-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Genome-Wide Association Study Identifies as a Novel Susceptibility Gene for Osteoporosis
Osteoporosis is a major public health problem. It is mainly characterized by low bone mineral density (BMD) and/or low-trauma osteoporotic fractures (OF), both of which have strong genetic determination. The specific genes influencing these phenotypic traits, however, are largely unknown. Using the Affymetrix 500K array set, we performed a case-control genome-wide association study (GWAS) in 700 elderly Chinese Han subjects (350 with hip OF and 350 healthy matched controls). A follow-up replication study was conducted to validate our major GWAS findings in an independent Chinese sample containing 390 cases with hip OF and 516 controls. We found that a SNP, rs13182402 within the ALDH7A1 gene on chromosome 5q31, was strongly associated with OF with evidence combined GWAS and replication studies (P = 2.08×10−9, odds ratio = 2.25). In order to explore the target risk factors and potential mechanism underlying hip OF risk, we further examined this candidate SNP's relevance to hip BMD both in Chinese and Caucasian populations involving 9,962 additional subjects. This SNP was confirmed as consistently associated with hip BMD even across ethnic boundaries, in both Chinese and Caucasians (combined P = 6.39×10−6), further attesting to its potential effect on osteoporosis. ALDH7A1 degrades and detoxifies acetaldehyde, which inhibits osteoblast proliferation and results in decreased bone formation. Our findings may provide new insights into the pathogenesis of osteoporosis.
Published in the journal: . PLoS Genet 6(1): e32767. doi:10.1371/journal.pgen.1000806
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1000806Summary
Osteoporosis is a major public health problem. It is mainly characterized by low bone mineral density (BMD) and/or low-trauma osteoporotic fractures (OF), both of which have strong genetic determination. The specific genes influencing these phenotypic traits, however, are largely unknown. Using the Affymetrix 500K array set, we performed a case-control genome-wide association study (GWAS) in 700 elderly Chinese Han subjects (350 with hip OF and 350 healthy matched controls). A follow-up replication study was conducted to validate our major GWAS findings in an independent Chinese sample containing 390 cases with hip OF and 516 controls. We found that a SNP, rs13182402 within the ALDH7A1 gene on chromosome 5q31, was strongly associated with OF with evidence combined GWAS and replication studies (P = 2.08×10−9, odds ratio = 2.25). In order to explore the target risk factors and potential mechanism underlying hip OF risk, we further examined this candidate SNP's relevance to hip BMD both in Chinese and Caucasian populations involving 9,962 additional subjects. This SNP was confirmed as consistently associated with hip BMD even across ethnic boundaries, in both Chinese and Caucasians (combined P = 6.39×10−6), further attesting to its potential effect on osteoporosis. ALDH7A1 degrades and detoxifies acetaldehyde, which inhibits osteoblast proliferation and results in decreased bone formation. Our findings may provide new insights into the pathogenesis of osteoporosis.
Introduction
Osteoporosis, characterized primarily by low bone mineral density (BMD), is a major public health problem because it increases susceptibility to low-trauma osteoporotic fractures (OF). Hip fractures, which are the most common and severe form of OF, are associated with high morbidity and mortality, as well as tremendous health care expenditures [1]. Due to an aging population, the annual incidence of hip fractures worldwide is predicted to be ∼6.27 million by the year 2050, with an estimated cost of ∼$131.5 billion [1]. The ultimate goal of osteoporosis research is to reduce the incidence and prevalence of OF.
Genetic factors play an important role in susceptibility to osteoporosis. Both BMD and OF have high genetic determinations [2],[3],[4],[5]. BMD has been identified as the major risk factor for susceptibility to OF and is currently the predominant study phenotype for osteoporosis. Variations in BMD account for ∼50–70% of the variation in total bone strength [6] and risk of OF [7]. Additional risk factors, including those not readily quantifiable (e.g. bone microstructure [8] and cartilage organization [9]), also contribute to the risk of OF. Most of genetic studies of osteoporosis have focused primarily on the surrogate phenotype BMD, whereas little effort has been expended on the study of OF per se as a focal phenotype or on the relevance of genes associated with BMD on OF [3]. The major obstacle to this approach has been assembling a homogeneous sample with a homogenously defined OF type. Genetic factors associated with variations in BMD and risk of OF overlap, to some extent, but are not all identical [2]. The ultimate goal of osteoporosis research is to reduce the incidence and prevalence of OF. Therefore, it is useful to conduct genetic studies of OF per se, in conjunction with other intermediate phenotypes (e.g. BMD) that influence the risk of OF. This approach can be used to identify quantifiable measures for early prevention and intervention before the adverse clinical outcome, OF, actually occurs.
So far, several specific genes contributing to osteoporosis (i.e. those impacting BMD or risk of OF) have been identified, such as ESR1 with OF risk, COL1A1 and VDR with BMD and vertebral fracture risk, OPG and LRP5 with BMD [10],[11],[12],[13],[14],[15]. However, the majority of genetic variants that influence osteoporosis remain unknown. With current high throughput SNP genotyping platforms and our knowledge about the distribution and correlation of SNPs in the human genome (e.g., haplotype structure), genome-wide association study (GWAS) has proven itself to be a feasible, powerful and effective approach for identifying novel genes associated with complex phenotypes. Four recent GWAS's [12],[13],[16],[17] have identified several specific genes for osteoporosis. In the current investigation, based on significant heritability of ∼50% for OF [2],[4], we utilized a GWAS to identify genetic variants underlying susceptibility to osteoporosis that are directly relevant to the risk of OF. Using the Affymetrix 500K array set, we successfully genotyped a study population of 700 elderly Chinese Han subjects consisting of 350 cases with homogeneous hip OF and 350 healthy matched controls. A follow-up replication study was performed in an independent Chinese sample consisting of 390 cases with hip OF and 516 controls. For SNPs that were identified for OF, we further examined their relationships with hip BMD in two ethnic groups (Chinese and Caucasians), involving additional 9,962 subjects, in order to determine whether the genetic basis for their contribution to the risk of OF might also be, at least partially, attributable to their effects on variation in BMD.
Results
GWAS Discovery Study
The study design included an initial exploratory stage in a Chinese Han sample of moderate size and follow-up replication and validation studies with much larger sample sizes in independent Chinese Han and Caucasian samples. Table 1 details the basic characteristics of the respective samples. In the GWAS discovery stage, a total of 281,533 SNPs passed our quality control criteria for GWAS analyses. A quantile-quantile (QQ) plot is presented in Figure 1. The χ2 distributions for the association tests across the SNPs tested showed little evidence of overall systematic bias (genomic inflation factor λ = 1.02). The highest χ2 was consistent with the presence of true association. We further performed the principal component analysis implemented in EIGENSTRAT to guard against possible population stratification. The first two principal components were not associated with case status (P values>0.05), further indicating that it is very unlikely that positive associations in this study would be attributable to confounding due to population structure. The association analyses by EIGENSTRAT confirmed, qualitatively, our main results and consequently, the results of the EIGENSTRAT analyses are not detailed here.
Fig. 1. Quantile-quantile (QQ) plot of genome-wide allelic association results. 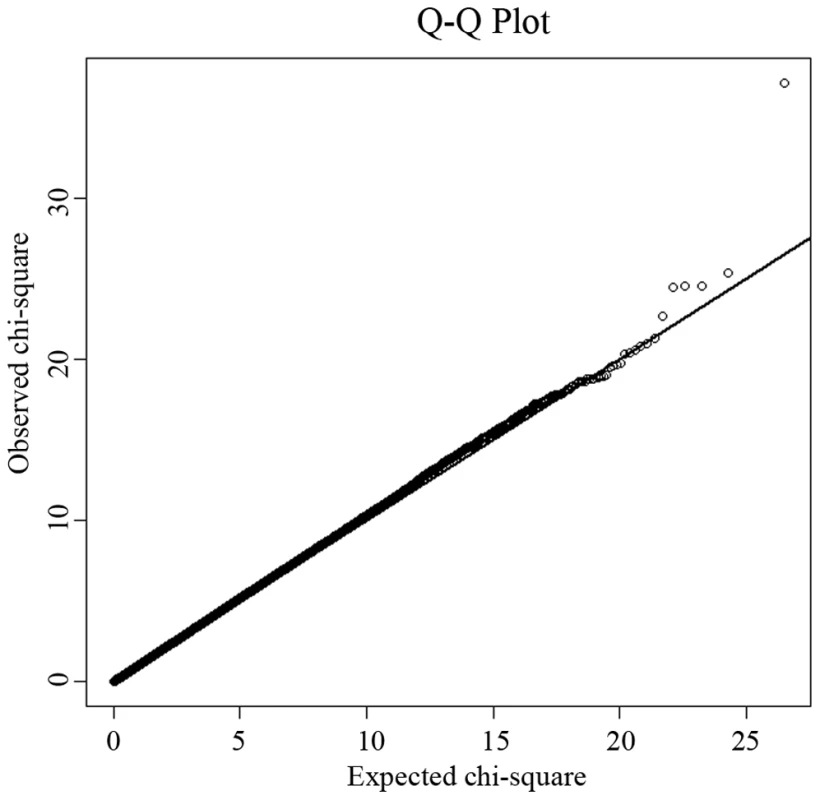
Under the null hypothesis of no association at any locus, the points would be expected to follow the slope line. Deviations from the slope line correspond to loci that deviate from the null hypothesis. Tab. 1. Characteristics of the study subjects. 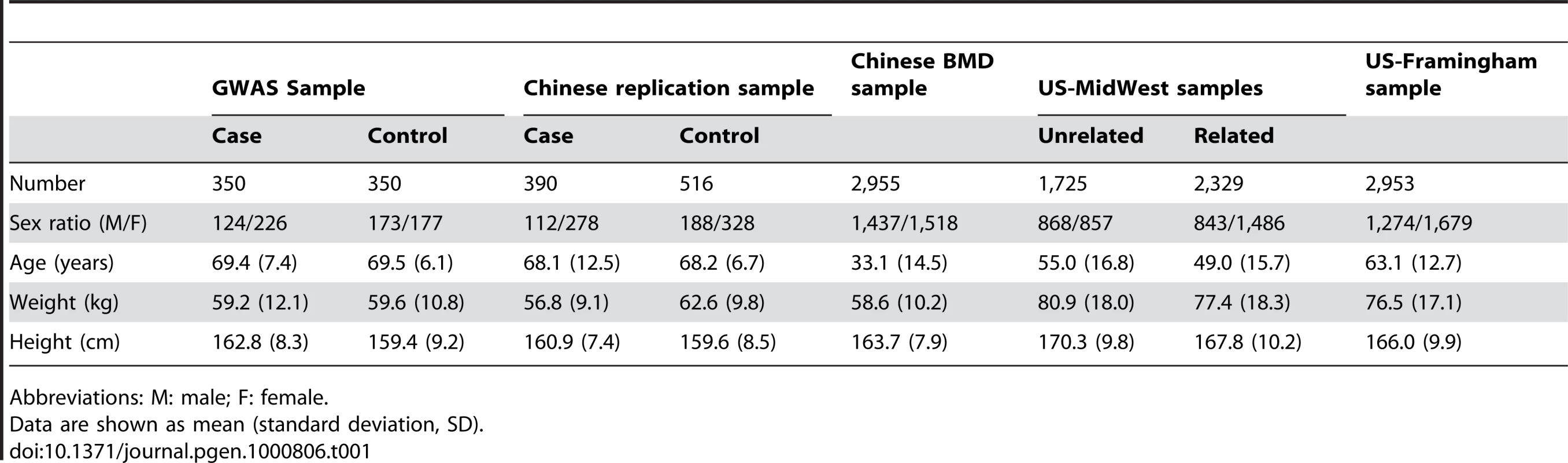
Abbreviations: M: male; F: female. Table 2 lists the most promising results from GWAS analyses. We identified five SNPs with P values<5×10−6 by allelic association analyses. After applying the Bonferroni correction for multiple testing, a single SNP, rs13182402, reached a genome-wide significance level (P<1.78×10−7). SNP rs13182402 achieved a P value of 8.53×10−9 in the allelic test (Bonferroni corrected P = 2.40×10−3). The odds ratio (OR) was 2.94 (95% confidence interval (CI): 2.02–4.30) for minor allele G. The frequency of the G allele was 0.162 in cases, and 0.061 in controls. When all covariates were considered simultaneously in a multivariate logistic regression model, this SNP remained a significant predictor of OF risk, independent of age, sex, height, and weight (P = 2.21×10−8).
Tab. 2. Summary of the GWAS and replication studies. 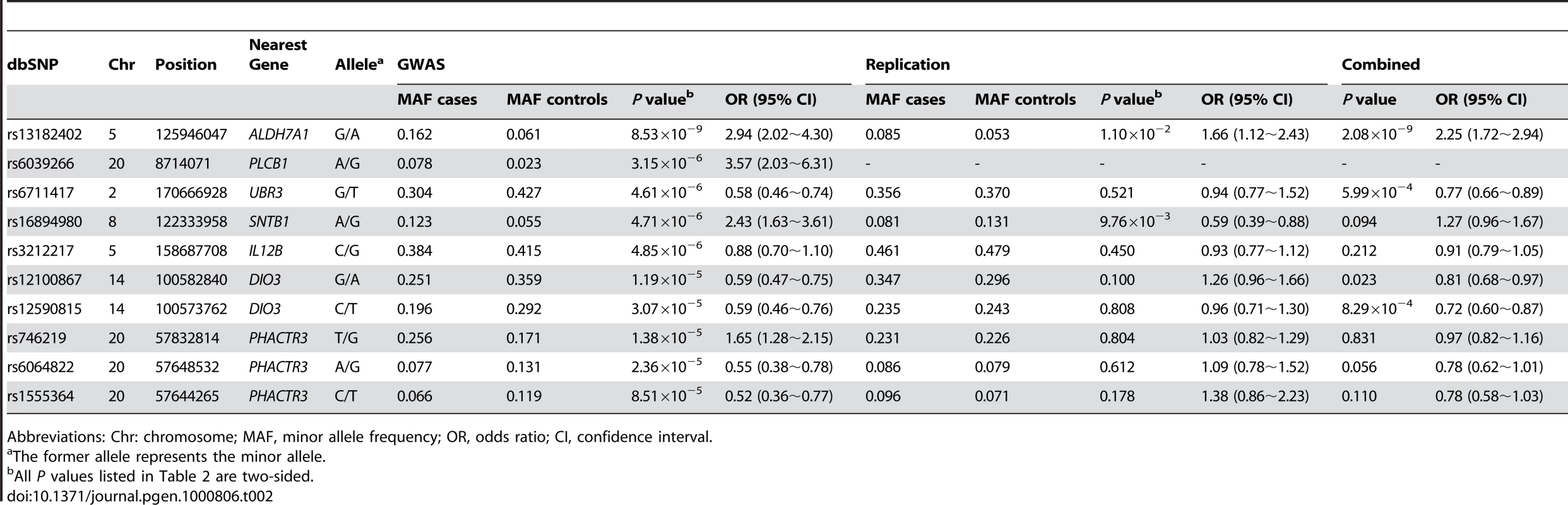
Abbreviations: Chr: chromosome; MAF, minor allele frequency; OR, odds ratio; CI, confidence interval. Assessment of Genome-Wide Findings
Replication in Chinese
Replication analyses were performed in an independent Chinese sample containing 390 cases with hip OF and 516 controls. Of the ten genotyped SNPs in the OF replication sample, two SNPs (rs13182402 and rs16894980) were nominally significant (Table 2). However, considering the effect direction, only SNP rs13182402 was successfully replicated (P = 1.10×10−2) and the effect was in the same direction as in the initial GWAS sample (OR: 1.66 for allele G, 95% CI: 1.12∼2.43). Combining the GWAS discovery and replication samples by meta-analyses, rs13182402 achieved a P value of 2.08×10−9 with an estimated OR of 2.25 (95% CI: 1.72∼2.94).
BMD validation in Chinese and Caucasians
To further explore the relationship between rs13182402 and osteoporosis risk, we performed association analyses with hip BMD in Chinese and Caucasian samples (Table 3). In the Chinese BMD sample, rs13182402 was associated with reduced hip BMD values (P = 2.35×10−2) and the effect size (β) was estimated to be ∼0.04 for each copy of the minor allele. This was consistent with its association with an increased risk of hip OF. The contribution of rs13182402 to BMD variation was estimated to be ∼0.68%.
Tab. 3. BMD validation association results for rs13182402. 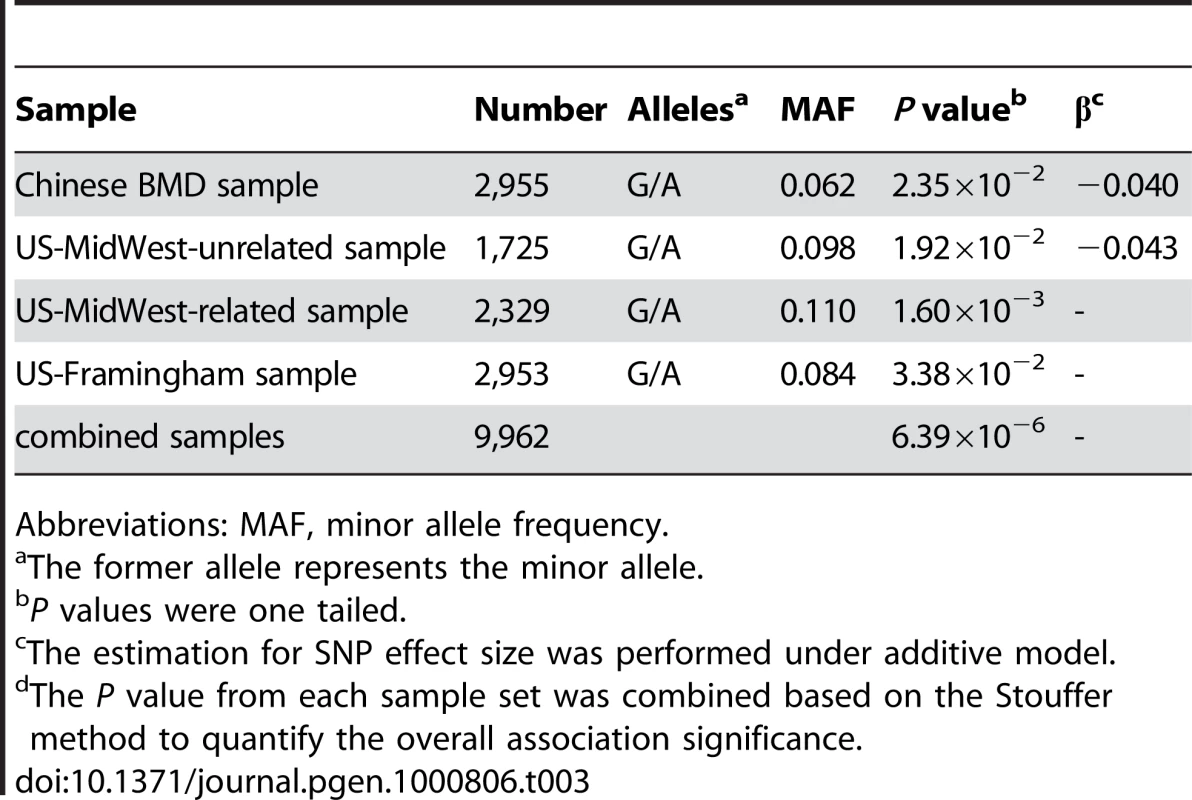
Abbreviations: MAF, minor allele frequency. Statistical significance of rs13182402 was consistently achieved in the US-MidWest Caucasian samples (unrelated sample: P = 1.92×10−2; related sample: P = 1.60×10−3), and the effect is in the same direction as in the Chinese BMD sample. The β was estimated to be 0.043 for each copy of the minor allele in the unrelated sample. The contribution of this SNP to BMD variation in the unrelated sample was estimated to be ∼0.75%.
We further examined the association signal in the US-Framingham Caucasian sample. SNP rs13182402 was consistently significantly associated with hip BMD in the US-Framingham sample (P = 3.38×10−2).
We also examined the associations between rs13182402 and spine BMD in the Chinese and Caucasian BMD samples. However, no significant results were found (data not shown), which might be due to the heterogeneity of BMD across different skeletal sites [18].
Finally, using meta-analysis, we combined all of the BMD validation results (one Chinese sample and three Caucasian samples) to yield a commonly used probability measure. The statistical significance for rs13182402 was significantly improved (P = 6.39×10−6). These findings, combined with the results of our GWAS studies, lend strong support for the conclusion that rs13182402 is associated with low hip BMD and increased risk for OF.
Fine Mapping for Gene Identification
Given the significant evidence for rs13182402, we imputed the genotypes of SNPs located surrounding this SNP based on our GWAS data and Asian HapMap data, and presented a regional association plot in Figure 2. The most significantly associated SNP, rs13182402 (GWAS: P = 8.53×10−9), is located 394 bp downstream from exon 5 of the ALDH7A1 gene (aldehyde dehydrogenase 7 family, member A1) on chromosome 5q31. According to the FASTSNP program (http://fastsnp.ibms.sinica.edu.tw), a change of “A→G” at rs13182402 may lead to removal of binding sites for transcription factors RORalp and CdxA.
Fig. 2. Regional Association Plot for rs13182402 on chromosome 5 in the GWAS stage. 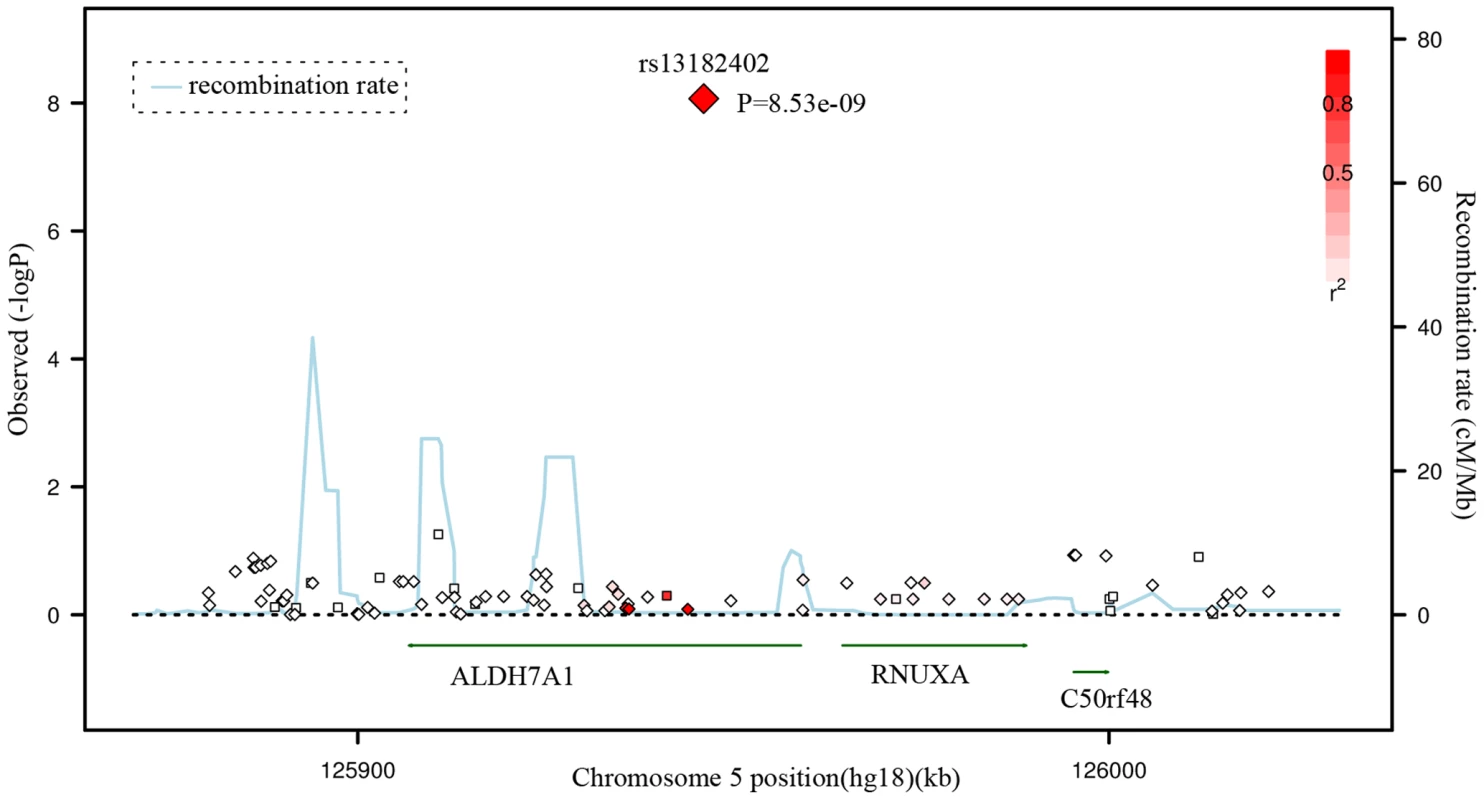
The color scheme of a white-to-red gradient reflects lower to higher LD values (r2). The scatter graph indicates the negative logarithm of P-value for each SNP. Discussion
In this study, we first performed a GWAS and follow-up replication on OF and identified a novel susceptibility gene (ALDH7A1) that significantly impacts the risk for OF per se. Next, we examined this gene's relationship with hip BMD both in Chinese and Caucasian populations, and this gene was consistently associated with hip BMD even across ethnic boundaries. The effect size on BMD was modest and lower than the effect size on OF risk. One interpretation of this differential effect would be that BMD is not the only risk factor for OF; other risk factors also contribute to the risk of OF. It is consistent with and supports our statement in the introduction. It might also be caused by the differences in power between the relatively small hip OF samples compared to the large BMD samples. In addition, because we didn't have BMD measurements for the hip OF cases, we couldn't adjust the OR for BMD to see if the risk would be attenuated by the adjustment. However, regardless of this differential effect, the significant association results we identified both for BMD and OF risk strongly support the potential contribution of ALDH7A1 to the pathogenesis of osteoporosis.
The ALDH7A1 gene encodes an enzyme of the acetaldehyde dehydrogenase superfamily, which degrades and detoxifies acetaldehyde generated by alcohol metabolism. Acetaldehyde has been shown to inhibit osteoblast proliferation and to decrease bone formation [19]. In addition, previous studies have identified that polymorphisms of the ALDH2 gene, another member of the acetaldehyde dehydrogenase family, are significantly associated with osteoporosis [20]. Our findings, combined with the above lines of evidence, suggest that ALDH7A1 might be a novel and potential candidate gene contributing to the risk of osteoporosis.
Using the genotyped and imputed genotypes in our GWAS sample of 700 Chinese, we examined the associations between hip OF and the key SNPs identified in previous GWAS on osteoporosis [12],[13],[16]. Table 4 summarizes the major results. Only two SNPs in RANKL were confirmed to be associated with hip OF in our sample, including rs9594759 (P = 0.020) and rs9594738 (P = 0.045). The data provided may serve as a reference for other investigators searching for replication for their GWAS results.
Tab. 4. Comparison of the previous GWAS for BMD and the current GWAS. 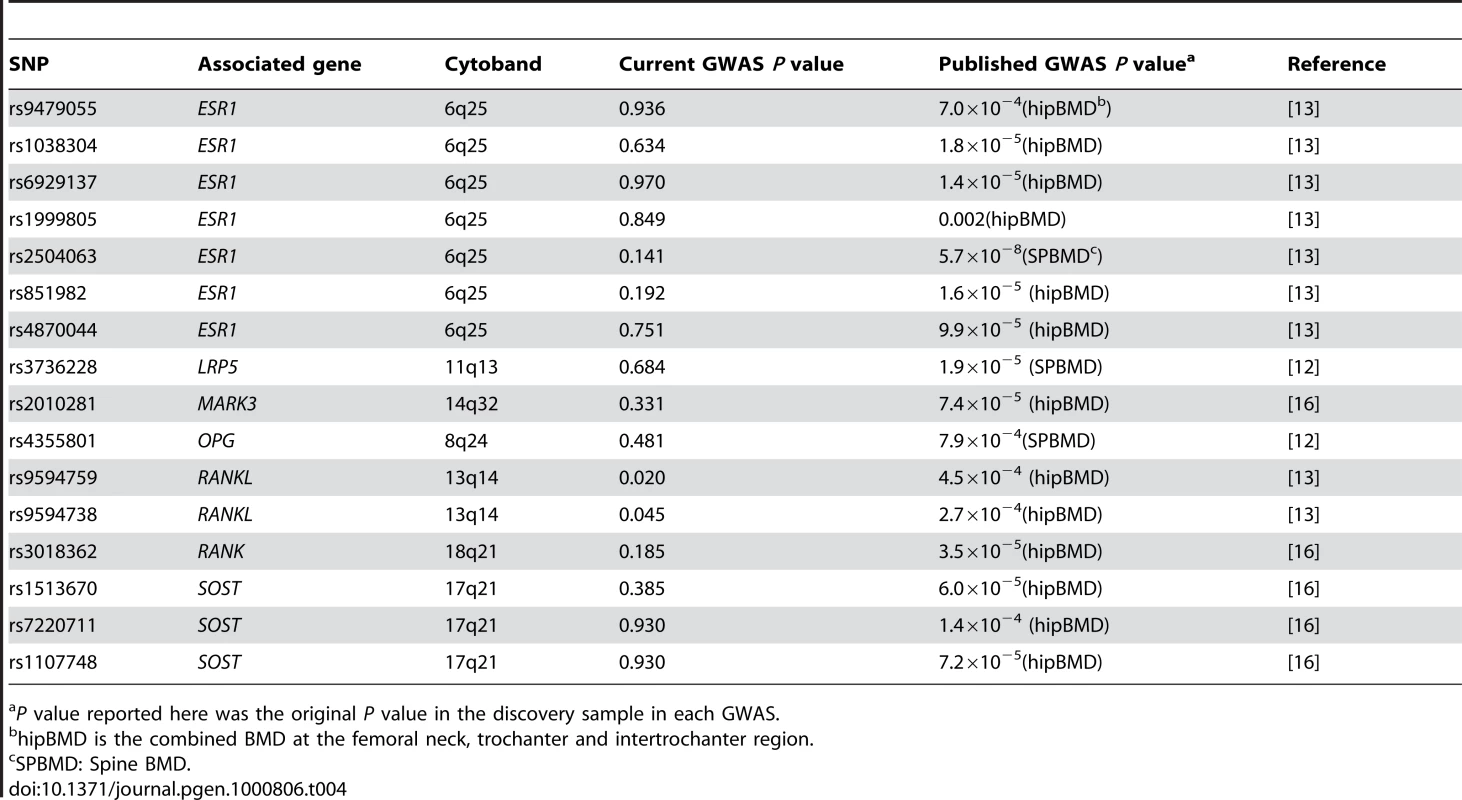
P value reported here was the original P value in the discovery sample in each GWAS. An apparent advantage of this study is that our GWAS sample came from a homogenous population with well defined homogeneous phenotype. The genomic control factor was quite close to 1.0 (λ = 1.02) (expected under no population stratification) and, analyses by EIGENSTRAT showed qualitatively supportive results. Thus, our association results are unlikely to be plagued by spurious associations due to population stratification. In particular, since the significant associations with BMD are shown in both Caucasian and Chinese samples, the results are even less likely to be due to population stratification/admixture.
A potential limitation of our study is the relatively small size of the GWAS sample and the replication sample, which might lead to over estimation of the effect size for the significant SNPs identified. However, hip fractures are the most severe OFs followed by high mortality rates, making subjects recruitment difficult. It took us several years to accumulate such a homogeneous hip OF sample. This study represents the best we can do under current conditions to identify genes for OF. Meanwhile, we are keeping the recruitment of hip OF subjects. As a future direction, a new GWAS needs to be implemented on a larger sample to identify more comprehensively novel genes for OF. In addition, since genetic and environmental backgrounds vary for different populations, replication across a wide range of populations is necessary to determine the generality of our findings to the broader population, or to specific ethnic groups or populations [21].
In summary, using data from over 11,500 individuals, we have identified and validated ALDH7A1 as a novel susceptibility candidate gene for osteoporosis. Further studies are warranted to explore the generality of our findings for ALDH7A1 identified by GWAS to other populations, and to determine the mechanisms by which this gene and its products contribute to the pathogenesis of osteoporosis.
Materials and Methods
Ethics Statement
The study was approved by the local institutional review boards or the office of research administration of all participating institutions. After signing an informed consent, all subjects received assistance completing a structured questionnaire including anthropometric variables, lifestyles, and medical history.
Study Samples
The study was initially performed with a GWAS discovery stage for SNPs of potential significance for OF in a Chinese Han, case-control sample. The significance of the SNPs identified in the discovery stage was subsequently confirmed through replication study in another independent Chinese case-control sample. For SNPs identified for OF, we further examined their relationships with hip BMD within/across ethnic groups in a Chinese unrelated BMD sample and three independent Caucasian samples. Table 1 details the basic characteristics of the respective samples, with additional descriptions below.
GWAS Discovery Sample
The sample for the initial GWAS consisted of 350 patients with osteoporotic (low trauma) hip fractures (including 124 males and 226 females) and 350 elderly controls (including 173 males and 177 females). Since fractures at different skeletal sites may have different underlying pathological mechanisms, we focused exclusively on hip fractures in order to minimize potential clinical and genetic heterogeneity of the study phenotype. All the subjects were unrelated northern Chinese Han adults living in the city of Xi'an and its neighboring areas. Affected individuals with low trauma hip fractures were recruited from the affiliated hospitals and their associated clinics of Xi'an Jiaotong University. Inclusion criteria for cases were (i) onset age of hip OF>55 years, to make sure all female subjects were postmenopausal, and the onset of OF was largely due to decreased BMD; (ii) age<80 years to minimize the effect due to age, since previous studies showed that approximately half of females aged 80 years or older have fractures [22]; (iii) fractures occurred with minimal or no trauma, usually due to falls from standing height or less; (iv) the fracture sites were at the femoral neck or inter-trochanter regions; (v) hip fracture was identified/confirmed through diagnosis of orthopedic surgeons/radiologists according to radiological reports and x-rays. Patients with pathological fractures and high-impact fractures (such as due to motor vehicle accidents) were excluded. Patients with chronic diseases before the onset of HF were also excluded.
Healthy control subjects were enrolled by use of local advertisements. They were geography - and age-matched to the cases. Inclusion/exclusion criteria for controls were: (i) age at exam must be >55 years, without any fracture histories (all female controls were postmenopausal); (ii) subjects with chronic diseases and conditions that might potentially affect bone mass, structure, or metabolism were excluded. Diseases/conditions resulting in exclusion included chronic disorders involving vital organs (heart, lung, liver, kidney, brain), serious metabolic diseases (diabetes, hypo - and hyper-parathyroidism, hyperthyroidism, etc.), other skeletal diseases (Paget's disease, osteogenesis imperfecta, rheumatoid arthritis, etc.), chronic use of drugs affecting bone metabolism (e.g., hormone replacement therapy, corticosteroid therapy, anti-convulsant drugs), and malnutrition conditions (such as chronic diarrhea, chronic ulcerative colitis); (iii) subjects taking anti-bone-resorptive or bone anabolic agents/drugs, such as bisphosphonates were excluded.
Chinese Replication Sample
For replication of our GWAS findings for hip OF, we used an independent Chinese sample containing 906 unrelated Han subjects (390 cases with hip OF and 516 controls). All subjects were drawn from the same geographic area as the above GWAS discovery sample, and the sample inclusion and exclusion criteria for cases and controls were the same as those adopted in the recruitment of the above GWAS sample.
For SNPs that were identified for OF, we further performed validation analyses to evaluate their relevance with hip BMD (with targeted experimental genotyping of candidate SNPs discovered in the initial GWAS) in two ethnic groups, including a Chinese sample and two US Mid-West Caucasian samples. We finally performed in silico validation to compare the association signals of our most promising GWAS results with those achieved in the Framingham Heart Study (FHS) [23].
Chinese BMD Sample
The Chinese BMD sample contained 2,955 unrelated ethnic Han adults. This sample came from Changsha, Hunan province, which is more than 1,000 km from Xi'an where the sample for the GWAS was recruited. The subjects were randomly selected from an established and expanding database with BMD measurements. The exclusion criteria were the same as those adopted in the recruitment of healthy control subjects in the GWAS sample, and have been detailed in our earlier publication [2].
US–MidWest Caucasian BMD Samples
The US-MidWest BMD samples with a total of 4,054 subjects consisted of two independent sample sets, including one sample of unrelated subjects and the other sample of nuclear families, which were all US Caucasians of Northern European origin living in Omaha, Nebraska, and its surrounding regions in Midwestern USA. They were normal healthy subjects defined by the same exclusion criteria as above in Chinese samples. The unrelated sample contained 1,725 subjects. The related sample contained 2,329 subjects from 593 nuclear families.
All hip BMD measurements for the above BMD samples were obtained with dual-energy X-ray absorptiometry using the same type of machine (Hologic 4500) under the same protocol defined by the manufacturer (Hologic Inc., Bedford, MA, USA). The machines were calibrated daily. The coefficients of variation (CV) of the hip BMD measurements were 1.33% for Chinese and 1.40% for Caucasians, respectively.
US-Framingham BMD Sample
The US-Framingham BMD sample is from the Framingham Osteoporosis Study, an ancillary study of the Framingham SNP Health Association Resource (SHARe) data sets [23]. Details and descriptions about the Framingham Osteoporosis Study have been previously reported [24]. Both genotype and phenotype data were downloaded from dbGaP database (http://www.ncbi.nlm.nih.gov/sites/entrez?db=gap). Data download and usage was authorized by SHARe data access committee (phs000007.v3.p2, phs000078.v3.p2). We have the data on 2,953 phenotyped Caucasian subjects, 448 from the Original cohort (160 men and 288 women) and 2,505 from the Offspring cohort (1,114 men and 1,391 women). The Original Cohort participants had BMD measures by dual x-ray absorptiometry machine (Lunar DPX-L) at the hip performed at exam 24. The Offspring Cohort participants were scanned with the same machine at exam 6/7. As reported before [24], the CV was 1.7% for femoral neck.
Genotyping and Quality Control
Genomic DNA was extracted from peripheral blood leukocytes using standard protocols. The genome-wide scan was performed using the Affymetrix Human Mapping 500K array set (Affymetrix, Santa Clara, CA, USA) according to the Affymetrix protocol. Data management and analyses were conducted using the Affymetrix GeneChip Operating System. Genotyping calls were determined from the fluorescent intensities using the DM algorithm with a 0.33 P-value setting [25] as well as the B-RLMM algorithm [26].
Quality control procedures were as follows. First, only samples with a minimum call rate of 95% were included. Due to efforts of repeat experiments, all samples (n = 700) met this criteria and the final mean BRLMM call rate reached a high level of 99.02%. Second, out of the initial full-set of 500,568 SNPs, we discarded: 1) SNPs with a call rate <90% in the total sample (n = 54,845); 2) those deviating from Hardy-Weinberg equilibrium (HWE) in controls (P<0.001, n = 22,002); 3) those having a minor allele frequency (MAF)<0.05 in the total sample (n = 142,188). Therefore, 281,533 SNPs were available for subsequent analyses.
Based on the initial GWAS results, we selected the 10 most promising SNPs for subsequent genotyping in the Chinese hip OF replication sample based on the following inclusion criteria: (i) P values≤5×10−6 in the GWAS allelic association analyses (5 SNPs); (ii) P values between 5×10−6 and 5×10−5, with neighboring SNPs having P values≤10−4 showing a consistent trend of association (5 SNPs). Genotypes were obtained using MALDI-TOF mass spectrometry on a Sequenom system (Sequenom, Inc., San Diego, CA) with iPLEX assay [27]. Primers were designed using MassARRAY Assay Design 3.1 software. Genotyping quality control procedures leading to SNP exclusion were call rate <90%, MAF<0.05 in the total sample and P<0.001 for deviations from HWE in controls. The average call rate was 98.7% for the Sequenom system and the corresponding consistency of genotyping (replication or concordance rates), as obtained by duplication samples, was 99.8%. Nine of the ten genotyped SNPs were qualified for subsequent association analyses.
The Chinese BMD sample was also genotyped using the Sequenom system, which was the same as that used for the OF replication sample. Genotyping of the two US-MidWest samples was performed by a service company KBioscience (Herts, UK) using a technology of competitive allele specific PCR (KASPar), which is detailed at the website (www.kbioscience.co.uk). The US-Framingham sample was genotyped using approximately 550,000 SNPs (Affymetrix 500K mapping array plus Affymetrix 50K supplemental array).
Statistical Analyses
For the GWAS analyses for OF risk, single-marker allelic association analysis was performed by comparing SNP allele counts among cases and controls with a χ2 test. ORs with the corresponding 95% CIs were also computed. For the interesting SNPs identified by allelic tests, we also used a multivariate logistic regression model to examine associations with OF risk, taking into account potential covariates such as age, sex, height, and weight. For the sex chromosome analyses, the affymetrix platform does not assay the Y chromosome. The X chromosome needs to be treated differently from the autosomes since males have only one copy of the X chromosome. As most loci on the X chromosome are subject to X chromosome inactivation, it is reasonable to treat males as if they were homozygous females, and then the assumption was the same as tests for autosomes. All association statistical analyses were carried out using HelixTree 5.3.1 software (Golden Helix, Bozeman, MT, USA). We adjusted for multiple testing by adopting the conservative Bonferroni correction. The genome-wide significance threshold was set at a P value of less than 1.78×10−7 (0.05/281,533 SNPs that passed our quality control check).
To correct for potential population stratification that may lead to spurious association results, we estimated the inflation factor (λ) for the GWAS sample using a method of genomic control [28]. λ was calculated as the median of the observed χ2 statistics divided by the median of the expected χ2 statistics for the genome-wide SNP set. This led to an λ of 1.02. Results presented in this study were based on adjusting χ2 statistics by dividing each of them by 1.02. The data were also analyzed by the principal component analyses implemented in EIGENSTRAT [29] for cross-checking the association results while controlling for admixture.
For OF replication analyses, the same allelic association analysis was performed by χ2 tests. For BMD validation analyses, significant parameters (P<0.05) such as age, sex, height and weight were used as covariates to adjust for the raw BMD values. For the unrelated samples, ANOVA was conducted to achieve the association tests. ANOVA is a model free test and more robust than assuming any genetic models. The independent variable was the genotype, which was divided into three levels corresponding to the three genotypes observed for each SNP (1,1; 1,2; 2,2). Since ANOVA can't give the effect size, we estimated the effect size of significant SNPs using the linear regression assuming the additive model in SAS (SAS Institute Inc., Cary, NC). For the related samples, we used the BMD residuals after adjustment to conduct family-based association tests under an additive model using FBAT program [30]. FBAT is a powerful approach to handle family sample, which tests for differences in probability of transmission of a genotype from parents to offspring based on phenotype. FBAT examines association within families, which is not affected by population stratification bias. To quantify the overall evidence of associations, we performed meta-analyses by using the Mantel-Haenszel method to calculate the P values and OR for the combined OF samples. For the BMD samples, we used a weighted Z-score method to calculate the combined P values. The individual Z-score (a standard normal deviate, the statistic associated with a P value) was weighted by the square root of the sample size of each sample. We added the individual weighted Z-score together and divided by the square root of the total sample size to obtain a combined Z-score and an associated combined P value (Stouffer method) [31]. If an individual result was nonsignificant and gave no other useful data for calculation of a Z-score, we set it as 0 to calculate the combined probability.
For the interested genomic regions, IMPUTE program [32] was utilized to impute the genotypes of all SNPs located in the regions based on Asian HapMap data. SNPTEST [32] was used to test for associations between the imputed SNPs and OF. SNAP was used to depict the regional association plot [33].
Zdroje
1. CooperC
CampionG
MeltonLJ3rd
1992 Hip fractures in the elderly: a world-wide projection. Osteoporos Int 2 285 289
2. DengHW
MahaneyMC
WilliamsJT
LiJ
ConwayT
2002 Relevance of the genes for bone mass variation to susceptibility to osteoporotic fractures and its implications to gene search for complex human diseases. Genet Epidemiol 22 12 25
3. LiuYJ
ShenH
XiaoP
XiongDH
LiLH
2006 Molecular genetic studies of gene identification for osteoporosis: a 2004 update. J Bone Miner Res 21 1511 1535
4. MichaelssonK
MelhusH
FermH
AhlbomA
PedersenNL
2005 Genetic liability to fractures in the elderly. Arch Intern Med 165 1825 1830
5. YangTL
ChenXD
GuoY
LeiSF
WangJT
2008 Genome-wide copy-number-variation study identified a susceptibility gene, UGT2B17, for osteoporosis. Am J Hum Genet 83 663 674
6. AmmannP
RizzoliR
2003 Bone strength and its determinants. Osteoporos Int 14 Suppl 3 S13 18
7. MarshallD
JohnellO
WedelH
1996 Meta-analysis of how well measures of bone mineral density predict occurrence of osteoporotic fractures. BMJ 312 1254 1259
8. HazenbergJG
TaylorD
LeeTC
2007 The role of osteocytes and bone microstructure in preventing osteoporotic fractures. Osteoporos Int 18 1 8
9. CarringtonJL
2005 Aging bone and cartilage: cross-cutting issues. Biochem Biophys Res Commun 328 700 708
10. IoannidisJP
RalstonSH
BennettST
BrandiML
GrinbergD
2004 Differential genetic effects of ESR1 gene polymorphisms on osteoporosis outcomes. JAMA 292 2105 2114
11. RalstonSH
UitterlindenAG
BrandiML
BalcellsS
LangdahlBL
2006 Large-scale evidence for the effect of the COLIA1 Sp1 polymorphism on osteoporosis outcomes: the GENOMOS study. PLoS Med 3 e90 doi:10.1371/journal.pmed.0030090
12. RichardsJB
RivadeneiraF
InouyeM
PastinenTM
SoranzoN
2008 Bone mineral density, osteoporosis, and osteoporotic fractures: a genome-wide association study. Lancet 371 1505 1512
13. StyrkarsdottirU
HalldorssonBV
GretarsdottirS
GudbjartssonDF
WaltersGB
2008 Multiple genetic loci for bone mineral density and fractures. N Engl J Med 358 2355 2365
14. UitterlindenAG
RalstonSH
BrandiML
CareyAH
GrinbergD
2006 The association between common vitamin D receptor gene variations and osteoporosis: a participant-level meta-analysis. Ann Intern Med 145 255 264
15. van MeursJB
TrikalinosTA
RalstonSH
BalcellsS
BrandiML
2008 Large-scale analysis of association between LRP5 and LRP6 variants and osteoporosis. JAMA 299 1277 1290
16. StyrkarsdottirU
HalldorssonBV
GretarsdottirS
GudbjartssonDF
WaltersGB
2009 New sequence variants associated with bone mineral density. Nat Genet 41 15 17
17. XiongDH
LiuXG
GuoYF
TanLJ
WangL
2009 Genome-wide association and follow-up replication studies identified ADAMTS18 and TGFBR3 as bone mass candidate genes in different ethnic groups. Am J Hum Genet 84 388 398
18. DengHW
LiJL
LiJ
DaviesKM
ReckerRR
1998 Heterogeneity of bone mineral density across skeletal sites and its clinical implications. J Clin Densitom 1 339 353
19. GiulianiN
GirasoleG
VescoviPP
PasseriG
PedrazzoniM
1999 Ethanol and acetaldehyde inhibit the formation of early osteoblast progenitors in murine and human bone marrow cultures. Alcohol Clin Exp Res 23 381 385
20. YamaguchiJ
HasegawaY
KawasakiM
MasuiT
KanohT
2006 ALDH2 polymorphisms and bone mineral density in an elderly Japanese population. Osteoporos Int 17 908 913
21. CooperRS
TayoB
ZhuX
2008 Genome-wide association studies: implications for multiethnic samples. Hum Mol Genet 17 R151 155
22. RossPD
1998 Risk factors for osteoporotic fracture. Endocrinol Metab Clin North Am 27 289 301
23. CupplesLA
ArrudaHT
BenjaminEJ
D'AgostinoRBSr
DemissieS
2007 The Framingham Heart Study 100K SNP genome-wide association study resource: overview of 17 phenotype working group reports. BMC Med Genet 8 Suppl 1 S1
24. HannanMT
FelsonDT
AndersonJJ
1992 Bone mineral density in elderly men and women: results from the Framingham osteoporosis study. J Bone Miner Res 7 547 553
25. DiX
MatsuzakiH
WebsterTA
HubbellE
LiuG
2005 Dynamic model based algorithms for screening and genotyping over 100 K SNPs on oligonucleotide microarrays. Bioinformatics 21 1958 1963
26. RabbeeN
SpeedTP
2006 A genotype calling algorithm for affymetrix SNP arrays. Bioinformatics 22 7 12
27. BraunA
RothR
McGinnissMJ
2003 Technology challenges in screening single gene disorders. Eur J Pediatr 162 Suppl 1 S13 16
28. DevlinB
RoederK
1999 Genomic control for association studies. Biometrics 55 997 1004
29. PriceAL
PattersonNJ
PlengeRM
WeinblattME
ShadickNA
2006 Principal components analysis corrects for stratification in genome-wide association studies. Nat Genet 38 904 909
30. LangeC
DeMeoDL
LairdNM
2002 Power and design considerations for a general class of family-based association tests: quantitative traits. Am J Hum Genet 71 1330 1341
31. RosenthalR
1991 Meta-analytic procedures for social research Calif Sage
32. MarchiniJ
HowieB
MyersS
McVeanG
DonnellyP
2007 A new multipoint method for genome-wide association studies by imputation of genotypes. Nat Genet 39 906 913
33. JohnsonAD
HandsakerRE
PulitSL
NizzariMM
O'DonnellCJ
2008 SNAP: a web-based tool for identification and annotation of proxy SNPs using HapMap. Bioinformatics 24 2938 2939
Štítky
Genetika Reprodukční medicína
Článek Activation of Mutant Enzyme Function by Proteasome Inhibitors and Treatments that Induce Hsp70Článek Maternal Ethanol Consumption Alters the Epigenotype and the Phenotype of Offspring in a Mouse ModelČlánek Genetic Dissection of Differential Signaling Threshold Requirements for the Wnt/β-Catenin PathwayČlánek Distinct Type of Transmission Barrier Revealed by Study of Multiple Prion Determinants of Rnq1
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2010 Číslo 1- Akutní intermitentní porfyrie
- Farmakogenetické testování pomáhá předcházet nežádoucím efektům léčiv
- Růst a vývoj dětí narozených pomocí IVF
- Pilotní studie: stres a úzkost v průběhu IVF cyklu
- Vliv melatoninu a cirkadiálního rytmu na ženskou reprodukci
-
Všechny články tohoto čísla
- Irradiation-Induced Genome Fragmentation Triggers Transposition of a Single Resident Insertion Sequence
- A Major Role of the RecFOR Pathway in DNA Double-Strand-Break Repair through ESDSA in
- Kidney Development in the Absence of and Requires
- Modeling of Environmental Effects in Genome-Wide Association Studies Identifies and as Novel Loci Influencing Serum Cholesterol Levels
- Inverse Correlation between Promoter Strength and Excision Activity in Class 1 Integrons
- Activation of Mutant Enzyme Function by Proteasome Inhibitors and Treatments that Induce Hsp70
- Postnatal Survival of Mice with Maternal Duplication of Distal Chromosome 7 Induced by a / Imprinting Control Region Lacking Insulator Function
- The Werner Syndrome Protein Functions Upstream of ATR and ATM in Response to DNA Replication Inhibition and Double-Strand DNA Breaks
- Maternal Ethanol Consumption Alters the Epigenotype and the Phenotype of Offspring in a Mouse Model
- Understanding Gene Sequence Variation in the Context of Transcription Regulation in Yeast
- miR-30 Regulates Mitochondrial Fission through Targeting p53 and the Dynamin-Related Protein-1 Pathway
- Elevated Levels of the Polo Kinase Cdc5 Override the Mec1/ATR Checkpoint in Budding Yeast by Acting at Different Steps of the Signaling Pathway
- Alternative Epigenetic Chromatin States of Polycomb Target Genes
- Co-Orientation of Replication and Transcription Preserves Genome Integrity
- A Comprehensive Map of Insulator Elements for the Genome
- Environmental and Genetic Determinants of Colony Morphology in Yeast
- U87MG Decoded: The Genomic Sequence of a Cytogenetically Aberrant Human Cancer Cell Line
- The MCM-Binding Protein ETG1 Aids Sister Chromatid Cohesion Required for Postreplicative Homologous Recombination Repair
- Genetic Dissection of Differential Signaling Threshold Requirements for the Wnt/β-Catenin Pathway
- Differential Localization and Independent Acquisition of the H3K9me2 and H3K9me3 Chromatin Modifications in the Adult Germ Line
- Genetic Crossovers Are Predicted Accurately by the Computed Human Recombination Map
- Collaborative Action of Brca1 and CtIP in Elimination of Covalent Modifications from Double-Strand Breaks to Facilitate Subsequent Break Repair
- Distinct Type of Transmission Barrier Revealed by Study of Multiple Prion Determinants of Rnq1
- Genome-Wide Association Study Identifies as a Novel Susceptibility Gene for Osteoporosis
- and Regulate Reproductive Habit in Rice
- Nonsense-Mediated Decay Enables Intron Gain in
- Altered Gene Expression and DNA Damage in Peripheral Blood Cells from Friedreich's Ataxia Patients: Cellular Model of Pathology
- The Systemic Imprint of Growth and Its Uses in Ecological (Meta)Genomics
- The Gift of Observation: An Interview with Mary Lyon
- Genotype and Gene Expression Associations with Immune Function in
- The Elongator Complex Regulates Neuronal α-tubulin Acetylation
- Rising from the Ashes: DNA Repair in
- Mis-Spliced Transcripts of Nicotinic Acetylcholine Receptor α6 Are Associated with Field Evolved Spinosad Resistance in (L.)
- BRIT1/MCPH1 Is Essential for Mitotic and Meiotic Recombination DNA Repair and Maintaining Genomic Stability in Mice
- Non-Coding Changes Cause Sex-Specific Wing Size Differences between Closely Related Species of
- Evidence for Pervasive Adaptive Protein Evolution in Wild Mice
- Evolutionary Mirages: Selection on Binding Site Composition Creates the Illusion of Conserved Grammars in Enhancers
- VEZF1 Elements Mediate Protection from DNA Methylation
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- A Major Role of the RecFOR Pathway in DNA Double-Strand-Break Repair through ESDSA in
- Kidney Development in the Absence of and Requires
- The Werner Syndrome Protein Functions Upstream of ATR and ATM in Response to DNA Replication Inhibition and Double-Strand DNA Breaks
- Alternative Epigenetic Chromatin States of Polycomb Target Genes
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



