subspecies.
There are a number of potential caveats associated with the findings in the present study. Although more copies of the CCL3L gene should translate into a higher level of CCL3L expression, we did not explore whether CCL3L copy number influences CCL3L mRNA expression in peripheral blood mononuclear cells (PBMCs) isolated from these experimental animals. Moreover, we only evaluated an association of CCL3L CNV with the clinical consequences of infection by one SIV isolate (SIVmac251). Since other SIV isolates may make use of a different array of co-receptors to infect cells, it is possible that the consequences of CCL3L copy number on SIV control may differ for different SIV isolates [24]. Finally, the interaction between chemokines and their receptors can be promiscuous; a single chemokine can bind multiple receptors and a single receptor can bind to multiple chemokines. CCR5 binds to multiple chemokine ligands as it subserves its biologic functions. These include MIP-1β (CCL4) and RANTES (CCL5). In humans, there are multiple CCL4L genes [25]; whether a similar diversity of these genes exist in rhesus monkeys and contribute to SIV pathogenesis either independently of, or in association with CCL3L in monkeys is not known.
CCL3L expression level was implicated in the control of HIV-1 replication and AIDS progression in humans as reported by Gonzalez et al. [7]. However, results from other recent studies suggest that CCL3L copy number is not associated with HIV-1 acquisition, viral loads or progression to AIDS following infection [12]–[14]. The present data are consistent with the latter findings, suggesting that CCL3L CNV is not associated with primate immunodeficiency virus containment following infection and, therefore, is not consequential in HIV/SIV pathogenesis.
Materials and Methods
Isolation of genomic DNA
Genomic DNA was isolated from freshly isolated PBMCs of 84 Indian-origin rhesus monkeys (66 animals inoculated with SIVmac and 18 uninfected animals) by using the QIAamp DNA kit (Qiagen). This group of monkeys included 56 Mamu-A*01− and 28 Mamu-A*01+ animals. Isolated total DNA quality was verified by average A260/A280 ratio of 1.84 (range1.77–1.98). These DNA samples were then aliquoted and stored at −20°C until use. We confirmed the integrity of selected stored DNA samples using gel electrophoresis.
Oligonucleotide primers and probes for real-time PCR
The qPCR primer and probe sequences for CCL3L were as described by Degenhardt et al. [16]. This primer set does not distinguish between CCL3 and the CCL3-like gene paralogs; therefore, CCL3 and its paralogs are referred to as CCL3L.
Estimation of CCL3L copy number using real-time PCR
We determined the gene copy number of rhesus monkey CCL3L using the method of Degenhardt et al. [15] with a few modifications. Briefly, qPCR was performed using the 7300 Real-Time PCR System (Applied Biosystems) detecting emitted fluorescence as FAM from the probe detecting CCL3L and VIC from the probe detecting the STAT6 gene during amplification. The qPCR included primers and probes with the Taqman PCR mastermix (Applied Biosystems). The amount of test DNA sample added to each PCR reaction was between 9–36 ng. Cycling conditions were: initial denaturation at 94°C for 2 min; followed by 40 cycles of 15 sec denaturation at 94°C; and 1 min annealing/extension at 60°C. The Stat6 gene was used as the internal control. Real-time qPCR results were analyzed using the SDS v1.4.0 software package (Applied Biosystems).
Generation of standard curves of CT value
Seven serial 1∶2 dilutions (50–0.78 ng) of genomic DNA from A431 cells known to have two copies of CCL3 and CCL3L1 pdg were used to generate standard of CT value against log DNA on each PCR plate (96 wells) for STAT6 present two copies per pdg and CCL3L. The values obtained for the target gene CCL3L in rhesus monkeys and the normalizer gene, STAT6, were similar, which makes STAT6 gene a good housekeeping standard to estimate of the copy numbers of CCL3L in rhesus monkeys. The square of the Pearson correlation coefficient (R2) for a standard curve of less than 99% was considered inadequate, and the corresponding PCR plate of DNA samples were repeated. The signal obtained for the test DNA samples always fell on the standard curve range. In Supplementary Figure 1 in Text S1, we reported the calibration curve for the A431 reference sample.
Absolute quantitation of CCL3L copy number based on reference samples
To estimate the absolute CCL3L copy number for each sample based on the real-time qPCR results as described by Degenhardt et al., we used the same reference sample, the A431 human cell line. The A431 cell line was shown to have two copies of CCL3L1 and two copies of CCL3 pdg, for a total copy number of four CCL3L using the qPCR assay. We determined the absolute CCL3L copy number in each sample by comparing qPCR results between the experimental and the reference samples. For each qPCR, triplicate wells were set up for STAT6 and CCL3L, CT was determined, and converted into template quantity using standard curves. Copy number is the ratio of the averaged template quantity across the replicates for CCL3L to the averaged template quantity of STAT6, multiplied by four (the diploid copy number of the A431 cell line including CCL3L1 and CCL3) or two, respectively. The resulting number was then rounded to the nearest integer value to estimate absolute copy number.
In vivo infection
Animals
Indian-origin rhesus monkeys used in the analysis were maintained according to the guidelines of the NIH Guide to the Care and Use of Laboratory Animals and the approval of the Institutional Animal Care and Use Committee of Harvard Medical School and the National Institutes of Health. All monkeys (n = 66) used in this retrospective analysis were part of 9 different SIV research experiments. All animals were received 1 ml of a 1∶3000 dilution of this stock by intravenous route. Animals from 2 experiments (n = 21) were excluded from the results of survival status analysis since they were euthanized or used in other experiments soon after day 70 (before any developed AIDS-related illness).
Challenge virus
A stock of uncloned SIVmac251 was expanded on human PBMC and titered in vivo in rhesus monkeys for use in intravenous challenge studies [26].
Plasma viral RNA assay
Assays were performed using an ultrasensitive branched DNA amplification assay (Bayer Diagnostics).
Statistical analysis
All statistical analyses were conducted using the XLSTAT software (Addinsoft) and the StarXact 8 (Cytel) for Fisher exact tests and stratified Fisher exact test, and nQuery Advisor 6.0 (Statistical Solutions) for power calculations for regression. The log10 SIV RNA levels in plasma at the time of peak and set-point were compared between groups by use of the exact Mann-Whitney U test as was comparing CCL3L copy number between Mamu-A*01 subgroups and between TRIM5 subgroups. The Kruskal-Wallis test was used to compare CCL3L copy number between 4 subgroups (divided by the expression of Mamu-A*01 and TRIM5 alleles by the monkeys). Linear regressions of the log10 plasma SIV RNA at the time of peak and set-point were used separately on each of three covariates (the binary variable Mamu-A*01 and TRIM5 alleles and the continuous variable CCL3L copy number) and also for models of two or three of these variables together. Fisher exact tests were used to compare the percent of monkeys that died by day 235 in various subgroups (defined by CCL3L copy number being below or above the median observed and by Mamu-A*01 and TRIM5 alleles). Exact estimated relative risk (RR, similar to odds ratio but odds ratio can only be estimated when the true prevalence is known) and 95% confidence intervals (CIs) for RR were provided. Exact tests of association of CCL3L copy number groups and the probability of dying by day 235 stratified by Mamu-A*01 and TRIM5 alleles and the related stratified RR and test of homogeneity of RR used the method of Zelen [27]. P values were two-tailed and considered significant when <0.05, no corrections for multiple comparisons were used.
Supporting Information
Zdroje
1. KaslowRA
CarringtonM
AppleR
ParkL
MuñozA
1996 Influence of combinations of human major histocompatibility complex genes on the course of HIV-1 infection. Nat Med 2 405 411
2. FellayJ
ShiannaKV
GeD
ColomboS
2007 A whole-genome association study of major determinants for host control of HIV-1. Science 317 944 947
3. MartinMP
GaoX
LeeJH
NelsonGW
DetelsR
2002 Epistatic interaction between KIR3DS1 and HLA-B delays the progression to AIDS. Nat Genet 31 429 434
4. DeanM
CarringtonM
WinklerC
HuttleyGA
SmithMW
1996 Genetic restriction of HIV-1 infection and progression to AIDS by a deletion allele of the CKR5 structural gene. Hemophilia Growth and Development Study, Multicenter AIDS Cohort Study, Multicenter Hemophilia Cohort Study, San Francisco City Cohort, ALIVE Study. Science 273 1856 1862
5. HuangY
PaxtonWA
WolinskySM
NeumannAU
ZhangL
1996 The role of a mutant CCR5 allele in HIV-1 transmission and disease progression. Nat Med 2 1240 1243
6. SmithMW
DeanM
CarringtonM
WinklerC
HuttleyGA
1997 Contrasting genetic influence of CCR2 and CCR5 variants on HIV-1 infection and disease progression. Hemophilia Growth and Development Study (HGDS), Multicenter AIDS Cohort Study (MACS), Multicenter Hemophilia Cohort Study (MHCS), San Francisco City Cohort (SFCC), ALIVE Study. Science 277 959 965
7. GonzalezE
KulkarniH
BolivarH
ManganoA
SanchezR
2005 The influence of CCL3L1 gene-containing segmental duplications on HIV-1/AIDS susceptibility. Science 307 1434 1440
8. AhujaSK
KulkarniH
CatanoG
AganBK
CamargoJF
2008 CCL3L1-CCR5 genotype influences durability of immune recovery during antiretroviral therapy of HIV-1-infected individuals. Nat Med 14 413 420
9. DolanMJ
KulkarniH
CamargoJF
HeW
SmithA
2007 CCL3L1 and CCR5 influence cell-mediated immunity and affect HIV-AIDS pathogenesis via viral entry-independent mechanisms. Nat Immunol 8 1324 1336
10. BaileyJA
GuZ
ClarkRA
ReinertK
SamonteRV
2002 Recent segmental duplications in the human genome. Science 297 1003 1007
11. TownsonJR
BarcellosLF
NibbsRJ
2002 Gene copy number regulates the production of the human chemokine CCL3-L1. Eur J Immunol 32 3016 3026
12. UrbanTJ
WeintrobAC
FellayJ
ColomboS
ShiannaKV
2009 CCL3L 1 and HIV/AIDS susceptibility. Nat Med 15 1110 1112
13. BhattacharyaT
StantonJ
KimEY
KunstmanKJ
PhairJP
2009 CCL3L1 and HIV/AIDS susceptibility. Nat Med 15 1112 1115
14. FieldSF
HowsonJM
MaierLM
WalkerS
WalkerNM
2009 Experimental aspects of copy number variant assays at CCL3L1. Nat Med 15 1115 1117
15. DegenhardtJD
de CandiaP
ChabotA
SchwartzS
HendersonL
2009 Copy number variation of CCL3-like genes affects rate of progression to simian-AIDS in Rhesus Macaques (Macaca mulatta). PLoS Genet 5 e1000346 doi:10.1371/journal.pgen.1000346
16. Reimann KA ParkerRA
SeamanMS
BeaudryK
BeddallM
2005 Pathogenicity of simian-human immunodeficiency virus SHIV-89.6P and SIVmac is attenuated in cynomolgus macaques and associated with early T-lymphocyte responses. J Virol 79 8878 8885
17. YasutomiY
ReimannKA
LordCI
MillerMD
LetvinNL
1993 Simian immunodeficiency virus-specific CD8+ lymphocyte response in acutely infected rhesus monkeys. J Virol 67 1707 1711
18. PalR
VenzonD
LetvinNL
SantraS
MontefioriDC
2002 ALVAC-SIV-gag-pol-env-based vaccination and macaque major histocompatibility complex class I (A*01) delay simian immunodeficiency virus SIVmac-induced immunodeficiency. J Virol 76 292 302
19. MothéBR
WeinfurterJ
WangC
RehrauerW
WilsonN
2003 Expression of the major histocompatibility complex class I molecule Mamu-A*01 is associated with control of simian immunodeficiency virus SIVmac239 replication. J Virol 77 2736 2740
20. LimSY
RogersT
ChanT
WhitneyJB
KimJH
2010 TRIM5α Modulates Immunodeficiency Virus Control in Rhesus Monkeys. PLoS Pathog 6 e1000738 doi:10.1371/journal.ppat.1000738
21. LifsonJD
NowakMA
GoldsteinS
RossioJL
KinterA
1997 The extent of early viral replication is a critical determinant of the natural history of simian immunodeficiency virus infection. J Virol 71(12) 9508 9514
22. LetvinNL
MascolaJR
SuY
GorgoneDA
BuzbyAP
2006 Preserved CD4+ Central Memory T Cells and Survival in Vaccinated SIV-Challenged Monkeys. Science 312 1530 1533
23. BurdoTH
MarcondesMC
LaniganCM
PenedoMC
FoxHS
2005 Susceptibility of Chinese rhesus monkeys to SIV infection. AIDS 19 1704 1706
24. ZhangY
LouB
LalRB
GettieA
MarxPA
2000 Use of inhibitors to evaluate coreceptor usage by simian and simian/human immunodeficiency viruses and human immunodeficiency virus type 2 in primary cells. J Virol 74 6893 6910
25. ModiWS
2004 CCL3L1 and CCL4L1 chemokine genes are located in a segmental duplication at chromosome 17q12. Genomics 83 735 738
26. LetvinNL
MascolaJR
SuY
GorgoneDA
BuzbyAP
2006 Preserved CD4+ Central Memory T Cells and Survival in Vaccinated SIV-Challenged Monkeys. Science 312 1530 1533
27. ZelenM
1971 The analysis of several 2×2 contingency tables. Biometrika 58 129 137
Štítky
Genetika Reprodukční medicína
Článek The Met 470 Allele Is Associated with Lower Birth Rates in Fertile Men from a Population IsolateČlánek Copy Number Variation and Transposable Elements Feature in Recent, Ongoing Adaptation at the Locus
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2010 Číslo 6- Růst a vývoj dětí narozených pomocí IVF
- Délka menstruačního cyklu jako marker ženské plodnosti
- Vztah užívání alkoholu a mužské fertility
- Šanci na úspěšný průběh těhotenství snižují nevhodné hladiny progesteronu vznikající při umělém oplodnění
- Vliv melatoninu a cirkadiálního rytmu na ženskou reprodukci
-
Všechny články tohoto čísla
- Translational Selection Is Ubiquitous in Prokaryotes
- Whole-Genome Sequencing of a Single Proband Together with Linkage Analysis Identifies a Mendelian Disease Gene
- Web-Based, Participant-Driven Studies Yield Novel Genetic Associations for Common Traits
- The Met 470 Allele Is Associated with Lower Birth Rates in Fertile Men from a Population Isolate
- Contributions of Status and Allele Expression, But Not Copy Number Variation, to the Control of SIVmac251 Replication in Indian-Origin Rhesus Monkeys
- A Genome-Wide Association Study of Optic Disc Parameters
- Amplification of a Cytochrome P450 Gene Is Associated with Resistance to Neonicotinoid Insecticides in the Aphid
- The Transcription Factor REST Is Lost in Aggressive Breast Cancer
- 14-3-3 Mediates Histone Cross-Talk during Transcription Elongation in
- Copy Number Variation and Transposable Elements Feature in Recent, Ongoing Adaptation at the Locus
- Use of Genome-Wide Expression Data to Mine the “Gray Zone” of GWA Studies Leads to Novel Candidate Obesity Genes
- Genome-Wide RNAi Screen Identifies Multiple Regulators of HIF–Dependent Transcription in Hypoxia
- The CYCLIN-A CYCA1;2/TAM Is Required for the Meiosis I to Meiosis II Transition and Cooperates with OSD1 for the Prophase to First Meiotic Division Transition
- Inactivation of hnRNP K by Expanded Intronic AUUCU Repeat Induces Apoptosis Via Translocation of PKCδ to Mitochondria in Spinocerebellar Ataxia 10
- Mice with Alopecia, Osteoporosis, and Systemic Amyloidosis Due to Mutation in , a Gene Coding for Palmitoyl Acyltransferase
- siRNA–Mediated Methylation of Telomeres
- Chromosome 4 Replicates in Two Phases That Correlate with Chromatin State
- Dynamic Switch of Negative Feedback Regulation in Akt–TOR Signaling
- On the Use of Variance per Genotype as a Tool to Identify Quantitative Trait Interaction Effects: A Report from the Women's Genome Health Study
- and Deficiency Cooperate in the Progression of Mouse Prostate Tumourigenesis
- An Integration of Genome-Wide Association Study and Gene Expression Profiling to Prioritize the Discovery of Novel Susceptibility Loci for Osteoporosis-Related Traits
- Consent and Internet-Enabled Human Genomics
- Understanding Adaptation in Large Populations
- Identification of a Functional Genetic Variant at 16q12.1 for Breast Cancer Risk: Results from the Asia Breast Cancer Consortium
- Evidence that Adaptation in Is Not Limited by Mutation at Single Sites
- The IG-DMR and the -DMR at Human Chromosome 14q32.2: Hierarchical Interaction and Distinct Functional Properties as Imprinting Control Centers
- Cushing's Syndrome and Fetal Features Resurgence in Adrenal Cortex–Specific Knockout Mice
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Web-Based, Participant-Driven Studies Yield Novel Genetic Associations for Common Traits
- The IG-DMR and the -DMR at Human Chromosome 14q32.2: Hierarchical Interaction and Distinct Functional Properties as Imprinting Control Centers
- Amplification of a Cytochrome P450 Gene Is Associated with Resistance to Neonicotinoid Insecticides in the Aphid
- Copy Number Variation and Transposable Elements Feature in Recent, Ongoing Adaptation at the Locus
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



