-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaThe Therapeutic Effects of Specific CDK4/6-inhibitors in Treating HR-positive, HER2-negative Advanced Breast Cancer
Authors: Mendoza Luis
Authors place of work: IQVIA Solutions a. s.
Published in the journal: Klin Onkol 2018; 31(4): 305-308
Category: krátké sdělení
doi: https://doi.org/10.14735/amko2018305Summary
The authors declare they have no potential confl icts of interest concerning drugs, products, or services used in the study.
The Editorial Board declares that the manuscript met the ICMJE recommendation for biomedical papers.
Introduction
The current oncology practice recommends that all newly diagnosed breast cancers (BC) be evaluated for PR (progestogen receptor) and OR (oestrogen receptor) protein expression by immunohistochemistry [1]. Approximately 70% of patients with metastatic BC have hormone receptor positive (HR+) disease and are commonly treated with hormone-based therapies that include a non-steroidal aromatase inhibitor (NSAI) such as letrozole, anastrozole, or exemestane [2,3]. However, some patients have intrinsic resistance or acquired tolerance to hormone or endocrine therapy, which hampers their survival prolongation [4]. Thus, novel and effective therapies are urgently required for the BC population treated with endocrine therapy.
Cancer derives from uncontrolled cell division, which results from dysregulation of the cell cycle progression, including four stages, G1 (Gap phase 1), S phase (DNA synthesis), G2 (Gap phase 2), and M phase (mitosis). Cyclin D1 is a major transcriptional target of the ER. The cyclin D1-CDK4-RB pathway regulates cell proliferation by controlling the G1 to S cell cycle checkpoint [5,6]. The OR factor (E2F) family of transcription factors are downstream targets of the RB protein, which function in the cell cycle control and contribute to tumour development [7]. The combination of RB and E2F causes suppression of E2F transcription modules through inducing recruitment of the chromatin remodelling proteins, histone modifiers, and repressive chromatin marks, resulting in cell cycle block [8]. Therefore, the essential roles of CDK4/6 in the cell cycle regulation make them effective targets for cancer therapeutic intervention, especially in BC [9–11]. Fig. 1 shows details of the mechanism of action of the CDK4/6-inhibitors.
Fig. 1. Mechanism of action of CDK4/6 inhibitors. 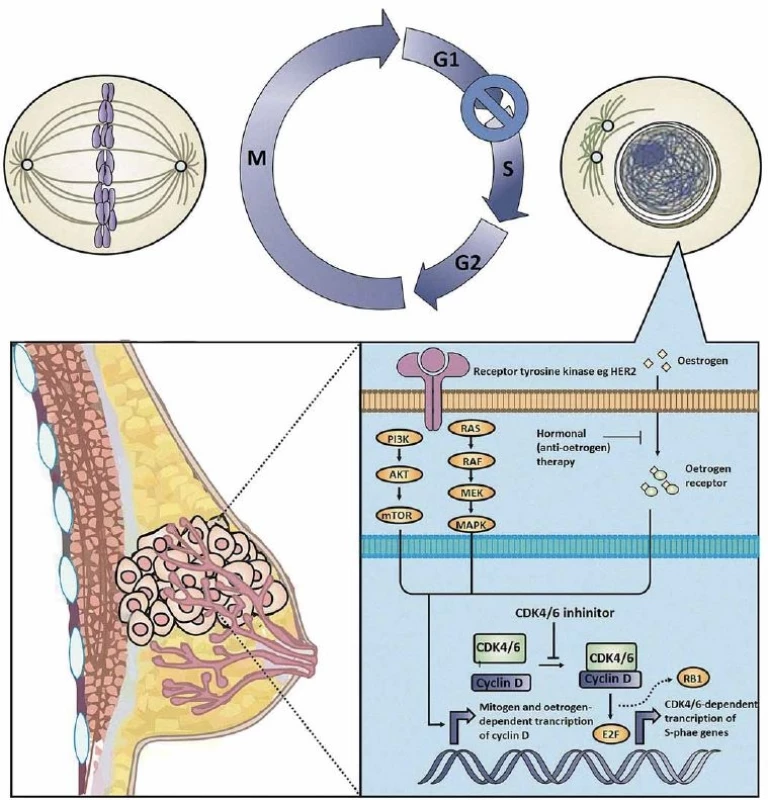
In brief, the CDK4/6-cyclin D axis is hyperactive in hormone receptor-positive breast cancer (BC) because estrogen receptor signaling induce the expression of cyclin D1. Selective CDK4/6 inhibitors prevent the assembly of the CDK4/6-cyclin D1 complex, which normally functions to phosphorylate the retinoblastoma (RB) protein in order to liberate transcription factor E2F from the inactive RB-E2F complex. Administration of anti-CDK4/6 agents therefore suppresses the bioavailability of the E2F transcription factor, resulting in decreased transcription and translation of S-phase-related gene sets, causing BC cells to undergo G1 arrest and/or death. The picture was a courtesy of Dr. Nicholas Syn from the National University of Singapore with small modifi cations. Clinical Trials
Palbociclib (Ibrance®, Pfizer), the most advanced specific CD4/6-inhibitor, was approved by the US FDA (United States Food and Drug Administration) based on the randomised phase II PALOMA-1 study, where palbociclib in combination with letrozole in the 1st line setting substantially improved median progression-free survival (mPSF) and yielded an absolute 10-month benefit [12]. In order to further confirm and extend the efficacy and safety data of PALOMA-1, the double-blind phase III PALOMA-2 study enrolled 666 postmenopausal patients with ER+, HER2 – advanced breast cancer (ABC) who had not received any treatment for this deadly disease [13]. A total of 444 patients were randomly assigned to receive letrozole (2.5 mg/d oral) + palbociclib (125 mg/d oral in 4-week cycles, 3 weeks of treatment followed by 1 week off), and the other patients received letrozole + placebo. The overall response rate (ORR) was 42.1% in the letrozole + palbociclib arm and 34.7% in the letrozole + placebo arm. In patients with measurable disease, the ORR was 55.3% and 44.4%, resp. Evaluation of the primary endpoint, mPFS, indicated that palbociclib in combination with letrozole dramatically prolonged mPFS (24.8 months) in comparison with letrozole + placebo (14.5 months) (HR 0.58; 95% CI 0.46–0.72; p < 0.001) [13]. In addition to the combination of palbociclib and letrozole, the efficacy of fulvestrant + palbociclib was also studied in HR+, HER2 – ABC that progressed in the process of prior hormone therapy [14]. In the PALOMA-3 study, 347 patients received palbociclib (with the same dosage scheme as PALOMA-2) + fulvestrant (500 mg intramuscularly on day 1 and 15, and on day 1 of subsequent cycles every 28 days) and 174 patients received fulvestrant + placebo. The ORR was 19% in the fulvestrant + palbociclib arm and 9% in the fulvestrant + placebo arm. In patients with measurable disease, fulvestrant + palbociclib achieved an ORR of 25 % compared with 11% in the control arm (Tab. 1). There was a clinically meaningful and statistically significant improvement in mPFS in patients receiving palbociclib + fulvestrant (9.5 months) in comparison with the placebo group (4.6 months) (HR 0.46; 95% CI 0.36–0.59; p < 0.001). The most common grade 3 or 4 adverse events observed in palbociclib-treated arms of the PALOMA-2 and -3 studies were neutropenia, leukopenia, and fatigue. The myelotoxicity effect > grade 3 or higher was observed in > 50% of the palbociclib-treated patients, but the rate of febrile neutropenia was low. The myelotoxicity effects were successfully managed with appropriate supportive care and dose reductions.
Tab. 1. Outcomes of treatment with CD4/6 inhibitors in HR+, HER2– advanced or metastatic breast cancer patients. 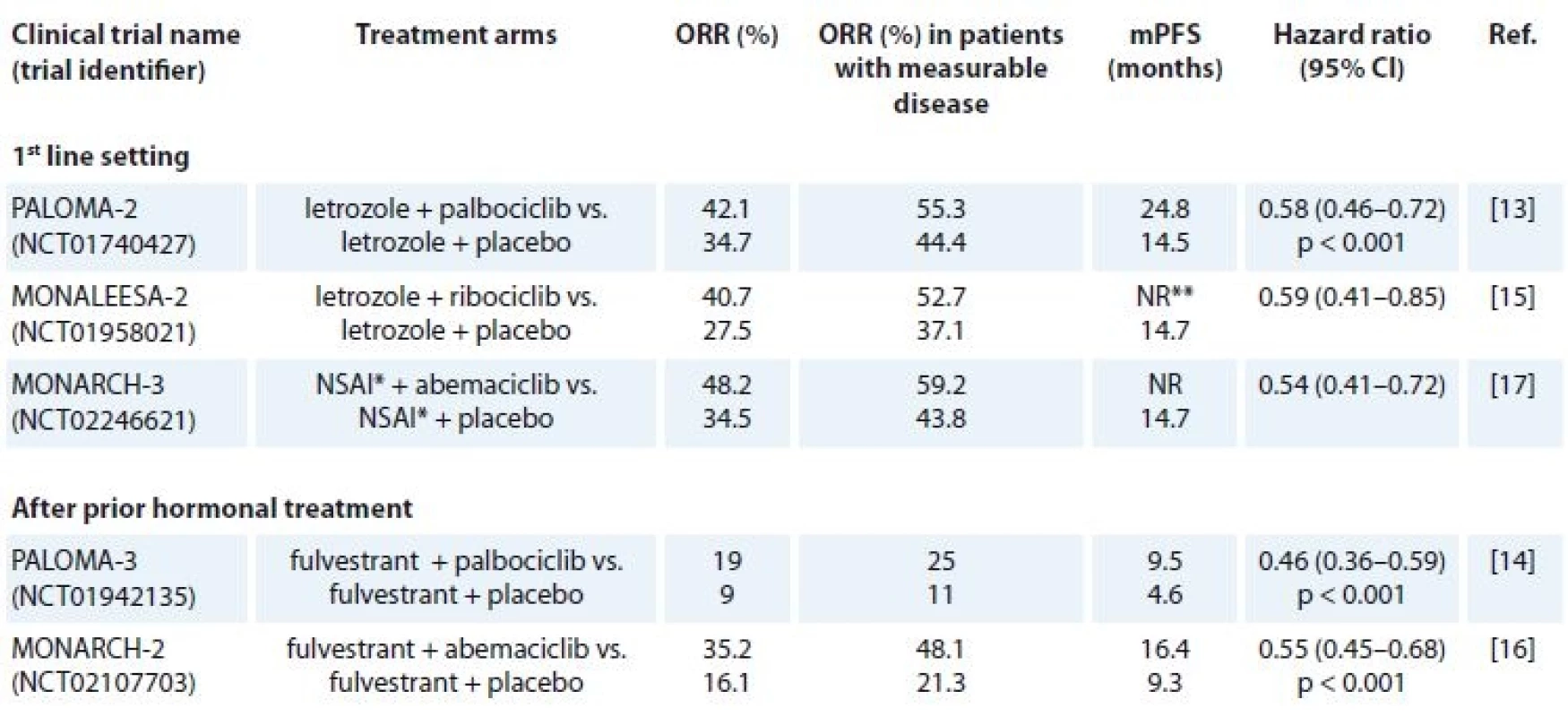
HR – hormonal receptor, HER2 – human epidermal growth factor receptor 2, NSAI – non-steroidal aromatase inhibitor, ORR – overall response rate, mPFS – median progression-free survival, NR – not reached
*letrozole or anastrozole
** after 24 months, the mPFS was 25.3 months for the ribociclib arm (updated data)Next to become FDA-approved was ribociclib (Kisqali®, Novartis), which when combined with letrozole in the MONALEESA-2 trial met its primary endpoint – after 18 months, mPFS was not reached in the ribociclib (600 mg/d oral in 4-week cycles, 3 weeks of treatment followed by 1 week off) arm vs. 14.7 months in the letrozole (2.5 mg/d oral) + placebo arm [15]. In this randomised, placebo-controlled, phase III trial, 668 postmenopausal (334 assigned to receive ribociclib +letrozole and 334 assigned to receive placebo + letrozole) women with HR+, HER2 – ABC who had not received previous systemic therapy for advanced disease were eligible. The data were updated, and after 24 months, the mPFS for the ribociclib arm showed 25.3 months. The mPFS of the placebo arm remained the same. The ORR was 40.7% in the letrozole + ribociclib arm and 27.5% in the letrozole + placebo arm. The ORR for patients with measurable disease was 52.7% vs. 37.1%, resp. [15] (Tab. 1). The most common grade 3 or 4 adverse events were neutropenia (59.3%), leukopenia (21%), hypertension (9.9%), lymphopenia (6.9%), and increase of liver enzymes in < 10% of the patients treated in the ribociclib arm. A few cases of febrile neutropenia were also reported.
A third selective CDK4/6 inhibitor is abemaciclib (Verzenio®, Eli Lilly and Company), which was granted a breakthrough designation after the positive results from the MONARCH-2 study in HR+, HER2 – ABC who had progressed while receiving neoadjuvant or adjuvant endocrine therapy [16]. The MONARCH-2 study enrolled 669 patients of whom 446 were randomly assigned to receive abemaciclib (150 mg 2× daily) + fulvestrant (500 mg intramuscularly on day 1 and 15, and on day 1 of subsequent cycles every 28 days) and 223 placebo + fulvestrant. The ORR was 35.2% in the abemaciclib arm and 16.1% in the control arm. Patients with measurable disease achieved an ORR of 48.1% in the abemiciclib arm and 21.3% in the control arm. Abemaciclib + fulvestrant significantly extended the mPFS vs. fulvestrant alone (16.4 vs. 9.3 months; HR 0.553; 95% CI 0.4449–0.681; p < 0.001) (Tab. 1). In September 2017, the US FDA approved abemaciclib based on the results of the phase III MONARCH-3 trial, which was a double-blind, randomised study of abemaciclib or placebo plus a NSAI in 493 postmenopausal women with HR+, HER2 – ABC who had no prior systemic therapy [17]. Patients were randomly assigned 2 : 1 to receive abemaciclib or placebo (150 mg 2× daily orally) or matching placebo plus a NSAI (either 1 mg anastrozole or 2.5 mg letrozole). The ORR was 59.2% in the abemaciclib + NSAI arm and 43.8% in the NSAI + placebo arm. Patients with measurable disease achieved an ORR of 59.2% in the abemiciclib arm and 43.8% in the control arm. The mPFS for the abemiciclib arm was not reached compared with 14.7 months in the placebo arm (0.54; 95% CI 0.41–0.72; p = 0.00021) (Tab. 1). In both MONARCH studies, the percentages of grade 3 or 4myelotoxicity events were lower than those observed in the palbociclib and ribociclib studies.
It is worth noting that individual trials have not been powered to detect a difference in the overall survival, and the follow-up data for survival of the trials are currently immature to conclude the real benefit for these patients in this aspect.
Conclusions
From the results presented above, it can be concluded that the addition of the three selective approved CDK4/6-inhibitors in CDK4/6-containing regimens enhances ORR and mPFS in the 1st line or relapsed setting for HR+, HER2 – ABC patients. For this reason, the National Comprehensive Cancer Network (NCCN) added the following recommendations in the NCCN guidelines version 4 (2017) for BC – the use of CDK4/6-containing regimens in the 1st line setting for the treatment of premenopausal and palbociclib or ribociclib for the treatment of postmenopausal HR+, HER2 – ABC patients; and CDK4/6-containing regimens in the 2nd line setting for pre - and postmenopausal HR+, HER2 – ABC patients [18]. It has to be noted that the concomitant use of CDK4/6 inhibitors to standard adjuvant treatments considerably aggravates the toxic effects of endocrine therapy. Serious haematologic toxicities are inevitably common, consistent with the on-target inhibition of CDKs 4 and 6, which are highly expressed in haematopoietic stem and progenitor cells.
Discussion
Despite the acceptance of anti-CDK4/6-containing regimens as a new therapeutic benchmark, several questions remain unresolved, especially whether the CDK4/6 inhibitor-containing regimens are more efficient compared with cytotoxic chemotherapy or what is the impact on the overall quality of life due to the toxicity. In fact, there is still a lot to know about the safety and efficacy of CDK4/6-containing regimens across various categories defined by the menopausal status, age, HER2 positivity and biomarkers (RB expression, p16 status). Ongoing clinical trials explore the potential of these agents outside the HR+, HER2 – ABC setting, including as adjuvant and neoadjuvant treatments and in combination withchemotherapy and targeted agents. The CDK4/6 inhibitors are being evaluated in women with triple-negative BCs, and since abemaciclib crosses the blood-brain barrier, it is also being evaluated as a treatment for brain me-tastases, including metastatic BC patients. Finally, CDK4/6 inhibition may result in favorable modulation of the immune microenvironment, and thus combination trials with immune checkpoint blockers have also been initiated.
The authors declare they have no potential conflicts of interest concerning drugs, products, or services used in the study.
The Editorial Board declares that the manuscript met the ICMJE recommendation for biomedical papers.
Luis Mendoza, MD, PhD.
IQVIA Solutions a. s. Senior Medical Advisor
Radlicka 714/113 Praha 5 158 00 Czech Republic
e-mail: luis.mendoza@iqvia.com
Submitted: 3. 2. 2018
Accepted: 28. 5. 2018
Zdroje
1. Hammond ME, Hayes DF, Dowsett M et al. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. J Clin Oncol 2010; 28 (16): 2784–2795. doi: 10.1200/JCO.2009.25.6529.
2. Thorpe SM, Rose C, Rasmussen BB et al. Prognostic value of steroid hormone receptors: multivariate analysis of systemically untreated patients with node negative primary breast cancer. Cancer Res 1987; 47 (22): 6126–6133.
3. Nomura Y, Miura S, Koyama H et al. Relative effect of steroid hormone receptors on the prognosis of patients with operable breast cancer. A univariate and multivariate analysis of 3089 japanese patients with breast cancer from the study group for the japanese breast cancer society on hormone receptors and prognosis in breast cancer. Cancer 1992; 69 (1): 153–164.
4. Milani A, Geuna E, Mittica G et al. Overcoming endocrine resistance in metastatic breast cancer. Current evidence and future directions. World J Clin Oncol 2014; 5 (5): 990–1001. doi: 10.5306/wjco.v5.i5.990.
5. Sherr CJ. Cancer cell cycles. Science 1996; 274 (5293): 1672–1677.
6. Shapiro GI. Cyclin-dependent kinase pathway as targets for cancer treatment. J Clin Oncol 2006; 24 (11): 1770–1783. doi: 10.1200/JCO.2005.03.7689.
7. Chen HZ, Tsai SY, Leone G. Emerging roles of E2Fs in cancer: an exit from cell cycle control. Nat Rev Cancer 2009; 9 (11): 785–797. doi: 10.1038/nrc2696.
8. Johnson J, Thijssen B, McDermott U et al. Targeting the RB-E2F pathway in breast cancer. Oncogene 2016; 35 (37): 4829–4835. doi: 10.1038/onc.2016.32.
9. Asghar U, Witkiewicz AK, Turner NC et al. The history and future of targeting cyclin-dependent kinases in cancer therapy. Nat Rev Drug Discov 2015; 14 (2): 130–146. doi: 10.1038/nrd4504.
10. Shah AN, Cristofanilli M. The growing role of CDK4/6 inhibitors in treating hormone receptor-positive advanced breast cancer. Curr Treat Options Oncol 2017; 18 (1): 6. doi: 10.1007/s11864-017-0443-7.
11. Sablin MP, Ricci F, Loirat D et al. Cell cycle inhibitors in endocrine receptor positive breast cancer. Bull Cancer 2017; 104 (2): 114–122. doi: 10.1016/j.bulcan.2016.12.005.
12. Finn RS, Crown JP, Lang I et al. The cyclin-dependent kinase 4/6 inhibitor palbociclib in combination with letrozole versus letrozole alone as first-line treatment of oestrogen receptor-positive, HER2-negative, advanced breast cancer (PALOMA-1/TRIO-18): a randomised phase 2 study. Lancet Oncol 2015; 16 (1): 25–35. doi: 10.1016/S1470-2045 (14) 71159-3.
13. Finn RS, Martin M, Rugo HS et al. Palbociclib and letrozole in advanced breast cancer. N Engl J Med 2016; 375 (20): 1925–1936. doi: 10.1056/NEJMoa1607303.
14. Cristofanilli M, Turner NC, Bondarenko I et al. Fulvestrant plus palbociclib versus fulvestrant plus placebo for treatment of hormone-receptor-positive, HER2-negative metastatic breast cancer that progressed on previous endocrine therapy (PALOMA-3): final analysis of the multicentre, double-blind, phase 3 randomised controlled trial. Lancet Oncol 2016; 17 (4): 425–439. doi: 10.1016/S1470-2045 (15) 00613-0.
15. Hortobagyi GN, Stemmer SM, Burris HA et al. Ribociclib as first-line therapy for HR-positive, advanced breast cancer. N Engl J Med 2016; 375 (18): 1738–1748. doi: 10.1056/NEJMoa1609709.
16. Sledge GW, Toi M, Neven P et al. MONARCH 2: abemaciclib in combination with fulvestrant in women with HR+/HER2-advanced breast cancer who had progressed while receiving endocrine therapy. J Clin Oncol 2017; 35 (25): 2875–2884. doi: 10.1200/JCO.2017.73.7585.
17. Goetz MP, Toi M, Campone M et al: MONARCH 3: abemaciclib as initial therapy for advanced breast cancer. J Clin Oncol 2017; 35 : 3628–3646. doi: 10.1200/JCO.2017.75.6155.
18. NCCN. [online]. Available from: https: //www.nccn.org.
Štítky
Dětská onkologie Chirurgie všeobecná Onkologie
Článek Precizovaná onkologie
Článek vyšel v časopiseKlinická onkologie
Nejčtenější tento týden
2018 Číslo 4- Metamizol jako analgetikum první volby: kdy, pro koho, jak a proč?
- Nejasný stín na plicích – kazuistika
- Nejlepší kůže je zdravá kůže: 3 úrovně ochrany v moderní péči o stomii
- Hojení análních fisur urychlí čípky a gel
-
Všechny články tohoto čísla
- Precizovaná onkologie
- Use of Salivary MicroRNAs for Diagnosis of Solid Cancers
- Expression Analysis of OIP5-AS1 in Non-Small Cell Lung Cancer
- A Combined Bioinformatics and Literature Based Approach for Identification of Long Non-coding RNAs That Modulate Vitamin D Receptor Signaling in Breast Cancer
- Monoclonal Gammopathy of Undetermined Significance (MGUS)Monoclonal Gammopathy of Undetermined Significance (MGUS)
- Carcinoma of Unknown Primary in Head and Neck Region
- Occurrence and Antibiotic Resistance of Enterobacteriaceae in Acute Leukemia Patients
- Malignant Melanomas of the Skin Arising on the Feet
- Metastases of a Breast Cancer to Skull Base
- Primary Branchiogenic Carcinoma
- Use of Fludarabine for the Treatment of Indolent Lymphoma with Chylothorax
- The Therapeutic Effects of Specific CDK4/6-inhibitors in Treating HR-positive, HER2-negative Advanced Breast Cancer
- Nové techniky IGRT – sledování povrchu těla pacienta (SIGRT)
- Klinická onkologie
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Monoclonal Gammopathy of Undetermined Significance (MGUS)Monoclonal Gammopathy of Undetermined Significance (MGUS)
- Carcinoma of Unknown Primary in Head and Neck Region
- Occurrence and Antibiotic Resistance of Enterobacteriaceae in Acute Leukemia Patients
- Use of Salivary MicroRNAs for Diagnosis of Solid Cancers
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



