-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Amplification of a Cytochrome P450 Gene Is Associated with Resistance to Neonicotinoid Insecticides in the Aphid
The aphid Myzus persicae is a globally significant crop pest that has evolved high levels of resistance to almost all classes of insecticide. To date, the neonicotinoids, an economically important class of insecticides that target nicotinic acetylcholine receptors (nAChRs), have remained an effective control measure; however, recent reports of resistance in M. persicae represent a threat to the long-term efficacy of this chemical class. In this study, the mechanisms underlying resistance to the neonicotinoid insecticides were investigated using biological, biochemical, and genomic approaches. Bioassays on a resistant M. persicae clone (5191A) suggested that P450-mediated detoxification plays a primary role in resistance, although additional mechanism(s) may also contribute. Microarray analysis, using an array populated with probes corresponding to all known detoxification genes in M. persicae, revealed constitutive over-expression (22-fold) of a single P450 gene (CYP6CY3); and quantitative PCR showed that the over-expression is due, at least in part, to gene amplification. This is the first report of a P450 gene amplification event associated with insecticide resistance in an agriculturally important insect pest. The microarray analysis also showed over-expression of several gene sequences that encode cuticular proteins (2–16-fold), and artificial feeding assays and in vivo penetration assays using radiolabeled insecticide provided direct evidence of a role for reduced cuticular penetration in neonicotinoid resistance. Conversely, receptor radioligand binding studies and nucleotide sequencing of nAChR subunit genes suggest that target-site changes are unlikely to contribute to resistance to neonicotinoid insecticides in M. persicae.
Published in the journal: . PLoS Genet 6(6): e32767. doi:10.1371/journal.pgen.1000999
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1000999Summary
The aphid Myzus persicae is a globally significant crop pest that has evolved high levels of resistance to almost all classes of insecticide. To date, the neonicotinoids, an economically important class of insecticides that target nicotinic acetylcholine receptors (nAChRs), have remained an effective control measure; however, recent reports of resistance in M. persicae represent a threat to the long-term efficacy of this chemical class. In this study, the mechanisms underlying resistance to the neonicotinoid insecticides were investigated using biological, biochemical, and genomic approaches. Bioassays on a resistant M. persicae clone (5191A) suggested that P450-mediated detoxification plays a primary role in resistance, although additional mechanism(s) may also contribute. Microarray analysis, using an array populated with probes corresponding to all known detoxification genes in M. persicae, revealed constitutive over-expression (22-fold) of a single P450 gene (CYP6CY3); and quantitative PCR showed that the over-expression is due, at least in part, to gene amplification. This is the first report of a P450 gene amplification event associated with insecticide resistance in an agriculturally important insect pest. The microarray analysis also showed over-expression of several gene sequences that encode cuticular proteins (2–16-fold), and artificial feeding assays and in vivo penetration assays using radiolabeled insecticide provided direct evidence of a role for reduced cuticular penetration in neonicotinoid resistance. Conversely, receptor radioligand binding studies and nucleotide sequencing of nAChR subunit genes suggest that target-site changes are unlikely to contribute to resistance to neonicotinoid insecticides in M. persicae.
Introduction
Insecticide resistance in crop pests has been a mounting constraint on crop protection since the introduction of synthetic insecticides in the 1940s, and has been shown to develop through three main mechanisms. These are 1) increased production of metabolic enzymes (such as esterases, glutathione transferases and P450-dependent monooxygenases) that break down or sequester the insecticide, 2) structural changes (mutations) in the gene encoding the insecticide target protein that make it less sensitive to the toxic effect of the insecticide (e.g. acetylcholinesterase for organophospates/carbamates, the voltage-gated sodium channel for pyrethroids, and the GABA receptor for cyclodienes) and 3) reduced penetration of insecticide through the cuticle [1].
The peach-potato aphid, Myzus persicae is an economically significant pest in many temperate regions of the world causing direct damage to a broad range of arable and horticultural crops and transmitting more than 100 plant viruses [2]. As a result this species has been treated extensively with insecticides and has developed multiple resistance to many classes of compounds including organophosphates, carbamates and pyrethroids. Three genetically independent mechanisms underlying resistance have been identified in M. persicae. Increased production of detoxifying carboxylesterases (E4 or FE4) confer broad-spectrum resistance to organophosphates and carbamates and results from gene amplification [3]. Two forms of target-site resistance have also evolved, a mutation in the gene encoding acetylcholinesterase (modified acetylcholinesterase, MACE) which results in insensitivity to dimethyl carbamates such as primicarb, and a mutation in the gene encoding the voltage-gated sodium channel (knockdown resistance, kdr) which confers resistance to pyrethroids [4]. As a result, neonicotinoids such as imidacloprid, clothianidin, thiamethoxam and thiacloprid, which are unaffected by these existing resistance mechanisms [5], are now the main class of insecticide used for M. persicae control. However, there have now been reports of resistance to the neonicotinoid imidacloprid in M. persicae from Europe, the USA and Japan [5]–[7], raising concerns for the long-term efficacy of this insecticide class.
Resistance to neonicotinoids is a significant problem in several insect species including the Colarado potato beetle (Leptinotarsa decemlineata), the brown planthopper (Nilaparvata lugens) and the tobacco whitefly (Bemisia tabaci) [5]. Neonicotinoids are nicotinic acetlycholine receptor (nAChR) agonists and resistance in a laboratory-selected colony of N. lugens was found to be conferred by a target-site modification (Y151S) within two alpha subunits of the nAChR [8]. However, to date, this remains the only example of target-site resistance and this mechanism is yet to be described in any field-collected insect population. Neonicotinoid resistance has been studied in greatest detail in B. tabaci, where resistance is associated with increased detoxification by P450s. Initially this detoxification mechanism was implicated by use of the P450 inhibitor piperonyl butoxide (PBO) and by biochemical assays with the model substrate 7-ethoxycoumarin [9]. Over-expression of a single P450 gene (CYP6CM1) has since been found to be associated with high levels of resistance to imidacloprid [10] and the role of this enzyme in resistance was demonstrated by studies of imidacloprid metabolism [11]. Over-expression of another P450 gene CYP6G1 has also been associated with moderate imidacloprid resistance in the fruit fly Drosophila melanogaster and results from the insertion of an Accord transposable element into the 5′ end of the CYP6G1 gene [12]. Although the mechanism(s) underlying neonicotinoid resistance in M. persicae have not been characterised there is evidence based on the differential effects of enzyme inhibitors that detoxification, via enhanced P450 activity, contributes to resistance [13].
P450s are a diverse class of enzymes with many functions ranging from biosynthesis to the metabolism of xenobiotics. Insect genomes have been found to contain from 46 to over 150 P450 genes, each encoding a different P450 enzyme [14], [15]. The rapid growth in genomics and post-genomic technologies has made it easier to study large and complex gene families such as the cytochrome P450 superfamily. Available resources include many full insect genome sequences, including the recently completed sequence of the pea aphid Acyrthosiphon pisum [16], expressed sequence tag (EST) libraries and tools such as microarrays and high-throughput transcriptome sequencing. Although the genome of M. persicae is yet to be sequenced, a number of other genomic resources are available. These include an EST library of 47,832 unique sequences and an oligonucleotide microarray populated with probes for nearly all available ESTs [17], [18]. A recent comparative analysis of detoxification enzymes in A. pisum and M. persicae revealed evidence of a substantial difference in the number of P450 genes in the two species, with this gene family at least 40% larger in M. persicae, where at least 115 unique P450 genes were identified [18]. In the work reported here, we have exploited the available genomic resources to investigate potential mechanisms of neonicotinoid resistance in a clone of M. persicae that shows 25–40 fold resistance to neonicotinoids.
Results
Topical bioassays
Four of five M. persicae clones (4106A, 4255A, 5142B and 1200Q) tested by a diagnostic dose bioassay (2.5 ng imidacloprid/aphid) showed complete susceptibility to imidacloprid with 100% mortality at the test dose. However, clone 5191A showed a significant decrease in mortality, with more than 50% surviving. The resistance profile of this clone was compared to the reference susceptible clone (4106A) by full dose-response bioassays with four neonicotinoid compounds, with and without pre-treatment with the P450 inhibitor PBO (see Table 1). Significant differences in LC50 values (the concentration required to kill 50% of the aphids tested) between 5191A and 4106A were observed for all of the insecticides tested, with the 5191A clone having a resistance factor between 27 and 41. Pre-treatment with PBO substantially synergised the effect of all of the insecticides tested, however, complete susceptibility was not restored with a resistance factor of 2.5–15 remaining.
Tab. 1. Results of full dose-response bioassays with a range of neonicotinoids to the <i>M. persicae</i> clones 4106A (susceptible) and 5191A (resistant) both with and without pre-treatment with the P450 inhibitor PBO. 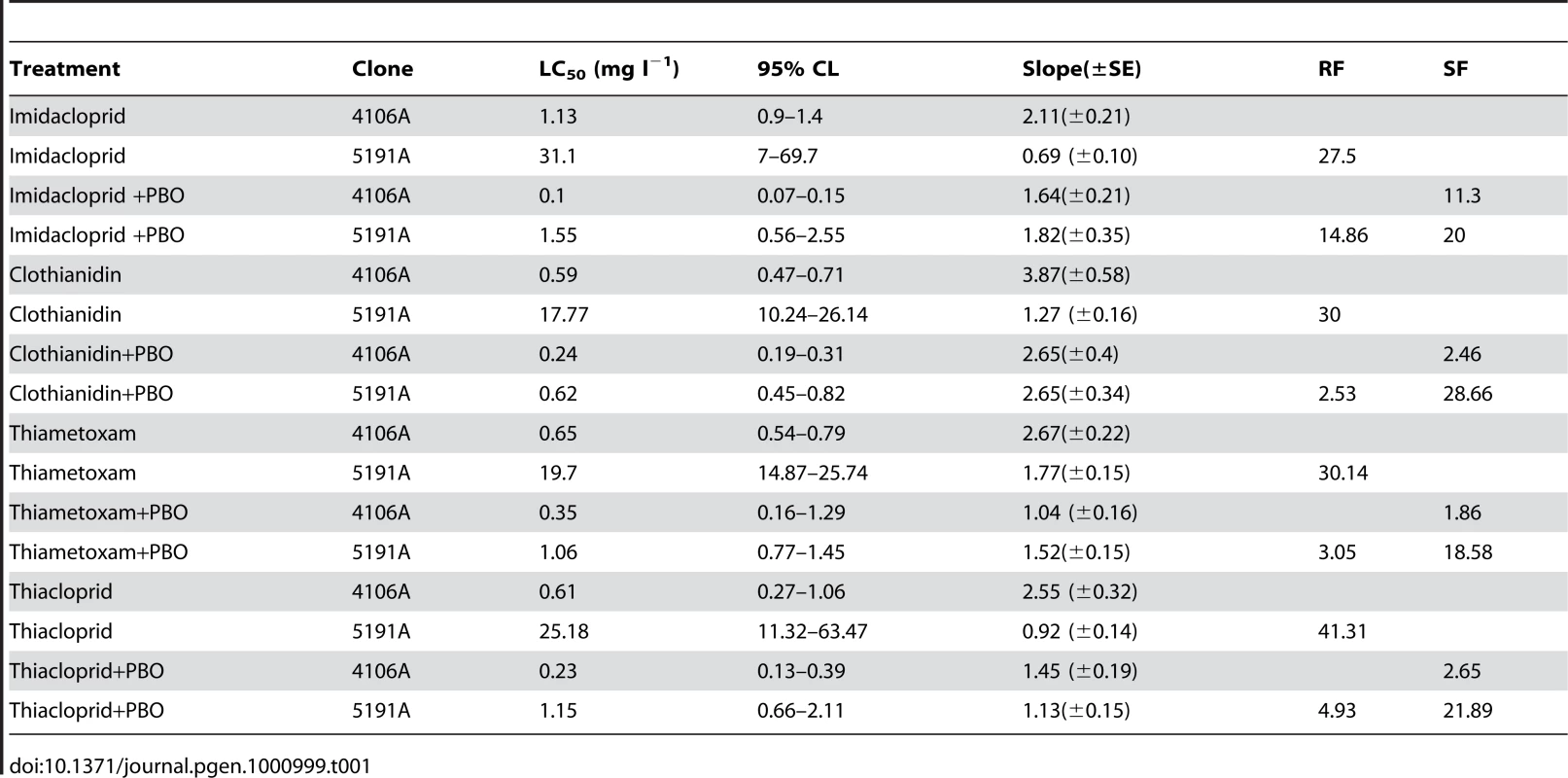
Microarray analysis
Microarray analysis identified 273 genes significantly differentially transcribed between the insecticide resistant clone 5191A and the susceptible clone 4106A. The full list of these genes along with Log2, calculated fold-change values and a description based on the closest BLAST hit is given in Table S1. 174 genes (86 of unknown function) had elevated expression in the 5191A clone and 93 (55 of unknown function) were under-transcribed in this clone relative to 4106A. Of the 88 over-expressed genes with a known function, half (44) were potential candidates for being involved in insecticide resistance and are shown in Table 2. These included genes encoding cytochrome P450s, carboxylesterase E4/FE4 and a large number of cuticular proteins.
Tab. 2. Selected genes identified by microarray as significantly differentially transcribed between the insecticide resistant <i>M. persicae</i> clone 5191 and the susceptible strain 4106a. 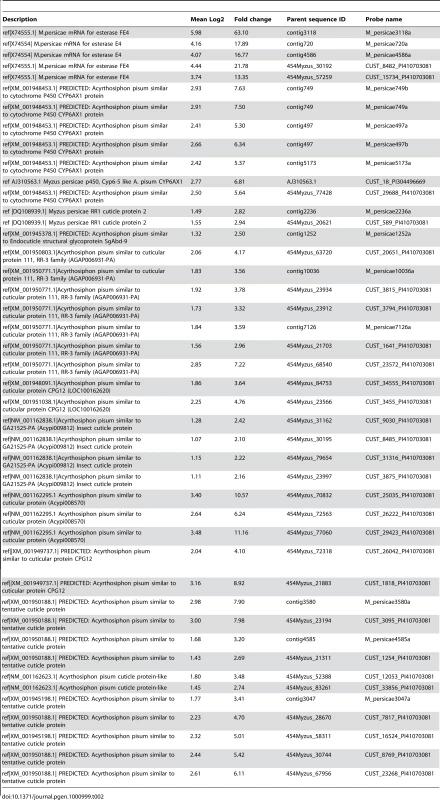
Five EST sequences encoding carboxylesterase FE4 and the closely related variant E4 were identified as being over-expressed, in the resistant clone. However the level of expression between these sequences was variable (ranging from 13–63 fold). This variation probably resulted from the fact that only one of the five ESTs (contig 3118) was a perfect match with FE4 at the probe site, shown previously to be the variant present in 5191A [13]. Therefore, the fold-change indicated by the probe designed on contig 3118 is likely to be the most accurate and this level corresponds well with the 64-fold increase in the esterase level previously reported in aphids with the FE4 genes and R3 levels of resistance [19].
Five EST sequences (Table 2) encoding cytochrome P450s were elevated in the 5191A strain (5.3–7.6 fold). In two cases duplicate probes corresponding to the same EST sequence were identified (contig749a/b and contig497a/b). All five partial EST sequences were most similar to a single cytochrome P450 gene of Acyrthosiphon pisum CYP6CY3 (77.3–88.7% at the nucleotide level) [15] and, as shown in Figure S1, align with different regions of the A. pisum gene and almost certainly correspond to the same gene in M. persicae.
Finally, a large number (32) of partial and full length EST gene sequences encoding cuticular proteins (CPs) were shown to be differentially transcribed between the 5191A and 4106A clones (Table 2). The changes in expression level of the 32 genes varied from 2.1–11.1 fold. The EST sequences on which these probes were designed vary considerably in length and it is difficult to ascertain the number of unique genes represented by these sequences. BLAST searching and a Hidden Markov Model tool on the CuticleDB WebSite (http://bioinformatics2.biol.uoa.gr/cuticleDB/index.jsp) [20] was used to characterise the different CPs shown to be over-expressed in the 5191A clone. All three members of the CPR group, as characterised by a conserved amino acid domain (the Rebers and Riddiford Consensus RR-1, RR-2 and RR-3), were represented along with several CPs of the cuticular protein glycine-rich (CPG) family.
Of the thirty eight genes of known function that were under-transcribed in 5191A relative to 4106A, only a limited number of detoxification genes were identified (see Table S1). These included four sequences encoding glutathione S-transferases (GSTs) with a negative fold change of -2 to -4 and a single sequence encoding a CYP6A2 type cytochrome P450 with a fold change of -6.4.
Real-time quantitative PCR was used to validate the microarray results by examining the expression profile of ten selected genes: the CYP6CY3 gene and nine of the over-expressed genes encoding cuticular proteins. In all cases, the over-transcription of the genes was confirmed (Table 3), although, as reported previously for the Agilent array platform, expression ratios obtained from RT-PCR were frequently higher than those generated by the microarrays [21]. The CYP6CY3 gene was found to be over-transcribed 22 fold (+/−0.91).
Tab. 3. Fold change in expression of CYP6CY3 and cuticular proteins in the insecticide resistant M. persicae clone 5191 (compared to the susceptible reference clone 4106A) as determined by quantitative PCR. 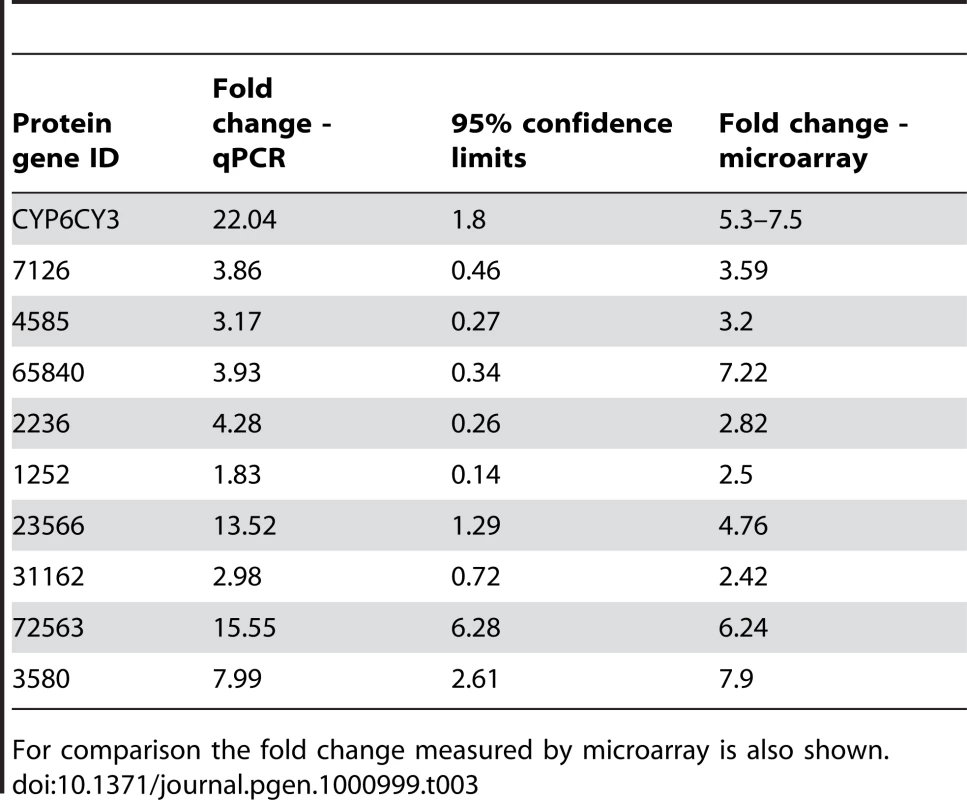
For comparison the fold change measured by microarray is also shown. CYP6CY3 gene copy number
Quantitative PCR was used to determine CYP6CY3 gene copy number using genomic DNA as template. Data were normalised using three genes; para (present in two copies in diploid insect genomes), ace (present in four copies in M. persicae) and actin (thought to be multi-copy). The clone 5191A showed a fold change in copy number of 9.4 (+/−0.3), 9.2 (+/−0.4) and 9.2 (+/−0.4) using each gene to normalise respectively compared to the 4106A clone. The mean cycle threshold values of three biological replicates in quantitative PCR of the CYP6CY3 and sodium channel genes in 4106A were essentially the same (CTs of 18.56 and 18.50 respectively) indicating that the diploid genome of this clone carries two copies of the CYP6CY3 gene and this is amplified to ∼18 copies in the resistant clone 5191A.
CYP6CY3 cDNA characterization
The CYP6CY3 sequence was initially derived from three non-overlapping EST sequences (479/5173 and 749 see Figure S1). The missing middle section and terminal 5′ UTR region of the gene were obtained by 5′ RACE and the complete mature mRNA sequence of 2087 bp is shown in Figure 1. This includes a 5′ UTR of 94 bp and a 3′ UTR of 460 bp. The cDNA has an open reading frame of 1533 bp encoding 511 amino acid residues. The predicted isoelectric point of the protein is 8.6 and the theoretical molecular weight is 59.13 kDa. CYP6CY3 shares similarity with other microsomal proteins with a strongly hydrophobic N-terminus (resembling a signal peptide sequence) acting as a transmembrane anchor. As shown in Figure 1, the encoded protein contains conserved domains characteristic of P450s such as the oxygen binding motif (helix I) ([A/G]GX[E/D]T[T/S], position 316), the helix K motif (EXXRXXP, position 374), the heme-binding “signature” motif (PFXXGXXXCXG, position 447) and a sequence motif (PXXFXP, position 429) specific to CYP6 members.
Fig. 1. Complete mRNA sequence of M. persicae CYP6CY3. 
5′ and 3′ untranslated regions are highlighted in grey. Sites of amino acid substitutions are boxed. Conserved domains common to cytochrome P450s or to CYP6 members are highlighted in blue such as the oxygen binding motif (position 316), the heme binding motif (position 447), the helix K motif (position 374) and a sequence motif (position 429) specific to CYP6 members. Variation in the coding sequence of CYP6CY3 copies in the two aphid clones was examined by both direct nucleotide sequencing and by cloning and sequencing the full cDNA ORF and/or 5′ RACE PCR products. In 4106A, direct sequencing revealed a very low level of variation in the sequence of the two copies with just one change (G/A) at position 319, indicating an E/K amino acid substitution. Interestingly, this change occurs in the oxygen binding motif, where the usual residue is either E or D (see above). However other insects also carry the K residue at this position with the A. pisum CYP314A1 and CYP314A2 P450s two examples. Attempts to direct sequence the CYP6CY3 gene in the 5191A clone gave poor sequence quality (characterised by multiple overlaid sequences). To overcome this, PCR products of the full length cDNA coding sequence and 5′ RACE products were cloned and sequenced to look for sequence variation in coding sequence and 5′ UTR/promoter regions. In general this showed that the coding sequences of the CYP6CY3 gene copies are conserved with only a single synonymous SNP observed in the full length cDNA clones. However, there were more differences in cloned 5 ′ RACE PCR products and an alignment of the 5′ UTR and N-terminal coding sequence of CYP6CY3 copy variants (Figure 2) revealed one variant where the 5′ UTR/promoter region had been replaced. There was also evidence of at least one expressed pseudogene of CYP6CY3 with a single base deletion which results in a frame shift and generates a stop codon one codon after the deletion (this frame shift variant probably explains the difficulty in obtaining clean sequence traces by direct sequencing). When the cDNA sequence of 4106A and 5191A are compared, only two completely conserved differences between the clones were observed, with 5191A having a synonymous (CGA/CGC) SNP at position 214 and a non-synonymous SNP (ACC/ATC) conferring a threonine to isoleucine substitution at position 24.
Fig. 2. Alignment of the 5′UTR and N-terminal coding sequence of CYP6CY3 copy variants in the M. persicae clone 5191a. 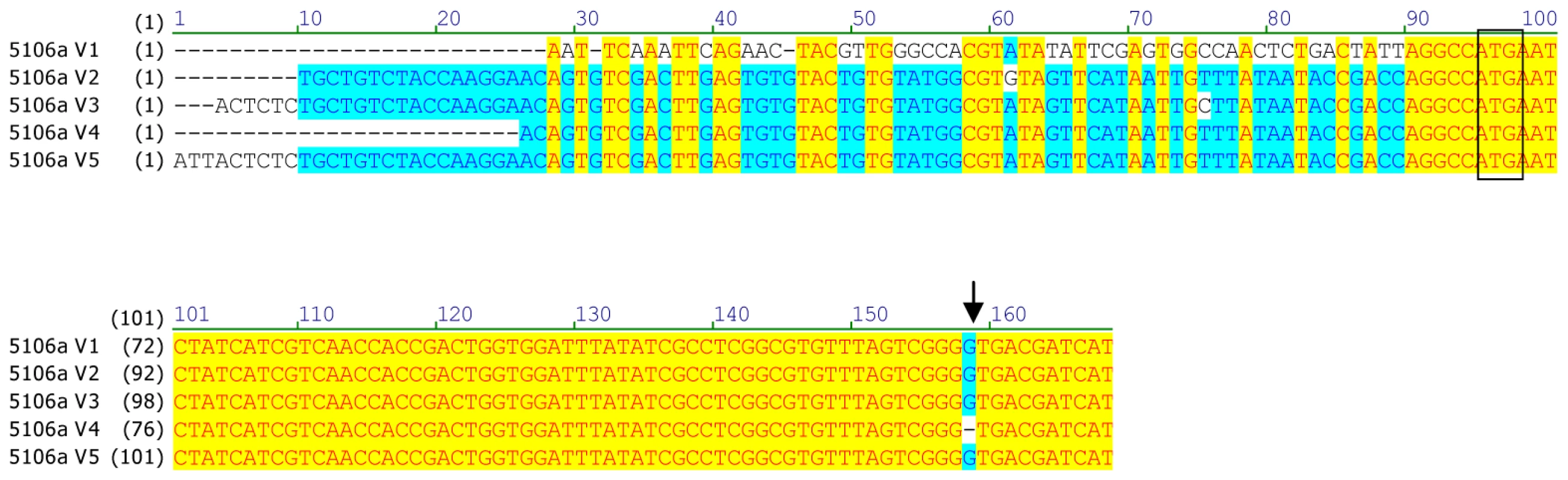
The start codon is boxed. An indel which introduces a stop codon in copy variant 4 is indicated by an arrow. Artificial feeding assays
Feeding the 5191A and 4106A M. persicae clones with insecticide using artificial feeding assays revealed significant differences in the calculated LC50 values compared to those derived from topical application. Differences were observed both between different neonicotinoids and between the two aphid clones (see Table 4). The LC50 in the feeding assays was 3.5 times lower for imidacloprid and 8.4 times lower for clothianidin and thiamethoxam for the susceptible clone than that calculated by topical bioassay. For the resistant clone the LC50 was 17 times lower for clothianidin. Unfortunately the LC50s for imidacloprid and thiamethoxam by feeding could not be calculated for the resistant clone because of anti-feedant effects (evident in the very shallow slope of the dose response line). As a consequence, the resistance ratio could only be calculated for clothianidin where it was approximately two-fold lower than calculated by topical bioassay. In all experiments the mortality of starvation controls was less than 10% when assessed at 48hs.
Tab. 4. Results of artificial feeding bioassays with a range of neonicotinoids to the <i>M. persicae</i> clones 4106A (susceptible) and 5191A (resistant). 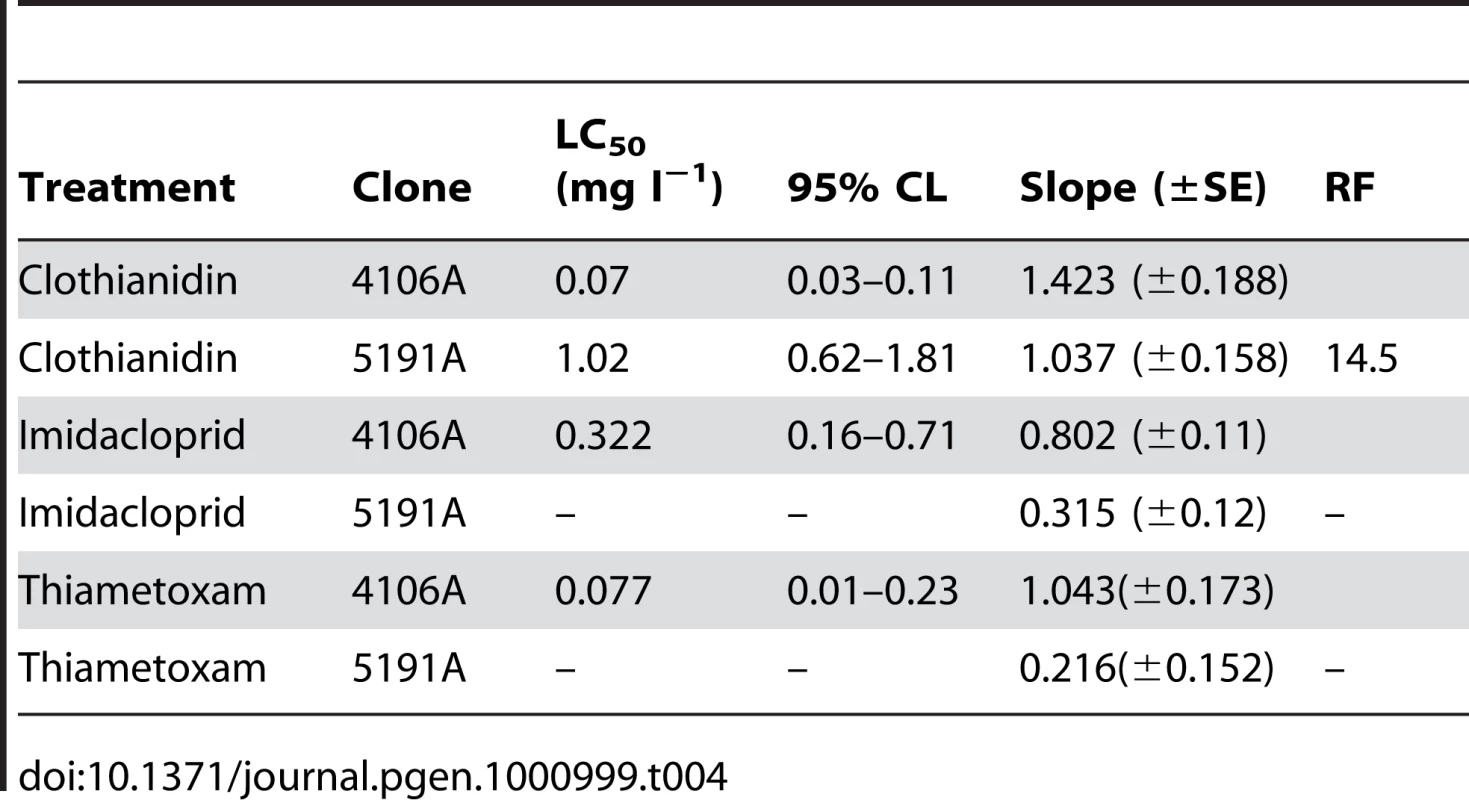
Penetration of imidacloprid
An in vivo penetration assay using [3H] imidacloprid revealed significant differences in the penetration of insecticide through the cuticle in the 4106A and 5191A M. persicae clones (see Figure 3). At the initial time points (30 min and 1 hour), the penetration of imidacloprid was relatively low, with no notable differences in the amount recovered by external rinsing between the susceptible and resistant clones. However, after 5 hours, the amount recovered from the susceptible clone had decreased sharply (to 63%), a trend that continued throughout the time course of the experiment, with only 22% of the initial imidacloprid dose recovered after 50 hours. In contrast, the levels of [3H] imidacloprid recovered from the resistant clone by external rinsing decreased at a much slower rate, with ∼88% of the applied dose being recovered after 5 hours and more than half the initial dose (56%) recovered after 50 hours, indicating a significant (p≤0.05) reduction in the rate of imidacloprid penetration through the cuticle compared to the susceptible clone. In future it may be interesting to relate how the penetration rate of imidacloprid observed in this study relates to the speed of action of this compound in resistant and susceptible clones (as determined by lethal time bioassays).
Fig. 3. Result of in vivo penetration assay using [3H] imidacloprid on the 4106A (susceptible) and 5191A (resistant) clones. ![Result of <i>in vivo</i> penetration assay using [<sup>3</sup>H] imidacloprid on the 4106A (susceptible) and 5191A (resistant) clones.](https://www.prolekare.cz/media/cache/resolve/media_object_image_small/media/image/c6f845236f14482cb53a32732e0d368b.png)
Graph shows the percentage of the initial [3H] imidacloprid dose recovered from the cuticle wash at different time points after application. [3H] imidacloprid binding assays
Radioligand binding assays of [3H] imidacloprid to several independent membrane preparations from M. persicae indicated a high degree of variability in the levels of imidacloprid binding to the nicotinic acetylcholine receptor (nAChR) (Figure 4). Although there were significant differences in specific binding of [3H] imidacloprid between some of the clones analyzed, (e.g. between clones 5142B/5191A and clones 4106A/4255A), a consistent correlation with the resistant phenotype was not observed (see Figure 4), providing no evidence of target-site insensitivity in the 5191A clone.
Fig. 4. Specific [3H] imidacloprid binding to membranes extracted from a range of M. persicae clones. ![Specific [<sup>3</sup>H] imidacloprid binding to membranes extracted from a range of <i>M. persicae</i> clones.](https://www.prolekare.cz/media/cache/resolve/media_object_image_small/media/image/d87479ad429fdd27631dc457117e397e.png)
Bars represent 95% confidence limits. Sequence analysis of nAChR subunits
The N-terminal region of the M. persicae nAChR α1-α4 and β1 subunits, encompassing the conserved domains previously implicated in neonicotinoid binding (loops A to F) were amplified by PCR from several individuals of the 4106A and 5191A clones and examined by nucleotide sequencing. Although a limited number of silent SNPs were detected, no non-synonymous changes were observed and the deduced amino acid sequence in the region studied was identical.
Discussion
Over-expression of one or more P450s currently appears to be, through direct or indirect evidence, the primary route for neonicotinoid resistance in insect pests. In the present study the involvement of P450s in neonicotinoid resistance in M. persicae was established by using the P450 inhibitor PBO, with a pre-treatment of PBO substantially synergising the effect of all the insecticides tested on a resistant M. persicae clone from Greece (5191A). A genomic approach was then used to quantify the expression of all known genes encoding detoxification enzymes in the resistant 5191A clone and a susceptible clone, 4106A, using a recently developed microarray populated with probes for >45 000 ESTs [17], [18]. A number of candidate genes with elevated expression were identified, including genes encoding a carboxylesterase, a P450 and several cuticular proteins.
The gene encoding the carboxylesterase FE4 was over-expressed 63-fold in the resistant clone, consistent with the 64-fold increase in the enzyme previously reported in aphids with FE4 genes at R3 levels [19]. A previous study examining the susceptibility of the 5191A clone to the pyrethroid α-cypermethrin reported a resistance factor of 33 to this compound which is similar to the level of resistance displayed by other aphid clones with the R3 level of esterase overproduction [13]. However, the enhanced level of carboxylesterase is unlikely to confer significant resistance to neonicotinoids since other M. persicae clones overproducing this enzyme show no resistance to imidacloprid [22].
A single P450 gene (represented by five ESTs) showed a 22-fold increased transcription in the resistant clone and the full-length cDNA sequence was most similar to a cytochrome P450 gene of A. pisum recently named as CYP6CY3 [15]. The CYP6 family has been implicated in insecticide resistance more often than any other P450 family and the M. persicae CYP6CY3 gene shares sequence identity with other CYP6 P450 genes implicated in neonicotinoid resistance in B. tabaci and D. melanogaster. To date, the increased expression of P450 genes in resistant insects has been shown to mainly arise through mutations and indels in cis-acting promoter sequences and/or trans-acting regulatory loci [23]. In this study we show that the enhanced transcription of M. persicae CYP6CY3 is due, at least in part, to amplification of the gene from 2 to ∼18 copies. Although duplication of P450 genes associated with insecticide resistance has been reported in An. funestus and D. melanogaster [24], [25] this study represents the first report of a P450 gene amplification event associated with insecticide resistance in an agriculturally important insect pest. In contrast, several species of crop pests including M. persicae have evolved resistance to insecticides through amplification of genes encoding esterases and glutathione-S-transferases [23]. There is also evidence from the recently sequenced A. pisum genome that aphids shows a propensity for gene duplication [16]. Amplification of esterase genes in M. persicae is linked to an autosomal 1,3 translocation event (E4) or involves multiple rearrangements (FE4) [4] but further work is required to characterise the mechanism by which the CYP6CY3 is amplified. Sequencing of the CYP6CY3 gene copies in the resistant strain showed a low level of variation in the coding sequence although there was evidence of one expressed pseudogene (caused by a single base deletion introducing a frame shift) and modification of the 5′ UTR sequence. Since the ∼9 fold increase in copy number may not fully explain the ∼22 fold increase in mRNA observed it is possible that additional mechanisms, such as upregulation of transcription, also contribute to over-expression of CYP6CY3 in clone 5191A. The finding of at least one copy of CYP6CY3 with a variant promoter sequence may be relevant and requires further investigation.
Although pre-treatment with PBO substantially synergised the effect of all the insecticides tested on the 5191A resistant clone, complete susceptibility was not restored (a resistance factor of between 2.5 and 15 remained) indicating that an additional mechanism(s) may play a role in the phenotypic resistance seen in this clone. However, the radioligand binding assays with [3H] imidacloprid to aphid membrane preparations and sequencing of the N-terminal (ligand binding) region of several nAChR subunits showed that target-site resistance is unlikely to be responsible. Conversely, the microarray analysis found a large number (32) of up-regulated sequences encoding cuticular proteins (CPs) in the resistant clone suggesting that a change in penetration of insecticide might be conferring some resistance. Although it is difficult to ascertain the exact number of unique genes represented by these ESTs (as many are short reads generated by 454 sequencing) all three members of the CPR group of CPs (the largest structural CP family in arthropods) were represented along with several CPs of the CPG family. Recent annotation of CPs in the pea aphid genome has revealed a family of 92 CPR genes [26]. Furthermore, in A. pisum CP genes were found to be clustered in the genome and may also be co-regulated [26] which may explain why several ESTs encoding CPs were identified as being over-expressed in our study. Insecticide resistance through decreased cuticular penetration has been demonstrated in several insect species including the red flour beetle (Tribolium castaneum) cotton bollworm (Helicoverpa armigera) and German Cockroach (Blattella germanica) [27]–[29]. In addition, over-expression of two cuticular precursor genes has been reported in pyrethroid resistant Anopheles gambiae [30].
The potential role of reduced cuticular penetration in neonicotinoid resistance in M. persicae was supported by our further biological and biochemical studies. Firstly, artificial feeding assays with the neonicotinoid clothianidin showed that the LC50s for the susceptible clone and the resistant clone were 8 and 17 times lower than those obtained with topical applications, suggesting that there is a difference in penetration. Secondly, in vivo penetration assays using [3H] imidacloprid revealed a significant reduction in the penetration of insecticide through the cuticle in the resistant clone. Taken together, these results suggest that over-expression of CPs may also play a role in resistance to neonicotinoid insecticides in M. persicae. However, the exact role CPs play in cuticular thickening and reduced penetration remains unknown and clearly warrants further investigation.
Although resistance is an almost inevitable outcome of intensive insecticide use it is possible to minimize its effect by adopting resistance management strategies which aim to prevent, or more usually slow, the development and spread of resistance. Such strategies often include minimizing the use of certain pesticides and rotating the usage of compounds with different target sites. In this regard this study provides valuable insights into the molecular basis of detoxification and the resistance mechanisms characterized here are new additions to those described previously in M. persicae which include MACE, kdr, and increased production of esterases (see introduction). Guidelines on managing resistance in this pest have been published by the Insecticide Resistance Action Group (IRAG) [31] and highlight insecticides with alternative modes of action such as the anti-feedants pymetrozine and flonicamid that may not be affected by these existing mechanisms and therefore represent potential alternatives for control of this pest.
In summary, we have shown through bioassays that P450-mediated detoxification likely plays a substantial role in neonicotinoid resistance in M. persicae but that other secondary mechanism(s) may be involved. The resistant phenotype is associated with the over-expression of a single P450 gene and we show that this is due, at least in part, to gene amplification. Over-expression of cuticular proteins and reduced penetration of insecticide may make an additional contribution but there is no evidence of a role for target-site insensitivity in the clone investigated.
Materials and Methods
Aphid clones and rearing
4106A is an insecticide-susceptible clone originally collected from potatoes in Scotland in 2000. Clone 5191A was collected from tobacco in Greece in 2007 and has the FE4 variant of enhanced esterase at R3 levels and a ∼50 fold resistance to the neonicotinoid insecticide imidacloprid [13]. Clones 4255A and 5142B were collected in the UK from oilseed rape and sugar beet respectively. Clone 1200Q was collected from peach in Argentina. All M. persicae clones were originally established from a single parthenogenetic female and were reared asexually on Chinese cabbage leaves (Brassica napus L var chinensis cv Tip-Top) in small plastic box-cages maintained at 18°C under a 16 : 8 h light:dark regime. Aphids of similar age were transferred regularly to new boxes in order to rear them as distinct cohorts.
Insecticides and synergists
Technical-grade insecticides (imidacloprid, clothianidin, thiamethoxam, thiacloprid) were purchased from Sigma (Sigma-Aldrich, UK) and technical piperonyl butoxide (PCP ‘Ultra’) was provided by Dr. Graham Moores. [3H] imidacloprid was either from a sample provided by Dr Ralf Nauen, Bayer CropScience (38 Ci/mmol) or purchased from American Radiolabeled Chemicals, Inc. (40 Ci/mmol).
Topical bioassays
Ten adult aphids were placed on the abaxial surface of a Chinese cabbage leaf disk sitting on 1% agar in a plastic tub (3 cm diameter). The walls of the tub above the leaf were coated with Fluon to prevent escape. The aphids were allowed to settle for an hour before being dosed individually with 0.25 µl acetone containing the insecticide to be tested using a microapplicator (Burkard Manufacturing Ltd, UK). For the synergism bioassays, aphids were initially dosed with 0.1% PBO solution in acetone followed 5 h later by the insecticide dose. For the initial screening of the clones a diagnostic dose of 10 ppm imidacloprid solution was used while for full dose-response bioassays a range of concentrations was applied. Control aphids were dosed with 0.25 µl acetone. Three replicates were used for each concentration tested and the responses were assessed after 72 h at 18°C under a 16 : 8 h light:dark regime. Aphids that were dead or seriously affected were classed as ‘dead’ and the concentrations required to kill 50% (LC50 values) were calculated by probit analysis using POLO software (LeOra Software, Petaluma, CA). Resistance ratios were calculated relative to the susceptible clone 4106A and synergism ratios were calculated by dividing the LC50 to an insecticide alone by the LC50 of the synergised clone (pre-treated with PBO) to the same insecticide.
Artificial feeding assays
The oral toxicity of imidacloprid, clothianidin and thiamethoxam was assessed using an artificial double-membrane feeding bioassay. Aphids (10 individuals) were placed in a vented plastic dish (4 cm diameter) and the opening was sealed with Parafilm. Aphids were then starved overnight before 0.4 ml sucrose (150 g litre−1) containing the appropriate insecticide concentration was pipetted onto the Parafilm and another layer stretched on top to form a sachet. In order to avoid false positive results due to the potential anti-feeding effect of the insecticide, a starvation control was set alongside the treatments which had no sucrose added. The dishes were covered with a yellow bag to enhance feeding activity and placed at 18°C under a 16 : 8 h light:dark regime. Mortality was scored after 48 h and data analysed as for topical application bioassays.
Microarray
The microarray used in this study was designed using the Agilent eArray platform (Agilent Technologies) by the Georg Jander Lab and is based on a previously described array containing probes for >10, 000 M. persicae unigenes produced by Sanger sequencing [17] augmented with an additional 30, 517 probe set designed on EST unigene sequences identified in a 454 sequencing project [18]. The final slide layout consists of four arrays of 45, 220 60-mer probes produced by Agilent by in situ oligonucleotide synthesis.
Total RNA was extracted from four pools of ten 15 day-old adult aphids using Trizol following the manufacturer's instructions. Genomic DNA was removed by DNase I digestion using DNA-free DNase Treatment and Removal Reagent (Ambion). The quality and quantity of the RNAs were assessed by spectrophotometery (Nanodrop Technologies) and by running an aliquot on a 1.5% agarose gel. For the latter RNA was mixed with 1× loading buffer (95% formamide; 0.025% xylene cyanol; 0.025% bromophenol blue; 18 mM EDTA; 0.025% SDS), heated for 5 minutes at 65°C and briefly chilled on ice prior to loading. Two micrograms of each RNA was used to generate labelled cRNA, which was hybridized to arrays and these were washed and scanned as described in the Agilent Quick Amp Labeling Protocol (Version 5.7) [32]. The microarray experiment consisted of four biological replicates and for each of these, two hybridisations were done in which the Cy3 and Cy5 labels were swapped between samples for a total of eight hybridisations between resistant and susceptible clones.
Microarrays were scanned with an Agilent G2565CA scanner, and fluorescent intensities of individual spots were obtained using the Agilent Feature Extraction software with default Agilent parameters. The TM4 suite of software from the Institute of Genomic Research was used for all subsequent analysis [33]. Each Agilent feature extraction text file was transferred to TIGR Express Converter to generate TIGR MultiExperiment Viewer files (.mev) as output. Data normalization, filtering, and flip-dye consistency checks were performed using TIGR MIDAS. Briefly, spot intensity values over 300 were transformed to the log2 scale and data were normalized using locally weighted linear regression (lowess) with 33% of data used for smoothing and a cut-off of 0.01 [34]. Normalized data were then imported into the MultiExperiment Viewer (MeV) software and statistical analysis performed with the SAM (Significance Analysis of Microarrays) module applying a False Discovery Rate (FDR) of zero to detect significantly differentially expressed genes [35]. Genes identified by SAM and showing a transcription ratio >2 fold in either direction were considered to be differentially transcribed between the two strains. Microarray data were submitted to the Gene Expression Omnibus (GEO) database (series no. GSE20542).
Quantitative RT–PCR
Quantitative RT-PCR was used to validate microarray data by examining the expression profile of selected genes. Primers were designed to amplify a fragment 90–150 bp in size and are listed in Table S2. Total RNA was prepared and DNase I treated as described and four micrograms was used for cDNA synthesis using Superscript III and random hexamers (Invitrogen) according to the manufacturer's instructions. PCR reactions (20 µl) contained 4 µl of cDNA (10 ng), 10 µl of SensiMix SYBR Kit (Bioline), and 0.25 µM of each primer. Samples were run on a Rotor-Gene 6000 (Corbett Research) using the temperature cycling conditions of: 10 minutes at 95°C followed by 40 cycles of 95°C for 15 seconds, 57°C for 15 seconds and 72°C for 20 seconds. A final melt-curve step was included post-PCR (ramping from 72°C–95°C by 1°C every 5 seconds) to confirm the absence of any non-specific amplification. The efficiency of PCR for each primer pair was assessed using a serial dilution of 100 ng to 0.01 ng of cDNA. Each qRT-PCR experiment consisted of three independent biological replicates with three technical replicates for each. Data were analysed according to the ΔΔCT method [36], using the geometric mean of three selected housekeeping genes (actin, para which encodes the voltage gated sodium channel, and ace, which encodes acetylcholinesterase) for normalisation according to the strategy described previously [37].
Determination of P450 gene copy number by quantitative PCR
Quantitative PCR was used to determine CYP6CY3 gene copy number essentially as described above but using genomic DNA as the template. For this DNA from three pools of five 15 day-old adult aphids were extracted using the DNeasy Plant DNA Mini Kit (Qiagen) and using RNase A to remove contaminating RNA. DNA quality and quantity was assessed by spectrophotometery (Nanodrop Technologies) and by running an aliquot on a 1.5% agarose gel. The DNA was then diluted to 2.5 ng/ul and 4 µl used in PCR as detailed above. Data were analysed according to the ΔΔCT method [36] and normalised independently using three genes, para (present in two copies in diploid insect genomes), ace (present in four copies in M. persicae) and actin where the exact gene copy number is unknown but is thought to be multi-copy [10].
Rapid amplification of cDNA ends and amplification of full length cDNA from CYP6CY3
Five prime RACE was carried out using the RLM-RACE kit (Ambion) following the manufacturer's instructions. RACE was performed on RNA extracted from both the 4106A and 5191A M. persicae clones. The gene-specific primers for this purpose (Mpcyp6 mid r, Mpcyp6 mid r2, Mpcyp6 5′ outer R and Mpcyp6 5′ inner R) are detailed in Table S2. PCR products were recovered from agarose gels, cloned using the Strataclone PCR Cloning kit (Stratagene) and sequenced using the ABI BigDye Terminator Cycle Sequencing kit and T3/T7 primers. To verify the assembly the full length coding sequence of CYP6CY3 was amplified by nested PCR using primers Full F1 and Full R1, followed by Full F2 and Full R2 (Table S1). PCR reactions (20 µl) contained 4 µl of cDNA (10 ng), 1.5 units of Pfu DNA polymerase (Promega) and 0.5 µM of each primer and were subjected to cycling conditions of: 2 minutes at 94°C followed by 35 cycles of 95°C for 30 seconds, 57°C for 30 seconds and 72°C for 4 minutes. PCR products were purified and cloned and sequenced as above or sequenced directly using primers Full F2, Full R2, Mpcyp6 mid r and Mpcyp6 mid r2 (Table S2).
Membrane preparation and radioligand binding assays
Aphids of mixed ages (∼0.5 g) were homogenised in ice cold 10 mM phosphate buffer (pH 7.4) with 0.32 M sucrose, 1 mM EDTA, 1 µM leupeptin, 1 µg/ml pepstatin, 250 µM PMSF and 2 µl/ml protease inhibitor cocktail P-8340 (Sigma-Aldrich, UK) using a Polytron homogeniser. The homogenate was centrifuged for 10 min at 700 g (4°C) and the resulting supernatant passed through four layers of synthetic mesh before being re-centrifuged for 1 h at 105000 g (4°C). The pellet was resuspended in 10 mM phosphate buffer (pH 7.4) and the protein concentration measured using the DC protein assay (Bio-Rad laboratories) with bovine serum albumin as a standard. For the binding assay, 300 µg of membranes were incubated with 3 nM [3H] imidacloprid in a total volume of 300 µl. Non-specific binding was determined by blocking the nAChRs with 1 mM carbamylcholine and 1 mM nicotine prior to the addition of [3H] imidacloprid. The samples were incubated for 2 hours at 4°C on a shaker, filtered through Whatman GF/B filters pre-soaked in 0.5% polyethylenimine and washed with 10 mM phosphate buffer (pH 7.4) using a cell harvester (Brandel, Bethesda, MD). The assay was repeated three times for each clone.
Sequence analysis of M. persicae nAChR subunits
Total RNA was isolated from individual 4106A and 5191A adult aphids using Trizol (Invitrogen) following the manufacturer's protocol. First strand cDNA was synthesised from total RNA (1 µg) using SuperScript II reverse transcriptase (Invitrogen) and oligo-dT primer (100 ng). PCR amplification was performed using PCR Master Mix (Promega) and gene-specific oligonucleotide primers designed to the published nAChRα1 (X81887), nAChRα2 (X81888), nAChRα3 (AJ236786), nAChRα4 (AJ236787) and nAChRβ1 (AJ251838) sequences (see Table S2). The cycling conditions were 2 min at 94°C followed by 35 cycles of 30 sec at 94°C, 30 sec at 50°C and 1 min at 72°C and a final extension step of 5 min at 72°C. Amplicons of the expected size were ethanol precipitated and sequenced directly in both directions using the BigDye mix (Applied Biosystems).
Imidacloprid penetration assay
For the [3H] imidacloprid penetration assay three replicates of ten aphids each were used for each time point for both the susceptible (4106A) and resistant clone (5191A). Individuals of both clones were dosed with a sub-lethal dose of imidacloprid (0.14 ppm). A 0.25 µl drop of acetone containing 0.14 ppm [3H] imidacloprid was applied to each individual using a microapplicator (Burkard Manufacturing Ltd, UK). Samples (three replicates) from each clone were taken after 0.5, 1, 5, 10, 20 and 50 h to measure the degree of insecticide penetration. Aphids from each replicate were pooled in a tube and washed three times with 50 µl methanol. The three washes containing the imidacloprid on the cuticle were pooled together in a scintillation vial. The radiolabeled imidacloprid levels in the washes were measured by liquid scintillation spectrometry by adding 4 ml scintillation liquid (Ultima Gold, Perkin Elmer Inc. MA) over the methanolic extract, mixing and counting for 10 minutes. In addition, ten drops (0.25 µl) of the dosing solution was added to a glass filter paper, placed in 4 ml scintillation liquid and counted for the total number of counts expected per replicate. Results were expressed as a percentage of the total imidacloprid added to the treatment.
Sequence analysis
Molecular mass and isoelectric point were predicted by Compute pI/Mw tool (http://us.expasy.org/tools/pi_tool.html). The N-terminal transmembrane anchor of deduced proteins was predicted by the TMHMM Server v. 1.0 (http://www.cbs.dtu.dk/services/TMHMM/). DNA and predicted protein sequences were assembled, analysed and aligned using the Vector NTI Advance 10 package (Invitrogen). The full P450 gene sequence identified in this study was named by Dr David Nelson (Department of Molecular Science, University of Tennessee, Memphis) in accordance with the P450 nomenclature committee convention [15].
Supporting Information
Zdroje
1. FeyereisenR
1995 Molecular biology of insecticide resistance. Toxicol Lett 82-3 83 90
2. BlackmanRL
EastopVF
2000 Aphids on the world's crops, an identification and information guide. Chichester, UK John Wiley & Sons Ltd.
3. FieldLM
DevonshireAL
FordeBG
1988 Molecular evidence that insecticide resistance in peach-potato aphids (Myzus persicae Sulz.) results from amplification of an esterase gene. Biochem J 251 309 312
4. DevonshireAL
FieldLM
FosterSP
MooresGD
WilliamsonMS
1998 The evolution of insecticide resistance in the peach-potato aphid, Myzus persicae. Philos Trans R Soc Lond B Biol Sci 353 1677 1684
5. NauenR
DenholmI
2005 Resistance of insect pests to neonicotinoid insecticides: Current status and future prospects. Arch Insect Biochem Physiol 58 200 215
6. MargaritopoulosJT
SkourasPJ
NikolaidouP
ManolikakiJ
MaritsaK
2007 Insecticide resistance status of Myzus persicae (Hemiptera: Aphididae) populations from peach and tobacco in mainland Greece. Pest Manag Sci 63 821 829
7. FosterSP
CoxD
OliphantL
MitchinsonS
DenholmI
2008 Correlated responses to neonicotinoid insecticides in clones of the peach-potato aphid, Myzus persicae (Hemiptera: Aphididae). Pest Manag Sci 64 1111 1114
8. LiuZW
WilliamsonMS
LansdellSJ
DenholmI
HanZJ
2005 A nicotinic acetylcholine receptor mutation conferring target-site resistance to imidacloprid in Nilaparvata lugens (brown planthopper). Proc Natl Acad Sci U S A 102 8420 8425
9. RauchN
NauenR
2003 Identification of biochemical markers linked to neonicotinoid cross resistance in Bemisia tabaci (Hemiptera: Aleyrodidae). Arch Insect Biochem Physiol 54 165 176
10. KarunkerI
BentingJ
LuekeB
PongeT
NauenR
2008 Over-expression of cytochrome P450 CYP6CM1 is associated with high resistance to imidacloprid in the B and Q biotypes of Bemisia tabaci (Hemiptera: Aleyrodidae). Insect Biochem Mol Biol 38 634 644
11. KarunkerI
MorouE
NikouD
NauenR
SertchookR
2009 Structural model and functional characterization of the Bemisia tabaci CYP6CM1vQ, a cytochrome P450 associated with high levels of imidacloprid resistance. Insect Biochem Mol Biol 39 697 706
12. DabornPJ
YenJL
BogwitzMR
Le GoffG
FeilE
2002 A single P450 allele associated with insecticide resistance in Drosophila. Science 297 2253 2256
13. PhilippouD
FieldLM
MooresGD
2009 Metabolic enzyme(s) confer imidacloprid resistance in a clone of Myzus persicae (Sulzer) (Hemiptera: Aphididae) from Greece. Pest Manag Sci 66 390 395
14. FeyereisenR
2006 Evolution of insect P450. Biochem Soc Trans 34 1252 1255
15. NelsonDR
2009 The cytochrome P450 homepage. Hum Genomics 4 59 65
16. The International Aphid Genomics Consortium 2010 Genome sequence of the pea aphid Acyrthosiphon pisum PLoS Biol 8 e1000313 doi:10.1371/journal.pbio.1000313
17. RamseyJS
WilsonACC
de VosM
SunQ
TamborindeguyC
2007 Genomic resources for Myzus persicae: EST sequencing, SNP identification, and microarray design. BMC Genomics 8 423
18. RamseyJS
RiderDS
WalshTK
De VosM
GordonKHJ
2010 Comparative analysis of detoxification enzymes in Acyrthosiphon pisum and Myzus persicae. Insect Mol Biol 19 155 164
19. FieldLM
BlackmanRL
Tyler-SmithC
DevonshireAL
1999 Relationship between amount of esterase and gene copy number in insecticide-resistant Myzus persicae (Sulzer). Biochem J 339 737 742
20. MagkriotiCK
SpyropoulosIC
IconomidouVA
WillisJH
HamodrakasSJ
2004 cuticleDB: a relational database of Arthropod cuticular proteins. BMC Bioinformatics 5 138
21. MarcombeS
PoupardinR
DarrietF
ReynaudS
BonnetJ
2009 Exploring the molecular basis of insecticide resistance in the dengue vector Aedes aegypti: a case study in Martinique Island (French West Indies). BMC Genomics 10 494
22. FosterSP
DenholmI
ThompsonR
2003 Variation in response to neonicotinoid insecticides in peach-potato aphids, Myzus persicae (Hemiptera: Aphididae). Pest Manag Sci 59 166 173
23. LiXC
SchulerMA
BerenbaumMR
2007 Molecular mechanisms of metabolic resistance to synthetic and natural xenobiotics. Annu Rev Entomol 52 231 253
24. WondjiCS
IrvingH
MorganJ
LoboNF
CollinsFH
2009 Two duplicated P450 genes are associated with pyrethroid resistance in Anopheles funestus, a major malaria vector. Genome Res 19 452 459
25. EmersonJJ
Cardoso-MoreiraM
BorevitzJO
LongM
2008 Natural selection shapes genome-wide patterns of copy-number polymorphism in Drosophila melanogaster. Science 320 1629 1631
26. GallotA
RispeC
LetermeN
GauthierJP
Jaubert-PossamaiS
2010 Cuticular proteins and seasonal photoperiodism in aphids. Insect Biochem Mol Biol doi:10.1016/j.ibmb.2009.12.001
27. VallesSM
DongK
BrennerRJ
2000 Mechanisms responsible for cypermethrin resistance in a strain of German cockroach, Blattella germanica. Pestic Biochem Physiol 66 195 205
28. AhmadM
DenholmI
BromilowRH
2006 Delayed cuticular penetration and enhanced metabolism of deltamethrin in pyrethroid-resistant strains of Helicoverpa armigera from China and Pakistan. Pest Manag Sci 62 805 810
29. WalterCM
PriceNR
1989 The uptake and penetration of pirimiphos-methyl into susceptible and resistant strains of the rust red flour beetle—Tribolium castaneum, herbst (coleoptera: tenebrionidae). Comp Biochem Physiol C Pharmacol Toxicol Endocrinol 94 419 423
30. DjouakaRF
BakareAA
CoulibalyON
AkogbetoMC
RansonH
2008 Expression of the cytochrome P450s, CYP6P3 and CYP6M2 are significantly elevated in multiple pyrethroid resistant populations of Anopheles gambiae s.s. from Southern Benin and Nigeria. BMC Genomics 9 538
31. Guidelines for preventing and managing insecticide resistance in aphids on potatoes. Version April 2008 ed: Insecticide Resistance Action Group (IRAG-UK)
32. Two-Color Microarray-Based Gene Expression analysis (Quick Amp Labeling). Version 5.7 ed: Agilent Technologies
33. SaeedAI
SharovV
WhiteJ
LiJ
LiangW
2003 TM4: A free, open-source system for microarray data management and analysis. Biotechniques 34 374 378
34. ClevelandWS
DevlinSJ
1988 Locally Weighted Regression - An approach to regression-Analysis by local fitting. J Am Stat Assoc 83 596 610
35. TusherVG
TibshiraniR
ChuG
2001 Significance analysis of microarrays applied to the ionizing radiation response. Proc Natl Acad Sci U S A 98 10515 10515
36. PfafflMW
2001 A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res 29 e45
37. VandesompeleJ
PreterKD
PattynF
PoppeB
RoyNV
2002 Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol 3 research0034
Štítky
Genetika Reprodukční medicína
Článek The Met 470 Allele Is Associated with Lower Birth Rates in Fertile Men from a Population IsolateČlánek Copy Number Variation and Transposable Elements Feature in Recent, Ongoing Adaptation at the Locus
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2010 Číslo 6- Růst a vývoj dětí narozených pomocí IVF
- Délka menstruačního cyklu jako marker ženské plodnosti
- Vztah užívání alkoholu a mužské fertility
- Šanci na úspěšný průběh těhotenství snižují nevhodné hladiny progesteronu vznikající při umělém oplodnění
- Vliv melatoninu a cirkadiálního rytmu na ženskou reprodukci
-
Všechny články tohoto čísla
- Translational Selection Is Ubiquitous in Prokaryotes
- Whole-Genome Sequencing of a Single Proband Together with Linkage Analysis Identifies a Mendelian Disease Gene
- Web-Based, Participant-Driven Studies Yield Novel Genetic Associations for Common Traits
- The Met 470 Allele Is Associated with Lower Birth Rates in Fertile Men from a Population Isolate
- Contributions of Status and Allele Expression, But Not Copy Number Variation, to the Control of SIVmac251 Replication in Indian-Origin Rhesus Monkeys
- A Genome-Wide Association Study of Optic Disc Parameters
- Amplification of a Cytochrome P450 Gene Is Associated with Resistance to Neonicotinoid Insecticides in the Aphid
- The Transcription Factor REST Is Lost in Aggressive Breast Cancer
- 14-3-3 Mediates Histone Cross-Talk during Transcription Elongation in
- Copy Number Variation and Transposable Elements Feature in Recent, Ongoing Adaptation at the Locus
- Use of Genome-Wide Expression Data to Mine the “Gray Zone” of GWA Studies Leads to Novel Candidate Obesity Genes
- Genome-Wide RNAi Screen Identifies Multiple Regulators of HIF–Dependent Transcription in Hypoxia
- The CYCLIN-A CYCA1;2/TAM Is Required for the Meiosis I to Meiosis II Transition and Cooperates with OSD1 for the Prophase to First Meiotic Division Transition
- Inactivation of hnRNP K by Expanded Intronic AUUCU Repeat Induces Apoptosis Via Translocation of PKCδ to Mitochondria in Spinocerebellar Ataxia 10
- Mice with Alopecia, Osteoporosis, and Systemic Amyloidosis Due to Mutation in , a Gene Coding for Palmitoyl Acyltransferase
- siRNA–Mediated Methylation of Telomeres
- Chromosome 4 Replicates in Two Phases That Correlate with Chromatin State
- Dynamic Switch of Negative Feedback Regulation in Akt–TOR Signaling
- On the Use of Variance per Genotype as a Tool to Identify Quantitative Trait Interaction Effects: A Report from the Women's Genome Health Study
- and Deficiency Cooperate in the Progression of Mouse Prostate Tumourigenesis
- An Integration of Genome-Wide Association Study and Gene Expression Profiling to Prioritize the Discovery of Novel Susceptibility Loci for Osteoporosis-Related Traits
- Consent and Internet-Enabled Human Genomics
- Understanding Adaptation in Large Populations
- Identification of a Functional Genetic Variant at 16q12.1 for Breast Cancer Risk: Results from the Asia Breast Cancer Consortium
- Evidence that Adaptation in Is Not Limited by Mutation at Single Sites
- The IG-DMR and the -DMR at Human Chromosome 14q32.2: Hierarchical Interaction and Distinct Functional Properties as Imprinting Control Centers
- Cushing's Syndrome and Fetal Features Resurgence in Adrenal Cortex–Specific Knockout Mice
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Web-Based, Participant-Driven Studies Yield Novel Genetic Associations for Common Traits
- The IG-DMR and the -DMR at Human Chromosome 14q32.2: Hierarchical Interaction and Distinct Functional Properties as Imprinting Control Centers
- Amplification of a Cytochrome P450 Gene Is Associated with Resistance to Neonicotinoid Insecticides in the Aphid
- Copy Number Variation and Transposable Elements Feature in Recent, Ongoing Adaptation at the Locus
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



